Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02769060 2012-02-17
=
- 1 -
SORBENTS FOR THE RECOVERY AND STRIPPING OF ACID GASES
FIELD OF THE INVENTION
The present invention relates generally to active amine composite sorbents for
capturing
acidic gases from, for example, a fluid stream, and more particularly to
sorbents, processes,
methods, systems, uses, apparatus, and the like for the adsorption and/or
absorption of such acid
gases from such a fluid stream.
BACKGROUND OF THE INVENTION
The combustion of fuels containing hydrogen and carbon, such as coal and
natural gas,
produces significant volumes of gaseous exhaust waste streams that contain one
or more
undesirable gaseous compounds such as one or more of the acid gases. Acid
gases such as
carbon dioxide (CO2), sulfur gases (e.g. SO2, H2S), and oxides of nitrogen
(NO), can cause
significant environmental pollution and health risks. There has been
increasing concern about the
damage caused by these contaminants, which has led to an increased demand to
reduce their
emission, including CO2.
Separation of acid gases, such as CO2 and H2S from gas streams can be achieved
via
chemical absorption or chemical/physical absorption processes. The most widely
used process
for CO2 separation and capture from acid gas-containing streams is the
chemical absorption
process utilizing liquid amine solutions. Aqueous solutions of
monoethanolamine (MEA) or
diethanolamine (DEA) are commonly used in the wet chemical absorption and low-
pressure
stripping of CO2. In this process, the CO2 reacts with the liquid amine
solution to form a
carbamate species. Upon heating, the carbamate species decomposes to release
the absorbed CO2
and regenerate the amine solution. This process can be costly and energy
intensive. For example,
the liquid amine solution has a limited life time due to its degradation
through oxidation.
Furthermore, the high corrosivity of the utilized amine makes it difficult to
use high
concentrations of the amine solutions. Typically, only 10-30 wt% solutions of
MEA are
employed to capture CO2 from the acid gas containing streams, which
necessitates the heating
and cooling of large volumes of water.
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-2-
Acid gas capture technology utilizing solid sorbents has increasingly received
attention
due to its potential for reducing corrosion, energy cost and mass/heat
transfer. Such technology
uses a porous solid sorbent to reversibly adsorb the CO2 and/or H2S from the
acid gas containing
streams.
Synthetic zeolite A and X types can be effective adsorbents for CO2. U.S. Pat.
3,981,698
to Leppard, U.S. Pat. 4,039,620 to Netteland, U.S. Pat. 4,711,645 to Kumar,
U.S. Pat. 4,986,835
to Uno, and U.S. Pat. 5,156,657 to Ravi describe the use of 5A, 10A and 13X as
CO2 sorbents. In
these processes, the molecular sieves physically adsorb the CO2 and are
regenerable at ambient
temperature and pressure. However, at ambient temperature, desorption cycles
are too short to
desorb all adsorbed CO2. Consequently, some of the adsorbed CO2 remains on the
molecular
sieve, which reduces its capacity.
In alternative embodiments of the solid sorbents, amino-containing moieties
have been
impregnated or grafted onto the surface of the solid supports. For instance,
WO 2004/054708
describes the use of a water-tolerant sorbent containing grafted amine on a
silica support. U.S.
Pat. App. Publ. No 2007/0149398 to Jones et al discloses a method of preparing
a CO2 solid
sorbent containing a hyperbranched amine polymer covalently bonded to at least
one surface
oxygen. At atmospheric pressure, the average CO2 capacity was said to be 4.4
mmol (CO2)/g
sorbent, or 0.1936 g CO2/g sorbent.
Liquid oxygenated amines, such as diisopropanolamine, have been used in
removing CO2
from acid gas containing streams. U.S. Pat. 4,044,100 describes the use of
liquid mixtures of
diisopropanolamine and polyethylene glycol in acid gas removal from gaseous
streams. The use
of the oxygenated compounds, such as polyols, glycols and ethers in CO2
absorption/desorption
cycles of solid sorbents has been examined and used in the space shuttle CO2
removal system.
In U.S. Pat. Nos. 6,364,938; 5,876,488; 5,492,683 and 5,376,614 describe a
solid sorbent
containing a high surface area solid polymer support and polyethyleneimine/
polyethylene
glycol. The concentration of both the polyethylenimine and polyol utilized was
from 1 wt% to 25
wt% of the total weight of the absorbent. Hicks et al, Journal of American
Chemical Society
130:2902 (2008), described a method for impregnating a mesoporous silica
support with
different amounts of polyethyleneimine. However, the resulting material was
very sticky due the
A8124670WO\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
,
-3-
addition of the polyethyleneimine to the internal and external surface of the
silica support. In
addition, a significant pressure drop was observed because of the clogging in
the absorbing
column. Therefore, the CO2 capacity could not be determined.
U.S. Pat. 7,795,175 to Olah describes a process for CO2 absorption using solid
nano-
particles of a silica support, polyethylenimine and polyol. The concentration
of the utilized
polyethylenimine was 25 wt% to 75 wt% from the total weight of the sorbent and
polyol was in
an amount up to 25 wt% of the total weight of the absorbent. The CO2 capacity
was 0.117 g
(CO2)/g of absorbent. Nevertheless, the small particle size of the sorbent
makes it difficult to
conduct the absorption/desorption cycles at high pressure due to the pressure
drop in the
absorption column.
As noted above, the prior art sorbents and methods have one or more
undesirable
characteristics.
SUMMARY OF THE INVENTION
As used herein, the term 'acid gas' refers to gases that form acidic solutions
when mixed
with water.
As used herein, "a" or "an" means "at least one" or "one or more".
The present disclosure provides a novel acid-gas sorbent comprising a
particular amine-
composite which may be directly introduced in a fluid stream to adsorb acid
gases such as CO2
or H2S therefrom, or may optionally be impregnated on a porous support through
which such
fluid may be passed to thereby "strip" such acid gases from within said fluid.
