Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02717255 2010-10-14
THREE ELEMENT COAXIAL VASO-OCCLUSIVE DEVICE
10
BACKGROUND
This invention relates to vaso-occlusive devices, such as vaso-occlusive coils
and the like, for the embolization of vascular aneurysms and similar vascular
abnormalities. Specifically, the invention is an improvement over existing two
layer
or two element coaxial vaso-occlusive' devices, particularly those having a
polymer
coating or covering. In particular, the present invention is a three layer or
three
element coaxial vaso-occlusive device that provides improved durability,
pushability,
and trackability inside a microcatheter. The characteristic termed
"trackability"
relates to the ease of advancing one interventional device within or over
another, and
it is related to friction and flexibility.
Vaso-occlusive devices are typically used within the vasculature of the human
body to block the flow of blood through a vessel through the formation of an
embolus. Vaso-occlusive devices are also used to form an embolus within an
aneurysm stemming from the vessel. Vaso-occlusive devices can be formed of one
or
more elements, generally delivered into the vasculature via a catheter or
similar
mechanism.
CA 02717255 2010-10-14
The embolization of blood vessels is desired in a number of clinical
situations.
For example, vascular embolization has been used to control vascular bleeding,
to
occlude the blood supply to tumors, and to occlude vascular aneurysms,
particularly
intracranial aneurysms. In recent years, vascular embolization for the
treatment of
aneurysms has received much attention. Several different treatment modalities
have
been employed in the prior art. One approach that has shown promise is the use
of
thrombogenic microcoils. These microcoils may be made of a biocompatible metal
alloy (typically platinum and tungsten) or a suitable polymer. If made of
metal, the
coil may be provided with Dacron fibers to increase thrombogenicity. The coil
is
deployed through a microcatheter to the vascular site. Examples of microcoils
are
disclosed in the following U.S. patents: 4,994,069 - Ritchart et al.;
5,133,731 - Butler
et al.; 5,226,911 - Chee et al.; 5,312,415 - Palermo; 5,382,259 - Phelps et
al.;
5,382,260 - Dormandy, Jr. et al.; 5,476,472 - Dormandy, Jr. et al.; 5,578,074 -
Mirigian; 5,582,619 - Ken; 5,624,461 - Mariant; 5,645,558 - Horton; 5,658,308 -
Snyder; and 5,718,711 - Berenstein et al.
A specific type of microcoil that has achieved a measure of success is the
Guglielmi Detachable Coil ("GDC"), described in U.S. Patent No. 5,122,136 -
Gug,lielmi et al. The GDC employs a platinum wire coil fixed to a stainless
steel
delivery wire by a solder connection. After the coil is placed inside an
aneurysm, an
electrical current is applied to the delivery wire, which electrolytically
disintegrates
the solder junction, thereby detaching the coil from the delivery wire. The
application of the current also creates a positive electrical charge on the
coil, which
attracts negatively-charged blood cells, platelets, and fibrinogen, thereby
increasing
the thrombogenicity of the coil. Several coils of different diameters and
lengths can
be packed into an aneurysm until the aneurysm is completely filled. The coils
thus
-2-.
CA 02717255 2010-10-14
create and hold a thrombus within the aneurysm, inhibiting its displacement
and its
fragmentation.
The advantages of the GDC procedure are the ability to withdraw and relocate
the coil if it migrates from its desired location, and the enhanced ability to
promote
the formation of a stable thrombus within the aneurysm.
A more recent development in the field of microcoil vaso-occlusive devices is
exemplified in US 6,299,619 ¨ Greene, Jr. et al. and US 6,602,261 ¨ Greene,
Jr. et
al., both assigned to the assignee of the subject invention. These patents
disclose
vaso-occlusive devices comprising a microcoil with one or more expansile
elements
disposed on the outer surface of the coil. The expansile elements may be
formed of
any of a number of expansile polymeric hydrogels, or alternatively,
environmentally-
sensitive polymers that expand in response to a change in an environmental
parameter (e.g., temperature or pH) when exposed to a physiological
environment,
such as the blood stream.
