Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02734932 2011-03-23
THERAPEUTIC IMPLANT
[00011
Technical Field
[0002] The present application relates to a biocompatible composite soft
tissue repair
surgical implant which comprises at least one therapeutic agent for use in
repair of hernias, for
example, and methods of making such surgical implants.
Background of Related Art
[0003] A hernia is basically a defect resulting in the protrusion of part of
an organ, tissue
or structure through the wall of a body cavity within which it is normally
contained.
[0004] Meshes may be applied subcutaneously (e.g., under the skin) internally
or
externally of the abdominal wall and may be either absorbable or nonabsorbable
depending on
the nature and severity of the particular defect or hernia being treated.
[0005] Both laparoscopic and open procedures have been preferred for the
treatment of
hernias with meshes. It is desirable to treat hernias, as when carrying out
any surgery, with as
little trauma to the patient as possible, reducing the post-operative pain for
the patient. Thus,
improvements to meshes, including reducing post-operative pain remain
desirable.
SUMMARY
[0006] The present disclosure is directed to a surgical implant including a
film for
releasing a first therapeutic agent, and a mesh for releasing a second
therapeutic agent. The
1
CA 02734932 2011-03-23
film covers at least a portion of the mesh. In certain embodiments, the mesh
is at least partially
embedded within the film, alternatively, the film may be positioned adjacent a
first surface of the
mesh. The first therapeutic agent is released at a first rate and the second
therapeutic agent is
released at a second rate.
[0007] In some embodiments, the film comprises a water soluble polymer. The
film may
also be rapidly degrading, wherein the film degrades from about less than 24
hours after
implantation, and in certain embodiments, from about less than one hour after
implantation.
[0008] Materials which may comprise the film include polyesters,
polysaccharides,
proteins, peptides, hydrophilic vinyls, polyamides, polyamines, polyalkylene
oxalates,
poly(anhydrides), polyamidoesters, copoly(ether-esters), poly(carbonates),
poly(hydroxyalkanoates), polyimide carbonates, poly(imino carbonates),
polyorthoesters,
polyoxaesters, polyphosphazenes, poly (propylene fumarates), polyurethanes,
polymer drugs
and combinations thereof. The film may also comprise glycerol or carboxymethyl
cellulose.
[0009] Materials which may comprise the mesh include polyolefins, polyesters,
proteins,
polysaccharides, and combinations thereof. The mesh may further include a
coating, which
optionally includes the second therapeutic agent. Alternatively, the mesh may
have at least one
degradable filament, the degradable filament optionally containing the second
therapeutic
agent.
[0010] The first therapeutic agent may be released in situ from about less
than 24 hours
after implantation, and in certain embodiments, from about less than one hour
after
implantation. The second therapeutic agent may be released in situ from about
more than 24
hours, and in certain embodiments, from about 24 hours to about fourteen days.
The first
therapeutic agent may be the same as or different than the second therapeutic
agent.
[0011] The first or second therapeutic agent may include anti-inflammatory
agents,
analgesic agents, anesthetic agents, antibiotic agents, angiogenic agents,
antispasmodic
agents, growth factors, gene-based therapies, proteins, peptides, nucleic
acids, polymers drugs,
2
CA 02734932 2011-03-23
and combinations thereof. In alternate embodiments, the first or the second
therapeutic agent
may include bupivacaine hydrochloride, bupivacaine, or capsaicin.
[0012] A method of treating tissue is disclosed, the method comprising the
steps of
implanting in the tissue a surgical implant, the surgical implant comprising a
film including a first
therapeutic agent and a mesh including a second therapeutic agent; the film
releasing the first
therapeutic agent to the tissue; and the mesh releasing the second therapeutic
agent to the
tissue.
[0013] A method of manufacturing a surgical implant is also disclosed,
including
providing, in a film, a first therapeutic agent; providing, in a mesh, a
second therapeutic agent
and applying the film to the mesh. An alternate method of manufacturing is
provided comprising
the steps of: providing, in a polymer solution, a first therapeutic agent;
providing, in a mesh, a
second therapeutic agent; applying the polymer solution to the mesh.
BRIEF DESCRIPTION OF DRAWINGS
[0014] The accompanying drawings, which are incorporated in and constitute a
part of
this specification, illustrate embodiments of the disclosure and, together
with a general
description of the disclosure given above, and the detailed description of the
embodiment(s)
given below, serve to explain the principles of the disclosure, wherein:
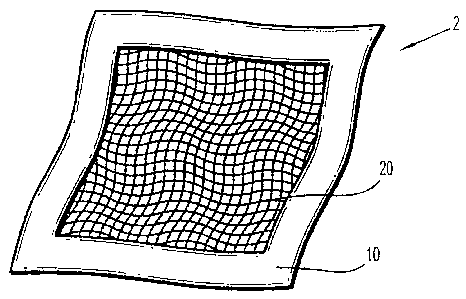
[0015] FIG. 1 illustrates one embodiment of an implant according to the
present
disclosure;
[0016] FIG. 2A illustrates a cross-sectional view of the implant of Figure 1;
[0017] FIG. 2B illustrates a schematic cross-sectional view of the implant of
Figure 2A;
[0018] FIG. 3 illustrates a plan view of another embodiment of an implant
according to
the present disclosure;
[0019] FIG. 4A illustrates a cross-sectional view of the implant of Figure 3;
[0020] FIG. 4B illustrates a schematic cross-sectional view of the implant of
Figure 3;
3
CA 02734932 2011-03-23
[0021] FIG. 5 illustrates a perspective view of one embodiment of a mold for
making
implants of the present disclosure;
[0022] FIG. 6 illustrates a sectional view of a portion of the mold of Figure
5,
[0023] FIG.7 illustrates incisional injury-induced hypersensitivity for
implants according
to the present disclosure; and
[0024] FIG. 8 illustrates in vivo bupivacaine HCI release from implants
according to the
present disclosure.
DETAILED DESCRIPTION OF EMBODIMENTS
[0025] The present invention relates to a therapeutic composite surgical
implant and
methods for making and using such a surgical implant. More specifically, the
implant includes a
film comprising at least one layer. The implant further includes a mesh which
may at least
partially in contact with or embedded within the film. In embodiments, the
mesh includes a first
release mechanism for releasing a first therapeutic agent and the film
includes a second release
mechanism for releasing a second therapeutic agent. In certain embodiments, at
least one of
the therapeutic agents comprises a local anesthetic.
[0026] According to the present disclosure, implants disclosed herein include
a mesh in
combination with a film, each comprising any suitable biocompatible material.
Suitable
materials should have sufficient tensile strength to support a fascial wall
(on injured tissue site)
during repair of a defect, be sufficiently inert to avoid foreign body
reactions when retained in
the human body for long periods of time and have suitably easy handling
characteristics for
insertion and placement in the desired location in the body.
[0027] Meshes disclosed herein generally comprise filaments, major spaces, and
pores.
The filaments of the mesh may be formed by at least two strands, the major
spaces formed
between the filaments providing the surgical implant with the necessary
strength, the filaments
arranged (i.e., woven or knit) such that pores are formed in the strands
themselves.
4
CA 02734932 2011-03-23
Alternatively, the filaments may be formed by a monofilament fiber (as opposed
to at least two
strands) that are arranged to form loops that give rise to the pores.
[0028] Suitable meshes for use in the present disclosure include, for example,
a
collagen composite mesh such as PARIETEXT" Composite Mesh (commercially
available from
Tyco Healthcare Group LG, d/b/a Covidien). PARIETEXTM Composite Mesh is a 3-
dimensional
polyester weave with a resorbable collagen film bonded on one side. Another
suitable mesh
includes Parietex ProgripTM self-fixating mesh (also commercially available
from Covidien).
