Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
COMPACT, HIGH-EFFECTIVENESS, GAS-TO-GAS COMPOUND RECUPERATOR
WITH LIQUID INTERMEDIARY
Cross-reference to related applications
This application claims the benefit of US application number 61/016,247 filed
December 21, 2007, and the benefit of US application number 61/034,148 filed
March
5, 2008, each of which is incorporated herein by reference for all purposes.
Field of the Invention
The field of this invention is heat exchangers, and more particularly,
compact,
gas-to-gas recuperation at high effectiveness for clean gases of similar heat
capacity
rates using compound recuperators with liquid intermediary.
Background of the Invention
Gas-to-gas recuperation with both high thermal effectiveness and order-of-
magnitude improvement in cost effectiveness is critical to addressing global
energy
needs, as shown in at least two co-pending patent applications. From a
manufacturing
perspective, the challenges arise from the fact that it is not practical to
produce heat
exchangers with closely-spaced fins on both the inside and outside of tubes,
and
alternative approaches thus far have had limited success.
An enormous number of heat exchangers have been well optimized for
numerous purposes over the past four decades. However, most have not been
directed at high thermal effectiveness F_ for cases where the heat capacity
rates in the
two streams are similar. The heat capacity rate Wof a stream is given by GCP
(its SI
units are W/K), where G is the mass flow rate (kg/s) and Cp is the specific
heat (J/kg-K).
By the standard definition of c (the ratio of heat transferred to the
theoretical limit), high
c is most easily achieved when Wmin (that of the weaker stream) is much less
than WmaX
(that of the stronger stream). However, exergy destruction can be minimized
only if
Wmin is close to WmaX. The terms "recuperator" and "regenerator" have usually
implied
the streams have similar Ws, and that will be the usage and regime of primary
focus in
this invention. However, the streams need not be in the same state - one may
be
liquid while one is a gas.
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
Common examples of cost-effective heat exchangers with high exergy loss
include automobile radiators and air-conditioning condensers. In the
automobile
radiator, for example, the warmed air leaves at a temperature much below that
at which
the hot water enters. Thus, most of the water's exergy (energy availability)
has been
destroyed, irrespective of precisely how one chooses to define it. Other
examples of
cost-effective compact exchangers for unrelated purposes include micro-
channel,
compact, fluid cooling systems, as seen for example in US Pat 6,907,921.
The subset of fluid heat exchangers directed at high c have mostly addressed
one of the following cases: condensing-vapor-to-liquid, condensing-vapor-to-
gas,
boiling-liquid-to-liquid, boiling-liquid-to-gas, liquid-to-gas, or liquid-to-
liquid. In all of
these cases, the fluid thermal conductivities, kt, W/m-K, are fairly large on
at least one
side (generally over 0.2 W/m-K), or phase change is present to drive small-
scale
turbulence on one side. A common gas-to-gas exchange application is in steam
power-plant superheaters. However, the steam here has high thermal
conductivity and
rather high density (for example, 0.067 W/m-K and 40 kg/m3 at 10 MPa, 650 K).
Moreover, high r- there is not an objective, as the flue gas will be used
subsequently for
boiling. Gas-to-gas exchange is also sometimes seen in air preheaters in steam
power
plants. Here, moderately high c may be seen, though usually the minimum flue-
gas
exhaust temperature is -400 K to limit corrosion from acid condensation, and
this limits
c of these recuperators.
Achieving high c in gas-to-gas exchange with low pumping power has been
challenging because volumetric specific heats are much lower than seen in
liquids and
thermal conductivities are usually low. Challenges are also seen in achieving
high c in
recuperators for organic liquids just above their pour point where viscosity
is quite high.
Doty, in U.S. Pat 4,676,305, disclosed a compact method of achieving highly
effective recuperation with low pressure drop for gases of similar Ws.
However, this
microtube recuperator has not yet been shown to be commercially competitive
with the
brazed plate-fin type, in wide usage in recuperated open Brayton cycles in the
30-250
kW range and occasionally up to 25 MW. See, for example, the microturbines
available
from Capstone Turbines Corporation, of Chatsworth, CA. These too have limited
cost
effectiveness and limitations in accommodating applications where there are
large
pressure differences (greater than -0.7 MPa) between the two streams at high
temperatures (above -750 K).
2
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
Optimized, compact high-c gas-to-gas recuperators require low flow velocities
(several percent of the sonic velocity), total flow-path exchange lengths in
the range of
0.1 to 2 m, and passage hydraulic diameters of 0.5 to 8 mm, with the larger
diameters
corresponding to pressures near 0.1 MPa and the smaller sizes corresponding to
pressures above 0.5 MPa. They have also required the use of construction
materials
having fairly low thermal conductivity, though that is not required in this
invention.
An alternative to paralleling tens of thousands of microtubes that has seen
rather
little usage but appears to be the most competitive for some compact
recuperation
applications is the rotating honeycomb regenerator, as used in some turbine
engines
where system mass is critical. Oda et al in US Pat 4,304,585 disclose an early
ceramic
design. Regenerators have seen very little usage largely because of the
difficulties in
obtaining adequate isolation between the high-pressure and low-pressure
streams and
because of the shedding of ceramic particles, leading to turbine abrasion.
Ceramic is usually selected for honeycomb regenerators in recuperated aero-
turbine applications because of the need for oxidation resistance at high
temperatures
and the advantage of low thermal conductivity in the flow direction. Rotating
ceramic
honeycomb regenerators have demonstrated c above 98% while the brazed plate-
fin
recuperators seldom achieve more than 87% c, primarily because of cost and
mass
optimization constraints. The honeycomb regenerators can be an order of
magnitude
more compact and an order of magnitude less costly for a given exchange power
and c
than plate-fin microturbine recuperators - which are an order of magnitude
more
compact than the gas-to-gas exchangers seen in most current chemical
engineering
and power generation applications.
Oxidation resistance is irrelevant in some applications, and there honeycomb
regenerators can be made at lower cost and with much higher reliability from a
low-
conductivity alloy honeycomb, such as silicon bronze, stainless steel, or some
magnesium or aluminum alloys. The thermal conductivity of silicon-nickel-
bronze can
be below 40 W/m-K, and 120 W/m-K is sufficiently low except perhaps for the
most
compact applications. For example, a magnesium alloy with thermal conductivity
-90
W/m-K has been used experimentally in a helicopter turboshaft engine. Titanium
alloys
would be better, and their relative cost should decrease over the next decade.
The
much higher thermal stress tolerance of metals compared to ceramics is
extremely
beneficial with respect to durability, as thermal stress is a primary factor
limiting ceramic
regenerator design and contributing to shedding of particles from ceramic
regenerators.
3
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
Regenerator cost for a given performance is typically near minimum when pore
diameters are about 0.7 mm for many mobile gas-gas exchange applications. The
relevant design theory, well understood for more than three decades, has
recently been
reviewed and updated by David G Wilson in "Design and Performance of a High-
Temperature Regenerator Having Very High Effectiveness, Low Leakage and
Negligible Seal Wear", paper GT 2006-90096, Turbo-Expo 2006. The use of a
metal
for the honeycomb, possibly with the innovations in Wilson's US Pat 5,259,444,
may
permit a satisfactory solution of the sealing and wear problems in larger
recuperators
where the pressure difference between the two streams is small.
However, the rotating honeycomb regenerator still has substantial limitations,
either where there are substantial pressure differences between the two
streams, or
where the size is small (below -100 kW), or where the lower-pressure stream is
above
-0.4 MPa. This last condition leads to greater difficulties in limiting
leakage and carry
over, and it leads to unreasonably low porosity requirements (or high
solidity) in the
honeycomb (for sufficient thermal storage). High solidity exacerbates axial
thermal
conduction losses and makes the regenerator more massive and perhaps more
prone
to stress-related failure. When two or more of the above conditions are
present
simultaneously, the honeycomb suffers markedly.
High-c recuperators are essential in many cryogenic processes. A common and
extremely effective design in cryocoolers uses micro-multi-port (MMP) tubing
with one
of the gases flowing in one direction through some of the "ports" (passages)
and the
other flowing in the opposite direction through the other ports. The viscous
losses in
very long lengths (4-20 m) of microtubes (under 1 mm ID, inside diameter) are
often
fully acceptable for gases at very high pressures (over 1 MPa) and low
temperatures
(below 140 K). Many cryogenic recuperators operate at such conditions, where
outstanding counterflow recuperators can be made from MMP tubing or a similar
construction. For many cryogenic applications outside the above conditions,
the novel
compound recuperator presented herein will be superior.
