Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02745763 2011-06-03
Description
METHOD FOR PRODUCING GRANULAR IRON
Technical Field
This invention relates to a method for producing
granular iron by: charging agglomerates formed from a raw
material mixture, which contains an iron oxide-containing
substance and a carbonaceous reducing agent, onto a
carbonaceous material spread on a hearth of a furnace;
and heating the agglomerates to thereby reduce and melt
iron oxides in the agglomerates.
Background Art
The direct reduced iron producing method has been
developed for making granular metallic iron from a raw
material mixture which contains an iron oxide source such
as iron ore or iron oxide (hereinafter referred to as
iron oxide-.containing substance) and a carbonaceous
reducing agent. With this producing method, the granular
metallic iron is made by: charging the raw material
mixture onto a hearth of a heating furnace; heating the
raw material mixture with the heat transferred by gas
from burners in the furnace or radiant heat to thereby
reduce iron oxides in the raw material mixture by the
carbonaceous reducing agent into reduced iron, carburize
1
CA 02745763 2011-06-03
and melt the reduced iron, followed by coalesce it to
granules while separating it from subgenerated slag; and
then cooling and solidifying it. This producing method
has been the subject of considerable practical research
of late, because it requires no large-scale facility such
as a blast furnace, and because it affords greater
flexibility in terms of resources such as not requiring
coke. However, the producing method has various problems
to be solved in order to be applied on an industrial
scale, including stability of operation, safety, cost,
quality of the granular iron (product), and so forth.
Since the granular iron made by the above-mentioned
direct reduced iron producing method is sent to an
existing steelmaking facility (such as an electric
furnace or a converter) and is used as an iron source, it
preferably has a low content of impurity elements. The
carbon content of the granular iron is preferably as high
as possible, without excessive range, in order to
increase its applicability as an iron source.
In an effort to improve the quality of granular iron,
the present applicant has proposed a granular iron having
a high Fe purity of 94 mass% or more and a carbon content
adjusted to between 1.0 and 4.5 masso in Japanese
Unexamined Patent Application Publication No. 2002-339009
(Patent Document 1). This granular iron is further
adjusted to have a sulfur content of 0.20 mass% or less,
2
CA 02745763 2011-06-03
a silicon content of 0.02 to 0.5 masso and a manganese
content of less than 0.3 mass-.. However, adjusting the
phosphorus content of the granular iron is not disclosed
in Patent Document 1. The reason for this is as follows:
since the behavior of phosphorus in the reduction process
of iron oxide is already clear from the chemical reaction
mechanism in blast furnace, it is recognized that almost
all phosphorus sourced from a material to be reduced
(that is, raw material) remains in a reduced product
(that is, metallic iron) under reductive atmosphere and
that the phosphorus does not move into subgenerated slag,
and thus it is also recognized that the phosphorus
content in the raw material has to be decreased, and/or
that the granular iron made by the producing method
disclosed in Patent Document 1 has to be subjected to a
further dephosphorization, in order to reduce the
phosphorus content of the granular iron.
In recent years, the grade of iron ore has tended to
be on the decline, and the amount of phosphorus contained
in mined iron ore is on the rise. Therefore, in the
future it will be increasingly difficult to procure raw
materials with a low phosphorus content. However, a
further dephosphorization subjected to the granular iron
made by the producing method disclosed in Patent Document
1 for reducing its phosphorus content leads to higher
cost.
3
CA 02745763 2011-06-03
Disclosure of the Invention
The present invention is developed based on the
above-mentioned background, and has an object to provide
a method for producing granular iron having a low
phosphorus content by: charging agglomerates formed from
a raw material mixture containing an iron oxide-
containing substance and a carbonaceous reducing agent
onto a carbonaceous material spread on a hearth of a
furnace; and heating the agglomerates to thereby reduce
and melt iron oxides in the agglomerates.
One aspect of the present invention is directed to a
method for producing granular iron comprising: charging
agglomerates formed from a raw material mixture
containing an iron oxide-containing substance and a
carbonaceous reducing agent onto a carbonaceous material
spread on a hearth of a furnace; and heating the
agglomerates to thereby reduce and melt iron oxides in
the agglomerates, wherein the temperature of the
agglomerates in the furnace is set in a range between
1200 C and 1500 C; the oxygen partial pressure in
atmospheric gas under which the agglomerates are heated
is set to 2.0 x 10-13 atm or more at standard state; and
the linear speed of the atmospheric gas in the furnace is
set to 4.5 cm/second or more.
4
CA 02745763 2011-06-03
The object, features, aspects and advantages of the
present invention will become clearer through reference
to the following detailed description and drawings.
Brief Description of the Drawings
Fig. 1 is a graph showing the relation between the
dephosphorization ratio and the gas linear speed under
different oxygen partial pressures.
Fig. 2 is a graph showing the relation between the
dephosphorization ratio and the gas linear speed.
Fig. 3 is a graph showing the relation between the
dephosphorization ratio and the oxygen partial pressure.
Fig. 4 is a graph showing the relation between the
dephosphorization ratio and the discharging time.
Fig. 5 is a graph showing the relation between the
dephosphorization ratio and the amount of fixed carbon
contained in the carbonaceous reducing agent blended to
the raw material mixture.
Best Mode for Carrying Out the Invention
The metallurgical process of producing granular iron
by: charging agglomerates formed from a raw material
mixture containing an iron oxide-containing substance and
a carbonaceous reducing agent onto a carbonaceous
material spread on a hearth of a furnace; and heating the
agglomerates to thereby reduce and melt iron oxides in
5
CA 02745763 2011-06-03
the agglomerates is usually carried out under reductive
atmosphere. The reason for this is as follows: when the
process is performed under oxidative atmosphere, the
reduction of the iron oxides contained in the
agglomerates comes to a standstill during the heating of
the agglomerates, and reduced iron cannot be obtained at
a high yield. On the other hand, when the process is
performed under reductive atmosphere, reduction of the
iron oxides proceeds well. However, almost none of the
phosphorus contained in the reduced iron move to slag
subgenerated in the reduction, and still remain in the
granular iron made by melting the reduced iron due to
this melting under reductive atmosphere. As a result,
granular iron with a high phosphorus content is obtained.
