Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02774488 2012-03-16
WO 2011/034791 PCT/US2010/048463
1
MERCURY REMOVAL FROM WATER
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application is a non-provisional application which claims benefit
under 35 USC
119(e) to U.S. Provisional Application Serial No. 61/243,879 filed September
18, 2009, entitled
"MERCURY REMOVAL FROM WATER," which is incorporated herein in its entirety.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR
DEVELOPMENT
[0002] None
FIELD OF THE INVENTION
[0003] Embodiments of the invention relate to methods and systems for removing
mercury from water.
BACKGROUND OF THE INVENTION
[0004] Recovered fluids from wells drilled into hydrocarbon reservoirs often
include
water. Separators remove the water from oil and gas products also produced.
However, the
water from some reservoirs contains mercury. The mercury in the water presents
environmental
and safety concerns and may prevent ability to discharge the water without
first being treated.
[0005] Techniques utilizing solid absorbents for mercury removal from the
produced
water tend to result in fouling of mercury removal beds. Other factors
limiting applicability of
prior approaches to remove mercury include expense and size requirements given
limited space
available when used at platforms. Due to mercury solubility in the water,
effectiveness problems
arise with some of the prior approaches since the mercury contaminating the
water tends to be
part of inorganic compounds or a mixture of the inorganic compounds and
elemental mercury.
[0006] Therefore, a need exists for improved methods and systems for removing
mercury
from water.
SUMMARY OF THE INVENTION
[0007] In one embodiment, a process of removing mercury from water includes
separating crude production into a gaseous hydrocarbon stream, a liquid
hydrocarbon stream and
an aqueous stream. Water forms a majority of the aqueous stream. Removing
mercury from a
contaminated gas stream including the gaseous hydrocarbon stream provides a
treated gas
CA 02774488 2012-03-16
WO 2011/034791 PCT/US2010/048463
2
stream. Further, contacting the treated gas stream with the aqueous stream
transfers mercury
from the aqueous stream to the treated gas stream such that mercury removal
from the aqueous
stream is independent from the liquid hydrocarbon stream.
[0008] According to one embodiment, a method of removing mercury from water
includes adding a reducing agent to an aqueous stream such that mercury-
containing compounds
in the aqueous stream are converted to form elemental mercury. The method
further includes
transferring the elemental mercury from the aqueous stream to a methane-
containing gas stream.
The transferring occurs upon contacting the gas stream with the aqueous stream
combined with
the reducing agent. In addition, the method includes removing the elemental
mercury from the
gas stream.
[0009] For one embodiment, a process of removing mercury from water includes
separating crude production into a gaseous hydrocarbon stream, a liquid
hydrocarbon stream and
an aqueous stream. Water forms a majority of the aqueous stream. Transferring
mercury from
the liquid hydrocarbon stream to a first portion of a treated gas stream
occurs by contacting the
first portion of the treated gas stream with the liquid hydrocarbon stream.
Furthermore,
transferring mercury from the aqueous stream to a second portion of the
treated gas stream by
contacting the second portion of the treated gas stream with the aqueous
stream is independent of
the first portion of the treated gas stream being contacted with the liquid
hydrocarbon stream.
The treated gas stream forms by removing mercury from the gaseous hydrocarbon
stream mixed
with the first and second portions of the treated gas stream recycled after
the contacting with the
liquid hydrocarbon and aqueous streams.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] The invention, together with further advantages thereof, may best be
understood
by reference to the following description taken in conjunction with the
accompanying drawings.
[0011] Figure 1 is a schematic of a production system for mercury removal from
water,
according to one embodiment of the invention.
[0012] Figure 2 is a schematic of a production system having elements shown in
Figure 1
with a subunit for removing mercury from a hydrocarbon liquid stream,
according to one
embodiment of the invention.
CA 02774488 2012-03-16
WO 2011/034791 PCT/US2010/048463
3
[0013] Figure 3 is a schematic of a production system having elements shown in
Figure 1
with a subunit for initial removal of mercury from a hydrocarbon and water
mixture, according
to one embodiment of the invention.
