Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
1
Method of culturing photosynthetic organisms
Field of the invention
The present invention relates to cultivation chambers and methods for the
production of
photosynthetic organisms, in particular microalgae.
Background of the invention
The culturing of photosynthetic organisms, particularly microalgae and
cyanobacteria,
has become the focus of much interest due to the multiple applications for
such
microorganisms. Firstly, the culturing of photosynthetic microorganisms can
utilise
waste carbon dioxide (COO) and nutrients (for example from sewerage or
agriculture
outputs) and, in the presence of light, convert these into biomass. Secondly,
the
produced biomass has the potential for a multitude of uses including: the
extraction of
oils, which may then be converted into biodiesel; as raw materials for the
bioplastics
industry; to extract nutraceutical, pharmaceutical and cosmetic products; for
animal feed
and as feedstock for biodiesel, pyrolysis and gasification plants.
Algae, such as microalgae, may be cultivated in both open and closed systems.
The
open systems include ponds and raceways or canals and the closed systems
include
photobioreactors made up, of enclosed tubes or other housings which allow
light to
penetrate to the medium containing the algae.
The open systems have the advantage of generally being cheaper to set up than
the
closed systems. However, the fact that these systems are open to the
environment
produces problems with lack of temperature control and potentially a greater
risk that
the culture becomes contaminated with undesirable organisms. Furthermore, the
mixing of the culture medium to maintain the distribution of nutrients and
gases may be
more difficult in an open pond or raceway system.
While there is greater control over temperature and nutrient supply in closed
photobioreactor systems, these types of systems often have the disadvantage of
high
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
2
costs to set up and a lack of flexibility. There are also difficulties in
efficiently
maintaining a suitable mixing and nutrient/temperature distribution in closed
bioreactor
systems.
The present invention aims to address one or more of the difficulties of the
systems
known in the art for culturing photosynthetic organisms, particularly
microalgae.
Summary of the invention
In a first aspect, the present invention provides a cultivation chamber for
the culture of
photosynthetic organisms including:
(a) a wall or walls defining:
a gas space; and
a culture medium containment area below the gas space;
(b) one or more gas inlets positioned within the culture medium containment
area
such that, in use, gas passes through the culture medium; and
(c) one or more gas outlets in communication with the gas space;
the cultivation chamber permitting exposure of the culture medium to natural
light and/or
including an artificial light source.
In a further aspect, the present invention provides a method of cultivating
photosynthetic
organisms including the steps of:
(a) providing a cultivation chamber including:
(i) a wall or walls defining:
a gas space; and
a culture medium containment area below the gas space;
(ii) one or more gas inlets positioned within the culture medium containment
area such that, in use, gas passes through the culture medium; and
(iii) one or more gas outlets in communication with the gas space;
(b) introducing into the cultivation chamber a culture medium and an inoculate
of
photosynthetic organisms, the cultivation chamber permitting exposure of the
culture medium to natural light and/or including an artificial light source;
(c) introducing gas through the gas inlets and allowing gas to pass out
through the
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
3
gas outlets, wherein the flow of gas thereby mixes the culture medium; and
(d) allowing the photosynthetic organisms to grow in the presence of light.
Detailed description
Gas may be passed into the cultivation chamber via the gas inlets. Where the
gas
inlets are situated below the surface of the culture medium containing the
photosynthetic organisms, gas may be bubbled into the culture medium. The
introduction of gas below the surface of the culture medium allows for the
mixing of the
culture medium and assists in the distribution of the gases, nutrients, light
and heat
throughout the culture medium. In a preferred embodiment, the gas is
introduced in a
substantially continuous manner while the photosynthetic organisms are
photosynthesising (in the presence of light).
In a preferred embodiment, the gas inlets are positioned along a base portion
of the
culture medium containment area.
In one embodiment, one or more walls of the cultivation chamber are composed
of a
flexible material, which may allow for the inflation of the cultivation
chamber. Such a
flexible material includes, but is not limited to, a plastic-type film. In a
preferred
embodiment, the cultivation chamber is in the form of an enclosed flexible
plastic
structure, such as a plastic bag-type structure.
In a preferred embodiment, one or more walls of the cultivation chamber are
light-
transmissible.
Where the cultivation chamber is inflatable, the inflation of the cultivation
chamber may
be maintained by the flow of gas achieved through the introduction of gas into
the
chamber through the gas inlets and out through the gas outlets. The gas may be
introduced into the cultivation chamber through one or more gas inlets
positioned above
and/or below the surface of the culture medium.
