Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02732747 2015-02-20
- 1 -
PROCESS FOR RECOVERING SULFUR FROM A FEEDSTREAM USING
ELECTROCHEMICAL MEANS
Field of the Invention
[0001] This invention relates to the desulfurization of a hydrocarbon
feedstock by
contacting said feedstock with an aqueous metal hydroxide solution, thus
resulting in
a desulfurized feedstock and an aqueous metal sulfide stream. In the present
invention, the aqueous metal sulfide stream is split into at least three
fractions and
each fraction is passed to a different electrochemical cell, connected in
series to
regenerate the metal hydroxide required in the desulfurization process and
producing
elemental sulfur as a by-product.
Background of the Invention
[0002] As the demand for hydrocarbon-based fuels has increased, the need
for
improved processes for desulfurizing hydrocarbon feedstocks of heavier
molecular
weight has increased as well as the need for increasing the conversion of the
heavy
portions of these feedstocks into more valuable, lighter fuel products. These
heavier,
"challenged" feedstocks include, but are not limited to, low API gravity, high
sulfur,
high viscosity crudes from such areas of the world as Canada, the Middle East,
Mexico,
Venezuela, and Russia, as well as less conventional refinery and petrochemical
feedstocks derived from such sources as tar sands bitumen, coal, and oil
shale. These
heavier crudes and derived crude feedstocks contain a significant amount of
heavy, high
molecular weight hydrocarbons. A considerable amount of the hydrocarbon of
these
heavy oil streams are often in the form of large multi-ring hydrocarbon
molecules
and/or a conglomerated association of large molecules containing a large
portion of the
sulfur, nitrogen and metals in the hydrocarbon stream. A significant portion
of the
sulfur contained in these heavy oils is in the form of heteroatoms in
polycyclic aromatic
CA 02732747 2011-02-01
WO 2010/016899 PCT/US2009/004480
- 2 -
molecules, comprised of sulfur compounds such as dibenzothiophenes, from which
the
sulfur is difficult to remove.
[0003] The high molecular weight, large multi-ring aromatic hydrocarbon
molecules
or associated heteroatom-containing (e.g., S, N, 0) multi-ring hydrocarbon
molecules in
the higher molecular weight oils are generally found in a solubility class of
molecules
termed as asphaltenes. A significant portion of the sulfur is contained within
the
structure of these asphaltenes or lower molecular weight polar molecules
termed as
"polars" or "resins". Due to the large aromatic structures of the asphaltenes,
the
contained sulfur can be refractory in nature and is not very susceptible to
removal by
conventional alkali salt solution complexes such as potassium hydroxide or
sodium
hydroxide solution treatments under conventional operating conditions. Other
intermediate refinery crude fractions, such as atmospheric resids, vacuum
resids, and
other similar intermediate feedstreams containing boiling point materials
above about
850 F (454 C) contain similar sulfur polycyclic heteroatom complexes and are
also
difficult to desulfurize by conventional methods. These heavy crudes, derived
refinery
feedstocks, and heavy residual intermediate hydrocarbon streams can contain
significant amounts of sulfur. Sulfur contents of in excess of 3 to 5 wt% are
not
uncommon for these streams and can often be concentrated to higher contents in
the
refinery heavy residual streams.
[0004] These high sulfur content hydrocarbon streams can be excessively
corrosive to equipment in refinery and petrochemical production and/or exceed
environmental limitations for use in processes such petroleum refining
processes. If
a significant amount of the sulfur is not removed from these feedstocks prior
to
refining, significant costs in capital equipment may be required to process
these
corrosive crudes and the sulfur is generally still required to be removed by
subsequent processes in order to meet intermediate and final product sulfur
specifications. Additionally, most conventional catalytic refining and
petrochemical
CA 02732747 2011-02-01
WO 2010/016899 PCT/US2009/004480
- 3 -
processes cannot be used on these heavy feedstocks and intermediates due to
their
use of fixed bed catalyst systems and the tendency of these heavy hydrocarbons
to
produce excessive coking and deactivation of the catalyst systems when in
contact
with such feedstreams. Also, due to the excessive hydrocarbon unsaturation and
cracking of carbon-to-carbon bonds experienced in these processes, significant
amounts of hydrogen are required to treat asphaltene containing feeds. The
high
consumption of hydrogen, which is a very costly treating agent, in these
processes
results in significant costs associated with the conventional catalytic
hydrotreating of
heavy oils for sulfur removal.