To lower the phosphorus content of granular iron, the
obtained granular iron is required to be supplied to an
electric furnace, for example, and subjected to a further
dephosphorization.
During reducing and melting the above-mentioned
agglomerates at a high temperature between 1200 and 1500 C,
the carbonaceous reducing agent causes reductive gas to
spew out of the interior of the agglomerates while the
iron oxides in the agglomerates are being reduced, in
contrast, almost no reductive gas is generated after the
reduction of the iron oxides are almost over and the
reduced iron melts and separates to granular iron and
6
CA 02745763 2011-06-03
subgenerated slag. Accordingly, the inventors considered
that the composition of the granular iron during the
period in which the reduced iron melts and separates to
granular iron and subgenerated slag is greatly influenced
by the composition of its atmospheric gas. In view of
this, the inventors conducted diligent study in the
vision that the suitable control of the atmospheric gas
under which the reduced iron melts and separates to
granular iron and subgenerated slag can adjust the
composition of the granular iron. As a result, the
inventors found that
(I) charging the agglomerates onto a carbonaceous
material spread on a hearth of a furnace and then heating
the agglomerate of which temperature is to be in a range
between 1200 C and 1500 C,
(II) setting the oxygen partial pressure in
atmospheric gas under which the agglomerates are heated
to 2.0 x 10-13 atm or more at standard state, and
(III) setting the linear speed of the atmospheric
gas in the furnace to 4.5 cm/second or more
make the phosphorus contained in the reduced iron move to
slag subgenerated in the reduction while the reduced iron
is melting. The inventors also found that granular iron
with a low phosphorus content can be produced based on
this knowledge, and thus accomplished the present
7
CA 02745763 2011-06-03
invention.
The present invention will now be described in terms
of the procedure of producing granular iron.
(I) Agglomerates are formed by agglomerating a raw
material mixture that contains a carbonaceous reducing
agent and an iron oxide-containing substance.
The above-mentioned iron oxide-containing substance
can be, for example, iron ore, iron sand, nonferrous
smelting slag, and so forth. The above-mentioned
carbonaceous reducing agent can be, for example, a
carbon-containing substance, more specifically, coal,
coke, or the like.
Other components can also be added to the above-
mentioned raw material mixture, such as a binder, an MgO
supplying substance, or a CaO supplying substance.
Binders can be, for example, polysaccharides (such as
wheat gluten and other starches). MgO supplying
substances can be, for example, MgO powder, magnesium-
containing substances extracted from natural ore,
seawater, or the like, and magnesium carbonate (MgCO3).
CaO supplying substances can be, for example, burnt lime
(CaO) and limestone (whose main component is CaCO3).
There are no particular limitations on the shape of
the agglomerates. For example, it can be in the form of
a pellet or a briquette. Nor are there any particular
limitations on the size of the agglomerates. In terms of
8
CA 02745763 2011-06-03
operations, the agglomerate size (maximum diameter) is
preferably 50 mm or smaller, and it is preferably about 5
mm or larger. This is because too large agglomerate size
decreases heat transfer to the lower part of the pellet
and results in poor productivity, additionally decreasing
agglomerating efficiency. Therefore, the agglomerate
size is preferably 50 mm or less.
Carbonaceous material is spread on the hearth in
advance in order to reduce the agglomerates. This is
because the carbonaceous material serves as a carbon
supply source when not enough carbon is contained in the
agglomerates, and also acts to protect the hearth.
It is recommended that the carbonaceous material
that is spread on the hearth have a maximum particle size
of 2 mm or less. Using a carbonaceous material with a
maximum particle size of 2 mm or less, can suppress that
molten slag runs down into the gaps in the carbonaceous
material. As a result, this prevents the molten slag
from reaching the surface of the hearth and corroding the
hearth. The lower limit to the maximum particle size of
the carbonaceous material is preferably about 0.5 mm, for
example. Using a carbonaceous material in which lower
limit to the maximum particle size is about 0.5 mm can
suppress that the agglomerates sink into the carbonaceous
material layer. As a result, this prevents a drop in
heating rate and a decrease in productivity. Spreading
9
CA 02745763 2011-06-03
the carbonaceous material on the hearth is preferably in
a thickness of about 1 to 5 mm, for example.
Then, the agglomerates that have been prepared are
charged to a hearth on which the carbonaceous material
spreads and heated so that the temperature of the
agglomerates becomes between 1200 and 1500 C, thereby the
iron oxides in the raw material mixture are reduced and
melted. The temperature of the agglomerates is
preferably 1250 C or more. Setting the temperature to
1250 C or more shortens the melting time of the granular
iron and slag, and also accelerates the separation of the
slag from the granular iron, allowing granular iron with
a higher iron purity to be obtained. On the other hand,
the temperature of the agglomerates is preferably 1450 C
or less. Setting the temperature to 1450 C or less does
not require the heating furnace to be a complicated
structure, and can suppress a decrease in thermal
efficiency. From the standpoints of the heating furnace
structure and energy use, the targeted metallic iron
nuggets are preferably produced at lower temperature.
When burners are used as the heating means in the furnace,
the temperature of the agglomerates can be adjusted by
controlling the combustion conditions of these burners.
There are no particular limitations on the type of
furnace used in the present invention, for example, a
CA 02745763 2011-06-03
heating furnace or a moving hearth furnace can be used.
