Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
METHODS AND APPARATUS FOR THERMAL DRILLING
RELATED APPLICATIONS
[0001] This application claims priority to U.S.S.N 61/103,859, filed October
8, 2008;
U.S.S.N. 61/140,477 filed December 23, 2008; U.S.S.N. 61/140,489, filed
December 23, 2008;
U.S.S.N. 61/140,512, filed December 23, 2008; and U.S.S.N. 61/236,958, filed
August 26,
2009, each of which is hereby incorporated by reference in its entirety.
FIELD
[0002] In various embodiments, this disclosure relates to methods and
apparatus for
conducting processes capable of spalling or penetrating a material such as
rock. For example,
the disclosed methods may be used for preparing boreholes for geothermal
energy systems.
BACKGROUND
[0003] Drilling very deep boreholes or enhancing existing wells in hard rock
far below the
earth's surface, e.g. 10,000 feet deep or more, is inherently incompatible
with traditional
mechanical or contact drilling or rock removal technologies. Low rates of
penetration, extreme
bit and drill string wear, and excessive time spent "tripping" to replace
damaged or worn bits
and drill string make conventional rotary and coiled tubing drilling
economically non-viable for
many deep, hard rock applications.
[0004] Several non-contact techniques have been developed for hard rock
drilling but may
be effective only in shallow and/or air filled boreholes. Most notably, air or
flame jet spallation
drilling uses a hot gas or flame directed against a rock surface to cause
spalling and removal of
the rock. This technique, however, is only feasible in shallow, air-filled
boreholes. To drill
deeper, a borehole must be filled with water or "mud" to provide mechanical
stability. In this
environment, flames are not viable in part because of the difficulty in
generating or maintaining
the required flame under the high pressure water column. For example, the high
pressures at
the bottom of deep, fluid-filled boreholes make behavior of the flames
extremely unstable and
difficult to maintain. Further, initiating combustion under these conditions
is extremely
challenging and typically requires an energy source to be provided at the
bottom of the
borehole. However, using an energy source such as a spark or glow plug would
require, e.g., a
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-2-
power cable to be run from the surface, which is not feasible in deep
applications. Other
energy sources such as flame holders are inherently unstable, especially at
such depths.
[0005] Further, most combustion reactions produce very high temperature
flames, typically
1800-3000 C or more. Such temperatures can destroy drilling components and
require careful
addition of cooling water to maintain a temperature that can be withstood by
downhole tools.
In addition, such high temperatures can melt rock (e.g., into an amorphous
glass) so that the
rock is then unspallable. Even a momentary interruption in cooling water can
transform rock
so that it can no longer be spalled and/or destroy downhole components, even
if a cooler
temperature is recovered. Small changes in the stand-off distance, or distance
from the
combustion to the rock surface, can result in dramatic changes in the nature
of the high
temperature flame impingement, which may result in a temperature too low for
spallation, or
temperatures high enough to soften or melt the rock. Such tight tolerances for
stand-off
distances are difficult to control at the bottom of a deep borehole.
[0006] Further, flame-based combustion systems require multiple conduits for
fuel, oxidant
and cooling or circulating water. Other approaches to spallation drilling such
as the use of
electrical heating require sufficient power down hole. In deep drilling
operations, multiple
conduits or supply of sufficient power through cables from the surface or
through
transformation of energy by hydraulic flow may not be feasible, or may be
simply impossible.
[0007] Combustion systems that require the use of gaseous oxidants, such as
air or oxygen,
are also unsuitable for deep fluid filled borehole conditions, in part because
the pressures
required to pump these gases against a hydrostatic column of a fluid filled
borehole are
sometimes impossible to achieve, and even if possible, have associated safety
risks.
[0008] While thermal spallation has promised to provide a solution to deep,
hard-rock
drilling, no methods have been able to adequately or feasibly provide the heat
required for
viable spallation drilling deep into a water filled borehole. If the challenge
of drilling deep
boreholes in hard rock is not solved, EGS may not become the much needed clean
alternative
to meeting our current and future global energy needs.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-3-
SUMMARY
[0009] The present disclosure relates, at least in part, to a method of
reducing near wellbore
impedance, or reducing the restriction to fluid flow in the immediate vicinity
(e.g. 1 inch to
about 3 feet) of an existing borehole wall) by providing a spallation system
to e.g. increase the
diameter of a section of an existing borehole or well, for example a
geothermal well.
[0010] For example, one aspect of the invention includes a method for spalling
a geological
rock formation. The method includes providing a housing comprising a reaction
chamber and a
catalyst element held within the reaction chamber, providing at least one jet
nozzle, contacting
one or more unreacted fluids or solids with the catalyst element, wherein the
catalyst element
facilitates the reaction of the unreacted fluid, thus generating a reacted
fluid, and emitting the
reacted fluid through the at least one nozzle. The at least one nozzle may be
directed to an
excavation site within or on the geological rock formation, thereby creating
spalls and/or a
reacted rock region.
[0011] In one embodiment, the unreacted fluid or solid is at a temperature of
about 350 C
or less. In one embodiment, the reacted fluid is about 500 C to about 1100 C
when formed.
The contacting may occur at a pressure of about 1 to about 200 MPa. The
unreacted fluid may
be substantially a liquid.
[0012] One embodiment further includes introducing a flow of water or drilling
mud into
the excavation site. One embodiment further includes heating the unreacted
fluid or solid. The
reacted fluid may interact with a heat exchanger disposed in a position
capable of heating the
unreacted fluid or solid.
[0013] In one embodiment, the method is capable of producing an about 1 inch
diameter
borehole in said geological formation at about 0.5 inches per minute of
reacted fluid flow. In
one embodiment, the method is capable of producing an about 8 inch diameter
borehole in said
geological formation at a rate of penetration of about 20 feet per hour or
more. The flow of
water or drilling mud may at least partially form an ascending fluid stream.
The ascending
fluid stream may at least partially remove the spall.
[0014] In one embodiment, the catalyst element may include a transition metal,
such as a
transition metal chosen from: platinum, lead, silver, palladium, nickel,
cobalt, copper,
chromium, manganese, iridium, gold, ruthenium and rhodium, or mixtures or
oxides or salts
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-4-
thereof. The transition metal may be disposed on a support. The catalyst
element may be
disposed on spheres, grains, pellets, or other appropriately configured
elements comprising
alumina. The catalyst element may have at least about 10 m2/g surface area of
catalyst. The
catalyst element may be heated.
[0015] In one embodiment, the unreacted fluid includes an aqueous solution.
The
unreacted fluid may be a miscible fluid mixture or a non-miscible fluid
mixture. The unreacted
fluid or solid may include an oxidant. The unreacted solid may include an
encapsulated
oxidant.
[0016] In one embodiment, the unreacted fluid or solid includes a fuel. The
fuel may be a
carbonaceous fuel. The fuel may include hydrocarbons. The fuel may be a liquid
fuel at room
temperature. The fuel may be a hydrocarbon gas, such as methane, ethane,
propane, butane
(e.g. natural gas (NG) and/or liquefied natural gas (LNG)) at room
temperature. In one
embodiment, the fuel is gasoline, diesel, kerosene, biodiesel, or alcohol. In
one embodiment,
the fuel includes an alcohol, an alkyl, alkenyl, alkynyl, an alkoxyalkyl, or
combinations thereof.
In one embodiment, the fuel is an alcohol fuel. In one embodiment, the
unreacted fluid may
include an alcohol fuel chosen from methanol, ethanol, propanol, or butanol.
[0017] In one embodiment, the oxidant may be chosen from oxygen, peroxide,
permanganate and combinations thereof. In one embodiment, the oxidant may be
hydrogen
peroxide or metal peroxide. In one embodiment, the unreacted fluid may include
hydrogen
peroxide or metal peroxide. The unreacted fluid may include an aqueous
solution comprising
about 2% to about 35% by weight hydrogen peroxide. The unreacted fluid may
include about
10% to about 20% by weight methanol or ethanol. The unreacted fluid may
include an aqueous
solution including about 10% to about 20% by weight hydrogen peroxide and
about 10% to
about 20% by weight methanol or ethanol. In one embodiment, the unreacted
fluid may have a
density similar to water.
[0018] The method may further include transporting the unreacted fluid to the
housing
through at least one conduit. The fuel and oxidant may be transported to the
housing through
separate conduits, or through the same conduit.
[0019] Another aspect of the invention includes a method for flamelessly
penetrating or
reacting rock. The methods includes contacting a composition comprising an
oxidant with a
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-5-
catalyst to flamelessly form a reacted fluid, and directing said reacted fluid
to said rock, thereby
effecting penetration of the rock and/or forming a reacted rock region.
[0020] In one embodiment, the contacting step occurs in the presence of a
fuel. In one
embodiment, the composition includes an alcohol fuel, such as ethanol or
methanol. The
oxidant may include oxygen or hydrogen peroxide.
[0021] In one embodiment, the method further includes drilling the reacted
rock region
with a drill bit. The contacting may occur at about 5,000 ft to about 40,000
ft below a surface
of the earth.
[0022] Another aspect of the invention includes a method for producing a
reacted fluid
flow capable of spallation of rock. The method includes contacting an
unreacted fluid with a
catalyst element in the presence of an oxidant thereby generating a reacted
fluid, and emitting
the reacted fluid through a nozzle, thereby producing the reacted fluid flow
capable of spalling
rock.
[0023] In one embodiment, the reacted fluid is at a temperature of about 500
C to about
900 C. In one embodiment, the reacted fluid produces a heat flux of about 0.1
to about 10
MW/m2 when said reacted fluid is in contact with the rock. The unreacted fluid
may be
substantially a liquid. The reacted fluid may be substantially a gas or a
supercritical fluid. The
unreacted fluid may include a fuel. The unreacted fluid may further include an
aqueous
solution. The unreacted fluid may be a miscible fluid mixture. The unreacted
fluid may
include an alcohol, such as an alcohol chosen from methanol, ethanol, propanol
or butanol. In
one embodiment, the oxidant may be oxygen. In one embodiment, the oxidant may
be a
peroxide. In one embodiment, the oxidant is hydrogen peroxide. In one
embodiment, the
unreacted fluid comprises the oxidant. In one embodiment, the catalyst
comprises a transition
metal, such as a transition metal chosen from silver, lead, gold, platinum,
palladium, or nickel.
The reacted fluid may include water.
[0024] Another aspect of the invention includes an apparatus for excavating a
borehole in a
geological formation. The apparatus includes a housing, a reaction chamber
within the
housing, a catalyst element held within the reaction chamber, and at least one
jet nozzle in fluid
communication with the reaction chamber.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-6-
[0025] In one embodiment, the apparatus further includes at least one conduit
in fluid
communication with the reaction chamber and adapted to transport an aqueous
solution to the
reaction chamber. In one embodiment, the apparatus further includes a heat
exchanger
positioned above the reaction chamber, wherein the heat exchanger is adapted
to transfer heat
between the aqueous solution being transported within the at least one conduit
and a fluid
passing around the heat exchanger. In one embodiment, the catalyst element may
include a
metal catalyst bed. The catalyst element may include a transition metal.
[0026] In one embodiment, the apparatus may further include a single jet
nozzle, or a
plurality of jet nozzles. The at least one jet nozzle may be directed
substantially along an
elongate axis of the apparatus. At least one of the plurality of jet nozzles
may be directed at an
acute angle to an elongate axis of the apparatus. The at least one jet nozzle
may have a
diameter ranging from approximately 0.01 inches to approximately two inches.
The single jet
nozzle may be a center jet nozzle or a non-rotating peripheral gap ring
nozzle.
[0027] These and other objects, along with advantages and features of the
present invention
herein disclosed, will become more apparent through reference to the following
description, the
accompanying drawings, and the claims. Furthermore, it is to be understood
that the features of
the various embodiments described herein are not mutually exclusive and can
exist in various
combinations and permutations.
BRIEF DESCRIPTION OF THE DRAWINGS
[0028] In the drawings, like reference characters generally refer to the same
parts
throughout the different views. Also, the drawings are not necessarily to
scale, emphasis
instead generally being placed upon illustrating the principles of the
invention. In the
following description, various embodiments of the present invention are
described with
reference to the following drawings, in which:
[0029] FIGS. lA-lE are schematic views of a spallation process, in accordance
with one
embodiment of the invention;
[0030] FIG. 2A is a schematic top view of a drill head for a thermal
spallation system, in
accordance with one embodiment of the invention;
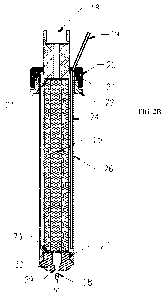
[0031] FIG. 2B is a sectional side view the drill head of FIG. 2A;
[0032] FIG. 2C is a schematic bottom view of the drill head of FIG. 2A;
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-7-
[0033] FIG. 2D is an end view of the drill head of FIG. 2A positioned against
a rock
interface;
[0034] FIG. 2E is a side view of the drill head of FIG. 2A positioned against
a rock
interface;
[0035] FIG. 3A is a schematic side view of a thermal-abrasive reaming system,
in
accordance with one embodiment of the invention;
[0036] FIG. 3B is a schematic sectional side view of the nozzle and reamer of
the thermal
spallation-abrasive reaming system of FIG. 3A;
[0037] FIG. 4A is a schematic side view of a composite thermal spallation and
tricone
roller bit drill system, in accordance with one embodiment of the invention;
[0038] FIG. 4B is a sectional side view of the nozzle and tricone drill bit
for the thermal
spallation and tricone roller bit drill system of FIG. 4A;
[0039] FIG. 4C is an end view of the nozzle and tricone drill bit of FIG. 4B;
[0040] FIG. 5 is a schematic sectional perspective view of a spallation system
and PDC
drag drill bit, in accordance with one embodiment of the invention;
[0041] FIG. 6A is a schematic sectional side view of a thermal spallation
system and a
milling/abrasive drill bit, along with an induction type heater system, in
accordance with one
embodiment of the invention;
[0042] FIG. 6B is an end view of the thermal spallation system and a
milling/abrasive drill
bit of FIG. 6A;
[0043] FIG. 7A is a schematic side view of a spallation system and hammer
drill bit, in
accordance with one embodiment of the invention;
[0044] FIG. 7B is an end view of the spallation system and hammer bit of FIG.
7A;
[0045] FIG. 8 is a graphical representation of thermal effects on the strength
of plagioclase
feldspar, in accordance with one embodiment of the invention;
[0046] FIG. 9 is a graphical representation of differential stress vs. strain
on natural quartz
crystals at various temperatures both dry and water saturated, in accordance
with one
embodiment of the invention;
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-8-
[0047] FIG. 10 is a graphical representation of an experimentally determined
melting curve
for water saturated granite mixture vs. pressure, in accordance with one
embodiment of the
invention;
[0048] FIG. 11 is a sectional side view of the convergent radial flow reactor;
[0049] FIG. 12A and 12B are schematics of convergent and divergent radial flow
reactors;
[0050] FIG. 13A, 13B, and 13C show views of a rock core confinement system for
laboratory drilling demonstrations;
[0051] FIG. 14 is an image of a cross section of a 24" x 24" x 36" Sierra
White Granite
block after being drilled, in accordance with one embodiment of the invention;
[0052] FIG. 15 shows a graph of wear rates of PDC and TSP cutters against hard
granite as
a function of temperature;
[0053] FIG. 16 shows a graph of the relative shear strength as a function of
the ultimate
temperature for two example granites;
[0054] FIG. 17 is an image of a 4" diameter, 6" long, rock core with a drill
head therein, in
accordance with one embodiment of the invention;
[0055] FIG. 18 is an image of a 4" diameter, 6" long rock core where an
initial predrilled
borehole (represented by the dotted line) is opened, increasing the borehole
diameter and
producing a thermally affected zone, in accordance with one embodiment of the
invention;
[0056] FIG. 19 A-D show schematic views of a fracture intersecting a wellbore:
(A) with
high near wellbore impedance; (B) globally opened; (C) to reduce the near
wellbore
impedance; and (D) with the fracture preferentially opened to produce to
reduce near wellbore
impedance;
[0057] FIG. 20 is an image of a slabbed Granodiorite sample subjected to
spallation drilling
followed by a dye penetrant which indicates a zone of microfracturing and
several distinct
linear fracture zones emanating perpendicular to the borehole region, in
accordance with one
embodiment of the invention; and
[0058] FIG. 21 shows a graph of spalled particle size distribution for an
example thermal
spallation drilling system.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-9-
DESCRIPTION
[0059] The present disclosure relates, at least in part, to methods and
systems for use in
spallation, fracturing, loosening, or excavation of material such as rock, for
example, methods
of making or excavating boreholes, and/or enlarging existing boreholes. Such
methods include
using a disclosed working fluid or reacted fluid, e.g. a working fluid capable
of producing a
heat flux of about 0.1 to about 50 MW/m2 when in contact with rock.
