Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-1-
"RHEOLOGICAL METHOD FOR THE HYDROMETALLURGICAL
RECOVERY OF BASE METALS FROM ORES"
Field of the Invention
The present invention relates to a method for the improved recovery of base
metals from sulphide and/or oxide ores. More particularly, though not
exclusively, the invention relates to a rheological method for the improved
application of a hydrometallurgical process for leaching of nickel from a
combination of nickel sulphide ores or concentrate and nickel oxide ores.
Background to the Invention
Nickel sulphide ores have traditionally been treated via a pyrometallurgical
smelting process, in order to recover nickel as a high grade nickel matte. The
nickel content of the matte can range from 60 to 80% nickel as a sulphide. In
Western Australia flash smelting and converting has been commercially
applied to produce a high grade nickel matte 70% nickel, from nickel sulphide
concentrates. The nickel sulphide concentrate is typically 12 to 18% nickel.
The high grade matte is subsequently refined utilising the Sherritt Gordon
process.
Hydrometallurgical processes such as leaching have historically not been
applied to nickel sulphide ores or concentrates, as smelting is commercially
competitive when compared to hydrometallurgical processes. Unlike the
Activox or Albion hydrometallurgical processes, smelting unlocks significant
energy credits that is converted to electrical energy and produces sulphuric
acid or sulphur as by-products. This co-generation approach improves the
overall competitiveness of pyrometallurgical process when compared to
hydrometallurgical processes.
Furthermore hydrometallurgical processes such as the Activox or the Albion
Process typically require fine grinding P90 or minus 10 microns, which
consumes energy. The energy released via the leaching process is lost to
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-2-
cooling towers or a simple flash system that does not capture any of the
energy released during leaching.
It is well documented that hydrometallurgical treatments such as High
Pressure Acid Leach (HPAL) plants operating in Western Australia have
added sulphides as either "transition" sulphide ore or non-smeltable
concentrates that cannot be treated via a conventional concentrator or
smelter. However, these plants are limited in their ability to add sulphides
due
to the reducing nature of the sulphide ores or concentrates. Typically these
hydrometallurgical plants are hydraulically limited and therefore unless the
slurry density or the ore grade is increased these hydrometallurgical plants
remain bottlenecked.
Furthermore as these hydrometallurgical plants are hydraulically limited,
increasing the slurry density or solids specific gravity has a significant
impact
on the plant capacity, unlocking sunk capital and more importantly reducing
the unit cost of production.
There are several commercial examples where high density or paste
thickening has been retrofitted to existing HPAL plants to improve the
existing
plant capacity by increasing slurry density though the application of improved
thickening technology. The typical improvement in density achieved by the
installation of high density or paste thickening is within a range of 2 to 4
w/w%
solids increase. The use of indirect heating is also a well known technically
and commercially proven method for increasing slurry density. Indirect
heating can be retrofitted into an existing hydrometallurgical plant to unlock
capital, introduced in the initial design to reduce the capital intensity of
new
plants. The unit cost of production in both instances is also reduced,
improving the plant competitiveness. However both of the above methods of
increasing slurry density are capital intensive and energy expensive.
The present invention aims to de-bottleneck existing hydrometallurgical plants
or reduce the capital intensity of proposed new plants by combining sulphide
ores or concentrate with oxide ores (such as laterite ore) in a milling
environment.
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-3-
The previous discussion of the background to the invention is intended to
facilitate an understanding of the present invention only. The discussion is
not
an acknowledgement or admission that any of the material referred to is or
was part of the common general knowledge as at the priority date of this
application. References to prior art in this specification are provided for
illustrative purposes only and are not to be taken as an admission that such
prior art is part of the common general knowledge in Australia or elsewhere.
Summary of the Invention
According to one aspect of the present invention there is provided a
rheological method for the hydrometallurgical recovery of base metals from
ores, the method comprising the steps of:
providing a sulphide ore or concentrate and a laterite or other oxide ore;
combining the sulphide ore or concentrate with the laterite or other oxide ore
and milling them together to form a combined slurry with improved rheological
characteristics.
Preferably the ratio of sulphide ore or concentrate to laterite or other oxide
ore
in the combining and milling step is in the range of about 1:1 to 1:40.
