Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02785228 2012-05-10
Translation of Specification of PCT/RU20111000320
ANTIMICROBIAL ACTION PHARMACEUTICAL COMPOSITION FOR
PARENTERAL ADMINISTRATION AND ITS' PRODUCTION PROCESS
This invention belongs to antimicrobial pharmaceutical preparations and its'
production
technologies. It can be used in medicine and veterinary science for treating
contagious and
inflammatory diseases, as well as being used in pharmaceutical industry for
medicinal products
manufacturing.
Currently most contagious and inflammatory diseases successful therapy is
based on using
different anti-infectives, including beta-lactam antibiotics.
Beta-lactam are preparations (natural and semisynthetic penicillins,
cephalosporins,
cephamycins, carbapenems and monobactams) with a beta-lactam ring as a
chemical structure
common fragment, which determines the antimicrobial activity and a series of
common
properties of this drug preparation group [1].
All beta-lactam possess a wide antimicrobial spectrum and a high level of
antimicrobial activity,
but many of them have a fast developing microbial resistance, because of their
specific ferments
production - beta-lactamase (extended spectrum beta-lactamase, chromosomal
beta-lactamase
class C, etc.), which hydrolyze the beta-lactam ring. This is what deprives
these preparation of
their antibacterial properties and leads to microbe resistant strains
development [2].
In the past decades there have been created specific beta-lactamase inhibitors
(clavulanic acid,
sulbactam, tazobactam and etc.) and on their basis there has been developed an
entire range of
effective combined antibacterial beta-lactam preparations of penicillin and
cephalosporin family
(amoxicillin/clavulanic acid, ampicillin/sulbactam, piperacillin/tazobactam,
cefoperazone/sulbactam and etc.) which are noted because of their increased
persistence to beta-
lactamase as well as their more apparent antibacterial activity [2, 3].
Nevertheless it ought to be remarked that many of these "inhibitor screened"
preparations
appeared to be insufficiently effective because in case of high beta-lactamase
production by
germs the inhibitors cannot fully protect the antibiotics from hydrolysis.
CA 02785228 2012-05-10
2
The carbapenems which are resistant to many beta-lactamase action cannot
entirely solve the
microbial resistance to the mentioned antibiotics problem. It happens because
many application
ways for treating serious infections lead to forming of multiply P. Aeruginosa
resistant strains
[3].
Besides, frequently the clinical betalactam ineffectiveness (or their low
effectiveness) in case of
infections induced by different microbes is associated not only with the
negative beta-lactamase
activity, but also with these preparations limited ability of local
concentration at contagious
inflammation locus and macrophage penetration, where many contagious and
inflammatory
diseases' activators are deposited. The antimicrobial resistance level depends
on their functional
status intensity [4, 5].
In the last few years it has been discovered that the use of different
nanoparticles as dosing
vehicle for different antibiotics delivery (as well as betalactam) inside the
bacteria and
macrophages to increase their concentration at the contagious inflammation
area and to increase
their antimicrobial properties as well as phagocytes (neutrophils and
macrophages) functional
activity stimulation and their additional recruitment to infected tissues, is
a very challenging
trend for modem experimental pharmacology and clinical medicine [6, 7, 8, 9,
10, 11, 12].
Here is the character of the mentioned invention. To increase the betalactam
therapeutic
effectiveness it is suggested to use the SiO2 (silica dioxide) nanoparticles,
which have different
pharmacologically beneficial biocompatibility, biodistribution, biodegradation
and low toxicity
properties (independent from looseness of the structure intensity), can serve
as antibiotics carrier
for endocellular macrophage delivery, which are concentrated at the
inflammatory tissues of
lungs, liver, kidneys, lien, absorbent glands, heart, skin, bladder and other
mammal organs (i.e.
considerably increase antibiotics concentration in the infected areas), and
also initiate the
immune system cells antimicrobial activity. This will help to authentically
increase the
germicides therapeutic effect during contagious inflammatory diseases
treatment [13, 14, 15, 16,
17, 18, 19, 20, 21].
