Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
SELF-DISPERSED PIGMENTS AND METHODS FOR
MAKING AND USING THE SAME
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application is a continuation-in-part of and claims priority to
U.S. Patent
Application No. 12/197,087, filed August 22, 2008, which claims priority under
35 U.S.C.
119(e) to U.S. Provisional Patent Application No. 60/957,596, filed August 23,
2007. This
application also claims priority under 35 U.S.C. 119(e) to U.S. Provisional
Patent Application
Nos. 60/957,596, 61/091,300, 61/094,307, and 61/154,686, filed August 23,
2007, August 22,
2008, September 4, 2008, and February 23, 2009, respectively. The entire
contents of these
applications are hereby incorporated by reference in their entireties.
FIELD OF USE
[0002] The present invention relates to a method of making self-dispersing
pigments. More
particularly, the present invention relates to the surface modification of
pigments. Pigments
whose surfaces are modified through covalent bonding are known in the industry
as self-
dispersing pigments. The surface modifications may be carried out in an
aqueous environment
and may be eco friendly. The invention further relates to end use applications
comprising
surface-modified pigments, including, without limitation, coatings, paints,
papers, adhesives,
latexes, toners, textiles, fibers, plastics, inks, and cosmetic applications.
Specific examples of
end uses include, without limitation, printing ink for paper, textiles,
fibers, metal deco and
plastics, wood stains, writing instruments, color filters, and mascaras. The
invention also relates
to inks such as inkjet inks.
BACKGROUND
[0003] Pigments offer several advantages over water-soluble dyes when it comes
to inks,
coatings, paints, papers, adhesives, latexes, toners, textiles, fibers, wood
stains, color filters,
plastics, and cosmetic applications. Pigments may exhibit at least one of
greater lightfastness,
waterfastness, optical density and edge acuity than water-soluble dyes.
Unfortunately, pigments
also have a greater propensity to settle during storage, thus initially
limiting their use in
demanding applications such as inkjet inks. The advent of media mills to grind
pigment particles
to sub-micron level combined with chemical additives for colloidal stability
has propelled the
1
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
use of pigment dispersions in inkjet ink formulations. However, chemical
additives can increase
the viscosity of dispersions such that it becomes difficult to jet the ink
from the small orifices in
an inkjet printhead. Moreover, chemical additives can add significant cost to
the preparation of
the materials listed above and are therefore economically unfavorable as well.
Chemical
additives, or dispersants, may not be bonded to the surface of the pigment and
therefore,
stabilization may be compromised. A need remains for improved ink
compositions, especially
for use in inkjet printers, which overcome at least some of the problems
typically associated with
current dye-based systems and pigment systems employing chemical additives. A
need also
remains for improved materials that use pigments, which overcome at least some
of the problems
typically associated with current dye based systems and pigment systems
employing chemical
additives.
SUMMARY
[0004] In one aspect, the invention may provide a modified pigment that may
include a
polymer. The pigment may be directly attached to a nitrogen atom. The nitrogen
atom may be
attached directly or indirectly to a group that may include -S-Z. S may be a
substituted or
unsubstituted alkyl group, substituted or unsubstituted aromatic group, or
polymer chain having a
molecular weight range from about 300 to about 20000. Z may be a hydrogen,
carboxyl,
sulfonyl, phenolic, phosphoryl, ammonium, trimethylammonium, or
tributylammonium group.
[0005] In another aspect, the invention may provide a modified pigment that
may include a
polymer. The pigment may be attached to an organic group through a carbon atom
that is part of
a N-C=N bond.
[0006] In yet another aspect, the invention may provide a cosmetic formulation
that may
include a pigment covalently bonded to an organic group.
[0007] In a further aspect, the invention may provide a method of modifying a
pigment
dispersion that may include reacting a substituted triazine with a pigment
dispersion.
[0008] In another aspect, the invention may provide a method of modifying a
pigment that
may include reacting cyanuric chloride with a secondary compound or a mixture
of secondary
2
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
compounds to displace at least one reactive chlorine to form a substituted
triazine and attaching
the substituted triazine and at least one polymer to a surface of a pigment.
[0009] In a further aspect, the invention may provide a method of modifying a
pigment that
may include displacing at least one reactive chorine of a cyanuric chloride
with at least one
polymer to form a substituted triazine. The substituted triazine may be
reacted with a pigment
dispersed in a medium.
[0010] In yet another aspect, the invention may provide a method of modifying
a pigment
that may include attaching at least one polymer to a pigment in a pigment
dispersion to form a
modified pigment. The modified pigment may have directly attached a nitrogen
atom. The
nitrogen atom may be directly or indirectly attached to an organic group.
[0011] In a further aspect, the invention may provide a modified pigment that
may include a
pigment having attached a polymer and at least one of a group that may include
N-S, a triazine
substituted with at least one group that may include N-S, and a combination
thereof. N may be a
nucleophilic group and S may be an organic group.
BRIEF DESCRIPTION OF THE DRAWINGS
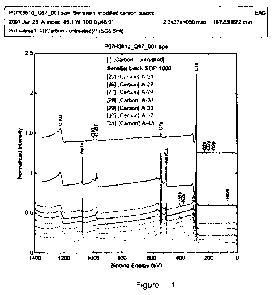
[0012] Fig. 1 displays low resolution X-Ray Photoelectron Spectroscopy (XPS)
spectra for
untreated carbon black sample, Sensijet SDP 1000 carbon, and carbon black
samples from
Examples 25 thru 31.
[0013] Fig. 2 displays high resolution CIs XPS spectra for untreated carbon
black sample,
Sensijet SDP 1000 carbon and carbon black samples from Examples 25 thru 31.
[0014] Fig. 3 displays high resolution NIs XPS spectra for carbon black
samples from
Examples 25 thru 31.
[0015] Fig. 4 displays high resolution Ols XPS spectra for untreated carbon
black sample,
Sensijet SDP 1000 carbon and carbon black samples from Examples 25 thru 31.
[0016] Fig. 5 displays high resolution S2p XPS spectra for untreated carbon
black sample,
Sensijet SDP 1000 carbon, and carbon black samples from Examples 25 thru 31.
3
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[0017] Fig. 6 displays high resolution Nals XPS spectra for Sensijet SDP 1000
carbon and
carbon black samples from Examples 25 thru 31.
[0018] Fig. 7 displays low resolution X-Ray Photoelectron Spectroscopy (XPS)
spectra for
untreated carbon black sample and carbon black samples from Examples 3, 8, 24
and 32.
[0019] Fig. 8 displays high resolution Cls XPS spectra for untreated carbon
black sample
and carbon black samples from Examples 3, 8, 24 and 32.
[0020] Fig. 9 displays high resolution Nls XPS spectra for carbon black
samples from
Examples 3, 8, 24 and 32.
[0021] Fig. 10 displays high resolution Ols XPS spectra for untreated carbon
black sample
and carbon black samples from Examples 3, 8, 24 and 32.
[0022] Fig. 11 displays high resolution S2p XPS spectra for untreated carbon
black sample
and carbon black samples from Examples 3, 8, 24 and 32.
[0023] Fig. 12 displays high resolution Nals XPS spectra for carbon black
samples from
Examples 3, 8, 24 and 32.
[0024] Fig. 13 displays low resolution XPS spectra for untreated Pigment Blue
15 sample
and Pigment Blue 15 samples from Examples 10, 14, and 21.
[0025] Fig. 14 displays high resolution Cls XPS spectra for untreated Pigment
Blue 15
sample and Pigment Blue 15 samples from Examples 10, 14, and 21.
[0026] Fig. 15 displays high resolution Nls XPS spectra for untreated Pigment
Blue 15
sample and Pigment Blue 15 samples from Examples 10, 14, and 21.
[0027] Fig. 16 displays high resolution Ols XPS spectra for untreated Pigment
Blue 15
sample and Pigment Blue 15 samples from Examples 10, 14, and 21.
[0028] Fig. 17 displays high resolution S2p XPS spectra for untreated Pigment
Blue 15
sample and Pigment Blue 15 samples from Examples 10, 14, and 21.
4
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[0029] Fig. 18 displays high resolution Nals XPS spectra for untreated Pigment
Blue 15
samples and Pigment Blue 15 sample from Examples 10 and 21.
[0030] Fig. 19 displays high resolution Cu2p XPS spectra for untreated Pigment
Blue 15
samples and Pigment Blue 15 sample from Examples 10, 14, and 21.
[0031] Fig. 20 displays low resolution XPS spectra for untreated Pigment Red
No. 122
sample, Pigment Red No. 122 samples from Examples 17 and 22 and Pigment Violet
No. 19
samples from Examples 6 and 7.
[0032] Fig. 21 displays high resolution Cls XPS spectra for untreated Pigment
Red No. 122
samples and Pigment Red No. 122 samples from Examples 17 and 22 and Pigment
Violet No. 19
samples from Examples 6 and 7.
[0033] Fig. 22 displays high resolution Nls XPS spectra for untreated Pigment
Red No. 122
sample and Pigment Red No. 122 samples from Examples 17 and 22 and Pigment
Violet No. 19
samples from Examples 6 and 7.
[0034] Fig. 23 displays high resolution Ols XPS spectra for untreated Pigment
Red No. 122
sample and Pigment Red No. 122 samples from Examples 17 and 22 and Pigment
Violet No. 19
samples from Examples 6 and 7.
[0035] Fig. 24 displays high resolution S2p XPS spectra for Pigment Red No.
122 sample
from Example 22 and Pigment Violet No. 19 samples from Examples 6 and 7
[0036] Fig. 25 displays high resolution Nals XPS spectra for Pigment Red No.
122 samples
from Examples 17 and 22 and Pigment Violet No. 19 samples from Examples 6 and
7.
[0037] Fig. 26 displays low resolution XPS spectra for untreated Pigment
Yellow No. 74
sample and for Pigment Yellow No. 74 samples from Examples 23 and 34.
[0038] Fig. 27 displays high resolution Cls XPS spectra for untreated Pigment
Yellow No.
74 sample and for Pigment Yellow No. 74 samples from Examples 23 and 34.
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[0039] Fig. 28 displays high resolution NIs XPS spectra for untreated Pigment
Yellow No.
74 sample and for Pigment Yellow No. 74 samples from Examples 23 and 34.
[0040] Fig. 29 displays high resolution Ols XPS spectra for untreated Pigment
Yellow No.
74 sample and for Pigment Yellow No. 74 samples from Examples 23 and 34.
[0041] Fig. 30 displays high resolution S2p XPS spectra for untreated Pigment
Yellow No.
74 sample and for Pigment Yellow No. 74 samples from Examples 23 and 34.
[0042] Fig. 31 displays high resolution Nals XPS spectra for untreated Pigment
Yellow No.
74 sample and for Pigment Yellow No. 74 samples from Examples 23 and 34.
[0043] Fig. 32 displays low resolution XPS spectra for untreated Pigment
Yellow No. 155
sample and for Pigment Yellow No. 155 samples from Examples 11 and 12.
[0044] Fig. 33 displays high resolution CIs XPS spectra for untreated Pigment
Yellow No.
155 samples and for Pigment Yellow No. 155 samples from Examples 11 and 12.
[0045] Fig. 34 displays high resolution NIs XPS spectra for untreated Pigment
Yellow No.
155 samples and for Pigment Yellow No. 155 samples from Examples 11 and 12.
[0046] Fig. 35 displays high resolution Ols XPS spectra for untreated Pigment
Yellow No.
155 samples and for Pigment Yellow No. 155 samples from Examples 11 and 12.
[0047] Fig. 36 displays high resolution S2p XPS spectra for Pigment Yellow No.
155
samples from Examples 11 and 12.
[0048] Fig. 37 displays an in vivo test of a mascara prepared according to the
present
invention compared with control mascaras (see Example 50).
DETAILED DESCRIPTION
[0049] Before any embodiments of the invention are explained in detail, it is
to be
understood that the invention is not limited in its application to the details
of construction and the
arrangement of components set forth in the following description. The
invention is capable of
other embodiments and of being practiced or of being carried out in various
ways. Also, it is to
6
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
be understood that the phraseology and terminology used herein is for the
purpose of description
and should not be regarded as limiting. The use of "including," "comprising,"
or "having" and
variations thereof herein is meant to encompass the items listed thereafter
and equivalents
thereof as well as additional items.
[0050] It also is understood that any numerical range recited herein includes
all values from
the lower value to the upper value. For example, if a concentration range is
stated as 1% to 50%,
it is intended that values such as 2% to 40%, 10% to 30%, or 1% to 3%, etc.,
are expressly
enumerated in this specification. These are only examples of what is
specifically intended, and
all possible combinations of numerical values between and including the lowest
value and the
highest value enumerated are to be considered to be expressly stated in this
application.
[0051] In one aspect, the invention provides a method of modifying a pigment.
The method
may include attaching an organic group with charged end groups (negative or
positive) through
the intermediacy of a reactive molecule to produce a surface stabilized
modified pigment.
Without being limited by theory, it is believed that the stabilization is
achieved by an even
distribution of similarly charged groups which are covalently attached on sub
micron sized
pigment particles by the forces of repulsion.
[0052] In yet another aspect, the invention provides a dispersion that
includes a self-
dispersing pigment that has been formed by a reaction of a pigment with a
reactive intermediate
that has been attached to suitable organic molecules as described above. The
selection of
reactive intermediates that are stable in an aqueous environment is another
aspect of the present
invention.
[0053] In a further aspect, the invention provides a dispersion that includes
a self-dispersing
pigment comprising about 0.01 to about 1.0 mMoles of S and about 0.01 to about
2.0 mMoles of
active hydrogen per gram of pigment, and water. In another aspect, the
invention provides a
dispersion that includes a self-dispersing pigment comprising about 0.06 to
about 0.7 mMoles of
S and about 0.07 to about 1.6 mMoles of active hydrogen per gram of pigment,
and water.
[0054] In another aspect, the invention provides a polymer, polymeric resin,
dispersant or
binder attachment to a pigment or modified pigment, which enhances at least
one durability
7
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
property such as highlighter, water, or rub resistance while enhancing
redispersibility. These
properties are relevant to certain of the applications discussed herein, such
as ink jet ink printing.
Fast print speeds and small jet volumes (2-5 pico liters) also dictate low
viscosity ink
formulations particularly for thermal ink jetting. Attaching the polymer,
polymeric resin,
dispersant or binder reduces the quantity requirement. Additionally, the
polymer stays with the
pigment, and therefore, low viscosity formulations produce comparable results.
[0055] Method for Making Surface-Modified or Self-Dispersing Pigments
[0056] One aspect of the present invention relates to a method for making
stable, self-
dispersing pigments.
[0057] As used herein, the term "pigment" means a colorant insoluble in a
solvent medium
that is used to impart color to a substrate such as plain or coated paper,
film and other types of
receiving media. The pigment may also impart color to cosmetic formulations.
Pigments may
be black as well as other colors.
[0058] As used herein, the term "self-dispersing" pigment means a pigment
having
stabilizing groups covalently attached to its surface such that the pigment
forms a stable aqueous
dispersion in the absence of any additional dispersing agents.
[0059] As used herein, the term "stable" means that on aging the dispersion
will undergo
minimal changes as demonstrated by less than 10% change in measured critical
properties (such
as at least one of mean particle size, viscosity, surface tension and pH) when
stored at ambient
temperature over a period of at least about three months to six months to two
years. Accelerated
test methods include a heat stability test at about 70 C for at least about
one week or a heat
stability test at about 70 C for at least about four weeks.
[0060] As used herein, the term "redispersible" means that a modified pigment
dispersion of
the present invention may be dried to form a powder, and that powder may be
redispersed in a
medium. The medium may be an aqueous or non-aqueous medium. In the present
invention, at
least about 90% of the modified pigment present in the powder may be
effectively redispersed in
water as a dry powder. "Effectively redispersed" means that the particles may
not be
agglomerated in the end product.
8
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[0061] As used herein, the term "attachment" may include direct or indirect
attachment.
[0062] In one embodiment, the invention provides a method for making a surface
modified
or self-dispersing pigment comprising a pigment having attached at least one
of a group
comprising N-S, an X substituted with at least one group comprising N-S, and a
combination
thereof. For example, the pigment may have attached at least one of -X-N-S-Z, -
N-S-Z and a
combination thereof. In some embodiments, Z may have an ionic end group with a
counter ion
M. The pigment being modified may be dispersed in a medium. The modified
pigment may
have further attached a polymer. In another embodiment, the invention provides
a method for
making a surface modified or self-dispersing pigment comprising attaching at
least one polymer
to a pigment dispersed in a medium.
[0063] X may include, without limitation, a triazinyl group, with 1,3,5
triazine being
preferred. N may be a nucleophilic group including, without limitation, an
amine, an imine,
pyridine, or thiol. S may include, without limitation, organic groups such as,
a substituted or
unsubstituted alkyl group, substituted or unsubstituted aryl group,
substituted or unsubstituted
aromatic group, or polymer chain having from about 1 to greater than 100
carbons or having a
molecular weight range from about 300 to about 20000, suitably about 300 to
about 8000. Z
may be a hydrogen, carboxyl, sulfonyl, phenolic, phosphoryl, ammonium,
trimethylammonium,
or tributylammonium group. Z may have an ionic end group with a counter ion M.