The amine-composite of the present invention may be formed as an adduct, from
a first
component comprising an amine compound at a concentration of from about 1 wt %
to about 75
wt % of the final composite; a second component comprising a hydrophilic
polymer and/or a
pre-polymer compound at a concentration of from about 1 wt% to about 30 wt %
of the final
composite; and a third component comprising a carboxylic acid, a cross-linking
agent, and/or a
coupling agent at a concentration of from about 0.01 wt % to about 30 wt % of
the final
composite.
A8124670WO\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-4-
The present disclosure further provides methods of the present amine-composite
sorbents,
uses of such sorbents, and processes of absorbing/adsorbing acid gases from a
fluid stream.
Accordingly, in a broad embodiment of the present invention, such invention
comprises
an acid-gas sorbent comprising an amine composite, said composite comprising:
(i) a first component comprising an amine compound at a concentration of
from
about 1 wt % to about 75 wt % of the final composite;
(ii) a second component comprising a hydrophilic polymer and/or a pre-
polymer
compound at a concentration of from about 1 wt% to about 30 wt % of the final
composite; and
(iii) a third component which serves as a coupling, crosslinking or
catalyzing agent to
induce a reaction between the first and second components, at a concentration
of from about
0.01 wt % to about 30 wt % of the final composite.
The amine is preferably a primary, secondary, or tertiary alkylamine, aromatic
amine,
polyamine, or a combination thereof.
In a preferred embodiment, the second component becomes chemically bonded to
the
first component.
In a greatly preferred embodiment, the adduct is formed within, situated on,
impregnated
in, or uniformly applied and dispersed within, a solid porous support. Such
solid porous support
preferably comprises silica, alumina, silica-alumina, zeolite, carbon,
precipitated oxides, ceria,
titania, or a combination thereof.
Use of an acid-gas sorbent according as per any of the above compositions for
the
absorption and/or adsorption of an acid gas also forms part of the invention.
In a further embodiment, the present invention comprises a method of preparing
an acid-
gas sorbent, comprising the steps of combining:
(i) a first component comprising an amine compound at a concentration of from
about 1 wt % to about 75 wt % of the final composite;
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-
..
- 5 -
(ii) a second component comprising a hydrophilic polymer and/or a pre-
polymer compound at a concentration of from about 1 wt% to about 30 wt % of
the
final composite; and
(iii) a third component which serves as a coupling, cross-
linking or catalyzing
agent to induce a reaction between the first and second components, at a
concentration of from about 0.01 wt % to about 30 wt % of the final composite.
The present invention further comprises a method of removing an acid gas from
a fluid
stream containing the acid gas, the method comprising:
a. providing an incoming fluid stream comprising the acid gas; and
b. bringing the fluid stream into contact with a sorbent as described above,
with or
without a porous solid support, under conditions suitable for absorption
and/or
adsorption of the acid gas by the sorbent;
wherein the fluid stream comprises a lesser amount of the acid gas after
contacting
the sorbent.
In a further refinement of the above method, the invention comprises a method
of
removing an acid gas from a fluid stream containing the acid gas, the method
comprising:
a. providing an incoming fluid stream comprising the acid gas;
b. exposing an adduct formed from the combination of:
i) a first component comprising an amine compound at a concentration of from
about 1 wt % to about 75 wt % of the final composite;
(ii) a second component comprising a hydrophilic polymer and/or a pre-polymer
compound at a concentration of from about 1 wt% to about 30 wt % of the final
composite; and
(iii) a third component which serves as a coupling, crosslinking or catalyzing
agent to induce a reaction between the first and second components, at a
concentration of from about 0.01 wt % to about 30 wt % of the final composite;
A8124670W0 \CAL_LAW \ 1 772878 \ 1
CA 02769060 2012-02-17
s
-6-
to said incoming fluid stream, under conditions suitable for absorption and/or
adsorption
of the acid gas by the adduct, so as to substantially or at least partially
remove such acid
gas from said fluid stream.
Thereafter, the acid gas may be de-adsorbed from the adduct by exposing the
adduct to
higher or lower temperatures or pressures.
Specifically, in a further refinement of the above method, where the fluid
stream is a
hydrocarbon-containing fluid stream recovered from an underground formation
containing the
acid gas carbon dioxide, and wherein it is desired to recover such carbon
dioxide from such fluid
stream and re-inject said carbon dioxide into said underground formation in a
closed loop
recovery system in respect of such carbon dioxide, such method further
comprises the
subsequent step, after step b) of:
c) exposing said adduct to a pressurized and heated stream of carbon dioxide,
to
force carbon dioxide within said adduct to be released into said pressurized
and heated
stream.
Such carbon dioxide recovered (ie stripped) from the fluid stream can then be
re-injected
downhole into the underground hydrocarbon-containing formation, thereby
creating a closed-
loop system for the injection and recovery of carbon dioxide during oil
recovery from the
underground formation.
The second component used in the above method of the present invention may
comprise
a polyol, and said third component comprise a cross-linking agent.
The method contemplates that the so-formed adduct may be dispersed directly in
said
fluid stream, including, in the case of a gaseous stream, spraying such adduct
in the form of
small droplets, into said acid gas stream. Alternatively, water may first be
added to said adduct
prior to dispersing said adduct and said water into the incoming fluid stream.
In an alternative embodiment of the above method, the adduct may be provided
on, or
formed within, pores of a solid porous support member, and the fluid stream
passed through said
solid porous support.
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-7-
In a still further embodiment of the above method, the second component is a
polyol
dissolved in a solvent which does not react with the amine composite, and the
adduct is
provided in solution into pores within a solid porous support, and the solvent
allowed to
evaporate leaving the adduct in the form of a thin residual film surrounding
or within the pores
of the solid porous support. Thereafter the fluid stream is passed through the
solid support
under conditions suitable for absorption and/or adsorption of the acid gas by
the adduct, to
thereby strip the acid gas, such as CO2 and/or H2S, from the fluid stream.
This summary does not necessarily describe the entire scope of the present
invention.
