Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02786368 2012-08-14
METHOD AND APPARATUS FOR DIAGNOSIS OF TUMOR ACTIVITY USING
TUMOR INTERSTITIAL FLUID PRESSURE
SCOPE OF THE INVENTION
The present invention provides for the simplified measurement of the rate of
fluid
flux away from tumor tissues as an indication of tumor type, aggressiveness
and/or
treatment effectiveness, and more preferably as indicating relative
differences between
tumor interstitial fluid pressure and that of normal tissues.
BACKGROUND OF THE INVENTION
Despite remarkable strides in the treatment of solid cancers, such as those in
the
breast and prostate, many cancers remain resistant to treatment. As new
therapies are
developed and medicines become increasingly individualized, a need exists for
effective
and early diagnosis and/or predictor of treatment effectiveness or treatment
response.
One physiological parameter which has demonstrated predictive value for tumor
type or aggressiveness, and response to chemotherapy andior radiotherapy is
tumor
interstitial fluid pressure (TIFP). Tumor
interstitial fluid pressure (TIFP) is a
physiological parameter that is elevated in aggressive tumors. TIFF decreases
as tumors
respond to treatments such as radiation therapy and chemotherapy. However at
present,
the clinical use of TIFP has been limited, as conventional techniques for TIFP
measurement typically rely on biopsy procedures which are both invasive and
which
provide only point-measures.
1
The inventors have previously described a treatise on the basis for TIFP in
the
publication Liu LJ, Brown SL, Ewing JR, Schlesinger M. "Phenomenological model
of
interstitial fluid pressure in solid tumor". Phys. Rev. E 2011; 83:021919. The
inventors
have appreciated that the predictive value of TIFP, and its measurement, may
advantageously be used in combination with tumor imaging methods, and more
preferably, non-invasive imaging methods, in the diagnosis of tumor type
and/or activity,
and/or evaluating cancer treatment effectiveness and/or therapy response.
SUMMARY OF THE INVENTION
Elevated tumor interstitial fluid pressure (TIFP) derives from fluid
accumulation
within the tumor due to increased capillary permeability. Blood vessels become
leaky in
response to proteins secreted by tumor cells, once known as vascular
permeability factor
and more recently termed vascular endothelial growth factor, VEGF. Blood
proteins
permeate leaky vasculature, resulting in a loss of oncotic pressure gradient
across the
blood vessel wall. Fluid travels across the microvessel wall into interstitium
as a
consequence and contributes to elevated TIFP. High TIFP impairs lymphatic
drainage
and may contribute to tumor vasculature being abnormal. Tumor blood vessels
are often
contorted, dilated, and saccular, and may further be longer, larger in
diameter, and denser
than normal microvessels. As tumors grow, the abnormal vasculature limits the
delivery
of metabolites (nutrition and oxygen supply) and removal of wastes (such as
lactic acid
causing tumors to become acidic) to the tumor center. As a result, there are
also regions
in the center of the tumor devoid of microvessels. Consequently, some tumors
with
elevated TIFP develop a necrotic core surrounded by a perfused and growing
rim.
2
CA 2786368 2019-07-02
CA 02786368 2012-08-14
,
The applicants have appreciated that TIFP will typically be either elevated
throughout the tumor, or slightly reduced from the tumor periphery toward the
tumor
center, and drops to near zero (relative to normal tissue) at the tumor-normal
tissue
boundary.
The permeable tumor vasculature and pressure gradient between tumor and
surrounding normal tissue allows for systemically administered imaging dyes,
radioactive
materials, or other contrast agents (hereafter collectively "contrast
agents"), to distinguish
tumors and cancerous cells from a patient's surrounding normal tissues. Such
contrast
agents are selected to allow for their concentration within the tumor, and
their subsequent
imaging to identify cancerous cells or tumors by suitable imaging apparatus.
Such
imaging apparatus may include without limitation magnetic resonance imaging
(MRI)
equipment, CT scanners, infrared scanners, sonographs or ultrasonic scanning
equipment
and/or conventional x-ray equipment.