If present, M
may be a halide, a negatively charged ion, ammonium, or a cation. In the case
of stabilization by
negative charge, ZM is an acidic tail group, wherein Z may be, without
limitation, carboxyl,
sulfonyl, phenolic, and phosphoryl and M may be either an ammonium or a
cation. In the case
of stabilization by positive charge, ZM may be a positively charged quaternary
ammonium type
tail group, wherein Z may be, without limitation, ammonium, trimethylammonium,
and
tributylammonium, and M may be a halide or any negatively charged ion.
Examples of
secondary compounds (N-S-ZM) include, without limitation, simple diamino
aromatics or
cationic polymers consisting of polyethyleneimines, polyguanidines, quaternary
ammonium
compounds etc.
[0064] In one embodiment, the method may comprise (1) optionally milling and
dispersing a
pigment to form an aqueous pigment dispersion (P)(R), the aqueous pigment
dispersion (P)(R)
9
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
comprising a) pigment (P), b) at least one of a polymer, a polymeric resin, a
dispersant or binder
(R) and c) water (alternatively, the aqueous pigment dispersion (P)(R) may be
a commercially-
available pigment dispersion such as those identified below and such as those
stabilized with
polymer additives); (2) reacting cyanuric chloride with about three
equivalents of a secondary
compound NSZ or a mixture of secondary compounds (NSZ, N1S1Z1 and N2S2Z2) to
displace all
reactive chlorines to form a substituted triazine; (3) reacting the
substituted triazine with the
aqueous pigment dispersion (P)(R) to form a self-dispersed pigment; and 4)
optionally purifying
the self-dispersed pigment to remove impurities, including the unattached
dispersant. The
resulting self-dispersed pigment may have attached at least one of a group
comprising N-S, a
triazine substituted with at least one group comprising N-S, and a combination
thereof. For
example, the modified pigment may have attached at least one of -X-N-S-Z, -X-
Ni-Si-Z1, -X-N2-
S2-Z2, -N-S-Z, -Ni-Si-Z1, and -N2-S2-Z2, depending on the secondary compounds
or mixture of
secondary compounds used. Optionally -X-N-S-Z, -X-Ni-Si-Z1, -X-N2-S2-Z2, may
be the same
or different, just as -N-S-Z, -Ni-Si-Z1, -N2-S2-Z2 maybe the same or
different. Additionally,
each X may be substituted with -N-S-Z groups that are the same or different,
and X may be
substituted with (R) (such as an amine-containing (R)). (R) may attach
directly to the pigment,
may attach to the pigment indirectly via X, or a combination thereof.
Indirectly via X means that
additional groups may or may not be present between (R) and X.
[0065] In another embodiment, the method for making a surface modified or self-
dispersing
pigment may comprise (1) reacting cyanuric chloride with about three
equivalents of a secondary
compound NSZ or a mixture of secondary compounds (NSZ, N1S1Z1 and N2S2Z2) to
displace all
reactive chlorines to form a substituted triazine; (2) reacting the
substituted triazine with a
pigment (P), which may or may not be a pigment dispersion (e.g., a dispersion
with or without
(R)), to form a self-dispersed pigment; (3) optionally mixing at least one (R)
with the self-
dispersed pigment of step (2); (4) optionally purifying the self-dispersed
pigment to remove
impurities, including the unattached (R), if applicable. The resulting self-
dispersed pigment may
have attached at least one of a group comprising N-S, a triazine substituted
with at least one
group comprising N-S, and a combination thereof. For example, the modified
pigment may have
attached at least one of -X-N-S-Z, -X-Ni-Si-Zi, -X-N2-S2-Z2, -N-S-Z, -Ni-Si-
Zi, and -N2-S2-Z2,
depending on the secondary compounds or mixture of secondary compounds used.
Optionally -
X-N-S-Z, -X-Ni-Si-Zi, -X-N2-S2-Z2, may be the same or different, just as -N-S-
Z, -Ni-Si-Zi, -
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
N2-S2-Z2 may be the same or different, and X may be substituted with (R) (such
as an amine-
containing (R)). Additionally, each X may be substituted with -N-S-Z groups
that are the same
or different. (R) may attach directly to the pigment, may attach to the
pigment indirectly via X,
or a combination thereof. Indirectly via X means that additional groups may or
may not be
present between (R) and X. Alternatively, mixing at least one (R) may be
conducted with step
(1) prior to step (2). Alternatively, reacting the substituted triazine with a
pigment (P) and
mixing at least one (R) may be conducted concurrently or substantially
concurrently.
[0066] During the substitution step, at least one chlorine of the cyanuric
chloride is
substituted with the secondary compound N-S-Z. This substitution may impart
charge and bulk
to the surface of the pigment. The substitution step may take place in an
aqueous media. The
choice of functional groups at the acidic tail is dictated by the final
application while the
functional groups at the basic head must have sufficient nucleophilicity to
displace the chlorine
in cyanuric chloride. The secondary compound may comprise polymers, amines,
amino acids,
alcohols, thiols, and combinations thereof. Examples of secondary compounds
include, without
limitation, amino benzoic acids, amino benzene sulfonic acids, amino phenols,
amino sulfonic
acids, polyethoxylated amino acids, sodium sulfanilate, sulfanilic acid,
sodium p-aminobenzoate,
p-aminophenol, ethyl 4-aminobenzoate, taurine, oleic acid (amino), sodium
aminooleate,
tetramethylammonium 4-aminobenzoate, and sodium 4-aminophenolate. Additional
secondary
compounds include organic polymeric substrates. Examples of organic polymeric
substrates
may include, without limitation, pentaethylenehexamine, linear alkyl and
branched ethoxy and
propoxy chain polymers with a known molecular weight range of 300-3000 MW,
available from
Huntsman Chemicals under the trade name "Surfonamines," linear polyethoxy
polymeric
amines, linear propoxy polymeric amines, and polyethyleneimines sold under the
trade name
"Epomines." Specific examples of organic polymeric substrates may include,
without limitation,
Surfonamine B30, L100, L300, B60, and L207 (Huntsman), pentaethylene hexamine
(Akzo
Nobel), and Epomin SP-012 (Nippon Shokubai).
[0067] As set forth above, R may be a polymer, a polymeric resin, a dispersant
or binder. In
one embodiment, a dispersant may be a polymer with functional groups that may
be activated to
form a radical and attach to the surface of the pigments. R may already
present in a raw pigment
dispersion; may be added to a raw pigment; added to a raw pigment dispersion;
or combinations
11
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
thereof. Specific examples of polymers include, but are not limited to
polystyrene-co-
maleicanhydride resins (SMA), poly(styrene-co-maleic anhydride) cumene
terminated resins,
PEI, PEHA, styrene-acrylic (SA), pentaethylenehexamine, linear alkyl and
branched ethoxy and
propoxy chain polymers with a known molecular weight range of 300-3000 MW,
available from
Huntsman Chemicals under the trade name "Surfonamines," linear polyethoxy
polymeric
amines, linear propoxy polymeric amines, styrene acrylic copolymers available
from BASF
under the trade name "Joncryls," and polyethyleneimines sold under the trade
name "Epomines,"
etc. Specific examples of polymers may include, without limitation, Joncryl
HPD 96, HPD 296,
HPD 196 (BASF), Surfonamine B30, L100, L300, B60, and L207 (Huntsman),
poly(styrene-co-
maleic anhydride) cumene terminated resin (molecular weights of 1700 and 1900)
(Aldrich),
pentaethylene hexamine (Akzo Nobel), and Epomin SP-012 (Nippon Shokubai). R
may attach
directly to the pigment, to the pigment via at least one of X-N-S-Z, or a
combination thereof.
[0068] Examples of commercial aqueous pigment dispersions (P)(R) that may be
used in the
present invention, include, but are not limited to, Sensijet Magenta PV 19,
Sensijet Cyan
PB15:3, Sensijet Yellow PY155, and Sensijet Black PB 094. Additional
examples of
commercial aqueous pigment dispersions (P)(R) include, but are not limited to,
Sensijet Black
SDP pigment dispersions (100, 1000 and 2000) available from Sensient Colors
Inc. Examples of
pigment dispersions (under the trade name Lemantex) available from Sensient
Imaging
Technologies - Specialty Inks and Colors (Switzerland) include, but are not
limited to, Cyan PB
15:3, Blue PB 60, Green PG 7, Magenta PR 122, Red PR 254, Orange PY 83, and
Yellow PY
120. Additional examples of pigment dispersions that may be modified using the
methods of the
present invention may be found in U.S. Patent No. 4,597,794 issued July 1,
1986, U.S. Patent
No. 5,172,133, issued December 15, 1992, and U.S. Patent No. 4,156,616, issued
May 29, 1979,
each of which is hereby incorporated by reference.
[0069] To help illustrate the invention, a specific example of the first
embodiment is
provided below, wherein P represents a pigment and R represents a polymer,
polymeric resin or
dispersant.
12
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
R
N;
C 'fi? 1`3 ier Eris#E~
[0070] In some embodiments, the modified pigment may comprise a polymer, and
the
pigment may be directly attached to a nitrogen atom, the nitrogen atom being
attached directly or
indirectly to an organic group. The organic group may comprise -S-Z. The
nitrogen atom may
be part of an amino group. The nitrogen atom may be part of an aromatic amino
acid such as,
without limitation, aminobenzoic acid, aminobenzenesulfonic acid, or
aminophenol. The
pigment may be covalently bonded to an aromatic substrate with an amine and
quarternary
ammonium end groups through an amine nitrogen. In other embodiments, the
modified pigment
may comprise a polymer, and the pigment may be attached to an organic group
through a carbon
atom that is part of a N-C=N bond. The N-C=N bond may be part of a triazine.
[0071] More generally speaking, surface modified pigments may be formed by
milling raw
pigments to a fine grind (typically less than 100 nm) and subsequently
attaching small organic
molecules as stabilizing groups. Surface modification chemistries, including
those described
herein, may also be used on raw pigment dispersions that comprise raw pigment,
a dispersant,
and water. A raw pigment may be dispersed as known in the art using a
dispersant to form a raw
pigment dispersion The raw pigment dispersion, rather than the raw pigment
(e.g., in powder
form), may be used in the surface modification techniques described herein, as
well as other
surface modification techniques that are well known in the art. In addition,
raw pigment
dispersions and raw pigment may be used together as starting materials in the
surface
modification techniques described herein. Any combination of raw pigments, raw
pigment
dispersions, surface modified pigments, and surface modified pigments from raw
pigment
dispersions, may be used as starting materials in the surface modification
techniques described
13
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
herein. Examples of other surface modification techniques that may be used
with this technique
include, but are not limited to, U.S. Patent Nos. 5,085,698, 5,310,778,
5,172,133, 4,156,616,
4,597,794B 1, and 6,406,143B 1, each of which is incorporated herein by
reference.
[0072] In using any of the previous surface modification chemistries to modify
a raw
pigment dispersion, the dispersant in the raw pigment dispersion, as well as
the compounds
described above, may attach to the surface of the raw pigment during the
surface modification.
In this way, dispersants capable of forming radicals and substituted reactive
intermediates may
be attached to the surface of the pigment simultaneously. This may form a
stable pigment
dispersion. Any remaining dispersant that does not attach to the surface of
the pigment during
the surface modification process, i.e., any dispersant that is only adsorbed
by the pigment, and
not attached, may be removed through a purification process.
[0073] In one embodiment, the commercial pigment dispersion may be modified
without any
milling required. If smaller particles are desired, then the dispersion may be
milled prior to or at
any point during the attachment process. For example, a Buhler micro media
mill may be used.
In a further embodiment, the dispersant may be added to a raw pigment and the
pigment and
dispersant may then be milled prior to or at any point during the attachment
process. In yet a
further embodiment, the dispersant may be added to a raw pigment and the
pigment and
dispersant may then be milled, or a raw pigment dispersion may be milled, and
before or at any
point during milling, additional polymer or substituted reactive intermediate
may be added. A
grind aid may also be milled with the raw pigment and dispersant. The amount
of dispersant
added may be controlled to affect the final amount of dispersant attached to
the surface of the
pigment. Milling performed prior to chemical treatment may allow the use of
common mill
chambers and parts while preventing re-agglomeration during the attachment
process.
[0074] Pigments
[0075] Pigments that may be surface modified according to the present
invention may
include, but are not limited to, azo pigment, phthalocyanine pigment,
anthraquinone pigment,
quinacridone pigment, thioindigo pigment, triphenylmethane lake pigment, and
oxazine lake
pigment. Specifically, those having yellow colors include, for example, C. I.
Pigment Yellow 1,
2, 3, 4, 5, 6, 10, 12, 13, 14, 16, 17, 65, 74, 83, 97, 120, 138, 150, 151 and
155. Those having red
14
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
colors include, for example, C. I. Pigment Red 1, 2, 3, 4, 5, 6, 7, 8, 9, 10,
11, 12, 13, 14, 15, 16,
17, 18, 21, 22, 23, 31, 32, 37, 38, 41, 48, 49, 50, 51, 52, 57, 58, 60, 64,
83, 88, 89, 90, 112, 114,
122, 123, 166, 188, 202, 254, C. I. Pigment Violet 19 and 23. Those having
blue colors include,
for example, C. I. Pigment Blue 1, 2, 15, 15:3, 15:4, 16, 25, 60, and 75.
Those having green
colors include, for example C.I. Pigment Green 7 and 36. Those having black
colors include, for
example, C. I. Pigment Black 1 and 7. Commercially available colored pigments
include, for
example, Pigment Red 122 and Pigment Violet 19 available from Lansco Colors,
Montvale, NJ
or BASF Color, Charlotte, NC or Clariant Colors, Charlotte, NC or Sun
Chemical, Cincinnati,
OH, Pigment Blue 15:3, Pigment 15:4, Pigment Yellow 74 and Pigment Yellow 97
(available
from BASF Color, Charlotte, NC or Clariant Colors, Charlotte, NC or Sun
Chemical, Cincinnati,
OH).
[0076] Suitable pigments also include carbon black. Carbon black is the
generic name for
carbon particles derived from the thermal decomposition or the incomplete
combustion of natural
gas and hydrocarbons, such as aromatic oils on coal tar basis, mineral oils,
coal tar distillate, and
acetylene. More than 100 individual grades of carbon black are available on
the market today,
each with its own distinctive set of characteristics and properties. Any
acidic carbon black,
neutral carbon black and alkaline carbon black may be beneficially subjected
to the treatment
disclosed in the present invention. This includes channel blacks, gas blacks,
lamp blacks,
thermal blacks, acetylene blacks and furnace blacks. More particularly,
suitable carbon blacks
include channel blacks. The quality of carbon black utilized will have an
impact on the critical
properties of the dispersion such as mean particle size, opacity, color shade,
stability, etc.
Examples of commercially available carbon blacks include, but are not limited
to, those available
from Cabot (Elftex 8, Black Pearls 490, Black Pearls 120, Monarch 120,
Monarch 700,
Monarch 880, Monarch 1000, Monarch 1100, Monarch 1300, Monarch 1400,
Mogul L,
Regal 99R, Regal 250R, Regal 300R, Regal 330R, Regal 400R, Regal 500R,
Regal
660R), Degussa (NIPex 150 IQ, NIPex 150, Printex 55, Printex 80, Printex
90, Printex A,
Printex G, Printex U, Printex V, Printex 140U, Printex 140V, Purex LS 35,
Corax HP
160, Thermal Black N 990, NIPex 160 IQ, NIPex 90, Special black 4, Special
black 4A,
Special black 5, Special black 6, Special black 100, Special black 250, Color
black FW 1, Color
black FW2, Color black FW2V, Color black FW 18, Color black FW200, Color black
S 150,
Color black S 160 and Color black S 170), Columbian (Raven 780, Raven 5000
U11, Raven
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
1255, Raven 2500 U, Raven 3600 U, Raven 3500, Raven 7000, Raven 1220 and
Raven
1225) and Mitsubishi Kagaku K.K. (MA8, MA11, MA77, MA100, MA220, MA230, MA600,
MCF88, #10B, #20B, #30, #33, #40, #44, #45, #45L, #50, #55, #95, #260, #900,
970#, #1000,
#2200B, #2300, #2350, #2400B, #2650, #2700, #4000B and CF9).
[0077] Other pigments that may be surface modified according to the present
invention may
include, without limitation, pigments that have been FDA approved. These may
be suitable for
cosmetic applications. Acceptable pigments that may be used in cosmetics may
be found in 21
C.F.R. 70-82, which are hereby incorporated by reference. Specific examples
of black
pigments (carbon blacks), include, without limitation, high purity carbon
black prepared by the
oil furnace process, D&C Black No. 2, and Unipure Black LC 902 (available from
Sensient
Cosmetic Technologies).
[0078] Other pigments that may be surface modified according to the present
invention may
include, pigments that have been previously oxidized, sulfonated, or a
combination thereof.
Oxidants include, without limitation, nitric acid, ozone, hydrogen peroxide,
persulfate,
hypohalite, or a combination thereof. Aqueous oxidation of carbon black using
sodium
hypochlorite is taught by U.S. Patent No. 2,439,442 issued April 13, 1948 and
U.S. Patent No.
3,347,632 issued October 17, 1967, each of which is hereby incorporated by
reference.
Hydrophilic groups comprising sulfonic acid are attached to pigments by
sulfonation with
sulfuric acid, oleum (fuming sulfuric acid) or a combination thereof.
