Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02754813 2011-09-08
WO 2010/104527 PCT/US2009/053505
LEAD-FREE BRASS ALLOY
This application claims priority to U.S. Utility Patent Application Serial No.
12/400,283, filed March 09, 2009 which is incorporated by reference herein in
its entirety.
TECHNICAL FIELD
[0001] The present invention relates to brass compositions with extremely low
to
no lead content. The compositions exhibit good machinability and strength
similar to that of
conventional leaded brass alloy free machining brass.
BACKGROUND OF THE INVENTION
[0002] It has been common practice to add up to 4.5% lead to brass
compositions
to improve the machinability of the resulting product. Lead, however, is a
toxic substance and
its use in the production of alloys is surrounded by legislation and expensive
control procedures.
For example, California adopted legislation which limits the amount of lead in
plumbing fixtures
to .25% or less beginning in 2010.
[0003] Furthermore, the lead phase in copper lead alloys can be affected by
corrosive attacks with hot organic or mineral oil. For example, when
temperature of such an
alloy rises, it has been known that the oil can break down to form peroxides
and organic gases
which effect a degree of leaching on the lead phase within the alloy. If this
leaching progresses
to any appreciable extent, the component, if it is a bearing or structural
component, may
eventually malfunction or fail.
[0004] There is, therefore, considerable advantage in reducing, or if
possible,
eliminating the contents of lead within powder metallurgy compositions.
Various proposals have
been put forward for doing this. The considerable proportions of lead
incorporated in powder
metallurgy materials in the past has resulted in ease of machinability and
durability of the
resulting product component. Replacement of part of the lead by bismuth has
been proposed in
International Application published under No. W091/14012. This results in
successful
replacement of part of the lead without significant reduction in the
machinability. It is, however,
accompanied by some reduction of transverse strength of the material. For many
purposes this
reduction in transverse strength is not a significant problem.
1
CA 02754813 2011-09-08
WO 2010/104527 PCT/US2009/053505
[0005] Another approach has been described in U.S. Patent 5,445,665. In this
product 0.1 to 1.5% graphite is added to the alloy allowing for reduction of
lead to 2% of the
alloy or less.
[0006] While the alloys described above yield substantially lead-free alloys,
they
do not possess the same machinability as the lead containing alloys. This
results in the need for
substantial retooling of the equipment used to produce end product, such as
plumbing equipment
and the like. In addition, the scrap produced during the manufacturing of the
lead products often
cannot be readily recycled by the end product manufacturer. Recycling
typically can only be
done by the manufacturer of the alloys. The cost of shipping the scrap back to
the initial foundry
increases the overall product cost of the end product.
[0007] Thus, there remains a need for a lead-free brass alloy which exhibits
machinability similar to that of lead containing products and that can be
recycled by the
customer.
BRIEF SUMMARY OF THE INVENTION
[0008] The present invention comprises a brass alloy containing from about
0.20%
to 1.5% tellurium as a substitute for lead, typically added to the brass
composition. In one series
of embodiments the tellurium ranges from about 0.4% to about 1.0%. The
resulting alloy
typically has a lead content of from less than about 0.025% to less than about
0.001% which is
considered "lead-free."
[0009] Brass alloys of the invention typically have a copper content of from
about
98% to about 57%, a zinc content of from about 43% to about 2%, a tellurium
content of from
about 1.0% to about 0.02%, a lead content of from about 0.025% to about
0.001%, and a
maximum phosphorous content of about 0.05%.
[0010] The resulting alloys exhibit excellent machinability and conductivity.
Depending on the composition of the alloy, the tensile strength will vary
between 240 MPa and
530 MPa and yield strength will vary from about 200 to about 450 MPa.
Conductivity will range
from about 28% to about 49% IACS. The machinability of the novel alloys of the
invention is
similar to that for lead containing compositions. This eliminates or reduces
the amount of
retooling needed to use the novel alloys to produce finished products such as
plumbing fixtures.
2
CA 02754813 2011-09-08
WO 2010/104527 PCT/US2009/053505
[0011] The composition of the novel alloys also allows the end product
manufacturers to recycle the scrap from the manufacturing process itself. This
eliminates the
need to return the scrap to the alloy manufacturer for recycling. Yet another
key feature of the
present invention is that the alloys containing less than about 15% zinc
exhibit excellent
dezincification resistance.