[0014] Figure 4 is a graph showing mercury concentration in water before and
after being
contacted with a stream of hydrocarbon gas, according to one embodiment of the
invention.
DETAILED DESCRIPTION OF THE INVENTION
[0015] Embodiments of the invention relate to removal of mercury from water.
The
removal relies on transferring mercury from an aqueous stream to a natural gas
stream upon
contacting the aqueous stream with the natural gas stream. Processing of the
natural gas stream
after used to strip the mercury from the aqueous stream removes the mercury
from the natural
gas stream.
[0016] In some embodiments, the water comes from crude production and is thus
recovered from reservoirs along with hydrocarbons that may be liquid and
gaseous. Mercury
concentrations in the water that is produced often prevent outputting the
water as waste due to
environmental issues and regulations. The removal of the mercury from the
water thereby
enables discharge of the water separated from the hydrocarbons. As used
herein, "mercury"
refers to mercury within or from compounds, such as mercuric chloride, mercury
oxide and
combinations thereof, containing mercury and at least one other element and/or
elemental
mercury. Location for removing the mercury depends on application and can be
performed
onsite at offshore platforms with limited space and facilities.
[0017] Figure 1 illustrates a system in which crude production removed from a
well
defines an input stream 100 introduced into a separator 102 for separation
into a hydrocarbon gas
stream 104, a hydrocarbon liquid "HC(L)" stream 108, and an aqueous stream 106
that are each
individually removed from the separator 102. Water forms a majority of the
aqueous stream
106. Mercury-containing gas, including in part at least a portion of the
hydrocarbon gas stream
104, feeds into a mercury removal unit (MRU) 118 for removal of mercury from
the mercury-
containing gas, thereby forming a treated gas stream 122 output from the MRU
118. The treated
gas stream 122 includes hydrocarbon gas "HC(G)," such as methane, and may
provide a supply
for natural gas usable in part for sales or as fuel.
[0018] Part of the treated gas stream 122 forms a recycle gas stream 120,
which is
introduced into a water-gas contactor 112 for contact with at least a portion
of the aqueous
CA 02774488 2012-03-16
WO 2011/034791 PCT/US2010/048463
4
stream 106 that also enters the water-gas contactor 112. Through such
contacting, at least a
portion of the mercury contained in the aqueous stream 106 transfers to the
recycle gas stream
120, thereby forming a water-passed gas stream 116 output from the water-gas
contactor 112 and
a treated water "H20" stream 114 output from the water-gas contactor 112. The
water-passed
gas stream 116 hence includes hydrocarbon gas and mercury "HC(G)+HG." For some
embodiments, the water-passed gas stream 116 mixes with the hydrocarbon gas
stream 104 and
provides a portion of the mercury-containing gas that feeds into the MRU 118.
[0019] In some embodiments, an optional chemical additive stream 110 mixes
with the
aqueous stream 106 to introduce a reducing agent into the aqueous stream 106
upstream from
passing of the recycle gas stream 120 in contact with the aqueous stream 106.
The reducing
agent breaks molecular bonds between mercury atoms and other elements in
mercury-containing
compounds. As used herein, the reducing agent may be provided as a liquid and
includes any
substance that forms a compound with such released non-mercury elements to
prevent
recombination with elemental mercury. Examples of the reducing agent include
stannous
chloride (SnC12i "SNCL2"), sodium borohydride, and hydrazine. Amount of the
reducing agent
introduced via the additive stream depends on concentration of mercury in the
aqueous stream
106 and may be sufficient to establish an excess mole ratio of the reducing
agent relative to the
mercury.
[0020] The reducing agent supplied through the additive stream 110 may
facilitate
effectiveness of sparging within the water-gas contactor 112 since mercury
removal ability via
the sparging is higher for elemental mercury relative to when not in elemental
form. Reducing
inorganic compounds, such as mercury oxide or mercuric chloride, in the
aqueous stream 106
tends to promote the mercury removal. Even though the mercury in the aqueous
stream 106 can
tend to remain in elemental form while at elevated formation temperatures, the
elemental
mercury may convert into mixed element compounds due to cooling of the aqueous
stream 106
and temperature influence on solubility of the elemental mercury. In
operation, the aqueous
stream 106 may cool upon coming out of the well making introduction of the
additive stream 110
desirable to reduce the inorganic compounds to the elemental mercury.
