Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
THERAPY SHOE
Technical Field
The present disclosure generally relates to systems and methods for providing
medical therapy and specifically to systems and methods for assisting the
stimulation of
blood flow, reducing edema, reducing leg cramping, treating plantar fasciitis
in the foot,
and/or promoting recovery from athletic excursion. More specifically, the
present disclosure
generally relates to a shoe configured to hold a removable and/or integrated
device that
applies pressure to a wearer's foot to treat various ailments and/or provide
athletic recovery
after physical exercise.
Background
In order to enhance circulation in a person's body, particularly in the feet
and legs,
periodic or cyclic compression of tissue, such as plexus regions of the foot,
at predetermined
timed intervals is beneficial. Under normal circumstances, blood moves up the
legs due to
muscle contraction and general movement of the feet or legs, such as when
walking. If a
person is immobilized, unable to move regularly, or has poor circulation
brought on by
disease, the natural blood return mechanism is impaired, and circulatory
problems such as
ulcers and deep vein thrombosis can occur.
To mitigate these problems, it is desirable to concentrate a compression force
against
veins throughout the legs and/or feet. Current systems are primarily based on
pneumatic
compression devices that squeeze the entire foot, calf, or thigh. These
systems require
significant power, and are inefficient because they provide high levels of
force across the
entire foot or leg rather than focusing in on those areas with the highest
concentration of
blood vessels. In addition, these systems may include air bags that can
rupture at the seam,
especially with high pressure within the bag.
In various current devices, tethered air lines limit mobility, and can lead to
injury
should the person attempt to walk while the device is in use. Further,
existing devices may
not be suited for continuous usage. Users cannot walk with them, or move away
from the
compression unit. The device must be removed before a user can walk.
Additionally,
current devices lack the ability to track and report user usage and
compliance. Also, most
pneumatic devices are quite noisy and can cause irritation of the skin leading
to ulcers.
Furthermore, current devices do not blend in well with ordinary clothing and
stand
out as medical devices. It would be advantageous to provide a therapy shoe
that could be
worn as ordinary clothing and not be an obvious medical aid.
1
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
Summary
In an exemplary embodiment, an item of footwear comprises a housing, a therapy
device disposed within a cavity in the housing, and a coupling portion. The
coupling portion
is configured to retain a portion of a foot.
In another exemplary embodiment, a method for compressing a foot comprises
providing an item of footwear having a therapy device entirely contained
therein, coupling
the item of footwear to the foot, and activating the therapy device.
In another exemplary embodiment, an athletic shoe comprises a housing
configured
with an aperture having a flexible aperture cover. The athletic shoe further
comprises a foot
compression system disposed within the housing. The foot compression system
comprises a
pressure pad extendable to at least partially displace the flexible aperture
cover into contact
with the venous plexus region of a foot. The housing is configured with an
opening on an
external side of the housing. The opening allows access to a control component
of the foot
compression system.
In another exemplary embodiment, an item of footwear comprises a housing
having
an aperture therethrough. The aperture is configured with a flexible aperture
cover. The
item of footwear further comprises a sole, and a therapy device disposed
between the
housing and the sole. The therapy device is configured to extend at least
partially through
the aperture to compress a portion of a foot. The item is footwear is
configured to fully
conceal the presence of the therapy device when the item of footwear is worn.
The contents of this summary section are provided only as a simplified
introduction
to the disclosure, and are not intended to be used to limit the scope of the
appended claims.
Brief Description of the Drawings
With reference to the following description and accompanying drawings:
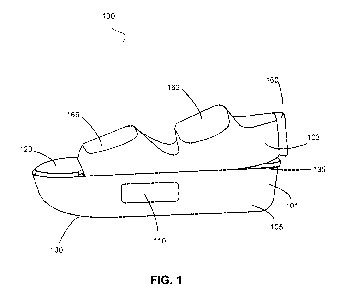
FIG. 1 illustrates a therapy shoe in accordance with an exemplary embodiment;
FIG. 2 illustrates a perspective view of the therapy shoe of FIG. 1;
FIG. 3 illustrates a top view of the therapy shoe of FIGS. 1 and 2;
FIG. 4 illustrates a foot compression system coupled to a therapy shoe in
accordance
with an exemplary embodiment;
FIG. 5 is a block diagram of a foot compression system coupled to a therapy
shoe in
accordance with an exemplary embodiment; and
FIG. 6 illustrates a therapy shoe coupled to a compression garment in
accordance
with an exemplary embodiment.
