Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02750809 2011-07-29
COMPOSITION AND METHOD FOR ATTRACTION OF EMERALD ASH
BORER AGRILUS PLANIPENNIS FAIRMAIRE (COLEOPTERA:
BUPRESTIDAE)
BACKGROUND OF THE INVENTION
The Emerald ash borer, Agrilus planipennis Fairrnaire, (Coleoptera:
Buprestidae) is an
invasive Palearctic species that has killed millions of ash trees (Fraxinus
spp. L.)
(Oleaceae) in the USA and Canada (Cappaert et al. 2005; Poland and McCullough
2006). Although initially detected near Detroit, Michigan in 2002, there is
evidence
that populations of this invasive species had been present in Michigan, USA
and
Ontario, Canada since the mid-1990s (Seigert et al. 2007). Since then, it has
spread
rapidly and has been detected in 15 states and two provinces, Ontario and
Quebec, in
Canada (EAB 2010). Movement of infested firewood and nursery stock has
exacerbated its spread and large scale devastation of ash trees is predicted
(Marchant
2006). Early detection of A. planipennis infestations has proven difficult
because
visual signs and symptoms, such as D-shape exit holes, epicormic shoots, bark
deformities, and thinning crowns, usually appear only on heavily infested
trees a year
or more after populations have been established (Cappaert et al. 2005; de
Groot et al.
2006, 2008; Poland and McCullough 2006). Development of a monitoring system is
critical for early detection of A. planipennis populations, which would aid in
management and control decisions. In order to maximize detection efficacy, a
better
understanding of the behavior and chemical ecology of adult A. planipennis is
needed.
1
CA 02750809 2011-07-29
Adult A. planipennis are typically active between 0600-1700 h, particularly
when the weather is warm and sunny (Yu 1992; Rodriguez-Saona et al. 2007),
with
mating occurring from 0900-1500 h and lasting for 20-90 min. Yu (1992)
observed
that adults preferred trees in open areas with direct sunlight and that during
rainy or
cloudy weather they tended to rest in cracks in the bark or on the foliage.
Adult
beetles, particularly males, spend much of their time in the canopy feeding
and flying
short distances (Lance et al. 2007; Lelito et al. 2007; Rodriguez-Saona et al.
2007).
Indeed, traps in the mid¨upper ash canopy capture more adults than traps hung
below
the canopy (Lance et al. 2007; Francese et al. 2007, 2008; Crook et al. 2008,
2009)
and traps in locations exposed to direct sunlight (i.e. on the edge or near a
gap)
generally catch more adults than those in shaded locations (Poland et al.
2005;
McCullough et al. 2006, 2009; Francese et al. 2008; Lyons et al. 2009).
Crook and Mastro (2010) reviewed the considerable progress made towards
developing a trap that is effective at capturing A. planipennis (Francese et
al. 2005,
2007, 2008, 2010; Crook et al. 2008, 2009; Lelito et al. 2007, 2008;
McCullough et al.
2008). Color has been identified as an important factor affecting trap
captures, with
purple shown to be highly attractive (Francese et al. 2005, 2008; Crook et al.
2008).
Purple traps typically catch more females than males (Francese et al. 2008;
Crook et
al. 2009), due to A. planipennis response to light in both the blue and red
range of the
visible spectrum (Crook et al. 2009). Currently, a sticky purple prism trap is
utilized
in surveys for A. planipennis in the United States (Francese et al. 2008;
Crook and
Mastro 2010). Adult A. planipennis also respond to light in the green range
(Crook et
al. 2009), with green traps capturing two to three times as many adults as
purple traps.
Green traps typically have a bias towards males in trap captures (Lance et al.
2007;
Rodriguez-Saona et al. 2007; Lelito et al. 2008; Crook et al. 2009). However,
green
2
CA 02750809 2011-07-29
traps typically catch more adults only when deployed high in the tree canopy.
Thus,
trap deployment, as well as color and lure combination, must be considered
when
evaluating traps for a monitoring program, as trap captures are likely
influenced by
adult preferences and behavioral activity patterns.
Numerous studies have described the chemical ecology of A. planipennis
(Crook and Mastro 2010) and two types of host volatiles have been demonstrated
to
be attractive: bark sesquiterpenes (Poland and McCullough 2006; Crook et al.
2008)
and green leaf volatiles (Poland et al. 2004, 2005, 2006, 2007; Rodriguez-
Saona et al.
2006; de Groot et al. 2008; Grant et al. 2010). Girdled or stressed ash
(Poland and
McCullough 2006; Crook et al. 2008) are attractive to both sexes, as are
Manuka and
Phoebe oils which contain, in part, the sesquiterpenes emitted by stressed
Fraxinus
spp. (Crook et al. 2008; Crook and Mastro 2010; Grant et al. 2010). Of the
green leaf
volatiles, one compound in particular, (3Z)-hexenol, is highly antennally
active and
attractive to males (de Groot et al. 2008; Grant et al 2010). These results
indicate that
specific host volatiles act as kairomones in the chemical ecology of A.
planipennis
and these compounds may provide useful detection tools.
Much of the literature on the mating behavior of buprestids (e.g. Rodriguez-
Saona et al. 2006; Lelito et al. 2007; Akers and Nielsen 1992; Gwynne and
Rentz
1983; Carlson and Knight 1969) has described the use of visual and tactile
cues for
mate location. For buprestids, including those in the genus Agrilus, host
location has
been described as occurring first by olfactory processes and then mate
location by
visual, or by vibratory and/or tactile cues (Carlson and Knight 1969).
However, Dunn
and Potter (1988) showed attraction of A. bilineatus (Weber) males to cages
containing females compared to host-logs only, suggesting the use of a female-
produced pheromone.
3
CA 02750809 2011-07-29
Limited progress has been made into the pheromone chemistry of A.
planipennis. Previous work suggested the presence of a contact pheromone
(Lelito et
al. 2007), subsequently identified as 9-methylpentacosane, which appears only
on the
cuticle of female A. planipennis at sexual maturity (7-10 d old) and
stimulates full
copulatory activity in males upon antenna! contact (Silk et al. 2009),
although 3-
methyltricosane may also be involved as an additional component (Lelito et al.
2009).
Bartelt et al. (2007) identified a volatile, antennally-active predominantly
female-
produced macrocyclic lactone, (3Z)-dodeeen-12-olide [(3Z)-lactone], which was
the
first putative volatile pheromone described for A. planipennis, but no
behavioral
activity was reported.
Pureswaran and Poland (2009) reported that males were able to locate and
identify females at close range using olfaction and an unidentified volatile
cue. Here,
we use GC-EAD in combination with field trapping and olfactometry to test
whether
(3Z)-lactone elicits behavioral responses in A. planipennis either alone or in
combination with host kairomones (bark sesquiterpenes or green leaf
volatiles). We
tested various lure combinations on both purple and green traps, as both
colors have
been shown to be attractive. We also tested the lactone stereoisomer, (3E)-
lactone,
for its effect on A. planipennis behavior because preliminary studies
suggested that
exposure to UV-light catalyzes the isomerization of (3Z) to the (3E)-lactone
and A.
planipennis adults are known to favor sunny locations.
SUMMARY OF THE INVENTION
This invention provides the first behavioral evidence for a volatile pheromone
of A. planipennis in combination with host foliar volatiles in association
with a trap of
4
CA 02750809 2011-07-29
a color in the green range of the visible light spectrum, contributing to the
knowledge
of the chemical ecology and the development of improved tools for the
detection of A.
planipennis infestations.
According to one aspect of the present invention, a composition for the
attraction of A. planipennis is provided, comprising
(a) (3Z)-dodecen-12-olide and
(b) ash foliar volatiles, associated with a trap of a color in the green range
of
the visible light spectrum
According to another aspect of the present invention, a method for the
attraction of A. planipennis is provided, comprising applying to an insect
habitat an
insect attracting amount of (3Z)-dodecen-12-olide and ash foliar volatiles,
associated
with a trap of a color in the green range of the visible light spectrum.
According to yet another aspect of the invention, the components (a) and (b)
of the composition are maintained separately until use, and associated with
the trap
for use. A kit form is also contemplated, including a septum for receiving the
lactone
dissolved in a solvent which evaporates before use leaving the lactone to emit
therefrom, and a bubble cap containing neat ash volatiles, associated with the
trap.
