Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02706414 2010-05-20
WO 2009/068735 PCT/F12008/050672
METHOD FOR PROCESSING PYRITIC CONCENTRATE CONTAINING
GOLD, COPPER AND ARSENIC
FIELD OF THE INVENTION
The invention relates to a method for processing pyritic copper sulphide
concentrate, in which, in addition to copper, there is arsenic and fine gold
or
invisible gold bound to the sulphide minerals. In the method, concentrate is
leached into a chloride-based solution. Raw material leaching is performed in
conditions where the copper, iron and arsenic of the raw material dissolve,
io but the gold-containing material remains undissolved. The chloride solution
obtained is routed to neutralisation, in which the iron and arsenic in it are
precipitated. The purified copper solution is routed to liquid-liquid
extraction.
In the stripping stage of extraction, copper is transferred to a sulphate
solution, which is routed to copper electrowinning. Gold is recovered from the
leaching residue by some known method.
BACKGROUND OF THE INVENTION
The problem with many gold-copper concentrates is that a considerable
amount of the gold is bound to pyrite as invisible gold. This is the case for
instance in arsenic-bearing gold-copper ores, in which up to 50 % of the gold
is in pyrite. The rest of the gold is either metallic, or bound to enargite
(Cu2AsS4), arsenopyrite (FeAsS) and other minerals. These kinds of ores
also contain copper, which is most often in the form of enargite or
chalcopyrite (CuFeS2). The beneficiation of the ores in question requires the
fabrication of the kind of bulk concentrate that include copper, free gold and
the maximum amount of pyrite. Arsenic treatment and the large amount of
pyritic sulphur set the major limitations on the creation of a profitable
overall
process for this type of concentrate.
3o A method is described in WO patent application 2004/035840, which relates
to the recovery of metals, in particular copper, from a copper-bearing raw
material, where the material is leached into a chloride-containing solution.
CA 02706414 2010-05-20
WO 2009/068735 PCT/F12008/050672
2
Raw material leaching is performed acidically at a pH value of at least 1.5
and a redox potential of 480 - 500 mV vs. Ag/AgCI, so that the copper in the
copper chloride solution exiting leaching is mostly divalent. At the same time
the sulphur contained in the concentrate is precipitated in elemental form,
and also the iron precipitates. The precious metals included in the
concentrate do not dissolve but go into the leaching residue. The leaching
residue is subjected to sulphur flotation, whereby a sulphur concentrate is
obtained, which also contains precious metals (gold + PGM). The majority of
the sulphur is separated from the sulphur concentrate by known methods
io and thus a PGM concentrate is formed containing precious metals. The
chloride solution formed in raw material leaching, which contains copper and
other possible valuable metals, is routed to liquid-liquid extraction. In
extraction copper is transferred first to the organic phase by means of
extraction, and in stripping to a sulphate solution, which is routed to copper
electrowinning. There is no mention in the method of how any arsenic that
may be contained in the raw material behaves or how gold-containing pyrite
is recovered.
US patent 4,023,964 describes a method for fabricating copper. In the
method copper sulphide concentrate is leached into a solution of copper (II)
chloride and sodium chloride. The NaCl concentration is 100-300 g/I and the
pH value at most 1, so that the iron dissolves. The solution obtained, in
which the majority of copper is monovalent, is divided into two parts, one of
which is subjected to iron precipitation as goethite by injecting air into the
solution. The cupric chloride solution formed during goethite precipitation is
routed back to concentrate leaching. The other part of the solution is
contacted with the extraction solution. During extraction, air is introduced
into
the solution in order to oxidise the monovalent copper to divalent. In
extraction the copper is bound to the organic phase and the cupric chloride
solution that is depleted with regard to copper is routed back to concentrate
leaching. In stripping, the organic phase and the copper bound to it are
contacted with an aqueous solution of sulphuric acid. The copper sulphate
CA 02706414 2010-05-20
WO 2009/068735 PCT/F12008/050672
3
solution that is formed is routed to fabrication of elemental copper and the
organic phase back to the extraction stage.
The method described in US patent 4,023,964 is practical in so far as the
copper sulphide concentrate is leached as chloride, which is routed to liquid-
liquid extraction and copper is recovered from extraction as copper sulphate
solution. The further processing of the copper sulphate solution for example
in electrowinning is a well-known technique and produces pure copper. The
method is made complicated by the fact that the solution is divided into two
io branches, in one of which the dissolved iron is precipitated and only the
other is routed to extraction. The copper in the copper chloride solution
exiting leaching is mainly monovalent, so it has to be oxidised separately
during extraction. When oxidation occurs during extraction, there is a danger
that the extractant will be oxidised at the same time, making it no longer fit
for use. It is recommended in the method that extraction be performed at a
temperature of 60 C, which in practice is far too high and causes the
destruction of the extractant. The processing of arsenic-bearing material is
not mentioned in the method, nor the behaviour of the gold contained in
pyrite or the behaviour of gold in general.
