Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
MAGNESIUM BASED ALLOY
FIELD OF THE INVENTION
The present invention relates to magnesium
based alloys and, more particularly, to magnesium based
alloys which can be cast by high pressure die casting
(HPDC).
BACKGROUND TO THE INVENTION
With the increasing need to limit fuel
consumption and reduce harmful emissions into the
atmosphere, automobile manufacturers are seeking to
develop more fuel efficient vehicles. Reducing the
overall weight of vehicles is a key to achieving this
goal. Major contributors to the weight of any vehicle are
the engine and other components of the powertrain. The
most significant component of the engine is the cylinder
block, which makes up 20 - 25% of the total engine
weight. In the past significant weight savings were made
by introducing aluminium alloy cylinder blocks to replace
traditional grey iron blocks, and further weight
reductions of the order of 40% could be achieved if a
magnesium alloy that could withstand the temperatures and
stresses generated during engine operation was used.
Development of such an alloy, which combines the desired
elevated temperature mechanical properties with a cost
effective production process, is necessary before viable
magnesium engine block manufacturing can be considered.
HPDC is a highly productive process for mass
production of light alloy components. While the casting
integrity of sand casting and low pressure/gravity
permanent mould castings is generally higher than HPDC,
HPDC is a less expensive technology for higher volume
mass production. HPDC is gaining popularity among
automobile manufacturers in North America and is the
predominant process used for casting aluminium alloy
engine blocks in Europe and Asia. In recent years, the
search for an elevated temperature magnesium alloy has
focused primarily on the HPDC processing route and
several alloys have been developed. HPDC is considered to
SUBSTITUTE SHEET (RULE 26) RO/AU
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
2
be a good option for achieving high productivity rates
and thus reducing the cost of manufacture.
W02006/105594 relates to a magnesium based
alloy consisting of, by weight:
1.5-4.0% rare earth element(s),
0.3- 0.8% zinc,
0.02-0.1% aluminium,
4- 25 ppm beryllium,
0-0.2% zirconium,
0-0.3% manganese,
0-0.5% yttrium,
0-0.1% calcium, and
the remainder being magnesium except for
incidental impurities.
Alloys according to W02006/105594 have
demonstrated excellent high temperature creep properties
but have proven somewhat difficult to die cast. The
present inventors have ascertained that fluidity and hot
tearing resistance during die casting and the oxidation
resistance of the molten alloy is improved by increasing
the proportion of lanthanum in alloys according to
W02006/105594.
Throughout this specification the expression
"rare earth" is to be understood to mean any element or
combination of elements with atomic numbers 57 to 71, ie.
lanthanum (La) to lutetium (Lu).
SUMMARY OF THE INVENTION
In a first aspect, the present invention
provides a magnesium based alloy consisting of, by
weight:
2-5% rare earth elements, wherein the alloy
contains lanthanum and cerium as rare earth elements and
the lanthanum content is greater than the cerium content;
0.2-0.8% zinc;
0.02-0.15% aluminium;
0-0.5% yttrium or gadolinium;
0-0.2% zirconium;
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
3
0-0.3% manganese;
0-0.1% calcium;
0-25 ppm beryllium; and
the remainder being magnesium except for
incidental impurities.
The total lanthanum and cerium content of the
alloy is preferably 1.5-3.5% by weight, more preferably
1.8-3.0%, and most preferably 2.0-2.80. Without wishing
to be bound by theory, the lanthanum and cerium improve
the castability and also the creep strength of the alloy.
Again, without wishing to be bound by theory, a greater
lanthanum content than cerium content further improves
the castability of the alloy, particularly the hot
tearing resistance of the alloy. Higher ratios of
lanthanum to cerium typically give the alloy greater
ductility and even greater resistance to hot tearing.
Typically, a higher total lanthanum and cerium content is
beneficial to the creep resistance of the alloy with a
concomitant reduction in the ductility of the alloy.
