Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2010/119264 PCT/GB2010/000773
IMIDAZOPYRAZINES FOR USE AS KINASE INHIBITORS
Field of the Invention
This invention relates to novel pharmaceutically-useful compounds, which
compounds are useful as inhibitors of protein or lipid kinases (such as
inhibitors
of the phosphoinositide 3'OH kinase (P13 kinase) family, particularly the P13K
class I sub-type, or, inhibitors of the mammalian target of rapamycin (mTOR)).
The compounds are of potential utility in the treatment of diseases such as
cancer. The invention also relates to the use of such compounds as
medicaments, to the use of such compounds for in vitro, in situ and in vivo
diagnosis or treatment of mammalian cells (or associated pathological
conditions), to pharmaceutical compositions containing them, and to synthetic
routes for their production.
Background of the Invention
The malfunctioning of protein kinases (PKs) is the hallmark of numerous
diseases. A large share of the oncogenes and proto-oncogenes involved in
human cancers code for PKs. The enhanced activities of PKs are also implicated
in many non-malignant diseases, such as benign prostate hyperplasia, familial
adenomatosis, polyposis, neuro-fibromatosis, psoriasis, vascular smooth cell
proliferation associated with atherosclerosis, pulmonary fibrosis, arthritis
glomerulonephritis and post-surgical stenosis and restenosis. PKs are also
implicated in inflammatory conditions and in the multiplication of viruses and
parasites. PKs may also play a major role in the pathogenesis and development
of neurodegenerative disorders.
For a general reference to PKs malfunctioning or disregulation see, for
instance,
Current Opinion in Chemical Biology 1999, 3, 459 - 465.
Phosphatidylinositol 3-kinases (P13Ks) are a family of lipid and
serine/threonine
kinases that catalyze the phosphorylation of the membrane lipid
phosphatidylinositol (PI) on the 3'-OH of the inositol ring to produce
1
-0&26
WO 2010/119264 PCT/GB2010/000773
phosphoinositol-3-phosphate (PIP), phosphoinositol-3,4-diphosphate (PIP2) and
phosphoinositol-3,4,5-triphosphate (PIP3), which act as recruitment sites for
various intracellular signalling proteins, which in turn form signalling
complexes to
relay extracellular signals to the cytoplasmic face of the plasma membrane.
These 3'-phosphoinositide subtypes function as second messengers in intra-
cellular signal transduction pathways (see e.g. Trends Biochem. Sci 22 87,267-
72
(1997) by Vanhaesebroeck et al.; Chem. Rev. 101 (8), 2365-80 (2001) by Leslie
et al (2001); Annu. Rev. Cell. Dev. Boil. 17, 615-75 (2001) by Katso et al;
and
Cell. Mol. Life Sci. 59 (5), 761-79 (2002) by Toker et al).
Multiple P13K isoforms categorized by their catalytic subunits, their
regulation by
corresponding regulatory subunits, expression patterns and signalling specific
funtions (pl1Oa, R, 6, y) perform this enzymatic reaction (Exp. Cell. Res. 25
(1),.
239-54 (1999) by Vanhaesebroeck and Katso et al., 2001, above).
The closely related isoforms p11 Oa and (3 are ubiquitously expressed, while 6
and
y are more specifically expressed in the haematopoietic cell system, smooth
muscle cells, myocytes and endothelial cells (see e.g. Trends Biochem. Sci. 22
(7),. 267-72 (1997) by Vanhaesebroeck et al). Their expression might also be
regulated in an inducible manner depending on the cellular, tissue type and
stimuli as well as disease context. Inductibility of protein expression
includes
synthesis of protein as well as protein stabilization that is in part
regulated by
association with regulatory subunits.
Eight mammalian P13Ks have been identified so far, including four class I
P13Ks.
Class Ia includes PI3Ka, PI3K(3 and PI3KS. All of the class la enzymes are
heterodimeric complexes comprising a catalytic subunit (p11Oa, p110(3 or
p1106)
associated with an SH2 domain containing p85 adapter subunit. Class Ia P13Ks
are activated through tyrosine kinase signalling and are involved in cell
proliferation and survival. Pl3Ka and PI3K(3 have also been implicated in
tumorigenesis in a variety of human cancers. Thus, pharmacological inhibitors
of
PI3Ka and P13K(3 are useful for treating various types of cancer.
P13Ky, the only member of the Class lb P13Ks, consists of a catalytic subunit
pl1Oy, which is associated with a p110 regulatory subunit. P13Ky is regulated
by
2
WO 2010/119264 PCT/GB2010/000773
G protein coupled receptors (GPCRs) via association with (3y subunits of
heterotrimeric G proteins. PI3Ky is expressed primarily in hematopoietic cells
and
cardiomyocytes and is involved in inflammation and mast cell function. Thus,
pharmacological inhibitors of PI3Ky are useful for treating a variety of
inflammatory diseases, allergies and cardiovascular diseases.
These observations show that deregulation of phosphoinositol-3-kinase and the
upstream and downstream components of this signalling pathway is one of the
most common deregulations associated with human cancers and proliferative
diseases (see e.g. Parsons et al., Nature 436:792 (2005); Hennessey et al.,
Nature Rev. Drug Discovery 4: 988-1004 (2005).
The mammalian target of rapamycin (mTOR) also known as FK506 binding
protein 12-rapamycin associated protein 1 (FRAP1) is a protein which in humans
is encoded by the FRAP1 gene. mTOR is a serine/threonine protein kinase that
regulates cell growth, cell proliferation, cell motility, cell survival,
protein
synthesis, and transcription. The inhibition of mTORs are believed to be
useful
for treating various diseases/conditions, such as cancer (for example, as
described in Easton et al. (2006). "mTOR and cancer therapy". Oncogene 25
(48): 6436-46).
The listing or discussion of an apparently prior-published document in this
specification should not necessarily be taken as an acknowledgement that the
document is part of the state of the art or is common general knowledge.
International patent applications WO 99/64401 and WO 02/10140 both disclose
compounds that may be useful as agonists or antagonists of somatostatin
receptors. These documents do not predominantly relate to imidazopyrazines.
International patent applications WO 2007/028051 and WO 2008/156614
disclose inter alia imidazopyrazines that may be useful as kinase inhibitors.
International patent application WO 2009/007029 discloses various compounds
that may be useful in the treatment of haematological diseases. However, none
of these documents predominantly relate to imidazopyrazines that are
substituted
at the 8-position with a cyclic group.
3
WO 2010/119264 PCT/GB2010/000773
International patent applications WO 2004/072080 and WO 2004/072081 both
disclose various imidazopyrazines, which may be useful as modulators of HSP90
complex or as modulators of a certain protein kinase. International patent
application WO 02/060492 also discloses various imidazopyrazines, which may
be useful as inhibitors of a certain protein kinase (JAK kinases). However,
all of
these documents predominantly relate to imidazopyrazines that are
unsubstituted
at the 2-, 3- and 5-position.
International patent application WO 2004/022562 discloses various
imidazopyrazines that may be useful as modulators of kinase activity. However,
this document predominantly relates to imidazopyrazines that are substituted
at
the 8-position with an amino group containing an aromatic ring and/or
imidazopyrazines that are unsubstituted at the 2-, 3-, and 4-position.
International patent application WO 02/062800 discloses various compounds for
use as antagonists on a corticotropin-releasing-factor receptor. International
patent application WO 88/04298 also discloses ceratin compounds for use as
medicaments. However, these documents do not predominantly relate to
imidazopyrazines substituted at the 6-position with an aromatic group and/or
relate to those imidazopyrazines unsubstituted at the 2-, 3-, and 4-position.
Disclosure of the Invention
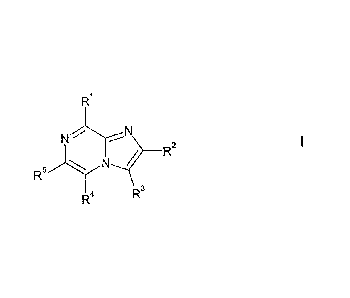
According to the invention, there is provided a compound of formula 1,
R
N N R2
RS N
4 R3
wherein:
R1 represents:
(a) -N(Rla)Rlb,
4
-0&26
WO 2010/119264 PCT/GB2010/000773
in which Ra and R'b are linked together to form, together with the nitrogen
atom to which they are necessarily attached, a 5- to 7-membered ring
optionally containing a further one or two heteroatoms (e.g. selected from
nitrogen, oxygen and sulfur), optionally containing one or two double
bonds, and which ring is optionally substituted by one or more
substituents selected from =0 and B';
(b) a heterocycloalkyl (e.g. a 3- to 7-membered) group (attached to the
requisite imidazopyrazine via a carbon atom), optionally substituted by
one or more substituents selected from =0 and B2;
(c) a monocyclic heteroaryl group optionally substituted by one or more
substituents selected from B3;
R2 and R3 independently represent:
(i) hydrogen;
(ii) Q1;
(iii) C1_12 alkyl optionally substituted by one or more substituents selected
from
=0, =S, =N(R1Oa) and Q2; or
R2 or R3 may represent a fragment of formula IA,
Ra
\N- (C(R15)2)m IA
Rb
m represents 0, 1, 2, 3, 4, 5 or 6;
each R15 represents hydrogen, halo (e.g. fluoro) or C1.6 alkyl optionally
substituted
by one or more substituents selected from E'; or
the two R15 groups may linked together to form (along with the requisite
carbon
atom to which those R15 groups are necessarily attached) a 3- to 6-membered
(spiro-cyclic) ring, which ring optionally contains one or more double bonds,
and
optionally contains a further heteroatom selected from nitrogen, sulfur and
5
WO 2010/119264 PCT/GB2010/000773
oxygen, and which ring is optionally substituted by one or more substituents
selected from E2;
Ra and Rb are linked together, along with the requisite nitrogen atom to which
they are necessarily attached, to form a first 3- to 7-membered cyclic group,
optionally containing one further heteroatom selected from nitrogen, sulfur
and
oxygen, and which ring:
(a) is fused to a second ring that is either a 3- to 7-membered saturated
heterocycloalkyl group containing one to four heteroatoms selected from
oxygen, sulfur and nitrogen (preferably oxygen and nitrogen), a 3- to 12-
membered saturated carbocyclic ring, or an unsaturated 5- to 12-
membered carbocyclic or heterocyclic ring (in which the heteroatoms are
preferably selected from sulfur and, especially, nitrogen and oxygen);
(b) comprises a linker group -(C(R" )2)P and/or -(C(R")2)r O-(C(R" )2)s
(wherein
p is 1 or 2; r is 0 or 1; s is 0 or 1; and each R" independently represents
hydrogen or C1_6 alkyl), linking together any two non-adjacent atoms of the
first 3- to 7-membered ring (i.e. forming a bridged structure); or
(c) comprises a second ring that is either a 3- to 12-membered saturated
carbocyclic ring or or a 3- to 7-membered saturated heterocycloalkyl
group containing one to four heteroatoms selected from oxygen and
nitrogen, and which second ring is linked together with the first ring via a
single carbon atom common to both rings (i.e. forming a spiro-cycle),
all of which cyclic groups, defined by the linkage of Ra and Rb, are
optionally
substituted by one or more substituents selected from =0 and E3;
R4 represents hydrogen or a substituent selected from halo, -CN, -OR10b
-N(R1ob)R11b, -C(O)N(R10b)R11b, -C(O)R10b, C1_6 alkyl and heterocycloalkyl
(e.g. a
3- to 7-membered heterocycloalkyl), which latter two groups are optionally
substituted by one or more substituents selected from E4 and =O;
but wherein at least one of R2, R3 and R4 represents a substituent other than
hydrogen;
6
WO 2010/119264 PCT/GB2010/000773
R5 represents aryl or heteroaryl (both of which are optionally substituted by
one or
more substituents selected from E);
each Q1 and Q2 independently represents, on each occasion when used herein:
halo, -CN, -NO2, -N(R10a)R11a, -OR10a, -C(=Y)-R10a, C(=Y)-OR10a,
-C(=Y)N(R10a)R11a, -OC(=Y)-R10a, _OC(=Y)-OR1oa, -OC(=Y)N(R10a)R11a
_OS(O)20R1oa, -OP(=Y)(OR1oa)(OR11 a), -OP(OR1oa)(OR'la), _N(R12a)C(=Y)R11a,
_N(R12a)C(=Y)OR11a, _N(R12a)C(=y)N(R1oa)R11a, -NR 12aS(O)2R1oa,
-NR 12aS(O)2N(R1oa)R11a, -S(O)2N(R1oa)R11a, -SC(=Y)R10a, _S(O)2R1oa, _SR10a
-S(O)R1oa C1-12 alkyl, heterocycloalkyl (which latter two-groups are
optionally
substituted by one or more substituents selected from =O, =S, =N(R10a) and
E6),
aryl or heteroaryl (which latter two groups are optionally substituted by one
or
more substituents selected from E7);
each B1, B2 and B3 independently represent halo, -NO2, -CN, -N(R1oa)R1la, -
OR10a,
-C(=Y)-R1oa, -C(=Y)-OR1oa, _C(=Y)N(R10a)R1la, -N(R12a)C(=Y)R11a,
-N(R12a)C(_Y)OR11a, -N(R12a)C(=Y)N(R1oa)R11a, -NR 12aS(O)2R1oa,
_NR12aS(O)2N(R1oa)R1la, -S(O)2N(R1oa)R1la, -SC(=Y)R1oa, _SC(=Y)OR1oa
-S(O)2R10a, C1-12 alkyl, heterocycloalkyl (which latter two groups are
optionally
substituted by one or more substituents selected from =0 and E8), aryl or
heteroaryl (which latter two groups are optionally substituted by one or more
substituents selected from E9);
or, any two B1 substituents, when attached to the same carbon atom (thereby
forming a spiro-cycle), may be linked together to form, a 3- to 12- membered
(e.g.
3- to 6-membered) ring, optionally containing one or more (e.g. one to three)
heteroatoms (preferably selected from sulfur, oxygen and nitrogen), which ring
optionally contains one or more (e.g. one to three) double bonds, and which
ring
is itself optionally substituted by one or more substituents selected from
halo, =0
and C1.3 alkyl optionally substituted by one or more fluoro atoms;
each R1oa R11a, R12a, R1ob R1 lb and R12b independently represent, on each
occasion when used herein, hydrogen, C1_12 alkyl, heterocycloalkyl (which
latter
two groups are optionally substituted by one or more substituents selected
from
7
-0&26
WO 2010/119264 PCT/GB2010/000773
=0, =S, =N(R20) and E10), aryl or heteroaryl (which latter two groups are
optionally
substituted by one or more substituents selected from E11); or
any relevant pair of R1 0a R11a and R12a (for example, when attached to the
same
atom, adjacent atom (i.e. 1,2-relationship) or to atoms that are two atom
atoms
apart, i.e. in a 1,3-relationship) and/or any pair of R'ob and R' lb may be
linked
together to form (e.g. along with the requisite nitrogen atom to which they
may be
attached) a 4- to 20- (e.g. 4- to 12-) membered ring, optionally containing
one or
more heteroatoms (for example, in addition to those that may already be
present,
e.g. (a) heteroatom(s) selected from oxygen, nitrogen and sulfur), optionally
containing one or more unsaturations (preferably, double bonds), and which
ring
is optionally substituted by one or more substituents selected from =O, =S,
=N(R20) and E12;
each E1, E2, E3, E4, E5, E6, E7, E8, E9, E10, E11 and E12 independently
represents,
on each occasion when used herein:
(i) Q4;
(ii) C1_12 alkyl optionally substituted by one or more substituents selected
from =0
and Q5; or
any two E1, E2, E3, E4, E5, E6, E7, E8, E9, E10, E11 or E12 groups, for
example on
C1_12 alkyl groups, e.g. when they are attached to the same or adjacent carbon
atoms or on aryl groups, e.g. when attached to adjacent carbon atoms, may be
linked together to form a 3- to 12-membered ring, optionally containing one or
more (e.g. one to three) unsaturations (preferably, double bonds), and which
ring
is optionally substituted by one or more substituents selected from =0 and J1;
each Q4 and Q5 independently represent, on each occasion when used herein:
halo, -CN, -NO2, -N(R20)R21, -OR20, -C(=Y)-R20, -C(=Y)-OR20,
-C(=Y)N(R20)R21, -OC(=Y)-R20, -OC(=Y)-OR20, -OC(=Y)N(R20)R21, -OS(O)2OR20,
-OP(=Y)(OR20)(OR21), -OP(OR20)(OR21), -N(R22)C(=Y)R21, -N(R22)C(=Y)OR21,
-N(R22)C(=Y)N(R20)R21, -NR22S(0)2R20, -NR 22S(0)2N(R20)R21, -S(0)2N(R20)R21,
-SC(=Y)R20, -S(0)2R20, -SR20, -S(O)R20, C1.6 alkyl, heterocycloalkyl (which
latter
two groups are optionally substituted by one or more substituents selected
from
8
WO 2010/119264 PCT/GB2010/000773
=0 and J2), aryl or heteroaryl (which latter two groups are optionally
substituted
by one or more substituents selected from J);
each Y independently represents, on each occasion when used herein, =0, =S,
=NR 23 or =N-CN;
each R20, R21, R22 and R23 independently represent, on each occasion when used
herein, hydrogen, C1.6 alkyl, heterocycloalkyl (which latter two groups are
optionally substituted by one or more substituents selected from J4 and =0),
aryl
or heteroaryl (which latter two groups are optionally substituted by one or
more
substituents selected from J5); or
any relevant pair of R20, R21 and R22, may (for example, when attached to the
same atom, adjacent atom (i.e. 1,2-relationship) or to atoms that are two atom
atoms apart, i.e. in a 1,3-relationship) be linked together to form (e.g.
along with
the requisite nitrogen atom to which they may be attached) a 4- to 20- (e.g. 4-
to
12-) membered ring, optionally containing one or more heteroatoms (for
example,
in addition to those that may already be present, e.g. (a) heteroatom(s)
selected
from oxygen, nitrogen and sulfur), optionally containing one or more
unsaturations (preferably, double bonds), and which ring is optionally
substituted
by one or more substituents selected from J6 and =0;
each J1, J2, J3, J4, J5 and J6 independently represents, on each occasion when
used herein:
(i) Q';
(ii) C.16 alkyl or heterocycloalkyl, both of which are optionally substituted
by one or
more substituents selected from =0 and Q8;
each Q7 and Q8 independently represents, on each occasion when used herein:
-CN or, preferably, halo, -N(R50)R51, -OR50, _C(=Ya)-RSO, _C(=Ya)_ORS
-C(=Ya)N(R50)R51, -N(R52)C(=Ya)R51, -NR52S(0)2R50, -S(0)2RSO -SR50, -S(0)R50
or
C1.6 alkyl optionally substituted by one or more fluoro atoms;
each Ya independently represents, on each occasion when used herein, =0, =S,
=NR 53 or =N-CN;
9
WO 2010/119264 PCT/GB2010/000773
each R50, R51, R52 and R53 independently represents, on each occasion when
used herein, hydrogen or C1_6 alkyl optionally substituted by one or more
substituents selected from fluoro, -OR60 and -N(R&1)R62; or
any relevant pair of R50, R51 and R52 may (for example when attached to the
same
or adjacent atoms) be linked together to form, a 3- to 8-membered ring,
optionally
containing one or more heteroatoms (for example, in addition to those that may
already be present, heteroatoms selected from oxygen, nitrogen and sulfur),
optionally containing one or more unsaturations (preferably, double bonds),
and
which ring is optionally substituted by one or more substituents selected from
=0
and C1_3 alkyl;
R60, R6' and R62 independently represent hydrogen or C1.6 alkyl optionally
substituted by one or more fluoro atoms;
or a pharmaceutically acceptable ester, amide, solvate or salt thereof,
which compounds, esters, amides, solvates and salts are referred to
hereinafter
as "the compounds of the invention".
Pharmaceutically-acceptable salts include acid addition salts and base
addition
salts. Such salts may be formed by conventional means, for example by reaction
of a free acid or a free base form of a compound of formula I with one or more
equivalents of an appropriate acid or base, optionally in a solvent, or in a
medium
in which the salt is insoluble, followed by removal of said solvent, or said
medium,
using standard techniques (e.g. in vacuo, by freeze-drying or by filtration).
Salts
may also be prepared by exchanging a counter-ion of a compound of the
invention in the form of a salt with another counter-ion, for example using a
suitable ion exchange resin.
By "pharmaceutically acceptable ester, amide, solvate or salt thereof', we
include
salts of pharmaceutically acceptable esters or amides, and solvates of
pharmaceutically acceptable esters, amides or salts. For instance,
pharmaceutically acceptable esters and amides such as those defined herein
may be mentioned, as well as pharmaceutically acceptable solvates or salts.
WO 2010/119264 PCT/GB2010/000773
Pharmaceutically acceptable esters and amides of the compounds of the
invention are also included within the scope of the invention.
Pharmaceutically
acceptable esters and amides of compounds of the invention may be formed from
corresponding compounds that have an appropriate group, for example an acid
group, converted to the appropriate ester or amide. For example,
pharmaceutically acceptable esters (of carboxylic acids of compounds of the
invention) that may be mentioned include optionally substituted C1.6 alkyl, C5-
10
aryl and/or C5-10 aryl-C1-6 alkyl- esters. Pharmaceutically acceptable amides
(of
carboxylic acids of compounds of the invention) that may be mentioned include
those of the formula -C(O)N(Rz1)Rz2, in which Rz1 and R12 independently
represent optionally substituted C1-6 alkyl, C5-10 aryl, or C5_10 aryl-C1.6
alkylene-.
Preferably, C1_6 alkyl groups that may be mentioned in the context of such
pharmaceutically acceptable esters and amides are not cyclic, e.g. linear
and/or
15- branched.
Further compounds of the invention that may be mentioned include carbamate,
carboxamido or ureido derivatives, e.g. such derivatives of existing amino
functional groups.
For the purposes of this invention, therefore, prodrugs of compounds of the
invention are also included within the scope of the invention.
The term "prodrug" of a relevant compound of the invention includes any
compound that, following oral or parenteral administration, is metabolised in
vivo
to form that compound in an experimentally-detectable amount, and within a
predetermined time (e.g. within a dosing interval of between 6 and 24 hours
(i.e.
once to four times daily)). For the avoidance of doubt, the term "parenteral"
administration includes all forms of administration other than oral
administration.
Prodrugs of compounds of the invention may be prepared by modifying functional
groups present on the compound in such a way that the modifications are
cleaved, in vivo when such prodrug is administered to a mammalian subject. The
modifications typically are achieved by synthesising the parent compound with
a
prodrug substituent. Prodrugs include compounds of the invention wherein a
11
WO 2010/119264 PCT/GB2010/000773
hydroxyl, amino, sulfhydryl, carboxy or carbonyl group in a compound of the
invention is bonded to any group that may be cleaved in vivo to regenerate the
free hydroxyl, amino, sulfhydryl, carboxy or carbonyl group, respectively.
Examples of prodrugs include, but are not limited to, esters and carbamates of
hydroxy functional groups, esters groups of carboxyl functional groups, N-acyl
derivatives and N-Mannich bases. General information on prodrugs may be found
e.g. in Bundegaard, H. "Design of Prodrugs" p. 1-92, Elesevier, New York-
Oxford
(1985).
Compounds of the invention may contain double bonds and may thus exist as E
(entgegen) and Z (zusammen) geometric isomers about each individual double
bond. Positional isomers may also be embraced by the compounds of the
invention. All such isomers (e.g. if a compound of the invention incorporates
a
double bond or a fused ring, the cis- and trans- forms, are embraced) and
mixtures thereof are included within the scope of the invention (e.g. single
positional isomers and mixtures of positional isomers may be included within
the
scope of the invention).
Compounds of the invention may also exhibit tautomerism. All tautomeric forms
(or tautomers) and mixtures thereof are included within the scope of the
invention. The term "tautomer" or "tautomeric form" refers to structural
isomers of
different energies which are interconvertible via a low energy barrier. For
example, proton tautomers (also known as prototropic tautomers) include
interconversions via migration of a proton, such as keto-enol and imine-
enamine
isomerisations. Valence tautomers include interconversions by reorganisation
of
some of the bonding electrons.
Compounds of the invention may also contain one or more asymmetric carbon
atoms and may therefore exhibit optical and/or diastereoisomerism.
Diastereoisomers may be separated using conventional techniques, e.g.
chromatography or fractional crystallisation. The various stereoisomers may be
isolated by separation of a racemic or other mixture of the compounds using
conventional, e.g. fractional crystallisation or HPLC, techniques.
Alternatively the
desired optical isomers may be made by reaction of the appropriate optically
12
-0&26
WO 2010/119264 PCT/GB2010/000773
active starting materials under conditions which will not cause racemisation
or
epimerisation (i.e. a 'chiral pool' method), by reaction of the appropriate
starting
material with a 'chiral auxiliary' which can subsequently be removed at a
suitable
stage, by derivatisation (i.e. a resolution, including a dynamic resolution),
for
example with a homochiral acid followed by separation of the diastereomeric
derivatives by conventional means such as chromatography, or by reaction with
an appropriate chiral reagent or chiral catalyst all under conditions known to
the
skilled person.
All stereoisomers (including but not limited to diastereoisomers, enantiomers
and
atropisomers) and mixtures thereof (e.g. racemic mixtures) are included within
the
scope of the invention.
In the structures shown herein, where the stereochemistry of any particular
chiral
atom is not specified, then all stereoisomers are contemplated and included as
the compounds of the invention. Where stereochemistry is specified by a solid
wedge or dashed line representing a particular configuration, then that
stereoisomer is so specified and defined.
The compounds of the present invention may exist in unsolvated as well as
solvated forms with pharmaceutically acceptable solvents such as water,
ethanol,
and the like, and it is intended that the invention embrace both solvated and
unsolvated forms.
The present invention also embraces isotopically-labeled compounds of the
present invention which are identical to those recited herein, but for the
fact that
one or more atoms are replaced by an atom having an atomic mass or mass
number different from the atomic mass or mass number usually found in nature
(or the most abundant one found in nature). All isotopes of any particular
atom or
element as specified herein are contemplated within the scope of the compounds
of the invention. Exemplary isotopes that can be incorporated into compounds
of
the invention include isotopes of hydrogen, carbon, nitrogen, oxygen,
phosphorus, sulfur, fluorine, chlorine and iodine, such as 2H, 3H, 11C, 13C,
140 ,
13N 150 170, 180, 32P, 33P, 35S 18F 36C1, 1231 and 1251. Certain isotopically-
labeled
compounds of the present invention (e.g., those labeled with 3H and 14C) are
13
-0&26
WO 2010/119264 PCT/GB2010/000773
useful in compound and for substrate tissue distribution assays. Tritiated
(3H)
and carbon-14 (14C) isotopes are useful for their ease of preparation and
detectability. Further, substitution with heavier isotopes such as deuterium
(i.e.,
2H may afford certain therapeutic advantages resulting from greater metabolic
stability (e.g., increased in vivo half-life or reduced dosage requirements)
and
hence may be preferred in some circumstances. Positron emitting isotopes such
as 150, 13N, 11C and 18F are useful for positron emission tomography (PET)
studies to examine substrate receptor occupancy. Isotopically labeled
compounds of the present invention can generally be prepared by following
procedures analogous to those disclosed in the Scheme 1 and/or in the
Examples herein below, by substituting an isotopically labeled reagent for a
non-
isotopically labeled reagent.
Unless otherwise specified, C1_q alkyl groups (where q is the upper limit of
the
range) defined herein may be straight-chain or, when there is a sufficient
number
(i.e. a minimum of two or three, as appropriate) of carbon atoms, be branched-
chain, and/or cyclic (so forming a C3_q cycloalkyl group). Such cycloalkyl
groups
may be monocyclic or bicyclic and may further be bridged. Further, when there
is
a sufficient number (i.e. a minimum of four) of carbon atoms, such groups may
also be part cyclic. Such alkyl groups may also be saturated or, when there is
a
sufficient number (i.e. a minimum of two) of carbon atoms, be unsaturated
(forming, for example, a C2_q alkenyl or a C2_q alkynyl group).
Unless otherwise stated, the term C1_q alkylene (where q is the upper limit of
the
range) defined herein may be straight-chain or, when there is a sufficient
number
of carbon atoms, be saturated or unsaturated (so forming, for example, an
alkenylene or alkynylene linker group). However, such C1_q alkylene groups may
not be branched.
C3_q cycloalkyl groups (where q is the upper limit of the range) that may be
specifically mentioned may be monocyclic or bicyclic alkyl groups, which
cycloalkyl groups may further be bridged (so forming, for example, fused ring
systems such as three fused cycloalkyl groups). Such cycloalkyl groups may be
saturated or unsaturated containing one or more double bonds (forming for
example a cycloalkenyl group). Substituents may be attached at any point on
the
14
-0&26
WO 2010/119264 PCT/GB2010/000773
cycloalkyl group. Further, where there is a sufficient number (i.e. a minimum
of
four) such cycloalkyl groups may also be part cyclic.
The term "halo", when used herein, preferably includes fluoro, chloro, bromo
and
iodo.
Heterocycloalkyl groups that may be mentioned include non-aromatic monocyclic
and bicyclic heterocycloalkyl groups in which at least one (e.g. one to four)
of the
atoms in the ring system is other than carbon (i.e. a heteroatom), and in
which
the total number of atoms in the ring system is between 3 and 20 (e.g. between
three and ten, e.g between 3 and 8, such as 5- to 8-). Such heterocycloalkyl
groups may also be bridged. Further, such heterocycloalkyl groups may be
saturated or unsaturated containing one or more double and/or triple bonds,
forming for example a C2_q heterocycloalkenyl (where q is the upper limit of
the
range) group. C2_q heterocycloalkyl groups that may be mentioned include 7-
azabicyclo[2.2.1 ]heptanyl, 6-azabicyclo[3. 1.1 ]heptanyl, 6-azabicyclo[3.2.1
]-
octanyl, 8-azabicyclo-[3.2. 1 ]octanyl, aziridinyl, azetidinyl,
dihydropyranyl,
dihydropyridyl, dihydropyrrolyl (including 2,5-dihydropyrrolyl), dioxolanyl
(including 1,3-dioxolanyl), dioxanyl (including 1,3-dioxanyl and 1,4-
dioxanyl),
dithianyl (including 1,4-dithianyl), dithiolanyl (including 1,3-dithiolanyl),
imidazolidinyl, imidazolinyl, morpholinyl, 7-oxabicyclo[2.2. 1 ]heptanyl, 6-
oxabicyclo-[3.2.1]octanyl, oxetanyl, oxiranyl, piperazinyl, piperidinyl,
pyranyl,
pyrazolidinyl, pyrrolidinonyl, pyrrolidinyl, pyrrolinyl, quinuclidinyl,
sulfolanyl, 3-
sulfolenyl, tetrahydropyranyl, tetrahydrofuranyl, tetrahydropyridyl (such as
1,2,3,4-tetrahydropyridyl and 1,2,3,6-tetrahydropyridyl), thietanyl,
thiiranyl,
thiolanyl, thiomorpholinyl, trithianyl (including 1,3,5-trithianyl), tropanyl
and the
like. Substituents on heterocycloalkyl groups may, where appropriate, be
located
on any atom in the ring system including a heteroatom. The point of attachment
of heterocycloalkyl groups may be via any atom in the ring system including
(where appropriate) a heteroatom (such as a nitrogen atom), or an atom on any
fused. carbocyclic ring that may be present as part of the ring system.
Heterocycloalkyl groups may also be in the N- or S- oxidised form.
Heterocycloalkyl mentioned herein may be stated to be specifically monocyclic
or
bicyclic. -
-0&26
WO 2010/119264 PCT/GB2010/000773
For the avoidance of doubt, the term "bicyclic" (e.g. when employed in the
context
of heterocycloalkyl groups) refers to groups in which the second ring of a two-
ring
system is formed between two adjacent atoms of the first ring. The term
"bridged" (e.g. when employed in the context of cycloalkyl or heterocycloalkyl
groups) refers to monocyclic or bicyclic groups in which two non-adjacent
atoms
are linked by either an alkylene or heteroalkylene chain (as appropriate).
Aryl groups that may be mentioned include C6_20i such as C6_12 (e.g. C6_10)
aryl
groups. Such groups may be monocyclic, bicyclic or tricyclic and have between
6
and 12 (e.g. 6 and 10) ring carbon atoms, in which at least one ring is
aromatic.
C6_10 aryl groups include phenyl, naphthyl and the like, such as 1,2,3,4-
tetrahydro-
naphthyl. The point of attachment of aryl groups may be via any atom of the
ring
system. For example, when the aryl group is polycyclic the point of attachment
may be via atom including an atom of a non-aromatic ring. However, when aryl
groups are polycyclic (e.g. bicyclic or tricyclic), they are preferably linked
to the
rest of the molecule via an aromatic ring.
Unless otherwise specified, the term "heteroaryl" when used herein refers to
an
aromatic group containing one or more heteroatom(s) (e.g. one to four
heteroatoms) preferably selected from N, 0 and S. Heteroaryl groups include
those which have between 5 and 20 members (e.g. between 5 and 10) and may
be monocyclic, bicyclic or tricyclic, provided that at least one of the rings
is
aromatic (so forming, for example, a mono-, bi-, or tricyclic heteroaromatic
group).
When the heteroaryl group is polycyclic the point of attachment may be via
atom
including an atom of a non-aromatic ring. However, when heteroaryl groups are
polycyclic (e.g. bicyclic or tricyclic), they are preferably linked to the
rest of the
molecule via an aromatic ring. Heteroaryl groups that may be mentioned include
3,4-dihydro-1H-isoquinolinyl, 1,3-dihydroisoindolyl, 1,3-dihydroisoindolyl
(e.g. 3,4-
dihydro-1H-isoquinolin-2-yl, 1,3-dihydroisoindol-2-yl, 1,3-dihydroisoindol-2-
yl; i.e.
heteroaryl groups that are linked via a non-aromatic ring), or, preferably,
acridinyl,
benzimidazolyl, benzodioxanyl, benzodioxepinyl, benzodioxolyl (including 1,3-
benzodioxolyl), benzofuranyl, benzofurazanyl, benzothiadiazolyl (including
2,1,3-
benzothiadiazolyl), benzothiazolyl, benzoxadiazolyl (including 2,1,3-
benzoxadiazolyl), benzoxazinyl (including 3,4-dihydro-2H-1,4-benzoxazinyl),
benzoxazolyl, benzomorpholinyl, benzoselenadiazolyl (including
16
WO 2010/119264 PCT/GB2010/000773
2,1,3-benzoselenadiazolyl), benzothienyl, carbazolyl, chromanyl, cinnolinyl,
furanyl, imidazolyl, imidazo[1,2-a]pyridyl, indazolyl, indolinyl, indolyl,
isobenzofuranyl, isochromanyl, isoindolinyl, isoindolyl, isoquinolinyl,
isothiaziolyl,
isothiochromanyl, isoxazolyl, naphthyridinyl (including 1,6-naphthyridinyl or,
preferably, 1,5-naphthyridinyl and 1,8-naphthyridinyl), oxadiazolyl (including
1,2,3-oxadiazolyl, 1,2,4-oxadiazolyl and 1,3,4-oxadiazolyl), oxazolyl,
phenazinyl,
phenothiazinyl, phthalazinyl, pteridinyl, purinyl, pyranyl, pyrazinyl,
pyrazolyl,
pyridazinyl, pyridyl, pyrimidinyl, pyrrolyl, quinazolinyl, quinolinyl,
quinolizinyl,
quinoxalinyl, tetrahydroisoquinolinyl (including 1,2,3,4-
tetrahydroisoquinolinyl and
5,6,7,8-tetrahydroisoquinolinyl), tetrahydroquinolinyl (including 1,2,3,4-
tetrahydroquinolinyl and 5,6,7,8-tetrahydroquinolinyl), tetrazolyl,
thiadiazolyl
(including 1,2,3-thiadiazolyl, 1,2,4-thiadiazolyl and 1,3,4-thiadiazolyl),
thiazolyl,
thiochromanyl, thiophenetyl, thienyl, triazolyl (including 1,2,3-triazolyl,
1,2,4-triazolyl and 1,3,4-triazolyl) and the like. Substituents on heteroaryl
groups
may, where appropriate, be located on any atom in the ring system including a
heteroatom. The point of attachment of heteroaryl, groups may be via any atom
in
the ring system including (where appropriate) a heteroatom (such as a nitrogen
atom), or an atom on any fused carbocyclic ring that may be present as part of
the ring system. Heteroaryl groups may also be in the N- or S- oxidised form.
Heteroaryl groups mentioned herein may be stated to be specifically monocyclic
or bicyclic. When heteroaryl groups are polycyclic in which there is a non-
aromatic ring present, then that non-aromatic ring may be substituted by one
or
more =0 group.
It may be specifically stated that the heteroaryl group is monocyclic or
bicyclic. In
the case where it is specified that the heteroaryl is bicyclic, then it may be
consist
of a five-, six- or seven-membered monocyclic ring (e.g. a monocyclic
heteroaryl
ring) fused with another a five-, six- or seven-membered ring (e.g. a
monocyclic
aryl or heteroaryl ring).
Heteroatoms that may be mentioned include phosphorus, silicon, boron and,
preferably, oxygen, nitrogen and sulfur.
For the avoidance of doubt, where it is stated herein that a group (e.g. a C1-
12
alkyl group) may be substituted by one or more substituents (e.g. selected
from
17
WO 2010/119264 PCT/GB2010/000773
E6), then those substituents (e.g. defined by E6) are independent of one
another.
That is, such groups may be substituted with the same substituent (e.g.
defined
by E6) or different substituents (defined by E).
For the avoidance of doubt, in cases in which the identity of two or more
substituents in a compound of the invention may be the same, the actual
identities of the respective substituents are not in any way interdependent.
For
example, in the situation in which there is more than one Q' (or e.g. E)
substituent present, then those Q1 (or e.g. E6) substituents may be the same
or
different. Further, in the case where there are two Q1 (or two E6)
substituents
present, in which one represents -OR"" (or e.g. -OR20, as appropriate) and the
other represents -C(O)2R10a (or e.g. -C(O)2R20, as appropriate), then those
R10a or
R20 groups are not to be regarded as being interdependent. Also, when e.g.
there
are two -OR' 0a substituents present, then those -OR' Da groups may be the
same
or different (i.e. each R10a group may be the same or different).
For the avoidance of doubt, when a term such as "E' to EY2i is employed
herein,
this will be understood by the skilled person to mean E1, E2, E3, E4, E5, E6,
E7, E8,
E9, E10, E11 and E12, inclusively.
All individual features (e.g. preferred features) mentioned herein may be
taken in
isolation or in combination with any other feature (including preferred
feature)
mentioned herein (hence, preferred features may be taken in conjunction with
other preferred features, or independently of them).
The skilled person will appreciate that compounds of the invention that are
the
subject of this invention include those that are stable. That is, compounds of
the
invention include those that are sufficiently robust to survive isolation from
e.g. a
reaction mixture to a useful degree of purity.
Compounds of the invention that may be mentioned include those in which:
R4 represents hydrogen or a substituent selected from halo, -CN, -OR'0b,
-N(R'Ob)R11b, -C(O)N(R'Ob)R1'b, C1_6 alkyl and heterocycloalkyl (e.g. a 3- to
7-
membered heterocycloalkyl), which latter two groups are optionally substituted
by
one or more substituents selected from E4 and =0.
18
-0&26
WO 2010/119264 PCT/GB2010/000773
Preferred compounds of the invention that may be mentioned include those in
which:
R1 does not represent an optionally substituted heteroaryl group (especially a
monocyclic heteroaryl group, e.g. a 5- or 6-membered heteroaryl group), such
as
a 5-membered heteroaryl group containing two heteroatoms (e.g. a pyrazolyl,
such as 1-pyrazolyl, or, especially, imidazolyl, such as 1-imidazolyl);
R' represents: (a) as defined herein, (b) as defined herein or (c) in which
the
heteroaryl group is a 5-membered heteroaryl group, in which the heteroatom(s)
is/are selected from oxygen and sulphur, or a 6-membered heteroaryl group;
R1 preferably represents (a) or (b);
R5 does not represent phenyl or 3-pyridyl (especially phenyl) (e.g. in which
the
latter two groups are substituted (for example, meta to the point of
attachment to
the imidazopyrazine), with e.g. a urea (e.g. -N(R22)C(=Y)N(R20)R21, in which Y
is
preferably =O).
Further preferred compounds of the invention that may be mentioned include
those in which, for example especially when R1 represents a group defined by
(c)
above, i.e. an optionally substituted monocyclic heteroaryl group (e.g. a 5-
membered heteroaryl group such as pyrazolyl (e.g. 1-pyrazolyl) or, especially,
imidazolyl (e.g. 1-imidazolyl)) and/or R5 represents phenyl or 3-pyridyl (e.g.
in
which the latter two groups are substituted, for example (e.g. meta to the
point of
attachment to the imidazopyrazine), with e.g. a urea (e.g. -
N(R22)C(=Y)N(R20)R21,
in which Y is preferably =0), then preferably:
R3 represents a substituent other than hydrogen;
when R3 represents a substituent other than hydrogen, then it is preferably
Q1, in
which Q1 represents heterocycloalkyl (e.g. piperidinyl, which heterocycloalkyl
group is attached to the imidazopyrazine via a carbon or, preferably, nitrogen
atom) or Q1 more preferably represents halo, -CN, -NO2, -N(R10a)R11a, -OR1oa
-C(=Y)-R1oa - C(=Y)-OR'oa, - C(=Y)N(R'oa)R11a -OC(=Y)-R'Oa, - OC(=Y)-OR1oa
-OC(=Y)N(R1oa)R11a _OS(O)20R'Oa, -OP(=Y)(OR1oa)(OR11a) _OP(OR1oa)(OR11a)
-N(R12a)C(_Y)R11a -N(R12a)C(_Y)OR11a -N(R12a)C(_Y)N(R1oa)R11a
-NR 12aS(O)2R1oa -NR 12aS(O)2N(R1oa)R11a -S(O)2N(R1oa)R1la -SC(=Y)R10a
-S(O)2R1oa, -SR")' or -S(O)R10' (and Q' preferably represents halo, -CN, -NO2,
19
-0&26
WO 2010/119264 PCT/GB2010/000773
-OR' 0a, -C(=Y)N(R'oa)R'la, -N(R'2a)C(=Y)R"a, -NR '2aS(O)Ooa, and, especially,
-N(R10a)R11a);
when R3 represents a substituent other than hydrogen, then it is preferably
does
not represent optionally substituted Cq_12 (e.g. C,_6) alkyl, heterocycloalkyl
(preferably linked to the imidazopyrazine via a carbon atom), aryl (e.g.
phenyl) or
heteroaryl; and/or
at least one of R2 and R4 do not represent hydrogen, i.e. either one of R2 and
R4
may represent hydrogen, and the other (or both) represent(s) a substituent as
defined herein.
Further preferred compounds of the invention that may be mentioned include
those in which, for example especially in the case when R' represents a group
defined by (c) above, i.e. an optionally substituted monocyclic heteroaryl
group
(e.g. a 5-membered heteroaryl group such as (e.g. 1-pyrazolyl) or, especially,
imidazolyl (e.g. 1-imidazolyl)) and/or R5 represents phenyl or 3-pyridyl (e.g.
in
which the latter two groups are substituted, for example, meta to the point of
attachment to the imidazopyrazine, with e.g. a urea such as
-N(R22)C(=Y)N(R20)R21, in which Y is preferably =O), then preferably:
B1, B2 and/or B3 (e.g. B3) do not represent or do not contain aromatic groups
(such as pyridyl, e.g. 4-pyridyl);
B1, B2 and/or B3 (e.g. B3) independently represent halo, -NO2, -CN, -
N(R1oa)R1'a,
-OR'oa, -C(=Y)-R1oa, -C(=Y)-OR'oa, -C(=Y)N(R'oa)R"a, -N(R12a)C(=Y)R"a,
-N(R'2a)C(=Y)OR1'a, -N(R'2a)C(_Y)N(R'oa)R"a, -NR12aS(0)2R'oa,
-NR12aS(O)2N(R1oa)R11a, -S(O)2N(R10a)R1Ia, -SC(=Y)R'oa, -SC(=Y)OR'oa,
-S(O)2R10a, C1_12 alkyl or heterocycloalkyl (which latter two groups are
optionally
substituted by one or more substituents selected from =0 and E), or, two B'
substituents may be linked together as herein defined (i.e./e.g. to form a
spiro-
cycle);
each R10a R11a, R12a, R'ob R1 lb and R12b (for example when there is a B3
group
present) independently represent, on each occasion when used herein,
hydrogen, C'_12 alkyl or heterocycloalkyl (which latter two groups are
optionally
substituted by one or more substituents selected from =O, =S, =N(R20) and
E10);
or any relevant pair of R10a R11a and R12a and/or any pair of R10b and R' lb
may be
linked together as defined herein, but wherein when e.g. there are one or more
E8, E10 and/or E12 groups present (which independently represent Q4 or C1_12
alkyl
WO 2010/119264 PCT/GB2010/000773
optionally substituted by one or more substituents selected from =0 and Q5),
then
those Q4 and Q5 groups do not represent, or contain, aromatic rings, i.e.
then:
each Q4 and Q5 independently represent, on each occasion when used herein:
halo, -CN, -NO2, -N(R20)R21, -OR20, -C(=Y)-R20, -C(=Y)-OR20,
-C(=Y)N(R20)R21, -OC(=Y)-R20, -OC(=Y)-OR20, -OC(=Y)N(R20)R21, -OS(O)2OR20,
OP(=Y)(OR20)(OR21), OP(OR20)(OR21), -N(R22)C(=Y)R21, -N(R22)C(=Y)OR21,
-N(R22)C(=Y)N(R20)R21, -NR 22S(0)2R2o -NR 22S(0)2N(R20)R21, -S(0)2N(R20)R21
-SC(=Y)R20, -S(0)2R20, -SR20, -S(O)R20, C1.6 alkyl or heterocycloalkyl (which
latter
two groups are optionally substituted by one or more substituents selected
from
=0 and J2);
each R20, R21, R22 and R23 independently represent hydrogen, C1.6 alkyl or
heterocycloalkyl (which latter two groups are optionally substituted by one or
more substituents selected from J4 and =O).
Further preferred compounds of the invention that may be mentioned include
those in which (for example, when R5 represents a monocyclic aromatic group,
such as phenyl or pyridyl, e.g. 3-pyridyl, e.g. in which the latter two groups
are
substituted, for example meta to the point of attachment to the
imidazopyrazine,
with e.g. a urea such as -N(R22)C(=Y)N(R20)R21, in which Y is preferably =0):
R3 preferably does not represent -C(=Y)-OR' Oa (particularly when, for
example, R1
represents a heteroaryl group, e.g. pyridyl);
R3 preferably represents (particularly when, for example, R1 represents a
heteroaryl group, e.g. pyridyl) hydrogen or, more preferably, a substituent
selected from heterocycloalkyl (e.g. one that is attached to the
imidazopyrazine
via a nitrogen atom) or, especially, halo, -CN, -NO2, -N(R1oa)R11a, -C(=Y)-
R1oa,
-C(=Y)N(R10a)R11a, -OC(=Y)-R1oa, -OC(=Y)-OR' oa -OC(=Y)N(R1oa)R11a
-OS(O)20R1oa, -OP(=Y)(OR10a)(OR11a) -OP(OR10a)(OR11a) -N(R12a)C(=Y)R11a
-N(R12a)C(_Y)OR11a -N(R12a)C(=Y)N(R1oa)R11a, -NR12aS(0)2R1oa
_NR12aS(O)2N(R10a)R11a -S(O)2N(R10a)R11a _SC(=Y)R1oa, -S(O)2R1oa -SR10a and
-S(O)R1oa or a fragment of formula IA as defined hereinbefore; and/or
when, for example, R1 represents a heteroaryl group (e.g. pyridyl), then R3
preferably does not represent, or contain, an aromatic group (especially when
R3
represents -N(R1oa)R11a, e.g. in which R10a and R11a are linked together to
form,
e.g. a piperazinyl group), i.e.:
21
WO 2010/119264 PCT/GB2010/000773
each R'oa R11a R12a R1ob, R"b and R12b (for example when R' represents a (c)
group, such as pyridyl) independently represent, on each occasion when used
herein, hydrogen, C1_12 alkyl or heterocycloalkyl (which latter two groups are
optionally substituted by one or more substituents selected from =O, =S,
=N(R20)
and E1); or any relevant pair of R10a R11a and R12a and/or any pair of R1ob
and
R1 lb may be linked together as defined herein (and therefore be substituted
by
one or more E12 groups), but wherein when there are one or more E'0 and/or E12
groups present, which independently represent Q4 or C1_12 alkyl optionally
substituted by one or more substituents selected from =0 and Q5, then those Q4
and Q5 groups do not represent, or contain, aromatic rings, i.e. then:
each Q4 and Q5 independently represent, on each occasion when used herein:
halo, -CN, -NO2, -N(R20)R21, -OR20, -C(=Y)-R20, -C(=Y)-OR20,
-C(=Y)N(R20)R2', -OC(=Y)-R20, -OC(=Y)-OR20, -OC(=Y)N(R20)R21, -OS(O)20R20,
-OP(=Y)(OR20)(OR21), -OP(OR20)(OR21), -N(R22)C(=Y)R21, -N(R22)C(=Y)OR21,
-N(R22)C(=Y)N(R20)R21, -NR22S(O)2R20, -NR22S(O)2N(R20)R21, -S(O)2N(R20)R21,
-SC(=Y)R20, -S(0)2R20, -SR20, -S(O)R20, C1_6 alkyl or heterocycloalkyl (which
latter
two groups are optionally substituted by one or more substituents selected
from
=0 and J2);
each R20, R21, R22 and R23 independently represent hydrogen, C..6 alkyl or
heterocycloalkyl (which latter two groups are optionally substituted by one or
more substituents selected from J4 and =O).
