Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02706274 2012-05-23
4127.1002 CA
SYSTEMS AND METHODS FOR REMOVING FINELY DISPERSED
PARTICULATE MATTER FROM A FLUID STREAM
10
FIELD OF THE APPLICATION
[0001] The application relates generally to the use of particles for removing
finely
dispersed particulate matter from fluid streams.
BACKGROUND
[0002] Fine materials generated from mining activities are often found well-
dispersed
in aqueous environments, such as wastewater. The finely dispersed materials
may
include such solids as various types of clay materials, recoverable materials,
fine sand
and silt. Separating these materials from the aqueous environment can be
difficult, as
they tend to retain significant amounts of water, even when separated out,
unless
special energy-intensive dewatering processes or long-term settling practices
are
employed.
[0003] An example of a high volume water consumption process is the processing
of
naturally occurring ores. During the processing of such ores, colloidal
particles, such
as clay and mineral fines, are released into the aqueous phase often due to
the
introduction of mechanical shear associated with the hydrocarbon-extraction
process.
In addition to mechanical shear, alkali water is sometimes added during
extraction,
creating an environment more suitable for colloidal suspensions. A common
method
1
CA 02706274 2012-05-23
4127.1002 CA
for disposal of the resulting "tailing" solutions, which contain fine
colloidal
suspensions of clay and minerals, water, sodium hydroxide and small amounts of
remaining hydrocarbon, is to store them in "tailings ponds." These ponds take
years to
settle out the contaminating fines, posing severe environmental challenges.
Tailings
ponds or similar liquid retention areas can contain aqueous suspensions of
fine
particles from mining operations and other industrial operations, for example
fine coal
particles from coal mining and fly ash from coal combustion, with the
potential for
environmental damage and catastrophic leakage. It is desirable to identify a
method for
treating tailings from mining operations to reduce the existing tailings
ponds, and/or to
prevent their further expansion.
[00041 Certain mining processes use a large volume of water, placing strains
on the
local water supply. It would be advantageous, therefore, to reuse the water
from
tailings streams, so that there is less need for fresh water in the
beneficiation process.
In addition, certain mining processes can create waste streams of large-
particle
inorganic solids. This residue is typically removed in initial separation
phases of
processing due to its size, insolubility and ease of sequestering. Disposal or
storage of
this waste material represents a problem for the mining industry. It would be
advantageous to modify this material so that it could be useful in-situ, for
example as
part of a treatment for the mining wastewater.
[00051 A typical approach to consolidating fine materials dispersed in water
involves
the use of coagulants or flocculants. This technology works by linking
together the
dispersed particles by use of multivalent metal salts (such as calcium salts,
aluminum
compounds or the like) or high molecular weight polymers such as partially
hydrolyzed
polyacrylamides. With the use of these agents, there is an overall size
increase in the
suspended particle mass; moreover, their surface charges are neutralized, so
that the
particles are destabilized. The overall result is an accelerated sedimentation
of the
treated particles. Following the treatment, though, a significant amount of
water
remains trapped with the sedimented particles. These technologies typically do
not
release enough water from the sedimented material that the material becomes
mechanically stable. In addition, the substances used for
flocculation/coagulation may
not be cost-effective, especially when large volumes of wastewater require
treatment,
2
CA 02706274 2012-05-23
4127.1002 CA
in that they require large volumes of flocculant and/or coagulant. While
ballasted
flocculation systems have also been described, these systems are inefficient
in
sufficiently removing many types of fine particles, such as those fine
particles that are
produced in wastewater from mining processes.
[0006] There remains an overall need in the art, therefore; for a treatment
system that
removes suspended particles from a fluid solution quickly, cheaply, and with
high
efficacy. It is also desirable that the treatment system yields a recovered
(or
recoverable) solid material that retains minimal water, so that it can be
readily
processed into a substance that is mechanically stable. It is further
desirable that the
treatment system yields clarified water that can be readily recycled for
further
industrial purposes.
[00071 An additional need in the art pertains to the management of existing
tailings
ponds. In their present form, they are environmental liabilities that may
require
extensive clean-up efforts in the future. It is desirable to prevent their
expansion. It is
further desirable to improve their existing state, so that their contents
settle more
efficiently and completely. A more thorough and rapid separation of solid
material
from liquid solution in the tailings pond could allow retrieval of recyclable
water and
compactible waste material, with an overall reduction of the footprint that
they occupy.
SUMMARY
The present invention is directed to systems and methods for removing finely
dispersed materials or particles from wastewater streams produced during
mining
operations.
In one embodiment, the invention is directed to a method of removing
particulate matter from a waste tailing fluid, comprising: providing an
activating
material capable of being affixed to the particulate matter; affixing the
activating
material to the particulate matter to form an activated particle; providing an
anchor
particle and providing a tethering material capable of being affixed to the
anchor
particle; and attaching the tethering material to the anchor particle followed
by attaching
the tethering material to the activated particle to form a removable complex
in the fluid;
3
CA 02706274 2012-05-23
4127.1002 CA
wherein the fluid is a waste tailing fluid derived from energy production or a
mining
process. In certain aspects, the mining process is coal mining or the mining
of an
inorganic ore. In additional aspects, the particulate matter is selected from
the group
consisting of coal combustion products, coal fines, clay particles and mineral
particles.
In an additional embodiment, the fluid is selected from the group consisting
of red mud
fluid stream, gangue, slurry containing fine particulate kaolin, tailings from
trona
mining and slurry produced by phosphate beneficiation. In certain aspects, the
anchor
particle comprises sand. In certain additional aspects, the tethering material
is material
is selected from the group consisting of chitosan, lupamin, branched
polyethyleneimine
(BPEI), polydimethyldiallylammonium chloride (PDAC), and
polydiallyldimethylammonium chloride (pDADMAC). In some embodiments, the
activated particle attaches to the tethering material by electrostatic
interaction, hydrogen
bonding or hydrophobic behavior. In some aspects, the anchor particle
comprises a
material indigenous to a mining process.
In an additional embodiment, the invention is a method of removing particulate
matter from a fluid, comprising providing a modified particle comprising a
particle
functionalized by attachment of at least one amine functional group;
dispersing the
modified particle within the fluid so that it contacts the particulate matter
to form a
removable complex in the fluid; and removing the removable complex from the
fluid
wherein the fluid is a waste tailing fluid derived from energy production or a
mining
process.
In a further aspect, the invention is directed to a system for removing coal
fines
from a fluid, comprising: a fluid containing a population of suspended coal
fines; an
activator polymer added to the fluid to complex with the suspended coal fines
to form
activated coal fines, the activated coal fines residing within the fluid
volume; an anchor
particle complexed with a tethering agent to form tether-bearing anchor
particles, the
tether-bearing anchor particles being mixed with the fluid volume to contact
the
activated coal fines, the tether-bearing anchor particles being capable of
complexing
with the activated coal fines to form complexes removable from the fluid,
wherein the complexes removable from the fluid comprise a composite material
comprising complexed coal fines and anchor particles. In some embodiments, the
4
CA 02706274 2012-05-23
4127.1002 CA
anchor particle comprises coal. In additional embodiments, the anchor particle
comprises a non-combustible material. In yet additional aspects, the anchor
particle
comprises a mineral.
The invention also encompasses a method for removing coal fines from a fluid,
comprising: providing an activator polymer capable of interacting with a
population of
coal fines suspended in a fluid; adding the activator polymer to the
population to form
activated coal fines; providing an anchor particle; complexing the anchor
particle with a
tethering agent capable of complexing with the activated coal fines, thereby
forming
tether-bearing anchor particles; mixing the tether-bearing anchor particles
with the
activated coal fines to form a complex removable from the fluid, the complex
comprising a composite material comprising coal fines and anchor particles,
and
removing the composite material from the fluid. In some embodiments, the
anchor
particle comprises coal. In additional embodiments, the anchor particle
comprises a
non-combustible material. In some embodiments, the anchor particle comprises a
combustible material. In yet additional aspects, the anchor particle comprises
a mineral.
In certain other aspects, anchor particle comprises a starch particle. The
invention
additionally encompasses an energy-bearing pellet produced according to this
method.
In some aspects, the energy-bearing pellet comprises a composite material
comprising
activated coal fines complexed to combustible tether-bearing anchor particles.
In
additional aspects, the energy-bearing pellet comprises a composite material
comprising
an energy-containing fine material and a combustible anchor particle in a
complex, the
complex further comprising an interacting pair of polyelectrolytes, wherein
the first of
the pair of polyelectrolytes is bound to the energy-containing fine material
and the
second of the pair of polyelectrolytes is bound to the combustible anchor
particle.
In some embodiments, the removable complex formed by a method of the
invention is removed by a process selected from the group consisting of
filtration,
centrifugation, skimming and gravitational settling.
In yet another embodiment, the invention is a system for removing particulate
matter from a fluid, comprising an activating material capable of being
affixed to the
particulate matter to form an activated particle; a tether-bearing anchor
particle capable
of attaching to the activated particle to form a removable complex in the
fluid; and
5
CA 02706274 2012-05-23
4127.1002 CA
a separator for separating the removable complex from the fluid, thereby
removing the
particulate matter; wherein the fluid is a waste tailing fluid derived from
energy
production or a mining process.
BRIEF DESCRIPTION OF FIGURES
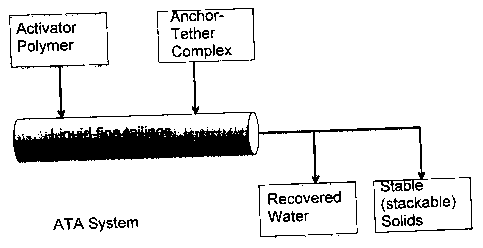
[0008] FIG. 1 is a schematic showing the activated-tethered-anchored (ATA)
system
comprising three basic components: an activator polymer, a tether polymer and
an
anchor particle; the ATA system is contacted with the liquid fine tailing
slurry resulting
in self-assembly of the solid material and the expulsion of water.
[0009] FIG. 2 is a reproduction of a photograph showing filtrates of and solid
material
filtered from samples containing activated coal slurry treated with tethered
filter cake
(FC) and two samples of coal processed refuse (CPR1 and CPR2).
[0010] FIG. 3A shows a suspension of clay fines containing 5% by weight
solids.
[0011] FIG. 3B shows the suspension of clay fines after adding an activator
polymer.
[0012] FIG. 4 shows an 85% by weight sand slurry.
[0013] FIG. 5 shows the result of mixing an activated clay fines stream with a
slurry
containing tether-bearing sand anchor particles.
[0014] FIG. 6A shows the separation of the mixture in FIG. 5 by gravity
filtration.
[0015] FIG. 6B shows the filtered solids from the filtration shown in FIG. 6A.
[0016] FIG. 7 presents Graph 1 showing Solids Content (%) and Turbidity (NTU)
as
a function of tether dosage (ppm).
[0017] FIG. 8 presents Graph 2 showing Solids Content (%) and Turbidity (NTU)
as
a function of activator dosage (ppm).
DETAILED DESCRIPTION
[0018] Disclosed herein are systems and methods for removing finely dispersed
materials or "fines" from wastewater streams produced during mining
operations. In
embodiments, the clay fines produced during phosphate beneficiation can be
removed
with these systems and methods. In embodiments, other types of fines can be
removed
where these contaminants are suspended in aqueous solutions.
6
CA 02706274 2012-05-23
4127.1002 CA
[0019] These systems and methods employ three subprocesses: (1) the
"activation" of
the wastewater stream bearing the fines by exposing it to a dose of a
flocculating
polymer that attaches to the fines; (2) the preparation of "anchor particles,"
fine
particles such as sand by treating them with a "tether" polymer that attaches
to the
anchor particles; and (3) adding the tether-bearing anchor particles to the
activated
wastewater stream containing the fines, so that the tether-bearing anchor
particles form
complexes with the activated fines. The activator polymer and the tether
polymer have
been selected so that they have a natural affinity with each other. Combining
the
activated fines with the tether-bearing anchor particles rapidly forms a solid
complex
that can be separated from the suspension fluid with a separator, resulting in
a stable
mass that can be easily and safely stored, along with clarified water that can
be used for
other industrial purposes. As used herein, the term "separator" refers to any
mechanism, device, or method that separates the solid complex from the
suspension
fluid, i.e., that separates the removable complexes of tether-bearing anchor
particle and
activated particles from the fluid. Following the separation process, the
stable mass
can be used for beneficial purposes, as can the clarified water. As an
example, the
clarified water could be recycled for use on-site in further processing and
beneficiation
of ores. As an example, the stable mass could be used for construction
purposes at the
mine operation (roads, walls, etc.), or could be used as a construction or
landfill
material offsite. Dewatering to separate the solids from the suspension fluid
can take
place in seconds, relying only on gravity filtration.
[0020] Disclosed herein are systems and methods for enhancing the settlement
rate of
dispersed fine materials by incorporating them within a coarser particulate
matrix, so
that solids can be removed from aqueous suspension as a material having
mechanical
stability. The systems and methods disclosed herein involve three components:
activating the fine particles, tethering them to anchor particles, and
sedimenting the
fine particle-anchor particle complex.
[0021] Generally speaking, the fines in the wastewater stream are "activated"
by
exposure to a dosing of flocculating polymer. Separately, the sand particles
or other
"anchor" particles are exposed to a polymer "tether." The activator and tether
are
chosen so they have a natural affinity towards each other. Combining the two
streams,
7
CA 02706274 2012-05-23
4127.1002 CA
the activated fines with tether-bearing anchors, produces a stable solid that
forms
rapidly. The solid can be separated from the clarified water in which it
resides by a
dewatering process, for example by gravity filtration, which can quickly yield
a mass
that can be easily and safely stored.
1. Activation
[00221 As used herein, the term "activation" refers to the interaction of an
activating
material, such as a polymer, with suspended particles in a liquid medium, such
as an
aqueous solution. In embodiments, high molecular weight polymers can be
introduced
into the particulate dispersion, so that these polymers interact, or complex,
with fine
particles. The polymer-particle complexes interact with other similar
complexes, or
with other particles, and form agglomerates.
[00231 This "activation" step can function as a pretreatment to prepare the
surface of
the fine particles for further interactions in the subsequent phases of the
disclosed
system and methods. For example, the activation step can prepare the surface
of the
fine particles to interact with other polymers that have been rationally
designed to
interact therewith in an optional, subsequent "tethering" step, as described
below. Not
to be bound by theory, it is believed that when the fine particles are coated
by an
activating material such as a polymer, these coated materials can adopt some
of the
surface properties of the polymer or other coating. This altered surface
character in
itself can be advantageous for sedimentation, consolidation and/or dewatering.
In
another embodiment, activation can be accomplished by chemical modification of
the
particles. For example, oxidants or bases/alkalis can increase the negative
surface
energy of particulates, and acids can decrease the negative surface energy or
even
induce a positive surface energy on suspended particulates. In another
embodiment,
electrochemical oxidation or reduction processes can be used to affect the
surface
charge on the particles. These chemical modifications can produce activated
particulates that have a higher affinity for tethered anchor particles as
described below.
[00241 Particles suitable for modification, or activation, can include organic
or
inorganic particles, or mixtures thereof. Inorganic particles can include one
or more
materials such as calcium carbonate, dolomite, calcium sulfate, kaolin, talc,
titanium
8
CA 02706274 2012-05-23
4127.1002 CA
dioxide, sand, diatomaceous earth, aluminum hydroxide, silica, other metal
oxides and
the like. Sand or other fine fractions of the solids, such as sand recovered
from the
mining process itself, is preferred. Organic particles can include one or more
materials
such as starch, modified starch, polymeric spheres (both solid and hollow),
and the
like. Particle sizes can range from a few nanometers to few hundred microns.
In
certain embodiments, macroscopic particles in the millimeter range may be
suitable.