Accordingly, in a further aspect, the present invention provides a method of
cultivating
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
4
photosynthetic organisms including the steps of:
(a) providing an inflatable cultivation chamber including:
(i) a wall or walls defining:
a gas space; and
a culture medium containment area below the gas space;
(ii) one or more gas inlets positioned within the culture medium containment
area such that, in use, gas passes through the culture medium; and
(iii) one or more gas outlets in communication with the gas space;
(b) introducing into the cultivation chamber a culture medium and an inoculate
of
photosynthetic organisms, the cultivation chamber permitting exposure of the
culture medium to natural light and/or including an artificial light source;
(c) introducing gas through the gas inlets and allowing gas to pass out
through the
outlets, wherein the flow of gas mixes the culture medium and maintains the
cultivation chamber in an inflated state; and
(d) allowing the photosynthetic organisms to grow in the presence of light.
Preferably a number of gas inlets are positioned throughout the cultivation
chamber. In
a preferred embodiment, the gas inlets are positioned at the base of the
cultivation
chamber, preferably along the length of the base of the cultivation chamber.
In a further preferred embodiment, the gas inlets are positioned along
conduits at the
base of the cultivation chamber, wherein the conduits are adapted to carry and
distribute the flow of gas. The gas inlets are preferably positioned along the
conduits at
intervals that allow for a substantially even distribution of gas flow along
the length of an
elongated cultivation chamber.
In a further embodiment, the gas outlets are designed to release excess gas
pressure
that may build up in the flexible cultivation chamber.
In a preferred embodiment, the gas outlets may include a valve system,
preferably a
one-way valve system. The use of one-way valves may reduce the risk of
contamination of the cultivation chamber from outside air.
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
In a further embodiment, gas is passed into the cultivation chamber through
gas inlets
both above the surface of the culture medium and below the surface of the
culture
medium. The introduction of the gas above the surface of the culture medium
allows for
a modification of the atmosphere in the cultivation chamber.
5 Suitable gases and/or liquid nutrients may be introduced into the
cultivation chamber of
the present invention to aid the growth of the photosynthetic organisms. Such
gases or
liquids may be selected from carbon dioxide (C02); fertilisers and waste from
aquaculture and agriculture (for example: trout, salmon, cattle, pig and
chicken farms).
The CO2 may be from any suitable source and may be from air or in a
concentrated
form. Examples of suitable concentrated sources of CO2 include, but are not
limited to,
flue gases, kiln and incineration gases and gases from anaerobic digestion. In
a
preferred embodiment, the source of CO2 is a flue gas, more preferably
desulphurised
flue gas (DFG).
The concentration of CO2 introduced into the cultivation chamber may be varied
by
varying the amount of air mixed with the CO2. For example, CO2-containing flue
gas
may be diluted with air, depending on the CO2 requirements of the
photosynthetic
organisms. During periods of darkness, for example at night when natural light
is used,
the amount of CO2 may be decreased while maintaining a constant gas flow by
increasing the amount of air in the mixture. The air to be mixed with the CO2
source
may be filtered to remove certain particulate matter, for example using a
particulate air
filter, more preferably a high efficiency particulate air (HEPA) filter.
The culture medium may be any suitable medium for the growth of the desired
photosynthetic organisms. The culture medium may be based on fresh or saline
water
and may include waste water from industrial processes or sewerage treatment
systems.
A source of light is required for the organisms to photosynthesise. Any
suitable source
of light may be used including natural light and artificial light or a
combination of natural
and artificial light. Artificial light may be provided by any suitable light
source. In one
embodiment, the artificial light source is provided by light-emitting diodes
(LEDs). An
artificial light source may be provided to extend the length of time per day
that the
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
6
organisms continue to photosynthesise beyond daylight hours. Accordingly, in
one
embodiment, the cultivation chambers and methods of the present invention are
adapted to alternate between using natural and artificial light as required.