[0005] Due to their high sulfur content, high viscosities, and low API
gravities,
these heavy hydrocarbon feedstreams cannot be readily transported over
existing
pipeline systems and are often severely discounted for use as a feedstock for
producing
higher value products. Another alternative utilized is to make these heavy
oils suitable
for pipeline transportation or petrochemical feed only after significant
dilution of the
heavy oil with expensive, lower sulfur hydrocarbon diluents.
[0006] As a result, many process methods have been utilized in the art to
desulfurize the very "heavy" or "high molecular weight" hydrocarbon containing
streams. Due to the problems discussed with the use of fixed bed catalyst
systems for
use in desulfurizing these heavy hydrocarbon streams, alkali metal hydroxides
have
been used in aqueous solutions and contacted with the heavy hydrocarbon stream
under specific conditions resulting in the formation of a desulfurized
hydrocarbon
product wherein a portion of the sulfur has been removed from the heavy
hydrocarbon stream and a spent alkali metal hydroxide. Herein, the spent metal
hydroxide is most predominantly in the forms of an alkali metal sulfide and/or
alkali
metal hydrosulfide.
[0007] A significant drawback to the alkali metal hydroxide desulfurization
process of the art is that the alkali metal hydroxides that are spent in the
processes
CA 02732747 2014-08-13
- 4 -
(i.e., either in the form of a sulfide or hydrosulfide) are not easily
converted back into their
active hydroxide forms. In most conventional processes for alkali metal
regeneration, the
alkali metals sulfides are typically steam stripped, removing the sulfur from
the alkali metal
compounds by forming hydrogen sulfide. Not only is this stripping process
typically
inefficient and costly, but it also results in the formation of hydrogen
sulfide (H2S) which in
turn must also under go an additional costly process for removal of the
elemental sulfur,
such as a typical Claus process. Besides requiring additional equipment and
expenses, the
use of steam stripping and subsequent Claus processing results in much of the
hydrogen
fraction of any hydrogen supplied to the hydrocarbon desulfurization process
being lost as
water in the H2S disposal process.
[0008] Therefore, there exists in the industry a need for an improved
process for removing
sulfur from bitumens, heavy crudes, derived crudes and refinery residual
streams utilizing a
metal hydroxide and a regenerating the spent metal hydroxide compounds for
reuse in the
desulfurization process.
[0008a] In one aspect, there is provided a process for recovering sulfur
and generating
hydrogen from a feedstream comprised of an aqueous solution of a metal
sulfide, which
process comprises: a) providing at least a first electrochemical cell, a
second electrochemical
cell, and a third electrochemical cell, all connected in series; b) dividing
the feedstream into at
least three fractions; c) introducing a first fraction of said feedstream into
said first
electrochemical cell along with an effective amount of water and an effective
amount of
oxygen resulting in the generation of elemental sulfur and a metal hydroxide,
and generating a
first electrical potential across said first electrochemical cell; d) removing
at least a portion of
said elemental sulfur and said metal hydroxide from said first electrochemical
cell; e) passing
electrons from the anode of said first electrochemical cell to the cathode of
said second
electrochemical cell to generate a second electrical potential across said
second electrochemical
cell; 0 introducing a second fraction of said feedstream to said second
electrochemical cell
along with an effective amount of water resulting in the generation of
elemental sulfur, a metal
hydroxide, and hydrogen; g) removing at least a portion of said elemental
sulfur and said metal
hydroxide and hydrogen from said second electrochemical cell; h) passing
electrons from the
CA 02732747 2014-08-13
- 4a -
anode of said second electrochemical cell to the cathode of said third
electrochemical cell to
generate a third electrical potential across said third electrochemical cell;
i) introducing a third
fraction of said feedstream into said third electrochemical cell along with an
effective amount
of water resulting in the generation of elemental sulfur, a metal hydroxide,
and hydrogen; j)
removing at least a portion of said elemental sulfur, said metal hydroxide,
and hydrogen from
said third electrochemical cell; and k) passing electrons from the anode of
said third
electrochemical cell to the cathode of at least one other electrochemical
cell; wherein an
electrical circuit around all of the electrochemical cells is completed with
the first
electrochemical cell.