The rare earth element content of the alloy may
optionally contain neodymium, in which embodiment the
rare earth element content is predominantly lanthanum,
cerium and neodymium. Without wishing to be bound by
theory, the inclusion of neodymium improves the creep
resistance of the alloy. However, the neodymium content
of the alloy may be reduced to improve the castability of
the alloy, in particular its resistance to hot tearing.
When present, the neodymium content is preferably 0.5-
2.0% by weight of the alloy, more preferably 0.5-1.5% by
weight, more preferably about 1% by weight.
Various of the rare earth elements are
typically derived from a lanthanum misch metal containing
lanthanum, cerium, optionally neodymium, a modest amount
of praseodymium (Pr) and trace amounts of other rare
earths. In another embodiment, the rare earth elements
can be derived from a cerium misch metal, together with
pure lanthanum to provide the greater lanthanum content
relative to the cerium content. For alloys that require a
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
4
low cerium content, the rare earth elements may be
derived from a commercial purity source of lanthanum.
The neodymium may be derived from one or both
of the above misch metals, a pure source of neodymium,
didymium (a neodymium rich neodymium-praseodymium alloy)
or any combination thereof.
Yttrium is an optional component which may be
included. Without wishing to be bound by theory, the
inclusion of yttrium is believed to be beneficial for
both melt protection and creep resistance. However, the
yttrium content of the alloy may be reduced to improve
the castability of the alloy, in particular its
resistance to hot tearing. When present, the yttrium
content is preferably 0.0050-0.5% by weight, more
preferably 0.01-0.4% by weight, more preferably 0.05-0.3%
by weight, and most preferably 0.1-0.2% by weight.
The lanthanum or cerium misch metal from which
the rare earth elements are derived may optionally also
contain yttrium. The yttrium content may thus be derived
from these misch metals. The yttrium content may also be
derived from a pure source of yttrium, a magnesium-
yttrium master alloy or any combination thereof with or
without the misch metals.
Gadolinium is an optional element which may be
included. Without wishing to be bound by theory, the
inclusion of gadolinium is believed to be beneficial to
both creep resistance and the oxidation resistance of the
melt. The gadolinium addition may be made instead of an
yttrium addition. The gadolinium addition may however be
made in combination with an yttrium addition. When
present, the gadolinium content is preferably 0.005%-0.5%
by weight, more preferably 0.01-0.4% by weight, more
preferably 0.05-0.3% by weight, and most preferably 0.1-
0.2% by weight.
Preferably, alloys according to the present
invention contain at least 94.0% magnesium, more
preferably 95-96% magnesium, and most preferably about
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
95.3-95.726 magnesium.
The zinc content is 0.2- 0.8o by weight,
preferably 0.2-0.6a, more preferably about 0.40.
The aluminium content is preferably 0.05-0.150
5 by weight, more preferably 0.08-0.120 by weight, more
preferably about 0.1o by weight. Without wishing to be
bound by theory, the inclusion of these small amounts of
aluminium in the alloys of the present invention is
believed to improve the creep properties of the alloys.
The beryllium content is 0-25 ppm. When
present, the beryllium content is preferably 4-20 ppm,
more preferably 4-15 ppm, more preferably 6-13 ppm, such
as 8-12 ppm although beryllium is preferably absent when
yttrium is present as yttrium has a similar effect to
beryllium at low yttrium levels. When present, beryllium
would typically be introduced by way of an aluminium-
beryllium master alloy, such as an Al-5a Be alloy.
Without wishing to be bound by theory, the inclusion of
beryllium is believed to improve the die castability of
the alloy. Again, without wishing to be bound by theory,
the inclusion of beryllium is also believed to improve
the oxidation resistance of the molten alloy and in
particular improves the retention of the rare earth
element(s) in the alloys against oxidation losses.
Reduction in iron content can be achieved by
addition of zirconium which precipitates iron from the
molten alloy. Accordingly, the zirconium contents
specified herein are residual zirconium contents.