Preferably a sulphide concentrate is used in the combining and milling step as
the specific gravity of sulphide concentrate is about twice that of a typical
laterite or other oxide ore.
Preferably water and/or pregnant leach solution (PLS) is added to the
sulphide ore or concentrate and the laterite or other oxide ore to form the
combined slurry in the combining and milling step.
Preferably the method further comprises the step of leaching the combined
slurry in a pressure acid leach circuit. Preferably the pressure acid leach
circuit comprises a series of pressure Pachuca tanks.
Typically the base metal is selected from the group consisting of nickel,
cobalt, copper, lead and zinc. Preferably the sulphide ore or concentrate is a
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-4-
nickel sulphide or concentrate and the laterite or other oxide ore is a nickel
laterite or other nickel oxide ore.
According to another aspect of the present invention there is provided a
rheological method for the hydrometallurgical recovery of nickel from ores,
the
method comprising the steps of:
providing a nickel sulphide ore or concentrate and a nickel laterite or other
nickel oxide ore;
combining the nickel sulphide ore or concentrate with the nickel laterite or
other nickel oxide ore and mixing them together to form a combined slurry
with improved rheological characteristics.
Preferably the step of combining the nickel sulphide ore or concentrate with
the nickel laterite or other nickel oxide ore and mixing them together
involves
milling the ores together.
The step of combining nickel sulphide ore or concentrate with nickel laterite
or
other nickel oxide ore and mixing them together to form a combined slurry
with improved rheological characteristics allows higher overall slurry
densities
to be achieved. This may allow for a reduction in capital and operating costs
in hydrometallurgical nickel processing plants.
The ratio of nickel sulphide ore or concentrate to nickel laterite or other
nickel
oxide ore in the combining and mixing step may be anywhere in the range of
about 1:1 to 1:40. More typically the ratio of nickel sulphide ore or
concentrate
to nickel laterite or other nickel oxide ore in the combining step is in the
range
of about 3:7 to 3:97.
Preferably the method further comprises the step of leaching the combined
slurry in a pressure acid leach circuit.
The method preferably further comprises a step of directing a nickel laterite
or
other nickel oxide ore to an atmospheric leach process and providing the PLS
from the atmospheric leach process to the combining and mixing step.
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-5-
The nickel sulphide ore or concentrate typically has a nickel concentration
within the range of about 1 to 10% Ni. Preferably the nickel sulphide ore or
concentrate has a nickel concentration within the range of about 2 to 4% Ni.
Typically the nickel laterite or oxide ore has a nickel concentration within
the
range of about 0.8 to 5% Ni. Preferably the nickel laterite or oxide ore has a
nickel concentration within the range of about 1 to 2% Ni.
Preferably the free acid concentration achieved in the pressure acid leach
circuit is maintained within the range of 30 to 80 g/l. Preferably, the
temperature within the acid leach circuit is maintained between about 160
and 260 C. More preferably, the temperature within the acid leach circuit is
maintained at about 220 to 250 C. Preferably, the oxygen over pressure
within the acid leach circuit is maintained between 100 to 1000 kPag.
According to a still further aspect of the present invention there is provided
a
method of improving the rheological characteristics of a laterite or other
oxide
ore, the method comprising:
providing a sulphide ore or concentrate and a laterite or other oxide ore;
combining the sulphide ore or concentrate with the laterite or other oxide ore
and mixing them together to form a slurry with higher density relative to a
slurry formed from the laterite or other oxide ore by itself.
Preferably the sulphide ore or concentrate is a nickel sulphide or concentrate
and the laterite or other oxide ore is a nickel laterite or other nickel oxide
ore.
Preferably the step of combining nickel sulphide ore or concentrate with the
nickel laterite or other nickel oxide ore and mixing them together involves
milling the ores together.
Preferably the ratio of nickel sulphide ore or concentrate to nickel laterite
or
other nickel oxide ore in the combining step is in the range of about 1:1 to
1:40. Typically, the ratio of nickel sulphide ore or concentrate: nickel
laterite or
other nickel oxide ore in the combining step is in the range of about 3:7 to
3:97.