While the microcoils with expansile elements have exhibit great promise in,
for example, embolizing aneurysms of a wide variety of sizes and
configurations, the
expansile elements increase the frictional forces between the vaso-occlusive
device
and a microcatheter through which the device is deployed. Furthermore,
depending
on the configuration and material of the expansile elements, the flexibility
of the
device may be reduced. These factors may result in a device that has less than
optimal pushability (resistance to buckling) and reduced trackability (as
defined
above).
There has thus been a long-felt, but as yet unsatisfied need for a microcoil
vaso-occlusive device that has all the advantages of the expansile element
type of
device, and that also exhibits enhanced pushability-and trackability, with
good
durability characteristics.
- 3 -
CA 02717255 2010-10-14
SUMMARY OF THE INVENTION
Broadly, the present invention is a vaso-occlusive device, comprising three
coaxial
elements: an elongate, flexible, filamentous inner element; a non-metallic
intermediate element comdally surrounding the inner element and in intimate
contact
therewith; and an outer element coaxially surrounding the intermediate element
and
in intimate contact therewith, the outer element including one or more
openings or
gaps through which the intermediate element is exposed.
In a preferred embodiment of the invention, the inner element is in the form
of
a helical coil made of a biocompatible, radiopaque metal, and the intermediate
element is a conformal coating or layer on the inner element, the conformal
coating
or layer being made of a soft polymeric material that is preferably an
expansile
polymer. Advantageously, the polymeric hydrogel is an environmentally-
responsive
hydrogel that expands upon exposure to the physiological environment, for
example,
of the blood stream. The polymer may advantageously be bio-absorbable or
biodegradable. Also in the preferred embodiment, the outer element is a
helical
"over-coil" that is loosely wound ("open-wound") over the intermediate
element,
except at proximal and distal end sections, where it is tightly wound ("close-
wound"). The close-wound proximal and distal end sections support the inner
element, protecting it from damage during deployment and any necessary
repositioning, while also securely binding the intermediate element to the
inner
element at the proximal and distal ends of the device and restraining the
hydrogel of
the intermediate element from expanding at the respective ends of the device.
The
open-wound section between the proximal and distal end sections creates a
single,
continuous helical opening through which the intermediate element expands. The
helical configuration of the opening forces the expanded polymeric
intermediate
- 4 -
CA 02717255 2010-10-14
element to assume the configuration of a chain of arcuate segments protruding
radially outwardly between the coils of the over-coil, rather than that of a
continuous
polymeric layer having a continuous, uninterrupted exterior surface. Because
each of
the arcuate segments contacts the interior surface of a microcatheter (e.g.,
during
deployment) primarily at or near a tangential contact point, the total contact
area of
the intermediate element is reduced as compared to a continuous axial
polymeric
element. This reduced contact area correspondingly reduces the aggregate
friction
between the polymeric layer and the microcatheter, thereby decreasing the
resistance
to manipulation of the device. The open-wound section also creates hinge
points
between the arcuate segments of the polymeric intermediate element, thereby
increasing the overall flexibility of the device.
It has been confirmed experimentally that the reduced friction and increased
flexibility afforded by the outer element, and by the interaction between the
outer
and intermediate elements, enhances the both the pushability and trackability
of a
device made in accordance with the present invention, as compared, for
example,
with prior art microcoil devices having expansile polymeric coatings or
elements on
or along their exterior surfaces.
The invention thus provides a microcoil vaso-occlusive device with an
expansile element that allows the device to embolize very efficiently a wide
variety of
vascular abnormalities, e.g., aneurysms of a wide variety of shapes, sizes,
and
locations, and yet that exhibits enhanced pushability and trackabifity as
compared to
the prior art.
BRIEF DESCRIPTION OF THE DRAWINGS
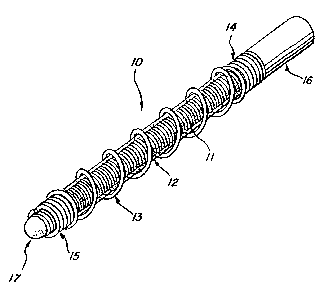
Fig. 1 is a perspective view of a vaso-occlusive device in accordance with a
preferred embodiment of the present invention;
- 5 -
CA 02717255 2010-10-14
Fig. 2 is an axial cross-sectional view of the device of Fig. 1;
Fig. 3 is a perspective view, similar to that of Fig. 1, showing the expansile
polymeric intermediate element in its expanded state;
Fig. 4 is an axial cross-sectional view of Fig. 3; and
Fig. 5 is a perspective view of an alternative embodiment of the invention.