Further preferred compounds of the invention that may be mentioned include
those in which:
an R' group may not be substituted by an aromatic ring (e.g. a heteroaryl
group);
B1, B2 and/or B3 (e.g. B1) do not represent or do not contain aromatic (e.g.
heteroaromatic) groups (especially when R1 represents an (a) group, i.e.
-N(R'a)RIb);
B1, B2 and B3 (e.g. B1) independently represent aryl (optionally substituted
by one
or more substituents selected from E9) or, preferably, halo, -NO2, -CN,
-N(R1oa)R11a -OR10a -C(=Y)-R1oa -C(=Y)-OR1oa -C(=Y)N(R'Oa)Rlla
-N(R12a)C(=Y)R11a, -N(R12a)C(_Y)OR'1a, -N(R12a)C(`Y)N(R1oa)R11a
-NR 12aS(0)2R1oa -NR 12aS(O)2N(R1oa)R'la -S(0)2N(R10a)R1la -SC(=Y)R1oa
-SC(=Y)OR10a -S(O)2R10a, C1.12 alkyl or heterocycloalkyl (which latter two
groups
are optionally substituted by one or more substituents selected from =0 and
E),
22
-0&26
WO 2010/119264 PCT/GB2010/000773
or, two B' substituents may be linked together (to form a spiro-cycle) as
herein
defined (this is especially the case when R' represents an (a) group, i.e.
-N(Rla)R1b);
each R1 0a R11a, R12a R1 0b R'1b and R12b (for example when a B' substituent
is
present containing such a moiety) independently represent, on each occasion
when used herein, aryl (optionally substituted by one or more substituents
selected from E") or, preferably, hydrogen, C1_12 alkyl or heterocycloalkyl
(which
latter two groups are optionally substituted by one or more substituents
selected
from =O, =S, =N(R20) and E10); or any relevant pair of R10a R11 and R12a
and/or
any pair of R'Ob and R11b may be linked together as defined herein (and
therefore
be substituted by one or more E12 groups), but wherein when there are one or
more E'0, E11 and/or E12 groups present, which independently represent Q4 or
C1_12 alkyl optionally substituted by one or more substituents selected from
=0
and Q5, then those Q4 and Q5 groups do not represent, or contain, aromatic
(e.g.
heteroaryl) rings, i.e. then:
each Q4 and Q5 independently represent, on each occasion when used herein:
aryl (optionally substituted by one or more substituents selected from J) or,
preferably, halo, -CN, -NO2, -N(R20)R21, -OR20, -C(=Y)-R20, -C(=Y)-OR20,
-C(=Y)N(R20)R21, -OC(=Y)-R20, -OC(=Y)-OR20, -OC(=Y)N(R20)R21, -OS(O)2OR20,
-OP(=Y)(0R20)(OR21), -OP(OR20)(OR21), -N(R22)C(=Y)R21, -N(R22)C(=Y)OR21,
-N(R22)C(=Y)N(R20)R21, -NR 22S(O)2R20, -NR 22S(O)2N(R20)R21, -S(O)2N(R20)R21
-SC(=Y)R20, -S(0)2R20, -SR20, -S(O)R20, C1.6 alkyl or heterocycloalkyl (which
latter
two groups are optionally substituted by one or more substituents selected
from
=0 and J2);
each R20, R21, R22 and R23 independently represent hydrogen, C1.6 alkyl or
heterocycloalkyl (which latter two groups are optionally substituted by one or
more substituents selected from J4 and =O);
R'a and R'b are preferably linked together to form a 5- or 6-membered ring as
defined herein;
when R' represents an (a) group, then, preferably, the ring formed by the
linkage
of the R1a and R1b groups contains at least one (further) heteroatom (in
addition to
the requisite nitrogen atom to which R1a and R'b are necessarily attached) as
defined herein;
when R' represents an (a) group, then the ring so formed (by the linkage of
the
R'a and R'b groups), preferably does not represent a 1-pyrrolidinyl or 1-
piperidinyl
23
-0&26
WO 2010/119264 PCT/GB2010/000773
ring (e.g. one in which that ring is substituted at the 2-position, by for
example an
a methyl group substituted by an amino moiety (a methylamino group, i.e.
substituted by B1, in which B' represents methyl substituted by E8, in which
E8
represents Q4 and Q4 represents -N(R20)R21) or, in which that ring is
substituted at
the 3-position by an amino moiety (i.e. by B1, in which B1 represents -
N(R'oa)R11a)
Further compounds of the invention that may be mentioned include those in
,which for example when R1 represents an (a) group (e.g. in which R1a and R1b
are
linked together to form a 5- or 6-membered ring, e.g. a 1-pyrrolidinyl or 1-
piperidinyl group), then preferably:
such groups are unsubstituted (i.e. do not contain any B1 groups);
when substituted (e.g. at the 3-position or, especially, at the 2-position
with a B1
group), then:
B1 preferably represents -N(R1oa)R11a, or, preferably, halo, -NO2, -CN,
-OR1oa -C(=Y)-R1oa -C(=Y)-OR' oa -C(=Y)N(R10a)R11a -N(R12a)C(=Y)Rlla
-N(R12a)C(_Y)OR1a -N(R12a)C(_Y)N(R1oa)R11a -NR 12aS (0)2R' 0a,
-NR 12aS(0)2N(R1oa)R11a, -S(O)2N(R10a)R1la -SC(=Y)R1oa -SC(=Y)OR1oa,
-S(O)2R'oa, heterocycloalkyl (optionally substituted by one or more
substituents selected from =0 and E8), aryl or heteroaryl (which latter two
groups are optionally substituted by one or more substituents selected
from E9);
when B' represents optionally substituted 01.12 alkyl (e.g. methyl), then
either that alkyl group is unsubstituted or, when substituted by one or
more =0 and/or E8 groups, then when E8 represents Q4, Q4 represents
halo, -CN, -NO2, -OR20, -C(=Y)-R20, -C(=Y)-OR20,
-C(=Y)N(R20)R21, -OC(=Y)-R20, -OC(=Y)-OR20, -OC(=Y)N(R20)R21,
-OS(O)2OR20, -OP(=Y)(OR20)(OR21), -OP(OR20)(OR21), -N(R22)C(=Y)R21
-N(R22)C(=Y)OR21, -N(R22)C(=Y)N(R2D)R21, -NR 22S(0)2R 20,
-NR 22S(O)2N(R2))R21, -S(O)2N(R20)R21, -SC(=Y)R20, -S(0)2R 20, -SR 20,
-S(O)R20, C1_6 alkyl, heterocycloalkyl (which latter two groups are
optionally substituted by one or more substituents selected from =0 and
J2), aryl or heteroaryl (which latter two groups are optionally substituted by
one or more substituents selected from J);
when substituted (e.g. at the 3-position with a B1 group), then:
24
0&26
WO 2010/119264 PCT/GB2010/000773
B1 preferably represents halo, -NO2, -CN, -OR10a, -C(=Y)-R'oa,
-C(=Y)-ORboa -C(=Y)N(Rboa)R'1a -N(R12a)C(=Y)R11a -N(R12a)C(=Y)ORIla
-N(R12a)C(_Y)N(R1oa)R11a, -NR 12aS(O)2R1oa -NR 12aS(O)2N(R1oa)R11a,
-S(O)2N(R1oa)R11a, -SC(=Y)R1oa, -SC(=Y)OR1oa, -S(O)2R1oa C1-12 alkyl,
heterocycloalkyl (which latter two groups are optionally substituted by one
or more substituents selected from =0 and E8), aryl or heteroaryl (which
latter two groups are optionally substituted by one or more substituents
selected from E).
Further preferred compounds of the invention that may be mentioned include
those in which:
at least one of R2 and R3 does not represent an aromatic group (i.e. an
optionally
substituted aryl or heteroaryl group);
at least one of R2 and R3 represents hydrogen or a substituent as defined
herein,
but wherein each Q1 and, optionally, Q2 are independently selected from halo,
-CN, -NO2, -N(R1oa)R11a, -OR10a, -C(=Y)-R1oa, -C(=Y)-OR1oa -C(=Y)N(R1oa)R11a
-OC(=Y)-R1oa -OC(=Y)-OR1oa, -OC(=Y)N(R1oa)R11a, -OS(O)2OR1oa
-OP(=Y)(OR1oa)(OR11a), -OP(OR1oa)(OR11a) -N(R12a)C(=Y)R11a
-N(R12a)C(_Y)OR11a, -N(R12a)C(_Y)N(R1oa)R11a, -NR12aS(O)2R1oa
-NR12aS(O)2N(R1oa)R11a, -S(O)2N(Rloa)R11a, -SC(=Y)R1oa, -S(O)2R1oa, -SR1oa,
-S(O)R10a, C1.12 alkyl or heterocycloalkyl (which latter two groups are
optionally
substituted by one or more substituents selected from =0, =S, =N(R10a) and E),
or a fragment of formula IA as defined hereinbefore;
more preferably neither R2 or R3 represent an aromatic group, i.e. these
groups
preferably represent hydrogen or a substituent as defined above.
Further preferred compounds of the invention that may be mentioned include
those in which, for example, when R1 represents amino (i.e. a group defined by
(a) above) or, in particular, when R1 represents a group defined by (c) above
(in
particular pyridyl or imidazolyl), then:
R5 does not represent optionally substituted phenyl, naphthyl, indolyl,
thiophenyl,
benzo[b]furanyl, benzo[b]thiophenyl, isoxazolyl, or:
O b~o O> ` ~ O
\ I
/ O /
and/or
-0&26
WO 2010/119264 PCT/GB2010/000773
R2 does not represent C1-C12 alkyl, -C(O)-O-R' 0a, -C(O)-N(R1oa)R11a and/or R3
does not represent H or C1-C6 alkyl; and/or
R2 represents hydrogen or Q1 (most preferably R2 represents hydrogen), in
which
Q1 is preferably selected from halo, -CN, -NO2, -N(R10a)R11a, -OR10a, -C(=Y)-
R10a,
-OC(=Y)-R10a, -OC(=Y)-OR10a, -OC(=Y)N(R1oa)R11a -OS(O)2OR10a,
OP(=Y)(OR1oa)(OR' 1a) -OP(OR10a)(OR'1a) _N(R12a)C(_Y)R11a
N(R12a)C(_Y)OR11a -N(R12a)C(_Y)N(R1oa)R1la -NR 12aS(O)2R1oa
-NR 12aS(O)2N(R1oa)R1la _S(O)2N(R1oa)R11a -SC(=Y)R1oa, _S(O)2R1oa -SR10a
-S(O)R1 0a heterocycloalkyl (optionally substituted by one or more
substituents
selected from =O, =S, =N(R1oa) and E6), aryl or heteroaryl (which latter two
groups are optionally substituted by one or more substituents selected from
E7);
R3 represents C7_12 alkyl (optionally substituted by one or more substituents
selected from =O, =S, =N(R10a) and Q), or R3 more preferably represents
hydrogen or Q1, in which Q1 preferably represents C7.12 alkyl (optionally
substituted by one or more substituents selected from =0, =S, =N(R10a) and E)
and in which Q1 more preferably represents halo, -CN, -NO2, -N(R1oa)R1la, -
OR10a,
-C(=Y)-R10a - C(=Y)-OR' oa - C(=Y)N(R1oa)R11a, -OC(=Y)-R1oa, - OC(=Y)-OR' oa
-OC(=Y)N(R1oa)R1a, -OS(O)20R1oa, _OP(=Y)(OR1oa)(OR11a), -OP(OR1oa)(OR11a),
-N(R12a)C(_Y)R11a, -N(R12a)C(_Y)OR11a, -N(R12a)C(_Y)N(R1oa)R1la,
-NR 12aS(O)2R1oa, -NR 12aS(O)2N(R1oa)R1la, -S(0)2N(R1oa)R1la, _SC(=Y)R10a,
-S(0)2R' Oa, -SR10a, -S(O)R10a, heterocycloalkyl (optionally substituted by
one or
more substituents selected from =0, =S, =N(R1Oa) and E), aryl or heteroaryl
(which latter two groups are optionally substituted by one or more
substituents
selected from E7); or R2 or R3 may represent a fragment of formula IA.
Preferred compounds of the invention that may be mentioned include those in
which, when R1 represents -N(Rla)R1b, then:
R1a and R1b are preferably linked together to form a saturated 5-, 6- or 7-
membered ring, optionally containing one further heteroatom (selected from
nitrogen or, preferably oxygen and sulfur), optionally substituted by one or
more
=0 substituents (especially so in the case where there is a sulfur atom
present,
which may be substituted with one or two =0 groups) and/or B1 substituents;
the ring formed when R1a and R1b are linked together may, when there is a
sulfur
atom present, be substituted by one or two =0 groups (e.g. such that the ring
26
-0&26
WO 2010/119264 PCT/GB2010/000773
contains a -S(O)- or -S(O)2- moiety), and, further, such a ring is preferably
not
substituted with any B' substituents;
when two B' groups are linked together to form a spiro-cyclic group, then such
a
cyclic moiety is preferably a saturated 3- to 7- (e.g. 3- to 6-) membered
ring,
optionally containing a heteroatom (but which ring is preferably carbocyclic;
and
may be substituted by one or more fluoro, =0 and methyl groups) and which
group is preferably attached to the 2- or 3-position of the ring formed by the
linkage of the integers R'a and R'b (for example, when R'a and Rib are linked
together to form a morpholinyl group);
the linkage of the R'a and R'b group preferably forms a N-morpholinyl, N-
thiomorpholinyl (in which the sulfur atom may be substituted with one or two
=0
groups), oxazepanyl (e.g. 1,4-oxazepanyl) or thiazepanyl (e.g. 1,4-
thiazepanyl)
group, all of which are optionally substituted as hereinbefore defined (e.g.
by one
or more =0 and/or B' substituents).
In certain embodiments, the present invention provides compounds of the
invention in which R' is typically:
N-morpholinyl which is unsubstituted or substituted, for instance by one or
more
B' and/or =0 substituents;
tetrahydropyranyl, tetrahydrofuranyl or C-morpholinyl, which is unsubstituted
or
substituted, for instance by one or more B2 and/or =0 substituents;
2-, 3- or 4-pyridyl, which is unsubstituted or substituted, for instance by
one or
more B3 substituents;
when R1 represents substituted morpholinyl, it is preferably selected from the
following structures:
27
-0&26
WO 2010/119264 PCT/GB2010/000773
C0 0rOH 0 0 (NH2 CO)NH2 co
N N N N o N -tw 411 -4-1 -4w 411 4W.
O
O N \ O (0)"ro" (OO
~H I / C ~ p N)\/\p
4- 1 411
O JOf
C N~ N Cr
N N~ N
-4- 4111 4A, 4&
O No J00'~'
4k 4-
In certain embodiments, the present invention provides compounds of the
invention in which:
R2 is -(CR6R7)mNR10R11 where m is 1, 2 or 3, and R10 and R" together with the
nitrogen to which they are attached form the C3-C20 heterocyclic ring; and R3
is H,
halo, CN, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R'6R17),
OR16 or NR'6R17;
R2 is -(CR6R7)nNR12S(O)2R10 where n is 1 or 2; R12, R6, and R7 are
independently
selected from H and C1-12 alkyl; and R10 is C1-C12 alkyl or C6-C20 aryl; and
R3 is H,
halo, CN, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17),
OR16 or NR16R17;
R2 is -(CR6R7)nOR10 where n is 1 or 2, and R10, R6 are independently selected
from H and C1-12 alkyl; and R3 is H, halo, CN, C1-6 alkyl, C3-C6 cycloalkyl,
C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R2 is -(CR6R7)nS(O)2R10 where n is 1 or 2; and R6, and R7 are H, R10 may be C1-
12
alkyl or C6-C20 aryl; and R3 is H, halo, CN, C1-6 alkyl, C3-C6 cycloalkyl, C2-
C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R2 is -(CR6R7)nS(O)2NR10R11 where n is 1 or 2; and R6, and R7 are H; and R3 is
H,
halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17)
OR16 or NR16R17;
28
-0&26
WO 2010/119264 PCT/GB2010/000773
R2 is -C(=Y)NR10R11 where Y is 0, and R10 and R" together with the nitrogen to
which they are attached form the C2-C20 heterocyclic ring. R10 and R11
together
with the nitrogen to which they are attached may form a C2-C20 heterocyclic
ring
selected from morpholinyl, piperidinyl, piperazinyl, and pyrrolidinyl; and R3
is H,
halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17)
OR16 or NR16R17;
R2 is -C(=Y)NR10R11 where Y is 0, and R10 and R11 are independently selected
from H and C1-C12 alkyl; and R3 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl,
C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R2 is -C(=Y)NR10R11 where Y is 0, and R10 and R11 are independently selected
from H and C3-C12 carbocyclyl, C2-C20 heterocyclyl, C6-C20 aryl, and C1-C20
heteroaryl; and R3 is H, halo, CN, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R2 is -NHR12 where R12 is C3-C12 carbocyclyl, C2-C20 heterocyclyl, C6-C20 aryl
or
C1-C20 heteroaryl, or, R12 may be phenyl or 4-pyridyl; and R3 is H, halo, CN,
C1-6
alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17), OR16 or
NR16R17;
R2 is -NR 12C(=Y)R11 where Y is 0, R12 is H or C1-C12 alkyl, and R11 is C1-C12
alkyl, C3-C12 carbocyclyl, C2-C20 heterocyclyl, C6-C20 aryl, or C1-C20
heteroaryl;
and R3 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl,
C(O)N(R16R17), OR16 or NR16R17;
R2 is -NR12S(O)2R10 where R12 is H or C1-C12 alkyl, and R10 is C1-C12 alkyl,
C3-C12
carbocyclyl, C2-C20 heterocyclyl, C6-C20 aryl, or C1-C20 heteroaryl; and R3 is
H,
halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17),
OR16 or NR16R17;
R2 is -S(O)2NR1oR11 where R10 and R11 together with the nitrogen to which they
are attached form a C2-C20 heterocyclyl ring selected from morpholinyl,
piperidinyl, piperazinyl, and pyrrolidinyl; and R3 is H, halo, ON, C1-6 alkyl,
C3-C6
cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R2 is -S(O)2NR10R11 where R10 and R11 are independently selected from H and
C1-C12 alkyl; R10 and R11 may be independently selected from H, substituted
ethyl,
and substituted propyl; and R3 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl,
C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R2 is C1-C12 alkyl, and R3 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
29
0&26
WO 2010/119264 PCT/GB2010/000773
R2 is C2-C8 alkenyl, and R3 is H, halo, CN, C1-6 alkyl, C3-C6 cycloalkyl, C2-
C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R2 is C2-C8 alkynyl (the C2-C8 alkynyl may be substituted with C2-C20
heterocyclyl,
which includes, but is not limited to, morpholinyl, piperidinyl, piperazinyl,
and
pyrrolidinyl); and R3 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R2 is C6-C20 aryl, such as phenyl; and R3 is H, halo, ON, C1-6 alkyl, C3-C6
cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17) OR16 NR16R17;
R2 is C3-C12 carbocyclyl; and R3 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl,
C2-C6
heterocycloalkyl, C(O)N(R16R17), OR'6 or NR16R17;
R2 is C2-C20 heterocyclyl; and R3 is H, halo, ON, C1-6 alkyl, C3-C6
cycloalkyl, C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R2 is C1-C20 heteroaryl; and R3 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl,
C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R2 is H;
R2 is methyl (CH3), cyclopropyl, CF3, CN or CONH2.
In certain embodiments of the invention:
R3 is -(CR6R7)mNR10R11 where m is 1, 2 or 3, and R10 and R11 together with the
nitrogen to which they are attached form the C3-C20 heterocyclic ring; and R2
is H,
halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17),
OR16 or NR16R17;
R3 is -(CR6R7)nNR12S(O)2R10 where n is 1 or 2; R12, R6, and R7 are
independently
selected from H and C1-12 alkyl; and R10 is C1-C12 alkyl or C6-C20 aryl; and
R2 is H,
halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17),
OR16 or NR16R17;
R3 is -(CR6R7)nOR10 where n is 1 or 2, and R10, R6 are independently selected
from H and C1-12 alkyl; and R2 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl,
C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R3 is -(CR6R7)nS(O)2R10 where n is 1 or 2; and R6, and R7 are H, R10 may be C1-
12
alkyl or C6-C20 aryl; and R2 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-
C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R3 is -(CR6R7)nS(O)2NR10R1'where n is 1 or 2; and R6, and R7 are H; and R2 is
H,
halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17)
OR16 or NR16R17;
-0&26
WO 2010/119264 PCT/GB2010/000773
R3 is -C(=Y)NR'0R" where Y is 0, and R10 and R" together with the nitrogen to
which they are attached form the C2-C20 heterocyclic ring; R10 and R" together
with the nitrogen to which they are attached may form a C2-C20 heterocyclic
ring
selected from morpholinyl, piperidinyl, piperazinyl, and pyrrolidinyl; and R2
is H,
halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17),
OR16 or NR16R17;
R3 is -C(=Y)NR10R11 where Y is 0, and R10 and R11 are independently selected
from H and 1-C12 alkyl; and R2 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl,
C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R3 is -C(=Y)NR10R11 where Y is 0, and R10 and R11 are independently selected
from H and C3-C12 carbocyclyl, C2-C20 heterocyclyl, C6-C20 aryl, and C1-C20
heteroaryl; and R2 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R3 is -NHR12 where R12 is C3-C12 carbocyclyl, C2-C20 heterocyclyl, C6-C20 aryl
or
C1-C20 heteroaryl, or, R12 may be phenyl or 4-pyridyl; and R2 is H, halo, ON,
C1-6
alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17), OR16 or
NR16R17;
R3 is -NR 12C(=Y)R11 where Y is 0, R12 is H or C1-C12 alkyl, and R11 is C1-C12
alkyl, C3-C12 carbocyclyl, C2-C20 heterocyclyl, C6-C20 aryl, or 1-C20
heteroaryl;
and R2 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl,
C(O)N(R16R17), OR16 or NR16R17;
R3 is -NR12S(O)2R10 where R12 is H or C1-C12 alkyl, and R10 is C1-C12 alkyl,
C3-C12
carbocyclyl, C2-C20 heterocyclyl, C6-C20 aryl, or 1-C20 heteroaryl; and R2 is
H,
halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17),
OR16 or NR16R17;
R3 is -S(O)2NR10R11 where R10 and R11 together with the nitrogen to which they
are attached form a C2-C20 heterocyclyl ring selected from morpholinyl,
piperidinyl, piperazinyl, and pyrrolidinyl; and R2 is H, halo, ON, C1-6 alkyl,
C3-C6
cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R3 is -S(O)2NR10R11 where R10 and R'1 are independently selected from H and
C1-C12 alkyl. R1 and R11 may be independently selected from H, substituted
ethyl,
and substituted propyl; and R2 is H, halo, ON, 1-6 alkyl, C3-C6 cycloalkyl, C2-
C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R3 is 1-C12 alkyl, and R2 is H, halo, ON, 1-6 alkyl, C3-C6 cycloalkyl, C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
31
0&26
WO 2010/119264 PCT/GB2010/000773
R3 is C2-C8 alkenyl, and R2 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl, C2-
C6
heterocycloalkyl, C(O)N(R'6R17), OR16 or NR16R17;
R3 is C2-C8 alkynyl (the C2-C$ alkynyl may be substituted with C2-C20
heterocyclyl,
which includes, but is not limited to, morpholinyl, piperidinyl, piperazinyl,
and
pyrrolidinyl); and R2 is H, halo, CN, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R3 is C6-C20 aryl, such as phenyl; and R2 is H, halo, ON, Cl-,, alkyl, C3-C6
cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R3 is C3-C12 carbocyclyl; and R2 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl,
C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R3 is C2-C20 heterocyclyl; and R2 is H, halo, ON, C1-6 alkyl, C3-C6
cycloalkyl, C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17;
R3 is C1-C20 heteroaryl; and R2 is H, halo, ON, C1-6 alkyl, C3-C6 cycloalkyl,
C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16 or NR16R17.
In the above two paragraphs (and in certain other paragraphs herein), any
relevant alkyl, cycloalkyl, heterocycloalkyl, aryl or heteroaryl groups may be
optionally substituted by relevant substituents defined herein (for example,
by a
substituent defined by Q1, Q2, E6, E7, Q4, Q5, J2 or J3 (e.g. by Q1, E6 and/or
E7)).
Further, unless otherwise specified in the above two paragraphs:
(i) each R16 and R17 respectively represents substituents R20 and R21 as
defined
herein (and more preferably, they respectively represent substituents R50 and
R51
as defined herein);
(ii) each R6 and R7 may independently represent a substituent as defined by
R15
herein (i.e. each may independently represent hydrogen, a substituent as
defined
herein, or, R6 and R7 may be linked together in the same manner as two R15
groups attached to the same carbon atom may be);
(iii) each R10, R11 and R12 respectively represents a substituent as defined
by the
substituents R1oa R11a and R12a
In certain embodiments, R2 or R3 represent a fragment of formula IA, as
hereinbefore depicted, wherein:
Ra and Rb form, together with the N atom to which they are attached, a group
of
the following formula:
32
-0&26
WO 2010/119264 PCT/GB2010/000773
0-
in which:
(a) ring A is a first 3- to 7-membered saturated N-containing heterocyclic
ring
which is fused to a second ring as hereinbefore defined to form a
heteropolycyclic ring system in which the first ring is selected from, but not
limited to, azetidine, pyrrolidine, piperidine, piperazine, morpholine,
thiomorpholine and homopiperazine, said group being fused to a second
ring as hereinbefore defined. The second ring is typically a 3- to 7-
membered saturated N-containing heterocyclic ring as defined above in
respect of the first ring, the second ring is a 5- to 12-membered
unsaturated heterocyclic group. More typically the second ring is a 5-, 6-
or 7- membered saturated N-containing heterocyclic ring or a 5- to 7-
membered unsaturated heterocyclic ring. Typical examples of the second
ring include azetidine, pyrrolidine, piperidine, piperazine, morpholine,
thiomorpholine, homopiperazine, pyrrole, imidazole, pyridine, pyridazine,
pyrimidine, pyrazine, tetrahydrofuran and tetrahydropyran. Examples of
the resulting heteropolycyclic system include octahydropyrrolo[1,2-
a]pyrazine and octahydro-pyrrolo[3,4-c]pyrrole. Specific examples of the
heteropolycyclic system include the following structures:
H N
N O
HN
(:~~
N~~ CN N
IrK
(b) ring A is a first 3- to 7-membered saturated N-containing heterocyclic
group as hereinbefore defined (which contains a linker group), which
includes, but is not limited to, a bridgehead group (i.e. a linker group
linking any two non-adjacent atoms of the first ring), thereby forming, for
example 3,8-diaza-bicyclo[3.2.1 ]octane, 2,5-diaza-bicyclo [2.2.1]heptane,
8-aza-bicyclo[3.2.1 ]octane, 2-aza-bicyclo[2.2.1 ]heptane, 3,6-diaza-
bicyclo[3. 1. 1 ]heptane, 6-aza-bicyclo[3. 1.1 ]heptane, 3, 9-diaza-
bicyclo[4.2.1]nonane and/or 2-oxa-7,9-diazabicyclo[3.3.1]nonane. Specific
examples of this group include the following structures:
33
-0&26
WO 2010/119264 PCT/GB2010/000773
HN7N - 4N7NH ~TN-~ N, N-- N~N4- O~ N N+
(c) ring A is a first 3- to 7-membered saturated N-containing heterocyclic
group as hereinbefore defined, which is spiro-fused at any available ring
carbon atom to a second 3- to 12- membered saturated carbocyclic ring,
typically to a 3- to 6- membered saturated carbocyclic ring, or to a 4- to 7-
membered saturated N-containg heterocyclic group. Examples include a
group in which the first ring is selected from azetidine, pyrrolidine,
piperidine and piperazine which is spiro-fused at a ring carbon atom to a
second ring selected from cyclopropane, cyclobutane, cyclopentane,
cyclohexane, azetidine, pyrrolidine, piperidine, piperazine and
tetrahydropyran. The ring so formed may, for instance, be a group
derived from 3,9-diazaspiro[5.5]undecane, 2,7-diazaspiro[3.5]nonane, 2,8-
diazaspiro[4.5]decane or 2,7-diazaspiro[4.4]nonane. Specific examples of
this group include the following structures:
ONH N~. PN
N
N N
H
P
Nj~ N N
H H N H O
N O N N-~
HN\~--~ Nf ~CN+N N- pN
H
N
In certain embodiments, R2 represent a fragments of formula IA as depicted
hereinbefore, in which Ra and Rb are as described above; and R3 is H, halo,
CN,
C1-6 alkyl, C3-C6 cycloalkyl, C2-C6 heterocycloalkyl, C(O)N(R16R17), OR'6,
NR16R17. The integers R16 and R17 are as defined herein.
In certain embodiments, R3 represent a fragment of formula IA as depicted
hereinbefore; and R2 is H, halo, CN, C1-6 alkyl, C3-C6 cycloalkyl, C2-C6
heterocycloalkyl, C(O)N(R16R17), OR16, NR16R17. The integers R16 and R17 are
as
defined herein.
34
-0&26
WO 2010/119264 PCT/GB2010/000773
Exemplary embodiments of R5 include, but are not limited to: pyrrole,
pyrazole,
triazole, tetrazole, thiazole, isothiazole, oxazole, isoxazole, isoindole, 1,3-
dihydro-
indol-2-one, pyridine-2-one, pyridine, pyridine-3-ol, imidazole, I H-indazole,
1 H-
indole, indolin-2-one, 1-(indolin-1-yl)ethanone, pyrimidine, pyridazine,
pyrazine
and isatin groups. 1 H-benzo[d][1,2,3]triazole, 1 H-pyrazolo [3,4-b]pyridine,
1 H-
pyrazolo[3,4-d]pyrimidine, 1 H-benzo[d]imidazole, 1 H-benzo[d]imidazol-2(3H)-
one, 1 H-pyrazolo[3,4-c]pyridine, 1 H-pyrazolo[4,3-d]pyrimidine, 5H-
pyrrolo[3,2-
d]pyrimidine, 2-amino-1 H-purin-6(9H)-one, quinoline, quinazoline,
quinoxaline,
isoquinoline, isoquinolin-1(2H)-one, 3,4-dihydroisoquinolin-1 (2H)-one, 3,4-
dihydroquinolin-2(1 H)-one, quinazolin-2(1 H)-one, quinoxalin-2(1 H)-one, 1,8-
napthyridine, pyrido[3,4-d]pyrimidine, and pyrido[3,2-b]pyrazine, 1,3-dihydro
benzimidazolone, benzimidazole, benzothiazole and benzothiadiazole, groups.
These groups may be unsubstituted or substituted.
The attachment site of the R5 group to the C-6 position of the requisite
imidazopyrazine ring of formula I may be at any carbon (carbon-linked) of the
R5
group (e.g. fused bicyclic C4-C20 heterocyclyl or fused bicyclic CI-C20
heteroaryl
group R5 group).
More exemplary embodiments of R5 include, but are not limited to, the
following
groups, where the wavy line indicates the site of attachment to the pyrazine
ring:
-0&26
WO 2010/119264 PCT/GB2010/000773
0
O N _
O N N NH NH NH
\ NH I \ NH I ~ I \
O N==N N
N N H N
N N N
sN N 0
NH N NH HN
H
Y Y
N N NI NON
IN N O
HN-N HN \ HN
NH NH
N N=\
O O NH l NH
N NH
I ~ N
N y 0 Ns / O
NH NH ~INH
/ \ NH NH NH
N
\ N N N~ N
N N N NH
iN / I \
N N
0
HN-N HN HN
NNH \ NH I \ / \
NON NON
0
N HN \ HN HN
NH NH I \ \
NN/
N
0 N
NNH NH NH / II N II
\ / \ \ N \ N N
O
NNH NH i / NH NH
NH
36
-0&26
WO 2010/119264 PCT/GB2010/000773
Preferred compounds of the invention include those in which:
R1 represents:
(a) -N(R1a)R1b, in which R" and RIb are linked together to form, together with
the nitrogen atom to which they are necessarily attached, a 5- to 7-
membered ring, optionally containing a further one or two heteroatoms
(e.g. selected from nitrogen, oxygen and sulfur), optionally containing one
or two double bonds, and which ring is optionally substituted by one or
more (e.g. by one to three) substituents selected from =0 and B1;
(b) a heterocycloalkyl (e.g. a 3- to 7-membered) heterocycloalkyl group
(attached to the requisite imidazopyrazine via a carbon atom), optionally
substituted by one or more (e.g. by one to three) substituents selected
from =0 and B2;
(c) a monocyclic heteroaryl group optionally substituted by one or more (e.g.
by one to three) substituents selected from B3;
when R1 represents . optionally substituted heterocycloalkyl, then that
heterocycloalkyl group preferably contains 1, 2 or 3 heteroatoms preferably
selected from nitrogen, oxygen and sulfur;
when R1 represents optionally substituted monocyclic heteroaryl, then that
heteroaryl group preferably contains 1, 2, 3 or 4 nitrogen heteroatoms and,
optionally, 1 or 2 additional heteroatoms preferably selected from nitrogen,
oxygen and sulfur;
R2 and R3 may represent a fragment of formula IA, although R2 and R3 more
preferably, and independently, represent C1_12 (e.g. C1.6) alkyl optionally
substituted by one or more substituents selected from =0 and Q2, or, R2 and R3
more preferably represent a substituent selected from Q1;
m represents 0, 1 or 2;
each R15 represents hydrogen or C1_6 (e.g. C1.3) alkyl, which latter group is
preferably unsubstituted;
when Ra and Rb are linked together, they form a first 5- or 6-membered cyclic
group, optionally containing one further heteroatom selected from nitrogen,
sulfur
and oxygen, which cyclic group is optionally substituted by one or more
substituents selected from =0 and, preferably, E3;
R4 represents hydrogen or a substituent selected from halo, -CN, -OR1 b,
-N(R1ob)R11b C1.6 alkyl and/or heterocycloalkyl (e.g. a 5- or 6-membered
37
-0&26
WO 2010/119264 PCT/GB2010/000773
heterocycloalkyl), which latter two groups are optionally substituted by one
or
more substituents selected from E4 and =0;
when R4 represents heterocycloalkyl, then it is preferably a 5- or 6-membered
heterocycloalkyl group containing one or two heteroatoms preferably selected
from nitrogen, oxygen and sulfur, which group is optionally substituted by one
or
more substituents selected from E4 and =0;
when R4 represents C1_6 alkyl, then that group is preferably an acyclic C1
alkyl
group, optionally substituted by one or more substituents selected from E4 and
=0;
when R5 represents aryl (e.g. phenyl), then that group may be unsubstituted
but
is preferably substituted by at least one (e.g. two or, preferably, one)
substituent(s) selected from E5;
when R5 represents monocyclic heteroaryl (e.g. a 5- or 6-membered heteroaryl
group), then that group preferably contains 1, 2, 3 or 4 nitrogen atoms and,
optionally 1 or 2 additional heteroatoms selected from oxygen and sulfur, and
which heteroaryl group is optionally substituted by one or more substituents
selected from E5;
when R5 represents bicyclic heteroaryl (e.g. a 8-, 9- or 10-membered
heteroaryl
group), then that group preferably consists of a 5- or 6-membered ring fused
to
another 5- or 6-membered ring (in which either one of those rings may contain
one or more (e.g. four, or, preferably one to three) heteroatoms), in which
the
total number of heteroatoms is preferably one to four, and which ring is
optionally
substituted by one or more (e.g. two or, preferably, one) substituent(s)
selected
from E5 (and, if there is a non-aromatic ring present in the bicyclic
heteroaryl
group, then such a group may also be substituted by one or more (e.g. one) =0
groups);
optional substituents (e.g. the first optional substituent) on the R5 group
(e.g.
when it represents aryl, such as phenyl) are preferably selected from -OR, -
SR,
-CH2OR, C02R, CF2OH, CH(CF3)OH, C(CF3)20H, -(CH2),NOR, -(CH2),NNR2i
-C(O)N(R)2i -NR2, -NRC(O)R, -NRC(O)NHR, -NRC(O)N(R)2i -S(O)yN(R)2,
-OC(O)R, OC(O)N(R)2, -NRS(O)yR, -NRC(O)N(R)2i ON, halogen and -NO2 (in
which each R is independently selected from H, Cj-C6 alkyl, C3-C1o cycloalkyl
and
a 5- to 12-membered aryl or heteroaryl group, the groups being unsubstituted
or
substituted (for example by one or more substituents as defined herein, e.g.
38
-0&26
WO 2010/119264 PCT/GB2010/000773
substituents on E5 moieties, e.g. =O, J2, J3, J4 and/or J5), w is 0, 1 or 2
and y is 1
or 2);
when R5 represents aryl (e.g. phenyl), then that group is substituted by one
or two
substituents (e.g. by a first substituent as defined above, and, optionally a
further
substituent (or a further two substituents) preferably selected from halo, C1-
12
alkyl, CN, NO2, ORd, SRd, NRd2, C(O)Rd, SORd, SO2Rd, SO2N(R)d2, NC(O)Rd and
CO2Rd (wherein each Rd is independently H or C1-C6 alkyl);
when R5 represents substituted aryl (e.g. phenyl), the the substituent may be
situated at the 2-, 3-, 4-, 5- or 6- position of the phenyl ring (typically it
is situated
at position 3 or 4; particularly preferred are phenyl groups substituted by -
OR d (in
which Rd is independently H or C1-C6 alkyl, e.g. methyl), e.g. -OH; in this
embodiment the -OR d group, or -OH group, is typically situated at the 3- or 4-
position of the phenyl ring, so forming a 3-hydroxyphenyl or 4-hydroxyphenyl
group or an isostere thereof, which is unsubstituted or substituted; an
isostere as
used herein is a functional group which possesses binding properties which are
the same as, or similar to, the 3-hydroxyphenyl or 4-hydroxyphenyl group in
the
context of the compounds of the invention; isosteres of 3-hydroxyphenyl and 4-
hydroxyphenyl groups are encompassed within definitions above for R5);
when R5 represents heteroaryl, it is unsubstituted or substituted (when
substituted, it may be substituted by one or more substitutents selected from
those listed in respect of substituents on R5, when R5 is a phenyl group;
typically,
the substituents are selected from OH and NH2);
each Q1 and Q2 independently represent -S(O)2R10a, -SR10a, -S(O)R1oa,
-S(0)2N(R1oa)R1la, or, preferably, halo, -CN, -NO2, -N(R1oa)R11a, -OR' Oa,
-C(=Y)-R1oa -C(=Y)-OR' oa, -C(=Y)N(R10a)R11a, -N(R12a)C(=Y)R1la,
-NR 12aS(O)2R10a, C1-12 (e.g. C1-6) alkyl, heterocycloalkyl (which latter two
groups
are optionally substituted by one or more substituents selected from =0 and
E),
aryl or heteroaryl (which latter two groups are optionally substituted by one
or
more substituents selected from E7);
any two B1 substituents may be linked together as hereinbefore defined, for
example to form a C3-6 cycloalkyl group, or, more preferably, B1, B2 and B3
independently represent halo, -NO2, -CN, -N(R10a)R11a, -OR' oa,
-C(=Y)-R' oa, -C(=Y)-OR1oa, -C(=Y)N(R1oa)R11a or C1-12 (e.g. C1_6) alkyl
optionally
substituted by one or more substituents selected from =0 and E8);
39
-0&26
WO 2010/119264 PCT/GB2010/000773
each Rl0a R'1a, R12a, R1 b, R1 1b and R12b independently represent, on each
occasion when used herein, hydrogen or C1-12 (e.g. C1.6) alkyl (which latter
group
is optionally substituted by one or more substituents selected from =0 and
E10);
or
any relevant pair of R1 a, R11a and R12a (e.g. R10a and R1"), and/or R1 0b and
R1lb
may, when attached to the same nitrogen atom, be linked together to form
(along
with the requisite nitrogen atom to which they are attached) a 3- to 12- (e.g.
4- to
12-) membered ring, optionally containing one or more (e.g. one to three)
double
bonds, and which ring is optionally substituted by one or more substituents
selected from E10 and =O;
each of E1, E2, E3, E4, E5, E6, E', E8, E9, E10, E11 and E12 independently
represents, on each occasion when used herein, Q4 or C1-16 alkyl (e.g. C1.6,
preferably, C1.3) alkyl optionally substituted by one or more substituents
selected
from =0 and Q5;
each Q4 and Q5 independently represent halo, -CN, -NO2, -N(R20)R21, -OR 211,
-C(=Y)-R20, -C(=Y)-OR20, -C(=Y)N(R20)R21, -N(R22)C(=Y)R21, -N(R22)C(=Y)OR21,
-N(R22)C(=Y)N(R20)R21, -NR 22S(0)2R2 , -NR 22S(0)2N(R20)R21, -S(0)2N(R20)R21
-S(0)2R20, -SR20, -S(O)R20 or C1.6 alkyl optionally substituted by one or more
fluoro atoms (and each Q5 more preferably represents halo, such as fluoro);
any two E1, E2, E3, E4, E5, E6, E7, EB, E9, E1D, E11 or E12 groups may not be
linked
together;
each R20, R21, R22 and R23 independently represent, on each occasion when used
herein, aryl (e.g. phenyl; preferably unsubstituted, but which may be
substituted
by one to three J5 groups) or, more preferably, hydrogen or C1.6 (e.g. C1-3)
alkyl
optionally substituted by one or more substituents selected from =0 and J4; or
any pair of R20 and R21, may, when attached to the same nitrogen atom, be
linked
together to form a 4- to 8-membered (e.g. 5- or 6-membered) ring, optionally
containing one further heteroatom selected from nitrogen and oxygen,
optionally
containing one double bond, and which ring is optionally substituted by one or
more substituents selected from J6 and =0;
each J1, J2, J3, J4, J5 and J6 independently represent C1.6 (e.g. C1.3) alkyl
optionally substituted by one or more substituents selected from =0 and Q8,
or,
more preferably, such groups independently represent a substituent selected
from Q7;
-0&26
WO 2010/119264 PCT/GB2010/000773
each Q7 and Q8 independently represents a substituent selected from fluoro,
-N(R50)R51, -OR50, -C(=Ya)-Rb0, -C(=Ya)-OR50, _C(=ya)N(R 50)R 51, -NR 52S(0)2R
50,
-S(O)2R50 and/or C1.6 alkyl optionally substituted by one or more fluoro
atoms;
each Y and Ya independently represent =S or, preferably, =O;
each R50, R51, R52 and R53 substituent independently represents, on each
occasion when used herein, hydrogen or C1.6 (e.g. C1_3) alkyl optionally
substituted by one or more substituents selected from fluoro;
when any relevant pair of R50, R51 and R52 are linked together, then those
pairs
that are attached to the same nitrogen atom may be linked together (i.e. any
pair
of R50 and R51), and the ring so formed is preferably a 5- or 6-membered ring,
optionally containing one further nitrogen or oxygen heteroatom, and which
ring is
optionally substituted by one or more substituents selected from =0 and C1.3
alkyl
(e.g. methyl);
R60, R61 and R62 independently represent hydrogen or C1.3 (e.g. C1_2) alkyl
optionally substituted by one or more fluoro atoms.
Preferred optional substituents on R1 and R5 (and, when they represent a
substituent other than hydrogen on R2, R3 and R4 groups) include:
=0 (e.g. in the case of alkyl, cycloalkyl or heterocycloalkyl groups);
-CN;
halo (e.g. fluoro, chloro or bromo);
C1.4 alkyl, which alkyl group may be cyclic, part-cyclic, unsaturated or,
preferably,
linear or branched (e.g. C1_4 alkyl (such as ethyl, n-propyl, isopropyl, t-
butyl or,
preferably, n-butyl or methyl), all of which are optionally substituted with
one or
more halo (e.g. fluoro) groups (so forming, for example, fluoromethyl,
difluoromethyl or, preferably, trifluoromethyl);
aryl (e.g. phenyl), if appropriate (e.g. when the substitutent is on an alkyl
group,
thereby forming e.g. a benzyl group);
-ORz1;
-C(O)Rz2;
-C(O)ORz3;
-N(RZ4)RZ5;
-S (0)2Rz6;
-S(0)2N(RZ7)RZs;
-N(R79)-C(O)-RZ1o;
41
-0&26
WO 2010/119264 PCT/GB2010/000773
-C (O)-N (Rz11) Rz12;
-N(RZ9)-C(O)-N(RZ1o);
wherein each Rz1 to Rz12 independently represents, on each occasion when used
herein, H or C1-4 alkyl (e.g. ethyl, n-propyl, t-butyl or, preferably, n-
butyl, methyl or
isopropyl) optionally substituted by one or more halo (e.g. fluoro) groups (so
forming e.g. a trifluoromethyl group). Further, any two Rz groups (e.g. Rz4
and
Rz5), when attached to the same nitrogen heteroatom may also be linked
together
to form a ring such as one hereinbefore defined in respect of corresponding
linkage of R10 and R11 or R10a and R11a groups.
Preferred compounds of the invention include those in which:
R1 represents: (i) -N(Ra)R1b; (ii) an optionally substituted 5- or 6-membered
heterocycloalkyl group (attached via a carbon atom); or (iii) an optionally
substituted 5- or 6-membered heteroaryl group;
when R1 represents -N(R1a)R1b, then R1a and Rib are preferably linked together
to
form a 6-membered ring containing a further oxygen heteroatom (so forming a
morpholinyl group), which ring may be unsubstituted, or may be substituted by
one or more B1 groups (such groups are preferably unsubstituted, i.e. most
preferably an unsubstituted morpholinyl group);
when R1 represents an optionally substituted 5- or 6-membered heterocycloalkyl
group, then such a group may contain two or preferably one heteroatom(s) (in
which the heteroatom is selected from sulfur, preferably nitrogen, and,
especially,
oxygen). Such a ring may be substituted with one or more substituents selected
from =0 and B2, but it is preferably unsubstituted. Further, it is preferably
6-
membered (e.g. a tetrahydropyranyl group);
when R1 represents optionally substituted 5- or 6-membered heteroaryl, then
such a group may contain two or, preferably, one heteroatom(s) (in which the
heteroatom is selected from sulfur, preferably oxygen and, especially,
nitrogen).
Such a ring may be substituted with one or more substituents selected from B3,
but it is preferably unsubstituted. Further, it is preferably 6-membered (e.g.
a
pyridyl group);
R2 and R3 independently represent hydrogen or a substituent selected from Q';
each Q1 and Q2 (e.g. each Q) independently represent(s) halo, -CN, -NO2,
-N(R1oa)R11a, -C(=Y)-R1oa, -C(=Y)-OR' oa, -C(=Y)N(R1oa)R1a, C112 alkyl or
42
-0&26
WO 2010/119264 PCT/GB2010/000773
heterocycloalkyl (which latter two groups are optionally substituted by one or
more substituents selected from =O, =S, =N(R10a) and E6);
R4 represents hydrogen or a substituent selected from -N(R10b)R11b and,
preferably, halo (e.g. chloro, bromo or iodo) and -CN;
at least one (e.g. one) of R2, R3 and R4 represents hydrogen;
R5 represents aryl (e.g. phenyl) or heteroaryl (e.g. a 5- or preferably a 6-
membered monocyclic heteroaryl group, or a 8-, 9- or 10-membered bicyclic
heteroaryl group), both of which are are optionally substituted by one or more
substituents selected from E5;
B1, B2 and B3 (e.g. B1) independently represent C1.6 (e.g. C1_3) alkyl
optionally
substituted by one or more substituents selected from =0 and E8;
each R1 0a R1 1a R12a, R1ob R' lb and R12b (e.g. each R1 0a and R1")
independently
represents hydrogen or C1.6 (e.g. C1_4) alkyl optionally substituted by one or
more
substituents selected from =0 and E10; or
any relevant pair of R' 0a R11a and R12a (e.g. R10a and R1") may (e.g. when
both
are attached to the same nitrogen atom) be linked together to form a 3- to 8-
(e.g.
4- to 8-) membered ring, optionally containing a further heteroatom, and
optionally substituted by one or more substituents selected from =0 and E12;
each E1, E2, E3, E4, E5, E6, E7, E8, E9, E10, E11 and E12 independently
represents
C1.12 alkyl optionally substituted by one or more substituents selected from
=0
and Q5, or, preferably (each E1 to E12 independently represent) Q4;
each Q4 and Q5 (e.g. each Q4) independently represent halo, -N(R20)R21, -OR20,
-C(=Y)-OR20, -C(=Y)N(R20)R21, -N(R22)C(=Y)R21, -N(R22)C(=Y)N(R20)R21,
-NR 22S(O)2R20, -S(O)2R20 and/or C1_6 alkyl (optionally substituted by one or
more
substituents selected from =0 and J2);
when E8 represents Q4, then Q4 preferably represents -N(R20)R21, -OR20,
-C(=Y)-OR20, -C(=Y)N(R20)R21 or -N(R22)C(=Y)R21;
each R20, R21, R22 and R23 (e.g. each R20 and R21) independently represents
heteroaryl, preferably, aryl (e.g. phenyl) (which latter two groups are
optionally
substituted by one or more substituents selected from J5), or, more
preferably,
hydrogen or C1.6 (e.g. C1.4) alkyl optionally substituted by one or more
substituents selected from =0 and J4; or
any relevant pair of R20, R21 and R22 (e.g. R20 and R21) may (e.g. when both
are
attached to the same nitrogen atom) may be linked together to form a 3- to 8-
43
-0&26
WO 2010/119264 PCT/GB2010/000773
(e.g. 4- to 8-) membered ring, optionally containing a further heteroatom, and
optionally substituted by one or more substituents selected from =0 and J6;
each J', J2, J3, J4, J5 and J6 independently represent C1.6 (e.g. C,_3) alkyl
optionally substituted by one or more substituents selected from Q8, or, J' to
J6
more preferably represent a substituent selected from Q7;
each Q' and Q$ independently represent halo, -N(R50)R51, -OR50, -C(=Ya)-OR 50,
-C(=Ya)-R50, -S(O)2R50 or C1.3 alkyl optionally substituted by one or more
fluoro
atoms;
Y and ya independently represent =0;
each R50, R51, R52 and R53 independently represents hydrogen or C1.6 (e.g.