It has been recognized that after the initial filling or exposure of a tumor
with a
suitable contrast agent, as a result of the leaky vasculature characteristics
of selected
tumor blood vessels, the size of the detected or imaged region of contrast
will increase
with time. In particular, the inventors have appreciated that due to fluid
flux from the
tumor into the patient's surrounding normal tissues, following the initial
concentration
and scanning of the contrast agent, the detected imaged area of the tumor will
over time
expand in a halo effect. The present invention provides in one possible
embodiment, an
apparatus and method for measuring the rate and/or extent of observed contrast-
enhanced
volume expansion and/or intensity change of the dyed tissue area as an
indication of one
or more of tumor type, tumor activity, treatment effectiveness and/or
treatment response.
3
CA 02786368 2012-08-14
In a more preferred embodiment, the inventors propose a mathematical basis for
measurement of TIFF distribution for use in tumor diagnosis and/or the
assessment of
treatment effectiveness is proposed.
In another embodiment, the invention provides a method and apparatus for use
in
measuring the rate of change in the contrast intensity and/or volume of dyed
tumor
tissues or cells as an indication TIFF.
A scanning apparatus, such as kinetic MRI, CT, x-ray or ultrasound imaging
unit
is preferably used to effect multiple images of a tumor in which a contrast
agent has been
localized over a selected time period to provide a measurement of TIFF. More
preferably, the rate of change in the imaged area and/or contrast intensity of
the dyed
tissues is further used to assess tumor aggressiveness and as an early
predictor of
response to cancer therapy. In particular, it has been appreciated that
following the
marking of tumor or cancerous tissues by the initial localization of an
imageable contrast
agent, such as a radioactive dye therein, the rate of change in one or more of
the area of
the imaged marker or detected contrast agent and/or its change in volumetric
area may be
used to provide an indication of tumor interstitial fluid pressure (TIFF).
Accordingly, in one aspect, the present invention resides in a method for the
diagnosis of tumor activity, a tumor property and/or tumor response in a
region of interest
in a patient, said region of interest including tumor and surrounding tissues,
said method
including, injecting a contrast agent which is detectable by an imaging
apparatus as a
marker into said patient, said contrast agent selected to initially
concentrate in tumor
tissues following injection, activating said imaging apparatus to obtain a
first image of
4
CA 02786368 2012-08-14
said region of interest at a first period of time, said first image including
a first imaged
marker representative of a distribution of said contrast agent in said tumor
and
surrounding tissues, activating said imaging apparatus to obtain a second
image of said
region of interest at a second subsequent period of time, said second image
including a
second imaged marker representative of a distribution of said contrast agent
in said tumor
and surrounding tissues, comparing at least one geometric property of the
first imaged
marker at said first period of time and said second imaged marker at said
second period
of time to identify the change in the distribution of the contrast agent in
the region of
interest between the first and second subsequent period of time.
In another aspect, the present invention resides in use of a system for the
non-
invasive diagnosis of tumor activity, tumor property and/or tumor response in
a region of
interest in a patient, said region of interest including tumor and surrounding
tissues, said
system including, an imaging apparatus for obtaining an image of said region
of interest,
an injectionable contrast agent selected to initially concentrate in tumor
tissues in said
region of interest, and when said region of interest is imaged by said imaging
apparatus,
said contrast agent appearing as an imaged marker for tumor cells, wherein
said use, said
imaging apparatus is activated to obtain a first image of said region of
interest, to produce
a first imaged marker representative of a distribution of said contrast agent
in said tumor
and surrounding tissues at a first period of time, and said imaging apparatus
activated to
obtain a second image of said region of interest, to produce a second imaged
marker
representative of a distribution of said contrast agent in said tumor and
surrounding
tissues at a second period of time, comparing at least one observed properties
of said first
imaged marker and said second imaged marker to assess the change in the
distribution of
the contrast agent in the region of interest.
