Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
ARTICLE AND METHOD FOR FOCUSED DELIVERY OF
THERAPEUTIC AND/OR DIAGNOSTIC MATERIALS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This Application claims benefit of and priority to Provisional
Application No.
61/017,815, filed December 31, 2007 and U.S. Patent Application No.
11/578,695,
filed October 17, 2006, both of which are hereby incorporated by reference in
their
entirety.
FIELD
10002] The present disclosure is generally directed to an article and method
for
delivering therapeutic and/or diagnostic materials and more particularly to an
article
and method for focused delivery of pharmaceuticals or other therapeutic
materials
and/or diagnostic materials to humans and other living organisms.
BACKGROUND
[0003] Many diseases, such as cancer, are often pernicious and very
aggressive.
Treatment is often complicated by the fact that some of the most effective
treatment
methods can have a deleterious impact on surrounding healthy tissue and cells.
As a
result, more recent efforts have moved toward therapies which attempt to
target only
unhealthy cells and thereby minimize the impact on healthy cells.
[0004] Hyperthermia is one such approach to cancer therapy. Hyperthermia
associated with radiotherapy or chemotherapy is a method for cancer treatment,
although the molecular mechanisms of this process are not well understood.
Hyperthermia exhibits various anti-tumor effects, including damage of tumor
vasculature.
100051 Cancer cells are more sensitive to higher body temperatures than are
normal
cells. Hyperthermia destroys cancer cells by raising the tumor temperature to
a "high
fever" range, similar to the way the body uses fever naturally when combating
other
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-2-
forms of disease. Because the body's means of dissipating heat is through
cooling
from blood circulation, sluggish or irregular blood flow leaves cancerous
tumor cells
vulnerable to destruction at elevated temperatures that are safe for
surrounding
healthy tissues with normal, efficient blood cooling systems.
[0006] Although not wishing to be bound by theory, scientists attribute the
destruction of cancer cells at hyperthermic temperatures to damage in the
plasma
membrane, the cytoskeleton and the cell nucleus. Cancer cells are vulnerable
to
hyperthermia therapy particularly due to their high acidity caused by the
inability to
properly expel waste created by anaerobic metabolism. Hyperthermia attacks
acidic
cells, disrupting the stability of cellular proteins and killing them.
[0007] Radiofrequency (RF) hyperthermia is a non-ionizing form of radiation
therapy
that can substantially improve results from cancer treatment. For chemotherapy
drugs
that depend on blood transport for delivery, hyperthermia used in combination
with
chemotherapy (thermo-chemotherapy) enhances blood flow in tumor tissues,
increasing the uptake of chemotherapy drugs in tumor membranes. Hyperthermia
also
induces disassembly of the cytoskeleton, which enlarges the tumor pores for
easier
drug entry. Once delivered, hyperthermic temperatures can be used as a drug
activator, accelerating chemical reactions through heat and drawing essential
oxygen
molecules to tumor tissue for chemical reaction with the drug. This technology
can be
designed to optimize those factors that are antagonistic to neoplastic growth.
[0008) Several therapies are associated with non-ionizing RF hyperthermic
therapy.
One is RF ablation where direct radio-stimulation of cancerous tissues creates
a local
intense heat enough to kill neoplastic cells. Another RF approach is to direct
RF at
nanoparticle targets localized in the tumor site. These nanospheres are
affixed with
antibodies to focus the delivery of the nanoparticle to the tumor site that
then becomes
the target of RF stimulation to directly deliver heat to the local tissue.
Still another
approach is to combine the separate actions of chemotherapeutic agents with
tissue
hyperthermia.
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
3-
SUMMARY
[00091 In an embodiment of the present disclosure, a microfiber extrudate
includes a
bio-compatible polymer matrix forming a body of the microfiber extrudate, an
exogenously excitable material arranged within the body, and an active load
arranged
within the body.
100101 In another embodiment of the present disclosure, a discrete exogenously
excitable domain includes an exogenously excitable material. The exogenously
excitable material is configured to be excited by an exogenous stimulus. The
exogenously excitable domain is arranged for positioning in a microfiber
extrudate.
The microfiber extrudate includes a bio-compatible polymer matrix forming a
body of
the microfiber extrudate, the exogenously excitable material in a discrete
domain
within the body.
100111 In another embodiment of the present disclosure, a discrete active load
domain
includes a therapeutic material. The therapeutic material is configured to be
released
into a living organism. The active load domain is arranged for positioning in
a
microfiber extrudate. The microfiber extrudate includes a bio-compatible
polymer
matrix forming a body of the microfiber extrudate, with the active load
arranged as a
discrete domain within the body.
[0012] In another embodiment of the present disclosure, a microfiber extrudate
delivery process includes medically identifying a region for treatment by the
active
load, administering a microfiber extrudate, and applying the exogenous
stimulus to
the region for treatment, thereby releasing an active load into the region for
treatment.
In the embodiment, the microfiber extrudate includes a bio-compatible polymer
matrix forming a body of the microfiber extrudate, an exogenously excitable
material
arranged within the body, and the active load arranged within the body.
100131 In another embodiment of the present disclosure, a microfiber extrudate
includes a bio-compatible polymer matrix forming a body of the microfiber
extrudate,
an exogenously excitable material arranged within the body, and an active load
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-4-
arranged within the body. In the embodiment, the bio-compatible polymer matrix
includes a polymer selected from the group consisting of poly(FAD-SA),
poly(CCP-
SA), poly(FA-SA), poly(EAD-SA), poly glycolide, poly lactic acid, copolymers
thereof, and combinations thereof. The exogenously excitable material
configured to
be excited by an exogenous stimulus is selected from the group of stimuli
consisting
of radiofrequency excitation, microwave excitation, terahertz excitation, mid
infrared
excitation, near infrared excitation, visible excitation, ultraviolet
excitation, x-
irradiation excitation, magnetic excitation, electron beam irradiation
excitation, and
combinations thereof. The active load has therapeutic properties.
