Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02703446 2010-06-03
Overcoming Hazardous Material Problems by Recycling
Techniques
Environmental hazardous material consist mainly House hold garbage, Electronic
waste, and steel scrap left over. House hold Garbage is in greatest tonnage.
Lead batteries
and car tires can be included in above mentioned second category
Garbage material is composed of hydrocarbons, carbohydrates, metals and non-
metals,
glass and ceramics, many diversified materials along with various percentages
of water
and air.
When metals and non metals along with glass and ceramics are removed, garbage
contains various percentage of C, H, 0, N, S, P, Cl and ash forming
ingredients.
Ash is a metallic residue which actually contained as complex compounds in
cellulose and is left over when cellulosic; a plastic type material is burnt
over.
An ash material contains oxides like Si02 (mainly), CaO, FeO, MnO, CrO, NiO,
TiO2,
CuO and alkali oxides. Some of these oxides may be in traces.
When C, H, N, S, P etc are gasified under reducing conditions, metals in ash
are
left over as oxides , which can be fractioned to individual oxides as Si02,
CaO, FeO,
MnO etc. Impurities in the gases like H2S, PH 3, Cl, NxO are removed and
gainfully
disposed. Remaining gases are high reduction gases CO, H2 (here after referred
as syn
gases). The sensible heat in these gases may be recovered as steam in heat
exchanger or
circulating inert gases (He, N2). Steam may be used in electricity production
or saved to
be latter used in shift reaction Electricity may be used by inert gases in gas
turbines.
Steam is used in continuous manner.
Steam + CO + H2 - H2 + some remaining CO + CO2 exothermic
Steam + CO + H2- (2nd step catalyst) H2+ CO2 exothermic
CO2 is removed and gainfully marketed.
CO + H2 are obtained appropriate stichiometric ratios and it is made to
undergo
Methenation reaction.
CO + H2 - (nickel catalyst) CH4 + H2O + Heat
Heat is used for generation of electricity, H2 0 is removed.
This reaction holds large prospect for generation of automobile fuel and is
gainfully promoted.
2
CA 02703446 2010-06-03
I have so far discussed garbage as a constant composition material. Garbage
has
high variable composition particularly in water content. It varies in chemical
composition
depending on geographical and seasonal factors.
The CO H2 gases after impurities and heat removal at about 300-400C go to heat
exchanger where moisture from garbage is removed. Exit gas CO,H2 are made to
exit at
50-75C when sent to market. When these gases are to go to shift reaction these
are
cooled to only 300 C
Garbage is deprived of its major water contents and exit garbage going to high
temperature reactor to react it with preheated or normal oxygen. The
temperature of the
exit gases from high temperature reactor is controlled by the control of
moisture in the
garbage charge or by in put of CO2
Temperature of exit product gases which are as reducing gas can be made to
reach
11000. By performing high temperature reaction high hydrocarbon like CH4, C21-
16 are
not formed.
Coal is similar in composition to garbage after metals; ceramics and glass
have been
removed from it.
Coal seams have water 30-40%. Where it is dried to approx. 10% it mainly
varies
from garbage in sulphur content and has high ash content up to 15%. Garbage
has low
sulphur and low ash contents.
A high quality coal with ash up to 5% and sulphur 1.5% has approximately
following composition by weight.
C- 72-78%; H- 2-2.5%;0 --4%;N- 2%
We can mix garbage and coal is a judicious ratio if so required.
When H2 is only produced and CO2 is marketed, H2 is used in a second type
garbage plant that is processing of electronic garbage for removing and
fractionation
various plastic.
Ethylene and Chlorofluoroethylenes Polycarbonates, Poly- imides type
polyethylene Poly type of plastics which Nylons, PET these of plastics which
vinyl melting higher than are relatively high may melting at
chloride(PVC) 200 but less than 250 melting plastics 500C or more these
Polypropylene (PP) C which will melt will melt with Zn.
all those plastic with first melting
melting up to 200C metals Sri, Se 300-
350
Removing and processing of melts are in the following sequence: Sri .,,
232C,Pb
328C,Zn .., 420C,Al- 660C,Cu -1083C,Fe - 1534C
Remaining is Fe + Glass + Ceramic, where Fe is magnetically picked up.
These days many different metals are used in coatings, soldering, welding, and
brazing.
These will be mostly going with Pb or Cu.
Plastic may be separated further by density means.