The basis for the innovation presented herein begins by learning from the
highly
developed liquid-to-gas exchangers best exemplified in air-conditioning (AC)
condensers and automobile radiators. To achieve the high c sometimes needed in
gas-
to-liquid recuperation, it is simply necessary to arrange 5 to 30 of such
exchangers in
series, with the liquid flowing serially from the first to the last and the
gas also flowing
serially, but from the last to the first. Such a counterFlow exchanger can be
an order of
4
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
magnitude less massive and less costly than conventional shell-and-tube gas-to-
liquid
counterflow exchangers of similar flow rates, pressure drops, and E. -
The dry-air condensers ubiquitous in AC condensers have been extremely well
optimized by numerous air-conditioning companies over the past four decades.
For
example, "80-ton" (280 kW of cooling) air conditioners are widely produced.
The air-
flow passage lengths in these condensers are often under 3 cm per row of
tubes; and
air-passages, though perhaps several centimeters wide, typically have
thicknesses of
-1.5 mm. This corresponds to a hydraulic diameter of -3 mm for the air flows,
which
interestingly is that predicted to be optimum at 0.1 MPa by the alternative
analysis
presented by Doty in US Pat 4,676,305. The condenser in such a unit typically
rejects
about 350 kWT at a bT (dry air) of about 10 C. Some large commercial freezer
systems
utilize refrigerant R744, C02, where condenser pressures can exceed 6 MPa, so
clearly
high tube-side pressures can be accommodated by cross-finned tubes produced by
automated manufacturing processes as used in AC condenser cores. These
exchanger cores are usually intended for use with two-phase flow tube-side
over a
significant portion of their length. However, predominately single-phase tube-
side liquid
flow, as seen for example in US Pat 3,922,880 in a design for use in an AC
unit, can
also be very cost effective.
In US Pat 4,831,844 Kadle discloses that for condensing two-phase tube-side
flow, substantial improvement is obtained by a step-down approach in which the
tube-
side vapor flow begins in two parallel tubes and then combines to a single
tube about
two-thirds of the way through the condensing process. Several advantages are
noted
for many AC applications, but the drawings therein also appear to show
interleaved
tube-side flow between parallel rows of finned tubes. Both step-down and
interleaved
tube-side flow would generally be disadvantageous for single-phase tube-side
liquid
flow in high-c exchange, as addressed herein; but with such patterns avoided,
common
AC condenser cores may be utilized for high-c recuperation.
Another common approach to improving tube-side heat transfer with a low-
velocity liquid of low kt is to use MMP tubing for the liquid-phase flow, as
discussed by
Guzowski et al (IMechE 1999, C543/083) and Guntly et al (US Pat 4,998,580). A
simpler method is to insert turbulators, such as open-pitch coil springs
inside the tubes.
This can be quite beneficial with single-phase tube-side flow of liquids under
certain
conditions.
5
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
The solution for order-of-magnitude improvement (compared to shell-and-tube
exchangers) in cost-effective high-c recuperation between clean gases shell-
side at
moderate temperatures and liquids tube-side is to simply use a series
arrangement of
several AC condenser cores (of proper design), serially connected inside a
pressure
vessel. As seemingly obvious and advantageous as the above approach is for
high-C
gas-liquid exchange, it does not appear to have been practiced as such -
liquid-only
tube-side flow through a series of thermally isolated cores. Related
exchangers, in
which the shell-side gas goes cross-wise back and forth several times over the
length
of cross-flow tubes, are commonplace; and often the tubes have fins (though
usually
spaced 3 to 15 mm). However, the above differences are of enormous importance
with
respect to manufacturing, compactness, and cost effectiveness.
Single- and multi-row cores similar to what are suitable for a component in
the
instant invention are produced by Armstrong under the product name DuraliteTM
Plate
Fin Coils. But apparently the value of thermally isolated serially connected
cores inside
a pressure vessel has not previously been appreciated as optimum to achieve
high-c.
Perhaps series arrangements of thermally isolated cores similar to those used
in
AC condensers have not been considered for high-c gas-liquid exchange because
most
large applications also require dealing with moisture, acids, and particulates
in the gas
stream. For many such cases, available shell-and-tube exchangers, developed
primarily for condensing shell-side steam, with typical tube diameters of 12
to 50 mm
and shell-side fins usually spaced -6 mm, may be the best option, especially
when the
gas pressure is below 0.12 MPa and high c is not desired.
The heat pipe is in some sense related to the compound exchanger disclosed
herein, as it too uses an intermediary fluid. However, the heat pipe uses a
self-pumped
two-phase fluid tube-side, and it is poorly suited to gas-gas recuperation. So
the
relationship to the heat pipe is tenuous at best. A complex, finned, device
cooler
shown in US Pat 7,296,619 may incorporate heat pipes, though that document
tries to
distort and confuse the standard meaning of "heat pipe". Regenerators are also
somewhat related, as they utilize an intermediary, but there it is a solid.
The standard air conditioner is most closely related to the inventive compound
recuperator, as it too provides heat transfer between two gases using a fluid
intermediary. There, however, the large majority of the heat transfer in each
exchanger
includes phase change, and a very energy-intensive vapor pump is required. It
is
possible that some air-to-air recuperators for heat recovery in buildings have
utilized
6
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
proprietary concepts somewhat related to those presented herein, but
apparently all
such have relied upon phase change in the fluid intermediary for most of the
heat
transfer, and there is no evidence that they have achieved high C.
Tube-side phase change has previously been desired because it greatly
increases tube-side heat transfer coefficient, ht, W/m2-K, and thus generally
allows
significant reduction in exchanger size. However, phase change is not desired
in the
instant invention, as it makes minimization of irreversibilities impractical
(because it
requires a very large number of intermediary loops). The instant invention
allows for
enormous reduction in exchanger size without phase change, and thus it also
readily
permits high c. Not surprisingly, commonly used "refrigerants" are the worst
type of
fluids that can be imagined for the applications envisaged by the instant
invention.
It is noteworthy that the chemical engineering process simulation software we
have evaluated is not capable of handling the case where a tube-side liquid
stream is
being heated by gas in a cross-flow finned-tube exchanger, as seen in the
instant
invention.
Two co-pending patent applications disclose enormous, emerging applications
for high-F- low-cost recuperation between clean gases where good solutions are
not
currently available: (A) where the hot gas stream enters above 550 K and at
more than
0.2 MPa, especially if the pressure difference between the streams exceeds 1
MPa, (B)
where some liquid condensation or frosting can be expected in one or both of
the gas
streams, and (C) where both gases are at pressures below 1 MPa, the pressure
difference exceeds 0.1 MPa, the temperatures are above 90 K, and cross-
contamination must be avoided. There also appears to be an enormous, emerging
application for high-c low-cost recuperation between viscous organic liquids.
The
invention presented herein addresses these and many other situations most
optimally.
The instant invention is, in practice, usually implemented as a minimum of two
separate modules with one or more liquid intermediary loops between them.
Naturally,
each independent module is usable as a fluid-to-liquid recuperator, where the
shell-side
fluid is usually a gas but may be a viscous liquid of low thermal
conductivity.
Relevant Art
1. MM Guzowski, FF Kraft, HR McCarbery, JC Noveskey, "Alloy and Process
Effects
on Brazed Automotive Condenser Tubing",
7
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
htt :/www. nt. hi /-kr ft"VTM 4 r. f, presented at IMechE 1999,
C543/083.
2. FD Doty, G Hosford, JB Spitzmesser, and JD Jones, "The Micro-Tube Strip
Heat
Exchanger", Heat Transfer Engr., 12, 3, 31-41, 1991.
3. DG Wilson and J Ballou, "Design and Performance of a High-Temperature
Regenerator Having Very High Effectiveness, Low Leakage and Negligible Seal
Wear", paper GT 2006-90096, Turbo-Expo 2006, Barcelona.
4. Trane Product Literature, "Installation, Operation, Maintenance: Series R",
hlt ://www.trane.com/webcache/rf/rotar `/g201i uid`/"20chillers4/Q20 rlc
/service/rtaa-
svx01 a-en09012005.pdf , RTAA-SVX01 A-EN, 2005.
5. FP Incropera and DP Dewitt, "Introduction to Heat Transfer", Wiley, NY,
2002.
6. RK Shah, AD Kraus, D Metzger, "Compact Heat Exchangers", Hemisphere Pub.,
NY, 1990.
7. LR Rudnick, "Synthetics, Mineral Oils, and Bio-based Lubricants: Chemistry
and
Technology", CRC, Boca Raton, 2006.
8. K Weissermel, HJ Arpe, Industrial Organic Chemistry, 4th ed., Wiley, 2003.
9. CH Bartholomew and RJ Farrauto, Industrial Catalytic Processes, Wiley,
2006.