However, it is to be noted that zirconium may be
incorporated at two different stages. Firstly, on
manufacture of the alloy and secondly, following
remelting of the alloy prior to casting. Preferably, the
zirconium content will be the minimum amount required to
achieve satisfactory iron removal. Typically, the
zirconium content will be less than 0.10.
Manganese is an optional component of the
alloy. When present, the manganese content will
typically be about 0.10.
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
6
Calcium (Ca) is an optional component which may
be included, especially in circumstances where adequate
melt protection through cover gas atmosphere control is
not possible. This is particularly the case when the
casting process does not involve a closed system.
Ideally, the incidental impurity content is
zero but it is to be appreciated that this is essentially
impossible. Accordingly, it is preferred that the
incidental impurity content is less than 0.15%, more
preferably less than 0.1%, more preferably less than
0.010, and still more preferably less than 0.001%.
In a second aspect, the present invention
provides an engine block for an internal combustion
engine produced by high pressure die casting an alloy
according to the first aspect of the present invention.
In a third aspect, the present invention
provides a component of an automotive powertrain formed
from an alloy according to the first aspect of the
present invention.
The component of the powertrain may be the
engine block or a portion of an engine such as a cover,
sump or brackets.
The component of the powertrain may be the
transmission housing or another transmission component.
Specific reference is made above to powertrains
but it is to be noted that alloys of the present
invention may find use in other elevated temperature
applications as well as in low temperature applications.
Specific reference is also made above to HPDC but it is
to be noted that alloys of the present invention may be
cast by techniques other than HPDC including
thixomoulding, thixocasting, permanent mould casting and
sand casting.
In a fourth aspect, the present invention
provides an article formed from an alloy according to the
first aspect of the present invention.
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
7
EXAMPLES
Example 1
A high-Nd variant die casting alloy has a
composition:
1.8 wt.% Nd
0.7 wt.% Ce
0.4 wt.% La
0.6 wt.% Zn
balance Mg
This alloy was removed from a proprietary cover
gas protection known as AM-cover by immersing a cylinder
with a 10mm diameter hole in the bottom. Dry air at 2
1/min was introduced to the top of the cylinder. The base
of the cylinder was immersed into the molten alloy to a
depth of 50mm and the condition of the surface of the
melt was observed.
For this high-Nd alloy, the new molten surface
turned black almost instantly and blooms of flaming
magnesium occurred shortly afterwards.
The addition of 53ppm of yttrium via a 43%
yttrium-57% magnesium master alloy to the melt
dramatically changed the oxidation behaviour of the melt.
When the cylinder was inserted into the melt, the melt
surface stayed bright and shiny for 50 seconds before
spot burning was initiated. For an addition of 250ppm
yttrium, the resistance to the onset of burning was also
excellent.
A similar effect is also experienced with the
addition of gadolinium to the melt instead of yttrium. A
gadolinium addition of 310ppm was sufficient to delay the
onset of spot burning in the cylinder test for 60 seconds
but is not as efficient as yttrium for this purpose.
Higher lanthanum variants of the alloy have
been observed to behave in a different manner to the
high-Nd variants. Test work was conducted on the
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
8
oxidation behaviour of a high-La variant of the alloy
containing:
1.6 wt.% La
0.9 wt.% Nd
1.1 wt.% Ce
0.6 wt.% Zn
balance Mg
The aforementioned cylinder test was again
used. In removing the melt from the protective atmosphere
and into dry air, the alloy remained bright and shiny
with no sign of oxidation or burning after 40 seconds.
This alloy had a similar melt protection behaviour to the
high-Nd variant of the alloy with the addition of 50-
100ppm of yttrium. Yttrium addition to this high-La
version of the alloy is not required for melt protection
purposes.
