Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
1
Description
TREATING ERYTHEMATOTELANGIECTATIC ROSACEA OR
PAPULOPUSTULAR ROSACEA WITH NARROW-BAND
INFRARED LIGHT RADIATION AND RADIATION KITS
THEREFOR
Technical Field
[1] The present invention generally relates to a method of treating erythem-
atotelangiectatic rosacea or papulopustular rosacea with narrow-band infrared
radiation, and to a kit therefor.
Background Art
[2] Rosacea is a chronic disease that affects the skin and sometimes the eyes.
Symptoms
include skin redness, pink bumps (papules), bumps containing pus(pustules),
pimples,
abnormal proliferation and dilation of superficial blood vessels
(telangiectasia), and, in
the advanced stages, thickened skin. The National Institute of Arthritis and
Musculo-
skeletal and Skin Diseases (NIAMS) reports that approximately 14 million
people in
the US suffer from rosacea. Several subtypes of rosacea are known in the art,
including
erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea
and
ocular rosacea.
[3] Typically, in erythematotelangiectatic type rosacea, central facial
flushing, often ac-
companied by burning or stinging, is the predominant sign. The redness usually
spares
the periocular skin. The erythematous areas of the face at times appear rough
presumably due to chronic, low-grade dermatitis with inflammation. Frequent
triggers
to flushing include acutely felt emotional stress, hot drinks, alcohol, spicy
foods,
exercise, cold or hot weather, and hot baths and showers. These patients also
report
that the burning or stinging is exacerbated when topical agents are applied.
[4] Typically, papulopustular rosacea is the classic presentation of rosacea.
Patients are
generally women of middle age who predominately present with a red central
portion
of their face that contains small erythematous papules surmounted by pustules.
One
may elicit a history of flushing. Telangiectasias are likely present but may
be difficult
to distinguish from the erythematous background in which they exist.
[5] The etiology of rosacea is not elucidated yet. Some possible causes that
have been
suggested to be related to development of rosacea are inherited abnormalities
in
cutaneous vascular homeostasis, exposure to sunlight, dermal matrix
degeneration,
chemical and ingested agents, abnormalities of sebaceous gland, certain
microbial
organisms such as Demodex and Helicobacterpylori. In addition, it has been
proposed
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
2
that those who blush frequently may be more likely to develop rosacea, and
research
has shown that rosacea is a disorder where blood vessels dilate too easily,
resulting in
flushing and redness. While the cause is unknown and there is no cure, the
signs and
symptoms of the disorder can be managed.
[6] So far, erythematotelangiectatic and papulopustular rosacea has been
treated with
various treatment modalities including laser treatments for telangiectatic
lesions, low
dose systemic antibiotics such as doxycycline, and topical agents such as
topical met-
ronidazole or azelaic acid, with variable success rate. Especially, pulsed dye
laser or
potassium titanyl phosphate (KTP) laser (which is typically based on selective
photo-
thermolysis) has been widely used for telangiectatic lesions regardless
whether it is
resulted from rosacea or other conditions. In selective photothermolysis,
typically,
chromophores (e.g. hemoglobin in blood vessels) absorb high-power energy of
pulses
of light from laser source. Then the light energy converts to heat energy and
the
resultant thermal injury causes destruction of the target chromophore. When
the
chromophore is hemoglobin of blood vessels, the vessels are also destroyed by
the
thermal damage or by resultant inflammatory process. Intense pulsed light
(IPL) can
also be used in a similar context as the pulsed dye laser or KTP laser in the
treatment
of rosacea.
[7] Although effective for many telangiectatic lesions of rosacea, laser or
IPL treatment
for rosacea can cause some side effects such as purpura, hyperpigmentation,
hypopig-
mentation, burn and scarring. These side effects are especially of concern to
dark-
skinned individuals and people who have tanned skin, as melanin is also one of
chro-
mophores that absorb light at the wavelength(s) employed such lasers and IPL.
Because these devices produce high-power, pulsed light energy that can create
photo-
thermolysis of, hence, thermal injury to skin components including melanin-
containing
epidermis and adjacent structures, they can cause these adverse effects
mentioned
above, and therefore, are not suitable for some patients with dark skin or
tanned skin.
Additionally, some rosacea lesions that have erythematous background and fine
vessels but not prominent dilated vessels sometimes tend to be resistant to
laser
treatment with pulsed dye lasers or KTP lasers, possibly due to lack of
sufficient chro-
mophores in blood vessels (e.g., hemoglobin) in such lesions and the presence
of
melanin particles in adjacent skin that compete for absorption of laser
energy. These
limitations of current laser and IPL treatment make it difficult to treat
erythem-
atotelangiectatic rosacea.