Attachment of sulfonic acid
groups directly on the surface of the pigment may also be achieved by
sulfonating with other
known chemical agents such as chlorosulfonic acid or by displacement of a
leaving group
attached to the pigment, such as halogen with a suitable reagent such as
sodium bisulfite.
Following the oxidation, sulfonation, or combination thereof of the pigment,
the surface of the
pigment may be treated using the methods of the current invention. The
oxidized or sulfonated
pigment may be dispersed in a dispersion prior to treating it with the methods
of the current
invention. Examples of commercially available surface modified pigment
dispersions include,
without limitation, Sensijet Black SDP 1000 carbon black dispersion and
Sensijet SDP 2000
dispersions (available from Sensient Colors Inc., St. Louis, MO). Other
commercially available
pigment dispersions available from Cabot Corporation as Cab-O-Jet self
dispersed pigments and
from Orient Chemicals as Bonjet black dispersions, may also be similarly
modified.
16
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[0079] Pigments are available in a variety of particle sizes. Generally,
smaller particle sizes
are associated with larger surface areas, and larger surface areas can
accommodate a higher
concentration of hydrophilic surface groups, which ultimately enhance the
dispersibility of the
pigment in aqueous-based media. Therefore, particle size can influence the
dispersibility of a
surface-modified pigment. For example, the average primary particle size of
carbon blacks in
the present invention may be less than about 50 nm, particularly less than
about 30 nm,
particularly less than about 20 nm, and more particularly less than about 10
nm. Aggregates of
carbon black particles may be less than about 200 nm, particularly less than
about 150 nm, and
more particularly less than about 100 nm. The surface area of carbon black
particles may be
greater than about 100 m2/g, particularly greater than about 150 m2/g, and
more particularly
greater than about 200 m2/g. Pigment particles with larger dimensions may be
comminuted to a
desired size either before or during surface modification using any number of
techniques known
to those skilled in the art. Such techniques may include, but are not limited
to, a ball mill, an
attritor, a flow jet mixer, an impeller mill, a colloidal mill and a sand mill
(e.g., one
commercially sold under the trade name `Super Mill', `Agitator Mill', `Dyno-
mill' or `Beads
Mill'). Mill media may include, but are not limited to, glass beads, zirconia
beads, plastic beads
and stainless steal beads. Mill media may comprise particles ranging in size
from about 0.01 mm
to about 5 mm, suitably from about 0.1 mm to about 3 mm. If the pigment is
easily crumbled, a
rotary homogenizer or an ultrasonic homogenizer may be used to reduce particle
size. In one
embodiment, a surface-modified black pigment is made from a commercial grade
carbon black
pigment consisting of primary particle sizes less than about 30 nm and
aggregates not more than
about 200 nm with a surface area greater than about 100 m2 /g.
[0080] In some instances, prior to the creation of the self-dispersing
pigments, the pigment
may be wetted and milled to nano sized particles and dispersed using a grind-
aid and/or a
polymeric resin. The pigment may be in powder or wet cake form prior to
milling with the aid of
a grind aid. The milling may take place prior to, at any point during, or
after the reaction with
the substituted reactive intermediate or additional polymer. After the
attachment reaction is
complete, unattached grind-aid /resin may be removed using purification
methods that are known
to those skilled in the art, forming a dispersion containing primarily the
modified pigment with
attached substrates and water. Examples of grind aids include, but are not
limited to Triton X-
100 (available from Ashland Inc., Dublin, OH), Igepal CA-630 (available from
Rhodia,
17
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Cranbury, NJ), Surfynol CT 121, 131, and 141 (available from Air Products,
Allentown, PA),
and Lemantex Binder (available from Sensient Imaging Technologies S.A.,
Switzerland).
[0081] In such instances, a radical initiator such as a persulfate moiety is
used to
disproportionate and facilitate the attachment process. In some embodiments,
the reaction may
be carried out at a temperature of about 25 C to about 90 C. The pigment may
be milled to less
than about 100 nm before, during, or after reacting the pigment with the
substituted triazine. A
defoamer may be added as needed to control foaming. Dye solutions and/or
surfactants may be
used as needed for wetting the pigment.
[0082] In embodiments where there are two slurries with different secondary
compounds, the
pigment is mixed with the slurries sequentially. The temperature of the
dispersion may be
maintained at about 0 C to about 15 C for a period of about 1 hour to about
2 hours. The
mixture of the reactive compound (e.g., substituted triazine) dispersion and
the pigment is then
heated to elevated temperatures for a period of up to about 2 days. A free
radical initiator such
as potassium persulfate may be added to promote the reaction. The reaction
temperature may be
at least about 40 C, particularly at least about 50 C, and more particularly
at least about 60 C.
Furthermore, the reaction temperature may be less than or equal to about 90
C, particularly less
than or equal to about 80 C, and more particularly less than or equal to
about 60 C. This
includes embodiments where the reaction temperature is about 50 C to about 60
C, more
particularly no more than 90 C. Generally, temperatures above 50 C are
required for the free
radical initiator to be effective. This includes embodiments where the
reaction time is from
about 16 hours to about 24 hours. The contents of the reaction vessel are
stirred during the
reaction to insure adequate mixing. The modified pigment may be filtered to
remove excess
reactants and impurities.
[0083] In one embodiment, the reactive compound (such as cyanuric chloride) is
reacted
with the secondary compound in an acidic pH (about 2 to about 5) range. The
acidic pH range
increases the stability of the reactive compound and decreases the degree of
undesirable
reactions such as hydrolysis and self-condensation. The reactive compound
reacts preferentially
with a base such as a primary amine even when an amino phenol is used as the
organic group.
The reaction can be directed primarily to the amino end by the proper choice
of the reaction
18
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
conditions such as pH, temperature, and dilution which is well known to those
skilled in the art.
For example, the pH may be from about 2 to about 5 and the temperature may be
from about 0
C to about 5 C.
[0084] Optionally, while reacting the pigment with the groups described above
(such as, for
example, X, X-N-S-Z, N-S-Z, and/or (R)), the particle size of the pigment can
be reduced by
performing the reaction in a bead mill. Due to the corrosivity of the
secondary compound,
proper materials of construction resistant to strong acids and bases may be
selected to prevent
metal leaching into the product.
[0085] Reaction of the pigments with reactive compounds or secondary groups
that include
acid derivatives may create acidic surface groups that can lower the pH of the
reaction mixture.
A decrease in pH may result in a destabilization of the modified pigment
dispersion or slurry of
reactive compound and secondary compound during the substitution and may also
result in an
increase in viscosity. Therefore, the pH may be adjusted, as needed, before
and during the
substitution with a basic reagent. The pH of the reaction mixture during
substitution may be
greater than or equal to about 7, particularly greater than or equal to about
8, and more
particularly greater than or equal to about 9. The pH may be adjusted by any
known method in
the art including, for example, the addition of base. Suitable bases may
include, but are not
limited to, alkali hydroxides and calcium free alkali hydroxides (e.g., NaOH,
KOH, LiOH,
NH4OH), alkali carbonates and bicarbonates (e.g., NaHCO3, KHCO3), and organic
bases (e.g.,
dimethylethanol amine and triethanol amine). In particular, a suitable pH
adjuster comprises
calcium free sodium hydroxide.
[0086] Surface Modified Pi,gment
[0087] After the reactions described above are complete, the self-dispersing
pigment may be
isolated from the reaction mixture as a dry powder. The resultant modified
pigment may be
purified by using any number of techniques known to those skilled in the art
to remove unreacted
raw materials, byproduct salts and other reaction impurities. Purification
techniques may
include, but are not limited to, filtration, centrifugation, or a combination
of the two. The
modified pigment may also be isolated, for example, by evaporation or it may
be recovered by
filtration and drying using techniques known to those skilled in the art.
19
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[0088] Alternatively, the self-dispersing pigment may be delivered as
concentrated aqueous
pigment dispersion. Dispersions of the self-dispersing pigments of the present
invention may be
purified to remove organic and inorganic impurities and other undesirable free
species which can
co-exist in the dispersion as a result of the manufacturing process.
Purification techniques may
include, but are not limited to, water washing, reverse osmosis, and
ultrafiltration. In some
embodiments, dissolved impurities may be removed by ultrafiltration until the
chloride and
sulfate content of the feed sample adjusted to 10% solids is less than about
150 ppm, particularly
less than about 100 ppm, and more particularly less than about 25 ppm. If
necessary, the pH of
the dispersion may be adjusted prior to purification. A sufficient amount of
acid or base may be
added to adjust the pH of the dispersion to at least about 7, particularly to
at least about 8, and
more particularly to at least about 9. This includes embodiments where the pH
of the dispersion
is about 7 to about 9. The dispersion may be concentrated if desired by
removal of some of the
water. In some embodiments, the dispersion is concentrated to at least about
8% solids, in others
to at least about 14% solids, and in yet others to at least about 20% solids.
This includes
embodiments where the dispersion is concentrated to about 8% to about 16%
solids. In other
embodiments, the dispersion is concentrated to at least about 10% solids, in
others to at least
about 18% solids, and in yet others to at least about 20% solids. This
includes embodiments
where the dispersion is concentrated to about 10% to about 17% solids.
[0089] In some embodiments, the dispersion may be dried and reconstituted to
at least about
40% solids. In other embodiments, the dispersion may be dried and
reconstituted to about 60%
to about 70% solids.
[0090] In some embodiments, pigments modified according to the present
invention may be
dispersed with unmodified pigment to form a dispersion comprising both
modified and
unmodified pigment.
[0091] A biocide may also be added to the dispersion to inhibit the growth of
microorganisms. Examples of suitable biocides include, but are not limited to,
sodium benzoate,
pentachlorophenol sodium, 2-pyridinethiol-l-oxide sodium, sodium sorbate,
sodium
dehydroacetate, benzisothiazolinone, 1,2-dibenzothiazolin- 3 -one,
methylisothiazolinone and
chloromethylisothiazolinone. Commercially available biocides include Proxel
CRL, Proxel
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
BDN, Proxel GXL, Proxel XL-2, and Proxel TN (available from Arch Chemicals,
Smyrna,
GA) and XBINX (available from PMC Specialties Group, Inc., Cincinnati, Ohio).
Typically, a
small amount, such as 0.05 to 5%, particularly 0.1 to 1%, and more
particularly 0.2 to 0.4% by
weight of biocide, is used in the dispersion. This includes 0.3 % by weight
biocide.
[0092] The dispersion may be filtered through filter cartridges as required
for the designated
end use of the dispersion. In some embodiments, the nominal pore size of the
filter cartridge is
less than or equal to about 5 microns, particularly less than or equal to
about 1 micron,
particularly less than or equal to about 0.5 micron, and more particularly
less than or equal to
about 0.2 micron.
[0093] In addition to powders and dispersions, the self-dispersing pigment may
also be
isolated as a water wet presscake. In presscake form, the self-dispersing
pigment is not
agglomerated to the extent that it is in dry form and thus the self-dispersing
pigment does not
require as much deagglomeration when used, for example, in the preparation of
inks.
[0094] If desired, the charge-balancing counterions associated with the
surface-modifying
groups as a result of the attachment/substitution process may be at least
partially substituted or
changed with the use of suitable base or salt form or exchanged or substituted
with other suitable
cations using known ion-exchange techniques such as ultrafiltration, reverse
osmosis, conversion
to acid form as an intermediate and the like. Examples of counterions include,
but are not
limited to, alkali metal ions (e.g., Na+, K+ and Li+), NR1R2R3H+, and
combinations thereof,
wherein R1, R2 and R3 may independently be H or CI-C5 alkyl groups that may be
unsubstituted
or substituted (e.g., tetraethylammonium ion (TEA), tetramethylammonium ion
(TMA),
ethanolammonium ion, triethanolammonium ion, tetrabutylammonium ion, etc).
[0095] Properties of Modified Pigments
[0096] The self-dispersing pigments may exhibit at least one of long-term and
high
temperature stability, higher water and highlighter fastness than expected of
a pigment particle
with attached sulfonic or carboxylic acid groups, and have a particle size
distribution suitable for
use in high speed jetting applications.
21
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[0097] The self-dispersing pigments may possess the following properties. The
% of solids
in the modified pigments may be from about 5-30, suitably about 10 - 30,
suitably about 10 - 22.
[0098] The pH of the modified pigment dispersion may be from about 5 to about
12,
suitably about 5 to about 10.
[0099] The viscosity of the modified pigment dispersion may be from about 1 to
about 11
cps, particularly about 2 to about 8 cps.
[00100] The surface tension of the modified pigment dispersion may be from
about 30 to
about 72 dynes/cm, suitably about 30 to about 60 dynes/cm.
[00101] The amount of Na and K in the modified pigment dispersion may be a
measure of
a newly attached anionic substrate (sulfanilic or 4-aminobenzoic acid or 4-
aminophenol as Na/K
forms). The amount of Na may be from about 100 to about 7500 ppm and the
amount of K may
be from about 30 to about 3000 ppm, suitably about 30 to about 2500 ppm.
[00102] The increase in the sulfur content in the modified pigment dispersion
may be due
to the introduction of a sulfonyl group and/or attachment of a sulfonated
substrate such as,
without limitation, sulfanilic acid. The amount of sulfur in the modified
pigments may be from
about 0 ppm to about 3000 ppm, suitably about 50 ppm to about 3000 ppm. In one
embodiment,
the amount of sulfur in the modified pigments may be about 50 ppm for 4-
aminobenzoic acid
and 4-aminophenol attachments. In another embodiment, the amount of sulfur in
the modified
pigments may be about 2000 ppm when a sulfanilic acid is attached to the
pigment.
[00103] Carbon black modified according to the present invention may comprise
about 0.3 to
about 1.7 mMoles, suitably about 0.403 to about 1.584 mMoles of active
hydrogen per gram of
pigment.
[00104] Cyan pigments modified according to the present invention may comprise
about 0 to
1 mMoles, suitably 0.03 to about 0.3 mMoles, suitably about 0.050 to about
0.112 mMoles of
sulfur per gram of pigment. Cyan pigments modified according to the present
invention may
comprise about 0.2 to about 0.9 mMoles, suitably about 0.395 to about 0.732
mMoles of active
hydrogen per gram of pigment.
22
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[00105] Magenta pigments modified according to the present invention may
comprise about 0
to 1 mMoles, suitably 0.02 to about 0.2 mMoles, suitably about 0.034 to about
0.140 mMoles of
sulfur per gram of pigment. Magenta pigments modified according to the present
invention may
comprise about 0.1 to about 1.2 mMoles, suitably about 0.196 to about 0.911
mMoles of active
hydrogen per gram of pigment.
[00106] Yellow pigments modified according to the present invention may
comprise about 0
to about 1 mMoles, suitably about 0.02 to about 1.0 mMoles, suitably about
0.065 to about 0.081
mMoles, suitably about 0.034 to about 0.075 mMoles of sulfur per gram of
pigment. Yellow
pigments modified according to the present invention may comprise about 0.1 to
about 1.0
mMoles, suitably about 0.196 to about 0.757 mMoles, suitably about 0.148 to
about 0.442
mMoles of active hydrogen per gram of pigment.
[00107] Violet pigments modified according to the present invention may
comprise about 0 to
1 mMoles, suitably 0.03 to 0.3, suitably about 0.022 to about 0.087 mMoles of
sulfur per gram of
pigment. Violet pigments modified according to the present invention may
comprise about 0.2
to about 0.4 mMoles, suitably about 0.283 to about 0.347 mMoles of active
hydrogen per gram
of pigment.
[00108] Pigments modified according to the present invention may be
redispersible in an
aqueous or non-aqueous medium.
[00109] The XPS results disclosed in Example 37 indicate that the surface
modification as
disclosed yields a modified carbon black with an increase in surface sodium,
as COONa, in
about 1.4 to 5.3 atomic%. The XPS results for untreated carbon blacks,
Sensijet SDP 1000
carbon and carbon blacks from Examples 3, 8, 24, 25-3 1, and 32 is displayed
in Figures 1-12.
[00110] The XPS results disclosed in Example 37 indicate that surface
modification as
disclosed yields a modified Pigment Blue No.15 with significantly higher
surface sodium content
(0.8 to 4.2 atomic %) compared to a low concentration of 0.1 atomic % in the
untreated pigment.
The XPS results for untreated Pigment Blue No. 15 and Pigment Blue No. 15 from
Examples 10,
14, and 21 are displayed in Figures 13-19.
23
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[00111] The XPS results disclosed in Example 37 indicate that the surface
modification as
disclosed yields a modified Pigment Red No.122 with a surface sodium present
at concentrations
in the range of 0.3 - 1.6 atomic% while in comparison the untreated pigment
has none. The XPS
results for untreated Pigment Red No. 122, Pigment Red No. 122 from Examples
17 and 22, and
Pigment Violet 19 from Examples 6 and 7 are displayed in Figures 20-25.
[00112] The XPS disclosed in Example 37 indicate that the surface modification
as
disclosed yields a modified Pigment Yellow No. 74 with a surface sodium in the
atomic ratio of
1.0 to 1.6% which is expected to be present as COONa/CSO3Na. In contrast, in
the untreated
pigment the surface sodium is only about 0.3. The XPS results for untreated
Pigment Yellow
No. 74 and Pigment Yellow No. 74 from Examples 23 and 34 are displayed in
Figures 26-3 1.