2
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
Detailed Description
Details of the present disclosure may be described herein in terms of various
components and processing steps. It should be appreciated that such components
and steps
may be realized by any number of hardware and/or software components
configured to
perform the specified functions. For example, a therapy device configured to
aid a portion
of a lower extremity and/or foot compression system, or a system for athletic
recovery, may
employ various medical treatment devices, input and/or output elements and the
like, which
may carry out a variety of functions under the control of one or more control
systems or
other control devices. In addition, principles of the present disclosure may
be practiced in
any number of medical or treatment contexts, and exemplary embodiments
relating to a deep
vein thrombosis treatment system as described herein are merely a few of the
exemplary
applications. For example, principles of the present disclosure may be
utilized in connection
with athletic recovery. Yet further, the principles, features and methods
discussed may be
applied to any suitable medical or other tissue or treatment application.
Moreover, principles of the present disclosure may suitably be combined with
principles of foot compression systems and methods disclosed in U.S. Patent
Application
No. 12/499,473 filed on July 8, 2009, now published as U.S. Patent Application
Publication
No. 2010/0010398 entitled "FOOT COMPRESSION SYSTEM", the contents of which are
hereby incorporated by reference in their entirety. Principles of the present
disclosure may
also suitably be combined with principles of foot compression systems and
methods
disclosed in U.S. Patent Application No. 13/004,754 filed on January 11, 2011
entitled
"FOOT COMPRESSION SYSTEM", the contents of which are hereby incorporated by
reference in their entirety.
An item of therapeutic footwear, for example therapy shoe 100, may be any
device
configured to aid in delivering a compressive force to a portion of a wearer,
for example a
human foot. With reference now to FIGS. 1-3, and in accordance with an
exemplary
embodiment, therapy shoe 100 comprises housing portion 101 and coupling
portion 102.
With further reference now to FIG. 1, and in accordance with an exemplary
embodiment, therapy shoe 100 may comprise housing portion 101 and coupling
portion 102.
In one exemplary embodiment, housing portion 101 retains a therapy device,
such as therapy
device 400 and/or other therapy devices capable of providing medical therapy
to a portion of
a wearer, such as a surface of the foot. In various exemplary embodiments,
therapy device
400 may comprise a foot compression system, for example as disclosed in U.S.
Patent
3
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
Application No. 13/004,754 filed on January 11, 2011 entitled "FOOT
COMPRESSION
SYSTEM". In one exemplary embodiment, a therapy device, such as therapy device
400, is
integral to therapy shoe 100. In one exemplary embodiment, a therapy device,
such as
therapy device 400, is removably coupled to therapy shoe 100.
In various exemplary embodiments, therapy shoe 100 may be configured for use
in
various medical applications, for example increasing blood flow, treating
and/or preventing
deep vein thrombosis, lower leg edema, ulcers, and/or the like. Moreover,
therapy shoe 100
may be configured for use in athletic recovery, for example in accordance with
principles of
athletic recovery as disclosed in U.S. Patent Application No. 13/004,754 filed
on January
11, 2011 entitled "FOOT COMPRESSION SYSTEM".
Although one exemplary embodiment of therapy shoe 100 is described and shown
as
a sandal-like item of footwear, principles of the present disclosure is not
limited to therapy
shoe 100 as explicitly shown. Specifically and without limitation, therapy
shoe 100 can be
any piece of footwear or clothing that is configured to at least partially
contain, fully
contain, and/or couple to therapy device 400. In that regard, therapy shoe 100
can be (but is
not necessarily limited to) in the form of a sock, slipper, sandal, tennis
shoe, running shoe,
athletic shoe, boot, dress shoe, high heel shoe, mule, slingback, clog, etc.
Further, therapy
shoe 100 can be in produced in various design(s) to appeal to various fashion
tastes. In
various exemplary embodiments, therapy shoe 100 is designed to look similar to
another
piece of footwear and/or be difficult to distinguish from (or even
indistinguishable from)
conventional footwear lacking functionality of therapy shoe 100.