Methods
[0060] For example, provided herein are systems and methods that may be
capable of
creating 20 feet of an e.g., 8 inch borehole in about hour, or 20 feet of a 4
inch borehole in
about an hour or less, or about a 0.2 inches of -i inch borehole in about 4
minutes. Also
provided herein are systems or methods for opening a length of existing
borehole, e.g. with an
original diameter of that may be as small as 4 inches, to a final diameter of
about 36 inches or
more, which in some embodiments may be accomplished in 12-24 hours, or days.
Contemplated systems and methods may be used to create boreholes, shafts,
caverns or tunnels
in a target material such as crystalline rock material, silicate rock, basalt,
granite, sandstone,
limestone, peridotite, or any other rocky material. Disclosed systems and
methods may also be
used for producing multilaterals from an existing borehole, which in turn may
be opened. In
certain embodiments, disclosed systems and methods may be used, for example,
to create
vertical boreholes, horizontal boreholes, deviated boreholes, angled
boreholes, larger diameter
boreholes, curved boreholes, or any combination thereof. Also provided herein
are systems and
methods that may spall rock at a rate of about 100 ft3/hour or more, which may
be useful for
example for the creation of tunnels, caverns, mineshafts, and the like.
[0061] For example, also provided herein are methods to reduce existing
wellbore
impedance and/or improve production of existing wells (e.g. EGS wells). Such
methods may
include, for example, increasing the diameter of at least portions (e.g. a
working, producing, or
production zone or portion - one or more sections that are typically
significantly downhole,
may be uncased, or cased with slotted or perforated casing, and where
substantially most of the
energy output or fluid production occurs, for example, in an EGS well) of an
existing wellbore.
[0062] The systems and methods disclosed herein may include sensors such as
gyroscopes,
magnetometers, and/or inclinometers, for monitoring the orientation of the
drilling systems.
Systems and methods may also include at least one of temperature and/or
pressure sensors,
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-10-
flow sensors, natural rock gamma ray sensors, resistivity/conductivity sensors
and rock and/or
pore space density sensors, to identify rock properties and hydrologic
conditions that may
influence the desired trajectory, for example, of the borehole/drill hole. For
example, sensors
may be provided to selectively monitor flow entry points and/or temperature
changes of fluids
that will influence the target which influences desired direction of drilling
or hole opening. In
one embodiment, the methods and systems described herein provide for deep
borehole drilling,
for example from approximately 1,000 feet to about 50,000 feet, or 5,000 feet
to about 50,000
feet, or about 10,000 feet to approximately 50,000 feet below the surface, or
more. In other
embodiments, methods and systems described herein provide for hole openings in
e.g.
production zones of a wellbore. One or more wellbore diameters may be
increased by about
0.1 to 10 feet or more. In other embodiments, for example, substantially
perpendicular holes
relative to a production zone of an existing well can be formed that may be
about 1 to about
1,000 feet or more in length. Also contemplated herein are the formation of
parallel/collinear
slots, multilaterals (similar to branching of a tree) or horizontal
deviations, which may be used
to increase production from e.g. a single, substantially vertical wellbore.
These multilaterals
may be further hole opened.
[0063] For example, provided herein are systems and/or methods that may be
configured
for drilling boreholes in hard rock for geothermal, enhanced or engineered
geothermal systems
(EGS), and/or oil and gas applications, natural gas production or enhanced oil
recovery or
unconventional oil production, using a disclosed working fluid to spall rock.
However, the
systems and methods described herein may also be used for other applications
such as, but not
limited to, exploratory boreholes, test boreholes, boreholes for scientific
study or resource
assessment, quarrying, ground source heat pumps, water wells, resource mining
(conventional
or solution mining), combined HDR (hot dry rock) solution mining, gas or
liquefied natural gas
(LNG) applications, CO2 sequestration capture or storage, storage of water or
other resources,
nuclear waste disposal, thermal or supercritical oxidations of wastes,
downhole chemical
processing and/or tunnel or cavern creation (new or in conjunction with an
existing well).
[0064] For example, methods are provided herein for increasing the diameter
along a
section of an existing geothermal well or borehole, for example, methods are
provided for
creating substantially axial (i.e. substantially parallel/collinear with the
wellbore) slots along
the length of a working portion or production zone of an existing borehole,
methods of
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-11-
perforating an existing borehole (e.g. creating holes substantially
perpendicular to the
wellbore); methods for creating radial branches off of and/or stemming from an
existing
borehole (e.g. intersecting a production zone); and/or methods of creating
one, two, or a
plurality of substantially axial slots along a length of an existing borehole,
wherein the methods
include using a disclosed working fluid. The axial slots or radial branches
may be oriented, in
some embodiments, so as to intersect the greatest number of fractures or to be
facing the
injection well. Also contemplated herein are methods for substantially
expanding the diameter
of a wellbore along a given length, or for removing a portion of material by
spallation, whereby
the spallation induces further fracturing, collapse or break-out of the rock
wall.
[0065] Methods contemplated herein also include hydrothermal reactions,
explosions or
detonations, which take place in the wellbore or fractures for only a finite
period. For example,
an unreacted fluid may be pumped into the wellbore and/or allowed to penetrate
the fractures.
A reaction may then be initiated by e.g. a catalyst "pill" sent down the drill
string or by
exposing a sample of catalyst in a downhole tool, initiating a hydrothermal
reaction and
causing spallation in fractures and macrofracturing in wellbore.
[0066] Alternatively, the wellbore may be cooled by traditional means of
circulating fluids.
An unreacted fluid which has a Self Accelerating Decomposition Temperature
(SADT) - a
temperature at which reaction runs away and propagates - that is below the
formation
temperature may then be injected into the wellbore and fractures. As the
formation is allowed
to recover from the cooling treatment, the reaction may initiate, with or
without the use of a
catalyst.
[0067] In some embodiments, two or more components of the unreacted fluid,
e.g. fuel and
oxidant may be delivered through the conduit in "slugs" so that there is no
chance of a
premature reaction in the conduit. Once the desired mixture of e.g. fuel and
oxidant have been
created in the wellbore, the reaction can be initiated by e.g. a catalyst
pill, exposing a catalyst in
the tool, auto-initiated, or by allowing the wellbore to warm. Since high
concentrations of e.g.
fuel and oxidant can be delivered by this "slug" flow, it may be possible to
produce an
unreacted fluid mixture e.g. above the detonation limits which allows for
propagation of the
reaction and shockwave throughout the producing zone and/or fractures,
creating spallation and
fracturing.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-12-
[0068] In general, as discussed herein, "spallation" refers to the breaking
away of surface
fragments of a material, e.g. rock "spall" refers to the fragments of material
formed by a
process of spallation. A thermal spallation process can refer to a spallation
process that uses a
working fluid other than air, such as working fluid that includes water (e.g.,
hydrothermal
spallation resulting from the creation of high temperature water from
hydrothermal oxidation
reaction as disclosed herein), water or oil based drilling muds, supercritical
fluids, and the like.
[0069] Disclosed herein, in an embodiment, is a spallation method that may use
a means,
for example, a hydrothermal means, a flameless means and/or a self-energized
means, e.g., a
means that does not use a separate energy source to initiate or generate a
chemical reaction to
produce a heated, working fluid and/or a means that does not include a flame.
For example, a
flameless chemical means may include a reaction such as a hydrothermal
oxidation reaction, or
a reaction that includes a physical change in the reacting fluids, e.g., a
phase change and/or
solvation. An exemplary hydrothermal oxidation reaction is the catalyzed
reaction of aqueous
methanol and aqueous peroxide. It is understood by a person skilled in the art
that a flameless
hydrothermal reaction refers to an exothermic reaction that produces heat but
does not produce
a flame. A flameless reacted fluid is the product of a flameless hydrothermal
reaction. For
example, a contemplated hydrothermal oxidation reaction may produce visible
light through
diffuse ionization, but does not produce light from a flame, as does
combustion. In some
embodiments, contemplated reactions are aqueous and flameless. Such reactions
are
substantially stable in the presence of water or increased temperature or
pressure.
Contemplated reactions are distributed through water so the reacted
temperature may be
produced at a desired temperature (e.g., below the limits of tool construction
or at a desired jet
temperature) without e.g. requiring mixing of cooling water. In some
embodiments,
contemplated fuel and/or oxidant may be delivered to the drill head down a
single conduit at
e.g., near pressure balance with the fluid in the borehole.
[0070] Such means may allow the application of a working fluid to a surface
zone of a
target material such as a hard and/or crystalline rock with substantially high
heat flux.
Provided herein, for example, are means to form a working fluid for e.g.
borehole creation or
borehole enlargement which may produce a heat transfer capability of about 0.1
to about 20
MW/m2, or about 1.0 to about 30 MW/ma, about 0.5 MW/m2 to about 8 MW/ma, about
0.1
MW/m2 to about 8 MW/ma, or about 2 MW/m2 to about 7 MW/ma, when in contact
with the
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
- 13-
material. For example, provided herein are means to form a working fluid may
produce a heat
flux of about 0.1 to about 10 MW/m2, or about 1.0 to about 10 MW/ma, about 0.5
MW/m2 to
about 8 MW/m2, or about 1 to about 8MW/m2 or about 2 MW/m2 to about 7 MW/m2,
when in
contact with the material.
[0071] In an alternative embodiment, provided herein are means for producing a
working
fluid having a heat flux of about 0.01 to about 10 kW/m2 when in contact with
material. Such a
heat flux may be used to form e.g., caverns, tunnels and mineshafts, or for
enlarging the
diameter of an existing borehole, for example, using a lower heat flux
process.
[0072] In some embodiments, the disclosed methods, means, and apparatus are
capable of
achieving and/or maintaining (in for example, a reaction chamber) or directing
a reacted fluid
towards e.g. a rock surface at a temperature that is not substantially higher
than a certain
desired temperature (for example not substantially higher that the desired
working fluid or the
limits of materials of construction of the system and/or apparatus), e.g. to
achieve and/or
maintain a reacted fluid temperature between about 500 C (or about 500 C
above the ambient
rock temperature), and about 900 C, or about the temperature of rock fusion
and/or brittle
ductile transition. In some embodiments, maintaining such a reacted fluid
temperature may be
more advantageous as compared to known techniques such as air spallation
and/or flame
spallation, which can use high combustion temperatures that can induce melting
or fusing of
rock or can damage downhole hardware. For example, FIG. 8 depicts brittle
ductile
measurements on feldspar samples under no loading and with overburden pressure
applied to
the material. It will be appreciated that the temperature that induces melting
or fusing of rock,
or the brittle/ductile transition may vary with the type and/or nature of
rock. For example, FIG.
9 depicts the relationship between differential stress and strain on natural
quartz crystals for
variations in temperatures and water content, while FIG. 10 shows how the
melting curve for
water saturated granite is affected by pressure. Furthermore, it can be
appreciated that using a
heat source which exceeds this temperature may lead to undesirable
transformation of the rock,
such as melting or softening. For example, if it occurred, such undesired
melting or softening
may impede further spallation.
[0073] In some embodiments, such a temperature and/or heat flux is necessary
for the
spallation of rock by e.g. creating enough heat flux to remove spalls while
e.g. substantially
maintaining a temperature that does not e.g. degrade materials of construction
and/or fuse or
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-14-
soften rock, minerals or grain boundaries which may make rock substantially
more difficult to
spall. For example, applying a working fluid having substantially high heat
flux when in
contact with rock may cause grains within the rock to expand and thereby
produce
microfractures within the rock. The growth of such microfractures may result
in a fractured
region that spalls, buckles and/or separates from the surface of the rock or
material. When such
spall is ejected from the rock surface, it exposes fresh material below the
spall, and the spall
process may continue. An exemplary spallation process is shown in FIG. 1. Such
spallation
processes may be easier when, for example, pre-existing stress in rock, e.g.
lithostatic loading
or deviatoric (non-uniform) loading, is present.
[0074] In the thermal spallation process of FIG. 1, a rock 1 has an exposed
surface 3 which
contains, near the surface, a small flaw 2 in the mineral structure. Heat is
applied to the rock
surface 3 by a high temperature source, such as a supersonic flame jet or
hydrothermal jet. The
rock 1 may be subjected to the natural stress found in the ground which acts
on the grain in all
directions, but is typically lowest in a direction perpendicular to the
exposed mineral surface.
As the mineral starts to expand from the applied heat, stresses parallel to
the exposed surface
increase, so the flaw 2 starts to grow 5 to relieve the stress. The flaw may
expand to a size 6
where the grain or portion of the grain 7 is separated from the rock 1,
thereby leaving a void 8
and a fresh surface for further heat transfer and spallation.
[0075] In some embodiments, the heat flux and/or temperature of the working
fluid may be
adjusted to produce or facilitate rock removal processes such as
macrofracturing, dissolution,
partial melting, softening, change in crystalline phase, decrystallization, or
the like. For
example, removal of large volumes of rock such as in the creation of caverns,
mine shafts or
tunnels, or larger hole opening processes, such as reducing near wellbore
impedance, may
require lower heat fluxes.
[0076] Substantially high heat fluxes may produce small spalls, which in turn
may improve
lift (and removal) from the borehole. For example, spalls produced by methods
disclosed
herein are, in some embodiments, approximately less than or about 0.1mm to
about 2.0 mm
thick and may have diameters less than or about 1-20 times, or about 1 to
about 5 times, their
thickness. In some embodiments, spalls may be produced that are less than or
about 0.1mm to
about 2.0mm in all dimensions. In some embodiments, spalls as large as 10 mm
may be
formed; these spalls have significant thermal damage and microfracturing which
may cause
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
- 15-
them to be broken down further in the flow streams or by mechanical forces in
the wellbore
during drilling.
[0077] In some embodiments, such as hole opening using lower heat fluxes,
created spalls
may be on the order of inches to several feet; these spalls may be left in
place, allowed to fall
into an existing cavern or "rat hole"( existing below the production zone), or
may be reduced
and/or removed by a secondary process such as mechanical drilling. Non-removal
of such
formed spalls may be advantageous, e.g. smaller conduits may be needed to
transport fluids to
and from the bottom of the hole. Substantial non-removal of spalls may be
particularly
advantageous if larger spalls are generated by lower heat fluxes. In other
embodiments, any
rock that is removed may intentionally makes the hole less stable, resulting
in break-out or
cave-ins, further expanding the diameter without requiring the complete
spallation of all of the
loosened material.
[0078] In some embodiments, seismic or acoustic monitoring of the fracturing
or the sound
in the section of the borehole may provide information as to the size and
extent of spalling and
the size or shape of the resulting borehole. In other embodiments, the methods
and apparatus
disclosed herein also provide for an additional down hole fluid, which may
improve buoyancy
or lift of cuttings (for example, improved buoyancy in aerated foams, liquid
water or drilling
mud as compared to air used in flame jet spallation) and may, in some
embodiments, assist in
transport of particles to the surface of the wellbore where they can be
separated from e.g., water
using standard oilfield (or geothermal) drilling technologies such as, but not
limited to, shaker
screens, mud pits, and hydro-cyclone de-sanders, and de-silters. In some
embodiments, the
methods of spallation disclosed herein produce substantially smaller cuttings
or spall in
comparison to conventional rotary drill cuttings. In another embodiment, the
methods of
spallation disclosed herein provide for substantial control over the size of
spalls formed, by e.g.
controlling heat flux and/or temperature e.g. of a heated or reacted fluid.
[0079] In another embodiment, application of a high heat flux (e.g. using a
reacted or
working fluid) on the surface of the target material may result in a thermally
affected zone or
reacted rock region. For example, a thermally-affected zone having reduced
mechanical
strength (due to e.g. microfracturing, macrofracturing, softening, and/or
annealing), which may
extend as much as about 1/4 inch or more below the rock surface, may be
created by a disclosed
reacted or working fluid inducing e.g. a substantially high heat flux.
Provided herein is a
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-16-
method for penetrating or reacting rock, e.g. a method for forming a reacted
rock region, which
may be suitable for penetration using conventional mechanical rock drills.
(For example, such
reacted rock region may be easier to drill using mechanical rock drills as
compared to a rock
region that has not been reacted). Such a method may therefore further include
mechanically
drilling, reaming, or otherwise removing the reacted rock, as described below.
For example,
removing the reacted rock may increase the diameter or improve the shape of
the well.
[0080] Near wellbore impedance may occur where fractures intersect a wellbore,
as shown,
e.g., in FIG. 19A. In one embodiment, a method of fracture enlargement is
provided, e.g. to
reduce wellbore impedance, by using a provided working fluid in a wellbore.