The XPS results for untreated Pigment Yellow No. 155 and for Pigment Yellow
No. 155 from
Examples 11 and 12 are displayed in Figures 32-36.
[00113] The level of Na is one measure of charge groups present on the
pigment. Higher
levels of Na may result from the surface modification of pigments. The levels
of Na disclosed in
the preceding paragraphs for modified pigments may indicate the ability to
create a stable
pigment dispersion with modified pigments of the present invention. The degree
to which a
modified pigment dispersion is stable may depend on the amount of charge
groups present on the
pigment, which can be indicated by the levels of sodium. These results may
indicate that
pigment dispersions prepared according to the present invention may be stable
as a result of the
mechanism of attachment.
[00114] Applications of Modified Pigments
[00115] The self-dispersing pigment according to the present invention may be
used in a
number of end use applications. These uses include, but are not limited to,
coatings, paints,
papers, adhesives, latexes, toners, textiles, fibers, plastics, and inks.
Specific examples include,
without limitation, printing ink for paper, textiles, fibers, metal deco and
plastics, wood stains,
writing instruments, and color filters. The self-dispersing pigments produced
by the process of
the invention are particularly well-suited for use in printing applications
and wood stains. In one
example, an inkjet ink incorporating a pigment of the present invention may be
useful in high
24
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
quality prints in an inkjet photo printer. The self-dispersing pigment
according to the present
invention may also be used in cosmetic applications, such as, without
limitation, mascaras, eye
liner, spray-on hair mascara, aqueous nail polish, and hair coloring or hair
dyes.
[00116] One aspect of the present invention relates to inkjet ink formulations
using the self-
dispersing pigment described above. Inkjet formulations containing such
pigments may do at
least one of the following: 1) provide uniform, bleed-free images with high
resolution and high
density on print media; 2) not cause nozzle clogging which typically occurs
due to drying of the
ink at a distal end of a nozzle; 3) rapidly dry on paper; 4) exhibit good
lightfastness and
waterfastness; 5) demonstrate good long-term storage stability; and 6)
demonstrate print
characteristics which are independent of the paper quality.
[00117] The ink compositions of the present invention may be prepared by
combining the
above modified pigments with an aqueous vehicle and any suitable additives.
The amount of
modified pigment (by weight) in the ink composition is at least about 0.1%,
particularly at least
about 10%, and more particularly at least about 20%. Furthermore, the amount
of modified
pigment (by weight) in the ink composition is less than or equal to about 12%,
particularly less
than or equal to about 8%, and more particularly less than or equal to about
5%. This includes
embodiments where the amount of modified pigment (by weight) in the ink
composition is
present in an amount ranging from about 2% to about 12%.
[00118] The aqueous vehicle may comprise water or water in combination with
one or more
water-soluble organic solvents. Water-soluble organic solvents may be combined
with water to
make up the aqueous vehicle. Water-soluble organic solvents may include
alcohols, polyhydric
alcohols such as ethylene glycol, ketones and ketone alcohols such as acetone
and diacetone
alcohol, ethers such as tetrahydrofuran and dioxane, lower alkyl ethers of
polyhydric alcohols,
such as ethylene glycol monomethyl (or monoethyl) ether, nitrogen-containing
solvents such as
pyrrolidone, N-methyl-2-pyrrolidone, sulfur-containing solvents such as
thiodiethanol, sugars
and derivatives thereof such as glucose, an oxyethylene adduct of glycerin;
and an oxyethylene
adduct of diglycerin. The water-soluble organic solvents may be used alone or
in combination.
If a mixture of water and a water-soluble organic solvent is used, the amount
of water-soluble
organic solvent (by weight) in the ink composition is at least about 5%,
particularly at least about
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
15%, and more particularly at least about 25%. Furthermore, the amount of
water-soluble
organic solvent (by weight) in the ink composition is less than or equal to
about 50%,
particularly less than or equal to about 30%, and more particularly less than
or equal to about
15%. This includes embodiments where the amount of water-soluble organic
solvent (by
weight) in the ink composition is about 5% to about 30%. The amount of water
in the ink
composition is at least about 40%, particularly at least about 50%, and more
particularly at least
about 60%. Furthermore, the amount of water (by weight) in the ink composition
is less than or
equal to about 90%, particularly less than or equal to about 80%, and more
particularly less than
or equal to about 70%. This includes embodiments where the amount of water (by
weight) in the
ink composition is about 40% to about 80%.
[00119] Additives may be incorporated into the aqueous vehicle to impart any
number of
desired properties, such as might be needed to adapt the ink to the
requirements of a particular
inkjet printer or to provide a balance of light stability, smear resistance,
viscosity, surface
tension, coating penetration, optical density, adhesion, highlighter
resistance or crust resistance.
Penetrants, for example, may be added to reduce bleed, improve wetting of the
print media, and
otherwise improve overall performance of the print image. Examples of
penetrants may include,
but are not limited to, alkyl alcohols having 1 to 4 carbon atoms, such as
ethanol, glycol ethers,
such as ethylene glycol monomethyl ether, diols such as 1,2-alkyl diols,
formamide, acetamide,
dimethylsulfoxide, sorbitol and sulfolane. The penetrants may be used alone or
in combination.
The amount of penetrant (by weight) in the ink composition ranges from 0% to
about 60%,
particularly from about 2% to about 40%, and more particularly from about 5%
to about 20%.
This includes embodiments where the amount of penetrant (by weight) in the ink
composition is
present in an amount ranging from about 10% to about 15%.
[00120] Surfactants may be added to the aqueous medium to reduce the surface
tension of the
ink composition. The surfactants may be anionic surfactants, non-ionic
surfactants and/or
cationic surfactants. Suitable surfactants may include those listed below and
in U.S. Patent No.
5,116,409 issued May 26, 1992, U.S. Patent No. 5,861,447 issued January 19,
1999, and U.S.
Patent No. 6,849,111 issued February 1, 2005, each of which is hereby
incorporated by
reference.
26
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[00121] Surfactants are commercially available under various well-known trade
names, such
as the PLURONIC series (BASF Corporation, Parsippany, N.J.), the TETRONIC
series
(BASF Corporation, Parsippany, N.J.), the ARQUAD series (Akzo Chemical Inc.,
Chicago,
Ill.), the TRITON series (Union Carbide Corp., Danbury, Conn.), the SURFONIC
series
(Texaco Chemical Company, Houston, Tex.), the ETHOQUAD series (Akzo Chemical
Inc.,
Chicago, Ill.), the ARMEEN series (Akzo Chemical Inc., Chicago, Ill.), the
ICONOL series
(BASF Corporation, Parsippany, N.J.), the SURFYNOL series (Air Products and
Chemicals,
Inc. Allentown, Pa.), and the ETHOMEEN series (Akzo Chemical Inc., Chicago,
Ill.), to name
a few.
[00122] The surfactants may be used alone or in combination. The amount of
surfactant (by
weight) in the ink composition may range from 0% to about 10%, particularly
from about 0.1%
to about 10%, and more particularly from about 0.3% to about 5%. This includes
embodiments
where the amount of surfactant (by weight) in the ink composition may range
from about 0.1%
to about 8%.
[00123] One or more humectants may be added to the aqueous vehicle to prevent
clogging,
caused by drying out during periods of latency, of inkjet nozzles. Humectants
may be selected
from materials having high hygroscopicity and water-solubility. Examples of
humectants
include, but are not limited to, polyols such as glycerol, lactams such as 2-
pyrrolidone, urea
compounds such as urea, 1,3-dimethylimidazolidinone, saccharides such as
sorbitol, 1,4-
cyclohexanedimethanol, 1-methyl-2-piperidone, N-ethylacetamide, 3-amino- l,2-
propanediol,
ethylene carbonate; butyrolacetone and Liponic EG- 1. There are no particular
limitations on the
amount used of the humectant, but in general the amount of humectant (by
weight) in the ink
composition may range from 0% to about 30%, particularly from about 1% to
about 15%, and
more particularly from about 5% to about 10%.
[00124] Polymers may be added to the ink composition to improve the water-
fastness, rub and
highlighter fastness of the images on print media. Suitable polymers may
include, but are not
limited to, polyvinyl alcohol, polyester, polyestermelamine, styrene-acrylic
acid copolymers,
styrene-maleic acid copolymers, styrene-maleic acid-alkyl acrylate copolymers,
styrene-
metacrylic acid copolymers, styrene-metacrylic acid-alkyl acrylate copolymers,
styrene-maleic
27
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
half ester copolymers, vinyl-naphthalene-acrylic acid copolymers, vinyl
naphthalene-maleic acid
copolymers and salts thereof. The amount of polymer (by weight) in the ink
composition may
range from 0% to about 5%, particularly from about 0.1% to about 3%, and more
particularly
from about 0.2% to about 2.5%. This includes embodiments where the amount of
polymer (by
weight) in the ink composition may range from about 0.1% to about 3.0%.
[00125] Ink compositions of the present invention may be buffered to a desired
pH using any
number of pH modifiers. Suitable pH modifiers may include alkali hydroxides,
alkali carbonates
and bicarbonates, triethylamine, dimethylethanolamine, triethanolamine, nitric
acid, hydrochloric
acid, and sulfuric acid. The pH modifiers may be used alone or in combination.
The amount of
pH modifier (by weight) in the ink composition may range from 0% to about
3.0%, particularly
from about 0.1% to about 2.0%, and more particularly from about 0.5% to about
1.5%. This
includes embodiments where the amount of pH modifier (by weight) in the ink
composition
ranges from about 0.2% to about 2.5%.
[00126] Preservatives, such as biocides and fungicides, may also be added to
the ink
composition. Examples of suitable preservatives include sodium benzoate,
pentachlorophenol
sodium, 2-pyridinethiol-l-oxide sodium, sodium sorbate, sodium dehydroacetate,
benzisothiazolinone, 1,2-dibenzothiazolin-3-one, methylisothiazolinone and
chloromethylisothiazolinone. Commercially available biocides include UCARCIDE
250
(available from Union Carbide Company), Proxel CRL, Proxel BDN, Proxel GXL,
Proxel
XL-2, Proxel TN (available from Arch Chemicals, Smyrna, GA ), Dowicides (Dow
Chemical,
Midland, Mich.), Nuosept (Huls America, Inc., Piscataway, N.J.), Omidines
(Olin Corp.,
Cheshire, Conn.), Nopcocides (Henkel Corp., Ambler, Pa.), Troysans (Troy
Chemical Corp.,
Newark, N.J.), and XBINX (PMC Specialties Group, Inc., Cincinnati, Ohio). The
preservatives
may be used alone or in combination. The amount of preservatives (by weight)
in the ink
composition may range from 0% to about 1.5%, particularly from about 0.05% to
about 1.0%,
and more particularly from about 0.1% to about 0.3%. This includes embodiments
where the
amount of preservative (by weight) in the ink composition may range from about
0.05% to about
0.5%.
28
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[00127] The ink composition may contain one or more viscosity modifiers.
Viscosity
modifiers may include rosin compounds, alginic acid compounds, polyvinyl
alcohol,
hydroxypropyl cellulose, carboxymethyl cellulose, hydroxyethyl cellulose,
methyl cellulose,
salts of polyacrylic acid, polyvinyl pyrrolidone, gum arabic and starch. The
amount of viscosity
modifier (by weight) in the ink composition may range from 0% to about 10%,
particularly from
about 0.5% to about 8%, and more particularly from about 1% to about 5%. This
includes
embodiments where the amount of viscosity modifier (by weight) in the ink
composition may
range from about 1% to about 7%.
[00128] Other additives which may be incorporated into the aqueous vehicle may
also include
antioxidants, ultraviolet absorbers, chelating agents, electric conductivity
adjusters, viscosity
modifiers, oxygen absorbers, anti-kogation agents, anti-curling agents, anti-
bleed agents,
defoamers, and buffers. The ink compositions of the present invention may
contain one or more
colorants in addition to the pigment dispersion of the present invention.
[00129] The ink compositions of the present invention are particularly suited
for use as an ink
composition for inkjet printing wherein droplets of the ink composition are
ejected from a
printing apparatus and deposited onto a substrate to generate an image.
Suitable printing
apparatus include, but are not limited to, Continuous Ink Jet (CIJ), Drop-on-
Demand Valve
(DoD Valve), Drop-on-Demand Piezo-Electric (DoD Piezo) and Thermal Ink Jet
(TIJ).
Similarly, any suitable substrate may be employed including plain papers,
bonded papers, coated
papers, transparency materials, textile materials, plastics, polymeric films
and inorganic
substrates. However, it should be recognized by those skilled in the art that
the above ink
compositions may also have use in other applications including, but not
limited to, general
writing utensil applications and stamp applications.
[00130] The ink compositions of the present invention may be used alone, or
with a color
underlay, to produce a black image or in combination with other ink
compositions to produce a
color image. In some embodiments, the ink composition of the present invention
is used in
combination with other ink composition(s), such as a cyan ink, a magenta ink
and/or a yellow
ink. In other embodiments, a cyan ink, a magenta ink and a yellow ink are
overprinted to form a
29
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
black image and this printing is used in combination with the printing of the
black ink of the
present invention.
Wood Stains
[00131] Another aspect of the present invention relates to aqueous
formulations using the self-
dispersing pigment described above as wood stains and coatings. Wood stain
formulations
containing such pigments may exhibit at least one of the following properties:
1) good wood
absorption and adhesion; 2) good transparency; and 3) excellent water and
light resistance.
[00132] Water resistance is measured by difference in measured DE* values of
wood stain in
dipped areas versus control. Lower DE* values may indicate higher water
resistance. If DE* is
small it may mean that there is minimal to no color change due to degradation
or loss. For
example, lower DE* values may indicate higher water resistance as seen with
carboxy modified
pigment dispersions. The DE* value of wood stains comprising the surface
modified pigment of
the present invention may be from about 0 to about 3, suitably about 0 to
about 1.5. Delta E is
the difference between two colors. L, a, and b values are measurements based
on spherical color.
+L=white, -L=black, +a=red, -a=green, +b=yellow, -b=blue. C is chroma
(saturation) and
H=Hue. Readings are measured using a spectrophotometer. Delta E _ I(Li-L2)2
+(ai-a2)2 + (bi-
b2)2.
Coatings
[00133] Coating formulations containing such pigments may exhibit at least one
of the
following properties: 1) good adhesion to substrates such as metal, paper,
glass, plastic, and
wood; 2) ease of application and drying; 3) good weather fastness, water and
light resistance; 4)
good gloss retention; and 5) good chemical and flocculation resistance.
[00134] As with water resistance, resistance to strong acids and bases of
coatings are
measured as the difference in DE* value of spotted versus control. The DE*
value of coatings
comprising the surface modified pigment of the present invention may be from
about 0 to about
48, suitably about 0 to about 5.
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Color Filters
[00135] Another aspect of the present invention relates to aqueous
formulations using the self-
dispersing pigment described above in color filters. Color filters find
application in display
imaging areas including, without limitation, desktop monitor/laptop screens,
LCD TV screens,
cell phone display panels, digital camera screens, and GPS panels. Color
filter formulations
containing pigments of the present invention may exhibit at least one of the
following properties:
1) good adhesion to glass and plastic film substrates; 2) good transparency;
3) ease of application
and drying; and 4) good heat and light resistance.
[00136] The transmission values of a specific color filter is measured to
determine its
usefulness. The color filters may have maximum transmittance in a narrow band
to provide the
most utility.
[00137] In one embodiment, color filter formulations comprising carbon black
may have no
transmission bands, color filter formulations comprising magenta pigment
dispersions may have
a lowest transmission in the about 520 to about 560 nm range, color filter
formulations
comprising yellow pigment dispersions may have a lowest transmission in the
about 400 to about
480 nm range, and color filter formulations comprising cyan pigment
dispersions may have the
lowest transmission in the about 600 to about 680 nm range.
Textile Printing
[00138] Another aspect of the present invention relates to aqueous
formulations using the self-
dispersing pigment described above in textile printing applications. Textile
printing formulations
containing pigments of the present invention may exhibit at least one of the
following properties:
1) good adhesion to textile fabrics such as cotton, nylon, polyester, wool,
polyacrylic, or blends
of the same; 2) ease of application and drying; 3) good water and light
resistance; and 4) good
washfastness.
[00139] The wash and water fastness properties of dyed textile may be measured
by the
difference in DE* value of a control versus a treated fabric.
31
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[00140] The DE* value of a textile printing composition comprising a surface
modified
pigment of the present invention may be from about 0 to about 12, suitably
from about 0.1 to
about 8Ø
Cosmetic Applications
Another aspect of the present invention relates to formulations using the self-
dispersing pigments
described above in cosmetic applications. Cosmetic applications may include
those directed to,
without limitation, the face, eyes, lips, hair, skin, and nails. Cosmetic
applications may include,
without limitation, mascaras, eye liner, spray-on hair mascara, aqueous nail
polish, brush-on-
brow, eye shadows, lipsticks, blushers and rouge, make-up, foundation, and
hair coloring or hair
dyes. The self dispersing pigment dispersions may be easy to incorporate into
any aqueous
phase portion of a cosmetic formula as they blend easily with polyols and
preservatives. Better
compatibility with silicones, esters (such as, without limitation, CCT), waxes
(such as, without
limitation, carnauba wax), and solvents (such as, without limitation,
isododecane) helps in the
emulsification and yields stable products. The self-dispersed pigments enable
a formulator to
create a product with higher color strength at equivalent pigment load than
with use of a
conventional pigment dispersion using a glycerine-water dispersion. The
fluidity of the product
allows the formulator the flexibility for even higher pigment loads which will
enhance the
product's pay-off leading to fewer strokes on application.