In one exemplary embodiment, coupling portion 102, coupled to housing portion
101, secures a therapy device, such as therapy device 400, to a portion of a
wearer, such as a
surface of the foot like the venous plexus region. Housing portion 101 and
coupling portion
102 may be made out of any suitable materials and/or fashioned in any suitable
architecture
and/or geometry, to perform their respective functions.
In one exemplary embodiment and with renewed reference to FIG. 1, housing
portion 101 is located in and/or integral to the base of a wearable therapy
shoe 100. In an
exemplary embodiment, housing portion 101 may be one of enclosed, partially
enclosed or
exposed. In one exemplary embodiment, housing portion 101 may comprise a
housing
cavity (not explicitly shown in FIG. 1) defined by a top housing top surface
135, a housing
bottom surface 130 and at least one housing side surface 105. In this
embodiment, the
dimensions of the housing cavity may be designed to hold a particular device,
for example
4
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
therapy device 400, which may be housed by housing portion 101. For example,
in one
exemplary embodiment, the housing cavity may be designed to be slightly larger
than a
provided therapy device 400. Thus, the housing cavity may effectively mirror
the exterior
dimensions of therapy device 400 housed by the housing cavity. Alternatively,
in one
exemplary embodiment, the housing cavity may be designed to mirror the
exterior
dimensions of therapy device 400 housed in the housing cavity with additional
cavity
portions configured to receive and/or retain additional elements. These
additional elements
may include power supplies, straps to retain a therapy device, spacers,
counter balances,
shock absorbers, cushioning, sensors, structural supports, and/or the like.
In one exemplary embodiment, housing side surface 105 may comprise one
contiguous surface that effectively wraps around and/or partially encloses
housing portion
101. In another exemplary embodiment, housing side surface 105 may comprise a
plurality
of surfaces that combine to form a portion of housing portion 101. Housing
side surface 105
may be made from any suitable material for providing effective containment
and/or
retention of a therapy device, such as therapy device 400. In another
exemplary
embodiment, housing side surface 105 may be made from any suitable material
for
providing effective structural support to support the weight of a user. For
example, in one
exemplary embodiment, housing side surface 105 is made from one or more of
plastic,
foam, metal, cork, wood, natural rubber, synthetic rubber, composite, or other
durable
material. Housing side surface 105 may be a constant width or may comprise a
variable
width. In one exemplary embodiment, the width of housing side surface 105 is a
function of
the height of therapy device 400. In one exemplary embodiment, the width of
housing side
surface 105, (i.e. the height of the housing portion 101) is as small as
possible. In one
exemplary embodiment, the heel area of therapy shoe 100 may comprise
additional heel
support. This heel support may be structural and/or configured to provide
shock absorption.
In one exemplary embodiment, a surface, such as housing side surface 105,
housing
top surface 135, and/or housing bottom surface 130, comprises an opening 110
to access a
portion of a provided therapy device, such as therapy device 400. In one
exemplary
embodiment, with renewed reference to FIG. 1, opening 110 is located on
housing side
surface 105. In one exemplary embodiment, opening 110 may comprise any
suitable
dimensions for accessing a provided therapy device, such as therapy device
400. This
accessing may be visual and/or physical. For instance, the dimensions and/or
location of
opening 110 may be related to the positioning of controls and/or indicators on
a provided
5
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
therapy device, such as therapy device 400. Such controls and/or indicators
may comprise
on/off buttons, lights, switches, and/or the like. Access to the same may be
provided via
opening 110.
In one exemplary embodiment, a cover (not shown in the figures) may be coupled
to
opening 110 to prevent debris and/or unwanted matter from entering housing
portion 101.
In one exemplary embodiment, the cover may be transparent. The cover may be
coupled to
opening 110 via any suitable components and/or methods, such as pressure fit,
latch, screw,
glue, epoxy, snap, and/or the like. Further, the cover may made from any
suitable material,
such as the same material as housing side surface 105, or a material other
than the same
material as housing side surface 105. For instance, the cover material may be
at least
partially transparent and/or flexible, for example in order to allow a user to
manipulate the
controls of therapy device 400, while still preventing debris from entering
the housing
portion 101.
In various exemplary embodiments, therapy shoe 100 may be configured absent
opening 110. In these exemplary embodiments, access to controls of therapy
device 400
may be provided via one or more wires passing through housing portion 101, via
wireless
communication with therapy device 400, and/or via any other suitable method,
as desired.
Moreover, in certain exemplary embodiments therapy device 400 may be
configured to be
powered and/or charged via an induction coupling.
In an exemplary embodiment, a gateway (not shown in the figures) is located on
housing portion 101, for example coupled to housing bottom surface 130. In an
exemplary
embodiment, the gateway comprises any suitable dimensions for accessing,
replacing,
installing and/or removing a provided therapy device, such as therapy device
400. For
instance, the dimensions of the gateway may be a function of the geometry of
the exterior
(housing) of therapy device 400. In one exemplary embodiment, the access panel
may be
coupled to the gateway to prevent debris and/or unwanted matter from entering
housing
portion 101 and/or to prevent a provided therapy device 400 from decoupling
from housing
portion 101. The access panel may be coupled to the gateway via any suitable
components
and/or methods, such as pressure fit, latch, screw, glue, epoxy, snap, and/or
the like. An
access panel (not shown) may be made from any suitable material, such as the
same material
as housing bottom surface 130 or a material other than the same material as
housing bottom
surface 130. For instance, in one embodiment, the access panel may be made
from the same
material and have the same surface texture as the outsole material.
6
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
In an exemplary embodiment, the exterior of housing bottom surface 130 (the
outsole material) may be textured. For instance, the exterior of housing
bottom surface 130
may be configured to comprise a tread pattern. In an exemplary embodiment, the
texture on
housing bottom surface 130 may be configured to improve traction of therapy
shoe 100.
Moreover, the texture on housing bottom surface 130 may be configured in any
suitable
pattern or configuration.
In an exemplary embodiment, with reference to FIGS. 2 and 3, aperture 150 is
located on housing top surface 135. Aperture 150 may extend from housing
potion 101 to
coupling portion 102. In one exemplary embodiment, aperture 150 may comprise
any
suitable dimensions to assist therapy device 400 in providing therapy. For
instance, the
dimensions of aperture 150 may be a function of the orientation and geometry
of therapy
elements of a provided therapy device 400. In various exemplary embodiments,
aperture
150 comprises an opening having an area from about 6 square centimeters to
about 40
square centimeters. In various exemplary embodiments, aperture 150 comprises
an opening
configured to facilitate a provided therapy device, such as therapy device
400, making
contact with the venous plexus region of the foot. For instance, aperture 150
may comprise
an opening configured to facilitate therapy device 400 applying a compressive
force to the
arch region of a foot.
In certain exemplary embodiments, housing top surface 135 maybe configured to
be
continuous (i.e., absent aperture 150). In these exemplary embodiments, at
least a portion of
housing top surface 135 may be configured to be flexible and/or deformable,
for example in
order to deform responsive to a force exerted by therapy device 400 and make
contact with
the venous plexus region of the foot.
In an exemplary embodiment, an aperture cover 155 may at least partially
couple to,
obstruct, and/or otherwise cover aperture 150 to prevent debris and/or
unwanted matter from
entering housing portion 101 while still facilitating contact of therapy
device 400 with a
foot. In general, aperture cover 155 is configured to be flexible and/or
deformable.
For instance, in an exemplary embodiment aperture cover 155 may stretch from a
first position and/or configuration where a force is not being provided by
therapy device 400
to a portion of a wearer, to a second position and/or configuration where a
force is being
provided by therapy device 400 to a wearer. Likewise, in an exemplary
embodiment,
aperture cover 155 may return from a second position and/or configuration
where a force is
being provided by therapy device 400 to a portion of a wearer, to a first
position and/or
7
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
configuration where a force is not being provided by therapy device 400 to a
wearer.
Aperture cover 155 may be made from any suitable material configured to
withstand
providing force to a portion of a wearer. For instance, aperture cover 155 may
be made
from latex, plastic, rubber, synthetic, cloth, fabric, and or the like. In one
exemplary
embodiment, aperture cover 155 may be washable.