Pressure in an
existing well may be controlled, in some embodiments, by e.g., "shutting in
the well", "zonal
isolation" or by "packing off' the length of the borehole being treated such
that the working
fluid is forced into or near fractures (e.g. identified fractures or fractures
along an isolated
zone), inducing spallation or geomechanical changes at the surface of the
fracture, enlarging
the fracture, and thereby resulting in an improvement in the flow of fluids
through the fracture,
as shown, e.g., in FIG. 19D. In other embodiments, the pressure in an existing
well may be
controlled to prevent flow of the fluid into the fractures, by either
maintaining neutrally or
"underbalanced" conditions. In other embodiments, the pressure may be varied
or cycled; this
may assist in blowing produced spalls or fractured rock out of the fractures
or away from the
borehole wall. Pressure or flow may also be cycled to allow for the
measurement of flow and
temperature from the borehole to determine how effective the treatment has
been, or if
additional treatment is necessary. In other embodiments, the wellbore may be
expanded more
globally, by removing the rock in and around the fracture, also leading to a
reduction in
wellbore impedance, as shown, e.g., in FIGS. 20B and 20C. In other
embodiments, the walls of
the borehole can be spalled to create features such as slots or perforations
that may be designed
to better intersect the existing fractures or to weaken the walls of the
wellbore in that location
so as to induce further collapse and expansion of the wellbore, leading to a
further reduction in
impedance. In some embodiments, the reacted fluid may comprise other chemicals
which may
assist in the process of reducing wellbore impedance, e.g. chemicals which
increase or decrease
the solubility of certain minerals. Incorporation of these chemicals either
from the unreacted
fluid or from a separate stream, may be used to prevent minerals from being
dissolved by the
high temperature fluid jet and/or or being redeposited in the cooler
fractures, or may be used to
facilitate dissolution of the minerals in either the spalls or along the
fracture walls. These
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-17-
chemicals may include alcohols e.g. methanol, or bases e.g. hydroxides, or
combinations of the
two, such as alcoxides. Alternatively, these chemicals may include acids, such
as HC1, HF or
the like.
[0081] The disclosed methods and apparatuses of e.g., spalling rock, can be
applied to any
formation of rock, for example, can be applied to a subterranean formation in
which the
hydrostatic head of fluid in the borehole produces a pressure at the bottom of
the borehole that
does not exceed the fracture pressure of the formation. In some embodiments,
during operation
of the disclosed methods, the pressure of a borehole may be maintained below
the formation's
fracture pressure or above the pressure of exposed permeable formations to
prevent inflow. For
example, a drilling mud may be used to vary the hydrostatic pressure in the
borehole or to
create partial isolation of the working zone.
[0082] The methods described herein may further include monitoring properties
(e.g. size,
shape, temperature and/or chemical composition) of the formed spalls and/or
may include
adjusting or monitoring e.g. a working fluid temperature and/or heat flux, to
e.g., optimize rate
of penetration or maintain a pre-determined or desired range of spall sizes.
Such measurements
may be performed by e.g., an optical measurement, seismic measurement, an
acoustic
measurement, a chemical measurement, and/or a mechanical measurement. For
example, fluid
flow and temperature sensors coupled with computational models may be used to
determine
heat flux at e.g. the bottom of the borehole. In some embodiments, chemistry
of the returning
fluid (e.g. fuel, oxidant or combustion products) may be monitored to e.g.
adjust the downhole
reaction conditions or as an indicator of system, e.g., combustion or
oxidation catalyst
efficiency. For example, CO, C02, formaldehyde, formic acid, NO,,, oxygen,
fuel (e.g. alkanes,
methanol or ethanol), or oxidant may be detected in returning fluids as e.g.
indicators of
condition of a catalyst used for oxidation reactions. In another embodiment,
fluid chemistry
(e.g. pH, dissolved minerals, suspended minerals, and agglomerates) may be
monitored in the
returning fluid, which may allow for adjusting additives in the working or
cooling-lift fluid to
reduce or enhance solid or mineral precipitation, agglomeration, dissolution.
Downhole
monitoring of temperature, heat flux, stand-off, and/or borehole geometry by
e.g. temperature
sensors, flow sensors, acoustic monitors, or calipers may allow for
optimization of the drilling
conditions. In other embodiments, standard oilfield and geothermal drilling
methods and
equipment for the measurement of the formation, orientation, and borehole
conditions, e.g.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
- 18 -
measurement while drilling (MWD) or logging while drilling (LWD) systems may
be used, as
well as directional drilling and drilling with casing or casing while drilling
technologies.
[0083] For example, in a disclosed method for hole opening of existing
wellbores, a drill
string deploying the heating system (e.g. the catalyst or combustion chamber
for producing the
reacted fluid) may also contain instrumentation to help identify and locate
the areas of the
working portions to be treated. Once the instrumentation identifies the
regions or fractures, a
drill string can then be pulled up the wellbore to align the jets or nozzles
with the areas to be
treated. A packer or heat shield may be used to separate the instrumentation
from the heat of
the spallation process and to isolate the zone of the borehole to be treated.
Working Fluids and Apparatus
[0084] In some embodiments, the working fluid includes a substantially aqueous
fluid, e.g.
water. Other exemplary fluids include oil or water based drilling mud. The
fluids may be
selected for optimum heat capacity and/or heat transfer properties. In
alternate embodiments, a
working fluid may include a gas such as neon or nitrogen. Contemplated working
fluids may
include by appropriate additives, e.g. viscosifiers, thermal stabilizers,
density modifying
additives such as barite, and those common in oil, gas and/or geothermal
drilling.
[0085] The working fluid may be directed through one or more nozzles, for
example, a
nozzle disposed in a drilling system. Such nozzles may be adapted to direct
the fluid
substantially along an elongate central axis, for example, in a pulsing (e.g.
cyclically pulsing)
flow or a substantially continuous flow. For example, in some embodiments, a
single, centrally
located, non-rotating thermal spallation system may have a reduced number of
moving parts
and reduced mechanical complexity that may result in a substantially
simplified and/or cost
effective system. Minimizing the moving parts within a thermal spallation
system, may allow
stronger and more robust materials to be used in construction of the system,
and therefore the
resulting structure may be better adapted to withstand the high pressures,
temperatures, and
mechanical wear and impact that is generated at the bottom of a borehole
during operation. In
another embodiment, a combination of centrally located and peripheral nozzles
can be used to
optimize heat flux across the surface of the rock, drilling rates, spall size
or borehole geometry.
[0086] For example, such as in hole opening applications provided herein, the
shape of the
openings may be controlled to make features in the walls of existing boreholes
such as
channels, perforations, slots, or multilaterals (multiple branches drilled out
from the existing
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-19-
wellbore). For example, the shape of the openings may be controlled by
controlling spall size,
or may be controlled by the orientation of the nozzles. For example, an
apparatus with at least
one substantially perpendicular nozzle may be slowly run along the length of a
production zone
of an existing borehole, creating a slot. Alternatively, a single
substantially perpendicular jet
may sit on one position in the existing borehole creating a perforation. An
apparatus with
multiple perpendicular jets (within the same or different apparatus) or if the
tool or apparatus is
rotated, a series of holes or parallel slots can be created. The pressure from
the surface pumps
and/or reaction may be used to move the nozzle e.g., towards the rock face to
maintain a small
stand-off. A ring or peripheral gap nozzle can create disc-like openings if
stationary (as shown,
e.g., in FIG. 19B), or open the diameter along the length of the wellbore if
translated. A less
directed or more even heat flux may be applied to open the hole more evenly in
all areas, or in
the areas of greatest existing stress. In an embodiment, methods of reducing
wellbore
impedance are provided that include the use of less focused or directed jets,
jets substantially
axial with the wellbore or with greater stand-off distances or lower heat
fluxes, to produce more
global spalling of the area of a production zone. In some embodiments,
"packers" or plugs
(e.g., cement or ceramic plugs) may be used to isolate the areas of a
production zone to be
treated.
[0087] Also provided herein are apparatuses for spalling rock, such as an
apparatus that
includes a fluid heating means adapted to heat a fluid to a temperature
greater than about 500 C
above the ambient temperature of a surrounding material and less than about
the temperature of
the brittle-ductile transition temperature of the material; and at least one
nozzle adapted to
direct the heated fluid onto a target location on the surface of the material,
wherein the fluid
produces a heat flux of about 0.1 to about 20 MW/m2 at an interface between
the fluid and the
target location, and thereby creating spalls of the material. The nozzles of
the disclosed
apparatuses and systems may include a high temperature resistant material,
e.g. a ceramic or
ceramic composites, metal-ceramic composites, stainless steels, austenitic
steels and
superalloys such as Hastelloy, Inconel, Waspaloy, Rene alloys (e.g. Rene 41,
Rene 80, Rene
95), Haynes alloys, Incoloy, MP98T, TMS alloys, and CMSX single crystal
alloys, metal
carbides, metal nitrides, alumina, silicon nitride, and the like. The
materials may also be coated
to improve their performance, oxidative and chemical stabilities, and/or wear
resistance.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-20-
Chemical Heating
[0088] For example, a disclosed spallation system or apparatus that is capable
of producing
a fluid for use in the disclosed methods and apparatuses may include at least
one jet nozzle, and
a housing including a reaction chamber and, optionally, a catalyst element
held within the
reaction chamber. In operation, unreacted fluids or solids can be contacted
with the catalyst
element within the housing, resulting in the unreacted fluid or solid
reacting, with the catalyst
element and generating a reacted fluid. This reacted fluid may then be emitted
through the at
least one jet nozzle and directed to an excavation site within the geological
rock formation,
thereby creating spalls and/or a reacted rock region. In some embodiments,
contemplated
unreacted fluid or solids react in the presence of a catalyst substantially
self-energized, e.g.,
does not require an additional energy or heat source such as e.g., a spark,
flame holder, flame,
or glow plug to initiate or maintain the reaction and produce the reacted
fluid.
[0089] For example, one or more unreacted fluids or solids (e.g. one or two
unreacted
fluids (e.g. liquids) (which may be the same or different), or one unreacted
fluid and one
unreacted solid, or one or two unreacted solids (which may be the same or
different), may be
contacted with the catalyst element, thereby forming or generating a reacted
or working fluid.
Such reacted fluid may be emitted through at least one nozzle (e.g. one center
nozzle, a ring or
peripheral gap nozzle, or a plurality of nozzles), where the at least one
nozzle is directed to an
excavation site (e.g. bottom hole or against the borehole wall) within or on
the geological rock
formation. The directed reacted fluid may create spalls which may or may not
then be
transported to the top of the hole and/or may create a reacted rock region
e.g., down hole. It
will be recognized by one skilled in the art that discrete spots on the
catalyst may, at times,
exceed the final temperature of the working fluid due to localized heating on
the catalytic
surface, but the reaction is self-energizing and does not require an
additional heat source to be
provided by e.g. a power cable from the surface or an unstable flame holder.
[0090] The unreacted fluid may, in one embodiment have a density similar to
water. This
may be advantageous, for example, in minimizing any pressure differences
between the
unreacted fluid and the fluids in the wellbore. For example, if the density of
the unreacted fluid
is slightly greater than the fluids in the wellbore, any required pumping
pressures for the
unreacted fluid may be reduced.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-21-
[0091] Contacting unreacted fluids or solids with the catalyst may occur at a
pressure of for
example, about 1 to about 200 MPa or 1 to about 400 MPa. The unreacted fluid
or solid may
be at a temperature of about 20 C to about 350 C. In some embodiments, at
least one of the
unreacted fluids is substantially liquid.
[0092] Contemplated catalysts include catalysts comprising transition metals
and/or noble
metals, e.g. lead, iron, silver, platinum, palladium, nickel, cobalt, copper,
iridium, gold,
samarium, cerium, vanadium, manganese, chromium, ruthenium, zinc, and/or
rhodium, and or
mixtures and/or alloys or salts thereof, and/or complexes, e.g. carbonyl
complexes thereof.
Contemplated catalysts include oxides and/or nitrides of e.g. metals. The
catalyst may, in one
embodiment, include lanthanum, zirconium, aluminum or cerium (e.g. lanthanum
cerium
manganese hexaaluminate, Zr-Al-oxides and Ce-oxides) or other mixed metal
oxide catalysts.
The catalyst may include promoters (e.g. cerium and/or palladium).
[0093] In some embodiments, the catalyst may be provided on a non-reactive
support,
and/or on a substantially porous support, or a support with channels (eg. a
honeycomb
structure). Such supports may include alumina, sol-gels such as sol-gel
derived alumina,
aerogels, carbon supports, solid oxides, solid nitrides, oxidatively stable
carbides, silica,
magnesium and/or oxides thereof, titanium zirconium, and/or zeolites, metals,
ceramics,
intermetallics, corrosion resistant metals (e.g. iron chromium alloys), or
alloy or composites
thereof, or other materials commonly used in catalytic supports. The supports
can be but are
not limited to powdered, granular, or fixed bed. In some embodiments, the
catalyst or catalytic
bed may further include inhibitors that inhibit e.g. plating or poisoning on
the surface of the
catalyst or catalytic support. In other embodiments, the catalyst may include
cation salts and/or
promoters such as ionic promoters or tin, nickel, silver, gold, cerium,
platinum, manganese
oxides, or salts. A contemplated catalyst may include other components such as
boron,
phosphorus, silica, selenium or tellurium. Catalysts or their supports may be
comprised of
nanoparticles.
[0094] In other embodiments, the catalyst may be configured as a bed over
which (or
through which) the unreacted fluid is flowed. In some embodiments, the
catalyst bed may be
sized and shaped to fit within an appropriate drill head housing, or the
catalyst bed may be
disposed in a different housing separate from the nozzle. In one embodiment,
the catalyst bed
may be substantially cylindrical, less than approximately three inches in
diameter and two feet
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-22-
in length. In an alternative embodiment larger or smaller catalyst beds may be
used. For
example, in one alternative embodiment a catalyst bed of approximately 0.5
inches in diameter
and 1-2 inches in length may be used. In other embodiments, axial or radial
flow reactors may
be used. In other embodiments, multiple catalyst beds may be used of the same
or different
designs. The catalyst bed may include a catalyst on a substantially non-
reactive support and/or
a porous support.
[0095] A catalytic support may include for example, a zeolite molecular sieve
of porous
extrudate, piece, pellets, powder, or spheres, and/or porous alumina, silica,
alumino-silicate
extrudate, pieces, pellets, powder, or spheres. Catalytic supports may be
chemically resistant to
any unreacted or reacted fluid. In one example embodiment, the catalyst bed
includes about
0.5% platinum on 1/16" alumina spheres having a surface area of at least
approximately 10
m2/g, or at least 100 m2/g (e.g. a surface area of about 5 m2/g to about 15
m2/g or more). In one
embodiment, the catalyst bed may be about 5% platinum with a promoter on
alumina grains
e.g., with a high surface area. In some embodiments, the catalyst or catalyst
bed may have
plates or sheets. In an alternative embodiment, other forms of catalysis are
contemplated (for
example using a hot surface or a slug of hydrogen peroxide to initiate the
reaction or bring the
catalyst bed up to temperature that may produce a substantially self-
sustaining reaction) may be
used in place of, or in addition to, catalytic reactions. In one embodiment,
the decomposition
of a peroxide over a catalyst generates free oxygen and heat which raises the
temperature of the
unreacted fluid to initiate or help initiate the reaction; the pressure of the
unreacted fluid may
be increased to raise the boiling point of the decomposed fluid to initiate or
assist initiation of
the reaction.
[0096] In an alternative embodiment, a catalyst bed can be used in conjunction
with a heat
exchanger to initiate the reaction and raise the temperature of a down flowing
unreacted fluid,
wherein once the system has an appropriate temperature and/or the reaction is
self-sustaining,
the catalyst bed may be by-passed and/or isolated by e.g. a thermally-actuated
mechanical
valve, which may improve catalytic longevity. A higher activity catalyst bed
may also be used
to "light off' the reaction, after which lower activity beds may be used to
maintain its high
activity. The use of higher pressures in the catalyst bed through e.g. choked
flow across the
nozzle, mud weight in the borehole, or back pressure at the wellhead, may
increase the reaction
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-23-
rates per unit catalyst and decrease the pressure drops across the catalyst
bed which may allow
for smaller catalyst bed volumes and e.g. axial reactor beds.
[0097] In some embodiments, the catalyst may be disposed on a moving rotating
element,
such as blades or screens on a hydraulically driven turbine, which may
increase the contact
between the catalyst and fluid. In another embodiment, the catalyst may be on
a support that
can be e.g., mechanically, thermally, or chemically removed, e.g. without
having to pull a drill
string out. For example, if the catalyst performance decreases or the catalyst
is poisoned, the
catalyst can be removed (e.g. by dissolution of alumina in hydrofluoric acid)
and a fresh
catalyst may be sent down in, e.g. in the form of a pill. The catalyst may be
supported on
carbon that is combusted once the reaction reaches full temperature.
[0098] The catalyst may be regenerated, by for example, passing an oxidant,
hydrogen or a
hydrogen source over the catalyst at temperature, by acid or base washes, or
any other
technique commonly used in catalytic combustion systems. Hydrogen or
additional oxidant
may be added continuously to the unreacted fluid to prevent e.g. coking while
also reducing the
light-off temperature.
[0099] A catalyst chamber may be a water cooled reactor. In another
embodiment, the
catalyst chamber may be a transpiring wall reactor from a porous material tube
that includes
metal or ceramics.