The mentioned invention solves the issue of creating an antimicrobial action
pharmaceutical
composition for injections on basis of using betalactam and silica dioxide
nanoparticles
antibiotics which possess a higher therapeutic effectiveness (comparing to the
standard
CA 02785228 2012-05-10
3
betalactam which are considered as a basis for this invention) for contagious
and inflammatory
diseases treatment.
To solve the assigned task it is suggested to use an antimicrobial action
pharmaceutical
composition for injections, which contains a betalactam antibiotic and finely
dispersed
nanostructured silica dioxide w/w (10-75) : 1.
The production process suggested to solve the assigned task is to obtain the
antimicrobial action
pharmaceutical composition for injections by mixing the betalactam antibiotics
with other
components. The betalactam antibiotic powder is mixed with the finely
dispersed nanostructured
silica dioxide powder w/w (10-75) : 1. The procured mixture is machined by
impact abrasive
method.
The therapeutic effectiveness of the proposed pharmaceutical composition will
increase if the
obtained mixture is machined by abrasive method in a way that the part of
finely dispersed
nanostructured silica dioxide particles of 5 microns would be no less than
25%.
To prepare the mentioned pharmaceutical composition, were used foreign
production antibiotics
provided by Russian pharmacological company LLC "ABOLmed" (penicillins:
carbenicillin;
cephalosporins: cefazolin, cefuroxime, cefotaxime, ceftriaxone, cefoperazone,
ceftazidime,
cefoperazone/sulbactam, cefepime; cephamycims: cefoxin; carbapenems:
meropenem;
monobactams: aztreonam). As a finely dispersed nanostructured silica dioxide
(hereafter referred
to as BHSiO2) was used "Polysorb" drug (pharmacological group: enterosorbing
solution; active
substance: colloidal silica dioxide), produced by Russian company CJSC
"Polysorb", containing
round shaped silica dioxide nanoparticles (dimension 5-20 nm) combined into
aggregates
(irregular microparticles) with dimension < 90 micron (registration number Ns
001140/01-
100908). There is s similar preparation produced by Ukrainian company CJSC
"Biopharma"
with a trade name "Silics" [121.
The composition formulation choice was based on convertible betalactam
molecules and nano-
as well as micro BHSiO2 particles sorption process, together with BHSiO2
particles reduction
during its' mixtures mechanical activation with betalactam substances by
impact abrasive
mechanization process.
CA 02785228 2012-05-10
4
The stated production process of the previously mentioned pharmaceutical
composition by
betalactam antibiotic powder mixture and BHSiO2 mechanical activation with
intensive impact
abrasive operations allow to increase the finely divided BHSiO2 particles
(less than 5 micron) on
which betalactam molecules are adsorbed and which are mostly phagocyted by
macrophages
[10,19].
To achieve this goal the mixture of the stated above materials in weight
rating, betalactam
antibiotic: BHSiO2 equal (10-75) : 1, is exposed to intensive impact abrasive
mechanical
activation process until the finely divided fraction weight rating is
increased up to 25%.
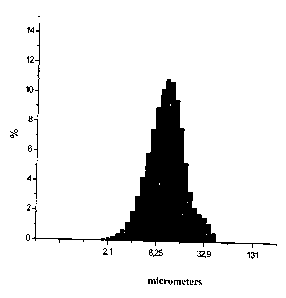
The data from the aqueous slurry fractional makeup in terms of ceftriaxone:
BHSiO2 equal 30
1, by the weight, measured by a laser granulometer Micro-Sizer 201is shown in
picture 1 and 2.
As you may see in pictures 1 and 2, the two hours analyzed composition
mechanical activation
leads to weight rating increase of its finely dispersed fraction (particles
dimension < 5 micron)
which contains not less than 25%.