Parietex Progrip TM is a polyester mesh which includes poly lactic acid (PLA)
microgrips. Other
suitable meshes include those sold under the names PARIETENE , PERMACOLT""
PARIETEXTM, SURGIPROTM (all commercially available from Covidien); PROLENETMV,
(commercially available from Ethicon, Inc.); MARLEX , DULEX , 3D MAX mesh,
PERFIX
plug, VENTRALEX , and KUGEL patch (all commercially available from C.R. Bard,
Inc.);
PROLITE TM, PROLITE ULTRA TM (all commercially available from Atrium Medical);
COMPOSIX , SEPRAMESH , and VISILEX (all commercially available from Davol,
Inc.);
and DUALMESH , MYCROMESH , and INFINIT mesh (all commercially available from
W.L.
Gore). Additionally, meshes within the scope and context of this disclosure
may include biologic
materials such as allografts, autografts, and xenografts.
[0029] According to one embodiment of the present disclosure, Parietex Pro-
gripTM Self-
fixating mesh may be employed. Pro-gripTM mesh includes a knit comprising a
monofilament
sheet forming, on one face of the knit, spiked/barbed naps which protrude
perpendicularly with
respect to said sheet. The naps each have a substantially rectilinear body
and, at the free end
of this body, a head of greater width than that of this body. The barbed naps
function as hooks,
which are capable of being fastened either to another prosthetic fabric
(belonging to the same
prosthesis or not) or directly to the biological tissues. In certain
embodiments, the fabric or
mesh may comprise barbed naps on each surface of the mesh.
CA 02734932 2011-03-23
[0030] The mesh component may be formed using any method suitable to forming
fibrous structures, including but not limited to knitting, weaving, knipling,
tatting, non-woven
techniques, wet-spinning, electro-spinning, gel-spinning, extrusion, co-
extrusion, and the like.
Suitable techniques for making mesh are within the purview of those skilled in
the art.
[0031] Implants according to the present disclosure further include at least
one film,
which in certain embodiments, is rapidly degrading. In one example, the
rapidly degrading
(biodegradable) film covers at least a portion of the mesh. As will be later
described, the film
may be positioned adjacent at least a first and/or second surface of the mesh
or, the mesh may
be at least partially embedded within the film. Similarly, the film may at
least partially penetrate
into the three dimensional construct of the mesh. Alternatively, the film may
be present on both
the first and second surfaces of the mesh.
[0032] Additionally, the film may comprise at least one layer, and in some
examples, the
film may have a multilaminar construct.
[0033] Implants disclosed herein may comprise, for example, synthetic
materials,
natural materials (e.g., biological) and combinations thereof. Suitable
polymers include,
polyolefins such as polyethylene (including ultra high molecular weight
polyethylene) and
polypropylene including atactic, isotactic, syndiotactic, and blends thereof;
polyethylene glycols;
polyethylene oxides; ultra high molecular weight polyethylene; copolymers of
polyethylene and
polypropylene; polyisobutylene and ethylene-alpha olefin copolymers;
fluorinated polyolefins
such as fluoroethylenes, fluoropropylenes, fluoroPEGSs, and
polytetrafluoroethylene;
polyamides such as nylon, Nylon 6, Nylon 6,6, Nylon 6,10, Nylon 11, Nylon 12,
and
polycaprolactam; polyamines; polyimines; polyesters such as polyethylene
terephthalate,
polyethylene naphthalate, polytrimethylene terephthalate, and polybutylene
terephthalate;
polyethers; polybutester; polytetramethylene ether glycol; 1,4-butanediol;
polyurethanes; acrylic
polymers; methacrylics; vinyl halide polymers and copolymers, such as
polyvinyl chloride;
polyvinyl alcohols; polyvinyl ethers such as polyvinyl methyl ether;
polyvinylidene halides such
6
CA 02734932 2011-03-23
as polyvinylidene fluoride and polyvinylidene chloride;
polychlorofluoroethylene;
polyacrylonitrile; poIyary Ietherketones; polyvinyl ketones; polyvinyl
aromatics such as
polystyrene; polyvinyl esters such as polyvinyl acetate; copolymers of vinyl
monomers with each
other and olefins, such as ethylene-methyl methacrylate copolymers;
acrylonitrile-styrene
copolymers; ABS resins; ethylene-vinyl acetate copolymers; alkyd resins;
polycarbonates;
polyoxymethylenes; polyphosphazine; polyimides; epoxy resins; aramids; rayon;
rayon-
triacetate; spandex; silicones; and copolymers and combinations thereof.
Additionally, non-
biodegradable polymers and monomers may be combined with each other to create
a core of a
fiber, for example a fiber possessing a core-sheath configuration. In certain
embodiments, at
least the mesh may comprise PET.
[0034] Other synthetic polymers which may be utilized in accordance with the
present
disclosure include, but are not limited to anionic, cationic and neutral
monomers and polymers
of vinyl polymers such as polyvinyl alcohol, polyvinyl methyl ether,
polyvinylpyrrolidone (PVP),
poly acrylic acid, styrene sulfonic acid, polyhydroxyethylmethylacrylate
(pHEMA) and
phospholipid vinyls; acrylic polymers such as sodium polyacrylate,
polyethylacrylate, and
polyacrylamide; polyethylene glycol, polypropylene oxide, and polypropylene
glycol and
homopolymers and copolymers thereof; phosphorylcholine functional acrylates
and
methacrylates; homopolymers and copolymers thereof.
[0035] Additionally, biodegradable synthetic or natural materials may be
employed. As
used herein, the term "biodegradable" includes both bioabsorbable and
bioresorbable materials.
By biodegradable, it is meant that the materials decompose, or lose structural
integrity under
body conditions (e.g., enzymatic degradation, hydrolysis) or are broken down
(physically or
chemically) under physiologic conditions in the body (e.g., dissolution) such
that the degradation
products are excretable or absorbable by the body.
[0036] Suitable bioabsorbable polymers may comprise implants of the present
disclosure including, but are not limited to polymers selected from the group
consisting of
7
CA 02734932 2011-03-23
aliphatic polyesters; polyamides; polyamines; polyalkylene oxalates;
poly(anhydrides);
polyamidoesters; copoly(ether-esters); poly(carbonates) including tyrosine
derived carbonates;
poly(hydroxyalkanoates) such as poly(hydroxybutyric acid), poly(hydroxyvaleric
acid), and
poly(hydroxybutyrate); polyimide carbonates; poly(imino carbonates) such as
poly (bisphenol A-
iminocarbonate and the like); polyorthoesters; polyoxaesters including those
containing amine
groups; polyphosphazenes; poly (propylene fumarates); polyurethanes; polymer
drugs such as
polydiflunisol, polyaspirin, and protein therapeutics; biologically modified
(e.g., protein,
peptide)bioabsorbable polymers; and copolymers, block copolymers,
homopolymers, blends,
and combinations thereof.