[0025] In embodiments, a particle, such as an amine-modified particle, may
comprise
materials such as lignocellulosic material, cellulosic material, vitreous
material,
cementitious material, carbonaceous material, plastics, elastomeric materials,
and the
like. In embodiments, cellulosic and lignocellulosic materials may include
wood
materials such as wood flakes, wood fibers, wood waste material, wood powder,
lignins, or fibers from woody plants.
[0026] Examples of inorganic particles include clays such as attapulgite and
bentonite. In embodiments, the inorganic compounds can be vitreous materials,
such
as ceramic particles, glass, fly ash and the like. The particles may be solid
or may be
partially or completely hollow. For example, glass or ceramic microspheres may
be
used as particles. Vitreous materials such as glass or ceramic may also be
formed as
fibers to be used as particles. Cementitious materials may include gypsum,
Portland
cement, blast furnace cement, alumina cement, silica cement, and the like.
Carbonaceous materials may include carbon black, graphite, carbon fibers,
carbon
microparticles, and carbon nanoparticles, for example carbon nanotubes.
[0027] In embodiments, the particle can be substantially larger than the fine
particulates it is separating out from the process stream. For example, for
the removal
of particulate matter with approximate diameters less than 50 microns,
particles may be
selected for modification having larger dimensions. In other embodiments, the
particle
can be substantially smaller than the particulate matter it is separating out
of the
process stream, with a number of such particles interacting in order to
complex with
the much larger particulate matter. Particles may also be selected for
modification that
have shapes adapted for easier settling when compared to the target
particulate matter:
spherical particles, for example, may advantageously be used to remove flake-
type
particulate matter. In other embodiments, dense particles may be selected for
9
CA 02706274 2012-05-23
4127.1002 CA
modification, so that they settle rapidly when complexed with the fine
particulate
matter in the process stream. In yet other embodiments, extremely buoyant
particles
may be selected for modification, so that they rise to the fluid surface after
complexing
with the fine particulate matter, allowing the complexes to be removed via a
skimming
process rather than a settling-out process. In embodiments where the modified
particles are used to form a filter, as in a filter cake, the particles
selected for
modification can be chosen for their low packing density or porosity.
Advantageously,
particles can be selected that are indigenous to a particular geographical
region where
the particulate removal process would take place. For example, sand can be
advantageously used as the particle to be modified for removing particulate
matter
from the waste stream of phosphate mining, because sand is a byproduct of
phosphate
beneficiation and is therefore found abundantly at phosphate mining sites.
[00281 In embodiments, plastic materials may be used as particles. Both
thermoset
and thermoplastic resins may be used to form plastic particles. Plastic
particles may be
shaped as solid bodies, hollow bodies or fibers, or any other suitable shape.
Plastic
particles can be formed from a variety of polymers. A polymer useful as a
plastic
particle may be a homopolymer or a copolymer. Copolymers can include block
copolymers, graft copolymers, and interpolymers. In embodiments, suitable
plastics
may include, for example, addition polymers (e.g., polymers of ethylenically
unsaturated monomers), polyesters, polyurethanes, aramid resins, acetal
resins,
formaldehyde resins, and the like. Addition polymers can include, for example,
polyolefins, polystyrene, and vinyl polymers. Polyolefins can include, in
embodiments, polymers prepared from C2-C10 olefin monomers, e.g., ethylene,
propylene, butylene, dicyclopentadiene, and the like. In embodiments,
poly(vinyl
chloride) polymers, acrylonitrile polymers, and the like can be used. In
embodiments,
useful polymers for the formation of particles may be formed by condensation
reaction
of a polyhydric compound (e.g., an alkylene glycol, a polyether alcohol, or
the like)
with one or more polycarboxylic acids. Polyethylene terephthalate is an
example of a
suitable polyester resin. Polyurethane resins can include polyether
polyurethanes and
polyester polyurethanes. Plastics may also be obtained for these uses from
waste
CA 02706274 2012-05-23
4127.1002 CA
plastic, such as post-consumer waste including plastic bags, containers,
bottles made of
high density polyethylene, polyethylene grocery store bags, and the like.
[00291 In embodiments, plastic particles can be formed as expandable polymeric
pellets. Such pellets may have any geometry useful for the specific
application,
whether spherical, cylindrical, ovoid, or irregular. Expandable pellets may be
pre-
expanded before using them. Pre-expansion can take place by heating the
pellets to a
temperature above their softening point until they deform and foam to produce
a loose
composition having a specific density and bulk. After pre-expansion, the
particles may
be molded into a particular shape and size. For example, they may be heated
with
steam to cause them to fuse together into a lightweight cellular material with
a size and
shape conforming to the mold cavity. Expanded pellets may be 2-4 times larger
than
unexpanded pellets. As examples, expandable polymeric pellets may be formed
from
polystyrenes and polyolefins. Expandable pellets are available in a variety of
unexpanded particle sizes. Pellet sizes, measured along the pellet's longest
axis, on a
weight average basis, can range from about 0.1 to 6 mm.
[00301 In embodiments, the expandable pellets may be formed by polymerizing
the
pellet material in an aqueous suspension in the presence of one or more
expanding
agents, or by adding the expanding agent to an aqueous suspension of finely
subdivided particles of the material. An expanding agent, also called a
"blowing
agent," is a gas or liquid that does not dissolve the expandable polymer and
which boils
below the softening point of the polymer. Blowing agents can include lower
alkanes
and halogenated lower alkanes, e.g., propane, butane, pentane, cyclopentane,
hexane,
cyclohexane, dichlorodifluoromethane, and trifluorochloromethane, and the
like.
Depending on the amount of blowing agent used and the technique for expansion,
a
range of expansion capabilities exist for any specific unexpanded pellet
system. The
expansion capability relates to how much a pellet can expand when heated to
its
expansion temperature. In embodiments, elastomeric materials can be used as
particles. Particles of natural or synthetic rubber can be used, for example.
[00311 In embodiments, the particle can be substantially larger than the fine
particulates it is separating out from the process stream. For example, for
the removal
of particulate matter with approximate diameters less than 50 microns,
particles may be
11
CA 02706274 2012-05-23
4127.1002 CA
selected for modification having larger dimensions. In other embodiments, the
particle
can be substantially smaller than the particulate matter it is separating out
of the
process stream, with a number of such particles interacting in order to
complex with
the much larger particulate matter. Particles may also be selected for
modification that
have shapes adapted for easier settling when compared to the target
particulate matter:
spherical particles, for example, may advantageously be used to remove flake-
type
particulate matter. In other embodiments, dense particles may be selected for
modification, so that they settle rapidly when complexed with the fine
particulate
matter in the process stream. In yet other embodiments, extremely buoyant
particles
may be selected for modification, so that they rise to the fluid surface after
complexing
with the fine particulate matter, allowing the complexes to be removed via a
skimming
process rather than a settling-out process. In embodiments where the modified
particles are used to form a filter, as in a filter cake, the particles
selected for
modification can be chosen for their low packing density or porosity.
Advantageously,
particles can be selected that are indigenous to a particular geographical
region where
the particulate removal process would take place. For example, sand can be
used as the
particle to be modified for removing particulate matter from the waste stream
(tailings)
in phosphate mining or other mining activities.
[0032] It is envisioned that the complexes formed from the modified particles
and the
particulate matter can be recovered and used for other applications. For
example, when
sand is used as the modified particle and it captures fine clay in tailings,
the sand/clay
combination can be used for road construction in the vicinity of the mining
sites, due to
the less compactable nature of the complexes compared to other locally
available
materials.
[0033] The "activation" step may be performed using flocculants or other
polymeric
substances. Preferably, the polymers or flocculants can be charged, including
anionic
or cationic polymers. In embodiments, anionic polymers can be used, including,
for
example, olefinic polymers, such as polymers made from polyacrylate,
polymethacrylate, partially hydrolyzed polyacrylamide, and salts, esters and
copolymers thereof (such as (sodium acrylate/acrylamide)
copolymers)polyacrylic
acid, polymethacrylic acid, sulfonated polymers, such as sulfonated
polystyrene, and
12
CA 02706274 2012-05-23
4127.1002 CA
salts, esters and copolymers thereof, and the like. Suitable polycations
include:
polyvinylamines, polyallylamines, polydiallyldimethylammoniums (e.g.,
thepolydiallyldimethylammonium chloride, branched or linear polyethyleneimine,
crosslinked amines (including epichlorohydrin/dimethylamine, and
epichlorohydrin/alkylenediamines), quaternary ammonium substituted polymers,
such
as (acrylamide/dimethylaminoethylacrylate methyl chloride quat) copolymers and
trimethylammoniummethylene- substituted polystyrene, polyvinylamine, and the
like.
Nonionic polymers suitable for hydrogen bonding interactions can include
polyethylene oxide, polypropylene oxide, polyhydroxyethylacrylate,
polyhydroxyethylmethacrylate, and the like. In embodiments, an activator such
as
polyethylene oxide can be used as an activator with a cationic tethering
material in
accordance with the description of tethering materials below. In embodiments,
activator polymers with hydrophobic modifications can be used. Flocculants
such as
those sold under the trademark MAGNAFLOC by Ciba Specialty Chemicals can be
used.
[0034] In embodiments, activators such as polymers or copolymers containing
carboxylate, sulfonate, phosphonate, or hydroxamate groups can be used. These
groups can be incorporated in the polymer as manufactured; alternatively they
can be
produced by neutralization of the corresponding acid groups, or generated by
hydrolysis of a precursor such as an ester, amide, anhydride, or nitrile
group. The
neutralization or hydrolysis step could be done on site prior to the point of
use, or it
could occur in situ in the process stream.
[0035] The activated particle can also be an amine functionalized or modified
particle. As used herein, the term "modified particle" can include any
particle that has
been modified by the attachment of one or more amine functional groups as
described
herein. The functional, group on the surface of the particle can be from
modification
using a multifunctional coupling agent or a polymer. The multifunctional
coupling
agent can be an amino silane coupling agent as an example. These molecules can
bond
to a particle surface (e.g., metal oxide surface) and then present their amine
group for
interaction with the particulate matter. In the case of a polymer, the polymer
on the
surface of the particles can be covalently bound to the surface or interact
with the
13
CA 02706274 2012-05-23
4127.1002 CA
surface of the particle and/or fiber using any number of other forces such as
electrostatic, hydrophobic, or hydrogen bonding interactions. In the case that
the
polymer is covalently bound to the surface, a multifunctional coupling agent
can be
used such as a silane coupling agent. Suitable coupling agents include
isocyano silanes
and epoxy silanes as examples. A polyamine can then react with an isocyano
silane or
epoxy silane for example. Polyamines include polyallyl amine, polyvinyl amine,
chitosan, and polyethylenimine.
[0036] In embodiments, polyamines (polymers containing primary, secondary,
tertiary, and/or quaternary amines) can also self-assemble onto the surface of
the
particles or fibers to functionalize them without the need of a coupling
agent. For
example, polyamines can self-assemble onto the surface of the particles
through
electrostatic interactions. They can also be precipitated onto the surface in
the case of
chitosan for example. Since chitosan is soluble in acidic aqueous conditions,
it can be
precipitated onto the surface of particles by suspending the particles in a
chitosan
solution and then raising the solution pH.
[0037] In embodiments, the amines or a majority of amines are charged. Some
polyamines, such as quaternary amines are fully charged regardless of the pH.
Other
amines can be charged or uncharged depending on the environment. The
polyamines
can be charged after addition onto the particles by treating them with an acid
solution
to protonate the amines. In embodiments, the acid solution can be non-aqueous
to
prevent the polyamine from going back into solution in the case where it is
not
covalently attached to the particle.
[0038] The polymers and particles can complex via forming one or more ionic
bonds,
covalent bonds, hydrogen bonding and combinations thereof, for example. Ionic
complexing is preferred.
[0039] To obtain activated fine materials, the activator could be introduced
into a
liquid medium through several different means. For example, a large mixing
tank could
be used to mix an activating material with tailings from mining operations
that contain
fine particulate materials. Alternatively, the activating material can be
added along a
transport pipeline and mixed, for example, by a static mixer or series of
baffles.
14
CA 02706274 2012-05-23
4127.1002 CA
Activated particles are produced that can be treated with one or more
subsequent steps
of tethering and anchor-separation.
[0040] The particles that can be activated are generally fine particles that
are resistant
to sedimentation. Examples of particles that can be filtered in accordance
with the
invention include metals, sand, inorganic, or organic particles. The particles
are
generally fine particles, such as particles having a mass mean diameter of
less than 50
microns or particle fraction that remains with the filtrate following a
filtration with, for
example, a 325 mesh filter. The particles to be removed in the processes
described
herein are also referred to as "fines."
2. Tethering
[0041] As used herein, the term "tethering" refers to an interaction between
an
activated fine particle and an anchor particle (for example, as described
below). The
anchor particle can be treated or coated with a tethering material. The
tethering
material, such as a polymer, forms a complex or coating on the surface of the
anchor
particles such that the tethered anchor particles have an affinity for the
activated fines.
In embodiments, the selection of tether and activator materials is intended to
make the
two solids streams complementary so that the activated fine particles become
tethered,
linked or otherwise attached to the anchor particle. When attached to
activated fine
particles via tethering, the anchor particles enhance the rate and
completeness of
sedimentation or removal of the fine particles from the fluid stream.
[0042] In accordance with these systems and methods, the tethering material
acts as a
complexing agent to affix the activated particles to an anchor material. In
embodiments, sand can be used as an anchor material, as may a number of other
substances, as set forth in more detail below. In embodiments, a tethering
material can
be any type of material that interacts strongly with the activating material
and that is
connectable to an anchor particle.
[00431 As used herein, the term "anchor particle" refers to a particle that
facilitates
the separation of fine particles. Generally, anchor particles have a density
that is
greater than the liquid process stream. For example, anchor particles that
have a
density of greater than 1.3 g/cc can be used. Additionally or alternatively,
the density
CA 02706274 2012-05-23
4127.1002 CA
of the anchor particles can be greater than the density of the fine particles
or activated
particles. Alternatively, the density is less than the dispersal medium, or
density of the
liquid or aqueous stream. Alternatively, the anchor particles are simply
larger than the
fine particles being removed. In embodiments, the anchor particles are chosen
so that,
after complexing with the fine particulate matter, the resulting complexes can
be
removed via a skimming process rather than a settling-out process, or they can
be
readily filtered out or otherwise skimmed off. In embodiments, the anchor
particles
can be chosen for their low packing density or potential for developing
porosity. A
difference in density or particle size can facilitate separating the solids
from the
medium.
[0044] For example, for the removal of particulate matter with an approximate
mass
mean diameter less than 50 microns, anchor particles may be selected having
larger
dimensions, e.g., a mass mean diameter of greater than 70 microns. An anchor
particle
for a given system can have a shape adapted for easier settling when compared
to the
target particulate matter: spherical particles, for example, may
advantageously be used
as anchor particles to remove particles with a flake or needle morphology. In
other
embodiments, increasing the density of the anchor particles may lead to more
rapid
settlement. Alternatively, less dense anchors may provide a means to float the
fine
particles, using a process to skim the surface for removal. In this
embodiment, one
may choose anchor particles having a density of less than about 0.9 g/cc, for
example,
0.5 g/cc, to remove fine particles from an aqueous process stream.
[0045] Advantageously, anchor particles can be selected that are indigenous to
a
particular geographical region where the particulate removal process would
take place.
For example, sand can be used as the anchor particle for use in removing fine
particulate matter from the waste stream (tailings) of phosphate mining.
[0046] Suitable anchor particles can be formed from organic or inorganic
materials,
or any mixture thereof. Particles suitable for use as anchor particles can
include
organic or inorganic particles, or mixtures thereof. In referring to an anchor
particle, it
is understood that such a particle can be made from a single substance or can
be made
from a composite. For example, coal can be used as an anchor particle in
combination
with another organic or inorganic anchor particle. Any combination of
inorganic or
16
CA 02706274 2012-05-23
4127.1002 CA
organic anchor particles can be used. Anchor particle combinations can be
introduced
as mixtures of heterogeneous materials. Anchor particles can be prepared as
agglomerations of heterogeneous materials, or other physical combinations
thereof.
