Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
THERMAL TRANSFER DEVICE AND ASSOCIATED SYSTEMS AND
METHODS
CROSS-REFERENCE TO RELATED APPLICATION(S)
[0001] The present application claims priority to and the benefit of U.S.
Patent
Application No. 61/304,403, filed on February 13, 2010 and titled FULL
SPECTRUM
ENERGY AND RESOURCE INDEPENDENCE. The present application is a
continuation in part of: U.S. Patent Application No. 12/857,546, filed on
August 16,
2010 and titled INCREASING THE EFFICIENCY OF SUPPLEMENTED OCEAN
THERMAL ENERGY CONVERSION (SOTEC) SYSTEMS, and U.S. Patent Application
No. 12/857,228, filed on August 16, 2010 and titled GAS HYDRATE CONVERSION
SYSTEM FOR HARVESTING HYDROCARBON HYDRATE DEPOSITS, each of which
claims priority to and the benefit of U.S. Provisional Application No.
61/304,403, filed
February 13, 2010 and titled FULL SPECTRUM ENERGY AND RESOURCE
INDEPENDENCE. U.S. Patent Application No. 12/857,546 and U.S. Patent
Application
No. 12/857,228 are also each a continuation-in-part of each of the following
applications: U.S. Patent Application No. 12/707,651, filed February 17, 2010
and titled
ELECTROLYTIC CELL AND METHOD OF USE THEREOF; PCT Application No. PCT/
US10/24497, filed February 17, 2010 and titled ELECTROLYTIC CELL AND METHOD
OF USE THEREOF; U.S. Patent Application No. 12/707,653, filed February 17,
2010
and titled APPARATUS AND METHOD FOR CONTROLLING NUCLEATION DURING
ELECTROLYSIS; PCT Application No. PCT/ US10/24498, filed February 17, 2010 and
titled APPARATUS AND METHOD FOR CONTROLLING NUCLEATION DURING
ELECTROLYSIS; U.S. Patent Application No. 12/707,656, filed February 17, 2010
and
titled APPARATUS AND METHOD FOR GAS CAPTURE DURING ELECTROLYSIS;
and PCT Application No. PCT/ US10/24499, filed February 17, 2010 and titled
APPARATUS AND METHOD FOR CONTROLLING NUCLEATION DURING
ELECTROLYSIS; each of which claims priority to and the benefit of the
following
applications: U.S. Provisional Patent Application No. 61/153,253, filed
February 17,
2009 and titled FULL SPECTRUM ENERGY; U.S. Provisional Patent Application No.
1
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
61/237,476, filed August 27, 2009 and titled ELECTROLYZER AND ENERGY
INDEPENDENCE TECHNOLOGIES; U.S. Provisional Application No. 61/304,403, filed
February 13, 2010 and titled FULL SPECTRUM ENERGY AND RESOURCE
INDEPENDENCE. Each of these applications is incorporated herein by reference
in its
entirety.
TECHNICAL FIELD
[0002] The present technology relates generally to thermal transfer devices
and
associated systems and methods.
BACKGROUND
[0003] Heat pipes transfer heat between a heat source and a heat sink
utilizing a
liquid-vapor phase change of a working fluid. For example, a working fluid
enclosed in
a conventional heat pipe contacts and absorbs heat from a hot interface such
that it
changes to a vapor phase. The vapor pressure drives the vapor phase working
fluid
through a conduit to a cold interface where the working fluid condenses to a
liquid
phase. The cold interface absorbs the latent heat from the phase change and
removes
it from the system. The liquid phase working fluid then returns to the hot
interface
using capillary action or gravity to continue the vaporization-condensation
cycle.
[0004] Heat pipes can generally transport large amounts of heat with
relatively small
temperature gradients and without mechanical moving parts. Thus, heat pipes
can
provide efficient heat transfer means. However, non-condensing gases can
diffuse
through the heat pipe's wall and thereby cause impurities in the working fluid
that
diminish the heat pipe's efficiency. Additionally, extreme temperatures can
cease the
vaporization-condensation cycle. For example, extreme heat can prevent the
working
fluid from condensing, whereas extreme cold can prevent the working fluid from
vaporizing. Accordingly, there is a need to improve the efficiency and
adaptability of
heat pipes and to harness the resultant thermal energy.
BRIEF DESCRIPTION OF THE DRAWINGS
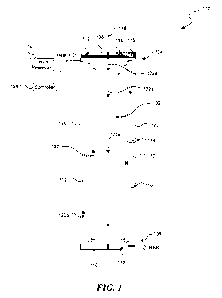
[0005] Figure 1 is a schematic cross-sectional view of a thermal transfer
device
configured in accordance with an embodiment of the present technology.
2
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
[0006] Figures 2A and 2B are schematic cross-sectional views of thermal
transfer
devices configured in accordance with other embodiments of the present
technology.
[0007] Figure 3A is a schematic cross-sectional view of a thermal transfer
device
operating in a first direction in accordance with a further embodiment of the
present
technology, and Figure 3B is a schematic cross-sectional view of the thermal
transfer
device of Figure 3A operating in a second direction opposite the first
direction.
[0008] Figures 4A and 4B are schematic plan views of thermal transfer devices
configured in accordance with embodiments of the present technology.
[0009] Figure 4C is a schematic cross-sectional view of a thermal transfer
device
configured in accordance with an additional embodiment of the present
technology.
[0010] Figure 5A is a schematic view of a thermal transfer system in a
representative
environment in accordance with an embodiment of the present technology, and
Figure
5B is an enlarged operational view of a portion of the thermal transfer system
of Figure
5A.
[0011] Figure 6A is a schematic view of a thermal transfer system in a
representative
environment in accordance with another embodiment of the present technology,
and
Figure 6B is an enlarged operational view of a portion of the thermal transfer
system of
Figure 6A.
[0012] Figure 7A is a schematic view of a thermal transfer system in a
representative
environment in accordance with yet another embodiment of the present
technology,
and Figures 7B and 7C are enlarged operational views of portions of the
thermal
transfer system of Figure 7A.
[0013] Figure 7D is a schematic view of a thermal transfer system in a
representative
environment in accordance with still another embodiment of the present
technology.
[0014] Figure 8 is a schematic view of a thermal transfer system in a
representative
environment in accordance with a further embodiment of the present technology.
[0015] Figure 9A is a cross-sectional view of a thermal transfer system in a
representative environment in accordance with an additional embodiment of the
present technology, and Figure 9B is an enlarged view of detail 9B of Figure
9A.
3
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
[0016] Figure 10 is a schematic cross-sectional view of a thermal transfer
device
configured in accordance with a further embodiment of the present technology.
[0017] Figure 11 is a schematic view of a thermal transfer system 1100 shown
in a
representative environment in accordance with yet another embodiment of the
present
technology.
DETAILED DESCRIPTION
[0018] The present disclosure describes thermal transfer devices, as well as
associated systems, assemblies, components, and methods regarding the same.
For
example, several of the embodiments described below are directed generally to
thermal transfer devices that include a working fluid or combination of
working fluids
that transfer heat utilizing a vaporization-condensation cycle. As used
herein, the term
working fluid can include any fluid that actuates the thermal transfer device.
In one
embodiment, for example, the working fluid is water. In other embodiments, the
working fluid can include ammonia, methanol, and/or other suitable working
fluids
selected based on available fluids and desired outputs of the thermal transfer
device.
Additionally, several embodiments described below refer to a vaporization-
condensation cycle that changes the working fluid between a vapor phase and a
liquid
phase. As used herein, the term vaporization-condensation cycle is construed
broadly
to refer to any phase change of the working fluid resulting in a transfer of
heat.
[0019] Certain details are set forth in the following description and in
Figures 1-11 to
provide a thorough understanding of various embodiments of the disclosure.
