Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02710642 2011-05-13
30310-65
Crystallization of lodixanol using ultrasound
TECHNICAL FIELD
This invention relates to the manufacture of iodixanol (1,3-bis(acetamido)-
N,N'-bis[3,5-
bis(2,3-dihydroxypropylaminocarbonyl)-2,4,6-triiodophenyl]-2-hydroxypropane),
more
specifically to the purification of iodixanol by crystallization.
BACKGROUND OF THE INVENTION
lodixanol is the non-proprietory name of the chemical drug substance of a non-
ionic X-ray
contrast agent marketed under the trade name VisipaqueTM. VisipaqueTM is one
of the most
used agents in diagnostic X-ray procedures and is manufactured in large
quantities.
The manufacture of such non-ionic X-ray contrast agents involves the
production of the
chemical drug substance (referred to as primary production) followed by
formulation into the
drug product (referred to as secondary production). Primary production of
iodixanol involves
a multi step chemical synthesis and a thorough purification process. For a
commercial drug
product it is important for the primary production to be efficient and
economical and to
provide a drug substance fulfilling the specifications, e.g. as expressed in
the US
Pharmacopeia.
A number of methods are known for the preparation of iodixanol. These are all
multi step
chemical synthetic processes and the cost of the final formulated product thus
mainly
depends on these processes. It is therefore important to optimize the
processes both for
economic and environmental reasons.
Three main chemical synthetic processes are known for the preparation of
iodixanol, all of
which start with 5-nitroisophthalic acid. In the first process described in EP
108638,
1
CA 02710642 2011-05-13
30310-65
the final intermediate 5-acetamido-N,N'-
bis(2,3-dihydroxypropyl)-2,4,6-triiodo-isophthalamide (hereinafter "Compound
A") is reacted
with a dimerisation agent such as epichlorohydrin to yield the drug substance,
see Scheme I.
HO O CHIO 0 OH
1-amino-2.3- HO NH O
CH3OH propanediol
HO \ I CH O OH /
NO2 NO2 HO J NH \ I
O O NO=
O
OH OH
HO NH 0 HO I NH O
Acetic
H2 OH / ICI 1 / anhydride
OH
HO ),, NH \ NH, Ho.. ) . NH \
NHj
O 0
OH OH
HO ,I,, NH O HO ),,k NH 0 0 NH OH
OH OH
Epichloro
OH 1 _ jY n OH HO NNH HO NH N - N NH OH
L OH
O 1 O~ O 1 O 0" ,. 0 O
Comnpoand A lodizanof
Scheme I
The overall yield in this process is relatively low and the purification of
the end product
iodixanol is expensive and time consuming. The purification process described
in EP patent
108638 involves purification by preparative liquid chromatography. The use of
preparative
liquid chromatography is a serious disadvantage in industrial processes in
particular due to
the high costs involved.
Several attempts have been made to find alternative manufacturing processes.
Attempts to
increase the yield of the chemical synthesis is published by Priebe et.al.
(Acta Radiol. 36
(1995), Suppl. 399, 21-31). This. publication describes another route which
avoids the difficult
last step of the process of Scheme 1. However, the route involves eight
reaction steps from
5-nitroisophthalic acid, which is undesirable, and one of the steps includes
chlorination with
2
CA 02710642 2010-07-20
PN0972
thionyl chloride, which is extremely corrosive. Also, the introduction of the
iodine atoms
takes place very early in the sequence, which is disadvantageous as iodine is
the most
expensive reagent in the process. The yield and final purification method for
this route have
not been reported.
The third route to iodixanol involves the synthesis of 5-amino-2,4,6-
triiodoisophthalic acid
(WO 96/37458) and then its dichloride (WO 96/37459), followed by conversion
into
Compound A (US 5705692) and finally dimerisation as in the process of Scheme
I. This
method thus has the same disadvantages as the first process, and also uses an
undesirable
acid chlorination step.
A common system for purification of the crude product in the final step of the
primary
production process, avoiding the liquid chromatography method, has been
purification by
crystallisation. To achieve the desired purity, the crude iodixanol produced
by the synthetic
chemical process is crystallized twice. The process is time consuming and
takes about 3
days for the first crystallization and about 2 days for the second one. Hence,
the
crystallisation process is very demanding in terms of time and equipment size,
it will take
several days to perform and is often a bottleneck in industrial scale
processes.
WO 99/18054 describes a process for the crystallization of i.a. iodixanol
where the
crystallization is effected with high thermal energy, specifically under
elevated pressure and
at a temperature above the boiling point of the solution at atmospheric
pressure.
WO 00/47549 describes a process for the preparation of iodixanol where
unreacted
Compound A is precipitated from the reaction mixture and recovered for reuse
in a later
batch.
It is hence a desire to shorten the crystallization time and also improve the
crystallization
step in order to increase the purity of the final product.
3
CA 02710642 2011-06-06
30310-65
BRIEF DESCRIPTION OF THE DRAWINGS
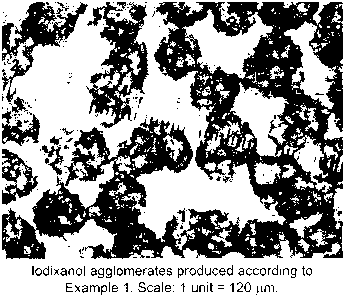
FIG. I shows agglomerates of iodixanol produced in a crystallization step
according to prior
art. Scale: 1 unit = 120 pm.
FIG. 2 shows iodixanol particles produced in a crystallization step according
to the present
invention. Scale: 1 unit = 60 pm.
SUMMARY OF THE INVENTION
The present invention provides improvements to the crystallization of
iodixanol.
Thus viewed from one aspect the invention provides a process for the
purification of a crude
product comprising iodixanol by crystallization, wherein the crystallization
solution is
exposed to ultrasound.
The process according to the present invention reduces the process time for
the
crystallization steps and improves the washing and hence the purity of the
final product.
4
CA 02710642 2012-05-07
30310-65PPH
In one aspect, this invention relates to an improved process for the
purification of a
crude reaction mixture resulting from the dimerization of 5-acetamido-N,N'-
bis(2,3-
dihydroxypropyl)-2,4,6-triiodo-isophtalamide (compound A) comprising
iodixanol, 5-
acetamido-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-isophtalamide (compound
A),
iohexol and impurities having longer retention times than iodixanol in
reversed phase
HPLC, comprising the step of treating said crude reaction mixture with
ultrasound
during the crystallization process of said crude reaction mixture to reduce
the
agglomeration of iodixanol crystals.
DETAILED DESCRIPTION OF THE INVENTION
The processing time for the first crystallization is substantially longer than
for the
second one because of the high concentration of impurities at this stage of
the
process. Due to the slow kinetics, both crystallizations are run at high
initial
supersaturation and during the crystallization process it is formed large
agglomerates, often of more than 100 pm in diameter. These agglomerates are
shown in Figure 1. Agglomeration significantly reduces the available total
surface
area for crystal growth and therefore prolongs the process time to achieve the
desired yield.
It has now surprisingly been found that it is possible to perform
deagglomeration
during the crystallization process by using ultrasound.
4a
CA 02710642 2010-07-20
PN0972
Thus viewed from one aspect, the invention provides a process for the
purification of a crude
product comprising iodixanol by crystallization, wherein the crystallization
solution is
deagglomerated by exposing said solution to ultrasound.
The use of ultrasound will significantly reduce the agglomeration of iodixanol
crystals and by
this reduce the process time for the crystallization steps. In cases where two
crystallization
steps are performed, the use of ultrasound will be able to reduce the process
time from
about three days to less than two days for the first crystallization and from
about two days to
less than 1 day for the second crystallization.
Further, the use of ultrasound in the crystallization step will also improve
the purity of the
final product. The purification process is finalized by filtering the
precipitated iodixanol,
preferably as unagglomerated crystals, from the solvents and finally washing
the crystals
with an alcohol such as methanol. The agglomerates of iodixanol crystals will
also entrap
mother liquor that needs to be removed by washing. By significantly reducing
the
agglomeration and hence the inclusion of mother liquor, a more effective
washing of the
crystals is achieved and also improved purity of the final product.
Figure 1 shows agglomerates produced during a crystallization process
according to prior
art. It can be seen that the agglomerates have a mean size in the area of
about 120 pm.
Figure 2 on the other hand shows particles produced under a process according
to the
present invention, and it can be seen that the particles are single crystals
or very small
agglomerates. The size of these particles is less then about 60 pm.