C1.4)
alkyl optionally substituted by one or more fluoro atoms;
each R60, R61 and R62 independently represents hydrogen or C1_2 alkyl (e.g.
methyl).
More preferred compounds of the invention include those in which:
R1 represents -N(R1a)R1b, in which R'a and R1b are linked together to form a 6-
membered ring containing a further oxygen heteroatom (so forming a morpholinyl
group), which ring may be unsubstituted, or may be substituted by one or more
B1
groups (such groups are preferably unsubstituted);
R2 and R3 independently represent(s) hydrogen or a substituent selected from
halo (e.g. bromo, chloro, iodo) -CN, -N(R1Oa)R11a, -C(=Y)OR10a, -C(=Y)-R1oa,
-C(=Y)-N(R'oa)R11a C1_6 alkyl (optionally substituted by one or more (e.g.
one)
substituent(s) selected from E6) and heterocycloalkyl (e.g. a 5- or,
preferably, a 6-
membered heterocycloalkyl group, which preferably contains one heteroatom
(e.g. nitrogen), and which may contain one unsaturation, e.g a double bond, so
forming e.g. a piperidinyl ring, e.g. 4-piperidinyl, for example in which the
1,2-
position optionally contains a double bond), which heterocycloalkyl group is
optionally substituted by one or more substituents selected from =0 and,
preferably, E6 (e.g. in which the E6 substituent is located on a nitrogen
heteroatom);
when R2 or R3 represents C1_12 (e.g. Cl-,,) alkyl, then it may be straight-
chained,
e.g. acyclic C1.3 alkyl (e.g. methyl) or C3_6 cycloalkyl (e.g. cyclopropyl),
all of which
are optionally substituted by one or more fluoro atoms (so forming for example
a
trifluoromethyl group);
R4 represents hydrogen, chloro, bromo, iodo or -CN;
44
-0&26
WO 2010/119264 PCT/GB2010/000773
one of R2 and R3 represents a substituent as defined herein, and the other
represents hydrogen or a substituent as defined herein;
R5 represents aryl (e.g. phenyl) or heteroaryl (e.g. a 5- or preferably a 6-
membered monocyclic heteroaryl group, or a 10- or, preferably, 9-membered
bicyclic heteroaryl group, in which, in both cases, there is one or two
heteroatom(s) present, preferably selected from nitrogen, so forming e.g.
pyridyl,
indazolyl, indolyl, pyrimidinyl, indolonyl or pyrrolopyridine, such as
pyrrolo[2,3]pyridine), both of which R5 groups are optionally substituted by
one or
more (e.g. one or two) substituents selected from E5;
each R1oa R11a R12a R1ob, R1 1b and R12b (e.g. each R10a and R11a)
independently
represents hydrogen or C1.4 (e.g. C1.3) alkyl (e.g. ethyl); or
any relevant pair of R1oa R11a and R12a (e.g. R10a and R11a) may (e.g. when
both
are attached to the same nitrogen atom) be linked together to form a 5- or,
preferably, a 6-membered ring, optionally containing a further heteroatom
(preferably selected from nitrogen and oxygen), which ring is preferably
saturated
(so forming, for example, a piperazinyl or morpholinyl group), and optionally
substituted by one or more substituents selected from =0 and E12 (which E12
substituent may be situated on a nitrogen heteroatom; and/or E12 is preferably
halo (e.g. fluoro) or C1_3 alkyl optionally substituted by one or more fluoro
atoms);
each E1, E2, E3, E4, E5, E6, E7, E8, E9, E10, E11 and E12 (e.g. each E5 and
E6)
independently represents a substituent selected from Q4;
each E5 independently represents halo (e.g. fluoro), -OR20, -N(R2o)R21,
-C(=Y)OR20, -C(=Y)N(R20)R21, -NR22S(O)2R2 and/or -N(R22)C(=Y)N(R20)R21;
each E6 independently represents -OR20 (in which R20 preferably represents
hydrogen), -N(R20)R21, -C(=Y)OR20, -C(=Y)N(R2D)R21, -S(0)2R20 and/or C1.6
alkyl
(e.g. C1.3 alkyl) optionally substituted by one or more fluoro atoms;
each Y represents, on each occasion when used herein, =S, or preferably =O;
each R20, R21, R22 and R23 (e.g. each R20 and R21) independently represents
hydrogen or C1_4 (e.g. C1.3) alkyl (e.g. tert-butyl, ethyl or methyl); or
any relevant pair of R20, R21 and R22 (e.g. R20 and R21) may (e.g. when both
are
attached to the same nitrogen atom) may be linked together to form a 5- or,
preferably, a 6-membered ring, optionally containing a further heteroatom
(preferably selected from nitrogen and oxygen), which ring is preferably
saturated
(so forming, for example, a piperazinyl or morpholinyl group), and optionally
-0&26
WO 2010/119264 PCT/GB2010/000773
substituted by one or more substituents selected from =0 and J6 (which J6
substituent may be situated on a nitrogen heteroatom);
R22 represents C1_3 alkyl or, preferably, hydrogen;
each J1, J2, J3, J4, J5 and J6 independently represent a substituent selected
from
Q7;
each Q7 and Q6 independently represent -C(=Ya)-OR B0, -C(=Ya)-R50, -S(0)2R50
or
C1.3 alkyl optionally substituted by one or more fluoro atoms;
each ya independently represents =S or, preferably, =0;
each R50 independently represents C1-4 alkyl (e.g. tert-butyl or methyl).
Particularly preferred compounds of the invention include those in which:
R1 represents: (a) -N(R1a)R1b, in which R1a and R1b are linked together to
form a
6-membered ring optionally containing a further heteroatom (e.g. an oxygen
heteroatom) (so forming e.g. a morpholinyl or piperidinyl group), which ring
may
be unsubstituted, or may be substituted by one or more B1 groups; (b) a
monocyclic heteroaryl group (preferably containing one or two heteroatoms
(preferably selected from nitrogen; so forming e.g. a pyrimidinyl or pyridyl
group)
optionally (and preferably) substituted by one or more (e.g. one) substituent
selected from B3; or (c) a 6-membered heterocycloalkyl group (containing two
or,
preferably, one heteroatom preferably selected from nitrogen and, especially,
oxygen, so forming for example a tetrahydropyranyl group);
B1, B2 and B3 (e.g. B3) preferably represents halo (e.g. fluoro), -N(R1oa)R11a
(e .g
-NH2) or C1_2 alkyl optionally substituted by one or more E$ group;
R2 and R3 independently represent(s) hydrogen, a fragment of formula IA, C1.6
alkyl (optionally substituted by one or more (e.g. one) substituent(s)
selected from
Q2) or a substituent selected from Q1;
Q1 represents halo (e.g. bromo, chloro, iodo) -CN, -N(R1oa)R11a, -C(=Y)OR1oa
-C(=Y)-R10a, -C(=Y)-N(R1oa)R11a C1.6 alkyl (optionally substituted by one or
more
(e.g. one) substituent(s) selected from E6) and heterocycloalkyl (e.g. a 5-, 7-
or,
preferably, a 6-membered heterocycloalkyl group, which preferably contains one
or two heteroatoms (e.g. selected from nitrogen, oxygen and sulfur), and which
may contain one unsaturation, e.g a double bond, so forming e.g. azepanyl or,
preferably, pierazinyl (e.g. 1-piperazinyl), morpholinyl, thiomorpholinyl,
piperidinyl
(e.g. 4-piperidinyl, for example in which the 1,2-position optionally contains
a
double bond) or tetrahydropyranyl (e.g. 4-tetrahydropyranyl), which
46
-0&26
WO 2010/119264 PCT/GB2010/000773
heterocycloalkyl group is optionally substituted by one or more substituents
selected from =0 (which may be present on a sulfur atom to form e.g. a -S(0)2-
moiety) and, preferably, E6 (e.g. in which the E6 substituent is located on a
nitrogen heteroatom);
when R2 or R3 represents a fragment of formula IA, then it is preferably R2
that
represents such a fragment;
when R2 or R3 represents a fragment of formula IA (in an embodiment of the
invention one of R2 and R3, e.g. R2, represents a fragment of formula IA),
then
preferably m represents 1 and each R15 independently represent hydrogen (so
forming a fragment -CH2-N(Ra)(Rb));
R a and Rb are linked together to form a 4-, 5- or 6-membered cyclic group
(preferably containing no further heteroatoms, and so forming a azetidinyl,
pyrrolidinyl, piperidinyl or piperazinyl group), which further comprises: (a)
a fused
6- or preferably 5-membered heterocycloalkyl (e.g. pyrrolidinyl) group
(preferably
containing one heteroatom, e.g. nitrogen, so forming e.g. a 5,5-fused
bicycle); (b)
a -CH2-CH2- linker group (thereby forming a bridged cyclic structure) or (c) a
4-,
5- or 6-membered heterocycloalkyl group (in which there is preferably one
nitrogen heteroatom, so forming e.g. pyrrolidinyl or piperidinyl) linked
together via
a single common carbon atom to form a spiro-cycle (e.g. 2,8-diaza-spiro[4,5]-
decane-8-yl, 2,8-diaza-spiro[4,5]-decane-2-yl, 3,9-diaza-spiro[5,5]undecane-3-
yl,
2,7-diaza-spiro[3.5]nonane-7-yl or 2,7-diaza-spiro[3.5]nonane-2-yl), which
rings
are optionally substituted by one or more substituents selected from =0 and E3
(for instance the second ring may be substituted with such substituents);
when R2 or R3 represents C1_12 (e.g. C1_6) alkyl, then it may be straight-
chained,
e.g. acyclic C1_3 alkyl (e.g. methyl) or C3_6 cycloalkyl (e.g. cyclopropyl),
all of which
are optionally substituted by one or more fluoro atoms (so forming for example
a
trifluoromethyl group);
R4 represents hydrogen, chloro, bromo, iodo, -CN, -C(O)RbOb (e.g. -C(O)H) or
methyl optionally substituted by one or more (e.g. one) substituent(s)
selected
from E4 (in which E4 preferably represents heteroaryl (e.g. imidazolyl) or,
especially, -OR20, so forming e.g. a -CH2OH group or a -CH2-heteroaryl
moiety);
one of R2 and R3 represents a substituent as defined herein, and the other
represents hydrogen or a substituent as defined herein;
R5 represents aryl (e.g. phenyl) or heteroaryl (e.g. a 5- or 6-membered
monocyclic heteroaryl group, or a 10- or, preferably, 9-membered bicyclic
47
-0&26
WO 2010/119264 PCT/GB2010/000773
heteroaryl group, in which, in both cases, there is one or two heteroatom(s)
present, preferably selected from nitrogen, so forming e.g. pyrazolyl,
pyridyl,
indazolyl, indolyl, pyrimidinyl, indolonyl or pyrrolopyridine, such as
pyrrolo[2,3]pyridine), both of which R5 groups are optionally substituted by
one or
more (e.g. one or two) substituents selected from E5;
each Q2 independently represents halo (e.g. fluoro; and hence when substituted
on alkyl, may form e.g. a -CF3 group), -OR' 0a (in which R1oa preferably
represents
hydrogen or C1.2 alkyl), -N(R10a)R10b, -C(=Y)OR10a, -C(=Y)R10a, -
C(=Y)N(R1oa)R106
-S(O)2R10a, C1.6 alkyl (e.g. C1.3 alkyl; optionally substituted by one or more
fluoro
atoms), heterocycloalkyl (optionally substituted by one or more substituents
selected from =0 and E6), aryl and/or heteroaryl (e.g. pyrimidinyl; which
latter two
aryl and heteroaryl groups are optionally substituted by one or more
substituents
selected from E7);
each R10a R11a R12a R1 0b R1 1b and R12b (e.g. each R10a and R11a)
independently
represents hydrogen or C1_6 alkyl (e.g. ethyl or propyl or C3_6 cycloalkyl,
such as
cyclohexyl) optionally substituted by one or more (e.g. one) substituent(s)
selected from E10; or
one of R10a and R11a may represent heterocyloalkyl (e.g. a 5- or preferably 6-
membered heterocycloalkyl group e.g. containing one heteroatom, so forming
e.g. a piperidinyl or a tetrahydropyranyl group; which heterocycloalkyl group
is
optionally substituted by one or more (e.g. one) substituent selected from
E10); or
any relevant pair of R10a R11a and R12a (e.g. R1 0a and R11a) may (e.g. when
both
are attached to the same nitrogen atom) be linked together to form a 5-, 6- or
7-
membered ring, optionally containing a further heteroatom (preferably selected
from nitrogen, oxygen and sulfur), which ring is preferably saturated (so
forming,
for example, a pyrrolidinyl, piperidinyl, azepanyl, piperazinyl, morpholinyl
or
thiomorpholinyl group), and optionally substituted by one or more substituents
selected from =0 and E12 (which E12 substituent may be situated on a nitrogen
heteroatom; and/or E12 is preferably halo (e.g. fluoro), -N(R20)R21 -OR20,
-C(O)OR20, -OC(O)R20, -C(O)R20, -S(0)2R20 or C1.3 alkyl optionally substituted
by
one or more fluoro atoms or substituents selected from Q5);
each E1, E2, E3, E4, E5, E6, E7, E8, E9, E10, E11 and E12 (e.g. each E5 and
E6)
independently represents a substituent selected from Q4 or C1_2 alkyl
optionally
substituted by one or more substituents selected from Q5 and =O;
48
0&26
WO 2010/119264 PCT/GB2010/000773
Q4 represents halo (e.g. fluoro or chloro), -CN, -OR20, -N(R20)R21, -
C(=Y)OR20,
-C(=Y)R20, -C(=Y)N(R20)R21, -N(R22)-C(=Y)-R21, -NR 22S(O)2R2o _S(O)2R211,
-N(R22)C(=Y)N(R20)R21, -OC(O)R20, C1.6 alkyl (e.g. C1.3 alkyl; optionally
substituted
by one or more fluoro atoms), aryl (which latter group, when attached to an
alkyl
group may form e.g. a benzyl moiety) or heteroaryl (e.g. imidazolyl), which
latter
two aryl and heteroaryl groups are optionally substituted by one or more J3
substituents;
Q5 represents halo (e.g. fluoro), -N(R20)R21, -OR20 and -O-C(O)R20;
each E3 independently represents -C(=Y)OR20 or -S(O)2R20;
each E4 independently represents halo (e.g. fluoro), -OR20 (e.g.
-OH) or heteroaryl (e.g. imidazolyl);
each E5 independently represents halo (e.g. fluoro or chloro), -CN, -OR20,
-N(R20)R21, -C(=Y)OR211, -C(=Y)N(R20)R21, -N(R22)-C(=Y)-R21, -NR 22S (O)2R 20,
-N(R22)C(=Y)N(R20)R21 and/or C1.6 alkyl (e.g. C1.3 alkyl) optionally
substituted by
one or more fluoro atoms;
each E6 independently represents halo (e.g. fluoro), -OR20 (in which R20
preferably represents hydrogen or C1.2 alkyl), -N(R20)R21, -C(=Y)OR20, -
C(=Y)R20,
-C(=Y)N(R20)R21, -S(O)2R20 and/or C1.6 alkyl (e.g. C1.3 alkyl) optionally
substituted
by one or more fluoro atoms;
each E7 independently represents -N(R20)R21;
each E8 independently represents -OR20 or -C(=Y)OR20;
each E10 (which is preferably located on a nitrogen heteroatom, when a
substituent on a heterocycloalkyl group) represents -S(O)2R20, -OR20,
-N(R20)R21, -N(R22)-C(O)-R21, -C(O)-OR20 or aryl (which latter group, when
attached to an alkyl group may form e.g. a benzyl moiety; and which may be
substituted by one or more J3 substituents);
each Y represents, on each occasion when used herein, =S, or preferably =O;
each R20, R21, R22 and R23 (e.g. each R20 and R21) independently represents
hydrogen, C14 (e.g. C1.3) alkyl (e.g. tert-butyl, ethyl or methyl; which alkyl
group is
optionally substituted by one or more substituents selected from J4) or aryl
(e.g.
phenyl; especially in the case of -S(O)2R20, and which aryl group is
optionally
substituted by one or more J5 substituents); or
any relevant pair of R20, R21 and R22 (e.g. R20 and R21) may (e.g. when both
are
attached to the same nitrogen atom) may be linked together to form a 5- or 6-
membered ring, optionally containing a further heteroatom (preferably selected
49
-0&26
WO 2010/119264 PCT/GB2010/000773
from nitrogen and oxygen), which ring is preferably saturated (so forming, for
example, a pyrrolidinyl, piperazinyl or morpholinyl group), and optionally
substituted by one or more substituents selected from =0 and J6 (which J6
substituent may be situated on a nitrogen heteroatom);
R22 represents C1.3 alkyl or, preferably, hydrogen;
each J1, J2, J3, J4, J5 and J6 independently represent a substituent selected
from
Q7:
each Q7 and Q8 independently represent halo, -N(R50)R81, -C(=Ya)-OR50
-C(=Ya)-N(R80)R51, -C(=Ya)-R50, -S(0)2R80 or C1.3 alkyl optionally substituted
by
one or more fluoro atoms;
each ya independently represents =S or, preferably, =O;
each R50 and R51 independently represents hydrogen, C1-4 alkyl (e.g. tert-
butyl or
methyl) or R50 and R51, when attached to the same carbon atom, may be linked
together to form a 5- or preferably, 6-membered ring (e.g. containing a
further
heteroatom, go forming e.g. piperazinyl) optionally substituted by methyl
(e.g.
which substituent is located in the additional nitrogen heteroatom).
Other preferred compounds of the invention that may be mentioned include:
R1 represents -N(R1a)R1b as hereinbefore defined (especially in which R1a and
R1b
are linked together to form a 6-membered ring optionally (and preferably)
containing a further heteroatom (e.g. oxygen), so forming e.g. a piperidinyl
or,
preferably, a morpholinyl group;
R2 represents a substituent other than hydrogen, and R3 and R4 independently
represent hydrogen or a substituent other than hydrogen;
R2 represents a substituent other than hydrogen;
R2 represents Q1 or C1_2 alkyl (e.g. methyl) optionally substituted by Q2
(e.g. at the
terminal position of the methyl group);
R3 and R4 independently represent C1_2 alkyl or, preferably, hydrogen or Q1
(e.g.
in which Q1 preferably represents halo (e.g. chloro) or heterocycloalkyl
optionally
substituted by one or more E6 groups);
at least one of R3 and R4 represent hydrogen;
R5 represents: (a) phenyl (which is preferably substituted e.g. by one E5
substituent located preferably at the meta position); (b) a 5- or 6-membered
(e.g.
6-membered) monocyclic heteroaryl group (e.g. containing one or two
heteroatoms preferably selected from nitrogen, so forming e.g. pyrimidinyl,
such
-0&26
WO 2010/119264 PCT/GB2010/000773
as 5-pyrimidinyl, or pyridyl, such as 3-pyridyl), which monocyclic heteroaryl
group
is optionally substituted e.g. by one or two E5 substituent(s) (e.g. located
at the 2-
position (and optionally 6-position), when R5 represents pyrimidinyl, or, at
the 6-
position when R5 represents 3-pyridyl; in each case a substituent is
preferably at
the postion para relative to the point of attachment to the requisite
imidazopyrazine of formula I); or (c) a 9- or 10-membered (e.g. 9-membered)
bicyclic heteroaryl group (e.g. indazolyl, such as 4-indazolyl, or azaindolyl,
such
as 7-azaindolyl i.e. pyrrolo[2,3-b]pyridyl, such as 7-azaindol-5yl), which
bicyclic
heteroaryl group is preferably unsubstituted;
Q1 represents -C(O)N(R1oa)R11a or -C(O)OR10a (e.g. in which R1oa is C1.2
alkyl);
Q2 represents fluoro, -N(R10a)R11a or heterocycloalkyl (e.g. piperazinyl or
morpholinyl) optionally (and preferably) substituted by one or more (e.g. one)
substituent(s) (preferably located on a nitrogen heteroatom) selected from =0
and, preferably, E6;
R1 0a and R11a (for instance when Q1 represents -C(O)N(R10a)R1la)
independently
represent hydrogen, acyclic C1_3 (e.g. C1_2) alkyl (e.g. methyl or ethyl)
(optionally
substituted by one or more (e.g. one) E10 substituent), C5_6 cycloalkyl (e.g.
cyclohexyl) (optionally substituted by one or more (e.g. one) E10 substituent)
or
heterocycloalkyl (e.g. a 5- or 6-membered heterocycloalkyl group (e.g.
containing
one heteroatom, so forming e.g. piperidinyl, such as 4-piperidinyl, or
tetrahydropyranyl, such as 4-tetrahydropyranyl) (optionally substituted by one
or
more (e.g. one) E10 substituent, which may be located on a nitrogen
heteroatom);
when Q2 represents -N(R10a)R1la, then R1 0a and R1 1a are preferably linked
together to form a 5- or preferably 6-membered ring preferably containing a
further (e.g. nitrogen, oxygen or sulfur) heteroatom (so forming, e.g.,
piperazinyl,
morpholinyl or thiomorpholinyl) optionally (and preferably) substituted by one
or
more (e.g. one) substituent(s) (optionally located on a nitrogen heteroatom)
selected from =O, E12 and C1.2 alkyl (e.g. methyl) optionally substituted by
one or
more fluoro atoms (and, e.g. in the case of rings containing S, with one or
more
(e.g. one or two) =O, which carbonyl group(s) are located on the S to form
e.g. a
-S(O)2- moiety);
when E5 represents a substituent on phenyl, then it is preferably Q4 (e.g. -
OR20);
when E5 represents a substituent on monocyclic heteroaryl, then it is
preferably
Q4 (e.g. -N(R20)R21) or C1.2 alkyl (e.g. methyl) optionally substituted by one
or
more fluoro atoms (so forming e.g. a -CF3 group);
51
-0&26
WO 2010/119264 PCT/GB2010/000773
E6 and E12 preferably represent Q4;
E10 represents Q4;
for instance when E5 represents Q4, then Q4 represents -OR20 or -N(R20)R21;
for instance when E6 and E12 represent Q4, then Q4 represents -S(O)2R20 (ea.g.
-S(O)2C1_4 alkyl), -C(O)R20 or -OC(O)R20;
E10 represents -N(R20)R21, -OR20 or -C(O)OR20;
for instance when E10 represents Q4 (and E10 is a substituent on an alkyl or
cycloalkyl group), then Q4 represents -N(R20)R21 or -OR20 (e.g. -OCH3 or -OH);
for instance when E10 represents Q4 (and E10 is a substituent on a
heterocycloalkyl group), then Q4 represents -C(O)OR20;
R20 and R21 independently represent hydrogen or C1_4 alkyl (e.g. methyl, ethyl
or
butyl (e.g isobutyl)), which alkyl group may (e.g. in the case when E12
represents
-C(O)R20) be substituted with a J4 substituent; or
for instance, when E10 represents -N(R2))R21, then R20 and R21 may be linked
together to form a 5- or preferably 6-membered ring, optionally containing a
further heteroatom (e.g. oxygen, so forming e.g. morpholinyl);
J4 represents Q7;
.Q7 represents -N(R50)R51;
R50 and R51 independently represent hydrogen, or, preferably, C1_2 alkyl (e.g.
methyl).
Most preferred compounds of the invention include those in which:
R1 represents 1-piperidinyl or, preferably, 4-morpholinyl;
R2 represents hydrogen or, preferably, methyl, -CF3, -CH2-[4-S(O)2CH3-
piperazinyl], -C(O)N(H)ethyl, -C(O)NH2, -C(O)Oethyl, -C(O)N(H)-CH2CH2-
N(CH3)2, -C(O)N(H)methyl, -CH2-[4-morpholinyl], -C(O)N(H)-CH2CH2-OCH3,
-C(O)N(H)-[(1-C(O)OCH2CH3)-piperidin-4-yl], -C(O)N(H)-[4-tetrahydropyranyl],
-C(O)N(H)-[4-OH-cyclohexyl], -CH2-[4-C(O)t-butyl-2,6-diemthyl-piperazinyl],
-CH2-[4-S(O)2CH3-2,6-dimethyl-piperazinyl], -CH2-[4-(S(O)2CH2CH3)-
piperazinyl],
-CH2-[4-(S(O)2CH2-C(H)(CH3)2)-piperazinyl], -CH2-[1,1-dioxo-thiomorpholinyl],
-CH2-[piperazinyl], -CH2-[4-(C(O)CH2N(CH3)2)-piperazinyl] or
-CH2-[4-(C(O)C(H)(CH3)-O-C(O)CH3)-piperazinyl];
R3 represents 4-piperidinyl (e.g. containing a double bond at the 3,4-position
and
a -C(O)-C(H)(CH3)2 substituent at the 1-position) or, preferably, hydrogen;
R4 represents hydrogen or halo (e.g. chloro);
52
-0&26
WO 2010/119264 PCT/GB2010/000773
at least one of R3 and R4 (preferably both) represent hydrogen;
R5 represents 3-hydroxyphenyl, 2-amino-5-pyrimidinyl, 4-indazolyl, 3-pyridyl,
6-
amino-pyridyl, 7-azaindol-5-yl (i.e. pyrrolo[2,3-b]pyrid-5-yl), 2-methyl-5-
pyrimidinyl, 2-amino-6-methyl-5-pyrimidinyl or 2-N(H)CH3-5-pyrimidinyl.
Particularly preferred compounds of the invention include those of the
examples
descibred hereinafter. For example:
ethyl 6-(3-hydroxyphenyl)-8-morpholinoimidazo[1,2-a]pyrazine-2-carboxylate (2-
01);
6-(3-hydroxyphenyl)-8-morpholinoimidazo[1,2-a]pyrazine-2-carboxylic acid (2-
02);
6-(3-hydroxyphenyl)-8-morpholinoimidazo[1,2-a]pyrazine-2-carbaldehyde (2-03);
3-(2-(hydroxymethyl)-8-morpholinoimidazo[1, 2-a]pyrazin-6-yl) phenol (2-04);
tert-butyl 4-((6-(3-hydroxyphenyl)-8-morpholinoimidazo[1,2-a]pyrazin-2-
yl)methyl)-
piperazine-1-carboxylate (2-05);
1-(4-((6-(3-hydroxyphenyl)-8-morpholinoimidazo[1, 2-a]pyrazin-2-yl)methyl)-
piperazin-1-yl)ethanone (2-06);
3-(2-((4-m ethyl piperazin-1-yl)methyl)-8-morpholinoimidazo[1, 2-a] pyrazin-6-
yl)phenol (2-07);
4-((6-(3-hydroxyphenyl)-8-morpholinoimidazo[1,2-a]pyrazin-2-
yl)methyl)piperazin-
2-one (2-08);
3-(8-morpholino-2-(morpholinomethyl)imidazo[1,2-a]pyrazin-6-yl)phenol (2-09);
1-(4-((6-(3-hydroxyphenyl)-8-morpholinoimidazo[1,2-a]pyrazin-2-yI)methyl)-
piperazin-1-yl)sulfonylmethane (2-10);
(6-(3-hydroxyphenyl)-8-morpholinoimidazo[1,2-a]pyrazin-2-yl)(4-sulfonylmethyl-
piperazin-1-yl)methanone (2-11);
3-(8-morpholinoimidazo[1,2-a]pyrazin-6-yl)phenoI (2-12);
3-(2-(trifluoromethyl)-8-morpholinoimidazo[1,2-a]pyrazin-6-yl) phenol (2-13);
3-(2-cyclopropyl-8-morpholinoimidazo[1,2-a]pyrazin-6-yl)phenol (2-14);
(6-(1 H-indazol-4-yl)-8-morpholinoimidazo[ 1, 2-a]pyrazin-2-yl)(4-m ethyl
piperazin-1-
yl)methanone (2-15);
(6-(1 H-indazol-4-yl)-8-morpholinoimidazo[1,2-a]pyrazin-2-yl)(4-sulfonylmethyl-
piperazin-1-yl)methanone (2-16);
6-(1 H-indazol-4-yl)-2-((4-sulfonylmethyl piperazin-1-yl)methyl)-8-morpholino-
imidazo[1,2-a]pyrazine (2-17);
53
-0&26
WO 2010/119264 PCT/GB2010/000773
(6-(1 H-indazol-4-yl)-8-morpholinoimidazo[1,2-a]pyrazin-2-yi)(piperazin-1-yl)-
methanone (2-18);
2-cyclopropyl-6-(1 H-indazol-4-yl)-8-morpholinoimidazo[1,2-a]pyrazine (2-19);
2-(trifluoromethyl)-6-(1 H-indazol-4-yl)-8-morpholinoimidazo[1,2-a]pyrazine (2-
20);
6-(1H-indazol-4-yl)-8-morpholinoimidazo[1,2-a]pyrazine-2-carboxamide (2-21);
6-(1 H-indazol-4-yl)-8-morpholinoimidazo[1,2-a]pyrazine-2-carbonitrile (2-22);
3-(2-methyl-8-morpholinoimidazo[1,2-a]pyrazin-6-yl)phenol (2-23);
6-(1 H-indazol-4-yl)-2-methyl-8-morpholinoimidazo[1,2-a]pyrazine (2-24);
6-(1 H-indol-5-yl)-2-methyl-8-morpholinoimidazo[1,2-a]pyrazine (2-25);
2-methyl-8-morpholino-6-(pyridin-3-yl)imidazo[1,2-a]pyrazine (2-26);
6-(5-methoxypyridin-3-yl)-2-methyl-8-morpholinoimidazo[1,2-a]pyrazine (2-27);
N-sulfonylmethyl-3-(2-methyl-8-morpholinoimidazo[1,2-a]pyrazin-6-yl)benzen-
amine (2-28);
1-methyl-3-(4-(2-methyl-8-morpholinoimidazo[1,2-a]pyrazin-6-yl)phenyl)urea (2-
29);
5-(2-methyl-8-morpholinoimidazo[1,2-a]pyrazin-6-yl)pyridin-3-oI (2-30);
2-methyl-8-morpholino-6-(pyridin-4-yl)imidazo[1,2-a]pyrazine (2-31);
6-(3-methoxyphenyl)-2-methyl-8-morpholinoimidazo[1,2-a]pyrazine (2-32);
5-chloro-2-methyl-8-morpholino-6-(pyridin-4-yl)imidazo[1,2-a]pyrazine (2-33);
5-chloro-2-methyl-8-morpholino-6-(pyridin-3-yl)imidazo[1,2-a]pyrazine (2-34);
8-morpholino-6-phenylimidazo[1,2-a]pyrazine-2-carboxamide (2-35);
6-(1 H-indazol-4-yl)-2-methyl-8-morpholinoimidazo[1,2-a]pyrazine-5-
carbonitrile
(2-36);
3-(3-(4-methylpiperazin-1-yl)-8-morpholinoimidazo[ 1, 2-a] pyrazi n-6-yl)
phenol (2-
37);
6-(1 H-indazol-4-yl)-3-(4-methylpiperazin-1-yi)-8-morpholinoim idazo[1,2-a]-
pyrazine (2-38);
3-(3-(4-sulfonylmethylpiperazin-1 -yl)-8-morpholinoimidazo[1,2-a]pyrazin-6-
yl)phenol (2-39); -
3-(8-morpholino-3-(piperazin-1-yl)imidazo[1,2-a]pyrazin-6-yl)phenol (2-40);
6-(1 H-indazol-4-yi)-3-(4-sulfonylmethylpiperazin-1-yl)-8-
morpholinoimidazo[1,2-
a]pyrazine (2-41);
3-bromo-6-(1 H-indazol-4-yl)-2-methyl-8-morpholinoimidazo[1,2-a]pyrazine (2-
42);
tert-butyl 2-(4-(6-(1 H-indazol-4-yl)-2-methyl-8-morpholinoimidazo[1,2-
a]pyrazin-3-
yl)-5,6-dihydropyridin-1(2H)-yl)acetate (2-43);
54
-0&26
WO 2010/119264 PCT/GB2010/000773
3-(1,2,3,6-tetrahydropyridin-4-yl)-6-(1 H-indazol-4-yl)-2-methyl-8-morpholino-
imidazo[1,2-a]pyrazine (2-44);
3-(1,2,3,6-tetrahydro-1-methylpyridin-4-yl)-6-(1 H-indazol-4-yl)-2-methyl-8-
morpholinoimidazo[1,2-a]pyrazine (2-45);
3-(1,2,3,6-tetrahydro-1-sulfonylmethyl pyridin-4-yl)-6-(1 H-indazol-4-yl)-2-
methyl-8-
morpholinoimidazo[1,2-a]pyrazine (2-46); -
tert-butyl 2-(4-(6-(1 H-indazol-4-yl)-2-methyl-8-morpholinoimidazo[1,2-
a]pyrazin-3-
yl)piperidin-1-yl)acetate (2-47);
5-chloro-6-(3-methoxyphenyl)-2-methyl-8-morpholinoimidazo[1, 2-a]pyrazine (2-
48);
3-(5-chloro-2-methyl-8-morpholinoimidazo[1,2-a]pyrazin-6-yl)phenol (2-49);
5-(2-((4-sulfonylmethylpiperazin-1-yl)methyl)-8-morpholinoimidazo[1,2-
a]pyrazin-
6-yI)pyrimidin-2-amine (2-50);
6-(1 H-indazol-4-yl)-8-morpholino-3-(piperazin-1 -yl)imidazo[1,2-a]pyrazine (2-
51);
ethyl 6-(1 H-indazol-4-yl)-8-morpholinoimidazo[1,2-a]pyrazine-2-carboxylate (2-
52);
5-iodo-6-(3-methoxyphenyl)-2-methyl-8-morpholinoimidazo[1,2-a]pyrazine (2-53);
5-chloro-6-(1 H-indazol-4-yl)-2-methyl-8-morpholinoimidazo(1,2-a]pyrazine (2-
54);
5-bromo-6-(1 H-indazol-4-yl)-2-methyl-8-morpholinoimidazo[1,2-a]pyrazine (2-
55);
5-iodo-2-methyl-8-morpholino-6-(pyridin-3-yl)imidazo[1,2-a]pyrazine (2-56);
6-(1 H-indazol-4-yl)-2-methyl-8-morpholino-3-(piperidin-4-yl)imidazo[1,2-a]-
pyrazine (2-57);
6-(1 H-indazol-4-yl)-2-methyl-3-(1-methylpiperidin-4-yl)-8-
morpholinoimidazo[1,2-
a]pyrazine (2-58);
6-(1 H-indazol-4-yl)-2-methyl-3-(1 -sulfonylmethylpiperidin-4-yl)-8-morpholino
imidazo[1,2-a]pyrazine (2-59);
5-chloro-6-(1 H-indazol-4-yl)-8-morpholino-2-((4-sulfonylmethylpiperazin-1-yl)-
methyl)imidazo[1,2-a]pyrazine (2-60);
6-(6-methoxypyridin-3-yl)-2-((4-sulfonylmethyl piperazin-1-yl)methyl)-8-
morpholinoimidazo[1,2-a]pyrazine (2-61);
6-(3-methoxyphenyl)-2-methyl-8-morpholinoimidazo[1,2-a]pyrazine-3-carbonitrile
(2-62);
tert-butyl 4-(6-(1 H-indazol-4-yl)-2-methyl-8-morpholinoimidazo[1,2-a]pyrazine-
3-
carboxamido)piperidine-1-carboxylate (2-63);
-0&26
WO 2010/119264 PCT/GB2010/000773
6-(1 H-i ndazol-4-yl)-2-methyl-8-m orpholino-N-(piperidin-4-yl)im idazo[1,2-a]-
pyrazine-3-carboxamide (2-64);
6-(5-methoxypyridin-3-yl)-2-((4-sulfonylmethyl piperazin-1-yl)methyl)-8-
morpholinoimidazo[1,2-a]pyrazine (2-65);
5-(2-((4-sulfonyl methylpiperazin-1-yl)methyl)-8-morpholinoimidazo[1,2-
a]pyrazin-
6-yl)pyridin-2-amine (2-66);
6-(2-methoxypyrimidin-5-yl)-2-((4-sulfonylmethylpiperazin-1-yl)methyl)-8-
morpholinoimidazo[1,2-a]pyrazine (2-67);
4-(2-((4-sulfonylmethylpiperazin-1 -yl)methyl)-8-morpholinoimidazo[1,2-
a]pyrazin-
6-yl)benzamide (2-68);
5-(2-((4-sulfonylmethylpiperazin-1 -yl)methyl)-8-morpholinoimidazo[1,2-
a]pyrazin-
6-yl)indolin-2-one (2-69);
6-(5-fluoro-1 H-indol-4-yl)-2-((4-sulfonylmethylpiperazin-1-yl)methyl)-8-
morpholino-
imidazo[1,2-a]pyrazine (2-70);
6-(1 H-indol-4-yl)-2-((4-sulfonylmethylpiperazin-1-yl)methyl)-8-morpholino
imidazo[1,2-a]pyrazine (2-71);
3-(2-((4-sulfonylmethyl piperazin-1-yl)methyl)-8-morpholinoimidazo[1,2-
a]pyrazin-
6-yl)benzamide (2-72);
6-(1 H-indazol-4-yl)-3-iodo-2-methyl-8-morpholinoimidazo[1,2-a]pyrazine (2-
73);
N-ethyl-6-(1 H-indazol-4-yl)-8-morpholinoimidazo[1,2-a]pyrazine-2-carboxamide
(2-74);
2-((4-sulfonylmethylpiperazin-1-yl)methyl)-8-morpholino-6-(1 H-pyrrolo[2,3-
b]pyridin-5-yl)imidazo[1,2-a]pyrazine (2-75).
Compounds of the invention may be made in accordance with techniques that are
well known to those skilled in the art, for example as described hereinafter.
According to a further aspect of the invention there is provided a process for
the
preparation of a compound of formula I which process comprises:
(i) reaction of a compound of formula IB,
56
-0&26
WO 2010/119264 PCT/GB2010/000773
R
N R2 IB
L N
R4 R3
wherein L' represents a suitable leaving group, such as iodo, bromo, chloro or
a
sulfonate group (e.g. -OS(O)2CF3, -OS(O)2CH3 or -OS(O)2PhMe), and R1, R2, R3,
and R4 are as hereinbefore defined, with a compound of formula IC,
R5-L2 IC
wherein L2 represents a suitable group such as -B(OH)2, -B(OR')2 or -Sn(R)3,
in which each RVd" independently represents a C1_6 alkyl group, or, in the
case of
-B(OR"'X)2, the respective R`"" groups may be linked together to form a 4- to
6-
membered cyclic group (such as a 4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl
group), thereby forming e.g. a pinacolato boronate ester group, (or L2 may
represent iodo, bromo or chloro, provided that L' and L2 are mutually
compatible)
and R5 is as hereinbefore defined. The reaction may be performed, for example
in the presence of a suitable catalyst system, e.g. a metal (or a salt or
complex
thereof) such as Pd, Cul, Pd/C, PdCI2, Pd(OAc)2i Pd(Ph3P)2CI2i Pd(Ph3P)4 (i.e.
palladium tetra kistripheny lphosphine), Pd2(dba)3 and/or NiCI2 (preferred
catalysts
include palladium) and a ligand such as PdC12(dppf).DCM, t-Bu3P, (C6H11)3P,
Ph3P, AsPh3, P(o-Tol)3, 1,2-bis(diphenylphosphino)ethane, 2,2'-bis(di-tert-
butyl-
phosphino)-1,1'-biphenyl, 2,2'-bis(diphenylphosphino)-1, 1'-bi-naphthyl, 1,1'-
bis(diphenyl-phosphino-ferrocene), 1,3-bis(diphenylphosphino)propane,
xantphos, or a mixture thereof (preferred ligands include PdCI2(dppf).DCM),
together with a suitable base such as, Na2CO3, K3PO4, Cs2CO3i NaOH, KOH,
K2C03, CsF, Et3N, (i-Pr)2NEt, t-BuONa or t-BuOK (or mixtures thereof;
preferred
bases include Na2CO3 and K2CO3) in a suitable solvent such as dioxane,
toluene,
ethanol, dimethylformamide, dimethoxyethane, ethylene glycol dimethyl ether,
water, dimethylsulfoxide, acetonitrile, dimethylacetamide, N-
methylpyrrolidinone,
tetrahydrofuran or mixtures thereof (preferred solvents include
dimethylformamide and dimethoxyethane). The reaction may be carried out for
example at room temperature or above (e.g. at a high temperature such as at
57
-0&26
WO 2010/119264 PCT/GB2010/000773
about the reflux temperature of the solvent system). Alternative reaction
conditions include microwave irradiation conditions, for example at elevated
temperature of about 130 C;
(ii) reaction of a compound of formula ID,
L3
N~ R2 ID
R5 Y N
R4 R3
wherein L3 represents a suitable leaving group, such as one hereinbefore
defined
in respect of L', and R2, R3, R4 and R5 are as hereinbefore defined, with a
compound of formula IE,
R'-L4 IE
wherein L4 represents a suitable leaving group, such as one hereinbefore
described in respect of L2, under reaction conditions such as those descibred
in
respect of process step (i) above, or, in the case where R' represents -
N(R'a)R'b
L4 may represents hydrogen (so forming an amine group), and the reaction may
be performed in the presence of an appropriate metal catalyst (or a salt or
complex thereof) such as Cu, Cu(OAc)2, Cul (or Cul/diamine complex), copper
tris(triphenylphosphine)bromide, Pd(OAc)2, tris(dibenzylideneacetone)-
dipalladium(0) (Pd2(dba)3) or NiCl2 and an optional additive such as Ph3P,
2,2'-
bis(diphenylphosphino)-1,1'-binaphthyl, xantphos, Nal or an appropriate crown
ether such as 18-crown-6-benzene, in the presence of an appropriate base such
as NaH, Et3N, pyridine, N,N-dimethylethylenediamine, Na2CO3, K2CO3, K3PO4,
Cs2CO3i t-BuONa or t-BuOK (or a mixture thereof, optionally in the presence of
4A molecular sieves), in a suitable solvent (e.g. dichloromethane, dioxane,
toluene, ethanol, isopropanol, dimethylformamide, ethylene glycol, ethylene
glycol dimethyl ether, water, dimethylsulfoxide, acetonitrile,
dimethylacetamide,
N-methylpyrrolidinone, tetrahydrofuran or a mixture thereof). This reaction
may
be performed at elevated temperature or under microwave irradiation reaction
conditions, for example as described in process step (i). The compound of
58
-0&26
WO 2010/119264 PCT/GB2010/000773
formula ID (e.g. in which L4 is chloro) may be prepared in situ, for example
from a
compound corresponding to a compound of formula ID, but in which L4
represents -OC1.3 alkyl (e.g. methoxy) by reaction in the presence of e.g. a
chlorinating agent (such as POCI3);
(iii) reaction of a compound of formula IF,
R'
NH2
N I IF
L 1 ---Iy N
4
wherein R', R4 and L' are as hereinbefore defined, with a compound of formula
IG,
R2-C(O)-C(R3) (H)-L5 I G
wherein L5 represents a suitable leaving group, such as one hereinbefore
defined
in respect of L' (and, especially, L5 represents halo, such as chloro or
bromo),
and R2 and R3 are as hereinbefore defined, under standard reaction conditions,
for example in the presence of a suitable reaction solvent such as DME or 2-
propanol, at a convenient temperature, typically heating at 90 C, followed by
reaction with a compound of formula IC as hereinbefore defined. The
compounds of formula IG may be a protected derivative thereof (e.g. it may be
a
carbonyl derivative, for instance R2-C(-OCH3)2-C(R3)(H)-L5, in which R2
preferably
represents H). Such an intermediate compound of formula IG in which R3
represents -N(R1oa)R11a (in which R1 la and R' la may be linked together to
form an
optionally piperazinyl group) and L5 may represent a suitable leaving group,
may
also be prepared by reaction of glyoxal with benzotraizole and an amine (e.g.
a
cyclic amine such as piperazine or a substituted derivative thereof), which
may be
an intermediate compound that is not isolated (e.g. which may be used in
situ);
(iv) reaction of a compound of formula IFA,
59
-0&26
WO 2010/119264 PCT/GB2010/000773
R'
NH2
N I FA
Y N
R
R4
wherein R1, R4 and R5 are as hereinbefore defined, with a compound of formula
IH,
5 R3-C(H)(Lsa)-C(H)(L6b)-R3 IH
wherein L6a and L6b independently represent a suitable leaving group, for
example
benzotriazole groups (or the like), and each R3 is as hereinbefore defined
(e.g.
each of the R3 groups are the same), which reaction may be performed following
similar conditions reported in literature (J. Org.Chem. 1990, 55, 3209-3213,
J.Org.Chem, 2003, 68, 4935-4937), in a suitable solvent, such as DCE, heating
at a convenient temperature, for a period of time to ensure completion of the
reaction, typically at reflux for 5h. Additionally an inorganic base can be
added to
ensure completion of the reaction;
(v) for compounds of formula I in which R3 or R4 represents bromo, iodo or
chloro,
reaction of a corresponding compound of formula I, in which R3 or R4 (as
appropriate) represents hydrogen, with a reagent that is a source of halide
ions (a
halogenating reagent). For instance, an electrophile that provides a source of
iodide ions includes iodine, diiodoethane, diiodotetrachloroethane or,
preferably,
N-iodosuccinimide, a source of bromide ions includes N-bromosuccinimide and
bromine, and a source of chloride ions includes N-chlorosuccinimide, chlorine
and iodine monochloride, for instance in the presence of a suitable solvent,
such
as CHCI3 or an alcohol (e.g. methanol), optionally in the presence of a
suitable
base, such as a weak inorganic base, e.g. sodium bicarbonate. Typically, the
reaction maybe performed by heating at a convenient temperature, either by
conventional heating under reflux or under microwave irradiation;
(vi) for compounds of formula I in which R3 or R4 represents a substituent
other
that hydrogen, or halo (e.g. bromo, iodo or chloro), reaction of a
corresponding
-0&26
WO 2010/119264 PCT/GB2010/000773
compound of formula I, in which R3 or R4 (as appropriate) represents bromo,
chloro or iodo, with a compound of formula IJ,
R3/4-L7 IJ
wherein R314 represents R3 or R4 (as appropriate), and L7 represents a
suitable
leaving group such as one hereinbefore described in respect of process step
(i)
or (ii) above. Alternatively, the skilled person will appreciate that
different
reagents and reaction steps may be employed, depending on the particular R3 or
R4 substituent required (for instance in order to introduce a -CN substituent,
zinc
cyanide (or the like) may be employed).
Other specific transformation steps that may be mentioned include:
(i) reductions of a carboxylic acid (or ester) to either an aldehyde or an
alcohol,
using appropriate reducing conditions (e.g. when R2 represents -C(O)OH (or an
ester thereof), it may be converted to a -C(O)H or -CH2-OH group, using DIBAL
and LiAIH4, respectively (or similar chemoselective reducing agents);
(ii) reductions of an aldehyde (-C(O)H) group to an alcohol group (-CH2OH),
using
appropriate reduction conditions such as those mentioned at point (i) above;
(iii) oxidations, for example of a moiety containing an alcohol group (e.g. -
CH2OH)
to an aldehyde (e.g. -C(O)H) or of a -S- moiety to a -S(O)- or -S(O)2- moiety
(or
the reverse reduction reaction), for example in the presence of a suitable
oxidising agent, e.g. Mn02 or mcpba or the like;
(iv) reductive amination of an aldehyde and an amine, under appropriate
reaction
conditions, for example in "one-pot" procedure in the presence of an
appropriate
reducing agent, such as sodium cyanoborohydride or, preferably, sodium
triacetoxyborohydride, or the like (and hence when e.g. R2 represents -C(O)H,
such a group may be converted to a -CH2-N(R1Oa)R1la group (or -CH2-N(Ra)(Rb),
i.e. a specific fragment of formula la), in which R1la and R1 la are as
hereinbefore
defined, and may be linked together as hereinbefore defined to form e.g. a 5-
or
6-membered ring optionally containing a further heteroatom such as oxygen or
nitrogen);
(v) formation of an amide or sulfonamide, for example by reaction of a
sulfonyl
choride with an amine or by an amide coupling reaction, i.e. the formation of
an
amide from a carboxylic acid (or ester thereof), for example -C(O)OH (or an
ester
61
0&26
WO 2010/119264 PCT/GB2010/000773
thereof), may be converted to -C(O)N(R102)R11a group (in which R1 0a and R11a
are
as hereinbefore defined, and may be linked together, e.g. as defined above),
and
which reaction may (e.g. for -000H) be performed in the presence of a suitable
coupling reagent (e.g. 1,1'-carbonyldiimidazole, N,W-dicyclohexylcarbodiimide,
or
the like) or, in the case of an ester (e.g. -C(O)OCH3 or -C(O)OCH2CH3), be
performed in the presence of e.g. trimethylaluminium, or, alternatively the
-C(O)OH group may first be activated to the corresponding acyl halide (e.g
-C(O)CI, by treatment with oxalyl chloride, thionyl chloride, phosphorous
pentachloride, phosphorous oxychloride, or the like), and, in all cases, the
relevant compound is reacted with a compound of formula HN(R1oa)R1a (in which
R' 0a and R11a are as hereinbefore defined), under standard conditions known
to
those skilled in the art (e.g. optionally in the presence of a suitable
solvent,
suitable base and/or in an inert atmosphere);
(vi) conversion of a primary amide to a nitrile functional group, for example
under
dehydration reaction conditions, e.g. in the presence of POCI3, or the like;
(vii) nucleophilic substitution (e.g. aromatic nucleophilic substitution)
reactions,
where any nucleophile replaces a leaving group, e.g. an amine may replace a
-S(O)CH3 leaving group, such reactions include "Mitsunobu"-type reactions (or
variants thereof), i.e. in which a -OH is the leaving group, which is
activated by
treatment with e.g. iodine and triphenylphosphine);
(viii) transformation of a methoxy group to a hydroxy group, by reaction in
the
presence of an appropriate reagent, such as boron fluoride-dimethyl sulfide
complex or BBr3 (e.g. in the presence of a suitable solvent such as
dichloromethane);
(ix) alkylation, acylation or sulfonylation reactions, which may be performed
in the
presence of base and solvent (such as those described hereinbefore);
(x) specific deprotection steps, such as deprotection of an N-Boc protecting
group
by reaction in the presence of an acid, or, a hydroxy group protected as a
silyl
ether (e.g. a tert-butyl-dimethylsilyl protecting group) may be deprotected by
reaction with a source of fluoride ions, e.g. by employing the reagent
tetrabutylammonium fluoride (TBAF). Further, a -O-C(O)-CH3 may be converted
to a -OH group by reaction with sodium methoxide in methanol, or similar
hydrolysis reactions may be performed;
62
-0&26
WO 2010/119264 PCT/GB2010/000773
(xi) hydrogenations, e.g. of a double bond to a single bond for instance under
standard hydrogenation reaction conditions, e.g. under a hydrogen atmosphere
in
the presence of a catalyst such as Pd/C;
(xii) Grignard reactions, e.g. the addition of a nucleophilic organometalic
reagent,
for instance the addition of MeMgCI to a carbonyl group;
(xiii) formation of a urea functional group by reaction of a isocyanate with
an
amine, e.g. when R5 represent phenyl substituted by -NH2, this may be
converted
to a -N(H)-C(O)-N(R20)R21 moiety.