For example, an anchor particle can be formed from a particle of one type of
biomass
combined with a particle of another type of biomass. In another example, an
anchor
particle can be formed from a combustible organic particle complexed, coated
or
otherwise admixed with other organic or inorganic anchor particle materials.
As an
example, a combustible organic material can be combined with particles of
ungelatinized starch. In embodiments, the starch can be gelatinized during a
thermal
drying step, optionally with the use of an alkali, to cause binding and
strengthening of
the composite fuel product.
[0047] In embodiments, the organic material selected as an anchor particle can
be a
coal particle, for example coal derived from coal mining or processing. As an
example, coal that is collected as filter cake (FC) coal can be used as anchor
particles.
This technology has the advantage of using materials that are readily
available on-site
during coal processing to treat the fines being produced there. Anchor
particles can be
energy-bearing (e.g., combustible) or non-energy-bearing (e.g., minerals), or
combinations thereof.
[0048] In accordance with these systems and methods, inorganic anchor
particles can
include one or more materials such as calcium carbonate, dolomite, calcium
sulfate,
kaolin, talc, titanium dioxide, sand, diatomaceous earth, aluminum hydroxide,
silica,
other metal oxides and the like. In embodiments, the coarse fraction of the
solids
recovered from the mining process itself can be used for anchor particles, for
example,
coal from coal mining. Organic particles can include one or more materials
such as
biomass, starch, modified starch, polymeric spheres (both solid and hollow),
and the
like. Particle sizes can range from a few nanometers to few hundred microns.
In
certain embodiments, macroscopic particles in the millimeter range may be
suitable.
[0049] In embodiments, a particle, such as an amine-modified particle, can
comprise
materials such as lignocellulosic material, cellulosic material, minerals,
vitreous
material, cementitious material, carbonaceous material, plastics, elastomeric
materials,
and the like. In embodiments, cellulosic and lignocellulosic materials may
include
17
CA 02706274 2012-05-23
4127.1002 CA
wood materials such as wood flakes, wood fibers, wood waste material, wood
powder,
lignins, or fibers from woody plants. Organic materials can include various
forms of
organic waste, including biomass and including particulate matter from post-
consumer
waste items such as old tires and carpeting materials.
[0050] Examples of inorganic particles include clays such as attapulgite and
bentonite. In embodiments, the inorganic compounds can be vitreous materials,
such
as ceramic particles, glass, fly ash and the like. The particles may be solid
or may be
partially or completely hollow. For example, glass or ceramic microspheres may
be
used as particles. Vitreous materials such as glass or ceramic may also be
formed as
fibers to be used as particles. Cementitious materials may include gypsum,
Portland
cement, blast furnace cement, alumina cement, silica cement, and the like.
Carbonaceous materials may include carbon black, graphite, carbon fibers,
carbon
microparticles, and carbon nanoparticles, for example carbon nanotubes.
[0051] In other embodiments, the inorganic material selected as an anchor
particle
can be produced during coal preparation and processing, as described above.
For
example, the inorganic material used as an anchor particle can be derived from
the
mineral waste products of coal processing, e.g., coal processing refuse (CPR).
Other
inorganic materials available on-site (sand, etc.) can be used as anchor
particles, either
alone or in combination with other inorganic or organic anchor particles. This
technology has the advantage of using materials that are readily available on-
site
during coal processing to treat the fines being produced there.
[0052] In embodiments, plastic materials may be used as particles. Both
thermoset
and thermoplastic resins may be used to form plastic particles. Plastic
particles may be
shaped as solid bodies, hollow bodies or fibers, or any other suitable shape.
Plastic
particles can be formed from a variety of polymers. A polymer useful as a
plastic
particle may be a homopolymer or a copolymer. Copolymers can include block
copolymers, graft copolymers, and interpolymers. In embodiments, suitable
plastics
may include, for example, addition polymers (e.g., polymers of ethylenically
unsaturated monomers), polyesters, polyurethanes, aramid resins, acetal
resins,
formaldehyde resins, and the like. Addition polymers can include, for example,
polyolefins, polystyrene, and vinyl polymers. Polyolefms can include, in
18
CA 02706274 2012-05-23
4127.1002 CA
embodiments, polymers prepared from C2-C10 olefin monomers, e.g., ethylene,
propylene, butylene, dicyclopentadiene, and the like. In embodiments,
poly(vinyl
chloride) polymers, acrylonitrile polymers, and the like can be used. In
embodiments,
useful polymers for the formation of particles may be formed by condensation
reaction
of a polyhydric compound (e.g., an alkylene glycol, a polyether alcohol, or
the like)
with one or more polycarboxylic acids. Polyethylene terephthalate is an
example of a
suitable polyester resin. Polyurethane resins can include polyether
polyurethanes and
polyester polyurethanes. Plastics may also be obtained for these uses from
waste
plastic, such as post-consumer waste including plastic bags, containers,
bottles made of
high density polyethylene, polyethylene grocery store bags, and the like. In
embodiments, elastomeric materials can be used as particles. Particles of
natural or
synthetic rubber can be used, for example.
[0053] Advantageously, anchor particles can be selected from biomass, so that
they
complex with the fines to form a biomass-fines composite solid. This process
can be
advantageous in producing a combustible complex, for example by complexing
coal
fines with a biomass tether. Biomass can be derived from vegetable sources or
animal
sources. Biomass can be derived from waste materials, including post-consumer
waste, animal or vegetable waste, agricultural waste, sewage, and the like. In
embodiments, the biomass sourced materials are to be processed so that they
form
particles of an appropriate size for tethering and combining with the
activated fines.
Particle sizes of, e.g., 0.01 - 50 millimeters are desirable. Processing
methods can
include grinding, milling, pumping, shearing, and the like. For example,
hammer
mills, ball mills, and rod mills can be used to reduce oversized materials to
an
appropriate size. In embodiments, additives might be used in the processing of
the
anchor particles to improve efficiency, reduce energy requirements, or
increase yield.
These processing additives include polymers, surfactants, and chemicals that
enhance
digestion or disintegration. Optionally, other treatment modalities, such as
exposure to
cryogenic liquids (e.g., liquid nitrogen or solid carbon dioxide) can be
employed to
facilitate forming anchor particles of appropriate size from biomass. It is
understood
that biomass-derived anchor particles can be formed as particles of any
morphology
(regular or irregular, plate-shaped, flakes, cylindrical, spherical, needle-
like, etc.) or
19
CA 02706274 2012-05-23
4127.1002 CA
can be formed as fibers. Fibrous materials may be advantageous in that they
facilitate
dewatering/filtration of the composite material being formed by these systems
and
methods, and they can add strength to such composite materials.
[0054] Vegetable sources of biomass can include fibrous material, particulate
material, amorphous material, or any other material of vegetable origin.
Vegetable
sources can be predominately cellulosic, e.g., derived from cotton, jute,
flax, hemp,
sisal, ramie, and the like. Vegetable sources can be derived from seeds or
seed cases,
such as cotton or kapok, or from nuts or nutshells, including without
limitation, peanut
shells, walnut shells, coconut shells, and the like. Vegetable sources can
include the
waste materials from agriculture, such as corn stalks, stalks from grain, hay,
straw, or
sugar cane (e.g., bagasse). Vegetable sources can include leaves, such as
sisal, agave,
deciduous leaves from trees, shrubs and the like, leaves or needles from
coniferous
plants, and leaves from grasses. Vegetable sources can include fibers derived
from the
skin or bast surrounding the stem of a plant, such as flax, jute, kenaf, hemp,
ramie,
rattan, soybean husks, corn husks, rice hulls, vines or banana plants.
Vegetable sources
can include fruits of plants or seeds, such as coconuts, peach pits, olive
pits, mango
seeds, corncobs or corncob byproducts ("bees wings") and the like. Vegetable
sources
can include the stalks or stems of a plant, such as wheat, rice, barley,
bamboo, and
grasses. Vegetable sources can include wood, wood processing products such as
sawdust, and wood, and wood byproducts such as lignin.
[00551 Animal sources of biomass can include materials from any part of a
vertebrate
or invertebrate animal, fish, bird, or insect. Such materials typically
comprise proteins,
e.g., animal fur, animal hair, animal hoofs, and the like. Animal sources can
include
any part of the animal's body, as might be produced as a waste product from
animal
husbandry, farming, meat production, fish production or the like, e.g.,
catgut, sinew,
hoofs, cartilaginous products, etc. Animal sources can include the dried
saliva or other
excretions of insects or their cocoons, e.g., silk obtained from silkworm
cocoons or
spider's silk. Animal sources can include dairy byproducts such as whey, whey
permeate solids, milk solids, and the like. Animal sources can be derived from
feathers
of birds or scales of fish.
CA 02706274 2012-05-23
4127.1002 CA
[00561 In embodiments, the anchor particle can be substantially larger than
the fine
particulates it is separating out from the process stream. For example, for
the removal
of fines with approximate diameters less than 50 microns, anchor particles may
be
selected for modification having larger dimensions. In other embodiments, the
particle
can be substantially smaller than the particulate matter it is separating out
of the
process stream, with a number of such particles interacting in order to
complex with
the much larger particulate matter. Particles may also be selected for
modification that
have shapes adapted for easier settling when compared to the target
particulate matter:
spherical particles, for example, may advantageously be used to remove flake-
type
particulate matter. In other embodiments, dense particles may be selected for
modification, so that they settle rapidly when complexed with the fine
particulate
matter in the process stream. In yet other embodiments, extremely buoyant
particles
may be selected for modification, so that they rise to the fluid surface after
complexing
with the fine particulate matter, allowing the complexes to be removed via a
skimming
process rather than a settling-out process. In embodiments where the modified
particles are used to form a filter, as in a filter cake, the particles
selected for
modification can be chosen for their low packing density or porosity.
Advantageously,
particles can be selected that are indigenous to a particular geographical
region where
the particulate removal process would take place. For example, sand can be
used as the
particle to be modified for removing particulate matter from the waste stream
(tailings)
in phosphate mining or other mining activities.
[00571 It is envisioned that the complexes formed from the modified particles
and the
particulate matter can be recovered and used for other applications. For
example, when
sand is used as the modified particle and it captures fine clay in tailings,
the sand/clay
combination can be used for road construction in the vicinity of the mining
sites, due to
the less compactable nature of the complexes compared to other locally
available
materials.
[00581 Anchor particle sizes (as measured as a mean diameter) can have a size
up to
few hundred microns, preferably greater than about 70 microns. In certain
embodiments, macroscopic anchor particles up to and greater than about l_ mm
may be
suitable. Recycled materials or waste, particularly recycled materials and
waste having
21
CA 02706274 2012-05-23
4127.1002 CA
a mechanical strength and durability suitable to produce a product useful in
building
roads and the like, or (in other embodiments) capable of combustion, are
particularly
advantageous.
[0059] As an example of a tethering material used with an anchor particle in
accordance with these systems and methods, chitosan can be precipitated onto
sand
particles, for example, via pH-switching behavior. The chitosan can have
affmity for
anionic systems that have been used to activate fine particles. Anchor
particles can be
complexed with tethering agents, such agents being selected so that they
interact with
the polymers used to activate the coal fines. In one example, partially
hydrolyzed
polyacrylamide polymers can be used to activate particles, resulting in a
particle with
anionic charge properties. The cationic charge of the chitosan will attract
the anionic
charge of the activated particles, to attach the sand particles to the
activated fine
particles.
[0060] In embodiments, various interactions such as electrostatic, hydrogen
bonding
or hydrophobic behavior can be used to affix an activated particle or particle
complex
to a tethering material complexed with an anchor particle.
[0061] In embodiments, the anchor particles can be combined with a
polycationic
polymer, for example a polyamine. One or more populations of anchor particles
may
be used, each being activated with a tethering agent selected for its
attraction to the
activated coal fines and/or to the other anchor particle's tether. The
tethering
functional group on the surface of the anchor particle can be from
modification using a
multifunctional coupling agent or a polymer. The multifunctional coupling
agent can
be an amino silane coupling agent as an example. These molecules can bond to
an
anchor particle's surface and then present their amine group for interaction
with the
activated coal fines. In the case of a tethering polymer, the polymer on the
surface of
the particles can be covalently bound to the surface or interact with the
surface of the
anchor particle and/or fiber using any number of other forces such as
electrostatic,
hydrophobic, or hydrogen bonding interactions. In the case that the polymer is
covalently bound to the surface, a multifunctional coupling agent can be used
such as a
30---- silane coupling agent. Suitable coupling agents include isocyano
silanes and-epoxy
22
CA 02706274 2012-05-23
4127.1002 CA
silanes as examples. A polyamine can then react with an isocyano silane or
epoxy
silane for example. Polyamines include polyallyl amine, polyvinyl amine,
chitosan,
and polyethylenimine.
[0062] In embodiments, polyamines (polymers containing primary, secondary,
tertiary, and/or quaternary amines) can also self-assemble onto the surface of
the
particles or fibers to functionalize them without the need of a coupling
agent. For
example, polyamines can self-assemble onto the surface of the particles
through
electrostatic interactions. They can also be precipitated onto the surface in
the case of
chitosan for example. Since chitosan is soluble in acidic aqueous conditions,
it can be
precipitated onto the surface of particles by suspending the particles in a
chitosan
solution and then raising the solution pH.
[0063] In embodiments, the amines or a majority of amines are charged. Some
polyamines, such as quaternary amines are fully charged regardless of the pH.
Other
amines can be charged or uncharged depending on the environment. The
polyamines
can be charged after addition onto the particles by treating them with an acid
solution
to protonate the amines. In embodiments, the acid solution can be non-aqueous
to
prevent the polyamine from going back into solution in the case where it is
not
covalently attached to the particle.
[0064] The polymers and particles can complex via forming one or more ionic
bonds,
covalent bonds, hydrogen bonding and combinations thereof, for example. Ionic
complexing is preferred.
[0065] As an example of a tethering material used with an anchor particle in
accordance with these systems and methods, chitosan can be precipitated onto
anchor
particles, for example, via pH-switching behavior. The chitosan as a tether
can have
affinity for anionic systems that have been used to activate fine particles.
In one
example, partially hydrolyzed polyacrylamide polymers can be used to activate
coal
fines, resulting in a particle with anionic charge properties. The cationic
charge of the
chitosan will attract the anionic charge of the activated particles, to attach
the anchor
particles to the activated coal fines. In the foregoing example, electrostatic
interactions
can govern the assembly of the activated fine particle complexes bearing the
anionic
23
CA 02706274 2012-05-23
4127.1002 CA
partially-hydrolyzed polyacrylamide polymer and the cationic anchor particles
complexed with the chitosan tethering material.
[00661 In embodiments, polymers such as linear or branched polyethyleneimine
can
be used as tethering materials. It would be understood that other anionic or
cationic
polymers could be used as tethering agents, for example
polydiallyldimethylammonium chloride (poly(DADMAC). In other embodiments,
cationic tethering agents such as epichlorohydrin dimethylamine (epi/DMA),
styrene
maleic anhydride imide (SMAI), polyethylene imide (PEI), polyvinylamine,
polyallylamine, amine-aldehyde condensates, poly(dimethylaminoethyl acrylate
methyl
chloride quaternary) polymers and the like can be used. Advantageously,
cationic
polymers useful as tethering agents can include quaternary ammonium or
phosphonium
groups. Advantageously, polymers with quaternary ammonium groups such as
poly(DADMAC) or epi/DMA can be used as tethering agents. In other embodiments,
polyvalent metal salts (e.g., calcium, magnesium, aluminum, iron salts, and
the like)
can be used as tethering agents. In other embodiments cationic surfactants
such as
dimethyldialkyl(C8-C22)ammonium halides, alkyl(C8-C22)trimethylammonium
halides, alkyl(C8-C22)dimethylbenzylammonium halides, cetyl pyridinium
chloride,
fatty amines, protonated or quaternized fatty amines, fatty amides and alkyl
phosphonium compounds can be used as tethering agents. In embodiments,
polymers
having hydrophobic modifications can be used as tethering agents.
