Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
METHOD FOR TREATING SUBTERRANEAN FORMATION
WITH DEGRADABLE MATERIAL
FIELD OF THE INVENTION
100011 The present invention relates to the art of treating subterranean
formations
and more particularly, to a method of delivering a fluid treatment composition
with
base mixture and a degradable material into a formation for low temperature
application. The invention is particularly applicable to methods of delivering
low
viscosity viscoelastic surfactant compositions that are capable of
transporting large
size proppants but break cleanly without the need for pre flushes or post
flushes.
BACKGROUND
[0002] Hydraulic fracturing of subterranean formations has long been
established
as an effective means to stimulate the production of hydrocarbon fluids from a
wellbore. In hydraulic fracturing, a well stimulation fluid (generally
referred to as a
fracturing fluid) is injected into and through a wellbore and against the
surface of a
subterranean formation penetrated by the wellbore at a pressure at least
sufficient to
create a fracture in the formation. Usually a "pad fluid" is injected first to
create the
fracture and then a fracturing fluid, often bearing granular propping agents,
is injected
at a pressure and rate sufficient to extend the fracture from the wellbore
deeper into
the formation. If a proppant is employed, the goal is generally to create a
proppant
filled zone from the tip of the fracture back to the wellbore. In any event,
the
hydraulically induced fracture is more permeable than the formation and it
acts as a
pathway or conduit for the hydrocarbon fluids in the formation to flow to the
wellbore
and then to the surface where they are collected.
100031 The fluids used as fracturing fluids have also been varied, but many
if not
most are aqueous based fluids that have been "viscosified" or thickened by the
addition of a natural or synthetic polymer (crosslinked or uncrosslinked) or a
viscoelastic surfactant (YES). The carrier fluid is usually water or a brine
(e.g., dilute
aqueous solutions of sodium chloride and/or potassium chloride).
1
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
[0004] The viscosifying
polymer is typically a solvatable (or hydratable)
polysaccharide, such as a galactomannan gum, a glycomannan gum, or a cellulose
derivative. Examples of such
polymers include guar, hydroxypropyl guar,
earboxymethyl guar, carboxymethylhydroxyethyl guar, hydroxyethyl cellulose,
carboxymethylhydroxyethyl cellulose, hydroxypropyl cellulose, xanthan,
polyacrylamides and other synthetic polymers. Of these, guar, hydroxypropyl
guar
and carboxymethylhydroxypropyl guar are typically preferred because of
commercial
availability and cost performance.
[0005] In many
instances, if not most, the viscosifying polymer is crosslinked
with a suitable crosslinking agent. The crosslinked polymer has an even higher
viscosity and is even more effective at carrying proppant into the fractured
formation.
The borate ion has been used extensively as a crosslinking agent, typically in
high pH
fluids, for guar, guar derivatives and other galactomannans. Other
crosslinking agents
include, for example, titanium, chromium, iron, aluminum, and zirconium.
[0006] Viscoelastic
surfactant fluids are normally made by mixing into the carrier
fluid appropriate amounts of suitable surfactants such as anionic, cationic,
nonionic
and zwitterionic surfactants. The viscosity of viscoelastic surfactant fluids
is
attributed to the three dimensional structure formed by the components in the
fluids.
When the concentration of viscoelastic surfactants significantly exceeds a
critical
concentration, surfactant molecules aggregate into micelles, which can become
highly
entangled to form a network exhibiting elastic behavior.
[0007] Viscoelastic
surfactant solutions are usually formed by the addition of
certain reagents to concentrated solutions of surfactants, frequently
consisting of long-
chain quaternary ammonium salts such as cetyltrimethylammonium bromide (CTAB).
Common reagents that generate viscoelasticity in the surfactant solutions are
salts
such as ammonium chloride, potassium chloride, sodium salicylate and sodium
isocyanate and non-ionic organic molecules such as chloroform. The electrolyte
content of surfactant solutions is also an important control on their
viscoelastic
behavior.
[0008] During hydraulic
fracturing treatments, control of fracture height growth
can be an important issue. In situations where the water table is close to the
fracturing
zone, or where the fracture zones have low stress barriers, where fracture
height
2
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
growth can result in screen outs, control of the fracture height may be
critical. A
common technique for the control of fracture height control is to use fluids
with lower
viscosity, such as YES surfactants. Lower viscosity fluids however, do not
transport
the large sized proppants effectively in the fracture.
[0009] One method of
addressing the issue has been the incorporation of fiber into
the surfactant fluids. However, the breaking of fiber and of fiber bearing YES
fracturing fluid can be still be problematic especially without pre or post
flushes.
