Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02740612 2011-04-14
WO 2010/047820 PCT/US2009/005774
1
SYSTEM USING UNUTILIZED HEAT FOR
COOLING AND/ OR POWER GENERATION
FIELD OF THE INVENTION
[0001] The present invention relates to methods and systems of employing
sorbent materials to provide refrigeration or to power a driver device, such
as a
drive shaft, particularly in chemical processing and petroleum refining
operations.
BACKGROUND OF THE INVENTION
[0002] Chemical processing operations, including petroleum refining and
petrochemical operations, are energy intensive. It is often necessary to
conduct
these operations at high temperatures using high temperature heat sources
including but not limited to steam. After the steam and other hot streams have
performed the intended functions, there remains unutilized energy. Refineries
and petrochemical facilities typically utilize only 70% of the input energy
needed to conduct processing of crude oil to products.
[0003] In an effort to increase energy efficiency, it is desirable to
recover and
utilize unutilized heat. One prior art method disclosed in U.S. Patent No.
5,823,003 to Rosser et al attempts to make use of waste heat and apply such
heat
to an adsorbent material in order to release an adsorbed gas at higher
pressure,
which in turn can be used in a refrigeration cycle that contains an expansion
valve. U.S. Patent No. 5,823,003 discloses the use of a zeolite-water pairing.
[0004] Current methods to obtain refrigeration from sorbent materials in
chemical process applications have limitations. Often the sorbent materials
and
gases employed in sorption systems require other process equipment such as
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
2
pumping devices, that are expensive to maintain, unreliable and require a
large
allocation of space. Such limitations often render the recovery of the
unutilized
heat economically unsustainable.
[0005]
Accordingly, there remains a need to make unutilized heat recovery
efforts more cost-effective by providing the opportunity to utilize lower and
higher grades of unutilized heat, to reduce equipment and space requirements
of
the process. There also remains a need to provide other uses, besides
refrigeration, of the fluid released from unutilized heat-charged sorbent
materials.
SUMMARY OF THE INVENTION
[0006] By
proper selection of the absorbent material and the fluid, sorption
systems can be provided that are efficiently powered by lower temperature
unutilized heat and require no supplemental equipment (e.g., compressors and
pumps).
[0007]
Accordingly, one embodiment of the present application provides a
sorption system including an adsorbent material and a fluid, in which the
sorbent
material and fluid in combination have a "pressure index" of at least 1.2
depending on the type of unutilized heat stream. For higher temperature
unutilized heat streams (e.g., between 600K and 1200K), the pressure index is
at
least 1.2. For lower temperature unutilized heat streams (e.g., less than
600K),
the sorbent material and fluid in combination have a pressure index of at
least
1.5. In a preferred embodiment, the pressure index is at least two, or at
least
three, or at least four, or at least six, or at least eight, depending upon
the
intended application. In another preferred embodiment, the pressure index is a
low grade heat pressure index. Generally, the pressure index is based on the
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
3
internal vessel pressure of a vessel that contains the adsorbent and fluid in
a
desorption mode under prescribed conditions that are described in greater
detail
below
[0008] The present application also provides a process for providing
cooling
or refrigeration including selecting a refrigerant fluid and an adsorbent
material
adsorbing the refrigerant fluid into the adsorbent material; heating the
adsorbent
material to desorb the refrigerant fluid from the adsorbent material and
directing
the desorbed refrigerant fluid to a device to expand the desorbed refrigerant
fluid
for refrigeration, in which the adsorbent material and fluid in combination
have a
pressure index of at least 1.2. While the present invention is described in
connection with refining and/or petrochemical applications, the present
invention is not introduced to be so limited. It is contemplated that the use
of the
pressure index, and the sorbent material and fluid combination as application
outside of the refining and petrochemical field, including but not limited to
use
as a passive cooling in a dwelling.
[0009] The present application also provides a process for generating
electricity or work that includes selecting an adsorbent material and a fluid,
adsorbing the fluid into the adsorbent material, heating the adsorbent
material to
desorb the fluid from the adsorbent material, and directing the desorbed fluid
to
drive a driver device to generate electricity or work. It is contemplated that
the
electricity generated may be used within the refinery or petrochemical plant
or
introduced into the electrical grid for use by the surrounding areas.