Intermediate compounds described herein are either commercially available, are
known in the literature, or may be obtained either by analogy with the
processes
described herein, or by conventional synthetic procedures, in accordance with
standard techniques, from available starting materials using appropriate
reagents
and reaction conditions. Further, processes to prepare compounds of formula I
may be described in the literature, for example in:
Werber,G. et al.; J. Heterocycl. Chem.; EN; 14; 1977; 823-827;
Andanappa K. Gadad et al. Bioorg. Med. Chem. 2004, 12, 5651-5659;
Paul Heinz et al. Monatshefte fur Chemie, 1977, 108, 665-680;
M.A. EI-Sherbeny et al. Boll. Chim. Farm. 1997, 136, 253-256;
Nicolaou, K. C.; Bulger, P. G.; Sarlah, D. Angew. Chem. Int. Ed. 2005, 44, 2-
49;
Bretonnet et al. J. Med. Chem. 2007, 50,1872;
Asuncion Marin et al. Farmaco 1992, 47 (1), 63-75;
Severinsen, R. et al. Tetrahedron 2005, 61, 5565-5575;
Nicolaou, K. C.; Bulger, P. G.; Sarlah, D. Angew. Chem. Int. Ed. 2005, 44, 2-
49;
M. Kuwahara et al., Chem. Pharm Bull., 1996, 44, 122;
Wipf, P.; Jung, J.-K. J. Org. Chem. 2000, 65(20), 6319-6337;
Shintani, R.; Okamoto, K. Org. Lett. 2005, 7 (21), 4757-4759;
Nicolaou, K. C.; Bulger, P. G.; Sarlah, D. Angew. Chem. Int. Ed. 2005, 44, 2-
49;
J. Kobe et al., Tetrahedron, 1968, 24, 239;
P.F. Fabio, A.F. Lanzilotti and S.A. Lang, Journal of Labelled Compounds and
Pharmaceuticals, 1978, 15, 407;
F.D. Bellamy and K. Ou, Tetrahedron Lett., 1985, 25, 839;
M. Kuwahara et al., Chem. Pharm Bull., 1996, 44, 122;
A.F. Abdel-Magid and C.A Maryanoff. Synthesis, 1990, 537;
63
-0&26
WO 2010/119264 PCT/GB2010/000773
M. Schlosser et al. Organometallics in Synthesis. A Manual, (M. Schlosser,
Ed.),
Wiley &Sons Ltd: Chichester, UK, 2002, and references cited therein;
L. Wengwei et al., Tetrahedron Lett., 2006, 47, 1941;
M. Plotkin et al. Tetrahedron Lett., 2000, 41, 2269;
Seyden-Penne, J. Reductions by the Alumino and Borohydrides, VCH, NY, 1991;
O. C. Dermer, Chem. Rev., 1934, 14, 385;
N. Defacqz, et al., Tetrahedron Lett., 2003, 44, 9111;
S.J. Gregson et al., J. Med. Chem., 2004, 47, 1161;
A. M. Abdel Magib, et al., J. Org. Chem., 1996, 61, 3849;
A.F. Abdel-Magid and C.A Maryanoff. Synthesis, 1990, 537;
T. Ikemoto and M. Wakimasu, Heterocycles, 2001, 55, 99;
E. Abignente et al., 11 Farmaco, 1990, 45, 1075;
T. Ikemoto et al., Tetrahedron, 2000, 56, 7915;
T. W. Greene and P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley,
NY, 1999;
S. Y. Han and Y.-A. Kim. Tetrahedron, 2004, 60, 2447;
J. A. H. Lainton et al., J. Comb. Chem., 2003, 5, 400; or
Wiggins, J. M. Synth. Commun., 1988, 18, 741.
For example, intermediate compounds, and compounds of the invention may be
prepared in accordance with the following scheme (Scheme I).
64
-0&26
WO 2010/119264 PCT/GB2010/000773
0 0I~ R1
NN R5-B(OR)2 NN 1. POCI3 N N
Br' NR2 Vlll R5 v ~~IN~R2 2. RI-B(OR)2 R5 NCI ~R2 R3 R3 XVII
xx Ax
for II-a MeONa XVIII
MeOH
Br R2-C( Br for ll-a Br R1
Ill-a
N~ I NHZ 111-a NN\~ N NXS N~ N R2 I. R1-Nu VII N
~N X vN R2 XIII Br' v N~ 2. R5-B(OR)2 VIII I` N R2
Br R2-C(=0)CH-R3-X Br RS, ~, ~l\
1-01 III-b R3 x
X
11-a (R3=H)
X XI
II-b (RxH
R1-Nu RI-Nu R3-B(OR)2
VII VII XIV
R1 forlV-b R1
R1
NH R2-C(=0)CH2X ~NN R5-B(OR)2 Ni N
Br" ZR2-C( IIO)CH-R3 X 8r" N~R2 VIII RS~N~ R2
R3 R3
III-b
V IV-a (R3=H) X11-a
IV-b (R3xH) X11-b
R5-B(OH)2
VIII for IV-a
R5-B(OR)2
R1 VIII
&--NNH 2
RSJ R1 R1 R1
XVI i N NXS i _N~ R4-X N, _N
~R2 N R2 Nom/ R2
Bat` ezt XX R5 All R XVI R5 \4
/\ (\ X R4
R3 R3 VI
IX XV
RI
NN N
R5" N
X11-b R3
R2= H
Compound 1-01 was reacted with an intermediate (III-a) of formula
R2-C(=O)-CH2-X or an intermediate (III-b) of formula R2-C(=O)-CH-R3-X, where
R2 and R3 are as hereinbefore defined and X represents a suitable leaving
group
(e.g. a halide), without solvent or in the presence of a suitable reaction
solvent
such as DME or 2-propanol, at a convenient temperature, typically heating at
90 C, to obtain compounds of formula (II-a) or formula (II-b).
Compounds of formula (II-a) can be reacted with a halogenating agent, such as
N-bromoSuccinimide, N-iodosuccininide, N-chlorosuccinimide or others, and X
represents an halogen group such as Cl, Br or Iodine atom, in the presence of
a
-0&26
WO 2010/119264 PCT/GB2010/000773
suitable reaction solvent such as CHCI3, typically heating at a convenient
temperature, either by conventional heating under reflux or under microwave
irradiation, for a period of time to ensure the completion of the reaction, to
obtain
compounds of formula (X).
Compounds of formula (X) can react with an intermediate (VII) of formula R'-
Nu,
where R' is as hereinbefore defined and Nu represents a nucleophilic group,
such as an amine (and R1-Nu together form the group that is to be linked to
the
imidazopyrazine) in a suitable solvent such as DCM, dioxane at room
temperature or by heating at a convenient temperature, for a period of time to
ensure the completion of the reaction. Further, reaction may be with an
intermediate (VIII) of formula R5-B(OR)2, which R is H or C1-C6 alkyl or the
two
groups OR form, together with the boron atom to which they are attached a
pinacolato boronate ester group, and where R5 is as defined before, in a
suitable
solvent such as DME or DMF, in the presence of a suitable base, such as an
inorganic aqueous base Na2CO3 or K2CO3, in the presence of a metal catalyst,
such as palladium, and a suitable ligand, such us PdCl2(dppf).DCM, Pd(PPh3)4
by
heating at a convenient temperature, such as 130 C under microwave irradiation
or reflux temperature under traditional heating, for a period of time that
allows the
completion of reaction, to obtain compounds of formula (XI).
Compounds of formula (XI) can react with an intermediate (XIV) of formula
R3-B(OR)2, in which the -B(OR)2 moeity is as defined above, and R3 is as
hereinbefore defined, under conditions such as those described hereinbefore
(e.g. reaction of (X) with (VIII); e.g. microwave irradiation conditions at
about
140 C may be deployed), to obtain compounds of formula (XII-a).
Compounds of formula (II-b) can react with an intermediate (VII) of formula
R1-Nu (as hereinbefore defined), in a suitable solvent such as DCM, dioxane at
room temperature or by heating, for a period of time to ensure the completion
of
the reaction to afford compounds of formula (IV-b).
Compounds of formula (IV-b) can react with an intermediate (VIII) of formula
R5-B(OR)2 as hereinbefore defined, under reaction conditions hereinbefore
66
-0&26
WO 2010/119264 PCT/GB2010/000773
described (e.g. the reaction of (X) with (VIII)), to obtain compounds of
formula
(XI I-b).
Compound 1-01 can react with an intermediate (VII) of formula R'-Nu (as
hereinbefore defined), at a convenient temperature, such us 120 C, for a
period
of time that allows the completion of reaction, to afford compound (V).
Compound (V) was reacted with an intermediate (III-a) of formula
R2-C(=O)-CH2-X or an intermediate (III-b) of formula R2-C(=O)-CH-R3-X, both of
which are as hereinbefore defined, under reaction conditions hereinbefore
described (e.g. the reaction of (1-01) with (III-a) or (III-b)), to obtain
compounds of
formula (IV-a).
Compounds of formula (IV-a) can react with an intermediate (VIII) of formula
R5-B(OR)2i as hereinbefore defined, e.g. under reaction conditions
hereinbefore
described (e.g. the reaction of (X) with (VIII)), to obtain compounds of
formula
(VI).
Compounds of formula (VI) can be reacted with a halogenating agent, for
example as described hereinbefore (e.g. reaction of (II-a) to (X)), to obtain
compounds of formula (IX).
The halogen atom X of compounds of formula (IX) can be substituted via a
coupling reaction with an intermediate (XVI) of formula R4-B(OR)2i in which
the
-B(OR)2 moiety is as hereinbefore defined, and R4 is as hereinbefore defined,
e.g. under reaction conditions hereinbefore described (e.g. the reaction of
(X)
with (VIII)), for a period of time that allows the completion of reaction, to
obtain
compounds of formula XV.
67
-0&26
WO 2010/119264 PCT/GB2010/000773
The halogen atom X of compounds of formula (IX) can be substituted via
coupling
reaction of a CN group, by treatment with Zn(CN)2, in a suitable solvent such
as
DMF, AcCN and in the presence of a Pd catalyst, such us Pd(PPh3)4 or
PdCI2(dppf)2. Additionally an inorganic aqueous base can be added such as
Na2CO3 aq. Heating at a convenient temperature, such as 130 C under
microwave irradiation or reflux temperature under traditional heating, for a
period
of time that allows the completion of reaction, to obtain compounds of formula
XV.
Compounds of formula (V) can react with an intermediate (VIII) of formula
R5-B(OR)2 as hereinbefore defined, e.g. under reaction conditions hereinbefore
described (e.g. the reaction of (X) with (VIII)), to obtain compounds of
formula
(XVI).
Compounds of formula (XVI) can react with an intermediate of formula XX, in
which Bzt is Benzotriazol, following similar conditions reported in literature
(J.
Org.Chem. 1990, 55, 3209-3213, J.Org.Chem, 2003, 68, 4935-4937), in a
suitable solvent, such as DCE, heating at a convenient temperature, in a
period
of time to ensure completion of the reaction, typically at reflux for 5h.
Additionally
an inorganic base can be added to ensure completion of the reaction.
Compounds of formula (ll-a) can react with sodium methoxide in the presence of
methanol, at room temperature or by heating at a convenient temperature, such
as 60 C, to obtain compounds of formula (XVII).
Compounds of formula (XVII) can react with an intermediate (VIII) of formula
R5-B(OR)2 as hereinbefore defined, e.g. under reaction conditions hereinbefore
described (e.g. the reaction of (X) with (VIII)), to obtain compounds of
formula
(XVIII).
Compound of formula (XVIII) can react with POC13 by heating, typycally to
reflux,
for a period of time to ensure the completion of the reaction, to afford the
replacement of the methoxy group by chlorine atom. Coupling of the chlorine
atom with an intermediate (XX) of formula R'-B(OR)2 in which the -B(OR)2
moiety
68
-0&26
WO 2010/119264 PCT/GB2010/000773
and R' are as hereinbefore defined, e.g. under reaction conditions
hereinbefore
described (e.g. the reaction of (X) with (VIII)), to obtain compounds of
formula
(XIX).
Those skilled in the art will appreciate that other synthetic routes may be
used to
synthesize the compounds of the invention. Although specific starting
materials
and reagents are depicted in the Scheme 1 and discussed below, other starting
materials and reagents can be easily substituted to provide a variety of
derivatives and/or reaction conditions.
The substituents R', R2, R3, R4 and R5 in final compounds of the invention or
relevant intermediates may be modified one or more times, after or during the
processes described above by way of methods that are well known to those
skilled in the art. Examples of such methods include substitutions,
reductions,
oxidations, alkylations, acylations, hydrolyses, esterifications,
etherifications,
halogenations or nitrations. Such reactions may result in the formation of a
symmetric or asymmetric final compound of the invention or intermediate. The
precursor groups can be changed to a different such group, or to the groups
defined in formula I, at any time during the reaction sequence.
For example, when R2, R3 and R4 groups such as CO2Et, CHO, CN and/or CH2CI,
are present, these groups can be further derivatized to other fragments
described
in R2, R3 and R4 in compounds of the invention, following synthetic protocols
very
well know to the person skilled in the art and/or according to the
experimental
part described in the patent. Other specific transformation steps that may be
mentioned include: the reduction of a nitro or azido group to an amino group;
the
hydrolysis of a nitrile group to a carboxylic acid group; and standard
nucleophilic
aromatic substitution reactions, for example in which an iodo-, preferably,
fluoro-
or bromo-phenyl group is converted into a cyanophenyl group by employing a
source of cyanide ions (e.g. by reaction with a compound which is a source of
cyano anions, e.g. sodium, copper (I), zinc or potassium cyanide, optionally
in the
presence of a palladium catalyst) as a reagent (alternatively, in this case,
palladium catalysed cyanation reaction conditions may also be employed).
69
-0&26
WO 2010/119264 PCT/GB2010/000773
Other transformations that may be mentioned include: the conversion of a halo
group (preferably iodo or bromo) to a 1-alkynyl group (e.g. by reaction with a
1-
alkyne), which latter reaction may be performed in the presence of a suitable
coupling catalyst (e.g. a palladium and/or a copper based catalyst) and a
suitable
base (e.g. a tri-(C1_6 alkyl)amine such as triethylamine, tributylamine or
ethyldiisopropylamine); the introduction of amino groups and hydroxy groups in
accordance with standard conditions using reagents known to those skilled in
the
art; the conversion of an amino group to a halo, azido or a cyano group, for
example via diazotisation (e.g. generated in situ by reaction with NaNO2 and a
strong acid, such as HCI or H2SO4, at low temperature such as at 0 C or below,
e.g. at about -5 C) followed by reaction with the appropriate nucleophile e.g.
a
source of the relevant anions, for example by reaction in the presence of a
halogen gas (e.g. bromine, iodine or chlorine), or a reagent that is a source
of
azido or cyanide anions, such as NaN3 or NaCN; the conversion of -C(O)OH to a
-NH2 group, under Schmidt reaction conditions, or variants thereof, for
example in
the presence of HN3 (which may be formed in by contacting NaN3 with a strong
acid such as H2SO4), or, for variants, by reaction with diphenyl phosphoryl
azide
((PhO)2P(O)N3) in the presence of an alcohol, such as tert-butanol, which may
result in the formation of a carbamate intermediate; the conversion of -
C(O)NH2
to -NH2, for example under Hofmann rearrangement reaction conditions, for
example in the presence of NaOBr (which may be formed by contacting NaOH
and Br2) which may result in the formation of a carbamate intermediate; the
conversion of -C(O)N3 (which compound itself may be prepared from the
corresponding acyl hydrazide under standard diazotisation reaction conditions,
e.g. in the presence of NaNO2 and a strong acid such as
H2SO4 or HCI) to -NH2, for example under Curtius rearrangement reaction
conditions, which may result in the formation of an intermediate isocyanate
(or a
carbamate if treated with an alcohol); the conversion of an alkyl carbamate to
-NH2, by hydrolysis, for example in the presence of water and base or under
acidic conditions, or, when a benzyl carbamate intermediate is formed, under
hydrogenation reaction conditions (e.g. catalytic hydrogenation reaction
conditions in the presence of a precious metal catalyst such as Pd);
halogenation
of an aromatic ring, for example by an electrophilic aromatic substitution
reaction
in the presence of halogen atoms (e.g. chlorine, bromine, etc, or an
equivalent
-0&26
WO 2010/119264 PCT/GB2010/000773
source thereof) and, if necessary an appropriate catalyst/Lewis acid (e.g.
AICI3 or
FeCl3).
Compounds of the invention bearing a carboxyester functional group may be
converted into a variety of derivatives according to methods well known in the
art
to convert carboxyester groups into carboxamides, N-substituted carboxamides,
N,N-disubstituted carboxamides, carboxylic acids, and the like. The operative
conditions are those widely known in the art and may comprise, for instance in
the conversion of a carboxyester group into a carboxamide group, the reaction
with ammonia or ammonium hydroxide in the presence of a suitable solvent such
as a lower alcohol, dimethylformamide or a mixture thereof; preferably the
reaction is carried out with ammonium hydroxide in a methanol/dimethyl-
formamide mixture, at a temperature ranging from about 500C to about 100 C.
Analogous operative conditions apply in the preparation of N-substituted or
N,N-
disubstituted carboxamides wherein a suitable primary or secondary amine is
used in place of ammonia or ammonium hydroxide. Likewise, carboxyester
groups may be converted into carboxylic acid derivatives through basic or
acidic
hydrolysis conditions, widely known in the art. Further, amino derivatives of
compounds of the invention may easily be converted into the corresponding
carbamate, carboxamido or ureido derivatives.
Compounds of the invention may be isolated from their reaction mixtures using
conventional techniques (e.g. recrystallisations).
It will be appreciated by those skilled in the art that, in the processes
described
above and hereinafter, the functional groups of intermediate compounds may
need to be protected by protecting groups.
The need for such protection will vary depending on the nature of the remote
functionality and the conditions of the preparation methods (and the need can
be
readily determined by one skilled in the art). Suitable amino-protecting
groups
include acetyl, trifluoroacetyl, t-butoxycarbonyl (BOC), benzyloxycarbonyl
(CBz)
and 9-fluorenylmethyleneoxycarbonyl (Fmoc). The need for such protection is
readily determined by one skilled in the art.
71
-0&26
WO 2010/119264 PCT/GB2010/000773
The protection and deprotection of functional groups may take place before or
after a reaction in the above-mentioned schemes.
Protecting groups may be removed in accordance with techniques that are well
known to those skilled in the art and as described hereinafter. For example,
protected compounds/intermediates described herein - may be converted
chemically to unprotected compounds using standard deprotection techniques.
The type of chemistry involved will dictate the need, and type, of protecting
groups as well as the sequence for accomplishing the synthesis.
The use of protecting groups is fully described in "Protective Groups in
Organic
Synthesis", 3`d edition, T.W. Greene & P.G.M. Wutz, Wiley-Interscience (1999).
Medical and Pharmaceutical Uses
Compounds of the invention are indicated as pharmaceuticals. According to a
further aspect of the invention there is provided a compound of the invention,
as
hereinbefore defined, for use as a pharmaceutical.
For the avoidance of doubt, although compounds of the invention may possess
pharmacological activity as such, certain pharmaceutically-acceptable (e.g.
"protected") derivatives of compounds of the invention may exist or be
prepared
which may not possess such activity, but may be administered parenterally or
orally and thereafter be metabolised in the body to form compounds of the
invention. Such compounds (which may possess some pharmacological activity,
provided that such activity is appreciably lower than that of the "active"
compounds to which they are metabolised) may therefore be described as
"prodrugs" of compounds of the invention.
A "prodrug of a compound of the invention" is as hereinbefore defined,
including
compounds that form a compound of the invention, in an experimentally-
detectable amount, within a predetermined time (e.g. about 1 hour), following
oral
or parenteral administration. All prodrugs of the compounds of the invention
are
included within the scope of the invention.
72
-0&26
WO 2010/119264 PCT/GB2010/000773
Furthermore, certain compounds of the invention may possess no or minimal
pharmacological activity as such, but may be administered parenterally or
orally,
and thereafter be metabolised in the body to form compounds of the invention
that possess pharmacological activity as such. Such compounds (which also
includes compounds that may possess some pharmacological activity, but that
activity is appreciably lower than that of the "active" compounds of the
invention
to which they are metabolised), may also be described as "prodrugs".
Thus, the compounds of the invention are useful because they possess
pharmacological activity, and/or are metabolised in the body following oral or
parenteral administration to form compounds which possess pharmacological
activity.
Compounds of the invention may inhibit protein or lipid kinases, such as a P13
kinase (especially a class I P13K) or mTOR, for example as may be shown in the
tests described below (for example, the test for P13Ka inhibition described
below)
and/or in tests known to the skilled person. Thus, the compounds of the
invention
may be useful in the treatment of those disorders in an individual in which
the
inhibition of such protein or lipid kinases (e.g. P13K, particularly class I
P13K,
and/or mTOR) is desired and/or required.
The term "inhibit" may refer to any measurable reduction and/or prevention of
catalytic kinase (e.g. P13K, particularly class I P13K, and/or mTOR) activity.
The
reduction and/or prevention of kinase activity may be measured by comparing
the
kinase activity in a sample containing a compound of the invention and an
equivalent sample of kinase (e.g. P13K, particularly class I P13K, and/or
mTOR) in
the absence of a compound of the invention, as would be apparent to those
skilled in the art. The measurable change may be objective (e.g. measurable by
some test or marker, for example in an in vitro or in vivo assay or test, such
as
one described hereinafter, or otherwise another suitable assay or test known
to
those skilled in the art) or subjective (e.g. the subject gives an indication
of or
feels an effect).
73
-0&26
WO 2010/119264 PCT/GB2010/000773
Compounds of the invention may be found to exhibit 50% inhibition of a protein
or
lipid kinase (e.g. P13K, such as class I P13K, and/or mTOR) at a concentration
of
100 pM or below (for example at a concentration of below 50 pM, or even below
pM, such as below 1 pM), when tested in an assay (or other test), for example
5 as described hereinafter, or otherwise another suitable assay or test known
to the
skilled person.
Compounds of the invention are thus expected to be useful in the treatment of
a
disorder in which a protein or lipid kinase (e.g. P13K, such as class I P13K,
and/or
10 mTOR) is known to play a role and which are characterised by or associated
with
an overall elevated activity of that kinase (due to, for example, increased
amount
of the kinase or increased catalytic activity of the kinase). Hence, compounds
of
the invention are expected to be useful in the treatment of a disease/disorder
arising from abnormal cell growth, function or behaviour associated with the
protein or lipid kinase (e.g. P13K, such as class I P13K, and/or mTOR). Such
conditions/disorders include cancer, immune disorders, cardiovascular
diseases,
viral infections, inflammation, metabolism/endocrine function disorders and
neurological disorders.
The disorders/conditions that the compounds of the invention may be useful in
treating hence includes cancer (such as lymphomas, solid tumours or a cancer
as
described hereinafter), obstructive airways diseases, allergic diseases,
inflammatory diseases (such as asthma, allergy and Chrohn's disease),
immunosuppression (such as transplantation rejection and autoimmune
diseases), disorders commonly connected with organ transplantation, AIDS-
related diseases and other associated diseases. Other associated diseases that
may be mentioned (particularly due to the key role of kinases in the
regulation of
cellular proliferation) include other cell proliferative disorders and/or non-
malignant diseases, such as benign prostate hyperplasia, familial
adenomatosis,
polyposis, neuro-fibromatosis, psoriasis, bone disorders, atherosclerosis,
vascular smooth cell proliferation associated with atherosclerosis, pulmonary
fibrosis, arthritis glomerulonephritis and post-surgical stenosis and
restenosis.
Other disease states that may be mentioned include cardiovascular disease,
stroke, diabetes, hepatomegaly, Alzheimer's disease, cystic fibrosis, hormone-
related diseases, immunodeficiency disorders, destructive' bone disorders,
74
-0&26
WO 2010/119264 PCT/GB2010/000773
infectious diseases, conditions associated with cell death, thrombin-induced
platelet aggregation, chronic myelogenous leukaemia, liver disease, pathologic
immune conditions involving T cell activation and CNS disorders.
As stated above, the compounds of the invention may be useful in the treatment
of cancer. More, specifically, the compounds of the invention may therefore be
useful in the treatment of a variety of cancer including, but not limited to:
carcinoma such as cancer of the bladder, breast, colon, kidney, liver, lung
(including non-small cell cancer and small cell lung cancer), esophagus, gall-
bladder, ovary, pancreas, stomach, cervix, thyroid, prostate, skin, squamous
cell
carcinoma, testis, genitourinary tract, larynx, glioblastoma, neuroblastoma,
keratoacanthoma, epidermoid carcinoma, large cell carcinoma, non-small cell
lung carcinoma, small cell lung carcinoma, lung adenocarcinoma, bone,
adenoma, adenocarcinoma, follicular carcinoma, undifferentiated carcinoma,
papilliary carcinoma, seminona, melanoma, sarcoma, bladder carcinoma, liver
carcinoma and biliary passages, kidney carcinoma, myeloid disorders, lymphoid
disorders, hairy cells, buccal cavity and pharynx (oral), lip, tongue, mouth,
pharynx, small intestine, colon-rectum, large intestine, rectum, brain and
central
nervous system, Hodgkin's and leukaemia; hematopoietic tumors of lymphoid
lineage, including leukemia, acute lymphocitic leukemia, acute lymphoblastic
leukemia, B-cell lymphoma, T-cell-lymphoma, Hodgkin's lymphoma, non-
Hodgkin's lymphoma, hairy cell lymphoma and Burkett's lymphoma;
hematopoietic tumors of myeloid lineage, including acute and chronic
myelogenous leukemias, myelodysplastic syndrome and promyelocytic leukemia;
tumors of mesenchymal origin, including fibrosarcoma and rhabdomyosarcoma;
tumors of the central and peripheral nervous system, including astrocytoma,
neuroblastoma, glioma and schwannomas; and other tumors, including
melanoma, seminoma, teratocarcinoma, osteosarcoma, xeroderma
pigmentosum, keratoxanthoma, thyroid follicular cancer and Kaposi's sarcoma.
Further, the protein or lipid kinases (e.g. P13K, such as class I P13K, and/or
mTOR) may also be implicated in the multiplication of viruses and parasites.
They may also play a major role in the pathogenesis and development of
neurodegenerative disorders. Hence, compounds of the invention may also be
-0&26
WO 2010/119264 PCT/GB2010/000773
useful in the treatment of viral' conditions, parasitic conditions, as well as
neurodegenerative disorders.
Compounds of the invention are indicated both in the therapeutic and/or
prophylactic treatment of the above-mentioned conditions.
According to a further aspect of the present invention, there is provided a
method
of treatment of a disease (e.g. cancer or another disease as mentioned herein)
which is associated with the inhibition of protein or lipid kinase (e.g. P13K,
such as
class I P13K, and/or mTOR) is desired and/or required (for example, a method
of
treatment of a disease/disorder arising from abnormal cell growth, function or
behaviour associated with protein or lipid kinases, e.g. P13K, such as class I
P13K, and/or mTOR), which method comprises administration of a therapeutically
effective amount of a compound of the invention, as hereinbefore defined, to a
patient suffering from, or susceptible to, such a condition.
"Patients" include mammalian (including human) patients. Hence, the method of
treatment discussed above may include the treatment of a human or animal body.
The term "effective amount" refers to an amount of a compound, which confers a
therapeutic effect on the treated patient. The effect may be objective (e.g.
measurable by some test or marker) or subjective (e.g. the subject gives an
indication of or feels an effect).
Compounds of the invention may be administered orally, intravenously,
subcutaneously, buccally, rectally, dermally, nasally, tracheally,
bronchially,
sublingually, by any other parenteral route or via inhalation, in a
pharmaceutically
acceptable dosage form.
Compounds of the invention may be administered alone, but are preferably
administered by way of known pharmaceutical formulations, including tablets,
capsules or elixirs for oral administration, suppositories for rectal
administration,
sterile solutions or suspensions for parenteral or intramuscular
administration,
and the like. The type of pharmaceutical formulation may be selected with due
regard to the intended route of administration and standard pharmaceutical
76
-0&26
WO 2010/119264 PCT/GB2010/000773
practice. Such pharmaceutically acceptable carriers may be chemically inert to
the active compounds and may have no detrimental side effects or toxicity
under
the conditions of use.
Such formulations may be prepared in accordance with standard and/or accepted
pharmaceutical practice. Otherwise, the preparation of suitable formulations
may
be achieved non-inventively by the skilled person using routine techniques
and/or
in accordance with standard and/or accepted pharmaceutical practice.
According to a further aspect of the invention there is thus provided a
pharmaceutical formulation including a compound of the invention, as
hereinbefore defined, in admixture with a pharmaceutically acceptable
adjuvant,
diluent and/or carrier.
Depending on e.g. potency and physical characteristics of the compound of the
invention (i.e. active ingredient), pharmaceutical formulations that may be
mentioned include those in which the active ingredient is present in at least
1%
(or at least 10%, at least 30% or at least 50%) by weight. That is, the ratio
of
active ingredient to the other components (i.e. the addition of adjuvant,
diluent
and carrier) of the pharmaceutical composition is at least 1:99 (or at least
10:90,
at least 30:70 or at least 50:50) by weight.
The amount of compound of the invention in the formulation will depend on the
severity of the condition, and on the patient, to be treated, as well as the
compound(s) which is/are employed, but may be determined non-inventively by
the skilled person.
The invention further provides a process for the preparation of a
pharmaceutical
formulation, as hereinbefore defined, which process comprises bringing into
association a compound of the invention, as hereinbefore defined, or a
pharmaceutically acceptable ester, amide, solvate or salt thereof with a
pharmaceutically-acceptable adjuvant, diluent or carrier.
Compounds of the invention may also be combined with other therapeutic agents
that are inhibitors of protein or lipid kinases (e.g. P13K, such as class I
P13K,
77
-0&26
WO 2010/119264 PCT/GB2010/000773
and/or mTOR) and/or useful in the treatment of a cancer and/or a proliferative
disease. Compounds of the invention may also be combined with other
therapies.
According to a further aspect of the invention, there is provided a
combination
product comprising:
(A) a compound of the invention, as hereinbefore defined; and
(B) another therapeutic agent that is useful in the treatment of cancer and/or
a
proliferative disease,
wherein each of components (A) and (B) is formulated in admixture with a
pharmaceutically-acceptable adjuvant, diluent or carrier.
Such combination products provide for the administration of a compound of the
invention in conjunction with the other therapeutic agent, and may thus be
presented either as separate formulations, wherein at least one of those
formulations comprises a compound of the invention, and at least one comprises
the other therapeutic agent, or may be presented (i.e. formulated) as a
combined
preparation (i.e. presented as a single formulation including a compound of
the
invention and the other therapeutic agent).
Thus, there is further provided:
(1) a pharmaceutical formulation including a compound of the invention, as
hereinbefore defined, another therapeutic agent that is useful in the
treatment of
cancer and/or a proliferative disease, and a pharmaceutically-acceptable
adjuvant, diluent or carrier; and
(2) a kit of parts comprising components:
(a) a pharmaceutical formulation including a compound of the invention, as
hereinbefore defined, in admixture with a pharmaceutically-acceptable
adjuvant, diluent or carrier; and
(b) a pharmaceutical formulation including another therapeutic agent that is
useful in the treatment of cancer and/or a proliferative disease in
admixture with a pharmaceutically-acceptable adjuvant, diluent or carrier,
78
0&26
WO 2010/119264 PCT/GB2010/000773
which components (a) and (b) are each provided in a form that is suitable for
administration in conjunction with the other.
The invention further provides a process for the preparation of a combination
product as hereinbefore defined, which process comprises bringing into
association a compound of the invention, as hereinbefore defined, or a
pharmaceutically acceptable ester, amide, solvate or salt thereof with the
other
therapeutic agent that is useful in the treatment of cancer and/or a
proliferative
disease, and at least one pharmaceutically-acceptable adjuvant, diluent or
carrier.
By "bringing into association", we mean that the two components are rendered
suitable for administration in conjunction with each other.
Thus, in relation to the process for the preparation of a kit of parts as
hereinbefore defined, by bringing the two components "into association with"
each other, we include that the two components of the kit of parts may be:
(i) provided as separate formulations (i.e. independently of one another),
which
are subsequently brought together for use in conjunction with each other in
combination therapy; or
(ii) packaged and presented together as separate components of a "combination
pack" for use in conjunction with each other in combination therapy.
Depending on the disorder, and the patient, to be treated, as well as the
route of
administration, compounds of the invention may be administered at varying
therapeutically effective doses to a patient in need thereof. However, the
dose
administered to a mammal, particularly a human, in the context of the present
invention should be sufficient to effect a therapeutic response in the mammal
over a reasonable timeframe. One skilled in the art will recognize that the
selection of the exact dose and composition and the most appropriate delivery
regimen will also be influenced by inter alia the pharmacological properties
of the
formulation, the nature and severity of the condition being treated, and the
physical condition and mental acuity of the recipient, as well as the potency
of the
specific compound, the age, condition, body weight, sex and response of the
patient to be treated, and the stage/severity of the disease.
79
-0&26
WO 2010/119264 PCT/GB2010/000773
Administration may be continuous or intermittent (e.g. by bolus injection).
The
dosage may also be determined by the timing and frequency of administration.
In
the case of oral or parenteral administration the dosage can vary from about
0.01
mg to about 1000 mg per day of a compound of the invention.
In any event, the medical practitioner, or other skilled person, will be able
to
determine routinely the actual dosage, which will be most suitable for an
individual patient. The above-mentioned dosages are exemplary of the average
case; there can, of course, be individual instances where higher or lower
dosage
ranges are merited, and such are within the scope of this invention.
Compounds of the invention may have the advantage that they are effective
inhibitors of protein or lipid kinases (e.g. P13K, such as class I P13K,
and/or
mTOR).
Compounds of the invention may also have the advantage that they may be more
efficacious than, be less toxic than, be longer acting than, be more potent
than,
produce fewer side effects than, be more easily absorbed than, and/or have a
better pharmacokinetic profile (e.g. higher oral bioavailability and/or lower
clearance) than, and/or have other useful pharmacological, physical, or
chemical
properties over, compounds known in the prior art, whether for use in the
above-
stated indications or otherwise.
Examples/Biological Tests
Determination of the activity of P13 kinase activity of compounds of the
invention
is possible by a number of direct and indirect detection methods. Certain
exemplary compounds described herein were prepared, characterized, and
tested for their P13K binding activity and in vitro activity against tumor
cells. The
range of P13K binding activities was less than 1 nM to about 10 M (i.e.
certain
compounds of the examples/invention had P13K binding activity IC50 values of
less than 10 nM). Compounds of the examples/invention had tumor cell-based
activity IC50 values less than 100 nM (see Table 4 below).
-0&26
WO 2010/119264 PCT/GB2010/000773
P13K activity assay
The kinase activity was measured by using the commercial ADP Hunter TM Plus
assay available from DiscoveRy (#33-016), which is a homogeneous assay to
measure the accumulation of ADP, a universal product of kinase activity. The
enzyme, P13K (p110a/p85a was purchased from Carna Biosciences (#07CBS-
0402A). The assay was done following the manufacturer recommendations with
slight modifications: Mainly the kinase buffer was replace by 50 mM HEPES, pH
7.5, 3 mM MgCI2i 100 mM NaCl, 1 mM EGTA, 0.04% CHAPS, 2 mM TCEP and
0.01 mg/ml BGG. The P13K was assayed in a titration experiment to determine
the optimal protein concentration for the inhibition assay. To calculate the
IC50 of
the ETP-compounds, serial 1:5 dilutions of the compounds were added to the
enzyme at a fixed concentration (2.5 gg/ml. The enzyme was preincubated with
the inhibitor and 30 M PIP2 substrate (P9763, Sigma) for 5 min and then ATP
was added to a final 50 M concentration. Reaction was carried out for 1 hour
at
C. Reagent A and B were sequentially added to the wells and plates were
incubated for 30 min at 37 C. Fluorescence counts were read in a Victor
instrument (Perkin Elmer) with the recommended settings (544 and 580 nm as
excitation and emission wavelengths, respectively). Values were normalized
20 against the control activity included for each enzyme (i.e., 100 % P13
kinase
activity, without compound). These values were plot against the inhibitor
concentration and were fit to a sigmoid dose-response curve by using the
Graphad software.
25 Cellular Mode of Action
Cell culture: The cell lines were obtained from the American Type Culture
Collection (ATCC). U20S (human osteosarcoma) was cultured in Dulbecco's
modified Eagle's medium (DMEM). PC3 (human prostate carcinoma), MCF7
(human breast cardinoma), HCT116 (human colon carcinoma), 768-0 (human
neuroblastoma), U251 (human glyoblastoma) were grown in RPMI. All media
were supplemented with 10% fetal bovine serum (FBS) (Sigma) and antibiotics-
antimycotics. Cell were maintained in a humidified incubator at 37 C with 5%
CO2
and passaged when confluent using trypsin/EDTA.
81
0&26
WO 2010/119264 PCT/GB2010/000773
U2foxRELOC and U2nesRELOC assay: The U2nesRELOC assay and the
U2foxRELOC assay have been described previously (1, 2). Briefly, cells were
seeded at a density of 1.0x 105 cells/ml into black-wall clear-bottom 96-well
microplates (BD Biosciences) After incubation at 37 C with 5% CO2 for 12
hours,
20 of each test compound were transferred from the mother plates to the assay
plates. Cells were incubated in the presence of the compounds for one hour.
Then cells were fixed and the nucleus stained with DAPI (Invitrogen). Finally
the
plates were washed with 1X PBS twice and stored at 4 C before analysis.
Compounds of the invention have a range of in vitro cell potency activities
from
about 1 nM to about 10 M.
Image acquirement and processing: Assay plates were read on the BD
PathwayTM 855 Bioimager equipped with a 488/10 nm EGFP excitation filter, a
380/10 nm DAPI excitation filter, a 515LP nm EGFP emission filter and a 435LP
nm DAPI emission filter. Images were acquired in the DAPI and GFP channels of
each well using 10x dry objective. The plates were exposed 0.066 ms (Gain 31)
to acquire DAPI images and 0.55 ms (Gain 30) for GFP images.
Data analysis: The BD Pathway Bioimager outputs its data in standard text
files.
Data were imported into the data analysis software BD Image Data Explorer. The
nuclear/cytoplasmic (Nuc/Cyt) ratios of fluorescence intensity were determined
by
dividing the fluorescence intensity of the nucleus by the cytoplasmic. A
threshold
ratio of greater than 1.8 was employed to define nuclear accumulation of
fluorescent signal for each cell. Based on this procedure we calculated the
percentage of cells per well displaying nuclear translocation or inhibition of
nuclear export. Compounds that induced a nuclear accumulation of the
fluorescent signal greater than 60% of that obtained from wells treated with
4nM
LMB were considered as hits. In order to estimate the quality of the HCS
assay,
the Z' factor was calculated by the equation: Z' = 1 - [(3 x std. dev. of
positive
controls) + (3 x std. dev. of negative controls) / (mean of positive controls)
-
(mean of negative controls)].
P13K signalling
AKT phosphorylation Inhibition. Western Blot Analysis: Subconfluent cells
were incubated under different conditions and washed twice with TBS prior to
82
-0&26
WO 2010/119264 PCT/GB2010/000773
lysis. Lysis buffer was added containing 50 mM Tris HCI, 150 mM NaCl, 1 % NP-
40, 2mM Na3VO4, 100 mM NaF, 20 mM Na4P2O7 and protease inhibitor cocktail
(Roche Molecular Biochemicals). The proteins were resolved on 10% SDS-PAGE
and transferred to nitrocellulose membrane (Schleicher & Schuell, Dassel,
Germany). The membranes were incubated overnight at 4 C with antibodies
specific for Akt, phospho-Ser-473-Akt (Cell Signaling Technology) and a-
tubulin
(Sigma), they were washed and then incubated with IRDye800 conjugated anti-
mouse and Alexa Fluor 680 goat anti-rabbit IgG secondary antibodies. The bands
were visualized using an Odyssey infrared imaging system (Li-Cor Biosciences).
Compounds of the invention have a range of in vitro cell potency activities
from
about 1 nM to about 10 M.
Cytotoxicity assessment
The compounds were tested on 96-well trays. Cells growing in a flask were
harvested just before they became confluent, counted using a haemocytometer
and diluted down with media adjusting the concentration to the required number
of cells per 0.2 ml (volume for each well). Cells were then seeded in 96-well
trays
at a density between 1000 and 4000 cells/well, depending of the cell size.
Cells
were left to plate down and grow for 24 hours before adding the drugs. Drugs
were weighed out and diluted with DMSO to get them into solution to a
concentration of 10mM. From here a "mother plate" with serial dilutions was
prepared at 200X the final concentration in the culture. The final
concentration of
DMSO in the tissue culture media should not exceed 0.5%. The appropriate
volume of the compound solution (usually 2 microlitres) was added
automatically
(Beckman FX 96 tip) to media to make it up to the final concentration for each
drug. The medium was removed from the cells and replaced with 0.2 ml of
medium dosed with drug. Each concentration was assayed in triplicate. Two sets
of control wells were left on each plate, containing either medium without
drug or
medium with the same concentration of DMSO. A third control set was obtained
with the cells untreated just before adding the drugs (seeding control, number
of
cells starting the culture). Cells were exposed to the drugs for 72 hours and
then
processed for MTT colorimetric read-out. Compounds of the invention have a
range of in vitro cell potency activities from about 1 nM to about 10 M.
83
-0&26
WO 2010/119264 PCT/GB2010/000773
mTOR assay
Mammalian target of rapamycin (mTOR) was assayed by monitoring
phosphorylation of GFP-4EBP using a homogeneous time-resolved fluorescence
resonante energy transfer format and assay reagents from Invitrogen. In the
presence of 10 pM ATP, 50 mM Hepes (pH 7.5), 0.01 % (v/v) Polysorbate 20, 10
mM MnCI2, 1mM EGTA, and 2.5 mM DTT, the mTOR-mediated phosphorylation
of 200 nM GFP-4E-BP1 was measured under initial rate conditions. After
incubation at room temperature for 60 min, the reaction was terminated by
addition of 10 mM EDTA, and phosphorylated GFP-4E-BP1 was detected with 2
nM Tb-anti-p4E-BP1 antibody before reading on a Perkin-Elmer Wallac 1420
Fluorescence Reader (exc 340; em 490/520).
Where compound names are given herein, they are typically generated with
ChemDraw.
The invention is illustrated by way of the following examples, in which the
following abbreviations (or chemical symbols) may be employed:
"dba" dibenzylidene acetone; "DCM" dichloromethane; "MeOH" methanol; "EtOH"
ethanol; "THF" tetrahydrofuran; "DMF" dimethylformamide; "CHC13" chloroform;
"DME" dimethoxyethane; "Et20" diethyl ether; "Hex" hexane; "EtOAc" ethyl
acetate; "Pd(PPh3)4" tetrakis(triphenylphosphine)palladium; "KOAc" potassium
acetate; "DIPEA" diisopropylethylamine; "Pd(PPh3)4"
tetrakis(triphenylphosphine)-
palladium; "Pd(dppf)Cl2.DCM" 1,1'-bis(diphenylphosphino)ferrocenepalladium(11)
dichloride, dichloromethane; "min." minutes; and "h." hours.
Examples and Experimental
The intermediate compounds of Table 1 were prepared according to the
procedures A-1, A-2 and A-3 described hereinafter. The intermediate
compounds of Table 2 were prepared according to the procedures A-4 to A-28
described hereinafter. The final examples of compounds of the invention were
prepared according to the procedures B-1 to B-26 (and A-13) described
hereinafter. Procedures of methods A and B are described in more detail in the
the experimental hereinafter. If an experimental procedure is not specifically
described, the synthesis is performed in accordance with the methods described
herein, optionally with reference to procedures known to the skilled person. A
84
-0&26
WO 2010/119264 PCT/GB2010/000773
procedure to prepare a final compound may or may not be accompanied by
characterising data for that final compound.
Table 1 - P razine Intermediates
R1
N NH2
R2 No. Exp. --R1 --R2
Meth.
1-01 A-1 --Br --Br
1-02 A-2 4L "O --Br
1-03 A-3 --N o
H-N
1-04 A-3 f c9 /---\ O HO V
1-53 Al --CI --I
-iL
1-54 A3
H2N N
-0&26
WO 2010/119264 PCT/GB2010/000773
Table 2 - Intermediates
R1
Ni N
R2
R5 )yN
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
Meth.
1-05 A4 --Br --Me --H --H --Br
1-06 A5 --Br --Me --Br --H --Br
1-07 A4 --Br --CO2Et --H --H --Br
1-08 A4 --Br --CF3 --H --H --Br
1-09 A6 --Br --CO2Et --Br --H --Br
1-10 A7 -N O --Me --H --H --Br
I-11 A7 O --Me --Br --H --Br
1-12 A7 --N O --CO2Et --H --H --Br
1-13 A8 O --CHO --H --H --Br
1-14 A9 --CH2OH --H --H --Br
1-15 A7 f- J --H --H --Br
N N -< 0
1-16 A10 fNCO N o --H --H --Br
1-17 All N\_/NH --H --H --Br
I-18 A10 f N NON - o --H --H --Br
0
1-19 Al0 N\_2 NON--~ --H --H --Br
1-20 A10 fN o N CN- --H --H --Br
1-21 A10 47N~O N"H --H --H --Br
0
86
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
R5 yN
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
Meth.
1-22 A10 U- cO --H --H --Br
1-23 A12 frO /T - --H --H --Br
1-24 A12 N\_2 O ~-JN %`O --H --H --Br
1-25 A12N 0 ON- 0 --H --H --Br
N
1-26 A7 NV___.Zo --CF3 --H --H --Br
OXNH
--H __Cl
1-27 A14 --N o --Me 6
1-28 A4 --Me --CO2Et --H __Cl
1-29 A13 --Me --CO2H --H __Cl
1-30 A15 - co --CONH2 --H --H --Br
1-35 A4 f- N O --H --H __Cl
1-36 A7 f_NO --CO2Et --H --H --Br
1-37 A16 -Nc --CO2Et --H --H I s
1-38 A16 -NO --CO2Et --H --H
H2N N
87
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
R5y N
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
Meth.
o~ o
1-39 A18 v N --H -- CHO __Cl
1-40 A9 p\ --H CH2OH __Cl
1-43 A4 __Cl --Me --H --H --I
1-44 A16 __Cl --Me --H --H
N=
NH2
1-45 A4 --H --H --H --Br
1-46 A4 __Cl --CO2Et --H --H --I
1-47 A6 __Cl --CO2Et --Br --H --I
1-48 A7 N o --CO2Et --Br --H --I
1-49 A9 fN o --CH2OH --Br --H --I
88
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
R2
N E N
R5 N
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
Meth.