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-6-
Throughout the specification, unless the context requires otherwise, the word
"comprise" or variations such as "comprises" or "comprising", will be
understood to imply the inclusion of a stated integer or group of integers but
not the exclusion of any other integer or group of integers. Likewise the word
"preferably" or variations such as "preferred", will be understood to imply
that
a stated integer or group of integers is desirable but not essential to the
working of the invention.
Brief Description of the Drawings
The nature of the invention will be better understood from the following
detailed description of several specific embodiments of the rheological
method for the hydrometallurgical recovery of a base metal according to the
invention, given by way of example only, with reference to the accompanying
drawings in which:
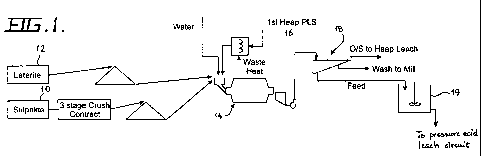
Figure 1 is a schematic diagram of a process circuit of a preferred
rheological
method for the hydrometallurgical recovery of nickel in accordance with the
present invention;
Figure 2 is a graphical presentation of rheology test results for laterite,
sulphide and a combined slurry in process water;
Figure 3 is a graphical presentation of rheology test results for laterite
slurry in
process water and two types of PLS;
Figure 4 is a graphical presentation of rheology test results for sulphide
slurry
in process water and two types of PLS;
Figure 5 is a graphical presentation of rheology test results for combined
slurry in process water and in PLS with first and second types of chemistry;
Figure 6 is a graphical presentation of rheology test results for three
different
blends of combined slurry in PLS with a first type of chemistry;
Figure 7 is a graphical presentation of rheology test results for three
different
blends of combined slurry in PLS with a second type of chemistry;
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-7-
Figure 8 is a graphical presentation of rheology test results for combined
slurry in process water and in PLS with a first type of chemistry with and
without shear; and,
Figure 9 is a graphical presentation of rheology test results for laterite
slurry in
process water and PLS.
Detailed Description of Preferred Embodiments
A preferred embodiment of the rheological method for the hydrometallurgical
recovery of a base metal according to the invention, as shown in schematic
form in Figure 1, relates to the leaching of nickel. The method preferably
comprises the step of combining nickel sulphide ore or concentrate 10 with
nickel laterite or other nickel oxide ore 12 and milling the combination in
the
milling circuit 14 with a pregnant leach solution (PLS) and/or water as the
case may be to form a combined slurry. The nickel sulphide ore or
concentrate 10 preferably has a nickel concentration within the range of about
1 to 10% Ni. Preferably, the nickel laterite or other nickel oxide ore 14
should
have a nickel concentration within the range of 0.8 to 5% Ni. More typically
the nickel sulphide ore or concentrate 10 has a nickel concentration within
the
range of about 2 to 4% Ni, and the nickel laterite or other nickel oxide ore
12
has a nickel concentration within the range of about 1 to 2% Ni.
The method preferably further comprises the step of directing a nickel
laterite
or other nickel oxide ore to an atmospheric leach process 16, which in the
embodiment of Figure 1 is a first heap leach process (not illustrated). The
clarified PLS from the first heap leach process is then directed to the
milling
circuit 14. The PLS is preferably heated prior to injection into the milling
circuit
14. The PLS may be derived from any suitable atmospheric leach process
and it not limited to heap leaching. However in the event that a suitable
source of PLS from an atmospheric leach process is not available, water may
be substituted for the PLS that is directed to the milling circuit.
Preferably the PLS from the first heap leach process 16 has a nickel
concentration of more than 4 g/l. Hence a significant benefit of adding the
PLS to the milling circuit 12 is that the head grade of ore passing through
the
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-8-
plant is doubled. This, together with acid credits, greatly improves the
economies of scale and efficiency of the plant.
Preferably the ratio of nickel sulphide ore or concentrate to nickel laterite
or
other nickel oxide ore in the combining step is in the range of about 1:1 to
1:40. More preferably the ratio of nickel sulphide ore (or concentrate) to
nickel
laterite ore (or other nickel oxide ore) is in the range of about 3:7 to 3:97.