Other aspects, features and advantages of the invention will be apparent to
those of ordinary skill
in the art upon review of the following description of specific embodiments of
the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic graph of the a carbon dioxide absorption process;
Figure 2 is a schematic graph of the carbon dioxide absorption using liquid
absorbing
fluid at atmospheric pressure;
Figure 3 is the FT-IR spectra of the sorbent described in Example 1, PVA and
PEI;
Figure 4 shows is a CO2 capacity comparison (at different pressures) of the
sorbent of
Example 3 versus a supported amine-polyol sorbent;
Figure 5 is a CO2 capacity comparison of the sorbent described in Example 3
versus
certain known sorbents;
Figure 6 represents the thermal stability of the sorbent described in Example
3;
Figure 7 is a graph illustrating repeated CO2 and H2S absorption-desorption
cycles of
Example 2;
Figure 8 shows the reaction of PEI with PVA in the presence of acetic acid;
Figure 9 shows the coupling of PVA with PEI using disuccinimidyl carbonate;
and
Figure 10 shows the coupling of PVA with PEI using N,N1-
Dicyclohexylcarbodiimide.
A8124670W0\CALLAW\ 1772878\1
CA 02769060 2012-02-17
-8-
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Use of examples in the specification, including examples of terms, is for
illustrative
purposes only and is not intended to limit the scope and meaning of the
embodiments of the
invention set out and described in the disclosure. Numeric ranges are
inclusive of the numbers
defining the range. In the specification, the word "comprising" is used as an
open-ended term,
substantially equivalent to the phrase "including, but not limited to," and
the word "comprises"
has a corresponding meaning.
The present invention provides active amine composite sorbents for capturing
acidic
gases. Acid gases may be absorbed and/or adsorbed by the sorbent and may be
desorbed at
preferably elevated pressure and/or temperature. For example, the desorption
may occur when
the acid gases are compressed for pipelining or deep well disposal thus
providing an energy
consumption advantage. The present sorbents may be employed in any suitable
form such as in
the liquid state or in a solid supported form.
Acidic gases can be captured from any suitable source. These may include small
gas
emissions or even large-scale processes, such as flue gas streams from
oilfield in-situ combustion
processes, power plants, coal or oil gasifiers and natural gas, as well as gas
streams from
hydrogen and ammonia production facilities.
In certain embodiments, the present absorbents allow for the absorption of
acidic gases,
including but not limited to CO2 and H2S gases, at capacities equivalent to or
higher than those
obtained using liquid amine systems.
Polyols that are chemically reactive toward amines can be used in combination
with
amines to provide an absorbent of higher acid gas absorption capacity. The
present adsorbates
comprise reactive adducts of amines, polyols and crosslinkers and the adducts
may be deposited
within porous solids which can provide structural integrity and a substrate
means of uniformly
exposing a gas stream to the amine composite. Active groups (such as primary,
secondary, and
tertiary amines, hydroxyl groups) are at least partially located inside the
pores and/or pore
channels of the solid substrate/support. The solid support may also provide a
higher surface area
for the gas/amine contact. For example, the present support may have a surface
area ranging
from 50-500 m2/g, and potentially up to 2000 m2/g. The pore size of the solid
support is
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-9-
preferably large enough to enable loading of adsorption active sites and to
allow passage of CO2,
H2S, or other acidic components. Exemplary solid porous supports include, but
are not limited to,
polymeric supports, alumina, carbon, molecular sieves, carbon nanotubes,
nanosilica,
organosilica, and mesoporous silica.
Any suitable amine may be used in manufacturing the present sorbents. Amines
include,
but are not limited to, primary, secondary, tertiary alkyl and alkanoamines,
aromatic amines,
polyamines, mixed amines, and suitable combinations thereof. It is preferred
that the sorbent
comprise primary, secondary and/or tertiary amines. These amines show good
activity toward
acidic gas absorption. Preferred amines include those with high
nitrogen/carbon ratios. Such
amines can provide a high density of active functional groups relative to
molecular weight, and a
high concentration of amine groups loaded on the support. Generally, in acidic
gas separation
processes the amine/amines utilized should have high absorption capacity, high
regeneration
ability, low volatility, and high thermal and oxidation stability.
Amines for use herein may be selected from, but are not limited to,
monoethanolamine
(MEA), diethanolamine (DEA), methyldiethanolamine (MDEA), triethanolamine,
tetraethylenepentaamine, polyethyleneimine, cyclic amines, and suitable
derivative/combinations
thereof. Polymeric amines, such as polyethyleneimines (PEI) may be useful
because of their
thermal stability, low volatility, high N/C ratio and high number of primary,
secondary and
tertiary amine groups. Polyethyleneimines of molecular weight greater than 600
are preferred.
Any suitable concentration of amine may be used in the present sorbent. For
example, the
amine concentration may be from about 1 wt % to about 75 wt% of the finished
sorbent.
The present sorbent may comprise one or more polyols. Any suitable polyol may
be used
including, but not limited to, ethylene glycol, polyethylene glycol, polyvinyl
alcohol, and
combinations thereof. Preferred polyols for use herein have low volatility,
high thermal stability,
and/or are chemically reactive toward amine groups. Polyols of any suitable
molecular weight
may be used such as about 1,000 AMU to 100,000 AMU. While not wishing to be
bound by
theory it is believed that the incorporation of the polyols in the pores and
pore channels of the
solid porous member improves the acid gas physical adsorption on the sorbent
by increasing the
volume of gas exposed to said amine composite, but may also enhance the
sorbent thermal
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-10-
stability. Moreover, it is believed that coupling of the amines and
hydrophilic polymer improves
their polymer/support cohesive properties and reduces volatility. This can
lead to high acidic gas
absorption capacity over a range of temperatures and pressures such as for
example, from 20 C
or lower to 100 C and atmospheric to 1500 psig. Enhanced thermal stability of
a sorbent will
allow for multiple absorption/desorption cycles with minimal loss of capacity.
The present sorbent may be prepared in any suitable manner. Various synthetic
methods
are known. Once prepared the sorbent may be examined for its thermal stability
and acidic gas
absorption capacity.
The present sorbent may be an active amine composite comprising a first
component, a
second component, and a third component.
The first component may include, but is not limited to, alkylamines, such as
monoethanolamine (MEA), diethanolamine (DEA), diisopropylamine (DIP);
arylamine;
alkylarylamine, cyclicamine, amine-containing polymers such as,
polyethyleneimine, polydiethyl
aminoethyl methacrylate, amine grafted poly vinyl alcohol, amine-grafted
polyethylene glycol,
amine-grafted sugar, amine-grafted polyesters, hyperbranched polyamine-grafted
polyol, or
combinations thereof. The amount of active absorption amine in the composite
may range from
about 1 wt% to about 75 wt%, preferably from about 10 wt% to about 40 wt %.