A rotary hearth furnace can be used, for example, as a
moving hearth furnace.
(II and III) The oxygen partial pressure in
atmospheric gas under which the agglomerates are heated
is set to 2.0 x 10-13 atm or more at standard state, and
that the linear speed of this gas is set to 4.5 cm/second
or more. As a result of various experiments, the
inventors found the followings: when the reduced iron is
melted under a slightly oxidative atmosphere, the
phosphorus contained in the reduced iron is oxidized, and
this phosphorus moves to the slag, and this decreases the
phosphorus content of the granular iron. More
specifically, when the oxygen partial pressure of the
atmospheric gas is less than 2.0 x 10-13 atm, or when the
gas linear speed is less than 4.5 cm/second, the
dephosphorization of the granular iron cannot be
accelerated, since not enough oxidative gas is contained
in the atmospheric gas near the surface of the
agglomerates. Therefore, the oxygen partial pressure of
the atmospheric gas under which the agglomerates are
heated is set to 2.0 x 10-13 atm or more at standard state,
and the gas linear speed is set to 4.5 cm/second or more.
The oxygen partial pressure of the atmospheric gas
is preferably 2.8 x 10-13 atm or more at standard state.
11
CA 02745763 2011-06-03
The higher is the oxygen partial pressure, the more the
dephosphorization of the granular iron is accelerated.
However, excessively high oxygen partial pressure causes
the granular iron to be re-oxidized, and this decreases
the iron purity (metallization ratio) . Therefore, the
oxygen partial pressure is preferably 4.8 x 10-13 atm or
less at standard state, and more preferably 4.0 x 10-13
atm or less at standard state.
The linear speed of the atmospheric gas in the
furnace is preferably 5 cm/second or more. The higher is
the gas linear speed, the more the dephosphorization of
the granular iron is accelerated. However, excessively
high gas linear speed causes the granular iron to be re-
oxidized, and this decreases the iron yield. Therefore,
the gas linear speed is preferably 13.5 cm/second or less,
and more preferably 9 cm/second or less.
The phrase "atmospheric gas under which the
agglomerates are heated" means the atmospheric gas near
the surface of the agglomerates. The phrase "near the
surface of the agglomerates" means the area up to a
height of 50 mm from the surface of the agglomerates.
Since the oxygen partial pressure and the linear speed of
the atmospheric gas in the furnace are often different at
the bottom portion of the furnace (near the hearth) and
the top portion of the furnace (near the roof), the
12
CA 02745763 2011-06-03
above-mentioned oxygen partial pressure and gas linear
speed are specified for the atmospheric gas near the
surface of the agglomerates, which affect the redox
reaction of the agglomerates.
The oxygen partial pressure of the atmospheric gas
under which the agglomerates are heated can be calculated
by taking a sample of the atmospheric gas near the
surface of the agglomerates, and analyzing the gas
composition. The linear speed of the atmospheric gas can
be measured with a pitot tube or the like.
The oxygen partial pressure of the atmospheric gas
can be controlled, for example, by: adjusting the amount
of oxygen fed to the burners; adjusting the amount of
fuel fed to the burners or the air ratio, etc.; or
adjusting the injection. of reductive gas. The linear
speed of the atmospheric gas can be controlled, for
example, by: adjusting the amount of gas fed to the
burners; adjusting the injection angle of the burners; or
varying the roof height.
The oxygen partial pressure and the linear speed of
the atmospheric gas are adjusted so as to be within the
above-mentioned ranges at latest from the point when the
melting of the reduced iron begins. This is because the
composition of the granular iron is actually affected by
the atmospheric gas composition more during melting than
during solid reduction.
13
CA 02745763 2011-06-03
Preferably, the linear speed of the atmospheric gas,
under which the agglomerates are heated, is controlled to
5.4 cm/second or less (including 0 cm/second) until the
iron oxides, which are contained in the raw material
mixture, begins to melt; and the linear speed of the
atmospheric gas, under which the agglomerates are heated,
is controlled to 4.5 cm/second or more, once the iron
oxides begins to melt. In the period before the iron
oxides begins to melt, the reduction reaction is very
actively occurring within the agglomerates, this causes a
difficult change in the composition of the atmospheric
gas near the surface of the agglomerates or inside the
agglomerates, even if the composition of the atmospheric
gas in the furnace is changed. Meanwhile, as the solid
reduction nears completion, melting of iron begins due to
the beginning of carburization in iron and the decrease
of the melting point of the resulting iron. When the
iron begins to melt, almost no gas is generated from the
agglomerates, and thus the composition of the iron is
greatly affected by the composition of the atmospheric
gas. Therefore, the linear speed of the atmospheric gas
under which the agglomerates are heated is preferably
controlled to suitable levels up in the period before the
iron oxides begins to melt and after the melting begins
respectively. Incidentally, the oxygen partial pressure
of the atmospheric gas in the period before the iron
14
CA 02745763 2011-06-03
oxides contained in the raw material mixture begins to
melt is preferably 2.8 x 10-13 atm or less.
Thus, in the present invention, it is preferable to
control the oxygen partial pressure and the linear speed
of the atmospheric gas at the time until the iron oxides
begins to melt and after the melting begins, for example,
when a moving hearth furnace is used as the heating
furnace, partitions can be suspended from the furnace
roof so that the inside of the furnace is divided into a
plurality of zones, and the oxygen partial pressure and
the linear speed of the atmospheric gas is controlled for
each of these zones.
As discussed above, suitable controlling of the
oxygen partial pressure and the linear speed of the
atmospheric gas in the period of reducing and melting can
proceed the dephosphorization of the granular iron more
effectively, and can produce granular iron with a lower
phosphorus content than reducing and melting just under
reductive atmosphere.