[0012] The foregoing has outlined rather broadly the features and technical
advantages of the present invention in order that the detailed description of
the invention that
follows may be better understood. Additional features and advantages of the
invention will be
described hereinafter which form the subject of the claims of the invention.
It should be
appreciated by those skilled in the art that the conception and specific
embodiment disclosed
may be readily utilized as a basis for modifying or designing other structures
for carrying out the
same purposes of the present invention. It should also be realized by those
skilled in the art that
such equivalent constructions do not depart from the spirit and scope of the
invention as set forth
in the appended claims. The novel features which are believed to be
characteristic of the
invention, both as to its organization and method of operation, together with
further objects and
advantages will be better understood from the following description when
considered in
connection with the accompanying figures. It is to be expressly understood,
however, that each
of the figures is provided for the purpose of illustration and description
only and is not intended
as a definition of the limits of the present invention.
BRIEF DESCRIPTION OF THE DRAWINGS
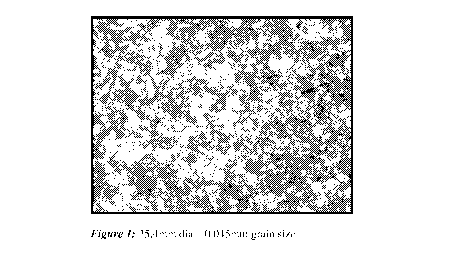
[0013] Figure 1 is a photomicrograph of the alloy used in Sample Cl after
draw.
[0014] Figure 2 is a photomicrograph of the alloy used in Sample C2 after
draw.
[0015] Figure 3 is a photomicrograph of the alloy used in Sample C3 after
draw.
DETAILED DESCRIPTION OF THE INVENTION
[0016] The brass alloys of the present invention are prepared by first melting
copper at a temperature of about 1050 C. Zinc and tellurium are then added to
the molten
copper. Brass alloy is then cast into billets utilizing horizontal or vertical
casting methods.
3
CA 02754813 2011-09-08
WO 2010/104527 PCT/US2009/053505
[0017] The copper used to make the alloys is typically copper cathode or high
grade uncontaminated and pure copper scrap comprising 99.95% minimum copper
and to .05%
impurities. Lead is a typical impurity, comprising less than 0.025% of the
copper used. In the
formation of the alloys of the invention, copper comprises from about 57.00 to
about 98.00% of
the alloy.
[0018] Zinc is the next major component comprising from about 2.00% to about
43.00% of the alloy.
[0019] Tellurium is used as a replacement for lead. Like lead, tellurium is
added to
improve machinability of the alloy without the negative contribution of lead.
Tellurium is added
in an amount ranging from about.20% to about 1.5% of the alloy. In one series
of embodiments,
the tellurium ranges from about 0.4 to about 1.0%. In one embodiment,
tellurium comprises
about .5% of the alloy. The amount of tellurium used will depend, in part, on
the amount of
copper used in the alloy, as copper levels increase the amount of tellurium
used with decrease.
Like lead, the addition of tellurium to the alloy creates discontinuities in
the copper and zinc
phases of the alloy like those shown in Figs 1-3. The good dispersion of these
discontinuities
leads to the improved machinability of the alloys.
[0020] One advantage of the present invention is that the alloys exhibit
machinability similar to that of lead containing alloys while using
significantly lower amounts of
tellurium.
[0021] Other materials which may be added to the brass alloys include arsenic,
nickel, manganese, silicon, and phosphorous. When phosphorous is used, the
amount present
will typically be less than 0.05% of the alloy.
[0022] The resulting alloys will generally exhibit excellent machinability and
conductivity as indicated by Ultimate Tensile Strength (UTS) ranging from
about 240 to about
530 MPa and a yield strength of from about 200 MPa to about 450 MPa as
determined using
ASTM method B140. The actual Tensile strength and Yield strength will depend,
in part, on the
actual composition of the alloy. Conductivity of the alloys will range from
about 28 to about
45% IACS.
EXAMPLES
4
CA 02754813 2011-09-08
WO 2010/104527 PCT/US2009/053505
[0023] A series of brass alloys were prepared where the added lead (typically
about
2%) was replaced with approximately 0.5% tellurium. The composition of each
alloy is shown
in Table 1.