[0037] More specifically, for the purpose of this invention, aliphatic
polyesters include,
but are not limited to, homopolymers and copolymers of lactide (including
lactic acid, D-,L- and
meso lactide); glycolide (including glycolic acid); epsilon-caprolactone, p-
dioxanone (1,4-dioxan-
2-one); trimethylene carbonate (1,3-dioxan-2-one); alkyl derivatives of
trimethylene carbonate;
A-valerolactone; [3-butyrolactone; y-butyrolactone; c-decalactone;
hydroxybutyrate;
hydroxyvalerate; 1,4-dioxepan-2-one (including its dimer 1,5,8,12-
tetraoxacyclotetradecane-
7,14-dione); 1,5-dioxepan-2-one; 6,6-dimethyl- 1,4-dioxan-2-one; 2,5-
diketomorpholine;
pivalolactone; a, a diethylpropiolactone; ethylene carbonate; ethylene
oxalate; 3-methyl-1,4-
dioxane-2,5-dione; 3,3-diethyl-1,4-dioxan-2,5-dione; 6,8-dioxabicycloctane-7-
one; and polymer
blends and copolymers thereof. In certain embodiments, the mesh may comprise
an aliphatic
polyester.
[0038] Other suitable biodegradable polymers include, but are not limited to,
poly(amino
acids) including proteins such as collagen (I, II and III), elastin, fibrin,
fibrinogen, silk, and
albumin; peptides including sequences for laminin and fibronectin (RGD);
polysaccharides such
as hyaluronic acid (HA), dextran, alginate, chitin, chitosan, and cellulose;
glycosaminoglycan;
gut; and combinations thereof. Collagen as used herein includes natural
collagen such as
8
CA 02734932 2011-03-23
animal derived collagen, gelatinized collagen, or synthetic collagen such as
human or bacterial
recombinant collagen.
[0039] Additionally, synthetically modified natural polymers such as cellulose
and
polysaccharide derivatives, including alkyl celluloses, hydroxyalkyl
celluloses, cellulose ethers,
cellulose esters, nitrocelluloses, and chitosan may be utilized. Examples of
suitable cellulose
derivatives include methyl cellulose, ethyl cellulose, hydroxypropyl
cellulose, hydroxypropyl
methyl cellulose, hydroxybutyl methyl cellulose, cellulose acetate, cellulose
propionate,
cellulose acetate butyrate, cellulose acetate phthalate, carboxymethyl
cellulose (CMC),
cellulose triacetate, and cellulose sulfate sodium salt. These may be
collectively referred to
herein, in embodiments, as "celluloses."
[0040] In certain embodiments, the film may comprise a polysaccharide such as
CMC
while the mesh portion comprises polyester or polypropylene.
[0041] Additionally, the surgical implant may comprise any or all of
emulsifying agents,
solubilizing agents, wetting agents, taste modifying agents, plasticizers,
active agents, water
soluble inert fillers, preservatives, buffering agents, coloring agents, and
stabilizers. Addition of
a plasticizer to the formulation can improve flexibility. The plasticizer or
mixture of plasticizers
may be polyethylene glycol, glycerol, sorbitol, sucrose, corn syrup, fructose,
dioctyl-sodium
sulfosuccinate, triethyl citrate, tributyl citrate, 1,2-propylenglycol, mono-,
di- or triacetates of
glycerol, or natural gums.
[0042] Turning now to Figure 1, one embodiment of an implant 2 according to
the
present disclosure is illustrated including a film 10, which surrounds or
encapsulates a mesh 20.
As illustrated, the film 10 is present on a first and second surface (20a,
20b) of the mesh 20.
More specifically, the film 10 comprises a water soluble polymer, which may be
provided as a
laminate film or sheet. The film 10 may be smooth or rough in surface texture.
Further, as
shown in Figure 2A, at least two films (1Oa and 1Ob) may be in contact with
the mesh 20. It is
further envisioned that more than one layer of mesh 20 may also be present in
the implant 2.
9
CA 02734932 2011-03-23
[0043] The films may comprise a single, laminate layer, or conversely, the
films may
comprise several layers, creating a multi-laminate film. Multi-laminate films
may comprise
similar or different materials. The multi-laminate films may also comprise
different polymer
chain orientations, i.e., they may have anisotropic properties, which when
combined (optionally
at various orientations relative to one another) create a stronger implant.
Additionally, films
disclosed herein may be continuous or discontinuous.
[0044] The film 10 is positioned adjacent the mesh 20 and as illustrated in
Figures 2A
and 2B, the film 10a, 10b is present of a first and second surface (20a, 20b)
of the mesh 20,
encapsulating the mesh 20 therein. The mesh 20 is illustrated as a
monofilament mesh 20;
however, implants comprising multifilament mesh are within the scope of the
present disclosure.
The monofilament mesh 20 includes spaces and/or pores which may be formed by
the
intersection of at least two filaments. The film 10 may also penetrate within
the pores or spaces
within or between the mesh 20, interlocking therewith.
[0045] Another embodiment of an implant according to the present disclosure is
illustrated in Figures 3, 4A, and 4B. An implant 100 comprises a rapidly
degrading film 110 in
direct contact with a mesh 120. The mesh 120 comprises monofilament threads
122 (illustrated
in the cross-sectional view, Figure 4). The rapidly degrading film 110 is in
direct contact with a
first side 120a of the mesh. The monofilament mesh 120 may include spaces
and/or pores
which, in embodiments, may be penetrable by the film 110. In other
embodiments, one layer of
the film 110 may be in direct contact with the mesh 120, covering at least a
portion of the first
surface 120a thereof. In some embodiments, the film 110 may penetrate pores of
the mesh knit
or weave 120.
[0046] In general, the film covers at least a portion of the mesh. The film
may be
positioned adjacent to at least a first surface of the mesh and in certain
embodiments, the film at
least partially penetrates into a first surface of the mesh. In yet alternate
embodiments, the film
may be embedded at least partially or entirely within the mesh construct (2-
dimensional or 3-
CA 02734932 2011-03-23
dimensional), penetrating into the pores and/or interstices of the mesh. It
should be understood
that above examples are non-limiting and other constructs are envisioned which
comprise the
combination of a film and a mesh.
[0047] As discussed hereinabove, the film may comprise a multi-laminar or
multilayer
construct. The multi-laminar construct may provide for different release rates
and kinetics. The
first therapeutic agent may be disposed therein at least one layer of the
multi-laminar film. In
one embodiment, the multi-laminar film includes a film having at least one
barrier layer disposed
thereon. The barrier layer may or may not include a bioactive agent.
Additionally, the multi-
laminar construct may utilize different polymers and different crystal
structures of varying
polymers to optimize drug delivery/release into the surrounding environment.
[0048] Films of the present disclosure may be rapidly degrading or rapidly
absorbing.
The rapidly degrading film at least partially degrades from about less than 24
hours after
implantation. The rapidly degrading film may entirely degrade from about less
than 24 hours
following implantation. In certain embodiments, the rapidly degrading film
degrades from about
less than one hour after implantation. The rapid degradation of the film may
enable a faster
delivery of the first therapeutic agent to the patient. In one non-limiting
example, bupivacaine
hydrochloride is combined with a CMC film (in solution using standard mixing
techniques).
Once implanted, the CMC film may hydrolyze in less than one hour in situ,
delivering a
predetermined payload of bupivacaine hydrochloride to the patient in less than
one hour.
[0049] Surgical implants include therapeutic agents which are delivered or
carried to the
tissue site and released over a specified time period. More specifically, the
first therapeutic
agent is delivered or carried to the implant/tissue site by the film
component. The film serves as
the delivery vehicle for transporting the first therapeutic agent into the
body. The first
therapeutic agent may be contained on or within the film utilizing a variety
of methods, for
example, the first therapeutic agent may be contained within
micro/nanospheres, liposomes,
carbon nanotubes, micro/nanoparticles, drug macromers, polymer drugs,
prodrugs, other nano
11
CA 02734932 2011-03-23
structures, and the like, and using various salt forms, incorporated in the
film. The first
therapeutic agent may also be impregnated within the film in the form of a
particulate
suspension or emulsion.