However,
other details describing well-known structures and systems often associated
with
thermal transfer devices and/or other aspects of heating and cooling systems
are not
set forth below to avoid unnecessarily obscuring the description of various
embodiments of the disclosure. Thus, it will be appreciated that several of
the details
set forth below are provided to describe the following embodiments in a manner
sufficient to enable a person skilled in the relevant art to make and use the
disclosed
embodiments. Several of the details and advantages described below, however,
may
not be necessary to practice certain embodiments of the disclosure. Many of
the
details, dimensions, angles, shapes, and other features shown in the Figures
are
merely illustrative of particular embodiments of the disclosure. Accordingly,
other
4
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
embodiments can have other details, dimensions, angles, and features without
departing from the spirit or scope of the present disclosure. In addition,
those of
ordinary skill in the art will appreciate that further embodiments of the
disclosure can be
practiced without several of the details described below.
[0020] Reference throughout this specification to "one embodiment" or "an
embodiment" means that a particular feature, structure, or characteristic
described in
connection with the embodiment is included in at least one embodiment of the
present
disclosure. Thus, the occurrences of the phrases "in one embodiment" or "in an
embodiment" in various places throughout this specification are not
necessarily all
referring to the same embodiment. Furthermore, the particular features,
structures, or
characteristics described with reference to a particular embodiment may be
combined
in any suitable manner in one or more other embodiments. Moreover, the
headings
provided herein are for convenience only and do not interpret the scope or
meaning of
the claimed disclosure.
[0021] Figure 1 is a schematic cross-sectional view of a thermal transfer
device 100
("device 100") configured in accordance with an embodiment of the present
technology. As shown in Figure 1, the device 100 can include a conduit 102
that has
an input portion 104, an output portion 106 opposite the input portion 104,
and a
sidewall 120 between the input and output portions 104 and 106. The device 100
can
further include a first end cap 108 at the input portion 104 and a second end
cap 110 at
the output portion 106. The device 100 can enclose a working fluid 122
(illustrated by
arrows) that changes between a vapor phase 122a and a liquid phase 122b during
a
vaporization-condensation cycle.
[0022] In selected embodiments, the device 100 can also include one or more
architectural constructs 112. Architectural constructs 112 are synthetic
matrix
characterizations of crystals that are primarily comprised of graphene,
graphite, boron
nitride, and/or another suitable crystal. The configuration and the treatment
of these
crystals heavily influence the properties that the architectural construct 112
will exhibit
when it experiences certain conditions. For example, as explained in further
detail
below, the device 100 can utilize architectural constructs 112 for their
thermal
properties, capillary properties, sorbtive properties, catalytic properties,
and
electromagnetic, optical, and acoustic properties. As shown in Figure 1, the
5
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
architectural construct 112 can be arranged as a plurality of substantially
parallel layers
114 spaced apart from one another by a gap 116. In various embodiments, the
layers
114 can be as thin as one atom. In other embodiments, the thickness of the
individual
layers 114 can be greater and/or less than one atom and the width of the gaps
116
between the layers 114 can vary. Methods of fabricating and configuring
architectural
constructs, such as the architectural constructs 112 shown in Figure 1, are
described in
U.S. Patent Application entitled "ARCHITECTURAL CONSTRUCT HAVING FOR
EXAMPLE A PLURALITY OF ARCHITECTURAL CRYSTALS" (Attorney Docket No.
69545-8701 US), filed concurrently herewith and incorporated by reference in
its
entirety.
[0023] As shown in Figure 1, the first end cap 108 can be installed proximate
to a
heat source (not shown) such that the first end cap 108 serves as a hot
interface that
vaporizes the working fluid 122. Accordingly, the first end cap 108 can
include a
material with a high thermal conductivity and/or transmissivity to absorb or
deliver heat
from the heat source. In the embodiment illustrated in Figure 1, for example,
the first
end cap 108 includes the architectural construct 112 made from a thermally
conductive
crystal (e.g., graphene). The architectural construct 112 can be arranged to
increase
its thermal conductively by configuring the layers 114 to have a high
concentration of
thermally conductive pathways (e.g., formed by the layers 114) substantially
parallel to
the influx of heat. For example, in the illustrated embodiment, the layers 114
generally
align with the incoming heat flow such that heat enters the architectural
construct 112
between the layers 114. This configuration exposes the greatest surface area
of the
layers 114 to the heat and thereby increases the heat absorbed by the
architectural
construct 112. Advantageously, despite having a much lower density than metal,
the
architectural construct 112 can conductively and/or radiatively transfer a
greater
amount of heat per unit area than solid silver, raw graphite, copper, or
aluminum. .
[0024] As further shown in Figure 1, the second end cap 110 can expel heat
from
the device 100 to a heat sink (not shown) such that the second end cap 110
serves as
a cold interface that condenses the working fluid 122. The second end cap 110,
like
the first end cap 108, can include a material with a high thermal conductivity
(e.g.,
copper, aluminum) and/or transmissivity to absorb and/or transmit latent heat
from the
working fluid 122. Accordingly, like the first end cap 108, the second end cap
110 can
6
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
include the architectural construct 112. However, rather than bringing heat
into the
device 100 like the first end cap 108, the second end cap 110 can convey
latent heat
out of the device 100. In various embodiments, the architectural constructs
112 of the
first and second end caps 108 and 110 can be made from the similar materials
and/or
arranged to have substantially similar thermal conductivities. In other
embodiments,
the architectural constructs 112 can include different materials, can be
arranged in
differing directions, and/or otherwise configured to provide differing thermal
conveyance capabilities including desired conductivities and transmissivities.
In further
embodiments, neither the first end cap 108 nor the second end cap 110 includes
the
architectural construct 112.
[0025] In selected embodiments, the first end cap 108 and/or the second end
cap
110 can include portions with varying thermal conductivities. For example, a
portion of
the first end cap 108 proximate to the conduit 102 can include a highly
thermally
conductive material (e.g., the architectural construct 112 configured to
promote thermal
conductivity, copper, etc.) such that it absorbs heat from the heat source and
vaporizes
the working fluid 122. Another portion of the first end cap 108 spaced apart
from the
conduit 102 can include a less thermally conductive material to insulate the
high
conductivity portion. In certain embodiments, for example, the insulative
portion can
include ceramic fibers, sealed dead air space, and/or other materials or
structures with
high radiant absorptivities and/or low thermal conductivities. In other
embodiments, the
insulative portion of the first end cap 108 can include the architectural
construct 112
arranged to include a low concentration of thermally conductive pathways
(e.g., the
layers 114 are spaced apart by large gaps 116) such that it has a low
availability for
conductively transferring heat.
[0026] In other embodiments, the configurations of the architectural
constructs 112
may vary from those shown in Figure 1 based on the dimensions of the device
100, the
temperature differential between the heat source and the heat sink, the
desired heat
transfer, the working fluid 122, and/or other suitable thermal transfer
characteristics.
For example, architectural constructs 112 having smaller surface areas may be
suited
for microscopic applications of the device 100 and/or high temperature
differentials,
whereas architectural constructs 112 having higher surface areas may be better
suited
for macroscopic applications of the device 100 and/or higher rates of heat
transfer.
7
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
The thermal conductivities of the architectural constructs 112 can also be
altered by
coating the layers 114 with dark colored coatings to increase heat absorption
and with
light colored coatings to reflect heat away and thereby decrease heat
absorption.
[0027] Referring still to Figure 1, the device 100 can return the liquid phase
122b of
the working fluid 122 to the input portion 104 by capillary action. The
sidewall 120 of
the conduit 102 can thus include a wick structure that exerts a capillary
pressure on the
liquid phase 122b to drive it toward a desired location (e.g., the input
portion 104). For
example, the sidewall 120 can include cellulose, ceramic wicking materials,
sintered or
glued metal powder, nanofibers, and/or other suitable wick structures or
materials that
provide capillary action.
[0028] In the embodiment shown in Figure 1, the architectural construct 112 is
aligned with the longitudinal axis 118 of the conduit 102 and configured to
exert the
necessary capillary pressure to direct the liquid phase 122b of the working
fluid 122 to
the input portion 104. The composition, dopants, spacing, and/or thicknesses
of the
layers 114 can be selected based on the surface tension required to provide
capillary
action for the working fluid 122. Advantageously, the architectural construct
112 can
apply sufficient capillary pressure on the liquid phase 122b to drive the
working fluid
122 short and long distances (e.g., millimeters to kilometers). Additionally,
in selected
embodiments, the surface tension of the layers 114 can be manipulated such
that the
architectural construct 112 rejects a preselected fluid. For example, the
architectural
construct 112 can be configured to have a surface tension that rejects any
liquid other
than the liquid phase 122b of the working fluid 122. In such an embodiment,
the
architectural construct 112 can function as a filter that prevents any fluid
other than the
working fluid 122 (e.g., fluids tainted by impurities that diffused into the
conduit 102)
from interfering with the vaporization-condensation cycle.