[0014] Exemplary embodiments may be used for selectively attacking cancer
cells by
administering a microfiber extrudate having an exogenously excitable material
that
may be excited to selectively attack cancer cells while leaving healthy cells
intact.
[0015] An advantage of the present disclosure includes selectively delivering
a
therapeutic material, which may, for example, be used for selective attack of
cancer
cells.
[0016] Another advantage of the present disclosure includes selectively
delivering a
diagnostic material, which may, for example, be used for identifying cancer
cells.
100171 Yet another advantage of the present disclosure includes the ability to
combine
two components that would otherwise impose compositional difficulties into the
same
structure.
[0018] Still another advantage is the diminished effect of an active
pharmaceutical
ingredient on matrix degradation and diffusion activity.
[0019] Another advantage is controlled diffusion of therapeutic and/or
diagnostic
material in conjunction with the release of material in response to an
exogenous
stimulus.
[0020] Other features and advantages of the present disclosure will be
apparent from
the following more detailed description of the preferred embodiment, taken in
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-5-
conjunction with the accompanying drawings which illustrate, by way of
example, the
principles of the disclosure.
BRIEF DESCRIPTION OF THE DRAWINGS
100211 Figure 1 shows a photograph of an exemplary embodiment of a microfiber
extrudate.
[0022] Figure 2 shows another exemplary embodiment of a microfiber extrudate.
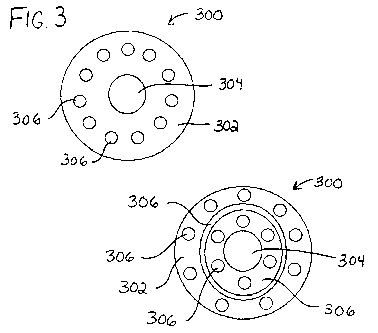
[0023] Figure 3 shows a cross-section of an exemplary embodiment of a
microfiber
extrudate.
[0024] Figure 4 shows a relationship between temperature and microwave dose
exposure time for microcells with differing materials according to several
exemplary
formulae.
[0025) Figure 5 shows a photograph of an exemplary embodiment of a microfiber
extrudate.
[0026] Figure 6 shows a photograph of an exemplary embodiment of a microfiber
extrudate.
[0027] Figure 7 shows plate counts for exemplary microcell formulae in
comparison
to microwave dose exposure time.
[0028] Figure 8 shows a cross-section of another exemplary embodiment of a
microfiber extrudate.
[0029] Figure 9 shows a cross-section of yet another exemplary embodiment of a
microfiber extrudate.
[0030] Wherever possible, the same reference numbers will be used throughout
the
drawings to represent the same parts.
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-6-
DETAILED DESCRIPTION
[0031] Figure 1 illustrates an exemplary embodiment of a microfiber extrudate
100.
The term "microfiber extrudate" as used herein includes microvectors,
microcells,
microspheres, artificial cells, and other suitable devices. Microfiber
extrudate 100
includes a matrix, an exogenously excitable material, and an active load. The
matrix
forms a body 102 of the microfiber extrudate. Body 102 defines the exterior of
microfiber extrudate 100. The body may be, but is not necessarily circular in
cross-
section and may be designed to have a diameter as small as about 5-10
micrometers or
up to about 300 micrometers or larger. As illustrated, body 102 has a diameter
D of
about 100 micrometers. The body may have a transverse thickness as small as
about 5
micrometers or may be elongate or spherical. As illustrated, body 102 has a
transverse
thickness T of about 10 micrometers.
[0032) Figure 2 illustrates another exemplary embodiment of a microfiber
extrudate
200. Here, microfiber 200 is elongate. Microfiber extrudate 200 may be
transversely
sliced along its cross-section to make a plurality of axial slices
substantially the same
as microfiber extrudate 100. Further, the microfiber extrudate may have a
predetermined size and geometry. The design of the microfiber extrudate is
spatially
resolvable, which permits a deliberate placement of active and passive
components
within the microfiber extrudate, as will be discussed in more detail herein.
Feature
size and shape are also controllable, which permits creation of the microfiber
extrudate in actual sizes and geometry that correspond to desired sizes and
geometries. The predetermined size and geometry may be intended to mimic the
size
of a cell. For example, the microfiber extrudate may be configured to have a
size and
geometry similar to a red blood cell or a white blood cell for a specific
animal
(including humans).
[0033] The construction of the micro fiber extrudate may be performed using a
micro-
extrusion fiber spinning process. In this process, a precision engineered die
defines
intended domains as nano-fiber regions that, when combined at the spinning
head,
anneal into one single fiber having any number of deliberately defined
internal
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-7-
domains. This produces a so-called "island-in-the-sea" arrangement of one or
more
different materials (e.g., active loads and/or exogenously excitable material)
as
"islands" within the matrix or "sea" of a base material. Suitable devices and
methods
for co-extruding a filament of different components in a pre-determined
spatial
arrangement are described, for example, in U.S. Patent Nos. 4,640,035;
5,162,074;
5,344,297; 5,466,410; 5,562,930; 5,551,588; and 6,861,142 and in WO
2007/134192,
all of which are herein incorporated by reference.
100341 The micro-extrusion process includes several extruder barrels that
intersect
into a specially designed "die head." Each barrel delivers a single component
for
subsequent combination within the die head. The die head is configured such
that the
matrix, the exogenously excitable materials, and the active load exiting the
multiple
extruder barrels enter a series of pixilated stacked die plates, called a die-
pack. A
unique die-pack may be provided for each different microfiber extrudate
design. The
total pixel bundle exiting the last plate may contain up to 21,000 or more
nano-fibers,
which coalesce at the spin head into a single fiber. Referring to Figures 3
and 5, a
cross-section of a fiber shows the "placement" of domains resulting from the
channel
directed engineering of the die pack plates.
[0035] Once the fibers are produced, they are then bundled into hanks and
prepared
into blocks, such as by using cellulose solutions in water as a potting media,
which
are then frozen. The hanks are so oriented to have all long axis structures
substantially
parallel. The frozen block is preferably mounted in a cryotome such that the
blade
edge cuts perpendicular to the fibers. Multiple transverse cuts at precise
thickness
may be made to produce the structure of microfiber extrudate 100.
