Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02697673 2014-07-31
78233-39
DESCRIPTION
BARLEY SYRUP PRODUCTION METHOD
Technical Field
[0001] The present invention relates to a method for producing barley
syrup.
Background Art
[0002] Syrup (rice jelly) obtained from grains such as barley as the
main raw material is a type of natural food with a good balance between
sweetness and flavor, and it is utilized as a raw material in additives
such as mirin, processed foods such as fermented foods and beverages
such as alcoholic beverages (for example, low-malt beer).
[0003] Examples of such syrups include barley syrup which contains
high concentrations of amino acids (see Patent document 1). Known
methods for producing syrup include the method described in Patent=
document 1 which comprises a liquefying step, a saccharification step
and a proteolysis step, and the method described in Patent document 2
which comprises a liquefying step and a saccharification step.
[Patent document 1] Japanese Unexamined Patent Publication No.
2006-262839
[Patent document 2] Japanese Unexamined Patent Publication No.
2005-323556
Disclosure of the Invention
[0004] Conventional methods for producing syrup such as those
described in Patent documents 1 and 2, however, yield syrup which has
high viscosity and is difficult to manage.
1
CA 02697673 2014-07-31
78233-39
[0005] The present invention has been accomplished in light of these
circumstances, and its object is to provide a method for producing
barley syrup with sufficiently low viscosity.
[0006] The invention provides a method for producing barley syrup
comprising decomposing barley or its milled product at a temperature of
20 C or higher and 80 C or lower in the presence of an a-amylase.
Throughout the present specification, "barley syrup" refers to syrup
=
obtained using barley as the starting material.
[0007] During the production of barley syrup, carbohydrates such as
starches from the barley starting material are decomposed by enzymes
such as an a-amylase to low molecular saccharides. Substances such
as P-glucans are extracted in addition to starches and low molecular
saccharides during the course of barley syrup production, and become
included in the barley syrup. Since the
temperature for the
decomposition of the method for producing barley syrup of the
invention is set to 20 C or higher and 80 C or lower, and an a-amylase
is present, the method of the invention can yield syrup with sufficiently
low viscosity and easy manageability. If the temperature for the
decomposition is lower than 20 C, decomposition of the carbohydrates
such as starches in the barley starting material will be inadequate. A
temperature of higher than 80 C, on the other hand, will increase the
viscosity of the obtained barley syrup and will hamper manageability.
[0008] The temperature for the decomposition is preferably 45 C or
higher and 80 C or lower from the viewpoint of obtaining barley syrup
which has sufficiently low viscosity and is rich infi-glucans.
2
CA 02697673 2010-02-24
FP08-0467-00CA-SB
[0009] From the viewpoint of obtaining barley syrup with especially
low viscosity, however, the temperature for the decomposition is
preferably 20 C or higher and 65 C or lower and most preferably 20 C
or higher and lower than 45 C.
If the temperature for the
decomposition is 65 C or lower and especially lower than 45 C, high
molecularization of P-glucans during production will be inhibited and
the viscosity of the obtained barley syrup will be notably reduced.
[0010] According to one aspect, therefore, the invention provides a
method for producing barley syrup comprising decomposing barley or
its milled product at a temperature of 45 C or higher and 80 C or lower
in the presence of an a-amylase (this mode of the method will
hereinafter be referred to as "production method (A)").
[0011] During the production of barley syrup, carbohydrates such as
starches from the barley starting material are decomposed by enzymes
such as an a-amylase to low molecular saccharides. Substances such
as 13-g1ucans are extracted in addition to starches and low molecular
saccharides during the course of barley syrup production, and become
included in the barley syrup.
Since the temperature for the
decomposition of production method (A) is set to 45 C or higher and
80 C or lower, and an a-amylase is present, production method (A) can
yield barley syrup which has sufficiently low viscosity and is rich in
P-glucans. From the viewpoint of realizing a more suitable balance
between viscosity and P-glucan content for the obtained barley syrup,
the temperature is more preferably 50 C or higher and 70 C or lower
and even more preferably 55 C or higher and 65 C or lower.
Throughout the present specification, "rich in 13-glucans" means that the
3
CA 02697673 2010-02-24
1
FP08-0467-00CA-SB
13-glucan concentration of the barley syrup is at least 0.01 mg/mL.
[0012] As mentioned above, barley syrups containing high
concentrations of amino acids have been known in the prior art (see
Patent document 1), but barley syrups containing high concentrations of
13-glucans have not been known.
[0013] In production method (A), the decomposition is accomplished
preferably in the presence of a 13-amylase. The presence of a
13-amylase allows a 13-amylase activity to be supplemented even when
the 13-amylase in the barley starting material has been inactivated by
heat, thus allowing more efficient production of saccharides.
[0014] In production method (A), the decomposition is accomplished
preferably in the presence of a pullulanase in order to hydrolyze the
a-1,6-glucoside bonds (branched portions) of the starch derived from
the barley starting material and more efficiently produce saccharides.
The presence of a pullulanase can yield more extensively saccharified
barley syrup.
[0015] Also, in production method (A), the decomposition is
accomplished preferably in the presence of a protease. The presence
of a protease can decompose the proteins to yield barley syrup
containing more amino acids.
[0016] The a-amylase, 13-amylase, pullulanase and protease used for the
decomposition preferably contains virtually or absolutely no
components that exhibit a 13-glucan-decomposing activity. Particularly
when a protease is present, it is preferred for the protease to contain
virtually or absolutely no components that exhibit a
13-glucan-decomposing activity. The use of such enzymes can yield
4
CA 02697673 2010-02-24
FP08-0467-00CA-SB
barley syrup containing even more 13-glucans.
[0017] When a f3-amylase is not added for the decomposition, the
production method (A) preferably comprises adding a j3-amylase to a
decomposed product obtained from the decomposition, for further
decomposition of the decomposed product. This will allow a
f3-amylase activity to be supplemented even when the 13-amylase in the
barley starting material has been inactivated by heat in the
decomposition, thus allowing more efficient production of saccharides.
[0018] The 13-amylase for the further decomposition preferably contains
virtually or absolutely no components that exhibit a
P-glucan-decomposing activity. The use of such enzymes can yield
barley syrup containing even more P-glucans.
[0019] According to another aspect, the invention provides a method
for producing barley syrup comprising decomposing barley or its milled
product at a temperature of 20 C or higher and 65 C or lower and
preferably 20 C or higher and less than 45 C, in the presence of an
a-amylase (this mode of the method will hereinafter be referred to as
"production method (B)").
[0020] Since the temperature for the decomposition of production
method (B) is set to 20 C or higher and 65 C or lower, and an
a-amylase is present, production method (B) can inhibit high
molecularization of P-glucans and notably lower the viscosity of the
obtained barley syrup.
[0021] In conventional methods for producing barley syrup, it has been
necessary to add a fresh 13-amylase after the liquefying step because the
3-amylase in the barley starting material is inactivated during the
5
CA 02697673 2010-02-24
FP08-0467-00CA-SB
liquefying step. In production method (B), however, the temperature
for the decomposition is 20 C or higher and 65 C or lower, and
therefore the 13-amylase activity in the barley can be adequately
maintained. It is thus possible to produce saccharides without adding
fresh enzymes such as a 13-amylase, so that barley syrup can be
produced with fewer steps than the prior art. This allows production
cost to be reduced and production efficiency to be increased in
production method (B).
[0022] The decomposition is accomplished preferably in the presence
of a pullulanase in order to more efficiently hydrolyze the
a-1,6-glucoside bonds (branched portions) of the starch and produce
saccharides. Since the temperature for the decomposition is 20 C or
higher and 65 C or lower in production method (B), it is possible to
simultaneously accomplish the decomposition with the a-amylase and
decomposition with the pullulanase. This also allows production cost
to be reduced and production efficiency to be increased in production
method (B).
[0023] The decomposition is accomplished preferably in the presence
of a protease to decompose the protein from the barley starting material
and produce amino acids. Since the temperature for the decomposition
is 20 C or higher and 65 C or lower in production method (B), it is
possible to simultaneously accomplish decomposition with the
a-amylase and decomposition with the protease. This also allows
production cost to be reduced and production efficiency to be increased
in production method (B).
[0024] Also, the decomposition is accomplished preferably in the
6
CA 02697673 2010-02-24
FP08-0467-00CA-SB
presence of a P-glucanase to decompose the P-glucans in the barley or
its milled product and thus further lower the viscosity of the obtained
barley syrup. Since the temperature for the decomposition is 20 C or
higher and 65 C or lower in production method (B), it is possible to
simultaneously accomplish decomposition with the a-amylase and
decomposition with the P-glucanase. This also allows production cost
to be reduced and production efficiency to be increased in production
method (B).