[00671 The efficacy of a tethering material, however, can depend on the
activating
material. A high affinity between the tethering material and the activating
material can
lead to a strong and/or rapid interaction there between. A suitable choice for
tether
material is one that can remain bound to the anchor surface, but can impart
surface
properties that are beneficial to a strong complex formation with the
activator polymer.
For example, a polyanionic activator can be matched with a polycationic tether
material or a polycationic activator can be matched with a polyanionic tether
material.
In one embodiment, a poly(sodium acrylate-co-acrylamide) activator is matched
with a
chitosan tether material.
24
CA 02706274 2012-05-23
4127.1002 CA
[0068] In hydrogen bonding terms, a hydrogen bond donor should be used in
conjunction with a hydrogen bond acceptor. In embodiments, the tether material
can
be complementary to the chosen activator, and both materials can possess a
strong
affinity to their respective deposition surfaces while retaining this surface
property.
[0069] In other embodiments, cationic-anionic interactions can be arranged
between
activated coal fines and tether-bearing anchor particles. The activator may be
a
cationic or an anionic material, as long as it has an affinity for the fine
particles to
which it attaches. The complementary tethering material can be selected to
have
affinity for the specific anchor particles being used in the system. In other
embodiments, hydrophobic interactions can be employed in the activation-
tethering
system.
[0070] It is envisioned that the complexes formed from the tether-bearing
anchor
particles and the activated coal fines can be recovered and used for other
applications.
For example, the complexes can be rapidly separated from water and can be
recovered
for compaction into coal pellets to be used for combustion. When a combustible
anchor particle is selected, the entire coal-anchor complex can be used for
energy
production. Other anchor particles can be selected to form specialized
composites with
the activated coal fines, as disclosed below in more detail.
[0071] Advantageously, anchor particles can be selected that are indigenous to
a
particular geographical region where the particulate removal process would
take place.
For example, sand can be used as the particle to be modified for removing
particulate
matter from the waste stream (tailings) in phosphate mining or other mining
activities.
[0072] Suitable anchor particles can be formed from organic or inorganic
materials,
or any mixture thereof Anchor particle sizes (as measured as a mass mean
diameter)
can have a size up to few hundred microns, preferably greater than about 70
microns.
In certain embodiments, macroscopic anchor particles up to and greater than
about 1
mm may be suitable. Recycled materials or waste, particularly recycled
materials and
waste having a mechanical strength and durability suitable to produce a
product useful
in building roads and the like are particularly advantageous.
CA 02706274 2012-05-23
4127.1002 CA
[0073] As an example of a tethering material used with an anchor particle in
accordance with these systems and methods, chitosan can be precipitated onto
sand
particles, for example, via pH-switching behavior. The chitosan can have
affinity for
anionic systems that have been used to activate fine particles. In one
example, partially
hydrolyzed polyacrylamide polymers can be used to activate particles,
resulting in a
particle with anionic charge properties. The cationic charge of the chitosans
will attract
the anionic charge of the activated particles, to attach the sand particles to
the activated
fine particles.
[0074] In embodiments, various interactions such as electrostatic, hydrogen
bonding
or hydrophobic behavior can be used to affix an activated particle or particle
complex
to a tethering material complexed with an anchor particle. In the foregoing
example,
electrostatic interactions can govern the assembly of the activated fine
particle
complexes bearing the anionic partially-hydrolyzed polyacrylamide polymer and
the
cationic sand particles complexed with the chitosan tethering material.
[0075] In embodiments, polymers such as linear or branched polyethyleneimine
can
be used as tethering materials. It would be understood that other anionic or
cationic
polymers could be used as tethering agents, for example
polydiallyldimethylammonium chloride. The efficacy of a tethering material,
however,
can depend on the activating material. A high affinity between the tethering
material
and the activating material can lead to a strong and/or rapid interaction
there between.
[0076] A suitable choice for tether material is one that can remain bound to
the
anchor surface, but can impart surface properties that are beneficial to a
strong complex
formation with the activator polymer. For example, a polyanionic activator can
be
matched with a polycationic tether material or a polycationic activator can be
matched
with a polyanionic tether material. In hydrogen bonding terms, a hydrogen bond
donor
should be used in conjunction with a hydrogen bond acceptor. In embodiments,
the
tether material can be complimentary to the chosen activator, and both
materials can
possess a strong affinity to their respective deposition surfaces while
retaining this
surface property.
[0077] In other embodiments, cationic-anionic interactions can be arranged
between
activated fine particles and tether-bearing anchor particles. The activator
may be a
26
CA 02706274 2012-05-23
4127.1002 CA
cationic or an anionic material, as long as it has an affinity for the fine
particles to
which it attaches. The complementary tethering material can be selected to
have
affinity for the specific anchor particles being used in the system. In other
embodiments, hydrophobic interactions can be employed in the activation-
tethering
system.
[00781 The anchor particle material is preferably added in an amount that
permits a
flowable slurry. For example, the particle material can be added in an amount
greater
than 1 gram/liter but less than the amount which results in a non-flowable
sludge,
amounts between about 1 to about 10 grams/liter, preferably 2 to 6 g/l are
often
suitable. In some embodiments, it may be desirable to maintain the
concentration of
the anchor particles to 20 g/l or higher. The anchor particles may be fresh
(unused)
material, recycled, cleaned ballast, or recycled, uncleaned ballast.
[00791 In embodiments, for example when sand is chosen as an anchor particle,
higher amounts of the particle material may be added. For example, sand can be
added
in a range between 1 - 300 gm/l, preferably between 50-300 gm/l, for example
at a
dosage level of 240 gm/l.
3. Removal of the Anchor-Tether-Activator Complexes
[00801 It is envisioned that the complexes formed from the anchor particles
and the
activated particulate matter can be recovered and used for other applications.
For
example, when sand is used as the modified particle and it captures fine clay
in tailings,
the dewatered sand/clay combination can be used for road construction in the
vicinity
of the mining sites, due to. the less compactable nature of the complexes
compared to
other locally available materials. As another example, a sand/clay complex
could be
used to fill in strip mining pits, such as would be found at phosphate mining
operations.
In other embodiments, complexes with anchor particles and fines could be used
in a
similar manner on-site to fill in abandoned mines, or the complexes could be
used off-
site for landfill or construction purposes. The uses of the solid material
produced by
the systems and methods disclosed herein will vary depending on the specific
constituents of the material.
27
CA 02706274 2012-05-23
4127.1002 CA
[0081] In embodiments, the interactions between the activated fine particles
and the
tether-bearing anchor particles can enhance the mechanical properties of the
complex
that they form. For example, an activated fine particle or collection thereof
can be
durably bound to one or more tether-bearing anchor particles, so that they do
not
segregate or move from the position that they take on the particles. This
property of
the complex can make it mechanically more stable.
[0082] Increased compatibility of the activated fine materials with a denser
(anchor)
matrix modified with the appropriate tether polymer can lead to further
mechanical
stability of the resulting composite material. This becomes quite important
when
dealing with tailings resulting from mining. This composite material can then
be
further utilized within the project for road building, dyke construction, or
even land
reclamation, rather than simply left in a pond to settle at a much slower
rate.
[0083] A variety of techniques are available for removing the activated-
tethered-
anchored (ATA) complexes from the fluid stream. For example, the tether-
bearing
anchor particles can be mixed into a stream carrying activated fine particles,
and the
complexes can then separated via a settling process such as gravity or
centrifugation.
In another method, the process stream carrying the activated fine particles
could flow
through a bed or filter cake of the tether-bearing anchor particles. In any of
these
methods, the modified particles interact with the fine particulates and pull
them out of
suspension so that later separation removes both modified particles and fine
particulates.
[0084] As would be appreciated by artisans of ordinary skill, a variety of
separation
processes could be used to remove the complexes of modified particles and fine
particulates. In the aforesaid removal processes, mechanical interventions for
separating the ATA complexes can be introduced, employing various devices as
separators (filters, skimmers, centrifuges, and the like). Or other separation
techniques
can be employed. For example, if the anchor particles had magnetic properties,
the
complexes formed by the interaction of tether-bearing anchor particles and
activated
fine particulates could be separated using a magnetic field. As another
example, if the
tether-bearing anchor particles were prepared so that they were electrically
conductive,
28
CA 02706274 2012-05-23
4127.1002 CA
the complexes formed by the interaction of tether-bearing anchor particles and
activated fine particulates could be separated using an electric field. As
would be
further appreciated by those of ordinary skill, tether-bearing anchor
particles could be
designed to complex with a specific type of activated particulate matter. The
systems
and methods disclosed herein could be used for complexing with organic waste
particles, for example. Other activation-tethering-anchoring systems may be
envisioned for removal of suspended particulate matter in fluid streams,
including
gaseous streams.
4. Specialized Composites
[00851 Composites can be formed with coal fines by selecting anchor particles
having
particular properties. For example, selecting a combustible anchor particle
allows the
coal-particle complex to be used for energy production. As another example,
selecting
an ungelatinized starch particle as an anchor particle allows the formation of
a coal-
particle complex that can be formed into an energy-bearing pellet that
responds to
heating by expanding and becoming porous, facilitating rapid and efficient
combustion.
[00861 In an embodiment, a fine powdered uncooked starch, e.g., ungelatinized,
can
be selected as anchor particles. A complementary pair of activator and
tethering agents
can be selected, whereby the starch particles can be coated with the tether
agent and the
coal fines can be coated with the activator. As an example, a polyelectrolyte
pair
including a polyanionic polymer and a polycationic polymer can be selected.
The
polyelectrolyte pair is selected to exhibit strong attraction to each other,
even when
surrounded with water molecules and other dissolved ions. Polyelectrolytes can
be
selected that are capable of spontaneous self-assembly on coal fines and
starch
particles, respectively, to deposit a monolayer or near-monolayer film on the
fines and
the particles.
[00871 In these embodiments, the tethering agent is disposed upon the starch
particles
to form a tether-bearing anchor. The activator is added to the coal fines. The
two fluid
streams can then be mixed together, whereby the charge-charge attraction
complexes
the tether-bearing anchor particles with the activated coal fines, expelling
intervening
water molecules and precipitating macroscopic coal-starch aggregates.
29
CA 02706274 2012-05-23
4127.1002 CA
[0088] In embodiments, polyanions including carboxymethyl cellulose (CMC),
carboxymethyl starch (CMS), pectin, xanthan gum, alginate, polyacrylic acid,
polymethacrylic acid, hydrolyzed polyacrylamide, styrene maleic anhydride
copolymer, certain proteins and peptides rich in amino acids containing
carboxylic acid
side groups, and the like, can be used in the system. In embodiments,
polycations
including polyethyleneimine, chitosan, polyvinylamine, polyallylamine,
polydimethyldiallylammonium chloride (PDAC), epi-dimethylamine (epi-DMA), and
certain proteins and peptides rich in amino acids with side amino groups, and
the like,
can be used in the system. Other polyanion-polycation pairs, including those
disclosed
above, will be apparent to those of ordinary skill in the art.
[0089] In an embodiment, the coal fine slurry can be treated with one type of
polyelectrolyte while a starch powder is lightly wetted with another (e.g.,
sprayed with
or a concentrated dispersion of the starch powder is added to a dilute
polyelectrolyte
solution). The charged starch is then mixed with the coal slurry containing
pre-coated
coal fines covered with counter-charged polyelectrolyte. Since both solids
have an
ultrathin polymer layer on their surface, the strong charge-charge attraction
immediately brings the disparate particles together, causing firm aggregation
and
precipitating cohesive pellets. Depending on the intensity of stirring,
pellets of different
but controlled size readily form. The consolidated aggregates can be easily
recovered
by passing the combined liquid stream over a coarse wire mesh. Clarified water
devoid
of either fine powder exits the system, posing little environmental concern.
Mechanical
pressure or vacuum can be applied to further dewater the solids. Since water
is largely
excluded from the interior of the pellets, little drying by heat is needed. In
and or semi-
arid areas, pellets can dry rapidly at room temperature. Other adjuncts to the
pellet
formation process, such as adding starches and other pelletizing ingredients,
are
consistent with these systems and methods, as would be understood by those of
ordinary skill in the art. In embodiments, additives to improve dewatering of
the solids
can be introduced using hydrophobic materials and practices known in the art.
[0090] In embodiments, the complexes formed from the ungelatinized-starch-coal-
fines composite can be shaped into energy-bearing pellets that possess a
unique
performance feature: upon modest heating, the starch greatly expands (foams)
leaving
CA 02706274 2012-05-23
4127.1002 CA
numerous interior channels for oxygen permeation. This rapid expansion can
cause the
pellets to disintegrate upon aggressive heating. Not to be bound by theory, it
is
understood that the thermal decomposition of the oxygen-rich sugar building
blocks of
starch can create oxygen radicals that can speed up combustion of nearby coal
particles
(which are typically difficult to burn due to their high aromaticity).
[0091] Other difficult-to-burn energy-containing particles can be similarly
treated by
the foregoing systems and methods so that they can be burned more efficiently.
Solutions bearing such energy-containing materials (e.g., coke from coking of
heavy
crude or bitumen, lignin from pulping, shredded or pulverized recycled
plastics/rubbers, fatty acids and waste oils/shortenings that are semi-solid-
like) can be
treated with activator polymers and complexed with tether-bearing anchor
particles,
e.g, starch particles as described above. Other tether-bearing anchor
particles can be
used to complex with the activated energy-containing material, such anchor
particles
being selected to enhance the combustion process or to effect other desirable
chemical
reactions. Alternatively, the energy-containing particles can be used as
anchor
particles, to be combined with other materials that have been activated, the
other
material being selected to enhance the combustion process or to effect other
desirable
chemical reactions.
[0092] In embodiments, more than two components can be complexed together
using
the foregoing systems and methods. One can therefore produce complexes of
multiple
materials designed to have desirable properties, such as more efficient
combustion.
For example, in an embodiment, lignin and coal dust can each be treated with
PDAC
while starch is activated with hydrolyzed polyacrylamide. When the dispersions
of
each component are brought together, a ternary complex can be formed, with
lignin/coal commingled and "glued" with starch particles that later expand
under heat.
The surface monolayer interaction among all the components serves to bind them
together and to provide cohesive strength to the pellet composite. In an
embodiment,
lignin and coke particles can be complexed with starch particles using these
systems
and methods.
[0093] In other embodiments, lignin and coal or coke can be combined as
complexes
without using coated starch particles. The lignin particles can be used as
anchor
31
CA 02706274 2012-05-23
4127.1002 CA
particles, to be coated with a tethering agent; the coal slurry (dilute or
otherwise) can
be pretreated with the complementary activator polymer. When the two fluid
streams
are combined, spontaneous aggregation ensues.
[0094] In yet other embodiments, these systems and methods can be used to
produce
composite pellets containing inorganic solids. For example, alkaline solids
(e.g.,
calcium oxide or magnesium oxide) can be compounded into the composite
pellets.
During pellet combustion, the aforesaid inorganic materials are converted to
the
respective carbonate, thus sequestering products of coal combustion such as
CO2 and
H2S. Thus the pelletized coal-based fuel formed in accordance with these
systems and
methods are able to capture some of their own undesirable combustion
byproducts.
Hence, pellets or other coal-based energy sources (e.g., briquettes) can be
made to
absorb noxious volatile products of combustion, an advantageous property for
applications in closed spaces, or example, or in situations where pollution
concerns are
of particular importance.
5. Exemplary Applications
a. Tailings Processing
[0095] Extraction of minerals from ores can produce fine, positively charged
particles
of clay or other materials that remain suspended in the effluent fluid stream.
