Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
HETEROGENEOUS HYDROGEN-CATALYST REACTOR
CROSS-REFERENCES TO RELATED APPLICATIONS
This application claims the benefit of priority of U.S. Provisional
Application Nos.
61/084,923, filed July 30, 2008; 61/086,316, filed August 5, 2008; 61/088,492,
filed August
13, 2008; 61/094,513, filed September 5, 2008; 61/098,514, filed September 19,
2008;
61/102,465, filed October 3, 2008; 61/104,534, filed October 10, 2008;
61/105,660, filed
October 15, 2008; 61/106,932, filed October 20, 2008; 61/109,088, filed
October 28, 2008;
61/110,253, filed October 31, 2008; 61/112,491, filed November 7, 2008;
61/114,735, filed
November 14, 2008; 61/116,966, filed November 21, 2008; 61/193,543, filed
December 5,
2008; 61/139,293, filed December 19, 2008; 61/145,022, filed January 15, 2009;
61/146,962,
filed January 23, 2009; 61/150,571, filed February 6, 2009; 61/152,500, filed
February 13,
2009; 61/156,328, filed February 27, 2009; 61/158,252, filed March 6, 2009;
61/160,145,
filed March 13, 2009; 61/164,151, filed March 27, 2009; 61/166,495, filed
April 3, 2009;
61/170,418, filed April 17, 2009; 61/174,346, filed April 30, 2009;
61/176,675, filed May 8,
2009; 61/178,796, filed May 15, 2009; 61/180,456, filed May 22, 2009;
61/182,468, filed
May 29, 2009; 61/186,660, filed June 12, 2009; 61/218,771, filed June 19,
2009; 61/220,911,
filed June 26, 2009; 61/222,721, filed July 2, 2009; and 61/226,541, filed
July 17, 2009, all of
which are herein incorporated by reference in their entirety.
SUMMARY OF DISCLOSED EMBODIMENTS:
The present disclosure is directed to catalyst systems comprising a hydrogen
catalyst
capable of causing atomic H in its n=1 state to form a lower-energy state, a
source of atomic
hydrogen, and other species capable of initiating and propagating the reaction
to form lower-
energy hydrogen. In certain embodiments, the present disclosure is directed to
a reaction
mixture comprising at least one source of atomic hydrogen and a catalyst or
source of catalyst
to support the catalysis of hydrogen to form hydrinos. The reactants and
reactions disclosed
herein for solid and liquid fuels are also reactants and reactions of
heterogeneous fuels
comprising a mixture of phases. The reaction mixture comprises at least two
components
chosen from a hydrogen catalyst or source of hydrogen catalyst and a source of
atomic
hydrogen, wherein at least one of the atomic hydrogen and the hydrogen
catalyst may be
formed by a reaction of the reaction mixture. In additional embodiments, the
reaction
mixture further comprises a support, which in certain embodiments can be
electrically
conductive, a solvent such as an organic solvent or inorganic solvent
including a molten salt,
a getter, and at least one reactant that by virtue of it undergoing a reaction
causes the catalysis
to be active.
The reaction to form hydrinos may be activated or initiated and propagated by
one or
more chemical reactions. These reactions can be chosen from (i) exothermic
reactions, which
1
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
provide the activation energy for the hydrino reaction, (ii) coupled
reactions, which provide
for at least one of a source of catalyst or atomic hydrogen to support the
hydrino reaction, (iii)
free radical reactions, which in certain embodiments, serve as an acceptor of
electrons from
the catalyst during the hydrino reaction, (iv) oxidation-reduction reactions,
which in certain
embodiments, serve as an acceptor of electrons from the catalyst during the
hydrino reaction,
(v) exchange reactions such as anion exchange including halide, sulfide,
hydride, arsenide,
oxide, phosphide, and nitride exchange that in an embodiment, facilitate the
action of the
catalyst to become ionized as it accepts energy from atomic hydrogen to form
hydrinos, and
(vi) getter, support, or matrix-assisted hydrino reactions, which may provide
at least one of a
chemical environment for the hydrino reaction, act to transfer electrons to
facilitate the H
catalyst function, undergoes a reversible phase or other physical change or
change in its
electronic state, and binds a lower-energy hydrogen product to increase at
least one of the
extent or rate of the hydrino reaction. In certain embodiments, the
electrically conductive
support enables the activation reaction.
In additional embodiments, the present disclosure is directed to a power
system
comprising at least two components chosen from: a source of catalyst or
catalyst; a source of
atomic hydrogen or atomic hydrogen; reactants to form the source of catalyst
or catalyst and
a source of atomic hydrogen or atomic hydrogen; one or more reactants to
initiate the
catalysis of atomic hydrogen; and a support to enable the catalyst,
wherein the power system can further comprise any of a reaction vessel, a
vacuum
pump, a power converter and systems such as separator systems, an
electrolyzer, thermal
systems for reversing an exchange reaction, and chemical synthesis reactors to
regenerate the
fuel from the reaction products.
In further embodiments, the present disclosure is directed to a system for
forming
compounds having hydrogen in lower-energy states comprising at least two
components
chosen from: a source of catalyst or catalyst; a source of atomic hydrogen or
atomic
hydrogen; reactants to form the source of catalyst or catalyst and a source of
atomic hydrogen
or atomic hydrogen; one or more reactants to initiate the catalysis of atomic
hydrogen; and a
support to enable the catalyst,
wherein the system for forming compounds having hydrogen in lower-energy
states
can further comprise any of a reaction vessel, a vacuum pump, and systems such
as separator
systems, an electrolyzer, thermal systems for reversing an exchange reaction,
and chemical
synthesis reactors to regenerate the fuel from the reaction products.
Other embodiments of the present disclosure are directed to a battery or fuel
cell
system for forming compounds having hydrogen in lower-energy states comprising
at least
two components chosen from: a source of catalyst or catalyst; a source of
atomic hydrogen or
atomic hydrogen; reactants to form the source of catalyst or catalyst and a
source of atomic
2
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
hydrogen or atomic hydrogen; one or more reactants to initiate the catalysis
of atomic
hydrogen; and a support to enable the catalyst,
wherein the battery or fuel cell system for forming compounds having hydrogen
in
lower-energy states can further comprise any of a reaction vessel, a vacuum
pump, and
systems such as separator systems, an electrolyzer, thermal systems for
reversing an
exchange reaction, and chemical synthesis reactors to regenerate the fuel from
the reaction
products.
BRIEF DESCRIPTION OF THE DRAWINGS
FIGURE 1 is a schematic drawing of an energy reactor and power plant in
accordance
with the present disclosure.
FIGURE 2 is a schematic drawing of an energy reactor and power plant for
recycling or
regenerating the fuel in accordance with the present disclosure.
FIGURE 3 is a schematic drawing of a power reactor in accordance with the
present
disclosure.
FIGURE 4 is a schematic drawing of a system for recycling or regenerating the
fuel in
accordance with the present disclosure.
FIGURE 5 is a schematic drawing of a discharge power and plasma cell and
reactor in
accordance with the present disclosure.
FIGURE 6 is a schematic drawing of a battery and fuel cell in accordance with
the
present disclosure.
DETAILED DESCRIPTION OF THE EMBODIMENTS OF THE DISCLOSURE
The present disclosure is directed to catalyst systems to release energy from
atomic
hydrogen to form lower energy states wherein the electron shell is at a closer
position relative
to the nucleus. The released power is harnessed for power generation and
additionally new
hydrogen species and compounds are desired products. These energy states are
predicted by
classical physical laws and require a catalyst to accept energy from the
hydrogen in order to
undergo the corresponding energy-releasing transition.
Classical physics gives closed-form solutions of the hydrogen atom, the
hydride ion,
the hydrogen molecular ion, and the hydrogen molecule and predicts
corresponding species
having fractional principal quantum numbers. Using Maxwell's equations, the
structure of
the electron was derived as a boundary-value problem wherein the electron
comprises the
source current of time-varying electromagnetic fields during transitions with
the constraint
that the bound n = 1 state electron cannot radiate energy. A reaction
predicted by the
solution of the H atom involves a resonant, nonradiative energy transfer from
otherwise
stable atomic hydrogen to a catalyst capable of accepting the energy to form
hydrogen in
3
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
lower-energy states than previously thought possible. Specifically, classical
physics predicts
that atomic hydrogen may undergo a catalytic reaction with certain atoms,
excimers, ions,
and diatomic hydrides which provide a reaction with a net enthalpy of an
integer multiple of
the potential energy of atomic hydrogen, Eh = 27.2 eV where Eh is one Hartree.
Specific
species (e.g. He', Art , Sr', K, Li, HO, and NaH) identifiable on the basis of
their
known electron energy levels are required to be present with atomic hydrogen
to catalyze the
process. The reaction involves a nonradiative energy transfer followed by q =
13.6 eV
continuum emission or q = 13.6 eV transfer to H to form extraordinarily hot,
excited-state H
and a hydrogen atom that is lower in energy than unreacted atomic hydrogen
that corresponds
to a fractional principal quantum number. That is, in the formula for the
principal energy
levels of the hydrogen atom:
E e2 13.598 eV (1)
n28;Tc a, n2
n =1, 2,3,... (2)
where aõ is the Bohr radius for the hydrogen atom (52.947 pm), e is the
magnitude
of the charge of the electron, and eo is the vacuum permittivity,
fractional quantum numbers:
n =1, , 3 , 4 , ..., 1 ; where p < 137 is an integer (3)
2 p
replace the well known parameter n = integer in the Rydberg equation for
hydrogen excited
states and represent lower-energy-state hydrogen atoms called "hydrinos." The
n =1 state of
hydrogen and the n = 1 states of hydrogen are nonradiative, but a transition
between
integer
two nonradiative states, say n =1 to n =1 / 2, is possible via a nonradiative
energy transfer.
Hydrogen is a special case of the stable states given by Eqs. (1) and (3)
wherein the
corresponding radius of the hydrogen or hydrino atom is given by
r=aH, (4)
p
where p =1, 2,3,.... In order to conserve energy, energy must be transferred
from the
hydrogen atom to the catalyst in units of an integer of the potential energy
of the hydrogen
atom in the normal n =1 state, and the radius transitions to aH . Hydrinos are
formed by
m+p
reacting an ordinary hydrogen atom with a suitable catalyst having a net
enthalpy of reaction
of
m=27.2 eV (5)
where m is an integer. It is believed that the rate of catalysis is increased
as the net enthalpy
of reaction is more closely matched to m = 27.2 eV . It has been found that
catalysts having a
net enthalpy of reaction within 10%, preferably 5%, of m = 27.2 eV are
suitable for most
applications.
4
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
The catalyst reactions involve two steps of energy release: a nonradiative
energy
transfer to the catalyst followed by additional energy release as the radius
decreases to the
corresponding stable final state. Thus, the general reaction is given by
m = 27.2 eV + Catq+ +H a" Cat(q+r)+ + re- + H * aH l+m27.2 eV
p (m + p)
(6)
H aH H aH +[(p+m)2-p2].13.6eV-m=27.2eV (7)
(m + p) (m + p)
Cat(q+r)+ + re Catq+ + m = 27.2 eV and (8)
the overall reaction is
H ap" -> H ( aHP) l+[(p+m)2 -p2].13.6 eV (9)
m+
q, r, m, and p are integers. H * ( aH ) has the radius of the hydrogen atom
m+ P)
(corresponding to the 1 in the denominator) and a central field equivalent to
(m + p) times
that of a proton, and H ( a.) is the corresponding stable state with the
radius of
m+ P)
1 ) that of H H. As the electron undergoes radial acceleration from the radius
of the
(m+P
hydrogen atom to a radius of ( 1 ) this distance, energy is released as
characteristic light
m+ P)
emission or as third-body kinetic energy. The emission may be in the form of
an extreme-
ultraviolet continuum radiation having an edge at [(p + m)2 - p2 -2m].13.6 eV
91.2
nm) and extending to longer wavelengths. In addition to radiation, a
[(P + m)2 - p2 - 2m]
resonant kinetic energy transfer to form fast H may occur. Subsequent
excitation of these
fast H (n =1) atoms by collisions with the background H2 followed by emission
of the
corresponding H (n = 3) fast atoms gives rise to broadened Balmer a emission.
Extraordinary Balmer a line broadening (>100 eV) is observed consistent with
predictions.
A suitable catalyst can therefore provide a net positive enthalpy of reaction
of
m = 27.2 eV. That is, the catalyst resonantly accepts the nonradiative energy
transfer from
hydrogen atoms and releases the energy to the surroundings to affect
electronic transitions to
fractional quantum energy levels. As a consequence of the nonradiative energy
transfer, the
hydrogen atom becomes unstable and emits further energy until it achieves a
lower-energy
nonradiative state having a principal energy level given by Eqs. (1) and (3).
Thus, the
catalysis releases energy from the hydrogen atom with a commensurate decrease
in size of
the hydrogen atom, n = naõ where n is given by Eq. (3). For example, the
catalysis of
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
H (n =1) to H (n =1 / 4) releases 204 eV , and the hydrogen radius decreases
from a,, to
4 aõ . The catalyst product, H (1 / p) , may also react with an electron to
form a hydrino
hydride ion H- (1 / p) , or two H (1 / p) may react to form the corresponding
molecular
hydrino H2 (1/ P) .
Specifically, the catalyst product, H (1 / p) , may also react with an
electron to form a
novel hydride ion H- (1/ p) with a binding energy EB :
E = h2 s(s+1) irpoe2h2 1 22 (10)
B 2 1+ s(s+1) 2 me a 3
õ 3 1+ s(s+1)
8,ueao a0
P P
where p = integer > 1, s =1 / 2, h is Planck's constant bar, u0 is the
permeability of vacuum,
me is the mass of the electron, pe is the reduced electron mass given by Pe
memp
=
m
e +mp
44
where mp is the mass of the proton, ao is the Bohr radius, and the ionic
radius is
r, = P (1 + s (s + 1)) . From Eq. (10), the calculated ionization energy of
the hydride ion is
0.75418 eV, and the experimental value is 6082.99 0.15 cm-' (0.75418 eV).
Upfield-shifted NMR peaks are direct evidence of the existence of lower-energy
state
hydrogen with a reduced radius relative to ordinary hydride ion and having an
increase in
diamagnetic shielding of the proton. The shift is given by the sum of that of
an ordinary
hydride ion H- and a component due to the lower -energy state:
AB', _ -p e2 (1+a27rp) = -(29.9+1.37p) ppm (11)
B 0
12meaa (1+j-i-l))
where for H- p = 0 and p = integer > 1 for H- (1 / p) and a is the fine
structure constant.
H (1 / p) may react with a proton and two H (1 / p) may react to form H2 (1 /
p)+
and H2 (1 / p) , respectively. The hydrogen molecular ion and molecular charge
and current
density functions, bond distances, and energies are solved from the Laplacian
in ellipsoidal
coordinates with the constraint of nonradiation.
(71- ~ )R~ (R~ a0) + (~ - ~ )R,, (RI) + 77)R4- ~ (R~ 0~) = 0 . (12)
The total energy E,. of the hydrogen molecular ion having a central field of
+pe at each
focus of the prolate spheroid molecular orbital is
6
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
F
2h
2 e (41n3-1-21n3) l+p mec 2E,. = -p 2 82re(,a,,
pee pee (13)
3 3
4g--_ 2aõ 82re(, 3aõ
_1hi p p
2 ,u
_ -p216.13392 eV - p30.118755 eV
where p is an integer, c is the speed of light in vacuum, and ,u is the
reduced nuclear mass.
The total energy of the hydrogen molecule having a central field of +pe at
each focus of the
prolate spheroid molecular orbital is
I e2
2h 4;Tc,,ao
o
e2
2r2 -T2 +' In 2+1-,,r2 1+p 2
8ire0ao 2 -1 mec
E,' -p2 pe2 pee
3 3
8,re - 1 + 1 )aO
vF2
p 8 iri _1h p
2 u
_ -p231.351 eV -p30.326469 eV
(14)
The bond dissociation energy, Eõ , of the hydrogen molecule H2 (1 / p) is the
difference between the total energy of the corresponding hydrogen atoms and ET
ED = E(2H (1 / p)) - ET (15)
where
E(2H(1/ p)) =-p227.20 eV (16)
ED is given by Eqs. (15-16) and (14):
ED =-p227.20eV-E,.
= -p2 27.20 eV - (-p231.351 eV - p30.326469 eV) . (17)
= p2 4.151 eV + p3 0.326469 eV
Calculated and experimental parameters of H2, D2, HZ , and Dz are given in
TABLE 1.
7
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
TABLE 1. The Maxwellian closed-form calculated and experimental parameters of
H2 1 D2 ,
HZ and DZ .
Parameter Calculated Experimental
H2 Bond Energy 4.478 eV 4.478 eV
D2 Bond Energy 4.556 eV 4.556 eV
HZ Bond Energy 2.654 eV 2.651 eV
DZ Bond Energy 2.696 eV 2.691 eV
H2 Total Energy 31.677 eV 31.675 eV
D2 Total Energy 31.760 eV 31.760 eV
H2 Ionization Energy 15.425 eV 15.426 eV
D2 Ionization Energy 15.463 eV 15.466 eV
HZ Ionization Energy 16.253 eV 16.250 eV
DZ Ionization Energy 16.299 eV 16.294 eV
HZ Magnetic Moment 9.274 X 10-24 JT -1 (Pe) 9.274 X 10-24 JT -' (NB
Absolute H2 Gas-Phase -28.0 ppm -28.0 ppm
NMR Shift
0.748 A
H2 Internuclear Distancea 12a, 0.741 A
0.748 A
D2 Internuclear Distancea 0.741 A
1.058 A
HZ Internuclear Distance 2a 1.06 A
0
1.058A
DZ Internuclear Distancea 2a 1.0559 A
H2 Vibrational Energy 0.517 eV 0.516 eV
D2 Vibrational Energy 0.371 eV 0.371 eV
H2 Wexe 120.4 cm-1 121.33 cm-'
D2 COeXe 60.93 cm-' 61.82 cm-1
HZ Vibrational Energy 0.270 eV 0.271 eV
DZ Vibrational Energy 0.193 eV 0.196 eV
H2 J=1 to J=0 Rotational Energya 0.0148 eV 0.01509 eV
D2 J=1 to J=0 Rotational Energya 0.00741 eV 0.00755 eV
HZ J=1 to J=0 Rotational Energy 0.00740 eV 0.00739 eV
DZ J=1 to J=0 Rotational Energya 0.00370 eV 0.003723 eV
a Not corrected for the slight reduction in internuclear distance due to EOSC
.
8
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
The NMR of catalysis-product gas provides a definitive test of the
theoretically
predicted chemical shift of H2(1/4). In general, the 'H NMR resonance of
H2(11P) is
predicted to be upfield from that of H2 due to the fractional radius in
elliptic coordinates
wherein the electrons are significantly closer to the nuclei. The predicted
shift, B1 , for
H2 (1/ p) is given by the sum of that of H2 and a term that depends on p =
integer > 1 for
H2 (1/ P):
AB', =-,uo 4-h1n r2 +1 e2 (1+;rap) (18)
B -1 36aome
OB,, _ -(28.01+0.64p) ppm (19)
B
where for H2 p = 0. The experimental absolute H2 gas-phase resonance shift of -
28.0 ppm
is in excellent agreement with the predicted absolute gas-phase shift of -
28.01 ppm (Eq.
(19)).
The vibrational energies, EVIb , for the v = 0 to v = 1 transition of hydrogen-
type
molecules H2 (1/ p) are
Evib = p20.515902 eV (20)
where p is an integer. The rotational energies, E,01, for the J to J +1
transition of
hydrogen-type molecules H2 (1/ p) are
E,ot = EJ+1-E, = j [J +1] = p2 (J +1)0.01509 eV (21)
where p is an integer, I is the moment of inertia.
The p2 dependence of the rotational energies results from an inverse p
dependence
of the internuclear distance and the corresponding impact on the moment of
inertia I . The
predicted internuclear distance 2c' for H2 (1 / p) is
2c' = a (22)
p
The data from a broad spectrum of investigational techniques strongly and
consistently indicates that hydrogen can exist in lower-energy states than
previously thought
possible. This data supports the existence of these lower-energy states called
hydrino, for
"small hydrogen," and the corresponding hydride ions and molecular hydrino.
Some of these
prior related studies supporting the possibility of a novel reaction of atomic
hydrogen, which
produces hydrogen in fractional quantum states that are at lower energies than
the traditional
"ground" (n =1) state, include extreme ultraviolet (EUV) spectroscopy,
characteristic
emission from catalysts and the hydride ion products, lower-energy hydrogen
emission,
chemically-formed plasmas, Balmer a line broadening, population inversion of H
lines,
elevated electron temperature, anomalous plasma afterglow duration, power
generation, and
analysis of novel chemical compounds.
9
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
The catalytic lower-energy hydrogen transitions of the present disclosure
require a
catalyst that may be in the form of an endothermic chemical reaction of an
integer m of the
potential energy of uncatalyzed atomic hydrogen, 27.2 eV , that accepts the
energy from
atomic H to cause the transition. The endothermic catalyst reaction may be the
ionization of
one or more electrons from a species such as an atom or ion (e.g. m = 3 for Li
-> Liz+) and
may further comprise the concerted reaction of a bond cleavage with ionization
of one or
more electrons from one or more of the partners of the initial bond (e.g. m =
2 for
NaH -> Na2+ +H ). He+ fulfills the catalyst criterion-a chemical or physical
process with
an enthalpy change equal to an integer multiple of 27.2 eV since it ionizes at
54.417 eV,
which is 2.27.2 eV. Two hydrogen atoms may also serve as the catalyst of the
same
enthalpy. Hydrogen atoms H (1 / p) p =1, 2,3,...137 can undergo further
transitions to
lower-energy states given by Eqs. (1) and (3) wherein the transition of one
atom is catalyzed
by a second that resonantly and nonradiatively accepts m = 27.2 eV with a
concomitant
opposite change in its potential energy. The overall general equation for the
transition of
H (1 / p) to H (1 / (p + m)) induced by a resonance transfer of m = 27.2 eV to
H (1 / p') is
represented by
H(1/p')+H(1/p)*H+H(1/(p+m))+[2pm+m2-pi2+1]=13.6 eV . (23)
Hydrogen atoms may serve as a catalyst wherein m =1 and m = 2 for one and two
atoms,
respectively, acting as a catalyst for another. The rate for the two-atom-
catalyst, 2H , may be
high when extraordinarily fast H collides with a molecule to form the 2H
wherein two atoms
resonantly and nonradiatively accept 54.4 eV from a third hydrogen atom of the
collision
partners.
With m = 2, the product of catalysts He+ and 2H is H (1/ 3) that reacts
rapidly to
form H(1/4), then molecular hydrino, H1(1/4), as a preferred state.
Specifically, in the
case of a high hydrogen atom concentration, the further transition given by
Eq. (23) of
H (1 / 3) (p = 3) to H (1 / 4) (p + m = 4) with H as the catalyst (p'= 1 ; m
=1) can be fast:
H(1/3)-">H(1/4)+95.2 eV . (24)
The corresponding molecular hydrino H 2 (1/ 4) and hydrino hydride ion H- (1 /
4) are final
products consistent with observation since the p = 4 quantum state has a
multipolarity
greater than that of a quadrupole giving it H(114) a long theoretical lifetime
for further
catalysis.
The nonradiative energy transfer to the catalysts, He+ and 2H , is predicted
to pump
the He+ ion energy levels and increase the electron excitation temperature of
H in helium-
hydrogen and hydrogen plasmas, respectively. For both catalysts, the
intermediate
H * [ 2 + 1 ] (Eq. (6) with m = 2) has the radius of the hydrogen atom
(corresponding to the 1
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
in the denominator) and a central field equivalent to 3 times that of a
proton, and H 3 is
the corresponding stable state with the radius of 1/3 that of H. As the
electron undergoes
radial acceleration from the radius of the hydrogen atom to a radius of 1/3
this distance,
energy is released as characteristic light emission or as third-body kinetic
energy. The
emission may be in the form of an extreme-ultraviolet continuum radiation
having an edge at
54.4 eV (22.8 nm) and extending to longer wavelengths. The emission may be in
the form
of an extreme-ultraviolet continuum radiation having an edge at 54.4 eV (22.8
nm) and
extending to longer wavelengths. Alternatively, fast H is predicted due to a
resonant kinetic-
energy transfer. A secondary continuum band is predicted arising from the
subsequently
rapid transition of the catalysis product [.LL] (Eqs. (4-7) and (23)) to the
[a,, 4 J state
wherein atomic hydrogen accepts 27.2 eV from L3J Extreme ultraviolet (EUV)
spectroscopy and high-resolution visible spectroscopy were recorded on
microwave and glow
and pulsed discharges of helium with hydrogen and hydrogen alone providing
catalysts He'
and 2H , respectively. Pumping of the He' ion lines occurred with the addition
of
hydrogen, and the excitation temperature of hydrogen plasmas under certain
conditions was
very high. The EUV continua at both 22.8 nm and 40.8 nm were observed and
extraordinary
(>50 eV) Balmer a line broadening were observed. H2 (1/4) was observed by
solution
NMR at 1.25 ppm on gases collected from helium-hydrogen, hydrogen, and water-
vapor-
assisted hydrogen plasmas and dissolved in CDC13.
Similarly, the reaction of Ar+ to Ar2+ has a net enthalpy of reaction of 27.63
eV,
which is equivalent to m =1 in Eqs. (4-7). When Ar+ served as the catalyst its
predicted
91.2 nm and 45.6 nm continua were observed as well as the other characteristic
signatures of
hydrino transitions, pumping of the catalyst excited states, fast H, and the
predicted gaseous
hydrino product H2(1/4) that was observed by solution NMR at 1.25 ppm.
Considering
these results and those of helium plasmas, the q . 13.6 eV continua with
thresholds at
54.4 eV (q = 4) and 40.8 eV (q = 3) for He' catalyst and at 27.2 eV (q = 2)
and
13.6 eV (q =1) for Ar+ catalyst have been observed. Much higher values of q
are possible
with transitions of hydrinos to lower states giving rise to high-energy
continuum radiation
over a broad spectral region.
In recent power generation and product characterization studies, atomic
lithium and
molecular NaH served as catalysts since they meet the catalyst criterion-a
chemical or
physical process with an enthalpy change equal to an integer multiple m of the
potential
energy of atomic hydrogen, 27.2 eV (e.g. m = 3 for Li and m = 2 for NaH ).
Specific
predictions based on closed-form equations for energy levels of the
corresponding hydrino
hydride ions H- (1 / 4) of novel alkali halido hydrino hydride compounds
11
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
(MH * X ; M = Li or Na, X = halide) and molecular hydrino H2 (1/ 4) were
tested using
chemically generated catalysis reactants.
First, Li catalyst was tested. Li and LiNH2 were used as a source of atomic
lithium
and hydrogen atoms. Using water-flow, batch calorimetry, the measured power
from 1g Li,
0.5g LiNHZ 1 10g LiBr, and 15g Pd / A1203 was about 160W with an energy
balance of
OH = -19.1 kJ . The observed energy balance was 4.4 times the maximum
theoretical based
on known chemistry. Next, Raney nickel (R-Ni) served as a dissociator when the
power
reaction mixture was used in chemical synthesis wherein LiBr acted as a getter
of the
catalysis product H(114) to form LiH *X as well as to trap H2 (1/ 4) in the
crystal. The
ToF-SIMs showed LiH * X peaks. The 1H MAS NMR LiH * Br and LiH *I showed a
large distinct upfield resonance at about -2.5 ppm that matched H- (1/ 4) in a
LiX matrix.
An NMR peak at 1.13 ppm matched interstitial H2 (1/ 4), and the rotation
frequency of
H2 (1/ 4) of 42 times that of ordinary H2 was observed at 1989 cm-' in the
FTIR spectrum.
The XPS spectrum recorded on the LiH * Br crystals showed peaks at about 9.5
eV and 12.3
eV that could not be assigned to any known elements based on the absence of
any other
primary element peaks, but matched the binding energy of H-(114) in two
chemical
environments. A further signature of the energetic process was the observation
of the
formation of a plasma called a resonant transfer- or rt-plasma at low
temperatures (e.g.
103 K) and very low field strengths of about 1-2 V/cm when atomic Li was
present with
atomic hydrogen. Time-dependent line broadening of the H Balmer a line was
observed
corresponding to extraordinarily fast H (>40 eV).
A compound of the present disclosure such as MH comprising hydrogen and at
least
one element M other than hydrogen serves as a source of hydrogen and a source
of catalyst to
form hydrinos. A catalytic reaction is provided by the breakage of the M - H
bond plus the
ionization of t electrons from the atom M each to a continuum energy level
such that the
sum of the bond energy and ionization energies of the t electrons is
approximately
m = 27.2 eV , where m is an integer. One such catalytic system involves
sodium. The bond
energy of NaH is 1.9245 eV, and the first and second ionization energies of Na
are
5.13908 eV and 47.2864 eV, respectively. Based on these energies NaH molecule
can
serve as a catalyst and H source, since the bond energy of NaH plus the double
ionization
(t = 2) of Na to Na2+ is 54.35 eV (2.27.22 eV). The catalyst reactions are
given by
54.35 eV + NaH -> N a 2+ + 2e- + H L 3 J + [32 -12 ] .13.6 eV (25)
Na2++2e +H ->NaH+54.35 eV . (26)
And the overall reaction is
H -> H r3l +[32 -12] . 13.6 eV. (27)
12
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
The product H (1/ 3) reacts rapidly to form H (1 / 4), then molecular hydrino,
H2 (1/ 4), as a
preferred state (Eq. (24)). The NaH catalyst reactions may be concerted since
the sum of
the bond energy of NaH , the double ionization (t = 2) of Na to Na 2+ , and
the potential
energy of H is 81.56 eV (3.27.2 eV ). The catalyst reactions are given by
81.56eV+NaH+H--+Na2++2e +Hfas,+e +H[ 4 +[42-12]=13.6 eV (28)
--* NaH + H + 81.56 eV. (29)
Na 2+ +2e- + H +Hfas, +e-
And the overall reaction is
H~HL4J+[42-12]13.6 eV, (30)
where H+ s, is a fast hydrogen atom having at least 13.6 eV of kinetic energy.
H (1 / 4)
forms stable halidohydrides and is a favored product together with the
corresponding
molecule formed by the reactions 2H(1/4) H, (1/ 4) and H- (114)+H+ H, (l/ 4).
Sodium hydride is typically in the form of an ionic crystalline compound
formed by
the reaction of gaseous hydrogen with metallic sodium. And, in the gaseous
state, sodium
comprises covalent Nat molecules with a bond energy of 74.8048 kJ/mole. It was
found that
when NaH (s) was heated at a very slow temperature ramp rate (0.1 C/min) under
a helium
atmosphere to form NaH (g) , the predicted exothermic reaction given by Eqs.
(25-27) was
observed at high temperature by differential scanning calorimetry (DSC). To
achieve high
power, a chemical system was designed to greatly increase the amount and rate
of formation
of NaH (g) . The reaction of NaOH and Na to Na2O and NaH (s) calculated from
the
heats of formation releases AH = -44.7 kI / mole NaOH :
NaOH + 2Na --* Na20 +NaH (s) AH = -44.7 kI / mole NaOH. (31)
This exothermic reaction can drive the formation of NaH (g) and was exploited
to drive the
very exothermic reaction given by Eqs. (25-27). The regenerative reaction in
the presence of
atomic hydrogen is
Na20 + H ->NaOH + Na AH = -11.6 kJ / mole NaOH (32)
NaH -+Na+H(1/3) AH =-10,500 kI /mole H (33)
and
NaH -+ Na + H (1 / 4) AH =-19,700 kI I mole H. (34)
NaH uniquely achieves high kinetics since the catalyst reaction relies on the
release
of the intrinsic H, which concomitantly undergoes the transition to form
H(113) that
further reacts to form H(114). High-temperature differential scanning
calorimetry (DSC)
was performed on ionic NaH under a helium atmosphere at an extremely slow
temperature
ramp rate (0.1 C/min) to increase the amount of molecular NaH formation. A
novel
exothermic effect of -177 k7 / moleNaH was observed in the temperature range
of 640 C to
825 C. To achieve high power, R-Ni having a surface area of about 100 m2 / g
was surface-
13
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
coated with NaOH and reacted with Na metal to form NaH . Using water-flow,
batch
calorimetry, the measured power from 15g of R-Ni was about 0.5 kW with an
energy balance
of AH = -36 kI compared to AH 0 kI from the R-Ni starting material, R-NiAl
alloy,
when reacted with Na metal. The observed energy balance of the NaH reaction
was
-1.6X104 kJ / mole H2 , over 66 times the -241.8 kI / mole H2 enthalpy of
combustion.
With an increase in NaOH doping to 0.5 wt%, the Al of the R-Ni intermetallic
served to
replace Na metal as a reductant to generate NaH catalyst. When heated to 60 C,
15g of the
composite catalyst material required no additive to release 11.7 kJ of excess
energy and
develop a power of 0.25 kW. Solution NMR on product gases dissolved in DMF-d7
showed
H2(1/4) at 1.2 ppm.
The ToF-SIMs showed sodium hydrino hydride, NaHx, peaks. The 1H MAS NMR
spectra of NaH * Br and NaH * Cl showed large distinct upfield resonance at -
3.6 ppm and
-4 ppm, respectively, that matched H-(1/4), and an NMR peak at 1.1 ppm matched
H2 (1/ 4). NaH * Cl from reaction of NaCl and the solid acid KHS04 as the only
source
of hydrogen comprised two fractional hydrogen states. The H-(114) NMR peak was
observed at -3.97 ppm, and the H-(113) peak was also present at -3.15 ppm. The
corresponding H2(1/4) and H2(113) peaks were observed at 1.15 ppm and 1.7 ppm,
respectively. 1H NMR of NaH * F dissolved in DMF-d7 showed isolated H2 (1/ 4)
and
H- (1 / 4) at 1.2 ppm and -3.86 ppm, respectively, wherein the absence of any
solid matrix
effect or the possibly of alternative assignments confirmed the solid NMR
assignments. The
XPS spectrum recorded on NaH * Br showed the H- (1 / 4) peaks at about 9.5 eV
and 12.3
eV that matched the results from LiH * Br and KH *I ; whereas, sodium hydrino
hydride
showed two fractional hydrogen states additionally having the H - (1 / 3) XPS
peak at 6 eV in
the absence of a halide peak. The predicted rotational transitions having
energies of 42 times
those of ordinary H2 were also observed from H2 (1/ 4) which was excited using
a 12.5 keV
electron beam.
These data such as NMR shifts, ToF-SIMs masses, XPS binding energies, FTIR,
and
emission spectrum are characteristic of and identify hydrino products of the
catalysts systems
that comprise an aspect of the present disclosure.
14
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
1. Hydrinos
A hydrogen atom having a binding energy given by
Binding Energy = 13.6 eV (35)
(1 / p)2
where p is an integer greater than 1, preferably from 2 to 137, is the product
of the H
catalysis reaction of the present disclosure. The binding energy of an atom,
ion, or molecule,
also known as the ionization energy, is the energy required to remove one
electron from the
atom, ion or molecule. A hydrogen atom having the binding energy given in Eq.
(35) is
hereafter referred to as a "hydrino atom" or "hydrino." The designation for a
hydrino of
radius a" ,where aõ is the radius of an ordinary hydrogen atom and p is an
integer, is
P
H . A hydrogen atom with a radius aõ is hereinafter referred to as "ordinary
hydrogen
P
atom" or "normal hydrogen atom." Ordinary atomic hydrogen is characterized by
its binding
energy of 13.6 eV.
Hydrinos are formed by reacting an ordinary hydrogen atom with a suitable
catalyst
having a net enthalpy of reaction of
m = 27.2 eV (36)
where m is an integer. It is believed that the rate of catalysis is increased
as the net enthalpy
of reaction is more closely matched to m = 27.2 eV. It has been found that
catalysts having a
net enthalpy of reaction within 10%, preferably 5%, of m = 27.2 eV are
suitable for most
applications.
This catalysis releases energy from the hydrogen atom with a commensurate
decrease
in size of the hydrogen atom, n = naõ . For example, the catalysis of H (n =1)
to
H(n =1 / 2) releases 40.8 eV , and the hydrogen radius decreases from aõ to 2
aõ . A
catalytic system is provided by the ionization of t electrons from an atom
each to a
continuum energy level such that the sum of the ionization energies of the t
electrons is
approximately m = 27.2 eV where m is an integer.
A further example to such catalytic systems given supra (Eqs. (6-9) involves
lithium
metal. The first and second ionization energies of lithium are 5.39172 eV and
75.64018 eV,
respectively. The double ionization (t = 2) reaction of Li to Liz+, then, has
a net enthalpy of
reaction of 81.0319 eV , which is equivalent to m = 3 in Eq. (36).
81.0319 eV+Li(m)+H - Liz++2e-+H a" +[(p+3)2-p21 .13.6 eV
ap " (p + 3)
(37)
Lie+ + 2e- ---> Li (m) + 81.0319 eV. (38)
And the overall reaction is
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
H P H (p+3) +[(p+3)2 - p21.13.6 eV. (39)
In another embodiment, the catalytic system involves cesium. The first and
second
ionization energies of cesium are 3.89390 eV and 23.15745 eV, respectively.
The double
ionization (t = 2) reaction of Cs to Cs2+, then, has a net enthalpy of
reaction of
27.05135 eV, which is equivalent to m = 1 in Eq. (36).
27.05135 eV+Cs(m)+H a_> Cs2++2e-+H all +[(p+1)2-p2].13.6 eV
P (P+1)
(40)
Cs2++2e -> Cs(m)+27.05135 eV . (41)
And the overall reaction is
H P -> H (P+1) +[(P+1)2 -p21 13.6 eV. (42)
An additional catalytic system involves potassium metal. The first, second,
and third
ionization energies of potassium are 4.34066 eV, 31.63 eV, 45.806 eV ,
respectively. The
triple ionization (t=3) reaction of K to K3+, then, has a net enthalpy of
reaction of
81.7767 eV, which is equivalent to m = 3 in Eq. (36).
81.7767 eV + K (m) + H aH -K3++3e +H all +[(p+3)2-p2]=13.6eV(43)
P (p+3)
K3+ +3e- - K(m)+81.7426 eV. (44)
And the overall reaction is
H P H (p+3) 1+[(p + 3)2 - p2] P13.6 eV. (45)
As a power source, the energy given off during catalysis is much greater than
the energy lost
to the catalyst. The energy released is large as compared to conventional
chemical reactions.
For example, when hydrogen and oxygen gases undergo combustion to form water
H2 (g) + 2 02 (g) -> H2O (1) (46)
the known enthalpy of formation of water is AH f = -286 kJ / mole or 1.48 eV
per hydrogen
atom. By contrast, each (n =1) ordinary hydrogen atom undergoing catalysis
releases a net
of 40.8 eV. Moreover, further catalytic transitions may occur: n = 1 - 1 , 1 --
> 1 , 1 -> 1
2 3 3 4 4 5
and so on. Once catalysis begins, hydrinos autocatalyze further in a process
called
disproportionation. This mechanism is similar to that of an inorganic ion
catalysis. But,
hydrino catalysis should have a higher reaction rate than that of the
inorganic ion catalyst due
to the better match of the enthalpy to m = 27.2 eV.
The hydrino hydride ion of the present disclosure can be formed by the
reaction of an
electron source with a hydrino, that is, a hydrogen atom having a binding
energy of about
16
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
13.62eV . where n = 1 and p is an integer greater than 1. The hydrino hydride
ion is
n p
represented by H- (n =1 / p) or H- (1/ P) :
H +e- ->H-(n=1/p) (47)
H a" +e- --> H-(1/ p). (48)
P
The hydrino hydride ion is distinguished from an ordinary hydride ion
comprising an
ordinary hydrogen nucleus and two electrons having a binding energy of about
0.8 eV. The
latter is hereafter referred to as "ordinary hydride ion" or "normal hydride
ion." The hydrino
hydride ion comprises a hydrogen nucleus including proteum, deuterium, or
tritium, and two
indistinguishable electrons at a binding energy according to Eqs. (49-50).
The binding energy of a hydrino hydride ion can be represented by the
following
formula:
z z z h Binding Energy = s (s +1) 2_ poe h 1 2 3 (49)
- 2 3
2 1+ S(S+1) me a 3 1+ s(S+1)
8pea0 ao
P P
where p is an integer greater than one, s =1 / 2 , ,r is pi, h is Planck's
constant bar, p0 is
the permeability of vacuum, me is the mass of the electron, pe is the reduced
electron mass
given by Pe = memp where mp is the mass of the proton, a1, is the radius of
the
m
e +mp
J
hydrogen atom, a,, is the Bohr radius, and e is the elementary charge. The
radii are given by
r2 = r, = a0 1+ s(s+1)); s = 1 . (50)
The binding energies of the hydrino hydride ion, H- (n =1 / p) as a function
of p ,
where p is an integer, are shown in TABLE 2.
17
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
TABLE 2. The representative binding energy of the hydrino hydride ion H- (n
=I/ p) as a
function of p , Eq. (49).
Hydride Ion Yl Binding Wavelength
(a0 )a Energy (eV)b (nm)
H (n =1) 1.8660 0.7542 1644
H (n = 1/ 2) 0.9330 3.047 406.9
H-(n=1/3) 0.6220 6.610 187.6
H (n =1/ 4) 0.4665 11.23 110.4
H (n =1 / 5) 0.3732 16.70 74.23
H (n =1/ 6) 0.3110 22.81 54.35
H (n = 1/ 7) 0.2666 29.34 42.25
H (n = 1/ 8) 0.2333 36.09 34.46
H (n =1/ 9) 0.2073 42.84 28.94
H (n =1/ 10) 0.1866 49.38 25.11
H (n =1/11) 0.1696 55.50 22.34
H (n =1 / 12) 0.1555 60.98 20.33
H (n =1 / 13) 0.1435 65.63 18.89
H (n =1 / 14) 0.1333 69.22 17.91
H (n =1/15) 0.1244 71.55 17.33
H-(n=1/16) 0.1166 72.40 17.12
H (n =1/17) 0.1098 71.56 17.33
H (n=1/18) 0.1037 68.83 18.01
H (n =I/ 19) 0.0982 63.98 19.38
H (n = 1/ 20) 0.0933 56.81 21.82
H (n =1 / 21) 0.0889 47.11 26.32
H (n = 1/ 22) 0.0848 34.66 35.76
H (n=1/23) 0.0811 19.26 64.36
H (n =1 / 24) 0.0778 0.6945 1785
a Eq. (50)
b Eq. (49)
According to the present disclosure, a hydrino hydride ion (H) having a
binding
18
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
energy according to Eqs. (49-50) that is greater than the binding of ordinary
hydride ion
(about 0.75 eV) for p = 2 up to 23, and less for p = 24 (H) is provided. For p
= 2 to
p = 24 of Eqs. (49-50), the hydride ion binding energies are respectively 3,
6.6, 11.2, 16.7,
22.8, 29.3, 36.1, 42.8, 49.4, 55.5, 61.0, 65.6, 69.2, 71.6, 72.4, 71.6, 68.8,
64.0, 56.8, 47.1,
34.7, 19.3, and 0.69 eV. Exemplary compositions comprising the novel hydride
ion are also
provided herein.
Exemplary compounds are also provided comprising one or more hydrino hydride
ions and one or more other elements. Such a compound is referred to as a
"hydrino hydride
compound."
Ordinary hydrogen species are characterized by the following binding energies
(a)
hydride ion, 0.754 eV ("ordinary hydride ion"); (b) hydrogen atom ("ordinary
hydrogen
atom"), 13.6 eV; (c) diatomic hydrogen molecule, 15.3 eV ("ordinary hydrogen
molecule");
(d) hydrogen molecular ion, 16.3 eV ("ordinary hydrogen molecular ion"); and
(e) H; , 22.6
eV ("ordinary trihydrogen molecular ion"). Herein, with reference to forms of
hydrogen,
"normal" and "ordinary" are synonymous.
According to a further embodiment of the present disclosure, a compound is
provided
comprising at least one increased binding energy hydrogen species such as (a)
a hydrogen
atom having a binding energy of about 13.6 eV , such as within a range of
about 0.9 to 1.1 )2 1
P
13.6 eV
times 2 where p is an integer from 2 to 137; (b) a hydride ion (H-) having a
binding
1
P
energy of about
) 1u2 h 2 2
= s(S +1 2_ e 1 2 3 such as
Binding Energy h 2
2 1+ S(S+1) me aH 3 1+ S(S+1)
8 uea0 p ao
P
within a range of about 0.9 to 1.1 times
2 h 2 2
= s(s+1) 2_ 1ue 1 2 where p is an
Binding Energy h 2
2 3 [~- 3 2 1+ -SUS +1) me aH 3 1+ S(S+1)
8,ueao P ao
P
integer from 2 to 24; (c) H4 (lip); (d) a trihydrino molecular ion, H3 (1 /
p), having a
19
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
binding energy of about 22.6 2 eV such as within a range of about 0.9 to 1.1
times
11
P
22.6 eV where is an integer from 2 to 137; (e) a dihydrino having a binding
energy of
1 2 p
P
about 15.3 eV such as within a range of about 0.9 to 1.1 times 15.3 eV where p
is an
1 )2 1 )2
P P
integer from 2 to 137; (f) a dihydrino molecular ion with a binding energy of
about
16.3 eV such as within a range of about 0.9 to 1.1 times 16.3 eV where p is an
integer,
1 )2 1 )2
P P
preferably an integer from 2 to 137.
According to a further embodiment of the present disclosure, a compound is
provided
comprising at least one increased binding energy hydrogen species such as (a)
a dihydrino
molecular ion having a total energy of about
2e2
2hi 47zs(,~2a~~~3
e2 (41n3-1-21n3) 1+p me
E7. =-p
2 87cs~aH Pee Pee mec
(51)
3 3
4zre0 2aH 8ge0 3a71
_1h P P
2
_ -p216.13392 eV -p30.118755 eV
such as within a range of about 0.9 to 1.1 times
2e2
4~ g,, (2aH )3
2h
h
e2 (41n3-1-21n3) 1+p me
E 2 8gE,,aH mec
E. -P
pee pee where p is an integer, h is
3 3
42aH 8irt0 3a,,
1h P P
2 ,u
_ -p216.13392 eV - p30.118755 eV
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
Planck's constant bar, me is the mass of the electron, c is the speed of light
in vacuum, and
,u is the reduced nuclear mass, and (b) a dihydrino molecule having a total
energy of about
ez
4.e0aI
2h
z
e
e
e 2~-~+? In 2+12 1+p m2
8reõaQ 2 -1 mec
z
ET = -p pee pee
3
8rceo P Cl + ~ )aO
87C,
1h p
2 ,u
_-p231.351 eV -p30.326469 eV
(52)
such as within a range of about 0.9 to 1.1 times
ez
2~i 4 i a0
e
ez
2~-r2 + In 2+1-~ 1+p me
8)Taõao 2 -1 mec 2
E' Pz Pez _ Pez where p is an
3 3
ao ~ 8~eo P C1+~aa
8,reo
-1h p
2 ,u
_ -p231.351 eV -p30.326469 eV
integer and a(, is the Bohr radius.
According to one embodiment of the present disclosure wherein the compound
comprises a negatively charged increased binding energy hydrogen species, the
compound
further comprises one or more cations, such as a proton, ordinary H2 +, or
ordinary H3 .
A method is provided herein for preparing compounds comprising at least one
hydrino hydride ion. Such compounds are hereinafter referred to as "hydrino
hydride
compounds." The method comprises reacting atomic hydrogen with a catalyst
having a net
enthalpy of reaction of about 2 =27 eV, where m is an integer greater than 1,
preferably an
integer less than 400, to produce an increased binding energy hydrogen atom
having a
binding energy of about 13.6 eV where p is an integer, preferably an integer
from 2 to 137.
1 )2
P
21
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
A further product of the catalysis is energy. The increased binding energy
hydrogen atom
can be reacted with an electron source, to produce an increased binding energy
hydride ion.
The increased binding energy hydride ion can be reacted with one or more
cations to produce
a compound comprising at least one increased binding energy hydride ion.
The novel hydrogen compositions of matter can comprise:
(a) at least one neutral, positive, or negative hydrogen species (hereinafter
"increased
binding energy hydrogen species") having a binding energy
(i) greater than the binding energy of the corresponding ordinary hydrogen
species, or
(ii) greater than the binding energy of any hydrogen species for which the
corresponding ordinary hydrogen species is unstable or is not observed because
the ordinary
hydrogen species' binding energy is less than thermal energies at ambient
conditions
(standard temperature and pressure, STP), or is negative; and
(b) at least one other element. The compounds of the present disclosure are
hereinafter referred to as "increased binding energy hydrogen compounds."
By "other element" in this context is meant an element other than an increased
binding energy hydrogen species. Thus, the other element can be an ordinary
hydrogen
species, or any element other than hydrogen. In one group of compounds, the
other element
and the increased binding energy hydrogen species are neutral. In another
group of
compounds, the other element and increased binding energy hydrogen species are
charged
such that the other element provides the balancing charge to form a neutral
compound. The
former group of compounds is characterized by molecular and coordinate
bonding; the latter
group is characterized by ionic bonding.
Also provided are novel compounds and molecular ions comprising
(a) at least one neutral, positive, or negative hydrogen species (hereinafter
"increased
binding energy hydrogen species") having a total energy
(i) greater than the total energy of the corresponding ordinary hydrogen
species, or
(ii) greater than the total energy of any hydrogen species for which the
corresponding ordinary hydrogen species is unstable or is not observed because
the ordinary
hydrogen species' total energy is less than thermal energies at ambient
conditions, or is
negative; and
(b) at least one other element.
The total energy of the hydrogen species is the sum of the energies to remove
all of the
electrons from the hydrogen species. The hydrogen species according to the
present
disclosure has a total energy greater than the total energy of the
corresponding ordinary
hydrogen species. The hydrogen species having an increased total energy
according to the
present disclosure is also referred to as an "increased binding energy
hydrogen species" even
22
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
though some embodiments of the hydrogen species having an increased total
energy may
have a first electron binding energy less that the first electron binding
energy of the
corresponding ordinary hydrogen species. For example, the hydride ion of Eqs.
(49-50) for
p = 24 has a first binding energy that is less than the first binding energy
of ordinary hydride
ion, while the total energy of the hydride ion of Eqs. (49-50) for p = 24 is
much greater than
the total energy of the corresponding ordinary hydride ion.
Also provided herein are novel compounds and molecular ions comprising
(a) a plurality of neutral, positive, or negative hydrogen species
(hereinafter
"increased binding energy hydrogen species") having a binding energy
(i) greater than the binding energy of the corresponding ordinary hydrogen
species, or
(ii) greater than the binding energy of any hydrogen species for which the
corresponding ordinary hydrogen species is unstable or is not observed because
the ordinary
hydrogen species' binding energy is less than thermal energies at ambient
conditions or is
negative; and
(b) optionally one other element. The compounds of the present disclosure are
hereinafter referred to as "increased binding energy hydrogen compounds."
The increased binding energy hydrogen species can be formed by reacting one or
more hydrino atoms with one or more of an electron, hydrino atom, a compound
containing
at least one of said increased binding energy hydrogen species, and at least
one other atom,
molecule, or ion other than an increased binding energy hydrogen species.
Also provided are novel compounds and molecular ions comprising
(a) a plurality of neutral, positive, or negative hydrogen species
(hereinafter
"increased binding energy hydrogen species") having a total energy
(i) greater than the total energy of ordinary molecular hydrogen, or
(ii) greater than the total energy of any hydrogen species for which the
corresponding ordinary hydrogen species is unstable or is not observed because
the ordinary
hydrogen species' total energy is less than thermal energies at ambient
conditions or is
negative; and
(b) optionally one other element. The compounds of the present disclosure are
hereinafter referred to as "increased binding energy hydrogen compounds".
In an embodiment, a compound is provided comprising at least one increased
binding
energy hydrogen species chosen from (a) hydride ion having a binding energy
according to
Eqs. (49-50) that is greater than the binding of ordinary hydride ion (about
0.8 eV) for p = 2
up to 23, and less for p = 24 ("increased binding energy hydride ion" or
"hydrino hydride
ion"); (b) hydrogen atom having a binding energy greater than the binding
energy of ordinary
hydrogen atom (about 13.6 eV) ("increased binding energy hydrogen atom" or
"hydrino"); (c)
hydrogen molecule having a first binding energy greater than about 15.3 eV
("increased
23
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
binding energy hydrogen molecule" or "dihydrino"); and (d) molecular hydrogen
ion having a
binding energy greater than about 16.3 eV ("increased binding energy molecular
hydrogen
ion" or "dihydrino molecular ion").
II. Power Reactor and System
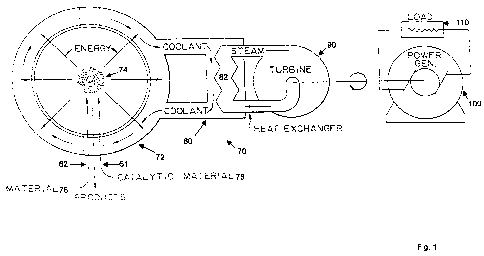
According to another embodiment of the present disclosure, a hydrogen catalyst
reactor for producing energy and lower-energy hydrogen species is provided. As
shown in
FIGURE 1, a hydrogen catalyst reactor 70 comprises a vessel 72 that contains
an energy
reaction mixture 74, a heat exchanger 80, and a power converter such as a
steam generator 82
and turbine 90. In an embodiment, the catalysis involves reacting atomic
hydrogen from the
source 76 with the catalyst 78 to form lower-energy hydrogen "hydrinos" and
produce power.
The heat exchanger 80 absorbs heat released by the catalysis reaction, when
the reaction
mixture, comprised of hydrogen and a catalyst, reacts to form lower-energy
hydrogen. The
heat exchanger exchanges heat with the steam generator 82 that absorbs heat
from the
exchanger 80 and produces steam. The energy reactor 70 further comprises a
turbine 90 that
receives steam from the steam generator 82 and supplies mechanical power to a
power
generator 100 that converts the steam energy into electrical energy, which can
be received by
a load 110 to produce work or for dissipation.
In an embodiment, the energy reaction mixture 74 comprises an energy releasing
material 76, such as a fuel supplied through supply passage 62. The reaction
mixture may
comprise a source of hydrogen isotope atoms or a source of molecular hydrogen
isotope, and
a source of catalyst 78 which resonantly remove approximately m = 27.2 eV to
form lower-
energy atomic hydrogen where m is an integer, preferably an integer less than
400, wherein
the reaction to lower energy states of hydrogen occurs by contact of the
hydrogen with the
catalyst. The catalyst may be in the molten, liquid, gaseous, or solid state.
The catalysis
releases energy in a form such as heat and forms at least one of lower-energy
hydrogen
isotope atoms, lower-energy hydrogen molecules, hydride ions, and lower-energy
hydrogen
compounds. Thus, the power cell also comprises a lower-energy hydrogen
chemical reactor.
The source of hydrogen can be hydrogen gas, dissociation of water including
thermal
dissociation, electrolysis of water, hydrogen from hydrides, or hydrogen from
metal-
hydrogen solutions. In another embodiment, molecular hydrogen of the energy
releasing
material 76 is dissociated into atomic hydrogen by a molecular hydrogen
dissociating catalyst
of the mixture 74. Such dissociating catalysts or dissociators may also absorb
hydrogen,
deuterium, or tritium atoms and/or molecules and include, for example, an
element,
compound, alloy, or mixture of noble metals such as palladium and platinum,
refractory
metals such as molybdenum and tungsten, transition metals such as nickel and
titanium, and
inner transition metals such as niobium and zirconium. Preferably, the
dissociator has a high
surface area such as a noble metal such as Pt, Pd, Ru, Ir, Re, or Rh, or Ni on
A1203, Si02, or
24
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
combinations thereof.
In an embodiment, a catalyst is provided by the ionization of t electrons from
an
atom or ion to a continuum energy level such that the sum of the ionization
energies of the t
electrons is approximately m = 27.2 eV where t and m are each an integer. A
catalyst may
also be provided by the transfer of t electrons between participating ions.
The transfer of t
electrons from one ion to another ion provides a net enthalpy of reaction
whereby the sum of
the t ionization energies of the electron-donating ion minus the ionization
energies of t
electrons of the electron-accepting ion equals approximately m = 27.2 eV where
t and m are
each an integer. In another embodiment, the catalyst comprises MH such as NaH
having
an atom M bound to hydrogen, and the enthalpy of m = 27.2 eV is provided by
the sum of
the M - H bond energy and the ionization energies of the t electrons.
In an embodiment, a source of catalyst comprises a catalytic material 78
supplied
through catalyst supply passage 61, that typically provides a net enthalpy of
approximately
2 . 27.2 eV plus or minus 1 eV. The catalysts comrpise atoms, ions, molecules,
and
hydrinos that accept energy from atomic hydrogen and hydrinos. In embodiments,
the
catalyst may comprise at least one species chosen from molecules of AIH, BiH,
C1H, CoH,
GeH, InH, NaH, RuH, SbH, SeH, SiH, SnH, C2 , N2 1 O2 , CO2 , NO2, and NO3 and
atoms or
ions of Li, Be, K, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Kr, Rb, Sr,
Nb, Mo, Pd, Sn,
Te, Cs, Ce, Pr, Sm, Gd, Dy, Pb, Pt, Kr, 2K+ , He+, Ti 2+ , Na+, Rb+, Sr+, Fe3+
, Mo 2+ ,
Mo4+ In3+ He+ Ar+ Xe+ Ar2+ and H+ and Ne+ and H+ .
In an embodiment of a power system, the heat is removed by a heat exchanger
having
a heat exchange medium. The heat exchanger may be a water wall and the medium
may be
water. The heat may be transferred directly for space and process heating.
Alternatively, the
heat exchanger medium such as water undergoes a phase change such as
conversion to steam.
This conversion may occur in a steam generator. The steam may be used to
generate
electricity in a heat engine such as a steam turbine and a generator.