[0025] The present invention further provides foods and beverages
comprising barley syrup obtained by the method of the invention.
Since the barley syrup obtained by the method of the invention has
sufficiently low viscosity, it can be applied for a wide range of different
foods and beverages. In particular, since the barley syrup obtained by
production method (A) is rich in f3-glucans, it can be applied for a wide
range of different functional foods and beverages. The term "foods
and beverages" includes, for example, solid food materials such as
bread, yogurt, cheese, confectioneries and snacks; seasonings such as
mirin, vinegar, miso, soy sauce and butter; and beverages such as soft
drinks, sake, beer, low-malt beer and Japanese spirits.
[0026] The present invention further provides a culture medium
comprising barley syrup obtained by the method of the invention.
Barley syrup obtained by the method of the invention can be used as a
culture medium and particularly a fermentation medium, can be utilized,
for example, for production of new fermented foods and beverages
implementing the functional substances included in barley.
[0027] If the temperature for the decomposition in production method
7
CA 02697673 2016-02-12
. 78233-39
i
(A) is 50 C or higher and 70 C or lower, it will be possible to obtain barley
syrup with a
P-glucan weight-average molecular weight of between 50,000 and 500,000.
[0028] Specifically, the invention further provides:
barley syrup comprising at least 0.01 mg/mL f3-glucans,
obtained by a method comprising decomposing barley or its milled product at a
temperature of 50 C or higher and 70 C or lower in the presence of an a-
amylase,
wherein the weight-average molecular weight of the P-glucans is between
50,000 and 500,000. The method may exclude heating to a temperature higher
than around
70 C in the presence of an a-amylase. The temperature range may also be 65 C
or lower, or
between 55 C and 65 C. The viscosity at 20 C of the barley syrup may be 2000
mPa=s/mg/mL of P-glucan or less.
[0029] The barley syrup of the invention has sufficiently low viscosity (1-20
mPa.$) and is
therefore easy to manage. It is also rich in P-glucans (0.01 mg/mL or more
with respect to the
total syrup), while also being rich in the various amino acids (glycine,
alanine, valine, leucine,
isoleucine, serine, threonine, lysine, arginine, aspartic acid, asparagine,
glutamic acid,
glutamine, methionine, phenylalanine, tyrosine, histidine, GABA, etc.). In
addition, even
with fermentation by yeast, for example, it does not adversely affect the
fermentation process
(extract decrease, floating yeast count, etc.).
[0030] Since the temperature for the decomposition is 50 C or higher and 70 C
or lower and
an a-amylase is present, the method for producing the barley syrup of the
invention can be
used to obtain barley syrup with a satisfactory balance between low viscosity
and high
P-glucan content.
[0031] From the viewpoint of realizing a more suitable balance between
viscosity and
P-glucan content in the obtained barley syrup, the
8
CA 02697673 2010-02-24
FP08-0467-00CA-SB
temperature for decomposing barley or milled product is preferably
55 C or higher and 65 C or lower.
[0032] This method used to obtain the barley syrup is a preferred mode
of production method (A) described above, and the above conditions
apply for production method (A), except that the temperature for the
decomposition is 50 C or higher and 70 C or lower (preferably 55 C or
higher and 65 C or lower).
[0033] If the temperature for the decomposition of production method
(A) is 50 C or higher and 70 C or lower, the obtained barley syrup will
have sufficiently low viscosity (between 1 mPa.s and 20 mPa.$) and will
be easy to manage. It is also rich in (3-glucans (0.01 mg/mL or more
with respect to the total syrup), while also being rich in the various
amino acids (glycine, alanine, valine, leucine, isoleucine, serine,
threonine, lysine, arginine, aspartic acid, asparagine, glutamic acid,
glutamine, methionine, phenylalanine, tyrosine, histidine, GABA, etc.).
In addition, even with fermentation by yeast, for example, it does not
adversely affect the fermentation process (extract decrease, floating
yeast, etc.). Thus, the barley syrup is suitable as a starting material for
effervescent alcoholic beverages, and it can be used to easily obtain
effervescent alcoholic beverages with high functionality, that are rich in
[3-glucans and amino acids.
[0034] Specifically, the invention further provides:
an effervescent alcoholic beverage obtained by a method
comprising fermenting barley syrup with yeast,
wherein the barley syrup is obtained by a method comprising
decomposing barley or its milled product at a temperature of 50 C or
9
CA 02697673 2016-02-12
78233-39
higher and 70 C or lower in the presence of an a-amylase, and comprises at
least 0.01 mg/mL
(3-glucans. The method may exclude heating to a temperature higher than around
70 C in the
presence of an a-amylase. The temperature range may also be 65 C or lower, or
between
55 C and 65 C. The viscosity of the barley syrup at 20 C may be 2000
mPa=s/mg/mL of 13-
glucan or less.
[0035] Since the temperature for the decomposition of the method used to
obtain the barley
syrup of the invention is 50 C or higher and 70 C or lower and an a-amylase is
present, the
method can be used to obtain barley syrup with a satisfactory balance between
low viscosity
and high13-glucan content.
[0036] From the viewpoint of realizing a more suitable balance between
viscosity and
13-glucan content in the obtained barley syrup, the temperature for
decomposing the barley or
milled product of the production method is preferably 55 C or higher and 65 C
or lower.
[0037] This method used to obtain the barley syrup is a preferred mode of
production
method (A) described above, and the above conditions apply for production
method (A),
except that the temperature for the decomposition is 50 C or more and 70 C or
lower
(preferably 55 C or higher and 65 C or lower).
[0038] The weight-average molecular weight of the P-glucans in the barley
syrup obtained by
the method described above will normally be between 50,000 and 500,000.
Effect of the Invention
[0039] According to the invention there are provided a method for producing
barley syrup
with sufficiently low viscosity, as well as foods and beverages and culture
media comprising
barley syrup which is obtained by the method. According to one aspect of the
invention there
are provided a method for producing barley syrup which has sufficiently low
viscosity and is
rich in 13-glucans, as well as foods and
CA 02697673 2010-02-24
,
FP08-0467-00CA-SB
beverages and culture media comprising barley syrup which is obtained
by the method.
Brief Description of the Drawings
[0040] Fig. 1 is a graph showing the f3-glucan concentration of a
13-glucan standard solution after reaction with different proteases.
Fig. 2 is a graph showing particle size distribution of the milled barley
flour.
Fig. 3 is a graph showing the concentrations of various amino acids in
malt liquor (Comparative Example 2) and syrup (Example 13) before
and after boiling.
Fig. 4 is a graph showing the time-dependent change in residual extract
volume of malt liquor (Comparative Example 2) and syrup (Example
13) during fermentation.
Fig. 5 is a graph showing the time-dependent change in floating yeast
count of malt liquor (Comparative Example 2) and syrup (Example 13)
during fermentation.
Fig. 6 is a graph showing the 0-glucan concentration in malt liquor
(Comparative Example 2) and syrup (Example 13) after fermentation.
Fig. 7 is a graph showing the concentrations of various free amino acids
in malt liquor (Comparative Example 2) and syrup (Example 13) after
fermentation.
Fig. 8 is a chromatogram obtained for a refrigerated sample of barley
syrup.
Fig. 9 is a chromatogram obtained for a frozen sample of barley syrup.
Fig. 10 is a molecular weight distribution curve for P-glucans obtained
for a refrigerated sample of barley syrup.
11
CA 02697673 2010-02-24
FP08-0467-00CA-SB
Fig. 11 is a molecular weight distribution curve for P-glucans obtained
for a frozen sample of barley syrup.
Fig. 12 is a calibration curve for the GPC column used in Example 14.
Best Modes for Carrying Out the Invention
[0041] Preferred embodiments of the invention will now be described
in detail.
[0042] The method for producing barley syrup of the invention
comprises decomposing barley or its milled product at 20 C or higher
and 80 C or lower in the presence of an a-amylase.
[0043] Since the temperature for the decomposition is set to 20 C or
higher and 80 C or lower, and an a-amylase is present, the method of
the invention can yield syrup with sufficiently low viscosity and easy
manageability. If the temperature for the decomposition is below
C, decomposition of the carbohydrates such as starches in the barley
15 starting material will be inadequate, and if it exceeds 80 C the
viscosity
of the obtained barley syrup will be increased resulting in more difficult
management.