[0021] For some embodiments, the treated water stream 114 passes through an
optional
filtration system 124 to remove suspended particulates from the treated water
stream 114. The
filtration system 124 operates based on size exclusion to trap or retain
particles above a certain
CA 02774488 2012-03-16
WO 2011/034791 PCT/US2010/048463
size, such as about 0.2 micron or about 0.4 micron. The cooling that is
inevitable after the input
stream 100 comes out of the well promotes adherence of the mercury to the
particulates.
Generation of a filtered water stream 126 flowing out of the filtration system
124 thus results in
further mercury removal since residual mercury still within the treated water
stream 114 is
associated with the particulates.
[0022] In some embodiments, the water-gas contactor 112 includes multiple
(e.g., 2, 4, 6
or more) theoretical stages of separation between vapor and liquid phases.
Either trays or
packing material of the water-gas contactor 112 may form the theoretical
stages by being in a
flow path of fluids described herein passing through the water-gas contactor
112. For example,
the packing material making up an internal part of the water-gas contactor 112
may include
random oriented objects or a shaped structure and may be made of metallic,
ceramic, plastic or
other solid material. For some embodiments, amount of the packing material
utilized depends on
a desired number of the stages provided by the packing material.
[0023] The MRU 118 defines a fixed bed including any mercury sorbent material
capable
of removing mercury from gases. In some embodiments, the treated gas stream
122 includes less
than about 20 weight percent (wt. %) of the mercury within the mercury-
containing gas, less than
about 10 wt. % of the mercury within the mercury-containing gas, or less than
about 1 wt. % of
the mercury within the mercury-containing gas. The treated water stream 114 or
the filtered
water stream 126 may contain less than about 50 wt. %, 10 wt. %, or 1 wt. % of
the mercury
contained in the aqueous stream 106. The aqueous stream 106 for some
embodiments contains
at least about 5 parts-per-billion (ppb), 100 ppb or 500 ppb mercury.
[0024] For some embodiments, the recycle gas stream 120 contacts the aqueous
stream
106 at ambient temperature, such as about 21 C, or from about 0 C to about
300 C; a pressure
in the range of from about 0.1 Bars to about 15 Bars, from about 0.5 Bars to
about 10 Bars, or
from about I Bar to about 5 Bars; and a gas to liquid ratio in the range of
from about 50 to about
300 standard cubic feet of gas/barrel of liquid (SCF/bbl) or from about 100 to
about 200
SCF/bbl.
[0025] Figure 2 illustrates a schematic of a production system that includes a
subunit for
removing mercury from the hydrocarbon liquid stream 108. The system
incorporates an oil-gas
contactor 200 of the subunit with elements already described herein with
respect to Figure 1 and
identified by common reference numbers. A first portion 220 of the treated gas
stream 122
CA 02774488 2012-03-16
WO 2011/034791 PCT/US2010/048463
6
enters the water-gas contactor 112 to generate the treated water stream 114. A
second portion
221 of the treated gas stream 122 flows into the oil-gas contactor 200 and is
introduced into the
hydrocarbon liquid stream 108 also input into the oil-gas contactor 200. Such
contacting
transfers mercury from the hydrocarbon liquid stream 108 to the second portion
221 of the
treated gas stream 122 and occurs subsequent to separation of the hydrocarbon
liquid stream 108
from the aqueous stream 106. Resulting effluent from the oil-gas contactor 200
includes
hydrocarbon liquids forming a treated oil stream 208 and hydrocarbon gases
contaminated with
mercury forming an oil-passed gas stream 216. The oil-passed gas stream 216
also passes
through the mercury removal unit 118 and is thereby regenerated to make up
part of the treated
gas stream 122.
