Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02783276 2012-07-17
METHOD AN SYSTEM FOR RECORDING DATA MONITORED DURING THE
MANUFACTURE OF MOLDED OPHTHALMIC LENSES
Related Application
This application is a divisional application of Canadian
Patent Application No. 2,481,649 filed April 4, 2003 as
International Application No. PCT/US03/10401.
Background Of The Invention
1. Field of the Invention
This invention generally relates to manufacturing ophthalmic
lenses, and more specifically to methods and systems for
recording data taken during the manufacturing of ophthalmic
lenses such as contact lenses.
BACKGROUND ART
Over the last several years, procedures have been developed to
mold contact lenses on a high speed automated basis, and for
example, such systems are disclosed in U.S. Patents 5,555,504
and 5,702,735 and European patent Publication no. 1052075A
entitled "Mold and Molding Machine For Making Ophthalmic
Devices." In these systems, generally, a group, or batch, of
lenses is formed by sandwiching a monomer between a set of
front and back mold sections. The monomer is polymerized, thus
forming the lenses, which are then removed from the mold
sections, further treated and packaged for consumer use.
1
CA 02783276 2012-07-17
' In this process, the mold sections and the lenses are
transported through a number of stations or zones. For
instance, the processing system may include filling, pre-
curing, polymerizing, de-molding, and hydration stations.
In order to be sure that the manufactured lenses are
suitable, various parameters must be maintained within
given ranges at each of these stations; and, accordingly,
these parameters are carefully monitored at the stations.
The number of monitored parameters can be quite large,
and for example, three to eight parameters may be
monitored at each station. Also, with previous systems,
all of these parameters were recorded so that a complete
history of the processing parameters was recorded and
available for every manufactured lens. Because of the
large number of lenses that are made using these
procedures and because of the large number of monitored
parameters, this resulted in an extremely large database.
For instance, a full print-out of the process parameters
recorded while a single batch of lenses was made might be
three pages long, and over the course of a year, 5000
batches of lenses may be manufactured on a system. This
results in an enormous amount of data, which is expensive
to organize and to store. Moreover, government
regulations require that a paper copy of each device
history record be provided for each batch of lenses when
the batch is shipped from the manufacturing site. Again,
because of the large number of lenses made and because of
the enormous amount of recorded data, finding the proper
paper record for each lens and matching that paper work
with the lens can be expensive, time consuming and also
can significantly delay release of the product.
2
CA 02783276 2012-07-17
In addition, because of the significance of data recorded
during the lens fabrication process, it is important to
prevent the inadvertent or intentional loss or alteration
of data. Heretofore, loss of data is usually solved with
audit trails implemented by using the built in
functionality of the database management system. A
problem with this approach, in the case of conventional
data base management systems, is that the audit trail
includes only the type of transaction (insert, update,
delete), the table affected, the time stamp of the
transaction, and the user id making the transaction.
This audit trail does not include a "before" and "after"
snapshot of the data affected. This eliminates the
ability of reports based on this data to provide a
complete picture of what happened during the fabrication
process.
SUMMARYOFTHEINVENTION
An object of this invention is to improve procedures for
recording the manufacturing histories of molded
ophthalmic lenses.
Another object of the present invention is to define a
reduced set of parameters that will provide a full
history of the manufacture of molded ophthalmic lenses.
A further object of this invention is to take advantage
of automated data collection capabilities to expedite
more rapid release of molded ophthalmic lenses without
sacrificing quality.
3
CA 02783276 2012-07-17
Another object of this invention is to prevent the
inadvertent or intentional loss of data due to user
influences on production database working tables.
A further object of the invention is to use the custom
trigger functionality built into commercially available
relational database management systems, to make a copy of
certain data into a second, or shadow, table when certain
predetermined events occur.