[00361 This process allows the co-fabrication of several material components
within
the "design space" of the microfiber extrudate. Microfiber extrudate 100 can
include
three to four material components; more or fewer may be incorporated. The
material
components can be spatially resolved and freely positioned by design within
the body
of the microfiber extrudate. It will be appreciated that the microfiber
extrudate may be
created by co-extruding pure materials for the matrix and each domain, but
more
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-8-
typically, the components of the microfiber extrudate may themselves be a
mixture of
material(s) with the desired properties (for example, the properties of the
exogenously
excitable materials and/or the active load) arranged in discrete domains or as
the
matrix, which may assist in the coextrusion of the materials.
100371 Figures 3, 8, and 9 show cross-sections of exemplary embodiments of a
microfiber extrudate 300. In the embodiments, microfiber extrudate 300 is
formed and
designed to arrange discrete domains 304, 306 of different materials or
combinations
of materials, such as an exogenously excitable material and/or an active load
within a
matrix 302. Each domain can harbor a preferred chemistry for a specific
action. Each
domain may include the exogenously excitable material, the active load, or a
combination of them or other materials. Each domain may also include a certain
percent of matrix material to facilitate excitement or to prevent excitement.
It will be
appreciated the number and location of discrete domains of different materials
is
exemplary and may be modified depending on the application.
[0038] Microfiber extrudate 300 may thus be constructed to include discrete
domains
with approved excipient materials that contain active pharmaceutical
ingredients
(API) or a combination of API and inactive or functional domains within the
microfiber extrudate. Outside of the domains, the microfiber extrudate may
additionally or alternatively include approved excipient materials which
contain AP[,
inactive materials or functional materials, or a combination of API and
inactive or
functional materials. As discussed above, the microfiber extrudate can be
designed to
have a wide range of sizes (e.g., about 5-10 m or up to about 300 m or
larger).
Consequently, a self-contained drug delivery device in accordance with
exemplary
embodiments in the size range of circulatory cells can be provided and
medically
administered intravenously or parenternally.
[00391 In an exemplary embodiment, a region for treatment is identified by
diagnostic
techniques. A microfiber extrudate contains both a therapeutic and an
exogenously
excitable material is administered to the region for treatment (in some cases,
beyond
the region for treatment). An exogenous stimulus is then applied to the region
of
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-9-
treatment (in some cases, beyond the region for treatment), thereby releasing
the
active load into the region for treatment. This process can decrease the
effect on
regions not identified for treatment. In another exemplary embodiment, this
process
increases the number of healthy cells left intact while attacking the
unhealthy cells. In
yet another exemplary embodiment, periodic pulses of the exogenous stimulus
are
applied while the microfiber extrudate is in situ. In administering pain
medication,
this can replace patient activated intravenous systems for administering pain
medicine
by providing the patient with control (or limited control) of a device
configured to
apply the exogenous stimulus. For example, when the patient pushes a button,
the
exogenous stimulus can be activated, thereby causing pain medicine in the
microfiber
extrudate to be released into the patient's body.
100401 The API, which may be the active load, may be any therapeutic material.
Active pharmaceutical ingredients may include, but are not limited to, ABVD,
AVICINE, Acetaminophen, Acridine carboxamide, Actinomycin, Alkylating
antineoplastic agent, 17-N-Allylamino-17-demethoxygeldanamycin, Aminopterin,
Amsacrine, Anthracycline, Antineoplastic, Antineoplaston, Antitumorigenic
herbs, 5-
Azacytidine, Azathioprine, BBR3464, BL22, Biosynthesis of doxorubicin,
Biricodar,
Bleomycin, Bortezomib, Bryostatin, Busulfan, Calyculin, Camptothecin,
Capecitabine, Carboplatin, Chlorambucil, Cisplatin, Cladribine, Clofarabine,
Cyclophosphamide, Cytarabine, Dacarbazine, Dasatinib, Daunorubicin,
Decitabine,
Dichloroacetic acid, Discodermolide, Docetaxel, Doxorubicin, Epirubicin,
Epothilone, Estramustine, Etoposide, Exatecan, Exisulind, Ferruginol,
Floxuridine,
Fludarabine, Fluorouracil, 5-Fluorouricil, Fosfestrol, Fotemustine,
Gemcitabine,
Hydroxyurea, Idarubicin, Ifosfamide, Imiquimod, Irinotecan, Irofulven,
Ixabepilone,
Lapatinib, Lenalidomide, Liposomal daunorubicin, Lurtotecan, Mafosfamide,
Masoprocol, Mechloretharnine, Melphalan, Mercaptopurine, Methotrexate,
Mitomycin, Mitotane, Mitoxantrone, Nelarabine, Nilotinib, Nitrogen mustard,
Oxaliplatin, PAC-1, Paclitaxel, Pawpaw, Pemetrexed, Pentostatin, Pipobroman,
Pixantrone, Polyaspirin, Plicamycin, Procarbazine, Proteasome inhibitor,
Raltitrexed,
Rebeccamycin, SN-38, Salinosporamide A, Satraplatin, Stanford V,
Streptozotocin,
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
- 10-
Swainsonine, Taxane, Tegafur-uracil, Temozolomide, ThioTEPA, Tioguanine,
Topotecan, Trabectedin, Tretinoin, Tris(2-chloroethyl)amine, Troxacitabine,
Uracil
mustard, Valrubicin, Vinblastine, Vincristine, Vinorelbine, Vorinostat,
Zosuquidar,
and combinations thereof.
100411 Other therapeutic materials such as anti-tumor antibodies (including
VEGH-A
or other monoclonal antibodies, for example), antibiotics, bio-agents, bio-
pharmaceuticals and/or other suitable therapeutic materials may be included.
Additionally or alternatively, diagnostic materials, matrix diffusion control
materials,
and/or other suitable materials may be included.
