Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02768407 2012-01-17
-1-
1 7-HYDROXY-1 7-PENTAFLUORE THYL-ESTRA-4,9(1 0)-DIEN-1 1-ARYL DERIVATIVES,
METHODS FOR THE PRODUCTION THEREOF AND USE THEREOF FOR TREATING
DISEASES
The invention relates to 17-hydroxy-17-pentafluoroethyl-estra-4,9(10)-dien-11-
aryl derivatives of Formula I
with progesterone antagonizing action and method of production thereof, use
thereof for the treatment
and/or prophylaxis of diseases and use thereof for the production of medicinal
products for the treatment
and/or prophylaxis of diseases, in particular of fibroids of the uterus (
myomas, uterine leiomyoma),
endometriosis, heavy menstrual bleeds, meningiomas, hormone-dependent breast
cancers and
complaints associated with the menopause or for fertility control and
emergency contraception.
These compounds are valuable pharmaceutical active substances. They can be
used among other things
for the production of pharmaceutical preparations for the treatment of
fibroids of the uterus or
endometriosis, heavy menstrual bleeds, meningiomas, hormone-dependent breast
cancers and of
complaints associated with the menopause or for fertility control and
emergency contraception. For the
treatment of fibroids of the uterus and endometriosis, the compounds according
to the invention can also
be administered sequentially in combination with gestagens. In such a
treatment regimen the compounds
according to the invention could be administered over a period of 1-6 months,
followed by a pause in
treatment or sequential treatment with a gestagen over a period of 2-6 weeks
or followed by treatment with
an oral contraceptive (0C-combinations) over the same period.
The efficacy of the compounds according to the invention as progesterone
receptor antagonist was
demonstrated in vitro in transactivation tests and in vivo in the rat
(termination of early pregnancy),
Compounds with antagonistic action on the progesterone receptor (competitive
progesterone receptor
antagonists) became known for the first time in 1982 (RU 486; EP57115) and
many of them have been
described since then. Progesterone receptor antagonists with a fluorinated 17a-
side chain were published
in WO 98/34947 and Fuhrmann et al., J. Med. Chem. 43, 5010 - 5016 (2000).
The compounds with fluorinated 17a-side chain described in WO 98/34947
generally have a very strong
antagonistic activity on the progesterone receptor. Compounds that are very
potent and therefore
preferred in WO 98/34947 are 1113-(4-acetylpheny1)-20,20,21,21,21-pentafluoro-
17-hydroxy-19-nor-17a-
preg na-4, 9-d ien-3-one, 1113-(4-acetylphenyI)-20,20,21,21,21-pentafluoro-17-
hydroxy-19-nor-17a-pregna-4-
en-3-one and 6'-acety1-9,1113-dihydro-1713-hydroxy-17a-(1,1,2,2,2-
pentafluoroethyl)-4'H-
naphth[31,21,11 0,9,11]ester-4-en-3-one. These compounds are transformed to a
considerable extent in
vivo to various metabolites, some with strong, and others with reduced
pharmacological activity.
Metabolism occurs mainly on the 4-substituent of the 11-phenyl residue.
Compounds are described in WO
2008/058767 that are, at least partly, metabolites of the compounds described
in WO 98/34947.
The problem to be solved by the present invention is to make highly potent
competitive progesterone
receptor antagonists available and thus create alternatives for the treatment
of gynaecological diseases.
CA 02768407 2012-01-17
- 2 -
It was found that the compounds according to the invention are especially
suitable for solving this problem.
In particular, compounds with alkylsulphanyl and alkylsulphonyl groups show
very strong antagonistic
activity on the progesterone receptor, i.e. they inhibit the action of
progesterone on its receptor.
It was also found that the compounds with alkylsulphonyl groups, compared e.g.
with alkanoyl groups,
have a far higher metabolic as well as chemical stability against temperature,
light and oxidative stress.
For example, the corn pound (116,176)-17-hydroxy-1144-
(methylsulphonyl)pheny1]-17-
(pentafluoroethypestra-4,9-dien-3-one (example 4) displays, compared with the
respective analogue with
an alkanoyl or hydroxyalkanoyl group (11f3-(4-acetylpheny1)-20,20,21,21,21-
pentafluoro-17-hydroxy-19-
nor-17a-pregna-4,9-dien-3-one or 20,20,21,21,21-pentafluoro-17-hyd roxy-113-[4-
(hydroxyacatyl)phenyl]-
19-nor-176-pregna-4,9-dien-3-one), a surprisingly high stability under thermal
load, under the influence of
UV light and a surprisingly low oxidation sensitivity.
Compounds with an alkylsulphonimidoyl group in position 4 of the 11-phenyl
ring have, despite sometimes
lower in vitro activity, a very strong action in vivo. These compounds are at
least partially prodrugs of the
corresponding sulphones, and the compounds with an alkylsulphonimidoyl group
have markedly better
solubility in water.
It is also surprising that both compounds with an alkylsulphonyl group, and
compounds with
alkylsulphonimidoyl groups, especially the corresPonding methyl compounds,
have a low inhibition
potential with respect to the investigated CYP-isoenzymes CYP1A2, CYP2C8,
CYP2C9, CYP2D6 and
CYP3A4, and up to a maximum concentration that is soluble or used in the assay
(minimum 10 pM,
maximum 20 pM), 50% inhibition was not reached in any case investigated.
These in vitro findings suggest, for the substances investigated, an
especially low risk of interactions with
co-administered medicinal products with respect to CYP-inhibition.
Moreover, for (116,1713)-17-hydroxy-1114-(methylsulphonyl)pheny1]-17-
(pentafluoroethyl)estra-4,9-dien-3-
one, an especially favourable safety profile (in acute and chronic tests) was
found in preclinical
investigations on rodents and non-rodents.
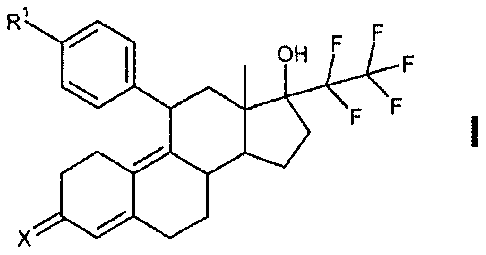
The present invention relates to 17-hydroxy-17-pentafluoroethyl-estra-4,9(10)-
dien-11-aryl derivatives with
the general chemical formula I:
R1
OH
EF
in which
CA 02768407 2012-01-17
- 3 -
,
stands either for a residue Y or for a phenyl ring substituted once or twice
with a residue
Y,
is selected from the group comprising SR', S(0)R3, S(0)2R3, S(0)(NH)R3,
S(0)(NR4)R3,
S(0)2NR9R10,
132 represents hydrogen or Ci-C6-alkyl or C7Cio-aralkyl or aryl,
R3 C1-C6-alkyl or aryl,
R4 a group S(0)2R5,
R6 phenyl or 4-methylphenyl
X an oxygen atom, NOR7 or NNHSO2R7 and
R7 is selected from the group comprising hydrogen, Ci-Cio-alkyl, aryl
Ro, Rlo independently of one another are selected from the
group comprising hydrogen, C1-C10-
alkyl or aryl or alternatively represent, together with the nitrogen atom, a 3-
to 8-
membered, saturated or unsaturated heterocyclic ring
and their salts, solvates or solvates of the salts, including all crystal
modifications.
Depending on their structure, the compounds according to the invention of
general formula I can exist in
stereoisomeric forms (enantiomers, diastereomers). The invention therefore
comprises the enantiomers or
diastereomers and their respective mixtures including racemates. The
stereoisomerically uniform constituents
can be isolated in a known way from said mixtures of enantiomers and/or
diastereomers.
Each of the stated substituents on the steroid backbone chain can be both in
an a-position and in a 8--
position. Moreover, the substituents on the steroid backbone chain, which
contain a double bond and in
which the double bond on each atom bears at least one substituent, which is
not hydrogen, can be both E-
and Z-configu red.
If the compounds according to the invention can exist in tautomeric forms, the
present invention comprises all
tautomeric forms.
Physiologically harmless salts of the compounds according to the invention are
preferred as salts within the
scope of the present invention. However, salts that are not suitable in
themselves for pharmaceutical uses,
but can for example be used for the isolation or purification of the compounds
according to the invention, are
also covered.
Physiologically harmless salts of the compounds according to the invention
comprise ¨ when they contain a
basic function ¨ salts with inorganic or organic acids, in particular of
mineral acids, carboxylic acids and
sulphonic acids, e.g. salts of hydrochloric acid, hydrobromic acid, sulphuric
acid, phosphoric acid,
methanesulphonic acid, ethanesulphonic acid, toluenesulphonic acid,
benzenesulphonic acid, naphthalene-
disulphonic acid, acetic acid, trifluoroacetic acid, propionic acid, lactic
acid, tartaric acid, malic acid, citric acid,
fumaric acid, maleic acid and benzoic acid.
Physiologically harmless salts of the compounds according to the invention
comprise ¨ when they contain an
acid function ¨ alkali metal salts, alkaline earth metal salts or ammonium
salts, such as can be obtained by
CA 02768407 2012-01-17
- 4 -
,
reaction with corresponding inorganic or organic bases. We may mention, for
example and preferably, alkali
metal salts (e.g. sodium and potassium salts), alkaline earth metal salts
(e.g. calcium and magnesium salts)
and ammonium salts, derived from ammonia or organic amines with 1 to 16 carbon
atoms, such as, for
example and preferably, ethylamine, diethylamine, triethylamine,
ethyldiisopropylamine, monoethanolamine,
diethanolamine, triethanolamine, bicyclo-hexylamine, dimethylamino-ethanol,
procaine, dibenzylamine, N-
methylmorpholine, arginine, lysine, ethylenediamine, N-methyl piperidine, N-
methyl glucamine, D-methyl
glucamine, ethyl glucamine, 1,6-hexadiamine, glucosamine, N-methylglycine, 2-
amino-1,3-propandiol, tris-
hydroxymethyl-aminomethane and 1-amino-2,3,4-butanetriol.