These and other objectives are attained With a method and
system for recording data monitored during the
manufacture of molded ophthalmic lenses. This method
comprises the steps of identifying a set of process
parameters used in said manufacture, identifying an
associated value range for each of the process
parameters, and monitoring each of the process parameters
during the manufacture of the lenses. If, during the
manufacture of one of the ophthalmic lenses, one of the
process parameters moves outside the associated value
range, then that lens is rejected. However, if, during
the manufacture of one of the ophthalmic lenses, all of
the process parameters stay within their associated
ranges, then that lens is identified as acceptable, and a
device history record is made for that lens. This device
history record includes a reference code for identifying
the set of process parameters.
For example, this reference code may identify a table,
kept in a separate database, that lists all of the
process parameters, and the associated value ranges, that
were monitored while the batch of lenses was
4
CA 02783276 2012-07-17
manufactured. In this way, the device history record
itself does not have to list those parameters or their
associated value ranges. The device history record may,
it may be noted, identify one, some or all of these
parameters, as well as their associated value ranges; but
with the above-mentioned reference code, the device
history record doe not have to list any of these
parameters or their value ranges.
A database, referred to as a shadow table, may be used to
protect the integrity of data recorded during, or
relating to, the manufacture process. More specifically,
in accordance with this feature, a shadow table is
formed, and data items from device history records are
copied into the shadow table in response to the
occurrence of predefined events. For example, these
predefined events may include whenever anyone, or anyone
outside a group of identified individuals accesses the
device history record to perform predefined operations,
such as alter or delete, on the data in the device
history record. Also, preferably, whenever any person
accesses the device history record to perform a given
operation on the data in that record, a value is placed
in the shadow table to identify that person, and a
designation is placed in the shadow table to identify the
operation performed by that person. A shadow table may
be used with device history records of the type discussed
immediately above, which contains comparatively minimal
information, as well as with previous, or other, types of
device history records, which contain much more
information.
5
CA 02783276 2012-07-17
Further benefits and advantages of the invention will
become apparent from a consideration of the following
detailed description, given with reference to the
accompanying drawings, which specify and show preferred
embodiments of the invention.
,
BRIEFDESCRIPTIONOFTHEDRAWINGS
Figure 1 is a diagrammatic top view of a contact lens
production line incorporating the present invention.
Figure 2 is a top plan view of a pallet that may be used
in the production line of Figure 1.
Figure 3 shows a contact lens mold assembly used in the
production line of Figure 1.
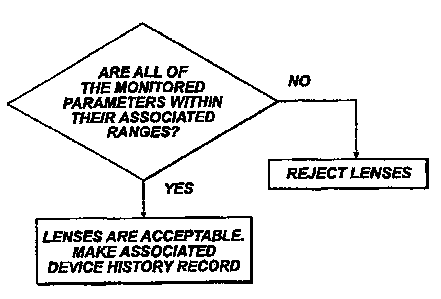
Figure 4 is a flow chart showing a procedure for making a
device history record for lenses manufactured in the
system of Figure 1.
Figure 5 is an overview of a system that may be used to
make the electronic device histories for the lenses.
Figure 6 illustrates the use of a shadow table in the
operation of the system of Figure 1.
Figure 7 shows software code that may be used to trigger
entries into the shadow table.
DETAILEDDESCRIPTIONOFTHEPREFERREDEMBODIMENTS
6
CA 02783276 2012-07-17
Figure 1 shows a lens production line system 10 implementing a
data recording procedure of the present invention. Operational
details of system 10 may be found in U.S. Patents 6,071,440,
5,702,735 and 5,555,504. Generally, system 10 comprises
various contiguously located stations including injection mold
assembly stations 12 and 14 for manufacturing thermoplastic
front and back curve contact lens mold sections, respectively.
Apparatus 16 is provided for transporting up to eight front
curve mold sections at a time from station 12 to a pallet 20,
positioned adjacent a first pallet conveyor 22, and apparatus
24 is provided for transporting up to eight back curve mold
sections at a time within a pallet 26 positioned adjacent a
second pallet conveyor 30. Both first and second pallet
conveyors 22 and 30 may be partially enclosed in a low-oxygen
enclosure.
A sequencing apparatus 32 for situating a pallet 20 containing
front curve contact lens mold sections adjacent a pallet 26
containing a corresponding number of complementary back curve
contact lens mold sections and onto a sequenced pallet
conveyor 34 is also provided to enable pallets 20 and 26 to be
conveyed alternately and sequentially into a filling and mold
assembly station 36.
The filling/mold assembly station 36 generally includes first,
second and third apparatuses 40, 42 and 44. First apparatus 40
is used to deposit, in a vacuum environment, a polymerizable
compound (monomer mixture) for forming a contact lens in the
concave portion of each front curve lens mold section in each
pallet 20.
7
CA 02783276 2012-07-17
Second apparatus 42 is provided for depositing a
surfactant along an annular rim portion of the front
curve to facilitate the later removal of the back curve
mold portion and the associated excess monomer ring from
the front curve mold section in a mold separation
apparatus located downstream of the filling station 36.
Third apparatus 44 is provided for assembling the
individual contact lens mold assemblies, which is done by
picking each back curve lens mold from pallet 26 and
placing it onto a corresponding associated front curve
lens mold located on carrier pallet 20 in an oriented
configuration. Additionally, after the back curves are
removed from the second pallet 26, a pallet recirculating
ram assembly 46 pushes the empty back curve pallets 26
back to the original back curve supply conveyor 30 for
receipt of a new set of back curve lens mold sections
from injection mold assembly 14.
The pallets 20, now containing completed mold assemblies,
exit the filling/mold assembly station 36 and are
conveyed along conveyor 50 to a pre-cure chamber 52. At
this chamber, the monomer solution contained in each mold
assembly is partially cured into a viscous gel-like
state, and the front and back curve lens mold sections
are subjected to a predetermined pressure to further
define the contact lens edges.
After exiting the precure chamber 52, the pallets
containing the precured lenses are transported along
conveyor 50 to a polymerization station 54, where the
precured lenses contained in the individual mold
8
CA 02783276 2012-07-17
assemblies are fully polymerized in UV ovens to form the
contact lens blank. Preferably, the sequenced pallet
conveyor 50 is split into two conveyors 50a and 50b to
enable a longer residence time in the polymerization
chamber as the mold assemblies are polymerized. Pusher
apparatus 62 is used to direct the travel of a
predetermined number of pallets containing the mold
assemblies from conveyor 50 to each of the two conveyors
50a and 50b.
After the polymerizable compound in each of the mold
assemblies is polymerized to form a contact lens blank at
the polymerization station 54, the pallets travel through
a demold buffer area 64, which provides temperature
adjustment to the mold assemblies exiting the ovens. The
pallets then travel along a dual walking beam 66 to a
back end of the system 20 that includes a mold separation
apparatus 70. Here, the back curve lens mold halves of
the mold assemblies are automatically separated from the
front curve lens mold halves to expose the polymerized
contact lens for conveyance to a downstream hydration
station 72. =
After the demold process, pusher assembly 74 pushes a
series of pallets 20 onto a reciprocating transfer pallet
apparatus 76 that conveys the pallets to the hydration
assembly 72. At the hydration assembly, the front curve
lens mold sections, now containing polymerized contact
lenses therein, are simultaneously removed from their
respective pallets and placed in an appropriate hydration
chamber (not shown) so that each contact lens may be
hydrated prior to packaging. The transfer apparatus
9
CA 02783276 2012-07-17
subsequently returns the empty pallets back to conveyor
78, where a pusher assembly 80 transfers the empty first
pallets back to conveyor 22. Conveyor 22, in turn,
transports the pallets back to a position adjacent
injection mold assembly 12 to receive a new batch of
front curve lens mold sections from that assembly.
A top view of a production line pallet 20 for carrying
production lens mold halves is shown in Figure 2.