PURPOSE OF THE INVENTION
Now a method has been developed for processing pyritic copper sulphide
concentrate that contains gold, copper and arsenic. Bulk concentrate
containing copper pyrite, pyrite, enargite and possibly other arsenic-bearing
minerals, such as arsenopyrite, is leached into a chloride-based aqueous
solution in conditions where copper, iron and arsenic dissolve, but pyrite and
gold remain in the leaching residue.
SUMMARY OF THE INVENTION
3o The invention relates to a method for processing pyritic copper sulphide
concentrate containing gold and/or platinum group metals (PGM) and
arsenic, whereby the concentrate is leached into an aqueous solution of
CA 02706414 2010-05-20
WO 2009/068735 PCT/F12008/050672
4
copper chloride and alkali chloride containing hydrochloric acid. It is
typical of
the method that the redox potential of the concentrate leaching is regulated
to be in the range of 400 - 600 mV vs. Ag/AgCI electrode by means of the
feed of an oxidising agent and the pH is adjusted to the range of 0.2 - 1,
whereupon the copper, iron and arsenic in the copper and arsenic minerals
of the concentrate dissolve and the gold and/or PGM bound to these
minerals is released. Pyrite and the gold and/or PGM it contains as well as
free gold and/or PGM and sulphur remain in the leaching residue. The cupric
chloride solution containing iron and arsenic that is formed is neutralised to
1o precipitate the iron and arsenic, after which the neutralised iron- and
arsenic-
free cupric chloride solution is routed to the liquid-liquid extraction stage,
where the copper is recovered into an aqueous solution of sulphuric acid,
and is routed to electrolysis for recovery of elemental copper. The raffinate
formed in liquid-liquid extraction is fed back to concentrate leaching.
According to one embodiment of the invention the leaching residue is
subjected to sulphur flotation. Sulphur concentrate or leaching residue
containing gold and/or PGM is preferably routed to pyrometallurgical
processing to recover the gold and/or PGM, in which case they are routed to
a smelter or roasted. The leaching residue may also be subjected to
hydro metallurgical processing in the form of pressure leaching.
The iron- and arsenic-containing cupric chloride solution generated in copper
sulphide concentrate leaching in accordance with the invention is neutralised
to a pH value of 2 - 2.5 in order to precipitate the iron and arsenic out of
the
solution. Neutralisation is preferably carried out by means of limestone, lime
or lye. Iron and arsenic are precipitated out of the solution as ferric
arsenate
and goethite or as ferric hydroxide.
3o The small amount of gold that may have dissolved in the leaching stage is
precipitated back into the leaching residue by means of a reducing agent or
adsorbent. A typical adsorbent is activated carbon.
CA 02706414 2010-05-20
WO 2009/068735 PCT/F12008/050672
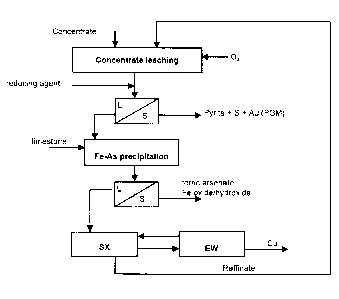
LIST OF DRAWINGS
The method according with the invention is described in the appended
Figure 1, which shows a flow chart of one of the preferred embodiments of
5 the invention.
DETAILED DESCRIPTION OF THE INVENTION
Pyritic bulk concentrate containing gold, copper and arsenic, is fed to a
leaching stage, in which leaching is performed using a solution of
io hydrochloric acid containing copper chloride and alkali chloride. Leaching
takes place generally in several stages, but for the sake of simplicity it is
shown in the flow chart as a single stage. Leaching is carried out as
countercurrent or concurrent leaching. The redox potential of the leaching is
regulated to be in the region of 400-600 mV vs. Ag/AgCI by means of oxygen
or some other oxidant. The acid concentration of the leaching stage is
adjusted to be in the range of 20 - 100 g/l, so that the pH value is below 1,
but preferably at least 0.2. In these conditions, iron and arsenic dissolve,
but
sulphur precipitates. In the description of the invention the general term
gold
refers to gold and/or platinum group metals (PGM).