Those forms of the compounds according to the invention that display, in the
solid or liquid state, adduct
formation with solvent molecules, are designated as solvates within the scope
of the invention. The solvent
can be present in stoichiometric or even non-stoichiometric proportions. In
the case of stoichiometric
solvates, they are also called hemi-, (semi-), mono-, sesqui-, di-, tri-,
tetra-, penta-, etc. solvates. Hydrates
are a special form of solvates, in which the coordination takes place with
water.
Moreover, the present invention also comprises prodrugs of the compounds
according to the invention. The
term "prodrugs" comprises compounds which, while they are in the body, are
converted to compounds
according to the invention, for example by enzymatic or hydrolytic processes.
Within the scope of the present invention, unless otherwise specified, the
substituents have the following
meaning:
Alkyl stands for linear or branched alkyl groups with 1-6 carbon atoms, for
example methyl, ethyl, propyl,
isopropyl, n-butyl, sec.-butyl, isobutyl, tert.-butyl, n-pentyl, isopentyl,
neopentyl, hexyl, heptyl, octyl, nonyl
and decyl.
Aryl stands for a mono- to tricyclic aromatic, substituted or unsubstituted
carbocyclic residue, for example
phenyl, naphthyl, which can be substituted one or more times with halogen (F,
Cl, Br, l), OH, 0-alkyl,
CO2H, CO2-alkyl, NH2, NH(Ci-Cio-alkyl), N(Ci-C10-alky1)2, in particular
N(CH3)2, NO2, N3, CN, C1-C10-alkyl,
Ci-Co-perfluoro-alkyl, Ci-Cio-acyl, Cl-Cio-acyloxy groups.
Heteroaryl stands for an aromatic, mono- or bicyclic residue with as a rule 5
to 10, preferably 5 to 6 ring
atoms and up to 5, preferably up to 4 heteroatoms from the series S, 0 and N,
for example and preferably
for benzofuranyl, benzothiophenyl, quinolinyl, furyl, imidazolyl, indazolyl,
indolyl, isoquinolinyl, oxazolyl,
pyridazinyl, pyridyl, pyrimidyl, pyrrolyl, thiazolyl, thienyl, pyrazolyl,
isoxazolyl, pyrazinyl, quinolyl or tetrazolyl.
Aralkyl stands for aralkyl groups that can contain up to 14 carbon atoms,
preferably 6-10 carbon atoms, in
the ring, and 1-8, preferably 1-4, carbon atoms in the alkyl chain. Aralkyl
residues that may be considered
are for example benzyl, phenylethyl, naphthylmethyl, naphthylethyl,
furylmethyl, thienylethyl, pyridylpropyl.
The rings can be substituted one or more times with halogen, OH, 0-alkyl,
CO2H, CO2-alkyl, NH2, NH(Ci-
Cio-alkyl), N(Ci-Cio-alky1)2, NO2, N3, ON, Ci-Cio-perfluoro-alkyl,
C1-C20-acyloxy
groups.
CA 02768407 2012-01-17
- 5
When residues in the compounds according to the invention are substituted,
unless otherwise specified,
the residues can be substituted one or more times. Within the scope of the
present invention, for all
residues that occur more than once, their meaning is independent of one
another. A substitution with one,
two or three identical or different substituents is preferred. Substitution
with one substituent is quite
especially preferred.
Compounds of formula (I) are preferred,
R1
OH
I)
O.
X
in which
R1 stands either for a residue Y or for a phenyl ring substituted once
with a residue Y and Y is
selected from the group comprising SR2, S(0)R3, S(0)2R3, S(0)(NH)R3,
S(0)(NR4)R3,
S(0)2NR9R10 and
R2 stands for Ci-Cs-alkyl, hydrogen or C7-C6-aralkyl, especially
preferably hydrogen, methyl, ethyl or
benzyl,
R3 stands for Ci-Cs-alkyl, preferably methyl or ethyl,
R4 stands fora group S(0)2R6 and
X represents oxygen,
R6 represents phenyl or 4-methylphenyl and
R9, Rlo independently of one another represent hydrogen or Ci-Cs-alkyl or
phenyl
and their salts, solvates or solvates of the salts.
Compounds of formula I are especially preferred in which FV: S(0)2R3, X: 0 and
R3: Ci-Cs-alkyl, in
particular those in which R3 denotes methyl.
Compounds of formula I are also especially preferred in which R1: S(0)(NH)R3,
X: 0 and R3: Cl-Cs-alkyl, in
particular those in which R3 denotes methyl. The preferred residue R1 can be
both in the R- and in the S-
configuration, and in any mixture ratio.
The following compounds are also preferred:
(111,1713)-17-Hydroxy-1144-(methylsulphanyl)pheny1]-17-(pentafluoroethyl)estra-
4,9-dien-3-one (Ex. 1)
(1113,1713)-11-14-(Ethylsulphanyl)pheny11-17-hydroxy-17-
(pentafluoroethyl)estra-4,9-dien-3-one (Ex. 2)
(1113,1713)-17-Hydroxy-11-14-[(RS)-methylsulphinyl]pheny11-17-
(pentafluoroethypestra-4,9-dien-3-one (Ex.
3)
(1113,1713)-17-Hydroxy-1144-(methylsulphonyl)pheny1]-17-(pentafluoroethypestra-
4,9-dien-3-one (Ex. 4)
CA 02768407 2016-11-10
30725-1113
- 6 -
(118,17(3)-1144-(Ethylsulphonyl)phenyll-17-hydroxy-17-(pentafluoroethypestra-
4,9-dien-3-one (Ex. 5)
(11(3,1713)-1144-(Benzylsulphanyl)pheny11-17-hydroxy-17-
(pentafluoroethyl)estra-4,9-dien-3-one (Ex. 6)
N-[{4-[(118,17(3))-17-Hydroxy-3-oxo-17-(pentafluoroethyl)estra-4,9-dien-11-
yllphenyl)(RS)(methyl) oxido-
6-sulphanylidene]-4-methylbenzene sulphonamide (Ex. 7)
(118,1713)-17-Hydroxy-1144-(RS-methylsulphonimidoyl)pheny11-17-
(pentafluoroethypestra-4,9-dien-3-one
(Ex. 8)
(11(3,1713)-17-Hydroxy-1144'-(methylsulphanyl)bipheny1-4-y1]-17-
(pentafluoroethyl)estra-4,9-dien-3-one
(Ex. 9)
(1113,178)-17-Hydroxy-1144'-(methylsulphonyl)bipheny1-4-y11-17-
(pentafluoroethyl)estra-4,9-dien-3-one
(Ex. 10)
N-R4'-[(11[3,1713)-17-Hydroxy-3-oxo-17-(pentafluoroethyl)estra-4,9-dien-11-
yl]bipheny1-4-yI)(RS)(methyl)
oxido-X6-sulphanylidene]-4-methylbenzene sulphonamide (Ex. 11)
(118,1713)-17-Hydroxy-11-[4'-(RS-methylsulphonimid oyObipheny1-4-y1)-17-
(pentaflu oroethypestra-4,9-dien-
3-one (Ex. 12)
(118,1713)-17-Hydroxy-17-(pentafluoroethyl)-11-(4'-sulphanylbiphenyl-4-
y1)estra-4,9-dien-3-one (Ex. 13)
4'-[(1113,17(3)-17-Hydroxy-3-oxo-17-(penta fluoroethyDestra-4,9-dien-11-A-N,N-
dim ethylbipheny1-4-
sulphonamide (Ex. 14)
4-[(11(3,1713)-17-Hydroxy-3-oxo-17-(pentafluoroethyl)estra-4,9-dien-11-y11-N,N-
dimethylbenzene
suiphonamide (Ex. 15)
The individually stated definitions of residues in the respective combinations
or preferred combinations of
residues are also replaced with any definitions of residues of some other
combination independently of the
respective stated combinations of the residues.
Combinations of two or more of the aforementioned preferred ranges are quite
especially preferred.
CA 02768407 2016-11-10
30725-1113
- 6a -
Also provided is the compound (1113,1713)-17-Hydroxy-1144-
(methylsulphonyl)pheny1]-17-(pentafluoroethyl)estra-4,9-dien-3-one
of formula
0,
`-s
OH
sot C7Fs
0011
It was found that the compounds according to the invention and/or derivatives
display
good progesterone-antagonizing action. It was found in several clinical
studies that
treatment with progesterone receptor antagonists (mifepristone, asoprisnil,
Proellex)
can lead to significant shrinking of fibroids of the uterus and a significant
reduction of
the symptoms associated with said fibroids of the uterus. Moreover, it was
shown in
clinical studies that during treatment with stated progesterone receptor
antagonists,
the symptoms caused by endometriosis (esecially pains) can be also reduced
considerably.
CA 02768407 2012-01-17
- 7 -
Scheme 1
OH 0 OH
Se rat
0 000
0 000
0.01r F F
1 2 3
R.
A
8
1, 2 or 3 -A-
o Compounds of general formula I
OH
Compounds of general formula II
(In the above formula, A and B have the following meaning: =0; -0H/-H or -0H/-
C2F5)
An outline of the production of compounds of general formula I is shown in
Scheme 1.
The compounds with the general chemical formula I are prepared starting from
(5'R,8'S,10'R,13'S,14'S,17'S)-5,5,13'-trimethy1-
1,21,7,8',12',13',14',15',16',17'-decahydro-6'H-spiro[1,3-
dioxane-2,3'45,10]epoxycyclopenta[a]phenanthren]-17'-ol (for production see
Tetrahedron Lett. 26, 2069-
2072 (1985) by analogy with the method described in WO 98/34947 and in WO
2008/058767. After
oxidation of the hydroxyl group in position 17 of the steroid backbone,
introduction of the 17a-
pentafluoroethyl side chain on the corresponding 17-keto compounds takes place
according to the
methods described in WO 98/34947 and in WO 2008/058767. Introduction of the
11[3-phenyl substituents
takes place by conjugated addition of arylgrignard or aryllithium reagents
under copper catalysis.