[0010] The present application also provides an adsorption system that
includes a vessel in communication with a heat source, the vessel containing
an
adsorbent material and a fluid, the sorbent material selected from zeolites,
silicagel, carbon, activated carbon, metal organic frameworks (M0Fs), and
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
4
zeolitic imidazolate frameworks (ZIFs), and the fluid selected from carbon
dioxide, methane, ethane, propane, butane, ammonia, freon and other known
refrigerants.
BRIEF DESCRIPTION OF THE DRAWINGS
[0011] The invention will now be described in conjunction with the
accompanying drawings in which:
Figure 1 is a schematic of an exemplary embodiment of the present
application which employs zeolite 13X as the adsorbent material and carbon
dioxide as the fluid and utilizes unutilized heat to achieve a temperature of
about
212 F.
Figure 2 is a Mollier Diagram annotated to show four points that
correspond to four stages of the exemplary embodiment described in Figure 1.
Figure 3 is a Mollier Diagram annotated to show alternative process
points based on the use of unutilized heat to achieve a temperature of about
450 F and alternative process points based on the use of higher sorbing
pressures.
Figure 4 is a graph which plots adsorbent capacity along the y-axis and
average heat of adsorption along the x-axis.
Figure 5 is a schematic of an adsorption system in accordance with an
embodiment of the present invention.
DETAILED DESCRIPTION OF THE INVENTION
[0012] The present invention will now be described in greater detail in
connection with the figures and the following terms. As used herein, the term
"sorbent material" refers to a material that reversibly binds to the fluid.
Sorbent
materials include, but are not limited to, adsorbents.
CA 02740612 2011-04-14
WO 2010/047820 PCT/US2009/005774
[0013] As used herein, the term "fluid" or "working fluid" refers to a
liquid
or gas that can reversibly bind to the sorbent material.
[0014] As used herein, the term "driver device" refers to a turbine, shaft
or
other suitable mechanism driven by a fluid to generate electricity or perform
work.
[0015] As used herein, the term "vessel" refers to an enclosed container
suitable for containing an adsorbent material and a fluid under suitable
conditions to permit adsorption and desorption of the fluid in the sorbent
material.
[0016] As used herein, the term "unutilized heat" or "unutilized heat
source"
refers to the residual or remaining heat source (e.g., steam) remaining
following
the processing operation after the heat source has been used for its primary
purpose in the refining or petrochemical processing operation. Unutilized heat
is
also referred to as waste heat. The unutilized heat or unutilized heat source
refers to a heat source that is no longer any use in the refining and/or
petrochemical processing operation and would traditionally be discarded. The
unutilized heat can be provided as a unutilized heat stream. For example, but
not
limitation, unutilized heat can include steam that was employed in a heat
exchanger used in petroleum and petrochemical processing, and is of no value
to
current processes and is being discarded.
[0017] As used herein, the term "pump" refers to a physical device that
assists in transporting fluids from one place to another.
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
6
[0018] In accordance with one aspect of the present application, a sorption
system is provided. The sorption system recovers unutilized heat from a
unutilized heat stream. The unutilized heat source may be used heat from a
heat
exchanger, or other process area of a chemical processing plant or
petrochemical
refining plant. The sorption system includes at least one vessel containing a
sorbent material or a mixture of sorbent materials and a working fluid or a
mixture of working fluids, and at least one unutilized heat source operatively
connected to the vessel, such that unutilized heat from the unutilized heat
source
can be transferred to the sorbent material and fluid contained within the
vessel.
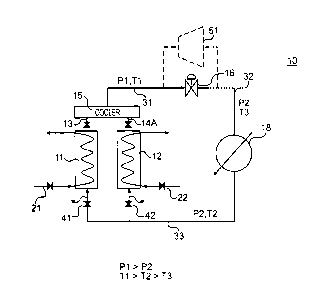
[0019] A sorption system 10 in accordance with one aspect of the present
invention is illustrated in Fig. 5. The sorption system 10 includes a first
adsorption vessel 11 and a second adsorption vessel 12. A unutilized heat
stream 21 passes through the first adsorption vessel 11. Unutilized heat
contained in the stream 21 passes through the walls of line containing the
stream
into the first adsorption vessel 11. A unutilized heat stream 22 passes
through
the second adsorption vessel 12. Unutilized heat contained in the stream 22
passes through the walls of line containing the stream into the second
adsorption
vessel 12. A unutilized heat stream 22 passes through the second adsorption
vessel 12. The unutilized heat streams 21 and 22 may supply from the same
unutilized heat source or the separate unutilized heat sources.