In a further aspect, the present invention provides a method of cultivating
photosynthetic
organisms including the steps of:
(a) providing a cultivation chamber including:
(i) a wall or walls defining:
a gas space; and
a culture medium containment area below the gas space;
(ii) one or more gas inlets and one or more gas outlets in communication with
the gas space; and
(iii) a gas flow control means ;
(b) introducing into the cultivation chamber a culture medium and an inoculate
of
photosynthetic organisms, the cultivation chamber permitting exposure of the
culture medium to natural light and/or including an artificial light source;
(c) controlling the flow of gas in through the gas inlets and out through the
gas
outlets using the gas flow control means, wherein the flow of gas drives
evaporation from the culture medium; and
(d) allowing the photosynthetic organisms to grow in the presence of light.
The gas flow control means is preferably a fan. The fan is preferably situated
at one
end of an elongate cultivation chamber.
In a preferred embodiment, the cultivation chamber is inflatable. In a further
embodiment the flow of gas maintains the cultivation chamber in an inflated
state.
It has been found that evaporation from the culture medium may enable a
control of the
temperature within the cultivation chamber. This assists in the culture of the
microorganisms, as temperature control is important to gaining optimal growth
and/or
optimal production of the relevant chemicals by the microorganisms (such as
triglycerides as ingredients for biodiesel).
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
7
To counteract any unwanted salinity increases associated with the evaporation
of the
water from the culture medium, additional water may be added to the culture
medium.
This additional water may be in any suitable form, for example as fresh water
or
aquaculture waste water.
A further mechanism for controlling the temperature of the culture medium
using the
method of the present invention is to control the rate at which gas is
introduced into the
cultivation chamber and/or the temperature of the introduced gas. For example,
heat
loss at lower than optimal ambient temperatures may be reduced by lowering the
amount of gas at a temperature lower then ambient temperature being introduced
into
the culture medium, thereby reducing the mixing of the medium and resultant
heat
exchange. Accordingly, in darkness the gas flow may be reduced and may be
stopped
completely to at least partially maintain the temperature of the cultivation
medium during
low night time ambient temperatures.
Furthermore, by varying the temperature and composition of the gas introduced
into the
cultivation chamber the temperature of the liquid culture medium may be
varied. For
example, if using enriched CO2 from flue gas as an input, the flue gas may be
maintained at a higher temperature to counteract the effect of low ambient
temperatures. Accordingly, the flue gas may be introduced at a higher
temperature
where the temperature of the culture medium needs to be increased. Conversely,
where the temperature of the liquid culture medium needs to be reduced, the
flue gas
may be cooled further before introducing to the cultivation chamber.
Alternatively, increasing the amount of air introduced into the cultivation
chamber may
aid in the cooling of the cultivation medium. This air may be introduced by
bubbling
from under the surface of the cultivation medium or by being passed over the
surface of
the cultivation medium.
Alternatively, the temperature of the cultivation medium may be controlled by
circulating
it directly or indirectly over a suitable heat exchanger, such a cooling tower
or a boiler.
The cultivation chamber may be of any suitable size to cultivate the required
amount of
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
8
the photosynthetic organisms.
In a preferred embodiment, the cultivation chamber may be about 1 metre to
about 10
metres, more preferably about 2 metres to about 6 metres in width. In a
particularly
preferred embodiment, the cultivation chamber of the present invention is
about 3
metres in width.
In a further preferred embodiment, the cultivation chamber is about 5 metres
to about
250 metres, more preferably about 10 metres to about 100 metres in length. In
a
particularly preferred embodiment, the cultivation chamber is about 50 metres
in length.
The culture medium can be present in the cultivation chamber in any suitable
volume for
the optimal cultivation of the photosynthetic organisms. In a preferred
embodiment, the
volume of culture medium is such that it is present in the cultivation chamber
to a depth
of about 20 centimetres to about 120 centimetres, more preferably about 30
centimetres
to about 100 centimetres. In a particularly preferred embodiment, the culture
medium is
at a depth of about 60 centimetres in the cultivation chamber.
In one embodiment, the level of the culture medium in the cultivation chamber
is
controlled by the regulation of the intake of culture medium through one or
more liquid
ports, which act as inlets and/or outlets for the passage of liquid in to and
out of the
cultivation chamber. The passage of the culture medium through the liquid
ports in one
or both directions is preferably regulated by one or more valves, which are
reactive to
the level of the culture medium in the cultivation chamber, thereby allowing
for the
emptying (for example, for harvesting the photosynthetic organisms) and
refilling of the
cultivation chamber. In a preferred embodiment the valves are ball valves.
In a further embodiment, the level of the culture medium in the cultivation
chamber is
measured by means of one or more pressure sensors.