An embodiment of an hydrogen catalyst energy and lower-energy-hydrogen species-
producing reactor 5, for recycling or regenerating the fuel in accordance with
the present
disclosure, is shown in FIGURE 2 and comprises a boiler 10 which contains a
fuel reaction
mixture 11 that may be a mixture of a source of hydrogen, a source of
catalyst, and optionally
a solvent that may be vaporized, a hydrogen source 12, steam pipes and steam
generator 13, a
power converter such as a turbine 14, a water condenser 16, a water-make-up
source 17, a
fuel recycler 18, and a hydrogen-dihydrino gas separator 19. At Step 1, the
fuel, such as one
that is gaseous, liquid, solid, or a heterogeneous mixture comprising multiple
phases,
comprising a source of catalyst and a source of hydrogen reacts to form
hydrinos and lower-
energy hydrogen products. At Step 2, the spent fuel is reprocessed to re-
supply the boiler 10
to maintain thermal power generation. The heat generated in the boiler 10
forms steam in the
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
pipes and steam generator 13 that is delivered to the turbine 14 that in turn
generates
electricity by powering a generator. At Step 3, the water is condensed by the
water
condensor 16. Any water loss may be made up by the water source 17 to complete
the cycle
to maintain thermal to electric power conversion. At Step 4, lower-energy
hydrogen products
such as hydrino hydride compounds and dihydrino gas may be removed, and
unreacted
hydrogen may be returned to the fuel recycler 18 or hydrogen source 12 to be
added back to
spent fuel to make-up recycled fuel. The gas products and unreacted hydrogen
may be
separated by hydrogen-dihydrino gas separator 19. Any product hydrino hydride
compounds
may be separated and removed using fuel recycler 18. The processing may be
performed in
the boiler or externally to the boiler with the fuel returned. Thus, the
system may further
comprise at least one of gas and mass transporters to move the reactants and
products to
achieve the spent fuel removal, regeneration, and re-supply. Hydrogen make-up
for that
spent in the formation of hydrinos is added from the source 12 during fuel
reprocessing and
may involve recycled, unconsumed hydrogen. The recycled fuel maintains the
production of
thermal power to drive the power plant to generate electricity.
The reactor may be run in a continuous mode with hydrogen addition and with
separation and addition or replacement to counter the minimum degradation of
the reactants.
Alternatively, the reacted fuel is continuously regenerated from the products.
In one
embodiment of the latter scheme, the reaction mixture comprises species that
can generate the
reactants of atomic or molecular catalyst and atomic hydrogen that further
react to form
hydrinos, and the product species formed by the generation of catalyst and
atomic hydrogen
can be regenerated by at least the step of reacting the products with
hydrogen. In an
embodiment, the reactor comprises a moving bed reactor that may further
comprise a
fluidized-reactor section wherein the reactants are continuously supplied and
side products
are removed and regenerated and returned to the reactor. In an embodiment, the
lower-
energy hydrogen products such as hydrino hydride compounds or dihydrino
molecules are
collected as the reactants are regenerated. Furthermore, the hydrino hydride
ions may be
formed into other compounds or converted into dihydrino molecules during the
regeneration
of the reactants.
The reactor may further comprise a separator to separate components of a
product
mixture such as by evaporation of the solvent if one is present. The separator
may, for
example, comprise sieves for mechanically separating by differences in
physical properties
such as size. The separator may also be a separator that exploits differences
in density of the
component of the mixture, such as a cyclone separator. For example, at least
two of the
groups chosen from carbon, a metal such as Eu, and an inorganic product such
as KBr can be
separated based on the differences in density in a suitable medium such as
forced inert gas
and also by centrifugal forces. The separation of components may also be based
on the
differential of the dielectric constant and chargeability. For example, carbon
may be
26
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
separated from metal based on the application of an electrostatic charge to
the former with
removal from the mixture by an electric field. In the case that one or more
components of a
mixture are magnetic, the separation may be achieved using magnets. The
mixture may be
agitated over a series of strong magnets alone or in combination with one or
more sieves to
cause the separation based on at least one of the stronger adherence or
attraction of the
magnetic particles to the magnet and a size difference of the two classes of
particles. In an
embodiment of the use of sieves and an applied magnetic field, the latter adds
an additional
force to that of gravity to draw the smaller magnetic particles through the
sieve while the
other particles of the mixture are retained on the sieve due to their larger
size.
The reactor may further comprise a separator to separate one or more
components
based on a differential phase change or reaction. In an embodiment, the phase
change
comprises melting using a heater, and the liquid is separated from the solid
by methods
known in the art such as gravity filtration, filtration using a pressurized
gas assist,
centrifugation, and by applying vacuum. The reaction may comprise
decomposition such as
hydride decomposition or reaction to from a hydride, and the separations may
be achieved by
melting the corresponding metal followed by its separation and by mechanically
separating
the hydride powder, respectively. The latter may be achieved by sieving. In an
embodiment,
the phase change or reaction may produce a desired reactant or intermediate.
In certain
embodiments, the regeneration including any desired separation steps may occur
inside or
outside of the reactor.
Other methods known by those skilled in the art that can be applied to the
separations
of the present disclosure by application of routine experimentation. In
general, mechanical
separations can be divided into four groups: sedimentation, centrifugal
separation, filtration,
and sieving. In one embodiment, the separation of the particles is achieved by
at least one of
sieving and use of classifiers. The size and shape of the particle may be
chosen in the starting
materials to achieve the desired separation of the products.
The power system may further comprise a catalyst condensor to maintain the
catalyst
vapor pressure by a temperature control which controls the temperature of a
surface at a
lower value than that of the reaction cell. The surface temperature is
maintained at a desired
value that provides the desired vapor pressure of the catalyst. In an
embodiment, the catalyst
condensor is a tube grid in the cell. In an embodiment with a heat exchanger,
the flow rate of
the heat transfer medium may be controlled at a rate that maintains the
condensor at the
desired lower temperature than the main heat exchanger. In an embodiment, the
working
medium is water, and the flow rate is higher at the condensor than the water
wall such that
the condensor is the lower, desired temperature. The separate streams of
working media may
be recombined and transferred for space and process heating or for conversion
to steam.
The cells of the present disclosure comprise the catalysts, reaction mixtures,
methods,
and systems disclosed herein wherein the particular cell serves as a reactor
and at least one
27
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
component to activate, initiate, propagate, and/or maintain the reaction and
regenerate the
reactants. According to the present disclosure, the cells comprise at least
one catalyst or a
source of catalyst, at least one source of atomic hydrogen, and a vessel. The
cells and
systems for their operation are known to those skilled in the art. The
electrolytic cell energy
reactor such as a eutectic-salt electrolysis cell, plasma electrolysis
reactor, barrier electrode
reactor, RF plasma reactor, pressurized gas energy reactor, gas discharge
energy reactor,
preferably pulsed discharge, and more preferably pulsed pinched plasma
discharge,
microwave cell energy reactor, and a combination of a glow discharge cell and
a microwave
and or RF plasma reactor of the present disclosure comprises: a source of
hydrogen; one of a
solid, molten, liquid, gaseous, and heterogeneous source of catalyst or
reactants in any of
these states to cause the hydrino reaction by a reaction amongst the
reactants; a vessel the
reactants or at least containing hydrogen and the catalyst wherein the
reaction to form lower-
energy hydrogen occurs by contact of the hydrogen with the catalyst or by
reaction of MH
catalyst; and optionally a component for removing the lower-energy hydrogen
product. In an
embodiment, the reaction to form lower-energy state hydrogen is facilitated by
an oxidation
reaction. The oxidation reaction may increase the reaction rate to form
hydrinos by at least
one of accepting electrons from the catalyst and neutralizing the highly-
charged cation
formed by accepting energy from atomic hydrogen. Thus, these cells may be
operated in a
manner that provides such an oxidation reaction. In an embodiment, the
electrolysis or
plasma cell may provide an oxidation reaction at the anode wherein hydrogen
provided by a
method such as sparging and catalyst react to form hydrinos via the
participating oxidation
reaction.
In an embodiment of a liquid fuel, the cell is operated at a temperature
wherein the
rate of decomposition of the solvent is negligible with respect to the power
to regenerate it
relative to the power of the cell. In the case, the temperature is below that
at which a
satisfactory efficiency of power conversion can be obtained by more
conventional methods
such as those using a steam cycle, a lower-boiling-point working medium may be
used. In
another embodiment, the temperature of a working medium may be increased using
a heat
pump. Thus, space and process heating may be supplied using the power cell
operating at a
temperature above ambient wherein a working medium is increased in temperature
with a
component such as a heat pump. With sufficient elevation of the temperature, a
liquid to gas
phase transition may occur, and the gas may be used for pressure volume (PV)
work. The PV
work may comprise powering a generator to produce electricity. The medium may
then be
condensed, and the condensed working medium may be returned to the reactor
cell to be re-
heated and recirculated in the power loop.
In an embodiment of the reactor, a heterogeneous catalyst mixture comprising a
liquid
and solid phase is flowed through the reactor. The flow may be achieved by
pumping. The
mixture may be a slurry. The mixture may be heated in a hot zone to cause the
catalysis of
28
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
hydrogen to hydrinos to release heat to maintain the hot zone. The products
may be flowed
out of the hot zone, and the reactant mixture may be regenerated from the
products. In
another embodiment, at least one solid of a heterogeneous mixture may be
flowed into the
reactor by gravity feed. A solvent may be flowed into the reactor separately
or in
combination with one or more solids. The reactant mixture may comprise at
least one of the
group of a dissociator, a high-surface-area (HSA) material, R-Ni, Ni, NaH, Na,
NaOH, and a
solvent.
In an embodiment, one or more reactants, preferably a source of halogen,
halogen gas,
source of oxygen, or solvent, are injected into a mixture of the other
reactants. The injection
is controlled to optimize the excess energy and power from the hydrino-forming
reaction.
The cell temperature at injection and rate of injection may be controlled to
achieve the
optimization. Other process parameters and mixing can be controlled to further
the
optimization using methods known to those skilled in the art of process
engineering.
For power conversion, each cell type may be interfaced with any of the known
converters of thermal energy or plasma to mechanical or electrical power which
include for
example, a heat engine, steam or gas turbine system, Sterling engine, or
thermionic or
thermoelectric converters. Further plasma converters comprise the magnetic
mirror
magnetohydrodynamic power converter, plasmadynamic power converter, gyrotron,
photon
bunching microwave power converter, charge drift power, or photoelectric
converter. In an
embodiment, the cell comprises at least one cylinder of an internal combustion
engine.
III. Hydrogen Gas Cell and Solid, Liquid, and Heterogeneous Fuel Reactor
According to an embodiment of the present disclosure, a reactor for producing
hydrinos and power may take the form of a reactor cell. A reactor of the
present disclosure is
shown in FIGURE 3. Reactant hydrinos are provided by a catalytic reaction with
catalyst.
Catalysis may occur in the gas phase or in solid or liquid state.
The reactor of FIGURE 3 comprises a reaction vessel 207 having a chamber 200
capable of containing a vacuum or pressures greater than atmospheric. A source
of hydrogen
221 communicating with chamber 200 delivers hydrogen to the chamber through
hydrogen
supply passage 242. A controller 222 is positioned to control the pressure and
flow of
hydrogen into the vessel through hydrogen supply passage 242. A pressure
sensor 223
monitors pressure in the vessel. A vacuum pump 256 is used to evacuate the
chamber
through a vacuum line 257.
In an embodiment, the catalysis occurs in the gas phase. The catalyst may be
made
gaseous by maintaining the cell temperature at an elevated temperature that,
in turn,
determines the vapor pressure of the catalyst. The atomic and/or molecular
hydrogen reactant
is also maintained at a desired pressure that may be in any pressure range. In
an embodiment,
the pressure is less than atmospheric, preferably in the range about 10
millitorr to about 100
29
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
Torr. In another embodiment, the pressure is determined by maintaining a
mixture of source
of catalyst such as a metal source and the corresponding hydride such as a
metal hydride in
the cell maintained at the desired operating temperature.
A source of suitable catalyst 250 for generating hydrino atoms can be placed
in a
catalyst reservoir 295, and gaseous catalyst can be formed by heating. The
reaction vessel
207 has a catalyst supply passage 241 for the passage of gaseous catalyst from
the catalyst
reservoir 295 to the reaction chamber 200. Alternatively, the catalyst may be
placed in a
chemically resistant open container, such as a boat, inside the reaction
vessel.
The source of hydrogen can be hydrogen gas and the molecular hydrogen.
Hydrogen
may be dissociated into atomic hydrogen by a molecular hydrogen dissociating
catalyst.
Such dissociating catalysts or dissociators include, for example, Raney nickel
(R-Ni),
precious or noble metals, and a precious or noble metal on a support. The
precious or noble
metal may be Pt, Pd, Ru, Ir, and Rh, and the support may be at least one of
Ti, Nb, A1203,
Si02 and combinations thereof. Further dissociators are Pt or Pd on carbon
that may
comprise a hydrogen spillover catalyst, nickel fiber mat, Pd sheet, Ti sponge,
Pt or Pd
electroplated on Ti or Ni sponge or mat, TiH, Pt black, and Pd black,
refractory metals such
as molybdenum and tungsten, transition metals such as nickel and titanium,
inner transition
metals such as niobium and zirconium, and other such materials known to those
skilled in the
art. In an embodiment, hydrogen is dissociated on Pt or Pd. The Pt or Pd may
be coated on a
support material such as titanium or A1203. In another embodiment, the
dissociator is a
refractory metal such as tungsten or molybdenum, and the dissociating material
may be
maintained at elevated temperature by temperature control component 230, which
may take
the form of a heating coil as shown in cross section in FIGURE 3. The heating
coil is
powered by a power supply 225. Preferably, the dissociating material is
maintained at the
operating temperature of the cell. The dissociator may further be operated at
a temperature
above the cell temperature to more effectively dissociate, and the elevated
temperature may
prevent the catalyst from condensing on the dissociator. Hydrogen dissociator
can also be
provided by a hot filament such as 280 powered by supply 285.
In an embodiment, the hydrogen dissociation occurs such that the dissociated
hydrogen atoms contact gaseous catalyst to produce hydrino atoms. The catalyst
vapor
pressure is maintained at the desired pressure by controlling the temperature
of the catalyst
reservoir 295 with a catalyst reservoir heater 298 powered by a power supply
272. When the
catalyst is contained in a boat inside the reactor, the catalyst vapor
pressure is maintained at
the desired value by controlling the temperature of the catalyst boat, by
adjusting the boat's
power supply. The cell temperature can be controlled at the desired operating
temperature by
the heating coil 230 that is powered by power supply 225. The cell (called a
permeation cell)
may further comprise an inner reaction chamber 200 and an outer hydrogen
reservoir 290
such that hydrogen may be supplied to the cell by diffusion of hydrogen
through the wall 291
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
separating the two chambers. The temperature of the wall may be controlled
with a heater to
control the rate of diffusion. The rate of diffusion may be further controlled
by controlling
the hydrogen pressure in the hydrogen reservoir.
To maintain the catalyst pressure at the desire level, the cell having
permeation as the
hydrogen source may be sealed. Alternatively, the cell further comprises high
temperature
valves at each inlet or outlet such that the valve contacting the reaction gas
mixture is
maintained at the desired temperature. The cell may further comprise a getter
or trap 255 to
selectively collect the lower-energy-hydrogen species and/or the increased-
binding-energy
hydrogen compounds and may further comprise a selective valve 206 for
releasing dihydrino
gas product.
In an embodiment, the reactants such as the solid fuel or heterogeneous-
catalyst fuel
mixture 260 is reacted in the vessel 200 by heating with heaters 230. A
further added
reactant such as at least one of an exothermic reactant, preferably having
fast kinetics, may be
flowed into the cell 200 through control valve 232 and connection 233. The
added reactant
may be a source of halogen, halogen, source of oxygen, or solvent. The
reactant 260 may
comprise a species that reacts with the added reactant. A halogen may be added
to form a
halide with reactant 260, or a source of oxygen may be added to reactant 260
to form an
oxide, for example.
The catalyst may be at least one of the group of atomic lithium, potassium, or
cesium,
NaH molecule, 2H, and hydrino atoms, wherein catalysis comprises a
disproportionation
reaction. Lithium catalyst may be made gaseous by maintaining the cell
temperature in about
the 500-1000 C range. Preferably, the cell is maintained in about the 500-750
C range.
The cell pressure may be maintained at less than atmospheric, preferably in
the range about
millitorr to about 100 Torr. Most preferably, at least one of the catalyst and
hydrogen
pressure is determined by maintaining a mixture of catalyst metal and the
corresponding
hydride such as lithium and lithium hydride, potassium and potassium hydride,
sodium and
sodium hydride, and cesium and cesium hydride in the cell maintained at the
desired
operating temperature. The catalyst in the gas phase may comprise lithium
atoms from the
metal or a source of lithium metal. Preferably, the lithium catalyst is
maintained at the
pressure determined by a mixture of lithium metal and lithium hydride at the
operating
temperature range of about 500-1000 C and most preferably, the pressure with
the cell at the
operating temperature range of about 500-750 C. In other embodiments, K, Cs,
and Na
replace Li wherein the catalyst is atomic K, atomic Cs, and molecular NaH.
In an embodiment of the gas cell reactor comprising a catalyst reservoir or
boat,
gaseous Na, NaH catalyst, or the gaseous catalyst such as Li, K, and Cs vapor
is maintained
in a super-heated condition in the cell relative to the vapor in the reservoir
or boat which is
the source of the cell vapor. In one embodiment, the superheated vapor reduces
the
condensation of catalyst on the hydrogen dissociator or the dissociator of at
least one of metal
31
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
and metal hydride molecules disclosed infra. In an embodiment comprising Li as
the catalyst
from a reservoir or boat, the reservoir or boat is maintained at a temperature
at which Li
vaporizes. H2 may be maintained at a pressure that is lower than that which
forms a
significant mole fraction of LiH at the reservoir temperature. The pressures
and temperatures
that achieve this condition can be determined from the data plots of H2
pressure versus LiH
mole fraction at given isotherms that are known in the art. In an embodiment,
the cell
reaction chamber containing a dissociator is operated at a higher temperature
such that the Li
does not condense on the walls or the dissociator. The H2 may flow from the
reservoir to the
cell to increase the catalyst transport rate. Flow such as from the catalyst
reservoir to the cell
and then out of the cell is a method to remove hydrino product to prevent
hydrino product
inhibition of the reaction. In other embodiments, K, Cs, and Na replace Li
wherein the
catalyst is atomic K, atomic Cs, and molecular NaH.
Hydrogen is supplied to the reaction from a source of hydrogen. For example,
the
hydrogen is supplied by permeation from a hydrogen reservoir. The pressure of
the hydrogen
reservoir may be in the range of 10 Torr to 10,000 Torr, preferably 100 Torr
to 1000 Torr,
and most preferably about atmospheric pressure. The cell may be operated in
the temperature
of about 100 C to 3000 C, preferably in the temperature of about 100 C to
1500 C, and
most preferably in the temperature of about 500 C to 800 C.
The source of hydrogen may be from decomposition of an added hydride. A cell
design that supplies H2 by permeation is one comprising an internal metal
hydride placed in a
sealed vessel wherein atomic H permeates out at high temperature. The vessel
may comprise
Pd, Ni, Ti, or Nb. In an embodiment, the hydride is placed in a sealed tube
such as a Nb tube
containing a hydride and sealed at both ends with seals such as Swagelocks. In
the sealed
case, the hydride could be an alkaline or alkaline earth hydride.
Alternatively, in this as well
as the internal-hydride-reagent case, the hydride could be at least one of the
group of saline
hydrides, titanium hydride, vanadium, niobium, and tantalum hydrides,
zirconium and
hafnium hydrides, rare earth hydrides, yttrium and scandium hydrides,
transition element
hydrides, intermetalic hydrides, and their alloys.
In an embodiment the hydride and the operating temperature 200 C, based on
each
hydride decomposition temperature, is chosen from at least one of the list of:
a rare earth hydride with an operating temperature of about 800 C; lanthanum
hydride with an operating temperature of about 700 C; gadolinium hydride with
an operating
temperature of about 750 C; neodymium hydride with an operating temperature
of about 750
C; yttrium hydride with an operating temperature of about 800 C; scandium
hydride with
an operating temperature of about 800 C; ytterbium hydride with an operating
temperature
of about 850-900 C; titanium hydride with an operating temperature of about
450 C; cerium
hydride with an operating temperature of about 950 C; praseodymium hydride
with an
operating temperature of about 700 C; zirconium-titanium (50%/50%) hydride
with an
32
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
operating temperature of about 600 C; an alkali metal/alkali metal hydride
mixture such as
Rb/RbH or K/KH with an operating temperature of about 450 C; and an alkaline
earth
metal/alkaline earth hydride mixture such as Ba/BaH2 with an operating
temperature of about
900-1000 C.
Metals in the gas state can comprise diatomic covalent molecules. An objective
of the
present disclosure is to provide atomic catalyst such as Li as well as K and
Cs. Thus, the
reactor may further comprise a dissociator of at least one of metal molecules
("MM") and
metal hydride molecules ("MH"). Preferably, the source of catalyst, the source
of H2, and the
dissociator of MM, MH, and HH, wherein M is the atomic catalyst are matched to
operate at
the desired cell conditions of temperature and reactant concentrations for
example. In the
case that a hydride source of H2 is used, in an embodiment, its decomposition
temperature is
in the range of the temperature that produces the desired vapor pressure of
the catalyst. In the
case of that the source of hydrogen is permeation from a hydrogen reservoir to
the reaction
chamber, preferable sources of catalysts for continuous operation are Sr and
Li metals since
each of their vapor pressures may be in the desired range of 0.01 to 100 Torr
at the
temperatures for which permeation occurs. In other embodiments of the
permeation cell, the
cell is operated at a high temperature permissive of permeation, then the cell
temperature is
lowered to a temperature which maintains the vapor pressure of the volatile
catalyst at the
desired pressure.
In an embodiment of a gas cell, a dissociator comprises a component to
generate
catalyst and H from sources. Surface catalysts such as Pt on Ti or Pd,
iridium, or rhodium
alone or on a substrate such as Ti may also serve the role as a dissociator of
molecules of
combinations of catalyst and hydrogen atoms. Preferably, the dissociator has a
high surface
area such as Pt/A1203 or Pd/A1203.
The H2 source can also be H2 gas. In this embodiment, the pressure can be
monitored
and controlled. This is possible with catalyst and catalyst sources such as K
or Cs metal and
LiNH2, respectively, since they are volatile at low temperature that is
permissive of using a
high-temperature valve. LiNH2 also lowers the necessary operating temperature
of the Li cell
and is less corrosive which is permissive of long-duration operation using a
feed through in
the case of plasma and filament cells wherein a filament serves as a hydrogen
dissociator.
Further embodiments of the gas cell hydrogen reactor having NaH as the
catalyst
comprise a filament with a dissociator in the reactor cell and Na in the
reservoir. H2 may be
flowed through the reservoir to main chamber. The power may be controlled by
controlling
the gas flow rate, H2 pressure, and Na vapor pressure. The latter may be
controlled by
controlling the reservoir temperature. In another embodiment, the hydrino
reaction is
initiated by heating with the external heater and an atomic H is provided by a
dissociator.
The reaction mixture may be agitated by methods known in the art such as
mechanical
agitation or mixing. The agitation system may comprise one or more
piezoelectric
33
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
transducers. Each piezoelectric transducer may provide ultrasonic agitation.
The reaction
cell may be vibrated and further contain agitation elements such as stainless
steel or tungsten
balls that are vibrated to agitate the reaction mixture. In another
embodiment, mechanical
agitation comprises ball milling. The reactant may also be mixed using these
methods,
preferably by ball milling.
In an embodiment, the catalyst is formed by mechanical agitation such as, for
example, at least one of vibration with agitation elements, ultrasonic
agitation, and ball
milling. The mechanical impact or compression of sound waves such as
ultrasound may
cause a reaction or a physical change in the reactants to cause the formation
of the catalyst,
preferably NaH molecules. The reactant mixture may or may not comprise a
solvent. The
reactants may be solids such as solid NaH that is mechanically agitated to
form NaH
molecules. Alternatively, the reaction mixture may comprise a liquid. The
mixture may have
at least one Na species. The Na species may be a component of a liquid
mixture, or it may be
in solution. In an embodiment, sodium metal is dispersed by high-speed
stirring of a
suspension of the metal in a solvent such as an ether, hydrocarbon,
fluorinated hydrocarbon,
aromatic, or heterocyclic aromatic solvent. The solvent temperature may be
held just above
the melting point of the metal.
IV. Fuels-Types
An embodiment of the present disclosure is directed to a fuel comprising a
reaction
mixture of at least a source of hydrogen and a source of catalyst to support
the catalysis of
hydrogen to form hydrinos in at least one of gaseous, liquid, and solid phases
of a possible
mixture of phases. The reactants and reactions given herein for solid and
liquid fuels are also
reactants and reactions of heterogeneous fuels comprising a mixture of phases.
An objective of the present disclosure is to provide atomic catalysts such as
Li as well
as K and Cs and molecular catalyst NaH. Metals form diatomic covalent
molecules. Thus, in
solid-fuels, liquid-fuels, and heterogeneous-fuels embodiments, the reactants
comprise alloys,
complexes, sources of complexes, mixtures, suspensions, and solutions that may
reversibly
form with a metal catalyst M and decompose or react to provide a catalyst such
as Li or NaH.
In another embodiment, at least one of the catalyst source and atomic hydrogen
source further
comprises at least one reactant that reacts to form at least one of the
catalyst and atomic
hydrogen. In another embodiment, the reaction mixture comprises NaH catalyst
or a source
of NaH catalyst or other catalyst such as Li or K that may form via the
reaction of one or
more reactants or species of the reaction mixture or may form by a physical
transformation.
The transformation may be solvation with a suitable solvent.
The reaction mixture may further comprise a solid to support the catalysis
reaction on
a surface. The catalyst or a source of catalyst such as NaH may be coated on
the surface.
The coating may be achieved by mixing a support such as activated carbon, TiC,
WC, R-Ni
34
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
with NaH by methods such as ball milling. The reaction mixture may comprise a
heterogeneous catalyst or a source of heterogeneous catalyst. In an
embodiment, the catalyst
such as NaH is coated on the support such as activated carbon, TiC, WC, or a
polymer by the
method of incipient wetness, preferably by using an aportic solvent such as an
ether. The
support may also comprise an inorganic compound such as an alkali halide,
preferably at
least one of NaF and HNaF2 wherein NaH serves as the catalyst and a
fluorinated solvent is
used.
In an embodiment of a liquid fuel, the reaction mixture comprises at least one
of a
source of catalyst, a catalyst, a source of hydrogen, and a solvent for the
catalyst. In other
embodiments, the present disclosure of a solid fuel and a liquid fuel further
comprises
combinations of both and further comprises gaseous phases as well. The
catalysis with the
reactants such as the catalyst and atomic hydrogen and sources thereof in
multiple phases is
called a heterogeneous reaction mixture and the fuel is called a heterogeneous
fuel. Thus, the
fuel comprises a reaction mixture of at least a source of hydrogen to undergo
transition to
hydrinos, states given by Eq, (35), and a catalyst to cause the transitions
having the reactants
in at least one of liquid, solid, and gaseous phases. Catalysis with the
catalyst in a different
phase from the reactants is generally known in the art as a heterogeneous
catalysis that is an
embodiment of the present disclosure. Heterogeneous catalysts provide a
surface for the
chemical reaction to take place on and comprise embodiments of the present
disclosure. The
reactants and reactions given herein for solid and liquid fuels are also
reactants and reactions
of heterogeneous fuels.
For any fuel of the present disclosure, the catalyst or source of catalyst
such as NaH
may be mixed with other components of the reaction mixture such as a support
such as a
HSA material by methods such as mechanical mixing or by ball milling. In all
cases
additional hydrogen may be added to maintain the reaction to form hydrinos.
The hydrogen
gas may be any desired pressure, preferably in the range of 0.1 to 200 atm.
Alternatives
sources of hydrogen comprise at least one of the group of NH4X (X is an anion,
preferably a
halide), NaBH4i NaA1H4, a borane, and a metal hydride such as an alkali metal
hydride,
alkaline earth metal hydride preferably MgH2, and a rare earth metal hydride
preferably LaH2
and GdH2.
A. Support
In certain embodiments, the solid, liquid, and heterogeneous fuels of the
present
disclosure comprise a support. The support comprises properties specific for
its function.
For example, in the case that the support functions as an electron acceptor or
conduit, the
support is preferably conductive. Additionally, in the case that the support
disperses the
reactants, the support preferably has a high surface area. In the former case,
the support such
as a HSA support may comprise a conductive polymer such as activated carbon,
graphene,
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
and heterocyclic polycyclic aromatic hydrocarbons that may be macromolecular.
The carbon
may preferably comprise activated carbon (AC), but may also comprise other
forms such as
mesoporous carbon, glassy carbon, coke, graphitic carbon, carbon with a
dissociator metal
such as Pt or Pd wherein the wt% is 0.1 to 5 wt%, transition metal powders
having preferably
one to ten carbon layers and more preferably three layers, and a metal or
alloy coated carbon,
preferably nanopowder, such as a transition metal preferably at least one of
Ni, Co, and Mn
coated carbon. A metal may be intercalated with the carbon. In the case that
the intercalated
metal is Na and the catalyst is NaH, preferably the Na intercalation is
saturated. Preferably,
the support has a high surface area. Common classes of organic conductive
polymers that
may serve as the support are at least one of the group of poly(acetylene)s,
poly(pyrrole)s,
poly(thiophene)s, poly(aniline)s, poly(fluorene)s, poly(3-alkylthiophene)s,
polytetrathiafulvalenes, polynaphthalenes, polyp-phenylene sulfide), and
poly(para-
phenylene vinylene)s. These linear backbone polymers are typically known in
the art as
polyacetylene, polyaniline, etc. "blacks" or "melanins". The support may be a
mixed
copolymer such as one of polyacetylene, polypyrrole, and polyaniline.
Preferably, the
conductive polymer support is at least one of typically derivatives of
polyacetylene,
polyaniline, and polypyrrole. Other support comprise other elements than
carbon such as the
conducting polymer polythiazyl ((S-N),,).
In another embodiment, the support is a semiconductor. The support may be a
Column IV element such as carbon, silicon, germanium, and a -gray tin. In
addition to
elemental materials such as silicon and germanium, the semiconductor support
comprises a
compound material such as gallium arsenide and indium phosphide, or alloys
such as silicon
germanium or aluminum arsenide. Conduction in materials such as silicon and
germanium
crystals can be enhanced in an embodiment by adding small amounts (e.g. 1-10
parts per
million) of dopants such as boron or phosphorus as the crystals are grown. The
doped
semiconductor may be ground into a powder to serve as a support.
In certain embodiments, the HSA support is a metal such as a transition metal,
noble
metal, intermetallic, rare earth, actinide, lanthanide, preferably one of La,
Pr, Nd, and Sm, Al,
Ga, In, Tl, Sn, Pb, metalloids, Si, Ge, As, Sb, Te, Y, Zr, Nb, Mo, Tc, Ru, Rh,
Pd, Ag, Cd, Hf,
Ta, W, Re, Os, Ir, Pt, Au, Hg, alkali metal, alkaline earth metal, and an
alloy comprising at
least two metals or elements of this group such as a lanthanide alloy,
preferably LaNi5 and Y-
Ni. The support may be a noble metal such as at least one of Pt, Pd, Au, Ir,
and Rh or a
supported noble metal such as Pt or Pd on titanium (Pt or Pd/Ti).
In other embodiments, the HSA material comprises at least one of cubic boron
nitride,
hexagonal boron nitride, wurtzite boron nitride powder, heterodiamond, boron
nitride
nanotubes, silicon nitride, aluminum nitride, titanium nitride (TiN), titanium
aluminum
nitride (TiA1N), tungsten nitride, a metal or alloy, preferably nanopowder,
coated with carbon
such as at least one of Co, Ni, Fe, Mn, and other transition metal powders
having preferably
36
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
one to ten carbon layers and more preferably three layers, metal or alloy
coated carbon,
preferably nanopowder, such as a transition metal preferably at least one of
Ni, Co, and Mn
coated carbon, a carbide, preferably a powder, beryllium oxide (BeO) powder,
rare earth
oxide powder such as La203, Zr203, A1203, sodium aluminate, and carbon such as
fullerene,
graphene, or nanotubes, preferably single-walled.
The carbide may comprise one or more of the bonding types: salt-like such as
calcium
carbide (CaC2), covalent compounds such as silicon carbide (SiC) and boron
carbide (B4C or
BC3), and interstitial compounds such as tungsten carbide. The carbide may be
an acetylide
such as Au2C2i ZnC2, and CdC2 or a methide such as Be2C, aluminum carbide
(A14C3), and
carbides of the type A3MC where A is mostly a rare earth or transition metal
such as Sc, Y,
La-Na,Gd-Lu, and M is a metallic or semimetallic main group element such as
Al, Ge, In, Ti,
Sri, and Pb. The carbide having C2- ions may comprise at least one of carbides
MZC2 with
the cation M' comprising an alkali metal or one of the coinage metals,
carbides M "CZ with
the cation M" comprising an alkaline earth metal, and preferably carbides M4"
(CZ )3 with
the cation M.. comprising Al, La, Pr, or Th. The carbide may comprise an ion
other than
C2- such as those of the group of YC2, ThC2, YbC2, UC2, Ce2C3, Pr2C3, and
Th2C3. The
carbide may comprise a sesquicarbide such as Mg2C3i Sc3C4, and Li4C3. The
carbide may
comprise a ternary carbide such as those containing lanthanide metals and
transition metals
that may further comprise C2 units such as Ln3M (CZ )2 where M is Fe, Co, Ni,
Ru, Rh, Os,
and Ir, Dy12Mn5C15, Ln3.67FeC6, Ln3Mn (C2 )2 (Ln=Gd and Tb), and ScCrC2. The
carbide
may further be of the classification "intermediate" transition metal carbide
such as iron
carbide (Fe3C or FeC2:Fe). The carbide may be at least one from the group of,
lanthanides
(MC2 and M2C3) such as lanthanum carbide (LaC2 or La2C3), yttrium carbide,
actinide
carbides, transition metal carbides such as scandium carbide, titanium carbide
(TiC),
vanadium carbide, chromium carbide, manganese carbide, and cobalt carbide,
niobium
carbide, molybdenum carbide, tantalum carbide, zirconium carbide, and hafnium
carbide.
Further suitable carbides comprise at least one of Ln2FeC4, Sc3CoC4, Ln3MC4
(M=Fe, Co, Ni,
Ru, Rh, Os, Ir), Ln3Mn2C6, Eu3.16NiC6, ScCrC2, Th2NiC2, Y2ReC2, Ln12M5C15
(M=Mn, Re),
YCoC, Y2ReC2, and other carbides known in the art.
In an embodiment, the support is an electrically-conductive carbide such as
TiC or
WC and HfC, Mo2C, TaC, YC2, ZrC, A14C3, and B4C. The support may be a metal
boride
such as MB2 borides including. The support or HSA material may be a boride,
preferably a
two-dimensional network boride that may be conducting such as MB2 wherein M is
a metal
such as at least one of Cr, Ti, Mg, Zr, and Gd (CrB2, TiB2, MgB2, ZrB2, GdB2).
In a carbon-HSA material embodiment, Na does not intercalate into the carbon
support or form an acetylide by reacting with the carbon. In an embodiment,
the catalyst or
source of catalyst, preferably NaH, is incorporated inside of the HSA material
such as
37
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
fullerene, carbon nanotubes, and zeolite. The HSA material may further
comprise graphite,
graphene, diamond-like carbon (DLC), hydrogenated diamond-like carbon (HDLC),
diamond
powder, graphitic carbon, glassy carbon, and carbon with other metals such as
at least one of
Co, Ni, Mn, Fe, Y, Pd, and Pt, or dopants comprising other elements such as
fluorinated
carbon, preferably fluorinated graphite, fluorinated diamond, or tetracarbon
fluoride (C4F).
Preferably the metals are a mixture of such as a mixture of Co, Ni, Mn. The
metals may be in
any wt% ratio. Preferably, the composition and weight percent (%) ratios are
about 20 to
25% Ni, 60 to 70% Co, and 5 to 15% Mn. The HSA material may be fluoride
passivated
such as fluoride coated metal or carbon or comprise a fluoride such as a metal
fluoride,
preferably an alkali or rare earth fluoride.
In another embodiment, the support has a pore size or interlayer spacing that
will
accommodate only one catalyst radius such as the atomic radius in the case of
Li or K and the
molecular dimensions in the case of NaH. In the Li case, the pore size or
interlayer spacing is
ideally between about 1.35 A and 3A. In the K case, the pore size or
interlayer spacing is
ideally between about 1.7 A and 3.5A. In the NaH case, the pore size or
interlayer spacing is
ideally between about 1.5 A and 5A. In an embodiment, the support provides
atomic catalyst
such as Li or K and single catalyst molecules such as NaH based on size
discrimination and
selection. A suitable support having a large surface area and an interlayer
separation distance
of about 3.5A is activated carbon. The activated carbon can be activated or
reactivated by
physical or chemical activation. The former activation may comprise
carbonization or
oxidation, and the latter activation may comprise impregnation with chemicals.
The reaction mixture may further comprise a support such as a polymer support.
The
polymer support may be chosen from poly(tetrafluoroethylene) such as TEFLONTM,
polyvinylferrocene, polystyrene, polypropylene, polyethylene, polyisoprene,
poly(aminophosphazene), a polymer comprising ether units such as polyethylene
glycol or
oxide and polypropylene glycol or oxide, preferably arylether, a polyether
polyol such as
poly(tetramethylene ether) glycol (PTMEG, polytetrahydrofuran, "Terathane",
"polyTHF"),
polyvinyl formal, and those from the reaction of epoxides such as polyethylene
oxide and
polypropylene oxide. In an embodiment, the HSA comprises fluorine. Exemplary
fluorinated HSAs are TEFLONTM, TEFLONTM-PFA, polyvinyl fluoride, PVF,
poly(vinylidene fluoride), poly(vinylidene fluoride-co-hexafluoropropylene),
and
perfluoroalkoxy polymers.
B. Solid Fuels
The solid fuel comprises a catalyst or source of catalyst to form hydrinos
such as at
least one catalyst chosen from LiH, Li, NaH, Na, KH, K, RbH, Rb, and CsH, a
source of
atomic hydrogen, and other solid chemical reactants that perform the one or
more of the
following functions (i) the reactants form the catalyst or atomic hydrogen by
undergoing a
38
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
reaction such as one between one or more components of the reaction mixture or
by
undergoing a physical or chemical change of at least one component of the
reaction mixture
and (ii) the reactants initiate, propagate, and maintain the catalysis
reaction to form hydrinos.
The many examples of solid fuels given in the present disclosure including the
reaction
mixtures of liquid fuels comprising a solvent except with the exception of the
solvent are not
meant to be exhaustive. Based on the present disclosure other reaction
mixtures are taught to
those skilled in the art.
In a sold fuel embodiment, the reaction mixture comprises a catalyst, a source
of
hydrogen, and at least one of a HSA support, getter, a dispersant, and an
inert gas. The
catalyst may be NaH. The inert gas may be at least one of a noble gas and
nitrogen.
Preferably the inert gas is a mixture of Ne and N2, more preferably, the
mixture is about 50%
Ne and 50% N2. The pressure may preferably be in the range of about 1 Torr to
100
atmosphere. Preferably, the pressure of a Ne- N2 mixture is one atmosphere.
The reaction
temperature is preferably in the range of about 100 to 900 C. The reaction
mixture may
further comprise at least one of Na and NaOH, and additionally a reductant
such as NaH, Sn,
Zn, Fe, and an alkali metal. In the case that the reaction mixture comprises
NaOH, preferably
H2 is also supplied, and H2 comprises a gas of any mixture in the case that
the reaction
mixture comprises one or more inert gases. The source of hydrogen may comprise
hydrogen
or a hydride and a dissociator such as Pt/Ti, hydrided Pt/Ti, Pd, Pt, or
Ru/A1203, Ni, Ti, or Nb
powder. At least one of the HSA support, getter, and dispersant may comprise
at least one of
the group of a metal powder such as Ni, Ti, or Nb powder, R-Ni, Zr02, A1203,
NaX (X=F, Cl,
Br, I), Na20, NaOH, and Na2CO3. In an embodiment, a metal catalyzes the
formation of
NaH molecules from a source such as a Na species and a source of H. The metal
may be a
transition, noble, intermetallic, rare earth, lanthanide, and actinide metal,
as well as others
such as aluminum, and tin.
C. Hydrino Reaction Activators
The hydrino reaction may be activated or initiated and propagated by one or
more
chemical other reactions. These reactions can be of several classes such as
(i) exothermic
reactions which provide the activation energy for the hydrino reaction, (ii)
coupled reactions
that provide for at least one of a source of catalyst or atomic hydrogen to
support the hydrino
reaction, (iii) free radical reactions that, in an embodiment, serve as an
acceptor of electrons
from the catalyst during the hydrino reaction, (iv) oxidation-reduction
reactions that, in an
embodiment, serve as an acceptor of electrons from the catalyst during the
hydrino reaction,
(v) exchange reactions such as anion exchange including halide, sulfide,
hydride, arsenide,
oxide, phosphide, and nitride exchange that in an embodiment, facilitate the
action of the
catalyst to become ionized as it accepts energy from atomic hydrogen to form
hydrinos, and
(vi) getter, support, or matrix-assisted hydrino reaction that may provide at
least one of a
39
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
chemical environment for the hydrino reaction, act to transfer electrons to
facilitate the H
catalyst function, undergoes a reversible phase or other physical change or
change in its
electronic state, and binds a lower-energy hydrogen product to increase at
least one of the
extent or rate of the hydrino reaction. In an embodiment, the reaction mixture
comprises a
support, preferably an electrically conductive support, to enable the
activation reaction.
In an embodiment a catalyst such as Li, K, and NaH serves to form hydrinos at
a
high rate by speeding up the rate limiting step, the removal of electrons from
the catalyst as it
is ionized by accepting the nonradiative resonant energy transfer from atomic
hydrogen to
form hydrinos. The typical metallic form of Li and K may be converted to the
atomic form
and the ionic form of NaH may be converted to the molecular form by using a
support or
HSA material such as activated carbon (AC), Pt/C, Pd/C, TiC, or WC to disperse
the catalyst
such as Li and K atoms and NaH molecules, respectively. Preferably, the
support has a
high surface area and conductivity considering the surface modification upon
reaction with
other species of the reaction mixture. The reaction to cause a transition of
atomic hydrogen
to form hydrinos requires a catalyst such as Li, K, or NaH and atomic hydrogen
wherein
NaH serves as a catalyst and source of atomic hydrogen in a concerted
reaction. The
reaction step of a nonradiative energy transfer of an integer multiple of 27.2
eV from atomic
hydrogen to the catalyst results in ionized catalyst and free electrons that
causes the reaction
to rapidly cease due to charge accumulation. The support such as AC may also
act as a
conductive electron acceptor, and final electron-acceptor reactants comprising
an oxidant,
free radicals or a source thereof, are added to the reaction mixture to
ultimately scavenge
electrons released from the catalyst reaction to form hydrinos. In addition a
reductant may be
added to the reaction mixture to facilitate the oxidation reaction. The
concerted electron-
acceptor reaction is preferably exothermic to heat the reactants and enhance
the rates. The
activation energy and propagation of the reaction may be provided by a fast,
exothermic,
oxidation or free radical reaction such as that of 02 or CF4 with Mg or Al
wherein radicals
such as CF and F and 02 and 0 serve to ultimately accept electrons from the
catalyst via
support such as AC. Other oxidants or sources of radicals singly or in
combination may be
chosen from the group of 02, 03, N20 NF3, M2S208 (M is an alkali metal), S,
CS2, and SO2,
Mn12, EuBr2, AgCl, and others given in the Electron Acceptor Reactions
section.
Preferably, the oxidant accepts at least two electrons. The corresponding
anion may
be OZ-, Sz-, C2S4 (tetrathiooxalate anion),SO3-, and SO4-. The two electrons
may be
accepted from a catalyst that becomes doubly ionized during catalysis such as
NaH and Li
(Eqs. (25-27) and (37-39)). The addition of an electron acceptor to the
reaction mixture or
reactor applies to all cell embodiments of the present disclosure such as the
solid fuel and
heterogeneous catalyst embodiments as well as electrolysis cells, and plasma
cells such as
glow discharge, RF, microwave, and barrier-electrode plasma cells and plasma
electrolysis
cells operated continuously or in pulsed mode. An electron conductive,
preferably
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
unreactive, support such as AC may also be added to the reactants of each of
these cell
embodiments. An embodiment of the microwave plasma cell comprises a hydrogen
dissociator such as a metal surface inside of the plasma chamber to support
hydrogen atoms.
In embodiments, mixtures of species, compounds, or materials of the reaction
mixture
such as a source of catalyst, a source of an energetic reaction such as a
metal and at least one
of a source of oxygen, a source of halogen, and a source of free radicals, and
a support may
be used in combinations. Reactive elements of compounds or materials of the
reaction
mixture may also be used in combinations. For example, the source of fluorine
or chlorine
may be a mixture of NXFy and NXCly, or the halogen may be intermixed such as
the in
compound NXFyCl,. The combinations could be determined by routine
experimentation by
those skilled in the art.
a. Exothermic Reactions
In an embodiment, the reaction mixture comprises a source of catalyst or a
catalyst
such as at least one of NaH, K, and Li and a source of hydrogen or hydrogen
and at least one
species that undergoes reaction. The reaction is preferably very exothermic
and preferably
has fast kinetics such that it provides the activation energy to the hydrino
catalyst reaction.
The reaction may be an oxidation reaction. Suitable oxidation reactions are
the reaction of
species comprising oxygen such as the solvent, preferably an ether solvent,
with a metal such
as at least one of Al, Ti, Be, Si, P, rare earth metals, alkali metals, and
alkaline earth metals.
More preferably, the exothermic reaction forms an alkali or alkaline earth
halide, preferably
MgF2, or halides of Al, Si, P, and rare earth metals. Suitable halide
reactions are the reaction
of a species comprising a halide such as the solvent, preferably a
fluorocarbon solvent, with
at least one of a metal and a metal hydride such as at least one of Al, rare
earth metals, alkali
metals, and alkaline earth metals. The metal or metal hydride may be the
catalyst or a source
of the catalyst such as NaH, K, or Li. The reaction mixture may comprise at
least NaH and
NaA1C14 or NaA1F4 having the products NaCI and NaF, respectively. The reaction
mixture
may comprise at least NaH a fluorosolvent having the product NaF.
In general, the product of the exothermic reaction to provide the activation
energy to
the hydrino reaction may be a metal oxide or a metal halide, preferably a
fluoride. Suitable
products are A1203, M203 (M=rare earth metal), Ti02, Ti203, Si02, PF3 or PF5,
A1F3, MgF2,
MF3 (M=rare earth metal), NaF, NaHF2, KF, KHF2, LiF, and LiHF2. In an
embodiment
wherein Ti undergoes the exothermic reaction, the catalyst is Ti2+ having a
second ionization
energy of 27.2 eV (m=1 in Eq. (5)). The reaction mixture may comprise at least
two of NaH,
Na, NaNH2, NaOH, Teflon, fluorinated carbon, and a source of Ti such as Pt/Ti
or Pd/Ti. In
an embodiment wherein Al undergoes the exothermic reaction, the catalyst is
AJH as given in
TABLE 3. The reaction mixture may comprise at least two of NaH, Al, carbon
powder, a
fluorocarbon, preferably a solvent such as hexafluorobenzene or
perfluoroheptane, Na,
41
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
NaOH, Li, LiH, K, KH, and R-Ni. Preferably, the products of the exothermic
reaction to
provide the activation energy are regenerated to form the reactants for
another cycle of
forming hydrinos and releasing the corresponding power. Preferably, metal
fluoride products
are regenerated to metals and fluorine gas by electrolysis. The electrolyte
may comprise a
eutetic mixture. The metal may be hydrided and the carbon product and any CH4
and
hydrocarbons products may be fluorinated to form the initial metal hydride and
fluorocarbon
solvent, respectively.
In an embodiments of the exothermic reaction to activate the hydrino
transition
reaction at least one of the group of a rare earth metal (M), Al, Ti, and Si
is oxidized to the
corresponding oxide such as M203, A1203, Ti203, and Si02, respectively. The
oxidant may
be an ether solvent such as 1,4-benzodioxane (BDO) and may further comprise a
fluorocarbon such as hexafluorobenzene (HFB) or perfluoroheptane to accelerate
the
oxidation reaction. In an exemplary reaction, the mixture comprises NaH,
activated carbon,
at least one of Si and Ti, and at least one of BDO and HFB. In the case of Si
as the reductant,
the product Si02 may be regenerated to Si by H2 reduction at high temperature
or by reaction
with carbon to form Si and CO and CO2. A certain embodiment of the reaction
mixture to
form hydrinos comprises a catalyst or a source of catalyst such as at least
one of Na, NaH, K,
KH, Li, and LiH, a source of exothermic reactants or exothermic reactants,
preferably having
fast kinetics, that activate the catalysis reaction of H to form hydrinos, and
a support. The
exothermic reactants may comprise a source of oxygen and a species that reacts
oxygen to
form an oxide. For x and y being integers, preferably the oxygen source is
H2O, 02, H202,
Mn02, an oxide, an oxide of carbon, preferably CO or C02, an oxide of
nitrogen, NxOy such
as N20 and NO2, an oxide of sulfur, SxOy, preferably an oxidant such as M2SxOy
(M is an
alkali metal) that may optionally be used with an oxidation catalyst such as
silver ion, Cl,,Oy
such as C120, and C102 preferably from NaC1O2, concentrated acids and their
mixtures such
as HN02i HNO3, H2SO4, H2S03i HCI, and HF, preferably, the acid forms nitronium
ion
(NO2 ), NaOCI, IxOy, preferably 1205, PxOy, SxOy, an oxyanion of an inorganic
compound
such as one of nitrite, nitrate, chlorate, sulfate, phosphate, a metal oxide
such as cobalt oxide,
and oxide or hydroxide of the catalyst such as NaOH, and perchlorate wherein
the cation is a
source of the catalyst such as Na, K, and Li, an oxygen-containing functional
group of an
organic compound such as an ether, preferably one of dimethoxyethane, dioxane,
and 1,4-
benzodioxane (BDO), and the reactant species may comprise at least one of the
group of a
rare earth metal (M), Al, Ti, and Si, and the corresponding oxide is M203,
A1203, Ti203, and
Si02i respectively. The reactant species may comprise the metal or element of
the oxide
products of at least one of the group of A1203 aluminum oxide, La203 lanthanum
oxide, MgO
magnesium oxide, Ti203 titanium oxide, Dy203 dysprosium oxide, Er203 erbium
oxide,
Eu203 europium oxide, LiOH lithium hydroxide, Ho203 holmium oxide, Li20
lithium oxide,
Lu203 lutetium oxide, Nb205 niobium oxide, Nd203 neodymium oxide, Si02 silicon
oxide,
42
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
Pr203 praseodymium oxide, Sc203 scandium oxide, SrSiO3 strontium metasilicate,
Sm203
samarium oxide, Tb203 terbium oxide, Tm203 thulium oxide, Y203 yttrium oxide,
and Ta205
tantalum oxide, B203 boron oxide, and zirconium oxide. The support may
comprise carbon,
preferably activated carbon. The metal or element may be at a least one of Al,
La, Mg, Ti,
Dy, Er, Eu, Li, Ho, Lu, Nb, Nd, Si, Pr, Sc, Sr, Sm, Th, Tm, Y, Ta, B, Zr, S,
P, C, and their
hydrides.
In another embodiment, the oxygen source may be at least one of an oxide such
as
M20 where M is an alkali metal, preferably Li20, Na20, and K20, a peroxide
such as M202
where M is an alkali metal, preferably Li202, Na202, and K202, and a
superoxide such as
MO2 where M is an alkali metal, preferably Li202, Na202, and K202. The ionic
peroxides
may further comprise those of Ca, Sr, or Ba.
In another embodiment, at least one of the source of oxygen and the source of
exothermic reactants or exothermic reactants, preferably having fast kinetics,
that activate the
catalysis reaction of H to form hydrinos comprises one or more of the group of
MNO3, MNO,
MNO2, M3N, M2NH, MNH2, MX, NH3, MBH4, MA1H4, M3A1H6, MOH, M2S, MHS, MFeSi,
M2CO3, MHC03, M2SO4, MHS04, M3P04i M2HPO4, MH2PO4, M2MoO4, MNb03, M2B407
(lithium tetraborate), MBO2, M2WO4, MA1C14, MGaC14, M2CrO4, M2Cr2O7, M2TiO3,
MZrO3,
MA102, MCo02, MGa02, M2GeO3, MMn204, M4SiO4, M2SiO3, MTaO3, MCuC14, MPdC14,
MV03, M103, MFeO2, M104,MC104, MScO, MTiO,,, MVO, MCrO,,, MCr20,,, MMn20,,,
MFeO, MCoO,,, MNiOn, MNi2O,,, MCuO,,, and MZnO,,, where M is Li, Na or K and
n=1,
2,3, or 4, an oxyanion, an oxyanion of a strong acid, an oxidant, a molecular
oxidant such as
V203, I205, Mn02, Re207, Cr03, Ru02, AgO, PdO, Pd02, PtO, Pt02, I204, I205,
1209, SO2,
SO3, C02, N20, NO, NO2, N203, N204, N205, C120, C102, C1203, C1206, C1207,
P02, P203,
and P205, NH4X wherein X is a nitrate or other suitable anion known to those
skilled in the
art such as one of the group comprising F, Cl-, Br , I-, N03-, N02 , 5042-,
HS04-, Co02-, I03-,
I04 , Ti03, Cr04, Fe02, P043 , HP042 , H2PO4, V03, C104 and Cr2072 and other
anions of
the reactants. The reaction mixture may additionally comprise a reductant. In
an
embodiment, N205 is formed from a reaction of a mixture of reactants such as
HNO3 and
P205 that reacts according to 2P205 + 12 HN03 to 4H3PO4 + 6N205.
In an embodiment wherein oxygen or a compound comprising oxygen participates
in
the exothermic reaction, 02 may serve as a catalyst or a source of a catalyst.
The bond
energy of the oxygen molecule is 5.165 eV, and the first, second, and third
ionization
energies of an oxygen atom are 13.61806 eV, 35.11730 eV, and 54.9355 eV,
respectively.
The reactions 02 _>0+02,, 02 -* 0 + 03+ , and 20 -> 20+ provide a net enthalpy
of about
2, 4, and 1 times Eh , respectively, and comprise catalyst reactions to from
hydrino by
accepting these energies from H to cause the formation of hydrinos.
Additionally, the source of an exothermic reaction to activate the hydrino
reaction
may be a metal alloy forming reaction, preferably between Pd and Al initiated
by melting the
43
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
Al. The exothermic reaction preferably produces energetic particles to
activate the hydrino-
forming reaction. The reactants may be a pyrogen or pyrotechnic composition.
In another
embodiment, the activation energy may be provided by operating the reactants
at a very high
temperature such as in the range of about 1000-5000 C, preferably in the range
of about
1500-2500 C. The reaction vessel may comprise a high-temperature stainless
steel alloy, a
refractory metal or alloy, alumina, or carbon. The elevated reactant
temperature may be
achieved by heating the reactor or by an exothermic reaction.
The exothermic reactants may comprise a halogen, preferably fluorine or
chlorine,
and a species that reacts with the fluorine or chlorine to form a fluoride or
chloride,
respectively. Suitable fluorine sources are fluorocarbons such as CF4,
hexafluorbenzene, and
hexadecafluoroheptane, xenon fluorides such as XeF2, XeF4, and XeF6, BXXy,
preferably BF3,
B2F4, BC13, or BBr3, SFx such as, fluorosilanes, fluorinated nitrogen, NxFy,
preferably NF3,
NF3O, SbFx, BiFx, preferably BiF5, NxCly, preferably NC13, SxXy, preferably
SC12 or SxFy (X
is a halogen; x and y are integers) such as SF4, SF6, or S2F10, fluorinated
phosphorous,
M2SiF6 wherein M is an alkali metal such as Na2SiF6 and K2SiF6 , MSiF6 wherein
M is an
alkaline earth metal such as MgSiF6, GaF3, PF5, MPF6 wherein M is an alkali
metal,
MHF2 wherein M is an alkali metal such as NaHF2 and KHF2, K2TaF,, KBF4 ,
K2MnF6 ,
and K2ZrF6 wherein other similar compounds are anticipated such as those
having another
alkali or alkaline earth metal substitution such as one of Li, Na, or K as the
alkali metal.
Suitable sources of chlorine are C12 gas, SbCl5, and chlorocarbons such as
CC14 and
chloroform. The reactant species may comprise at least one of the group of an
alkali or
alkaline earth metal or hydride, a rare earth metal (M), Al, Si, Ti, and P
that forms the
corresponding fluoride or chloride. Preferably the reactant alkali metal
corresponds to that of
the catalyst, the alkaline earth hydride is MgH2, the rare earth is La, and Al
is a nanopowder.
The support may comprise carbon, preferably activated carbon, mesoporous
carbon, and the
carbon using in Li ion batteries. The reactants may be in any molar ratios.
Preferably, the
reactant species and the fluorine or chlorine are in about the stoichiometric
ratio as the
elements of the fluoride or chlorine, the catalyst is in excess, preferably in
about the same
molar ratio as the element that reacts with the fluorine or chlorine, and the
support is in
excess.
The exothermic reactants may comprise a halogen gas, preferably chlorine or
bromine, or a source of halogen gas such as HF, HC1, HBr, HI, preferably CF4
or CC14, and a
species that reacts with the halogen to form a halide. The source of halogen
may also be a
source of oxygen such as CXOyX, wherein X is halogen, and x, y, and r are
integers and are
known in the art. The reactant species may comprise at least one of the group
of an alkali or
alkaline earth metal or hydride, a rare earth metal, Al, Si, and P that forms
the corresponding
halide. Preferably the reactant alkali metal corresponds to that of the
catalyst, the alkaline
earth hydride is MgH2, the rare earth is La, and Al is a nanopowder. The
support may
44
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
comprise carbon, preferably activated carbon. The reactants may be in any
molar ratios.