Compounds of general formula II are obtained, in which R8 can have all
meanings already stated for R1
and additionally can be a hydroxy, benzyloxy, Ci-Cio-alkanoyloxy,
benzoyloxy, silyloxyl,
alkoxyalkyloxy group a Cl, Br, I or a group CmFm+1S03 with m=1-4 and A and/or
B stand either for a
carbonyl group or for a 1713-0H/17a-H group or for a 1713-0H/17a-C2F5 group.
From compounds of
general formula II it is then possible to obtain the compounds of general
formula I. For this, functional
groups are optionally further modified. We may mention in particular the
oxidation of sulphides to
sulphoxides or sulphones by methods known by a person skilled in the art and
the formation of the
sulphoximines from sulphides by adding Chloramine-T-Trihydrate and subsequent
oxidation to the
corresponding protected sulphoximine, which is then liberated e.g. by acid
cleavage. As an alternative,
however, it is also possible to use methods known by a person skilled in the
art starting from
corresponding sulphoxides. For compounds in which there is a biphenyl residue
in position llp of the
steroid backbone, this c an take place either directly by conjugated addition
of the diarylgrignard or
diaryllithium reagent under copper catalysis or alternatively e.g. by
palladium-catalysed coupling reactions
CA 02768407 2012-01-17
- 8 -
, =
on the corresponding functionalized 11p-phenyl derivatives, e.g. phenyl
triflate or phenyl nonaflate.
Generally, both the 113-phenyl residue and the 173-pentafluoroethyl side chain
can be introduced first.
Functional groups, especially the 3-keto group, are optionally protected in
the meantime, e.g. as ketal. As
ketal protecting groups, we may for example mention the ethylenedioxy or 2,2-
dimethylpropylene-1,2-dioxy
group. Hydroxyl groups are for example protected in the form of methoxymethyl,
methoxyethyl,
tetrahydropyranyl, benzyl, or silyl ethers.
At a suitable stage, the protecting groups are then split off by methods known
by a person skilled in the art.
During cleavage of the 3-ketal to the 3-keto group of the steroid backbone, a
5a-hydroxyl group optionally
still present is eliminated, so that compounds of general formula 1 are
formed.
Unless the production of the starting compounds is described here, these are
known by a person skilled in
the art or can be produced analogously to known compounds or methods described
here. The mixtures of
isomers can be separated into the individual compounds by usual methods, for
example crystallization,
chromatography or salt formation.
The production of the salts takes place in the usual way, by adding the
equivalent amount or an excess of
a base or acid, which is optionally in solution, to a solution of the compound
with the general chemical
formula I, optionally separating the precipitate or processing the solution in
the usual way.
The resulting compounds of formula (I) are optionally converted, with the
corresponding (i) solvents and/or
(ii) bases or acids to their solvates, salts and/or solvates of the salts.
The general definitions of residues given above or stated in preferred ranges
apply both to the end
products of formula (I) and correspondingly also to the starting substances or
intermediates needed in
each case for production.
The compounds according to the invention display an unforeseeable, valuable
pharmacological,
pharmacokinetic and pharmacodynamic profile of action.
They are therefore suitable for use as medicinal products for the treatment
and/or prophylaxis of diseases
in humans and animals.
The pharmaceutical efficacy of the compounds according to the invention can be
explained by their action as
progesterone receptor antagonists, and thus by their antagonizing action on
the progesterone receptor.
Another object of the present invention is the use of the compounds according
to the invention for the
treatment and/or prophylaxis of diseases based on hormone-dependent
hyperproliferative processes,
preferably of gynaecological diseases, in particular of fibroids of the
uterus, endometriosis or hormone-
dependent breast cancers.
Another object of the present invention is the use of the compounds according
to the invention for the
treatment and/or prophylaxis of diseases, in particular the aforementioned
diseases.
CA 02768407 2012-01-17
- 9 -
Another object of the present invention comprises the compounds according to
the invention for use in a
method of treatment and/or prophylaxis of fibroids of the uterus,
endometriosis and hormone-dependent
breast cancers.
Another object of the present invention is the use of the compounds according
to the invention for the
production of a medicinal product for the treatment and/or prophylaxis of
diseases, in particular the
aforementioned diseases.
Another object of the present invention is a method of treatment and/or
prophylaxis of diseases, in
particular the aforementioned diseases, using 0.1-100 mg of the compounds
according to the invention per
day per patient in the treatment of fibroids of the uterus or endometriosis
and for contraceptive use or of
0.1-500 mg of the compounds according to the invention per day per patient in
tumour diseases (e.g.
meningiomaµ or hormone-dependent tumours, e.g. breast cancer) and for
emergency contraception.
Another object of the present invention comprises medicinal products
containing at least one compound
according to the invention and at least one or more other active substances,
in particular for the treatment
and/or prophylaxis of the aforementioned diseases.
For the treatment of tumour diseases, for example the following active
substances/classes of active
substances can be administered either simultaneously or sequentially: SERMs,
SERDs, anti-oestrogens,
aromatase inhibitors, kinase inhibitors, angiogenesis inhibitors and/or
cytostatics.
For the treatment of fibroids of the uterus or endometriosis, the compounds
according to the invention can
be combined simultaneously or sequentially with gestagens or combinations of
oestrogens and gestagens.
Progesterone receptor antagonists/gestagen regimens are d isclosed in WO
96/15794 (Spicer et al.,
Balance Pharm. Inc.), WO 96/03130 (Stockemann et al., Schering AG) and
PCT/EP2009/003249 (Moller
et al., Bayer Schering Pharma AG). Regimens ¨ optionally repeated ¨ in which
the progesterone receptor
antagonist is administered over a period of two to four months, followed by
the administration of the
gestagen for a period of one to four weeks, are very suitable for the
treatment of fibroids of the uterus and
endometriosis. Administration of the progesterone receptor antagonist for 84
days, followed by
administration of the gestagen for 14 days ¨ optionally repeated ¨ is
especially suitable.
Simultaneous or sequential administration of the compounds according to the
invention e.g. with SERMs,
SERDs and oestrogens can be considered for the treatment of complaints
associated with the
menopause.
SERMs (selective estrogen receptor modulators) are compounds that are tissue
selective and have either
an anti-oestrogenic or oestrogenic action, for example on the uterus they
inhibit the action of oestrogen,
but on bone they have a neutral or oestrogen-like aetion. Examples are
clomifene, raloxifene, tamoxifen,
torimifene, bazedoxifene, lasofoxifene and ormeloxifene.
Selective estrogen receptor destabilizers (SERD) are pharmaceuticals which
completely antagonize the
oestrogen receptor ('pure anti-oestrogens' without oestrogenic active
component) and lead to down-
CA 02768407 2012-01-17
- 1 0 -
regulation of the receptor (for example fulvestrant, ZK-703 and ZK-253
(Hoffmann J et al., J Nati Cancer
lnst 2004, 96:210-218) and compounds described in VVO 98/007740, WO 99/33855
and WO 03/045972.
Anti-oestrogens are compounds that completely antagonize the oestrogen
receptor, for example
fulvestrant.
Aromatase inhibitors inhibit the enzyme aromatase and ther efore the
aromatization of androgens to
oestrogens. These include, among others, anastrozole, letrozole, exemestane,
vorozole, formestans and
fadrozole.
Kinase inhibitors are enzymes that transfer a phosphate residue from ATP to
other substrates, and in
particular to hydroxyl groups there, e.g. sorafenib (Nexavar) or imatinib
(Gleevec).
Angiogenesis inhibitors, e.g. Avastin, reduce or block the supply of vessels
and therefore the blood supply
to a tumour.
Cytostatics, e.g. cisplatin, taxol, Taxotere are natural or synthetic
substances that inhibit cell growth or cell
division.
Gestagens are, in the sense of the present invention, either the natural
progesterone itself or synthetic
derivatives, which like progesterone itself bind to the progesterone receptor
and, at dosages above the
ovulation inhibiting dose, inhibit ovulation. As examples of the synthetic
derivatives, we may mention
drospirenone, gestodene, levonorgestrel, cyproterone acetate, desogestrel and
3-ketodesogestrel,
norethisterone, norethisterone acetate and dienogest.
Combinations of gestagens and oestrogens are the combinations of active
substances that are contained
in the oral contraceptives that are known per se, for example Yasmin, Femovan,
Triquilar, Marvelon, YAZ
etc.
The compounds according to the invention can act systemically and/or locally.
For this purpose they can
be applied in a suitable way, e.g. by the oral, intrauterine, intravaginal,
parenteral, pulmonary, nasal,
sublingual, lingual, buccal, rectal, dermal, transdermal, conjunctival, or
otic route or as an implant or stent.
Intrauterine means in particular application by means of an IUS (intrauterine
system) or IUD (intrauterine
device). Intravaginal application can be effected by means of, among others,
IVR/VRS (intravaginal
ring/vaginal ring system).
Forms for intrauterine or intravaginal application (cf. e.g. VVO 01/47490,
especially page 1, line 10 to line 5,
line 13 and line 7, line 19 to line 58, line 6, or for vaginal rings: VVO
06/010097, especially page 10, line 22
to page 14, line 28) can contain the compounds according to the invention and
non-silicone and/or silicone
polymers, in particular also siloxane-based elastomers (cf. VVO 01/47490,
especially page 7, line 19 ¨
page 15, line 15).
For these routes of administration, the compounds according to the invention
can be administered in
suitable dosage forms.
CA 02768407 2012-01-17
= = - 11 -
Quick-release and/or modified-release dosage forms functioning according to
the prior art are suitable for
oral administration, containing the compounds according to the invention in
crystalline and/or amorphous
and/or dissolved form, e.g. tablets (uncoated or coated tablets, for example
with enteric coatings or
delayed-dissolving or insoluble coatings, which control the release of the
compound according to the
invention), tablets or films/wafers that quickly disintegrate in the oral
cavity, films/lyophilizates, capsules
(for example hard-gelatin or soft-gelatin capsules), coated tablets, granules,
pellets, powders, emulsions,
suspensions, aerosols or solutions.