10. E Prabhu, "Solar Trough Organic Rankine Electricity System (STORES)",
NREL/SR-550-39433, http://www.nrel.gcv/decs/fy06osti/39433.~rdf , 2006.
11. DESIGN II for Windows, Version 9.4, 2007, by WinSim Inc., documentation
available from http://www.lulu.corrÃ/includes/download.php2
fC I D= 390777&fM I D=810115 .
12. Armstrong DuraliteTM Plate Fin Coils, product information, Granby, Quebec,
2008,
htt ://www.armstron ginternaticnal.cem/files/common/all roductscatalo /
latefincoiIs
~df
U.S. PATENT DOCUMENTS
3,922,880 12/1975 Morris .......................................62/498
3,994,337 11/1976 Asselman et al .........................165/119
4,304,585 12/1981 Oda et al ...................................65/43
4,645,700 2/1987 Matsuhisa et al .........................428/116
4,676,305 6/1987 Doty ..........................................165/158
4,831,844 5/1989 Kadle .........................................62/507
5,259,444 9/1993 Wilson .......................................165/8
5,435,154 7/1995 N ish ig uch i ..................................62/476
6,907,921 6/2005 I n s l ey . . . . . . . . . .. . . . . . .. . . . . . . . ..
. . . . . . . . . . . . . .165/ 170
6,957,689 10/2005 Ambros et al ..............................165/41
7,225,621 6/2006 Zimron et a1 ................................60/651
7,296,619 11/2007 Hegde ........................................165/104.33
U.S. PATENT Application Publication
US 2006/0211777 9/2006 Severinsky
Summary of the Invention
8
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
A liquid-loop compound recuperator is disclosed for high-c heat exchange
between a
first shell-side fluid stream and a second shell-side fluid stream of similar
thermal
capacity rates (W/K). The compound recuperator is comprised of at least two
fluid-to-
liquid (FL) recuperator modules for transfer of heat from a shell-side fluid,
usually a gas,
to an intermediary tube-side heat transfer liquid (HTL). Each FL module
includes a
plurality of thermally isolated, serially connected, adjacent exchanger cores
inside a
pressure vessel. The cores are rows of finned tubes for cross-flow transfer of
heat, and
they are arranged in series to effectively achieve counterflow exchange
between the
HTL and the shell-side stream. The HTL may be water, an organic liquid, a
molten
alloy, or a molten salt. Alumina-dispersion-strengthened-metal fins,
superalloy tubes,
and a lead-bismuth-tin alloy HTL may be used for high temperatures. Cumene may
be
used as the HTL in cryogenic applications.-
Brief Description of the Drawings
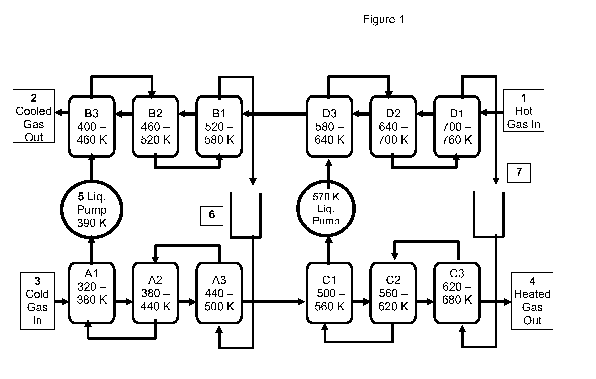
Figure 1 illustrates schematically a multi-stage, liquid-loop, compound
recuperator.
Figure 2 illustrates the preferred liquid routing for a portion of a compound
exchanger.
Figure 3 is a perspective, cut-away view of a typical fluid-liquid exchanger
module.
Figure 4 illustrates a typical, single-row, finned-tube core.
Figure 5 illustrates a serpentine pattern in a finned-tube core.
Figure 6 illustrates five thermally isolated series tubes.
Figure 7 illustrates a radial-flow version of an FL module.
Detailed Description of the Preferred Embodiment
Figure 1 illustrates a 4x3 array of 12 liquid-gas cross-flow exchanger cores
with
2 liquid pumps and two different heat transfer liquids as an example of a
method of
achieving high-c recuperation between two isolated fluids of low thermal
conductivity,
gas-I and gas-2, identified in the figure using hollow lines. These fluids
have mean
thermal conductivity less than 0.4 W/m-K (that of H2 at -720 K) and will
usually be
gases with kt less than 0.06 W/m-K. Thus, for improved clarity, they are
generally
9
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
referred to as gases herein, though applications where these fluid streams
would be
viscous organic liquids are seen in a co-pending patent application. Both gas-
1 and
gas-2 are shell-side, sometimes also called "fin-side". In this example, gas-1
is the hot
source stream, and gas-2 is the cold stream being heated to nearly the entry
temperature of gas-1. Often, the hotter gas will be at lower pressure than the
cooler
gas, but the reverse relationship is also possible.
In the example of Figure 1, there are four sets of exchangers (A, B, C, D).
The
heat transfer liquids (HTLs) are identified in the figure with heavy solid
lines. Here,
each is directed serially through three cross-flow exchanger cores for each
gas stream.
The HTLs are all tube-side.
In this example, gas-1 enters 1 fin-side into exchanger labeled D1 at 760 K
and
exits 2 fin-side from exchanger B3 at 400 K. Gas-2 enters 3 fin-side into
exchanger
labeled A1 at 320 K and exits 4 fin-side from exchanger C3 at perhaps 680 K.
For such
temperatures with similar Ws, c would be about 78% by the standard definition.
Here, each gas stream passes through 6 cross-flow exchanger cores, three on
each side of each liquid loop. In practice, this will often be a minimum
number, though
it also depends on how one defines a cross-flow exchanger core. For example, a
typical AC condenser "core" contains 2, 3, or 4 rows of finned tubes, often
connected
serially. Hence, a typical, 3-row, serial "AC core" could perform the three
serial
exchanges as shown in Figure 1 for each side of each loop. Herein, 3 rows of
thermally
isolated finned tubes, serially connected, is considered to be three cross-
flow
exchanger cores in series. For the rows to be considered thermally isolated,
it is
required that the fin metal not be continuous from one row to the next (at
least on most
of the fins) and that the tube flow pattern not be interleaved - that is, that
the tubes not
return back to a first row after leaving that row and going to a second row.
From a
functional perspective, the rows may be considered to be thermally isolated if
the
thermal conduction of the metal between adjacent rows is less than twice the
thermal
conduction of the fluids (the sum of the shell-side and tube-side) between the
rows.
For variety in presentation, a thermally isolated, serially connected, cross-
flow
exchanger core may be referred to as a "finned tube ro\&/'. The complete
serial group
of tube rows in a single HTL loop for one of the gases will be referred to as
a "core set".
The core sets will be inside a pressure vessel to contain the shell-side
pressure, and
often all the sets associated with the first gas stream would be inside one
pressure
vessel, and those associated with the second gas stream would be inside a
second
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
pressure vessel. For example, sets B and D of Figure 1 would normally be
inside one
pressure vessel and sets A and C would normally be inside a second pressure
vessel.
The pressure vessel with the cores it contains may be referred to as a fluid-
to-liquid
(FL) recuperator module or a gas-to-liquid (GL) recuperator module, as the
shell-side
fluid will usually be a gas.
The combination of two FL recuperator modules coupled with an intermediary
HTL may be referred to as a liquid-loop recuperator or a compound recuperator.
At
least one liquid pump 5 and surge tank 6 are also required for each compound
recuperator. Figure 1 illustrates a dual-loop compound recuperator.
For minimization of 6T-related irreversibilities, the thermal capacity rate
WL=GLCPLof the HTL through a core set in a compound recuperator should be
close to
the geometric mean WS of the thermal capacity rates W1 and W2 for the two
shell-side
gas streams, G1Cp1, and G2Cp2,
WL - Ws=(W1W2)0.5 [1]
Moreover, the ratio W1/W2 should be fairly close to 1, though the compound
recuperator
will also be advantageous for other conditions. Normally, WL would be between
0.7Ws
and 1.4WS. Of course, GL is proportional to npvd2, where n is the number of
parallel
tubes in a core, p is the fluid density, v is the flow velocity, and d is the
tube inside
diameter (mm).