When a sticky trap is used, no insecticide is included. However, if a non-
sticky trap is used, an appropriate insecticide is used.
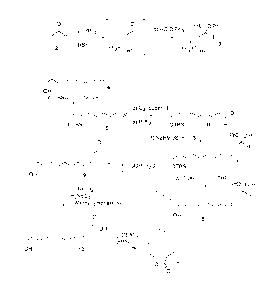
BRIEF DESCRIPTION OF THE DRAWING
Figure 1 is a flow diagram illustrating a process according to the invention
for
the synthesis of (3Z)-dodecen-12-olide.
CA 02750809 2011-07-29
Figure 2 is a flow diagram illustrating a process according to the invention
for
the synthesis of (3E)-dodecen-12-olide.
Figure 3 is a graph illustrating electroantennographic (EAG) dose-response
curves of male and female A. planipennis antennae to (3Z)-dodece-12-olide
(3ZLac)
and (3E)-dodece-12-olide (3ELac) according to dosages applied to stimuli
cartridges.
EAG dose-responses (mean SEM) are presented relative to a positive control
standard (geranyl acetone, 1 p.g applied dose).
Figure 4 illustrates GC-FID/EAD responses of male and female A. planipennis
antennae. The FID trace is a synthetic mixture of (3E)- and (3Z)-lactones
Figures 5a-d are graphs illustrating proportions of male A. planipennis
crawling up the test vs. control arms of a Y-tube olfactometer in 12
independent trials
in response to: (a) (3E)-lactone, (3Z)-lactone or a 60:40 combination; (b)
Phoebe oil
(25 ul) alone or combined with either (3E)-lactone or (3Z)-lactone); (c)
Phoebe oil
(2.5 i.t1) alone or combined with (3E)-lactone or (3Z)-lactone; and (d) (3Z)-
hexenol
alone or combined with either (3E)-lactone or (3Z)-lactone. For each stimulus,
the test
treatment was compared with the control using a chi-square goodness of fit
test.
Figure 6 are bar graphs illustrating mean ( SE) catches of male and female A.
planipennis on purple sticky prism traps baited with various combinations of
(3Z)-
and/or (3E)-lactone and host volatiles in field experiments carried out at two
sites in
(a) 2008 and two sites (b, c) in 2009. Sites were analyzed separately in 2009
due to
the differences in sex ratio. Note differences in scale of X-axis. Prior to
analyses, data
were transformed using a natural log (n+1), however untransformed data are
presented. Error bars reflect + or ¨ one standard error of the least squares
means. In
2008 (Fig. 2a), (3E)-lactone was not tested except that it was present in the
synthetic
(32)-lactone at 2%.
6
CA 02750809 2011-07-29
Figure 7 are bar graphs illustrating mean ( SE) catches of male and female A.
planipennis on green sticky prism traps baited with the different attractant
combinations at (a) Anika and McKellar sites combined and (b) sites in
Michigan,
USA. Plotted values reflect the least squares means of 12 replicate blocks in
total
(untransformed data). Statistics (P > F) apply to natural log (n+1)-
transformed data
following ANOVA. Error bars reflect + or ¨ one standard error of the least
squares
means.
DETAILED DESCRIPTION OF THE INVENTION
Methods and Materials
Source of insects. Trees with larval A. planipennis were felled near Windsor
and Sarnia, Ontario; infested logs were transported to the Great Lakes
Forestry Centre
in Sault Ste Marie, Ontario. Storage and rearing protocols have been
previously
reported (Silk et al. 2009). Emerged adults were sexed and virgin males and
females
were kept on a 16:8 h L:D cycle and supplied with water and foliage of
evergreen ash,
Fraxinus tthdei (Wenzig) Linglesh.
Volatile collection. Volatiles were collected from two groups of virgin adult
males (n=18 and n=8) and two groups of virgin adult females (n=17 and n=18)
feeding on ash leaves in separate 250 ml glass chambers (16:8 L:D at 22 C).
Adults
were 10 d old when placed in the chambers in groups of 6-8 at one time; and
were
replaced as they died over the volatile collection period. Filtered air was
drawn from
the chambers at ¨0.1 L/min onto a pre-conditioned Super-Q filter (-200 mg)
for 10-
11 d. Volatiles were eluted using methylene chloride (3 x 2mL) and
concentrated to
10-20111 under dry nitrogen.
7
CA 02750809 2011-07-29
Analytical techniques and purification. Synthetic samples and extracts were
analyzed by GC/MS on a Hewlett-Packard 5890 GC and a 5971 mass selective
detector in the electron impact (El, 70eV) mode (Silk et al. 2007). The column
used
for analysis was a Supelco SPB-5 capillary (30 m x 0.32 mm x 0.25 gm film) in
the
splitless mode with helium as carrier gas. The injection port was at 220 C and
the
oven temperature was programmed from 70 C, held for 1 mm and then increased at
C/min to 240 C and held for 30 mm. Compounds were purified by flash
chromatography on silica gel and, when required, by Kugelrohr distillation.
NMR (1H and 13C) was carried out on a Varian Innova 300 MHz spectrometer
in CDC13 with TMS as internal standard. IR spectra were recorded as thin
liquid films
on KBr discs with a Perkin Elmer 727B 1R-spectrometer.
Chemical synthesis. The macrocyclic lactone, (3Z)-dodecen-12-olide (1)
(Fig. 1), was synthesized according to the procedure described by Boden et al.
(1993)
and used by Bartelt et al. (2007) with the addition of a tert-
butyldimethylsilyl (TBS)
protecting group (which doubled the yield of the Wittig step). This involved
ozonolysis of a TBS-protected alkenol (5) into a protected hydroxyaldehyde
(6),
Wittig reaction with a Wittig salt containing a protected aldehyde (3),
removal of the
TBS group to give 8, then hydrolysis of the acetal to give a (37)-unsaturated
aldehyde
9, Lindgren oxidation (Lindgren and Nilsson 1973) to a carboxylic acid (10)
and
finally a Mitsunobu esterification (Kurihara et al. 1976) to effect the
macrolactonisation. The synthesis
of (3Z)-dodecen-12-olide was, therefore,
successfully accomplished with the IR spectra, El (70 eV) mass spectra and 1H
and
13C NMR spectra closely matching those reported (Boden et al. 1993). Formation
of
(2E)-dodecen-12-olide and (3E)-dodecen-12-olide were found to be intrinsic to
the
synthesis at approximately 3% each. The (2E)-product, characterized by 1H NMR,
8
CA 02750809 2011-07-29
was readily separated from the desired (32)-lactone by column chromatography.
The
(3E)-lactone, however, could not be separated from the (3Z)-lactone. IH NMR
supported the presence of ca. 3% of (3E)-lactone in the product.
The 3E-lactone [(3E)-dodecen-12-olide] (11) (Fig. 2) synthesis was
successfully accomplished by a Julia-Kocienski olefination according to the
methodology described by Blakemore et al. (1998). The Julia-Kocienski
olefination
of aldehyde 17 proceeded with 34 % yield and ca 97 % E stereochemistry (Fig.
2) to
give olefin 18a. Thus, protection of alkenol 4 with ethyl vinyl ether (EVE)
proceeded
smoothly to give 16, and ozonolysis with reductive workup gave aldehyde 17.
The
phenyltetrazole (PT) sulfone 15 was synthesized by deprotonating 1-pheny1-1H-
tetrazole-5-thiol 13 with sodium hydride and coupling it with commercially
available
12 to give thioether 14. mCPBA oxidation of 14 furnished the PT sulfone 15.
After
the Julia-Kocienski olefination, double hydrolysis of the two acetals of 18a
gave 19
and Lindgren oxidation of 19 gave the hydroxyacid 20. Finally, as reported by
Boden
et al. (1993), activation of the hydroxyl group using the Mitsunobu method
modified
according to Steglich (Justus and Steglich 1991) gave (3E)-dodecen-12-olide 11
in an
overall yield of 14 % from alkenol 4.