[0020] The first and second adsorption vessels 11 and 12 are operatively
connected to a pressure damper/cooler 15. A valve assembly 13 is interposed
between the first adsorption vessel 11 and the cooler 15. The valve assembly
13
functions like a back pressure regulator, which permits the working fluid to
escape from the first adsorption vessel 11 at a predetermined or pre-set
pressure.
The predetermined or pre-set pressure may range from ¨170 psig to ¨3400psig,
which is dependent upon the amount of sorbent material contained in the vessel
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
7
and the temperature of the waste stream. A valve assembly 14 is interposed
between the second adsorption vessel 12 and the cooler 15. Like the first
valve
assembly 13, the second valve assembly 14 functions like a back pressure
regulator, which permits the working fluid in the second adsorption vessel to
escape from the second adsorption vessel 12 at the pre-set pressure. A line 31
extends from the cooler 15. The working fluid contained in the cooler 15 and
the line 31 has a pressure of P1 and a temperature of Ti.
100211 In accordance with one aspect of the present invention, the line 31
is
connected to an expansion valve 16. When the working fluid passes through the
expansion valve 16 into the line 32, the temperature of the working fluid
drops
from T1 to T3 such that Ti > T3 and the pressure of the working fluid drops
from P1 to P2 such that P1 > P2. With such an arrangement, the working fluid
can be used to provide cooling for a heat exchanger 18, which is operatively
coupled to line 32. As the working fluid passes through the heat exchanger 18,
the temperature of the working fluid increases from T3 to T2 such that T2 > T3
and T1 > T2 in response to withdrawing heat from the other fluid passing
through the heat exchanger 18. The pressure of the working fluid remains close
to P2.
100221 The working fluid is returned to the first adsorption vessel 11 and
the
second adsorption vessel 12 via a return line 33. The temperature of the
working
fluid is T2 and the pressure is P2. A valve 41 controls the flow of working
fluid
from the return line 33 to the first adsorption vessel 11. A valve 42 controls
the
flow of working fluid from the return line 33 to the second adsorption vessel
12.
When the working fluid is returned to the first adsorption vessel 11, the
working
fluid is adsorbed onto the sorbent material contained in the first adsorption
vessel 11. When the working fluid is returned to the second adsorption vessel
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
8
12, the working fluid is adsorbed onto the sorbent material contained in the
second adsorption vessel 12.
[0023] The operation of the system 10 will now be described in greater
detail. In accordance with the present invention, the first and second
adsorption
vessels 11 and 12 operate in tandem. The working fluid flows into the first
adsorption vessel 11 when the valve 41 is open. The valve 41 remains open
until
equilibrium is established within the first vessel 11. The unutilized heat
stream
21 passes through the first vessel 11 such that the sorbent material and the
working fluid are heated, which results in the desorption of the working fluid
from the sorbent material. This increases the pressure of the working fluid
contained in the first vessel 11. Once the pre-set pressure is reached, the
working fluid is released from the first vessel 11 via valve assembly 13, such
that the working fluid is released into the cooler 15 and the line 31. The
working
fluid has a pressure of P1 and a temperature of T1 within line 31. When the
expansion valve 16 is operated, the pressure and temperature of the working
fluid drops to a pressure of P2 and a temperature of T3 in the line 32.
[0024] The working fluid is passed through the heat exchanger 18 to cool
the
fluid contained therein. In accordance with the present invention, the heat
exchanger 18 is used to cool a process stream for a refining or petrochemical
processing operation. With such an arrangement, the unutilized heat, which
normally would be lost, is recaptured and used to perform cooling of another
process stream. It is also contemplated that the working fluid can be used to
cool water to provide cooling water to an overhead condenser in a distillation
tower. It is also contemplated that the cooling performed by the working fluid
may be used to recover gas molecules from a fuel stream. The present invention
is not intended to be limited for use in process streams in refining and
petrochemical processing applications. It is contemplated that the heat
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
9
exchanger can be used in connection with a building cooling system located in
one of the buildings located at the facility such that the unutilized heat can
be
used to cool one or more of the buildings.