[00141] The properties of mascara comprising self-dispersed pigments of the
present
invention can be evaluated visually by applying the mascara evenly to the skin
and comparing it
side-by-side with mascaras that do not comprise self-dispersed pigments of the
present invention.
EXAMPLES
[00142] Exemplary embodiments of the present invention are provided in the
following
examples. The following examples are presented to illustrate the present
invention and to assist
one of ordinary skill in making and using the same. The examples are not
intended in any way to
otherwise limit the scope of the invention.
32
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Example 1
[00143] Pigment Dispersion (example of preparation of a cyanuryl tris adduct
with sulfanilic
acid and use in the surface modification of a pigment).
[00144] A solution of sulfanilic acid (114 g) in DI water (310 g), calcium
free sodium
hydroxide (32 g) and sodium bicarbonate (55 g) at a pH of 8.5 was added to a
stirred mixture of
cyanuric chloride (40.2 g, available from Lonza Walkersville, Inc.,
Walkersville, Maryland), ice
(570 g) and DI water (480 g) in three stages controlling the temperature < 0
C, < 3 C and <10
C, respectively. After the addition, the pH was 7.1 and the reaction mixture
was heated to 90
C over 4.5 hours to get 1000 g of a clear liquid.
Example 2
[00145] Pigment Dispersion (example of preparation of a cyanuryl tris adduct
with 4-
aminobenzoic acid and use in the surface modification of a pigment).
[00146] A solution of 4-aminobenzoic acid (90.1 g) in DI water (300 g),
calcium free sodium
hydroxide (30 g) and sodium bicarbonate (55 g) at a pH of 7.2 was added to a
stirred mixture of
cyanuric chloride (40.2 g, available from Lonza Walkersville, Inc.,
Walkersville, Maryland), ice
(550 g) and DI water (500 g) in three stages controlling the temperature < 0
C, < 3 C and <10
C, respectively. After the addition, the pH was 7.1 and the reaction mixture
was heated to 92
C over 3 hours to get 901 g of a clear liquid.
Example 3
[00147] Pigment Dispersion (example of converting a polymer stabilized
dispersion to a self
dispersed pigment dispersion with cyanuryl tris adduct with 4-aminobenzoic
acid/ sulfanilic
acid).
[00148] A resin stabilized 15% dispersion of Pigment Black (Carbon black)
Sensijet Black
PB 094 available from Sensient Imaging Technologies, Inc., 300 g, was slowly
added to a
mixture of 157 g of the Tris 4-ABA reagent described in Example 2 and 300 g of
DI water.
33
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[00149] After one half hour, the reaction mixture was heated to 51 C. A
solution of 16.8 g
potassium persulfate and 15 g sodium bicarbonate in hot, 50 C DI water (300
g) was introduced
slowly while the pH was maintained between 7.5 and 9.0 with the addition of
calcium free
sodium hydroxide. After the addition of potassium persulfate solution, the
reaction mixture was
heated to 80 C [Step 1]. The dissolved impurities were removed by
ultrafiltration until the
chloride and sulfate content of the feed sample were less than 50 ppm. The
product was then
concentrated to 16.7% solids and mixed with (0.3%, wt/wt) Proxel GXL
(available from Arch
Chemicals, Smyrna, GA). Finally, the product (300 g) was centrifuged at 10,000
rpm for 5
minutes and then filtered through a 0.7 micron GF filter.
Examples 4-7
[00150] Examples 4-7 were prepared following the same process as set forth
above for
Example 3, except in some instances, the Tris reagent from Example 1 was used
(shown in the
table).
Table 1. Examples of attaching molecules to a polymer dispersed pigment via a
Cyanuric
adduct.
Example Pigment Tris Adduct NaHCO3 K2S208 Equivalent Step 1
[#] Type (g) Type (g) (g) (g) SA/4- (g) 0 C h
ABA
4 PB 15 300 4-ABA 177.8 16.7 16.8 4-ABA 10.26 90 0.5
PB15 300 SA 215 20 24 SA 14.1 95 0.5
6 PV19 500 SA 100 11 32 SA 11.4 85 3
7 PV 19 300 4-ABA 90 10 11 4-ABA 8.85 83 0.5
1 Sensijet Black PB 094 from Sensient Imaging Technologies, Inc
2 Sensijet Cyan PB 15:3 from Sensient Imaging Technologies, Inc.
3 Sensijet Magenta PV19 from Sensient Imaging Technologies, Inc
34
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Example 8
[00151] Pigment Dispersion [example of milling to (<100 nm) and converting a
polymer
stabilized dispersion to a self stabilized dispersion using a cyanuryl tris
adduct with 4-
aminobenzoic acid/ sulfanilic acid].
[00152] A resin stabilized 15% dispersion of Pigment Black (Carbon black)
Sensijet Black
PB 094, available from Sensient Imaging Technologies, Inc., 5 Kg, was milled
with Buhler
Micro Media P1 Perl Mill with 0.1 mm YTZ ceramic media for 4 hours at 43 C.
As required
additional styrene acrylic copolymer dispersant Joncryl 678 (available from
BASF) was added
(358 g) to prevent gross agglomeration.
[00153] A part (400 g) was slowly added to a mixture of 80.8 g of the Tris 4-
ABA reagent
described in Example 2 and 725 g of DI water.
[00154] After one half hour, the reaction mixture was heated to 47 C. A
solution of 20 g
potassium persulfate and 18 g sodium bicarbonate in hot, 50 C DI water (300
g) was introduced
slowly while the pH was maintained between 7.5 and 9.0 with the addition of
calcium free
sodium hydroxide (17 g). After the addition of potassium persulfate solution,
the reaction
mixture was heated to 95 C [Step 1]. The dissolved impurities were removed by
ultrafiltration
until the chloride and sulfate content of the feed sample were less than 50
ppm. The product was
then concentrated to 16.7% solids and mixed with (0.3%, wt/wt) Proxel GXL
(available from
Arch Chemicals, Smyrna, GA). Finally, the product (352 g) was centrifuged at
10,000 rpm for 5
minutes and then filtered through a 0.7 micron GF filter.
Examples 9-13
[00155] Examples 9-13 were prepared following the same process as set forth
above for
Example 8, except in example 12, the Tris reagent from Example 1 was used
(shown in the
table).
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Table 2. Examples of attaching molecules to a polymer dispersed pigment after
particle
size reduction with a micro media mill, using a Cyanuric adduct.
Example Pigment Tris Adduct NaHCO3 K2S208 Equivalent Step 1
[#] Type (g) Type (g) (g) (g) SA/4- (g) 0 C h
ABA
9 Carbon' 500 4-ABA 223.2 45 49 4-ABA 22.3 85 0.5
PB 15 300 4-ABA 177.8 16.7 16.8 4-ABA 10.26 90 0.5
11 PY155 312 4-ABA 90 13.6 11 4-ABA 6.0 80 0.5
12 PY155 200 SA 332 20.1 11 SA 20 90 0.5
13 PV 19 600 4-ABA 73 5.5 16 4-ABA 7.3 85 1
4 Sensijet Yellow PY155 from Sensient Imaging Technologies, Inc
Examples 14-20
[00156] Examples 14-21 were prepared following the same process as set forth
for Example 3,
using Lemantex pigment dispersions available from Sensient Imaging
Technologies - Specialty
Inks and colors, Switzerland.
Table 3. Additional examples of attaching molecules to a polymer dispersed
pigment via a
Cyanuric adduct.
Example Pigment Tris Adduct NaHCO3 K2S208 4-ABA Step 1
[#] Equivalent
Type (g) (g) (g) (g) (g) C h
14 PB 15 463 60 4.2 13.5 6 55 16
PB60 496 66.3 5.0 16.2 6.6 55 16
16 PG7 519 66.3 5.4 16.2 6.6 54 16
17 '-R122 400 101.8 7.1 22 10.1 55 16
18 '-R254 440 71.8 5.6 17.6 7.2 54 16
19 PY8310 403 65.3 5.0 16.2 6.5 53 16
PY12011 601 66.5 5.1 16.3 6.7 56 16
5 Cyan PB 15:3 [11.2%] from Sensient Specialty Inks and Colors, Switzerland
6 Blue PB 60 [13.5%] from Sensient Specialty Inks and Colors, Switzerland
7 Green PG7 [10.7%] from Sensient Specialty Inks and Colors, Switzerland
36
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
8 Magenta PR122 [12.9%] from Sensient Specialty Inks and Colors, Switzerland
9 Red PR254 [11.6%] from Sensient Specialty Inks and Colors, Switzerland
Orange PY83 [13.3%] from Sensient Specialty Inks and Colors, Switzerland
11 Yellow PY120 [10%] from Sensient Specialty Inks and Colors, Switzerland
Example 21
[00157] Tris 4-ABA reagent described in Example 2 (200g at 10.2% conc) is used
to wet
commercial Pigment Blue No. 15 wet cake12 (100 g @ 100% solids) available from
Clariant
Colors (Charlotte, NC) along with 100 g of Sensijet Direct Blue 199 (available
from Sensient
Colors Inc, St. Louis, MO). The mixture is heated to 50 C and stirred with 10
g of 25% solution
of calcium free sodium hydroxide, an aqueous solution containing 1 g of
Joncryl HPD 296 resin
(available from BASF), and 20 g of poly(styrene-co-maleic anhydride) cumene
terminated resin
MW -1700 (available from Aldrich chemicals) to get an uniform mix. It is then
milled with
Hockmeyer Basket Mill with 0.2 mm YTZ ceramic media for 12 hours. A mixture of
potassium
persulfate (20 g) and sodium bicarbonate (13 g) is added to facilitate
attachment. As needed, a
defoamer to control the foaming and calcium free sodium hydroxide solution to
hold the pH 9-10
are added.
[00158] The milled product above is then removed from the mill, combined with
mill rinses
and heated overnight at 50-55 C to complete the reaction. The dissolved
impurities are removed
by ultrafiltration until the chloride and sulfate content of the feed sample
are less than 50 ppm.
The product is then concentrated to 18% solids and mixed with (0.3%, wt/wt)
Proxel GXL
(available from Arch Chemicals, Smyrna, GA). Finally, the product is
centrifuged at 5,000 rpm
for 20 minutes and then filtered through a 0.7 micron GF filter.
Examples 22-24
[00159] Examples 22-24 were prepared following the same process as set forth
above for
Example 21 using different colored pigments as shown in the Table 4. The
Sensijet Direct Blue
199 was replaced with Sensijet Acid Red 289 Na solution in example #22 and
with 20 g of
Surfynol CT-131 in example #23.
37
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Table 4.
Example Pigment 4-ABA NaHCO3 K2S208 Final conc
[#] equivalent of pigment
Type (g) (g) (g) (g) %
22 PR122 13 120 60 0 39.6 15.34
23 PY74 14 120 20 0 21 15.53
24 Carbon 100 60 0 40 12.7
12 Pigment Blue No. 15:3 wet cake, 45% solids, from Clariant Colors
(Charlotte,
NC)
13 Pigment Red 122 from CIBA (Newport, DE)
14 Pigment Yellow 74 from SUN (Parsippany, NJ)
15 Carbon Black powder from Degussa, (Akron, OH)
Example 25
[00160] Example of attaching Surfonamines to oxidized self-dispersed carbon.
[00161] Sensijet Black SDP1000 carbon16 dispersion (40 g @ 100%) is diluted to
5%
concentration and mixed with the tris reagent17 in a 1L beaker. The mixture is
stirred with a
regular overhead stirrer at 300-500 rpm while it is heated to 50 C on a hot
plate. To the heated
pigment mixture, is added dropwise, a solution of potassium persulfate (6.81
g) and sodium
bicarbonate (2.12 g) while adjusting the pH to 8-9 with Ca free sodium
hydroxide. The reaction
mixture is stirred at 50 C for 20 hours and purified by ultrafiltration until
the chloride and
sulfate content of the feed sample are less than 50 ppm. The product is then
concentrated to 11.1
% solids and mixed with (0.3%, wt/wt) Proxel GXL (available from Arch
Chemicals, Smyrna,
GA).
16 Oxidized Carbon Black self dispersed liquid @ 14-14.5%, available from
Sensient Colors Inc (St. Louis, MO)
38
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Examples 26-31
[00162] Example of attaching Surfonamines and PEHA to oxidized self-dispersed
carbon.
Examples 26-31 are prepared using Sensijet SDP100016 oxidized carbon black
pigment and tris
reagents containing various Surfonamines and pentaethylhexamine as given in
Table 5.
Table 5.
Example Pigment Tris Adduct NaHCO3 K2S208 Final conc
[#] SDP 1000 of pigment
(g) (g) (g) (g) %
26 40 7.9618 2.12 6.81 8.21
27 40 4.9519 0.54 1.62 15.1
28 40 4.4820 0.61 1.95 15.83
29 40 .0921 1.01 3.24 12.03
30 40 .022 2.74 8.81 12.86
31 40 8923 1 2.12 6.81 10.35
17 Tris adduct is obtained by reacting cyanuric chloride with 1 equiv.
Surfonamine
B-30 and 2 equiv. 4-aminobenzoic acid.
18 Tris adduct is obtained by reacting cyanuric chloride with 1 equiv.
Surfonamine
B-60 and 2 equiv. 4-aminobenzoic acid.
19 Tris adduct is obtained by reacting cyanuric chloride with 1 equiv.
Surfonamine
B-30 and 2 equiv Surfonamine L-100.
20 Tris adduct is obtained by reacting cyanuric chloride with 3 equiv.
Surfonamine
B-60.
21 Tris adduct is obtained by reacting cyanuric chloride with 3 equiv.
Surfonamine
B-30.
22 Tris adduct is obtained by reacting cyanuric chloride with 3 equiv. 4-
aminobenzoic acid. The reaction mixture also contained Joncryl HPD 196 (11.94g
as a 36%
solution from BASF).
23 Tris adduct is obtained by reacting cyanuric chloride with 1 equiv. PEHA
(pentaethylhexamine) and 2 equiv 4-aminobenzoic acid.
39
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Example 32
[00163] Pigment Dispersion [example of milling a pigment with a polymer
stabilizer and a
polymer of styrene co-acrylic acid type converting to a self stabilized
dispersion by the use of
cyanuryl tris adduct with 4-aminobenzoic acid].
[00164] Tris 4-ABA reagent described in Example 2 (400g at 15% conc) is used
to wet 100g
of commercial gas carbon black available from Degussa (Akron, OH), with a
primary particle
size of 20 nm and B.E.T surface area of 160 m2 /g, along with 100 g of
Sensijet Direct Blue 199
(available from Sensient Colors Inc, St. Louis, MO). The mixture is heated to
50 C and stirred
with 10 g of 25% solution of calcium free sodium hydroxide, an aqueous
solution containing 1 g
of Joncryl HPD 296 resin (available from BASF), and 55.6g of Joncryl HPD 196
(available from
BASF as a 36% solution) to get an uniform mix. It is then milled with
Hockmeyer Basket Mill
with 0.4 mm YTZ ceramic media for 14 hours. Potassium persulfate (40 g) is
added to facilitate
attachment. As needed, a defoamer to control the foaming and calcium free
sodium hydroxide
solution to hold the pH 9-10 are added.
[00165] The milled product above is then removed from the mill, combined with
mill rinses
purified by ultrafiltration until the chloride and sulfate content of the feed
sample are less than 50
ppm. The product is then concentrated to 15.6% solids and mixed with (0.3%,
wt/wt) Proxel
GXL (available from Arch Chemicals, Smyrna, GA). Finally, the product is
centrifuged at
10,000 rpm for 20 minutes and then filtered through a 0.7 micron GF filter.
Example 33
[00166] Example of attaching polyethyleneimine [PEI] via tris reagents to
conventionally
dispersed pigment(s)
[00167] A resin stabilized 20% dispersion of Sensijet Magenta PV 193 (300 g)
was slowly
added to a mixture of 90 g of the Tris 4-ABA reagent described in Example 2
and 250 g of DI
water.
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[00168] A clear solution of Epomine SP-012 (79.5 g, 1200 MW, available from
Nippon
Shokubai) in 300 g DI water was cooled to 8 C and mixed with cyanuric
chloride (5 g, available
from Lonza Walkersville, Inc., Walkersville, Maryland) to form 690.6 g of tris
epomine reagent,
analogues to tris 4-ABA preparation described in Example#2. A part (48 g) of
the tris epomine
reagent was diluted with DI water (140 g), heated to 38 C and then added to
the treated pigment
dispersion above. The reaction mixture was then heated to 51 C and a solution
of 13 g
potassium persulfate and 11 g sodium bicarbonate in hot, 50 C DI water (300
g) was introduced
slowly while the pH was maintained between 10 and 10.5 with the addition of
calcium free
sodium hydroxide. After the addition of potassium persulfate solution, the
reaction mixture was
heated to 80 C for one hour. The reaction mixture was held at 55-58 C for 20
hours. The
dissolved impurities were removed by ultrafiltration until the chloride and
sulfate content of the
feed sample were less than 50 ppm. The product was then concentrated to 15.5%
solids and
mixed with (0.3%, wt/wt) Proxel GXL (available from Arch Chemicals, Smyrna,
GA). Finally,
the product (300 g) was centrifuged at 10,000 rpm for 20 minutes and then
filtered through a 0.7
micron GF filter.
