Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
TITLE
Methods for Detecting Metabolic States
by Laser Ablation Electrospray Ionization Mass Spectrometry
GOVERNMENTAL INTEREST
Portions of this invention were made with United States government support
under Grant
Nos. 0415521 and 0719232 awarded by the National Science Foundation. The
government has
certain rights in the invention.
BACKGROUND
The apparatuses and methods described herein generally relate to ionization
sources for
mass spectrometers and methods of mass spectrometry, and in particular, laser
ablation
electrospray ionization mass spectrometry as well as methods of making and
using the same.
Infectious diseases and metabolic disorders cause death, disability, and
social and
economic distress for millions of people throughout the world. Examples of the
causative agents
of infectious diseases include, but are not limited to, human immunodeficiency
virus I (HIV-1),
human immunodeficiency virus 2 (HIV-2), human T-lymphotropic virus type 1
(HTLV1), ),
human T-lymphotropic virus type 2 (HTLV2), human T-lymphotropic virus type 3
(HTLV3),
human papillomavirus (HPV), hepatitis A, hepatitis B, hepatitis C, viral
encephalitis, herpes
virus, paramyxoviruses, influenza, and severe acute respiratory syndrome
(SARS). Examples of
metabolic disorders include, but are not limited to, diabetes, muscular
dystrophy,
phenylketonuria (PKU), Tay Sachs disease, leukodystrophies, lysosomal
disorders, Wilson's
disease, Lesch-Nyhan syndrome, urea cycle disorder, amyloidosis and lipid
storage diseases.
Genomic, proteomic, and metabolomic technologies have been used to identify
biomarkers and
other indicators of infectious diseases and metabolic disorders. Rapid
monitoring of the
metabolome may be more useful than monitoring the transcriptome or proteome
because the
metabolic composition of a cell or organism provides its actual biochemical
condition.
Infectious diseases and metabolic disorders may alter the metabolism of a
living cell or
organism. In other words, the metabolism of a healthy cell may be different
than the
metabolism of an unhealthy cell of the same type. Metabolism generally refers
to the chemical
processes of a living cell or organism that support and maintain life. The
products of these
chemical processes may be generally referred to as metabolites. The metabolism
and/or
metabolites of a living cell or organism may change depending on its
biological state,
developmental stage, history, and/or environment. Viruses, for example, may
alter the
metabolism of an infected cell. The metabolisms and/or metabolites of a
virally infected cell
and an uninfected cell of the same type may be different. The identification
and analysis of
- 1 -
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
metabolites may facilitate the detection, prevention, and/or treatment of
infectious diseases and
metabolic disorders.
Mass spectrometry is an analytical technique that has been successfully used
in
chemistry, biology, and other fields for qualitative and quantitative
analysis. The identification
and analysis of metabolites by conventional methods of mass spectrometry may
be problematic.
For example, matrix-assisted laser desorption ionization (MALDI) and
electrospray ionization
(ESI), may suffer from time consuming and complex sample preparation, and in
situ analysis of
a sample may be difficult. Atmospheric pressure mass spectrometry methods,
such as direct
analysis in real time (DART), desorption electrospray ionization (DESI),
atmospheric pressure
infrared matrix-assisted laser desorption ionization (AP IR-MALDI), desorption
atmospheric
pressure chemical ionization (DAPCI), matrix-assisted laser desorption
electrospray ionization
(MALDESI), and electrospray laser desorption ionization (ELDI), may suffer
from other
limitations, including, but not limited to, complex and time consuming
separation techniques, a
narrower range of samples that may be analyzed, higher detection limits,
sensitivity to surface
properties, sampling errors, and/or lack of imaging and quantization
capabilities. Mass
spectrometry may be combined with separation techniques, such as gas
chromatography, high
performance liquid chromatography and capillary electrophoresis, however,
these techniques
may increase the analysis time and/or cost.
Therefore, more efficient and/or cost-effective mass spectrometers and methods
of
making and using the same are desirable.
SUMMARY
In certain embodiments, more efficient and/or cost-effective mass
spectrometers and
methods of making and using the same are described.
According to certain embodiments, a method of mass spectrometry may generally
comprise subjecting a sample comprising at least one indicator to laser
ablation electrospray
ionization mass spectrometry; determining a relative intensity of the
indicator; and comparing
the relative intensity of the indicator to a standard indicator intensity.