[0100] The catalyst chamber may have distinct zones. For example, different
zones may be
responsible for different chemical reactions, destruction or binding of
catalyst poisons, or for
different temperatures or to reduce the amount of the most expensive catalyst
(e.g. noble metal)
that is needed, or to provide zones of less expensive, sacrificial catalysts.
The relative flow
through different zones may be changed depending on the temperature of the
catalyst chamber
or over time. Different zones, for example, may have substantially the same
catalyst and
geometry or different catalyst and geometry. For example, sending the
unreacted fluid over
one bed at a time until the bed is no longer active can extend the working
life of a tool before it
needs to be pulled from the hole to replace the catalyst.
[0101] In one embodiment, the unreacted fluid is an aqueous fluid. In other
embodiments,
an unreacted fluid may be liquid and may include water, oil, water or oil
based drilling muds,
aerated fluids, and/or supercritical C02, or any other appropriate liquid for
use as e.g. the
working fluid. In one embodiment water can be separated downhole from the
unreacted fluid
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-24-
by cyclone separators or other appropriate fluid separation systems and
methods. For example,
an unreacted fluid may be liquid, gaseous, or a supercritical fluid (e.g. H2O
at temperatures
above about 375 C and 3200 PSI (approximately 7400' water column).
[0102] For example, the unreacted fluid may include water and/or an oxidant
and/or a fuel.
In operation, the unreacted fluid may be, e.g., pumped to a drill head
assembly of a disclosed
spallation system. In the drill head, the unreacted fluid can be, for example,
passed over a
catalyst configured (or otherwise put in contact with the catalyst) to e.g.,
cause the flameless
reaction with an oxidant and/or a fuel that may be present in e.g. the
unreacted fluid. Such a
reaction may produce a reacted fluid, e.g. a fluid at an elevated temperature,
that may then be
directed out of an e.g., distal jet nozzle of the spallation drill head
assembly and impinge upon a
target rock surface, creating thermally damaged rock and/or spalled rock. The
reacted fluid, in
some embodiments, may include water in gaseous (steam) or supercritical form,
for example,
may be a gas when in first contact with rock. After contacting the rock, the
expelled water, gas
or supercritical fluid can then, in some embodiments, flow up the borehole,
carrying the spalled
rock with it. In some embodiments, the reacted (hot) fluid is allowed to
travel up the borehole
to further spall the borehole walls and expand the diameter of the borehole.
In other
embodiments, the reacted fluid is cooled e.g. just above the drilling assembly
by a heat
exchanger and/or cooling-lift fluid, thereby substantially stopping the
spallation reaction. In
other embodiments, the reacted fluid is directed through a "shroud" which may
reduce its
interaction with the sides of the rock wall, and also substantially stopping
the spallation
reaction. In an alternative embodiment, some of the reacted fluid does not
travel up the
wellbore but rather enters the rock or formation through e.g. fractures. In
some embodiments,
the spalls or rock fragments are not carried up the wellbore but are allowed
to fall further into
the hole or remain on the borehole wall.
[0103] In one embodiment, a non-reacted or unreacted fluid includes a fuel
and/or oxidant.
For example, the unreacted fluid may include two or more components that are
miscible with
each other. In another embodiment, an unreacted fluid and/or an unreacted
solid is present, for
example, an unreacted solid may include an oxidant (e.g. a solid encapsulated
oxidant), or an
unreacted substantially solid fuel, e.g. a wax. An unreacted solid may be
dispersed, dissolved,
undissolved or encapsulated within a solid. In one embodiment at least one of
the fuel and/or
oxidant may change state or dissolve, decompose, or otherwise react during its
transport along
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-25-
the borehole to the drill head, or upon reaching a drill head. A catalyst or
accelerant may be
added to the unreacted fluid, wherein the catalyst can be activated at the
bottom of the hole by
heat or mechanical force, with or without the use of a secondary permanent
catalyst. The
working fluid may also contain an inhibitor to prevent the reaction from
occurring along the
length of a drill string.
[0104] In certain embodiments, a nonreacted fluid is pumped down hole to a
drill head at
the distal end of the borehole at approximately 1- 50 or 5-50 gallons per
minute, e.g. about 20
gallons/minute. In one embodiment, an unreacted fluid may be pumped down one
or more
small diameter tubes that may be nested inside of a traditional steel coiled
tubing system. Such
small diameter tube or tubes may have one or more periodic check valves so as
to prevent the
unreacted fluid from back-flowing and to limit uncontrolled reactions from
propagating up the
nested tube.
[0105] In an alternative embodiment, any appropriate tubing system for
transporting the
aqueous solution to the catalyst or drilling head assembly may be utilized. In
some
embodiments, the fuel and oxidant are transported to the catalyst or drilling
head assembly
through one conduit, or in separate conduits. For example, fuel/oxidant
mixtures which are
stable at desired concentrations can be transported together in one tube. This
may, for example,
have advantages over transporting the fuel and oxidant separately in that it
would require one
less conduit to pass material to the distal end of the borehole. It may also
simplify storage,
mixing, or handling procedures on the surface. Fuels or oxidants which may be
carried in the
bulk cooling-lift water (and separated at the bottom of the hole) to also
reduce the number of
conduits.
[0106] In one embodiment, the fuel and oxidant may be combined in a number of
different
ways to allow for transportation of the fuel and oxidant down the same
conduit. For example,
fuel and oxidant may be transported down a single conduit through use of a
single molecule
("single-source") or network/complex. The chemical heat source can be a
monopropellant,
such as hydrogen peroxide, nitrous oxide, or hydrazine. Alternatively, fuel
and oxidant may be
transported down a single conduit through use of methods including, but not
limited to, slug
flow (i.e. gases and/or liquids sent one after another), dissolved gases, or
bubble flow (i.e. small
bubbles suspended in a fluid and transported along with the fluid). In an
alternative
embodiment, the fuel and oxidant may be transported down the same conduit as
two solid
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-26-
materials in one or more "pills". In a further alternative embodiment, one or
more of the fuel
and/or oxidant may be transported in an encapsulated form such as, but not
limited to, a
material, such as a peroxide, encapsulated by e.g., wax.
[0107] In some embodiments, fuel and oxidant may be sent down one conduit in
two
separate fluid phases. For example, the fuel may be carried in an oil-based
phase, and the
oxidant in the water based phase. At the bottom of the hole, the two phases
can be, for
example, homogenated, or the fuel and/or oxidant can be separated from its
respective phase by
means of a hydrocylcone or other separation device and then combined with its
reactant.
[0108] Contemplated fuels include carbonaceous fuel, such as a fossil fuel
(e.g. coal,
biomass), gasoline, natural gas (e.g. liquefied natural gas) diesel, biodiesel
or kerosene. For
example, fuels contemplated for use in the disclosed methods include alcohols,
alkyls,
cycloalkyls, alkenes, alkynyls, ethers, alkoxyalkyls, (e.g. CH3CH2O CH2CH3,),
dioxanes,
glycols, diols, ketones, acetone, aldehydes and/or aromatic organic compounds
such as benzene
or naphthalene, or combinations thereof. Hydrocarbons may be used as fuel, and
include
alkanes (e.g. C1-C20 alkanes) such as methane, ethane, propane, butane,
pentane, hexane,
heptane, octane, and higher alkyl fuels such as naptha, kerosene, paraffin,
hydrocarbon
oligomers, and /or other waxes. Other contemplated fuels include ethylene
vinyl acetate
(EVA), polyvinyl chloride (PVC), boranes (such as B2H6 or B5H9), carboranes,
ammonia,
kerosene, diesel, fuel oil, bio-based oils, such as biodiesel, starch, sugars,
carbohydrates, or
other oxyhydrocarbons. A fuel may be, or include, hydrogen, hydrogen
generating compounds,
or hydrogen containing polymers such as polyethylene, polypropylene, or
paraffin polymers. A
fuel may also be, or include, reactive metals such as aluminum, beryllium, and
coated or
encapsulated sodium.
[0109] For example, contemplated fuels include alcohol fuels (e.g. C1-C8
alcohols) such as
methanol, ethanol, propanol, and/or butanol, or mixtures thereof, which in
some embodiments
may be optionally substituted by one or more halogens. In certain embodiments,
the fuel may
be substantially miscible in water, e.g. methanol, ethanol or benzene.
[0110] Contemplated oxidants include air, oxygen, peroxides, (e.g., hydrogen
peroxide or
methyl ethyl ketone peroxide) percarbonates, permanganates, permanganate
salts, as well as
combinations thereof. For example, contemplated oxidants include inorganic
and/or organic
peroxides such as peroxides of alkali metal peroxides, e.g. lithium, sodium,
and/or potassium
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-27-
peroxides, e.g. sodium peroxide and/or barium peroxide. Alkyl peroxides such
as t-butyl
peroxide and benzoyl are contemplated. Oxidants contemplated herein may
include
hypochlorite and/or hypohalite compounds, halogens such as iodine, chlorite,
chlorate or
perchlorate compounds, hexavalent chromium compounds, sulfoxides, ozone,
nitric acid, N20,
and/or persulfuric acid. Other possible oxidants include F2, OF2, 02/F2
mixtures, N2F4, CIF5,
CIF3, N,,Oy, IRFNA IIIa: 83.4% HNO3, NO2, H2O, 0.6% HE IRFNA IV HAD: 54.3%
HNO3, NO2, 1% H2O, 0.7% HF, RP-1, CioH18, and CH3NHNH9.
[0111] As disclosed herein the peroxide may be in e.g. aqueous form, or may be
in a solid
form e.g. pellets that may include urea. An unreacted fluid that includes an
e.g. oxidant, e.g.
hydrogen peroxide, may also include corrosion inhibitors and/or passivating
agents and/or anti-
foaming agents and/or surfactants and/or surface tension modifying agents. For
example, an
unreacted fluid may include stabilizers such as phosphoric or phosphonic acid
or sodium
pyrophosphate or tin compounds. In an embodiment, an oxidant, e.g. high
pressure or liquid
oxygen may be metered into a fuel stream (e.g. methane or methanol stream);
mixing can take
place either at the surface or in the drill head. The mixture may then travel
into the drill head.
In one embodiment the drilling head is configured to withstand bottom hole
pressures of
upwards of about 100 to 4000 PSI, 1000 to about 4000 PSI, or about 1000 to
about 30000 PSI
(e.g. about 1 to about 200 MPa), e.g. the pressures present at the bottom of a
deep wellbore.
[0112] In some embodiments, a provided unreacted fluid may include an aqueous
solution
comprising by weight of about 5% to about 52% oxidant, e.g. hydrogen peroxide,
or about 30%
to about 40% oxidant, or about 5% to about 50% oxidant, and may include about
5% to about
20% fuel, e.g. methanol, or about 10% to about 20% fuel, e g. 10% to about 15%
fuel, or even
about 5% to about 50% fuel. For example, an unreacted fluid may include about
2% to about
40% by weight hydrogen peroxide. In another embodiment, the unreacted fluid
may include
about 10% to about 20% by weight methanol or ethanol. In an exemplary
embodiment, the
unreacted fluid includes about 15% methanol or ethanol and about a
stoichiometric amount of
air, oxygen, or peroxide (e.g. hydrogen peroxide). In another exemplary
embodiment, the
unreacted fluid includes 38% by weight hydrogen peroxide and about 12% by
weight methanol,
or e.g. about a 4:1 weight ratio of hydrogen peroxide/methanol, e.g. about a
5:1 to about a 1:1
weight ratio of hydrogen peroxide/methanol.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-28-
[0113] In an exemplary embodiment, the unreacted fluid is slightly oxidant
rich to assure
complete combustion of the hydrocarbons to reduce the amount of by-products
caused by
incomplete combustion, such as carbon monoxide, formaldehyde, and/or formic
acid. In other
embodiments, the unreacted fluid may be T-Stoff (80% hydrogen peroxide, H202
as the
oxidizer) and C-Stoff (methanol, CH3OH, and hydrazine hydrate, N2H4=nH2O) as
the fuel);
nitric acid (HNO3) and kerosene; inhibited red fuming nitric acid (IRFNA, HNO3
+ N204) and
unsymmetric dimethyl hydrazine (UDMH, (CH3)2N2H2), nitric acid 73% with
dinitrogen
tetroxide 27% (AK27), and kerosene/gasoline mixture, hydrogen peroxide and
kerosene;
hydrazine (N2H4) and red fuming nitric acid; Aerozine 50 and dinitrogen
tetroxide,
unsymmetric dimethylhydrazine (UDMH) and dinitrogen tetroxide; or
monomethylhydrazine
(MMH, (CH3)HN2H2) and dinitrogen tetroxide. In another embodiment, the
unreacted fluid
may include 50-98% hydrogen peroxide. The products from decomposing the 50-98%
peroxide (e.g. H2O and/or 02) over a catalyst (e.g. platinum, silver, or
palladium), may then be
allowed to react with a fuel (e.g. methanol). The heat from the decomposition
of the hydrogen
peroxide, combined with downhole temperatures and pressures and/or the use of
a heat
exchanger, may auto-initiate or sustain the reaction of fuel and oxidant, such
as peroxide and/or
oxygen with methanol and/or ethanol.
[0114] An unreacted fluid or solid, when contacted with the catalyst, may
generate a
reacted fluid, e.g. a fluid for use in the thermal systems disclosed herein.
The reacted fluid may
include water and may also include nitrogen, carbon dioxide and/or carbon
monoxide, as well
as smaller amounts of unreacted fuels and/or oxidants and/or side products.
For example, an
unreacted fluid that includes methanol and hydrogen peroxide, reacting with a
catalyst,
produces exothermically water and carbon dioxide. In some embodiments, little
or no heat,
and/or other initiator (e.g. spark, glow plug, or flame holder), is required
to initiate the reaction.
In some embodiments, contacting the unreacted fluid and catalyst produces
substantially
continuously reacted fluid.
[0115] In some embodiments, the reacted or working fluid, e.g., hot water, is
focused out of
the jet nozzle of the drill head assembly and directed against the target rock
surface. In one
embodiment, the jet temperature (reacted fluid temperature) and/or heat flux
may be controlled
by adjusting the mixture of the aqueous solution (for example, by increasing
the methanol
and/or oxygen concentration to increase the jet temperature). In another
embodiment, the jet
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-29-
temperature and/or heat flux may be controlled by increasing the flow rate of
the unreacted and
e.g., hence reacted fluid. In another embodiment, the jet temperature and/or
heat flux may be
controlled by adjusting the flow rate of the unreacted fluid to adjust for
complete or incomplete
reaction. The jet temperature and/or heat flux may also be controlled by, for
example,
adjusting the flow rate of the unreacted fluid to reduce the amount of heat
exchange between
the reacted and unreacted fluids.
[0116] A drill assembly may include a drill head with a nozzle. An exemplary
drill head
may have a diameter of approximately 3/4 inches with a 0.1 inch center nozzle
through which
the reacted fluid is expelled. In alternative embodiments, nozzles with
different configurations
and/or geometries may be utilized, such as a larger or smaller nozzle
diameter. For example,
the drill head may be about 5 to about 15, or 4 to about 29 times the diameter
of the nozzle. In
one embodiment, the drill head assembly may include a plurality of jet nozzles
directed in
either the same or different directions from a distal portion of the drill
head assembly. In
another embodiment, the drill head assembly includes one center jet nozzle.
Rock "spalls" (e.g.
grains or platelets of less than about 0.025 inch to about 0.1 inch) can be
ejected and may be
swept up the borehole by the reacted fluid (after the reacted fluid contacts
the rock). In one
embodiment, a larger flow of cooling-lift water (e.g., traveling in the
annulus between the
nested tube and coiled tubing), can be introduced after the heat exchanger (if
used), to cool the
fluid and help transport the spalls to the surface.
[0117] In one embodiment, a heat exchanger is placed above the catalyst bed so
that some
of heat of the upflowing (e.g. reacted) fluid is transferred to the down
flowing (e.g. unreacted)
fluid, both conserving energy and preheating the solution prior to the e.g.
the catalyst bed,
heater, or drill head. In an exemplary embodiment, a nested drill string may
act as a heat
exchanger. In some embodiments, the catalyst may be preheated by sending some
chemical,
e.g. an oxidant (e.g. peroxide) in the down-flowing fluid, with or without
fuel, which may in
some embodiments, initiate a reaction, for example heating the catalyst. For
example, heat
provided by a heat exchanger to a down flowing fluid may provide enough heat
to initiate the
combustion reaction without the need for a catalyst, which may allow flow to
be directed away
from the catalyst bed (and thus may preserve or prolong the useful lifetime of
the catalyst). In
some embodiments, hot gas may be used to dry the catalyst bed prior to contact
with the fuel
and oxidant.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-30-
[0118] In another embodiment, approximately 0.12 gallons per minute of a 15-
20%
aqueous solution, such as, but not limited to an aqueous methanol solution, is
pumped through
a preheater to bring the temperature up to 290 C. In an alternative
embodiment, a greater or
lesser volume of aqueous solution may be pumped. In further alternative
embodiments the
preheater may bring the temperature of the aqueous solution up to a greater or
lesser
temperature, as required. In a further alternative embodiment, no preheater is
required
[0119] In one embodiment spallation takes place with stand-off distances (i.e.
the distance
from the nozzle exit at which the target surface is placed) ranging from
approximately 0.2-10.0
inches. In an alternative embodiment, stand-off distances of less than 0.2
inches or greater than
inches may be achieved. This may, for example, allow a one inch diameter hole
to be
drilled at a rate of greater than 0.5 inches per minute. In one embodiment,
the standoff distance
is varied, either periodically or randomly, in a controlled or relatively
uncontrolled manner, or
in response to a downhole measurement or physical, mechanical, electrical
thermal, or
chemical condition. This variation in standoff may improve the tools ability
to reliably under
ream or to produce a borehole of consistent or desired geometry. Standoff
distance, for
example, may be controlled by acoustic monitoring, e.g. analysis of the sound
of the jet can be
used to determine the shape of the bottom of the hole and distance between the
nozzle and the
bottom. Parameters of the jet, (e.g., nozzle geometry, flow, temperature,
stand-off) can be
adjusted to optimize drilling, either through communications to the surface or
by downhole
processors or actuators. The backpressure of the flow through the nozzle may
also be used for
feedback to adjust e.g., the geometry of the nozzle, the flow rate, the stand-
off, and/or the rate
of drill string displacement.