Preferably the nickel laterite or nickel oxide ore for the atmospheric leach
is a
saprolite smectite ore and the laterite or oxide ore used for the combined
leach is a limonite ore.
The viscosity of laterite ores is impacted by additives such as free acid or
total
dissolved solids. Limonites typically exhibit a reduction in viscosity when
solutions from a heap leach operation are slurried with limonite ores. That
is,
for a given weight percent, solids milling in PLS reduces the viscosity of the
pulp. However with saprolite or smectite ores slurrying in PLS will increase
the viscosity for a given weight percent solids. Adding sulphides to all
laterite
ores, whether limonite, saprolite or smectite, acts to significantly reduce
the
viscosity and is considered innovative. By appropriate selection of the
relative
proportions of both kinds of minerals in the combined ores, milling at optimum
density can be achieved. Therefore saprolite or smectite is the preferred
laterite ore for the atmospheric leach, and limonite is the preferred laterite
ore
for milling in atmospheric PLS due to the improvement in slurry density
achieved.
The milled combined ore from the milling circuit 14 is then subject to a
screening step in screening circuit 18. Oversize ore is directed from the
screening circuit 18 back to the first heap leach process 16. Undersize ore is
fed from the screening circuit 18 to a slurry tank 19, and the combined slurry
is then pumped by high pressure slurry pumps to a pressure acid leach circuit
(not illustrated). The pressure acid leach circuit may comprise a series of
pressure Pachuca tanks. Wash from the screening circuit 18 is returned to the
milling circuit 14.
Figures 2 and 9 in the accompanying drawings clearly highlight the positive
impact that adding sulphide ore has on laterite rheology. The improvement in
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-9-
the rheological characteristics of the combined slurry has a significant
effect
on the economics of the mineral recovery process. The density (% solids) of
the slurry is a measure of the ore per unit volume. The higher the density the
more ore can be processed per unit volume. Therefore the aim is to maximise
the density of the slurry without increasing the viscosity to such an extent
that
the slurry cannot be pumped through the process plant. A typical slurry pump
may be rated, for example, to a maximum slurry viscosity of 75 Pa. If the
slurry viscosity exceeds this figure the pump may fail.
Milling of the combined nickel sulphide ore or concentrate 10 with nickel
laterite or other nickel oxide ore 12, preferably in the proportions specified
above, has a dramatic effect on the rheology of the combined slurry. The step
of combining nickel sulphide ore or concentrate with nickel laterite or other
nickel oxide ore and milling them together to form a slurry with improved
rheological characteristics allows higher overall slurry densities to be
achieved. This allows for a reduction in capital and operating costs in
hydrometallurgical plants.
The milling is typically carried out using the PLS from the first heap leach
process instead of, or in addition to, water. The clarified PLS from the first
heap leach process preferably has a ferric iron concentration within the range
of 10 to 60 g/l. Preferably the PLS from the first heap leach process 10 has a
free acid concentration of less than 30g/l.
Rheology Tests
Tests were conducted on the rheology of the combined slurry using various
ratios (blends) of nickel laterite ore to nickel sulphide ore or concentrate
with
process water (PW) and PLS. Slurries formed using two different chemistries
of PLS were also tested. Chemical analyses of the ores and liquors used and
the results of the rheology tests are summarised below:
Chemical Analysis:
Analysis of the major elements in the respective laterite and sulphide ores
and the various blends used in the tests are as follows:
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-10-
Laterite: Ni 1.29%, Fe 12.4%, Si 20.4%, Mg 5.22, Al 4.35%
Sulphide: Ni 2.05%, Fe 18.3%, Si 22.1%, Mg 3.49, Al 2.02%
US Blend 80:20: Ni 1.46%, Fe 13.3%, Si 21 %, Mg 4.88, Al 4.02%
US Blend 70:30: Ni 1.54%, Fe 14.4%, Si 21%, Mg 4.64, Al 3.64%
L/S Blend 60:40: Ni 1.67%, Fe 15.1 %, Si 21.6%, Mg 4.42, Al 3.48%
Calculated analyses of the above blends are very close to actual analysis
Liquor Analysis:
PLS: Free acid 19 g/L, Ni 4.5 g/L, Fe 41.6 g/L, Mg 19.5 g/L, Al 9 g/L, Na 2.8
g/L, Ca 0.36 g/L
Process Water: Mg 1.2 g/L, Ca 0.38 g/L, Na 10.8 g/L, CI 19.4 g/L
The following chemistries 1 and 2 of PLS were employed in the tests:
Chemistry 1: Fe 41.6 g/L and Free acid 19 g/L
Chemistry 2: Fe 41.6 g/L and Free acid 34 g/L
Analysis of all the other major elements are very much the same as the PLS
analysis.
