Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
TITLE OF THE INVENTION:
SYSTEM AND METHOD FOR IMPROVED HEAT RECOVERY FROM FLUE GASES
WITH HIGH SO3 CONCENTRATIONS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This Application claims the benefit of U.S. Provisional Application No.
61/356,765, filed on June 21, 2010, and U.S. NonProvisional Application No.
13/090,651
filed April 20, 2011. The disclosure of these Applications are hereby
incorporated by
reference.
BACKGROUND OF THE INVENTION
[0002] The present invention is directed to systems and methods of power
generation.
More particularly, the present invention is directedto systems and methods of
power
generation having neutralizing sorbent injection and second stage heat
recovery.
[0003] Energy demand is constantly increasing. As the energy demand increases,
efficiency for fossil fuel energy sources increases in importance. Generally,
the efficiency
of fossil fuel powered plants can be impacted by the amount of heat that can
be
recovered from flue gas.
[0004] In some fossil fuel powered plants, flue gas may be cooled by
transferring heat
to combustion air, thereby improving efficiency of combustion in a boiler. For
example,
Swedish patent SE 448117 B, which is hereby incorporated by reference in its
entirety,
discloses cooling flue gas below an acid dew point, thereby condensing
sulfuric acid.
The sulfuric acid can cause heat exchangers and other process elements to
foul. To
prevent fouling, expensive alloys and corrosion resistant material are used.
[0005] EP 0102770 A2, which is hereby incorporated by reference in its
entirety,
discloses condensing sulfuric acid by cooling flue gas below an acid dew
point. To
prevent fouling, heat exchangers include special designs and protective
coatings, both of
which may be expensive.
-1-
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
[0006] International patent application WO 2006/087416A1, which is hereby
incorporated by reference in its entirety, discloses heat recovery from flue
gas through a
fluidized bed baler combusting sulfurous fuel. The process results in flue gas
being
cooled belowthe acid and water dew points. To prevent fouling, heat exchanger
tubes
are made of plastics and other acid resistant materials, which may be
expensive.
[0007] Japanese patent application JP 2002162020 A, which is hereby
incorporated by
reference in its entirety, discloses two stage heat recovery with the second
stage heating
the flue gas directed to a stack, thereby mitigating visual plume concerns.
The additional
heat recovered from the flue gas in the second stage is not converted into
power and is
only used to reheat the flue gas leaving the stack. Thus, the second stage
does not
improve the efficiency of the power plant.
[0008] What is needed is a system and method for improving efficiency in a
fossil fuel
power plant by increasing the low level heat recovery from the flue gas
without relying
upon expensive alloys and corrosion resistant materials to prevent fouling of
heat
exchangers and other process elements.
BRIEF SUMMARY OF THE INVENTION
[0009] The instant invention solves problems associated with conventional
practices by
providing a system and method for controlling and removing SO3 in a sulfur
containing
flue gas.
[0010] In an exemplary embodiment, a power generation system for high sulfur
fuel
combustion includes a boiler for combusting a sulfur containing fuel to form a
sulfur
containing flue gas, a heat exchanger arranged and disposed to transfer heat
from the
sulfur containing flue gas to combustion air, a sorbent injector arranged and
disposed to
inject sorbent into the sulfur containing flue gas, and a second stage heat
recovery
mechanism arranged and disposed to transfer heat from the sulfur containing
flue gas.
The heat exchanger maintains a temperature of the sulfur containing flue gas
above a
predetermined temperature, the predetermined temperature relating to the acid
condensation temperature. The sorbent reduces concentration of sulfur in the
sulfur
containing flue gas, thereby reducing the acid condensation temperature of the
sulfur
containing flue gas.