Preferably, pallets 20 and 26 are interchangeable so that
they may accommodate either front curve or back curve
contact lens mold halves, and the production line pallets
may be formed of any suitable material, such as aluminum
or stainless steel. Each pallet 20 also contains a
plurality of recesses 82 for receiving a complementary
pair of front and back curve mold halves that define the
shape of the final desired lens. Figure 3 shows one such
mold assembly 84 shown seated within a recess 82 of the
pallet. The contact lenses are formed by placing an
amount of polymerizable composition, generally on the
order of about 70 milligrams, in each front curve mold
half 86 seated within a pallet recess at the filling mold
assembly 36. Then, the back curve mold half 90 is placed
onto the polymerizable composition.
With reference again to Figure 1, the production line
tracking and quality control system includes a control
subsystem 100, which may include a computer or one or
more programmable logic controller (PLC) and a plurality
of sensor devices. These sensor devices generate process
condition information at particular stations of the
facility 10 for receipt by the computer or PLC, which
CA 02783276 2012-07-17
controls the processes performed to the pallet carrying mold
halves or mold assemblies at the particular stations. The
respective PLC processes the received information and, when
appropriate, generates control signals for corrective action,
and/or generates error flags indicating that other types of
intervention or correction may be needed.
In the embodiment of system 10 illustrated in Figure 1,
control subsystem 100 includes at least three PLCs and
associated circuitry and software for providing tracking and
control of the production line pallet system. A first PLC 102a
controls and tracks pallet movement from the injection mold
stations up to and including the filling/mold assembly
stations. A second PLC 102b provides quality control of and
tracks pallet transport through the precure, UV
polymerization, and mold separation stations. A third PLC 102c
is provided for retaining the identification of pallets at the
hydration assembly, where the contact lenses are removed from
the pallets for subsequent processing. Additional PLCs may be
provided for controlling the various aspects of hydration,
post hydration, lens inspection, and packaging stations, as
described in U.S. Patent 5,836,323 for "Automated Method and
Apparatus for Hydrating Soft Contact Lenses."
Memory storage devices 104a, b and c are provided for each PLC
102a, b and c, respectively, and have adequate addressing and
storage capabilities for each respective PLC to access and
process data in the form of time
11
CA 02783276 2012-07-17
information and process condition status information.
Specifically, the process condition status information
constitutes information indicating whether or not
particular contact lens products are acceptable - that
is, whether process conditions involving a particular
pallet carrying contact lens mold halves or contact lens
mold assemblies up to a particular point in time, have
been performed in accordance with prescribed limits and
tolerances. This information is used to determine
whether the products carried by that specific pallet are
acceptable. The specific pallet will be rejected by
appropriate means provided in the system 10 if a product
or products carried by the pallet are determined to be
out of process specification parameters. It may be noted
that the product specification parameters are determined
prior to producing saleable products off a manufacturing
line by extensively running the individual steps of the
line at various process conditions, e.g., temperature and
pressures, etc. and testing the product of those steps to
determine if the process conditions will ultimately
produce an acceptable product. From this testing, the
acceptable operational 'ranges are determined.
A data acquisition system collects the individual process
parameter values gathered by each PLC for particular
process operations, and inputs this information into a
cell supervisor that associates the process parameters
and conditions at the various process stations with the
specific pallets.
As mentioned above, the number of parameters monitored in
the operation of facility 10 can be quite large, and for
12
CA 02783276 2012-07-17
_
example, three to eight parameters may be monitored at
each station in the facility. Also, with previous
systems, all of these parameters were recorded so that a
complete history of the processing parameters was
recorded and available for every manufactured lens. With
the present invention, instead of recording all of the
values of all of the monitored parameters, a simpler data
recording procedure is preferably employed. With
reference to Figure 4, one feature of this simpler
_
approach is, as represented at steps 122 and 124, that if
while a batch of lenses is being made in a particular
area of the machine, any process parameter moves outside
its associated, acceptable range, the lenses in that
group of pallets are rejected. The advantage of this
process is that instead of rejecting an entire batch of
lenses, only those lenses made outside the process
parameters will be rejected. When lenses are rejected,
system 10 could be modified to maintain a single
reference code that would indicate why the batch of
lenses were rejected, e.g., a code that would indicate
that a temperature was outside the operational ranges at
injection molding, or a different code if the lens spent
too much time in the uv tunnel, or a different code if
the hydration water temperature was outside the
appropriate range, etc. Keeping a record of why lenses
are rejected would help to make the proper repairs; but
this information would not be stored in a device history
record because that is for saleable product, and instead,
this information would be stored in an alternative
database.
13
CA 02783276 2012-07-17
A second feature of this simpler approach is used when a
lens is found acceptable, or more precisely, when a batch
of lenses are found acceptable - which is the case, it
may be noted, if during the manufacture of the batch, all
of the monitored processing parameters stay within their
associated respective ranges. In this event, as
represented by step 126, a specific device history record
of that batch is made and maintained, and that device
history record includes a reference code for identifying
a set of the process parameters. For example, this code
may identify a table, kept in a separate database, that
lists all of the process parameters and the associated
value ranges that were monitored while the batch of
lenses were manufactured..
In this way, the device history record itself does not
have to list those parameters or their associated value
ranges. The device history record, however, may identify
one, some, or all of these parameters, as well as their
associated value ranges; but with the above-mentioned
reference code, the device history record does not have
to list any of these parameters or their value ranges.
Also, it may be noted, the fact that this record exists
indicates that the monitored parameters were all within
their associated ranges during the manufacturing process.
It may also be noted that the device history record may
include data items about the lenses or their manufacture
in addition to the above-mentioned reference code. For
instance, the device history record may include a lot
number, the date of manufacture, and other information.
Figure 5 is an illustrated overview of a preferred
electronic device history record and product release
14
CA 02783276 2012-07-17
subsystem 140 for system 10. The subsystem 140 includes
four main types of components: database 142, a data
collection application 144, a data transfer application
146 and a user interface application 150. These
components are housed either on client machines or on
network servers, depending on their function and
interface needs, and Figure 5 also illustrates the
relationships of system components across client machines
and network servers.
The data collection application 144 is provided to work
with the specific model of manufacturing machine used on
the production lines. It collects data on the processing
and status of the product for the device history record
as the product moves through each stage of the
manufacturing process. Preferably, each production
machine is assigned a dedicated copy of the data
collection application, configured and housed on the
client PC attached to the machine itself. This data
collection application 144 works in tandem with the
human-machine interface 152 that provides system
monitoring and operational and troubleshooting
capabilities for that machine. Each data collection
application 144 is accompanied by a local database 154
used to store data temporarily, in the event of a loss of
connectivity with the network. This backup capability
prevents data loss and minimizes machine downtime. Data
stored to the temporary database 154 is uploaded to the
network database 142 upon reconnection to the network.
The data transfer application 146, which is preferably
housed on the network servers, provides the system 140 an
CA 02783276 2012-07-17
interface to external systems 156 such as accounting and
distribution systems. Application 146 preferably
operates without human intervention, driven by suitable
process-defined algorithms transferring transactional
data stored within the database 142 to the desired
external systems.
The user interface application 150 is used to enforce the
workflow established by management for the release of
lenses from Manufacturing to Distribution, by ensuring
that the data meets quality criteria. For example,
sterilizer run records for a batch must be reviewed prior
. to the release of the product of Distribution. Until all
sterilizer run records for the batch have been reviewed
to confirm to an acceptable status, the batch cannot be
released. These workflow management capabilities
minimize the dependency upon physical records that
existed in the previous, manual process. As physical
records can be time-consuming and prone to human error,
automating the process improves the accessibility,
maintainability and accuracy of live data generated in
the course of the manufacturing process. Features within
these applications 150 interface with external data
systems that record data from the inspection and testing
of product, to enable the eDHR/PR system to provide a
"one-stop-shopping" visibility solution to expedite
product release.
In accordance with another feature of system 10, a
procedure is provided to preserve the integrity of data
recorded during or relating to the manufacture process.
In particular, this procedure may be used to prevent the
16
CA 02783276 2012-07-17
inadvertent or intentional loss of data due to user
influences on the production database working tables.
Generally, and with reference to Figure 6, this can be
done forming a shadow table, and copying data from device
history records into the shadow table in response to the
occurrence of predefined events. For example, these
predefined events may include whenever anyone, or anyone
outside a group of identified individuals accesses one of
the device history records to perform predefined
operations, such as alter or delete, on any of the data
in the device history record. The ability of users to
access or to alter data in the shadow table is limited
and controlled so that this data can be relied upon to
get a complete picture of an instance of the device
history record.
More specifically, data from a device history record can
be copied into the shadow table by using the custom
trigger functionality built into commercially available
relational database management systems (DBSM), such as
Oracle 8i, at the data definition language (DDL) level.
Oracle 8i, for example, may be deployed on a commercially
available Sun E5000 Enterprise server running a Solaris
version 5.6 operating system.
Triggers are explicitly written by a programmer to
accomplish a desired task upon the occurrence of some
specific event in the DBMS. For example, a custom
written, mandatory trigger may be executed every time a
record is inserted, updated, or deleted on any of the
tables related to the DHR. The trigger makes a copy of
the data into the shadow table that has the same fields
17
. CA 02783276 2012-07-17
as the working table, as well as fields for the type of
transaction, user id making the transaction and a
sequence number for the transaction. The shadow tables
are view only to the user and therefore not directly
modifiable by the user.
The database administrators are the only users who have
password protected access to the shadow tables in an
update or delete capacity. Thus, the data in the shadow
tables can be relied on to get a complete picture of an
instance of the DHR data at any point in time. Since the
DHR reports are based on the data in the shadow tables,
we have assurance that the DHR is reliable.
In the case of an insert or update against a working
table, the trigger preferably inserts a complete copy of
the record being inserted or updated into the shadow
table, along with an appropriate designation such as, for
example, an "I" for insert, or a "U" for update, the user
id and a sequence number. This results in the last
record (that is, the latest in time before the insert or
update) for the item in the working table being the same
information as the matching record in the shadow table.
If a record is deleted from the working table, the data
are still available in the shadow table. However, the
data in the working table will not contain the rows
deleted. The shadow table will contain a copy of the
record just prior to the delete, along with, for example,
a "D" for delete, the user id and a sequence number.
Figure 7 shows software code for one suitable trigger
that may be used to make and manage a shadow table.
18
CA 02783276 2012-07-17
Similar triggers can be used on other tables for DHR
traceability.
A shadow table, it may be noted, may be used with device
history records of the type discussed above in connection
with Figure 4, which contain comparatively minimal
information, and with previous, or other types, of device
history records, which contain much more information.
The use of a shadow table, in the above-described way,
has a number of significant advantages. For instance,
the shadow table readily provides the DHR data on demand.
The risk of loss or change of important data in the
device history record is greatly reduced in that an
intentional or inadvertent influence on the data could
occur only by the database administrators. Developers
and analysts cannot get at these shadow tables directly.
All transactions record the user id and type of
transaction along with the transaction so that there is
traceability of who made what types of changes. Since
the DHR report is based on these shadow tables, there is
a very high level of confidence in the reports based on
the data. The shadow table gives a reliable, traceable
repository of DHR data that also provides a data analysis
trouble shooting tool when we have occasional errors in
the process.
While it is apparent that the invention herein disclosed
is well calculated to fulfill the objects stated above,
it will be appreciated that numerous modifications and
embodiments may be devised by those skilled in the art,
and it is intended that the appended claims cover all
19
CA 02783276 2012-07-17
such modifications and embodiments as fall within the
true spirit and scope of the present invention.