[8] The treatment of papulopustular rosacea usually involves uses of topical
agent such
as topical metronidazole, azelaic acid, and low dose oral antibiotics such as
doxycycline. The mechanism of their efficacy is not fully understood, but anti-
inflammatory effect of such agents is believed to play a role. However, their
use may
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
3
cause some side effects such as skin irritation and bacterial resistance to
the antibiotics
used. The outcome of these treatments is variable according to the treatment
protocols
and the patients' condition.
[9] Thus, there is a need for developing a method for treating rosacea, such
as erythem-
atotelangiectatic type rosacea and papulopustular rosacea, in particular in a
non-
invasive manner, e.g., without photothermolysis of the skin under treatment or
skin ir-
ritation.
Disclosure of Invention
Technical Problem
[10] The present invention generally relates to a method of treating erythem-
atotelangiectatic rosacea or papulopustular rosacea with narrow-band infrared
radiation, and to a kit therefor.
[11] In one embodiment, the present invention is directed to a method of
treating erythem-
atotelangiectatic rosacea or papulopustular rosacea in a subject. The method
comprises
exposing the subject's skin in need thereof to narrow-band infrared radiation
at a
wavelength(s) in a range of between 790 nm and 900 nm and having a band width
of
between 0 nm and 20 nm, in an effective dose to treat erythematotelangiectatic
rosacea
or papulopustular rosacea and essentially not to cause photothermolysis of the
skin.
[12] In another embodiment, the present invention is directed to a method of
treating
erythematotelangiectatic rosacea or papulopustular rosacea in a subject. The
method
comprises exposing the subject's skin in need thereof to narrow-band infrared
radiation
at a wavelength(s) in a range of between 790 nm and 900 nm and having a band
width
of between 0.1 nm and 20 nm, in an effective dose to treat
erythematotelangiectatic
rosacea or papulopustular rosacea.
[13] In yet another embodiment, the present invention is directed to a kit
comprising a
radiation source generating narrow-band infrared radiation at a wavelength(s)
in a
range of between 790 nm and 900 nm, the narrow-band infrared radiation having
a
band width of between 0 nm and 20 nm and having a power density of between 1
mW/
cm2 and 100 mW/cm2, and a manual instructing a user how to use the narrow-band
infrared radiation for treating erythematotelangiectatic rosacea or
papulopustular
rosacea.
Technical Solution
[14] The foregoing will be apparent from the following more particular
description of
example embodiments of the invention, as illustrated in the accompanying
drawings in
which like reference characters refer to the same parts throughout the
different views.
The drawings are not necessarily to scale, emphasis instead being placed upon
il-
lustrating embodiments of the present invention.
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
4
[15] The invention employs narrow-band radiation at a wavelength(s) in a range
of
between 790 nm and 900 nm and having a band width of between 0 nm and 20 nm,
or
alternatively between 0.1 nm and 20 nm, in an effective dose to treat erythem-
atotelangiectatic rosacea or papulopustular rosacea in a subject.
[16] Rosacea is generally defined by persistent erythema of the central
portion of the face
lasting for at least 3 months. Supporting criteria include flushing, papules,
pustules,
and telangiectasias on the convex surfaces. Secondary characteristics are
burning and
stinging, edema, plaques, a dry appearance, ocular manifestations, and
phymatous
changes. The prevalence of these findings designates the subclassification of
the
presentation and, additionally, the therapeutic options. The diagnosis of
rosacea is a
clinical diagnosis. Before making the diagnosis of rosacea, skin biopsy may be
necessary to exclude other disease states that mimic the clinical presentation
of
rosacea, for example to exclude polycythemia vera, connective tissue diseases
(e.g.,
lupus erythematosus, dermatomyositis, mixed connective tissue disease),
carcinoid,
mastocytosis, long-term application of topical steroids, contact dermatitis
and photo-
sensitivity.
[17] Typically, in erythematotelangiectatic type rosacea, central facial
flushing, often ac-
companied by burning or stinging, is the predominant sign. The redness usually
spares
the periocular skin. The erythematous areas of the face at times appear rough
presumably due to chronic, low-grade dermatitis with inflammation. Frequent
triggers
to flushing include acutely felt emotional stress, hot drinks, alcohol, spicy
foods,
exercise, cold or hot weather, and hot baths and showers. These patients also
report
that the burning or stinging is exacerbated when topical agents are applied.
[18] Typically, papulopustular rosacea is the classic presentation of rosacea.
Patients are
generally women of middle age who predominately present with a red central
portion
of their face that contains small erythematous papules surmounted by pustules.