Parenteral application can take place while avoiding an absorption step (e.g.
intravenous, intraarterial,
intracardial, intraspinal or intralumbar) or with inclusion of absorption
(e.g. intramuscular, subcutaneous,
intradermal, percutaneous or intraperitoneal). Injection and infusion
preparations in the form of solutions,
suspensions, emulsions, lyophilizates or sterile powders, among others, are
suitable as dosage forms for
parenteral administration.
For the other routes of administration, the following are suitable, e.g.
inhalation dosage forms (including
powder inhalers, nebulizers), nasal drops, solutions, and sprays; tablets for
lingual, sublingual or buccal
administration, films/wafers or capsules, suppositories, ear or eye
preparations, vaginal capsules, aqueous
suspensions (lotions, shaking mixtures), lipophilic suspensions, ointments,
creams, transdermal
therapeutic systems (for example patches), milk, pastes, foams, dusting
powders, implants or stents.
The compounds according to the invention can be converted to the
aforementioned dosage forms. This
can be carried out in a manner that is known per se, by mixing with inert, non-
toxic, pharmaceutically
suitable excipients. These excipients include, among others, carrier
substances (for example
microcrystalline cellulose, lactose, mannitol), solvents (e.g. liquid
polyethylene glycols), emulsifiers and
dispersants or wetting agents (for example sodium dodecylsulphate,
polyoxysorbitan oleate), binders (for
example polyvinylpyrrolidone), synthetic and natural polymers (for example
albumin), stabilizers (e.g.
antioxidants, for example ascorbic acid), colouring matter (e.g. inorganic
pigments, for example iron
oxides) and taste and/or odour correctants.
Another object of the present invention comprises medicinal products that
contain at least one compound
according to the invention, usually together with one or more inert, non-
toxic, pharmaceutically suitable
excipients, and use thereof for the aforementioned purposes.
Nevertheless, it may optionally be necessary to deviate from the stated
amounts, namely depending on
body weight, route of administration, individual response to the active
substance, type of preparation and
point of time or interval when application takes place. Thus, in some cases it
may be sufficient to use less
than the aforementioned minimum amount, whereas in other cases the stated
upper limit must be exceeded.
In the case of the administration of larger amounts it may be advisable to
distribute these in several individual
doses throughout the day.
CA 02768407 2012-01-17
- 12 -
The percentages in the following tests and examples are, unless stated
otherwise, percentages by weight;
parts are parts by weight. Proportions of solvents, dilution ratios and
concentration figures for liquid/liquid
solutions always refer to volume.
The following examples serve to explain the invention without limiting it in
any way.
Example 1:
(1113,1713)-17-hydroxy-1144-(methylsulphanyl)pheny1]-17-
(pentafiuoroethyl)estra-4,9-dien-3-one
S 40
OH ,F,,kF
db/Oee
0
a) (5'R,8'S,101R,13'S,14'S,17'S)-5,5,13'-trimethy1-17'-(pentafluoroethyl)-
1',21,71,81,12',131,141,15',16',17'-
decahydro-6'H-spiro[1,3-dioxane-2,3'45,101epoxycyclopenta[a]phenanthrene]-17'-
ol
OH F/0
Oe )<F
0 000 7C011100
50 g of (5'R,8'S,101R,131S,14'S)-5,5,13'-trimethy1-1',2,61,7,81,12',13',
14',15', 16'-decahyd ro-17'H-s piro[1, 3-
dioxane-2,3'45,10Jepoxycyclopenta[a]phenanthrenj-171-one (for preparation see
Tetrahedron Lett. 26,
2069-2072 (1985) was added to 116 g of condensed pentafluoroiodoethane in 500
ml absolute toluene at
¨70 C. 290 ml of a 1.5-molar solution of methyllithium-lithium bromide complex
in diethyl ether was added
to this at the same temperature. It was then stirred for one hour at 0 C. The
reaction mixture was then
added to saturated aqueous ammonium chloride solution and was extracted with
ethyl acetate. The
organic phase was washed with saturated aqueous sodium chloride solution,
dried over sodium sulphate
and concentrated under vacuum. The raw product was dissolved in 200 ml acetone
and 450 ml water was
added. The precipitated product was filtered off and dried in vacuum.
Yield 61.6g
1H-NMR (400 MHz, CDCI3): 6= 6.04 brd (1H); 3.60 d (1H); 3.35-3.50 m (3H); 2.51
dbr (1H); 1.06 s (3H);
0.93 s (3H); 0.85 s (3H).
b) (5R, 8S,11R,13S, 14S,17S)-5',5',13-trimethy1-1144-
(methylsulphanyl)pheny1]-17-(pentafiu oroethyl)-
1,2,6,7,8,11,12,13,14,15,16,17-dodecahydrospiro[cyclopenta[a]phenanthrene-3,2'-
11 ,3]d ioxane]-5, 17(4H)-
diol
CA 02768407 2012-01-17
- 13 -
F
s FTF
OH JF
O
0.77 lt
F
0 001:111 o
61-1
1.23 g magnesium shavings were suspended in 5 ml THF and 50 pl dibromoethane
was added, while
stirring. A solution of 10.31 g of 1-bromo-4-(methylthiopheny)benzene in 60 ml
THF was added to the
suspension in such a way that the reaction temperature did not go above 55 C.
Then it was stirred for a
further hour. The resultant solution was then cooled to 0 C. 151 mg CuCI was
added and it was stirred for
a further 15 minutes at 0 C. Then a solution of 5 g of the substance described
in example la) in 50 ml
THF was added. Then the reaction mixture was allowed to reach 23 C in the
space of approx. 3 hours,
with stirring, and then it was stirred at this temperature for a further 10
hours. Then saturated aqueous
NH4CI solution was added to the reaction mixture, with external cooling.
Stirring was continued for 30
minutes and it was then extracted several times with ethyl acetate. The
combined organic phases were
washed with saturated sodium chloride solution and dried over sodium sulphate.
The raw product was
purified by silica gel chromatography followed by crystallization from a
mixture of dichloromethane and
diisopropyl ether. This gave 5.72 g of the title compound.
1H-NMR (300 MHz, CDCI3): ö= 7.50 d (2H); 7.30 d (2H); 4.41 s (1H); 4.28 dbr
(1H); 3.40-3.60 m (4H);
2.51 s (3H); 1.05 s (3H); 0.87s (3H); 0.53 s (3H).
c)
(1113,170)-17-hydroxy-11 44-(m ethylsulphanyl)phenyI]-17-(pentafiuo
roethypestra-4,9-d ien-3-one
00 F F F
OH kk
OH
OS
0
O
H
500 mg of the compound described in lb) was dissolved in 15 ml methanol. 360
pl of semi-concentrated
sulphuric acid was added and stirring was continued for 3 hours at 23 C. Then
the reaction mixture was
poured into saturated aqueous sodium hydrogen carbonate solution. It was
extracted several times with
ethyl acetate. The combined or ganic phases were washed with saturated aqueous
sodium chloride
solution, dried over sodium sulphate and concentrated under vacuum. The raw
product was purified by
silica gel chromatography. This gave 297 mg of the title compound.
1H-NMR (300 MHz, CDCI3): 5= 7.20 d (2H); 7.13 d (2H); 5.80 sbr (1H); 4.45 dbr
(1H); 2.51 s (3H); 0.68 s
(3H).
CA 02768407 2012-01-17
.
- 14 -
Example 2
(113,1713)-1144-(ethylsulphanyl)pheny1]-17-hydroxy-17-(pentafluoroethyDestra-
4,9-dien-3-one
10'0.
a)
(5R,8S,11R,13S,14S,17S)-1144-(ethylsulphanyl)pheny1]-5',5',13-trimethy1-17-
(pentafluoroethyl)-
1,2,6,7,8,11,12,13,14,15,16,17-dodecahydrospiro[cyclopenta[a]phenanthrene-
3,2'41,3]dioxane]-5,17(4H)-
diol
01-1kFA_F=
FF =
oe. A oSS
OH
As in example 1b), 2.7 g of the title compound was prepared from 3 g of the
compound described in la),
888 mg magnesium shavings, 91 mg CuCI and 7.94 g of 1-bromo-4-
(ethylthiopheny)benzene in THF.
1H-NMR (300 MHz, CDCI3): 6= 7.50 d (2H); 7.38 d (2H); 4.43 s (1H); 4.39 dbr
(1H); 3.40-3.60 m (3H);
2.95 q (2H); 1.30 t (3H); 1.07s (3H); 0.87 s (3H); 0.53 s (3H).
b)
(1113,1713)-1144-(ethylsulphanyl)pheny1]-17-hydroxy-17-(pentafluoroethyl)estra-
4,9-dien-3-one
õpoi
0
OH
As in example 1c), 125 mg of the title compound was prepared from 200 mg of
the compound prepared in
2a) by reaction with semi-concentrated sulphuric acid in methanol.
1H-NMR (300 MHz, CDCI3): 6= 7.21 d (2H); 7.08 d (2H); 5.78 sbr (1H); 4.43 dbr
(1H); 2.93 q (2H); 1.29 t
(3H); 0.60 s (3H).
Example 3
(113,1713)-17-Hydroxy-11-(4-[(RS)-methylsulphinyl]pheny11-17-
(pentafluoroethyl)estra-4,9-dien-3-one
CA 02768407 2012-01-17
-15-
-S
F F
1
/ipee 0111
0 0
180 pl of 30% hydrogen peroxide solution was added to 0.5 ml of
trifluoroacetic acid at 23 C. It was stirred
for 30 minutes and then the mixture was added to a suspension, cooled to 10 C,
of 533 mg of the
compound prepared in example 1c), in 1.8 ml of trifluoroacetic acid. It was
stirred for a further 2 hours at
10 C. Then the reaction mixture was poured into ice water. It was stirred for
a further 2 hours and then the
precipitated product was filtered off. The raw product obtained was purified
by silica gel chromatography.
This gave 146 mg of the tine compound and 123 mg of the compound described in
example 4.
1H-NMR (400 MHz, CDCI3): 6= 7.58 d (2H); 7.38 d (2H); 5.80 sbr (1H); 4.50 dbr
(1H); 2.71 s (3H); 0.58 s
(3H) + 0.56s (3H) (mixture of the diastereomers).