3
CA 02703446 2010-06-03
Polyethylene is the lightest, PVC is next heavy, and Fluro-chloro-hydrocarbons
are
heavier than PVC.
More effort is required to separate PET, nylons, polycarbonates.
Temperature of the staircase furnace is increased gradually by electric
heaters.
Hydrogen is blown upward through refractory filters so that these are not
plugged.
Hydrogen is drawn from upper floor; it is cooled to condense any volatile
metals and
recycled after external heating and adding some made up hydrogen.
Hydrogen atmosphere is required to save Zn, Al from oxidation with CO.
When iron is finally collected in exchanging holders it is cooled under
Hydrogen.
The exit material is separated by electromagnetic and transported.
Electronic material has Silicon chips which will melt in the same range as Ni,
Co
but due to low density as compared to Ni will go along steel, when cooled it
will separate
with glass.
Germanium is also used as chip material it will melt and join with melted
copper
where it will be removed from copper by electrolytic techniques. In electronic
scraps we
have new soldering alloys new brazing alloys, new circuit material. All these
join with Pb
fraction or copper fraction.
The third type of scrap is left over scraps when prime quality material is
separated
by visual techniques. Left over scrap is paints + colour materials in paints,
steel pieces
and all other metallic ingredients.
Separation of Garbage and Coal Ash into Individual Oxide
Coal and garbage ash will consist of SiO2, CaO, FeO, A1203, Mn02, Cr2 O3,TiO2
and alkali oxides.
These are reacted with Na2CO3 at temperatures of approximately 13000 and burnt
material dropped into water tank. Water solution is filtered.
Na2SiO3, NaA1O2, NaCr2O3 being water soluble will separate. CaSi03, NaFe02 are
again put into water and CO2 is passed Fe304 formed will be separated by wet
magnetic
separators. Na2SiO3, NaCrO4, NaA1O2 are changed to pure A12O3 by calcium
desiliconization. Some other sodium salt like CuO, ZnO, and Mn02 are also
formed.
These are discharged from the bottom of the cyclone separator in the step
separating Cat
Si03. These mixtures go to a heavy media separator when CO2 is also
introduced.
Magnetic fractions FeO, MnO, CrO are separated by wet magnetic separator.
Some residue material settles at the bottom of magnetic separator. It will be
mainly ZnO, CuO, TiO2, and NiO. These will be treated by acetic acid, HCl to
make
these water soluble and used as micro nutrients in fields.
Phase separations:
4
CA 02703446 2010-06-03
When two metals or more than two metals separate together and they are soluble
together, they can be separated to individual metals by different densities
vaporization or
different phase formation. When one phases namely oxide phase is lighter and
float on
the surface then floating phase can be made stable by external additions like
CaO, NaOH.
Table 1
Metals, metal oxide density g/cu.m melting point c boiling point C
Al 2.3 660 2529
A1203 3.95 2072 2977
Cu 8.94 1084 2562
CuO 6.31 1201 2000
Cr 6.3 1807 2671
Cr2O3 5.22 2435 4000
Fe 7.87 1538 2862
FeO 5.7 1378
Fe304 5.2 More than 1700 Decomposes
Ge 5.6 938 2833
GeO 4.28 400 1200
Mn 5.95 1246 2061
MnO 5.37 1945 ----
Sri 6.94 231 2602
SnO 6.45 1080
Ni 7.81 1453 2732
NiO 6.67 1955 ---
Pb 10.66 327 1749
PbO 9.64 888 1477
Zn 7.57 419 907
ZnO 5.6 1975 Decomposes
Iron which dissolves Mn, Cr, Ni, C, Si can be made as interstitial free metal
by
melting and passing oxygen.
Fe Mn - MnO,Cr + 0 - CrO,Ni - NiO, C - CO,S - SO2
In the following is shown some preferred embodiment of gasification of
garbage.
If the situation is that only garbage disposal is required then the end
product are CO,H2
(synthetic gas) and electricity.
If it is desired to produce artificial natural gas The steam produced and Hot
CO,H2 are
reacted over shift catalyst and also the ratio are 3mole H2 and One mole CO.
The gases
are then passed over Methenation catalyst with heat removal from the catalyst
bed the
product is synthetic natural gaseCH4. ) Also significant amount of electricity
is produced.
This approach could be desirable in areas where natural gas is used as
automobile fuels.
The shift conversion may be carried that H2 is only end product (apart from
minor
impurities
This H2 is used as protective media in the electronic garbage or other steel
left over
garbage.