In the present invention, it is preferred to adjust
the percentage of the amount of fixed carbon contained in
the carbonaceous reducing agent blended to the raw
material mixture to be in a range between 98 mass% and
102 mass-06 with respect to the amount of fixed carbon
needed to reduce the iron oxides contained in the iron
oxide-containing substance. The reason for this is as
CA 02745763 2011-06-03
follows: when the percentage of the amount of fixed
carbon contained in the carbonaceous reducing agent is
less than 98 mass-6 with respect to the amount of fixed
carbon needed to reduce the iron oxides, this makes lack
of carbon, and leads an inadequate reduction of the iron
oxides, even though reductive gas (CO gas) rises up from
the carbonaceous material spread on the hearth, as
discussed below. The required percentage of the amount
of fixed carbon contained in the carbonaceous reducing
agent is preferably 98 mass% or more, and more preferably
98.5 mass. or more, with respect to the amount of fixed
carbon needed to reduce the iron oxides. However, when
the amount of fixed carbon contained in the carbonaceous
reducing agent is excessively large, the reductive gas
(CO gas) still continues to rise up from the agglomerates
by reacting with the atmospheric gas even after the
reduction is finished. This decreases the oxygen partial
pressure in melting the reduced iron, as discussed below,
and thus makes the dephosphorization ratio of the reduced
iron be lower. Therefore, the required percentage of the
amount of fixed carbon contained in the carbonaceous
reducing agent is preferably 102 mass% or less, and more
preferably 101 mass. or less, with respect to the amount
of fixed carbon needed to reduce the iron oxides.
In the present invention, it is especially
recommended that the amount of fixed carbon contained in
16
CA 02745763 2011-06-03
the carbonaceous reducing agent be adjusted somewhat on
the low side with respect to the amount of fixed carbon
needed to reduce the iron oxides. The reason for this is
as follows: the unreduced portions of the iron oxides are
reduced by the carbonaceous material spread on the hearth,
since the agglomerates are on the carbonaceous material
in the present invention, although an insufficient amount
of fixed carbon contained in the carbonaceous reducing
agent seems to cause inadequate reduction of the granular
iron.
Specifically, the iron oxides (FeOX) contained in the
agglomerates are reduced by the carbon (C) contained in
the carbonaceous reducing agent and by the carbonaceous
material spread on the hearth, according to the reduction
reactions in the following formulas (1) and (2), to form
granular iron.
FeOX + xCO -* Fe + xCO2 (1)
FeO, + xC -> Fe + xCO (2)
As a result of various experiments, the inventors
found that the reduction reaction proceeds in the
proportions indicated by the following formula (3) when a
moles of the FeOX of Formula (1) react, and when b moles
of the FeOX of Formula (2) react. That is, Formula (3)
indicates the number of oxygen atoms reduced by one
carbon atom. In the reduction of FeOX, the inventors
17
CA 02745763 2011-06-03
estimate that about 380 of the total occurs by direct
reduction by carbon (C), and about 720 of the total
occurs by indirect reduction by reducing gas (CO gas).
1.0 <_ 1 + a/ (a + b) < 1.5 (3)
Therefore, even though the amount of carbon, which
is calculated as one carbon atom needed to reduce one
oxygen atom contained in iron oxide, is slightly on the
low side (for example, by blending carbonaceous reducing
agent a little less into the raw material mixture), iron
oxides in agglomerates are still adequately reduced.
Also, adjusting the amount of fixed carbon contained
in the carbonaceous reducing agent on the lower side with
respect to the amount of fixed carbon needed to reduce
the iron oxides causes to form more iron oxides (FeO)
contained in subgenerated slag in the reduction, and
drives the dephosphorization reaction faster in melting
the reduced iron. Therefore, the percentage of the
amount of fixed carbon contained in the carbonaceous
reducing agent is more preferably 100 mass% or less with
respect to the amount of fixed carbon needed to reduce
the iron oxides.
The amount of fixed carbon needed to reduce the iron
oxide may be calculated from the composition of the raw
material mixture.
The granular iron is required to be carburized so
that it contains about 3 mass% carbon in order to
18
CA 02745763 2011-06-03
decrease the melting point of the granular iron during
separating the melted granular iron from the slag.
However, when the amount of fixed carbon contained in the
carbonaceous reducing agent blended to the raw material
mixture is set to be slightly insufficient with respect
to the amount of fixed carbon needed to reduce the iron
oxides, the granular iron does not contain enough fixed
carbon, and this results that the granular iron cannot be
melted. Accordingly, spreading a carbonaceous material
on the hearth and setting the amount of fixed carbon
contained in this carbonaceous material to be in excess
over the amount of fixed carbon needed to reduce the iron
oxides increase the amount of fixed carbon supplied to
the granular iron, and this can make the molten granular
iron be separated from the slag.
The percentage of the amount of fixed carbon
contained in the carbonaceous material that is spread on
the hearth is preferably adjusted to within a range
between 2 mass. and 5 mass. with respect to the amount of
fixed carbon needed to reduce the iron oxides. There are
no particular limitations on the type of carbonaceous
material that is spread on the hearth, for example, the
carbon-containing material used as the above-mentioned
carbonaceous reducing agent can be used.
In the present invention, the above-mentioned
agglomerates is also preferred to adjust the composition
19
CA 02745763 2011-06-03
of the raw material mixture so that the basicity of slag
subgenerated in reducing the iron oxides is in a range
between 1.0 and 1.6. The reason for this is as follows:
when the slag basicity is less than 1.0, the
dephosphorization reaction in melting the reduced iron
does not proceed and thus the phosphorus content of the
granular iron cannot be reduced. Therefore, the basicity
is preferably 1.3 or more, and more preferably 1.4 or
more. However, too high slag basicity causes too high
melting point of the slag, and the resulting slag does
not melt when the reduced iron is melted, making it
difficult to separate the granular iron from the slag.