DESCRIPTION OF THE INVENTION
Referring to Figures 1-4, a vaso-occlusive device 10, in accordance with a
preferred embodiment of the invention, comprises three elongate, coaxial
elements:
an inner core element 11, a non-metallic intermediate element 12, and a non-
expansile outer element 13 that covers at least a portion of the intermediate
element.
The intermediate element 12 is in intimate contact with both the inner element
11
and the outer element 13.
The inner element 11 is formed of a flexible, elongate filament or wire that
is
preferably made of a material that allows visualization under various medical
imaging means, such as X-ray, MRI, or ultrasound. Preferably, the inner
element 11
is formed from a length of wire made of any of various biocompatible,
radiopaque
metals, such as platinum, tantalum, tungsten, gold, titanium, nitinol,
stainless steel,
Elgiloy (cobalt-chromium-nickel), or other suitable alloys known in the art.
Alternatively, it can be made from or include non-metallic materials, such
polymers,
collagen, proteins, drugs, and biologic materials, bioactive agents,
therapeutic
compounds, or combinations of these materials. If made of a non-radiopaque
material, it should advantageously be doped or impregnated or chemically
modified
to be visible with one or more imaging techniques. Alternatively, it can be
made of a
material that is highly visible by means of MRI or ultrasound. The inner
element 11
can be formed in various configurations, including, but not limited to, coils,
rods,
-6-
CA 02717255 2013-11-18
tubes, cables, braids, cut tubes, or other elongate, flexible forms. As shown,
it is in
the form of a helical coil, which may be preferred. In one specific
embodiment, it is
formed at least in part of a multi-filar coil configuration, as described in
the co-
owned and co-pending US Application No. 10/189,934; filed July 2, 2002.
The intermediate element 12 may be formed as a coating, wrapping, tubular
sleeve, or other construction to create a substantially continuous surface
coa2dally
around the inner element 11. Alternatively, it can be formed into a cylinder
and then
skewered onto the inner core element 11, as described in the co-owned and co-
pending US Application No. 10/157,621; filed May 29, 2002.
The intermediate element 12 preferably covers
all of the length of the inner element 11, except for short proximal and
distal sections.
The intermediate element 12 may be made of any of various suitable,
substantially non-metallic, biocompatible materials, including polymers,
biopolymers, biologic materials, and combinations of these materials. Suitable
polymers include cellulose, polypropylene, polyvinylpyrrolidone, polyacrylics,
polylactides, polyamides, polyvinyl alcohol, polyester, polyurethane,
polyglycolic
acid, polyfluorocarbons, hydrogels, and silicones. Exemplary biologic
materials
include alginates, hyaluronic acid, fibrin, collagen and silk. Optionally, the
intermediate element 12 can be impregnated, grafted, bound, or modified to
deliver
therapeutic compounds, proteins, genes, bioactive agents, or cellular
material. See,
e.g., US 5,658,308 and International Publications Nos. WO 99/65401 and WO
00/274457 In one
preferred embodiment, the intermediate element 12 is made of a state-of-the-
art
bioabsorbable or biodegradable polymer, such as, for example, those described
in US
-7-
CA 02717255 2013-11-18
=
Published Applications Nos. 2002/0040239 and 2002/0020417.
In another preferred embodiment, the
intermediate element 12 is made of a soft conformal material, and more
preferably of
an expansile material such as a hydrogel.
The most preferred material is an environmentally responsive hydrogel, such
as that described in co-owned and co-pending US Application No. 09/804,9354
Specifically, the hydrogels
described in Application No. 09/804,935 are of a type that undergoes
controlled
volumetric expansion in response to changes in such environmental parameters
as
pH or temperature. These hydrogels are prepared by forming a liquid mixture
that
contains (a) at least one monomer and/or polymer, at least a portion of which
is
sensitive to changes in an environmental parameter; (b) a cross-linking agent;
and (c)
a polymerization initiator. If desired, a porosigen (e.g., NaC1, ice crystals,
or
sucrose) may be added to the mixture, and then removed from the resultant
solid
hydrogel to provide a hydrogel with sufficient porosity to permit cellular
ingrowth.
