Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
MEMBRANE ELECTRODE ASSEMBLY FOR FUEL CELL, FUEL CELL, AND FUEL
CELL SYSTEM
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0001] The present invention relates to a membrane electrode assembly for a
fuel cell,
a fuel cell, and a fuel cell system, which have an anode catalyst layer.
2. Description of the Related Art
[0002] A membrane electrode assembly for a fuel cell generally includes an ion
conducting membrane that has two sides in the- thickness direction, an anode
catalyst
layer laminated on one side of the ion conducting membrane, a cathode catalyst
layer
laminated on the other side of the ion conducting membrane, an anode diffusion
layer
laminated on the outer side of the anode catalyst layer, and a cathode
diffusion layer
laminated on the outer side of the cathode catalyst layer.
[0003] An anode fluid supplied to an anode may contain carbon monoxide that
interferes with catalytic activity in the catalyst layer. In this case, as the
duration of
power generation increases, catalytic activity gradually reduces. This may
cause a
decrease in power generated by the fuel cell.
[0004] In light of the above situation, Japanese Patent Application
Publication No.
8-203537 (JP-A-8-203537) describes a fuel cell that includes a membrane
electrode
assembly in which a layer that oxidizes carbon monoxide is provided at portion
of the
catalyst layer, adjacent to the diffusion layer.
[0005] In addition, Japanese Patent Application Publication No. 2004-186049
(JP-2004-186049) describes a membrane electrode assembly in which, focusing on
porosity, both an anode catalyst layer and a cathode catalyst layer each have
an increased
porosity at a portion adjacent to a diffusion layer. Furthermore, Japanese
Patent
1
CONFIRMATION COPY
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
Application Publication No. 7-85874 (JP-A-7-85874) describes a membrane
electrode
assembly in which the amount of ruthenium supported is greater at the
downstream side
than at the upstream side to thereby suppress carbon monoxide (CO) poisoning
at the
downstream side.
[0006] According to the above described technologies, when the duration of
power
generation extends over a long period of time, a decrease in power generated
by the fuel
cell can be somewhat suppressed but it is not sufficient.
SUMMARY OF THE INVENTION
[0007] The invention provides a membrane electrode assembly for a fuel cell, a
fuel
cell, and 'a fuel cell system that advantageously suppress a decrease in
generated power
even when the duration of power generation extends over a long period of time.
[0008] A first aspect of the invention provides a membrane electrode assembly
for a
fuel cell. The membrane electrode assembly includes: an ion conducting
membrane that
has two sides in a thickness direction thereof; a porous anode catalyst layer
that is
laminated on one side of the ion conducting membrane and that has an anode
catalyst that
accelerates anode reaction; a porous cathode catalyst layer that is laminated
on the other
side of the ion conducting membrane and that has a cathode catalyst that
accelerates
cathode reaction; an anode diffusion layer that is laminated on an outer side
of the anode
catalyst layer and that allows an anode fluid to pass therethrough; and a
cathode diffusion
layer that is laminated on an outer side of the cathode catalyst layer and
that allows a
cathode fluid to pass therethrough. The anode catalyst in the anode catalyst
layer
contains a poisoning-suppression catalytic component that reduces poisoning of
carbon
monoxide contained in the anode fluid. The anode catalyst layer includes a
first catalyst
layer portion and a second catalyst layer portion. The first catalyst layer
portion is
located closer to the anode diffusion layer than the second catalyst layer
portion, and the
second catalyst layer portion is located closer to the ion conducting membrane
than the
first catalyst layer portion. The density of the first catalyst layer portion
is smaller than
the density of the second catalyst layer portion.
2
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
[0009] Carbon monoxide may be contained in an anode fluid. Carbon monoxide
reduces catalytic activity of the anode catalyst in the anode catalyst layer.
Moreover,
when raw fuel is reformed into an anode fluid, carbon monoxide may be
contained in the
anode fluid. In this case, when the duration of service of the fuel cell
extends for a long
period of time, poisoning may occur in the anode catalyst and, as a result,
the catalytic
activity may be reduced. This may cause a decrease in power generated by the
fuel cell.
Then, in order to maintain desirable catalytic activity of the anode catalyst
in the anode
catalyst layer, the anode catalyst includes a catalyst component that reduces
poisoning of
carbon monoxide contained in the anode fluid (hereinafter, also referred to as
poisoning-suppression catalytic component).
[0010] The anode catalyst layer has a porous structure So as to allow the
anode fluid
to pass therethrough. Although it may be identified in the manufacturing
process, in the
complete membrane electrode assembly, the first catalyst layer portion and the
second
catalyst layer portion are integrated and it may be difficult to identify them
clearly. The
first catalyst layer portion represents portion of the anode catalyst layer,
located closer to
the anode diffusion layer than the second catalyst layer portion. On the other
hand, the
second catalyst layer portion represents portion of the anode catalyst layer,
located closer
to the ion conducting membrane than the first catalyst layer portion.
[0011] As an anode fluid is supplied from the anode diffusion layer to the
anode
catalyst layer, the anode fluid initially flows from the anode diffusion layer
into the first
catalyst layer portion and then flows into the second catalyst layer portion,
thus being
transferred toward the ion conducting membrane side. The second catalyst layer
portion
is located closer to the ion conducting membrane than the first catalyst layer
portion.
Thus, the second catalyst layer portion is able to effectively contribute to
power
generation reaction at the anode side. Hence, it is undesirable that catalytic
activity of
the second catalyst layer portion is reduced due to carbon monoxide in terms
of ensuring
generating power.
[0012] Here, the density p1 of the porous first catalyst layer portion is
smaller than
the density p2 of the porous second catalyst layer portion. Thus, the specific
surface of
3
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
the first catalyst layer portion increases as compared with the specific
surface of the
second catalyst layer portion. Even when the duration of service extends over
a long
period of time, a decrease in power generated by the fuel cell is suppressed.
This may
be presumably due to the following reason.
[0013] The density pl of the first catalyst layer portion is smaller than the
density p2
of the second catalyst layer portion. Thus, the surface area per unit volume
of the
porous first catalyst layer portion increases as compared with the surface
area per unit
volume of the porous second catalyst layer portion. For this reason, when an
anode
fluid is supplied to the anode catalyst layer, in comparison with the case in
which the
density of the first catalyst layer portion is excessively large, the
poisoning-suppression
catalytic component contained in the first catalyst layer portion is more
likely to contact
carbon monoxide contained in the anode fluid. Thus, the poisoning-suppression
catalytic component efficiently works. Furthermore, because the density p2 of
the
second catalyst layer portion is larger than the density pl of the first
catalyst layer portion,
it can be expected to restrict circulation of poisoning carbon monoxide
contained in the
anode fluid to the second catalyst layer portion.
[0014] The pore diameter 4)1 of the first catalyst layer portion is, for
example,
smaller than or substantially equal to the pore diameter 4)2 of the second
catalyst layer
portion (4)l < 4)2 or 4)1 - 4)2). Within the relationship of 4)1 < +2 or +1 =
4)2, 4)1/4)2, for
example, ranges from 0.1 to less than 1, ranges from 0.5 to 0.9, ranges from
0.5 to 0.8,
ranges from 0.8 to less than 1, or ranges from 0.9 to less than 1. The pore
diameter may
be based on a median diameter.
[0015] The inventors conducted the test under the conditions that the
relationship of
pl < p2 was applied to the anode catalyst layer that may be subject to carbon
monoxide,
and the relationship similar to that of the anode catalyst layer, that is, the
relationship that
the density plc of the first catalyst layer portion is smaller than the
density p2c of the
second catalyst layer portion, was applied to the cathode catalyst layer that
is basically
not subject to carbon monoxide. The results of the test showed that as the
duration of
power generation increases, a decrease in power generated by the fuel cell
increases.
4
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
[00161 A second aspect of the invention provides a membrane electrode assembly
for
a fuel cell. The membrane electrode assembly includes: an ion conducting
membrane
that has two sides in a thickness direction thereof; a porous anode catalyst
layer that is
laminated on one side of the ion conducting membrane and that has an anode
catalyst that
accelerates anode reaction; a porous cathode catalyst layer that is laminated
on the other
side of the ion conducting membrane and that has a cathode catalyst that
accelerates
cathode reaction; an anode diffusion layer that is laminated on an outer side
of the anode
catalyst layer and that allows an anode fluid to pass therethrough; and a
cathode diffusion
layer that is laminated on an outer side of the cathode catalyst layer and
that allows a
cathode fluid to pass therethrough. The anode catalyst of the anode catalyst
layer
contains a poisoning-suppression catalytic component that reduces poisoning of
carbon
monoxide contained in an anode fluid. The anode catalyst layer includes a
first catalyst
layer portion and a second catalyst layer portion. The first catalyst layer
portion is
located closer to the anode diffusion layer than the second catalyst layer
portion, and the
second catalyst layer portion is located closer to the ion conducting membrane
than the
first catalyst layer portion. The pore diameter of the first catalyst layer
portion is
smaller than the pore diameter of the second catalyst layer portion.