100421 The exogenously excitable material is selected to be excited by an
exogenous
stimulus. The exogenous stimuli include, but are not limited to,
radiofrequency
excitation, microwave excitation, terahertz excitation, mid infrared
excitation, near
infrared excitation, visible excitation, ultraviolet excitation, x-irradiation
excitation,
magnetic excitation, electron beam irradiation excitation, and combinations
thereof.
Upon receiving the exogenous stimulus, the exogenously excitable material can
be
excited. The exogenously excitable material may be arranged within the domains
in
the microfiber extrudate or may be mixed within the microfiber matrix. Various
therapies may combine exogenously excitable materials in the microfiber
extrudate
along with the API.
100431 The microfiber extrudate may include a radiofrequency (RF) sensitive
additive
as the exogenously excitable material and a degradable polymer as a bio-
compatible
matrix that can be administered. The exogenously excitable material may be
exogenously excited in situ at the local site of tumor angiogenesis, such as a
receptor
specific region in advancing vascular tissue binding VEGF to facilitate
localized
heating and thereby denaturing angiogenesis factors and/or destroying abnormal
cells
at the advancing site. Where the API is the active load, the excitation may be
configured to expedite breakdown of the matrix, thus releasing the
pharmaceutical
more quickly. In RF active embodiments, the microfiber matrix may be
formulated
with a known additive having a known radio frequency, lambda max or excitation
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-11-
frequency, which can then be exogenously excited. In another approach, the
natural
RF response of the cell in the absence of a specific radiosensitive additive
is
determined by some spectroscopic mechanism like NMR, and a tunable RF
generator
may be used to administer the exogenous non-ionizing radiation.
[0044] An exemplary embodiment of the microfiber matrix includes a
radiosensitive
active pharmaceutical drug arranged within a polylactide/polyglycolide
copolymer
prepared as one of four extrudable components. A second component includes the
copolymer and an antibody. A third component includes the copolymer and a
chemotherapeutic agent. A fourth material is neat copolymer. In another
exemplary
embodiment, the API is 5-fluorouracil (5-FU), doxorubricin, or acetaminophen.
[0045] The matrix of the microfiber extrudate may be any suitable
thermoplastic
material that is biologically compatible. Generally, suitable bio-compatible
matrix
material generally falls into one of two primary categories, diffusive or
degradable. In
primarily diffusive matrix materials, active load components diffuse from its
initial
domain, through the matrix, and eventually into the environment (e.g.,
bloodstream or
tissue) over time, the rate of which may be enhanced or retarded through
exogenous
stimulation when an exogenously excitable material is also present. The
stimulation
may also modify the diffusive profile to increase the amount transmitted.
Exemplary
diffusive matrix material includes ethyl cellulose polymer, such as that sold
by Dow
Chemical under the tradename Ethocel.
[0046] Degradable material breaks down in body over time, which can be
initiated or
the rate enhanced, by stimulation in the presence of an exogenously excitable
material. Exemplary degradable polymers include poly(FAD-SA), poly(CPP-SA),
poly(FA-SA), poly(EAD-SA), poly glycolide, poly lactic acid, copolymers
thereof,
and combinations thereof. In one embodiment, the microfiber extrudate has a
bio-
compatible polymer matrix including a polyglycolide/lactide copolymer. In an
exemplary embodiment, the matrix is vascular-infusible and bio-compatible
material
that can be administered parenternally or intravenously into a tumor site to
deliver a
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
- 12-
chemotherapeutic agent released over time as the matrix breaks down. Such a
system
may also be coupled with antibody technology.
[00471 Referring again to Figure 3, microfiber extrudate 300 includes a matrix
depicted as a bio-compatible polymer matrix 302 and a first discrete domain
304 at
the core that may contain a suitable bio-active material that may be selected
depending upon the desired therapy. As shown in Figures 3, 8, and 9, the
arrangement
of discrete domains 304, 306 and/or polymer matrix 302 can be varied. Varying
the
arrangement of the discrete domains and/or the polymer matrix can permit
additional
control of diffusion of materials from the microfiber extrudate and/or
degradation of
the matrix. Discrete domains 306 of a second material may include the
exogenously
excitable material depicted as "exogenous activators" demonstrating the
ability to
custom model the microfiber extrudate to include radiosensitive materials.
Referring
to Figure 9, in an exemplary embodiment, microfiber extrudate 300 can be
arranged
for material in discrete domain 304 to travel through polymer matrix 302
and/or
discrete domain 306, thereby permitting staged reactions of materials
traveling
through the various domains. The staged reactions can be controlled by
diffusion
and/or by degradation due to the application of exogenous stimulus. Domains
susceptible to differing exogenous stimuli can permit the reaction to be
further
controlled by providing the differing exogenous stimuli at differing times or
in
differing amounts. In this embodiment, in addition to the timing of the
release of
material from microfiber extrudate 300, the pathway of the reaction can be
controlled.
[00481 Additionally or alternatively, domains 306 may include "immunospecific
targeting agents" which permits the microfiber extrudate to include antibodies
as an
active load. While exemplary embodiments are described with respect to cancer
therapy, it is contemplated that localized delivery of therapeutic materials
in
accordance with exemplary embodiments would be useful in the treatment of
other
diseases, conditions, and disorders by providing different compositions of
therapeutic
materials, by adjusting the microfiber extrudate size, or other modifications,
all of
which are within the scope of the invention.
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-13-
10049] Similarly, the microfiber extrudate may be used for delivering other
materials
into an animal (including humans) for therapeutic and/or diagnostic purposes.
For
example, nutrients, vitamins, toxins, poisons, tracers, and/or other
components may be
included within the domains of the microfiber extrudate to be released upon
excitation
of the exogenously excitable material. In an exemplary embodiment, toxins may
be
administered to canines for the purpose of euthanizing. In another exemplary
embodiment, a harmless dye that is sensitive to gamma radiation may be
administered
for the purpose of monitoring exposure to gamma radiation.