Example 34
[00169] Pigment Dispersion [example of milling a pigment with a grind aid, a
polymer
stabilizer, and converting to a self stabilized dispersion by the use of
cyanuryl tris adduct with 4-
aminobenzoic acid].
[00170] Surfynol CT-131 (available from Air Products, Allentown, PA) (20g)
diluted with
300 g DI water is used to wet and grind commercial Pigment Yellow 7414 (100
g). It is then
milled with Hockmeyer Basket Mill with 0.4 mm YTZ ceramic media for 5 hours.
The milled
pigment is then treated with tris 4-ABA reagent described in Example 2 (81.4 g
at 15.0% conc)
according to procedure described in example 3-7. The final product (579.4 g)
was centrifuged at
10,000 rpm for 10 minutes and then filtered through a 0.7 micron GF filter.
Example 35
[00171] The example 34 was repeated with Tamol SN (available from Rohm and
Haas,
Philadelphia, PA) as grind aid replacing Surfynol CT-131 and special grade24
of PY 74.
41
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
24 Hansa Brilliant Yellow from Clariant Colors (Charlotte, NC)
Example 36
[00172] The physical properties of the modified pigments from the examples
above are set
forth in the following table.
Table 6.
Example Pigment Solids pH Cl SO4 Viscosity
[#] Type (%) ppm ppm Cps
3 Carbon' 16.7 8.5 4 7 3.38
4 PB 15 14.3 7.9 1 5 2.7
PB 15 14.6 7.7 5 4 2.8
6 PV19 16.1 8.2 1 19 2.86
7 PV19 15.6 8.3 16 11 2.6
8 Carbon' 15.9 9.4 7 2 7.42
9 Carbon 18.2 9.2 38 11 10.8
PB 15 27- 17.6 8.3 20 9 9.15
11 PY155 14.8 8.1 2 4 2.89
12 PY155 14.7 8.2 1 3 2.90
13 PV 19 14.9 8.5 9 25 5.69
14 PB 15 24.6 8.5 5 4 5.77
PB60 16.4 8.3 10 98 4.52
16 PG7 13.2 9.7 5 12 2.04
17 PR122 17.7 8.1 300 182 4.27
18 PR2549 17.0 6.1 1 7 3.75
19 PY83 19.5 8.3 15 3 5.2
PY 120 16.6 7.8 40 8 3.2
21 PB15 12 18.3 9.7 6 1 4.9
22 PR122 13 15.3 8.9 1 3 2.2
23 P774 15.5 9.9 9 51 2.19
24 Carbon 77- 16.4 9.3 26 28 5.52
Carbon 11.1 9.7 32 136 2.4
26 Carbon 8.2 9.6 7 38 1.8
27 Carbon 15.1 9.2 5 <1 2.56
42
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
28 Carbon 16 15.8 9.3 4 3 4.08
29 Carbon 12.0 9.4 <1 12 1.88
30 Carbon 12.9 9.5 2 1 3.85
31 Carbon 16 10.4 11.1 16 10 1.60
32 Carbon 12.5 9.0 8 25 3.44
33 PV19 15.1 8.9 4 2 2.88
34 PY74 14 14.5 8.7 7 37 1.66
35 PY74 24 14.4 8.3 2 4 1.97
Table 6 Continued. Analytical Results of Pigment Dispersions.
Example Pigment Condu Surface Na K S Heavy
[#] ctivity tension metals25
Type S Dynes/ ppm Ppm ppm ppm
cm
3 Carbon' 2460 56.8 1131 699 487 38.8
4 PB 15 2500 43 869 660 293 35.4
PB 15 2500 41 725 630 722 31.4
6 PV19 2120 34.2 887 679 456 28.7
7 PV 19 2000 39 746 449 132 33.3
8 Carbon' 3820 61.4 2863 1438 473 144
9 Carbon' 3750 62.5 3103 1586 605 178.9
PB 15 2870 41.4 1328 923 279 70.6
11 PY155 3820 36.8 1099 698 159 8.7
12 PY155 3720 39.7 360 256 356 45.9
13 PV19 1793 42.5 880 460 169 58.2
14 *PB15 4710 46.8 459 3000* 477 185.6
PB60 2840 34.3 829 195 2 20.3
16 PG7 7 1422 39.8 587 33 186 63
17 PR122 1327 46.6 543 433 192 95.2
18 PR2549 949 49.1 277 280 217 8.8
19 PY8310 1403 44.6 462 358 189 147
PY12011 954 42 477 438 215 62
21 PB 15 12 4140 51.3 2796 469 653 55.3
22 PR122 13 4970 54.3 2859 577 685 24.2
23 PY74 14 4270 39.3 2382 526 327 33.9
24 Carbon 15 5370 40.1 2600 1060 - 16.7
Carbon 16 1181 38.0 1933 1206 - 51.7
43
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
26 Carbon 16 1082 38.2 1798 1172 - 23.0
27 Carbon 16 1660 34.2 2986 908 - 25.4
28 Carbon 16 1464 40.8 4047 1204 - 27.4
29 Carbon 16 886 37.0 2676 1427 - 23.3
30 Carbon 77- 2970 42.7 3230 1673 - 21.8
31 Carbon 16 1730 66.8 3429 598 - 24.3
32 Carbon 15 4730 45.6 2261 913 - 18.0
33 PV19 1889 35.1 825 297 109 21.7
34 PY74 14 1359 40.8 591 107 375 21.4
35 PY74 2450 59.4 1252 283 1596 22.2
25 Sum of Ca, Mg and Fe present as a contaminant in the raw materials and/or
formed during the milling process.
* A potassium chelate called Belcene was used to remove heavy metals.
Example 37
X-Ray Photoelectron Spectroscopy (XPS) Analyses
[00173] XPS data were collected and analyzed for Black samples, Cyan samples,
Magenta
samples, and Yellow samples (Table 7). Dried samples of purified "Tris"
reagents were also
analyzed for identifying the nature of the groups attached to the pigment
surface. The numbers
in brackets in the table refer to example numbers.
Table 7. XPS of pigment samples.
Sample Source
[-]Carbon Black Gas carbon black, available from Degussa,
Akron, OR
[-]Sensijet black SDP 1000 Inkjet Grade Carbon Dispersion from Sensient
Colors Inc, St. Louis, MO.
[3] [Carbon] A-01 Dispersion from Example#3 with 4-ABA
attachment
[8] [Carbon] A-04 Dispersion from Example#8 with 4-ABA
attachment
[24] [Carbon] A-51 Dispersion from Example#24, 4-ABA and SMA
attachment
44
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[25] [Carbon] A-21 Dispersion from Example#25 oxidized carbon
with 4ABA and Surfonamine B 30 attachment
[26] [Carbon] A-27 Dispersion from Example#26 oxidized carbon
with 4ABA and Surfonamine B 60 attachment
[27] [Carbon] A-29 Dispersion from Example#27 oxidized carbon
with Surfonamine B 30 and L-100 attachment
[28] [Carbon] A-31 Dispersion from Example#28 oxidized carbon
with Surfonamine B 60 attachment
[29] [Carbon] A-33 Dispersion from Example#29 oxidized carbon
with Surfonamine B 30 attachment
[30] [Carbon] A-37 Dispersion from Example#30 oxidized carbon
with Styrene-Acrylate attachment
[31] [Carbon] A-43 Dispersion from Example#31 oxidized carbon
with PEHA attachment
[32] [Carbon] A-53 Dispersion from Example#32 raw carbon with
Styrene-Acrylate attachment
[ ] PB 15 - untreated Inkjet Grade Pigment Blue 15:3 from BASF
[10] [PB 15] A-02 Dispersion from Example#10 with 4-ABA
attachment
[14] [PB 15] A-03 Dispersion from Example#14 with 4-ABA
attachment
[21] [PB 15] A-05 Dispersion from Example#21 with 4-ABA and
SMA attachment
[ ] [PR 122 - untreated] Inkjet Grade Pigment Red 122 from CIBA
[17] [PR 122] A-06 Dispersion from Example#17 with 4-ABA
attachment
[22] [PR 122] A-57 Dispersion from Example#22 with 4-ABA and
SMA attachment
[6] [PV 19] S-03 Dispersion from Example#6 with SA
attachment
[7] [PV 19] A-01 Dispersion from Example#7 with 4-ABA
attachment
[ ] [PY 74 - untreated] Inkjet Grade Pigment Yellow 74 from SUN
[23] [PY 74] A-49 Dispersion from Example#23 with 4-ABA and
SMA attachment
[34] [PY 74] A-34 Dispersion from Example#34 with 4-ABA and
alkyne attachment
[ ] [PY 155 - untreated] Inkjet Grade Pigment Yellow 155 from Clariant
[11 [PY 155] A-14 Dispersion from Example#11 with 4-ABA
attachment
[12] [PY 155] 5-11 Dispersion from Example#12 with SA
attachment
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[00174] The XPS data were acquired by EAG Labs (in Chanhassen, MN) using a
probe beam
of focused, monochromatic Al Ka, radiation. The x-rays generate photoelectrons
that are energy
analyzed and counted to reveal the atomic composition and chemistry of the
sample surface. The
escape depth of the photoelectrons limits the depth of analysis to the outer -
50 A. The data
presented includes low resolution survey scans, which give the full spectrum
between 0 and
1400eV binding energy. Also included in the data are high resolution spectra
from selected
elements, which provide chemical state information. The spectra are used to
obtain surface
composition by integrating the areas under the photoelectron peaks and
applying empirical
sensitivity factors. The XPS data is presented in Figures 1-36.
Table 8. Analytical Conditions.
Instrument: Physical Electronics 5802 Multitechnique,
Quantum 2000 Scanning XPS
X-ray Source: Monochromatic Al Ka, 1486.6eV
Analysis Area: 1.5mm x 0.6mm - 5802, 1.2mm x 0.2mm - Quantum 2000
Take-off Angle: 450
Charge Correction: C-C, C-H in Cis spectra set to 284.8eV
Charge Neutralization: Low energy electron and ion floods
Tables for Carbon Black Samples
[00175] The following tables were normalized to 100% of the elements detected.
XPS does
not detect H or He. Detection limits are typically between 0.05% and 1.0% for
other elements.
A dash "-" indicates the element was not detected. High 0 (more than 10
atomic%) and Na
(more than 3 atomic%) for modified samples is indicative of a surface COONa
bond introduced
by oxidation. The S content as sulfide is typical of carbon black which
appears to be partially
oxidized under modification conditions. The levels of N, Na and K present in
all samples,
except the unreacted carbon, is a measure of charge groups present either as
amino benzoic or
46
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
maleic acid or acrylic acid or surface carboxylic or sulfonic acid groups as
corresponding sodium
or potassium salts.
Table 9-1. XPS Surface Concentrations of Carbon Black Samples (Atomic %).
Group A (Attachment to Carboxy modified)
Sample C N 0 Na S Cl K
[-] [Carbon - untreated] 97.5 - 2.4 - 0.11 0.03 -
Sensijet Black SDP 1000 81.4 - 13.0 5.3 0.11 0.19 -
[25] [Carbon] A-21 79.6 2.6 13.2 3.5 0.10 0.30 0.7
[26] [Carbon] A-27 78.8 0.5 15.1 4.7 0.04 0.14 0.8
[27] [Carbon] A-29 79.0 1.4 14.9 3.9 0.05 0.26 0.5
[28] [Carbon] A-31 80.0 0.3 13.8 4.9 0.05 0.11 0.9
[29] [Carbon] A-33 80.7 1.0 13.5 3.9 0.04 0.11 0.7
[30] [Carbon] A-37 83.0 0.8 11.8 3.8 0.1 0.1 0.5
[31] [Carbon] A-43 82.9 1.3 10.9 4.4 0.1 0.2 0.2
Group B (Attachment to Carbon Black)
Sample C N 0 Na S Cl K
[-] [Carbon - untreated] 97.5 - 2.4 - 0.11 0.03 -
[3] [Carbon] A-01 91.4 0.6 6.5 1.4 0.13 - -
[8] [Carbon] A-04 86.7 0.9 10.1 2.0 0.07 0.06 -
[24] [Carbon] A-51 83.3 2.4 9.3 3.5 0.8 0.1 0.4
[32] [Carbon] A-53 86.9 2.6 7.0 2.3 0.6 0.1 0.2
Table 9-2. Carbon Chemistries of Carbon Black Samples (% of total Q.
Group A (Attachment to Carboxy modified)
Aromatic Shake-
Sample C-C,H C-O* C=O COONa, O-C=O up
[-] [Carbon -
untreated] 86 3 0.7 0.2 10
Sensijet Black SDP 1000 77 7 2 5 9
[25] [Carbon] A-21 79 8 2 6 5
[26] [Carbon] A-27 78 6 3 6 8
[27] [Carbon] A-29 75 9 5 5 6
[28] [Carbon] A-31 81 6 2 5 6
[29] [Carbon] A-33 80 7 2 5 6
47
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[30] [Carbon] A-37 84 7 3 5 1
[31] [Carbon] A-43 80 8 4 5 3
Group B (Attachment to Carbon Black)
Aromatic Shake-
Sample C-C,H C-O* C=O COONa, O-C=O up
[-] [Carbon -
untreated] 86 3 0.7 0.2 10
[3] [Carbon] A-01 91 4 0.3 1.6 3
[8] [Carbon] A-04 90 5 - 2.6 2
[24] [Carbon] A-51 86 8 0.3 4 2
[32] [Carbon] A-53 88 8 1 2 1
Table 9-3. Nitrogen Chemistries of Carbon Black Samples (% of total N).
Group A (Attachment to Carboxy modified)
Sample N-C=N NH
[-] [Carbon - untreated] - -
Sensijet Black SDP 1000 - -
[25] [Carbon] A-21 49 51
[26] [Carbon] A-27 48 52
[27] [Carbon] A-29 45 55
[28] [Carbon] A-31 42 58
[29] [Carbon] A-33 43 57
[30] [Carbon] A-37 37 63
[31] [Carbon] A-43 40 60
Group B (Attachment to Carbon Black)
Sample N-C=N NH NO3
[-] [Carbon - untreated] - - -
[7] [Carbon] A-1 40 60 -
[6] [Carbon] A-004 31 43 26
[24] [Carbon] A-51 71 29 -
[32] [Carbon] A-53 74 26 -
48
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Table 9-4. Oxygen Chemistries of Carbon Black Samples (% of total 0).
Group A (Attachment to Carboxy modified)
Sample C=O, COONa, Sox C-O
[-] [Carbon - untreated] 32 68
Sensijet Black SDP 1000 65 35
[25] [Carbon] A-21 49 51
[26] [Carbon] A-27 58 42
[27] [Carbon] A-29 43 57
[28] [Carbon] A-31 55 45
[29] [Carbon] A-33 52 48
[30] [Carbon] A-37 60 40
[31] [Carbon] A-43 63 37
Group B (Attachment to Carbon Black)
Sample C=O, COONa, Sox C-O
[-] [Carbon - untreated] 32 68
[3] [Carbon] A-01 44 56
[8] [Carbon] A-04 50 50
[24] [Carbon] A-51 72 28
[32] [Carbon] A-53 61 39
Table 9-5. Sulfur Chemistries of Carbon Black Samples (% of total S).
Group A (Attachment to Carboxy modified)
Sample Sulfides SOx
[ ] [Carbon - untreated] 69 31
Sensijet Black SDP 1000 82 18
[25] [Carbon] A-21 49 51
[26] [Carbon] A-27 100 0
[27] [Carbon] A-29 100 0
[28] [Carbon] A-31 100 0
[29] [Carbon] A-33 100 0
[30] [Carbon] A-37 59 41
[31] [Carbon] A-43 61 39
49
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Group B (Attachment to Carbon Black)
Sample Sulfides SOx
[-] [Carbon - untreated] 69 31
[3] [Carbon] A-01 53 47
[8] [Carbon] A-04 100 -
[24] [Carbon] A-51 6 94
[32] [Carbon] A-53 11 89
[00176] The S present in untreated carbon as sulfides was largely oxidized to
sulfate/sulfone
in all treated samples, adding to the surface charge groups.
Tables for PB 15 samples
Table 10-1. XPS Surface Concentrations of PB 15 Samples (Atomic %).
Sample C N 0 Na S Cl Cu
[-] [PB 15 - untreated] 78.7 17.3 1.6 0.1 0.09 - 2.3
[10] [PB 15] A-02 77.9 13.1 6.6 0.8 0.05 - 1.6
[14] [PB 15] A-03 72.3 11.8 11.7 - 0.12 - 1.5
[21] [PB 15] A-05 70.0 13.1 10.0 4.2 0.25 0.12 2.0
Table 10-2. Carbon Chemistries of PB 15 Samples (% of total Q.