[0044] According to one aspect, the method for producing barley syrup
of the invention is a method comprising decomposing barley or its
20 milled product at a temperature of 45 C or higher and 80 C or lower in
the presence of an a-amylase (production method (A)). According to
another aspect, the method for producing barley syrup of the invention
is a method comprising decomposing barley or its milled product at a
temperature of 20 C or higher and 65 C or lower and preferably 20 C
or higher and lower than 45 C, in the presence of an a-amylase
("production method (B)"). Preferred modes of production method (A)
12
CA 02697673 2010-02-24
FP08-0467-00CA-SB
and (B) will now be explained in order.
[0045] [Production method (A)]
In production method (A), first barley or its milled product is
decomposed in an aqueous solvent in the presence of an a-amylase to
obtain mash (decomposing step). In this decomposing step,
carbohydrates such as starch in barley starting material are decomposed
into low molecular saccharides, and the functional substances such as
13-glucans are extracted.
[0046] The barley used may be any type of barley including two-row,
six-row, naked or hulled barley. Any tissue or fraction from barley
seeds may be used as the barley starting material, including the whole
grain, milled barley grains and bran.
[0047] Syrup is produced using barley as the starting material in
production method (A), and cereals such as wheat, oats, rye, rice or the
like may be used instead of or in addition to barley for the starting
material, to produce syrup otherwise in the same manner as production
method (A).
[0048] As a-amylases there may be mentioned any publicly known
ones such as the commercially available amylase AD "AMANO 1"
(product of Amano Enzyme, Ltd.), KLEISTASE T1 OS (product of
Daiwa Fine Chemicals Co., Ltd.) or KLEISTASE YC 15S (product of
Daiwa Fine Chemicals Co., Ltd.). The addition amounts of such
a-amylases may be appropriately adjusted according to the a-amylase
activity, and for example, they may be used at between 0.01 part by
weight and 1 part by weight with respect to 100 parts by weight as the
total of the barley or its milled product.
13
CA 02697673 2010-02-24
FP08-0467-00CA-SB
[0049] In the production method described above, a 13-amylase is
preferably added during the decomposing step. Addition of a
13-amylase allows a 13-amylase activity to be supplemented even when
the 13-amylase in the barley starting material has been inactivated by
heat, thus allowing more efficient production of saccharides.
[0050] When a 13-amylase is not added during the decomposing step,
the production method (A) preferably includes addition of a (3-amylase
to the decomposed product obtained from the decomposing step, for
further decomposition of the decomposed product (additional
decomposing step). This will allow a 13-amylase activity to be
supplemented even when the (3-amylase in the barley starting material
has been inactivated by heat in the decomposing step, thus allowing
more efficient production of saccharides.
[0051] Any conventionally known 13-amylases may be used, such as the
commercially available products by Tokyo Kasei Kogyo Co., Ltd., for
example. When a 13-amylase is added, the amount of addition is
preferably between 0.01 part by weight and 1 part by weight with
respect to 100 parts by weight as the total of the barley or its milled
product.
[0052] In the decomposing step and/or the additional decomposing step,
a pullulanase is preferably added in order to hydrolyze the
a-1,6-glucoside bonds (branched portions) of the starch derived from
the barley starting material and more efficiently produce saccharides.
Any conventionally known pullulanase may be used. When a
pullulanase is added, the amount of addition is preferably 0.01-1 part by
weight with respect to 100 parts by weight as the total of the barley or
14
CA 02697673 2010-02-24
,
FP08-0467-00CA-SB
its milled product.
[0053] In the decomposing step and/or the additional decomposing step,
a protease is preferably added to decompose the protein from the barley
starting material and produce amino acids. Any conventionally known
protease may be used. When a protease is added, the amount of
addition is preferably 0.01-1 part by weight with respect to 100 parts by
weight as the total of the barley or its milled product. Examples of
amino acids produced by addition of proteases include GABA and
glutamic acid. The barley syrup obtained by the production method of
the invention contains amino acids such as GABA and glutamic acid in
addition to P-glucans, and it can be suitably used in various functional
foods, fermentation media and the like.
[0054] The a-amylase, P-amylase, pullulanase and protease may be in
admixture with components that exhibit a P-glucan-decomposing
activity, but preferably, virtually or absolutely no components that
exhibit a P-glucan-decomposing activity are included, and most
preferably the protease contains virtually or absolutely no components
that exhibit a f3-glucan-decomposing activity. Examples of proteases
containing virtually or absolutely no components that exhibit a
13-glucan-decomposing activity include the protease S "AMANO 3G"
(product of Amano Enzyme, Ltd.), THERMOASE PC10F, and PROTIN
AC1OF (products of Daiwa Fine Chemicals Co., Ltd.), and papain.
[0055] When enzymes such as 13-amylases, pullulanases or proteases
are added, there are no particular restrictions on the order of addition of
the enzymes, and they may be added simultaneously with the a-amylase
or before or after addition of the a-amylase. Also, two or more
CA 02697673 2010-02-24
FP08-0467-00CA-SB
different a-amylases, f3-amylases, pullulanases or proteases may be
combined and added as a mixture.
[0056] (Decomposing step)
The temperature for decomposing barley or its milled product in the
decomposing step of production method (A) is 45 C or higher and 80 C
or lower. If the temperature is 45 C or higher and 80 C or lower, it
will be possible to obtain barley syrup which has a sufficiently low
viscosity and is rich in P-glucans. A temperature of higher than 80 C,
on the other hand, will increase the viscosity of the obtained barley
syrup and will hamper manageability. From the viewpoint of realizing
a more suitable balance between viscosity and 13-glucan content for the
obtained barley syrup, the temperature is more preferably 50 C or
higher and 70 C or lower and even more preferably 55 C or higher and
65 C or lower. The temperature for decomposing barley or milled
product may be higher than 60 C and 75 C or lower, or higher than
65 C and 75 C or lower, according to the required 13-glucan content.
[0057] The reaction time in the decomposing step may be appropriately
adjusted according to the a-amylase activity and reaction scale, and may
be, for example, between 30 minutes and 24 hours.
[0058] The barley concentration in the decomposing step is preferably
between 0.5 wt% and 80 wt%, more preferably between 2 wt% and 60
wt% and even more preferably between 2.5 wt% and 40 wt% with
respect to the water.
[0059] The decomposing step may be based on a batch process or a
continuous process. For a batch process, appropriate stirring is
preferably conducted during the decomposing step. For a continuous
16
CA 02697673 2010-02-24
FP08-0467-00CA-SB
process, the barley starting material and water are mixed beforehand,
and heating is performed with a prescribed temperature and residence
time while conveying the liquid with a pump, to decompose the barley
or its milled product and obtain a mash.
[0060] (Additional decomposing step)
The temperature for further decomposition of the decomposed product
obtained by the decomposing step, in the additional decomposing step,
is preferably 45 C or higher and 80 C or lower, more preferably 50 C
or higher and 70 C or lower and even more preferably 55 C or higher
and 65 C or lower. The reaction time in the additional decomposing
step may be appropriately adjusted according to the (3-amylase activity
and reaction scale, and may be, for example, between 30 minutes and 24
hours.
[0061] The additional decomposing step may be based on a batch
process or a continuous process. For a batch process, appropriate
stirring is preferably conducted during the additional decomposing step.
For a continuous process, the mash obtained from the decomposing step
is conveyed with a pump while being heated with a prescribed
temperature and residence time, to further decompose the decomposed
product.
[0062] (Post-decomposing step)
Next, the insoluble portion is removed from the mash obtained from the
decomposing step or additional decomposing step, using centrifugal
separation or a filter press. The remaining soluble portion is filtered
using diatomaceous earth, active carbon or the like as an auxiliary agent
and further purified by microfiltration, to obtain the desired barley
17
CA 02697673 2010-02-24
FP08-0467-00CA-SB
syrup.
[0063] (Pre-processing step)
Production method (A) may also comprise a pre-processing step in
which the barley or its milled product is pre-processed at a temperature
of 20 C or higher and 40 C or lower, before the decomposing step.
[0064] In the pre-processing step, the barley or its milled product is
reacted for 30 minutes to 24 hours in a water solvent at a temperature
of, for example, 20 C or higher and 40 C or lower. This can activate
the endogenous enzymes in the barley and increase the content of amino
acids (a GABA, a glutamic acid, etc.), peptides and proteins in the
obtained barley syrup.
[0065] (Barley syrup)
The barley syrup obtained by production method (A) has a sufficiently
low viscosity and is easy to manage. It is also rich in P-glucans (0.01
mg/mL or more with respect to the total syrup), while also containing
various amino acids (glycine, alanine, valine, leucine, isoleucine, serine,
threonine, lysine, arginine, aspartic acid, asparagine, glutamic acid,
glutamine, methionine, phenylalanine, tyrosine, histidine, GABA, etc.).
The types and contents of functional substances in the barley syrup can
be modified by varying the type of barley starting material or the types
of enzymes used in the decomposing step.