1-50 A20 U --CHO --Br --H --I
1-51 A10 4-N N~ N-5\ --Br --H --I
1-55 A4 --H --H --Br
0,,/
0
1-56 A4 LT --CO2Et --Me --H --Br
0
1-57 A4 N --CH2CI --H --H --Br
--H --H --Br
1-58 A21
o-r-o
0~ H
1-59 A21 LN,/ 9 --H -- --Br
OH
I-59A A21 N CND I H --H --Br
0_~JNIII
89
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
E R2
R5 yN
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
Meth.
rll~
1-60 A22 CN --H --H --Br
0
0
1-61 A23 N N--H --H --Br
o
1-62 A24 CN --H --H --Br
NCc
N
1-63 A25 ON CN) --H --H --Br
o"JID
1-64 A26 O" CN) --H --H --Br
o1,11o
-tl
SNIT
1-65 A10 N --H --H --Br
N
Oc$_0
01-66 A10 LN1 --H --H --Br
N O
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
Ni N
R2
R5 \N
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
Meth.
o N
1-67 All LN1 C --H --H --Br
N
H
O
1-68 A27 N --H --H --Br
0
O
1-69 A3 --H --Me --H
NH2
O
1-70 A28 ON, --H --Me --H --Br
91
-0&26
WO 2010/119264 PCT/GB2010/000773
Table 3 -Final Products prepared in accordance with the procedures described
herein
R1
Ni N
R2
\
R5
R4 R3
No. Ex p. --RR1 --R2 --R3 --R4 --R5
O HO V
2-01 BI N --COZEt --H --H
HO V
2-02 B1 ~N --CO2H --H --H
O HO V
2-03 BI N --CHO --H --H
O HO V
2-04 B1 N --CH2OH --H --H ,
O HO
2-05 B1 ~N N --H --H
)\ ~=o
O HO
2-06 BI O --H --H
O
O N HO
2-07 B1 N --H --H 1
ON HOV
2-08 B7 P --H --H
H
HO V
2-09 BI 1 --H --H
ON,~e
92
-0&26
WO 2010/119264 PCT/GB2010/000773
RI
N, N
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
ON N - HO
2-10 BI ~ o --H -H
=o
0
0~ - HO
2-11 BI (j --H -H V
~N N 110
s=O
ON,~e 2-12 B4 --Me --I --H
~
O HO
2-13 BI --CF3 --H --H
0 HO
2-14 BI --H --H V
N
o
2-15 BI ON,~e (N N --H --H
~N
\ H-N
0
1 N
2-16 BI O ON --H --H
N =110
=
o H-N
2-17 B1 N o --H --H
S=O 0 H-N
o
o
2-18 BI N C) --H --H
N N-N
H H
93
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R5 yN E R3 R2
R4
No. Exp. --R1 --R2 --R3 --R4 --R5
O (
2-19 BI --H --H
N /
N-N 0N,/ 2-20 BI
-CF3 --H --H
H
-
N-N
2-21 B2 1 --CONH2 --H --H
0 N-N
O
2-22 B3 N --CN --H --H
N-N
--Me Me --H --H HO
2-23 BI
ON,~~
-24 B1 --Me --H --H I
2
ON,~e
N-N
O i H
2-25 BI --Me --H --H L J
N
2-26 BI --Me --H --H
v ~ N
O
--Me --H --H
2-27 B1 N J
IN
94
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
N
R5 ly E?
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
O~
O`,NH
2-28 B1 v N --Me --H --H p
0
2-29 BI ~N --Me --H --H HH
O HO
2-30 B11 --Me --H --H
N
O
2-31 BI --Me --H --H N
O
O
2-32 BI N --Me --H --H
2-33 B4 --Me --H __Cl
ON
O
2-34 B4 --Me --H -.-Cl
(N)~
O --CONH2
2-35 B2 v N --H --H
N --Me --H --CN I i
2-36 B14 O
N-N
0,~e N Ho
2-37 B5 --H --H
9
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
R2
N iN
N
R5
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
ON, 2-38 B5 --H (N) __H
N
N-N
--H (N) --H HO \
N
2-39 B5 O N
0 N~ - Ho
2-40 B5 --H C -H
N
H
O
2-41 B5 --H (N) --H N
0?S N-N
O
2-42 B1 --Me --Br --H
v N~
N-N
O \
2-43 B6 --Me N --H
oo /
IK N-N
O \
2-44 B7 --Me --H 6N
H N-N
2-45 B8 N --Me --H I i
N
N-N
96
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
E R2
N
R5
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
O \ \
2-46 B9 --Me --H
v N N
O~S~O N-N
2-47 B10 o ~ --Me C~N --H I
v N Olltlo
N-N
O~
2-48 B4 v N --Me --H __Cl
HO
O
I i
2-49 B11 --Me --H __Cl
O~ -
2-50 BI ~ -H --H
~ 1110 HZN N
S=0
ON~ HNN
--H H
2-51 B5
N-N
\
2-52 B13 f ~ / --CO2Et --H --H
H-N
O~ I\
2-53 B4 v N --Me --H --I
97
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
-
N N
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
O \
2-54 B4 --Me --H --CI I i
v N~ /
N-N
O \
2-55 B4 --Me --H --Br I i
ff N-N
O~
2-56 B4 --Me --H --I
v ~ N
O \
2-57 B12 --Me --H I
H N-N
O \
2-58 B8 --Me --H
6N
N-N
O \
--H I
2-59 B9 --Me N
6
Ozzl
"Is s -10 N-N
ON,, 2-60 B4 o --H -CI
% -o N-N
2-61 BI 0 0N O _H --H o N
/s=o
\ /'
2-62 B14 --Me --CN --H
ON,~r o \
98
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N, N
E R2
R5--'y N
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
o
2-63 B1 --Me N --H I
v N o~
0 N-N
0
2-64 B12 --Me N --H I
N ~
H N-N
2-65 BI 0--H--H
N o
%
S=O 0--H--H 2-66 B1 I r
N` Io H2N
N
` =0
2-67 BI
Q 0 --H --H
oN
\ 0
S=o
--H --H
2-68 BI ~N ~N, NO
04
0 NH2
N
o
2-69 BI 0 0 --H --H H
/"/ s=o
2-70 B1 0 -H --H
/S=0 N 6
`"
H
99
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
2-71 BI ON --H --H
"I N /
/S=0 H
O
I s
1\= --H --H H2N
2-72 B1 ~N,~e o
o
S
2-73 B4 --Me --H --I I i
0,~e
N-N
O --CONHEt
2-74 B2 LN --H --H
N-N
i-\N / I \
2-75 BI IN o --H --H
ii H N
S=O
0
O 1~4
2-76 B9 --Me N --H
v N "'
-0 N-N
2-77 B12 --Me --CONHZ --H
N-N
O N
2-78 B5 --H --H
NHZ N-N
100
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N/ N
R2
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
2-79 B15 --CONHMe --H --H
N-N
0 (N) I
2-81 BI --H --H N
~/N N HN O
Oi-0
O \
2-82 BI (N) --H --H
N
HN,,e
O+O /NH
O
2-83 BI (N) --H --H F N
O'i~0 F F NH2
O
2-84 B14 (NN) --H --CN
010 N-N
2-86 B15 --CONMe2 --H --H
N-N
O~ O /
2-87 B16 ON VkN~ --H --H /
N-N
101
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
R5yN
R4 R3
--R3 --R4 --R5
No. Exp. --R1 -R2
O
2-88 B11 --Me --CN --H I i
OH
89 BI N (N)
--H --H
2-
O+0 NH2
2-90 BI N CI N) --H --H
N-N
O'i-.o
2-91 B13 --CONH2 --H --H
ON,
N
O
2-92 B13 l N --CONH2 --H --H
O
2-93 B13 N --CONH2 --H --H N-(
NH2
ON,:~e 2-94 B13 --CONH2 --H --H 6ON
H
O F
2-95 B13 --CONH2 --H --H
H
102
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N ~-N
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
O ~N
2-96 B4 N --CONH2 --H --Cl
N<
NH2
2-97 B17 --H --H
ON 2-98 B17 )== --H --H
H-N
2-99 B17 N~, --H H
H-N
2-100 B17 ON,~e > --H H /
A- H
0,~e 2-101 B4 --CONH2 --H --Cl
bN
0,~e 2-102 B4 --CONH2 --H --Cl t/~\N
ci
O/
2-103 B4 N\ --CONH2 --H --H ~ \
\ N
H
103
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
N
R5
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
ci
O
2-104 B4 --CONH2 --H --Cl
66N
H
cl
2-105 B4 --CONH2 --H --H \ \
ON,~e F
N
H
cl
Q F
2-106 B4 --CONH2 --H --Cl \ \
N
H
-107 B13 v N --H --H 2 O O I
H-N
O / \N
2-108 B4 N (N) --H --Cl
N
0-_I 0 NH2
(N) --H --Cl N
2-109 B4 N,
v N
0-_I 0
2-110 B4 (N)
--H --Cl
6,,1 N 0
0'10
O I ~ `N
2-111 B7 ~N,,/ `~ --H --H
" H-N
104
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
N
R5
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
O / \N
2-112 B4 N (N) N--H --Cl
N
010 o-
2-113 B4 N (NN) --H --Cl i
0N`o H
H o
2-114 B4 (N) --H --Cl N
N
N ~ oS o N I
H
CI
2-115 B4 O ( ) --H --Cl N
So N
o
N
H
2-116 B4 CN ) --H --Cl
N
o'h`o NH2
O N y ~ \
2-117 B7 3 --H --H
~N
N H_N
H
0 N
2-118 B7 N --H --H
N N-N
H
105
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
R5 N E R4 R3
No. Exp. --RI - --R2 --R3 --R4 --R5
2-119 B2 ON --CONH2 --H --H 2-120 B2 ()N --CONH2 --H --H
N=C
NH 2
O
2-121 B4 N (N) --H --Cl
0 1 0 O NH2
F
\
O
2-122 B4 ~N (NN) --H --H i ci
o N
H
F
O
2-123 B4 ~N (NN) --H --Cl
o' 1 H
O H
F
N
2-124 B4 N (N) --H --Cl
ci
o`o N
H
"
O
2-125 B1 (NN) --H --H NII
N
0 0
106
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N, N
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
2-126 BI (N) --H --H N~ \
v N N
o T' o
O 2-127 BI (N) --H --H
N N /
o'j`o H-N
O T N
2-128 B17 ON --H --H
6N,I
Z0 H_N
O
2-129 BI 7 --H --H
v N N~S\
H-N
2-130 B17 --H
NH N-N
Jiiiii;iLf 2-131 BI7 N --H --H
NH N N
0
--Me --H
2-132 B1
NN
NH2 NH2
107
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
R5 N
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
0~
2-133 B5 v N --H --H
HZN P,
H-N
0~ N I /
2-134 B17 N --H --H
-S=O HF-N
N
2-135 B4
CN,,/ (N) --H --Cl
N
O 1
2-136 B4 N CNJ --H --Cl N.N
0,f'-0
2-137 BI --C02Et --H --H
CN,)e
H--N
O 7N 2-138 B1 --CO2Et --H --H
=-C
NH2
o
2-139 B16 0 H --H --H
H-N
0 2-140 B16 H"O --H --H
N / C I
H-N
108
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
E R2
N
R5
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
0 o
2-141 B16 H-\_N --H --H
H-N
N
2-142 B16 o H' --H --H
N\~
}` 00 H
--H
2-143 B7 N --Me N N
H --C
NH2
" --
2-144 B16 ON, H --H
CHZOH N-N
H
o')
2-145 B17 N, --H --H
N N-N
/So
0
2-146 B16 N H^~ j --H --H
N
H --H --H
IIX
ON 2-147 B16
N
0
2-148 B16 0 H--H --H
109
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
2-149 B17 0 --H --H
N H-N
"S"o 0
2-150 B17 --H --H
ZN) H-N
,s` o
0
~
2-151 B4 N () --H --Cl
0~'\0 N
O
2-152 BI (NN) --H --H
0 TO
N hN' 2-153 B1 N (N) --H i O
01--0
O o ~
2-154 B16 Hti -' --H --H
N
O~
2-155 B18 H \ --H N q--
N-N
N N !/ 110
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N, N
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
ON,~~ 2-156 B10 --Me 6N --H
'Y N-N
o H
N
2-157 B16 ~N H --H --H I i
N-N
0
2-158 B16 N H ^~" --H --H
N
o
2-159 B16H--H --H
N
0
2-160 B16 H~~ I " --H --H 7N
NH2
0
--H --H
2-161 B16 ON HN-
NH2
0
2-162 B16 NH--H --H N \,N
-N H2
0 --H --H
2-163 B16 --
N1 H~ ~ N=~
NH2
111
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
o o I Jo 7N 2-164 B16 " --H --H
=
NH2
N
2-165 B13 () --Me --H NON
o'h`o NH2
/ \N
2-166 B13 --Me --H --H
N-
N NH2
2-167 B4 ~ CN --H --Cl
N) hN O
i
0'1`0
2-168 B13 \ --Me --H --H I i
N N-N
N
o --H --H
/
2-169 B17 N= C
z
b,-r~` o NH2
0 0 NC 2-170 B17 ~ --H --H Z N-S//
o N
NH2
O
-- /
2-171 B1 l N
H --H N
~/ / NHz
112
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N EN
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
O / \N
2-172 BI N --CF3 --H --H N={
NH2
N
O
2-173 B4 N\~ EN' --H --Cl
-6
o Imo N
2-174 B1 0j --H --H --H N~N
NH2
2-175 BI 0 N --H --H N- \N
COJ C H2
2-176 BI oj N N A --H --H N
NH NH2
2
2-177 B19 0j N Ho --H --H N_~\\/N
NH2
o 2-178 B1 N (N) N--Br --H N-
0-_I 0 NH2
o \N
2-179 B5 --H CN ND --H /N _
O~~ H
-S z
113
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
Ni N
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
O N / \N
2-180 B14 ~N C) --H --CN
N N
O-O NH2
O \N
/-
2-181 BI N --Me --H --H N
NH2
o~
2-182 B16 ON "ON o,-,, --H --H
N-N
2-183 BI N (N) N--Br --H
o S, `o N-N
O 7N 2-184 B16 --CONHMe --H --H NH2
\N
N_<
2-185 B4 ON,~e --Me --H --CI
~ NH2
O
2-186 B16 ~N--H --H
N-N
O / \N
2-187 B14 LN1 --Me --H --CN N=~
NH2
114
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
R5y N
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
0 H /
2-188 B16 ON "CNyo,/ --H --H N
O NH2
O / \N
2-189 B4 ~N --CONHMe --H --Cl
N
NH2
/ \N
2-190 B13 O --Me --H --H
N=C
NH2
O
o H / \N
2-191 B20 _O --H --H N~
`` O NH2
O\~
O HN,,=
2-192 B20 N --H --H ~N
OH
N
NH2
0
O N
/ \N
2-193 B20 LN ~N --H --H
N=C
O NH2
XO
O
2-194 B4N/--'O~ --H --Cl H N
NH2
O~ O / \N
2-195 BI --H --H
N~
N_-
NH2
115
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
Ni N
R5 \ N E R3 R2
R4
No. Exp. --R1 --R2 --R3 --R4 --R5
`
2-196 B1 O(N) --H --H N
N~
o'j`o
2-197 BI N CN --H --H
0 1 0 _ NH
N
O
2-198 B1 ~N CN) N--H --H
N
oh`o NH2
2-199 BI C) --H --H _ N
N N-<
NH
o'h`o ~
2-200 B7 N (NN) --H --H N
i N
o1 o NH2
O 2-201 B7 N (N) N--H --H N-
01 0
N
116
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
R2
N :iN
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
F F
F
O /
2-202 B1 N CN N --H --H \N
N=C
o'j`o
NH2
`
O
2-203 B1 (NN) --H --H NH2
~ N
0 0
N
2-204 BI N (N) --H --H / \
' N-
o's`o
O / \
2-205 BI N (NN) --H --H F
i N-
0 I' 0 NH2
O
2-206 B4 N (NN) --H --Cl N-
' \\
01~s~0 N
O N
2-207 B4 (N) --H --Cl
N-
v N
NH
0 1 0 /
117
-0&26
WO 2010/119264 PCT/GB2010/000773
RI
N N
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
0 / ~N
2-208 B4 N (N) --H --Cl i N={
o'h`o NH2
F F
F
N
2-209 B4 N () --H --CI ~N
o'h`o N-=-(
NH2
O
-210 B4 N (N)
\ NH2
N--H --Cl N
2
o'~+0 -
1 0-
2 -211 B4 ~N C:) --H --Cl N-
0-_I 0
O 2-212 B4 N C:) --H --CI N
~
o4'~O N
2-213 B4 (N) --H --Cl
o,i,o NNH
118
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
R2
N E
N
__ N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
O / \N
2-214 B1 --CO2Et --Me --H
N N
NH2
O ~~ o
2-215 B16 ~i `N--iO= --Me --H
N\~ H N<
~` NH2
2-216 B20 I7ri kN-~ _1 __Cl --H
ON,~e 0
H N-={
NH2
2-217 B15NH2 --Me --H N-\N
NH2
N
2-218 B13 0~ N" H --H /_\N
oho
NH2
O N \N
2-219 B9 N ~NY --H --H N /
o%$i--o NH2
O / \N
2-220 B13 CNND --H --H
0 J 0 NH2
119
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
N
R5
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
N
/
N --H --H
2-221 B13
~N o ,I N
NH2
O~ \N
2-222 B13 LN\~ C ) --H --H N /--(
Sa
o o NH2
OH
/ \N
2-223 B13 L --Me --H --H
N\ N
NH2
O O
/ \N
2-224 B13 --Me --H --H
0
N--C
L.N1 NH2
0 (N)
2-225 B13 " -H --H
N-
o'
NH2
O~
2-226 B13 ~N1 () --H --H N-
` H / NH2
0
2-227 B13 LN1 --Me N<
NH2
120
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N EN
R2
N
R5
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
O N / \N
2-228 B13" --H --H N={
0 1 0 NH2
O N / \N
2-229 B13
9 --H --H
" N--C
OH NH2
o _
2-230 B13 -H --H
~", N
Nl~ NH2
N
O
N
2-231 B13 ~" --H --H
I N=C
o! lir
NH
2
0
N
2-232 B13 OC N~ --H --H
~ " NH2
Z
SNIT
6 --H --H
2-233 B13 "
N <
Oct NHZ
N
2-234 B21 o~1 CD --H --H \N
N=~
oN NH2
121
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
N
R5
R4 R3
No. Exp. --RI --R2 --R3 --R4 --R5
~ N /
2-235 B13 N 0 --H --H
0.:_\o NH2
o
2-236 B12 N N --H --H
() /N--C
H NH2
2-237 B22 f- U --Me --CO2Et --H
H-N
2-238 B23 fcO --Me --CO2H --H
H-N
2-239 B1 ~ U --CHO --H --H
H-N
2-240 BI N O\ --H CH2OH N-N
H
O
2-241 B24 --Me N --H
~/
O' Y N-N
N
2-242 BI ~N CN) --H --H N \,N
I! NH2
0
0
O~ 9CL
2-243 B25 ~N1 --H --Me --H
NH
H
122
-0&26
WO 2010/119264 PCT/GB2010/000773
R1
N N
R2
N
R5
R4 R3
No. Exp. --R1 --R2 --R3 --R4 --R5
0
O I off
2-244 B23 v N --H --Me --H i NH
0
O 011
2-245 B26 --H --Me --H NH
0
72-246 A13 ~/ --CO2H --H --H
v N N--C
NH2
Experimental Part
Preparation of the intermediates
Method Al
Preparation of intermediate 1-01.
NH2
Br
N
N Y
Br
To a mixture of 2-amino pyrazine (50 g, 0.5 mol) in chloroform (1000 ml)
cooled
to 0 C was added pyridine (100 ml, 1.21 mol) and bromine (54 ml, 1.05 mmol)
dropwise. The mixture was stirred at rt for 16h, then water was added. The
organic phase was extracted, dried (MgSO4), filtered and evaporated to obtain
I-
01, 48 g (Y: 36 %) of a yellow solid which was dried in vacuo.
123
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of intermediate 1-53
NH-
CI
N
N ,/
I
To a mixture of 2-amino-3-chloropyrazine (3.627 g, 28.00 mmol) in acetonitrile
(20 mL), N-iodosuccinimide (6.929 g, 30.800 mmol) and trifluoroacetic acid
(2.2
mL) were added. The reaction mixture was stirred at it for 18 h. EtOAc was
added and the mixture was washed with Na2S2O3i dried, filtered and evaporated.
The residue was purified in by column chromatography (EtOAc:Cyclohexane,
0:100 to 40:60) to render 5.1 g of Intermediate 1-53 (71%).
Method A2
Preparation of intermediate 1-02.
0
N
N NH2
N
Br
A solution of intermediate 1-01(15 g, 59.3 mmol) in morpholine (15 ml, 178
mmol)
was heated at 120 C in a Parr reactor for 48h. A brown solid appears. The
solid
was suspended in DCM and washed with NaHCO3 aq. sat (twice). The organic
phase was dried (NaSO4), filtered and evaporated to dryness to obtain 1-02,
14.8 g
of a brown solid (Y: 96 %)
124
-0&26
WO 2010/119264 PCT/GB2010/000773
Method A3
Preparation of intermediate 1-03.
C:)
N N -- YINH,
HN N
A mixture of intermediate 1-02 (360 mg, 1.35 mmol), indazole-4-boronic acid
hydrochloride (600 mg, 2.97 mmol), K2C03 (2 mL of saturated solution),
PdC12(dppf).DCM (112 mg, 0.135 mmol) in DME (5 mL) was heated under
microwave irradiation for 10 min at 130 C. The reaction mixture was filtered
through a celite pad, washing with DCM. The filtrate was dried over Na2SO4 and
concentrated. The crude was purified by flash column chromatography (Isolute
Si
11 10 g cartridge) eluting with a gradient of DCM/MeOH (from 100% to 90:10) to
yield 250 mg of the intermediate 1-03 pure (Y: 62 %).
Preparation of intermediate 1-69.
0
N
N" I' N
\ \ N ~
H2N
A mixture of Intermediate 1-70 (45 mg, 0.15 mmol), 4-(4,4,5,5-tetramethyl-
1,3,2-
dioxaborolan-2-yl)aniline (40 mg, 0.18 mmol), PdCI2(dppf) (12 mg, 0.02 mmol)
and Na2CO3 (sat aq sol; 0.75 mL) in 1,2-DME (0.75 mL) was heated under
microwave irradiation at 130 C for 1 h. The mixture was diluted with
DCM:MeOH,
adsorbed on celite and purified by chromatography (Isolute 5g; MeOH:DCM,
0:100 to 20:809 to give Intermediate 1-69 (50 mg, 100%) as a yellow solid.
125
0&26
WO 2010/119264 PCT/GB2010/000773
Method A4
Preparation of intermediate 1-05.
Br
N N
Br~N~
Intermediate 1-01 (2 g, 7.9 mmol) was solved in 2-chloroacetone (3 ml,). The
reaction was heated in a sealed tube at 90 C for 16h. A precipitate appears.
Then
Et20 was added. The precipitate was filtered off as a salt. The resulting
solid was
suspended in DCM and treated with an aqueous saturated solution of Na2CO3.
The organic phase was extracted, dried (MgSO4), filtered and evaporated to
obtain the intermediate 1-05 (1.2 g of a brown solid, Y: 35 %)
Preparation of intermediate 1-28.
(0)
N
NrN
CI' vIIN__ ~
O
0
Intermediate 1-02 (1.2 g, 4.7 mmol) and ethyl 2-chloroacetoacetate (2.3 g,
14.2
mmol) were suspended in EtOH (12 mL). The mixture was heated under
microwave irradiation for I h at 150 C. After cooling down to room
temperature,
petroleum ether was added and the solid formed was filtered off. The filtrate
was
evaporated under reduced pressure and the residue was purified by flash
chromatography on silica gel (c-Hex /EtAOAc 8:2) to obtain a solid that was
washed with petroleum ether to give the desired product 1-28 (231 mg, Y: 33%).
Preparation of intermediate 1-35.
(0)
N
Ni I N
CI O
IN\~ //
O
Intermediate 1-02 (2 g, 7.72 mmol) and methyl 4-chloroacetoacetate (3.56 mL,
30.88 mmol) were heated in two sealed tubes (half of material in each tube) at
90 C for 2h. Volatiles were removed under reduced pressure and the residue was
126
-0&26
WO 2010/119264 PCT/GB2010/000773
purified by flash chromatography on silica gel (c-Hex /EtAOAc 10:0 to 6:4) to
obtain a solid that was washed with diethyl ether to render the desired
product I-
35 (1.17g, Y: 49%).
Preparation of intermediate 1-57.
(0)
N
N/N
~IN'~ CI
Br
A mixture of Intermediate 1-02 (8.17 g, 31.52 mmol) and 1,3-dichloroacetone
(6.0
g, 47.29 mmol) in 2-propanol (15 ml-) was heated in a sealed tube at 55 C for
2
days. On cooling, the mixture was filtered and rinsed with Et20` and MeOH. The
solid was purified by flash chromatography on silica gel (MeOH:DCM, 5:95) and
the product obtained was washed with MeOH and dried to give Intermediate 1-57
(3.97 g, 38%).
Method A5
Preparation of intermediate 1-06.
Br
NN
11 IIN''
Br
Br
Intermediate 1-05 (0.62 g, 2.13 mmol) was dissolved in CHCI3 (4 mL) and N-
bromosuccinimide (455 mg, 2.56 mmol) was added. The reaction mixture was
heated under microwave irradiation at 120 C for 1 h. On cooling, the mixture
was
adsorbed in silica and purified by Biotage column chromatography (DCM/MeOH
from 100% to 95:5) to give intermediate 1-06 (720 mg, Y: 91 %) as a yellow
solid.
127
-0&26
WO 2010/119264 PCT/GB2010/000773
Method A6
Preparation of intermediate 1-09.
Br
N O 0
N-"~0~14'
Br
/
Br
Intermediate 1-07 (1.00 g, 2.87 mmol) was dissolved in DCM (28 ml-) and N-
bromosuccinimide (0.61 g, 3.44 mmol) and trifluoroacetic acid (0.25mL) were
added. The reaction mixture was stirring at rt for 16h and then heated at 600C
for
2h more. The reaction mixture was cooled, and washed with water. The organic
phase was dried (Na2SO4), filtered and the solvent removed in vacuum. The
residue was purified by biotage with a gradient cyclohexane/EtOAc: from 100%
to
50:50 The desired fractions were collected to obtained 1.15 g of a white solid
as
intermediate 1-09 (Y: 94 %).
Method A7
Preparation of intermediate 1-10.
(0)
II
N~N
~NI*
Br
A mixture of Intermediate 1-05 (1.75 g, 6.015 mmol), morpholine (0.526 mL,
6.015
mmol) and DCM (20 ml-) was stirred at it for 16h. Additional morpholine (0.526
mL, 6.015 mmol) was added and the mixture was stirred at it for 18h more.
Na2CO3 sat. aq. was added. The organic phase was separated, dried (Na2SO4),
filtered and evaporated till dryness to obtain 1.8 g of intermediate 1-10 (Y:
quantitative). The resulting product was used in the next step without further
purification.
128
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of intermediate I-11.
(0)
N
W' \ N
Br
Br
Intermediate 1-06 (0.72 g, 1.95 mmol) was dissolved in DCM (6 ml-) and
morpholine (0.68 mL, 7.79 mmol) was added in one portion. The reaction mixture
was stirred at rt for 3 h. The mixture was purified, together with a second
batch of
the same reaction, by column chromatography (DCM/MeOH from 100% to 50:50)
to give the expected product intermediate I-11 (980 mg, Y: 76%) as a clear
yellow
solid.
Preparation of intermediate 1-48
(0)
N
N O
Br
To a mixture of Intermediate 1-47 (2.25 g, 5.22 mmol) in acetonitrile (20 ml-)
morpholine (0.59 mL, 6.79 mmol) and N,N-diisopropylethylamine (1.36 mL, 7.84
mmol) were added. The reaction mixture was heated under microwave irradiation
at 160 C for 30 min. On cooling, NH4CI was added and the mixture was extracted
with DCM. The organic layer was dried (Na2SO4), filtered and evaporated. The
residue was precipitated with Et2O and MeOH to render Intermediate 1-48
(1.875g, 75%) as a white solid. The filtrate was evaporated and purified by
column chromatography (Cyclohexane:EtOAc, 100:0 to 60:40) to render 620 mg
of Intermediate 1-48 (24%).
129
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of intermediate 1-36.
N
N~ N 0
i 0
Intermediate 1-07 (0.50 g, 1.40 mmol) was suspended in DCE (8 mL) and
piperidine (0.18 mL, 1.7 mmol) was added dropwise. The reaction mixture was
heated at 60 C for 16 h. Additional piperidine (0.05 mL) was added and heating
conitinued for 2h. The suspension at RT was filtered affording a solid
(imurities)
and the filtrate that was concentrated. The resulting residue was triturated
with
Et20 to render another solid (impurities) and the filtrate that was
concentrated to
afford the required intermediate 1-36 as light orange solid (400 mg, Y: 80%).
Method A8
Preparation of intermediate 1-13.
C0)
N:;, NH
Ngyp
Br
To a solution of intermediate 1-12 (2 g, 5.6 mmol) in DCM (50 mL) was added
dropwise DIBAL (3.8mL, 1 M in toluene, 22.75mmol) at -78 C stirring at that
temperature for 40 min. The reaction was quenched with cold methanol and
stirred for 10 min. more. The mixture was poured into a biphasic mixture of
saturated NaHCO3 and DCM and allowed to warm to room temperature with
occasional stirring. It was then passed though a Celite bed to remove a
gelatinous mass, and the bed was throughly washed with DCM. After the organic
layer was separated, the aqueous layer was extrated with DCM. The combined
organic phase was dried (Na2SO4), filtered and concentrated under reduced
pressure to obtain a light yellow solid, 1.674 g, Y: 96 % as intermediate 1-13
which was used in next reaction step without further purification.
130
-0&26
WO 2010/119264 PCT/GB2010/000773
Method A9
Preparation of intermediate 1-14.
0
N
N 'N} SOH
~IN
Br
To a stirred slurry of L1AIH4 (556 mg, 14.64-mmol) in dry THE was slowly added
intermediate 1-12 (5.63 mmol) in THE (28 mL) at 0 C. After the addition, the
reaction mixture was stirred at room temperature for 2h and quenched with
saturated NH4CI/NH4OH. The mixture was poured into CHCI3/MeOH (3:1) and it
was then passed though a Celite bed to remove a gelatinous mass, and the bed
was throughly washed with CHCI3. The organic layer was washed with saturated
NaCl. The organic phase was dried (Na2SO4), filtered and evaporated under
reduced pressure to obtain the expected product 1-14 as a light yellow solid
(1.02
g, Y: 57% yield) which was used in next reaction step without further
purification.
Preparation of intermediate 1-40
(0)
N
N';~N
CI N~O
~
HO
A solution of Intermediate 1-39 (1g, 2.952 mmol) in THE (10 mL) was slowly
added to a stirred slurry of NaBH4 (123 mg, 3.247 mmol) in dry THE (11 mL) at
0 C. The mixture was stirred 2h at rt. The solvent was removed and the residue
was suspended in H2O and extracted with EtOAc. The organic layer was dried
(Na2SO4), filtered and evaporated concentrated. The residue was used in the
next
experiment without further purification.
131
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of intermediate 1-49
(0)
N
N N
IINI OH
Br
To a solution of Intermediate 1-48 (1.3 g, 2.7 mmol) in DCM (25 mL) was added
diisobutylaluminum hydride (1 M in toluene) (2.7 mL, 2.7 mmol) drowise at 0 C.
The reaction mixture was stirred at rt for 16h and more DIBAL (2.7 mL) was
added. Stirring was continued at rt for 2 days and another eq. of DIBAL was
added (2.7 mL). After 2 days the reaction was quenched with cold MeOH, stirred
for 10 min and poured into a biphasic mixture of H20/DCM. The suspension was
filtered off to render Intermediate 1-49 (0.86 g). The organic layer was
extracted
with DCM, dried, filtered and evaporated to render Intermediate 1-49 (320 mg)
as
a white solid.
Method Al 0
Preparation of intermediate 1-16
0 \\ o
N ~
CN
BrNi ~N _)
~N >
A mixture of intermediate 1-13 (520 mg, 1.67 mmol), 1-boc-piperazine (405 mg,
2.17 mmol) and trimethyl orthoformate (1.83 mL, 16.71 mmol) was stirred in 1,2-
dichloroethane (14 mL) for 6h at room temperature. Then sodium
triacetoxyborohydride (425 mg, 2.0 mmol) was added and the reaction mixture
was stirred for 48h at room temperature. The mixture was then quenched with
brine and extrated with DCM. The organic phase was dried (Na2SO4), filtered
and
evaporated in vacuo. The residue was purified by silica gel column
chromatography in Biotage by eluting it with cyclohexane/ ethyl acetate and
then
with DCM/MeOH to obtain intermediate 1-16, 475 mg, Y: 60 % as a light yellow
solid.
132 .
-0&26
WO 2010/119264 PCT/GB2010/000773
Method Al 1
Preparation of intermediate 1-17.
~ H
N
NN N
Br
Intermediate 1-16 (0.380 mg, 0.789 mmol) was dissolved in DCM (10 ml-) and 2N
HCI (2mL) was added and the reaction was stirred at rt for 16h. Because only
starting material was observed, the solvent was evaporated and THE 3 mL and
3mL HCI (2N) were added and the reaction mixture was stirred for 2h. The
solvent was removed in vacuo to obtain intermediate 1-17 as a chlorohydrate
salt
(307 mg, Y: 93%) which was used in the next reaction without further
purification.
Method Al 2
Preparation of intermediate 1-23.
0
N /
N
N
N~/N
/\/N/:p
Br
Methylpiperazine (0.282mmo1, 32 L) and AIMe3 2M in hexanes (0.282mmo1,
0.14mL) in dry DCM (4mL) was stirred at rt for 15min. Then intermediate 1-12
(100mg, 0.282 mmol) was added and the mixture was stirred at rt for 3h and
then
at 40 C overnight. The reaction was quenched with sat sol of ammonium chloride
and diluted with DCM. The organic phase was dried (Na2SO4), filtered and
evaporated to afford a residue which was triturated with Et20-DCM
precipitating
an off white solid as an impurity. The filtrate was purified by flash
chromatography
(Biotage Hex-EtOAc from 100 % to 70:30 and then DCM-MeOH/NH3 7N 80:20 to
obtain 67 mg (Y: 48 %) of required product as intermediate 1-23.
133
-0&26
WO 2010/119264 PCT/GB2010/000773
Method A13
Preparation of intermediate 1-29
CO)
N
NN
CI' v NE
OH
O
2N NaOH (0.85 ml-) was added to a stirred mixture of intermediate 1-28 in
MeOH.
The reaction was stirred at 50 C for 1.5h and at reflux for 20 min. The
solvent
was evaporated and water was added and the pH was adjusted to 4 by addition
of AcOH. The mixture was diluted with EtOAc (until a clear solution was
obtained
(ca 250 mL). The layers were separated and the aqueous layer was extracted
twice with EtOAc. The organic layer was dried and evaporated. The residue was
azeotropically dried with toluene to give 261 mg (Y: 100%) of desired product
1-29
which was used in next reaction step without further purification.
Method A14
Preparation of intermediate 1-27
CND
N/\ N
N
CI4_~N:
0 bN
O O
4-Amine-1-BOC piperidine was added to a stirred mixture of intermediate 1-29,
DIPEA and HATU in DMF. The reaction was stirred at rt for 4h The reaction
mixture was directly chromatographed on silica gel (biotage c-Hex/ EtOAc 10 to
100% EtOAc) to obtain the desired product (233 mg, Y: for two steps: 69 %).
134
-0&26
WO 2010/119264 PCT/GB2010/000773
Method A15
Preparation of Intermediate 1-30
C:) N/N/~NHz
/\/ INS O
Br
Intermediate 1-12 (240 mg) in a seal tube, was suspended in a solution of
MeOH/NH3 7N. The reaction mixture was heated at 100 C for 16h. The solvent
was evaporated to dryness and the residue was washed with MeOH and Et20.
the resulting yellow solid was dried in vacuo to obtain 200 mg of desired
product
1-30. Alternatively, a precipitate may appear, which may be filtered off to
obtain
the desired product 1-30.
Method A16
Preparation of Intermediate 1-44
ci
NN
N
I
H2N N
A mixture of Intermediate 1-43 (0.15 g, 0.51 mmol), 2-aminopyrimidine-5-
boronic
acid, pinacol ester (136 mg, 0.613 mmol) and PdCI2(dppf) (42 mg, 0.051 mmol)
and sat. sol. Na2CO3 (1.96 ml-) in 1,2-DME (1.96 ml-) was stirred at r.t. for
1h 30
min. DCM was added and the mixture was washed with H2O and sat. NaCl. The
organics were dried (Na2SO4), filtered and evaporated. The residue was
purified
by column chromatography (DCM:MeOH, 99:1 to 90:10) to render Intermediate I-
44 (10 mg, 8%) as a beige solid.
135
-0&26
WO 2010/119264 PCT/GB2010/000773
Method A18
Preparation of intermediate 1-39
(0)
N
NN
\ N~O
CI~ ~
CHO O
To a solution of Intermediate 1-35 (3.76 g, 12.11 mmol) in DMF (120 ml-) was
added POCI3 (3.38 mL, 36.34 mmol) at -20 C. The mixture was stirred at rt
overnight under N2 and diluted with H20/ice. The white solid was filtered off
and
dried to render 2.95 g (63%) of Intermediate 1-39. It was used in the next
experiment without further purification.
Method A20
Preparation of intermediate 1-50
(0)
N
N/Y' N O
'/NCH
I
Br
To a solution of Intermediate 1-49 (1.18 g, 2.7 mmol) in CHCI3 (54 ml-)
activated
Mn02 (4.0 g, 45.93 mmol) was added. The reaction mixture was refluxed for 8 h.
On cooling, the mixture was filtered through celite. The filtrate was
evaporated to
render Intermediate 1-50 (0.67 g). It was used in the next reaction without
further
purification.
136
-0&26
WO 2010/119264 PCT/GB2010/000773
Method A21
Preparation of intermediate 1-58
0
N
N/ rN
JJJ
Br N
N
'O
O-:~-S\
To a suspension of Intermediate 1-57 (0.232 g, 0.7 mmol) and K2C03 (0.193 g,
1.4 mmol) in Acetonitrile (20 ml-) was added 8-methanesulfonyl-3,8-diaza-
bicyclo
[3.2.1]octane ((0.133 g, 0.7 mmol). The reaction mixture was refluxed for 24 h
and concentrated. The residue was suspended in DCM and washed with brine.
The organic layer was dried, filtered and evaporated. The residue was
triturated
from MeOH to give Intermediate 1-58 (0.189 g, 56%) as a white solid.
Method A22
Preparation of intermediate 1-60
(0)
N
NN
Br
II~ 'ON
O
O O-1\
To a solution of Intermediate 1-17 (100 mg, 0.297 mmol), BOP (158 mg, 0.356
mmol) and (s)-(-)-2-acetoxypropionic acid (41 mg, 0.356 mmol) in CH2C12 (3
mL),
Et3N (0.083- mL, 0.594 mmol) was added. The mixture was stirred at RT for 2
days. CH2CI2 was added and the mixture was washed with water. The organic
layer was dried over Na2SO4, filtered and evaporated. The residue was purified
by column chromatography (Biotage, CH2CI2:MeOH, 100:0 to 60:40) to give
Intermediate 1-60 (130 mg, 88%) as a colourless oil.
137
-0&26
WO 2010/119264 PCT/GB2010/000773
Method A23
Preparation of intermediate 1-61
(0)
N
N O
Br ~N c
A 2M solution of trimethylaluminum in hexanes (5.5 mL, 11.02 mmol) was added
to a mixture of N,O-dimethylhydroxylamine hydrochloride (1.075 g, 11.02 mmol)
in DCM (10 ml-) and the reaction was stirred at it for 40 min. A solution of
Intermediate 1-12 (0.783 g, 2.20 mmol) in DCM (16 ml-) was added and the
reaction mixture was stirred at 40 C for 2 h. On cooling, the mixture was
carefully
quenched with 1 N HCI and diluted with DCM. After 30 min stirring layers were
separated and the aqueous layer was extracted with DCM (x2). The combined
organic layers were washed with brine, dried, filtered and evaporated. The
residue was purified by column chromatography (Biotage, cHex/EtOAc 50:50 to
0:100) to give Intermediate 1-61(545 mg, 67%).
Method A24
Preparation of intermediate 1-62
(0)
N
NN O
Br
To a mixture of Intermediate 1-61 (545 mg, 1.47 mmol) in THE (15 ml-) was
added
MeMgBr (2.2 mL, 2.2 mmol) at 0 C. The reaction was stirred at 0 C for 1h.
Additional MeMgBr (1.1 mL, 1.1 mmol) was added and the reaction was stirred at
0 C for 1 h. The mixture was quenched with sat NH4CI, and extracted with
EtOAc
(x3). The combined organic layers were dried, filtered and evaporated to give
Intermediate 1-62 (458 mg, 96%).
138
-0&26
WO 2010/119264 PCT/GB2010/000773
Method A25
Preparation of intermediate 1-63
C:)
N
N. ''N % I CN
N
Br
>=O
To a mixture of Intermediate 1-62 (0.1 g, 0.308 mmol) and 1-Boc-piperazine
(0.115 g, 0.615 mmol) in DCM (4 ml-) was added Ti(iPrO)4 (0.182 mL, 0.615
mmol). The reaction was stirred at reflux for 2h. Et2AICN (0.62 mL, 0.615
mmol)
was added and the reaction mixture was stirred at reflux for 5h. On cooling,
the
mixture was quenched with sat =NaHCO3 and extracted with EtOAc (x3). The
combined organic layers were washed with brine, dried, filtered and
evaporated.
The residue was purified by column chromatography (biotage, cHex/EtOAc 10:90
to 0:100) to give Intermediate 1-63 (100 mg, 63%).
Method A26
Preparation of intermediate 1-64
C:)
NN
>
Br 0
>=C
To a stirred solution of MeMgBr (1 mL, 0.96 mmol) was added a solution of
Intermediate 1-63 (50 mg, 0.096 mmol) in THE (1.5 ml-) at 0 C. The reaction
was
stirred at 0 C for 4 h and the mixture was poured onto ice cold sat NH4CI. The
mixture was extracted with EtOAc and the combined organic layers were dried,
filtered and evaporated. The residue was purified by column chromatography
(biotage, cHex/EtOAc 10:90 to 0:100) to give Intermediate 1-64 (22 mg, 45%).
139
-0&26
WO 2010/119264 PCT/GB2010/000773
Method A27
Preparation of intermediate 1-68
(0)
N
N~N
,N
Br CN)
O 'S
0
MsCI (0.046 mL, 0.589 mmol) was added to solution of Intermediate 1-67 (0.175
g, 0.393 mmol) and TEA (0.274 mL, 1.96 mmol) in DCM (4 ml-) at 0 C. The
reaction mixture was stirred at rt for 3 h and poured onto sat NaHCO3. The
mixture was extracted with DCM and the combined organic layers were dried,
filtered and evaporated. The residue was purified on silica gel (DCM:MeOH,
90:10) to afford Intermediate 1-68 (133 mg, 70%).
Method A28
Preparation of intermediate 1-70
(0)
N
NN
~.~ NII''
Br
To a solution of Intermediate 1-02 (150 mg, 0.58 mmol) in Toluene (6.8 mL), 2-
chloro-1,1-dimethoxypropane (0.758 mL, 5.8 mmol) and p-toluensulfonic acid (18
mg, 0.09 mmol) were added. The reaction mixture was refluxed for 24 h and
additional amounts of 2-chloro-1,1-dimethoxypropane (10 eq) and p-
toluensulfonic acid (0.16 eq) were added. The reaction mixture was refluxed
for
15 h and the solvent was removed. The residue was purified by column
chromatography (Isolute 10g; AcOEt-cyclohexane 0:100 to 50:50) to give the
Intermediate 1-70 (55 mg, 32%) as a beige solid.
140
-0&26
WO 2010/119264 PCT/GB2010/000773
Example B1
Preparation of Final product 2-01
(0)
N
NN~O
HO N1J `~O
A mixture of intermediate 1-12 (100 mg, 0.282 mmol), 3-hydroxyphenylboronic
acid (85 mg, 0.685 mmol), and PdC12(dppf)=DCM (23 mg. 0.028 mmol) in DME
(1.2 mL) was added a saturated aqueous solution of sodium carbonate (1 mL).
The mixture was heated to 130 C under microwave irradiation for 3 min. The
reaction mixture was cooled, diluted with chloroform, washed with brine. The
organic phase was dried (Na2SO4), filtered and the solvent removed in vacuo.
The residue was purifired by biotage chromatography with a gradient
cyclohexane/EtOAc: from 100 % to 50:50. The desired fractions were collected
and the solvent evaporated. The resulting solid was crystallised with MeOH to
obtain a white solid as final product 2-01 (44 mg, Y: 68% yield).
Preparation of Final product 2-10
(0)
N~~
N/ fN
H O \ \ ~IN~'J `N
~_N \
--S=O
O
A mixture of intermediate 1-18 (200 mg, 0.435 mmol), 3-hydroxyphenylboronic
acid (132mg, 0.985 mmol), and PdCl2(dppf).DCM (36 mg. 0.044 mmol) in DME
(1.8 mL) was added an aqueous saturated solution of potasium carbonate (1 mL).
The mixture was heated to 130 C under microwave irradiation for 3 min. The
reaction mixture was cooled, diluted with chloroform, washed with brine. The
organic phase was dried (Na2SO4), filtered and evaporated. The residue was
purified by biotage chromatography and eluted with a gradient DCM/MeOH: from
100 % to 50:50. The desired fractions were collected and the resulting residue
141
-0&26
WO 2010/119264 PCT/GB2010/000773
was purified again with EtOAc and then EtOAc/MeOH 20:1. The desired fractions
were collected to obtain a white solid, 27 mg, Y: 13 %, as final product 2-10.
Preparation of Final product 2-11
O
N
N N 0
HO \ \ E!/ N
/ ~~
N
--S=O
0
Intermediate 1-24 (165mg, 0.349mmo1), 3-hydroxyphenylboronic acid
(0.523mmol, 72mg) and PdC12(dppf).DCM (0.035mmol, 29mg) were suspended
in a saturated solution of sodium carbonate (1.8mL) and 1,2-DME (1.8mL). The
mixture was heated under microwave irradiation at 130 C for 10min. The mixture
was diluted with DCM and washed with water. The organic layer was dried
(Na2SO4), filtered and evaporated. The resulting residue was purified by
Biotage
chromatography (DCM-EtOAc from 50:50 to 100 % of EtOAc) to afford a product
still impure which was repurified using DCM-MeOH 95:5. The resulting oil was
precipitated with DCM-MeOH-Et2O (approx. 10:1:5) to give the desired product 2-
11 (63mg, Y: 36%).
Preparation of Final product 2-13
(O)
N
N~N F F
HO NJ<F
A mixture of intermediate 1-26 (225 mg, 0.641 mmol), 3-hydroxyphenylboronic
acid (194 mg, 1.410 mmol), and PdC12(dppf).DCM (53 mg, 0.064 mmol) in DME
(2.8 ml-) was added a saturated aqueous solution of potassium carbonate
(0.5mL). The mixture was heated to 130 C under microwave irradiation for 3
min.
The reaction mixture was cooled, diluted with DCM, washed with brine. The
142
-0&26
WO 2010/119264 PCT/GB2010/000773
organic phase was dried (Na2SO4), filtered and the solvent removed in vacuum.
The resulting residue was purified by biotage chromatography and eluted with a
gradient EtOAc/MeOH: from 100% to 50:50. The desired fractions were collected
to yield a yellow solid which was crystallised in MeOH to obtain the desired
product 2-13 (150 mg, Y: 64 %).
Preparation of Final product 2-14
C:)
N
Ni E
HO NI
Intermediate 1-15 (210 mg, 0.650 mmol), 3-hydroxyphenylboronic acid (0.975
mmol, 134 mg) and PdC12(dppf).DCM (0.065 mmol, 54 mg) were suspended in
saturated solution of sodium carbonate (2.6mL) and 1,2-DME (2.6mL). The
mixture was heated under microwave irradiation at 130 C for 10 min. The
mixture
was diluted with DCM and washed with water. The organic layer was dried
(Na2SO4), filtered and the solvent was evaporated in vacuo. The resulting
residue
was purified by flash chromatography (DCM-EtOAc from 100 % to 40:60) to
afford the desired product 2-14 (69 mg, Y: 31 %) as a white solid.
Preparation of Final product 2-15
0
N
N_ N/~N O
HN IIN: `N
N
Intermediate 1-23 (50mg, 0.122mmol), indazole-4-boronic acid hydrochloride
(0.183mo1, 36mg) and PdC12(dppf).DCM (0.012mmol, 10mg) were suspended in
a saturated solution of sodium carbonate (0.6 ml-) and 1,2-DME (0.6mL). The
mixture was heated under microwave irradiation at 130 C for 10min. The mixture
was diluted with DCM and washed with brine. The organic layer was dried
143
-0&26
WO 2010/119264 PCT/GB2010/000773
(Na2SO4), filtered and the solvent was evaporated. The resulting residue was
purified by flash chromatography (DCM-MeOH/NH3 7N from 100% to 90:10). The
desired fractions were collected to obtain final product 2-15 (48mg, Y: 88%)
as a
white solid.
Preparation of Final product 2-16
(0)
N
N NN O
HN N `Q
--S=O
0
Intermediate 1-24 (55mg, 0.116mmol), indazole-4-boronic acid hydrochloride
(0.174mmol, 35mg) and PdCI2(dppf).DCM (0.012mmol, 10mg) were suspended
in a saturated solution of sodium carbonate (0.6 mL) and 1,2-DME (0.6mL). The
mixture was heated under microwave irradiation at 130 C for 10 min. The
mixture
was diluted with DCM and washed with water. The organic layer was dried
(Na2SO4), filtered and the solvent evaporated in vacuo. The resulting residue
was
purified by flash chromatography (EtOAc-MeOH from 100% to 98:2) to obtain the
desired final product 2-16 as a white solid (32mg, Y: 54%).