TABLE 1
Sample Cu Pb Zn Te P Sn
A Balance <0.01 5.10 0.5 0.011 ----
B Balance 0.00 8.82 0.57 .001 ----
C 83.03 0.06 Balance .052 0.05 0.11
D 59.41 0.02 Balance 0.17 0.05 ----
[0024] The billets were then changed into an extrusion press at a temperature
ranging from about 780 C to about 860 C. The billets were then hot extruded
through a variety
of dies and at different pressures to produce numerous sizes. Each shot was
lubricated prior to
extrusion and the extrusion dies were preheated. The results are shown in
Table 2.
TABLE 2
Sample Final Draw Size Shot Temp Pressure Length
Al 31.75mm 808 C 234MPa 12m
A2 75.40mm 822 C 268MPa 18.5m
A3 19.05mm 803 C 305MPa 34m
B1 31.75mm 794 C 267MPa 12m
B2 25.40mm 800 C 289MPa 18.5m
B3 19.05mm 806 C 304MPa 34m
B4 12.70mm 870 C 265MPa 67m
C1a 25.40mm 830 C 298MPa 18.4m
CA 02754813 2011-09-08
WO 2010/104527 PCT/US2009/053505
C1b 25.40mm 867 C 28OMPa 18.4m
C2a 50.80mm 750 C 230MPa 21.5m
C3a 22.23mm AF 830 C 312MPa 21.5m
Hex
C3b 22.23mm AF 837 C 324MPa 21.5m
Hex
D1a 50.8mm 640 C 128MPa 4.6m
D1b 50.80mm 637 C 142MPa 4.6m
D2a 25.4mm 650 C 234MPa 18.4m
D2b 25.4mm 660 C 214MPa 18.4m
D3a 22.23mm AF 648 C 235MPa 21.5m
Hex
D3b 22.23mm 621 C 276MPa 21.5m
[0025] The bars were then passed through a bath of sulfuric pickling acid and
then
cold drawn so as to induce the correct mechanical properties and grain size
requirements. Also
this process ensures that the correct size tolerances are met. The cold
drawing operation was
accomplished effortlessly. The products were then tested for tensile strength,
hardness,
conductivity, and machinability. The results are shown in Table 3.
TABLE 3
Sample Reduction Ultimate Tensile Yield Strength ELONGATION HARDNESS
in Area Strength (MPa) (MPa) (Rb)
(%)
Al 11.24 267.9 210.3 30% 56
A2 12.8 296.5 341.3 24% 57
A3 1_.71 322 279.2 16% 60
B1 11.24 302.7 241.3 26% 59
B2 12.8 322 259.2 24% 63
6
CA 02754813 2011-09-08
WO 2010/104527 PCT/US2009/053505
B3 17.71 322.7 268.9 21% 64
B4 28.32 393.7 393 12% 69
Cl 12.8 350.2 291.9 20% 51
C2 11.13 354.9 295.8 20% 52
C3 13 358.8 294.1 22% 53
D1 14.10 487.8 378.2 29% 75
D2 14.10 531.6 443 20% 78
D3 14.44 485.3 407.8 19% 76
[0026] Conductivity tests were then conducted on various samples. Conductivity
diminishes as the ratio of zinc content increases. The results ranged from at
least about 28% to
about 49% maximum.
[0027] Photomicrographs of Samples Cl, C2 and C3 were taken after draw and are
shown in FIGS 1-3. The micro structure in the alloys were uniform indicating
good dispersion
of the tellurium throughout the alloy.
[0028] Although the present invention and its advantages have been described
in
detail, it should be understood that various changes, substitutions and
alterations can be made
herein without departing from the spirit and scope of the invention as defined
by the appended
claims. Moreover, the scope of the present application is not intended to be
limited to the
particular embodiments of the process, machine, manufacture, composition of
matter, means,
methods and steps described in the specification. As one of ordinary skill in
the art will readily
appreciate from the disclosure of the present invention, processes, machines,
manufacture,
compositions of matter, means, methods, or steps, presently existing or later
to be developed that
perform substantially the same function or achieve substantially the same
result as the
corresponding embodiments described herein may be utilized according to the
present invention.
Accordingly, the appended claims are intended to include within their scope
such processes,
machines, manufacture, compositions of matter, means, methods, or steps.
7