[0050] The second therapeutic agent is delivered or carried to the
implant/tissue site by
the mesh component. The mesh serves as the delivery vehicle for transporting
the second
therapeutic agent. The second therapeutic agent may be contained on or within
the mesh. For
example, the mesh may comprise a coating which includes the second therapeutic
agent. In
another non-limiting example, the second therapeutic agent may be compounded
within the
polymer resin, comprising at least one filament of the mesh. In another
example, the
therapeutic agent may be disposed around or within interstices of the mesh. In
yet an alternate
example, at least one filament of the mesh may comprise a polymer drug,
wherein upon
degradation of the filament, the polymer drug is hydrolyzed and released into
the surrounding
tissue in either monomer or polymer form. In alternate embodiments, the second
therapeutic
agent may be contained within microspheres or microparticles. Similar to the
first therapeutic
agent, the second therapeutic agent may also be impregnated within a coating
in the form of a
particulate suspension or emulsion.
[0051] Therapeutic agents of the present disclosure are released into the
surrounding
tissue at various elution or release rates (dose/unit time). More
specifically, the first therapeutic
agent may be released at a first rate and the second therapeutic agent may be
released at a
second rate. In other embodiments, the first and second therapeutic agents may
be released at
the same rate. Similarly, the first and second therapeutic agents may have the
same release
rate, however, the first therapeutic agent may be released over less or more
hours/days/weeks
as compared to the second therapeutic agent (longer or shorter persistence).
For example, at
37 C, a first therapeutic agent such as bupivacaine hydrochloride may have a
release rate of
7.5 mg/hour for a total of 10 hours (releasing 75 mg), while the second
therapeutic agent such
12
CA 02734932 2011-03-23
as bupivacaine (free base) may have a release rate of 7.5 mg/hour for a total
of 72 hours
(releasing 540 mg).
[0052] The first therapeutic agent may be released in situ from about less
than 24 hours
after implantation, in certain embodiments, from less than about 1 hour after
implantation. The
second therapeutic agent may be released in situ from about greater than 24
hours, and in
certain embodiments, from about 3 days (72 hours) to about 14 days after
implantation. In
certain embodiments, the second therapeutic agent may begin releasing in situ
less than about
24 hours and continue to release for about 14 days following implantation.
[0053] More specifically, when the first and second therapeutic agents
comprise
bupivacaine hydrochloride, the first therapeutic agent may have a delivery of
7.5-10
milligrams/hour for a total time period of less than 24 hours, while the
second therapeutic agent
may have a delivery of 7.5 milligrams/hour, for a total time period of greater
than 24 hours.
[0054] In certain embodiments, at least the first therapeutic agent comprises
bupivacaine hydrochloride. In other embodiments, at least the second
therapeutic agent
comprises bupivacaine (free base).
[0055] In certain embodiments, at least the first therapeutic agent comprises
capsaicin.
In other embodiments, at least the second therapeutic agent comprises
capsaicin.
[0056] In other embodiments, the therapeutic agents have different release
rates. The
term rate as used herein should be understood to relate to a therapeutic
payload/unit time. For
example, the second release rate may be slower compared to the first release
rate. Upon
implantation, both the first and second therapeutic agents may begin to elute
into the
surrounding tissue. The first therapeutic agent is released into tissue
immediately upon
implantation (bolus release), while the second therapeutic agent is delivered
at a slower rate,
which may be through a sustained or controlled release over the following
days/weeks.
[0057] Conversely, in other embodiments, the first therapeutic agent may have
a slower
release rate as compared to the second therapeutic agent.
13
CA 02734932 2011-03-23
[0058] As previously stated, in certain embodiments, the first and second
therapeutic
agents are released over time periods ranging from minutes to weeks. For
example, the first
therapeutic agent may comprise a local anesthetic, to assist in providing
local pain relief during
surgery, the first therapeutic agent having a persistence of less than 6
hours. The second
therapeutic agent may comprise an analgesic to relieve longer term pain
associated with
healing or inflammation, The second therapeutic agent may have a persistence
of from about a
few hours (or immediately following surgery), to several weeks post operation.
In certain
embodiments, the second therapeutic agent may have a persistence of from about
24 hours to
about 7 days following surgery.
[0059] In one embodiment, where the film comprises a fast degrading film, due
to fast
hydrolysis or degradation of the film component, the first therapeutic agent
may have a faster
release rate, such as a bolus release compared to the second agent disposed on
or within the
mesh. In certain embodiments, the film component may at least partially
degrade before the
second therapeutic agent is eluted into the surrounding environment. In other
words, the first
therapeutic agent may shield, protect or otherwise provide a barrier to the
release of the second
therapeutic agent. It is also envisioned that the release of the first
therapeutic agent may trigger
environmental changes (e.g., pH and Tonicity) which signal the release of the
second therapeutic
agent. In yet alternate embodiments, the second therapeutic agent may diffuse
through the film,
releasing into the surrounding environment.
[0060] Concentrations and doses of therapeutic agents of the present
disclosure may
vary depending on drug choice or patient condition. For example, one patient
may require more
or less of a specific therapeutic agent as compared to another. The dosing
rates of different
therapeutic agents may vary while efficacy remains similar. For example, a
lower concentration
of a first therapeutic agent may be required for a first therapeutic effect,
while a higher
concentration of a second therapeutic agent may be required for a second
therapeutic effect.
14
CA 02734932 2011-03-23
[0061] The first and second therapeutics may have a bolus release or sustained
delivery/release into the surrounding environment, the release kinetics of
which may correspond
to zero order, first order, second order, third order, nth order and
combinations thereof.
Additionally, releases may be diffusion, partition, or solubility controlled.
[0062] In general, therapeutic agents may be incorporated into the implant
during
manufacture or formation of the implant, such as by free solution, suspension,
liposomal
delivery, microspheres, etc., or by coating a surface of the implant, or
selective regions thereof,
such as by polymer coating, dry coating, freeze drying, applying directly to
the mesh or implant
surface; ionically, covalently, or affinity binding to functionalize the
components of the implant.
Thus, at least one therapeutic agent may be combined with a component of the
implant i.e., the
mesh and/or film, to provide release of the therapeutic agent during
implantation and in some
embodiments, release of the therapeutic agent via degradation of the implant.
In some
embodiments, as the implant degrades or hydrolyzes in situ, the therapeutic
agents are
released. In other embodiments, therapeutic agents may be included in the film
component (or
a selective region(s) thereof) for rapid release of the bioactive agent.
[0063] The delivery mechanism for the first therapeutic agent is the film,
which in certain
embodiments, is a rapidly degrading film. The film may comprise a water
soluble polymer
which, upon implantation, begins dissolution, releasing the first therapeutic
agent. It should be
understood, as similarly stated above, that the term degrading includes
decomposition,
enzymatic degradation, hydrolysis or dissolution, wherein the materials are
broken down
(physically or chemically) under physiologic conditions in the body and the
degradation products
are excretable or absorbable by the body.
[0064] The first therapeutic agent may be formulated into the polymer film in
the form of
an emulsion, suspension or other heterogeneous mixture; or mixed in as a
homogeneous
solution prior to, during, or after film formation. Solvents for use in
creating films of the present
CA 02734932 2011-03-23
disclosure include polar, non-polar solvents, buffers, and the like. Films may
be made from
solutions using methods such as film casting, which are later described.