Preferably, the reactant species and the halogen are in about an equal
stoichiometric ratio, the
catalyst is in excess, preferably in about the same molar ratio as the element
that reacts with
the halogen, and the support is in excess. In an embodiment, the reactants
comprise, a source
of catalyst or a catalyst such as Na, NaH, K, KH, Li, LiH, and H2, a halogen
gas, preferably,
chlorine or bromine gas, at least one of Mg, MgH2, a rare earth, preferably
La, Gd, or Pr, Al,
and a support, preferably carbon such as activated carbon.
b. Free Radical Reactions
In an embodiment, the exothermic reaction is a free radical reaction,
preferably a
halide or oxygen free radical reaction. The source of halide radicals may be a
halogen,
preferably F2 or C12i or a fluorocarbon, preferably CF4. A source of F free
radicals is S2F10.
The reaction mixture comprising a halogen gas may further comprise a free
radical initiator.
The reactor may comprise a source of ultraviolet light to form free radials,
preferably halogen
free radicals and more preferably chlorine or fluorine free radicals. The free
radical initiators
are those commonly known in the art such as peroxides, azo compounds and a
source of
metal ions such as a metal salt, preferably, a cobalt halide such as CoC12
that is a source of
Co2+ or FeSO4 which is a source of Fee+. The latter are preferably reacted
with an oxygen
species such as H202 or 02. The radical may be neutral.
The source of oxygen may comprise a source of atomic oxygen. The oxygen may be
singlet oxygen. In an embodiment, singlet oxygen is formed from the reaction
of NaOCI
with H202. In an embodiment, the source of oxygen comprises 02 and may further
comprise
a source of free radicals or a free radical initiator to propagate a free
radical reaction,
preferably a free radical reaction of 0 atoms. The free radical source or
source of oxygen
may be at least one of ozone or an ozonide. In an embodiment, the reactor
comprises an
ozone source such as an electrical discharge in oxygen to provide ozone to the
reaction
mixture.
The free radical source or source of oxygen may further comprise at least one
of a
peroxo compound, a peroxide, H202, a compound containing an azo group, N20,
NaOCI,
Fenton's reagent, or a similar reagent, OH radical or a source thereof,
perxenate ion or a
source thereof such as an alkali or alkaline earth perxenate, preferably,
sodium perxenate
(Na4XeO6) or potassium perxenate (K4XeO6), xenon tetraoxide (Xe04), and
perxenic acid
(H4XeO6), and a source of metal ions such as a metal salt. The metal salt may
be at least one
of FeSO4, A1C13, TiC13, and, preferably, a cobalt halide such as CoC12 that is
a source of Coe+.
In an embodiment, free radicals such as Cl are formed from a halogen such as
C12 in
the reaction mixture such as NaH + MgH2 + support such as activated carbon
(AC) + halogen
gas such as C12. The free radicals may be formed by the reaction of a mixture
of C12 and a
hydrocarbon such as CH4 at an elevated temperature such as greater than 200
C. The
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
halogen may be in molar excess relative to the hydrocarbon. The chlorocarbon
product and
Cl radicals may react with the reductant to provide the activation energy and
pathway for
forming hydrinos. The carbon product may be regenerated using the synthesis
gas (syngas)
and Fischer-Tropsch reactions or by direct hydrogen reduction of carbon to
methane. The
reaction mixture may comprise a mixture of 02 and Cl2 at an elevated
temperature such as
greater than 200 C. The mixture may react to form Cl,,Oy (x and y are
integers) such as CIO,
C120, and C102. The reaction mixture may comprise H2 and C12 at an elevated
temperature
such as greater than 200 C that may react to form HCI. The reaction mixture
may comprise
H2 and 02 with a recombiner such as Pt/Ti, Pt/C, or Pd/C at a slightly
elevated temperature
such as greater than 50 C that may react to form H2O. The recombiner may
operate at
elevated pressure such as in the range of greater than one atmosphere,
preferably in the range
of about 2 to 100 atmospheres. The reaction mixture may be nonstoichiometric
to favor free
radical and singlet oxygen formation. The system may further comprise a source
of
ultraviolet light or plasma to form free radicals such as a RF, microwave, or
glow discharge,
preferably high-voltage pulsed, plasma source. The reactants may further
comprise a catalyst
to form at least one of atomic free radicals such as Cl, 0, and H, singlet
oxygen, and ozone.
The catalyst may be a noble metal such as Pt. In an embodiment to form Cl
radicals, the Pt
catalyst is maintained at an temperature greater than the decomposition
temperature of
platinum chlorides such as PtC12, PtC13, and PtC14 which have decomposition
temperatures of
581 C, 435 C, and 327 C, respectively. In an embodiment, Pt may be recovered
from a
product mixture comprising metal halides by dissolving the metal halides in a
suitable solvent
in which the Pt, Pd or their halides are not soluble and removing the
solution. The solid that
may comprise carbon and Pt or Pd halide may be heated to form Pt or Pd on
carbon by
decomposition of the corresponding halide.
In an embodiment, N20, NO2, or NO gas is added reaction mixture. N20 and NO2
may serve as a source of NO radical. In another embodiment, the NO radical is
produced in
the cell, preferably by the oxidation of NH3. The reaction may be the reaction
of NH3 with
02 on platinum or platinum-rhodium at elevated temperature. NO, NO2, and N20
can be
generated by known industrial methods such as by the Haber process followed by
the
Ostwald process. In one embodiment, the exemplary sequence of steps are:
N2 ber > NH3 o NO, N20, NO2 . (53)
process process
Specifically, the Haber process may be used to produce NH3 from N2 and H2 at
elevated temperature and pressure using a catalyst such as a -iron containing
some oxide.
The Ostwald process may be used to oxidize the ammonia to NO, NO2, and N20 at
a catalyst
such as a hot platinum or platinum-rhodium catalyst. Alkali nitrates can be
regenerated using
the methods disclosed supra.
46
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
The system and reaction mixture may initiate and support a combustion reaction
to
provide at least one of singlet oxygen and free radicals. The combustion
reactants may be
nonstoichiometric to favor free radical and singlet oxygen formation that
react with the other
hydrino reaction reactants. In an embodiment, an explosive reaction is
suppressed to favor a
prolonged steady reaction, or an explosive reaction is caused by the
appropriate reactants and
molar ratios to achieve the desired hydrino reaction rate. In an embodiment,
the cell
comprises at least one cylinder of an internal combustion engine.
c. Electron Acceptor Reactions
In an embodiment, the reaction mixture further comprises an electron acceptor.
The
electron acceptor may act as a sink for the electrons ionized from the
catalyst when energy is
transferred to it from atomic hydrogen during the catalytic reaction to form
hydrinos. The
electron acceptor may be a at least one of a conducting polymer or metal
support, an oxidant
such as group VI elements, molecules, and compounds, a free radical, a species
that forms a
stable free radical, and a species with a high electron affinity such as
halogen atoms, 02, C,
CF1,2,3 or 4, Si, S, PXSy, CS29 SXNy and these compounds further comprising 0
and H, Au, At,
A1XOy (x and y are integers), preferably A102 that in an embodiment is an
intermediate of the
reaction of Al(OH)3 with Al of R-Ni, CIO, C12, F2, A102, B2N, CrC2, C2H,
CuC12, CuBr2,
MnX3 (X = halide), MoX3 (X = halide), NiX3 (X = halide), RuF4, 5, or 6, ScX4
(X = halide),
W03, and other atoms and molecules with a high electron affinity as known by
those skilled
in the art. In an embodiment, the support acts as an electron acceptor from
the catalyst as it is
ionized by accepting the nonradiative resonant energy transfer from atomic
hydrogen.
Preferably, the support is at least one of conductive and forms stable free
radicals. Suitable
such supports are conductive polymers. The support may form a negative ion
over a
macrostructure such as carbon of Li ion batteries that form C6 ions. In
another embodiment,
the support is a semiconductor, preferably doped to enhance the conductivity.
The reaction
mixture further comprises free radicals or a source thereof such as 0. OH, 02,
03, H202, F,
Cl, and NO that may serve as a scavenger for the free radicals formed by the
support during
catalysis. In an embodiment, the free radical such as NO may form a complex
with the
catalyst or source of catalyst such an alkali metal. In another embodiment,
the support has
unpaired electrons. The support may be paramagnetic such as a rare earth
element or
compound such as Er203. In an embodiment, the catalyst or source of catalyst
such as Li,
NaH, K. Rb, or Cs is impregnated into the electron acceptor such as a support
and the other
components of the reaction mixture are add. Preferably, the support is AC with
intercalated
NaH or Na.
d. Oxidation-Reduction Reactions
47
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
In an embodiment, the hydrino reaction is activated by an oxidation-reduction
reaction. In an exemplary embodiment, the reaction mixture comprises at least
two species of
the group of a catalyst, a source of hydrogen, an oxidant, a reductant, and a
support. The
reaction mixture may also comprise a Lewis acid such as Group 13 trihalides,
preferably at
least one of AiC13, BF3, BC13, and BBr3. In certain embodiments, each reaction
mixture
comprises at least one species chosen from the following genus of components
(i) - (iii).
(i) A catalyst chosen from Li, LiH , K, KH , NaH , Rb, RbH, Cs, and CsH.
(ii) A source of hydrogen chosen from H2 gas, a source of H2 gas, or a
hydride.
(iii) And an oxidant chosen from a metal compound such as one of halides,
phosphides, borides, oxides, hydroxides, silicides, nitrides, arsenides,
selenides, tellurides,
antimonides, carbides, sulfides, hydrides, carbonate, hydrogen carbonate,
sulfates, hydrogen
sulfates, phosphates, hydrogen phosphates, dihydrogen phosphates, nitrates,
nitrites,
permanganates, chlorates, perchlorates, chlorites, perchlorites,
hypochlorites, bromates,
perbromates, bromites, perbromites, iodates, periodates, iodites, periodites,
chromates,
dichromates, tellurates, selenates, arsenates, silicates, borates, colbalt
oxides, tellurium
oxides, and other oxyanions such as those of halogens, P, B, Si, N, As, S, Te,
Sb, C, S, P, Mn,
Cr, Co, and Te wherein the metal preferably comprises a transition metal, Sn,
Ga, In, an
alkali metal or alkaline earth metal; the oxidant further comprising a lead
compound such as a
lead halide, a germanium compound such as a halide, oxide, or sulfide such as
GeF2, GeC12,
GeBr2, GeI2i GeO, GeP, GeS, GeI4, and GeC14, fluorocarbon such as CF4 or
C1CF3,
chlorocarbon such as CC14, 02, MNO3 , MC104, M02 , NF3 , N20, NO, NO2, a boron-
nitrogen compound such as B3N3H6, a sulfur compound such as SF6, S , S02 1
SO3, S205C12,
F5SOF, M2S208, SXXy such as S2C12, SC12, S2Br2, or S2F2, CS2, SOXXy such as
SOC12, SOF2,
S02F2, or SOBr2, XXX'y such as C1F5, XXX'yOZ such as C1O2F, C102F2, C1OF3,
C1O3F, and
C102F3, boron-nitrogen compound such as B3N3H6, Se, Te, Bi, As, Sb, Bi, TeX,
preferably
TeF4, TeF6, TeOX, preferably Te02 or Te03, SeXX, preferably SeF6, SeOX,
preferably Se02 or
Se03, a tellurium oxide, halide, or other tellurium compound such as Te02,
Te03, Te(OH)6,
TeBr2, TeC12, TeBr4, TeC14, TeF4, Tet4, TeF6, CoTe, or NiTe, a selenium oxide,
halide,
sulfide, or other selenium compound such as Se02, Se03, Se2Br2, Se2Cl2, SeBr4,
SeC14, SeF4,
SeF6, SeOBr2, SeOC12i SeOF2, SeO2F2, SeS2, Se2S6, Se4S4, or Se6S2, P, P205,
P2S5, PXXy such
as PF3, PC13, PBr3, P13, PF5, PC15, PBr4F, or PC14F, PO, Xy such as POBr3,
P013, POC13 or
POF3, PSXXy (M is an alkali metal, x, y and z are integers, X and X' are
halogen) such as
PSBr3, PSF3, PSC13, a phosphorous-nitrogen compound such as P3N5, (C12PN)3,
(C12PN)4, or
(Br2PN)X, an arsenic oxide, halide, sulfide, selenide, or telluride or other
arsenic compound
such as AlAs, AS2I4, As2Se, AS4S4, AsBr3, AsC13, AsF3, As13, AS203, As2Se3,
AS2S3, As2Te3i
AsC15, AsF5, As205, As2Se5, or As2S5i an antimony oxide, halide, sulfide,
sulfate, selenide,
arsenide, or other antimony compound such as SbAs, SbBr3, SbC13, SbF3, SbI3,
Sb203,
SbOCI, Sb2Se3, Sb2(SO4)3, Sb2S3, Sb2Te3, Sb204, SbC15, SbF5, SbC12F3, Sb205,
or Sb2S5, an
48
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
bismuth oxide, halide, sulfide, selenide, or other bismuth compound such as
BiAs04, BiBr3,
BiC13, BiF3, BiF5, Bi(OH)3, BiI3i Bi203, BiOBr, BiOC1, BiOI, Bi2Se3, Bi2S3,
Bi2Te3, or Bi204,
SiC14, SiBr4, a metal oxide, hydroxide, or halide such as a transition metal
halide such as
CrC13, ZnF2, ZnBr2, Zn12, MnC12, MnBr2, Mn12, CoBr2, Co12, CoC12, NiC12,
NiBr2, NiF2,
FeF2, FeC12, FeBr2, FeC13, TiF3, CuBr, CuBr2, VF3, and CuC12i a metal halide
such as SnF2,
SnC12, SnBr2, SnI2, SnF4, SnC14, SnBr4, Sn14, InF, InCl, InBr, InI, AgCI, AgI,
ALF3, A1Br3,
A1I3, YF3, CdC12, CdBr2, CdI2, InC13, ZrC14, NbF5, TaC15, MoC13, MoCl5, NbC15,
AsC13,
TiBr4, SeC12, SeC14, InF3, InCl3, PbF4, Te14i WC16, OSCl3, GaC13, PtC13,
ReC13, RhC13, RuC13,
metal oxide or hydroxide such as Y203, FeO, Fe203, or NbO, NiO, Ni203, SnO,
Sn02, Ag20,
AgO, Ga20, As203, Se02, Te02, In(OH)3, Sn(OH)2, In(OH)3, Ga(OH)3, and Bi(OH)3,
C02,
As2Se3, SF6, S, SbF3, CF4, NF3, a permanganate such as KMnO4 and NaMnO4, P205,
a nitrate
such as LiNO3, NaNO3 and KNO3, and a boron halide such as BBr3 and B13, a
group 13
halide, preferably an indium halide such as InBr2, InC12, and InI3, a silver
halide, preferably
AgCI or AgI, a lead halide, a cadmium halide, a zirconoium halide, preferably
a transition
metal oxide, sulfide, or halide (Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, or Zn with
F, Cl, Br or I), a
second or third transition series halide, preferably YF3, oxide, sulfide
preferably Y2S3, or
hydroxide, preferably those of Y, Zr, Nb, Mo, Tc, Ag, Cd, Hf, Ta, W, Os, such
as NbX3,
NbX5, or TaX5 in the case of halides, a metal sulfide such as Li2S, ZnS, FeS,
NiS, MnS,
Cu2S, CuS, and SnS, an alkaline earth halide such as BaBr2, BaC12, BaI2,
SrBr2, Sr12, CaBr2,
Ca12, MgBr2, or Mg12, a rare earth halide such as EuBr3, LaF3, LaBr3, CeBr3,
GdF3, GdBr3,
preferably in the II state such as one of CeI2, EuF2, EuC12, EuBr2, Eu12,
DyI2, NdI2, SmI2,
YbI2, and Tm12, a metal boride such as a europium boride, an MB2 boride such
as CrB2, TiB2,
MgB2, ZrB2, and GdB2 an alkali halide such as LiCl, RbCl, or CsI, and a metal
phosphide
such as Ca3P2, a noble metal halide, oxide, sulfide such as PtC12, PtBr2,
Pt12, PtC14, PdC12,
PbBr2, and PbI2, a rare earth sulfide such as CeS, other suitable rare earths
are those of La
and Gd, a metal and an anion such as Na2TeO4, Na2TeO3, Co(CN)2, CoSb, CoAs,
Co2P, CoO,
CoSe, CoTe, NiSb, NiAs, NiSe, Ni2Si, MgSe, a rare earth telluride such as
EuTe, a rare earth
selenide such as EuSe, a rare earth nitride such as EuN, a metal nitride such
as AIN, GdN,
and Mg3N2, a compound containing at least two atoms from the group of oxygen
and
different halogen atoms such as F20, C120, C102, C1206, C1207, C1F, C1F3,
C1OF3, C1F5,
C1O2F, C102F3, C1O3F, BrF3, BrF5, 1205, IBr, ICI, IC13, IF, IF3, IF5, IF7, and
a metal second
or third transition series halide such as OsF6, PtF6, or IrF6, an alkali metal
compound such as
a halide, oxide or sulfide, and a compound that can form a metal upon
reduction such as an
alkali, alkaline earth, transition, rare earth, Group 13, preferably In, and
Group 14, preferably
Sn, a metal hydride such as a rare earth hydride, alkaline earth hydride, or
alkali hydride
wherein the catalyst or source of catalyst may be a metal such as an alkali
metal when the
oxidant is a hydride, preferably a metal hydride. Suitable oxidants are metal
halides, sulfides,
oxides, hydroxides, selenides, and phosphides such as alkaline earth halides
such as BaBr2,
49
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
BaC12, BaI2, CaBr2, MgBr2, or Mgl2, a rare earth halide such as EuBr2, EuBr3,
EuF3, LaF3,
GdF3 GdBr3, LaF3, LaBr3i CeBr3, a second or third series transition metal
halide such as YF3,
a metal boride such as CrB2 or TiB2, an alkali halide such as LiCl, RbCl, or
Csl, a metal
sulfide such as Li2S, ZnS, Y2S3, FeS, MnS, Cu2S, CuS, and Sb2S5, a metal
phosphide such as
Ca3P2, a transition metal halide such as CrC13, ZnF2, ZnBr2, ZnI2, MnC12,
MnBr2, Mn12,
CoBr2, CoI2, CoC12, NiBr2, NiF2, FeF2, FeC12, FeBr2, TiF3, CuBr, VF3, and
CuC12, a metal
halide such as SnBr2, Sn12, InF, InCl, InBr, Inl, AgCI, AgI, A1I3, YF3, CdCl2,
CdBr2, CdI2,
InCl3, ZrCl4, NbF5, TaC15, MoC13, MoC15i NbC15, AsC13, TiBr4, SeCl2, SeC14,
InF3, PbF4, and
TeI4, metal oxide or hydroxide such as Y203, FeO, NbO, In(OH)3, As203, Se02,
Te02, BI3,
C02, As2Se3, metal nitride such a Mg3N2, or A1N, metal phosphide such as
Ca3P2, SF6, S,
SbF3, CF4, NF3, KMnO4, NaMnO4, P205, LiNO3, NaNO3, KNO3, and a metal boride
such
asBBr3. Suitable oxidants include at least one of the list of BaBr2, BaC12,
EuBr2, EuF3, YF3,
CrB2, TiB2, LiCl, RbCl, Csl, Li2S, ZnS, Y2S3, Ca3P2, MnI2, CoI2, NiBr2, ZnBr2,
FeBr2, SnI2,
InCI, AgCI, Y203, Te02, C02, SF6, S, CF4, NaMnO4, P205, LiNO3. Suitable
oxidants include
at least one of the list of EuBr2, BaBr2, CrB2, Mn12, and AgCI. Suitable
sulfide oxidants
comprise at least one Li2S, ZnS, and Y2S3. In certain embodiments, the oxide
oxidant is
Y203.
In additional embodiments, each reaction mixture comprises at least one
species
chosen from the following genus of components (i) - (iii) described above, and
further
comprises (iv) at least one reductant chosen from a metal such as an alkali,
alkaline earth,
transition, second and third series transition, and rare earth metals and
aluminum. Preferably
the reductant is one from the group of Al, Mg, MgH2 1 Si, La, B, Zr, and Ti
powders, and
H.
In further embodiments, each reaction mixture comprises at least one species
chosen
from the following genus of components (i) - (iv) described above, and further
comprises (v)
a support, such as a conducting support chosen from AC, 1% Pt or Pd on carbon
(Pt/C, Pd/C),
and a carbide, preferably TiC or WC.
The reactants may be in any molar ratio, but preferably they are in about
equal molar
ratios.
A suitable reaction system comprising (i) a catalyst or a source of catalyst,
(ii) a
source of hydrogen, (iii) an oxidant, (iv) a reductant, and (v) a support
comprises NaH or KH
as the catalyst or source of catalyst and source of H, one of BaBr2, BaC12,
MgBr2, MgI2,
CaBr2, EuBr2, EuF3, YF3, CrB2, TiB2, LiCl, RbCl, CsI, Li2S, ZnS, Y2S3, Ca3P2,
Mn12, CoI2,
NiBr2, ZnBr2, FeBr2, SnI2, InCl, AgCI, Y203, Te02, C02, SF6, S, CF4, NaMnO4,
P205,
LiNO3i as the oxidant, Mg or MgH2 as the reductant wherein MgH2 may also serve
as the
source of H, and AC, TiC, or WC as the support. In the case that a tin halide
is the oxidant,
Sn product may serve as at least one of the reductant and conductive support
in the catalysis
mechanism.
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
In another suitable reaction system comprising (i) a catalyst or a source of
catalyst,
(ii) a source of hydrogen, (iii) an oxidant, and (iv) a support comprises NaH
or KH as the
catalyst or source of catalyst and source of H, one of EuBr2, BaBr2, CrB2,
MnI2, and AgC1 as
the oxidant, and AC, TiC, or WC as the support. The reactants may be in any
molar ratio, but
preferably they are in about equal molar ratios.
The catalyst, the source of hydrogen, the oxidant, the reductant, and the
support may
be in any desired molar ratio. In an embodiment having the reactants, the
catalyst comprising
KH or NaH, the oxidant comprising at least one of CrB2, AgC12, and a metal
halide from the
group of an alkaline earth, transition metal, or rare earth halide, preferably
a bromide or
iodide, such as EuBr2, BaBr2, and Mn12, the reductant comprising Mg or MgH2,
and the
support comprising AC, TiC, or WC, the molar ratios are about the same. Rare
earth halides
may be formed by the direct reaction of the corresponding halogen with the
metal or the
hydrogen halide such as HBr. The dihalide may be formed from the trihalide by
H2
reduction.
Additional oxidants are those that have a high dipole moment or form an
intermediate
with a high dipole moment. Preferably, the species with a high dipole moment
readily
accepts electrons from the catalyst during the catalysis reaction. The species
may have a high
electron affinity. In an embodiment, electron acceptors have a half-filled or
about half-filled
electron shell such as Sn, Mn, and Gd or Eu compounds having half-filled spa,
3d, and 4f
shells, respectively. Representative oxidants of the latter type are metals
corresponding to
LaF3, LaBr3, GdF3, GdC13, GdBr3, EuBr2, EuI2, EuC12, EuF2, EuBr3, EuI3, EuC13,
and EuF3.
In an embodiment, the oxidant comprises a compound of a nonmetal such as at
least one of P,
S, Si, and C that preferably has a high oxidation state and further comprises
atoms with a
high electronegativity such as at least one of F, Cl, or 0. In another
embodiment, the oxidant
comprises a compound of a metal such as at least one of Sn and Fe that has a
low oxidation
state such as II and further comprises atoms with a low electronegativity such
as at least one
of Br or I. A singly-negatively charged ion such as MnO, , C1044, or N03 is
favored over a
doubly-negatively charged one such as C03 or SO4- . In an embodiment, the
oxidant
comprises a compound such as a metal halide corresponding to a metal with a
low melting
point such that it may be melted as a reaction product and removed from the
cell. Suitable
oxidants of low-melting-point metals are halides of In, Ga, Ag, and Sn. The
reactants may be
in any molar ratio, but preferably they are in about equal molar ratios.
In an embodiment, the reaction mixture comprises (i) a catalyst or a source of
catalyst
comprising a metal or a hydride from the Group I elements, (ii) a source of
hydrogen such as
H2 gas or a source of H2 gas, or a hydride, (iii) an oxidant comprising an
atom or ion or a
compound comprising at least one of the elements from Groups 13, 14, 15, 16,
and 17;
preferably chosen from the group of F, Cl, Br, I, B, C, N, 0, Al, Si, P, S,
Se, and Te, (iv) a
reductant comprising an element or hydride, preferably one or more element or
hydride
51
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
chosen Mg, MgH2, Al, Si, B, Zr, and a rare earth metal such as La, and (v) a
support that is
preferably conductive and preferably does not react to form another compound
with other
species of the reaction mixture. Suitable supports preferably comprise carbon
such as AC,
graphene, carbon impregnated with a metal such as Pt or Pd/C, and a carbide,
preferably TiC
or WC.
In an embodiment, the reaction mixture comprises (i) a catalyst or a source of
catalyst
comprising a metal or a hydride from the Group I elements, (ii) a source of
hydrogen such as
H2 gas or a source of H2 gas, or a hydride, (iii) an oxidant comprising a
halide, oxide, or
sulfide compound, preferably a metal halide, oxide, or sulfide, more
preferably a halide of the
elements from Groups IA, IIA, 3d, 4d, 5d, 6d, 7d, 8d, 9d, 10d, 11d, 12d, and
lanthanides, and
most preferably a transition metal halide or lanthanide halide, (iv) a
reductant comprising an
element or hydride, preferably one or more element or hydride chosen from Mg,
MgH2, Al,
Si, B, Zr, and a rare earth metal such as La, and (v) a support that is
preferably conductive
and preferably does not react to form another compound with other species of
the reaction
mixture. Suitable supports preferably comprise carbon such as AC, carbon
impregnated with
a metal such as Pt or Pd/C, and a carbide, preferably TiC or WC.
e. Exchange Reactions, Thermally Reversible Reactions, and Regeneration
In an embodiment, the oxidant and at least one of the reductant, the source of
catalyst,
and the catalyst may undergo a reversible reaction. In an embodiment, the
oxidant is a
halide, preferably a metal halide, more preferably at least one of a
transition metal, tin,
indium, alkali metal, alkaline earth metal, and rare earth halide, most
preferably a rare earth
halide. The reversible reaction is preferably a halide exchange reaction.
Preferably, the
energy of the reaction is low such that the halide may be reversibly exchanged
between the
oxidant and the at least one of the reductant, source of catalyst, and
catalyst at a temperature
between ambient and 3000 C, preferably between ambient and 1000 C. The
reaction
equilibrium may be shifted to drive the hydrino reaction. The shift may be by
a temperature
change or reaction concentration or ratio change. The reaction may be
sustained by addition
of hydrogen. In a representative reaction, the exchange is
n1M0xXx+n2Mcat/red F n1MfX+n2Mcat1redxy (54)
where ni, n2, x, and y are integers, X is a halide, and M0 is the metal of the
oxidant, Mred/cat is
the metal of the at least one of the reductant, source of catalyst, and
catalyst. In an
embodiment, one or more of the reactants is a hydride and the reaction further
involves a
reversible hydride exchange in addition to a halide exchange. The reversible
reaction may be
controlled by controlling the hydrogen pressure in addition to other reaction
conditions such
as the temperature and concentration of reactants. An exemplary reaction is
n 1M0xXx + n2Mcat/red 1 F niMoxH + n2Mcat/redKy. = (55)
52
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
In an embodiment, the oxidant such as an alkali metal halide, alkaline earth
metal
halide, or a rare earth halide, preferably RbCl, BaBr2, BaC12, EuX2 or GdX3
wherein X is
halide or sulfide, most preferably EuBr2, is reacted with the catalyst or
source of catalyst,
preferably NaH or KH, and optionally a reductant, preferably Mg or MgH2, to
form MOX or
MoxH2 and the halide or sulfide of the catalyst such as NaX or KX. The rare
earth halide may
be regenerated by selectively removing the catalyst or source of catalyst and
optionally the
reductant. In an embodiment, MoxH2 may be thermally decomposed and the
hydrogen gas
removed by methods such as pumping. The halide exchange (Eqs. (54-55)) forms
the metal
of the catalyst. The metal may be removed as a molten liquid or as an
evaporated or
sublimed gas leaving the metal halide such as the alkaline earth or rare earth
halide. The
liquid may be removed, for example, by methods such as centrifugation or by a
pressurized
inert gas stream. The catalyst or source of catalyst may be rehydrided where
appropriate to
regenerate the original reactants that are recombined into the originally
mixture with the rare
earth halide and the support. In the case that Mg or MgH2 is used as the
reductant, Mg may
be first removed by forming the hydride with H2 addition, melting the hydride,
and removing
the liquid. In an embodiment wherein X=F, MgF2 product may be converted to
MgH2 by F
exchange with the rare earth such as EuH2 wherein molten MgH2 is continuously
removed.
The reaction may be carried out under high pressure H2 to favor the formation
and selective
removal of MgH2. The reductant may be rehydrided and added to the other
regenerated
reactants to form the original reaction mixture. In another embodiment, the
exchange
reaction is between metal sulfides or oxides of the oxidant and the at least
one of the
reductant, source of catalyst, and catalyst. An exemplary system of each type
is 1.66g KH +
1g Mg + 2.74g Y2S3 + 4g AC and 1g NaH + 1g Mg + 2.26g Y203 + 4g AC.
The selective removal of the catalyst, source of catalyst, or the reductant
may be
continuous wherein the catalyst, source of catalyst, or the reductant may be
recycled at least
partially with in the reactor. The reactor may further comprise a still or
reflux component to
remove the catalyst such as still 34 of FIGURE 4, source of catalyst, or the
reductant and
return it to the cell. Optionally it may be hydrided or further reacted and
this product may be
returned. The reaction temperature may be cycled between two extremes to
continuously
recycle the reactants by an equilibrium shift. In an embodiment, the system
heat exchanger
has the capacity to rapidly change the cell temperature between a high and low
value to shift
the equilibrium back and forth to propagate the hydrino reaction.
The regeneration reaction may comprise a catalytic reaction with an added
species
such as hydrogen. In an embodiment, the source of catalyst and H is KH and the
oxidant is
EuBr2. The thermally driven regeneration reaction may be
2KBr + Eu to EuBr2 + 2K (56)
or
2KBr + EuH2 to EuBr2 + 2KH. (57)
53
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
Alternatively, H2 may serve as a regeneration catalyst of the catalyst or
source of
catalyst and oxidant such as KH and EuBr2, respectively:
3KBr + 1/2H2 + EuH2 to EuBr3 + 3KH. (58)
Then, EuBr2 is formed from EuBr3 by H2 reduction. A possible route is
EuBr3 + 1/21-12 to EuBr2 + HBr. (59)
The HBr may be recycled:
HBr + KH to KBr + H2 (60)
with the net reaction being:
2KBr + EuH2 to EuBr2 + 2KH. (61)
The rate of the thermally driven regeneration reaction can be increased by
using a
different pathway with a lower energy known to those skilled in the art:
2KBr + H2 + Eu to EuBr2 + 2KH (62)
3KBr + 3/2H2 + Eu to EuBr3 + 3KH or (63)
EuBr3 + 1/2H2 to EuBr2 + HBr. (64)
The reaction given by Eq. (62) is possible since an equilibrium exists between
a metal and the
corresponding hydride in the presence of H2 such as
Eu + H2 EuH2. (65)
The reaction pathway may involve intermediate steps of lower energy known to
those skilled
in the art such as
2KBr + Mg + H2 to MgBr2 + 2KH and (66)
MgBr2 + Eu + H2 to EuBr2 + MgH2. (67)
The KH or K metal may be removed as a molten liquid or as an evaporated or
sublimed gas leaving the metal halide such as the alkaline earth or rare earth
halide. The
liquid may be removed by methods such as centrifugation or by a pressurized
inert gas
stream. In other embodiments, another catalyst or catalyst source such as NaH,
LiH, RbH,
CsH, Na, Li, Rb, Cs may substitute for KH or K, and the oxidant may comprise
another metal
halide such as another rare earth halide or an alkaline earth halide,
preferably BaC12 or BaBr2.
In other embodiments, the thermally reversible reaction comprises further
exchange
reactions, preferable between two species each comprising at least one metal
atom. The
exchange may be between a metal of the catalyst such as an alkali metal and
the metal of the
exchange partner such as an oxidant. The exchange may also be between the
oxidant and the
reductant. The exchanged species may be a anion such as a halide, hydride,
oxide, sulfide,
nitride, boride, carbide, silicide, arsenide, selenide, telluride, phosphide,
nitrate, hydrogen
sulfide, carbonate, sulfate, hydrogen sulfate, phosphate, hydrogen phosphate,
dihydrogen
phosphate, perchlorate, chromate, dichromate, cobalt oxide, and other
oxyanions and anions
known to those skilled in the art. The at least one of an exchange-partners
may be comprise
an alkali metal, alkaline earth metal, transition metal, second series
transition metal, third
series transition metal, noble metal, rare earth metal, Al, Ga, In, Sn, As,
Se, and Te. Suitable
54
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
exchanged anions are halide, oxide, sulfide, nitride, phosphide, and boride.
Suitable metals
for exchange are alkali, preferably Na or K, alkaline earth metal, preferably
Mg or Ba, and a
rare earth metal, preferably Eu or Dy, each as the metal or hydride. Exemplary
catalyst
reactants and with an exemplary exchange reaction are given infra. These
reactions are not
meant to be exhaustive and further examples would be known to those skilled in
the art.
= 4g AC3-3 + 1g Mg + 1.66g KH + 2.5g Dy12, Ein:135.0 kJ, dE: 6.1 kJ, TSC:
none,
Tmax: 403 C, theoretical is 1.89 kJ, gain is 3.22 times,
DyBr2 + 2K -+ 2KBr + Dy. (68)
= 4g AC3-3 + 1g Mg + 1g NaH + 2.09g EuF3, Ein:185.1 kJ, dE: 8.0 kJ, TSC: none,
Tmax: 463 C, theoretical is 1.69 U, gain is 4.73 times,
EuF3 + 1.5Mg F 1.5MgF2+ Eu (69)
EuF3 + 3NaH 3NaF+ Eu H2. (70)
= KH 8.3 gm+ Mg 5.0 gm + CAII-300 20.0gm + CrB2 3.7gm, Ein:317 kJ, dE: 19 kJ,
no TSC with Tmax-340 C, theoretical energy is endothermic 0.05 kJ, gain is
infinite,
CrB2 + Mg r MgB2. (71)
= 0.70 g of TiB2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated
carbon powder (AC3-4) was finished. The energy gain was 5.1 kJ, but no cell
temperature
burst was observed. The maximum cell temperature was 431 C, theoretical is 0.
TiB2 + Mg ; MgB2. (72)
= 0.42 g of LiCl, 1.66 g of KH, 1 g of Mg powder and 4 g of AC3-4 was
finished. The
energy gain was 5.4 kJ, but no cell temperature burst was observed. The
maximum cell
temperature was 412 C, theoretical is 0, the gain is infinity.
LiCI + KH - KCl + LiH. (73)
= 1.21 g of RbCl, 1.66 g of KH, 1 g of Mg powder and 4 g of AC3-4, energy gain
was
6.0 kJ, but no cell temperature burst was observed. The maximum cell
temperature was 442
C, theoretical is 0.
RbCI + KH - KCl + RbH. (74)
= 4g AC3-5 + 1g Mg + 1.66g KH + 0.87g LiBr; Ein: 146.0 kJ; dE: 6.24 kJ; TSC:
not
observed; Tmax: 439 C, theoretical is endothermic,
LiBr + KH KBr + LiH(75).
= KH 8.3 gm+ Mg_ 5.0 gm + CAII-300 20.0gm + YF3 7.3 gm; Ein: 320 kJ; dE: 17
kJ;
no TSC with Tmax- 340 C; Energy Gain - 4.5 X (X-0.74kJ * 5=3.7kJ),
YF3 + 1.5Mg + 2KH 1.5MgF2 + YH2 + 2K. (76)
= NaH 5.0 gm+ Mg 5.0 gm + CAII-300 20.0gm + BaBr2 14.85 gm (Dried); Ein: 328
kJ; dE: 16 kJ; no TSC with Tmax- 320 C; Energy Gain 160X (X_0.02kJ*5=0.1 kJ),
BaBr2 + 2NaH F 2NaBr + BaH2. (77)
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + BaC12 10.4 gm; Ein: 331 kJ; dE: 18
kJ No TSC with Tmax- 320 C. Energy Gain - 6.9X (X-0.52x5=2.6 kJ)
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
BaC12 + 2KH - 2KC1 + BaH2. (78)
= NaH 5.0 gm+ Mg 5.0 gm + CAII-300 20.0gm + Mg12 13.9 gm; Ein: 315 kJ; dE: 16
kJ No TSC with Tmax- 340 C. Energy Gain - 1.8X (X-1.75x5=8.75 kJ)
MgI2 + 2NaH + 2NaI + MgH2. (79)
= 4g AC3-2 + 1g Mg + 1g NaH + 0.97g ZnS; Ein:132.1kJ; dE: 7.5kJ; TSC: none;
Tmax: 370 C, theoretical is 1.4 kJ, gain is 5.33 times,
ZnS + 2NaH 2NaHS + Zn (80)
ZnS + Mg MgS + Zn. (81)
= 2.74 g of Y2S3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated
carbon powder (dried at 300 C), energy gain was 5.2 kJ, but no cell
temperature burst was
observed. The maximum cell temperature was 444 C, theoretical is 0.41 kJ,
gain is 12.64
times,
Y2S3 + 3KH 3KHS + 2Y (82)
Y2S3 + 6KH + 3Mg 3K2S + 2Y + 3MgH2 (83)
Y2S3 + 3Mg 3MgS + 2Y. (84)
= 4g AC3-5 + 1g Mg + 1.66g KH + 1.82g Ca3P2; Ein:133.0 kJ; dE: 5.8 kJ; TSC:
none;
Tmax: 407 C, the theoretical is endothermic, the gain is infinity.
= 20g AC3-5 + 5g Mg + 8.3g KH + 9.1g Ca3P2, Ein:282.1kJ, dE:18.lkJ, TSC: none,
Tmax: 320 C, theoretical is endothermic, the gain is infinity.
Ca3P2 + 3Mg + Mg3P2+ 3Ca. (85)
In an embodiment, the thermally regenerative reaction system comprises:
(i) at least one catalyst or a source of catalyst chosen from NaH and KH;
(ii) at least one source of hydrogen chosen from NaH, KH, and MgH2;
(iii) at least one oxidant chosen from an alkaline earth halide such as BaBr2,
BaC12,
BaI2i CaBr2, MgBr2, or MgI2, a rare earth halide such as EuBr2, EuBr3, EuF3,
DyI2, LaF3, or
GdF3, a second or third series transition metal halide such as YF3, a metal
boride such as
CrB2 or TiB2, an alkali halide such as LiCI, RbCI, or CsI, a metal sulfide
such as Li2S, ZnS or
Y2S3i a metal oxide such as Y203, and a metal phosphide such as Ca3P2;
(iv) at least one reductant chosen from Mg and MgH2; and
(v) a support chosen from AC, TiC, and WC.
f. Getter, Support, or Matrix-Assisted Hydrino Reaction
In another embodiment, the exchange reaction is endothermic. In such an
embodiment, the metal compound may serve as at least one of a favorable
support or matrix
for the hydrino reaction or getter for the product to enhance the hydrino
reaction rate.
Exemplary catalyst reactants and with an exemplary support, matrix, or getter
are given infra.
These reactions are not meant to be exhaustive and further examples would be
known to
those skilled in the art.
56
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= 4g AC3-5 + 1g Mg + 1.66g KH + 2.23g Mg3As2, Ein:139.0 kJ, dE: 6.5 kJ, TSC:
none, Tmax: 393 C, the theoretical is endothermic, the gain is infinity.
= 20g AC3-5 + 5g Mg + 8.3g KH + 11.2g Mg3As2,Ein:298.6 kJ, dE:21.8 kJ, TSC:
none, Tmax: 315 C, theoretical is endothermic, the gain is infinity.
= 1.01 g of Mg3N2, 1.66 g of KH, 1 g of Mg powder and 4 g of AC3-4 in a 1"
heavy
duty cell, energy gain was 5.2 kJ, but no cell temperature burst was observed.
The maximum
cell temperature was 401 C, theoretical is 0, the gain is infinity.
= 0.41 g of A1N, 1.66 g of KH, 1 g of Mg powder and 4 g of AC3-5 in a 1" heavy
duty
cell, energy gain was 4.9 kJ, but no cell temperature burst was observed. The
maximum cell
temperature was 407 C, theoretical is endothermic.
In an embodiment, the thermally regenerative reaction system comprises at
least two
components chosen from (i)-(v):
(i) at least one catalyst or a source of catalyst chosen from NaH, KH, and
MgH2;
(ii) at least one source of hydrogen chosen from NaH and KH;
(iii) at least one oxidant, matrix, second support, or getter chosen from a
metal
arsenide such as Mg3As2 and a metal nitride such as Mg3N2 or A1N;
(iv) at least one reductant chosen from Mg and MgH2; and
(v) at least one support chosen from AC, TiC, or WC.
D. Liquid Fuels: Organic and Molten Solvent Systems
Further embodiments comprise a molten solid such as a molten salt or a liquid
solvent
contained in chamber 200. The liquid solvent may be vaporized by operating the
cell at a
temperature above the boiling point of the solvent. The reactants such as the
catalyst may be
dissolved or suspended in the solvent or reactants that form the catalyst and
H may be
suspended or dissolved in the solvent. A vaporized solvent may act as a gas
with the catalyst
to increase the rate of the hydrogen catalyst reaction to form hydrinos. The
molten solid or
vaporized solvent may be maintained by applying heat with heater 230. The
reaction mixture
may further comprise a solid support such as a HSA material. The reaction may
occur at the
surface due to the interaction of a molten solid, a liquid, or a gaseous
solvent with the catalyst
and hydrogen such as K or Li plus H or NaH. In an embodiment using a
heterogeneous
catalyst, a solvent of the mixture may increase the catalyst reaction rate.
In embodiments comprising hydrogen gas, the H2 may be bubbled through the
solution. In another embodiment, the cell is pressurized to increase the
concentration of
dissolved H2. In a further embodiment, the reactants are stirred, preferably
at high speed and
at a temperature that is about the boiling point of the organic solvent and
about the melting
point of the inorganic solvent.
The organic solvent reaction mixture may be heated, preferably in the
temperature
range of about 26 C to 400 C, more preferably in the range of about 100 C to
300 C. The
57
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
inorganic solvent mixture may be heated to a temperature above that at which
the solvent is
liquid and below a temperature that causes total decomposition of the NaH
molecules.
a. Organic Solvents
The organic solvent may comprise one or more of the moieties that can be
modified to
further solvents by addition of functional groups. The moieties may comprise
at least one of
a hydrocarbon such as an alkane, cyclic alkane, alkene, cyclic alkene, alkyne,
aromatic,
heterocyclic, and combinations thereof, ether, halogenated hydrocarbon
(fluoro, chloro,
bromo, iodo hydrocarbon), preferably fluorinated, amine, sulfide, nitrile,
phosphoramide (e.g.
OP(N(CH3)2)3), and aminophosphazene. The groups may comprise at least one of
alkyl,
cycloalkyl, alkoxycarbonyl, cyano, carbamoyl, heterocyclic rings containing C,
0, N, S,
sulfo, sulfamoyl, alkoxysulfonyl, phosphono, hydroxyl, halogen, alkoxy,
alkylthiol, acyloxy,
aryl, alkenyl, aliphatic, acyl, carboxyl, amino, cyanoalkoxy, diazonium,
carboxyalkylcarboxamido, alkenylthio, cyanoalkoxycarbonyl,
carbamoylalkoxycarbonyl,
alkoxy carbonylamino, cyanoalkylamino, alkoxycarbonylalkylamino,
sulfoalkylamino,
alkylsulfamoylaklylamino, oxido, hydroxy alkyl, carboxy alkylcarbonyloxy,
cyanoalkyl,
carboxyalkylthio, arylamino, heteroarylamino, alkoxycarbonyl,
alkylcarbonyloxy,
cyanoalkoxy, alkoxycarbonylalkoxy, carbamoylalkoxy, carbamoylalkyl
carbonyloxy,
sulfoalkoxy, nitro, alkoxyaryl, halogenaryl, amino aryl, alkylaminoaryl,
tolyl, alkenylaryl,
allylaryl, alkenyloxyaryl, allyloxyaryl, cyanoaryl, carbamoylaryl,
carboxyaryl,
alkoxycarbonylaryl, alkylcarbonyoxyaryl, sulfoaryl, alkoxysulfoaryl,
sulfamoylaryl, and
nitroaryl. Preferably, the groups comprise at least one of alkyl, cycloalkyl,
alkoxy, cyano,
heterocyclic rings containing C, 0, N, S, sulfo, phosphono, halogen, alkoxy,
alkylthiol, aryl,
alkenyl, aliphatic, acyl, alkyl amino, alkenylthio, arylamino,
heteroarylamino, halogenaryl,
amino aryl, alkylaminoaryl, alkenylaryl, allylaryl, alkenyloxyaryl,
allyloxyaryl, and
cyanoaryl groups.
The catalyst may be at least one of NaH molecules, Li, and K. In the latter
case, LiH
and KH may serve as the source of catalyst. The solvent may be an organic
solvent. The
solvent may be substantially vaporized at the operating temperature of the
cell that is
preferably above the boiling point of the solvent. Preferably, the solvent is
polar. The
solvent may be an aprotic solvent. Polar aprotic solvents are solvents that
share ion dissolving
power with protic solvents such as water, methanol, ethanol, formic acid,
hydrogen fluoride
and ammonia, but lack an acidic hydrogen. These solvents generally have high
dielectric
constants and high polarity. Examples are dimethyl sulfoxide,
dimethylformamide, 1,4-
dioxane, and hexamethylphosphoramide.
In one embodiment of the present disclosure, the solvent comprises an ether
such as at
least one of the group of 1,4-dioxane, 1,3-dioxane, trioxane,
acetylacetaldehyde dimethyl
acetal, 1,4-benzodioxane, 3-dimethylaminoanisole, 2,2-dimethyl-1,3-dioxolane,
1,2-
58
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
dimethoxyethane, N-N-dimethylformamide dimethyl acetal, N-N-dimethylformamide
ethylene acetal, diethyl ether, diisopropyl ether, methylal
(dimethoxymethane),
tetrahydropyran dibenzodioxane, n-butyl ethyl ether, di-n-butyl ether, allyl
ethyl ether,
diethylene glycol dibutyl ether, bis(2-ethylhexyl) ether, sec-butyl ethyl
ether, dicyclohexyl
ether, diethylene glycol diethyl ether, 3,4-dihydro-lH-2-benzopyran, 2,2'-
dimethoxybiphenyl, 1,6-dimethoxyhexane, substituted aromatic ethers such as
methoxy
benzene, methoxy toluene, 2,5-dimethoxytoluene, diphenoxybenzene such as 1,4-
diphenoxybenzene, allyl phenyl ether, dibenzyl ether, benzyl phenyl ether, n-
butyl phenyl
ether, trimethoxytoluene such as 3,4,5-trimethoxytoluene, 2,2'-dinaphthyl
ether, 2-[2-
(benzyloxy)ethyl]-5,5-dimethyl-1,3-dioxane, 1,3-benzodioxole, veratrole (1,2-
dimethoxybenzene), anisole, bis(phenyl) ether, 1,4-dioxin, dibenzodioxin or
dibenzo[1,4]dioxin, divinyl ether, crown ethers such as dicyclohexano-18-crown-
6, dibenzo-
18-crown-6, 15-crown-5, and 18-crown-6, bis(4-methylphenyl) ether, bis(2-
cyanoethyl)
ether, bis(2-dimethylaminoethyl)ether, and bis[2-(vinyloxy)ethyl] ether. In an
embodiment
comprising Na and a source of hydrogen, an ether is an exemplary solvent since
Na is
somewhat soluble in ether, and also stabilizes sodium ions. These features
favor the hydrino
reaction. Additionally to NaH, K or Li may serve as the catalyst of a reaction
mixture further
comprising an ether solvent.
In an embodiment, the solvent or HSA material comprises functional groups with
a
high bond moment such as C-O, C=O, C = N and C-F. The molecules of the solvent
or HSA
material may have a high dipole moment. Preferably, the solvent or HSA
comprises at least
one of an ether, nitrile, or halogenated hydrocarbons, preferably having very
stable bonds,
preferably polar, such as fluorinated hydrocarbons. Preferably, the
fluorocarbon solvent has
the formula CF2,,+2 and may also have some H in place of F or may be aromatic.
In another
embodiment, the solvent or HSA comprises as at least one of the group of
fluorinated organic
molecules, fluorinated hydrocarbons, fluorinated alkoxy compounds, and
fluorinated ethers.
Exemplary fluorinated solvents are 1,2-dimethoxy-4-fluorobenzene,
hexaflorobenzene,
perfluoroheptane, octafluoronaphthalene, octafluorotoluene, 2H-perfluoro-
5,8,11,14-
tetramethyl-3,6,9,12,15-pentaoxaoctadecane, perfluoro-5,8,11,14-tetramethyl-
3,6,9,12,15-
pentaoxaoctadecane, perfluoro(tetradecahydrophenanthrene), and perfluoro-1,3,5-
trimethylcyclohexane. Exemplary fluorinated HSAs are TEFLONTM, TEFLONTM-PFA,
polyvinyl fluoride, PVF, poly(vinylidene fluoride), poly(vinylidene fluoride-
co-
hexafluoropropylene), and perfluoroalkoxy polymers. A suitable reaction
mixture comprises
octafluoronaphthalene, NaH, and and a support such as Ac, TiC, WC, or R-Ni.
The reactants
can be in any desired proportions such as octafluoronaphthalene (45 wt%), NaH
(10 wt%),
and R-Ni (45 wt%).
Another exemplary solvent is a fluorocarbon such as one having the formula
C,FZõ+2 ,
and it may also have some H in place of F or may be aromatic. In an
embodiment, the
59
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
fluorinated solvent comprises at least one of the group and derivatives of
perfluoro-methane,
perfluoro-ethane, perfluoro-propane, perfluoro-heptane, perfluoro-pentane,
perfluoro-hexane,
and perfluoro-cyclohexane as well as other straight and branched chain
perfluoro-alkanes and
partially F substituted alkanes, bis(difluoromethyl) ether, 1,3-
bis(trifluoromethyl)benzene,
1,4-bis(trifluoromethyl)benzene, 2,2',3,3',4,4',5,5',6,6'-decafluoro-1,1'-
biphenyl, o-
difluorobenzene, m-difluorobenzene, p-difluorobenzene, 4,4'-difluoro-1,1'-
biphenyl, 1,1-
difluorocyclohexane, 1,1-difluoroethane, 1,2-difluoroethane, 1,1-
difluorethene, cis-1,2-
difluoroethene, trans-l,2-difluoroethene, difluoromethane, 2-(difluoromethoxy)-
1,1,1-
trifluoroethane, 2,2-difluoropropane, fluorobenzene, 2-fluoro-1,1'-biohenyl, 4-
fluoro-1,1'-
biohenyl, 1-fluorobutane, 2-fluorobutane, fluorocyclohexane, 1-
fluorocyclohexene, 1-
fluorodecane, fluoroethane, fluoroethene, 1-fluoroheptane, 1-fluorohexane,
fluoromethane, 1-
fluoro-2-methoxybenzene, 1-fluoro-3-methoxybenzene, 1-fluoro-4-methoxybenzene,
(fluoromethyl)benzene, 2-fluoro-2-methylpropane, 1-fluoronaphthalene, 2-
fluoronaphthalene,
1-fluorooctane, 1-fluoropentane, 1-fluoropropane, 2-fluoropropane, cis-l-
fluoropropene,
trans- l-fluoropropene, 2-fluoropropene, 3-fluoropropene, 2-fluoropyridine, 3-
fluoropyridine,
2-fluorotoluene, 3-fluorotoluene, 4-fluorotoluene, 1-fluoro-2-
(trifluoromethyl)benzene, 1-
fluoro-3-(trifluoromethyl)benzene, 1-fluoro-4-(trifluoromethyl)benzene,
1,1,1,2,3,3,3-
heptafluoropropane, hexafluorobenzene, 1,1,2,3,4,4-hexafluoro-1,3-butadiene,
1,1,1,4,4,4-
hexafluoro-2-butyne, hexafluorocyclobutene, hexafluoroethane, 1,1,1,2,3,3-
hexafluoropropane, methyl pentafluorethyl ether, pentafluorobenzene,
pentafluoroethane,
pentafluoromethoxybenzene, 1, 1, 1,2,2-pentafluoropropane, 2,3,4,5,6-
pentafluorotoluene,
1,1,2,4,4-pentafluoro-3-(trifluoromethyl)-1,3-butadiene, perfluorobutane,
perfluoro-2-butene,
perfluoro-2-butyltetrahydrofuran, perfluorocyclobutane, perfluorocyclohexane,
perfluorocyclohexene, perfluorodecalin, perfluorodecane,
perfluorodimethoxymethane,
perfluoro-2,3-dimethylbutane, perfluoroethyl ethyl ether, perfluoroethyl2,2,2-
trifluoroethyl
ether, perfluoroheptane, perfluoro-1-heptane, perfluorohexane, perfluoro-l-
hexene,
perfluoroisobutane, perfluoroisobutene, perfluoroisopropyl methyl ether,
perfluoromethylcyclohexane, perfluoro-2-methylpentane, perfluoro-3-
methylpentane,
perfluoronaphthalene, perfluorononane, perfluorooctane, perfluorooctylsulfonyl
fluoride,
perfluorooxetane, perfluoropentane, perfluoropropane, perfluoropropene,
perfluoropropyl
methyl ether, perfluoropyridine, perfluorotoluene, perfluorotripropylamine,
1,1,1,2-
tetrafluoroethane, 1,1,2,2-tetrafluoroethane, 1,2,2,2-tetrafluoroethyl
difluoromethyl ether,
tetrafluoromethane, triflumizole, trifluoperazine, 1,2,4-trifluorobenzene,
1,3,5-
trifluorobenzene, 1, 1, 1 -trifluoroethane, 1,1,2-trifluoroethane,
trifluoroethene, 2,2,2-
trifluoroethyl methyl ether, trifluoromethane, trifluoromethyl difluoromethyl
ether,
trifluoromethyl 1,1,2,2-tetrafluoroethyl ether, 1,1,1-trifluoropropane, 3,3,3-
trifluoropropene,
3,3,3-trifluoro-l-propyne, triflupromazine, undecafluorocyclohexane,
pentafluorbenzonitrile,
trifluoroacetonitrile, (trifluoromethyl)benzene, 3-
(trifluoromethyl)benzonitrile, 4-
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
(trifluoromethyl)benzonitrile, trifluoro(trifluoromethyl)loxirane, and
tris(perfluorobutyl)lamine.
