Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02758529 2011-10-12
WO 2010/120876 PCT/US2010/031026
U. S. PATENT APPLICATION
SYSTEM AND METHOD FOR USING SUPER CRITICAL
STATE CARBON DIOXIDE (C02)
FOR HYDROCARBON RECOVERY AND TRANSPORT
CROSS REFERENCE TO RELATED APPLICATION
[0001] This application claims the benefit of Provisional U.S. Patent
Application
No. 61/124,596 filed April 17, 2008.
STATEMENT REGARDING FEDERALLY FUNDED RESEARCH AND
DEVELOPMENT
[0002] The invention described in this patent application was not the subject
of
federally sponsored research or development.
FIELD
[0003] The system and method of the present invention pertains to the recovery
and transport of extracted or processed hydrocarbons; more particularly, the
system
and method of the present invention pertains to the use of super critical
state CO2 in the
recovery and transport of extracted or processed hydrocarbons.
BACKGROUND
[0004] Extracted or processed hydrocarbons, for example, heavy hydrocarbons,
especially like bitumen or those heavy hydrocarbons mined or produced from
"oil
sands," require an additional element to be combined with the heavy
hydrocarbon, to
enable the heavy hydrocarbon to be transported via pipeline. This additional
element or
additive, more commonly called a "diluent," is injected into and/or otherwise
combined
with the heavy hydrocarbon, to form a combination known in the industry as a
"dilbit."
[0005] The use of the diluents is necessary to transport heavy hydrocarbons to
upgraders and other processing facilities to make usable products. However,
the very
CA 02758529 2011-10-12
WO 2010/120876 PCT/US2010/031026
high cost of diluents, as represented by the direct investment to acquire
diluents and
then process these diluents, as well as the opportunity cost of not marketing
the diluents
for sale in their own markets, directly impacts the recovery and
transportation cost
associated with producing heavy hydrocarbons, extracted from oil sands as well
as
making useful products from the heavy hydrocarbons.
[0006] Diluents currently in use in the transport of heavy hydrocarbons,
include
various lighted hydrocarbons such as condensate. With the growth in the
production of
heavy hydrocarbons from oil sands expected to both continue and grow, the
demand for
condensate (a high value, very light hydrocarbon product) will exceed
available supply.
As a result of the anticipated shortage or lack of availability of condensate,
other
diluents must be considered for use in the dilbit to make up for the
anticipated shortfall
in the supply of available condensate. Principal among the other diluents
being
considered are natural gas liquids or as they are more commonly called in the
industry,
"NGLs." NGLs, like condensate, are very valuable light end hydrocarbons which
have
their own value and are marketed, traded and transported in their own markets.
[0007] Accordingly, there remains a need in the art for a low cost, easily
accessible diluent to be used in the transport of heavy hydrocarbons such as
those
heavy hydrocarbons produced from oil sands.
SUMMARY
[0008] The system and method of the present invention provides a low cost,
easily accessible product which can be used in the transport of extracted or
processed
hydrocarbons such as those heavy hydrocarbons produced from oil sands.
CA 02758529 2011-10-12
WO 2010/120876 PCT/US2010/031026
[0009] According to the system and method of the present invention, C02 is
compressed and transformed into a super critical state. Once in a super
critical state,
the CO2 is added to a heavy hydrocarbon. The combination of the super critical
state
C02 facilitates the removal of the heavy hydrocarbon from a ground formation,
such as
oil sands.
[0010] Once the heavy hydrocarbon is removed from the ground formation, such
as oil sands, the combination of the heavy hydrocarbon, with the super
critical state CO2
may be transported in a pressurized pipeline or tank to a predetermined
delivery
destination. Specifically, the super critical state C02 acts as a carrying or
suspension
agent for the heavy hydrocarbon.