The practical c limit (for similar Ws) is essentially determined by the total
number
of rows, nr, of isolated, serially connected, finned tubes (or cores) and the
"number of
heat transfer units", NTU, where
NTU = hcsAxlWs , [2]
where AX is the heat transfer surface area. The c suggested for Figure 1 is
probably
above a cost-effective limit for just 12 total cores with liquid
intermediaries, though it is
certainly possible. On the other hand, with 16 cores per set, four sets, and
two liquid
loops, a practical limit of about 94% would be expected. The same practical
limit would
be expected with a single liquid loop and 32 cores per set. Such a design
would be
preferred when the temperature difference between the hot source gas and the
cold
source gas is rather small, as this requires only one liquid pump. Having
multiple loops,
11
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
as shown in the multi-stage compound recuperator of Figure 1, allows for the
use of
different HTLs in different temperature ranges, which allows for improved
performance
in large recuperators operating over a large temperature range.
Figure 1, though drawn acceptably by diagramming conventions and chosen for
its clarity, does not convey flow details that improve maximum practical
effectiveness
per tube row. The fluid routing shown in Figure 2 better conveys liquid
routing details
that significantly improve effectiveness per stage. There, the liquid enters
each row
from the same side relative to the shell-side flow, which is always
distributed across the
face of the tube rows, as indicated by using parallel gas-flow arrows. The
object is to
make the direction of the thermal gradient along each row the same and
maintain a
fairly uniform change in the gas temperature per row across the face of each
core.
Such a tube-side flow pattern is uncommon in AC condenser cores, as c there is
not so
important.
Suitable finned-tube AC condenser or evaporator cores, though generally
without the most optimum tube-side flow routing, are readily available for
efficient heat
transfers at power levels from about a hundred watts to tens of kilowatts, and
heat
transfers of hundreds of megawatts can be handled just as cost effectively by
paralleling tens of thousands of suitable AC cores. AC condenser cores are
available
at low cost because efficient production methods have been so highly optimized
from
the competitive pressures of high-volume manufacturing over the past four
decades.
Accommodating high shell-side pressures is straightforward - one simply places
the
assembly in a large pressure vessel with suitable baffles, as seen in U.S. Pat
4,676,305, for example, and addressed later in more detail. Although AC
condenser
cores are usually intended for operation near 310 K, they are sometimes
constructed
using copper tubing with aluminum or copper fins brazed on using a filler
material
having liquidus near 870 K. It is also not too uncommon to use 90Cu-10Ni alloy
C706
for the tubing with copper fins. In larger sizes these cores typically use
tubing of 9 to 13
mm diameter, and the fin pitch (center-to-center spacing) is often under 2 mm.
Fin
length in the direction of air flow is typically -25 mm per row, though
sometimes up to
80 mm per row. Fin pitch in the FL recuperator up to 8 mm may be desired if
the shell-
side fluid is a very viscous liquid, such as an oil just above its pour point.
Only minor modifications of available cores are needed to permit operation to
about 700 K at limited pressures with non-oxidizing clean gases. Moreover,
operation
to 900 K in many non-oxidizing conditions is possible by simply changing to an
12
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
alumina-dispersion-strengthened copper such as C15720 (0.4%AI203, bal Cu) for
the
fins and to the common 70Cu-30Ni alloy C715 for the tubing (-70 MPa yield
strength at
-900 K for C715, compared to -750 K for alloy C706).
The benefits of this approach may not be immediately clear to those accustomed
to evaluating heat transfer primarily on the basis of surface area, as (A)
changing from
a conventional shell-and-tube exchanger to a typical AC condenser core may
increase
the shell-side surface area per volume by only a factor of 5 to 10 (from -200
m2/m3 to
1000 or perhaps even 2000 m2/m3), (B) the tube-side "compactness ratio" may
decrease by a factor of 2 or more, and (C) the heat has to be transferred
twice. What
may be overlooked is that the shell-side heat transfer coefficient, ht, W/m2-
K, will also
typically increase by a factor of 5 to 10 because the passage thicknesses are
decreased, so the shell-side total benefit can be a factor of 25 to 100. By
selection of
an optimum HTL and flow velocity, the tube-side ht can easily be made over 30
times
(possibly even more than 200 times) that of most gases - usually without
adding tube-
side turbulators. Hence, the novel compound-exchanger can permit an order of
magnitude improvement in compactness compared to shell-and-tube exchangers for
gas-gas exchange (for comparable powers, flow rates, E, and pumping losses)
even
though the heat has to be transferred twice.
The increased complexity associated with compound recuperators using a liquid
intermediary would not be justified below some size threshold. That cutoff is
dependent
on many variables, including desired c, temperatures, gas compositions, the
importance
of mass reduction, cleanliness of the gas streams, and gas pressure
differences. It will
also depend on availability of appropriate finned-tube cores for the relevant
conditions,
a factor that is likely to change markedly over time. Even today, it seems
that
compound recuperators would be preferred for many cases with non-oxidizing
gases if
c above 70% is desired at (A) temperatures below 700 K, (B) exchange power
levels
above 20 kW, and (C) mean gas pressures above 0.05 MPa. Suitable cores for
competitive compound exchangers for a much wider range of conditions should
become available.
A few more comments are useful to help elucidate the value and hence
inventiveness of the instant invention. The shell-side thermal specific
conductance, W/
kgK, under the typical shell-side flow conditions (largely laminar) will be
inverse with the
square of the pitch; but the mass will be nearly independent of the pitch for
a given core
volume. Clearly, as materials become steadily more expensive, there will be a
strong
13
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
incentive to minimize the pitch to permit ever higher heat transfer per
exchanger mass.
Of course, the shell-side pressure drop will increase inversely with the pitch
for a given
flow velocity. However, most applications will be with shell-side pressure
well above
0.2 MPa, and the shell-side pumping power losses will often be inverse with
the square
of the gas density. Hence, smaller fin pitch than is generally seen in AC
condenser
cores will often be optimum, as long as the shell-side flow-section area AS
(the frontal
section area, not Ax) is kept large and the flow path length is kept short, as
discussed
later in more detail.
Minimum channel thickness in most prior-art, compact, high-effectiveness
exchangers is ultimately limited by the need to establish highly uniform flow.
Hence,
manufacturing tolerances limit minimum spacing. Channel thickness tolerance is
not as
critical in the instant invention because flow mixing can readily occur
between
successive thermally isolated cores.
Current AC condenser practice (-2 mm fin pitch) is probably close to optimum
in
the compound exchanger for mean gas pressures of -0.3 MPa, mean kt of -0.04
W/m-
K, and F of 75-90%. The fin pitch can often be reduced (for further reductions
in
exchanger mass and cost) at higher gas pressures or low temperatures. However,
there are limits, as the fin thickness must be sufficient to provide the
needed thermal
conduction and stiffness, and corrosion lifetime may be an issue in some
cases. A
strong advantage of the liquid-loop compound exchanger compared to
regenerators is
that the gas-passage thickness of the high-pressure gas stream may readily be
made
much less than that for the low-pressure gas stream, as desired for maximum
performance.
It is not uncommon in AC condensers for the tubes to have internal features
such as ribs, fins, or undulations to increase htL, though this adds to tubing
cost and
increases stress concentrations. Such surface enhancement is mostly beneficial
in the
initial portion of a condenser where no condensation is occurring (the tube-
side vapor is
still superheated) and in the final portion (subcooling) where the liquid
velocities are
very low. Surface enhancements are much less beneficial in the compound
recuperator because the tube-side flow is liquid-only, of essentially constant
velocity,
which may be better optimized.
Heat-Transfer Liquids (HTLs). The primary requirements in the HTL are
chemical stability at the relevant conditions, low viscosity, low vapor
pressure, high
14
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
thermal conductivity, fairly low cost, low health hazard, and high
autoignition
temperature (AIT). It is also beneficial to have freezing point above the
minimum start-
up temperature, though thaw-out measures can be implemented. The AIT is also
of
minor importance, as inert or reducing-gas pressurization over the HTL would
normally
be incorporated; but it is still of some concern, should liquid leaks develop.
Water,
organic fluids, molten alloys, or molten salts will normally be selected,
based mostly on
the temperature range. Table 1 presents some pertinent data, some of which are
estimates, on some HTLs at 500 K. The column labeled "Risks" gives a single,
overall
indication of the three hazards normally considered - health, flammability,
and
reactivity.