In yet a further aspect, the present invention resides in a method for the
diagnosis
of tumor activity and/or a tumor property in a region of interest in a
patient, said region of
interest including tumor and surrounding tissues, said method including,
injecting a
contrast agent which is detectable by an imaging apparatus as a marker into
said patient,
said contrast agent selected to initially concentrate in tumor tissues
following injection,
activating said imaging apparatus to obtain a first image of said region of
interest at a first
period of time, said first image including a first imaged marker
representative of a
distribution of said contrast agent in said tumor and surrounding tissues,
activating said
imaging apparatus to obtain a second image of said region of interest at a
second
subsequent period of time, said second image including a second imaged marker
representative of a distribution of said contrast agent in said tumor and
surrounding
tissues, comparing at least one geometric property of the first imaged marker
at said first
period of time and said second imaged marker at said second period of time to
identify a
rate of change in the distribution of the contrast agent in the region of
interest between
the first and second subsequent period of time, and correlating the rate of
change in the
distribution of the contrast agent to at least one of tumor interstitial fluid
pressure and
tumor fluid flux, and wherein the diagnosis of tumor activity comprises
classifying said
tumor as a benign tumor or a malignant tumor based on the rate of change in
distribution
of the contrast agent.
In yet another aspect, the present invention resides in use of a system for
the non-
invasive diagnosis of tumor activity and/or tumor property in a region of
interest in a
6
CA 2786368 2019-01-07
patient, said region of interest including tumor and surrounding tissues, said
system
including, an imaging apparatus for obtaining an image of said region of
interest, an
injectionable contrast agent selected to initially concentrate in tumor
tissues in said region
of interest, and when said region of interest is imaged by said imaging
apparatus, said
contrast agent appearing as an imaged marker for tumor cells, wherein said
use, after
injecting said contrast agent concentrating in said tumor, activating said
imaging
apparatus to obtain a first image of said region of interest, to produce a
first imaged
marker representative of a distribution of said contrast agent in said tumor
and
surrounding tissues at a first period of time, and activating said imaging
apparatus to
obtain a second image of said region of interest, to produce a second imaged
marker
representative of a distribution of said contrast agent in said tumor and
surrounding
tissues at a second period of time, comparing at least one observed property
of said first
imaged marker and said second imaged marker to assess a rate of change in the
distribution of the contrast agent in the region of interest, wherein the
observed property
is a geometric property, and further correlating the rate of change in said
geometric
property over time to at least one of an interstitial fluid pressure of said
tumor, a
vasculature permeability of said tumor and a tumor pressure gradient, and
wherein the
diagnosis of tumor activity comprises classifying said tumor as a benign tumor
or a
malignant tumor based on the rate of change in distribution of the contrast
agent
In another preferred aspect, the method is for the diagnosis of said tumor
property,
wherein the tumor property is selected from the group consisting of tumor
vasculature
permeability and tumor pressure gradient.
BRIEF DESCRIPTION OF THE DRAWINGS
6a
CA 2786368 2019-07-02
Reference may be had to the following detailed description taken together with
the accompanying drawings in which:
6b
CA 2786368 2019-07-02
CA 02786368 2012-08-14
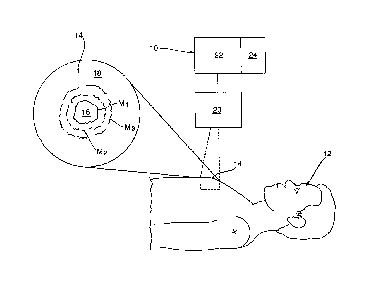
Figure 1 shows schematically a system for use in the non-invasive
diagnosis of tumor properties in accordance with a preferred embodiment
of the invention; and
Figure 2 shows an imaged tumor illustrating the change in the detected
area of contrast agent over an elapsed period of three minutes to fifteen
minutes following agent injection.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Reference may be had to Figure 1 which illustrates schematically an apparatus
10
for use in tumor identification and/or diagnosis in a patient 12. As will be
described, the
apparatus 10 is used in conjunction with a suitable contrast agent which is
selected both
for injection into the patient 12 to initially collect and concentrate within
tumor or
cancerous tissues (hereinafter collectively a tumor 16), and to be detectable
by the
apparatus 10 as a tumor marker to provide a detectable or imaged marker M
thereof. In a
preferred embodiment, the apparatus 10 is provided with an electromagnetic
radiation
(EMR) source 20 which is operable to produce optical images of a region of
interest 14 in
the patient 12, and which is selected as a site likely including a solid tumor
16 together
with its surrounding tissue 18.