Mineralogical Analysis:
The mineralogy of the nickel laterite and nickel sulphide ores employed in the
tests was as follows:
Laterite: Smectite (nontronite) is the major phase
Maghemite, goethite, hematite, chlorite, hornblend, quartz are
minor to moderate phases
Sulphide: Quartz, pyrrhotite, feldspar, hornblend, chlorite are major
phases
Pentlandite, pyrite, chalcopyrite, muscovite talc are minor to
moderate phases
Rheology:
(i) Laterite, sulphide and US blend 70:30 in PW
Yield stress at 100Pa: Laterite in PW -51 % w/w
Sulphide in PW -82% w/w
Blend in PW -56.6%
(ii) Laterite in PW, PLS Chemistry 1 and PLS Chemistry 2
Yield stress at 1 00Pa: Laterite in PW -51 % w/w
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-11-
Laterite in PLS Chem 1 -42.5% w/w
Laterite in PLS Chem 2 -45% w/w
(iii) Sulphide in PW, PLS Chemistry 1 and PLS Chemistry 2
Yield stress at 100Pa: Sulphide in PW -82% w/w
Sulphide in PLS Chem 1 -77.8% w/w
Sulphide in PLS Chem 2 -78.3% w/w
(iv) US Blend 70:30 in PW and Chemistries 1 and 2
Yield stress at 100Pa: Blend in PW -56.6% w/w
Blend in PLS Chem 1 -48.5% w/w
Blend in PLS Chem 2 -51.8% w/w
(v) Laterite, sulphide and US blends 80:20, 70:30 and 60:40 in PLS Chem 1
Yield stress at 100Pa: Laterite in PLS Chem 1 -42.5% w/w
Blend 80:20 in PLS Chem 1 -46.2% w/w
Blend 70:30 in PLS Chem 1 -48.5% w/w
Blend 60:40 in PLS Chem 1 -56% w/w
Sulphide in PLS Chem 1 -77.8% w/w
(vi) Laterite, sulphide and US blends 80:20, 70:30 and 60:40 in PLS Chem 2
Yield stress at 100Pa: Laterite in PLS Chem 2 -45% w/w
Blend 80:20 in PLS Chem 2 -46% w/w
Blend 70:30 in PLS Chem 2 -51.8% w/w
Blend 60:40 in PLS Chem 2 -55.5% w/w
Sulphide in PLS Chem 2 - 78.3% w/w
The rheological results illustrate the significant improvement in density of
the
combined slurry (blend) that can be achieved by the use of sulphides to
modify the viscosity (as measured by the yield stress) of the laterite ores.
With each of the blends there is a substantial increase in the density of the
slurry compared to the laterite by itself in slurry. The more sulphide is
added
to the blend the greater the density. Since the primary objective is the
leaching of nickel from laterite or other nickel oxide ores, a compromise
between preferred density and the proportion of sulphide ore added is
necessary. Preferably a blend of laterite and sulphide in the ratio of about
70:30 or 7:3 achieves an acceptable compromise, i.e. blend comprising 70%
laterite ore and 30% sulphide ore. However the blend of nickel sulphide to
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-12-
nickel laterite may typically vary within the range 1:1 to 1:40. More
typically
the ratio of nickel sulphide to nickel laterite varies within the range of
about
3:7 to 3:97.