Example 2
Ten alloys were prepared and chemical analyses
of the alloys are set out in Table 1 below. The rare
earths were added as a cerium-based misch metal (which
contained cerium, lanthanum and some neodymium) and
elemental lanthanum and neodymium. The yttrium and zinc
were added in their elemental forms. The beryllium was
added as an aluminium-beryllium master alloy. The
aluminium was added as this master alloy supplemented
with elemental aluminium or where beryllium was not
added, as elemental aluminium alone. The zirconium was
added through a proprietary Mg-Zr master alloy known as
AM-cast. The balance of the alloys was magnesium except
for incidental impurities. Standard melt handling
procedures were used throughout preparation of the
alloys.
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
9
wt.. wt.% wt.% o wt.% ppm wt.% ppm wt.. Zr
Alloy Nd Ce La wt.o Y Zn Be Al Fe (total)
A 1.47 0.49 1.71 <0.005 0.59 <1 0.008 7 0.097
B 1.50 0.50 1.73 0.052 0.61 <1 0.008 8 0.080
C 1.35 0.47 1.70 0.037 0.60 <1 0.030 6 0.052
D 1.34 0.46 1.73 0.033 0.61 <1 0.055 5 0.040
E 1.33 0.46 1.73 0.027 0.61 <1 0.10 3 0.018
F 1.38 0.47 1.73 0.016 0.61 <1 0.59 7 0.018
G 0.88 1.13 1.87 <0.01 0.41 4 0.07 13 NA
H 0.84 1.13 1.84 0.23 0.46 12 0.05 19 NA
1 1.62 0.66 0.37 <0.005 0.50 2 0.02 12 NA
J 1.69 0.28 0.68 <0.005 0.43 3 0.05 22 NA
Table 1 - Alloys Prepared (NA: not analysed)
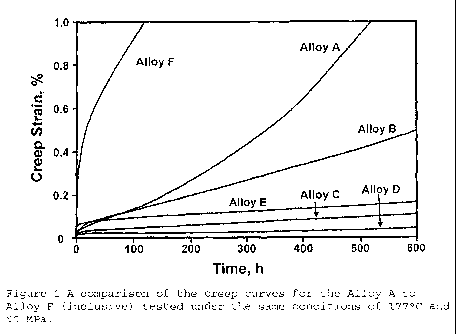
Figure 1 shows the creep results for 177 C and 90MPa for
Alloys A, B, C, D, E and F. This set of creep curves
illustrates the dramatic effect that compositional
variations had on creep performance in alloys of the
present invention. The control alloy (Alloy A) displayed
a relatively poor creep resistance under the imposed test
conditions, entering into tertiary creep quite early in
the test (<50 hours) and ending with 1.3% creep strain
when the test was terminated at 600 hours. This was
consistent with previous results for other alloy variants
that contained no Al/Be addition for melt protection.
With the addition of yttrium (-0.05 wt.%) the
creep response improved substantially (Alloy B). Although
both Alloy A and Alloy B reached 0.1% creep strain at
approximately the same time, 62 hours and 60 hours
respectively, the onset of tertiary creep was delayed
until much later in the test for Alloy B.
The addition of a small amount of aluminium
(-0.03 wt.%) produced a significant improvement in the
creep response (Alloy C). This alloy did not reach 0.10
creep strain under the imposed test conditions until -500
hours and did not appear to have gone into tertiary creep
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
up to the time of the termination of the test (600 h).
With an additional amount of aluminium (-0.06 wt.%) a
further improvement in the creep properties was observed
(Alloy D), which did not reach 0.1o creep strain at all
5 during the duration of the test (0.04% creep strain after
600 hours). With a further increase in the aluminium
content (Alloy E, -0.1 wt.%) the creep resistance began
to decline (0.1% creep strain in -190 hours), although
this was still considered to be relatively good. Finally,
10 with a significant increase in the aluminium content
(Alloy F, -0.6 wt.%) the creep response of the alloy
deteriorated totally. Alloy F was considered to have
very poor creep resistance under the imposed test
conditions. These results confirm that aluminium is an
important micro-alloying addition in obtaining excellent
creep properties.