Sample C-C,H N-C=N* CN-Cu COONa/CSO3Na Aromatic Shake-up
[-] [PB 15 - untreated] 67 22 4.7 1.1 5
[10] [PB 15] A-02 68 23 3.7 1.5 4
[14] [PB 15] A-03 45 26 26 - 3
[21] [PB 15] A-05 64 25 4 4 3
*C-O bonding may also contribute to the intensity of this band.
Table 10-3. Nitrogen Chemistries of PB 15 Samples (% of total N).
Sample N-C=N CN-Cu Aromatic Shake-up
[-] [PB 15 - untreated] 79 9 12
[10] [PB 15] A-02 77 8 15
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[14] [PB 15] A-03 76 16 9
[21] [PB 15] A-05 87 7 6
Table 10-4. Oxygen Chemistries of PB 15 Samples (% of total 0).
Sample C=O, COONa, Sox C-O
[-] [PB 15 - untreated] 69 31
[10] [PB 15] A-02 38 62
[14] [PB 15] A-03 4 96
[21] [PB 15] A-05 69 31
Tables for PR 122 / PV19 Samples
Table 11-1. XPS Surface Concentrations of PR 122/ PV19 Samples (Atomic %).
Sample C N 0 Na S Cl
[-] [PR 122 - untreated] 84.4 8.0 7.7 - - -
[17] [PR 122] A-06 79.0 5.0 15.6 0.3 - -
[22] [PR 122] A-57 82.0 6.1 10.2 1.6 0.14 -
[6] [PV 19] S-03 80.6 5.1 12.9 0.7 0.4 0.2
[7][PV 19] A-01 82.4 6.7 10.2 0.6 - -
Table 11-2. Carbon Chemistries of PR 122/PV 19 Samples (% of total Q.
C=O, COONa, Aromatic
Sample C-C,H C2NH # C-O O-C-O CSO3Na Shake-up
[-] [PR 122 - untreated] 66 24 - 3 - 7
[17] [PR 122] A-06 55 14 26 2 - 3
[22] [PR 122] A-57 70 21 - 1 2 6
[6] [PV 19] S-03 71 13 6 6 1 3
[7] [PV 19] A-01 54 18 12 11 2 3
H
# C2NH denotes each of the C atoms bonded in the following group: -C-N-C-
Table 11-3. Oxygen Chemistries of PR 122/PV 19 Samples (% of total 0).
Sample C=O, COONa, SOx C-O Aromatic Shake-up
[ ] [PR 122 - untreated] 73 15 11
51
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[17] [PR 122] A-06 21 78 1
[22] [PR 122] A-57 65 25 10
[6] [PV 19] S-03 42 55 3
[7][PV 19] A-01 43 50 7
Tables for PY 74 Samples
Table 12-1. XPS Surface Concentrations of PY 74 Samples (Atomic %).
Sample C N 0 Na S
[-] [PY 74 - untreated] 64.6 13.8 20.8 0.3 0.3
[23] [PY 741 A-49 69.6 9.5 19.1 1.6 0.1
[34] [PY 74] A-34 66.8 9.7 21.5 1.0 0.3
Table 12-2. Carbon Chemistries of PY 74 Samples (% of total Q.
Sample C-C,H C-NH* C-O C=O COONa/CSO3Na Aromatic Shake-up
[-] [PY 74 - untreated] 45 17 21 11 1.8 4
[23] [PY 74] A-49 72.5 8.3 12.4 5.7 - 1.2
[34] [PY 74] A-34 63.7 9.4 18.8 6.6 - 1.6
*C-O bonding may also contribute to the intensity of this band.
Table 12-3. Nitrogen Chemistries of PY 74 Samples (% of total N).
Sample C-N NO2 NO3
[-] [PY 74 - untreated] 71 9 20
[23] [PY 74] A-49 76 8 16
[34] [PY 74] A-34 78 8 14
Table 12-4. Oxygen Chemistries of PY 74 Samples (% of total 0).
Sample C=O, COONa, SOx C-O, NOx
[-] [PY 74 - untreated] 41 59
[23] [PY 74] A-49 39 61
[34] [PY 74] A-34 33 67
52
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Tables for PY 155 samples
Table 13-1: XPS Surface Concentrations of PY 155 samples (Atomic %)
Sample C N 0 Na S
[-] [PY 155 - untreated] 67.2 10.8 22.0 - -
[5] [PY 155] A-14 70.1 8.3 21.1 0.4 0.04
[4] [PY 155] 5-11 68.2 9.5 21.9 0.4 0.03
Table 13-2: Carbon Chemistries of PY 155 samples (% of total C)
0-C=011'
Sample C-C,H C-N* C=O COONa, SO3Na Aromatic Shake-up
[-] [PY 155 - untreated] 57 17 9 14 3
[5] [PY 155] A-14 60 18 8 12 2
[4] [PY 155] S-11 57 19 9 12 3
*C-O bonding may also contribute to the intensity of this band.
# O-C=O is likely the main component of this band, as Na and S concentrations
are very
low
Table 13-3: Oxygen Chemistries of PY 155 samples (% of total 0)
Sample C=O, COONa, Sulfate C-0
[-] [PY 155 - untreated] 54 46
[5] [PY 155] A-14 54 46
[4] [PY 155] 5-11 55 45
[00177] The XPS results indicate that the surface modification as disclosed
yields a modified
carbon black with an increase in surface nitrogen, as an NH/N-C=N group
distributed almost
equally, in about 0.3 to 2.6 atomic%. It is bonded in NH and N-C=N groups of
comparable
concentrations. A smaller contribution of NO3 group is also observed on one
sample [8]
[Carbon] A-04.
[00178] The XPS results indicate that the surface modification as disclosed
yields a modified
carbon black with a surface oxygen in the atomic ratio of 6.5 to 15.1% wherein
- 43 to 72% of
the oxygen is present as C=O, COONa, or SOx group and the balance (-57 to 28%)
as a C-O
53
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
group. In contrast, the surface oxygen in the untreated carbon black is only
about 2.4 atomic %
and is distributed as 32% as a C=O, COONa or SOx group and the balance (68%)
as a C-O
group.The XPS results indicate that the surface modification as disclosed
yields a modified
carbon black with an increase in surface sodium, as COONa, in about 1.4 to 5.3
atomic%.
[00179] The XPS results for untreated carbon blacks, Sensijet SDP 1000 carbon
and carbon
blacks from Examples 3, 8, 24, 25-31, and 32 is displayed in Figures 1-12.
[00180] The XPS results indicate that surface modification as disclosed yields
a modified
Pigment Blue No.15 with significantly higher surface sodium content (0.8 to
4.2 atomic %)
compared to a low concentration of 0.1 atomic % in the untreated pigment. The
XPS results for
untreated Pigment Blue No. 15 and Pigment Blue No. 15 from Examples 10, 14,
and 21 are
displayed in Figures 13-19.
[00181] The XPS results indicate that the surface modification as disclosed
yields a modified
Pigment Red No.122 with a surface sodium present at concentrations in the
range of 0.3 - 1.6
atomic% while in comparison the untreated pigment has none. The XPS results
for untreated
Pigment Red No. 122, Pigment Red No. 122 from Examples 17 and 22, and Pigment
Violet 19
from Examples 6 and 7 are displayed in Figures 20-25.
[00182] The XPS indicate that the surface modification as disclosed yields a
modified
Pigment Yellow No. 74 with a surface sodium in the atomic ratio of 1.0 to 1.6%
which is
expected to be present as COONa/CSO3Na. In contrast, in the untreated pigment
the surface
sodium is only about 0.3. The XPS results for untreated Pigment Yellow No. 74
and Pigment
Yellow No. 74 from Examples 23 and 34 are displayed in Figures 26-31. The XPS
results for
untreated Pigment Yellow No. 155 and for Pigment Yellow No. 155 from Examples
11 and 12
are displayed in Figures 32-36.
54
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Example 38
Table 14. Elemental analysis (% C,H,N, & S).
Sample - [Ex#] [S] [H]
[Pigment Type] C H N S26 mmol/g Na26 K26 mmol/g
1 [-] [PB 15 -untreated] 66.78 3.09 18.42 0.25 0.078 - - -
2 [21] [PB 1512]A-05 62.04 3.52 14.87 0.36 0.112 1.53 0.26 0.732
3 [10] [PB 152]A-02 65.28 4.34 13.62 0.16 0.050 0.75 0.52 0.459
4 [14] [PB 155]A-03 62.45 4.09 14.78 0.19 0.059 0.19 1.22 0.395
[-] [PR12213 - untreated] 76.79 4.72 8.16 - - - - -
6 [22] [PR 12213]A-57 71.47 4.80 6.15 0.45 0.140 1.87 0.38 0.911
7 [17] [PR 1228]A-06 71.95 5.50 6.57 0.11 0.034 0.31 0.24 0.196
8 [6] [PV 193]S-03 71.68 4.64 7.34 0.28 0.087 0.55 .42 0.347
9 [7] [PV 193]A-01 71.64 4.7 7.08 0.08 0.025 0.48 0.29 0.283
[33] [PV 193]A-02 73.67 4.93 7.62 0.07 0.022 0.55 0.20 0.290
11 [-] [PY 747- untreated] 52.98 4.47 13.53 0.31 0.097 - - -
12 [23] [PY 7414]A-49 54.11 5.04 10.91 0.21 0.065 1.54 0.34 0.757
13 [34] [PY 7414]A-34 53.04 4.68 12.55 0.26 0.081 0.41 0.07 0.196
14 [-] [PY 155- untreated] 56.69 4.35 11.55 0.19 0.059 - - -
[11] [PY 1554]A-14 57.53 5.05 8.88 0.11 0.034 0.74 0.47 0.442
16 [12] [PY 1554]S-11 57.44 4.79 9.14 0.24 0.075 0.24 0.17 0.148
17 [-] [Carbon15 - untreated] 91.35 1.15 0.10 0.32 0.100 - - -
18 [-] [Carbon16 - oxidized] 75.12 2.03 - 0.19 0.059 2.93 0.01 1.277
19 [3] [Carbon']A-01 86.89 1.48 0.50 0.29 0.090 0.68 0.42 0.403
[8] [Carbon']A-04 77.51 2.02 1.01 0.30 0.094 1.80 0.90 1.013
21 [24] [Carbon 15]A-51 79.45 1.51 0.90 - - 1.59 0.65 0.858
22 [25] [Carbon 16]A-21 75.46 1.97 0.94 - - 1.74 1.09 1.036
23 [26] [Carbon 16]A-27 76.33 1.40 0.29 - - 2.19 1.43 1.318
24 [27] [Carbon 16]A-29 75.28 1.98 0.64 - - 1.98 0.60 1.015
[28] [Carbon 16]A-31 78.59 1.33 0.12 - - 2.56 0.76 1.308
26 [29] [Carbon 16]A-33 76.26 1.86 0.34 - - 2.23 1.19 1.274
27 [30] [Carbon 16]A-37 74.55 1.88 <0.50 - - 2.50 1.30 1.420
28 [31] [Carbon 16]A-43 76.44 1.27 0.89 - - 3.30 0.58 1.584
29 [32] [Carbon 15]A-53 79.51 2.04 0.88 - - 1.81 0.73 0.974
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
26 The sulfur, sodium and potassium were calculated @ 100% solids from ICP
metal
analysis of the original dispersion.
[00183] The results of the elemental analysis indicate that the surface
modification as
disclosed yields a modified Pigment Blue No. 15 with 0.050-0.112 mMoles of S
and 0.395-0.732
mMoles of active hydrogen per gram of pigment.
[00184] The results of the elemental analysis indicate that the surface
modification as
disclosed yields a modified Pigment Red No. 122 with 0.034-0.140 mMoles of S
and 0.196-
0.911 mMoles of active hydrogen per gram of pigment.
[00185] The results of the elemental analysis indicate that the surface
modification as
disclosed yields a modified Pigment Yellow No. 74 with 0.065-0.081 mMoles of S
and 0.196-
0.757 mMoles of active hydrogen per gram of pigment
[00186] The results of the elemental analysis indicate that the surface
modification as
disclosed yields a modified Pigment Yellow No. 155 with 0.034-0.075 mMoles of
S and 0.148-
0.442 mMoles of active hydrogen per gram of pigment
[00187] The results of the elemental analysis indicate that the surface
modification as
disclosed yields a modified Pigment Violet No. 19 with 0.022-0.087 mMoles of S
and 0.283-
0.347 mMoles of active hydrogen per gram of pigment
[00188] The results of the elemental analysis indicate that the surface
modification as
disclosed yields a modified Carbon Black with 0.403-1.584 mMoles of active
hydrogen per gram
of pigment.
Example 39
Particle Size Measurement
[00189] Samples comprising 8-15% solids were prepared by diluting one drop of
sample to 15
ml deionized water and loading into a 1 cm disposable cuvette, avoiding air
bubbles. Malvern
56
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Zetasizer Nano series Model ZEN3600 was then used to measure mean particle
size in the
sample.
Table 15. Particle Size Measurements and Stability data of Pigment
Dispersions.
Example Pigment Viscosity Particle Size pH
[#] Type Initial Week Week Initial Week Week Initial Final
1 3 1 3
22 PR122- 2.20 2.19 2.21 100.3 100.9 102 8.9 8.5
17 PR 122 4.27 3.57 3.62 129.4 127.5 126.2 8.1 7.0
6 '-FR 122 2.86 2.66 2.83 137.8 134.7 137 8.2 7.9
14 PB 15 5.77 3.92 3.89 131.5 129.7 133.2 8.5 -
PB 15 2.80 3.09 3.11 136.3 135.1 131.9 7.7 7.8
PB 15 9.15 6.05 5.67 102 102 109 8.3 8.2
11 PY155 2.89 2.71 2.42 150 152 154 8.1 7.3
12 PY155 2.90 2.90 2.80 151 165 178 8.2 7.4
23 PY74 14 2.44 3.45 3.64 99 113 130 9.9 9.1
34 PY74 14 1.97 2.06 2.03 125 128 129 8.7 8.0
32 Carbon 3.44 - 3.69 120 111 116 9.0 8.6
Example 40
Redispersion Studies
[00190] The following dispersions (Ex#22-24) were dried and redispersed in DI
water of
pH 7.5 as a powder, as described below.
[00191] About 0.5 g of the dry powder was mixed with DI water (pH=7.5), made
up to
about 80.0 g and sonicated for 5 minutes. A part of the dispersions were then
filtered through
0.7 micron GF/F (available from Fisher Scientific) 25 mm diameter syringe
filters and the weight
of the residue and filtrate, after drying were recorded. The results in Table
16 shows that > 93%
the modified pigments were effectively redispersed in neutral water. The
average particle size
(D50, nm) shows that even under these extreme conditions the particles do
resist agglomeration.
57
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Table 16. Redispersion of modified pigments from Examples 22, 23 and 24.
Example #22 Example # 23 Example # 24
Weight of dried powder (g) 0.5112 0.5065 0.5038
Weight of mix in DI water (g) 80.004 80.1351 80.0226
Weight of sample filtered 29.2631 29.7757 30.3676
Average Particle Size (D50) nm 135 152 136
Dry weight of filtrate 0.1827 0.1823 0.1780
Dry weight of residue 0.0014 0.0009 0.0032
% Redispersed 97.71 96.87 93.10
Example 41
[00192] The following ink base was made according to the procedure described
below and
used to make final inks with black dispersions.
Table 17. Ink Base I formulation.
Ingredients % by Weight
Water, deionized 9.6
2-Pyrrolidone water blend 10.0
1,5-pentanediol 5.0
PEG 600 Carb. Polyethylene Glycol 4.0
Nipacide BIT 20 0.3
Surfynol 104E solution 0.1
1,2-hexanediol 1.0
[00193] First, 9.6% by weight of water was added to a clean vessel. A mixing
device was
then placed inside the vessel to agitate the water and provide mixing while
the other ingredients
are added. Mixing was achieved by using a magnetic stirring device. Next, 10%
by weight of 2-
pyrrolidone, 5% by weight of 1,5-pentanediol, 4% by weight of PEG 600, and 1%
by weight of
1,2-hexanediol were added to the vessel. These were allowed to dissolve. Then,
0.1% by weight
of Surfynol 104E solution and 0.3% by weight of Nipacide BIT 20 were added and
allowed to
dissolve.
58
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Example 42
[00194] The following inks were made according to the procedure described
below using
pigment dispersion from Example #24.
Table 18. Inks A-C.
Ink A Ink B Ink C
Matt Black Photo Black Light Grey
Water, deionized (g) 35.58 47.38 65.3
Dispersion (g) 34.42 22.62 4.70
Inkbase (g) 30.00 30.00 30.00
Surfynol 465 (g) 0.17 0.17 0.17
Surfynol 440 (g) 0.12 0.12 0.12
[00195] A second vessel was prepared by adding calculated % by weight of DI
water to the
pigment dispersion to the vessel per Table 18. A magnetic stirring device was
then placed into
the vessel. Next the ink base, followed by surfynol surfactants (Air Products
& Chemicals, Inc.,
Allentown, PA), were slowly added to the pigment dispersion in the second
vessel. The
dispersion was mixed during this process. After all of the diluent has been
added, the ink was
mixed for about 1 hour, or until it was completely homogenous. After mixing,
the ink was
filtered using a 1 micron glass filter (available from Whatman, Kent,
England).
Example 43
[00196] The following ink base was made according to the procedure described
below and
used to make final inks with color dispersions.