[0120] An example drill head assembly, a small scale axial flow reactor, for a
spallation
system is shown in FIGS. 3A to 3C. In this embodiment, a catalytic heater
drilling spallation
system 31 may be used to create high temperature high pressure fluids in a
reaction chamber or
cell 26, initiated by a stream of hot water mixed with 20% methanol to which
gaseous oxygen
is added. In alternative embodiments, a higher or lower percentage methanol
may be used.
This stream of fluid flows into the cell 26 through an inlet fitting 18. In
one embodiment, the
cell body 26 is constructed with an insulating gap 24 filled with an
insulating material, such as,
but not limited to, nitrogen gas at the same, or substantially the same,
pressure as the fluid
flowing into the cell 26. This gap 24 may assist in preventing heat loss from
the reaction
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-31-
chamber within the cell 26 into the cooling water surrounding the cell 26, and
also helps
maintain the cell integrity at the high temperatures of the reaction occurs.
The nitrogen enters
the gap through a tube fitting 19 and into a collar 20. A replaceable o-ring
seal 21 allows the
inner region to thermally expand without loss of the nitrogen pressure
blanket. A threaded nut
22 secures the o-ring in place. In alternative embodiments, alternative
insulating materials and
systems may be utilized in place of, or in addition to, the nitrogen gas
layer.
[0121] The reaction chamber within the central region of the cell 26 is filled
with a catalyst,
such as, but not limited to, platinum coated alumina spheres 25, that are held
in place by two
stainless steel filter screens 23. In an alternative embodiment, other
appropriate materials
and/or means of positioning and holding the catalyst may be used. In
operation, the reacted
fluid passes out of the reaction chamber, after reacting with the catalyst 25,
at an elevated
temperature. A nozzle body 27, such as a threaded nozzle body, focuses the
high temperature
jet 28 of reacted fluid out of a nozzle exit 29 onto a target location on a
rock surface. The
nozzle body 27 may be, for example, screwed into place on the distal end of
the system 31
using the two drilled holes 30 and a spanner wrench.
[0122] FIGS. 3D and 3E show the system 31 in operation. Prior to starting the
system 31, a
granite block 39 is predrilled with a small borehole 40. A seal-interface
block 36 isolates the
nozzle 27 from the coolant fluid, and provides a means for venting spalls and
oxidation
fluids/gases from the borehole. The interface block 36 may, for example, have
a cap 33 which
is held in place using a number of screws 34. The cap retains in place a thin
metal washer and
ceramic felt pad 35 which makes a sliding seal for the system 31, thereby
preventing inflow of
coolant. The interface block 36 may be sealed to the outside using, for
example, an o-ring 38.
A jet 37 of hot reacted fluid exits the nozzle exit 29 and enters the
predrilled borehole 40,
where it spalls the rock at the distal end of the borehole and flows upward
and out of the
interface block through the chimney tube 32.
[0123] Another example, as depicted in FIG. 11, is a convergent radial flow
reactor housed
within a 2 7/8" OD drill head for producing 4" holes in granite using the
laboratory test system
or deployed on a coiled tubing unit. This system is comprised of a steam
generation assembly
132 containing a catalyst bed 135, a drill head 136, and a connector 134 that
couples the unit to
other downhole subassemblies and the drill string. Unreacted fluid is pumped
down a single
capillary in the drill string, through 133, and into the steam generation
assembly 132 where it
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-32-
flows through a catalyst bed and reacts producing reacted that exits out a
nozzle 136. Pressures
and temperatures inside the steam generation assembly 132 are measured at
specific locations
137, 138 which can be used to monitor the performance of the system. Flow
schematics of this
steam generation assembly 140, 144 for a thermal spallation drilling system
are shown in FIG.
12A and FIG. 12B. A converging flow design is shown in FIG. 12A. Fuel and
oxidant enter
the cell 141 and flow across a catalyst bed 142 where they react producing the
working fluid
which exits down a tube 143 to the drill nozzle (not shown). A diverging flow
design is shown
in FIG. 12B. Fuel and oxidant enter the cell 145 and flow across a catalyst
bed 146 where they
react producing working fluid which exits down and annulus which converges to
a tube 147
that leads to a drill nozzle (not shown). For surface demonstrations of the
drill head shown in
FIG. 11, an example of a spallation drilling test system rock core confinement
apparatus 148 is
shown in FIG. 13A, FIG. 13B, and FIG. 13C. The system can be used to simulate
spallation
drilling at the surface where there is low stress on the rock. The system is
comprised of a steel
concrete mold 149 that encases a rock sample 156 which is surrounded by
concrete 157. A
wellhead 151 is secured to the rock sample prior to the sample being encased
in concrete. The
entire system rests on a pallet 150 for ease of transportation. Bolts 153 on
the side on the
concrete mold 149 can be tightened after the concrete has hardened in order to
induce a
compressive stress on the rock sample. A drill 158 enters as shown. Cooling
water or drilling
mud is pumped through injection tubes 152 and enters the wellbore at injection
points 154. A
flow barrier 155 prevents the cooling water from entering the hot thermal
spallation region
downhole while the drill is in operation. Unreacted fluid is pumped into the
drill through a tube
159 and reacted fluid exits the drill nozzle 160.
Thermochemical
[0124] In an alternative embodiment, a working fluid including an aqueous
fluid
comprising water and hydroxides of Group I elements of The Periodic Table of
Elements, and
mixtures thereof, may be used. For example, an aqueous fluid may include a
hydroxyl ion
concentration of the hydroxides of Group I elements of The Periodic Table of
Elements and
mixtures thereof at ambient conditions is in the range of about 0.025 to 30
moles of hydroxyl
ion per kilogram of water. In some embodiments, an upper limit of the range
can be
determined by the solubility of the Group I hydroxide. For example, a fluid
may include about
0.1 to about 52 grams sodium hydroxide per 100 grams of solution at room
temperature (but
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-33-
may include more at higher temperatures). In some embodiments, the fluid may
comprise
alcohols such as methanol or ethanol with hydroxides, which produce alkoxides.
Such
alkoxides may help solubilize minerals in rock.
[0125] In some embodiments, concentrated aqueous or alcohol solutions of
hydroxides of
alkali metals can react with subsurface rock formations and may be capable of
forming one or
more water soluble complexes with at least one of Si or Al. For
aluminosilicate rocks, the high
alkoxide or hydroxyl ion concentration in the fluid may provides the dual
benefit of (i)
enhancing the dissolution rate by fully ionizing the chemical surface groups
on the formation
rock, thus maximizing the density of surface sites vulnerable to hydrolysis,
and (ii) enhancing
solubility of reaction products by forming thermally stable soluble complexes.
Such fluids may
dissolve rock and consume hydroxide stochiometrically until e.g., the hydroxyl
ion
concentration drops to near 0.01 moles of hydroxyl ion per kilogram of water
or alcohol.
Materials to achieve hydroxyl ion concentration above 0.01 moles of hydroxyl
ion per kilogram
of water include, but are not limited to alkali metal and alkaline earth metal
components such
as hydroxides, silicates, carbonates, bicarbonates, mixtures thereof and the
like. In example
material is sodium hydroxide. Other solutes may be added in any desired
quantity to achieve
other objectives, as long as the hydroxyl ion concentration is maintained
Coupled Thermal and Mechanical Systems
[0126] One aspect of the present invention relates, at least in part, to
drilling systems, and
associated methods of use, that includes a heat source to thermally affect a
target material and a
mechanical drilling system. The drilling systems may be used to create
boreholes or increase
the diameter of existing boreholes in any of the target materials described
herein including, but
not limited to, crystalline rock material, silicate rock, basalt, granite,
sandstone, limestone, or
any other rocky material. The drilling systems may be used to create vertical
boreholes,
horizontal boreholes, angled boreholes, curved boreholes, as well as slots,
perforations, fracture
enlargement, or other forms of hole opening, or any combination thereof. In
one embodiment,
the methods and systems described herein provide for improved deep borehole
drilling, for
example from approximately 10,000 feet to approximately 50,000 feet below the
surface, or
more.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-34-
[0127] A borehole may be created, for example, through the combined use of a
heated fluid
and a mechanical drilling and/or reaming or milling system. Combining a
mechanical drilling
system with e.g. a thermal drilling system such as those described above may
overcome certain
limitations of thermal systems alone, by, for example, the combination may
provide for
controlling stand-off and/or rate of penetration or bit advancement,
penetrating unspallable or
thermally-insensitive or unspallable zones, comminuting larger pieces of rock
that may be
produced or fall from the borehole wall, penetrating fractures which have
inflowing or potential
for outflowing fluids. Combining the use of a heat source to thermally affect
a target material
with a mechanical drilling system may overcome certain limitations of
conventional
mechanical drilling systems alone by, for example, preventing the wear and
fatigue to the drill
bit that is produced through traditional mechanical drilling technologies.
More particularly, by
utilizing one or more heat sources to thermally affect a rock portion in
advance of one or more
conventional drilling and/or milling systems, the mechanical and physical
strength of the rock
to be drilled and/or milled can be reduced forward of, and/or simultaneously
with, the
mechanical drilling process. This may allow for increased penetration rates
with reduced bit
wear, vibration and drill string fatigue, and uncontrolled trajectory
deviations compared to
conventional drilling processes. For example, new cutter materials such as TSP
can operate at
temperatures above 1000 C, as shown, e.g., in FIG. 15, where hard rocks such
as granites are
significantly softened, as shown, e.g., in FIGS. 8, 9, 10, and 16. Therefore,
a thermal jet which
reduces the rock strength by, e.g. partially spalling and/or microfracturing
and/or softening
combined with a mechanical drilling process using a high temperature bit
material, has the
possibility of a corresponding ROP exceeding that of either process along. As
a result, the
efficiency of conventional mechanical drilling methods may be significantly
increased by the
use of a heat source to modify the properties of the rock in advance of the
mechanical drilling
system.
[0128] In one embodiment, the mechanical drilling and/or reaming system may,
for
example, include a traditional mechanical, chemical, or other appropriate
drilling and/or
reaming mechanism. Embodiments of the invention may, for example, incorporate
any
appropriate mechanical bit design, including, but not limited to, roller cone
bits, tricone bits,
polycrystalline diamond compact (PDC), reaming bits, milling bits, hammer
drill bits or coring
bits, or other appropriate drilling bits. The design of these bits, including
cutting and rock
reduction surfaces, can be optimized so that the depth-of-cut and rate-of-
penetration can be
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-35-
maximized while keeping the wear, vibration, and trajectory deviations within
acceptable
limits. Materials and novel designs, including high temperature metals and
alternative methods
for inclusion of cutting surfaces, may be optimized for use under these
relatively high
temperature conditions. The use of high temperatures may also allow for the
use of ultra-hard
materials that tend to be brittle at lower temperatures. In an alternative
embodiment, the
drilling system may include other physical or chemical processes such as, but
not limited to,
sonication, sonic drilling, laser drilling, arc/plasma, particle assisted
drilling, chemical
dissolution, or other appropriate physical or chemical processes of use in
drilling applications
in addition to, or in place of, a mechanical drilling system.
[0129] In order to thermally affect the rock to be drilled and/or reamed or
milled by the
mechanical drilling system, one or more heat sources may additionally be
incorporated into the
system. This heat source may include any appropriate heat source adapted to
thermally affect a
rock through spallation, microfracturing, macrofracturing, dissolution,
partial melting,
softening, modification of grain boundaries, change in crystalline phase,
decrystallization,
erosion, or the like. For example, certain materials such as shales and clays
may be modified
(e.g., dehydrated at high temperatures) to reduce or eliminate bit baling.
[0130] In one embodiment of the invention, a combined thermal and mechanical
borehole
creation system may include a spallation drilling mechanism, such as, but not
limited to, any of
the thermal spallation systems described herein, with mechanical drilling
mechanism such as,
but not limited to, a drilling, reaming, milling, and/or hole opening process.
A downhole
chemical reaction (e.g. hydrothermal oxidation of methanol and peroxide over a
catalyst) may
provide both thermal energy as well as the mechanical energy (e.g. expansion
of the hot fluid to
e.g. drive a hammer).
[0131] In one embodiment, a small pilot borehole may be formed, e.g. with the
thermally
produced pilot borehole being substantially smaller than the target diameter
of the final
borehole. The pilot borehole may thereafter be milled, drilled, or otherwise
enlarged, by a
mechanical system such as a reaming system, or other appropriate hole opening
system, to form
the final borehole of the required diameter. This method may, for example,
allow for more
precise control of borehole geometry, and provide substantial cost and time
benefits for
producing the final reamed borehole. The pilot hole may serve as a guide,
stay, or centralizer
for the reaming bit. In addition, removal of rock from the circumference of a
lead borehole
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-36-
(that has been created by spallation system) through a reaming process may be,
for example,
faster, easier, and/or produce less bit wear than traditional drilling of the
entire borehole. The
spallation drilling mechanism and reaming mechanism may be part of a single
device, or be
separate devices. The pilot borehole may be used, for example, as an
exploratory, test,
monitoring, or scientific borehole to e.g. determine the quality of the
resource and evaluate if a
larger borehole should be created.
[0132] The use of a working fluid for e.g., creation of a lead borehole, may
affect one or
more properties (e.g. a thermal, mechanical, chemical or physical property) of
the material at
the surface of the pilot borehole wall. This may, in turn, make it easier for
the reaming system
to ream the surface of the lead borehole to create the final borehole. In one
embodiment, the
reaming operation may also remove rock that is not structurally stable. Such
rock could, if not
removed, fall into the hole, bridge the hole, or form ledges that prevent the
advance of casing or
stick the casing before it is on-depth. Bridges that form in the casing
annulus can e.g. divert or
disrupt the placement of cement which may jeopardize the success of well
completion. The
reduced mechanical strength of the thermally affected zone, if not removed,
may also reduce
the overall integrity of a completed well.
[0133] In each of the embodiments described above, a working fluid, such as
those
described herein, may be used to weaken and/or remove the rock at a distal end
of a borehole
prior to, or simultaneously with, the drilling, reaming, and/or milling action
of a mechanical bit
coupled to the thermal spallation system. In different embodiments of the
invention, a working
fluid can be configured to spall or thermally affect the entire bottom surface
of the distal end of
the borehole. In an alternative embodiment, the thermally-affected zone
produced by a
working fluid does not cover the entire surface under the drill bit. Rather,
the fluid stream can
be directed so as to target certain regions under the bit to be weakened.
Damage to or removal
of these regions can cause structural weakening of the remainder of the
surface so that it may
be easily removed by a separate feature on the drill bit. In another
embodiment, a working
fluid may be focused toward the sides of the borehole, with or without
additional working fluid
being focused toward the bottom of the borehole.
[0134] In various embodiments of the invention, the mechanical drilling and/or
milling or
reaming operation may be carried out concurrently with a thermal drilling
operation, e.g. use of
a working fluid. For example, a mechanical drilling/reaming element may be
located either
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-37-
substantially close to the thermal treatment operation and/or substantially
offset along the
drilling assembly, thereby allowing the mechanical drilling process to be
carried out
concurrently, or substantially concurrently, with a thermal drilling
operation. The mechanical
drilling elements, (e.g. drill bits or reaming bits) may therefore remove the
thermally modified
portion of the geological formation and/or thermally unmodified rock
surrounding the
thermally modified rock, thereby creating the borehole and, in some
embodiments, improving
the geometry or integrity of a wall of the borehole created by the spallation
system or other
thermal treatment system.