As a result, the slag ends up being mixed in the granular
iron, and this degrades in quality of the granular iron.
Therefore, the basicity is preferably 1.6 or less.
The basicity of slag is the value [(CaO)/(Si02)]
calculated from the CaO content and the Si02 content in
the slag.
Examples
The present invention will now be described in
further detail through examples. These examples are not
intended to limit the present invention, various
modifications are possible without departing from the
scope of the invention described above and below, and
these modifications are included in the technical scope
CA 02745763 2011-06-03
of the present invention.
In the examples, each agglomerates were made from
raw material mixtures each containing a carbonaceous
reducing agent and an iron oxide-containing substance,
and then, each granular irons were made by: charging each
agglomerates onto a carbonaceous material spread on a
hearth of a heating furnace; and heating the agglomerates
to thereby reduce and melt the iron oxides in the
agglomerates in a laboratory. The compositions of the
agglomerates and the conditions of reduction and melting
were varied. Specifically, as follows.
Two kinds of iron oxide-containing substance were
used: iron ore (n) with a low phosphorus content; and
iron ore (hpb) with a high phosphorus content. Table 1
below shows the compositions of the iron ore (n) and the
iron ore (hpb). Two kinds of carbonaceous reducing agent
were also used: coal (p) with a low phosphorus content;
and coal (b) with a high phosphorus content. Table 2
below shows the compositions of the coal (p) and the coal
(b).
The iron ore each shown in Table 1 and the coal each
shown in Table 2 were blended with additives, and then,
each pelletized agglomerates (test material) with
particle sizes of 18 to 20 mm were produced. The blended
additives were wheat gluten that was added as a binder,
MgO, CaO, etc. Table 3 below shows the blending ratio of
21
CA 02745763 2011-06-03
each test materials (percentages of weight values).
Table 3 shows the target values for the percentage
of the amount of fixed carbon contained in the
carbonaceous reducing agent blended to the raw material
mixture with respect to the amount of fixed carbon needed
to reduce the iron oxides. Table 3 also shows the target
values for the basicity of slag subgenerated during
reduction.
Table 4 below shows the compositions of the test
materials. In Table 4, test material (1) is pellets with
a low phosphorus content, and test materials (2) to (5)
are pellets with a high phosphorus content.
Each granular irons were produced by: charging each
test materials shown in Table 4 into the furnace of which
hearth was spread with a carbonaceous material; heating
them to thereby reduce and melt the iron oxides in the
raw material mixture; and discharging products to a
cooling zone at the point when the granular iron and slag
had completely separated. The number of samples of each
test materials charged into the furnace was 30. 130 g of
smokeless coal with a maximum particle size of 2 mm or
less was spread over the hearth as a carbonaceous
material. Extra carbonaceous material was spread around
the edges to protect the hearth.
The test materials charged into the furnace were
heated with a heater provided to the furnace, so that the
22
CA 02745763 2011-06-03
temperature of the test materials would reach 1450 C.
Inside the furnace, the linear speed of the
atmospheric gas under which the test materials were
heated (the linear speed of the atmospheric gas near the
test materials) was controlled to be in a range between
1.35 and 20.27 cm/second, and the oxygen partial pressure
of the atmospheric gas under which the test materials
were heated (the oxygen partial pressure of the
atmospheric gas near the test materials) was controlled
to be in a range between 0 and 5.057 x 10-13 atm. Tables
S and 6 below show the gas linear speeds and oxygen
partial pressures. The gas linear speeds were the value
at standard state.
The gas linear speeds were calculated from the
amount of gas supplied and the cross sectional area at
the sample placement portion inside the furnace. The
oxygen partial pressures were calculated by the following
procedure.
The following formula (4) expresses a carbon
combustion reaction, and the standard generation free
energy AF in this reaction is expressed by the following
formula (5).
C (graphite) + 02 (g) = CO2 (g) (4)
AF = -94640 + 0.05 x T (cal/mol) (5)
Meanwhile, the standard generation free energy AF of
23
CA 02745763 2011-06-03
this reaction is expressed by the following formula (6),
using the partial pressure PC02 of the atmospheric gas
accounted for by carbon dioxide gas, and the partial
pressure P02 of the atmospheric gas accounted for by
oxygen gas.
AF = -RT x log(PC02/P02) (6)
The absolute temperature of 1450 C is:
1450 ( C) + 273 = 1723 (K),
therefore the relation between the carbon dioxide partial
pressure and the oxygen partial pressure in the
atmospheric gas at 1450 C is found as follows from the
above formulas (5) and (6).
-94640 + 0.05 x 1723 = -4.575 x 1723 x log (P002/P02)
log (PC02/P02) = 11.995
PCO2/PO2 = 9.887 x 1011
Here, the partial pressure of the atmospheric gas
accounted for by carbon dioxide gas is measured, for
example, when PC02 = 0.5 is measured, then
P02 = 5.0571 x 10-13
is obtained as the partial pressure of the atmospheric
gas accounted for by oxygen gas.
Tables 5 and 6 show the compositions of the
resulting granular irons, and the compositions of the
subgenerated slags when granular irons were produced. Of
the compositions of the granular irons shown in Tables 5
24
CA 02745763 2011-06-03
and 6, each amount of iron is the value calculated by
subtracting the amount of alloy elements and impurities
from the total (100 mass%).
In Table 6, No. 30 is the result of discharging the
granular iron one minute before the point at which the
separation of slag and granular iron was complete, and No.
31 is the result of discharging the granular iron from
the furnace after waiting for three minutes from the
point at which the separation of slag and granular iron
was complete. In Tables S and 6, everything other than
Nos. 30 and 31 is the result of discharging the granular
iron from the furnace at the point when one minute had
elapsed from the point at which the separation of slag
and granular iron was complete.