Spectral data for (3E)-lactone [(3E)-dodecen-12-olide)] 11:
NMR (CDC13, 400 MHz): 8 5.47 ¨ 5.62 (10 line symmetrical multiplet,
2H), 4.12 (AA'XX', 2H), 2.98 (d, 2H, J = 7.0 Hz), 2.05 (m, 2H), 1.57 (m,
2H), 1.29 ¨ 1.42 (m, 10H); I3C NMR (CDC13, 100 MHz): 8 172.0, 135.4,
123.2, 64.5, 39.0, 31.4, 27.2, 26.34, 26.28, 25.7, 24.9, 23.6. IR (neat): cm-I
3027 (w), 2928 (s), 2855 (s), 1733 (s), 1666 (w), 1457 (w), 1375 (w), 1348
(w), 1246 (s), 1143 (m), 1111 (m), 1039 (m); MS (El) Major peaks: 41 (base
peak), 54, 67, 81, 95, 109, 121, 136, 150, 168, 178, 196.
9
CA 02750809 2011-07-29
Schlosser modification of the Wittig reaction (Schlosser and Christmann 1966)
was
initially employed in an attempt to make 18b starting from Wittig salt 3 and
aldehyde
17, however, the E-selectivity of the reaction was very capricious, with 80 %
stereochemical purity being the best result out of a dozen attempts at the
reaction.
This was deemed to be unacceptable, and the much better ¨97 % stereochemical
purity obtained with the Julia-Kocienski olefination which gave 18a was much
more
satisfactory. Reagents and conditions of the syntheses of (3Z)-lactone and
(3E)-
lactone are as follows.
Fig. 1 Synthesis of (3Z)-Dodecen-12-olide 1 ((3Z)-lactone) (after Boden et al.
1993).
a) 2-Propanol, HBr, CH2C12. PPh3, -10 C ¨ RT b) HC(0'1303, one pot c) TBSC1,
imidazole, DMF, RT d) 03. Sudan III, CH2C12, -78 C e) PPh3, -78 C ¨ RT f) 3 +
NaHMDS, PhCH3 / THF (4: 1), 0 C ¨ RT, then 6, -99 C ¨ RT g) TBAF, THF, RT
h) Ts0H, wet THF, reflux i) NaC102, H2NSO3H, 1-methylcyclohexene, CH2C12 /
H20 (1: 3), 0 C - RT j) DEAD, PPh3, PhCH3, RT. HBr = Hydrobromic acid, CH2C12
Dichloromethane, PPh3 =
Triphenylphosphine, HC(01Pr)3 =
Triisopropylorthoformate, TBSC1 = tert-butyldimethylsilyl chloride, DMF =
Dimethylformamide, 03 = Ozone, NaHMDS = Sodium Hexamethyldisilylamide,
PhCH3 = Toluene, THF = Tetrahydrofuran, TBAF = tetrabutylammonium fluoride,
Ts0H = para-Tolunesulfonic acid, NaC102 = Sodium chlorite, H2NSO3H = Sulfamic
acid, DEAD = Diethyl azodicarboxylate.
Fig. 2 Synthesis of (3E)-Dodecen-12-olide 11 ((3E)-lactone); modified Julia-
Kocienski olefination. k) 13 + NaH, DMF, 0 C ¨ 60 C, then 12, NaI, 60 C 1)
mCPBA, NaHCO3, CH2C12, RT m) EVE, PPTS, CH2C12, RT n) 15 + KHMDS,
CA 02750809 2011-07-29
DME, -55 C, then 17, -55 C ¨ RT. NaH = Sodium hydride, Nal = Sodium iodide,
mCPBA = meta-Chloroperoxybenzoic acid, NaHCO3 = Sodium bicarbonate, EVE =
Ethyl vinyl ether, PPTS = Pyridinium para-toluenesulfonate, KHMDS = Potassium
hexamethyldisilylamide, DME = 1, 2-Dimethoxyethane.
GC-EAD analysis and EAG dose-response study. EAG analyses were made
by methods and equipment generally described by Cosse and Badelt (2000). EAG
connections were made by inserting a glass-pipette silver-grounding electrode
into the
back of an excised beetle head. A second glass-pipette silver-recording probe
was
placed in contact with the distal end of one antenna. Both pipettes were
filled with
Beadle-Ephrussi (Ephrussi and Beadle 1936) saline.
For the EAG-dose-response study, (3Z)- and (3E)-lactones were purified
(99.9% purity by GC/MS) by high performance liquid chromatography (HPLC) using
a Waters 515 pump, a Waters R401 refractive index detector, and a 25 cm by
0.46 cm
i.d. silica column (Adsorbosphere Silica 5 p.m, Alltech, Deerfield, IL),
treated with
silver nitrate as described by Heath and Sonnett (1980). Solvent was 8% ether
in
hexane. Ten micro liters of serially diluted solutions (methylene chloride) of
synthetic (3Z)-lactone and (3E)-lactone were applied to filter paper strips
(0.5 cm x 5
cm, Whatman no.1). The filter paper strips were placed in 14-cm-long Pasteur
pipettes, hereafter referred to as stimulus cartridges, after 5 min at room
temperature.
Stimulus doses tested were 0.01, 0.1, 1, 10, and 100 p,g. Male and female
antennae
were exposed to single 0.2s puffs of odor-bearing air at 5 ml/s by placing the
tip of an
stimulus cartridge into a hole of a glass tube (0.7 cm ID x 20 cm), 10 cm from
the
outlet and 11 cm away from the antennal preparation. Airflow through the glass
tube
was humidified and set at 10 ml/s. Puff duration and airflow speeds were
maintained
11
CA 02750809 2011-07-29
by a stimulus flow controller (SFC-2, Syntech, Hilversum, The Netherlands).
Stimuli
cartridges were selected in random order, beginning with the lowest dosages
and
working upward to the highest dosages. Each puffed dosage was preceded and
followed by a puff from a solvent blank cartridge (filter paper plus solvent).
To
compensate for possible deterioration of the antennal preparation, a standard
control
compound, geranyl acetone (1 lug dose) preceded dosages of stimuli compound.
EAG
amplitudes were normalized according to the responses to geranyl acetone by
dividing
the amplitude of the EAG generated by the test compounds by that of geranyl
acetone.
Dose-response series were replicated, using different antennal preparation for
each
replication, and the EAG responses were expressed as a percentage of the EAG
responses to geranyl acetone. Each antennal preparation was tested with
freshly
prepared sets of stimuli cartridges. Male and female EAG responses were
submitted
to analysis of variance (ANOVA) using Statistica for Windows software
(StatSoft Inc.
Tulsa, OK)).
A Varian CP-3380 gas chromatograph with FID detector was modified for use
with a GC-EAD signal recording device (IDAC-232). EAG data were analyzed using
Syntech GC-EAD software v.2.6 (SYNTECH, The Netherlands). The column used
for analysis was a Supelco SPB-5 capillary (30 m x 0.32 mm x 0.25 m film) in
the
splitless mode with helium as carrier gas. The injection port was at 220 C and
the
oven temperature was programmed from 70 C, held for 1 min and then increased
at
C/min to 240 C and held for 30 min. A number of GC-EAD runs on male and
female volatiles were carried out. Both the (3Z)-lactone and (3E)-lactone were
diluted
to 1 0[1g/m1 in hexane; 1 I of diluted pheromone was injected for each GC-EAD
run.
Ten nanograms was injected for the GC-EAD analysis consisting of 90% (3Z)-
intone and 10% (3E)-lactone using a DB-1 (15m x 0.25mm ID, 1 m film)
capillary
12
CA 02750809 2011-07-29
column (J&W Scientific, Folsom, CA). The GC oven temperature program was 50 C
for 1 min, then increased at 20 C/min and held at 280 C for 2 min. The GC-EAD
responses of five male and five female EAB antennae were analyzed.