[0066] The obtained barley syrup can be appropriately processed into a
form suitable for the desired purpose. Such processing may be, for
example, concentration or sterilization and pulverization treatment.
Sterilization and pulverization have been difficult, particularly with
high-viscosity barley syrup obtained by prior art production methods.
18
CA 02697673 2010-02-24
FP08-0467-00CA-SB
However, sterilization and pulverization treatment can be easily
accomplished with barley syrup obtained by production method (A).
[0067] The barley syrup obtained by production method (A) can be
suitably used in foods and beverages including, for example, solid food
materials such as bread, yogurt, cheese, confectioneries and snacks;
seasonings such as mirin, vinegar, miso, soy sauce and butter; and
beverages such as soft drinks, sake, beer, low-malt beer and Japanese
spirits.
[0068] Barley syrup obtained by production method (A) can also be
used as a culture medium and particularly a fermentation medium, and
can be utilized, for example, for production of new fermented foods and
beverages implementing the functional substances included in barley.
[0069] If the temperature in the decomposing step of production
method (A) is 50 C or higher and 70 Cor lower, the obtained barley
syrup will have sufficiently low viscosity (between 1 mPa-s and 20
mPa.$) and will be easy to manage. It is also rich in 13-glucans (0.01
mg/mL or more with respect to the total syrup), while also being rich in
the various amino acids (glycine, alanine, valine, leucine, isoleucine,
serine, threonine, lysine, arginine, aspartic acid, asparagine, glutamic
acid, glutamine, methionine, phenylalanine, tyrosine, histidine, GABA,
etc.). In addition, even with fermentation by yeast, for example, it
does not adversely affect the fermentation process (extract decrease,
floating yeast count, etc.). The barley syrup is therefore suitable as a
starting material for effervescent alcoholic beverages, for example, and
it can be used to easily obtain effervescent alcoholic beverages with
high functionality that are rich in P-glucans and amino acids.
19
CA 02697673 2010-02-24
FP08-0467-00CA-SB
[0070] If the temperature of the decomposing step is 50 C or higher and
70 C or lower, the weight-average molecular weight Mw of the
P-glucans in the barley syrup will usually be between 50,000 and
500,000. Barley syrup having 13-glucans with a weight-average
molecular weight Mw within this range has low viscosity and is
especially suitable for utilization in foods and beverages. From the
viewpoint of easier use in foods and beverages, the weight-average
molecular weight Mw of the P-glucans is preferably, for example,
between 100,000 and 300,000, and more preferably between 100,000
and 200,000.
[0071] From the same viewpoint of easier use in foods and beverages,
the number-average molecular weight Mn of the P-glucans in the barley
syrup is preferably, for example, between 30,000 and 300,000, more
preferably between 50,000 and 200,000, and even more preferably
between 50,000 and 150,000.
[0072] From the same viewpoint of easier use in foods and beverages,
the molecular weight distribution of the P-glucans in the barley syrup is
preferably monodispersed, and the ratio of the weight-average
molecular weight Mw and number-average molecular weight Mn of the
P-glucans (Mw/Mn) is preferably, for example, between 1 and 16, more
preferably between 1 and 10, even more preferably between 1 and 5, yet
more preferably between 1 and 3, and most preferably between 1 and 2.
[0073] The molecular weights (Mw, Mn) of the P-glucans in the barley
syrup can be measured by GPC, osmotic pressure or the like, but GPC is
preferred from the viewpoint of convenience. For measurement by
GPC, for example, the pre-column method or post-column method is
CA 02697673 2010-02-24
FP08-0467-00CA-SB
preferably also used for specific derivatization of the P-glucans, from
the viewpoint of eliminating the effects of the other components in the
barley syrup.
[0074] (Effervescent alcoholic beverage)
The production method used to obtain an effervescent alcoholic
beverage of the invention comprises a fermenting step in which the
barley syrup is fermented with yeast. In the fermenting step, the
sugars (extract portion) in the barley syrup are decomposed by the yeast
and alcohol fermentation occurs.
[0075] The production method described above preferably comprises a
lager step in which the sugars (extract portion) remaining in the
fermentate obtained from the fermenting step are re-fermented at low
temperature and matured. Also, a filtrating step is preferably carried
out to remove the yeast and turbid substances from the aged liquor
obtained from the lager step. The conditions for the fermenting step
(yeast species, culture medium, culture medium aeration rate,
fermentation temperature and fermentation time) may be appropriately
selected depending on the desired effervescent alcoholic beverage.
[0076] In the production method described above, a step of, for
example, adding hop to the barley syrup and heating it may be carried
out before the fermenting step. In this case, the heat-treated barley
syrup is supplied to the fermenting step to obtain an effervescent
alcoholic beverage with beer taste (low-malt beer).
[0077] An effervescent alcoholic beverage of the invention is rich in
P-glucans and amino acids (glycine, alanine, valine, leucine, isoleucine,
serine, threonine, lysine, arginine, aspartic acid, asparagine, glutamic
21
CA 02697673 2010-02-24
FP08-0467-00CA-SB
acid, glutamine, methionine, phenylalanine, tyrosine, histidine, GABA,
etc.), and is suitable for use as a functional beverage.
[0078] [Production method (B)]
In production method (B), first barley or its milled product is
decomposed in an aqueous solvent in the presence of an a-amylase to
obtain mash (decomposing step). In this decomposing step,
carbohydrates such as starch in the barley starting material are
decomposed into low molecular saccharides.
[0079] The barley used may be any type of barley including two-row,
six-row, naked or hulled barley. Any tissue or fraction from barley
seeds may be used as the barley starting material, including the whole
grain, milled barley grains and bran.
[0080] Syrup is produced using barley as the starting material in
production method (B), and cereals such as wheat, oats, rye, rice or the
like may be used instead of or in addition to barley for the starting
material, to produce syrup otherwise in the same manner as production
method (B).
[0081] Examples of a-amylases include any publicly known ones such
as the commercially available amylase AD "AMANO 1" (product of
Amano Enzyme, Ltd.), KLEISTASE Ti OS (product of Daiwa Fine
Chemicals Co., Ltd.) or KLEISTASE YC 15S (product of Daiwa Fine
Chemicals Co., Ltd.). The addition amounts of such a-amylases may
be appropriately adjusted according to the a-amylase activity, and for
example, they may be used at between 0.01 part by weight and 1 part by
weight with respect to 100 parts by weight as the total of the barley or
its milled product. Components that exhibit a P-glucanase activity
22
CA 02697673 2010-02-24
FP08-0467-00CA-SB
may also be added as a-amylases.
[0082] A P-glucanase is also preferably added in the decomposing step
to decompose the p-glucans in the barley starting material and thus
further lower the viscosity. Any conventionally known f3-glucanases
may be used. When a P-glucanase is added, the amount of addition is
preferably between 0.01 part by weight and 1 part by weight with
respect to 100 parts by weight as the total of the barley or its milled
product.
[0083] A pullulanase is preferably added in the decomposing step in
order to hydrolyze the a-1,6-glucoside bonds (branched portions) of the
starch derived from the barley starting material and more efficiently
produce saccharides. Any conventionally known pullulanases may be
used. When a pullulanase is added, the amount of addition is
preferably between 0.01 part by weight and 1 part by weight with
respect to 100 parts by weight as the total of the barley or its milled
product. Components that exhibit a P-glucanase activity may also be
added as pullulanases.
[0084] In the decomposing step, a protease is preferably added to
decompose the protein from the barley starting material and produce
amino acids. Any conventionally known proteases may be used.
When a protease is added, the amount of addition is preferably between
0.01 part by weight and 1 part by weight with respect to 100 parts by
weight as the total of the barley or its milled product. The barley syrup
obtained by the production method of the invention contains amino
acids such as GABA and glutamic acid, and it can be suitably used in
various functional foods, fermentation media and the like.
23
CA 02697673 2010-02-24
FP08-0467-00CA-SB
Components that exhibit a p-glucanase activity may also be added as
proteases.
[0085] When enzymes such as P-glucanases, pullulanases or proteases
are added, there are no particular restrictions on the order of addition of
the enzymes, and they may be added simultaneously with the a-amylase
or before or after addition of the a-amylase. Also, two or more
different a-amylases, P-glucanases, pullulanases or proteases may be
combined and added as a mixture.