One
may elicit a history of flushing. Telangiectasias are likely present but may
be difficult
to distinguish from the erythematous background in which they exist.
[19] "Narrow-band" radiation, as used herein, means radiation at a wavelength
or
wavelengths having a band width between 0 nm and 20 nm, such as between 0.1 nm
and 20 nm. It is noted that the term "between 0 nm and 20 nm" includes "0 nm"
and
"20 nn." For example, narrow-band radiation at a wavelength(s) having a band
width
of 20 nm means that the radiation at the wavelength(s) has, for example, a
deviation of
nm. Similarly, narrow-band radiation at a wavelength(s) having a band width of
0
nm means that the radiation at the wavelength(s) has a deviation of 0 nm.
[20] As used herein a "subject" is a mammal, preferably a human. Subject and
patient are
used interchangeably.
[21] "Treatment" or "treating" refers to both therapeutic and prophylactic
treatment, and
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
also includes any improvement of the condition being treated with the narrow-
band
infrared radiation treatment compared with the absence of such treatment.
[22] As used herein, the "effective dose" is a quantity that results in a
beneficial clinical
outcome of, or exerts an influence on, the condition being treated with the
narrow-band
infrared radiation treatment compared with the absence of such treatment. For
example, the "effective dose" can be a dose sufficient to treat
erythematotelangiectatic
rosacea or papulopustular rosacea in a subject after each dose or after a
plurality of
consecutive such doses. When a plurality of consecutive doses are employed,
the doses
are typically repeated at intervals of from 0.5 day (twice per day) to 10
days. Spe-
cifically, the doses are repeated at intervals of from one day to 4 days, such
as 2 days
or 3 days. Alternatively, each irradiant dose is up to 7 days apart, such as 1
day apart, 2
days apart, 3 days apart or 4 days apart. Alternatively, each irradiant dose
is 1 day
apart, 2 days apart or 3 days apart. The intervals can be the same length or
different
lengths. In one specific embodiment, the intervals are the same length.
[23] Generally, an effective dose of the narrow-band infrared radiation
depends, in each
case, upon several factors, e.g., the skin types, age, gender and condition of
the subject
to be treated, among others.
[24] Generally, the effective dose essentially does not cause photothermolysis
of the skin.
Selective photothermolysis is a photothermolytic reaction by which a target
chromophore is selectively damaged or destroyed by light, resulting in
destruction of
the target chromophore or necrosis of the cells that contain the target
chromophore.
Photothermolysis generally occurs when the following three fundamental
conditions
are met:
[25] Wavelength: specific wavelength that can be absorbed by the target
molecule;
[26] Pulse duration: pulse duration of the pulsed light from a laser that is
shorter than the
thermal relaxation time (TRT) of the target (TRT: TRT is the time taken for
the target
to dissipate about 63% of the incident thermal energy); and
[27] Fluence (energy density, J/cm2): a sufficient fluence (energy density;
the amount of
energy per unit area) to create the thermal damage enough to destroy the
target.
[28] In one example, meeting the above-mentioned three conditions, when the
pulsed
light from a laser is absorbed by a given target within time duration shorter
than the
TRT of the target (thus, the pulse duration of the pulsed light should be
shorter than the
TRT of the target), the target cannot dissipate the heat energy to the
adjacent structures
before the sufficient amount of energy to destroy it accumulates in it, and
therefore, is
destroyed by the thermal damage.
[29] Typically, photothermolysis can occur with a light source that can
produce short
pulses. Generally, the TRT (thermal relaxation time) is so short that only
pulsed light
sources, such as lasers or intense pulsed light (IPL), can produce light that
has pulse
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
6
duration sufficiently shorter than TRT of the given target (e.g., TRT of blood
vessels).
[301 In general, a fluence that causes photothermolysis varies depending upon
the types of
the target, TRT of the target, depth of the target, type of lasers, skin
phototypes of the
subjects, and many other things, because, at least in part, power sufficient
for photo-
thermolysis varies depending upon the target, laser type, depth of the target,
skin
phototypes, etc. Such power that can cause photothermolysis generally cannot
be
produced with an light-emitting diode (LED) light source or with a light
source which
cannot produce pulses of light. Thus, with an LED light source or a low level
laser or a
low level light therapy device, which cannot produce high-power pulses of
light,
selective photothermolysis of any give components of the skin, including blood
vessels, generally does not occur.
[311 In one embodiment, the narrow-band infrared radiation employed in the
invention
does not meet at least one of the above-mentioned three requirements for photo-
thermolysis.
[321 In another embodiment, the narrow-band infrared radiation employed in the
invention is generated from an LED light source or a low level laser.