From the received powder like composition you can prepare an injection sol for
parenteral
insertion (water it down by any means appropriate for betalactam), composed of
finely dispersed
BHSiO2 particles with inversibly sorbed any betalactan molecules on its
surface.
The table N21 contains data (received by high performance liquid
chromatography method -
HPLC) about different betalactam antibiotics sorption rate on BHSiO2 particles
after mechanical
activation of antibiotic composition : BHSiO2, equal 30 : 1, which shows us
that the finely
dispersed nanostructured silica dioxide can be used for parenteral
administration as a dosing
vehicle for antibiotics and other pharmacons which are capable of sorbing on
the nano- and
microparticles of this inanimate matter to make its' delivery to the
inflammation areas, tumor
growth areas, regeneration areas, cicatrization areas, scaring areas and etc.
That means make the
delivery into the areas with increased macrophages presence to purposefully
increase local
concentration (as well as cellicolous) the pharmaceutical concentration and
its therapeutic effect.
Table N21
Betalactam sorption rate by BHSiO2* particles
CA 02785228 2012-05-10
Composition formuation, Sorbed antibiotic q-ty : BHSiO2 q-ty,
m/a time** mg (weight %)
Cefazolin:BHSi02 (30:1), 8,1 mg : 16,7 mg (48%)
m/a 2 hours
Ceftriaxone:BHSi02 (30:1), 14,5 mg : 16,7 mg (85%)
m/a 2 hours
Cefotaxime:BHSi02 (30:1), 9,4 mg : 16,7 mg (55%)
m/a 2 hours
Cefuroxime:BHSi02 (30:1), 7,4 mg : 16,7 mg (44%)
m/a 2 hours
Cefepime:BHSi02 (30:1), 16,1 mg : 16,7 mg (96%)
m/a 2 iaca
Cefoperazone:BHSi02 (30:1), 12,2 mg 16,7 mg (73%)
m/a 2 hours
Cefoperazone/sulbactam: BHSiO2 13,9 mg : 16,7 mg (83%)
(30:1), m/a 2 hours
Ceftazidime:BHSi02 (30:1), 9,6 mg : 16,7 mg (53%)
m/a 2 hours
Cefoxotin:BHSi02 (30:1), 8,5 mg : 16,7 mg (51%)
m/a 2 hours
Meropenem:BHSi02 (30:1), 10,6 mg : 16,7 mg (63%)
m/a 2 hours
Aztreonam:BHSi02 (30:1), 9, 7 mg : 16,7 mg (58%)
m/a 2 hours
Carbenicillin:BHSiO2 (30:1), 11,2 mg : 16,7 mg (67%)
m/a 2 hours
* - finely dispersed nanostructured silica dioxide **- mechanical activation
Introducing of the finely dispersed nanostructured silica dioxide equal
betalactam : BHSiO2
from 10: 1 to 75:1 regarding its' weight is determined by the combination of 2
factors: 1) during
BHSiO2 more than 10% increase from the composition weight in case of
laboratory animals, they
suffer from the small capillary tube blockage of solid viscus; 2) in case of
BHSiO2 content
decrease for more than 1% of the composition weight (in particular during the
mice treatment of
CA 02785228 2012-05-10
6
bacterial sepsis) it's therapeutic efficiency doesn't differ from the initial
antibiotic basic
efficiency.
To receive the composition mechanochemical method was used, which comprehends
the solid
components mixture processing by intensive mechanical impacts - pressure and
shearing
deformations, mostly realized in different kind of mills which perform impact
abrasing actions
on the substances. The mixture of the solid betalactam antibiotic substance
and finely dispersed
nanostructured silica dioxide taken in the ratio from 10:1 to 75:1 by weight,
are exposed to bead
mills mechanical activation. The used mixture preparation method helps in a
certain way to
avoid chemical degradation and achieve powder components full homogeneity in
comparison
with making the mixture by a simple components mixing, or evaporating their
solutions, and as
consequence causes a high pharmacological activity of pharmaceutical
composition.