In various exemplary embodiments, aperture cover 155 comprises material
configured to disperse pressure across a portion of a wearer. For instance, in
one exemplary
embodiment, aperture cover 155 comprises a non-solid material, such as a gel
pack. This
non-solid material may assist in the prevention of skin breakdown. In another
exemplary
embodiment, a soft material, such a cushioning, cotton, padding, foam, gel
pack, gauze,
and/or the like, may be coupled to and/or integral to aperture cover 155. In
various
exemplary embodiments, this soft material coupled to aperture cover 155 may
aid in
preventing skin breakdown. Moreover, aperture cover 155 may be coupled to
aperture 150
via any suitable components and/or methods, such as pressure fit, latch,
screw, glue, epoxy,
snap, and/or the like.
In an exemplary embodiment, coupling portion 102 comprises surface 120. In one
embodiment, surface 120 is coupled to the exterior surface of housing top
surface 135.
Surface 120 may be configured to at least partially receive and/or support a
portion of a
wearer, such as the sole of a foot. Accordingly, surface 120 may be configured
with any
suitable shape or dimension. In an exemplary embodiment, surface 120 is larger
than a
provided foot. Moreover, surface 120 may be made from any suitable material,
such as
foam, leather, sponge, rubber, plastic, synthetic, cork and/or the like.
In various exemplary embodiments, surface 120 is configured to provide a
comfortable surface for touching a foot. For instance, surface 120 may
comprise an insole
configured to provide shock absorption. In various embodiments, the insole
coupled to
surface 120 may be removable, such as for washing and/or cleaning. Moreover,
in various
embodiments, custom orthotics may be integral to and/or coupled to surface
120.
In an exemplary embodiment, coupling portion 102 comprises couplers to receive
and retain a portion of a wearer, such as a foot, ankle and/or portion of a
lower extremity.
The couplers may be custom sized material, belts, elastics, buckles, snaps,
ties, hook and
loop style fasteners (e.g., VELCRO brand fasteners provided by Velcro USA,
Inc.),
buttons, zipper, sheaths, and/or the like.
8
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
In an exemplary embodiment, the couplers comprise adjustable straps, such as
straps
160, 163, 165, configured to receive and retain a portion of a foot, such as
dorsal surface, the
heel, and/or the like, as is known. Moreover, therapy shoe 100 may comprise
any suitable
number of straps. In one exemplary embodiment, each strap 160, 163, 165
comprises a first
side and a second side. The first side and/or second side are configured to
comprise a
portion of a fastener, such as a first hook and loop fastener portion and/or a
second hook and
loop fastener portion configured to mate with and couple to an opposite hook
and loop
fastener portion, on at least one of a top surface and/or or bottom surface of
the first side
and/or second side.
In an exemplary embodiment, with regard to strap 160, 163, 165, the first side
is
configured to have an opening which a portion of the second side may be fed
through. A
portion of second side of strap 160, 163, 165 is fed though an opening in a
first side of strap
160, 163, 165 and is adjustably anchored using a fastener, such as a hook and
loop fastener
to a portion of first side of strap 160, 163, 165. In this embodiment, a
portion of first side of
strap 160, 163, 165 overlaps second side of strap 160, 163, 165 and is
adjustably anchored
using a fastener, such as a hook and loop fastener.
Straps (e.g. strap 160, 163, 165) may be located in any suitable location. In
one
embodiment strap 160 may located to receive and retain the rear portion of an
ankle. In one
embodiment strap 163 may located to receive and retain the front portion of an
ankle and/ a
top surface of the foot. In one embodiment strap 165 may located to receive
and retain a top
surface of the foot. In another exemplary embodiment, a soft material, such a
cushioning,
cotton, padding, foam, gel pack, gauze, and/or the like, may be coupled to the
inside surface
of strap 160, 163, 165, to provide comfort for a user. Moreover, straps (e.g.
strap 160, 163,
165) may be configured to accept variances in individual feet (e.g., height of
arch, curvature
of arch, width, length, and/or the like).
In other exemplary embodiments, straps (e.g. strap 160, 163, 165) are
eliminated and
other known footwear constructions are provided. These examples include, but
are not
limited to, laces, buttons, tongues, designer straps (such as those on a high
heel shoe), and
other similar constructions. Moreover, any material now known or used in the
future to
construct footwear may suitably be used, and is considered to fall within the
scope of the
present disclosure.