The photosynthetic organisms may be selected from any suitable organisms and
may
be cultured as a single species in monoculture or two or more species in the
same
cultivation chamber. Photosynthetic organisms that produce useful ingredients
for the
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
9
chemical, biodiesel, pharmaceutical or nutraceutical industries are preferred.
Suitable
photosynthetic microorganisms include cyanobacteria (blue-green algae) and
algae,
preferably microalgae. The microorganisms may grow in fresh or salt water.
Examples
of photosynthetic microorganisms that may produce useful
ingredients/feedstocks
include, but are not limited to, those belonging to the following genera:
Chlamydomoas;
Chaetoceros; Cladophora; Chaetomorpha; Dunaliella; Haematococcus; Isochrysis;
Nannochloropsis; Porphyridum; Picochlorum (synonym Nannochloris);
Pleurochrysis;,
Rhodomoas, Spriulina. The method of the present invention may also be utilised
to
culture macroalgae, for example those of the genus Ulva.
The photosynthetic organisms produced according to the present invention have
a
number of potential uses. Oil (e.g. triglycerides) may be extracted from the
microorganism and this oil may be used for: biodiesel production (e.g., using
known
transesterification processes); as a raw material for the production of
plastics and for
the synthesis of jet and other fuels. The cake component of the biomass that
is left after
the extraction of oil may be used as: feed for the livestock industry;
fertilizer production;
biomass for bio-plastic production or biomass for energy production and/or
pyrolysis.
Photosynthetic organisms may also produce other useful products, such as
nutraceuticals (e.g. omega 3 and 6 fatty acids; antioxidants, such as
astaxanthin and
pigments, such as 13-carotene), phycocolloids, triglycerides and other
ingredients for the
pharmaceutical and cosmetics industries.
Accordingly, in a further aspect the present invention provides a product
extracted from
photosynthetic organisms produced in accordance with the method of the present
invention. In one embodiment, the product is selected from the group
consisting of an
oil; glycerol; omega 3 and 6 fatty acids; astaxanthin; and 13-carotene. In
another
embodiment, the product is biomass cake, such as algae cake.
Where the cultivation chamber is inflatable, gas outlets may be provided above
the level
of the cultivation medium to release the pressure that is built up through the
gas which
has been bubbled through the cultivation medium, for example from the base of
the
cultivation chamber.
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
Accordingly, in a further aspect, the present invention provides an inflatable
cultivation
chamber for the culturing of photosynthetic organisms including:
(a) a wall or walls defining:
a gas space; and
5 a culture medium containment area below the gas space;
(b) one or more gas inlets positioned within the culture medium containment
area
and one more gas outlets in communication with the gas space;
(c) wherein, in use, gas passes through the culture medium and into the gas
space
causing the chamber to inflate; and
10 (d) wherein, in use, excess gas passes out through the gas outlets;
the cultivation chamber permitting exposure of the culture medium to natural
light and/or
including an artificial light source.
In one embodiment, the cultivation chamber includes one or more liquid ports
to allow
for the introduction and removal of the culture medium. Preferably, the one or
more
liquid ports include a regulation means to control the introduction and
removal of the
culture medium from the cultivation chamber. More preferably, the regulation
means is
a valve, such as a ball valve.
The cultivation chambers of the present invention may be joined together to
form a
series of cultivation chambers in a continuous system for the cultivation of
the
photosynthetic organisms. Any suitable number of cultivation chambers may be
connected via a manifold, whereby the movement of culture medium and gas may
be
centrally regulated for each manifold. The central regulation may be achieved,
for
example, by an automated valve that controls the flow of the culture medium. A
central
regulation of the level of culture medium in the cultivation chambers may also
be
achieved by using one or more pressure sensors to monitor the level of the
culture
medium, which can then allow an automated response to fill or empty the
cultivation
chambers to the required level.
A manifold may be utilised to flow connect any suitable number of cultivation
chambers
according to the present invention. Preferably 10 to 200 cultivation chambers
may be
joined; more preferably 20 to 60.
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
11
Photosynthetic organisms convert carbon dioxide, water and nutrients into
biomass in
the presence of light. Therefore, the growth of these photosynthetic organisms
enables
carbon dioxide emitted as, for example, flue gas from a power plant, refinery
or cement
kiln, liquid natural gas production or coal seam gas, to be recycled as
biomass rather
than being released into the atmosphere.