[0008b] In another aspect, there is provided a process for desulfurizing a
sulfur-containing
heavy-oil feedstock comprising: a) contacting said heavy-oil feedstock with an
aqueous metal
hydroxide solution wherein the metal is selected from the alkali metals and
the alkaline-earth
metals, thereby converting at least a portion of the metal hydroxides into
metal sulfides; b)
separating the mixture from step a) into a desulfurized heavy-oil product and
an aqueous metal
sulfide-containing stream wherein the desulfurized heavy-oil product has a
lower sulfur content
by wt % than said heavy-oil feedstock; c) providing at least a first
electrochemical cell, a
second electrochemical cell, and a third electrochemical cell, all connected
in series; d) dividing
the aqueous metal sulfide-containing stream into at least three fractions; e)
introducing a first
fraction of said aqueous metal sulfide-containing stream into said first
electrochemical cell
along with an effective amount of water and an effective amount of oxygen
resulting in the
generation of elemental sulfur and a metal hydroxide, and generating a first
electrical potential
across said first electrochemical cell; f) removing at least a portion of said
elemental sulfur and
said metal hydroxide from said first electrochemical cell; g) passing
electrons from the anode
of said first electrochemical cell to the cathode of said second
electrochemical cell to generate a
second electrical potential across said second electrochemical cell; h)
introducing a second
fraction of said aqueous metal sulfide-containing stream to said second
electrochemical cell
along with an effective amount of water resulting in the generation of
elemental sulfur, a metal
hydroxide, and hydrogen; i) removing at least a portion of said elemental
sulfur and said metal
hydroxide and hydrogen from said second electrochemical cell; j) passing
electrons from the
anode of said second electrochemical cell to the cathode of said third
electrochemical cell to
CA 02732747 2014-08-13
- 4b -
generate a third electrical potential across said third electrochemical cell;
k) introducing a third
fraction of said aqueous metal sulfide-containing stream into said third
electrochemical cell
along with an effective amount of water resulting in the generation of
elemental sulfur, a metal
hydroxide, and hydrogen; 1) removing at least a portion of said elemental
sulfur, said metal
hydroxide, and hydrogen from said third electrochemical cell; and m) passing
electrons from
the anode of said third electrochemical cell to the cathode of at least one
other electrochemical
cell; wherein an electrical circuit around all of the electrochemical cells is
completed with the
first electrochemical cell.
Summary of the Invention
100091 In one
embodiment in accordance with the present invention there is provided a
process for recovering sulfur and generating hydrogen from a feedstream
comprised of an
aqueous solution of a metal sulfide, which process comprises:
a) providing at least a first electrochemical cell, a second electrochemical
cell, and a
third electrochemical cell, all connected in series;
b) dividing the feedstream into at least three fractions;
c) introducing a first fraction of said feedstream into said first
electrochemical cell
along with an effective amount of water and an effective amount of oxygen
resulting in the
generation of elemental sulfur and a metal hydroxide, and generating a first
electrical
potential across said first electrochemical cell;
CA 02732747 2011-02-01
WO 2010/016899 PCT/US2009/004480
- 5 -
d) removing at least a portion of said elemental sulfur and said metal
hydroxide from said first electrochemical cell;
e) passing electrons from the anode of said first electrochemical cell to the
cathode of said second electrochemical cell to generate a second electrical
potential
across said second electrochemical cell;
f) introducing a second fraction of said feedstream to said second
electrochemical cell along with an effective amount of water resulting in the
generation of elemental sulfur, a metal hydroxide, and hydrogen;
g) removing at least a portion of said elemental sulfur and said metal
hydroxide and hydrogen from said second electrochemical cell;
h) passing electrons from the anode of said second electrochemical cell to the
cathode of said third electrochemical cell to generate a third electrical
potential
across said third electrochemical cell;
i) introducing a third fraction of said feedstream into said third
electrochemical cell along with an effective amount of water resulting in the
generation of elemental sulfur, a metal hydroxide, and hydrogen;
j) removing at least a portion of said elemental sulfur, said metal hydroxide,
and hydrogen from said third electrochemical cell; and
k) passing electrons from the anode of said third electrochemical cell to the
cathode of at least one other electrochemical cell;
wherein an electrical circuit around all of the electrochemical cells is
completed with the first electrochemical cell.