The controlled rate of expansion is provided through the incorporation of
ethylenically unsaturated monomers with ionizable functional groups (e.g.,
amines,
carboxylic acids). For example, if acrylic acid is incorporated into the
crosslinked
network, the hydrogel is incubated in a low pH solution to protonate the
carboxylic
acids. After the excess low pH solution is rinsed away and the hydrogel dried,
the
hydrogel can be introduced through a microcatheter filled with saline at
physiological pH or with blood. The hydrogel cannot expand until the
carboxylic
acid groups deprotonate. Conversely, if an amine-containing monomer is
incorporated into the crosslinked network, the hydrogel is incubated in a high
pH
solution to deprotonate amines. After the excess high pH solution is rinsed
away
and the hydrogel dried, the hydrogel can be introduced through a
rnicrocatheter filled
- 8 -
CA 02717255 2010-10-14
with saline at physiological pH or with blood. The hydrogel cannot expand
until the
amine groups protonate.
More specifically, in a preferred formulation of the hydrogel, the monomer
solution is comprised of ethylenically unsaturated monomers, an ethylenically
unsaturated crosslinking agent, a porosigen, and a solvent. At least a
portion,
preferably 10% - 50%, and more preferably 10% - 30%, of the monomers selected
must be pH sensitive. The preferred pH sensitive monomer is acrylic acid.
Methacrylic acid and derivatives of both acids will also impart pH
sensitivity. Since
the mechanical properties of hydrogels prepared exclusively with these acids
are
poor, a monomer to provide additional mechanical properties should be
selected. A
preferred monomer for providing mechanical properties is acrylamide, which may
be
used in combination with one or more of the above-mentioned pH sensitive
monomers to impart additional compressive strength or other mechanical
properties.
Preferred concentrations of the monomers in the solvent range from 20% w/w to
30% w/w.
The crosslinking agent can be any multifunctional ethylenically unsaturated
compound, preferably N, N'-methylenebisacrylamide. If biodegradation of the
hydrogel material is desired, a biodegradable crosslinking agent should be
selected.
The concentrations of the crosslinking agent in the solvent should be less
than about
1% w/w, and preferably less than about 0.1% w/w.
The porosity of the hydrogel material is provided by a supersaturated
suspension of a porosigen in the monomer solution. A porosigen that is not
soluble
in the monomer solution, but is soluble in the washing solution can also be
used.
Sodium chloride is the preferred porosigen, but potassium chloride, ice,
sucrose, and
sodium bicarbonate can also be used. It is preferred to control the particle
size of the
porosigen to less than about 25 microns, more preferably less than about 10
microns.
- 9 -
CA 02717255 2010-10-14
The small particle size aids in the suspension of the porosigen in the
solvent.
Preferred concentrations of the porosigen range from about 5% w/w to about 50%
w/w, more preferably about 10% w/w to about 20% w/w, in the monomer solution.
Alternatively, the porosigen can be omitted and a non-porous hydrogel can be
fabricated.
The solvent, if necessary, is selected based on the solubilities of the
monomers,
crosslinking agent, and porosigen. If a liquid monomer (e.g. 2-hydroxyethyl
methacrylate) is used, a solvent is not necessary. A preferred solvent is
water, but
ethyl alcohol can also be used. Preferred concentrations of the solvent range
from
about 20% w/w to about 80% w/w, more preferably about 50% w/w to about 80%
w/w.
The crosslink density substantially affects the mechanical properties of these
hydrogel materials. The crosslink density (and hence the mechanical
properties) can
best be manipulated through changes in the monomer concentration, crosslinldng
agent concentration, and solvent concentration. The crosslinking of the
monomer
can be achieved through reduction-oxidation, radiation, and heat. Radiation
crosslinking of the monomer solution can be achieved with ultraviolet light
and
visible light with suitable initiators or ionizing radiation (e.g. electron
beam or
gamma ray) without initiators. A preferred type of crosslinking initiator is
one that
acts via reduction-oxidation. Specific examples of such red/ox initiators that
may be
used in this embodiment of the invention are ammonium persulfate and N,N,N',N'-
tetramethylethylenediamine.
After the polymerization is complete, the hydrogen is washed with water,
alcohol or other suitable washing solution(s) to remove the porosigen(s), any
unreacted, residual monomer(s) and any unincorporated oligomers. Preferably
this
is accomplished by initially washing the hydrogel in distilled water.