Accordingly, in a further aspect the present invention provides a method for
the
conversion of carbon dioxide to algal biomass including the steps of:
(a) providing a cultivation chamber including:
(i) a wall or walls defining:
a gas space; and
a culture medium containment area below the gas space;
(ii) one or more gas inlets positioned within the culture medium
containment area such that, in use, gas passes through the culture
medium; and
(iii) one or more gas outlets in communication with the gas space;
(b) introducing into the cultivation chamber a culture medium and an inoculate
of algae, the cultivation chamber permitting exposure of the culture
medium to natural light and/or including an artificial light source;
(c) introducing carbon dioxide-containing gas through the gas inlets and
allowing excess gas to pass out through the gas outlets, wherein the flow
of gas thereby produced mixes the culture medium; and
(d) allowing the algae to grow in the presence of light to produce algal
biomass.
In a further aspect, the present invention provides a method of recycling
emitted carbon
dioxide by utilising the emitted carbon dioxide as an input in the production
of
photosynthetic organisms using the method of the present invention. The
emitted
carbon dioxide may be flue gas, kiln gas, incineration gas and gas from
anaerobic
digestion.
Where the C02 is provided from flue gas, the flue gas is preferably cooled and
partly
scrubbed of pollutants such as SOx and NOx, dust, heavy metals etc before it
is
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
12
introduced into the cultivation chamber. Heavy metals, SOx and NOx and dust
remaining in the flue gas after partial scrubbing may provide micronutrients
required for
the growth of the photosynthetic organism. Such micronutrients may then be
added to
the culture medium, either directly or with additional treatment, e.g. the
selective
removal of heavy metals.
In the figures:
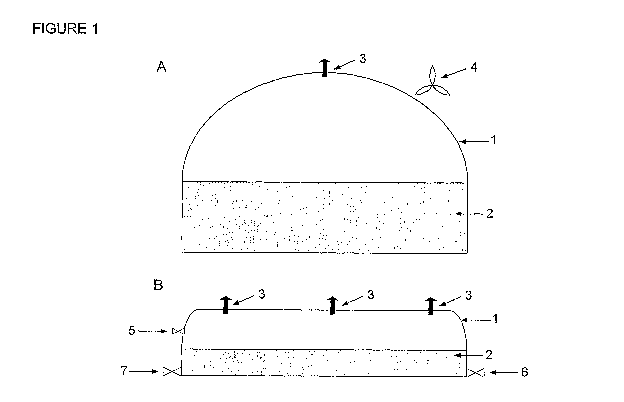
Figure 1: (A) Bag cultivation chamber 1 front view and (B) side view,
including: a
flexible bag (1) containing a culture medium for growing algae (2); gas outlet
(3); fan (4);
gas inlet (5); cultivation medium outlet (6); and cultivation medium inlet
(7).
Figure 2: (A) Bag cultivation chamber 2 front view and (B) side view,
including: a
flexible bag (1) containing a culture medium for growing algae (2); gas outlet
(3);gas
bubbling tracks (4) with pinprick holes (5); gas inlets (6); cultivation
medium outlet (7);
cultivation medium inlet (8); draining outlet (9) and ball valve (10) to
regulate ports (7),
(8) and (9).
Figure 3: (A) Bag cultivation chamber 3 front view and (B) side view,
including: a
flexible bag (1) containing a culture medium for growing algae (2); gas outlet
(3);gas
bubbling tracks (4) with pinprick holes (5); gas inlets (6); cultivation
medium outlet (7);
cultivation medium inlet (10) with pressure sensor (8) and ball valve.
Figure 4: Top view of gas bubbling tracks (5) in-the base of a bag cultivation
chamber
having a gas inlet (1) with compression fitting (2), a conduit (3) to
transport the gas to
restrictive flow orifices (4) and the end of each track (5) and pinprick holes
(6) to allow
the exit of gas.
Figure 5: Cell density (cells mL-1) from day 1 (inoculation) to day 20.
Average
standard deviation, n = 3.
Figure 6: Time course of nutrient concentrations in the bag cultivation
chamber. A)
nitrite, B) nitrate (red squares) and phosphate (black triangles). Average
standard
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
13
deviation, n = 3.
Figure 7: Fluctuation of A) pH, B) temperature, and C) conductivity over
culture time of
Nannochloropsis oculata in the bag. WP-81: handheld TPS pH- and conductivity-
meter,
manual: handheld thermometer.