[0086] (Decomposing step)
The temperature for decomposing the barley or its milled product in the
decomposing step of production method (B), is 20 C or higher and
65 C or lower and preferably 20 C or higher and less than 45 C from
the viewpoint of obtaining barley syrup of lower viscosity. If the
temperature is 65 C or lower and especially lower than 45 C, high
molecularization of P-glucans during production will be inhibited and
the viscosity of the obtained barley syrup will be notably reduced. If
the temperature is lower than 20 C, on the other hand, decomposition of
the carbohydrates such as starches in the barley starting material will be
inadequate. The temperature for decomposing the barley or its milled
product may be, for example, 45 C or higher and 65 C or lower, or
50 C or higher and 60 C or lower.
[0087] The reaction time in the decomposing step may be appropriately
adjusted according to the a-amylase activity and reaction scale, and may
be, for example, between 30 minutes and 24 hours.
[0088] The barley concentration in the decomposing step is preferably
between 0.5 wt% and 80 wt%, more preferably between 2 wt% and 60
24
CA 02697673 2010-02-24
FP08-0467-00CA-SB
wt%, and even more preferably between 2.5 wt% and 40 wt% with
respect to the water.
[0089] The decomposing step may be based on a batch process or a
continuous process. For a batch process, appropriate stirring is
preferably conducted during the decomposing step. For a continuous
process, the barley starting material and water are mixed beforehand,
and heating is performed with a prescribed temperature and residence
time while conveying the liquid with a pump, to decompose the barley
or its milled product and obtain a mash.
[0090] (Post-decomposing step)
Next, the insoluble portion is removed from the mash obtained from the
decomposing step, using centrifugal separation or a filter press. The
remaining soluble portion is filtered using diatomaceous earth, active
carbon or the like as an auxiliary agent and further purified by
microfiltration, to obtain the desired barley syrup.
[0091] (Pre-processing step)
Production method (B) may also comprise a pre-processing step in
which the barley or its milled product is pre-processed at a temperature
of 20 C higher and 40 C or lower, before the decomposing step.
[0092] In the pre-processing step, the barley or its milled product is
reacted for 30 minutes to 24 hours in a water solvent at a temperature
of, for example, 20 C or higher and 40 C or lower. This can activate
the endogenous enzymes in the barley and increase the content of amino
acids (GABA, glutamic acid, etc.), peptides and proteins in the obtained
barley syrup.
[0093] (Barley syrup)
CA 02697673 2010-02-24
=
FP08-0467-00CA-SB
The barley syrup obtained from production method (B) contains amino
acids such as GABA and glutamic acid. The types and contents of
amino acids in the barley syrup can be appropriately modified by
varying the type of barley starting material or the types of enzymes used
in the decomposing step.
[0094] The obtained barley syrup can be appropriately processed into a
form suitable for the desired purpose. Such processing may be, for
example, concentration or sterilization and pulverization treatment.
Sterilization and pulverization have been difficult, particularly with
high-viscosity barley syrup obtained by prior art production methods.
However, sterilization and pulverization treatment can be easily
accomplished with barley syrup obtained by production method (B).
[0095] The barley syrup obtained by production method (B) can be
suitably used in foods and beverages including, for example, solid food
materials such as bread, yogurt, cheese, confectioneries and snacks;
seasonings such as mirin, vinegar, miso, soy sauce and butter; and
beverages such as soft drinks, sake, beer, low-malt beer and Japanese
spirits.
[0096] Barley syrup obtained by production method (B) can also be
used as a culture medium and particularly a fermentation medium, and
can be utilized, for example, for production of new fermented foods and
beverages implementing the functional substances included in barley.
Examples
[0097] The invention will now be explained in greater detail based on
examples and comparative examples. However, the present invention
is not limited to the examples described below.
26
CA 02697673 2010-02-24
a
FP08-0467-00CA-SB
[0098] [Evaluation of a p-glucan-decomposing activity of enzymes]
In order to examine whether each enzyme (protease) had a
p-glucan-decomposing activity, a 13-glucan standard solution was mixed
with each enzyme and reaction was conducted at 50 C for 16.5 hours,
after which the P-glucan concentration was measured.
[0099] First, a frozen-stored P-glucan (Standard: Calibration Standard
(Solution 250 mL, 300 mg/L, P-Glucan Analyzer, Carlsberg System
(Contains: Barley P-glucan, Thimerosal))) by FOSS (FOSS Analytical
AB, Sweden) was thawed at 70 C for 1 hour just prior to use, and
diluted with water to 150 ppm to prepare a p-glucan standard solution.
[0100] The evaluation samples shown in Table I were then prepared.
Of the enzymes listed in Table 1, 125 Kg of each of the powdered
enzymes No. 4 to 16 and 18 was dissolved in 1000 !IL of water, and 10
KL of the obtained solution was diluted with 990 1.1L of water, after
which 10 IAL of the obtained enzyme solution was added to 1000 IIL of
the P-glucan standard solution to prepare an evaluation sample.
[0101] Of the enzymes listed in Table 1, 125 [IL of the liquid enzyme of
No. 17 was dissolved in 1000 ilL of water, and 10 pL of the obtained
solution was diluted with 990 1AL of water, after which 10 1AL of the
obtained enzyme solution was added to 1000 tiL of the P-glucan
standard solution to prepare an evaluation sample.
[0102] Samples No. 1-3 listed in Table 1 are control samples, using
only the P-glucan standard solution without enzyme addition.
[0103] [Table 1]
27
CA 02697673 2010-02-24
FP08-0467-00CA-SB
No. Sample
1 STD150, 5 C (no heat treatment)
2 STD150, 5 C
3 STD150, 50 C
4 Umamizyme G
Newlase F3G
6 Protease A "AMANO" G
7 Protease M "AMANO" G
8 Protease N "AMANO" G
9 Protease P "AMANO" 3G
Protease S "AMANO" G
11 Proleather FG-F
12 Peptidase R
13 Samoase PC1OF
14 Protine AC1OF
Protine PC1OF
16 Glutaminase Daiwa ClOOS
17 YL-NL "AMANO"
18 Papain
[0104] Samples No. 1 to 18 were processed. Samples No. 1 and 2
were stored at 5 C for 16.5 hours, sample No. 3 was stored at 50 C for
16.5 hours, and samples No. 4 to 18 were stored at 50 C for 16.5 hours
5 after addition of enzyme solution. Samples No. 2 to 18 were heat
treated at 100 C for 5 minutes and then centrifuged at 5 C, 13,200 rpm
for 15 minutes, and the supernatant of the sample was supplied for
measurement of the 13-glucan concentration. Sample No. 1 was
supplied for measurement of the P-glucan concentration without heat
10 treatment or centrifugation.
[0105] Samples No. 1 to 18 treated in the manner described above were
filtered with a 0.45 pm filter, and then the apparatus described below
was used in a 20 C measurement chamber for measurement of the
28
CA 02697673 2010-02-24
FP08-0467-00CA-SB
13-glucan concentration. The results are shown in Fig. 1.
= Two high-pressure pumps:
Shodex (Showa Denko K.K.) DS-4 (water: 1.0 mL/min)
HITACHI L-6000 Pump (reaction mixture: 2.0 mL/min)
= Autosamplers:
No. 1: Autosampler Model AS-09, product of System
Instruments, Co., Ltd.
No. 2: Autosampler Model 33, product of System Instruments,
Co., Ltd.
= Fluorescence detector: Shimadzu High Performance Liquid
Chromatography Fluorescence Detector RF-10AXL (excitation
wavelength: 360 nm, fluorescence wavelength: 420 nm)
= Column thermostatic bath: Shodex (product of Showa Denko K.K.)
OVEN A0-30C
= Deaeration apparatus: ERC-3215 by ERC, Inc.
= Data processor: Chromatocorder 21 by System Instruments, Co., Ltd.
= Mixing coil: Teflon tube with 0.5 mm inner diameter and 0.5 mL
empty volume, coiled to a diameter of 7 cm
= Gel filtration column: Shodex SUGAR BT-603
Column size: 69 X 50 mm
Column end-connecting screw: Push-screw type, No.10-32UNF
Column material: SUS 316
Filler: Polyhydroxy methacrylate
Exclusion limit molecular weight: 1 X 105 (pullulan)
[0106] From Fig. 1 it is clear that using enzymes No. 4 to 9 and 16
resulted in extensive decomposition of P-glucans, and therefore that the
29
CA 02697673 2010-02-24
FP08-0467-00CA-SB
enzymes contain components exhibiting a P-glucan-decomposing
activity. In contrast, using enzymes No. 10, 13, 14 and 18 resulted in
virtually no decomposition of 13-g1ucans, and therefore these enzymes
contained virtually no components exhibiting a P-glucan-decomposing
activity.