The
effluent fluid stream can be directed to a mechanical separator such as a
cyclone that
can separate the fluid stream into two components, an overflow fluid
comprising fine
tails that contains the fine (< approximately 50 micron) particles, and an
underflow
fluid stream that contains coarse tails, mainly sand, with a small amount of
fine clay
particles.
[0096] In embodiments, the systems and methods disclosed herein can treat each
fluid
stream, an overflow fluid and/or an underflow fluid. An activating agent, such
as a
polyanion as described above, can preferably be introduced into the overflow
fluid
stream, resulting in a flocculation of the fine particles therein, often
forming a soft,
spongy mass. The underflow fluid can be used for the preparation of tether-
bearing
anchor particles. However, it will be clear that other sources for anchor
particles (e.g.,
sand) can also be used. In certain tailings fluids, the sand within the
underflow fluid
32
CA 02706274 2012-05-23
4127.1002 CA
itself can act as an "anchor particle," as described above. A cationic
tethering agent, as
described above, can be introduced into the underflow fluid so that it self-
assembles
onto the surface of the anchor particles, creating a plurality of tether-
bearing anchor
particles.
100971 Following this treatment to each fluid stream, the two fluid streams
can be re-
mixed in a batch, semi-batch or continuous fashion. The tether-bearing anchor
particles can interact, preferably electrostatically, with the activated,
preferably
flocculating, fine particles, forming large agglomerations of solid material
that can be
readily removed from or settled in the resulting fluid mixture.
[00981 In embodiments, the aforesaid systems and methods are amenable to
incorporation within existing tailings separation systems. For example, a
treatment
process can be added in-line to each of the separate flows from the overflow
and
underflow fluids; treated fluids then re-converge to form a single fluid path
from which
the resulting agglomerations can be removed. Removal of the agglomerations can
take
place, for example, by filtration, centrifugation, or other type of mechanical
separation.
[00991 In one embodiment, the fluid path containing the agglomerated solids
can be
subsequently treated by a conveyor belt system, analogous to those systems
used in the
papermaking industry. In an exemplary conveyor belt system, the mixture of
fluids
and agglomerated solids resulting from the electrostatic interactions
described above
can enter the system via a headbox. A moving belt containing a mechanical
separator
can move through the headbox, or the contents of the headbox are dispensed
onto the
moving belt, so that the wet agglomerates are dispersed along the moving belt.
One
type of mechanical separator can be a filter with a pore size smaller than the
average
size of the agglomerated particles. The size of the agglomerated particles can
vary,
depending upon the size of the constituent anchor particles (i.e., sand). For
example,
for systems where the sand component has a size between 50/70 mesh, an 80 mesh
filter can be used. Other adaptations can be envisioned by artisans having
ordinary
skill in the art. Agglomerated particles can be transported on the moving belt
and
further dewatered. Water removed from the agglomerated particles and residual
water
from the headbox from which agglomerates have been removed can be collected in
33
CA 02706274 2012-05-23
4127.1002 CA
whole or in part within the system and optionally recycled for use in
subsequent
processing.
[00100] In embodiments, the filtration mechanism can be an integral part of
the
moving belt. In such embodiments, the captured agglomerates can be physically
removed from the moving belt so that the filter can be cleaned and regenerated
for
further activity. In other embodiments, the filtration mechanism can be
removable
from the moving belt. In such embodiments, the spent filter can be removed
from the
belt and a new filter can be applied. In such embodiments, the spent filter
can
optionally serve as a container for the agglomerated particles that have been
removed.
[00101] Advantageously, as the agglomerated particles are arrayed along the
moving
belt, they can be dewatered and/or dried. These processes can be performed,
for
example, using heat, air currents, or vacuums. Agglomerates that have been
dewatered
and dried can be formed as solid masses, suitable for landfill, construction
purposes, or
the like.
[00102] Desirably, the in-line tailings processing described above is
optimized to
capitalize upon the robustness and efficiency of the electrostatic interaction
between
the activated tailings and the tether-bearing anchor particles.
Advantageously, the
water is quickly removed from the fresh tailings during the in-line tailings
processing,
permitting its convenient recycling into the processing systems.
b. Remediation of Treatment Ponds
[00103] The systems and methods disclosed herein can be used for treatment of
tailings at a facility remote from the mining and beneficiation facility or in
a pond.
Similar principles are involved: the fluid stream bearing the fine tailings
can be treated
with an anionic activating agent, preferably initiating flocculation. A tether-
bearing
anchor particle system can then be introduced into the activated tailings
stream, or the
activated tailings stream can be introduced into a tether-bearing anchor
particle system.
In embodiments, a tailings stream containing fines can be treated with an
activating
agent, as described above, and applied to a stationary or moving bed of tether-
bearing
anchor particles. For example, a stationary bed of tether-bearing anchor
particles can
be arranged as a flat bed over which the activated tailings stream is poured.
The tether-
bearing anchor particles can be within a container or housing, so that they
can act as a
34
CA 02706274 2012-05-23
4127.1002 CA
filter to trap the activated tailings passing through it. On a larger scale,
the tether-
bearing anchor particles can be disposed on a large surface, such as a flat or
inclined
surface (e.g., a beach), so that the activated tailings can flow over and
through it, e.g.
directionally toward a pond.
[00104] As an example, sand particles retrieved from theunderflow fluid stream
can be
used as the anchor particles to which a cationic tether is attached. A mass of
these
tether-bearing anchor particles can be arranged to create a surface of a
desired
thickness, forming an "artificial beach" to which or across which the
activated tailings
can be applied. As would be appreciated by those of ordinary skill in the art,
the
application of the activated tailings to the tether-bearing anchor particles
can be
performed by spraying, pouring, pumping, layering, flowing, or otherwise
bringing the
fluid bearing the activated tailings into contact with the tether-bearing
anchor particles.
The activated tailings are then associated with the tether-bearing anchor
particles while
the remainder of the fluid flows across the surface and into a collection pond
or
container.
[00105] In embodiments, an adaptation of the activator-tether-anchor systems
disclosed herein can be applied to the remediation of existing tailings ponds
for mining
operations. Tailings ponds can comprise different layers of materials,
reflecting the
gravity-induced settlement of fresh tailings after long residence periods in
the pond.
For example, the top layer in the tailings pond can comprise clarified water.
The next
layer is a fluid suspension of fine particles like fine tailings. The fluid
becomes denser
and denser, often settling into a stable suspension of fluid fine tailings
that has
undergone self-weight consolidation/dewatering, where the suspended particles
have
not yet settled out. The bottom layer is formed predominately from material
that has
settled by gravity. Desirably, the strata of the tailings pond containing
suspended
particles can be treated to separate the water that they contain from the fine
particles
suspended therein. The resultant clarified water can be drawn off and the
solid material
can be reclaimed. This could reduce the overall size of the tailings ponds, or
prevent
them from growing larger as fresh untreated tailings are added.
[00106] In embodiments, the systems and methods disclosed herein can be
adapted to
treat tailings ponds. In an embodiment, an activating agent, for example, one
of the
35,
CA 02706274 2012-05-23
4127.1002 CA
anionic polymers disclosed herein can be added to a pond, or to a particle-
bearing layer
within a tailings pond, such as by injection with optional stirring or
agitation. Tether-
bearing anchor particles can then be added to the pond or layer containing the
activated
fine particles. For example, the tether-bearing anchor particles can be added
to the
pond from above, so that they descend through the activated layer. As the
activated
layer is exposed to the tether-bearing anchor particles, the flocculated fines
can adhere
to the anchor particles and be pulled down to the bottom of the pond by
gravity,
leaving behind clarified water. The tailings pond can thus be separated into
two
components, a top layer of clarified water, and a bottom layer of congealed
solid
material. The top layer of clarified water can then be recycled for use, for
example in
further ore processing. The bottom layer of solids can be retrieved, dewatered
and used
for construction purposes, landfill, and the like.
c. Treating Waste or Process Streams
[00107] Particles modified in accordance with these systems and methods may be
added to fluid streams to complex with the particulate matter suspended
therein so that
the complex can be removed from the fluid. In embodiments, the modified
particles
and the particulate matter may interact through electrostatic, hydrophobic,
covalent or
any other type of interaction whereby the modified particles and the
particulate matter
form complexes that are able to be separated from the fluid stream. The
modified
particles can be introduced to the process or waste stream using a variety of
techniques
so that they complex with the particulate matter to form a removable complex.
A
variety of techniques are also available for removing the complexes from the
fluid
stream. For example, the modified particles can be mixed into the stream and
then
separated via a settling process such as gravity or centrifugation. If buoyant
or low-
density modified particles are used, they can be mixed with the stream and
then
separated by skimming them off the surface. In another method, the process
stream
could flow through a bed or filter cake of the modified particles. In any of
these
methods, the modified particles interact with the fine particulates and pull
them out of
suspension so that later separation removes both modified particles and fine
particulates.
36.
CA 02706274 2012-05-23
4127.1002 CA
[00108] The particles described herein can be utilized to sequester and
suspend fines
and pollutants from waste tailings. The technology can be used for the
treatment of
waste slurry as it is generated or can be used for the remediation of existing
tailings
ponds. As discussed below, massive amounts of waste tailings are generated in
the
course of energy production and other mining endeavors. Such wastes or waste
fluids
can include, but are not limited to, oilfield drilling waste, fine coal
tailings and coal
combustion residues. Mining endeavors producing wastes and waste fluids
include,
but are not limited to, processing and beneficiation of ores such as bauxite,
phosphate,
taconite, kaolin, trona, potash and the like. Mining endeavors having a waste
slurry
stream of fine particulate matter, can also include without limitation the
following
mining processes: sand and gravel, nepheline syenite, feldspar, ball clay,
kaolin,
olivine, dolomite, calcium carbonate containing minerals, bentonite clay,
magnetite and
other iron ores, barite, and talc.
[00109] As examples, the systems and methods disclosed herein can be applied
to
waste materials such as would be produced by drilling in oil fields, by mining
for coal,
by burning coal, or by mining other organic materials. Oilfield drilling
wastes include
rock cuttings, drilling fluids, well stimulation/fracturing fluids, brines,
and petroleum
residual. In a 1995 survey, 68% of these wastes were disposed onsite by
evaporation in
retention ponds and burial. The majority of drilling fluids are held in open
pits, but the
trend is towards a closed system with a storage tank replacing the reserve
pit. Fine coal
tailings are a waste product of coal preparation plants, where coal is crushed
and
washed to make a suitable fuel with low sulfur and ash content. The washing
process
generates a slurry of finely divided particles of clay, coal, and other
impurities. This
material has accumulated as hundreds of ponds in coal producing areas, often
resulting
in accidental discharges. Coal combustion products include fly ash, bottom
ash, boiler
slag, flue gas desulfurization material, and other scrubber wastes. The coal
ash flood
of December 2008 released 300 million gallons of fly ash sludge and water from
a
TVA coal fired power plant, damaging 15 homes in Kingston, TN and polluting
the
Emory River. A number of inorganic mines generate waste materials in fluid
streams
that can also be separated using the systems and methods disclosed herein.
37
CA 02706274 2012-05-23
4127.1002 CA
1) Coal Mininy, Waste
[00110] Coal as it is recovered from the mine (termed "run-of-mine" or ROM
coal)
comes in a variety of sizes and shapes and contains mineral impurities from
which it
must be separated. Preparing the ROM coal for other uses, involving processes
known
as coal preparation or cleaning, aims to sort the coal according to size, and
aims to
separate it from its mineral content. The mineral content of coal is the
noncombustible
inorganic fraction, comprised of minerals that are either detrital or
authigenic in origin
and that are introduced into the coal in the first or second phases of
coalification.
Minerals can be found in the ROM coal as combinations of larger inclusions
within the
coal lumps and ultrafine crystals disseminated throughout the coal lumps.
[00111] Asa first step in coal cleaning, the coal is crushed to reduce its
size and to free
it up from the larger mineral inclusions. Assisting in this process is the
fact that the
coal tends to break more easily than the minerals, so that the coal can be
liberated from
some of the surrounding minerals by size reduction techniques using crushers,
rotary
breakers or other similar devices. Size differences are exploited to sort the
crushed
coal into different categories of pellet sizes, some of which can be used
immediately if
the coal is of sufficient quality. In addition, the larger lumps of coal (- 10
- 150 mm in
length) can be treated with a technology called dense-medium separation, where
the
organic coal is floated free of impurities by immersing the crushed material
in a high-
density liquid; because the coal is less dense, it floats to the surface,
while the heavier
mineral matter will sink to be removed as waste.
[00112] Further crushing may be necessary if the coal is more intimately
associated
with minerals. The smaller-sized coal fragments can then be treated with froth
flotation to separate the coal from the minerals that surround it. Using this
technique,
fine coal fragments can be mixed with water and other additives to form a
slurry, which
is then exposed to streams of air bubbles introduced into the mixture. The
coal is
carried to the surface in the froth, where it can be skimmed off, screened and
dewatered
for commercial uses, while the minerals sink to the bottom. The dewatered mass
of
fine coal obtained through this process is termed FC, for "filter cake." Coal
particles in
the filter cake are typically about the size of sand particles.
38
CA 02706274 2012-05-23
4127.1002 CA
[00113] The mineral material separated from the coal during these processes is
dewatered, using for example vibratory screens, and then compacted for
disposal or for
further mineral recovery efforts. This waste mineral material is called coal
refuse, or
coal processing refuse (CPR). Depending on the type and source of the coal,
the ratio
of CPR to filter cake can be as high as 5:1 by weight. It may contain
particles that
range from microns in size to millimeters in size. The CPR may be further
treated to
remove useful minerals from it, or it may be disposed of as a waste material.
[00114] After these water-driven separation processes, fine particles remain
in the
slurry, called "fines." The fines from coal processing are similar in behavior
to the
fines produced by coal extraction. Fine materials generated from such mining
activities are often found well-dispersed in aqueous environments, such as
wastewater.
The finely dispersed materials from coal mining, termed "coal fines," are
suspended in
water during coal extraction and processing. Separating the coal fines from
the
suspending medium is difficult, as the fines tend to remain suspended unless
energy-
intensive processes are employed to recover them. In coal mining and
processing,
significant quantities of coal fines are created that require disposal and
handling.
About 15-20% of the mined tonnage can be left as residual fines, in sizes
ranging from
powder to small granules. There is presently no direct utility for these
fines, so that
they are a source of waste and inefficiency in the industry. Moreover, their
handling
and storage are hazardous and expensive.
[00115] Coal fines can be converted into pellets to facilitate disposal,
transportation
and handling. Coal-fired power plants can burn coal pellets as the fuel of
choice.
Pelletizing the coal fines generally requires adding an adhesive binder to the
slurry
containing coal fines, and using high temperatures or pressures to form the
dry,
consolidated pellets. Such steps are typically employed to agglomerate coal
because
coal particles do not naturally adhere to each other unless particle size is
carefully
controlled and extremely high pressures are used (over 20,000 psi for
bituminous coal,
for example). As an alternative to high pressure, an adhesive binder such as
asphalt
can be applied to bind the coal particles together. The adhesive can be
expensive itself,
and its use requires that a system incorporate equipment specifically to
prepare and
meter the adhesive, adding additional expense.
39
CA 02706274 2012-05-23
4127.1002 CA
[00116] Pellet manufacture presently requires both shaping and drying. Water-
soluble
or water-dispersible binders are difficult to dry, and the resulting pellets
are difficult to
dewater. Once in pellet form, the coal product is densely consolidated, so
that oxygen
for combustion penetrates with difficulty. In other words, the high
interfacial area
characteristic of fines is drastically reduced by pellet formation, and the
great
combustion efficiency inherent in powder burning is lost.
[00117] Currently, then, pelletization permits fines to be disposed of in a
form that is
useful for combustion purposes and convenient for transport and handling.