Example 4
(1113,1713)-17-Hydroxy-1144-(methylsulphonyl)pheny1]-17-
(pentafluoroethyl)estra-4,9-dien-3-one
S
OH rs F F F
OF
,0
OH 0
5 g of the compound described in example 1 b) was dissolved in a mixture of
140 ml THF and 140 ml
methanol. A solution of 20 g Oxone in 94 ml water was slowly added dropwise
at 0 C. Then it was stirred
for a further 3.5 hours at 0 C. Then a mixture of water and dichloromethane
was added to the reaction
mixture. The phases were separated and the aqueous phase was extracted several
times with
dichloromethane. The combined organic phases were washed with saturated
aqueous sodium chloride
solution, dried over sodium sulphate and concentrated under vacuum. The raw
product was purified by
silica gel chromatography. This gave 3.8 g of the title compound.
1H-NMR (300 MHz, CDCI3): 6= 7.86 d (2H); 7.40 d (2H); 5.81 sbr (1H); 4.50 dbr
(1H); 3.07 s (3H); 0.51 s
(3H).
Example 5
(11[3,1713)-1144-(ethylsulphonyl)pheny1]-17-hydroxy-17-(pentafluoroethypestra-
4,9-dien-3-one
CA 02768407 2012-01-17
-16-
;
miLk;
,==
.40*
OH 0
As in example 4), after purification by silica gel chromatography, 183 mg of
the title compound was
obtained by reaction of 400 mg of the compound described in example 2a) with
1.56 g Oxone in a mixture
of 10 ml THF and 10 ml methanol.
1H-NMR (400 MHz, CDCI3): 6= 7.82 d (2H); 7.40 d (2H); 5.80 sbr (1H); 4.52 dbr
(1H); 3.13 q (2H); 1.28 t
(3H); 0.51 s (3H).
Example 6
(1113,1713)-11-[4-(benzylsulphanyl)pheny1]-17-hydroxy-17-
(pentafluoroethyDestra-4,9-dien-3-one
S.
Oi
0
a)
(5R,8S,11R,13S,14S,17S)-1144-(benzylsulphanyl)pheny1]-5',5',13-trimethy1-17-
(pentafluo roethyl)-
1,2,6,7,8,11,12,13,14,15,16,17-dodecahydrospiro[cyclopenta[a]phenanthrene-
3,2'41,3]dioxane]-5,17(4H)-
diol
F 40 s
OHJe....FA_
00 OH
F F
0 cflrli ________________ 0 O.
OH
As in example lb), 6.65 g of the title compound was prepared from 8.5 g of the
compound described in
la), 2.64 g magnesium shavings, 171 mg CuCI and 30.36 g of 1-benzylsulphany1-4-
bromobenzene in
THF.
1H-NMR (300 MHz, CDCI3): 6= 7.13-7.30 m (7H); 7.10 d (2H); 4.44 s (1H); 4.27
dbr (1H); 4.05 s (2H);
3.40-3.60 m (4H); 1.05s (3H); 0.87 s (3H); 0.51 s (3H).
b)
CA 02768407 2012-01-17
- 17 -
(11 p,1713)-1114-(benzylsulphanyl)phenyl]-17-hydroxy-17-
(pentafluoroethyl)estra-4,9-dien-3-one
401 s OHk F F F
k
S *
0.0 OH 00.
0
As in example 1c), 1.02 g of the title compound was prepared from 1.62 g of
the compound described in
example 6a) by reaction with semi-concentrated sulphuric acid in methanol.
1H-NMR (300 MHz, CDCI3); 6= 7.15-7.40 m (7H); 7.06 d (2H); 5.78 sbr (1H); 4.40
dbr (1H); 4.08 s (2H);
0.59 s (3H).
Example 7
N-R4-[(1113,1713)-17-hydroxy-3-oxo-17-(pentafluoroethypestra-4,9-dien-11-
yl]phenyl}(RS)(methyl) oxido-X6-
sulphanylidene]-4-methylbenzene sulphonamide
0=s.0
0,11 F F F
401Atilk H'F<F
/egriW
0
a)
N-[{4-[(5R,8S, 11R,139,149,17S)-5,17-Dihydroxy-5',5',13-trimethy1-17-(pe
ntafluoroethyl)-
1 5 1,2,4 ,5,6,7,8,11,12,13,14,15,16,17-
tetradecahydrospiro[cyclopenta[a]phenanthrene-3,2'-[1,3]dioxane]-11-
yliphenyl}(RS)(methyl)-M-sulphanylidene]-4-methylbenzene sulphonamide
,s
OH* 0=S=0
=". F F NI
F F
O. 111 XCF
j0
00
OH
3 g of the substance described in example 1b) was suspended in 80 ml
acetonitrile. 1.64 g of Chloramine-
T-Trihydrate was added and stirring was continued for 20 hours at 23 C. Then
the reaction mixture was
diluted with 70 ml dichloromethane. After filtering off precipitated sodium
chloride, it was concentrated in
vacuum. The raw product was purified by silica gel chromatography. This gave
3.16 g of the title
compound.
CA 02768407 2012-01-17
- 1 8 -1H-NMR (300 MHz, CDCI3): 6= 7.74 d (2H); 7.49 d (2H); 7.38 d (2H); 7.18
d (2H); 4,40 s (1H); 4.33 dbr
(1H); 3.40-3.70 m (4H); 2.80 (3H); 2.37 s (3H), 1.05 s (3H); 0.89 s (3H); 0.45
s (3H) (mixture of
diastereomers).
b) N-R4-[(5R,8S,11R,13S,14S,17S)-5,17-dihydroxy-5',5',13-trimethyl-17-
(pentafluoroethyl)-
1,2,4,5,6,7,8,11,12,13,14,15,16,17-
tetradecahydrospiro[cyclopenta[a]phenanthrene-3,2'41,3)dioxane]-11-
yliphenyll(RS)(methyl) oxido-A6-sulphanylidene]-4-methylbenzene sulphonamide
40
0=S=0
NI 0.S=0
11
F F F
11
C*,S F F
!µleFcF
O.
OH ee
3.16 g of the compound obtained in 7a) was dissolved in 2.5 ml acetonitrile
and 1.6 ml methanol. 1.22 g of
10 sodium carbonate and 234 ml of 30% hydrogen peroxide solution were
added. Then it was stirred for 2.5
hours at 23 C. The reaction mixture was then poured into water. It was
extracted several times with
dichloromethane. The combined organic phases were washed with saturated
aqueous sodium chloride
solution, dried over sodium sulphate and concentrated under vacuum. The raw
product was purified by
silica gel chromatography. This gave 2.56 g of the title compound.
15 1H-NMR (300 MHz, CDCI3): 6= 7.78-8.00 m (4H); 7.51 d (2H); 7.31 d (2H);
4.50s (1H); 4.44 dbr (1H);
3.45-3.67 m (7H); 2.46 s (3H); 1.09 s (3H); 0.91 s (3H); 0.51 s (3H) (mixture
of diastereomers).
c) N-[{4-[(1113,1713)-17-hydroxy-3-oxo-17-(pentafluoroethypestra-4,9-dien-11-
yl]phenyl)(RS)(methyl) oxido-
k6-sulphanylidene]-4-methylbenzene sulphonamide
40
0=5=0
NI 0=7=0
04
F 0, 1,F 11
F F F
411
oFF
ee
o 00 =
OH 0
As in example 1c), 2.2 g of the title compound was prepared from 2.72 g of the
compound prepared in 7b)
by reaction with semi-concentrated sulphuric acid in methanol.
CA 02768407 2012-01-17
- 19 -
1H-NMR (300 MHz, CDC13): 6= 7.95 d (2H); 7.86 d (2H); 7.45 d (2H); 7.28 d
(2H); 5.81 sbr (1H); 4.51 dbr
(1H); 3.41 s (3H); 2.40 s (3H); 0.51 s (3H) (mixture of diastereomers).
Example 8
(118,178)-17-Hydroxy-1144-(RS-methylsulphonimidoyl)pheny11-17-
(pentafluoroethyl)estra-4,9-dien-3-one
110
01=0 11% ,,0
F F F
o,g
F 401 Ol:"F(F
C''HXF'F et.
0
0
500 mg of the compound prepared in example 7c) was dissolved in 10 ml
chloroform. 1.15 ml of
concentrated sulphuric acid was added at 0 C and it was stirred for 7 hours at
0 C. Then the reaction
mixture was poured into saturated aqueous sodium hydrogen carbonate solution.
It was then made basic
by adding 5% NaOH. It was extracted several times with dichloromethane. The
combined organic phases
were washed with saturated aqueous sodium chloride solution, dried over sodium
sulphate and
concentrated under vacuum. The raw product was purified by silica gel
chromatography. This gave
306 mg of the title compound.
1H-NMR (300 MHz, CDC13): 6=7.91 d (2H); 7.39 d (2H); 5.81 sbr (1H); 4.50 dbr
(1H); 3.12s (3H) + 3.10 s
(3H); 0.56 s (3H) + 0.40 s (3H) (mixture of diastereomers).
Example 9
(1113,17(3)-17-Hydroxy-1144'-(methylsulphanyl)bipheny1-4-y11-17-
(pentafluoroethypestra-4,9-dien-3-one
,s 40
40 7,7
esti-
0 eio F
a) (5R,8S,11R,13S,14S,17S)-11-(4-(benzyloxy)pheny11-5',51,13-
trimethyl-17-(pentafluoroethyl)-
1,2,6,7,8,11,12,13,14,15,16117-dodecahydrospirolcyclopenta[a]phenanthrene-3,2'-
[1,3]dioxane]-5,17(4H)-
diol
OH F F
40 OH f F
o =
41,
CA 02768407 2012-01-17
-20-
2.47 g magnesium shavings were suspended in 5 ml THF and 50 pl dibromoethane
was added, while
stirring. A solution of 26.7 g of 1-bromo-4-(phenylmethoxy)benzene in 115 ml
THF was slowly added to the
suspension at 65 C. The resultant solution was cooled to 0 C. 301 mg CuCI was
added to it. It was stirred
for 10 minutes at 0 C and then a solution of 10 g of the substance described
in example la) in 70 ml THF
was added slowly. The reaction mixture was allowed to warm to 23 C while
stirring in the space of approx.