100251 After the passing through the heat exchanger 18, the working fluid
enters return line 33. The temperature of the working fluid is T3 and the
pressure remains close to P2. The valve 41 is closed such that the working
fluid
does not return to the first vessel 11; rather, the valve 42 is open such that
that
working fluid can flow into the second adsorption vessel 12. The valve 42
remains open until equilibrium is established within the second vessel 12. The
unutilized heat stream 22 passes through the second vessel 12 such that the
sorbent material and the working fluid are heated, which results in the
desorption
of the working fluid from the sorbent material. This increases the pressure of
the
working fluid contained in the second vessel 12. Once the pre-set pressure is
reached, the working fluid is released from the second vessel 12 via valve
assembly 13, such that the working fluid is released into the cooler 15 and
the
line 31. The working fluid has a pressure of P1 and a temperature of Ti within
line 31. The working fluid passes through the system, as described above.
After
passing through the heat exchanger, the working fluid is returned to the first
adsorption vessel 11.
100261 The first and second adsorption vessels 11 and 12 operated in tandem
such that one is operating in an adsorption mode when the other is operating
in a
desorption mode and vice versa. With such an arrangement, the first and second
vessels 11 and 12 operate to provide a continuous supply of working fluid to
the
cooler 15 and line 31 at pressure Pl.
100271 In accordance with an aspect of the present invention, the expansion
valve 16 can be replaced with a driver device 51 (e.g., a turbine). The
working
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
fluid passes through the driver device to either generate electricity or
perform
work by driving a shaft or other suitable mechanism, whereby the temperature
and pressure of the working fluid would decrease as described above. The
working fluid could then be used in the heat exchanger 18. It is also
contemplated that expansion valve 16 and the driver device 51 can be used in
tandem in order to perform both power generation and refrigeration.
[0028] The sorption system includes a sorbent material or a mixture sorbent
materials and a working fluid or a mixture of working fluids. The sorbent
material and fluid in combination have a pressure index of at least 1.2, or at
least
1.5, or at least 2, or at least 3, or at least 4, or at least 6, or at least
8. In various
embodiments, the pressure index may be a low level heat pressure index (e.g.,
for unutilized heat applications below 600K), or a high level heat pressure
index
(e.g., for unutilized heat applications between 600K and 1200K). Various
combinations or sorbent materials and fluids are considered to be within the
scope of the present invention provided such combinations satisfy the desired
pressure index. It should be noted that a combination that is suitable for
application with a higher temperature unutilized heat stream may not be
applicable for a lower temperature unutilized heat stream. The determination
of
the pressure index is described below in greater detail.
[0029] The adsorbent material in the sorption system has an average heat of
sorption (Q) between about 2 kcal/mole and about 20 kcal/mole, or more
preferably between about 3 kcal/mole and about 10 kcal/mole.
[0030] In one embodiment of the present application, the working fluid is
selected from carbon dioxide, methane, ethane, propane, butane, ammonia, freon
or other suitable refrigerant. The adsorbent material is selected from
zeolites,
silicagel, adsorbing polymers, carbon, activated carbon, metal organic
CA 02740612 2011-04-14
WO 2010/047820 PCT/US2009/005774
11
frameworks (M0Fs), and zeolitic imidazolate frameworks (ZIFs). In one
embodiment the fluid is carbon dioxide and/or the adsorbent material is a
zeolite.
In one embodiment the fluid is carbon dioxide and the zeolite is a zeolite X,
preferably a zeolite 13X.
[0031] In accordance with another aspect of the present application, a
process for providing refrigeration is provided. The process for providing
refrigeration includes, selecting a refrigerant fluid and a sorbent material,
sorbing the refrigerant fluid into the sorbent material, heating the sorbent
material to desorb the refrigerant fluid from the sorbent material, and
directing
the desorbed refrigerant fluid to an instrument to expand the desorbed
refrigerant
fluid for refrigeration. The process preferably employs a sorbent system, as
described above. The sorbent material and fluid in combination have a pressure
index of at least 1.5, or at least 2, or at least 3, or at least 4, or at
least 6, or at
least 8. In various embodiments, the pressure index may be a low level heat
pressure index, or a high level heat pressure index.
[0032] In accordance with an aspect of the present invention, the sorbent
system and the processes described herein do not require the use of a pump or
additional components to facilitate movement of the working fluid through the
system.