Alternatively, the
narrow-band infrared radiation employed in the invention is generated from a
low level
light therapy device which does not generate pulses of light that has power
high
enough to cause photothermolysis of skin components.
[331 Typically, in the disclosed methods, the skin of the subject is exposed
to a plurality
of exposures. The exposures can be repeated for any time period, as long as
the subject
does not experience any side effect, such as photosensitity. The plurality of
exposures
are collectively referred to as a "treatment period." The treatment period can
be
between one week and 12 weeks, or between two weeks and 8 weeks, such as two,
three, four, five or six weeks. Alternatively, the treatment period can be
longer than 12
weeks.
[341 In one embodiment, the skin is exposed to the narrow-band infrared
radiation one,
two, three, four, five, six or seven times per week during the treatment
period. In
another embodiment, the skin is exposed to the narrow-band infrared radiation
four,
five, six or seven times per week during the treatment period.
[351 In another embodiment, a layer of a gel, cream or lotion is applied on
the skin prior
to the skin exposure to the narrow-band infrared radiation. Thus, in this
embodiment,
the skin is exposed to the narrow-band infrared radiation through the gel,
cream or
lotion layer. In one specific embodiment, the gel, cream or lotion has at
least 70%
transparency at the narrow-band infrared radiation. In another specific
embodiment,
the gel, cream or lotion has at least 90% transparency at the narrow-band
infrared
radiation. In yet another specific embodiment, a layer of a transparent gel
having, for
example, at least 70% transparency, particularly at least 90% transparency, at
the
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
7
narrow-band infrared radiation is applied to the skin prior to the skin
exposure to the
narrow-band infrared radiation. In yet another specific embodiment, the
transparent gel
is water-based. In yet another specific embodiment, the water-based
transparent gel
comprises hyaluronic acid. In yet another specific embodiment, the water-based
transparent gel consists essentially of water and hyaluronic acid.
[36] In the invention, the narrow-band infrared radiation is at a
wavelength(s) in a range
of between 790 nm and 900 nm. Alternatively, the narrow-band infrared
radiation is at
a wavelength(s) in a range of between 800 nm and 860 nm. Alternatively, the
narrow-
band infrared radiation is between 820 nm and 840 nm. Alternatively, the
narrow-band
infrared radiation is at a wavelength(s) in a range of between 825 nm and 835
nm. Al-
ternatively, the narrow-band infrared radiation is at 830 nm.
[37] Typically, the narrow-band infrared radiation has a band width of between
0 nm and
20 nm, or between 0.1 nm and 20 nm. Specifically, in any one of the
embodiments
described in the previous paragraph, the band width of the narrow-band
infrared
radiation is between 0 nm and 15 nm, such as 0 nm, 6 nm, 10 nm, 12 nm or 15
nm.
More specifically, in any one of the embodiments described in the previous
paragraph,
the band width of the narrow-band infrared radiation is between 0 nm and 12
nm. Al-
ternatively, in any one of the embodiments described in the previous
paragraph, the
band width of the narrow-band infrared radiation is between 0.1 nm and 12 nm,
such
as 10 nm. Alternatively, in any one of the embodiments described in the
previous
paragraph, the band width of the narrow-band infrared radiation is between 0.1
nm and
1 nm.
[38] The narrow-band infrared radiation employed in the invention generally
has power
density in a range of between 1 mW/cm2 and 100 mW/cm2. In one specific em-
bodiment, the power density is in a range of between 1 mW/cm2 and 75 mW/cm2.
In
another specific embodiment, the power density is in a range of between 1
mW/cm2
and 50 mW/cm2. In yet another specific embodiment, the power density is in a
range of
between 1 mW/cm2 and 30 mW/cm2. In yet another specific embodiment, the power
density is in a range of between 1 mW/cm2 and 15 mW/cm2.
[39] In any one of the embodiments described above, including the embodiments
described in the three previous paragraphs, specifically, the narrow-band
infrared
radiation employed in the invention has energy density in a range of between 3
J/cm2
and 180 J/cm2. Alternatively, the energy density is in a range of between 3
J/cm2 and
150 J/cm2. Alternatively, the energy density is in a range of between 3 J/cm2
and 120 J/
cm2. Alternatively, the energy density is in a range of between 3 J/cm2 and
100 J/cm2.
Alternatively, the energy density is in a range of between 3 J/cm2 and 70
J/cm2. Al-
ternatively, the energy density is in a range of between 3 J/cm2 and 50 J/cm2.
Al-
ternatively, the energy density is in a range of between 3 J/cm2 and 30 J/cm2.
