Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02765409 2012-01-19
54236-1D
- 1 -
HYDROXAMIC ACID COMPOUNDS COMPRISING A SULFONAMIDE LINKAGE
AS HDAC INHIBITORS
This is a division of Canadian Patent Application Serial No. 2,423,744 filed
on
September 27, 2001.
It is to be understood that the expression "the present invention" or the like
used in
this specification encompasses not only the subject-matter of this divisional
application but that of the parent also.
TECHNICAL FIELD
This invention pertains generally to the field of biologically active
compounds, and
more specifically to certain active hydirixamic acid compounds which inhibit
HDAC
(histone deacetylase) activity. The present invention also pertains to
pharmaceutical compositions comprising, such compounds, and the use of such
compounds and compositions, both in vitro and in vivo, to inhibit HDAC, and,
e.g.,
to inhibit proliferative conditions, such as cancer and psoriasis.
BACKGROUND
DNA in eukaiyotic cells is tightly complexed with proteins (histones) to form
chromatin. Hisiones are small, positively charged proteins which are rich in
basic
amino acids (positively charged at physiological pH), which contact the
phosphate
groups (negatively charged at physiological pH) of DNA. There are five main
classes of histones, H1, H2A, H2B, H3, and H4. The amino acid sequences of
histones H2A, H2B, H3, and H4 show remarkable conservation between species,
whereas H1 varies somewhat, and in some cases is replaced by another histone,
e.g., H5. Four pairs of each of H2A, H26,113, and H4 together form a disk-
shaped octomeric protein core, around which DNA (about 140 base pairs) is
wound to form a nudeosome. Individual nucleosomes are connected by short
CA 02765409 2012-01-19
WO 02/30879 PCT/G B01/04326
- 2 -
stretches of linker DNA associated with another histone molecule (e.g., H1, or
in
certain cases, H5) to form a structure resembling a beaded string, which is
itself
arranged in a helical stack, known as a solenoid.
The majority of histones are synthesised during the S phase of the cell cycle,
and
newly synthesised histones quickly enter the nucleus to become associated with
DNA. Within minutes of its synthesis, new DNA becomes associated with
histones in nucleosomal structures.
A small fraction of histones, more specifically, the amino side chains
thereof, are
enzymatically modified by post-translational addition of methyl, acetyl, or
phosphate groups, neutralising the positive charge of the side chain, or
converting
it to a negative charge. For example, lysine and arginine groups may be
methylated, lysine groups may be acetylated, and serine groups may be
phosphorylated. For lysine, the -(CH2)4-NH2 sidechain may be acetylated, for
example by an acetyltransferase enzyme, to give the amide
-(CH2)4-NHC(=0)CH3. Methylation, acetylation, and phosphorylation of amino
termini of histones which extend from the nucleosomal core affects chromatin
structure and gene expression. (See, for example, Spencer and Davie, 1999).
Acetylation and deacetylation of histones is associated with transcriptional
events
leading to cell proliferation and/or differentiation. Regulation of the
function of
transcription factors is also mediated through acetylation. Recent reviews of
histone deacetylation include Kouzarides, 1999 and Pazin et al., 1997.
The correlation between the acetylation status of histones and the
transcription of
genes has been known for over 30 years (see, for example, Howe et al., 1999).
Certain enzymes, specifically acetylases (e.g., histone acetyltransferase,
HAT)
and deacetylases (e.g., histone deacetylase, HDAC), which regulate the
acetylation state of histones have been identified in many organisms and have
been implicated in the regulation of numerous genes, confirming the link
between
acetylation and transcription. See, for example, Davie, 1998. In general,
histone
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 3 -
acetylation correlates with transcriptional activation, whereas histone
deacetylation is associated with gene repression.
A growing number of histone deacetylases (HDACs) have been identified (see,
for
example, Ng and Bird, 2000). The first deacetylase, HDAC1, was identified in
1996 (see, for example, Tauton et al., 1996). Subsequently, two other nuclear
mammalian deacetylases has been found, HDAC2 and HDAC3 (see, for example,
Yang et al., 1996, 1997, and Emiliani et al., 1998). See also, Grozinger et
al.,
1999; Kao et al., 2000; and Van den Wyngaert et al., 2000.
Eight human HDACs have been cloned so far:
HDAC1 (Genbank Accession No. NP 004955)
HDAC2 (Genbank Accession No. NP 001518)
HDAC3 (Genbank Accession No. 015739)
HDAC4 (Genbank Accession No. AAD29046)
HDAC5 (Genbank Accession No. NP 005465)
HDAC6 (Genbank Accession No. NP 006035)
HDAC7 (Genbank Accession No. AAF63491)
HDAC8 (Genbank Accession No. AAF73428)
These eight human HDACs fall in two distinct classes: HDACs 1,2,3 and 8 are in
class I, and HDACs 4,5,6 and 7 are in class II.
There are a number of histone deacetylases in yeast, including the following:
RPD3 (Genbank Accession No. NP 014069)
HDA1 (Genbank Accession No. P53973)
HOS1 (Genbank Accession No. Q12214)
HOS2 (Genbank Accession No. P53096)
HOS3 (Genbank Accession No. Q02959)
There are also numerous plant deacetylases, for example, HD2, in Zea mays
(Genbank Accession No. AF254073_1).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 4 -
HDACs function as part of large multiprotein complexes, which are tethered to
the
promoter and repress transcription. Well characterised transcriptional
repressors
such as Mad (Laherty et al., 1997), pRb (Brehm et al., 1998), nuclear
receptors
(Wong et al., 1998) and YY1 (Yang et al., 1997) associate with HDAC complexes
to exert their repressor function.
The study of inhibitors of histone deacetylases indicates that these enzymes
play
an important role in cell proliferation and differentiation. The inhibitor
Trichostatin
A (TSA) (Yoshida et al., 1990a) causes cell cycle arrest at both G1 and G2
phases (Yoshida and Beppu, 1988), reverts the transformed phenotype of
different cell lines, and induces differentiation of Friend leukaemia cells
and others
(Yoshida et al., 1990b). TSA (and SAHA) have been reported to inhibit cell
growth, induce terminal differentiation, and prevent the formation of tumours
in
mice (Finnin et al., 1999).
Trichostatin A (TSA)
Me2N
Me Me
C OH
0 0
Suberoylanilide Hydroxamic Acid (SAHA)
0
N,
C OH
H II
Cell cycle arrest by TSA correlates with an increased expression of gelsolin
(Hoshikawa et al., 1994), an actin regulatory protein that is down regulated
in
malignant breast cancer (Mielnicki et al., 1999). Similar effects on cell
cycle and
differentiation have been observed with a number of deacetylase inhibitors
(Kim et
al., 1999).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 5 -
Trichostatin A has also been reported to be useful in the treatment of
fibrosis, e.g.,
liver fibrosis and liver cirrhosis. See, e.g., Geerts et al., 1998.
Recently, certain compounds that induce differentiation have been reported to
inhibit histone deacetylases. Several experimental antitumour compounds, such
as trichostatin A (TSA), trapoxin, suberoylanilide hydroxamic acid (SAHA), and
phenylbutyrate have been reported to act, at least in part, by inhibiting
histone
deacetylase (see, e.g., Yoshida et al., 1990; Richon et al., 1998; Kijima et
at.,
1993). Additionally, diallyl sulfide and related molecules (see, e.g., Lea et
at.,
1999), oxamflatin (see, e.g., Kim et at., 1999), MS-27-275, a synthetic
benzamide
derivative (see, e.g., Saito et al., 1999; Suzuki et al., 1999; note that MS-
27-275
was later re-named as MS-275), butyrate derivatives (see, e.g., Lea and
Tulsyan,
1995), FR901228 (see, e.g., Nokajima et at., 1998), depudecin (see, e.g., Kwon
et
at., 1998), and m-carboxycinnamic acid bishydroxamide (see, e.g., Richon et
at.,
1998) have been reported to inhibit histone deacetylases. In vitro, some of
these
compounds are reported to inhibit the growth of fibroblast cells by causing
cell
cycle arrest in the GI and G2 phases, and can lead to the terminal
differentiation
and loss of transforming potential of a variety of transformed cell lines
(see, e.g.,
Richon et at, 1996; Kim et at., 1999; Yoshida et at., 1995; Yoshida & Beppu,
1988). In vivo, phenybutyrate is reported to be effective in the treatment of
acute
promyelocytic leukemia in conjunction with retinoic acid (see, e.g., Warrell
et al.,
1998). SAHA is reported to be effective in preventing the formation of mammary
tumours in rats, and lung tumours in mice (see, e.g., Desai et at., 1999).
The clear involvement of HDACs in the control of cell proliferation and
differentiation suggest that aberrant HDAC activity may play a role in cancer.
The
most direct demonstration that deacetylases contribute to cancer development
comes from the analysis of different acute promyelocytic leukaemias (APL). In
most APL patients, a translocation of chromosomes 15 and 17 (t(15;17)) results
in
the expression of a fusion protein containing the N-terminal portion of PML
gene
product linked to most of RARa (retinoic acid receptor). In some cases, a
different
translocation (t(11;17)) causes the fusion between the zinc finger protein
PLZF
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 6 -
and RARa. In the absence of ligand, the wild type RARa represses target genes
by tethering HDAC repressor complexes to the promoter DNA. During normal
hematopoiesis, retinoic acid (RA) binds RARa and displaces the repressor
complex, allowing expression of genes implicated in myeloid differentiation.
The
RARa fusion proteins occurring in APL patients are no longer responsive to
physiological levels of RA and they interfere with the expression of the RA-
inducible genes that promote myeloid differentiation. This results in a clonal
expansion of promyelocytic cells and development of leukaemia. In vitro
experiments have shown that TSA is capable of restoring RA-responsiveness to
the fusion RARa proteins and of allowing myeloid differentiation. These
results
establish a link between HDACs and oncogenesis and suggest that HDACs are
potential targets for pharmaceutical intervention in APL patients. (See, for
example, Kitamura et at., 2000; David et at., 1998; Lin et al., 1998).
Furthermore, different lines of evidence suggest that HDACs may be important
therapeutic targets in other types of cancer. Cell lines derived from many
different
cancers (prostate, colorectal, breast, neuronal, hepatic) are induced to
differentiate by HDAC inhibitors (Yoshida and Horinouchi, 1999). A number of
HDAC inhibitors have been studied in animal models of cancer. They reduce
tumour growth and prolong the lifespan of mice bearing different types of
transplanted tumours, including melanoma, leukaemia, colon, lung and gastric
carcinomas, etc. (Ueda et al., 1994; Kim et at., 1999).
Psoriasis is a common chronic disfiguring skin disease which is characterised
by
well-demarcated, red, hardened scaly plaques: these may be limited or
widespread. The prevalence rate of psoriasis is approximately 2%, i.e.,
12.5 million sufferers in the triad countries (US/Europe/Japan). While the
disease
is rarely fatal, it clearly has serious detrimental effects upon the quality
of life of
the patient: this is further compounded by the lack of effective therapies.
Present
treatments are either ineffective, cosmetically unacceptable, or possess
undesired
side effects. There is therefore a large unmet clinical need for effective and
safe
drugs for this condition.
CA 02765409 2012-01-19
=
54236-1
- 7 -
Psoriasis is a disease of complex etiology. Whilst there is clearly a genetic
component, with a number of gene loci being involved, there are also undefined
environmental triggers. Whatever the ultimate cause of psoriasis, at the
cellular
level, it is characterised by local 1-cell mediated inflammation, by
keratinocyte
hyperproliferation, and by localised angiogenesis. These are all processes in
which histone deacetylases have been implicated (see, e.g., Saunders et al.,
1999; Bernhard et at, 1999; Takahashi et al, 1996; Kim et al , 2001).
Therefore
HDAC inhibitors may be of use in therapy for psoriasis. Candidate drugs may be
screened, for example, using proliferation assays with T-cells and/or
keratinocytes.
Thus, one aim of the present invention is the provision of compounds which are
potent inhibitors of histone deacetylases (HDACs). There is a pressing need
for
such compounds, particularly for use as antiproliferatives, for example,
anti-cancer agents, agents for the treatment of psoriasis, etc.
Such molecules desirably have one or more of the following properties and/or
effects:
(a) easily gain access to and act upon tumour cells;
(b) down-regulate HDAC activity;
(c) inhibit the formation of HDAC complexes;
(d) inhibit the interactions of HDAC complexes;
(e) inhibit tumour cell proliferation;
(e) promote tumour cell apoptosis;
(f) inhibit tumour growth; and,
(g) complement the activity of traditional chemotherapeutic agents.
A number of hytimxamic acid compounds have been described.
Amides
CA 02765409 2012-01-19
54236-1
- 8 -
Hashimoto et al., 1989 describe hydroxamic acid compounds which are claimed to
inhibit cell proliferation. Some of the compounds are hwiroxamic acid
compounds
having a substituted phenyl-diorie group linked to a hydrox3mic acid group
(-CONHOH) via an aryl-substituted alkylene group.
Ohtani et al., 1993 describe a number of hydroxamic acid compounds which are
claimed to be inhibitors of ras transformation. A few of the compounds are
hydroanic acid compounds having a phenylacylamido group (-NHCOPh) linked to
a iiydronbacid group (-CONHOH) via a phenylene-meta-alkylene group having a
carbon-carbon triple bond. See, for example, compounds 1-29 (page 69), 1-39
(page 87), and 1-41 (page 90). Compound 1-41, shown below, employs an aryl
leader.
õ
Me0 C,
N
,N,
C OH
Me0 II
Onishi et al., 1996, describe several hydroxamic acid compounds which have a
phenyl (or substituted phenyl) group linked via an oxazole group to a
hydnoxamic
acid group. These compounds were reported to inhibit a deacetylase enzyme
critical in the biosynthesis of lipid A (a component of the outer membrance of
Gram-negative bacteria).
Parsons et al., 1998 describe a number of hydroxamic acid compounds which are
claimed to selectively prevent the growth of a variety of human tumour cell
lines.
Some of the compounds are hylicx3mic acid compounds having an arylamide
group linked to a hyluernic acid group via a methylene or substituted
methylene
group (see, for example, pages 16 and 17).
Some of the compounds are hydrocamic acid compounds having a phenylamido
group (-CONHPh) linked to a hydrommic acid group (-CONHOH) via a long alkylene
CA 02765409 2012-01-19
54236-1
- 9 -
chain, -(CH2)n-, wherein n is from 4 to 7 (see, for example, pages 47, 48, and
58
therein).
Some of the compounds are hydiommic acid compounds having an aryl group
linked via a short chain to an amide group (-CON H-), which in turn is linked
via a
short chain (e.g., 3 atoms or less) to a hydroxamio acid group (-CONHOH). See,
for
example, page 16, 2nd formula; page 46, 4th formula; page 51, compound 7; and
page 61, 2nd formula.
x2 o
_o 0
H II
$10 N
C,N,OH
OYcYH
/
n=0,1,2 0 R2
R1
CI
0
H 0 1101
.1µ(
0 0
HN 0 0111 HN 0
H II
Me
(INS NCVOH
¨/
Richon et al., 1998 describe several hydroxamic acid compounds, including
SAHA, which apparently inhibit HDAC activity, and induce terminal
differentiation
and/or apoptosis in various transformed cells (see, for example, Table 1
therein).
Suzuki et al., 1998 describe a number of hydroxamic acid compounds which are
claimed to have antitumour activity. Some of the compounds are hydroxamic acid
compounds having a substituted phenylamido group (-CONHPh) linked to a
nyclimmic acid (-CONHOH) group via a phenylene-meta-ethenylene or phenylene-
para-ethylene group (see, for example, pages 8 and 9, compounds 31-50).
Breslow et al., 1994, 1995, 1997 describe a number of hydroxamic acid
compounds which are claimed to selectively induce terminal differentiation of
neoplastic cells.
CA 02765409 2012-01-19
54236-1
- 10 -
Some of the compounds are hdoait acid compounds having a substituted
phenylacylamido group (-NHCOPh) linked to a hyclioarnic acid (-CONHOH) group
via a long alkylene chain, -(CH2)n-, wherein n is from 4 to 8
Some of the compounds are hydroxarric acid compounds having a substituted
phenylamido group (-CONHPh) or phenylacylamido group (-NHCOPh) linked to a
hydroomic acid (-CONHOH) group via a long alkylene chain, -(CH2)n-, wherein n
is
from 4 to 8 (see, for example, columns 7 and 13 of Breslow et at., 1997), or
via a
phenylene group (see, for example, columns 24, 30-31 and compounds 20-55 in
Table l'of Breslow et al., 1997).
One of the compounds is a hydroxamic acid compound having benzylamido group
(-CONHCH2Ph) linked to a hydrovfnic acid group (-CONHOH) via a -(CH2)6- group
(see, for example, compound 19 in Table 1, at column 37 of Breslow et al.,
1997).
Jung et at., 1997, 1999, describe several aromatic hydroxamic acid compounds
which apparently inhibit HDAC. Some of the compounds have a phenylamido
group (PhCONH-). One compound, a peptide analog, is shown below (see, e.g.,
compound 6 in Jung et at., 1997; compound 4 in Jung et at., 1999).
Me0 0
0
C OH
20
Kato et at., 1998, describe a number of aromatic hydroxamic acid compounds,
comprising an aryl group linked via an alkylene group to a hydrox3mic acid
group,
which are apparently active in the treatment of neurodegenerative conditions.
25 One compound, 4-1 at columns 63-64, has a phenylamido group
(PhCONH-)
linked via a -(CH2)5- group to a .dioait acid group.
Glick et at., 1999, describe the apparent apoptotic and differentiating
effects of
m-carboxy-cinnamic acid bishydroxamide (CBHA) on various tumour cell lines.
(
CA 02765409 2012-01-19
54236-1D
- 11 -
Massa et at., 2001, describe various hydroxamic acid compounds which have a
benzoyl (or substituted benzoyl) group linked via a pyrrolyl group and an
C2alkylene group (-CH=CH- or -CH2CH2-) to a hytoxarric acid group. The
compounds apparently showed HDAC inhibitory activity in the micromolar range.
Sulfonamides
Oxamflatin, also known as (2E)-5[3-[(phenylsulfonyl)amino] phenyl]-pent-2-en-4-
ynohydroxamic acid, shown below, has been reported to have in vitro
antiproliferative activity against various mouse and human tumour cell lines,
and
in vivo antitumour activity against B16 melanoma (see, e.g., Sonoda et al.,
1996;
Kim et al., 1999).
Oxamflatin
OH
s,
N
H
Ohtani et al., 1993, describe a number of hydroxamic acid compounds which are
claimed to be inhibitors of ras transformation. Many of the compounds are
hykoomic acid -compounds which have a sulfonamide group, and which employ
an acid leader which is: a phenylene-ortho-alkylene (e.g., 1-10); phenylene-
meta-
alkylene (e.g., 1-24); phenylene-para-alkylene (e.g., 1-12); or napthylen-1,2-
diy1
(e.g., 1-20). However, in every case, the sulfonamide group is -SO2NR-, as
opposed to -NRS02-. Also, in every case, the terminal aryl group is linked
directly
to the -SO2NR- sulfonamide group, without an intervening aryl leader. Ohtani
et
at., 1996, describe similar compounds.
Richon et at., 2001, describe various branched compounds which apparently
inhibit histone deacetylase. See the table at pages 96-101 therein. Some of
the
CA 02765409 2012-01-19
54236-1
- 12 -
compounds are hydrocarnic acid compounds having a hydrommt acid group
(-CONHOH) linked to a branch point, from which two aryl groups are appended. A
few linear hydroxamic acid compounds are also described, including a single
-SO2NH- sulfonamide hydroomic acid with a -(CH2)5- acid leader (compound 671).
Delorme et al., 2001, describe various hydioamic acid compounds, including
compounds having, inter alia, a sulfonamide group. Of the 108 compounds in the
table at pages 114-123 therein, 88 are hydroxamic acids (-CONHOH), and the
remainder are terminal amides, -CONHR. Of the 881-Orocamic acid compounds,
54 have a sulfonamide linkage.
Of the 54 sulfonamide hydirocamic acids, 51 are indicated to have a -SO2NR-
sulfonamide group, and 3 (compounds 98, 161, and 162) are indicated to have a -
NRS02- sulfonamide group.
All of the 54 sulfonamide hydocamic acids employ a phenylene-alkylene acid
leader
group (analogous to Q2 herein). Of the 54 compounds, 52 employ a phenylene-
para-alkylene group, and only 2 (compounds 41 and 26) employ a phenylene-
meta-alkylene group (-Ph-CH2- and -Ph-(CH2)4-, respectively). Compounds 41
and 26 both have a -SO2NR- sulfonamide group, as opposed to a -NRS02-
sulfonamide group; the former has a benzothiophenyl group, and the latter has
a
phenyl group.
All but one of the 54 sulfonamide hydroxamt acids have an aryl group linked
directly
to the sulfonamide; compound 100 has a benzyl group (Ph-CH2-) linked a -
SO2NR- sulfonamide group linked to phenylene-para-ethylene.
SUMMARY OF THE INVENTION
One aspect of the invention pertains to active hydroxamic acid compounds, as
described herein, which inhibit HDAC activity.
CA 02765409 2012-01-19
WO 02/30879 PCT/G1301/04326
=
- 13 -
Another aspect of the invention pertains to active compounds, as described
herein, which treat a proliferative condition, such as cancer or psoriasis.
Another aspect of the invention pertains to active compounds, as described
herein, which treat conditions which are known to be mediated by HDAC, or
which
are known to be treated by HDAC inhibitors (such as; e.g., trichostatin A).
Another aspect of the present invention pertains to a composition comprising a
compound as described herein and a pharmaceutically acceptable carrier.
Another aspect of the present invention pertains to methods of inhibiting HDAC
in
a cell, comprising contacting said cell with an effective amount of an active
compound, as described herein.
Another aspect of the present invention pertains to methods of inhibiting cell
proliferation, comprising contacting a cell with an effective amount of an
active
compound, as described herein, whether in vitro or in vivo.
Another aspect of the present invention pertains to methods of treating a
proliferative condition in a patient comprising administering to said patient
a
therapeutically-effective amount of an active compound, as described herein.
In one preferred embodiment, the proliferative condition is cancer. In one
preferred embodiment, the proliferative condition is psoriasis.
Another aspect of the present invention pertains to methods of treating
a condition in a patient which is known to be mediated by HDAC, or which is
known to be treated by HDAC inhibitors (such as, e.g., trichostatin A),
comprising
administering to said patient a therapeutically-effective amount of an active
compound, as described herein.
Another aspect of the present invention pertains to an active compound, as
described herein, for use in a method of treatment of the human or animal
body.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 14 -
Another aspect of the present invention pertains to use of an active compound,
as
described herein, for the manufacture of a medicament for use in the treatment
of
a proliferative condition. In one preferred embodiment, the proliferative
condition
is cancer. In one preferred embodiment, the proliferative condition is
psoriasis.
Another aspect of the present invention pertains to use of an active compound
for
the manufacture of a medicament, for example, for the treatment of conditions
which are known to be mediated by HDAC, or which are known to be treated by
HDAC inhibitors (such as, e.g., trichostatin A), as discussed herein.
Another aspect of the present invention pertains to a kit comprising (a) the
active
compound, preferably provided as a pharmaceutical composition and in a
suitable
container and/or with suitable packaging; and (b) instructions for use, for
example,
written instructions on how to administer the active compound.
Another aspect of the present invention pertains to compounds obtainable by a
method of synthesis as described herein, or a method comprising a method of
synthesis as described herein.
Another aspect of the present invention pertains to compounds obtained by a
method of synthesis as described herein, or a method comprising a method of
synthesis as described herein.
=
Another aspect of the present invention pertains to novel intermediates, as
described herein, which are suitable for use in the methods of synthesis
described
herein.
Another aspect of the present invention pertains to the use of such novel
intermediates, as described herein, in the methods of synthesis described
herein.
CA 02765409 2013-09-27
=
54236-1D
- 14a -
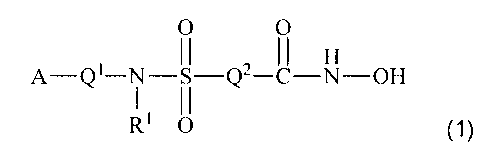
In another aspect, the invention relates to a compound selected from
compounds of the following formula and pharmaceutically acceptable salts,
solvates,
amides, esters, ethers, chemically protected forms and prodrugs thereof:
0 0
H
A ________________________ Q'¨N--S--Q2¨C--N--OH
RI 0 (1)
wherein:
A is a C5-20 aryl group, wherein ring atoms of the C520 aryl group
optionally comprise one or more heteroatoms selected from the group consisting
of
oxygen, nitrogen, and sulfur, and wherein the C5_20 aryl group is optionally
substituted
with:
one or more substituents independently selected from:
-F, -Cl, -Br, -I,
-OH,
-0Me, -0Et,
-SH, -SMe, -SEt,
-C(=0)Me, -C(=0)0H, -C(=0)0Me,
-C(=)ONH2, -C(=)ONHMe,
-NH2, -NMe2, NEt2, -N(nPr)2, -W02,
-CN,
-NO2,
-Me, -Et,
CA 02765409 2013-09-27
54236-1D
- 14b -
-CF3, -0CF3,
-CH2OH, -CH2CH2OH,
-CH2NH2, -CH2CH2NH2,
-Ph,
-0(tBu), -OCH2Ph,
-C(=0)0Et, -C(=0)0(tBu),
-C(=0)NMe2, -C(=0)NHEt,
-NHMe, -NHEt, -NH(iPr), -NH(nPr),
-N(nBu)2, -N(tBu)2,
-nPr, -iPr, -nBu, -tBu,
-CHF2, -CH2F, -CCI3, -CBr3, -CH2CH2F, -CH2CHF2, -CH2CF3,
-CH(OH)CH2OH, and
-CH2CH2NMe2;
Q1 is a covalent bond or a C1-7 alkylene group, wherein the C1-7
alkylene group is optionally aliphatic or alicyclic, or a combination thereof,
and
wherein the C1-7 alkylene group is optionally saturated, partially
unsaturated, or fully
unsaturated;
R1 is hydrogen or a C1-7 alkyl group, wherein the C1_7 alkyl group is
optionally aliphatic or alicyclic, or a combination thereof, and wherein the
C1_7 alkyl
group is optionally saturated, partially unsaturated or fully unsaturated;
and,
Q2 is a linear or branched, saturated or unsaturated, optionally
substituted aliphatic C4-7 alkylene or alicyclic, with the proviso that:
CA 02765409 2013-09-27
=
54236-1D
- 14c -
(i) the number of bonds connecting the sulfur atom of the sulfonamide
group and the carbonyl carbon atom of the hydroxamic acid group in Formula 1
is not
less than three;
(ii) if the number of bonds connecting the sulfur atom of the sulfonamide
(iii) if the number of bonds connecting the sulfur atom of the
sulfonamide group and the carbonyl carbon atom of the hydroxamic acid group in
CA 02765409 2012-01-19
54236-1
- 15 -
As will be appreciated by one of skill in the art, features and preferred
embodiments of one aspect of the invention will also pertain to other aspects
of
the invention.
DETAILED DESCRIPTION OF THE INVENTION
Compounds
In one embodiment, the present invention pertains to hydroxamic acid compounds
of
, II
A ______________________ Q1 __ J ____ c-H-OH (1)
wherein:
A is an aryl group;
Q1 is a covalent bond or an aryl leader group;
J is a sulfonamide linkage selected from:
II II
__________________________ S N ____________________ N S __
I, 11 II
OR R 0
R1 is a sulfonamido substituent; and,
Q2 is an acid leader group;
with the proviso that if J is:
_____________________________________ S N
II I,
OR '
then Q1 is an aryl leader group;
and pharmaceutically acceptable salts, solvates, amides, esters, ethers,
chemically protected forms, and prodrugs thereof.
In preferred embodiments, the hydnarnic acid group, -C(=0)NHOH, is unmodified
(e.g., is not an ester).
CA 02765409 2012-01-19
54236-1
- 16 -
In one preferred embodiment, the present invention pertains to hydragmb acid
compounds of the formula:
A¨Q1 _______________________ J Q2¨C-N-OH (1)
wherein:
A is an aryl group;
Q1 is an aryl leader group;
J is a sulfonamide linkage selected from:
0
II
¨S-N-
11 11
0 R
al is an sulfonamido substituent; and,
Q2 is an acid leader group.
In one preferred embodiment, the present invention pertains to hyloxamic acid
compounds of the formula:
,
A Q1 _______________________ J¨Q=C-11-0H (1)
wherein:
A is an aryl group;
Q1 is a covalent bond or an aryl leader group;
J is a sulfonamide linkage selected from:
II
R
R1 is an sulfonamido substituent; and,
Q2 is an acid leader group.
In one preferred embodiment, Q1 is an aryl leader group, J is -SO2NR1-, and
the
compounds have the following formula:
II 2
A Q1 _______________________ SN¨I Q¨C-H-OH (2)
II
0 R
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 17 -
In one preferred embodiment, Q1 is a covalent bond or an aryl leader group, J
is
-NR1S02-, and the compounds have the following formula:
2 II
A-C)' _____________________ N1-Q- ____ C_-N_-OH (3)
1, II
R o
In one preferred embodiment, Q1 is an aryl leader group, J is -NR1S02-, and
the
compounds have the following formula:
o
A-Q1 _________________________________ C-N-OH (3)
1 II
R', 0
In one preferred embodiment, Q1 is a covalent bond, J is -NR1S02-, and the
compounds have the following formula:
0
,
A N S Cr ___________________________ C-N-OH (4)
11 11
R 0
In one embodiment, where Q1 is an aryl leader, the aryl group, A, is linked to
Q1
via a covalent single bond.
In one embodiment, where Q1 is a cyclic aryl leader, the aryl group, A, may be
fused to Q1 and so the moiety A-Q1- forms a fused polycyclic structure. For
example, the moiety 2,3-dihydro-1H-indene-2-yl, derived from indan (2,3-
dihydro-
1H-indene), is considered to be a phenyl group (A) fused to a C5cycloalkyl
group
(Q1):
3
lee 2
1
In such cases, the tridentate aryl leader, Q1, may be denoted as:
CA 02765409 2012-01-19
WO 02/30879
PCT/C1301/04326
/
In a similar example, the moiety 9H-fluorene-9-yl, derived from fluorene, is
considered to be two phenyl groups (either of which is A), fused to a
C5cycloalkyl
group, which forms part of Q1:
\A /
=
In such cases, the pentadentate aryl leader, Q1, may be denoted as:
The Aryl Group, A
The aryl group, A, is a C5_20ary1 group, and is optionally substituted.
In one preferred embodiment, A is a C5_20heteroaryl group, and is optionally
substituted. In one preferred embodiment, A is a monocyclic C5_20heteroaryl
group, and is optionally substituted. In one preferred embodiment, A is a
monocyclic C5_6heteroaryl group, and is optionally substituted.
In one preferred embodiment, A is a C5_20carboaryl group, and is optionally
substituted. In one preferred embodiment, A is a monocyclic C5_20carboaryl
group, and is optionally substituted. In one preferred embodiment, A is a
monocyclic C5_6carboaryl group, and is optionally substituted. In one
preferred
embodiment, A is a phenyl group, and is optionally substituted.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 19 -
In one preferred embodiment, A is a C5-20aryl group derived from one of the
following: benzene, pyridine, furan, indole, pyrrole, imidazole, naphthalene,
quinoline, benzimidazole, benzothiofuran, fluorene, acridine, and carbazole.
In one preferred embodiment, A is an optionally substituted phenyl group of
the
formula:
3' 2'
RA ir
wherein n is an integer from 0 to 5, and each RA is independently a
substituent as
defined herein.
Thus, in one preferred embodiment, A is an optionally substituted phenyl
group,
Q1 is an aryl leader group, J is -SO2NR1-, and the compounds have the
following
formula:
RA, Q1 __ g _N ___Q2 8--OH (5)
0 R
In one preferred embodiment, A is an optionally substituted phenyl group, Q1
is a
covalent bond or an aryl leader group, J is -NR1S02-, and the compounds have
the following formula:
3' 2'
2
4'
RAõ 01 __ N S _____ C-N-OH (6)
5,
In one preferred embodiment, A is an optionally substituted phenyl group, Q1
is an
aryl leader group, J is -NR1S02-, and the compounds have the following
formula:
3 2'
0 0
11 Cil-N-S Q2 C-11-01-1 (6)
,
RAri 5, 6, R 0
CA 02765409 2012-01-19
WO 02/30879 PCT/CB01/04326
=
- 20 -
In one preferred embodiment, A is an optionally substituted phenyl group, Q1
is a
covalent bond, J is -NR1S02-, and the compounds have the following formula:
3' 2'
, (7) I I
N-S C-N-OH
I,
RA,R' o
In one preferred embodiment, n is an integer from 0 to 5. In one preferred
embodiment, n is an integer from 0 to 4. In one preferred embodiment, n is an
integer from 0 to 3. In one preferred embodiment, n is an integer from 0 to 2.
In
one preferred embodiment, n is 0 or 1.
In one preferred embodiment, n is an integer from 1 to 5.
In one preferred embodiment, n is an integer from 1 to 4.
In one preferred embodiment, n is an integer from 1 to 3.
In one preferred embodiment, n is 1 or 2.
In one preferred embodiment, n is 5.
In one preferred embodiment, n is 4.
In one preferred embodiment, n is 3.
In one preferred embodiment, n is 2.
In one preferred embodiment, n is 1.
In one preferred embodiment, n is 0.
If the phenyl group has less than the full complement of ring substituents,
RA, they
may be arranged in any combination. For example, if n is 1, RA may be in the
2'-,
3'-, 4'-, 5'-, or 6'-position. Similarly, if n is 2, the two RA groups may be
in, for
example, the 2',3'-, 2',4'-, 2',5'-, 2',6'-, 3',4'-, or 3',5'-positions. If n
is 3, the three
RA groups may be in, for example, the 2',3',4'-, 2',3',5'-, 2',3',6'-, or
3',4',5'-
positions.
In one preferred embodiment, n is 1, and the RA group is in the 4'-position.
In one preferred embodiment, n is 2, and one RA group is in the 4'-position,
and
the other RA group is in the 2'-position.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 21 -
In one preferred embodiment, n is 2, and one RA group is in the 4'-position,
and
the other RA group is in the 3'-position.
Each aryl substituent, RA, is a substituent as defined herein.
Examples of preferred aryl substituebts, RA, include, but are not limited to,
the
following: fluoro, chloro, bromo, iodo, methyl, ethyl, isopropyl, t-butyl,
cyano,
trifluoromethyl, hydroxy, methoxy, ethoxy, isopropoxy, trifluoromethoxy,
phenoxy,
methylthio, trifluoromethylthio, hydroxymethyl, amino, dimethylamino,
diethylamino, morpholino, amido (unsubstituted, i.e., -CONH2), acetamido,
acetyl,
nitro, sulfonamido (unsubstituted, i.e., -SO2NH2), and phenyl.
In one preferred embodiment, A is a substituted phenyl group selected from:
para-(fluoro)phenyl; ortho-(fluoro)phenyl; meta-(fluoro)phenyl;
para-(chloro)phenyl; ortho-(chloro)phenyl; meta-(chloro)phenyl;
para-(bromo)phenyl; ortho-(bromo)phenyl; meta-(bromo)phenyl;
para-(iodo)phenyl; ortho-(iodo)phenyl; meta-(iodo)phenyl;
para-(methyl)phenyl; ortho-(methyl)phenyl; meta-(methyl)phenyl;
para-(ethyl)phenyl; ortho-(ethyl)phenyl; meta-(ethyl)phenyl;
para-(isopropyl)phenyl; ortho-(isopropyl)phenyl; meta-(isopropyl)phenyl;
para-(t-butyl)phenyl; ortho-(t-butyl)phenyl; meta-(t-butyl)phenyl;
para-(cyano)phenyl; ortho-(cyano)phenyl; meta-(cyano)phenyl;
para-(trifluoromethyl)phenyl; ortho-(trifluoromethyl)phenyl; meta-
(trifluoromethyl)phenyl;
para-(hydroxy)phenyl; ortho-(hydroxy)phenyl; meta-(hydroxy)phenyl;
para-(methoxy)phenyl; ortho-(methoxy)phenyl; meta-(methoxy)phenyl;
para-(ethoxy)phenyl; ortho-(ethoxy)phenyl; meta-(ethoxy)phenyl;
para-(isopropoxy)phenyl; ortho-(isopropoxy)phenyl;
meta-(isopropoxy)phenyl;
para-(trifluoromethoxy)phenyl; ortho-(trifluoromethoxy)phenyl;
meta-(trifluoromethoxy)phenyl;
para-(phenoxy)phenyl; ortho-(phenoxy)phenyl; meta-(phenoxy)phenyl;
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
=
- 22 -
para-(methylthio)phenyl; ortho-(methylthio)phenyl; meta-(methylthio)phenyl;
para-(trifluoromethylthio)phenyl; ortho-(trifluoromethylthio)phenyl;
meta-(trifluoromethylthio)phenyl;
para-(hydroxymethyl)phenyl; ortho-(hydroxymethyl)phenyl;
meta-(hydroxymethyl)phenyl;
para-(amino)phenyl; ortho-(amino)phenyl; meta-(amino)phenyl;
para-(dimethylamino)phenyl; ortho-(dimethylamino)phenyl;
meta-(dimethylamino)phenyl;
para-(diethylamino)phenyl; ortho-(diethylamino)phenyl;
meta-(diethylamino)phenyl;
para-(nnorpholino)phenyl; ortho-(morpholino)phenyl;
meta-(morpholino)phenyl;
para-(amido)phenyl; ortho-(amido)phenyl; meta-(amido)phenyl;
para-(acetamido)phenyl; ortho-(acetamido)phenyl;
meta-(acetamido)phenyl;
para-(acetyl)phenyl; ortho-(acetyl)phenyl; meta-(acetyl)phenyl;
para-(nitro)phenyl; ortho-(nitro)phenyl; meta-(nitro)phenyl;
para-(sulfonamido)phenyl; ortho-(sulfonamido)phenyl;
meta-(sulfonamido)phenyl; and,
para-(phenyl)phenyl; ortho-(phenyl)phenyl; meta-(phenyl)phenyl.
In one preferred embodiment, A is a substituted phenyl group selected from:
para-(fluoro)phenyl;
para-(chloro)phenyl;
para-(bromo)phenyl;
para-(iodo)phenyl;
para-(methyl)phenyl;
para-(ethyl)phenyl;
para-(isopropyl)phenyl;
para-(t-butyl)phenyl;
para-(cyano)phenyl;
para-(trifluoromethyl)phenyl;
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
-23 -
para-(hydroxy)phenyl;
para-(methoxy)phenyl;
para-(ethoxy)phenyl;
para-(isopropoxy)phenyl;
para-(trifluoromethoxy)phenyl;
para-(phenoxy)phenyl;
para-(methylthio)phenyl;
para-(trifluoromethylthio)phenyl;
para-(hydroxymethyl)phenyl;
para-(amino)phenyl;
para-(dimethylamino)phenyl;
para-(diethylamino)phenyl;
para-(morpholino)phenyl;
para-(amido)phenyl;
para-(acetamido)phenyl;
para-(acetyl)phenyl;
para-(nitro)phenyl;
para-(sulfonamido)phenyl; and,
para-(phenyl)phenyl.
In one preferred embodiment, A is a substituted phenyl group selected from:
ortho,para-di(methoxy)phenyl;
ortho,para-di(halo)phenyl;
ortho,para-di(fluoro)phenyl;
ortho-(methoxy),para-(methyl)phenyl;
ortho-(methoxy),para-(trifluoromethyl)phenyl;
ortho-(trifluoromethyl),para-(halo)phenyl;
ortho,meta-di(trifluoromethyl)phenyl;
ortho-(halo),meta-(trifluoromethyl)phenyl;
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 24 -
meta,para-di(halo)phenyl;
meta,para-di(hydroxy)phenyl;
meta,para-di(methyl)phenyl;
meta,para-di(methoxy)phenyl;
meta-(halo),para-(nitro)phenyl;
3',5'-di(trifluoromethyl)phenyl;
3'-(trifluoromethyl),51-(methoxy)phenyl;
3'-(trifluoromethyl),5'-(halo)phenyl;
2'-(halo),5'-(methyl)phenyl;
2',6'-di(methyl)phenyl;
2',6'-di(halo)phenyl;
26'-di(isopropyl)phenyl;
Z,4',6'-tri(halo)phenyl;
3',4',5'-tri(halo)phenyl;
3',4',5'-tri(methoxy)phenyl;
2',5'-di(halo)-4'-(hydroxy)phenyl; and
3'-(trifluoromethyl),51,61-di(halo)phenyl.
The Aryl Leader Group, Q1
As mentioned above, in some embodiments, Q1 is a covalent bond or an aryl
leader group; in some embodiments, Q1 is a covalent bond; in some
embodiments, Q1 is an aryl leader group.
In one preferred embodiment, Q1 is a covalent bond.
In one preferred embodiment, Q1 is a Ciqalkylene group and is optionally
substituted.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 25 -
In one preferred embodiment, Q1 is a covalent bond or a Ci_7alkylene group and
is
optionally substituted.
group. In one preferred ernbodiment, Q1 is a saturated Ci_7alkylene group.
In one preferred embodiment, Q1 is a covalent bond or a partially unsaturated
Cijalkylene group. In one preferred embodiment, Q1 is a partially unsaturated
In one preferred embodiment, Q1 is a covalent bond or an aliphatic Cigalkylene
group. In one preferred embodiment, Q1 is an aliphatic Ci_7alkylene group.
In one preferred embodiment, Q1 is a linear CiJalkylene group.
In one preferred embodiment, Q1 is a covalent bond or a branched Ci_7alkylene
group. In one preferred embodiment, Q1 is a branched C1.7alkylene group.
In one preferred embodiment, Q1 is a covalent bond or an alicyclic CiJalkylene
group. In one preferred embodiment, Q1 is an alicyclic CiJalkylene group.
In one preferred embodiment, Q1 is a covalent bond or a saturated aliphatic
In one preferred embodiment, Q1 is a covalent bond or a saturated linear
C1_7alkylene group. In one preferred embodiment, Q1 is a saturated linear
C1_7alkylene group.
CA 02765409 2012-01-19
WO 02/39879 PCT/GB01/04326
=
-26 -
In one preferred embodiment, Q1 is a covalent bond or a saturated branched
C1_7alkylene group. In one preferred embodiment, Q1 is a saturated branched
C1..7alkylene group.
In one preferred embodiment, Q1 is a covalent bond or a saturated alicyclic
C1..7alkylene group. In one preferred embodiment, Q1 is a saturated alicyclic
Ciqalkylene group.
In one preferred embodiment, Q1 is a covalent bond or a partially unsaturated
aliphatic Ciqalkylene group. In one preferred embodiment, Q1 is a partially
unsaturated aliphatic C1.7alkylene group.
In one preferred embodiment, Q1 is a covalent bond or a partially unsaturated
linear C1_7alkylene group. In one preferred embodiment, Q1 is a partially
unsaturated linear Ciqalkylene group.
In one preferred embodiment, Q1 is a covalent bond or a partially unsaturated
branched Cigalkylene group. In one preferred embodiment, Q1 is a partially
unsaturated branched Ci_7alkylene group.
In one preferred embodiment, Q1 is a covalent bond or a partially unsaturated
alicyclic Ciqalkylene group. In one preferred embodiment, Q1 is a partially
unsaturated alicyclic Cigalkylene group.
The Arvl Leader Group, Q1: Backbone Length
In one embodiment, the aryl leader group, Q1, has a backbone of at least 2
carbon
atoms; that is, the shortest chain of atoms linking the aryl group, A, and the
sulfonamide group, J, has 2 or more atoms, more specifically, 2 or more carbon
atoms. In this way, groups such as methylene (-CH2-) and substituted methylene
(-CR2- and -CHR-) are excluded.
CA 02765409 2012-01-19
WO 02/39879
PCT/C1301/04326
- 27 -
If there are two or more paths linking the aryl group, A, and the sulfonamide
group, J, then the shortest path is relevant. For example, in the embodiments
shown below, where the moiety A-Q1- is derived from indan (2,3-dihydro-1H-
indene), A is considered to be a phenyl group fused to Q1, a C5cycloalkyl
group:
At
O. 2
In each case, there are two paths to the aryl group. In the first case, one
path has
1 carbon atom, and the other path has 3 carbon atoms, and so the relevant
backbone length is 1. In the second case, both paths have 2 carbon atoms, and
so the relevant backbone length is 2.
If the group A-Q1- has two or more aryl groups, the aryl group furthest from
the
sulfonamide group, J, as measured by counting chain atoms, is identified as A;
the relevant backbone is then the shortest chain of atoms linking that aryl
group
and the sulfonamide group, J. For example, where the group A-Q1- is as shown
below, the phenyl group marked "1" is identified as the A, Q1 is -CH2CH(Ph)-
(i.e.,
substituted ethylene), and the backbone length is 2.
If the sulfonamide group is -NR1S02- (as opposed to -SO2NR1-), and
substituent,
R1, discussed below, is or comprises an aryl group (or two or more aryl
groups),
then the aryl group furthest from the sulfonamide group nitrogen atom, as
measured by counting chain atoms, is identified as A. For example, where the
group A-Q1-NR1S02- is as shown below, the phenyl group marked "1" is
identified
as the A, Q1 is -CH2-, and the backbone length is 1.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 28 -
o
\\ A
.s
N \\0
1.1
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 3 carbon atoms.
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 4 carbon atoms.
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 5 carbon atoms.
In one embodiment, the aryl leader group, Q1, has a backbone of:
from 2 to 7 carbon atoms;
from 2 to 6 carbon atoms; or,
from 2 to 5 carbon atoms.
In one embodiment, the aryl leader group, Q1, has a backbone of:
from 3 to 7 carbon atoms;
from 3 to 6 carbon atoms; or,
from 3 to 5 carbon atoms.
In one embodiment, the aryl leader group, Q1, has a backbone of:
from 4 to 7 carbon atoms;
from 4 to 6 carbon atoms; or,
from 4 to 5 carbon atoms.
In one embodiment, the aryl leader group, Q1, has a backbone of 2 carbon
atoms.
In one embodiment, the aryl leader group, Q1, has a backbone of 3 carbon
atoms.
In one embodiment, the aryl leader group, Q1, has a backbone of 4 carbon
atoms.
In one embodiment, the aryl leader group, Q1, has a backbone of 5 carbon
atoms.
CA 02765409 2012-01-19
WO 02/30879 PCT/CI301/04326
- 29 -
The Aryl Leader Group, Q1: Alkylene
In one embodiment, the aryl leader group, Q1, is an alkylene group, and has a
backbone of at least 2 carbon atoms.
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 2 carbon atoms, and is a C2.7alkylene group.
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 3 carbon atoms, and is a C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a saturated C2_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a saturated C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a partially unsaturated C2..7a1kylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a partially unsaturated C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is an aliphatic C2_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is an aliphatic C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a linear C2_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a linear C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a branched C2_7alkylene group.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 30 -
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a branched C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is an alicyclic C2_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is an alicyclic C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a saturated aliphatic C2_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a saturated aliphatic C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a saturated linear C2_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a saturated linear C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a saturated branched C2.7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a saturated branched C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a saturated alicyclic C2_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a saturated alicyclic Cwalkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a partially unsaturated aliphatic C2Jalkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a partially unsaturated aliphatic C37alkylene group.
CA 02765409 2012-01-19
WO 02/30879 PCT/CB01/04326
- 31 -
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a partially unsaturated linear C2_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a partially unsaturated linear C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a partially unsaturated branched C2_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a partially unsaturated branched C3_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 2 carbon atoms, and
is a partially unsaturated acyclic C2_7alkylene group.
In one preferred embodiment, Q1 has a backbone of at least 3 carbon atoms, and
is a partially unsaturated alicyclic C3_7alkylene group.
The Aryl Leader Group, 01: Backbone Length of 0 or 2 or more
In one preferred embodiment, the aryl leader group, Q1, is either: a covalent
bond,
or: has a backbone of at least 2 carbon atoms.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a C2_7alkylene group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a saturated C2_7alkylene group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a partially unsaturated C2_7alkylene group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is an aliphatic C2_7alkylene group.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 32 -
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a linear C2.7alkylene group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a branched C2jalkylene group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is an alicyclic C2_7alkylene group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a saturated aliphatic C2_7alkylene group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a saturated linear C2_7alkylene group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a saturated branched C2_7alkylene group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a saturated alicyclic C2_7alkylene group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a partially unsaturated aliphatic C2_7alkylene
group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a partially unsaturated linear C24alkylene
group.
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a partially unsaturated branched C2_7alkylene
group.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 33 -
In one preferred embodiment, Q1 is either: a covalent bond, or: has a backbone
of
at least 2 carbon atoms and is a partially unsaturated alicyclic C2.7alkylene
group.
Note that, as discussed below in the context of isomers, where unsaturation
permits isomers, e.g., cis- and trans, E- and Z-, etc., and combinations
thereof, a
reference to one isomer is to be considered a reference to all such isomers,
unless otherwise specified.
The Aryl Leader Group, Q1: Substituents
In one embodiment, Q1 is unsubstituted.
In one embodiment, Q1 is optionally substituted.
In one embodiment, Q1 is substituted.
Examples of substituents on Q1 include, but are not limited to, those
described
under the heading "Substituents" below.
In one preferred embodiment, substituents on Q1, if present, are independently
selected from: halo, hydroxy, ether (e.g., Ci_7alkoxy), C5_20aryl, acyl,
amido, and
oxo.
In one preferred embodiment, substituents on Q1, if present, are independently
selected from -F, -CI, -Br, -I, -OH, -0Me, -0Et, -0Pr, -Ph, and =0.
In one preferred embodiment, substituents on Q1, if present, are -OH or -Ph.
In one preferred embodiment, substituents on Q1, if present, are -Ph.
For example, in one embodiment, Q1 is unsubsituted ethylene, and is -CH2-CH2-;
in one embodiment, Q1 is oxo (=0) subsituted ethylene, and is -C(=0)-CH2-; in
one embodiment, Q1 is hydroxy (-OH) subsituted ethylene, and is -CH(OH)-CH2-;
in one embodiment, Q1 is phenyl (-Ph) substituted ethylene, and is -CH2CH(Ph)-
.
CA 02765409 2012-01-19
WO 02/3(1879 PCT/GB01/04326
- 34 -
The Aryl Leader Group, Q1: Certain Embodiments
Note that, for embodiments excluding, e.g., a covalent bond, certain backbone
lengths, etc., it is to be understood that the corresponding species listed
below are
similarly excluded from the respective embodiments discussed below.