[0107] [Preparation of milled barley flour]
Whole grain CDC Fibar (2006 Canada product) was milled with a
cyclone mill to prepare a barley syrup starting material. After placing
50 g of the milled barley flour on a 75 gm mesh, 150 ilm mesh, 300 gm
mesh, 600 gm mesh, 1000 gm mesh or 2000 gm mesh sieve, it was
sieved for 5 minutes to measure the particle size of the milled barley
flour. The results are shown in Fig. 2. The crude protein in the
milled barley flour was also quantified by the Kjeldahl method, and the
anhydrous value was found to be 18.5%.
[0108] [Preparation of enzymes]
There were prepared KLEISTASE YC 15S (trade name of Daiwa Fine
Chemicals Co., Ltd.) as an a-amylase, the protease S "AMANO G"
(trade name of Amano Enzyme, Ltd.) as a protease, a 13-amylase by
Tokyo Kasei Kogyo Co., Ltd. as a 13-amylase and a pullulanase by
Amano Enzyme, Ltd. as a pullulanase. In each of Examples 1 to 11
below, there was used 40 RI, of solution obtained by dissolving 25 mg
of each of the enzymes in 1000 gI, of water.
[0109] [Example 1]
After placing 40 mL of water in a 50 mL Falcon tube, the water was
incubated at 50 C (preheating). Next, 1.0 g of the milled barley flour
was added, the a-amylase was added to 0.1% (w/w) with respect to the
CA 02697673 2010-02-24
,
FP08-0467-00CA-SB
barley, and agitating decomposition was carried out for 4 hours with a
shaker in an air incubator, with the internal temperature kept at 50 C
(decomposing step). This was then rapidly cooled in an ice bath and
centrifuged at 800 rpm for 15 minutes, after which the supernatant was
filtered with filter paper (product of Advantec) to obtain barley syrup.
[0110] [Example 2]
Barley syrup was obtained in the same manner as Example 1, except
that a protease was added in addition to an a-amylase.
[0111] [Example 3]
Barley syrup was obtained in the same manner as Example 1, except
that the incubation temperature and internal air incubator temperature
were changed from 50 C to 60 C.
[0112] [Example 4]
Barley syrup was obtained in the same manner as Example 1, except
that the incubation temperature and internal air incubator temperature
were changed from 50 C to 60 C and a protease was further added in
addition to an a-amylase.
[0113] [Example 5]
Barley syrup was obtained in the same manner as Example 1, except
that the incubation temperature and internal air incubator temperature
were changed from 50 C to 70 C.
[0114] [Example 6]
Barley syrup was obtained in the same manner as Example 1, except
that the incubation temperature and internal air incubator temperature
were changed from 50 C to 70 C and a protease was further added in
addition to an a-amylase.
31
CA 02697673 2010-02-24
=
FP08-0467-00CA-SB
[0115] [Barley syrup evaluation (1)]
The viscosity, filtration rate, f3-glucan concentration, SN (Soluble
Nitrogen), amino acid concentration and Brix value were measured for
each of the barley syrups obtained in Examples 1 to 6.
[0116] (Viscosity measurement)
An Ubbellohde Brookfield viscometer was used to measure the 20.00 C
viscosity of each of the barley syrups obtained in Examples 1 to 6, as
stock solution samples or water-diluted stock solution samples. The
results are shown in Table 2.
[0117] (Filtration rate evaluation)
The time required for filtration in each of Examples 1 to 6 was
measured. A filtration time of no longer than 15 minutes was
evaluated as "A", a time of between 15 minutes and 30 minutes was
evaluated as "B", and a time of longer than 30 minutes was evaluated as
"C". The results are shown in Table 2.
[0118] (Measurement of P-glucan concentration)
Each barley syrup obtained in Examples 1 to 6 was diluted 7.5-fold with
water and then filtered with a 0.45 pm filter, and the p-glucan
concentration of each filtered product was measured in a 20 C
measuring chamber. The results are shown in Table 2. The apparatus
used for measurement was the same used for "Evaluation of a
P-glucan-decomposing activity of enzymes" described above.
[0119] (SN measurement)
The SN values of filtrates of each of the barley syrups obtained in
Examples 1 to 6 were measured by the Kjeldahl method. The results
are shown in Table 2.
32
CA 02697673 2010-02-24
=
FP08-0467-00CA-SB
[0120] (Brix measurement)
The Brix values of filtrates of each of the barley syrups obtained in
Examples 1 to 6 were measured with an ATAGO RX-5000. The
results are shown in Table 2.
[0121] (Amino acid concentration measurement)
Each barley syrup obtained in Examples 1 to 6 was filtered with an
Ultracel YM-10 Regenerated Cellulose 10,000 MWCO (Millipore) and
the filtrate was diluted with water, after which an AccQ FLUOR
REAGENT KIT (Waters Col) was used for derivatization by the AccQ
Tag method to measure the amino acid concentration. The results are
shown in Table 2. In the table, "Total a.a." indicates the total free
amino acids of the constituent amino acids of the protein, except for
tryptophan which is undetectable, "GABA" indicates y-aminobutyric
acid, and "Glu" indicates glutamic acid. The following apparatus and
conditions were employed for measurement.
= Apparatus: 2695 Separation Module: Column heater: 2475 Multi X
fluorescence detector: Empower Personal
= Mobile phase A: 166 mM sodium acetate/5.6 mM triethylamine, pH
5.7
= Mobile phase B: 166 mM sodium acetate/5.6 mM triethylamine, pH
6.8
= Mobile phase C: Acetonitrile
= Mobile phase D: Water
= Column: AccQ-Tag Amino Acid Analysis Column (3.9 X 150 mm) +
Sentry Nova C18
= Column temperature: 39 C
33
v)
.
Viscosity Filtration 13-Glucan SN Total
a.a. GABA Glu Brix
1\..)
0 =
w (mPa=S) rate
(mg/mL) (mg/ 100mL) (g/mL) (pg/mL) (vg/mI.,) (%) N) E> 2
0 r+.
--t
o':"-t= 5
1-11
r
-1 Example 1 1.35 A 1.44 11.0 152.9 11.8
9.4 1.62 $t 8 0
x
ID
Example 2 1.53 A 1.46 40.1 352.1
11.8 15.8 1.88
, x CD.
_
Example 3 1.88 A 1.78 10.2 150.6
11.4 10.3 1.72
c),
.c)
---.)
c>
Example 4 1.73 A 1.81 42.0 379.1
11.7 19.9 1.97
0
Example 5 3.68 B 1.91 10.3 132.8
10.0 12.0 1.74 I,
(5,
.
Example 6 3.95 B 1.89 44.2 295.6
10.6 20.4 1.99 t....)
.c)
,
(5,
,
ul
us,
L...)
g 0"
H
0
-P.
I
C.)
0
.
IV
I
IV
FP
8
oil
0 d
00
ci:D
41,
01
il
0
0
n
el)
to
CA 02697673 2010-02-24
=
FP08-0467-00CA-SB
After placing 40 mL of water in a 50 mL Falcon tube, the water was
incubated at 60 C (preheating). Next, 1.0 g of the milled barley flour
was added, the a-amylase was added to 0.1% (w/w) with respect to the
barley, and agitating decomposition was carried out for 24 hours with a
shaker in an air incubator, with the internal temperature kept at 60 C
(decomposing step). This was then rapidly cooled in an ice bath and
centrifuged at 800 rpm for 15 minutes, after which the supernatant was
filtered with filter paper (product of Advantec) to obtain barley syrup.
[0124] [Example 8]
Barley syrup was obtained in the same manner as Example 7, except
that a 0-amylase was added in addition to an a-amylase.
[0125] [Example 9]
Barley syrup was obtained in the same manner as Example 7, except
that a pullulanase was added in addition to an a-amylase.
[0126] [Example 10]
Barley syrup was obtained in the same manner as Example 7, except
that a 0-amylase and a pullulanase were added in addition to an
a-amylase.
[0127] [Example 11]
Barley syrup was obtained in the same manner as Example 7, except
that a 0-amy1ase, a pullulanase and a protease were added in addition to
an a-amylase.
[0128] [Barley syrup evaluation (2)]
The 0-glucan concentration, amino acid concentration, Brix value and
sugar concentration were measured for each of the barley syrups
obtained in Examples 7 to 11. The f3-glucan concentration, amino acid
CA 02697673 2010-02-24
FP08-0467-00CA-SB
concentration and Brix value were measured by the same method as for
"Barley syrup evaluation (1)". The results are shown in Table 3.
[0129] (Sugar concentration measurement)
Filtrates of each of the barley syrups obtained in Examples 7 to 11 were
heat treated at 100 C for 10 minutes and then rapidly cooled in an ice
bath. This was then centrifuged at 15,000 rpm, 5 C for 15 minutes,
and the supernatant was diluted with 0.1% benzoic acid and supplied for
measurement of the sugar concentration. The results are shown in
Table 3. The following apparatus and conditions were employed for
the measurements.