3 hours and was then stirred at this temperature for a further 10 hours. Then
saturated aqueous NH4CI
solution was added to the reaction mixture, with external cooling. It was
stirred for a further 30 minutes and
then extracted several times with ethyl acetate. The combined organic phases
were washed with saturated
sodium chloride solution and dried over sodium sulphate. The raw product was
purified by silica gel
chromatography followed by crystallization from a mixture of dichloromethane
and diisopropyl ether. This
gave 9.7 g of the title compound.
1H-NMR (400 MHz, CDCI3): 6:--= 7.30-7.50 m (5H); 7.12 d (2H); 6.88 d (2H);
5.02 s (2H); 4.43 s (1H); 4.28
dbr (1H); 3.50-3.60 m (3H); 3.42 d (1H); 1.06 s (3H); 0.87 s (3H); 0.56 s
(3H).
b) (5R,8S,11R,13S,14S,17S)-11-[4-(benzyloxy)pheny1]-5',5',13-trimethy1-17-
(pentafluoroethyl)-
1,2,6,7,8,11,12,13,14,15,16,17-dodecahydrospiro[cyclopenta[a]phenanthrene-3,2'-
[1,3]dioxane]-5,17(4H)-
diol
OH F
, HO
OH, r F
So F
0 eio F
oH
5.53 g ammonium formate and 972 mg palladium on activated charcoal (10%) were
added to a solution of
9.72 g of the compound described in 9a) in 100 ml methanol. It was stirred for
2 hours at 23 C and then
filtered on Celite . The filtrate was concentrated under vacuum. This gave 8.5
g of raw product, which was
used in the next stage without purification.
1H-NMR (300 MHz, CDCI3): 6=. 7.05 d (2H); 6.70 d (2H); 4.43 sbr (1H); 4.27 dbr
(1H); 3.50-3.58 m (3H);
3.41 sbr (1H); 1.94 s (3H); 0.86s (3H); 0.54 s (3H).
c) 4-
[(5R,8S,11R,13S,14S,17S)-5,17-Dihyd roxy-5', 51,13-trimethy1-17-(pentaflu
oroethyl)-
1,2,4 ,5,6,7,8,11,12,13,14,15,16,17-
tetradecahydrospiro[cyclopenta[a]phenanthrene-3,2'-[1,3]dioxan]-11-
yl]pheny1-1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulphonate
CA 02768407 2012-01-17
- 21 -
HO F FF F
OH F T
OH F F
F F FO 0
tF
0 F
0 00
OH
14.64 ml of a 1.6-molar solution of n-butyllithium in hexane was added at 0 C
to a solution of 9.16 g of the
compound described in 9b) in 100 ml absolute THF. It was stirred for 30
minutes at 0 C and then 5.62 ml
perfluorobutane-1-sulphonyl fluoride was added slowly. Then it was stirred for
a further 1.5 hours at 0 C.
Then the reaction mixture was poured into a mixture of 300 ml of saturated
sodium hydrogen carbonate
solution and 90 ml of 2N sodium hydroxide solution. It was stirred for 45
minutes and then extracted
several times with ethyl acetate. The combined organic phases were washed with
saturated sodium
chloride solution and dried over sodium sulphate. The raw product obtained was
purified by silica gel
chromatography. This gave 10.1 g of the title compound.
1H-NMR (400 MHz, CDCI3): 6= 7.28 d (2H); 7.18 d (2H); 4.42 s (1H); 4.34 dbr
(1H); 3.50-3.58 m (3H);
3.42 d (1H); 1.05s (3H); 0.86 s (3H); 0.50 s (3H).
d) (5R,8S,11R,13S,14S,17S)-5',5',13-trimeth y1-1144'-(methylsulp
hanyl)bipheny1-4-y1]-17-
(pentafluoroethyl)-1,2,6,7,8,11,12,13,14,15,16,17-dodecahyd rospiro[cyclo
penta [a]phenanth rene-3,2'-
[1,3]dioxane]-5,17(4H)-diol
F FF F
OH F 110
F F F 0 0
OH F F
0 SO = Fi-F)-F
OH
2 ml of a 2-molar aqueous sodium carbonate solution, 131 mg of lithium
chloride, 240 mg of 4-
(methylthio)phenylboronic acid and 192 mg of
tetrakis(triphenylphosphine)palladium were added to a
solution of 1.2 g of the compound described in 9c) in a mixture of 12 ml
toluene and 6 ml ethanol. Then it
was boiled under reflux for 2 hours. Then a mixture of ethyl acetate and water
was added to the reaction
mixture. It was extracted several times with ethyl acetate. The combined
organic phases were washed with
saturated aqueous sodium chloride solution, dried over sodium sulphate and
concentrated under vacuum.
The raw product was purified by silica gel chromatography. This gave 927 mg of
the title compound.
1H-NMR (300 MHz, CDCI3): 6= 7.45-7.55 m (4H); 7.30 d (2H); 7.27 d (2H); 4.45 s
(1H); 4.35 dbr (1H);
3.40-3.60 m (4H); 2.50 s (3H); 1.07 s (3H); 0.97 s (3H); 0.58 s (3H).
e)
(1113,1713)-17-hyd roxy-11-14'-(methylsulphanyl)bipheny1-4-y1]-17-
(pentafluoroethypestra-4,9-dien-3-one
CA 02768407 2012-01-17
- 22 -
A s
err7
0 **II F
0
0H
As in example 1c), 82 mg of the title compound was prepared from 120 mg of the
compound prepared in
9d) by reaction with semi-concentrated sulphuric acid in methanol.
1H-NMR (400 MHz, CDCI3): 6= 7.45-7.58 m (4H); 7.30 d (2H); 7.24 d (2H); 5.80
sbr (1H); 4.50 dbr (1H);
2.50 s (3H); 0.62 s (3H).
Example 10
(1113,1713)-17-hydroxy-1114'-(methylsulphonyl)bipheny1-4-y1]-17-
(pentafluoroethypestra-4,9-dien-3-one
0
/
4111 OH F
0.1110.+F
0
a) (5R,8S,11R,13S,14S,17S)-5',5',13-trimethy1-11 44'-
(methylsulphonyl)bipheny1-4-y1]-17-
(pentafluoroethyl)-1,2,6,7,8,11,12,13,14,15,16,17-
dodecahydrospiro[cyclopenta[a]phenanthrene-3,2'-
[1,3]dioxane]-5,17(4H)-diol
F FF F S=OH F 41
F ?(..;./(\F ..?:==-c, 40
40 OH r
4011, F F
0 04
OH
OH
As in example 9d), 256 mg of the title compound was prepared from 500 mg of
the compound described in
example 9c) and (4-methylsulphonylphenyl)boronic
acid in the presence of
tetrakis(triphenylphosphine)palladiurn, lithium chloride, 2-molar aqueous
sodium carbonate solution in a
mixture of toluene and ethanol.
1H-NMR (300 MHz, CDCI3): 6= 8.03 d (2H); 7.80 d (2H); 7.58 d (2H); 7.39 d
(2H); 4.48 s (1H); 4.45 dbr
(1H); 3.45-3.65 m (4H); 3.12 s (3H); 1.10 s (3H); 0.91 s (3H); 0.62s (3H).
b) (11p,17(3)-17-hydroxy-1144'-(methylsulphonyl)bipheny1-4-y1]-17-
(pentafluoroethypestra-4,9-dien-3-one
CA 02768407 2012-01-17
= -23-
0,0
0, /?
*OF
OH Fi
0 dk 1101.11''F F
F
As in example 1c), 62 mg of the title compound was prepared from 110 mg of the
compound prepared in
10a) by reaction with semi-concentrated sulphuric acid in methanol.
1H-NMR (400 MHz, CDCI3): 6= 8.00 d (2H); 7.75 d (2H); 7.55 d (2H); 7.30 d
(2H); 5.80 sbr (1H); 4.50 dbr
(1H); 3.09 s (3H); 0.65 s (3H).
Example 11
N-R4'-[(1113,1713)-17-hydroxy-3-oxo-17-(pentafluoroethyl)estra-4,9-dien-11-
ylibipheny1-4-yll(RS)(methyl)
oxido-X6-sulphanylidene]-4-methylbenzene sulphonamide
o=s_.
0,7s el
40 HiF.
1101. F
10 0 SS F
a) N-R4'-[(5R,8S,11R,13S,14S,17S)-5,17-dihydroxy-5',5',13-trimethyl-17-
(pentafluoroethyl)-
1,2,4,5,6,7,8, 11,12,13,14,15,16,17-
tetradecahydrospiro[cyclopenta[a]phenanthrene-3,2'-[1,3]dioxane]-11-
ylibipheny1-4-y1)(RS)(methyl)- X4-sulphanylidene]-4-methylbenzene sulphonamide
07¨
40
e
40 e"
F 40 C*1 (FF
0 SO*
7NvA
As in example 7a), 715 mg of the title compound was prepared from 800 mg of
the compound prepared in
example 9d) with Chloramine-T-Trihydrate in acetonitrile.
1H-NMR (300 MHz, CDCI3): 6= 7.65-7.80 (6H); 7.47 d (2H); 7.30 d (2H); 7.18 d
(2H); 4.45 s (1H); 4.39 dbr
(1H); 3.40-3.60m (4H); 2.87 (3H); 2.35 s (3H), 1.03 s (3H); 0.87 s (3H); 0.56
s (3H) (mixture of
diastereomers).
CA 02768407 2012-01-17
= - 24 -
b) N-[(4'-[(5R,8S,11R,13S,14S,17S)-5,17-dihydroxy-5',5',13-trimethy1-17-
(pentafluoroethyl)-
1,2,4,5,6,7,8,11,12,13,14,15,16,17-
tetradecahydrospiro[cyclopenta[a]phenanthrene-3,2'41,3]dioxan]-11-
Abipheny1-4-yll(RS)(methyl)oxido-X6-sulphanylideneJ-4-methylbenzene
sulphonamide
1101 1St
0=0, o=r
,Is 5c%7's 40
00 c.Ht.
Sio
0 F F F F
OH C*1
As in example 7b), 638 mg of the title compound was obtained from 709 mg of
the compound obtained in
example 11a) by reaction with 30% hydrogen peroxide solution and sodium
carbonate in a mixture of
acetonitrile and methanol.
1H-NMR (300 MHz, CDC13): 6= 8.04 d (2H); 7.87 d (2H); 7.78 d (2H); 7.50 d
(2H); 7.35 d (2H); 7.27 d
(2H); 4.46 s (1H); 4.40 dbr (1H); 3.40-3.60 m (4H); 3.46s (3H); 2.39s (3H);
1.07 s (3H); 0.87 s (3H); 0.56 s
(3H) (mixture of diastereomers).
c) N-R4'-[(1113,1713)-17-hydroxy-3-oxo-17-(pentafluoroethyl)estra-4,9-dien-11-
yl]bipheny1-4-y1)(RS)(methyl)-
oxido-X6-sulphanylidene]-4-methylbenzene sulphonamide
=
0=.
oo 40 e
40 40 iik"
F F
F 4010 F
0
As in example 1c), 523 mg of the title compound was prepared from 633 mg of
the compound prepared in
11b) by reaction with semi-concentrated sulphuric acid in methanol.
1H-NMR (300 MHz, CDC13): 6= 8.06 d (2H); 7.87 d (2H); 7.78 d (2H); 7.52 d
(2H); 7.20-7.35 m (4H); 5.80
sbr (1H); 4.51 dbr (1H); 3.45 s (3H); 2.39 s (3H); 0.62 s (3H) (mixture of
diastereomers).
Example 12
(11[3,1713)-17-hydroxy-1144'-(RS-methylsulphonimidoyl)bipheny1-4-y1]-17-
(pentafluoroethyl)estra-4,9-dien-
3-one
CA 02768407 2012-01-17
= - 25 -
*
o=s=0
HN, 4,0
07S
F F
OH F
OH ki<F
111"(4'T
F
F
0
As in example 8, 325 mg of the title compound was obtained from 500 mg of the
compound prepared in
example 11c) by reaction with concentrated sulphuric acid in chloroform.
1H-NMR (300 MHz, CDC13): 6-= 8.07 d (2H); 7.74 d (2H); 7.55 d (2H); 7.30 d
(2H); 5.80 sbr (1H); 4.51 dbr
(1H); 3.15 s (3H); 0.64 s (3H) (mixture of diastereomers).
Example 13
(118,1713)-17-hydroxy-17-(pentafluoroethyl)-11-(4'-sulphanylbipheny1-4-
yl)estra-4,9-dien-3-one
HS=
is
F F F
" F
Oi
0
a) (5R,8S,11R,13S,14S,17S)-5',5',13-trimethy1-17-(pentafluoroethyl)-11-(4'-
sulphanylbiphenyl-4-y1)-
1,2,6,7,8,11,12,13,14,15,16,17-dodecahydrospiroKyclopenta[a]phenanthrene-
3,2'41,3]dioxane]-5,17(4H)-
diol
HS .
F s
F FF F
40 OH F
40 OHFjF
111, F
¨/C0 oH
0 0.
0
---7C0 OH
As in example 9d), 478 mg of the title compound was prepared from 1 g of the
compound described in
example 9c) and (4-mercaptophenyl)boronic acid in the presence of
tetrakis(triphenylphosphine)palladium,
lithium chloride, 2-molar aqueous sodium carbonate solution in a mixture of
toluene and ethanol.
1H-NMR (400 MHz, CDCI3): 6.= 7.14-7.32 m (8H); 4.42 s (1H); 4.30 dbr (1H);
3.40-3,60 m (4H); 1.05 s
(3H); 0.88 s (3H); 0.54 s (3H).
b) (118,170)-17-hydroxy-17-(pentafluoroethyl)-11-(4'-sulphanylbipheny1-4-
yl)estra-4,9-dien-3-one
CA 02768407 2012-01-17
= - 26 -
HS HS
j(kF F
F
0 SO0
-7C0 OH
As in example 1c), 103 mg of the title compound was prepared from 200 mg of
the compound prepared in
13a) by reaction with semi-concentrated sulphuric acid in methanol.
1H-NMR (400 MHz, CDCI3): El= 7.20-7.38 m (6H); 7.11d (2H); 5.78 sbr (1H); 4.42
dbr (1H); 0.61 s (3H).
Example 14
4'-[(1113,178)-17-hydroxy-3-oxo-17-(pentafluoroethypestra-4,9-dien-11-y1]-N,N-
dimethylbipheny1-4-
sulphonamide
=
OH F
+F
OS F
0
a) 4'-[(5R,8S,11R,13S,14S,17S)-5,17-dihydroxy-5'5,13-trimethy1-17-
(pentafluoroethyl)-
1,2,4,5,6,7,8,11,12,13,14,15,16,17-
tetradecahydrospiro[cyclopenta[a]phenanthrene-3,2'11,31dioxane]-11-
yli-N,N-dimethylbipheny1-4-sulphonamide
0v?
FFFFF N010
40 OH F F O=oH.,FF
F F 0
IOC F F-.
0 00 A es
0 614
As in example 9d), 235 mg of the title compound was prepared from 300 mg of
the compound described in
example 9c) and [4-[(dimethylamino)sulphonyl]phenyliboronic acid in the
presence of
tetrakis(triphenylphosphine)palladium, lithium chloride, 2-molar aqueous
sodium carbonate solution in a
mixture of toluene and ethanol.
1H-NMR (300 MHz, CDCI3): 6= 7.83 d (2H); 7.73 d (2H); 7.52 d (2H); 7.33 d
(2H); 4.47 s (1H); 4.39 dbr
(1H); 3.40-3.60 m (4H); 2.75 s (6H); 1.06 s (3H); 0.88 s (3H); 0.57s (3H).
b) 4'-[(1113,1713)-17-hydroxy-3-oxo-17-(pentafluoroethypestra-4,9-dien-11-
y1]-N,N-dimethylbipheny1-4-
sulphonamide
CA 02768407 2012-01-17
- 27 -
0 0
''/N'S
014F F F-11/ 140
OH 0 .tt' F 7
F
eF
0 OS F
6H
As in example 1c), 113 mg of the title compound was prepared from 230 mg of
the compound prepared in
14a) by reaction with semi-concentrated sulphuric acid in methanol.
1H-NMR (400 MHz, CDCI3): ö= 7.83 d (2H); 7.72 d (2H); 7.55 d (2H); 7.30 d
(2H); 5.80 sbr (1H); 4.52 dbr
(1H); 2.75 s (6H); 0.64 s (3H).
Example 15
4-[(110,1713)-17-Hydroxy-3-oxo-17-(pentafluoroethyl)estra-4,9-dien-11-yli-N,N-
dimethylbenzene
sulphonamide
-7" 40 ri
00 F
0
a) 4-[(5R,8S,11R, 13S,14S,17S)-5, 17-Dihydroxy-5', 5',13-
trimethy1-17-(pentafluoroethyl)-
1,2,4 ,5,6,7,8, 11,12,13,14,15,16,17-
tetradecahydrospiro[cyclopenta[a]phenanthrene-3,2'-[1,3]d ioxan]-11-
yI]-N,N-dimethylbenzene sulphonamide
0 0
='
OH F 4) OH F
F
0
.00 0 00.1P F
OH
5.1 ml of a 2-molar solution of diisopropylmagnesium chloride in diethyl ether
was diluted with 10 ml THF,
with cooling (-10 C). Then 8.12 ml of a 2.5-molar solution of n-butyllithium
in hexane was added dropwise
at -10 C in the space of 30 minutes. It was stirred for a further 2 hours and
then 15.1 mg CuCI was added.
After stirring for a further 5 minutes, a solution of 500 mg of the substance
described in example 1a) in
5 ml THF was added. It was stirred for a further 3 hours at -10 C and then
heated slowly to 23 C. It was
stirred for a further 12 hours at 23 C. Then saturated aqueous NH4CI solution
was added to the reaction
mixture, with external cooling. It was stirred for a further 30 minutes and
then extracted several times with
ethyl acetate. The combined organic phases were washed with saturated sodium
chloride solution and
dried over sodium sulphate. The raw product was purified by silica gel
chromatography. This gave 214 mg
of the title compound.
CA 02768407 2012-01-17
- 28 -1H-NMR (300 MHz, CDC13): 6= 7.65 d (2H); 7.40 d (2H); 4.45 s (1H); 4.38
dbr (1H); 3.40-3.60 m (4H);
2.69 s (6H); 1.03 s (3H); 0.89 s (3H); 0.49 s (3H).
b) 4-[(1113,1713)-
17-Hydroxy-3-oxo-17-(pentafluoroethyl)estra-4,9-dien-11-y1]-N,N-
dimethylbenzene
sulphonamide
0, ,0
F op OH F
rlIFIF4'F 11111+F
As in example 1c), 74 mg of the title compound was prepared from 100 mg of the
compound prepared in
15a) by reaction with semi-concentrated sulphuric acid in methanol.