CA 02703446 2010-06-03
If the plant is a complex producing all these items their relative production
can be
adjusted for maximum profitability.
In the following I describe labelled production facilities of all these plants
and
processes.
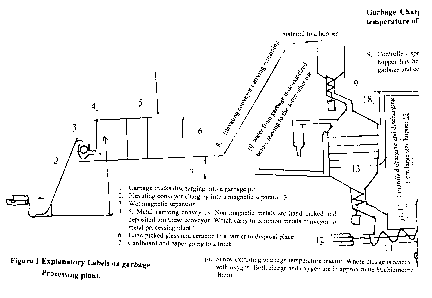
Figure 1 Explanatory Labels on garbage Processing plant.
L Garbage trucks discharging into a garbage pit
2. Elevating conveyor charging into a magnetic separator. 3.
3. Wet magnetic separator.
4. 5. Metal carrying conveyors. Non magnetic metals are hand picked and
deposited ion these conveyor, which carry to common metals conveyor to
metal processing plant.
6. Hand picked glass and ceramic to a carrier to disposal place.
7. Cardboard and paper going to a truck.
8. Elevating conveyor carrying remaining material to a hopper
9. Controlled speed screw carrying material to a large size hopper. This
hopper has heat exchangers pipes inside which vaporize water from the
garbage and cool down the exit CO, H2 gas.
10. Water from garbage is de-odorized before leaving to the some other use.
11. 12. Controlled charging and discharging from large size hopper 13.
14. Screw extruding to a high temperature reactor. Where charge is reacted
with oxygen. Both charge and oxygen are in approximate stichiometric
ratio
15. A high temperature reactor is a steel shell with refractory lining.
In the lower half are tuyeres for oxygen input. These tuyeres can be
ordinary water-cooled tuyeres or plasma tuyeres for oxygen heating.
15. Nearby is oxygen plant supplying oxygen to high temperature reactor.
16. Oxygen plant is pressure swing absorbing type due to investment capital
treason. Other wise cryogenic plant would have been better.
17 At the top of high temperature reactor is top refractory lined pipe.
18 This carries the gases to high temperature impurities removing
arrangement. Here S, P, Cl, NxO. Are removed these gases are further led
to heat removal by 1. Steam production. 2. By circulating gases
19 The gases CO, H2 go to garbage hopper for moisture removal.
Garbage charge can be gasified to CO, H2, H2S, PH3, HCI, and NxO. At
temperature of
1100 C H4,C2H6 are not formed.
Figure 2. Shift gas reactions carrying over system connected with hot
Potassium
carbonate removal Of C02, H2S.
1 Purified gases CO, H2 ait about 300 C an steam are brought from plant of
Figure 1.
Gaseous composition at various point are shown in attached table on figure 2.
Table 1.
Typical 1 2 3 4
operating
conditions
6
CA 02703446 2010-06-03
H2 47 45 64-62 72-70- 74-72
CO 47-48 14-16 5-6 2-3
CO2 0-3 20-21 21-22 24-26
N2+ CH4+ Bal. Bal Bal Bal
Others
H20/Drry gas 0.6-1 .47-0.8 .46 .81
ratio
Temperature 650-700 765-810 420-465 450-500
Pressure PSIG 200 400 200-400 200-400 200-400
Figure 3. Simplified flow diagram of carrying out Methenation reaction,
between CO
and H2.
1 H2 and CO ratio for Methenation reaction are produced in the gas shifting
reaction.
2. The formation of CH4 and H2O is highly exothermic reaction and catalyst
cooling is
required here it is done by circulating N2 after cooling in a gas turbine
electricity
generation reaction.
Three Methenation reactors are shown feed one gas turbine
20 CO + 60H2 =20CH4 + 20 H2O
From 3 tons of coal we get about 3x20 kg.mole CH4 and 1.44 MW electricity
Figure 4. Separating of metal and plastics from electronic garbage
Shown is slanting stair case furnace with internally heated electric means, a
positive
hydrogen atmosphere is kept in the furnace.
1.Double lock charging system either a conventional double bell
system or when charge is relatively large size a double plate sliding system
Cut car
body in four pieces.
2.Steel shell with refractory lining, a refractory filter is at the bottom.
Furnace is sliding downward with stairways. Melt collectors one before the
stair step and
one after the step after the charge have tumbled down. Both melt collector are
joined
3.A charge pusher at every step
4.A hydrogen circulation system to collect volatile metals, and reuse the
hydrogen after
heating.