As a quantitative criterion of the minimum necessary mechanical impact dose it
is comfortable to
use the granulometry method of the composition suspension. It is necessary
that the mass
fraction of the particles less than 5 micron was more than 25%. On the other
hand it is necessary
to avoid the excessive mechanical processing which can cause betalactam
chemical degradation
which level can be controlled by the known analytical methods, such as HPLC.
Powder mixtures mechanical processing is performed in rotary, vibrational and
planetary mills.
As grinding bodies you can use balls, cores and etc.
Laboratory animals (mice) pharmacological tests of the compositions showed,
that the
mentioned compositions prepared by the mentioned method have a higher
therapeutic efficiency
while treating bacterial sepsis, provoked by Staphylococcus aureus,
Escherichia coli and
Pseudomonas aeruginosa, comparing to the initial antibiotics.
In such manner, using the mentioned pharmaceutical compositions and their
production process
provide the stated below advantages:
1) Clinically significant increase of the effectiveness and quality of the
antimicrobial
therapy of semi-acute and acute infection inflammatory diseases, death rate
reduction;
2) Ecological safety, lack of wastes and low price of pharmaceutical
production
technology.
The offered invention is illustrated by examples listed below.
CA 02785228 2012-05-10
7
Example N1. Solid composition production: betalactam antibiotic - finely
dispersed
nanostructured silica dioxide.
The mixture of the betalactam antibiotic and BHSiO2 in weight ratio 10:1,
20:1; 30:1 and 40:1
are being processed in an orbicular rotary mill for 1, 2 and 4 hours. The data
of the water
suspension granulometric composition as well as HPLC analysis of the
antibiotic content (in %
from the initial substance) are listed in the table N2.
Table N92
Water suspensions granulometric composition and antibiotics content in
different
composition variations
Composition content, Dimension and content % of Antibiotic
time m/a* BHSi02particles** content
%<3 %<5 %< 10 (%)
micron micron micron
Initial BHSiO2 0,5 5,3 25,7 -
Cefotaxime:BHSi02 (10:1), 13,4 30,4 57,3 89
m/a 1 hour
Cefotaxime:BHSi02 (20:1), m/a 16,6 33,9 59,1 95
1 hour
Cefotaxime: BHSi02 (40: 1), m/a 13,1 27,7 47,9 97
1 hour
Cefotaxi me: B HS i02 (30: 1), 14,7 30,6 54,1 99
m/a 2 hours
Cefuroxime: BHSi02 (30: 1), m/a 22,6 35,2 50,2 97
2 hours
Ceftazidime:BHSi02 (30: 1), m/a 14,3 25,3 37,0 98
2 hours
Ceftazidime:BHSi02 (30:1), m/a 23,8 38,9 56,2 96
4 hours
Cefepime: BHSi02 (30: 1), 23,8 38,8 57,7 92
CA 02785228 2012-05-10
8
m/a 2 hours
Ceftriaxone:BHSi02 (30:1), m/a 24,2 43,9 66,2 97
1 hour
Ceftriaxone:BHSi02 (30:1), 19,4 34,5 52,4 99
m/a 2 hours
Ceftriaxone: BHSi02 (30: 1), m/a 14,5 26,4 41,7 95
4 hours
Ceftriaxone:BHSi02 (40: 1), m/a 23,4 41,2 59,1 98
1 hour
Aztreonam:BHSi02 (30:1), 21,7 39,4 53,6 97
m/a 2 hours
Meropenem: BHSiO2 (30:1), 19,1 32,9 47,3 98
m/a 2 hours
Aztreonam: BHSiO2 (30:1), 19,8 31,1 49,5 97
m/a 2 hours
Carbenicillin: BHSiO2 (30:1), 22,3 38,9 51,4 96
m/a 2 hours
* - finely dispersed nanostructured silica dioxide **- mechanical activation
As you can see from table N22 the chosen conditions of the composition
production afford to
increase until a certain value (not less than 25% from the total weight) the
part of the finely
dispersed BHSiO2 fraction (particles size less than 5 micron) and to avoid the
antibiotic chemical
degradation.