In one preferred embodiment, Q1 is selected from the following:
a covalent bond;
-(CH2)n- where n is an integer from 1 to 7;
-CH(CH3)-;
-CH(CH3)CH2- and -CH2CH(CH3)-;
-CH(CH3)CH2CH2-, -CH2CH(CH3)CH2-, and -CH2CH2CH(CH3)-;
-CH(CH3)CH2CH2CH2-, -CH2CH(CH3)CH2CH2-, -CH2CH2CH(CH3)CH2-, and
-CH2CH2CH2CH(CH3)-;
-CH(CH3)CH2CH2CH2CH2-, -CH2CH(CH3)CH2CH2CH2-,
-CH2CH2CH(CH3)CH2CH2-, -CH2CH2CH2CH(CH3)CH2-, and
-CH2CH2CH2CH2CH(CH3)-;
-CH(CH2CH3)-;
-CH(CH2CH3)CH2- and -CH2CH(CH2CH3)-;
-CH(CH2CH3)CH2CH2-, -CH2CH(CH2CH3)CH2-, and -CH2CH2CH(CH2CH3)-;
-CH(CH2CH3)CH2CH2CH2-, -CH2CH(CH2CH3)CH2CH2-,
-CH2CH2CH(CH2CH3)CH2-, and -CH2CH2CH2CH(CH2CH3)-;
-CH(CH2CH3)CH2CH2CH2CH2-, -CH2CH(CH2CH3)CH2CH2CH2-,
-CH2CH2CH(CH2CH3)CH2CH2-, -CH2CH2CH2CH(CH2CH3)CH2-, and
-CH2CH2CH2CH2CH(CH2CH3)-;
-CH=CH-;
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
=
- 35 -
-CH=CHCH2- and -CH2CH=CH-;
-CH=CHCH2CH2-, -CH2CH=CHCH2-, and -CH2CH2CH=CH-;
-CH=CHCH2CH2CH2-, -CH2CH=CHCH2CH2-, -CH2CH2CH=CHCH2-, and
-CH2CH2CH2CH=CH-;
-CH=CHCH2CH2CH2CH2-, -CH2CH=CHCH2CH2CH2-,
-CH2CH2CH=CHCH2CH2-, -CI2CH2CH2CH=CHCH2-, and
-CH2CH2CH2CH2CH=CH-;
-C(CH3)=CH- and -CH=C(CH3)-;
-C(CH3)=CHCH2-, -CH=C(CH3)Ci2-, and -CH=CHCH(CH3)-;
-CH(CH3)CH=CH-, -CH2C(CH3)=CH-, and -CH2CH=C(CH3)-;
-CH=CHCH=CH-;
-CH=CHCH=CHCH2-, -CH2CH=CHCH=CH-, and -CH=CHCH2CH=CH-;
-CH=CHCH=CHCH2CH2-, -CH=CHCH2CH=CHCH2-,
-CH=CHCH2CH2CH=CH-, -CH2CH=CHCH=CHCH2-, -CH2CH=CHCH2CH=CH-,
and -CH2CH2CH=CHCH=CH-;
-C(CH3)=CHCH=CH-, -CH=C(CH3)CH=CH-, -CH=CHC(CH3)=CH-, and
-CH=CHCH=C(CH3)-;
-CEO-;
-CE-CCH2-, -CH2CE-C-; -CECCH(CH3)-, and -CH(CH3)CE-C-;
-C-ECCH2CH2-, -CH2CECCH2-, and -CH2CH2CEC-;
-CE--CCH(CH3)CH2- and -CECCH2CH(CH3)-;
-CH(CH3)CE-CCH2- and -CH2CECCH(CH3)-;
-CH(CH3)CH2CEC- and -CH2CH(CH3)CEC-;
-CECCH=CH-, -CH=CHCEC-, and -CECCEC-;
-CECCH2CH2CH2- and -CH2CH2CH2CEC-;
-CECCH2CH2CH2CH2- and -CH2CH2CH2CH2CEC-;
-CECCH=CHCH=CH-, -CH=CHCEC-CH=CH-, and -CH=CHCH=CHCEC-;
CA 02765409 2012-01-19
WO 02/30879
PCT/C1301/04326
=
- 36 -
-C(CH3)=CHCEC-, -CH=C(CH3)CEC-, -CECC(CH3)=CH-, and
-CECCH=C(CH3)-;
cyclopentylene and cyclopentenylene; and,
cyclohexylene, cyclohexenylene, and cyclohexadienylene.
In one preferred embodiment, Q1 is selected from:
a covalent bond;
-CH2-, -(CH2)2-, -(CH2)3-, -(CH2)4-, -(CH2)5-, and -(CH2)6-;
-CH(CH3)CH2CH2CH2CH2-, -CH2CH(CH3)CH2CH2CH2-,
-CH2CH2CH2CH(CH3)CH2-, and -CH2CH2CH2CH2CH(CH3)-;
-CH=CH-;
-CH=CH-CH=CH-;
-CH=CHCH2CH2CH2- and -CH2CH2CH2CH=CH-;
-CH=CHCH2CH2CH2CH2- and -CH2CH2CH2CH2CH=CH-;
-C(CH3)=CHCH=CH-, -CH=C(CH3)CH=CH-, -CH=CHC(CH3)=CH-, and
-CH=CHCH=C(CH3)-;
(cyclopent-1,3-ylene) (4-cyclopenten-1,3-ylene)
pH
*
(cyclohex-1,4-ylene) (2-cyclohexen-1,4-ylene)
(2 ,5-cyclohexadien-1,4-ylene) (cyclohex-
1,4-ylene-methylene)
/
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
=
- 37 -
(methylene-cyclohex-1,4-ylene)
In. one preferred embodiment, al is selected from:
a covalent bond;
-CH2-, -(CH2)2-, -(CH2)3-, -(CH2)4-, -(CH2)5-,
-CH=CH-;
-CH=CH-CH=CH-;
-C(CH3)=CHCH=CH-, -CH=C(CH3)CH=CH-, -CH=CHC(CH3)=CH-, and
-CH=CHCH=C(CH3)-;
-CH=CHCH2CH2CH2- and -CH2CH2CH2CH=CH-; and,
(cyclopent-1,3-ylene) (4-cyclopenten-1,3-ylene)
*
In one preferred embodiment, Q1 is selected from: a covalent bond, -CH2-,
-CH2CH2-, -CH2CH2CH2-, -CH=CH-, and -CH=CH-CH=CH-.
In one preferred embodiment, Q1 is selected from: a covalent bond, -CH2-,
-CH2CH2-, -CH2CH2CH2-, -CH=CH-, -CH=CH-CH=CH-, and C5cycloalkyl
(e.g., cyclopentylene and cyclopentenylene, e.g., as in indan, fluorene,
etc.).
The Sulfonamido Substituent, R1
The sulfonamido substituent, R1, is hydrogen, C1_7alkyl (including, e.g.,
C5_20ary1-
C1_7a1ky1), C3_20heterocyclyl, or C5.20ary1.
Note that R1 is a monodentate species. It is not intended that R1 be
additionally
linked to A, Q1, and/or Q2, thereby forming a cyclic group.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 38 -
In one preferred embodiment, R1 is hydrogen, Cijalkyl, or C5_20ary1.
In one preferred embodiment, R1 is hydrogen or CiJalkyl.
In one preferred embodiment, R1 is hydrogen, saturated Cijalkyl, or C5_20aryl.
= In one preferred embodiment, R1 is hydrogen or saturated C1_7a1ky1.
In one preferred embodiment, R1 is hydrogen, saturated aliphatic CiJalkyl,
or C5_20a ryl.
In one preferred embodiment, R1 is hydrogen or saturated aliphatic C1_7alkyl.
In one preferred embodiment, R1 is -H, -Me, -Et, -nPr, -iPr, -nBu, -sBu, -tBu,
-Ph,
or -Bn.
In one preferred embodiment, R1 is -H, -Me, -Et, -nPr, -iPr, -nBu, -sBu, or -
tBu.
In one preferred embodiment, R1 is -H, -Me, -Et, -Ph, or -Bn.
In one preferred embodiment, R1 is -H, -Me, or -Et.
In one preferred embodiment, R1 is -H.
The Acid Leader Group, Q2
The acid leader group, Q2, is C1_7alkylene; C5_2oarylene; C5_20arylene-
Ci_7alkylene;
C17alkylene-05_20arylene; or an ether linkage (i.e. -R2-X-R3-); and is
optionally
substituted.
In one preferred embodiment, Q2 is Cigalkylene; C5_2oarylene;
C5_20arylene-C17alkylene; or C17alkylene-05.20arylene; and is optionally
substituted.
In one embodiment, Q2 is unsubstituted.
In one embodiment, Q2 is optionally substituted.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 39 -
In one embodiment, Q2 is substituted.
The Acid Leader Group, Q2: Alkylene
In one preferred embodiment, the acid leader group, Q2, is C1_7alkylene and is
optionally substituted.
In one preferred embodiment, Q2 is a Ciqalkylene group.
In one preferred embodiment, Q2 is a saturated C1_7alkylene group.
In one preferred embodiment, Q2 is a partially unsaturated C1_7alkylene group.
In one preferred embodiment, Q2 is an aliphatic CiJalkylene group.
In one preferred embodiment, Q2 is a linear C1..7alkylene group.
In one preferred embodiment, Q2 is a branched C1_7alkylene group.
In one preferred embodiment, Q2 is a saturated aliphatic C1.7alkylene group.
In one preferred embodiment, Q2 is a saturated linear CiJalkylene group.
In one preferred embodiment, Q2 is a saturated branched CiJalkylene group.
In one preferred embodiment, Q2 is a saturated alicyclic Cigalkylene group.
group.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
-40 -
In one preferred embodiment, Q2 is a partially unsaturated linear CiJalkylene
group.
In one preferred embodiment, Q2 is a partially unsaturated branched
Ciqalkylene
group.
In one preferred embodiment, Q2 is a partially unsaturated alicyclic
Cijalkylene
group.
Note that, for embodiments excluding, e.g., unsaturation, etc., it is to be
understood that the corresponding species listed below are similarly excluded
from the respective embodiments discussed below.
In one preferred embodiment, Q2 is selected from:
-(CH2),- where n is an integer from 1 to 7;
-CH(CH3)-;
-CH(CH3)CH2- and -CH2CH(CH3)-;
-CH(CH3)CH2CH2-, -CH2CH(CH3)CH2-, and -CH2CH2CH(C1-13)-;
-CH(CH3)CH2CH2CH2-, -CH2CH(CH3)CH2CH2-, -CH2CH2CH(CH3)CH2-, and
-CH2CH2CH2CH(CH3)-;
-CH(CH3)CH2CH2CH2CH2-, -CH2CH(CH3)CH2CH2CH2-,
-CH2CH2CH(CH3)CH2CH2-, -CH2CH2CH2CH(CH3)CH2-, and
-CH2CH2CH2CH2CH(C13)-;
-CH(CH2CH3)-;
-CH(CH2CH3)CH2- and -CH2CH(CH2CH3)-;
-CH(CH2CH3)CH2CH2-, -CH2CH(CH2CH3)CH2-, and -CH2CH2CH(CH2C13)-;
-CI(CH2CH3)CH2CH2CH2-, -CH2CH(CH2CH3)CH2CI-12-,
-CH2CH2CH(CH2CH3)0H2-, and -CH2CH2CH2CH(CH2CH3)-;
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 41 -
-CH(CH2CH3)CH2CH2CH2CH2-, -CH2CH(CH2CH3)CH2CH2CH2-,
-CH2CH2CH(CH2CH3)CH2CH2-, -CH2CH2CH2CH(CH2CH3)CH2-, and
-CH2CH2CH2CH2CH(CH2CH3)-;
-CH=CH-;
-CH=CHCH2- and -CH2CH=CH-;
-CH=CHCH2CH2-, -CH2CH=CHCH2-, and -CH2CH2CH=CH-;
-CH=CHCH2CH2CH2-, -CH2CH=CHCH2CH2-, -CH2CH2CH=CHCH2-, and
-CH2CH2CH2CH=CH-;
-CH=CHCH2CH2CH2CH2-, -CH2CH=CHCH2CH2CH2-,
-CH2CH2CH=CHCH2CH2-, -CH2CH2CH2CH=CHCH2-, and
-CH2CH2CH2CH2CH=CH-;
-C(CH3)=CH- and -CH=C(CH3)-;
-C(CH3)=CHCH2-, -CH=C(CH3)CH2-, and -CH=CHCH(CH3)-;
-CH(CH3)CH=CH-, -CH2C(CH3)=CH-, and -CH2CH=C(CH3)-;
-CH=CHCH=CH-;
-CH=CHCH=CHCH2-, -CH2CH=CHCH=CH-, and -CH=CHCH2CH=CH-;
-CH=CHCH=CHCH2CH2-, -CH=CHCH2CH=CHCH2-, and
-CH=CHCH2CH2CH=CH-, -CH2CH=CHCH=CHCH2-, -CH2CH=CHCH2CH=CH-,
and -CH2CH2CH=CHCH=CH-;
-C(CH3)=CHCH=CH-, -CH=C(CH3)CH=CH-, -CH=CHC(CH3)=CH-, and
-CH=CHCH=C(CH3)-;
-CEO-;
-CECCH2-, -CH2CEC-; -CECCH(CH3)-, and -CH(CH3)CEC-;
-CECCH2CH2-, -CH2CECCH2-, and -CH2CH2CEC-;
-CECCH(CH3)CH2- and -CECCH2CH(CH3)-;
-CH(CH3)CECCH2- and -CH2CECCH(CH3)-;
-CH(CH3)CH2CEC- and -CH2CH(CH3)CEC-;
CA 02765409 2012-01-19
WO 02/30879
PCT/G1301/04326
- 42 -
-CECCH=CH-, -CF=CHCEC-, and -CECCEC-;
-CECCH2CH2CH2- and -CH2CH2CH2CEC-;
-CECCH2CH2CH2CH2- and -CH2CH2CH2CH2CEC-;
-CECCH=CHCH=CH-, -CH=CHCEC-CH=CH-, and -CH=CHCH=CHCEC-;
-C(CH3)=CHCEC-, -CH=C(CH3)CEC-, -CECC(CH3)=CH-, and
-CECCH=C(CH3)-;
cyclopentylene and cyclopentenylene; and,
cyclohexylene, cyclohexenylene, and cyclohexadienylene.
In one preferred embodiment, Q2 is selected from:
-CH2-, -(CH2)2-, -(CH2)3-, -(CH2)4-, -(CH2)5-, and -(CH2)6-;
-CH(CH3)CH2CH2CH2CH2-, -CH2CH(CH3)CH2CH2CH2-,
-CH2CH2CH2CH(CH3)CH2-, and -CH2CH2CH2CH2CH(CH3)-;
-CH=CHCH2CH2CH2- and -CH2CH2CH2CH=CH-;
-CH=CHCH2CH2CH2CH2- and -CH2CH2CH2CH2CH=CH-;
(cyclopent-1,3-ylene) (4-cyclopenten-1,3-ylene)
(cyclohex-1,4-ylene) (2-cyclohexen-1,4-ylene)
(2,5-cyclohexadien-1,4-ylene) .(cyclohex-1,4-ylene-methylene)
(methylene-cyclohex-1,4-ylene)
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
=
-43 -
In one preferred embodiment, Q2 is selected from:
-CH(CH3)CH2CH2CH2CH2- and -CH2CH2CH2CH2CH(CH3)-;
-CH2CH2CH2CH=CH-; and,
-CH2CH2CH2CH2CH=CH-.
The Acid Leader Group, 02: Arylene
In one preferred embodiment, the acid leader group, Q2, is C5_20arylene, and
is
optionally substituted.
In one preferred embodiment, Q2 is C5_2oarylene. In one preferred embodiment,
Q2 is C5_6arylene. In one preferred embodiment, Q2 is phenylene.
The Acid Leader Group, Q2:
Alkvlene-Arylene and Arylene-Alkylene
In one preferred embodiment, the acid leader group, Q2, is
C5_20arylene-C17alkylene or C17alkylene-05_20arylene, and is optionally
substituted.
In one preferred embodiment, Q2 is C5_6arylene-C1_7alkylene or
Ci7alkylene-05_6arylene, and is optionally substituted.
In one preferred embodiment, Q2 is C17alkylene-05_20arylene. In one preferred
embodiment, Q2 is C1_7alkylene-05_6arylene.
In one preferred embodiment, Q2 is C5_20arylene-Ci7alkylene. In one preferred
embodiment, Q2 is C5_6arylene-C17alkylene.
In one preferred embodiment, Q2 is Ci_7alkylene-phenylene. In one preferred
embodiment, Q2 is methylene-phenylene, ethylene-phenylene, propylene-
phenylene, and ethenylene-phenylene (also known as vinylene-phenylene).
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
=
-44 -
In one preferred embodiment, Q2 is phenylene-C1..7alkylene. In one preferred
embodiment, Q2 is phenylene-methylene, phenylene-ethylene, phenylene-
propylene, or phenylene-ethenylene (also known as phenylene-vinylene).
In the above alkylene-phenylene and phenylene-alkylene groups, the phenylene
linkage may be ortho, meta, or para, and the phenylene group is optionally
substituted with from 1 to 4 aryl substituents, RB:
RBm
B
Rnn
R Bm
ortho meta para
In one preferred embodiment, the phenylene linkage is meta or para. In one
preferred embodiment, the phenylene linkage is para. In one preferred
embodiment, the phenylene linkage is meta.
In one preferred embodiment, m is an integer from 0 to 4.
In one preferred embodiment, m is an integer from 0 to 3.
In one preferred embodiment, m is an integer from 0 to 2.
In one preferred embodiment, m is 0 or 1.
In one preferred embodiment, m is an integer from 1 to 4.
In one preferred embodiment, m is an integer from 1 to 3.
In one preferred embodiment, m is 1 or 2.
In one preferred embodiment, m is 4.
In one preferred embodiment, m is 3.
In one preferred embodiment, m is 2.
In one preferred embodiment, m is 1.
In one preferred embodiment, m is 0.
Each aryl substituent, RB, is a substituent as defined herein.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 45 -
Examples of preferred aryl substituents, RB, include, but are not limited to,
the
following: fluoro, chloro, methyl, ethyl, isopropyl, t-butyl, trifluoromethyl,
hydroxy,
methoxy, ethoxy, isopropoxy, methylthio, amino, dimethylamino, diethylamino,
morpholino, acetamido, nitro, and phenyl.
In one preferred embodiment, the phenylene linkage is meta, and Q2 has the
following formula, wherein R 2 is Ci_7alkylene and is optionally substitued
(referred to herein as "phenylene-meta-Cijalkylene"):
RBm
4111 ____________________________________
In one preferred embodiment, R 2 is a saturated C1..7alkylene group.
In one preferred embodiment, R 2 is a partially unsaturated C1_7alkylene
group.
In one preferred embodiment, R 2 is an aliphatic C1_7alkylene group.
In one preferred embodiment, RQ2 is a linear CiJalkylene group.
In one preferred embodiment, R 2 is a branched Cijalkylene group.
In one preferred embodiment, R 2 is an alicyclic CiJalkylene group.
In one preferred embodiment, RQ2 is a saturated aliphatic CiJalkylene group.
In one preferred embodiment, R 2 is a saturated linear C17alkylene group.
In one preferred embodiment, R 2 is a saturated branched Cvialkylene group.
In one preferred embodiment, RC)2 is a saturated alicyclic Cijalkylene group.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 46 -
In one preferred embodiment, R 2 is a partially unsaturated aliphatic
Ci_7alkylene
group.
In one preferred embodiment, R 2 is a partially unsaturated linear
C1_7alkylene
group.
In one preferred embodiment, R 2 is a partially unsaturated branched
C1_7alkylene
group.
In one preferred embodiment, R 2 is a partially unsaturated alicyclic
CiJalkylene
group.
In one preferred embodiment, RQ2 is selected from:
-(CH2)n- where n is an integer from 1 to 7;
-CH(CH3)-;
-CH(CH3)CH2- and -CH2CH(CH3)-;
-CH(CH3)CH2CH2-, -CH2CH(CH3)CH2-, and -CH2CH2CH(CH3)-;
-CH(CH3)CH2CH2CH2-, -CH2CH(CH3)CH2CH2-, -CH2CH2CH(CH3)CH2-, and
-CH2CH2CH2CH(CH3)-;
-CH(CH3)CH2CH2CH2CH2-, -CH2CH(CH3)CH2CH2CH2-,
-CH2CH2CH(CH3)CH2CH2-, -CH2CH2CH2CH(CH3)CH2-, and
-CH2CH2CH2CH2CH(CH3)-;
-CH(CH2CH3)-;
-CH(CH2CH3)CH2- and -CH2CH(CH2CH3)-;
-CH(CH2CH3)CH2CH2-, -CH2CH(CH2CH3)CH2-, and -CH2CH2CH(CH2CF13)-;
-CH(CH2CH3)CH2CH2CH2-, -CH2CH(CH2CH3)CH2CH2-,
-CH2CH2CH(CH2CH3)CH2-, and -CH2CH2CH2CH(CH2CH3)-;
-CH(CH2CH3)CH2CH2CH2CH2-, -CH2CH(CH2CH3)CH2CH2CH2-,
-CH2CH2CH(CH2CH3)CH2CH2-, -CH2CH2CH2CH(CH2CH3)CH2-, and
-CH2CH2CH2CH2CH(CH2CH3)-;
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
-47 -
-CH=CH-;
-CH=CHCH2- and -CH2CH=CH-;
-CH=CHCH2CH2-, -CH2CH=CHCH2-, and -CH2CH2CH=CH-;
-CH=CHCH2CH2CH2-, -CH2CH=CHCH2CH2-, -CH2CH2CH=CHCH2-, and
-CH2CH2CH2CH=CH-;
-CH=CHCH2CH2CH2CH2-, -CH2CH=CHCH2CH2CI-12-,
-CH2CH2CH=CHCH2CH2-, -CH2CH2CH2CH=CHCH2-, and
-CH2CH2CH2CH2CH=CH-;
-C(CH3)=CH- and -CH=C(CH3)-;
-C(CH3)=CHCH2-, -CH=C(CH3)CH2-, and -CH=CHCH(CH3)-;
-CH(CH3)CH=CH-, -CH2C(CH3)=CH-, and -CH2CH=C(CH3)-;
-CH=CHCH=CH-;
-CH=CHCH=CHCH2-, -CH2CH=CHCH=CH-, and -CH=CHCH2CH=CH-;
-CH=CHCH=CHCH2CH2-, -CH=CHCH2CH=CHCH2-, and
-CH=CHCH2CH2CH=CH-, -CH2CH=CHCH=CHCH2-, -CH2CH=CHCH2CH=CH-,
and -CH2CH2CH=CHCH=CH-;
-C(CH3)=CHCH=CH-, -CH=C(CH3)CH=CH-, -CH=CHC(CH3)=CH-, and
-CH=CHCH=C(CH3)-;
-CEO-;
-CECCH2-, -CH2CEC-; -C_CCH(CH3)-, and -CH(CH3)CEC-;
-CE-CCH2CH2-, -CH2CECCH2-, and -CH2CH2CF----C-;
-CECCH(CH3)CH2- and -CECCH2CH(CH3)-;
-CH(CH3)CECCH2- and -CH2CECCH(CH3)-;
-CH(CH3)CH2C-Y_C- and -CH2CH(CH3)CEC-;
-CECCH=CH-, -CH=CHCEC-, and -CECCE-C-;
-CECCH2CH2CH2- and -CH2CH2CH2CEC-;
-CE-*CCH2CH2CH2CH2- and -CH2CH2CH2CH2CEC-;
CA 02765409 2012-01-19
WO 02/30879
PCT/C1301/04326
-48 -
-CECCH=CHCH=CH-, -CH=CHCEC-CH=CH-, and -CH=CHCH=CHCEC-;
-C(CH3)=CHCE-C-, -CH=C(CH3)CEC-, -C-ECC(0H3)=CH-, and
-CECCH=C(CH3)-;
cyclopentylene and cyclopentenylene; and,
cyclohexylene, cyclohexenylene, and cyclohexadienylene.
In one preferred embodiment, R 2 is selected from:
-CH2-, -(CH2)2-, -(CH2)3-, -(CH2)4-, -(CH2)5-, and -(CH2)6-;
-CH=CH-, -CH=CH-CH=CH-;
In one preferred embodiment, RQ2 is cis or trans -CH=CH-.
In one preferred embodiment, IR 2 is cis -CH=CH-.
In one preferred embodiment, IR 2 is trans -CH=CH-.
In one preferred embodiment, RQ2 is -CH=CH-, and Q2 is (referred to herein as
"phenylene-meta-trans-ethylene"):
RBm
In one preferred embodiment, m is 0, and Q2 is (referred to herein as
"unsubstituted phenylene-meta-trans-ethylene"):
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
-49 -
The Acid Leader Group, Q2: Ether
In one embodiment, Q2 is an ether linkage, -R2-X-R3-, wherein X is an ether
heteroatom, and is -0- or -S- and each of R2 and R3 is independently an ether
group.
Each of the ether groups, R2 and R3, is independently a Ciqalkylene group, and
is
optionally substituted.
In one embodiment, each of R2 and R3 is unsubstituted. In one embodiment,
each of R2 and R3 is optionally substituted. In one embodiment, each of R2 and
R3 is substituted.
In one preferred embodiment, R2 and/or R3 is a saturated CiJalkylene group.
In one preferred embodiment, R2 and/or R3 is a partially unsaturated
C1.7alkylene
group.
In one preferred embodiment, R2 and/or R3 is an aliphatic C1_7alkylene group.
In one preferred embodiment, R2 and/or R3 is a linear C1.7alkylene group.
In one preferred embodiment, R2 and/or R3 is a branched Cijalkylene group.
In one preferred embodiment, R2 and/or R3 is an alicyclic CiJalkylene group.
In one preferred embodiment, R2 and/or R3 is a saturated aliphatic
C1_7alkylene
group.
In one preferred embodiment, R2 and/or R3 is a saturated linear CiJalkylene
group.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 50 -
In one preferred embodiment, R2 and/or R3 is a saturated branched Ci_7alkylene
group.
In one preferred embodiment, R2 and/or R3 is a saturated alicyclic CiJalkylene
group.
In one preferred embodiment, R2 and/or R3 is a partially unsaturated aliphatic
C1_7alkylene group.
In one preferred embodiment, R2 and/or R3 is a partially unsaturated linear
Ci_7alkylene group.
In one preferred embodiment, R2 and/or R3 is a partially unsaturated branched
CiJalkylene group.
In one preferred embodiment, R2 and/or R3 is a partially unsaturated alicyclic
CiJalkylene group.
In one preferred embodiment, R2 and/or R3 is selected from:
-(CH2)n- where n is an integer from 1 to 7;
-CH(CH3)-;
-CH(CH3)CH2- and -CH2CH(CH3)-;
-CH(CH3)CH2CH2-, -CH2CH(CH3)CH2-, and -CH2CH2CH(CH3)-;
-CH(CH3)CH2CH2CH2-, -CH2CH(CH3)CH2CH2-, -CH2CH2CH(CH3)CH2-, and
-CH2CH2CH2CH(CH3)-;
-CH(CH3)CH2CH2CH2CH2-, -CH2CH(CH3)CH2CH2CH2-,
-CH2CH2CH(CH3)CH2CH2-, -CH2CH2CH2CH(CH3)CH2-, and
-CH2CH2CH2CH2CH(CH3)-;
-CH(CH2CH3)-;
CA 02765409 2012-01-19
WO 02/30879 PCT/C1301/04326
- 51 -
-CH(CH2CH3)CH2- and -CH2CH(CH2CH3)-;
-CH(CH2CH3)CH2CH2-, -CH2CH(CH2CH3)CH2-, and -CH2CH2CH(CH2CH3)-;
-CH(CH2CH3)CH2CH2CH2-, -CH2CH(CH2CH3)CH2CF12-,
-CH2CH2CH(CH2CH3)CH2-, and -CH2CH2CH2CH(CH2CH3)-;
-CH(CH2CH3)CH2CH2CH2CH2-, -CH2CH(CH2CH3)CH2CH2CF12-,
-CH2CH2CH(CH2CH3)CH2CH2-, -CH2CH2CH2CH(CH2CH3)CH2-, and
-CH2CH2CH2CH2CH(CH2CH3)-;
-CH=CH-;
-CH=CHCH2- and -CH2CH=CH-;
-CH=CHCH2CH2-, -CH2CH=CHCH2-, and -CH2CH2CH=CH-;
-CH=CHCH2CH2CH2-, -CH2CH=CHCH2CH2-, -CH2CH2CH=CHCH2-, and
-CH2CH2CH2CH=CH-;
-CH=CHCH2CH2CH2CH2-, -CH2CH=CHCH2CH2CH2-,
-CH2CH2CH=CHCH2CH2-, -CH2CH2CH2CH=CHCH2-, and
-CH2CH2CH2CH2CH=CH-;
-C(CH3)=CH- and -CH=C(CH3)-;
-C(CH3)=CHCH2-, -CH=C(CH3)CH2-, and -CH=CHCH(CH3)-;
-CH(CH3)CH=CH-, -CH2C(CH3)=CH-, and -CH2CH=C(CH3)-;
-CH=CHCH=CH-;
-CH=CHCH=CHCH2-, -CH2CH=CHCH=CH-, and -CH=CHCH2CH=CH-;
-CH=CHCH=CHCH2CH2-, -CH=CHCH2CH=CHCH2-, and
-CH=CHCH2CH2CH=CH-, -CH2CH=CHCH=CHCH2-, -CH2CH=CHCH2CH=CH-,
and -CH2CH2CH=CHCH=CH-;
-C(CH3)=CHCH=CH-, -CH=C(CH3)CH=CH-, -CH=CHC(CH3)=CH-, and
-CH=CHCH=C(CH3)-;
-CEC-;
-CECCH2-, -CH2CEC-; -CE-CCH(CH3)-, and -CH(CH3)CEC-;
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 52 -
-CECCH2CH2-, -CH2CECCH2-, and -CH2CH2CEC-;
-CECCH(CH3)CH2- and -CECCH2CH(CH3)-;
-CH(CH3)CF--:CCH2- and -CH2CECCH(CH3)-;
-CH(0H3)CH2CEC- and -CH2CH(CH3)CEC-;
-CECCH=CH-, -CH=CHCEC-, and -CECCEC-;
-CECCH2CH2CH2- and -CH2CH2CH2CEC-;
-C-ECCH2CH2CH2CH2- and -CH2CH2CH2CH2CEC-;
-CECCH=CHCH=CH-, -Cl=CHCEC-CH=CH-, and -CH=CHCH=CHCEC-;
-C(CH3)=CHCEC-, -CH=C(CH3)CE-C-, -CECC(CH3)=CH-, and
-CECCH=C(CH3)-;
cyclopentylene and cyclopentenylene; and,
cyclohexylene, cyclohexenylene, and cyclohexadienylene.
In one preferred embodiment, R2 and/or R3 is selected from:
-CH2-, -(CH2)2-, -(CH2)3-, -(CH2)4-, -(CH2)5-, and -(CH2)6-;
-CH(CH3)CH2CH2CH2CH2-, -CH2CH(CH3)CH2CH2CH2-,
-CH2CH2CH2CH(CH3)CH2-, and -CH2CH2CH2CH2CH(0H3)-;
-CH=CHCH2CH2CH2- and -CH2CH2CH2CH=CH-;
-CH=CHCH2CH2CH2CH2- and -CH2CH2CH2CH2CH=CH-;
(cyclopent-1,3-ylene) (4-cyclopenten-1,3-ylene)
(cyclohex-1,4-ylene) (2-cyclohexen-1,4-ylene)
¨OH ¨OH
(2,5-cyclohexadien-1,4-ylene) (cyclohex-1,4-ylene-methylene)
¨OH
CA 02765409 2012-01-19
WO 02/30879
PCT/C1301/04326
- 53 -
(methylene-cyclohex-1,4-ylene)
\-0-1
In one preferred embodiment, each of R2 and R3 is a saturated Ciqalkylene
group.
In one preferred embodiment, each of R2 and R3 is selected from -(CH2)n-,
wherein n is an integer from 1 to 5.
In one preferred embodiment, the group R2-X-R3 is selected from the following:
-CH2-0-CH2- and -CH2-S-CH2-;
-CH2-0-CH2CH2- and -CH2-S-CH2CH2-;
-CH2CH2-0-CH2- and -CH2CH2-S-CH2-;
-CH2-0-CH2CH2CH2- and -Cl2-S-CH2CH2CH2-;
-CH2CH2-0-CH2CH2- and -CH2CH2-S-CH2CH2-;
-CH2CH2CH2-0-CH2- and -CH2CH2CH2-S-CH2-;
-CH2-0-CH2CH2CH2CH2- and -CH2-S-CH2CH2CH2CH2-;
-CH2CH2-0-CH2CH2CH2- and -CH2CH2-S-CH2CH2CH2-;
-CH2CH2CH2-0-CH2CH2- and -CI-12CH2CH2-S-CH2CH2-;
-CH2CH2CH2CH2-0-CH2- and -CH2CH2CH2CH2-S-0H2-;
-CH2-0-CH2CH2CH2CH2CH2- and -CH2-S-CH2CH2CH2CH2CH2-;
-CH2CH2-0-CH2CH2CH2CH2- and -CH2CH2-S-CH2CH2CH2CH2-;
-CH2CH2CH2-0-CH2CH2CH2- and -CH2CH2CH2-S-CH2CH2CH2-;
-CH2CH2CH2CH2-0-CH2CH2- and -CH2CH2CH2CH2-S-CH20H2-;
-CH2CH2CH2CH2CH2-0-CH2- and -CH2CH2CH2CH2CH2-S-CFI2-;
In one preferred embodiment, the group R2-X-R3 is selected from the following:
-CH2-0-CH2- and -CH2-S-CH2-.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 54 -
In one preferred embodiment, the group R2-X-R3 is selected from the following:
-CH2-0-CH2CH2- and -CH2-S-CH2CH2-;
-CH2CH2-0-CH2- and -CH2CH2-S-CH2-.
In one preferred embodiment, the group R2-X-R3 is selected from the following:
-CH2-0-CH2CH2CH2- and -CH2-S-CH2CH2CH2-;
-CH2CH2-0-CH2CH2- and -CH2CH2-S-CH2CH2-;
-CH2CH2CH2-0-CH2- and -CH2CH2CH2-S-CH2-.
In one preferred embodiment, the group R2-X-R3 is selected from the following:
-CH2-0-CH2CH2CH2CH2- and -CH2-S-CH2CH2CH2CH2-;
-CH2CH2-0-CH2CH2CH2- and -CH2CH2-S-CH2CH2CH2-;
-CH2CH2CH2-0-CH2CH2- and -CH2CH2CH2-S-CH2CH2-;
-CH2CH2CH2CH2-0-CH2- and -CH2CH2CH2CH2-S-CH2-.
In one preferred embodiment, the group R2-X-R3 is selected from the following:
-CH2-0-CH2CH2CH2CH2CH2- and -CH2-S-CH2CH2CH2CH2CH2-;
-CH2CH2-0-CH2CH2CH2CH2- and -CH2CH2-S-CH2CH2CH2CH2-;
-CH2CH2CH2-0-CH2CH2CH2- and -CH2CH2CH2-S-CH2CH2CH2-;
-CH2CH2CH2CH2-0-CH2CH2- and -CH2CH2CH2CH2-S-CH2CH2-;
-CH2CH2CH2CH2CH2-0-CH2- and -CH2CH2CH2CH2CH2-S-CH2-.
Certain Embodiments
In one preferred embodiment, Q1 is a covalent bond or an aryl leader group, J
is
-NR1S02-, Q2 is meta-phenylene-CiJalkylene, and the compounds have the
following formula:
Fern
0 (8)
I I 01 I I
A __________________ Q1 _____ N S R 2 C N-OH
1 , 11
R 0
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 55 -
In one preferred embodiment, Q1 is a covalent bond, J is -NR1S02-, Q2 is meta-
phenylene-Cvialkylene, and the compounds have the following formula:
RBõ,
o =(9)
Q2
A _________________________ N S R-C-N-OH
, II H=
R
In one preferred embodiment, Q1 is an aryl leader group, J is -NR1S02-, Q2 is
meta-phenylene-C1..7alkylene, and the compounds have the following formula:
RBm
(8)
õ 410
A-QL-N-S Q2
R-C-N-OH
I II
R 0
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 2 carbon atoms.
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 3 carbon atoms.
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 4 carbon atoms.
In one preferred embodiment, the aryl leader group, Ql, has a backbone of at
least 5 carbon atoms.
In one preferred embodiment, Q1 is -CH2CH2-, J is -NR1S02-, Q2 is meta-
phenylene-C1_7alkylene, and the compounds have the following formula:
RB.
o 411 (10)
02
N-S R-C-N-OH
I HI
R ID
In one preferred embodiment, Q1 is a covalent bond or an aryl leader group, J
is
-NR1S02-, Q2 is phenylene-meta-trans-ethylene, and the compounds have the
,following formula:
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 56 -
RBm
0 (11)
II Oil
A __________________ Q1 __ N S C-N-OH
I II
R 0
In one preferred embodiment, Q1 is a covalent bond, J is -NR1S02-, Q2 is.
phenylene-meta-trans-ethylene, and the compounds have the following formula:
RBm
(12)
A ____________________ N S C-N-OH
I II
R 0
In one preferred embodiment, Q1 is an aryl leader group, J is -NR1S02-, Q2 is
phenylene-meta-trans-ethylene, and the compounds have the following formula:
RBm
o = (11)
A __________________ Q1 __ N-S C-N-OH
I II
R 0
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 2 carbon atoms.
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 3 carbon atoms.
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 4 carbon atoms.
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 5 carbon atoms.
In one preferred embodiment, Q1 is -CH2CH2-, J is -NR1S02-, Q2 is phenylene-
meta-trans-ethylene, and the compounds have the following formula:
CA 02765409 2012-01-19
WO 02/30879
PCT/C1301/04326
- 57 -
Fern
O 0 (13)
11
N-S C-N-OH
R 0
In one preferred embodiment, A is an optionally substituted phenyl group, Q1
is a
covalent bond or an aryl leader group, J is -NR1S02-, Q2 is phenylene-meta-
trans-
ethylene, and the compounds have the following formula:
RBm
(14)
4.11 Q1 ____________________ N-S C-N-OH
01
RA R
In one preferred embodiment, A is an optionally substituted phenyl group, Q1
is a
covalent bond, J is -NR1S02-, Q2 is phenylene-meta-trans-ethylene, and the
compounds have the following formula:
RBm
3' 2' 0 (15)
4.4) C-N-OH
1
RA 1 R 0
In one preferred embodiment, A is an optionally substituted phenyl group, Q1
is an
aryl leader group, J is -NR1S02-, Q2 is phenylene-meta-trans-ethylene, and the
compounds have the following formula:
RBm
Q1 N-ISI 11 (14)
4 ' 40 C-N-OH
, 11
RA R. 0
ri 5' 6'
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 2 carbon atoms.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 58 -
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 3 carbon atoms.
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 4 carbon atoms.
In one preferred embodiment, the aryl leader group, Q1, has a backbone of at
least 5 carbon atoms.
In one preferred embodiment, A is an optionally substituted phenyl group, Q1
is
-CH2CH2-, J is -NR1S02-, Q2 is phenylene-meta-trans-ethylene, and the
compounds have the following formula:
R
3 .'
RAn N 40 2'
0 (16)
5' N¨S C¨N¨OH
6' I II
R 0
Examples of Specific Embodiments
Examples of compounds with J as -SO2NR1- and no Q1 group (i.e., where Q1 is a
covalent bond) are shown below, for comparison purposes.
SIC'N N,
OH PX089342
OH
0
Me
111
2e s, l II N I\LOH PX089344
0 H
0
0
0
N,OH
3
s, PX106499
110II N
OH
CA 02765409 2012-01-19
WO 02/30879 PCT/GBOI
/04326
- 59 -
o
II H
s, N,
4 II N OH PX106522
401 0 H
0
1 0 H
N/ PX117432
õN OH .
OH
H
0
Me
I
0
6 Meõ illo
,5)PX117780
0 ,sõ SI 7- 0.,
q N OH
OH
0
Si
7 1 ,o[1 PX117781
i/ N OH
0 H
0
OH
\\ ,N si
0 S\\0 H
8 7- N,
OH PX117793
lei 0
OH
H
l
\\ ,N ao e S\\0
H
9Me 7 N,
OH PX117794
0
0
,
Me0
Some individual embodiments of the present invention include the following
compounds.
_ _______________________________________________________________
40 I 001 ICI
'
ON PX089343
c; N -- r '
0
11 el 0\\ la / 11 PX105684
,s 'OH
H \\0 0
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 60 -
12
Si % 0 11
S ''OH PX105685
N \\
1 0 0
Me
Me0
13 , OH
S
0\\s 110
11 . PX105844
N \\0 0
Me
14 elOH PX106508
N \\0 0
Br
15 Si R\s SI 11, OH PX106509
N \\0 0
CI
16 le \\S 01 .--- I, OH PX106510
N \\
H 0 0
()\\ lel / H
17 OH PX106511
40 N\ 0
18
% SI 11
,s OH PX106512
N % 0
el 01
's
19 , H PX116238
N \\ .- N,
H 0 OH
0 .
0 / EN1,
OH
20 o--=s=o o PX116242
1
= NH
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 61 -
211111 0 H
N,
S OH PX117225
lel Ill \ \O 0
C)\\ 101 _. IN.., .
22 0 0 FNA k OH PX117226
.,
N
<o 0
0 0\\ 401 ,i,
23 PX117227
\\
H 0 0
OMe
24 S\ d,OH PX117228
H 0
=Nõ s N,
H
OH
25 PX117233
0
0
0,\ H
N,
26 40 S OH PX117234
N \\
H 0 0
_
H 0
N 0 H
27 *lel 0//s N,
OH PX117235
0
NH 0 H
0
28 40 ,
os N,OH PX117236
0
0
N% H
N,
29 SI S OH PX117245
\\
H 0 0
0 H
\\ . _. N
30 ,
NNS\\ OH PX117250
1 H 0 0
-
CA 02765409 2012-01-19
WO 02/30879
PCT/C1301/04326
- 62 -
Me
I 0
N // H
31 le
N, PX117260
OH
0
0
32 le o
\\S H
N,OH PX117410
N \\
'
1 0 0
Me
H
(10 I:)\\ N,
33 ,S OH PX117411
N \\
1 0 0
Me
11110H
0\\ N,
S OH
N\ \
34 o o PX117412
lel
401 R\ H
N,OH
S
N \\
35 o o PX117414
S
0
H
36 N OH
el & N, PX117429
0 H
0
37 540 0.\ 410,-- Fri,
S
PX117445
N \\ OH
H 0 0
38 lel ii
o
.-- //s,N 1101OH
H
-
,- N, PX117446
0 H
0
-
CA 02765409 2012-01-19
WO 02/30879
PCT/C1301/04326
- 63 -
OH ,OH
0-----S=0 0
39
NH PX117447
Okl,OH
40 0=---S=0 0 PX117448
N,
Si Me
HN, //0
41 PX117450
40
N,
OH
0
0
HN,
42
Oi 0
PX117453
N,
OH
0
0OH
4 \\ N
3 ,
OH PX117710
N
H 0 0
0
44 cZ\PX117712
OH
Me0
H u 0
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 64 -fa ,-- ii.,_
45 OH PX117713
N \\
H 0 0
Ph
Os, 5H
N
40 H 0 II
0 PX117715
46
1001
47 5 (:)\\ 5 ,-- EN1, PX117734
OH
Br N \\
H 0 0
48 IIII
aR\ 0kii3OH PX117735
N \\
H 0 0
H 0
N ii
SI .
49
le) 0 H
/ N,
OH PX117736
o
Olo,
50 v 40 .__ Fd, PX117773
s
0 OH
\\c) o
lei n
51 el -\\ el _-Fri,
,S
N \\ OH PX117774
H o o
F3co 5 0 5
H
52 \\ ,-- N, PX117775
,S OH
N \\
H 0 0
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 65 -
c)\\ IN.,,' ki,
Me0 . S OH
53 N \\ PX117778
H 0 0
Me0
OMe
OMe
Me0 0
54 \ 5H
.õ N, PX117779
,s OH
N \\
H 0 0
Me
O
55 0\ H PX117782
Me õo 5S (I -' NLOH
N \\
HO 0
R
I. \ el / M,
56 S OH PX117787
N"
H 0 0
F
57 1 c)\\ (10 ,- Eni, PX117788
s OH
F N \\
H 0 0
F F
-..õ...--
0 0
58 PX117789
\\ 1110 ...- kilõ
,S OH
N \\
H 0
0 Fo\\ 0 pi,
59
N \\,.,
H u 0
F
OF,
60 14110 c)\\ 5i'd,
,s OH PX117791
N \\
HO o
,0
Me _
P
61 loi le ol SI H PX117792
/ N.
OH
0
CA 02765409 2012-01-19
WO 02/30879
PCT/G1301/04326
- 66 -
H 0
N., //
/S
62 la o/ is Br
H PX117795
/ N.
OH
0
CI 0
63 \\ OP ,--- kl.õ PX117796
,S OH
N \\
H 0 0
Et
\
ON ei
H
64 N \\ OH
N PX117798
H 0 0
NC 411
65 (:)\\ el ,-= II,
N
,s\\ OH
H 0 0
'
Me0
66 el (31""s lei 1\11`0H
N \\
HO 0
67
F,C 0
o"" la ,, 11,0H
.,S
H u 0
,
CFµ"S eli 0 H
68 ,--- N.
,S OH
N' \õ
H u 0
0
69 I.R\ IP .,..-- 11,
S
N'\" OH
H 0 0
0
70 D1' \\ 0 ,.. 0,
N\ OH
OH
H 0
HP2 N, /
s
71 cir 401 0\\ le Ifr\i3OH
N,s\\,,
H u 0
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 67 -
72 40 0\\ 40 7 i
d
NS ,OH
,. \\
H 0 0
HO 40
73 N \\ ENI
R\ SI ,-- ,
,s - OH
,_,
H ,-, 0
74 110 0\\ op 7 ii3OH
S
NC N \`õ
H ,-, 0
el R\ le75 H ,.s\ ,7 N,
OH
N \
H 0 0
0
el R\ 100 - [1
7 ,
76 H2N N ,,,S OH
H \\,_, k., 0
o
77 SI v., ki,
HO S OH
N \\,.,
H Li o
78 111111 c)\\ SI EN1,
cF3,s S OH
N \\,õ
H u 0
el FN1,
OH
N \\
H 0 0
CF,
_
ei 0\\ 40 N 7 ,
80 ,,S OH
\\
H 0 0
CI
_
el R\ 11101 / kli õ
81 ,S OH
N \\
H 0 0
HO
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 68 -
Me 40
82 N inii,
,S\\ OH
Me
H 0
F
83
Fr&
õ--S\
F N OH
\,.,
H Li 0
F 40
84 R\ 40 .,- LI OH
,
CI N \\,,,
H Li 0
CI 0
0\\ le ...., Fd OH
,
85 ,S
N \\,_,
H Li 0
CF3
Br el 0
\\ \ 11101 --' H,
86 OH
,S
N \
H 0 0
CI
CI is 0
87 \\ 410 --- kl
N OH
,s\\,_,
H k-, 0
CI
CF3 .
R\ 1101 -- Ed,
88 ,,S OH
N \\
H 0 0
OMe
F 41
H
R\ lial
89 N / N,
,S OH
\\
H 0 0
CF3
Br el 0
90 \\ le --- ld.OH
,S
N \\
H 0 0
F
F,
H
91 R\s SI OH
.,-- N.,
N-- \\,_,
H L, 0
F
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 69 -
F
92 111111 R\ OH
4111 ,7- kii,
F N,s\\õ
H u 0
_
CF3
93 =R\ SI H
.,--" N,
--S OH
CF3 N \\õ
H u 0
CF3
94 01 R\ 1101.7- H.,
,S OH
N \\
H 0 0
CF3
CF3
95 111111R\ 5 õ--- Fd,
S OH
N \\
H 0 0
F
CF3
96 5o5\\ r\l,
S OH
N \\
H 0 0
Br
F
el R\ le--- FN1õ
OH
N \\
H 0 0
F
SI MC,
\\ 401 / Id,
OH
98 ,S
N \\
H 0 0
Me
0 iPOr H
99 \\ 1101 ,--- 1\1,,
.-S OH
N \\
H 0 0
iPr
F Br
100
11111 H
O\\ la / N,
OH
H u 0
Br
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 70 -
CI ______________________________________________________________
HO el
101 R\ IP / FNI,
S OH
N \\
H 0 0
CI
CI
CI .
,...S, OH
H 0 0
SOS .... kii,
õS OH
N \\
103 o 0
40
.0\\ op ..., fd,
,õS OH
104 N \\
0 0
\-,-'
el R\ le ,,- INI,
S OH
N \\
105 o 0
-õ,
N
1 R\ laH
,-- N,
106 ,,S OH
110 il \\0 0
107 Nal
I -, R\ III Fd,
I.
NS\\ OH
H ID 0
NC
_., Fd,
108 ,,S OH
la H \\c) 0
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
-71-
109 11, II)
N N
N \\ E,
,S OH
--/ H 0 0
N
0 H
110 \ IO N r\
\\S\\õ*. LOH
H L, 0
R\\ la 7 N,
111 S OH
T'-'Y'''' '''' HN \\O 0
F
R\ 40 7 N,
40
112 ,,S OH ri \\,0 0
Br
R\\ 11101 7 N,
OH
113 5 N \\(3,
0
110
O H
\\ le / N,
S OH
114 5 N \\0
0
115 a (:)\\ 5 / F1\11 ,
OH
. N\'
H 0 0
HO ei
116 \\ 111101 ,- ri,
,S OH
HO N \\
H 0 0
117
lei0
\\ 5 H
7 ,N,
--S\ OH
N \
H 0 0
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 72 -
S
118 14111 0 H
\\ 1110 7-- N,
õ
N \\,_, OH
H u 0
OH
119 1011110 H
\\ 11101 7-- Nõ
õS =
N \` OH
H 0 0
0
H
N H
120
. 1 R\ [10 õ. N,
7S
N \\
H 0 0 OH
_
121411 N
I R\c, * / 0 H
N ---- N \
H H 0 0 _
,
122 I (:)\\, le r1.01-1
H ,-,r,
0
123 HN \\s 5 7- N,
OH
N \\,.,
H u 0
0\\ 1101 / NI,
124 5
N \\
H 0 0 OH
125
(:)\\ 1101 .- Li, 0 H
,S
N \\
H 0 0
Chemical Terms
The term "carbo," "carbyl," "hydrocarbo," and "hydrocarbyl," as used herein,
5 pertain to compounds and/or groups which have only carbon and hydrogen
atoms.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 73 -
The term "hetero," as used herein, pertains to compounds and/or groups which
have at least one heteroatom, for example, multivalent heteroatoms (which are
also suitable as ring heteroatoms) such as boron, silicon, nitrogen,
phosphorus,
oxygen, and sulfur, and monovalent heteroatoms, such as fluorine, chlorine,
bromine, and iodine.
The term "saturated," as used herein, pertains to compounds and/or groups
which
do not have any carbon-carbon double bonds or carbon-carbon triple bonds.
The term "unsaturated," as used herein, pertains to compounds and/or groups
which have at least one carbon-carbon double bond or carbon-carbon triple
bond.
The term "aliphatic," as used herein, pertains to compounds and/or groups
which
are linear or branched, but not cyclic (also known as "acyclic" or "open-
chain"
groups).
The term "cyclic," as used herein, pertains to compounds and/or groups which
have one ring, or two or more rings (e.g., Spiro, fused, bridged).
The term "ring," as used herein, pertains to a closed ring of from 3 to 10
covalently linked atoms, more preferably 3 to 8 covalently linked atoms.
The term "aromatic ring," as used herein, pertains to a closed ring of from 3
to 10
covalently linked atoms, more preferably 5 to 8 covalently linked atoms, which
ring
is aromatic.
The term "heterocyclic ring," as used herein, pertains to a closed ring of
from 3 to
10 covalently linked atoms, more preferably 3 to 8 covalently linked atoms,
wherein at least one of the ring atoms is a multivalent ring heteroatom, for
example, nitrogen, phosphorus, silicon, oxygen, and sulfur, though more
commonly nitrogen, oxygen, and sulfur.
CA 02765409 2012-01-19
WO 02/30879 PCT/CB01/04326
- 74 -
The term "alicyclic," as used herein, pertains to compounds and/or groups
which
have one ring, or two or more rings (e.g., Spiro, fused, bridged), wherein
said
ring(s) are not aromatic.
The term "aromatic," as used herein, pertains to compounds and/or groups which
have one ring, or two or more ring (e.g., fused), wherein at least one of said
ring(s) is aromatic.
The term "heterocyclic," as used herein, pertains to cyclic compounds and/or
groups which have one heterocyclic ring, or two or more heterocyclic rings
(e.g.,
Spiro, fused, bridged), wherein said ring(s) may be alicyclic or aromatic.
The term "heteroaromatic," as used herein, pertains to cyclic compounds and/or
groups which have one heterocyclic ring, or two or more heterocyclic rings
(e.g.,
fused), wherein said ring(s) is aromatic.
Substituents
The phrase "optionally substituted," as used herein, pertains to a parent
group
which may be unsubstituted or which may be substituted.
Unless otherwise specified, the term "substituted," as used herein, pertains
to a
parent group which bears one or more substituents. The term "substituent" is
used herein in the conventional sense and refers to a chemical moiety which is
covalently attached to, appended to, or if appropriate, fused to, a parent
group. A
wide variety of substituents are well known, and methods for their formation
and
introduction into a variety of parent groups are also well known.
In one preferred embodiment, the substituent(s), often referred to herein as
R, are
independently selected from: halo; hydroxy; ether (e.g., Ci_7alkoxy); formyl;
acyl
(e.g., CiJalkylacyl , C5_20arylacyl); acylhalide; carboxy; ester; acyloxy;
amido;
acylamido; thioamido; tetrazolyl; amino; nitro; nitroso; azido; cyano;
isocyano;
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
-75 -
cyanato; isocyanato; thiocyano; isothiocyano; sulfhydryl; thioether (e.g.,
Cijalkylthio); sulfonic acid; sulfonate; sulfone; sulfonyloxy; sulfinyloxy;
sulfamino;
sulfonamino; sulfinamino; sulfamyl; sulfonamido; Cijalkyl (including, e.g.,
C14haloalkyl, Ciqhydroxyalkyl, C1_7carboxyalkyl, Ciqaminoalkyl, C5_20aryl-
Cijalkyl); C3.20heterocycly1; or C5_20aryl (including, e.g., C5_20carboaryl,
C5_20heteroaryl, C17alkyl-05_20aryi and C5.20haloary1)).
In one preferred embodiment, the substituent(s), often referred to herein as
R, are
independently selected from:
-F, -Cl, -Br, and -I;
-OH;
-0Me, -0Et, -0(tBu), and -OCH2Ph;
-SH;
-SMe, -SEt, -S(tBu), and -SCH2Ph;
-C(0)H;
-C(0)Me, -C(=0)Et, -C(=0)(tBu), and -C(0)Ph;
-C(=0)0H;
-C(=0)0Me, -C(=0)0Et, and -C(=0)0(tBu);
-C(=0)NH2, -C(=0)NHMe, -C(=0)NMe2, and -C(=0)NHEt;
-NHC(=0)Me, -NHC(=0)Et, -NHC(=0)Ph, succinimidyl, and maleimidyl;
-NH2, -NHMe, -NHEt, -NH(iPr), -NH(nPr), -NMe2, -NEt2, -N(iPr)2, -N(nPr)2,
-N(nBu)2, and -N(tB02;
-CN;
-NO2;
-Me, -Et, -nPr, -iPr, -nBu, -tBu;
-CF3, -CHF2, -CH2F, -CCI3, -CBr3, -CH2CH2F, -CH2CHF2, and -CH2CF3;
-0CF3, -OCHF2, -OCH2F, -OCCI3, -OCBr3, -OCH2CH2F, -OCH2CHF2, and
-OCH2CF3;
-CH2OH, -CH2CH2OH, and -CH(OH)CH2OH;
-CH2NH2,-CH2CH2NH2, and -CH2CH2NMe2; and,
optionally substituted phenyl.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 76 -
In one preferred embodiment, the substituent(s), often referred to herein as
R, are
independently selected from: -F, -Cl, -Br, -I, -OH, -0Me, -0Et, -SH, -SMe, -
SEt,
-C(=0)Me, -C(=0)0H, -C(=0)0Me, -CONH2, -CONHMe, -NH2, -NMe2, -NEt2,
-N(nPr)2, -N(iPr)2, -ON, -NO2, -Me, -Et, -CF3, -0CF3, -CH2OH, -CH2CH2OH,
-CH2NH2, -CH2CH2NH2, and -Ph.
In one preferred embodiment, the substituent(s), often referred to herein as
R, are
independently selected from: hydroxy; ether (e.g., Cigalkoxy); ester; amido;
amino; and, C1..7alkyl (including, e.g., C1_7haloalkyl, C1..7hydroxyalkyl,
Cijcarboxyalkyl, Ci.qaminoalkyl, C5_20ary1-Ci7alkyl).
In one preferred embodiment, the substituent(s), often referred to herein as
R, are
independently selected from:
-OH;
-0Me, -0Et, -0(tBu), and -OCH2Ph;
-C(=0)0Me, -C(=0)0Et, and -C(=0)0(tBu);
-C(=0)NH2, -C(=0)NHMe, -C(=0)NMe2, and -C(=0)NHEt;
-NH2, -NHMe, -NHEt, -NH(iPr), -NH(nPr), -NMe2, -NEt2, -N(iPr)2, -N(nPr)2,
-N(nBu)2, and -N(tBu)2;
-Me, -Et, -nPr, -iPr, -nBu, -tBu;
-CF3, -CHF2, -CH2F, -0013, -CBr3, -CH2CH2F, -CH2CHF2, and -CH2CF3;
-CH2OH, -CH2CH2OH, and -CH(OH)CH2OH; and,
-CH2NH2,-CH2CH2NH2, and -CH2CH2NMe2.
The substituents are described in more detail below.
Cijalkyl: The term "C1_7a1ky1," as used herein, pertains to a monovalent
moiety
obtained by removing a hydrogen atom from a Ciqhydrocarbon compound having
from 1 to 7 carbon atoms, which may be aliphatic or alicyclic, or a
combination
thereof, and which may be saturated, partially unsaturated, or fully
unsaturated.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 77 -
Examples of (unsubstituted) saturated linear Cijalkyl groups include, but are
not
limited to, methyl, ethyl, n-propyl, n-butyl, and n-pentyl (amyl).
Examples of (unsubstituted) saturated branched C1_7a1ky1 groups include, but
are
not limited to, iso-propyl, iso-butyl, sec-butyl, tert-butyl, and neo-pentyl.
Examples of saturated alicyclic (also carbocyclic) Cijalkyl groups (also
referred to
as "C3_7cycloalkyl" groups) include, but are not limited to, unsubstituted
groups
such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and norbornane, as
well
as substituted groups (e.g., groups which comprise such groups), such as
methylcyclopropyl, dimethylcyclopropyl, methylcyclobutyl, dimethylcyclobutyl,
methylcyclopentyl, dimethylcyclopentyl, methylcyclohexyl, dimethylcyclohexyl,
cyclopropylmethyl and cyclohexylmethyl.
Examples of (unsubstituted) unsaturated C17a1ky1 groups which have one or more
carbon-carbon double bonds (also referred to as "C2_7alkenyl" groups) include,
but
are not limited to, ethenyl (vinyl, -CH=CH2), 2-propenyl (allyl, -CH-CH=CH2),
isopropenyl (-C(CH3)=CH2), butenyl, pentenyl, and hexenyl.
Examples of (unsubstituted) unsaturated Ci_7alkyl groups which have one or
more
carbon-carbon triple bonds (also referred to as "C2..7alkynyl" groups)
include, but
are not limited to, ethynyl (ethinyl) and 2-propynyl (propargyl).
Examples of unsaturated alicyclic (also carbocyclic) C1_7alkyl groups which
have
one or more carbon-carbon double bonds (also referred to as "C3_7cycloalkenyl"
groups) include, but are not limited to, unsubstituted groups such as
cyclopropenyl, cyclobutenyl, cyclopentenyl, and cyclohexenyl, as well as
substituted groups (e.g., groups which comprise such groups) such as
cyclopropenylmethyl and cyclohexenylmethyl.
Additional examples of substituted C3_7cycloalkyl groups include, but are not
limited to, those with one or more other rings fused thereto, for example,
those
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 78 -
derived from: indene (09), indan (2,3-dihydro-1H-indene) (C9), tetraline
(1,2,3,4-
tetrahydronaphthalene (C10), adamantane (C10), decalin (decahydronaphthalene)
(C12), fluorene (C13), phenalene (C13). For example, 2H-inden-2-y1 is a
C5cycloalkyl group with a substituent (phenyl) fused thereto.
C3_20heterocyclyl: The term "C3_20heterocyclyl," as used herein, pertains to a
monovalent moiety obtained by removing a hydrogen atom from a ring atom of a
C3_20heterocyclic compound, said compound having one ring, or two or more
rings
(e.g., Spiro, fused, bridged), and having from 3 to 20 ring atoms, of which
from 1
to 10 are ring heteroatoms, and wherein at least one of said ring(s) is a
heterocyclic ring. Preferably, each ring has from 3 to 7 ring atoms, of which
from
1 to 4 are ring heteroatoms.
In this context, the prefixes (e.g., C3-20, C3-7, C6-6, etc.) denote the
number of ring
atoms, or range of number of ring atoms, whether carbon atoms or heteroatoms.
For example, the term "C5_6heterocyclyl," as used herein, pertains to a
heterocyclyl group having 5 or 6 ring atoms. Examples of groups of
heterocyclyl
groups include C3.20heterocyclyl, C3_7heterocyclyl, C5_7heterocyclyl.
Examples of (non-aromatic) monocyclic heterocyclyl groups include, but are not
limited to, those derived from:
N1: aziridine (03), azetidine (Ca), pyrrolidine (tetrahydropyrrole) (C5),
pyrroline
(e.g., 3-pyrroline, 2,5-dihydropyrrole) (C5), 2H-pyrrole or 3H-pyrrole
(isopyrrole,
isoazole) (05), piperidine (C6), dihydropyridine (06), tetrahydropyridine
(06),
azepine (C7);
01: oxirane (C3), oxetane (04), oxolane (tetrahydrofuran) (CO, oxole
(dihydrofuran) (C5), oxane (tetrahydropyran) (C6), dihydropyran (C6), pyran
(Cs),
oxepin (C7);
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 79 -
Si: thiirane (C3), thietane (C4), thiolane (tetrahydrothiophene) (C5), thiane
(tetrahydrothiopyran) (06), thiepane (C7);
02: dioxolane (05), dioxane (C6), and dioxepane (C7);
03: trioxane (00;
N2: iMidaZOlidirle (CO, pyrazolidine (diazolidine) (C5), imidazoline (C5),
pyrazoline
(dihydropyrazole) (C5), piperazine (Cs);
N101: tetrahydrooxazole (C5), dihydrooxazole (C5), tetrahydroisoxazole (05),
dihydroisoxazole (05), morpholine (C6), tetrahydrooxazine (06), dihydrooxazine
(C6), oxazine (06);
NiSi: thiazoline (05), thiazolidine (C5), thiomorpholine (Cs);
N201: oxadiazine (Cs);
01S1: oxathiole (C5) and oxathiane (thioxane) (06); and,
NiOiSi: oxathiazine (C6).
Examplesof substituted (non-aromatic) monocyclic heterocyclyl groups include
saccharides, in cyclic form, for example, furanoses (05), such as
arabinofuranose,
lyxofuranose, ribofuranose, and xylofuranse, and pyranoses (06), such as
allopyranose, altropyranose, glucopyranose, mannopyranose, gulopyranose,
idopyranose, galactopyranose, and talopyranose.
Examples of heterocyclyl groups which are also heteroaryl groups are described
below with aryl groups.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 80 -
C5.20aryl: The term "C5_20aryl," as used herein, pertains to a monovalent
moiety
obtained by removing a hydrogen atom from an aromatic ring atom of a
C5.20aromatic compound, said compound having one ring, or two or more rings
(e.g., fused), and having from 5 to 20 ring atoms, and wherein at least one of
said
ring(s) is an aromatic ring. Preferably, each ring has from 5 to 7 ring atoms.
In this context, the prefixes (e.g., C3-201 C5-71 C5-6, etc.) denote the
number of ring
atoms, or range of number of ring atoms, whether carbon atoms or heteroatoms.
For example, the term "C5_6aryl," as used herein, pertains to an aryl group
having
5 or 6 ring atoms. Examples of groups of aryl groups include C3_20aryl,
C5_7aryl,
C5_6aryl.
The ring atoms may be all carbon atoms, as in "carboaryl groups" (e.g.,
C5_20carboary1).
Examples of carboaryl groups include, but are not limited to, those derived
from
benzene (i.e., phenyl) (CO, naphthalene (C10), azulene (C10), anthracene
(C14),
phenanthrene (C14), naphthacene (C18), and pyrene (Cm).
Examples of aryl groups which comprise fused rings, at least one of which is
an
aromatic ring, include, but are not limited to, groups derived from indene
(C9),
isoindene (CO, and fluorene (C13).
Alternatively, the ring atoms may include one or more heteroatoms, including
but
not limited to oxygen, nitrogen, and sulfur, as in "heteroaryl groups." In
this case,
the group may conveniently be referred to as a "C5_20heteroaryl" group,
wherein
"C5_20" denotes ring atoms, whether carbon atoms or heteroatoms. Preferably,
each ring has from 5 to 7 ring atoms, of which from 0 to 4 are ring
heteroatoms.
Examples of monocyclic heteroaryl groups include, but are not limited to,
those
derived from:
Ni: pyrrole (azole) (05), pyridine (azine) (C6);
01: furan (oxole) (Cs);
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 81 -
S1: thiophene (thiole) (C5);
N101: oxazole (C5), isoxazole (C5), isoxazine (C6);
N201: oxadiazole (furazan) (C5);
N301: oxatriazole (Cs);
NISI: thiazole (C5), isothiazole (C5);
N2: imidazole (1,3-diazole) (C5), pyrazole (1,2-diazole) (C5), pyridazine
(1,2-diazine) (C6), pyrimidine (1,3-diazine) (C6) (e.g., cytosine, thymine,
uracil),
pyrazine (1,4-diazine) (C6);
N3: triazole (C5), triazine (C6); and,
N4: tetrazole (C5).
Examples of heterocyclic groups (some of which are also heteroaryl groups)
which comprise fused rings, include, but are not limited to:
C9heterocyclic groups (with 2 fused rings) derived from benzofuran (01),
isobenzofuran (01), indole (N1), isoindole (N1), purine (N4) (e.g., adenine,
guanine), benzimidazole (N2), benzoxazole (N101), benzisoxazole (N101),
benzodioxole (02), benzofurazan (N201), benzotriazole (N3), benzothiofuran
(Si),
benzothiazole (NISI), benzothiadiazole (N2S);
Cioheterocyclic groups (with 2 fused rings) derived from benzodioxan (02),
quinoline (N1), isoquinoline (N1), benzoxazine (N101), benzodiazine (N2),
pyridopyridine (N2), quinoxaline (N2), quinazoline (N2);
C13heterocyclic groups (with 3 fused rings) derived from carbazole (N1),
dibenzofuran (01), dibenzothiophene (Si); and,
C14heterocyclic groups (with 3 fused rings) derived from acridine (N1),
xanthene (01), phenoxathiin (01S1), phenazine (N2), phenoxazine (N101),
phenothiazine (NISI), thianthrene (S2), phenanthridine (Ni), phenanthroline
(N2),
phenazine (N2).
Heterocyclic groups (including heteroaryl groups) which have a nitrogen ring
atom
in the form of an -NH- group may be N-substituted, that is, as -NR-. For
example,
pyrrole may be N-methyl substituted, to give N-methypyrrole. Examples of N-
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 82 -
substitutents include, but are not limited to C1_7alkyl, C3_20heterocyclyl,
C5_20aryl,
and acyl groups.
Heterocyclic groups (including heteroaryl groups) which have a nitrogen ring
atom
in the form of an -N= group may be substituted in the form of an N-oxide, that
is,
as -N(-->0)= (also denoted -N+(--0")=). For example, quinoline may be
substituted to give quinoline N-oxide; pyridine to give pyridine N-oxide;
benzofurazan to give benzofurazan N-oxide (also known as benzofuroxan).
Cyclic groups may additionally bear one or more oxo (=0) groups on ring carbon
atoms. Monocyclic examples of such groups include, but are not limited to,
those
derived from:
C5: cyclopentanone, cyclopentenone, cyclopentadienone;
C6: cyclohexanone, cyclohexenone, cyclohexadienone;
01: furanone (C5), pyrone (C6);
N1: pyrrolidone (pyrrolidinone) (C5), piperidinone (piperidone) (C6),
piperidinedione
(C6),
N2: imidazolidone (imidazolidinone) (C5), pyrazolone (pyrazolinone) (C5),
piperazinone (06), piperazinedione (C6), pyridazinone (C6), pyrimidinone (C6)
(e.g., cytosine), pyrimidinedione (C6) (e.g., thymine, uracil), barbituric
acid (06);
NiSi: thiazolone (C5), isothiazolone (05);
N101: oxazolinone (C5).
Polycyclic examples of such groups include, but are not limited to, those
derived
from:
Cg: indenedione;
N1: oxindole (Cs);
01: benzopyrone (e.g., coumarin, isocoumarin, chromone) (Cio);
N101: benzoxazolinone (C9), benzoxazolinone (C10);
N2: quinazolinedione (C10);
N4: purinone (C9) (e.g., guanine).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 83 -
SO more examples of cyclic groups which bear one or more oxo (=0) groups on
ring carbon atoms include, but are not limited to, those derived from:
cyclic anhydrides (-C(=0)-0-C(=0)- in a ring), including but not limited to
maleic anhydride (05), succinic anhydride (C5), and glutaric anhydride (06);
cyclic carbonates (-0-C(=0)-0- in a ring), such as ethylene carbonate (C5)
and 1,2-propylene carbonate (05);
imides (-C(=0)-NR-C(=0)- in a ring), including but not limited to,
succinimide (C5), maleimide (05), phthalimide, and glutarimide (C6);
lactones (cyclic esters, -0-C(=0)- in a ring), including, but not limited to,
8-propiolactone, y-butyrolactone, 6-valerolactone (2-piperidone), and
E-caprolactone;
lactams (cyclic amides, -NR-C(=0)- in a ring), including, but not limited to,
8-propiolactam (C4), y-butyrolactam (2-pyrrolidone) (C5), 6-valerolactam (06),
and
E-caprolactam (C7);
cyclic carbamates (-0-C(=0)-NR- in a ring), such as 2-oxazolidone (C5);
cyclic ureas (-NR-C(=0)-NR- in a ring), such as 2-imidazolidone (05) and
pyrimidine-2,4-dione (e.g., thymine, uracil) (C6).
The above Ci_7alkyl, C3_20heterocyclyl, and C5_20aryl groups, whether alone or
part
of another substituent, may themselves optionally be substituted with one or
more
groups selected from themselves and the additional substituents listed below.
Hydrogen: -H. Note that if the substituent at a particular position is
hydrogen, it
may be convenient to refer to the compound as being "unsubstituted" at that
position.
Halo: -F, -Cl, -Br, and -I.
Hydroxy: -OH.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 84 -
Ether: -OR, wherein R is an ether substituent, for example, a CiJalkyl group
(also
referred to as a CiJalkoxy group, discussed below), a C3_20heterocyclylgroup
(also referred to as a C3_20hetercyclyloxy group), or a C5_20ary1 group (also
referred
to as a C5_20aryloxy group), preferably a C1_7alkyl group.
C1_7alkoxy: -OR, wherein R is a Ci_7alkyl group. Examples of Cigalkoxy groups
include, but are not limited to, -OCH3 (methoxy), -OCH2CH3 (ethoxy) and
-0C(CH3)3 (tert-butoxy).
Oxo (keto, -one): =0. Examples of cyclic compounds and/or groups having, as a
substituent, an oxo group (=0) include, but are not limited to, carbocyclics
such as
cyclopentanone and cyclohexanone; heterocyclics, such as pyrone, pyrrolidone,
pyrazolone, pyrazolinone, piperidone, piperidinedione, piperazinedione, and
imidazolidone; cyclic anhydrides, including but not limited to maleic
anhydride and
succinic anhydride; cyclic carbonates, such as propylene carbonate; imides,
including but not limited to, succinimide and maleimide; lactones (cyclic
esters,
-0-C(=0)- in a ring), including, but not limited to, (3-propiolactone, y-
butyrolactone,
5-valerolactone, and c-caprolactone; and lactams (cyclic amides, -NH-C(=0)- in
a
ring), including, but not limited to, I3-propiolactam, y-butyrolactam, 6-
valerolactam,
and c-caprolactam.
Imino (imine): =NR, wherein R is an imino substituent, for example, hydrogen,
Ci:Talky( group, a C3_20heterocyclylgroup, or a C5_20arylgroup, preferably
hydrogen or a C1_7alkyl group. Examples of imino groups include, but are not
limited to, =NH, =NMe, =NEt, and =NPh.
Formyl (carbaldehyde, carboxaldehyde): -C(=0)H.
Acyl (keto): -C(=0)R, wherein R is an acyl substituent, for example, a
C1_7alkyl
group (also referred to as C1_7alkylacyl or Ci7alkanoy1), a C3_20heterocycly1
group
(also referred to as C3_20heterocyclylacyl), or a C5_20ary1 group (also
referred to as
C5_20arylacyl), preferably a C1_7alkyl group. Examples of acyl groups include,
but
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 85 -
are not limited to, -C(=0)CH3 (acetyl), -C(=0)CH2CH3 (propionyl), -
C(=0)C(CH3)3
(butyryl), and -C(0)Ph (benzoyl, phenone).
Acylhalide (haloformyl, halocarbonyl): -C(=0)X, wherein X is -F, -Cl, -Br, or -
I,
preferably -Cl, -Br, or -I.
Carboxy (carboxylic acid): -COOH.
Ester (carboxylate, carboxylic acid ester, oxycarbonyl): -C(=0)0R, wherein R
is
an ester substituent, for example, a Cijalkyl group, a C3_20heterocyclylgroup,
or a
C5.20ary1 group, preferably a Ciqalkyl group. Examples of ester groups
include,
but are not limited to, -C(=0)0CH3, -C(=0)0CH2CH3, -C(=0)0C(CH3)3, and -
C(=0)0Ph.
Acyloxy (reverse ester): -0C(=0)R, wherein R is an acyloxy substituent, for
example, a C1_7alkyl group, a C3.20heterocyclylgroup, or a C5_20aryl group,
preferably a Cijalkyl group. Examples of acyloxy groups include, but are not
limited to, -0C(=0)CH3 (acetoxy), -0C(=0)CH2CH3, -0C(=0)C(C1-13)3,
-0C(=0)Ph, and -0C(=0)CH2Ph.
Amido (carbamoyl, carbamyl, aminocarbonyl, carboxamide): -C(=0)NR1R2,
wherein R1 and R2 are independently amino substituents, as defined for amino
groups. Examples of amido groups include, but are not limited to, -C(=0)NH2,
-C(=0)NHCH3, -C(=0)NH(CH3)2, -C(=0)NHCH2CH3, and -C(=0)N(CH2CH3)2, as
well as amido groups in which R1 and R2, together with the nitrogen atom to
which
they are attached, form a heterocyclic structure as in, for example,
piperidinocarbonyl, morpholinocarbonyl, thiomorpholinocarbonyl, and
piperazinocarbonyl.
Acylamido (acylamino): -NR1C(=0)R2, wherein R1 is an amide substituent, for
example, a Cigalkyl group, a C3_20heterocyclylgroup, or a C5_20aryl group,
preferably a Ci_palkyl group, and R2 is an acyl substituent, for example, a
Ciqalkyl
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 86 -
group, a C3_2oheterocyclylgroup, or a C5_20ary1 group, preferably a Giqalkyl
group.
Examples of acylamido groups include, but are not limited to, -NHC(=0)CH3 ,
-NHC(=0)CH2CH3, and -NHC(=0)Ph. R1 and R2 may together form a cyclic
structure, as in, for example, for example, succinimidyl, maleimidyl, and
phthalimidyl:
0 0
Nr0 0¨ro
succinimidyl maleimidyl phthalimidyl
Thioamido (thiocarbamyl): -C(=S)NR1R2, wherein R1 and R2 are independently
amino substituents, as defined for amino groups. Examples of amido groups
include, but are not limited to, -C(=S)NH2, -C(=S)NHCH3, -C(=S)NH(CH3)2, and
-C(=S)NHCH2CH3.
Tetrazolyl: a five membered aromatic ring having four nitrogen atoms and one
carbon atom,
II
¨N
N
Amino: -NR1R2, wherein R1 and R2 are independently amino substituents, for
example, hydrogen, a C1_7alkyl group (also referred to as C1..7alkylamino or
di-
Ci_7alkylamino), a C3_20heterocyclylgroup, or a C5_20ary1 group, preferably H
or a
Ci..7alkyl group, or, in the case of a "cyclic" amino group, R1 and R2, taken
together with the nitrogen atom to which they are attached, form a
heterocyclic
ring having from 4 to 8 ring atoms. Examples of amino groups include, but are
not
limited to, -NH2, -NHCH3, -NHCH(CH3)2, -N(CH3)2, -N(CH2CH3)2, and -NHPh.
Examples of cyclic amino groups include, but are not limited to, aziridino,
azetidino, piperidino, piperazino, morpholino, and thiomorpholino.
Nitro: -NO2.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 87 -
Nitroso: -NO.
Azido: -N3.
=
Cyano (nitrile, carbonitrile): -CN.
lsocyano: -NC.
Cyanato: -OCN.
lsocyanato: -NCO.
Thiocyano (thiocyanato): -SCN.
lsothiocyano (isothiocyanato): -NCS.
Sulfhydryl (thiol, mercapto): -SH.
Thioether (sulfide): -SR, wherein R is a thioether substituent, for example, a
CiJalkyl group (also referred to as a Ci_7alkylthio group), a
C3_20heterocycly1
group, or a C5_20ary1 group, preferably a Cijalkyl group. Examples of
C1_7alkylthio
groups include, but are not limited to, -SCH3 and -SCH2CH3.
Sulfonic acid (sulfo): -S(=0)20H.
Sulfonate (sulfonic acid ester): -S(=0)20R, wherein R is a sulfonate
substituent,
for example, a C1_7a1ky1 group, a C3_20heterocyclylgroup, or a C5_20aryl
group,
preferably a CiJalkyl group. Examples of sulfonate groups include, but are not
limited to, -S(=0)20CH3 and -S(=0)200H2CH3.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 88 -
Sulfone (sulfonyl): -S(=0)2R, wherein R is a sulfone substituent, for example,
a
Cijalkyl group, a C3_20heterocyclylgroup, or a C5_20aryl group, preferably a
C1_7alkyl group. Examples of sulfone groups include, but are not limited to,
-S(=0)2CH3 (methanesulfonyl, mesyl), -S(=0)2CF3, -S(=0)2CH2CH3, and 4-
methylphenylsulfonyl (tosyl).
Sulfonyloxy: -0S(=0)2R, wherein R is a sulfonyloxy substituent, for example, a
CiJalkyl group, a C3_20heterocyclylgroup, or a C5_20ary1 group, preferably a
Cijalkyl group. Examples of sulfonyloxy groups include, but are not limited
to,
-0S(=0)2CH3 and -0S(=0)2CH2CH3.
Sulfinyloxy: -0S(=0)R, wherein R is a sulfinyloxy substituent, for example, a
C1_7alkyl group, a C3_20heterocyclylgroup, or a C5_20ary1 group, preferably a
Ci_7alkyl group. Examples of sulfinyloxy groups include, but are not limited
to,
-0S(=0)CH3 and -0S(=0)CH2CH3.
Sulfamino: -NR1S(=0)20H, wherein R1 is an amino substituent, as defined for
amino groups. Examples of sulfamino groups include, but are not limited to,
-NHS(0)20H and -N(CH3)S(=0)20H.
Sulfonamino: -NR1S(=0)2R, wherein R1 is an amino substituent, as defined for
amino groups, and R is a sulfonamino substituent, for example, a CiJalkyl
group,
a C3_20heterocyclylgroup, or a C5_20aryl group, preferably a Cijalkyl group.
Examples of sulfonamino groups include, but are not limited to, -NHS(=0)2CH3
and -N(CH3)S(=0)2C6H5.
Sulfinamino: -NR1S(=0)R, wherein R1 is an amino substituent, as defined for
amino groups, and R is a sulfinamino substituent, for example, a Cijalkyl
group, a
C3_20heterocyclylgroup, or a C5_20aryl group, preferably a CiJalkyl group.
Examples of sulfinamino groups include, but are not limited to, -NHS(=0)CH3
and
-N(CH3)S(=0)C6H5.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 89 -
Sulfamyl: -S(=0)NR1R2, wherein R1 and R2 are independently amino substituents,
as defined for amino groups. Examples of sulfamyl groups include, but are not
limited to, -S(=0)NH2, -S(=0)NH(CH3), -S(=0)N(CH3)2, -S(=0)NH(CH2CH3),
-S(=0)N(CH2CH3)2, and -S(=0)NHPh.
Sulfonamido: -S(=0)2NR1R2, wherein R1 and R2 are independently amino
substituents, as defined for amino groups. Examples of sulfonamido groups
include, but are not limited to, -S(=0)2NH2, -S(=0)2NH(CH3), -S(=0)2N(CH3)2,
-S(=0)2NH(CH2CH3), -S(=0)2N(CH2CH3)2, and -S(=0)2NHPh.
As mentioned above, a Cijalkyl group may be substituted with, for example,
hydroxy (also referred to as a C1..7hydroxyalkyl group), Cigalkoxy (also
referred to
as a Ci_7alkoxyalkyl group), amino (also referred to as a Ciqaminoalkyl
group),
halo (also referred to as a Ci.qhaloalkyl group), carboxy (also referred to as
a
Cijcarboxyalkyl group), and C5_20ary1 (also referred to as a C5_20aryl-
C1_7a1ky1
group).
Similarly, a C5_20ary1 group may be substituted with, for example, hydroxy
(also
referred to as a C5_20hydroxyaryl group), halo (also referred to as a
C5_20haloaryl
group), amino (also referred to as a C5_20aminoaryl group, e.g., as in
aniline),
C1_7alky1 (also referred to as a Ci7alkyl-05_20ary1 group, e.g., as in
toluene), and
Ci_7alkoxy (also referred to as a C17alkoxy-05_20ary1 group, e.g., as in
anisole).
These and other specific examples of such substituted groups are also
discussed
below.
Cighaloalkyl group: The term "Ciqhaloalkyl group," as used herein, pertains to
a
Ci_7alkyl group in which at least one hydrogen atom (e.g., 1, 2, 3) has been
replaced with a halogen atom (e.g., F, Cl, Br, l). If more than one hydrogen
atom
has been replaced with a halogen atom, the halogen atoms may independently be
the same or different. Every hydrogen atom may be replaced with a halogen
atom, in which case the group may conveniently be referred to as a
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 90 -
Ci_7perhaloalkyl group." Examples of C1_7haloalkyl groups include, but are not
limited to, -CF3, -CHF2, -CH2F, -CCI3, -CBr3, -CH2CH2F, -CH2CHF2, and -CH2CF3.
C1.7hydroxyalkyl: The term "C1_7hydroxyalkyl group," as used herein, pertains
to a
Ci_7alkyl group in which at least one hydrogen atom has been replaced with a
hydroxy group. Examples of C1..7hydroxyalkyl groups include, but are not
limited
to, -CH2OH,-CH2CH2OH, and -CH(OH)CH2OH.
C1_7carboxyalkyl: The term "C1..7carboxyalkyl group," as used herein, pertains
to a
C1_7alkyl group in which at least one hydrogen atom has been replaced with a
carboxy group. Examples of C1_7carboxyalkyl groups include, but are not
limited
to, -CH2COOH and -CH2CH2COOH.
Ciqaminoalkyl: The term "Ciqaminoalkyl group," as used herein, pertains to a
Cigalkyl group in which at least one hydrogen atom has been replaced with an
amino group. Examples of Cigaminoalkyl groups include, but are not limited to,
-CH2NH2,-CH2CH2NH2, and -CH2CH2N(CH3)2.
C1_7alkyl-05_20ary1: The term "C17a1ky1-05_20ary1," as used herein, describes
certain
C5.20aryl groups which have been substituted with a Ci_7alkyl group. Examples
of
such groups include, but are not limited to, tolyl (as in toluene), xylyl (as
in
xylene), mesityl (as in mesitylene), styryl (as in styrene), and cumenyl (as
in
cumene).
C5_20ary1-C1Jalkyl: The term "C5_20aryl-C1Jalkyl," as used herein, describers
certain C1_7alkyl groups which have been substituted with a C5_20aryl group.
Examples of such groups include, but are not limited to, benzyl
(phenylmethyl),
tolylmethyl, phenylethyl, and triphenylmethyl (trityl).
C5_20haloaryl: The term "C5_20haloaryl," as used herein, describes certain
C5_20ary1
groups which have been substituted with one or more halo groups. Examples of
such groups include, but are not limited to, halophenyl (e.g., fluorophenyl,
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 91 -
chlorophenyl, bromophenyl, or iodophenyl, whether ortho-, meta-, or para-
substituted), dihalophenyl, trihalophenyl, tetrahalophenyl, and
pentahalophenyl.
Bidentate Substituents
Some substituents are bidentate, that is, have two points for covalent
attachment.
For example, a bidentate group may be covalently bound to two different atoms
on two different groups, thereby acting as a linker therebetween.
Alternatively, a
bidentate group may be covalently bound to two different atoms on the same
group, thereby forming, together with the two atoms to which it is attached
(and
any intervening atoms, if present) a cyclic or ring structure. In this way,
the
bidentate substituent may give rise to a heterocyclic group/compound and/or an
aromatic group/compound. Typically, the ring has from 3 to 8 ring atoms, which
ring atoms are carbon or divalent heteroatoms (e.g., boron, silicon, nitrogen,
phosphorus, oxygen, and sulfur, typically nitrogen, oxygen, and sulfur), and
wherein the bonds between said ring atoms are single or double bonds, as
permitted by the valencies of the ring atoms. Typically, the bidentate group
is
covalently bound to vicinal atoms, that is, adjacent atoms, in the parent
group.
C1_7alkylene: The term "C1_7alkylene," as used herein, pertains to a bidentate
moiety obtained by removing two hydrogen atoms, either both from the same
carbon atom, or one from each of two different carbon atoms, of a
C1_7hydrocarbon compound having from 1 to 7 carbon atoms, which may be
aliphatic or alicyclic, or a combination thereof, and which may be saturated,
partially unsaturated, or fully unsaturated.
Examples of linear saturated C1_7alkylene groups include, but are not limited
to,
-(CH2),-,- where n is an integer from 1 to 7, for example, -CH2- (methylene),
-CH2CH2- (ethylene), -CH2CH2CH2- (Propylene), and -CH2CH2CH2CH2-
(butylene).
CA 02765409 2012-01-19
WO 02/30879 PCT/CB01/04326
- 92 -
Examples of branched saturated Ci_7alkylene groups include, but are not
limited
to, -OH(CH3)-, -CH(CH3)CH2-, -CH(CH3)CH2CH2-, -OH(CH3)CH2CH2CH2-,
-CH2CH(CH3)CH2-, -CH2CH(CH3)CH2CH2-, -CH(CH2CH3)-, -CH(CH2CH3)CH2-,
and -CH2CH(CH2CH3)CH2-=
Examples of linear partially unsaturated C1_7alkylene groups include, but are
not
limited to, -CH=CH- (vinylene), -CH=CH-CH2-, -CH=CH-CH2-CH2-,
-CH=CH-CH2-CH2-0H2-, -CH=CH-CH=CH-, -CH=CH-CH=CH-CH2-, -CH=CH-
CH=CH-CH2-0H2-, -CH=CH-CH2-CH=CH-, and -CH=CH-CH2-CH2-CH=CH-.
Examples of branched partially unsaturated C1_7alkylene groups include, but
are
not limited to, -C(CH3)=CH-, -C(CH3)=0H-CH2-, and -CH=CH-CH(CH3)-.
Examples of alicyclic saturated Cijalkylene groups include, but are not
limited to,
cyclopentylene (e.g., cyclopent-1,3-ylene), and cyclohexylene (e.g., cyclohex-
1,4-
ylene).
Examples of alicyclic partially unsaturated Cijalkylene groups include, but
are not
limited to, cyclopentenylene (e.g., 4-cyclopenten-1,3-ylene), cyclohexenylene
(e.g., 2-cyclohexen-1,4-ylene, 3-cyclohexen-1,2-ylene, 2,5-cyclohexadien-1,4-
ylene).
C5_20arylene: The term "C5.20arylene," as used herein, pertains to a bidentate
moiety obtained by removing two hydrogen atoms, one from each of two different
ring atoms of a C5_20aromatic compound, said compound having one ring, or two
or more rings (e.g., fused), and having from 5 to 20 ring atoms, and wherein
at
least one of said ring(s) is an aromatic ring. Preferably, each ring has from
5 to 7
ring atoms.
The ring atoms may be all carbon atoms, as in "carboarylene groups," in which
case the group may conveniently be referred to as a "C5_20carboarylene" group.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 93 -
Alternatively, the ring atoms may include one or more heteroatoms, including
but
not limited to oxygen, nitrogen, and sulfur, as in "heteroarylene groups." In
this
case, the group may conveniently be referred to as a "C5_2oheteroarylene"
group,
wherein "C5_20" denotes ring atoms, whether carbon atoms or heteroatoms.
Preferably, each ring has from 5 to 7 ring atoms, of which from 0 to 4 are
ring
heteroatoms.
Examples of C5_20arylene groups which do not have ring heteroatoms
(i.e., C5_20carboarylene groups) include, but are not limited to, those
derived from
benzene (i.e., phenyl) (06), naphthalene (C10), anthracene (C14), phenanthrene
(014), and pyrene (016).
Examples of C5_20heteroarylene groups include, but are not limited to,
C5heteroarylene groups derived from furan (oxole), thiophene (thiole), pyrrole
(azole), imidazole (1,3-diazole), pyrazole (1,2-diazole), triazole, oxazole,
isoxazole, thiazole, isothiazole, oxadiazole, and oxatriazole; and
C5heteroarylene
groups derived from isoxazine, pyridine (azine), pyridazine (1,2-diazine),
pyrimidine (1,3-diazine; e.g., cytosine, thymine, uracil), pyrazine (1,4-
diazine),
triazine, tetrazole, and oxadiazole (furazan).
C5_20Arylene-C1..7alkylene: The term "C5_20arylene-C17alkylene," as used
herein,
pertains to a bidentate moiety comprising a C5_20arylene moiety, -Arylene-,
linked
to a C1_7alkylene moiety, -Alkylene-, that is, -Arylene-Alkylene-.
Examples of C5_20arylene-C17alkylene groups include, but are not limited to,
phenylene-methylene, phenylene-ethylene, phenylene-propylene, and phenylene-
ethenylene (also known as phenylene-vinylene).
C5_20Alkylene-C17arylene: The term "C5_20alkylene-C17arylene," as used herein,
pertains to a bidentate moiety comprising a C5_20alkylene moiety, -Alkylene-,
linked to a CiJarylene moiety, -Arylene-, that is, -Alkylene-Arylene-.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
-94 -
Examples of C5_20alkylene-C1_7arylene groups include, but are not limited to,
methylene-phenylene, ethylene-phenylene, propylene-phenylene, and
ethenylene-phenylene (also known as vinylene-phenylene).
Included in the above are the well known ionic, salt, solvate (e.g., hydrate),
and
protected forms of these substituents. For example, a reference to carboxylic
acid
(-COON) also includes carboxylate (-COO). Similarly, a reference to an amino
group includes a salt, for example, a hydrochloride salt, of the amino group.
A
reference to a hydroxyl group also includes conventional protected forms of a
hydroxyl group. Similarly, a reference to an amino group also includes
conventional protected forms of an amino group.
Acronyms
For convenience, many chemical moieties are represented herein using well
known abbreviations, including but not limited to, methyl (Me), ethyl (Et), n-
propyl
(nPr), iso-propyl (iPr), n-butyl (nBu), tert-butyl (tBu), n-hexyl (nHex),
cyclohexyl
(cHex), phenyl (Ph), biphenyl (biPh), benzyl (Bn), naphthyl (naph), methoxy
(Me0), ethoxy (Et0), benzoyl (Bz), and acetyl (Ac).
For convenience, many chemical compounds are represented herein using well
known abbreviations, including but not limited to, methanol (Me0H), ethanol
(Et0H), iso-propanol (i-PrOH), methyl ethyl ketone (MEK), acetic acid (AcOH),
dichloronnethane (methylene chloride, DCM), trifluoroacetic acid (TEA),
dimethylformamide (DMF), and tetrahydrofuran (THF).
Isomers, Salts, Solvates, Protected Forms, and Prodruos
A certain compound may exist in one or more particular geometric, optical,
enantiomeric, diasteriomeric, epimeric, stereoisomeric, tautomeric,
conformational, or anomeric forms, including but not limited to, cis- and
trans-
forms; E- and Z-forms; c-, t-, and r- forms; endo- and exo-forms; R-, S-, and
CA 02765409 2012-01-19
WO 02130879 PCT/GB01/04326
- 95 -
meso-forms; D- and L-forms; (+) and (-) forms; keto-, enol-, and enolate-
forms;
syn- and anti-forms; synclinal- and anticlinal-forms; a- and (3-forms; axial
and
equatorial forms; boat-, chair-, twist-, envelope-, and halfchair-forms; and
combinations thereof, hereinafter collectively referred to as "isomers" (or
"isomeric
forms").
Note that, except as discussed below for tautomeric forms, specifically
excluded
from the term "isomers," as used herein, are structural (or constitutional)
isomers
(i.e., isomers which differ in the connections between atoms rather than
merely by
the position of atoms in space). For example, a reference to a methoxy group,
-OCH3, is not to be construed as a reference to its structural isomer, a
hydroxymethyl group, -CH2OH. Similarly, a reference to ortho-chlorophenyl is
not
to be construed as a reference to its structural isomer, meta-chlorophenyl.
However, a reference to a class of structures may well include structurally
isomeric forms falling within that class (e.g., Cijalkyl includes n-propyl and
iso-
propyl; butyl includes n-, iso-, sec-, and tert-butyl; methoxyphenyl includes
ortho-,
meta-, and para-methoxyphenyl).
The above exclusion does not pertain to tautomeric forms, for example, keto-,
enol-, and enolate-forms, as in, for example, the following tautomeric pairs:
keto/enol (illustrated below), imine/enamine, amide/imino alcohol,
amidine/amidine, nitroso/oxime, thioketone/enethiol, N-nitroso/hydroxyazo, and
nitro/aci-nitro.
,0 ,OH H+
--C-C1 C=C C=C
\ / \ 11+
Note that specifically included in the term "isomer" are compounds with one or
more isotopic substitutions. For example, H may be in any isotopic form,
including
1H, 2H (D), and 8H (T); C may be in any isotopic form, including 120, 130, and
140;
0 may be in any isotopic form, including 180 and 180; and the like.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 96 -
Unless otherwise specified, a reference to a particular compound includes all
such
isomeric forms, including racemic and other mixtures thereof. Methods for the
preparation (e.g., asymmetric synthesis) and separation (e.g., fractional
crystallisation and chromatographic means) of such isomeric forms are either
known in the art or are readily obtained by adapting the methods taught herein
in
a known manner.
Unless otherwise specified, a reference to a particular compound also includes
ionic, salt, solvate (e.g., hydrate), protected forms, and prodrugs thereof,
for
example, as discussed below.
It may be convenient or desirable to prepare, purify, and/or handle a
corresponding salt of the active compound, for example, a pharmaceutically-
acceptable salt. Examples of pharmaceutically acceptable salts are discussed
in
Berge et al., 1977, "Pharmaceutically Acceptable Salts," J. Pharm. Sc., Vol.
66,
pp. 1-19.
For example, if the compound is anionic, or has a functional group which may
be
anionic (e.g., -COOH may be -COO), then a salt may be formed with a suitable
cation. Examples of suitable inorganic cations include, but are not limited
to, alkali
metal ions such as No+ and K+, alkaline earth cations such as Ca2+ and Mg2+,
and other cations such as Al+3. Examples of suitable organic cations include,
but
are not limited to, ammonium ion (i.e., NH4) and substituted ammonium ions
(e.g., NH3R+, NH2R2+, NHR3+, NR4+). Examples of some suitable substituted
ammonium ions are those derived from: ethylamine, diethylamine,
ethylenediamine, ethanolamine, diethanolamine, piperazine. An example of a
common quaternary ammonium ion is N(CH3)4+.
If the compound is cationic, or has a functional group which may be cationic
(e.g.,
-NH2 may be -NH3), then a salt may be formed with a suitable anion. Examples
of suitable inorganic anions include, but are not limited to, those derived
from the
following inorganic acids: hydrochloric, hydrobromic, hydroiodic, sulfuric,
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 97 -
sulfurous, nitric, nitrous, phosphoric, and phosphorous. Examples of suitable
organic anions include, but are not limited to, anions from the following
organic
acids: acetic, propionic, succinic, gycolic, stearic, lactic, malic, tartaric,
citric,
ascorbic, maleic, hydroxymaleic, phenylacetic, glutamic, benzoic, salicylic,
sulfanilic, 2-acetyoxybenzoic, fumaric, toluenesulfonic, methanesulfonic,
ethanesulfonic, ethane disulfonic, oxalic, isethionic, and valeric.
It may be convenient or desirable to prepare, purify, and/or handle a
corresponding solvate of the active compound. The term "solvate" is used
herein
in the conventional sense to refer to a complex of solute (e.g., active
compound,
salt of active compound) and solvent. If the solvent is water, the solvate may
be
conveniently referred to as a hydrate, for example, a mono-hydrate, a di-
hydrate,
a tri-hydrate, etc.
It may be convenient or desirable to prepare, purify, and/or handle the active
compound in a chemically protected form. The term "chemically protected form,"
as used herein, pertains to a compound in which one or more reactive
functional
groups are protected from undesirable chemical reactions, that is, are in the
form
of a protected or protecting group (also known as a masked or masking group).
By protecting a reactive functional group, reactions involving other
unprotected
reactive functional groups can be performed, without affecting the protected
group; the protecting group may be removed, usually in a subsequent step,
without substantially affecting the remainder of the molecule. See, for
example,
Protective Groups in Organic Synthesis (T. Green and P. Wuts, Wiley, 1991),
and
Protective Groups in Organic Synthesis (T. Green and P. Wuts; 3rd Edition;
John
Wiley and Sons, 1999).
For example, a hydroxy group may be protected as an ether (-OR) or an ester
(-0C(=0)R), for example, as: a t-butyl ether; a benzyl, benzhydryl
(diphenylmethyl), or trityl (triphenylmethyl) ether; a trimethylsilyl or
t-butyldimethylsilyl ether; or an acetyl ester (-0C(=0)CH3, -0Ac).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 98 -
For example, an aldehyde or ketone group may be protected as an acetal or
ketal,
respectively, in which the carbonyl group (>0=0) is converted to a diether
(>C(OR)2), by reaction with, for example, a primary alcohol. The aldehyde or
ketone group is readily regenerated by hydrolysis using a large excess of
water in
the presence of acid.
For example, an amine group may be protected, for example, as an amide
(-NRCO-R) or a urethane (-NRCO-OR), for example, as: a methyl amide
(-NHCO-CH3); a benzyloxy amide (-NHCO-0CH206H5, -NH-Cbz); as a t-butoxy
amide (-NHCO-0C(CH3)3, -NH-Boc); a 2-biphenyl-2-propoxy amide
(-NHCO-0C(CH3)2C6H4C6H5, -NH-Bpoc), as a 9-fluorenylmethoxy amide
(-NH-Fmoc), as a 6-nitroveratryloxy amide (-NH-Nvoc), as a
2-trimethylsilylethyloxy amide (-NH-Teoc), as a 2,2,2-trichloroethyloxy amide
(-NH-Troc), as an allyloxy amide (-NH-Alloc), as a 2(-phenylsulfonyl)ethyloxy
amide (-NH-Psec); or, in suitable cases (e.g., cyclic amines), as a nitroxide
radical
(>N-0.).
For example, a carboxylic acid group may be protected as an ester or an amide,
for example, as: a benzyl ester; a t-butyl ester; a methyl ester; or a methyl
amide.
For example, a thiol group may be protected as a thioether (-SR), for example,
as:
a benzyl thioether; an acetamidomethyl ether (-S-CH2NHC(=0)CH3).
It may be convenient or desirable to prepare, purify, and/or handle the active
compound in the form of a prodrug. The term "prodrug," as used herein,
pertains
to a compound which, when metabolised, yields the desired active compound.
Typically, the prodrug is inactive, or less active than the active compound,
but
may provide advantageous handling, administration, or metabolic properties.
For
example, some prodrugs are esters of the active compound; during metabolysis,
the ester group is cleaved to yield the active drug. Also, some prodrugs are
activated enzymatically to yield the active compound, or a compound which,
upon
further chemical reaction, yields the active compound. For example, the
prodrug
CA 02765409 2012-01-19
54236-1
- 99 -
may be a sugar derivative or other glycoside conjugate, or may be an amino
acid
ester derivative.
Synthesis
Several methods for the chemical synthesis of compounds of the present
invention are described herein. These methods may be modified and/or adapted
in known ways in order to facilitate the synthesis of additional compounds
within
the scope of the present invention.
The compounds of the present invention may be prepared, for example, by the
methods described herein, or by adapting these or other well known methods in
well known ways.
In one method, an arylaldehyde is reacted with oleum to form a sulfonyl-
arylaldehyde product. The aldehyde group is then reacted with a phosphono
ester, to form a pendant carboxylic acid ester. The sulfonyl group is then
reacted
with SOCl2 to form a sulfonyl halide group. The product is then reacted with
an
amine (e.g., an aryl amine) to form the corresponding sulfonamide. The
carboxylic acid ester is then deprotected by reaction with base, and
subsequently
converted to an acyl halide. The acyl halide is reacted with hydroxylamine to
form
the corresponding hydracimic acid.
One example of this approach is illustrated below, in Scheme 1, wherein the
reaction conditions are as follows: (i) H2SO4 + SO3, 30 C at mixing, mixing 40
C
for 10 hours, mixing at room temperature overnight, add cold H20, add CaCO3;
(ii) K2CO3, (Me0)2P(=0)CH2COOMe, H20, room temperature, 30 min.; (iii) thionyl
chloride, benzene, DMF, reflux, one hour; (iv) aniline, pyridine, DCM, 50 C,
1 hour; (v) NaOH, Me0H; (vi) oxalyl chloride, DMF, DCM, 40 C, 1 hour;
(vii) hydroxylamine hydrochloride and NaHCO3 in THF, room temperature, 1 hour.
=
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 100 -
Scheme 1
S
CHO
1
Na03S CHO
(ii)
Na03S COOMe =
(iii) 3
110
CIS02 COOMe
io
(iv) 4,14 0\,s
N COOMe
0
(v) 5,15
0.\
N COOH
I 0
(vi) =
6,16
(:)\\
COCI
N
0
(vii)
Olt R 7,17\ N,
OH
N
I 0 0
By using amines instead of aniline, the corresponding products may be
obtained.
The use of aniline, 4-methoxyaniline, 4-methylaniline, 4-bromoaniline,
4-chloroaniline, 4-benzylamine, and 4-phenethyamine, among others, is
described
in the Examples below.
In another method, a suitable amino acid (e.g., w-amino acid) having a
protected
carboxylic acid (e.g., as an ester) and an unprotected amino group is reacted
with
a sulfonyl chloride compound (e.g., RSO2C1) to give the corresponding
sulfonamide having a protected carboxylic acid. The protected carboxylic acid
is
then deprotected using base to give the free carboxylic acid, which is then
reacted
CA 02765409 2012-01-19
54236-1
- 101 -
with, for example, hydroxylamine 2-chlorotrityl resin followed by acid (e.g.,
trifluoroacetic acid), to give the desired hydnamic acid.
One example of this approach is illustrated below, in Scheme 2, wherein the
reaction conditions are as follows: (i) RSO2CI, Pyridine, DCM, room
temperature,
12 hours; (ii) 1 M LiOH or 1 M NaOH, dioxane, room temperature, 3-48 hours;
(iii)
hydroxylamine 2-chlorotrityl resin, HOAt, HATU, DIPEA, DCM, room temperature,
16 hours; and (iv) TFA/DCM (5:95, v/v), room temperature, 1.5 hours.
Scheme 2
(i)
Et0 Et0 1110
NH2 9 NHSO2R
OD HO 7 10
10 NHSO
2R
0
(iii) and (iv) HO,
N 010
11 NHSO2R
=
Additional methods for the synthesis of compounds of the present invention are
illustrated below and are exemplified in the examples below.
Scheme 3A
02N
21 OH HCl/Me0H 02N 10 22
OMe
0 0
SnCl2 SI 23
OMe
0
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 102 -
Scheme 3B
R... 0
0
,/ Me 4. - __ g RN/S//
H2N 0
N OMe
23 o 24 H
25 0
R,
/N7 OH 26
0H
0
13,1) 11111
N ./ OH 27
0 H
0
R, 11101
S,
N OH 28
H
0
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 103 -
Scheme 4
SO3Na SO3Na 0
CHO (Me0)2P(0)CH2COOMe
la 31 K2c03/ H20
la 32 OMe
SO2CI 0
SOCl2
401 33 OMe
R2
I
R SO2 0
RiNFIR2
=
la 34 OMe
R2
I
õ1\1,.
R1 SO2 0
NaOH
lel 35 OH
Er
Ri r\ISCD2 0
(Cod)2
SI 36 01
12
NH2OHif\I
___________________________________ R SC)2 0
N,OH
40 37 H
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 104
Scheme 5
N cooH
41 Hso3ci N cooH
42
o
COOH
PhNH2 N,s 43
o 0
0
(COCI)2 N Cl 44
o
N_OH
NH2OH N, 45
0 0
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 105 -
Scheme 6
NH2
410
0 H 01
Nõ. X
. 1110
00
x
ciso2 51
H 41111
e
--. -. CH2OH l N;A\ 53 ----.
(Ph3P)4Pd OH,
00
HO
Dess-Martin
0 N':,,A\ 54 CHO
00
(Me0)213(0)CH2COOMe H 4111
H -..
NaH / THF 7
, 5-; \ \
/s 55
o o COOCH3
H SI
,,--
NaOH fa a 56 COOH
_
O0
HO
N,,, '. ,---
(COO)2 4101 57 Coo
i/S\N
....
O 0
HO H
NH2OH ... $A\ 58 OH
O0 N`OH
0
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
=
- 106 -
Scheme 7A
Na2S03
0 0
Br<-COOR1 Et0H / H20 \\//
, , n
61 ______ . Na0--S-Lt-COOR1
62
pa o 0
5\\//
___________________________________ , ,--S " COOR1
CI = ----------
, n
63
Scheme 7B
00 00
R2-NH2
CI--S-;,_yn-000R1 1 FrS-,' COORI
63 "H n 64
NH2OH
0 0 0
Na0Me / Me0H , \\//
_____________________________________ o= R------N--S-!_____),--------N-OH
H i n H
67
Scheme 7C
00 00
\\ // Ph-NH-Me \\//
ci_-S-H--COOR1 . Ph---Ni--S--,'COOR1
63 i
Me 65
NH2OH 0õ0 0
Na0Me / Me0H
_____________________________________ i Ph=---__N--S-,. r_1,--------N-OH
I ' H
Me
68
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 107 -
Scheme 7D
00 00
\\// Ph-NH2 \\//
CI---00R1 ___________________________ .- Ph---w-S<_\_-COOR1
- , n
63 H ' i n 64
00
\\ II
PhCH2Br / NaH Ph.---N--S-H-r-ICOOR1
. . I 66
Ph----'
00 0
NH2OH \\
Na0Me 1 Me0H .....põ
Ph--N_.-S N-OH
. I
H
Ph---- n
69
Scheme 8
40 02N
02N *
71 HCl/Me0H 72
7- OH _________________________________________ ,--= OMe
0 0
H2N 5
SnCl2 73
. ,,,. OMe
R'? o H 0
S, \\ .,N 5
ii CI S 74
0 R \\
' 0 7' OMe
0
NH,OH a H
Na0Me/Me0H \\ ,N
,S
' R \\ =75 H
OH
0
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 108 -
Scheme 9
n s
H2N o
=81
OH R40
0 0 11
S \ 82
___________________________________________________ Rn 40, \O 4101 OH
0
H
\\ õIN
(COCI)2 =O 583 a
0 h
,.N
NH2OH S\\ 84 H
, Rn 0 1101 N,
OH
Scheme 10
91 R,
H2N
o//s-ca 92
.HCI 0
CDI, Et3N 0
__________________________ - 93
H
0
NH2OH ,0
Me0Na
/11\1N'OH 94
H
Uses
The present invention provides active compounds which are capable of
inhibiting
HDAC (for example, inhibiting HDAC activity, inhibiting formation of HDAC
complexes, inhibiting activity of HDAC complexes), as well as methods of
inhibiting HDAC activity, comprising contacting a cell with an effective
amount of
an active compound, whether in vitro or in vivo.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 109 -
The term "active," as used herein, pertains to compounds which are capable of
inhibiting HDAC activity, and specifically includes both compounds with
intrinsic
activity (drugs) as well as prodrugs of such compounds, which prodrugs may
themselves exhibit little or no intrinsic activity.
One of ordinary skill in the art is readily able to determine whether or not a
candidate compound is active, that is, capable of inhibiting HDAC activity.
For
example, assays which may conveniently be used to assess the inhibition
offered
by a particular compound are described in the examples below.
For example, a sample of cells (e.g., from a tumour) may be grown in vitro and
a
candidate compound brought into contact with the cells, and the effect of the
compound on those cells observed. As examples of "effect," the morphological
status of the cells may be determined (e.g., alive or dead), or the expression
levels of genes regulated by HDAC. Where the candidate compound is found to
exert an influence on the cells, this may be used as a prognostic or
diagnostic
marker of the efficacy of the compound in methods of treating a patient
carrying
cells of the same type (e.g., the tumour or a tumour of the same cellular
type).
In one aspect, the present invention provides antiproliferative agents. The
term
"antiproliferative agent" as used herein, pertains to a compound which treats
a
proliferative condition (i.e., a compound which is useful in the treatment of
a
proliferative condition).
The terms "cell proliferation," "proliferative condition," "proliferative
disorder," and
"proliferative disease," are used interchangeably herein and pertain to an
unwanted or uncontrolled cellular proliferation of excessive or abnormal cells
which is undesired, such as, neoplastic or hyperplastic growth, whether in
vitro or
in vivo. Examples of proliferative conditions include, but are not limited to,
pre-malignant and malignant cellular proliferation, including but not limited
to,
malignant neoplasms and tumours, cancers, leukemias, psoriasis, bone diseases,
fibroproliferative disorders (e.g., of connective tissues), and
atherosclerosis. Any
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
=
- 110 -
type of cell may be treated, including but not limited to, lung, colon,
breast,
ovarian, prostate, liver, pancreas, brain, and skin.
Antiproliferative compounds of the present invention have application in the
treatment of cancer, and so the present invention further provides anticancer
agents. The term "anticancer agent" as used herein, pertains to a compound
which treats a cancer (i.e., a compound which is useful in the treatment of a
cancer). The anti-cancer effect may arise through one or more mechanisms,
including but not limited to, the regulation of cell proliferation, the
inhibition of
angiogenesis (the formation of new blood vessels), the inhibition of
metastasis
(the spread of a tumour from its origin), the inhibition of invasion (the
spread of
tumour cells into neighbouring normal structures), or the promotion of
apoptosis
(programmed cell death).
The compounds of the present invention may also be used in the treatment of
conditions which are known to be mediated by HDAC, or which are known to be
treated by HDAC inhibitors (such as, e.g., trichostatin A). Examples of such
conditions include, but are not limited to, the following:
Cancer (see, e.g., Vigushin et al., 2001).
Psoriasis (see, e.g., lavarone et al., 1999).
Fibroproliferative disorders (e.g., liver fibrosis) (see, e.g., Niki et al.,
1999; Corneil
et al., 1998).
Smooth muscle proliferative disorder (e.g., atherosclerosis, restenosis) (see,
e.g.,
Kimura et al., 1994).
Neurodegenative diseases (e.g., Alzheimer's, Parkinson's, Huntington's chorea,
amyotropic lateral sclerosis, spino-cerebellar degeneration) (see, e.g.,
Kuusisto et
al., 2001).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 111 -
Inflammatory disease (e.g., osteoarthritis, rheumatoid arthritis) (see, e.g.,
Dangond et al., 1998; Takahashi et a)., 1996).
Diseases involving angiogenesis (e.g., cancer, rheumatoid arthritis,
psoriasis,
diabetic retinopathy) (see, e.g., Kim et al., 2001).
Haematopoietic disorders (e.g., anaemia, sickle cell anaemia, thalassaeimia)
(see, e.g., McCaffrey et al., 1997).
Fungal infection (see, e.g., Bernstein et al., 2000; Tsuji et al., 1976).
Parasitic infection (e.g., malaria, trypanosomiasis, helm inthiasis, protozoal
infections (see, e.g., Andrews et al., 2000).
Bacterial infection (see, e.g., Onishi et al., 1996).
Viral infection (see, e.g., Chang et al., 2000).
Conditions treatable by immune modulation (e.g., multiple sclerosis,
autoimmune
diabetes, lupus, atopic dermatitis, allergies, asthma, allergic rhinitis,
inflammatory
bowel disease; and for improving grafting of transplants) (see, e.g., Dangond
et
a)., 1998; Takahashi et al., 1996).
The invention further provides active compounds for use in a method of
treatment
of the human or animal body. Such a method may comprise administering to
such a subject a therapeutically-effective amount of an active compound,
preferably in the form of a pharmaceutical composition.
The term "treatment," as used herein in the context of treating a condition,
pertains generally to treatment and therapy, whether of a human or an animal
(e.g., in veterinary applications), in which some desired therapeutic effect
is
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 112 -
achieved, for example, the inhibition of the progress of the condition, and
includes
a reduction in the rate of progress, a halt in the rate of progress,
amelioration of
the condition, and cure of the condition. Treatment as a prophylactic measure
is
also included.
The term "therapeutically-effective amount," as used herein, pertains to that
amount of an active compound, or a material, composition or dosage from
comprising an active compound, which is effective for producing some desired
therapeutic effect, commensurate with a reasonable benefit/risk ratio.
The term "treatment" includes combination treatments and therapies, in which
two
or more treatments or therapies are combined, for example, sequentially or
simultaneously. Examples of treatments and therapies include, but are not
limited
to, chemotherapy (the administration of active agents, including, e.g., drugs,
antibodies (e.g., as in immunotherapy), prodrugs (e.g., as in photodynamic
therapy, GDEPT, ADEPT, etc.); surgery; radiation therapy; and gene therapy.
The invention further provides the use of an active compound for the
manufacture
of a medicament, for example, for the treatment of a proliferative condition,
as
discussed above.
The invention further provides the use of an active compound for the
manufacture
of a medicament, for example, for the treatment of conditions which are known
to
be mediated by HDAC, or which are known to be treated by HDAC inhibitors
(such as, e.g., trichostatin A), as discussed above.
The invention further provides a method for inhibiting HDAC in a cell
comprising
said cell with an effective amount of an active compound.
The invention further provides a method of treatment of the human or animal
body, the method comprising administering to a subject in need of treatment a
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 113 -
therapeutically-effective amount of an active compound, preferably in the form
of
a pharmaceutical composition.
Active compounds may also be used, as described above, in combination
therapies, that is, in conjunction with other agents, for example, cytotoxic
agents.
Active compounds may also be used as part of an in vitro assay, for example,
in
order to determine whether a candidate host is likely to benefit from
treatment with
the compound in question.
Active compounds may also be used as a standard, for example, in an assay, in
order to identify other active compounds, other antiproliferative agents, etc.
The compounds of the present invention may also be used in methods of
improving protein production by cultured cells (see, e.g., Furukawa et al.,
1998).
Routes of Administration
The active compound or pharmaceutical composition comprising the active
compound may be administered to a subject by any convenient route of
administration, whether systemically/ peripherally or topically (i.e., at the
site of
desired action).
Routes of administration include, but are not limited to, oral (e.g, by
ingestion);
buccal; sublingual; transdermal (including, e.g., by a patch, plaster, etc.);
transmucosal (including, e.g., by a patch, plaster, etc.); intranasal (e.g.,
by nasal
spray); ocular (e.g., by eyedrops); pulmonary (e.g., by inhalation or
insufflation
therapy using, e.g., via an aerosol, e.g., through the mouth or nose); rectal
(e.g.,
by suppository or enema); vaginal (e.g., by pessary); parenteral, for example,
by
injection, including subcutaneous, intradermal, intramuscular, intravenous,
intraarterial, intracardiac, intrathecal, intraspinal, intracapsular,
subcapsular,
intraorbital, intraperitoneal, intratracheal, subcuticular, intraarticular,
subarachnoid,
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 114 -
and intrasternal; by implant of a depot or reservoir, for example,
subcutaneously
or intramuscularly.
The Subject
The subject may be a prokaryote (e.g., bacteria) or a eukaryote (e.g.,
protoctista,
fungi, plants, animals).
The subject may be a protoctista, an alga, or a protozoan.
The subject may be a plant, an angiosperm, a dicotyledon, a monocotyledon, a
gymnosperm, a conifer, a ginkgo, a cycad, a fern, a horsetail, a clubmoss, a
liverwort, or a moss.
The subject may be an animal.
The subject may be a chordate, an invertebrate, an echinoderm (e.g., starfish,
sea
urchins, brittlestars), an arthropod, an annelid (segmented worms) (e.g.,
earthworms, lugworms, leeches), a mollusk (cephalopods (e.g., squids, octopi),
pelecypods (e.g., oysters, mussels, clams), gastropods (e.g., snails, slugs)),
a
nematode (round worms), a platyhelminthes (flatworms) (e.g., planarians,
flukes,
tapeworms), a cnidaria (e.g., jelly fish, sea anemones, corals), or a porifera
(e.g.,
sponges).
The subject may be an arthropod, an insect (e.g., beetles, butterflies,
moths), a
chilopoda (centipedes), a diplopoda (millipedes), a crustacean (e.g., shrimps,
crabs, lobsters), or an arachnid (e.g., spiders, scorpions, mites).
The subject may be a chordate, a vertebrate, a mammal, a bird, a reptile
(e.g.,
snakes, lizards, crocodiles), an amphibian (e.g., frogs, toads), a bony fish
(e.g.,
salmon, plaice, eel, lungfish), a cartilaginous fish (e.g., sharks, rays), or
a jawless
fish (e.g., lampreys, hagfish).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 115 -
The subject may be a mammal, a placental mammal, a marsupial (e.g., kangaroo,
wombat), a monotreme (e.g., duckbilled platypus), a rodent (e.g., a guinea
pig, a
hamster, a rat, a mouse), murine (e.g., a mouse), a lagomorph (e.g., a
rabbit),
avian (e.g., a bird), canine (e.g., a dog), feline (e.g., a cat), equine
(e.g., a horse),
porcine (e.g., a pig), ovine (e.g:, a sheep), bovine (e.g., a cow), a primate,
simian
(e.g., a monkey or ape), a monkey (e.g., marmoset, baboon), an ape (e.g.,
gorilla,
chimpanzee, orangutang, gibbon), or a human.
Furthermore, the subject may be any of its forms of development, for example,
a
spore, a seed, an egg, a larva, a pupa, or a foetus.
In one preferred embodiment, the subject is a human.
Formulations
While it is possible for the active ingredient to be administered alone, it is
preferable to present it as a pharmaceutical composition (e.g., formulation)
comprising at least one active ingredient, as defined above, together with one
or
more pharmaceutically acceptable carriers, excipients, buffers, adjuvants,
stabilisers, or other materials well known to those skilled in the art and
optionally
other therapeutic agents.
Thus, the present invention further provides pharmaceutical compositions, as
defined above, and methods of making a pharmaceutical composition comprising
admixing at least one active ingredient, as defined above, together with one
or
more pharmaceutically acceptable carriers, excipients, buffers, adjuvants,
stabilisers, or other materials, as described herein.
The term "pharmaceutically acceptable" as used herein pertains to compounds,
materials, compositions, and/or dosage forms which are, within the scope of
sound medical judgement, suitable for use in contact with the tissues of a
subject
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 116 -
(e.g., human) without excessive toxicity, irritation, allergic response, or
other
problem or complication, commensurate with a reasonable benefit/risk ratio.
Each
carrier, excipient, etc. must also be "acceptable" in the sense of being
compatible
with the other ingredients of the formulation.
The formulations may conveniently be presented in unit dosage form and may be
prepared by any methods well known in the art of pharmacy. Such methods
include the step of bringing into association the active ingredient with the
carrier
which constitutes one or more accessory ingredients. In general, the
formulations
are prepared by uniformly and intimately bringing into association the active
ingredient with liquid carriers or finely divided solid carriers or both, and
then if
necessary shaping the product.
Formulations may be in the form of liquids, solutions, suspensions, emulsions,
tablets, losenges, granules, powders, capsules, cachets, pills, ampoules,
suppositories, pessaries, ointments, gels, pastes, creams, sprays, foams,
lotions,
oils, boluses, electuaries, or aerosols.
Formulations suitable for oral administration (e.g., by ingestion) may be
presented
as discrete units such as capsules, cachets or tablets, each containing a
predetermined amount of the active ingredient; as a powder or granules; as a
solution or suspension in an aqueous or non-aqueous liquid; or as an oil-in-
water
liquid emulsion or a water-in-oil liquid emulsion; as a bolus; as an
electuary; or as
a paste.
A tablet may be made by compression or moulding, optionally with one or more
accessory ingredients. Compressed tablets may be prepared by compressing in a
suitable machine the active ingredient in a free-flowing form such as a powder
or
granules, optionally mixed with a binder (e.g., povidone, gelatin,
hydroxypropylmethyl cellulose), lubricant, inert diluent, preservative,
disintegrant
(e.g., sodium starch glycolate, cross-linked povidone, cross-linked sodium
carboxymethyl cellulose), surface-active or dispersing agent. Moulded tablets
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 117 -
may be made by moulding in a suitable machine a mixture of the powdered
compound moistened with an inert liquid diluent. The tablets may optionally be
coated or scored and may be formulated so as to provide slow or controlled
release of the active ingredient therein using, for example,
hydroxypropylmethyl
cellulose in varying proportions to provide the desired release profile.
Tablets
may optionally be provided with an enteric coating, to provide release in
parts of
the gut other than the stomach.
Formulations suitable for topical administration (e.g., transdermal,
intranasal,
ocular, buccal, and sublingual) may be formulated as an ointment, cream,
suspension, lotion, powder, solution, paste, gel, spray, aerosol, or oil.
Alternatively, a formulation may comprise a patch or a dressing such as a
bandage or adhesive plaster impregnated with active ingredients and optionally
one or more excipients or diluents.
Formulations suitable for topical administration in the mouth include losenges
comprising the active ingredient in a flavored basis, usually sucrose and
acacia or
tragacanth; pastilles comprising the active ingredient in an inert basis such
as
gelatin and glycerin, or sucrose and acacia; and mouthwashes comprising the
active ingredient in a suitable liquid carrier.
Formulations suitable for topical administration to the eye also include eye
drops
wherein the active ingredient is dissolved or suspended in a suitable carrier,
especially an aqueous solvent for the active ingredient.
Formulations suitable for nasal administration, wherein the carrier is a
solid,
include a coarse powder having a particle size, for example, in the range of
about
20 to about 500 microns which is administered in the manner in which snuff is
taken, i.e., by rapid inhalation through the nasal passage from a container of
the
powder held close up to the nose. Suitable formulations wherein the carrier is
a
liquid for administration as, for example, nasal spray, nasal drops, or by
aerosol
CA 02765409 2012-01-19
..A236-1
- 118 -
administration by nebuliser, include aqueous or oily solutions of the active
ingredient.
Formulations suitable for topical administration via the skin include
ointments,
creams, and emulsions. When formulated in an ointment, the active ingredient
may optionally be employed with either a paraffinic or a water-miscible
ointment
base. Alternatively, the active ingredients may be formulated in a cream with
an
oil-in-water cream base. If desired, the aqueous phase of the cream base may
include, for example, at least about 30% w/w of a polyhydric alcohol, i.e., an
alcohol having two or more hydroxyl groups such as propylene glycol, butane-
1,3-
diol, mannitol, sorbitol, glycerol and polyethylene glycol and mixtures
thereof. The
topical formulations may desirably include a compound which enhances
absorption or penetration of the active ingredient through the skin or other
affected areas. Examples of such dermal penetration enhancers include
dimethylsulfoxide and related analogues.
When formulated as a topical emulsion, the oily phase may optionally comprise
merely an emulsifier (otherwise known as an emulgent), or it may comprises a
mixture of at least one emulsifier with a fat or an oil or with both a fat and
an oil.
Preferably, a hydrophilic emulsifier is included together with a lipophilic
emulsifier
which acts as a stabiliser. It is also preferred to include both an oil and a
fat.
Together, the emulsifier(s) with or without stabiliser(s) make up the so-
called
emulsifying wax, and the wax together with the oil and/or fat make up the so-
called emulsifying ointment base which forms the oily dispersed phase of the
cream formulations.
Suitable emulgents and emulsion stabilisers include Tween 60, Span 80,
cetostearyl alcohol, myristyl alcohol, glyceryl monostearate and sodium lauryl
sulfate. The choice of suitable oils or fats for the formulation is based on
achieving the desired cosmetic properties, since the solubility of the active
compound in most oils likely to be used in pharmaceutical emulsion
formulations
may be very low. -Thus the cream should preferably be a non-greasy, non-
*Trade -mark
CA 02765409 2012-01-19
-4236-1
- 119 -
staining and washable product with suitable consistency to avoid leakage from
tubes or other containers. Straight or branched chain, mono- or dibasic alkyl
esters such as di-isoadipate, isocetyl stearate, propylene glycol diester of
coconut
fatty acids, isopropyl myristate, decyl oleate, isopropyl palmitate, butyl
stearate, 2-
*
ethylhexyl palmitate or a blend of branched chain esters known as Crodamol CAP
may be used, the last three being preferred esters. These may be used alone or
in combination depending on the properties required. Alternatively, high
melting
point lipids such as white soft paraffin and/or liquid paraffin or other
mineral oils
can be used.
Formulations suitable for rectal administration may be presented as a
suppository
with a suitable base comprising, for example, cocoa butter or a salicylate.
Formulations suitable for vaginal administration may be presented as
pessaries,
tampons, creams, gels, pastes, foams or spray formulations containing in
addition
to the active ingredient, such carriers as are known in the art to be
appropriate.
Formulations suitable for parenteral administration (e.g., by injection,
including
cutaneous, subcutaneous, intramuscular, intravenous and intradermal), include
aqueous and non-aqueous isotonic, pyrogen-free, sterile injection solutions
which
may contain anti-oxidants, buffers, preservatives, stabilisers, bacteriostats
and
solutes which render the formulation isotonic with the blood of the intended
recipient; and aqueous and non-aqueous sterile suspensions which may include
suspending agents and thickening agents, and liposomes or other
microparticulate systems which are designed to target the compound to blood
components or one or more organs. Examples of suitable isotonic vehicles for
use in such formulations include Sodium Chloride Injection, Ringer's Solution,
or
Lactated Ringer's Injection. Typically, the concentration of the active
ingredient in
the solution is from about 1 ng/ml to about 10 pg/ml, for example from about
10
ng/ml to about 1 pgirnl. The formulations may be presented in unit-dose or
multi-
dose sealed containers, for example, ampoules and vials, and may be stored in
a
freese-dried (lyophilised) condition requiring only the addition of the
sterile liquid
*Trade-mark -
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 120 -
carrier, for example water for injections, immediately prior to use.
Extemporaneous injection solutions and suspensions may be prepared from
sterile powders, granules, and tablets. Formulations may be in the form of
liposomes or other microparticulate systems which are designed to target the
active compound to blood components or one or more organs.
Dosage
It will be appreciated that appropriate dosages of the active compounds, and
compositions comprising the active compounds, can vary from patient to
patient.
Determining the optimal dosage will generally involve the balancing of the
level of
therapeutic benefit against any risk or deleterious side effects of the
treatments of
the present invention. The selected dosage level will depend on a variety of
factors including, but not limited to, the activity of the particular
compound, the
route of administration, the time of administration, the rate of excretion of
the
compound, the duration of the treatment, other drugs, compounds, and/or
materials used in combination, and the age, sex, weight, condition, general
health,
and prior medical history of the patient. The amount of compound and route of
administration will ultimately be at the discretion of the physician, although
generally the dosage will be to achieve local concentrations at the site of
action
which achieve the desired effect.
Administration in vivo can be effected in one dose, continuously or
intermittently
throughout the course of treatment. Methods of determining the most effective
means and dosage of administration are well known to those of skill in the art
and
will vary with the formulation used for therapy, the purpose of the therapy,
the
target cell being treated, and the subject being treated. Single or multiple
administrations can be carried out with the dose level and pattern being
selected
by the treating physician.
In general, a suitable dose of the active compound is in the range of about
0.1 to
about 250 mg per kilogram body weight of the subject per day. Where the active
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 121 -
ingredient is a salt, an ester, prodrug, or the like, the amount administered
is
calculated on the basis the parent compound and so the actual weight to be
used
is increased proportionately.
Kits
One aspect of the invention pertains to a kit comprising (a) the active
ingredient,
preferably provided in a suitable container and/or with suitable packaging;
and
(b) instructions for use, for example, written instructions on how to
administer the
active compound.
The written instructions may also include a list of indications for which the
active
ingredient is a suitable treatment.
CA 02765409 2012-01-19
54236-1
- 122 -
EXAMPLES
The following are examples are provided solely to illustrate the present
invention
and are not intended to limit the scope of the invention, as described herein.
General
1H NMR spectra were recorded at ambient temperature with WH-90/DS or
Mercury 200 (Varian) spectrometers. The HPLC measurements were performed
on a Gilson Model 302 system equipped with a spectrophotometer. Elemental
analyses were obtained with a Carlo Erba EA 1108 instrument. Melting points
were measured on a "Boetius" or "Fisher" micro melting point apparatus and are
uncorrected. Silicagel, 0.035-0.070 mm, (Acros) was employed for column
chromatography. All the solvents were purified before use by routine
techniques.
To isolate reaction products, the solvents were removed by evaporation using a
vacuum rotary evaporator, the water bath temperature not exceeding 40 C.
Various reagents were purchased from Sigma-Aldrich (The Old Brickyard, New
Road, Gillingham, Dorset, UK), Acros Organics (Janssens Pharmaceuticalaan 3A,
2440 Geel, Belgium), Lancaster Synthesis Ltd. (Eastgate, White Lund,
Morecambe, Lancashire, LA3 3DY, UK), and Maybridge plc (Trevillett, Tingagel,
Cornwall, PL34 OHW, UK).
Example 1
3-Formylbenzenesulfonic.acid, sodium salt (1)
H
Na0
0
Oleum (5 ml) was placed in a reaction vessel and benzaldehyde (2.00 g, 18.84
mmol) was slowly added not exceeding the temperature of the reaction mixture
more than 30 C, The Obtained solution was stirred at 40 C for ten hours and at
*Trade-mark
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
=
- 123 -
ambient temperature overnight. The reaction mixture was poured into ice and
extracted with ethyl acetate. The aqueous phase was treated with CaCO3 until
the
evolution of CO2 ceased (pH-6-7), then the precipitated CaSO4was filtered off
and washed with water. The filtrate was treated with Na2CO3 until the pH of
the
reaction medium increased to pH 8, obtained CaCO3 was filtered off and water
solution was evaporated in vacuum. The residue was washed with methanol, the
washings were evaporated and the residue was dried in desiccator over P205
affording the title compound (2.00 g, 51%). 1H NMR (D20), 6: 7.56-8.40 (4H,
m);
10.04 ppm (1H, s).
Example 2
3-(3-Sulfophenyl)acrylic acid methyl ester, sodium salt (2)
'S I 0,
,S Me
Na0
0
Sodium salt of 3-formylbenzenesulfonic acid (1) (1.009, 4.80 mmol), potassium
carbonate (1.329, 9.56 mmol), trimethyl phosphonoacetate (1.05 g, 5.77 mmol)
and water (2 ml) were stirred at ambient temperature for 30 min., precipitated
solid was filtered and washed with methanol. The filtrate was evaporated and
the
title compound (2) was obtained as a white solid (0.70 g, 55%). 1H NMR (DMSO-
d6, HMDSO), 8: 3.68 (3H, s); 6.51 (1H, d, J=16.0 Hz); 7.30-7.88 (5H, m).
Example 3
3-(3-Chlorosulfonylphenyl)acrylic acid methyl ester (3)
O\\OO ,
,s
CI me' \\
0
To the sodium salt of 3-(3-sulfophenyl)acrylic acid methyl ester (2) (0.670 g,
2.53
mmol) benzene (2 ml), thionyl chloride (1.508 g, 0.9 ml, 12.67 mmol) and 3
drops
of dimethylformamide were added and the resultant suspension was stirred at
reflux for one hour. The reaction mixture was evaporated, the residue was
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 124 -
dissolved in benzene (3 ml), filtered and the filtrate was evaporated to give
the
title compound (0.640 g, 97%).
Example 4
3-(3-Phenylsulfamoylphenyl)acrylic acid methyl ester (4a)
(:)\\ 1110I 0,
,s -Me
No
A solution of 3-(3-chlorosulfonylphenyl)acrylic acid methyl ester (3) (0.640
g, 2.45
mmol) in dichloromethane (2 ml) was added to a mixture of aniline (0.465 g,
4.99
mmol) and pyridine (1 ml), and the resultant solution was stirred at 50 C for
one
hour. The reaction mixture was evaporated and the residue was partitioned
between ethyl acetate and 10% HCI. The organic layer was washed successively
with water, saturated NaCl, and dried (Na2SO4). The solvent was removed and
the residue was chromatographed on silica gel with chloroform-ethyl acetate
(7:1,
v/v) as eluent. The obtained product was washed with diethyl ether to give the
title
compound (0.226 g, 29%). 1H NMR (CDCI3, HMDSO), 8: 3.72 (3H, s); 6.34 (1H, d,
J=16.0 Hz); 6.68 (1H, br s); 6.92-7.89 (10H, m).
Example 5
3-(3-Phenylsulfamoylphenyl)acrylic acid (5a)
% OH
.s
N\\O
3-(3-Phenylsulfamoylphenyl)acrylic acid methyl ester (4a) (0.220 g, 0.69 mmol)
was dissolved in methanol (3 ml), 1N NaOH (2.08 ml, 2.08 mmol) was added and
the resultant solution was stirred at ambient temperature overnight. The
reaction
mixture was partitioned between ethyl acetate and water. The aqueous layer was
acidified with 10% HCI and stirred for 30 min. The precipitated solid was
filtered,
washed with water and dried in desiccator over P205 to give the title compound
as
a white solid (0.173 g, 82%).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 125 -
Example 6
3-(3-Phenylsulfamoylphenyl)acryloyl chloride (6a)
1401 ()\\ CI
S
\\
N, ci
To a suspension of 3-(3-phenylsulfamoylphenyl)acrylic acid (5a) (0.173 g, 0.57
mmol) in dichloromethane (2.3 ml) oxalyl chloride (0.17 ml, 1.95 mmol) and one
drop of dimethylformamide were added. The reaction mixture was stirred at 40 C
for one hour and concentrated under reduced pressure to give crude title
compound (0.185 g).
Example 7
N-Hydroxy-3-(3-phenylsulfamoylphenyl)acrylamide (7a) (PX105684)
c)\\
vs OH
N
To a suspension of hydroxylamine hydrochloride (0.200 g, 2.87 mmol) in
tetrahydrofuran (3.5 ml) a saturated NaHCO3 solution (2.5 ml) was added and
the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a 3-(3-phenylsulfamoylphenyl)acryloyl chloride (6a) (0.185 g) solution
in
tetrahydrofuran (2.3 ml) was added and stirred at ambient temperature for one
hour. The reaction mixture was partitioned between ethyl acetate and 2N HC1.
The organic layer was washed successively with water and saturated NaCI, the
solvent was removed and the residue was washed with acetonitrile and diethyl
ether.
The title compound was obtained as a white solid (0.066 g, 36%), m.p. 172 C.
1H
NMR (DMSO-d6, HMDSO), 6:6.49 (1H, d, J=16.0 Hz); 7.18-8.05 (10H, m); 9.16
(1H, br s); 10.34(1H, s); 10.85 ppm (1H, br s). HPLC analysis on Symmetry C18
column: impurities 4% (column size 3.9x150 mm; mobile phase acetonitrile -
0.1M
phosphate buffer (pH 2.5), 40:60; sample concentration 1mg/m1; flow rate 0.8
ml/
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 126 -
min; detector UV 220 nm). Anal. Calcd for C15H14N204S, `)/0: C 56.59, H 4.43,
N
8.80. Found, Vo: C 56.28, H 4.44, N 8.56.
Example 8
3[3-(Methylphenylsulfamoyl)phenyliacrylic acid methyl ester (4b)
(:)\\
N COOMe
1 0
Me
A solution of 3-(3-chlorosulfonylphenyl)acrylic acid methyl ester (3) (0.440
g, 1.68
mmol) in dichloromethane (2 ml) was added to a mixture of N-methylaniline
(0.364
g, 3.40 mmol) and pyridine (0.5 ml), and the resultant solution was stirred at
50 C
for one hour. The reaction mixture was evaporated and the residue was
partitioned between ethyl acetate and 10% HCI. The organic layer was washed
successively with water, saturated NaCI, and dried (Na2SO4). The solvent was
removed and the residue was chromatographed on silica gel with chloroform-
ethyl
acetate (15:1, v/v) as eluent. The obtained product was washed with diethyl
ether
to give the title compound (0.155 g, 28%). 1H NMR (CDCI3, HMDSO), 6: 3.12 (3H,
s); 3.74 (3H, s); 6.34 (1H, d, J=16.0 Hz); 6.97-7.74 (10H, m).
Example 9
3[3-(Methylphenylsulfamoyl)phenyl]acrylic acid (5b)
R\
COOH
N
1 0
Me
3[3-(Methylphenylsulfamoyl)phenyl]acrylic acid methyl ester (4b) (0.150 g,
0.45
mmol) was suspended in methanol (2 ml), 1 N NaOH solution (1.35 ml, 1.35
mmol) was added and the resultant solution was stirred at ambient temperature
overnight. The reaction mixture was partitioned between ethyl acetate and
water.
The aqueous layer was acidified with 10% HCI and extracted with ethyl acetate.
The organic layer was washed successively with water, saturated NaCI, and
dried
(Na2SO4). The solvent was removed to give the title compound (0.135 g, 94%).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 127 -
Example 10
3[3-Methylphenylsulfamoyl)phenyl]acryloyl chloride (6b)
% 11110
coci
I 0
Me
To a suspension of 3[3-(methylphenylsulfamoyl)phenyl] acrylic acid (5b) (0.135
g,
0.42 mmol) in dichloromethane (2.3 ml) oxalyl chloride (0.17 ml, 1.95 mmol)
and
one drop of dimethylformarnide were added. The reaction mixture was stirred at
40 C for one hour and concentrated under reduced pressure to give crude title
compound (0.142 g).
Example 11
N-Hydroxy43-(3-methylphenylsulfamoyl)phenyl]acrylamide (7b) (PX105685)
14111 c)\\
'*OH
I 0 0
Me
To a suspension of hydroxylamine hydrochloride (0.200 g, 2.87 mmol) in
tetrahydrofuran (3.5 ml) a saturated NaHCO3 solution (2.5 ml) was added and
the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a 3[3-(methylphenylsulfamoyl)phenyllacryloyl chloride (6b) (0.142 g)
solution in tetrahydrofuran (2.3 ml) was added and stirred at ambient
temperature
for one hour. The reaction mixture was partitioned between ethyl acetate and
2N
HC1. The organic layer was washed successively with water and saturated NaCI,
the solvent was removed and the residue was washed with diethyl ether.
The title compound was obtained as a white solid (0.062 mg, 42%), m.p. 152 C.
1H NMR (DMSO-d6, HMDSO), 6: 3.16 (3H, s); 6.47 (1H, d, J=16.0 Hz); 7.03-7.96
(10H, m); 9.09 (1H, br s); 10.78 ppm (1H, br s). HPLC analysis on Symmetry C18
column; impurities 1.7% (column size 3.9x150 mm; mobile phase acetonitrile -
0.1M phosphate buffer (pH 2.5), 40:60; sample concentration 1mg/m1; flow rate
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
=
- 128 -
1.0 ml/ min; detector UV 220 nm). Anal. Calcd for C161-116N204S, (YO: C 57.82,
H
4.85, N 8.43. Found, %: C 57.82, H 4.83, N 8.35.
Example 12
343-(4-Methoxyphenylsulfamoy1)-phenylAacrylic acid methyl ester (4c)
= Me0
0\\=0,
Me
\\0
A solution of 3-(3-chlorosulfonylphenyl)acrylic acid methyl ester (3) (2.0g,
7.23
mmol) in dioxane (10 ml) was added to a mixture of 4-methoxyaniline (0.89 g,
7.23 mmol) in dioxane (15 ml) and NaHCO3 (1.2g, 14.5 mmol) in water (20 ml),
and the resultant solution was stirred at room temperature for one hour. The
reaction mixture was evaporated and the residue was partitioned between ethyl
acetate and 2N HO!. The organic layer was washed successively with water,
saturated NaCl, and dried (Na2SO4). The solvent was removed and the residue
was chromatographed on silica gel with dichloromethane-ethyl acetate (20:1,
v/v)
as eluent. The obtained product was washed with diethyl ether to the title
compound (2.0 g, 80%). 1H NMR (DMSO-d6, HMDSO), 6: 3.65 (3H, s); 3.74 (3H,
s); 6.65 (1H, d, J=16.0 Hz); 6.72-7.20 (4H, m); 7.56-8.18 (5H, m); 9.96 (1H,
br s).
Example 13
343-(4-Methoxyphenylsulfamoy1)-phenyl)]-acrylic acid (5c)
Me0
\\ OH
S\
\c)
To a suspension of 343-(4-methoxyphenylsulfamoy1)-phenyl)Facrylic acid methyl
ester (4c) (1.0g, 2.88 mmol) in methanol (15 ml) 1N NaOH solution (8.63 ml,
8.63
mmol) was added and the resultant mixture was stirred at ambient temperature
overnight. The reaction mixture was partitioned between ethyl acetate and
water.
The aqueous layer was acidified with 2N HC1 solution and stirred for 30 min.
The
precipitated solid was filtered, washed with water and dried in desiccator
over
P205 to give the title compound as a white solid (0.95 g, 99%).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 129 -
Example 14
313-(4-Methoxyphenylsulfamoy1)-phenyeacryloyl chloride (6c)
Me0 es 0
ci
.s
\\c,
To a suspension of 343-(4-methoxyphenylsulfamoy1)-phenyl)l-acrylic acid (5c)
(0.95 g, 2.85 mmol) in dichloromethane (12.0 ml) oxalyl chloride (0.88 ml,
10.07
mmol) and one drop of dimethylformamide were added. The reaction mixture was
stirred at 40 C for one hour and concentrated under reduced pressure to give
crude title compound (1.01 g).
Example 15
N-Hydroxy-343-(4-methoxyphenylsulfamoy1)-phenylkacrylamide (7c) (PX105844)
Me0
0
ICI
"S
\\0
To a suspension of hydroxylamine hydrochloride (0.99 g, 14.38 mmol) in
tetrahydrofuran (17.0 ml) a saturated NaHCO3 solution (12.0 ml) was added and
the resultant mixture was stirred at ambient temperature for 10 min. To the
reaction mixture a solution of 343-(4-methoxyphenylsulfamoy1)-phenyl)-acryloyl
chloride (6c) (1.01 g) in tetrahydrofuran (12.0 ml) was added and the mixture
was
stirred at ambient temperature for one hour. The reaction mixture was
partitioned
between ethyl acetate and 2N HCI. The organic layer was washed successively
with water and saturated NaCl, then the solvent was removed.
The residue was crystallised from ethyl acetate-methanol affording the title
compound (0.77 g, 77%), m.p. 186 C. 1H NMR (DMSO-d6, HMDSO), 6: 3.67 (s,
3H); 6.49 (d, J=16.0 Hz, 1H); 6.72-8.03 (m, 9H); 9.14 (br s, 1H); 9.91 (s,
1H);
10.85 (br s, 1H). HPLC analysis on Symmetry C18 column: impurities 2.5%
(column size 3.9x150 mm; mobile phase acetonitrile - 0.1M phosphate buffer (pH
2.5), 30:70; sample concentration 0.25 mg/ml; flow rate 1.0 ml/ min; detector
UV
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 130 -
220 nm). Anal. Calcd for C161-116N205S, C 55.16, H 4.63, N 8.04, S 9.20.
Found, cYo: C 55.07, H 4.60, N 7.94, S 9.01.
Example 16
3-(3-p-Tolylsulfamoyl-phenyl)-acrylic acid methyl ester (4d)
Me
0\\ 0,
,S Me
\\0
A solution of 3-(3-chlorosulfonylphenyI)-acrylic acid methyl ester (3) (2.0g,
7.23
mmol) in dioxane (10 ml) was added to a mixture of 4-methylaniline (0.77 g,
7.23
mmol) in dioxane (20 ml) and NaHCO3 (1.2g, 14.5 mmol) in water (20 ml), and
the
resultant solution was stirred at room temperature for one hour. The reaction
mixture was evaporated and the residue was partitioned between ethyl acetate
and 2N HCI. The organic layer was washed successively with water, saturated
NaCI, and dried (Na2SO4). The solvent was removed and the residue was
chromatographed on silica gel with dichloromethane-ethyl acetate (20:1, v/v)
as
eluent. The obtained product was washed with diethyl ether to give the title
compound (1.9 g, 79%). 1H NMR (DMSO-d6, HMDSO), 6: 2.16 (3H, s); 3.69 (3H,
s); 6.65 (1H, d, J=16.0 Hz); 7.00 (4H, s); 7.49-8.11 (5H, m); 10.14 (1H, br
s).
Example 17
3-(3-p-Toly)sulfamoyl-phenyl)-acrylic acid (5d)
Me
o\\S 1.1 OH
1- 0
To a suspension of 3-(3- p-tolylsulfamoyl-phenyl)-acrylic acid methyl ester
(4d)
(0.89g, 2.70 mmol) in methanol (12 ml) IN NaOH solution (8.10 ml, 8.10 mmol)
was added and the resultant mixture was stirred at ambient temperature
overnight. The reaction mixture was partitioned between ethyl acetate and
water.
The aqueous layer was acidified with 2N HCI solution and stirred for 30 min.
The
precipitated solid was filtered, washed with water and dried in desiccator
over
P206 to give the title compound as a white solid (0.75 g, 87%).
CA 02765409 2012-01-19
u4236-1
- 131 -
Example 18
3-(3-p-Tolylsulfamoyl-phenyl)-acryloyl chloride (6d)
Me
No
o
ci
,s
0
To a suspension of 3-(3-p-tolylsulfamoyl-phenyl)-acrylic acid (5d) (0.75 g,
2.36
mmol) in dichloromethane (10.0 ml) oxalyl chloride (0.62 ml, 7.08 mmol) and
one
drop of dimethylformamide were added. The reaction mixture was stirred at 40 C
for one hour and concentrated under reduced pressure to give crude title
compound (0.79g).
Example 19
N-Hydroxy-3-(3-p-tolylsulfamoy1)-phenyl)-acrylamide (7d) (PX106508)
Me
o
!SL11,
,S\ OH
No o
To a suspension of hydroxylamine hydrochloride (0.82 g, 11.80 mmol) in
tetrahydrofuran (10.0 ml) a saturated NaHCO3 solution (12.0 ml) was added and
the resultant mixture was stirred at ambient temperature for 10 min. To the
reaction mixture a solution of 3-(3-p-tolylsulfamoyI)-phenyl)-acryloyl
chloride (6d)
(0.79 g) in tetrahydrofuran (12.0 ml) was added and the mixture was stirred at
ambient temperature for one hour. The reaction mixture was partitioned between
ethyl acetate and 2N HCI. The organic layer was washed successively with water
and saturated NaCI, and the solvent was removed.
The residue was crystallised from ethyl acetate giving the title compound
(0.67 g,
85%), m.p. 200 C. 1H NMR (DMSO-d6, HMDSO) 6: 2.16 (s, 3H); 6.47 (d, 1H,
J=16.0 Hz); 6.98 (s, 4H); 7.29-7.98 (m, 5H); 9.09 (br s, 1H); 10.09 (s, 1H);
10.76
(br s, 1H). HPLC analysis on Zorbax SB- C18 column: impurities 4% (column size
4.6x150 mm; mobile phase acetonitrile - 0.1% H3PO4, gradient from 4010 100%;
sample concentration 0:6 mg/ml; flow rate 1.5 ml/ min; detector UV 270 nm).
Anal.
*Trade -mark
CA 02765409 2012-01-19
WO 02/30879 PCT/G B01/04326
- 132 -
Calcd for C161-116N204S, %: C 57.82, H 4.85, N 8.43. Found, 70: C 57.61, H
4.93, N
8.16.
Example 20
343-(4-Bromo-phenylsulfamoyl-phenyl)]-acrylic acid methyl ester (4e)
Br siMe
\\O 0
A solution of 3-(3-chlorosulfonylphenyI)-acrylic acid methyl ester (3) (1.85g,
6.50
mmol) in dioxane (10 ml) was added to a mixture of 4-bromoaniline (1.12 g,
6.50
mmol) in dioxane (20 ml) and NaHCO3 (1.10g, 13.09 mmol) in water (15 ml), and
the resultant solution was stirred at room temperature for one hour. The
reaction
mixture was evaporated and the residue was partitioned between ethyl acetate
and 2N HCI. The organic layer was washed successively with water, saturated
NaCI, and dried (Na2SO4). The solvent was removed and the residue was
chromatographed on silica gel with dichloromethane-ethyl acetate (20:1, v/v)
as
eluent. The obtained product was washed with diethyl ether to give the title
compound (1.62 g, 63 %). 1H NMR (DMSO-d6, HMDS0), 6: 3.76 (3H, s); 6.69
(1H, d, J=16.0 Hz); 6.98-7.23 (2H, m); 7.32-8.07 (7H, m); 10.47 (1H,br s).
Example 21
3-[(3-(4-Bromo-phenylsulfamoyl-phenyl)]-acrylic acid (5e)
Br
S\S OH
\\O
To a suspension of 343-(4-bromo-phenylsu1famoyl-phenyl)]-acrylic acid methyl
ester (4e) (0.80g, 2.02 mmol) in methanol (10 ml) 1N NaOH solution (6.00 ml,
6.00 mmol) was added and the resultant mixture was stirred at ambient
temperature overnight. The reaction mixture was partitioned between ethyl
acetate and water. The aqueous layer was acidified with 2N HCI and stirred for
30
min. The precipitated solid was filtered, washed with water and dried in
desiccator
over P205 to give the title compound as a white solid (0.64 g, 84%).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 133 -
Example 22
343-(4-Bromo-phenylsulfamoyl-phenyl)]-acryloyl chloride (6e)
Br 0 401
HO o
.s
To a suspension of 343-(4-bromo-phenylsulfamoyl-phenyl)Facrylic acid (5e)
(0.64
g, 1.67 mmol) in dichloromethane (8.0 ml) oxalyl chloride (0.44 ml, 5.02 mmol)
and one drop of dimethylformamide were added. The reaction mixture was stirred
at 40 C for one hour and concentrated under reduced pressure to give crude
title
compound (0.67 g).
Example 23
N-Hydroxy-343-(4-bromo-phenylsulfamoy1)-phenyl)]-acrylamide (7e) (PX106509)
Br
411 \\S\ 11,
OH
(3
To a suspension of hydroxylamine hydrochloride (0.58 g, 8.35 mmol) in
tetrahydrofuran (8.0 ml) a saturated NaHCO3 solution (8.0 ml) was added and
the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a 3-[3-(4-bromo-phenyisulfamoy1)-phenyl)]-acryloyl chloride (6e) (0.67
g)
solution in tetrahydrofuran (8.0 ml) was added and stirred at ambient
temperature
for one hour. The reaction mixture was partitioned between ethyl acetate and
2N
HCI. The organic layer was washed successively with water and saturated NaCI,
and the solvent was removed.
The residue was crystallised from ethyl acetate giving the title compound
(0.52 g,
78%), m.p. 204 C. 1H NMR (DMSO-d6, HMDSO), 5: 6.49 (d, 1H, J=16.0 Hz); 7.05
(d, 2H, J=9.0 Hz); 7.34-7.98 (m, 7H); 9.09 (br s, 1H); 10.47 (s, 1H); 10.80
(br s,
1H). HPLC analysis on Zorbax SB- C18 column: impurities 5% (column size
4.6x150 mm; mobile phase acetonitrile - 0.1% H3PO4, gradient from 40 to 100%;
sample concentration 0.9 mg/ml; flow rate 1.5 ml/ min; detector UV 270 nm).
Anal.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 134 -
Calcd for C151-113BrN204S, C 45.35, H 3.30, N 7.05. Found, (Yo: C 45.73, H
3.33,
N 6.81.
Example 24
343-(4-Chloro-phenylsulfamoyl-phenyeacrylic acid methyl ester (4f)
of
0,\ 0,
,S Me
N
A solution of 3-(3-chlorosu(fonylphenyl) acrylic acid methyl ester (3) (1.10
g, 4.22
mmol) in dioxane (10 ml) was added to a mixture of 4-chloroaniline (0.53 g,
4.22
mmol) in dioxane (10 ml) and NaHCO3 (0.50g, 5.95 mmol) in water (10 ml), and
the resultant solution was stirred at room temperature for one hour. The
reaction
mixture was evaporated and the residue was partitioned between ethyl acetate
and 2N HCI. The organic layer was washed successively with water, saturated
NaCI, and dried (Na2SO4). The solvent was removed and the residue was
chromatographed on silica gel with dichloromethane-ethyl acetate (20:1, vi v)
as
eluent. The obtained product was washed with diethyl ether to give the title
compound (1.01 g, 71 /0).
Example 25
3-[(3-(4-Chloro-phenylsulfamoyl-phenyl)]-acrylic acid (5f)
ci el 0
OH
N \\0
To a suspension of 343-(4-chloro-phenylsulfamoyl-phenyl)Facrylic acid methyl
ester (4f) (0.77g, 2.12 mmol) in methanol (10 ml) IN NaOH solution (6.57 ml,
6.57
mmol) was added and the resultant solution was stirred at ambient temperature
overnight. The reaction mixture was partitioned between ethyl acetate and
water.
The aqueous layer was acidified with 2N HCI and stirred for 30 min. The
precipitated solid was filtered, washed with water and dried in desiccator
over
P205 to give the title compound as a white solid (0.64 g, 86%).
CA 02765409 2012-01-19
WO 02/39879 PCT/GB01/04326
=
- 135 -
Example 26
343-(4-Chloro-phenylsulfamoyl-phenyl)}-acryloyl chloride (6f)
14111 R\s õr CI
\\O
To a suspension of 3-[3-(4-chloro-phenylsulfamoyl-phenyl)]-acrylic acid (5f)
(0.64
g, 1.89 mmol) in dichloromethane (8.0 ml) oxalyl chloride (0.50 ml, 5.68 mmol)
and one drop of dimethylformamide were added. The reaction mixture was stirred
at 40 C for one hour and concentrated under reduced pressure to give crude
title
compound (0.65 g).
Example 27
N-Hydroxy-343-(4-chloro-phenylsulfamoy1)-phenyl)]-acrylamide (7f) (PX106510)
CI
"s
\\O
To a suspension of hydroxylamine hydrochloride (0.66 g, 9.45 mmol) in
tetrahydrofuran (12.0 nil) a saturated NaHCO3 solution (8.0 ml) was added and
the resultant mixture was stirred at ambient temperature for 10 min. To the
reaction mixture a 343-(4-chloro-phenylsulfamoy1)-phenyl)}-acryloyl chloride
(6f)
(0.65 g) solution in tetrahydrofuran (8.0 ml) was added and the mixture was
stirred
at ambient temperature for one hour. The reaction mixture was partitioned
between ethyl acetate and 2N HCI. The organic layer was washed successively
with water and saturated NaCl, and the solvent was removed.
The residue was crystallised from acetonitrile giving the title compound (0.47
g, 75
%), m.p. 198 C. 1H NMR (DMSO-d6, HMDSO), 5:6.49 (d, 1H, J=16.0 Hz); 6.98-
8.05 (m, 9H); 9.16 (br s, 1H); 10.49 (s, 1H); 10.85 (s, 1H). HPLC analysis on
Zorbax SB- C18 column: impurities 5% (column size 4.6x150 mm; mobile phase
acetonitrile - 0.1% H3PO4, gradient from 30 to 100%; sample concentration 0.2
mg/ml; flow rate 1.5 ml/nnin; detector UV 270 nm). Anal. Calcd for
C15H13C1N204S,
%: 051.07, H 3.71, N 7.94, S 9.09. Found, %: C 50.96, H 3.62, N 7.76, S 9.00.
CA 02765409 2012-01-19
WO 02/30879
PCPCB01/04326
- 136 -
Example 28
3-(3-Benzylsulfamoyl-phenyl)-acrylic acid methyl ester (4g)
R\ 0,
-Me
r \\43 0
mmol) in dioxane (5.0 ml) was added to a mixture of 4-benzylamine (0.17 ml,
1.53
mmol) in dioxane (1.0 ml) and NaHCO3 (0.26g, 3.06 mmol) in water (3.0 ml), and
the resultant solution was stirred at room temperature for one hour. The
reaction
mixture was evaporated and the residue was partitioned between ethyl acetate
Example 29
3-(3-Benzylsulfamoyl-phenyl)acrylic acid (5g)
\\s OH
401
MD a
To a suspension of 3-(3- benzylsulfamoyl-phenyl)-acrylic acid methyl ester
(4g)
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 137 -
Example 30
3-(3-Benzylsulfamoyl-phenyl)-aoryloyl chloride (6g)
o 401
ci
N,s\\13
To a suspension of 3-(3-(benzylsulfamoyl-phenyl)-acrylic acid (5g) (0.16 g,
0.52
mmol) in dichloromethane (2.0 ml) oxalyl chloride (0.16 ml, 1.79 mmol) and one
drop of dimethylformamide were added. The reaction mixture was stirred at 40 C
for one hour and concentrated under reduced pressure to give crude title
compound (0.17 g).
Example 31
N-Hydroxy-3-(3-benzylsulfamoy1)-phenyl)acrylamide (7g) (PX106511)
%
,s --OH
N
To a suspension of hydroxylamine hydrochloride (0.18 g, 2.60 mmol) in
tetrahydrofuran (3.0 ml) a saturated NaHCO3 solution (2.5 ml) was added and
the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a 3-(3- benzylsulfamoy1)-phenyl)acryloyl chloride (6g) (0.17 g)
solution in
tetrahydrofuran (2.0 ml) was added and stirred at ambient temperature for one
hour. The reaction mixture was partitioned between ethyl acetate and 2N HCI.
The
organic layer was washed successively with water and saturated NaCI, and the
solvent was removed.
The residue was crystallised from ethyl acetate giving the title compound
(0.12 g,
68%), m.p. 179 C. 1H NMR (DMSO-d6, HMDS0), 6: 4.02 (d, 2H, J= 6.4 Hz); 6.53
(d, 1H, J=16.0 Hz); 7.25 (s, 5H); 7.39-8.03 (m, 5H); 8.20 (t, 1H, J= 6.4 Hz);
9.12
(br s, 11-l); 10.80 (br s, 1H). HPLC analysis on Zorbax BB-C18 column:
impurities
5% (column size 4.6x150 mm; mobile phase acetonitrile - 0.1% H3PO4, gradient
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 138 -
from 30 to 100%; sample concentration 0.5 mg/ml; flow rate 1.5 ml/min;
detector
UV 230 nm). Anal. Calcd for C161-116N204S, %: C 57.82, H 4.85, N 8.43, S 9.6,
Found, %: C 57.59, H 4.82, N 8.14, S 9.6.
Example 32
3-(3-Phenethylsulfamoyl-phenyl)-acrylic acid methyl ester (4h)
411 (:),\ õ. 0,
Me
lb 0
A solution of 3-(3-chlorosulfonylphenyI)-acrylic acid methyl ester (3) (0.40g,
1.53
mmol) in dioxane (5.0 ml) was added to a mixture of 4-phenethylamine (0.19 ml,
1.53 mmol) in dioxane (1.0 ml) and NaHCO3 (0.26 g, 3.06 mmol) in water (3.0
ml)
and the resultant solution was stirred at room temperature for one hour. The
reaction mixture was evaporated and the residue was partitioned between ethyl
acetate and 2N HCI. The organic layer was washed successively with water,
saturated NaCI, and dried (Na2SO4). The solvent was removed and the residue
was chromatographed on silica gel with petroleum ether-ethyl acetate (2:1,
v/v) as
eluent. The obtained product was washed with diethyl ether to give the title
compound (0.43 g, 82 %). 1H NMR (DMSO-d6, HMDS0), 6: 2.69 (2H, m); 2.98
(2H, m); 3.72 (3H, s); 6.72 (1H, d, J=16.0 Hz); 7.05-7.43 (5H, m); 7.54-8.14
(6H,
m).
Example 33
3-(3-Phenethylsulfamoyl-phenyl)-acrylic acid (5h)
11101 OH
,\\O
To a suspension of 3-(3-phenethylsulfamoyl-phenyl)-acrylic acid methyl ester
(4h)
(0.20g, 0.58 mmol) in methanol (3.0 ml) IN NaOH solution (1.75 ml, 1.75 mmol)
was added and the resultant solution was stirred at ambient temperature
overnight. The reaction mixture was partitioned between ethyl acetate and
water.
The aqueous layer was acidified with 2N HCI solution and extracted with ethyl
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 139 -
acetate. The organic layer was washed successively with water, saturated NaCI,
and dried (Na2SO4). The solvent was removed and the residue was washed with
ether to give the title compound as a white solid (0.15 g, 77%).
Example 34
3-(3-Phenethylsulfamoyl-phenyl)acryloyl chloride (6h)
0
\\
,s
N\\
O
a suspension of 3-(3-phenethylsulfamoyl-phenyl)-acrylic acid (5h) (0.15 g,
0.45
mmol) in dichloromethane (2.0 ml) oxalyl chloride (0.14 ml, 1.57 mmol) and one
drop of dimethylformamide were added. The reaction mixture was stirred at 40 C
for one hour and concentrated under reduced pressure to give crude title
compound (0.16 g).
Example 35
N-Hydroxy-3-(3-phenethylsulfamoyI)-phenyl)-acrylamide (7h) (PX106512)
= 0\\ 1110
\\O
To a suspension of hydroxylamine hydrochloride (0.16 g, 2.25 mmol) in
tetrahydrofuran (3.0 ml) a saturated NaHCO3 solution (2.0 ml) was added and
the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a 3-(3-phenethylsulfamoy1)-phenyl)-acryloyl chloride (6h) (0.16 g)
solution
in tetrahydrofuran (2.0 ml) was added and stirred at ambient temperature for
one
hour. The reaction mixture was partitioned between ethyl acetate and 2N HCI.
The
organic layer was washed successively with water and saturated NaCl, and the
solvent was removed.
The residue was crystallised from dichloromethane-ether giving the title
compound (0.10 g, 66%), m.p. 114 C. 1H NMR (DMSO-d6, HMDS0), 6: 2.67 (m,
2H); 3.00 (m, 2H); 6.55 (d, 1H, J=16.0 Hz); 7.00-8.05 (m, 11H); 9.12 (br s,
1H);
CA 02765409 2012-01-19
54236-1
-140-
10.78 (br s, 1H). HPLC analysis on Zorbax SB-C18 column: impurities 5% (column
size 4.6x150 mm; mobile phase acetonitrile - 0.1% H3PO4, gradient from 30 to =
100%; sample concentration 1.0 mg/ml; flow rate 1.5 mi/min; detector: UV 230
nm). Anal. Calcd for C17H18N204S, %: C 58.94, H 5.24, N 8.09, S 9.26. Found,
`)/0:
C 58.81, H 5.16, N 8.00, S 9.05.
Example 36
343-(3-Methoxy-phenylsulfamoy1)-phenyll-acrylic acid methyl ester (4i)
A solution of 3-(3-chlorosulfonylphenyI)-acrylic acid methyl ester (3) (0.4 g,
1.53
mmol) in dioxane (5 ml) was added to a mixture of 3-methoxyphenylamine (0.189
g, 1.53 mmol) in dioxane (1 ml) and NaHCO3 (0.25 g, 3.06 mmol) in water (3
ml),
and the resultant solution was stirred at room temperature until the
completion of
the reaction (control by TLC). The reaction mixture was evaporated and the
residue was partitioned between ethyl acetate and 2N HCI. The organic layer
was
washed successively with water, saturated NaCl, and dried (Na2SO4). The
solvent
was removed-an-d the residue was chromatographed on silica gel with petroleum
ether-ethyl acetate (2:1, v/v) as eluent. The obtained product was washed with
diethyl ether to give the title compound (0.44 g, 82%) as a white solid. 1H
NMR
(DMSO-d6, HMDSO), 5: 3.60 (3H, s), 3.71 (3H, s); 6.52-6.74 (3H, m); 6.63 (1H,
d,
J==-.16.0 Hz); 7.07 (1H, m); 7.43 ¨8.05 (5H, m); 10.27 ppm (1H, br s).
Example 37
343-(3-Methoxy-phenylsulfamoy1)-phenylFacrylic acid (51)
To a suspension of 3-[3-(3-methoxyphenyl-sulfamoy1)-phenyll-acrylic acid
methyl
ester (41) (0.42 g, 1.2 mmol) in methanol (5.5 ml) IN NaOH solution (3.6 ml,
3.6
mmol) was added and the resultant mixture was stirred at ambient temperature
overnight. The reaction mixture was partitioned between ethyl acetate and
water.
The aqueous layer was acidified with 2N HCI solution and stirred for 30 min.
The
precipitated solid was filtered, washed with water and dried in desiccator
over
P205. The title compound was obtained as a white solid (0.38 g, 95%). 1H NMR
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 141 -
(DMSO-d6, HMDSO), 5 3.65 (31-I, s); 6.40-6.78 (41-1, m); 7.16 (1H, m); 7.45
¨8.09
(5H, m); 10.32 (1H, br s).
Example 38
343-(3-Methoxy-phenylsulfamoy1)-phenyl]-acryloyl chloride (61)
To a suspension of 343-(3-methoxyphenyl-sulfamoy1)-phenyn-acrylic acid (51)
(0.38 g, 1.14 mmol) in dichloromethane (4 ml) oxalyl chloride (0.3 ml, 3.43
mmol)
and one drop of dimethylformamide were added. The reaction mixture was stirred
at 40 C for one hour and concentrated under reduced pressure to give crude
title
compound (0.40 g, 100%).
Example 39
N-Hydroxy-343-(3-methoxy-phenylsulfamoy1)-phenyl]-acrylamide (71) (PX117712)
401 c)\\ Fri,OH
Me0 N
HO 0
To a suspension of hydroxylamine hydrochloride (0.39 g, 5.7 mmol) in
tetrahydrofuran (6 ml) a saturated NaHCO3 solution (4.5 ml) was added and the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a solution of crude 343-(3-methoxy-phenylsulfamoy1)-phenylFacryloyl
chloride (61) (0.40 g) in tetrahydrofuran (4 ml) was added and the mixture was
stirred at ambient temperature for one hour. The reaction mixture was
partitioned
between ethyl acetate and 2N HCI. The organic layer was washed successively
with water and saturated NaCI, then the solvent was removed. The residue was
crystallised from ethyl acetate-acetonitrile affording the title compound
(0.15 g,
39%) as a lightly pink crystals. M.p. 137 C. 1H NMR (DMSO-d6, HMDSO) 6: 3.65
(3H, s); 6.38-6.78 (4H, m); 6.98-7.27 (1H, m); 7.34-8.03 (5H, m); 9.14 (1H, br
s);
10.30 (1H, s); 10.83 (1H, br s). HPLC analysis on Symmetry C6 column:
impurities
5% (column size 3.9x150 mm; mobile phase acetonitrile - 0.1M phosphate buffer
(pH 2.5), 40:60; sample concentration 0.5 mg/m1; flow rate 1.2 ml/ min;
detector
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 142 -
UV 254 nm). Anal. Calcd for C161-116N206S containing 1 % of inorganic
impurities,
%: C 54.67, H 4.50, N 8.09. Found, %: C 54.61, H 4.58, N 7.96.
Example 40
343-(Biphenyl-2-ylsulfamoy1)-phenyl]-acrylic acid methyl ester (4j)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylphenyl)acrylic acid methyl ester (3) and 2-aminobiphenyl, as a
white
solid, yield 48%, 1H NMR (DMSO-d6, HMDSO), 8: 3.65 (3H, s); 6.56 (1H, d,
Example 41
343-(Biphenyl-2-ylsulfamoy1)-phenyll-acrylic acid (5j)
Using an analogous method, the title compound was obtained from 343-(bipheny1-
2-ylsulfamoy1)-phenylFacrylic acid methyl ester (4j) and sodium hydroxide,
yield
89%. 1H NMR (DMSO-d6, HMDSO), 8: 6.47 (1H, d, J= 16.0 Hz); 6.98 ¨8.03 (14H,
m); 9.54 (1H, br s).
Example 42
3[3-(Bipheny1-2-ylsulfamoy1)-pheny1]-acryloyl chloride (6j)
Using an analogous method, the title compound was obtained from 343-(biphenyl-
2-ylsulfamoyl)-phenyl1-acrylic acid (5j) and oxalyl chloride in a form of a
crude
product, yield ca. 97%.
Example 43
313-(Biphenyl-2-ylsulfamoy1)-phenyl]-N-hydroxy-acrylamide (7j) (PX117713)
140
,S OH
N
H 0 0
Ph
CA 02765409 2012-01-19
WO 112/30879 PCT/G BO 1 /04326
- 143 -
Using an analogous method, the title compound was obtained from 343-(bipheny1-
2-ylsulfamoy1)-phenyll-acryloyl chloride (6j)and hydroxylamine hydrochloride,
yield
47%, foam. 1H NMR (DMSO-d6, HMDSO), 5: 6.43 (1H, d, J=16.0 Hz); 6.94-7.85
(14H, m); 9.07 (1H, br s); 9.58 (1H, br s); 10.78 (1H, br s). HPLC analysis on
Symmetry C8 column: impurities 6.4% (column size 3.9x150 mm; mobile phase
acetonitrile-0.1M phosphate buffer, pH 2.5, 50:50; sample concentration 0.5
mg/ml; flow rate 1.0 ml/min; detector UV 254 nm). Anal. Calcd for C211-
118N204S *
0.5 H20, %: 62.52, H 4.75, N 6.94. Found, %: C 62.58, H 4.66, N 6.65.
Example 44
343-(Biphenyl-4-ylsulfamoy1)-phenyTacrylic acid methyl ester (4k)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylpheny1)-acrylic acid methyl ester (3) and 4-aminobiphenyl as a
white
solid, yield 88%, 1H NMR (DMSO-d5, HMDSO), 8: 3.71 (3H, s); 6.67 (1H, d,
J=16.0 Hz); 7.07 ¨8.09 (14H, m); 10.36 ppm (1H, br s).
Example 45
343-(Biphenyl-4-yisulfamoy1)-phenylFacrylic acid (5k)
Using an analogous method, the title compound was obtained from 343-(biphenyl-
4-ylsulfamoy1)-phenyll-acrylic acid methyl ester (4k) and sodium hydroxide,
yield
88%. 1H NMR (DMSO-d6, HMDSO), 5: 6.56 (1H, d, J--= 16.0 Hz); 7.09-8.12 (14H,
m); 10.38 ppm (1H, br s).
Example 46
343-(Biphenyl-4-ylsulfamoy1)-phenyl]-acryloyl chloride (6k)
Using an analogous method, the title compound was obtained from 313-(biphenyl-
4-ylsulfamoyI)-phenyl]-acrylic acid (5k) and oxalyl chloride in a form of a
crude
product, yield ca. 98%.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 144 -
Example 47
3[3-(Bipheny1-4-ylsulfamoy1)-pheny11-N-hydroxy-acrylamide (7k) (PX117715)
Os, 10
C OH
'0
0
Using an analogous method, the title compound was obtained from 3-[3-(biphenyl-
4-ylsulfamoy1)-phenyl]-acryloyl chloride (6k) and hydroxylamine hydrochloride,
yield 78%. M.p. 188 C. 1H NMR (DMSO-d6, HMDSO), 6: 6.49 (1H, d, J= 16.0 Hz);
7.07-8.07 (14H, m); 9.09 (1H, br s); 10.35 (1H, br s); 10.80 (1H, br s). HPLC
analysis on Symmetry C8 column: impurities 2.2% (column size 3.9x150 mm;
mobile phase acetonitrile-0.1M phosphate buffer, pH 2.5, 40:60; sample
concentration 0.5 mg/ml; flow rate 1.5 ml/min; detector UV 254 nm). Anal.
Calcd
for C21Fl18N204S * 0.2 H20, %: C 63.37, H 4.66, N 7.04. Found, %: C 63.42, H
4.57, N 6.95.
Example 48
343-(3-Bromo-phenylsulfamoy1)-pheny1]-acrylic acid methyl ester (41)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylpheny1)-acrylic acid methyl ester (3) and 3-bromoaniline as a
white
solid, yield 79%, 1H NMR (DMSO-d6, HMDSO), 5: 3.73 (3H, s); 6.65 (1H, d,
J=16.0 Hz); 6.98-7.34(4H, m); 7.49 ¨8.07 (5H, m); 10.52 ppm (1H, br s).
Example 49
343-(3-Bromo-phenylsulfamoy1)-pheny1]-acrylic acid (51)
Using an analogous method, the title compound was obtained from 313-(3-bromo-
phenylsulfamoy1)-phenyli-acrylic acid methyl ester (41) and sodium hydroxide,
yield 85%.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 145 -
Example 50
343-(3-Bromo-phenylsulfamoy1)-phenyl}-acryloyl chloride (61)
Using an analogous method, the title compound was obtained from 3-[3-(3-bromo-
phenylsulfamoy1)-phenyl]acrylic acid (51) and oxalyl chloride in a form of a
crude
product, yield ca. 98%.
Example 51
343-(3-Bromo-phenylsulfamoy1)-phenyll-N-hydroxy-acrylamide (71) (PX117734)
kil,OH
S
Br N \\
N o
Using an analogous method, the title compound was obtained from 343-(3-bromo-
phenylsulfamoy1)-phenylFacryloyl chloride (61) and hydroxylamine
hydrochloride,
yield 24%. M.p. 135.5-136.5 C. 1H NMR (DMSO-d6, HMDS0), 8: 6.53 (1H, d,
J=15.6 Hz); 7.07-7.28 (4H, m); 7.48 (1H, d, J=15.6 Hz); 7.60 (1H, t, J=7.6
Hz);
7.72 (1H, d, J=7.6 Hz); 7.81 (1H, d, J=7.6 Hz); 7.94 (1H, s); 9.15 (1H, br s);
10.60
(1H, br s); 10.84 (1H, br s). HPLC analysis on Symmetry C8 column: impurities
2.5% (column size 3.9x150 mm; mobile phase acetonitrile-0.1M phosphate buffer,
pH 2.5, 50:50; sample concentration 0.5 mg/ml; flow rate 0.8 ml/min; detector
UV
220 nm). Anal. Calcd for C161-113BrN204S, %: C 45.35, H 3.30, N 7.05. Found, C
45.38, H 3.03, N 6.96.
Example 52
343-(1ndan-2-ylsulfamoy1)-phenyll-acrylic acid methyl ester (4m)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylpheny1)-acrylic acid methyl ester (3) and 2-aminoindane
hydrochloride as a white solid, yield 80%, 1H NMR (DMSO-d6, HMDSO), 5: 2.65-
2.93 (4H, m); 3.71 (3H, s); 3.93 (1H, m); 6.71 (1H, d, J=16.0 Hz); 7.09 (4H,
s);
7.49 ¨8.27 ppm (6H, m).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 146 -
Example 53
343-(lndan-2-ylsulfamoy1)-phenylFacrylic acid (5m)
Using an analogous method, the title compound was obtained from 3-[3-(indan-2-
ylsulfamoy1)-phenyl]-acrylic acid methyl ester (4m) and sodium hydroxide,
yield
86%.
Example 54
3[3-(Indan-2-ylsulfamoy1)-phenylFacryloyl chloride (6m)
Using an analogous method, the title compound was obtained from 343-(indan-2-
ylsulfamoyI)-phenyll-acrylic acid (5m) and oxalyl chloride in a form of a
crude
product, yield ca. 98%.
Example 55
N-Hydroxy-3[3-(indan-2-ylsulfamoy1)-phenyTacrylamide (7m) (PX117735)
c)\\0 H
N
H 0
Using an analogous method, the title compound was obtained from 3-[3-(indan-2-
ylsulfamoy1)-phenyl]-acryloyl chloride (6m) and hydroxylamine hydrochloride,
yield
63%. M.p. 164 C (from acetonitrile). 1H NMR (DMSO-de, HMDSO), 5: 2.72 (2H,
dd, J=15.8 and 7.0 Hz); 2.94 (2H, dd, J=15.8 and 7.4 Hz); 3.83-4.03 (1H, m);
6.59
(1H, d, J=15.9 Hz); 7.04-7.19 (4H, m); 7.55 (1H, d, J=15.9 Hz); 7.66 (1H, t,
J=7.7
Hz); 7.84 (1H, d, J=7.2 Hz); 7.84 (1H, d, J=8.2 Hz); 8.02 (1H, s); 8.11 (1H,
br d,
J=6.6 Hz); 9.15 (1H, br s); 10.84 (1H, br s). HPLC analysis on Symmetry 08
column: impurities 1% (column size 3.9x150 mm; mobile phase acetonitrile-0.1M
phosphate buffer, pH 2.5, 45:55; sample concentration 0.5 mg/ml; flow rate 1.0
ml/min; detector UV 254 nm). Anal. Calcd for C18H18N204S * 0.25 H20, A: C
59.57, H 5.14, N 7.72. Found C 59.51, H 5.01, N 7.54.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 147 -
Example 56
3[3-(Benzhydryl-sulfamoy1)-phenylFacrylic acid methyl ester (4n)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylphenyl)-acrylic acid methyl ester (3) and aminodiphenylmethane
as
a white solid, yield 73%, 1H NMR (DMSO-d6, HMDSO), 5: 3.72 (3H, s); 5.60 (1H,
d, J=9.0 Hz); 6.52 (1H, d, J=16.0 Hz); 7.00-7.83 (15H, m); 8.76 ppm (1H, d,
J=9.0 Hz).
Example 57
3[3-(Benzhydryl-sulfamoy1)-phenyl}-acrylic acid (5n)
Using an analogous method, the title compound was obtained from 343-
(benzhydryl-sulfamoyI)-phenyl]-acrylic acid methyl ester (4n) and sodium
hydroxide, yield 78%. 1H NMR (DMSO-dÃ, HMDSO), 6: 5.60 (1H, d, J=9.0 Hz);
6.43 (1H, d, J= 16.0 Hz); 6.94 ¨7.83 (15H, m); 8.80 ppm (1H, d, J=9.0 Hz).
Example 58
3[3-(Benzhydryl-sulfamoy1)-phenyl]-acryloyi chloride (6n)
Using an analogous method, the title compound was obtained from 3-[3-
(benzhydryl-sulfamoy1)-phenyg-acrylic acid (5n) and oxalyl chloride in a form
of a
crude product, yield ca. 98%.
Example 59
3-[3-(Benzhydryl-sulfamoy1)-phenyl]-N-hydroxy-acrylamide (7n) (PX117773)
lel o H
\\
,S OH
lel I N'\O o
Using an analogous method, the title compound was obtained from 343-
, (benzhydryl-sulfamoy1)-phenyll-acryloyl chloride (6n) and hydroxylamine
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 148 -
hydrochloride, yield 68%. M.p. 180 C. 1H NMR (DMSO-d6, HMDSO), 8: 5.60 (1H,
d, J= 9.0 Hz); 6.43 (1H, d, J= 16.0 Hz); 6.98-7.83 (15H, m); 8.85 (1H, d, J=
9.0
Hz); 9.14 (1H, br s); 10.80 (1H, br s). HPLC analysis on Symmetry C8 column:
impurities <1% (column size 3.9x150 mm; mobile phase acetonitrile-0.1M
phosphate buffer, pH 2.5, 45:55; sample concentration 0.5 mg/ml; flow rate 1.4
ml/min; detector UV 220 nm). Anal. Calcd for C22H20N204S, C 64.69;
H 4.94,
N 6.86. Found C 64.60, H 4.94, N 6.77.
Example 60
343-(1,2-Diphenyl-ethylsulfamoy1)-phenyll-acrylic acid methyl ester (4o)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylpheny1)-acrylic acid methyl ester (3) and 1,2-diphenylamine as a
white solid, yield 96%, 1H NMR (DMSO-d6, HMDSO), 8: 2.83 (2H, d, J=9.0 Hz);
3.78 (3H, s); 4.49 (1H, q, J=9.0 Hz); 6.54 (1H, d, J=16.0 Hz); 6.94 ¨7.83
(15H, m);
8.38 ppm (1H, d, J=9.0 Hz).
Example 61
343-(1,2-Diphenyl-ethylsulfamoy1)-phenylFacrylic acid (5o)
Using an analogous method, the title compound was obtained from 34341,2-
diphenyl-ethylsulfamoylyphenylFacrylic acid methyl ester (4o) and sodium
hydroxide, yield 70%. 1H NMR (DMSO-d6, HMDSO), 8: 2.85 (2H, d, J=9.0 Hz);
4.49 (1H, q, J=9.0 Hz); 6.40 (1H, d, J= 16.0 Hz); 6.85-7.78 (15H, m); 8.38 ppm
(1H, d, J=9.0 Hz).
Example 62
313-(1,2-Diphenyl-ethylsulfamoy1)-phenylFacryloyl chloride (6o)
Using an analogous method, the title compound was obtained from 343-(1,2-
diphenyl-ethylsulfamoy1)-phenyll-acrylic acid (5o) and oxalyl chloride in a
form of a
crude product, yield ca. 98%.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 149 -
Example 63
343-(1,2-Diphenyl-ethylsulfamoy1)-phenyl]-N-hydroxy-acrylamide (7o) (PX117774)
Slo
410 N,
N OH
H
Using an analogous method, the title compound was obtained from 343-(1 ,2-
diphenyl-ethylsulfamoy1)-phenyll-acryloyl chloride (6o) and hydroxylamine
hydrochloride, yield 72%. M.p. 150 C. 1H NMR (DMSO-d6, HMDSO), 8: 2.83 (2H,
d, J=9.0 Hz); 4.47 (1H, q, J= 9.0 Hz); 6.38 (1H, d, J= 16.0 Hz); 6.92-7.65
(15H,
m); 8.38 (1H, d, J= 9.0 Hz); 9.12 (1H, br s); 10.80 (1H, br s). HPLC analysis
on
Symmetry C8 column: impurities 1% (column size 3.9x150 mm; mobile phase
acetonitrile-0.1M phosphate buffer, pH 2.5, 45:55; sample concentration 0.5
mg/ml; flow rate 1.4 ml/min; detector UV 220 nm). Anal. Calcd for C23H22N204S,
(Yo: C 65.39, H 5.25, N 6.63. Found C 64.97, H 5.14, N 6.57.
Example 64
343-(4-Trifluoromethoxy-phenyisulfamoy1)-phenyll-acrylic acid methyl ester
(4p)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylpheny1)-acrylic acid methyl ester (3) and 4-
trifluoromethoxyaniline as
a white solid, yield 82%, 1H NMR (CDCI3, TMS) 6: 3.82 (3H, s); 6.47 (1H, d,
J=16.0 Hz); 6.89 ¨7.98 ppm (10H, m).
Example 65
3-[3-(4-Trifluoromethoxy-phenylsulfamoy1)-pheny1]-acrylic acid (5p)
Using an analogous method, the title compound was obtained from 3-13-(4-
trifluoromethoxy-phenylsulfamoy1)-pheny1]-acrylic acid methyl ester (4p) and
sodium hydroxide, yield 94%. 1H NMR (DMSO-d6, HMDSO), 8: 6.54 (11-1, d, J=
16.0 Hz); 7.23 (4H, s) 7.47 ¨8.14 (6H, m); 10.54 ppm (1H, br s).
CA 02765409 2012-01-19
WO 02/311879
PCT/GB01/04326
- 150 -
Example 66
343-(4-trifluoronnethoxy-phenylsulfamoy1)-phenyll-acryloyl chloride (6p)
Using an analogous method, the title compound was obtained from 34344-
trifluoromethoxy-phenylsulfamoy1)-phenyll-acrylic acid (5p) and oxalyl
chloride in a
form of a crude product, yield ca. 98%.
Example 67
N-Hydroxy-343-(4-trifluoromethoxy-phenylsulfamoy1)-phenyl]-acrylamide (7p)
(PX117775)
F3C0
0 (00
N,
OH
N
HO
Using an analogous method, the title compound was obtained from 34344-
trifluoromethoxy-phenylsulfamoyI)-phenyl]-acryloyl chloride (6p) and
hydroxylamine hydrochloride, yield 46%. M.p. 131 C. 1H NMR (DMSO-c16,
HMDSO), 5: 6.49 (1H, d, J= 16.0 Hz); 7.03-8.05 (9H, m); 8.98 (1H, br s); 10.54
(1H, br s); 10.78 (1H, br s). HPLC analysis on Symmetry CB column: impurities
3.5% (column size 3.9x150 mm; mobile phase acetonitrile-0.1M phosphate buffer,
pH 2.5, 45:55; sample concentration 0.5 mg/ml; flow rate 1.4 ml/min; detector
UV
220 nm). Anal. Calcd for C161-113F3N205S, C 47.76, H 3.26, N 6.96. Found C
47.68, H 3.15, N 6.91.
Example 68
343-(3,4,5-Trimethoxy-benzylsulfamoy1)-phenylFacrylic acid methyl ester (4q)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylphenyl)acrylic acid methyl ester (3) and 3,4,5-
trimethoxybenzylamine as a white solid, yield 85%, 1H NMR (CDCI3, TMS) 5:
3.72 (6H, s); 3.78 (3H, s); 3.83 (31-1, s); 4.14 (2H, d, J=8.0 Hz); 5.07 (1H,
t, J=8.0
Hz); 6.38 (2H, s); 6.49 (1H, d, J=16.0 Hz); 7.36-8.07 ppm (5H, m).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 151 -
Example 69
343-(3,4,5-Trimethoxy-benzylsulfamoy1)-phenyn-acrylic acid (5q)
Using an analogous method, the title compound was obtained from 313-(3,4,5-
trimethoxy-benzylsulfamoy1)-pheny11-acrylic acid methyl ester (4q) and sodium
hydroxide, yield 90%. 1H NMR (DMSO-d6, HMDSO), 6: 3.52 (3H, s); 3.65 (6H, s);
3.98 (2H, d, J=8.0 Hz); 6.43 (2H, s); 6.49 (1H, d, J= 16.0 Hz); 7.38-8.27 ppm
(6H, m).
Example 70
3-[3-(3,4,5-Trimethoxy-benzylsulfamoy1)-phenyl]-acryloyl chloride (6q)
Using an analogous method, the title compound was obtained from 3-[3-(3,4,5-
trimethoxy-benzylsulfamoy1)-phenylFacrylic acid (5q) and oxalyl chloride in a
form
of a crude product, yield ca. 100%.
Example 71
N-Hydroxy-343-(3,4,5-trimethoxy-benzylsulfamoy1)-phenyll-acrylamide (7q)
(PX117778)
N,
Me0 C-:\S\\ 1.1 OH
N 0
Me0
OMe
Using an analogous method, the title compound was obtained from 3-[3-(3,4,5-
trimethoxy-benzylsulfamoy1)-phenyl]-acryloyl chloride (6q) and hydroxylamine
hydrochloride, yield 19%, foam. 1H NMR (DMSO-d6, HMDSO), 6: 3.54 (3H, s);
3.65 (6H, s); 3.98 (2H, m); 6.46 (2H, s); 6.56 (1H, d, J=15.0 Hz); 7.32-7.98
(5H,
m); 8.18 (1H, br t, J=5.5 Hz); 9.12 (1H, br s); 10.78 (1H, br s). HPLC
analysis on
Symmetry C8 column: impurities 7% (column size 3.9x150 mm; mobile phase
acetonitrile-0.1M phosphate buffer, pH 2.5, 30:70; sample concentration 0.5
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 152 -
mg/m1; flow rate 1.4 ml/min; detector UV 220 nm). Anal. Calcd for C16H22N207S
*
0.25 Et0Ac, containing 1.6% of inorganic impurities, %: C 53.18, H 5.36, N
6.20.
Found C 53.13, H 5.31, N 6.02.
Example 72
3-{312-(3,4-Dimethoxy-phenyl)-ethylsulfamoyll-pheny1}-acrylic acid methyl
ester
(4r)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylphenyI)-acrylic acid methyl ester (3) and 2-(3,4-
dimethoxyphenyl)ethylamine as a white solid, yield 81%, 1H NMR (CDCI3, TMS)
6: 2.72 (2H, t, J=7.0 Hz); 3.20 (2H, q, J=7.0 Hz); 3.80 (9H, s); 4.49 (1H, t,
J=7.0
Hz); 6.36-6.87 (4H, m); 7.38-8.00 ppm (5H, m).
Example 73
3-{342-(3,4-Dimethoxy-pheny1)-ethylsulfamoy1]-phenyll-acrylic acid (5r)
Using an analogous method, the title compound was obtained from 3-{342-(3,4-
dimethoxy-pheny1)-ethylsulfamoyll-phenyll-acrylic acid methyl ester (4r) and
sodium hydroxide, yield 87%. 1H NMR (DMSO-d6, HMDSO), 6: 2.58 (2H, t,
partially overlapped with a signal of DMS0); 2.83-3.20 (2H, m, partially
overlapped with a water signal of DMS0); 3.72 (6H, s); 6.43 ¨6.89 (4H, m);
7.49 ¨
8.09 ppm (6H, m).
Example 74
3-{342-(3,4-Dimethoxy-pheny1)-ethylsulfamoy1]-phenyl}-acryloyl chloride (6r)
Using an analogous method, the title compound was obtained from 3-{342-(3,4-
dimethoxy-pheny1)-ethylsulfamoy1J-phenyll-acrylic acid (5r) and oxalyl
chloride in a
form of a crude product, yield ca. 100%.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 153 -
Example 75
3-{342-(3,4-Dimethoxy-phenyl)-ethylsulfamoy1]-pheny1)-N-hydroxy-acrylamide
(7r)
(PX117779)
OMe
Me0 001
11101 NOH=
N
H 0 0
Using an analogous method, the title compound was obtained from 3-{342-(3,4-
dimethoxy-phenyl)-ethylsulfamoy1]-phenyl)-acryloyl chloride (6r) and
hydroxylamine hydrochloride, yield 32%, foam. 1H NMR (DMSO-d6, HMDSO), 8:
2.58 (2H, t, partially overlapped with a signal of DMSO, J=7.0 Hz); 2.85-3.16
(2H,
m); 3.67 (6H, s); 6.38-6.94 (4H, m); 7.38-8.05 (6H, m); 9.16 (1H, br s); 10.76
(1H,
br s). HPLC analysis on Symmetry C8 column: impurities 3.6% (column size
3.9x150 mm; mobile phase acetonitrile-0.1M phosphate buffer, pH 2.5, 30:70;
sample concentration 0.5 mg/ml; flow rate 1.5 ml/min; detector UV 254 nm).
Anal.
Calcd for C16H22N206S containing 4.3 % of inorganic impurities, %: C 53.73, H
5.22, N 6.60. Found C 53.75, H 5.24, N 6.45.
Example 76
313-(3,4-Dimethoxy-phenylsulfamoy1)-phenyl]-acrylic acid methyl ester (4s)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylpheny1)-acrylic acid methyl ester (3) and 3,4-dimethoxyaniline
as a
white solid, yield 90%, 1H NMR (DMSO-d6, HMDSO), 8: 3.60 (3H, s); 3.65 (3H,
s);
3.76 (3H, s); 6.45-6.85 (4H, m); 7.47 ¨8.05 (5H, m); 9.92 ppm (1H, br s).
Example 77
343-(3,4-Dimethoxy-phenylsulfamoy1)-ohenyl]-acrylic acid (5s)
Using an analogous method, the title compound was obtained from 343-(3,4-
dimethoxy-phenylsulfamoy1)-phenyTacrylic acid methyl ester (4s) and sodium
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 154 -
hydroxide, yield 90%. 1H NMR (DMSO-d6, HMDSO), ö: 3.60 (3H, s); 3.65 (31-1,
s);
6.29 ¨6.89 (4H, m); 7.47-8.09 (5H, m); 9.93 ppm (1H, br s).
Example 78
343-(3,4-Dimethoxy-phenylsulfamoy1)-phenyl]-acryloyl chloride (6s)
Using an analogous method, the title compound was obtained from 343-(3,4-
dimethoxy-phenylsulfamoy1)-phenyl]-acrylic acid (5s) and oxalyl chloride in a
form
of a crude product, yield ca. 100%.
Example 79
343-(3,4-Dimethoxy-phenylsu)famoy1)-pheny1]-N-hydroxy-acrylamide (7s)
(PX117782)
Me
0
Me Si \\S
N,
OH
H
Using an analogous method, the title compound was obtained from 343-(3,4-
dimethoxy-phenylsulfamoy1)-pheny1J-acryloyl chloride (V112) and hydroxylamine
hydrochloride, yield 45%. M.p. 191 C. 1H NMR (DMSO-d6, HMDSO), 6: 3.60 (3H,,
s); 3.65 (3H, s); 6.34-6.87 (4H, m); 7.32-8.03 (5H, m); 9.09 (1H, br s); 9.92
(1H, br
s); 10.80 (1H, br s). HPLC analysis on Symmetry C8 column: impurities 6%
(column size 3.9x150 mm; mobile phase acetonitrile-0.1M phosphate buffer, pH
2.5, 30:70; sample concentration 0.5 mg/ml; flow rate 1.3 ml/min; detector UV
220
nm). Anal. Calcd for C17H18N206S, C 53.96, H 4.79, N 7.40. Found C 53.84,
H 4.78, N 7.25.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 155 -
Example 80
343-(4-Difluoromethoxy-phenylsulfamoy1)-phenyl]-acrylic acid methyl ester (4t)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylphenyI)-acrylic acid methyl ester (3) and 4-
difluoromethoxyphenylamine as a white solid, yield 79%.
Example 81
343-(4-Difluoromethoxy-phenylsulfamoy1)-phenyll-acrylic acid (5t)
Using an analogous method, the title compound was obtained from 313-(4-
difluoromethoxy-phenylsulfamoy1)-phenyll-acrylic acid methyl ester (4t) and
sodium hydroxide, yield 71%. 1H NMR (DMSO-d6, HMDSO), 8: 6.56 (1H, d,
J=16.0 Hz); 7.11 (4H, s); 7.47-8.04 (6H, m).
Example 82
343-(4-Difluoromethoxy-phenylsulfamoyl)-phenyll-acryloyl chloride (6t)
Using an analogous method, the title compound was obtained from 313-(4-
difluoromethoxy-phenylsulfamoy1)-phenyl]-acrylic acid (5t) and oxalyl
chloride, ca.
yield of the crude product 98% (yellow oil).
Example 83
343-(4-Difluoromethoxy-phenylsulfamoy1)-phenyll-N-hydroxy-acrylamide (7t)
(PX117789)
F.y.F
0 el
,S
N OH
H
Using an analogous method, the title compound was obtained from 343-(4-
difluoromethoxy-phenylsulfamoy1)-phenylFacryloyl chloride (6t) and
hydroxylamine hydrochloride, yield 65%. M.p. 91-93 C. 1H NMR (DMS0-4
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 156 -
HMDSO) 5: 6.47 (1H, d, J=16.0 Hz); 6.96 (4H, s); 7.31-7.93 (6H, m). HPLC
analysis on Symmetry C8 column: impurities 3.5 % (column size 3.9 x 150 mm;
mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5), 40:60; detector UV
220 nm; flow rate 1.4 ml/min; sample concentration 0.5 mg/ml). Anal. Calcd for
C16H14N205F2S* 0.2 H20 * 0.5 Et0H, C 49.68, H 4.27, N 6.82, S 7.80. Found,
%: C 49.46, H 3.95, N 6.65, S 7.39.
Example 84
343-(9-Ethy1-9H-carbazol-3-ylsulfamoy1)-phenylFacrylic acid methyl ester (4u)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylpheny1)-acrylic acid methyl ester (3) and 9-ethy1-9H-carbazol-3-
ylamine in a form of yellow solid, yield 86%. 1H NMR (CDCI3, HMDSO), 8: 1.38
(3H, t, J=7.0 Hz); 3.78 (3H, s); 4.33 (2H, q, J=7.0 Hz); 6.33 (1H, d, J=16.0
Hz);
6.58 (1H, s); 7.02-8.04 (12H, m).
Example 85
343-(9-Ethy1-9H-carbazol-3-ylsulfamoy1)-phenylFacrylic acid (5u)
Using an analogous method, the title compound was obtained from 343-(9-ethy1-
91-1-carbazol-3-ylsulfamoy1)-pheny13-acrylic acid methyl ester (4u) and sodium
hydroxide, yield 65%.
Example 86
343-(9-Ethyl-9H-carbazol-3-ylsulfamoy1)-phenyl}-acryloyi chloride (6u)
Using an analogous method, the title compound was obtained from 343-(9-ethy1-
9H-carbazol-3-ylsulfamoy1)-phenylFacrylic acid (5u) and oxalyl chloride, ca.
yield
of the crude product 98% (yellow oil).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/0-1326
- 157 -
Example 87
343-(9-Ethyl-9H-carbazol-3-ylsulfamoy1)-phenyll-N-hydroxy-acrylamide (7u)
(PX117798)
E\
\\s 110 N,
N
H 0 = OH
Using an analogous method, the title compound was obtained from 343-(9-ethyl-
9H-carbazol-3-ylsulfamoy1)-phenylFacryloyl chloride (6u) and hydroxylamine
hydrochloride, yield 42%. M.p. 130-133 C. 1H NMR (DMSO-d6, HMDSO) 8: 1.20
(3H, t, J=6.6 Hz); 4.34 (2H, q, J=6.6 Hz); 6.42 (1H, d, J=16 Hz); 6.93-8.07
(13H,
m); 9.07 (1H, br. s); 10.3 (1H, br. s). HPLC analysis on Symmetry C8 column:
impurities 10 % (column size 3.9 x 150 mm; mobile phase acetonitrile ¨ 0.1M
phosphate buffer (pH 2.5), 40:60; detector UV 254 nm; flow rate 1.0 ml/min;
sample concentration 1 mg/ml). Anal. Calcd for C231-121N304S * 1 H20, /0: C
60.91,
H 5.11, N 9.27, S 7.07. Found, Vo: C 61.01, H 5.15, N 8.75, S 6.65.
Example 88
343-(2,6-Difluoro-pheny(sulfamoy1)-phenyll-acrylic acid methyl ester (4v)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylphenyl)acrylic acid methyl ester (3) and 2,4-difluorophenylamine
in a
form of yellow crystals, yield 70%. 1H NMR (CDCI3, HMDSO), 5: 3.82 (3H, s);
6.49
(1H, d, J=16.0 Hz); 7.00 (1H, t, J=8 Hz); 5.89-6.69 (7H, m).
Example 89
313-(2,4-Difluoro-phenylsulfamoy1)-phenyll-acrylic acid (5v)
Using an analogous method, the title compound was obtained from 343-(2,4-
difluoro-phenylsulfamoy1)-phenyll-acrylic acid methyl ester (4v) and sodium
hydroxide in a form of white solid, yield 66%. 1H NMR (DMSO-d6, HMDSO), 8:
6.56 (1H, d, J=16.0 Hz); 6.96-8.09 (9H, m); 10.13 (1H, br. s).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 158 -
Example 90
343-(2,4-Difluoro-phenylsulfamoy1)-phenyl]-acryloyl chloride (6v)
Using an analogous method, the title compound was obtained from 343-(2,4-
difluoro-phenylsulfamoy1)-phenylFacrylic acid (5v) and oxalyl chloride, ca.
yield of
the crude product 98% (yellow oil).
Example 91
343-(2,4-Difluoro-phenylsulfamoy1)-phenyl]-N-hydroxy-acrylamide (7v)
(PX117790)
401
,S
N OH
H0 0
Using an analogous method, the title compound was obtained from 343-(2,4-
difluoro-phenylsulfamoy1)-phenyll-acryloyl chloride (6v) and hydroxylamine
hydrochloride, yield 26%. M.p. 79-82 C. 1H NMR (DMSO-d6, HMDSO) 5: 6.47
(1H, d, J=16.0Hz); 6.89-7.89 (8H, m); 9.07 (1H, br. s); 10.02 (1H, br. s);
10.73
(1H, br s). HPLC analysis on Symmetry C8 column: impurities 7.5 % (column size
3.9 x 150 mm; mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5),
35:65;
detector UV 220 nm; flow rate 1.4 ml/min; sample concentration 0.5 mg/ml).
Anal.
Calcd for C161-112N204F2S 1 Et0H, /0: C 51.00, H 4.53, N 7.00, S 8.01. Found,
%:
C 50.84, H 4.60, N 6.78, S 7.76.
Example 92
313-(2-Fluoro-phenylsulfamoy1)-phenylFacrylic acid methyl ester (4w)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonyl-phenyl)acrylic acid methyl ester (3) and 2-fluorophenylamine in
a
form of white crystals, yield 65%. 1H NMR (CDCI3, HMDSO), 6: 3.80 (3H, s);
6.44
(1H, d, J=16.0 Hz); 6.71-7.22 (4H, m); 7.44-7.93 (6H, m).
CA 02765409 2012-01-19
WO 02/30879
PCPCB01/04326
- 159 -
Example 93
343-(2-Fluoro-phenylsulfamoy1)-phenyl]-acrylic acid (5w)
Using an analogous method, the title compound was obtained from 343-(2-fluoro-
phenylsulfamoyI)-phenyl]-acrylic acid methyl ester (4w) and sodium hydroxide,
yield 50%. 1H NMR (DMSO-d6, HMDSO), 5: 6.58.(1 H, d, J=16.0 Hz); 7.04-7.36
(4H, m); 7.51-8.09 (5H, m).
Example 94
343-(2-Fluoro-phenylsulfamoy1)-phenyl]-acryloyl chloride (6w)
Using an analogous method, the title compound was obtained from 313-(2-fluoro-
phenylsulfamoy1)-phenylFacrylic acid (5w) and oxalyl chloride, ca. yield of
the
crude product 98% (yellow oil).
Example 95
343-(2-Fluoro-phenylsulfamoy1)-phenyl}N-hydroxy-acrylamide (7w) (PX117787)
11111(:)\\
,S OH
N
H 0 0
Using an analogous method, the title compound was obtained from 343-(2-fluoro-
phenylsulfamoy1)-phenyl}-acryloyl chloride (6w) and hydroxylamine
hydrochloride,
yield 30%. M.p. 102-103 C. 1H NMR (DMSO-d6, HMDSO), 45: 6.44 (1H, d, J=16.0
Hz); 6.96-7.24 (4H, m); 7.43 (1H, d, J=16.0 Hz); 7.49-7.91 (4H, m); 9.04 (1H,
br
s); 10.13 (1H, br s); 10.73 (1H, br s). HPLC analysis on Symmetry C8 column:
impurities 4.5% (column size 3.9 x 150 mm; mobile phase acetonitrile ¨ 0.1M
phosphate buffer (pH 2.5), 35:65; detector UV 220 nm; flow rate 1.4 ml/min;
sample concentration 0.5 mg/ml). Anal. Calcd for C161-113N204FS* 0.9 Et0H, %:
C
53.41, H 4.91, N 7.41. Found, A: C 53.79, H 4.62, N 7.13.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 160 -
Example 96
343-(3-Fluoro-phenylsulfamoy1)-phenylFacrylic acid methyl ester (4x)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonyl-phenyl)acrylic acid methyl ester (3) and 3-fluorophenylamine in
a
form of white crystals, yield 80%. 1H NMR (CDC13, HMDSO), 5: 3.78 (3H, s);
6.42
(1H, d, J=16.0 Hz); 6.64-8.02 (10H m).
Example 97
3p-(3-Fluoro-phenylsulfamoy1)-phenyl]-acrylic acid (5x)
Using an analogous method, the title compound was obtained from 343-(3-fluoro-
phenylsulfamoy1)-phenylFacrylic acid methyl ester (4x) and sodium hydroxide,
yield 60%. 1H NMR (DMSO-d6, HMDSO), 5: 6.56 (1H, d, J=16.0 Hz); 6.80-7.36
(4H, m); 7.49-8.09 (6H, m).
Example 98
343-(3-Fluoro-phenylsulfamoy1)-phenylFacryloyl chloride (6x)
Using an analogous method, the title compound was obtained from 3-[3-(3-fluoro-
phenylsulfamoyl)-phenyl]-acrylic acid (5x) and oxaly1 dichloride, ca. yield of
the
crude product 99% (yellow oil).
Example 99
343-(3-Fluoro-phenylsulfamoy1)-phenyll-N-hydroxy-acrylamide (7x) (PX117788)
\\s 401
N,
OH
N
H 0 0
Using an analogous method, the title compound was obtained from 343-(3-fluoro-
phenylsulfamoy1)-phenyll-acryloyl chloride (6x) and hydroxylamine
hydrochloride,
yield 65%. M.p. 130-133 C. 1H NMR (DMSO-d6, HMDSO) 5: 6.52 (1H, d, J=15.8
Hz); 6.75-6.97 (4H, m); 7.17-7.32 (1H, m); 7.47 (1H, d, J=15.8 Hz); 7.58 (1H,
t,
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 161 -
J=7.8 Hz); 7.67-7.85(2H, m); 7.94 (1H, s); 9.19(1H, br s); 10.89 (1H, br s).
HPLC
analysis on Symmetry C8 column: impurities 5.5 % (column size 3.9 x 150 mm;
mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5), 40:60; detector UV
254 nm; flow rate 1.5 ml/min; sample concentration 0.5 mg/ml). Anal. Calcd for
C16F113N204FS* 0.65 Et0H, %: C 53.45, H 4.65, N 7.65, S 8.75. Found, %: C
53.54, H 4.32, N 7.37, S 8.50.
Example 100
343-(2-Methoxy-5-trifluoromethyl-phenylsulfamoy1)-phenyTacrylic acid methyl
ester (4y)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylpheny1)-acrylic acid methyl ester (3) and 2-methoxy-5-
(trifluoromethyl)aniline as a white solid, yield 55%. 1H NMR (CDC13, HMDSO),
5:
3.68 (3H, s), 3.80 (3H, s); 6.39 (1H, d, J=16.0 Hz); 6.77 (1H, d, J=8.4 Hz);
7.11
(1H, s); 7.20-7.95 ppm (7H, m).
Example 101
343-(2-Methoxy-5-trifluoromethyl-phenylsulfamoy1)-phenyll-acrylic acid (5y)
Using an analogous method, the title compound was obtained from 343-(2-
methoxy-5-trifluoromethyl-phenylsulfamoy1)-phenyll-acrylic acid methyl ester
(4y)
and sodium hydroxide, yield 80%. 1H NMR (DMSO-d6, HMDSO), .5: 3.60 (3H, s);
6.54 (1H, d, J=16.0 Hz); 7.07 (1H, d, J=8.4 Hz); 7.45 ¨7.97 (8H, m); 9.70 ppm
(1H, br s).
Example 102
3-[3-(2-Methoxy-5-trifluoromethyl-phenylsulfamoy1)-phenyl]-acryloyl chloride
(6y)
Using an analogous method, the title compound was obtained from 343-(2-
methoxy-5-trifluoromethyl-phenylsulfamoy1)-pheny1J-acrylic acid (5y) and
oxalyl
chloride, ca. yield of the crude product 98% (yellow oil).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 162 -
Example 103
N-Hydroxy-343-(2-methoxy-5-trifluoromethyl-phenylsulfamoy1)-phenyll-acrylamide
(7y) (PX117791)
c,3
411 0,\ N,OH
N
H0 0
Me
Using an analogous method, the title compound was obtained from 34342-
methoxy-5-trifluoromethyl-phenylsulfamoy1)-phenyli-acryloyl chloride (6y) and
hydroxylamine hydrochloride, yield 64%. M.p. 207 C (dec.). 1H NMR (DMSO-c16,
HMDSO) 5: 3.57 (3H, s); 6.52 (1H, d, J= 15.8 Hz); 7.12 (1H, d, J=8.4 Hz); 7.36-
8.09 (7H, m); 9.11 (1H, br s); 9.98 (1H, s); 10.82 (1H, s). HPLC analysis on
Symmetry 08 column: impurities 1.8% (column size 3.9 x 150 mm; mobile phase
acetonitrile ¨ 0.1M phosphate buffer (pH 2.5), 50:50; detector UV 254 nm; flow
rate 0.9 ml/min; sample concentration 0.5 mg/ml). Anal. Calcd for
C17H15F3N205S,
C 49.04, H 3.63, N 6.78. Found, A: C 49.39, H 3.41, N 6.66.
Example 104
3-{3-[(Furan-2-ylmethyl)-sulfamoyl]-phenyl}-acrylic acid methyl ester (4z)
Using an analogous method, the title compound was obtained from 3-(3-
chlorosulfonylphenyI)-acrylic acid methyl ester (3) and furfurilamine as a
white
solid, yield 87%. 1H NMR (DMSO-d6, HMDSO), 5: 3.73 (3H, s); 4.05 (2H, d, J=
6.4Hz); 6.20 (2H, m); 6.71 (1H, d, J=16.0 Hz); 7.38-8.38 (7H, m).
Example 105
3-{3-[(Furan-2-ylmethyl)-sulfamoyl]-phenyl}-acrylic acid (5z)
Using an analogous method, the title compound was obtained from 3-{3-Rfuran-2-
ylmethyl)-sulfamoy1Fphenylyacrylic acid methyl ester (4z) and sodium
hydroxide,
yield 89%.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 163 -
Example 106
3(3-[(Furan-2-ylmethyl)-sulfamoy1]-pheny1}-N-hydroxyacrylamide (7z) (PX117710)
0
\\ N.,OH
OH \\O = 0
0
To a solution of 3-{3-Rfuran-2-ylmethyl)-sulfamoyll-phenyl}-acrylic acid (5z)
(0.17
g, 0.55 mmol) in tetrahydrofuran (2.0 ml) at 0 C temperature
ethylchloroformate
(0.072g ,0.66 mmol) and triethylamine (0.1 ml, 0.72 mmol) were added and the
resulting mixture was stirred for 15 min. To a stirred solution of KOH
(0.058g, 1.04
mmol) in methanol (0.25m1) a solution of hydroxylamine hydrohloride (0.072g,
1.04 mmol) in methanol (0.7m1) was added at 0 C. The mixture was stirred for
15
min., the precipitated KC1 was removed and the filtrate was added to the first
solution..The reaction mixture was stirred at room temperature for 2 hours.
Then
the mixture was partitioned between 1N KH2PO4 solution and ethyl acetate. The
organic layer was washed with water, saturated NaCl, and dried (Na2SO4). The
solvent was evaporated and the residue was washed successively with
dichloromathane and ethyl acetate affording the title compound (0.057 g, 32%).
M.p. 165 C. 1H NMR (DMSO-d6, HMDSO) 8: 4.03 (2H, d, J=6.4 Hz); 6.23 (2H, m);
6.54 (1H, d, J=16.0 Hz); 7.38-8.05 (6H, m); 8.20 (1H, t, J=6.4 Hz); 9.09 (11-
1, br s);
10.83 (1H, br s). HPLC analysis on Zorbax SB-C18 column: impurities 8 %
(column size 4.6 x 150 mm; mobile phase methanol - 0.1% 1-13PO4, gradient from
to 90%; detector UV 270 nm; flow rate 1.5 ml/min; sample concentration 1.0
mg/mi). Anal. Calcd for C14F114N205S, %: C 52.17, H 4.38, N 8.69. Found, /0:
C
51.87, H 4.39, N 8.41.
25 Example 107
3-(4-(((Phenylmethyl)sulfonyl)amino)phenyl)acrylic acid ethyl ester (9)
0/ C00Et
CA 02765409 2012-01-19
WO 02/30879 PCT/G1101/04326
- 164 -
a-Toluenesulfonyl chloride (1.0 g, 5.2 mmol) was added to a mixture of 4-
aminocinnamic acid ethyl ester (1.0 g, 5.2 mmol), pyridine (0.42 ml, 5.2 mmol)
and dichloromethane (10 ml) and the resultant solution was stirred at ambient
temperature for twelve hours. The solution was then heated at reflux for a
further
eight hours.
The mixture was allowed to cool to ambient temperature and was diluted with
dichloromethane (100 ml) and was washed with 10 % aqueous citric acid (20 ml),
saturated aqueous sodium hydrogen carbonate (20 ml), and water (2 x 20 ml).
The organic extract was dried (MgSO4), filtered and the solvent was removed
under reduced pressure.
The crude product was purified by column chromatography on silica gel using a
gradient of ethyl acetate B hexane (1:10) to ethyl acetate as the eluent to
afford
the title compound as a yellow solid (0.80 g, 45%), tR 5.18 (254 nm, 3.0
5% ACN/95% H20 + 0.2% TFA to 95% ACN/5% H20 + 0.2% TFA over 3.5 min
then 2.5 min at 95% ACN/5% H20 + 0.2% TFA), mtz [ES] 368 [M - Na]t
Example 108
3-(4-(((Phenylmethyl)sulfonyl)amino)phenyl)acrylic acid (10)
4) I.
1101 KI
0 11 COOH
A 1 M aqueous solution of lithium hydroxide (2.9 ml, 2.9 mmol) was added to a
solution of 3-(4-(((phenylmethyl) sulfonyl) amino)phenyl)acrylic acid ethyl
ester (9)
(500 mg, 1.45 mmol) in dioxane (4 ml). The resultant solution was stirred at
ambient temperature for two hours. Additional 1 M aqueous lithium hydroxide
(2.9
ml, 2.9 mmol) was added and the reaction mixture was stirred at ambient
temperature for one hour. The solution was stored at + 4 C for sixteen hours.
The solvent was removed under reduced pressure and ethyl acetate (15 ml) was
added to the residue. The resultant mixture was washed with water (2 x 10 m1).
CA 02765409 2012-01-19
WO 02/30879 PCT/C1301/04326
- 165 -
The aqueous extracts were combined and acidified to ¨ pH 4 with a 1 M aqueous
solution of hydrochloric acid. The acidified solution was extracted with ethyl
acetate (4 x 10 m1). The combined organic extracts were washed with water (10
ml), dried (MgS0.4) and the solvent was removed under reduced pressure.
The crude product was purified by column chromatography on silica gel using of
ethyl acetate as the eluent to afford to afford the title compound as a yellow
solid
(320 mg, 70%), tR 4.56 (254 nm, 3.0 mlmin1, 5% ACN/95`)/0 H20 + 0.2% TFA to
95% ACN/5`)/0 H20 + 0.2% TFA over 3.5 min then 2.5 min at 95% ACN/5% H20 +
0.2% TFA), m/z [ES] 316 [M + TFA] and 430 [M + TFAr.
Example 109
3-(4-(((Phenylmethyl)sulfonyl)amino)phenyl)acrylic acid hydroxyamide (11)
(PX089343)
0 H
N-Fmoc-hydroxylamine 2-chlorotrityl resin (0.80 g, 0.57 mmol) (Calbiochem-
Novabiochem Corp., Nottingham, UK) was swollen with a solution of piperidine
in
dichloromethane (20/80, v/v) (5 ml) and then agitated at ambient temperature
for
two hours. The resin was filtered and was washed with 1-methylpyrrolidinone (5
ml), alternately with methanol (4 x 5 ml) and dichloromethane (4 x 5 ml) and
finally
with diethyl ether (5 m1).
The resin was placed in a reaction vessel and was swollen with dichloromethane
(2 ml). The swollen resin was treated with 3-(4-(((Phenylmethyl)sulfonyl)
amino)phenyl)acrylic acid (10) (90 mg, 0.28 mmol), 1-hydroxy-7-
azabenzotriazole
(HOAt) (Aldrich, Dorset, UK) (77 mg, 0.57 mmol), 0-(7-azabenzotriazol-1-y1)-
N,N,W,N-tetramethyluronium hexafluorophosphate (HATU) (216 mg, 0.57 mmol)
(Aldrich, Dorset, UK), N,N-diisopropylethylamine (198 pl, 1.14 mmol) and a
mixture of dichloromethane and N,N-dimethylformamide (4:1, v/v) (5 m1). The
resultant mixture was agitated at ambient temperature for sixteen hours.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 166 -
The resin was filtered and was washed with 1-methylpyrrolidinone (5 ml),
alternately with methanol (4 x 5 ml) and dichloromethane (4 x 5 ml) and
finally with
diethyl ether (5 ml). The resin was placed in a reaction vessel and was
swollen
with dichloromethane (2 ml). The swollen resin was treated with a solution of
trifluoroacetic acid in dichloromethane (5 / 95, v/v) (3 ml) and the resultant
mixture
was agitated at ambient temperature for ninety minutes. The mixture was
filtered
and the resin was washed with methanol (2 x 5 m1). The solvent was removed
from the combined filtrates under reduced pressure.
The crude product obtained was purified by preparative hplc using a 150 x 21.2
mm 5 pm Hypersil7 Elite C18 column eluting with a gradient of 5% ACN/95`)/0
H20
+ 0.2% TFA to 95% ACN/5 /0 H20 + 0.2% TFA over 10 minutes. The flow rate
was 25 mlmin-1 and the detector was set at 254 nm. The fractions that
contained
the desired product were concentrated under reduced pressure and the resultant
residue was lyophilised from a mixture of dioxane and water. The title
compound
was obtained as a white solid (1.2 mg, 14%), tR 4.11 (254 nm, 3.0 mlmin-1, 5%
ACN/95% H20 + 0.2% TFA to 95% ACN/5% H20 + 0.2% TFA over 3.5 min then
2.5 min at 95% ACN/5`)/0 H20 + 0.2% TFA), mtz [ES] 317 [M - HT and 311 [M +
H].
Example 110
3-{3-[(Naphthalen-1-ylmethyl)-sulfamoy1]-pheny1}-acrylic acid methyl ester
(14a)
el la
,s õ- OMe
. rf \\O o
A solution of 3-(3-chlorosulfonylphenyl)acrylic acid methyl ester (3) (0.4 g,
1.53
mmol) in dioxane (5 ml) was added to a mixture of 1-naphthalenemethylamine
(0.24 g, 1.53 mmol) in dioxane (1 ml) and NaHCO3 (0.25 g, 3.06 mmol) in water
(3 ml), and the resultant solution was stirred at room temperature until the
completion of the reaction (control by TLC). The reaction mixture was
evaporated
and the residue was partitioned between ethyl acetate and 2N HCI. The organic
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 167 -
layer was washed successively with water, saturated NaCI, and dried (Na2SO4).
The solvent was removed and the residue was chromatographed on silica gel with
petroleum ether-ethyl acetate (2:1, v/v) as eluent. The obtained product was
washed with diethyl ether to give the title compound (0.44 g, 76%) as a white
solid. 1H NMR (DMSO-d6, HMDSO), 5: 3.74 (3H, s); 4.47 (2H, d, J=6.0 Hz); 6.69
(1H, d, J=16.0 Hz); 7.32-8.32 (13H, m).
Example 111
3-{3-[(Naphthalen-1-ylmethyl)-sulfamoy11-phenyll-acrylic acid (15a)
Si 0 op ...., OH
,S
401 HO 0
.
To a suspension of 3-{3-[(naphthalen-1-ylmethyl)-sulfamoyll-phenyl}-acrylic
acid
methyl ester (14a) (0.44 g, 1.15 mmol) in methanol (5 ml) 1N NaOH solution
(3.45
ml, 3.45 mmol) was added and the resultant mixture was stirred at ambient
temperature overnight. The reaction mixture was partitioned between ethyl
acetate and water. The aqueous layer was acidified with 2N HCI solution and
stirred for 30 min. The precipitated solid was filtered, washed with water and
dried
in desiccator over P205. The title compound was obtained as a white solid
(0.32 g,
76%).
Example 112
3-{3-[(Naphthalen-1-ylmethyl)-sulfamoyll-phenyll-acryloyl chloride (16a)
lel \\ IP ,--- CI
el r%
o
To a suspension of 3(3-Rnaphthalen-1-ylmethyl)-sulfamoy1J-phenyl}-acrylic acid
(15a) (0.32 g, 0.87 mmol) in dichloromethane (4 ml) oxalyl chloride (0.22 ml,
2.61
mmol) and one drop of dimethylformamide were added. The reaction mixture was
stirred at 40 C for one hour and concentrated under reduced pressure to give
the
title compound (0.33 g, 98%).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 168 -
Example 113
N-Hydroxy-3-t3-[(naphthalen-1-ylmethyl)-sulfamoy1]-phenyll-acrylamide (17a)
(PX117225)
S. 410
OH
To a suspension of hydroxylamine hydrochloride (0.30 g, 4.35 mmol) in
tetrahydrofuran (6 ml) a saturated NaHCO3 solution (4 ml) was added and the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a solution of crude 3-{3-[(naphthalen-l-ylmethyl)-sulfamoyl]-phenyly
acryloyl chloride (16a) (0.33 g) in tetrahydrofuran (4 ml) was added and the
mixture was stirred at ambient temperature for one hour. The reaction mixture
was partitioned between ethyl acetate and 2N HCI. The organic layer was washed
successively with water and saturated NaCI, then the solvent was removed. The
residue was crystallised from ethyl acetate-acetonitrile affording the title
compound (0.13 g, 40%) as a lightly pink crystals. M.p. 177 C. 1H NMR (DMSO-
d6, HMDSO) 6: 4.45 (2H, d, J= 6.0 Hz); 6.58 (1H, d, J=16.0 Hz); 7.29-8.38
(13H,
m); 9.12 (1H, br s); 10.83 (1H, br s). HPLC analysis on Symmetry C8 column:
impurities 1.5% (column size 3.9x150 mm; mobile phase acetonitrile - 0.1M
phosphate buffer (pH 2.5), 40:60; sample concentration 0.25 mg/ml; flow rate
1.2
ml/ min; detector UV 220 nm). Anal. Calcd for C20H18N204S, %: C 62.54, H 4.70,
N 7.21. Found, A: C 62.81, H 4.74, N 7.32.
Example 114
3-(3-[(Pyridin-3-ylmethyl)-sulfamoyl]-phenyl}-acrylic acid methyl ester (14b)
0,\ OMe
I H 0 0
A solution of 3-(3-chlorosulfonylphenyl)acrylic acid methyl ester (3) (0.40 g,
1.53
mmol) in dioxane (5 ml) was added to a mixture of 3-(aminomethyppyridine (0.16
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01 /04326
- 169 -
g, 1.48 mmol) in dioxane (1 ml) and NaHCO3 (0.37 g, 4.49 mmol) in water (3
ml),
and the resultant solution was stirred at room temperature until the
completion of
the reaction (control by TLC). The reaction mixture was evaporated and the
residue was partitioned between ethyl acetate and water. The organic layer was
washed successively with water, saturated NaCI, and dried (Na2SO4). The
solvent
was removed and the residue was chromatographed on silica gel with
dichloromethane-methanol (20:1, v/v) as eluent. The obtained product was
washed with diethyl ether to give the title compound (0.35 g, 71%) as a white
solid. 1H NMR (DMSO-d6, HMDS0),S: 3.76 (3H, s); 4.09 (2H, d, J=6.0 Hz); 6.72
(1H, d, J=16.2 Hz); 7.29 (1H, dd, J=8.0 and 5.0 Hz); 7.51-8.12 (6H, m); 8.27
(1H,
br t, J=6.0 Hz); 8.31-8.50 (2H, m).
Example 115
3-{3-[(Pyridin-3-ylmethyl)-sulfamoyl]-phenyl}-acrylic acid (15b)
11101 ,-- OH
N N
HO 0
To a suspension of 3-{3-[(pyridin-3-ylmethyl)-sulfamoyl]-phenyl}acrylic acid
methyl ester (14b) (0.35 g, 1.05 mmol) in methanol (4.3 ml) IN NaOH solution
(3.15 ml, 3.15 mmol) was added and the resultant mixture was stirred at
ambient
temperature overnight. The reaction mixture was partitioned between ethyl
acetate and water. The aqueous layer was acidified with 2N HCI solution to pH-
5
of the reaction medium and stirred for 30 min. The precipitated solid was
filtered,
washed with water and dried in desiccator over P205. The title compound was
obtained as a white solid (0.28 g, 84%).
Example 116
3-{3-1(Pyridin-3-ylmethyl)-sulfamoyll-phenyl}-acryloyl chloride (16b)
\\ [el CI
N i\/NS\\
I
HO 0
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 170 -
To a suspension of 3-{3-[(pyridin-3-ylmethyl)-sulfamoy1]-phenyl}-acrylic acid
(15b)
(0.28 g, 0.88 mmol) in dichloromethane (3.5 ml) oxalyl chloride (0.23 ml, 2.64
mmol) and one drop of dimethylformamide were added. The reaction mixture was
stirred at 40 C for one hour and concentrated under reduced pressure to give
crude title compound (0.29 g, 98%).
Example 117
N-Hydroxy-3-{3-[(pyridin-3-ylmethyl)-sulfamoy1]-phenyll-acrylamide (17b)
(PX117250)
o
\\ 401 7- k-11.
0H
S
N(1 \\10
0
\
To a suspension of hydroxylamine hydrochloride (0.31 g, 4.40 mmol) in
tetrahydrofuran (5 ml) a saturated NaHCO3 solution (6.8 ml) was added and the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a solution of crude 3-{3-[(pyridin-3-ylmethyl)-sulfamoyll-phenyl)-
acryloyl
chloride (16b) (0.29 g, 0.86 mmol) in tetrahydrofuran (5 ml) was added and the
mixture was stirred at ambient temperature for one hour. The reaction mixture
was poured into water, the obtained solution was acidified with 2N HCI to pH-5
of
the reaction medium and extracted with ethyl acetate. The organic layer was
washed successively with water and saturated NaCI, then the solvent was
removed. The residue was washed with hot ethyl acetate and methanol affording
the title compound (0.12 g, 37%). M.p. 191 C. 'H NMR (DMSO-d6, HMDSO) 5:
4.05 (2H, d, J=6.4 Hz); 6.56 (1H, d, J=16.0 Hz); 7.16-8.05 (7H, m); 8.16-8.49
(3H,
m); 9.12 (1H, br s); 10.80 (1H, br s). HPLC analysis on Symmetry Cig column:
impurities 8% (column size 3.9x150 mm; mobile phase acetonitrile - 0.1M
phosphate buffer (pH 2.5), 10:90; sample concentration 0.4 mg/ml; flow rate
1.3
ml/min; detector UV 270 nm). Anal. Calcd for C15H15N304S containing 0.5 % of
inorganic impurities, 'A: C 53.77, H 4.51, N 12.54. Found, Vo: C 53.72, H
4.33, N
12.41.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
-171 -
Example 118
313-(2-Methoxy-phenylsulfamoy1)-phenyll-acrylic acid methyl ester (14c)
= 0\\ (401 OMe
N
H 0 0
. OMe
A solution of 3-(3-chlorosulfonylphenyl)acrylic acid methyl ester (3) (0.40 g,
1.53
mmol) in dioxane (5 ml) was added to a mixture of o-anisidine (0.19 g, 1.54
mmol)
in dioxane (1 ml) and NaHCO3 (0.26 g, 3.06 mmol) in water (3 ml), and the
resultant solution was stirred at room temperature until the completion of the
reaction (control by TLC). The reaction mixture was evaporated and the residue
was partitioned between ethyl acetate and water. The organic layer was washed
successively with water, saturated NaCI, and dried (Na2SO4). The solvent was
removed and the residue was chromatographed on silica gel with petroleum ether
¨ ethyl acetate (2:1, v/v) as eluent. The obtained product was washed with
diethyl
ether to give the title compound (0.42 g, 79%) as a white solid. 1H NMR (DMSO-
d6, HMDSO), 6: 3.43 (3H, s); 3.72 (3H, s); 6.60 (1H, d, J=16.0 Hz); 6.72-7.27
(4H,
m); 7.45-8.12 (5H, m); 9.47 (1H, s).
Example 119
343-(2-Methoxy-phenylsulfamoy1)-phenyl]-acrylic acid (15c)
411 c)\\s OH
N
H 0 0
OMe
To a suspension of 313-(2-methoxy-phenylsulfamoy1)-phenyll-acrylic acid methyl
ester (14c) (0.42 g, 1.20 mmol) in methanol (5.5 ml) 1N NaOH solution (3.6 ml,
3.60 mmol) was added and the resultant mixture was stirred at ambient
temperature overnight. The reaction mixture was partitioned between ethyl
acetate and water. The aqueous layer was acidified with 2N HCI solution and
extracted with ethyl acetate. The extract was washed with saturated NaCI and
dried (Na2SO4). The solvent was removed and the residue was dried in
desiccator
over P205 to give the title compound as a white solid (0.37 g, 92%).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 172 -
Example 120
343-(2-Methoxy-phenylsulfamoy1)-phenylFacryloyl chloride (16c)
14110 % 401 CI
N
H 0 0
OMe
To a suspension of 343-(2-methoxy-phenylsulfamoy1)-phenyll-acrylic acid (15c)
(0.369, 1.04 mmol) in dichloromethane (4 ml) oxalyl chloride (0.27 ml, 3.12
mmol)
and one drop of dimethylformamide were added. The reaction mixture was stirred
at 40 C for one hour and concentrated under reduced pressure to give crude
title
compound (0.37 g, 97%).
Example 121
N-Hydroxy-343-(2-methoxy-phenylsulfamoy1)-phenylFacrylamide (17c)
(PX117227)
N
H 0 0
OMe
To a suspension of hydroxylamine hydrochloride (0.36 g, 5.20 mmol) in
tetrahydrofuran (6 ml) a saturated NaHCO3 solution (4.5 ml) was added and the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a solution of crude 343-(2-methoxy-phenylsulfamoy1)-phenyl]-acryloyl
chloride (16c) (0.37 g, 1.05 mmol) in tetrahydrofuran (5 ml) was added and the
mixture was stirred at ambient temperature for one hour. The reaction mixture
was poured into water, the obtained solution was acidified with 2N HCI and
extracted with ethyl acetate. The organic layer was washed successively with
water and saturated NaCl, then the solvent was removed. The residue was
crystallised from ethyl acetate and washed with diethyl ether affording the
title
compound (0.23 g, 64%). M.p. 181 C. 1H NMR (DMSO-d6, HMDSO) 8: 3.45 (3H,
s); 6.49 (1H, d, J=16.0 Hz); 6.76-7.96 (9H, m); 9.09 (1H, br s); 9.54 (1H, s);
10.78
(1H, br s). HPLC analysis on Symmetry CB column: impurities 1.3% (column size
CA 02765409 2012-01-19
WO 02/39879 PCT/GB01/04326
- 173-
3.9x150 mm; mobile phase acetonitrile - 0.1M phosphate buffer (pH 2.5), 35:65;
sample concentration 0.15 mg/ml; flow rate 1.2 ml/min; detector UV 230 nm).
Anal. Calcd for C16H1eN205S, %: C 55.16, H 4.63, N 8.04. Found, %: C 55.14, H
4.52, N 7.99.
Example 122
3-[3-(Naphthalen-1-ylsulfamoy1)-phenyl]-acrylic acid methyl ester (14d)
0 lel
/ OMe
SI \S
la 11 % 0
A solution of 3-(3-chlorosulfonylphenyl)acrylic acid methyl ester (3) (0.4 g,
1.53
mmol) in dioxane (5 ml) was added to a mixture of 1-aminonaphthalene (0.22 g,
1.53 mmol) in dioxane (1 ml) and NaHCO3 (0.26 g, 3.09 mmol) in water (3 ml),
and the resultant solution was stirred at room temperature until the
completion of
the reaction (control by TLC). The reaction mixture was evaporated and the
residue was partitioned between ethyl acetate and 2N HCI. The organic layer
was
washed successively with water, saturated NaCI, and dried (Na2SO4). The
solvent
was removed and the residue was chromatographed on silica gel with petroleum
ether-ethyl acetate (gradient from 2:1 to 1:1, v/v) as eluent. The obtained
product
was washed with diethyl ether to give the title compound (0.29 g, 51%) as a
white
solid. 1H NMR (DMSO-deõ HMDSO), 6: 3.69 (3H, s); 6.56 (1H, d, J=16.0 Hz); 7.16
(1H, dd, J=7.0 and 1.4 Hz); 7.27-8.14 (11H, m); 10.25 (1H, s).
Example 123
3[3-(Naphthalen-1-ylsulfamoy1)-phenyll-acrylic acid (15d)
OH
S
40
N \\
H 0 0
To a suspension of 3[3-(naphthalen-1-ylsulfamoy1)-phenyTacrylic acid methyl
ester (14d) (0.29 g, 0.79 mmol) in methanol (3 ml) IN NaOH solution (2.4 ml,
2.4
mmol) was added and the resultant mixture was stirred at ambient temperature
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/0-1326
- 174 -
overnight. The reaction mixture was partitioned between ethyl acetate and
water.
The aqueous layer was acidified with 2N HCI solution and stirred for 30 min.
The
precipitated solid was filtered, washed with water and dried in desiccator
over
P205. The title compound was obtained as a white solid (0.22 g, 79%).
Example 124
343-(Naphthalen-1-ylsulfamoy1)-phenylFacryloyl chloride (16d)
0\\ 4101ci
\0
To a suspension of 343-(naphthalen-1-ylsulfamoy1)-phenyl]-acrylic acid (15d)
(0.22 g, 0.62 mmol) in dichloromethane (2.5 ml) oxalyl chloride (0.16 ml, 1.86
mmol) and one drop of dimethylformamide were added. The reaction mixture was
stirred at 40 C for one hour and concentrated under reduced pressure to give
crude title compound (0.23 g, 99%).
Example 125
N-Hydroxy-343-(naphthalen-1-ylsulfamoy1)-phenyl]-acrylamide (17d) (PX117228)
\\
,S OH
HO Q
To a suspension of hydroxylamine hydrochloride (0.215 g, 3.1 mmol) in
tetrahydrofuran (3.5 ml) a saturated NaHCO3 solution (2.7 ml) was added and
the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a solution of crude 343-(naphthalen-1-ylsulfamoy1)-phenylFacryloyl
chloride (16d) (0.23 g) in tetrahydrofuran (2.5 ml) was added and the mixture
was
stirred at ambient temperature for one hour. The reaction mixture was
partitioned
between ethyl acetate and 2N HCI. The organic layer was washed successively
with water and saturated NaCl, then the solvent was removed. The residue was
crystallised from ethyl acetate affording the title compound (0.054 g, 24%).
M.p.
180 C. 1H NMR (DMSO-d6, HMDSO) 6: 6.45 (1H, d, J=16.0 Hz); 7.14 (1H, dd,
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 175 -
J=7.0 and 1.4 Hz); 7.31-8.14 (11H, m); 9.09 (1H, br s); 10.27 (1H, s); 10.76
(1H,
br s). HPLC analysis on Symmetry C18 column: impurities 4% (column size
3.9x150 mm; mobile phase acetonitrile - 0.1M phosphate buffer (pH 2.5), 40:60;
sample concentration 0.3 mg/ml; flow rate 1.2 ml/ min; detector UV 220 nm).
Anal.
Calcd for C19H16N204S, %: C 61.94, H 4.38, N 7.60. Found, A: C 61.18, H 4.32,
N
7.54.
Example 126
3[3-(Naphthalen-2-ylsulfamoy1)-phenyll-acrylic acid methyl ester (14e)
ONO R\s 401 OMe
N
H 0 0
A solution of 3-(3-chlorosulfonylphenyl)acrylic acid methyl ester (3) (1.0 g,
3.83
mmol) in dioxane (10 ml) was added to a mixture of 2-aminonaphthalene (0.55 g,
3.83 mmol) and NaHCO3 (0.48 g, 5.71 mmol) in water (6 ml), and the resultant
solution was stirred at room temperature until the completion of the reaction
(control by TLC). The reaction mixture was evaporated and the residue was
partitioned between ethyl acetate and 2N HCI. The organic layer was washed
successively with water, saturated NaCI, and dried (Na2SO4). The solvent was
removed and the residue was chromatographed on silica gel with petroleum ether-
ethyl acetate (3:2, v/v) as eluent. The obtained product was crystallised from
petroleum ether-ethyl acetate to give the title compound (0.52 g, 34%) as a
white
solid. 1H NMR (DMSO-d6, HMDS0), 6: 3.73 (3H, s); 6.67 (1H, d, J=16.0 Hz); 7.21-
8.07 (11H, m); 8.16 (1H, s); 10.55 (1H, s).
Example 127
3[3-(Naphthalen-2-ylsulfamoy1)-phenyll-acrylic acid (15e)
OH
N
To a suspension of 3[3-(naphthalen-2-ylsulfamoy1)-pheny1]-acrylic acid methyl
ester (14e) (0.25 g, 0.68 mmol) in methanol (3.5 ml) 2N NaOH solution (1.0 ml,
CA 02765409 2012-01-19
WO 02/30879 PCT/G1301/04326
- 176 -
2.0 mmol) was added and the resultant mixture was stirred at ambient
temperature overnight. The reaction mixture was partitioned between ethyl
acetate and water. The aqueous layer was acidified with 2N HCI solution and
stirred for 30 min. The precipitated solid was filtered, washed with water and
dried
in desiccator over P205. The title compound was obtained as a white solid
(0.21 g,
87%). 1H NIV1R (DMSO-d6, HMDSO), 5: 6.56 (1H, d, J=16.0 Hz); 7.21-8.01 (11H,
m); 8.12 (1H, s); 10.56 (1H, br s); 12.54 (1H, br s).
Example 128
3[3-(Naphthalen-2-ylsulfamoy1)-phenyTacryloyl chloride (16e)
SO c3,s
CI
N
H 0
To a suspension of 3-[3-(naphthalen-2-ylsulfamoy1)-phenyll-acrylic acid (15e)
(0.21 g, 0.57 mmol) in dichloromethane (2.5 ml) oxalyl chloride (0.15 ml, 1.71
mmol) and one drop of dimethylformamide were added. The reaction mixture was
stirred at 40 C for one hour and concentrated under reduced pressure to give
crude title compound (0.21 g, 95%).
Example 129
N-Hydroxy-3[3-(naphthalen-2-ylsulfamoy1)-phenyli-acrylamide (17e) (PX117445)
S ip 0\\
,S OH
N
H 0
To a suspension of hydroxylamine hydrochloride (0.2 g, 2.85 mmol) in
tetrahydrofuran (3.5 ml) a saturated NaHCO3 solution (2.3 ml) was added and
the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a solution of crude 3[3-(naphthalen-2-ylsulfamoy1)-phenyl]-acryloyl
chloride (16e) (0.21 g) in tetrahydrofuran (2.5 ml) was added and the mixture
was
stirred at ambient temperature for one hour. The reaction mixture was
partitioned
between ethyl acetate and 2N HCI. The organic layer was washed successively
with water and saturated NaCI, then the solvent was removed. The residue was
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 177 -
washed with diethyl ether and potroleum ether-ethyl acetate (3:1) affording
the
title compound (0.14 g, 68%). M.p. 164 C. 1H NMR (DMSO-d5, HMDSO) 6: 6.49
(1H, d, J=16.0 Hz); 7.16-7.89 (12H, m); 7.98 (1H, br s); 10.52 (1H, s); 10.76
(1H,
br s). HPLC analysis on Symmetry C18 column: impurities 5% (column size
3.9x150 mm; mobile phase acetonitrile - 0.1M phosphate buffer (pH 2.5), 50:50;
=
sample concentration 0.5 mg/ml; flow rate 0.8 ml/min; detector UV 220 nm).
Anal.
Calcd for C19H16N204S, c1/0: C 61.94, H 4.38, N 7.60. Found, ck: C 61.44, H
4.39, N
7.48.
Example 130
3-(3-Nitro-phenyl)-acrylic acid methyl ester (22)
OMe
02N
0
Acetyl chloride (6.5 ml, 0.09 mol) was added dropwise to methanol (130 ml) at -
C temperature. The reaction mixture was stirred for 30 min. simultaneously
15 allowing to warm up to 0 C. 3-(3-Nitro-phenyl)-acrylic acid (21) (25 g,
0.13 mol)
was added by small portions to the mixture at 0 C and the resulting reaction
mixture was stirred overnight at ambient temperature. The forming precipitate
was
filtered, washed with methanol and dried affording the title compound in a
form of
white crystals (26.58 g, 98%).
Example 131
3-(3-Amino-phenyl)-acrylic acid methyl ester (23)
401 OMe
H2N
0
A mixture of 3-(3-nitro-phenyl)-acrylic acid methyl ester (22) (10.0 g, 48
mmol)
and SnC12=21-120 (54 g, 240 nnmol) in anhydrous ethanol (200 ml) was heated at
80 C for 1 hour. The reaction mixture was allowed to cool to room temperature,
then the solvent was partially evaporated by vacuum rotary evaporator (up to
ca.
1/2 volume). The residue was poured in ice water, neutralised (pH-7) with
CA 02765409 2012-01-19
WO 02/3()879 PCT/GB01/04326
- 178 -
saturated Na2CO3 and the resulting mixture was extracted with ethyl acetate.
The
organic extract was washed with saturated NaCI and dried (Na2SO4). The extract
was filtrated through a small amount of silicagel and evaporated to give pure
title
compound in a form of white crystals (8.5 g, 99%). 1H NMR (CDCI3, HMDSO), 5:
3.69 (2H, br s); 3.79 (3H, s); 6.39 (1H, d, J=16.0 Hz); 6.61-7.03 (3H, m);
7.18 (1H,
t, J=7.6 Hz); 7.62 (1H, d, J=16.0 Hz).
Example 132
3-13-[(E)-2-Phenylethenesulfonylamino]phenyllacrylic acid methyl ester (25a)
1111 ,p
s,N OMe
H
0
A solution of (E)-2-phenylethenesulfonyl chloride (24a) (0.59 g, 2.82 mmol) in
dioxane (3 ml) was added to a mixture of 3-(3-aminophenyI)-acrylic acid methyl
ester (23) (0.50 g, 2.82 mmol) in dioxane (12 ml) and NaHCO3 (0.36 g, 4.28
mmol) in water (8 ml), and the resultant solution was stirred at room
temperature
until the completion of the reaction (control by TLC). The reaction mixture
was
evaporated and the residue was partitioned between ethyl acetate and 2N HCI.
The organic layer was washed successively with water, saturated NaCl, and
dried
(Na2SO4). The solvent was removed and the residue was chromatographed on
silica gel with chloroform-ethyl acetate (100:2, v/v) as eluent to give the
title
compound (0.68 g, 70%) as a white solid. 1H NMR (CDCI3, HMDSO), 6: 3.78 (3H,
s); 6.39 (1H, d, J=16.0 Hz); 6.77 (1H, d, J=15.8 Hz); 6.78 (1H, s); 7.17-7.48
(9H,
m); 7.49 (1H, d, J=15.8 Hz); 7.58 (1H, d, J=16.0 Hz).
Example 133
343-[(E)-2-Phenylethenesulfonylamino]phenyllacrylic acid (26a)
OH
H
0
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
= - 179 -
To a suspension of 3-{3-[(E)-2-phenylethenesulfonylamino]phenyl}acrylic acid
methyl ester (25a) (0.30 g, 0.87 mmol) in methanol (4 ml) IN NaOH solution
(2.62
ml, 2.62 mmol) was added and the resultant mixture was stirred at ambient
temperature overnight. The reaction mixture was partitioned between ethyl
acetate and water. The aqueous layer was acidified with 2N HCI solution and
extraeted with ethyl acetate. The extract was washed with saturated NaCI and
dried (Na2SO4). The solvent was evaporated and the residue was dried in
desiccator over P205. The tite compound was obtained as a white solid (0.26 g,
90%). 1H NMR (DMSO-d6, HMDSO) 8: 6.41 (1H, d, J=16.0 Hz); 7.12-7.51 (9H,
m); 7.55-7.81 (3H, m); 10.16 (1H, br s), 12.32 (1H, br s).
Example 134
3-{3-[(E)-2-Phenylethenesulfonylamino]phenyllacryloyl chloride (27a)
40 I) 401 ,..-
o a
To a suspension of 3-{3-[(E)-2-phenylethenesulfonylamino]phenyllacrylic acid
(26a) (0.26 g, 0.79 mmol) in dichloromethane (3.5 ml) oxalyl chloride (0.21
ml,
2.37 mmol) and one drop of dimethylformamide were added. The reaction mixture
was stirred at 40 C for one hour and concentrated under reduced pressure to
give
crude title compound (0.27 g, 98%).
Example 135
N-Hydroxy-3-{3-[(E)-2-phenylethenesulfonylaminolphenyl}acrylamide (28a)
(PX117446)
1.1 ,p (101 H
OH
0 H
0
To a suspension of hydroxylamine hydrochloride (0.27 g, 3.88 mmol) in
tetrahydrofuran (5 ml) a saturated NaHCO3 solution (3 ml) was added and the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 180 -
mixture a solution of crude 3-{3-[(E)-2-
phenylethenesulfonylamino]phenyllacryloyl
chloride (27a) (0.27 g, 0.77 mmol) in tetrahydrofuran (3.5 ml) was added and
the
mixture was stirred at ambient temperature for one hour. The reaction mixture
was partitioned between ethyl acetate and 2N HCI. The organic layer was washed
successively with water and saturated NaCI, then the solvent was removed. The
residue was crystallised from ethyl acetate and washed with diethyl ether
affording
the title compound (0.115 g, 42%) as white crystals. M.p. 171 C. 1H NMR (DMSO-
d6, HMDSO) 6: 6.38 (d, J=16.0 Hz, 1H); 7.07-7.80 (m, 12H); 9.03 (br s, 1H);
10.16
(s, 1H); 10.76 (br s, 1H). HPLC analysis on Symmetry C18 column: impurities 1%
(column size 3.9x150 mm; mobile phase acetonitrile - 0.1M phosphate buffer (pH
2.5), 35:65; sample concentration 0.4 mg/ml; flow rate 1.2 ml/ min; detector
UV
254 nm). Anal. Calcd for C17H16N204S, %; C 59.29, H 4.68, N 8.13. Found, %: C
59.13, H 4.70, N 7.92.
Example 136
343-(3,4-Dimethoxy-benzenesulfonylamino)-phenyll-acrylic acid methyl ester
(25b)
Me0
4) 40Me0 S, OMe
N
v H
Using an analogous method, the title compound was obtained from 3,4-
dimethoxybenzenesulphonyl chloride (24b) and 3-(3-aminophenyl)acrylic acid
methyl ester (23) as a white solid, yield 77%. 1H NMR (DMSO-d6, HMDSO), 6:
3.69(3H, s); 3.72 (3H, s); 3.78 (3H, s); 6.45 (1H, d, J=16.0 Hz); 6.94-7.67
(8H,
m); 10.23 ppm (1H, br s).
Example 137
343-(3,4-Dimethoxy-benzenesulfonylamino)-phenyli-acrylic acid (26b)
Me0
1:) 401
Me0
,4N/ OH
H
0
CA 02765409 2012-01-19
WO 02/30879
PCT/G1301/04326
- 181 -
Using an analogous method, the title compound was obtained from 343-(3,4-
dimethoxy-benzenesulfonylamino)-phenyll-acrylic acid methyl ester (25b) and
sodium hydroxide, ca. yield of the crude product 95%.
Example 138
343-(3,4-Dimethoxy-benzenesulfonylamino)-phenyl]-acryloyi chloride (27b)
Me0
(101
Me0 S, N CI
=
H
Using an analogous method, the title compound was obtained from 343-(3,4-
dimethoxy-benzenesulfonylamino)-phenyl]-acrylic acid (26b) and oxalyl
chloride,
ca. yield of the crude product 98% (yellow oil).
Example 139
343-(3,4-Dimethoxy-benzenesulfonylamino)-phenyli-N-hydroxy-acrylamide (28b)
(PX117780)
Me0
0
// N
Me0 Sõ Si .7. ,
OH
OH.
Using an analogous method, the title compound was obtained from 343-(3,4-
dimethoxy-benzenesulfonylamino)-phenyl]-acryloyl chloride (27b) and
hydroxylamine hydrochloride, yield 32%. M.p. 158 C. 1H NMR (DMSO-d6,
HMDSO) 6: 3.72 (311, s); 3.80 (3H, s); 6.36 (1H, d, J= 16.0 Hz); 6.89-7,52 (81-
1, m);
9.03 (1H, br s); 10.16 (1H, br s); 10.78 (1H, br s). HPLC analysis on Symmetry
C8
column: impurities 2.5 % (column size 3.9 x 150 mm; mobile phase acetonitrile
¨
0.1M phosphate buffer (pH 2.5), 30:70; detector UV 254 nm; flow rate 1.3
ml/min;
sample concentration 0.5 mg/ml). Anal. Calcd for C17H18N206S,1%; C 53.96, H
4.79, N 7.40. Found, /0: C 53.74, H 4.71, N 7.35.
CA 02765409 2012-01-19
WO 02/30879 PCT/G1301/04326
- 182 -
Example 140
3-[3-(Biphenyl-4-sulfonylamino)-phenyl]acrylic acid methyl ester (25c)
Ph 40/5)
s, OMe
N
u H
0
=
Using an analogous method, the title compound was obtained from biphenyl-4-
sulfonyl chloride (24c) and 3-(3-aminophenyl)acrylic acid methyl ester (23) as
a
white solid, yield 78%. 1H NMR (DMSO-d6, HMDSO), 6: 3.71 (31-1, s); 6.43 (1H,
d,
J=16.0 Hz); 7.12-8.11 (14 H, m); 10.49 ppm (1H, br s ).
Example 141
3[3-(Bipheny1-4-sulfonylamino)-phenyll-acrylic acid (26c)
Ph el,p
,sõ =OH
N
u H
0
Using an analogous method, the title compound was obtained from 343-(bipheny1-
4-sulfonylamino)-phenylFacrylic acid methyl ester (25c) and sodium hydroxide,
ca. yield of the crude product 87%.
Example 142
3[3-(Bipheny1-4-sulfonylamino)-phenylFacryloyl chloride (27c)
Ph,,p
s, Oct
N
H
Using an analogous method, the title compound was obtained from 3-[3-(biphenyl-
4-sulfonylamino)-phenyl]acrylic acid (26c) and oxalyl chloride, ca. yield of
the
crude product 98% (yellow oil).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 183 -
Example 143
3-[3-(Biphenyl-4-sulfonylamino)-phenyl]N-hydroxy-acrylamide (28c) (PX117781)
Ph si
s,
N OH
0 H
0 =
Using an analogous method, the title compound was obtained from 3-[3-(biphenyl-
4-sulfonylamino)-phenyl]-acryloyl chloride (27c) and hydroxylamine
hydrochloride,
yield 20%. M.p. 115 C. 1H NMR (DMSO-d6, HMDSO) 6: 6.38 (1H, d, J= 16.0 Hz);
6.98-7.65 (10H, m); 7.87 (4H, s); 9.03 (1H, br s); 10.45(1H, br s); 10.78 (1H,
br
s). HPLC analysis on Symmetry C8 column: impurities 2.5 `)/0 (column size 3.9
x
150 mm; mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5), 50:50;
detector UV 254 nm; flow rate 1.0 ml/min; sample concentration 0.5 mg/ml).
Anal.
Calcd for C21H18N204S containing 1.3 `)/0 of inorganic impurities, ck: C
63.11, H
4.54, N 7.01. Found, A: C 63.16, H 4.53, N 6.93.
Example 144
343-(Toluene-4-sulfonylamino)-pheny1]-acrylic acid methyl ester (25d)
Me 40,p
s, N OMe
0 H
0
Using an analogous method, the title compound was obtained from tolylsulfonyl
chloride (24d) and 3-(3-aminophenyl)acrylic acid methyl ester (23) as a white
solid, yield 78%. 1H NMR (CDCI3, TMS), 8: 2.38 (3H, s); 3.78 (3H, s); 6.34
(1H, d,
J=16.0 Hz); 6.80 (1H, br, s); 7.00-7.76 (9H, m).
Example 145
343-(Toluene-4-sulfonylamino)-phenyll-acrylic acid (26d)
Me 40,p
s, OH
N
0 H
0
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 184 -
Using an analogous method, the title compound was obtained from 343-(toluene-
4-sulfonylamino)-phenylFacrylic acid methyl ester (25d) and sodium hydroxide,
ca. yield of the crude product 91%.
Example 146
=
343-(Toluene-4-sulfonylamino)-phenyl]-acryloyl chloride (27d)
Me
,p
s,
N ci
0H
Using an analogous method, the title compound was obtained from 343-(toluene-
4-sulfonylamino)-phenyli-acrylic acid (26d) and oxaly1 chloride, ca. yield of
the
crude product 98% (yellow oil).
Example 147
N-Hydroxy-313-(toluene-4-sulfonylamino)-phenyl]-acrylamide (28d) (PX089342)
Me 400
,4 11,0H
N
OH
0
Using an analogous method, the title compound was obtained from 313-(toluene-
4-sulfonylamino)-phenyll-acryloyl chloride (27d) and hydroxylamine
hydrochloride,
yield 82%. M.p. 147 C. 1H NMR (DMSO-d6, HMDSO) 5: 2.32 (s, 3H); 6.36 (d,
J=16.0 Hz, 1H); 6.94-7.76 (m, 9H); 9.03 (br s, 1H); 10.32 (s, 1H); 10.78 ppm
(br s,
1H). HPLC analysis on Symmetry C18 column: impurities <1 % (column size 3.9 x
150 mm; mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5), 35:65;
detector UV 220 rim; flow rate 1.0 mlimin; sample concentration 1.0 mg/ml).
Anal.
Calcd for C16H16N204S, %: C 57.82, H 4.85, N 8.43. Found, 70: C 57.73, H 4.86,
N
8.36.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 185 -
Example 148
3-[3-(Benzene-4-sulfonylamino)-phenyl]acrylic acid methyl ester (25e)
,sii N
v H
= 0
Using an analogous method, the title compound was obtained from
benzenesulfonyl chloride (24e) and 3-(3-aminophenyl)acrylic acid methyl ester
(23) as a white solid, yield 85%. 1H NMR (CDCI3, TMS), 6: 3.78 (3H, s); 6.34
(1H,
d, J=16.0 Hz); 6.74 (1H, br, s); 6.98-7.83 (10H, m).
Example 149
3-[3-(Benzene-4-sulfonylamino)-phenyl]acrylic acid (26e)
el 1) OH 40
i/ 'tµl
0 H
o
Using an analogous method, the title compound was obtained from 313-
(benzene-4-sulfonylamino)-phenylyacrylic acid methyl ester (25e) and sodium
hydroxide, ca. yield of the crude product 88%.
Example 150
343-(Benzene-4-sulfonylamino)-phenyl}-acryloyl chloride (27e)
N
0 H
o
Using an analogous method, the title compound was obtained from 3-13-
(benzene-4-sulfonylamino)-phenyI]-acrylic acid (26e) and oxalyl chloride, ca.
yield
of the crude product 98% (yellow oil).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 186 -
Example 151
3-(3-Benzenesulfonylamino-phenyl)-N-hydroxy-acrylamide (PX089344)
el 40
0
lei
// N OH
0 H
0 .
Using an analogous method, the title compound was obtained from 343-
(benzene-4-sulfonylamino)-phenyl]acryloyl chloride (27e) and hydroxylamine
hydrochloride, yield 86%. M.p. 172 C. 1H NMR (DMSO-d6, HMDSO) 6: 6.35 (d,
J=16.0 Hz, 1H); 6.96-7.92 (m, 10H); 9.03 (br s, 1H); 10.38 (s, 1H); 10.78 ppm
(br
s, 1H). HPLC analysis on Symmetry C18 column: impurities <3 % (column size 3.9
x 150 mm; mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5), 35:65;
detector UV 220 nm; flow rate 0.8 mlimin; sample concentration 1.0 mg/ml).
Anal.
Calcd for C161-114N204S, %; C 56.59, H 4.43, N 8.80. Found, %: C 56.48, H
4.57, N
8.45.
Example 152
Sodium 2-(2-methoxycarbonyl-vinyl)benzenesulfonate (32)
OMe
SO,Na o
A mixture of sodium 2-formylbenzenesulfonate hydrate (31) (tech., purity 75%;
1.33 g, 4.79 mmol), potassium carbonate (1.32 g, 9.56 mmol), and trimethyl
phosphonoacetate (1.05 g, 5.77 mmol) in water (2.5 ml) was vigorously stirred
at
ambient temperature for 1 hour. The precipitate was filtered and carefully
washed
with methanol. The methanol extract was evaporated to give the title compound
(0.66 g, 52%) as a white solid. 1H NMR (DMSO-d6, HMDSO), 6: 3.72 (3H, s); 6.43
(1H, d, J=16.0 Hz); 7.18-7.96 (4H, m); 8.83 (1H, d, J=16.0 Hz).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 187 -
Example 153
3-(2-Chlorosulfonylphenyl)acrylic acid methyl ester (33)
OMe
0=-S=0 0
=
CI
To a solution of 2-(2-methoxycarbonyl-vinyl)benzenesulfonate (32) (0.63 g,
2.38
mmol) in benzene (2 ml) thionyl chloride (1.439, 12.00 mmol) and three drops
of
dimethylformamide were added, and the resultant suspension was stirred at
reflux
temperature for 1.5 hours. The reaction mixture was evaporated and the residue
was dissolved in benzene (5 m1). The benzene solution was filtered and the
filtrate
was evaporated to give the title compound (0.47 g, 71%) as an oil.
Example 154
3-(2-Phenylsulfamoyl-phenyl)-acrylic acid methyl ester (34a)
OMe
0:=S=0 0
is NH
To a mixture of aniline (0.33 g, 3.53 mmol) and pyridine (1 ml) a solution of
3-(2-
chlorosulfonylphenypacrylic acid methyl ester (33) (0.45 g, 1.72 mmol) in
dichloromethane (3 ml) was added and the resultant solution was stirred at 50
C
for 1 hour. The reaction mixture was evaporated and the residue was
partitioned
between ethyl acetate and 10% HD. The organic layer was washed successively
with water, saturated NaCl and dried (Na2SO4). The solvent was removed and the
residue was chromatographed on silica gel with ethyl acetate ¨ chloroform
(1:7,
v/v) as eluent. The obtained product was washed with diethyl ether to give the
title
compound (0.339, 60%). 1H NMR (CDCI3, HMDSO), 6: 3.86 (3H, s); 6.27 (1H, d,
J=16.0 Hz); 6.69 (1H, br s); 6.87-7.67 (8H, m); 7.94-8.13 (1H, m); 8.49 (1H,
d,
J=16.0 Hz).
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 188 -
Example 155
3-(2-Phenylsulfamoyl-phenyl)acrylic acid (35a)
OH
07----S=0 0
si NH
3-(2-phenylsulfamoyl-phenyl)-acrylic acid methyl ester (34a) (0.30 g, 0.94
mmol)
was dissolved in methanol (4 ml), IN NaOH solution (2.82 ml, 2.82 mmol) was
added and the resultant solution was stirred at ambient temperature overnight.
The reaction mixture was partitioned between ethyl acetate and water. The
aqueous layer was acidified with 10% HCI solution and stirred at ambient
temperature for 1 hour. The precipitated solid was filtered, washed with water
and
dried in desiccator over P205. The title compound (0.2 g, 70%) was obtained as
a
white solid. 1H NMR (DMSO-c16, HMDS0), 6: 6.40 (1H, d, J=16.0 Hz); 6.93-7.32
(5H, in); 7.45-8.00 (5H, m); 8.47 (1H, d, J=16.0 Hz); 10.59(1H, br s).