In another embodiment, the solvent comprises a hydrocarbon such as those
having
functional groups for the list of straight and branched-chain alkanes,
alkenes, alkynes, and
aromatics. The hydrocarbon solvent may be at least one of or derivatives of
the group
comprising acenaphthene, acenaphthylene, allylbenzene, 1-allylcyclohexene,
allylcyclopentane, anthracene, benz[a]anthracene, benzene, benzo[g]chrysene,
benzo[g]chrysene, benzo[b]fluoranthene, benzo[j]fluoranthene,
benzo[k]fluoranthene, 11H-
benzo[a] fluorine, 11H- benzo[b] fluorine, benzo[ghi]perylene,
benzo[c]phenanthrene,
benzo[a]pyrene, benzo[e]pyrene, benzo[b]triphenylene, 9,9'-bianthracene,
bicyclo[2.2.1]heptane, bicyclo[4.1.0]heptane, bicyclo[2,2,1]hept-2-ene, 1,1'-
bicyclopentyl,
1,1'-binaphthalene, 2,2'-binaphthalene, biphenyl, 1,3-bis(1-
methylethenyl)benzene, (trans)-
1,3-butadienylbenzene, 1,3-butadiyne, butane, 1-butene, cis-2-butene, trans-2-
butene, (trans-
1-butenyl)benzene, 2-butenylbenzene, 3-butenylbenzene, 1-buten-3-yne,
butylbenzene, sec-
butylbenzene, ( ), tert-butylbenzene, 2-butyl-1,1'-biphenyl, butylcyclohexane,
sec-
butylcyclohexane, tert-butylcyclohexane, butylcyclopentane, 1-tert-butyl-3,5-
dimethylbenzene, 5-butyldocosane, 11-butyldocosane, 1-tert-butyl-4-
ethylbenzene, 1-tert-
butyl-2-methylbenzene, 1-tert-butyl-3-methylbenzene, 1-tert-butyl-4-
methylbenzene, 1-
butylnaphthalene, 2-butylnaphthalene, 5-butylnonane, camphene, (+), camphene,
(-), 3-
carene, (+), a-carotene, (3-carotene, (3,`I'-carotene, `P,`I'-carotene, `P,`I'-
caroten-16-ol,
cholestane, (5a), cholestane, (5(3), cyclobutane, cyclobutene, cyclodecane,
cyclododecane,
1,5,9-cyclododecatriene, cis-cyclododecene, trans-cyclododecene, 1,3-
cycloheptadiene,
cycloheptane, 1,3,5-cycloheptatriene, cycloheptene, 1,3-cyclohexadiene, 1,4-
cyclohexadiene,
cyclohexane, cyclohexene, 1-cyclohexen-1-ylbenzene, cylohexylbenzene,
cyclohexylcyclohexane, cyclononane, 1,4-cyclooctadiene, cis, cis- 1,5-
cyclooctadiene,
cycloocatane, 1,3,5,7-cyclooctatetraene, 1,3,5-cyclooctatriene, cis-
cyclooctene, trans-
cyclooctene, cyclooctyne, cyclopentadecane, 1,3-cyclopentadiene, cyclopentane,
cyclopentene, cyclopentylbenzene, 1,3-decadiene, 1,9-decadiene, cis-
decahydronaphthalene,
trans-decahydronaphthalene, decane, 1-decene, cis-2-decene, trans-2-decene,
cis-5-decene,
trans-5-decene, decylbenzene, decylcyclohexane, decylcyclopentane, 11-
decylheneicosane,
1-decylnaphthalene, 1-decyne, 5-decyne, dibenz[a,h]anthracene,
dibenz[a,j]anthracene,
dibenzo[b,k]chrysene, dibenzo[a,e]pyrene, dibenzo[a,h]pyrene,
dibenzo[a,i]pyrene,
dibenzo[a,l]pyrene, o-diethylbenzene, m-diethylbenzene, p-diethylbenzene, 1,1-
diethylcyclohexane, 1,2-dihydrobenz[j]aceanthrylene, 9,10-dihydro-9,10[1',2']-
benzenoanthracene, 16,17-dihydro-15H-cyclopenta[a]phenanthrene, 2,3-dihydro-l-
methyl-
1H-indene, 1,2-dihydronaphthalene, 1,4-dihydronaphthalene, 9,10-
dihydrophenanthrene, 2,3-
dihydro-1,1,3-trimethyl-3-phenyl-1H-indene, 1,2-diisopropylbenzene, 1,3-
diisopropylbenzene, 1,4-diisopropylbenzene, 2,6-diisopropylnaphthalene, 7,12-
61
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
dimethylbenz[a]anthracene, 2,2'-dimethylbiphenyl, 2,3-dimethyl- 1,3-butadiene,
2,2-
dimethylbutane, 2,3-dimethylbutane, 2,3-dimethyl-l-butene, 3,3-dime thyl-1-
butene, 2,3-
dimethyl-2-butene, 3,3-dimethyl-l-butyne, 1,1-dimethylcyclohexane, cis-1,3-
dimethylcyclohexane, trans-l,3-dimethylcyclohexane, cis- 1,4-
dimethylcyclohexane, trans-
1,4-dimethylcyclohexane, 1,2-dimethylcyclohexene, 1,3-dimethylcyclohexene, 1,1-
dimethylcyclopentane, cis- 1,2-dimethylcyclopentane, trans-l,2-
dimethylcyclopentane, cis-
1,3-dimethylcyclopentane, trans- l,3-dimethylcyclopentane, 1,2-
dimethylcyclopentene, 1,5-
dimethylecyclopentene, 1,2-dimethylenecyclohexane, 2,6-dimethyl-1,5-
heptadiene, 2,2-
dimethylheptane, 2,3-dimethylheptane, 2,4-dimethylheptane, 2,5-
dimethylheptane, 2,6-
dimethylheptane, 3,3-dimethylheptane, 3,4-dimethylheptane, 3,5-
dimethylheptane, 4,4-
dimethylheptane, 2,5-dimethyl-1,5-hexadiene, 2,5-dimethyl-2,4-hexadiene, 2,2-
dimethylhexane, 2,3-dimethylhexane, 2,4-dimethylhexane, 2,5-dimethylhexane,
3,3-
dimethylhexane, 3,4-dimethylhexane, 2,3-dimethyl-l-hexene, 5,5-dimethyl-l-
hexene, 2,3-
dimethyl-2-hexene, 2,5-dimethyl-2-hexene, cis-2,2-dimethyl-3-hexene, trans-2,2-
dime thyl-3-
hexene, 1-(1,5-dimethyl-4-hexenyl)-4-methylbenzene, 1,1-dimethylindan, 1,4-
dimethyl-7-
isopropylazulene, 1,6-dimethyl-4-isopropylnaphthalene, 2,4-dimethyl-3-
isopropylpentane,
1,2-dimethylnaphthalene, 1,3-dimethylnaphthalene, 1,4-dimethylnaphthalene, 1,5-
dimethylnaphthalene, 1,6-dimethylnaphthalene, 1,7-dimethylnaphthalene, 1,8-
dimethylnaphthalene, 2,3-dimethylnaphthalene, 2,6-dimethylnaphthalene, 2,7-
dimethylnaphthalene, 3,7-dimethyl-1,6-octadiene, 2,2-dimethyloctane, 2,3-
dimethyloctane,
2,4-dimethyloctane, 2,5-dimethyloctane, 2,6-dimethyloctane, 2,7-
dimethyloctane, 3,4-
dimethyloctane, 3,6-dimethyloctane, cis-3,7-dimethyl-1,3,6-octatriene, trans-
3,7-dimethyl-
1,3,6-octariene, 3,7-dimethyl-1,3,7-octariene, cis, cisi-2,6-dimethyl-2,4,6-
octariene,
trans, trans-2,6-dimethyl,2,4,6-octariene, 3,7-dimethyl-l-octene, dimethyl-1,3-
pentad iene,
2,2-dimethylpentane, 2,3-dimethylpentane, 2,4-dimethylpentane, 3,3-
dimethylpentane, 2,3-
dimethyl-1-pentene, 2,4-dimethyl-l-pentene, 3,3-dimethyl-l-pentene, 3,4-
dimethyl-l-
pentene, 2,3-dimethyl-2-pentene, 2,4-dimethyl-2-pentene, cis-3,4-dimethyl-2-
pentene, cis-
3,4-dimethyl-2-pentene, trans-3,4-dimethyl-2-pentene, cis-4,4-dimethyl-2-
pentene, trans-4,4-
dimethyl-2-pentene, 4,4-dimethyl-l-pentyne, 4,4-dimethyl-2-pentyne,(1,1-
dimethylpropyl)benzene, (2,2-dimethylpropyl)benzene, 2,7-dimethylpyrene, 9,10-
diphenylanthracene, trans,trans-1,4-diphenyl-1,3-butadiene, 1,4-diphenyl-1,3-
butadiyne, 1,1-
diphenylbutane, 1,2-diphenylbutane, 1,4-diphenylbutane, 1,3-diphenyl-l-butene,
1,1-
diphenylethane, 1,2-diphenylethane, 1,1-diphenylethene, 1,6-diphenyl-1,3,5-
hexatriene,
diphenylmethane, 1,3-diphenylpropane, 2,2-diphenylpropane, 1,1-diphenyl-l-
propene, 1,2-
di(p-tolyl)ethane, o-divinylbenzene, m-divinylbenzene, p-divinylbenzene,
docosane, 1-
docosene, 5,7-dodecadiyne, dodecane, dodecylcyclohexane, 1-dodecyne, 6-
dodecyne,
dotriacontane, eicosane, ergostane, (5a), ergostane, (5[3), ethane,
ethylbenzene,
ethylcyclohexane, 1-ethylcyclohexene, ethylcyclopentane, 1-ethylcyclopentene,
1-ethyl-2,4-
62
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
dimethylbenzene, 1 -ethyl -3,5 -dimethylbenzene, 2-ethyl-1,3-dimethylbenzene,
3-ethyl-1,2-
dimethylbenzene, 4-ethyl-1,2-dimethylbenzene, 3-ethyl-2,2-dimethylpentane, 3-
ethyl-2,3-
dimethylpentane, 3-ethylheptane, 4-ethylheptane, 3-ethylhexane,
ethylidenecyclohexane, 1-
ethyl-2-isopropylbenzene, 2-ethyl-3-methyl-l-butene, trans-l-ethyl-4-
methylcyclohexane, 1-
ethyl-1 -methylcyclopentane, cis- 1 -ethyl-2-methylcyclopentane, trans- l-
ethyl -2-
methylcyclopentane, cis-l-ethyl-3-methylcyclopentane, trans- l-ethyl -3-
methylcyclopentane,
3-ethyl-4-methylhexane, 4-ethyl-2methylhexane, 3-ethyl-2-methylpentane, 3-
ethyl-3-
methylpentane, 3-ethyl-2-methyl-l-pentene, 1-ethylnaphthalene, 2-
ethylnaphthalene, 3-
ethyloctane, 4-ethyloctane, 3-ethylpentane, 2-ethyl-l-pentene, 3-ethyl-l-
pentene, 3-ethyl-l-
pentene, 3-ethyl-2-pentene, 2-ethylstyrene, 3-ethylstyrene, 4-ethylstyrene, 2-
ethyltoluene, 3-
ethyltoluene, 4-ethyltoluene, 1-ethyl-2,4,5-trimethylbenzene, 2-ethyl-1,3,5-
trimethylbenzene,
Fluoranthene, Fulvene, heneicosane, hentriacontane, heptacosane, heptadecane,
1-
heptadecene, heptadecylbenzene, 1,6-heptadiene, 1,6-heptadiyne, 2,2,4,4,6,8,8-
heptamethylnonane, heptane, 1-heptene, cis-2-heptene, trans-2-heptene, cis-3-
heptene, trans-
3-heptene, heptylcyclohexane, heptylcyclopentane, 1-heptyne, 2-heptyne, 3-
heptyne,
hexacene, hexacosane, hexadecane, 1-hexadecene, hexadecylbenzene, 1-
hexadecyne, cis-1,3-
hexadiene, trans-l,3-hexadiene, cis-1,4-hexadiene, trans-l,4-hexadiene, 1,5-
hexadiene,
cis, cis-2,4-hexadiene, trans, cis-2,4-hexadiene, trans, trans-2,4-hexadiene,
1,5-hexadien-3-
yne, 1,5-hexadiyne, 2,4-hexadiyne, hexaethylbenzene, cis- 1,2,3,5,6,8a-
hexahydro-4,7-
dimethyl-l-isopropylnaphthalene, (is), hexamethylbenzene, 2,6,10,15,19,23-
hexamethyltetracosane, hexane, hexatriacontane, cis-1,3,5-hexatriene, trans-
1,3,5-hexatriene,
1-hexene, cis-2-hexene, trans-2-hexene, cis-3-hexene, trans-3-hexene,
hexylbenzene,
hexylcyclohexane, hexylcyclopentane, 1-hexylnapthalene, 1-hexyl-1,2,3,4-
tetrahydronaphthalene, 1-hexyne, 2-hexyne, 3-hexyne, indan, indeno[1,2,3-
cd]pyrene,
isobutane, isobutene, isobutylbenzene, isobutylcyclohexane,
isobutylcyclopentane,
isopentane, isopentylbenzene, isopropenylbenzene, p-
isopropenylizopropylbenzene, p-
isopropenylstyrene, isopropylcyclohexane, 4-isopropylheptane, 1-isopropyl-2-
methylbenzene, 1-isopropyl-3-methylbenzene, 1-isopropyl-4-methylbenzene, 5-
isopropyl-2-
methyl-1,3-cyclohexadiene, (R), 1-isopropylnaphthalene, 2-
isopropylnaphthalene, d-
limonene, I-limonene, [2,2]metacyclophane, 1-methylanthracene, 2-
methylanthracene, 9-
methylanthracene, 7-methylbenz[a]anthracene, 8-methylbenz[a]anthracene, 9-
methylbenz[a]anthracene, 10-methylbenz[a]anthracene, 12-
methylbenz[a]anthracene, 1-
methyl-2-benzylbenzene, 1-methyl-4-benzylbenzene, 2-methylbiphenyl, 3-
methylbiphenyl,
4-methylbiphenyl, 3-methyl-1,2-butadiene, 2-methyl-1,3-butadiene, 2-methyl-l-
butene, 3-
methyl-l-butene, 2-methyl-2-butene, 2-methyl-l-buten-3-yne, 3-methyl-l-butyne,
3-
methylchrysene, 5-methylchrysene, 6-methylchrysene, 2-methyl-1,3-
cyclohexadiene, 1-
methylcyclohexene, 3-methylcyclohexene, ( ), 4-methylcyclohexene, 1-methyl-1,3-
cyclopentadiene, methylcyclopentane, 1-methylcyclopentene, 3-
methylcyclopentene, 4-
63
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
methylcyclopentene, 2-methyldecane, 3-methyldecane, 4-methyldecane, 4-methyl-
2,4-
diphenyl-1-pentene, methylenecyclohexane, 3-methyleneheptane, 4-methylene-1-
isopropylcyclohexene, 5-(1-methylethylidene)-1,3-cyclopentadiene, 1-methyl-9H-
fluorene,
9-methyl-9H-fluorene, 2-methylheptane, 3-methylheptane, 4-methylheptane, 2-
methyl-l-
heptene, 6-methyl-l-heptene, 2-methyl-2-heptene, cis-3-methyl-2-heptene, 2-
methylhexane,
3-methylhexane, 2-methyl-l-hexene, 3-methyl-l-hexene, 4-methyl-l-hexene, 5-
methyl-l-
hexene, 2-methyl-2-hexene, cis-3-methyl-2-hexene, cis-4-methyl-2-hexene, trans-
4-methyl-
2-hexene, cis-5-methyl-2-hexene, trans-5-methyl-2-hexene, cis-2-methyl-3-
hexene, trans-2-
methyl-3-hexene, cis-3-methyl-3-hexene, trans-3-methyl-3-hexene, 5-methyl-l-
hexyne, 5-
methyl-2-hexyne, 2-methyl-3-hexyne, cis- l-methyl-4-isopropylcyclohexane,
trans-l-methyl-
4-isopropylcyclohexane, 1-methyl-4-isopropylcyclohexene, 1-methyl-7-
isopropylphenanthrene, 3-methyl-4-methylenehexane, 1-methyl-4-(5-methyl-l-
methylene-4-
hexenyl)cyclohexene, (S), 1-methyl-4-(1-methylvinyl)benzene, 1-
methylnaphthalene, 2-
methylnaphthalene, 2-methylnonane, 3-methylnonane, 4-methylnonane, 5-
methylnonane, 2-
methyl-l-nonene, 2-methyl-2-norbornene, 2-methyloctane, 3-methylocatane, 4-
methyloctane,
2-methyl-l-octene, 7-methyl-l-octene, cis-2-methyl-1,3-pentadiene, 3-methyl-
1,3-
pentadiene, 4-methyl-1,3-pentadiene, 2-methylpentane, 3-methylpentane, 2-
methyl-1-
pentene, 3-methyl-l-pentene, 4-methyl-l-pentene, 2-methyl-2-pentene, 3-methyl-
cis-2-
pentene, 3-methyl-trans-2-pentene, 4-methyl-cis-2-pentene, 4-methyl-trans-2-
pentene, 3-
methyl-3-penten-1-yne, 4-methyl-l-pentyne, 4-methyl-2-pentyne, 1-
methylphenanthrene, 3-
methylphenanthrene, 4-methylphenanthrene, 2-methyl-l-propene, tetramer, cis-(1-
methyl-l-
propenyl)benzene, trans-(1-methyl-l-propenyl)benzene, 1 -methyl-2-
propylbenzene, 1-
methyl-3-propylbenzene, 1-methylpyrene, 2-methylpyrene, 2-methylstyrene, 3-
methylstyrene, 4-methylstyrene, 2-methylundecane, 3-methylundecane, 1-methyl-4-
vinylcyclohexane, (3-myrcene, naphthacene, naphthalene, nonadecane, 1,8-
nonadiene, 1,8-
nonadiyne, nonane, 1-nonene, nonylbenzene, nonylcyclohexane,
nonylcyclopentane, 1-
nonylnaphthalene, 1-nonyne, octacosane, octadecahydrochrysene, octadecane, 1-
octadecene,
octadecylbenzene, octadecylcyclohexane, 1,7-octadiene, 1,7-octadiyne,
1,2,3,4,5,6,7,8-
octahydroanthracene, octahydroindene, 1,2,3,4,5,6,7,8-octahydrophenanthrene,
octane,
1,3,5,7-octatetraene, 1-octene, cis-2-octene, cis-3-octene, trans-3-octene,
cis-4-octene, trans-
4-octene, 1-octen-3-yne, octylbenzene, octylcyclohexane, octylcyclopentane, 1-
octyne, 2-
octyne, 3-octyne, 4-octyne, 1,3-pentadiyne, pentaethylbenzene,
pentamethylbenzene,
2,2,4,6,6-pentamethylheptane, 2,2,4,6,6-pentamethyl-3-heptene, 2,2,3,3,4-
pentamethylpentane, 2,2,3,4,4-pentamethylpentane, pentane, pentaphene,
pentatriacontane, 1-
pentene, cis-2-pentene, trans-2-pentene, 1-penten-3-yne, 1-penten-4-yne, cis-3-
penten-1-yne,
trans-3-penten-1-yne, pentylbenzene, pentylcyclohexane, pentylcyclopentane, 1-
pentylnaphthalene, 1-pentyne, 2-pentyne, perylene, a-phellandrene, (3-
phellandrene,
phenanthrene, phenylacetylene, 9-phenylanthracene, 2-phenyl-1,3-butadiene, 2-
phenyl-1-
64
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
butene, 1-phenyl-lH-indene, 1 -phenylnaphthalene, 2-phenylnaphthalene, 5'-
phenyl-
1,1':3',1"-terphenyl, picene, propane, propene, cis- 1 -propenylbenzene, trans-
l-
propenylbenzene, propylbenzene, propylcyclohexane, propylcyclopentane, 4-
propylheptane,
1-propylnaphthalene, pyrene, 1,1':4',1":4",1-quaterphenyl, spiro[5.5]undecane,
squalene,
cis-stilbene, trans-stilbene, styrene, o-terphenyl, m-terphenyl, p-terphenyl,
a-terpinene, y-
terpinene, tetracosane, tetradecahydrophenanthrene, tetradecane,
tetradecylbenzene,
tetradecylcyclohexane, 1,2,3,5-tetraethylbenzene, 1,2,3,4-tetrahydro-1,5-
dimethylnaphthalene, 1,2,3,4-tetrahydro-l-methylnaphthalene, 1,2,3,4-
tetrahydro5-
methylnaphthalene, 1,2,3,4-tetrahydro-6-methylnaphthalene, 1,2,3,4-
tetrahydronaphthalene,
1,2,3,4-tetrahydrophenanthrene, 1,2,3,4-tetrahydro-1,1,6-trimethylnaphthalene,
1,2,3,4-
tetramethylbenzene, 1,2,3,5-tetramethylbenzene, 1,2,4,5-tetramethylbenzene,
2,2,3,3-
tetramethylbutane, 1,2,3,4-tetramethylcyclohexane, 1,1,3,3-
tetramethylcyclopentane, 1,1,2,2-
tetramethylcyclopropane, 2,2,3,3-tetramethylhexane, 2,2,5,5-tetramethylhexane,
3,3,4,4-
tetramethylhexane, 2,2,3,3-tetramethylpentane, 2,2,3,4-tetramethylpentane,
2,2,4,4-
tetramethylpentane, 2,3,3,4-tetramethylpentane, 1,1,4,4-tetraphenyl-1,3-
butadiene, 1,1,2,2-
tetraphenylethane, 1,1,2,2-tetraphenylethene, tetraphenylmethane, 5,6,11,12-
tetraphenylnaphthacene, triacontane, tricosane, tricyclo[3.3.13'7]decane,
tridecane, 1-
tridecene, tridecylbenzene, tridecylcyclohexane, 1-tridecyne, 1,2,3-
triethylbenzene, 1,2,4-
triethylbenzene, 1,3,5-triethylbenzene, 1,2,4-triisopropylbenzene, 1,3,5-
triisopropylbenzene,
1,2,3-trimethylbenzene, 1,2,4-trimethylbenzene, 1,3,5-trimethylbenzene, 1,7,7-
trimethylbicyclo[2.2. 1]heptane, 1,7,7-trimethylbicyclo[2.2.1]hept-2-ene,
2,2,3-
trimethylbutane, 2,3,3-trimethyl-1-butene, 1,1,2-trimethylcyclohexane, 1,1,3-
trimethylcyclopentane, 1a,2a,4(3-1,2,4-trimethylcyclopentane, 2,2,6-
trimethylheptane, 2,5,5-
trimethylheptane, 3,3,5-trimethylheptane, 3,4,5-trimethylheptane, 2,2,3-
trimethylhexane,
2,2,4-trimethylhexane, 2,3,3-trimethylpentane, 2,3,4-trimethylpentane, 2,3,3-
trimethyl-l-
pentene, 2,4,4-trimethyl-l-pentene, 2,3,4-trimethyl-2-pentene, 1,1,2-
triphenylethane, 1,1,2-
triphenylethene, triphenylmethane, tritriacontane, 1,10-undecadiyne,
undecane,1-undecane,
cis-2-undecane, trans-2-undecane, cis-4-undecane, trans-4-undecane, cis-5-
undecane, trans-
5-undecane, undecylbenzene, 1-undecyne, 2-undecyne, vinylcyclohexane, 1-
vinylcyclohexane, 4-vinylcyclohexane, vinylcyclopentane, 6-vinyl-6-methyl-l-
isopropyl-3-1-
(1-methylethylidene)cyclohexene, (S), 1-vinylnaphthalene, 2-vinylnaphthalene,
2-vinyl-5-
norbornene, o-xylene, m-xylene, and p-xylene.
In another embodiment, the solvent comprises as at least one of the group of
amines
such as tributylamine, triethyamine, triisopropylamine, N,N-dimethylaniline,
tris(N,N-
dimethylaniline), allyldiethylamine, allyldimethylamine, benzo[f]quinoline,
bis[4-
(dimethylamino)phenyl] methane, 4,4bis-(dimethylamino)triphenylmethane,
butyldimethylamine, hydrocarbons solvents such as alkanes, alkenes, and
alkynes such as
pentane, hexane, heptane, octane, cyclopentane, cyclohexane, dipentene,
methylcyclohexane,
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
2-methylpentane, octane, tetrahydrofuran (THF), pinene, styrene,terpinene, and
mineral oil,
aromatics and heterocyclic aromatic such as toluene, o-xylene, m-xylene, p-
xylene,
ethylbenzene, cumene (isopropylbenzene), p-cymene (1-methyl-4-
isopropylbenzene),
mesitylene (1,3,5-trimethylbenzene), propylbenzene, pseudocumene (1,2,4-
trimethylbenzene), naphthalene, decalin (cis and trans decahydronaphthalene),
tetralin
(1,2,3,4-tetrahydronaphthalene), pyrrole, furan, 2,5-diphenylfuran, thiophene,
imidazole,
pyridine, pyrimidine, pyrazine, quinoline, isoquinoline, indole, acridine, 1,2-
dimethylindole,
9,9'-dixanthylidene, 2,6-lutidine (2,6-dimethylpyridine), 2-picoline (2-
methylpyridine), and
nitriles such as acetonitrile and propanenitrile. In an embodiment, the amino
group is bound
to aryl. Suitable amino solvents are N,N-dimethylanline analogs such as N-
benzyl-N-
ethylaniline, preferably with multiple alkylated amino groups 0 on an aryl
such as 1, 3, 5-tris-
(N,N-dimethylamino)benzene.
In another embodiment, the solvent comprises as at least one of the group and
derivatives of dimethylformamide (DMF), dimethylacetamide (DMA),
dimethylsulfoxide
(DMSO), 1,3-dimethyl-2-imidazolidinone (DMI), hexamethylphosphoramide (HMPA),
N-
methyl-2-pyrrolidone (NMP), 4-dimethylaminobenzaldehyde, acetone, dimethyl
acetone-1,3-
dicarboxylate, 3',4'-dimethylacetophenone, dimethyl methylphosphonate,
hexamethylcyclotrisiloxane, hexamethylphosphorous triamide, tributyl
phosphite, tributyl
borate, triethyl borate, tri-n-butyl borate, triphenylboron, triethyl
phosphite,
triethylphosphine, tri-n-butylphosphine, trimethyl borate, trimethylene
borate, trimethyl
phosphite, triphenyl phosphite, tris(phenyl)phosphine, organometallic such as
ferrocene,
nickelocene, organometallics, dimethyl selenium, dimethyl telluride,
tretraethyl lead,
ethyltrimethyllead, tretra-n-butyllead, phenylthiobenzene, and diphenyl
seleninde, trimethyl
stibine, tetra-n-butylgermanium, tetrapropyl titanate, tetrabutyl titanate,
tributyl aluminate,
tributyl aluminum, triethyl stibine, trimethylarsine, trimethyl indium, and
triphenylstibine,
alkyl sulfides such as diethyl sulfide and bis(phenyl) sulfide, alkyl
selenides such as diethyl
selenides, alkyl tellurides such as diethyl telluride, diethylsulfoxide, allyl
ethyl ether,
aluminum ethanolate, aluminum ethoxide, aluminum sec-butoxide, trimethyl
borate, triethyl
borate, tripropyl borate, tributyl borate, trihexyl borate, triphenylstibine,
1,3-benzodioxole,
benzofuran, 2H-1-benzopyran, benzothiazole, benzo[b]thiophene, benzoxazole, N-
benzyl-N-
ethylaniline, benzyl ethyl ether, benzyl methyl ether, benzyl phenyl ether,
2,2'-bipyridine,
1,3-bis(1-methyl-4-piperidyl)propane, bis(4-methylphenyl) ether, bis(phenyl)
ether, bis(4-
methylphenyl) sulfide, bis(methylthio)methane, 1,2-bis(N-morpholino)ethane,
2,2'-
bithiophene, 1-(2-butoxyethoxy)-2-propanol, 1 -butoxy-4-methylbenzene, 4-[3-(4-
butoxyphenoxy)propyl]morpholine, butyl ethyl ether, sec-butyl ether, t-butyl
ethyl ether,
butyl ethyl sulfide, t-butyl ethyl sulfide, butyl isobutyl ether, t-butyl
isobutyl ether, t-butyl
isopropyl ether, 1-t-butyl-4-methoxybenzene, butyl methyl ether, sec-butyl
methyl ether,
butyl phenyl ether, N-butylpiperidine, butyl propyl ether, butyl vinyl ether,
t-butyl vinyl
66
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
ether, dibenzyl ether, 1,4-dibutoxybenzene, 1,2-dibutoxyethane,
dibutoxymethane, dibutyl
ether, di-sec-butyl ether, di-tert-butyl ether, dicyclomine hydrochloride,
diethyl ether,
dicyclopentyl ether, 1,2-diethoxybenzene, 1,4-diethoxybenzene, 1,1-diethoxy-
N,N-
dimethylmethanamine, 1,1-diethoxyethane, 1,2-diethoxyethane, diethoxymethane,
2-
(diethoxymethyl)furan, 1,1-diethoxypentane, 1,1-diethoxypropane, 2,2-
diethoxypropane, 3,3-
diethoxy-1-propene, 3,3-diethoxy-l-propyne, N,N-diethylaniline, diethylene
glycol dibutyl
ether, diethylene glycol diethyl ether, diethylene glycol dimethyl ether,
diethyl telluride,
difurfuryl ether, diheptyl ether, dihexyl ether, 2,3-dihydro-1,4-benzodioxin,
2,3-
dihydrobenzofuran, 3,4-dihydro-lH-2-benzopyran, 3,4-dihydro-2H-1-benzopyran,
2,5-
dihydro-2,5-dimethoxyfuran, 2,3-dihydro-1,4-dioxin, 3,6-dihydro-4-methyl-2H-
pyran, 4,5-
dihydro-2-methylthiazole, 3,4-dihydro-2H-pyran, 3,6-dihydro-2H-pyran,
diisopentyl ether,
diisopropyl ether, 1,2-dimethoxybenzene, 1,3-dimethoxybenzene, 1,4-
dimethoxybenzene,
1,2-dimethoxyethane, 4,8-dimethoxyfuro[2,3-b]quinoline, dimethoxymethane, 1,2-
dimethoxy-4-methylbenzene, 1,3-dimethoxy-5-methylbenzene, 1,4-dimethoxy-2-
methylbenzene, 1,2-dimethyl-lH-imidazole, 1,3-dimethyl-IH-indole, dimethyl
selenide, 1,3-
dioxane, 1,3-dioxepane, 1,3-dioxolane, 1,2-diphenoxythane, diphenyl selenide,
1,2-
dipropoxyethane, dipropoxymethane, dipropyl ether, divinyl ether, divinyl
sulfide, 3-ethoxy-
N,N-diethylaniline, 2-ethoxy-3,4-dihydro-2H-pyran, 1-ethoxy-2-methoxyethane,
ethyltrimethyllead, Indolizine, 4-methoxypyridine, 6-methoxyquinoline, 1-
methyl-3-
phenoxybenzene, 1-methyl-4-(phenylthio)benzene, methyltriethyllead, 1,4-
oxathiane,
oxazole, oxepane, pteridine, tetraethoxygermane, titanium(IV) n-butoxide,
tetrapropyl
titanate, tributyl aluminate, tributylaluminum, tributyl borate, tributyl
phosphite, 1,3,5-
triethoxybenzene, triethyl borate, triethylphosphine, triethyl phosphite,
triethylstibine,
trimethylindiuim, trimethyl phosphite, trimethylstibine, triphenylstibine, N-
(1-cyclopenten-1-
yl)pyrrolidine, cyclopentyl methyl sulfide, decamethylcyclopentasiloxane,
decamethyltetrasiloxane, NA-diallyl-2-propen-l-amine, diallyl sulfide,
dibenzofuran,
benzo[b]thiophene, dibenzothiophene, dibenzyl sulfide, N,N-dibutylaniline, 2,6-
di-tert-
butylpyridine, dibutyl sulfide, di-sec-butyl sulfide, di-tert-butyl sulfide,
didecyl ether,
diethylmethylamine, N,N-diethyl-2-methylaniline, N,N-diethyl-4-methylaniline,
N,N-diethyl-
1-naphthalenamine, NN-diethyl-10H-phenothiazine-l0-ethanamine, N,N-diethyl-a-
phenylbenzenemethanamine, diethyl sulfide, diheptyl sulfide, dihexyl sulfide,
2,3-
dihydrofuran, 2,5-dihydrofuran, 2,3-dihydro-2-methylbenzofuran, 2,3-
dihydrothiophene, 2,5-
dihydrothiophene, diisobutyl sulfide, diisopentyl sulfide, diisopropyl
sulfide, 1,2-dimethoxy-
4-allylbenzene, 4,7-dimethoxy-5-allyl-1,3-benzodioxole, 4,4'-dimethoxy-1,1'-
biphenyl, 1,1-
dimethoxydodecane, (2,2-dimethoxyethyl)benzene, 1,1-dimethoxyhexadecane, 1,2-
dimethoxy-4-(1-propenyl)benzene, 4,5-dimethoxy-6-(2-propenyl)-1,3benzodioxole,
1,2-
dimethoxy-4-vinylbenzene, 2-(p-dimethylaminostyryl)benzothiazole, 2,6-
dimethylanisole,
3,5-dimethylanisole, 2,5-dimethylbenzoxazole, N,N-dimethylbenzylamine, N,N-
dimethyl-N'-
67
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
benzyl- N'-2-pyridinyl-1,2-ethanediamine, 4'-dimethyl-2,2'-bipyridine,
dimethyldecylamine,
dimethyl ether, (1,1-dimethylethoxy)benzene, 2,5-dimethylfuran, N,N-dimethyl-l-
naphthylamine, N,N-dimethyl-2-naphthylamine, 2,9-dimethyl-1,10-phenanthroline,
1,4-
dimethylpiperazine, 1,2-dimethylpiperidine, NN-dimethyl-l-propanamine, 2,3-
dimethylpyrazine, 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, 1,3-dimethyl-lH-
pyrazole,
N,N-dimethyl-2-pyridinamine, N,N-dimethyl-4-pyridinamine, 2,3-
dimethylpyridine, 2,4-
dimethylpyridine, 2,5-dimethylpyridine, 2,6-dimethylpyridine, 3,4-
dimethylpyridine, 3,5-
dimethylpyridine, 4,6-dimethylpyrimidine, 1,2-dimethylpyrrolidine, 2,4-
dimethylquinoline,
2,6-dimethylquinoline, 2,7-dimethylquinoline, 2,3-dimethylquinoxaline,
dimethyl sulfide,
dimethyl telluride, 2,5-dimethyl-1,3,4-thiadiazole, 2,7-dimethylthianthrene,
2,4-
dimethylthiazole, 4,5-dimethylthiazole, 2,3-dimethylthiophene, 2,4-
dimethylthiophene, 2,5-
dimethylthiophene, 3,4-dimethylthiophene, 2,6-dimethyl-4-tridecylmorpholine,
dinonyl ether,
dioctyl ether, dioctyl sulfide, dipentyl ether, dipentyl sulfide, 2,5-
diphenyloxazole, 1-(3,3-
diphenylpropyl)piperidine, 1,4-bis(4-methyl-5-phenloxazol-2-yl)benzene,
diphenyl sulfide,
N,N-dipropylaniline, dipropyl sulfide, 1,3-dithiane, 1,4-dithiane, 1,3-
dithiolane, 1-
dodecylpiperidine, dothiepin, doxepin, doxylamine, 1-ethoxy-3-methylbenzene, 1-
ethoxy-4-
methylbenzene, 2-ethoxy-2-methylbutane,l-ethoxynaphthalene, 2-
ethoxynaphthalene, 2-
ethyl- 1H-benzimidazole, 9-ethyl-9H-carbazole, ethyldimethylamine, 3-ethyl-2,5-
dimethylpyrazine, 2-ethylfuran, ethyl hexyl ether, 1-ethyl-1H-imidazole, ethyl
isopentyl
ether, ethyl isopropyl ether, N-ethyl-N-isopropyl-2-propanamine, ethyl
isopropyl sulfide, 1-
ethyl-4-methoxybenzene, N-ethyl-N-methylaniline, 1-ethyl-2-methyl-lH-
benzimidazole, 2-
ethyl-2-methyl-1,3-dioxolane, ethyl methyl ether, 2-ethyl-5-methylpyrazine, 3-
ethyl-4-
methylpyridine, 4-ethyl-2-methylpyridine, ethyl methyl sulfide, N-
ethylmorpholine, 1-
ethylpiperidine, ethyl propyl ether, 2-(1-ethylpropyl)pyridine, 4-(1-
ethylpropyl)pyridine,
ethyl propyl sulfide, 2-ethylpyrazine, 2-ethylpyridine, 3-ethylpyridine, 4-
ethylpyridine, 1-
ethyl-1H-pyrrole, 2-ethyltetrahydrofuran, (ethylthio)benzene, ethyl
thiocyanate, 1-(ethylthio)-
4-methylbenzene, 2-ethylthiophene, ethyl vinyl ether, hexabutyldistannoxane,
hexadecyldimethylamine, hexadecyl vinyl ether, 2,3,4,6,7,8-
hexahydropyrrolo[1,2-
a]pyrimidine, hexahydro-1,3,5-triphenyl-1,3,5-triazine, hydrocotarnine,
hydrohydrastinine,
imipramine, isobutyldimethylamine, isopropyl methyl ether, isopropyl methyl
sulfide,
isorpopyl propyl sulfide, (isopropylthio)benzene, isopropyl vinyl ether,
mebhydroline, 2-
methoxy-1,1'-biphenyl, 4-methoxy-1,1'-biphenyl, 1-methoxy-1,3-butadiene, 2-
methoxy-1,3-
butadiene, 1-methoxy-l-buten-3-yne, methoxycyclohexane, (2-
methoxyethoxy)ethene, 2-(2-
methoxyethyl)pyridine, 2-methoxyfuran, 4-methoxyfuro[2,3-b]quinoline, 2-
methoxy-2-
methlybutane, 2-(methoxymethyl)furan, 1-methoxynaphthalene, 2-
methoxynaphthalene,
trans-l-methoxy-4-(2-phenylvinyl)benzene, 2-methoxy-l-propene, 3-methoxy-l-
propene,
trans- l-methoxy-4-(1-propenyl)benzene, 1-methoxy-4-(2-propenyl)benzene, 1-
methoxy-4-
propylbenzene, 2-methoxypyridine, 3-methoxypyridine, 3-methoxypyridine, (2-
68
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
methoxyvinyl)benzene, 2-methylanisole, 3-methylanisole, 4-methylanisole, 1-
methyl-lH-
benzimazole, 2-methylbenzofuran, 2-methylbenzothiazole, 2-methylbenzoxazole, 4-
methyl-
N,N-bis(4-methylphenyl)aniline, [(3-methylbutoxy)methyl]benzene, 1-[2-(3-
methylbutoxy)-
2-phenylethyl]pyrrolidine, methyl tert-butyl ether, 3-methyl-9H-carbazole, 9-
methyl-9H-
carbazole, 2-methyl-N,N-dimethylaniline, 3-methyl-N,N-dimethylaniline, 4-
methyl-N,N-
dimethylaniline, methyldioctylamine, 4-methyl-1,3-dioxane, 2-methyl-1,3-
dioxolane,
methyldiphenylamine, 1-(1-methylethoxy)butane, 2-[2-(1-
methylethoxy)ethyl]pyridine, 1-(1-
methylethoxy)propane, 2-methylfuran, 3-methylfuran, 1-methylimidazol, 1-methyl-
lH-
indole, 1-methylisoquinoline, 3-methylisoquinoline, 4-methylisoxazole, 5-
methylisoxazole,
4-methylmorpholine, methyl-l-naphthylamine, 2-methyloxazole, 4-methyloxazole,
5-
methyloxazole, 2-methyl-2-oxazoline, 3-(4-methyl-3-pentenyl)furan, methyl
pentyl ether,
methyl pentyl sulfide, methyl tert-pentyl sulfide, 10-methyl-lOH-
phenothiazine, N-methyl-N-
phenylbenzenemethanamine, 1-methyl-N-phenyl-N-benzyl-4-piperidinamine, 2-
methyl-5-
phenylpyridine, 1-methylpiperidine, 4-(2-methylpropenyl)morpholine, methyl
propyl ether,
1-methyl-2-propylpiperidine, (S), methyl propyl sulfide, N-methyl-N-2-
propynylbenzenemethanamine, 2-methylpyrazine, 1-methyl-lH-pyrazole, 3-
methylpyridine,
4-methylpyridine, 2-methylpyrimidine, 4-methylpyrimidine, 5-methylpyrimidine,
1-
methylpyrrole, N-methylpyrrolidine, 3-(1-methyl-2-pyrrolidinyl)pyridine,( ), 2-
methylquinoline, 3-methylquinoline, 4-methylquinoline, 5-methylquinoline, 6-
methylquinoline, 7-methylquinoline, 8-methylquinoline, 2-methylquinoxaline, 2-
methyltetrahydrofuran, 2-methylthiazole, 4-methylthiazole,
(methylthio)benzene,
(methylthio)ethene, [(methylthio)methyl]benzene, 2-methylthiophene, 3-
methylthiophene, 3-
(methylthio)-1-propene, methysticin, 2-(4-morpholinothio)benzothiazole,
myristicin, 1,5-
naphthyridine, 1,6-naphthyridine, nicotelline, octyl phenyl ether,
orphenadrine, papaverine,
2-(3-pentenyl)pyridine, perazine, phenanthridine, 1,7-phenanthroline, 1,10-
phenanthroline,
4,7-phenanthroline, phenazine, phendimetrazine, phenindamine, 9-
phenylacridine, N-phenyl-
N-benzylbenzenemethanamine, 2-(2-phenylethyl)pyridine, 2-phenylfuran, 1-phenyl-
lH-
imidazole, 4-phenylmorpholine, 1-phenylpiperidine, phenyl propyl ether, 4-(3-
phenylpropyl)pyridine, 2-phenylpyridine, 3-phenylpyridine, 4-phenylpyridine, 1-
phenyl-lH-
pyrrole, 1-phenylpyrroleidine, 2-phenylquinoline, phenyl vinyl ether,
piprotal, promazine,
promethazine, trans-5-(1-propenyl)-1,3-benzodioxole, 5-propyl-1,3-
benzodioxole, 2-
propylpyridine, 4-propylpyridine, (propylthio)benzene, propyl vinyl ether, 4H-
pyran,
pyrantel, pyrilamine, quinazoline, safrole, 2,2':6',2"-terpyridine, 2,2':5,2"-
terthiophene,
tetrabutyl titanate, tetraethoxymethane, tetraethylene glycol dimethyl ether,
N,N,N',N'-
tetraethyl-l,2-ethanediamine, 1,2,3,4-tetrahydro-6,7-dimethoxy-1,2-
dimethylisoquinoline,
( ), 4,5,6,7-tetrahydro-3,6-dimethylbenzofuran, cis-tetrahydro-2,5-
dimethylthiophene,
3,4,5,6-tetrahydro-7-methoxy-2H-azepine, 1,2,3,6- tetrahydro-l-methyl-4-
phenylpyridine,
tetrahydro-3-methyl-2H-thipyran, 2,3,4,5-tetrahydro-6-propylpyridine,
tetrahydropyran,
69
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
5,6,7,8-tetrahydroquinoline, tetrahydrothiophene, N,N,2,6-tetramethylaniline,
N,N,N',N'-
tetramethyl-1,4-benzenediamine, N, N N',N'-tetramethyl-[1,1'-biphenyl]-4,4'-
diamine,
N,N,N',N'-tetramethyl-1,4-butanediamine, NNN',N'-tetramethyl-l,2-
ethanediamine,
N, N, N',N'-tetramethyl-1,6-hexanediamine, thenaldine, thenyldiamine,
thiacyclohexane,
1,2,5-thiadiazole, thianthrene, thiazole, thiepane, thiethylperazine,
thioridazine, 9H-
thioxanthene, tipepidine, tributylamine, 1,1,1-triethoxyethane,
triethoxymethane, 1,1,1-
triethoxypropane, triethylaluminum, triethylamine, triethylarsine, triethylene
glycol dimethyl
ether, trifenmorph, trihexylamine, trihexyl borate, triisobutyl aluminate,
triisobutylaluminum, triisobutylamine, triisopentylamine,
triisopropoxymethane, triisopropyl
borate, triisopropyl phosphite, 1,3,5-trimethoxybenzene, trimethoxyboroxin,
1,1,1-
trimethoxyethane, trimethoxymethane, trimethyl aluminum, trimethylamine,
trimethylarsine,
trimethylborane, trimethyl borate, 1,2,4-trimethylpiperazine,
trimethylpyrazine, 2,3,6-
trimethylpyridine, 2,4,6-trimethylpyridine, 1,2,5-trimethyl-1H-pyrrole, NN2-
trimethyl-6-
quinolinamine, triphenylarsine, triphenyl phosphite, 2,4,6-triphenyl-1,3,5-
triazine,
triprolidine, tripropylamine, tripropylborane, tripropyl borate, tripropyl
phosphite, tris(4-
dimethylaminophenyl)methane, tris(ethylthio)methane, tris(2-
methylphenyl)phosphine,
tris(3-methylphenyl)phosphine, tris(4-methylphenyl)phosphine, 2,46-tris(2-
pyridinyl)-1,3,5-
tiazine, tris(o-tolyl)phosphite, 9-vinyl-9H-carbazole, 2-vinylfuran, 1-vinyl-2-
methoxybenzene, 1 -vinyl -3 -methoxybenzene, 1-vinyl-4-methoxybenzene, 2-
vinylpyridine, 3-
vinylpyridine, 4-vinylpyridine, 9H-xanthene, dibenzofuran, 3,4-dihydro-2H-
benzopyran,
alverine, aluminum 2-butoxide, aluminum isopropoxide, antazoline, 1-
benzylpiperidine, 2-
benzylpyridne, 4-benzylpyridne, 1-benzvl-1H-pyrrole, (benzylthio)benzene, 2,2'-
bipyridine,
2,3'-bipyridine, 2,4'-bipyridine, 3,3'-bipyridine, 4,4'-bipyridine, 2,2'-
biquinoline, 1,3-bis(1-
methyl-4-piperidyl)propane, butyl methyl sulfide, t-butyl methyl sulfide, 4-
butyl morpholine,
4-t-butylpyridine, 2-butylthiophene, cusparine, cyclizine, 4-(3-cyclohexen-1-
yl)pyridine,
cyclohexyldiethylamine, cyclohexyldimethylamine, silicon-based solvents such
as silanes,
disilanes, siloxanes, and disiloaxanes preferably hexamethyldisiloxane,
(CH3)3SiOCH2CH2CH3, and (CH3)2Si(OCHCH2CH3)2, halogenated silanes, siloxanes,
and
disiloxanes, preferably fluorinated, and ionic liquids such as imidazolium and
alkyl
imidazolium salts, preferably methylimidazolium chloride and other similar
compounds.
Further fluorosolvents or sources thereof comprise tetrafluorosilane,
hexafluorodisilane,
Siõ F2i+2 such as Si16F34 , M2SiF6 wherein M is an alkali metal such as
Na2SiF6 and K2SiF6 ,
MSiF6 wherein M is an alkaline earth metal such as MgSiF6, GaF3, PF5 , and
MPF6 wherein
M is an alkali metal.
The solvent may comprise a polymer. The polymer solvent may provide a low
vapor
pressure at the operating temperature of the cell, preferably the polymer is a
liquid at the
operating temperature of the cell. One such polymeric solvent is polypropylene
glycol or
polypropylene oxide.
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
Other solvents are those known in the art having the property that they
solvate NaH
molecules. Mixtures of solvents may be in any molar ratio. Suitable solvents
comprise at
least one of the group of toluene, naphthalene, heaxfluorbenzene, 1,4-dioxane,
1,3-dioxane,
trioxane, 1,4-benzodioxane, 1,2-dimethoxyethane, and N,N-dimethylaniline,
bis(phenyl)
ether, 1,4-dioxin, dibenzodioxin or dibenzo[1,4]dioxin, and divinyl ether.
In an embodiment comprising a liquid solvent, the catalyst NaH is at least one
of a
component of the reaction mixture and is formed from the reaction mixture. The
reaction
mixture may further comprise at least one of the group of NaH, Na, NH3, NaNH2,
Na2NH,
Na3N, H2O, NaOH, NaX (X is an anion, preferably a halide), NaBH4, NaAlH4, Ni,
Pt black,
Pd black, R-Ni, R-Ni doped with a Na species such as at least one of Na, NaOH,
and NaH, a
HSA support, getter, a dispersant, a source of hydrogen such as H2, and a
hydrogen
dissociator. Preferably, the support does not form an oxide with components of
the reaction
mixture such NaOH and the solvent such an ether, preferably BDO. In this case,
the support
may be a noble metal such as at least one of Pt, Pd, Au, Ir, and Rh or a
supported noble metal
such as Pt or Pd on titanium (Pt or Pd/Ti).
An exemplary reaction mixture comprises NaH or a source of NaH, at least one
of
high-surface-area nickel powder, high-surface-area cobalt powder, and a rare
earth metal
powder, preferably La, and an ether solvent, preferably, 1,4-benzodioxane
(BDO).
In an embodiment, the reaction mixture comprises NaH + solvent + support
wherein
(1) the support comprises at least one support chosen from reduced high-
surface-area Ni
powder, La powder, and carbon such as nanotubes, preferably single-walled,
graphite,
graphene, diamond-like carbon (DLC), hydrogenated diamond-like carbon (HDLC),
diamond
powder, graphitic carbon, glassy carbon, and carbon with other metals such as
Pd or
Pt/carbon or dopants comprising other elements such as fluorinated carbon,
preferably
fluorinated graphite or fluorinated diamond; and (2) the solvent comprises an
ether such as
1,4-dibenzodioxane (BDO), dimethoxyethane (DME), 1,4-dioxane, and
biphenylether, N,N-
dimethylaniline (DMAn), perfluorinated alkane or aryl such as
hexafluorbenzene,
hexamethylphosphoramide (HMPA), protic amine, and toluene. In other
embodiments, at
least one of Na, K, KH, Li, and LiH replaces NaH. In an embodiment, the
reaction mixture
comprises species from the group of Na, NaH, NaF, a solvent, preferably a
fluorinated
carbon-based solvent, and a HSA material such as carbon, preferably single-
walled
nanotubes.
Suitable reaction mixtures comprises at least one of the group of (1) NaH,
hexafluorobenzene, and at least one of single-walled nanotubes, Pr powder,
activated carbon,
and mesoporous carbon doped with Al, La, Y, or Ni powder or the corresponding
carbide, (2)
NaH or KH, 1,4-dibenzodioxane (BDO), and at least one of La powder, Nd powder,
and a
carbide of Al, La, Y, and Ni, (3) NaH, dioxane, and Co or Nd powder, (4) NaH,
NaOH,
BDO, and Teflon powder. The weight percentages may be in any proportions,
preferably
71
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
they are about equivalent. In another embodiment, the reaction mixture
comprises species
chosen from Na, NaH, a solvent, preferably an ether solvent, and a HSA
material such as a
metal, preferably a rare earth. A suitable reaction mixture comprises NaH, 1,4-
dibenzodioxane (BDO), and La. The weight percentages may be in any
proportions,
preferably they are about 10/45/45 wt%, respectively. In another exemplary
power cell
embodiment, the reaction mixture comprises NaH, R-Ni or high-surface-area Ni
powder, and
an ether solvent. In certain chemical cell embodiments, the reaction mixture
further
comprises a getter for hydrino hydride ions and molecular hydrino such as an
alkali halide,
preferably a sodium halide such as at least one of NaF, NaCl, NaBr, and Na!.
In an embodiment, the solvent has a halogen functional group, preferably
fluorine. A
suitable reaction mixture comprises at least one of hexafluorobenzene and
octafluoronaphthalene added to a catalyst such as NaH, and mixed with a
support such as
activated carbon, a fluoropolymer or R-Ni. In an embodiment, the reaction
mixture
comprises one or more species from the group of Na, NaH, a solvent, preferably
a fluorinated
solvent, and a HSA material. The HSA material may comprise at least one of a
metal or alloy
coated with carbon such as at least one of Co, Ni, Fe, Mn, and other
transition metal powders,
preferably nanopowder, having preferably one to ten carbon layers and more
preferably three
layers according to methods known by those skilled in the art; metal or alloy
coated carbon,
preferably nanopowder, such as a transition metal preferably at least one of
Ni, Co, and Mn
coated carbon, and a fluoride, preferably a metal fluoride. Preferably, the
metal is capable of
being coated with an unreactive layer of fluoride such as steel, nickel,
copper, or Monel
metal. The coated metal may be a powder having a high surface area. Other
suitable metals
are the rare earths such as La with a fluoride coating that may comprise LaFX
such as LaF3.
In certain embodiments, the metal fluoride is more stable than MF wherein M is
the catalyst
or a source of catalyst such as Li, Na, and K. In a further embodiment, the
reaction mixture
further comprises a fluoride such as a metal fluoride. The fluoride may
comprise the metal of
the catalyst such as NaF, KF, and LiF and may further comprise transition,
noble,
intermetallic, rare earth, lanthanide, preferably La or Gd, and actinide
metal, Al, Ga, In, Tl,
Sn, Pb, metalloids, B, Si, Ge, As, Sb, Te, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag,
Cd, Hf, Ta, W,
Re, Os, Ir, Pt, Au, Hg, alkali metal, and alkaline earth metals. The fluoride
may comprise a
getter as well as a HSA material. In an embodiment, the metal may comprise an
alloy such as
LaNi5 and Ni-Y alloy or a carbide, preferably resistant to forming an
inorganic fluoride.
A suitable fluorinated solvent for regeneration is CF4. A suitable support or
HSA
material for a fluorinated solvent with NaH catalysts is NaF. In an
embodiment, the reaction
mixture comprises at least NaH, CF4, and NaF. Other fluorine-based supports or
getters
comprise M2SiF6 wherein M is an alkali metal such as Na2SiF6 and K2SiF6, MSiF6
wherein
M is an alkaline earth metal such as MgSiF6, GaF3, PF5, MPF6 wherein M is an
alkali
metal, MHF2 wherein M is an alkali metal such as NaHF2 and KHF2, K2TaF7, KBF4,
72
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
K2MnF6 , and K2ZrF6 wherein other similar compounds are anticipated such as
those having
another alkali or alkaline earth metal substitution such as one of Li, Na, or
K as the alkali
metal.
In an embodiment, the solvent comprises fluorine and at least one other
element
wherein the at least one-other-element-based fluorides are stable
thermodynamically or
kinetically to NaH reaction and are preferably liquid at the cell operating
temperature which
can be between 200 C to 700 C. The other element may be Si, Te, Se, or Sb. The
solvent
may be SiXFy wherein x and y are integers. In another embodiment, the solvent
chemistry
with NaH or any other reactant of the reaction mixture is reversible chemistry
such as a
reversible reaction of NaF and H2 to a fluorinated solvent, preferably
comprising carbon, and
NaH. In an embodiment comprising NaH and a fluorinated solvent, H2 is supplied
to the
fluorinated solvent reaction such that NaH is less reactive than Na towards
any C-F bonds
and the H2 reduces the amount of Na.
In an embodiment, at least one of a fluorinated solvent and a HSA material are
protected from attack to form NaF. Fluorocarbons are stable to strong bases,
and in an
embodiment, the source of NaH catalysts is a strong base. The source may be at
least one of
Na, NaH, NaNH2, NH3, NaOH, Na20, and a source of hydrogen such as at least one
of a
hydride and H2 and dissociator. Exemplary reactions to form the catalyst NaH,
some
regenerative, are given by Eqs. (158-161), (168), and (177-183), set forth
below. A cycle of
NaOH to form the NaH catalyst is given by Eqs. (158-161). The reaction given
by Eq. (158)
may limit the amount of Na to react with a fluorocarbon solvent. A reductant
may be added
to the reaction mixture having NaOH to form NaH and an oxide of the reductant.
The
reactant may be recycled by reduction of the oxide with hydrogen that may
further yield
NaOH. The hydrogen may be dissociated by a dissociator. The reductant may be a
metal
having a corresponding oxide that can be reduced by hydrogen such as Cr, Fe,
Sn, and Zn.
Alternatively, the oxide such as ZnO can be reduced to the metal by heating to
high
temperature such as about 1750 C. In other embodiments, the fluorinated
solvent may be
replaced by another type such as at least one of an ether, preferably one of
dibenzodioxin,
dibenzo-1,4-dioxane, dioxane, and dimethoxyethane, and a hydrocarbon such as
at least one
of toluene, xylene, benzene, naphthalene, naphthacene, phenanthrene, chrysene,
fluoranthene,
and pyrene. The support may be a metal preferably at least one of La, Pr, Co,
and Nd.
A suitable reaction mixture comprises NaH or a source of NaH, a solvent,
preferably a
fluorocarbon such as CF4, hexafluorobenzene (HFB), or perfluoroheptane, a
support,
preferably comprising carbon and a metal, and optionally hydrogen. The carbon
may
preferably comprise activated carbon (AC), but may also comprise other forms
such as glassy
carbon, coke, graphitic carbon, and carbon with a dissociator metal such as Pt
or Pd wherein
the wt% is 0.1 to 5 wt%. The metal may be in the form of at least one of a
metal powder,
hydride, or carbide, such as at least one of the group of an alkali metal, an
alkaline earth
73
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
metal, preferably Mg as MgH2, Al as a metal or carbide such as A14C3, a rare
earth metal or
carbide, preferably La, a metal or alloy, preferably nanopowder, coated with
carbon such as
at least one of Co, Ni, Fe, Mn, and other transition metal powders having
preferably one to
ten carbon layers and more preferably three layers, and a metal or alloy
coated carbon,
preferably nanopowder, such as a transition metal preferably at least one of
Ni, Co, and Mn
coated carbon. The metal may be intercalated with the carbon. In the case that
the
intercalated metal is Na and the catalyst is NaH, preferably the Na
intercalation is saturated.
The reactants can be in any desired proportions such as (1) NaH (14 wt%), HFB
(14 wt%),
AC (58 wt%), and MgH2 (14 wt%); (2) NaH (14 wt%), HFB (14 wt%), AC (58 wt%),
and Al
(14 wt%); (3) NaH (14 wt%), HFB (14 wt%), AC (58 wt%), and A14C3 (14 wt%); (4)
NaH
(14 wt%), HFB (14 wt%), AC (58 wt%), and carbon-coated Co nanopowder (14 wt%);
(5)
NaH (14 wt%), HFB (14 wt%), AC (58 wt%), and La (14 wt%). In other
embodiments, AC,
activated carbon, is replaced by mesoporous carbon, and in others the solvent
is increased,
preferably by a factor of two to three relative to the other reactants. In
other embodiments,
another catalyst such as K or Li replaces NaH catalyst.
In one general embodiments, the reaction mixture comprises a component called
a
protecting agent or blocking agent that at least partially suppresses an
undesired reaction of
one component of the mixture with another. Preferably, the protecting agent or
blocking
agent is nonreactive with a solvent or a support. Strong bases are nonreactive
towards
fluorocarbons; whereas, Na is. Thus, in an embodiment, at least one of H2,
NaOH, NaNH2
and NH3 may be added to the reaction mixture as a blocking agent to react with
any Na
formed during the reaction to form hydrino to prevent it from reacting with a
support such as
a fluorocarbon support. An exemplary reaction mixture comprises NaH, a
blocking agent
such as at least one of NaOH, NaNH2, NH3, H2, a solvent such as at least one
of BDO, crown
ether, polypropylene oxide, CF4, and HFB, and a support comprising at least a
fluorocarbon
such as Teflon powder. Exemplary protective agents are hydride and carbide.
The protected
reactant may be a metal support. The reaction may comprise NaH, and ether
solvent such as
BDO, and a metal hydride such as a rare earth metal hydride or a carbide such
as at least one
of Al, rare earth, and transition metal carbides.
In a second general embodiments, the reaction mixture is substantially stable
over
long duration towards reaction amongst components other than forming hydrinos.
Preferably, the solvent such as a polar solvent is nonreactive with the
catalyst or a support.
For example, an ether solvent is nonreactive towards NaH as a source of
catalyst, a
fluorocarbon support, or a rare earth powder, hydride or carbide at a suitably
low reaction
temperature such as less than 350 C. Thus, an exemplary reaction mixture
comprises NaH,
an ether solvent such as BDO, dioxane, or a crown ether, and a rare metal
powder support
such as La powder. Another support comprises an alloy resistant to reaction
with the solvent
such as LaNi5.
74
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
In a third general embodiments, the reaction mixture comprises reactants that
form
hydrinos at a high yield as a side reaction amongst components also occurs.