Preparation of Final product 2-17
(0)
N
N N ~N
HN Nom'/
N
--5=O
0
A mixture of intermediate 1-18 (160 mg, 0.348 mmol), PdC12(dppf).DCM (cat.
amount), a saturated solution of K2CO3 (0.5 mL), indazole-4-boronic acid
hydrochloride (150 mg, 0.766 mmol), in DME (3.5 mL) was heated under
144
-0&26
WO 2010/119264 PCT/GB2010/000773
microwave irradiation at 130 C for 10 min. The mixture was diluted with DCM
(30
mL), washed with brine (40 mL). The organic phase was dried (Na2SO4), filtered
and concentrated. The crude was purified by Biotage flash column
chromatography eluting with a gradient of EtOAc/MeOH (from 100% to 60:40),
the resulting solid was triturated with MeOH and filtered to obtain the
desired
product 2-17 (43 mg) as a white solid.
Preparation of Final product 2-18
C:)
N NN O
HN INN
N
H
Intermediate 1-25 (150mg, 0.303mmol), indazole-4-boronic acid hydrochloride
(1.5 equiv, 0.454mmo1, 90mg) and PdC12(dppf)2. DCM (0.lequiv, 0.03mmol,
25mg) were suspended in sat sol of sodium carbonate (1.5mL) and 1,2-DME
(1.5mL). The mixture was heated under microwave irradiation at 130 C for
10min.
The mixture was diluted with DCM and washed with water. The organic layer was
dried over sodium sulfate to yield the crude product which was purified by
flash
chromatography (EtOAc-MeOH 0-5%) to afford the required (120mg as yellow
solid, 75%). This product (120mg, 0.225mmol) was suspended in dry methanol
(2.25mL) and AmberlystR(5) (400mg) was added. The mixture was slowly stirred
at it for 48 h. The resin was washed with MeOH, and then with MeOH-NH3 7N.
This phase was collected and evaporated to obtain final product 2-18 as a
syrup
(83 mg, Y: 85 %).
145
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of Final product 2-19
(0)
N
N N N
HN L
Intermediate 1-15 (140mg, 0.433mmol), 4-indazoleboronic acid (1.5 equiv,
0.650mmol, 129 mg) and PdCl2(dppf).DCM (0.043mmol, 36mg) were suspended
in a saturated solution of sodium carbonate (2mL) and 1,2-DME (2mL). The
mixture was heated under microwave irradiation at 130 C for 10min. The mixture
was diluted with DCM and washed with water. The organic layer was dried
(Na2SO4), filtered and evaporated. The residue was purified by flash
chromatography (DCM-EtOAc from 80:20 to 100 % on EtOAc) and then by HPLC
to afford final product 2-19 (40mg, Y: 26 %).
Preparation of Final product 2-20
(0)
N
N N ~N F
HN N / F F
A reaction mixture of intermediate 1-26 (140 mg, 0.4 mmol), indazole-4-boronic
acid hydrochloride (175 mg, 0.87 mmol), K2CO3 (300 mg), PdC12(dppf).DCM (cat
amount) in DME (3 mL) and water (1 mL), was heated under microwave
irradiation at 130 C for 10 min. The dark reaction mixture was diluted with
DCM
(25 mL), washed with saturated solution of NaHCO3 (2 x 30 ml-) and brine (30
mL). The organic phase was dried (Na2SO4), filtered and concentrated. The
resulting residue was purified by flash column chromatography eluting with a
gradient system of DCM/MeOH (from 100 % to 97:3). The desired fractions were
collected and precipitated with DCM/cyclohexane, to obtain the final product 2-
20
(40 mg, Y: 26 %).
146
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of Final product 2-23
(0)
IN
N/
~ N
HO N
__ II~
~
A mixture of intermediate 1-10 (100 mg, 0.337 mmol), 3-hydroxyphenylboronic
acid (0.102 g, 0.740 mmol), and PdC12(dppf).DCM (28 mg, 0.034 mmol) in DME
(1.463 mL) was added an aquous saturated solution of potassium carbonate
(0.5mL). The mixture was heated to 130 C under microwave irradiation for 10
min. The reaction mixture was cooled, diluted with DCM, washed with brine. The
organic phase was dried (Na2SO4), filtered and the solvent removed in vacuum.
The residue was purified by biotage chromatography and eluted with a gradient
EtOAc/MeOH from 100% to 50:50. The desired fractions were collected and the
residue was crystallised in DCM to obtain the desired product 2-23 (44 mg, Y:
42
%).
Preparation of Final product 2-24
(0)
N
N_ N-
HN N:/
A mixture of intermediate 1-10 (700 mg, 2.356 mmol), indazole-4-boronic acid
hydrochloride (701 mg, 3.534 mmol) and PdCl2(dppf).DCM (190 mg. 0.235 mmol)
in DME (11 ml-) was added a saturated aqueous solution of potassium carbonate
(11 mL). The mixture was heated to 130 C in the microwave for 0.5 h. The
reaction mixture was diluted with EtOAc and washed with water. The organic
phase was separated, dried (Na2SO4), filtered and evaporated to give a brown
oil.
This residue was purified column chromatography (hexane/EtOAc mixtures) to
give the desired product 2-24 as green foam (456 mg, 58% yield).
147
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of Final product 2-27
(0)
N
N, N
O \ Ne
N
A mixture of intermediate 1-10 (200 mg, 0.673 mmol), 3-methoxypyridine-5-
boronic acid pinacol ester (348 mg, 1.481 mmol), and PdCI2(dppf).DCM (56 mg.
0.067 mmol) in DME (2.9 ml-) was added a saturated solution of sodium
carbonate (1 mL). The mixture was heated to 130 C under microwave irradiation
for 10 min. The reaction mixture was cooled, diluted with DCM, washed with
brine. The organic phase was dried (Na2SO4), filtered and the solvent removed
in
vacuum. The residue was purified by biotage chromatography twice and eluted
with a gradient EtOAc/MeOH from 100% to 50:50. The desired fractions were
collected to obtain 120 mg of the desired product 2-27 as a yellow solid (Y:
55%).
Preparation of Final product 2-42
0
N
N_ N N
HN N
Br
Intermediate I-11 (1.36 g, 3.62 mmol) was suspended in DME (5 ml-) and
indazole-4-boronic acid.HCI (0.86 g, 4.34 mmol), PdC12(dppf).DCM (300 mg, 0.36
mmol), K2CO3 (1.5 g, 10.85 mmol) and H2O (2.5 ml-) were added. The reaction
mixture was heated under microwave irradiation at 130 C for 30 min. On
cooling,
the mixture was evaporated and the residue was purified by column
chromatography (DCM/MeOH from 100 % to 98:2) to give the expected product
2-42 (230 mg, Y: 15%) as a yellow solid.
148
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of Final product 2-71
(0)
N
NN
\ \ N'~N
H N
i r C)
N
-S=O
0
A mixture of intermediate 1-18 (100 mg, 0.218 mmol), PdCI2(dppf).DCM (cat.
amount), a saturated solution of K2CO3 (1 mL), indol-4-boronic acid
hydrochloride
(53 mg, 0.327 mmol), in DME (1 mL) was heated under microwave irradiation at
130 C for 10 min. The mixture was diluted with DCM (30 mL), washed with brine
(40 mL). The organic phase was dried (Na2SO4), filtered and concentrated. The
crude was purified by Biotage flash column chromatography eluting with a
gradient of DCM/MeOH (from 100% to 50:50) to obtain the desired product 2-71
(39 mg) as a white solid.
Preparation of Final product 2-70
0
N
N N
HN Nom'/ `
F N
N
--5=0
0
A mixture of intermediate 1-18 (100 mg, 0.218 mmol), PdC12(dppf).DCM (cat.
amount), a saturated solution of K2C03 (0.5 mL), 5-fluoro-4-(4,4,5,5-
tetramethyl-
1,3,2-dioxaborolan-2-yl)-1-tert-butyldimethylsilyl-indole (98 mg, 0.26 mmol,
CAS:
1072009-08-5), in DME (1 mL) was heated under microwave irradiation at 130 C
for 1h. The organic phase was concentrated. The crude was purified by Biotage
149
-0&26
WO 2010/119264 PCT/GB2010/000773
flash column chromatography eluting with a gradient of DCM/MeOH (from 100%
to 90:10) to obtain the desired product 2-70 (39 mg) as a white solid.
Preparation of Final product 2-35
(0)
N
N N 0
N~1/ NH2
Intermediate 1-30 (50 mg, 0.15 mmol) was dissolved in DME (1 ml-) and
phenylboronic acid (22 mg, 0.18 mmol), K2CO3 (64 mg, 0.46 mmol), PdCl2(dppf)-
DCM (13 mg, 15 umol) and H2O (0.5 ml-) were added. The mixture was heated
under microwave irradiation at 130 C for 1 h. On cooling, the mixture was
purified by column chromatography (Biotage, 25-S, 5% to 10% MeOH in DCM),
and the product obtained was precipitated with Et20 and filtered to give the
expected product 2-35 (45 mg, Y: 91 %) as a white solid.
Preparation of final product 2-63.
CN/
Fi N_ N O-
N
PdCl2(dppf) was added to a degassed mixture of intermediate 1-27 (100 mg, 0.21
mmol), indazol 4-boronic acid hydrochloride (0.091 g, 0.43 mmol) and an
aqueous saturated solution of Na2CO3 (0.25 ml-) in DME (1 mL). The vial was
sealed and heated at 130 C under microwaves for 10 min. The mixture was
diluted with EtOAc, washed with water, brine, dried and evaporated. The
residue
was purified by Biotage chromatography in DCM/MeOH 2 to 10% MeOH) to
obtain 52 mg of desired compound 2-63.
150
0&26
WO 2010/119264 PCT/GB2010/000773
Example B2
Preparation of Final product 2-21
(0)
N
N N :N
HN \ N NH2
A suspension of final compound 2-52 (160 mg, 0.5 mmol) in MeOH/NH3 7N was
heated in a sealed tube at 90 C for 16h. A precipitate appears which was
filtered
off to obtain 75 mg of the desired product 2-21 as a brown solid (Y: 41 %)
Preparation of Final product 2-77
(0)
N
_ N~N
HN N
/ O NH2
To a mixture of intermediate 1-32 (70 mg, 0.185 mmol) with dry DMF (3 drops)
in
benzene (2 mL) was added oxalyl chloride (2 equiv, 0.370mmol, 31 uL). The
mixture was stirred at rt for 3h, then same amount of reagents was added and
stirring continued for 1h. No reaction observed so volatiles were removed
under
reduced pressure and the residue was dissolved in dioxane (2 mL) and NH3 in
dioxane 0.5N (2 mL) was added. The mixture was stirred at it overnight and
solvent was evaporated under vacuum. The residue was purified by preparative
HPLC affording 3 mg of final product 2-77 (Y: 4%).
151
-0&26
WO 2010/119264 PCT/GB2010/000773
Example B3
Preparation of Final product 2-22
(0)
N
N Ni 71 N
-
HN \ \ N~
Final product 2-21 (50 mg, 0.138 mmol) in POC13 (2 ml) was heated to reflux
for
2h. The solvent was evaporated in vacuo and the residue was suspended in
DCM and Na2SO4 aq. solution. The organic phase was extracted, dried (MgSO4),
filtered and evaporated to obtain a light brown solid which was washed with
Et20.
The precipitate was filtered and dried to obtain the desired product 2-22 (25
mg,
Y: 53 %) as a pure solid.
Example B4
Preparation of Final product 2-33
(0)
N
Ni N
\ Nom'/
N CI
A mixture of final product 2-31 (50 mg, 0.169 mmol) and NCS (18 mg, 0.135
mmol, 0.8 eq) in THE (2 mL) was heated at 60 C for 18h. A saturated solution
of
NaHCO3 was added and the mixture was extracted with EtOAc. The organic
phase was separated, dried (Na2SO4), filtered and evaporated till dryness. The
residue was purified by using a sep-pack in a manifold, eluent:
cyclohexane/EtOAc, 2/1. The desired fractions were collected and the solvent
was evaporated till dryness to obtain 15mg, Y: 27% of desired product 2-33.
152
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of Final product 2-54
(0)
N
N_ W1 YN
HN N~
CI
A mixture of final product 2-24 (60 mg, 0.179 mmol) and NCS (31 mg, 0.233
mmol) in dioxane (2 ml-) was heated at 50 C for 18h. The organic phase was
evaporated till dryness. The residue was purified by using column
chromatograpy
(hexane/EtOAc mixtures) and then by HPLC. The desired fractions were
collected and the solvent was evaporated till dryness to obtain 9 mg, Y: 14 %
of
desired product 2-54.
Preparation of Final product 2-56
(0)
N
N N~--
N \ N>
I
A mixture of final product 2-31 (0.2 g, 0.677 mmol) and NIS (233 mg, 1.016
mmol) in THE (4 mL) was heated at 65 C for 18h. A saturated solution of
NaHCO3 was added and the mixture was extracted with EtOAc. The organic
phase was separated, dried (Na2SO4), filtered and evaporated till dryness. The
residue was purified by using a sep-pack in a manifold and then by HPLC. The
desired fractions were collected and the solvent was evaporated till dryness
to
obtain 2 mg of desired product 2-56.
153
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of Final product 2-60
C:)
N~
N ON /O
N-N
A mixture of final product 2-17 (45 mg, 0.1 mmol) and NCS (20 mg, 0.15 mmol)
in
acetonitrile (2 ml-) was stirred at room temperature for 4h. A saturated
solution of
NaHCO3 was added and the mixture was extracted with EtOAc. The organic
phase was separated, dried (Na2SO4), filtered and evaporated till dryness. The
residue was purified by column chromatograpy (DCM/MeOH 100% to 95:5) and
then by HPLC. The desired fractions were collected and the solvent was
evaporated till dryness to obtain 10 mg, of desired product 2-60.
Preparation of Final product 2-12
C:)
N/ N
O \ \ rN
To a solution of final product 2-32 (1.249 mmol) in THE (4.5 ml-) was added
NIS
(1.249 mmol) and the reaction mixture was stirred at it for 24h. Excess of NIS
(0.31 mmol, 70 mg) was added stirring the reaction for 16h more. A saturatated
aqueous solution of NaHCO3 and DCM was added. The organic phase was
extracted, dried (Na2SO4), filtered and evaporated. The residue was purified
by
flash chromatography in Biotage, eluent: CH2CI2-AcOEt/CH2CI2 to obtain 38.6 mg
of final product 2-12 and 41 mg of the corresponding regioisomer, final
product
2.53.
154
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of Final product 2-96
(O)
N
N 0
NNH
N 2
i CI
H2N N
Final product 2-93 (30mg, 88umol) was suspended in acetonitrile (2 mL) and
NCS (12mg, 88umol) was added. The mixture was stirred at rt for 15h and
filtered
to render a solid that was reprecipitated (DMSO/MeOH/formic acid) affording
the
final product 2-96 (15mg, 42%) as a yellow solid with purity of 90%
(contaminated
with 10% starting material).
Preparation of Final product 2-108
Co)
N
NN
-I-T- -N
N__
NI
cl
H2N N N
/SO
Final product 2-50 (50mg, 0.11 mmol) was suspended in DCM (1 mL) and NCS
(14mg, 0.11 mmol) was added. The mixture was stirred at rt for 20h. The
suspension was filtered and rinsed with DCM to render the final product 2-108
(41 mg, 76%) as white solid.
155
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of Final product 2-112
(0)
NII
NN
IIN__~ N
N
0' N CI ~N
o
Final product 2-67 (35mg, 72 mol) was suspended in DCM (1 mL) and NCS
(10mg, 72umol) was added. The mixture was stirred at rt for 20h. NaHCO3 sat
sol
was added to the mixture and it was extracted with DCM (x2). The combined
organic layer was dried, filtered and concentrated. The residue was
precipitated
with diethyl ether to afford the final product 2-112 (35mg, 93%) as white
solid
Example B5
Preparation of Final product 2-38
(0)
N
N_ NN
HN N 0 T
N~
1N
Benzotriazole (0.7 g, 5.9 mmol) and 1-m ethylpiperazine (0.660 mL, 5.9 mmol)
were stirred in ethanol (20 mL) at rt for 10 min. Glyoxal (0.360 mL of 40%
aqueous solution, 2.9 mmol) was added to the reaction mixture, and the
stirring
was continued for 16 h. The light yellow solution was concentrated under
vacuum
and precipitated. A light yellow-crystal solid appeared when the resulted oil
was
washed with diethyl ether to yield a 1.6 g of a solid which was used in next
reaction step without further purification. 210 mg (0.44 mmol) of this solid
and
intermediate 1-03 (130 mg, 0.44 mmol) were dissolved in DCE and refluxed for 5
h. The reaction mixture was cooled to rt and then KOH (powder, 250 mg) was
added. The mixture was stirred for 20 min at rt, filtered off and washed
(DCM).
156
-0&26
WO 2010/119264 PCT/GB2010/000773
The solvent was concentrated under reduced pressure. The resulting residue was
purified by flash column chromatography eluting with a gradient of DCM/MeOH
(from 100% to 96:4), yielding final product 2-38, 30 mg, Y: 16 %.
Preparation of Final product 2-41
(0)
N
N NN
HN N
N~
N
'
O=,S-
O
A mixture of benzotriazole (300 mg, 2.43 mmol) 4-methylsulfonylpiperazine (400
mg, 2.43 mmol) in EtOH (20 mL) was stirred for 20 min. Glyoxal (0.160 mL of a
40% w solution in water, 1.2 mmol) was added, and the resultant mixture was
stirred for 16 In. The white solid formed was filtered off, washing with EtOH
and
diethylether to yield 280 mg that was used in next reaction step without
further
purification. Another batch of this reaction was progressed. 497 mg (0.844
mmol) of this solid and intermediate 1-03 were heated in DCE under reflux for
6h.
The reaction mixture was cooled to rt, KOH (156 mg podwer) was added and the
resulted mixture was stirred at rt for 1 h. The reaction mixture was filtered
off and
the filtrate was concentred under vacumn to yield a residue which was purified
by
flash column chromatography (eluting with a gradient of DCM/MeOH/NH3 7N
(from 100% to 95:5), to yield desired final product 2-41 (50 mg, Y: 12.3 %).
157
-0&26
WO 2010/119264 PCT/GB2010/000773
Example B6
Preparation of Final product 2-43
0
NN
N_ N// YN
HN IN
N
O ~- O
Final product 2-42 (230 mg, 0.56 mmol) was dissolved in 1,4-dioxane (3 mL) and
Pd(PPh3)4 (64 mg, 56 umol), Cs2CO3 (363 mg, 1.11 mmol), 1-N-boc-4-(4,4,5,5-
tetramethyl-[1,3,2]dioxaborolan-2-yl)-3,6-dihydro-2H-pyridine (198 mg, 0.64
mmol) and H2O (2 mL) were added. The mixture was heated under microwave
irradiation at 140 C for 1 h. On cooling, the solvents were removed and the
residue was purified by column chromatography (DCM/MeOH from 98:2 to 94:6)
to give the final product 2-43 (260 mg, Y: 91%) as a yellow solid.
Example B7
Preparation of Final product 2-44
(0)
~N
N N// -N
HN N
N
H
Final product 2-43 (200 mg, 0.39 mmol) was dissolved in MeOH (7 ml-) and
Amberlyst(r) 15 (1 g, 4.7 mmol) was added. The reaction mixture was stirred at
rt
for 24 h and filtered. The resin was suspended in MeOH/NH3 7N (10 mL), stirred
for 10 min and filtered. This treatment was repeated 3 times. The filtrates
were
together evaporated and the residue was precipitated from DCM (5 mL) and
filtered to give the expected product 2-44 (43 mg, Y: 27%), as a white solid.
158
-0&26
WO 2010/119264 PCT/GB2010/000773
Example B8
Preparation of Final product 2-45
(0)
N
N N _N
HN N
N
Final product 2-44 (35 mg, 84 umol) was suspended in DCM (1.5 ml-) and
formaldehyde (0.1 mL, 1.26 mmol) and sodium cyanoborohydride (32 mg, 0.5
mmol) were added. The reaction mixture was stirred at it for 20 h. The
reaction
was adsorbed in silica and purified by column chromatography (DCM/MeOH from
96:4 to 70:30) and then by HPLC to give the expected product 2-45 (3.2 mg) as
a
white solid.
Preparation of Final product 2-58
(0)
NN
N_ N/ -N
HN N
N
Final product 2-57 (20 mg, 48 umol) was suspended in DCM (1.5 mL) and
formaldehyde (52 l, 0.72 mmol) and sodium cyanoborohydride (18 mg, 0.29
mmol) were added. The reaction mixture was stirred at it for 1 h. The reaction
was adsorbed in silica and purified by column chromatography (DCM/MeOH from
96:4 to 70:30) and then by HPLC to give the expected product 2-58 (9 mg) as a
white solid.
159
-0&26
WO 2010/119264 PCT/GB2010/000773
Example B9
Preparation of Final product 2-46
(0)
NN
N N// YN
HN N
N
O oS-
O
Final product 2-44 (30 mg, 72 umol) was suspended in acetonitrile (1 mL) and
DIPEA (19 L, 0.11 mmol) and MeSO2CI (6 L, 79 umol) were added. The
solution was stirred at rt for 2.5 h. Excess of McSO2CI (3 L, 0.5 eq) was
added.
The reaction mixture was stirred for 3 days and evaporated. The residue was
dissolved in HCI 1M (10 mL) and extracted with DCM (3 x 7 mL). The organic
phase was dried (Na2SO4), filtered and the solvent evaporated in vacuo. The
resulting residue was purified by HPLC to give the expected product 2-46 (10
mg,
Y: 28%) as a white solid.
Example B10
Preparation of Final product 2-47
(0)
N
N N
HN N
N
O ~- O
Final product 2-43 (50 mg, 97 umol) was dissolved in MeOH (100 ml-) and
hydrogenated in the H-Cube (Pd/C 10%, 60 C, Full H2, 1 mL/min). The resulting
solution was evaporated and the residue was purified by HPLC to give the
expected product 2-47 (14 mg, Y: 28%) as a white solid.
160
-0&26
WO 2010/119264 PCT/GB2010/000773
Example B11
Preparation of Final product 2-30
CO)
NN
Ni rN
HO NJ
N
Boron fluoride-dimethyl sulfide complex (0.226 mL, 2.151 mmol) was added to a
stirred solution of final product 2-27 (70 mg, 0.2165mmol) in DCM (1.3 mL) at
rt.
The mixture was stirred at rt for 24 h. Additional amount of boron fluoride-
dimethyl sulfide complex (2.1 mL) was added, and the mixture was stirred at rt
for
48 h more. A saturated solution of NaHCO3 was added and the mixture was
extracted with DCM/MeOH 90:1. The organic phase was separated, dried
(Na2SO4), filtered and evaporated to dryness. The residue was purified by
biotage
chromatography and eluted with a gradient DCM/MeOH from 100 % to 50:50.
The desired fractions were collected to obtain 20 mg of desired product 2-30
as a
solid (Y: 30 %).
Preparation of Final product 2-88
0
NII
N N,~
HO IIN:
CN
Boron trifluoride-dimethyl sulfide complex (0.084 mL, 0.8 mmol) was added to a
stirred solution of final product 2-62 (28 mg, 0.08 mmol) in DCM (1.5 mL) at
rt.
The mixture was stirred at rt for 24 h. Additional amount of boron fluoride-
dimethyl sulfide complex (total of 0.3 ml-) was added, and the mixture was
stirred
at rt for 48 h more. Then, THE (1 ml-) was added and the mixture was heated at
50 c for 53h. A saturated solution of NaHCO3 was added and the mixture was
extracted with DCM/MeOH 90:1. The organic phase was separated, dried
161
-0&26
WO 2010/119264 PCT/GB2010/000773
(Na2SO4), filtered and evaporated to dryness. The residue was purified by
biotage
chromatography and eluted with a gradient DCM/MeOH from 100% to 95:5. The
desired fractions were collected to obtain 7 mg of desired product 2-88 as a
solid
(Y: 26%).
Example B12
Preparation of Final product 2-57
(0)
N
N N
N
I __
HN N
V'zz~
N
H
Final Product 2-47 (174 mg, 0.34 mmol) was dissolvend in MeOH (7 ml) and
Amberlyst 15 (1g) was added. The reaction mixture was stirred at room
temperature for 24h and filtered. The resin was suspended in MeOH/NH3 7N
stirred for 10 min. and the organic phase was collected. The solvent was
evaporated and the residue was precipitated with MeOH, and then purified by
HPLC to obtain 9 mg as a formiate salt of Final product 2-57.
Preparation of Final product 2-236
o
CND
NN
N N N
~
H2N ~N H
A 4M solution of HCI in dioxane (1 mL) was added at 0 C to Final product 2-232
(12 mg, 0.023 mmol). The reaction mixture was stirred at it for 3 h. Solvents
were
removed and the residue was purified by column chromatography (Isolute SCX-2
cartridge, MeOH to NH3 7N in MeOH) to give Final product 2-236 (9 mg, 92%).
162
-0&26
WO 2010/119264 PCT/GB2010/000773
Example B13
Preparation of Final product 2-52
(0)
N
N NNO
HN NS'/ O
To a reaction mixture of intermediate 1-12 (150 mg, 0.42 mmol), indazole-4-
boronic acid hydrochloride (0.93 mmol, 0.150 mg), and PdCl2(dppf).DCM (35 mg,
0.042 mmol) in DME (2 ml), was added a saturated solution of potassium
carbonate (0.5 ml). The mixture was heated at 1300C under microwave
irradiation
for 10 min. A precipitate appears which was filtered, washed with DCM and
dried.
The resulting solid (0.160 mg, Y: 96 %) is the expected final compound 2-52
and
was used in next reaction step without further purification.
Preparation of Final product 2-92
(0)
N
NO
N N NH2
Intermediate 1-30 (100mg, 0.31 mmol) was dissolved in DME (2 ml-) and pyridine-
3-boronic acid (45mg, 0.37mmol), K2C03 (127mg, 0.92mmol), PdC12(dppf)=DCM
and water (1 ml-) were added. The reaction mixture was heated under microwave
irradiation at 130 C for 1h. The volatiles were removed under vacuum and the
residue was purified by flash chromatography (DCM-MeOH 95:5 to 90:10). The
product obtained was precipitated in MeOH affording the final product 2-92 as
off-
white solid (96mg, 97%).
163
-0&26
WO 2010/119264 PCT/GB2010/000773
Preparation of Final product 2-93
CO)
N
N N O
N \ \ IIN/" NH2
H2N N
Intermediate 1-30 (100mg, 0.31mmol) was dissolved in DME (2 mL) and 2-
aminopyrimidine-5-boronic acid pinacol ester (81 mg, 0.37mmol), K2CO3 (1 27mg,
0.92mmol), PdC12(dppf)=DCM and water (1 mL) were added. The reaction mixture
was heated under microwave irradiation at 1300C for 1h. The volatiles were
removed under vacuum and the residue was purified by flash chromatography
(DCM-MeOH 95:5 to 90:10). The product obtained was precipitated in MeOH
affording the final product 2-93 as off-white solid (96mg, 97%).
Preparation of Final product 2-165
C0)
N
N~N
N 'N N
H2N~ N O
/S=O
Final product 2-178 (50 mg, 0.087 mmol) was dissolved in 1,4-dioxane (0.3 mL)
and Pd(PPh3)4 (10 mg, 0.009 mmol), Cs2CO3 (57 mg, 0.174 mmol),
methylboronic acid (6 mg, 0.1 mmol) and water (0.2 mL) were added. The mixture
was heated under microwave irradiation at 140 C for 1 h. Water was added and
the mixture was extracted with DCM. The organics were dried (Na2SO4), filtered
and evaporated. The residue was purified by column chromatography-TLC in the
Chromatotron (DCM:MeOH, 15:1) twice. The desired fractions were collected and
evaporated to obtain Final compound 2-165 (22 mg, 52%).
164
-0&26
WO 2010/119264 PCT/GB2010/000773
Example B14
Preparation of Final product 2-62
0
C)
N
N N
0 N
CN
To a solution of final Product 2-12 (0.087 mmol) in DMF dry (I ml) was added
zinc
cyanide (0.091 mmol), tris(dibenzylidenaceton)dipalladium (Pd2dba3) (0.004
mmol), 1,1'-bis(diphenylphosphino)ferrocene (DPPF) (0.011 mmol). The mixture
was heated at 1401C for 1 h under microwave irradiation. The solution was
diluted
with ethyl acetate, washed with water and a saturated solution of NaCI. The
organic phase was dried (Na2SO4), filtered and the solvent evaporated. The
residue was purified by flash column chromatography, eluent: CH2CI2-
AcOEt/CH2CI2 1:100-1:50 to obtain 22.9 mg as a white solid of compound 2-62.
Preparation of Final product 2-36
C:)
N. N ~%
i
HN N~
CN
A mixture of final product 2-73 (38 mg, 0.163 mmol), Zn(CN)2 (10 mg, 0.087
mmol), diphenylphosphineferrocene (6 mg, 0.01 mmol) and Pd2(dba)3 (4 mg,
0.004 mmo) in DMF (0.5 mL) was heated for 1 h at 120 C under microwave
irradiation. Then, more Zn(CN)2 (10 mg, 0.087 mmol), dppf (6 mg, 0.01 mmol,
0.125 eq) and Pd2(dba)3 (4 mg, 0.004 mmol, 0.05 eq) were added and the
mixture was heated 1.5 h at 120 C under microwave irradiation. This excess was
added twice. The solvent was removed in vacuo and the residue was purified by
column chromatography (EtOAc and EtOAc/MeOH mixtures) and then by HPLC
to obtain 1.2 mg of desired product 2-36.
165
-0&26
WO 2010/119264 PCT/GB2010/000773
Example B15
Preparation of Final product 2-79
(O)
N
N N N O
HN N~J
I "
Final product 2-52 (25mg, 0.064mmol) was suspended in EtOH (1.5mL) and
methyl amine (2M in THF, 1.27 mmol, 0.7mL) was added. The reaction mixture
was heated in a sealed tube at 1000C for 18h. The reaction mixture was then
directly adsorbed in silica to be purified by column chromatography (5% to 10%
of
MeOH in DCM) rendering 5mg of the final product 2-79 as a white solid (Y. 21
%).
Preparation of Final product 2-217
C:)
N~N 0
N IN_ NH2
H 2 N N
Final product 2-214 (300 mg, 0.78 mmol) was suspended in MeOH/NH3 7N (10
ml-) and heated under microwave irradiation at 130 C for 24 h. The mixture
was
evaporated and purified by column chromatography (MeOH in DCM, 100:0 to
40:60) rendering 80 mg of final product 2-217 as a white solid (Y. 29%).
166
-0&26
WO 2010/119264 PCT/GB2010/000773
Example B16
Preparation of Final product 2-87
(0)
N
N N% YEN O
HN N v N
Pyrrolidine (0.54 mmol, 45 uL) was dissolved in EtOH (5 ml-) in a sealed tube
and
AIMe3 (0.54 mmol, 0.26 mL) was added. The mixture was stirred at rt for 15min
and then, the final product 2-52 (0.27 mmol, 105 mg) was added. The reaction
mixture was stirred at rt for 1h and 4h at 40 C. On cooling, the reaction was
carefully quenched with NH4CI sat sol and extracted with CHCI3-iPrOH 1:1 (x3).
The combined organic layer was dried, filtered and concentrated. The crude
product was purified by flash chromatography (DCM-MeOH 96:4 to 90:10)
rendering the final product 2-87 (15mg, 13%) as white solid.
Preparation of Final product 2-139
(0)
NII
N_ NN 0
HN \ \ IINN
~
H
/N--
Final product 2-52 (0.127 mmol, 50 mg) was dissolved in EtOH (3 mL) and N,N-
dimethyl-1,3-propanediamine (1.27 mmol, 0.16 ml-) and AIMe3 (1.27 mmol, 0.64
ml-) were added. The mixture was heated at 150 C for 3 days and under
microwave irradiation at 180 C for 1 h. On cooling, the reaction was
carefully
quenched with NH4CI sat. sol. and extracted with DCM (x2). The combined
organic layers were dried, filtered and concentrated. The crude product was
purified by flash chromatography (DCM-MeOH:NH3(7N); 100:0 to 80:20)
rendering the final product 2-139 (18 mg, 31%) as white solid.
167
0&26
WO 2010/119264 PCT/GB2010/000773
Example B17
Preparation of Final product 2-97
(0)
NN
N N YN
HN N/N
N
o o
A mixture of intermediate 1-34 (100mg, 0.28mmol), AcOH (40uL, 0.52mmol), 2,8-
diaza-spiro[4. 5]decane-2-carboxylic acid tert-butyl ester hydrochloride
(90mg,
0.29mmol) in DCE (5mL) was stirred at rt for 40 min. Then, NaBH(OAc)3 (90mg,
0.40mmol) was added and stirring continued for 5h. The reaction mixture was
quenched by adding 4N aq sol of KOH and it was extracted with EtOAc (x2). The
combined organic layer was washed with brine, dried (Na2SO4), filtered and
concentrated the crude product (150 mg, 93%) that was used in next reaction
step without further purification. Part of this crude product (50mg) was
further
purified by preparative HPLC rendering the final product 2-97 (13 mg).
Method B18
Preparation of Final product 2-155
(0)
N
HN N~, // N\//~
N
A mixture of iodine (34 mg, 0.133 mmol), triphenylphosphine (29 mg, 0.111
mmol) and imidazole (9 mg, 0.133 mmol) in DMF (2 mL) was stirred at RTfor 1h.
Then, Final product 2-144 (55 mg, 0.111 mmol) was added and the mixture was
168
-0&26
WO 2010/119264 PCT/GB2010/000773
stirred at 70 C overnight. The mixture was evaporated and the residue was
purified by using a sep-pack in a manifold (DCM:MeOH, 92:8) to render 10 mg of
Final product 2-155 (16%).
Method B19
Preparation of Final product 2-177
(0)
N
N~11 N
N IINH
H2N N
Final product 2-138 (0.3 g, 0.81 mmol) was suspended in THE (6 mL) and
MeMgCI (3M, 2.7 mL, 8.1 mmol) was slowly added at 0 C. The reaction mixture
was stirred for 4 h and then carefully quenched with H2O. The resulting
mixture
was purified by column chromatography (DCM:MeOH, 95:5 to 85:15). The
product obtained was precipitated with DCM and drops of MeOH and filtered to
render Final product 2-177 (20 mg, 7%) as a yellow solid. The filtrate was
evaporated and purified by column chromatography (DCM:MeOH, 95:5 to 85:15)
and by prep-HPLC to give Final product 2-177 (38 mg, 13%) as a yellow solid.
Method B20
Preparation of Final product 2-191
C)
N
N~N O
N NN-GO
H
H2N N
To a solution of Intermediate 1-52 (50 mg, 0.146 mmol), BOP (78 mg, 0.176
mmol) and 4-aminotetrahydropyran.HCI (0.024 mL, 0.176 mmol) in DCM (1.5 ml-)
was added Et3N (0.041 mL, 0.293 mmol). The mixture was stirred at rt for 2h.
DCM was added and the mixture was washed with water. The organic phase was
dried, filtered and evaporated. The residue was purified by column
169
-0&26
WO 2010/119264 PCT/GB2010/000773
chromatography (DCM:MeOH, 100:0 to 60-40) and by prep-HPLC to render 4 mg
(6 %) of Final product 2-191 as a white solid.
Method B21
Preparation of Final product 2-234
(o)
N
N~N
N N
H2N N
OH
0
Final product 2-231 (40 mg, 0.078 mmol) was suspended in sodium methoxide
0.5 M in MeOH, 3 ml-) and the reaction mixture was stirred at RT for 45 min.
H2O
(3 ml-) was added, the solution was slightly acidified with HCI and extracted
with
n-BuOH. The organics were dried over Na2SO4, filtered and evaporated. The
residue was purified by chromatotron (DCM:MeOH, 10:1). The residue was
dissolved in MeOH (4 mL), amberlyst (0.3 g) was added and the mixture was
stirred at rt for 2 h, filtered and washed with MeOH. The resine was suspended
in
NH3/MeOH (7 N, 35 ml-) and stirred for 1 h. The mixture was filtered and the
filtrate was evaporated. The residue was purified by chromatotron (DCM/MeOH,
10:1) to give Final product 2-234 (12 mg, 33%) as a white solid.
Method B22
Preparation of Final product 2-237
(0)
N
NrN
\ IIN__
N-N
Intermediate 1-28 (500 mg, 1.540 mmol), indazole-4-boronic acid hydrochloride
(3.387mmo1, 672 mg) and PdCl2(dppf).DCM (0.154 mmol, 127 mg) were
suspended in a saturated solution of sodium carbonate (1.5 ml-) and 1,2-DME
170
-0&26
WO 2010/119264 PCT/GB2010/000773
(7mL). The mixture was heated under microwave irradiation at 130 C for 10 min.
The mixture was diluted with EtOAc and washed with water. The organic layer
was dried (Na2SO4), filtered and the solvent evaporated in vacuo. The
resulting
residue was purified by flash chromatography (DCM-MeOH from 100:0 to 96:4) to
obtain the Final Product 2-237 as a white solid (88 mg, Y: 14%).
Method B23
Preparation of Final product 2-238
(o)
N
Ni N
NI
0 off
H--N
Final Product 2-237 (88 mg, 0.217 mmol) was suspended in MeOH (2 mL) and
treated with 2N NaOH (0.24 mL, 0.48 mmol). The reaction mixture was refluxed
for 4 h. Solvents were evaporated and the residue was dissolved in EtOAc,
treated with AcOH and washed with water. The organic layer was dried, filtered
and evaporated to give the Final Product 2-238 (82 mg, 100%).
Method B24 -
Preparation of Final product 2-241
(0)
N
N N N
HN N
N
d-~
To a solution of Final product 2-44 (50 mg, 0.12 mmol) and N,N-
diisopropylethylamine (0.031 mL, 0.181 mmol) in acetonitrile (2 mL) was added
isobutyryl chloride (0.014 mL, 0.132 mmol). The mixture was stirred at rt for
4 h
and evaporated. H2O was added and the mixture was extracted with DCM. The
171
-0&26
WO 2010/119264 PCT/GB2010/000773
organic layer was dried (Na2SO4), filtered and evaporated to render Final
Product
2-241 (51 mg, 87%).
Method B25
Preparation of Final product 2-243
(0)
N
N N
N ~ / I O I N
~N \ N~N
H H
O
A mixture of Final Compound 2-245/Intermediate 1-69 (62 mg, 0.13 mmol), 1-
methylpiperazine (0.019 mL, 0.17 mmol), TEA (0.024 mL, 0.17 mmol), HOBT (26
mg, 0.17 mmol) and EDCI (33 mg, 0.17 mmol) in THE (1 mL) was stirred at rt
overnight and evaporated. The residue was purified by column chromatography
(Isolute 5 g; MeOH:DCM, 1:99 to 20:80 and Flash-NH2 5g; MeOH:DCM, 0:100 to
2:98) to give the Final Product 2-243 (49 mg, 67%) as a white solid.
Method B26
Preparation of Final product 2-245
0
NII
NN
N
II
O \ I NN
H H
O
172
-0&26
WO 2010/119264 PCT/GB2010/000773
To a solution of Intermediate 1-69 (50 mg, 0.16 mmol) in DCM (1.3 mL) was
added methyl 4-isocyanatobenzoate (31 mg, 0.18 mmol). The reaction mixture
was stirred at rt for 5 h. Cyclohexane was added and the mixture was filtered
to
give Final Product 2-245 (46 mg) as a beige solid. The filtrate was evaporated
and the residue was purified by column chromatography (Isolute 5g; MeOH:DCM,
0:100 to 5:95) to give Final product 2-245 (25 mg) as a light yellow solid.
Total
yield: 91 %.
General Procedure
The HPLC measurement was performed using a HP 1100 from Agilent
Technologies comprising a pump (binary) with degasser, an autosampler, a
column oven, a diode-array detector (DAD) and a column as specified in the
respective methods below. Flow from the column was split to a MS spectrometer.
The MS detector was configured with an electrospray ionization source or
API/APCI. Nitrogen was used as the nebulizer gas. The source temperature was
maintained at 150 C. Data acquisition was performed with ChemStation LC/MSD
quad, software.
Method 1
Reversed phase HPLC was carried out on a RP-C18 Gemini column (150 x 4.6
mm, 5 um); 10 min. linear gradient of 50- 100% acetonitrile in water + 100 %
acetonitrile in water 2 min ): 210 nm and 254 or DAD.
Method 2
Reversed phase HPLC was carried out on a Gemini-NX C18 (100 x 2.0 mm;
5um), Solvent A: water with 0.1% formic acid; Solvent B: acetonitrile with
0.1%
formic acid. Gradient: 5% of B to 100% of B within 8 min at 50 C, DAD.
Method 3
Reversed phase HPLC was carried out on a Gemini-NX C18 (100 x 2.0 mm;
5um), Solvent A: water with 0.1 % formic acid; Solvent B: acetonitrile with
0.1 %
formic acid. Gradient: 5% of B to 40% of B within 8 min at 50 C, DAD.
173
-0&26
WO 2010/119264 PCT/GB2010/000773
Method 4
Reversed phase HPLC was carried out on a Gemini-NX C18 (100 x 2.0 mm;
5um), Solvent A: water with 0.1% formic acid; Solvent B: acetonitrile with
0.1%
formic acid. Gradient: 0% of B to 30% of B within 8 min at 50 C, DAD.
Method 5
Reversed phase HPLC was carried out on a Gemini C18 (50 x 2.0 mm; 3um),
Solvent A: water with 0.1 % formic acid; Solvent B: acetonitrile with 0.1 %
formic
acid. Gradient: 10% of B to 95% of B within 4 min at 50 C, DAD.
Table 4: Analytical data and P13Ka activity - Rt means retention time (in
minutes), [M+H]+ means the protonated mass of the compound, method refers to
the method used for (LC)MS.
Biological activity in P13Ka for certain examples is represented in Table 4 by
semi-quantative results: IC50 >1jM (+), IC50 <100 nM (+++), 100 nM<IC50< 1
tM (++). Biological activity in Pl3Ka for certain examples is also represented
in
Table 4 by quantative results.
P13Ka
Cpd. 1H NMR (300 MHz; 8 in ppm, J in
Nr. Rt [M+1] Meth. IC50
Hz)
( M)
DMSO 6 9.52 (s, I H), 8.49 (d, J = 16.6,
2-01 4.882 369.1 1 0.158 2H), 7.32 (m, 3H), 6.78 (dd, J = 7.9, 1.5,
(++) 1 H), 4.32 (dt, J = 13.0, 6.4, 6H), 3.80 (m,
4H), 1.32 (t, J = 7.1, 3H).
DMSO 6 9.58 (s, 1 H), 8.38 (s, 1 H), 7.99 (s,
2-02 9.199 341.1 1 19 1 H), 7.31 (m, 2H), 7.15 (t, J = 7.8, 1 H),
6.69 (d, J = 7.8, 1 H), 4.22 (m, 4H), 3.72 (m,
4H).
DMSO) 6 10.01 (s, 1H), 9.53 (s, 1H), 8.60
2-03 10.43 325.1 1 10 (s, 1 H), 8.51 (s, 1 H), 7.38 (m, 2H), 7.25 (t,
3 J = 7.8, 1 H), 6.78 (d, J = 7.6, 1 H), 4.32 (s,
4H), 3.81 (s, 4H).
1H NMR (300 MHz, DMSO) 6 9.46 (s, 1 H),
2-04 8.396 327.1 1 0.639 8.50 (s, 1 H), 7.81 (s, 1 H), 7.38 (m, 2H),
(++) 7.23 (t, J = 7.9, 1 H), 6.75 (d, J = 7.8, 1 H),
4.59 (s, 2H), 4.23 (m, 4H), 3.78 (m, 4H).
174
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. Rt [M+11-" Meth. IC50 1H NMR (300 MHz; 6 in ppm, J in
Nr. Hz)
( M)
DMSO 6 9.48 (s, 1 H), 8.45 (s, 1 H), 7.82 (s,
1 H), 7.36 (m, 2H), 7.24 (d, J = 7.7, 1 H),
2-05 7.277 495.3 1 0.476 6.74 (d, J = 7.5, 1 H), 4.23 (m, 4H), 3.78 (m,
4H), 3.62 (s, 2H), 3.35 (m, 4H), 2.40 (m,
4H), 1.38 (s, 9H).
DMSO 6 9.48 (s, 1 H), 8.45 (s, 1 H), 7.83 (s,
0.451 1 H), 7.36 (dd, J = 11.7, 5.0, 2H), 7.22 (t, J
2-06 4.707 437.2 1 = 7.9, 1 H), 6.74 (dd, J = 7.9, 1.6, 1 H), 4.23
(++)
(m, 4H), 3.77 (m, 4H), 3.62 (s, 2H), 3.40
(m, 4H), 2.41-2.39 (m, 4H), 1.96 (s, 3H).
DMSO 6 9.48 (s, 1 H), 8.45 (s, I H), 7.80 (s,
1 H), 7.37 (dd, J = 12.2, 5.0, 2H), 7.22 (t, J
2-07 4.577 409.2 1 27 = 7.9, 1 H), 6.74 (d, J = 9.5, 1 H), 4.24 (m,
4H), 3.78 (m, 4H), 3.58 (s, 2H), 2.42-2.31
(m, 4H), 2.14 (s, 4H), 1.39 (s, 3H).
DMSO 6 9.48 (s, 1 H), 8.46 (s, 1 H), 7.86 (s,
0.54 1 H), 7.74 (s, 1 H), 7.38 (dd, J = 11.8, 5.0,
2-08 4.714 409.2 1 2H), 7.23 (t, J = 7.9, 1 H), 6.75 (dd, J = 8.0,
(++) 1.7, 1 H), 4.24 (m, 4H), 3.78 (m, 4H), 3.69
(s, 2H), 3.16 (m, 2H), 2.64 (t, J = 5.3, 2H).
DMSO 6 9.48 (s, 1 H), 8.45 (s, 1 H), 7.83 (s,
2-09 4.601 396.2 1 0.444 1 H), 7.38 (m, 2H), 7.22 (t, J = 7.9, 1 H),
(++) 6.74 (dd, J = 7.5, 2.0, 1 H), 4.24 (m, 4H),
3.78 (m, 4H), 3.58 (m, 6H), 2.45 (m, 4H).
DMSO 6 9.48 (s, 1 H), 8.46 (s, 1 H), 7.83 (s,
0.037 1 H), 7.37 (dd, J = 11.9, 4.9, 2H), 7.23 (t, J
2-10 4.409 473.2 1 = 7.8, 1 H), 6.75 (dd, J = 7.9, 1.7, 1 H), 4.24
(+++)
(m, 4H), 3.78 (m, 4H), 3.67 (s, 2H), 3.12
(m, 4H), 2.87 (s, 3H), 2.56 (m, 4H).
DMSO 6 9.51 (s, 1 H), 8.49 (s, 1 H), 8.34 (s,
2-11 6.401 487 1 0.044 1 H), 7.37 (m, 2H), 7.25 (t, J = 7.8, 1 H),
6.77 (d, J = 8.1, 1H), 4.25 (s, 6H), 3.80 (s,
6H), 3.22 (s, 4H), 2.92 (s, 3H).
CDCI3 6 7.67 (d, J =0.7, 1H), 7.35 (t, J =7.9
2-12 5.599 397.1 1 ++ Hz, 1 H), 7.22 (m, 1 H), 7.18 (m, 1 H), 6.95
(ddd, J =8.2, 2.6, 0.9 Hz, 1 H), 4.28 (m, 4H),
3.85 (s, 3H), 3.84 (m, 4H), 2.46 (s, 3H).
2-13 7.691 365.1 1 0.108 DMSO 6 9.53 (s, 1 H), 8.50 (d, J = 13.9,
2H), 7.38 (dd, J = 9.6, 4.9, 2H), 7.26 (t, J =
175
-0&26
WO 2010/119264 PCT/GB2010/000773
PI3Ka
Cpd. + 1H NMR (300 MHz; 6 in ppm, J in
Rt [M+1] Meth. IC50
Nr. Hz)
( M)
(++) 7.8, 1 H), 6.79 (dd, J = 7.9, 1.6, 1 H), 4.25
(m, 4H), 3.80 (m, 4H).
DMSO 6 9.46 (s, 1 H), 8.38 (s, 1 H), 7.69 (s,
0.34 1 H), 7.37 (dd, J = 12.3, 5.0, 2H), 7.21 (t, J
2-14 7.025 337 1 = 7.9, 1 H), 6.73 (dd, J = 7.9, 1.6, 1 H), 4.21
(m, 4H), 3.77 (m, 4H), 2.03 (ddd, J = 13.2,
8.3, 4.9, 1 H), 0.92 (m, 2H), 0.79 (m, 2H).
DMSO 6 13.25 (s, 1 H), 8.65 (s, 1 H), 8.55
0.741 (s, 1 H), 8.40 (s, 1 H), 7.67 (d, J = 6.8, 1 H),
2-15 3.131 447.2 1 7.58 (m, 1 H), 7.45 (m, 1 H), 4.29 (s, 4H),
(++) 4.08 (s, 2H), 3.82 (s, 4H), 3.70 (s, 2H),
2.48 (s, 4H), 2.28 (s, 3H).
DMSO 6 13.24 (s, 1 H), 8.65 (s, 1 H), 8.55
0.254 (s, I H), 8.44 (s, I H), 7.67 (d, J = 7.0, 1 H),
2-16 4.152 511.2 1 7.58 (d, J = 8.3, 1 H), 7.45 (t, J = 7.7, 1 H),
(++)
4.29 (s, 6H), 3.82 (s, 6H), 3.23 (s, 4H),
2.92 (s, 3H).