The (turbulent-flow) tube-side heat transfer coefficient may be calculated by,
htL = Bi Goa kto6 (CP/ )0.4 d-1.8 [3]
where u is the dynamic viscosity (cP, centipoise, which is identical to 1 mPa-
s, or 0.001
kg/m-s) and B, is a dimensioned factor that is nearly constant over a wide
range of
conditions but varies with surface features and other exchanger design
details. (Note:
a fluid with u =1 cP and p=1000 kg/m3 has kinematic viscosity,,u/p, of 1 cSt,
centistokes.) A few simple manipulations and calculations are useful:
G08 = B2 (p v)o.s d1.6 [4]
htL = B (p v)o.s kto.6 (CP/,u)0.4 d -0.2 [5]
FH = po.8 kto.6 (CP/u)0.4 [6]
htL = B vo.8 FH d-0.2 [7]
where the B's are dimensioned constants, and FH is a convenient, composite
fluid
property. A typical magnitude of B at a Reynolds number of 10,000 to 20,000
inside
smooth tubes is -5.6, assuming the parameters are in the units shown above.
For
40wt engine oil at 500 K, for v=10 m/s in tubes of 0.0077 m ID (Re 0 15,000),
this gives
htL 0 9000 W/m2-K. For comparison, FP Incropera gives a representative value
of
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
overall ht for air in cross-flow with water inside finned tubes as -35 W/m2-K,
and
maximum overall htof 6000 W/m2-K for steam condensers.
From eq. 7 and the above example, it might appear that one simply needs to
increase the HTL flow velocity to make htL very large compared to mean shell-
side heat-
transfer coefficient hs (as desired for economic optimization), but of course
that
consumes power - which increases almost as the cube of v. The pumping power
also
increases with increasing p, u, and flow length. Considering this, a better
HTL figure of
merit (composite fluid property) for its selection than the above FH is the
following FM,
Table 1. HTL Properties at 500 K
pour CP, k~, FD ~tj Name point, n.b.p. K IT, kg/m3 K/kg cP W/m- Risks H M kDt
K acetone 185 329 138 411 3.44 0.05 0.08 2 146 1430 2200
ethanol 200 352 336 475 3.43 0.06 0.09 1 164 14602400
butanol 210 380 399 581 3.78 0.0940.10 1 179 1260 2300
water 274 373 835 4.57 0.11 0.646 0 143 5130 ~200
toluene 190 384 08 640 2.51 0.12 0.077 2 127 357 1030
cumene 130 425 397 661 4.57 0.15 0.108 2 188 10902220
ethylene glycol 260 470 373 935 3.16 0.34 0.2 1 221 369 1740
1-but Inaphthalene 260 561 00 824 2.1 0.35 0.093 2 106 269 50
Delo 100 30wt 243 570 550 670 2.5 0.3 0.09 0 100 300 500
PAO, Delo 400 5W40 230 580 320 670 2.5 0.3 0.09 0 100 300 500
Delo 6170 40wt 255 620 340 680 2.5 0.35 0.09 0 36 260 1.40
POE, Mobil 254 212 640 372 700 2.5 0.4 0.1 1 39 250 1.40
dioctyl phthalate 250 657 180 798 2.1 0.5 0.11 1 38 200 365
1-dodec I-na hthalene 305 676 00 795 2.5 0.41 0.092 1 103 250 1.45
tri-o-cresyl phosphate 260 693 580 950 2.2 0.4 0.11 2 136 340 680
TBPP-100 phosphate 270 708 195 900 2.2 0.5 0.13 1 123 255 515
ol hen I ether 5P4E 280 749 60 970 1.9 0.6 0.13 1 114 200 00
60NaNO3-40KNOs 480 870 70 1950 1.4 4.5 0.45 2 166 17 270
55Bi-45Pb 400 1800 10000 0.15 2.7 4 2 1150300 2220
38Pb-37Bi-25Sn 400 1900 9000 0.18 2.5 8 1 1770520 5200
FM = kro.6 (pCP)o.s/ [8]
The combination of the need to achieve htL hs at moderate v and flow length,
along with good W matching, imposes constraints on the tube diameters and the
tube
paralleling scheme. The HTL would usually have nearly constant velocity
throughout
most of the cores, so tube diameters would be nearly constant throughout.
However, in
16
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
cores containing several parallel tubes, it may be beneficial for them to
combine at their
core entrances and exits to simplify tubing inter-connections between cores.
Clearly,
the HTL velocities in the interconnections could be very different from the
typical value
in the cores.
As seen in Table 1, FM is low for organics compared to that for water or
molten
alloys, but it is usually higher than that of molten salts - a concept that
has previously
been misunderstood. Other advantages of organics may include no freezing
problems,
no metal erosion, lower corrosion, lower density, lower toxicity, lower cost,
lower
viscosity, and simpler disposal problems. Pressurized water can be used well
beyond
500 K, but exchanger costs are increased because of the very high stresses. An
organic of low vapor pressure is often better, though in some applications
lower-boiling
fluids, such as ethanol or even acetone, could meet the specific requirements
and be
preferred. Note that the relative merits of the HTLs are temperature
dependent.
Silicone fluids (such as Dow Corning 550, AIT of 755 K, but not suitable for
long
term usage above 550 K) and low-grade hydrocarbon (HC) mixtures, such as Exxon
Caloria HT-43 (AIT of 627 K) have been used. Some more attractive organic
fluids with
n.b.p. and AIT both above 660 K, pour point below 320 K, and acceptable
chemical
stability and safety are: (A) polyphenyl ethers (PPEs, aerospace lubricants
and
diffusion-pump oils, 5-ring type 5P4E has AIT-880 K, n.b.p.=749 K, 290 K pour
point,
AGf -2 kJ/g, non toxic, has been used in short-term vapor-phase lubrication up
to 870
K), (B) polyol esters (POEs, most type-2 aviation turbine oils, AIT usually -
670 K, but
AIT and n.b.p. can be over 740 K), (C) polyalphaolefins (PAOs, a major
component in
type 5W50 synthetic engine oil, 16 cSt at 100 C, AIT often -650 K, but AIT can
be -700
K in heavy PAOs), (D) phosphate esters (used in aviation hydraulic fluids),
(E) phenyl
silicones, (F) fluorocarbons, (G) polymer esters (PEs), (H) phthalates, and
(I) mixtures
of the above and high-boiling alkylated polynuclear aromatics. See Table 1 for
data on
two alkylated polynuclear aromatics.
Highly branched alkanes are preferred to n-alkanes in engine lubrication
applications, as they have much better oxidation resistance, much lower
viscosity for a
given boiling point, and are more resistant to dehydrogenation and cracking.
The
relative price of such synthetic oils, similar to PAOs, should drop
substantially over the
coming decade.
Inexpensive tin-lead alloys may be acceptable as an HTL at high temperatures
with stainless or superalloy tubing. The solubility of iron in tin is about
0.1 % at 650 K,
17
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
and this may lead to excessive exchanger erosion with low-alloy steel tubing
(even after
the molten alloy becomes saturated with iron, as there will be some thermal
gradients in
the liquid). The solubility of iron in both bismuth and lead is at least an
order of
magnitude lower than in tin. However, alloys of more than 50% bismuth expand
upon
freezing (if not immediately, then after several days), and this could produce
unacceptably high stresses within the exchangers. Lead-bismuth-tin alloys of
relatively
low tin content should be fine with some low-cost steel alloys for the tubing.
The
38Pb-37Bi-25Sn alloy shown in Table 1 has an excellent balance of low iron
solubility,
low vapor pressure, low toxicity, high FM, low cost, and low liquidus
temperature, though
perhaps lower Bi and Sn contents with increased Pb and minor additions of
antimony
(Sb) would give an even better balance.
Molten salts, especially mixtures of NaNO3, KNO3, NaNO2, and Ca(N03)2, have
often been used for HTLs. Some have freezing points lower than those of some
lead
alloys, but their upper temperature limits are lower. For example,
KNO3decomposes at
670 K and NaNO3 decomposes at 650 K, though some mixtures, such as the
eutectic
listed in Table 1, have higher stable temperature limits. There are some
security risks,
as all can easily be used to make powerful explosives of limited stability.
Moreover,
their NEPA health ratings are usually "2, highly hazardous", and their FM is
quite inferior
to other options. When hot, they react vigorously with most pump lubricants
and
elastomeric seals, and they slowly attack many alloys at grain boundaries.
Another
complication with salts, alloys, and heavy polynuclear aromatics is that they
are solid at
room temperature.
The thermal conductivities of the gases expected in some emerging applications
typically range from 0.04 to 0.06 W/m-K at 500 K (for CO, C4H,o, air, and some
H2/C02
mixtures of interest), gas densities are often -5 kg/m3, Cp is often 1 to 3
kJ/kg-K, and
is typically 0.01-0.03 cP. For gases under the relevant shell-side
(substantially laminar)
conditions, a composite fluid property more useful than FM for estimating the
ease with
which heat transfer may be achieved is
FG = kr (pCP)21,u [9]
A useful expression for comparing liquid and gas heat transfer fluids for
similar flow
geometries (same hydraulic diameters, flow lengths, etc.) is (FmFG f5 and this
suggests the heat-transfer challenges for the gases could be two to three
orders of
18
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
magnitude greater than for liquids for similar geometries. A simpler
parameter, Fo, for
comparing diverse fluids is included in Table 1 and discussed in the last
section with
reference to applications for shell-side liquids. The important point here is
that there is
usually little need to worry about tube-side heat-transfer enhancement. This
allows
enormous manufacturing simplifications. The focus needs to be primarily on
reducing
passage thickness and increasing surface area on the shell-side.