[0029] In other embodiments, the selective capillary action of the
architectural
construct 112 separates substances at far lower temperatures than conventional
distillation technologies. The faster separation of substances by the
architectural
construct 112 can reduce or eliminates substance degradation caused if the
substance
reaches higher temperatures within the device 100. For example, a potentially
harmful
substance can be removed from the working fluid 122 by the selective capillary
action
8
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
of the architectural construct 112 before the working fluid 122 reaches the
higher
temperatures proximate to the input portion 104.
[0030] The conduit 102 and the first and second end caps 108 and 110 can be
sealed together using suitable fasteners able to withstand the temperature
differentials
of the device 100. In other embodiments, the device 100 is formed integrally.
For
example, the device 100 can be molded using one or more materials. A vacuum
can
be used to remove any air within the conduit 102, and then the conduit 102 can
be
filled with a small volume of the working fluid 122 chosen to match the
operating
temperatures.
[0031] In operation, the device 100 utilizes a vaporization-condensation cycle
of the
working fluid 122 to transfer heat. More specifically, the first end cap 108
can absorb
heat from the heat source, and the working fluid 122 can in turn absorb the
heat from
the first end cap 108 to produce the vapor phase 122a. The pressure
differential
caused by the phase change of the working fluid 122 can drive the vapor phase
122a
of the working fluid 122 to fill the space available and thus deliver the
working fluid 122
through the conduit 102 to the output portion 104. At the output portion 104,
the
second end cap 110 can absorb heat from the working fluid 122 to change the
working
fluid 122 to the liquid phase 122b. The latent heat from the condensation of
the
working fluid 122 can be transferred out of the device 100 via the second end
cap 110.
In general, the heat influx to the first end cap 108 substantially equals the
heat
removed by the second end cap 110. As further shown in Figure 1, capillary
action
provided by the architectural construct 112 or other wick structure can return
the liquid
phase 122b of the working fluid 122 to the input portion 104. In selected
embodiments,
the termini of the layers 114 can be staggered or angled toward the conduit
102 to
facilitate entry of the liquid phase 122b between the layers 114 and/or to
facilitate
conversion of the liquid phase 122b to the vapor phase 122b at the input
portion 104.
At the input portion 104, the working fluid 122 can again vaporize and
continue to
circulate through the conduit 102 by means of the vaporization-condensation
cycle.
[0032] The device 100 can also operate the vaporization-condensation cycle
described above in the reverse direction. For example, when the heat source
and heat
sink are reversed, the first end cap 108 can serve as the cold interface and
the second
end cap 110 can serve as the hot interface. Accordingly, the input and output
portions
9
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
104 and 106 are inverted such that the working fluid 122 vaporizes proximate
to the
second end cap 110, condenses proximate to the first end cap 108, and returns
to the
second end cap 110 using the capillary action provided by the sidewall 120.
The
reversibility of the device 100 allows the device 100 to be installed
irrespective of the
positions of the heat source and heat sink. Additionally, the device 100 can
accommodate environments in which the locations of the heat source and the
heat sink
may reverse. For example, as described further below, the device 100 can
operate in
one direction during the summer to utilize solar energy and the device 100 can
reverse
direction during the winter to utilize heat stored during the previous summer.
[0033] Embodiments of the device 100 including the architectural construct 112
at
the first end cap 108 and/or second end cap 110 have higher thermal
conductivity per
unit area than conventional conductors. This increased thermal conductivity
can
increase process rate and the temperature differential between the first and
second
end caps 108 and 110 to produce greater and more efficient heat transfer.
Additionally, embodiments including the architectural construct 112 at the
first and/or
second end caps 108 and 110 require less surface area to absorb the heat
necessary
to effectuate the vaporization-condensation cycle. Thus, the device 100 can be
more
compact than a conventional heat pipe that transfers an equivalent amount of
heat and
provide considerable cost reduction.
[0034] Referring still to Figure 1, in various embodiments, the device 100 can
further
include a liquid reservoir 124 in fluid communication with the conduit 102
such that the
liquid reservoir 124 can collect and store at least a portion of the working
fluid 122. As
shown in Figure 1, the liquid reservoir 124 can be coupled to the input
portion 104 of
the conduit 102 via a pipe or other suitable tubular shaped structure. The
liquid phase
122b can thus flow from the sidewall 102 (e.g., the architectural construct
112, wick
structure, etc.) into the liquid reservoir 124. In other embodiments, the
liquid reservoir
124 is in fluid communication with another portion of the conduit 102 (e.g.,
the output
portion 106) such that the liquid reservoir 124 collects the working fluid 122
in the vapor
phase 122a or in mixed phases.
[0035] The liquid reservoir 124 allows the device 100 to operate in at least
two
modes: a heat accumulation mode and a heat transfer mode. During the heat
accumulation mode, the vaporization-condensation cycle of the working fluid
122 can
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
be slowed or halted by funneling the working fluid 122 from the conduit 102 to
the liquid
reservoir 124. The first end cap 108 can then function as a thermal
accumulator that
absorbs heat without the vaporization-condensation cycle dissipating the
accumulated
heat. After the first end cap 108 accumulates a desired amount of heat and/or
the heat
source (e.g., the sun) no longer supplies heat, the device 100 can change to
the heat
transfer mode by funneling the working fluid 122 into the conduit 102. The
heat stored
in first end cap 108 can vaporize the incoming working fluid 122 and the
pressure
differential can drive the vapor phase 122a toward the output portion 106 of
the conduit
102 to restart the vaporization-condensation cycle described above. In certain
embodiments, the restart of the vaporization-condensation cycle can be
monitored to
analyze characteristics (e.g., composition, vapor pressure, latent heat,
efficiency) of the
working fluid 122.
[0036] As shown in Figure 1, a controller 126 can be operably coupled to the
liquid
reservoir 124 to modulate the rate at which the working fluid 122 enters the
conduit 102
and/or adjust the volume of the working fluid 122 flowing into or out of the
conduit 102.
The controller 126 can thereby change the pressure within the conduit 102 such
that
the device 100 can operate at varying temperature differentials between the
heat
source and sink. Thus, the device 100 can provide a constant heat flux despite
a
degrading heat source (e.g., first end cap 108) or intermittent vaporization-
condensation cycles.
[0037] Figures 2A and 2B are schematic cross-sectional views of thermal
transfer
devices 200 ("devices 200") in accordance with other embodiments of the
present
technology. Several features of the devices 200 are generally similar to the
features of
the device 100 shown in Figure 1. For example, each device 200 can include the
conduit 102, the sidewall 120, and the first and second end caps 108 and 110.
The
device 200 also transfers heat from a heat source to a heat sink utilizing a
vaporization-
condensation cycle of the working fluid 122 generally similar to that
described with
reference to Figure 1. Additionally, as shown in Figures 2A and 2B, the device
200 can
further include the liquid reservoir 124 and the controller 126 such that the
device 200
can operate in the heat accumulation mode and the heat transfer mode.
[0038] The devices 200 shown in Figures 2A and 2B can utilize gravity, rather
than
the capillary action described in Figure 1, to return the liquid phase 122b of
the working
11
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
fluid 122 to the input portion 104. Thus, as shown in Figures 2A and 2B, the
heat
inflow is below the heat output such that gravity can drive the liquid phase
122b down
the sidewall 120 to the input portion 104. Thus, as shown in Figure 2A, the
sidewall
120 need only include an impermeable membrane 228, rather than a wick
structure
necessary for capillary action, to seal the working fluid 122 within the
conduit 102. The
impermeable membrane 228 can be made from a polymer such as polyethylene, a
metal or metal alloy such as copper and stainless steel, and/or other suitable
impermeable materials. In other embodiments, the devices 200 can utilize other
sources of acceleration (e.g., centrifugal force, capillary action) to return
the liquid
phase 122b to the input portion 104 such that the positions of the input and
output
portions 104 and 106 are not gravitationally dependent.