Polylactic acid (PLA) fibers have been shown to degrade into soluble materials
under
temperature and with time. However, all applications are limited to
temperatures
above 82 C based on the rate of degradation. At temperatures below 82 C, PLA
fibers degrade too slowly to be useful for those oilfield applications. It
would be
helpful to have a YES fluid which would transport the large sized proppants
effectively and still break under low temperature conditions (below 82 C, for
example
50 C or 60 C), leaving little or no residue solids in the fracture.
SUMMARY
100101 In one embodiment, the invention provides a method for treating a
subterranean formation penetrated by a wellbore which comprises providing a
treatment fluid comprising a viscoelastic surfactant having at least one
degradable
linkage, a hydrolysable material, and a pH control material, wherein the pH
control
material has a pH equal or greater than about 9 and comprises a strongly
alkaline
material and an oxidizing agent; and injecting into the subterranean formation
the
treatment fluid.
100111 In another embodiment, the invention provides a method for treating a
subterranean formation penetrated by a wellbore which comprises injecting into
the
subterranean formation a treatment fluid made of a viscoelastic surfactant
having at
least one degradable linkage, a hydrolysable material and a pH control
material,
wherein the pH control material is an amine additive.
100121 In some embodiments, the hydrolysable material is a hydrolysable fiber
for
example selected from the group consisting of polyesters, polyamides, and
polylactides. The hydrolysable fiber and the viscoelastic surfactant may form
non-
solid products upon hydrolysis.
3
[0013] In some embodiments, the oxidizing agent and/or strongly alkaline
material
and/or the amine additive may be encapsulated.
[0014] In another embodiment, the strongly alkaline material has a pH of at
least
about 11. The strongly alkaline material may be selected from the group
consisting of metal
hydroxide, metal oxide, calcium hydroxide, metal carbonates, and metal
bicarbonates. The
metal hydroxide can be NaOH, Ca(OH)2, Mg(OH)2 or KOH and the metal oxide can
be CaO,
MgO or ZnO. The oxidizing agent may be persulfate ammonium or calcium
peroxide. The
amine additive may be selected from the group consisting of urea,
dimethylolurea,1,1-diethylurea,1,1,3,3-tetramethylurea,1,3-diethylurea,
hydroxyurea,1,3-diallylurea, ethylurea,1,1 -dimethylurea.4-
dimethylaminopyridine (DMAP)
and 1,8-diazabicylo(5.4.0)undec-7-ene (DBU). The amine additive may further
have a salt,
for example potassium carbonate.
[0014a] In another embodiment, the invention provides a method for treating
a
subterranean formation penetrated by a wellbore comprising: providing a
treatment fluid
comprising a viscoelastic surfactant having at least one degradable linkage, a
hydrolysable
material, and a pH control material, wherein the pH control material comprises
at least one
metal oxide and an oxidizing agent selected from the group consisting of
persulfate
ammonium and calcium peroxide; injecting the treatment fluid into the
subterranean
formation , wherein the subterranean formation has a temperature between about
50 C and
about 66 C; and maintaining the subterranean formation at said temperature for
a period of
time, wherein the at least one metal oxide and the oxidizing agent act to
break the
hydrolysable material after the period of time.
[0014b] In another embodiment, the invention provides a method for treating
a
subterranean formation penetrated by a wellbore which comprises injecting into
the
subterranean formation a treatment fluid comprising a viscoelastic surfactant
having at least
one degradable linkage, a hydrolysable material and a pH control material,
wherein the pH
control material comprises an amine additive selected from the group
consisting of urea,
dimethylolurea,1,1-diethylurea,1,1,3,3-tetramethylurea,1,3-diethylurea,
hydroxyurea.1,3-diallylurea, and ethylurea,1,1-dimethylurea, at least one
metal oxide and an
oxidizing agent selected from the group consisting of persulfate ammonium and
calcium
4
CA 2714802 2017-12-05
peroxide, wherein the at least one metal oxide and the oxidizing agent act to
break the
hydrolysable material after a period of time.
[0015] Unless otherwise specifically stated, all percentages herein are
percentages by
weight.
BRIEF DESCRIPTION OF THE DRAWINGS
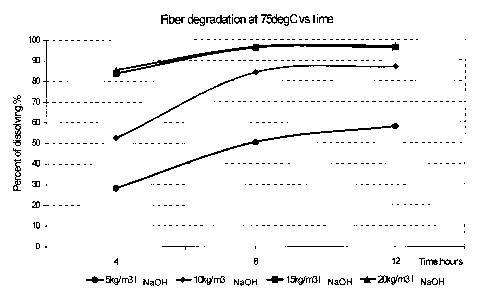
[0016] Figure 1 is a graph plotting fiber dissolution over time in hours at
75 C.
[0017] Figure 2 is a graph plotting PLA fiber degradation after 18h30min at
50 C.
[0018] Figure 3 is a graph plotting PLA fiber degradation after 4h at 50 C.
[0019] Figure 4 is a graph plotting PLA fiber degradation with different
oxidizing
agents at 50 C.
[0020] Figure 5 is a graph plotting conductivity of Fores 12/18 proppants
with fiber and
different amount of sodium hydroxide.
[0021] Figure 6 is a graph plotting degradation of fibers with various urea
additives and
amine additives at 66 C and 100 C.
[0022] Figure 7 is a graph plotting degradation of fibers as a function of
DBU concentration at 50 C and 66 C.