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
8
[401 The skin exposure to the narrow-band infrared radiation per each dose can
last for
any suitable time period as long as it can cause treatment of
erythematotelangiectatic
rosacea or papulopustular rosacea of the patient's skin after each dose or
after a
plurality of such doses, and essentially not to cause photothermolysis of skin
components. In one example, the skin exposure to the narrow-band infrared
radiation
per each dose lasts for less than 20 minutes, such as between 5 minutes and 20
minutes, or between 5 minutes and 15 minutes. Alternatively, the skin exposure
to the
narrow-band infrared radiation per each dose can last for more than 20
minutes, for
example, between 20 minutes and 60 minutes or between 20 minutes and 40
minutes.
[411 For the narrow-band infrared radiation employed in the invention, any
suitable
radiation source can be employed, including relatively low-power laser and low
level
light therapy devices and LEDs known in the art. Specifically, the narrow-band
infrared radiation employed in the invention is non-coherent radiation. More
spe-
cifically, the narrow-band infrared radiation employed in the invention is
generated by
an LED device.
[421 Generally, prior to performing the narrow-band infrared radiation
treatment of the
invention, it is generally checked whether or not the subject to be treated
has any
conditions where exposure to light may affect the health of her/his skin, such
as photo-
sensitive condition, especially in regards to any possibility of
photosensitivity.
Examples of such conditions include: recent history (e.g., within one weak) of
systemic or topical photodynamic therapy involving any photosensitizer that
has the
absorption peaks within or near the range of the near infrared light and the
use of such
photosensitizer for any other purposes; any photosensitive condition, such as
disease
(e.g. systemic lupus erythematosus, certain types of porphyria (erythropoietic
porphyria, erythropoietic protoporphyria, porphyria cutanea tarda, variegate
porphyria,
hereditary coproporphyria, hepatoerythropoietic porphyria), polymorphous light
eruption, hydroa vacciniforme, and other conditions that can cause
photosensitivity),
drugs (e.g. tetracycline, fluoroquinolones, ibuprofen, amiodarone,
phenothiazine,
furosemide, hydrochlorothiazide, retinoic acid, isotretinoin, etc.). For
example, it is
generally checked whether or not the subject to be treated has photosensitive
condition,
especially in regards to any possibility of photosensitivity, such as disease
(e.g.
systemic lupus erythematosus, certain types of porphyria (erythropoietic
porphyria,
erythropoietic protoporphyria, porphyria cutanea tarda, variegate porphyria,
hereditary
coproporphyria, hepatoerythropoietic porphyria), polymorphous light eruption,
hydroa
vacciniforme, and other conditions that can cause photosensitivity), drugs
(e.g. tet-
racycline, fluoroquinolones, ibuprofen, amiodarone, phenothiazine, furosemide,
hydro-
chlorothiazide, retinoic acid, isotretinoin, etc.), recent history of
photodynamic therapy
using 5-aminolevulinic acid or methyl-5-aminolevulinic acid or photofrin or
other pho-
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
9
tosensitizers. If the subject has one or more of these conditions or other
photosensitive
conditions, it is recommended for the subject to consult her/his doctor
regarding
whether or not, and/or when, she/he can take the narrow-band infrared
radiation
treatment of the invention. Also, even if the subject does not have any of the
above-
mentioned conditions, but if the subject has any photosensitivity condition to
the
visible light (e.g., between 400 nm and 670 nm), it is generally recommended
for the
subject to consult her/his doctor regarding whether or not, and/or when,
she/he can
take the narrow-band infrared radiation treatment of the invention.
[43] The invention also includes a kit comprising a radiation device that
includes a
radiation source generating the narrow-band infrared radiation employed in the
narrow-band infrared radiation methods described above. Specifically, the
narrow-
band infrared radiation has power density in a range of between 1 mW/cm2 and
100
mW/cm2. Alternatively, the power density is between 1 mW/cm2 and 75 mW/cm2. Al-
ternatively, the power density is between 1 mW/cm2 and 50 mW/cm2.
Alternatively,
the power density is between 1 mW/cm2 and 30 mW/cm2. Alternatively, the power
density is between 1 mW/cm2 and 15 mW/cm2. The kit further comprises a manual
in-
structing a user how to use the narrow-band infrared radiation for the narrow-
band
infrared irradiation treatment to treat erythematotelangiectatic rosacea or
papu-
lopustular rosacea on the skin of a subject. Features, including specific
features, of the
narrow-band infrared irradiation treatment using the kit are as described
above for the
methods of the invention.