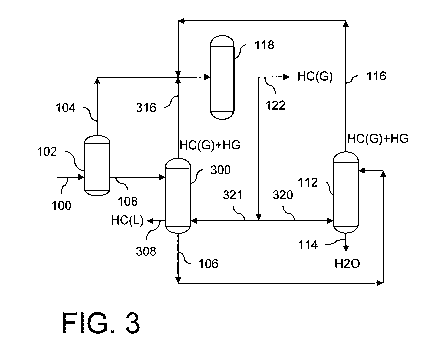
[00261 Figure 3 shows a schematic of a production system with an exemplary
alternative
configuration such that a subunit provides initial removal of mercury from a
hydrocarbon and
water liquid mixture. U.S. Patent Application No. 12/538,606, which is herein
incorporated by
reference in its entirety, further describes such exemplary techniques
depicted by the subunits in
Figures 2 and 3 for liquid hydrocarbon processing to remove mercury. Similar
to other
embodiments, the water-gas contactor 112 generates the treated water stream
114 utilizing a first
portion 320 of the treated gas stream 122. In addition to elements already
described herein
having like reference numbers, the system further incorporates an emulsion-gas
contactor 300 of
the subunit. The separator 102 may only provide separation for two phases
leaving water in the
hydrocarbon liquid stream 108 that feeds into the emulsion-gas contactor 300.
A second portion
321 of the treated gas stream 122 flows into the emulsion-gas contactor 300
where introduced
into the hydrocarbon liquid stream 108. Such contacting transfers mercury from
the hydrocarbon
liquid stream 108 to the second portion 321 of the treated gas stream 122 and
occurs prior to
separation of the aqueous stream 106 out of the hydrocarbon liquid stream 108.
Three individual
resulting effluents from the oil-gas contactor 200 include hydrocarbon liquids
forming a treated
oil stream 308, hydrocarbon gases contaminated with mercury forming an
emulsion-passed gas
stream 316, and the aqueous stream 106, which then feeds to the water-gas
contactor 112 for
further water treatment as set forth herein. The emulsion-passed gas stream
316 passes through
the mercury removal unit 118 and is thereby regenerated to make up part of the
treated gas
stream 122.
CA 02774488 2012-03-16
WO 2011/034791 PCT/US2010/048463
7
[0027] Independence with respect to removing mercury from the aqueous stream
enables
mercury to be removed from the water alone or tailoring amount of mercury to
be removed from
each of the water and the hydrocarbon liquids as desired. Referring to Figures
2 and 3, the oil-
gas contactor 200 and the emulsion-gas contactor 300 enable reducing mercury
concentration in
the hydrocarbon liquid stream 108 independent of utilizing the water-gas
contactor 112 to
remove the mercury from the aqueous stream 106. Thresholds for mercury
concentrations in the
hydrocarbon liquids depend on economics and marketability to refineries.
However, mercury
concentration in the water may need to meet separate set requirements
necessitating individual
treatment of the aqueous stream 106. Furthermore, independent processing of
the aqueous
stream 106 to remove mercury makes possible optional addition of the reducing
agent, optional
use of the filtration system 124, and conducting treatment without having to
ensure certain
temperatures of the aqueous stream 106 during the contacting to remove the
mercury.
[0028] Figure 4 depicts a graph showing mercury concentration in water before
and after
being contacted with a stream of hydrocarbon gas. Prior to being sparged with
the gas, the water
contained 489 micrograms per liter ( g/1) of total mercury and 1.4 g/1 of
dissolved mercury.
The water after being sparged with the gas for 20 minutes contained no
dissolved mercury and
had only 10 gg/1 of the total mercury remaining. Since the 10 g/1 of the
total mercury
remaining was associated with suspended particles greater than 0.45 microns in
size, filtering
provided an option for removing residual mercury from the water following the
sparging.
Results thus demonstrated effectiveness of such techniques for removing
mercury from actual
produced water.
[0029] The preferred embodiment of the present invention has been disclosed
and
illustrated. However, the invention is intended to be as broad as defined in
the claims below.
Those skilled in the art may be able to study the preferred embodiments and
identify other ways
to practice the invention that are not exactly as described herein. It is the
intent of the inventors
that variations and equivalents of the invention are within the scope of the
claims below and the
description, abstract and drawings are not to be used to limit the scope of
the invention.