[0050] In an exemplary embodiment, the body of the microfiber extrudate is an
artificial cell-like article for focused therapeutic treatments. One such
focused
therapeutic treatment is hyperthermic cancer therapy for humans or other
animals.
The embodiments combine the feature aspects of focused chemotherapy and RF-
sensitivity into a single cell-like device that approximates the cellular
dimensions of
the circulatory system. The artificial cell approach involves combining drug
delivery
and RF-sensitivity in the microfiber extrudate.
100511 In another exemplary embodiment, the body of the microfiber extrudate
is an
artificial cell the size of a red or white blood cell and includes API that
can degrade
over time. The matrix may be selected to expedite or extend the breakdown of
the
matrix. The matrix may include the exogenously excitable material and, thus,
be
broken down by exogenous stimulus, thereby releasing the API. Placement of the
API
(or another active load) and/or the exogenously excitable material active
loads may be
achieved by using high definition micro-extrusion technology capable of
spatially
resolving local domains within the microfiber extrudate, as described above.
]0052] In a combined system, the microfiber extrudate may deliver a
radiofrequency
sensitive body and a chemotherapeutic drug. The matrix can be eliminated by
resorption following RF excitement. Thus, a single delivery microfiber
extrudate
acting as an artificial cell combines a controlled drug delivery vehicle
(e.g., a red or
white blood cell) based on degradable FDA compliant drug delivery polymers
(such
as but not limited to polyglycolide copolymers), a radiosensitive target
material or a
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-14-
radiosensitive chemotherapeutic agent (such as, but not limited to, a
fluorinated
species), a non-radiosensitive chemotherapeutic agent (such as, but not
limited to, 5-
fluorouracil), and an optional antibody (such as but not limited to anti-VEGH
antibodies) as separate domains of the extrudate. Another embodiment includes
a
system with indigenous acidic properties like those of cancer cells.
100531 In an exemplary embodiment in which acetaminophen is used as the API
active load, the microfiber extrudate can be used for treating melanoma.
Because
acetaminophen is toxic to the liver, by providing an exogenous stimulus to a
region of
the body (instead of the whole body) for localized melanoma treatment, the
amount of
acetaminophen processed by the liver may be lower.
100541 In an exemplary embodiment, a stable, multifunctional microcell acting
as a
nanocarrier can transport superparamagnetic iron oxide non-particles (SPIONs)
and/or
nanoparticle domains for simultaneous diagnostic imaging, hyperthermia or
specific
therapeutic action, a combination of anti-VEFG antibodies and anti-
angiopoietin
factors for targeted disruption of angiogenesis, a chemotherapeutic agent, and
a
microenvironment pH antagonist in a single microfiber extrudate.
100551 In other exemplary embodiments, the microfiber extrudate can be
delivered to
plants and other living organisms. The exemplary embodiments incorporate
ballistic
techniques, such as those commonly employed in genetic transformation of crops
and
other plants for example (e.g., via a gene gun, although the delivery methods
described herein are generally not a genetic transformation process per se),
to
permanently embed microfiber extrudates in plant tissue, which are secured
through
the use of bio-derived adhesions, such as Agrobacterium sp., provided as the
discrete
domains within the matrix.
100561 In yet another exemplary embodiment, the microfiber extrudate can be
treated
to transform its shape and/or geometry. The change in shape and/or geometry
can
include producing a biomimetic delivery system in the natural range of
circulatory
cells, transforming the entire shape and/or geometry of the microfiber
extrudate (for
example, transforming the matrix of the microfiber extrudate), and/or
transforming
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
- 15-
the shape and/or geometry of a portion of the microfiber extrudate (for
example,
transforming the domains in the matrix of the microfiber extrudate). For
example, the
microfiber extrudate can be transformed from a disc-like microfiber extrudate
100 as
shown in Figure I to a sphere-like structure 600 shown in Figure 6. The matrix
of the
microfiber extrudate can be configured for transformation in a 50% ethanol and
50%
water solution or any other suitable solution. Additionally or alternatively
poly
ethylene glycol (PEG) can be used. The matrix of the microfiber extrudate can
be
configured to have increased osmotic potential and may include hypertonic
materials,
for example, salt, that permit the microfiber extrudate to transform or swell
under
selected conditions. The transformation into the sphere-like structure may
increase the
efficacy of a thermally-sensitive active pharmaceutical ingredient.
Unexpectedly, the
sphere-like microfiber extrudate can generally maintain its sphere-like
geometry after
being dried. The sphere-like structure can be configured to transform or swell
with
specific elements of the microfiber extrudate.
100571 The process of converting into the sphere-like structure can permit the
microfiber extrudate to incorporate other materials introduced after
extrusion. For
example, PEG can be used for performing "PEGilation" that brings a suitable
material, for example a nano-particle, into the micro fiber extrudate. To
increase the
ability to incorporate other material into the microfiber extrudate, the
geometry of the
microfiber extrudate may be configured to provide increase surface area and/or
decreased surface area. This may be achieved by modifying the extrusion
process or
by modifying the microfiber extrudate after it is extruded. In an exemplary
embodiment, PEGilation can be used for bringing binding agents, such as
macrophages, into the microfiber extrudate, thereby permitting the binding
agents to
be released through diffusion and/or degradation of the matrix. In another
exemplary
embodiment, PEGilation can used for bringing in material, structures, or nano-
particles that prevent white blood cells from attacking the microfiber
extrudate.
Additionally or alternatively, the microfiber extrudate may be aerosolized.
[00581 In other embodiments, the sphere-like structure can be used as or in
conjunction with insecticides, fertilizers, degradable applications of
controlled release
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-16-
bio-active compounds, taggants, lubricants, sound dampeners, insulators,
and/or other
suitable applications.