-2-
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
[0011] In another exemplary embodiment, a power generation system for high
sulfur
fuel combustion includes a boiler for combusting a sulfur containing fuel to
form a sulfur
containing flue gas, a heat exchanger arranged and disposed to transfer heat
from the
sulfur containing flue gas to combustion air, a sorbent injector arranged and
disposed to
inject sorbent into the sulfur containing flue gas, and a second stage heat
recovery
mechanism arranged and disposed to transfer heat from the sulfur containing
flue gas to
air directed to the heat exchanger. In the embodiment, the heat exchanger
maintains a
temperature of the flue gas above a predetermined temperature, the
predetermined
temperature relating to the acid condensation temperature, the predetermined
temperature corresponds to a sulfuric acid dew point or is based upon a
correlation, the
sorbent comprises at least one sorbent selected from the group consisting of
limestone,
lime, trona, sodium bisulfate, magnesium hydroxide, and combinations thereof,
the
application of the sorbent results in the sulfur containing flue gas having a
S03
concentration of less than about 50 ppm volume, and the application of the
sorbent
lowers SO3 concentration in the sulfur containing flue gas by at least 80%.
[0012] In another exemplary embodiment, a method of generating power includes
combusting a sulfur containing fuel to form a sulfur containing flue gas, in a
heat
exchanger, transferring heat from the sulfur containing flue gas to combustion
air,
maintaining a temperature of the sulfur containing flue gas leaving the heat
exchanger
above a predetermined temperature, the predetermined temperature relating to
the acid
condensation temperature, injecting sorbent into the sulfur containing flue
gas, wherein
the sorbent reduces concentration of sulfur in the sulfur containing flue gas,
thereby
reducing the acid condensation temperature of the sulfur containing flue gas,
and
transferring heat from the sulfur containing flue gas in a second stage heat
recovery
mechanism.
BRIEF DESCRIPTION OF SEVERAL VIEWS OF THE DRAWINGS
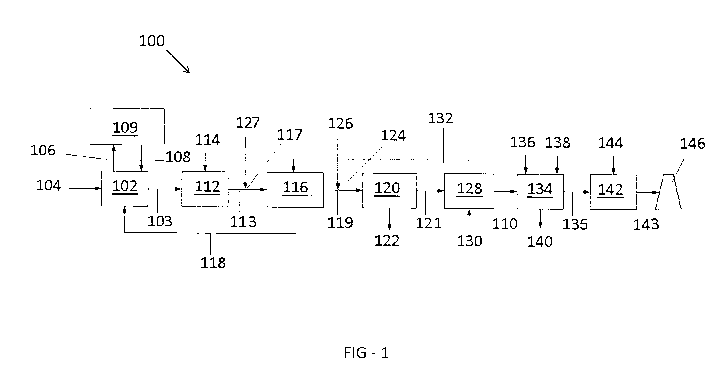
[0013] Figure 1 shows an exemplary power generation system according to the
disclosure.
[0014] Figure 2 shows the sulfuric acid dew point as a function of the SO 3
and water
vapor concentrations in flue gas.
-3-
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
[0015] Figure 3 shows another exemplary power generation system according to
the
disclosure.
[0016] Wherever possible, the same reference numbers will be used throughout
the
drawings to represent the same parts.
DETAILED DESCRIPTION OF THE INVENTION
[0017] Provided is a system and method of power generation having neutralizing
sorbent injection and heat recovery. Embodiments of the disclosure may improve
efficiency in fossil fuel power plants by recovering the low level heat in
flue gas wihout
relying upon expensive allays and corrosion resistant materials to prevent
fouling of heat
exchangers and other process elements.
[0018] An exemplary power generation system 100 may include a boiler 102, a
selective catalytic reduction unit 112, a first air preheater 116, a sorbent
injection location
124, a particulate removal mechanism 120, a second stage heat recovery
mechanism
(for example, a second air preheater 128), a flue gas desulfurization unit
134, a wet
electrostatic precipitator 142, and a stack 146.