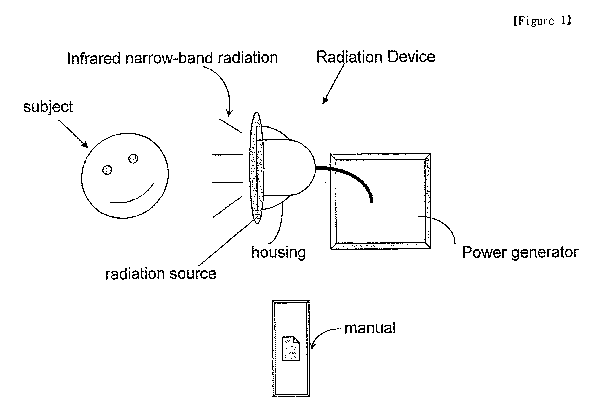
[44] FIG. 1 shows one embodiment of a kit of the invention, comprising a
radiation
device, such as an LED device, and a manual instructing a user how to use the
narrow-
band infrared radiation for the narrow-band infrared irradiation treatment to
treat eryth-
ematotelangiectatic rosacea or papulopustular rosacea on the skin of a
subject. The
housing of the radiation device of the figure can include any suitable
radiation source,
such as one or more LEDs.
[45] In one embodiment of a kit of the invention, the radiation device is an
LED light
device or a low level laser. In another embodiment of a kit of the invention,
the
radiation device is a low level light therapy device which does not generates
pulses of
light whose power is high enough to cause photothermolysis of skin components.
[46] In a specific embodiment, the radiation source is a non-coherent
radiation source,
such as an LED device that includes one or more LEDs.
[47] In another embodiment, the manual included in a kit of the invention
further
comprises instructions about distance between the skin of the subject and the
radiation
source during the narrow-band infrared radiation treatment, duration time per
single
treatment of the narrow-band infrared radiation and frequency of the narrow-
band
infrared radiation treatment, and warning about conditions where exposure to
the
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
narrow-band infrared irradiation may affect the health of the subject s skin.
Specific
examples of such conditions are as described above. In a further specific
embodiment,
the warning also recommends users or subjects that they should seek
professional
advice as to whether the subject(s) to be treated have any photosensitive
condition
prior to using the kit for the narrow-band infrared radiation treatment, if
they do not
have prior knowledge about this.
[481 In a specific embodiment, the kit further comprises a pair of goggles
that are spe-
cifically designed to protect the retinae of the eyes of the subject to be
treated with the
narrow-band infrared radiation from direct illumination at the wavelength(s)
of the
narrow-band infrared radiation. Specifically, the goggles have color and/or
optical
density to essentially block light at the wavelength(s) of the narrow-band
infrared
radiation.
[491 In another specific embodiment, the manual of the kit includes warning
that direct
exposure of the eyes to the narrow-band infrared may harm the eyes. In yet
another
specific embodiment, the manual further provides guidance that any people who
do not
wear suitable protective goggles must not be exposed to the narrow-band
infrared
radiation. One example of such protecting guidance is to recommend a user to
use the
narrow-band infrared irradiation treatment alone in a room which is closed
(locked or
not), and/or to put a warning sign on the door of the room that a suitable
protective
goggles should be worn before entering the room.
[501 In yet another specific embodiment, a kit of this invention comprises a
protective
shield (e.g., protective shield 10 of FIGs. 2, 4, 5) in addition to a pair of
goggles that
are specifically designed to protect the retinae of the eyes of the subject to
be treated
with the narrow-band infrared radiation from direct illumination at the
wavelength(s)
of the narrow-band infrared radiation. The protective shield generally
includes a
material that blocks the narrow-band infrared irradiation so that the narrow-
band
infrared irradiation can not spread to outside of the area where the narrow-
band
infrared radiation treatment is being performed. Any suitable material known
in the art
that blocks infrared irradiation can be employed for the protective shield of
the
invention. One suitable example of such materials is aluminum. The protective
shield
can be of either a hard material (e.g., metal plate) or a soft material (e.g.,
a cloth
containing aluminum foil).
[511 In yet another specific embodiment, as shown in FIG. 2 and 3, a kit of
this invention
comprises a pair of goggles 20 and radiation device 30. As shown in FIG. 3,
goggles
include one or more detection components 22. Radiation device 30 includes
radiation source 31 and one or more detector(s) 34 that can detect or sense a
signal
from, or the presence of, the detection components of the protective goggles
22. In a
further specific embodiment, detector(s) 34 is placed at radiation source 31
(e.g.,
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
11
radiation head) of radiation device 30. One example of this embodiment is
shown in
FIGs. 2-4. As shown in FIG. 3, goggles 20 include one or more detection
components
22. Detection component(s) 22 of goggles 20 can contain any component that can
be
detected or sensed by detector(s) 34. Alternatively, detector(s) 34 includes a
component that can be activated by a signal transmitted from detection
component(s)
22 of goggles 20.