[0017] According to the above aspect, the anode catalyst of the anode catalyst
layer
contains a poisoning-suppression catalytic component that reduces poisoning of
carbon
monoxide contained in an anode fluid. The first catalyst layer portion and the
second
catalyst layer portion each have a porous structure so as to allow the anode
fluid to pass
therethrough. The second catalyst layer portion is located closer to the ion
conducting
membrane than the first catalyst layer portion. Thus, the second catalyst
layer portion is
able to effectively contribute to power generation reaction at the anode side.
Hence, it is
undesirable that catalytic activity of the second catalyst layer portion is
reduced due to
carbon monoxide.
[0018] Here, the pore diameter 41 of the first catalyst layer portion is
smaller than
the pore diameter 42 of the second catalyst layer portion. Thus, the specific
surface of
the porous first catalyst layer portion increases as compared with the
specific surface of
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
the porous second catalyst layer portion. Hence, even when the duration of
service
extends over a long period of time, a decrease in power generated by the fuel
cell is
suppressed.
[0019] The reason why a decrease in power generated by the fuel cell is
suppressed
as described above may be presumed as follows. The pore diameter ~1 of the
porous
first catalyst layer portion is smaller than the pore diameter ~2 of the
porous second
catalyst layer portion. Thus, the specific surface of the first catalyst layer
portion
increases as compared with the specific surface of the second catalyst layer
portion. For
this reason, when an anode fluid is supplied to the anode catalyst layer, in
comparison
with the case in which the pore diameter ~1 of the first catalyst layer
portion is
excessively large, the poisoning-suppression catalytic component contained in
the first
catalyst layer portion is more likely to contact carbon monoxide contained in
the anode
fluid. As a result, the poisoning-suppression catalytic component efficiently
works.
Thus, the first catalyst layer portion has improved capability to reduce
poisoning of
carbon monoxide contained in a reaction fluid to be supplied to the anode. For
this
reason, the second catalyst layer portion is more likely to exert an ability
to effectively
contribute to power generation reaction. Note that the density pl of the first
catalyst
layer portion may be smaller than or substantially equal to the density p2 of
the second
catalyst layer portion (pl < p2 or pl. = p2). Within the relationship of pl <
p2 or pl.
p2, pl/p2, for example, ranges from 0.1 to less than 1, ranges from 0.2 to
0.9, ranges
from 0.3 to 0.7, ranges from 0.7 to 0.8 or ranges from 0.8 to less than 1.
[0020] A third aspect of the invention provides a fuel cell. The fuel cell
includes:
the membrane electrode assembly according to the first aspect or the second
aspect; an
anode flow distribution member that is located on an outer side of the anode
diffusion
layer of the membrane electrode assembly and that supplies the anode fluid to
the
membrane electrode assembly; and a cathode flow distribution member that is
located on
an outer side of the cathode diffusion layer of the membrane electrode
assembly and that
supplies the cathode fluid to the membrane electrode assembly. The same
function as
that of the first aspect or the second aspect may be obtained. Therefore, even
when the
6
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
duration of service extends over a long period of time, a decrease in power
generated by
the fuel cell is suppressed.
[0021] A fourth aspect of the invention provides a fuel cell system. The fuel
cell
system includes: a reformer that reforms raw fuel into an anode fluid; and the
fuel cell
according to the third aspect. The fuel cell includes an anode that is
supplied with the
anode fluid reformed in the reformer; and a cathode that is supplied with the
cathode
fluid. The same function as that of the first aspect, the second aspect or the
third aspect
may be obtained. Therefore, even when the duration of service extends over a
long
period of time, a decrease in power generated by the fuel cell is suppressed.
[0022] According to the aspects of the invention, even when the duration of
power
generation of the fuel cell extends over a long period of, time, a decrease in
power
generated by the fuel cell is suppressed.
BRIEF DESCRIPTION OF THE DRAWINGS
[0023] The features, advantages, and technical and industrial significance of
this
invention will be described in the following detailed description of example
embodiments
of the invention with reference to the accompanying drawings, in which like
numerals
denote like elements, and wherein:
FIG. 1 is a conceptual cross-sectional view that schematically shows an
membrane
electrode assembly (MEA) according to a first embodiment of the invention;
FIG. 2 is a conceptual cross-sectional view that schematically shows a process
of
manufacturing the MEA according to the first embodiment of the invention;
FIG. 3 is a conceptual cross-sectional view that schematically shows the
internal
structure of the MEA according to the first embodiment of the invention.
FIG. 4 is a conceptual cross-sectional view that schematically shows a process
of
manufacturing the MEA according to a fourth embodiment of the invention;
FIG. 5 is a conceptual cross-sectional view that schematically shows a process
of
manufacturing the MEA according to a fifth embodiment of the invention;
FIG. 6 is a conceptual cross-sectional view that schematically shows a process
of
7
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
manufacturing the MEA according to a sixth embodiment of the invention;
FIG. 7 is a conceptual cross-sectional view that schematically shows a process
of
manufacturing the MEA according to a seventh embodiment of the invention;
FIG. 8 is a conceptual cross-sectional view that schematically shows a process
of
manufacturing the MEA according to an eighth embodiment of the invention;
FIG. 9 is a cross-sectional view of a sample of a fuel cell;
FIG. 10 is a graph of the test results that shows the relationship between
duration and a
cell voltage;
FIG. 11 is a graph of the experimental results that shows the relationship
between
duration and a voltage difference; and
FIG. 12 is a view that shows an example in which the embodiments of the
invention is
applied to a fuel cell system.
DETAILED DESCRIPTION OF EMBODIMENTS
First Embodiment
[0024] A membrane electrode assembly (MEA) for a fuel cell includes an anode
catalyst layer and a cathode catalyst layer. The anode catalyst layer has an
anode
catalyst that accelerates anode reaction. The cathode catalyst layer has a
catalyst that
accelerates cathode reaction. The anode catalyst of the anode catalyst layer
contains a
poisoning-suppression catalytic component that reduces poisoning of carbon
monoxide
contained in an anode fluid. The poisoning-suppression catalytic component,
for
example, includes at least one of ruthenium, tin, osmium, rhodium, palladium,
nickel,
copper, cobalt, manganese, zinc, iridium, and iron. Thus, the anode catalyst
layer
contains the poisoning-suppression catalytic component in addition to a
regular catalytic
component (for example, platinum). Generally, a cathode fluid does not contain
carbon
monoxide. For this reason, the cathode catalyst of the cathode catalyst layer
does not
need to contain the poisoning-suppression catalytic component.
[0025] According to the first embodiment, the anode catalyst layer includes a
first
catalyst layer portion located adjacent to an anode diffusion layer and a
second catalyst
8
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
layer portion located adjacent to an ion conducting membrane. In the
manufacturing
process, it is applicable that the first catalyst layer portion and the second
catalyst layer
portion are separately formed and, after that, they are laminated and
integrated as the
anode catalyst layer. Alternatively, it is applicable that, in the
manufacturing process,
the anode catalyst layer is formed in a monolayer structure and then the side
closer to the
anode diffusion layer than the second catalyst layer portion is regarded as
the first
catalyst layer portion and the side closer to the ion conducting membrane than
the first
catalyst layer portion is regarded as the second catalyst layer portion.
[0026] Between the anode catalyst layer and the cathode catalyst layer, only
in the
anode catalyst layer, the density pl of the porous first catalyst layer
portion is smaller
than the density p2 of the porous second catalyst layer portion (pl < p2). In
this case,
pl/p2, for example, ranges from 0.1 to less than 1, ranges from 0.3 to 0.7, or
ranges from
0.5 to 0.8. However, pl/p2 is not limited to them. Note that p2 desirably
ranges from
500 to 5000 mg/cm3 and, more desirably, ranges from 1500 to 1700 mg/cm3;
however, p2
is not limited to them.
[0027] In the porous anode catalyst layer, p1 is smaller than p2, and the
specific
surface of the first catalyst layer portion increases as compared with the
specific surface
of the porous second catalyst layer portion.
[0028] Generally, as the density of the porous anode catalyst layer increases,
the
porosity decreases. Conversely, as the density decreases, the porosity
increases. Thus,
when pl is smaller than p2, where the porosity (pore volume ratio) of the
first catalyst
layer portion is ?.1 and the porosity of the second catalyst layer portion is
k2, the porosity
?.1 of the first catalyst layer portion is higher than the porosity ?.2 of the
second catalyst
layer portion (X1 > X2). Generally, when pl is larger than p2, Xl is lower
than k2.