The second component may comprise a hydrophilic component that reacts with the
amine
groups in the first component. The hydrophilic component may include, but is
not limited to,
carbohydrates (e.g. sugar), ethylene glycol, polyethylene glycol, polyvinyl
ester, polyvinyl
alcohol, or combinations thereof The amount of second component in the present
composite
may range from about 1 wt% to about 75 wt%, preferably from about 20 wt% to
about 40 wt%,
more preferably from about 5 wt% to about 20 wt%, and most preferably from
about 1 wt% to
about 5 wt.
The third component of the present composite may comprise a coupling,
crosslinking or
catalyzing agent to induce a reaction between the first and second components.
The third
component may include, but is not limited to, mineral acids, carboxylic acids
containing 1-4
carbon atoms (steric hindrance), di-carboxylic acids, poly-functional or di-
functional aldehydes
(e.g. formaldehyde, glutaraldehyde, glyoxal), or combinations thereof. The
concentration of the
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-11-
third component may range from about 0.1 wt% to about 30 wt%, from about 5 wt%
to about 15
wt%, from about 1.5 wt% to about 10 wt%. The addition of the third component
when forming
the composite enhances the adhesion of the polyol component to a solid porous
support, if a
porous solid support is further employed. The third component is a catalyst or
a coupling agent
that promotes the coupling of active groups in the amine and/or polyol, and
does not necessarily
get incorporated into the final composite (eg. see composite formed as end
product in Figure 8
which lacks such third component). In a first embodiment, an amine-based
sorbent can be
prepared by a conventional impregnation process of a pre-prepared active amine
composite as
described herein or by reacting the three components of the active amine
composite on the
porous solid support.
Synthesis of the pre-prepared active amine composite may be carried out via a
refluxing
route of a solution containing amine, any polyol and carboxylic acid
components, for example
for 5 hours and at 70 C. The active amine composite may then precipitated
using a proper mixed
solvent process. When the impregnation method is employed, the solid porous
support can be
impregnated with a solution of the pre-prepared active amine composite
preferably utilizing the
incipient wetness process. The amine-supported sorbent may then dried by
evaporation.
In a further embodiment, the present amine-based sorbent may by synthesized by
reacting
the active amine component with a second component catalytically inside the
pores of the solid
support. For example, polyol may be dissolved in a suitable solvent and a
solid porous support
then impregnated with the prepared polyol solution preferably utilizing the
incipient wetness
process. The solvent may then removed via, for example, a slow evaporation
method to form a
thin film of the polyol inside the pores and pore channels of the solid
support. Amine solution
may then be prepared by dissolving the amine in a suitable solvent. For
example, where the
amine is polyethylene amine (PEI), in the branched form PEI is soluble in
water or ethanol. In
the linear form, PEI is soluble in hot water, methanol, ethanol, or
chloroform. The supported
polyol sorbent may be dispersed in a suitable solvent and maintained dispersed
by continuous
stirring. The amine solution and supported polyol sorbent are combined, and a
suitable amount of
a third component added. The reaction mixture can be refluxed, for example,
for 5 hours at 70 C
with stirring, or at an appropriate time and temperature. The amine-based
sorbent may then
filtered, washed several times with a solvent to remove the un-reacted amine
and dried, for
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-12-
example, for 5 hours at 105 C. The reaction temperature can be varied based on
the amine,
polyol and employed solvent boiling points. The selected solvent, such as
water, alcohols or
chloroform, and preferably water, does not react with either amine or polyol
under the reaction
conditions.
In a further embodiment, amine based sorbent can be synthesized by forming a
layer of
cross-linked hydrogel on a porous support material. In an aspect of this
embodiment, a solid
porous support is impregnated with a solution containing a polyamine and
hydrophilic polymer.
This may be achieved by, but not limited to, the incipient wetness method. If
necessary, solvent
in the pores of the support can be removed by slow evaporation, for example at
105 C for 5
hours. A cross linking agent, containing aldehyde groups, may be dissolved in
a suitable solvent.
The cross linking agent solution can be combined with the mixed-polymers
supported sorbent.
For example by stirring for 4 hours. The solid sorbent may be then filtered,
washed several times
with an appropriate solvent and then dried, for example, for 5 hours at 105 C.
In a further embodiment, hydrophilic polymer contains hydroxyl groups can be
coupled
with amine groups on the amine and/or polyamine using disuccinimidyl carbonate
coupling
agent.
In a further embodiment, hydrophilic polymer can be coupled with the
amine/polyamine
using a coupling agent. For example, hydrophilic polymer, such as polyvinyl
alcohol, is reacted
with succinic anhydride to attach a carboxylic acid group to the PVA chain. A
primary amine
group in the (poly)amine may be coupled with the carbonyl group on the PVA
chain to produce a
PVA-PEI amide linkage using a coupling agent such as, but not limited to,
dicyclohexyl
carbodiimide.
In a further embodiment, pre-synthesized active amine composite can be
utilized for the
CO2 absorption in an aqueous solution. For example, pre-synthesized active
amine composite
can be dissolved in an appropriate solvent e.g. one that does not
substantially react with the
amine composite.
The present sorbents can be used for removing acidic gases, such as, but are
not limited
to, CO2 and/or H2S from gaseous streams. The sorbents are preferably thermally
stable over a
range of operating temperatures and pressures. Porous solid sorbent can be
employed as powder,
A8124670WO\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-13-
pellets, extrudates, spheres, monoliths, or other physical forms. Once
synthesized, the sorbent
may be employed in sorption/desorption cyclic processes using a fixed bed,
fluidized bed, or
other appropriate reactor.
The present sorbents may be employed via contacting said sorbent with a
gaseous stream
containing acidic gases and possibly water vapour where the acid gases
physically adsorb and/or
chemically react with the active amine on the sorbent. The process may be
conducted at any
suitable temperature such as from ambient to about 100 C. The process may be
conducted at any
suitable pressure such as up to about 1500 psig.