[0039] As shown in Figure 2B, in other embodiments, the sidewall 120 can
further
include the architectural construct 112. For example, the architectural
construct 112
can be arranged such that the layers 114 are oriented orthogonal to the
longitudinal
axis 118 of the conduit 102 to form thermally conductive passageways that
transfer
heat away from the conduit 102. Thus, as the liquid phase 122b flows along the
sidewall 120, the architectural construct 112 can draw heat from the liquid
phase 122b,
along the layers 114, and away from the sidewall 120 of the device 200. This
can
increase the temperature differential between the input and output portions
104 and
106 to increase the rate of heat transfer and/or facilitate the vaporization-
condensation
cycle when the temperature gradient would otherwise be insufficient. In other
embodiments, the layers 114 can be oriented at a different angle with respect
to the
longitudinal axis 118 to transfer heat in a different direction. In certain
embodiments,
the architectural construct 112 can be positioned radially outward of the
impermeable
membrane 228. In other embodiments, the impermeable membrane 228 can be
radially outward of architectural construct 112 or the architectural construct
112 itself
can provide a sufficiently impervious wall to seal the working fluid 122
within the
conduit 102.
[0040] The first and second end caps 108 and 110 shown in Figures 2A and 2B
can also include the architectural construct 112. As shown in Figures 2A and
2B, the
layers 114 of the architectural constructs 112 are generally aligned with the
direction
heat input and heat output to provide thermally conductive passageways that
efficiently
12
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
transfer heat. Additionally, the architectural constructs 112 of the first
and/or second
end caps 108 and 110 can be configured to apply a capillary pressure for a
particular
substance entering or exiting the conduit. For example, the composition,
spacing,
dopants, and/or thicknesses of the layers 114 of the architectural constructs
112 can
be modulated to selectively draw a particular substance between the layers
114. In
selected embodiments, the architectural construct 112 can include a first zone
of layers
114 that are configured for a first substance and a second zone of layers 114
that are
configured for a second substance to selectively remove and/or add two or more
desired substances from the conduit 102.
[0041] In further embodiments, the second end cap 110 can utilize the sorbtive
properties of the architectural constructs 112 to selectively load a desired
constituent of
the working fluid 122 between the layers 114. The construction of the
architectural
construct 112 can be manipulated to obtain the requisite surface tension to
load almost
any element or soluble. For example, the layers 114 can be preloaded with
predetermined dopants or materials to adjust the surface tension of adsorption
along
these surfaces. In certain embodiments, the layers 114 can be preloaded with
CO2
such that the architectural construct 112 can selectively mine CO2 from the
working
fluid 122 as heat releases through the second end cap 110. In other
embodiments, the
layers 114 can be spaced apart from one another by a predetermined distance,
include
a certain coating, and/or otherwise be arranged to selectively load the
desired
constituent. In some embodiments, the desired constituent adsorbs onto the
surfaces
of individual layers 114, while in other embodiments the desired constituent
absorbs
into zones between the layers 114. In further embodiments, substances can be
purposefully fed into the conduit 102 from the input portion 104 (e.g.,
through the first
end cap 108) such that the added substance can combine or react with the
working
fluid 122 to produce the desired constituent. Thus, the architectural
construct 112 at
the second end cap 110 can facilitate selective mining of constituents.
Additionally, the
architectural construct 112 can remove impurities and/or other undesirable
solubles
that may have entered the conduit 102 and potentially interfere with the
efficiency of
the device 200.
[0042] Similarly, in selected embodiments, the architectural construct 112 at
the
first end cap 110 can also selectively load desired compounds and/or elements
to
13
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
prevent them from ever entering the conduit 102. For example, the
architectural
construct 112 can filter out paraffins that can impede or otherwise interfere
with the
heat transfer of the device 200. In other embodiments, the devices 200 can
include
other filters that may be used to prevent certain materials from entering the
conduit
102.
[0043] Moreover, similar to selective loading of compounds and elements, the
architectural construct 112 at the first and second end caps 108 and 110 may
also be
configured to absorb radiant energy of a desired wavelength. For example, the
layers
114 can have a certain thickness, composition, spacing to absorb a particular
wavelength of radiant energy. In selected embodiments, the architectural
construct
112 absorbs radiant energy of a first wavelength and converts it into radiant
energy of a
second wavelength, retransmitting at least some of the absorbed energy. For
example,
the layers 114 may be configured to absorb ultraviolet radiation and convert
the
ultraviolet radiation into infrared radiation.
[0044] Additionally, the layers 114 can also catalyze a reaction by
transferring
heat to a zone where the reaction is to occur. In other implementations, the
layers 114
catalyze a reaction by transferring heat away from a zone where a reaction is
to occur.
For example, heat may be conductively transferred into the layers 114 (e.g.,
as
discussed in U.S. Patent Application No. 12/857,515, filed August 16, 2010,
entitled
"APPARATUSES AND METHODS FOR STORING AND/OR FILTERING A
SUBSTANCE" which is incorporated by reference herein in its entirety) to
supply heat
to an endothermic reaction within a support tube of the layers 114. In some
implementations, the layers 114 catalyze a reaction by removing a product of
the
reaction from the zone where the reaction is to occur. For example, the layers
114 may
absorb alcohol from a biochemical reaction within a central support tube in
which
alcohol is a byproduct, thereby expelling the alcohol on outer edges of the
layers 114,
and prolonging the life of a microbe involved in the biochemical reaction.
[0045] Figure 3A is schematic cross-sectional view of a thermal transfer
device 300
("device 300") operating in a first direction in accordance with a further
embodiment of
the present technology, and Figure 3B is a schematic cross-sectional view of
the
device 300 of Figure 3A operating in a second direction opposite the first
direction.
Several features of the device 300 are generally similar to the features of
the devices
14
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
100 and 200 shown in Figures 1-2B. For example, the device 300 can include the
conduit 102, the first and second end caps 108 and 110, and the architectural
construct
112. As shown in Figures 3A and 3B, the sidewall 120 of the device 300 can
include
two architectural constructs 112: a first architectural construct 112a having
layers 114
oriented parallel to the longitudinal axis 118 of the conduit 102 and a second
architectural construct 112b radially inward from the first architectural
construct 112a
and having layers 114 oriented perpendicular to the longitudinal axis 118. The
layers
114 of the first architectural construct 112a can perform a capillary action,
and the
layers 114 of the second architectural construct 112b can form thermally
conductive
passageways that transfer heat away from the side of the conduit 102 and
thereby
increase the temperature differential between the input and output portions
104 and
106.
[0046] Similar to the device 100 shown in Figure 1, the device 300 can also
operate
when the direction of heat flow changes and the input and output portions 104
and 106
are inverted. As shown in Figure 3A, for example, the device 300 can absorb
heat at
the first end cap 108 to vaporize the working fluid 122 at the input portion
104, transfer
the heat via the vapor phase 122a of the working fluid 122 through the conduit
102,
and expel heat from the second end cap 110 to condense the working fluid 122
at the
output portion 106. As further shown in Figure 3A, the liquid phase 122b of
the working
fluid 122 can move between the layers 114 of the first architectural construct
112b by
capillary action as described above with reference to Figure 1. In other
embodiments,
the sidewall 120 can include a different capillary structure (e.g., cellulose)
that can drive
the liquid phase 122b from the output portion 106 to the input portion 104. As
shown in
Figure 3B, the conditions can be reversed such that heat enters the device 300
proximate to the second end cap 110 and exits the device 300 proximate to the
first
end cap 108. Advantageously, as discussed above, the dual-direction vapor-
condensation cycle of the working fluid 122 accommodates environments in which
the
locations of the heat source and the heat sink reverse.
[0047] Figures 4A-4C are schematic views of thermal transfer devices 400A-C,
respectively, configured in accordance with embodiments of the present
technology.