[0019] Referring to FIG. 1, boiler 102 is arranged and disposed to receive a
fuel (for
example, provided by a fuel stream 104) to generate steam (for example,
transported by
steam stream 106) and convert the steam into power. Fuel may be a fossil fuel
(for
example, coal, oil, and/or petcoke, etc.). Any suitable system for converting
steam into
power may be used. For example, boiler 102 converts water (for example,
provided by a
water stream 108) into the steam, and the steam is converted into mechanical
energy to
power a steam turbine or generator 109. Any suitable steam turbine or
generator 109
may be used in conjunction with boiler 102. Generally, steam turbine or
generator 109
receives the steam from boiler 102 via steam stream 106, the steam heats an
element
such as a liquid-filled heat exchanger (not shown), and water is formed and
returned via
water stream 108 to boiler 102 to be converted into the steam. The process is
repeated
with the introduction of more fuel. In the conversion of the steam into
mechanical energy,
boiler 102 produces a flue gas that is transported to further processing
equipment via a
flue gas conduit 103.
[0020] The amount of heat energy transferred to the steam may be controlled by
the
design of boiler 102 and/or by environmental emission control equipment
located
-4-
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
downstream of boiler 102. In one embodiment, boiler 102 may use preheated air
(from
any suitable source) at a temperature of about 300 F to about 600 F (about 149
C to
about 316 C). The heat may be transferred to water to generate the steam used
to
produce power. In one embodiment, the flue gas generated by boiler 102 may be
at
about 550 F to 800 F (about 288 C to about 427 C).
[0021] The flue gas leaving boiler 102 may include nitrogen oxides (NOx),
sulfur oxides
(SOx), H2O, and/or particulate. The amount of the SO3 generated in boiler 102
may
depend upon various factors including, but not limited to, the sulfur content
of the fuel,
combustion process conditions, and other process characteristics. For
combusting high
sulfur containing fuels, the concentration of SO3 in the flue gas present in
flue gas
conduit 103 can be as high as 300-400 parts per million (ppm) by volume in the
flue gas
and the acid dew point can be as high as about 340 F (about 171 C). System
100 may
include environmental control equipment for reducing or eliminating NOx, SOx,
and/or
particulate. Pre-combustion, combustion and/or post combustion emission
control
technologies may form environmental control equipment.
[0022] Flue gas is provided b selective catalytic reduction (SCR) unit 112 via
flue gas
conduit 103 as part of the environmental control equipment. SCR unit 112 is in
fluid
communication with boiler 102 and is arranged and disposed to apply a
reductant (for
example, from reductant stream 114) to flue gas. SCR unit 112 may reduce NOx
in the
flue gas generated during combustion into N2 and H2O. The reduction may be
performed
by applying the reductant (for example, anhydrous ammonia, aqueous ammonia,
urea,
cyanuric acid, and/or ammonium sulfate) in the presence of a catalyst (for
example,
ceramic carriers including active catalytic components, such as, oxides of
base metals,
zeolites, and/or precious metals) to the flue gas within SCR unit 112. In
embodiments
using urea as the reductant, additional mechanisms for removing CO2 may be
included.
In one embodiment, SCR unit 112 may reduce NOx by about 60% to about 95%. In
the
embodiment, the flue gas leaving SCR unit 112 via a flue gas conduit 113
includes about
5% to about 40% of the NOx in the flue gas leaving boiler 102 via flue gas
conduit 103
and the concentration of the SO3 may be increased due to SO2 oxidizing to SO3
in the
presence of SCR catalyst. Thus, flue gas leaving SCR unit 112 may include
particulate,
H2O, an increased concentration of SO3i and a decreased concentration of NOx.
[0023] The efficiency of system 100 may relate to the amount of heat that can
be
recovered from the flue gas to form the steam. Including air preheater 116 in
system 100
-5-
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
may provide such heat recovery. Generally, including air preheater 116 may
transfer
heat to combustion air in boiler 102. By heating the combustion air in boiler
102, more
heat energy from the combustion of fuel in boiler 102 can be used to form the
steam,
thereby improving combustion efficiency.