Example 1
A chamber for the cultivation of photosynthetic organisms was created using a
bag
culture system as shown in Figure 1.
The operation of bag cultivation chamber 1 is as follows:
1. A fan (4) inflates the empty cultivation chamber (without culture medium
(2)) to
operational volume, with all excess pressure exiting through the gas outlet
(3). The
fan is continuously running so as to ensure the bag (1) does not deflate.
2. The empty cultivation chamber is inoculated with 10 000 I of microalgae
culture
(0.2% algae) produced in a separate photobioreactor and topped up with 10 000
I
filtered and treated recycled saline waste water.
3. CO2 is injected continuously during daylight hours through the gas inlet
(5). The
microalgae absorb the required quantities of CO2 and the excess is released
through
the always open gas outlet (3).
4. An additional 20 000 I of recycled saline waste water is added, bringing
the total
capacity to 40 000 I of culture medium.
5. This process continues for another 24 hours until total harvesting capacity
reaches
100 000 I. At this stage, the level of the culture medium (2) in the
cultivation
chamber is 60 cm.
6. After the algae have reached maximum harvest capacity (72 hours), 50 0000 I
is
harvested from the cultivation medium outlet (6).
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
14
7. 50 000 I of recycled saline waste water is returned to the cultivation
chamber via the
cultivation medium inlet (7), bringing the total culture medium volume back to
100
0001.
8. The harvesting and return cycle repeats once every 24 hours, while
maintaining
continuous CO2 injection during daylight hours.
Example 2
The bag cultivation chamber described in Example 1 was modified as shown in
Figure
2.
The operation of bag cultivation chamber 2 is as follows:
1. Cultivation chamber 2 inoculation follows the same procedure as Example 1
steps 2,
4 and 5 to bring the harvesting capacity to 100 000 I within 72 hours.
2. CO2 is pre-mixed with a high efficiency particulate air (HEPA) filtered air
stream and
fed through gas inlets (6) to gas bubbling tracks (4). These tracks are
pinpricked (5)
at suitable intervals to allow even air/CO2 distribution along the length of
the bag
cultivation chamber. This bubbling operates continuously, with the CO2
component
reduced overnight.
The air/C02 injection acts to slowly inflate the bag (1) and maintain
circulation of the
algae in the culture medium (2). Excess pressure is released through the one-
way
valve regulated gas outlet (3). This creates a closed loop system to minimise
the
contamination risk.
3. After the 72 hours of culturing the microalgae, 50 0001 is harvested from
the ball-
valve-regulated (10) cultivation medium outlet (7), which is positioned at 30
cm in
height. Once the cultivation medium reaches 30 cm, a signal is sent to the
automation system that the cultivation chamber is at 50 0001 capacity.
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
4. 50 0001 of treated recycled saline waste water is returned to the
cultivation chamber
via the ball valve-regulated (10) cultivation medium inlet (8), sending a back
pressure signal to the automation system that the cultivation chamber is now
at 100
0001.
5 5. The ball valve-regulated (10) draining outlet (9) allows the complete
draining of
cultivation chamber in case of contamination or for a regular cleaning
routine. The
remaining cultivation medium is either drained to the harvesting system for
processing or, in the case of contamination, to the UV treatment system.
Example 3
10 The bag cultivation chamber described in Example 1 was further modified as
shown in
Figure 3.
The operation of bag cultivation chamber 3 is as follows:
1. Cultivation chamber 3 inoculation follows the same procedure as Example 1
steps 2,
4 and 5 to bring the harvesting capacity to 100 000 I within 72 hours.
15 2. CO2 is pre-mixed with a high efficiency particulate air (HEPA) filtered
air stream and
fed through gas inlets (6) to gas bubbling tracks (4). These tracks are
pinpricked (5)
at suitable intervals to allow even air/CO2 distribution along the length of
the
cultivation chamber. This bubbling operates continuously, with the CO2
component
reduced overnight.
The air/CO2 injection acts to slowly inflate the bag (1) and maintain
circulation of the
algae in the culture medium (2). Excess pressure is released through the one-
way
valve regulated gas outlet (3). This creates a closed loop system to minimise
the
contamination risk.
3. After the 72 hours of culturing the microalgae, 50 0001 is harvested from
the
cultivation chamber through the ball valve-regulated harvesting outlet (7).