EXAMPLES
100591 A multifunctional, polymeric microfiber extrudate having a dual action
payload was created using high definition microextrusion (HDME). An example of
HDME is described in the previously referenced publication WO 2007/134192. The
microfiber extrudate was modeled for substantially simultaneous noninvasive
anti-
tumor hyperthermia and drug release. A 75 micrometer (diameter) by 10
micrometer
(thickness) microfiber extrudate in the form was a microcell was created by
HDME
and included an active load of the active pharmaceutical ingredient
acetaminophen, an
exogenously excitable material of a hyperthermia agent superparamagnetic iron
oxide
non-particle (SPION), and a bio-compatible polymer matrix of ethyl cellulose
drug
delivery polymer (for example, Ethocel ) (Ethocel is a registered trademark
of
Dow Chemical, Co., Midland, MI). The SPION was susceptible to exogenous
excitation from RF,
100601 A second microcell included an active load of acetaminophen as an API,
an
exogenously excitable material of SPION combined with polylactic acid (PLA),
and
several different bio-compatible polymer mixtures. The SPION combined with PLA
was susceptible to exogenous excitation from microwave RF.
[00611 The SPION particle size included in the microcells was 30 micrometers
obtained from Rockwood Co. of St. Louis, MO. The API was USP 99.95%
acetaminophen obtained from Sigma-Aldrich, Milwaukee, WI.
100621 Ethocel was used as a core stable "passive observer" constituent
carrier
matrix in an effort to eliminate any contribution a degrading polymer would
have on
hyperthermia response and drug elution from the matrix. The use of a "passive
observer" isolated the hyperthermia and drug elution from any matrix
contribution
under the influence of RF. Three 75 micrometer by 10 micrometer microcell
device
samples were prepared by HDME: (1) Ethocel and SPION identified as Example 1,
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-17-
(2) Ethocei , SPION, and 10% API identified as Example 2, and (3) neat Ethocel
identified as Example 3 for comparison.
100631 Master batch drug delivery polymer feed stock extrusion pellets were
prepared
for each of the Examples. At extrusion temperatures the melt flow index of the
final
polymer feed stocks was 60 grams per minute. The extrusion temperature was set
at
about 190 C (about 374 F). For Example 2, the final theoretical concentration
of
SPION and API per microcell was determined to be 1.8% by weight and 1.0% by
weight, respectively in an Ethocel matrix showing a loss of 0.8% during
cutting.
However, as a result of processing loss, the API as determined by UV methods,
was
0.85% by weight based on a bulk 500 milligram sampling of microcells.
[00641 The micro-spatial cross-sectional resolution and general cross-section
die
design of the microcells were intended to include selected high concentration
spatially
resolved domains of SPION/polymer adjacent to API/polymer for microwave
induction and local heating within the microcells. The configuration of the
die plate
through which the molten thermoplastic polymer base stock passed during HDME
was produced by photolithography to include about 21,000 individual
nanofibrils
eventually spun to a final total cross-section design of about 75 micrometers
on a
heated Godet role and collected on a high speed fiber bobbin.
100651 Fiber on the bobbins was cut perpendicular to fiber spooling to release
8
inches in length parallel fiber hanks. Hanks were bundled in parallel and
potted in an
aqueous 1% sodium cellulose solution and frozen into about -20 F (about -28.8
C)
aqueous cellulose bricks. The frozen bricks of parallel fiber hanks were then
transversely sliced to about 10 micrometer thickness using a Leika Cryo
microtome
Model CM-3600 operating at about -10 F (about 23.3 C). About 20 grams of each
sample was collected. The slices were collected and sieved to 75 micrometers
final
particle size at room temperature through Retch vertical sieve stacks under
continuous
aqueous flow. The final particles collected were dried under vacuum for about
24
hours.
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-18-
[00661 The microcells were suspended as an aqueous sample while exposed to
microwave RF. Both water and mineral oil were evaluated as a liquid medium to
expose microcell samples. 500 milligram samples of each microcell component
were
dispersed in both water and mineral oil and exposed to 130 watts of microwave
RF
for 60 seconds to determine the preferred fluid medium to carry out the
experiment.
As shown in Table 1, temperatures were recorded and illustrate that water had
the
broadest temperature range response.
Table 1. Isolated Temperature Response of Component Materials
(130 Watts for 60 seconds of exposure)
Dielectric Initial P1 60 AT Thermal
Material Constant OF seconds OF OF Conductivity
lOg Mineral Oil 2.1 68 68 0 0.138 Watts/MK
lOg Deionized Water 80.4 68 98 30 0.600 Watts/M=K
500mg Ethocel 7 in
IOg Water 68 92 24 -
500mg Acetaminophen
in IOg Water 68 90 22 -
500mg Castor Oil in
10g Water 68 90 22 -
500mg SPION in lOg
Water 68 115 47 -
500mg Example 2 in
Water 68 109 41 -
500mg Ethocel 7 in
10 Mineral Oil 68 68 0 -
500mg Acetaminophen
in 10 Mineral Oil 68 68 0 -
500mg Castor Oil in
IOg Mineral Oil 68 68 0 -
500mg SPION in lOg
Mineral Oil 68 75 7 -
500mg Example 2 in
Og Mineral Oil 68 73 5 -
[00671 Surface analysis of samples was performed on a Hitachi Scanning
Electron
Microscope Model S-30ON with IXRF System Energy Dispersive Spectrometer
(EDS). Fiber and microcell slices were examined for the presence of emerging
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-19-
acetaminophen crystals before and after microwave exposure. EDS was used to
define
spatial position of SPION in the polymer matrix of a representative individual
microcell.
[0068] Microwave RF exposure was performed in a Panasonic 1300 Watt Inverter
Technology Microwave Oven Model NN-SN667W at 130 Watts. The oven was
retrofitted with all quartz platforms to reduce/eliminate dipole induction
heating from
standard glass structures. All containment and experimental material other
than water
or albumen in contact with the experimental samples was microwave transparent.
Containment devices such as test tubes, slides, and cover slips were either
quartz or
microwave transparent plastics. Microwave oven use was limited to less than 90
minutes per test period to reduce/prevent incidental heating of the oven
chamber. A
500 milliliter water "reservoir-load" was maintained within the oven chamber
to
absorb microwave energy and prevent magnetron damage. All tests were run in an
environmentally-controlled room at about 65 F (about 18.3 C) and 50% RH.