[0065] The second therapeutic agent is delivered to the surrounding tissue via
the
mesh. The second therapeutic agent may be in the form of a coating on the
mesh, trapped
within interstices or pores in the mesh, compounded into the resin or
otherwise incorporated
therein. The second therapeutic agent may also comprise suspensions or
emulsions,
microparticles, fibers and the like, which may be combined or otherwise
incorporated into the
woven or knit mesh. For example, the controlled release of the second
therapeutic agent may
correspond to the degradation of at least one filament of the mesh. Coating
materials may
comprise polymers not limited to those listed herein.
[0066] It should be understood that as described herein, the therapeutic
agents are both
localized methods of drug delivery, however, the therapeutic agent(s) may also
be distributed to
the surrounding tissues and organs (such as surrounding vasculature) and even
dispersed
systemically.
[0067] Suitable first and second therapeutic agents employed in the present
disclosure
may include analgesics, anesthetics, anti-inflammatory agents (steroidal and
non-steroidal),
antispasmodic agents, growth factors, gene-based therapeutic agents and
combinations
thereof. The first therapeutic agent may be the same as or different than the
second therapeutic
agent. The therapeutic agents may be the same class of agents, i.e., both the
first and second
therapeutics may comprise analgesics.
[0068] More specifically, analgesics such as narcotic analgesic therapeutic
agents
include, but are not limited to: alfentanil, allylprodine, alphaprodine,
anileridine, benzylmorphine,
bezitramide, buprenorphine, butorphanol, clonitazene, codeine, codeine methyl
bromide,
codeine phosphate, codeine sulfate, desomorphine, dextromoramide, dezocine,
diampromide,
dihydrocodeine, dihydrocodeinone enol acetate, dihydromorphine, dimenoxadol,
dimepheptanol, dimethylthiambutene, dioxaphetyl butyrate, dipipanone,
eptazocine,
16
CA 02734932 2011-03-23
ethoheptazine, ethylmethylthiambutene, ethylmorphine, etonitazene, fentanyl,
hydrocodone,
hydromorphone, hydroxypethidine, isomethadone, ketobemidone, levorphanol,
lofentanil,
meperidine, meptazinol, metazocine, methadone hydrochloride, metopon,
morphine, myrophine,
nalbuphine, narceine, nicomorphine, norlevorphanol, normethadone, normorphine,
norpipanone, opium, oxycodone, oxymorphone, papaveretum, pentazocine,
phenadoxone,
phenazocine, pheoperidine, piminodine, piritramide, proheptazine, promedol,
properidine,
propiram, propoxyphene, rumifentanil, sufentanil, tilidine, and
pharmaceutically acceptable salts
thereof.
[0069] Exemplary non-narcotic analgesic agents that may be combined with the
implants of the invention include, but are not limited to, aceclofenac,
acetaminophen,
acetaminosalol, acetanilide, acetylsalicylsalicylic acid (aspirin),
alclofenac, alminoprofen,
aloxiprin, aluminum bis(acetylsalicylate), aminochlorthenoxazin, 2-amino-4-
picoline,
aminopropylon, aminopyrine, ammonium salicylate, amtolmetin guacil,
antipyrine, antipyrine
salicylate, antrafenine, apazone, benorylate, benoxaprofen, benzpiperylon,
benzydamine,
bermoprofen, brofenac, p-bromoacetanilide, 5-bromosalicylic acid acetate,
bucetin, bufexamac,
bumadizon, butacetin, calcium acetylsalicylate, capsaicin, carbamazepine,
carbiphene,
carsalam, celecoxib, chloralantipyrine, chlorthenoxazin(e), choline
salicylate, cinchophen,
ciramadol, clometacin, croproparnide, crotethamide, dexoxadrol, diclofenac,
difenamizole,
diflunisal, dihydroxyaluminum acetylsalicylate, dipyrocetyl, dipyrone,
emorfazone, enfenamic
acid, epirizole, etersalate, ethenzamide, ethoxazene, etodolac, felbinac,
fenoprofen,
floctafenine, flufenamic acid, fluoresone, flupirtine, fluproquazone,
flurbiprofen, fosfosal,
gabapentin, gentisic acid, glafenine, ibufenac, ibuprofen, imidazole
salicylate, indomethacin,
indoprofen, isofezolac, isoladol, isonixin, ketoprofen, ketorolac, p-
lactophenetide, lefetamine,
loxoprofen, lysine acetylsalicylate, magnesium acetylsalicylate,
methotrimeprazine, metofoline,
miroprofen, morazone, morpholine salicylate, naproxen, nefopam, nifenazone, 5'
nitro-2'
propoxyacetanilide, parsalmide, perisoxal, phenacetin, phenazopyridine
hydrochloride,
17
CA 02734932 2011-03-23
phenocoll, phenopyrazone, phenyl acetylsalicylate, phenyl salicylate,
phenyramidol,
pipebuzone, piperylone, pregabalin, prodilidine, propacetamol, propyphenazone,
proxazole,
quinine salicylate, ramifenazone, rimazolium metilsulfate, salacetamide,
salicin, salicylamide,
salicylamide o-acetic acid, salicylsulfuric acid, salsalte, salverine,
simetride, sodium salicylate,
sulfamipyrine, suprofen, talniflumate, tenoxicam, terofenamate, tetradrine,
tinoridine, tolfenamic
acid, tolpronine, tramadol, TRPA1 modulators, TRPM8 modulators, TRPV1
modulators, viminol,
xenbucin, zomepirac, and pharmaceutically acceptable salts thereof.
[0070] Exemplary local anesthetic therapeutic agents include, but are not
limited to,
ambucaine, amolanone, amylocalne hydrochloride, benoxinate, benzocaine,
betoxycaine,
biphenamine, bupivacaine, butacaine, butaben, butanilicaine, butethamine,
butoxycaine,
carticaine, chloroprocaine hydrochloride, cocaethylene, cocaine,
cyclomethycaine, dibucaine
hydrochloride, dimethisoquin, dimethocaine, diperadon hydrochloride,
dyclonine, ecgonidine,
ecgonine, ethyl chloride, beta-eucaine, euprocin, fenalcomine, fomocaine,
hexylcaine
hydrochloride, hydroxytetracaine, isobutyl p-aminobenzoate, leucinocaine
mesylate,
levobupivacaine, levoxadrol, lidocaine, mepivacaine, meprylcaine,
metabutoxycaine, methyl
chloride, myrtecaine, naepaine, octacaine, orthocaine, oxethazaine,
parethoxycaine,
phenacaine hydrochloride, phenol, piperocaine, piridocaine, polidocanol,
pramoxine, prilocaine,
procaine, propanocaine, proparacaine, propipocaine, propoxycaine
hydrochloride,
pseudococaine, pyrrocaine, ropivacaine, salicyl alcohol, tetracaine
hydrochloride, tolycaine,
trimecaine, zolamine, and pharmaceutically acceptable salts thereof.