A more detailed comparison of the rheology of the laterite, sulphide and US
combined slurry using various liquors and blends may be gained from the
graphical presentations of the test results in Figures 2 to 9. Figure 2
illustrates
the improvement in density for the same viscosity that can be achieved using
a 70:30 blend of combined slurry compared to laterite by itself in process
water. The much higher densities of sulphide slurry by itself is also
illustrated
for comparison. Figure 3 illustrates the change in density for the same
viscosity that occurs using PLS (Chemistries 1 and 2) to form slurry using the
laterite ore by itself compared to using process water (PW) to form the
slurry.
These results show that for a moderate reduction in density, simply combining
the laterite with the PLS can achieve a significant improvement in the head
grade of ore passing through the process. Figure 3 also shows that increasing
the free acid concentration (Chemistry 2) in the PLS results in an increase in
the density of the laterite slurry with the same viscosity.
Figure 4 illustrates the change in density for the same viscosity that occurs
using PLS to form slurry from the sulphide ore by itself compared to using
PW. The results are similar to that shown in Figure 3, except at the higher
densities of the sulphide slurry. Figure 5 illustrates the change in density
for
the same viscosity that occurs using PLS to form combined slurry with a
70:30 L/S blend compared to using PW. These results show that for a
moderate reduction in density, combining a 70:30 L/S blend with the PLS can
achieve a significant improvement in the head grade of ore passing through
the process. Figure 5 again shows that increasing the free acid concentration
in the PLS (Chemistry 2) results in an increase in the density of the combined
slurry with the same viscosity.
Figures 6 and 7 are similar to Figure 2 and illustrate the improvement in
density for the same viscosity that can be achieved using three different
blends of a combined slurry in PLS compared to laterite by itself and sulphide
by itself. The three blends employed for the combined slurry are L/S 80:20,
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-13-
L/S 70:30 and L/S 60:40. In Figure 6 the results relate to a slurry formed in
a
PLS with Chemistry 1 (see Liquor Analysis above), and in Figure 7 the results
relate to slurry formed in a PLS with Chemistry 2. A comparison of Figure 6
with Figure 7 reveals that increasing the free acid concentration in the PLS
in
most cases has the effect of increasing the density of the combined slurry for
a specified viscosity. However the effect is most marked in the combined
slurry with a L/S 70:30 blend and L/S 80:20 blend.
Figure 8 is similar to Figure 5 except that only the results for a combined
slurry with a 70:30 US blend using PLS with Chemistry 1 are shown. Figure 8
illustrates the affect that shearing has on the density of the combined slurry
(
a marked reduction) for the same viscosity. Figure 9 is similar to Figure 2
except that is also includes the results for Laterite in PLS and a 70:30 blend
in
PLS for comparison. Figure 9 illustrates the positive impact that adding
sulphide ore has on laterite rheology. Combining the sulphide with the
laterite
in a 70/30 blend results in a marked increase in the density of the combined
slurry with the same viscosity, whether in PW or PLS.
Now that preferred embodiments of a hydrometallurgical method for leaching
nickel in a combined pressure acid leach have been described in detail, it
will
be apparent that the embodiments provide a number of advantages over the
prior art, including the following:
(i) Combining sulphide ore or concentrate with oxide ore in a
milling environment produces a combined slurry with modified
viscosity and higher density relative to slurry formed from a
laterite or other oxide ore by itself.
(ii) The increased slurry density achieved by combining sulphide
ore or concentrate with oxide ore significantly reduces the unit
cost of production and improves the competitiveness of the
hydrometallurgical process plant.
(iii) As existing or new hydrometallurgical plants are hydraulically
limited, adding a sulphide concentrate (with a typical
concentration of 8% nickel) to a laterite or other oxide ore
CA 02727557 2010-12-10
WO 2009/149522 PCT/AU2009/000755
-14-
results in an immediate 15 to 20% increase in throughput on a
displaced volume for volume basis due to the differences in
specific gravity.
(iv) With this increased throughput, compounded by the improved
rheological properties typically >4% w/w and nickel grade 8%
versus 1.5%, nickel the benefits of the process are significant.
It will be readily apparent to persons skilled in the relevant art that
various
modifications and improvements may be made to the foregoing embodiments,
in addition to those already described, without departing from the basic
inventive concepts of the present invention. Therefore, it will be appreciated
that the scope of the invention is not limited to the specific embodiments
described.