[0029] Hereinafter, the first embodiment will be described in greater detail
with
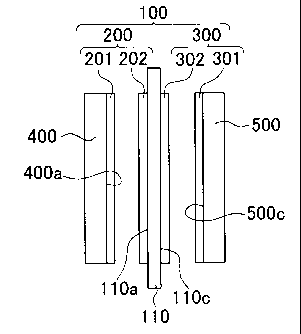
reference to the accompanying drawings. FIG. 1 is a conceptual view of an
membrane
electrode assembly (MEA) 100 according to the first embodiment. FIG. 2 is a
conceptual view of a process of manufacturing the MEA 100. FIG. 3 is a
conceptual
view of the internal structure of the MEA 100. As shown in FIG. 1, the MEA 100
9
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
includes an ion conducting membrane 110, a porous anode catalyst layer 200, a
porous
cathode catalyst layer 300, an anode diffusion layer 400, and a cathode
diffusion layer
500. The ion conducting membrane 110 is made of a fluorocarbon-based solid
polymer
material (for example, perfluorosulfonate resin) or a hydrocarbon-based solid
polymer
material, and has a thickness of, for example, 20 to 50 m. The ion conducting
membrane 110 has two sides (faces) in the thickness direction (arrow t
direction). The
anode catalyst layer 200 is located on one side of the ion conducting membrane
110, and
has a thickness of, for example, 5 to 15 m. The cathode catalyst layer 300 is
located
on the other side of the ion conducting membrane 110, and has a thickness of,
for
example, 5 to 15 m. The anode diffusion layer 400 is located on the outer
side of the
anode catalyst layer 200. The cathode diffusion layer 500 is located on the
outer side of
the cathode catalyst layer 300. Here, the thickness of the ion conducting
membrane 110
is, for example, smaller than or equal to 80 m and, more specifically, ranges
from 20 to
50 m. The thickness of the anode catalyst layer 200 is, for example, smaller
than or
equal to 50 p,m and, more specifically, ranges from 5 to 15 m. The thickness
of the
cathode catalyst layer 300 is, for example, 50 m and, more specifically,
ranges from 5 to
15 m. However, the thicknesses are not limited to the above described values.
[0030] The anode diffusion layer 400 desirably allows an anode fluid (anode
gas) to
permeate therethrough, and is desirably formed of a porous fiber-accumulated
body,
which is formed of fibers such as conductive fibers, or a porous foam. The
cathode
diffusion layer 500 desirably allows a cathode fluid (cathode gas) to permeate
therethrough, and is desirably formed of a porous fiber-accumulated body,
which is
formed of fibers such as conductive fibers, or a porous foam. The conductive
fiber is,
for example, a carbon fiber.
[0031] As shown in FIG. 3, the ion conducting membrane 110 is a solid polymer
electrolyte and has an ion conducting property (proton conducting property).
The anode
catalyst layer 200 includes an anode catalyst 221 (for example, noble metal
catalyst) that
accelerates anode reaction, a conductive carrier 222 that supports the anode
catalyst 221,
and an ion conducting material 223. The anode catalyst layer 200 has a porous
structure
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
and has a large number of pores that allow anode gas (for example, hydrogen
gas or gas
containing hydrogen) as an anode fluid to pass therethrough. The cathode
catalyst layer
300 includes a cathode catalyst 321 (for example, platinum) that accelerates
cathode
reaction, a conductive carrier 322 that supports the cathode catalyst 321, and
an ion
conducting material 323. The cathode catalyst layer 300 has a porous structure
and has
a large number of pores that allow cathode gas (for example, gas containing
oxygen, such
as air) as a cathode fluid to pass therethrough. The carrier is, for example,
conductive
carbon-based microcarriers, such as carbon black. The carbon black is, for
example,
acetylene black, furnace black, lamp black, or thermal black. The carrier may
be a
conductive fiber such as a carbon nanofiber or a carbon fiber. The
microcarriers may be
agglomerated.
[0032] As described above, the components that constitute the anode catalyst
layer
200 include the catalyst, the carrier that supports the catalyst, and the ion
conducting
material. Similarly, the components that constitute the cathode catalyst layer
300
include the catalyst, the carrier that supports the catalyst, and the ion
conducting material.
The ion conducting material (proton conducting material) may be a fluorocarbon-
based
material or a hydrocarbon-based material. The fluorocarbon-based material, for
example, has a perfluoroalkylene group as a principal chain skeleton and a
functional
group, such as a sulfonic acid group, as a side chain of perfluorovinylether.
The anode
catalyst layer 200 and the cathode catalyst layer 300 employ the same type of
carrier and
the same type of ion conducting material. Thus, the difference between the
anode
catalyst layer 200 and the cathode catalyst layer 300 is that the catalyst of
the anode
catalyst layer 200, which may be subject to carbon monoxide, contains the
following
poisoning-suppression catalytic component, whereas the catalyst of the cathode
catalyst
layer 300, which is basically not subject to carbon monoxide, does not contain
the
poisoning-suppression catalytic component.
[0033] Here, in the anode catalyst layer 200 (see FIG. 2, which will be
described
later), if the solid content that constitutes a first catalyst layer portion
201 is 100 percent
by mass, the composition of the solid content is, for example, such that the
sum of the
11
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
catalyst and the carrier ranges from 50 to 80 percent by mass or ranges from
60 to 70
percent by mass and the ion conducting material ranges from 20 to 50 percent
by mass or
ranges from 30 to 40 percent by mass. On the other hand, if the solid content
that
constitutes the second catalyst layer portion 202 is 100 percent by mass, the
composition
of the solid content is, for example, such that the sum of the catalyst and
the carrier
ranges from 50 to 80 percent by mass or ranges from 60 to 70 percent by mass
and the
ion conducting material ranges from 20 to 50 percent by mass or ranges from 30
to 40
percent by mass.
[0034] The anode fluid (anode gas) may contain carbon monoxide (poisoning
material). There is a possibility that carbon monoxide may reduce catalytic
activity of
the electrode catalyst (for example, platinum or palladium). For this reason,
the anode
catalyst 221 in the anode catalyst layer 200 contains the poisoning-
suppression catalytic
component that is able to reduce poisoning of carbon monoxide. The
poisoning-suppression catalytic component desirably removes carbon monoxide by
oxidizing the carbon monoxide. Instead, the poisoning-suppression catalytic
component
just needs to reduce poisoning of carbon monoxide through another mechanism.
[0035] The above poisoning-suppression catalytic component, for example,
includes
at least one of ruthenium, tin, osmium, rhodium, palladium, nickel, copper,
cobalt,
manganese, zinc, iridium, and iron. Thus, the anode catalyst 221 compounded
into the
anode catalyst layer 200 contains the above described poisoning-suppression
catalytic
component and platinum. In this case, the poisoning-suppression catalytic
component
and platinum may be present one by one, or may be alloyed.
[0036] As shown in FIG. 2, the anode catalyst layer 200 includes the porous
first
catalyst layer portion 201 laminated on the surface 400a of the anode
diffusion layer 400
and the porous second catalyst layer portion 202 laminated on the surface 110a
of the ion
conducting membrane 110. Here, the density pl of the first catalyst layer
portion 201 is
smaller than the density p2 of the second catalyst layer portion 202 (p1 <
p2). In this
case, pl/p2 ranges from 0.1 to less than 1, ranges from 0.2 to 0.9 and, more
specifically,
ranges from 0.3 to 0.7. However, pl/p2 is not limited to them. p2 ranges from
about
12
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
500 to about 5000 mg/cm3, ranges from about 1000 to about 3000 mg/cm3 and,
furthermore, ranges from about 1500 to 1700 mg/cm3.
[0037] Generally, as the density of the anode catalyst layer 200 increases,
the
porosity decreases. Conversely, as the density decreases, the porosity
increases. Thus,
where the porosity of the first catalyst layer portion 201 is k1 and the
porosity of the
second catalyst layer portion 202 is k2, the porosity k1 of the first catalyst
layer portion
201 is higher than the porosity k2 of the second catalyst layer portion 202
(Al > A,2).
[0038] The first catalyst layer portion 201 is desirably formed by spray
coating,
pore-forming agent addition, or fiber blending in order to decrease the
density of the first
catalyst layer portion 201. The second catalyst layer portion 202 is formed by
applicator coating in order to keep the density of the second catalyst layer
portion 202.
In this way, the first catalyst layer portion 201 and the second catalyst
layer portion 202,
for example, have the same or approximate compositions while the coating
methods are
varied from each other.
[0039] Here, where the diameter of a pore formed in the first catalyst layer
portion
201 is +1 and the diameter of a pore formed in the porous second catalyst
layer portion
202 is 4)2, +1 is smaller than +2 (+1 < 4)2). 4)1/4)2, for example, ranges
from about 0.3 to
about 0.8 and, more specifically, ranges from about 0.4 to about 0.7. Here,
while +1 is
smaller than 4)2, 4)2, for example, ranges from 30 to 100 nm, ranges from 40
to 90 nm and,
furthermore, ranges from 50 to 70 nm. The pore diameter in the embodiments and
examples may be based on a median diameter measured by a mercury porosimeter.
[0040] As described above, according to the present embodiment, because the
density of the anode catalyst layer 200 is set to the relationship that pl is
smaller than p2,
the specific surface of the porous first catalyst layer portion 201 increases
as compared
with the specific surface of the porous second catalyst layer portion 202.
Furthermore,
the pore diameter is set to the relationship that +1 is smaller than 4)2.
Thus, the specific
surface of the porous first catalyst layer portion 201 further increases as
compared with
the specific surface of the porous second catalyst layer portion 202.