However,
the pellets do not burn efficiently in a combustion chamber. It is known in
the art to
coat wet pellets with a hydrophobic material during processing so that
residual water is
trapped in the interior of the pellet; when such pellets are introduced into a
boiler, the
interior water vaporizes rapidly so that the pellet bursts, releasing powdered
coal for
combustion. However, the high heat of vaporization for water lowers the
overall
power output of a plant using such technology. In addition, a coating step is
required,
adding to the expense of manufacturing.
[00118] In addition to coal fines waste, an enormous amount of biomass waste
is
generated annually. Wood waste is produced by lumber mills, for example, with
wasted wood accounting for about ten percent of processed lumber. Wood waste
can
also be found in forests as deadwood, living biomass, or residua from timber
harvesting. Lignocellulosic waste is produced by agriculture (e.g., corn
stalks, wheat,
hays, grasses, sugar cane bagasse, soybeans) and by processing (e.g., cotton
gins).
Feathers remaining from poultry farming require disposal as waste. Waste from
animal
husbandry includes organic material such as manure, feedstock and bedding.
Additional organic waste is produced by cattle, hog, chicken, turkey and fish
farming.
Industrial products such as carpeting and automobile tires end up as waste
that must be
disposed of.
[00119] In embodiments, the systems and methods disclosed herein can use coal
from
coal processing sources to remove coal fines from a mixture and form a coal-on-
coal
composite particle. In embodiments, coal from filter cake can be used to
attract,
consolidate and/or organize coal fines in mixtures, thereby forming a
composite
particle substantially formed from coal. Such a composite particle,
advantageously,
CA 02706274 2012-05-23
4127.1002 CA
can be an efficient source of energy. In embodiments, composite coal-on-coal
particles
can be formed that are then combined with other adulterants such as sand,
minerals or
water to decrease the energy content of the final product. Such modification
may be
carried out, for example, to meet the specifications of a particular customer
for an
energy source delivering a known quantity of energy. In certain cases, for
example, a
customer's contract calls for receiving a coal-based energy source that
provides 1200
BTU per ton; if the composite coal-on-coal particle pellets provide 1300 BTU
per ton,
they can be adulterated so that the delivered energy content is lowered.
[001201 In embodiments, the systems and methods disclosed herein can use
particulate
waste material from coal processing to remove coal fines from a mixture and
form a
composite particle. In embodiments, waste materials such as that found in coal
processing refuse (CPR) or other mineral wastes can be used to attract,
consolidate
and/or organize coal fines in mixtures, forming composite particles with the
coal fines
that put the waste materials to beneficial uses. In embodiments, these systems
and
methods have the advantage of using materials (whether energy-yielding like
the coal
in filter cake (FC) or non-energy-yielding like the minerals in CPR or other
waste
materials) that are found abundantly on site where coal is mined and
processed.
[001211 In accordance with these systems and methods, FC and CPR can be used
as
anchoring particles for treating coal fines dispersed in slurries in a process
that is rapid
and robust, yielding clarified water and geotechnically stable solids that are
easy to
handle and stackable. These systems and methods can result in near-immediate
recovery of coal fines from aqueous suspensions, producing solids that have
very low
initial (i.e., pre-drying) moisture levels. Sequestration of coal fines as
composite
particles with CPR can allow stockpiling and disposal of this waste material.
Sequestration of coal fines as composite particles with FC can produce
combustible
pellets that can convert energy sources now discarded into useable fuel.
[00122] In embodiments, the systems and methods disclosed herein can use the
activator-tether-anchor particle (ATA) technology for pelletizing coal to
yield pellets
that are dense during handling and transport, but that combust efficiently and
completely. In preparing pellets from coal fines, dewatering takes place
spontaneously,
rapidly and substantially completely; in embodiments, heat and/or pressure is
not
41
CA 02706274 2012-05-23
4127.1002 CA
required. The dewatering process exploits strong molecular forces between
charged
species.
[00123] In embodiments, the pellets can become porous upon exposure to heat,
achieving the high combustion efficiency found in powdery fuel. In such
embodiments, the porosity can be imparted due to heat-induced foaming of
components within the pellet matrix. The disclosed systems and methods can
produce
a pellet comprising components that expand upon heating, creating interior
pores and
channels that allow oxygen penetration. Such a highly perforated and expanded
structure can optimize combustion. The self-expanding feature of the pellets
contributes to combustion efficiency by virtue of its behavior as a de facto
oxygenator.
In embodiments, oxygen for combustion with the pellets can be taken up by coal
particle surfaces by diffusion through the heat-induced porosity of the pellet
matrix,
and by fragmentation of the matrix structure.
[001241 Pellets in accordance with these systems and methods are suitable for
use in,
for example, power generation facilities. The enhanced efficiency of the
instant pellets
can yield greater power generation, and less unwanted byproducts (e.g.,
various
noxious effluent gases and/or colloidal solids).
[00125] In accordance with these systems and methods, pellets can be produced
that
are composites of coal and biomass. In embodiments, composite pellets can be
formed
having a self-expanding feature that creates porosity, so that the pellets can
undergo
efficient combustion. Finally the process consolidates coal slurry without the
need of
intricate mechanical assist and the expelled water is clarified. Fines
originally
dispersed in the slurry are nearly completely captured and incorporated into
the pellets.
[00126] In embodiments, the systems and methods disclosed herein can enhance
the
settlement rate of dispersed coal fines materials by incorporating them within
a coarser
particulate matrix, so that coal solids can be removed from aqueous suspension
as a
material suitable for pelletizing. The systems and methods disclosed herein
involve
three components: preparing tether-bearing anchor particles, activating the
coal fines,
and complexing the activated coal fines with the tether-bearing anchor
particles to form
a removable complex.
42
CA 02706274 2012-05-23
4127.1002 CA
[00127] In embodiments, the systems and methods disclosed herein can remove
coal
fines from a fluid, where the fluid contains a population of suspended coal
fines. The
system comprises an activator polymer added to the fluid to complex with the
suspended coal fines to form activated coal fines, the activated coal fines
residing
within the fluid volume, and further comprises an anchor particle complexed
with a
tethering agent to form tether-bearing anchor particles. In this system, the
tether-
bearing anchor particles are mixed with the fluid volume to contact the
activated coal
fines, the tether-bearing anchor particles being capable of complexing with
the
activated coal fines to form complexes removable from the fluid. In accordance
with
this system, the complexes removable from the fluid comprise a composite
material
that includes complexed coal fines and anchor particles. In embodiments, the
anchor
particle comprises biomass. In embodiments, the anchor particle comprises
starch. In
embodiments, the anchor particle comprises a combustible material. In
embodiments,
the methods for removing coal fines from a fluid comprise providing an
activator
polymer capable of interacting with a population of coal fines suspended in a
fluid;
adding the activator polymer to the population to form activated coal fines;
providing
an anchor particle; complexing the anchor particle with a tethering agent
capable of
complexing with the activated coal fines, thereby forming tether-bearing
anchor
particles; mixing the tether-bearing anchor particles with the activated coal
fines to
form a complex removable from the fluid, the complex comprising a composite
material comprising coal fines and anchor particles, and removing the
composite
material from the fluid. In embodiments, the anchor particle comprises biomass
or
starch or combustible materials.
[00128] In accordance with these systems and methods, energy-bearing pellets
can be
produced that are composite materials comprising an energy-containing fine
material
and a combustible anchor particle in a complex. The complex can include an
interacting pair of polyelectrolytes, wherein the first of the pair of
polyelectrolytes is
bound to the energy-containing fine material and the second of the pair of
polyelectrolytes is bound to the combustible anchor particle. In embodiments,
the
energy-containing fine material comprises coal fines. In embodiments, the
anchor
particle comprises biomass or starch.
43
CA 02706274 2012-05-23
4127.1002 CA
2) Coal Combustion Products
[00129] One of the significant wastes produced during coal combustion is fly
ash. The
ash content of coal can range from 5 wt% for high-grade coal up to 50 wt% for
poor
quality coal. Over 131 million tons of fly ash is generated annually in the US
alone.
Current regulations mandate that fly ash be captured from exhaust gas streams,
typically by electrostatic precipitators.
[00130] Fly ash is primarily composed of silicon dioxide, aluminum oxide, iron
oxides,
and calcium oxide, though its composition varies depending on the input coal
and
combustion conditions. Fly ash particles are usually spherical with diameters
in the
range of 0.1 - 100 m. Depending on the composition of the fly ash, it may
possess
pozzolanic or even self-cementing properties. These properties allow fly ash
to be
reused in concrete, embankments, and a variety of building and construction
materials.
[001311 Up to 47% of fly ash generated in the US ends up being beneficially
reused.
The remaining 53% of fly ash generated in the US is disposed of in landfills
(in dry
powder-like form) or in massive man-made impoundment areas (in slurry form).
Problems with landfill disposal include seepage into the environment and the
ability of
the fly ash to become airborne in the form of hazardous dusts. Trace amounts
of toxic
elements are frequently present in fly ash, including but not limited to the
following:
arsenic, barium, chromium, lead, manganese, selenium, strontium, and zinc.
Slurry
impoundment avoids dust issues; however groundwater contamination can occur
and,
more significantly, massive and immediate environmental damage can occur if an
impoundment dam ruptures. A prime example occurred in 2008 when a Tennessee
Valley Authority fly ash impoundment dam ruptured and released approximately
1.1
billion gallons of fly ash slurry into the environment.
[00132] In embodiments, the systems and methods disclosed herein can be used
to
consolidate the fine coal combustion products like fly ash. A fluid stream
containing
the fly ash or similar fine particles can be treated with an activator, and a
tether-bearing
anchor particle can be added to the activated fluid stream. The activator
binds to the
fine particles, and the tethers on the anchor particles binds to the activator-
fine particle
units. A complex is formed between the activator-fine particle units and the
tether-
44
CA 02706274 2012-05-23 -
4127.1002 CA
bearing anchor particles. Such complexes can be readily removed from the fluid
stream. Few alternative disposal techniques exist for fly ash.
3) Inorganic Mining Waste
[001331 A number of mining operations yield wastewater streams containing fine
particles produced during the processing or beneficiation of ores. As an
example, the
production of aluminum from bauxite ore according to the commonly-used Bayer
process takes place by treating the crushed or ground ore with a hot sodium
hydroxide
solution to produce alumina (A1203), which can be reduced to yield aluminum.
The
insoluble part of the bauxite ore is carried away as an alkaline aqueous
slurry called
"red mud." Red mud is a complex material with characteristics that depend on
the
bauxite from which it is derived, and on the process parameters that produce
it.
Common characteristics of red mud include a water suspension of fine particles
suspended in a highly alkaline water solution, mainly composed of iron oxides,
but
having a variety of elements and mineralogical phases. The red mud fluid
stream,
containing about 7-9% solids, is typically sequestered in a containment area
(an old
excavated mine or a manmade lake called a tailings pond) so that the solids
can settle
out by gravity. About two tons of red mud is produced per ton of metallic
aluminum.
The magnitude of red mud associated with aluminum production poses a
significant
environmental challenge for countries where bauxite is refined. A small
country like
Jamaica, for example, where bauxite refinement is a leading industry, lacks
open land
suitable for disposal of the hazardous red mud; moreover, containment problems
such
as leakage, groundwater seepage and rupture of tailings pond dikes makes
disposal of
this material even more hazardous.
[001341 As another example, iron is produced from an ore called taconite that
contains
magnetite, an amalgam of iron oxides with about 25-30% iron. To extract the
iron
from the ore, the ore is crushed into fine particles so that the iron can be
removed from
the non-ferromagnetic material in the ore by a magnetic separator. The iron
recovered
by the magnetic separator is then processed into "pellets" containing about
65% iron
that can be used for industrial purposes like steel-making. Ore material not
picked up
by the magnetic separator is considered waste material, or gangue, and is
discarded.
Gangue typically includes non-ferrous rocks, low-grade ore, waste material,
sand, rock
CA 02706274 2012-05-23
4127.1002 CA
and other impurities that surround the iron in the ore. For every ton of
pellets
produced, about 2.7 tons of gangue is also produced. The waste is removed from
the
beneficiation site as a slurry of suspended fine particles, termed tailings.
About 2/3 of
the tailings are classified as "fine tailings," composed of extremely fine
rock particles
more than 90% of which are smaller than 75 microns, or -200 mesh); typically,
the
fine tailings they have little practical use at the mines, and end up
sequestered in
containment areas such as tailings ponds.
[00135] Another mining operation with similar wastewater handling issues is
the
production of kaolin. Kaolin ("china clay") is a white claylike material
composed
mainly of a hydrated aluminum silicate admixed with other clay minerals.
Kaolin,
used for a variety of industrial applications, is mined and then processed;
dry processes
and wet processes are available. Wet processes, used extensively to produce
additives
for the paper industry, yield a slurry that is fractionated into coarse and
fine fractions
using a variety of mechanical means like centrifuges, hydrocyclones and
hydroseparators. Despite repeated processing, a fraction of the slurry
contains fine
particulate kaolin that cannot be separated from other fine particulate waste
residues.
This material is deemed waste, and is sequestered in containment areas, either
manmade lagoons or spent kaolin mines.
[00136) Trona (trisodium hydrogendicarbonate dihydrate) is a mineral that is
mined in
the United States as a source of sodium carbonate. After the trona is mined,
it is
processed by exposing it to aqueous solvents so that the sodium carbonate can
be
recovered. The insoluble materials in the trona, including oil shales,
mudstone and
claystone, is carried away as tailings for disposal. Tailings, containing
suspended fine
particles in a fluid stream, may be transported to confinement areas, like
tailings ponds;
alternatively, tailings may be pumped into abandoned areas of the mine, with
retaining
walls or other barriers being constructed as needed to prevent the tailings
from entering
mine areas that are still active.
[00137] Phosphatic ore (fluorapatite) mining is a major worldwide industry,
with over
150 million tons of ore mined annually. Domestic mining produces around 30
million
tons of ore, about 75% of which comes from Florida. During the extraction of
46
CA 02706274 2012-05-23
4127.1002 CA
phosphate from the mined ore, a process called beneficiation, significant
quantities of
waste clay and sand are generated. The approximate ratio of the extracted ore
is 1:1:1
of fluorapatite to clay to sand. Thus, with the 30 million tons of ore being
mined,
around 10 million tons of waste clay and 10 million tons of waste sand must be
disposed of annually in the U.S.
[00138] The clay that is produced by beneficiation exists in a 3-5% (by
weight) slurry.
The current practice of clay disposal is to store the clay slurry in large
ponds known as
clay settling areas (CSAs), where the clay is allowed to separate from the
water
suspension by gravity over long periods of time, i.e., several decades. For a
typical
phosphate mine, up to 60% of the surface area of the mine ends up as CSAs.
Estimates
are that around 5,000 acres of land is turned into CSAs annually in central
Florida.
Left untreated it can take several decades before CSAs become stable enough
for reuse
to be considered. Because of the huge environmental and economic impacts of
CSAs,
a simple, robust, and cost-effective method for treating the clay slurry waste
is needed.
[00139] While other methods for separating clay fines from wastewater slurries
have
been tried for phosphate mining, they have proven to be difficult and costly.
For
example, the Dewatering Instantaneously with Pulp Recycle (DIPR) process has
been
under investigation for over 20 years at the Florida Institute of Phosphate
Research
(FIPR), disclosed in US Pat. No. 5,449,464. According to this disclosure, clay
slurry is
treated with a flocculant and a pulp material to dewater the slurry. While
this approach
has been studied for over two decades, its high cost, partly due to capital
costs of
equipment to dewater the treated slurry to high solids content, has prevented
its
adoption. There remains a need in the art, therefore, for an effective and
economical
approach to treating the clay-bearing wastewater slurry that is produced
during
phosphate beneficiation.
[00140] Research in treating wastewater produced by extracting bitumen from
oil
sands ore has demonstrated that tailings from these operations can be treated
in a three-
step process to consolidate suspended clay fines into solid masses that can be
readily
removed from the fluid stream. These systems and methods are disclosed in
International Application No.: PCT/US09/54278.