The center temperatures of the test materials were
measured and found to be: approximately 1300 C at the
point one minute before the separation of slag and
granular iron was complete (No. 30); approximately 1400 C
at the point when one minute had elapsed since the
completion of the separation of slag and granular iron;
and approximately 1450 C at a point three minutes after
the completion of the separation of slag and granular
iron (No. 31).
Also, the CO2 gas proportion near the test materials
was substantially constant from the point one minute
CA 02745763 2011-06-03
before the completion of the separation of slag and
granular iron, up to the point three minutes after the
completion of the separation of slag and granular iron.
Meanwhile, some CO gas was noted to rise up from the test
material at a point one minute before the completion of
the separation of slag and granular iron, but no CO gas
was seen to rise up from the test material once the
separation of slag and granular iron was partially
completed.
The dephosphorization ratio was calculated from the
composition of the granular iron and the composition of
the test material by the following formula.
Dephosphorization ratio(%) = [1 - (amount of phosphorus
contained in the resulting granular iron/total amount of
iron contained in the resulting granular iron)/(amount of
phosphorus contained in test material/total amount of
iron contained in test material)] x 100
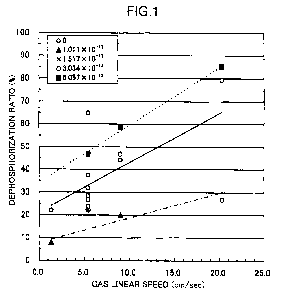
Fig. 1 shows the relation between the
dephosphorization ratio and the gas linear speed under
different oxygen partial pressures, based on the data in
Tables 4 and 5. In Fig. 1, the mark 0 indicates the
result at an oxygen partial pressure of 0 atm, the mark
indicates the result at an oxygen partial pressure of
26
CA 02745763 2011-06-03
1.011 x 10-13 atm, the mark x indicates the result at an
oxygen partial pressure of 1.517 x 10-13 atm, the mark 0
indicates the result at an oxygen partial pressure of
3.034 x 10-13 atm, and the mark ^ indicates the result at
an oxygen partial pressure of 5.057 x 10-13 atm.
As is clear from Fig. 1, when the atmospheric gas
contains oxygen, the higher is the linear speed of the
atmospheric gas under which the test material is heated,
the higher is the dephosphorization ratio. For example,
with test material 3, which had a gas linear speed of
5.41/sec, it can be seen that the dephosphorization ratio
rises when the oxygen partial pressure of the atmospheric
gas is increased from 1.517 x 10-13 atm to 3.034 x 10-13
atm, and that with a given test material and at a given
gas linear speed, the dephosphorization ratio rises when
the oxygen partial pressure of the atmospheric gas is
increased. When the oxygen partial pressure of the
atmospheric gas is 0 atm (that is, under a nitrogen gas
atmosphere), the dephosphorization is not affected by the
gas linear speed. When the gas linear speed is less than
5 cm/second, the result for dephosphorization ratio is
reversed from that when the oxygen partial pressure of
the atmospheric gas is 1.517 x 10-13 atm, but these are
considered to be affected by sample variance or
phosphorus analysis error.
27
CA 02745763 2011-06-03
Based on the above results, increasing the gas
linear speed and the oxygen partial pressure of the
atmospheric gas to the specified values or higher is an
effective way to raise the dephosphorization ratio.
Fig. 2 shows the relation between the
dephosphorization ratio and the gas linear speed in Nos.
24, 25 and 32, out of the results in Table 6 when the
oxygen partial pressure was 3.034 x 10-13 atm. A
comparison of Fig. 2 with Fig. 1 reveals that even though
the amount of phosphorus contained in the test material
changes, at the condition that the oxygen partial
pressure is constant, the dephosphorization ratio rises
along with the gas linear speed. Although not shown in
the drawings, the same thing can be understood from Nos.
2, 4 and 6, for example.
Fig. 3 shows the relation between the
dephosphorization ratio and the oxygen partial pressure
in Nos. 25, 27, 28 and 29, out of the results in Table 6
when the gas linear speed was 5.41 cm/second. As is
clear from Fig. 3, when the gas linear speed is constant,
the dephosphorization ratio rises along with the oxygen
partial pressure. Also, when the oxygen partial pressure
is 1.517 x 10-13 atm, it can be seen that there is almost
no change in the dephosphorization ratio. Although not
shown in the drawings, it can be understood from Nos. 3,
28
CA 02745763 2011-06-03
4 and 5, for example, that when the gas linear speed is
constant, the dephosphorization ratio rises along with
the oxygen partial pressure.
Fig. 4 shows the relation between the
dephosphorization ratio and the time discharging granular
iron in Nos. 25, 30 and 31, out of the results in Table 6
when the oxygen partial pressure was 3.034 x 10-13 atm and
the gas linear speed was 5.41 cm/second. Fig. 4 shows
the dephosphorization ratio of variation compared with
the variation of the time discharging the granular iron
which was separated from the slag out of the furnace from
the clock time when the slag and granular iron had been
completely separated was set 0 minute, after the reduced
irons were melted. As is clear from Fig. 4, the
dephosphorization ratio drops when heating is continued
after the slag and granular iron have been separated.
The highest dephosphorization ratio in Fig. 4 is
when the discharging time is "-1 minute," and this "-1
minute" means that the granular iron was discharged from
the furnace before the granular and slag were separated,
which is a condition that cannot be employed in actual
practice.
Fig. 5 shows the relation between the
dephosphorization ratio and the amount of fixed carbon
contained in the carbonaceous reducing agent blended to
the raw material mixture for Nos. 21, 22 and 25, out of
29
CA 02745763 2011-06-03
the results shown in Table 6. As is clear from Fig. 5,
the dephosphorization ratio is advantageously higher when
the amount of fixed carbon contained in the carbonaceous
reducing agent blended to the raw material mixture is set
on the low side with respect to the amount of fixed
carbon needed to reduce the iron oxides.