[0010] In a preferred embodiments, the metal hydroxide is selected from an
alkali
hydroxide, an alkaline-earth metal hydroxide, or a combination thereof.
[0011] Also in accordance with the present invention is a process for
desulfurizing
a sulfur-containing heavy-oil feedstock comprising:
=
CA 02732747 2011-02-01
WO 2010/016899 PCT/US2009/004480
- 6 -
a) contacting said heavy-oil feedstock with an aqueous metal hydroxide
solution wherein the metal is selected from the alkali metals and the alkaline-
earth
metals, thereby converting at least a portion of the metal hydroxides into
metal
sulfides;
b) separating the mixture from step a) into a desulfurized heavy-oil product
and an aqueous metal sulfide-containing stream wherein the desulfurized heavy-
oil
product has a lower sulfur content by wt% than said heavy-oil feedstock;
c) providing at least a first electrochemical cell, a second electrochemical
cell,
and a third electrochemical cell, all connected in series;
d) dividing the aqueous metal sulfide-containing stream into at least three
fractions;
e) introducing a first fraction of said aqueous metal sulfide-containing
stream
into said first electrochemical cell along with an effective amount of water
and an
effective amount of oxygen resulting in the generation of elemental sulfur and
a
metal hydroxide, and generating a first electrical potential across said first
electrochemical cell;
f) removing at least a portion of said elemental sulfur and said metal
hydroxide from said first electrochemical cell;
g) passing electrons from the anode of said first electrochemical cell to the
cathode of said second electrochemical cell to generate a second electrical
potential
across said second electrochemical cell;
h) introducing a second fraction of said aqueous metal sulfide-containing
stream to said second electrochemical cell along with an effective amount of
water
resulting in the generation of elemental sulfur, a metal hydroxide, and
hydrogen;
i) removing at least a portion of said elemental sulfur and said metal
hydroxide and hydrogen from said second electrochemical cell;
CA 02732747 2011-02-01
WO 2010/016899 PCT/US2009/004480
- 7 -
j) passing electrons from the anode of said second electrochemical cell to the
cathode of said third electrochemical cell to generate a third electrical
potential
across said third electrochemical cell;
k) introducing a third fraction of said aqueous metal sulfide-containing
stream
into said third electrochemical cell along with an effective amount of water
resulting
in the generation of elemental sulfur, a metal hydroxide, and hydrogen;
I) removing at least a portion of said elemental sulfur, said metal hydroxide,
and hydrogen from said third electrochemical cell; and
m) passing electrons from the anode of said third electrochemical cell to the
cathode of at least one other electrochemical cell;
wherein an electrical circuit around all of the electrochemical cells is
completed with the first electrochemical cell.
Brief Description of the Figure
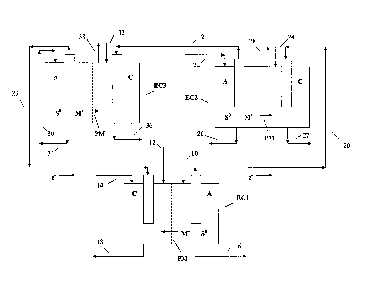
[0012] The Figure herein is a simplified diagram of the electrochemical
system of
the present invention wherein an aqueous metal sulfide solution is split into
three
fractions and each fraction is sent to a separate electrochemical cell wherein
all three
cells are connected in series. This results in the generation of elemental
sulfur,
hydrogen, and the hydroxide of the metal of the aqueous metal sulfide
solution.
Detailed Description of the Invention
[0013] The process of the present invention, in its broadest sense, relates
to
treating an aqueous stream having one or more metal sulfides dissolved
therein.