Table 19. Ink Base II formulation.
Ingredients % by Weight
Water 12.3
Glycerine 14
PEG 600 2
Butyl Carbitol 3
59
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
TEA 0.1
Cobratec 0.3
Xbinx 19G 0.3
Ethanol 2
Butanol 1
[00197] First, 12.3% by weight of water was added to a clean vessel. A mixing
device was
then placed inside the vessel to agitate the water and provide mixing while
the other ingredients
are added. Mixing was achieved by using a magnetic stirring device. Next, 14%
by weight of
glycerine, 2% by weight of PEG 600, 3% by weight of butyl carbitol, 2% by
weight of ethanol,
and 1% by weight of butanol were added to the vessel. These were allowed to
dissolve. Then,
0.1% by weight of triethanolamine was added and allowed to dissolve. Finally,
0.3% by weight
of Cobratec solution and 0.3% by weight of Xbinx 19G were added and allowed to
dissolve.
Example 44
[00198] The following inks were made according to the procedure described
below.
Table 20. Inks D-L.
Ink Ink Ink Ink Ink
D E F G H
Yellow Dark Light Dark Light
Cyan Cyan Magenta Magenta
Pigment Dispersion Example Example Example Example Example
from: #23 #21 #21 #22 #22
Water, deionized(g) 41.00 38.13 48.90 35.71 55.04
Dispersion (g) 24.00 26.87 16.10 29.29 9.96
Ink base (g) 35.00 35.00 35.00 35.00 35.00
Surfynol 465 (g) 0.087 0.087 0.087 0.17 0.17
Surfynol 440 (g) 0.058 0.058 0.058 0.12 0.12
[00199] A second vessel was prepared by adding the calculated percentage by
weight of DI
water to the pigment dispersion to the vessel per Table 20. A magnetic
stirring device was then
placed into the vessel. Next the ink base, followed by surfynol surfactants
(Air Products &
Chemicals, Inc., Allentown, PA), were slowly added to the pigment dispersion
in the second
vessel. The dispersion was mixed during this process. After all of the diluent
has been added,
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
the ink was mixed for about 1 hour, or until it was completely homogenous.
After mixing, the
ink was filtered using a 1 micron glass filter (available from Whatman, Kent,
England).
Example 45
Print Performance - Print testing of ink set made with dispersions from
examples 21-24
with mixed 4-ABA and SMA attachment
[00200] Test pages were printed with an Epson C88+ printer Model B251A and HP
Photosmart Plus B9180 printer (known to use pigmented ink sets) using four
different commonly
used copy papers. The printed pages, identified by ink set and media, were
analyzed by the
Center for Integrated Manufacturing, Rochester Institute of Technology,
Rochester, NY. Image
Quality was measured with ImageXpert Full Motion System. Optical Density was
measured with
X-rite 939 Spectrodensitometer. Ozone Exposure was measured using RIT custom
ozone
chamber and Sutherland Rub test was done with Sutherland rub fixture.
Highlighter A is Sanford
Yellow Major Accent and Highlighter B is Avery Dennison Fluorescent Yellow Hi-
Liter .
Ozone fading is defined by RIT as follows: "The color change is described by
calculating the
Delta E 2000 (AEOO) and reporting according to ASTM D2244-02 Calculation of
Color
Tolerances and Color Differences from Instrumentally Measured Color
Coordinates." Mottle is
determined as follows: "A solid color block is broken into regions of interest
(ROI) and the
grayness is measured in each region. The average and standard deviation is
calculated for the
entire solid block. The larger the standard deviation, the more mottle in the
sample."
[00201] The print performance characteristics of the color set using ink A, D,
E and G printed
with Epson C88+ printer is identified below:
Table 21
HP MP- ColorLok Xerox 4200
Black Yellow Cyan Magenta Black Yellow Cyan Magenta
Optical 1.16 0.626 0.833 0.952 0.993 0.632 0.81 0.818
Density
Rub 0.04 0.01 0.03 0.01 0.02 0.01 0.03 0.01
Resistance
(OD Diff)
Highlighter A 0.115 - 0.049 0.055 0.024 - 0.002 0.017
61
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Resistance
(OD Diff)
Highlighter B 0.039 - 0.015 0.021 0.009 - 0.006 0.017
Resistance
(OD Diff)
Water 0.015 0.007 0.015 0.022 0.005 0 0.006 0.009
resistance (OD
Diff)
Ozone Fade 1.037 0.369 2.11 1.792 1.11 0.754 3.173 1.954
Mottle 2.353 1.913 2.169 2.201 1.924 1.477 1.746 1.536
Table 21 (Continued)
Office Depot 104 Hammerill GW
Black Yellow Cyan Magenta Black Yellow Cyan Magenta
Optical Density 1.068 0.674 0.873 0.898 0.963 0.655 0.833 0.826
Rub Resistance 0.01 0.01 0.02 0.01 0.03 0.01 0.03 0.02
(OD Diff)
Highlighter A 0.01 - 0.012 0.019 0.049 - 0.038 0.03
Resistance (OD
Diff)
Highlighter B 0.011 - 0.007 0.011 0.036 - 0.022 0.021
Resistance (OD
Diff)
Water 0.006 0 0.001 0.005 0.006 0.003 0.004 0.009
resistance (OD
Diff)
Ozone Fade 0.884 0.53 3.507 1.827 0.881 0.482 2.518 1.732
Mottle 1.699 1.871 1.757 1.427 2.777 1.833 1.92 2.985
[00202] The print performance characteristics of the color set using ink A, B,
C, D, E, F and G
printed with HP Photosmart Pro B9180 printer is identified below:
62
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Table 22
HP MP- ColorLok Xerox 4200
Black Yellow Cyan Magenta Black Yellow Cyan Magenta
Optical Density 1.099 1.084 0.97 0.928 0.839 0.901 0.842 0.79
Rub Resistance 0.05 0.01 0.06 0.02 0.02 0.01 0.04 0.01
(OD Diff)
Highlighter A 0.135 - 0.085 0.108 0 - 0.019 0
Resistance (OD
Diff)
Highlighter B 0.051 - 0.041 0.019 0.01 - 0.021 0.002
Resistance (OD
Diff)
Water 0.01 0.008 0.012 0.018 0.005 0.002 0.006 0.014
resistance (OD
Diff)
Ozone Fade 1.798 1.101 2.703 2.497 1.28 1.112 2.167 1.656
Mottle 1.595 1.141 1.768 2.85 2.064 1.681 1.336 2.071
Table 22 (Continued)
Office Depot 104 Hammerill GW
Black Yellow Cyan Magenta Black Yellow Cyan Magenta
Optical Density 0.906 0.917 0.906 0.911 0.781 0.835 0.848 0.834
Rub Resistance 0.01 0.01 0.04 0.01 0.03 0.01 0.05 0.01
(OD Diff)
Highlighter A 0.017 - 0.011 0.017 0.037 - 0.032 0.023
Resistance (OD
Diff)
Highlighter B 0.005 - 0.013 0.007 0.02 - 0.032 0.009
Resistance (OD
Diff)
Water 0.004 0.002 0.003 0.011 0.007 0.003 0.004 0.011
resistance (OD
Diff)
Ozone Fade 1.063 0.983 2.693 1.68 1.398 0.839 1.94 1.417
Mottle 1.671 1.534 1.413 1.88 3.161 3.35 2.53 2.773
[00203] The print results show that the pigment dispersions produced by the
process described
in the corresponding experiments produce dispersions suitable to make high
quality pigmented
inkjet inks. Side by side comparison of these prints with print output from
the dispersions made
63
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
by the process disclosed in U.S. Patent Publication No. US20090050014A1,
published Feb 26,
2009 which is hereby incorporated by reference, shows that these prints are
more vibrant and
equally durable.
Example 46
Wood Stain Application Performance
[00204] The following wood stains were prepared and tested at 6% dry pigment
loading with
a resin solution consisting of 18% Joncryl 95 (available from BASF) and the
balance de-ionized
water. Waterfastness comparison of drawdowns on Leneta Form 3NT-3 using a wire
wound
rod#7 (available from Paul N. Gardner Company, Pompano Beach, FL) was done
with 1"x4"
strips. Half of each strip was dipped in de-ionized water for one minute. The
strips were
allowed to dry at ambient temperature. The color difference (DE*) was read
with Datacolor
SF600 PLUS-CT colorimeter. Lower DE* indicates better waterfastness.
Table 23. Wood stain comparison.
Example Pigment Dipped area vs. Control
[#] Type DL* Da* Db* DC* DH* DE*
24 Carbon 15 0.00 0.00 -0.01 -0.01 0.00 0.01
22 PR122 13 0.34 0.42 -0.23 0.47 -0.10 0.59
23 PY74 -0.37 0.22 -1.15 -1.16 -0.17 1.23
21 PB15 -0.27 0.25 -0.09 -0.02 0.27 0.38
Example 47
Coating Performance
[00205] The following coating formulations (Masstone) were prepared and tested
at 6% dry
pigment loading with a resin solution consisting of 25% acrylic vehicle
(available from Valspar,
Wheeling, IL) and the balance de-ionized water. Each Masstone color was mixed
with a latex
based tint base (available from Sherwin Williams, Cleveland, OH) at 1:10 ratio
for the tint
preparation. The drawdown was prepared on Leneta form 2A using a 6.0 mil wire
wound rod.
Chemical resistance was measured separately by spotting 10 drops of 10%
hydrochloric acid and
64
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
drops of 10% sodium hydroxide solution on a Masstone drawdown. The degree of
chemical
resistance is measured by taking the DE* value between the spotted area versus
the control area.
Table 24. Coating resistance to strong acid (10% Hydrochloric acid).
Example Pigment Spotted area vs Control
[#] Type DL* Da* Db* DC* DH* DE*
24 Carbon 0.11 0.01 -0.19 -0.11 -0.16 0.22
22 PR122 -0.43 -1.67 -0.76 -1.79 -0.41 1.88
23 PY74 -0.73 0.01 -1.15 -1.13 -0.23 1.36
21 PB15 12 0.12 -0.63 1.38 -1.47 0.35 1.52
Table 25. Coating resistance to strong base (10% Sodium hydroxide).
Example Pigment Spotted area vs Control
[#] Type DL* Da* Db* DC* DH* DE*
24 Carbon 15 1.15 -0.02 -0.04 -0.04 -0.01 1.15
22 PR122 13 0.25 3.93 1.46 4.17 0.48 4.20
23 PY74 -22.86 20.31 -35.84 -22.93 -34.22 47.11
21 PB15 12 -0.95 -1.53 1.49 -2.11 -0.34 2.34
Example 48
Color Filter Application Performance
[00206] The following color filter formulations were prepared and tested at 6%
dry pigment
loading adjusted to 75% of the total with de-ionized water and then mixed with
a vehicle (25%)
consisting of 30% Valspar acrylic vehicle, 30% Joncryl 1972 (available from
BASF) and 40% 1-
methoxy-2-propanol (Propylene Glycol Monomethyl Ether). Transmission values of
the color
filter coatings on a transparent olefin polymer substrate using a wire wound
rod #7 (Paul N.
Gardner Company, Pompano Beach, FL) were measured after drying at ambient
temperature.
Table 26. Transmission Values of Color Filter Coatings.
Example Pigment % Transmittance (nm)
[#] Type 400 440 480 520 560 600 640 680
24 Carbon 1.54 2.20 2.98 3.90 4.81 5.55 6.47 7.45
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
22 PR122 62.05 66.44 57.49 36.60 23.90 65.52 80.51 83.28
23 PY74 8.51 2.07 5.05 59.92 74.48 79.85 82.76 84.53
21 PB15 43.99 73.00 81.92 71.97 22.55 3.83 3.38 6.03
Example 49
Textile Printing Application Performance
[00207] The following printing pastes were prepared and tested at 6% dry
pigment loading
with Delta Ceramcoat Textile Medium (33%) (available from Delta), Valspar
Acrylic Vehicle
(5%) and the balance de-ionized water. The drawdowns of the print pastes on a
white cotton
fabric were prepared using a 6.0 mil wire wound rod. After drying at ambient
temperature the
prints were heat fixed at 140 C for 10 minutes in an oven. The fabric was cut
into 1"x4" strips
and half of each strip (1"x2") was immersed in boiling de-ionized water for
five minutes.
Afterwards, the exposed strips were washed in cold tap water for one minute
and allowed to dry
at ambient temperature. The washfastness and waterfastness were assessed by
measuring the
total color difference (DE*) between control and treated fabric.
Table 27. Wash and Water Fastness Evaluation.
Example Pigment Washed Fabric vs Control
[#] Type DL* Da* Db* DC* DH* DE*
24 Carbon -0.04 0.02 0.21 0.21 0.06 0.22 15 22 PR122 13 1.37 10.54 2.60 10.75
1.47 10.94
23 PY74 4.20 -0.57 7.77 7.66 1.41 8.85
21 PB 15 12 -0.80 0.28 -0.34 0.40 0.18 0.92
Example 50
Cosmetic Application Performance
[00208] The following mascaras (AG8-106A and Glycerine-Water Control) were
prepared
according to the procedures described below and were tested as visual color
strength.
66
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
[00209] The wax base included Caprylyl Methicone (Dow Coming FZ-3196), Lauryl
PEG/PEG-18/18 Methicone (Dow Corning DC 5200) and C30-45 Alkyldimethylsilyl
Polypropylsilsesquioxane (Dow Corning DC SW-885 C30 Resin Wax) as emollient,
emulsifier
and film former respectively.
[00210] The pigment dispersions tested included the following:
1. TW 1829 Original formula (isododecane control) has Isoblack 902 AT20 which
is 20%
Unipure Black LC 902 in Isododecane. (Isoblack 902 AT20 and Unipure Black LC
902
are available from Sensient Cosmetic Technologies.)
2. Glycerine-Water control (AG8-112A) has Noir Covarine W9793 which is 25%>
Unipure
Black LC 902 in Water/Glycerin (Noir Covarine W9793 is available from Sensient
Cosmetic Technologies.)
3. AG8-106A has Example #28 which is 15.85% SDP Carbon Black dispersion in
water.
[00211] The procedure followed included the following steps:
(1) Preparation of Phase A by mixing with a propeller blade at 85 C the
ingredients
indicated in Table 28 with a propeller blade to get a homogeneous mix.
(2) Add Phase B with mixing.
(3) Prepare Phase C, mix at 60 C to get a homogeneous mix.
(4) Add Phase C to bulk, emulsify using Turrax homogenizer for 2 minutes.
(5) Add and mix Phase D to bulk and de-aerate.
(6) Pour into an appropriate container at 60 C.
Table 28. Formula using dispersions from Example 28 and Glycerine-Water
Control.
Example Glycerine-Water
#28 Pi~Zment
Dispersion
Phase A AG8-106A AG8-112A
%W/W %W/W
Dow Corning FZ-3196 4.00 4.00
Dow Corning 5200 Formulation Aid 6.00 6.00
Camuaba Wax 2.50 2.50
Dow Corning SW-8005 C30 Resin Wax 4.00 4.00
67
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
Caprylic/Capric Triglyceride 3.00 3.00
Phase B
Covabead PMMA 2MUSI (available from 10.00 10.00
Sensient)
Phase C
SDP Carbon Dispersion (1.60 % dry) 10.11 -
Noir Covarine W9793 (1.60% dry) - 6.40
Preservative (Germaben II) 1.00 1.00
Propylene glycol 2.50 2.50
Pure Water 11.89 15.60
Covacryl P12 (available from Sensient) 25.00 25.00
Phase D
Isododecane 20.00 20.00
[00212] The procedure below was followed for the preparation of the
isododecane dispersion
control:
Table 29. Formula for isododecane dispersion control (TW 1829).
% W/W
Phase Dow Corning FZ-3196 4.00
A
Dow Corning 5200 Formulation Aid 6.00
Camauba Wax # 104F 2.50
Dow Corning SW-8005 C30 Resin Wax 4.00
Caprylic/Capric Triglyceride 3.00
Phase B Covabead 2 MUSI 10.00
Phase Isoblack 902 AT20 8.00
C
Phase Isododecane 20.00
D
Phase E Pure water 14.00
Propylene glycol 2.50
Preservative 1.00
Phase F Covacryl P12 25.00
(1) Prepare, heat and mix Phase A (same as in Table 28) to 75 C until
homogenous.
(2) Add Phase B (same as in Table 28) to A while mixing using a propeller
blade
mixer.
68
CA 02735049 2011-02-22
WO 2010/022377 PCT/US2009/054700
(3) Add Phase C (Isoblack 902 AT20, 8.0 g) to Bulk under stirring. Maintain
temperature for < 2 minutes.
(4) Cool Bulk to 65 C and add Phase D (Isododecane, 20.0 g), mix until
homogenous.
(5) Add Phase E (pure water 14.0 g, propylene glycol and preservative 1.0 g)
to bulk,
emulsify using Turrax homogenizer for 2 minutes.
(6) Add Phase F (Covacryl P12, 25.0 g) to bulk and mix.
(7) Pour into an appropriate container at 55 C.
[00213] The three mascaras (AG8-106A, Glycerine-Water Control and Isododecane
Control
(see above for the preparation) were evaluated for in vivo color performance,
as shown in Figure
37. The color of the mascara made with Example # 28 (AG8-106A) is deeper than
the other two
samples.
69