The EMR source 20 is preferably selected as an MRI apparatus which is operable
to detect and output an image or data (hereinafter collectively referred to as
an image) of
the injected contrast agent. The EMR
source 20 is operated by way of a
processor/controller 22 having memory 24 to scan and obtain over time periods
t1, t2, t3,
tn, multiple optical images of the region of interest 14.
7
CA 02786368 2012-08-14
In use, the contrast agent is initially injected into the patient 12, and a
first period
of time (t1) is peimitted to pass which is selected to achieve the optimum
concentration of
the contrast agent within the tumor 16. Typically the first time period t1 is
selected at
between about 0.5 and 15 minutes, however, longer or shorter periods of time
may be
necessary depending on the tumor and/or contrast agent type, and the loci of
the injection.
Following the passage of time t1 after initial contrast agent injection into
the
patient 12, the processor/controller 22 is used to activate the EMR source 20
to obtain
and store in the memory 24 multiple images of the tumor 16 and surrounding
tissue 18
over a selected timeframe. Most preferably images are obtained at
predetermined and/or
measured intervals between 0.5 and 60 minutes apart, and most preferably
between about
3 and 15 minutes following the initial contrast agent injection. In this
manner the
apparatus 10 produces multiple images of the region of interest 14, which show
the
concentration and dispersion over time of the contrast agent as an imaged
marker MI, M2,
M3, ... Mn at each respective time t1,
.2, -3, = = =tn=
The processor/controller 22 is then activated to optically analyze in each
obtained
image, a geometric property, and preferably an area of the imaged marker MI,
M2, M3.= =
Mr, (as shown by the detected contrast agent), and to further calculate the
rate of the
change in marker area over time. The rate at which the detected contrast area
is observed
to expand is then correlated either by the processor/controller 22 with data
prestored in
the memory 24 or by a medical professional with predetermined anticipated
dispersion or
expansion rates for benign and malignant classes of like tumors, to provide an
indication
of tumor type and/or activity.
8
CA 02786368 2012-08-14
Whilst a simplified embodiment of the invention describes the apparatus 10 as
including an imaging apparatus such as an EMR source 20 operable to provide
images
showing the change in area of the scanned imaged markers MI, M2, M3. ¨Mn (or
detected
enhanced portion of the images), more preferably the imaging apparatus 10 is
provided
with three-dimensional scanning capabilities. In such a configuration, the EMR
source
20 is operable to obtain three-dimensional images of the area of interest 14
including the
tumor 16 and surrounding tissue 18. The processor/controller 22 is operable to
calculate
the rate of change in the volume area of each imaged marker MI, M2, M3.. .M by
comparing the geometry of the contrast-enhanced tissues shown in successively
scanned
images of the region of interest 14. The imaged markers M are used to
calculate the rate
of volume expansion of the contrast-enhanced region highlighted by the
contrast agent
over time, may thus be used to calculate fluid flux in or from the tumor 16
and/or tumor
interstitial fluid pressure.
Scientific Principle
Without being bound by a specific scientific theory, it is understood that
tumors
are provided with distinct regions including a core, with or without necrotic
tissue,
having radius r11 and a well perfused periphery characterized by leaky
vasculature. As a
tumor grows, the tumor center enlarges and the periphery remains approximately
the
same width. This is the classical "orange rind model" of a tumor.
The pressure profile is from high (relative to natmal tissue) interstitial
fluid
pressure, IFP, in tumor core and low IFP in surrounding normal tissue. TIFP
results from
fluid collecting in the interstitial spaces surrounding leaky vasculature.
In the
surrounding normal tissues, the fluid is carried away by lymphatics or by the
processes of
9
CA 02786368 2012-08-14
convection and/or diffusion. Within the tumor the lymphatics system is
impaired.
Consequently, a pressure gradient exists in the intermediary region between
tumor core
and tumor periphery.
The net fluid flux Js that leaks from tumor blood vessels follows Starling's
law,
the relationship connecting TIFF, p, with the surface area, A, of the blood
vessels, the
vascular fluid pressure, pv, the osmotic pressure difference, 2rv--z, between
the plasma in
the blood vessel and interstitial fluid, the osmotic reflection coefficient,
a, and the
hydraulic conductivity, L, of the blood vessel. The net driving pressure is
defined as (p,-
p)-o-(7rõ--71-). Starling's law defines the net fluid flux as
proportional to the product of
the surface area A of the blood vessels and the net driving pressure. The
proportionality
coefficient is the hydraulic conductivity L.