Once the sorbent has reached capacity, the sorbent is typically be regenerated
and the
absorbed acidic gas removed. This allows the sorbent to be reused with
consequent cost and
efficiency advantages.
The desorption of the acidic gas may be conducted in any suitable manner. For
example,
the desorption can be conducted at an elevated temperature from ambient to
about 130 C or
higher using processes such as, but not limited to, pressure swing, gas purge,
vacuum, lean gas
sweep, temperature swing, liquid sweep, or combinations thereof Certain of the
present sorbents
enable acidic gas sorption/desorption cycles at various temperatures and
pressures. The
sorption/desorption cycles can be performed using different pressure
protocols, for example:
Desorption pressure equal to Absorption pressure; Desorption pressure less
than Absorption
pressure; Desorption pressure greater than Absorption pressure
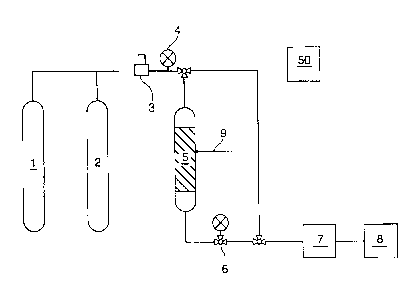
Figure 1 is a schematic depiction of an acid gas stripping process 50 of the
present
invention, as used in the Examples referred to herein, where the acid gas
contained in the fluid
stream is carbon dioxide contained in cylinder 1, and where a nitrogen
(sweeping gas) supply is
provided by cylinder 2 containing N2.. A mass flow controller 3, a pressure
gauge 4, and a
sorbent bed 5 is provided in the fluid stream, wherein the sorbent is prepared
in accordance as
by the method of the present invention, and comprises:
(i) a first component comprising an amine compound at a concentration of from
about 1 wt % to about 75 wt % of the final composite;
A8124670WO\CALLAW\ 1772878\1
CA 02769060 2012-02-17
-14-
(ii) a second component comprising a hydrophilic polymer and/or a pre-polymer
compound at a concentration of from about 1 wt% to about 30 wt % of the final
composite; and
(iii) a third component which serves as a coupling, cross-linking or
catalyzing agent
to induce a reaction between the first and second components, at a
concentration of
from about 0.01 wt % to about 30 wt % of the final composite.
A back pressure regulator 6, a CO2 auto analyzer 7, a gas chromatograph 8, are
further provided
within the fluid stream used in the Examples referred to herein. A
thermocouple 9 was used to
determine temperature of the sorbent.
Figure 2 is a schematic depiction of the carbon dioxide absorption (stripping
) process of
the present invention using liquid absorbing fluid at atmospheric pressure,
where a fluid stream
containing CO2 gas 1 is injected into a tubular absorber 2. Treated gas 3,
with CO2 at least
partly or substantially removed, flows out of tubular absorber 2. Rich
solution 4 containing CO2
is passed through heat exchanger 5 using circulating pump 6, and is thereafter
passed through
boiler 7, into tubular desorption tank 8. Boiler 9 supplies heated and
pressurized rich solution 4
to desorption tank 8. Stripped (desorbed) CO2 flows through condenser 10, into
drum 11, and
thereafter for collection or further usage via outlet 12. Circulating pump 13
circulates a portion
of collected CO2 under pressure back to desorption tank 8, to thereby act as
the sweep fluid in
the desorption step in desorption tank 8. Circulating pump 14 circulates
sorbent stripped of all
CO2 through heat exchanger 15 back to tubular absorber 2.
Figure 3 is a graph of the FT-IR spectra of the sorbent described in Example
1(line 3),
polyvinyl alcohol (PVA) (line 2), and polyethylenimine (PEI) (line 3).
Figure 4 is a CO2 capacity comparison (at different pressures) of the sorbent
described in
Example 3 (3.4 wt. % of PVA, 8.9 Wt. % PEI and 87.7 wt. % Aerolyst 3046, line
1) versus a
known supported amine-polyol sorbent (line 2).
Figure 5 is a CO2 capacity comparison of the sorbent described in Example 3,
(3 wt. %
of PVA, 9.3 Wt. % PEI and 88 wt. % Aerolyst 3046, (line 1) versus a known
sorbent comprised
of 9.4 wt % PEI and 90,6 wt% Aerolyst 3046 (line 2) and another known sorbent
comprised of
33.6 wt% PEI and 66.4 wt% Aerolyst 3046 (line 3).
A8124670WO\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
,
,
-15-
Figure 6 is a graph of CO2 concentration over days , and shows the thermal
stability of
the sorbent described in Example 3.
Figure 7 is a graph illustrates repeated CO2 (column 1) and H2S (column 2)
absorption-
desorption cycles of Example 2 where the absorption modes were performed at
100 psig, and
the desorption mode was performed at different pressures of 100, 200, 300, and
400 psig.
It is contemplated that any embodiment, aspect, example, method, composition,
or
element discussed in this specification may be implemented or combined in any
suitable manner
with any other embodiment, aspect, example, method, composition, or element.
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as is commonly understood by one of ordinary skill in the art to which
this invention
belongs. If a definition set forth in this section is contrary to or otherwise
inconsistent with a
definition set forth in the patents, applications, published applications and
other publications that
are herein incorporated by reference, the definition set forth in this section
prevails over the
definition that is incorporated herein by reference. Citation of references
herein is not to be
construed nor considered as an admission that such references are prior art to
the present
invention.
The invention includes all embodiments, modifications and variations
substantially as
hereinbefore described and with reference to the examples and Figures. It will
be apparent to
persons skilled in the art that a number of variations and modifications can
be made without
departing from the scope of the invention as defined in the claims. Examples
of such
modifications include the substitution of known equivalents for any aspect of
the invention in
order to achieve the same result in substantially the same way.
Embodiments of the invention are illustrated, in part, by the following non-
limiting
methods and examples.
Example 1 (Active components coupled in solution, then dispersed in a support)
The following example illustrates the synthesis of a polyethylenimine-polyol
sorbent
composed of 13.9 wt% polyethylenimine, 24.6 wt% polyvinyl alcohol, 1.3 wt %
acetate, 3.2
wt% H2O and 57 wt% porous support.