DESCRIPTION OF THE DRAWING FIGURES
The various embodiments described herein may be better understood by
considering the
following description in conjunction with the accompanying drawing figures.
FIGS. 1-2 include illustrations of LAESI ion sources for mass spectrometers in
accordance with various embodiments described herein.
FIG. 3A and 3B include photographs illustrating an ablation plume (LA) and an
electrospray plume (ES) in accordance with various embodiments described
herein.
-2-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
FIGS. 4-5 include flow diagrams illustrating methods of mass spectrometry in
accordance with various embodiments described herein.
FIGS. 6A and 6B and 7A-C include tables listing representative data from
methods of
mass spectrometry in accordance with various embodiments described herein.
FIGS. 8A and 8B include representative mass spectra in accordance with various
embodiments described herein.
FIGS. 9A-D include representative mass spectra in accordance with various
embodiments described herein.
FIGS. 1 OA-C include representative mass spectra in accordance with various
embodiments described herein.
FIG. 11 includes a flow diagram illustrating the metabolic pathways involving
putrescine, spermidine, and spermine.
FIG. 12 includes a chart comparing CEM and C81 cells in accordance with
various
embodiments described herein.
FIGS. 13A and 13B include representative mass spectra in accordance with
various
embodiments described herein.
FIGS. 14A-C include charts comparing CEM and C81 cells in accordance with
various
embodiments described herein.
FIGS. 15-16 include tables listing representative data from methods of mass
spectrometry in accordance with various embodiments described herein.
FIG. 17 includes a flow diagram illustrating creatine and polyamine
biosynthesis
pathways in T lymphocytes.
FIGS. 18A and 18B includes representative mass spectra in accordance with
various
embodiments described herein.
FIG. 19 includes a flow diagram illustrating a lipid metabolism pathway in T
lymphocytes.
FIGS. 20A and 20B include a flow diagram illustrating metabolites detected in
accordance with various embodiments described herein.
DESCRIPTION OF CERTAIN EMBODIMENTS
A. Definitions
As generally used herein, the terms "consisting essentially of and "consisting
of are
embodied in the term "comprising".
As generally used herein, the articles "one", "a", "an" and "the" refer to "at
least one" or
"one or more", unless otherwise indicated.
-3-
RECTIFIED SHEET (RULE 91)
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
As generally used herein, the terms "including" and "having" mean
"comprising".
As used herein, the terms "LAESI" and "LAESI-MS" refer to laser ablation
electrospray
ionization mass spectrometry.
As used herein, the term "infection" refers to the invasion by, multiplication
and/or
presence of a virus or bacteria in a cell, tissue, organ and/or organism.
As used herein, the term "metabolic disorder" refers to any pathological
condition in a
cell, tissue, organ and/or organism resulting from an alteration in an
organism's metabolism.
As used herein, the term "metabolome" refers to the total or partial set of
metabolites in a
cell, tissue, organ and/or organism at a specific time. An infection and/or
metabolic disorder
may cause certain metabolites to be upregulated or downregulated.
As used herein, the term "pattern" refers to a set of metabolites and their
intensities
measured by a diagnostic method. The set of metabolites may be the total or
partial set of
metabolites in a cell, tissue, organ and/or organism at a specific time
As generally used herein, the terms "about" and "approximately" refer to an
acceptable
degree of error for the quantity measured, given the nature or precision of
the measurements.
Typical exemplary degrees of error may be within 20%, 10%, or 5% of a given
value or range of
values. Alternatively, and particularly in biological systems, the terms
"about" and
"approximately" refer to values within an order of magnitude, potentially
within 5-fold or 2-fold
of a given value.
All numerical quantities stated herein are approximate unless stated
otherwise; meaning
that the term "about" may be inferred when not expressly stated. The numerical
quantities
disclosed herein are to be understood as not being strictly limited to the
exact numerical values
recited. Instead, unless stated otherwise, each numerical value is intended to
mean both the
recited value and a functionally equivalent range surrounding that value. At
the very least, and
not as an attempt to limit the application of the doctrine of equivalents to
the scope of the claims,
each numerical parameter should at least be construed in light of the number
of reported
significant digits and by applying ordinary rounding techniques.
Notwithstanding the
approximations of numerical quantities stated herein, the numerical quantities
described in
specific examples of actual measured values are reported as precisely as
possible.
All numerical ranges stated herein include all sub-ranges subsumed therein.