[0041] Thus, when an anode fluid is supplied to the anode catalyst layer 200,
in
13
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
comparison with the case in which the density of the first catalyst layer
portion 201 is
large, the poisoning-suppression catalytic component contained in the first
catalyst layer
portion 201 is more likely to contact carbon monoxide in the anode fluid that
flows a gas
passage (fluid passage) of the first catalyst layer portion 201. Furthermore,
because the
density of the second catalyst layer portion 202 is larger than the density of
the first
catalyst layer portion 201, it can also be expected to restrict circulation of
carbon
monoxide contained in the anode fluid. As a result, in comparison with the
case in
which the density of the first catalyst layer portion 201 is large, the first
catalyst layer
portion 201 has improved capability to reduce poisoning of carbon monoxide
contained
in the anode fluid.
[0042] Thus, at the time when the anode fluid reaches the second catalyst
layer
portion 202 via the first catalyst layer portion 201, poisoning of carbon
monoxide is
reduced. For this reason, catalyst activity in the second catalyst layer
portion 202 that
effectively contributes to power generation reaction is desirably exerted.
Hence, even
when the duration of service extends over a long period of time, a decrease in
power
generated by the fuel cell is suppressed.
[0043] The cathode catalyst layer 300 will be described. The cathode catalyst
layer
300 is formed by applicator coating, and includes a porous first cathode
catalyst layer
portion 301 laminated on the surface 500c of the cathode diffusion layer 500
and a porous
second cathode catalyst layer portion 302 laminated on the surface 110c of the
ion
conducting membrane 110. In the cathode catalyst layer 300, the density plc of
the first
catalyst layer portion 301 is not purposely varied from the density p2c of the
second
catalyst layer portion 302, and the densities of both are basically about the
same.
plc/p2c ranges from 0.85 to 1.15 and, more specifically, ranges from 0.95 to
1.05. In
addition, the pore diameter 4lc of the first catalyst layer portion 301 is not
purposely
varied from the pore diameter 42c of the second catalyst layer portion 302,
and the pore
diameters of them are basically about the same. 4lc/~2c ranges from 0.85 to
1.15 and,
more specifically, ranges from 0.95'to 1.05.
Second Embodiment
14
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
[0044] According to a second embodiment of the invention, between the anode
catalyst layer and the cathode catalyst layer, where, in the anode catalyst
layer only, the
pore diameter 4)1 of the porous first catalyst layer portion is smaller than
the pore
diameter 4)2 of the porous second catalyst layer portion (4)1 < 4)2). 4)2
desirably ranges
from 30 to 100 nm, ranges from 40 to 90 rum and, furthermore, ranges from 50
to 70 nm.
The ratio of pore diameters 4)1/4)2 desirably ranges from 0.1 to less than 1,
ranges from
0.2 to 0.95, ranges from 0.3 to 0.95, ranges from 0.4 to 0.95 and,
furthermore, ranges
from 0.5 to 0.9. Note that the pore diameter may be based on a median diameter
measured by a mercury porosimeter; instead, it may be based on a mode diameter
(most
frequent diameter).
[0045] The illustrated first catalyst layer portion of the anode catalyst
layer has a
small density owing to at least one of spray coating in which catalyst ink
(catalyst paste)
is applied by spraying, pore-forming agent addition in which pore-forming
agent is added
to the first catalyst layer portion, or a structure that the first catalyst
layer portion
incorporates microfibers. Spray coating can increase micropores in comparison
with
applicator coating, and it is advantageous in decreasing the density of the
porous first
catalyst layer portion. When pore-forming agent is added at the time of
manufacturing
the first catalyst layer portion, pores owing to the pore-forming agent are
formed in the
first catalyst layer portion. Thus, it is advantageous in decreasing the
density of the
porous first catalyst layer portion.
[0046] The pore-forming agent is, for example, a material, such as polyvinyl
alcohol,
that dissolves in water or a material, such as pulp, that is burnt,
evaporated, or liquefied
by heating. If microfibers are contained in the first catalyst layer portion,
it is easy to
decrease the density of the porous first catalyst layer portion. The length of
a microfiber,
for example, ranges from 1 to 300 m, ranges from 2 to 100 m and, more
specifically,
ranges from 5 to 50 m. The microfiber is desirably conductive, and is, for
example,
carbon nanotube (including carbon nanohorn) or carbon nanofiber.
[0047] When the anode fluid that contains carbon monoxide (poisoning material)
flows through the anode catalyst layer, an anode active material (for example,
hydrogen)
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
contained in the anode fluid is progressively consumed for power generation
reaction as
it is transferred toward the downstream side. Thus,. the concentration of
anode active
material (for example, hydrogen) is gradually reduced from the upstream side
of the
anode catalyst layer toward the downstream side thereof. This means that the
concentration of carbon monoxide gradually increases from the upstream side of
the
anode catalyst layer toward the downstream side thereof and, therefore, the
poisoning is
more likely to occur at the downstream side of the anode catalyst layer than
at the
upstream side thereof. Then, in the illustrated anode catalyst layer, the
amount of
poisoning-suppression catalytic component supported per unit area, which
reduces
poisoning of carbon monoxide contained in an anode fluid, is greater at the
downstream
side than at the upstream side. That is, in the anode catalyst layer, the
anode fluid flows
from the upstream side toward the downstream side, and in the illustrated
anode catalyst
layer, the amount of poisoning-suppression catalytic component supported per
unit area is
increased at the upstream side as compared with at the downstream side. Note
that the
upstream side indicates an inlet side region of the anode fluid and indicates
a relative
region in a direction in which the anode fluid flows. The downstream side
indicates an
outlet side region of the anode fluid and indicates a relative region in a
direction in which
the anode fluid flows.
[0048] Hereinafter, the second embodiment will be described in detail with
reference
to the accompanying drawings. The present embodiment basically has a similar
configuration, function and advantageous effects to those of the first
embodiment, so FIG.
1 and FIG. 2 are also used for reference. Hereinafter, different portions will
be mainly
described. The density pl of the porous first catalyst layer portion 201 is
smaller than
the density p2 of the porous second catalyst layer portion 202 (pl < p2). p2
may range
from 500 to 5000 mg/cm3 and, more specifically, may range from 1500 to 1700
mg/cm3.
The pore diameter +1 of the first catalyst layer portion 201 is substantially
equal to the
pore diameter +2 of the porous second catalyst layer portion 202 (41 - +2).
Here, +1/42
ranges from 0.85 to 1.15. +2 may range from 30 to 100 nm and, furthermore, may
range
from 50 to 70 nm. The pore diameter may be based on a median diameter.
16
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
[0049] In the present embodiment as well, as in the case of the first
embodiment, the
density of the anode catalyst layer 200 is set to the relationship that pl is
smaller than p2.
Thus, the poisoning-suppression catalytic component contained in the first
catalyst layer
portion 201 is more likely to contact carbon monoxide in the anode fluid that
flows
through the fluid passage of the first catalyst layer portion 201. As a
result, in
comparison with the case in which the density of the first catalyst layer
portion 201 is
large, the first catalyst layer portion 201 has improved capability to reduce
poisoning of
carbon monoxide contained in the anode fluid. Thus, poisoning of carbon
monoxide is
reduced in the second catalyst layer portion 202. For this reason, the second
catalyst
layer portion 202 is more likely to exert an ability to effectively contribute
to power
generation reaction. Hence, even when the duration of service extends over a
long
period of time, a decrease in power generated by the fuel cell is suppressed.
[0050] Furthermore, the cathode catalyst layer 300 will be described. The
cathode
catalyst layer 300 is formed not by spray coating but by applicator coating,
and includes
the porous first catalyst layer portion 301 located adjacent to the cathode
diffusion layer
500 and the porous second catalyst layer portion 302 located adjacent to the
ion
conducting membrane 110. In the cathode catalyst layer 300, the density plc of
the first
catalyst layer portion 301 is not purposely varied from the density p2c of the
second
catalyst layer portion 302, and the densities of both are basically about the
same. Thus,
plc/p2c ranges from 0.85 to 1.15. Note that the pore diameter of the first
catalyst layer
portion 301 is not purposely varied from the pore diameter of the second
catalyst layer
portion 302, and the pore diameters of them are basically about the same.
Thus,
4lc/42c ranges from 0.85 to 1.15. The pore diameter may be based on a median
diameter.
Third Embodiment
[0051] The present embodiment basically has a similar configuration, function
and
advantageous effects to those of the first embodiment. Hereinafter, different
portions
will be mainly described, so FIG. 1 and FIG. 2 are also used for reference.
Here, in the
anode catalyst layer 200, the pore diameter ~1 of the porous first catalyst
layer portion
17
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
201 is smaller than the pore diameter 4)2 of the porous second catalyst layer
portion 202
(4)1 < 4)2). 41/4)2 ranges from 0.5 to 0.85. +2 may range from 30 to 100 nm
and,
furthermore, may range from 50 to 70 nm. The pore diameter may be based on a
median diameter. Note that the density pl of the first catalyst layer portion
201 is
substantially equal to the density p2 of the second catalyst layer portion
202.