Effect of light on (3Z)-lactone. To determine whether light would promote
the isomerization from (3Z) to (3E)-lactone, 20mg of (3Z)-lactone was placed
neat on
a glass slide 10 mm below a UV light (UVG-54 handheld UV-lamp, 254nm, 6w; UVP
Upland California, USA) for three d. Subsamples (taken as ¨ 1 mg in a pipette)
were
analyzed by GC/MS at regular intervals and the ratio of (3E):(3Z)-lactone was
recorded. In addition, 6 mg of each of (3Z)- and (3E)-lactones (neat) were
coated on
the quartz surface of a cuvette and exposed outdoors to sunlight at 11 C mean
temperature for 9 d for an average of 5 h a day. Finally, (3Z)-lactone was
coated (4
mg) on the dorsal surface of abdomen and elytra of 3 female EAB cadavers that
were
exposed to sunlight for 6 h per day for 1, 2 or 3 d at 10 C mean temperature;
cadavers
were stored at 4 C between sunny days. The lactones were removed from cuvettes
and cadavers with hexane washing and analysed by GC/MS to determine the E : Z
ratio.
Two-choice olfactometer assays. A Y-tube olfactometer (Analytical
Research Systems Inc, Gainsville, Florida) was used to test for attraction of
A.
planipennis to lactone isomers and host volatiles. The glass olfactometer (1.5
cm i.d.)
had an 11 cm main stem that branched into two 9-cm arms. Each arm was
connected
to a cylinder that contained the stimulus. Charcoal filtered air was passed
into each
aim at a flow rate of 1.2 L/min. Treatments included the pheromone alone: (3Z)-
lactone (101.tg); (3E)-lactone (10[tg); and 60:40 (3E):(3Z)-lactone (10pg).
Next, we
tested bark sesquiterpenes and a green leaf volatile alone: Phoebe oil (25pg
and
2.5ptg) and (32)-hexenol (5 lag). We then tested the pheromone combined with
bark
13
CA 02750809 2011-07-29
sesquiterpenes: (3Z)-lactone (1 Ogg) + Phoebe oil (at both 25 g and 2.5m);
(3E)-
lactone (10 g) + Phoebe oil (at both 25gg and 2.5m). Finally, we tested the
pheromone combined with the green leaf volatile: (3Z)-lactone (10 g) + (3Z)-
hexenol
(5 jig); and (3E)-lactone (lOgg) + (32)-hexenol (5 jig). Each stimulus (lptl
for single
compound treatments and a total of 41 for two-compound treatments) was diluted
in
hexane, placed on a strip of filter paper and given one minute for the solvent
to
evaporate before being placed in the olfactometer. A second filter paper,
treated with
the equivalent volume of solvent was placed in the other arm of the
olfactometer to
serve as the control. The apparatus was rinsed with acetone after each
treatment, and
the arm attached to the test stimulus was randomized between replicates.
For each treatment, we tested increasing numbers of adults until we obtained a
minimum of 12 beetles responding to the stimuli (either positively or
negatively). To
obtain this minimum, we tested 15-54 beetles per treatment. For each trial, a
single A.
planipennis (mature virgin male or female, > 10 days old) was given ten
minutes to
choose between the two stimuli; adults were used only once in the bioassay. A
choice
was recorded when the beetle passed a "finish line", 7 cm beyond the branching
point
of each arm. 'No choice' was recorded if the beetle failed to pass either
finish line
after the ten minutes. Beetles that did not select either the stimulus or the
control (i.e.,
no choice) were excluded from a subsequent chi-square goodness of fit test
used to
test whether the ratio of beetles choosing the stimulus vs. the hexane control
differed
significantly from 1:1. A chi-square test was conducted for each independent
trial.
Field trapping. Three trapping experiments were carried out in green ash
plantations (F. pennsylvanica Marsch) with low-to-moderate A. planipennis
populations about 40 km southeast of Sarnia, Ontario (42 58' 0 N, 82 24 0 W)
in
2008, 2009 and 2010. Trees at these sites were generally healthy in appearance
with
14
CA 02750809 2011-07-29
low or no signs of decline, and only a small number of trees had obvious
signs/symptoms of infestation by A. planipennis. In Ontario sites, trees were
20-25
years old, 4-6 m tall, 10-15 cm in diameter, and spaced about 2 m apart within
a row
and 2.5 m between rows. In 2010, the trapping experiment was replicated at
four sites
in Michigan, USA, in addition to the sites in Ontario. Sites in Michigan were
10-100
years old, 10-30 m tall, 15-70 cm in diameter, and located in a mixed woodlot.
Corrugated plastic "prism" traps (0.30cm x 35.00 cm x 58.75 cm) were coated
with
stickem (Crook et al. 2008) (Synergy Semiochemicals Corp., Burnaby, BC) and
hung
using rig spreaders (Zing Products, Westport Massachusetts, USA). Purple traps
were
suspended from metal stands at a height of 1.5 m (2008-2009), whereas green
traps
were hung in the mid-canopy from ropes tied between two trees at 2.5 m in
Ontario
and at 6 m height in Michigan (2010). In Michigan, traps were hung from a
single
line thrown over the lowest canopy branch. Light green traps (approx 540nm
wavelength) were the same as used by Francese et al. (2010). Traps were set
within
1.5-2 m of trees, spaced 20-30 m apart, in a randomized complete block design.
Traps
were checked every 2 weeks and A. planipennis were collected, counted and
sexed.
Experiment 1, conducted in Ontario in 2008, was designed to test for
attractiveness of (3Z)-lactone (Bartelt et al. 2007), alone and in combination
with two
types of host volatiles: bark sesquiterpenes (Crook et al. 2008) and a binary
blend of
green leaf volatiles ((3Z)-hexenol and (2E)-liexenol) (Poland et al. 2005, de
Groot et
al. 2008). We used purple prism traps, which at the time of this experiment
were
shown to be more attractive than traps of other colors (Francese et al. 2005),
and
which had been used successfully in other recent trapping experiments for A.
planipennis (Crook et al. 2008, de Groot et al. 2008). Traps were baited with
one of
the following treatments: (3Z)-lactone; Phoebe oil (Synergy Semiochemicals
Corp.,
CA 02750809 2011-07-29
Burnaby, BC); (3Z)-lactone + Phoebe oil; green leaf volatiles (GLVs)
consisting of
two bubblecaps, one containing (3Z)-hexenol and the other containing (2E)-
hexenol
(ConTech, BC); (3Z)-Lactone + GLVs; and unbaited controls. We selected Phoebe
oil because it contained two additional sesquiterpenes that had been detected
in ash
trees and appeared to be more attractive than Manuka oil (Crook et al. 2008)
and the
(3Z)-hexenol and (2E)-hexenol combination based on results from de Groot et
al.
(2008). Release rates at 20 C were estimated by weight loss as ca. 50 mg/d, 17
mg/d
and 16 mg/d for Phoebe oil, (3Z)-hexenol, and (2E)-hexenol, respectively. (3Z)-
lactone was emitted at ca. 80 pg/d at 20 C from red rubber septa (Wheaton)
impregnated with 5.0 mg per lure. Traps were out 10-24 June 2008, replicated
with 3
blocks at one site (Site A: Conservation area) and 7 blocks at the second site
(Site B:
Union Gas site). Lures were not changed during the experiment.
Experiment 2, conducted in Ontario in 2009, was designed to test the
attractiveness of (3E)- vs. (3Z)-lactone, alone and in combination with Phoebe
oil,
based on results from 2008. Purple prism traps were baited with the following
lure
treatments: (3Z)-lactone; (3E)-lactone; Phoebe oil; (3Z)-lactone + Phoebe oil;
(3E)-
lactone + Phoebe oil; and unbaited controls. As in 2008, release rate of
phoebe oil
was ca. 50 mg/d at 20 C. The lactone lure consisted of a 1.5 ml PCR tube
containing
50 mg of either (3E)- or (3Z)-lactone; a pipe cleaner wick was placed into the
vial
through a 1.0 mm hole with 2.0 mm of the wick protruding through the top of
the tube
(release rate = ¨0.5 mg /d at 20 C. Traps were in the field from 2 June-4
August
2009, with 7 blocks at one site (Site B: Union Gas site) and 8 blocks at the
other (Site
C: Anika Mills site). Lures were not changed during the experiment.
Experiment 3, conducted in 2010, was designed to test the effect of the single
green leaf volatile, (3Z)-hexenol (de Groot et al. 2008, Grant et al. 2010),
as a
16
CA 02750809 2011-07-29
potential kairomone in combination with either (3Z)- or (3E)-lactone. We used
green
sticky prism traps deployed in the ash canopy, which had recently been
demonstrated
to capture more A. planipennis than purple traps (Francese et al. 2008; Crook
et al.