[0023] Figure 8 is a graph plotting degradation of fibers with DMAP as a
function of
DBU concentration at 50 C and 66 C,
4a
CA 2714802 2017-12-05
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
[0024] Figure 9 is a graph plotting degradation of fibers with DMAP at 50 C
for 58
hours.
[0025] Figure 10 is a graph plotting degradation of fibers with DMAP at 66 C
for 21
hours.
[0026] Figure 11 is a graph plotting degradation of fibers with DBU at 50 C
for 68
hours.
[0027] Figure 12 is a graph plotting degradation of fibers with DBU at 66 C
for 21
hours.
[0028] Figure 13 is a graph plotting degradation of fibers with 1,1-
diethylurea at 50 C
for 5 hours.
[0029] Figure 14 is a graph plotting degradation of fibers with 1,1-
diethylurea at 66 C
for 21 hours.
DETAILED DESCRIPTION
[0030] At the outset, it should be noted that in the development of any such
actual
embodiment, numerous implementation-specific decisions must be made to achieve
the developer's specific goals, such as compliance with system related and
business
related constraints, which will vary from one implementation to another.
Moreover, it
will be appreciated that such a development effort might be complex and time
consuming but would nevertheless be a routine undertaking for those of
ordinary skill
in the art having the benefit of this disclosure. The description and examples
are
presented solely for the purpose of illustrating the preferred embodiments of
the
invention and should not be construed as a limitation to the scope and
applicability of
the invention. While the compositions of the present invention are described
herein as
comprising certain materials, it should be understood that the composition
could
optionally comprise two or more chemically different materials. In addition,
the
composition can also comprise some components other than the ones already
cited.
[0031] In the summary of the invention and this description, each numerical
value
should be read once as modified by the term "about" (unless already expressly
so
modified), and then read again as not so modified unless otherwise indicated
in
context. Also, in the summary of the invention and this detailed description,
it should
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
be understood that a concentration range listed or described as being useful,
suitable,
or the like, is intended that any and every concentration within the range,
including
the end points, is to be considered as having been stated. For example, "a
range of
from 1 to 10" is to be read as indicating each and every possible number along
the
continuum between about 1 and about 10. Thus, even if specific data points
within
the range, or even no data points within the range, are explicitly identified
or refer to
only a few specific data points, it is to be understood that inventors
appreciate and
understand that any and all data points within the range are to be considered
to have
been specified, and that inventors have disclosed and enabled the entire range
and all
points within the range.
[0032] A first embodiment is an oilfield treatment method including providing
a fluid
viscosified with a viscoelastic surfactant, including a degradable material
and a base
mixture for hydrolysis of PLA at low temperature.
[0033] According to some embodiments, degradable material is a degradable
fiber or
degradable particle. For example, degradable fibers or particles made of
degradable
polymers are used. The differing molecular structures of the degradable
materials that
are suitable for the present embodiments give a wide range of possibilities
regarding
regulating the degradation rate of the degradable material. The degradability
of a
polymer depends at least in part on its backbone structure. One of the more
common
structural characteristics is the presence of hydrolyzable and/or oxidizable
linkages in
the backbone. The rates of degradation of, for example, polyesters, are
dependent on
the type of repeat unit, composition, sequence, length, molecular geometry,
molecular
weight, morphology (e.g., crystallinity, size of spherulites, and
orientation),
hydrophilicity, surface area, and additives. Also, the environment to which
the
polymer is subjected may affect how the polymer degrades, e.g., temperature,
presence of moisture, oxygen, microorganisms, enzymes, pH, and the like. One
of
ordinary skill in the art, with the benefit of this disclosure, will be able
to determine
what the optimum polymer would be for a given application considering the
characteristics of the polymer utilized and the environment to which it will
be
subjected.
[0034] Suitable examples of polymers that may be used in accordance with the
embodiments herewith include, but are not limited to, homopolymers, random
aliphatic polyester copolymers, block aliphatic polyester copolymers, star
aliphatic
6
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
polyester copolymers, or hyperbranched aliphatic polyester copolymers. Such
suitable
polymers may be prepared by polycondensation reactions, ring-opening
polymerizations, free radical polymerizations, anionic polymerizations,
carbocationic
polymerizations, coordinative ring-opening polymerization for, such as,
lactones, and
any other suitable process. Specific examples of suitable polymers include
polysaccharides such as dextran or cellulose; chitins; chitosans; proteins;
aliphatic
polyesters; poly(lactides); poly(glycolides); poly(e-caprolactones);
poly(hydroxy ester
ethers); poly(hydroxybutyrates); polyanhydrides; polycarbonates;
poly(orthoesters);
poly(acetals); poly(acrylates); poly(alkylacrylates); poly(amino acids);
poly(ethylene
oxide); poly ether esters; polyester amides; polyamides; polyphosphazenes; and
copolymers or blends thereof. Other degradable polymers that are subject to
hydrolytic degradation also may be suitable. Of these suitable polymers,
aliphatic
polyesters are preferred. Of the suitable aliphatic polyesters, polyesters of
a or 13
hydroxy acids are preferred. Poly(lactide) is most preferred. Poly(lactide) is
synthesized either from lactic acid by a condensation reaction or more
commonly by
ring-opening polymerization of cyclic lactide monomer. The lactide monomer
exists
generally in three different forms: two stereoisomers L-and D-lactide; and D,L-
lactide
(meso-lactide). The chirality of the lactide units provides a means to adjust,
inter alia,
degradation rates, as well as the physical and mechanical properties after the
lactide is
polymerized. Poly(L-lactide), for instance, is a semicrystalline polymer with
a
relatively slow hydrolysis rate. This could be desirable in applications where
slow
degradation of the degradable material is desired. Poly(D,L-lactide) is an
amorphous
polymer with a much faster hydrolysis rate. This may be suitable for other
applications. The stereoisomers of lactic acid may be used individually or
combined
for use in the compositions and methods of the present embodiments.