[0024] As also shown in FIG. 1, air preheater 116 is arranged and disposed to
transfer
heat from the flue gas to combustion air provided by air inlet 132. The flue
gas may
transfer heat to the combustion air in air preheater 116. Upon transferring
heat in air
preheater 116, the air in combustion air inlet line 118 is heaied. The heated
combustion
air may be provided to boiler 102 for increasing efficiency of combustion. In
one
embodiment, the air in combustion air inlet line 132 may be pre-heated prior
to reaching
air preheater 116.
[0025] The flue gas entering air preheater 116 may contain particulate.
Particulate may
include fly ash and/or other solid or semi-solid combustion products carried
by the flue
gas. The heat exchanger may be configured to operate in the presence of
particulate (or
other high-solids conditions). For example, the heat exchanger may be
configured to
operate despite fouling that may be caused by particulate. In one embodiment,
the heat
exchanger may be a rotating regenerative heat exchanger (for example,
Ljungstrom type
heat exchangers or other suitable regenerative heat exchangers). In the
rotating
regenerative heat exchanger, the flue gas and the air may flow counter-
currently, thereby
permitting the heat exchanger to have a flue gas side and an air side. The
heat
exchangers may include a round basket having corrugated sheet metal plates
that rotate
inside a round housing betweei the flue gas and the air provided to air
preheater 116.
The corrugated sheet metal plates may be heated by flue gas. The corrugated
sheet
metal plates may then rotate and transfer heat to the air in the air side of
the heat
exchanger.
[0026] Flue gas entering air preheater 116 via flue gas conduit 113 may be
cooled by
the exchange of heat in the air preheater 116 but is maintained above a
predetermined
temperature. The predetermined temperature corresponds to an acid dew point
(for
example, a sulfuric acid dew point), thereby reducing or eliminating corrosion
and fouling
within air preheater 116. The acid dew point is a temperature at which acid
condensation
begins. The sulfuric acid dew point is the temperature at which sulfuric acid
begins to
condense. As shown in FIG. 2, the sulfuric acid dew point depends uponthe
concentration of SO3 and H2O. In addition, the sulfuric acid dew pant may
depend upon
-6-
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
the pressure of flue gas. FIG. 2 specifically shows H2SO4 dew point as a
function of SO3
concentration in the flue gas at three different concentrations of H2O in flue
gas at a flue
gas pressure of 14.3 psia. The acid dew point increases as SO3 and H2O
concentrations
increase.
[0027] As used herein, the term "corresponds," "corresponding," and
grammatical
variations thereof refer to a relationship of the predetermined temperature
and
preselected operating conditions. The predetermined temperature corresponds to
the
acid dew point of preselected operating conditions. The flue gas may be
maintained
above the predetermined temperature independent of whether the preselected
operating
conditions are present. Thus, the predetermined temperature may correspond to
an acid
dew point but not be the actual acid dew point.
[0028] In one embodiment, the predetermined temperature may be based upon a
dew
point correlation. The dew point correlation may be any suitable dew point
correlation,
including, but not limited to, the Kiang correlation. As used herein, the
"Kiang correlation"
is a mathematic formula for predicting the dew point of an acid. For example,
the Kiang
correlation for sulfuric acid is the following: 1,000 / TDP = 2.276 - 0.0294
In (PH2O) -
0.0858 In (PH2sn4) + 0.0062 In (PH?O) In (PH2SO4). In the Kiang correlation,
TDP is the acid
dew point (in Kelvin), and P is the partial pressure (in units mm Hg).
[0029] In maintaining the flue gas above the predetermined temperature (for
example,
the sulfuric acid dew point), corrosion and fouling may be reduced or
eliminated, despite
the sulfur in fuel oxidizing into SO2 and SO3 in boiler 102. In one
embodiment,
maintaining the predetermined temperature may prevent the decrease in
temperature of
the flue gas (for example, in air preheater 116) from condensing gaseous H2SO4
at the
sulfuric acid dew point. For example, maintaining the predetermined
temperature above
about 500 F (about 260 C) may prevent the SO3 in the flue gas from reacting
with H2O in
the flue gas to form H2SO4.