[0135] The system may be adapted to remove both spalled or thermally affected
rock and
non-spallable rock. In addition, the system may be adapted to reduce the size
of rock pieces
that are too large to be removed from the borehole in a circulating fluid. As
a result, the
mechanical drilling system, in combination with the thermal treatment system,
may be used to
create boreholes in a number of different geological formations including a
number of different
properties. For example, a coiled tubing deployed thermal spallation drill
head can be
combined with a coiled tubing deployed mud-motor drill; in formations where
the thermal
spallation process is not effective, the mud motor may be used to turn a
conventional coiled
tubing drill bit. Likewise, a drill pipe deployed hydraulically driven turbo-
generator can be
used to produce electricity for resistance heating elements used to initiate
thermal spallation or
treatment of the rock. A thermally-stable rotary drill bit serves to maintain
proper stand-off of
the jet during pure spallation drilling, assist in some sections via
thermomechanical drilling,
and be the sole mechanism for drilling in others. This is particularly
advantageous over prior,
uncoupled, systems, wherein, for example, a thermal treatment or thermal
drilling system may
need to be removed from the borehole if unspallable rock is found at the
bottom of the
borehole, or created by over-heating the rock, and temporarily replaced by a
mechanical
drilling system. This removal of a drilling system, and insertion of another
type of drilling
system, whenever materials with different properties are met may be extremely
costly and time
consuming. By coupling a thermal system with a mechanical system within a
single drilling
system, the need to replace the system when different materials are met may be
avoided.
[0136] In an alternative embodiment, the mechanical drilling process may be
performed as
a secondary operation while some tubing or pipe remains in the hole. In a
further embodiment,
the mechanical drilling process may be performed as a secondary operation
after the thermal
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-38-
drilling assembly has been removed. In one embodiment, different processes,
such as a thermal
drilling process and a mechanical drilling and/or reaming process, may be
performed
concurrently along different portions of a single casing interval or wellbore.
[0137] In one embodiment, one or more thermal treatment nozzles can be
distributed
throughout the front of a mechanical drill bit, or through slots radially
extending from an outlet
port. The nozzles can also be shrouded with a protective gas or fluid stream
to reduce cooling
and mixing with the drilling fluid and/or increase the potential for thermally
damaging the rock
surface. Gas shrouds, fluid streams, solid insulation such as a ceramic or
syntactic ceramic,
vacuum gaps, or gas or fluid filled gaps can also be used to protect the
materials of construction
or mechanical drilling equipment from high temperatures.
[0138] In one embodiment, the drilling process includes rotary or coiled
tubing drilling. As
a result, a thermal jet, or a portion thereof, may be configured to rotate. In
an alternative
embodiment, one or more thermal jets, or a portion thereof, may be fixed, for
example, through
either a center or peripheral ring jet.
[0139] In some of the embodiments described herein, a thermal system including
a single
nozzle may be incorporated into a mechanical drilling system. The single
nozzle may be
located centrally along a central elongate axis of the system. As a result,
the thermal system
may include a fixed, non-rotating, structure. A mechanical drilling and/or
reaming or milling
mechanism may then by positioned over or in the thermal system, and rotate
around or in the
thermal system, to mechanically drill and/or ream the borehole being created
in conjunction
with the thermal system. Providing a single, centrally located, non-rotating
thermal system
may be advantageous, for example, in simplifying the structure of the system
by reducing the
number of necessary moving parts and reducing the mechanical complexity of the
overall
system. This may, for example, reduce the cost of the system while also
allowing for a more
structurally sound and sturdy borehole creating tool. In one embodiment, by
minimizing the
moving parts within the thermal system, stronger and more robust materials may
be used in the
construction of the thermal system, and the resulting structure may therefore
be better adapted
to withstand the high pressures, temperatures, impact, and mechanical wear
that are generated
at the bottom of a borehole during drilling operations.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-39-
[0140] In one embodiment, a heat source may be incorporated into a mechanical
drilling
system such that the distal end of the mechanical drilling system extends a
specified distance
from the distal end of the heat source. As a result, the impingement of the
distal end of the
mechanical drilling system against the target portion of the rock results in
the substantially
constant stand-off distance between the rock surface and the heat source. This
may be
advantageous, for example, in applications where a set distance is required
between the target
surface and the distal end of the heat source to ensure that the temperature,
flow, and heat flux
produced at the surface of the target portion of the rock is within the
required limits for
efficient spallation. Also provided herein are methods that may achieve e.g.,
softening of rock
at a radius proportional to the wear rate of e.g. mechanical cutters such that
the life of the
cutters is more uniform.
[0141] An example drilling system is shown in FIG. 3A and FIG. 3B. In this
embodiment,
the drilling system 400 includes a pilot hole thermal spalling system 54 and
borehole reamer 55
in conjunction with coiled tube drill rig system 410. The pilot hole thermal
spalling system 54
is powered by a fuel and oxidant fed through a nested tube 42 contained in a
motor driven shaft
41. The reactants move through the assembly to a pilot drill reaction chamber
47. The reaction
chamber 47 is filled with a catalyst to initiate a thermal reaction with the
fluid passing
therethrough to change at least one property of the fluid such as, but not
limited to, a
temperature, a pressure, or a state of the fluid. In one example, the reaction
between the fluid
and the catalyst increases the temperature and decreases the density of the
fluid. As a result of
the thermal reaction, a jet 50 of hot gases/liquids is directed out of a
nozzle 49 at the distal end
of the chamber 47. The reaction chamber 47 may, in one embodiment, be
thermally insulated
from the main body by, e.g. a gas filled cavity 48. The exit jet 50 spalls the
rock at the distal
end of the borehole, thereby drilling a hole in the rock 52 and creating a
damaged zone 51
around the bore.
[0142] The spalled rock can then be carried away from the target location at
the end of the
borehole by the recirculating fluid or drilling mud within the borehole. The
nozzle portion 49
may, in one embodiment, be constructed from a high temperature resistant
material such as, but
not limited to, at least one of a ceramic, ceramic composite, high temperature
steel alloy, or the
like.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-40-
[0143] The pilot spallation sub assembly 54 is attached to a rotating reamer
sub-assembly
55 which carves away the damaged rock. The reamer 55 has multiple blades 43
having
attached carbide or diamond compacts 44 to cut away at the damaged rock zone
51. Coolant,
such as, but not limited to, a water or drilling mud, may be introduced just
below the reamer
blades 43 with imbedded compacts 44 through one or more outlets 45 to help
cool the assembly
and remove cuttings.
[0144] In one embodiment, where the system is attached to a coiled tube drill
rig 410, the
downhole assembly, or a portion thereof, may need to be rotated through the
use of a downhole
motor 56 attached, for example, to a connector 57 and then to the nested
coiled tube 66 and
powered by high pressure fluid supplied by surface pumps 70.
[0145] The hard rock 58 found at depth can be effectively drilled by this
system. In one
embodiment, shallow depth rock 59 can be drilled, cased 61, and cemented 60 to
prevent loss
or introduction of fluid during drilling. Drilling fluids including drilling
mud water and spalls
are removed from the borehole through a flow line 62 to be separated and
possibly recirculated.
A rubber packoff in a stripper head 63 diverts the returns into the flow line
away from the drill
rig 410. On the surface, the coiled tube rig 400 contains a coiled tubing
injector 64a which is
used to drive the coiled tube within the borehole, a tube straightener 64b and
a gooseneck 65
which is used to guide the tubing from the injector 64 into or off of the reel
67. Fluid,
including e.g. reactants, can be fed in from a source 69 through a rotating
coupling 68 into the
reel assembly 67.
[0146] One example drilling system may include a drill string based thermally
assisted
tricone drilling system. An example thermally assisted tricone drilling system
500 is shown in
FIGS. 5A-5C. In this embodiment, heat to power a downhole spallation system
such as, for
example, a hydrothermal spallation drill system, can be provided by electrical
resistance
heating. A tricone bit 510 is incorporated into a distal end of the drilling
system 500. In one
embodiment, the tricone bit 510 has multiple rotating rollers 80a which
incorporate hard
segments, constructed, for example, from carbide, steel or ceramic segments,
that are used to
grind and wear away at the rock and are held in place by sleeve or roller
bearings 80b.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-41-
[0147] In one embodiment, electrical power may be generated using a downhole
turbine 83
in conjunction with an electrical generator 82. Power from the generator 82 is
carried to a
heater 75 through one or more power cables 71. Water 72 is pumped into the
heater and boiled
producing superheated fluid at high pressure that is ejected through one or
more nozzles 79 in
the drill bit. The heater 75 may include an insulating gap 74, as described
above. Drilling mud
and/or coolant is pumped down through an annular region 73 and into the
borehole through one
or more conduits 78. A surface assembly 90 may be attached to the tricone bit
510. The
surface assembly 90 may include a conductor pipe and conductor casing 87
cemented in place
86 in a surface rock portion 85 to protect the potable water zones and provide
a high pressure
seal to the earth. A segmented drill string 88 is driven into the ground and
rotated by the drill
rig 90 and connected to a drilling fluid circulating pump 91.
[0148] In alternative embodiments of the invention, a drilling system may
include a
spallation system, such as any of the spallation systems described herein,
coupled to other types
of mechanical drill bit, such as a PDC drill bit, diamond-impregnated coring
bit, or hammer
drill bit. Example drilling systems including a thermal spallation system
coupled to various
drill bits are shown in FIGS. 6-8B.
[0149] For example, FIG. 5 shows a PDC bit 600 incorporating a spallation
system such as
a hydrothermal spallation system. In this embodiment, fluid, including water,
fuel, and
oxidant, is introduced through an inlet tube 92 into a reaction chamber 95.
The reaction
chamber 95 may be insulated by, e.g. a pressurized air gap 96. Upon passing
into the chamber
95, the reactants within the fluid contact a catalyst located within the
chamber 95 and react,
producing high temperature reacted fluid. The reacted fluid exits through one
or more
openings 100 as jets directed against a target rock face. The spallation
system is contained in
the drill body 94 of the PDC bit 600 and connected to a drill string at a
threaded tool joint or
threaded connection 93. Drilling mud or coolant is pumped down through an
annular gap 97
and down to one or more outlet feeders 101 and vents 102 close to the bottom
of the drill bit
600. Rotation of the bit engages flutes 98 mounted on which the compacts 99,
such as, but not
limited to carbide or PDC compacts, cut away at the thermally affected target
rock surface.
The compacts 99 are cutting elements set in the matrix of the bit body on
ridges, sometimes
called blades, with flutes between the blades for mud flow and cuttings
passage to the annulus.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-42-
[0150] In an exemplary embodiment, nozzles 100 leading a PDC drill bit 600 may
be sized
to soften the rock just ahead of each cutter element (compacts) 99. Drilling
through the
presoftened rock will reduce the wear on the tool 600, especially the compacts
99.
[0151] FIGS. 7A and 7B show a drilling system 700 including an
abrasive/grinding bit
incorporating a hydrothermal spallation system. In this embodiment, water is
pumped
downhole through an opening 103 in a segmented drill string 104 into a
downhole turbine or
motor 105 located within a subassembly 106. The motor 105 is connected by a
shaft to a water
cooled rotating magnet assembly 107 contained within a housing 108. The magnet
assembly
107 surrounds a non-rotating metal core 109 having a series of holes to allow
a fluid to flow
therethrough to remove heat generated by induction from the rotating magnets
107. This
resulting super-heated fluid exits into a chamber 110 which may be insulated
by an air gap 111
from a coolant fluid channel 112. The heated fluid exits through one or more
nozzles 113 to
interact with a target rock surface. Coolant is directed from coolant exit
ports 114. An
abrasive material, such as, but not limited to diamond, are surface set into
or impregnated in a
plurality of cutter segments (pads) 115. In operation, the super-heated fluid
exiting the nozzles
113 and impinges upon the target rock surface, thereby damaging the rock and
assisting the
cutting of the rock by the cutter segments (pads) 115.
[0152] FIGS. 8A and 8B show a drilling system 800 including a thermal
spallation system
coupled to a hammer drill bit. In general, a hammer drill is a drill with a
hammering action.
The hammering action provides a short, rapid hammer thrust to pulverize
relatively brittle
material and provide quicker drilling with less effort. In one embodiment, the
hammer drill
may additionally include a rotating motion that may be used separately or in
combination with
the hammering motion. When used in the hammer mode, the tool provides a
drilling function
similar to a jackhammer.
[0153] In the embodiment of FIGS. 8A and 8B, coolant and/or drilling fluid is
introduced
into a bit 800 through a drill string connector 116 (e.g. a connection to a
drilling assembly that
includes drill collars to provide a hammer with a large and stiff inertial
load to push off of.)
The drill string connector 116 connects to the drill assembly. An upper valve
plunger 118 and
return spring 119 is integrated into the hammer bit 800 to rapidly press a
driver 121 into an
anvil 124, thereby driving the distal end of the anvil 124 from a distal end
128 of the bit 800 to
transmit a blow to a target rock surface. The driver 121 may include seals
120, 122, and a
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-43-
return spring 123. The anvil 124 is attached to the body of the bit 800
through a guide nut 125,
which also prevents rotation of the bit. Integral to the anvil 124 is a
thermal combustion
chamber 127 which is fed a fluid including a fuel, water, and an oxidant from
the surface
through a separate tube 117. The combustion chamber 127 may be thermally
insulated
through, for example, a pressurized air gap 126. Hot fluid/gas exits the
chamber 127 through
one or more jets 131 distributed across the drill face. The distal end of the
drill bit 128 is
cooled by water or drilling mud exiting through exit ports 129. Stress to the
thermally altered
rock is created by the hammering action combined with drill string rotation
through the carbide
buttons 130.
[0154] In other embodiments, improved well control may also be achieved
through the use
of a hydrostatic column of a fluid such as, but not limited to, water or
geothermal drilling mud,
to increase hydrostatic pressure e.g. to balance formation pressure in exposed
formation using,
e.g., deep surface or intermediate casing and high pressure blowout prevention
equipment
installed on a wellhead. Thermal spallation, coupled with high velocity liquid
flow through
nozzles, may produce high pressure jets, pulsating jets or abrasive jets to
produce a dual
spallation/jet drilling system. Such dual systems may include a combination of
hot and cold
jets or include operating spallation jets at higher flow rates than needed to
produce spallation
(and thus have a jet drilling process substantially directly ahead of the
nozzle and a spallation
process in the wall jet that forms beyond the radius of the jet produced
hole.). For example, the
use of high temperature fluids may greatly reduce the pressure required to
achieve jet drilling in
high strength rock. Additionally, the use of fluids with temperatures below
the brittle-ductile
transition of the rock may prevent the rock from being overheated and becoming
unspallable.
Alternatively, the rock may be heated above the ductile-brittle transition far
enough to soften
the rock enough that it can be swept away or drilled like soft to medium
sediments. This may
be advantageous, for example, for materials, such as basalts, which are
typically less prone to
spallation and not significantly damaged by heating to a temperature below the
ductile-brittle
transition.
[0155] A thermal degradation process or spallation formation may not be used
continuously. Rather, certain embodiments of the invention may include pulsed
heat treatment,
such as a cyclically pulsed heat treatment. In a further alternative
embodiment, the heat
treatment may be alternated with a cooling treatment. Such alternation may
increase the
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-44-
damage to the rock or may help moderate the temperature of the drilling
mechanism and
materials of construction while still imparting high temperature, at times,
against the rock
surface. In one embodiment, the thermal spallation jet(s), or other
appropriate heat source, may
be activated and turned off as required, thereby allowing the use of the
spallation system to
assist in the penetration through certain sections of a target rock, while
allowing the thermal
spallation process to be turned off when penetrating other sections or target
rock, for example
where thermal spallation is either not required or advantageous.
[0156] One embodiment of the invention includes a drill bit design for use
with a thermally
assisted mechanical drilling method. In one embodiment, for example in very
deep/hot
formations, the thermal treatment can be a cooling process, where a very low
temperature jet
causes microfracture of the surface through a reduction in temperature.
[0157] In one embodiment, the bulk of the fluid flow through the drilling
assembly - e.g.
the portion used for cooling and cuttings lift - may be relatively cool, while
only a small
portion - e.g. that used for thermal degradation - is hot. As a result, some,
or all, of the cold
fluid can be used to provide cooling to at least a portion of the drilling
device. For example,
cold fluid may be sent through or around the mechanical drilling structure to
reduce its
temperature and improve survivability. In one embodiment, cold water may be
sent through
flow channels in a traditional PDC or tricone bit, while the hot portion of
the fluid is insulated
directed substantially down against the rock. The channels transporting the
hot water may be
isolated from the bit by a layer of insulation, such as, but not limited to, a
substantially solid,
liquid, gas, or vacuum insulation layer, or a combination of the different
insulation layers. In
one embodiment, the relative ratio of hot/cold can be adjusted to balance the
performance of
the two drilling mechanisms.