Example N22. Determination of the therapeutic efficiency of antimicrobial
preparations and
pharmaceutical compositions.
There has been a research of betalactam antibiotics (Cefazolin, Cefuroxime,
Cefotaxime,
Ceftriaxone, Cefoperazone, Cefoperazone/sulbactam, Ceftazidime, Cefepime,
Cefoxitin,
Aztreonam, Meropenem, Carbenicillin) and their compositions mechanized for 2
hours and
composed of a miture antibiotic/ BHSiO2 in weight ratio 30:1, consequently
(Cefazolin/ BHSiO2,
Cefuroxime/ BHSiO2, Cefotaxime/ BHSiO2, Ceftriaxone/ BHSiO2, Cefoperazone/
BHSiO2,
Cefoperazone/sulbactam/ BHSiO2, Ceftazidime/ BHSiO2, Cefepime/ BHSiO2,
Cefoxitin/
BHSiO2, Aztreonam/ BHSiO2, Meropenem/ BHSiO2, Carbenicillin/ BHSiO2).
CA 02785228 2012-05-10
9
To determine therapeutic efficiency of betalactam and their pharmaceutical
compositions
including BHSiO2, we used experimental sepsis models and a statistical
processing method of
the received data ( x2) according to [22, 23].
Microorganisms: Staphylococcus aureus (ATCC N2 25923 F-49), Escherichia coli
(ATCC
No,25922 F-50), Pseudomonas aeruginosa (ATCC N27853 F-51).
Animals: for the experiments we used hybrid mice (CBA X C57Black/6)CBF1
according to the
"Regulations for test animals use" (USSR Ministry of health order supplement
N2755 from
12.08. 1977).
Experimental sepsis models:
The mice have been injected 0,8ml of Pseudomonas aeruginosa daily culture
suspension with a
dosage 5x108 CFU/mouse or Staphylococcus aureus daily culture suspension with
a dosage
1010 CFU/mouse or Escherichia coli daily culture suspension with a dosage
8x108 CFU/mouse.
The control group has been injected with 0,8m1 of normal saline solution (0,9%
sodium chloride
solution). In a day after being infected the test mice have been daily (during
3 days) intravenous
injected with 100mg/kg of antibiotics or different pharmaceutical compositions
(antibiotic/
BHSiO2) watered down with 0,25m1 of normal saline solution. The control group
of mice has
been injected using the same scheme with normal saline solution 0,25mg.
Antibacterial therapy efficiency was evaluated basing on the quantity of the
surviving animals on
the 7th day after being infected [22, 23].
The received data shown in table Ns3 reflect the results of 3 independent
experiments (for each
preparation research were used not less than 30 test animals in total).