The amount of alkali chloride in the solution used in leaching is around 100
g/l. The alkali is preferably sodium. The amount of alkali chloride is
relatively
low, because the copper in solution is divalent, so the required alkali
chloride
concentration is also low. Leaching conditions are regulated to be such that
the iron in the sulphide minerals contained in the concentrate, apart from the
pyrite, dissolves, as does the arsenic contained in the concentrate. The gold
does not dissolve, but is released as the enargite and other arsenic minerals
decompose, and the gold enters the leaching residue.
3o The reactions occurring in the leaching method accordant with the invention
are described by means of the following reaction equations:
CA 02706414 2010-05-20
WO 2009/068735 PCT/F12008/050672
6
2Cu3AsS4+12H+ + 51/2 0 2= 6Cu2+ + 2H3AsO4 + 8S + 3H20 (1)
CuFeS2 + 4H+ + 02 = Cu2+ + Fe 2+ + 2S + 2H20 (2)
FeAsS + 202 + 3H+ = S + Fe 2+ + H3AsO4 (3)
If a small amount of gold has dissolved in the concentrate leaching step, it
is
precipitated back into the leaching residue by means of a suitable reducing
agent or adsorbent, for example activated carbon. After this, liquid/solids
io separation is performed, whereby the sulphur, pyrite and gold contained in
the solids are separated from the solution. The arsenic-free leaching residue
thus obtained is treated in an appropriate way to recover the gold. In
addition
to sulphur, the leaching residue comprises gold, pyrite and the gold
contained in it that is released as a result of the decomposition of the
minerals, but not iron and arsenic. Therefore the quantity of leaching residue
is relatively small and there are many possible ways to process it further.
Sulphur flotation can be performed on the leaching residue (not shown in the
drawing), whereby concentrate is obtained that is formed of pyrite and
sulphur, containing nearly all the gold of the concentrate used as feedstock.
Depending on the composition of the leaching residue or the type of further
processing, the leaching residue may be treated without a sulphur
concentration stage. The gold content of the generated sulphur concentrate
and/or of the leaching residue is so large, that it can be routed to
pyrometallurgical processing. This may take place for instance in a smelter,
in which case the recovery yield of precious metals is high. The other
possible further processing method is roasting, in which pyrite is oxidised
and sulphur is burnt and gold is leached from the calcine with cyanide.
Leaching waste can likewise be processed hydrometallurgically by pressure
leaching in an autoclave, whereupon the pyrite decomposes and the gold
can be leached into cyanide with a good yield.
CA 02706414 2010-05-20
WO 2009/068735 PCT/F12008/050672
7
When leaching is performed at the high acid concentration described above,
the solution obtained in solids separation has to be neutralised before
routing
it to the extraction stage. In the method accordant with the invention, iron
and arsenic removal from the solution are also carried out during
neutralisation by precipitating them as ferric arsenate (FeAsO4-2H20). Since
the amount of iron is generally greater than that of arsenic, the excess iron
is
precipitated in the same stage as goethite (FeOOH) or ferric hydroxide
(Fe(OH)3). Precipitation is performed by neutralising the solution to a pH
value of about 2 - 2.5. Neutralisation is carried out by means of a suitable
io neutralising agent such as limestone, lime or lye (NaOH).
The divalent copper chloride solution, cleaned of impurities and solids, is
routed to extraction. Copper recovery from the organic solution is performed
into an aqueous solution of sulphuric acid, which is fed to electrowinning to
recover elemental copper. Since the known copper extractants are mostly
selective with regard to divalent copper, the copper chloride solution can be
fed directly to extraction without an oxidation stage.
The extraction stage is depicted in the drawing as a single step, but it
consists of the extraction and stripping stages normally included in
extraction. In the extraction stage, an aqueous solution of cupric chloride is
contacted with an organic extractant and the copper is made to transfer to
the organic phase. The extraction stage includes the normal mixing and
settling section, although they are not shown in detail in the drawing. Any
known copper extractant is suitable as the extractant, such as oximes, which
are diluted with an appropriate solvent, for example kerosene.
The chloride-containing aqueous solution of the extraction stage, the
raffinate, which is depleted with regard to copper and has a raised acid
concentration, is routed back to concentrate leaching. The organic solution
exiting the extraction stages is conveyed via scrubbing to stripping. In
stripping, the organic solution containing divalent copper ions is contacted
CA 02706414 2010-05-20
WO 2009/068735 PCT/F12008/050672
8
with an aqueous solution of sulphuric acid and the copper transfers to the
aqueous phase as sulphate, from where it is recovered by electrowinning.
When copper recovery takes place in copper electrolysis, the electrolysis
return acid can be used as the aqueous solution of sulphuric acid in
stripping.