[0158] One embodiment of the invention includes a spallation system including
control
systems, and associated methods, adapted, for example, to control the diameter
of the wellbore
produced by a e.g., hydrothermal jet, maintain the desired well hole
trajectory, control the
distance between the nozzle and the bottom of the hole (i.e. the "stand-off"),
and/or ensure a
sufficient temperature differential so as to induce spallation. These control
systems may
include software and/or hardware based control elements designed to ensure
optimum
performance of the thermal drilling system.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-45-
[0159] Disclosed methods may include introducing a flow of water into the
borehole. This
flow of water may be used, for example, to at least partially form an
ascending fluid stream to
carry loose material such as, but not limited to, the spalled, drilled, or
otherwise loose rock
from the bottom of the borehole. The returning fluid may also travel up the
borehole in reverse
circulation, e.g., where the fluid can be directed upward through a separate
tube or annulus in
the main drill string. The water flow may also be used to provide cooling for
one or more parts
of the system and/or surrounding rock. The provided cooling may be produced by
at least one
of temperature cycling, thermal protection, and a circulated cooling fluid.
[0160] In one embodiment, a heat exchanger may be coupled to a portion of the
system
above the nozzle of the thermal spallation system. This heat exchanger may be
used, for
example, to exchange heat between a working or heating fluid (e.g. a reacted
fluid), spallation
fluid, and loose material ascending through the borehole and the fluid being
pumped to the
thermal spallation system, e.g. an unreacted fluid, within a conduit extending
from the surface
to the thermal spallation system.
[0161] In one embodiment, one or more of properties of working fluid and jet
may be
selected to ensure that that the required conditions are met for optimum
spallation. These jet
properties may include, but are not limited to, a temperature, a heat flux, an
exciting jet
velocity, a heat capacity, a heat transfer coefficient, a Reynolds number, a
Nusselt number, a
density, a viscosity, and/or e.g., a mass flow rate. For example, these
properties may be
obtained through selection of the specific fluids used, by mixing of multiple
fluids, and/or by
treatment of the fluid through heating, cooling, pressurizing, chemically
treating, or otherwise
adjusting the composition of the working fluid. Exemplary ranges, without
being limiting, for
a thermal system for borehole creation from 1,000-30,000 feet, using a working
fluid, may
include those provided in Table 1 below. Such parameters may be determined by
using a
disclosed working fluid in several different or similar rock formations, as
exemplified below,
and assessing preferable ranges.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-46-
Property Borehole creation
Temperature (C) 400-1200
Total Heat Output (MW/m2) 0.1-100 (e.g. about 1-10)
Heat Flux (MW/m) 0.1-100
Mass Flow (lbs/min) 0-500
Exiting Jet Velocity (m/s) 0-700 (e.g. about 400-700)
Heat Capacity of the Working Fluid (kJ/kg*K) 2.26-5
Heat Transfer Coefficient of Working Fluid (kW/m2*K) 38-56
Reynolds Number 0.5x 106 - 2.5x 106 (for 1" hole)
(for the single, non-rotating center jet with round nozzle with 12x106 - 60x
106 (for 24" hole)
diameter = 1/16 of hole diameter)
Nusselt Number 30-45 (for 1" hole)
740-1040 (for 24" hole)
Density of working fluid at temperature/pressure (g/cm) 0.01-0.1
Viscosity of working fluid at temperature/pressure (cp) 0.025-0.045
Induced Strain in Rock (%) 0-30
Spall Size, 80% of total mass (mm) 0.001-3
Table 1: Example property ranges for Hydrothermal Spallation drilling of
boreholes.
[0162] For example, a temperature at least that of the onset of rapid thermal
spallation but
below the, e.g. brittle ductile transition of the rock may be maintained.
[0163] The total heat output - the thermal power of the drill divided by the
cross sectional
area of the borehole to be drilled - may be kept, for example, between 0.1 and
100 MW/m2.
The heat flux - a product of the heat transfer coefficient and the temperature
difference between
the wall jet and the rock surface - may be kept, for example, between 0.1 and
100 MW/m2. In
certain embodiments, if too low a value of heat flux is used, a thermal
gradient may propagate
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-47-
and build in the rock, reducing the relative strain of the surface rock to the
underlying layer,
thereby reducing or preventing spallation. In one embodiment, it is possible
to increase the
heat flux by increasing the Reynolds number - a dimensionless number that
gives a measure of
the ratio of inertial forces to viscous forces - in the nozzle exit. In
certain embodiments, the
heat flux of a thermal jet for spallation drilling may be increased without
having the jet exceed
the temperature e.g. brittle-ductile transition of the rock, by increasing the
mass flow, and/or
reducing the nozzle diameter (to increase the exiting jet velocity).
Increasing the velocity or
mass flow of the jet may also provide a mechanical or erosive means of
removing material or
spalls from the rock surface, clearing and providing a freshly exposed surface
for further
spallation, and/or help with spall and cuttings lift.
[0164] The Nusselt number - a dimensionless ratio of convective to conductive
heat
transfer across (normal to) the rock-fluid boundary - may, in a non-limiting
example, for a
working fluid in one or more of the disclosed systems, be between about 30 and
1040,
depending on hole size. In one embodiment, working fluid properties can be
optimized so as to
produce an induced strain within the grains of the rock of between about 0-
30%, thereby
generating enough stress to cause structural failure, which may make use of
existing flaws,
discontinuities, or grain boundaries in the rock and/or in-situ stresses
[0165] Spall sizes may, in one embodiment, be optimized so that 80% of the
transported
spalls maintain a range of 0.001-3 mm. If the produced spalls are too large,
they may not be
lifted by the drilling fluid and may plug small openings in heat exchangers
and internal returns
tubes used in reverse circulation. If the produced spalls are too small, it
may be an indication
that the heat flux is too high, causing excessive microfracturing beyond what
is needed for
drilling and cuttings lift, thereby wasting energy and sacrificing efficiency,
as well as
increasing mineral dissolution. Spall size may also be controlled to help plug
fractures leading
to lost circulation or intrusion of fluids during drilling, or to attempt not
to plug fractures in
producing zones during e.g. hole opening for enhanced wellbore impedance.
[0166] In one embodiment, at least one property of the spalls and/or working
fluid (e.g.
reacted fluid) may be monitored to provide information relating to the
spallation process. For
example, the spall size, shape, chemical composition, and/or number of created
spalls may be
monitored to provide information on the efficiency of a spallation process. In
addition, or in
the alternative, one or more properties of the reacted fluid may be monitored
to provide
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-48-
information on the efficiency of the catalytic reaction between the unreacted
fluid and the
catalyst. By monitoring one or more of these properties, information on the
spallation process,
such as, but not limited to, the efficiency of the heating reaction, the rate
of spallation, the
composition of the spalled rock, the temperature of the fluid leaving the
nozzle, and/or the heat
flux at the target surface may be deduced.
[0167] In an embodiment, the properties of the fluids may be used to inform
the adjustment
or addition of any additives into the unreacted or cooling-lift water streams.
Such additives
may include cleaning agents (e.g. to remove deposits from a catalyst, nozzle
or heat
exchanger), and additives that increase or decrease tendencies for materials
in returning fluids
to crystallize, precipitate, or agglomerate. Contemplated cleaning agents may
include solids
that are significantly abrasive to unwanted deposits but not to the ceramic or
metal of the
nozzle. A cleaning agent may be added continuously to a flow, or sent down
periodically.
Additives may also assist in the opening of existing fractures in production
zones, or by
preventing the produced spalls and minerals from plugging the existing
fractures by e.g.
mineral redeposition.
[0168] The monitoring may be carried out using at least one of a thermal
measurement, an
optical measurement, an acoustic measurement, a chemical measurement, and a
mechanical
measurement (e.g. a flow meter). For example, a laser-based optical system may
be used to
measure one or more properties of the spalls exiting the borehole. In
alternative embodiment,
any appropriate measurement device may be used.
[0169] If a change in one or more properties is observed, a property of the
fluid and/or
spallation system may be adjusted to compensate for the observed change and
ensure optimum
spallation. This adjustment may be made, for example, by adjusting one or more
properties of
the unreacted fluid being sent down the borehole to adjust the fluid
temperature and/or heat
flux created by the spallation process to maintain e.g., a pre-determined
spall size. The
unreacted fluid may be adjusted by changing a parameter such as, but not
limited to, a chemical
composition, a fluid mixture, a pressure, and/or a temperature.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-49-
[0170] In one embodiment, control of the Reynolds number of the fluid jet at
the exit of the
nozzle by, e.g. controlling the mass flow exiting the nozzle, controlling the
nozzle size, and/or
controlling the viscosity of the fluid, may assist in controlling the heat
flux at the surface of the
rock at the target location.
[0171] The spalls and/or reacted fluid may be monitored at the surface (i.e.
after traveling
from the distal end of the borehole to the surface in the ascending fluid
stream). In an
alternative embodiment, the spalls and/or reacted fluid may be monitored at a
location part way
down the borehole and/or at the distal end of the borehole. In one embodiment
the spalls
and/or reacted fluid are monitored at a single location. In an alternative
embodiment, the spalls
and/or reacted fluid are monitored at multiple locations.
[0172] One embodiment disclosed herein includes a method for excavation of a
borehole in
a geological formation by using a heat source, such as, but not limited to, a
thermal drilling
system to create a pilot borehole in a geological formation, measuring at
least one property of
the geology of the pilot borehole, evaluating the at least one measured
property to determine
whether to enlarge the pilot borehole, and enlarging the pilot borehole if the
at least one
measured property meets a set requirement. The pilot borehole may be enlarged
by inserting at
least one of a spallation drilling system and a mechanical drilling system
into the pilot
borehole.
[0173] This method may be advantageous in situations where a pilot borehole is
to be
formed in order to test the properties of the geology to determine whether
further drilling and
completion is warranted. The smaller pilot borehole is cheaper to drill than a
larger diameter
borehole, but may still allow access to the subterranean geology for testing.
The pilot borehole
may also be used as a guide hole for the larger borehole drilling, and may
weaken the structure
of the rock surrounding the pilot borehole to facilitate easier drilling of
the larger borehole.
[0174] The evaluating step may include evaluating whether the geological
formation is
suitable for use as, for example, an injection or production borehole for at
least one of a
geothermal system, oil and gas, mining, excavation, or CO2 or nuclear
sequestration or storage.
As discussed above, one or more properties of the geology of the pilot
borehole may be
evaluated by evaluating at least one property of spalls and/or the fluid (e.g.
the reacted
spallation fluid, a cooling fluid, and a drilling mud) exiting the borehole.
In various
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-50-
embodiment, any of the drilling systems described herein may be used to create
the pilot
borehole and/or larger borehole.
Self-Casing
[0175] The fluids used in the systems described herein, and/or the loose
materials created
by the process described herein, can, in one embodiment, strengthen and seal
the walls against
structural collapse and wellbore fluid loss, thereby greatly extending time
interval between
casing of the borehole. This may happen through processes such as, but not
limited to,
precipitation of materials on the surface walls of the borehole and/or
depositing of loose
materials within cracks and other cavities on the walls of the borehole.
[0176] In some applications, however, it may be desirable to install casing in
addition to
any self-casing processes produced by the systems and methods described
herein. For larger
diameter borehole, for example, casing may be accomplished employing
conventional
telescoping casing strings using methods familiar to those skilled in the art.
For small diameter
boreholes, the slim borehole can be cased, for example, using an expandable
casing string that
is inserted into the borehole and then radially expanded. The casing may be
made of a
malleable material, and when it is placed in the borehole, it can be radially
expanded against
the borehole wall upon application of an internal radial load.
[0177] The examples which follow are intended in no way to limit the scope of
this
invention but are provided to illustrate the methods and apparatus of the
present invention.
Many other embodiments of this invention will be apparent to one skilled in
the art.
Example 1
[0178] An example method of testing the efficiency of a thermal spallation
system is
described below. This method may be used to test any appropriate spallation
system on a
material.
[0179] In the embodiment of Example 1, a Sierra White granite rock core
measuring 4" in
diameter and 6" long was prepared by pre-drilling a 0.75" diameter hole 0.5"
deep on the top
surface. The core was then loaded into a stainless steel pressure vessel. A
preheater was
assembled by winding a 20' long section of 0.125" ID stainless steel tubing in
a machined
groove around a 4" brass block in which contained a series of rod heaters.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-51-
[0180] The thermal spallation system included a 0.5" ID x 3" long catalyst
chamber which
exits through a single, 0.09" diameter non-rotating nozzle located along the
central axis. The
catalyst chamber is filled to a height of roughly 1.5" with 0.5% platinum on
1/16" spheres
having a surface area of 100 m2/g. A series of stainless steel screens,
spacers, and diffusers
allow fluid to pass through while holding the catalyst bed in place. The drill
head is insulated
from surrounding cooling water by a 0.040" gap pressurized with nitrogen.
[0181] Before the start of a test, the drill head is driven to the bottom of
the predrilled hole
and a depth is read off of a dial indicator. The drill head is then retracted
approximately 1.5"
from the bottom of the hole into a large cooling water chamber.
[0182] The hydrostatic pressure in the vessel is then raised to 1600 PSI by
means of a back-
pressure dome regulator. An axial load of 6000 PSI and confining pressure of
3000 PSI are
applied by separate pumps acting upon the core to simulate deep geological
formation
conditions. An air actuated pump is used to deliver 3 g/s of a 20% by volume
methanol in
deionized water through the preheater which raises the temperature of the
unreacted fluid to
250-300 C. A high pressure oxygen flow is metered into the preheated aqueous
methanol
solution at sub-stoichiometric ratios.
[0183] The thermal spallation system may use a methyl alcohol fuel, and an 02
oxidant.
The aqueous methanol/02 solution travels through the spacers, screens, and
diffuser and over or
through the catalyst bed. The catalyst is not preheated and does not need an
additional heat
source such as a glow plug, spark, or flame for the reaction to initiated or
maintained. The
substantially flameless catalytic oxidation of the methanol produces heat
within the water
which raises the temperature of the fluid to 800-900 C.
[0184] The high temperature fluid exiting the nozzle into the cooling chamber
is initially
diverted and cooled by a 4 GPM water flow. The flow of aqueous methanol is
increased to 9
g/s over 2 minutes while simultaneously adjusting the oxygen flow. The drill
head is then
driven by a high pressure fluid pump at a rate of 1.0"/min through a stainless
steel seal,
isolating it from the cooling water, and into the predrilled hole to a
standoff of 0.25" from the
rock surface, as measured by the dial indicator. The displacement of the drill
head is then
reduced to 0.5"/min. The drill head penetrates into the rock until it reaches
the full stroke of
the equipment, roughly 1.5" below the predrilled rock surface. In one
embodiment, the drill
head is then held at this position to demonstrate the ability of the center
jet nozzle to drill in
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-52-
advance of the drill head and under ream. In an alternative embodiment, the
drill head need not
be held consistently at the bottom. Fluid and spalls exit the borehole into
the cooling water
above the rock via a 0.189" tube approximately 1.5" in length. The bulk fluid
then passes
through a series of screens which remove the bulk of the spalls before the
bulk fluid passes the
back pressure dome regulator and then through a low pressure hydrocyclone to
remove very
small size spalls. The spalls from may be separated from the bulk fluid by
filtering through a
200 mesh screen which retained approximately 88% of mass of the excavated
rock. Size
analysis may be performed by laser light scattering.
[0185] After being held for 10 minutes at this depth, the drill head is
rapidly retracted
through the borehole seal, allowing cooling water to fill the hole and the jet
to be diverted,
quenching the spallation process. Aqueous methanol and oxygen flow rates are
gradually
reduced and the preheater is turned off.
[0186] The sample may then be removed from the cell. The volume of excavated
rock may
thereafter be determined from the mass of water required to fill the volume of
the new
borehole, less the volume of the predrilled hole. The rock core may then be
dried and weighed.
A image of a rock core sectioned axially following the test with the drill
head that produced the
borehole is shown in FIG. 17. A graph showing spalled particle size
distribution for the system
of Example 1 can be seen in FIG. 21.