Figure 2 shows the creep results for 177 C and
90MPa for Alloys G and H. Both Alloys G and H had delayed
tertiary creep to beyond the duration of the test. The
creep resistance of Alloy H, as shown in Figure 2,
compared favourably to Alloy X prepared in accordance
with W02006/105594 and having a composition by weight of:
0.68% zinc,
1.89% neodymium,
0.56% cerium,
0.33% lanthanum,
<0.005% yttrium,
0.05% aluminium,
<5 ppm iron
12 ppm beryllium
with the balance magnesium except for
incidental impurities.
Tensile properties were measured in accordance
with ASTM E8 at 20 and 177 C in air using an Instron
Universal Testing Machine. Samples were held at
temperature for 10 minutes prior to testing. The test
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
11
specimens had a circular cross section (5.6mm diameter),
with a gauge length of 25mm.
Tensile test results for various samples of the
alloys are set out in Table 2.
Table 2 - Tensile Test Data
20 C 177 C
Alloy 0.20 0 0.20 0
Proof, UTS, MPa Elong. Proof, UTS, MPa Elong.
MPa MPa
A 166.8 175.6 1.3 129.1 158.7 6.6 +
1.6 0.6 0.5 6.2 11.8 2.8
B 165.2 171.7 1.4 125.5 153.4 5.3
3.1 4.8 0.5 4.1 8.1 1.5
C 160.4 171.7 1.5 t 124.2 150.0 --- 5.4
5.8 7.7 0.5 2.1 0.6 0.7
D 158.5 175.4 1.8 123.4 143.0 t 3.9
4.5 2.9 0.6 3.2 5.2 0.5
E 150.8 170.0 1.5 121.3 145.2 4.8
1.9 5.5 0.6 3.6 5.8 1.8
F 140.0 173.4 1.7 106.1 130.9 3.7 ~-
1.4 4.7 0.6 1.5 3.4 0.9
G 175.5 183.7 2.4 118.7 151.8 6.7
2.6 4.1 0.9 1.3 2.6 0.8
H 176.2 179.3 2.0 132.4 167.6 7.5
1.6 1.8 0.3 1.8 3.8 1.1
It is noted that Alloy G and Alloy H in
particular both had very good castability. The processing
window for which sound castings can be obtained is much
wider for these two alloys than for Alloy X referred to
above. For good casting quality an alloy requires a low
susceptibility to hot tearing, good die filling
characteristics and reduced susceptibility to the
formation of defects at the intersection of flow fronts
in the die.
A castability test die was developed to assess
the castability of a wide range of alloys in high
pressure die casting (HPDC). Castings from the die are
shown in Figure 3. The die was designed to have a complex
shape such that it would be extremely difficult to
produce good quality high pressure die castings using
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
12
this die. Figure 3(a) shows the channels of a three-part
gating system on the right hand side of the casting
(known in the art as "runners") through which the molten
alloy flows into the die. The "overflows" can be seen on
the opposing side (the left hand side) of the casting to
the runners. The overflows and runners are broken off
after casting.
The castability test die was used to produce a
casting of Alloy H. The as-cast surface quality of this
casting of Alloy H is shown in Figure 3(b).
Example 3
Alloys I, J and H (see Table 1, Example 2) were
cast by high pressure die casting using the castability
test die referred to above in Example 2 to study the
effect of lanthanum and cerium on the castability of the
alloy.
Figure 4 shows the internal defect structure of
the same section of the castings of (a) Alloy I, (b)
Alloy J and (c) Alloy H. Alloy I (0.66% wt cerium, 0.370
wt lanthanum) was found to have a large amount of
internal cracking after casting. By changing the
lanthanum to cerium ratio to greater than 1:1 in Alloy J
(0.68% wt lanthanum, 0.28% wt cerium) the amount of
internal cracking can'be seen in Figure 4(b) to have been
reduced and the overall quality of the casting improved.