Table Ns3
Bacterial sepsis antimicrobial therapy efficiency
Tested antibiotics and Mice survival rate on the 7` day of infection**
compositions* Staphylococcus Escherichia Pseudomonas xz
aureus coli aeruginosa
CA 02785228 2012-05-10
Normal saline solution 0% (0/30) 0% (0/30) 0% (0/30) -
(control)
Cefazolin 37,5% (12/32) - *** P<0,01
CefazolinBHSiO2 83,9% (26/31) - -
Cefuroxime 40,0% (14/35) 43,7% (14/32) - P<0,01
CefuroximeBHSi02 84,4% (27/32) 81,2% (26/32) -
Cefotaxime 40,0 % (12/30) 43,3% (13/30) - P<0,01
Cefotaxime/BHSiO2 86,7% (26/30) 83,3% (25/30) -
Ceftriaxone 46,7% (14/30) 41,9% (13/31) - P<0,01
CeftriaxoneBHSi02 90,0% (27/30) 87,5% (28/32) -
Cefoperazone - 45,2% (14/31) 40,0% (12/30) P<0,01
Cefoperazone/BHSiO2 - 90,0% (27/30) 80,6% (25/31)
Ceftazidime - 38,7% (15/31) 43,3% (13/30) P<0,01
Ceftazidime/BHSiO2 - 84,8% (28/33) 86,7% (26/30)
Cefepime 46,7% (14/30) 43,7% (14/32) 46,7% (14/30) P<0,01
CefepimeBHSi02 90,0% (27/30) 85,3% (29/34) 90,3% (28/31)
Cefoxitin 35,2% (15/34) 46,7% (14/30) - P<0,01
CefoxitinBHSi02 87,5% (28/32) 83,3% (25/30) -
Aztreonam - 77,5% (31/40) 74,4% (32/43) P<0,01
Aztreonam/BHSiO2 - 95,0% (38/40) 95,2% (40/42)
Meropenem 73,3% (22/30) 78,0% (32/41) 73,8% (31/42) P<0,01
Meropenem/BHSi02 90,6% (29/32) 95,0% (38/40) 95,1% (39/41)
Carbenicillin 46,7% (14/30) 43,3 % (13/30) 43,3% (13/30) P<0,01
Carbenicillin BHSiO2 83,3% (25/30) 86,7% (26/30) 90,0% (27/30)
Cefoperazone/sulbactam 56,7% (17/30) 58,1% (18/31) 59,3% (19/32) P<0,01
Cefoperazone/sulbactam 86,7% (26/30) 93,3% (28/30) 93,5% (29/31)
BHSiO2
*- mixtures composed of betalactam antibiotic: finely dispersed nanostructured
silica dioxide (BHSiO2) in weight
ratio 30:1
** survival rate/infected animals rate measured in % and absolute values
***- tests were not conducted because microorganisms relatively low-grade
sensitivity to initial antibiotics
As you may see in Table NN3 all suggested antimicrobial action pharmaceutical
compositions
(betalactsmBHSi02) definitely possess an increased therapeutic efficiency (1,2
- 2 times higher)
comparing to simple betalactam in case of lab animals sepsis treatment,
provoked by
CA 02785228 2012-05-10
11
Pseudomonas aeruginosa, Staphylococcus aureus or Escherichia coli. These
results mostly
concern compositions with cefalosporins, cefamicyns and penicillins used as
betalactam.
Used Literature
1. Antibacterial pharmacons. Preparations standartization methods. - M.: JSC
<<Medicine>>
Publishing>>, 2004. - 944 p.
2. M.D. Mashkovsky // Pharmacons: Tome 2. - 14`h edition. M.: LLC <<Novaya
Volna
Publishing>>, 2001. - 608 p.
3. Patent RU Ns 2377985 MTIK A61K31/43
4. Rational antibacterial pharmacopy // Practicians Guidance. Under the
general editorship of
V.P. Yakovlev, S.V. Yakovlev. - M.: Litterra, 2003. - 1008 p.
5. A.M. Mayansky // Microbiology for physitians (patogenetic microbiology
essays). - Nizhny
Novgorod: Nizhny Novgorod State Medical Academy Publishing, 1999. - 400 p.
6. Abeylath S.C., Turos E. Drug delivery approaches to overcome bacterial
resistance to 13-
lactam antibiotics // Expert Opinion on Drug Delivery. - 2008. - Vol.5. -
P.931-949.
7. Bastus N.G., Sanchez-Tillo E., Pujals S. et al. Peptides conjugated to gold
nanopar-ticles
induce macrophage activation // Molecular Immunology. - 2009. - Vol.46. -
P.743-748.