The reactants
may be regenerated to run another cycle to form hydrinos. An exemplary
reaction mixture
comprises NaH, a fluorocarbon solvent such as CF4, and a support such as at
least one of
Teflon, fluorinated graphite, activated carbon, graphene, and mesoporous
carbon plus at least
one of Al, La, Co, Ni, Mn, Y, and Fe powder and their carbides. Preferably the
metal and
carbide comprise a mixture such as one of Ni, Co, Mn. The metals and carbides
may be in
any wt% ratio. Preferably, the composition and weight percent (%) ratios are
about 20 to
25% Ni, 60 to 70% Co, and 5 to 15% Mn. In another, the metal and carbide
comprise a
mixture with other elements such as one of Ni, Co, Mn, Fe, S, and Ca. The
metals and
carbides and other elements may be in any wt% ratio. Preferably, the
composition and
weight percent (%) ratios are about 20 5% Ni, 65 5% Co, 10 5% Mn, 1 5%
Fe, 1% 2
S, and 0.5 2% Ca. In other embodiments, the carbon support comprises a high-
surface-area
carbon such as activated carbon or mesoporous carbon and at least one metal
that forms a less
thermodynamically stable fluoride than NaF such as nickel, iron, iridium,
vanadium, lead,
molybdenum, and tungsten.
Other embodiments comprise reaction mixtures involving any combination of
these
three general embodiments based on these, any combination of, or any
alternative reaction
strategy or pathway.
In an embodiment, the source or sources to provide the catalyst and atomic
hydrogen
comprise at least one of amides such as LiNH2, imides such as Li2NH, nitrides
such as Li3N,
and catalyst metal with NH3. Reactions of these species provide both Li atoms
and atomic
hydrogen. Additionally, K, Cs, and Na may replace Li, and the catalyst is
atomic K, atomic
Cs, and molecular NaH. In another embodiment of a reaction mixture comprising
a liquid
solvent, the catalyst is Li. The reaction mixture may further comprise species
of the group of
Li, LiNH2, Li2NH, Li3N, LiNO3, LiX, NH4X (X is an anion, preferably a halide),
NH3, R-Ni,
a HSA support, getter, a dispersant, a source of hydrogen such as H2, and a
hydrogen
dissociator.
b. Inorganic Solvents
In another embodiment, the reaction mixture comprises at least one inorganic
solvent.
The solvent may additionally comprise a molten inorganic compound such as a
molten salt.
The inorganic solvent may be molten NaOH. In an embodiment, the reaction
mixture
comprises a catalyst, a source of hydrogen, and an inorganic solvent for the
catalyst. The
catalyst may be at least one of NaH molecules, Li, and K. The solvent may be
at least one of
a molten or fused salt or eutectic such as at least one of the molten salts of
the group of alkali
halides and alkaline earth halides. The inorganic solvent of the NaH catalyst
reaction mixture
may comprise a low-melting eutectic of a mixture of alkali halides such as
NaCl and KCI.
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
The solvent may be a low-melting point salt, preferably a Na salt such as at
least one of Nal
(660 C), NaA1C14 (160 C), NaA1F4, and compound of the same class as NaMX4
wherein M
is a metal and X is a halide having a metal halide that is more stable than
NaX. The reaction
mixture may further comprise a support such as R-Ni.
The inorganic solvent of the Li catalyst reaction mixture may comprise a low-
melting
eutectic of a mixture of alkali halides such as LiCl and KCI. The molten salt
solvent may
comprise a fluorine-based solvent that is stable to NaH. The melting point of
LaF3 is 1493 C
and the melting point of NaF is 996 T. A ball-milled mixture in appropriate
ratios, with
optionally other fluorides, comprises a fluoride-salt solvent that is stable
to NaH and melts
preferably in the range of 600 C-700 C. In a molten-salt embodiment, the
reaction mixture
comprises NaH + salt mixture such as NaF-KF-LiF (11.5-42.0-46.5) MP=454 C or
NaH +
salt mixture such as LiF-KF (52%-48%) MP=492 C.
V. Regeneration Systems and Reactions
A schematic drawing of a system for recycling or regenerating the fuel in
accordance
with the present disclosure is shown in FIGURE 4. In an embodiment, the
byproducts of the
hydrino reaction comprise a metal halide MX, preferably NaX or KX. Then, the
fuel recycler
18 (Figure 4) comprises a separator 21 to separate inorganic compounds such as
NaX from
the support. In an embodiment, the separator or a component thereof comprises
a shifter or
cyclone separator 22 that performs the separation based on density differences
of the species.
A further separator or component thereof comprises a magnetic separator 23
wherein
magnetic particles such as nickel or iron are pulled out by a magnet while
nonmagnetic
material such as MX flow through the separator. In another embodiment, the
separator or a
component thereof comprises a differential product solubilization or
suspension system 24
comprising a component solvent wash 25 that dissolves or suspends at least one
component
to a greater extent than another to permit the separation, and may further
comprise a
compound recovery system 26 such as a solvent evaporator 27 and compound
collector 28.
Alternatively, the recovery system comprises a precipitator 29 and a compound
dryer and
collector 30. In an embodiment, waste heat from the turbine 14 and water
condensor 16
shown in Figure 4 is used to heat at least one of the evaporator 27 and dryer
30 (Figure 4).
Heat for any other of the stages of the recycler 18 (Figure 4) may comprise
the waste heat.
The fuel recycler 18 (Figure 4) further comprises an electrolyzer 31 that
electrolyzes
the recovered MX to metal and halogen gas or other halogenated or halide
product. In an
embodiment, the electrolysis occurs within the power reactor 36, preferably
from a melt such
as a eutectic melt. The electrolysis gas and metal products are separately
collected at highly
volatile gas collector 32 and a metal collector 33 that may further comprise a
metal still or
separator 34 in the case of a mixture of metals, respectively. If the initial
reactant is a
hydride, the metal is hydrided by a hydriding reactor 35 comprising a cell 36
capable of
76
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
pressures less than, greater than, and equal to atmospheric, an inlet and
outlet 37 for the metal
and hydride, an inlet for hydrogen gas 38 and its valve 39, a hydrogen gas
supply 40, a gas
outlet 41 and its valve 42, a pump 43, a heater 44, and pressure and
temperature gauges 45.
In an embodiment, the hydrogen supply 40 comprises an aqueous electrolyzer
having a
hydrogen and oxygen gas separator. The isolated metal product is at least
partially
halogenated in a halogenation reactor 46 comprising a cell 47 capable of
pressures less than,
greater than, and equal to atmospheric, an inlet for the carbon and outlet for
the halogenated
product 48, an inlet for fluorine gas 49 and its valve 50, a halogen gas
supply 51, a gas outlet
52 and its valve 53, a pump 54, a heater 55, and pressure and temperature
gauges 56.
Preferably, the reactor also contains catalysts and other reactants to cause
the metal 57 to
become the halide of the desired oxidation state and stoichiometry as the
product. The at
least two of the metal or metal hydride, metal halide, support, and other
initial reactants are
recycled to the boiler 10 after being mixed in a mixer 58 for another power-
generation cycle.
In exemplary hydrino and regeneration reactions, the reaction mixture
comprises NaH
catalyst, Mg, Mn12, and support, activated carbon, WC or TiC. In an
embodiment, the source
of exothermic reaction is the oxidation reaction of metal hydrides by Mn12
such as
2KH + Mn12 -+ 2KI + Mn + H2 (86)
Mg + Mn12 -+ MgI2 + Mn . (87)
KI and MgI2 may be electrolyzed to 12, K, and Mg from a molten salt. The
molten
electrolysis may be performed using a Downs cell or modified Downs cell. Mn
may be
separated using a mechanical separator and optionally sieves. Unreacted Mg or
MgH2 may
be separated by melting and by separation of solid and liquid phases. The
iodides for the
electrolysis may be from the rinse of the reaction products with a suitable
solvent such as
deoxygenated water. The solution may be filtered to remove the support such as
AC and
optionally the transition metal. The solid may be centrifuged and dried,
preferably using
waste heat from the power system. Alternative, the halides may be separated by
melting
them followed by separation of the liquid and solid phases. In another
embodiment, the
lighter AC may initially be separated from the other reaction products by a
method such as
cyclone separation. K and Mg are immiscible, and the separated metals such as
K may be
hydrided with H2 gas, preferably from the electrolysis of H20. The metal
iodide may be
formed by know reactions with the separated metal or with the metal,
unseparated from AC.
In an embodiment, Mn is reacted with HI to form Mn12, and H2 that is recycled
and reacted
with 12 to form HI. In other embodiments, other metals, preferably a
transition metal,
replaces Mn. Another reductant such as Al may replace Mg. Another halide,
preferably
chloride may replace iodide. LiH, KH, RbH, or CsH may replace NaH.
In exemplary hydrino and regeneration reactions, the reaction mixture
comprises NaH
catalyst, Mg, AgCI, and support, activated carbon. In an embodiment, the
source of
exothermic reaction is the oxidation reaction of metal hydrides by AgCI such
as
77
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
KH + AgCI - KCl + Ag + 1/ 2H2 (88)
Mg + 2AgC1 -> MgCl2 + 2Ag. (89)
KCl and MgC12 may be electrolyzed to C12, K, and Mg from a molten salt. The
molten
electrolysis may be performed using a Downs cell or modified Downs cell. Ag
may be
separated using a mechanical separator and optionally sieves. Unreacted Mg or
MgH2 may
be separated by melting and by separation of solid and liquid phases. The
chlorides for the
electrolysis may be from the rinse of the reaction products with a suitable
solvent such as
deoxygenated water. The solution may be filtered to remove the support such as
AC and
optionally the Ag metal. The solid may be centrifuged and dried, preferably
using waste heat
from the power system. Alternative, the halides may be separated by melting
them followed
by separation of the liquid and solid phases. In another embodiment, the
lighter AC may
initially be separated from the other reaction products by a method such as
cyclone
separation. K and Mg are immiscible, and the separated metals such as K may be
hydrided
with H2 gas, preferably from the electrolysis of H2O. The metal chloride may
be formed by
know reactions with the separated metal or with the metal, unseparated from
AC. In an
embodiment, Ag is reacted with C12 to form AgCI, and H2 that is recycled and
reacted with I2
to form HI. In other embodiments, other metals, preferably a transition metal
or In, replaces
Ag. Another reductant such as Al may replace Mg. Another halide, preferably
chloride may
replace iodide. LiH, KH, RbH, or CsH may replace NaH.
In an embodiment, the reaction mixture is regenerated from hydrino reaction
products. In exemplary hydrino and regeneration reactions, the solid fuel
reaction mixture
comprises KH or NaH catalyst, Mg or MgH2, and alkaline earth halide such as
BaBr2, and
support, activated carbon, WC, or preferably TiC. In an embodiment, the source
of
exothermic reaction is the oxidation reaction of metal hydrides or metals by
BaBr2 such as
2KH + Mg + BaBr2 -> 2KBr + Ba + MgH2 (90)
2NaH +Mg +BaBr2 -> 2NaBr +Ba +MgH2. (91)
The melting points of Ba, magnesium, MgH2, NaBr, and KBr are 727 C, 650 C,
327 C, 747
C, and 734 C, respectively. Thus, MgH2 can be separated from barium and any
Ba-Mg
intermetalic by maintaining the MgH2 with optional addition of H2,
preferentially melting the
MgH2, and separating the liquid from the reaction-product mixture. Optionally,
it may be
thermally decomposed to Mg. Next, the remaining reaction products may be added
to an
electrolysis melt. Solid support and Ba precipitates to form preferably
separable layers.
Alternatively, Ba may be separated as a liquid by melting. Then, NaBr or KBr
may be
electrolyzed to form the alkali metal and Br2. The latter is reacted with Ba
to form BaBr2.
Alternatively, Ba is the anode, and BaBr2 forms directly in the anode
compartment. The
alkali metal may be hydrided following electrolysis or formed in the cathode
compartment
during electrolysis by bubbling H2 in this compartment. Then, MgH2 or Mg, NaH
or KH,
BaBr2, and support are retuned to the reaction mixture. In other embodiments,
another
78
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
alkaline earth halide replaces BaBr2, preferably BaC12. In another embodiment,
the
regeneration reactions may occur without electrolysis due to the small energy
difference
between the reactants and products. The reactions given by Eqs. (90-91) may be
reversed by
changing the reactions condition such as temperature or hydrogen pressure.
Alternatively, a
molten or volatile species such as K or Na may be selectively removed to drive
the reaction
backwards to regenerate a reactant or a species that can be further reacted
and added back to
the cell to form the original reaction mixture. In another embodiment, the
volatile species
may be continually refluxed to maintain the reversible reaction between the
catalyst or source
of catalyst such as NaH, KH, Na, or K and the initial oxidant such as an
alkaline earth halide
or rare earth halide. In an embodiment, the reflux is achieved using a still
such as still 34
shown in FIGURE 4. In another embodiment, the reaction conditions such as the
temperature
or hydrogen pressure may be changed to reverse the reaction. In this case, the
reaction is
initially run in the forward direction to form hydrinos and the reaction
mixture products.
Then, the products other than lower-energy hydrogen are converted to the
initial reactants.
This may be performed by changing the reaction conditions and possibly adding
or removing
at least partially the same or other products or reactant as those initially
used or formed.
Thus, the forward and regeneration reactions are carried out in alternating
cycles. Hydrogen
may be added to replace that consumed in the formation of hydrinos. In another
embodiment, reaction conditions are maintained such as an elevated temperature
wherein the
reversible reaction is optimized such that both the forward and reverse
reactions occur in a
manner that achieves the desired, preferably maximum, rate of hydrino
formation.
In exemplary hydrino and regeneration reactions, the solid fuel reaction
mixture
comprises NaH catalyst, Mg, FeBr2, and support, activated carbon. In an
embodiment, the
source of exothermic reaction is the oxidation reaction of metal hydrides by
FeBr2 such as
2NaH + FeBr2 - 2NaBr + Fe + H2 (92)
Mg + FeBr2 - MgBr2 + Fe. (93)
NaBr and MgBr2 may be electrolyzed to Br2, Na, and Mg from a molten salt. The
molten
electrolysis may be performed using a Downs cell or modified Downs cell. Fe is
ferromagnetic and may be separated magnetically using a mechanical separator
and
optionally sieves. In another embodiment, ferromagnetic Ni may replace Fe.
Unreacted Mg
or MgH2 may be separated by melting and by separation of solid and liquid
phases. The
bromides for the electrolysis may be from the rinse of the reaction products
with a suitable
solvent such as deoxygenated water. The solution may be filtered to remove the
support such
as AC and optionally the transition metal. The solid may be centrifuged and
dried, preferably
using waste heat from the power system. Alternative, the halides may be
separated by
melting them followed by separation of the liquid and solid phases. In another
embodiment,
the lighter AC may initially be separated from the other reaction products by
a method such
as cyclone separation. Na and Mg are immiscible, and the separated metals such
as Na may
79
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
be hydrided with H2 gas, preferably from the electrolysis of H2O. The metal
bromide may be
formed by know reactions with the separated metal or with the metal,
unseparated from AC.
In an embodiment, Fe is reacted with HBr to form FeBr2, and H2 that is
recycled and reacted
with Br2 to form HBr. In other embodiments, other metals, preferably a
transition metal,
replaces Fe. Another reductant such as Al may replace Mg. Another halide,
preferably
chloride may replace bromide. LiH, KH, RbH, or CsH may replace NaH.
In exemplary hydrino and regeneration reactions, the solid fuel reaction
mixture
comprises KH or NaH catalyst, Mg or MgH2, SnBr2, and support, activated
carbon, WC, or
TiC. In an embodiment, the source of exothermic reaction is the oxidation
reaction of metal
hydrides or metals by SnBr2 such as
2KH + SnBr2 -> 2KBr + Sn + H2 (94)
2NaH+SnBr2 -+2NaBr+Sn+H2 (95)
Mg+SnBrz -> MgBr2 + Sn. (96)
The melting points of tin, magnesium, MgH2, NaBr, and KBr are 119 C, 650 C,
327 C,
747 C, and 734 C, respectively. Tin-magnesium alloy will melt above a
temperature such
as 400 C for about 5wt% Mg as given in its alloys phase diagram. In an
embodiment, tin
and magnesium metals and alloys are separated from the support and halides by
melting the
metals and alloys and separating the liquid and solid phases. The alloy may be
reacted with
H2 at a temperature that forms MgH2 solid and tin metal. The solid and liquid
phases may be
separated to give MgH2 and tin. The MgH2 may be thermally decomposed to Mg and
H2.
Alternatively, H2 may be added to the reaction products in situ at a
temperature selective to
convert any unreacted Mg and any Sn-Mg alloy to solid MgH2 and liquid tin. The
tin may be
selectively removed. Then, MgH2 may be heated and removed as a liquid. Next,
halides may
be removed from the support by methods such (1) melting them and separation of
the phases,
(2) cyclone separation based on density differences wherein a dense support
such as WC is
preferred, or (3) sieving based on size differences. Alternatively, the
halides may be
dissolved in a suitable solvent, and the liquid and solid phases separated by
methods such as
filtering. The liquid may be evaporated and then the halides may be
electrolyzed from the
melt to Na or K and possibly Mg metals that are immiscible and each separated.
In another
embodiment K is formed by reduction of the halide using Na metal that is
regenerated by
electrolysis of a sodium halide, preferably the same halide as formed in the
hydrino reactor.
In addition, halogen gas such as Br2 is collected from the electrolysis melt
and reacted with
isolated Sn to form SnBr2 that is recycled for another cycle of the hydrino
reaction together
with NaH or KH, and Mg or MgH2 wherein the hydrides are formed by hydriding
with H2
gas. In an embodiment, HBr is formed and reacted with Sn to from SnBr2. HBr
may be
formed by reaction of Br2 and H2 or during electrolysis by bubbling H2 at the
anode that has
an advantage of lowering the electrolysis energy. In other embodiment another
metal
replaces Sn, preferably a transition metal, and another halide may replace Br
such as I.
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
In another embodiment, at the initial step, all of the reaction products are
reacted with
aqueous HBr, and the solution is concentrated to precipitate SnBr2 from MgBr2
and KBr
solution. Other suitable solvents and separation methods may be used to
separate the salts.
MgBr2 and KBr are then electrolyzed to Mg and K. Alternatively, Mg or MgH2 is
first
removed using mechanical or by selective solvent methods such that only KBr
need be
electrolyzed. In an embodiment, Sn is removed as a melt from solid MgH2 that
may be
formed by adding H2 during or after the hydrino reaction. MgH2 or Mg, KBr, and
support are
then added to the electrolysis melt. The support settles in a sedimentary zone
due to its large
particle size. MgH2 and KBr form part of the melt and separate based on
density. Mg and K
are immiscible, and K also forms a separate phase such that Mg and K are
collected
separately. The anode may be Sn such that K, Mg, and SnBr2 are the
electrolysis products.
The anode may be liquid tin or liquid tin may be sparged at the anode to react
with bromine
and form SnBr2. In this case the energy gap for regeneration is the compound
gap versus the
higher elemental gap corresponding to elemental products at both electrodes.
In a further
embodiment, the reactants comprise KH, support, and SnI2 or SnBr2. The Sn may
be
removed as a liquid, and the remaining products such as KX and support may be
added to the
electrolysis melt wherein the support separates based on density. In this
case, a dense support
such as WC is preferred.
The reactants may comprise an oxygen compound to form an oxide product such as
an oxide of the catalyst or source of catalyst such as that of NaH, Li, or K
and an oxide of the
reductant such as that of Mg, MgH2, Al, Ti, B, Zr, or La. In an embodiment,
the reactants are
regenerated by reacting the oxide with an acid such as a hydrogen halide acid,
preferably
HCI, to form the corresponding halide such as the chloride. In an embodiment,
an oxidized
carbon species such as carbonate, hydrogen carbonate, a carboxylic acid
species such as
oxalic acid or oxalate may be reduced by a metal or a metal hydride.
Preferably, at least one
of Li, K, Na, LiH, KH, NaH, Al, Mg, and MgH2 reacts with the species
comprising carbon
and oxygen and forms the corresponding metal oxide or hydroxide and carbon.
Each
corresponding metal may be regenerated by electrolysis. The electrolysis may
be performed
using a molten salt such as that of a eutectic mixture. The halogen gas
electrolysis product
such as chlorine gas may be used to form the corresponding acid such as HCl as
part of a
regeneration cycle. The hydrogen halide acid HX may be formed by reacting the
halogen gas
with hydrogen gas and by optionally dissolving the hydrogen halide gas into
water.
Preferably the hydrogen gas is formed by electrolysis of water. The oxygen may
be a
reactant of the hydrino reaction mixture or may be reacted to form the source
of oxygen of
the hydrino reaction mixture. The step of reacting the oxide hydrino reaction
product with
acid may comprise rinsing the product with acid to form a solution comprising
the metal
salts. In an embodiment, the hydrino reaction mixture and the corresponding
product mixture
comprises a support such as carbon, preferably activated carbon. The metal
oxides may be
81
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
separated from the support by dissolving them in aqueous acid. Thus, the
product may be
rinsed with acid and may further be filtered to separate the components of the
reaction
mixture. The water may be removed by evaporation using heat, preferably waste
heat from
the power system, and the salts such as metal chlorides may be added to the
electrolysis
mixture to form the metals and halogen gas. In an embodiment, any methane or
hydrocarbon
product may be reformed to hydrogen and optionally carbon or carbon dioxide.
Alternatively, the methane was be separated from the gas product mixture and
sold as a
commercial product. In another embodiment, the methane may be formed into
other
hydrocarbon products by methods known in the art such as Fischer-Tropsch
reactions. The
formation of methane may be suppressed by adding an interfering gas such as an
inert gas
and by maintaining unfavorable conditions such as a reduced hydrogen pressure
or
temperature.
In another embodiment, metal oxides are directly electrolyzed from a eutectic
mixture. Oxides such as MgO may be reacted to water to form hydroxides such as
Mg(OH)2-
In an embodiment, the hydroxide is reduced. The reductant may be an alkaline
metal or
hydride such as Na or NaH. The product hydroxide may be electrolyzed directly
as a molten
salt. Hydrino reaction products such as alkali metal hydroxides may also be
used as a
commercial product and the corresponding halides acquired. The halides may
then be
electrolyzed to halogen gas and metal. The halogen gas may be used as a
commercial
industrial gas. The metal may be hydrided with hydrogen gas, preferably for
the electrolysis
of water, and supplied to the reactor as a part of the hydrino reaction
mixture.
The reductant such as an alkali metal can be regenerated from the product
comprising
a corresponding compound, preferably NaOH or Na20, using methods and systems
known to
those skilled in the art. One method comprises electrolysis in a mixture such
as a eutectic
mixture. In a further embodiment, the reductant product may comprise at least
some oxide
such as a reductant metal oxide (e.g. MgO). The hydroxide or oxide may be
dissolved in a
weak acid such as hydrochloric acid to form the corresponding salt such as
NaCl or MgC12.
The treatment with acid may also be an anhydrous reaction. The gases may be
streaming at
low pressure. The salt may be treated with a product reductant such as an
alkali or alkaline
earth metal to form the original reductant. In an embodiment, the second
reductant is an
alkaline earth metal, preferably Ca wherein NaCI or MgC12 is reduced to Na or
Mg metal.
The additional product of CaC13 is recovered and recycled as well. In
alternative
embodiment, the oxide is reduced with H2 at high temperature.
In exemplary hydrino and regeneration reactions, the reaction mixture
comprises NaH
catalyst, MgH2, 02, and support, activated carbon. In an embodiment, the
source of
exothermic reaction is the oxidation reaction of metal hydrides by 02 such as
MgH2+02 ->Mg(OH)2 (97)
MgH2+1.502+C->MgCO3+H2 (98)
82
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
NaH + 3 / 202 + C -> NaHCO3 (99)
2NaH +02 -> 2NaOH. (100)
Any MgO product may be converted to the hydroxide by reaction with water
MgO+H20 -> Mg(OH)2. (101)
Sodium or magnesium carbonate, hydrogen carbonate, and other species
comprising carbon
and oxygen may be reduced with Na or NaH:
NaH+Na2CO3 -> 3NaOH+C+1/H2 (102)
NaH+1/3MgCO3 -+NaOH+1/3C+1/3Mg (103)
Mg(OH)2 can be reduced to Mg using Na or NaH:
2Na+Mg(OH)2 -> 2NaOH+Mg. (104)
Then, NaOH can be electrolyzed to Na metal and NaH and 02 directly from the
melt. The
Castner process may be used. A suitable cathode and anode for a basic solution
is nickel.
The anode may also be carbon, a noble metal such as Pt, a support such as Ti
coated with a
noble metal such as Pt, or a dimensionally stable anode. In another
embodiment, NaOH is
converted to NaCl by reaction with HCl wherein the NaCl electrolysis gas C12
may be reacted
with H2 from the electrolysis of water to form the HCI. The molten NaCl
electrolysis may be
performed using a Downs cell or modified Downs cell. Alternatively, HCl may be
produced
by chloralkali electrolysis. The aqueous NaCl for this electrolysis may be
from the rinse of
the reaction products with aqueous HCI. The solution may be filtered to remove
the support
such as AC that may be centrifuged and dried, preferably using waste heat from
the power
system.
In an embodiment, the reaction step comprise, (1) rinse the products with
aqueous
HCl to form metal chlorides from species such as hydroxides, oxides, and
carbonates, (2)
convert any evolved CO2 to water and C by H2 reduction using the water gas
shift reaction
and the Fischer Tropsch reaction wherein the C is recycled as the support at
step 10 and the
water may be used at steps, 1, 4, or 5, (3) filter and dry the support such as
AC wherein the
drying may include the step of centrifugation, (4) electrolyze water to H2 and
02 to supply
steps 8 to 10, (5) optionally form H2 and HCl from the electrolysis of aqueous
NaCl to supply
steps 1 and 9, (6) isolate and dry the metal chlorides, (7) electrolyze a melt
of the metal
chloride to metals and chlorine, (8) form HCl by reaction of C12 and H2 to
supply step 1, (9)
hydride any metal to form the corresponding starting reactant by reaction with
hydrogen, and
(10) form the initial reaction mixture with the addition of 02 from step 4 or
alternatively
using 02 isolated from the atmosphere.
In another embodiment, at least one of magnesium oxide and magnesium hydroxide
are electrolyzed from a melt to Mg and 02. The melt may be a NaOH melt wherein
Na may
also be electrolyzed. In an embodiment, carbon oxides such as carbonates and
hydrogen
carbonates may be decomposed to at least one of CO and CO2 that may be added
to the
reaction mixture as a source of oxygen. Alternatively, the carbon oxide
species such as CO2
83
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
and CO may be reduced to carbon and water by hydrogen. CO2 and CO and may be
reduced
by the water gas shift reaction and the Fischer Tropsch reaction.
In exemplary hydrino and regeneration reactions, the reaction mixture
comprises NaH
catalyst, MgH2, CF4, and support, activated carbon. In an embodiment, the
source of
exothermic reaction is the oxidation reaction of metal hydrides by CF4 such as
2MgH2+CF4 -C+2MgFZ+2H2 (105)
2MgHZ +CF4 -* CH4 +2MgFZ (106)
4NaH + CF4 - C + 4NaF + 2H2 (107)
4NaH + CF4 -* CH4 + 4NaF. (108)
NaF and MgF2 may be electrolyzed to F2, Na, and Mg from a molten salt that may
additionally comprise HE Na and Mg are immiscible, and the separated metals
may be
hydrided with H2 gas, preferably from the electrolysis of H2O. The F2 gas may
be reacted
with carbon and any CH4 reaction product to regenerate CF4. Alternatively and
preferably,
the anode of the electrolysis cell comprises carbon, and the current and
electrolysis conditions
are maintained such that CF4 is the anode electrolysis product.
In exemplary hydrino and regeneration reactions, the reaction mixture
comprises NaH
catalyst, MgH2, P205 (P4010), and support, activated carbon. In an embodiment,
the source of
exothermic reaction is the oxidation reaction of metal hydrides by P205 such
as
5MgH2 + P205 -* 5MgO + 2P + 5H2 (109)
5NaH+P205 -5NaOH+2P. (110)
Phosphorous can be converted to P205 by combustion in 02
2P+2.502 -> P205. (111)
The MgO product may be converted to the hydroxide by reaction with water
MgO+H20-*Mg(OH)2. (112)
Mg(OH)2 can be reduced to Mg using Na or NaH:
2Na+Mg(OH)2 -2NaOH+Mg. (113)
Then, NaOH can be electrolyzed to Na metal and NaH and 02 directly from the
melt, or it
may be converted to NaCl by reaction with HCI wherein the NaCl electrolysis
gas C12 may be
reacted with H2 from the electrolysis of water to from the HCI. In
embodiments, metals such
as Na and Mg may be converted to the corresponding hydrides by reaction with
H2,
preferably from the electrolysis of water.
In exemplary hydrino and regeneration reactions, the solid fuel reaction
mixture
comprises NaH catalyst, MgH2, NaNO3, and support, activated carbon. In an
embodiment,
the source of exothermic reaction is the oxidation reaction of metal hydrides
by NaNO3 such
as
NaNO3 + NaH + C - Na2CO3 +1/ 2N2 +1/ 2H2 (114)
NaNO3 +1/ 2H2 + 2NaH - 3NaOH + 1 / 2N2 (115)
NaNO3 + 3MgH2 - 3MgO + NaH + I/ 2N2 + 5 / 2H2 . (116)
84
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
Sodium or magnesium carbonate, hydrogen carbonate, and other species
comprising carbon
and oxygen may be reduced with Na or NaH:
NaH+Na2CO3 ->3NaOH+C+1/H2 (117)
NaH + 1/ 3MgCO3 -> NaOH+1/3C+1/3Mg. (118)
Carbonates can also be decomposed from aqueous media to the hydroxides and CO2
Na2CO3 +H20 -> 2NaOH +CO2 . (119)
Evolved CO2 may be reacted to water and C by H2 reduction using the water gas
shift
reaction and the Fischer Tropsch reaction
CO2+H2 -> CO+H2O (120)
CO+H2 ->C+H20. (121)
The MgO product may be converted to the hydroxide by reaction with water
MgO+H2O-> Mg(OH)2. (122)
Mg(OH)2 can be reduced to Mg using Na or NaH:
2Na+Mg(OH)2 -> 2NaOH+Mg. (123)
Alkali nitrates can be regenerated using the methods known to those skilled in
the art. In an
embodiment, NO2, can be generated by known industrial methods such as by the
Haber
process followed by the Ostwald process. In one embodiment, the exemplary
sequence of
steps are:
N2 112 02 NO (124)
2 Ilh NH3 o2 .
process process
Specifically, the Haber process may be used to produce NH3 from N2 and H2 at
elevated
temperature and pressure using a catalyst such as a -iron containing some
oxide. The
Ostwald process may be used to oxidize the ammonia to NO2, at a catalyst such
as a hot
platinum or platinum-rhodium catalyst. The heat may be waste heat from the
power system.
NO2 may be dissolved in water to form nitric acid that is reacted with NaOH,
Na2CO3, or
NaHCO3 to form sodium nitrate. Then, the remaining NaOH can be electrolyzed to
Na metal
and NaH and 02 directly from the melt, or it may be converted to NaCl by
reaction with HCl
wherein the NaCl electrolysis gas C12 may be reacted with H2 from the
electrolysis of water
to from the HCI. In embodiments, metals such as Na and Mg may be converted to
the
corresponding hydrides by reaction with H2, preferably from the electrolysis
of water. In
other embodiments, Li and K replace Na.
In exemplary hydrino and regeneration reactions, the reaction mixture
comprises NaH
catalyst, MgH2, SF6, and support, activated carbon. In an embodiment, the
source of
exothermic reaction is the oxidation reaction of metal hydrides by SF6 such as
4MgH2 +SF6 -> 3MgF2 +4H2 +MgS (125)
7NaH+SF6 ->6NaF+3H2+NaHS. (126)
NaF and MgF2 and the sulfides may be electrolyzed to Na and Mg from a molten
salt that
may additionally comprise HF. The fluorine electrolysis gas may react with the
sulfides to
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
form SF6 gas that may be removed dynamically. The separation of SF6 from F2
may be by
methods known in the art such as cryo-distillation, membrane separation, or
chromatography
using a medium such as molecular sieves. NaHS melts at 350 C and may be part
of the
molten electrolysis mixture. Any MgS product may be reacted with Na to form
NaHS
wherein the reaction may occur in situ during electrolysis. S and metals may
be products
formed during electrolysis. Alternatively, the metals may be in minority such
that the more
stable fluorides are formed, or F2 may be added to form the fluorides.
3MgH2+SF6 -.3MgF2+3H2+S (127)
6NaH +SF6 -+ 6NaF +3H2 +S . (128)
NaF and MgF2 may be electrolyzed to F2, Na, and Mg from a molten salt that may
additionally comprise HE Na and Mg are immiscible, and the separated metals
may be
hydrided with H2 gas, preferably, the make up is from the electrolysis of H2O.
The F2 gas
may be reacted with sulfur to regenerate SF6.
In exemplary hydrino and regeneration reactions, the reaction mixture
comprises NaH
catalyst, MgH2, NF3, and support, activated carbon. In an embodiment, the
source of
exothermic reaction is the oxidation reaction of metal hydrides by NF3 such as
3MgH2+2NF3 ->3MgF2+3H2+N2 (129)
6MgH2 + 2NF3 - 3MgF2 +Mg3N2 + 6H2 (130)
3NaH+NF3 -3NaF+1/2N2+1.5H2. (131)
NaF and MgF2 may be electrolyzed to F2, Na, and Mg from a molten salt that may
additionally comprise HE The conversion of Mg3N2 to MgF2 may occur in the
melt. Na
and Mg are immiscible, and the separated metals may be hydrided with H2 gas,
preferably
from the electrolysis of H2O. The F2 gas may be reacted with NH3, preferably
in a copper-
packed reactor, to form NF3. Ammonia may be created from the Haber process.
Alternatively, NF3 may be formed by the electrolysis of NH4F in anhydrous HE
In exemplary hydrino and regeneration reactions, the solid fuel reaction
mixture
comprises NaH catalyst, MgH2, Na2S208 and support, activated carbon. In an
embodiment,
the source of exothermic reaction is the oxidation reaction of metal hydrides
by Na2S208 such
as
8MgH2 + Na2S2O8 -> 2MgS + 2NaOH + 6MgO + 6H2 (132)
7MgH2 +Na2S2O8 + C - 2MgS + Na2CO3 +5MgO+7H2 (133)
1ONaH + Na2S2O8 -+ 2Na2S + 8NaOH + H2 (134)
9NaH + Na2S2O8 + C - 2Na2S + Na2CO3 + 5NaOH + 2H2 . (135)
Any MgO product may be converted to the hydroxide by reaction with water
MgO+H20-Mg(OH)2. (136)
Sodium or magnesium carbonate, hydrogen carbonate, and other species
comprising carbon
and oxygen may be reduced with Na or NaH:
NaH+Na2CO3 -+3NaOH+C+1/H2 (137)
86
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
NaH+1/3MgC03 ->NaOH+1/3C+1/3Mg . (138)
MgS can be combusted in oxygen, hydrolyzed, exchanged with Na to form sodium
sulfate,
and electrolyzed to Na2S20s
2MgS +10H20 + 2NaOH -f Na2S2O8 + 2Mg(OH)2 + 9H2 . (139)
Na2S can be combusted in oxygen, hydrolyzed to sodium sulfate, and
electrolyzed to form
Na2S2Os
2Na2S +10H20 -> Na2S2O8 + 2NaOH + 9H2 (140)
Mg(OH)2 can be reduced to Mg using Na or NaH:
2Na +Mg (OH)2 -> 2Na OH +Mg . (141)
Then, NaOH can be electrolyzed to Na metal and NaH and 02 directly from the
melt, or it
may be converted to NaCl by reaction with HC1 wherein the NaCl electrolysis
gas C12 may be
reacted with H2 from the electrolysis of water to from the HC1.
In exemplary hydrino and regeneration reactions, the solid fuel reaction
mixture
comprises NaH catalyst, MgH2, S, and support, activated carbon. In an
embodiment, the
source of exothermic reaction is the oxidation reaction of metal hydrides by S
such as
MgH2 +S -~ MgS +H2 (142)
2NaH+S -+Na2S+H2. (143)
The magnesium sulfide may be converted to the hydroxide by reaction with water
MgS + 2H20 Mg (OH )2 +H2S . (144)
H2S may be decomposed at elevated temperature or used to covert SO2 to S.
Sodium sulfide
can be converted to the hydroxide by combustion and hydrolysis
Na2S + 1.502 - Na20 + SO2
Na20 + H2O - 2NaOH (145)
Mg(OH)2 can be reduced to Mg using Na or NaH:
2Na +Mg (OH)2 -> 2Na OH +Mg . (146)
Then, NaOH can be electrolyzed to Na metal and NaH and 02 directly from the
melt, or it
may be converted to NaCI by reaction with HCI wherein the NaCl electrolysis
gas C12 may be
reacted with H2 from the electrolysis of water to from the HCI. SO2 can be
reduced at
elevated temperature using H2
SO2 + 2H2S - 3S + 2H20. (147)
In embodiments, metals such as Na and Mg may be converted to the corresponding
hydrides
by reaction with H2, preferably from the electrolysis of water. In other
embodiments, the S
and metal may be regenerated by electrolysis from a melt.
In exemplary hydrino and regeneration reactions, the reaction mixture
comprises NaH
catalyst, MgH2, N20, and support, activated carbon. In an embodiment, the
source of
exothermic reaction is the oxidation reaction of metal hydrides by N20 such as
4MgH2 + N20 -f MgO + Mg3N2 + 4H2 (148)
NaH+3N20+C-NaHCO3+3N2+1/2H2. (149)
87
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
The MgO product may be converted to the hydroxide by reaction with water
MgO+H20-Mg(OH)2. (150)
Magnesium nitride may also be hydrolyzed to magnesium hydroxide:
Mg3N2 + 6H20 - 3Mg (OH )2 + 3H2 + N2 . (151)
Sodium carbonate, hydrogen carbonate, and other species comprising carbon and
oxygen
may be reduced with Na or NaH:
NaH+Na2CO3 -*3NaOH+C+1/H2. (152)
Mg(OH)2 can be reduced to Mg using Na or NaH:
2Na +Mg (OH )2 - 2NaOH +Mg . (153)
Then, NaOH can be electrolyzed to Na metal and NaH and 02 directly from the
melt, or it
may be converted to NaCI by reaction with HCI wherein the NaCI electrolysis
gas C12 may be
reacted with H2 from the electrolysis of water to from the HCI. Ammonia
created from the
Haber process is oxidized (Eq. (124)) and the temperature is controlled to
favor production of
N20 that is separated from other gasses of the steady state reaction product
mixture.
In exemplary hydrino and regeneration reactions, the reaction mixture
comprises NaH
catalyst, MgH2, C12, and support, such as activated carbon, WC or TiC. The
reactor may
further comprise a source of high-energy light, preferably ultraviolet light
to dissociate C12 to
initiate the hydrino reaction. In an embodiment, the source of exothermic
reaction is the
oxidation reaction of metal hydrides by C12 such as
2NaH +C12 -+ 2NaC1+H2 (154)
MgH2+C12 -+MgC12+H2. (155)
NaCI and MgC12 may be electrolyzed to C12, Na, and Mg from a molten salt. The
molten
NaCI electrolysis may be performed using a Downs cell or modified Downs cell.
The NaCl
for this electrolysis may be from the rinse of the reaction products with
aqueous solution.
The solution may be filtered to remove the support such as AC that may be
centrifuged and
dried, preferably using waste heat from the power system. Na and Mg are
immiscible, and
the separated metals may be hydrided with H2 gas, preferably from the
electrolysis of H20-
An exemplary result follows:
= 4g WC + 1g MgH2 + 1g NaH + 0.01mol C12 initiated with UV lamp to dissociate
C12 to Cl, Ein:162.9 U, dE:16.0 U, TSC: 23-42 C, Tmax: 85 C, theoretical is
7.10 kJ, gain
is 2.25 times.
The reactants comprising a catalyst or a catalyst source such as NaH, K, or Li
or their
hydrides, a reductant such as an alkaline metal or hydride, preferably Mg,
MgH2, or Al, and
an oxidant such as NF3 can be regenerated by electrolysis. Preferably, metal
fluoride
products are regenerated to metals and fluorine gas by electrolysis. The
electrolyte may
comprise a eutectic mixture. The mixture may further comprise HF. NF3 may be
regenerated
by the electrolysis of NH4F in anhydrous HF. In another embodiment, NH3 is
reacted with F2
in a reactor such as a copper-packed reactor. F2 may be generated by
electrolysis using a
88
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
dimensionally stable anode or a carbon anode using conditions that favor F2
production. SF6
may be regenerated by reaction of S with F2. Any metal nitride that may form
in the hydrino
reaction may be regenerated by at least one of thermal decomposition, H2
reduction,
oxidation to the oxide or hydroxide and reaction to the halide followed by
electrolysis, and
reaction with halogen gas during molten electrolysis of a metal halide. NC13
can be formed
by reaction of ammonia and chlorine gas or by reaction of ammonium salts such
as NH4CI
with chlorine gas. The chlorine gas may be from the electrolysis of chloride
salts such as
those from the product reaction mixture. The NH3 may be formed using the Haber
process
wherein the hydrogen may be from electrolysis, preferably of water. In an
embodiment, NC13
is formed in situ in the reactor by the reaction of at least one of NH3 and an
ammonium salt
such as NH4CI with C12 gas. In an embodiment, BiF5 can be regenerated by
reaction of BiF3
with F2 formed from electrolysis of metal fluorides.
In an embodiment wherein the a source of oxygen or halogen optionally serves
as a
reactant of an exothermic activation reaction, an oxide or halide product is
preferably
regenerated by electrolysis. The electrolyte may comprise a eutectic mixture
such as a
mixture of A1203 and Na3AJF6i MgF2, NaF, and HF; Na3AJF6i NaF, SiF4, and HF;
and AJF3,
NaF, and HE The electrolysis of SiF4 to Si and F2 may be from a alkali
fluoride eutectic
mixture. Since Mg and Na have low miscibility, they can be separated in phases
of the melts.
Since Al and Na have low miscibility, they can be separated in phases of the
melts. In
another embodiment, the electrolysis products can be separated by
distillation. In further
embodiment, Ti203 is regenerated by reaction with C and C12 to form CO and
TiC14 that is
further reacted with Mg to form Ti and MgC12. Mg and C12 may be regenerated by
electrolysis. In the case that MgO is the product, Mg can be regenerated by
the Pidgeon
process. In an embodiment, MgO is reacted with Si to form Si02 and Mg gas that
is
condensed. The product Si02 may be regenerated to Si by H2 reduction at high
temperature
or by reaction with carbon to form Si and CO and CO2. In another embodiment,
Si is
regenerated by electrolysis using a method such as the electrolysis of solid
oxides in molten
calcium chloride. In an embodiment, chlorate or perchlorate such as an alkali
chlorate or
perchlorate is regenerated by electrolytic oxidation. Brine may be
electrolytically oxidized to
chlorate and perchlorate.
To regenerate the reactants, any oxide coating on a metal support that may be
formed
may be removed by dilute acid following separation from the reactant or
product mixture. In
another embodiment, the carbide is generated from the oxide by reaction with
carbon with
release of carbon monoxide or dioxide.
In the case that the reaction mixture comprises a solvent, the solvent may be
separated
from other reactants or products to be regenerated by removing the solvent
using evaporation
or by filtration or centrifugation with retention of the solids. In the case
that other volatile
components such as alkali metals are present, they may be selectively removed
by heating to
89
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
a suitably elevated temperature such that they are evaporated. For example, a
metal such that
Na metal is collected by distillation and a support such as carbon remains.
The Na may be
rehydrided to NaH and returned to the carbon with solvent added to regenerate
the reaction
mixture. Isolated solids such as R-Ni may be regenerated separately as well.
The separated
R-Ni may be hydrided by exposure to hydrogen gas at a pressure in the range of
0.1 to 300
atm
The solvent may be regenerated in the case that it decomposes during the
catalyst
reaction to form hydrinos. For example, the decomposition products of DMF may
be
dimethylamine, carbon monoxide, formic acid, sodium formate, and formaldhyde.
In an
embodiment, dimethyl formamide is produced either with catalyzed reaction of
dimethyl
amine and carbon monoxide in methanol or the reaction of methyl formate with
dimethyl
amine. It may also be prepared by reacting dimethylamine with formic acid.
In an embodiment, an exemplary ether solvent may be regenerated from the
products
of the reaction mixture. Preferably, the reaction mixture and conditions are
chosen such that
reaction rate of ether is minimized relative to the rate to form hydrinos such
that any ether
degradation is insignificant relative to the energy produced from the hydrino
reaction. Thus,
ether may be added back as needed with the ether degradation product removed.
Alternatively, the ether and reaction conditions may be chosen such that the
ether reaction
product may be isolated and the ether regenerated.
An embodiment comprises at least one of the following: the HSA is a fluoride,
the
HSA is a metal, and the solvent is fluorinated. A metal fluoride may be a
reaction product.
The metal and fluorine gas may be generated by electrolysis. The electrolyte
may comprise
the fluoride such as NaF, MgF2, A1F3, or LaF3 and may additionally comprise at
least one
other species such as HF and other salts that lowers the melting point of the
fluoride, such as
those disclosed in U.S. Pat. No. 5,427,657. Excess HF may dissolve LaF3. The
electrodes
may be carbon such as graphite and may also form fluorocarbons as desired
degradation
products. In an embodiment, at least one of the metal or alloy, preferably
nanopowder,
coated with carbon such as carbon-coated Co, Ni, Fe, other transition metal
powders, or
alloys, and the metal-coated carbon, preferably nanopowder, such as carbon
coated with a
transition metal or alloy, preferably at least one of Ni, Co, Fe, and Mn
coated carbon,
comprise particles that are magnetic. The magnetic particles may be separated
from a
mixture such as a mixture of a fluoride such as NaF and carbon by using a
magnet. The
collected particles may be recycled as part of the reaction mixture to form
hydrinos.
In an embodiment, the catalyst or source of catalyst such as NaH and the
fluorinated
solvent is regenerated from the products comprising NaF by separation of the
products
followed by electrolysis. The method of isolation of NaF may be rinsing the
mixture with a
polar solvent with a low boiling point followed by one or more of filtration
and evaporation
to give NaF solid. The electrolysis may be molten-salt electrolysis. The
molten salt may be
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
a mixture such as eutectic mixture. Preferably, the mixture comprises NaF and
HF as
known in the art. Sodium metal and fluorine gas may be collected from the
electrolysis. Na
may be reacted with H to form NaH. Fluorine gas may be reacted with a
hydrocarbon to
form a fluorinated hydrocarbon that may serve as the solvent. HF fluorination
product can be
returned to the electrolysis mixture. Alternatively, a hydrocarbon and a
carbon product such
as benzene and graphitic carbon, respectively, can be fluorinated and returned
to the reaction
mixture. Carbon can be cracked to smaller fluorinated fragments with a lower
melting point
to serve as the solvent by methods known in the art. The solvent may comprise
a mixture.
The degree of fluorination can be used as a method to control the hydrogen
catalysis reaction
rate. In an embodiment, CF4 is produced by electrolysis of a molten fluoride
salt, preferably
an alkali fluoride, using a carbon electrode or by reaction of carbon dioxide
with fluorine gas.
Any CH4 and hydrocarbons products may also be fluorinated to CF4 and
fluorcarbons.
Suitable fluorinated HSA materials and methods to fluorinated carbon to form
said
HSA materials may those known in the art such as those disclosed in U.S. Pat.
No. 3,929,920,
U.S. Pat. No. 3,925,492, U.S. Pat. No. 3,925,263, and U.S. Pat. No. 4,886,921.
Further
methods comprise the preparation of poly-dicarbon monofluoride as disclosed in
U.S. Pat.
No. 4,139,474, a process for the continuous fluorination of carbon as
disclosed in U.S. Pat.
No. 4,447,663, a process for producing a graphite fluoride comprising mainly
polydicarbon
monofluoride represented by the formula (C2F)õ as disclosed in U.S. Pat. No.
4,423,261, a
process for preparing polycarbonmonofluoride as disclosed in U.S. Pat. No.
3,925,263, a
process for the preparation of graphite fluoride as disclosed in U.S. Pat. No.
3,872,032, a
process for preparing poly-dicarbon monofluoride as disclosed in U.S. Pat. No.
4,243,615, a
method for the preparation of graphite fluoride by contact reaction between
carbon and
fluorine gas as disclosed in U.S. Pat. No. 4,438,086, the synthesis of
fluorographite as
disclosed in U.S. Pat. No. 3,929,918 , the process for preparing
polycarbonmonofluoride as
disclosed in U.S. Pat. No. 3,925,492, and a mechanism for providing new
synthetic
approaches to graphite-fluorine chemistry as disclosed by Lagow et al., J. C.
S. Dalton, 1268
(1974) wherein the materials disclosed therein comprise the HSA materials. As
a kind of
material of reactors, Monel metal, nickel, steel, or copper may be employed in
consideration
of the corrosion by fluorine gas. The carbon materials include amorphous
carbons such as
carbon black, petroleum coke, petroleum pitch coke and charcoal, and
crystalline carbons
such as natural graphite, graphene, and artificial graphite, fullerene and
nanotubes, preferably
single-walled. Preferably Na does not intercalate into the carbon support or
form an
acetylide. Such carbon materials can be employed in various forms. In general
preferably,
the powdery carbon materials have an average particle size of not more than 50
microns, but
greater is suitable as well. In addition to the powdery carbon materials,
other forms are
suitable. The carbon materials may be in the form of blocks, spheres, bars and
fibers. The
91
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
reaction may performed in a reactor chosen from a fluidized bed-type reactor,
a rotary kiln-
type reactor and a tray tower-type reactor.
In another embodiment, the fluorinated carbon is regenerated using an
additive.
Carbon can also be fluorinated by inorganic reactants such as CoF3 outside of
the cell or in
situ. The reaction mixture may further comprise a source of inorganic
fluorinating reactant
such as one of Co, CoF, CoF2, and CoF3 that may be added to the reactor and
regenerated or
it may be formed during the operation of the cell from the reactant mixture to
form hydrinos
and possibly another reagent such as F2 gas with optionally a fluorination
catalytic metal such
as Pt or Pd. The additive may be NH3 that may form NH4F. At least one of
carbon and
hydrocarbon may react with NH4F to become fluorinated. In an embodiment, the
reaction
mixture further comprises HNaF2 that may react with carbon to fluorinate it.
The
fluorocarbon may be formed in situ or externally to the hydrino reactor. The
fluorocarbon
may serve as a solvent or HSA material.
In an embodiment wherein at least one of the solvent, support, or getter
comprises
fluorine, products comprise possibly carbon, in cases such that the solvent or
support is a
fluorinated organic, as well as fluorides of the catalyst metal such as NaHF2,
and NaF. This
is in addition to lower-energy hydrogen products such as molecular hydrino gas
that may be
vented or collected. Using F2, the carbon may be etched away as CF4 gas that
may be used as
a reactant in another cycle of the reaction to make power. The remaining
products of NaF
and NaHF2 may be electrolyzed to Na and F2. The Na may be reacted with
hydrogen to form
NaH and the F2 may be used to etch carbon product. The NaH, remaining NaF, and
CF4 may
be combined to run another cycle of the power-production reaction to form
hydrinos. In
other embodiments, Li, K, Rb, or Cs may replace Na.
VI. Other Liquid and Heterogeneous Fuel Embodiments
In the present disclosure a "liquid-solvent embodiment" comprises any reaction
mixture and the corresponding fuel comprising a liquid solvent such as a
liquid fuel and a
heterogeneous fuel.
In another embodiment comprising a liquid solvent, one of atomic sodium and
molecular NaH is provided by a reaction between a metallic, ionic, or
molecular form of Na
and at least one other compound or element. The source of Na or NaH may be at
least one of
metallic Na, an inorganic compound comprising Na such as NaOH, and other
suitable Na
compounds such as NaNH2, Na2CO3, and Na20, NaX (X is a halide), and NaH(s).
The other
element may be H, a displacing agent, or a reducing agent. The reaction
mixture may
comprise at least one of (1) a solvent, (2) a source of sodium such as at
least one of Na(m),
NaH, NaNH2, Na2CO3, Na20, NaOH, NaOH doped-R-Ni, NaX (X is a halide), and NaX
doped R-Ni, (3) a source of hydrogen such as H2 gas and a dissociator and a
hydride, (4) a
displacing agent such as an alkali or alkaline earth metal, preferably Li, and
(5) a reducing
92
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
agent such as at least one of a metal such as an alkaline metal, alkaline
earth metal, a
lanthanide, a transition metal such as Ti, aluminum, B, a metal alloy such as
AlHg, NaPb,
NaAl, LiAl, and a source of a metal alone or in combination with reducing
agent such as an
alkaline earth halide, a transition metal halide, a lanthanide halide, and
aluminum halide.
Preferably, the alkali metal reductant is Na. Other suitable reductants
comprise metal
hydrides such as LiBH4, NaBH4, LiA1H4, or NaA1H4. Preferably, the reducing
agent reacts
with NaOH to form a NaH molecules and a Na product such as Na, NaH(s), and
Na20. The
source of NaH may be R-Ni comprising NaOH and a reactant such as a reductant
to form
NaH catalyst such as an alkali or alkaline earth metal or the Al intermetallic
of R-Ni. Further
exemplary reagents are an alkaline or alkaline earth metal and an oxidant such
as AIX3,
MgX2, LaX3, CeX3, and TiXõ where X is a halide, preferably Br or I.
Additionally, the
reaction mixture may comprise another compound comprising a getter or a
dispersant such as
at least one of Na2CO3, Na3SO4, and Na3PO4 that may be doped into the
dissociator such as
R-Ni. The reaction mixture may further comprise a support wherein the support
may be
doped with at least one reactant of the mixture. The support may have
preferably a large
surface area that favors the production of NaH catalyst from the reaction
mixture. The
support may comprise at least one of the group of R-Ni, Al, Sn, A1203 such as
gamma, beta,
or alpha alumina, sodium aluminate (beta-aluminas have other ions present such
as Na' and
possess the idealized composition Na20.11A1203 ), lanthanide oxides such as
M203
(preferably M= La, Sm, Dy, Pr, Th, Gd, and Er), Si, silica, silicates,
zeolites, lanthanides,
transition metals, metal alloys such as alkali and alkali earth alloys with
Na, rare earth metals,
Si02-A1203 or Si02 supported Ni, and other supported metals such as at least
one of alumina
supported platinum, palladium, or ruthenium. The support may have a high
surface area and
comprise a high-surface-area (HSA) materials such as R-Ni, zeolites,
silicates, aluminates,
aluminas, alumina nanoparticles, porous A1203, Pt, Ru, or Pd/A1203, carbon, Pt
or Pd/C,
inorganic compounds such as Na2CO3, silica and zeolite materials, preferably Y
zeolite
powder, and carbon such as fullerene or nanotubes. In an embodiment, the
support such as
A1203 (and the A1203 support of the dissociator if present) reacts with the
reductant such as a
lanthanide to form a surface-modified support. In an embodiment, the surface
Al exchanges
with the lanthanide to form a lanthanide-substituted support. This support may
be doped with
a source of NaH molecules such as NaOH and reacted with a reductant such as a
lanthanide.
The subsequent reaction of the lanthanide-substituted support with the
lanthanide will not
significantly change it, and the doped NaOH on the surface can be reduced to
NaH catalyst
by reaction with the reductant lanthanide.
In an embodiment comprising a liquid solvent, wherein the reaction mixture
comprises a source of NaH catalyst, the source of NaH may be an alloy of Na
and a source of
hydrogen. The alloy may comprise at least one of those known in the art such
as an alloy of
sodium metal and one or more other alkaline or alkaline earth metals,
transition metals, Al,
93
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
Sn, Bi, Ag, In, Pb, Hg, Si, Zr, B, Pt, Pd, or other metals and the H source
may be H2 or a
hydride.
The reagents such as the source of NaH molecules, the source of sodium, the
source
of NaH, the source of hydrogen, the displacing agent, and the reducing agent
are in any
desired molar ratio. Each is in a molar ratio of greater than 0 and less than
100%. Preferably,
the molar ratios are similar.
In a liquid-solvent embodiment, the reaction mixture comprises at least one
species of
the group comprising a solvent, Na or a source of Na, NaH or a source of NaH,
a metal
hydride or source of a metal hydride, a reactant or source of a reactant to
form a metal
hydride, a hydrogen dissociator, and a source of hydrogen. The reaction
mixture may further
comprise a support. A reactant to form a metal hydride may comprise a
lanthanide, preferably
La or Gd. In an embodiment, La may reversibly react with NaH to form LaHõ
(n=1,2,3). In
an embodiment, the hydride exchange reaction forms NaH catalyst. The
reversible general
reaction may be given by
NaH+M,;:tNa +MH (156)
The reaction given by Eq. (156) applies to other MH -type catalysts given in
TABLE 3. The
reaction may proceed with the formation of hydrogen that may be dissociated to
form atomic
hydrogen that reacts with Na to form NaH catalyst. The dissociator is
preferably at least one
of Pt, Pd, or Ru/A1203 powder, Pt/Ti, and R-Ni. Preferentially, the
dissociator support such
as A1203 comprises at least surface La substitution for Al or comprises Pt,
Pd, or Ru/M203
powder wherein M is a lanthanide. The dissociator may be separated from the
rest of the
reaction mixture wherein the separator passes atomic H.
A suitable liquid-solvent embodiment comprises the reaction mixture of a
solvent,
NaH, La, and Pd on A1203 powder wherein the reaction mixture may be
regenerated in an
embodiment by removing the solvent, adding H2, separating NaH and lanthanum
hydride by
sieving, heating lanthanum hydride to form La, and mixing La and NaH.
Alternatively, the
regeneration involves the steps of separating Na and lanthanum hydride by
melting Na and
removing the liquid, heating lanthanum hydride to form La, hydriding Na to
NaH, mixing La
and NaH, and adding the solvent. The mixing of La and NaH may be by ball
milling.