[0033] Another aspect of the present application provides a process for
generating electricity or work. The process for generating electricity or work
includes selecting a sorbent material or a mixture of sorbent materials and a
fluid
or a mixture of working fluids, sorbing the fluid into the sorbent material,
heating the sorbent material to desorb the fluid from the sorbent material,
and
directing the desorbed fluid to drive a driver device to generate electricity
or
work. The sorbent material and fluid in combination have a desorbed:sorbed
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
12
pressure index of at least 1.2 (for high temperature unutilized heat
applications),
at least 1.5, or at least 2, or at least 3, or at least 4, or at least 6, or
at least 8. The
process preferably utilizes a sorbent system, described above.
Pressure Index
100341 Embodiments of the present application employ a "pressure index"
that can be determined at various desorbing temperatures, which is used to
determine suitable combinations of a sorbent material and a fluid. The
pressure
index is determined by the following method. One hundred (100) grams of
sorbent material are placed in a 1 liter vessel designed to be isolated from
associated equipment with existing valves on both ends of the vessel. The
vessel
also has indicators to measure inside pressure and temperature. The vessel is
flushed and filled with a pure fluid (e.g., CO2) at one atmospheric pressure.
The
sorbent material adsorbs fluid and the sorbent may heat up. The vessel is
equilibrated at 298 K and 1 atmospheric pressure, this sorbing pressure being
defined as PI = 1Ø The vessel is heated to a pre-selected desorbing
temperature
(e.g. 348 K). When the vessel and sorbent material reach the pre-selected
desorbing temperature, the internal vessel pressure is measured to determine
PF.
The pressure index is defined as the ratio of PF to Pi.
[0035] As noted above, preferred embodiments of the present application
make use of a lower temperature of unutilized heat. In order to select a
sorbent
material/fluid combination that is preferred for use with low level heat (e.g.
sorption systems that utilize low grade unutilized heat), it is often
desirable or
necessary to ascertain at least the low level heat pressure index, as
determined
above. A pressure index of at least 1.5 is generally appropriate for use in
low
level unutilized heat applications. Nevertheless, other embodiments of the
present invention may use high level heat sources. Thus in these embodiments,
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
13
it is desirable to select a high level heat pressure index. In such cases,
combinations of sorbent material and working fluid may have a pressure index
as low as 1.2. =
Exemplary Embodiment Using Zeolite 13X and CO2
[0036] For purposes of illustration and not limitation, a zeolite 13X/CO2
sorption chilling system 100 is provided in one representative embodiment of
the
present application, as depicted schematically in Figure 1. A Mother Diagram
for carbon dioxide at various temperatures and pressures for this embodiment
is
shown in Figures 2 and 3 for reference. In this embodiment, two vessels 111
and 112 are maintained in an adsorption mode and a desorption mode,
respectively. When on vessel is in the adsorption mode, the other vessel is in
the
desorption mode and vice versa. In the present embodiment, the sorbent
material is zeolite 13X. The working fluid is CO2. For the vessel in the
adsorption mode, carbon dioxide is adsorbed by the zeolite 13X at a pressure
of
about 140 psi and a temperature of about 95 F. These conditions are denoted in
Figure 2 as Stage 1.
[0037] After adsorption is complete, the adsorbent bed is isolated by
operating the relevant valve (e.g., valve 141 for vessel 111 or valve 142 for
vessel 112) and heated using unutilized heat from petroleum refining or
chemical processes. The adsorption mode may last for several seconds (e.g., 10
seconds) to several minutes. The duration of the adsorption mode varies based
upon the adsorbent material and fluid selected. Unutilized heat is applied to
the
vessel in order to desorb the CO2, thus initiating the desorption mode. Using
the
unutilized heat, the vessel is heated to about 212 F in this particular
embodiment. A pressurized stream is generated due to desorption of CO2 from
the 13X sorbent material as the adsorbent bed heats to 212 F. In response to
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
14
operation of a back pressure regulator valve ((i.e., valve 113 for vessel 111
or
valve 114 for vessel 112), high pressure CO2 is released from the vessel to
pressure damper or cooler 115 at a preset pressure (e.g., ¨ 1400 psig), which
is
denoted in Figure 2 as stage 2. The temperature of the CO2 is approximately
212 F.
[0038] The pressurized CO2 stream is cooled to the pressure damper/cooler
115 to approximately 110 F, which is denoted as stage 3 in Figure 2. As a
result, the pressure of the cooled CO2 stream in the line 131 is approximately
1380psi (P1) and the temperature is approximately 110 F. The cooled working
fluid stream is subsequently expanded adiabatically using an expansion valve
116 to about 140 psi (P2) and -40 C (T3), which is denoted as stage 4 in
Figure
2. The expansion valve 116 may be a flow restrictor or a needle valve to
restrict
but not stop flow. This cooled stream can be used as a high quality
refrigeration
load for many different applications within refineries or similar facilities
where
unutilized heat is readily available. For example, the refrigerated CO2 can be
directed to a heat exchanger 118 to chill process streams within refineries
and
chemical plants.