[00691 Microcell aqueous solution sample temperature increases were recorded
with a
Digi-Tech Digital Thermocouple K-1 probe. The temperature increases showed
microcell dispersion is a function of microwave dose. Infrared thermography
images
of isolated microcells dispersed albumen and aqueous solutions and exposed to
130
Watts microwave radiation were compared and recorded using a FUR S65 HS
infrared camera. Thermographic analysis suggested that SPION filled microcells
responded to microwave RF with a more relevant heating profile for
hyperthermia
therapy than an unfilled microcell in aqueous and albumin solutions. The
thermographic analysis showed a sustained generation of heat after microwave
RF
was discontinued.
100701 Sample vials containing microcells were prepared for microwave
exposure.
First, 10 milliliters of deionized water containing 0.01% Igepal CO-360 non-
ionic
surfactant was placed into a 20 ml scintillation vial. Second, 500 milligrams
of the
microcell sample was added to each vial and dispersed. Duplicate samples for
each
130 Watt interval point were prepared. The samples were vortexed to
uniformity. The
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-20-
samples included a control of aqueous, 500 grams of Example 2 identified
above, and
500 grams of Example 3 identified above. The samples were exposed to 130 Watts
of
microwave radiation for twelve 30 second intervals with 30 seconds of no
exposure
between each interval. Temperatures were recorded in the 30 seconds of no
exposure
using the DigiTec Thermocouple. Samples were averaged and additional thermal
imaging thermographic evaluations were performed as isolated samples using the
FUR infrared camera for microcells dispersed in albumen compared to
water/surfactant.
[00711 Sample vials were prepared in an identical manner and analyzed for API
elution. The elution of API from the microcell samples was studied as a
passive
aqueous diffusion and compared to active microwave induced elution. The active
event was induced by 130 Watts microwave RF for 30 second intervals with 30
seconds of no exposure between each interval for a total of 3 minutes.
Temperatures
were recorded in the 30 seconds of no exposure.
100721 As shown in Figure 4, after 60 seconds of exposure, a differential of
17 F was
recorded between microcells containing SPION compared to microcells without
SPION. SPION containing microcells reached 109 F, whereas microcells without
SPION reached 92 F. The 109 F temperature is within the hyperthermia clinical
range for cell death.
[00731 As shown in Table 2, about 91% of the 24 hour passive API elution level
was
reached in 10 minutes. About 84% of the original 1% acetaminophen API in the
microcell with SPION was retained in the microcell after processing. Microwave
heating of the microcell with SPION may have promoted elution of the API from
the
microcell matrix. The percentages in Table 2, below, show the amount of API
diffused from each sample. Table 2 shows the relative percentage comparison of
microcell API diffusion driven by active microwave RF compared to API elution
by
passive diffusion. The 98 F 24 hour elution exposure was set for comparison.
The
table suggests that the 10 minute microwave RF exposure of microcells may
elute
API within a few percentage of the 24 hour passive diffusion.
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-21-
Table 2. The relative percentage comparison of microcell API elution driven by
active
microwave radiation showing matrix diffusion
Time Relative Percentage based
Temperature/Energy (in minutes) on 24 hours at 98 F
98 F Passive Immediate 11%
98 F Passive 10 25%
98 F Passive 24 100% (standard benchmark)
130 Watts - Active 3 59%
130 Watts - Active 5 59%
130 Watts - Active 10 91%
[0074] UV analysis of elution samples was determined from a standard curve.
Samples were evaluated using a Perkin Elmer UV-Vis Model Lambda 900. Microcell
aqueous samples were exposed to radiotherapy and 98 F (about 36.6 C) to
observe
acetaminophen elution from the delivery polymer matrix and compared to aqueous
samples exposed to microwave RF for 3 minutes, 5 minutes, and 10 minutes for
30
second intervals with 30 seconds of no exposure between each interval.
[0075] Aliquots of aqueous sample were evaluated by UV spectroscopy from a
standard curve analysis. Aliquot samples were drawn for UV scanning. Following
thermal or microwave exposure, samples were centrifuged at 2500 grams for 5
minutes and swept with a stir bar magnet to ensure complete pick-up of
microcells
containing SPION and transferred to a quartz cuvette for analysis.
100761 Normal phase 10 centimeter by 5 centimeter plates (specifically, Biotag
Flash
KP-Sil brand with Indicator TLC plates from VWR) with fluorescent indicator
were
developed in ethyl acetate. Visualization was either under long wave UV (365
nanometer) exposure or iodine crystal vapor staining. Drummond calibrated
micropipettes were used for transfers. Thin layer chromatography was performed
as a
qualitative test on all samples of materials through processing to insure the
integrity
of acetaminophen through the handling of the microcell.
[00771 FT-IR analysis was performed on Smiths Detection FT-IR Model
IlluminatlR Microscope Smiths Detection ATR FT-IR Model IdentifyIR
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-22-
Spectrometer. Microcell and fiber acetaminophen migration was followed by FT-
IR
ATR spectroscopy using a ZnSe crystal or specular reflectance. Because ATR is
a
surface technique, "path length" is virtual and a function of the ATR crystal
refractive
index of the contact pressure. All samples were compressed by the automatic
stage to
ensure full uniform contact at setting 95 (absorbance values are relative to
controls).
Dry samples of master batch pellets, fiber or dry microcell were placed in a
20
milliliter scintillation vial and exposed to radiotherapy, about 98 F (about
36.6 C),
and about 110 F (about 43.3 C) for 96 hours and then surface scanned for the
emergence of acetaminophen bands. Microcell samples were also stimulated as
neat
samples in the microwave for 90 seconds and examined by ATR. Surfaces of the
microcells were analyzed under an SEM for detection of crystals.