In a liquid-solvent embodiment, a high-surface-area material such as R-Ni is
doped
with NaX (X=F, Cl, Br, I). The doped R-Ni is reacted with a reagent that will
displace the
halide to form at least one of Na and NaH. In an embodiment, the reactant is
at least an alkali
or alkaline earth metal, preferably at least one of K, Rb, Cs. In another
embodiment, the
reactant is an alkaline or alkaline earth hydride, preferably at least one of
KH, RbH, CsH,
MgH2 and CaH2. The reactant may be both an alkali metal and an alkaline earth
hydride.
The reversible general reaction may be given by
NaX+MH; NaH+MX (157)
94
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
A. NaOH Catalyst Reactions to Form NaH Catalyst
The reaction of NaOH and Na to Na2O and NaH is
NaOH+2Na -> Na20+NaH (158)
The exothermic reaction can drive the formation of NaH(g). Thus, Na metal can
serve as a
reductant to form catalyst NaH(g). Other examples of suitable reductants that
have a similar
highly exothermic reduction reaction with the NaH source are alkali metals,
alkaline earth
metals such as at least one of Mg and Ca, metal hydrides such as LiBH4, NaBH4,
LiA1H4, or
NaA1H4, B, Al, transition metals such as Ti, lanthanides such as at least one
of La, Sm, Dy,
Pr, Tb, Gd, and Er, preferably La, Th, and Sm. Preferably, the reaction
mixture comprises a
solvent, a high-surface-area material (HSA material) having a dopant such as
NaOH
comprising a source of NaH catalyst. Preferably, conversion of the dopant on
the material
with a high surface area to the catalyst is achieved. The conversion may occur
by a reduction
reaction. In addition to Na, other preferred reductants are other alkali
metals, Ti, a
lanthanide, or Al. Preferably, the reaction mixture comprises NaOH doped into
a HSA
material preferably R-Ni wherein the reductant is Na or the intermetallic Al.
The reaction
mixture may further comprise a source of H such as a hydride or H2 gas and a
dissociator. In
certain embodiments, the H source is hydrided R-Ni.
In a liquid-solvent embodiment, Na20 formed as a product of a reaction to
generate
NaH catalyst such as that given by Eq. (158), is reacted with a source of
hydrogen to form
NaOH that can further serve as a source of NaH catalyst. In an embodiment, a
regenerative
reaction of NaOH from Eq. (158) in the presence of atomic hydrogen is
Na2O + H ->NaOH +Na All = -11.6 k1 / mole NaOH (159)
NaH->Na+H(1/3) AH=-10,500k1/moleH (160)
and
NaH->Na+H(1/4) AH=-19,700kJ/moleH. (161)
Thus, a small amount of NaOH and Na with a source of atomic hydrogen or atomic
hydrogen serves as a catalytic source of the NaH catalyst, that in turn forms
a large yield of
hydrinos via multiple cycles of regenerative reactions such as those given by
Eqs. (158-161).
In an embodiment, from the reaction given by Eq. (162), Al(OH)3 can serve as a
source of
NaOH and NaH wherein with Na and H, the reactions given by Eqs. (158-161)
proceed to
form hydrinos.
3Na+Al(OH)3 -NaOH+NaA102+NaH+1/2H2. (162)
In a liquid-solvent embodiment, the Al of the intermetallic serves as the
reductant to form
NaH catalyst The balanced reaction is given by
3NaOH + 2A1-> A1203 + 3NaH (163)
This exothermic reaction can drive the formation of NaH (g) to drive the very
exothermic
reaction given by Eqs. (25-30) wherein the regeneration of NaH occurs from Na
in the
presence of atomic hydrogen.
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
Two suitable liquid-solvent embodiments comprise the first reaction mixture of
Na
and R-Ni comprising about 0.5 wt% NaOH wherein Na serves as the reductant and
a second
reaction mixture of R-Ni comprising about 0.5 wt% NaOH wherein intermetallic
Al serves as
the reductant. The reaction mixture may be regenerated by adding NaOH and NaH
that may
serve as an H source and a reductant.
In a liquid-solvent embodiment, of the energy reactor, the source of NaH such
as
NaOH is regenerated by addition of a source of hydrogen such as at least one
of a hydride
and hydrogen gas and a dissociator. The hydride and dissociator may be
hydrided R-Ni. In
another embodiment, the source of NaH such as NaOH-doped R-Ni is regenerated
by at least
one of rehydriding, addition of NaH, and addition of NaOH wherein the addition
may be by
physical mixing. With the solvent first removed, the mixing may be performed
mechanically
by methods such as by ball milling.
In a liquid-solvent embodiment, the reaction mixture further comprises oxide-
forming
reactants that react with NaOH or Na20 to form a very stable oxide and NaH.
Such reactants
comprises a cerium, magnesium, lanthanide, titanium, or aluminum or their
compounds such
as AiX3, MgX2, LaX3, CeX3, and TiXõ where X is a halide, preferably Br or I
and a reducing
compound such as an alkali or alkaline earth metal. In an embodiment, the
source of NaH
catalyst comprises R-Ni comprising a sodium compound such as NaOH on its
surface. Then,
the reaction of NaOH with the oxide-forming reactants such as AiX3, MgX2,
LaX3, CeX3, and
TiX, and alkali metal M forms NaH, MX, and A1203, MgO, La203, Ce203, and
Ti203,
respectively.
In a liquid-solvent embodiment, the reaction mixture comprises NaOH doped R-Ni
and an alkaline or alkaline earth metal added to form at least one of Na and
NaH molecules.
The Na may further react with H from a source such as H2 gas or a hydride such
as R-Ni to
form NaH catalyst. The subsequent catalysis reaction of NaH forms H states
given by Eq.
(35). The addition of an alkali or alkaline earth metal M may reduce Na' to Na
by the
reactions:
NaOH + M to MOH + Na (164)
2NaOH + M to M(OH)2 + 2Na. (165)
M may also react with NaOH to form H as well as Na
2NaOH + M to Na20 + H2 + MO (166)
Na20 + M to M20 + 2Na. (167)
Then, the catalyst NaH may be formed by the reaction
Na + H to NaH (168)
by reacting with H from reactions such as that given by Eq. (166) as well as
from R-Ni and
any added source of H. Na is a suitable reductant since it is a further source
of NaH.
Hydrogen may be added to reduce NaOH and form NaH catalyst:
NaOH + H2 to NaH + H2O. (169)
96
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
The H in R-Ni may reduce NaOH to Na metal, and water that may be removed by
pumping.
An organic solvent may first be removed before the reduction or a molten
inorganic solvent
may be used.
In a liquid-solvent embodiment, the reaction mixture comprises one or more
compounds that react with a source of NaH to form NaH catalyst. The source may
be NaOH.
The compounds may comprise at least one of a LiNH2, Li2NH, and Li3N. The
reaction
mixture may further comprise a source of hydrogen such as H2. In embodiments,
the reaction
of sodium hydroxide and lithium amide to form NaH and lithium hydroxide is
NaOH+LiNH2 -*LiOH+NaH+1/2N2+LiH. (170)
The reaction of sodium hydroxide and lithium imide to form NaH and lithium
hydroxide is
NaOH+Li2NH -*Li2O+NaH+1/2N2+1/2H2. (171)
And, the reaction of sodium hydroxide and lithium nitride to form NaH and
lithium oxide is
NaOH+Li3N -*Li20+NaH+1/2N2 +Li. (172)
B. Alkaline Earth Hydroxide Catalyst Reactions to Form NaH Catalyst
In a liquid-solvent embodiment, a source of H is provided to a source of Na to
form
the catalyst NaH. The Na source may be the metal. The source of H may be a
hydroxide.
The hydroxide may be at least one of alkali, alkaline earth hydroxide, a
transition metal
hydroxide, and Al(OH)3. In an embodiment, Na reacts with a hydroxide to form
the
corresponding oxide and NaH catalyst. In an embodiment wherein the hydroxide
is
Mg(OH)2, the product is MgO. In an embodiment wherein the hydroxide is
Ca(OH)2, the
product is CaO. Alkaline earth oxides may be reacted with water to regenerate
the
hydroxide. The hydroxide can be collected as a precipitate by methods such as
filtration and
centrifugation.
For example, in an embodiment, the reaction to form NaH catalyst and
regeneration
cycle for Mg(OH)2, are given by the reactions:
3Na+Mg(OH)2 -*2NaH+MgO+Na2O (173)
MgO+H20 -+Mg(OH)2. (174)
In a liquid-solvent embodiment, the reaction to form NaH catalyst and
regeneration
cycle for Ca(OH)2, are given by the reactions:
4Na+Ca(OH)2 -*2NaH+CaO+Na20 (175)
CaO + H2O -> Ca (OH)2' (176)
C. Na/N Alloy Reactions to Form NaH Catalyst
Alkali metal in the solid and liquid states is a metal. In order to generate M
or MH
catalyst, M is an alkali metal, the reaction mixture of the liquid or
heterogeneous fuel
97
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
comprises M/N alloy reactants. In an embodiment, the reaction mixture, liquid-
fuel
reactions, heterogeneous-fuel reactions, and regeneration reactions comprise
those of the
M/N system, wherein the fuel generates at least one of the catalyst and atomic
hydrogen.
In an embodiment, the reaction mixture comprises one or more compounds that
react
with a source of NaH to form NaH catalyst. The reaction mixture may comprise
at least one
of the group of Na, NaH, NaNH2, Na2NH, Na3N, NH3, a dissociator, a hydrogen
source such
as H2 gas or a hydride, a support, and a getter such as NaX (X is a halide).
The dissociator is
preferably Pt, Ru, or Pd/Al203 powder. The dissociator may comprise Pt or Pd
on a high
surface area support suitably inert to Na. The dissociator may be Pt or Pd on
carbon or
Pd/A1203. The latter support may comprise a protective surface coating of a
material such as
NaAlO2. The reactants may be present in any wt%.
A suitable liquid-solvent embodiment comprises the reaction mixture of a
solvent, Na
or NaH, NaNH2, and Pd on A1203 powder wherein the reaction mixture may be
regenerated
by addition of H2.
In an embodiment, NaNH2 is added to the reaction mixture. NaNH2 generates NaH
according to the reversible reactions
Na2 +NaNH2 -> NaH +Na2NH (177)
and
2NaH+NaNH2 -NaH(g)+Na2NH+H2. (178)
In the hydrino reaction cycle, Na-Na and NaNH2 react to form NaH molecule and
Na2NH, and the NaH forms hydrino and Na. Thus, the reaction is reversible
according to the
reactions:
Na2NH +H2 - NaNH2 +NaH (179)
and
Na2NH + Na + H - NaNH2 + Na2 . (180)
In an embodiment, NaH of Eq. (179) is molecular such that this reaction is
another to
generate the catalyst.
The reaction of sodium amide and hydrogen to form ammonia and sodium hydride
is
H2 +NaNH2 - NH3 +NaH . (181)
In a liquid-solvent embodiment, this reaction is reversible. The reaction can
be driven to
form NaH by increasing the H2 concentration. Alternatively, the forward
reaction can be
driven via the formation of atomic H using a dissociator. The reaction is
given by
2H + NaNH2 -> NH3 + NaH. (182)
The exothermic reaction can drive the formation of NaH(g).
In a liquid-solvent embodiment, NaH catalyst is generated from a reaction of
NaNH2
and hydrogen, preferably atomic hydrogen as given in reaction Eqs. (181-182).
The ratios of
reactants may be any desired amount. Preferably the ratios are about
stoichiometric to those
of Eqs. (181-182). The reactions to form catalyst are reversible with the
addition of a source
98
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
of H such as H2 gas or a hydride to replace that reacted to form hydrinos
wherein the catalyst
reactions are given by Eqs. (25-30), and sodium amide forms with additional
NaH catalyst by
the reaction of ammonia with Na:
NH3 + Na2 -> NaNH2 + NaH. (183)
In a liquid-solvent embodiment, a HSA material is doped with NaNH2. The doped
HSA material is reacted with a reagent that will displace the amide group to
form at least one
of Na and NaH. In an embodiment, the reactant is an alkali or alkaline earth
metal,
preferably Li. In another embodiment, the reactant is an alkaline or alkaline
earth hydride,
preferably LiH. The reactant may be both an alkali metal and an alkaline earth
hydride. A
source of H such as H2 gas may be further provided in addition to that
provided by any other
reagent of the reaction mixture such as a hydride, HSA material, and
displacing reagent.
In a liquid-solvent embodiment, sodium amide undergoes reaction with lithium
to
form lithium amide, imide, or nitride and Na or NaH catalyst. The reaction of
sodium amide
and lithium to form lithium imide and NaH is
2Li +NaNH2 ---> Li2NH +NaH . (184)
The reaction of sodium amide and lithium hydride to form lithium amide and NaH
is
LiH + NaNH2 -> LiNH2 + NaH . (185)
The reaction of sodium amide, lithium, and hydrogen to form lithium amide and
NaH is
Li+1/2H2+NaNH2 --->LiNH2+NaH. (186)
In a liquid-solvent embodiment, the reaction of the mixture forms Na, and the
reactants
further comprise a source of H that reacts with Na to form catalyst NaH by a
reaction such as
the following:
Li + NaNH2 to LiNH2 + Na (187)
and
Na + H to NaH (188)
LiH + NaNH2 to LiNH2 + NaH. (189)
In a liquid-solvent embodiment, the reactants comprise NaNH2, a reactant to
displace
the amide group of NaNH2 such as an alkali or alkaline earth metal, preferably
Li, and may
additionally comprise a source of H such as at least one of MH (M=Li, Na, K,
Rb, Cs, Mg,
Ca, Sr, and Ba), H2 and a hydrogen dissociator, and a hydride.
The reagents of the reaction mixture such as solvent, M, MH, NaH, NaNH2, HSA
material, hydride, and the dissociator are in any desired molar ratio. Each of
M, MH, NaNH2,
and the dissociator are in molar ratios of greater than 0 and less than 100%,
preferably the
molar ratios are similar.
Other embodiments of liquid-solvent systems to generate molecular catalyst NaH
involve Na and NaBH4 or NH4X (X is an anion such as halide). Molecular NaH
catalyst can
be generated by reaction of Nat and NaBH4:
Nat + NaBH4 to NaBH3 + Na + NaH. (190)
99
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
NH4X can generate NaNH2 and H2
Nat + NH4X to NaX + NaNH2 + H2. (191)
Then, NaH catalyst can be generated according to the reaction of Eqs. (177-
189). In
another liquid-solvent embodiment, the reaction mechanism for the Na/N system
to form
hydrino catalyst NaH is
NH4X + Na-Na to NaH + NH3 + NaX. (192)
D. Additional MH-Type Catalysts and Reactions
Another catalytic system of the type MH involves aluminum. The bond energy of
AIH is 2.98 eV. The first and second ionization energies of Al are 5.985768 eV
and
18.82855 eV, respectively. Based on these energies AIH molecule can serve as a
catalyst and
H source since the bond energy of AIH plus the double ionization (t = 2) of Al
to A12+, is
27.79 eV (27.2 eV) which is equivalent to m =1 in Eq. (36). The catalyst
reactions are given
by
+[(2)2 _12].13.6 eV (193)
27.79 eV +A1H -> A12++2e +H (2)
A12+ + 2e- +H A1H + 27.79 eV, and (194)
the overall reaction is
H _>H [ti]+[(2)2 _12].13.6 eV. (195)
In a liquid-solvent embodiment, the reaction mixture comprises at least one of
AIH
molecules and a source of AIH molecules. A source of AIH molecules may
comprise Al
metal and a source of hydrogen, preferably atomic hydrogen. The source of
hydrogen may
be a hydride, preferably R-Ni. In another embodiment, the catalyst AIH is
generated by the
reaction of an oxide or hydroxide of Al with a reductant. The reductant
comprises at least
one of the NaOH reductants given previously. In an embodiment, a source of H
is provided
to a source of Al to form the catalyst AIH. The Al source may be the metal.
The source of H
may be a hydroxide. The hydroxide may be at least one of alkali, alkaline
earth hydroxide, a
transition metal hydroxide, and AI(OH)3-
Raney nickel can be prepared by the following two reaction steps:
Ni + 3Al -* NiA13 (or Ni2Al3) (196)
NiA1,, (skeleton, porous Ni)
NiAl3 + 2NaOH + 6HZO (197)
+2Na[Al(OH )4 ] + 3H2
Na[Al(OH)4] is readily dissolved in concentrated NaOH. It can be washed in de-
oxygenated
water. The prepared Ni contains Al (-10 wt%, that may vary), is porous, and
has a large
surface area. It contains large amounts of H, both in the Ni lattice and in
the form of Ni-AIHX
(x=1,2,3).
R-Ni may be reacted with another element to cause the chemical release of AIH
100
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
molecules which then undergo catalysis according to reactions given by Eqs.
(193-195). In
an embodiment, the A1H release is caused by a reduction reaction, etching, or
alloy
formation. One such other element M is an alkali or alkaline earth metal which
reacts with
the Ni portion of R-Ni to cause the A1HX component to release A1H molecules
that
subsequently under go catalysis. In an embodiment, M may react with Al
hydroxides or
oxides to form Al metal that may further react with H to form A1H. The
reaction can be
initiated by heating, and the rate may be controlled by controlling the
temperature. Solvent,
M (alkali or alkaline earth metal), and R-Ni are in any desired molar ratio.
Each of solvent,
M, and R-Ni are in molar ratios of greater than 0 and less than 100%.
Preferably the molar
ratio of M and R-Ni are similar.
In a liquid-solvent embodiment, the source of A1H comprises R-Ni and other
Raney
metals or alloys of Al known in the art such as R-Ni or an alloy comprising at
least one of Ni,
Cu, Si, Fe, Ru, Co, Pd, Pt, and other elements and compounds. The R-Ni or
alloy may
further comprise promoters such as at least one of Zn, Mo, Fe, and Cr. The R-
Ni may be at
least one of W. R. Grace Raney 2400, Raney 2800, Raney 2813, Raney 3201, Raney
4200, or
an etched or Na doped embodiment of these materials. In another liquid-solvent
embodiment
of the A1H catalyst system, the source of catalyst comprises a Ni/Al alloy
wherein the Al to
Ni ratio is in the range of about 10-90%, preferably about 10-50%, and more
preferably about
10-30%. The source of catalyst may comprise palladium or platinum and further
comprise Al
as a Raney metal.
Another catalytic system of the type MH involves chlorine. The bond energy of
HCl
is 4.4703 eV. The first, second, and third ionization energies of Cl are
12.96764 eV, 23.814
eV, and 39.61 eV, respectively. Based on these energies HCl can serve as a
catalyst and H
source since the bond energy of HCl plus the triple ionization (t = 3) of Cl
to Cl", is 80.86
eV (3.27.2 eV ) which is equivalent to m = 3 in Eq. (36). The catalyst
reactions are given by
80.86 eV + HCI _> C13+ + 3e- + H [-~H) + [(4) 2 _121 .13.6 eV (198)
C13+ + 3e- + H - HC1 + 80.86 eV , and (199)
the overall reaction is
H _ H (4) + [(4) 2 _121 .13.6 eV. (200)
In a liquid-solvent embodiment, the reaction mixture comprises HCl or a source
of
HCI. A source may be NH4C1 or a solid acid and a chloride such as an alkali or
alkaline earth
chloride. The solid acid may be at least one of MHSO4, MHCO3, MH2PO4, and
MHPO4
wherein M is a cation such as an alkali or alkaline earth cation. Other such
solid acids are
known to those skilled in the art. In an embodiment, the reaction mixture
comprises a strong
acid such as H2SO4 and an ionic compound such as NaCl. The reaction of the
acid with the
ionic compound such as NaCl generates HCl to serve as a hydrino catalyst and H
source.
101
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
In general, MH type hydrogen catalysts to produce hydrinos provided by the
breakage
of the M-H bond plus the ionization of t electrons from the atom M each to a
continuum
energy level such that the sum of the bond energy and ionization energies of
the t electrons is
approximately in .27.2 eV where m is an integer are given in TABLE 3. Each MH
catalyst
is given in the first column and the corresponding M-H bond energy is given in
column two.
The atom M of the MH species given in the first column is ionized to provide
the net
enthalpy of reaction of m = 27.2 eV with the addition of the bond energy in
column two. The
enthalpy of the catalyst is given in the eighth column where m is given in the
ninth column.
The electrons that participate in ionization are given with the ionization
potential (also called
ionization energy or binding energy). For example, the bond energy of NaH ,
1.9245 eV , is
given in column two. The ionization potential of the nth electron of the atom
or ion is
designated by IP and is given by the CRC. That is for example,
Na + 5.13908 eV -+ Na+ + e- and Na' + 47.2864 eV - Na 2+ + e-. The first
ionization
potential, IP, = 5.13908 eV, and the second ionization potential, IP2 =
47.2864 eV , are
given in the second and third columns, respectively. The net enthalpy of
reaction for the
breakage of the NaH bond and the double ionization of Na is 54.35 eV as given
in the
eighth column, and m = 2 in Eq. (36) as given in the ninth column.
Additionally, H can react
with each of the MH molecules given in TABLE 3 to form a hydrino having a
quantum
number p increased by one (Eq. (35)) relative to the catalyst reaction product
of MH alone as
given by exemplary Eq. (23).
102
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
TABLE 3. MH type hydrogen catalysts capable of providing a net enthalpy of
reaction of
approximately m .27.2 eV.
Catalyst M-H IP1 IP2 IP3 IP4 IP5 Enthalpy m
Bond
Energy
AIH 2.98 5.985768 18.82855 27.79 1
BiH 2.936 7.2855 16.703 26.92 1
CIH 4.4703 12.96763 23.8136 39.61 80.86 3
CoH 2.538 7.88101 17.084 27.50 1
GeH 2.728 7.89943 15.93461 26.56 1
InH 2.520 5.78636 18.8703 27.18 1
NaH 1.925 5.139076 47.2864 54.35 2
RuH 2.311 7.36050 16.76 26.43 1
SbH 2.484 8.60839 16.63 27.72 1
SeH 3.239 9.75239 21.19 30.8204 42.9450 107.95 4
SiH 3.040 8.15168 16.34584 27.54 1
SnH 2.736 7.34392 14.6322 30.50260 55.21 2
103
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
In other liquid-solvent embodiments of the MH type catalyst, the reactants
comprise
sources of SbH, SiH, SnH, and InH. In embodiments providing the catalyst MH,
the sources
comprise at least one of M and a source of H2 and MH,, such as at least one of
Sb, Si, Sn, and
In and a source of H2, and SbH3,, SiH4, SnH4,and InH3.
The liquid-solvent reaction mixture may further comprise a source of H and a
source
of catalyst wherein the source of at least one of H and catalyst may be a
solid acid or NH4X
where X is a halide, preferably Cl to form HCl catalyst. Preferably, the
reaction mixture may
comprise at least one of solvent, NH4X, a solid acid, NaX, LiX, KX, NaH, LiH,
KH, Na, Li,
K, a support, a hydrogen dissociator and H2 where X is a halide, preferably
Cl. The solid
acid may be NaHSO4, KHSO4, LiHSO4, NaHCO3, KHCO3, LiHCO3, Na2HPO4, K2HPO4,
Li2HPO4, NaH2PO4, KH2PO4, and LiH2PO4. The catalyst may be at least one of
NaH, Li, K,
and HCI. The reaction mixture may further comprise at least one of a
dissociator and a
support.
In each case of a source of MH comprising an M alloy such as AIH and Al,
respectively, the alloy may be hydrided with a source of H2 such as H2 gas. H2
can be
supplied to the alloy during the reaction, or H2 may be supplied to form the
alloy of a desired
H content with the H pressure changed during the reaction. In this case, the
initial H2
pressure may be about zero. The alloy may be activated by the addition of a
metal such as an
alkali or alkaline earth metal. For MH catalysts and sources of MH, the
hydrogen gas may be
maintained in the range of about 1 Torr to 100 atm, preferably about 100 Torr
to 10 atm,
more preferably about 500 Torr to 2 atm. In other embodiments, the source of
hydrogen is
from a hydride such as an alkali or alkaline earth metal hydride or a
transition metal hydride.
Atomic hydrogen in high density can undergo three-body-collision reactions to
form
hydrinos wherein one H atom undergoes the transition to form states given by
Eq. (35) when
two additional H atoms ionize. The reaction are given by
27.21 eV+2H[a1]+H[a1]--> 2H++2e-+H a2) +[(2)2_12].13.6eV (201)
2H+ + 2e- -+ 2H [aH ] + 27.21 eV, and (202)
the overall reaction is
H[a, H [-~H-] ) +[(2)2 _12.13.6 eV. (203)
In another embodiment, the reaction are given by
54.4 eV + 2H [aH ] + H [aH ] -* 2H fast + 2e- + H )+[(3) 2 _12] .13.6 eV (204)
ast + 2e- -* 2H IaH ] + 54.4 eV , and (205)
2H+
the overall reaction is
H [a, H )+ [(3) 2 _121. 13.6 eV. (206)
104
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
In a liquid-solvent embodiment, the material that provides H atoms in high
density is
R-Ni. The atomic H may be from at least one of the decomposition of H within R-
Ni and the
dissociation of H2 from an H2 source such as H2 gas supplied to the cell. R-Ni
may be
reacted with an alkali or alkaline earth metal M to enhance the production of
layers of atomic
H to cause the catalysis. R-Ni isolated from the solvent mixture can be
regenerated by
evaporating the metal M followed by addition of hydrogen to rehydride the R-
Ni.
VII. Additional H Auto-Catalyst Reactions
In another catalyst reaction involving solely H atoms, two hot H2 molecules
collide
and dissociate such that three H atoms serve as a catalyst of 3.27.2 eV for
the fourth.
Then, the reaction between four hydrogen atoms whereby three atoms resonantly
and
nonradiatively accept 81.6 eV from the fourth hydrogen atom such that 3H
serves as the
catalyst is given by r l
81.6 eV+3H+H->3Hfu51+3e-+H 4 +[42_12].13.6eV (207)
3Hfasl +3e- -+ 3H +81.6 eV, and (208)
the overall reaction is
H --> H[ 4 ]+[42 _12].13.6 eV. (209)
The extreme-ultraviolet continuum radiation band due to the H a intermediate
of Eq.
3+1
(207) is predicted to have short wavelength cutoff at 122.4 eV (10.1 nm) and
extend to
longer wavelengths.
In general, the transition of H to H aH due by the acceptance of
p=m+1
m = 27.2 eV gives a continuum band with a short wavelength cutoff at energy E
ll
of
( p=m+1
given by
Er l = m2.13.6 eV corresponding to 91.22 nm (210)
H-->H[ ay Il m
l p=m+1 Jl
and extends to longer wavelengths than the corresponding cutoff.
Another catalyst reaction involving a collision of hot H with H2 can occur
wherein
each of two of the H atoms accept 13.6 eV to from the third to become ionized
to serve as a
catalyst of 27.2 eV for the third. Then, the reaction between the hydrogen
atoms whereby
two atoms resonantly and nonradiatively accept 27.2 eV from the third hydrogen
atom such
that 2H serves as the catalyst is given by rr l
27.2 eV+2H+H-> 2H++2e-+HLa-"J+[22_12].13.6 eV (211)
2
105
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
2H+ + 2e- 2H + 27.2 eV, and (212)
the overall reaction is
H_H[ 2 J+[22-12]=13.6 eV. (213)
The extreme-ultraviolet continuum radiation band due to the H * a intermediate
of Eq.
1+1
(211) is predicted to have short wavelength cutoff at 13.6 eV (91.2 nm) and
extend to longer
wavelengths. High densities are permissive of another reaction to give the
91.2 continuum
band wherein a H atom serves as a catalyst by accepting 27.2 eV from a second
hydrogen
atom.
In the presence of a high field, an ionized electron can transition to a
fractional state
directly with the binding energy released as a continuum band with a short
wavelength cutoff
at the binding energy of the final-state hydrino atom. The transitions for H
(1 / 2) and
H (1 / 3) are given by l
H' +e- H[ 4+ [ 22_ 0 2 ]. 1 3 . 6 4 l+[22-02].13.6 eV (214)
H++e-H[ 4 l+[32-02].13.6 eV. (215)
The extreme-ultraviolet continuum radiation bands are predicted to have short
wavelength
cutoffs at 54.4 eV (22.8 nm) and 122.4 eV (10.1 nm), respectively, and extend
to longer
wavelengths. Due to the multipolarity and corresponding selection rules H (1 /
4) is a prefer
state. The extreme-ultraviolet continuum radiation is predicted to have a
short wavelength
cutoff at 217.6 eV (5.7 nm) and extending to longer wavelengths.
The ionization potential of molecular hydrino H2 (1/ p) is
IP, =E7. (HZ (1/ p))-ET (H2 (1/p))
= -p216.13392 eV - p30.118755 eV - (-p231.351 eV _P3 0.326469 eV) (216)
= p215.2171 eV +p30.207714 eV
The molecular hydrino H2 (1 / p) bond energy, Eõ , is given by:
Eõ =-P 2 27.20 eV -ET
= -p2 27.20 eV - (-p2 31.351 eV - p3 0.326469 eV) (217)
= p24.151 eV +p30.326469 eV
Another aspect of the present disclosure comprises a light source of EUV
radiation. The light
source comprises molecular hydrino gas and a component to excite molecular
hydrino gas to
the ionization threshold. The de-excitation energy is given by Eq. (216). The
excitation may
be with a particle beam, preferably an electron beam. The molecular hydrino
gas may be
trapped in a matrix, preferably an alkali or alkaline earth halide crystal.
The crystal may be
bombarded with an electron beam at high energy such as about 12 kV to cause
the excitation
106
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
followed by de-excitation emission. In another embodiment, the de-excitation
further results
in the breaking of the molecular hydrino bond. The emitted energy is then
given by the
difference in the energies given by Eqs. (216) and (217):
Eemission = p211.0661 eV - p30.118755 eV. (218)
For p=4, the radiation is 7.3 nm (169.5 eV), which is in the extreme
ultraviolet (EUV). This
light could be for EUV lithography to make microelectronic devices.
In embodiments disclose herein, at least one of a source of Rb+ such as Rb or
hydride
or a source of Cs such as Cs metal or hydride may serve as a source of Rb+ or
Cs catalyst,
respectively.
The hydrino hydride ion may react with an oxidant such as oxygen or sulfur to
form
molecular hydrino. Exemplary reactions are
2H-(1/p)+S-4H,(1/p)+S2- (219)
2H- (1/ p)+02 H2 (1/ p)+O2 . (220)
Thus, in an embodiment of a hydrino chemical reaction, the hydrino hydride may
be
converted to molecular hydrino when it is the desired product.
VIII. Hydrogen Gas Discharge Power and Plasma Cell and Reactor
A hydrogen gas discharge power and plasma cell and reactor of the present
disclosure
is shown in FIGURE 5. The hydrogen gas discharge power and plasma cell and
reactor of
FIGURE 5, includes a gas discharge cell 307 comprising a hydrogen gas-filled
glow
discharge vacuum vessel 315 having a chamber 300. A hydrogen source 322
supplies
hydrogen to the chamber 300 through control valve 325 via a hydrogen supply
passage 342.
A catalyst is contained in the cell chamber 300. A voltage and current source
330 causes
current to pass between a cathode 305 and an anode 320. The current may be
reversible.
In an embodiment, the material of cathode 305 may be a source of catalyst such
as Fe,
Dy, Be, or Pd. In another embodiment of the hydrogen gas discharge power and
plasma cell
and reactor, the wall of vessel 313 is conducting and serves as the cathode
that replaces
electrode 305, and the anode 320 may be hollow such as a stainless steel
hollow anode. The
discharge may vaporize the catalyst source to catalyst. Molecular hydrogen may
be
dissociated by the discharge to form hydrogen atoms for generation of hydrinos
and energy.
Additional dissociation may be provided by a hydrogen dissociator in the
chamber.
Another embodiment of the hydrogen gas discharge power and plasma cell and
reactor where catalysis occurs in the gas phase utilizes a controllable
gaseous catalyst. The
gaseous hydrogen atoms for conversion to hydrinos are provided by a discharge
of molecular
hydrogen gas. The gas discharge cell 307 has a catalyst supply passage 341 for
the passage
of the gaseous catalyst 350 from catalyst reservoir 395 to the reaction
chamber 300. The
catalyst reservoir 395 is heated by a catalyst reservoir heater 392 having a
power supply 372
to provide the gaseous catalyst to the reaction chamber 300. The catalyst
vapor pressure is
107
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
controlled by controlling the temperature of the catalyst reservoir 395, by
adjusting the heater
392 through its power supply 372. The reactor further comprises a selective
venting valve
301. A chemically resistant open container, such as a stainless steel,
tungsten or ceramic
boat, positioned inside the gas discharge cell may contain the catalyst. The
catalyst in the
catalyst boat may be heated with a boat heater using an associated power
supply to provide
the gaseous catalyst to the reaction chamber. Alternatively, the glow gas
discharge cell is
operated at an elevated temperature such that the catalyst in the boat is
sublimed, boiled, or
volatilized into the gas phase. The catalyst vapor pressure is controlled by
controlling the
temperature of the boat or the discharge cell by adjusting the heater with its
power supply.
To prevent the catalyst from condensing in the cell, the temperature is
maintained above the
temperature of the catalyst source, catalyst reservoir 395 or catalyst boat.
In an embodiment, the catalysis occurs in the gas phase, lithium is the
catalyst, and a
source of atomic lithium such as lithium metal or a lithium compound such as
LiNH2 is made
gaseous by maintaining the cell temperature in the range of about 300-1000 C.
Most
preferably, the cell is maintained in the range of about 500-750 C. The
atomic and/or
molecular hydrogen reactant may be maintained at a pressure less than
atmospheric,
preferably in the range of about 10 millitorr to about 100 Torr. Most
preferably, the pressure
is determined by maintaining a mixture of lithium metal and lithium hydride in
the cell
maintained at the desired operating temperature. The operating temperature
range is
preferably in the range of about 300-1000 C and most preferably, the pressure
is that
achieved with the cell at the operating temperature range of about 300-750 C.
The cell can
be controlled at the desired operating temperature by the heating coil such as
380 of FIGURE
that is powered by power supply 385. The cell may further comprise an inner
reaction
chamber 300 and an outer hydrogen reservoir 390 such that hydrogen may be
supplied to the
cell by diffusion of hydrogen through the wall 313 separating the two
chambers. The
temperature of the wall may be controlled with a heater to control the rate of
diffusion. The
rate of diffusion may be further controlled by controlling the hydrogen
pressure in the
hydrogen reservoir.
In another embodiment of a system having a reaction mixture comprising species
of
the group of Li, LiNH2, Li2NH, Li3N, LiNO3i LiX, NH4X (X is a halide), NH3,
LiBH4,
LiA1H4i and H2, at least one of the reactants is regenerated by adding one or
more of the
reagents and by a plasma regeneration. The plasma may be one of the gases such
as NH3 and
H2. The plasma may be maintained in situ (in the reaction cell) or in an
external cell in
communication with the reaction cell. In other embodiments, K, Cs, and Na
replace Li
wherein the catalyst is atomic K, atomic Cs, and molecular NaH.
To maintain the catalyst pressure at the desire level, the cell having
permeation as the
hydrogen source may be sealed. Alternatively, the cell further comprises high
temperature
108
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
valves at each inlet or outlet such that the valve contacting the reaction gas
mixture is
maintained at the desired temperature.
The plasma cell temperature can be controlled independently over a broad range
by
insulating the cell and by applying supplemental heater power with heater 380.
Thus, the
catalyst vapor pressure can be controlled independently of the plasma power.
The discharge voltage may be in the range of about 100 to 10,000 volts. The
current
may be in any desired range at the desired voltage. Furthermore, the plasma
may be pulsed at
any desired frequency range, offset voltage, peak voltage, peak power, and
waveform.
In another embodiment, the plasma may occur in a liquid medium such as a
solvent of
the catalyst or of reactants of species that are a source of the catalyst.
IX. Fuel Cell and Battery
In embodiments of a fuel cell and a battery 400 shown in FIGURE 6, the hydrino
reactants comprising a solid fuel or a heterogeneous catalyst comprise the
reactant for
corresponding cell half reactions. During operation, the catalyst reacts with
atomic hydrogen,
and the energy transfer results in the ionization of the catalyst. This
reaction may occur in the
anode compartment 402 such that the anode 410 ultimately accepts the ionized-
electron
current. At least one of Li, K, and NaH may serve as the catalysts to form
hydrinos. The
reaction step of a nonradiative energy transfer of an integer multiple of 27.2
eV from atomic
hydrogen to the catalyst results in ionized catalyst and free electrons. The
support such as
AC may serve as a conductive electron acceptor in electrical contact with the
anode. The
final electron-acceptor reactants comprise an oxidant such as free radicals,
or a source
thereof, and a source of a positively-charged counter ion that are the
components of the
cathode-cell reaction mixture that ultimately scavenge electrons released from
the catalyst
reaction to form hydrinos. The oxidant or cathode-cell reaction mixture is
located in the
cathode compartment 401 having cathode 405. Preferably the oxidant is at least
one of
oxygen or a source of oxygen, a halogen, preferably F2 or C12, or a source of
halogen, CF4,
SF6, and NF3. During operation, the counterion such as the ion of the catalyst
may migrate to
the anode compartment to the cathode compartment, preferably through a salt
bridge 420.
Each cell reaction may be supplied by additional reactant or products may be
removed
through passages 460 and 461 to sources of reactants or reservoirs for product
storage 430
and 431.
In certain embodiments, the power, chemical, battery and fuel cell systems
disclosed
herein that regenerate the reactants and maintain the reaction to form lower-
energy hydrogen
can be closed except that only hydrogen consumed in forming hydrinos need be
replaced
wherein the consumed hydrogen fuel may be obtained from the electrolyis of
water.
X. Chemical Reactor
109
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
The present disclosure is also directed to other reactors for producing
increased
binding energy hydrogen compounds of the present disclosure, such as dihydrino
molecules
and hydrino hydride compounds. Further products of the catalysis are power and
optionally
plasma and light depending on the cell type. Such a reactor is hereinafter
referred to as a
"hydrogen reactor" or "hydrogen cell." The hydrogen reactor comprises a cell
for making
hydrinos. The cell for making hydrinos may take the form of a chemical reactor
or gas fuel
cell such as a gas discharge cell, a plasma torch cell, or microwave power
cell. Exemplary
embodiments of the cell for making hydrinos may take the form of a liquid-fuel
cell, a solid-
fuel cell, and a heterogeneous-fuel cell. Each of these cells comprises: (i) a
source of atomic
hydrogen; (ii) at least one catalyst chosen from a solid catalyst , a molten
catalyst , a liquid
catalyst , a gaseous catalyst, or mixtures thereof for making hydrinos; and
(iii) a vessel for
reacting hydrogen and the catalyst for making hydrinos. As used herein and as
contemplated
by the present disclosure, the term "hydrogen," unless specified otherwise,
includes not only
proteum (W), but also deuterium (2H) and tritium (3H ). In the case of the use
of
deuterium as a reactant of the hydrino reaction, relatively trace amounts of
tritium or helium
products of the heterogeneous fuels and solid fuels are expected.
In an embodiment of the chemical reactor to synthesize compounds comprising
lower-energy hydrogen such as hydrino hydride compounds, iron hydrino hydride
film is
synthesized using an iron salt having Fe in a positive oxidation state that
can react with
H- (1 / p) by displacement of the iron counterion, preferably iron carbide, an
iron oxide, or a
volatile iron salt such as Fe12 or Feb. The catalyst can be K, NaH, or Li. The
H can be from
H2 and a dissociator such as R-Ni or Pt/A1203- In another embodiment, iron
hydrino hydride
is formed from an iron source such as an iron halide that decomposes at the
reactor operating
temperature, a catalyst such as NaH, Li, or K, and a source of hydrogen such
as H2 gas and a
dissociator such as R-Ni. Manganese hydrino hydride may be formed from a
manganese
source such as an organometallic such as Mn(11)2,4-pentanedionate that
decomposes at the
reactor operating temperature, a catalyst such as NaH, Li, or K, and a source
of hydrogen
such as H2 gas and a dissociator such as R-Ni. In an embodiment, the reactor
is maintained in
the temperature range of about 25 C to 800 C, preferably in the range of about
400 C to
500 C.
Since alkali metals are covalent diatomic molecules in the gas phase, in an
embodiment, the catalyst to form increased-binding-energy hydrogen compounds
is formed
from a source by a reaction with at least one other element. The catalyst such
as K or Li may
be generated by the dispersion of K or Li metal in an alkali halide such as
the KX or LiX to
form KHX LiHX wherein X is halide. The catalyst K or Li may also be generated
by the
reaction of vaporized K2 or Lie with atomic H to form KH and K or LiH and Li,
respectively.
The increased-binding-energy hydrogen compounds may be MHX wherein M is an
alkali
metal, H is hydrino hydride, and X is a singly negatively charged ion,
preferably X is one of a
110
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
halide and HCO, . In an embodiment, the reaction mixture to form KHI or KHCI
wherein H
is hydrino hydride comprises K metal covered with the KX (X=C1, I) and a
dissociator,
preferably nickel metal such as nickel screen and R-Ni, respectively. The
reaction is carried
out by maintaining the reaction mixture at an elevated temperature preferably
in the range of
400-700 C with the addition of hydrogen. Preferably the hydrogen pressure is
maintained at
a gauge pressure of about 5 PSI. Thus, MX is placed over the K such that K
atoms migrate
through the halide lattice and the halide serves to disperse K and act as a
dissociator for K2
that reacts at the interface with H from the dissociator such as nickel screen
or R-Ni to form
KHX.
A suitable reaction mixture for the synthesis of hydrino hydride compounds
comprises at least two species of the group of a catalyst, a source of
hydrogen, an oxidant, a
reductant, and a support wherein the oxidant is a source of at least one of
sulfur,
phosphorous, and oxygen such as SF6, S , S02 1 503, S205C12i F5SOF, MZSZO. ,
S,,Xy such
as S2CI2, SC12, S2Br2, S2F2, CS2, Sb2S5, SOXXy such as SOC12, SOF2, S02F2,
SOBr2, P, P205,
P2S5, PXXy such as PF3, PC13, PBr3, PI3, PF5, PC15, PBr4F, or PC14F, POXXy
such as POBr3,
P013, POC13 or POF3, PSXXy such as PSBr3, PSF3, PSC13, a phosphorous-nitrogen
compound
such as P3N5, (C12PN)3, or (C12PN)4, (Br2PN)X (M is an alkali metal, x and y
are integers, X
is halogen), 02, N2O, and Te02. The oxidant may further comprise a source of a
halide,
preferable fluorine, such as CF4, NF3, or CrF2. The mixture may also comprise
a getter as a
source of phosphorous or sulfur such as MgS, and MHS (M is an alkali metal). A
suitable
getter is an atom or compound that gives rise to an upfield shifted NMR peak
with ordinary H
and a hydrino hydride peak that is upfield of the ordinary H peak. Suitable
getters comprise
elemental S, P, 0, Se, and Te or comprise compounds comprising S, P, 0, Se,
and Te. A
general property of a suitable getter for hydrino hydride ions is that it
forms chains, cages, or
rings in elemental form, in doped elemental form, or with other elements that
traps and
stabilizes hydrino hydride ions. Preferably, the H-(1/p) can be observed in
solid or solution
NMR. In another, embodiment, either NaH or HCI serves as the catalyst. A
suitable reaction
mixture comprises MX and M'HSO4 wherein M and M' are alkali metals, preferably
Na and
K, respectively, and X is a halogen, preferably Cl.
The reaction mixtures comprising at least one of (1) NaH catalyst, MgH2, SF6,
and
activated carbon (AC), (2) NaH catalyst, MgH2, S, and activated carbon (AC),
(3) NaH
catalyst, MgH2, K2S208, Ag, and AC, (4) KH catalyst, MgH2, K2S208, and AC, (5)
MH
catalyst (M=Li, Na, K), Al or MgH2, 02, K2S208i and AC, (6) KH catalyst, Al,
CF4, and AC,
(7) NaH catalyst, Al, NF3, and AC, (8) KH catalyst, MgH2, N20, and AC, (9) NaH
catalyst,
MgH2, 02, and activated carbon (AC), (10) NaH catalyst, MgH2, CF4, and AC,
(11) MH
catalyst, MgH2, (M=Li, Na, or K) P205 (P4010), and AC, (12) MH catalyst, MgH2,
MNO3,
(M=Li, Na, or K) and AC, (13) NaH or KH catalyst, Mg, Ca, or Sr, a transition
metal halide,
preferably, FeC12, FeBr2, NiBr2, Mn12, or a rare earth halide such as EuBr2,
and AC, and (14)
111
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
NaH catalyst, Al, CS2, and AC are suitable systems for generating power and
also for
producing lower-energy hydrogen compounds. In other embodiments of the
exemplary
reaction mixtures given supra, the catalyst cation comprises one of Li, Na, K,
Rb, or Cs and
the other species of the reaction mixture are chosen from those of reactions 1
through 14.
The reactants may be in any desired ratios.
The hydrino reaction product is at least one of a hydrogen molecule and a
hydride ion
having a proton NMR peak shifted upfield of that or ordinary molecular
hydrogen or
hydrogen hydride, respectively. In an embodiment, the hydrogen product is
bound to an
element other than hydrogen wherein the proton NMR peak is shifted upfield of
that of the
ordinary molecule, species, or compound that has the same molecular formula as
the product,
or the ordinary molecule, species, or compound is not stable at room
temperature.
In an embodiment, power and increased binding energy hydrogen compounds are
produced by a reaction mixture comprising two or more of the following
species; LiNO3,
NaNO3, KNO3, LiH, NaH, KH, Li, Na, K, H2, a support such as carbon, for
example
activated carbon, a metal or metal hydride reductant, preferably MgH2. The
reactants can be
in any molar ratio. Preferably the reaction mixture comprises 9.3 mole % MH,
8.6 mole %
MgH2, 74 mole % AC, and 7.86 mole % MNO3 (M is Li, Na, or K) wherein the molar
% of
each species can be varied within a range of plus or minus a factor of 10 of
that given for
each species. The product molecular hydrino and hydrino hydride ion having a
preferred 1/4
state may be observed using liquid NMR at about 1.22 ppm and -3.85 ppm,
respectively,
following extraction of the product mixture with an NMR solvent, preferably
deuterated
DFM. The product M2CO3 may serve as a getter for hydrino hydride ion to form a
compound
such as MHMHCO3.
In another embodiment, power and increased binding energy hydrogen compounds
are produced by a reaction mixture comprising two or more of the following
species; LiH,
NaH, KH, Li, Na, K, H2, a metal or metal hydride reductant, preferably MgH2 or
Al powder,
preferably nanopowder, a support such as carbon, preferably activated carbon,
and a source
of fluorine such as a fluorine gas or a fluorocarbon, preferably CF4 or
hexafluorobenzene
(HFB). The reactants can be in any molar ratio. Preferably the reaction
mixture comprises
9.8 mole % MH, 9.1 mole % MgH2 or 9 mole % Al nanopowder, 79 mole % AC, and
2.4
mole % CF4 or HFB (M is Li, Na, or K) wherein the molar % of each species can
be varied
within a range of plus or minus a factor of 10 of that given for each species.
The product
molecular hydrino and hydrino hydride ion having a preferred 1/4 state may be
observed
using liquid NMR at about 1.22 ppm and -3.86 ppm, respectively, following
extraction of the
product mixture with an NMR solvent, preferably deuterated DFM or CDC13.
In another embodiment, power and increased binding energy hydrogen compounds
are produced by a reaction mixture comprising two or more of the following
species; LiH,
NaH, KH, Li, Na, K, H2, a metal or metal hydride reductant, preferably MgH2 or
Al powder,
112
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
a support such as carbon, preferably activated carbon, and a source of
fluorine, preferably
SF6. The reactants can be in any molar ratio. Preferably the reaction mixture
comprises 10
mole % MH, 9.1 mole % MgH2 or 9 mole % Al powder, 78.8 mole % AC, and 24 mole
%
SF6 (M is Li, Na, or K) wherein the molar % of each species can be varied
within a range of
plus or minus a factor of 10 of that given for each species. A suitable
reaction mixture
comprises NaH, MgH2 or Mg, AC, and SF6 in these molar ratios. The product
molecular
hydrino and hydrino hydride ion having a preferred 1/4 state may be observed
using liquid
NMR at about 1.22 ppm and -3.86 ppm, respectively, following extraction of the
product
mixture with an NMR solvent, preferably deuterated DFM or CDC13.
In another embodiment, power and increased binding energy hydrogen compounds
are produced by a reaction mixture comprising two or more of the following
species; LiH,
NaH, KH, Li, Na, K, H2, a metal or metal hydride reductant, preferably MgH2 or
Al powder,
a support such as carbon, preferably activated carbon, and a source of at
least one of sulfur,
phosphorous, and oxygen, preferably S or P powder, SF6, CS2, P205, and MNO3 (M
is an
alkali metal). The reactants can be in any molar ratio. Preferably the
reaction mixture
comprises 8.1 mole % MH, 7.5 mole % MgH2 or Al powder, 65 mole % AC, and 19.5
mole
% S (M is Li, Na, or K) wherein the molar % of each species can be varied
within a range of
plus or minus a factor of 10 of that given for each species. A suitable
reaction mixture
comprises NaH, MgH2 or Mg, AC, and S powder in these molar ratios. The product
molecular hydrino and hydrino hydride ion having a preferred 1/4 state may be
observed
using liquid NMR at about 1.22 ppm and -3.86 ppm, respectively, following
extraction of the
product mixture with an NMR solvent, preferably deuterated DFM or CDC13.
In another embodiment, power and increased binding energy hydrogen compounds
are produced by a reaction mixture comprising NaHS. The hydrino hydride ion
may be
isolated from NaHS. In an embodiment, a solid state reaction occurs within
NaHS to form H-
(1/4) that may be further reacted with a source of protons such as a solvent,
preferably H2O,
to form H2(1/4).
In an embodiment, hydrino hydride compounds may be purified. The purification
method may comprise at least one of extraction and recrystallization using a
suitable solvent.
The method may further comprise chromatography and other techniques for
separation of
inorganic compounds known to those skilled in the art.
In a liquid-fuel embodiment, the solvent has a halogen functional group,
preferably
fluorine. A suitable reaction mixture comprises at least one of
hexafluorobenzene and
octafluoronaphthalene added to a catalyst such as NaH, and mixed with a
support such as
activated carbon, a fluoropolymer or R-Ni. The reaction mixture may comprise
an energetic
material that may be used in applications that are known by those skilled in
the art. Suitable
applications due to the high-energy balance are a propellants and piston-
engine fuel. In an
embodiment, a desired product is at least one of fullerene and nanotubes that
are collected.
113
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
In an embodiment, molecular hydrino H2(1/p), preferably H2(1/4), is a product
that is
further reduced to form the corresponding hydrides ions that may be used in
applications such
as hydride batteries and surface coatings. The molecular hydrino bond may be
broken by a
collisional method. H2(1/p) may be dissociated via energetic collisions with
ions or electrons
in a plasma or beam. The dissociated hydrino atoms may then react to form the
desired
hydride ions.
In a further embodiment, molecular hydrino H2(1/p), preferably H2(1/4), is a
product
that is used as a Magnetic Resonance Imaging (MRI) contrast agent. The agent
may be
inhaled to image the lungs wherein its upfield chemical shift relative to
ordinary H permits it
to be distinguishable and thus selective. In another embodiment, at least one
of the lower-
energy hydrogen compound and lower-energy hydrogen species such as H-(1/p) is
a
pharmaceutical agent comprising at least one of the group of antilipidemic
drugs,
anticholesterol drugs, contraceptive agents, anticoagulants, anti-inflamatory
agents, immuno-
suppressive drugs, antiarrhythmic agents, antineoplastic drugs,
antihypertensive drugs,
epinephrine blocking agents, cardiac inotropic drugs, antidepressant drugs,
diuretics,
antifungal agents, antibacterial drugs, anxiolytic agents, sedatives, muscle
relaxants,
anticonvulsants, agents for the treatment of ulcer disease, agents for the
treatment of asthma
and hypersensitivity reactions, antithroboembolic agents, agents for the
treatment of muscular
dystrophy, agents to effect a therapeutic abortion, agents for the treatment
of anemia, agents
to improve allograft survival, agents for the treatment of disorders of purine
metabolism,
agents for the treatment of ischemic heart disease, agents for the treatment
of opiate
withdrawal, agents which activate the effects of secondary messenger inositol
triphosphate,
agents to block spinal reflexes, and antiviral agents including a drug for the
treatment of
AIDS. In a formulation that occurs naturally, the least one of the lower-
energy hydrogen
compound and lower-energy hydrogen species is made to have a desired
concentration such
as a higher concentration than naturally occurring.
XI. Experimental
A. Water-Flow, Batch Calorimetry
The energy and power balance of the catalyst reaction mixtures listed on the
right-
hand side of each entry infra was obtained using cylindrical stainless steel
reactors of
approximately 130.3 cm3 volume (1.5" inside diameter (ID), 4.5" length, and
0.2" wall
thickness) or 1988 cm3 volume (3.75" inside diameter (ID), 11" length, and
0.375" wall
thickness) and a water flow calorimeter comprising a vacuum chamber containing
each cell
and an external water coolant coil that collected 99+% of the energy released
in the cell to
achieved an error < 1%. The energy recovery was determined by integrating the
total
output power F,. over time. The power was given by
PT = mCPOT (221)
114
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
where m was the mass flow rate, Cn was the specific heat of water, and AT was
the
absolute change in temperature between the inlet and outlet. The reaction was
initiated by
applying precision power to external heaters. Specially, 100-200 W of power
(130.3 cm'
cell) or 800-1000 W (1988 cm3 cell) was supplied to the heater. During this
heating period,
the reagents reached a hydrino reaction threshold temperature wherein the
onset of reaction
was typically confirmed by a rapid rise in cell temperature. Once the cell
temperature
reached about 400-500 C the input power was set to zero. After 50 minutes,
the program
directed the power to zero. To increase the rate of heat transfer to the
coolant, the chamber
was re-pressurized with 1000 Torr of helium, and the maximum change in water
temperature
(outlet minus inlet) was approximately 1.2 C. The assembly was allowed to
fully reach
equilibrium over a 24-hour period as confirmed by the observation of full
equilibrium in the
flow thermistors.
In each test, the energy input and energy output were calculated by
integration of the
corresponding power. The thermal energy in the coolant flow in each time
increment was
calculated using Eq. (221) by multiplying volume flow rate of water by the
water density at
19 C (0.998 kg/liter), the specific heat of water (4.181 kJ/kg C), the
corrected temperature
difference, and the time interval. Values were summed over the entire
experiment to obtain
the total energy output. The total energy from the cell ET must equal the
energy input Ejn
and any net energy Ene1 . Thus, the net energy was given by
Ene, = E,. - E. . (222)
From the energy balance, any excess heat EeX was determined relative to the
maximum
theoretical E., by
Eex = Enet - Emt (223)
The calibration test results demonstrated a heat coupling of better than 98%
of the
resistive input to the output coolant, and zero excess heat controls
demonstrated that the with
calibration correction applied, the calorimeter was accurate to within less
than 1% error. The
results are given as follows where Tmax is the maximum cell temperature, Ein
is the input
energy, and dE is the measured output energy in excess of the input energy.
All energies are
exothermic. Positive values where given represent the magnitude of the energy.
Metal Halides, Oxides, and Sulfides
= 20g AC3-5 + 5g Mg + 8.3g KH + 11.2g Mg3As2, 298.6kJ, dE:21.8 kJ, TSC: none,
Tmax:
315 C, theoretical is endothermic, the gain is infinity.
= 20g AC3-5 + 5g Mg + 8.3g KH + 9.1g Ca3P2, Ein: 282.1 U, dE:18.1 kJ, TSC:
none, Tmax:
320 C is endothermic, the gain is infinity.
= Rowan Validation KH 7.47gm + Mg 4.5gm + TiC 18.0gm + EuBr2 14.04gm, Ein:
321.1 kJ
115
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
dE: 40.5 kJ, Tmax- 340 C, Energy Gain - 6.5X (1.37 kJ x 4.5 = 6.16 kJ).
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + TiB2 3.5gm, Ein: 299 kJ, dE: 10
kJ, No
TSC with Tmax- 320 C. Energy Gain - X (X-0 kJ; 1 "cell: Excess energy-5.1
kJ).
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + RbCI 6.05gm, Ein: 311 kJ, dE: 18
kJ, No
TSC with Tmax-340 C, Energy Gain - X (X-0 kJ; 1"Cell: Excess energy-6.0 kJ).
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + Li2S 2.3gm, Ein: 323 kJ, dE: 12
kJ, No
TSC with Tmax-. 340 C. Energy Gain - X (X-0 kJ; 1 "cell: Excess energy-5.0
kJ).
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + Mg3N2 5.05gm, Ein: 323 kJ, dE: 11
kJ, No
TSC with Tmax - 330 C. Energy Gain - (X-0 kJ; 1" cell: Excess energy-5.2 kJ).
= 4g AC3-5 + 1g Mg + 1.66g KH + 3.55g PtBr2, Ein: 95.0 kJ, dE:15.7 U, TSC: 108-
327 C,
Tmax: 346 C, theoretical is 6.66 kJ, gain is 2.36 times.
= 4g AC3-5 + 1g Mg + 1g NaH + 3.55g PtBr2, Ein: 94.0 kJ, dE:14.3 kJ, TSC: 100-
256 C,
Tmax: 326 C, theoretical is 6.03 U, gain is 2.37 times.
= 4g WC + 1g MgH2 + 1g NaH + 0.01mol C12 initiated with UV lamp to dissociate
C12 to Cl,
Ein:162.9 U, dE:16.0 U, TSC: 23-42 C, Tmax: 85 C, theoretical is 7.10 kJ,
gain is 2.25
times.
= 4g AC3-5 + 1g Mg + 1.66g KH + 2.66g PdBr2, Ein: 113.0 U, dE: 11.7kJ, TSC:
133-276
C, Tmax: 370 C, theoretical is 6.43 kJ, gain is 1.82 times.