Example 2 Repeatability and Other Rock Types
[0187] An experiment as in Example 1 was been repeated on Sierra White
Granite, as
shown in Table 2:
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-53-
run# 1 2 3 4 5
hole volume (cc) 104.1 84.9 55.1 42.6 57.3
hole depth (cm) 10.26 8.27 6.016 6.49 6.16
final drill nozzle depth (cm) 4.66 4.47 3.67 4.33 4.655
final stand off (cm) 5.6 3.8 2.346 2.16 1.505
penetration rate(pump setting) 200 400 600 800 990
run time(sec) 300 150 125 73 62
pen rate (cm/min) 0.9525 1.905 2.8575 3.81 4.6736
quarrying rate (cc/min) 20.82 33.96 26.448 35.0137 55.45161
avg hole area (cm) 10.1462 10.26602 9.15891 6.563945 9.301948
avg hole diameter (cm) 3.594239 3.6154 3.414893 2.890931 3.441456
avg hole diam (in) 1.415055 1.423386 1.344446 1.138162 1.354904
quarrying rate (m /hr) 0.001249 0.002038 0.001587 0.002101 0.003327
Table 2: Additional hydrothermal spallation drilling of boreholes in Sierra
White Granite
[0188] The process was conducted on other rock types including Sioux
Quartzite, Wausau
Granite, Berea Sandstone, and granodiorites, as shown in Table 3, as well as
Barre, and
Westerly granites:
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-54-
run# 6 7 8 9 10
wausau souix berea
rock type granite quartzite sandstone granodiorite granodiorite
hole volume (cc) 202.3 199.1 195.7 133.5 131.7
hole depth (cm) 10.202 11.121 11.32 8.72 9.243
final drill nozzle depth (cm) 4.48818 4.445 4.572 3.62204 4.198
final stand off (cm) 5.71382 6.676 6.748 5.09796 5.045
penetration rate (pump setting) 9 18 27 9 9
run time (sec) 179 138 69 167 183
penetration rate (cm/min) 2.5 5 7.5 2.5 2.5
quarrying rate (cc/min) 67.81 86.57 170.17 47.96 43.18
avg hole area (cm2) 19.83 17.90 17.29 36.86 14.25
avg hole diameter(cm) 5.02 4.77 4.69 6.85 4.26
avg hole diam (in) 1.98 1.88 1.85 2.70 1.68
Table 3: Example results for hydrothermal spallation drilling of boreholes in
other rock types
[0189] Other tests were conducted on Sierra White Granite while independently
varying a
number of parameters including temperature, mass flow, axial stress, confining
stress, nozzle
diameter, jet velocity, heat flux, rate of drill head displacement. Table 1,
above, indicates
determined parameters used to enable hydrothermal spallation in one embodiment
of the
invention.
[0190] Other tests following Example 1 were conducted with hydrostatic
pressures
including near ambient, 1500 PSI (subcritical), and 3500 PSI (supercritical),
to demonstrate the
viability of this system from shallow to deep wellbores.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-55-
Example 3 Borehole Drilling- 4" diameter in hard rock
[0191] A 4" diameter hole is pre-drilled to a depth of 5" in Sierra White
granite rock block
measuring 24x24" square and 36" tall. A drill head interface is placed in the
pre-drilled hole
and sealed in place with high temperature cement. The block is centered in
cylindrical steel
mold 38" diameter, 44" in length, with a 0.375" wall. This mold had been split
down the side
and support railings were welded onto the outside edge. Bolts are used to
clamp the two halves
of the mold together. Concrete is poured to fill the empty volume between the
rock block and
mold. The concrete is allowed to cure for 10 days, after which time the bolts
are tightened to
provide 150 psi clamping pressure on the sample. A diagram of the apparatus is
shown in
FIGS. 14A-C.
[0192] Approximately 450 g of Instant Steam catalyst obtained from Oxford
Catalyst PLC
is loaded into a converging radial flow reactor and placed inside a 2 7/8" OD
drill head, as
shown in FIG. 11. The drill head is slid into the drill head interface. Before
the start of a test,
the drill head is driven to the bottom of the predrilled hole and a depth is
read off of the
computer controls. The drill head is then retracted approximately 10" from the
bottom of the
hole to allow cooling water from the drill head interface to enter the bottom
of the hole. A
mixture of 38% hydrogen peroxide and 12% methanol by weight is pumped into the
catalyst
bed at 3200mL/min. Neither the catalyst nor the fuel/oxidant fluid is
preheated, and no
additional heat source such as a glow plug, spark, or flame for the reaction
is used. The
mixture "lights off' over the catalyst bed producing a 800 C jet of steam
which exits a single,
0.189" diameter non rotating nozzle located along the central axis.
[0193] The drill head interface is advanced quickly through a stainless steel
seal in the drill
head interface, isolating it from the cooling water, and into the predrilled
hole a to a distance of
5" off the bottom of the hole; the advance rate is then reduced to a setpoint
drilling rate of 10'/h
by a stepper motor, gear reducer, drive screw, ball nut, and static and
sliding support members.
A load cell is included to measure the drive force transmitted to the drill
assembly. The drill is
advanced to its full stroke, roughly 13" below the depth of the predrilled
hole.
[0194] The reaction is immediately quenched by stopping the flow of the
reactants, and the
drill is removed to reveal a hole that extends 5" past the final depth of
nozzle exit. The sample
is removed from the concrete and sectioned to display the hole that is
created, as shown in FIG.
14.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-56-
Example 4 Field Drilling
[0195] A thermal spallation system can be deployed on a customized AmKin 800 V
track
mounted coiled tubing unit. A 20' long 2 7/8-3 1/2" OD bottom hole assembly is
prepared from
instrumentation and controls subassembly (or "sub"), a release sub, a dynamic
barrier sub,
stabilizers and centralizers, and an iteration of the steam generation sub
described in Example
4. The steam generation sub houses an axial catalyst bed 2 1/2" in diameter
and 12" long filled
with Oxford Catalysts Instant Steam catalyst. The bottom hole assembly is
attached to a
Tenaris HS-90 2.00" steel coiled tubing with a 0.134" wall through a connector
sub. Inside of
the coiled tubing, a 3/8" OD nitric-acid passivated stainless steel capillary
is housed to
transport the unreacted fluid to the steam generation sub, and a 5/16" 7-
conductor wireline
cable is used for communication in the instrumentation controls sub.
[0196] A starter well is drilled into competent rock and lined with 4" ID
casing. At the top
of the casing is mounted a wellhead diverter with stripper rubber. The bottom
hole assembly
and coiled tubing is run through a wellhead diverter seal to the bottom of a
water-filled 300'
hole.
[0197] The unreacted fluid is prepared at the surface by continuously metering
52% high
test peroxide, reagent grade methanol, and deionized water into a mix tank to
produce 38%
peroxide and 12% methanol. The mixture is pumped through the capillary at 1
gallon per
minute to the catalyst bed where it self-energizes and reacts with the
catalyst element without
the need for an external energy source (such as a spark, glow plug or flame
holder) thus
generating a 800 C reacted fluid, without an inherently unstable flame or the
need for cooling
water to protect the materials of construction or overheating of the rock.
This reacted fluid is
then emitting through a 0.189" nozzle and directed at the bottom of the hole,
causing rapid
spallation of the rock. The coiled tubing is fed into the hole at a rate of
20'/h by means of the
coiled tubing injector on the AmKin 800 V continuously drilling a 4" borehole
in the solid
granite. Spalls are swept through the dynamic seal assembly where they meet a
50 gallon per
minute flow of water flow, which has travelled down inside the 2" coiled
tubing and exited a
series of upward pointing jets, to cool the reacted fluid and carry the spalls
to the surface. At
the surface, the spalls are removed by a series of "shakers", cyclones, and
filters, the water is
cooled by a 200 kW mud cooler, and continuously recirculated.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-57-
Example 5 Multilaterals with hole opening
[0198] A system as described in Example 4 can be used to create multilaterals.
At the
desired depth, the bottom hole assembly is deviated, the spallation jet is
directed at the wall of
the borehole causing the drill to create a hole off-axis from the existing
borehole. The bottom
hole assembly is advanced using the coiled tubing injector and intersects
additional fracture
networks which can provide flow to the main wellbore. When the final target
depth ("TD") is
reached, the unreacted fluid is directed through a second catalyst bed that is
in fluid
communication with 6 jets oriented normal to the axis of the bottom hole
assembly and spaced
60 degrees apart around the circumference of the tool. The unreacted fluid is
pumped again
and reacted fluid exits the circumferential jets, expanding the diameter of
the wellbore as the
bottom of the hole assembly is withdrawn on the coiled tubing. Periodically,
this hole opening
process is paused and the well is allowed to produce fluid, blowing produced
spalls and loose
rock from the fractures. Flow sensors including "spinners", and thermocouples
are used to
infer the flow rate from a given fracture. If additional hole opening is
required, the hole
opening is restarted. In certain sections of the well where larger/global hole
opening is desired,
the bottom hole assembly can be held in place, causing extensive spallation,
macrofracturing,
breakout and collapse of sections in the producing zone.
Example 6 Hole opening of a 0.75" borehole
[0199] Using the procedure of Example 1, a Sierra White granite rock core
measuring 4" in
diameter and 6" long was prepared by pre-drilling a 0.75" diameter hole 4"
deep on the top
surface. The core was then loaded into a stainless steel pressure vessel
described in Example 1.
[0200] The thermal spallation system includes a 0.5" ID x 3" long catalyst
chamber which
exits through a single, 0.04" diameter non-rotating nozzle oriented
perpendicular to the existing
predrilled hole. The catalyst chamber is filled to a height of roughly 1.5"
with 0.5% platinum
on 1/16" spheres having a surface area of 100 m2/g. A series of stainless
steel screens, spacers,
and diffusers allow fluid to pass through while holding the catalyst bed in
place. The drill head
is insulated from surrounding cooling water by a 0.040" gap pressurized with
nitrogen. The
drill head is held in a large cooling water chamber during start up.
[0201] The hydrostatic pressure in the vessel is then raised to 1600 PSI by
means of a back-
pressure dome regulator. An axial load of 4500 PSI and confining pressure of
3000 PSI are
applied by separate pumps acting upon the core to simulate deep geological
formation
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-58-
conditions. An air actuated pump is used to deliver 3 g/s of a 20% by volume
methanol in
deionized water through the preheater which raises the temperature of the
unreacted fluid to
250-300 C. A high pressure oxygen flow is metered into the preheated aqueous
methanol
solution at sub-stoichiometric ratios.
[0202] The thermal spallation system uses methyl alcohol fuel, and an 02
oxidant. The
aqueous methanol/02 solution travels through the spacers, screens, and
diffuser and over or
through the catalyst bed. The catalyst is not preheated and no additional heat
source is used.
The catalytic oxidation of the methanol produces heat within the water which
raises the
temperature of the fluid to 800-900 C.
[0203] The high temperature fluid exiting the nozzle into the cooling chamber
is initially
diverted and cooled by a 4 GPM water flow. The flow of aqueous methanol is
increased to 9
g/s over 2 minutes while simultaneously adjusting the oxygen flow. The drill
head is then
driven by a high pressure fluid pump at a rate of 7.5 cm/min through a
stainless steel seal,
isolating it from the cooling water, and into the predrilled hole. The reacted
fluid spalls the
wall of the borehole until it reaches the full stroke of the equipment,
roughly 1.5" below the
predrilled rock surface. Fluid and spalls exit the borehole into the cooling
water above the rock
via a 0.189" tube approximately 1.5" in length. The bulk fluid then passes
through a series of
screens which remove the bulk of the spalls before the bulk fluid passes the
back pressure dome
regulator and into a large collection tank.
[0204] The drill head is rapidly retracted through the borehole seal, allowing
cooling water
to fill the hole and the jet to be diverted, quenching the spallation process.
Aqueous methanol
and oxygen flow rates are gradually reduced and the preheater is turned off.
[0205] The sample is then removed from the cell. A large slot is formed along
the length of
the predrilled hole in the same orientation as the jet, increasing the
diameter by roughly 2x.
[0206] Effective experiments, following Example 5, holding the jet stationary
to open the
hole globally; using axial jets, multiple jets, and diffuse heating; and where
rock is intentionally
fractured either or parallel or normal to either the existing borehole or the
jets have also been
conducted. In one embodiment, as shown in FIG. 18, using a vertical spallation
jet in a
predrilled 7/8" hole 1" deep (shown as dashed lines) into a 4" diameter rock
core increased the
diameter by roughly 2x and created a thermally affected zone (shown by arrow)
of highly
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-59-
altered materials with reduced strength, as determined by SEM-EDAX, thin
sections,
microscopy, punch and modified Chercar testing.
Example 7 Thermal and Mechanical Drilling
[0207] Spalls and/or a reacted rock region can be formed as described above. A
reamer
element, including one or more reamer elements mounted to the housing and
located back from
the distal portion of the thermal spallation system, can then be used to ream
the thermally
effected rock at the outer sides of the borehole created by the thermal
spallation system to
enlarge and/or shape the borehole, as required.
Example 8 Thermal Heating and TSP Drag bit
[0208] Spalls and/or a reacted rock region can be formed as described above. A
drag bit
with TSP cutters is then used to remove the thermally effected rock from the
borehole more
easily than if the rock was not heated.
Example 9 Rock sample tests
[0209] Thin sections: samples extracted from rocks in Examples 1-4 were cut
into small
sections using diamond blades and sent to a thin section preparation
laboratory. The samples
were evacuated and saturated with a blue epoxy to identify pores and
fractures. The samples
were polished and then mounted to a glass slide and the section ground down to
a thickness
required using a transmission microscope with polarizing lens to determine
mineral structure
alteration and other microscopic features.
[0210] Microscopic observations on the regions near the borehole suggest
thermal
fracturing of grains especially quartz and feldspars but little or no
alteration of these minerals is
apparent in the micrographs.
[0211] Binocular microscope: samples were inspected with a binocular
microscope looking
for evidence of alteration fractures and other feature associated with changes
in the physical or
chemical properties due to the rapid heating accompanying hydrothermal
spallation. Radial
crack were identified in many of the samples that have the appearance of being
filled with
small quartz remnants (spalls). A general bleaching of the thermally altered
surface suggests
removal of iron and other color generating compounds.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-60-
[0212] Punch tests: a small spring loaded punch (pointed tool steel) was used
to remove
small amounts of rock. The spring force on each punch when triggered is
approximately 15
pounds total. The removed rock was collected and the total amount weighed. A
series of
punches tests (20 ea) were used on each sample on the thermally affected zone
and on virgin
rock, and results shown below:
Rock Removed (grams
from 20 punches)
Thermally-Affected %
Rock Type Untreated Zone Increase
Sierra White
Granite 0.014 0.084 600%
Red Wausau
Granite 0.019 0.044 232%
Diorite 0.017 0.057 335%
Souix Quartzite 0.017 0.027 159%
Berea Sandstone 0.053 0.127 240%
Dye penetrant: a visual dye penetrant was applied to the surface of the
thermally altered rocks
to see the extent and depth of the fracturing/alteration. After application
the rocks were
visually inspected with the binocular microscope. FIG. 20 shows an image of an
example
diorite sample indicating the depth of penetration of the dye into the altered
zone and the flow
of dye into two smaller fracture zone perpendicular to the altered region. In
various
embodiments of the invention, dye penetration from about 0.7 cm, at the
regions closest to
where the jet is impacting the rock, to approximately 1.5 cm further up the
annulus, where the
rock has been exposed to the superheated fluid longer, may be achieved.
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-61-
References
[0213] All publications and patents mentioned herein, including those items
listed below,
are hereby incorporated by reference in their entirety as if each individual
publication or patent
was specifically and individually incorporated by reference. In case of
conflict, the present
application, including any definitions herein, will control.
US5,771,984; US7,742,603; US7,025,940; US2008/0093125
"Feldspars and Feldspathoids, Structures, Properties, and Occurrences:
Structures, Properties
and Occurrences," by William L. Brown, North Atlantic Treaty Organization
Scientific Affairs
Division, Published by Springer, 1983.
"Hydrolytic weakening of quartz and other silicates," by D.T. Griggs, Geo-
phys. J. Roy.
Astron. Soc., 1967.
"Origin of granite in the light of experimental studies," by Tuttle, O.F. and
N.L. Bowen, Geol.
Soc. Am. Mem. 74, 1958.
Equivalents
[0214] While specific embodiments of the subject invention have been
discussed, the above
specification is illustrative and not restrictive. Many variations of the
invention will become
apparent to those skilled in the art upon review of this specification. The
full scope of the
invention should be determined by reference to the claims, along with their
full scope of
equivalents, and the specification, along with such variations.
[0215] Unless otherwise indicated, all numbers expressing quantities of
ingredients,
reaction conditions, and so forth used in the specification and claims are to
be understood as
being modified in all instances by the term "about." Accordingly, unless
indicated to the
contrary, the numerical parameters set forth in this specification and
attached claims are
approximations that may vary depending upon the desired properties sought to
be obtained by
the present invention.
[0216] The terms "a" and "an" and "the" used in the context of describing the
invention
(especially in the context of the following claims) are to be construed to
cover both the singular
and the plural, unless otherwise indicated herein or clearly contradicted by
context. Recitation
of ranges of values herein is merely intended to serve as a shorthand method
of referring
CA 02740048 2011-04-08
WO 2010/042720 PCT/US2009/060003
-62-
individually to each separate value falling within the range. Unless otherwise
indicated herein,
each individual value is incorporated into the specification as if it were
individually recited
herein. All methods described herein can be performed in any suitable order
unless otherwise
indicated herein or otherwise clearly contradicted by context. The use of any
and all examples,
or exemplary language (e.g. "such as") provided herein is intended merely to
better illuminate
the invention and does not pose a limitation on the scope of the invention
otherwise claimed.
No language in the specification should be construed as indicating any non-
claimed element
essential to the practice of the invention.
[0217] Having described certain embodiments of the invention, it will be
apparent to those
of ordinary skill in the art that other embodiments incorporating the concepts
disclosed herein
may be used without departing from the spirit and scope of the invention.
Accordingly, the
described embodiments are to be considered in all respects as only
illustrative and not
restrictive.
[0218] What is claimed is: