Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02775366 2012-03-26
P
AP
Electrolytic reaction system for producing gaseous hydrogen and oxygen
The invention relates to an electrolytic reaction system for generating
gaseous hydrogen and
oxygen, of the type specified in claim 1 or 2.
The invention specifically relates to a system for generating gaseous hydrogen
and oxygen in
a highly efficient manner by means of an electrolysis process in a reaction or
resonance
chamber, the sought and resultant aim being to make optimum use of the
electrical energy as a
means of splitting water into gaseous hydrogen and oxygen. The invention
further relates to
the use of these gases, in particular to the use of the energy-carrying
hydrogen, for chemical
combustion or oxidation. In particular, water is broken down by electrolysis
into gaseous hy-
drogen and oxygen, after which the chemical energy carrier hydrogen is
converted into ther-
mal energy or kinetic energy by a combustion process. Water is broken down
into said gases
with a positive and as efficient as possible energy balance. Furthermore,
large quantities of
electrolytically generated gaseous hydrogen and oxygen can be produced with
this electrolysis
process within relatively short periods of time.
The technology proposed by the invention reduces to a minimum the electrical
energy used or
needed in order to split water into hydrogen and oxygen in order to obtain an
as efficient as
possible or positive energy balance in producing the chemical energy carrier
and in order to
make economical and at the same time environmentally friendly use of the
gaseous fuel hy-
drogen and the thermal or kinetic energy obtained from it.
The technology proposed by the invention was devised with the aim of
generating hydrogen
gas and oxygen gas, preferably from naturally occurring water or from aqueous,
electrolytic
solutions, and to do so in a quantity which enables the generated chemical
energy carrier hy-
drogen to be supplied to a consumer without the need for any high-volume or
complex tempo-
rary storage, in particular to supply a consumer device or a converter device.
The correspond-
ing consumer device then converts this chemical energy carrier or fuel into
the respective en-
ergy form needed by means of a combustion process, in particular into thermal
or kinetic en-
ergy or alternatively into electrical energy.
CA 02775366 2012-03-26
-2-
The chemical energy carrier obtained by the invention in the form of hydrogen
gas, in particu-
lar gaseous hydrogen in conjunction with gaseous oxygen, thus enables energy
to be used and
converted without the emission values which usually occur during the
combustion of fossil
fuels. When using the system proposed by the invention, only steam or
condensed water and
other trace elements occur in addition to the respective form of energy
desired. The by-
products of the thermal combustion of hydrogen gas, in particular when using
its energy, are
known to be significantly more environmentally friendly than is the case with
fossil fuels. The
primary waste product from the combustion process of hydrogen is specifically
only steam or
water, which can be discharged to the environment without any problem. This
waste product
is therefore cleaner than many other types of water which occur and the
electrolytically gen-
erated oxygen is purer and more concentrated than the rest of the air in the
environment.
The system proposed by the invention and the method features proposed by the
invention are
the result of numerous series of tests and experiments with the most varied of
design struc-
tures and operating modes of these structures for producing hydrogen based on
the principle
of electrolysis, which has been known in terms of its physical principles for
more than a cen-
tury.
In theory, the electrolysis of water is a very simple, known principle,
whereby water can be
made to split into gaseous hydrogen and oxygen by means of two or several
electrodes dis-
posed in an electrolyte or water bath and by applying electrical energy, in
particular DC volt-
age. This process is basically nothing new. However, the known processes are
relatively inef-
ficient because they have required significantly more primary energy for
splitting purposes
than the gases subsequently generated by using the thermal or chemical energy
of the gener-
ated gases or by a combustion process of the generated gases. Until now,
therefore, a some-
what negative or poor energy balance was obtained. On the other hand, it was
necessary to
apply such a high amount of electrical energy that the resultant advantages
were not percepti-
ble or disappeared because electrical energy is generated to a high degree by
burning fossil
fuels. From an environmental point of view, therefore, the systems known from
the prior art
did not bring any outstanding advantages. For this reason, the use of hydrogen
and its energy
potential has never been pursued in practice or has been so in only very
limited applications.
CA 02775366 2012-03-26
-3-
Numerous types of apparatus for electrolysis are known from the prior art.
However, none of
these devices is clearly in a position to be used for a broad range of
applications. For example,
these known designs are clearly not satisfactory as a means of supplying
energy to motor ve-
hicles, power generators or heating systems because drive or supply systems
based on electro-
lytically obtained hydrogen or a hydrogen-oxygen mixture have generally not
been available
at all or are still only at the testing stage.
The technology proposed by the invention now makes it possible, with a special
structure and
with special features, to supply gaseous hydrogen and oxygen in the respective
quantity re-
quired from water or from water-based solutions, i.e. without having to
address the problems
of technically complex or high-volume storage and with a quick reaction. In
particular, when
generating the chemical energy carrier, in particular during the process of
electrolytically ob-
taining hydrogen gas, a positive energy balance is obtained whilst assuring
that chemical en-
ergy can be generated with a minimal input of primary energy. The thermal or
heat energy
which can ultimately be generated from emission-free combustion of hydrogen
and oxygen
can therefore be used in a very versatile manner. Almost all appliances in the
home or in in-
dustry, such as ovens, grills, heaters, air-conditioning systems and also
power generators, can
be operated with this chemical energy and thus offer a conversion into
electrical, kinetic
and/or thermal energy or a conversion into other forms of energy. Hydrogen and
oxygen can
also be used to operate virtually all conventional internal combustion
engines.
Electrolysis technology, in particular the electrolytic reaction system
proposed by the inven-
tion, offers the chance to use chemical energy or thermal or heat energy from
hydrogen and
oxygen without causing major damage to the environment such as occurs in the
case of the
standard combustion of fossil fuels used these days.
The corresponding technology is safer than the systems known to date for
operating motors,
generating power, and for heating and similar purposes. In order to operate,
these systems
respectively need fuels which are contained in tanks or a system of pipework.
An incompara-
bly high quantity of combustion energy is stored and held ready in these
components. In the
case of a breakdown, which is increasingly common in practical applications,
this often caus-
es serious problems. In particular, supplying the fuel directly sometimes
leads to unexpected
CA 02775366 2012-03-26
-4-
consequences. It is usually relatively difficult to deal with such breakdowns
or relatively
technically complex solutions are needed.
In the case of the system proposed by the invention, only a relatively low, in
particular a sig-
nificantly smaller, quantity of combustible gas is supplied in the system. The
single supply is
held in tanks or in pipes in the form of relatively uncritical aqueous
solutions or in the form of
pure water, which poses no chemical or environmental problems and which is
naturally not
combustible. Furthermore, effective safety systems may be used in conjunction
with the gen-
eration process, in particular the reaction or resonance chamber, in a simple
manner, and are
reliable and cost-effective. The electrolysis system proposed by the
invention, which is par-
ticularly efficient and fast in terms of reaction, means that only relatively
small quantities of
gas have to be supplied. In particular, a storage or buffer volume comprising
the reaction
chamber and the system of incoming pipework is all that is needed in most
cases. As a result,
this electrolysis system and the specified device for converting energy are
easy to control and
the system proposed by the invention can be very reliably staged.
The underlying objective of this invention is to propose an improved
electrolytic reaction sys-
tem. In particular, the aim is to obtain an electrolytic system for breaking
down water or
aqueous solutions into gaseous hydrogen and oxygen, which offers the highest
possible effi-
ciency and as high a degree of effectiveness as possible in terms of the
quantity of electrical
energy which has to be input and the quantity of generated or converted
chemical or thermal
or kinetic energy.
This objective is achieved by the invention on the basis of an electrolytic
reaction system us-
ing the features defined in claim 1 and, independently of this, by an
electrolytic reaction sys-
tem based on the features defined in claim 2.
A surprising advantage obtained as a result of the features defined in claim 1
or 2 resides in
the fact that such an electrolytic reaction system offers an improved, in
particular positive,
energy balance so that by inputting a relatively small quantity of primary
energy, in particular
electrical energy, a relatively high quantity of energy can be obtained in the
form of the chem-
ical energy carrier hydrogen or in the form of a gaseous hydrogen-oxygen
mixture. This is
primarily achieved due to the structural combination and the technical
interaction between the
CA 02775366 2012-03-26
-5-
respective electrode arrangement and the at least one electromagnetic coil
disposed preferably
above and/or underneath the electrode arrangement. Due to the oscillations
superimposed on
one another and due to the combined effects of the electric fields and
magnetic fields of the at
least one electromagnetic coil and electrode arrangement, optimum conditions
are obtained
for generating hydrogen and oxygen or an appropriate mixture based on a
positive energy
balance. A surprising, unforeseeable effect resides in the fact that vibration
or resonant or
quasi resonant effects and interactions occur, which have a very positive
effect on the degree
of efficiency of the conversion or splitting process.
One surprising, advantageous interaction amongst others is that gas bubbles
occurring during
the electrolysis process, in particular the respective hydrogen and oxygen
bubbles, are more
efficiently detached from the electrode surfaces and accelerated. In addition,
shorter release
times of the respective gases from the electrolyte are obtained. What this
means is that the
electrodes and their effective surfaces that are available are available for
the conversion proc-
ess to the maximum degree and there is always the most intensive contact with
the electrolyte.
In particular, gas boundary layers between the electrodes and the electrolyte
are kept as small
as possible or broken down as rapidly as possible. Moreover, discharge of the
proportion of
gas contained in the electrolyte is assisted and accelerated so that the
effectiveness and effi-
ciency of the electrolysis process is kept as high as possible. Overall, this
results in an im-
proved electrolytic reaction system, which supplies relatively high quantities
of electrolyti-
cally obtained gaseous hydrogen and oxygen within relatively short process
times. In addi-
tion, the electrolysis system proposed by the invention can be built to a
relatively inexpensive
design and thus results in a highly economic system that is practical to use.
The effects and details of actions outlined below as well as those mentioned
above should be
construed as examples and no claim is made that they are complete.
Furthermore, not all of
the different effects described need occur. No weight is placed on these
effects and details of
actions and the explanations of the various interactions should be regarded as
the most likely
in some cases. To a certain extent, phenomena and interactions occur which
cannot or can be
barely explained, and the technical reasons for them will not be obvious to
the person gener-
ally skilled in this field or will be difficult to explain. The corresponding
results are partly
based on numerous series of tests and on empirical adjustments made to
parameters of the
electrolytic system.
CA 02775366 2012-03-26
=
-6-
Also of advantage is another embodiment defined in claim 3, because it results
in a body
shape and orientation which is particularly effective in terms of flow
technology with a view
to obtaining defined and specifically directed flows in the electrolyte and in
portions of the
chamber for the gases as they collect. It is also possible to obtain
relatively compact electro-
lytic reaction systems with a relatively high degree of efficiency.
Another embodiment defined in claim 4 is of advantage because it results in a
sort of con-
tainer-in-container arrangement, which also has a positive effect on the
efficiency of the elec-
trolysis process. In particular, this offers a sub-division into a container
for the electrolyte and
for accommodating the electrodes and a container or chamber arrangement
surrounding this
container to accommodate said components and for collecting the gases which
occur.
An embodiment defined in claim 5 is also of advantage because it offers a
degasifying cross
section that is as large as possible, which contributes to a degasification
time that is as short as
possible and a degasification process that is as intensive as possible.
Furthermore, a container
for the electrolyte is obtained which offers an unobstructed and large
overflow for the electro-
lyte liquid and/or for any electrolyte foam which might occur. Such an
electrolyte foam usu-
ally forms on the electrolyte liquid, in particular on the surface of the
electrolyte bath, and to a
certain extent prevents the gas elements in the electrolyte from escaping. Due
to a continuous
break-up or prevention of a ring of foam on the electrolyte bath, which can be
achieved in
particular by using a simple discharge line for it, the efficiency of the
system can be kept as
high as possible.
Also as a result of the claimed features, it is advantageously relatively easy
to provide a de-
fined electrolyte circuit. In particular, electrolyte liquid can be
continuously or intermittently
fed into and discharged from the holding container, and the excess quantity of
electrolyte liq-
uid is able to flow out over the top edge of the container again like a
waterfall and can option-
ally be recycled to the holding or electrolyte container after a cleaning
and/or cooling and/or
treatment process. Accordingly, the electrolyte liquid can be easily
recirculated which,
amongst other things, results in intensive and rapid degasification. In
particular, this results in
a reaction or holding container in which the expansion or increase in volume
of the electrolyte
induced by the electrolytic process can be easily compensated and regulated by
means of the
CA 02775366 2012-03-26
-7-
overflow edge of the holding container. Alternatively or in combination with
this, the surplus
quantity of electrolyte liquid which occurs due to a continuous or
discontinuous supply of
electrolyte to the holding container is able to flow out of the electrolyte
container again in a
defined manner and in one advantageous embodiment fed back to the holding
container again.
This also results in a sort of "electrolyte waterfall" over the external
and/or over the internal
walls of the holding container. This electrolyte discharge or electrolyte
outflow can therefore
take place on external surfaces of the holding container and/or on central,
internal wall por-
tions of the holding container because the holding container for the
electrolyte has a body
shape based on a hollow cylinder or several hollow cylinders, in particular
based on a cas-
caded design with holding containers disposed coaxially one inside the other.
The features defined in claim 6 also result in a design that is conducive from
the point of view
of flow technology and improves the efficiency and reaction time of the
electrolytic reaction
system.
Also of particular advantage are the features defined in claim 7 and/or 8
because a particularly
good electrolysis action is achieved and a technical interaction built up is
as intensive as pos-
sible. In particular, the electromagnetic field of the at least one
electromagnetic coil acts on
the electrode arrangement and on the electrolyte in a particularly intensive
manner, thereby
improving progress or efficiency during the electrolytic process. Firstly, for
example, the elec-
tromagnetic field of the at least one electromagnetic coil has a positive
effect on the break-
down process. Secondly, the mechanical vibrations which occur in the at least
one electro-
magnetic coil are transmitted as directly as possible to the electrolyte and
to the electrode ar-
rangement. This improves and accelerates the process of detaching the gas
bubbles from the
electrodes and the degasification process from the electrolyte. These effects
are accompanied
by an improvement to the electrolytic reaction system, in particular an
increase in efficiency
and performance.
Also of advantage is an embodiment defined in claim 9 because an
electromagnetic coil of
this type builds up an electromagnetic field which has a positive effect on
the electrolytic pro-
cess, and in particular increases its efficiency. In particular, this results
in a relatively intimate
and relatively uniform contact of the electrode arrangement with the
electromagnetic field of
this coil, which creates a pulsating field or generates an alternating field.
In this respect, it
CA 02775366 2012-03-26
-8-
should be pointed out that the electrode arrangement co-operates with and
faces only one end
or only one pole of the electromagnetic coil, in particular the south or north
pole. By prefer-
ence, the north pole end of the electromagnetic coil is preferably disposed as
close as possible
to the top end of the electrode arrangement. Alternatively, however, it would
also be conceiv-
able for the south pole of the electromagnetic coil to be positioned or
oriented closest to the
electrode arrangement.
The design described in claim 10 or 11 represents an advantageous and
particularly effective
embodiment of the electromagnetic coil. Consequently, the effectiveness and
overall perform-
ance of the electrolytic reaction system can be favorably influenced.
Also of advantage is the feature defined in claim 12 because a highly
efficient separation of
the water molecules into the respective gases is obtained, namely hydrogen and
oxygen.
Also of particular advantage is an embodiment defined in claim 13 because the
electrolytic
process is assisted or set up much more efficiently. Due to the pulsating
energy supply of the
electromagnetic coil, the coil is periodically or a-periodically switched off,
as a result of
which its magnetic field breaks up at least partially or completely, and a
much stronger mag-
netic field with reversed polarity or orientation is triggered. When the
energy supply is
switched on again, a substantially stronger field is emitted because the
consecutive fields are
at least partially summed or cumulated with very pulse until a maximum field
intensity is ob-
tained. Due to the effect of reversing the magnetic fields every time the
energy supply is
switched off, the molecules of the electrolyte are displaced in vibration so
that an unstable or
virtually unstable molecular status is obtained and the splitting or
conversion into the gaseous
states, namely into gaseous hydrogen and oxygen, is optimized.
Also of advantage is the embodiment defined in claim 14 because the electrodes
of the elec-
trode arrangement are also made to vibrate due to the alternating magnetic
fields, which caus-
es the adhered gas bubbles to be detached more rapidly. In addition, an
interaction or a reac-
tion occurs between the electric or electrostatic field between the electrodes
and the superim-
posed electromagnetic field of the at least one electromagnetic coil. As a
result of this super-
imposition, a swinging effect is produced at least some of the tine, which in
turn assists the
splitting process. The electric or electrostatic field between the anodic and
cathodic electrodes
CA 02775366 2012-03-26
-9-
therefore has superimposed on it an electromagnetic field generated by at
least one coil dis-
posed above and/or underneath the electrodes. Based on one advantageous
embodiment, the
magnetic field, in particular the electrical energy supply of the at least one
electromagnetic
coil, is dimensioned so as to be relatively low frequency compared with the
electric field of
the electrode arrangement and compared with the energy supply for the
electrode arrange-
ment. Based on a dimensioning which has been found to be expedient, the ratio
of the rela-
tively low-frequency energy supply for the electromagnetic coil and the
relatively high-
frequency energy supply for the electrode arrangement is approximately 1:1000.
Also of particular advantage is an embodiment defined in claim 15 because the
detachment or
degasification process in the electrolyte liquid is improved and accelerated.
In particular, a
circulation or a flow can be generated as a result, by means of which the gas
bubbles are more
effectively detached from the electrode surfaces, in particular relatively
thoroughly and rap-
idly. Furthermore, the degasification process is assisted in terms of the gas
bubbles disposed
in the electrolyte liquid in a gas chamber disposed above the electrolyte
liquid. The electrolyte
is filled and/or topped in the bottom portion of the reaction chamber or
holding container, and
is so periodically, a-periodically and/or on a controlled basis if necessary.
The essential aspect
is that due to this intake and/or top-up, turbulence or a flow is created in
the electrolyte.
The advantageous effects and technical actions described above are also
achieved by the fea-
tures defined in claim 16, independently or in combination. The means used to
cause turbu-
lence in the electrolyte and for creating a flow in the electrolyte may
therefore be the electro-
lyte itself and/or gaseous media could be added, for example air or nitrogen.
If other, non-
combustible gases are added, such as ambient air or nitrogen for example, the
combustion
value of the electrolytically generated hydrogen gases can advantageously be
regulated, in
particular reduced. By admixing non-combustible gases directly in the
electrolyte in this way,
therefore, turbulence or a flow effect is created in the electrolyte bath on
the one hand and the
combustion value or combustion rate of the electrolytically generated hydrogen
gas is reduced
on the other hand. As a result, the quantity of energy or explosivity, in
particular the combus-
tion rate of the electrolytically generated gases or gas mixture, can be
reduced to a level suit-
able for use in virtually standard internal combustion engines easily and with
relatively few
problems.
CA 02775366 2012-03-26
-10-
Also of advantage is another embodiment defined in claim 17 because a sort of
spray or diffu-
sor effect is produced, which causes a flow distribution in the electrolyte
which is as uniform
and intimate as possible. In particular, this causes a degasification that is
as complete and uni-
form as possible in terms of the gas bubbles disposed in the electrolyte and
in terms of the gas
bubbles adhered to the electrode surfaces. Furthermore, this enables the
density of foreign
gas, in particular the quantity of gases blasted or introduced into the
electrolyte for a defined
electrolyte volume, to be kept low and homogenized, thereby keeping the
electrolysis per-
formance high.
Another embodiment for shortening the degasification times from the liquid and
for establish-
ing more intensive contact between the electrolyte and the electrode plates is
obtained using
the features defined in claim 18.
As a result of the features defined in claim 19, however, the degasifications
effect and the
degasifications performance of the electrolytic reaction systems is improved.
Especially if the
electrolyte liquid continuously or intermittently flows over the overflow
edge, a sort of elec-
trolyte fall or "waterfall" is obtained, resulting in an intensive and
effective degasification
feature, as already explained above. A corresponding overflow or discharge of
the electrolyte
can be achieved by a forced intake or top-up of electrolyte liquid and/or may
be caused or
induced or determined due to the expansion in the volume of the electrolyte
liquid during the
electrolysis process.
A structurally simple construction of an overflow edge is obtained on the
basis of the features
defined in claim 20. This also results in a relatively homogenous and uniform
electrolyte
overflow so that the most intensive possible degasification or separation is
obtained between
the electrolyte liquid and the gases or gas bubbles contained in the
electrolyte liquid. Amongst
other things, this is made possible by the spread of the electrolyte liquid
over a relatively large
surface area.
Also of advantage is an embodiment defined in claim 21 because there is always
an intensive
degasification and a sufficiently large gas chamber is available. Furthermore,
this makes it
possible to prevent an over-pressure in the reaction chamber and prevent a
defined pressure
value from being exceeded. In particular, a specific pressure level is
maintained inside the
CA 02775366 2012-03-26
-11-
reaction chamber as a result, at which the expansion of the electrolyte liquid
caused by elec-
trolysis is at least more or less compensated or offset by discharging a
defined amount of elec-
trolysis liquid. In particular, a defined degasification volume is maintained
inside the reaction
chamber as a result and a defined gas pressure in the gas chamber of the
reaction chamber is
not exceeded.
Also of advantage is an embodiment defined in claim 22 because quantities of
gas contained
in the overflowing or discharged electrolyte are kept in the system and are
therefore not lost,
as it were. Furthermore, a turbulence or flow builds up in the electrolyte
container due to the
fact that the electrolyte is recycled, as a result of which the outflow or
removal of the quanti-
ties of gas from the liquid electrolyte is improved and accelerated.
As a result of the features defined in claim 23, hydrogen gas which primarily
collects in the
top portion of the reaction chamber is easily and reliably sucked out or
discharged via the
electrolyte outflow. In particular, this prevents the electrolytically
obtained hydrogen gas from
being fed away via the discharge or intake for the electrolytic liquid and
getting into a coolant
circuit for the electrolyte. The electrolytically generated hydrogen gas or
hydrogen-oxygen
mixture is therefore always available to the respective consumer or user of
the hydrogen and
oxygen gases. This also makes allowance for more stringent safety requirements
because any
discharge of hydrogen gas into passages and regions other than those
specifically provided in
the gas outlet region can be effectively prevented or minimized and can be so
using simple
technical means.
Also of particular advantage are the features defined in claim 24 because a
recirculation is
obtained in the electrolyte liquid, which accelerates and improves a
degasification process.
Another major advantage resides in the fact that it enables a simple system to
be used to regu-
late the electrolyte liquid. In particular, this enables a simple system for
cooling or limiting
the temperature for the electrolyte liquid. The corresponding cooling process
is operated by
applying a relatively small amount of energy because the usual ambient
temperature is suffi-
cient to keep the electrolyte liquid at a temperature level that is conducive
to an electrolysis
process or in a satisfactory temperature range as a rule. An advantageous
temperature range
prevails when the electrolyte liquid is kept within a temperature range below
60 C, preferably
in a temperature range of between 20 C and 50 C, in particular between 28 C
and 43 C.
CA 02775366 2012-03-26
-12-
Also of particular advantage are the features defined in claim 25. Firstly,
this assures cooling
and/or turbulence of the electrolyte liquid and hence an increase in
degasification rate and
degasification efficiency in terms of the quantities of electrolytically
generated gas in the elec-
trolyte liquid. Secondly, however, a simple system of regulating the
combustion or energy
value of the gas mixture in the electrolytic reaction system is obtained. In
particular, by regu-
lating the quantity of ambient air or gaseous nitrogen introduced, its
quantity of energy or
combustion value, in particular its combustion rate, can be adjusted so that
problem-free com-
bustion is made possible in standard consumers, such as in internal combustion
engines or
heating devices , for example. The gases introduced therefore produce a dual
effect or a mul-
tiple effect and the cumulative effects have a surprisingly high positive
impact.
Also of advantage is a feature defined in claim 26. Again, the performance of
the electrolytic
reaction system is increased in a surprisingly simple and effective or
efficient manner. In par-
ticular, the quantity of hydrogen gas or gaseous oxygen generated or released
can be im-
proved as a result. This is attributable to the accelerated degasification and
the more intensive
detachment of gas bubbles.
Another advantageous embodiment is defined in claim 27. A multiple use and an
advanta-
geous application is obtained as a result. In particular, the negative
pressure which is built up
by a consumer or its unit, e.g. a vacuum pump or a charging device for the
combustion cham-
ber (e.g. a turbocharger), is also used as a means of assisting or
accelerating degasification or
the detaching of gas in the electrolytic reaction system. The respective
negative pressure built
up by the respective consumer or its fuel intake can be kept in a specific
range regarded as
optimum using any regulating systems known from the prior art.
Another advantageous embodiment can be obtained by the features defined in
claim 28 and/or
29. In particular, this results in a conducive flow or creates a defined flow
direction in the
electrolyte extending from the bottom end portions of the electrodes in the
direction towards
to top end portions.
As a result of the features defined in claim 30, the electrolyte liquid can be
accelerated in the
portions between the electrodes, especially if the rate of the electrolyte
flow underneath the
CA 02775366 2012-03-26
-13-
electrode arrangement is relatively low. A Venturi effect is therefore
produced and hence an
increase in the flow rate between the individual electrodes. This also
improves detachment
performance, in particular the rate of detachment per unit of time, as well as
the intensity of
detachment or separation of gas bubbles.
Also of particular advantage are the features defined in claim 31. In
particular, such a multiple
arrangement of electrodes nested with one another assures increased
electrolytic performance
for a relatively compact structural volume. Another result is a multi-layered
capacitor effect
because the electric fields between the individual electrode pairs each have
at least slightly
different properties, which can be conducive to a highly effective
electrolysis process.
Since the tube electrodes lying farther to the inside are at an increasingly
large distance from
one another, the respective gap volume created between the different electrode
pairs is at least
partially compensated. In particular, the gap volumes between the outwardly
lying electrodes
are of the same or approximately the same design compared with the gap volumes
between
electrode pairs lying centrally or farther inwards. Empirical tests have shown
that this enables
a high electrolysis performance to be obtained.
The features defined in claim 32 are also of advantage because at least
individual electrodes
of the electrode arrangement can be forced into a mechanical vibrating
movement with a rela-
tively low electrical power and with a relatively low magnetic field strength.
In particular, the
detachment efficiency or degasification rate is increased in a simple manner
and the perform-
ance of the electrolytic reaction system as a whole increased.
The features defined in claim 33 are of advantage because even at relatively
weak electro-
magnetic field strengths, a relatively intensive mechanical vibration can be
generated at least
on individual electrodes of the electrode arrangement. Furthermore, flow and
overflow pas-
sages are obtained as a result, which further improves degasification of the
gas bubbles from
the electrolyte liquid.
The features defined in claim 34 are of advantage because zones in which a
relatively strong
or intensive electromagnetic field can be generated are defined as a result,
and zones are also
created in which the intensity of this field is lower, relatively speaking.
These non-
CA 02775366 2012-03-26
-14-
homogeneous field strengths, i.e. increasing and decreasing field strengths,
have a positive
effect on the effectiveness and overall performance of the electrolytic
reaction system.
Due to the features defined in claim 35, a favorable ratio is obtained between
the angle of
extension of part-windings and the winding gaps disposed in between. In
particular, a practi-
cal number of part-windings is obtained, distributed around the ring
circumference of the
electromagnetic coil as a result.
Also of advantage are the features defined in claim 36 because a sufficient
field intensity or a
magnetic field strong enough to influence and accelerate the electrolytic
processes is advanta-
geously generated.
Also of advantage are the features defined in claim 37 because the magnetic
field strength or
magnetic flux density varies or rises and falls alternately in the
circumferential direction of
the torus-shaped coil. This has a positive effect in terms of removing the
binding forces be-
tween the atoms of the electrolyte, in particular a water molecule, thereby
improving the elec-
trolytic performance of the specified reaction system.
Finally, the features defined in claim 38 are of advantage because the
magnetic field lines are
able to act on the electrode arrangement and on the electrolyte in a
concentrated manner.
To provide a clearer understanding of the invention, it will be explained in
more detail below
with reference to the appended drawings.
These are highly simplified, schematic diagrams illustrating the following:
Fig. 1 is an operating diagram of one embodiment of the electrolytic reaction
system,
illustrating a plurality of technical design and embodiment options;
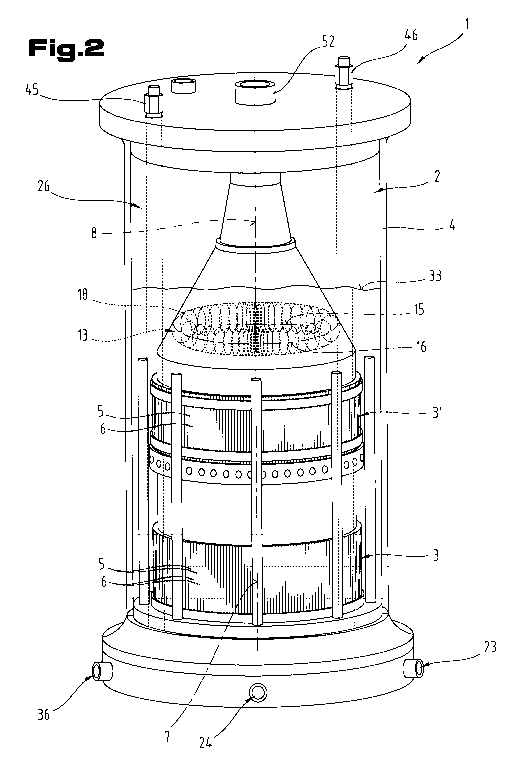
Fig. 2 shows a perspective view of a first embodiment of the electrolytic
reaction sys-
tem;
CA 02775366 2012-03-26
-15-
Fig. 3 is a plan view illustrating an electrode arrangement with plate-shaped
electrodes
fanned out in a star-shaped arrangement;
Fig. 4 is a plan view of another embodiment of a star-shaped electrode
arrangement
comprising plate-shaped electrodes based on a wedge or segment shape as viewed
in cross section;
Fig. 5 shows an embodiment of an electromagnetic coil such as used in the
electrolytic
reaction system;
Fig. 6 is a longitudinal section showing another embodiment of an electrolytic
reaction
system;
Fig. 7 shows the electrolytic reaction system based on Fig. 6, viewed in
section along
line VII - VII indicated in Fig. 6;
Fig. 8 is a plan view of another embodiment of an electrode arrangement inside
an elec-
trolytic reaction system;
Fig. 9 shows another embodiment of an electromagnetic coil such as may be used
to
advantage in the electrolytic reaction system.
Firstly, it should be pointed out that the same parts described in the
different embodiments are
denoted by the same reference numbers and the same component names and the
disclosures
made throughout the description can be transposed in terms of meaning to same
parts bearing
the same reference numbers or same component names. Furthermore, the positions
chosen for
the purposes of the description, such as top, bottom, side, etc., relate to
the drawing specifi-
cally being described and can be transposed in terms of meaning to a new
position when an-
other position is being described. Individual features or combinations of
features from the
different embodiments illustrated and described may be construed as
independent inventive
solutions or solutions proposed by the invention in their own right.
CA 02775366 2012-03-26
-16-
All the figures relating to ranges of values in the description should be
construed as meaning
that they include any and all part-ranges, in which case, for example, the
range of 1 to 10
should be understood as including all part-ranges starting from the lower
limit of I to the up-
per limit of 10, i.e. all part-ranges starting with a lower limit of 1 or more
and ending with an
upper limit of 10 or less, e.g. Ito 1.7, or 3.2 to 8.1 or 5.5 to 10.
Fig. 1 is a schematic operating diagram of an embodiment of the electrolytic
reaction system
1 with a view to illustrating its main, technical design. It should explicitly
be pointed out that
not all the features illustrated in it constitute part of the subject matter
of the invention. Indi-
vidual ones of the design features or process features illustrated in the
diagram of Fig. I may
naturally also be applied to the examples of embodiment that will be described
below.
The specified electrolytic reaction system 1 is used to generate gaseous
hydrogen and oxygen
by applying the electrolysis method. In particular, an electrolyte, in
particular water or an
aqueous electrolyte, in particular a mixture of water and an additive such as
sulfuric acid to
increase its conductivity for example, are split by an electrolytic process
into gaseous hydro-
gen and gaseous oxygen or converted into a corresponding gas mixture by means
of the elec-
trolytic reaction system 1 during its operation.
In a manner known per se, such an electrolytic reaction system 1 comprises at
least one reac-
tion chamber 2 for accommodating or supplying an aqueous or water-based
electrolyte, as
well as at least one electrode arrangement 3 made up of a plurality of anodic
and cathodic
electrodes.
The reaction chamber 2 is preferably provided in the form of an essentially
hollow cylindrical
holding container 4, in which at least one electrode arrangement 3 is
disposed. Based on a
first embodiment, this electrode arrangement 3 comprises a plurality of plate-
shaped elec-
trodes 5, 6 fanned out in a star-shaped arrangement. Mutually adjacent
electrode plates 5, 6
thus alternately form a cathode and an anode. The consecutive alternating pole
arrangement of
the individual electrodes 5, 6 to form consecutive cathodes and anodes in
electrolytic systems
is known. Instead of the plate-shaped electrodes 5, 6 fanned out in a star
shape, it would also
be possible to opt for electrodes of the type with a hollow body, in
particular prismatic or tu-
bular electrodes based on another embodiment, which will be described below.
CA 02775366 2012-03-26
-17-
In this embodiment with electrode plates 5, 6 fanned out in a star-shaped
arrangement or ex-
tending in a radiating arrangement, a virtual fanning axis 7 of this electrode
arrangement 3 is
oriented or positioned essentially on a virtual cylinder or vertical axis 8
and essentially con-
gruently with the cylinder or vertical axis 8 of the holding container 4, as
may be seen by
comparing Figs. 2 and 3. The individual plate-shaped electrodes 5, 6 are
vertically oriented,
i.e. the flat faces of the individual electrode plates 5, 6 are oriented in
the manner of walls and
are spaced apart from one another by a relatively short distance of 0.5 mm to
15 mm, prefera-
bly 1 mm to 5 mm. A thickness of the plate-shaped electrodes 5, 6 is 0.1 mm to
5 mm, pref-
erably approximately 1 mm.
As may best be seen from Fig. 3, the distance 9, 9' which lies between
adjacent electrode
plates 5, 6 of the star-shaped or fan-shaped electrode arrangement 3 varies.
This varying dis-
tance 9, 9' between directly adjacent electrode plates 5, 6 is a result of the
star-shaped or fan-
shaped arrangement of the individual, plate-shaped electrodes 5, 6 by
reference to a common
virtual fanning axis 7 of this electrode arrangement 3. In particular, the
individual electrode
plates 5, 6 extend from the common virtual fanning axis 7 in the radial
direction towards the
fanning axis 7. Seen in plan view - as is the case in Fig. 3 - the electrodes
5, 6 are therefore
oriented in a V-shaped arrangement. Consequently, there is a spread angle 10,
in particular a
so-called mid-point angle or a dimension a between directly adjacent electrode
plates 5, 6
respectively, depending on the number of pairs of electrode plates 5, 6
disposed around the
fanning axis 7 in a circle or radiating arrangement, as may clearly be seen
from Fig. 3. Due to
this star-shaped arrangement of the respective electrode plates 5, 6 and the
varying distances
9, 9' which occur depending on the distance from the fanning axis 7, the
effectiveness of the
electrolysis process is assisted. In particular, better allowance can be made
for the different
water qualities or different conductivities of the electrolyte due to the
varying distance 9, 9'
and due to the defined spread angle 10 between adjacent electrode plates 5, 6.
An especially
highly efficient or higher performance electrolysis process can be implemented
if different or
gradually fluctuating or drifting water qualities prevail or if their
conductivity differs. In other
words, the specified star-shaped layout is relatively insensitive in terms of
varying water qual-
ities or in terms of varying conductivity or with respect to other physical
properties which
change during the duration or course of the electrolysis process. Furthermore,
these features
assist or are conducive to degasification of the electrolysis products, in
particular hydrogen
CA 02775366 2012-03-26
-18-
and oxygen, from the electrode arrangement 3. This results in higher
efficiency and a higher
electrolysis performance within a defined period of time. Based on one
practical embodiment,
the distance 9 between adjacent electrodes 5, 6 in an end portion lying
closest to the fanning
axis 7 is approximately 0.6 mm and the distance 9' in the end portion remote
from the fanning
axis 7 is approximately 4 mm.
Seen in plan view, the star-shaped electrode arrangement 3 is preferably
circular in terms of
its contour. However, a polygonal contour would also be conceivable. Based on
one particu-
larly practical embodiment, the star-shaped or fan-shaped electrode
arrangement 3 is of a cir-
cular design when seen in plan view, as may best be seen from Fig. 3. In
particular, a cylin-
drical or tubular gap 11 may be provided around the fanning axis 7 which may
be completely
filled with the electrolyte and/or which may at least partially function as a
discharge chamber
or overflow or discharge passage for surplus or overflowing electrolyte liquid
or for electro-
lyte foam, as will be explained in more detail below. In other words, the
individual electrode
plates 5, 6 are fanned or disposed consecutively around the fanning axis 7,
preferably keeping
a defined radial distance 12 and are therefore oriented radially with respect
to the fanning axis
7, as best illustrated in Fig. 3. Viewed as a whole, an electrode arrangement
3 based on this
design has an essentially hollow cylindrical body, as may be seen by comparing
Figs. 2 and 3.
This hollow cylindrical electrode body has a plurality of electrode plates 5,
6 with different
poles layered in a lamellar arrangement but spaced at a distance apart,
extending in a fence or
radiating arrangement around the common cylinder or fanning axis 7. The
individual plate-
shaped electrodes 5, 6 therefore look like imaginary beams of the star-shaped
electrode ar-
rangement 3 radiating out from the fanning axis 7, as it were, when seen in
plan view.
The individual electrode plates 5, 6 have a uniform or constant thickness or
width by refer-
ence to the mutually opposing flat faces of the plate electrodes. Instead of
the design based on
plate-shaped electrodes 5, 6, it would also be possible to opt for electrodes
5, 6 based on the
shape of a circle segment when the electrode arrangement 3 is seen in plan
view, in particular
circle segment-shaped anodes and cathodes, as schematically illustrated in
Fig. 4 by way of
example.
These electrodes 5, 6 with the shape of a circle segment when seen in plan
view or cross sec-
tion are also disposed about a common fanning axis 7. The individual circle
segment-shaped
CA 02775366 2012-03-26
-19-
electrodes 5, 6 are preferably disposed at a radial distance 12 from the
fanning axis 7. This
also results in a star-shaped or fan-shaped arrangement of the circle segment-
shaped or ap-
proximately circle segment-shaped electrode plates 5, 6 when seen in cross
section - as illus-
trated in 4. This electrode arrangement 3 therefore also has an essentially
hollow cylindrical
body shape because a cylindrical or tubular gap 11 is preferably formed around
the virtual or
imaginary fanning axis 7. Unlike the embodiment illustrated in Fig. 3,
however, a distance 9
between adjacent electrodes 5, 6 is constant or approximately constant in
terms of different
radial distances from the fanning axis 7, as may be seen from Fig. 4.
Disposed in the axial direction of the virtual cylinder or vertical axis 8,
i.e. in the axial direc-
tion of the vertical axis of the holding container 4, is at least one
electromagnetic coil 13,
preferably disposed at least above and/or underneath the electrode arrangement
3, which is
based on the star-shaped design. The electromagnetic field generated by this
electromagnetic
coil 13 when exposed to electrical energy acts on the electrolyte and also on
the electrode
arrangement 3 in the reaction chamber 2. In other words, the coil 13 is
disposed or dimen-
sioned so that the field lines of the electromagnetic field intersect or
influence the electrolyte
and also the anodic and cathodic electrodes 5, 6 of the electrode arrangement
3.
By preference, the at least one electrode arrangement 3 is completely
submersed in the elec-
trolyte, which is preferably provided in the form of water or an aqueous
solution. However,
the at least one electromagnetic coil 13 is preferably also disposed below a
regular or mini-
mum liquid level 14 for the electrolyte. In other words, the electromagnetic
coil 13 for gener-
ating an electromagnetic field is preferably also disposed at least
predominantly, preferably
completely, submersed in the electrolyte. This is important in terms of
transmitting vibrations
or high frequency vibrations to the electrolyte on the one hand and at least
indirectly also to
the anodic and cathodic electrodes 5, 6 on the other hand so as to detach gas
bubbles from the
electrodes 5, 6 and assist or accelerate degasification of the hydrogen and
oxygen bubbles
from the liquid electrolyte. In particular, the electromagnetic field of the
at least one coil 13
causes the anodic and cathodic electrodes 5, 6 of the electrode arrangement 3
to be mechani-
cally vibrated in order to assist the process of detaching gas bubbles which
occur, in particular
the respective oxygen and hydrogen bubbles, from the anodic and cathodic
electrodes 5, 6. In
addition, the electromagnetic field of the at least one electromagnetic coil
13 causes ionization
and enhances or intensifies the electrolytic process.
CA 02775366 2012-03-26
-20-
The anodic and cathodic electrodes 5, 6 are made from a ferromagnetic
material, in particular
one which can be influenced by magnetic fields, e.g. metals containing iron
and/or precious
metals, for example so-called Nirosta metal, or from any other stainless
steel. Due to the high-
frequency, mechanical vibrations of the electromagnetic coil 13, which are of
a relatively low
amplitude, the process of detaching the gas from the electrodes 5, 6 is
enhanced or acceler-
ated. At the same time, the active surface of the electrodes 5, 6 is held as
high as possible rela-
tive to the electrolyte in order to keep high or maximize the effectiveness or
productivity of
the electrolytic process or electrode surfaces of the electrodes 5, 6. This
accelerates the elec-
trolysis process and improves or maximizes the breaking down process as a
function of a de-
fined period. In other words, the electrolytic performance or breakdown
performance of the
electrolytic reaction system 1 can be improved or enhanced. In particular, the
conversion or
breaking down work per unit of time is increased by the described features so
that even with
compact reaction systems 1 with a relatively small volume, an efficient
discharge of hydrogen
and oxygen gas can be obtained by reference to a corresponding gas mixture.
The specified
electrolytic reaction system 1 therefore offers intensive reactions or rapid
reactions. The at
least one electromagnetic coil 13 at least partially submersed in the
electrolyte therefore offers
a synergy effect because it causes ionization one the one hand and acts as a
means of generat-
ing vibrations for the electrolyte and for the electrodes 5, 6.
Based on one advantageous alternative or embodiment, another electrode
arrangement 3'
comprising a plurality of anodic and cathodic electrodes 5, 6 is disposed
above the at least one
electromagnetic coil 13. This other electrode arrangement 3' disposed above
the electromag-
netic coil 13 is also preferably completely, in particular as completely as
possible, submersed
in the liquid, in particular the aqueous electrolyte inside the reaction
chamber 2.
As schematically illustrated by way of example in Fig. 1, the electromagnetic
fields of the
electromagnetic coil 13 when exposed to energy act on the electrodes 5, 6 of
the electrode
arrangement 3, 3' disposed underneath and/or above causing them to vibrate,
and when ex-
posed to energy the electromagnetic coil 13 also acts on the electrolyte due
to vibrations or
induces vibrations so that gas bubbles are detached from the electrodes 5, 6
and a movement
of the gas bubbles in the electrolyte is intensified or enhanced.
CA 02775366 2012-03-26
-21-
Alternatively, it would also be conceivable to dispose the electromagnetic
coil 13 underneath
the electrode arrangement 3, in particular in the base portion of the reaction
chamber 2 or
holding container 4 accommodating the electrolyte.
The electrode arrangement 3 is preferably disposed at a vertical distance from
the base portion
or base plate of the reaction chamber 2. Accordingly, there is a defined
electrolyte volume
disposed underneath the electrode arrangement 3 or a defined quantity of
electrolyte is able to
accumulate underneath the electrode arrangement as a result so that a flow
passage is created
underneath the electrode arrangement 3 close to the base. An electromagnetic
coil 13' posi-
tioned towards the cylinder or vertical axis 8 in the axial direction
underneath the electrode
arrangement 3 is preferably likewise positioned at a distance from the base
portion of the re-
action chamber 2 to enable a flow to be created in the electrolyte inside the
electrode ar-
rangement 3 starting from the base portion and moving upwards in the vertical
direction, in
particular in the direction towards the gas chamber of the electrolytic
reaction system 1.
Based on one advantageous embodiment, which may be seen from a comparison of
Figures 1
and 5, the at least one electromagnetic coil 13 as seen in plan view is
essentially of an annular
shape. A central or mid-point 15 of this torus-shaped electromagnetic coil 13
therefore lies on
or close to the cylinder or vertical axis 8 of the holding container 4 or on
or close to the fan-
ning axis 7 of the electrode arrangement 3. In other words, the essentially
disk-shaped mid-
plane 16 of the coil 12 is oriented transversely to, in particular at a right
angle to, the cylinder
or vertical axis 8 or at a right angle the fanning axis 7, as may best be seen
from Fig. 1.
A coil body 17 of the coil 13 is based on an annular or torus shape. This coil
body 17 is pref-
erably made from a non-magnetizable material, in particular from plastic or
such like. In other
words, the electromagnetic coil 13 is preferably designed without an iron
core, and in particu-
lar is provided in the form of an air reactor. This coil body 17 supports at
least one coil wind-
ing 18 comprising a plurality of turns, in particular hundreds or thousands of
turns, wound
around the coil body 17. Instead of opting for a design based on a coil body
17, however, it
would also be possible for the at least one coil winding 18 to be based on a
self-supporting
design, i.e. formed without a coil body 17, in which case it is of an
intrinsically stable design,
as it were.
CA 02775366 2012-03-26
-22-
The individual turns of the coil winding 18 are oriented radially or
essentially radially with
respect to the annular coil 13. In particular, the individual turns extend in
a circle or coil
around the bead-type coil body 17, as best illustrated in Fig. 5. Based on a
preferred embodi-
ment, four part-windings 19, 19', 19", 19"' are provided, wound around the
circumference of
the coil body 17 or coil 13 distributed at a distance from one another. The
individual part-
windings 19-19"' are connected in series. A winding gap 20, 20', 20"is
preferably left free
between the individual part-windings 19-19"'.
Based on one advantageous embodiment, three coil windings are provided, each
disposed
offset from the coil axis or central or mid-point 15 by 45 , wound one on top
of the other. In
particular, this results in an at least three-layered coil winding 18, the
winding gaps 20, 20',
20" of which are disposed one after the other and offset from one another in
the circumferen-
tial direction of the torus-shaped coil 13.
Based on one advantageous embodiment, the at least one electromagnetic coil 13
is connected
to the electrode arrangement 3 so as to disperse load and is supported so that
it takes the load
away from the electrode arrangement 3. This means that the at least one
electromagnetic coil
13 is not mechanically connected directly to the reaction chamber 2 and
instead is mechani-
cally connected as directly as possible to the electrode arrangement 3. This
makes it possible
for the vibrations to be transmitted as intensively as possible to the
electrode arrangement 3.
In the case of the embodiment illustrated in Fig. 2, the electromagnetic coil
13 is accommo-
dated in a hollow conical or funnel-shaped retaining element, which retaining
element is sup-
ported on the top face of the electrode arrangement 3. Mechanical vibrations
or vibrations of
the electromagnetic coil 13 are therefore transmitted to the electrode
arrangement 3 and vice
versa. In the case of the embodiment illustrated in Figs. 6, 7, the at least
one electromagnetic
coil 13 is secured and supported by means of a clamp-type support or retaining
mechanism on
the top face of the electrode arrangement 3 so that it takes load.
The electrodes 5, 6 are expediently retained or mounted so that they are able
to oscillate in the
electrolyte bath as freely as possible. To this end, it is practical to opt
for a one-ended or
tongue-type retaining or mounting system. Another conceivable alternative is
to retain the
electrodes 5, 6 on at most two mutually opposite edge portions or terminal
ends of the elec-
trodes 5, 6, as illustrated by way of example in Fig. 2.
CA 02775366 2012-03-26
- 23 -
The individual anodic and cathodic electrodes 5, 6 of the electrode
arrangement 3 are supplied
with electrical energy in a manner known per se from a first electrical energy
source 21. The
first energy source 21 is preferably designed to provide the anodic and
cathodic electrodes 5,
6 with a pulsating energy supply.
The at least one electromagnetic coil 13 is supplied with electrical energy by
another electri-
cal energy source 22. The other electrical energy source 22 is preferably
designed to provide
the at least one electromagnetic coil 13 with a pulsating energy supply.
The first energy source 21 and the other energy source 22 supply the
electrodes 5, 6 respec-
tively the coil 13, preferably with a pulsating DC voltage of varying
amplitude level and de-
fined pulse pauses between the individual voltage or energy pulses in each
case. The energy
sources 21, 22 are preferably provided in the form of electrical energy
transformers, in par-
ticular transformer circuits or signal generators, of a type long known from
the prior art. The
respective energy sources 21, 22 are supplied with electrical energy from a
public power sup-
ply network or preferably from a DC voltage source, in particular from an
electrochemical
voltage source, e.g. an accumulator. The electrical energy supplier of the
energy sources 21,
22 is preferably an accumulator, in particular at least one lead accumulator
with a terminal
voltage of 12V respectively 24V. In particular, the energy supplier may be the
12V/24V on-
board network of an automotive vehicle.
As a result of one advantageous feature, an energy frequency of the first
energy source 21
supplying energy to the anodic and cathodic electrodes 5, 6 compared with an
energy fre-
quency of the second energy source 22 supplying energy to the at least one
electromagnetic
coil 13 is selected so that the electrolytic reaction system 1 operates close
to or at its reso-
nance frequency, at least some of the time. In particular, the respective
energy frequencies of
the first energy source 21 and the other energy source 22 are adapted to one
another so that
the electrolytic system operates in a resonant or quasi resonant state,
thereby offering a highly
efficient and highly active breakdown of the electrolyte into gaseous hydrogen
and oxygen.
As a result, amongst other things, the degree or efficiency with which the
respective gas bub-
bles are detached from the anodic and cathodic electrodes 5, 6 is
significantly influenced. In
particular, the effect of the electric or electromagnetic fields in the
reaction chamber 2 assists
CA 02775366 2012-03-26
-24-
and accelerates an electrolytic splitting process on the one hand. On the
other hand, a vibra-
tion or oscillation is generated due to the electromagnetic coupling of forces
and vibrations in
the electrolyte and/or in the metallic, in particular ferromagnetic,
electrodes 5, 6, which is
conducive to detaching gas and hence the breakdown and splitting process.
The pulse frequency of the first energy source 21 supplying the anodic and
cathodic elec-
trodes 5, 6 is a multiple higher than the pulse or energy frequency of the
second energy source
22 supplying the at least one electromagnetic coil 13. The supply frequency of
the first energy
source 21 is at least from a hundred times up to approximately ten thousand or
a hundred
thousand times that of the supply frequency of the second energy source 22,
preferably ap-
proximately a thousand times, The frequency ratio between the electrical
energy supply for
the electrode arrangement 3 and the electrical energy supply for the at least
one electromag-
netic coil 13 is therefore preferably approximately 1000:1. For example, the
energy frequency
for the coil 13 is approximately 30 Hz and the energy frequency for the anodic
and cathodic
electrodes 5, 6 is approximately 30 kHz. Naturally, other base or frequency
values could be
set or generated at the energy sources 21, 22.
A voltage level of the first energy source 21 supplying the anodic and
cathodic electrodes 5, 6
may be several 100 V or several 1000 V, in particular up to 50 kV, but
preferably less than 10
kV.
The respective voltage or frequency values will primarily depend on the
structural arrange-
ment and geometric dimensions of the respective components inside the reaction
chamber 2
and can be empirically adjusted or adapted in a manner familiar to the skilled
person.
Based on one advantageous embodiment, at least one inlet orifice 23 for
filling up and/or con-
tinuously or intermittently topping up electrolyte liquid, in particular the
electrolyte capacity
or holding container 4 for the electrolyte, is disposed in the bottom portion
of the reaction
chamber 2. Due to the electrolyte which is or can be fed in at the bottom
portion, in particular
the base portion of the electrolyte bath , the electrolyte becomes turbulent
or swirls, which
advantageously assists and accelerates detachment of the gas bubbles from the
anodic and
cathodic electrodes 5, 6.
CA 02775366 2012-03-26
V
-25-
Alternatively or in combination with this, at least one means 24 for creating
turbulence in the
electrolyte, in particular for creating a flow in the electrolyte, for example
a turbulent flow,
may be provided in the reaction chamber 2, in particular in the holding
container 4 for the
electrolyte. This turbulence-creating means 24 may be any means known from the
prior art for
creating flows or turbulence in a liquid bath. In one advantageous embodiment,
the means 24
for creating turbulence in the electrolyte is provided in the form of intake
and/or outlet noz-
zles 25 for the electrolyte running into the reaction chamber. A plurality of
intake and/or out-
let nozzles 25 is preferably provided for the electrolyte, which preferably co-
operate with the
holding container 4 for the electrolyte. Depending on the turbulence or
distribution of the re-
spective turbulent forces required, the number of these intake and/or outlet
nozzles 25 may be
varied considerably to suit the relevant requirements. Also depending on the
diameter of these
intake and/or outlet nozzles 25, at least two or also hundreds of such intake
and/or outlet noz-
zles 25 may be provided, and they are preferably disposed in the base region
of the holding
container 4 for the electrolyte. Based on one advantageous embodiment, at
least individual
ones of the effective axes of a plurality of intake and/or outlet nozzles 25
are inclined with
respect to the base portion. In particular, the effective axes of the intake
and/or outlet nozzles
may be oriented at an angle with respect to the cylinder or vertical axis 8 of
the reaction
chamber 2 in order to build up an intrinsic turbulence or extensive flow in
the electrolyte bath,
which is conducive to removing the hydrogen respectively oxygen bubbles from
the anodic
20 and cathodic electrodes 5, 6 and from the interior of the electrolyte in
the direction towards
the top to the degasification zone, in particular a gas chamber 26 of the
reaction chamber 2.
Instead of creating pronounced turbulence or flow in the electrolyte by
introducing liquid or
gas, it would naturally also be possible to provide the means 24 for creating
turbulence in the
25 electrolyte in the form of at least one agitator, which is immersed in the
electrolyte liquid.
Based on one advantageous feature, the means 24 for creating a flow in the
electrolyte is de-
signed so that an approximately screw-shaped flow is created around the
cylinder or vertical
axis 8 of the holding container 4 and reaction chamber 2, in which case the
direction in which
this screw-shaped flow is propagated extends from the base portion of the
electrolyte in the
direction towards the surface of the electrolyte bath.
Based on one advantageous embodiment, at least one overflow edge 27 is
provided in the
reaction chamber 2, which is designed to mark a maximum liquid level 28 of the
electrolyte.
CA 02775366 2012-03-26
-26-
Based on one advantageous embodiment, this at least one overflow edge 27 is
provided in the
form of at least one top boundary edge 29 of a hollow cylindrical or hollow
prismatic electro-
lyte container 30. This electrolyte container 30 preferably has a vertically
oriented cylinder
axis 31, which is preferably congruent with the cylinder or vertical axis 8 of
the reaction
chamber 2 or at least approximately congruent with it. The at least one
overflow edge 27 may,
as an alternative or in addition to the top boundary edge 29 of the
electrolyte container 30, be
provided in the form of at least one bore or some other orifice in the wall of
the electrolyte
container 30. However, the top portion of the electrolyte container 30 is
preferably as open as
possible, in particular across the entire cross-sectional surface, to assist
with efficient separa-
tion and removal of foam 32 which usually occurs during the electrolysis
process, in particu-
lar a ring of foam which forms on the electrolyte. Especially if the liquid or
electrolyte level
lies at the same height as the overflow edge 37, removal of the foam 32 from
the electrolyte
will be efficient. An initial filling level 33 of the electrolyte preferably
lies slightly below the
overflow edge 27. During an active electrolytic process, the volume of
electrolyte increases
significantly, primarily due to the formation of gas bubbles in the
electrolyte. This means that
during operation of the electrolytic reaction system 1, the electrolyte level
in the reaction
chamber 2, in particular in the holding or electrolyte container 4, 30, rises.
It is for this reason
that an initial filling level 33 for the electrolyte preferably lies below the
overflow edge 27 of
the electrolyte container 30. The overflow edge 27 in any event defines the
maximum possible
electrolyte level in the electrolyte container 30. When this maximum
electrolyte level is
reached or exceeded, the electrolyte foam or the ring of foam is efficiently
removed.
Based on the embodiment illustrated as an example, the ring of foam or the
foam 32 or also
the overflowing or surplus electrolyte liquid is discharged from the central
region of the elec-
trolyte container 30 in the outward direction, in particular in the radial
direction towards the
vertical or cylinder axis 8, 31. Based on an alternative or combined
embodiment, it is also
possible for foam 32 and the electrolyte flowing over the at least one
overflow edge 27 to be
discharged via a discharge passage 34 disposed in a central region of the
electrolyte container
30, as indicated by broken lines. In this central or centrally disposed
discharge passage 34,
electrolyte foam or electrolyte spilling over the overflow edge 27' can be
directed in the
downward direction and preferably gated back into the electrolyte container
30, as will be
explained in more detail below.
CA 02775366 2012-03-26
-27-
A collection portion 35 for electrolyte or electrolyte foam which has flowed
over the overflow
edge 27 is preferably provided in the base portion of the reaction chamber 2.
This collection
portion 35 extends across a defined vertical height of the reaction chamber 2
and prevents or
reduces the electrolytically obtained gases from escaping through an outlet
orifice 36, used to
feed the electrolyte out of the reaction chamber 2 in a controlled manner.
This collection por-
tion 35 may be provided in the form of a defined electrolyte level in the base
portion of the
reaction chamber 2 or by some other siphon-type gas barrier. The collection
portion 35 or
corresponding liquid siphon primarily ensures that the reaction chamber 2 is
closed in a gas-
tight manner as far as possible and that hydrogen and oxygen gas is prevented
as far as possi-
ble from escaping or being discharged through an outlet orifice 36 for the
electrolyte close to
the base. The siphon-type collection portion 35 for electrolyte liquid and for
separated electro-
lyte foam flowing over the overflow edge 27 therefore closes the outlet
orifice 36 off so that it
is relatively gas-tight, whereas the electrolyte liquid can still be
discharged from the reaction
chamber 2 through the at least one outlet orifice 36. Particular care must be
taken to ensure
that a defined liquid level exists or is built up within the collection
portion 35 in order to pro-
duce a sufficiently gas-tight gas barrier.
The liquid level in the collection portion 35 is preferably lower than the
regular filling level
33 for the electrolyte inside the electrolyte container 30. As illustrated,
the collection portion
35 may be disposed around the electrolyte container 30 or, if the surplus
electrolyte is intro-
duced centrally into a centrally disposed discharge passage 34, in the central
region of the
electrolyte container 30, as indicated by broken lines in the embodiment
illustrated. Alterna-
tively, it would naturally also be possible to opt for a combined outer and
inner collection
system or else a cascaded electrolyte collection system in order to separate
and degasify elec-
trolyte foam and electrolyte liquid by means of at least one collection
portion 35 for electro-
lyte liquid.
It is also expedient to provide at least one return line 37 for the
electrolyte flowing over the
overflow edge 27 of the holding or electrolyte container 4, 30. The
electrolyte is fed back into
the hollow cylindrical or hollow prismatic electrolyte container 30 or into
the reaction cham-
ber 2 by means of this return line 37. Within the at least one line
incorporating the return line
37 for the electrolyte, it is also preferable to provide a liquid tank 38, in
particular a water
container 39, in which a certain quantity of electrolyte, in particular a
liquid electrolyte in the
CA 02775366 2012-03-26
-28-
form of water, can be held in supply or buffered. Electrolyte liquid is fed
from this liquid
tank 38 to the electrolytic process inside the reaction chamber 2 continuously
or intermit-
tently. The at least one return line 37 extends more or less through or via
the liquid tank 38.
This means that the return line 37 opens into the liquid tank on the one hand
and the return
line 37 continues on from the liquid tank 38 again in the direction towards
the reaction cham-
ber 2 to provide a means of filling or topping up the electrolytic liquid in
the holding or elec-
trolyte container 4, 30. This electrolyte circuit 41 between the reaction
chamber 2 and the liq-
uid tank 38 respectively the water container 39 is comparable with the intake
and return lines
of fuel supply systems used in internal combustion engines from a hydraulic
point of view.
At least one filter device 40 for filtering out residues, in particular
impurities, in the electro-
lyte or in the electrolytically treated water may be disposed in the return
line 37. In order to
create an active or forced water or electrolyte circuit 41, at least one
liquid pump 42 may be
incorporated in the return line 37 or in the intake line for the electrolyte
delivered to the reac-
tion chamber 2. It is of practical advantage if the return line 37 also serves
as a cooling device
43 for the electrolyte or comprises a cooling device 43. This cooling device
43 may be the
pipe connections of the return line 37 per se and/or may be provided in the
form of an addi-
tional heat exchanger, in particular an air/liquid exchanger, e.g. cooling
fins. This heat ex-
changer 44 or cooling fins may be provided in the pipe connection and/or on
the liquid tank
38 or water container 39. Based on a preferred embodiment, the cooling device
43 is dimen-
sioned and the return line 37 is dimensioned so that the temperature of the
electrolyte is kept
within a range of between 20 C and 60 C, in particular in a range of between
28 C and 50 C,
preferably at 35 C to 43 C. It is primarily within the specified temperature
range of the elec-
trolyte that the electrolysis process is optimized and relatively more
efficient. In particular,
only a relatively small quantity of power in terms of electrical energy is
needed in this tem-
perature range. The cooling device 43 may naturally also be provided in the
form of other
passively and/or actively operating cooling devices selected from the many
designs known
from the prior art.
Based on one advantageous embodiment, the electrolytic reaction system 1
therefore has a
continuous or discontinuous intake 45 and discharge 46 for the electrolyte. In
particular, this
intake 45 and discharge 46 of the electrolyte provides or creates a time-based
gradual re-
placement or top-up of the electrolyte containing water or comprising water in
the reaction
CA 02775366 2012-03-26
-29-
chamber 2 or in its electrolyte container 30. In this respect, it is
preferable to create a closed
electrolyte circuit 41 in which the liquid tank 38 and the at least one liquid
pump 42 is incor-
porated.
Based on one advantageous feature intended to improve the system, at least one
passage ori-
fice 47 for ambient air 48 to be introduced into the reaction chamber 2, in
particular into the
holding container 4 for the electrolyte, is provided, preferably in the base
portion and/or in the
wall region of the reaction chamber 2. Alternatively or in addition to this,
the at least one pas-
sage orifice 47 may also be provided as a means of feeding nitrogen or other
non-combustible
gases into then holding container 4, in particular into the electrolyte
container 30. The at least
one passage orifice 47 then opens directly into the electrolyte bath, which is
disposed in the
reaction chamber 2, in particular in the electrolyte container 30, during
operation of the reac-
tion system 1. A plurality of passage orifices 47 for ambient air 48 and/or
nitrogen is provided
in a distributed arrangement, preferably in the base portion and/or wall
region of the electro-
lyte container 30. In particular, ambient air 48 and/or nitrogen is fed or
introduced directly
into the electrolyte so that a liquid or gas mixture and a flow or turbulence
is created in the
electrolyte. A regulating means 49 may optionally be provided, in particular a
valve arrange-
ment or similar, which is designed to regulate the quantity and/or pressure of
the ambient air
48 or nitrogen flowing into the electrolyte. This process of introducing
ambient air 48 or ni-
trogen or other non-combustible gases preferably takes place under pressure.
In other words,
the ambient air 48 or oxygen is actively blown into the electrolyte. Another
option would be
to generate a negative pressure in the reaction chamber 2 to enable the
appropriate gases or
gas mixtures, such as air, to be sucked in. As a result of the passage
orifices 47 described
above as a means of introducing or blowing ambient air 48 or nitrogen directly
into the elec-
trolyte, the process of detaching oxygen and hydrogen bubbles adhered to the
electrode ar-
rangement 3 is assisted on the one hand. In addition, introducing this air or
nitrogen into the
electrolyte can be used as a means of creating turbulence or mixing the
electrolyte. This has a
positive effect in terms of the electrolytic performance, in particular in
terms of the efficiency
of the electrolytic reaction system 1.
It is preferable to provide a multiple arrangement of passage orifices 47 by
means of which air
or nitrogen can be introduced into the holding container 4 for the electrolyte
on a selective
and distributed basis. Based on one advantageous embodiment, these passage
orifices 47 are
CA 02775366 2012-03-26
-30-
positioned in the base portion of the reaction chamber 2, in particular
underneath der electrode
arrangement 3.
Based on one advantageous feature intended to improve the system, the
electrolytic reaction
system 1 is provided with at least one means 50 for generating negative
pressure inside the
reaction chamber 2, in particular in its gas chamber 26. This negative
pressure should be in-
terpreted by reference to atmospheric ambient pressure. In other words, means
50 generating
the negative pressure inside the reaction chamber 2, in particular in the gas
chamber 26, create
defined negative pressure conditions. Based on a first embodiment, this means
50 may be
provided in the form of a vacuum pump. Based on one advantageous embodiment,
this means
50 for generating negative pressure may be provided in the form of a consumer
for the chemi-
cal energy carrier hydrogen, connected to the reaction chamber 2. This
consumer, which in
the case of one advantageous embodiment is provided in the form of an internal
combustion
engine 51, in particular a petrol, gas or diesel engine, converts the chemical
energy of the hy-
drogen into kinetic energy by releasing thermal energy. The consumer may
naturally also be
provided in the form of any heating or generator system for generating power.
Based on one
advantageous embodiment, negative pressure is built up in the reaction chamber
2 by estab-
lishing a flow connection 52 between the reaction chamber 2, in particular its
gas chamber 26,
and a fuel intake line 53, in particular the intake passage of an internal
combustion engine 51
or some other combustion system for converting the chemical energy of the
hydrogen-oxygen
mixture into thermal or kinetic energy. This also increases degasification
performance with
respect to the electrolyte and the electrode arrangement 3 and increases the
electrolysis per-
formance which can be achieved with the electrolytic reaction system 1.
Figs. 6, 7 illustrate another embodiment of the electrolytic reaction system 1
for generating
gaseous hydrogen and oxygen. This may also be construed as an independent
embodiment of
the reaction system 1 proposed by the invention in its own right. The same
reference numbers
and component names are used to denote the same parts as those used in the
drawings above.
To avoid unnecessary repetition, reference may be made to the detailed
description of the pre-
vious drawings given above. It is explicitly pointed out that not all the
features and design
features illustrated in these drawings necessarily form part of the reaction
system 1 proposed
by the invention. Moreover, features may be combined with features of the
invention de-
scribed with reference to the drawings above.
CA 02775366 2012-03-26
-31 -
This electrolytic reaction system 1 also comprises a reaction chamber 2 for
accommodating an
electrolyte, such as water, an aqueous solution or a water mixture together
with additives to
increase conductivity, for example. Also disposed in the reaction chamber 2 is
at least one
electrode arrangement 3, comprising a plurality of anodic and cathodic
electrodes 5, 6. In the
case of this embodiment, the electrode arrangement 3 is provided in the form
of at least two,
preferably more than at least three, tubular electrodes 5, 6 disposed
coaxially or approxi-
mately coaxially one inside the other. In the embodiment illustrated as an
example, there are
five tubular electrodes 5, 6 disposed coaxially, nested one inside the other,
in particular in-
serted one inside the other. In this connection, it should be pointed out that
electrodes 5, 6
with circular or annular or elliptical cross sections are preferred. However,
instead of tubular
electrodes 5, 6 with a hollow cylindrical body shape, it would naturally also
be possible to use
tubular electrodes 5, 6 with a prismatic body shape, in particular with
square, rectangular or
any other polygonal cross section. The individual electrodes 5, 6 form
preferably alternating
or consecutive anodes and cathodes respectively in the electrolytic reaction
system 1.
The wall surfaces of the mutually adjacent tubular electrodes 5, 6, which may
be cylindrical
or made up of several prismatic surfaces oriented at an angle to one another,
are spaced at a
distance apart from one another. In particular, defined gaps 54 respectively
55 are disposed
between the respective cylindrical or wall surfaces, in particular between the
internal and ex-
ternal faces of the respective electrodes 5, 6. Based on one advantageous
feature, a distance
54 or a gap dimension between the tubular or hollow prismatic, mutually nested
electrodes 5,
6 of an outer pair of electrodes 5, 6 increases or become larger in size than
an electrode 5, 6 or
a pair of electrodes 5, 6 of this tubular electrode arrangement 3 disposed
further inwards, in
particular closer to a central tube axis 56. In other words, gaps 55 between
tubular or hollow
prismatic electrodes 5, 6 at the center of the electrode arrangement 3 are
preferably of bigger
dimensions than the gaps 54 between outer or pairs of electrodes 5, 6
surrounding the inner
electrodes 5 .
The individual, virtual tube axes 56 of the tubular electrodes 5, 6 are
preferably vertically ori-
ented. This being the case, the distal end portions of the tubular electrodes
5, 6 are respec-
tively of an open design. The individual tubular electrodes 5, 6 preferably
have a constant
cross-sectional surface by reference to their length or height.
CA 02775366 2012-03-26
-32-
The essential aspect is that between the wall or cylinder surfaces of the
tubular or hollow
prismatic electrodes 5, 6, at least one at least approximately hollow
cylindrical or prismatic
gap 57, 58 is provided. The fact that there is at least one gap 57, 58 between
the various elec-
trodes 5, 6 of the electrode arrangement 3 means that the formation of gas
bubbles is made
possible and assisted. In particular, gas bubbles which occur and adhere to
the anodic and
cathodic electrodes 5, 6 during the electrolysis process can be efficiently
fed away into a gas
chamber 26 lying above the electrolyte. A sort of suction and carrying effect
occurs as a result
and assists the release of gas bubbles from the electrolyte. This effect is
reinforced by the
electrolyte volume disposed underneath the electrode arrangement 3 and by a
Venturi effect
inside the tubular electrode arrangement 3.
In particular, the at least one approximately hollow cylindrical or prismatic
gap 57, 58 be-
tween adjacent electrodes 5, 6 creates a sort of chimney flue effect for the
gas bubbles and
thus increases the rate at which they are released as well as the
degasification efficiency. This
effect is further enhanced by the cascaded or multiple arrangement of
electrodes or electrode
pairs 5, 6.
At least one electromagnetic coil 13 is disposed at least above the tubular
electrode arrange-
ment 3 by reference to the virtual central tube axis 56, as described above.
The essential as-
pect is that when energy is applied to this electromagnetic coil 13, the
preferably alternating
or pulsating electromagnetic fields which occur or are generated act on the
electrolyte and
also on the electrode arrangement 3. In particular, the field lines intersect
both the electrode
arrangement 3 and the electrolyte volume in the electrolytic reaction system 1
with sufficient
intensity. Alternatively or in combination with an electromagnetic coil 13
lying above the
electrode arrangement 3, at least one electromagnetic coil 13 may also be
provided under-
neath the electrode arrangement 3.
Amongst other things, the at least one electromagnetic coil 13 causes the
electrode arrange-
ment 3 to mechanically vibrate or vibrate, which assists and accelerates
release of the gas
bubbles from the electrolyte. In addition, the electric field of the
electromagnetic coil 13 also
has a positive effect on the electrolytic conversion and splitting process
above all.
CA 02775366 2012-03-26
-33-
Based on one advantageous embodiment, the reaction chamber 2 of the
electrolytic reaction
system 1 has an essentially hollow cylindrical or hollow prismatic body shape.
The virtual
cylinder or vertical axis 8, in particular the wall surface of the reaction
chamber 2, is verti-
cally or at least approximately vertically oriented, as may be seen in Fig. 6
or Fig. 2 for exam-
ple.
As may also best be seen from Figs. 2 and 6, it is of practical advantage if
the reaction cham-
ber 2 comprises or has an essentially hollow cylindrical or hollow prismatic
holding container
4, in which the at least one star-shaped or tubular electrode arrangement 3 is
disposed. Based
on the embodiment illustrated in Figs. 1, 2, the holding container 4 for the
electrolyte and for
the at least one electrode arrangement 3 is of an open design at the top end
portion. In addi-
tion, its wall or cylinder surface is spaced at a distance apart from the
inner faces of the reac-
tion chamber 2, as may best be seen from Fig. 1. This offers a simple way of
providing the
separation or collection portion 35 described above. Based on one advantageous
feature, the
virtual fanning axis 7 of the star-shaped electrode arrangement 3 and the
virtual tube axis 56
of the tubular electrode arrangement are essentially congruent with the
virtual cylinder axis 8
or congruent with the virtual cylinder axis 8 of the holding container 4 and
reaction chamber
2, as may be seen in particular from the diagrams of Figs. 1 and 6.
Fig. 8 is another schematic diagram illustrating an electrode arrangement 3.
In this case, the
holding container 4 and reaction chamber 2 are hollow cylindrical, in
particular circular in
terms of their cross section. Based on an alternative embodiment indicated by
broken lines,
the reaction chamber 2 or holding container 4 may also have a different hollow
prismatic
body shape, in particular a cross-sectional shape with corners, although it is
of advantage to
opt for rounded corners or edges. Provided in the interior of the reaction
chamber 2 is a plu-
rality of electrode arrangements 3, 3'. In particular, a bundle of tube
electrodes is provided,
and the individual electrode pairs 5, 6 are disposed in a distributed
arrangement inside the
holding container 4 for the electrolyte. In particular, a first electrode
arrangement 3 is dis-
posed at the center of the holding container 4 and, disposed in a circle
around this central
electrode arrangement 3, is a plurality of other electrode arrangements 3'. It
would also be
possible to use mixed shapes of electrodes. For example, tube electrodes 5, 6
with a circular
cross section and tube electrodes 5, 6 with a square cross section could be
combined, for ex-
ample as a means of obtaining a higher packing density inside the holding
container 4.
CA 02775366 2012-03-26
-34-
As regards the dimensioning of the tubular or hollow prismatic electrodes 5,
6, it is expedient
to ensure that their stiffness values do not exceed a defined upper threshold
value as far as
possible. In particular, the wall thicknesses 59, 60 of the electrodes 5, 6
should be selected so
that the electromagnetic field of the at least one coil 13 induces mechanical
vibrations in the
electrode arrangement 3 or at least individual electrodes 5, 6. Since the
electrodes 5, 6 are
made from electrically conducting, in particular ferromagnetic, material, the
electromagnetic
alternating field or the electromagnetically pulsating field of the at least
one coil 13 has the
effect of inducing vibrations or oscillations. This is conducive to efficient
detachment of gas
bubbles and the capacity of the gas bubbles to be released from the
electrolyte. In particular,
the material elasticity or the wall thickness 59, 60 of the respective
electrodes 5, 6 should be
selected so that the most intensive vibrations possible are induced by the
electromagnetic coil
13.
Based on one advantageous embodiment and with a view to enhancing this
detachment proc-
ess, the at least one plate-shaped electrode 5, 6 - Fig. 1 - or the at least
one tubular or hollow
prismatic electrode 5, 6 - Fig. 6 - may be provided with at least one slot 61,
62 or a plurality
of orifices or perforations. In particular, the respective electrodes 5, 6
have at least one me-
chanical weakening or reduction in stiffness, such as slots 61, 62 or orifices
or cut-outs in the
material or material recesses, so as to vibrate more mechanically intensively
under the influ-
ence of the electromagnetic field of the at least one electromagnetic coil 13.
These features
also enhance the performance and reaction time of the electrolytic reaction
system 1 in terms
of efficiency in producing hydrogen. An intensive inducement of vibrations or
one involving
little loss for the electrodes 5, 6 is also obtained by opting for a load-
transmitting support, in
particular due to an as rigid as possible mechanical connection between the at
least one elec-
tromagnetic coil 3 and at least one electrode 5, 6 of the electrode
arrangement 3. This me-
chanical connection or retaining device is preferably electrically insulating.
The quantity of hydrogen and oxygen which can be produced by the electrolytic
reaction sys-
tem 1 specified above is sufficient, without having to store the chemical
energy carrier hydro-
gen temporarily, to run an internal combustion engine 51 with a power of 30 to
100 kW, for
example, uninterrupted. In particular, the specified electrolytic reaction
system 1 is energy
efficient and powerful to the degree that the electrolytically obtained
quantity of hydrogen is
CA 02775366 2012-03-26
-35-
sufficient to supply engines in standard automotive vehicles with a sufficient
quantity of pow-
er or fuel in the form of a hydrogen-oxygen mixture. In particular, the
specified electrochemi-
cal conversion system, i.e. the electrolytic reaction system 1, is capable of
producing a quan-
tity of a hydrogen-oxygen mixture high enough to generate sufficient kinetic
energy when
combusted in internal combustion engines 51, in particular in petrol or gas or
diesel engines,
to drive standard commercial automotive vehicles with the usual or requisite
power. The es-
sential aspect of this is that the specified electrolytic reaction system 1
enables standard op-
eration of the respective automotive vehicle without the need for temporarily
storing or tem-
porarily buffering large quantities of hydrogen gas. A capacity of the gas
chamber 26 and the
flow connection 52 to the consumer is typically less than 0.5 m3. In
particular, a capacity of
the gas chamber 26 of less than 0.1 m3 is sufficient to supply an internal
combustion engine
51 with a maximum output power of 50 kW "on demand" with the requisite fuel,
in particular
with a hydrogen/oxygen mixture. This is a major safety feature because the
quantity of ignit-
able gaseous hydrogen present inside the electrolytic reaction system 1 is
relatively small. The
risks incurred by this electrolytic reaction system 1 are therefore relatively
low and the poten-
tial for danger can easily be addressed and mastered. In particular, the
specified electrolytic
reaction system 1 can be easily inspected with a view to meeting stringent
safety require-
ments. This is due above all to the "on demand" supply or requisite
availability of the hydro-
gen gas or hydrogen-oxygen mixture required in each case. However, this
requires a high de-
gree of efficiency and power and reaction capacity, all of which are provided
by the specified
reaction system 1. In particular, a sufficient quantity or sufficient volume
of hydrogen gas can
be generated after a relatively short warm-up or run-up phase of the
electrolytic reaction sys-
tem 1 to start, continuously run and supply a consumer with an output power of
50 kW or
more. The volume needed to mount the electrolytic reaction system 1, in
particular the reac-
tion chamber 2, is less than 0.5 m3, in particular less than 0.25 m3,
typically only approxi-
mately 0.02 m3.
As defined in the claims, the electrode arrangement 3 comprises several
electrode plates ex-
tending in a star-shaped arrangement or at least one bundle of tubular
electrodes coaxially
nested one inside the other. This enables optimum electrolysis performance to
be obtained.
However, it would also be conceivable to produce similar actions or effects
with other elec-
trode arrangements known from the prior art, for example with a cascaded or
serial arrange-
ment of plate-shaped electrodes, so that the claimed electrode arrangements
need not neces-
CA 02775366 2012-03-26
-36-
sarily be the ones used. In particular, only relatively low impairment of
performance and effi-
ciency can be anticipated if using electrode arrangements of different types.
Fig. 9 illustrates another embodiment of the at least one electromagnetic coil
13 which can
advantageously be used with the electrolytic reaction system 1 in the manner
explained above.
This embodiment of the electromagnetic coil 13 can therefore be used in
combination with the
features described above to obtain an advantageous electrolytic reaction
system 1. In the sec-
tions below, the same reference numbers and component names are used as those
used for the
previous drawings. To avoid unnecessary repetition, reference may be made to
the detailed
description of the drawings given above.
The schematically illustrated electromagnetic coil 13 represents an
alternative to the embodi-
ment illustrated in Fig. 5 and, in keeping with the explanations given with
reference to Fig-
ures 1, 2 and 6, is preferably disposed above and/or underneath a star-shaped
or tubular elec-
trode arrangement 3 so that its electromagnetics field acts on the electrolyte
on the one hand
and on the electrode arrangement 3 on the other hand when supplied with
electrical energy.
The at least one electromagnetic coil 13 provided is essentially torus-shaped
or annular and
comprises a plurality of part-windings 19, 19', 19", 19... electrically
connected in series. The
individual part-windings 19, 19', 19", 19"` of the electromagnetic coil 13
extend respectively
across a circumferential angle 63 constituting only a fraction of the total
ring circumference
64, i.e. a fraction of the 360 angle of the torus-shaped electromagnetic coil
13. The circum-
ferential angle 63 of the individual part-windings 19, 19', 19", 19"`
connected in series is
typically between 20 and 50 , in particular between 25 and 45 , preferably
approximately
30 by reference to the full ring circumference 64 of the coil 13.
The part-windings 19, 19', 19", 19... connected in series one after the other
in the circumfer-
ential direction of the annular coil 13 form a free angle 65 with respect to
one another, which
corresponds to the winding gaps 20, 20', 20", 20"` described above. No
electromagnetic coil
is disposed within this free angle 65 between directly consecutive part-
windings 19, 19', 19",
19... and instead there is virtually an empty space without an electromagnetic
coil body. This
free angle 65 between directly consecutive part-windings 19, 19', 19", 19"`
connected in
series is expediently between 10 and 30 , in particular between 15 and 25 ,
preferably ap-
CA 02775366 2012-03-26
-37-
proximately 20 . This free angle 65 or the corresponding winding gap 20, 20',
20", 20"` de-
fines zones within the electromagnetic coil 13 in which different
electromagnetic conditions
prevail than in those zones of the electromagnetic coil 13 where the serially
arranged part-
windings 19, 19', 19", 19... are disposed or positioned one after the other.
The gaps without
windings defined by the free angle 65 between the individual part-windings 19,
19', 19",
19... create a diversity within the electromagnetic field which is generated
or can be gener-
ated by the electromagnetic coil 13, which is conducive to the electrolytic
process in the elec-
trolytic reaction system 1.
A particularly effective electromagnetic field is generated or can be
generated by the electro-
magnetic coil 13 if the circumferential angle 63 of the individual part-
windings 19, 19', 19",
19... and the free angle 65 between the individual part-windings 19, 19', 19",
19... are se-
lected so that after more than one complete ring circumference, i.e. on
exceeding 360 of
winding extension, an offset angle 66 is formed between part-windings 19, 19',
19", 19"`
wound one on top of the other. In other words, as a result, the part-windings
19, 19', 19",
19" ` of the first turn around the annular or torus-shaped coil 13 are offset
from the part-
windings 19, 19', 19", 19... of the second or every other ring of part-
windings 19, 194, 19",
19'4' by an offset angle 66. Consequently, the part-windings 19, 19', 19",
19... lying one
above the other in the circumferential direction of the annular coil 13 are
always offset or
shifted relative to one another so that there is preferably no 100% overlap
between part-
windings 19, 19', 19", 19"' wound one on top of the other.
Based on one practical embodiment, a number of consecutive part-windings 19,
19', 194',
19` "connected in series is selected so that approximately three full rings
are formed, i.e. the
part-windings 19, 19', 19", 19"` connected in series extend approximately
across 1080 of
the annular or torus-shaped coil 13.
Based on one practical embodiment, the individual part-windings 19, 19', 19",
19'4' are
wound in one layer, in which case the part-windings 19, 19', 19", 19"` formed
after a com-
plete turn round the ring are wound with the appropriate offset angle 66 but
essentially with-
out an air gap across part-windings 19, 19', 19", 19"` lying underneath or
inside.
CA 02775366 2012-03-26
-38-
The electromagnetic coil 13 preferably has no core, in particular does not
have an electro-
magnetically active core. In particular, the electromagnetic coil 13 is
provided in the form of
an air reactor so that the electromagnetic field generated acts to a high
degree on the electro-
lyte and on the electrode arrangement 3 and thus influences the physical and
chemical proc-
esses in the electrolytic reaction system 1 to a high degree.
A part-winding 19, 19', 19", 19... comprises a plurality of turns, in
particular dozens, hun-
dreds or thousands of turns made from an isolated conductor, in particular a
copper wire iso-
lated by means of lacquer. The preferably two-layered, in particular three-
layered electromag-
netic coil 13 comprising mutually spaced apart part-windings 19, 19', 19",
19"` serially con-
nected to one another therefore has a first coil terminal 67 and another coil
terminal 68, be-
tween which the mutually spaced apart part-windings 19, 19', 19", 19... extend
in a circle.
Via these coil terminals 67, 68, the electromagnetic coil 13 is connected to
the electrical en-
ergy source 22, as explained in the earlier parts of the description.
Accordingly, a diameter of
the outer part-windings 19, 19', 19", 19"` is bigger than a diameter of the
inner part-
windings 19, 19', 19", 19... of the annular or torus-shaped electromagnetic
coil 13.
Instead of the schematically illustrated electrical connecting bracket between
the directly con-
secutive part-windings 19, 194, 19", 19`", it would naturally also be possible
to wind the in-
dividual part-windings 19, 19', 19", 19... without interruption or in one
piece, in particular
from a one-piece electrical conductor, thereby obviating the need for the
connecting bracket
disposed in between.
The embodiments illustrated as examples represent possible variants of the
electrolytic reac-
tion system 1, and it should be pointed out at this stage that the invention
is not specifically
limited to the variants specifically illustrated, and instead the individual
variants may be used
in different combinations with one another and these possible variations lie
within the reach
of the person skilled in this technical field given the disclosed technical
teaching. Accord-
ingly, all conceivable variants which can be obtained by combining individual
details of the
variants described and illustrated are possible and fall within the scope of
the invention.
For the sake of good order, finally, it should be pointed out that, in order
to provide a clearer
understanding of the structure of the electrolytic reaction system 1, it and
its constituent parts
CA 02775366 2012-03-26
-39-
are illustrated to a certain extent out of scale and/or on an enlarged scale
and/or on a reduced
scale.
The objective underlying the independent inventive solutions may be found in
the description.
Above all, the individual embodiments of the subject matter illustrated in
Figs. 1; 2; 3; 4; 5; 6,
7; 8; 9 constitute independent solutions proposed by the invention in their
own right. The ob-
jectives and associated solutions proposed by the invention may be found in
the detailed de-
scriptions of these drawings.
15
25
CA 02775366 2012-03-26
-40-
List of reference numbers
1 Reaction system
2 Reaction chamber 36 Outlet orifice
3 Electrode arrangement
3' Electrode arrangement 37 Return line
4 Holding container 38 Liquid tank
5 Electrode (anodic) 39 Water container
40 Filter device
6 Electrode (cathodic)
axis 41 Electrolyte circuit
7 Fanning
42 Liquid Cylinder or vertical axis id pump
9, 9' Distance 43 Cooling device
10 Spread angle 44 Heat exchanger
45 Intake
11 Gap 46 Discharge
12 Radial distance
13 Electromagnetic coil 47 Passage orifice
14 Liquid level (min.) 48 Ambient air
15 Central or mid-point 49 Regulating means
50 Means (negative pressure generat-
16 Mid-plane ing)
17 Coil body
18 Coil winding 51 Internal combustion engine
19 Part-winding 52 Connection
19' Part-winding 53 Fuel intake line
54 Distance
19" Part-winding 55 Distance
19"' Part-winding
20 Winding gap 56 Tube axis
20' Winding gap 57 Gap
20" Winding gap 58 Gap
59 Wall thickness
21 Energy source 60 Wall thickness
22 Energy source
23 Inlet orifice 6 Slot
24 Means (turbulence) 62 2 Slot
25 Intake and/or outlet nozzles 63 Circumferential angle
64 Ring circumference
26 Gas chamber 65 Free angle
27 Overflow edge
28 Liquid level (max.) 66 Offset angle
29 Boundary edge 67 Coil terminal
30 Electrolyte container 68 Coil terminal
31 Cylinder axis
32 Foam
33 Filling level
34 Discharge passage
35 Collection portion