2009) particularly when baited with (3Z)-hexenol (Grant et al. 2010).
Treatments
tested were: (3Z)-lactone; (3E)-lactone; (3Z)-hexenol; (3Z)-lactone + (3Z)-
hexenol;
(3E)-lactone + (3Z)-hexenol; and unbaited controls. (31)- and (3E)-lactone
were
loaded at a source dosage of 1.0 mg each and emitted ¨22 ttg/d at 25 C from
red
rubber septa (Wheaton). The source dosage of 1.0 mg is taken from a solution
of the
lactone in hexane, which is absorbed into the red rubber septum. The solvent
evaporates ro the septum, which then emits the lactone at an effective rate of
¨22
ttg,/d at 25 C . This experiment was replicated in Ontario and the Michigan.
In
Ontario, traps were out 1 June-14 July 2010 with 7 blocks at one site (Anika
Mills
site) and 5 blocks at another site (McKellar conservation area). Traps were
hung at
2.5m above the ground in the bottom edge of the canopy. In Michigan, traps
were out
from 25 May ¨ 7 July at four different sites. All traps in Michigan in 2010
were
deployed below the canopy; the trees were 10-30 m in height. The lactone lures
were
replaced every two weeks; the other lures were unchanged.
The effect of each attractant on mean catch of female and male A. planipennis
was analyzed independently using ANOVA and a randomized complete block design.
Sites were analyzed separately in 2009 due to differences in sex ratios. In
2010, sites
in Ontario were analyzed separately from those in Michigan due to the
considerable
differences in stand conditions and height of traps with respect to the ash
canopy. In
all three experiments, a priori hypotheses about the treatments were tested
with
contrasts; tests were conducted as one-sided tests for increases in trap
captures. The
first contrasts tested whether a single-component lure ((3E)-or (3Z)-lactone,
Phoebe
17
CA 02750809 2011-07-29
=
oil or GLV) caught more beetles than the unbaited control; a second set of
contrasts
compared captures of two-component lures vs. single component lures to test
for the
effect of adding the second component. Residuals were tested for homogeneity
of
variance and normality, and a /n(y+1) transformation was used where necessary.
We
present the untransformed least squares treatment means and their standard
errors,
along with statistics (P>F) from ANOVA of transformed data. For the two
component traps, the foliar volatiles, in this case comprising (3Z)-hexenol,
is provided
in a separate emitter ie. a 'bubble cap" emitter from Contech of Vancouver,
Canada,
associated with the trap and .containing a source concentration of 2-3 g of
neat
material, which emits 40-60 mg per day.
18
CA 02750809 2011-07-29
Results
GC/MS of collected volatiles. GC/MS analysis of extracts from female
volatiles confirmed the presence of the (3Z)-lactone with retention time and
El-mass
spectra identical with the synthetic material. The (3E)-lactone, if present,
was below
the detection limit (ca. <200 picograms injected) and could not be confirmed
as being
emitted by females in the laboratory. Neither lactone was detected in
volatiles
collected from male A. planipennis.
EAG dose-response study and GC-EAD analysis.
The EAG dose-response curves of male and female A. planipennis antennae
for the two isomers of synthetic lactone are presented in Fig. 3. Female
antennae did
not respond differently to the (3Z)- and (3E)-lactone (F1,149= 0.01, P =
0.91). Similar
results were obtained with the male antennae (F1,149= 2.3, P = 0.14). However,
female
antennae were more responsive to both (3Z)-lactone (F1,149 = 45.3, P<0.0001)
and
(3E)-lactone (F1,149 = 39.8, P<0.0001) than male antennae, particularly at
higher
doses. The mean responses of A. planipennis antennae to the geranyl acetone
standard
(1 tig applied dose) was -0.06 0.03 mV ( SD, n = 80, 15 antennal
preparations),
while those to the solvent/air controls measured -0.03 + 0.03 mV (+ SD, n =
45, 15
antenna! preparations).
GC/EAD analysis showed responses at the retention time of (3Z)-lactone (not
(3E)-lactone) produced by females only confirming previously published results
(Bartelt et al. 2007). This was confirmed by GC/MS analysis. GC-FID/EAD
responses of male and female A. planipennis antennae is shown in Fig. 4 to a
synthetic mixture of (3E)- and (32)-lactones; note the significant responses
to both
stereoisomers.
19
CA 02750809 2011-07-29
Effect of light on (3Z)-lactone. Exposure to UV-light had a considerable
impact on the ratio of (3E):(3Z)-lactone The initial lactone sample had a
(3E):(3Z)
ratio of 0.028 which increased with time of exposure to UV light, reaching a
ratio of
0.60 after three d. GC/MS confirmed that exposure to UV-light resulted in
isomerization without producing any other secondary products except a small
amount
(< 1%) of the conjugated isomer. Preliminary studies found that under our
normal
laboratory fluorescent lighting conditions, (3Z)-lactone is very stable and
did not
readily isomerize to the (3E)-lactone. In addition, storing (3Z)-lactone in a
pyrex
container filtered out the UV-light, also preventing photoisomerisation.
Exposure of
either lactone isomer in a quartz cuvette or on the surface of female A.
planipennis
cadavers in direct sunlight resulted in very slow isomerization even after 2-3
days.
Y-tube olfactometer assays. In the Y-tube olfactometer assay, males were
significantly attracted to the (3E)-lactone (C2= 6.76, n = 25, P = 0.009), but
not the
(3Z)-lactone ()C2= 2.88, n = 17, P = 0.09) or the 60:40 ratio (x2 = 0.17, n =
24, P =
0.68) (Fig. 5a). Low doses of Phoebe oil were attractive to males (x2= 5.54, n
= 26, P
= 0.018) (Fig. 5c), whereas higher doses were significantly repellant (,2 =
7.12, n =
17, P = 0.008) (Fig. 5b). Combining either lactone isomer with a low dose of
Phoebe
oil was not attractive to males (x2= 0.0, n = 38, P = 1.0 and x2= 0.08, 12 =
48, P =
0.773, for (3E) and (3Z)-lactone, respectively). Similarly, combining (3E)-
lactone
with the high dose of Phoebe oil was not attractive (x2 = 0.11, n = 9, P =
0.74) and
(3Z)-lactone combined with high dose of Phoebe oil was significantly repellant
(x2 =
8.33, n = 12, P = 0.004). Finally, males were highly attracted to (3Z)-hexenol
(x2 --
9.0, n = 25, P = 0.003) (Fig. 5d), the (3Z)-lactone + (3Z)-hexenol combination
(x2=5.4, n=15, P=0.02) (Fig. 5d), but not the (3E)-lactone + (3Z)-hexenol
combination
(x2 = 0.059, n = 17, P = 0.88). Females were slightly attracted to a low dose
of
CA 02750809 2011-07-29
Phoebe oil (70% responded) (x2 = 3.52, n = 23, P = 0.061) and to the (3Z)-
hexenol
(75% responded) (x2 = 6.00, n = 24, P = 0.014), but did not respond in
sufficient
numbers for analysis in any other treatment.
Field trapping. In experiment 1 (2008), both host volatile treatments
increased trap captures compared to unbaited controls (Fig. 6a). Phoebe oil
increased
trap captures of both sexes (P<0.01) (Contrast 4 vs. 6; Fig. 6a); the GLVs
increased
trap capture significantly for males (P<0.01); female capture was only
marginally
increased (P<0.06) (Contrast 3 vs. 6; Fig. 6a). The (3Z)-lactone was not
significantly
attractive on its own (Contrast 5 vs. 6; P = 0.06) and there was only a
marginal mean
catch of males when combined with Phoebe oil (P = 0.06; Contrast 2 vs. 4).
There
was no evidence of increases in trap captures for the lactone + GLV
combination on
purple traps (Contrast 1 vs. 3; Fig. 6a).