[521 In a further specific embodiment, detector(s) 34 can sense an area where
the eyes of
the subject would be placed, for example, an area facing the upper half of
radiation
source 31 (e.g., radiation head) of radiation device 30, and detect the
presence or
absence of goggles 20 by detecting the detection components 22 of goggles 20.
In this
embodiment, the radiation source 31 of radiation device 30 can be activated by
controller(s) 35 (which is in communication with detector(s) 34) only when
detector(s)
34 detects or senses the presence of the detection components 22 of goggles 20
in the
eye area of the subject's face.
[531 In another, further specific embodiment, detector(s) 34 can scan the
contour of the
subject's face, detect the presence or absence of goggle 20 on the subject's
face. In this
embodiment, controller(s) 35 (which is in communication with detector(s) 34)
activates the radiation source 31 of radiation device 30 only when detector(s)
34
detects or senses the presence of goggles 20 on the subject's face.
[541 In yet another specific embodiment, as shown in FIG. 2 and 3, a kit of
this invention
comprises protective shield 10, a pair of goggles 20 and radiation device 30
that is
designed such that it cannot be activated without properly being connected to
protective shield 10. In one specific example of such designs, protective
shield 10 and
radiation device 30 include one or more connecting spots 12 and 32,
respectively.
Radiation device 30 also includes one or more detectors 34. Detector(s) 34
detect or
sense a signal from, or the presence of detection component(s) 22 of
protective goggles
22. Features, including specific features, of protective shield 10, goggles 20
and
detectors 34 of radiation device 30 are as described above.
[551 In yet another specific embodiment, radiation device 30 further includes
controller(s)
35 (see FIG. 2) that controls the activation of the radiation source 31 of
radiation
device 30. The controller(s) 35 is in communication with detector(s) 34 and
connecting
spots of the radiation source and the protective shield 12 and 32,
electronically, via
signal(s) or via any other means known in the art. For example, controller(s)
35 can
receive information (e.g., electronic information) as to the presence or
absence of
protective goggles from detector(s) 34.
[561 In a further specific embodiment, controller(s) 35 receives information
(e.g.,
electronic information), or signals, from detector(s) 34, determines whether
or not
connecting spots 12 and 32 are properly connected to each other, and controls
the ac-
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
12
tivation of the radiation source 31 of radiation device 30. For example, when
detector(s) 34 detects or senses the presence of detection components 22 of
protective
goggles 20 in the proper location, that is, in the area facing the upper half
of the
radiation source 31 of the radiation device 30, or scans the subject's face
and detects
the presence of goggles 20 in the proper position, that is, the eye area of
the subject's
face, the controller(s) 35 receives this information from the detector(s) 34
and allows
the activation of the radiation source 31 of the radiation device 30.
Alternatively, when
the controller(s) 35 does not receive the information of the presence of
goggles 20 in a
proper position, the proper position as mentioned above, the controller(s)
does not
activate the radiation source 31 of radiation device 30. For further example,
when
connecting spots 12 and 32 are connected properly to each other, controller(s)
35
allows the activation of the radiation source. Alternatively, when connecting
spots 12
and 32 are not connected properly to each other, controller(s) 35 disallows
the ac-
tivation of the radiation source.
[57] Any suitable electronic sensor known in the art can be employed in the
invention for
the sensor(s) of detector(s) 34. Any suitable electronic controller known in
the art can
be employed in the invention for the controller(s) 35 of radiation device 30.
[58] In yet another specific embodiment, a kit of this invention comprises
protective
shield 10 that includes one or connecting spots 12; a pair of goggles 20 that
includes
one or more detection component(s) 22; and radiation device 30 that includes
radiation
source 31, one or more connecting spots 32, one or more detector(s) 34, one or
more
controller(s) 35 (see FIG. 2-4). Features, including specific features of
protective shield
10, goggles 20, radiation device 30, radiation source 31, connecting spots 12
and 32,
detection components 22, detectors 34, and controller(s) 35 are as described
above.
FIG. 5 shows a schematic view of a subject under the narrow-band infrared
radiation
treatment with the kit including shield 10 that includes one or connecting
spots 12; a
pair of goggles 20 that includes one or more detection component(s) 22; and
radiation
device 30 that includes radiation source 31, one or more connecting spots 32,
one or
more detector(s) 34, and one or more controller(s) 35.