FL Module Implementations. As noted previously, the core sets will be inside
a pressure vessel sufficient for the shell-side pressure. Often, all the sets
associated
with the first shell-side stream (usually a gas) would be inside one pressure
vessel, and
those associated with the second shell-side stream would be inside a second
pressure
vessel. The pressure vessel with the cores it contains is referred to as a
fluid-to-liquid
(FL) recuperator module, and a typical embodiment is shown crudely in Figure
3.
A typical FL recuperator module might contain 30 series-connected, thermally
isolated finned-tube cores 31 (though the figure shows just 8 cores for better
clarity),
each having typical external dimensions of about 1 m x 1 m x 0.03 m. The shell-
side
entrance and exit ports 32, 33 are normally at the opposite ends from the tube-
side
entrance and exit ports, 34, 35. A typical core is better illustrated in
Figure 4, though
again not likely to scale. Each 1 m x 1 m core might typically have 40
parallel finned
tubes 41, each of 8 mm ID and 10 mm OD, each traversing the full width, with
typical
center-to-center spacing of 25 mm, and tube-side entrance and exit manifolds
42, 43.
Figure 4, on the other hand, shows 20 tubes and 64 fins, which is closer to
being typical
for a 30 cm x 30 cm core, though even there the number of fins would likely be
greater
than shown by a factor of 2 to 4.
If the fin thickness is 0.5 mm and the fin pitch 1.6 mm, then the example
shell-
side flow-section area AS is about 0.7 m2 and the tube-side flow-section area
AT is
-0.002 m2. Hence, As is -350 times AT. For the typical core dimensions noted
earlier
(1 m x 1 m x 0.03 m), the mean shell-side flow length Ls is about 0.03 m per
core, and
the mean tube-side flow-length LT is 1 m per core. Hence, LT is about 30 times
Ls.
Note that this ratio is independent of the number of cores when they are
serially
connected, as both flow lengths increase by the same factor. The flow-section
area
ratio is also independent of the number of serially connected cores. There may
be a
substantial gap between adjacent cores, as depicted in Figure 3, in the shell-
side flow
19
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
direction for pressure equalization across the face of the cores and some
transverse
mixing, but the shell-side flow is substantially axial with respect to the
pressure vessel.
The tube-side HTL flow is shown in Figure 3 as being ducted 36 from an exit
manifold on one side of a core diagonally across to the entrance manifold on
the next
core. Note that the HTL enters all the cores on the same side and exits all
the cores on
the opposite side. The diagonal HTL ducting pattern is one way to improve tube-
side
flow homogeneity. Other measures may also be taken, and often a primary
measure
will be judicious selection of the diameter of the tubes 41 such that the flow
velocity
within them will achieve pressure drops that are large compared to the
pressure drop in
the manifold while simultaneously meeting the other previously noted
requirements with
respect to pumping power, ht, and WL. Support structure for the cores is not
shown,
though clearly some is needed. The flow-cage 37 for constraining the shell-
side flow
within the cores is only partially shown. For the conditions normally
addressed, the
shell-side volumetric flow rate will generally be relatively high (especially
when
compared to tube-side), so shell-side pressure drops from inlet 32 to outlet
33 must
necessarily be low (to achieve low pumping power) and differential stresses on
the
cage can easily be handled. The pressure vessel would preferably have a burst
pressure greater than twice the mean shell-side relative pressure and
generally much
greater than 0.3 MPa.
One may define an HTL conductance YF [W] between adjacent cores as
YF = Td WL [10]
where Td is the mean temperature difference between adjacent cores. Adjacent
cores
are herein considered to be effectively thermally isolated if the heat
conducted between
cores through solid materials is less than one-third of YF. Such a condition
is not easily
met if more than 20% of the fins are continuous from one core to the adjacent
core in
the shell-side flow direction, but such a condition is easily met if none of
the fins are
continuous between adjacent cores and the tube pattern is not interleaved
between
adjacent cores. However, adequate thermal isolation will sometimes be possible
if up
to 30% of the fins are largely continuous, except for holes for transverse
pressure
equalization, between adjacent rows.
It is not necessary for all tube rows to be thermally isolated. A "compound
core"
may have several rows of finned tubes thermally coupled by continuous fins
between
them for improved core robustness. However, the practical effectiveness limit
is
strongly dependent on the total number of thermally isolated cores in series.
Hence, it
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
will often be desirable for this number to be more than 20, though there will
be some
cases when as few as two thermally isolated cores per FL module are
sufficient. It is
unlikely that a compound core would contain more than four rows of thermally
coupled
rows of finned tubes. In most cases, each thermally isolated core would be a
single
row of finned tubes, as shown in Figures 3 and 4.
Shell-side flow homogeneity is also essential for high c, at least when the Ws
are similar. In most cases, allowing for pressure equalization across the
faces, as is
readily achieved when none of the fins are continuous between adjacent cores,
will be
sufficient, as shown in Figure 3. In the prior art, all the fins are usually
continuous
between adjacent rows, such as seen in the Armstrong DuraliteT' Plate Fin
Coils
products. A minor fraction could still be continuous. In such a case, pressure
equalization across the faces of thermally isolated cores can readily be
achieved if
holes or cut-outs are included in fins joining adjacent cores.
With series-connected cross-flow exchangers, the flow homogeneity may be
further improved by inserting turbulent mixers in the gas flow streams between
cores.
(This obviates the benefit from the flow routing illustrated in Figure 2, but
is better than
the alternative of channeling -where, because of the viscosity dependence on
temperature, the shell-side velocity may become higher than mean on one side
of all
the cores when the shell-side gas is being heated a large amount in each
core.) The
use of separate, series-connected FL modules further simplifies the insertion
of
turbulent mixers into the shell-side stream.
Figure 5 illustrates a portion of a core with a serpentine pattern that may be
desired to better meet the HTL velocity and pressure-drop objectives in some
cases. If
the tube-side flow for the core of Figure 4 were instead handled by 10
parallel tubes of
8 mm ID, each traversing the full width 5 times in a serpentine pattern with
center-to-
center spacing of 20 mm, the tube-side flow-section area then would be 5E-4
m2. With
a reasonable allowance for the bends at each end, each tube may then need to
be -6
m long. In this case, LT would be about 200 times LS, and Aswould be -1400
times AT.
As in Figure 4, the shell-side flow in Figure 5 is normal to the plane of
these figures.
A method of arranging thermally isolated, serially connected finned tubes
without
manifolds between them is shown in Figure 6. Such an arrangement may have
manufacturing advantages in some cases. An option is to stack a large number
of
serpentine finned tubes normal to the plane of Figure 6, with the shell-side
flow as
indicated. For the shell-side flow direction assumed in Figures 4 and 5, the
fins would
21
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
normally be continuous between the tubes as shown within a core. However, the
fins
could not be continuous between the tubes for the flow direction shown in
Figure 6 and
achieve thermal isolation between these serially connected tubes. Conventional
usage
may not refer to such an arrangement of a single, serpentine finned tube as a
"core".
When a large number of the serpentine finned tubes as shown in Figure 6 are
stacked
normal to the plane of the Figure, five thermally isolated planar cores are
effectively
formed.
As discussed earlier, it will sometimes be preferable to utilize more than one
liquid loop. Hence, in some applications, there may be two or even three
liquid loops
servicing the cores in a single FL module. In some applications, it may be
preferable to
utilize separate pressure vessels for the high-temperature cores, mid-
temperature
cores, and low-temperature cores, and in such cases in particular a small
number of
thermally isolated cores per FL module may be sufficient. In larger
applications, it will
often be desirable to arrange modules in parallel, as it may not be optimum to
manufacture modules larger than can easily be transported by truck. Note that
paralleling also does not affect the ratios AS/AT or LT/LS, but the ratio
ASILS steadily
increases with capacity in an optimum design.