Preferred metals of the metal sulfide are the alkali and alkaline-earth metals
with the
alkali metals being more preferred and sodium being most preferred. Although
the
aqueous metal sulfide stream can be from any source, it is preferred that it
be a
stream resulting from the caustic treatment of sulfur-containing hydrocarbon
feedstreams, and most preferably the aqueous metal sulfide stream is a
resulting from
CA 02732747 2011-02-01
WO 2010/016899 PCT/US2009/004480
- 8 -
the caustic treatment of sulfur-containing heavy oil feedstreams. As utilized
herein,
the terms "hydrocarbon feedstream", "hydrocarbon feedstream, and "hydrocarbon-
containing feedstream" are considered equivalents and defined herein as any
stream
containing at least 75 wt% hydrocarbons. The terms "heavy oil" and "heavy oil
feedstream" as used herein are considered equivalents and are defined herein
as a
hydrocarbon-containing feedstream having at least about 10 wt% of hydrocarbon
material boiling in excess of about 1050 F. In more preferred embodiments of
the
present invention, the heavy oil feedstream to be treated has at least about
25 wt% of
hydrocarbon material boiling above about 1050 F. Non-limiting examples of such
heavy oil feedstreams include, but are not limited to, whole, topped or froth-
treated
bitumens; heavy oils; whole or topped crude oils; and residua. These
feedstreams
include crude oils obtained from any area of the world, as well as heavy gas
oils, oils
derived from shale, bitumens obtained from tar sands, syncrude derived from
tar
sands, coal oils, asphaltenes, and mixtures thereof. Additionally, both
atmospheric
residuum (boiling above about 650 F) and vacuum residuum (boiling above about
1050 F) may considered as heavy oils as utilized in the present invention. The
preferred feedstream to be treated in accordance with the present invention is
bitumens. Such heavy oil feedstreams also typically contain an appreciable
amount
of so-called "hard" or "refractory" sulfur such as dibenzothiophenes (DBTs)
that are
very difficult to remove by conventional means. In preferred embodiments of
the
present invention, the heavy oil feedstream to be treated has a sulfur content
of at
least 3 wt%, even more preferably, at least 5 wt%.
[0014] The aqueous metal sulfide stream will typically result from
desulfurizing
and upgrading heavy oil by treatment with a metal hydroxide, preferably in the
presence of hydrogen. Effective conditions for desulfurization and upgrading
of
heavy oil using a metal hydroxide include temperatures in the range of about
150 F
to about 500 F, preferably from about 200 F to about 400 F, and pressures in
the
range of about 15 psia to about 800 psia, and reaction times from about 0.1 to
about
CA 02732747 2011-02-01
WO 2010/016899 PCT/US2009/004480
-9-
hours. A molar ratio of hydroxide to total sulfur (as "S") in the feed of
about 0.5
to about 5 is preferred, although lesser or greater amounts of hydroxide may
also be
effective. The metal hydroxide is effective in removing a substantial fraction
of the
sulfur from the heavy oil while also reducing its viscosity, density, and the
fraction
boiling above about 1050 F. Hydrogen, which is optional, but preferred, in the
desulfurization process, is effective in attaining greater reduction of
viscosity,
density, and fraction boiling above about 1050 F than can be achieved by
treatment
with metal hydroxide alone.
[0015] As a result of the reaction with the sulfur contained in the heavy
oil, the
metal hydroxide is converted to metal sulfide and metal hydrosulfide. It is
one object
of this invention to provide a means to recover and regenerate the metal
hydroxide
from the resulting metal sulfide or metal hydrosulfide. It is also an object
of this
invention to provide a means for the generation of hydrogen as a result of the
recovery and regeneration of the metal hydroxide. It will be understood that
the term
"metal sulfide", as used herein, for simplicity purposes also includes metal
hydrosulfides. It is a further object of this invention to provide a means for
the
conversion of at least a portion of the metal sulfide or hydrosulfide to
elemental
sulfur. In a preferred embodiment of the present invention, at least about 50
wt% of
the combined metal sulfides and hydrosulfides are converted in the
electrochemical
cells of the present invention to metal hydroxides.
[0016] This invention is better understood with reference to the sole
Figure herein.