47
CA 02706274 2012-05-23
4127.1002 CA
Modifications of such systems and methods can be advantageously applied
to the treatment of fluid wastewater streams that beneficiation processes
for mined ores produce.
[00141] In embodiments, the systems and methods disclosed herein can be
applied to
the treatment of wastewater streams containing fine particles produced during
the
processing or beneficiation of ores. The systems and methods disclosed herein
can be
combined with routine modifications of the fluid stream in anticipation of
treatment, in
the course of treatment, or following treatment. For example, pH adjustments
of the
fluid stream can be carried out. In embodiments, the systems and methods
disclosed
herein can be adapted to and optimized for the needs of a specific mining
industry for
treatment of particulate suspensions in fluid streams of waste products.
[00142] For example, following the production of aluminum, e.g., from bauxite
ore
according to the commonly-used Bayer process, the insoluble part of the
bauxite ore is
carried away as an alkaline aqueous slurry called "red mud." Red mud typically
comprises a water suspension of fine particles suspended in a highly alkaline
water
solution, mainly composed of iron oxides, but having a variety of elements and
mineralogical phases. The fluid stream can be treated with an activator in
accordance
with these systems and methods, and can be contacted with tether-bearing
anchor
particles. As a result of this treatment, the fines in the fluid stream can be
sequestered
as solids and separated from the fluid itself. In embodiments, the sequestered
solids
can be consolidated into a mass that can be used for a variety of beneficial
applications.
In embodiments, anchor particles can be used that are indigenous to the mining
area, or
that are economically introduced into the mining area for use with these
processes.
[00143] As another example, the systems and methods disclosed herein can be
applied
to waste produced during the beneficiation of iron, for example, iron produced
from
taconite. As iron is produced from the ore, waste material called gangue is
generated.
The gangue is removed from the beneficiation site as a slurry of suspended
fine
particles, termed tailings. About 2/3 of the tailings are classified as "fine
tailings," a
waste material suitable for treatment by the systems and methods disclosed
herein. In
embodiments, the fluid stream containing the fine tailings can be treated with
an
48
CA 02706274 2012-05-23
4127.1002 CA
activator in accordance with these systems and methods, and can be contacted
with
tether-bearing anchor particles. As a result of this treatment, the fines in
the fluid
stream can be sequestered as solids and separated from the fluid itself. In
embodiments, the sequestered solids can be consolidated into a mass that can
be used
for a variety of beneficial applications. In embodiments, anchor particles can
be used
that are indigenous to the mining area, or that are economically introduced
into the
mining area for use with these processes.
[001441 As another example, the systems and methods disclosed herein can be
applied
to waste produced during the beneficiation of kaolin. The processing of kaolin
yields a
slurry that can be separated into a fraction that contains fine particulate
kaolin that
cannot be readily removed from the fluid stream. This fluid stream is suitable
for
treatment by the systems and methods disclosed herein. In embodiments, the
fluid
stream containing the fine tailings can be treated with an activator in
accordance with
these systems and methods, and can be contacted with tether-bearing anchor
particles.
As a result of this treatment, the fines in the fluid stream can be
sequestered as solids
and separated from the fluid itself. In embodiments, the sequestered solids
can be
consolidated into a mass that can be used for a variety of beneficial
applications. In
embodiments, anchor particles can be used that are indigenous to the mining
area, or
that are economically introduced into the mining area for use with these
processes.
[00145] As another example, the systems and methods disclosed herein can be
applied
to the waste produced during the mining of trona. Following the mining and
beneficiation of trona, insoluble materials carried away as waste can include
fine
particulate tailings transported in a fluid stream. This fluid stream is
suitable for
treatment by the systems and methods disclosed herein. In embodiments, the
fluid
stream containing the fine tailings can be treated with an activator in
accordance with
these systems and methods, and can be contacted with tether-bearing anchor
particles.
As a result of this treatment, the fines in the fluid stream can be
sequestered as solids
and separated from the fluid itself. In embodiments, the sequestered solids
can be
consolidated into a mass that can be used for a variety of beneficial
applications. In
embodiments, anchor particles can be used that are indigenous to the mining
area, or
that are economically introduced into the mining area for use with these
processes.
49
CA 02706274 2012-05-23
4127.1002 CA
[00146] As another example, the systems and methods disclosed herein can be
applied
to the waste produced during the mining of phosphate. During the beneficiation
of
phosphate ore, waste materials including fine clay particles (clay fines) are
produced
and are carried away in a fluid waste stream or slurry. This fluid stream is
suitable for
treatment by the systems and methods disclosed herein. In embodiments, the
fluid
stream containing the fine tailings can be treated with an activator in
accordance with
these systems and methods, and can be contacted with tether-bearing anchor
particles.
As a result of this treatment, the fines in the fluid stream can be
sequestered as solids
and separated from the fluid itself. In embodiments, the sequestered solids
can be
consolidated into a mass that can be used for a variety of beneficial
applications. In
embodiments, anchor particles can be used that are indigenous to the mining
area, or
that are economically introduced into the mining area for use with these
processes.
[00147] In embodiments, for example, the systems and methods disclosed herein
provide methods for treating and disposing of phosphatic clays, in conjunction
with the
sand waste also generated during phosphatic ore beneficiation. In other
embodiments,
the systems and methods disclosed herein provide methods for treating and
disposing
of fines collected from tailings streams. Advantageously, coarse waste from
mining
operations can be used as anchor particles, or waste-like materials (sand,
crushed rock,
or other waste materials) can be brought on-site to be used for anchor
particles.
[00148] As another example, potash mining operations result in wastewater
handling
issues that can be advantageously addressed with the systems and methods
disclosed
herein. Potash is the general name for potassium salts, including potassium
carbonate,
and is mined for agricultural (fertilizer) use. A large portion of the mined
potash ore
ends up as a waste, either as a solid or slurry, called potash tailings. The
potash
tailings slurry is an aqueous saturated salt/brine stream that contains waste
ore, clays,
and other fine materials. The most common method for disposal is to pump the
potash
tailings into above-ground impoundment areas or mined underground pits. The
large
volumes of tailings and high salinity pose significant disposal issues.
Additionally,
large amounts of salt simply end up in these waste streams. Environmental
concerns
are adding increased pressure for potash mining companies to find alternatives
to
tailings ponds as a disposal practice.
CA 02706274 2012-05-23
4127.1002 CA
[00149] A number of other mining operations produce fine particulate waste
carried in
fluid streams. Such fluid streams are suitable for treatment by the systems
and methods
disclosed herein. Modification of the fluid stream before, during or after
application of
these systems and methods may be advantageous. For example, pH of the fluid
stream
can be adjusted. Examples of additional mineral mining operations that have a
waste
slurry stream of fine particulate matter can include the following mining
processes: sand
and gravel, nepheline syenite, feldspar, ball clay, kaolin, olivine, dolomite,
calcium
carbonate containing minerals, bentonite clay, magnetite and other iron ores,
barite, and
talc.
EXAMPLES
Examples 1-7
The following materials were used in the Examples 1-7 below:
= Washed Sea Sand, 50+70 Mesh, Sigma Aldrich, St. Louis, MO
= Chitosan CG 800, Primex, Siglufjodur, Iceland
= Branched Polyethyleneimine (BPEI) (50% w/v), Sigma Aldrich, St. Louis, MO
= Polyvinyl Amine - Lupamin 1595, Lupamin 9095, BASF, Ludwigshafen,
Germany
= Poly(diallyldimethylammonium chloride) (pDAC) (20% w/v), Sigma Aldrich,
St. Louis, MO
= FD&C Blue #1, Sigma Aldrich, St. Louis, MO
= Hydrochloric Acid, Sigma Aldrich, St. Louis, MO
= Tailings Solution from a low-grade tar sand
= Dicalite, Diatomaceous Earth, Grefco Minerals, Inc., Burney, CA
= 3-Isocyanatopropyltriethoxysilane, Gelest, Morrisville, PA
= Sodium Hydroxide, Sigma Aldrich, St. Louis, Mo
= Isopropyl Alcohol (IPA), Sigma Aldrich, St. Louis, MO
Example 1: BPEI coated Diatomaceous Earth
[00150] Diatomaceous earth (DE) particles coupled with BPEI are created using
a
silane coupling agent. 100 g of DE along with 1000 mL isopropyl alcohol (IPA)
and a
magnetic stir bar is placed into an Erlenmeyer flask. 1 gm 3-
Isocyanatopropyltriethoxysilane is added to this solution and allowed to react
for 2
hours. After 2 hours, 2 mL of BPEI is added and stirred for an additional 5
hours
before filtering and washing the particles with IPA 2x's and deionized water
(DI
51
CA 02706274 2012-05-23
4127.1002 CA
water). The particles are then filtered and washed with a 0.12 M HCl solution
in
isopropanol (IPA) then dried.
Example 2: 1 % Chitosan CG800 Stock Solution
[00151] The chitosan stock solution is created by dispersing 10 g of chitosan
(flakes) in
1000 mL of deionized water. To this solution is added hydrochloric acid until
a final
pH of 5 is achieved by slowly and incrementally adding 12 M HCI while
continuously
monitoring the pH. This solution becomes a stock solution for chitosan
deposition.
Example 3: Diatomaceous Earth - 1% chitosan coating
[00152] 10 g of diatomaceous earth is added to 100 mL deionized water with a
stir bar
to create a 10% slurry. To this slurry is added 10 mL's of the 1 % chitosan
stock
solution of CG800. The slurry is allowed to stir for 1 hour. Once the slurry
becomes
homogeneous the polymer is precipitated out of solution by the slow addition
of 0.1 N
sodium hydroxide until the pH stabilizes above 7 and the chitosan precipitates
onto the
particles of diatomaceous earth. The slurry is filtered and washed with a 0.05
M HCl
solution in isopropanol (IPA) then dried.
Example 4: Particle performance on tailings solution
[00153] Coated and uncoated diatomaceous earth particles were used in
experiments to
test their ability to settle dispersed clay fines in an aqueous solution. The
following
procedure was used for each type of particle, and a control experiment was
also
performed where the particle addition step was omitted.
[00154] One gram of particles was added to a centrifugation tube. Using a
syringe, the
centrifugation tube was then filled with 45 ml of tailing solution containing
dispersed
clay. One more tube was filled with just the tailings solution and no
diatomaceous
earth particles. The tube was manually shaken for 30 seconds and than placed
on a flat
countertop. The tube was then observed for ten minutes allowing the clay fines
to settle
out.
[00155] Results:
[00156] No DE addition (control samples): Tailing solution showed no
significant
improvement in cloudiness.
.52
CA 02706274 2012-05-23
4127.1002 CA
[00157] DE Coated with Chitosan: Tailing solution was significantly less
cloudy
compared to control samples.
[00158] DE Coated with BPEI: Tailing solution was significantly less cloudy
compared to control samples.
[00159] DE Uncoated: Tailing solution showed no significant improvement in
cloudiness compared to control samples.
Example 5: Preparation of polycation-coated Washed Sea Sand
[00160] Washed sea sand is coated with each of the following polycations:
chitosan,
lupamin, BPEI, and PDAC. To perform the coating, an aqueous solution was made
of
the candidate polycation at 0.01M concentration, based on its molecular
weight. 50 g
washed sea sand was then placed in a 250 ml jar, to which was added 100 ml of
the
candidate polycation solution. The jar was then sealed and rolled for three
hours. After
this, the sand was isolated from the solution via vacuum filtration, and the
sand was
washed to remove excess polymer. The coated sea sand was then measured for
cation
content by solution depletion of an anionic dye (FD&C Blue #1) which confirmed
deposition and cationic nature of the polymeric coating. The sea sand coated
with the
candidate polymer was then used as a tether-attached anchor particle in
interaction with
fine particulate matter that was activated by treating it with an activating
agent.
Example 6: Use of Polymer-coated Sea Sand to Remove Fine Particles from
Solution
[00161] In this Example, a 45 ml. dispersion of fine materials (7% solids)
from an oil
sands tailings stream is treated with an activating polymer (Magnafloc LT30,
70ppm).
The fines were mixed thoroughly with the activating polymer. 10 gm of sea sand
that
had been coated with PDAC according to the methods of Example 1 were added to
the
solution containing the activated fines. This mixture is agitated and is
immediately
poured through a stainless steel filter, size 70 mesh. After a brief period of
dewatering,
a mechanically stable solid is retrieved. The filtrate is also analyzed for
total solids, and
is found to have a total solids content of less than 1 %.
Example 7: Use of Sea Sand without Polymer Coating to Remove Fine Particles
from Solution (Control)
53
CA 02706274 2012-05-23
4127.1002 CA
[00162] In this Example, a 45 ml. dispersion of fine materials (7% solids) is
treated
with an activating polymer (Magnafloc LT30, 70ppm). The fines were mixed
thoroughly with the activating polymer. 10 gm of uncoated sea sand were added
to the
solution containing the activated fines. This mixture is agitated and is
immediately
poured through a stainless steel filter, size 70 mesh. The filtrate is
analyzed for total
solids, and is found to have a total solids content of 2.6%.
Examples 8-18
The following materials were used in Examples 8-18 below:
= Commercially available poly(acrylamide) (50% hydrolyzed), 15M MW
= Poly(diallyldimethylammonium chloride) (pDADMAC) (20% w/v), Sigma
Aldrich, St. Louis, MO
= Coal slurry from a coal washing plant
= Bagasse from the Louisiana sugar industry
= Peanut shells from Whole Foods Grocery Store
= Coal solids, 0.01-0.2 cm size fraction
= Lignin powder, Sigma Aldrich, St. Louis, MO
= Paper pulp from a bleached kraft mill
= Corn starch Shaw's Grocery Store brand
= Grass clippings from Cambridge, MA
= Coal mine samples of filter cake and coal processing refuse
Example 8: Activated Coal Slurry
[00163] Coal slurry was thoroughly mixed to ensure that a uniformly dispersed,
homogeneous suspension is present. The coal slurry that was used contains 22%
dry
solids. To the slurry, an activator, 50% hydrolyzed poly(acrylamide), was
added to
yield a 113ppm (activator to coal solids) concentration. The coal slurry with
activator
is gently mixed until visible flocculations ("flocs") are formed.
Example 9: Activated Coal Slurry + Tethered Bagasse
[00164] Commercial bagasse was dried and mechanically chopped or blended to
produce solids of 1 cm in length or smaller. The dried, chopped bagasse was
mixed
with water and tethered with 500 ppm of pDADMAC. Activated coal slurry
prepared
in accordance with Example 8 was combined with the tethered bagasse in a ratio
of
0.06:1 (bagasse to coal slurry dry solids). The tethered bagasse plus
activated coal
slurry solution was mixed for -10 seconds and poured into a 250=mL graduated
cylinder and allowed to settle for 15 minutes. The settling rate corresponded
to
54
CA 02706274 2012-05-23
4127.1002 CA
approximately 8 ft/hr. The bed height compacted to approximately 63% of the
initial
volume of the mixture, and the turbidity of the supernatant was 221
Nephelometric
Turbidity Units (NTU). A sample of the bagasse-coal solids was dabbed dry with
paper
towels, and the remaining wet solids contained approximately 54% dry solids.
The
solids can be air dried or dried by some other means to produce readily usable
fuel.
Example 10: Activated Coal Slurry + Tethered Peanut Shells
[00165] Commercial peanut shells were mechanically chopped or blended to
produce
solids of 2cm in size or smaller. The chopped peanut shells were mixed with
water and
tethered with 500 ppm of pDADMAC. Activated coal slurry prepared in accordance
with Example 8 was combined with the tethered peanut shells in a ratio of
0.06:1
(peanut shells to coal slurry dry solids). The tethered peanut shells plus
activated coal
slurry solution was mixed for -10 seconds and poured into a 250 mL graduated
cylinder and allowed to settle for 15 minutes. The settling rate corresponded
to
approximately 4 ft/hr. The bed height compacted to approximately 60% of the
initial
volume of the mixture, and the turbidity of the supernatant was 27 NTU. A
sample of
the peanut shells-coal solids was dabbed dry with paper towels, and the
remaining wet
solids contained approximately 51 % dry solids. The solids can be air dried or
dried by
some other means to produce readily usable fuel.