In experiment 2 (2009 site 1 and site 2), Phoebe oil again increased trap
captures compared to unbaited controls for both sexes at both sites (P<0.01)
(Contrast
3 vs. 6; Fig. 6b, c). However, neither (3Z) nor (3E)-lactone alone, or in
combination
with Phoebe oil, significantly increased the number of male or female A.
planipennis
captured on purple traps (Contrast 1 or 2 vs. 3; Fig. 6b, c) as compared to
the Phoebe
oil alone. At one site (Union Gas), captures of females were 4 x and 5 x
greater than
captures of males for blank traps and treatments containing Phoebe oil,
respectively
(Fig. 6b). In contrast, trap captures were male-biased at the other site
(Anika Mills).
In experiment 3 (2010), as in experiment 2, the (3E)-lactone isomer by itself
did not affect trap catch (Contrast 4 vs. 6; Fig. 7a and b); however, there
was a slight
increase in trap captures when the (3Z)-lactone was used alone on green traps
deployed in the canopy at sites in Ontario (Contrast 5 vs. 6; P<0.02) (Fig.
7a). Most
importantly, there was a significant increase in captures of males at sites in
Ontario
21
CA 02750809 2011-07-29
when (3Z)-hexenol was combined with either (3Z)-lactone or (3E)-lactone
(Contrast 1
or 2 vs. 3; P<0.01) (Fig. 7a). A similar trend was observed at sites in
Michigan (Fig.
7b), although differences were not significant (P = 0.16); captures of males
on traps
baited with (3Z)-lactone+(3Z)-hexenol was ca. 50% greater than traps baited
with
(37)-hexenol alone. Most lure treatments did not significantly affect capture
of
female A. planipennis (P>0.31) (Fig. 7a and b), except mean female catch in
traps
baited with (3E)-lactone + (3Z)-hexenol was slightly lower than that in traps
baited
with (3Z)-hexenol alone in Michigan (P = 0.03) (Fig. 7b).
Discussion
We provide the first evidence for a pheromone in a buprestid beetle that
increases attraction of males to a host volatile. Our data confirms that
female A.
planipennis emit (3Z)-lactone, as observed by Bartelt et al. (2007), and
demonstrates
that it increases mean catch of male A. planipennis on green prism sticky
traps when
combined with the green leaf volatile, (3Z)-hexenol, and when deployed in the
tree
canopy. Captures of males with the (3Z)-lactone + (3Z)-hexenol was at least 50-
100%
greater compared to the (3Z)-hexenol alone in Michigan and Ontario,
respectively.
The (3E)-lactone + (3Z)-hexenol was inconsistent increasing captures of males
by
60% in Ontario only. Our results are similar to the increases in trap captures
observed
for the combination of host kairomones and the male¨produced pheromones in
Tetropium fuseurn (Fabricius) (Coleoptera: Cerambycidae) (Silk et al. 2007;
Sweeney
et al. 2010) and Anaplophora glabripennis (Motschuslky) (Nehme et al. 2010).
Indeed, (3Z)-hexenol has been demonstrated to synergize pheromone attraction
and
function as a kairomone for a number of other beetles species (Dickens et al.
1990;
Ruther et al. 2000, 2002; Ruther and Mayer 2005; Reinecke et al. 2006). (3Z)-
dodecen-12-olide was previously reported as the major component of the male-
22
CA 02750809 2011-07-29
produced pheromone of the flat grain beetle Cryptolestes push/us (Schanherr)
(Coleoptera: Cucujidae) (Millar et al. 1985).
Concerning the Ontario vs. Michigan trapping data, the inconsistent results
are
likely due to differences related to tree sizes and where mating activity
takes place. In
Michigan, trees were 10-30 m high with traps hanging at ca. 6 m. Thus, in most
cases
this was well below the canopy. In contrast, in Ontario the green traps were
placed in
mid-canopy of 4-6 m tall trees. Most of the mating activity of A. planipennis
has been
shown to occur in the canopy and in sunshine (Lance et al. 2007; Lelito et al.
2007;
Rodriguez-Saona et al. 2007). Thus, trap color, lure combination, and trap
deployment (i.e., trap height) may all influence attraction to the putative
pheromone
compounds.
Our study indicates that the type of host volatile affects attraction by A.
planipennis to the pheromone: i.e., the lactone increased male attraction when
combined with (3Z)-hexenol but not with Phoebe oil. (3Z)-hexenol elicits
significant
antenna! responses (Rodriguez-Saona et at. 2006; de Groot et al. 2008) and
consistently increased trap captures over the controls regardless of trap
color (de
Groot et al. 2008; Grant et al. 2010), indicating its importance as a host
kairomone for
A. planipennis. Adding other green leaf volatiles to (3Z)-hexenol tends to
reduce trap
captures of A. planipennis (Crook et al. 2008; de Groot et at. 2008; Grant et
al. 2010),
which could explain the lack of effect between our two-component GLV lure and
the
(3Z)-lactone in 2008. Our observation of increased attraction of the pheromone
+
green leaf volatile combination further suggests that A. planipennis females
may call
more frequently on host foliage than on host bark. Observations by others
(Lance et
al. 2007; Lelito et al. 2007; Rodriguez-Saona et al. 2007) that flight
activity of male
A. planipennis tends to be greatest in the upper canopy of host trees lends
some
23
CA 02750809 2011-07-29
support to our contention, but some mating has also been observed on the
trunks of
host trees (Rodriguez-Saona et al. 2007; Lelito et al. 2007).
Exposure of (3Z)-lactone to UV light in the laboratory caused a significant
isomerization to the (3E)-lactone. A. planipennis adults tend to be most
active in the
upper canopy of host trees (Lelito et al. 2007) when the weather is warm and
sunny
(Yu 1992) so adults are naturally well exposed to sunlight. Whether or not
female A.
planipennis are exposed to sufficient UV radiation to cause partial
isomerization of
the (3Z)-lactone to the (3E)-lactone is unknown. However, the synthesis of
insect
pheromones mediated by sunlight is not unprecedented. Staples et al. (2009)
recently
identified a female-produced sex-pheromone of the pamphiliid sawfly,
Acantholyda
erythrocephala (L.) ((Z)-6, 14-pentadecadienal) and showed that females also
produce
(Z, .7)-1, 9, 15-pentacosatriene, which is a precursor to the sex-pheromone.
Bartell et al. (2007) noted that the (3Z)-lactone was detected with the
greatest
emission from females 2-4 d post emergence, which corresponds to the time when
A.
planipennis are sexually immature. These authors suggest that this may, in
part, be
due to declining beetle health (i.e. high mortality in the collection
chamber). Our data
suggest that 3d exposure to natural sunlight on the surface of cadavers of
females is
not sufficient to cause photoisomerization. Our olfactometer observations
indicated
that the (3E)-lactone but not the (3.Z)-lactone was attractive to males, and
our field
experiments indicate that trap captures may be significantly increased by the
combination of either (3Z)- or (3E)-lactones plus (3Z)-hexenol. There is a
need for
further research to test whether light is an important determinant in the
mating activity
of A. planipennis and to determine what role the lactone stereoisomers play in
the
mating behavior.
24
CA 02750809 2011-07-29
In summary, Bartelt et al. (2007) identified a macrocyclic (3Z)-lactone that
was hypothesized to act as a pheromone. Here we report the first evidence that
(3Z)-
lactone can significantly increase male trap catch when combined with the
green leaf
volatile, (3Z)-hexenol, in green traps deployed in the canopy. This provides
evidence
that indeed, the (32)-lactone is a pheromone component. It appears that two
cue
modalities are required by A. planipennis in the mate-finding process: a
visual cue
(green) and a two-component olfactory cue: a foliage volatile (kairomone),
(3Z)-
hexenol, and the pheromone, (3Z)-lactone. It is this combination we recommend
to
develop monitoring and early detection tools recognizing that some further
improvements may come from fine-tuning each of the three components. Further
research is required to optimize the kairomone component of a lure for A.
planipennis,
including release rate and ratios of chemical components. Further study is
also needed
to elucidate the possible biological relevance of (3Z)- and (3E)-lactone given
their
sex-specific effects on A. planipennis behavior. The mechanism of a possible
photolytic interconversion of (3Z)- and (3E)-lactone is presently being
studied. The
effect of light on the mating behavior and pheromone production of A.
planipennis
may also be a key determinant which may translate to other Agrilus species.
CA 02750809 2011-07-29
References Cited
Akers, R.C., and D. G. Nielsen. 1992. Mating behavior of the bronze birch
borer
(Coleoptera: Buprestidae). J. Entomol. Sci. 27: 44-49.