[0039] After performing the refrigeration operation within the exchanger
118, the carbon dioxide of this representative embodiment can have a
temperature of about 60 F to 100 F (T2) and a pressure of about 140psi (P2).
The carbon dioxide working fluid is then recycled back to one of the vessels
for
use in a subsequent adsorption mode.
[0040] The CO2/zeolite 13X system has a pressure index of greater than 3.5.
The pressure index is determined in accordance with the procedure set forth
above.
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
[0041] Alternatively, higher temperature heat can be applied to desorb more
working fluid molecules from the adsorption bed. As shown in Figure 3, and for
purposes of illustration and not limitation, stage 2 is now stage 2A, in which
a
higher-temperature unutilized heat source is used to heat the bed to 450 F,
instead of 212 F. This pressurized stream is to be cooled to 110 F before
expansion. It, therefore, will require much higher amount of cooling media at
stage 2. The efficiency of this alternative system based on a 450 F heat
source,
using the selection of zeolite 13X and carbon dioxide, will be significantly
lower
as it requires higher level of heating and cooling. It is understood, however,
that
a selection of sorbent material and fluid based on a higher level heat
pressure
index can produce a sorption system that is better suited for a higher quality
of
heat.
[0042] Alternatively, a system can be operated at lower pressure
differentials
during adiabatic expansion. Figure 3 demonstrates a system where stages 1-B
and 4-B are at higher adsorption pressures. This will also reduce the
efficiency
of the system. By not expanding the fluid to a lower pressure cooling may be
limited. Nevertheless, such an embodiment can be useful for providing
refrigeration.
[0043] For purposes of the above discussion, each vessel can be a shell-in-
tube type configuration with adsorbents in the tube(s). The vessel may have an
inner diameter of about 5 ft and contains tubes having a length of about 20
ft.
Other vessel sizes are considered to be well within the scope of the present
invention. Furthermore, the present invention is not limited to shell-in-tube
heat
exchangers, other exchangers and other vessels may be selected based on
ordinary skill in the art and are considered to be well within the scope of
the
present invention.
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
16
[0044] This representative embodiment is provided for exemplary purposes;
neither the application nor the invention is limited to the specific
embodiments
discussed above, or elsewhere in the application. For example, other adsorbent
materials and fluids can be used in the place of, or in addition to, zeolite
13X and CO2.
[0045] Adsorbent Materials
[0046] As noted above, and as used in this application, the term "sorbent
material" or "adsorbent material" refers to a material that reversibly binds
the
fluid. Sorbent materials include adsorbents.
[0047] Sorbent materials that can be used in embodiments of the present
invention include, but are not limited to, metal-organic framework-based (MOF-
based) sorbents, zeolitic imidazole framework (ZIF) sorbent materials,
zeolites
and carbon.
[0048] MOF-based sorbents include, but are not limited to, MOF-based
sorbents with a plurality of metal, metal oxide, metal cluster or metal oxide
cluster building units. As disclosed in International Published Application
No.
WO 2007/111738, which is hereby incorporated by reference, the metal can be
selected from the transition metals in the periodic table, and beryllium.
Exemplary metals include zinc (Zn), cadmium (Cd), mercury (Hg), beryllium
(Be) and copper (Cu). The metal building units can be linked by organic
compounds to form a porous structure, where the organic compounds for linking
the adjacent metal building units can include 1,3,5- benzenetribenzoate (BTB);
1,4-benzenedicarboxylate (BDC); cyclobutyl 1,4- benzenedicarboxylate (CB
BDC); 2-amino 1,4 benzenedicarboxylate (H2N BDC); tetrahydropyrene 2,7-
dicarboxylate (HPDC); terphenyl dicarboxylate (TPDC); 2,6 naphthalene
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
17
dicarboxylate (2,6-NDC); pyrene 2,7-dicarboxylate (PDC); biphenyl
dicarboxylate (BDC); or any dicarboxylate having phenyl compounds.