Any kind of ultrasound probes can be used, as a single probe or a battery of
probes.
The ultrasound probe(s) can be mounted into the crystallizer or in-line to the
crystallizer.
When mounted in-line to the crystallizer the crystallization solution is
circulated between the
crystallizer and the ultrasound probe(s).
The crystallization solution can be continuously exposed to ultrasound
throughout the
crystallization, but normally a certain period at the start of the
crystallization is sufficient to
achieve the desired results.
The crude product referred to in the present invention can be obtained from
the processes
known from the state of the art, e.g. from the dimerisation process
illustrated in Scheme I
5
CA 02710642 2010-07-20
PN0972
above. The dimerisation step itself may be carried out as described in
European patent
108638 and WO 98/23296, for example using epichlorohydrin, 1,3-dichloro-2-
hydroxypropane or 1,3-dibromo - 2-hydroxypropane as the dimerisation agent,
with
epichlorohydrin being most preferred. The reaction is usually carried out in a
solvent such as
2-methoxyethanol, methanol, 1-methoxy-2-propanol or a mixture of 2-
methoxyethanol or 1-
methoxy-2-propanol and water, and generally results in the conversion of 40 to
60% of
Compound A to iodixanol.
Hence, in a second aspect of the invention it is provided a process for the
manufacture of
iodixanol comprising the steps of:
a) reacting 5-acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-
isophthalamide
with a dimerisation agent in the presence of a solvent;
b) purifying the reaction product from step a) in a crystallization procedure;
c) deagglomerating the crystallization solution in the crystallization
procedure by
exposing said solution to ultrasound;
d) filtering and washing the product from step c).
The crude product from the dimerisation and following work-up steps is
preferably in
aqueous solution with small traces of organic solvent. The crude product
normally contains
about 75-90 weight% iodixanol, 3-10 weight% iohexol, 0-7 weight% Compound A,
and also
minor amounts of other impurities. The most important impurities in the
reaction with regard
to work-up consequences are the so-called backpeaks. This term refers to
retention times in
reversed phase HPLC, where the backpeaks have slightly longer retention times
than
iodixanol itself. Most of the backpeaks are either trimers or O-alkylated
dimers. This crude
product is preferably the starting material for the further purification by
crystallization
according to the present invention.
The invention is illustrated further by the following examples that are not to
be construed as
limiting the invention in scope to the specific procedures or products
described in them.
Example 1 shows a conventional crystallization process according to prior art
without the
use of ultrasound. Figure 1 shows agglomerates of iodixanol produced (1 unit =
120 pm).
After 12 hours the process reaches a yield of 2%. From the comparative example
2 showing
the same process with the use of ultrasound, it can be seen that a yield of
25% is reached
after the same period of time. Figure 2 shows iodixanol particles produced (1
unit = 60 pm).
6
CA 02710642 2011-05-13
30310-65
EXAMPLES
EXAMPLE 1
305 g of crude product containing 253 g iodixanol and 17.8 g Compound A, 22.5
g iohexol
and 5.1 g backpeaks was dissolved in a mixture of water and 1-methoxy-2-
propanol (PM) in
a 1 liter vessel equipped with a stirrer (with a magnet driven shaft),
condenser and heating
jacket. The amount of water and PM in the solution was 105 g and 442 g
respectively. The
solution was heated to'reflux at atmospheric pressure and seeded with 2.4 g
seed particles
of iodixanol. The yield of iodixanol at 12 hours after seeding was 2%. Figure
1 shows
iodixanol agglomerates produced.
EXAMPLE 2
305 g of crude product containing 253 g iodixanol and 17.8 g Compound A, 22.5
g iohexol
and 5.1 g backpeaks was dissolved in a mixture of water and 1-mehtoxy-2-
propanol (PM) in
a 1 liter vessel equipped with a stirrer (with a magnet driven shaft),
condenser, ultrasound
probe and heating jacket. The amount of water and PM in the solution was 105 g
and 442 g
respectively. The solution was heated to reflux at atmospheric pressure and
seeded with 2.4
g seed particles of iodixanol. The yield of iodixanol at 12 hours after
seeding was 25%.
Figure 2 shows iodixanol particles produced.
7