For
example, a range of "1 to 10" is intended to include all sub-ranges between
and including the
recited minimum value of I and the recited maximum value of 10. Any maximum
numerical
limitation recited herein is intended to include all lower numerical
limitations. Any minimum
numerical limitation recited herein is intended to include all higher
numerical limitations.
-4-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
In the following description, certain details are set forth in order to
provide a better
understanding of various embodiments of mass spectrometers and methods for
making and
using the same. However, one skilled in the art will understand that the
embodiments described
herein may be practiced without these details. In other instances, well-known
structures and
methods associated with mass spectrometers and methods of mass spectrometry
may not be
shown or described in detail to avoid unnecessarily obscuring descriptions of
the embodiments
of this disclosure.
This disclosure describes various features, aspects, and advantages of various
embodiments of mass spectrometers and methods for making and using the same.
It is
understood, however, that this disclosure embraces numerous alternative
embodiments that may
be accomplished by combining any of the various features, aspects, and
advantages of the
various embodiments described herein in any combination or sub-combination
that one of
ordinary skill in the art may find useful.
B. Overview
Infection of a cell or organism causes extensive changes at the gene, protein,
and
metabolite levels. These changes are usually followed by gene-expression
profiling and
proteomic analysis. Viruses, for example, rely on the metabolic network of
their cellular hosts
for survival and replication. Exploring the metabolic consequences of a viral
infection may
provide insight into the causes and/or treatment of viral infection. The
insight gained by such
studies may depend on the target sample, treatment procedures, and detection
techniques used.
Conventionally, biofluids, such as blood and urine, have been used to follow
metabolic changes
after infection, but in many cases they may complicate the analysis due to the
pooling of
changes in different cell types and the variations between individuals.
Ultimately, direct
analysis of a sample is a more straightforward way to understand the actual
disease-associated
metabolic changes in and at the site of an infection. In such cases, a direct
detection technique
may offer key advantages.
Metabolites are small molecules of diverse physico-chemical properties with
greatly
different abundance levels that make their identification and analysis
challenging. Typically,
optical spectrometry, such as Fourier transform infrared spectrometry, nuclear
magnetic
resonance (NMR), and mass spectrometric techniques in combination with
separation
techniques, such as gas chromatography, high performance liquid chromatography
(HPLC) and
capillary electrophoresis, have been used for metabolomic studies. Mass
spectrometry (MS) is a
versatile technique that, combined with chromatographic separations, may
provide qualitative
and quantitative analyses of complex samples with high selectivity and
sensitivity, as well as a
-5-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
broad dynamic range. Conventional mass spectrometric methods, however, are
time consuming
and involve extensive sample preparation. The application of direct sampling
methods, such as
flow injection electrospray ionization (ESI), may avoid chromatographic
separation, but not the
extensive sample preparation that may affect sample integrity and, in some
cases, may lead to
metabolite degradation, Therefore, these techniques generally restrict the
choice of samples and
discourage their in situ analysis.
Some of these problems may be mitigated by the use of atmospheric pressure ion
sources. Recent advances in atmospheric pressure ion sources, such as direct
analysis in real
time (DART), desorption electrospray ionization (DESI), atmospheric pressure
infrared matrix-
assisted laser desorption ionization (AP IR-MALDI), and laser ablation
electrospray ionization
(LAESI) may enable direct analysis of cell and tissue samples without
extensive sample
preparation. Analysis of cells, cell cultures, and cell extracts using DART,
DESI, and MALDI
techniques may have their own limitations, such as coverage of analytes,
sampling of the surface
only, and quantitation restrictions. According to certain embodiments, LAESI-
MS may provide
for in situ cell and tissue analysis, and may sample the entire volume of the
cells for metabolites
and lipid components with tissue imaging and quantitation capabilities.
Previous studies of viral infection indicate that glycolysis, the citrate
(TCA) cycle,
pyrimidine nucleotide biosynthesis, and lipid metabolism are the main
metabolic changes
generally involved with viral infection. The metabolic intermediates of these
pathways may
increase in response to the infection, e.g., faster uptake of glucose and
glutamine, greater
accumulation of citrate, and increasing excretion of lactate and glutamate in
infected cells. The
efflux from infected cells may be enriched in metabolites related to
nucleotide and fatty acid
biosynthesis. The fatty acid biosynthesis in infected cells may be considered
an antiviral
response. Previous studies of HIV infected cells indicate a reduction of
glutathione levels,
changes in lipid metabolism, and polyamine pools (putrescine, spermidine and
spermine).