In a tumor, L and A are much greater, than those in the normal tissue, whereas
7tv¨Ir is smaller. This makes the net fluid flux J in a tumor much greater
than that in
normal tissue. If both outer and inner spherical surfaces of the vascularized
region are
closed and no fluid can flow out, the net fluid flux is zero at a steady
state. Further, IFP
in this region is at maximum value, which is expressed as the difference
between the
vascular fluid pressure p, and cs(7rv¨a)--the product of the osmotic
reflection coefficient cr
and the osmotic pressure difference ?r-'r between the plasma and interstitial
fluid. Once
TIT? reaches this value, no fluid flows out from blood vessels. When there are
openings
on each spherical surface of the vascularized region, the pressure will be
modified from
the two surfaces to the central area of that region. With the openings
increasing, the
pressure decreases. In the maximum pressure area, fluid remains stationary
since there is
no pressure difference. The maximum pressure region becomes narrower when the
CA 02786368 2012-08-14
openings become larger. Correspondingly, the flow rate across these two
surfaces
becomes larger. The maximum pressure region will narrow down to a point when
the
openings widen to a critical value. If the openings continue to increase, then
the highest
pressure pc becomes smaller than pm. The bigger the openings are, the smaller
the Po.
The maximum value of IFP at steady state is between 0 and pm. The exact value
pc)
depends on the conditions, such as the pressure pv inside blood vessels, the
osmotic
pressure difference 71-v-71" between the plasma and interstitial fluid, the
lymphatic drainage
ability and fluid flow rate from the openings. The pressure at the surface of
necrotic core
depends on the conditions within the necrotic core pm, and the pressure at the
periphery
of a tumor depends on the conditions of the environment.
TIFF in the central area
TIFF variation in the central area depends on po, the pressure barrier, and
the fluid
conditions in the region. Initially, pressure in the necrotic core area is
smaller than the
pressure pc. The leaked fluid flows into this area. Since drainage
(lymphatics) does not
readily occur, more and more fluid accumulates within the core, gradually
increasing the
pressure in the region and reaching po after the fluid fills up the entire
area. Once pc is
reached, no more fluid can flow into the area, with the central area
maintaining a constant
pressure po, and which is as high as that of the pressure barrier. With the
central area
filled with fluids, all leaked fluid flows to the outside. The pressure in the
necrotic core
thus should be the same as that at the surface, r =rn where the fluid flows
in. The relation
between IFP and the fluid velocity is constrained by Darcy's law, which states
that the
fluid velocity is proportional to the negative gradient of TIFP. The
proportionality
coefficient K is defined as the hydraulic conductivity of the interstitium.
The more fluid
11
CA 02786368 2012-08-14
accumulates in this region, the higher the pressure will be. Correspondingly,
the pressure
difference between po and p(rn) becomes smaller and smaller. Therefore, the
fluid
velocity across the surface (r ----rn) of the necrotic core will also be
smaller. When p(r11)
equals to po, the pressure difference is zero so no more fluid flows in.
Assuming the
radius that corresponds to the pressure barrier po is ro, the whole region
inside ro reaches
pressure po. The pressure difference po-pi(1) decreases from po-pin(0)
exponentially with
time. This TIFP variation may also be applied to a contrast agent if one is
used. At
steady state, TIFP in the region within ro is uniformly po. The exact value of
po, which is
between 0 and pm, will depend on the conditions at the periphery of the tumor.
TIFP in the periphery
In the periphery, the difference between the tumor radius R and ro (which
corresponds to the pressure barrier po) is small. Darcy's law gives an
approximate linear
relationship between the fluid velocity change and IFP change from 7-0 to R.
Since fluid
velocity u(ro) at ro is zero, the fluid velocity u(R) at the periphery is
proportional to the
ratio of the IFP variation po-p(R) to the difference R-ro. This relationship
connects po
with the fluid velocity u(R) and IFP p(R), which is balanced with the IFP of
the
environment.