Although the present invention can be utilized with more than three
electrochemical
cells with such modifications as would obvious to one of skill in the art, the
present
invention is illustrated herein using a three cell configuration. The Figure
shows a
system comprised of three divided electrochemical cells Ed, EC2, and EC3. The
electrochemical cells are connected in series. It is preferred that each
electrochemical cell be divided with a cation permeable membrane PM. Any
cation
CA 02732747 2011-02-01
WO 2010/016899 PCT/US2009/004480
- 10 -
permeable membrane can be used to separate the compartments of the
electrochemical cells. These cation permeable membranes typically have fixed
negative charges distributed in a polymer matrix and are permeable to
positively
charged ions. The membranes can be membranes of hydrocarbon and halocarbon
polymers containing acid and acid derivative functional groups. Particularly
suitable
acid polymers are perhalocarbon polymers containing sulfonic, sulfoamide and
carboxylic acid groups. The membranes may be a multi-layered structure of
different
polymers and contain fillers, reinforcements and chemical modifiers. The
preferred
membranes are substantially chemically stable to process conditions and
mechanically suitable for design and economical operation of the instant
electrochemical process.
[0017] The feed to the electrochemical cells of this invention will be a
by-product
aqueous stream comprised of metal sulfides, metal hydrosulfides, or both
(i.e., the
. "aqueous metal sulfide stream"). Preferably, this aqueous metal sulfide
stream
results from the treatment of a hydrocarbon feedstream or heavy oil feedstream
with
a metal hydroxide to remove sulfur. Preferably, the metal is an alkali metal
or an
alkali-earth metal. Although the present invention is not so limited, in a
preferred
embodiment, the metal hydroxide is sodium hydroxide, while in another
preferred
embodiment, the metal hydroxide is potassium hydroxide. The metal hydroxide
may
also be comprised of a combination of different metal hydroxides, including,
but not
limited to, a combination of sodium hydroxide and potassium hydroxide. The
desulfurized heavy oil stream that has been treated with aqueous metal
hydroxides is
collected and at least a portion of the resulting aqueous metal sulfide stream
is
divided into three fractions. Although not required, preferably, the aqueous
metal
sulfide waste stream is divided into three substantially equal fractions. By
substantially equal it is meant that the no one of the flow rates of the three
streams to
CA 02732747 2011-02-01
WO 2010/016899
PCT/US2009/004480
- 11 -
the individual cells as illustrated deviates from the average fractional flow
rate of the
overall aqueous metal sulfide stream by more than about 25 vol%.
[0018]
Continuing with the Figure, a first fraction of the aqueous metal sulfide
stream is passed, via line 10, to the anode (A) side of first electrochemical
cell EC1.
An "effective amount of oxygen" is introduced into the cathode (C) side of
said
electrochemical cell via line 12. By "effective amount of oxygen" it is meant
herein
at least that minimum amount needed to power the cell system and to provide at
least
a stoichiometric amount of oxygen, wherein the stoichiometric amount of
oxygen, as
02, is one-half the molar rate of molar rate of sulfur as S fed to the
individual cell.
The oxygen may be provided via a purified oxygen source or may be provided via
an
oxygen-containing source such as, but not limited to, air. Water is added, via
line 14,
to the cathode side of first electrochemical cell EC1 in an amount needed as
make-up
water for the water consumed during the reduction of oxygen to hydroxide. The
net
overall reaction occurring on the cathode side of the cell is:
Y2 02+ H20 + 2e- ---> 2 OH-
and at the anode side:
S-2 --> S +2e
wherein it is understood that the above reactions are a simplification of a
complex
series of reactions that result in the transformation of the reactants into
the products.
Furthermore, it is well-known that elemental sulfur exists as an octamer (S8)
at
typical processing conditions, but it is shown herein as a monomeric species
for
convenience. The above reactions occur at or near the surface of the
respective
electrodes. As is known in the art, the electrodes can be optimized by
constructing
them of catalytic or non-catalytic materials that favor the above reactions.