Additionally,
they may be copolymerized with, for example, glycolide or other monomers like
s-
caprolactone, 1,5-dioxepan-2-one, trimethylene carbonate, or other suitable
monomers to obtain polymers with different properties or degradation times.
Additionally, the lactic acid stereoisomers can be modified by blending high
and low
molecular weight polylactide or by blending polylactide with other aliphatic
polyesters. For example, the degradation rate of polylactic acid may be
affected by
blending, for example, high and low molecular weight polylactides; mixtures of
polylactide and lactide monomer; or by blending polylactide with other
aliphatic
polyesters.
7
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
[0035] One guideline for choosing which composite particles to use in a
particular
application is what degradation products will result. Another guideline is the
conditions surrounding a particular application. In choosing the appropriate
degradable material, one should consider the degradation products that will
result. For
instance, some may form an acid upon degradation, and the presence of the acid
may
be undesirable; others may form degradation products that would be insoluble,
and
these may be undesirable. Moreover, these degradation products should not
adversely
affect other operations or components.
[0036] The physical properties of degradable polymers may depend on several
factors
such as the composition of the repeat units, flexibility of the chain,
presence of polar
groups, molecular mass, degree of branching, crystallinity, orientation, etc.
For
example, short chain branches reduce the degree of crystallinity of polymers
while
long chain branches lower the melt viscosity and impart, inter alia,
extensional
viscosity with tension-stiffening behavior. The properties of the material
utilized can
be further tailored by blending, and copolymerizing it with another polymer,
or by a
change in the macromolecular architecture (e.g., hyper-branched polymers, star-
shaped, or dendrimers, etc.). The properties of any such suitable degradable
polymers
(such as hydrophilicity, rate of biodegration, etc.) can be tailored by
introducing
functional groups along the polymer chains. One of ordinary skill in the art,
with the
benefit of this disclosure, will be able to determine the appropriate
functional groups
to introduce to the polymer chains to achieve the desired effect.
[0037] In one embodiment, the method employs degradable fiber when exposed to
high pH conditions for a period of time. Examples of such fibers include, but
are not
limited to polyesters, polyamides, polylactides and the like.
[0038] In one embodiment, the method employs polylactic acid, which undergoes
a
hydrolysis to form a liquid when exposed to a high pH environment as shown in
the
following reaction scheme:
8
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
CH3 CH3 CH3
Ki
\ 0 n H20 0 m
Solid Solid 0 Solid 0
H201 K1
OH CH3
K2
nix
ri2v
H3C
0 0
Liquid
Lactic Acid K2> K1
Scheme 1: Hydrolysis reaction of polylactic fibers
100391 In order to provide a pH environment suitable for the hydrolysis of the
fiber to
occur at low temperature (as low as 40 C and up to 85 C), a base mixture is
used. The
base mixture can be a pH control agent.
[0040] Useful pH control agents will vary with the specific degradable fiber
selected
for use, but generally may include those agents which are strongly alkaline
materials
that may provide a high pH environment. Generally, pH control agents having a
pH of
9 or more are considered to be strongly alkaline materials. Examples of such
strongly
alkaline materials include, but are not limited to, metal hydroxides, metal
oxides,
calcium hydroxide, metal carbonates or bicarbonates, and the like. For
example, the
strong alkaline material can be CaO, Ca(OH)2, MgO as well as liquid additives
such
NaOH and KOH.
[0041] The pH control agent may also contain oxidizing agents such as
(NH4)2S204
and Ca02. The oxidizing agents were found to increase rate of fiber
degradation when
used in conjunction with metal oxide.
[0042] The pH control agent may also contain amines base additives such as
urea and
its derivatives, as well as nucleophilic amines such as 4-
dimethylaminopyridine
(DMAP) and 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU). In one embodiment, the pH
control agent may also contain a combination of amines with potassium
carbonate
(K2CO3).