In an exemplary embodiment, a covering, such as a waterproof covering, may be
coupled to therapy shoe 100. For example, a bag or shroud may be coupled
around therapy
9
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
shoe 100 to prevent unwanted elements such as sand, water, dirt, debris,
and/or particulate
from coming into contact with therapy shoe 100. In various exemplary
embodiments,
therapy shoe 100 may at least partially comprise natural rubber, synthetic
rubber, polymer
composites, or other waterproof and/or water-resistant materials.
In various exemplary embodiments, therapy shoe 100 is coupled to an external
object. For instance, the external object may be a fixed surface, such as a
wedge in a fixed
position coupled to the ground. The external object may be an elevated
surface, such as a
footrest. Alternatively, the external object may be coupled to and/or comprise
a movable
object such as a portion of a bed, chair, hospital bed, cart, or wheelchair.
The coupling may
be by any suitable means, such as magnetic, mechanical, hook and loop
fastener, strapping,
fastening, lacing, tying, and/or the like.
In accordance with an exemplary embodiment, therapy shoe 100 is configured to
be
fabricated integral to a shoe, boot, sandal, or other footwear. Moreover,
therapy shoe 100
may be configured with various sizes, including industry standard sizes. For
instance,
therapy shoe 100 may be formed in a size compatible to and related to a foot
size such as at
least one of. USA men's sizes: 6 through 16 (in any suitable width such as C,
D, E, and/or
the like); USA women's sizes: 4 through 14 (in any suitable width such as
narrow, average,
wide, extra wide, and/or the like); USA children's sizes: 6 through 13 and I
through 3;
European men's sizes 35 through 51; Europeans women's sizes 35 through 48.5;
universal:
small, medium, large, XL, XXL, and/or the like. Although specific shoe sizes
have been
recited herein, any size item of footwear is considered to falls within the
scope of the present
disclosure.
Moreover, in accordance with an exemplary embodiment, therapy shoe 100 is
configured to be one-size-fits-all. For instance, portions of therapy shoe
100, such as
coupling portion 102 and/or housing 101, may be expandable and/or retractable
to
accommodate a variety of foot sizes, widths, and lengths. In various exemplary
embodiments, therapy shoe 100 is configured to be one of shaped to receive a
right foot,
shaped to receive a left foot, or shaped to receive a right or left foot. In
certain exemplary
embodiments, therapy shoe 100 may be provided in pairs, for example two
therapy shoes
100, one each for the right foot and the left foot.
With reference now to FIG. 4, in accordance with an exemplary embodiment, a
user
(and/or caregiver, third person, etc.) may insert therapy device 400 into a
cavity of therapy
shoe 100 (not shown). Straps 160, 163, 165 (from a first closed position) of
therapy shoe
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
100 are opened. A portion of a user, such as a lower extremity (e.g. foot), is
inserted
between a first and second side of straps 160, 163, 165, and comes into
contact with surface
120. The location of the portion of a user with respect to surface 120 and/or
other
components of therapy shoe 120 may be a function of the location of therapy
shoe 100's
elements and/or a user's individualized anatomy. Once a portion of a user is
in a desired
position, straps 160, 163, 165 may be adjusted and/or closed (e.g., from a
second position to
a first closed position). Therapy device 400 may be activated through opening
110.
In various exemplary embodiments, therapy shoe 100 is configured to withstand
force provided by a user while therapy shoe 100 is coupled to the user. For
instance, a user
may provide a force to therapy shoe 100 by standing or walking while coupled
to therapy
shoe 100. A user may move at any pace while coupled to therapy shoe 100. It
will be
appreciated that, in certain embodiments, therapy shoe 100 is configured to
allow a user to
remain mobile; however, in other exemplary embodiments, therapy shoe 100 is
configured
for use by a user who remains non-ambulatory while coupled to therapy shoe
100.
For example, in certain exemplary embodiments therapy shoe 100 is configured
as a
"walking" shoe. In these embodiments, therapy shoe 100 may be utilized by a
wearer in a
manner similar to a conventional shoe. In these embodiments, therapy device
400 may be
configured to be inactive when the wearer is walking, for example in order to
prevent wearer
discomfort, damage to therapy device 400, and/or the like. In other exemplary
embodiments, therapy show 100 is configured as a "non-walking" shoe. In these
embodiments, therapy shoe 100 may be utilized by a wearer when non-ambulatory.