[59] Although only few examples are illustrated in FIGs. 2-4, any other
protective
measure known in the art that can protect the eyes of the subject under the
narrow-
band infrared radiation treatment from any hazard from the narrow-band
infrared
radiation can also be employed in the invention.
[60] A kit of the invention can further comprise a gel, cream, or lotion
having at least 70%
transparency at the narrow-band infrared radiation. Specific examples of
suitable,
transparent gels, creams or lotions are as described above for the methods of
the
invention. Specifically, the gel, cream or lotion included in the kit has at
least 90%
transparency at the narrow-band infrared radiation. More specifically, a
transparent gel
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
13
having at least 70% transparency, particularly at least 90% transparency, at
the narrow-
band infrared radiation is employed. Even more specifically, a water-based
transparent
gel having at least 70% transparency, particularly at least 90% transparency,
at the
narrow-band infrared radiation is employed.
[61] The kits of the invention can be portable. Such a portable kit of the
invention can be
used as a home-therapy kit so that a user is the subject to be treated with
the narrow-
band infrared radiation. Alternatively, such a portable kit of the invention
can also be
used in a professional medical clinic for treating erythematotelangiectatic or
papu-
lopustular rosacea.
Brief Description of the Drawings
[62] FIG. 1 depicts a kit of the invention that includes a radiation device
generating
narrow-band infrared radiation employed in the invention, and a manual
instructing a
user how to use the narrow-band infrared radiation for treating a subject with
erythem-
atotelangiectatic rosacea or papulopustular rosacea.
[63] FIG. 2 is a schematic drawing of one embodiment of a radiation device and
a
protection shield that can be employed in the invention.
[64] FIG. 3 is a schematic drawing of protective goggles that can be employed
in the
invention for protecting the retinae of the eyes of a subject to be treated
with the
narrow-band infrared radiation of the invention from direct illumination at
the
wavelength(s) of the narrow-band infrared radiation.
[65] FIG. 4 is a schematic drawing of another embodiment of a radiation
device, a
protection shield and a pair of protective goggles, which can be employed in
the
invention.
[66] FIG. 5 is a schematic drawing of a patient under one embodiment of the
narrow-band
infrared radiation treatment of the invention.
[67] FIG. 6 is a photograph showing a cheek of a patient, who has
telangiectasia on the
cheek, prior to the narrow-band infrared radiation treatment of the invention.
[68] FIGs. 7 and 8 are follow-up photos of the cheek of the patient of FIG. 6
three months
and seventeen months after the narrow-band infrared radiation treatment of the
invention, respectively, which show substantial reduction of telangiectasia on
the
patient's cheek after the narrow-band infrared radiation treatment.
Mode for the Invention
[69] The invention is illustrated by the following examples which are not
intended to be
limiting in any way.
[70] Example: Treatment of Telangiectasia and Inflammatory Papules with Narrow
Band Infrared light at the wavelength of 830 nm
[71] A light source for the phototherapy system consisted of a base and an
irradiating
CA 02721354 2010-10-13
WO 2009/128570 PCT/KR2008/002102
14
head which emitted quasimonochromatic light of wavelength at 830nm from
adjustable
planar arrays of LEDs. The irradiating head (Omnilux plusTM, Photo
Therapeutics Ltd.,
Fazeley, UK) comprised five articulated panels containing 108 LEDs each, so
that they
could be adjusted to fit the contour of the patient's face optimally. The
wavelength
used was 830 5 nm with symmetrical peak. The irradiance was 55 mW/cm2 at a
distance of 1 to 10 centimeters from the light source. The radiant fluences,
or doses,
during a single treatment for twenty minutes were 66 J/cm2.
[721 The patient was treated with this light source twice a week for four
weeks at a three
to four day interval between each session. The distance between the
irradiating head
and the patient's nose was about 3-5 cm. Goggles were worn to protect the
retinae from
direct illumination. Follow up evaluation was done at 3 months and 17 months
after
the final treatment. FIG. 6 shows a cheek of the patient prior to the narrow-
band
infrared radiation treatment. FIGs. 7 and 8 are follow-up photos of the cheek
of the
patient of FIG. 6 three months and seventeen months after the narrow-band
infrared
radiation treatment. As shown in FIGs. 7 and 8, telangiectasia on the
patient's cheek
was substantially reduced after the narrow-band infrared radiation treatment.
Also, it
was observed that inflammatory papules, found around the cheeks and the chin
of the
patient prior to the narrow-band infrared radiation treatment, were also
reduced after
the treatment (data not shown).
[731 While this invention has been particularly shown and described with
references to
example embodiments thereof, it will be understood by those skilled in the art
that
various changes in form and details may be made therein without departing from
the
scope of the invention encompassed by the appended claims.