On the other hand, it can be seen that there is a
further drop in the dephosphorization ratio when the
percentage of the amount of fixed carbon contained in the
carbonaceous reducing agent blended to the raw material
mixture is over 102 mass. with respect to the amount of
fixed carbon needed to reduce the iron oxides. This is
considered to be attributable to the fact that since a
large amount of reductive gas rises up even in the
process of melting the reduced iron, the effect of
increasing the gas linear speed is lost.
As is clear from the results for No. 22, even though
the amount of fixed carbon contained in the carbonaceous
reducing agent blended to the raw material mixture is set
on the low side with respect to the amount of fixed
carbon needed to reduce the iron oxides contained in the
test material, adjusting the amount of carbon contained
in the carbonaceous material spread on the hearth to be
in a range between 2 and 5 mass-06 with respect to the
amount of fixed carbon needed to reduce the iron oxides
causes stable reduction of the iron oxides remaining
CA 02745763 2011-06-03
after dephosphorization has proceeded by the carbonaceous
material spread on the hearth.
Table 1
COMPOSITION (mass%)
IRON
ORE TOTAL
TYPE AMOUNT FeO Si02 CaO A1203 MgO S P
OF IRON
(n) 67.64 29.13 4.85 0.44 0.23 0.47 0.004 0.018
(hpb) 62.97 0.47 3.25 0.04 2.08 0.04 0.030 0.13
Table 2
COAL TYPE COMPOSITION (mass%)
VOLATILE ASH FIXED TOTAL TOTAL TOTAL AMOUNT
CONTENT CONTENT CARBON AMOUNT AMOUNT OF PHOSPHORUS
OF SULFUR OF CARBON (CALCULATED VALUE)
16.79 4.64 78.57 0.595 86.46 0.00243
(p) COMPOSITION OF ASH CONTENT (mass%)
Fe203 SiO2 Ga0 j AI203 MgO Ti02 P205
15.75 43.55 3.98 27.03 j 1.85 1.67 0.12
COAL TYPE COMPOSITION (mass%)
VOLATILE ASH FIXED TOTAL TOTAL TOTAL AMOUNT
CONTENT CONTENT CARBON AMOUNT AMOUNT OF PHOSPHORUS
OF SULFUR OF CARBON (CALCULATED VALUE)
14.03 4.75 81.22 0.458 83.31 0.03254
(b) COMPOSITION OF ASH CONTENT (mass%)
Fe203 Si02 CaO A1203 MgO TiO2 P2O5
4.26 57.25 2.16 23.93 0.59 0.87 1.57
Table 3
31
CA 02745763 2011-06-03
BLENDING RATIO (mass%) TARGET VALUE
TEST IRON ORE TYPE COAL TYPE AMOUNT OF
MATERIAL ADDITIVES CARBON BASICITY
(n) (hpb) (p) (b) (mass%)
(1) 71.581 - 16.871 - 11.55 101.00 1.26
(2) - 72.470 -- 17.098 10.43 103.20 1.26
(3) - 73.454 - 16.102 10.44 101.00 1.26
(4) - 74.327 - 15.209 10.46 99.00 1.26
(5) - 72.668 16.972 - 10.36 10320 1.26
Table 4
TEST COMPOSITION (mass%)
MATERIAL TOTAL TOTAL
AMOUNT OF MOUNT OF Si02 A1203 CaO MgO F P S
CARBON IRON
(1) 15.62 48.60 4.01 0.48 5.96 0.97 0.35 0.017 0.104
(2) 15.90 46.01 2.92 1.67 4.17 0.87 0.36 0.100 0.094
(3) 15.89 46.63 3.31 1.75 4.11 0.80 0.32 0.099 0.094
(4) 14.32 47.29 2.98 1.72 4.20 0.87 0.36 0.100 0.087
(5) 16.14 46.55 3.41 1.72 4.31 0.84 0.36 0.094 0.133
Table 5
32
CA 02745763 2011-06-03
OXYGEN
TEST GAS LINEAR PARTIAL COMPOSITION OF GRANULAR IRON (mass%) COMPOSITION OF
SLAG (mass%) DEPHOSPHOPoZA110N
No. MATERIAL SPEED PRESSURE BASICITY RA110
(cm/sec) x 10-13(atm) C L P S Fe FeO P S (%)
1 (1) 20.27 5.057 2.9 0.005 0.134 96,92 4.96 0.110 0.244 1,47 85.25
2 (1) 20.21 3.034 3.04 0.007 0.12 96.77 2.84 0.099 0.313 1.53 79.32
3 (1) 9.01 5.057 3.18 0.014 0.114 96.64 1.9 0.068 0.379 1.52 58.59
4 (1) 9.01 3.034 3 0.018 0.091 96,83 0.98 0.037 0.486 1.43 46.86
(1) 9,01 1.011 3.49 0.027 0.038 96.39 0.27 0.011 0.712 1.54 19.92
6 (1) 5.41 3.034 323 0.023 0,056 96.63 0.45 0.020 0.589 1.43 31.95
7 (1) 1.35 1.011 3.64 0.031 0.028 96.21 0.24 0.007 0.762 1.57 7.89
8 (1) 20.27 5.057 2.15 0.005 0.164 97.65 6.83 0.086 0.292 1.41 85.36
9 (1) 20.27 3.034 2.53 0.005 0.155 97.28 3.5 0.090 0.317 1.47 85.31
(1) 9.01 3.034 2.65 0.019 0.111 97.19 0.72 0.032 0.501 1.44 44.11
11 (1) 5.41 3.034 2.97 0.024 0.079 96.89 0.43 0.014 0.668 1.42 29.18
Table 6
TEST GAS LINEAR OXYGEN COMPOSITION OF GRANULAR IRON (mass%) COMPOSITION OF
SLAG (mass%) BASICITY IEPHOSPHOHQA1I3
No. MATERIAL SPEED PARTIAL PRESSURE RATIO
(cm/sec) x 10-11 (atm) C P S Fe FeO P S (%)
21 (2) 5,41 1034 3.00 0.16 0.089 96.72 0.17 0.180 0.336 1.52 23.89
22 (4) 5.41 3.034 290 0,07 0.121 96.88 3.94 0.640 0.198 1.50 64.85
23 (5) 5.41 3.034 2.66 0.14 0.110 97.06 0.46 0.240 0.453 1.62 28.57
24 (3) 1.35 3.034 2.96 0.16 0.073 96.78 0.47 0.136 0,378 1.47 22.13
25 (3) 5.41 3.034 2.87 0.14 0.113 96.85 0.42 0.150 0.514 1.61 31.91
26 (3) 2027 3.034 2.91 0.15 0.103 96.81 0.81 0.250 0292 0.68 27.02
27 (3) 5.41 1.517 2.62 0.16 0.081 97.11 0.54 0.181 0.367 1.48 22.39
28 (3) 5.41 0 2.78 0.16 0.063 96.95 0.44 0.110 0.487 1.49 22.27
29 (3) 5.41 5.057 2.79 0.11 0.109 96.96 2.16 0.390 0.225 1.48 46.56
30 (3) 5.41 3.034 1.79 0.13 0.091 97.86 1.41 0.300 0.312 1.49 37.43