A8124670W0\CALLAW\ 1772878\1
CA 02769060 2012-02-17
-16-
Synthesis of the active amine composite
Approximately 5 g of polyvinyl alcohol (99% hydrolyzed, MW=85000-124000),
Aldrich) was dissolved in 35 ml of distilled water at a temperature of 90 C
for 3 hours. To the
polyvinyl alcohol solution, 10 g of polyethylenimine solution
(Polyethylenimine, 50% solution
in water, Mn=1200, Mw 1300, Aldrich) was added drop-wise while stirring.
Subsequently, a
small amount of glacial acetic acid (0.45 g) was added to the mixture. The PEI-
PVA mixture was
stirred and refluxed at 70 C for 5 hours. The reaction mixture was then cooled
down to 50 C and
solid PEI-PVA product was precipitated utilizing 50 ml of mixed acetone/water
solution
containing 10% of acetone. The sticky PEI-PVA product was then filtered and
washed several
times with the mixed solvent to remove the un-reacted PEI. The white PEI-PVA
solid product
was dried at 50 C and -65 kPa for 6 hours. The weight of the white plastic-
like product was 8.37
and its density was 0.5170 g/ml.
In this Example 1 the addition of the acetic acid has twofold functionality.
First, the
acetate group enhances the adsorption of the PVA on the silica surface (as per
Malgorzata
Wisniewska, Colloid Polym Sci 289:341-344 (2011)). Second, the acid induces
the complexation
between the hydroxyl group of the PVA and cationic group of the PEI forming
ionically cross
linked PVA-PEI composite. Furthermore, the acid catalyzed reaction of the PVA
with PEI may
also result in eliminating a water molecule and producing PVA-PEI composite
illustrated in
Figure 8.
The active amine composite was analyzed using FT-IR spectroscopy for the
presence of
the PEI and acetate group, Figure 2. The two additional peaks appeared at 1261
and 1587 can be
attributed to the NH3 + deformation (see P. Srinivasa Rao, Separation and
Purification
Technology 48:244-254 (2006) and U.S. 7,863,258). In addition, the elemental
analysis of the
precipitated active amine composite (C= 50.72 wt%, N2=10.24 wt %, H2=8.91 wt
%, and 0=
30.13) was in a good agreement with the calculated values (C=50.95 wt%,
N2=9.81 wt%,
H2=9.96 wt%, and 0=29.28 wt%). From the elemental analysis, the active amine
composite
contains PEI-32.18%, PVA=57.14, CH3-000-=3.07, H20=7.61%. From the above the
cross-
linked nature and chemical composition of the adduct can be inferred.
Synthesis of the sorbent
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-17-
The silica support (Aerolyst 3038) was ground and utilized to synthesize a
supported
PEI-PVA sorbent composed of 43 wt. % of PEI-PVA composite (24.6 wt. % PVA,
13.9 wt. %
PEI, 1.3% Acetate and 3.2 wt% H20) and 57 wt. % Aerolyst 3038.
3.4 g of the synthesized PEI-PVA composite was dissolved in 14.5 ml of
distilled water.
Approximately 4.5g of Aerolyst 3038 support was impregnated with the obtained
PEI-PVA
viscous solution utilizing the incipient wetness process. Subsequently, the
sorbent was dried at
105 C for 5 hours. The supported PEI-PVA sorbent was then crushed to form the
powder
sorbent.
Example 2 (Active components dispersed in a support and then coupled)
This example describes the the preparation of a supported PEI-PVA sorbent
composed of
3 wt. % PVA, 11 wt. % PEI and 86 wt. % Aerolyst 3038.
Aerolyst 3038 support was ground and dried at 105 C for 2 hours before use.
13.62 g of
the dry Aerolyst support was impregnated with 22.1 ml of aqueous solution that
contained 2.5
wt. % PVA (0.55 g PVA) using the incipient wetness process. The supported PVA
sorbent was
dried at 105 C for 5 hours. Approximately 5.51 g of the synthesized supported
PVA sorbent was
then refluxed for 5 hours at 70 C with 0.75 g of glacial acetic acid and 83.5
ml of 10 wt. % PEI
solution while stirring. The PEI/PVA/ Aerolyst 3038 sorbent was cooled to room
temperature,
filtered and then dried at 105 C for 5 hours. The synthesized sorbent was
ground before use.
Example 3
Example 3 is similar to Example 2 except the silica support was Aerolyst 3046.
The
surface characteristics of the silica support are given in Table (1). The
sorbent was composed of
3.4 wt. % of PVA, 8.9 Wt. % PEI and 87.7 wt. % Aerolyst 3046.
Example 4
Example 4 is similar to Example 3 except MEA was employed. The sorbent was
composed of 3.4 wt. % PVA, 5.5 wt. % MEA and 91.1 wt. % Aerolyst 3046.
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-18-
Example 5
This Example describes the preparation of a supported cross-linked PEI-PVA
sorbent
composed of 7.5 wt. % PVA, 7.5 wt. % PEI, 10 wt% aldehyde and 75 wt. %
mesoporous silica
support (SBA-15).
Synthesis of the mesoporous silica support
The mesoporous silica support was synthesized similarly to reported procedures
(see US
2007/0149398 and D. Zhao, Q. HuO, J. Feng, B. F. Chmelka, G.D. Stucky, J. Am.
Chem, Soc.
120:6024, 1998).
Preparation of the sorbent
lOg of 2.5 wt % PVA aqueous solution was mixed with lOg of 2.5 wt % of PEI
aqueous
solution and stirred for a few minutes to produce a homogenous solution.
Approximately 2.5g of
SBA-15 silica support was impregnated with PEI-PVA homogenous mixture
employing the
incipient wetness method. The supported PEI-PVA sorbent was then dried at 105
C for 10 hours
in a convection oven. 2.5g of glyoxal (40% wt% in water) was mixed with 37.5g
of acetone.