Further improvement in the castability was found for
Alloy H which has a greater total lanthanum and cerium
content (1.7% wt lanthanum, 1.1o wt cerium) as well as a
ratio of lanthanum to cerium above 1:1 and a reduced
neodymium content (0.7% wt neodymium compared to 1.62% wt
neodymium in Alloy I and 1.69% wt in Alloy J). Almost no
internal cracking was observed for the casting of Alloy
H. It can also be seen in Figure 4(c) that Alloy H has a
good resistance to the formation of internal flow defects
and hot tearing.
Without wishing to be bound by theory, the
probable reason for this second observation can be
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
13
explained with reference to Figure 5 which shows the
temperature versus fraction solid curves for Alloys I and
H based on Gulliver-Scheil model calculations using the
phase diagrams of magnesium with each of the individual
rare earth elements assuming complete mixing within the
alloy. Alloy H, which has a higher lanthanum content
than Alloy I can be seen to have a shorter freezing
range. This is known to reduce the susceptibility of the
alloy to hot tearing. Alloy H also has an increased
amount of eutectic over Alloy I. This is evidenced by the
last part of solidification of the Alloys which is
occurring at the same temperature. For Alloy H this
occurs for a greater fraction of the alloy and thus for a
longer period of time as compared to Alloy I. This
further reduces the susceptibility of Alloy H to hot
tearing. It is noted that lanthanum is more efficient
than cerium in changing the solidification
characteristics to reduce the alloy's susceptibility to
hot tearing. This is because for alloys with the same
total cerium plus lanthanum contents, the eutectic
proportion is greater in solidifying lanthanum-rich
alloys and the eutectic temperature is also higher.
Again, without wishing to be bound by theory, a
reduction in flow lines when high pressure die casting
using Alloy H as compared to Alloy I is also likely to be
responsible for the reduction in internal cracking in
Alloy H. Flow lines are formed during HPDC where flows of
molten alloy from runners into the die meet the flow of
other runners. Oxidation of the alloy occurs on the
surfaces of these flows which meet to form the visible
flow lines of oxidised alloy within the casting. Without
wishing to be bound by theory, it is believed that the
higher yttrium content in Alloy H is responsible for this
effect as this improves the recovery rate of beryllium
from the master alloy addition and also influences the
beryllium's oxidation rate from the molten alloy.
Figure 6 illustrates the improved surface
appearance of HPDC castings from (a) Alloy I and (b)
CA 02711753 2010-07-08
WO 2009/086585 PCT/AU2008/001902
14
Alloy H, with the higher lanthanum and beryllium content
alloy (Alloy H) having a much improved surface
appearance.
Example 4
Five further alloys were prepared to study the
effects of the neodymium addition. The alloys were
prepared in accordance with the procedures describe above
in Example 2. Table 3 below provide the chemical analysis
of these further alloys (K-P).
Table 3 Alloys Prepared
Alloy wt.% wt.% wt.% wt.% wt. 06 ppm wt.% ppm wt.% Zr
Nd Ce La Y Zn Be Al Fe (total)
K 0.01 0.52 1.49 0.05 0.41 <1 0.05 73 0.0
L 0.22 0.84 1.80 0.01 0.41 <1 0.023 108 0.0
M 0.45 0.53 1.52 0.03 0.41 <1 0.05 86 0.0
N 0.73 0.46 1.42 0.02 0.42 <1 0.04 107 0.0
P 0.93 0.39 1.42 0.04 0.42 <1 0.032 121 0.0
Figure 7 shows the creep results for Alloy K to Alloy P
at 177 C and 90 MPa. It can be seen from Figure 7 that
the creep response improves with an increase in the
neodymium content of the alloy (refer to Table 3). Alloy
K, Alloy M, Alloy N and Alloy P also have very similar
compositions in all the other alloying elements except
for the neodymium content. The curves indicate that the
neodymium content in the alloy should be greater than
about 0.5 wt.% in order to obtain a creep response that
is suitable for elevated temperature applications.