5.Charge cooling circulating hydrogen system
6Magnetic separation of iron.
7 Plastic melts are collected in separate filter collectors.
8 Refractory filter and collector.
9 Collectors for volatile metals
1OManetic separator
11 Hydrogen cooling of iron
Figure 5. Separation of a metal mixture when it has separated together in a
step. It is an
illustrative example.
Separation of Sri, Pb, Zn, Cu,
1. The mixture is heated and Zn will be volatized.
2. The remaining mixture is transferred in an other vessel Oxidizing gases
CO/CO2
mixture is blown and charge is heated PbO is volatilized.
7
CA 02703446 2010-06-03
3. Cu with noble metals in it is cooled Cu and noble metal are solidified.
4. Sn remain as liquid.
Figure 6. Refractory filter.
Upper layer and lower layers are refractory plates with small whole in these.
The
hole size could be 1mm. Embedded between is a steel wire Gauze of 300 mesh.
Below this filter plate may be bricks with large size holes for support.
Figure7.
Figure shows how to cast to desired film or shape.
Shown is a piston and cylinder of high strength steel with refractory lining
inside.
There is inlet for the melt. The melt may be liquid metal plus some slag,
metal and
plastic, metal only. On the extrusion side is a water cooled die of required
configuration.
Flat or H shape. When the piston closes the inlet hole every thing is enclosed
inside. The
extruded metal with a thin coating travels on a floor for cooling.
From earlir basic consideration and preferred practical designs following
summarized
conclusions may be drawn:
1) I have invented series of unit process leading to comprehensive process for
the
gainful utilization of hazardous waste, namely household garbage, electronic
waste
and steel scrap leftover.
2) Whereby high energy, high temperature syn gases (CO, H2) are produced which
can be directly used for other purposes; that the sensible heat energy is
taken
away from these gases by creation of steam, by heating of circulating gases or
by
heating of charge material. By way of heat exchangers.
3) Where these high energy gases themselves can be used as source of energy by
way of combustion, by reaction with other gases and materials.
4) Where syn gases and steam produced in single or multiple nuits can be made
to
undergo shift reactions by passing over catalysts. When gases compositions
is H2, CO2 this can be separated to pure H2 and CO2. when it is H2 CO CO2 it
can
be separated into H2 CO and CO2.where by H2 and CO can be formed in desired
ratios.
5) Where a appropriately adjusted ratio of H2 and CO and by passing over a
catalyst
and cooling the catalyst internally by circulating gases like He and N2
electricity
can be produced with simultaneous production of CH4 and H2O (Methenation)
where H2O is removed and dried CH4 is normal methane gas.
6) When H2 is the only reaction product it is lead to electronic garbage
processing
plant. Where H2 is preheated or otherwise is used to separate plastics and
metals
from each others.
7) Plastics up to certain type are separated as individual type polyethylenes
from
polyvinyl chloride and Flourochloro type plastic. High melting range plastics
will
melt along with metals.
8) Whereby plastics will be melted and drained up to certain range, there
after
melting range of plastics and metal may over lap and they will drain together.
Heat is provided by electric heaters inside the staircase furnace. Hydrogen
atmosphere and circulation kept inside the furnace to save any metal from
8
CA 02703446 2010-06-03
compound formation ZnO, A1203, A13C. After separation of Ni and Co as a one
group steal falls into an alternative holding hopper vessels where it is
cooled to
room temperature. Steel along with glass, silicon, and ceramic is discharged
on a
conveyor and magnetically separated to fall into separate containers.
9) Whereby hydrogen is used counter current and is recycled to keep the
filters pore
open. Where the refractory filter is a thin refractory plate with small holes
and
fine steel wire gauge is embedded between these plates.
10) When more than one metal separate to gather these are separated by density
means, vaporization, phase separation and conventional electrolysis processes.
11) where by moisture in the garbage is adjusted to obtain a desired
temperature of
the product gases. Where as instead of moisture CO2 may be introduced.
Explanatory Notes:
When good quality steel material is separated by hand picking, a material is
left
which is mixture of metals and plastics. It is steel scrap left over.
Electronic waste is synonymous with electronic garbage.
Stair case furnace is a slanting down ward refractory line furnace where steps
are made to
slide downward and tumbles to separate the melted material. Furnace is under
atmosphere of hydrogen gas.
Stichiometric According to a chemical formula
Heavy media separation. Separation in a media which approximately floats the
constituents.
9