Specifically, pl/p2 ranges from 0.95 to 1.05 (pl = p2). Here, p2 may range
from 500 to
5000 mg/cm3 and, more specifically, may range from 1500 to 1700 mg/cm3.
Furthermore, the porosity X1 of the first catalyst layer portion 201 is
substantially equal
to the porosity k2 of the second catalyst layer portion 202 (X1/,2 ranges from
0.95 to
1.05, X1 = X2).
[0052] According to the present embodiment, the pore diameter of the porous
anode
catalyst layer 200 is set to the relationship that +1 is smaller than +2 as
described above.
Thus, the specific surface of the porous first catalyst layer portion 201
increases as
compared with the specific surface of the porous second catalyst layer portion
202. As a
result, in comparison with the case in which the pore diameter of the first
catalyst layer
portion 201 is large and the specific surface thereof is small, the poisoning-
suppression
catalytic component contained in the first catalyst layer portion 201 is more
likely to
contact carbon monoxide in the anode fluid that flows through the fluid
passage of the
first catalyst layer portion 201. Thus, poisoning of carbon monoxide is
reduced in the
second catalyst layer portion 202. For this reason, the second catalyst layer
portion 202
is more likely to exert an ability to effectively contribute to power
generation reaction
and, therefore, a decrease in power generated by the fuel cell is suppressed.
[0053] According to the present embodiment, the cathode catalyst layer 300 is
formed not by spray coating but by applicator coating, and includes the porous
first
catalyst layer portion 301 located adjacent to the cathode diffusion layer 500
and the
porous second catalyst layer portion 302 located adjacent to the ion
conducting
membrane 110. In the cathode catalyst layer 300, the density plc of the first
catalyst
layer portion 301 is not purposely varied from the density p2c of the second
catalyst layer
portion 302, and the densities of both are basically about the same. Thus,
plc/p2c
18
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
ranges from 0.85 to 1.15. p2 may range from 500 to 5000 mg/cm3 and, more
specifically, may range from 1900 to 2100 mg/cm3.
[0054] Furthermore, the pore diameter of the first catalyst layer portion 301
is not
purposely varied from the pore diameter of the second catalyst layer portion
302, and the
pore diameters of them are basically about the same (4lc = ~2c). 41c/~2c
ranges from
0.85 to 1.15. The pore diameter may be based on a median diameter.
[0055] A manufacturing method will be specifically described in fourth and
fifth
embodiments.
Fourth Embodiment
[0056] FIG. 4 shows the fourth embodiment. The present embodiment basically
has
a similar configuration, function and advantageous effects to those of the
first
embodiment. Hereinafter, different portions will be mainly described.
According to
the present embodiment, as is different from the first embodiment, the cathode
catalyst
layer 300 that is not subject to carbon monoxide is formed but not by
laminating the first
catalyst layer portion 301 and the second catalyst layer portion 302. The
cathode
catalyst layer 300 is formed as a monolayer on the surface 110c of the ion
conducting
membrane 110, facing the cathode diffusion layer 500, in the manufacturing
process. In
this state, the MEA 100 is integrated by hot pressing. Because the cathode
catalyst layer
300 is a monolayer in the manufacturing process, an excessive thickness may be
suppressed. Although the cathode catalyst layer 300 is a monolayer in the
manufacturing process, in the bonded and complete membrane electrode assembly
100,
the cathode catalyst layer 300 may be regarded separately as the first
catalyst layer
portion 301 located close to the ion conducting membrane 100 and the second
catalyst
layer portion 302 located close to the cathode diffusion layer 500.
Fifth Embodiment
[0057] FIG. 5 shows the fifth embodiment. The present embodiment basically has
a
similar configuration, function and advantageous effects to those of the first
embodiment.
Hereinafter; different portions will be mainly described. The fifth embodiment
differs
from the first embodiment in that the cathode catalyst layer 300 is formed but
not by
19
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
laminating the first catalyst layer portion 301 and the second catalyst layer
portion 302.
In the manufacturing process, the cathode catalyst layer 300 is formed as a
monolayer on
the surface 500c of the cathode diffusion layer 500, facing the ion conducting
membrane
110. In this state, the MEA 100 is integrated by hot pressing. Because the
cathode
catalyst layer 300 is a monolayer in the manufacturing process, an excessive
thickness
may be suppressed.
[0058] The distribution of the amount of ruthenium in the anode catalyst layer
will
be described in sixth to eighth embodiments.
Sixth Embodiment
[0059] FIG. 6 shows the sixth embodiment. The present embodiment basically has
a similar configuration, function and advantageous effects to those of the
first
embodiment. Hereinafter, different portions will be mainly described. When a
gaseous anode fluid that contains carbon monoxide flows through the anode
catalyst layer
200, an anode active material (hydrogen) contained in the anode fluid is
progressively
consumed for power generation reaction. Thus, the concentration of anode
active
material (hydrogen) is gradually reduced from the upstream side of the anode
catalyst
layer 200 toward the downstream side thereof. This means that the
concentration of
carbon monoxide gradually increases from the upstream side of the anode
catalyst layer
200 toward the downstream side thereof. Thus, it is desirable to take measures
against
poisoning at the downstream side of the anode catalyst layer 200. According to
the
present embodiment, in the first catalyst layer portion 201 that constitutes
the anode
catalyst layer 200, the amount of the above described poisoning-suppression
catalytic
component (for example, ruthenium) supported gradually increases from an
upstream
side 201u toward a downstream side 201d. That is, the amount of
poisoning-suppression catalytic component (for example, ruthenium) supported
is greater
at the downstream side 201d than at the upstream side 201u. In FIG. 6, the
amount of
poisoning-suppression catalytic component (for example, ruthenium) supported
is
schematically shown in a stepwise manner.
[0060] In contrast, in the second catalyst layer portion 202, the amount of
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
poisoning-suppression catalytic component (for example, ruthenium) per unit
area (mass
per unit area) is substantially equal from an upstream side 202u toward a
downstream
side 202d. The "substantially equal" means that the ratio of the amount of
poisoning-suppression catalytic component (for example, ruthenium) supported
per unit
area (downstream side/upstream side) ranges from 0.9 to 1.1 and,-more
specifically,
ranges from 0.95 to 1.05.
[0061] According to the present embodiment as described above, in the first
catalyst
layer portion 201 to which an anode fluid is supplied prior to the second
catalyst layer
portion 202, the amount of the above described poisoning-suppression catalytic
component (for example, ruthenium) supported per unit area is greater at the
downstream
side 201d than at the upstream side 201u. Thus, it is advantageous in
suppressing
poisoning of carbon monoxide.
Seventh Embodiment
[0062] FIG. 7 shows the seventh embodiment. The present embodiment basically
has a similar configuration, function and advantageous effects to those of the
sixth
embodiment. Hereinafter, different portions will be mainly described.
According to
the present embodiment, in the second catalyst layer portion 202 that
constitutes the
anode catalyst layer 200, the amount of the above described poisoning-
suppression
catalytic component (for example, ruthenium) supported per unit area gradually
increases
from the upstream side 202u toward the downstream side 202d. In the first
catalyst
layer portion 201, the amount of poisoning-suppression catalytic component
(for example,
ruthenium) supported is substantially equal from the upstream side 201u toward
the
downstream side 201d. The "substantially equal" means that the ratio (mass
ratio) of the
amount of poisoning-suppression catalytic component (for example, ruthenium)
supported per unit area (downstream side/upstream side) ranges from 0.9 to 1.1
and, more
specifically, ranges from 0.95 to 1.05. According to the present embodiment as
described above, in the second catalyst layer portion 202 that is located
close to the ion
conducting membrane 110 and that largely contributes to power generation
reaction, the
amount of the above described poisoning-suppression catalytic component (for
example,
21
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
ruthenium) supported is greater at the downstream side 202d than at the
upstream side
202u. Thus, it is advantageous in suppressing poisoning of carbon monoxide.
Eighth Embodiment
[0063] FIG. 8 shows the eighth embodiment. The present embodiment basically
has a similar configuration, function and advantageous effects to those of the
sixth
embodiment shown in FIG. 6. Thus, pl is smaller than p2. Hereinafter,
different
portions will be mainly described. In the first catalyst layer portion 201
that constitutes
the anode catalyst layer 200, the amount of the above described poisoning-
suppression
catalytic component (for example, ruthenium) supported gradually increases
from the
upstream side 201u toward the downstream side 201d. In addition, in the first
catalyst
layer portion 201, the density plu of the upstream side 201u is slightly
smaller than the
density p1d of the downstream side 201d. plu/pld substantially ranges from 0.8
to 0.97.
The pore diameter 4lu of the upstream side 201u is substantially equal to the
pore
diameter Old of the downstream side 201d. In the present embodiment as well,
it is
advantageous in suppressing poisoning of carbon monoxide.
[0064] Hereinafter, examples will be described together with comparative
examples.
Catalyst Ink Formation
[0065] Catalyst ink used in examples and in comparative examples will be
described.