In these
embodiments, therapy device 400 may be configured to be constantly active,
active at a
scheduled interval, and/or the like.
With reference now to FIG. 6, in various exemplary embodiments therapy shoe
100
may be coupled to and/or utilized in connection with a compression garment,
for example
compression sock 680. Compression sock 680 may be configured to work in a
complementary manner with therapy device 400, for example in order to treat
and/or prevent
deep vein thrombosis, to facilitate athletic recovery, and/or the like. In an
exemplary
embodiment, compression sock 680 is releasably coupled to therapy shoe 100 via
one or
more of zippers, snaps, straps, buttons, hooks, hook and loop fasteners,
and/or the like. In
other exemplary embodiments, compression sock 680 is permanently coupled to
therapy
shoe 100, for example via gluing, stitching, and/or the like.
11
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
Compression sock 680 may comprise any suitable flexible material and may be
configured with any suitable dimensions, shapes, curves, stitching, and/or the
like, as
desired, in order to at least partially receive and/or compress a portion of a
limb. Moreover,
compression sock 680 may be configured with any suitable level of compression,
for
example from between about 5 mmHg to about 50 mmHg. Compression sock 680 may
be
configured as knee-high, as thigh-high, as pantyhose, and/or in any other
suitable
configuration.
Because compression sock 680, when wom by a user, compresses the surface
veins,
arteries, muscles, and other soft tissue, circulating blood is forced through
narrower
circulatory channels, increasing the arterial pressure. This distribution of
blood in the limb
of a wearer can facilitate improved results (for example, increased blood
circulation,
decreased pooling of blood in the feet and lower extremities, etc.) responsive
to operation of
therapy device 400. Stated another way, when compression sock 680 and therapy
device
400 work in tandem, blood flow is further concentrated and increased, with the
result of
facilitating athletic recovery, reducing deep vein thrombosis, reducing edema,
and/or the
like.
The present disclosure has been described above with reference to various
exemplary
embodiments. However, those skilled in the art will recognize that changes and
modifications may be made to the exemplary embodiments without departing from
the
scope of the present disclosure. For example, the various operational steps,
as well as the
components for carrying out the operational steps, may be implemented in
alternate ways
depending upon the particular application or in consideration of any number of
cost
functions associated with the operation of the system, e.g., one or more of
the steps may be
deleted, modified, or combined with other steps. Further, it should be noted
that while the
methods and systems for aiding compression described above are suitable for
use on the
foot, similar approaches may be used on the hand, calf, extremities or other
areas of the
body. These and other changes or modifications are intended to be included
within the
scope of the present disclosure.
In the foregoing specification, the disclosure has been described with
reference to
various embodiments. However, one of ordinary skill in the art appreciates
that various
modifications and changes can be made without departing from the scope of the
present
disclosure as set forth in the claims below. Accordingly, the specification is
to be regarded
in an illustrative rather than a restrictive sense, and all such modifications
are intended to be
12
CA 02792111 2012-09-04
WO 2011/109725 PCT/US2011/027220
included within the scope of the present disclosure. Likewise, benefits, other
advantages,
and solutions to problems have been described above with regard to various
embodiments.
However, benefits, advantages, solutions to problems, and any element(s) that
may cause
any benefit, advantage, or solution to occur or become more pronounced are not
to be
construed as a critical, required, or essential feature or element of any or
all the claims. As
used herein, the terms "comprises," "comprising," or any other variation
thereof, are
intended to cover a non-exclusive inclusion, such that a process, method,
article, or
apparatus that comprises a list of elements does not include only those
elements but may
include other elements not expressly listed or inherent to such process,
method, article, or
apparatus. Also, as used herein, the terms "coupled," "coupling," or any other
variation
thereof, are intended to cover a physical connection, an electrical
connection, a magnetic
connection, an optical connection, a communicative connection, a functional
connection,
and/or any other connection. Further, when language similar to "at least one
of A, B, or C"
is used in the claims, the phrase is intended to mean any of the following:
(1) at least one of
A; (2) at least one of B; (3) at least one of C; (4) at least one of A and at
least one of B; (5)
at least one of B and at least one of C; (6) at least one of A and at least
one of C; or (7) at
least one of A, at least one of B, and at least one of C.
13