Example 11: Activated Coal Slurry + Tethered Coal
[00166] Coal chunks were mechanically crushed to produce solids of 0.2 cm in
length
or smaller. The crushed coal was mixed with water and tethered with 500 ppm of
pDADMAC. Activated coal slurry prepared in accordance with Example 8 was
combined with the tethered crushed coal in a ratio of 0.51:1 (crushed coal to
coal slurry
dry solids). The tethered crushed coal plus activated coal slurry solution was
mixed for
-10 seconds and poured into a 250 mL graduated cylinder and allowed to settle
for 15
minutes. The settling rate corresponded to approximately 11 ft/hr. The bed
height
compacted to approximately 56% of the initial volume of the mixture, and the
turbidity
of the supernatant was 473 NTU. A sample of the coal-coal solids was dabbed
dry with
paper towels, and the remaining wet solids contained approximately 61 % dry
solids.
The solids can be air dried or dried by some other means to produce readily
usable fuel.
CA 02706274 2012-05-23
4127.1002 CA
Example 12: Activated Coal Slurry + Tethered Li nin
[00167] Lignin powder was mixed with water and tethered with 500 ppm of
pDADMAC. Activated coal slurry prepared in accordance with Example 8 was
combined with the tethered lignin in a ratio of 0.51:1 (lignin to coal slurry
dry solids).
The tethered lignin plus activated coal slurry solution was mixed for -10
seconds and
poured into a 250 mL graduated cylinder and allowed to settle for 15 minutes.
The
settling rate corresponded to approximately 6 ft/hr. The bed height compacted
to
approximately 65% of the initial volume of the mixture. A sample of the lignin-
coal
solids was dabbed dry with paper towels, and the remaining wet solids
contained
approximately 57% dry solids. The solids can be air dried or dried by some
other
means to produce readily usable fuel.
Example 13: Activated Coal Slurry + Tethered Pulp
[00168] Commercial paper pulp was mixed with water overnight and tethered with
500
ppm of pDADMAC. Activated coal slurry prepared in accordance with Example 8
was combined with the tethered paper pulp in a ratio of 0.58:1 (wet pulp to
coal slurry
dry solids). The tethered pulp plus activated coal slurry solution was mixed
for -10
seconds and poured into a 250 mL graduated cylinder and allowed to settle for
15
minutes. The settling rate corresponded to approximately 5 ft/hr. The bed
height
compacted to approximately 71 % of the initial volume of the mixture, and the
turbidity
of the supernatant was 109 NTU. A sample of the pulp-coal solids was dabbed
dry with
paper towels, and the remaining wet solids contained approximately 51 % dry
solids.
The solids can be air dried or dried by some other means to produce readily
usable fuel.
Example 14: Activated Coal Slurry + Tethered Starch
[00169] Commercial corn starch was mixed with water and tethered with 500 ppm
of
pDADMAC. Activated coal slurry prepared in accordance with Example 8 was
combined with the tethered starch in a ratio of 0.53:1 (starch to coal slurry
dry solids).
The tethered starch plus activated coal slurry solution was mixed for -10
seconds and
poured into a 250 mL graduated cylinder and allowed to settle for 15 minutes.
The
settling rate corresponded to approximately 2 ft/hr. The bed height compacted
to
approximately 69% of the initial volume of the mixture, and the turbidity of
the
supernatant was 803 NTU. A sample of the starch-coal solids was dabbed dry
with
56
CA 02706274 2012-05-23
4127.1002 CA
paper towels, and the remaining wet solids contained approximately 56% dry
solids.
The solids can be air dried or dried by some other means to produce readily
usable fuel.
Example 15: Activated Coal Slurry + Tethered Grass Clippings
[001701 Grass clippings were dried and mechanically chopped or blended to
produce
solids of 1cm in length or smaller. The dried, chopped grass clippings were
mixed with
water and tethered with 500 ppm of pDADMAC. Activated coal slurry prepared in
accordance with Example 8 was combined with the tethered grass clippings in a
ratio
of 0.06:1 (grass clippings to coal slurry dry solids). The tethered grass
clippings plus
activated coal slurry solution was mixed for -10 seconds and poured into a 250
mL
graduated cylinder and allowed to settle for 15 minutes. The settling rate
corresponded
to approximately 7 ft/hr. The bed height compacted to approximately 64% of the
initial
volume of the mixture, and the turbidity of the supernatant was 387 NTU. A
sample of
the grass clippings-coal solids was dabbed dry with paper towels, and the
remaining
wet solids contained approximately 52% dry solids. The solids can be air dried
or dried
by some other means to produce readily usable fuel.
Example 16: Activated Coal Slurry + Tethered Peanut Shells. Filtration
[001711 A solution containing tethered peanut shells plus activated coal
slurry solution,
all prepared in accordance with Example 11, was mixed for -10 seconds and
poured
into a filtration unit with a 80 mesh stainless steel screen. When mild vacuum
was
applied to the mixture, the filtration process took 40 seconds. The turbidity
of the
filtrate was 75 NTU. A sample of the retentate (peanut shells-coal. solids)
contained
approximately 48% dry solids. The solids can be air dried or dried by some
other
means to produce readily usable fuel.
Example 17: Activated Coal Slurry + Tethered Pulp. Filtration
[001721 A solution of tethered pulp plus activated coal slurry solution, all
prepared in
accordance with Example 13, was mixed for -10 seconds and poured into a
filtration
unit with a 80 mesh stainless steel screen. When mild vacuum was applied to
the
mixture, the filtration process took 37 seconds. The turbidity of the filtrate
was 122
NTU. A sample of the retentate (pulp-coal solids) contained approximately 48%
dry
57
CA 02706274 2012-05-23
4127.1002 CA
solids. The solids can be air dried or dried by some other means to produce
readily
usable fuel.
Example 18: Activated Coal Slurry + Tethered Filter Cake Coal (FC) or Coal
processing refuse (CPR)
[00173] For each experiment in this Example, a sample of FC or CPR was used as
anchors. For each sample, a dilute solution of the tethering polymer (p-
DADMAC)
was added at 500 ppm based on solids, and mixed. Activated coal slurry was
prepared
in accordance with Example 8. The tethered FC or CPR was added to the
activated
coal slurry and was gently mixed. The mixture was then gravity filtered
through a
filtration unit having a 80 mesh stainless steel screen. The time of
filtration for each
sample was measured starting from the time that the activated coaL'tethered
CPR or FC
mixture was poured on the filter mesh. The solids residing on the mesh were
analyzed
while resident on the screen, using a moisture analyzer. An aliquot of the
resident
solids was blotted dry with paper towels to remove externally adherent water
drops and
was then analyzed to determine the dry solids content. The results of these
experiments are set forth in Table I below.
TABLE I
Sample Ancbor:Fines Filtration Filtrate Dry Solids Dry Solids
(g:g) Time Turbidity M
Dabbed
(s) (NM (%)
FC-1 0.5:1 67 23 59 66
CPR-1 0,5:1 152 274 60 65
CPR-2 1:1 66 20 66 79
[00174] For each sample, the solids resident on the filter mesh were compact
and self-
adherent. For certain samples, the solids could be easily scraped or removed
from the
filter in one or two pieces. The filtrate for all samples had low turbidity
values, with
samples FC- I and CPR-2 having extremely low turbidity values of 23 NTU and 20
NTU, respectively.
[00175] After performing the measurements above, the integrity and
cohesiveness of
the solid material on the filter mesh was tested by pouring a large amount of
water onto
58
CA 02706274 2012-05-23
4127.1002 CA
the solids resident on the mesh. Vacuum was applied and water was refiltered
through
the solids. Filtration time and turbidity of filtrate were measured, and the
solid samples
were examined. For each sample, the resident solids appeared stable and
cohesive. The
CPR-2 sample's second filtration time was under three minutes and the filtrate
had an
even lower turbidity of 8.5 NTU, while the FC-1 sample took 17 minutes to
filter and
the filtrate had a slightly higher turbidity value of 34 NTU. These
experiments suggest
that the consolidated solids prepared in accordance with this protocol retain
their
integrity even after exposure to water washing, as might occur, for example,
with
heavy rains, and there is no evidence of significant redispersion of the
particles in the
water.
[00176] For each sample, the remaining solids were oven-dried and examined
thereafter for consistency and cohesiveness. For each sample, a solid and
apparently
geotechnically stable dried mass resulted from oven-drying.
Examples 19-27
The following materials were used in Examples 19-27 below:
= Poly(diallyldimethylammonium chloride) (PDAC), 20% in Water, Sigma-
Aldrich, St. Louis, MO
= Magnafloc LT30, CibaBASF, Ludwigshafen, Germany
= Sand, Sigma-Aldrich, St. Louis, MO
= Clay fines slurry, BASF montmorillonite (F- 100)
Example 19: Basic Procedure
[00177] A clay fines suspension, shown in FIG. 3A, was prepared from a 25 wt%
slurry of montmorrilonite in water made by mixing in a Silverson L4RT-A
homogenizer at 5,000 rpm for one hour. The 25 wt% slurry was diluted down to a
5%
solids by weight of clay to simulate the clay fines suspension (tailings)
produced
during phosphate beneficiation. A 250 gm. sample of the 5 wt% clay fines
suspension
was activated by adding an amount of a 0.1 % solution of Magnafloc LT30 as the
activator polymer, as detailed in the Examples below. After the activator was
added,
the sample was agitated by pouring it between two beakers (up to six times) to
ensure
good mixing. Flocs were evident following this mixing, as shown in FIG. 3B.
Separately, a slurry of sand "anchor particles" in water was prepared by
adding sand to
59
CA 02706274 2012-05-23
4127.1002 CA
water as detailed in the Examples below to produce an 85% by weight sand
slurry, as
shown in FIG. 4. Various amounts of 1% PDAC were added to the sand slurry as
the
tether polymer, so that tether-bearing anchor particles were produced. The
activated
clay fines and the tether-bearing anchor particles were combined in a jar and
sealed
with a lid. The jar was inverted five times to mix the two fluid streams. The
contents
of the jar were then poured onto an 80-mesh screen and allowed to gravity-
filter for
one minute. After one minute, a sample of the filtered-out solids was analyzed
on an
A&D ML-50 moisture balance to determine the solids content. The turbidity of
the
filtrate was also determined.
Example 20: 1:1 sand-to-clay
[001781 The following materials were used in accordance with the procedure set
forth
in Example 19:
Clay Fines Stream
a. 250 g of 5% clay fines suspension
b. 12.5 g of 0.1 % activator polymer
Sand Stream
a. 12.5 g of sand
b. 2.2 g of water
c. 1.25 g of I% tether polymer
[001791 The filtered-out solid sample contained 30.1% solids, and the filtrate
had a
turbidity of 87 NTU. FIG. 5 shows the mixed fluid streams in the jar
immediately after
combination. FIG. 6A shows the mixture following filtration, with recovered
solids
and clear filtrate. FIG. 6B shows the recovered solids.
Example 21: No sand stream
[001801 The following materials were used in accordance with the procedure set
forth
in Example 19:
Clay Fines Stream
a. 250 g of 5% clay fines suspension
b. 12.5 g of 0.1% activator polymer
(No sand stream)
CA 02706274 2012-05-23
4127.1002 CA
[00181] No solids were retained on the 80-mesh screen. After settling for one
minute
the supernatant had a turbidity above detection limits (>1000 NTU).
Example 22: Tethered sand, no activator
[00182] The following materials were used in accordance with the procedure set
forth
in Example 19:
Clay Fines Stream
a. 250 g of 5% clay fines suspension
Sand Stream
a. 12.5 g of sand
b. 2.2 g of water
c. 1.25 g of 1 % tether polymer
[00183) No solids were retained on the 80-mesh screen. After settling for one
minute
the supernatant had a turbidity above detection limits (>1000 NTU).
Example 23: Only tether polymer, without sand
[00184] The following materials were used in accordance with the procedure set
forth
in Example 19:
Clay Fines Stream
a. 250 g of 5% clay fines suspension
b. 12.5 g of 0.1 % activator polymer
Sand Stream: 1.25 g of 1 % tether polymer alone, without attachment to sand
[00185] The filtered-out solid contained 13.2% solids, and the supernatant had
a
turbidity of 13.5 NTU. Only about 10% of the generated solids were retained on
the
80-mesh screen.
Exam le 24: Plain sand, no tether
[00186] The following materials were used in accordance with the procedure set
forth
in Example 19:
Clay Fines Stream
a. 250 g of 5% clay fines suspension
b. 12.5 g of 0.1 % activator polymer
61
CA 02706274 2012-05-23
4127.1002 CA
Sand Stream: 12.5 g of sand
[00187] The filtered-out solid contained 27.2% solids, and the turbidity of
the filtrate
was 509 NTU. Only about 5% of the solids were retained on the 80-mesh screen.
Example 25: 1:1 sand-to-clay, low polymer dosing
[00188] The following materials were used in accordance with the procedure set
forth
in Example 19:
Clay Fines Stream
a. 250 g of 5% clay fines suspension
b. 6.3 g of 0.1 % activator
Sand Stream
a. 12.5 g of sand
b. 2.2 g of water
c. 0.3 g of 1% tether
[00189] The filtered-out solid contained 33.7% solids and the supernatant had
a
turbidity of 77 NTU. Thus it is possible with low polymer dosing to generate
solids
with good solids content and clear water in the supernatant.
Example 26: 1:1 sand-to-clay, constant activator polymer amount, varying
amounts of tether polymer
[00190] The following materials were used in accordance with the procedure set
forth
in Example 19:
Clay Fines Stream
a. 250 g of 5% clay fines suspension slurry
b. 12.5 g of 0.1 % activator
Sand Stream
a. 12.5gofsand
b. 2.2 g of water
c. 1 % tether solution, at doses of about 250ppm, about 500ppm, about 1000
ppm and about 2000 ppm.
[00191] The results are shown in Graph l on FIG. 7. At a constant activator
dosage of
1000 ppm, the solids generated by using varying amounts of tether to modify
the sand
62
CA 02706274 2012-05-23
4127.1002 CA
produce decrease in solids content above a tether dosage of around 500ppm.
Turbidity
values vary in a less consistent manner, but can still be manipulated by
varying tether
dosage. This demonstrates that varying the tether dosage can improve the
solids
retrieval from the clay fines stream.
Example 27: 1:1 sand-to-clay, constant tether polymer amount, varying amount
of activator polymer
[001921 The following materials were used in accordance with the procedure set
forth
in Example 19:
Clay Fines Stream
a. 250 g of 5% clay fines suspension
b. 0.1 % activator solution, at doses of about 250ppm, about 500ppm, about
1000 ppm, and about 2000 ppm
Sand Stream
a. 12.5 g of sand
b. 2.2 g of water
c. 1.25 g of 1% tether
[00193] The results are shown in Graph 2 on FIG. 8. At a constant tether
dosage of
1000 ppm, the solids generated by using varying amounts of activator increase
with
increasing activator dosage, but appear to level out around by 2,000 ppm.
Thus, the
activator dosage can also be used as a way to modulate the solids content of
the
consolidated clay.
EQUIVALENTS
[00194) While specific embodiments of the subject invention have been
discussed, the
above specification is illustrative and not restrictive. Many variations of
the invention
will become apparent to those skilled in the art upon review of this
specification.
Unless otherwise indicated, all numbers expressing quantities of ingredients,
reaction
conditions, and so forth used in the specification and claims are to be
understood as
being modified in all instances by the term "about." Accordingly, unless
indicated to
the contrary, the numerical parameters set forth herein are approximations
that can vary
depending upon the desired properties sought to be obtained by the present
invention.
63