= 4g AC3-5 + 1g Mg + 1g NaH + 2.66g PdBr2, Ein: 116.0 kJ, dE: 9.4 U, TSC: 110-
217 C,
Tmax: 361 C, theoretical is 5.81 U, gain is 1.63 times.
= 4g AC3-5 + 1g Mg + 1.66g KH + 3.60g Pd12, Ein: 142.0 kJ, dE: 7.8 kJ, TSC:
177-342 C,
Tmax: 403 C, theoretical is 5.53 U, gain is 1.41 times.
= 0.41 g of AIN + 1.66 g of KH + 1 g of Mg powder + 4 g of AC3-5 in a 1" heavy
duty cell,
energy gain was 4.9 kJ, but no cell temperature burst was observed. The
maximum cell
temperature was 407 C, theoretical is endothermic.
116
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + CrB2 3.7gm, Ein: 317 kJ, dE:19 kJ,
No
TSC with Tmax-340 C, theoretical energy is endothermic 0.05 kJ, gain is
infinite.
= KH 8.3 gm + NEW Mg 5.0 gm + CAII-300 20.0gm + AgCI_9.36 gm, Ein: 99 kJ, dE:
43 kJ,
Small TSC at -250 C with Tmax-340 C. Energy Gain -2.3X (X=18.88 kJ).
- KH 8.3 gm+ Mg 5.0 gm + NEW TiC (G06UO55) 20.0gm + AgCI 7.2 gm, Ein: 315 kJ,
dE:
25 kJ, Small TSC at -250 C with Tmax-340 C. Energy Gain - 1.72X (X=14.52
kJ).
= KH 8.3 gm+ Mg 5.0 gm + CAII-300 20.0gm + Y203 11.3 gm (Gain -4X with TiC),
Ein:
353 kJ, dE: 23 kJ, No TSC with Tmax- 350 C. Energy Gain -4X (X_1.18kJ*5=5.9kJ
).
= KH 4.15 gm + Mg 2.5 gm + CAII-300 10.0gm + EuBr3 9.8 gm, Ein: 323 kJ, dE: 27
kJ, No
TSC with Tmax- 350 C. Energy Gain - 2.26 X (X= 11.93 kJ ).
= 4g AC3-5 + 1g Mg + 1g NaH + 2.23g Mg3As2, 133.0 kJ, dE: 5.8 kJ, TSC: none,
Tmax:
371 C, the theoretical is endothermic, the gain is infinity.
= 4g AC3-5 + 1g Mg + 1.66g KH + 2.23g Mg3As2, Ein: 139.0 kJ, dE: 6.5 kJ, TSC:
none,
Tmax: 393 C, the theoretical is endothermic, the gain is infinity.
= 4g AC3-5 + 1g Mg + 1.66g KH + 1.82g Ca3P2, Ein: 133.0 kJ, dE: 5.8 kJ, TSC:
none,
Tmax: 407 C, the theoretical is endothermic, the gain is infinity.
- 4g AC3-5 + 1g Mg + 1g NaH + 3.97g WC16; Ein: 99.0 U; dE: 21.84 kJ; TSC: 100-
342 C;
Tmax: 375 C, theoretical is 16.7, the gain is 1.3 times.
= 2.60 g of CsI, 1.66 g of KH, 1 g of Mg powder and 4 g of AC3-4 in a 1" heavy
duty cell
was finished. The energy gain was 4.9 kJ, but no cell temperature burst was
observed. The
maximum cell temperature was 406 C, theoretical is 0, the gain is infinity.
= 0.42 g of LiCl, 1.66 g of KH, 1 g of Mg powder and 4 g of AC3-4 was
finished. The energy
gain was 5.4 kJ, but no cell temperature burst was observed. The maximum cell
temperature
was 412 C, theoretical is 0, the gain is infinity.
= 4g AC3-4 + 1g Mg + 1g NaH + 1.21g RbCI, Ein:136.0 kJ, dE: 5.2 kJ, TSC: none,
Tmax:
372 C, theoretical is 0 kJ, gain is infinite.
117
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= KH 8.3 gm+ Mg 5.0 gm + CAII-300 20.Ogm+ CaBr2 10.0 gm, Ein: 323 kJ, dE: 27
kJ, No
TSC with Tmax- 340 C. Energy Gain - 3.0 X (X-1.71kJ * 5= 8.55 U).
= KH 8.3 gm+ Mg 5.0 gm + CAII-300 20.Ogm+ YF3 7.3 gm, Ein: 320 U, dE: 17 U, No
TSC
with Tmax- 340 C. Energy Gain - 4.5 X (X-0.74kJ * 5=3.7kJ ).
= KH 8.3 gm+ Mg 5.0 gm + TiC 20.0gm + Dried SnBr2 14.0 gm, Ein: 299 kJ, dE: 36
kJ,
Small TSC at -130 C with Tmax-350 C. Energy Gain - 1.23X (X-5.85kJx5=29.25
kJ).
= KH 8.3 gm+ Mg 5.0 gm + TiC 20.0gm + EuBr2 15.6 gm, Ein: 291 kJ, dE: 45 kJ,
Small
TSC at -50 C with Tmax- 320 C. Energy Gain - 32X (X-0.28kJx5=1.4kJ) and Gain
is
-6.5X(1.37kJx5=6.85kJ).
= KH 8.3 gm+ Mg 5.0 gm + CAII-300 20.0gm + Dried ZnBr2 11.25 gm, Ein: 288 kJ,
dE: 45
kJ,Small TSC at -200 C with Tmax- 350 C. Energy Gain - 2.1X (X-
4.19kJx5=20.9kJ).
= NaH 5.0 gm + Mg 5.0 gm + CAII-300 20.0gm + SF6, Ein: 77.7 kJ, dE: 105 kJ,
Tmax-400
C. Energy Gain - 1.43X (X for 0.03mole SF6-73 kJ).
= NaH 5.0 gm + Mg 5.0 gm + CAII-300 20.0gm + SF6, Ein: 217 U, dE: 84 kJ, Tmax-
400
C. Energy Gain - 1.15X (X for 0.03mole SF6--73 kJ).
= KH 8.3 gm + Mg_ 5.0 gm + CAII-300 20.0gm + AgC1 7.2 gm, Ein: 357 kJ, dE: 25
kJ,
Small TSC at -250 C with Tmax-340 C. Energy Gain - 1.72X (X-14.52 kJ).
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + AgC1 7.2 gm, Ein: 487 kJ, dE: 34
kJ, Small
TSC at -250 C with Tmax-340 C. Energy Gain - 2.34X (X14.52 kJ).
= 20g AC3-4 + 8.3g Ca + 5g NaH + 15.5g Mn12, Ein: 181.5 kJ, dE: 61.3 kJ, TSC:
159-233
C, Tmax: 283 C, theoretical is 29.5 kJ, gain is 2.08 times.
= 4g AC3-4 + 1.66g Ca + 1.66g KH + 3.09g Mn12, Ein: 113.0 U, dE: 15.8 kJ, TSC:
228-384
C, Tmax: 395 C, theoretical is 6.68 kJ, gain is 2.37 times.
= 4g AC3-4 + 1g Mg + 1.66g KH + 0.46g Li2S, Ein: 144.0 kJ, dE: 5.0 kJ, TSC:
none, Tmax:
419 C, theoretical is endothermic.
= 1.01 g of Mg3N2, 1.66 g of KH, 1 g of Mg powder and 4 g of AC3-4 in a 1"
heavy duty
118
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
cell, energy gain was 5.2 kJ, but no cell temperature burst was observed. The
maximum cell
temperature was 401 C, theoretical is 0.
= 1.21 g of RbCl, 1.66 g of KH, 1 g of Mg powder and 4 g of AC3-4, energy gain
was 6.0 kJ,
but no cell temperature burst was observed. The maximum cell temperature was
442 C,
theoretical is 0.
- 2.24 g of Zn3N2, 1.66 g of KH, 1 g of Mg powder and 4 g of AC3-4 was
finished. The
energy gain was 5.5 kJ, but no cell temperature burst was observed. The
maximum cell
temperature was 410 C, theoretical is 4.41 kJ, gain is 1.25 times.
= 4g AC3-4 + lg Mg + 1g NaH + 1.77g PdC12, Ein: 89.0kJ, dE:10.5kJ, TSC: 83-204
C,
Tmax: 306 C, theoretical is 6.14 kJ, gain is 1.7 times.
= 0.74 g of CrB2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3-4) in a 1" heavy duty cell, energy gain was 4.3 kJ, but no cell
temperature burst
was observed. The maximum cell temperature was 404 C, theoretical is 0.
= 0.70 g of TiB2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3-4) was finished. The energy gain was 5.1 kJ, but no cell
temperature burst was
observed. The maximum cell temperature was 431 C, theoretical is 0.
- NaH 5.0 gm+ Mg 5.0 gm + CAII-300 20.Ogm+ BaBr2 14.85 gm (Dried), Ein: 328
kJ, dE:
16 kJ, No TSC with Tmax- 320 C. Energy Gain 160X (X~0.02kJ*5=0.1 kJ).
- NaH 1.0 gm+ Mg 1.0 gm + CAII-300 4.0gm+ BaBr2 2.97 gm (Dried), Ein: 140 U,
dE: 3
kJ, No TSC with Tmax- 360 C. Energy Gain -150X (X-0.02kJ).
= NaH 5.0 gm+ Mg 5.0 gm + CAII-300 20.Ogm+ Mg12 13.9 gm, Ein: 315 kJ, dE: 16
kJ, No
TSC with Tmax- 340 C. Energy Gain - 1.8X (X-1.75x5=8.75 kJ).
= KH 8.3 gm+ Mg 5.0 gm + CAII-300 20.0gm+ MgBr2 9.2 gm, Ein: 334 kJ, dE: 24
kJ, No
TSC with Tmax- 340 C. Energy Gain - 2.1X (X-2.23x5 =11.5 U).
= 20g AC3-3 + 8.3g KH + 7.2g AgCI, Ein:286.6 kJ, dE:29.5 kJ, TSC: 327-391 C,
Tmax: 394
C, theoretical is 13.57 kJ, gain is 2.17 times.
= 4g AC3-3 + lg MgH2 + 1.66g KH + 1.44g AgCI, Ein: 151.0 kJ, dE: 4.8 kJ, TSC:
none,
119
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
Tmax: 397 C, theoretical is 2.53 kJ, gain is 1.89 times.
= 4g AC3-3 + 1g Mg + 1g NaH + 1.48g Ca3N2, Ein: 140.0 kJ, dE: 4.9 kJ, TSC:
none, Tmax:
392 C, theoretical is 2.01 kJ, gain is 2.21 times.
= 4g AC3-3 + 1g Mg + 1g NaH + 1.86g InC12, Ein: 125.0 kJ, dE: 7.9 kJ, TSC: 163-
259 C,
Tmax: 374 C, theoretical is 4.22 U, gain is 1.87 times.
= 4g AC3-3 + 1g Mg + 1.66g KH + 1.86g InC12, Ein: 105.0 kJ, dE: 7.5 kJ, TSC:
186-302 C,
Tmax: 370 C, theoretical is 4.7 U, gain is 1.59 times.
= 4g AC3-3 + 1g Mg + 1.66g KH + 2.5g Dy12, Ein:135.0 kJ, dE: 6.1 kJ, TSC:
none, Tmax:
403 C, theoretical is 1.89 kJ, gain is 3.22 times.
= 3.92 g of EuBr3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-I11 300
activated carbon
powder (AC3-3) in a 1" heavy duty cell, energy gain was 10.5 kJ, but no cell
temperature
burst was observed. The maximum cell temperature was 429 C, theoretical is
3.4 kJ, gain is
3 times.
= 4.56 g of As13, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-I11 300
activated carbon
powder (AC3-3), energy gain was 13.5 kJ, and the cell temperature burst was
166 C(237 -
403 C). The maximum cell temperature was 425 C, theoretical is 8.65 kJ, gain
is 1.56 times.
= 4g AC3-3 + 1g Mg + 1g NaH + 2.09g EuF3, Ein: 185.1 U, dE: 8.0 kJ, TSC: none,
Tmax:
463 C, theoretical is 1.69 kJ, gain is 4.73 times.
= 4g AC3-3 + 1g Mg + 1.66g KH + 1.27g AgF; Ein: 127.0 kJ; dE: 6.04 kJ; TSC: 84-
190 C;
Tmax: 369 C, theoretical is 3.58 U, gain is 1.69 times.
= 4g AC3-3 + 1g Mg + 1g NaH + 3.92g EuBr3; Ein: 162.5 kJ; dE: 7.54 kJ; TSC:
not
observed; Tmax: 471 C, theoretical is 3.41 kJ, gain is 2.21 times.
= 2.09 g of EuF3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-I11 300
activated carbon
powder (AC3-3) in a 1" heavy duty cell, energy gain was 5.5 kJ, but no cell
temperature burst
was observed. The maximum cell temperature was 417 C, theoretical is 1.71 kJ,
gain is
3.25 times.
= 3.29 g of YBr3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-I11 300
activated carbon
120
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
powder (AC3-3), energy gain was 7.0 kJ, but no cell temperature burst was
observed. The
maximum cell temperature was 441 C, theoretical is 4.16 kJ, gain is 1.68
times.
= NaH 5.0 gm+ Mg 5.0 gm + CAII-300 20.Ogm+ Ba12 19.5 gm, Ein: 334 kJ, dE: 13
kJ, No
TSC with Tmax- 350 C. Energy Gain - 2.95 X (X-0.88 kJ x5 =4.4 kJ).
= KH 8.3 gm+ Mg 5.0 gm + CAII-300 20.0gm + BaC12 10.4 gm, Ein: 331 kJ, dE: 18
kJ, No
TSC with Tmax- 320 C. Energy Gain - 6.9X (X-0.52x5=2.6 kJ).
= KH 8.3 gm+ Mg 5.0 gm + TiC 20.0gm + LaF3 9.8 gm, Ein: 338 kJ, dE: 7 kJ, No
TSC with
Tmax- 320 C. Energy Gain - 1.9X (X3.65 kJ).
= NaH 5.0 gm+ Mg 5.0 gm + CAII-300 20.0gm + BaBr2 14.85 gm (Dried), Ein: 280
kJ, dE:
kJ, No TSC with Tmax- 320 C. Energy Gain - 100 X (X-0.01=0.02x5 kJ).
= KH 8.3 gm+ Mg 5.0 gm + CAII-300 20.Ogm+ BaBr2 14.85 gm (Dried), Ein: 267 kJ,
dE: 8
kJ, No TSC with Tmax- 360 C. Energy Gain - 2.5 X (X-3.2 kJ).
= NaH 5.0 gm+ Mg 5.0 gm + TiC 20.Ogm+ ZnS 4.85 gm, Ein: 319 kJ, dE: 12 kJ, No
TSC
with Tmax- 340 C. Energy Gain - 1.5 X (X-8.0 kJ).
= KH 8.3 gm+ Mg 5.0 gm + TiC 20.Ogm+ AgCI 7.2 gm (Dried on 070109), Ein: 219
kJ, dE:
26 kJ, Small TSC at -250 C with Tmax-340 C. Energy Gain - 1.8X (X14.52 kJ).
= KH 8.3 gm+ Mg 5.0 gm + TiC 20.Ogm+ Y203 11.3 gm, Ein: 339 kJ, dE: 24 kJ,
Small TSC
at -300 C with Tmax- 350 C. Energy Gain - 4.0 X (X-.5.9kJ with NaH).
= 4g AC3-3 + 1g Mg + 1g NaH + 1.95g YC13, Ein:137.0 U, dE: 7.1kJ, TSC: none,
Tmax:
384 C, theoretical is 3.3 kJ, the gain is 2.15 times.
= 4.70 g of Y13, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3- 1) in a 1" heavy duty cell, energy gain was 6.9 kJ, but no cell
temperature burst
was observed. The maximum cell temperature was 426 C, theoretical is 3.37 kJ,
the gain is
2.04 times.
= 1.51 g of Sn02, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3-1), energy gain was 9.4 kJ, but no cell temperature burst was
observed. The
maximum cell temperature was 460 C, theoretical is 7.06 U, the gain is 1.33
times.
121
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= 4.56 g of AsI3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3-1), energy gain was 11.5 kJ, and the cell temperature burst was
144 C (221 -
365 C). The maximum cell temperature was 463 C, theoretical is 8.65 kJ, the
gain is 1.33
times.
= 3.09 g of Mn12, 1.66 g of KH, 1 g of Mg powder and 4 g of STiC-1(TiC from
Sigma
Aldrich), energy gain was 9.6 kJ, and the cell temperature burst was 137 C
(38 - 175 C).
The maximum cell temperature was 396 C, theoretical is 3.73 kJ, the gain is
2.57 times.
= 3.99 g of SeBr4, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-I11 300
activated carbon
powder (AC3-1), energy gain was 20.9 kJ, and the cell temperature burst was
224 C (47 -
271 C). The maximum cell temperature was 383 C, theoretical is 16.93 kJ, the
gain is 1.23
times.
= 20g AC3-3 + 5g Mg + 8.3g KH + 11.65g AgI, Ein: 238.6 kJ, dE: 31.7 kJ, TSC:
230-316 C,
Tmax: 317 C, theoretical is 12.3 kJ, gain is 2.57 times.
= 4g AC3-3 + 1g Mg + 1.66g KH + 0.91g CoS, Ein: 145.1 kJ, dE: 8.7 kJ, TSC:
none, Tmax:
420 C, theoretical is 2.63 kJ, gain is 3.3 times.
- 4g AC3-3 + 1g Mg + 1.66g KH + 1.84g MgBr2; Ein: 134.1 kJ; dE: 5.75 kJ; TSC:
not
observed; Tmax: 400 C, theoretical is 2.23 kJ, gain is 2.58 times.
= 5.02 g of Sb13, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-111 300
activated carbon
powder (AC3-1), energy gain was 12.2 kJ, and the cell temperature burst was
154 C (141 -
295 C). The maximum cell temperature was 379 C, theoretical is 9.71 kJ, gain
is 1.26 times
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + AgCI 7.2 gm, Ein: 304 kJ, dE: 30 kJ,
Small TSC
at -275 C with Tmax- 340 C. Energy Gain - 2.1X (X-14.52 kJ).
= KH 1.66 gm + Mg 1.0 gm + TiC 5.0gm + BaBr2 2.97 gm Loaded BaBr2-KH-Mg-TiC,
Ein:
130 kJ, dE: 2 kJ, No TSC with Tmax- 360 C, theoretical is 0.64 kJ, gain is 3
times.
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + CuS 4.8 gm, Ein: 318 kJ, dE: 30 kJ,
Small TSC at
-250 C with Tmax- 360 C. Energy Gain - 2.1X (X14.4 kJ).
- KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + MnS 4.35 gm, Ein: 326 kJ, dE: 14 kJ, No
TSC
122
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
with Tmax- 350 C. Energy Gain - 2.2X (X-6.3 kJ).
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + GdF3 10.7 gm, Ein: 339 kJ, dE: 7 kJ, No
TSC
with Tmax- 360 C. Energy Gain - 2.54X (X2.75 kJ).
= 20g AC3-2 + 5g Mg + 8.3g KH + 7.2g AgCl, Ein:327.1 kJ, dE:40.4 kJ, TSC: 288-
318 C,
Tmax: 326 C, theoretical is 14.52, gain is 2.78 times.
= 20g AC3-2 + 5g Mg + 8.3g KH + 7.2g CuBr, Ein: 205.1 kJ, dE:22.5kJ, TSC: 216-
268 C,
Tmax: 280 C, theoretical is 13.46, gain is 1.67 times.
= 4g AC3-2 + 1g Mg + 1g NaH + 1.46g YF3, Ein:157.0 kJ, dE: 4.3 kJ, TSC: none,
Tmax:
405 C, theoretical is 0.77, gain is 5.65 times.
= 4g AC3-2 + 1g Mg + 1.66g KH + 1.46g YF3, Ein:137.0 kJ, dE: 5.6 kJ, TSC:
none, Tmax:
398 C, theoretical is 0.74, gain is 7.54 times.
= 11.3 g of Y203, 5 g of NaH, 5 g of Mg powder and 20 g of CA-I11 300
activated carbon
powder (AC3-2) in a 2" heavy duty cell, energy gain was 24.5 kJ, but no cell
temperature
burst was observed. The maximum cell temperature was 386 C, theoretical is
5.9, gain is
4.2 times.
= 4g AC3-2 + 1g Mg + 1g NaH + 3.91g Ba12, Ein:135.0 kJ, dE: 5.3 U, TSC: none,
Tmax:
378 C, theoretical is 0.1 kJ, gain is 51 times.
= 4g AC3-2 + 1g Mg + 1.66g KH + 3.91g Ba12, Ein:123.1 kJ, dE: 3.3 kJ, TSC:
none, Tmax:
390 C, theoretical is 0.88 kJ, gain is 3.8 times.
= 4g AC3-2 + 1g Mg + 1.66g KH + 2.08g BaC12, Ein:141.0 kJ, dE: 5.5 kJ, TSC:
none, Tmax:
403 C, theoretical is 0.52 kJ, gain is 10.5 times.
= 4g AC3-2 + 1g Mg + 1.66g KH + 3.42g SrI2; Ein: 128.2 kJ; dE: 4.35 kJ; TSC:
not
observed; Tmax: 383 C, theoretical is 1.62 kJ, gain is 3.3 times.
= 4.04 g of Sb2S5, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3-2) was finished. The energy gain was 18.0 kJ, and the cell
temperature burst
was 251 C (224 - 475 C). The maximum cell temperature was 481 C,
theoretical is 12.7
kJ, gain is 1.4 times.
123
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= 4g AC3-2 + 1g Mg + 1g NaH + 0.97g ZnS, Ein: 132.1 kJ, dE: 7.5kJ, TSC: none,
Tmax: 370
C, theoretical is 1.4 kJ, gain is 5.33 times.
= 4g AC3-2 + 1g Mg + 1g NaH + 3.12g EuBr2, Ein:135.0 kJ, dE: 5.0kJ, TSC: 114-
182 C,
Tmax: 371 C, theoretical is endothermic +0.35 kJ, gain is infinite.
= 4g AC3-2 + Ig Mg + 1.66g KH + 3.12g EuBr2, Ein:122.0 kJ, dE: 9.4kJ, TSC: 73-
135 C,
Tmax: 385 C, theoretical is 0.28 kJ, gain is 34 times.
= 4g CA3-2 + 1g Mg + 1.66g KH + 3.67g PbBr2; Ein: 126.0 kJ; dE: 6.98 kJ; TSC:
270-408
C; Tmax: 421 C, theoretical is 5.17 kJ, gain is 1.35 times.
= 4g CA3-2 + 1g Mg + Ig NaH + 1.27g AgF; Ein: 125.0 kJ; dE: 7.21 kJ; TSC: 74-
175 C;
Tmax: 372 C, theoretical is 3.58 kJ, gain is 2 times.
= 1.80 g of GdBr3 (0.01 mol GdBr3 is 3.97 g, but there was no enough GdBr3),
1.66 g of KH,
1 g of Mg powder and 4 g of CA-III 300 activated carbon powder (AC3-1), energy
gain was
2.8 kJ, but no cell temperature burst was observed. The maximum cell
temperature was 431
C, theoretical is 1.84 kJ, gain is 1.52 times.
= 0.97 g of ZnS, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3-1), energy gain was 4.0 kJ, but no cell temperature burst was
observed. The
maximum cell temperature was 444 C, theoretical is 1.61 kJ, gain is 2.49
times.
= 3.92 g of B13 (in PP vial), 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III
300 activated
carbon powder (AC3-1), energy gain was 13.2 kJ, and the cell temperature slope
change was
87 C (152 - 239 C). The maximum cell temperature was 465 C, theoretical is
9.7 kJ, gain
is 1.36 times.
= 4g AC3-2 + Ig Mg + Ig NaH + 3.2g HfC14, Ein:131.0 kJ, dE:10.5 kJ, TSC: 277-
439 C,
Tmax: 440 C, theoretical is 8.1 kJ, gain is 1.29 times.
= 4g AC3-2 + 1g Mg + 1.66g KH + 3.2g HfC14, Ein:125.0 kJ, dE:11.5 kJ, TSC: 254-
357 C,
Tmax: 405 C, theoretical is 9.06 kJ, gain is 1.27 times.
= 4g CA3-2 + Ig Mg + 1.66g KH + 2.97g BaBr2; Ein: 132.1 kJ; dE: 4.65 kJ; TSC:
not
observed; Tmax: 361 C, theoretical is 0.64 kJ, gain is 7.24 times.
124
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= 4g CA3-2 + 1g Mg + 1.66g KH + 2.35g AgI; Ein: 142.9 kJ; dE: 7.32 kJ; TSC:
not observed;
Tmax: 420 C, theoretical is 2.46 kJ, gain is 2.98 times.
= 4.12 g of P13, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3-1) was finished. The energy gain was 13.8 kJ, and the cell
temperature burst
was 189 C (184 - 373 C). The maximum cell temperature was 438 C,
theoretical is 11.1
kJ, gain is 1.24 times.
= 1.57 g of SnF2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3-1), energy gain was 7.9 kJ, and the cell temperature slope change
was 72 C
(149 - 221 C). The maximum cell temperature was 407 C, theoretical is 5.28
kJ, gain is 1.5
times.
= 1.96 g of LaF3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3-1), energy gain was 4.2 kJ, but no cell temperature burst was
observed. The
maximum cell temperature was 442 C, theoretical is 0.68 kJ, gain is 6.16
times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.78g MgI2, Ein: 129.0 kJ, dE: 6.6 kJ, TSC:
none,
Tmax: 371 C, theoretical is 1.75 kJ, gain is 3.8 times.
= 4g CAIII-300 + 1g Mg + 1.66g KH + 2.48g SrBr2, Ein:137.0 kJ, dE: 6.1 kJ,
TSC: none,
Tmax: 402 C, theoretical is 1.35 kJ, gain is 4.54 times.
= 4g CA3-2 + 1g Mg + 1.66g KH + 2.Og CaBr2; Ein: 147.0 kJ; dE: 6.33 kJ; TSC:
not
observed; Tmax: 445 C, theoretical is 1.71 kJ, gain is 3.7 times.
= 4g CA3-2 + 1g Mg + 1g NaH + 2.97g BaBr2; Ein: 140.1 kJ; dE: 8.01 kJ; TSC:
not
observed; Tmax: 405 C, theoretical is 0.02 kJ, gain is 483 times.
= 0.90 g of CrF2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3-1) was finished. The energy gain was 4.7 kJ, but no cell
temperature burst was
observed. The maximum cell temperature was 415 C, theoretical is 3.46 kJ,
gain is 1.36
times.
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + InCI 7.5 gm, Ein 275 kJ, dE: 26 kJ, No
TSC with
Tmax- 340 C. Energy Gain - 2.2 X (X11.45 kJ).
125
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + InI 12.1 gm, Ein 320 kJ, dE: 12 kJ, No
TSC with
Tmax- 340 C. Energy Gain - 1.25 X (X-9.6 kJ).
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + InBr 9.75 gm, Ein 323 kJ, dE: 17 U, No
TSC
with Tmax- 340 C. Energy Gain - 1.7X (X-10 kJ).
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + Mn12 15.45 gm VALIDATION Experiment for
Dr. Peter Jansson, Ein 292 kJ, dE: 45 U, Small TSC at -30 C with Tmax- 340
C. Energy
Gain - 2.43X (X18.5 kJ).
- KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + FeBr2 10.8 gm (FeBr2 from STREM
Chemicals)
VALIDATION Experiment for Dr. Peter Jansson, Ein: 308 kJ, dE: 46 kJ, TSC at -
220 C
with Tmax- 330 C. Energy Gain -1.84X (X-25 kJ).
- KH 8.3 gm + Mg_ 5.0 gm + TiC 20.0gm + Co12_15.65 gm, Ein: 243 kJ, dE: 55 kJ,
Small
TSC at -170 C with Tmax-330 C, theoretical is 26.35 kJ, gain is 2.08 times.
- KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + NiBr2 11.0gm, Ein: 270 kJ, dE: 45 kJ,
TSC at
-220 C with Tmax- 340 C, theoretical is 23 kJ, gain is 1.95 times.
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + FeBr2 10.8 gm (FeBr2 from STREM
Chemicals),
Ein: 291 kJ, dE: 38 kJ, TSC at -200 C with Tmax- 330 C, theoretical is 25
kJ, gain is 1.52
times.
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + ZnBr2_11.25 gm, Ein 302 kJ, dE: 42
kJ,
Small TSC at - 200 C with Tmax- 375 C. Energy Gain - 2X (X-20.9 kJ).
= KH 8.30 gm + Mg 5.0 gm + TiC 20.0gm + GdBr3 19.85gm, Ein: 308 kJ, dE: 26 kJ,
TSC at
-250 C with Tmax-340 C. Energy Gain- 1.3X (X-20.3 kJ).
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + MnS 4.35 gm, Ein: 349 kJ, dE: 24
kJ, TSC
at - 260 C with Tmax- 350 C. Energy Gain - 3.6 X (X-6.6 kJ).
= 4g CAIII-300 + 1g Mg + 1g NaH + 3.79g LaBr3, Ein:143.0 kJ, dE:4.8 kJ, TSC:
none,
Tmax: 392 C, theoretical is 2.46 kJ, gain is 1.96 times.
= 4g CAIII-300 + 1g Mg + 1.66g KH + 3.80g CeBr3, Ein: 145.0 kJ, dE:7.6 kJ,
TSC: none,
Tmax: 413 C, theoretical is 3.84 kJ, gain is 1.97 times.
126
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= 4g CAIII-300 + 1g Mg + 1.66g KH + 1.44g AgCl; Ein: 136.2 kJ; dE: 7.14 kJ;
TSC: not
observed; Tmax: 420 C, theoretical is 2.90 kJ, gain is 2.46 times.
= 4g CAIII-300 + 1g Mg + 1.66g KH + 1.60g Cu2S, Ein:137.0 kJ, dE:5.5 kJ, TSC:
none,
Tmax: 405 C, theoretical is 2.67 kJ, gain is 2.06 times.
= 2.54 g of TeI4 (0.01 mol TeI4 is 6.35 g, but no enough TeI4), 1.66 g of KH,
1 g of Mg
powder and 4 g of CA-III 300 activated carbon powder (AC3-1), energy gain was
8.3 kJ, and
the cell temperature burst was 113 C (202 - 315 C). The maximum cell
temperature was
395 C, theoretical is 5.61 U, the gain is 1.48 times.
- 2.51 g of BBr3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (AC3-1), energy gain was 12.4 kJ. The cell temperature slope change was
52 C (77 -
129 C), and the cell temperature burst was 88 C (245 - 333 C). The maximum
cell
temperature was 438 C, theoretical is 9.28 kJ, the gain is 1.34 times.
= 4g CAIII-300 + 1g Mg + 1.Og NaH + 3.59g TaC15, Ein:102.0 kJ, dE:16.9 kJ,
TSC: 80-293
C, Tmax: 366 C, theoretical is 11.89 kJ, gain is 1.42 times.
= 2.72 g of CdBr2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 6.6 kJ, and the cell temperature
burst was 56 C
(253 - 309 C). The maximum cell temperature was 414 C, theoretical is 4.31
kJ, gain is
1.53 times.
= 2.73 g of MoC15, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 20.1 kJ, and the cell temperature
burst was 240 C
(67 - 307 C). The maximum cell temperature was 511 C, theoretical is 15.04
kJ, gain is
1.34 times.
= 2.75 g of InBr2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 7.3 kJ, but no cell temperature
burst was observed.
The maximum cell temperature was 481 C, theoretical is 4.46 U, gain is 1.64
times.
= 1.88 g of NbF5, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 15.5 kJ, but no cell temperature
burst was
observed. The maximum cell temperature was 448 C, theoretical is 11.36 kJ,
gain is 1.36
times.
127
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= 2.33 g of ZrC14, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-111 300
activated carbon
powder (dried at 300 C), energy gain was 12.9 kJ, and the cell temperature
burst was 156 C
(311 - 467 C). The maximum cell temperature was 472 C, theoretical is 8.82
kJ, gain is
1.46 times.
= 3.66 g of CdI2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 6.7 kJ, and the cell temperature
slope change was
74 C (125 - 199 C). The maximum cell temperature was 417 C, theoretical is
4.12 kJ,
gain is 1.62 times.
= 4g CAIII-300 + 1g Mg + 1.66g KH + 2.64g GdC13; Ein: 127.0 kJ; dE: 4.82 kJ;
TSC: not
observed; Tmax: 395 C, theoretical is 3.54 kJ, gain is 1.36 times.
= KH 8.3 gm+ Mg 5.0 gm + CAII-300 20.Ogm+ Ind 7.5 gm, Ein: 305 kJ, dE: 32 kJ,
Small
TSC at - 150 C with Tmax-350 C. Energy Gain - 2.8X (X11.5 kJ).
= KH 8.3 gm+ Mg 5.0 gm +WC 20.Ogm+ Co12 15.65 gm, Ein: 306 kJ, dE: 41 kJ,
Small TSC
at -200C with Tmax-350 C. Energy Gain - 1.55 X (X26.4 kJ).
= NaH 5.0 gm+ Mg 5.0 gm +WC 20.Ogm+GdBr3 19.85gm, Ein 309 kJ, dE: 28 kJ, Small
TSC
at -250 C with Tmax-340 C. Energy Gain- 1.8X (X-15.6 kJ).
= KH_4.98 gm+ Mg_ 3.0 gm + CAII-300_12.Ogm+ InBr 5.85 gm 3X system, Ein: 297
kJ,
dE: 13 kJ, Small TSC at - 200 C with Tmax-- 330 C. Energy Gain - 1.3X (X10
kJ).
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.26g Y203, Ein:133.1kJ, dE: 5.2kJ, TSC:
none, Tmax:
384 C, theoretical is 1.18 kJ, the gain is 4.44 times.
= 4.11 g of ZrBr4, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 11.2 kJ, and the cell temperature
burst was 154 C
(280 - 434 C). The maximum cell temperature was 444 C, theoretical is 9.31
kJ, gain is
1.2 times.
= 5.99 g of Zr14, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 11.3 kJ, and the cell temperature
burst was 200 C
(214 - 414 C). The maximum cell temperature was 454 C, theoretical is 9.4
kJ, gain is 1.2
times.
128
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= 2.70 g of NbC15, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-111 300
activated carbon
powder (dried at 300 C), energy gain was 16.4 kJ, and the cell temperature
burst was 213 C
(137 - 350 C). The maximum cell temperature was 395 C, theoretical is 13.40
kJ, gain is
1.22 times.
= 2.02 g of MoC13, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-111 300
activated carbon
powder (dried at 300 C), energy gain was 12.1 kJ, but no cell temperature
burst was
observed. The maximum cell temperature was 536 C, theoretical is 8.48 kJ,
gain is 1.43
times.
= 3.13 g of Ni12, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 8.0 kJ, and the cell temperature
burst was 33 C
(335 - 368 C). The maximum cell temperature was 438 C, theoretical is 5.89
kJ, gain is
1.36 times.
= 3.87 g of As2Se3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 12.3 kJ, and the cell temperature
burst was 241 C
(195 - 436 C). The maximum cell temperature was 446 C, theoretical is 8.4
kJ, gain is
1.46 times.
= 2.74 g of Y2S3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 5.2 kJ, but no cell temperature
burst was observed.
The maximum cell temperature was 444 C, theoretical is 0.41 kJ, gain is 12.64
times.
= 4g CAIII-300 + 1g Mg + 1.66g KH + 3.79g LaBr3, Ein:147.1 kJ, dE:7.1 kJ, TSC:
none,
Tmax: 443 C, theoretical is 3.39 U, gain is 2 times.
= 4g CAIII-300 + 1g Mg + 1.66g KH + 2.15g MnBr2; Ein: 124.0 kJ; dE: 5.55 kJ;
TSC: 360-
405 C; Tmax: 411 C, theoretical is 3.63 kJ, gain is 1.53 times.
= 2.60 g of Bi(OH)3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-111 300
activated carbon
powder (dried at 300 C), energy gain was 14.8 kJ, and the cell temperature
burst was 173 C
(202 - 375 C). The maximum cell temperature was 452 C, theoretical is 12.23
kJ, the gain
is 1.2 times.
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + Sn12 18.5 gm Strem, Ein: 244 kJ, dE: 53
kJ, TSC
at - 150 C with Tmax- 330 C, theoretical is 28.1 kJ, gain is 1.9 times.
129
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + FeBr2 10.8 gm, Ein: 335 kJ, dE: 43 kJ,
TSC at
-250 C with Tmax- 375 C, theoretical is 22 kJ, gain is 1.95 times.
= KH 8.3 gm + Mg 5.0 gm + WC 20.0gm + FeBr2 10.8 gm, Ein: 335 kJ, dE: 32 kJ,
TSC at
230 C with Tmax- 360 C, theoretical is 22 kJ, gain is 1.45 times.
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + Mn12 15.45 gm Strem, Ein: 269 kJ, dE:
49 kJ,
Small TSC at -50 C with Tmax-350 C. Energy Gain- 3.4X (X-14.8kJ).
= 4g CAIII-300 + 1.66g Ca + 1g NaH + 3.09g MnI2; Ein: 112.0 kJ; dE: 9.98 kJ;
TSC: 178-
374 C; Tmax: 383 C, theoretical is 5.90 kJ, gain is 1.69 times.
= 0.96 g of CuS, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 5.5 kJ, but no cell temperature
burst was observed.
The maximum cell temperature was 409 C, theoretical is 2.93 kJ, the gain is
1.88 times.
= 0.87 g of MnS, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 4.7 kJ, but no cell temperature
burst was observed.
The maximum cell temperature was 412 C, theoretical is 1.32 kJ, the gain is
3.57 times.
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + Mn12 15.45 gm, Ein: 269 kJ, dE: 49 kJ,
Small
TSC at -50 C with Tmax-350 C, theoretical is 18.65 U, gain is 2.6 times.
= NaH 5.0 gm + Mg 5.0 gm + TiC 20.0gm + NiBr2 11.0 gm, Ein: 245 kJ, dE: 43 kJ,
TSC at
200 C with Tmax- 310 C, theoretical is 26 kJ, gain is 1.6 times.
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + MnC12 6.3 gm, Ein: 333 kJ, dE: 34
kJ, TSC
at -250 C with Tmax- 340 C, theoretical is 17.6 kJ, gain is 2 times.
= 2.42 g of InI, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 4.4 kJ, but no cell temperature
burst was observed.
The maximum cell temperature was 438 C, theoretical is 1.92 kJ, the gain is
2.3 times.
= 1.72 g of InF3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 9.2 kJ, but no cell temperature
burst was observed.
The maximum cell temperature was 446 C, theoretical is 5 kJ, gain is 1.85
times.
130
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= 4g CAIII-300 + 1g Mg + 1g NaH + 1.98g As203, Ein:110.5kJ, dE:17.1 kJ,
TSC:325-452
C, Tmax: 471 C, theoretical is 11.48 kJ, gain is 1.49 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 4.66g Bi203, Ein:152.OkJ, dE:17.7kJ, TSC:185-
403 C,
Tmax: 481 C, theoretical is 13.8 kJ, gain is 1.28 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.02g MoC13; Ein: 118.0 kJ; dE: 11.10 kJ;
TSC: 342-
496 C; Tmax: 496C, theoretical is 7.76, gain is 1.43 times.
= 2.83 g of PbF4, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-I11 300
activated carbon
powder (dried at 300 C), energy gain was 13.9 kJ, and the cell temperature
burst was 245 C
(217 - 462 C). The maximum cell temperature was 464 C, theoretical is 13.38
kJ, gain is
1.32 times.
= 2.78 g of PbC12, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 6.8 kJ, but no cell temperature burst
was observed.
The maximum cell temperature was 488 C, theoretical is 5.22 kJ, gain is 1.3
times.
= 4g CAIII-300 + 1.66g KH + 2.19g NiBr2, Ein:136.OkJ, dE: 7.5 kJ, TSC:275-
350C, Tmax:
385C, theoretical is 4.6 kJ, gain is 1.6 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.74g MoC15, Ein: 96.0 U, dE:19.OkJ, TSC: 86-
334C,
Tmax: 373C, theoretical is 14.06 kJ, gain is 1.35 times.
= 4g CAIII-300 + 1.66g Ca + 1g NaH + 2.19g NiBr2; Ein: 127.1 U; dE: 10.69 kJ;
TSC: 300-
420C; Tmax: 10.69C, theoretical is 7.67 kJ, gain is 1.39 times.
= 5.90 g of Bi13, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-111 300
activated carbon
powder (dried at 300 C), energy gain was 10.9 kJ, and the cell temperature
slope change was
70 C (217 - 287 C). The maximum cell temperature was 458 C, theoretical is
8.87 kJ, gain
is 1.23 times.
= 1.79 g of SbF3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-I11 300
activated carbon
powder (dried at 300 C), energy gain was 11.7 kJ, and the cell temperature
burst was 169 C
(138 - 307 C). The maximum cell temperature was 454 C, theoretical is 9.21 kJ,
gain is 1.27
times.
= 4g CAIII-300 + 1.66g Ca + 1g NaH + 3.09g MnI2, Ein:111.0 kJ, dE:12.6 kJ,
TSC:178-
131
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
340C, Tmax: 373C, theoretical is 5.9 kJ, the gain is 2.13 times.
= 4g CAIII-300 + 1.66g Ca + 1g NaH + 1.34g CuC12; Ein: 135.2 kJ; dE: 12.26 kJ;
TSC: 250-
390C; Tmax: 437C, theoretical is 8.55 kJ, the gain is 1.43 times.
= 1.50 g of InCI, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 5.1 kJ, but no cell temperature burst
was observed.
The maximum cell temperature was 410 C, theoretical is 2.29 kJ, the gain is
2.22 times.
= 2.21 g of InC13, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 10.9 kJ and the cell temperature
burst was 191 C
(235 -426 Q. The maximum cell temperature was 431 C, theoretical is 7.11 kJ,
the gain is
1.5 times.
= 1.95 g of InBr, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 6.0 kJ, but no cell temperature burst
was observed.
The maximum cell temperature was 435 C, theoretical is 2 kJ, the gain is 3
times.
= 3.55 g of InBr3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 9.1 kJ, and the cell temperature
burst was 152 C
(156 - 308 C). The maximum cell temperature was 386 C, theoretical is 6.92 kJ,
the gain is
1.3 times.
= 4g CAIII-300 + 1.66g KH + 3.79g Sn12, Ein: 169.1 kJ, dE: 6.0 kJ, TSC:200-
289C, Tmax:
431C, theoretical is 4.03 kJ, the gain is 1.49 times.
= KH 8.3 gm + Mg 5.0 gm + WC 20.0gm + MnBr2 10.75 gm, Ein: 309 kJ, dE: 35 kJ,
No
TSC with Tmax-335C. Energy Gain-1.9X (X-18.1 kJ).
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + MnBr2 10.75 gm, Ein: 280 kJ, dE:
41 U,
TSC at -280C with Tmax-350C. Energy Gain - 2.2 X (X-18.1 kJ).
= KH 1.66 gm + Mg 1.0 gm + TiC 4.0gm + TiF3 1.05 gm 5X Cell#1086 with CAII-
300,
Ein: 143 kJ, dE: 6 U, No TSC with Tmax-280C, theoretical is 2.5 kJ, gain is
2.4 times.
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + FeF2 4.7 gm, Ein: 280 kJ, dE: 40
kJ, TSC at
-260C with Tmax-340C, theoretical is 20.65 kJ, gain is 1.93 times.
132
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + CuF2 5.1 gm, Ein: 203 kJ, dE: 57
kJ, TSC
at -125C with Tmax-280C, theoretical is 29 kJ, gain is 1.96 times.
= KH 83.0gm + Mg 50.0gm + WC 200.0gm + Sn12 185 gm URS, Ein: 1310 kJ, dE: 428
kJ,
TSC at-140C with Tmax-350C, theoretical is 200 kJ, gain is 2.14 times.
061009KAWFCI#1102 NaH 1.0 gm+ Mg 1.0 gm + WC 4.Ogm+GdBr3_3.97gm, Ein: 148
kJ, dE: 7 U, Small TSC at -300C with Tmax-420C. Energy Gain-3.5 X (X-2 kJ).
= KH 8.3 gm+ Mg 5.0 gm + CAII-300 20.0gm + FeO 3.6 gm, Ein: 355 kJ, dE: 24 U,
Small
TSC at -260C with Tmax-360C. Energy Gain-1.45 X (X-16.6 kJ).
= KH 83.0gm+Mg 50.0gm + WC 200.0gm+Snl2 185 gm ROWAN, Ein: 1379 kJ, dE: 416
kJ,
TSC at-140C with Tmax-350C, theoretical is 200 kJ, gain is 2 times.
= KH 8.3 gm+ Mg 5.0 gm + CAII-300 20.0gm + Co12 15.65 gm, Ein: 361 kJ, dE: 69
kJ, TSC
at -200C with Tmax-410C, theoretical is 26.35 kJ, gain is 2.6 times.
= KH 8.3 gm+ 5.0 gm + CAII 300 20.0gm + FeS 4.4 gm, Ein: 312 kJ, dE: 22 kJ, No
TSC
with Tmax--350C. Energy Gain -1.7 X (X12.3 kJ).
= KH 8.3 gm+ WC 40.0gm + SnI2 18.5 gm, Ein: 315 kJ, dE: 27 kJ, Small TSC at -
140C with
Tmax--340C. Energy Gain-1.35 X (X-20 kJ).
= NaH 5.0 gm+ Mg 5.0 gm + WC 20.0gm + Mn12 15.45 gm, Ein: 108 kJ, dE: 30 kJ,
TSC at
-70C with Tmax-170C, theoretical is 14.8 kJ, gain is 2 times.
= NaH 5.0 gm+ Mg 5.0 gm + WC 20.0gm + NiBr2 11.0 gm, Ein: 248 kJ, dE: 34 kJ,
TSC at
-170C with Tmax-300C. Energy Gain-1.7 X (X-20 kJ) , theoretical is 26.25 kJ,
gain is 1.3
times.
= KH 8.3 gm + Mg 5.0 gm + WC 20.0gm + NiBr2 11.0 gm, Ein: 291 kJ, dE: 30 kJ,
Small
TSC at -250C with Tmax-340C. Energy Gain-1.5 X (X-20 kJ), theoretical is 26.25
U, gain
is 1.14 times.
= NaH 5.0 gm+ Mg 5.0 gm + WC 20.Ogm+ NiBr2 11.0 gm Repeat of Cell#1105, Ein:
242 kJ,
dE: 33 kJ, TSC at -70C with Tmax--280C. Energy Gain-1.65 X (X-20 kJ).
133
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= NaH 5.0 gm + Mg 5.0 gm + CAII-300 20.0gm + InC13 11.1 gm, Ein: 189 kJ, dE:
48 kJ,
Small TSC at -80C with Tmax- 260 C. Energy Gain- 1.5X (X-31 kJ).
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + Mn12 15.45 gm, Ein: 248 kJ, dE: 46
kJ,
Small TSC at -2000 with Tmax- 325C. Energy Gain- 3 X (X-14.8 kJ).
= 2.96 g of FeBr3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-111 300
activated carbon
powder (dried at 300 C), energy gain was 12.5 kJ, and the cell temperature
burst was 77 C
(72 -149 Q. The maximum cell temperature was 418 C, theoretical is 8.35 kJ,
the gain is 1.5
times.
= 0.72 g of FeO, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 6.7 kJ, but no cell temperature burst
was observed.
The maximum cell temperature was 448 C, theoretical is 3.3 kJ, the gain is 2
times.
= 1.26 g of MnC12, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 8.6 kJ, but no cell temperature burst
was observed.
The maximum cell temperature was 437 C, theoretical is 3.52 kJ, the gain is
2.45 times.
= 1.13 g of FeF3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 12.6 kJ, but no cell temperature
burst was
observed. The maximum cell temperature was 618 C, theoretical is 6.44 kJ, the
energy gain is
1.96 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 3.97g GdBr3, Ein:143.1 kJ, dE: 5.4 kJ,
TSC:none,
Tmax: 403C, theoretical is 1.99 kJ, the gain is 2.73 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 1.57g SnF2; Ein: 139.0 kJ; dE: 7.24 kJ; TSC:
not
observed; Tmax: 413C, theoretical is 5.28kJ, gain is 1.37 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 4.04g Sb2S5,Ein:125.0 kJ, dE:19.3 kJ,
TSC:421-651C,
Tmax: 651C, theoretical is 12.37kJ, gain is 1.56 times.
= 1.36 g of ZnC12, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 6.6 kJ, but no cell temperature burst
was observed.
The maximum cell temperature was 402 C, theoretical is 4.34kJ, gain is 1.52
times.
= 1.03 g of ZnF2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
134
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
powder (dried at 300 C), energy gain was 6.5 kJ, but no cell temperature burst
was observed.
The maximum cell temperature was 427 C, theoretical is 3.76kJ, gain is 1.73
times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.22g InC13, Experimental dE: -12.6kJ
Reaction
considered: InC13(c) + 3NaH(c) + 1.5Mg(c) = 3NaC1(c) + In(c) + 1.5MgH2(c) Q=-
640.45kJ/reaction theoretical chemical reaction energy: -6.4kJ, Excess heat: -
6.2kJ, 2.0X
excess heat.
= 1.08 g of VF3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 9.5 kJ, but no cell temperature burst
was observed.
The maximum cell temperature was 447 C, theoretical is 4.9 kJ, the gain is
1.94 times.
= 8.3g KH + 5.Og Mg + 20.0g AC (11-300) + 5.4g VF3, Ein: 286 U, dE: 58 kJ,
theoretical is
24.5 kJ, gain is 2.3 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 1.72g InF3, Ein:134.0 kJ, dE: 8.1 kJ,
TSC:none, Tmax:
391C, theoretical is 5 kJ, gain is 1.62 times.
= 4g CAIII-300 + 1g Mg + 1.66g KH + 1.02g CuF2, Experimental dE: -9.4 kJ
Reaction
considered: CuF2(c) + Mg(c) = MgF2(c) + Cu(c) Q=-581.5kJ/reaction theoretical
chemical
reaction energy: -5.82kJ, Excess heat: -3.59kJ, 1.6X excess heat.
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.83g PbF4, Experimental dE: -17.6 kJ
Reaction
considered: PbF4(c) + 2Mg(c) + 4NaH(c) = 2MgH2(c) + 4NaF(c) + Pb(c) Q=-
1290.OkJ/reaction theoretical chemical reaction energy: -12.9kJ, Excess heat: -
4.7kJ 1.4X
excess heat.
= KH 1.66 gm + Mg 1.0 gm + TiC 4.0gm + Sn14 6.26gm, Ein: 97 U, dE: 17 kJ, TSC
at
-150C with Tmax-370C, theoretical is 10.1 U, the gain is 1.7 times.
= 4g CAIII-300 + 1g Mg + 1.66g KH + 3.7g TiBr4, Experimental dE: -16.1kJ
Reaction
considered: TiBr4(c) + 4KH(c) + 2Mg(c) + C(s) = 4KBr(c) + TiC(c) + 2MgH2(c) Q=-
1062.3kJ/reaction theoretical chemical reaction energy: -10.7kJ, Excess heat: -
5.4kJ 1.5X
excess heat.
B13
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.4g B13, Ein:128.1 U, dE: 7.9 kJ, TSC:180-
263C,
Tmax: 365C, theoretical is 5.55 kJ, the gain is 1.4 times.
135
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
MnBr2
= 4g CAIII-300 + 1g Mg + 1.66g KH + 2.15g MnBr2, Experimental dE: -7.OkJ
Reaction
considered: MnBr2(c) + 2KH(c) + Mg(c) = 2KBr(c) + Mn(c) + MgH2(c) Q=-
362.6kJ/reaction theoretical chemical reaction energy: -3.63kJ, Excess heat: -
3.4kJ 1.9X
excess heat.
= KH 8.3 gm + Mg 5.0 gm + WC 20.0gm + MnBr2 10.75 gm, Ein: 309 kJ, dE: 35 kJ,
No
TSC with Tmax-335C. Energy Gain-1.9X (X18.1 kJ).
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + MnBr2 10.75 gm, Ein: 280 kJ, dE:
41 U,
TSC at -280C with Tmax--350C. Energy Gain - 2.2 X (X18.1 kJ).
FeF2
= 4g CAIII-300 + 1g Mg + 1.66g KH + 0.94g FeF2, Experimental dE: -9.8kJ
Reaction
considered: FeF2(c) + Mg(c) = MgF2(c) + Fe(c) Q=-412.9kJ/reaction theoretical
chemical
reaction energy: -4.13kJ, Excess heat: -5.67kJ, 2.4X excess heat.
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + FeF2 4.7 gm, Ein: 280 kJ, dE: 40
kJ, TSC
at -260C with Tmax-340C, theoretical is 20.65 kJ, the gain is 1.94 times.
TiF3
= KH 1.66 gm + Mg 1.0 gm + TiC 4.0gm + TiF3 1.05 gm (5X Cell#1086 with CAII-
300),
Ein: 143 kJ, dE: 6 kJ, No TSC with Tmax-280C, theoretical is 2.5, the gain is
2.4 times.
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + TiF3 5.25 gm, Ein: 268 kJ, dE: 7
kJ, No
TSC with Tmax-280C. No Energy Gain (X-21.7 kJ).
CuF2
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + CuF2 5.1 gm, Ein: 203 kJ, dE: 57
U, TSC
at -125C with Tmax-280C, the theoretical is 29.1 kJ, the gain is 2 times.
Mn12
= NaH 4.0 gm + Mg 4.0 gm + CAII-300 16.0gm + Mn12 12.36 gm (4X Scale up), Ein:
253
kJ, dE: 30 U, No TSC with Tmax-300C, theoretical is 11.8 kJ, gain is 2.5
times.
= The heat measurement with 3.09 g of Mn12, 1.66 g of KH, 1 g of Mg powder and
4 g of
CA-III 300 activated carbon powder (dried at 300 C), energy gain was 8.8 kJ,
and the cell
136
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
temperature burst was 92 C (172 - 264 C). The maximum cell temperature was 410
C,
theoretical is 2.96 kJ, gain is 3 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 3.09g Mn12, Ein:126.1 kJ, dE: 8.0 kJ,
TSC:157-241C,
Tmax: 385C, theoretical is 2.96 kJ, the gain is 2.69 times.
ZnBr2
- 2.25 g of ZnBr2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 10.3 kJ, and the cell temperature
burst was 82 C
(253 - 335 C). The maximum cell temperature was 456 C, theoretical is 3.56 kJ,
gain is 2.9
times.
= NaH 5.0 gm + Mg 5.0 gm + CAII-300 20.0gm + ZnBr2 11.25 gm, Ein: 291 kJ, dE:
26 kJ,
No TSC with Tmax-330C, theoretical is 17.8 kJ, gain is 1.46 times.
CoCl2
- 1.3 g of CoC12, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 10.4 kJ, and the cell temperature
slope change was
105 C (316 - 421 C). The maximum cell temperature was 450 C, theoretical is
5.2 kJ, gain is
2 times.
= 1.3 g of CoC12, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 9.6 kJ, and the cell temperature
burst was 181 C
(295 - 476 Q. The maximum cell temperature was 478 C, theoretical is 5.2 kJ,
the gain is
1.89 times.
SnBr2
= 2.8 g of SnBr2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 14.2 kJ, and the temperature burst
was 148 C(148 -
296 C). The maximum cell temperature was 376 C, theoretical is 3.75 kJ, gain
is 3.78 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.79g SnBr2, Ein:116.0 kJ, dE: 7.7 kJ,
TSC:135-236C,
Tmax: 370C, theoretical is 3.75 kJ, the gain is 2 times.
- KH 8.3 gm + Mg Powder 5.0 gm + CAll 300 20.0gm + SnBr2 11.4 gm, Ein: 211 kJ,
dE: 41
kJ, TSC at -170C with Tmax-3000; theoretical is 15.5 kJ, the gain is 2.6
times.
= KH 8.3 gm + Mg 5.0 gm + TiC 20.0gm + SnBr2 14.0 gm , Ein 229 kJ, dE: 46 kJ,
TSC at
137
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
-150C with Tmax-310C and Gain-2.4X (X-19kJ), theoretical is 18.8 kJ, gain is
2.4 times.
= KH 1.66 gm + Mg 1.0 gm + WC 4.0gm + SnBr2 2.8 gm, Ein: 101 kJ, dE: 10 kJ,
TSC at
-150C with Tmax-350C, theoretical is 3.75 kJ, the gain is 2.66 times.
= 4g CAIII-300 + 1.66g KH + 2.79g SnBr2, Ein:132.0 kJ, dE: 9.6 kJ, TSC:168-
263, Tmax:
381C, theoretical is 4.29 kJ, the gain is 2.25 times.
= 1g Mg + 1.66g KH + 2.79g SnBr2; Ein: 123.0 kJ; dE: 7.82 U; TSC: 125-220C;
Tmax:
386C, theoretical is 5.85 kJ, the gain is 1.33 times.
Sn12
= KH 6.64gm + Mg Powder 4.0 gm + TiC 18.0gm + Sn12 14.8 gm, Ein: 232 kJ, dE:
47 kJ,
TSC at -150C with Tmax-280C. Energy Gain - 3.6X (X-12.8 kJ), theoretical is
12.6 kJ, the
gain is 3.7 times.
= 3.7 g of Sn12, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-111 300
activated carbon
powder (dried at 300 C), energy gain was 11.9 kJ, but no temperature burst was
observed.
The maximum cell temperature was 455 C, theoretical is 3.2 kJ, gain is 3.7
times.
= KH 1.6 gm + Mg Powder 1.0 gm + TiC 4.0gm + Sn12 3.7 gm, Ein: 162 kJ, dE: 13
kJ; TSC
at 100C with Tmax-490C; theoretical is 3.2 kJ, gain is 4 times.