The
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
16
required volume is determine by measuring volume in reference to a pressure
head
sensor (8).
4. 50 0001 of treated recycled saline waste water is returned to the
cultivation chamber
via the ball valve-regulated (9) cultivation medium inlet (10) with pressure
head
sensor (8), sending a back pressure signal to the automation system that the
cultivation chamber is now at 100 0001.
Further detail (in top view) of a gas bubbling setup that may be included in
the modified
cultivation chamber is provided in Figure 4. This figure shows six gas
bubbling tracks
(5) with pinprick holes (6) which are fed with gas introduced through the gas
inlet (1)
and compression fitting (2) via a gas conduit (3) and restrictive flow
orifices (4) at the
end of each track. The restrictive flow orifices serve to divide the gas flow
evenly
between the air distributor vanes. The flow rate of the gas through the gas
inlet is
approximately 100 kg/hr and through the restrictive flow orifice 17 kg/hr.
Example 4
The growth of the microalga Nannochloropsis oculata was tested using the bag
cultivation chamber described in Example 1. This culture bag was 10m in length
and
3m in width and fitted with a six-bladed fan at one end to keep the bag
inflated and drive
evaporation. Along the top of the bag, four holes (13 cm diameter) allowed hot
air and
vapor to escape. This evaporation assisted in maintaining the algal culture at
more
stable temperatures.
In this trial, both freshwater and filtered marine aquaculture waste (A3)
water was added
to the culture to account for salinity increases and evaporative loss of
liquid. The bag
cultivation chamber was filled to approximately 0.30 m in depth, resulting in
a final
culture volume of slightly less than 9 m3. The algae were cultivated in sea
water than
had been filtered through 20 pm, 5pm and 1 pm filters.
Aeration and C02 enrichment was provided through tubing designed for the gas
diffusion via delivery into liquid media. This tubing had an outer diameter of
25 mm, an
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
17
inner diameter of 10 mm and a porous wall of 7.5 mm thickness.
The bag cultivation chamber system was inoculated with Nannochloropsis oculata
to a
comparatively low cell density of 2.1 x 104 cells mL"1 and not filled up to
full capacity
volume. Already after 24h, cell densities had increased dramatically, and the
bag was
filled up to its maximum depth on day 2. The growth of the culture up to the
harvest of
the algae on day 20 is shown in Figure 5.
Nutrient consumption
There was a steady increase in nitrite from the day of inoculation (0.5 mg L-)
to day 8
(2.5 mg L-1) (Figure 6 A). After a few days at a steady concentration, nitrite
peaked at
3.7 mg L"1 on day 13, and then was rapidly utilized. Within a few days,
nitrite was
depleted and remained so until the culture crashed. Nitrate was a high 90 mg L-
1 at the
beginning of the period, and was steadily being utilized (Figure 6 B). From
day 13,
nitrate concentration remained stable around 10 mg L-1. There was an increase
in
phosphate the first few days (through addition of filtered A3 water to top the
system up)
(Figure 6 B). From day 3, phosphate was being noticeably assimilated and
fluctuated
between 2 mg L-1 and totally deplete. No nutrients were added to the bag
system,
however fresh filtered A3 water was regularly added along with freshwater to
compensate for evaporation. The added A3 water accounts for the regular, small
increases in nutrient concentrations.
Physical and chemical parameters
In the culture, pH quickly rose to over 9 in the first three days (Figure 7).
After day 3, a
CO2 supply was connected and pH could now be regulated by adding CO2 when a
value
above 8.4 was recorded.
Photosynthetic activity was high in the bag in the beginning of the period,
with rapid
changes in pH due to uptake of CO2 during photosynthesis, leading to large
fluctuations
in pH.
CA 02760336 2011-10-24
WO 2010/132917 PCT/AU2009/001255
18
Temperature fluctuated in diet rhythm, with the highest temperatures measured
in the
afternoon (4pm) (Figure 7 B). Similar to the tank system, temperatures rarely
rose
above 30 C, and were quite stable.
Conductivity in the bag fluctuated between 32 and 36 mS due to evaporation,
and both
freshwater and additional filtered A3 water was regularly added (Figure 7 C).
It will be understood that the invention disclosed and defined in this
specification
extends to all alternative combinations of two or more of the individual
features
mentioned or evident from the text or drawings. All of these different
combinations
constitute various alternative aspects of the invention.