9
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
[0043] In a first embodiment, the pH control agent is made of a strongly
alkaline
material and an oxidizing agent. In a second embodiment, the pH control agent
is
made of an amine additive. The amine additive can be an amine base and/or a
nucleophilic amine. In one embodiment, the amine additive may also be a amine
and a
salt. In a third embodiment, the pH control agent is made of a strongly
alkaline
material, an oxidizing agent and an amine additive.
[0044] The amount of pH control agent required to provide hydrolysis at low
temperature will vary with the particular control agent selected and with the
system,
but generally, the pH control agent may comprise from about 0.5 weight percent
to
about 15 weight percent of the treatment fluid. In one embodiment, the fluid
may
contain from about 1 weight percent to about 10 weight percent. In another
embodiment, the fluid may contain about 3 weight percent to about 10 weight
percent.
In yet another embodiment, the fluid may contain from about 3 weight percent
to
about 7 weight percent.
[0045] When fluids are viscosified by the addition of viscoelastic surfactant
systems,
the viscosity increase is believed to be due to the formation of micelles, for
example
worm-like micelles, which entangle to give structure to the fluid that leads
to the
viscosity. In addition to the viscosity itself, an important aspect of a
fluid's properties
is the degree of viscosity-recovery or re-healing when the fluid is subjected
to high
shear and the shear is then reduced. For VES fluids, shear may disrupt the
micelle
structure, after which the structure reforms. Controlling the degree of
reassembling
(re-healing) is necessary to maximize performance of the surfactant system for
different applications. For example, in hydraulic fracturing it is critical
for the fluid to
regain viscosity as quickly as possible after exiting the high-shear region in
the
tubulars and entering the low-shear environment in the hydraulic fracture. On
the
other hand, it is beneficial in coiled tubing cleanouts to impart a slight
delay in
regaining full viscosity in order to "jet" the solids more efficiently from
the bottom of
the wellbore into the annulus. Once in the annulus the regained viscosity
ensures that
the solids are effectively transported to the surface. Controlling the
viscosity-
recovery and the time required for such recovery is therefore desirable.
[0046] Many viscoelastic surfactants may be used in this application.
Surfactants with
a degradable linkage in the molecule will hydrolyse to separate the
hydrophilic head
CA 2714802 2017-03-13
54138-15
and the hydrophobic tail. While not wishing to be bound by theory, it is
believed that
such separation will degrade the mic.ellea formed by the VBS surfactant.
10047] Exemplary cationic viseoe. logic surfhcouds include the amine salts and
quaternary amine salts disclosed in U.S. Patent Nos. -5,979,551, and 6,435,277
which
have a common Assignee as the present application,
100451 In one embodiment, the viacoelastic surfactant has an amide linkage In
the
head group, according to the scheme
0
Ncei0
11 11 11!
=
MA XX Examples of suitable cationic visonelastio suriktants include cationic-
,
surfactants having the structure:
R]N(R2)(R3)(R4) X-
in which RI has from about 14 to about 26 carbon atoms and may be branched or
straight chained, nomad; saturated or unsaturated., end may contain a
carbonyl, an
amid; a rettolunide, an Imide, or an amine; R2, Ita, and R4 are each
independently
" hydrogen or a C1 to about C6 aliphatic group which may be the seine or
different,
iv:inched or straight chained, saturated or unsaturated and one or more than
one Of
which may he substituted with a group that renders the R2, R3, and lt4 group
more
hydrophilic; the R2, R3 and 124 groups may be incorporated into a heterocyclic
5- or
6-member ring structure which includes the nitrogen atom; the 112, R3 and R4
groups
may he the same or different; and r iv an anion. Matures of such compounds are
also suitable. As a further example, Iti is fi-om about 18 to about 22 carbon
afralP
and may contain a carbonyl, an amide, or an amine, and it2, 113, and R4 are
the same
as one another and contain from 1 to about 3 carbon atoms. Cationic
surfactants
11
CA 2714802 2017-03-13
54138-15
having the structure RIN+(112)(R3)(R4) X- may optionally contain ainines
having the
structure 1414(R2)(R3). It is well known that commercially available cationic
quaternary amine stufactants often ordain the eorresponcling amines cut which
Rt.
R2, and R3 in the oationia =Octant and In the amine have the. same struoture).
As
received commercially available VIES surfactant concentrate formulations. for
example cationio YES surfactant formulations, may also optionally contain one
or
more menthol's of the group consisting of solvents, mutual solvents, organics
acids,
organic acid salts, inorganic salts, and ollgomem, polymers, co-polymers, and
mixtures of these members. They may also contain performance enhancers, such
as=
viscosity enhancers, for example polysulfonates, for example polysulfbnio
acids, as
described in copending 118. Patent Application Publication No. 2003-0134751
which
has a common Assignee as the present application.