TIFP Measurement
The value of po may be detelinined by measuring p(R) and u(R). As such, u(K)
may be measured non-invasively using a contrast enhanced imaging modality, as
by way
of non-limiting example, computed tomography (CT) or magnetic resonance
imaging
(MRI), or ultrasound (US) either of these together with a suitable contrast
agent.
12
TIFP in the periphery and intermediary region can be determined based on
corresponding environmental conditions. When fluid velocity at tumor periphery
is
greater than a critical fluid velocity ue(R), p(R) is zero; otherwise, p(R) is
greater than
zero. For instance, where for example /30=15 mmHg, K=4.13x10-8cm2/mm HgSec,
r0=0.9cm, R=1.0cm, tic(R) = 0.124 pm/sec or 0.5mm/hour, which corresponds to
the
results for isolated tumors, as for example is described in Baxter LT, Jain
RK.
"Transport of fluid and macromolecules in tumors I. Role of interstitial
pressure and
convection". Microvasc Res 1989; 37(1): 77-104. Previous authors have
estimated that
fluid velocity at the periphery of isolated tumors is 0.13-0.2,um/sec (see
Baxter above).
There is a critical flow rate Qõ which is defined as 47tR2uc(R), coinciding
with the critical
fluid velocity, and which for a tumor with radius R=1.0cm, Qc=1.56x10-4m1/sec.
TIFP
p(R) at the periphery of the tumor is zero if the lymphatic drainage's ability
is large
enough to ensure the maximum drainage (i.e. Q., is greater than Qc). When the
drainage
ability Q,,, is smaller than the critical flow rate Qõ the drainage ability of
the tumor is
small so that TIFP at the periphery will be high. When the TIFP at the
periphery is too
high for the given conditions, the tumor must find a way to release the
pressure by
creating channels that connect with normal tissue. Here the pressure release
may result in
the break of the normal structure at the interface or make it complicated. Qc
may be a
factor for determining whether it is an isolated or embedded tumor. In this
case, the fluid
flux Q at a tumor edge 47-1-R2u(R) is greater than the drainage ability Q,,,
but smaller than
the critical flow rate Q. Some fluid crosses over the edge (r=R) and flows
into the
normal tissue. Lymphatics are plenty and functional in normal tissue, so some
fluid is
drained
13
CA 2786368 2019-07-02
CA 02786368 2012-08-14
away. Similar to the Starling's law, the net fluid flux drained from the
lymphatics is
proportional to the surface area A(rm) and the pressure difference between
TIFP and the
pressure PL in lymphatics. The proportionality coefficient is LL, which is
defined as the
hydraulic conductivity of lymphatics. The rm is the maximum radius of fluid
that the
tumor can spread. The maximum radius corresponds to the radius from which the
pressure becomes the same as the pressure of the normal tissue. When balanced,
the
pressure pi, in lymphatics should be the same as that in the environment p,õ
AL(rm) is the
total surface area of the lymphatics within radius rm. At steady state, the
radius is a fixed
value; therefore, the A L(rm) is fixed. Since fluid does not tend to collect
outside the
tumor, the total fluid flux across the tumor edge should be conserved.
Combined with
Darcy's law, the distribution of TIFP outside the tumor may be determined. In
particular,
TIFP in the region from ro to R can be determined by considering the
continuity
conditions.
Time dependent TIFF
Besides being spatially different, the net fluid flux in a tumor is time
dependent.
In normal tissues with functional vasculature and lymphatics, the interstitial
fluid is
balanced, that is, all capillaries have the same L,cr, pressure difference pr-
p and osmotic
pressure difference a-17 Ir. In contrast, in tumor tissues, the capillaries
are abnormal and
lymphatics are absent. Consequently, L, cr and 7-1-v-g are not unifoiin but
rather
heterogeneous, though the pv may stay the same. The total fluid flux at time t
is the sum
of fluid flux from different capillaries at time t. Permeable microvessels
have an osmotic
pressure difference 7tv-7 approaching zero. Leaky capillaries near the
tumor/normal
tissue boundary have conductivity greater than that of the capillaries in the
central region.