[0071] Other therapeutic agents which may be utilized in accordance with the
present
disclosure include drugs, amino acids, peptides, polypeptides, proteins,
polysaccharides,
muteins, immunoglobulins, antibodies, cytokines (e.g., lymphokines, monokines,
chemokines),
blood clotting factors, hemopoletic factors, interleukins (1 through 18),
interferons ((3-IFN, a-IFN
and y-IFN), erythropoietin, nucleases, tumor necrosis factor, colony
stimulating factors (e.g.,
GCSF, GM-CSF, MCSF), insulin, anti-tumor agents and tumor suppressors, blood
proteins,
18
CA 02734932 2011-03-23
fibrin, thrombin, fibrinogen, synthetic thrombin, synthetic fibrin, synthetic
fibrinogen,
gonadotropins (e.g., FSH, LH, CG, etc.), hormones and hormone analogs (e.g.,
growth
hormone, luteinizing hormone releasing factor ), vaccines (e.g., tumoral,
bacterial and viral
antigens); somatostatin; antigens; blood coagulation factors; growth factors
(e.g., nerve growth
factor, insulin-like growth factor); bone morphogenic proteins, TGF-B, protein
inhibitors, protein
antagonists, and protein agonists; nucleic acids, such as antisense molecules,
DNA, RNA,
RNAi, sRNA; oligonucleotides; polynucleotides; cells (including stem cells,
adult, embryonic, or
induced) viruses, and ribozymes.
[0072] In embodiments, the therapeutic agent may include at least one of the
following
drugs, including combinations and alternative forms of the drugs such as
alternative salt forms,
free acid form, free base forms, pro-drugs and hydrates:
analgesics/antipyretics (e.g., aspirin,
acetaminophen, ibuprofen, naproxen sodium, buprenorphine, propoxyphene
hydrochloride,
propoxyphene napsylate, meperidine hydrochloride, hydromorphone hydrochloide,
morphine,
oxycodone, codeine, dihydrocodeine bitartrate, pentazocine, hydrocodone
bitartrate,
levorphanol, diflunisal, trolamine salicylate, nalbuphine hydrochloride,
mefenamic acid,
butorphanol, choline salicylate, butalbital, phenyltoloxamine citrate,
diphenhydramine citrate,
methotrimeprazine, cinnamedrine hydrochloride, and meprobamate);
antiasthamatics (e.g.,
ketotifen and traxanox); antibiotics (e.g., neomycin, streptomycin,
chloramphenicol,
cephalosporin, ampicillin, penicillin, tetracycline, and ciprofloxacin);
antidepressants (e.g.,
nefopam, oxypertine, doxepin, amoxapine, trazodone, amitriptyline,
maprotiline, phenelzine,
duloxetine, desipramine, nortriptyline, tranylcypromine, fluoxetine, doxepin,
imipramine,
imipramine pamoate, isocarboxazid, trimipramine, and protriptyline);
antidiabetics (e.g.,
biguanides and sulfonylurea derivatives); antifungal agents (e.g.,
griseofulvin, ketoconazole,
itraconizole, amphotericin B, nystatin, and candicidin); antihypertensive
agents (e.g., propanolol,
propafenone, oxyprenolol, nifedipine, reserpine, trimethaphan,
phenoxybenzamine, pargyline
hydrochloride, deserpidine, diazoxide, guanethidine monosulfate, minoxidil,
rescinnamine,
19
CA 02734932 2011-03-23
sodium nitroprusside, rauwolfia serpentina, alseroxylon, and phentolamine);
anti-inflammatories
(e.g., (non-steroidal) indomethacin, ketoprofen, aspirin, diclofenac,
ketorolac, flurbiprofen,
naproxen, ibuprofen, ramifenazone, piroxicam, celecoxib, rofecoxib,
(steroidal) cortisone,
dexamethasone, fluazacort, hydrocortisone, prednisolone, and prednisone);
antineoplastics
(e.g., cyclophosphamide, actinomycin, bleomycin, dactinomycin, daunorubicin,
doxorubicin,
epirubicin, mitomycin, methotrexate, fluorouracil, gemcitabine, carboplatin,
carmustine (BCNU),
methyl-CCNU, cisplatin, etoposide, camptothecin and derivatives thereof,
phenesterine,
paclitaxel and derivatives thereof, docetaxel and derivatives thereof,
vinblastine, vincristine,
goserelin, leuprolide, tamoxifen, interferon alfa, retinoic acid (ATRA),
nitrogen mustard alkylating
agents, and piposulfan); antianxiety agents (e.g., lorazepam, buspirone,
prazepam,
chlord iazepoxide, oxazepam, clorazepate dipotassium, diazepam, hydroxyzine
pamoate,
hydroxyzine hydrochloride, alprazolam, droperidol, halazepam, chlormezanone,
and
dantrolene); immunosuppressive agents (e.g., cyclosporine, azathioprine,
mizoribine, and
FK506 (tacrolimus)); antimigraine agents (e.g., triptans such as sumatriptan,
ergotamine,
propanolol, isometheptene mucate, and dichioralphenazone); sedatives/hypnotics
(e.g.,
barbiturates such as pentobarbital, pentobarbital, and secobarbital; and
benzodiazapines such
as flurazepam hydrochloride, triazolam, and midazolam); antianginal agents
(e.g., beta-
adrenergic blockers; calcium channel blockers such as nifedipine, and
diltiazem; and nitrates
such as nitroglycerin, isosorbide dinitrate, pentearythritol tetranitrate, and
erythrityl tetranitrate);
antipsychotic agents (e.g., haloperidol, loxapine succinate, loxapine
hydrochloride, thioridazine,
thioridazine hydrochloride, thiothixene, fluphenazine, fluphenazine decanoate,
fluphenazine
enanthate, trifluoperazine, chlorpromazine, perphenazine, lithium citrate, and
prochlorperazine);
antimanic agents (e.g., lithium carbonate); antiarrhythmics (e.g., bretylium
tosylate, esmolol,
verapamil, amiodarone, encainide, digoxin, digitoxin, mexiletine, disopyramide
phosphate,
procainamide, quinidine sulfate, quinidine gluconate, quinidine
polygalacturonate, flecainide
acetate, tocainide, and lidocaine); antiarthritic agents (e.g.,
phenylbutazone, sulindac,
CA 02734932 2011-03-23
penicillanine, salsalate, piroxicam, azathioprine, indomethacin,
meclofenamate, gold sodium
thiomalate, ketoprofen, auranofin, aurothioglucose, and tolmetin sodium);
antigout agents (e.g.,
coichicine, and allopurinol); anticoagulants (e.g., heparin, heparin sodium,
and warfarin sodium);
thrombolytic agents (e.g., urokinase, streptokinase, and alteplase);
antifibrinolytic agents (e.g.,
aminocaproic acid); hemorheologic agents (e.g., pentoxifylline); antiplatelet
agents (e.g.,
aspirin); anticonvulsants (e.g., valproic acid, divalproex sodium, phenytoin,
phenytoin sodium,
clonazepam, primidone, phenobarbitol, carbamazepine, amobarbital sodium,
methsuximide,
metharbital, mephobarbital, mephenytoin, phensuximide, paramethadione,
ethotoin,
phenacemide, secobarbitol sodium, clorazepate dipotassium, and trimethadione);
antiparkinson
agents (e.g., ethosuximide); antihistamines/antipruritics (e.g., hydroxyzine,
diphenhydramine,
chlorpheniramine, brompheniramine maleate, cyproheptadine hydrochloride,
terfenadine,
clemastine fumarate, triprolidine, carbinoxamine, diphenylpyraline,
phenindamine, azatadine,
tripelennamine, dexchlorphenirarnine maleate, methdilazine, and); agents
useful for calcium
regulation (e.g., calcitonin, and parathyroid hormone); antibacterial agents
(e.g., amikacin