[0049] Specific materials MOF-based sorbent materials include: MOF-177, a
material having a general formula of Zn40(1, 3, 5-benzenetribenzoate)2; MOF-5,
also known as IRMOF-I, a material having a general formula of Zn40(1,4-
benzenedicarboxylate)3; IRMOF-6, a material having a general formula of
Zn40(cyclobutyl 1,4-benzenedicarboxylate); IRMOF-3, a material having a
general formula of Zn40(2-amino 1,4 benzenedicarboxylate)3; and IRMOF-11, a
material having a general formula of Zn40(terphenyl dicarboxylate)3,or
Zn40(tetrahydropyrene 2,7-dicarboxylate)3; and IRMOF-8, a material having a
general formula of Zn40(2,6 naphthalene dicarboxylate)3 and Cu-BTC-MOF, a
material having a general formula of C18H6Cu3012 (copper benzene-1,3,5-
tricarboxylate).
[0050] Exemplary zeolitic imidazole framework (ZIF) sorbent materials
include, but are not limited to, ZIF-68, ZIF-60, ZIF-70, ZIF-95, ZIF-100
developed at the University of California at Los Angeles and generally
discussed
in Nature 453, 207-211 (8 May 2008), hereby incorporated by reference in its
entirety.
[0051] Zeolite adsorbent materials include, but are not limited to,
aluminosilicates that are represented by the formula M210A1203=3/SiO2'wH20,
where y is 2 or greater, M is the charge balancing cation, such as sodium,
potassium, magnesium and calcium, N is the cation valence, and w represents
the moles of water contained in the zeolitic voids. Examples of zeolites that
can
be included in the methods and systems of the present application include
natural
and synthetic zeolites.
CA 02740612 2014-02-18
-18-
[0052] Natural zeolites include, but are not limited to, chabazite (CAS
Registry No. 12251-32-0; typical formula Ca2[(A102)4(Si02)8] 13H20),
mordenite (CAS Registry No. 12173-98-7; typical formula
Na8(A102)8(Si02)40124120), erionite (CAS Registry No. 12150-42-8; typical
formula (Ca, Mg, Na2, K2)4.5RA102)9(Si02)27] 27E120), fauiasite (CAS Registry
No. 12173-28-3, typical formula (Ca, Mg, Na2,
1(2)29.5[("2)59(Si02)133] 235H20), clinoptilolite (CAS Registry No. 12321-85-
6, typical formula Na6[(A102)6(Si02)30i 24H20) and phillipsite (typical
formula:
(0.5 Ca, Na, K)3 RA102)3(S i02)516H20).
[0053] Synthetic zeolites include, but are not limited to, zeolite A
(typical
formula: Na12RA102)12(S102)12127H20), zeolite X (CAS Registry No.68989-23-
1; typical formula; Na86[A102)86(Si02)1061264H20), zeolite Y (typical formula:
Na56[(A102)56(Si02)136] 250H20), zeolite L (typical formula:
K91(A102)9(Si02)27122H20), zeolite omega (typical formula:
Na6.8TMAI 6[A102)8(SiO2)28].21H20, where TMA is tetramethylammonium) and
ZSM-5 (typical formula: (Na, TPARA102)3(Si02)931 16H20, where TPA is
tetrapropylammonium).
[0054] Zeolites that can be used in the embodiments of the present
application also include the zeolites disclosed in the Encyclopedia of
Chemical
Technology by Kirk-Othmer, Volume 16, Fourth Edition, under the heading
"Molecular Sieves".
[0055] Synthetic zeolite sorbent materials are commercially available, such
as under the Sylosiv brand from W.R. Grace and Co. (Columbia, Md.) and
from Chengdu Beyond Chemical (Sichuan, P.R. China). For example, Sylosiv
Al0 is one commercially available zeolite 13 X product.
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
19
Fluids
100561 As noted above, the term fluid refers to a liquid or gas that
reversibly
binds to the adsorbent material. Non-limiting examples of fluids that can be
used in accordance with the present application include carbon dioxide,
methane,
ethane, propane, butane, ammonia, freon and other suitable refrigerants
satisfying the above-described pressure index.