Subsequently, the dry supported PEI-PVA sorbent was dispersed in the prepared
2.5 wt%
glyoxal solution. The mixture was stirred for 4 hours at room temperature to
form a cross-linked
PEI-PVA thin film inside the mesopores of the silica support. The sorbent was
filtered and
washed several times with acetone and then dried at 105 C for 5 hours.
Example 6
This Example concerns the measurement of the acidic gas absorption capacity
using a
fixed bed flow system.
CO2 and/or H2S capacity were obtained by a down-flow tubular absorber. The
system
comprised, as shown in Figure 1, a gas feeding section, a down-flow pressure
absorber, heating
elements with a temperature controller for controlling the regeneration
temperature and a CO2
auto analyzer and/or gas chromatograph.
In a typical run, a few grams of the sorbent particles under investigation
were enclosed
between two glass wool zones. Feed and sweep gas flows were controlled by a
mass flow
controller, and the pressure of the tubular absorber was controlled via a back
pressure control
A8124670W0\CALLAW\ 1772878\1
CA 02769060 2012-02-17
-19-
valve placed on the outlet gas stream. The breakthrough time of the acidic gas
was determined
by a CO2 auto-analyzer and/or micro gas chromatograph equipped with an
automated stream
selection valve. The absorber was pretreated with a N2 gas stream with a flow
of 10 cc/min and a
temperature of 130 C for 2 hours. The acidic gas absorption modes were
performed at room
temperature and different pressures, while desorption modes were conducted at
a constant
temperature of 130 C and different pressures using N2 or CO2 gas. The CO2 and
H2S desorption
time was varied based on the sweep gas flow. Table 2 shows the absorption
measurements of
some sorbents. Figure 4, line 1 refers to the sorbent according to Example 3
and line 2 refers to a
known amine sorbent comprising 11 wt% polyethyleneimine and 16 wt%
polyethylene glycol
and 73 wt% Aerolyst 3046 (U.S. 5,376,614, Birbara). In Figure 3, each
absorption/desorption
cycle was conducted at a pressure of 10, 100, 200, 300, 400 and 500 psig,
respectively. From
Figure 3, it can be seen that at a pressure of 400 psig and ambient
temperature, the CO2 capacity
of the present invention was 77.7% higher than the comparator sorbent.
Figure 5, line 1 refers to the sorbent according to Example 3, line 2 refers
to a known
sorbent (see, X. Xu et al, Microporous Mesoporous Mater, 62:29, 2003)
comprised of 9.4 wt%
PEI and 90.6 wt% Aerolyst 3046, and line 3 refers to a known sorbent comprised
of 33.6 wt%
PEI and 66.4 wt % Aerolyst 3046. From Figure 5, the CO2 absorption capacity of
the sorbent
according to Example 3 was 21.5% higher at a pressure of 500 psig than that
obtained for a
known sorbent composed the same amount of PEI and Aerolyst 3046 support.
However, the CO2
absorption capacity of the known sorbent comprised of a high amount of PEI
(33.6 wt%)
remained fairly constant but still less than that obtained from Example 3 at
absorption pressures
ranging from 10 psig to 400 psig.
Example 7
The sorbent of Example 3 was subjected to a thermal stability test. The
tubular absorber
was charged with 4.4g of the sorbent described in Example 3, and the pressure
of the tubular
absorber was then increased to 400 psig employing a CO2/N2 gas mixture
containing 22.4 %
CO2. The temperature of the tubular absorber was increased to 130 C. After two
weeks, the
tubular absorber flushed with N2 gas at 400 psig to remove the CO2 from the
tubular absorber,
cooled down to room temperature, and the absorption mode was then repeated at
the same
pressure. Subsequently, the temperature of the tubular absorber was increased
to 130 C and the
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-20-
absorption mode was repeated after 21 and 30 days. Figure 6 shows the CO2
absorption capacity
for several cycles. From Figure 6, the sorbents are seen to have high thermal
stability and are
capable of repeated absorption/desorption cycles without diminished absorption
capacity.
Example 8
This example describes the CO2 and H2S desorption at a pressure higher than
the
absorption pressure.
The sorbent according to Example 2 was put through absorption/desorption
cycles where
the absorption modes were performed at a constant pressure of 100 psig and the
desorption
modes were performed using a flow of N2 gas at a temperature of 130 C and
pressures of 100,
200, 300, 400 psig, respectively. Figure 7 shows that the CO2 absorption
capacity was constant
at pressures up to 300 psi, and diminished slightly at 400 psi, while H2S
absorption capacity was
fairly constant over the pressure range. This demonstrates that the sorbents
are capable of high-
pressure desorption , even when absorption occurred at low pressure, which
enables substantial
savings in compression energy when recovering acid gases like CO2 and H2S for
high-pressure
uses, such as disposal by injection into subterranean formations or for
pipelining.
Example 9
This example demonstrates the utilization of the amine composite in aqueous
solution. In
this Example the active amine composite was synthesized as described in
Example 2 and the
absorbing fluid was manufactured by dissolving 50 g of the synthesized active
amine composite
in 450 g of water at 90 C and stirring until homogenous. The concentration of
the active amine
composite in the manufactured absorbent was 10 wt% of the final absorbent. In
order to increase
the CO2 absorption capacity of the absorbing fluid according to this Example,
attempts were
made to manufacture aqueous solutions comprising higher concentrations of the
active amine
composite than 10 wt % of the final absorbent. However, the viscosity of the
aqueous absorbing
fluid increased and the volumetric flow of the feed stream decreased
significantly.
Example 10
In this Example the active amine composite was synthesized as described in
Example 2,
except that the molecular weight of the utilized polyvinyl alcohol was lower
than the molecular
A8124670W0\CALLAW\ 1772878\1
CA 02769060 2012-02-17
-21-
weight of the polyvinyl alcohol employed in Example 2 and was in the range of
from 13000 to
23000 AMU. Therefore, the synthesized active amine composite according to this
example was
of a lower molecular weight than the active amine composite according to
Example 2.
Accordingly, aqueous solutions comprising higher concentrations of the active
amine composite
than 10 wt % of the final absorbent can be manufactured. The absorbing fluid
was manufactured
by dissolving 75 g of the active amine composite synthesized according to this
Example in 425 g
of water at 90 C and stirring until homogeneous. The concentration of the
active amine
composite in the manufactured sorbent was 15 wt % of the final sorbent.