31 (3) 5.41 3.034 3.29 0.15 0.111 96.42 0.80 0200 0.237 1.47 26.72
32 (3) 9.01 3.034 2.69 0.11 0.113 97.06 2.82 0.510 0.223 1.48 46.62
33 (5) 5.41 3.034 2.82 0.14 0.113 96.88 0.48 0.140 0.554 1.61 28.44
5
As described above, one aspect of the present
33
CA 02745763 2011-06-03
invention is a method for producing granular iron
comprising: charging agglomerates formed from a raw
material mixture containing an iron oxide-containing
substance and a carbonaceous reducing agent onto a
carbonaceous material spread on a hearth of a furnace;
and heating the agglomerates to thereby reduce and melt
iron oxides in the agglomerates, wherein the temperature
of the agglomerates in the furnace is set in a range
between 1200 C and 1500 C; the oxygen partial pressure in
atmospheric gas under which the agglomerates are heated
is set to 2.0 x 10-13 atm or more at standard state; and
the linear speed of the atmospheric gas in the furnace is
set to 4.5 cm/second or more.
According to the present invention, since the
reduced agglomerates are melted in a state in which the
oxygen partial pressure of the atmospheric gas and the
gas linear speed and are controlled to the above-
mentioned conditions, the phosphorus contained in the
reduced iron can be moved to the subgenerated slag during
reduction. As a result, the granular iron made by
melting the reduced iron contains less phosphorus.
In the above-mentioned method for producing granular
iron, it is preferable that the composition of the raw
material mixture is adjusted so that the percentage of
the amount of fixed carbon contained in the carbonaceous
34
CA 02745763 2011-06-03
reducing agent is in a range between 98 masse and 102
mass. with respect to the amount of fixed carbon needed
to reduce the iron oxides. This causes the reduction of
iron oxides to proceed more actively and the granular
iron with lower phosphorus content to be produced.
In the above-mentioned method for producing granular
iron, it is preferable that the composition of the raw
material mixture is adjusted so that the basicity of slag
subgenerated in reducing the iron oxides is in a range
between 1.0 and 1.6. This causes the dephosphorization
reaction to proceed faster and the granular iron with
lower phosphorus content to be produced.
In the above-mentioned method for producing granular
iron, it is preferable that the percentage of the amount
of fixed carbon contained in the carbonaceous reducing
agent is in a range between 98 mass. and 100 mass. with
respect to the amount of fixed carbon needed to reduce
the iron oxides. This causes the amount of fixed carbon
contained in the carbonaceous reducing agent to be on the
low side with respect to the amount of fixed carbon
needed to reduce the iron oxides, and thus more iron
oxides (FeO) contained in the subgenerated slag during
reduction to be produced. As a result, this accelerates
the dephosphorization reaction during the melting of the
reduced iron, therefore, the dephosphorization ratio of
the reduced iron can be further increased.
CA 02745763 2011-06-03
In the above-mentioned method for producing
granular iron, it is preferable that the linear speed of
the atmospheric gas is set to 5.4 cm/second or less
(including 0 cm/second) until the iron oxides begins to
melt; and the linear speed of the atmospheric gas is set
to 4.5 cm/second or more after the iron oxides begins to
melt. Adjusting the linear speed of the atmospheric gas
under which the agglomerates are heated, both until the
iron oxides begins to melt and after the melting has
begun, allows the reduction reaction to proceed actively
in the agglomerates up until the melting of the iron
oxide begins, and allows the melting of the iron to
proceed stably after melting has begun.
In the above-mentioned method for producing
granular iron, it is preferable that the percentage of
the amount of fixed carbon contained in the carbonaceous
material which is spread on the hearth is set in a range
between 2 mass. and 5 mass. with respect to the amount of
fixed carbon needed to reduce the iron oxides; and the
maximum particle size of the carbonaceous material is set
to 2 mm or less. This increases the amount of fixed
carbon supplied to the granular iron, allows the molten
granular iron to separate from slag, and also prevents
the molten slag from running down into the crevices in
the carbonaceous material and corroding the hearth.
36
CA 02745763 2011-06-03
Industrial Applicability
Granular iron with a low phosphorus content can be
made stably by using the method of the present invention
for producing granular iron.
37