100781 Yeast culture Saccharomyces cerevisiae ATCC #9763 (from American Type
Culture Collection, Rockville, MD) was incubated in a VWR Scientific Model
1510E
incubator at about 74 F (about 23.3 C). Cultures were incubated at 74 F (about
23.3 C) following each challenge in the fashion of the standard cell viability
plate
count test. As shown in Figure 7, microcells containing SPION killed yeast
cells with
greater efficiency compared to unfilled co-cultured microcells. Figure 7 shows
plate
counts for Example 1 and Example 3.
[00791 Log phase yeast cultures were prepared in Yeast Peptone Dextrose (YPD)
broth or log phase broth transferred to YPD agar or saline solutions. A
preliminary
evaluation of microwave heating was evaluated for co-culture exposure.
Likewise, a
preliminary serial dilution standard plate count was performed to determine a
workable region. Cultures were diluted to 104 and prepared for microwave
exposure.
Culture sample optical densities were read on a Thermo Scientific Spectronic
20
Series Spectrometer as a reference point to growth. Cells from active cultures
were
harvested and centrifuged. Broth was decanted and cells rewashed four times
with
saline solution. Washed cells were transferred to 0.85% saline solution and
the
solution was adjusted to about 0.20 to 0.25. The microcell-yeast sample co-
exposures
were incubated for 48 hours and plates were counted.
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-23-
[00801 Rabbit/anti-Saccharomyces cerevisiae antibody (from Affinity
BioReagents,
Golden, CO.) was used in the preparation of two sets of samples. Example 1, as
described above, was prepared for analysis with a first sample of the
microcell
without antibody and a second sample with antibody in the microcells. Both
samples
were pretreated to an acid microetch to enhance absorption. About 2 grams of
the
microcells described in Example I were bathed in a solution of 2 milliliters
of 0.01
motes HCL in 100 milliliters of deionized water for 15 seconds followed by 5
times
rinsing in deionized water. Two 500 milligram samples of microetched
microcells
were transferred to 20 milliliter vials.
[00811 10 milliliters of 2% D-+glucose in 10 millimoles HEPES (GH-solution)
were
added to the first sample. 100 microliters of anti-Saccharomyces antibody in
100
milliliters of 2% D-+glucose in 10 millimoles HEPES (anti-GH solution) were
added
to the second sample. Both samples were bathed in solution for two hours at
room
temperature and then centrifuged. The second sample was washed free of
antibody
three times in 0.85% saline, and both samples were vacuum dried for 30 minutes
with
a magnet holder to prevent movement of microcells.
10082] After drying, about 250 milligrams of each sample were transferred into
silica
well slides and 1 milliliter of active culture in YPD broth was added to each
well.
Slides were incubated for 30 minutes at about 74 F (about 23.3 C) and examined
under visible microscopy. Differential absorption of yeast cells to the
untreated and
treated microcells was analyzed by Nikon L-IM digital light microscopy under
blue
light filtration, SEM Hitachi Scanning Electron Microscope Model S-3000N with
IXRF Systems Energy Dispersive Spectrometer, and Western blot (described
below).
100831 In performing Western blot analysis, the primary antibody was diluted
with
PBS to a concentration of 0.1 milligrams per milliliter. 25 microliters of
NuPAGE
LDS Sample Buffer (4X) was added to 75 microliters of diluted antibody. 50
microliters of lX PBS was added to the microcells and vortexed for about 3
minutes.
The solution was then centrifuged at about 14000 rpm for 5 minutes at
radiotherapy.
25 microliters of the supernatant was then added to 25 microliters of NuPAGE
LDS
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-24-
Sample Buffer (4X). After gel loading was completed, the lower buffer chamber
of
the Mini-Cell apparatus was filled with about 600 milliliters of MES Running
Buffer.
The electrodes were aligned on the lid to the gel box and connected to the EPS
power
supply. The gel running parameters for the 12% Bis-Tris Gels were 200 volts
and 12
milliamps for a period of 35 minutes. The blotting parameters were 25 volts
and 160
milliamps for a period of 60 minutes.
[0084] In another example, PLA Microcells were prepared by HDME with and
without SPION. Samples were divided up into two groups. Microcells were placed
in
PBS solution at pH 6.4, 7.0, and 7.4 and incubated at about 115 F (about 46.1
C).
Each group remained in solution throughout their exposure except when samples
were
extracted for FT-IR analysis. Convection oven exposure samples were run first
to
establish baseline microstructural changes. Microwave samples were suspended
from
exposure when their spectral changes matched convection oven microstructural
changes. The first group incubated in a convection oven over time in solution.
Samples of each group were retrieved and examined every 48 hours for a period
of 21
days to compare spectral changes. The second group was incubated in solution
and
periodically exposed to RF pulses of 138 Watts for 1 minute intervals up to 60
minutes. Both the microcell polymer and the isolated solids from the buffered
solution
were examined by FTIR.
100851 Spectra were taken on a Smith's Identifier ATR FT-IR spectrometer with
a
diamond ATR crystal from 4000 cm-1 to 600 cm' at 4 cm -1 resolution and 64
scans.
Data was examined using Gram's software. Samples were air dried for FT-lR
examination. Solid microcell samples were placed directly onto the ATR and the
spectrum was recorded. Solid samples from liquid residue were also recorded.
One
milliliter liquid aliquots were transferred to an aluminum dish and the liquid
dried to a
solid residue. The ATR FT-IR was performed on the aluminum surface. The
examples
and analytical test suggested that SPIONS in combination with PLA accelerated
matrix degradation and that the introduction of RF provided further
accelerated matrix
degradation.
CA 02708514 2010-06-08
WO 2009/088777 PCT/US2008/088176
Attorney Docket No.: 02910-0014
-25-
[0086] While the disclosure has been described with reference to a preferred
embodiment, it will be understood by those skilled in the art that various
changes may
be made and equivalents may be substituted for elements thereof without
departing
from the scope of the disclosure. In addition, many modifications may be made
to
adapt a particular situation or material to the teachings of the disclosure
without
departing from the essential scope thereof. Therefore, it is intended that the
disclosure
not be limited to the particular embodiment disclosed as the best mode
contemplated
for carrying out this disclosure, but that the disclosure will include all
embodiments
falling within the scope of the appended claims.