sulfate, aztreonam, chloramphenicol, chloramphenicol palirtate, ciprofloxacin,
clindamycin,
clindamycin palmitate, clindamycin phosphate, metronidazole, metronidazole
hydrochloride,
gentamicin sulfate, lincomycin hydrochloride, tobramycin sulfate, vancomycin
hydrochloride,
polymyxin B sulfate, colistimethate sodium, and colistin sulfate); antiviral
agents (e.g., interferon
alpha, beta or gamma, zidovudine, amantadine hydrochloride, ribavirin, and
acyclovir);
antimicrobials (e.g., cephalosporins such as cefazolin sodium, cephradine,
cefaclor, cephapirin
sodium, ceftizoxime sodium, cefoperazone sodium, cefotetan disodium,
cefuroxime e azotil,
cefotaxime sodium, cefadroxil monohydrate, cephalexin, cephalothin sodium,
cephalexin
hydrochloride monohydrate, cefamandole nafate, cefoxitin sodium, cefonicid
sodium,
ceforanide, ceftriaxone sodium, ceftazidime, cefadroxil, cephradine, and
cefuroxime sodium;
penicillins such as ampicillin, amoxicillin, penicillin G benzathine,
cyclacillin, ampicillin sodium,
penicillin G potassium, penicillin V potassium, piperacillin sodium, oxacillin
sodium,
21
CA 02734932 2011-03-23
bacampicillin hydrochloride, cloxacillin sodium, ticarcillin disodium,
azlocillin sodium,
carbenicillin indanyl sodium, penicillin G procaine, methicillin sodium, and
nafcillin sodium;
erythromycins such as erythromycin ethylsuccinate, erythromycin, erythromycin
estolate,
erythromycin lactobionate, erythromycin stearate, and erythromycin
ethylsuccinate; and
tetracyclines such as tetracycline hydrochloride, doxycycline hyclate, and
minocycline
hydrochloride, azithromycin, clarithromycin); anti-infectives (e.g., GM-CSF);
bronchodilators
(e.g., sympathomimetics such as epinephrine hydrochloride, metaproterenol
sulfate, terbutaline
sulfate, isoetharine, isoetharine mesylate, isoetharine hydrochloride,
albuterol sulfate, albuterol,
bitolterolmesylate, isoproterenol hydrochloride, terbutaline sulfate,
epinephrine bitartrate,
metaproterenol sulfate, epinephrine, and epinephrine bitartrate;
anticholinergic agents such as
ipratropium bromide; xanthines such as aminophylline, dyphylline,
metaproterenol sulfate, and
aminophylline; mast cell stabilizers such as cromolyn sodium; inhalant
corticosteroids such as
beclomethasone dipropionate (BDP), and beclomethasone dipropionate
monohydrate;
salbutamol; ipratropium bromide; budesonide; ketotifen; salmeterol; xinafoate;
terbutaline
sulfate; triamcinolone; theophylline; nedocromil sodium; metaproterenol
sulfate; albuterol;
flunisolide; fluticasone proprionate; steroidal compounds and hormones (e.g.,
androgens such
as danazol, testosterone cypionate, fluoxymesterone, ethyltestosterone,
testosterone enathate,
methyltestosterone, fluoxymesterone, and testosterone cypionate; estrogens
such as estradiol,
estropipate, and conjugated estrogens; progestins such as methoxyprogesterone
acetate, and
norethindrone acetate; corticosteroids such as triamcinolone, betamethasone,
betamethasone
sodium phosphate, dexamethasone, dexamethasone sodium phosphate, dexamethasone
acetate, prednisone, methyiprednisolone acetate suspension, triamcinolone
acetonide,
methyiprednisolone, prednisolone sodium phosphate, methylprednisolone sodium
succinate,
hydrocortisone sodium succinate, triamcinolone hexacetonide, hydrocortisone,
hydrocortisone
cypionate, prednisolone, fludrocortisone acetate, paramethasone acetate,
prednisolone
tebutate, prednisolone acetate, prednisolone sodium phosphate, and
hydrocortisone sodium
22
CA 02734932 2011-03-23
succinate; and thyroid hormones such as levothyroxine sodium); hypoglycemic
agents (e.g.,
human insulin, purified beef insulin, purified pork insulin, glyburide,
chlorpropamide, glipizide,
tolbutarnide, and tolazamide); hypolipidemic agents (e.g., clofibrate,
dextrothyroxine sodium,
probucol, pravastitin, atorvastatin, lovastatin, and niacin); proteins (e.g.,
DNase, alginase,
superoxide dismutase, and lipase); nucleic acids (e.g., sense or anti-sense
nucleic acids
encoding any therapeutically useful protein, including any of the proteins
described herein);
agents useful for erythropoiesis stimulation (e.g., erythropoietin);
antiulcer/antireflux agents
(e.g., famotidine, cimetidine, and ranitidine hydrochloride);
antinauseants/antiemetics (e.g_,
meclizine hydrochloride, nabilone, prochiorperazine, dimenhydrinate,
promethazine
hydrochloride, thiethylperazine, and scopolamine); proton pump inhibitors
(e.g., omeprazole);
erectile dysfunction therapies (e.g., sildenafil, vardenafil, tadalafil, and
alprostadil); as well as
other drugs useful in the compositions and methods described herein include
mitotane,
halonitrosoureas, anthrocyclines, ellipticine, ceftriaxone, ketoconazole,
ceftazidime, oxaprozin,
albuterol, valacyclovir, urofollitropin, famciclovir, flutamide, enalapril,
mefformin, itraconazole,
buspirone, gabapentin, fosinopril, tramadol, acarbose, lorazepan, follitropin,
glipizide,
omeprazole, fluoxetine, lisinopril, tramsdol, levofloxacin, zafirlukast,
interferon, growth hormone,
interleukin, erythropoietin, granulocyte stimulating factor, nizatidine,
bupropion, perindopril,
erbumine, adenosine, alendronate, alprostadil, benazepril, betaxolol,
bieomycin sulfate,
dexfenfluramine, diltiazem, fentanyl, flecainid, gemcitabine, glatiramer
acetate, granisetron,
lamivudine, mangafodipir trisodium, mesalamine, metoprolol fumarate,
metronidazole, miglitol,
moexipril, monteleukast, octreotide acetate, olopatadine, paricalcitol,
somatropin, sumatriptan
succinate, tacrine, verapamil, nabumetone, trovafloxacin, dolasetron,
zidovudine, finasteride,
tobramycin, isradipine, toicapone, enoxaparin, fluconazole, lansoprazole,
terbinafine,
pamidronate, didanosine, diclofenac, cisapride, venlafaxine, troglitazone,
fluvastatin, losartan,
imiglucerase, donepezil, olanzapine, valsartan, fexofenadine, calcitonin, and
ipratropium
23
CA 02734932 2011-03-23
bromide. In some embodiments, the drug may be water soluble. In some
embodiments, the
drug may not be water soluble.
[0073] The rate of release of a therapeutic agent can be controlled by any
means within
the purview of one skilled in the art. Some examples include, but are not
limited to, the depth of
the therapeutic agent from the surface of the film, the size of the
therapeutic agent, the
hydrophilicity/hydrophobicity of the therapeutic agent, and the strength of
physical and physical-
chemical interaction between the therapeutic agent, the rapidly degrading film
and/or the mesh
material. By properly controlling some of these factors, a controlled release
of a therapeutic
agent from the implant of the present disclosure can be achieved.