Paso] Another suitable cationic VE8 is eruoyl his(2-bydroxyethyl) methyl
ammonium chloride, (fElvalAC), also known as (Z)-13 docosanyl-N-N- bin C2-
hydroxyetkyl) methyl ammonhmt nhloride. It is commonly obtained from
manufacturers as &mixture containing about 60 weight percent surfactant in a
mixture
of iso-propand, ethylene glycol and water. In this patent, when we refer to
"RIMAC" we mean such a solution. Other suitable amine salts and quatamary
amine
salts include (either alone or in combination), eruoyl trlmethyl anunorlium'
chloride;
N-methyl.N.N-bis(2.-hydroxyethyl) rapeseed ammonium chloride; oley1 methyl
bis(bydroxyethyl) ammonium chloride; entoylamidopropyittimethylamine chloride,
octadcoyi methyl bis(hydroxyathyl) ammonium bromide; octadeoyi
tris(hydroxyathyl)
ammonium bromide; oetadecyl dimethyl hydroxyethyl ammonium bromide; cetyl
dimethyl hydroxyethyl auunonlum bromide; coil methyl bis(hydroxyathyl)
ammonium salicylate; cetyl methyl bis(hydroxyethyi) ammonium 3,4õ-
dichlorobenzoate; cetyl iris(hydroxyethyl) ammonium iodide; cosyl diroethyl
hydroxyethyl ammonium bromide; cosyl methyl bis(hydroxyethyl) ammonium
chloride; oosyl tris(hydroxyethyl) ronmonium bromide; dicosyl dimethyl
hydroxyethyl ammonium bromide; dicosyl methyl his(hydroxyethyl) ammonium
chloride; dicosyl tris(hydroxyethyl) ammonium bromide; hexadecyl ethyl
12
CA 2714802 2017-03-13
54138-15
bis(hydroxyethyl) ammonium chloride; hexadeoyl isopropyl his(hydroxyethyl)
artunonium iodide; and cetylamino, N-octadeayi pyridinitnn chloride.
100S11 Zwitterionlc viscoalastie Reactants are also suitable. lixemplary
zwitterionlo
viscoelastic surtketants include those &earthed in U.S. Patent No. 6,703.352
which
has a common Assignee as the present application.
Exemplary zwittcrionic surfactants have the structure:
0 R2
=
In which III Is a hydrocarbyl group that may be branched or straight chained,
aromatio, aliphatic or olefulle and contains fkom about 14 to about 26 carbon
atoms
and may include an amine; 11.2 Is hydrogen or an alkyl group having from Ito
about 4
carbon atoms: 113 is a hydrocarbyl group having from 1 to about 5 carbon
atoms; and
Is an electron withdrawing group. More particularly, the zwitterlonic
surfactant
may have the betalne struoture;
Iji H3C CH3 0
\ . I
N,
In which. 11 is a hydrocarbyl group that may be branched or strafes chained,
aromatic,
aliphatic or triclinic and has from about 14 to about 26 carbon atoms and may
contain
an amine; n about 2 to about 4; and p Ito about 5. Mixtures of these compounds
may also be used.
ppm Two examples of suitable betaines are, respectively, BBT-0-30 and BET-S-
40, The YES stntmtant in BET-0-30 is oleylamidopropyi betaIne. It Is
designated
BET-0-30 here, because as obtained from the supplier (Rhoda, be. Crankily, NeW
Jersey, U. S. A.) it is called Noggin!) BET-0-30; it contains an ley' acid
amide
= group (including a C171133 alkene tail group) and is supplied as about
30% active
13
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
surfactant; the remainder is substantially water, sodium chloride, glycerol
and
propane-1,2-diol. An analogous suitable material, BET-E-40, was used in the
experiments described below; one chemical name is erucylamidopropyl betaine.
BET-E-40 is also available from Rhodia; it contains a erucic acid amide group
(including a C21H41 alkene tail group) and is supplied as about 40% active
ingredient,
with the remainder substantially water, sodium chloride, and iso-propanol. BET
surfactants, and others that are suitable, are described in U. S. Patent No.
6,703,352.
[0053] Certain co-surfactants may be useful in extending the brine tolerance,
to
increase the gel strength, to reduce the shear rehealing time, and/or to
reduce the shear
sensitivity of zwitterionic VES fluid systems, such as betaine VES fluids. An
example given in U. S. Patent No. 6,703,352 is sodium dodecylbenzene sulfonate
(SDBS). Another example is polynaphthalene sulfonate. Zwitterionic VES
surfactants may be used with or without this type of co-surfactant, for
example those
having a SDBS-like structure having a saturated or unsaturated, branched or
straight-
chained C6 to C16 chain; further examples of this type of co-surfactant are
those
having a saturated or unsaturated, branched or straight-chained C8 to C16
chain. Other
suitable examples of this type of co-surfactant, especially for BET-0-30, are
certain
chelating agents such as trisodium hydroxyethylethylenediamine triacetate.
Many
suitable additives are known for improving the performance of gelled VES
surfactant
systems; any may be used; they should be tested for compatibility with the
compositions and methods of the present embodiments before use; simple
laboratory
experiments for such testing are well known.
[0054] Zwitterionic surfactant viscoelastic systems typically contain one or
more
members of the group consisting of organic acids, organic acid salts,
inorganic salts,
and oligomers, polymers, co-polymers, and mixtures of these members. This
member
is typically present in only a minor amount and need not be present at all.