Referring to Figures 4A-C together, several features of the devices 400A-C are
generally similar to the features of the devices 100, 200, and 300 shown in
Figures 1-
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
3B. For example, the devices 400A-C can include the conduit 102, the first and
second
end caps 108 and 110, the architectural constructs 112, and the liquid
reservoir 124
(reference numbers not shown in Figures 4A and 4B for clarity). The devices
400A-C
shown in Figures 4A-C rotate at an angular velocity w, and thus undergo a
centrifugal
force. In the embodiments shown in Figures 4A and 4B, the devices 400A-B can
be
spaced apart from an axis of rotation 430. Referring to Figure 4A, for
example, when
the heat influx is radially outward from the heat output (i.e., the input
portion is radially
outward from the output portion), the device 400A can utilize centrifugal
force to return
the liquid phase 122b of the working fluid 122 radially outward to the input
portion 104.
When the heat output is radially outward from the heat input, such as the
embodiment
shown in Figure 4B, the device 400B must utilize a capillary action or another
force to
overcome the centripetal force and drive the liquid phase 122b radially inward
to the
input portion.
[0048] As the shown in Figure 4C, in other embodiments, the axis of rotation
430 can
be spaced along the length of the device 400C. In the embodiment shown in
Figure
4C, heat enters the device 400C at both the first and second end caps 108 and
110,
and heat exits the device 400C at the axis of rotation 430. As shown in Figure
4A, this
configuration creates a double vaporization-condensation cycle of the working
fluid
122. For example, the working fluid 122 moves through the conduit 102 until it
reaches
the axis of rotation 430. From there, the device 400C expels from the output
portion
106 such that the working fluid 122 condenses and returns to the input portion
104 via
the centripetal force. In other embodiments, the input portion 104 and the
output
portion 106 are inverted such that the double vaporization-condensation cycle
operates
in reverse of that shown in Figure 4C.
[0049] In operation, the devices 400A-C shown in Figures 4A-4C can effectuate
heat
transfer in rotating environments, such as windmills, wheels, and/or other
rotating
devices. In certain embodiments, the device 400A-C can be installed in a
centrifuge.
The working fluid 122 can be plasma, blood, and/or other bodily fluids, and
the
architectural construct 112 can be included at the second end cap 110 to
selectively
mine the constituents of bodily fluid to measure the levels of the constituent
and/or aid
in diagnosis. In other embodiments, the devices 400A-C can utilize other
16
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
characteristics of the architectural constructs 112 in conjunction with the
rotating
environment.
[0050] Figure 5A is a schematic view of a thermal transfer system 500 ("system
500") shown in a representative environment in accordance with an embodiment
of the
present technology, and Figure 5B is an enlarged operational view of a portion
of the
system 500 of Figure 5A. The system 500 can include a solar collector 552
proximate
to the surface of a body of water, such as the ocean, a movable pickup bell
554
proximate to a gas hydrate deposit 553, and an appendage 556 connecting the
solar
collector 552 and the bell 554. The appendage 556 can include a thermal
transfer
device 550 ("device 550") that has generally similar features as the device
100
described above with reference to Figure 1. For example, as shown in Figure
5B, the
device 550 can move the vapor phase 122a of the working fluid 122 down the
conduit
102 and return the liquid phase 122b via capillary action. In other
embodiments, the
liquid phase can be returned to the input portion 104 using another suitable
method.
[0051] In the embodiment shown in Figure 5A, the device 550 can be utilized to
transfer heat from the solar collector 552 to the bell 554 to heat the gas
hydrate deposit
553. The heated gas hydrate deposit 553 can release the gas hydrate (e.g.,
methane
hydrate) up a conduit 558 to a methane recovery director 560. Accordingly, the
system
500 can harness solar energy, transfer it via the device 550 to the methane
hydrate
deposit 553, and initiate the release of the methane hydrate. Further
operation of such
a methane hydrate collection system is described in U.S. Patent Application
No.
12/857,228, entitled GAS HYDRATE CONVERSION SYSTEM FOR HARVESTING
HYDROCARBON HYDRATE DEPOSITS, filed August 16, 2010, which is herein
incorporated by reference in its entirety.
[0052] It is also contemplated that the heating of water that is a product of
the
decomposition of gas hydrates may be accomplished using a system such as that
which is disclosed in U.S. Patent Application No. 12/857,546, filed on August
16, 2010,
and entitled INCREASING THE EFFICIENCY OF SUPPLEMENTED OCEAN
THERMAL ENERGY CONVERSION (SOTEC) SYSTEMS, which is incorporated by
reference in its entirety as if fully set forth herein. In this instance it is
optionally
intended to evaporate such collected water for further energy conversion and
17
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
purification of water inventories first collected in conjunction with
decomposition of gas
hyd rates.
[0053] Figure 6A is a schematic view of a thermal transfer system 600 ("system
600") shown in another representative environment in accordance with an
embodiment
of the present technology, and Figure 6B is an enlarged operational view of a
portion of
the system 600 of Figure 6A. The system 600 can include a thermal transfer
device
650 ("device 650") that absorbs heat from a geothermal formation 660 and
expels heat
to a factory, building, or other structure 662. The device 650 can be
generally similar to
the devices 200 described with reference to Figures 2A and 2B. For example, as
shown in Figure 6B, the device 650 can drive the vapor phase 122a of the
working fluid
122 up the conduit 102 and return the liquid phase 122b to a hot interface
(e.g., the
first end cap 108, not shown) via a gravitational force. In operation, the
device 650 can
capture the thermal energy supplied by the geothermal formation 660 and
transfer it to
the structure 662 where it can be used to provide heat, electricity, and/or
otherwise
utilize the thermal energy transferred to the structure 662. In other
embodiments, the
system 600 can be used to transfer heat away from the structure 662 and/or
other
formation. For example, the system 600 can be installed such that the
structure 662
transmits heat to the device 650 and transfers it to another structure,
engine, and/or
other location spaced apart from the structure 662. As another example, the
system
600 can be installed such that the device 650 transfers heat away from
permafrost and
into a heat sink not negatively affected by additional heat (e.g., outer
space).
[0054] Figure 7A is a schematic view of a thermal transfer system 700 ("system
700") shown in yet another representative environment in accordance with an
embodiment of the present technology, and Figures 7B and 7C are enlarged
operational views of portions of the system 700 of Figure 7A. The system 700
can
include a thermal transfer device 750 ("device 750") that includes features
generally
similar as the devices 100 and 300 described above with reference to Figures
1, 3A,
and 3B such that the device 750 can operate the vaporization-condensation
cycle in
both directions. For example, as shown in Figure 7B, under a first condition,
the device
750 can drive the vapor phase 122a of the working fluid 122 down the conduit
102 and
return the liquid phase 122b to the hot interface by capillary action. As
shown in Figure
7C, under the second condition the device 750 can drive the vapor phase 122a
of the
18
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
working fluid 122 in the reverse direction, up the conduit 102 and return the
liquid
phase 122b to the hot interface using capillary action and/or gravitational
force.
[0055] This dual-direction system 700 can be used in environments with
reversing or
otherwise changing temperature differentials. As shown in Figure 7A, for
example, the
system 700 can operate under the first condition during warmer seasons to
absorb
solar energy via a solar collector 766. An aquifer 768 positioned at the
output portion
106 of the conduit 102 can function as a natural thermal accumulator that can
store the
heat transferred to it from the system 700. As seasons change, the system 700
can
reverse directions and operate under the second condition to transfer the heat
of the
aquifer 768 to transfer the stored heat to a factory 767 and/or other
structure or device
that can utilize the thermal energy. Thus, the dual-directional system 700
provides an
efficient way to capture solar energy and store it for a later use (e.g.,
electricity during
the winter). Additionally, in certain embodiments, the portion of the device
750 at the
aquifer 768 (e.g., the first or second end caps described above) can include
an
architectural construct (e.g., the architectural constructs 112 described
above) that can
use its capillary and/or sorbtive properties to selectively filter toxins from
aquifer and
thereby rehabilitate a previously hazardous aquifer.