Example 156
3-(2-Phenylsulfamoyl-phenyl)-acryloyl chloride (36a)
CI
0=-S=0 0
lp NH
To a suspension of 3-(2-phenylsulfamoyl-phenyl)acrylic acid (35a) (0.18 g,
0.59
mmol) in dichloromethane (3.0 ml) oxalyl chloride (0.18 ml, 2.06 mmol) and one
drop of dimethylformamide were added. The reaction mixture was stirred at 40 C
for one hour and concentrated under reduced pressure to give crude title
compound (0.19g, 99%).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 189 -
Example 157
N-Hydroxy-3-(2-phenylsulfamoylphenyl)acrylamide (37a) (PX116242)
IN,OH
0=S=0 0
40 NH
To a suspension of hydroxylamine hydrochloride (0.21 g, 3.01 mmol) in
tetrahydrofuran (4.0 ml) a saturated NaHCO3 solution (2.6 ml) was added and
the
resultant mixture was stirred at ambient temperature for 25 min. To the
reaction
mixture a 3-(2-phenylsulfamoyl-phenyl)acryloyl chloride (36a) (0.19 g, 0.59
mmol)
solution in tetrahydrofuran (2.5 ml) was added and the mixture was stirred at
ambient temperature for 2 hours. The reaction mixture was partitioned between
ethyl acetate and 2N HCI. The organic layer was washed successively with water
and saturated NaCI, and the solvent was removed. The residue was washed with
diethyl ether and crystallised from acetonitrile to give the title compound
(0.056 g,
30%) as white crystals, m.p. 205-206.5 C. 1H NMR (DMSO-d6, HMDSO), 6: 6.37
(1H, d, J=16.0 Hz); 6.99-8.04 (10H, m); 8.23 (1H, d, J=16.0 Hz); 10.55 (1H,
s);
10.83 (1H, br s). HPLC analysis on Symmetry 018 column: impurities 6.4%
(column size 3.9x150 mm; mobile phase acetonitrile-0.1M phosphate buffer, pH
2.5, 30:70; sample concentration 0.2 mg/ml; flow rate 1.2 ml/min; detector UV
220
nm). Anal. Calcd for C161-114N204S *0.1 H20, (Yo: C 56.28, H 8.75, N 4.47.
Found,
ct/0: C 55.63, H 9.07, N 4.36.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 190 -
Example 158
342-(Naphthalen-1-ylsulfannoy1)-phenyll-acrylic acid methyl ester (34b)
1110 OMe
0=S=0 0
NH
OA,
Using an analogous method, the title compound was obtained from 3-(2-
chlorosulfonylphenyl)acrylic acid methyl ester (33) and 1-aminonaphthalene,
yield
59%. 1H NMR (CDCI3, HMDSO), 6: 3.76 (3H, s); 6.22 (1H, d, J=16.0 Hz); 6.88-
7.85 (11H, m); 7.94-8.12 (1H, m); 8.51 (1H, d, J=16.0 Hz).
Example 159
342-(Naphthalen-1-ylsulfamoy1)-phenylFacrylic acid (35b)
1101 OH
0=S=0 0
le NH
Using an analogous method, the title compound was obtained from 342-
(naphthalen-1-ylsulfamoy1)-phenyl]-acrylic acid methyl ester (34b) and sodium
hydroxide, yield 41%. 1H NMR (DMSO-d6, HMDSO), 6: 6.16 (1H, d, J=16.0 Hz);
7.18-7.95 (12H, m); 8.29 (1H, d, J=16.0 Hz); 10.54 (1H, br s).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 191 -
Example 160
342-(Naphthalen-1-ylsulfamoy1)-phenyli-acryloyl chloride (36b)
CI
0=S=0 0
NH
11011
Using an analogous method, the title compound was obtained from 342-
(naphthalen-1-ylsulfamoy1)-phenyl]-acrylic acid (35b) and oxalyl chloride in a
form
of a crude product, yield ca. 98%.
Example 161
N-Hydroxy-342-(naphthalen-1-ylsulfamoy1)-phenylFacrylamide (37b) (PX117447)
li&OH
0=--S=0 0
NH
Using an analogous method, the title compound was obtained from 342-
(naphthalen-1-ylsulfamo0-phenylFacryloyl chloride (36b) and hydroxylamine
hydrochloride, yield 38%, m.p. 186-187 C. 1H NMR (DMSO-d6, HMDSO), 8: 6.29
(1H, d, J=15.0 Hz); 7.17-8.16 (11H, m); 8.36 (1H, d, J=15.0 Hz); 9.14 (1H, br
s);
10.57 (1H, s); 10.83 (1H, s). HPLC analysis on Symmetry C8 column: impurities
6.4% (column size 3.9x150 mm; mobile phase acetonitrile-0.1M phosphate buffer,
pH 2.5, 35:65; sample concentration 0.5 mg/ml; flow rate 1.6 ml/min; detector
UV
220 nm). Anal. Calcd for C161-116N204S *0.4 H20,13/0: C 60.76, H 4.51, N 7.46.
Found, (Yo: C 60.46, H 4.35, N 7.69.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 192 -
Example 162
312-(Methyl-phenyl-sulfamoy1)-phenylFacrylic acid methyl ester (34c)
401 OMe
0=S=0 0
N, =
(1110 Me
Using an analogous method, the title compound was obtained from 3-(2-
chlorosulfonylphenyl)acrylic acid methyl ester (33) and N-methylaniline, yield
54%.
1H NMR (DMSO-c15, HMDSO), 6: 3.13 (3H, s); 3.67 (3H, s); 6.29 (1H, d, J=16.0
Hz); 7.01-7.45 (5H, m); 7.52-8.09 (5H, m).
Example 163
342-(Methyl-phenyl-sulfamoy1)-phenyll-acrylic acid (35c)
OH
0=S=0 0
N,
la Me
Using an analogous method, the title compound was obtained from 342-(methyl-
phenyl-sulfamoy1)-phenyli-acrylic acid methyl ester (34c) and sodium
hydroxide,
yield 48%. 1H NMR (DMSO-deõ HMDSO), 6:3.17 (3H, s); 6.28 (1H, d, J=16.0 Hz);
7.06-7.42 (5H, m); 7.53-8.20 (6H, m).
Example 164
3-[2-(Methyl-phenyl-sulfamoyI)-phenyl]-acryloyl chloride (36c)
CI
0=S=0 0
N,
= Me
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 193 -
Using an analogous method, the title compound was obtained from 312-(methyl-
phenyl-sulfamoy1)-phenyl]-acrylic acid (35c) and oxalyl chloride in a form of
the
crude product, yield ca. 99%.
Example 165
N-Hydroxy-342-(methyl-phenyl-sulfamoy1)-phenyl]-acrylamide (37c) (PX117448)
OH
OH
0=S=0 0
N,
lb Me
Using an analogous method, the title compound was obtained from 342-(methyl-
phenyl-sulfamoy1)-phenylFacryloyl chloride (36c) and hydroxylamine
hydrochloride, yield 40%, m.p. 144.5-145.5 C. 1H NMR (DMSO-d6, HMDSO), 8:
3.16 (3H, s); 6.32 (1H, d, J=16.0 Hz); 7.00-7.86 (9H, m); 8.09 (1H, d, J=16.0
Hz);
9.12 (1H, br s); 10.80 (1H, s). HPLC analysis on Zorbax SB 018 column:
impurities
1.0% (column size 4.6x150 mm; mobile phase methanol-0.1% H3PO4, gradient
from 50:50 to 90:10; sample concentration 0.5 mg/ml; flow rate 1.5 ml/min;
detector UV 230 nm). Anal. Calcd for C16H16N204S * 0.7 H20, %: C 55.70, H
5.08,
N 8.12. Found, A: C 55.17, H 4.65, N 8.05.
Example 166
3-(4-Chlorosulfonyl-phenyl)-acrylic acid (42)
ck, I/O
õ
0 s
OH
To neat chlorosulfonic acid (5.3 ml, 80 mmol) at 0-5 C temperature slowly
cinnamic acid (41) (1.47 g, 10 nnmol) was added. As the reaction proceeded
hydrogen chloride gas evolved. The reaction mixture was stirred successively
at
0 C for 1 hour, at ambient temperature for 2 hours and at 40-42 C for 2 hours.
The dark, viscous syrup was poured onto ice, the precipitated solid was
filtered
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
=
- 194 -
and washed with water. The title compound (0.5 g, 20%) as a white solid was
obtained. 1H NMR (DMSO-d6, HMDSO), 8: 6.55 (1H, d, J=16 Hz); 7.58 (1H, d,
J=16.0 Hz); 7.65 (4H, s); 8.15 (1H, br s).
Example 167
3-(4-Phenylsulfamoyl-phenyl)acrylic acid (43a)
H 0
N
401
0
OH
To a mixture of aniline (0.35 g, 3.75 mmol) and pyridine (1 ml) a solution of
3-(4-
chlorosulfonyl-phenyl)-acrylic acid (42) (0.45 g, 1.82 mmol) in
dichloromethane (3
ml) was added and the resultant solution was stirred at 40 C for 1 hour. The
reaction mixture was evaporated and the residue was partitioned between ethyl
acetate and 6N HCI. The organic layer was washed successively with water,
saturated NaCI and dried (Na2SO4). The solvent was evaporated under reduced
pressure to give the title compound (0.30 g, 54%). 1H NMR (DMSO-d6, HMDSO),
5: 6.60 (1H, d, J=16.0 Hz); 6.93-7.43 (5H, m); 7.60 (1H, d, J=16.0 Hz); 7.79
(2H,
d, J=8.0 Hz); 7.87 (2H, d, J=8.0 Hz); 10.35 (1H, s).
Example 168
3-(4-Phenylsulfamoyl-phenyl)acryloyl chloride (44a)
H
N
0
CI
To a suspension of 3-(4-phenylsulfamoyl-phenyl)-acrylic acid (43a) (0.25 g,
0.82
mmol) in dichloromethane (4.7 ml) oxalyl chloride (0.32 ml, 3.68 mmol) and one
drop of dimethylformamide were added. The reaction mixture was stirred at 40 C
for one hour and concentrated under reduced pressure to give crude title
compound (0.24g, 92%).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 195 -
Example 169
N-Hydroxy-3-(4-phenylsulfamoylphenyI)-acrylamide (45a) (PX117450)
H 0
N.,/
0
N.,
OH
To a suspension of hydroxylamine hydrochloride (0.21 g, 3.01 mmol) in
tetrahydrofuran (4.0 ml) a saturated NaHCO3 solution (2.6 ml) was added and
the
resultant mixture was stirred at ambient temperature for 25 min. To the
reaction
mixture a 3-(4-phenylsulfamoyl-phenyl)-acryloyl chloride (44a) (0.19 g, 0.59
mmol)
solution in tetrahydrofuran (2.5 ml) was added and the mixture was stirred at
ambient temperature for 2 hours. The reaction mixture was partitioned between
ethyl acetate and 2N HCI. The organic layer was washed successively with water
and saturated NaCI, and the solvent was removed. The residue was washed with
diethyl ether to give the title compound (0.074 g, 39%) as white crystals,
m.p. 176-
177.5 C. 1H NMR (DMSO-d6, HMDSO), 6: 6.54 (1H, d, J=16.0 Hz); 6.96-7.32 (5H,
m); 7.47 (1H, d, J=16.0 Hz); 7.76 (4H, s); 9.14 (1H, br s); 10.29 (1H, br s);
10.86
(1H, s). Anal. Calcd for C16H14N204S, C 56.59, H 4.43, N 8.80. Found, ck:
55.82, H 4.38, N 9.01.
Example 170
3[4-(Naphthalen-2-ylsulfamoy1)-phenyl]-acrylic acid (43b)
H 0
N
14040
./ OH
Using an analogous method, the title compound was obtained from 3-(4-
chlorosulfonyl-phenyl)-acrylic acid (42) and 2-aminonaphthalene, yield 49%. 1H
NMR (DMSO-d6, HMDSO), 5:6.62 (1H, d, J=16.0 Hz); 7.19 (1H, dd, J=8.0 and
2.0 Hz); 7.34-8.14 (11H, m); 10.32 (1H, br s).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 196 -
Example 171
3[4-(Naphthalen-2-ylsulfamoy1)-phenylFacryloyl chloride (44b)
H 0
OsN //
0 lelCI
Using an analogous method, the title compound was obtained from 3-[4-
(naphthalen-2-ylsulfamoy1)-phenyl]acrylic acid (43b) and oxalyl chloride, ca.
yield
of the crude product 98% (yellow oil).
Example 172
N-Hydroxy-3[4-(naphthalen-2-ylsulfamoy1)-phenyTacrylamide (45b) (PX117736)
H
N //
040 0//S
OH
N.
0
Using an analogous method, the title compound was obtained from 314-
(naphthalen-2-ylsulfamoy1)-phenylFacryloyl chloride (44b) and hydroxylamine
hydrochloride, yield 25%. M.p. 198.5-199.5 C. 1H NMR (DMSO-d6, HMDSO), 6:
6.54 (1H, d, J= 16.0 Hz); 7.16 (1H, dd, J=8.0 and 2.0 Hz); 7.29-8.12 (11H, m);
9.11 (1H, br s); 10.07(1H, s); 10.87 (1H, s). HPLC analysis on Symmetry C8
column: impurities 1.8 % (column size 3.9 x 150 mm; mobile phase acetonitrile -
0.1M phosphate buffer (pH 2.5), 35:65; detector UV 254 nm; flow rate 1.5
ml/min;
sample concentration 0.5 mg/ml). Anal. Calcd for C161-116N204S * 0.2 H20, c/0:
C
61.34, H 4.44, N 7.53. Found, %: C 60.96, H 4.28, N 7.56.
Example 173
3[4-(Bipheny1-4-ylsulfamoy1)-phenyl]-acrylic acid (43c)
H 0
N
100
0
Ph OH
0
CA 02765409 2012-01-19
- 197 -
Using an analogous method, the title compound was obtained from 3-(4-
chlorosulfonyl-phenyl)-acrylic acid (42) and 4-aminobiphenyl, yield 67%. 1H
NMR
(DMSO-d6, HMDSO), 5: 6.62 (1H, d, J=16.0 Hz); 7.19 (2H, d, J=8.0 Hz); 7.25-
7.75
(9H, m); 7.77-7.95 (4H, m); 10.46 (1H, br s).
Example 174
344-(Biphenyl-4-ylsulfamoy1)-phenyl]-acryloyi chloride (44c)
H0
Ph 1.1 diS
401 CI
Using an analogous method, the title compound was obtained from 344-(biphenyl-
4-ylsulfamoy1)-phenyl]-acrylic acid (43c) and oxalyl chloride, ca. yield of
the crude
product 79% (yellow oil).
Example 175
N-Hydroxy-3-[4-(biphenyl-4-ylsulfamoy1)-phenyl]-acrylamide (45c) (PX117792)
HO
N
CTtI
Ph N.,
OH
Using an analogous method, the title compound was obtained from 344-(biphenyl-
4-ylsulfamoy1)-phenyl]-acryloyl chloride (44c) and hydroxylamine
hydrochloride,
yield 32%. M.p. 211-211.5 C. 1H NMR (DMSO-d6, HMDSO), 8: 6.53 (1H, d,
J=16.0 Hz); 7.19 (2H, d, J=8.0 Hz); 7.32-7.69 (8H, m); 7.72-7.92 (4H, m); 9.09
(1H, br s); 10.45 (1H, s); 10.85 (1H, br s). HPLC analysis on Zorbax SB-C18
column: impurities 3% (column size 4.6 x 150 mm; mobile phase acetonitrile r-
0.1% H3PO4, gradient from 50 to 100% (10 min); detector UV 254 nm; flow rate
1.0 ml/min; sample concentration 0.65 mg/ml). Anal. Calcd for C21H18N204S, %:
C
63.94, H 4.60, N 7.10. Found, `1/0: C 63.51, H 4.37, N 7.11.
*Trade-mark
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 198 -
Example 176
344-(4-Bromo-phenylsulfamoy1)-phenyl]-acrylic acid (43d)
H 0
N
0/P
Br OH
= o
Using an analogous method, the title compound was obtained from 3-(4-
chlorosulfonyl-phenyl)-acrylic acid (42) and 4-bromoaniline, yield 66%. 1H NMR
(DMSO-d6, HMDSO), 5: 6.60 (1H, d, J=16.0 Hz); 7.09 (2H, d, J=8.0 Hz); 7.44
(2H,
d, J=8.0 Hz); 7.60 (1H, d, J=16.0 Hz); 7.73-7.85 (4H, m); 10.49 (1H, br s).
Example 177
344-(4-Bromo-phenylsulfamoy1)-phenyli-acryloyl chloride (44d)
H 0
Is
Br lel
CI
Using an analogous method, the title compound was obtained from 3-[4-(4-bromo-
phenylsulfamoy1)-phenyli-acrylic acid (43d) and oxalyl chloride, ca. yield of
the
crude product 91% (yellow oil).
Example 178
N-Hydroxy-344-(4-bromo-phenylsulfamoyl)-phenyll- acrylamide (45d) (PX117795)
H0
N
Br 0 401
N-'0H
Using an analogous method, the title compound was obtained from 344-(4-bromo-
phenylsulfamoyI)-phenyl]-acryloyl chloride (44d) and hydroxylamine
hydrochloride, yield 59%. M.p. 219-220.5 C. 1H NMR (DMSO-d6, HMDSO), 5:
6.54 (1H, d, J=16.0 Hz); 7.05 (2H, d, J=8.0 Hz); 7.43 (21-I, d, J=8.0 Hz);
7.49 (1H,
d, J=16.0 Hz); 7.63-7.87(4H, m); 9.11 (1H, br s); 10.45(1H, s); 10.83 (1H, br
s).
CA 02765409 2012-01-19
s.r2361
- 199 -
HPLC analysis on Zorba)Z SB-C18 column: impurities 3% (column size 4.6 x 150
mm; mobile phase acetonitrile ¨0.1% H3PO4,gradient from 30 to 100% (15 min);
detector UV 254 nm; flow rate 1.0 ml/mm; sample concentration 0.65 mg/m1).
Anal. Calcd for C15H13BrN204S, C 45.35, H 3.30, N 7.05. Found, `)/0: C
45.44, H
3.28, N 7.05.
Example 179
344-(4-Chloro-phenylsulfamoy1)-phenyll-acrylic acid (43e)
H
=0
CI OH
0
Using an analogous method, the title compound was obtained from 3-(4-
chlorosulfonyl-phenyl)-acrylic acid (42) and 4-chloroaniline, yield 83%. 1H
NMR
(DMSO-d5, HMDSO), 5: 6.63 (1H, d, J=16.0 Hz); 7.09 (2H, d, J=8.0 Hz); 7.34
(2H,
d, J=8.0 Hz); 7.58 (2H, d, J8.0 Hz); 7.72 (2H, d, J=8.0 Hz); 7.84 (2H, d,
J=8.0
Hz);10.47 (1H, br s).
Example 180
344-(4-Chloro-phenylsulfamoy1)-phenyl]-acryloyi chloride (44e)
0
N.,
Cl 401 0 401
OH
0
Using an analogous method, the title compound was obtained from 344-(4-chloro-
phenylsulfamoy1)-phenyl]acrylic acid (43e) and oxalyl chloride, ca. yield of
the
crude product 71% (yellow oil).
*Trade -mark
CA 02765409 2012-01-19
54236-1
- 200 -
Example 181
N-Hydroxy-344-(4-chloro-phenylsulfannoy1)-phenyl]-acrylamide (45e) (PX117796)
H
N,
PS0 Si
CI N,
OH
0
Using an analogous method, the title compound was obtained from 314-(4-chloro-
phenylsulfamoyI)-phenyl]acryloyl chloride (44e) and hydroxylamine
hydrochloride,
yield 33%. M.p. 201-202 C. 1H NMR (DMSO-d6, HMDSO), 5: 6.52 (1H, d, J=16.0
Hz); 7.08 (2H, d, J=8.0 Hz); 7.29 (2H, d, J=8.0 Hz); 7.45 (1H, d, J=16.0 Hz);
7.63-
7.89(5H, m); 10.43(1H, br s); 10.83(1H, br s). HPLC analysis on Zorbax SE3-C18
column: impurities 61% (column size 4.6 x 150 mm; mobile phase acetonitrile ¨
0.1% H3PO4, gradient from 30 to 100% (15 min); detector UV 254 nm; flow rate
1.0 ml/min; sample concentration 0.5 mg/ml). Anal. Calcd for C161-113CIN204S,
70:
C 51.07, H 3.71, N 7.94. Found, %: C 51.14, H 3.70, N 7.86.
Example 182
3-Bromo-N-phenyl-benzenesulfonamide (52a)
% 1101
Br
N
H 0
3-Bromobenzenesulfonyl chloride (51a) (1.0 g, 3.9 mmol),was added to a mixture
of aniline (0.47 g, 5.1 mmol) in acetonitrile (10 ml) and sodium carbonate
(1.3g,
12.3 mmol) in water (10 m1). The mixture was stirred at ambient temperature
for 1
hour and the reaction product was extracted with ethyl acetate (30 m1). The
extract was dried (Na2SO4) and solvents were removed under reduced pressure
to give the title compound (1.15 g, 94%) as an oil witch solidified upon
standing.
M.p. 98-100 C. 1H NMR (DMSO-d6, HMDSO) 5: 6.94-7.48 (5H, m, C6F16); 7.50-
7.96 (4H, m, C6F14); 10.36 (1H, s, NH).
*Trademark
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 201 -
Example 183
3-(3-Hydroxyprop-1-ynyI)-N-phenylbenzenesulfonamide (53a)
0\\
,s
N OH
H 0
A mixture of 3-bromo-N-phenyl-benzenesulfonamide (52a) (1.0 g, 3.2 mmol),
benzene (2.4 ml), tetrakis(triphenylphosphine)palladium(0) (0.4 g, 0.34 mmol),
copper iodide (0.032 g, 0.16 mmol), triethylamine (2.4 ml, 17.2 mmol), and
propargyl alcohol (1.0 ml, 17.2 mmol)) was refluxed under argon for 30 min.
The
reaction mixture was diluted with 5% HCI (50 ml) and product was extracted
with
ethyl acetate (50 ml). The extract was washed successively with 5% NaHCO3,
water and dried (Na2SO4). The solvents were removed under reduced pressure
and the product was purified on silica gel with ethyl acetate ¨ hexane (1:1,
v/v) as
eluent. The title compound (0.59 g, 64%) was obtained as an oil. 1H NMR (DMSO-
d6, HMDSO) 6:4.29 (2H, d, J=6.0 Hz, CH2); 5.36 (1H, t, J=6.0 Hz, OH); 6.94-
7.32
(5H, m, C6H5); 7.35-7.91 (4H, m, C6H4); 10.32 (1H, s, NH).
Example 184
3-(3-0xoprop-1-yny1)-N-phenylbenzenesulfonamide (54a)
=R\
N
H 0 CHO
3-(3-Hydroxyprop-1-yny1)-N-phenylbenzenesulfonamide (53a) (0.55 g, 1.9 mmol)
was dissolved in a solution of Dess-Martin reagent in methylene chloride
(0.157
g/ml) (8.2 ml) and the resultant mixture was stirred at ambient temperature
for 30
min. The mixture was partitioned between water (50 ml) and ether (50 ml), and
ether solution was washed successively with 5% Na2CO3, water, and dried
(Na2SO4). The solvents were removed under reduced pressure to give the title
compound (0.47 g, 72%) as an oil. The crude product 54a was used in the
further
step without an additional purification. 1H NMR (DMSO-d6, HMDSO) 5: 6.96-7.41
(5H, m, C6H5); 7.54-8.07 (4H, m, C6I-14); 9.45 (1H, s, CH); 10.41 (1H, s, NH).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 202 -
Example 185
(E)-5-(3-Phenyisulfamoylphenyl)pent-2-en-4-ynoic acid methyl ester (55a)
SOS. N\
,
N OMe
H0
=
To a solution of trimethyl phosphonoacetate (0.81 g, 4.5 mmol) in dry
tetrahydrofuran (20 ml) under an argon atmosphere at 15-20 C sodium hydride
(0.12 g, 5.0 mmol) was added. The mixture was stirred at ambient temperature
for
1 hour, and a solution of 3-(3-oxoprop-1-yny1)-N-phenylbenzenesulfonamide
(54a)
(0.44g, 1.5 mmol) in dry tetrahydrofuran (20 ml) was added dropwise at 15-20
C.
The reaction mixture was stirred at ambient temperature for 1 hour and
quenched
by 3% HC1 (20 ml). The product was extracted with ethyl acetate (50 ml), the
extract was washed with 5% NaHCO3, water and dried (Na2SO4). The solvents
were removed under reduced pressure and the residue was chromatographed on
silica gel with ethyl acetate - hexane (1:2, v/v) as eluent to give the title
compound
(0.39 g, 74%) as a white solid. M.p. 134-136 C. 1H NMR (DMSO-d6, HMDSO) 6:
3.73 (3H, s, CH3); 6.49 (1H, d, J=15.5 Hz, CH); 7.03 (1H, d, J=15.5 Hz, CH);
7.01-
7.38 (5H, m, C6F16); 7.41-7.89 (4H, m, C6H4); 10.34 (1H, s, NH).
Example 186
(E)-5-(3-Phenylsulfamoylphenyl)pent-2-en-4-ynoic acid (56a)
\\
,s
N OH
H 0
To a solution of E-5-(3-phenylsulfamoylphenyl)pent-2-en-4-ynoic acid methyl
ester
(55a) (0.34 g, 1 mmol) in methanol (3 ml) 1N solution of sodium hydroxide (3
ml)
was added and the mixture was stirred at ambient temperature for 3 hours.
Methanol was removed under reduced pressure, to the residue water (5 ml) was
added and the mixture was acidified with 3% HCI. The precipitate was filtered,
washed with water, and dried to give the title compound (0.31 g, 95%) as white
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 203 -
crystals. M.p. 188-190 C. 1H NMR (DMSO-d6, HMDSO) 8: 6.36 (1H, d, J=15.8 Hz,
CH); 6.92 (1H, d, J=15.8 Hz, CH); 7.01-7.36 (5H, m, C6H6); 7.38-7.89 (4H, m,
C6H4); 10.32 (1H, s, NH).
Example 187
(E)-5-(3-Phenylsulfamoylphenyl)pent-2-en-4-ynoic acid hydroxyamide (58a)
(PX116238)
=R\
,s
N N,
H 0 OH
To a solution of (E)-5-(3-phenylsulfamoylphenyl)pent-2-en-4-ynoic acid (56a)
(0.25g 0.77 mmol) in methylene chloride (5 ml) oxalyl chloride (0.42g 3.1
mmol)
was added. The resultant mixture was stirred for 1 hour at ambient temperature
and the solvents were removed under reduced pressure. The crude product (57a)
was dissolved in acetonitrile (5 ml) and the obtained solution to a mixture of
hydroxylamine hydrochloride (0.3 g, 4.3 mmol) and NaHCO3 (0.3 g, 3.6 mmol) in
water (8 ml) was added. The reaction mixture was stirred for 10 min. and the
product was extracted with ethyl acetate (30 ml). The extract was washed with
10% Na2CO3, and the aqueous phase was acidified with 3% HCI. The precipitate
was filtered and dried to give the title compound (0.12 g, (46%). M.p 88-90 C.
1H
NMR (DMSO-d6, HMDSO) 5: 6.41 (1H, d, J=15.8 Hz, CH); 6.82 (1H, d, J=15.8 Hz,
CH); 6.92-7.41 (5H, m, C61-16); 7.47-8.01 (4H, m, C6H4); 8.94-11.21 (3H, br s,
NH,
NH, OH). Anal. Calcd for C17H14N204S * 0.4H20: C 58.58, H 4.27, N 8.01. Found:
C 58.12, H 4.03, N 7.80.
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
=
- 204 -
Example 188
4-lodo-N-phenyl-benzenesulfonamide (52b)
0
2 le
Using an analogous method, the title compound was obtained from 4-
5. iodobenzenesulfonyl chloride (51b) and aniline, yield 86%, m.p. 135-137
C. 1H
NMR (DMSO-d6, HMDSO) 5: 6.85-7.36 (5H, m, C6H6); 7.52 (2H, d, J=8.5 Hz,
C6H2); 7.89 (2H, d, J=8.5 Hz, C6H2); 10.32 (1H, s, NH).
Example 189
4-(3-Hydroxyprop-1-yny1)-N-phenylbenzenesulfonamide (53b)
FINI /IP
0/
OH
Using an analogous method, the title compound was obtained from 4-iodo-N-
phenyl-benzenesulfonamide (52b) and propargyl alcohol, yield 86%, m.p. 161-
163 C. 1H NMR (DMSO-d6, HMDSO) 5: 4.29 (2H, d, J=6.0 Hz, CH2); 5.38 (1H, t,
J=6.0 Hz, OH); 6.92-7.38 (5H, m, C6F15); 7.54 (2H, d, J=9.0 Hz, C6H2); 7.73
(2H,
d, J=9.0 Hz, C6H2); 10.29 (1H, s, NH).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 205 -
Example 190
4-(3-0xoprop-1-yny1)-N-phenylbenzenesulfonamide (54b)
HN, IP
0f/
CHO
Using an analogous method, the title compound was obtained from 4-(3-
hydroxyprop-1-yny))-N-phenylbenzenesulfonamide (53b) and Dess-Martin
reagent, yield 70%, m.p. 161-163 C. 1H NMR (DMSO-d6, HMDSO) 8: 6.92-7.41
(5H, m, C6H6); 7.83 (4H, s, C6H4); 9.43 (1H, s, CH); 10.42 (1H, s, NH).
Example 191
E-5-(4-Phenylsulfamoylphenyl)pent-2-en-4-ynoic acid methyl ester (55b)
HN, /P
0/
OMe
0
Using an analogous method, the title compound was obtained from 4-(3-oxoprop-
1-yny1)-N-phenylbenzenesulfonamide (54b) and trimethyl phosphonoacetate, yield
49%, m.p. 153-155 C. 1H NMR (DMSO-c16, HMDSO) 8: 3.72 (3H, s, CH3); 6.49
(1H, d, J=16.0 Hz, CH); 6.98 (1H, d, J=16.0 Hz, CH); 6.92-7.38 (5H, m, C6H6);
7.64 (2H, d, J=9.0 Hz, C6H2); 7.76 (2H, d, J=9.0 Hz, C6H2); 10.32 (1H, s, NH).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 206 -
Example 192
5-(4-Phenylsulfamoylphenyl)pent-2-en-4-ynoic acid (56b)
0
0 40 0
OH
Using an analogous method, the title compound was obtained from E-5-(4-
phenylsulfamoylphenyl)pent-2-en-4-ynoic acid methyl ester (55b), yield 81%,
m.p. 234-236 C. 1H NMR (DMSO-d6, HMDSO) 8: 6.39 (1H, d, J=16.0 Hz, CH);
6.95 (1H, d, J=16.0 Hz, CH); 6.94-7.39 (5H, m, C6H6); 7.69 (2H, d, J=9.0 Hz,
C6H2); 7.83 (2H, d, J=9.0 Hz, C6H2); 10.36 (1H, s, NH), 12.77 (1H, br s, OH).
Example 193
E-5-(4-Phenylsulfamoylphenyl)pent-2-en-4-ynoic acid hydroxyamide (58b)
(PX117453)
N.,,
OH
Using an analogous method, the title compound was obtained from 5-(4-
phenylsulfamoylphenyl)pent-2-en-4-ynoic acid (56b) via (E)-544-
phenylsulfamoylpheny1]-2-penten-4-ynoyl chloride (57b), yield 59%, m.p. 161-
163 C. 1H NMR (DMSO-d6, HMDSO) 8:6.38 (1H, d, J=16.0 Hz, CH); 6.78 (1H, d,
J=16.0 Hz, CH); 6.89-7.43 (5H, m, 06H6); 7.67 (2H, d, J=9.0 Hz, 06H2); 7.78
(2H,
d, J=9.0 Hz, C6H2); 10.05 (3H, br s, NH, NH, OH). Anal. Calcd for C17H14N204S
0.25H20: C 58.86, H 4.21, N 8.08. Found: C 58.36, H 3.93, N 7.82.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 207 -
Example 194
Sodium 6-ethoxy-6-oxo-1-hexanesulfonate (62b)
OEt
Na0
0
To a solution of ethyl 6-bromohexanoate (61b) (2.48 g, 11.0 mmol) in ethanol
(6
ml) a solution of sodium sulfite (2.16 g, 20.6 mmol) in water (9 ml) was added
and
the resulting mixture was refluxed for 1 hour. The reaction mixture was
evaporated under reduced pressure and the obtained solid was extracted with
boiling ethanol in Soxhlet extraction apparatus for 15-20 hours. The extract
was
evaporated and the residue was crystallised from ethanol-diethyl ether (1:10)
giving the title compound (2.71 g, 99%) in a form of a white solid material.
1H
NMR (DMSO-d6, HMDSO), 6: 1.05-1.78 (6H, m); 1.17 (3H, t, J=7.2 Hz); 2.26 (4H,
t, J=7.5 Hz); 4.05 (2H, q, J=7.2 Hz).
Example 195
Ethyl 6-(chlorosulfonyl)hexanoate (63b)
0
OEt
CKs\\0
Sodium 6-ethoxy-6-oxo-1-hexanesulfonate (62b) (1.68 g, 6.8 mmol) was mixed
with phosphorus pentachloride and the mixture was carefully pestled in a
mortar.
After the reaction came to the end (the foaming of the reaction mixture
ceased)
the mixture was extracted with dry benzene (50 ml). The extract was evaporated
under reduced pressure and the residue was dried in vacuum to give crude title
compound (1.03 g, 61%) as a hygroscopic oil. The chloride (63b) was used in
further reactions without additional purification.
Example 196
Ethyl 6-(anilinosulfonyl)hexanoate (64b)
R\ OEt
N
H 0 0
CA 02765409 2012-01-19
WO 02/30879 PC T/G B01/04326
- 208 -
To a solution of ethyl 6-(chlorosulfonyl)hexanoate (63h) (0.5 g, 2.0 mmol) in
benzene (5 ml) aniline (0.8 g, 8.5 mmol) was added and the resulting solution
was
stirred at ambient temperature for 24 hours. The reaction mixture was
partitioned
between ethyl acetate and IN HCI. The organic layer was washed successively
with water, saturated NaCI, and dried (Na2SO4). The solvent was evaporated and
the residue was chroMatographed on silica gel with petroleum ether - tert-
butylmethyl ether (3:2, v/v) as eluent to give the title compound (0.45 g,
75%) as
an oil. 1H NMR (DMSO-d6, HMDSO), 5: 1.03-1.81 (6H, m); 1.15 (3H, t, J=7.1 Hz);
2.23 (2H, t, J=6.8 Hz); 3.07 (2H, t, J=7.6 Hz); 4.04 (2H, q, J=7.1 Hz); 7.00-
7.47
(5H, m); 9.76 (1H, s).
Example 197
6-(AnilinosulfonyI)-N-hydroxyhexanamide (67b) (PX117234)
,`\s=----\/\/\/N'-oH
N
HO 0
To a mixture of ethyl 6-(anilinosulfonyl)hexanoate (64b) and hydroxylamine
hydrochloride (0.43 g, 6.2 mmol) in methanol (5 ml) the 3.43 N solution of
sodium
methylate (2.62 ml, 9.0 mmol) in methanol was added and the reaction was
stirred
at ambient temperature for 40 min. The reaction mixture was poured into
saturated NaH2PO4 (15 ml) and extracted with ethyl acetate. The extract was
washed successively with water, saturated NaCI, and dried (Na2SO4). The
solvent
was evaporated, the residue was washed with diethyl ether and crystallised
from
ethyl acetate. The title compound (0.3 g, 69%) was obtained as white crystals,
m.p. 97-98 C. 1H NMR (DMSO-d6, HMDSO), 5: 1.02-1.78 (m, 6H, CH2); 1.90 (br t,
2H, J=6.4 Hz, CH2); 3.06 (t, 2H, J=7.0 Hz, CH2); 6.94-7.60 (m, 5H, arom.);
8.66
(br s, 1H, NH); 9.76 (br s, 1H, NH); 10.36 (br s, 1H, OH). HPLC analysis on
Symmetry C8 column: impurities 3.5% (column size 3.9 x 150 mm; mobile phase
acetonitrile ¨ 0.1M phosphate buffer (pH 2.5), 30:70; detector UV 220 nm; flow
rate 1.1 ml/min; sample concentration 0.4 mg/ml). Anal. Calcd for C12H18N204S,
%: 050.33, H 6.34, N 9.78, S 11.20. Found, %: C 50.10, H 6.22, N 9.83, S
11.10.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 209 -
Example 198
Sodium 5-ethoxy-5-oxo-1-pentanesulfonate (62a)
0
Na0,
0
0
Using an analogous method, the title compound was obtained from ethyl 5-
bromopentanoate (61a) and sodium sulfite in a form of white crystals, yield
98%.
1H NMR (DMSO-d6, HMDSO), 6: 1.17 (3H, t, J=7.0 Hz); 1.37-1.73 (4H, m); 2.12-
2.56 (4H, m, partially overlapped with a signal of DM30); 4.04 (2H, q, J=7.0
Hz).
Example 199
Ethyl 6-(chlorosulfonyl)pentanoate (63a)
0
CI., i/
0
o
Using an analogous method, the title compound was obtained from sodium 5-
ethoxy-5-oxo-1-pentanesulfonate (62a) and phosphorus pentachloride, ca. yield
of
the crude product 90% (hygroscopic oil).
Example 200
Ethyl 6-(anilinosulfonyl)pentanoate (64a)
H 0
N...
40 r0 Et
0
0
Using an analogous method, the title compound was obtained from ethyl 6-
(chlorosulfonyl)pentanoate (63a) and aniline as an oil, yield 38%. 1H NMR
(DMSO-d6, HMDSO), 6: 1.15 (3H, t, J=7.0 Hz); 1.48-1.81 (4H, m); 2.27 (2H, t,
J=6.2 Hz); 3.09 (2H, t, J=6.7 Hz); 4.04 (2H, q, J=7.0,Hz); 6.98-7.48 (5H, m);
9.78
I
(1H, s).
CA 02765409 2012-01-19
WO 02/30879 PCT/CB01/04326
=
- 210 -
Example 201
5-(AnilinosulfonyI)-N-hydroxypentanamide (67a) (PX117233)
/I
P
401 OH
0
Using an analogous method, the title compound was obtained from ethyl 6-
(anilinosulfonyl)pentanoate (64a) and hydroxylamine hydrochloride, yield 49%,
m.p. 128-129 C (from ethyl acetate). 1H NMR (DMSO-d6, HMDSO) 8: 1.37-1.78
(m, 4H, CH2); 1.92 (t, 2H, J=5.9 Hz, CH2); 3.07 (t, 2H, J=7.0 Hz, CH2); 6.97-
7.47
(m, 5H, C6H6); 8.69 (s, 1H, NH); 9.78 (s, 1H, NH); 10.33 (s, 1H, OH). HPLC
analysis on Symmetry C8 column: impurities 1.2 % (column size 3.9 x 150 mm;
mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5), 25:75; detector UV
220 nm; flow rate 1.2 ml/min; sample concentration 0.5 mg/ml). Anal. Calcd for
C11H16N204S, %: C 48.52, H 5.92, N 10.29, S 11.77. Found, %: C 48.57, H
5.92, N 10.21, S 11.65.
Example 202
Ethyl 5-[(2-naphthylamino)sulfonyl]pentanoate (64e)
H 0
N II
Using an analogous method, the title compound was obtained from ethyl
6-(chlorosulfonyl)pentanoate (63a) and 2-naphthylamine as brown crystals,
yield
20%. 1H NMR (DMSO-d6, HMDSO), 8: 1.11 (3H, t, J=7.1 Hz); 1.35-1.88 (4H, m);
2.25 (2H, t, J=6.2 Hz); 3.18 (2H, t, J=6.7 Hz); 3.99 (2H, q, J=7.1 Hz); 7.27-
7.97
(7H, m); 10.03 (1H, s).
Example 203
N-Hydroxy-5-[(2-naphthylamino)sulfonylipentanamide (67e) (PX117235)
H 0
N
110101 OH
0
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
-211 -
Using an analogous method, the title compound was obtained from ethyl 54(2-
naphthylamino)sulfonyl]pentanoate (64e) and hydroxylamine hydrochloride, yield
55%, m.p. 163-164 C (from ethyl acetate). 1H NMR (DMSO-d6, HMDSO) 6: 1.39-
1.78 (m, 4H, CH2); 1.93 (t, 2H, J=6.4 Hz, CH2); 3.16 (m, 2H, overlapped with a
H20 signal from DMSO-d6, CH2); 7.30-7.61 (m, 3H, arom.); 7.67 (1H, d, J=2.0
Hz,
arom.); 7.76-7.99 (m, 3H, arom.); 8.67 (br s, 1H, NH); 10.00 (br s, 1H, NH);
10.31
(br s, 1H, OH). HPLC analysis on Symmetry C18 column: impurities 1 % (column
size 3.9 x 150 mm; mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5),
35:65; detector UV 230 nm; flow rate 1.1 ml/min; sample concentration 0.5
mg/ml). Anal. Calcd for C161-118N204S, C 55.89, H 5.63, N 8.69, S 9.95.
Found,
%: C 55.83, H 5.52, N 8.68, S 9.95.
Example 204
Sodium 7-rnethoxy-7-oxo-1-heptanesulfonate (62c)
0
Na0,
OMe
0
Using an analogous method, the title compound was obtained from methyl 7-
bromoheptanoate (61c) and sodium sulfite as white crystals, yield 98%. 1H NMR
(DMSO-d6, HMDSO), 6: 1.05-1.76 (8H, m); 2.27 (4H, t, partially overlapped with
a
signal of DMSO, J=6.6 Hz); 3.58 (3H, s).
Example 205
Methyl 7-(chlorosulfonyl)heptanoate (63c)
OMe
0
0
Using an analogous method, the title compound was obtained from sodium 7-
methoxy-7-oxo-1-heptanesulfonate (62c) and phosphorus pentachloride, ca. yield
of the crude product 73% (hygroscopic oil).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/0-1326
- 212 -
Example 206
Methyl 7-(anilinosulfonyl)heptanoate (64c)
H 0
N,
40 1 OMe
0
Using an analogous method, the title compound was obtained from methyl 7-
(chlorosulfonyl)heptanoate (63c) and aniline as an oil, yield 53%. 1H NMR
(DMSO-d6, HMDSO), 8: 1.05-1.83 (8H, m); 2.24 (2H, t, J=6.8 Hz); 3.06 (2H, t,
J=7.4 Hz); 3.57 (3H, s); 6.97-7.45 (5H, m); 9.76 (1H, s).
Example 207
5-(Anilinosulfony1)-N-hydroxyheptanamide (67c) (PX117236)
H 0
N,
140
OS N,
OH
Using an analogous method, the title compound was obtained from methyl 7-
(anilinosulfonyl)heptanoate (64c) and hydroxylamine hydrochloride, yield 74%,
m.p. 94-95 C (from ethyl acetate). 1H NMR (DMSO-d6, HMDSO) 8: 1.07-1.51 (m,
6H, CH2); 1.53-1.73 (m, 2H, CH2); 1.89 (t, 2H, J=7.2 Hz, CH2); 3.04 (t, 2H,
J=7.6
Hz, CH2); 7.03-7.40 (m, 5H, C6I-16); 8.67 (s, 1H, NH); 9.78 (s, 1H, NH); 10.33
(s,
11-1, OH). HPLC analysis on Symmetry C8 column: impurities 3.5% (column size
3.9 x 150 mm; mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5),
35:65;
detector UV 220 nm; flow rate 0.9 ml/min; sample concentration 0.3 mg/ml).
Anal.
Calcd for C13H201\1204S, /0: C 51.98, H 6.71, N 9.33, S 10.67. Found, /0:
051.83,
H 6.64, N 9.23, S 10.65.
Example 208
Sodium 8-methoxy-8-oxo-1-octanesulfonate (62d)
OMe
,S
Na0
0 0
Using an analogous method, the title compound was obtained from methyl 8-
bromooctanoate (61d) and sodium sulfite as white crystals, yield 98%. 1H NMR
CA 02765409 2012-01-19
WO 02/30879 PCT/CB01/04326
- 213 -
(DMSO-d6, HMDSO), 6: 1.00-1.75 (10H, m); 2.28 (4H, t, partially overlapped
with
a signal of DMSO, J=7.8 Hz); 3.58 (3H, s).
Example 209
Methyl 8-(chlorosulfonyl)octanoate (63d)
0,
OMe
CI \\
0
Using an analogous method, the title compound was obtained from sodium 8-
methoxy-8-oxo-1-octanesulfonate (62d) and phosphorus pentachloride, ca. yield
of the crude product 73% (hygroscopic oil).
Example 210
Methyl 8-(anilinosulfonyl)octanoate (64d)
=R\ OMe
N
H 0
Using an analogous method, the title compound was obtained from methyl 8-
(chlorosulfonyl)octanoate (63d) and aniline as an oil, yield 54%. 1H NMR (DMSO-
d6, HMDSO), 6:1.01-1.80 (10H, m); 2.25 (2H, t, J=6.9 Hz); 3.06 (2H, t, J=7.5
Hz);
3.57 (3H, s); 6.99-7.46 (5H, m); 9.75 (1H, s).
Example 211
5-(AnilinosulfonyI)-N-hydroxyoctanamide (67d) (PX117245)
R\ N,OH
N
H 0
Using an analogous method, the title compound was obtained from methyl 8-
(anilinosulfonyl)octanoate (64d) and hydroxylamine hydrochloride, yield 76%,
m.p.
87-88 C (from ethyl acetate). 1H NMR (DMSO-d6, HMDSO) 6:1.08-1.51 (m, 8H,
CH2); 1.52-1.73 (m, 2H, CH2); 1.90 (t, 2H, J=7.2 Hz, CH2); 3.05 (t, 2H, J=7.6
Hz,
CH2); 7.02-7.39 (m, 5H, C6H6); 8.66 (s, 1H, NH); 9.74 (s, 1H, NH); 10.32 (s,
1H,
OH). HPLC analysis on Symmetry 08 column: impurities 3% (column size 3.9 x
CA 02765409 2012-01-19
WO 02/30879 PC T/G
B01/04326
=
-214-
150 mm; mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5), 35:65;
detector UV 220 nm; flow rate 1.1 ml/min; sample concentration 0.4 mg/ml).
Anal.
Calcd for C14H22N204S, %: C 53.48, H 7.05, N 8.91, S 10.20. Found, %: C 53.23,
H 7.05, N 8.82, S 10.25.
Example 212
Methyl 7-Rmethylanilino)sulfonypeptanoate (65c)
Me
I 0
= OMe
0
0
Using an analogous method, the title compound was obtained from methyl
7-(chlorosulfonyl)heptanoate (63c) and N-methylaniline as white crystals,
yield
70%. 1H NMR (DMSO-d6, HMDSO), 8: 1.10-1.77 (8H, m); 2.26 (2H, t, J=6.8 Hz);
3.11 (2H, t, J=7.4 Hz); 3.25 (3H, s); 3.57 (3H, s); 7.24-7.51 (5H, m).
Example 213
N-Hydroxy-7-[(methylanilino)sulfonyl]heptanamide (68c) (PX117260)
Me
I Ni?
0 N,OH
0
Using an analogous method, the title compound was obtained from methyl 7-
(methylanilinosulfonyl)heptanoate (65c) and hydroxyla mine hydrochloride,
yield
59%, m.p. 69-70 C (from ethyl acetate). 1H NMR (DMSO-d6, HMDSO) 8: 1.11-
1.70 (m, 8H, CH2); 1.91 (t, 2H, J=7.2 Hz, CH2); 3.09 (t, 2H, J=7.7 Hz, CH2);
3.25
(s, 3H, CH3); 7.21-7.45 (m, 5H, C6I-15); 8.65 (br s, 1H, NH); 10.32 (s, 1H,
OH).
HPLC analysis on Symmetry C18 column: impurities <1% (column size 3.9 x 150
mm; mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5), 30:70;
detector
UV 220 nm; flow rate 1.1 ml/min; sample concentration 0.5 mg/ml). Anal. Calcd
for C14H22N204S, %: C 53.48, H 7.05, N 8.91, S 10.20. Found, (Y0: C 53.44, H
7.05, N 8.86, S 10.13.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 215 -
Example 214
Ethyl 6-[(methylanilino)sulfonyl]hexanoate (65b)
=c)\\ OEt
N
0 0
Me
Using an analogous method, the title compound was obtained from ethyl 6-
(chlorosulfonyl)hexanoate (63b) and N-methylaniline as an oil, yield 43%. 1H
NMR
(DMSO-d6, HMDSO), 5: 1.10-1.77 (8H, m); 2.26 (2H, t, J=6.8 Hz); 3.11 (2H, t,
J=7.4 Hz); 3.25 (3H, s); 3.57 (3H, s); 7.24-7.51 (5H, m).
Example 215
N-Hydroxy-6-Rmethylanilino)sulfonyl]hexanamide (68b) (PX117410)
=
N,
OH
N
0 0
Me
Using an analogous method, the title compound was obtained from ethyl 6-
(methylanilinosulfonyl)hexanoate (65b) and hydroxylamine hydrochloride, yield
40%, m.p. 121-122 C (from ethyl acetate). 1H NMR (DMSO-d6, HMDSO) 8: 1.13-
1.72 (m, 6H, CH2); 1.91 (t, 2H, J=7.0 Hz, CH2); 3.09 (t, 2H, J=7.6 Hz, CH2);
3.25
(s, 3H, CH3); 7.22-7.46 (m, 5H, C6H6); 8.68 (s, 1H, NH); 10.35 (s, 1H, OH).
HPLC
analysis on Zorbax SB-C18 column: impurities ¨6% (column size 4.6 x 150 mm;
mobile phase methanol ¨ 0.1% H3PO4, gradient from 50:50 to 90:10; detector UV
230 nm; flow rate 1.5 ml/min; sample concentration 0.5 mg/ml). Anal. Calcd for
C13H20N204S, %: C 51.98, H 6.71, N 9.33, S 10.67. Found, %: C 51.76, H 6.63, N
9.29, S 10.63.
Example 216
Methyl 8-Rmethylanilino)sulfonyl]octanoate (65d)
= \\s OMe
N
0 0
Me
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 216 -
Using an analogous method, the title compound was obtained from methyl 8-
(chlorosulfonyl)octanoate (63d) and N-methylaniline as an oil, yield 68%. 1H
NMR
(DMSO-d6, HMDSO), 6: 1.05-1.76 (10H, m); 2.27 (2H, t, J=7.0 Hz); 3.11 (2H, t,
J=7.3 Hz); 3.25 (3H, s); 3.57 (3H, s); 7.23-7.51 (5H, m).
Example 217
N-Hydroxy-8-[(methylanilino)sulfonyl]octanamide (68d) (PX117411)
11101 c)\\
N,
N7S OH
1 0
Me
Using an analogous method, the title compound was obtained from methyl 8-
(methylanilinosulfonyl)octanoate (65d) and hydroxylamine hydrochloride, yield
66%, m.p. 65.5-66.5 C (from ethyl acetate). 1H NMR (DMSO-d6, HMDSO) 6: 1.06-
1.72 (m, 10H, CH2); 1.91 (t, 2H, J=7.2 Hz, CH2); 3.09 (t, 2H, J=7.6 Hz, CH2);
3.25
(s, 3H, CH3); 7.21-7.50 (m, 5H, C6I-15); 8.64 (s, 1H, NH); 10.31 (s, 1H, OH).
HPLC
analysis on Zorbax SB-C18 column: impurities -6% (column size 4.6 x 150 mm;
mobile phase methanol - 0.1% H3PO4, gradient from 50:50 to 90:10; detector UV
230 nm; flow rate 1.5 ml/min; sample concentration 0.7 mg/ml). Anal. Calcd for
C161-124N204S, %: C 54.86, H 7.37, N 8.53, S 9.76. Found, %: C 54.68, H 7.30,
N
8.55, S 9.70.
Example 218
Ethyl 6-1(benzylanilino)sulfonylThexanoate (66b)
SI
7S
N
0 0
To a cold solution (ice bath) of ethyl 6-(anilinosulfonyl)hexanoate (64b)
(0.86 g,
2.88 mmol) in 1,2-dimethoxyethane (5 ml) a 60% suspension of sodium hydride in
mineral oil (0.12 g, 3.0 mmol) and a solution of benzylbromide (0.49 g, 2.88
mmol) in 1,2-dimethoxyethane (3 ml) were added, and the resulting solution was
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 217 -
stirred at ambient temperature for 24 hours. The reaction mixture was poured
into
water and the resulting mixture was extracted with ethyl acetate. The organic
layer
was washed successively with water, saturated NaCI, and dried (Na2SO4). The
solvent was evaporated and the residue was chromatographed on silica gel with
petroleum ether - tert-butylmethyl ether (3:2, v/v) as eluent to give the
title
compound (0.56 g, 50%) as an oil. 1H NMR (DMSO-d6, HMDSO), 5: 1.16 (3H, t,
J=7.0 Hz); 1.21-1.87 (6H, m); 2.27 (2H, t, J=6.6 Hz); 3.21 (2H, t, partially
overlapped with a signal of H20, J=7.6 Hz); 4.05 (2H, q, J=7.0 Hz); 4.89 (2H,
s);
7.14-7.58 (10H, m).