[0030] Maintaining the predetermined temperature may control the amount of
heat
recovered from the flue gas based upon the sulfuric acid dew point. In one
embodiment,
maintaining the temperature of the flue gas above the predetermined
temperature may
prevent sulfuric acid condensate from reacting with the reductant introduced
in SCR unit
112. For example, maintaining the predetermined temperature may prevent
ammonia
from reacting with the sulfuric acid condensate to form ammonium bisulfate,
which may
foul the heat exchanger. In the embodiment, preventing the fouling of the heat
exchanger
-7-
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
may prevent a reduction in heat tansfer, may prevent drops in pressure, may
reduce or
eliminate use of expensive anti-fouling and/or anti-corrosion materials (for
example, in
ducts for flue gas, air preheater 116, particulate removal mechanism 120,
and/or air
preheater 128), and/or may prevent shutdown of boiler 102 for cleaning and/or
repair of
air preheater 116.
[0031] In one embodiment, maintaining the temperature of the flue gas above
the
predetermined temperature may permit the formation of a small amount of
sulfuric acid
condensate on the heat exchange surface. In the embodiment, which may rely
upon the
Ljungstrom type heat exchanger, the basket element of the heat exchanger
rotates from
air inlet 132 to the flue gas, thereby contacting the flue gas with cold metal
plates in the
heat exchanger. The temperature of the cold metal plates in the heat exchanger
may be
below the sulfuric acid dew point, thereby forming some sulfuric acid
condensate.
[0032] As shown in FIG. 1, the flue gas may exit air preheater 116 via flue
gas conduit
119 having been cooled to the predetermined temperature. A sorbent 126 is
applied to
flue gas at sorbent injection location 124 or any other suitable location. In
one
embodiment, sorbent injection location 124 may be positioned along flue gas
conduit
119. Sorbent injection location 124 may include or be part of vessels and/or
equipment
for enhanced contact between sorbent 126 and the flue gas. Additionally or
alternatively,
sorbent injection location 124 may be positioned to increase the residence
time to allow
sufficient time for the reactions.
[0033] Sorbent 126 may be any suitable neutralizing agent(s). For example,
sorbent
126 may comprise at least one member selected from the group consisting of
limestone,
lime, trona, sodium bisulfate, and/or magnesium hydroxide. The amount of
sorbent 126
applied to the flue gas, the specific sorbent to be applied, the method of
applying sorbent
126 may be adjusted to correspond to the selected sorbent.
[0034] With the addition of sorbent 126, the amount of SO3 in the flue gas may
be
reduced. For example, if trona is used as sorbent 126, triona is calcined with
the
following reaction and then the resultant Na2CO3 reacts with SO3 to form
Na2SO4:
Calcination: 2 Na2CO3 = NaHCO3 . H2O - 3 Na2CO3 + CO2 + 5 H2O
SO3 Removal: Na2CO3 + SO3 - Na2SO4 + CO2
-8-
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
[0035] As shown in FIG. 2, reducing the amount of SO3 in the flue gas reduces
the
sulfuric acid dew point. By reducing the sulfuric acid dew point, a lower
temperature may
be maintained in the flue gas without (or substantially devoid of) formation
(precipitation)
of sulfuric acid, thereby increasing heat transfer and increasing efficiency
by increasing
the temperature of combustion air inlet line 118. In one embodiment, sorbent
126 may be
applied at an amount resulting in SO3 concentration being less than about 50
ppm
volume, reduced from 150-200 ppm volume in the flue gas present in flue gas
conduit
103 and 300-400 ppm volume in the flue gas present in flue gas conduit 113
(for
example, about a 84% to 80% reduction in SO3, or above 84% reduction in SO3).