For very large modules, a hexagonal arrangement of the cores as shown in
Figure 7 (depicted without the containment vessel) may be preferred, as it
permits a
larger ratio of As/Ls within practical trucking constraints. Here, the shell-
side fluid flow is
generally radial, and the core arrangement of Figure 6 is assumed, though the
arrangement of Figure 4 could also be used. Similar arrangements of finned-
tube
cores, except square rather than hexagonal, are commonplace in the AC
industry,
where shell-side air flow through a condenser exhausts to atmosphere. However,
the
prior-art condensers (A) utilize tube-side phase change for most of the
enthalpy
change, (B) are not enclosed in a pressure vessel, and (C) may not include
serially
connected thermally isolated cores.
For the hexagonal arrangement shown in Figure 7, the shell-side face flows are
all functionally in parallel. Hence, the tube-side flows must also be
functionally in
parallel. In other words, all of the innermost cores would connect to the same
HTL port,
and all of the outmost cores would connect to the same HTL port. Obviously,
pentagonal, octagonal, or other circumferential arrangements of cores could
also work
well. The pressure vessel would normally have its axis aligned perpendicular
to the
shell-side flows through the cores.
22
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
Cores of significantly different characteristics may also be combined, either
in
series or in parallel, with predictable results, though the analysis is more
complex.
Clearly, many variations in dimensions and patterns are possible, but
generally AS
would be more than 100 times AT and LT would be more than 10 times Ls. Such
ratios
appear to be well outside the prior art in multi-pass, finned-tube, shell-and-
tube heat
exchangers.
Core Modifications for Severe Conditions. For high performance at
demanding conditions (high temperatures, oxidizing atmospheres, or large
differences
in pressures between the two gases), appropriate changes in choice of
materials for the
tubing, fins, and braze are required. The tubing material is selected
primarily for yield
strength at the required temperature, formability, brazability, and corrosion
resistance.
The fin material is selected primarily for thermal conductivity, cost,
corrosion resistance,
melting point, and brazability. In some cases, the fins have been pressed on
rather
than brazed on, but this approach is less desirable for extremes of
temperature, for
closely spaced thin fins, or if much vibration is likely.
Alumina-dispersion-strengthened copper, aluminum, or nickel are particularly
good choices for the fins, though cobalt and alloys are also possible for high
temperature fins. While most superalloys have poor thermal conductivity
compared to
pure metals near room temperature, some with superior oxidation and corrosion
resistance, such as Haynes 214 (16Cr, 4.5AL, 3Fe, 0.2Y, bal-Ni), have fairly
good
thermal conductivity at high temperatures (32.4 W/m-K at 1255 K).
Some superalloys, such as Haynes 188 (38Co, 22Cr, 22Ni, 14.5W, 2Fe, lMn,
0.3Si, 0.1C, 0.07La), have good brazability and formability in the annealed
state as well
as outstanding oxidation resistance and high-temperature strength (-1400 K for
70
MPa yield strength in alloy 188). An alloy similar to Haynes 188 would be well
suited
for high-temperature exchanger tubing, though modifications to reduce cost and
improve formability and brazability, particularly by reducing Co, W, and Cr,
may be
preferred. The tubing material can have poor thermal conductivity with little
consequence on performance. If the hydrostatic pressure on the HTL(s) is
maintained
near the mean of the pressures of the gas streams, preferably within a factor
of two of
this mean, the stresses on the tubing are reduced.
Brazes compatible with the higher temperatures and materials are required.
Nickel-plated dispersion-strengthened-copper fins could be brazed to such
using filler
23
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
BNi-7 (890 C liquidus, 85Ni, 14Cr, 10P). Superalloy or alumina-dispersion-
strengthened nickel fins could be brazed to Haynes 188 or similar tubing using
BNi-5
(1135 C liquidus, 70Ni, 19Cr, 10Si) for operation at still higher
temperatures. Methods
for applying chromium platings to the finned tube rows can be developed, based
on the
prior art.
Organic HTLs at High Temperatures. An organic HTL may be used quite
satisfactorily at a much higher temperature than that for which it has
normally been
recommended if suitable measures are taken. First of all, it is most important
that the
surfaces in contact with the hot oil (tubing interiors, etc.,) be
catalytically deactivated
with a thin layer (0.1 micron is sufficient) of coke - carbon and very heavy
condensed
polynuclear aromatics. Thermal (non-catalytic) reactions require much higher
temperatures than catalytic, and most metallic or oxide surfaces have some
catalytic
activity. Secondly, since water catalyzes reactions on many metal surfaces, it
is
important to maintain the liquid pressure well above the maximum external gas
pressure (the greater of the pressures in shell-side gas-1, gas-2, and
ambient) at all
times to prevent ingress of air and moisture through minute leaks. Of course,
it is
important to insure that any organic HTL is initially de-gassed of dissolved
02 and H20.
In general, there are four primary types of thermal reactions that will
dominate for
most of the heavy HCs likely to be used for a high-temperature HTL: cracking,
dehydrogenation, de-isomerization, and aromatic polymerization or
condensation. All
but de-isomerization (conversion from a highly branched to a less branched
structure)
are somewhat inhibited by moderate H2 and CH4 concentrations within the HTL -
or
perhaps it is more proper to say that high H2 and CHa concentrations increase
the rate
of the reverse of many undesired reactions.
As previously noted, a small surge tank is required for the HTL to accommodate
expansion and contraction. To extend the lifetime and useable temperature
limit, the
gas overhead 7 in this reservoir should have an H2 partial pressures of at
least 0.01
MPa and possibly as much as 5 MPa, though excessive H2 partial pressures will
increase cracking (especially of n-alkanes) and hydrogenation of aromatics
into lower-
boiling cyclics. Hence, it may be desirable to also have significant methane
partial
pressure, possibly as much as 15 MPa, as it is less reactive. For some HTLs,
such as
water, glycols, phthalates, silicones, polyol esters, and polyphenyl ethers,
partial
pressurization with argon and perhaps N2 may be preferred. Maintaining an
excessive
24
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
total pressure on the HTL increases the cost of the high-temperature cores and
exacerbates problems with dynamic seals, but an HTL static pressure about 0.1
to 1
MPa above the higher of the shell-side gas pressures would normally add little
to the
system cost.
The concentrations of CH4 and H2 dissolved within the HTL are determined
largely by their partial pressures and the liquid temperature in the HTL
reservoir.
Solubilities of H2 in HCs (A) are generally higher for alkanes than for
aromatics, (B) they
increase with increasing temperature, (C) they approximate a Henry's law
behavior,
and (D) they decrease slowly with increasing molecular mass of the HTL. The
solubility
of H2, in moles H2 per kg liquid per MPa, at 460 K are 0.068 and 0.044 for
hexadecane
(C16H34) and tetralin (C1oH12) respectively, for example. Solubilities at 520
K are about
30% higher. Solubilities in very heavy oils are about half that for
hexadecane.
Methane solubility is much higher (by perhaps a factor of 20 at 460 K) and
much less
dependent on temperature. When the HTL cools during power-down, it may
effervesce
Hz.
It will normally be preferable to have the liquid pumps at the low-temperature
points in the loops, as shown in Figure 1, as this simplifies problems
associated with
dynamic seals. It may also be preferable to have the reservoir near the low-
temperature point in the loop to avoid H2 super-saturation within the HTL at
its cooler
points in the loop, as super-saturation could lead to hydrogen effervescence
in the
cooler exchangers and reduced heat transfer. However, some level of H2 super-
saturation is usually quite stable in HCs, and this may further inhibit
production of coke
precursors with some HTLs. Hence, it may be preferable to have the surge tank
at a
higher temperature point in the loop, even though this increases its cost a
little.
Even with the above measures, operating at temperatures near the upper
practical limits will result in the production of reaction products, both
light and heavy,
that are undesirable beyond a certain level but are quite tolerable at low
levels. In most
cases, this will simply mean that periodic HTL changes will be required. For
large
installations there are other options. Cracking produces light alkenes, some
of which
will be hydrogenated to light gases such as C2H6, C3H8i and C4H1o, which are
less than
optimum for reservoir pressurization. An easy way to deal with such is to
continually,
slowly vent some pressurization gas and maintain the desired pressure with
fresh gas
of optimum mixture. Of course, membranes and other separations methods could
be
used to separate the vented gas into useful product streams if desired. Some
of the
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
alkenes will alkylate with other alkanes or aromatics to heavy HCs and coke
precursors
in the HTL. One way to maintain the HTL at an acceptable composition is to
steadily
bleed HTL from the reservoir and maintain the desired level with fresh supply.
Various
separations methods could be applied to the used fluid for reclamation. More
examples
of reaction-product separation processes are disclosed in a co-pending patent
application on Dual-source Organic Rankine Cycles.