[0011] At the predetermined delivery destination, the pressurized pipeline or
tank containing the combination of the super critical state C02 and the heavy
hydrocarbon is de-pressurized. Such de-pressurization of the pressurized
pipeline
causes the combination of the heavy hydrocarbon, and the C02 to return to a
dual gas-
liquid state. The gaseous C02, is then separated from the heavy hydrocarbon.
The
heavy hydrocarbon may then be transformed into useful products. The CO2 may
then
be caused to re-enter a super critical state for re-use or storage.
BRIEF DESCRIPTION OF THE DRAWING FIGURE
[0012] A still better understanding of the system and method of the present
invention may be had by reference to the drawing figure wherein:
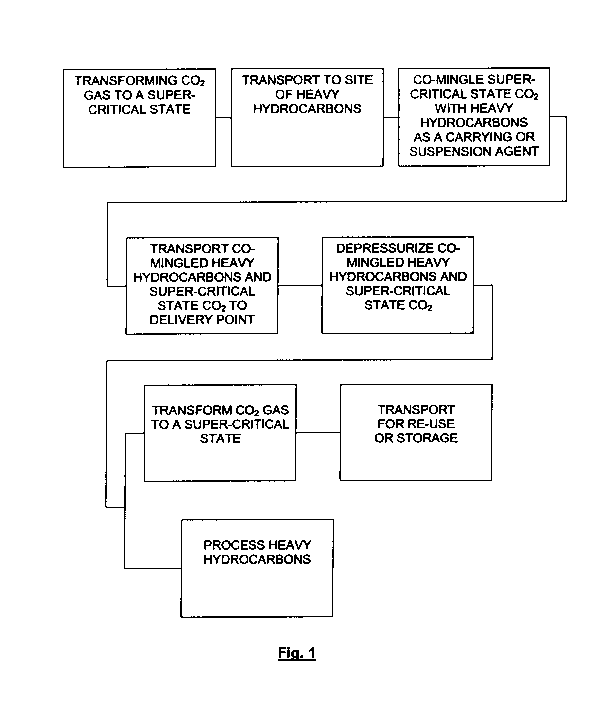
[0013] Figure 1 is a flow diagram of the system and method of the present
invention.
DESCRIPTION OF THE EMBODIMENTS
[0014] Technical and Historical Background
CA 02758529 2011-10-12
WO 2010/120876 PCT/US2010/031026
[0015] Carbon dioxide, or as hereinafter referred to as C02, is a unique
molecule. CO2 is found in many common applications such as: an additive for
soft
drinks to add the "fizz"; dry ice for keeping food cold; and a component in
chemical
processes, for example, the urea process for making fertilizer. CO2 is also
importantly
used, in a specialty sense, in the oil and gas industry as an injectant for
tertiary oil
recovery for what is called in the industry, C02-EOR (enhanced oil recovery).
[0016] The unique application of CO2 in the oil and gas industry began in the
early 1970's. Part of this key development was the construction of an
expansive and
unique infrastructure for handling C02 at locations where naturally occurring
C02 was
discovered. These "naturally sourced" CO2 reservoirs were predominantly at
four
formations or "domes" discovered in the Untied States. These four formations
or domes
are: McElmo Dome (SW Colorado), Sheep Mountain (SE Colorado), Bravo Dome
(Eastern New Mexico), and Jackson Dome (near Jackson, Mississippi). CO2 from
these
four formations or domes served as the first source from which major CO2
transport
pipelines infrastructures were built. These C02 transport pipelines connect
natural CO2
sources to major oil fields at which C02-EOR is conducted.
[0017] Over time, additional anthropogenic sources such as C02 sources from
man-made processes based on the separation of CH4 or methane from C02, also
became more widely available. This increase in availability of CO2 from
anthropogenic
sources resulted in both anthropogenic and naturally sourced CO2 becoming
available
for use in C02 EOR. The designation of CO2 from these two sources is denoted
by
suffix "a" or "n". C02a designates CO2 from an anthropogenic source and CO2n
CA 02758529 2011-10-12
WO 2010/120876 PCT/US2010/031026
designates CO2 from a natural source. This nomenclature will be applied herein
in all
references to CO2.