[0074] Films of the present disclosure may be prepared using casting
techniques. In
some embodiments, implants of the present disclosure may be formed using a
mold such as
one illustrated in Figures 5 and 6. The mold 200 has a base 210 and side walls
220. Although
the base 210 and side walls 220 are shown generally rectangular in shape, they
may comprise
other shapes. The mold 220 includes shims 230 which are disposed parallel to
each other on
an upper surface of the base 210. The molds may also include a non-stick
coating or surface,
such as silicone or polyester terephthalate, for ease of manufacturing. It
should be noted that
any number of shims 230 may be employed, as well as any desired alignment to
create
implants of various shapes. The mesh may be combined with a polymer solution
or cast film
and placed in the mold. In some embodiments, the implant may need to cure or
dry for a
specified amount of time under set temperature, humidity and pressure. The
height of the
shims and the base may vary so that the mesh can be placed nearer to one
surface or placed a
set distance away from the implant surface.
[0075] In other embodiments, films may be pre-cast and later combined with the
mesh
component to create an implant. The films may be adhered of otherwise combined
with the
mesh using techniques not limited to solvent welding, heat staking,
compression fitting, or the
use of adhesives, glues, sealants, epoxies or combinations thereof. For
example, the mesh
24
CA 02734932 2011-03-23
may be coated with a monomer solution and then placed/pressed adjacent the
mesh, creating
an implant of the present. If two layers of film are desired, a similar
technique may be used to
apply the second film layer.
[0076] Upon implantation of the implant in vivo, the film solubilizes in the
aqueous
environment, releasing the first therapeutic agent. In some embodiments, the
first therapeutic is
released from about less than one day, in further embodiments, from about less
than one hour.
Sometime thereafter, the second therapeutic agent is released through a second
mechanism,
and in preferred embodiments, the second therapeutic agent is released from
the mesh. The
release of the second therapeutic agent is from about more than one day, and
in further
embodiments, from about three days to about fourteen days.
[0077] Implants of the present disclosure must have sufficient structural
integrity and
physical properties to facilitate the surgeon's ease of handling in the
operating room as well as
positioning in vivo. Materials selection in addition to
processing/manufacturing constraints can
be used to alter/control the strength and physical properties of the surgical
implant.
[0078] Desired properties of composite mesh include flexible enough,
conforming to
tissue and being repositionable, yet stiff enough to be unfolded after
insertion, perhaps through
a laparoscopic port. Additionally, other materials such as a polymer coating
or polymer fibers
may be incorporated into the mesh to increase the mesh stiffness at least for
insertion and
implantation. A mesh according to the present disclosure can be inserted
through a small
incision (e.g., from about 1 cm to about 2 cm in length) with the use of a
laparoscopic
deployment device, trocar, or other device. The mesh may be rolled or folded
so as to fit within
the device for transfer into the body cavity.
[0079] More broadly, the present disclosure recognizes that the implant can
have any
shape that conforms to an anatomical surface of a human or animal body that
may be subject to
a defect to be repaired by the implant.
CA 02734932 2011-03-23
[0080] In another embodiment, the surgical implant of the present disclosure
may
comprise a backing strip which may releasably attach to the implant. The
backing strip may be
formed from a range of materials, including plastics, and may releasably
attach using an
adhesive.
(0081] The releasable attachment of a backing strip to the implant may provide
a more
rigid and less flexible surgical implant, which may be more easily handled by
a surgeon.
Following suitable placement of the surgical implant, the backing strip can be
removed from the
surgical implant, the surgical implant being retained in the body and the
backing material being
removed by the surgeon. The surgical implant can therefore benefit from
reduced mass while
still providing characteristics required for surgical handling.
EXAMPLE 1
[0082] A 2% weight/volume stock solution is created by combining medium
viscosity
CIVIC (MW=150k-400k g/mol) and deionized water using a mechanical stirrer. The
CMC
solution is then combined with glycerol in a 5:1 ratio (CMC: glycerol), and
left stirring with a stir
bar for about 240 minutes. Next, bupivacaine (MW=324.89, from Sigma Aldrich)
is added to the
CMC/glycerol solution using a mechanical stir bar (at ambient temperature).
The concentration
of the bupivacaine in solution is about 0.25-0.75% wt/vol.
[0083] Next, a sheet of polyester mesh is dip coated in a bupivacaine solution
and dried
in the oven overnight at 40 Celsius and 40 % relative humidity.
[0084] The bupivacaine-coated polyester mesh is then placed in a silicone-
coated mold,
sized to fit the mesh. The bupivacaine/CMC/glycerol solution is poured over
the mesh, creating
a uniform film, and then placed in an oven overnight at a temperature of about
45 Celsius.
[0085] A mesh according to the present disclosure can be inserted through a
small
incision (e.g., from about 1 cm to about 2 cm in length) or tissue puncture
with the use of a
laparoscopic deployment device, such as a needle or trocar. The mesh may be
rolled or folded
26
CA 02734932 2011-03-23
so as to fit within the device for transfer into the body cavity. In
embodiments utilizing an
absorbable film, the absorbable film may provide sufficient stiffness to the
mesh upon exiting the
transfer device, to re-open the rolled or folded mesh into its original
geometric shape.
EXAMPLE 2
[0086] Mesh/film compositions as prepared in Example 1 were evaluated for in
vivo
bupivacaine HCI release and efficacy using rodent subcutaneous and back
incisional acute
postoperative pain preclinical models, respectively. Briefly, Sprague-Dawley
rats were
anesthetized and a 2 cm incision was made through the skin of the back on
midline and a small
pocket created by blunt dissection. Mesh/film and/or film and mesh alone test
articles were
gently implanted into the incisional site using forceps and were located
directly under the
incision. The incisions were closed with suture and all rats were carefully
monitored after
surgery. Control groups consist of one implant control group and two systemic
treatment
groups. Implant control groups receive mesh/films with no drug. The systemic
controls receive
either systemic morphine (3 mg/kg) or saline (2 ml/kg) at the end of surgery
and again at 47 hr
following surgery (1 hr prior to 48 hr testing). In Vivo bupivacaine HCI
release was assessed by
evaluating residual bupivacaine at various time points following explantation
(mass balance of
remaining bupivacaine HCI) using high pressure liquid chromatography.
Behavioral testing was
used to assess thermal response latency following application of thermal
stimulus to incision
sites via a diode laser. The thermal stimulus was applied to the left side of
the incision and the
latency (in time,seconds) was measured as response to distinct twitching or
rippling of the
musculature under the skin of the stimulated area. Three response latency
readings were
preformed for each subject at each time point.
[0087] Results for representative bupivacaine HCI-eluting CMC/glycerol mesh
integrated
films demonstrated the ability to completely prevent injury-induced
hypersensitivity in the back
incisional model of acute pain and in dose dependant manner. The in vivo
release profile of
27
CA 02734932 2011-03-23
bupivacaine HCI correlated with behavioral efficacy at early time points with
drug release being
mostly complete by 8 his post-implantation (Figures 7 and 8). Figure 7.
illustrates incisional
injury-induced hypersensitivity for CMC/glycerol/bupivacaine HCI films. Figure
8. illustrates in
Vivo bupivacaine HCI release from CMC/glycerol films of various payloads (0.3
to 3 mg/cm2).
[0088] While several embodiments of the disclosure have been described, it is
not
intended that the disclosure be limited thereto, as it is intended that the
disclosure be as broad
in scope as the art will allow and that the specification be read likewise.
Therefore, the above
description should not be construed as limiting, but merely as
exemplifications of embodiments
of the present disclosure. Various modifications and variations of the porous
substrate and the
reinforcement component of the implant will be apparent to those skilled in
the art from the
foregoing detailed description. Such modifications and variations are intended
to come within
the scope and spirit of the claims appended hereto.
28