- 10-
CA 02717255 2010-10-14
As discussed above, the control of the expansion rate of the hydrogel is
achieved through the protonation/deprotonation of ionizable functional groups
present on the hydrogel network. Once the hydrogel has been prepared and the
excess monomer and porosigen have been washed away, the steps to control the
rate
of expansion can be performed.
In embodiments where pH sensitive monomers with carboxylic acid groups
have been incorporated into the hydrogel network, the hydrogel is incubated in
a low
pH solution. The free protons in the solution protonate the carboxylic acid
groups
on the hydrogel network. The duration and temperature of the incubation and
the
pH of the solution influence the amount of control on the expansion rate.
Generally,
the duration and temperature of the incubation are directly proportional to
the
amount of expansion control, while the solution pH is inversely proportional.
It has
been determined that the water content of the treating solution also affects
the
expansion control. In this regard, the hydrogel is able to expand more in the
treating
solution and it is presumed that an increased number of carboxylic acid groups
are
available for protonation. An optimization of water content and pH is required
for
maximum control on the expansion rate. After the incubation is concluded, the
excess treating solution is washed away and the hydrogel material is dried.
The
hydrogel treated with the low pH solution has been observed to dry down to a
smaller dimension than the untreated hydrogel. This is a desired effect since
delivery
of these hydrogel materials through a microcatheter is desired.
If pH sensitive monomers with amine groups were incorporated into the
hydrogel network, the hydrogel is incubated in high pH solution. Deprotonation
occurs on the amine groups of the hydrogel network at high pH. The duration
and
temperature of the incubation, and the pH of the solution, influence the
amount of
control on the expansion rate. Generally, the duration, temperature, and
solution
- 11 -
CA 02717255 2010-10-14
pH of the incubation are directly proportional to the amount of expansion
control.
After the incubation is concluded, the excess treating solution is washed away
and
the hydrogel material is dried. ,
For the embodiment of the vaso-occlusive device having an intermediate
element formed of an expansile polymeric hydrogel, when the intermediate
element
12 expands, the areas of the soft, conformal intermediate element 12 that are
not
covered or constrained by the outer element 13 extend radially outward through
the
openings or gaps, or between the coils of the outer element 13 (as described
below) to
form an undulating outer surface comprising a chain of arcuate segments, as a
result
of the constraint imposed by the outer element 13. Because the arcuate
segments of
the undulating outer surface contact the interior wall surface of a
microcatheter
through which the device is deployed only at or near tangential contact points
proximate the apex of each segment, this undulating or arcuate configuration
provides reduced friction as compared to a continuous or smooth surface of the
same
material.
The outer element 13 is a flexible, elongate, substantially tubular member, at
least a substantial portion of the length of which, and preferably most of the
length of
which, includes or defines at least one opening or gap to allow the exposure
and/or
protrusion of the intermediate element 12. Suitable configurations for the
outer
element 13 include helical coils, braids, and slotted or spiral-cut tubes. The
outer
element 13 may be made of any suitable biocompatible metal or polymer,
including
those listed above for the inner element 11. For those embodiments using a
soft,
conformal intermediate element 12, the outer element 13 should have sufficient
radial strength to compress or restrain the intermediate element 12.
In the most preferred embodiment, the device comprises an inner core element
11 formed of a tightly-wound ("close-wound") helical coil of a biocompatible
metal
-12-
CA 02717255 2010-10-14
wire (e.g., platinum alloy), an intermediate element 12 of a hydrophilic
expansile
polymer (e.g., hydrogel), and an outer element 13 in the form of a
biocompatible
metal or polymer helical coil that is open-wound for most of its length, with
a close-
wound proximal end section 14 and a close-wound distal end section 15. The
open-
wound portion of the outer element defines a single, continuous, helical
opening or
gap. A coupling element 16 is advantageously attached to the proximal end of
the
inner element 11 for detachable attachment to a deployment device (not shown).
A
rounded distal obturator tip 17 may be attached to the distal end of the inner
element
11.