First, a mixture for anode was dispersed by a homogenizer through bead milling
to form
anode catalyst ink. The mixture contained 6 g of catalyst-supporting carbon
particles
(Tanaka Kikinzoku Kogyo K.K., Product Name: TEC62E58) that supports an anode
catalyst on a carbon carrier (carbon black), 16 g of solution that contains 20
percent by
mass of ion conducting material (Du Pont, NafionTm), 23 g of ethanol
(dispersion
medium), and 55 g of distilled water (dispersion medium). If the anode
catalyst-supporting carbon is 100 percent by mass, the composition was 28
percent by
mass of platinum, 29 percent by mass of ruthenium, and the remainder carbon.
Thus,
the amount of platinum and the amount of ruthenium were substantially equal.
Ruthenium facilitates oxidization of carbon monoxide contained in the anode
gas.
[0066] A mixture for cathode was dispersed by a homogenizer through bead
milling
22
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
to form cathode catalyst ink. The mixture contained 14 g of catalyst-
supporting carbon
particles (Tanaka Kikinzoku Kogyo K.K., Product Name: TEC62E58) that supports
a
cathode catalyst on a carbon carrier (carbon black), 15 g of solution that
contains 20
percent by mass of ion conducting material (NafionT"), 26 g of ethanol
(dispersion
medium), and 45 g of distilled water (dispersion medium). If the cathode
catalyst-supporting carbon is 100 percent by mass, the composition was 70
percent by
mass of platinum and the remainder carbon. Ruthenium was not substantially
contained
therein. As described above, the components of the anode catalyst ink and the
components of the cathode catalyst ink are almost the same except ruthenium.
Note that
after the catalyst layers are formed, ethanol and distilled water evaporate.
(1) First Example
(1-1) Formation of First Catalyst Layer Portion 201 of Anode Catalyst Layer
200
[0067] The anode diffusion layer 400 employed commercially-available carbon
paper (Toray Industries, Inc., 200 m in thickness). Then, the anode catalyst
ink was
applied on the surface 400a of the anode diffusion layer 400, facing the ion
conducting
membrane 110, with a spray coater to thereby form the first catalyst layer
portion 201.
Spray coating increases the porosity of the first catalyst layer portion 201
in order to
decrease the density of the first catalyst layer portion 201. The spray
coating was
performed with a nozzle aperture of 1.0 mm, at a height of 85 mm and at an
atomization
pressure of 0.3 MPa. In this case, in the first catalyst layer portion 201,
the amount of
platinum (Pt) supported was 0.2 mg Pt/cm2, and the amount of ruthenium (Ru)
supported
was 0.2 mg Ru/cm2. In the coated first catalyst layer portion 201, the density
of
platinum was 150 mg Pt/cm3 on average, and the density of ruthenium was 150 mg
Ru/cm3 on average. In the first catalyst layer portion 201, the density p1 was
830
mg/cm3, and the pore diameter 41 was 42 nm. The pore diameter was based on a
median diameter. The density p1 was obtained -as follows. The difference in
weight
(coated amount) between before and after coating the anode first catalyst
layer portion
201 was measured. Next, the difference in weight (coated amount) was converted
into
the weight per unit area of the first catalyst layer portion 201 (0.2 mg
Pt/cm2). The
23
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
density (150 mg Pt/cm3) was calculated from the thickness observed with an
SEM. The
calculated density was converted into a solid content density (150 mg Pt/cm3 =
0.18
830 mg/cm3) using the ratio of Pt to solid content (= 18 percent by mass).
Note that
ethanol and distilled water evaporate, so they were not included in the mass
of the
catalyst layer portions 201 and 202.
(1-2) . Formation of Second Catalyst Layer Portion 202 of Anode Catalyst Layer
200
[0068] The anode catalyst ink was applied on the surface of a Teflon sheet
(fluororesin sheet) with an applicator coater to thereby form the second
catalyst layer
portion 202. Applicator coating is to ensure the porosity of the second
catalyst layer
portion 202 while the density of the second catalyst layer portion 202 is
larger than the
density pl of the first catalyst layer portion 201. The applicator coating was
performed
with a gap of 200 p.m set between the applicator blade and the Teflon sheet.
In this case,
in the second catalyst layer portion 202, the amount of platinum supported was
0.2 mg
Pt/cm2, and the amount of ruthenium supported was 0.2 mg Ru/cm2 The second
catalyst layer portion 202 applied on the Teflon sheet as described above was
transferred
to the surface 110a of one side of the ion conducting membrane 110 (thickness:
30 m).
The transfer was performed at a temperature of 150 C and at a pressure of 8
MPa. The
conditions of the transfer are not limited to them.
[0069] Here, in the second catalyst layer portion 202 transferred to the ion
conducting membrane 110, the density of platinum was 290 mg Pt/cm3 on average,
and
the density of ruthenium was 290 mg Ru/cm3 on average. In the second catalyst
layer
portion 202 of the anode catalyst layer 200, the density p2 was 1600 mg/cm3,
and the
pore diameter 42 was 60 nm. Here, pl/p2 was 0.5. X1/42 was 42/60, which is
approximately equal to 0.7. According to the first example, in the anode
catalyst layer
200, the density pl of the first catalyst layer portion 201 was smaller than
the density p2
of the second catalyst layer portion 202. The pore diameter ~1 (42 nm) of the
first
catalyst layer portion 201 was smaller than the pore diameter 42 (60 nm) of
the second
catalyst layer portion 202. As described above, the components of the first
catalyst
layer portion 201 and the components of the second catalyst layer portion 202
are almost
24
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
the same except ruthenium.
(1-3) Formation of First Catalyst Layer Portion 301 of Cathode Catalyst Layer
300
[0070] The cathode diffusion layer 500 employed commercially-available carbon
paper (Toray Industries, Inc., 200 m in thickness). The cathode catalyst ink
was
applied on the surface 500c of the cathode diffusion layer 500, facing the ion
conducting
membrane 110, with an applicator coater to thereby form the first catalyst
layer portion
301 of the cathode catalyst layer 300. The applicator coating was performed
with a gap
of 400 m set between the applicator blade and the cathode diffusion layer
500. In this
case, in the first catalyst layer portion 301, the amount of platinum
supported was 1.0 mg
Pt/cm2. In the first catalyst layer portion 301, the density of platinum was
1100 mg
Pt/cm3 on average. In the first catalyst layer portion 301, the density pcl
was 2000
mg/cm3, and the pore diameter (median diameter) 4cl was 68 nm. Note that the
density
and pore diameter for the cathode side have a suffix of "c" in reference
numerals.
(1-4) Formation of Second Catalyst Layer Portion 302 of Cathode Catalyst Layer
300
[0071] The cathode catalyst ink was applied on the surface of a Teflon sheet
with an
applicator coater to thereby form the second catalyst layer portion 302. The
applicator
coating was performed with a gap of 200 m set between the applicator blade
and the
Teflon sheet. In this case,, in the second catalyst layer portion 302, the
amount of
platinum supported was 1.0 mg Pt/cm2. The second catalyst layer portion 302
applied
on the Teflon sheet as described above was transferred to the surface 110c of
one side of
the ion conducting membrane 110 (thickness: 30 m). The transfer was performed
at a
temperature of 150 C and at a pressure of 8 MPa. Note that the conditions of
the
transfer are not limited to them. In the second catalyst layer portion 302
transferred to
the ion conducting membrane 110, the density of platinum was 1100 mg Pt/cm3 on
average. In the second catalyst layer portion 302, the density pc2 was 2000
mg/cm3,
and the pore diameter 4c2 was 65 nm.
(2) Second Example
[0072] In the second example, the anode catalyst ink and cathode catalyst ink
similar
to those of the first example were used.
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
(2-1) Formation of First Catalyst Layer Portion 201 of Anode Catalyst Layer
200
[0073] The anode catalyst ink was applied on the surface 400a of the anode
diffusion
layer 400, facing the ion conducting membrane 110, with a spray coater to
thereby form
the first catalyst layer portion 201. The spray coating was performed with a
nozzle
aperture of 1.0 mm, at a height of 85 mm and at an atomization pressure of 0.3
MPa, as in
the similar manner to the first example. However, the anode diffusion layer
400 is
divided into three equal parts in the direction in which anode gas flows, and
then the
amount of catalyst (platinum-ruthenium) supported was increased from the
upstream side
toward the downstream side in a stepwise manner. That is, in the first
catalyst layer
portion 201, in the upstream region, the amount of platinum supported was 0.08
mg
Pt/cm2 and the amount of ruthenium supported was 0.08 mg Ru/cm2; in the
midstream
region, the amount of platinum supported was 0.2 mg Pt/cm2 and the amount of
ruthenium supported was 0.2 mg Ru/cm2; and in the downstream region, the
amount of
platinum supported was 0.32 mg Pt/cm2 and the amount of ruthenium supported
was 0.32
mg Ru/cm2. The ratio of the amount of catalyst supported at the downstream
side to the
amount of catalyst supported at the upstream side was 0.32/0.08, which is
equal to 4.