However, these studies were carried out using conventional MS-based techniques
involving both
sample extraction and chromatographic separation. According to certain
embodiments, LAESI-
MS provides rapid and direct identification of metabolic changes in HTLV I
infected T
lymphocytes and reduces and/or eliminates the need for extensive sample
preparation.
Human T cell leukemia virus type I (HTLV 1), a member of the delta-
retroviridae
subfamily, was the first human pathogenic retrovirus discovered. HTLV 1 may
contribute to
cancer development. Infection with HTLV 1 may result in the development of
adult T-cell
leukemia (ATL), a CD4+ T lymphoproliferative malignancy. Estimates of
worldwide HTLV 1
infections may be between 15 million to 25 million individuals. However,
infected individuals
-6-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
develop ATL after a long latent period and at a 3-5% incidence rate. Infection
with HTLV1 may
result in HTLV 1 -associated myelopathy/tropical spastic paraparesis (HAM/TSP)
and several
inflammatory diseases, including polymyositis, uveitis, and lymphocyte
alveolitis. The
development of ATL from HTLV 1 infection may be a multi-hit occurrence with
initial
transformation due to the viral protein Tax. Recent studies have indicated
that the use of novel
treatments, including monoclonal antibodies against the interleukin-2 receptor
(IL-2R) and the
combination therapy of interferon-alpha (IFN-a) and zidovudine (AZT), to be
effective, but only
in a small percentage of ATL patients. Therefore, new therapies for the
treatment of ATL, and
in particular, HTLV 1 infection, are desirable.
According to certain embodiments, mass spectrometers and methods of mass
spectrometry for identifying and analyzing changes in the metabolic profiles
of a cell, tissue,
and/or organism after infection and/or development of a metabolic disorder.
The metabolic
changes in virally infected cells and tissues may be monitored using high
throughput
metabolomic and complementary transcriptomic and proteomic technologies, such
as LAESI-
MS, as described herein. In certain embodiments, LAESI-MS may be used to
identify and
analyze changes in the metabolic profiles of a cell, tissue, and/or organism
after HTLV 1
infection, HTLV3 infection, Tax 1 expression, and Taxi expression as well as
comparing the
metabolic profiles of cells and tissues transfected with either HTLV3
molecular clone or Taxi
and HTLV 1 transformed cells. Understanding the role of Tax in destabilizing
key regulators,
such as proteins, in metabolism and cell cycle control, may help identify
molecular markers that
contribute to ATL development and define new therapeutic strategies. Certain
embodiments
comprise in situ metabolite profiling of infected cells. Certain embodiments
comprise
facilitating the identification of virus-induced perturbations in the
biochemical processes of a
host cell.
Certain embodiments of the mass spectrometers and methods of making and using
the
same described herein may provide certain advantages over other approaches of
mass
spectrometric analysis. The advantages may include, but are not limited to, in
situ analysis,
simultaneous detection of multiple samples, independent optimization of
ablation conditions and
ionization conditions, a wider dynamic range of samples that may be used,
operation under
ambient conditions, simpler sample preparation, minimal sample manipulation,
minimal sample
degradation, improved sampling time, positional sensitivity, improved
sensitivity to surface
properties, and/or improved detection limits.
C. Laser Ablation Electrospray Ionization Mass Spectrometry
-7-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
According to certain embodiments, a mass spectrometer for laser ablation
electrospray
ionization mass spectrometry may generally comprise a laser system, an
electrospray apparatus,
and a mass spectrometer. The laser system may comprise a laser and a focusing
system
comprising fiber optics, coupling lenses, and/or focusing lenses, and an x-y-z
translation stage
having a sample mount. The laser may be selected from the group consisting of
an Er:YAG
laser, an Nd:YAG laser driven optical parametric oscillator and a free
electron laser. The
electrospray apparatus may comprise an electrospray ionization emitter having
a power supply
and a syringe pump. The mass spectrometric ion source may comprise a solid
state camera. The
mass spectrometric ion source may comprise a shroud to enclose the sample, the
sample holder,
and/or the electrospray emitter. The translation stage and the sample
environment may be
temperature controlled and/or atmosphere controlled. This is to maintain
sample integrity and to
avoid condensation of moisture from the environment. The atmosphere may
comprise ambient
atmosphere. The temperature may ranges from -10 C to 60 C. The relative
humidity may range
from 10% to 90%.