[0056] Figure 7D is a schematic view of the system 700 shown in Figures 7A-7C
in
another representative environment in accordance with an embodiment of the
present
technology. As shown in Figure 7D, the device 750 can be installed between a
dwelling 780 and an insulated structure 782 in the surface of the ground. The
insulated
structure 782 can be filled with sand, gravel, rocks, water, and/or other
suitable
materials that can absorb and store heat. In operation, the system 700 can
absorb
heat with a solar collector 784, transfer heat to the insulated structure 782
via the
device 750, and accumulate the heat in the insulated structure 782. The heat
stored in
the insulated structure 782 can later be used to provide heat or other forms
of energy
to the dwelling 780. Accordingly, as discussed above, the dual-direction
system 700
provides an efficient way to accumulate heat for later use.
[0057] Figure 8A is an enlarged schematic cross-sectional view of a thermal
transfer
system 800a ("system 800a") in a representative environment in accordance with
a
further embodiment of the present technology. The system 800a can include a
thermal
transfer device 850 ("device 850") that has features generally similar to the
devices
19
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
described above. For example, as shown in Figure 8A, the device 850 can
include the
architectural construct 112 with layers 114 arranged orthogonally to the
sidewall 120 to
transfer heat away from the conduit 102. As shown in Figure 8A, the system
800a can
also include one or more external conduits 890 positioned along at least a
portion of
the device 850. The external conduits 890 can include openings 891 in fluid
communication with the environment outside of the device 850. In some
embodiments,
the conduits 890 can be made from the architectural construct 112 and
configured to
selectively draw in desired substances from outside the conduit 102. For
example, the
architectural construct 112 can use capillary action to drive a preselected
liquid through
the external conduits 890 and/or use sorbtive properties to adsorb a
preselected
constituent from the liquid. The preselected liquids and/or constituents can
be
collected in a harvest located along any portion of the external conduits 890
(e.g.,
proximate to either of the end caps). In other embodiments, the external
conduits 890
can be made from other materials (e.g., plastic tubing, wick structures, etc.)
to draw in
chemicals, minerals, and/or other substances from outside the device 850.
[0058] As shown in Figure 8A, the system 800a can absorb heat from at least
two
heat sources spaced apart from one another and expels heat toward a single
heat sink
to generate two vaporization-condensation cycles within the device 850. In the
embodiment illustrated in Figure 8A, for example, the device 850 is installed
between a
solar collector 882 and a submarine geothermal formation 884 and releases heat
at a
submarine heat sink (e.g., proximate to an ocean floor 886). The system 800a
thus
includes one vaporization-condensation cycle spaced above the ocean floor 886
and
one spaced below the ocean floor 886. Advantageously, the heat outputs from
the two
vaporization-condensation cycles can combine to generate a greater heat output
from
the system 800a than either cycle could individually. In selected embodiments,
the
system 800a can harvest thermal energy released from the device 850 to power
turbines, another engine, and/or other suitable devices above or below the
water.
[0059] The system 800a can also utilize the increased heat output of the dual
vaporization-condensation cycles to release gas hydrates (e.g., methane
hydrates)
from their present state (i.e., ice crystals) such as described in U.S. Patent
Application
No. 12/857,228, entitled GAS HYDRATE CONVERSION SYSTEM FOR HARVESTING
HYDROCARBON HYDRATE DEPOSITS, filed August 16, 2010. As shown in Figure
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
8A, for example, the system 800a can be positioned proximate to a deposit 888
of gas
hydrates at the ocean floor 886 such that the heat output of the system 800a
can
increase the local temperature of the deposit 888, melt the gas hydrate ice
crystals,
and release the gas hydrates. The gas hydrates can be drawn through the
external
conduits 890 to a harvest where they can be used for fuel, manufacturing
materials,
and/or other suitable applications. In some embodiments, carbon dioxide can
drive the
released gas hydrate through the external conduits 890. In other embodiments,
the
architectural construct 112 can be configured to selectively draw up the gas
hydrate
using capillary action. In other embodiments, the gas hydrates can be drawn
through
the external conduits 890 by a pump and/or other suitable liquid driving
device.
[0060] Advantageously, the increased heat output of the system 800a can
increase
the local temperature of the deposit 888 faster and higher than a single
vaporization-
condensation cycle system to more efficiently harvest the gas hydrates.
Additionally,
as shown in Figure 8A, the heat transferred outward from the architectural
construct
112 positioned at the sidewall 120 of the conduit 102 can transfer additional
heat to the
deposit 888 to further speed the release of the gas hydrates. The increased
heat
output of the system 800a can also increase the local temperature of a greater
area of
the deposit 888. For example, in some embodiments, the system 800a warms
several
square miles of the deposit 888 at one time. Therefore, the dual vaporization-
condensation cycle increases the zone of influence that the system 800a can
have
over the deposit 888.
[0061] Figure 8B is a schematic view of a thermal transfer system 800b
("system
800b") in a representative environment in accordance with an embodiment of the
disclosure. The system 800b can include generally similar features as the
system
800a discussed above. For example, the system 800b can include the device 850
and
the external conduit 890 configured to draw in desired fluids from the
external
environment. Additionally, the system 800b can be installed between two heat
sources
(e.g., the solar collector 882 and the geothermal formation 884) spaced apart
from one
another and a heat sink (e.g., proximate to the ocean floor 886) therebetween
to
effectuate two vaporization-condensation cycles that have a combined heat
output.
Similar to the system 800a described above, the system 800b shown in Figure 8B
can
transfer heat from the device 850 to a methane hydrate deposit 894. As
discussed
21
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
above, the dual vaporization-condensation cycle device 850b has a broad zone
of
influence over the methane deposit 894 such that the system 800b can
efficiently
harvest methane above and/or below the surface of the water.
[0062] In the embodiment illustrated in Figure 8B, the system 800b further
includes a
barrier film 896a over the zone of influence of the system 800b and a methane
conduit
898 configured to receive methane from beneath the barrier film 896a. The
barrier film
896a can be made of a non-pervious film, such as polyethylene, that prevents
methane
from escaping from the system 800b and releasing dangerous greenhouse gases
into
the atmosphere. In selected embodiments, the barrier film 896 can be
configured to
distribute heat released from the device 850 to further increase the zone of
influence of
the system 800b. As further shown in Figure 8B, the system 800b can also
include
second barrier film 896b at the surface of the water to further ensure methane
does not
escape the system 800b. As further shown in Figure 8B, the system 800b can
include
an optional permeable film 897 that can permit methane to pass through it and
block
carbon dioxide and water such that only methane flows between the barrier film
896a
and the methane permeable film 897 to the methane conduit 898. Accordingly,
the
methane can flow through the methane conduit 898 where the methane can be
harvested for fuel, carbon materials, and/or other suitable purposes. The
water and
carbon dioxide blocked by the methane permeable layer 897 can flow up the
external
conduit 890 using lift from the carbon dioxide and/or capillary action. In
selected
embodiments, the external conduit 890 can be made from an architectural
construct
loaded with carbon dioxide such that the architectural construct 112 adsorbs
carbon
dioxide as it travels through the external conduit 890 and only the water is
delivered
from the external conduit 890. In other embodiments, the system 800b can be
installed
such that the external conduit 890, rather than the methane conduit 898, draws
up the
methane hydrate. In other embodiments, the system 800b can be used to harvest
another gas hydrate and/or other substance released by heating the ocean floor
886
and/or other geothermal formation.
[0063] In selected embodiments, the system 800b can include an underwater
methane harvest that can be used to drive a turbine 895 used to accelerate the
flow of
the working fluid 122 through the device 850. In other embodiments, the
methane can
be used to drive other underwater systems. In further embodiments, the system
800
22
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
can include a thermal deposit at the heat output of the system 800b to store
heat for
subsequent methane hydrate collection and/or drive systems above and/or below
the
surface of the water. For example, the thermal harvest can collect heat
released from
the system 800b and transport it via conduits to portions of the methane
deposit 894
spaced beyond the zone of influence of the system 800b and/or other methane
deposits.