In the above-described most preferred embodiment, the hydrogel of the
intemiediate element 12 expands or swells upon exposure to an aqueous
environment (e.g., blood). Preferably, the hydrogel expands to between about
two
timq and about 20 times its original volume. As shown in Figs 3 and 4, the
swollen
or expanded intermediate element 12 protrudes through the helical opening or
gap
defined between the coils of the open-wound section of the outer element 13 to
form
an undulating, convexly-curved surface defining a chain of arcuate or rounded
segments, each having a diameter that is substantially greater than the
diameter of
the outer element 13. The open-wound section of the coil forming the outer
element
13 preferably has a coil pitch that is at least one-half the diameter of the
outer
element 13. The coil is preferably made from a wire that has a diameter of no
more
than about 0.15 mm.
The helical outer element 13 described above may be considered as defining a
single, helical opening or gap, or it may be viewed as defining a plurality of
connected openings or gaps, each defined between an adjacent pair of windings
of
the coil of the outer element 13. Alternatively, if the outer element 13 is
formed as a
slotted tube, for example, the outer element 13 will be seen to define a
plurality of
- 13 -
CA 02717255 2010-10-14
discrete openings or gaps in its axial middle section that are functionally
equivalent
to the helical opening(s) defined in the illustrated embodiment.
The device 10 can be constructed with various radial thickness of each coaxial
element to provide different handling characteristics. Preferably, the inner
element
11 has a diameter of between about 0.075 mm and 0.75 mm; the intermediate
element 12 has a thickness of between about 0.025 mm and 1.00 mm; and the
outer
element 13 has a thickness of between about 0.025 mm and 0.25 Dam. For the
embodiments that use an expansile intermediate element 12, these thicknesses
are
measured in the non-expanded state. Preferably, the outer diameter of the
outer
element 13 is actually somewhat less than the expanded or swollen diameter of
the
intermediate element 12, so that the latter will readily expand through the
openings
or gaps in the outer element 13.
Figure 5 shows a vaso-occlusive device 10' in accordance with an alternative
embodiment of the invention. This embodiment includes an outer element 13'
with
a distal section 15' that is not close wound, but is, instead, made with small
gaps of
approximately 5% to 100% of the diameter of the wire or filament of which the
outer
element 13' is made. These gaps make the distal section 15' of the device 10'
more
flexible in the area where the outer element 13' overlaps the inner element
11'.
In the embodiment shown in Fig. 5, the proximal ends of both the inner
element 11' and the outer element 13' are both advantageously attached to a
coupling element 16' by soldering or welding. The attachment of both the inner
element 11' and the outer element 13' to the coupling element 16' makes the
proximal end of the device 10' more resistant to deformation during deployment
and
implantation.
As indicated above, the present invention provides good trackability in a
microcatheter. In =other words, it is easily advanced through a catheter
without
- 14-
CA 02717255 2010-10-14
binding against or moving the catheter. This advantage is achieved through
reduced
friction and reduced buckling at the ends of the device. The force required to
advance the device through a typical microcatheter would normally be less than
about 0.7 lbs.
The device is preferably detachable from a flexible, elongate delivery
apparatus
(not shown), such as a wire, a pusher tube, or the like. Exemplary detachment
systems known in the art include electrolytic, mechanical, electromechanical,
thermal, ultrasonic, and hydraulic detachment mechanisms. The device may be
formed into a secondary configuration, such as a helical coil, a sphere, an
ovoid, or
any other suitable two- or three-dimensional shape known in the art of vaso-
occlusive devices. Alternatively, the device can be left in a relatively
straight
configuration with or without a curvature at the end such as a "J"
configuration).
The device is useful for the occlusion and/or embolization of blood vessels,
other vascular spaces such as aneurysms, and other tubular or saccular organs
or
spaces throughout the body. Specific applications where it may be useful
include the
occlusion-of cerebral aneurysms, aortic aneurysms, fistulas, fallopian tubes,
cardiac
septa' defects, patent foramen ovale, and the left atrial appendage of the
heart. For
some of these applications, it may be preferable to use devices with
dimensions larger
than those specified above.
Although preferred embodiments of the invention have been described in this'
specification and the accompanying drawings, it will be appreciated that a
number of
variations and modifications may suggest themselves to those skilled in the
pertinent
arts. Thus, the scope of the present invention is not limited to the specific
embodiments and examples described herein, but should be deemed to encompass
alternative embodiments and equivalents, as determined by a fair reading of
the
claims that follow.
- 15 -