Example 219
6-[(Benzylanilino)sulfony1]-N-hydroxyhexanamide (69b) (PX117414)
=
0õ
N,
,S OH
N
0 0
Using an analogous method, the title compound was obtained from ethyl 6-
[(benzylanilino)sulfonyl]hexanoate (66b) and hydroxylamine hydrochloride,
yield
93%, m.p. 129-129.5 C (from ethyl acetate). 1H NMR (DMSO-d6, HMDSO) 6:
1.11-1.56 (m, 4H, CH2); 1.61-1.79 (m, 2H, CH2); 1.93 (t, 2H, J=7.2 Hz, CH2);
3.19
(t, 2H, J=7.5 Hz, CH2); 4.89 (s, 2H, CH2Ph); 7.16-7.41 (m, 10H, 2C6H6); 8.67
(s,
1H, NH); 10.36 (s, 1H, OH). HPLC analysis on Symmetry 018 column: impurities
3% (column size 3.9 x 150 mm; acetonitrile ¨ 0.1M phosphate buffer (pH 2.5),
40:60; detector UV 220 nm; flow rate 1.2 ml/min; sample concentration 0.5
mg/ml). Anal. Calcd for C19H24N204S, %: C 60.62, H 6.43, N 7.44, S 8.52.
Found,
%: C 60.37, H 6.35, N 7.45, S 8.46.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 218 -
Example 220
Methyl 84(benzylanilino)sulfonylloctanoate (66d)
(1101 OMe
N
Using an analogous method, the title compound was obtained from methyl 8-
(anilinosulfonyl)octanoate (64d), 60% suspension of sodium hydride in mineral
oil,
and benzylamine as white crystals, yield 23%. 1H NMR (DMSO-d6, HMDS0), 6:
1.06-1.87 (10H, m); 2.28 (2H, t, J=6.8 Hz); 3.21 (2H, t, J=7.8 Hz); 3.58 (3H,
s);
4.89 (2H, s); 7.14-7.45 (10H, m).
Example 221
8-[(Benzylanilino)sulfonyll-N-hydroxyoctanamide (69d) (PX117412)
,S N OH
0 0
Using an analogous method, the title compound was obtained from methyl 8-
[(benzylanilino)sulfonyljoctanoate (66d) and hydroxylamine hydrochloride,
yield
83%, m.p. 119-119.5 C (from ethyl acetate). 1H NMR (DMSO-d6, HMDSO) 5:
1.11-1.57 (m, 8H, CH2); 1.60-1.81 (m, 2H, CH2); 1.93 (t, 2H, J=7.2 Hz, CH2);
3.20
(t, 2H, J=7.5 Hz, CH2); 4.89 (s, 2H, CH2Ph); 7.17-7.41 (m, 10H, 2C6H5); 8.67
(s,
1H, NH); 10.34 (s, 1H, OH). HPLC analysis on Symmetry C8 column: impurities
5.6% (column size 3.9 x 150 mm; acetonitrile ¨ 0.1M phosphate buffer (pH 2.5),
50:50; detector UV 220 nm; flow rate 1.3 ml/min; sample concentration 0.5
mg/ml). Anal. Calcd for C211-1281\1204S * 0.25 H20, /0: C 61.67, H 7.02, N
6.85, S
7.84. Found, Yo: C 61.50, H 6.87, N 6.85, S 7.89.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 219 -
Example 222
3-(4-Nitro-phenyl)-acrylic acid methyl ester (72)
0
OMe
02N
Thionyl chloride (28.8 ml, 0.4 mol) was added dropwise to methanol (450 ml) at
-
10 C temperature. To the obtained solution was added 3-(4-nitrophenyI)-acrylic
acid (71) (38.63 g, 0.2 mol) and the reaction mixture was stirred at 0 C for 3
hours, at ambient temperature for 24 hours and at 40 C for 1 hour. The
resulting
precipitate was filtered, washed with methanol (2 x 10 ml) and dried affording
the
title compound in a form of yellow crystals (39.55 g, 96%). 1H NMR (DMSO-d6,
HMDSO), 6:3.69 (2H, br s); 3.77 (3H, s); 6.87 (1H, d, J=16.0 Hz); 7.67-8.39
(5H,
m).
Example 223
3-(4-Amino-phenyl)-acrylic acid methyl ester (73)
OMe
HN
2
A mixture of 3-(4-nitro-phenyl)-acrylic acid methyl ester (72) (39.54 g, 0.191
mol)
and SnC12=2H20 (220 g, 0.98 mol) in anhydrous ethanol (300 ml) was heated at
50 C for 1 hour and at 75 C for 1 hour. The reaction mixture was allowed to
cool
to 10 C, treated with 20% NaOH solution to pH 8-9, and extracted with ethyl
acetate (3 x 200 m1). The organic extract was washed with saturated NaCl (3 x
150 ml), dried (MgSO4), and evaporated under reduced pressure.
Recrystallization from isopropanol (180 ml) afforded pure title compound in a
form
of yellowish crystals (17.938 g, 53%). 1H NMR (DMSO-d6, HMDSO), 8: 3.64 (3H,
s); 5.73 (2H, s); 6.22 (1H, d, J=16.0 Hz); 6.57 (2H, d, J=8.0 Hz); 7.38 (2H,
d,
J=8.0 Hz); 7.50 (1H, d, J=16.0 Hz).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 220 -
Example 224
3-(4-Benzenesulfonylamino-phenyl)-acrylic acid methyl ester (74)
0
OMe
8,
Si OH
To a suspension of 3-(4-amino-phenyl)-acrylic acid methyl ester (73) (1.740 g,
6.18 mmol) in methylene chloride (10 ml) benzenesulfonyl chloride (1.094 g,
6.20
mmol) and pyridine (0.563 g,.7.00 mmol) were added. The resulting suspension
was stirred at 15 C for 24 hours and filtrated. The precipitate was washed
with
methylene chloride (10 ml), NaHCO3 solution (10 ml), and water (2 x 20 ml).
The
obtained solid was dried to give the title compound (1.962 g, 75%). 1H NMR
(DMSO-d6, HMDSO), 5: 3.71 (3H, s); 6.51 (1H, d, J=16.0 Hz); 7.57-8.11 (10H,
m);
10.59 (1H, s).
Example 225
3-(4-Benzenesulfonylaminopheny1)-N-hydroxyacrylamide (75) (PX106499)
0
o
II H
OH
To a mixture, consisting of dioxane (25 ml), methanol (3 ml), and water (1
ml),
hydroxylamine hydrochloride (0.834 g, 12 mmol) and NaOH (0.960 g, 24 mmol)
followed by 3-(4-benzenesulfonylamino-phenyl)-acrylic acid methyl ester (74)
(1.735 g, 4.1 mmol)) were added. The resulting mixture was vigorously stirred
at
ambient temperature for 24 hours and evaporated under reduced pressure. The
residue was mixed with warm (50 C) water and filtered. The aqueous solution
was
acidified with hydrochloric acid to pH 4 and filtered. The precipitate was
washed
with water (2 x 10 ml), ethyl acetate (10 ml), and crystallised from
acetonitrile (15
ml) to give title compound as a yellow solid (0.405 g, 31%). M.p. 189-191 C.
1H
NMR (DMSO-d6, HMDSO) 5: 6.30 (d, 1H, J=15.8 Hz); 7.12 (d, 2H, J=8.6 Hz); 7.32
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 221 -
(d, 1H, J=15.8 Hz); 7.45 (d, 2H, J=8.4 Hz); 7.48-7.86 (6H, m); 9.01 (s, 1H);
10.56
(s, 1H); 10.72 (s, 1H). HPLC analysis on Zorbax SB-C18 column: impurities 1.8%
(column size 4.6x150 mm; mobile phase acetonitrile - 0.1% H3PO4, gradient from
30:70 to 100:0; sample concentration 1.0 mg/mi; detector UV 220 nm). Anal.
Calcd for C16H14N204S, C 56.59, H 4.43,
N 8.80, S 10.07. Found, %: C 56.03,
H 4.24, N 8.66, S 10.02.
Example 226
344-(Biphenyl-4-sulfonylamino)-phenyl}-acrylic acid (82a)
111
µs' 401
OH
0
410
To a solution of 3-(4-aminophenyl)-acrylic acid hydrochloride (81) (0.3 g, 1.5
mmol) in dioxane (10 ml) and 0.63 M NaHCO3 (9.56 ml, 6.0 mmol) biphenyl-4-
sulfochloride (0.5 g, 1.78 mmol) was added and the resulting mixture was
stirred
at room temperature for 60 min. The reaction mixture was partitioned between
ethyl acetate and 2N NCI. The organic layer was washed successively with
water,
saturated NaCl, and dried (Na2SO4). The solvent was evaporated and the residue
was crystallised from acetonitrile to give the title compound (0.27 g, 47%).
1H
NMR (DMSO-d6, HMDSO), 8: 6.37 (1H, d, J=16.0 Hz); 7.19 (2H, d, J=8.0 Hz);
7.36-7.80 (8H, m); 7.86 (5H, m); 10.66 (1H, br s).
Example 227
3-14-(Biphenyl-4-sulfonylamino)-phenyl)-acryloyl chloride (83a)
OH
S\\0 140
./ CI
0
To a suspension of 3-[4-(biphenyl-4-sulfonylamino)-phenyl]-acrylic acid (82a)
(0.27 g, 0.71 mmol) in dichloromethane (3 ml) oxalyl chloride (0.3 ml, 3.39
mmol)
and one drop of dimethylformamide were added. The reaction mixture was stirred
CA 02765409 2012-01-19
WO 02/30879
PCT/GB01/04326
- 222 -
at 40 C for one hour and concentrated under reduced pressure to give crude
title
compound (0.277 g, 98%).
Example 228
(E)-N-Hydroxy-344-(4-biphenylsulfonylamino)-phenyl]-2-propenamide (84a)
(PX117793)
OH
\\A
s% 1110
0 OH
To a suspension of hydroxylamine hydrochloride (0.27 g, 3.88 mmol) in
tetrahydrofuran (5 ml) saturated NaHCO3 solution (3 ml) was added and the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a solution of crude 343-(3-methoxy-phenylsulfamoy1)-phenyl]-acryloyl
chloride (83a) (0.27 g, 0.68 mmol) in tetrahydrofuran (3.5 ml) was added and
the
mixture was stirred at ambient temperature for one hour. The reaction mixture
was partitioned between ethyl acetate and 2N HCI. The organic layer was washed
successively with water and saturated NaCI, then the solvent was removed. The
residue was crystallised from acetonitrile and washed with diethyl ether
affording
the title compound as a white solid (0.1 g, 37%). M.p. 190-191.5 C. 1H NMR
(DMSO-d6, HMDSO) 5: 6.29 (1H, d, J=16.0 Hz); 7.16 (2H, d, J=8.0 Hz); 7.24-7.78
(8H, m); 7.86 (4H, m); 8.94 (1H, br s); 10.57 (1H, s); 10.66 (1H, br s). HPLC
analysis on Zorbax SB-C18 column: impurities 4% (column size 4.6x150 mm;
mobile phase acetonitrile ¨0.1% H3PO4, gradient from 30 to 100%; sample
concentration 0.2 mg/ml; flow rate 1.0 ml/ min; detector UV 254 nm). Anal.
Calcd
for C21H18N2048, C 63.94, H 4.60, N 7.10. Found, %: C C 63.64, H 4.45, N
7.00.
CA 02765409 2012-01-19
WO 02/30879 PCPCB01/04326
- 223 -
Example 229
344-(3,4-Dimethoxy-benzenesulfonylamino)-phenylFacrylic acid (82b)
OH
,N
Me0 S11101
401 \0 OH
Me0
Using an analogous method, the title compound was obtained from 3-(4-
aminophenyI)-acrylic acid hydrochloride (81) and 3,4-dimethoxybenzenesulfonyl
chloride as a white solid, yield 56%. 1H NMR (DMSO-d6, HMDSO), 6: 3.74 (3H,
s);
3.77 (3H, s); 6.34 (1H, d, J=16.0 Hz); 6.94-7.69 (8H, m); 10.36 (1H, br s).
Example 230
3-[4-(3,4-Dimethoxy-benzenesulfonylamino)-phenyl]-acryloyl chloride (83b)
0
,N
Me0 S \
\O CI
Me0
Using an analogous method, the title compound was obtained from 344-(3,4-
dimethoxy-benzenesulfonylamino)-phenylFacrylic acid (82b) and oxalyl chloride,
yield of the crude product ca. 76%.
Example 231
(E)-N-Hydroxy-344-(3,4-dimethoxyphenylsulfonylamino)-phenyl]-2-propenamide
(84b) (PX117794)
11
\\ A 401
Me0 401 S\\
0 N,
Me0 OH
0
Using an analogous method, the title compound was obtained from 3-[4-(3,4-
dimethoxy-benzenesulfonylamino)-phenyTacryloyl chloride (83b) and
hydroxylamine hydrochloride, yield 35%. M.p. 178.5-179 C. 1H NMR (DMSO-d6,
HMDSO), 8: 3.72 (3H, s); 3.78 (3H, 5); 6.32 (1H, d, J=16.0 Hz); 7.00-7.65 (8H,
m);
8.98 (1H, br s); 10.32 (1H, br s); 10.69 (1H, s). HPLC analysis on Zorbax SB-
C18
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 224 -
column: impurities 3.5% (column size 4.6x150 mm; mobile phase acetonitrile ¨
0,1
M phosphate buffer (pH 2.5), 25:75; sample concentration 0.5 mg/ml; flow rate
1.0
ml/ min; detector UV 254 nm). Anal. Calcd for C17H18N206S, cYo: C 53.96, H
4.79,
N 7.40. Found, %: C 53.58, H 4.56, N 7.62.
Example 232
6-Benzenesulfonylaminohexanoic acid methyl ester (93a)
0
OMe
(11101
0H
0
Benzenesulfonyl chloride (92a) (0.88 g, 5.0 mmol) was added to the mixture of
methyl 6-aminohexanoate hydrochloride (91) (1.82 g, 10 mmol) in acetonitrile
(10
ml) and sodium carbonate (2.6 g, 24.6 mmol) in water (10 m1). The mixture was
stirred for 6 hours at ambient temperature, and the product was extracted with
ethyl acetate (30 ml). The extract was dried (Na2SO4) and solvents were
removed
under reduced pressure. The product was chromatographed on silica gel with
ethyl acetate ¨ hexane (1:2) as eluent. The title compound was obtained as oil
(1.28 g, 90%). 1H NMR 6H. (90 MHz, DMSO-d6) 6:0.90-1.63 (6H, m, CH2); 2.21
(2H, t, J=7.0 Hz, CH2); 2.71 (2H, q, J=6.0 Hz, CH2N); 3.58 (3H, s, CH3); 7.40-
7.72
(3H, m, C6I-13); 7.72-7.89 (2H, m, C6I-12).
Example 233
6-Benzenesulfonylaminohexanoic acid hydroxyamide (94a) (PX106522)
0
N,
te 811 OH
0
By an analogous method, the title compound was obtained from 6-benzene-
sulfonylaminohexanoic acid methyl ester (93a). Yield 47%, m.p. 80-82 C. 1H
NMR 8H (90 MHz, DMSO-d6) 8: 0.98-1.58 (6H, m, CH2); 1.87 (2H, t, J=7.5 Hz,
CH2); 2.69 (2H, q, J=6.0 Hz, CH2N); 7.38-7.69 (4H, m, C6H3, NH); 7.69-7.87
(2H,
m, C6H2); 8.58 (1H, s, NH), 10.27 (1H, s, OH). HPLC analysis on Symmetry C18
column: impurities <1 A (column size 3.9 x 150 mm; mobile phase acetonitrile
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 225 -
0.1M phosphate buffer (pH 2.5), 25:75; detector UV 220 nm; sample
concentration 1.0 mg/ml). Anal. Calcd for C12H18N204S: C 50.33, H 6.34, N
9.78.
Found: C 50.48, H 6.25, N 9.69.
Example 234
6-(E-2-Phenylethenesulfonylamino)hexanoic acid methyl ester (93b)
411 OMe
N
0 H
By an analogous method, the title compound was obtained from 2-phenyl-
ethenesulfonyl chloride (92b) and and methyl 6-aminohexanoate hydrochloride
(91) by the method of example 2, yield 56%, m.p. 47-49 C. 1H NMR ohl. (90 MHz,
DMSO-d6) 5: 0.98-1.66 (6H, m, CH2); 1.91 (2H, t, J=6.5 Hz, CH2); 2.83 (2H, t,
J=6.0 Hz, CH2); 3.59 (3H, s, CH3); 7.14 (1H, d, J=16.0 Hz, CH); 7.33 (1H, d,
J=16.0 Hz, CH); 7.33-7.89 (5H, m, C6H6).
Example 235
6-(2-Phenylethenesulfonylamino)hexanoic acid hydroxyamide (94b) (PX117429)
/P
S,
N,
N OH
0 H
0
By an analogous method, the title compound was obtained from 6-(E-2-
phenylethenesulfonylamino)hexanoic acid methyl ester (93b). Yield 62%, m.p.
107-109 C. 1H NMR 01-1(90 MHz, DMSO-d6) 5: 1.03-1.670 (6H, m, CH2); 2.25
(2H, t, J=6.6 Hz, CH2); 2.86 (2H, t, J=6.5 Hz, CH2); 7.13 (1H, d, J=16.0 Hz,
CH);
7.36 (1H, d, J=16.0 Hz, CH); 7.36-7.87 (5H, m, C6H6); 8.38-9.43 (3H, br s, NH,
NH, OH). HPLC analysis on Symmetry 018 column: impurities <1 % (column size
3.9 x 150 mm; mobile phase acetonitrile ¨ 0.1M phosphate buffer (pH 2.5),
30:70;
detector UV 230 nm; sample concentration 0.11 mg/ml). Anal. Calcd for
C14H20N204S: C 53.83, H 6.45, N 8.97. Found: C 53.30, H 6.32, N 8.53.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 226 -
Example 236
6-(Pyridine-3-sulfonylamino)hexanoic acid methyl ester (93c)
n 0
OMe
N
0 H
0
Pyridine-3-sulfonyl chloride hydrochloride (92c) (1.8 g, 5.0 mmol) was added
to a
solution of methyl 6-aminohexanoate hydrochloride (91) (1.82 g, 10 mmol) and
triethylamine (3.03 g, 30 mmol) in acetonitrile (30 ml). The mixture was
stirred for
1 hour at ambient temperature, filtered and solvents were removed under
reduced
pressure. The oily product was dissolved in water (15 ml) and extracted with
ethyl
ether (50 m1). The extract was dried (Na2SO4) and the solvents were removed
under reduced pressure. The title compound (1.09 g, 76%) was obtained as oil
and was used for the next step without an additional purification. 1H NMR 6FI.
(90
MHz, DMSO-d6) 5: 0.80-1.51 (6H, m, CH2); 1.83 (2H, t, J=6.5 Hz, CH2); 2.76
(2H,
t, J=6.5 Hz, CH2N); 3.58 (3H, s, CH3); 7.54 (1H, dd, J=5.0 Hz, J=8.2 Hz, C61-
1N);
8.12 (1H, dt, J=2.0 Hz, J=8.2 Hz, C61-1N); 8.61 (1H, dd, J=2.0 Hz, J=5.0 Hz,
C61-1N); 8.81 (1H, d, J=2.0 Hz, C6HN).
Example 237
6-(Pyridine-3-sulfonylamino)hexanoic acid hydroxyamide oxalate (94c)
(PX117432)
0
N,OH
N
0 H
A solution of sodium methylate (12 mmol) in methanol (10 ml) was added to a
solution of hydroxylamine hydrochloride (0.56 g, 8 mmol) in methanol (16 m1).
The
mixture was stirred for 10 min, and NaCl was filtered off. 6-(Pyridine-3-
sulfonylamino)hexanoic acid methyl ester (93c) (0.58 g, 2 mmol) was added to
the
filtrate and the mixture was left to stand overnight at ambient temperature.
The
precipitate was filtered off, dissolved in water (20 ml) and oxalic acid (0.36
g, 4
mmol) was added to the solution. Water was removed under reduced pressure
CA 027 65409 2012-01-19
WO 02/30879 PCT/G B01/04326
=
- 227 -
and the product was crystallised from methanol. The title compound (0.33 g,
44%) was obtained as white solid. M.p. 132-134 C. 1H NMR oF1. (90 MHz, DMSO-
d6) 8: 0.78-1.49 (6H, m, CH2); 1.83 (2H, t, J=6.5 Hz, CH2); 2.76 (2H, t, J=6.5
Hz,
CH2N); 7.54 (1H, dd, J=5.0 Hz, J=8.2 Hz, C61-IN); 8.12 (1H, dt, J=2.0 Hz,
J=8.2
Hz, C6F1N); 8.61 (1H, dd, J=2.0 Hz, J=5.0 Hz, C61-1N); 8.81 (1H, d, J=2.0 Hz,
C6FIN). HPLC analysis on Symmetry C18 column: impurities <1 % (column size 3.9
x 150 mm; mobile phase acetonitrile - 0.1M phosphate buffer (pH 2.5), 5:95;
detector UV 254 nm; sample concentration 1.0 mg/ml). Anal. Calcd for
C11H17N304S * (COOH)2: 041.38, H 5.07, N 11.13. Found: C 41.53, H 5.10, N
19.83.
Example 238
3-13-[(Benzo[1,31dioxol-5-ylmethyl)-sulfamoyll-pheny1}-acrylic acid methyl
ester
io
OMe
N
H 0
A solution of 3-(3-chlorosulfonylphenyl)acrylic acid methyl ester (0.4 g, 1.53
mmol)
in dioxane (5 ml) was added to a mixture of piperonylamine (0.23 g, 1.52 mmol)
in
dioxane (1 ml) and NaHCO3 (0.25 g, 3.06 mmol) in water (3 ml), and the
resultant solution was stirred at room temperature until the completion of the
reaction (control by TLC). The reaction mixture was evaporated and the residue
was partitioned between ethyl acetate and 2N HCI. The organic layer was washed
successively with water, saturated NaCI, and dried (Na2SO4). The solvent was
removed and the residue was chromatographed on silica gel with petroleum ether-
ethyl acetate (2:1, v/v) as eluent. The obtained product was washed with
diethyl
ether to give the title compound (0.47 g, 81%) as a white solid. 1H NMR (DMS0-
d6, HMDSO), 6: 3.72 (3H, s); 3.96 (2H, d, J=6.4 Hz); 5.94 (2H, s); 6.66-6.85
(3H,
m); 6.71 (1H, d, J=16.4 Hz); 7.49-8.07 (5H, m); 8.14 (1H, br t, J=6.4 Hz).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 228 -
Example 239
3-{3-[(Benzo[1,3]dioxo1-5-ylmethyl)-sulfamoylj-phenyl}-acrylic acid
0
\\ OH
<0
N
H 0
0 el
=
To a suspension of 3-{3-Rbenzo[1,3]dioxol-5-ylmethyl)-sulfamoyli-phenyl}-
acrylic
acid methyl ester (0.47 g, 1.25 mmol) in methanol (6 ml) 1N NaOH solution
(3.75
ml, 3.75 mmol) was added and the resultant mixture was stirred at ambient
temperature overnight. The reaction mixture was partitioned between ethyl
acetate and water. The aqueous layer was acidified with 2N HCI solution and
stirred for 30 min. The precipitated solid was filtered, washed with water and
dried
in desiccator over P205. The title compound was obtained as a white solid
(0.39 g,
87%).
Example 240
3-{3-[(Benzo[1,3]clioxol-5-ylmethyl)-sulfamoyl]-phenyl}-acryloyl chloride
\\ CI
(o
0
H 0 0
To a suspension of 3-{3-Rbenzo[1,3]dioxol-5-ylmethyl)-sulfamoy1Fphenylyacrylic
acid (0.39 g, 1.08 mmol) in dichloromethane (4 ml) oxalyl chloride (0.28 ml,
3.24
mmol) and one drop of dimethylformamide were added. The reaction mixture was
stirred at 40 C for one hour and concentrated under reduced pressure to give
crude title compound (0.41 g, quant.).
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
=
- 229 -
Example 241
343-[(Benzo[1,3]clioxol-5-ylmethyl)-sulfamoy1)-phenyl}-N-hydroxy-acrylamide
(PX117226)
\\ N,
OH
o 0
=
To a suspension of hydroxylamine hydrochloride (0.37 g, 5.40 mmol) in
tetrahydrofuran (6 ml) a saturated NaHCO3 solution (4.5 ml) was added and the
resultant mixture was stirred at ambient temperature for 10 min. To the
reaction
mixture a solution of crude 3-{3-[(benzo[1,31clioxol-5-ylmethyl)-sulfamoyll-
pheny1)-
acryloyl chloride (0.41 g) in tetrahydrofuran (4 ml) was added and the mixture
was stirred at ambient temperature for one hour. The reaction mixture was
partitioned between ethyl acetate and 2N HCI. The organic layer was washed
successively with water and saturated NaCI, then the solvent was removed. The
residue was crystallised from ethyl acetate and washed with diethyl ether
affording
the title compound (0.14 g, 35%). M.p. 163 C. 1H NMR (DMSO-d6, HMDSO) 5:
3.92 (2H, d, J= 6.4 Hz); 5.92 (2H, s); 6.49 (1H, d, J=16.0 Hz); 6.67 (3H, s);
7.34-
7.89 (5H, m); 8.12 (1H, t, J= 6.4 Hz); 9.07 (1H, br s); 10.78 (1H, br s). HPLC
analysis on Symmetry C8 column: impurities 3.5% (column size 3.9x150 mm;
mobile phase acetonitrile - 0.1M phosphate buffer (pH 2.5), 30:70; sample
concentration 0.25 mg/m1; flow rate 1.2 ml/min; detector UV 254 nm). Anal.
Calcd
for C17H16N206S, %: C 54.25, H 4.28, N 7.44. Found, %: C 54.19, H 4.20, N
7.33.
CA 02765409 2012-01-19
54236-1
- 230 -
Biological Activity
Candidate compounds were assessed for their ability to inhibit deacetylase
activity
(biochemical assays) and to inhibit cell proliferation (cell-based
antiproliferation
assays), as described below.
Primary Assay: Deacetylase Activity
Briefly, this assay relies on the release of radioactive acetate from a
radioactively
labelled histone fragment by the action of HDAC enzyme. Test compounds,
which inhibit HDAC, reduce the yield of radioactive acetate. Signal (e.g.,
scintillation counts) measured in the presence and absence of a test compound
provide an indication of that compound's ability to inhibit HDAC activity.
Decreased activity indicates increased inhibition by the test compound.
The histone fragment was an N-terminal sequence from histone H4, and it was
labelled with radioactively labelled acetyl groups using tritiated
acetylcoenzyme A
(coA) in conjunction with an enzyme which is the histone acetyltransferase
domain of the transcriptional coactivator p300. 0.33 mg of peptide H4 (the N-
terminal 20 amino acids of histone H4, synthesised using conventional methods)
were incubated with His6-tagged p300 histone acetyltransferase domain (amino
acids 1195-1673, expressed in E. coli strain BLR(DE3)pLysS (Novagen, Cat. No.
69451-3) and 3H-acetyl coA (10 pL of 3.95 Ci/mmol; from Amersham) in a total
volume of 300 pL of HAT buffer (50 mM TrisC1 pH 8, 5% glycerol, 50 mM KC1,
0.1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol (DTT) and
= 1 mM 4-(2-aminoethyl)-benzenesulfonylfluoride (AEBSF)). The mixture was -
incubated at 30 C for 45 min after which the His-p300 was removed using nickel-
trinitriloacetic acid agarose (Qiagen, Cat No. 30210). The acetylated peptide
was
then separated from free acetyl coA by size exclusion chromatography on
Sephadex G-15 (Sigma G-15-120), using distilled H20 as the mobile phase.
*Trade -mark
CA 02765409 2012-01-19
WO (2/30879
PCT/GB01/04326
- 231 -
After purification of the radiolabelled histone fragment, it was incubated
with a
source of HDAC (e.g., an extract of HeLa cells (a rich source of HDAC),
recombinantly produced HDAC1 or HDAC2) and any released acetate was
extracted into an organic phase and quantitatively determined using
scintillation
-- counting. By including a test compound with the source of HDAC, that
compound's ability to inhibit the HDAC was determined.
HeLa Cell Extract
-- The HeLa cell extract was made from HeLa cells (ATCC Ref. No. CCL-2) by
freeze-thawing three times in 60 mM TrisCI pH 8.0, 450 mM NaCI, 30% glycerol.
Two cell volumes of extraction buffer were used, and particulate material was
centrifuged out (20800 g, 4 C, 10 min). The supernatant extract having
deacetylase activity was aliquotted and frozen for storage.
Recombinantly Produced HDAC1 and HDAC2
Recombinant plasmids were prepared as follows.
-- Full length human HDAC1 was cloned by PCR using a Agt11 Jurkat cDNA library
(Clontech-HL5012b). The amplified fragment was inserted into the EcoRI-Sall
sites of pFlag-CTC vector (Sigma-E5394), in frame with the Flag tag. A second
PCR was carried out in order to amplify a fragment containing the HDAC1
sequence fused to the Flag tag. The resulting fragment was subcloned into the
-- EcoRI-Sac1 sites of the baculovirus transfer vector pAcHTL-C (Pharmingen-
21466P).
Full length human HDAC2 was subcloned into pAcHLT-A baculovirus transfer
vector (Pharmingen-21464P) by PCR amplification of the EcoRI-Sac1 fragment
-- from a HDAC2-pFlag-CTC construct.
Recombinant protein expression and purification was performed as follows.
CA 02765409 2012-01-19
j4236-1
- 232 -
HDAC1 and HDAC2 recombinant baculoviruses were constructed using
BaculoGold Transfection Kit (Pharmingen-554740). Transfer vectors were co-
transfected into SF9 insect cells (Pharmingen-21300C). Amplification of
recombinant viruses was performed according to the Pharmingen Instruction
Manual. SF9 cells were maintained in serum-free SF900 medium
(Gibco 10902-096).
For protein production, 2x107 cells were infected with the appropriate
recombinant
virus for 3 days. Cells were then harvested and spun at 3,000 rpm for 5
minutes.
They were then washed twice in PBS and resuspended in 2 pellet volumes of
lysis
buffer (25 mM HEPES pH 7.9, 0.1 mM EDTA, 400 mM KCI, 10% glycerol, 0.1%
NP-40, 1 mM AEBSF). Resuspended cells were frozen on dry ice and thawed at
37 C 3 times and centrifuged for 10 minutes at 14,000 rpm. The supernatant was
collected and incubated with 300 pl of 50% Ni-NTA agarose bead slurry (Qiagen-
30210). Incubation was carried out at 4 C for 1 hour on a rotating wheel. The
slurry was then centrifuged at 500 g for 5 minutes. Beads were washed twice in
1
ml of wash buffer (25 mM HEPES pH7.9, 0.1 mM EDTA, 150 mM KCI, 10%
glycerol, 0.1% NP-40, 1 mM AEBSF). Protein was eluted 3 times in 300 pl
elution
buffer (25 mM HEPES pH 7.9, 0.1 mM EDTA, 250 mM KCI, 10% glycerol, 0.1%
NP-40, 1 mM AEBSF) containing increasing concentrations of imidazole: 0.2 M,
0.5 Nil- and 1 M. Each elution was performed for 5 minutes at room
temperature.
Eluted protein was kept in 50% glycerol at -70 C.
Assay Method
=
A source of HDAC (e.g., 2 pL of crude HeLa extract, 5 pL of HDAC1 or (-IDAC2;
in
elution buffer, as above) was incubated with 3 pL of radioactively labelled
peptide
along with appropriate dilutions of candidate compounds (1.5 pL) in a total
volume
of 150 pL of buffer (20 mM Tris pH 7.4, 10% glycerol). The reaction was
carried
out at 37 C for one hour, after which the reaction was stopped by adding 20 pL
of
1 M HCI / 0.4 M sodium acetate. Then, 750 pL of ethyl acetate was added, the
*Trade-mark
CA 02765409 2012-01-19
54236-1
- 233 -
samples vortexed and, after centrifugation (14000 rpm, 5 min), 600 pL from the
upper phase were transferred to a vial containing 3 mL of scintillation liquid
(UltimaGold, Packard, Cat. No. 6013329). Radioactivity was measured using a
Tri-Carbc 2100TR Liquid Scintillation Analyzer (Packard).
Percent activity (% activity) for each test compound was calculated as:
% activity = { (Sc - B)/ (S - B) x 100
wherein Sc denotes signal measured in the presence of enzyme and the
compound being tested, S denotes signal measured in the presence of enzyme
but in the absence of the compound being tested, and B denotes the background
signal measured in the absence of both enzyme and compbund being tested. The
IC50 corresponds to the concentration which achieves 50% activity.
IC50 data for several compounds of the present invention, as determined using
this assay, are also shown in Table 1, below.
Measurement of cell viability in the presence of increasing concentration of
test
compound at different time points is used to assess both cytotoxicity and the
effect of the compound on cell proliferation.
Secondary Assay: Cell Proliferation
Compounds with HDAC inhibition activity, as determined using the primary
assay,
were subsequently evaluated using secondary cell-based assays. The following
cell lines were used:
HeLa - Human cervical adenocarcinoma cell line (ATCC ref. No. CCL-2).
K11 ¨ HPV E7 transformed human keratinocyte line provided by Pidder
Jansen-Duerr, Institut fiir Biomedizinische Alternsforschung, Innsbruck,
Austria.
*Trade-mark
CA 02765409 2012-01-19
WO 02/30879 PCT/C1301/04326
- 234 -
NHEK-Ad ¨ Primary human adult keratinocyte line (Cambrex Corp., East
Rutherford, NJ, USA).
JURKAT ¨ Human T-cell line (ATCC no. TIB-152).
Assay Method
Cells were cultured, exposed to candidate compounds, and incubated for a time,
and the number of viable cells was then assessed using the Cell Proliferation
Reagent WST-1 from Boeh ringer Mannheim (Cat. No. 1 644 807), described
below.
Cells were plated in 96-well plates at 3-10x103 cells/well in 100 pL of
culture
medium. The following day, different concentrations of candidate compounds
were added and the cells incubated at 37 C for 48 h. Subsequently, 10 pL/well
of
WST-1 reagent was added and the cells reincubated for 1 hour. After the
incubation time, absorbance was measured.
WST-1 is a tetrazolium salt which is cleaved to formazan dye by cellular
enzymes.
An expansion in the number of viable cells results in an increase in the
overall
activity of mitochondrial dehydrogenases in the sample. This augmentation in
the
enzyme activity leads to an increase in the amount of formazan dye formed,
which
directly correlates to the number of metabolically active cells in the
culture. The
formazan dye produced is quantified by a scanning multiwell spectrophotometer
by measuring the absorbance of the dye solution at 450 nm wavelength
(reference wavelength 690 nm).
Percent activity (% activity) in reducing the number of viable cells was
calculated
for each test compound as:
% activity = (Sc - B) / (S - B) } x 100
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 235 -
wherein Sc denotes signal measured in the presence of the compound being
tested, S denotes signal measured in the absence of the compound being
tested,
and B denotes the background signal measured in blank wells containing medium
only. The IC50 corresponds to the concentration which achieves 50% activity.
1050 values were calculated using the software package Prism 3.0 (GraphPad
Software Inc., San Diego, CA) , setting top value at 100 and bottom value at
0.
IC50 data for several compounds of the present invention, as determined using
this assay, are also shown in Table 2, below.
Measurement of cell viability in the presence of increasing concentration of
test
compound at different time points is used to assess both cytotoxicity and the
effect of the compound on cell proliferation.
Biological Data
IC50 (or percent activity) data for several compounds of the present
invention, as
determined using the assays described above are summarised in Table 1 and
Table 2, below.
Table 1
Biochemical Assay Data
Compound HDAC Inhibition
(IC50 unless otherwise specified)
No. Ref. HeLa HDAC1 HDAC2
TSA 5 15 17
Oxamflatin 38
1 PX089342 125 50
2 PX089344 89 172
3 PX106499 35
4 PX106522 1580
5 PX117432 24%@ 500
6 PX117780 125
7 PX117781 58
8 PX117793 50
9 PX117794 24
CA 02765409 2012-01-19
WO 02/3(1879
PCT/GB01/04326
- 236 -
Table 1
'
Biochemical Assay Data
Compound HDAC Inhibition
(1050 unless otherwise specified)
No. Ref. HeLa HDAC1 HDAC2
_
PX089343 24%@ 1 WV! , - -
11 PX105684 19.5- 124
12 PX105685 238- 600
_
13 PX105844 15 29 - _
14 PX106508 31 90 -
,
PX106509 6 --
r _
16 PX106510 12- -
17 , PX106511 35- -
18 PX106512 22 458 -
_
19 PX116238 14 - -
PX116242 9%@500 - -
_
21 PX117225 640 - -
_
22 PX117226 26.3 - -
23 PX117227 50 - -
, 24 PX117228 7 - -
PX117233 21%@ 500 - -
_
26 PX117234 59%@ 500 - -
27 PX117235 40%@ 500 - -
28 PX117236 54%@ 500 , - -
, 29 PX117245 16 - -
_
PX117250 192 - -
_
31 PX117260 , 35%@ 500 . -
32 PX117410 40%@ 500 - -
33 PX117411 39%@ 500 - -
_ _
34 PX117412 54%@ 500 - -
PX117414 46%@ 500 - -
36 PX117429 73%@ 500 - - _
- -
37 PX117445 2
_ _ _
38 PX117446 18 - -
. 39 PX117447 , 3%@500 - -
PX117448 3%@500 - -
41 _ PX117450 _ 20 - -
42 PX117453 45 - -
43 PX117710 125 - -
44 PX117712 14 - -
_
PX117713 138 - -
46 PX117715 10 - -
_
_
47 PX117734 8 - -
_
48 PX117735 , 6 - -
_
49 PX117736 6 - -
. _
PX117773 67 - -
CA 02765409 2012-01-19
PCT/C1301/04326
WO 02/30879
- 237 -
,
Table 1
Biochemical Assay Data
Compound HDAC Inhibition
(IC50 unless otherwise specified)
No. Ref. HeLa HDAC1 HDAC2 -
51 PX117774 396 - -
52 PX117775 16 - - .
53 PX117778 >400 - _
-
54 PX117779 250 - -
-
55 PX117782 38 - -
56 PX117787 67 - -
57 PX117788 36 - -
58 PX117789 30 - -
59 PX117790 175 - -
- -
60 PX117791 250 - -
-
61 PX117792 48 - -
62 PX117795 13 - -
63 PX117796 19 - -
64 PX117798 50 - -
Table 2
Cell-Based Antiproliferation Assay Data
Compound Cell Proliferation Inhibition WST-1
(1050 unless otherwise specified)
No. Ref. HeLa K11 NHEK-AD , Jurkat
TSA 0.350 0.38 0.2 0.042
Oxamflatin 1.1 4.56 3.53 0.260
MS-275 - 9.16 3.1 0.365 .
SAHA , - 6.82 5.37 , 0.750
1 PX089342 4.1- - -
2 PX089344 8.9- - -
3 PX106499 3.8- - -
4 PX106522 16.7- - -
PX117432 - - - -
6 PX117780 16.8 10.5 - 4.0
7 PX117781 3.4 2.2 - 0.8
8 PX117793 2.0 2.7- 0.5
_
9 PX117794 3.3 2.3 - 0.6
PX089343 - - - -
11 PX105684 2.2 2.4 1.5 0.2
12 PX105685 7.3- - - .
13 PX105844 0.4- - -
_
14 PX106508 1.6 3.5 - 0.30
_
PX106509 2.0 2.0 - 0.33
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 238 -
Table 2
Cell-Based Antiproliferation Assay Data
Compound Cell Proliferation Inhibition WST-1
(IC50 unless otherwise specified)
No. Ref. HeLa K11 NHEK-AD Jurkat
16 PX106510 2.3 4.2 - 0.25
17 PX106511 0.38 2.5 - 0.235
18 PX106512 1.9 2.4 - 0.21
19 PX116238 0.8 - - -
20 PX116242 - - - -
21 PX117225 11.9 26%@20 pM _ - 3.3
22 PX117226 0.5 2.8 - 0.10
23 PX117227 1.2 4.7 - 0.36
24 PX117228 0.8 1.4 1.2 0.15
25 , PX117233 - - - -
26 PX117234 - - - -
27 PX117235 - - - -
28 PX117236 - - - -
29 PX117245 0.31 - 0.52 1.1
30 PX117250 7.8 - 1.0
31 PX117260 - - - -
32 PX117410 - - - -
33 PX117411 - - - -
34 PX117412 - - - -
35 PX117414 . - - - -
36 PX117429 - - - -
37 PX117445 1.1 1.2 0.75 0.13
38 PX117446 6.0 3.7 - 0.43
39 PX117447 77.8 - - -
40 PX117448 88.9 - - -
41 PX117450 1.6 - - -
42 PX117453 5.7 4.2 - 1.1
43 PX117710 5.0 4.0 - 0.42
44 PX117712 1.1 0.65 - 0.13
45 PX117713 5.1 9.2- 0.62
46 PX117715 1.5 0.93- 0.29
47 PX117734 2.1 0.88- 0.079
48 PX117735 - 3.10.074
-
49 PX117736 _ - 0.80- 0.12
50 PX117773 3.4 6.2- 1.2
_
51 PX117774 6.4 7.0- 1.0
_
52 PX117775 2.1 5.3- , 0.53
53 PX117778 , - >30- >10
54 PX117779 9.6 1.4- 1.1
55 PX117782 2.9 15.6- 0.35
56 PX117787 2.6 1.2- 0.50
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 239 -
Table 2
Cell-Based Antiproliferation Assay Data
Compound Cell Proliferation Inhibition WST-1
(IC50 unless otherwise specified)
No. Ref. HeLa K11 NHEK-AD Jurkat
57 PX117788 2.0 1.7 0.29
58 PX117789 1.1 0.8 0,3
59 PX117790 12.5 8.0 2.1 -
60 PX117791 3.6 6.7 1.3
61 PX117792 1.4 0.4 0.43
62 PX117795 3.4 1.5 0.51
63 PX117796 2.6 1.2 0.56
64 PX117798 0.9 0.35 3.6
Activity
(1) (A) As mentioned above, in one embodiment, the compounds employ, as J, a
(2) (B1) As mentioned above, in one embodiment, the compounds employ, as Q2,
(3) (B2) As mentioned above, in one embodiment, the compounds employ, as 02,
(4) (Cl) As mentioned above, in one embodiment, the compounds employ, as Q1,
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 240 -
(5) (C2) As mentioned above, in one embodiment, the compounds employ, as Q1,
a covalent bond. Such compounds enjoy the surprising and unexpected property
of superior activity as compared to analogs which comprise, as Q1, an aryl
leader
having a backbone of one carbon atom.
(6) (C3) As mentioned above, in one embodiment, the compounds employ, as Q1,
an aryl leader having a backbone of at least two carbon atoms. Such compounds
enjoy the surprising and unexpected property of superior activity as compared
to
analogs which comprise, as Q1, an aryl leader having a backbone of one carbon
atom, and often as compared to analogs which comprise, as Q1, a covalent bond.
(7) (A+B1) As mentioned above, in one embodiment, the compounds employ, as
J, a "reverse" sulfonamide linkage (i.e., -NHS02-); and as Q2, a phenylene-
meta-
CiJalkylene linkage. Such compounds enjoy the surprising and unexpected
property of superior activity as compared to analogs which do not employ these
groups.
(8) (A+B2) As mentioned above, in one embodiment, the compounds employ, as
J, a "reverse" sulfonamide linkage (i.e., -NHS02-); and as Q2, a phenylene-
meta-
ethylene linkage. Such compounds enjoy the surprising and unexpected property
of superior activity as compared to analogs which do not employ these groups.
(9) (A+C1) As mentioned above, in one embodiment, the compounds employ, as
J, a "reverse" sulfonamide linkage (i.e., -NHS02-); and as Q1, either: a
covalent
bond, or: an aryl leader having a backbone of at least two carbon atoms. Such
compounds enjoy the surprising and unexpected property of superior activity as
compared to analogs which do not employ these groups.
(10) (A+C2) As mentioned above, in one embodiment, the compounds employ, as
J, a "reverse" sulfonamide linkage (i.e., -NHS02-); and as Q1, a covalent
bond.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 241 -
Such compounds enjoy the surprising and unexpected property of superior
activity
as compared to analogs which do not employ these groups.
(11) (A+C3) As mentioned above, in one embodiment, the compounds employ, as
J, a "reverse" sulfonamide linkage (i.e., -NHS02-); and as Q1, an aryl leader
having a backbone of at least two carbon atoms. Such compounds enjoy the
surprising and unexpected property of superior activity as compared to analogs
which do not employ these groups.
(12) (B1+C1) As mentioned above, in one embodiment, the compounds employ,
as Q2, a phenylene-meta-Ciqalkylene linkage; and, as Q1, either: a covalent
bond,
or: an aryl leader having a backbone of at least two carbon atoms. Such
compounds enjoy the surprising and unexpected property of superior activity as
compared to analogs which do not employ these groups.
(13) (B1+C2) As mentioned above, in one embodiment, the compounds employ,
as Q2, a phenylene-meta-Ciqalkylene linkage; and, as Q1, a covalent bond. Such
compounds enjoy the surprising and unexpected property of superior activity as
compared to analogs which do not employ these groups.
(14) (B1+C3) As mentioned above, in one embodiment, the compounds employ,
as Q2, a phenylene-meta-CiJalkylene linkage; and, as Ql, an aryl leader having
a
backbone of at least two carbon atoms. Such compounds enjoy the surprising
and unexpected property of superior activity as compared to analogs which do
not
employ these groups.
(15) (B2+C1) As mentioned above, in one embodiment, the compounds employ,
as Q2, a phenylene-meta-ethylene linkage; and, as Q1, either: a covalent bond,
or:
an aryl leader having a backbone of at least two carbon atoms. Such compounds
enjoy the surprising and unexpected property of superior activity as compared
to
analogs which do not employ these groups.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 242 -
(16) (B2+C2) As mentioned above, in one embodiment, the compounds employ,
as Q2, a phenylene-meta-ethylene linkage; and, as Q1, a covalent bond. Such
compounds enjoy the surprising and unexpected property of superior activity as
compared to analogs which do not employ these groups.
(17) (B2+C3) As mentioned above, in one embodiment, the compounds employ,
as Q2, a phenylene-meta-ethylene linkage; and, as Q1, an aryl leader having a
backbone of at least two carbon atoms. Such compounds enjoy the surprising
and unexpected property of superior activity as compared to analogs which do
not
employ these groups.
(18) (A+B1+C1) As mentioned above, in one embodiment, the compounds
employ, as J, a "reverse" sulfonamide linkage (i.e., -NHS02-); as Q2, a
phenylene-
meta-Ciqalkylene linkage; and, as Q1, either: a covalent bond, or: an aryl
leader
having a backbone of at least two carbon atoms. Such compounds enjoy the
surprising and unexpected property of superior activity as compared to analogs
which do not employ these groups.
(19) (A+B1+C2) As mentioned above, in one embodiment, the compounds
employ, as J, a "reverse" sulfonamide linkage (i.e., -NHS02-); as Q2, a
phenylene-
meta-Ciqalkylene linkage; and, as Q1, a covalent bond. Such compounds enjoy
the surprising and unexpected property of superior activity as compared to
analogs which do not employ these groups.
(20) (A+B1 +C3) As mentioned above, in one embodiment, the compounds
employ, as J, a "reverse" sulfonamide linkage (i.e., -NHS02-); as Q2, a
phenylene-
meta-C1..7alkylene linkage; and, as Q1, an aryl leader having a backbone of at
least two carbon atoms. Such compounds enjoy the surprising and unexpected
property of superior activity as compared to analogs which do not employ these
groups.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 243 -
(21) (A+B2+C1) As mentioned above, in one embodiment, the compounds
employ, as J, a "reverse" sulfonamide linkage (i.e., -NHS02-); as Q2, a
phenylene-
meta-ethylene linkage; and, as Q1, either: a covalent bond, or: an aryl leader
having a backbone of at least two carbon atoms. Such compounds enjoy the
surprising and unexpected property of superior activity as compared to analogs
which do not employ these groups.
(22) (A+62+C2) As mentioned above, in one embodiment, the compounds
employ, as J, a "reverse" sulfonamide linkage (i.e., -NHS02-); as Q2, a
phenylene-
meta-ethylene linkage; and, as Q1, a covalent bond. Such compounds enjoy the
surprising and unexpected property of superior activity as compared to analogs
which do not employ these groups.
(23) (A+B2+C3) As mentioned above, in one embodiment, the compounds
employ, as J, a "reverse" sulfonamide linkage (i.e., -NHS02-); as Q2, a
phenylene-
meta-ethylene linkage; and, as Q1, an aryl leader having a backbone of at
least
two carbon atoms. Such compounds enjoy the surprising and unexpected
property of superior activity as compared to analogs which do not employ these
groups.
Comparative Data for Sulfonamide Direction
Comparative data for sets of compounds, where the only difference in chemical
structure is the sulfonamide direction, are shown below.
Compounds which employ, as J, a "reverse" sulfonamide linkage (i.e., -NES02-)
surprisingly and unexpectedly have superior activity as compared to their
"forward" sulfonamide (i.e., -SO2NH-) analogs.
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 244 -
H
* N Jr- (30F1
o
Compound Qi J o/m/p HeLa 1050
PX117234 -NHS02- - 59`)/0 @ 500 nM
PX106522 - -SO2NH- - _ 1.6 pM
-
01101 H.OH
J =
o
Compound Q1 J o/m/p HeLa IC50
-
PX105684 , -NHS02- m 20 nM
PX089344 - -SO2NH- m 89 nM ,
la
Si j o OH
-
_ Compound Q1 J 1 o/m/p HeLa IC50
PX106511 -CH2- -NHS02- m 35 nM _
, PX089343 -CF-I2- -SO2NH- , m 24% @ 1 pM
* J 5
H
OH
o
Compound Q1 J o/m/p HeLa IC50
PX117450 - -NHS02- P 20 nM
PX106499 - -SO2NH- P _ 35 nM ,
Me 0 Of
H
/ N,OH
J
o
Compound r- Q1 : J o/m/p _ HeLa 1050
PX106508 - -NHS02- m 31 nM
¨
PX089342 - ' -SO2NH- m 125 nM _
CA 02765409 2012-01-19
WO 02/30879 PCT/GB01/04326
- 245 -
SI J
N,
OH
0
Compound Q1 o/m/p HeLa IC50
PX116238 -NHS02- m= 14 nM
Oxamflatin -SO2NH- m 38 nM
Comparative Data for Phenylene-Alkvlene Acid Leader Orientation
Comparative data for sets of compounds, where the only difference in chemical
structure is the ortho/meta/para orientation of the phenylene-alkylene acid
leader,
are shown below.
In some embodiments, compounds which employ, as Q2, a phenylene-meta-
Ci_7alkylene linkage surprisingly and unexpectedly have superior activity as
compared to their ortho and para analogs.
For compounds with a "forward" sulfonamide linkage, para analogs are more
active than meta analogs. Surprisingly and unexpectedly, for compounds with a
"reverse" sulfonamide linkage, meta analogs are as active, or more active,
than
para analogs. Thus, compounds which employ both, as J, a "reverse"
sulfonamide linkage (i.e., -NHS02-) and, as Q2, a phenylene-meta-C1_7alkylene
linkage, surprisingly and unexpectedly have superior activity as compared to
their
"forward" analogs.
j
N,
OH
0
Compound QiJ o/m/p HeLa IC50
PX117447 -NHS02- o 3% @ 500 nM
PX117228 -NHS02- m 7 nM
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.