[0064] As further shown in Figure 8B, the system 800b can further include an
oxygen
conduit 899 and an engine 801. The oxygen conduit 899 can drive oxygen from
above
the water or another oxygen source and deliver it to the engine 801 installed
below the
barrier layer 896a. The engine 803 can burn the oxygen delivered by the oxygen
conduit 899 and the hydrogen produced as the system 800b (i.e., CH4 + HEAT - C
+
2H2) to provide hot steam to the methane deposit 894. The additional heat from
the
engine 803 can liberate additional methane. The engine 801 can be any suitable
engine that delivers hot steam, such as a turbine.
[0065] Figure 9A is a cross-sectional view of a thermal transfer system 900
("system 900") in an additional representative environment in accordance with
an
embodiment of the present technology, and Figure 9B is an enlarged view of
detail 9B
of Figure 9A. The system 900 can include a thermal transfer device 950
("device 950")
that includes features generally similar to the devices described above. The
system
900 shown in Figures 9A and 9B is installed in a microscopic environment,
rather than
the macroscopic systems shown in Figures 5A-8B, for use as a sensor or other
type of
monitor as described in U.S. Patent Application entitled METHODS, DEVICES, AND
SYSTEMS FOR DETECTING PROPERTIES OF TARGET SAMPLES (Attorney Docket
No. 69545-8801US1), filed February 14, 2011, concurrently herewith and
incorporated
by reference in its entirety. In other embodiments, the system 900 can be used
for
other microscopic applications that benefit from heat transfer.
[0066] In the embodiment illustrated in Figures 9A and 9B together, a tube 903
and a fitting 905 are sealed together. For example, the tube 903 and the
fitting 905 are
sealed together by tightening a nut 907. One or more devices 950 can be
positioned
between a tube 903 and the fitting 907 to test for incipient leaks of a fluid
909 running
through the tube 903. For example, the devices 950 can sense the presence of
the
fluid 909 and/or the composition of the fluid 909. In selected embodiments,
the device
23
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
950 can include a sensor positioned within an architectural construct (e.g.,
the
architectural construct 112 described above). The architectural construct can
be
configured to selectively adsorb a predetermined constituent of the fluid 909
such that
the sensor can determine the presence and/or trend in the presence of the
predetermined constituent. In other embodiments, the architectural construct
can be
configured to selectively transfer a target sample of the fluid 909 or a
constituent
thereof to a reservoir (e.g., the liquid reservoir 124 described above) that
includes a
sensor to monitor or otherwise test the sample. In further embodiments, the
devices
950 can be otherwise positioned to monitor other aspects of the system 900.
[0067] Figure 10 is a schematic view of a thermal transfer device 1000
configured in
accordance with a further embodiment of the present technology. The device
1000 can
include features and functions generally similar to the devices described
above.
However, the device 1000 shown in Figure 10 has a different aspect ratio than
the
devices shown above. More specifically, the first and second end caps 108 and
110
and the sidewall 120 are closer in length such that the device 1000 forms a
wide
conduit 102. Such an aspect ratio is well suited for transferring heat through
a room.
For example, the device 1000 can be used for dry cleaning. Garments can be
positioned within the conduit 102, and the vapor phase 122a of the working
fluid 122
(e.g., C02) can capture dirt, oils, and other filth from the garments as it
moves through
the conduit 102. The filth can be filtered from the device 1000 at the second
end cap
110 with the architectural construct 112 and/or another suitable filter. Thus,
rather than
conventional dry cleaning methods that use toxic chemicals to clean clothes,
the heat
transfer provided by the device can be utilized to clean clothes. In other
embodiments,
the device 1000 can be used for other suitable heat transfer methods and/or
the aspect
ratio of the device 1000 can have other suitable variations.
[0068] Figure 11 is a schematic view of a thermal transfer system 1100
("system
1100") shown in a representative environment in accordance with yet another
embodiment of the present technology. The system 1100 shown in Figure 11 can
include a thermal transfer device 1150 ("device 1150") that has features
generally
similar to the thermal transfer devices described above. For example, the
device 1150
can transfer heat utilizing a vaporization-condensation cycle of the working
fluid 122
within the conduit 102. As shown in Figure 11, the system 1100 can further
include a
24
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
solar collector 1121 configured to concentrate heat and deliver it to a first
pipe 1123. A
pump 1125 can be operably coupled to the first pipe 1123 to drive a fluid
(e.g., the
working fluid 122) within the first pipe 1123 to a first heat exchanger 1127
proximate to
the input portion 104 of the device 1150. The first heat exchanger 127 can
heat and
vaporize the fluid within the first pipe 1123 and thereby deliver heat to the
input portion
104 of the device 1150. As shown in Figure 11, the working fluid 122 can
vaporize at
the input portion 104 and circulate through the device 1150 to release heat at
the
output portion 106. The device 1150 can utilize the released heat for domestic
water
heating, crop drying, and other suitable applications.
[0069] In selected embodiments, the working fluid 122 flows through the first
pipe
1121 such that the device 1150 can apply capillary pressure to the working
fluid 122
using the architectural construct 112 such that the working fluid 122 is drawn
into the
conduit 102. In other embodiments, the vaporized fluid emitted by the heat
exchanger
1127 can be filtered by the architectural construct 112 to selectively admit
one or more
desired substances (e.g., chemicals that catalyze with the working fluid 122)
into the
conduit 102.
[0070] As shown in Figure 11, the system 1100 can further include a second
heat
source 1129 (i.e., separate from the solar collector 1121) that can be used in
conjunction with the solar collector 1121 to increase the heat influx to the
device 1150
and/or to replace the solar collector 1121 when solar heating is unavailable
or not
desired. The second heat source 1129 can be a wind generator as shown in
Figure
11, resistive or inductive heating by grid power, and/or other suitable heat
transmitting
devices. In the embodiment illustrated in Figure 11, the second heat source
1129 is
coupled to a second pipe 1133 and a second heat exchanger 1131 that transfer
heat to
the input portion 104 of the device 1150. In other embodiments, the second
heat
source 1129 is connected to the first pipe 1121 and the first heat exchanger
1123.
[0071] Additionally, as shown in Figure 11, the system 1100 can further
include a
supplementary processing portion 1135 positioned proximate to the input
portion 104
such that heat is transmitted from the first and/or second heat exchangers
1127 and
1131 to the supplementary processing portion 1135. The supplementary
processing
portion 1135 can be used to provide additional manufacturing and/or services
to the
system 1100. For example, the supplementary processing portion 1135 can be
used
2s
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
for drying fruit, dehydrating maple syrup to provide surplus water, and/or
removing
preselected substances such as flavinoids by the architectural construct 112.
[0072] The present application incorporates by reference in its entirety the
subject
matter of the following applications: U.S. Patent Application, entitled
METHODS AND
APPARATUSES FOR DETECTION OF PROPERTIES OF FLUID CONVEYANCE
SYSTEMS (Attorney Docket No. 69545-8801US1); U.S. Patent Application, entitled
ARCHITECTURAL CONSTRUCT HAVING FOR EXAMPLE A PLURALITY OF
ARCHITECTURAL CRYSTALS (Attorney Docket No. 69545-8701 US); U.S. Patent
Application No. 12/857,546, filed on August 16, 2010, and entitled INCREASING
THE
EFFICIENCY OF SUPPLEMENTED OCEAN THERMAL ENERGY CONVERSION
(SOTEC) SYSTEMS; U.S. Patent Application No. 12/857,228, entitled GAS HYDRATE
CONVERSION SYSTEM FOR HARVESTING HYDROCARBON HYDRATE
DEPOSITS, filed August 16, 2010, all of which are herein incorporated by
reference in
their entirety.
[0073] From the foregoing, it will be appreciated that specific embodiments of
the
disclosure have been described herein for purposes of illustration, but that
various
modifications may be made without deviating from the spirit and scope of the
invention.
For example, any of the thermal transfer devices discussed above can have a
different
aspect ratio (e.g., between the sidewall 120 and the first and second end caps
108 and
110) than those shown in Figures 1-11 to accommodate differing applications.