[00181 It is important to note, that what is referred to as either C02a or
C02n is
not usually pure CO2; that is CO2 without other gases or elemental components.
Rather, C02X is the term to describe a somewhat "mixed" gas, or C02 containing
small
amounts of nitrogen, oxygen, hydrogen, sulfide and other gases. Some C02n
sources
are 99% or greater pure CO2. Some C02a sources contain varying amounts of H2S
gas.
[0019] In all cases herein, the terms C02a or C02n will be used to primarily
indicate the distinction in source from which they are obtained. In all cases,
both C02
and C02n indicate a C02 gas or gas mix with varying constituent component
gases
such as 02, N2, H2S and other gases as the case may be. The CO2 used as a
diluent
can either be 100% pure CO2 or be a C02 gas mix consisting of the various gas
components present as a result of the process through which the C02 is
extracted or
otherwise captured.
[0020] A unique feature of the evolved C02-EOR industry is the manner in which
CO2 is transported. Unlike natural gas (methane) or hydrogen gas which are
transported in a gaseous state, all C02, be it in its relatively pure and/or
in its mixed gas
state, is transported differently. Specifically, the C02a/ C02n gas and/or
mixed gas is
first dehydrated to achieve a dryer gas with little or no water or water vapor
content.
This dehydration process effectively lessens the chances of the CO2X forming
carbonic
acid. Such dehydration is important because carbonic acid causes corrosion to
form in
the pipe or tank transporting the CO2X gas. Second, and most significantly,
CO2 is
CA 02758529 2011-10-12
WO 2010/120876 PCT/US2010/031026
typically compressed to a pressure at which it takes on a "super critical"
state for
transport.
[0021] Super critical state C02" is the term used to describe CO2 that is in a
fluid
state while also being at or above both its critical temperature and pressure.
In its super
critical state, CO2 exhibits some uncommon properties. Specifically, CO2
usually
behaves as a gas in air at STP or as a solid called "dry ice" when the C02 gas
is frozen.
If the temperature and pressure are both increased from STP to be at or above
the
critical point for CO2, CO2 can adopt properties some where between a gas and
a liquid.
More specifically, super critical CO2 behaves as a super critical fluid above
its critical
temperature (31.1 C) and critical pressure (73 atm), expanding to fill its
container like a
gas but with a density like that of a liquid.
[0022] Consequently, C02 (both a and n) is different from gases from a
transport
and infrastructure perspective. Specifically, CO2 (a and n) in the pure or
mixed gas
state needs to be caused to enter a super critical state. More particularly a
means for
causing CO2 to enter a super critical state such as a compressor is used to
cause the
C02 to achieve a "super critical" state. In the super critical state, CO2
behaves like a
liquid. And, like most liquids, super critical state CO2 can be passed through
a series of
pumps where the pressure of the super critical state CO2 can be increased at
each
pump. However, the CO2 gas must first be compressed or otherwise cooled to
reach a
super critical state. New process technologies are providing methods by which
the
amount of compression required for CO2 to reach its super critical state is
minimized
through cryogenic processing. In cryogenic processing, a C02 gas/mix is passed
through cryogenic units whereupon the density of the CO2 gas/mix increases and
less
CA 02758529 2011-10-12
WO 2010/120876 PCT/US2010/031026
compression of the CO2 gas is subsequently required. It is important to note
that
reaching the "super critical state" is the key transport attribute.
(0023] Once CO2 in its super critical state, the CO2 gas/mix is usually run
through pumps to increase its pressure up to 2000 psi, and in some cases
higher
pressures, depending on the pressure rating of the transport pipe or tank.