[0074] As a result, in the first catalyst layer portion 201 that constitutes
the anode
catalyst layer 200, the amount of platinum supported was 0.2 mg Pt/cm2 on
average, and
the amount of ruthenium supported was 0.2 mg Ru/cm2 on average.
[0075] In the first catalyst layer portion 201, as in the case of the first
example, the
density of platinum was 150 mg Pt/cm3 on average, and the density of ruthenium
was 150
mg Ru/cm3 on average. In the first catalyst layer portion 201, the density pl
was 830
mg/cm3, and, furthermore, the pore diameter ~1 was 42 nm as in the case of the
first
example.
(2-2) Formation of Second Catalyst Layer Portion 202 of Anode Catalyst Layer
200
[0076] The second catalyst layer portion 202 of the anode catalyst layer 200
was
formed as in the similar manner to that of the first example. According to the
second
example, in the second catalyst layer portion 202 of the anode catalyst layer
200, the
density p2 was 1600 mg/cm3, and the pore diameter +2 was 60 nm. Thus, p1 was
26
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
smaller than p2 (p1 < p2), and pl/p2 was 0.5. Furthermore, +1 was smaller than
+2 (4)1
< 4)2), and 4)1/4)2 was 42nm/60nm, which is equal to 0.7.
(2-3) Formation of First Catalyst Layer Portion 301 of Cathode Catalyst Layer
300
[0077] The first catalyst layer portion 301 that constitutes the cathode
catalyst layer
300 was formed under the same conditions as those of the first catalyst layer
portion 301
according to the first example. The second catalyst layer portion 302 that
constitutes the
cathode catalyst layer 300. was also formed under the same conditions as those
of the
second catalyst layer portion 302 according to the first example.
(3) First Comparative Example
[0078] In the first comparative example, the anode catalyst ink and cathode
catalyst
ink similar to those of the first example were used.
(3-1) Formation of First Catalyst Layer Portion 201 of Anode Catalyst Layer
200
according to First Comparative Example
[0079] In the first comparative example, the anode catalyst ink was applied on
the
surface 400a of the anode diffusion layer 400, facing the ion conducting
membrane 110,
with a spray coater to thereby form the first catalyst layer portion 201. The
applicator
coating was performed with a gap of 350 m set between the applicator blade
and the
Teflon sheet. In this case, in the first catalyst layer portion 201, the
amount of platinum
supported was 0.2 mg Pt/cm2, and the amount of ruthenium supported was 0.2 mg
Ru/cmZ. In the first catalyst layer portion 201, the density of platinum was
290 mg
Pt/cm3 on average, and the density of ruthenium was 290 mg Ru/cm3 on average.
According to the first comparative example, in the first catalyst layer
portion 201 of the
anode catalyst layer 200, the density pl was 1600 mg/cm3, and the pore
diameter (median
diameter) +1 of the first catalyst layer portion 201 was 60 nm, which is
larger than that of
the first example or the second example.
(3-2) Formation of Second Catalyst Layer Portion 202 of Anode Catalyst Layer
200
according to First Comparative Example
[0080] The second catalyst layer portion 202 of the anode catalyst layer 200
according to the first comparative example was formed with an applicator
coater under
27
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
the same conditions as those of the second catalyst layer portion 202
according to the first
example. According to the first comparative example, in the first catalyst
layer portion
201 of the anode catalyst layer 200, the density pl was 1600 mg/cm3, which is
substantially equal to the density p2 of the second catalyst layer portion
202, and,
furthermore, the pore diameter 41 (60 nm) was substantially equal to the pore
diameter
4)2 (60 nm) of the second catalyst layer portion 202. Thus, pl/p2 was 1.0, and
4)1/4)2
was 60/60, which is equal to 1.
(3-3) Formation of First Catalyst Layer Portion 301 of Cathode Catalyst Layer
300
according to First Comparative Example
[0081] The first catalyst layer portion 301 that constitutes the cathode
catalyst layer
300 according to the first comparative example was formed under the same
conditions as
those of the first catalyst layer portion 301 according to the first example.
In the first
comparative example, the second catalyst layer portion 302 that constitutes
the cathode
catalyst layer 300 was also formed under the same conditions as those of the
second
catalyst layer portion 302 according to the first example.
(4) Second Comparative Example
[0082] In the second comparative example, the anode catalyst ink and cathode
catalyst ink similar to those of the first example were used. Then, under the
same
conditions as those of the first example, the first catalyst layer portion 201
and second
catalyst layer portion 202 of the anode catalyst layer 200 were formed. Thus,
as in the
case of the first example, pl/p2 was 0.5. 4)1/4)2 was 42/60, which is
approximately
equal to 0.7.
(4-1) Formation of First Catalyst Layer Portion 301 of Cathode Catalyst Layer
300
according to Second Comparative Example
[0083] The cathode diffusion layer 500 employed commercially-available carbon
paper (Toray Industries, Inc., 200 m in thickness). Then, the cathode
catalyst ink was
applied on the surface 500c of the cathode diffusion layer 500, facing the ion
conducting
membrane 110, with a spray coater to thereby form the first catalyst layer
portion 301.
The spray coating was performed with a nozzle aperture of 0.6 mm, at a height
of 85 mm
28
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
and at an atomization pressure of 0.3 MPa. In this case, in the first catalyst
layer portion
301, the amount of platinum (Pt) supported was 0.1 mg Pt/cm'`. In the coated
first
catalyst layer portion 301, the density of platinum was 770 mg Pt/cm3 on
average. The
density plc of the first catalyst layer portion 301 was 1400 mg/cm3. The pore
diameter
plc was 45 nm in median diameter.
(4-2) Formation of Second Catalyst Layer Portion 302 of Cathode Catalyst Layer
300
according to Second Comparative Example
[0084] In the second comparative example, the second catalyst layer portion
301 of
the cathode catalyst layer 300 was formed as in the similar manner to that of
the first
example. Thus, the density p2c of the second catalyst layer portion 302 was
2000
mg/cm3. The pore diameter 4 2c was 65 nm in median diameter. The ratio of the
pore
diameters 4lc/42c was, 45/65, which is approximately equal to 0.7.
(5) Formation of MEA 100
[0085] As can be understood from FIG. 1 and FIG. 2, the anode-side first
catalyst
layer portion 201 was laminated on the anode diffusion layer 400, the ion
conducting
membrane 110 was held between the anode-side second catalyst layer portion 202
and the
cathode-side second catalyst layer portion 302, the cathode-side first
catalyst layer
portion 301 was laminated on the cathode diffusion layer 500, and then the
anode
diffusion layer 400, the ion conducting membrane 110 and the cathode diffusion
layer
500 were laminated to thereby form a laminated body. Then, the laminated body
was
pressurized in the thickness direction (laminated direction) under the
predetermined hot
pressing conditions (at a temperature of 140 C and at a pressure of 8 MPa) to
closely
adhere the layers to one another, thus forming the MEA 100. The hot pressing
conditions are not limited to them.
[0086] The size of the MEA 100 was 90 mm by 150 mm. The above MEA 100 was
held between a carbon-based anode flow distribution member 600 and a carbon-
based
cathode flow distribution member 700 to thereby form a sample of a fuel cell
(see FIG. 9).
The anode flow distribution member 600 includes groove-like passages 601 that
allow
anode gas to flow, an inlet 602, and an outlet 603. The cathode flow
distribution
29
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
member 700 includes passages 701 that allow cathode gas to flow, an inlet 702,
and an
outlet 703.
[0087] In the fuel cell, hydrogen gas (anode gas) that contains carbon
monoxide
having a predetermined concentration was supplied to the anode diffusion layer
400 of
the MEA 100 via the passages 601 of the anode flow distribution member 600.
Similarly, air (cathode gas) was supplied to the cathode diffusion layer 500
of the MEA
100 via the passages 701 of the cathode flow distribution member 700. In this
manner,
power generation reaction was initiated. Hydrogen gas was supplied at a
pressure of 1
atm (absolute pressure), and air was supplied at a pressure of 1 atm (absolute
pressure).
[0088] In this case, durability test was conducted at 0.34 A/cm2. The hydrogen
utilization was adjusted to 90%, the air utilization to 50%, the dew point of
anode gas to
60 C, and the dew point of cathode gas to 65 C. Furthermore, the electric
current
density was set to 0.26 A/cm2, and the cell stack temperature was set to 65 C.
After that,
the voltage characteristics of the fuel cell were measured. The measured
results are
shown in FIG. 10.
[0089] FIG. 10 shows the relationship between duration and a cell voltage when
anode gas contains 20 ppm (mass ratio) of carbon monoxide. The characteristic
line W1
represents the first example. The characteristic line W2 represents the second
example.
The characteristic line W3 represents the first comparative example. The
characteristic
line W4 represents the second comparative example. As shown by the
characteristic
lines W3 and W4 in FIG. 10, as duration during which the fuel cell generates
power
increases, a decrease in cell voltage is large in the first and second
comparative examples.