14
CA 02786368 2012-08-14
The osmotic pressure difference may possibly also be smaller in this area. The
total fluid
flux near the tumor edge is therefore expected to be much greater than that in
the central
area, assuming that the total fluid flux near the edge represents the total
fluid flux of the
tumor. In this narrow region, the value of the different vascular parameters
is
homogeneous.
Noninvasive measurement
The distribution of TIFF was derived under steady state conditions based on
the
rate of contrast agent flux from the tumor into the normal tissue as a
function of time.
A minimally-invasive measurement of TIFF is possible based on the
aforementioned model. An exemplary approach was an MRI protocol which could be
performed as a modification of current imaging practices.
Often MRI diagnostic tests use a vascular contrast agent to delineate a
suspicious
mass prior to a biopsy procedure. Depending on the imaging sequence used, the
contrasted volume of tissues in which the contrast agent is concentrated may
vary and in
fact appear to increase with duration (measured in minutes) after its initial
administration.
This phenomenon is shown in Figure 2. In particular, Figure 2 illustrates the
consequence of high TIFF on contrast agent kinetics in a rat 9L cerebral
glioma acquired
using a 7 Tesla MRI. The five panels illustrate ratio images of 12* to Ti
relaxivities, so
called, "Gamma-2 images", taken at minute 3 through minute 15 following the
injection
of the contrast agent (GadomerTM) and which from left to right were acquired
at 2.5
minute intervals. The movement of the contrast agent wave front (blue circular
region) in
normal tissue is clearly visualized across this time period. Figure 2 shows a
pulse
sequence of five images of a tumor region in which the contrast agent is
concentrated and
CA 02786368 2012-08-14
image analysis technique sensitive to the presence of contrast. The result
shown in
Figure 2 reflect a contrast agent wave front which appears to increase in
volume as a
function of time, as the contrast agent streams outwardly at a velocity u(R)
from initially
concentrated within tumor and into surrounding normal tissue. The rate of
contrast agent
flux is proportional to the TIFF relative to that of surrounding normal tissue
(i.e. usually
near zero).
Though a spherical three-dimensional model was used in the clinical testing,
the
invention is not limited to the specific geometry. Contrast agent flux at
irregular
boundaries can be modeled at various gradient directions perpendicular to the
tumor
boundary. However, as an approximation, at a distance away from most tumors, a
simple
spherical model may be advantageously adequately approximate the contrast
agent
kinetics and movement.
The present invention is suitable for use with any imaging modality capable of
monitoring the dynamics of a contrast agent to determine TIFP, including MRI,
CT, US,
PET and SPECT.
The current technique further may also advantageously be used to augment
image-guided radiation therapy, since regions of a tumor identified to be more
aggressive
than other regions, may be isolated and treated accordingly. The present
invention thus
shows promise as a simple, new imaging apparatus and method which could be
rapidly
implemented on a variety of clinical machines and has the potential to
identify or predict
tumor response to cancer treatment.
Although the detailed description describes the use of GadomerTM as the
contrast
agent used in tumor imaging, the invention is not so limited. It is to be
appreciated that
16
the present invention is also contemplated for a use with a variety of
different types of
radioactive and nonradioactive contrast agents, including, without
restriction, radioactive
iodine dyes, blue dye, Patentblau V, iron-based contrast agents, mircobubble
contrast
agents and phosphorescent contrast agents selected to allow dye loading within
cancer
tissues or tumors.
While the preferred embodiment of the invention describes the apparatus 10 as
operable to calculate the rate of expansion of the area or volume of the
imaged marker
(contrast-enhanced tissue region), the invention is not so limited. In an
alternate
construction, the processor/controller 22 may be used to measure and output
the rate of
change of the intensity of part or all of the imaged markers over time. Such a
change in
contrast would be extrapolated as showing the dissipation and migration of the
contrast
agent from its initial concentration within the tumor 16.
While the detailed description describes and illustrates various preferred
embodiments, the invention is not limited strictly to the precise embodiments
which are
disclosed. Modifications and variations will now occur to persons skilled in
the art. The
scope of the claims is not limited by the preferred embodiments set forth in
the detailed
description.
17
CA 2786368 2019-07-02