DMSO 6 13.20 (s, 1 H), 8.58 (d, J = 15.8,
2H), 7.99 (d, J = 37.6, 1 H), 7.52 (m, 3H),
2-17 3.171 497.20 1 0.095 4.27 (d, J = 4.4, 4H), 3.81 (m, 4H), 3.70 (s,
2H), 3.14 (t, J = 9.4, 4H), 2.87 (s, 3H), 2.58
(s, 4H).
DMSO 6 8.64 (s, 1 H), 8.55 (s, 1 H), 8.40 (s,
1 H), 8.30 (s, 1 H), 7.66 (d, J = 7.1, 1 H),
2-18 3.745 432.5 1 0.182 7.58 (d, J = 8.3, 1 H), 7.44 (m, 1 H), 4.89 (br
(++) s, 1 H), 4.28 (br s, 4H), 4.07 (br s, 1 H), 3.81
(br s, 4H), 3.70 (br s, I H), 2.92 (brs, 4H)
(rotamers observed)
DMSO) 6 13.26 (s, 1 H), 8.53 (s, 3H), 7.79
(s, 1 H), 7.62 (d, J = 7.1, 1 H), 7.55 (d, J =
2-19 4.151 361.2 1 0.438 8.2, 1 H), 7.42 (d, J = 7.5, 1 H), 4.24 (s, 4H),
3.79 (s, 4H), 2.06 (m, 1 H), 0.94 (m, 2H),
0.81 (m, 2H).
DMSO 6 13.26 (s, 1H), 8.59 (m, 3H), 7.67
2-20 4.63 389.10 1 1.8 (d, J = 7.1, 1 H), 7.60 (d, J = 8.3, 1 H), 7.45
(m, 1 H), 4.28 (s, 4H), 3.82 (m, 4H).
DMSO 6 13.20 (s, 1 H), 8.58 (s, 1 H), 8.50
2-21 5.25 364.1 1 - (s, 1 H), 8.34 (s, 1 H), 7.83 (s, 1 H), 7.61 (d,
J = 7.1, 1 H), 7.52 (d, J = 8.2, 1 H), 7.39 (m,
176
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. 'H NMR (300 MHz; S in ppm, J in
Rt [M+1]+ Meth. IC50
Nr. Hz)
( M)
2H), 4.27 (s, 4H), 3.75 (s, 4H).
DMSO 6 13.19 (s, 1 H), 8.73 (s, 1 H), 8.58
2-22 6.72 346 1 0.618 (s, 1 H), 8.47 (s, 1 H), 7.61 (d, J = 7.1, 1 H),
(++) 7.53 (d, J = 8.2, 1 H), 7.38 (t, J = 7.7, 1 H),
4.21 (s, 4H), 3.75 (m, 4H).
DMSO 6 9.45 (s, 1 H), 8.43 (s, 1 H), 7.69 (d,
2-23 5.438 311.1 1 0.096 J = 0.7, 1 H), 7.38 (m, 2H), 7.22 (t, J = 7.9,
(+++} 1 H), 6.74 (dd, J = 8.0, 1.6, 1 H), 4.23 (m,
4H), 3.78 (m, 4H).
DMSO 6 13.19 (s, 1 H), 8.58 (s, 1 H), 8.55
2-24 5.576 335.1 1 2.6 (s, 1 H), 7.79 (s, 1 H), 7.64 (d, J = 7.1, 1 H),
(+} 7.54 (d, J = 8.2, 1 H), 7.42 (m, 1 H), 4.26 (m,
4H), 3.80 (m, 4H), 2.37 (s, 3H).
DMSO 611.18 (s, 1H), 8.48 (s, 1H), 8.05
2-25 6.033 334.1 1 10 (d, J = 0.7, 1 H), 7.70 (d, J = 0.7, 1 H), 7.58
(d, J = 1.4, 2H), 7.37 (m, I H), 6.43 (m, 1 H),
4.26 (m, 4H), 3.80 (m, 4H), 2.35 (m, 3H).
DMSO 6 9.09 (m, 1 H), 8.56 (s, 1 H), 8.48
(dd, J = 4.8, 1.6, 1 H), 8.24 (m, 1 H), 7.64 (d,
2-26 3.350 296.1 1 10 J = 0.8, 1 H), 7.40 (ddd, J = 8.0, 4.7, 0.7,
1 H), 4.19 (m, 4H), 3.71 (m, 4H), 2.29 (s,
3H).
DMSO 6 8.78 (s, 1 H), 8.65 (s, 1 H), 8.26 (s,
2-27 4.651 326.1 1 3.9 1 H), 7.85 (s, 1 H), 7.69 (s, 1 H), 4.24 (d, J =
(+) 4.4, 4H), 3.90 (s, 3H), 3.77 (m, 4H), 2.36
(s, 3H).
DMSO 6 9.89 (s, 1 H), 8.53 (s, 1 H), 7.96 (s,
I H), 7.79 (s, 1 H), 7.72 (d, J = 7.7, 1 H),
2-28 5.758 388.1 1 50 7.45 (t, J = 7.9, 1 H), 7.26 (d, J = 7.9, 1 H),
4.32 (s, 4H), 3.85 (d, J = 4.4, 4H), 3.08 (s,
3H), 2.41 (s, 3H).
DMSO 6 8.62 (s, 1 H), 8.39 (s, 1 H), 7.82 (d,
2-29 4.843 367.2 1 3 J = 8.3, 2H), 7.66 (s, 1 H), 7.46 (d, J = 8.2,
3H), 6.03 (s, 1 H), 4.22 (s, 4H), 3.77 (s, 4H),
2.65 (d, J = 3.5, 3H), 2.34 (s, 3H).
DMSO 6 9.97 (s, 1 H), 8.61 (s, 1 H), 8.57 (s,
2-30 3.726 312.1 1 0.235 1 H), 8.09 (d, J = 2.4, 1 H), 7.70 (d, J = 3.5,
(++) 2H), 4.24 (d, J = 4.5, 4H), 3.78 (m, 4H),
2.35 (s, 3H).
177
-0&26
WO 2010/119264 PCT/GB2010/000773
PI3Ka
Cpd. Rt [M+1] Meth. IC50 lH NMR (300 MHz; S in ppm, J in
N r. Hz)
( M)
DMSO 6 8.69 (s, 1 H), 8.56 (d, J = 5.9, 2H),
2-31 3.512 296.1 1 10 7.87 (d, J = 6.0, 2H), 7.66 (s, 1 H), 4.20 (m,
4H), 3.71 (m, 4H), 2.30 (s, 3H).
CDCI3 6 7.84 (s, 1 H), 7.48 (m, 1 H), 7.42 (d,
J = 7.9, 1 H), 7.31 (t, J = 7.9, 1 H), 7.26 (s,
2-32 NMR - 1 H), 6.87 (dd, J = 8.1, 1.8, 1 H), 4.32 (m,
4H), 3.88 (m, 4H), 3.85 (s, 3H), 2.41 (s,
3H).
CDCI3 6 8.62 (dd, J = 4.6, 1.6, 2H), 7.68
2-33 4.509 330.1 1 - (dd, J = 4.5, 1.6, 2H), 7.55 (m, 1 H), 4.20
(m, 4H), 3.79 (dd, J =10.4, 5.7, 4H), 2.38
(t, J = 0.5, 3H).
CDC13 6 8.99 (d, J = 1.8, 1 H), 8.55 (dd, J =
2-34 5.260 330.1 1 4.8, 1.6, 1 H), 8.04 (m, 1 H), 7.50 (d, J = 0.6,
-
1 H), 7.31 (m, 1 H), 4.22 (m, 4H), 3.80 (m,
4H), 2.40 (d, J = 0.5, 3H).
DMSO 6 8.57 (s, 1 H), 8.29 (s, 1 H), 7.97 (d,
2-35 4.78 324.1 2 J = 7.4, 2H), 7.87 (s, 1 H), 7.47 (t, J = 7.3,
-
3H), 7.37 (t, J = 7.2, 1 H), 4.31 (s, 4H), 3.80
(d, J = 4.4, 4H).
MeOD 6 8.26 (s, 1 H), 7.77 (s, 1 H), 7.70 (d,
2-36 4.31 360.1 2 J = 8.9, 1 H), 7.62 (d, J = 7.1, 2H), 7.52 (d,
-
J = 8.2, 1 H), 4.54 (m, 4H), 3.86 (m, 4H),
2.47 (s, 3H).
DMSO 6 9.45 (s, 1 H), 7.79 (s, 1 H), 7.42 (d,
J = 6.9, 2H), 7.23 (m, 2H), 6.75 (d, J = 7.4,
2-37 3.37 395.20 1 ++
1 H), 4.26 (m, 4H), 3.77 (m, 4H), 3.02 (m,
4H), 2.56 (m, 4H), 2.27 (s, 3H).
CDCI3 6 10.16 (s, 1 H), 8.51 (s, 1 H), 7.80
(s, 1 H), 7.55 (d, J = 6.6, 1 H), 7.42 (m, 2H),
2-38 3.06 419.2 1 ++
7.17 (s, 1 H), 4.32 (m ,4H), 3.84 (m, 4H),
3.07 (m, 4H), 2.59 (s, 4H), 2.34 (s, 3H).
DMSO 6 9.46 (s, 1 H), 7.88 (s, 1 H), 7.40
2-39 4.12 459.20 1 ++ (m, 3H), 7.23 (t, J = 7.8, 1 H), 6.76 (d, J =
7.3, 1 H), 4.27 (s, 4H), 3.77 (s, 4H), 3.38 (s,
4H), 3.13 (s, 4H), 2.98 (s, 3H).
DMSO 6 9.45 (s, 1 H), 7.81 (s, 1 H), 7.42
2-40 2.90 381.20 1 ++ (m, 2H), 7.23 (m, 2H), 6.75 (d, J = 8.8, 1 H),
4.26 (s, 4H), 3.77 (m, 4H), 2.93 (s, 8H).
X178
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. 'H NMR (300 MHz; 8 in ppm, J in
Nr. Rt [M+1] Meth. IC50
Hz)
( M)
DMSO 6 13.20 (s, 1H), 8.45 (s, 1H), 7.97
(s, 1 H), 7.61 (dd, J = 7.1, 0.6, 1 H), 7.57 (d,
2-41 6.065 483.2 1 ++ J = 8.3, 1 H), 7.44 (m, 1 H), 7.40 (d, J = 3.8,
1 H), 4.30 (s, 4H), 3.80 (m, 4H), 3.37 (m,
4H), 3.17 (m, 4H), 2.98 (s, 3H).
DMSO 6 13.22 (s, 1 H), 8.46 (s, 1 H), 8.05
2-42 7.644 413.1 1 _ (s, 1 H), 7.61 (dd, J = 16.1, 7.7, 2H), 7.43 (t,
J = 7.7, 1 H), 4.27 (s, 4H), 3.80 (s, 4H), 2.38
(s, 3H).
DMSO 6 13.12 (s, 1 H), 8.35 (s, 1 H), 7.96
(s, I H), 7.51 (dd, J = 11.0, 7.7, 2H), 7.34
2-43 8.144 516.3 1 - (m, 1 H), 6.02 (s, 1 H), 4.21 (s, 4H), 4.03 (s,
2H), 3.74 (s, 4H), 3.57 (s, 2H), 2.38 (s, 2H),
2.28 (s, 3H), 1.38 (s, 9H).
DMSO 6 13.13 (s, 1 H), 8.34 (s, 1 H), 7.95
0.731 (s, 1 H), 7.50 (m, 2H), 7.35 (t, J = 7.8, 1 H),
2-44 3.836 416.2 1 6.01 (s, 1 H), 4.21 (s, 4H), 3.74 (m, 4H),
(++) 3.39 (s, 2H), 2.93 (s, 2H), 2.28 (s, 3H),
2.23 (s, 2H).
DMSO 6 8.33 (s, 1 H), 8.12 (s, 1 H), 7.91 (s,
1 H), 7.51 (d, J = 5.0, 1 H),7.49 (d, J = 6.2,
2-45 7.145 430.2 1 1 H), 7.35 (dd, J = 8.3, 7.1, 1 H), 5.98 (s,
-
1 H), 4.21 (m, 4H), 3.73 (m, 4H), 3.11 (s,
2H), 2.64 (m, 2H), 2.38 (m, 2H), 2.31 (s,
3H), 2.27 (s, 3H).
DMSO 6 13.25 (bs, 1 H), 8.43 (s, 1 H), 8.22
(s, 1 H), 7.56 (t, J = 7.0, 2H), 7.41 (dd, J =
2-46 6.306 494.2 1 - 8.3, 7.2, 1 H), 6.12 (s, 1 H), 4.25 (m, 4H),
3.98 (d, J = 2.6, 2H), 3.81 (m, 4H), 3.49 (m,
2H), 3.00 (s, 3H), 2.55 (m, 2H), 2.36 (s,
3H).
DMSO 6 13.17 (s, 1 H), 8.49 (s, 1 H), 8.26
(s, 1 H), 7.62 (d, J = 7.1, 1 H), 7.56 (d, J =
2-47 7.661 518.3 1 _ 8.2, 1 H), 7.42 (m, 1 H), 4.26 (s, 4H), 4.09
(d, J = 12.2, 2H), 3.79 (s, 4H), 3.47 (s, 1 H),
2.95 (s, 2H), 2.41 (s, 3H), 1.80 (s, 4H),
1.42 (s, 9H).
2-48 3.697 359.1 1 CDCI3 5 7.57 (d, J = 0.7, 1 H), 7.36 (m, 3H),
-
6.95 (dt, J = 6.5, 2.6, 1 H), 4.28 (m, 4H),
179
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. 'H NMR (300 MHz; = 8 in ppm, J in
Rt [M+1]+ Meth. IC50
Nr. Hz)
( M)
3.87 (m, 7H), 2.47 (d, J = 0.5, 3H).
DMSO 5 9.54 (s, 1 H), 7.87 (s, 1 H), 7.25 (t,
2-49 1.596 345.1 1 ++ J = 8.1, 1 H), 7.15 (dd, J = 5.1, 3.0, 2H),
6.80 (m, 1 H), 4.17 (m, 4H), 3.74 (m, 4H),
2.39 (s, 3H).
DMSO 5 8.77 (s, 2H), 8.41 (s, 1 H), 7.76 (s,
2-50 2.33 474.2 2 0.001 1 H), 6.83 (s, 2H), 4.22 (d, J = 4.5, 4H),
3.77 (m, 4H), 3.66 (s, 2H), 3.11 (d, J = 4.8,
4H), 2.87 (s, 3H), 2.55 (s, 4H).
DMSO 5 13.21 (s, 1 H), 8.42 (s, 1 H), 7.92
2-51 4.95 405.2 1 _ (s, 1 H), 7.59 (dd, J 16.1, 7.7, 2H), 7.43
(m, 1 H), 7.29 (s, 1 H), 4.30 (s, 4H), 3.79 (m,
4H), 2.94 (m, 8H).
DMSO 5 8.66 - 8.32 (m, 2H), 7.74 - 7.15
2-52 NMR 0.676 (m, 3H), 4.29 (d, J = 7.3, 5H), 3.76 (s, 4H),
1.25 (t, J = 15.7, 3H).
CDCI3 5 7.67 (d, J =0.7 Hz, 1 H), 7.35 (t, J
=7.9 Hz, 1 H), 7.22 (m, 1 H), 7.18 (m, 1 H),
2-53 NMR - 6.95 (ddd, J =8.2, 2.6, 0.9 Hz, 1 H), 4.28
(m, 4H), 3.87 (s, 3H), 3.85 (m, 4H), 2.46 (s,
3H).
0.266 CDCI3 6 10.06 (s, 1 H), 8.19 (d, J = 0.6,
2-54 1.42 369.1 2 1 H), 7.61 (s, 1 H), 7.50 (m, 3H), 4.30 (m,
(++) 4H), 3.83 (m, 4H), 2.49 (m, 3H).
CDCI3 5 8.13 (s, 1 H), 7.66 (s, 1 H), 7.54 (dt,
2-55 1.43 413.0 2 12 J = 7.2, 3.6, 1 H), 7.47 (m, 2H), 4.29 (m,
4H), 3.86 (m, 4H), 2.49 (s, 3H).
CDCI3 6 9.10 (d, J = 1.8, 1 H), 8.54 (dd, J =
2-56 4.192 422.0 2 0.253 4.8, 1.6, 1 H), 8.16 (m, 1 H), 7.87 (m, 1 H),
7.31 (dd, J = 7.9, 4.4, 1 H), 4.25 (m, 4H),
3.80 (m, 4H), 2.40 (s, 3H).
DMSO 6 8.50 (d, J = 0.7, 1 H), 8.44 (s, 1 H),
8.32 (s, 1 H), 7.63 (d, J = 7.0, 1 H), 7.56 (d,
0.144 J = 8.3, 1 H), 7.42 (dd, J = 8.2, 7.2, 1 H),
2-57 3.89 418.2 1 4.27 (m, 4H), 3.79 (m, 4H), 3.51 (t, J =
(++~ 12.2, 1 H), 3.25 (dd, J = 15.8, 8.2, 2H), 2.95
(t, J = 11.7, 2H), 2.43 (s, 3H), 2.15 (t, J =
11.6, 2H), 1.82 (m, 2H).
180
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. 'H NMR (300 MHz; 8 in ppm, J in
Rt [M+1] Meth. IC50
Nr. Hz)
( M)
DMSO 6 8.50 (s, 1 H), 8.26 (s, 1 H), 8.20 (d,
J = 7.4, 2H), 7.63 (d, J = 7.1, 1 H), 7.55 (t, J
= 9.4, 1 H), 7.43 (dd, J = 8.2, 7.3, 1 H), 4.26
2-58 2.80 432.2 2 ++ (m, 4H), 3.79 (m, 4H), 3.33 (t, J = 12.3,
1 H), 3.17 (d, J = 11.2, 2H), 2.57 (t, J =
11.1, 2H), 2.49 (s, 4H), 2.44 (s, 3H), 2.12
(m, 2H), 1.86 (d, J = 12.1, 2H).
DMSO 6 13.17 (s, 1H), 8.50 (s, 1H), 8.27
(s, 1 H), 7.63 (d, J = 7.1, 1 H), 7.56 (d, J =
2-59 6.37 496.2 1 _ 8.3, 1 H), 7.43 (dd, J = 8.2, 7.2, 1 H), 4.27
(s, 4H), 3.80 (m, 4H), 3.72 (d, J = 11.5,
2H), 2.98 (d, J = 13.4, 2H), 2.94 (s, 3H),
2.45 (s, 3H), 1.96 (s, 4H).
DMSO 6 13.19 (s, 1 H),,8.08 (s, 1 H), 8.00
(s, 1 H), 7.61 (d, J = 8. 1, 1 H), 7.45 (m, 1 H),
2-60 4.70 4.97 2 ++ 7.38 (dd, J = 7.1, 1.0, 1 H), 4.19 (m, 4H),
3.76 (m, 4H), 3.14 (m, 4H), 2.88 (s, 3H),
2.59 (m, 4H).
CDCI3 6 8.71 (d, J = 2.4, 1 H), 8.08 (dd, J =
8.6, 2.5, 1 H), 7.82 (s, 1 H), 7.49 (s, 1 H),
2-61 3.14 488.2 2 4.5 6.83 (d, J = 8.7, 1 H), 4.37 (m, 4H), 4.00 (s,
3H), 3.90 (m, 4H), 3.77 (s, 2H), 3.30 (m,
4H), 2.80 (s, 3H), 2.71 (m, 4H).
CDCI3 6 7.99 (s, 1 H), 7.48 (m, 2H), 7.37 (t,
2-62 2.28 350.1 2 - J = 8.1, 1 H), 6.95 (m, 1 H), 4.35 (m, 4H),
3.89 (m, 7H), 2.57 (s, 3H).
DMSO 6 13.25 (s, 1 H), 8.83 (s, 1 H), 8.43
(s, 1 H), 8.09 (d, J = 7.7, 1 H), 7.62 (d, J =
7.1, 1 H), 7.58 (d, J = 8.3, 1 H), 7.44 (dd, J =
2-63 7.53 561.3 1 - 8.1, 7.4, 1 H), 4.33 - 4.21 (m, 4H), 4.13 -
4.00 (m, 1 H), 3.93 (d, J = 13.1, 2H), 3.86 -
3.76 (m, 4H), 2.98 - 2.85 (m, 2H), 2.55 (s,
3H), 1.89 (d, J = 10.2, 2H), 1.55 -1.42 (m,
2H), 1.41 (s, 9H).
DMSO 6 13.27 (s, 1 H), 8.81 (s, 1 H), 8.58 -
8.48 (m, 1 H), 8.43 (s, I H), 8.32 (d, J = 7.5,
2-64 2.94 461.2 2 + 1 H), 8.28 - 8.20 (m, 1 H), 7.63 (d, J = 7.1,
1 H), 7.59 (d, J = 8.3, 1 H), 7.45 (dd, J = 8.2,
7.2, 1 H), 4.32-4.24 (m, 4H), 4.20 - 4.10 (m,
181
D9-26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. 1H NMR (300 MHz; 8 in ppm, J in
Rt [M+1] Meth. 1C50
Nr. Hz)
( M)
1 H), 3.86 - 3.78 (m, 4H), 3.15 - 3.00 (m,
2H), 2.58 (s, 3H), 2.16 - 2.03 (m, 2H), 1.85
- 1.68 (m, 2H). Two protons are missing,
most likely under the water signal
CDCI368.71 (d,J=1.7, 1H),8.31 (d,J=
2.8, 1 H), 7.95 (s, I H), 7.77 (dd, J = 2.7,
2-65 0.37 488.2 2 + 1.8, 1 H), 7.51 (s, 1 H), 4.38 (m, 4H), 3.96
(s, 3H), 3.91 (m, 4H), 3.77 (s, 2H), 3.30 (m,
4H), 2.80 (s, 3H), 2.72 (m, 4H).
DMSO 6 8.51 (d, J = 2.1, 1 H), 8.33 (s, 1 H),
7.90 (dd, J = 8.7, 2.4, 1 H), 7.76 (s, 1 H),
2-66 0.367 473.2 2 0.169 6.50 (d, J = 8.6, 1 H), 6.10 (s, 2H), 4.22 (s,
4H), 3.78 (d, J = 4.6, 4H), 3.65 (s, 2H),
3.11 (s, 4H), 2.87 (s, 3H), 2.54 (d, J = 8.2,
4H).
DMSO 6 9.12 (s, 2H), 8.57 (s, 1 H), 7.81 (s,
2-67 3.44& 489.2 2 ++ 1 H), 4.25 (d, J = 4.4, 4H), 3.97 (s, 3H),
3.99 3.77 (m, 4H), 3.68 (s, 2H), 3.11 (d, J = 4.8,
4H), 2.87 (s, 3H), 2.56 (s, 4H).
DMSO) 6 8.65 (s, 1 H), 8.04 (d, J = 8.5,
2.54& 2H), 8.00 (s, 1 H), 7.94 (d, J = 8.5, 2H),
2-68 500.2 2 11 7.83 (s, 1 H), 7.38 (s, 1 H), 4.27 (s, 4H),
2.64 3.79 (m, 4H), 3.68 (s, 2H), 3.12 (s, 4H),
2.87 (s, 3H), 2.56 (s, 4H).
DMSO 6 10.47 (s, 1 H), 8.43 (s, 1 H), 7.81
(s, 1 H), 7.80 (d, J = 5.5, 1 H), 7.78 (m, 1 H),
2-69 2.75 512.2 2 - 6.88 (d, J = 8.0, 1 H), 4.23 (d, J = 4.5, 4H),
3.78 (m, 4H), 3.66 (s, 2H), 3.55 (s, 2H),
3.11 (d, J = 4.7, 4H), 2.87 (s, 3H), 2.56 (s,
4H).
DMSO 6 11.25 (s, 1 H), 8.24 (d, J = 2.1,
1 H), 7.93 (s, 1 H), 7.41 (m, 2H), 7.02 (dd, J
2-70 3.18 514 2 - = 11.2, 8.8, 1 H), 6.70 (s, 1 H), 4.21 (s, 4H),
3.78 (m, 4H), 3.69 (s, 2H), 3.12 (s, 4H),
2.87 (s, 3H), 2.57 (s, 4H).
CDCI3 5 8.51 (s, 1 H), 7.97 (s, 1 H), 7.53
2-71 4.15& 496.2 2 (dd, J = 7.3, 0.6, 1 H), 7.50 (s, 1 H), 7.43 (d,
-
4.73 J = 8.1, 1 H), 7.28 (m, 2H), 6.98 (s, 1 H),
4.37 (m, 4H), 3.89 (m, 4H), 3.77 (s, 2H),
182
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. R{ ~M+~~+ Meth. IC50 1H NMR (300 MHz; 6 in ppm, J in
Nr. Hz)
( M)
3.27 (m, 4H), 2.77 (s, 3H), 2.71 (m, 4H).
CDCI3 5 8.37 (s, 1 H), 8.12 (d, J = 7.8, 1 H),
3.53& 8.00 (s, 1 H), 7.75 (d, J = 7.6, 1 H), 7.51 (dd,
2-72 500.2 2 - J = 13.2, 5.3, 2H), 4.37 (m, 4H), 3.90 (m,
3.89 4H), 3.76 (m, 2H), 3.29 (m, 4H), 2.79 (s,
3H), 2.71 (m, 4H).
CDCI3 6 10.14 (m, 1 H), 8.06 (d, J = 0.9,
1 H), 7.70 (d, J = 0.7, 1 H), 7.54 (dt, J = 8.1,
2-73 5.37 461.0 2 - 1.0, 1 H), 7.46 (t, J = 7.6, 1 H), 7.40 (dd, J =
7.0, 1.2, 1 H), 4.29 (m, 4H), 3.84 (m, 4H),
2.48 (d, J = 0.4, 3H).
DMSO 6 13.23 (s, 1 H), 8.65 (s, 1 H), 8.56
(s, 1 H), 8.44 (t, J = 6.1, 1 H), 8.40 (s, 1 H),
2-74 4.55 392.1 2 0.092 7.67 (d, J = 7.1, 1 H), 7.58 (d, J = 8.3, 1 H),
7.44 (m, 1 H), 4.34 (s, 4H), 3.83 (m, 4H),
3.40 (s, 2H), 1.15 (t, J = 7.1, 3H).
DMSO 6 11.65 (s, 1 H), 8.75 (d, J = 1.9,
1 H), 8.49 (s, 1 H), 8.41 (d, J = 1.7, 1 H),
2-75 2.81 497.2 2 0.113 7.75 (s, 1 H), 7.43 (m, 1 H), 6.45 (dd, J =
3.3, 1.8, 1 H), 4.21 (s, 4H), 3.73 (s, 4H),
3.61 (s, 2H), 3.06 (s, 4H), 2.80 (s, 3H),
2.50 (s, 4H).
DMSO 6 13.27 (s, 1 H), 8.82 (s, 1 H), 8.44
(s, 1 H), 8.18 (d, J = 7.7, 1 H), 7.60 (dd, J =
11.7, 7.9, 2H), 7.45 (m, 1 H), 4.29 (d, J =
2-76 4.67 539.1 2 - 7.8, 4H), 4.03 (s, 1 H), 3.82 (s, 4H), 3.58 (d,
J= 12.1, 2H), 2.95 (m, 2H), 2.92 (s, 3H),
2.58 (s, 3H), 2.02 (d, J = 10.9, 2H), 1.66
(dd, J = 20.4, 11.1, 2H).
DMSO 6 13.25 (s, 1 H), 9.06 (s, 1 H), 8.44
2-77 4.12 378.1 2 - (s, 1 H), 7.60 (dd, J = 11.9, 7.7, 3H), 7.45
(dd, J = 8.3, 7.2, 1 H), 4.27 (m, 4H), 3.81
(m, 4H), 2.61 (s, 3H).
DMSO 6 13.23 (s, 1H), 8.42 (d, J = 0.8,
1 H), 7.87 (s, 1 H), 7.63 (d, J = 7.0, 1 H),
2-78 2.78 419.2 2 - 7.57 (d, J = 8.3, 1 H), 7.43 (dd, J = 8.3, 7.2,
1 H), 7.28 (s, 1 H), 4.30 (m, 4H), 3.79 (m,
4H), 3.32 (s, 2H), 3.25 (dd, J = 28.1, 16.1,
2H), 2.80 (dd, J = 20.2, 9.4, 2H), 1.86 (d, J
183
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. Rt [M+1] Meth. IC50 1H NMR (300 MHz; S in ppm, J in
N r. Hz)
( M)
= 10.1, 2H), 1.53 (m, 2H).
DMSO 5 13.23 (s, 1 H), 8.64 (s, 1 H), 8.56
(s, 1 H), 8.40 (s, I H), 8.38 (d, J 5.0, 1 H),
2-79 4.23 378.1 2 0.11 7.67 (d, J = 7.1, 1 H), 7.58 (d, J = 8.3, 1 H),
7.44 (dd, J = 8.2, 7.3, 1 H), 4.34 (d, J = 4.4,
4H), 3.83 (m, 4H), 2.84 (d, J = 4.8, 3H).
DMSO 6 10.58 (s, 1 H), 8.87 (s, 1 H), 8.56
3.54 (s, 1 H), 8.28 (d, J = 9.1, 1 H), 8.14 (d, J =
2-81 and 515.2 3 0.21 8.9, 1 H), 7.81 (s, 1 H), 4.25 (s, 4H), 3.78 (s,
3.88 4H), 3.67 (s, 2H), 3.12 (s, 4H), 2.87 (s, 3H),
2.55 (s, 4H), 2.11 (s, 3H).
DMSO 6 8.61 (s, 1 H), 8.41 (s, 1 H), 7.80
3.99 (m, 3H), 7.46 (d, J = 8.7, 2H), 6.02 (d, J =
2-82 and 529.2 3 0.158 4.7, 1H), 4.23 (s, 4H), 3.77 (m, 4H), 3.65
4.26 (s, 2H), 3.11 (s, 4H), 2.86 (s, 3H), 2.64 (d,
J = 4.6, 3H), 2.54 (d, J = 4.6, 4H).
CDCI3 6 8.74 (d, J = 1.7, 1 H), 8.22 (d, J =
4.79 1.9, 1 H), 7.81 (s, 1 H), 7.47 (s, 1 H), 5.08 (s,
2-83 and 541.2 3 0.21 2H), 4.36 (m, 4H), 3.90 (m, 4H), 3.76 (s,
4.96 2H), 3.29 (m, 4H), 2.79 (s, 3H), 2.70 (m,
4H).
DMSO 6 13.33 (s, 1 H), 8.23 (s, 1 H), 7.95
4.43 (s, 1 H), 7.72 (d, J = 8.0, 1 H), 7.52 (dd, J =
2-84 and 522.2 3 - 13.2, 7.0, 2H), 4.46 (s, 4H), 3.80 (m, 4H),
4.75 3.74 (s, 2H), 3.13 (s, 4H), 2.88 (s, 3H),
2.59 (s, 4H).
CDCI3 6 8.49 (s, 1 H), 8.04 (s, 1 H), 7.93 (s,
2-86 4.40 392.1 1 0.891 1 H), 7.47 (m, 2H), 7.39 (m, 2H), 4.36 (m,
4H), 3.84 (m, 4H), 3.47 (s, 3H), 3.10 (s,
3H).
DMSO 6 13.24 (s, 1 H), 8.63 (s, 1 H), 8.56
(s, 1 H), 8.43 (s, 1 H), 7.66 (d, J = 7.1, 1 H),
2-87 4.87 418.2 1 0.355 7.58 (d, J = 8.3, 1 H), 7.44 (dd, J = 8.2, 7.3,
1 H), 4.30 (d, J = 4.3, 4H), 3.95 (t, J = 6.6,
2H), 3.81 (m, 4H), 3.53 (t, J = 6.7, 2H),
1.87 (m, 4H).
DMSO 6 9.60 (s, 1 H), 8.26 (s, 1 H), 7.48 (d,
2-88 0.98 336.1 1 - J = 6.8, 2H), 7.25 (t, J = 8.1, 1 H), 6.80 (d, J
= 8.6, 1 H), 4.23 (s, 4H), 3.79 (d, J = 4.3,
184
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. Rt [M+1]+ Meth. IC50 'H NMR (300 MHz; = 8 in ppm, J in
Nr. ( Hz)
M)
4H).
CDCI3 6 7.78 (s, 1 H), 7.71 (d, J = 8.5, 2H),
2-89 0.39 472.2 3 33 7.43 (s, 1 H), 6.74 (d, J = 8.5, 2H), 4.33 (m,
4H), 3.89 (m, 4H), 3.74 (s, 2H), 3.28 (m,
4H), 2.78 (s, 3H), 2.70 (m, 4H).
2.21 DMSO 6 12.93 (s, 1 H), 8.22 (s, I H), 8.11
2-90 and 447.2 3 6 (s, 1 H), 7.89 (s, 1 H), 7.74 (s, 1 H), 4.22 (m,
4H), 3.76 (m, 4H), 3.65 (s, 2H), 3.11 (m,
3.02 4H), 2.87 (s, 3H), 2.55 (m, 4H).
DMSO 6 8.79 (s, I H), 8.65 (dd, J = 4.6,
2-91 2.69 325.1 3 3.9 1.6, 2H), 8.31 (s, 1 H), 7.93 (dd, J = 4.6,
1.6, 3H), 7.51 (s, 1 H), 4.33 (s, 4H), 3.79
(m, 4H).
DMSO 6 9.16 (d, J = 1.7, 1 H), 8.67 (s, 1 H),
2-92 3.29 325.1 3 - 8.57 (dd, J = 4.7, 1.6, 1 H), 8.31 (m, 2H),
7.90 (s, 1 H), 7.49 (dd, J = 7.4, 4.8, 2H),
4.33 (s, 4H), 3.79 (m, 4H).
DMSO 6 8.78 (s, 2H), 8.44 (s, 1H), 8.21 (s,
2-93 3.01 341.1 1 - 1 H), 7.86 (s, 1 H), 7.46 (s, 1 H), 6.88 (s, 2H),
4.29 (s, 4H), 3.77 (m, 4H).
DMSO 6 11.27 (s, 1 H), 8.49 (s, 1 H), 8.42
2-94 4.37 363.1 1 0.944 (s,1 H), 7.86 (s,1 H), 7.56 (d, J = 6.8, 1 H),
7.45 (t, J = 6.0, 3H), 7.18 (t, J = 7.7, 1 H),
6.94 (s, 1 H), 4.32 (s, 4H), 3.80 (m, 4H).
DMSO 6 11.27 (s, 1 H), 8.41 (s, 1 H), 8.29
2-95 4.28 381.1 1 0.891 (t, J = 3.7, 1 H), 7.87 (s, 1 H), 7.43 (m, 3H),
7.03 (dd, J = 11.3, 8.8, 1 H), 6.72 (s, 1 H),
4.27 (s, 4H), 3.78 (m, 4H).
DMSO 6 8.66 (s, 2H), 8.35 (s, 1 H), 7.98 (s,
2-96 3.32 375.1 1 - 1 H), 7.56 (s, 1 H), 6.99 (s, 2H), 4.25 (s, 4H),
3.76 (m, 4H).
DMSO 6 13.21 (s, 1 H), 8.60 (s, 1 H), 8.55
(s, 1 H), 7.90 (s, 1 H), 7.64 (d, J = 7.0, 1 H),
7.55 (d, J = 8.3, 1 H), 7.42 (dd, J = 8.2, 7.3,
2-97 0.29 573.3 4 4.5 1H), 4.27 (m, 4H), 3.80 (m, 4H), 3.62 (s,
2H), 3.26 (t, J = 6.8, 4H), 3.05 (s, 2H), 2.40
(s, 2H), 1.66 (dd, J= 12.0, 6.1, 2H), 1.49
(s, 4H), 1.38 (s, 9H).
185
-0&26
WO 2010/119264 PCT/GB2010/000773
PI3Ka
Cpd. 1H NMR (300 MHz; 6 in ppm, J in
Rt [M+1] Meth. IC50
Nr. Hz)
( M)
DMSO 6 13.19 (s, 1 H), 8.61 (s, 1 H), 8.55
(s, 1 H), 7.93 (s, 1 H), 7.64 (d, J = 7.0, 1 H),
2-98 3.73 587.4 1 7.2 7.56 (d, J = 8.3, 1 H), 7.43 (m, 1 H), 4.27 (m,
4H), 3.80 (m, 4H), 3.73 (s, 2H), 3.28 (s,
4H), 2.54 (s, 4H), 1.48 (s, 4H), 1.38 (s,
1 OH), 1.35 (s, 4H).
DMSO 6 13.21 (s, 1 H), 8.60 (s, 1 H), 8.55
(s, 1 H), 7.91 (s, 1 H), 7.64 (d, J = 7.0, 1 H),
2-99 3.45 545.3 1 7.9 7.55 (d, J = 8.3, 1 H), 7.42 (m, 1 H), 4.27 (s,
4H), 3.81 (m, 4H), 3.71 (s, 2H), 3.48 (m,
4H), 3.10 (d, J = 10.9, 2H), 2.76 (s, 2H),
2.58 (dd, J = 14.6, 8.4, 2H), 1.38 (s, 9H).
DMSO 6 13.21 (s, 1 H), 8.60 (s, 1 H), 8.55
(s, 1 H), 7.90 (s, 1 H), 7.65 (d, J = 7.1, 1 H),
2-100 3.68 573.3 1 5.3 7.55 (d, J = 8.3, 1 H), 7.42 (m, 1 H), 4.26 (m,
4H), 3.80 (m, 4H), 3.71 (s, 2H), 3.28 (m,
4H), 2.64 (t, J = 6.8, 2H), 2.47 (s, 2H), 1.59
(t, J = 6.8, 2H), 1.44 (s, 4H), 1.38 (s, 9H).
DMSO 6 8.70 (dd, J = 4.5, 1.6, 2H), 8.42
2-101 2.60 359.1 1 - (s, 1 H), 8.02 (s, 1 H), 7.81 (m, 2H), 7.59 (s,
1 H), 4.27 (s, 4H), 3.77 (m, 4H).
DMSO 6 8.96 (d, J = 1.7, 1 H), 8.63 (dd, J =
2-102 3.01 359.1 1 - 4.8, 1.5, 1 H), 8.40 (s, 1 H), 8.17 (m, 1 H),
8.01 (s, 1 H), 7.58 (s, 1 H), 7.53 (dd, J = 7.9,
4.8, 1 H), 4.26 (s, 4H), 3.76 (m, 4H).
DMSO 6 11.57 (s, 1 H), 8.36 (s, 1 H), 8.06
(s, 1 H), 7.86 (s, I H), 7.54 (d, J = 2.6, 1 H),
2-103 4.27 397.1 1 34 7.48 (m, 1 H), 7.44 (m, 1 H), 7.22 (m, 1 H),
7.16 (d, J = 6.1, 1 H), 4.23 (s, 4H), 3.76 (m,
4H).
DMSO 6 11.58 (s, 1 H), 8.36 (m, 1 H), 8.01
2-104 4.74 431.1 1 93 (s, 1 H), 7.58 (s, 1 H), 7.53 (s, 1 H), 7.52 (s,
1 H), 7.25 (m, 1 H), 7.11 (dd, J = 7.2, 0.8,
1 H), 4.17 (m, 4H), 3.73 (t, J = 4.7, 4H).
DMSO 6 11.62 (s, 1 H), 8.35 (s, 1 H), 8.09
2-105 4.34 415.1 1 123 (s,1 H), 7.88 (s, 1 H), 7.58 (d, J = 2.7, 1 H),
7.49 (dd, J = 8.9, 4.3, 2H), 7.11 (m, 1 H),
4.20 (s, 4H), 3.73 (t, J = 4.6, 4H).
186
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. R~ [M+1]+ Meth. IC50 1H NMR (300 MHz; 8 in ppm, J in
N r. Hz)
( M)
DMSO 6 11.68 (s, 1 H), 8.38 (s, 1 H), 8.03
2-106 4.76 449.1 1 28 (s, 1 H), 7.59 (s, 2H), 7.54 (dd, J = 8.9, 4.3,
1 H), 7.14 (m, 1 H), 4.17 (m, 4H), 3.73 (t, J =
4.7, 4H).
DMSO 5 13.13 (s, 1 H), 8.57 (s, 1 H), 8.49
(s, 1 H), 7.90 (s, 1 H), 7.59 (d, J = 7.1, 1 H),
2-107 4.61 393.1 1 2.6 7.49 (d, J = 8.3, 1 H), 7.36 (m, 1 H), 4.18 (m,
4H), 3.81 (s, 2H), 3.74 (m, 4H), 3.59 (s,
3H).
2.43 DMSO 6 8.57 (s, 2H), 7.87 (s, 1 H), 6.89 (s,
2-108 and 508.2 1 2H), 4.11 (m, 4H), 3.69 (m, 4H), 3.62 (d, J
= 14.9, 2H), 3.05 (m, 4H), 2.80 (s, 3H),
2.58 2.48 (m, 4H).
DMSO 6 8.57 (s, 1 H), 8.06 (m, 2H), 6.93
2-109 3.49 522.2 1 1 (d, J = 7.2, 1 H), 4.19 (s, 4H), 3.91 (s, 3H),
3.75 (s, 6H), 3.16 (s, 2H), 2.89 (s, 3H),
2.54 (s, 2H).
CDCI3 6 8.68 (d, J = 1.6, 1 H), 8.35 (d, J =
2.8, 1 H), 7.79 (s, 1 H), 7.63 (dd, J = 2.6,
2-110 2.96 522.2 1 - 1.8, 1 H), 4.31 (m, 4H), 3.94 (s, 3H), 3.88
(m, 4H), 3.83 (s, 2H), 3.33 (s, 4H), 2.80 (s,
3H), 2.75 (s, 4H).
DMSO 6 8.60 (s, I H), 8.55 (d, J = 0.5, 1 H),
8.38 (s, 1 H), 7.89 (s, 1 H), 7.64 (d, J = 7.0,
2-111 2.39 487.3 3 0.813 1 H), 7.56 (d, J = 8.3, 1 H), 7.42 (dd, J = 8.2,
7.3, 1 H), 4.26 (m, 4H), 3.80 (m, 4H), 3.63
(s, 2H), 2.96 (s, 4H), 2.45 (s, 4H), 1.54 (s,
4H), 1.48 (s, 4H).
DMSO 6 8.94 (s, 2H), 7.95 (s, 1 H), 4.15 (s,
2-112 3.10 523.2 1 - 4H), 3.93 (s, 3H), 3.69 (m, 6H), 3.06 (s,
3H), 2.82 (s, 4H), 2.51 (m, 4H).
DMSO 6 10.52 (s, 1 H), 7.97 (s, 1 H), 7.58
2-113 2.95 546.2 1 (m, 2H), 6.91 (d, J = 8.6, 1 H), 4.20 (s, 4H),
3.76 (m, 6H), 3.55 (s, 2H), 3.16 (s, 4H),
2.87 (s, 3H), 2.65 (s, 4H).
DMSO 6 11.81 (s, 1 H), 8.57 (d, J = 2.1,
2-114 3.15 531.2 2 - 1 H), 8.29 (d, J = 2.0, 1 H), 7.97 (s, 1 H),
7.54 (m, 1 H), 6.54 (dd, J = 3.4, 1.8, 1 H),
4.20 (m, 4H), 3.76 (m, 4H), 3.73 (s, 2H),
187
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. Rt [M+1] Meth. IC50 1H NMR (300 MHz; S in ppm, J in
Nr. Hz)
( M)
3.14 (t, J = 7.7, 4H), 2.87 (s, 3H), 2.56 (m,
4H).
DMSO 6 12.18 (s, 1 H), 8.66 (d, J = 2.0,
1 H), 8.22 (d, J = 2.1, 1 H), 7.99 (s, 1 H),
2-115 3.66 565.2 2 - 7.77 (s, 1 H), 4.21 (s, 4H), 3.77 (m, 4H),
3.74 (s, 2H), 3.12 (m, 4H), 2.87 (s, 3H),
2.58 (s, 4H).
DMSO 6 8.34 (d, J = 2.2, 1 H), 7.92 (s, 1 H),
7.77 (dd, J = 8.6, 2.4, 1 H), 6.52 (d, J = 8.7,
2-116 1.67 507.0 2 - 1 H), 6.22 (s, 2H), 4.17 (m, 4H), 3.75 (m,
4H), 3.71 (s, 2H), 3.11 (m, 4H), 2.87 (s,
3H), 2.54 (m, 4H).
DMSO 6 8.60 (s, I H), 8.55 (d, J = 0.6, 1 H),
7.90 (s, 1 H), 7.64 (d, J = 7.0, 1 H), 7.56 (d,
2-117 0.32 445.2 3 0.851 J = 8.3, 1 H), 7.42 (dd, J = 8.2, 7.3, 1 H),
4.27 (m, 4H), 3.80 (m, 4H), 3.72 (s, 2H),
2.96 (m, 4H), 2.65 (t, J = 6.7, 2H), 1.62 (m,
4H).
DMSO 6 8.60 (s, I H), 8.56 (d, J = 0.6, 1 H),
0.32 8.35 (s, 1 H), 7.91 (s, 1 H), 7.64 (d, J = 7.1,
2-118 and 473.2 3 1.5 1 H), 7.56 (d, J = 8.3, 1 H), 7.42 (dd, J = 8.1,
7.3, 1 H), 4.27 (m, 4H), 3.80 (m, 4H), 3.72
2.30 (s, 2H), 2.96 (m, 4H), 2.65 (t, J = 6.8, 2H),
1.62 (m, 6H).
DMSO 6 9.15 (m, 1 H), 8.60 (m, 1 H), 8.57
2-119 3.40 323.2 2 0.093 (m, 1 H), 8.30 (m, 1 H), 8.27 (m, 1 H), 7.77
(m, 1 H), 7.50 (m, 2H), 4.32 (m, 4H), 1.69
(m, 6H).
DMSO 6 8.76 (s, 2H), 8.37 (s, 1 H), 8.19 (s,
2-120 3.82 339.2 2 1.3 1 H), 7.73 (s, 1 H), 7.45 (s, 1 H), 6.86 (s, 2H),
4.28 (s, 4H), 1.67 (s, 6H).
DMSO 6 8.04 (s, 1 H), 7.98 (s, 1 H), 7.96 (d,
2-121 2.91 534.2 2 - J = 8.4, 2H), 7.83 (d, J = 8.3, 2H), 7.44 (s,
1 H), 4.20 (s, 4H), 3.76 (s, 4H), 3.72 (s, 2H),
3.13 (s, 4H), 2.87 (s, 3H), 2.57 (s, 4H).
DMSO 6 11.60 (s, 1 H), 8.07 (s, 1 H), 7.86
2-122 3.18 548.2 2 50 (s, 1 H), 7.57 (d, J = 2.6, 1 H), 7.48 (dd, J =
8.9, 4.3, 1H), 7.10 (m, 1H), 4.13 (s, 4H),
3.72 (t, J = 4.6, 4H), 3.69 (s, 2H), 3.13 (s,
188
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. Rt [M+1] Meth. 1C50 lH NMR (300 MHz; 6 in ppm, J in
Nr. Hz)
( M)
4H), 2.87 (s, 3H), 2.58 (s, 4H).
DMSO 6 11.31 (s, 1 H), 7.96 (s, 1 H), 7.48
(dd, J = 9.0, 4.0, 1 H), 7.42 (t, J = 2.7, 1 H),
2-123 3.46 548.2 2 - 7.03 (dd, J = 10.3, 8.9, 1 H), 6.29 (s, 1 H),
4.11 (m, 4H), 3.73 (m, 4H), 3.72 (s, 2H),
3.13 (m, 4H), 2.87 (s, 3H), 2.59 (s, 4H).
DMSO 6 11.66 (s, 1H), 7.96 (s, 1H), 7.59
(d, J = 2.6, 1 H), 7.53 (dd, J = 8.9, 4.3, 1 H),
2-124 3.58 582.2 2 - 7.13 (m, 1 H), 4.12 (ddd, J = 18.3, 13.5, 8.9,
4H), 3.72 (m, 4H), 3.70 (s, 2H), 3.13 (d, J =
4.7, 4H), 2.87 (s, 3H), 2.60 (s, 4H).
CDCI3 6 9.23 (s, 2H), 9.20 (s, 1 H), 7.95 (s,
2-125 3.31 459.2 3 - 1 H), 7.52 (s, 1 H), 4.40 (m, 4H), 3.90 (m,
4H), 3.77 (s, 2H), 3.29 (m, 4H), 2.79 (s,
3H), 2.71 (m, 4H).
CDCI3 6 8.97 (s, 1 H), 8.09 (d, J = 5.6, 1 H),
7.90 (s, 1 H), 7.49 (s, 1 H), 7.24 (m, 1 H),
2-126 2.21 472.2 3 0.776 4.36 (s, 4H), 3.89 (s, 4H), 3.76 (s, 2H),
3.28 (s, 4H), 2.78 (s, 3H), 2.70 (s, 4H),
2.60 (s, 3H).
CDCI3 6 10.38 (s, I H), 8.31 (s, 1H), 8.13
(s, 1 H), 7.90 (m, 2H), 7.52 (d, J = 8.8, 1 H),
2-127 4.27 497.2 3 37 7.46 (s, 1H), 4.35 (m, 4H), 3.88 (m, 4H),
3.74 (s, 2H), 3.26 (m, 4H), 2.76 (s, 3H),
2.69 (m, 4H).