Selection of Sorbent Materials and Fluids
100571 In accordance with another aspect of the invention, a method is
provided for selecting a sorbent material and a fluid for use in combination
in a
waste-heat sorbent system within a chemical processing or petrochemical
refining operation. The method generally includes providing an adsorbent
material in a vessel, introducing a fluid into the vessel to a predetermined
adsorbing pressure, allowing the chamber to reach equilibrium at the
predetermined adsorbing pressure (e.g. 1 atm), securing the chamber to prevent
the escape of fluid, heating the secured chamber to a predetermined
temperature
(e.g., 348K), measuring internal pressure within the secured chamber after the
sorbent material has reached the predetermined temperature and selecting the
sorbent and the fluid for use in combination if the measured internal pressure
within the secured chamber is at least 1.5 times the adsorbing pressure. In
one
embodiment, the sorbent material and the fluid for use in combination is
selected
if the measured internal pressure within the secured chamber is at least two
times, or at least three times, or at least four times, or at least six times,
or at
least eight times the sorbing pressure. The adsorption system can be used to
provide refrigeration, to drive a turbine to provide electricity or to drive
a.work
shaft or other driver to perform work.
CA 02740612 2011-04-14
WO 2010/047820
PCT/US2009/005774
Heat of Sorption
[0058] Preferably, the sorbent material and fluid couple has an average
heat
of sorption (Q) from about 2 kcal/mole to about 20 kcal/mole, and more
preferably from about 4 kcal/mole to about 10 kcal/mole for heat sources up to
600K. The heat of sorption should be between 2kcal/mole to about 40 kcal/mole
if a higher temperature heat source (e.g., great than 600K and up to 1200K) is
available. The sorbent material should also have a high capacity for the
fluid.
[0059] Figure 4 demonstrates the influence of these two factors in the
selection of a fluid and sorbing material. As shown on the left side of the
Figure
4, labeled the "Low Heat of Sorption Region," the sorbent/fluid couple
generally
has too low of a heat of sorption to provide a suitable pressure "spark" and
such
a sorbent/fluid is not a proper couple. The bottom of Figure 4, labeled "Low
Capacity Region" demonstrates a region in which the choice of sorbent material
provides for a low capacity of the fluid such that, regardless of the heat of
sorption, the fluid cannot drive the sorption system. The right side of Figure
4,
labeled "High Heat of Sorption Region" demonstrates a region in which the
choice of the sorbent provides too high of a heat of sorption to provide
sufficient
pressure to drive the sorption system. Hence, the preferred region is labeled
accordingly.
Uses of Adsorbent Systems of the Present Application
[0060] The adsorbent systems of the present application can be used in
various applications provided the setting allows for the presence of a vessel
that
contains a sorbent material, a supply of fluid, a heat supply and means to
effectively direct the desorbed fluid to an expansion device to provide
refrigeration or a driver device to provide electricity or work. For example,
the
CA 02740612 2014-02-18
- 21 -
desorbed gas may be directed to a Joule-Thompson expansion valve, to provide
refrigeration. Alternatively, the desorbed fluid can be directed to a turbine
to
provide electricity or a work shaft to run a machine to provide work.
[0061] Possible applications for sorption systems of the present
application
include residential (for generating air conditioning in the summer and a heat
pump in the winter), vehicular (where the on-board air conditioning utilizes
exhaust heat) and industrial (refining and chemical plants).
[0062] In a preferred embodiment of the present application, the adsorbent
system is used within a chemical or petrochemical plant, and the desorbed
fluid
is used to provide refrigeration to aid in other process areas, particularly
areas
that rely on temperature differences to separate components of a mixture. For
example, the refrigeration can be used to recover liquefied petroleum gas
(LPG,
C3+) from flue gases going up a stack, or the refrigeration can be used to
operate
condensers to improve the effectiveness of vacuum distillation columns,
particularly in the summer months.
[0063] By proper selection of the adsorbent and fluid, the sorbent system
can
make effective use of lower grade heat than previously provided by adsorption
systems in the prior art. For example, in one embodiment of the present
application, the heat supply is "unutilized heat" which has a temperature of
from
about 70 C to about 300 C, more preferably from about 90 C to about 180 C.
[0064] The present invention is not to be limited in scope by the specific
embodiments described herein. Indeed, various modifications of the invention
in
addition to those described herein will become apparent to those skilled in
the art
from the foregoing description and the accompanying figures. The scope of the
CA 02740612 2014-02-18
- 22 -
claims should not be limited by the embodiments set out herein but should be
given the broadest interpretation consistent with the description as a whole.
[0065] It is further
to be understood that all values are approximate, and are
provided for description.