The
amount of sorbent 126 used may be based upon economic and efficiency
considerations. In one embodiment, the cost of sorbent 126 may be balanced
with the
cost savings associated with increased efficiency resulting from the amount of
SO3. For
example, as the SO3 concentration decreases, more contact time and more
sorbent may
be used. The increased contact time and increased sorbent increase operating
costs.
The return based upon the heat recovered may be balanced with the increased
operating costs.
[0036] Referring again to FIG. 1, an additional optional sorbent injection
location 117
may be included in system 100. Optional sorbent injection location 117 may be
upstream
of air preheater 116. Optional sorbent injection location 117 along flue gas
conduit 113
may apply the same sorbent as sorbent injection location 124 or any other
suitable
sorbent(s) for sulfuric acid dew point reduction. In one embodiment, optional
sorbent
injection location 117 applies sorbent 127 configured for reducing fouling in
air preheater
116 and preventing slagging and/or corrosive material being included in
combustion air
inlet line 118 with carry over. For example, in the embodiment, sorbent 127
may be a
magnesium based sorbent. In one embodiment, sorbent(s) 127 may be selectively
applied by sorbent injection location 117 and/or sorbent injection location
124. Such
selective application of sorbent(s) 127 may permit additional control of
system 100. For
example, increased application of sorbent(s) 127 in sorbent injection location
117 may
reduce slagging in boiler 102 in conjunction with a decreased application of
sorbent(s)
126 in sorbent injection location 124 to achieve the desired sulfuric acid dew
point and/or
corresponding/correlated temperature. Other suitable modifications to the
amount or type
of sorbent(s) being applied to sorbent injection location 117 and/or sorbent
injection
location 124 may include increased SO3 reduction as the flue gas cools in air
preheater
116 and prevent fouling.
-9-
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
[0037] Particulate removal mechanism 120 is arranged and disposed to receive
the
flue gas from air preheater 116. A portion or all of a SO3 neutralization
product resulting
from the application of sorbent(s) 126, any excess sorbent 126, and/or
particulate may
be removed by any suitable mechanism. For example, the SO3 neutralization
product,
sorbent(s) 126, and/or particulate may form particulate waste 122, which may
be
removed by particulate removal mechanism 120. In one embodiment, particulate
removal mechanism 120 may include particulate material collection equipment
(not
shown) such as, an electro-static precipitator (ESP) and/or a filter medium
(bag house).
The flue gas may be treated by the particulate material collection equipment
to remove a
predetermined amount of particulate waste 122 in the flue gas. For example, in
one
embodiment, about 99% of particulate is removed from the flue gas as
particulate waste
122. In another embodiment, all particulate above a predetermined size (for
example,
about 10 microns) is removed from the flue gas as particulate waste 122.
[0038] The flue gas with reduced or eliminated particulate may travel from
particulate
removal mechanism 120 to a second air preheater 128 for second stage heat
recovery.
Air preheater 128 may be any suitable preheater. Air preheater 128 may be the
same
type of air preheater as air preheater 116. Air preheater 128 is arranged and
disposed to
directly or indirectly receive the flue gas via flue gas conduit 121 from
particulate removal
mechanism 120 and to receive air 130.
[0039] Referring to FIG. 1, air preheater 128 may be arranged and disposed for
cooling
flue gas provided by particulate removal mechanism 120. Although the
temperature of
the flue gas may be reduced by air preheater 128 transferring heat from the
flue gas to
air 130, the temperature of the flue gas is maintained above the sulfuric acid
dew point
and/or corresponding/correlated temperature. Upon heating air 130, a stream of
preheated air may be provided via combustion air inlet line 132 to any
suitable
component. For example, all or a portion of the preheated air may be provided
to air
preheater 116 and/or boiler 102, thereby increasing efficiency of combustion.