Bartelt, R., A. A. Cosse, B.W. Zilkowski, and I. Fraser. 2007. Antennally
active
macrolide from the emerald ash borer Agritus planipennis emitted
predominantly by females. J. Chem. Ecol. 33:1299-1302.
Blakemore, P. R., W.J. Cole, P. J. Kociefiski, and A. Morley. 1998. A
stereoselective synthesis of trans-1, 2-di substituted alkenes based on the
condensation of aldehydes with metallated 1-phenyl-1H-tetrazol-5-y1 sulfones.
Synlett. 26-28.
Boden, C. D. J., J. Chambers, and D. R. Stevens. 1993. A concise, efficient
and
flexible strategy for the synthesis of the pheromones of Oryzaephilus and
Oyptolestes grain beetles. Synthesis. 4: 411-420.
Cappaert, D., D. G. McCullough, T. M. Poland, and N.W. Siegert. 2005. Emerald
ash borer in North America: a research and regulatory challenge. Am.
Entomol. 51:152-165.
Carlson, R.W., and F. B. Knight. 1969. Biology, taxonomy, and evolution of
sympatric Agrilus beetles (Coleoptera: Buprestidae). Contr. Am. Entomol.
Inst. 3: 1-105.
Cosse, A. A., and R. J. Bartell. 2000. Male-produced aggregation pheromone of
Colopterus truncatus: structure, electrophysiological and behavioral activity.
J.
Chem. Ecol. 26:1735-1748.
Crook, D. J., A. Krimian, J. Francese, 1. Fraser, T. M. Poland, A.J. Sawyer,
and
V. C. Mastro. 2008. Development of a host-based semiochemical lure for
26
trapping emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae).
Environ. Entomol. 37: 356-365.
Crook, D. J., J. A. Francese, K. E. Zylstra, I. Fraser, A. J. Sawyer, D. W.
BartelIs, D.
R. Lance, and V. C. Mastro. 2009. Laboratory and field response of emerald
ash borer (Coleoptera: Buprestidae), to selected regions of the
electromagnetic
spectrum. J. Econ. Entomo1.102: 2160-2169.
Crook, D.J., and V.C. Mastro. 2010. Chemical ecology of the emerald ash borer.
J.
Chem. Ecol. 36: 101-112.
de Groot, P., W. D. Biggs, D. B. Lyons, T. Scarr, E. Czwerwinski, H. J. Evans,
W. Ingram, and K. Marchant. 2006. A visual guide to detecting emerald ash
borer damage. Natural Resources Canada and Ontario Ministry of Natural
Resources, Sault Ste. Marie, Ontario, Canada.
de Groot, P., G.G. Grant, T. M. Poland, R. Scharbach, L. Buchan, R.W. Nott, L.
MacDonald, and D. Pitt. 2008. Electrophysiological response and attraction
of emerald ash borer to green leaf volatiles (GLVs) emitted by host foliage.
J.
Chem. Ecol. 34: 1170-1179.
Dickens, J. C., E. B Jang, D. M. Light, and A. R. Alford. 1990. Enhancement of
insect pheromone responses by green leaf volatiles. Naturwissenschaften 77:
29-31.
Dunn, J. P., and D. A. Potter. 1988. Evidence for sexual attraction by the two-
lined
chestnut borer, A grilus bilineatus (Weber) (Coleoptera: Buprestidae). Can.
Entomol. 120:1037-1039
27
CA 2750809 2019-02-18
CA 02750809 2011-07-29
=
Ephrussi, B., and G. W. Beadle. 1936. A technique of transplantation for
Drosophila. Am. Nat. 70:218-225.
Francese, J. A., V.C. Mastro, J. B. Oliver, D.R. Lance, N. Youssef, and S. G.
Lavalee. 2005. Evaluation of colors for trapping Agrilus planipennis
(Coleoptera: Buprestidae). J. Entomol. Sci. 40: 93-95.
Francese, J. A., I. Fraser, D. R. Lance, and V. C. Mastro. 2007. Developing
survey
techniques for emerald ash borer: the role of trap height and design, pp. 72-
73.
In Proceedings: Emerald ash borer research and technology development
meeting, US Department of Agriculture Forest Health Technology Enterprise
Team FHTET-2005-16, 26-27 September 2005, Pittsburgh, Pennsylvania,
USA.
Francese, J. A., D. J. Crook, I. Fraser, D. R. Lance, A. J. Sawyer, and V.C.
Mastro. 2008. Further investigations in developing a trap for emerald ash
borer: how trap height affects capture rates. In: Emerald Ash Borer Research
and Development Meeting FHTET 2007-08. Comp. by Mastro, V, Lance D,
Reardon R, Parra G, USDA, Forest Service, Morgantown, WV, p. 79-80.
Francese, J. A., D. J. Crook, I. Fraser, D. R. Lance, A. J. Sawyer, and V. C.
Mastro. 2010. Optimization of trap color for the emerald ash borer, Agrilus
planipennis (Coleoptera: Buprestidae). J. Econ. Entomol. 103: 1235-1241.
Grant, G.G., K. L. Ryall, D. B. Lyons, and M. M. Abou-Zaid. 2010. Differential
response of male and female emerald ash borers (Col., Buprestidae) to (Z)-3-
hexenol and Manuka oil. J. Appl. Entomol. 134: 26-33.
Gwynne, D.T., and D.C.F. Rentz. 1983. Beetles on the bottle: male buprestids
mistake stubbies for females (Coleoptera). J. Austral. Entomol. Soc. 23: 79-
80.
28
CA 02750809 2011-07-29
4
Heath, R. R., and P. E. Sonnet. 1980. Technique for in situ coating of Ag
onto
silica gel in HPLC columns for the separation of geometrical isomers. J. Liq.
Chromatogr. 3:1129-1135.
Justus, K., and W. Steglich. 1991. First synthesis of a strained 14-membered
biaryl
lactone ether by macrolactonization. Tetrahedron Letters. 32: 5781-5784.
Kurihara, T., Y. Nakajima, and 0. Mitsunobu. 1976. Synthesis of Lactones and
cycloalkanes. Cyclization of w-hydroxy acids and ethyl a-cyano-
w-
hydroxycarboxylates. Tetrahedron Letters. 28: 2455-2458.
Lance, D. R., I. Fraser, and V. C. Mastro. 2007. Activity and microhabitat-
selection
patterns for emerald ash borer and their implications for the development of
trapping systems. In: Emerald Ash Borer Research and Asian Longhomed
Beetle Research and Technology Development Meeting, FHTET 2007-04.
Comp. by Mastro V, Lance D, Reardon R, Parra G, USDA, Forest Service,
Morgantown, WV, p. 77-78.
Lelito, J. P., I. Fraser, V.C. Mastro, J. H. Tumlinson, K. Boriiczky, and T.
C.
Baker. 2007. Visually mediated 'paratrooper copulations' in the mating
behaviour of Agrilus planipennis (Coleoptera: Buprestidae), a highly
destructive invasive pest of North American ash trees. J. Insect. Behay.
20:537-552.
Lelito, J. P., I. Fraser, V.C. Mastro, J. H. Tumlinson, and T. C. Baker. 2008.
Novel visual-cue-based sticky traps for monitoring of emerald ash borers,
Agrilus planipennis (Col., Buprestidae). J. Appl. Entomol. 132: 668-674.
Lelito, J. P., K. Boriiczky, T. H. Jones, I. Frazer, V. C. Mastro, J. H.
Turnlinson,
and T.C. Baker. 2009. Behavioral Evidence for a Contact Pheromone
29
CA 02750809 2011-07-29
Component of the Emerald Ash Borer, Agrilus planipennis Fairrnaire. J.
Chem. Ecol., 35:104-110.
Lindgren, B. 0., and T. Nilsson. 1973. Preparation of carboxylic acids from
aldehydes (including hydroylated benzaldehydes) by oxidation with chlorite.
Acta Chemica Scandinavica. 27: 888-890.