= KH 8.3 gm + Mg Powder 5.0 gm + CAII 300 20.0gm + Sn12 18.5 gm, Ein: 221 kJ,
dE: 47
kJ, TSC at -170C with Tmax-300C, theoretical is 15.9 kJ, the gain is 3 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 3.73g Sn12; Ein: 121.9 kJ; dE: 7.56 kJ; TSC:
not
observed; Tmax: 391C, theoretical is 3.2 kJ, the gain is 2.36 times.
= 1.66g KH + 3.79g Sn12, Ein:114.OkJ, dE: 8.8kJ, TSC:161-259C, Tmax: 359C,
theoretical is
4 kJ, the gain is 2.17 times.
SnC12
= NaH 5.0 gm + Mg 5.0 gm + CAII-300 20.0gm + SnC12 9.6 gm, Ein: 181 kJ, dE: 30
kJ,
TSC at -140C with Tmax-280C, theoretical is 19 kJ, the gain is 1.57 times.
138
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
NiBr2
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.19g NiBr2; Ein: 126.0 kJ; dE: 12.01 kJ;
TSC: 290-
370C; Tmax: 417C, theoretical is 4 kJ, gain is 3 times.
= NaH 1.0 gm + MgH2 Powder 1.0 gm + TiC 4.0gm) Mix +NiBr2_2.2 gm, Ein: 121 kJ,
dE:
11 kJ, Temp. slope Jump at 260C with Tmax-390C, theoretical is 4 kJ, gain is
2.75 times.
= 4g CAIII-300 + 1g Al + 1g NaH + 2.19g NiBr2; Ein: 122.0 kJ; dE: 7.78 kJ;
TSC: not
observed; Tmax: 392C, theoretical is 4 kJ, gain is 1.95 times.
= 4g CAIII-300 + 1g Mg + 0.33g LiH + 2.19g NiBr2; Ein: 128.0 kJ; dE: 10.72 kJ;
TSC: 270-
436C; Tmax: 440C, theoretical is 4 kJ, gain is 2.68 times
= 4g CAIII-300 + 1g Mg + 1.66g KH + 2.19g NiBr2; Ein: 126.0 kJ; dE: 10.45 kJ;
TSC: 285-
423C; Tmax: 423C, theoretical is 4 kJ, gain is 2.6 times.
= 4g CAIII-300 + 1g MgH2 + 1g NaH + 2.19g NiBr2; Ein: 138.1 kJ; dE: 8.12 kJ;
TSC: not
observed; Tmax: 425C, theoretical is 4 kJ, gain is 2 times.
= NaH 5.0 gm + Mg Powder 5.0 gm + Activated Carbon CAII 300 20.0gm) Mix +
NiBr2 11.0
gm (Theoretical 23.6 kJ), Ein: 224 U, dE: 53 kJ, Temp. slope Jump at 160C with
Tmax--280C, theoretical is 20 kJ, the gain is 2.65 times.
= NaH 1.0 gm + Mg 1.0 gm + WC 4.0gm + NiBr2 2.2gm, Ein: 197 kJ, dE: 11 kJ,
Small TSC
at -2000 with Tmax-500C ; theoretical is 4 kJ, the gain is 2.75 times.
= NaH 50.0gm + Mg 50.0gm + CAII-300 200.0gm + NiBr2 109.5 gm, Ein: 1990 kJ,
dE: 577
kJ, TSC at -140C with Tmax-980C, theoretical is 199 kJ, gain is 2.9 times.
= no Mg control: 4g CAIII-300 + 1g NaH + 2.19g NiBr2; Ein: 134.0 kJ; dE: 5.37
kJ; TSC:
not observed; Tmax: 375C, theoretical is 3.98kJ, the gain is 1.35 times.
= control: 1g Mg + 1g NaH + 2.19g NiBr2; Ein: 129.0 kJ; dE: 5.13 kJ; TSC: 195-
310C;
Tmax: 416C, theoretical is 5.25kJ.
= control: 19 NaH + 2.19g NiBr2; Ein: 138.2 kJ; dE: -0.18 kJ; TSC: not
observed; Tmax:
377C, theoretical is 3.98kJ.
139
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
CuCl2
= 4g CAIII-300 + 1g Mg + 1g NaH + 1.34g CuC12, Ein:119.0 kJ, dE:10.5kJ,
TSC:250-381C,
Tmax: 393C, theoretical is 4.9 kJ, gain is 2.15 times.
= 4g CAIII-300 + Ig Al + 1g NaH + 1.34g CuC12, Ein:126.0 kJ, dE: 7.4 kJ,
TSC:229-354C,
Tmax: 418C, theoretical is 4.9 kJ, gain is 1.5 times.
= 4g CAIII-300 + 1g MgH2 + Ig NaH + 1.34g CuC12, Ein:144.0 kJ, dE: 8.3 kJ,
TSC:229-
314C, Tmax: 409C, theoretical is 4.9 kJ, the gain is 1.69 times.
= NaH 5.0 gm + Mg Powder 5.0 gm + Activated Carbon CAII 300 20.0gm) Mix +
CuC12
10.75gm (Theoretical is 45 kJ), Ein: 268 kJ, dE: 80 kJ, Temp. slope Jump at
210C with
Tmax-360C, theoretical is 39 kJ, the gain is 2 times.
= 1.4 g of CuCI2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 14.6 kJ,
and the
temperature burst was 190 C(188 - 378 Q. The maximum cell temperature was 437
C,
theoretical is 4.9 kJ, the gain is 3 times.
= KH 8.3 gm + Mg Powder 5.0 gm + CAII-300 20.0gm + CuC12 6.7 gm, Ein: 255 kJ,
dE: 55
kJ, TSC at -200C with Tmax-320C, theoretical is 24.5 kJ, the gain is 2.24
times
CuCI
= 4g CAIII-300 + Ig Mg + 1g NaH + 1g CuCI; Ein: 128.1 kJ; dE: 4.94 U; TSC: not
observed;
Tmax: 395C, theoretical is 2.18 kJ, the gain is 2.26 times.
Co12
= 4g CAIII-300 + Ig Mg + 1g NaH + 3.13g Co12, Ein:141.1kJ, dE:9.7kJ, TSC:none,
Tmax:
411C Reaction considered: 2NaH(c) + Co12(c) + Mg(c) = 2NaI(c) + Co(c) +
MgH2(c) Q=-
449.8kJ/reaction theoretical chemical reaction energy: -4.50kJ, Excess heat: -
5.18kJ, the
gain is 1.9 times.
= 3.13 g of Co12, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 10.7 kJ, and the cell temperature
burst was 117 C
(248 - 365 C). The maximum cell temperature was 438 C, theoretical is 5.27kJ,
gain is 2.03
times.
Zn12
140
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= 4g CAIII-300 + 1g Mg + 1g NaH + 3.19g Zn12, Ein: 157.1 kJ, dE: 5.8 kJ,
TSC:none, Tmax:
330C Reaction considered: 2NaH(c) + Znl2(c) + Mg(c) = 2NaI(c) + Zn(c) +
MgH2(c) Q=-
330.47kJ/reaction theoretical chemical reaction energy: -3.30kJ, Excess heat: -
2.50U, the
gain 1.75 times.
= 3.19 g of Zn12, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 5.9 kJ, and the cell temperature
slope change was
79 C (180 - 259 Q. The maximum cell temperature was 423 C, theoretical is
4.29kJ, gain is
1.38 times.
NiF2
= 4g CAIII-300 + 1g Mg + 1g NaH + 0.97g NiF2, Ein:135.0 kJ, dE:7.9 kJ, TSC:253-
335C,
Tmax: 385C Reaction considered: 2NaH(c) + NiF2(c) + Mg(c) = 2NaF(c) + Ni(c) +
MgH2(c)
Q=-464.4kJ/reaction theoretical chemical reaction energy: -4.64kJ, Excess
heat: -3.24kJ, the
gain is 1.7 times.
= 0.97 g of NiF2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 8.7 kJ, and the cell temperature
slope change was
63 C (256 - 319 Q. The maximum cell temperature was 410 C, theoretical is 5.25
kJ, the
gain is 1.66 times.
CoBr2
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.19g CoBr2, Ein:140.0 kJ, dE:7.6 kJ,
TSC:none,
Tmax: 461C Reaction considered: 2NaH(c) + CoBr2(c) + Mg(c) = 2NaBr(c) + Co(c)
+
MgH2(c) Q=-464kJ/reaction theoretical chemical reaction energy: -4.64kJ,
Excess heat: -
2.9kJ, the gain is 1.64 times.
= 2.19 g of CoBr2, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 10.4 kJ, and the cell temperature
burst was 110 C
(306 - 416 Q. The maximum cell temperature was 450 C, theoretical is 5.27kJ,
gain is 1.97
times.
= 2.19 g of CoBr2, 1.66 g of KH, 1 g of Mg powder
and 4 g of CA-III 300 activated carbon powder (dried at 300 C), energy gain
was 10.2 kJ, but
no cell temperature burst was observed. The maximum cell temperature was 446
C,
theoretical is 5.27 kJ, the gain is 1.94 times.
FeC12
141
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= 4g CAIII-300 + 1g Mg + 1g NaH + 1.27g FeC12, Ein: 155.0 kJ, dE:10.5 kJ,
TSC:none,
Tmax: 450C, theoretical is 3.68 kJ, gain is 2.85 times.
= 4g CAIII-300 + 1g Al + 1g NaH + 1.27g FeC12, Ein: 141.7 kJ, dE: 7.0 kJ,
TSC:none, Tmax:
440C, theoretical is 3.68 kJ, gain is 1.9 times.
= 1.3 g of FeC12, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 11.5 kJ,
and the
temperature burst was 142 C(287 - 429 C). The maximum cell temperature was 448
C,
theoretical is 4.1 kJ, the gain is 2.8 times.
= NaH_5.0 gm+ Mg Powder_ 5.0 gm + Activated Carbon CAII 30020.09m) Mix
+FeC12_6.35gm, Ein: 296 U, dE: 37 kJ, Temp. slope Jump at 220C with Tmax-330C,
theoretical is 18.4 kJ, the gain is 2 times.
FeC13
= 2.7 g of FeC13, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 21.3 kJ, and the cell temperature
burst was 205 C
(147 - 352 C) The maximum cell temperature was 445 C, theoretical is 10.8 kJ,
the gain is
1.97 times.
= NaH 1.0 gm + Mg Powder 1.0 gm + TiC 4.0gm + FeC13 1.6 gm, Ein: 88 kJ, dE: 14
kJ; TSC
at 80C with Tmax--350C, theoretical is 6.65 kJ, gain is 2.1 times.
= KH 8.3 gm + MgH2 Powder 5.0 gm + CAII 300 20.0gm + FeC13 8.1 gm, Ein: 253
kJ, dE:
52 kJ/; No TSC with Tmax-300C, theoretical is 33 kJ, gain is 1.56 times.
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + FeC12 6.5 gm, Ein: 299 kJ, dE: 44
kJ, No
TSC with Tmax-350C, theoretical is 18.9 kJ, gain is 2.3 times.
FeBr2
= 4g CAIII-300 + lg Mg + 1.66g KH + 2.16g FeBr2; Ein: 144.0 kJ; dE: 9.90 kJ;
TSC: not
observed; Tmax: 455C, theoretical is 3.6 kJ, gain is 2.75 times.
= 4g CAIII-300 + 1g MgH2 + 1g NaH + 2.16g FeBr2; Ein: 142.0 kJ; dE: 8.81 kJ;
TSC: not
observed; Tmax: 428C, theoretical is 3.6 kJ, gain is 2.44 times.
= 4g CAIII-300 + lg MgH2 + 0.33g LiH + 2.16g FeBr2; Ein: 164.0 kJ; dE: 8.68
kJ; TSC: not
142
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
observed; Tmax: 450C, theoretical is 3.6 kJ, the gain is 2.4 times.
= 4g CAIII-300 + Ig MgH2 + 1.66g KH + 2.16g FeBr2; Ein: 159.8 kJ; dE: 9.07 kJ;
TSC: not
observed; Tmax: 459C, theoretical is 3.6 kJ, the gain is 2.5 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.96g FeBr2, Experimental dE: -6.7kJ
Reaction
considered: 2NaH(c) + FeBr2(c) + Mg(c) = 2NaBr(c) + Fe(c) + MgH2(c) Q=-
435.lkJ/reaction
theoretical chemical reaction energy: -4.35kJ, Excess heat: -2.35kJ, 1.54X
excess heat.
NiC12
= 4g CAIII-300 + 1g Mg + 1g NaH + 1.30g NiC12, Ein: 112.0 kJ, dE: 9.7 kJ,
TSC:230-368C,
Tmax: 376C, theoretical is 4 kJ, gain is 2.4 times.
= 1.3 g of NiC12, 0.33 g of LiH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 9.2 kJ, and
the
temperature slope change was 100 C(205 - 305 Q. The maximum cell temperature
was 432
C, theoretical is 4 kJ, the gain is 2.3 times.
= 1.3 g of NiC12, 0.33 g of LiH, 1 g of Al powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 8.0 kJ, and
the
temperature slope change was 85 C(206 - 291 C). The maximum cell temperature
was 447 C,
theoretical is 4 kJ, the gain is 2 times.
CuBr
= 4g CAIII-300 + 1g Mg + 1g NaH + 1.44g CuBr; Ein: 125.0 kJ; dE: 4.67 kJ; TSC:
not
observed; Tmax: 382C, theoretical is 2 kJ, the gain is 2.33 times.
= 4g CAIII-300 + 1g Mg + 1.66g KH + 1.44g CuBr, Experimental dE: -7.6kJ
Reaction
considered: CuBr(c) + KH(c) + 0.5Mg(c) = KBr(c) + Cu(c) + 0.5MgH2(c) Q=-
269.2kJ/reaction theoretical chemical reaction energy: -2.70kJ, Excess heat: -
4.9OkJ 2.8X
excess heat.
143
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
CuBr2
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.23g CuBr2; Ein: 118.1 kJ; dE: 8.04 kJ;
TSC: 108-
180C; Tmax: 369C, theoretical is 4.68 kJ, the gain is 1.7 times.
SnF4
= 2.0 g of SnF4, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 18.4 kJ, but no temperature burst was
observed.
The maximum cell temperature was 576 C, theoretical is 9.3 kJ, the gain is
1.98 times
A113
= 4.1 g of A113, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 10.1 kJ, but no temperature burst was
observed.
The maximum cell temperature was 412 C, theoretical is 6.68 kJ, the gain is
1.51 times.
= KH 8.3 gm + Mg 5.0 gm + CAII-300 20.0gm + A113 20.5 gm, Ein: 318 kJ, dE: 48
U,
theoretical is 33.4 kJ, gain is 1.4 times.
SiC14
= 1.7 g of SiC14, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 12.6 kJ, and the temperature burst
was 68 C(366 -
434 C. The maximum cell temperature was 473 C, theoretical is 7.32 kJ, gain is
1.72 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 0.01 mol SiC14 (1.15 cc); Ein: 114.0 kJ; dE:
14.19 kJ;
TSC: 260-410C; Tmax: 423C, theoretical is 7.32 kJ, the gain is 1.94 times.
A1Br3
= 2.7 g of A1Br3, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 7.5 kJ, but no temperature burst was
observed. The
maximum cell temperature was 412 C, theoretical is 4.46 kJ, the gain is 1.68
times.
FeC13
= 2.7 g of FeC13, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 21.3 kJ, and the cell temperature
burst was 205 C
(147 - 352 C) The maximum cell temperature was 445 C, theoretical is 10.8 kJ,
the gain is
1.97 times.
144
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
SeBr4
= 4g CAIII-300 + Ig Mg + Ig NaH + 3.99g SeBr4; Ein: 112.0 kJ; dE: 23.40 kJ;
TSC: 132-
448C; Tmax: 448C, theoretical is 15.7 kJ, the gain is 1.5 times.
SnBr4
= 4g CAIII-300 + 1g Mg + lg NaH + 4.38g SnBr4; Ein: 98.0 kJ; dE: 12.44 kJ;
TSC: 120-
270C; Tmax: 359C, theoretical is 8.4 kJ, the gain is 1.48 times.
= KH 8.3 gm + Mg Powder 5.0 gm + CAII 300 20.0gm + SnBr4 22.0 gm, Ein: 163 kJ,
dE: 78
kJ; TSC at 60C with Tmax-290C, theoretical is 42 kJ, gain is 1.86 times.
SiBr4
= 3.5 g of SiBr4, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C), energy gain was 11.9 kJ, and the temperature burst
was 99 C(304 -
403 Q. The maximum cell temperature was 449 C, theoretical is 7.62 kJ, the
gain is 1.56
times.
TeBr4
= 4g CAIII-300 + lg Mg + Ig NaH + 4.47g TeBr4, Ein: 99.0 kJ, dE:18.4 kJ,
TSC:186-411C,
Tmax: 418C, theoretical is 11.3 kJ, gain is 1.63 times.
= 4g CAIII-300 + 1g Al + Ig NaH + 4.47g TeBr4, Ein: 101.0 kJ, dE: 14.7 kJ,
TSC:144-305C,
Tmax: 374C, theoretical is 11.4 kJ, gain is 1.29 times.
= 4.5 g of TeBr4, 1.66 g of KH, 1 g of MgH2 powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 19.1 kJ,
and the
temperature burst was 218 C(172 - 390 Q. The maximum cell temperature was 410
C,
theoretical is 12.65 kJ, gain is 1.5 times.
= 4.5 g of TeBr4, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 23.5 kJ,
and the
temperature burst was 247 C(184 - 431 Q. The maximum cell temperature was 436
C
theoretical is 12.4 kJ, gain is 1.89 times.
= KH 6.64 gm + Mg Powder 4.0 gm + Activated Carbon CAII 300 16gm) + TeBr4 18
gm( kJ
Theoretical) (80% of 5X scaleup), Ein: 213 kJ, dE: 77 kJ, Temp. slope Jump at
140C with
Tmax-320C, theoretical is 48.4 kJ, the gain is 1.59 times
145
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
TeC14
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.7g TeC14; Ein: 99.0 kJ; dE: 16.76 kJ; TSC:
114-3000;
Tmax: 385C, theoretical is 13 kJ, gain is 1.29 times.
= 2.7 g of TeC14, 0.33 g of LiH, 1 g of MgH2 powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 20.4 kJ,
and the
temperature burst was 140 C(138 - 278 C). The maximum cell temperature was 399
C,
theoretical is 12.1 kJ, the gain is 1.69 times.
= 2.7 g of TeC14, 0.33 g of LiH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 17.2 kJ,
and the
temperature burst was 240 C(137 - 377 Q. The maximum cell temperature was 398
C,
theoretical is 12.8 kJ, the gain is 1.34 times.
= 2.7 g of TeC14, 1.66 g of KH, 1 g of MgH2 powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell. energy gain was 15.6 kJ,
and the
temperature burst was 216 C(139 - 355 Q. The maximum cell temperature was 358
C,
theoretical is 12.1 kJ, the gain is 1.29 times.
= 2.7 g of TeC14, 1.66 g of KH, 1 g of Al powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 19.4 kJ,
and the
temperature burst was 202 C(89 - 291 C). The maximum cell temperature was 543
C,
theoretical is 10.9 kJ, gain is 1.78 times.
= 2.7 g of TeC14, 0.33 g of LiH, 1 g of Al powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 19.0 kJ,
and the
temperature burst was 288 C(155 - 443 Q. The maximum cell temperature was 443
C,
theoretical is 10.9 kJ, gain is 1.74 times.
= 2.7 g of TeC14, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 17.7 kJ,
and the
temperature burst was 208 C(84 - 292 C). The maximum cell temperature was 396
C,
theoretical is 13 kJ, gain is 1.36 times.
= 2.7 g of TeC14, 1.66 g of KH, 1 g of Al powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 18.7 kJ,
and the
temperature burst was 224 C(112 - 336 C). The maximum cell temperature was 398
C,
theoretical is 12 kJ, gain is 1.56 times.
146
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
SeC14
= 4g CAIII-300 + 1g Mg + 1g NaH + 2.21g SeC14; Ein: 93.0 kJ; dE: 22.14 kJ;
TSC: 141-
435C; Tmax: 435C, theoretical is 15 kJ, the gain is 1.48 times.
= 4g CAIII-300 + lg Mg + 1.66g KH + 2.20g SeC14,
Experimental dE: -25.2kJ Reaction considered: SeC14(c) + 4KH(c) + 3Mg(c) =
4KCI(c) +
MgSe(c) + 2MgH2(c) Q=-1750.4kJ/reaction
theoretical chemical reaction energy: -17.5kJ, Excess heat: -7.7kJ, 1.44X
excess heat.
CF4
= NaH 50gm + Al 50gm+Activated Carbon CAII300 200 gm + CF4 0.3 mole; 45 PSIG
Reservoir Cell Volume: 2221.8CC, Ein: 2190 kJ, dE: 482 kJ, Temp. jump at 200C
with
Tmax-760C, theoretical is 345 kJ, gain is 1.4 times.
= NaH 50.0 gm + Mg Powder 50 gm + Activated Carbon CAII-300 200 gm+ CF4_75-9.9
PSIG after Evacuation. Volume of the reservoir is 1800 CC and for this
pressure drop,
n=0.356 mole and Theoretical Energy is -392 kJ, Ein: 1810 kJ, dE: 765 kJ, Temp
Slope
Jump at 170 C with Tmax-1000C and gain is 765/392 = 1.95 X.
= NaH 1.0gm + (Mg Powder 1.0 gm +Activated Carbon CAII 300 4gm) Ball Mill +
CF4
0.0123 mole and Theoretical Energy -13.6kJ), Ein: 143 kJ, dE: 25 U, Temp.
slope jump at
250 C with Tmax-500C and Energy Gain -1.8 X.
= NaH 1.0gm + (Mg Powder 1.0 gm + Activated
Carbon CAII-300 4gm 4gm) Ball Mill +CF4 -0.01 mole Theoretical Energy -10.2
kJ, Ein:
121 kJ, dE: 18 U, Temp. slope jump at 260 C with Tmax-500C and Energy Gain -
1.7 X.
= NaH 1.Ogm+ (Mg Powder 1.0 gm + ctivated Carbon CAII-300 4gm 4gm) Ball Mill
+CF4
0.006 mole and Theoretical Energy -7.2 kJ), Ein: 133 kJ, dE: 15 U, Temp. slope
jump at
300 C with Tmax-440C and Energy Gain -2.0 X.
= 4g CAIII-300 + 1g MgH2 + 3.55g Rb + 0.0082 mol CF4 + 0.0063 mol H2; Ein:
76.0 kJ;
dE: 20.72 kJ; TSC: 30-2000; Tmax: 348C, theoretical is 10 kJ, gain is 2 times.
SF6
= NaH 50gm + MgH2_50gm+Activated Carbon CAII300 200 gm + SF6 0.29 mole; 43
PSIG
Reservoir Cell Volume: 2221.8CC, Ein: 1760 kJ, dE: 920 kJ, Temp. slope jump at
-140C
147
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
with Tmax-1100 C, theoretical is 638 kJ, gain is 1.44 times.
= 4g CAIII-300 + 1g MgH2 + 1g NaH + 0.0094 mol SF6; Ein: 96.7 kJ; dE: 33.14
kJ; TSC:
110-455C; Tmax: 455C, theoretical is 20.65 kJ, the excess is 12.5 kJ, the gain
is 1.6 times.
= NaH 1.0 gm + Al Powder 1.0 gm + Activated Carbon CAII 300 4gm) Ball Mill
+SF6 0.01
mole and Theoretical Energy -20kJ), Ein:95 kJ, dE: 30 kJ, Temperature slope
change at
-100C with Tmax-400C, theoretical is 20.4 kJ, excess is 9.6 kJ, the gain is
1.47 times.
= NaH 1.0 gm + MgH2 Powder 1.0 gm + Activated Carbon CAII 300 4gm) Ball Mill +
SF6
0.01 mole and Theoretical Energy -22kJ), Ein: 85 kJ, dE: 28 kJ, Temperature
slope change at
-110C with Tmax-410C, theoretical is 22 kJ, excess is 6 kJ, the gain is 1.27
times.
= NaH 1.0 gm + Al nano Powder 1.0 gm + Activated Carbon CAR 300 4gm) Ball Mill
+ SF6
0.005 mole, Ein: 107 kJ, dE: 21 kJ, Temperature slope change at -160C with
Tmax-380C,
theoretical is 10.2 kJ, the gain is 2 times
= NaH 1.0 gm + Mg Powder 1.0 gm + Activated Carbon CAII 300 4gm) Ball Mill +
SF6
0.005 mole, Ein: 104 kJ, dE: 18 kJ, Temperature slope change at -150C with
Tmax-370C,
theoretical is 12.5 kJ, the excess is 5.5 kJ, the gain is 1.44 times.
= NaH 1.0 gm + MgH2 Powder 1.0 gm + Activated Carbon CAR 300 4gm) Ball Mill +
SF6
0.0025 mole and Theoretical Energy -5.5kJ), Ein: 100 kJ, dE: 10 kJ,
Temperature slope
change at -160C with Tmax--335C, theoretical is 5.5 kJ, the gain is 1.8 times.
= 4g CAIII-300 + 0.5g B + 1g NaH + 0.0047 mol SF6; Ein: 112.0 kJ; dE: 15.14
kJ; TSC: 210-
350C; Tmax: 409C, theoretical is 10.12 kJ, excess is 5 kJ, gain is 1.49 times.
= 4g CAIII-300 + 1g MgH2 + 1.66g KH + 0.00929 mol SF6 (Cell temperature rose
up to 29C
upon SF6 fill); Ein: 66.0 kJ; dE: 26.11 kJ; TSC: 37-375C; Tmax: 375C,
theoretical is 20.4 kJ,
gain is 1.28 times.
= 4g CAIII-300 + 1g Mg + 0.33g LiH + 0.00929 mol SF6 (Cell temperature rose up
to 26C
upon SF6 fill); Ein: 128.0 kJ; dE: 32.45 U; TSC: 275-540C; Tmax: 550C,
theoretical is 23.2
kJ, gain is 1.4 times.
= 4g CAIII-300 + 1g S + 1g NaH + 0.0106mol SF6(online), Ein: 86.OkJ,
dE:18.1kJ, TSC: 51-
313C, Tmax: 354C, theoretical is 11.2 kJ, gain is 1.6.
148
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= NaH 5.0 gm + MgH2 5.0gm + Activated Carbon CAII 300 20.0gm) Ball Mill + SF6
40
PSIG; 0.026 mole ON LINE (Theoretical Energy -57kJ) 2" cell, Ein: 224 kJ, dE:
86 kJ,
Temp jump at 150C with Tmax-350C, theoretical is 57 U, gain is 1.5 times.
Te02
= 4g CAIII-300 + lg MgH2 + 1g NaH + 1.6g Te02; Ein: 325.1 kJ; dE: 18.46 kJ;
TSC: 210-
440C; Tmax: 440C, theoretical is 9.67 kJ, the excess is 8.8 kJ, the gain is
1.9 times.
= 4g CAIII-300 + 2g MgH2 + 2g NaH + 3.2g Te02, Ein: 103.OkJ, dE:31.6kJ,
TSC:185-491C,
Tmax: 498C, theoretical is 17.28 kJ, gain is 1.83 times.
= 1.6 g of Te02, 0.33 g of LiH, 1 g of Al powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 18.1 kJ,
but no
temperature burst was observed. The maximum cell temperature was 637 C,
theoretical 8.66
kJ, gain is 2.1 times.
= 1.6 g of Te02, 1.66 g of KH, 1 g of MgH2 powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 22.0 kJ,
and the
temperature
burst was 233 C(316 - 549 C). The maximum cell temperature was 554 C,
theoretical 8.64 kJ,
gain is 2.55 times.
= 1.6 g of Te02, 1.66 g of KH, 1 g of Mg powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 20.3 U, and
the
temperature burst was 274 C(268 - 542 Q. The maximum cell temperature was 549
C,
theoretical is 10.9 kJ, gain is 1.86.
= NaH 5.0 gm + MgH2 Powder 5.0 gm + Activated Carbon CAII 300 20gm) Ball Mill
+
Te02 8.0gm, Ein: 253 U, dE: 77 U, Temp. slope Jump at 200C with Tmax-400C,
theoretical is 48.35 kJ, gain is 1.6 times.
= NaH 1.0 gm + MgH2 Powder 1.0 gm + Activated Carbon CAII 300 4.0gm) Ball Mill
+
Te02 1.6gm, Ein: 110 U, dE: 16 U, Temp. slope Jump at 190C with Tmax-400C,
theoretical is 9.67 kJ, gain is 1.65 times.
= KH 1.66 gm + MgH2 Powder 1.0 gm + Activated Carbon CAII 300 4.0gm) Ball Mill
+
Te02 1.6gm, Ein: 119 U, dE: 19 kJ, Temp. slope Jump at 340C with Tmax-570C,
149
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
theoretical is 9.67 kJ, the gain is 2 times.
= 4g CAIII-300 + 1g NaH + 1.6g Te02, Ein:116.OkJ, dE: 11.0kJ, TSC:207-352C,
Tmax:
381C, theoretical is 6.6 kJ, the gain is 1.67 times.
= KH 1.66 gm + MgH2 Powder 1.0 gm + TiC 4.0gm + Te02 1.6gm, Ein: 133 kJ, dE:
15 kJ,
Temp. slope Jump at 280C with Tmax-460C, theoretical is 8.64 kJ, the gain is
1.745 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 1.60g Te02, Experimental dE: -17.OkJ
Reaction
considered: Te02(c) + 3Mg(c) + 2NaH(c) = 2MgO(c) + Na2Te(c) + MgH2(c) Q=-
1192.7kJ/reaction theoretical chemical reaction energy: -11.9kJ, Excess heat: -
5.lkJ.1.43X
excess heat.
P205
- 1.66 g of KH, 2 g of P205 and 1 g of MgH2 and 4 g of CA-III 300 activated
carbon powder
(dried at 300 C) in 1 inch heavy duty cell, energy gain was 21.2 kJ, and
temperature burst
was 242 C (299 - 541 Q. The maximum cell temperature was 549 C, the
theoretical is 10.8
kJ, the excess is 10.35 kJ, the gain is 1.96 times
032609GC4: 031909RCWF4 / 1.66 g KH + 2 g P205 + 1 g MgH2 + 4 g CA 111-300 in
DMF-
d7 (as received), strong -3.86 ppm peak.
- 4g CAIII-300 + 1g MgH2 + 1.66g KH + 2g P205, Ein: 138.0 U, dE:21.6 U,
TSC:320-
616C, Tmax: 616C, theoretical is 11.5 kJ, excess is 10.1 kJ, gain is 1.9
times.
= KH 8.3 gm + MgH2 Powder 5.0 gm + Activated Carbon CAII 300 20gm) Ball Mill +
P205
10.0gm, Ein: 272 kJ, dE: 98 kJ, Jump at 250C with Tmax-450C, theoretical is 54
kJ, gain is
1.81 times.
- KH 1.66 gm + MgH2 Powder 1.0 gm + Activated Carbon CAII 300 4gm) Ball Mill
+P205
2.0gm, Ein:130 kJ, dE: 21 kJ, Jump at 300C with Tmax-550C, theoretical is 10.8
kJ, gain is
1.94 times.
= KH 1.66 gm + MgH2 Powder 1.0 gm + TiC 4.0gm +P205 2.0 gm, Ein: 129 kJ, dE:
21 kJ,
Temp. slope Jump at 270C with Tmax-600C theoretical is 10.8 kJ, the gain is
1.95 times.
NaMn04
= 4g CAIII-300 + 1g Si + 1g NaH + 3.5g NaMn04; Ein: 123.0 kJ; dE: 26.25 kJ;
TSC: 45-
330C; Tmax: 465C, theoretical is 17.6 kJ, excess is 8.7 kJ, gain is 1.5 times.
150
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= 4g CAIII-300 + Ig Al + 1g NaH + 3.5g NaMnO4; Ein: 120.0 kJ; dE: 32.41 kJ;
TSC: 44-
373C; Tmax: 433C, theoretical is 20.5 kJ, excess is 7.7 kJ, gain is 1.58
times.
= 4g CAIII-300 + lg Mg + lg NaH + 3.5g NaMnO4; Ein: 66.0 kJ; dE: 32.27 kJ;
TSC: 74-
430C; Tmax: 430C, theoretical is 17.4 kJ, excess is 14.9 kJ, gain is 1.85
times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 3.5g NaMnO4, Ein:72.0kJ, dE:34.lkJ, TSC:49-
362C,
Tmax: 364C, theoretical is 17.4 kJ, excess is 16.7 kJ, gain is 2.
= KH 8.3 gm + Mg Powder 5.0 gm + Activated Carbon CAII 300 20gm) Ball Mill +
NaMnO4 17.5gm, Ein: 130 kJ, dE: 160 kJ, Temp. slope Jump at 70C with Tmax-
350C,
theoretical is 87 kJ, gain is 1.84 times
= KH 8.3 gm + Al Powder 5.0 gm + Activated Carbon CAII 300 20gm) Ball Mill +
NaMnO4
17.5gm, Ein: 134 kJ, dE: 171 kJ, Temp. slope Jump at 50C with Tmax-350C,
theoretical is
102.5 kJ, gain is 1.66 times.
= NaH 1.0 gm + Mg Powder 1.0 gm + Activated Carbon CAII 300 4.0gm) Ball Mill +
NaMnO4 3.5gm (Theoretical -17.4 kJ), Ein: 54 kJ, dE: 32 kJ, Temp. slope Jump
at 60C with
Tmax~450C, theoretical is 17.4 kJ, gain is 1.8 times.
= KH 1.66 gm + Mg Powder 1.0 gm + TiC 4.0gm + NaMnO4 3.5gm, Ein: 65 kJ, dE: 30
kJ,
Temp. slope Jump at 70C with Tmax--410C, theoretical is 17.4 kJ, the gain is
1.7 times.
Nitrate
= 2. g of NaH, 3 g of NaNO3 and the mixture of 1 g of Ti powder and 4 g of
activated C
powder (dried at 300 C) in 1 inch cell, energy gain was 33.2 kJ, and
temperature burst was
418 C
(110 - 528 C). The maximum cell temperature was 530 C, theoretical is 24.8 kJ,
excess is 8.4
kJ, gain is 1.3 times.
= 3. g of NaH, 3 g of NaNO3 and the mixture of 1 g of Al nanopowder and 4 g of
activated C
powder (dried at 300 C) in 1 inch cell, energy gain was 42.3 kJ, and
temperature burst was
384 C (150 - 534 Q. The maximum cell temperature was 540 C, theoretical is
33.3 kJ, excess
is 9 kJ, gain is 1.27 times
= 2, 1 g of NaH, 3 g of NaNO3 and the mixture of 1 g of MgH2 and 4 g of
activated C
151
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
powder (dried at 300 C) in linch cell, energy gain was 43.4 kJ, and
temperature burst was
382C(67-
449 C). The maximum cell temperature was 451 C, theoretical is 28.6 kJ, excess
is 14.8 kJ,
gain is 1.52 times.
= 0.33 g of LiH, 1.7 g of LiNO3 and the mixture of 1 g of MgH2 and 4 g of
activated C
powder (dried at 300 C) in 1 inch heavy duty cell, the energy gain was 40.1
kJ, and
temperature burst was 337 C (92 - 429 Q. The maximum cell temperature was 431
C,
theoretical is 21.6 kJ, excess is 18.5 kJ, gain is 1.86 times.
= 0.33 g of LiH, 1.7 g of LiNO3 and the mixture of 1 g of Ti and 4 g of
activated C powder
(dried at 300 C) in 1 inch cell, energy gain was 36.5 kJ, and temperature
burst was 319 C (83
-402 Q. The maximum cell temperature was 450 C, theoretical is 18.4 kJ, excess
is 18 kJ,
gain is 2 times.
= 4g CAIII-300 + 1g MgH2 + lg NaH + 2.42g LiNO3; Ein: 75.0 U; dE: 39.01 kJ;
TSC: 57-
492C; Tmax: 492C, theoretical is 28.5 kJ, excess is 10.5 kJ, gain is 1.37
times
= 4g CAIII-300 + 1g Al + 1g NaH + 2.42g LiNO3; Ein: 81.2 kJ; dE: 41.89 kJ;
TSC: 73-528C;
Tmax: 528C, theoretical is 34.6 kJ, excess is 7.3 kJ, gain is 1.21 times.
C104
- 4g CAIII-300 + lg MgH2 + 2g NaC1O4 + lg NaH; Ein: 86.0 kJ; dE: 38.88 kJ;
TSC: 130-
551C; Tmax: 551C, theoretical is 30.7 kJ, excess is 8.2 kJ, gain is 1.27
times.
= 4g CAIII-300 + lg Al + lg NaH + 4.29g NaC1O4; Ein: 88.0 kJ; dE: 58.24 kJ;
TSC: 119-
615C; Tmax: 615C, theoretical is 47.1 kJ, excess is 11.14 kJ, gain is 1.23
times.
= 4g CAIII-300 + lg MgH2 + lg NaH + 4.29g NaC1O4; Ein: 98.0 kJ; dE: 56.26 kJ;
TSC:
113-571C; Tmax: 571C, theoretical is 36.2 kJ, excess is 20.1 kJ, gain is 1.55
times.
K2S208
= 4g CAIII-300 + 1g MgH2 + 1.66g KH + 2.7g K2S208, Ein: 121.0 kJ, dE: 27.4 kJ,
TSC:178-462C, Tmax: 468C, theoretical is 19.6 kJ, excess is 7.8 kJ, the gain
is 1.40 times.
S02
= 4g CAIII-300 + 1g MgH2 + lg NaH + 0.0146 mol S02, Ein: 58.0 kJ, dE: 20.7 U,
TSC:42-
287C, Tmax: 309C, theoretical 15 kJ, excess is 5.7 kJ, the gain is 1.38 times.
152
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
S
= 4g CAIII-300 + 1g MgH2 + 1g NaH + 3.2g S, Ein: 67.0 kJ, dE:22.7 kJ, TSC:49-
356C,
Tmax: 366C, theoretical is 17.9 kJ, excess is 4.8 kJ, the gain is 1.27 times.
= 1.3 g of S powder, 1.66 g of KH, 1 g of Si powder and 4 g of CA-III 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 13.7 kJ,
and the
temperature burst was 129 C(66 - 195 C). The maximum cell temperature was 415
C,
theoretical is 7.5 kJ, excess is 1.82 times.
= 3.2 g of S powder, 0.33 g of LiH, 1 g of Al powder and 4 g of CA-IV 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 27.1 kJ,
and the
temperature burst was 301 C(163 - 464 C). The maximum cell temperature was 484
C,
theoretical is 20.9 kJ, excess is 6.2 kJ, gain is 1.3 times.
= 3.2 g of S powder, 0.33 g of LiH, 1 g of Si powder and 4 g of CA-IV 300
activated carbon
powder (dried at 300 C) in 1 inch heavy duty cell, energy gain was 17.7 kJ,
and the
temperature burst was 233 C(212 - 445 C). The maximum cell temperature was 451
C,
theoretical is 13.7 kJ, excess is 4 kJ, gain is 1.3 times.
= 4g CAIII-300 + 1g Si + 1.66g KH + 1.3g S, Ein: 81.0 kJ, dE:10.8 kJ, TSC: 52-
196C, Tmax:
326C, theoretical is 7.4 kJ, gain is 1.45 times.
SnF4
= 4g CAIII-300 + 1g Mg + 1g NaH + 1.95g SnF4; Ein: 130.2 U; dE: 13.89 kJ; TSC:
375-
520C; Tmax: 525C, , theoretical is 9.3 kJ, the gain is 1.5 times.
= 4g CAIII-300 + 1g Mg + 1g NaH + 1.95g SnF4; Ein: 130.2 kJ; dE: 13.89 kJ;
TSC: 375-
520C; Tmax: 525C, , theoretical is 9.3 kJ, the gain is 1.5 times.
Se02
= 4g CAIII-300 + 2g MgH2 + 2g NaH + 2.2g Se02, Ein: 82.OkJ, dE: 29.5kJ, TSC:99-
388C,
Tmax: 393C, theoretical is 20.5 kJ, gain is 1.4 times.
CS2
= NaH 1.0 gm + (Al Powder 1.0 gm + Activated Carbon CAII 300 4gm) Ball Mill +
CS2 1.2
ml in PP Vial, Ein: 72 kJ, dE: 18 kJ, Temp. Slope jump at - 80C with Tmax-
320C,
theoretical is 11.4 kJ, gain is 1.58 times.
153
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
= NaH 1.0 gm + MgH2 Powder 1.0 gm + Activated Carbon CAII 300 4gm) Ball Mill +
CS2
1.2 ml in PP Vial, Ein: 82 kJ, dE: 18 kJ, Temp. Slope jump at - 80C with Tmax-
330C,
theoretical is 12.6 kJ, gain is 1.4 times.
C02
= 4g CAIII-300 + 1g MgH2 + 1g NaH + 0.00953 mol C02(Cell temperature rose up
to 45C
upon C02 fill); Ein: 188.4 kJ; dE: 10.37 kJ; TSC: 80-120C; Tmax: 508C,
theoretical is 6.3
kJ, the gain is 1.65 times.
PF5
= 4g CAIII-300 + 1g Al + 1g NaH + 0.010 mol PF5; Ein: 127.0 kJ; dE: 15.65 kJ;
TSC: 210-
371C; Tmax: 371C, theoretical is 10 kJ, excess is 6.45 kJ, the gain is 1.57
times.
= 4g CAIII-300 + 1g Al + 1g NaH + 0.01 mol PF5, Ein:101.OkJ, dE:15.7kJ,
TSC:178-370C,
Tmax: 391C, theoretical is 10 kJ, the gain is 1.57 times.
NF3
= NaH 1.0gm + (Mg Powder 1.0 gm + Activated Carbon CAII-300 4gm) Ball Mill
+NF3
0.011 mole and Theoretical Energy -U), Ein: 136 U, dE: 28 kJ, Temp. slope jump
at 70 C
with Tmax-470C, theoretical is 19.6 kJ, gain is 1.4 times.
154
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
PCl5
= 4g CAIII-300 + 1g MgH2 + 2.08g PC15 + 1g NaH; Ein: 90.0 kJ; dE: 20.29 kJ;
TSC: 180-
379C; Tmax: 391C, theoretical is 13.92 kJ, the gain is 1.45 times.
P2S5
= 4g CAIII-300 + 1g MgH2 + 1g NaH + 2.22g P2S5; Ein: 105.0 kJ; dE: 13.79 kJ;
TSC: 150-
363C; Tmax: 398C, theoretical is 10.5 kJ, the excess is 3.3 kJ, the gain is
1.3 times.
= NaH 1.0 gm + Al Powder 1.0 gm + Activated Carbon CAII 300 4gm) Ball Mill +
P2S5 2.22
gm), Ein: 110 kJ, dE: 14 kJ, Temp. Slope jump at - 170C with Tmax-425C,
theoretical is
10.1 kJ, gain is 1.39 times.
Oxide
= 4g AC + 1g MgH2 + 1.66g KH + 1.35g K02, Ein:86.0 kJ, dE: 21.0 kJ, TSC:157-
408C,
Tmax: 416C, theoretical is 15.4 kJ, gain is 1.36 times.
Mn04
= 4g CAIII-300 + 1g Mg + 1g NaH + 3.5g Mn02; Ein: 108.0 kJ; dE: 22.11 kJ; TSC:
170-
498C; Tmax: 498C, theoretical is 18.4 kJ, excess is 3.7 kJ, gain is 1.2 times.
N20
= 4g Pt/C + 1g Mg + 1g NaH + 0.0198mo1 N20, Ein:72.0 kJ, dE:22.2 kJ, TSC:73-
346C,
Tmax: 361C, theoretical is 16.2 kJ, gain is 1.37 times.
HFB
= NaH 1.0gm + (Aluminum Nano Powder 1gm + Activated Carbon (AC) 5gm)Ball
Milled +
HFB 1 ml, Ein: 108 kJ, dE 35 kJ, Temp. jump of 450 C at 90 C.
= NaH 1.0gm + (La Sgm+Activated Carbon 59m) Ballmilled + HexaFluoro Benzene 1
ml,
Ein: 109 kJ, dE:38 kJ, Temp. jump of 400 C at 90 C.
= (4g activated carbon (AC) + 1g MgH2)Ball Milled + 1ml HFB + 1g NaH, Ein:
150.0 kJ, dE:
45.1 kJ, TSC:-50-240, Tmax -250 C.
= Blend (4g AC + 1g MgH2) + 1ml HFB + 1g NaH, Ein: 150.0 kJ, dE: 35.0 kJ, TSC:
54-255
C, 45-241 C, 48-199 C; Tmax: 258 C, 247 C, 206 C (three tandem cells).
= 1.66 g of KH, 1 ml of hexadecafluoroheptane (HDFH), and the mixture of 4 g
of activated
155
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
C powder and 1 g of MgH2 in a 1 inch cell, dE: 34.3 kJ, and the burst was 419
C (145 - 564
C), Tmax -575 C.
B. Solution NMR
Representative reaction mixtures for forming hydrino comprise (i) at least one
catalyst
such as one chosen from LiH , KH, and NaH , (ii) at least one oxidant such as
one chosen
from NiBr2, Mn12, AgCI, EuBr2, SF6, S, CF4, NFL, LiNO3, M2S201 with Ag, and
P205,
(iii) at least one reductant such as one chosen from Mg powder, or MgH2, Al
powder, or
aluminum nano-powder (Al NP), Sr, and Ca, and (iv) at least one support such
as one
chosen from AC and TiC. 50 mg of reaction product of the reaction mixtures
were added to
1.5 ml of deuterated N,N-dimethylformamide-d7 (DCON (CD3 )2 , DMF-d7, (99.5%
Cambridge Isotope Laboratories, Inc.) in a vial that was sealed with a glass
TEFLONTM
valve, agitated, and allowed to dissolve over a 12 hour-period in a glove box
under an argon
atmosphere. The solution in the absence of any solid was transferred to an NMR
tube (5 mm
OD, 23 cm length, Wilmad) by a gas-tight connection, followed by flame-sealing
of the tube.
The NMR spectra were recorded with a 500 MHz Bruker NMR spectrometer that was
deuterium locked. The chemical shifts were referenced to the solvent frequency
such as
DMF-d7 at 8.03 ppm relative to tetramethylsilane (TMS).
The hydrino hydride ion H-(1/4) was predicted to be observed at about -3.86
ppm and
molecular hydrino H2(1/4) was predicted to be observed at 1.25 ppm relative to
TMS. The
position of occurrence of these peaks with the shift and intensity for a
specific reaction
mixture are given in TABLE 4.
TABLE 4. The 1H solution NMR following DMF-d7 solvent extraction of the
product of the
heterogeneous hydrino catalyst systems comprising reactants (I) catalyst such
as LiH, KH, or
NaH, (ii) reductant such as Al, Al NP, Mg, or MgH2, and (iii) oxidant such as
CF4, N20, NF3,
K2S208, FeS04, 02, LiNO3, P205, SF6, S, CS2, NiBr2, Te02, NaMNO4, SnF4, and
SnI4 mixed
with (iv) a support such as AC or Pt/C.
H2 (1/4) Peak position and H- (1/4) Peak position and
Reactants
Intensity Intensity
1.66 g KH, 1 g Al, 4 g AC, and 0.01 moles CF4 1.22 ppm strong minus 3.85 ppm
strong
1 g NaH, 1 g Al, 4 g AC, and 0.01 moles CF4 1.23 ppm strong
1 g NaH, 1 g MgH2, 4 g AC, and 0.01 moles CF4 1.22 ppm strong
1 g NaH, 1g MgH2, 4 g AC and 0.004 mole CF4 1.22 ppm strong
1 g NaH, 1g Mg, 4 g AC and 52 milli mole CF4 1.21 ppm medium
1 g NaH, 1 g Al, 4 g AC and 52 milli mole CF4 1.21 ppm strong
1 g NaH, 1 g MgH2, 4 g Pt/C and 0.01 mole CF4 1.27 ppm medium minus 3.86 ppm
medium
1 g NaH, 1 g Al, 4 g Pt/C and 0.002 mole CF4 1.21 ppm strong
0.5 g NaH, 0.5 g Mg, 2 g AC and 52 millimole CF4 1.22 ppm strong
0.5 g NaH, 0.5 g Al, 2 g AC and 0.002 mole CF4 1.21 ppm strong
156
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
1.66 g KH, 1 g MgH2, 4 g AC, 0.01 moles N20 1.22 ppm very strong minus 3.85
ppm medium
1 g NaH, 1 g Al, 4 g AC, 0.002 moles N20 1.21 ppm strong
1 g NaH, 1 g Al, 4 g AC, 0.004 moles N20 1.21 ppm strong
1 g NaH, 1 g MgH2, 4 g AC, 0.002 moles N20 1.21 ppm strong
1 g NaH, 1 g MgH2, 4 g AC, 0.004 moles N20 1.22 ppm medium
1 g NaH, 1 g MgH2, 4 g AC and 0.01 mole N20 1.24 ppm strong
1 g NaH, I g MgH2, 4 g AC and 0.018 mole N20 1.24 ppm strong minus 3.84 ppm
strong
0.33 g LiH, 1 g Al, 4 g Al and 0.004 mole N20 1.22 ppm medium minus 3.85 ppm
strong
1 g NaH, 1 g MgH2, 4 g Pd/C(1%) and 0.01 mole N20 1.24 ppm very strong
1 g NaH, 4 g AC and 0.004 mole N20 1.21 ppm very strong
1 g NaH, 1 g MgH2, 5 g Er203, 4 g Ac and 0.01 mole
N20 1.23 ppm strong
1 g NaH, 1 g Al, 5 g Er203, 4 g Ac and 0.01 mole N20 1.24 ppm strong
1 g NaH, I g Mg, 4 g Ac and 0.004 mole N20 1.23 ppm strong
0.5 g NaH, 0.5 g MgH2, 4 g AC and 0.004 mole N20 1.22 ppm strong
0.33 g LiH, 1 g Al, 4 g AC,0.21 g K2S208 and 0.01 mole
02 1.26 ppm medum minus 3.85 ppm very strong
0.33 g LiH, 1 g Al, 4 g AC and 0.01 mole 02 1.27 ppm medium minus 3.85 ppm
strong
0.33 g LiH, 1 g MgH2, 4 g AC,0.21 g K2S208 and 0.01
mole 02 1.27 ppm medium minus 3.85 ppm very strong
1 g NaH, 1 g MgH2, 4 g AC, 0.15 g FeSO4 and 0.01
mole 02 1.24 ppm strong
1.66 g KH, 1 g Mg, 4 g AC and 0.004 mole 02 1.21 ppm strong
1 g NaH, 1 g Si, 4 g AC and 0.01 mole 02 1.21 ppm strong
1 g NaH, 10 g Pt/Ti, 1 g MgH2, 4 g AC, 0.01 mole NH3
and 0.01 mole 02 1.22 ppm very strong
0.5 g NaH, 0.5 g Al, 4 g AC and 0.002 mole NF3 1.22 ppm medium minus 3.85 ppm
strong
0.5 NaH, 0.5 g MgH2, 4 g AC and 0.004 mole NF3 1.21 ppm very strong
1 g NaH, 1 g Al, 4 g AC and 0.002 mole NF3 1.21 ppm strong
0.5 g NaH, 0.5 g MgH2, 4 g AC and 0.004 mole NF3 minus 3.85 ppm medium
0.5 NaH, 0.5 g MgH2, 4 g AC and 0.002 mole NF3 1.22 ppm strong
1.66 g KH, 2.5 g LiNO3, 4 g AC and 1 g MgH2 1.22 ppm strong minus 3.85 ppm
strong
1 g NaH, 3 g NaNO3, 4 g AC and 1 g MgH2 minus 3.84 ppm medium
1 g NaH, 2.5 g LiNO3, 4 g AC and 1 g MgH2 minus 3.84 ppm medium
1.66 g KH, 2.5 g LiNO3 and 1 g MgH2 1.22 ppm very strong
1.66 g KH, 2 g P205, 4 g AC and 1 g MgH2 1.28 ppm very strong minus 3.86 ppm
strong
0.33 g LiH, 2 g P205, 4 g AC and 1 g MgH2 minus 3.85 ppm medium
1 g NaH, 2 g P205, 4 g AC and 1 g MgH2 minus 3.85 ppm medium
1 g NaH, 2 g P205, 4 g AC and 1 g Al 1.20 ppm strong minus 3.85 ppm medium
1.66 g KH, 1 g MgC12, 4 g AC, 4.5 g K02 and 0.1 g
CoC12 1.23 ppm very strong minus 3.85 ppm medium
1 g NaH, 1 g MgH2, 4 g AC and 0.0094 mole SF6 minus 3.84 ppm very strong
1 g NaH, 0.5 g B, 4 g AC and 0.0047 mole SF6 minus 3.85 ppm strong
1 g NaH, 1 g Mg, 4 g AC and 0.01 mole SF6 minus 3.86 ppm strong
1 g NaH, 1 g Al, 4 g AC and 0.005 mole SF6 1.20 ppm strong minus 3.86 ppm weak
1.66 g KH, 1 g Si, 4 g AC and 0.0092 mole SF6 minus 3.86 ppm very strong
1.66 g KH, 1 g Al, 4 g AC and 0.0092 mole SF6 minus 3.86 ppm very strong
157
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
1.66 g KH, 1 g MgH2, 4 g AC and 0.0092 mole SF6 minus 3.86 ppm very strong
0.33 g LiH, 1 g MgH2, 4 g AC and 0.009 mole SF6 minus 3.82 ppm very strong
0.33 g LiH, 1 g Mg, 4 g AC and 0.009 mole SF6 minus 3.84 ppm medium
0.33 g LiH, 1 g La, 4 g AC and 0.0094 mole SF6 minus 3.75 ppm board
1.66 g KH, 1 g MgH2, 4 g AC and 0.0093 mole SF6 1.21 ppm strong minus 3.86 ppm
weak
1 g NaH, 5 g La, 4 g AC and 0.0047 mole SF6 1.21 ppm medium minus 3.86 ppm
weak
1 g NaH, 1 g MgH2, 4 g AC and 3.2 g S minus 2.83 ppm very strong
minus 2.83 ppm strong and
1 g NaH, 1 g MgH2, 4 g AC and 3.2 g S (outside) board
0.33 g LiH, 1 g Si, 4 g AC and 1.3 g S minus 3.81 ppm very strong
0.33 g LiH, 1 g Al, 4 g AC and 1.3 g S minus 3.81 ppm very strong
1.66 g KH, 1 g Al, 4 g AC and 1.3 g S minus 3.47 ppm very strong
1.66 g KH, 1 g Al, 4 g AC and 1.3 g S minus 3.86 ppm very strong
1.66 g KH, 1 g Si, 4 g AC and 1.3 g S minus 3.55 ppm very strong
1.66 g KH, 1 g Si, 4 g AC and 1.3 g S minus 3.85 ppm strong
1.66 g KH, 1 g MgH2, 4 g AC and 2.7 g K2S208 1.24 ppm strong minus 3.85 ppm
very strong
1 g NaH, 1 g Al, 4 g AC and 1.2 ml CS2 minus 3.85 ppm very strong
1 g NaH, 1 g MgH2, 4 g AC and 1.2 ml CS2 minus 3.85 ppm very strong
1 g NaH, 1 g MgH2, 4 g AC and 0.0146 mol S02 1.21 ppm medim minus 3.86 ppm
medium
1 g NaH, 1 g MgH2, 4 g AC and 2.2 g NiBr2 1.23 ppm strong
1 g NaH, 1 g Mg, 4 g AC and 2.2 g NiBr2 1.25 ppm medium
1 g NaH, 4 g AC and 2.2 g NiBr2 1.24 ppm very strong
1.66 g KH, 4 g AC and 2.2 g NiBr2 1.22 ppm very strong
1 g NaH, 1.66 g Ca, 4 g AC and 2.2 g NiBr2 1.24 ppm very strong
1 g NaH, 3.67 g Sr, 4 g AC and 3.1 g MnI2 1.24 ppm very strong
83 g KH, 50 g Mg, 200 g TiC and 154.5 g Mn12 1.24 ppm strong
1 g NaH, 1.66 g Ca, 4 g AC and 3.1 g MnI2 1.23 ppm very strong
1 g NaH, 4 g AC and 1.6 g Te02 1.21 ppm strong minus 3.85 ppm strong
2 g NaH, 2 g MgH2, 4 g AC and 3.2 g Te02 1.21 ppm medium
1.66 g KH, 1 g MgH2, 4 g AC and 1.6 g Te02 1.21 ppm strong
0.33 g LiH, 1 g MgH2, 4 g AC and 1.6 g Te02 1.22 ppm medium
1 g NaH, 1 g Mg, 4 g AC and 3.5 g NaMn04 1.21 ppm medium
8.3 g KH, 5 g Mg, 20 g AC and 17.5 g NaMn04 1.21 ppm strong
1.66 g KH, 1 g Mg, 4 g AC and 2.0 g SnF4 1.23 ppm medium
1.66 g KH, 1 g Mg, 4 g AC and 6.3 g SnI4 1.21 ppm medium
1.66 g KH, 4 g AC and 3.79 g SnI2 1.24 ppm very strong
1 g NaH, 1 g Mg, 4 g AC and 1.57 g SnF2 1.22 ppm strong
83 g KH, 50 g Mg, 200 g WC and 185 g SnI2 1.23 ppm medium
1 g NaH, 1.66 g Ca, 4 g AC and 1.34 g CuC12 1.22 ppm very strong
1 g NaH, 1 g Mg, 4 g AC and 0.96 g CuS 1.21 ppm strong
8.3 g KH + 5 g Mg + 20 g CA 11-300 + 14.85 g BaBr2 1.22 ppm strong
g NaH + 5 g Mg + 20 g CA 11-300 + 14.85 g BaBr2 1.22 ppm medium
20 g AC 3-3 + 8.3 g KH + 7.2 g AgC1 1.22 ppm medium
158
CA 02730712 2011-01-13
WO 2010/014684 PCT/US2009/052072
3.09 g MnI2 + 1.66 g KH + 1 g Mg + 4 g S TiC-1 1.25 ppm medium
159