In one
embodiment, air 130 may be pre-heated prior to reaching air preheater 128. In
one
embodiment, air 130 may be provided to air preheater 128 at a temperature
above the
sulfuric acid dew point, thereby preventing condensation of any remaining SO3
and
sharing similar benefits identified with reference to air preheater 116. In
one
embodiment, air preheater 128 may be configured for low solids. For example,
air
preheater 128 may include a tubular heat exchanger.
-10-
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
[0040] As shown in FIG. 3, prior to the flue gas traveling to flue gas
desulfurization unit
134, wet electrostatic precipitator 142, and/or stack 146, in one embodiment,
the flue gas
may travel through one or more flue gas heat exchangers 306 operably connected
to air
preheater 128 and forming the second stage heat recovery. The second stage
heat
recovery may be performed indirectly by drawing a heat transfer medium (for
example,
water) circulated within heat transfer medium circulation loop 302 with a pump
304 from
the one or more flue gas heat exchangers 306 to air preheater 128. The one or
more flue
gas heat exchangers 306 may reduce the temperature of the flue gas prior to
providing
the flue gas to further processing. Heat transfer medium in circulation loop
302 may heat
air 130 in air preheater 128 and provide air for use as described above via
combustion
air inlet line 132. Upon the heat transfer medium cooling, the cooled heat
transfer
medium may recirculale back to the one or more flue gas heat exchangers 306.
[0041] As shown in FIGS. 1 or 3, the flue gas may be provided to flue gas
desulphurization (FGD) unit 134 via flue gas conduit 110. FGD unit 134 is
arranged and
disposed to receive the flue gas from the second stage heat recovery mechanism
(for
example, from air preheater 128 in FIG. 1 or from flue gas heat exchangers 306
in FIG.
3). Within FGD unit 134, the SO2 in the flue gas may react with oxidation air
136 and
reagent 138 (for example, a limestone slurry) to form waste product 140 (for
example,
gypsum). In an exemplary embodiment, the limestone forced oxidation process is
used.
In one embodiment, FGD unit 134 may remove over 90% of the SO2 in the flue
gas. In
one embodiment, FGD unit 134 may include corrosion resistant material at the
flue gas
entrance where reagent 138is applied to the flue gas and neutralizes the acid
in the flue
gas.
[0042] Within FGD unit 134, cooling of the flue gas may remove a portion of
the
remaining SO3 in the flue gas by condensing as sulfuric acid. In one
embodiment, a
portion of SO3 is removed with waste product 140. The flue gas may then be
optionally
provided for further processing via flue gas conduit 135. For example, if SO3
in the flue
gas is below a predetermined concentration and/or amount, the flue gas may be
released via flue gas conduit 143 to the atmosphere through stack 146. If SO3
in flue gas
is above a predetermined amount, the flue gas may be provided to wet
electrostatic
precipitator 142.
[0043] Wet electrostatic precipitator (WESP) 142 is arranged and disposed to
receive
wash water 144 and to provide the flue gas to stack 146 via flue gas conduit
143. WESP
-11-
CA 02797260 2012-10-23
WO 2011/162871 PCT/US2011/034419
142 may treat remaining SO3 (for example, a sulfuric acid mist) and remaining
particulate
(for example, very fine particulate, such as particulate smaller than about 10
micrometers). WESP 142 may remove the remaining SO3 and the remaining
particulate
by charged electrodes. The electrodes attract the remaining SO3 and the
remaining
particulate. The remaining SO3 and the remaining particulate are washed
substantially
continuously from the charged electrodes with wash water 144 to dilute and
remove the
remaining SO3 and the remaining particulate.
[0044] While the invention has been described with reference to a preferred
embodiment, it will be understood by those skilled in the art that various
changes may be
made and equivalent may be substituted for elements thereof without departing
from
the scope of the invention. In addition, many modifications may be made to
adapt a
particular situation or material to the teachings of the invention without
departing from the
essential scope thereof. Therefore, it is intended that the invention not be
limited to the
particular embodiment disclosed as the best mode contemplated for carrying out
this
invention, but that the invention will include all embodiments falling within
the scope of
the appended clams.
-12-