= Apparatus: DIONEX DX-300
= Mobile phase A: 0.1 M Sodium hydroxide
= Mobile phase B: 0.1 M Sodium hydroxide/1 M sodium acetate
= Column: CarboPac PA1
= Injection rate: 15 [IL
[0130] [Table 3]
36
1-5
c7) P-Glucan Total a.a. GA.BA Glu Total
Glucose Maltose Brix
(mg/mL) (1.1.g/mL) (1.4g/mL) (tig/mL)
(i.L.g(mL) (/mL) (%)
(sugarsi.i.g/mL)
Example 7 2.17 173.7 9.1 15.2 9484.9
613.5 5218.3 1.77
Example 8 2.17 177.9 9.2 15.6 9479.1
601.3 5950.2 1.78
Example 9 2.11 1047.3 13.3 52.0 13304.9
407.9 8847.4 2.28
Example 10 2.11 1048.0 13.5 51.8 13823.7
341.9 9319.0 2.26
Example 11 2.12 1279.4 10.4 60.8 13954.0
370.3 9452.5 2.36
0
0
FP"
GC
Cr \
(5?)
CIA
CA 02697673 2010-02-24
FP08-0467-00CA-SB
After placing 400 mL of water in a 500 mL tube, the water was
incubated at 60 C (preheating). Next, 50 g of the milled barley flour
was added, the a-amylase, 0-amylase and protease were added to 0.1%
(w/w) with respect to the barley, and agitating decomposition was
carried out for 24 hours with a shaker in an air incubator, with the
internal temperature kept at 60 C (decomposing step). This was then
rapidly cooled in an ice bath and centrifuged at 800 rpm for 15 minutes,
after which the supernatant was filtered with filter paper (product of
Advantec) to obtain barley syrup.
[0132] [Comparative Example 1]
After placing 400 mL of water in a 500 mL tube, the water was
incubated at 55 C (preheating). Next, 50 g of the milled barley flour
was added, the a-amylase was added to 0.05% (w/w) with respect to the
barley, and agitating decomposition was carried out for 1 hour with a
shaker in an air incubator, with the internal temperature kept at 55 C
(decomposing step). The temperature was then raised to 90 C over a
period of 1 hour, and then a-amylases were further added to 0.05%
(w/w) with respect to the barley and reaction was conducted for 1 hour
to obtain a liquefied solution. The liquefied solution was then cooled
to 60 C, 0-amy1ases were added to 0.1% (w/w) with respect to the
barley, and reaction was conducted at 60 C for 24 hours to obtain a
mash. The protease was then added to 0.1% (w/w) with respect to the
barley and reaction was conducted at 60 C for 24 hours. The reaction
mixture was filtered with filter paper to obtain barley syrup.
[0133] [Barley syrup evaluation (3)]
The viscosities, 13-glucan concentrations, SN and Brix values of the
38
CA 02697673 2010-02-24
FP08-0467-00CA-SB
barley syrups obtained in Example 12 and Comparative Example 1 were
measured by the same methods as "Barley syrup evaluation (1)" above.
The results are shown in Table 4.
[0134] [Table 4]
Viscosity 0-g1ucan SN Brix
(mPa. S)
(mg/mL) (mg/100 mL) (%)
Example 12 6.53 7.8 238.7 9.99
Comp. Ex. 1 18.56 9.8 209.4 9.84
[0135] [Example 13]
(Preparation of syrup)
To 123 g of milled barley seeds obtained by milling whole-grain barley
(2006 Hokkaido Ryofu) seeds with a cyclone mill, there was added a
mixture containing 140 mg of a-amylases (KLEISTASE YC 15S,
product of Daiwa Fine Chemicals Co., Ltd.), 140 mg of 0-amylases
(A0448, product of Tokyo Kasei Kogyo Co., Ltd.), 140 mg of protease
(THERMOASE PC10F, product of Daiwa Fine Chemicals Co., Ltd.),
140 [iL of pullulanase stock solution and 700 mL of water, and the
mixture was agitated at 60 C for 24 hours. The mixture was then
centrifuged at 5000 rpm for 30 minutes to obtain an unboiled syrup
(supernatant). The 0-glucan concentration, nitrogen content and free
amino acid concentration of the unboiled syrup were measured. The
results are shown in Table 5, Table 6 and Fig. 3.
[0136] After adding 0.5 g/250 mL of hops to the unboiled syrup, it was
boiled at 105 C for 90 minutes, and then water was added to a Brix of
11.0%, to obtain boiled syrup. The 13-g1ucan concentration, nitrogen
content and free amino acid concentration of the boiled syrup were
39
CA 02697673 2010-02-24
FP08-0467-00CA-SB
measured. The results are shown in Table 5, Table 6 and Fig. 3.
[0137] The p-glucan concentration, nitrogen content (SN) and free
amino acid concentration were measured by the same method as for
"Barley syrup evaluation (1)".
[0138] (Syrup fermentation)
After adding bottom beer yeast (S. pastorianus) to the aforementioned
barley syrup (boiled syrup), it was fermented at a temperature between
C and 12 C for 6 days. The fermentation conditions were as
follows.
10 = Extract concentration: about 11%
= Barley syrup volume: about 2.5 L
= Barley syrup dissolved oxygen concentration: between 5 ppm and 10
PPm
= Bottom beer yeast input: approximately 12 g of wet yeast cells
[0139] The change in residual extract volume and floating yeast count
of the barley syrup in the fermenting step was monitored. The results
are shown in Fig. 4 and Fig. 5.
[0140] The 13-glucan concentration and free amino acid concentration of
the fermented barley syrup were measured. The results are shown in
Table 7, Fig. 6 and Fig. 7. The 13-glucan concentration and free amino
acid concentration were measured by the same method as for "Barley
syrup evaluation (1)".
[0141] [Comparative Example 2]
(Malt liquor preparation)
To 60 g of milled barley malt obtained by milling barley (2006
Hokkaido Ryofu) malt with a cyclone mill there was added 230 mL of
CA 02697673 2010-02-24
,
FP08-0467-00CA-SB
water, and saccharification was carried out under the following
temperature conditions.
Holding at 48 C for 20 minutes ---> heating to 65 C at 1 C/min
---> holding at 65 C for 80 minutes ---> heating to 75 C at 1 C/min
--> holding at 75 C for 10 minutes
[0142] The obtained mash was adjusted upward with water to a total
weight of 400 g (60 g malt/340 g water = 0.176 g malt/1 mL water), and
then filtered to obtain unboiled malt liquor (filtrate). The P-glucan
concentration, nitrogen content and free amino acid concentration of the
unboiled malt liquor were measured. The results are shown in Table 5,
Table 6 and Fig. 3.
[0143] After adding 0.5 g/250 mL of hops to the unboiled malt liquor, it
was boiled at 105 C for 90 minutes, and then water was added to a Brix
of 11.0%, to obtain boiled malt liquor. The P-glucan concentration,
nitrogen content and free amino acid concentration of the boiled malt
liquor were measured. The results are shown in Table 5, Table 6 and
Fig. 3.
[0144] The 13-glucan concentration, nitrogen content (SN) and free
amino acid concentration were measured by the same method as for
"Barley syrup evaluation (1)".
[0145] (Malt liquor fermentation)
After adding bottom beer yeast (S. pastorianus) to the aforementioned
malt liquor (boiled malt liquor), it was fermented at a temperature of
between 10 C and 12 C for 6 days. The fermentation conditions were
as follows.
= Extract concentration: about 11%
41
CA 02697673 2010-02-24
FP08-0467-00CA-SB
= Malt liquor volume: about 2.5 L
= Malt liquor dissolved oxygen concentration: between 5 ppm and 10
ppm
= Bottom beer yeast input: approximately 12 g of wet yeast cells
[0146] The change in residual extract volume and floating yeast count
of the malt liquor in the fermenting step was monitored. The results
are shown in Fig. 4 and Fig. 5.
[0147] The P-glucan concentration and free amino acid concentration of
the fermented malt liquor were measured. The results are shown in
Table 7, Table 8, Fig. 6 and Fig. 7. The 13-glucan concentration and
free amino acid concentration were measured by the same method as for
"Barley syrup evaluation (1)".