8. Pinto-Alphandary H., Andremont A., Couvreur P. Targeted delivery of
antibiotics using
liposomes and nanoparticles: research and applications // International
Journal of
Antimicrobial Agents. - 2000. - Vol.13. - P.155-168.
9. Ulbrich W., Lamprech A. Targeted drug-delivery approaches by
nanoparticulate carriers in
the therapy of inflammatory diseases // Journal Royal Society Interface. -
2010. - Vol.7,
Suppl. 1. - P.S55-S66.
10. A.E. Guliaev, B.A. Ermekbaeva, G.Y. Kivman and etc. Nanoparticles as
targeted antibiotic
transport (review) // Chemical and pharmaceutical magazine. - 1998. - N23. -
P.3-6.
11. Rosemary M.J., MacLaren I., Pradeep T. Investigation of antibacterial
properties of
ciprofloxacin@Si02. // Langmuir. - 2006. - Vol.22. - P.10125-10129.
12. Rai A., Prabhune A., Perry C.C. Antibiotic mediated synthesis of gold
nanoparticles with
potent antimicrobial activity and their application in antimicrobial coatings
// Journal of
Materials Chemistry. - 2010. - Vol.20. - P.6789-6798.
13. Park J-H., Gu L., Maltzahn G. et al. Biodegradable luminescent porous
silica nanoparticles
for in vivo applications // Nature Materials. - 2009. - Vol.8. - P.331-336.
14. Pernis B. Silica and the immune system // Acta Biomed. - 2005. - Vol.76,
Suppl. 2.- P.38-
44.
CA 02785228 2012-05-10
12
15. Tasciotti E., Liu X., Bhavane R. Et et al. Mesoporous silica particles as
a multistage delivery
system for imaging and therapeutic applications // Nature Nanotechnology. -
2008. - Vol.3.
- P.151-157.
16. Seleem M.N., Munusamy P., Ranjan A et al. Silica-antibiotic hybrid
nanoparticles for
targeting intracellular pathogens // Antimicrobial Agents and Chemotherapy. -
2009. -
Vol.53. - P.4270-4274.
17. Clinical chemistry and silica dioxide clinical use // Edited by NAS of
Ukrane academician
F.F. Chuyko - Kiev: Naukova Dumka>>, 2003. - 416 p.
18. Chuiko A., Pentyuk A., Shtat'ko E., Chuiko N. Medical aspects of
application of highly
disperse amorphous silica // Surface Chemistry in Biomedical and Environmental
Science.
Edited by J.P.Blitz and V. Gun'ko.Il. Mathematics, Physics and Chemistry. -
2006. -
Vol.228. - P.191-204.
19. Lucarelli M., Gatti A.M., Savarino G. et al. Innate defence functions of
macrophages can be
biased by nano-sized ceramic and metallic particles // European Cytokine
Network. - 2004. -
Vol.15. - P.339-346.
20. Zolnik B.S., Gonzalez-Fernandez A., Sadrieh N., Dobrovolskaia V.
Minireview:
Nanoparticles and the immune system // Endocrinology. - 2010. - Vol.151. -
P.458-465.
21. N.A. Piataev, F.N. Beliaev, M.D. Romanov, I.S. Kotlov // Pharmacons
directed celll
assosiated transport. - Saransk: Mordovia University Publishing, 2007. - 140
p.
22. Eckhardt C., Fickweiler K., Schaumann R. et al. Therapeutic efficacy of
moxifloxacin in a
murine model of severe systemic mixed infection with E.coli and B.fragilis //
Anaerobe. -
2003. - Vol.9. - P.157-160.
23. Schaumann R., Blatz R., Beer J. et al. Effect of moxifloxacin versus
imipenem/cilastatin
treatment on the mortality of mice infected intravenously with different
strains of
Bacteroides fragilis and Escherichia coli // Journal of Antimicrobial
Chemotherapy. - 2004.
- Vol.53. - P.318-324.