Lyons, D. B., P. de Groot, G. C. Jones, and R. Scharbach. 2009. Host selection
by
Agrilus planipennis (Coleoptera: Buprestidae): inferences from sticky-band
trapping. Can. Entomol. 141: 40-52
Marchant, K. R. 2006. Managing the emerald ash borer in Canada-2005, p.2. In
Proceedings: Emerald ash borer research and technology development
meeting, US Department of Agriculture Forest Health Technology Enterprise
Team FHTET-2005-16, 26-27 September 2005, Pittsburgh, Pennsylvania,
USA.
McCullough, D. G., T. M. Poland, and D. L. Cappaert. 2006. Attraction of
emerald
ash borer to trap trees: Effects of stress agents and trap height, pp. 61-62.
In
Proceedings: Emerald ash borer research and technology development
meeting, US Department of Agriculture Forest Health Technology Enterprise
Team 26-27 September 2005, Pittsburgh, Pennsylvania, USA.
McCullough, D.G., T.M. Poland, A.C. Anulewicz, and D.L. Cappaert. 2008.
Double-deckers and towers: Emerald ash borer traps in 2007, pp. 73-75. In V.
Mastro, D. Lance, R. Reardon and G. Parra (eds.). Proceedings of the emerald
ash borer and asian longhorned beetle research and technology development
meeting, Pittsburgh, Pa., 23-24 October 2007. FTTET-2008-07, USDA Forest
Service Forest Health Technology Enterprise Team, Morgantown, W. Va.
CA 02750809 2011-07-29
=
McCullough, D.G., T. M. Poland, and D. L. Cappaert. 2009. Emerald ash borer
(Coleoptera: Buprestidae) attraction to stressed or baited ash trees. Environ.
Entomol. 38: 1668-1679.
Millar, J. G., H.D. Pierce, JR., A. M. Pierce, A. C. Oehlschlager, J. H.
Borden,
and A.V. Barak. 1985. Aggregation pheromones of the flat grain beetle
Crytoplestes pusillus (Coleoptera: Cucujidae). J. Chem. Ecol. 11:1053-1070.
Nehme, M. E., M. A. Keena, A. Zhang, T. C. Baker, Z. Zhu, and K. Hoover.
2010. Evaluating the use of Male-Produced Pheromone Components and Plant
Volatiles in Two Trap Designs to Monitor Anoplophora glabripennis.
Environ. Entomol. 39:169-176.
Poland, T. M., and D. G. McCullough. 2006. Emerald ash borer: Invasion of the
urban forest and the threat to North America's ash resource. J. For. 104:118-
124.
Poland T. M., P. de Groot, G. Grant, L. Macdonald, and D. G. McCullough.
2004. Developing attractants and trapping techniques for the emerald ash
borer. In: Emerald Ash Borer Research and Technology Development
Meeting, FHTET-2004-02. Comp. by Mastro V and Reardon R, USDA Forest
Service, Morgantown, WV p. 15-16.
Poland, T. M., D. G. McCullough, P. de Groot, G. G. Grant, L. MacDonald, and
D. L. Cappaert. 2005. Progress toward developing trapping techniques for the
emerald ash borer, pp.53-54. In Proceedings: Emerald Ash Borer Research
and Technology Development Meeting, US Department of Agriculture Forest
Health Technology Enterprise Team, Morgantown, West Virginia, USA.
Poland T. M., C. Rodriguez-Saona, G. G. Grant, L. Buchan, P. de Groot, J.
Miller, and D. G. McCullough. 2006. Trapping and detection of emerald ash
31
CA 02750809 2011-07-29
borer: Identification of stress-induced volatiles and tests of attraction in
the lab
and field. In: Emerald Ash Borer Research Technology Development Meeting,
FHTET-2005-16. Comp. by Mastro V, Reardon R, Parra G, USDA Forest
Service, Morgantown WV, p. 64-65.
Poland, T. M., D. S. Pureswaren, G. G. Grant, and P. de Groot. 2007. Field
attraction of emerald ash borer to antenally and behaviorally active ash
volatiles, pp.80-81. In Proceedings: Emerald Ash Borer Research and
Technology Development Meeting, US Department of Agriculture Forest
Health Technology Enterprise Team 20 October ¨ 2 November, 2006,
Cincinnati, Ohio, USA.
Pureswaran, D. S., and T. M. Poland. 2009 The role of olfactory cues in short-
range
mate finding by the emerald ash borer, Agrilus planipennis, Fairmaire
(Coleoptera: Cerambycidae). J. Insect. Behay. 22: 205-216.
Reinecke A., J. Ruther, C. J. Mayer, and M. Hilker. 2006. Optimized trap lure
for
male Melolontha cockchafers. J. Appl. Entomol. 130, 171-176.
Rodriguez-Saona, C., T. M. Poland, J. R. Miller, L. L. Stelinski, G.G. Grant,
P.
de Groot, L. Buchan, and L. MacDonald. 2006. Behavioral and
electrophysiological responses of the emerald ash borer, Agrilus planipennis,
to induced volatiles of Manchurian Ash, Fraxinus mandshurica.
Chemoecology 16:75-86.
Rodriguez-Saona, C. R., J. R. Miller, T. M. Poland, T. M. Kuhn, G.W. Otis, T.
Turk, and D. L. Ward. 2007. Behaviors of adult Agrilus planipennis
(Coleoptera: Buprestidae). Great Lakes Entomol. 40:1-16.
32
= CA 02750809 2011-07-29
Ruther J., A. Reinecke, K. Thiemann, T. Tolasch, W. Francke, and M. Hilker.
2000. Mate finding in the forest cockchafer, Melolontha hippocastani,
mediated by volatiles from plants and females. Physiol. Entomol. 25: 172-179.
Ruther J., A. Reinecke, and M. Hilker. 2002. Plant volatiles in the sexual
communication of Melolontha hzppocastani: response toward time dependent
bouquets and novel function of (Z)-3-hexen-1-ol as a sexual kairomone. Ecol.
Entomol. 27, 76-83.
Ruther J., and C. J. Mayer. 2005. Response of garden chafer, Phylloertha
horticola,
to plant volatiles: from screening to application. Entomol. Exp. Appl. 115: 51-
59.
Schlosser M, and K. F. Christmann. 1966. Trans-Selective Olefin Syntheses.
Angew. Chem. Internet. Edit. 5:126.
Seigert, N. W., D. G. McCullough, A. M. Liebhold, and F. W. Telewski. 2007.
Resurrected from the ashes: A historical reconstruction of emerald ash borer
dynamics through dendrochronological analysis. In: Emerald ash borer and
Asian longhorned beetle research and technology development meeting.
FHTET-2007-04. Comp. by Mastro V, Lance D, Reardon R, Parra G, Forest
Health Technology Enterprise Team, Morgantown, WV, 18-19.
Silk, P.J., K. Ryall, B. Lyons, J. D. Sweeney, and J. Wu. 2009. A Contact Sex
Pheromone Component of the Emerald Ash Borer Agrilus planipennis
Fairmaire (Coleoptera: Buprestidae). Naturwissenschaften. 96: 601- 608.
Silk, P. J., J. D. Sweeney, J. Wu, J. Price, J. Gutowski, and E. G. Kettela.
2007.
Evidence for a male-produced pheromone in Tetropium fuscum (F.) and
Tetroplum cinnamopterum (Kirby) (Coleoptera: Cerambycidae).
Naturwissenschaften. 94:697-701.
33
CA 02750809 2011-07-29
Staples J. K., R. J. Bartelt, A. A. Cosse, and D. W. Whitman. 2009. Sex
Pheromone of the Pine False Webworm Acantholyda erythrocephala. J.
Chem. Ecol. 35:1448-1460.
Sweeney, J. D, P. J. Silk, J. M. Gutowski, J. Wu, M . Lemay, P. D. Mayo, and
D.
Magee. 2010. Effect of Chirality, Release Rate and Host Volatiles on
Response of Tetropium fuscum (F.), Tetropium cinnamopterum (L.) Kirby and
Tetropium castaneum (L.) to the Aggregation Pheromone, Fuscumol. J. Chem.
Ecol., 36:1309-1321.
Yu, C.M. 1992. Agrilus marcopoli Obenberger (Coleoptera: Buprestidae), pp. 400-
401./n: G. Ziao (ed.), Forest insects of China, 211d edition. China Forestry
Publishing House, Beijing, China.
34