The
organic acid is typically a sulfonic acid or a carboxylic acid and the anionic
counter-
ion of the organic acid salts are typically sulfonates or carboxylates.
Representative
of such organic molecules include various aromatic sulfonates and carboxylates
such
as p-toluene sulfonate, naphthalene sulfonate, chlorobenzoic acid, salicylic
acid,
phthalic acid and the like, where such counter-ions are water-soluble. Most
preferred
are salicylate, phthalate, p-toluene sulfonate, hydroxynaphthalene
carboxylates, e.g. 5-
hydroxy-l-naphtho ic acid, 6-hydroxy-1-naphthoic acid, 7-hydroxy- 1 -naphthoic
acid,
14
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
1-hydroxy-2-naphthoic acid, preferably 3-hydroxy-2-naphthoic acid, 5-hydroxy-2-
naphthoic acid, 7-hydroxy-2-naphthoic acid, and 1, 3-dihydroxy-2-naphthoic
acid and
3, 4-dichlorobenzoate. The organic acid or salt thereof typically aids the
development
of increased viscosity that is characteristic of preferred fluids. The organic
acid or
salt thereof is typically present in the zwitterionic viscoelastic fluid
(after the
viscoelastic surfactant has concentrated sufficiently to viscosify the fluid)
at a weight
concentration of from about 0.1% to about 10%, more typically from about 0.1%
to
about 7%, and even more typically from about 0.1% to about 6%.
[0055] Inorganic salts that are particularly suitable for use in the
zwitterionic
viscoelastic fluid include water-soluble potassium, sodium, and ammonium
salts, such
as potassium chloride and ammonium chloride. Additionally, calcium chloride,
calcium bromide and zinc halide salts may also be used. The inorganic salts
may aid
in the development of increased viscosity which is characteristic of preferred
fluids.
Further, the inorganic salt may assist in maintaining the stability of a
geologic
formation to which the fluid is exposed. Formation stability and in particular
clay
stability (by inhibiting hydration of the clay) is achieved at a concentration
level of a
few percent by weight. The inorganic salt is typically present in the
zwitterionic
viscoelastic fluid (after the viscoelastic surfactant has concentrated
sufficiently to
viscosify the fluid) at a weight concentration of from about 0.1% to about
30%, more
typically from about 0.1% to about 10%, and even more typically from about
0.1% to
about 8%. Organic salts, e.g. trimethylammonium hydrochloride and
tetramethylammonium chloride, may also be used in addition to, or as a
replacement
for, the inorganic salts. Optionally, these systems may be formed in dense
brines,
including brines containing polyvalent cations.
[0056] As an alternative to the organic salts and inorganic salts, or as a
partial
substitute therefore, one can use a medium to long chain alcohol (preferably
an
alkanol), preferably having five to ten carbon atoms, or an alcohol ethoxylate
(preferably an alkanol ethoxylate) preferably of a 12 to 16 carbon alcohol and
having
1 to 6, preferably 1-4, oxyethylene units.
[0057] Amphoteric viscoelastic surfactants are also suitable. Exemplary
amphoteric
viscoelastic surfactants include those described in U.S. Patent No. 6,703,352,
for
example amine oxides. One useful amine oxide surfactant has the formula:
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
R2
R3
wherein R1, R2, and R3 are independently selected from alkyl, alkenyl,
arylalkyl, or
hydroxyalkyl groups wherein each of said alkyl groups contain from about 8 to
about
24 carbon atoms and may be branched or straight chained and saturated or
unsaturated
[0058] Mixtures of zwitterionic surfactants and amphoteric surfactants are
also
suitable. An example, called BET-E-40/A0 here, is a mixture of about 13% iso-
propanol, about 5% 1-butanol, about 15% ethylene glycol monobutyl ether, about
4%
sodium chloride, about 30% water, about 30% cocamidopropyl betaine, and about
2%
cocamidopropylamine oxide.
[0059] The fluid may be used, for example in oilfield treatments. As examples,
the
fluid may be used as a pad fluid and as a carrier fluid in hydraulic
fracturing, as a
carrier fluid for lost circulation control agents, and as a carrier fluid for
gravel
packing.
[0060] The optimal concentration of a given rheology enhancing additive for a
given
choice of VES surfactant fluid system at a given concentration and
temperature, and
with given other materials present, can be determined by simple experiments.
The
total viscoelastic surfactant concentration must be sufficient to form a
viscoelastic gel
under conditions at which the surfactants have sufficient aggregation
tendency. The
appropriate amounts of surfactant and rheology enhancer are those necessary to
achieve the desired viscosity and shear recovery time as determined by
experiment.
In general, the amount of surfactant (as active ingredient) is from about 1 to
about 10
%. Commercially available surfactant concentrates may contain some materials
that
we have found may be used as rheology enhancers, for example for concentrate
freezing point depression, but normally the amount of such material is not
sufficient,
when the concentrate is diluted, in the final fluid. The amount of rheology
enhancer
used, in addition to any that may be already present in the as-received
surfactant
concentrate, is from about 0.1 to about 6%, for example from about 0.25 to
about
3.5%, most particularly from about 0.25 to about 1.75%. Mixtures of
surfactants
and/or mixtures of rheology enhancers may be used.