[0148] [Table 5]
Malt liquor (Comp. Ex. 2) Syrup (Example 13)
Before boiling After boiling Before boiling After boiling
13-Glucan
114 138 5103 4472
Nitrogen content
126.9 124.3 202.8 198.6
(mg/100mL)
[0149] [Table 6]
42
CA 02697673 2010-02-24
FP08-0467-00CA-SB
Amino acid Malt liquor (Comp. Ex. 2) Syrup (Example 13)
(mg/L) Before boiling After boiling Before boiling After boiling
Gly 45.3 38.8 84.1 76.0
Ala 161.4 140.4 277.2 242.0
Val 177.4 151.8 298.2 229.0
Leu 238.5 202.3 476.6 394.5
Ile 103.9 88.6 189.9 159.1
Ser 101.6 86.4 175.5 156.8
Thr 107.9 99.4 185.1 161.0
Lys 140.1 108.8 290.1 211.0
Arg 244.7 191.8 419.1 331.8
Asp 94.8 84.7 104.1 93.6
Asn 149.4 154.2 102.0 122.2
Glu 75.3 61.9 202.6 167.2
Gln 312.0 14.5 308.9 20.3
Met 55.0 46.3 220.9 150.1
Phe 193.5 167.9 338.1 267.8
Tyr 145.2 123.4 263.3 192.7
Pro 788.2 659.5 345.3 279.6
His 83.2 70.0 117.2 95.3
Total 3217.5 2490.7 4398.3 3350.0
[0150] [Table 7]
Malt liquor (Comp. Ex. 2) Syrup (Example 13)
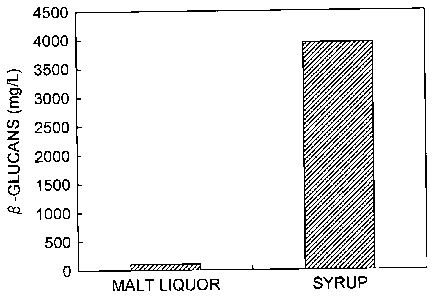
p-Glucan (mg/L) 106 3945
[0151] [Table 8]
43
CA 02697673 2010-02-24
FP08-0467-00CA-SB
Amino acid Malt liquor Syrup
(mg/L) (Comp. Ex. 2) (Example 13)
Gly 42.5 72.1
Ala 174.5 296.3
Val 103.9 209.7
Leu 73.0 260.3
Ile 40.0 108.1
Ser 9.3 17.3
Thr 11.2 14.7
Lys 22.5 98.5
Arg 104.8 231.6
Asp 55.9 66.4
Asn 31.3 21.0
Glu 56.6 130.4
Gin 21.3 24.6
Met 9.5 71.3
Phe 108.9 198.9
Tyr 106.1 160.5
Pro 732.9 261.1
His 43.7 64.3
Total 1747.8 2307.3
[0152] As seen in Table 5, the P-glucan concentration of the syrup
(Example 13) was markedly higher than the malt liquor (Comparative
Example 2), both before and after boiling. Also, the nitrogen content
of the syrup (Example 13) was higher than the malt liquor (Comparative
Example 2), both before and after boiling.
[0153] As seen from Table 6 and Fig. 3, the concentration of each free
amino acid (except for proline) and the total free amino acid content of
44
CA 02697673 2010-02-24
FP08-0467-00CA-SB
the syrup (Example 13) were higher than the malt liquor (Comparative
Example 2), both before and after boiling. In particular, the
branched-chain amino acids (Val, Leu, Ile), Ala, Lys, Arg, Glu and Met
were notably higher in the syrup (Example 13) than in the malt liquor
(Comparative Example 2).
[0154] As seen from Fig. 4 and Fig. 5, no significant difference was
found between the syrup (Example 13) and the malt liquor
(Comparative Example 2) in terms of extract decrease (the rate of
reduction of the extract) or floating yeast count during the fermentation
process.
[0155] As seen in Table 7 and Fig. 6, the [3-glucan concentration of the
syrup (Example 13) was markedly higher than the malt liquor
(Comparative Example 2) after fermentation.
[0156] As seen from Table 8 and Fig. 7, the concentration of each free
amino acid (except for proline) and the total free amino acid content of
the syrup (Example 13) were higher than the malt liquor (Comparative
Example 2) after fermentation. In particular, the branched-chain
amino acids (Val, Leu, Ile), Ala, Lys, Arg, Glu and Met were notably
higher in the syrup (Example 13) than in the malt liquor (Comparative
Example 2).
[0157] These examples and comparative examples demonstrated that
the barley syrup of the invention is rich in P-glucans and amino acids
(especially Val, Leu, Ile, Ala, Lys, Arg, Glu and Met), and has
fermentative power equivalent to malt liquor obtained by conventional
methods. It was also demonstrated that the barley syrup of the
invention is suitable as a starting material for highly functional
CA 02697673 2010-02-24
FP08-0467-00CA-SB
effervescent alcoholic beverages, for example.
[0158] [Example 14]
(Preparation of barley syrup)
To 123 g of milled barley seeds obtained by milling whole-grain barley
(2006 Hokkaido Ryofii) seeds with a cyclone mill, there was added a
mixture containing 140 mg of a-amylases (KLEISTASE YC 15S,
product of Daiwa Fine Chemicals Co., Ltd.), 140 mg of 0-amylases
(A0448, product of Tokyo Kasei Kogyo Co., Ltd.), 140 mg of protease
(THERMOASE PC10F, product of Daiwa Fine Chemicals Co., Ltd.),
140 pL of pullulanase stock solution and 700 mL of water, and the
mixture was agitated at 60 C for 24 hours. The mixture was then
centrifuged at 5000 rpm for 30 minutes to obtain barley syrup
(supernatant).
[0159] (Measurement of 0-glucan molecular weight distribution)
The obtained barley syrup was used for measurement of the 0-glucan
molecular weight distribution by GPC. The following two different
samples were used (refrigerated sample and frozen sample), and each
was subjected to GPC analysis under the conditions described below
(analysis of the frozen sample was carried out to examine the
cryopreservability of the barley syrup). Two pumps (pumps A and B)
were used, and eluents A and B were passed through each pump A and
B.
[0160] = Refrigerated sample: A sample (filtrate) obtained by
refrigerating the barley syrup (supernatant) and then filtering it with a
0.45 gm filter at room temperature just before analysis.
= Frozen sample: A sample (filtrate) obtained by freezing the barley
46
CA 02697673 2010-02-24
,
FP08-0467-00CA-SB
syrup (supernatant) at -18 C and then thawing it at room temperature
and filtering with a 0A5 p.m filter just before analysis.
= Oven temperature: 40 C
= Column: Shodex OHPak SB-806HQ (molecular weight exclusion:
20,000,000) + Shodex OHPak SB-804HQ (molecular weight exclusion:
1,000,000) + Shodex OHPak SB-803HQ (molecular weight exclusion:
100,000).
= Mixing coil: Stainless steel tube with 0.5 mm inner diameter and 0.5
mL empty volume.
= Eluent A: Ultrapure water
Flow rate: 1 mL/min
= Eluent B: Ultrapure water (for RI analysis); Calcoflow solution (for FL
analysis).
Flow rate: 1 mL/min
= HPLC apparatus: LC-10 Series by Shimadzu Corp.
System controller: SCL-10Avp
Pump: LC-10ATvp
Oven: CTO-10ACvp
Auto sampler: SIL-10ADvp
Detector: RID-10A,RF-10AxL
= Analysis software: GPC analysis software for Class-VP, Class-VP
= Detector: Differential refractive index (RI) detector (temperature:
40 C); fluorescence (FL) detector (excitation wavelength of 360 nm,
fluorescence wavelength of 420 nm)
= Injection rate: 100 piL
= Analysis time: 40 minutes
47
CA 02697673 2010-02-24
FP08-0467-00CA-SB
[0161] The results are shown in Table 9 and Figs. 8 to 11. Table 9
shows the (3-glucan weight-average molecular weights (Mw),
number-average molecular weights (Mn) and their ratios (Mw/Mn), for
refrigerated samples and frozen samples of barley syrup. Fig. 8 and
Fig. 9 are chromatograms obtained for refrigerated samples and frozen
samples of barley syrup. Fig. 10 and Fig. 11 are I3-glucan molecular
weight distribution curves obtained for refrigerated samples and frozen
samples of barley syrup. The molecular weight distribution curves
were determined from the results of FL analysis, using a calibration
curve drawn with a 0.2% (w/v) aqueous solution of standard pullulan
(Shodex) [molecular weight (M): 5800, 12200, 23700, 48,000, 100,000,
186,000, 380,000, 853,000] as the standard solution (Fig. 12). The
results of FL analysis are considered to reflect the molecular weight
distribution of f3-glucans that specifically react with Calcoflow.
[0162] [Table 9]
Weight-average Number-average
Barley syrup molecular weight molecular weight Mw/Mn
(Mw) (Mn)
Refrigerated sample 142,200 92,500 1.54
Frozen sample 142,200 94,400 1.51
48