Example 11
This Example concerns measurement of the acidic gas absorption capacity of the
active
amine composite solutions.
Figure 2 is a schematic of the acidic gas absorption apparatus. The apparatus
comprises a
gas and liquid feeding sections, a tubular absorber, a liquid trap, a back
pressure control valve
and a CO2 auto-analyzer.
A glass tubular absorber of 1 cm internal diameter and 59.5 cm length was
employed to
measure the CO2 capacity of the active amine composite solutions at
atmospheric pressure, while
a stainless steel tubular absorber was utilized to measure the CO2 capacity at
pressures higher
than atmospheric pressure. The glass absorber was packed with glass beads to
provide a proper
contact between the CO2 containing gas stream and active amine composite
solution. The
active amine composite solution and CO2 containing gas stream were supplied to
the glass
tubular absorber via an HPLC pump and a mass flow controller, respectively.
The active amine
composite solutions were fed to the absorber from top to bottom, while the CO2
containing gas
or desorption sweep gas (N2) was fed to the absorber from bottom to top. The
absorption modes
were conducted at atmospheric pressure and ambient temperature, while
desorption modes were
performed at 90 C using a flow of N2 gas as a sweep gas. Table 3 shows the CO2
absorption
capacity of the active amine composite solutions according to Example 9 and
Example 10 at
atmospheric pressure. From Table 3, the CO2 capacity of the sorbent according
to Example 10
increased 36.3 % compared with that calculated for the absorbing fluid
according to Example 9.
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-22-
A thick-walled 316 stainless steel tube of 1.41 cm internal diameter and 59.5
cm length
was utilized for measuring the CO2 capacity at pressures higher than the
atmospheric pressure.
The stainless steel tubular absorber was charged with 50 gm of the active
amine composite
solution according to Example 9 and the CO2-containing gas stream was supplied
to the stainless
tubular absorber from bottom to top by a mass flow controller. The CO2
absorption modes were
conducted at ambient temperature and pressures of 100 and 500 psig,
respectively. The CO2
desorption modes were performed at a temperature of 90 C and at pressures
similar to the
absorption pressures. The CO2 capacity was measure at ambient temperature and
a pressure of
100 and 500 psig. Table 4 shows the CO2 absorption capacity of the active
amine composite
solution according to Example 9 at different pressures. The CO2 capacity of
the active amine
composite solution increased with increasing the pressure.
Selection of stripping (ie desorption sweep) gas
Although nitrogen was used as the stripping gas (ie desorption sweep gas) in
the above
Examples and as shown in Figure 1, other gases can be utilized. For example,
if the acid gas
being stripped from a fluid stream (containing hydrocarbons from a hydrocarbon-
containing
formation) is CO2 , and which CO2 is desired to be utilized (or re-used) for
re-injection into a
hydrocarbon¨containing formation for diluent purposes and thereby enhanced
recovery of oil
from within the hydrocarbon-containing formation , it would be desirable to
maintain the
concentration of CO2 in the stripping (ie desorption sweep) gas as high as
possible: In this case
hot CO2 can be selected as the stripping gas so there will be no dilution of
the CO2. This
approach was adopted in the process/system shown in Figure 2 herein.
Table 1: Physical characteristics of the silica support
Support Aerolyst 3038 Aerolyst 3046
Basic compound Aerosil 380 Aerosil 380
Shape Extrudates Rings
Diameter, mm 2.5 OD 4.7, ID 2.0
Surface area, m2/g 270 180
Pore volume, ml/g 0.9-1.0 0.8-0.9
Tapped density, g/1 400-460 340-400
Si content 99.8 99.8
A8124670W0\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-23-
Table 2: CO2 absorption capacity of some of the solid sorbents
Absorption P, psig 10 100 200 300 400 500 Ex.
#
Feed gas flow, cc/min 17.65 17.65 17.65 17.65 17.65 17.65
CO2 Conc. % 21.14 21.14 21.14 21.14 21.14 21.14 1
CO2 mmol/g Absorbent 0.42 0.73 0.89 1.06 1.38 0.42
Feed gas flow, cc/min 14.1 13.1 10.7 10.7 10.7 13.0
CO2 Conc. % 10.79 10.79 11.84 11.84 11.84 11.84
2
CO2 mmol/g Absorbent 0.815 0.974 1.275 1.491 1.554 1.792
Feed gas flow, cc/min 17.65 17.65 17.65 17.65 17.65 17.65
CO2 Conc. % 24.4 24.4 24.4 24.4 24.4 24.4 4
CO2 mmol/g Absorbent 0.51 0.94 1.20 1.38 1.77 2.30
Feed gas flow, cc/min 18.18 18.18 18.18 17.65 17.65 17.65
CO2 Conc. % 24.4 24.4 24.4 24.4 24.4 24.4 5
CO2 mmol/g Absorbent 0.309 0.965 1.639 2.316 2.957 3.562
Table 3: CO2 absorption capacity of the absorbing fluids at atmospheric
pressure
Example 9 Example 1010
Mass of the absorbent 150 100
Active amine composite, wt % 10 15
Sorbent flow rate, ml/min 0.3 0.3
Feed gas flow, cc/min 6.19 6.19
CO2 Conc. % 6.5 16.75 15
CO2 mmoUgm of absorbing fluid 0.067 0.091
A8124670WO\CAL_LAW\ 1772878\1
CA 02769060 2012-02-17
-24-
Table 4: CO2 absorption capacity of the absorbing fluid according to Example 9
at
different pressures
Absorption P, psig 100 500
Mass of the absorbent, gm 50 50
Active amine composite, wt % 10 10 10
Feed gas flow, cc/min 10.71 10.71
CO2 Conc. % 19.66 19.66
CO2 mmol/gm of absorbing fluid 0.067 0.179
The scope of the claims should not be limited by the preferred embodiments set
forth in
the foregoing examples, but should be given the broadest interpretation
consistent with the
description as a whole, and the claims are not to be limited to the preferred
or exemplified
embodiments of the invention.
A8124670WO\CALLAW\ 1772878\1