16
CA 02714802 2010-09-15
= Attorney Docket Number 56.1327
EXAMPLES
[ 0061 ] The present embodiments can be further understood from
the
following examples:
[0062] Figure 1 shows the results of PLA fiber (3.6kg/m3) degradation in
polymer
solution with different amount of NaOH added, at temperature of 75 C. Table 1
shows the results of fiber degradation in fluids at 60 C prepared with
3.6kg/m3 PLA
fiber and different amounts of CaO and Ca(OH)2. As can be seen, higher
concentration of CaO and Ca(OH)2 resulted in faster degradation of PLA fibers.
Chemical Concentration, g/L Degradation time,
days
CaO 1 > 10 days
Ca0 3 7
CaO 5 2
Ca(OH)2 1 > 10 days
Ca(OH)2 3 7
Ca(OH)2 5 2
Table 1
[0063] Additives such as CaO or Ca(OH)2 can either be made large mesh size or
encapsulated to avoid fast dissolution in fracturing fluids during pumping and
flowback (dissolution constant for Ca(OH)2 is 6x10-6 m013/L3). Figure 2
demonstrates
PLA fiber degradation at low temperature (bottles heated from room temperature
to
50 C) in the presence of Ca(OH)2 (fine powder and encapsulated in paraffin
(hydroxide:paraffin ratio is 1:1)). Also, degradation in the presence of
encapsulated
Ca(OH)2 with hexane (oil mimic) is shown.
[0064] The use of pre-heated sample at 50 C, Ca(OH)2 slurry showed even higher
degradation rates (Figure 3). Also, positive influences of peroxide type
oxidizers on
the degradation rate were also observed.
[0065] Figure 4 shows that MgO did not have significant impact on PLA
degradation
at 50 C. However, when mixed with oxidizing agents such as ammonium persulfate
and calcium peroxide, MgO significantly increases the rate of degradation of
PLA.
Interestingly, addition of the non-generating oxidizer sodium bromate to MgO
did not
have any impact on PLA degradation at the same temperature. It is expected
that the
amount of fiber degraded will have a significant impact on the fracture
conductivity
as shown in Figure 5.
17
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
[0066] A series of amines derivatives were also evaluated in order to increase
the
degradation rate of the polymer. Nucleophilic bases were chosen and in
particular
amines bases. Amongst them, urea and its derivatives were assessed. Indeed,
urea and
its derivatives self-decompose and liberate ammonia which reacts rapidly with
ester
bonds, leading to amide terminated oligomers. The nucleophilic attack of the
amine
together with a high pH environment accelerates the degradation rate of the
fibers as
shown in Figure 6. In all of the experiments a loading of fibers of 1kg/m3 of
fluid was
used.
[0067] Some derivatives such as 4-dimethylaminopyridine (DMAP) and 1,8-
Diazabicyclo[5.4.0]undec-7-ene (DBU) accelerate significantly the degradation
rate at
66 C. Further experiments were then carried out to further study the action of
DMAP
and DBU on the degradation of fibers at low temperatures. Figure 7, shows the
influence of DBU (with various concentrations) on the degradation of the
fibers at
50 C and 66 C. It appears that a concentration of 0.5mol/L gives full
degradation at
66 C after 21h. Much higher concentrations are required to achieve full
degradation at
50 C.
[0068] The results obtained with the addition of DMAP are presented in Figure
8. As
compared to DBU, DMAP accelerates more significantly the degradation at low
temperatures. A concentration of 0.2mo1/L to 0.5mo1/L of DMAP gives
significant
degradation of the fibers. The combination of these derivatives with the
presence of a
base such as K2CO3 was also investigated and results are shown on Figures 9
and 10.
[0069] Figure 9 and 10 show the influence of the presence of various amounts
of
K2CO3 together with DMAP (at 0.01M). The experiments were performed at 66 C
for
21h and 50 C for 68h. Results show that there is an increase of degradation of
the
fibers when using the combination of the two products. K2CO3 is a base which
helps
maintaining a high pH environment. As the degradation of the polymer occurs,
lactic
acid is generated which decreases the overall pH of the solution. In acidic
environment protonation of the amine can occurs which will then limit its
activity on
the degradation process. Therefore keeping the pH alkaline is a much better
option to
insure faster degradation.
[0070] Similar results were obtained with DBU and 1,1-diethyl urea as shown in
Figures 11, 12, 13 and 14.
18
CA 02714802 2010-09-15
Attorney Docket Number 56.1327
100711 The foregoing disclosure and description of the invention is
illustrative and
explanatory thereof and it can be readily appreciated by those skilled in the
art that
various changes in the size, shape and materials, as well as in the details of
the
illustrated construction or combinations of the elements described herein can
be made
without departing from the spirit of the invention.
19