CDCI3 6 8.54 (d, J = 0.7, 1 H), 7.94 (s, 1 H),
2.71 7.48 (m, 3H), 7.39 (dd, J = 8.2, 6.9, 1H),
2-128 and 523.3 2 - 4.34 (m, 4H), 3.84 (m, 4H), 3.79 (s, 2H),
3.38 (dd, J = 9.6, 6.6, 2H), 3.16 (dd, J =
2.86 9.5, 2.4, 2H), 2.87 (m, 4H), 2.82 (s, 3H),
2.53 (d, J = 6.0, 2H).
CDCI3 6 8.54 (d, J = 0.7, 1 H), 7.96 (s, 1 H),
2.73 7.49 (m, 3H), 7.41 (dd, J = 8.3, 6.9, 1H),
2-129 and 551.3 2 2.5 4.35 (m, 4H), 3.86 (m, 4H), 3.81 (s, 2H),
3.03 3.16 (dd, J = 12.8, 6.3, 4H), 2.78 (m, 2H),
2.74 (s, 3H), 2.55 (s, 2H), 1.69 (m, 6H).
2.57 CDCI3 6 8.53 (m, I H), 7.90 (t, J = 10.1,
2-130 487.3 2 1.4 1 H), 7.49 (m, 3H), 7.38 (m, 1 H), 6.23 (m,
and 1 H), 4.33 (m, 4H), 3.85 (m, 4H), 3.73 (s,
189
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. Rt [M+1]+ Meth. IC50 1H NMR (300 MHz; 8 in ppm, J in
Nr. Hz)
( M)
2.80 2H), 3.31 (m, 2H), 3.00 (m, 2H), 2.27 (m,
2H), 2.03 (m, 4H), 1.48 (m, 2H).
CDCI3 6 10.84 (s, I H), 8.53 (d, J = 0.8,
2.57 1 H), 7.96 (s, 1 H), 7.49 (m, 3H), 7.41 (dd, J
2-131 and 487.3 2 14 = 8.3, 6.9, 1 H), 5.89 (s, 1 H), 4.35 (m, 4H),
3.86 (m, 4H), 3.71 (s, 2H), 3.18 (s, 2H),
2.71 2.55 (d, J = 20.1, 4H), 2.21 (s, 2H), 1.71 (t,
J = 5.4, 4H).
DMSO 6 9.66 (s, 2H), 8.93 (s, 1H), 8.92 (s,
2-132 2.74 320.2 2 - 2H), 7.87 (s, 1 H), 7.23 (s, 2H), 6.90 (s, 2H),
2.46 (s, 3H).
DMSO 6 13.24 (s, 1H), 8.47 (s, 1H), 8.05
(s, 1 H), 7.66 (d, J = 7.0, 1 H), 7.57 (d, J =
8.2, 1 H), 7.44 (d, J = 7.4, 1 H), 7.31 (s, 1 H),
2-133 4.47 419.3 3 - 4.29 (s, 4H), 3.80 (m, 4H), 3.27 (d, J = 9.8,
2H), 2.96 (dt, J = 17.2, 9.6, 2H), 2.82 (t, J =
8.7, 1 H), 1.92 (m, 2H), 1.63 (dd, J = 56.7,
6.4, 2H).
DMSO 6 13.20 (s, 1 H), 8.59 (s, 1 H), 8.56
(s, 1 H), 7.89 (s, 1 H), 7.64 (d, J = 7.1, 1 H),
2-134 3.04 551.3 2 5.2 7.55 (d, J = 8.3, 1 H), 7.42 (dd, J = 8.2, 7.3,
1 H), 4.27 (m, 4H), 3.80 (m, 4H), 3.58 (s,
6H), 2.99 (s, 3H), 2.40 (s, 4H), 1.71 (s, 4H),
1.39 (s, 2H).
CDCI3 6 8.91 (d, J = 2.0 Hz, 1 H), 7.97 (dd,
J = 8.1, 2.3 Hz, 1 H), 7.71 (s, 1 H), 7.22 (d, J
2-135 0.56 506.2 3 - = 8.1 Hz, 1H), 4.27 (m, 4H), 3.83 (m, 4H),
3.76 (s, 2H), 3.26 (m, 4H), 2.76 (s, 3H),
2.68 (m, 4H), 2.60 (s, 3H).
3.43 CDCI3 6 9.23 (s, 1 H), 9.20 (s, 2H), 7.76 (s,
2-136 and 493.2 3 1H), 4.32 (m, 4H), 3.87 (m, 4H), 3.80 (s,
-
2H), 3.30 (m, 4H), 2.79 (s, 3H), 2.71 (m,
3.85 4H).
DMSO 6 13.15 (s, 1 H), 8.60 (s, 2H), 8.53
2-137 5.00 393.2 2 0.676 (s, 1 H), 7.62 (dd, J = 26.6, 4.9 Hz, 2H),
7.44 (m, 1 H), 4.34 (s, 6H), 3.83 (s, 4H),
1.35 (t, J = 6.1 Hz, 3H).
2-138 3.99 370.2 2 0.012 CDCI3 6 8.78 (s, 2H), 8.06 (s, 1 H), 7.75 (s,
1 H), 5.14 (s, 2H), 4.40 (s, 6H), 3.87 (s, 4H),
190
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. H NMR (300 MHz; 8 in ppm, J in
Rt [M+1] Meth. IC50
Nr. Hz)
(.tM)
1.40 (t, J = 7.0 Hz, 3H).
DMSO 6 8.65 (s, 1H), 8.56 (s, 1H), 8.41
2.75 (m, 1 H), 7.67 (d, J = 7.1 Hz, 1 H), 7.59 (d, J
2-139 and 449.3 2 - = 8.7 Hz, 1H), 7.44 (dd, J = 8.2, 7.3 Hz,
1H), 4.34 (m, 4H), 3.84 (m, 4H), 3.33 (m,
2.89 2H), 2.30 (m, 2H), 2.16 (s, 6H), 1.70 (d, J =
7.0 Hz, 2H).
DMSO 6 13.24 (s, 1 H), 8.65 (s, 1 H), 8.56
(s, 1 H), 8.41 (s, 1 H), 7.68 (d, J = 6.5 Hz,
2-140 4.60 436.2 2 - 1 H), 7.58 (d, J = 8.4 Hz, 1 H), 7.44 (dd, J =
8.3, 7.3 Hz, 1 H), 4.34 (m, 4H), 3.83 (m,
4H), 3.38 (m, 4H), 3.26 (s, 3H), 1.80 (m,
2H).
DMSO 6 13.24 (s, 1 H), 8.65 (s, 1 H), 8.56
3.94 (s, 1H), 8.42 (s, 1H), 7.68 (d, J = 7.1 Hz,
2-141 and 435.3 3 0.065 1 H), 7.58 (d, J = 8.2 Hz, 1 H), 7.44 (dd, J =
8.2, 7.2 Hz, 1 H), 4.34 (m, 4H), 3.84 (m,
4.24 4H), 3.41 (dd, J = 12.7, 6.1 Hz, 2H), 2.27
(m, 2H), 2.24 (s, 6H).
DMSO 6 13.24 (s, 1H), 8.65 (s, 1H), 8.56
(s, 1 H), 8.42 (s, 1 H), 7.68 (d, J = 6.8 Hz,
2-142 2.90 477.3 2 0.038 1 H), 7.58 (d, J = 8.5 Hz, 1 H), 7.44 (m, 1 H),
4.34 (s, 4H), 3.84 (m, 4H), 3.59 (m, 4H),
3.43 (dd, J = 12.1, 6.7 Hz, 2H), 2.45 (m,
6H).
DMSO 6 8.83 (s, 2H), 7.99 (s, 1 H), 6.81 (s,
2-143 3.05 393.2 3 2H), 6.00 (m, 1H), 4.22 (m, 4H), 3.75 (m,
-
4H), 3.35 (m, 2H), 3.15 (m, 2H), 2.34 (m,
2H), 2.30 (s, 3H).
DMSO 6 13.15 (s, 1 H), 8.54 (t, J = 5.9 Hz,
1 H), 8.08 (s, 1 H), 7.96 (s, 1 H), 7.59 (d, J =
2-144 4.33 498.3 2 8.3 Hz, 1 H), 7.42 (dd, J = 8.2, 7.2 Hz, 1 H),
-
7.29 (m, 5H), 5.45 (s, 1 H), 4.67 (s, 2H),
4.32 (d, J = 5.9 Hz, 2H), 4.19 (m, 4H), 3.74
(m, 6H).
DMSO 6 13.20 (s, 1H), 8.60 (s, 1H), 8.55
2-145 2.91 537.3 2 3 (d, J = 0.7 Hz, 1 H), 7.90 (s, 1 H), 7.64 (d, J
= 7.1 Hz, 1 H), 7.55 (d, J = 8.3 Hz, 1 H),
7.42 (dd, J = 8.2, 7.2 Hz, 1 H), 4.27 (m, 4H),
191
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. H NMR (300 MHz; 6 in ppm, J in
Nr. Rt [M+1] Meth. IC50
Hz)
( M)
3.81 (m, 4H), 3.59 (m, 6H), 2.98 (s, 3H),
2.40 (s, 4H), 1.72 (t, J = 4.8 Hz, 4H).
DMSO 6 9.16 (s, 1 H), 8.67 (s, 1 H), 8.58 (d,
J = 4.1 Hz, 1 H), 8.43 (t, J = 5.3 Hz, 1 H),
2-146 3.24 397.2 2 0.295 8.33 (s, 1 H), 8.31 (s, 1 H), 7.50 (dd, J = 7.6,
5.0 Hz, 1 H), 4.33 (s, 4H), 3.81 (s, 4H), 3.39
(m, 4H), 3.25 (s, 3H), 1.78 (m, 2H).
DMSO 6 9.16 (dd, J = 2.2, 0.6 Hz, 1 H),
8.68 (s, 1 H), 8.58 (dd, J = 4.8, 1.6 Hz, 1 H),
0.38 8.33 (q, J = 1.9 Hz, 1 H), 8.30 (s, 2H), 7.50
2-147 and 396.2 3 0.832 (ddd, J = 8.0, 4.8, 0.6 Hz, 1H), 4.33 (m,
1.62 4H), 3.81 (m, 4H), 3.39 (dd, J = 13.2, 6.7
Hz, 2H), 2.42 (t, J = 6.9 Hz, 2H), 2.19 (s,
6H).
DMSO 6 9.16 (d, J = 1.7 Hz, 1 H), 8.67 (s,
1 H), 8.58 (dd, J = 4.7, 1.6 Hz, 1 H), 8.41 (t,
2-148 3.04 383.2 2 0.617 J = 5.0 Hz, 1 H), 8.31 (m, 2H), 7.50 (dd, J =
8.0, 4.8 Hz, 1 H), 4.33 (m, 4H), 3.81 (m,
4H), 3.48 (m, 4H), 3.28 (s, 3H).
DMSO 6 13.20 (s, 1H), 8.61 (s, 1H), 8.55
(d, J = 0.8 Hz, 1 H), 7.91 (s, 1 H), 7.64 (dd, J
2-149 3.08 565.3 2 1.3 = 7.1, 0.6 Hz, 1 H), 7.55 (d, J = 8.3 Hz, 1 H),
7.43 (dd, J = 8.3, 7.2 Hz, 1 H), 4.28 (m, 4H),
3.81 (m, 4H), 3.66 (s, 2H), 3.08 (m, 4H),
2.84 (s, 3H), 2.50 (s, 4H), 1.49 (s, 8H).
DMSO 6 13.21 (s, 1H), 8.61 (s, 1H), 8.55
(d, J = 0.8 Hz, 1 H), 7.90 (s, 1 H), 7.65 (dd, J
2-150 2.97 537.3 2 2.8 = 7.1, 0.5 Hz, 1 H), 7.56 (d, J = 8.3 Hz, 1 H),
7.43 (dd, J = 8.3, 7.2 Hz, 1 H), 4.27 (m, 4H),
3.80 (m, 6H), 3.13 (s, 4H), 3.05 (m, 4H),
2.82 (s, 3H), 1.77 (m, 4H).
DMSO 6 11.22 (s, 1H), 8.19 (s, 1H), 7.93
(s, 1 H), 7.47 (s, 2H), 7.41 (m, 1 H), 6.51 (m,
2-151 5.73 530.2 3 - 1 H), 4.20 (m, 4H), 3.77 (m, 4H), 3.72 (s,
2H), 3.13 (m, 4H), 2.87 (s, 3H), 2.57 (m,
4H).
CDCI3 6 8.55 (s, 1H), 8.43 (d, J = 5.0 Hz,
2-152 0.32 472.2 3 0.468 1 H), 7.55 (s, 1 H), 7.46 (s, 1 H), 7.16 (d, J =
5.0 Hz, 1 H), 4.26 (m, 4H), 3.82 (m, 4H),
192
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. 1H NMR (300 MHz; 8 in ppm, J in
Rt [M+1]+ Meth. IC50
Nr. Hz)
(!iM)
3.73 (s, 2H), 3.24 (m, 4H), 2.75 (s, 3H),
2.67 (m, 4H), 2.42 (s, 3H).
CDCI3 6 8.12 (s, 1H), 7.50 (s, 1H), 7.44 (s,
1 H), 6.63 (s, 1 H), 4.26 (m, 4H), 3.93 (s,
2-153 3.07 502.2 2 2.4 3H), 3.83 (m, 4H), 3.74 (s, 2H), 3.26 (m,
4H), 2.76 (s, 3H), 2.68 (m, 4H), 2.37 (s,
3H).
DMSO 6 9.16 (d, J = 1.7, 1 H), 8.67 (s, 1 H),
8.58 (dd, J = 4.7, 1.6, 1 H), 8.41 (t, J = 5.0,
2-154 3.04 383.2 2 0.166 1H), 8.31 (m, 2H), 7.50 (dd, J = 8.0, 4.8,
1 H), 4.33 (m, 4H), 3.81 (m, 4H), 3.48 (s,
4H), 3.28 (s, 3H).
DMSO 6 13.23 (s, 1 H), 8.49 (t, J = 5.9,
1 H), 8.07 (s, 1 H), 7.84 (s, 1 H), 7.78 (s, 1 H),
7.62 (d, J = 8.4, 1 H), 7.46 - 7.37 (m, 1 H),
2-155 3.08 548.2 2 - 7.37 - 7.20 (m, 5H), 7.16 (d, J = 6.8, 1 H),
6.96 (d, J = 3.9, 2H), 5.48 (s, 2H), 4.29 (d,
J = 5.9, 2H), 4.22 (s, 4H), 3.81 - 3.69 (m,
4H), 3.65 (s, 2H).
CDCI3 6 8.53 (s, 1H), 7.80 (s, 1H), 7.62 -
7.34 (m, 3H), 4.89 (s, 1 H), 4.36 (m, 4H),
2-156 4.98 488.3 2 - 4.12 (m, 1 H), 3.89 (m, 4H), 3.25 (m, 2H),
2.86 (m, 1 H), 2.62 (m, 1 H), 2.46 (s, 3H),
1.90 (m, 4H), 1.16 (m, 6H).
DMSO 6 13.23 (s, 1H), 8.64 (s, 1H), 8.80
(m, 2H), 8.42 (s, 1H), 8.05 (m, 1H), 7.67 (d,
2-157 3.93 449.2 2 0.776 J = 6.8, 1 H), 7.57 (d, J = 8.3, 1 H), 7.45 (m,
1 H), 4.34 (m, 4H), 3.85 (m, 4H), 3.35 (m,
2H), 3.26 (m, 2H), 1.82 (s, 3H).
DMSO 6 9.17 (d, J = 1.6, 1 H), 8.67 (s, 1 H),
8.57 (m, 2H), 8.32 (m, 2H), 8.05 (m, 1 H),
2-158 2.75 410.2 2 2.3 7.50 (dd, J = 7.7, 5.1, 1H), 4.34 (m, 4H),
3.81 (m, 4H), 3.33 (m, 2H), 3.24 (m, 2H),
1.80 (s, 3H).
DMSO 6 9.16 (d, J= 1.6, 1 H), 8.68 (s, 1 H),
8.58 (dd, J = 4.7, 1.6, 1H), 8.28 (m, 3H),
2-159 0.37 438.2 3 0.398 7.50 (dd, J = 8.0, 4.2, 1H), 4.33 (s, 4H),
3.78 (m, 4H), 3.55 (m, 4H), 3.42 (m, 2H),
2.49 (m, 2H), 2.45 (m, 4H).
193
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. 1H NMR (300 MHz; 6 in ppm, J in
Nr. Rt [M+1] Meth. IC50
Hz)
( M)
DMSO 6 8.79 (s, 2H), 8.57 (s, 1H), 8.46 (s,
2-160 0.37 426.2 3 - 1 H), 8.23 (s, 1 H), 6.89 (s, 2H), 4.31 (s, 4H),
3.81 (s, 4H), 2.35 (s, 2H), 2.25 (s, 6H),
1.69 (m, 2H), 1.23 (s, 2H).
DMSO 6 8.77 (s, 2H), 8.44 (s, 1 H), 8.39
2-161 3.73 413.1 2 - (m, 1 H), 8.21 (s, 1 H), 6.88 (s, 2H), 4.29 (s,
4H), 3.79 (s, 4H), 3.38 (m, 4H), 3.25 (s,
3H), 1.75 (m, 2H).
0.39 DMSO 6 8.79 (s, 2H), 8.46 (s, 1H), 8.30 (s,
2-162 and 412.3 3 1 H), 8.23 (s, 1 H), 6.89 (s, 2H), 4.30 (s, 4H),
-
3.80 (s, 4H), 3.39 (s, 2H), 2.42 (s, 2H),
2.74 2.20 (s, 6H).
DMSO 6 8.78 (s, 2H), 8.45 (s, 1H), 8.36 (s,
2-163 5.57 399.2 3 - 1H), 8.23 (s, 1H), 6.88 (s, 2H), 4.29 (m,
4H), 4.80 (m, 4H), 3.46 (m, 4H), 3.27 (s,
3H).
0.37 DMSO 6 8.77 (s, 2H), 8.45 (s, I H), 8.28
2-164 and 454.3 3 0.011 (m, 1H), 8.20 (s, 1H), 6.88 (s, 2H), 4.29 (s,
4H), 3.79 (s, 4H), 3.59 (s, 4H), 3.49 (m,
2.85 2H), 2.43 (m, 6H).
DMSO 6 8.81 (s, 2H), 8.09 (s, 1H), 6.75 (s,
2-165 0.38 488.3 3 2H), 4.16 (s, 4H), 3.69 (m, 4H), 3.58 (s,
-
2H), 3.02 (s, 4H), 2.78 (s, 3H), 2.44 (m,
7H).
DMSO 6 9.14 (s, 1 H), 8.96 (s, 2H), 8.80 (s,
2-166 2.48 304.1 2 1.8 4H), 7.98 (s, 1 H), 6.96 (s, 2H), 2.50 (s, 3H).
CDCI3 6 8.10 (s, 1H), 7.70 (s, 1H), 6.68 (s,
2-167 3.49 536.2 2 - 1 H), 4.24 (m, 4H), 3.96 (s, 3H), 3.82 (m,
4H), 3.75 (s, 2H), 3.28 (m, 4H), 2.80 (s,
3H), 2.71 (m, 4H), 2.23 (s, 2H).
DMSO 6 13.31 (s, 1H), 9.33 (s, 1H), 8.81
2-168 3.21 327.2 2 26 (m, 4H), 8.67 (s, 1 H), 8.11 (s, 1 H), 7.81 (d,
J = 7.1, 1 H), 7.64 (d, J = 8.3, 1 H), 7.52 (m,
1 H), 2.53 (s, 3H).
0.33 DMSO 6 8.76 (s, 2H), 8.40 (s, 1H), 7.70 (s,
2-169 and 514.3 3 0.11 1 H), 6.82 (s, 2H), 4.22 (m, 4H), 3.76 (m,
4H), 3.69 (s, 2H), 3.32 (s, 4H), 3.04 (s, 4H),
3.07 2.81 (s, 3H), 1.74 (m, 4H).
194
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. 1H NMR (300 MHz; 8 in ppm, J in
Rt [M+1] Meth. IC50
Nr. Hz)
( M)
DMSO S 8.76 (s, 2H), 8.40 (s, 1 H), 7.75 (s,
1 H), 6.82 (s, 2H), 4.22 (m, 4H), 3.76 (m,
2-170 2.14 500.3 2 - 4H), 3.68 (s, 2H), 3.38 (m, 2H), 2.96 (dd, J
= 9.9, 3.8, 2H), 2.90 (s, 3H), 2.81 (m, 2H),
2.59 (m, 2H).
DMSO S 8.76 (s, 2H), 8.34 (s, 1 H), 7.63 (s,
2-171 4.13 338.2 2 0.05 1 H), 6.81 (s, 2H), 4.19 (m, 4H), 3.75 (m,
4H), 2.03 (m, 1 H), 0.91 (m, 2H), 0.79 (m,
2H).
DMSO 6 8.79 (s, 2H), 8.47 (d, J = 0.8, 1 H),
2-172 4.72 366.2 2 0.032 8.44 (s, 1 H), 6.92 (s, 2H), 4.23 (m, 4H),
3.78 (m, 4H).
0.33 CDCI3 6 8.54 (s, 2H), 7.74 (s, 1 H), 7.30 (d,
2-173 and 506.2 3 - J = 4.2, 1H), 4.25 (m, 4H), 3.86 (s, 2H),
3.83 (m, 4H), 3.32 (m, 4H), 2.79 (s, 7H),
2.59 2.32 (s, 3H).
DMSO S 8.78 (s, 2H), 8.46 (s, 1 H), 7.89 (d,
2-174 3.11 298.2 2 0.191 J = 1.0, 1 H), 7.56 (d, J = 1.0, 1 H), 6.84 (s,
2H), 4.26 (m, 4H), 3.76 (m, 4H).
0.32 DMSO 6 8.76 (s, 2H), 8.40 (s, 1 H), 7.75 (s,
2-175 and 397.2 3 0.083 1 H), 6.83 (s, 2H), 4.22 (m, 4H), 3.76 (m,
2.40 4H), 3.57 (m, 6H), 2.44 (m, 4H).
DMSO 5 8.75 (s, 2H), 8'.37 (s, 1 H), 8.19 (d,
2-176 4.29 405.2 3 - J = 6.4, 2H), 7.58 (s, 1 H), 6.82 (s, 2H), 6.46
(s, 2H), 4.21 (d, J = 4.4, 4H), 3.85 (s, 2H),
3.76 (m, 4H).
DMSO 6 8.77 (s, 2H), 8.42 (s, 1 H), 7.67 (s,
2-177 3.45 356.2 2 - 1 H), 6.82 (s, 2H), 5.09 (s, 1 H), 4.23 (s, 4H),
3.76 (s, 4H), 1.48 (s, 6H).
DMSO 6 8.81 (s, 2H), 8.04 (s, 1 H), 6.82 (s,
2-178 2.72 553.2 2 - 2H), 4.18 (s, 4H), 3.71 (m, 4H), 3.60 (s,
2H), 3.03 (s, 4H), 2.79 (s, 3H), 2.48 (d, J =
4.7, 4H).
DMSO 6 8.88 (s, 2H), 7.91 (s, 1 H), 7.30 (s,
2-179 3.76 460.2 2 - 1 H), 6.89 (s, 2H), 4.25 (s, 4H), 3.75 (m,
4H), 3.39 (m, 4H), 3.12 (m, 4H), 2.99 (s,
3H).
2-180 0.32 499.2 3 - DMSO 5 8.74 (s, 2H), 7.88 (s, 1 H), 7.23 (s,
195
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. Rt [M+1]+ Meth. IC50 1H NMR (300 MHz; 8 in ppm, J in
N r. Hz)
( M)
and 2H), 4.44 (s, 4H), 3.77 (s, 4H), 3.70 (s, 2H),
3.18 3.12 (s, 4H), 2.87 (s, 3H), 2.56 (s, 4H).
DMSO 6 8.76 (s, 2H), 8.37 (s, 1 H), 7.61 (s,
2-181 3.17 312.2 2 0.11 1 H), 6.80 (s, 2H), 4.21 (s, 4H), 3.75 (s, 4H),
2.33 (s, 3H).
DMSO 6 13.24 (s, 1 H), 8.64 (s, 1 H), 8.55
(s, 1 H), 8.43 (s, 1 H), 8.12 (d, J = 8.6, 1 H),
2-182 5.08 519.2 2 0.245 7.67 (d, J = 7.1, 1 H), 7.58 (d, J = 8.1, 1 H),
7.43 (d, J = 8.2, 1 H), 4.33 (s, 4H), 4.05 (m,
6H), 3.83 (m, 4H), 2.91 (m, 1 H), 1.77 (m,
2H), 1.56 (m, 2H), 1.20 (t, J = 7.1, 3H).
DMSO 6 13.22 (s, 1H), 8.46 (s, 1H), 8.11
(s, 1 H), 7.65 (d, J = 7.1, 1 H), 7.60 (d, J =
2-183 3.45 576.2 2 0.17 8.3, 1 H), 7.46 (d, J = 7.3, 1 H), 4.30 (m,
4H), 3.82 (m, 4H), 3.71 (s, 2H), 3.11 (m,
4H), 2.86 (s, 3H), 2.58 (m, 4H).
DMSO 6 8.64 (s, 2H), 8.30 (s, 1 H), 8.22 (s,
2-184 3.05 355.2 2 - 1H), 8.07 (s, 1H), 6.74 (s, 2H), 4.16 (m,
4H), 3.65 (m, 4H), 2.68 (d, J = 3.9, 3H).
DMSO 6 8.62 (s, 2H), 7.85 (d, J = 0.8, 1 H),
2-185 4.10 346.2 2 - 6.94 (s, 2H), 4.17 (m, 4H), 3.74 (m, 4H),
2.38 (d, J = 0.5, 3H).
DMSO 6 13.17 (s, 1 H), 8.58 (s, 1 H), 8.49
(s, 1 H), 8.35 (s, 1 H), 8.31 (m, 1 H), 7.61 (d,
2-186 4.15 422.2 2 0.066 J = 7.2, 1 H), 7.51 (d, J = 8.2, 1 H), 7.39 (d,
J = 7.2, 1H), 4.27 (m, 4H), 3.77 (m, 4H),
3.42 (m, 4H), 3.21 (s, 4H).
DMSO 6 8.74 (s, 2H), 7.83 (s, 1 H), 7.20 (s,
2-187 4.02 337.2 2 - 2H), 4.43 (s, 4H), 3.76 (m, 4H), 2.37 (s,
3H).
DMSO 6 8.77 (s, 2H), 8.44 (s, 1 H), 8.25 (s,
1H), 8.09 (d, J = 8.5, 1H), 6.88 (s, 2H),
2-188 4.03 496.2 2 0.003 4.28 (s, 4H), 4.04 (m, 5H), 3.79 (m, 4H),
2.87 (s, 2H), 1.78 (d, J = 9.9, 2H), 1.57 (dt,
J = 12.2, 8.4, 2H), 1.19 (t, J = 7.1, 3H).
DMSO 6 8.65 (s, 2H), 8.46 (s, 1H), 8.33 (s,
2-189 3.39 389.2 2 - 1 H), 6.99 (s, 2H), 4.26 (s, 4H), 3.78 (m,
4H), 2.83 (d, J = 4.8, 3H).
196
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. 'H NMR (300 MHz; 8 in ppm, J in
Nr. Rt [M+1] Meth. 1C50
Hz)
( M)
CDCI3 6 8.83 (d, J = 1.8, 2H), 8.14 (s, 1 H),
7.44 (s, 1H), 5.43 (s, 2H), 4.10 (dd, J =
2-190 3.11 311.2 2 - 11.4, 3.4, 2H), 3.84 (m, 1H), 3.67 (t, J =
11.5, 2H), 2.49 (s, 3H), 2.15 (m, 2H), 1.90
(m, 2H).
DMSO 6 8.71 (s, 2H), 8.38 (s, 1H), 8.18 (s,
1 H), 8.03 (d, J = 8.4, 1 H), 6.81 (s, 2H),
2-191 3.53 425.2 2 0.013 4.22 (m, 4H), 3.96 (s, 1 H), 3.83 (d, J =
11.1, 2H), 3.73 (m, 4H), 3.33 (m, 2H), 1.66
(m, 4H).
DMSO 6 8.70 (s, 2H), 8.37 (s, 1H), 8.15 (s,
1H), 7.83 (d, J = 8.5, 1H), 6.80 (s, 2H),
2-192 3.40 439.2 2 0.01 4.50 (d, J = 4.4, 1 H), 4.20 (s, 4H), 3.72 (m,
4H), 3.33 (m, 1 H), 1.75 (m, 4H), 1.42 (m,
2H), 1.18 (m, 2H).
DMSO 6 8.77 (s, 2H), 8.44 (s, 1 H), 8.22 (s,
2-193 4.64 510.3 2 - 1 H), 6.89 (s, 2H), 4.24 (m, 4H), 4.02 (m,
2H), 3.78 (m, 4H), 3.63 (m, 2H), 3.41 (m,
4H), 1.42 (s, 9H).
DMSO 6 8.66 (s, 2H), 8.50 (s, 1H), 8.36 (s,
2-194 3.77 433.3 2 - 1H), 7.00 (s, 2H), 4.25 (m, 4H), 3.78 (m,
4H), 3.48 (m, 4H), 3.28 (s, 3H).
DMSO 6 8.76 (s, 2H), 8.37 (s, 1H), 7.65 (s,
1 H), 6.81 (s, 2H), 4.23 (m, 4H), 3.92 (d, J =
2-195 4.52 382.2 2 - 9.5, 2H), 3.77 (m, 4H), 3.46 (t, J = 10.8,
2H), 2.93 (m, 1 H), 1.91 (d, J = 12.5, 2H),
1.69 (m, 2H).
DMSO 6 9.20 (s, 2H), 8.67 (s, 1H), 7.83 (s,
2-196 2.58 473.2 2 0.021 1 H), 4.26 (m, 4H), 3.78 (m, 4H), 3.68 (s,
2H), 3.12 (m, 4H), 2.87 (s, 3H), 2.66 (s,
3H), 2.56 (m, 4H).
DMSO 6 13.13 (s, 1H), 8.64 (s, 1H), 8.16
(s, 1 H), 8.08 (s, 1 H), 7.85 (s, 1 H), 7.81 (d,
2-197 2.96 497.2 2 - J = 8.6, 1 H), 7.69 (d, J = 8.5, 1 H), 4.28 (m,
4H), 3.81 (m, 4H), 3.68 (s, 2H), 3.12 (s,
4H), 2.87 (s, 3H), 2.57 (s, 4H).
DMSO 6 8.39 (s, 1 H), 8.35 (s, 1 H), 7.76 (s,
2-198 0.37 487.2 2 - 2H), 5.96 (s, 2H), 4.22 (m, 4H), 3.77 (m,
4H), 3.66 (s, 2H), 3.11 (m, 4H), 2.87 (s,
197
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. 'H NMR (300 MHz; 8 in ppm, J in
Rt [M+1] Meth. 1C50
Nr. Hz)
( M)
3H), 2.56 (m, 4H), 2.11 (s, 3H).
DMSO 6 8.82 (s, 2H), 8.40 (s, 1 H), 7.77 (s,
0.37 1 H), 7.31 (q, J = 4.9, 1 H), 4.23 (m, 4H),
2-199 and 488.2 2 - 3.76 (m, 4H), 3.66 (s, 2H), 3.10 (m, 4H),
2.47 2.87 (s, 3H), 2.84 (d, J = 4.8, 3H), 2.53 (m,
4H).
0.36 DMSO 6 8.24 (s, 1 H), 8.05 (s, 1 H), 7.81 (s,
2-200 and 488.2 2 0.032 1 H), 6.67 (s, 2H), 4.17 (m, 4H), 3.75 (m,
4H), 3.67 (s, 2H), 3.11 (m, 4H), 2.87 (s,
1.99 3H), 2.55 (m, 4H), 2.39 (s, 3H).
DMSO 6 9.33 (d, J = 2.3, 1 H), 8.84 (s, I H),
0.35 8.56 (dd, J = 8.2, 2.2, 1 H), 8.13 (d, J = 8.2,
2-201 and 483.2 2 3.3 1 H), 7.86 (s, 1 H), 4.28 (s, 4H), 3.78 (m,
2.88 4H), 3.69 (s, 2H), 3.12 (m, 4H), 2.87 (s,
3H), 2.56 (m, 4H).
0.36 DMSO 6 8.56 (s, 1 H), 8.08 (s, 1 H), 7.86 (s,
2-202 and 542.2 2 - 1 H), 7.45 (s, 2H), 4.16 (m, 4H), 3.72 (m,
4H), 3.67 (s, 2H), 3.11 (m, 4H), 2.87 (s,
2.75 3H), 2.52 (m, 4H).
DMSO 6 8.55 (s, 1 H), 7.94 (d, J = 5.4, 1 H),
7.86 (s, 1 H), 7.07 (s, 1 H), 6.97 (d, J = 6.9,
2-203 0.36 473.2 2 - 1 H), 5.97 (s, 2H), 4.26 (m, 4H), 3.78 (m,
4H), 3.67 (s, 2H), 3.12 (m, 4H), 2.87 (s,
3H), 2.56 (m, 4H).
DMSO 6 9.11 (s, 1H), 8.60 (s, 1H), 8.43 (d,
J = 5.7, 1 H), 7.95 (s, 1 H), 7.18 (d, J = 5.8,
2-204 0.35 488.2 2 10 1 H), 4.21 (m, 4H), 3.99 (s, 3H), 3.79 (m,
4H), 3.67 (s, 2H), 3.12 (m, 4H), 2.87 (s,
3H), 2.56 (m, 4H).
0.36 DMSO 6 8.43 (s, 1 H), 8.38 (s, 1 H), 7.85 (d,
2-205 and 491.2 2 J = 12.7, 1 H), 7.75 (s, 1 H), 6.39 (s, 2H),
-
4.23 (m, 4H), 3.77 (m, 4H), 3.66 (s, 2H),
2.23 3.11 (m, 4H), 2.87 (s, 3H), 2.52 (m, 4H).
DMSO 6 9.16 (d, J = 1.4, 1H),8.44(dd,J=
8.1, 2.2, 1 H), 8.18 (d, J = 8.2, 1 H), 8.05 (s,
2-206 3.20 517.2 2 3.7 1 H), 4.22 (m, 4H), 3.76 (m, 4H), 3.73 (s,
2H), 3.12 (m, 4H), 2.87 (s, 3H), 2.56 (m,
4H).
198
-0&26
WO 2010/119264 PCT/GB2010/000773
PI3Ka
Cpd. Rt [M+1]+ Meth. IC50 'H NMR (300 MHz; = 8 in ppm, J in
Nr. Hz)
( M)
2.72 DMSO 6 8.70 (s, 2H), 7.95 (s, 1 H), 7.45
2-207 and 522.2 2 0.011 (m, 1H), 4.19 (m, 4H), 3.75 (m, 4H), 3.71
(s, 2H), 3.12 (m, 4H), 2.86 (s, 3H), 2.83 (d,
2.85 J = 6.3, 3H), 2.56 (m, 4H).
0.36 DMSO 6 8.15 (s, 1H), 7.93 (s, 1H), 6.76 (s,
2-208 and 522.2 2 - 2H), 4.15 (m, 4H), 3.74 (m, 4H), 3.72 (s,
2H), 3.12 (m, 4H), 2.87 (s, 3H), 2.57 (m,
2.32 4H), 2.19 (s, 3H).
2.96 DMSO 6 8.56 (s, 1H), 7.96 (s, 1H), 7.59 (s,
2-209 and 576.2 2 - 2H), 4.14 (m, 4H), 3.72 (m, 6H), 3.12 (m,
4H), 2.87 (s, 3H), 2.54 (m, 4H).
3.09
0.37 DMSO 6 7.98 (m, 2H), 6.84 (m, 2H), 6.04
2-210 and 507.2 2 _ (s, 2H), 4.20 (m, 4H), 3.76 (m, 4H), 3.72 (s,
2H), 3.12 (m, 4H), 2.87 (s, 3H), 2.53 (m,
1.70 4H).
DMSO 6 8.53 (d, J = 5.8, 1 H), 8.40 (s, 1 H),
2-211 0.36 522.2 2 7.94 (s, 1 H), 7.20 (d, J = 5.9, 1 H), 4.14 (m,
-
4H), 3.86 (s, 3H), 3.73 (m, 6H), 3.12 (m,
4H), 2.87 (s, 3H), 2.57 (m, 4H).
MeOD 6 9.13 (s, 2H), 8.02 (s, 1 H), 4.30 (m,
2-212 2.85 507.2 2 _ 4H), 3.85 (dd, J = 9.2, 4.5, 4H), 3.82 (s,
2H), 3.27 (m, 4H), 2.86 (s, 3H), 2.77,(s,
3H), 2.71 (m, 4H).
MeOD 6 8.10 (s, 1H), 7.99 (s, 1H), 7.92 (s,
2-213 3.24 531.2 2 1 H), 7.84 (d, J = 8.4, 1 H), 7.56 (d, J = 8.2,
-
1H), 4.27 (m, 4H), 3.83 (m, 6H), 3.27 (m,
4H), 2.84 (s, 3H), 2.68 (m, 4H).
DMSO 6 8.91 (s, 2H), 8.23 (s, 1 H), 6.89 (s,
2-214 4.16 384.2 2 - 2H), 4.32 (m, 6H), 3.78 (m, 4H), 2.74 (s,
3H), 1.33 (t, J = 7.1, 3H).
DMSO 6 8.90 (s, 2H), 8.27 (s, 1H), 8.20 (s,
2-215 0.35 413.2 2 1H), 6.86 (s, 2H), 4.28 (m, 4H), 3.78 (m,
-
4H), 3.45 (m, 4H), 3.28 (s, 3H), 2.75 (s,
3H).
DMSO 6 8.91 (s, 2H), 8.44 (s, 1 H), 8.20 (s,
2-216 3.76 433.2 2 - 1H), 6.92 (s, 2H), 4.29 (s, 4H), 3.79 (s, 4H),
3.45 (m, 4H), 3.25 (s, 3H).
2-217 4.79 355.2 3 - DMSO 6 8.90 (s, 2H), 8.18 (s, 1 H), 7.77 (s,
199
-0&26
WO 2010/119264 PCT/GB2010/000773
PI3Ka
Cpd. 'H NMR (300 MHz; 6 in ppm, J in
Rt [M+1] Meth. IC60
Nr. Hz)
( M)
1 H), 7.35 (s, 1 H), 6.85 (s, 2H), 4.28 (s, 4H),
3.77 (m, 4H), 2.74 (s, 3H).
3.53 DMSO 6 8.75 (s, 2H), 8.38 (s, 1H), 7.78 (s,
2-218 and 524.3 2 0.028 1 H), 6.82 (s, 2H), 4.25 (s, 4H), 4.00 (s, 2H),
3.76 (s, 6H), 2.36 (s, 2H), 1.34 (s, 9H),
3.76 1.15 (d, J = 5.8, 6H).
DMSO 6 8.76 (s, 2H), 8.39 (s, 1H), 7.80 (s,
2-219 0.39 502.2 3 0.003 1 H), 6.82 (s, 2H), 4.25 (s, 4H), 4.02 (s, 2H),
3.76 (m, 4H), 3.34 (m, 2H), 2.84 (s, 3H),
2.53 (m, 2H), 1.20 (d, J = 5.8, 6H).
DMSO 6 8.77 (s, 2H), 8.41 (s, 1H), 7.76 (s,
2-220 0.43 488.2 3 0.013 1 H), 6.83 (s, 2H), 4.23 (m, 4H), 3.77 (m,
4H), 3.65 (s, 2H), 3.18 (m, 4H), 3.04 (q, J =
7.4, 2H), 2.51 (m, 4H), 1.20 (t, J = 7.4, 3H).
DMSO 6 8.76 (s, 2H), 8.41 (s, 1H), 7.76 (s,
3.73 1 H), 6.83 (s, 2H), 4.22 (m, 4H), 3.76 (m,
2-221 and 516.3 3 0.011 4H), 3.66 (s, 2H), 3.14 (m, 4H), 2.88 (m,
4.03 2H), 2.51 (m, 4H), 2.11 (m, 1H), 1.01 (d, J
= 6.5, 6H).
DMSO 6 8.57 (s, 2H), 8.21 (s, 1H), 7.60 (s,
2-222 4.07 445.1 3 0.082 1 H), 6.63 (s, 2H), 4.03 (s, 4H), 3.61 (s, 2H),
3.56 (m, 4H), 2.89 (m, 4H), 2.76 (m, 4H).
DMSO 6 8.80 (s, 2H), 8.35 (s, 1H), 7.60 (s,
1 H), 6.79 (s, 2H), 5.43 (s, 1 H), 4.83 (t, J =
2-223 2.74 342.2 2 - 5.2, 1 H), 4.07 (d, J = 11.5, 1 H), 3.95 (d, J =
8.9, 1H), 3.80 (m, 1H), 3.59 (m, 3H), 3.36
(m, 2H), 2.33 (s, 3H).
DMSO 6 8.77 (s, 2H), 8.39 (s, 1H), 7.62 (s,
1 H), 6.80 (s, 2H), 5.51 (s, 2H), 3.93 (m,
2-224 3.60 384.2 2 25.4 1H), 3.80 (m, 1H), 3.70 (m, 1H), 3.59 (m,
1H), 3.46 (s, 3H), 3.34 (m, 1H), 2.86 (m,
1 H), 2.72 (m, 1 H), 2.32 (s, 3H).
DMSO 6 8.74 (s, 2H), 8.35 (s, 1 H), 7.70
2-225 4.23 536.3 3 - (m, 6H), 6.82 (s, 2H), 4.20 (s, 4H), 3.74 (m,
4H), 3.60 (s, 2H), 2.90 (s, 4H), 2.50 (m,
4H).
DMSO 6 8.76 (s, 2H), 8.40 (s, 1 H), 7.72 (s,
2-226 0.36 396.3 4 0.077 1 H), 6.82 (s, 2H), 4.22 (s, 4H), 3.76 (m,
4H), 3.55 (s, 2H), 3.35 (s, 1H), 2.69 (m,
200
-0&26
WO 2010/119264 PCT/GB2010/000773
PI3Ka
Cpd. 1H NMR (300 MHz; 8 in ppm, J in
Nr. Rt [M+1]+ Meth. IC50
Hz)
( M)
4H), 2.37 (m, 4H).
CDCI3 6 8.74 (s, 2H), 7.77 (s, 1 H), 7.27 (s,
1H), 5.59 (s, 2H), 4.53 (d, J = 11.7, 1H),
2-227 3.58 370.2 2 6.09 4.05 (t, J = 12.2, 1H), 3.95 (dd, J = 11.8,
3.7, 1H), 3.81 (m, 1H), 3.76 (s, 3H), 3.63
(m, 1 H), 2.42 (s, 3H).
DMSO 6 8.77 (s, 2H), 8.40 (s, 1H), 7.80 (s,
0.35 1H), 6.83 (s, 2H), 4.21 (m, 4H), 3.76 (m,
2-228 and 500.4 3 0.108 4H), 3.63 (s, 2H), 3.38 (s, 2H), 3.19 (dd, J
= 10.7, 2.3, 2H), 2.96 (d, J = 10.0, 2H),
2.84 2.85 (s, 3H), 1.99 (m, 2H), 1.66 (q, J = 6.1,
2H).
DMSO 6 8.76 (s, 2H), 8.40 (s, 1 H), 7.72 (s,
0.35 1H), 6.82 (s, 2H), 4.52 (d, J = 4.1, 1H),
2-229 and 411.3 3 0.114 4.22 (s, 4H), 3.76 (m, 4H), 3.56 (s, 2H),
0.87 3.42 (m, 1 H), 2.75 (m, 2H), 2.10 (t, J = 9.7,
2H), 1.69 (m, 2H), 1.40 (m, 2H).
DMSO 6 8.77 (s, 2H), 8.41 (s, 1H), 7.76 (s,
1 H), 6.83 (s, 2H), 4.23 (s, 4H), 3.76 (m,
2-230 0.34 481.4 3 0.034 4H), 3.62 (s, 2H), 3.52 (m, 2H), 3.44 (m,
2H), 3.05 (s, 2H), 2.43 (m, 4H), 2.16 (s,
6H).
DMSO 6 8.77 (s, 2H), 8.41 (s, 1 H), 7.77 (s,
1H), 6.83 (s, 2H), 5.34 (q, J = 6.8, 1H),
2-231 0.34 510.4 3 0.085 4.23 (s, 4H), 3.77 (m, 4H), 3.64 (s, 2H),
3.45 (m, 4H), 2.49 (m, 2H), 2.41 (m, 2H),
2.02 (s, 3H), 1.28 (d, J = 6.7, 3H).
0.42 524.2 MeOD 6 8.80 (s, 2H), 8.18 (s, 1 H), 7.70 (s,
2-232 and and 2 _ 1H), 7.54 (m, 2H), 4.31 (s, 4H), 3.85 (s,
4H), 3.40 (s, 4H), 2.52 (s, 4H), 1.54 (s, 6H),
3.03 424.2 1.42 (s, 9H).
DMSO 6 8.80 (s, 2H), 8.44 (s, 1 H), 7.77 (s,
2.40 1 H), 6.86 (s, 2H), 4.25 (m, 4H), 3.79 (m,
2-233 and 502.2 3 - 6H), 3.59 (s, 3H), 2.87 (m, 3H), 2.72 (m,
2.87 2H), 2.28 (s, 3H), 1.91 (m, 2H), 1.54 (m,
2H).
DMSO 6 8.77 (s, 2H), 8.42 (s, 1 H), 7.77 (s,
2-234 0.39 468.2 3 - 1H), 6.85 (s, 2H), 4.85 (d, J = 7.0, I H),
4.40 (m, 1 H), 4.23 (m, 4H), 3.78 (m, 4H),
201
-0&26
WO 2010/119264 PCT/GB2010/000773
P13Ka
Cpd. H NMR (300 MHz; 6 in ppm, J in
Rt [M+1] Meth. IC50
Nr. Hz)
( M)
3.64 (s, 2H), 3.44 (m, 4H), 2.40 (m, 4H),
1.16 (d, J = 6.5, 3H).
0.41 DMSO 6 8.76 (s, 2H), 8.42 (s, 1 H), 7.76 (s,
2-235 and 488.2 3 - 1 H), 6.84 (s, 2H), 4.23 (m, 4H), 3.78 (m,
6H), 3.34 (m, 4H), 2.89 (s, 3H), 2.73 (m,
2.71 4H), 1.79 (m, 2H).
MeOD 6 8.71 (s, 2H), 8.07 (s, 1 H), 7.58 (s,
2-236 0.41 424.2 3 - 1 H), 4.20 (m, 4H), 3.75 (m, 4H), 3.21 (s,
2H), 2.73 (m, 4H), 2.50 (m, 4H), 1.39 (s,
6H).
DMSO 6 13.29 (s, 1H), 9.17 (s, 1H), 8.45
2-237 4.70 407.1 5 - (s, 1 H), 7.63 (m, 2H), 7.47 (m, 1 H), 4.42 (q,
J = 7.1, 2H), 4.28 (m, 4H), 3.82 (m, 4H),
2.64 (s, 3H), 1.40 (t, J = 7.1, 3H).
2-238 4.07 379.1 5 -
2-239 3.92 349.1 5 -
2-240 3.65 423.2 5 -
2-241 4.34 486.3 5 -
0.40 DMSO 6 8.77 (s, 2H), 8.37 (s, 1 H), 7.72 (s,
2-242 and 502.3 3 0.016 1 H), 6.82 (s, 2H), 4.25 (s, 4H), 3.77 (m,
4H), 3.07 (s, 4H), 2.84 (s, 3H), 2.54 (m,
3.01 4H), 1.47 (s, 6H).
DMSO 6 8.89 (d, J = 10.8, 2H), 8.18 (s,
I H), 8.02 (d, J = 8.7, 2H), 7.54 (dd, J = 8.5,
2-243 2.97 555.1 2 - 6.5, 4H), 7.34 (d, J = 8.9, 2H), 4.27 (m,
4H), 3.78 (m, 4H), 3.49 (m, 4H), 2.50 (s,
3H), 2.31 (m, 4H), 2.19 (s, 3H).
DMSO 6 9.22 (s, 1 H), 9.07 (s, 1 H), 8.22 (s,
2-244 4.58 473.4 2 0.144 1 H), 8.03 (d, J = 8.6, 2H), 7.88 (d, J = 8.7,
2H), 7.57 (m, 5H), 7.41 (s, 1H), 4.24 (m,
4H), 3.78 (m, 4H), 2.50 (s, 3H).
DMSO 6 9.14 (s, 1 H), 8.96 (s, 1 H), 8.19 (s,
1 H), 8.02 (d, J = 8.7, 2H), 7.90 (d, J = 8.7,
2-245 5.33 487.3 2 - 2H), 7.58 (m, 5H), 7.36 (s, 1 H), 4.27 (m,
4H), 3.81 (s, 3H), 3.78 (m, 4H), 2.50 (s,
3H).
202
-0&26
WO 2010/119264 PCT/GB2010/000773
Table 5. IC50 of AKT Phosphorylation Inhibition of some representative
compounds of the examples ( M), as tested in the cell assay procedure
described above.
Cpd. Nr. p-AKT cell Cpd. Nr. p-AKT cell
2-01 0.132 2-171 0.379
2-10 0.048 2-172 0.149
2-13 0.714 2-175 0.469
2-16 0.503 2-186 0.281
2-17 0.391 2-188 0.060
2-50 0.008 2-191 0.034
2-66 0.014 2-192 0.070
2-67 0.198 2-196 0.134
2-74 0.037 2-219 0.244
2-75 0.077 2-220 0.098
2-79 0.042 2-221 0.135
2-119 0.087 2-222 0.095
2-142 0.116
2-154 1.05
2-164 0.034
203