1H-NMR (400 MHz, CDC13): 6= 7.69 d (2H); 7.38 d (2H); 5.80 sbr (1H); 4.04 dbr
(1H); 2.68 s (6H); 0.52 s
(3H)
Example 16 Progesterone receptor¨antagonistic action in stable transfectants
of human
neuroblastoma cells (SK-N-MC cells) with the human progesterone A or
progesterone B receptor
and an MTV-LUC reporter construct
SK-N-MC cells (human neuroblastoma cells), which have been stably transfected
with plasmids,
which express the human progesterone receptor B (pRChPR-B-neo) or the human
progesterone receptor
A (pRChPR-A-neo) and a reporter construct (pMMTV-LUC), were incubated for 24
hours either in the
absence (negative control) or in the presence of increasing amounts of the
respective test compound
(0.01 nmo1/1, 0.1 nmo1/1, 1 nmo1/1, 10 nmo1/1, 100 nmo1/1 and 1 pmo1/1), in
order to determine the agonistic
efficacy. As positive control of reporter gene induction, the cells were
treated with the synthetic gestagen
promegestone (0.01 nmo1/1, 0.1 nmo1/1, 1 nmo1/1, 10 nmo1/1, 100 nmo1/1 and 1
pmo1/1). For determination of
the antagonistic activity, the cells were treated with 0.1 nmo1/1 promegestone
and additionally with
increasing amounts of the respective test compound (0.01 nmo1/1, 0.1 nmo1/1, 1
nmo1/1, 10 nmo1/1,
100 nmo1/1 and 1 pmo1/1). The activity of the LUC reporter gene (LUC =
luciferase) was determined in the
= 25 cell lysates and was measured as RLU (relative light
units). All measured values are given as percentage
efficacy and as EC50 or 1050 concentrations.
1113-(4-Acetylpheny1)-20,20,21,21,21-pentafluoro-17-hydroxy-19-nor-17a-pregna-
4,9-dien-3-one and
20,20,21,21,21-pentafluoro-17-hydroxy-11 B-[4-(hyd roxyacetyl)pheny1]-19-nor-
17a-pregna-4,9-dien-3-one,
very potent and therefore preferred examples from W098/34947 and
W02008/058767, were tested as
comparative compounds along with the test compound.
CA 02768407 2012-01-17
- - 29 -
- a) agonistic activity:
None of the stated test compounds shows agonistic activity.
b) antagonistic activity:
All of the stated compounds show 100% antagonistic efficacy.
The antagonistic efficacy of the compounds is presented in Table 1.
Progesterone receptor A (PR- Progesterone receptor B (PR-
Al 131
Compound Potency Efficacy Potency Efficacy
I050 [nmo1/1] EN IC50 [nmo1/1]
EN
1113-(4-Acetylpheny1)-20,20, 21,21,21- 0.014 100 0.02
100
pentafluoro-17-hydroxy-19-nor-17a-pregna-
4,9-dien-3-one
. 20,20,21,21,21-Pentafluoro-17-hydroxy- 0.18 100 . 0.28
100
111344-(hydroxyacatyl)pheny1]-19-nor-17a-
pregna-4,9-dien-3-one dien-3-one
,
,
Example 1 0.011 100 0.012 100
Example 2 0.01 100 0.01 100
Example 3 0.11 100 0.12 100
Example 4 0.096 100 0.087 100
Example 5 0.1 100 0.09 100
Example 6 0.2 100 0.23 100
Example 7 1.0 100 0.8 100 '
Example 8 0.9 100 0.9 100
Example 9 0.01 100 0.01 100
Example 10 0.011 100 0.013 100
Example 11 0.01 100 0.01 100
Example 12 0.08 100 0.08 100
Example 13 0.072 100 0.072 100
Example 14 0.01 100 0.01 100
Example 15 0.1 100 0,2 100
CA 02768407 2012-01-17
- 30 -
Example 17 Abortion test on female rats
The action of progesterone and of the progesterone receptor is a fundamental
precondition for
successful pregnancy or gestation in mammals. The progesterone-antagonistic
action of the compounds
according to the invention was tested on pregnant rats (6 rats per group) on
day 5 to 7 post coitum with
conventional housing and feeding conditions.
After successful hand mating, the pregnant animals (presence of sperm in the
vaginal smear on day 1 of
pregnancy = dl p.c.) were randomized and divided into the treatment group and
the control group. The
animals then each received subcutaneously or orally 0.15; 0.5; 1.5 or 5 mg/kg
of the test compound or
1.0 ml/kg of vehicle (benzyl benzoate/castor oil: 1+4 [v/v]) daily from day 5
to day 7 (d5 ¨ d7 p.c.).
Autopsy was carried out on day 9 (d9 p.c.). As a characteristic of
progesterone receptor
antagonistic, action, the uterus was examined for the presence of nidation
sites. Complete absence, or also
the presence of pathological, haemorrhagic or otherwise abnormal nidation
sites on day 9 (d9 p.c.) was
assessed as abortion. The results of the tests are shown in Table 3.
Table 3: Results for the rat (termination of early pregnancy)
Test compound according to Daily dose Abortion rate
[mg/kg] s.c. or p.o. [%]
Vehicle 0
Example 1 0.5 80
(116,176)-17-hydroxy-1144- 1.5 100
(methylsulphanyl)phenyI]-17-
(pentafluoroethypestra-4,9-dien-3- 5.0 100
one
Example 4 0.15 40
(116,176)-17-hydroxy-11-[4-
0.5 100
(methylsulphonyl)phenyI]-17-
(pentafluoroethyl)estra-4,9-dien-3- 1.5 100
one
5.0 100
Example 8 0.15 40
(11(3,17(3)-17-hydroxy-11-[4-(RS-
methylsulphonimidoy1)-phenyl]-17-
0.5 100
(pentafluoroethyl)estra-4,9-dien-3- 1.5 100
one
5.0 100
CA 02768407 2012-01-17
- 3 1 -
Example 18) Metabolic stability of (11(3,1713)-17-hydroxy-1144-
(methylsulphonyl)phenyl]-17-
(pentafluoroethyl)estra-4,9-dien-3-one and (11(3,17p)-17-hydroxy-1144'-
(methylsulphony1)-bipheny1-
4-y1]-17-(pentafluoroethyl)estra-4,9-dien-3-one in human liver microsomes
(HLM)
Isolated human liver microsomes ( HLM) wer e used for assessing the metabolic
stability of
compounds of general formula I.
Incubations were carried out with 2.4 ml of HLM solution (0,5 mg/ml protein
content), 30 pl of the test
compound (final concentration 1 pM) and 0.6 ml of the cofactor mixture (=
NADPH-generating system of 3
IU glucose-6-phosphate dehydrogenase, 14.6 mg glucose-6-phosphate, 1.2 mg
NADP) at 37 C in 100 mM
phosphate buffer at pH 7.4. Samples are taken at 6 time points (2-60 min),
precipitated with an equal
volume of methanol and the recovery of the test substances used in the
supernatant is determined by LC-
MS/MS analysis. The intrinsic clearance of the substance in the liver
microsome preparation can be
calculated from the half-life found for the breakdown of the substance. Based
on this, together with various
physiological characteristics according to the well-stirred model, it is then
possible to predict a (metabolic)
in vivo clearance with respect to phase I reactions. The (metabolic) in vivo
clearance in humans predicted
correspondingly for the test compounds (1113,1713)-17-hydroxy-1114-
(methylsulphonyl)pheny11-17-
(pentaff uoroethyl)estra-4,9-dien-3-one and (11[3,1713)-17-hydroxy-1144'-
(methylsulphonyl)bipheny1-4-y11-
17-(pentafluoroethyl)-estra-4,9-dien-3-one was very low: 0.1 Uh/kg and <0.01
L/h/kg, respectively.
Example 19) Permeation of
(11(3,1713)-17-hydroxy-1114-(methylsulphonyl)pheny1]-17-
(pentafluoroethyl)estra-4,9-dien-3-one in Caco-2 cells.
For the permeation studies, Caco-2 cells with a cell count of 300000 cells/ml
were cultivated on Transwell
Clear filter inserts (polyester, pore size 0.4 pm) in 12-well cell culture
plates for at least 14 days in cell
culture medium (1.5 ml) at 37 C, 5% CO2 and 95% air humidity. Before the test,
to verify the
"compactness" of the cell monolayer, the transepithelial resistance (TEER
value) was determined, which
must be greater than 300 0 cm2. Then the cell culture medium was replaced with
hot transport buffer
(0.5 ml apical, 1.5 ml basolateral) and the cells were equilibrated in it for
5 min. The permeability test was
performed in duplicate at a substance concentration of 2 pM. At the start of
the experiment, 100 pl
(Ap0 min) was taken from the apical compartment and 100 pl of ice-cold
stopping solution was added to it
immediately. The filters were then incubated at 37 C with gentle shaking for
90 min, then 100 pl was taken
again from the apical side (Ap90 min) and 400 pl from the basolateral side
(Bas90 min) and in each case
the same volume of stopping solution was added. After further dilution of the
samples with 4 times the
volume of stopping solution/transport buffer (1+1) they were sedimented
overnight at -20 C and the
supernatant was analysed by LCMS/MS. The Papp value of the substances was
calculated from the
following formula.
CA 02768407 2012-01-17
- 32 -
Vres ACres
Papp= =
A' C10 don At
Vres: buffer volume on the receptor side; A: filter area = 1 cm2; CA don:
concentration of substance on the
donor side; ACres/L,t: change in concentration of substance over time on the
receptor side
(1113,17B)-17-Hydroxy-1144-(methylsulphonyl)pheny11-17-(pentafluoroethypestra-
4,9-dien-3-one showed a
very high permeation of 104 nm/s in this assay.
Example 20) Investigation of the action on the cardiovascular system (incl.
ECG) of anaesthetized
beagle dogs
(1113,17p)-17-Hydroxy-11-[4-(methylsulphonyl)pheny1]-17-
(pentafluoroethyl)estra-4,9-dien-3-one, dissolved
in a mixture of PEG 400 and HP-B.-CD (60% PEG 400, 40% HP-13-CD 30%), was
administered
intravenously to anaesthetized female beagle dogs. The body weight of the dogs
was > 9 kg. 3 dogs were
treated per group and additionally 3 dogs in the control group. 0.1; 0.33 and
1 mg/kg of the substance was
administered in 3 consecutive infusions, in each case over a period of 30
minutes. The maximum amount
of vehicle was 0.4 ml per kg for 30 minutes. Blood samples were taken from the
animals at various time
points. The highest plasma level (average for all 3 animals) was 1650 ng/ml at
the end of the third infusion.
In the tested dose range, in comparison with the control, no biologically
relevant effects on the
cardiovascular system (pulmonary artery pressure, systemic arterial blood
pressure, heart rate, ECG) were
observed.