Pumps for
the CO2 gas/mix are generally inexpensive to operate. Therefore, the movement
of
super critical CO2 has comparatively lower overall transportation costs than
C02 gas
while still enabling transport and delivery to an injection point - especially
for CO2EOR
use and geologic sequestration at a much higher pressure. It is this key
characteristic
of C02a and C02n upon which the system and method of the present invention is
predicated. Once in its super critical state, the C02 may be moved in a means
for
transport such as a pipeline or in tanks on a transport vehicle to the
location of the
heavy hydrocarbon. Heretofore, this important transportation characteristic of
CO2 in a
super critical state has not been used with a heavy hydrocarbon, such as
bitumen.
[0024] Disclosed System and Method
[0025] According to the system and method of the present invention, C02 from
any source (either anthropogenic or natural), in either pure or mixed gas
form, is
compressed or otherwise taken to a super critical state. The super critical
state CO2 is
then used as a carrying or suspension agent for the transport of bitumen
and/or other
heavy hydrocarbons via a pipeline or other transportation means (even
including
petcoke where CO2 could serve as a carrying or suspension agent or what is
more
commonly referred to for petcoke to be a "slurry" mix). The super critical
state CO2 is
added to the very heavy hydrocarbons, in either of the following combinations:
CA 02758529 2011-10-12
WO 2010/120876 PCT/US2010/031026
100% CO2 - pure "carbonbit"; or
a predetermined mixture consisting of some percentage of either condensate,
NGLs, or other very light hydrocarbon or non-hydrocarbon fluids - wherein the
super critical state CO2 constitutes a minimum of 5% or greater of the total
added
carrying or suspension agent - either by volume or by weight.
[0026] Specific processing techniques will vary depending on site, operation,
and existing process facilities as to how the super critical state C02 and
heavy
hydrocarbon, such as bitumen, and possibly other chemical products are
commingled
and injected as a combination into the pipeline for transport. Such means for
co-
mingling the super critical state CO2 and the heavy hydrocarbons are well
known to
those of ordinary skills in the art.
[0027] Regardless of the specific engineered process, the energy savings along
with the market and economic efficiencies will be significant. By using super
critical
state CO2 as a carrying or suspension agent in the dilbit (e.g. "carbit" with
C02 or
carbon) or even as partial replacement by volume of current diluents, an
inexpensive
technique/process will exist by which C02a/ C02n may be transported for CO2
FOR
and/or geologic sequestration in approved aquifers and formations.
[0028] The newly constituted dilbit including C02a or C02n and a heavy
hydrocarbon is then moved through a means for transport such as a pipeline or
in tanks
on a transport vehicle to a final delivery destination. Upon receipt at the
final delivery
destination, the pipe containing the combination of super critical state CO2
and the
heavy hydrocarbon will be depressurized so that the combined super critical
state CO2
and heavy hydrocarbon would return to a dual gas-liquid phase. At that point,
the C02
CA 02758529 2011-10-12
WO 2010/120876 PCT/US2010/031026
would be separating from the heavy hydrocarbon. The heavy hydrocarbon would
drop
out of formation and be processed at the processing destination or upgrader.
The
separated C02, while in a gaseous phase, would be handled to maintain as high
a
pressure rating as possible. Maintaining a high pressure rating enables
inexpensive re-
compression and/or pumping of the CO2 back to a super critical state wherein
the super
critical state CO2 would be transported via a pipeline or tank to oil and/or
gas fields for
EOR/EGR, coal fields for enhanced coal bed methane, or saline
reservoirs/dormant
oil/gas fields or other geological formations where the CO2 can be permanently
sequestered and/or stored.
[0029] The complete process would then result in a full carbon cycle from (i)
initial production/mining or processing of the heavy hydrocarbon in the
extraction phase
to; (ii) pre-transport processing phase, to (iii) a final storage or
sequestration phase.
[0030] While the system and method of the disclosed invention has been
disclosed according to its preferred and alternate embodiments, those of
ordinary skill in
the art will understand that modifications may be made to the disclosed
invention
without departing form the system and method of the present invention. Such
modifications shall be included within the scope and meaning of the appended
claims.