In summary, the following are required to operate with organics at high
temperatures:
1. Deactivate all surfaces in contact with the hot HTL.
2. Maintain sufficient HTL pressure to prevent ingress of air and moisture
through
minute leaks.
3. Maintain an optimum gas mixture pressurizing the HTL.
4. Remove primary HTL reaction products before they lead to excessive coking.
5. Select a fluid with high chemical stability with optimum gas
pressurization.
For the temperatures indicated in the example of Figure 1 with appropriate gas
pressurization over the HTLs, the HTL for sets A and B could be dioctyl
phthalate, a
PAO oil, or a POE oil. For sets C and D, a molten alloy, a molten salt, PPE-
5P4E, or
possibly an alkylated polynuclear aromatic could be used.
Cryogenic Applications. While some of the largest applications may be at
elevated temperatures in chemical processes and power plants, there will also
likely be
enormous applications at cryogenic temperatures, as very high c in exchange
between
gases is often required there. Moreover, gas viscosities (and hence pressure
drops)
there are often so low that it is very difficult to establish the uniform flow
conditions that
are essential for high c. As previously noted, the use of separate, series-
connected
cores or FL modules allows simple insertion of turbulent mixers into the shell-
side
stream between modules.
For cryogenic applications, the fin pitch can be further reduced - because (A)
viscous losses are much smaller, (B) the fin-metal thermal conductivity can be
an order
of magnitude higher, (C) the gas thermal conductivity is often much lower, (D)
the HTL
may have a higher FM, and (E) corrosion is more readily controlled.
One HTL, cumene (isoproplybenzene, C9H12), is listed in Table 1 that is
particularly advantageous down to 130 K, and others are suitable for lower
temperatures. Propane, for example is usable down to 90 K and is easily
liquefied at
room temperature, as its critical temperature Tc is 370 K. For lower
temperatures,
26
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
gases with Tc well below 300 K are required, and this complicates start-up
somewhat,
as a rather large compressed-gas reservoir is needed. Oxygen (Tc = 155 K) is
an
excellent HTL for the range of 60-130 K. Fluorine oxide, F20 (Tc = 215 K), is
suitable
for the 55-170 K range, and other gases can be used over other, narrow ranges.
For
example, H2 (Tc = 33 K) can be used over the 15-30 K range. However, very high
pressures are required to condense these gases near the upper ends of their
maximum
liquid ranges, and this increases exchanger cost.
In principle, a gas could be used as the heat transfer intermediary, where the
obvious choice for the 35-60 K range would be hydrogen. However, high (tube-
side) htL
with low pumping power cannot be achieved with a gas as the intermediary above
its
Tc, as its density is much too low at practical pressures. The best way to
increase htL
with gases is to use MMP tubing, which indeed works beautifully at very high
pressures.
The minimum competitive size of the compound exchanger for cryogenic
applications will be smaller than for most high-temperature applications -
because
mass is often much more critical and mean temperature difference between the
gas
streams may need to be an order of magnitude smaller. The compound exchanger
seems likely to be preferred in many cryogenic recuperators down to 90 K at
exchange
powers above 1 kW for gas pressures below 0.5 MPa.
Compact Recuperator Variations. An advantage not yet noted for the
compound exchanger is that it can greatly reduce the ducting costs in large
plants
where the heat generated in one process is needed in another process hundreds
of
meters or even tens of kilometers away. In such a case, it will sometimes be
easier to
achieve optimum thermal balancing - matching the gas Wand temperature to those
of
the HTL within each module - by splitting and re-combining the HTL streams at
numerous points. When streams are combined, their temperatures should be
similar
for minimal exergy destruction. A portion of an HTL may be split out from an
intermediate point in an exchanger module to exchange energy with another
process
and then be re-combined at an appropriate point where the temperature is
similar. -
Of course, it is not uncommon to transfer heat long distances using either
phase
change (usually water) or liquids (including many of those mentioned earlier
as good
HTLs for compound recuperators). For example, Severinsky in US publication
2006/02 1 1 777 notes that it can be advantageous to transfer heat throughout
a large
plant using a number of different phase-change heat-transfer fluids (HTFs).
27
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
While it is important to emphasize that exergy destruction is more readily
minimized by avoiding substantial phase change when there is a large
temperature
difference between the hot source gas and the cold source gas (so the number
of HTL
loops can be reduced), a minor amount of boiling and condensing may take place
within the HTLs without departing from the spirit of this invention. Hence,
the HTL may
be referred to as an HTF, as is customary in the prior art, though in a high-c
recuperator
the enthalpy associated with phase change would be small compared to that
associated with temperature change.
The description of the shell-side fluids as "clean gases" in earlier
discussions
requires further clarification. It is anticipated that in many cases the
amount of
condensation, acid formation, ice formation, corrosion, and particulates will
be minor,
though such are not precluded. When fouling mechanisms are negligible, the fin
pitch
may be reduced for improved compactness. However, the FL recuperator will
still be
advantageous in many applications where these mechanisms are substantial -
though
perhaps not where they are strongly dominant.
Fouling will often be significant in only one of the gas streams, and often
only at
either the hot or cold end of that stream. A strong advantage of the compound
recuperator is that it may readily permit individual modules to be switched
off-line for
rejuvenation (defrosting, cleaning, re-plating, etc.) while a fresh module is
put into
service. In some case, the fouled module may need to be shipped back to the
factory
for service, but often it will simply need to be drained, defrosted, burned
out, or solvent
washed. In many cases, it will simply be necessary to orient those modules in
which
significant condensation occurs so that the condensate readily drains while in
use - as
for example the draining of moisture from the common AC evaporator on a humid
day.
The compound exchanger will often permit a dramatic reduction in the number of
replacement exchanger modules that will need to be kept on hand in a large
process
plant.
Large applications are also anticipated where the shell-side fluids are
viscous
organic liquids, since such exchanges also benefit from the very short flow
passages
that can more easily be obtained in the inventive module. While the benefits
may be
greatest with oils of high viscosity, even moderate-viscosity oils, such as
1,3-
diphenylpropane at 310 K, where =4.4 cP, kt= 0.12 W/m-K, p= 968 kg/m3, and CP
= 2
kJ/kg-K, would benefit when high effectiveness is needed, especially if
effervescence is
also present in one of the streams. In such a case, a phase separator or flash
drum
28
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
can be inserted between modules or even between cores to separate the evolved
shell-
side gas so the fluid's volumetric flow rate (and hence velocity) remains low -
to limit
viscous losses.
A composite fluid property that is dimensionally much simpler than (FMFG )0-5
and
nearly as valid for comparing diverse fluids for similar flow geometries is
Fo=ktpCPl,u. [11]
The HTL in Table 1 having the lowest FD (i.e., least desirability) at 500 K is
(again) the
salt, where Fo = 2.7E5 J2/(s-m4-K2-cP) in these mixed reduced units, which
herein will
be abbreviated Dt (for Doty). (In SI units, 1 Dt = 1000 J2/(kg-m3-K2).) For
comparison
(again at 500 K), Fo is seen to be -440 kDt for 40wt engine oil and 22 MDt for
water.
In contrast, the shell-side fluids have lower Fo. A typical value for the gas
conditions indicated earlier (500 K, 5 kg/m3, 0.05 W/m-K, etc.) would be -25
kDt. Some
liquids for which high-performance heat recovery will be needed have Fo well
below
those of a preferred HTL, and in such cases, a compound recuperator can be
advantageous, particularly if the temperature permits the use of a tube-side
HTL of very
high Fo, such as water or a molten alloy.
For diphenylmethane at 310 K for example, Fo is -100 kDt, and for 1,3-
diphenylpropane Fo is 52 kDt. For heavy oils, Fo can be an order of magnitude
smaller
yet, even at temperatures where substantial heat recuperation may be needed in
some
situations.
The FL recuperator will be useful for heat recovery in many fluids where Fo is
less than 200 kDt at the operating conditions, which generally implies >1 cP
for
organic liquids. When the shell-side fluid has rather high Fo (as for some low-
viscosity
liquids and gases at very high pressures), a tube-side HTF would be needed
with very
high Fo, such as water or a molten alloy. However, a tube-side HTF with Fo as
low as
200 kDt would be satisfactory when operating with shell-side fluids of rather
low Fo.
Preferably, the tube-side fluid would have FD more than 10 times that of the
shell-side
fluids (which of course can be very different, and at very different
conditions).
Although this invention has been described herein with reference to specific
embodiments, it will be recognized that changes and modifications may be made
29
CA 02698337 2010-03-02
WO 2009/082504 PCT/US2008/067008
without departing from the spirit of the present invention. All such
modifications and
changes are intended to be included within the scope of the following claims.