It may be presumed that the anode catalyst layer 200 is influenced by catalyst
poisoning
due to carbon monoxide. In contrast, as shown by the characteristic lines W1
and W2, a
decrease in cell voltage is smaller in the first and second examples than in
the first and
second comparative examples. It may be presumed that catalyst poisoning due to
carbon monoxide is suppressed.
[0090] Furthermore, the case in which pure hydrogen gas that contains no
carbon
monoxide is supplied to the anode and the case in which hydrogen gas that
contains 100
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
ppm of carbon monoxide in mass ratio is supplied to the anode were tested. A
voltage
difference AV between both cases was measured. Then, the relationship between
duration during which the fuel cell generates power and a voltage difference
AV between
both cases was obtained. The results are shown in FIG. 11. The characteristic
line W1
represents the first example. The characteristic line W2 represents the second
example.
The characteristic line W3 represents the first comparative example. The
characteristic
line W4 represents the second comparative example. As shown in FIG. 11, the
voltage
difference AV is large in the first and second comparative examples. Moreover,
the
voltage difference AV is considerably large in the first comparative example.
[0091] As shown in the above described test results, in the second comparative
example, in the anode catalyst layer 200 that is subject to carbon monoxide,
4)1 is smaller
than 4)2, and also in the cathode catalyst layer 300 that is not subject to
carbon monoxide,
the relationship similar to the anode catalyst layer 200 is applied, that is,
the density plc
of the first catalyst layer portion 301 is smaller than the density p2c of the
second catalyst
layer portion 302.
[0092] However, according to the second comparative example, as can be seen
from
the above test results, a decrease in power generated by the fuel cell was
large. Here, it
is advantageous that the relationship that 4)1 is smaller than 4)2 is applied
to the anode
catalyst layer 200 that may be influenced by catalyst poisoning due to carbon
monoxide
being supplied thereto. On the other hand, it is not advantageous that the
structure
similar to that of the anode catalyst layer 200 that is subject to carbon
monoxide is
applied to the cathode catalyst layer 300 that will not be influenced by
catalyst poisoning
because of no carbon monoxide being supplied thereto.
[0093] It is not clear at the present moment why the cathode catalyst layer
300 will
not be influenced by poisoning. However, the first catalyst layer portion 301
and the
second catalyst layer portion 302 are laminated to form the cathode catalyst
layer 300, so
the thickness of the cathode catalyst layer 300 tends to increase. This can
presumably
restrict mobility of active material in the cathode catalyst layer 300.
[0094] Hereinafter, an example of a fuel cell system to which the embodiments
of
31
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
the invention are applied will be described with reference to FIG. 12. The
fuel cell
system includes a reformer 2, a raw fuel supply passage 3, an aqueous raw
material
supply passage 5, and a control unit 6. The reformer 2 generates fuel gas in
such a
manner that raw fuel undergoes reforming reaction. The raw fuel supply passage
3 is
connected to an inlet 2i of the reformer 2. The aqueous raw material supply
passage 5 is
connected to an inlet 2r of the reformer 2. The control unit 6 controls the
raw fuel
supply passage 3 and the aqueous raw material supply passage 5. Thus, the
control unit
6 controls devices equipped for the raw fuel supply passage 3 and devices
equipped for
the aqueous raw material supply passage 5.
[0095] As shown in FIG. 12, the raw fuel supply passage 3 is used to supply
gaseous
raw fuel (hydrocarbon-based raw gas such as natural gas) from a raw fuel
source 38 to
the reformer 2 for reforming raw fuel during operation of the fuel cell
system. The raw
fuel supply passage 3 includes a main passage 30 that connects the raw fuel
source 38
with the inlet 2i of the reformer 2 and a combustion passage 40 provided in
parallel with
the main passage 30. The combustion passage 40 communicates with the raw fuel
source 38 and an inlet 20i of a combustion unit 20. The raw fuel source 38, a
main
valve 33, a desulfurizer 34, a raw gas pump 35, and an inlet valve 37 are
serially provided
in the main passage 30 from the upstream side to the downstream side. The main
valve
33 is formed of two serially arranged valves 33a and 33c. The combustion
passage 40 is
provided with a combustion gas pump 42.
[0096] As shown in FIG. 12, an anode gas passage 7 is provided so as to
connect an
outlet 2p of the reformer 2 with an inlet 70i of a fuel cell stack 70. The
anode gas
passage 7 is used to supply anode gas, reformed in a reforming unit 21, to the
fuel cell
stack 70. The fuel cell stack 70 is supplied with cathode gas from a cathode
gas passage
71, and generates electric energy by power generation reaction. The anode gas
passage
7 includes an outlet valve 72 that opens or closes the side of the outlet 2p
of the reformer
2 and a pressure sensor 73 that detects an internal pressure P1 in the
reformer 2. The
aqueous raw material supply passage 5 is used to supply aqueous raw material
to the
reformer 2 for reforming reaction during operation of the fuel cell system. A
raw
32
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
material water source 50, a raw material water pump 51, a feedwater valve 53,
and an
evaporator 23 are serially arranged in the aqueous raw material supply passage
5 from the
upstream side to the downstream side.
[0097] As shown in FIG. 12, a CO purification passage 8 is provided so as to
be
connected to a CO purification unit 24 of the reformer 2. The CO purification
passage 8
is provided with an air pump 80 and an air valve 82 serially from the upstream
side to the
downstream side. Carbon monoxide (CO) is oxidized by oxygen contained in air
supplied from the CO purification passage 8 to the CO purification unit 24 to
become
carbon dioxide (CO2). In this manner, the CO component contained in reformed
gas is
removed.
[0098] During steady operation of the fuel cell stack 70, while the main valve
33 is
open, the combustion gas pump 42 operates. Thus, gaseous raw fuel is supplied
from
the raw fuel source 38 to the combustion unit 20. By so doing, combustion
reaction
occurs in the combustion unit 20, and the reforming unit 21 and evaporator 23
of the
reformer 2 are heated to a high temperature. Furthermore, while the inlet
valve 37 and
the outlet valve 72 are open, the raw gas pump 35 operates. As a result,
gaseous raw
fuel is supplied from the raw fuel source 38 to the reforming unit 21 of the
reformer 2.
In addition, in the aqueous raw material supply passage 5, while the feedwater
valve 53 is
open, the raw material water pump 51 operates. Thus, an aqueous raw material,
as a
liquid, is supplied to the evaporator 23 via the feedwater valve 53. Because
the
evaporator 23 is heated to a high temperature, an aqueous raw material, as a
liquid, is
heated to become a vapor in the evaporator 23, and the vapor is supplied to
the reforming
unit 21. As a result, in the reforming unit 21, raw fuel undergoes reforming
reaction
utilizing the vapor.
[0099] Hydrogen-rich anode gas is transferred to the CO purification unit 24.
In the
CO purification unit 24, CO contained in the fuel gas is oxidized and removed
from the
fuel gas. The anode gas, from which CO has been removed, is supplied from the
outlet
2p of the reformer 2 via the anode gas passage 7 to the fuel cell stack 70,
and then used
for power generation reaction in the stack 70 together with oxidant gas.
33
CA 02706829 2010-05-26
WO 2009/068958 PCT/IB2008/003205
[0100] As described above, anode gas generated by reforming reaction from raw
fuel
mostly contains CO. The amount of CO is reduced in the CO purification unit
24;
however, the anode gas mostly still contains a small amount of CO. In terms of
this
point, the fuel cells that constitute the stack 70 are formed in accordance
with any one of
the above described embodiments or examples, so measures are taken against CO.
Thus,
even when the duration of service extends over a long period of time, a
decrease in power
generated by the fuel cells is suppressed.
[0101] The invention is not limited to the embodiments or examples described
above; it may be changed appropriately without departing from the scope of the
invention.
The following technical ideas may be derived from the above description.
[0102] The membrane electrode assembly for a fuel cell according to the above
embodiments includes an ion conducting membrane that has two sides in the
thickness
direction; a porous anode catalyst layer that is arranged on one side of the
ion conducting
membrane and that has an anode catalyst that accelerates anode reaction; a
porous
cathode catalyst layer that is arranged on the other side of the ion
conducting membrane
and that has a cathode catalyst that accelerates cathode reaction; an anode
diffusion layer
that is arranged on the outer side of the anode catalyst layer and that allows
an anode
fluid to pass therethrough; and a cathode diffusion layer that is arranged on
the outer side
of the cathode catalyst layer and that allows a cathode fluid to pass
therethrough. The
anode catalyst layer includes a first catalyst layer portion and a second
catalyst layer
portion. The first catalyst layer portion is located closer to the anode
diffusion layer
than the second catalyst layer portion, and the second catalyst layer portion
is located
closer to the ion conducting membrane than the first catalyst layer portion.
Then, for
example, the first catalyst layer portion may be formed by spray coating, and
the second
catalyst layer portion may be formed by applicator coating.
[0103] The invention may be utilized in a fuel cell system, such as a
stationary fuel
cell system, a vehicle fuel cell system, a fuel cell system for an electrical
apparatus, a fuel
cell system for an electronic apparatus or a portable fuel cell system.
34