Certain
aspects of the new technology described in the context of particular
embodiments may
be combined or eliminated in other embodiments. For example, the thermal
transfer
devices shown in Figures 3A-4C and 6A-10 can include the liquid reservoir
and/or
controller described with reference to Figure 1. Additionally, while
advantages
associated with certain embodiments of the new technology have been described
in
the context of those embodiments, other embodiments may also exhibit such
advantages, but not all of the embodiments within the scope of the technology
necessarily exhibit such advantages. Accordingly, the disclosure and
associated
technology can encompass other embodiments not expressly shown or described
herein. Moreover, unless the context clearly requires otherwise, throughout
the
description and the claims, the words "comprise," "comprising," and the like
are to be
construed in an inclusive sense as opposed to an exclusive or exhaustive
sense; that is
26
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
to say, in a sense of "including, but not limited to." Words using the
singular or plural
number also include the plural or singular number, respectively. When the
claims use
the word "or" in reference to a list of two or more items, that word covers
all of the
following interpretations of the word: any of the items in the list, all of
the items in the
list, and any combination of the items in the list.
[0074] Features of the various embodiments described above can be combined to
provide further embodiments. All of the U.S. patents, U.S. patent application
publications, U.S. patent applications, foreign patents, foreign patent
applications and
non-patent publications referred to in this specification and/or listed in the
Application
Data Sheet are incorporated herein by reference, in their entirety. Aspects of
the
disclosure can be modified, if necessary, to employ fuel injectors and
ignition devices
with various configurations, and concepts of the various patents,
applications, and
publications to provide yet further embodiments of the disclosure.
[0075] These and other changes can be made to the disclosure in light of the
above-detailed description. In general, in the following claims, the terms
used should
not be construed to limit the disclosure to the specific embodiments disclosed
in the
specification and the claims, but should be construed to include all systems
and
methods that operate in accordance with the claims. Accordingly, the invention
is not
limited by the disclosure, but instead its scope is to be determined broadly
by the
following claims.
[0076] To the extent not previously incorporated herein by reference, the
present
application incorporates by reference in their entirety the subject matter of
each of the
following materials: U.S. Patent Application No. 12/857,553, filed on August
16, 2010
and titled SUSTAINABLE ECONOMIC DEVELOPMENT THROUGH INTEGRATED
PRODUCTION OF RENEWABLE ENERGY, MATERIALS RESOURCES, AND
NUTRIENT REGIMES; U.S. Patent Application No. 12/857,553, filed on August 16,
2010 and titled SYSTEMS AND METHODS FOR SUSTAINABLE ECONOMIC
DEVELOPMENT THROUGH INTEGRATED FULL SPECTRUM PRODUCTION OF
RENEWABLE ENERGY; U.S. Patent Application No. 12/857,554, filed on August 16,
2010 and titled SYSTEMS AND METHODS FOR SUSTAINABLE ECONOMIC
DEVELOPMENT THROUGH INTEGRATED FULL SPECTRUM PRODUCTION OF
RENEWABLE MATERIAL RESOURCES USING SOLAR THERMAL; U.S. Patent
27
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
Application No. 12/857,502, filed on August 16, 2010 and titled ENERGY SYSTEM
FOR DWELLING SUPPORT; Attorney Docket No. 69545-8505.US00, filed on
February 14, 2011 and titled DELIVERY SYSTEMS WITH IN-LINE SELECTIVE
EXTRACTION DEVICES AND ASSOCIATED METHODS OF OPERATION; U.S.
Patent Application No. 61/401,699, filed on August 16, 2010 and titled
COMPREHENSIVE COST MODELING OF AUTOGENOUS SYSTEMS AND
PROCESSES FOR THE PRODUCTION OF ENERGY, MATERIAL RESOURCES AND
NUTRIENT REGIMES; Attorney Docket No. 69545-8601.US00, filed on February 14,
2011 and titled CHEMICAL PROCESSES AND REACTORS FOR EFFICIENTLY
PRODUCING HYDROGEN FUELS AND STRUCTURAL MATERIALS, AND
ASSOCIATED SYSTEMS AND METHODS; Attorney Docket No. 69545-8602.USOO,
filed on February 14, 2011 and titled REACTOR VESSELS WITH TRANSMISSIVE
SURFACES FOR PRODUCING HYDROGEN-BASED FUELS AND STRUCTURAL
ELEMENTS, AND ASSOCIATED SYSTEMS AND METHODS; Attorney Docket No.
69545-8603.USOO, filed on February 14, 2011 and titled CHEMICAL REACTORS
WITH RE-RADIATING SURFACES AND ASSOCIATED SYSTEMS AND METHODS;
Attorney Docket No. 69545-8605.USOO, filed on February 14, 2011 and titled
CHEMICAL REACTORS WITH ANNULARLY POSITIONED DELIVERY AND
REMOVAL DEVICES, AND ASSOCIATED SYSTEMS AND METHODS; Attorney
Docket No. 69545-8606.US00, filed on February 14, 2011 and titled REACTORS FOR
CONDUCTING THERMOCHEMICAL PROCESSES WITH SOLAR HEAT INPUT, AND
ASSOCIATED SYSTEMS AND METHODS; Attorney Docket No. 69545-8608.US00,
filed on February 14, 2011 and titled INDUCTION FOR THERMOCHEMICAL
PROCESS, AND ASSOCIATED SYSTEMS AND METHODS; Attorney Docket No.
69545-8611.US00, filed on February 14, 2011 and titled COUPLED
THERMOCHEMICAL REACTORS AND ENGINES, AND ASSOCIATED SYSTEMS
AND METHODS; U.S. Patent Application No. 61/385,508, filed on September 22,
2010
and titled REDUCING AND HARVESTING DRAG ENERGY ON MOBILE ENGINES
USING THERMAL CHEMICAL REGENERATION; Attorney Docket No. 69545-
8616.USOO, filed on February 14, 2011 and titled REACTOR VESSELS WITH
PRESSURE AND HEAT TRANSFER FEATURES FOR PRODUCING HYDROGEN-
BASED FUELS AND STRUCTURAL ELEMENTS, AND ASSOCIATED SYSTEMS
AND METHODS; Attorney Docket No. 69545-8701.USOO, filed on February 14, 2011
28
CA 02789703 2012-08-13
WO 2011/100731 PCT/US2011/024814
and titled ARCHITECTURAL CONSTRUCT HAVING FOR EXAMPLE A PLURALITY
OF ARCHITECTURAL CRYSTALS; U.S. Patent Application No. 12/806,634, filed on
August 16, 2010 and titled METHODS AND APPARATUSES FOR DETECTION OF
PROPERTIES OF FLUID CONVEYANCE SYSTEMS; Attorney Docket No. 69545-
8801.US01, filed on February 14, 2011 and titled METHODS, DEVICES, AND
SYSTEMS FOR DETECTING PROPERTIES OF TARGET SAMPLES; Attorney Docket
No. 69545-9002.USOO, filed on February 14, 2011 and titled SYSTEM FOR
PROCESSING BIOMASS INTO HYDROCARBONS, ALCOHOL VAPORS,
HYDROGEN, CARBON, ETC.; Attorney Docket No. 69545-9004.USOO, filed on
February 14, 2011 and titled CARBON RECYCLING AND REINVESTMENT USING
THERMOCHEMICAL REGENERATION; Attorney Docket No. 69545-9006.USOO, filed
on February 14, 2011 and titled OXYGENATED FUEL; U.S. Patent Application No.
61/237,419, filed on August 27, 2009 and titled CARBON SEQUESTRATION; U.S.
Patent Application No. 61/237,425, filed on August 27, 2009 and titled
OXYGENATED
FUEL PRODUCTION; Attorney Docket No. 69545-9102.USOO, filed on February 14,
2011 and titled MULTI-PURPOSE RENEWABLE FUEL FOR ISOLATING
CONTAMINANTS AND STORING ENERGY; U.S. Patent Application No. 61/421,189,
filed on December 8, 2010 and titled LIQUID FUELS FROM HYDROGEN, OXIDES OF
CARBON, AND/OR NITROGEN; AND PRODUCTION OF CARBON FOR
MANUFACTURING DURABLE GOODS; and Attorney Docket No. 69545-9105.USOO,
filed on February 14, 2011 and titled ENGINEERED FUEL STORAGE,
RESPECIATION AND TRANSPORT.
29