CA 02732747 2011-02-01
WO 2010/016899 PCT/US2009/004480
- 12 -
[0019] The metal sulfide, preferably an alkali metal sulfide, undergoes an
oxidation reaction at the anode side of electrochemical cell EC1 wherein metal
cations M+ and elemental sulfur S are produced. Electrons are generated and
collect
at the anode and pass via electrically conductive line 20 to the cathode of
second
electrochemical cell EC2. The metal cations (M+) permeate through the cation
permeable membrane PM where they balance the negative charge of the hydroxide
ion that is produced at the cathode side, thus regenerating the metal
hydroxide
(MOH), which is removed from the cell via line 18. At least a portion of the
elemental sulfur is removed via line 16.
[0020] For purposes of the example, a "unit voltage" is generated in EC1
and
enough power is typically generated to drive electrochemical cells EC2 and EC3
by
a flow of electrons from the anode side of first electrochemical cell EC1
along
electrically conductive line 20 to the cathode of second electrochemical cell
EC2. In
a preferred embodiment of the present invention the unit voltage is preferably
from
about 0.5 to about 10 volts, more preferably from about 0.5 to 5 volts. The
second
fraction of the aqueous metal sulfide stream is introduced into the anode side
of the
second electrochemical cell EC2 via line 22 and water is introduced via line
24.
Preferably, the amount of water introduced is about twice the molar amount of
sulfide fed to the cell. The metal sulfide undergoes an oxidation reaction at
the
anode, as in electrochemical cell EC1, which results again in metal cations M+
that
permeate through cation permeable membrane PM and elemental sulfur S which is
collected via line 26. The permeating metal cations balance the negative
charge of
the hydroxide that is produced on the cathode side of the cell, thus
regenerating the
metal hydroxide (MOH), which is removed via line 27. Excess hydrogen produced
by the reduction of water at the cathode side of second electrochemical cell
EC2 is
removed via line 28. In this configuration, a portion of the power generated
in the
first electrochemical cell EC1 provides the electrical power for the second
CA 02732747 2011-02-01
WO 2010/016899 PCT/US2009/004480
- 13 -
electrochemical cell EC2. In this configuration, a portion of the power
generated in
the first electrochemical cell EC1 also provides the electrical power for the
third
electrochemical cell EC3 via the transport of electrons via electrically
conductive
line 21 from the anode of electrochemical cell EC2 to the cathode of
electrochemical
cell EC3.
[0021] In this configuration, the third fraction of the aqueous metal
sulfide stream
is introduced into the anode side of third electrochemical cell EC3 via line
30 and an
effective amount of water via line 32. Again, the metal sulfide undergoes an
oxidation reaction that produces metal cations M+ and elemental sulfur S . The
elemental sulfur is collected via line 34 and the metal cations permeate
through
membrane PM of the third electrochemical cell EC3 wherein the metal cations
combine with hydroxide ions to regenerate additional metal hydroxide (MOH),
which is collected via line 36. The overall electrical loop is closed with a
transfer of
electrons via electrically conductive line 23 from the anode of
electrochemical cell
EC3 to the cathode of electrochemical cell Ed. Excess hydrogen produced in the
cathode side of electrochemical cell EC3 is collected via line 38.
[0022] As shown in the Figure hereof, the electrical potential generated in
the first
cell is ideally sufficient to drive the hydroxide regeneration reactions in
all three
cells. In the event that cell resistances (for example: ohmic, diffusional,
over-
potential) are such that the potential generated in the first cell is not
sufficient, then
additional electrical potential can be supplied from an external power source
(not
shown) by connecting it in series with the electronic circuit of the cells.
External
power may also be usefully applied to increase the rates of reactions in the
cells.
[0023] Although the present invention has been described in terms of
specific
embodiments, it is not so limited. Suitable alterations and modifications for
CA 02732747 2015-02-20
- 14 -
operation under specific conditions will be apparent to those skilled in the
art. Such
modifications that may be obvious to one of skill in the art include, but are
not
limited to, the use of more than three electrochemical cells as shown as well
as
providing additional external power as required to maintain the energy
required for
reactions and/or improve the system performance. It is therefore intended that
the
following claims be interpreted as covering all such alterations and
modifications.
The scope of the claims should not be limited by particular embodiments set
forth
herein, but should be construed in a manner consistent with the specification
as a
whole.