In certain embodiments, the atmosphere and/or the electrospray solution may
comprise a
reactive component to facilitate the ionization and/or fragmentation of
certain constituents of the
sample. For example, the electrospray solution may comprise Li2SO4 to
facilitate the structural
identification of lipids by inducing structure specific fragmentation in
collision induced
dissociation experiments. Examples of reactive gases include, but are not
limited to, ammonia,
SO2, and NO2.
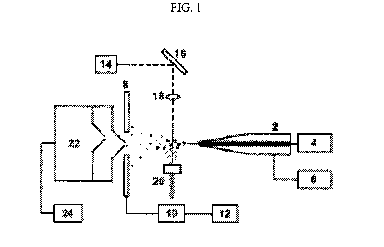
Referring to FIG. 1, in certain embodiments, a mass spectrometer comprising a
LAESI
ion source may generally comprise an electrospray capillary 2, an optional
liquid supply with
pump 4, a high voltage power supply 6, a counter electrode 8, an oscilloscope
10, a recording
device 12, e.g., personal computer, a laser 14, such as an Er:YAG laser or
Nd:YAG laser driven
optical parametric oscillator, a beam steering device 16, e.g., a mirror, a
focusing device 18, e.g.,
lens or sharpened optical fiber, a sample holder with x-y-z positioning stage
20, a mass
spectrometer 22, and a recording device 24, e.g., a personal computer.
Referring to FIG. 2, in certain embodiments, a mass spectrometer comprising a
LAESI
ion source may generally comprise an electrospray capillary (E), an optional a
liquid supply with
pump (SP), a high voltage power supply (HV), a laser, such as an Er:YAG laser
or Nd:YAG
laser driven optical parametric oscillator, beam steering devices, e.g., a
mirror (M), a focusing
device, e.g., lens or sharpened optical fiber, a sample holder with x-y-z
positioning stage (TS), a
long-distance video microscope (FMM), a second video microscope (CSM), and a
mass
spectrometer (MS). In certain embodiments, a mass spectrometer comprising a
LAESI ion
-8-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
source may comprise an etched optical fiber tip (F) to generate mid-IR
ablation products that
may be intercepted by the electrospray plume and post-ionized to form ions
sampled by the mass
spectrometer (MS), a long-distance video microscope (fiber monitor, FMM) to
maintain a
constant distance between the fiber tip and the sample surface (S), a sample
placed on a three-
axis translation stage (TS), and a second video microscope (cell spotting
microscope, CSM) to
target the sample. The electrospray may be produced by applying high voltage
(HV) to the
capillary emitter (E) and by maintaining a constant solution flow rate by a
syringe pump (SP).
Pulses from the laser may be coupled to the optical fiber, adjusted by a fiber
chuck (C) and a
five-axis fiber mount (FM), using two Au-coated mirrors (M) and a CaF2 lens
(L). The LAESI-
MS device may comprise a recording device, e.g., a personal computer. The mass
spectrometer
comprising a LAESI ion source may be configured for single cell analysis. The
LAESI-MS
method for a single cell may comprise using micromanipulators and reducing the
laser spot size
from 5 m to 200 m.
In certain embodiments, an ablation plume (LA) may intersect an electrospray
plume
(ES). Referring to FIGS. 3A and 3B, the electrosprayed droplets travel
downstream from the
emitter (from left to right). The electrosprayed droplets are intercepted by
particulates traveling
upward from the ablation plume. The ablation plume may comprise 1 m to 3 m
particles.
Without wishing to be bound to any particular theory, at the intersection of
the two plumes,
some of the ablated particulates may fuse with the electrospray droplets to
form charged droplets
that contain some of the ablated material, and ultimately produce ions in an
ESI process. The
electrospray emitter may be operated in a pulsating spraying regime and/or a
cone-jet regime.
As shown in FIG. 3A, the pulsating spraying regime may offer a lower duty
cycle and produce
larger electrospray droplets resulting in lower ionization efficiency and
LAESI signal. As
shown in FIG. 3B, the cone-jet regime may produce smaller electrospray
droplets resulting in
higher ionization efficiency and LAESI signal.
In certain embodiments, the laser may comprise an infrared laser. The infrared
laser may
operate at a wavelength from 2600 nm to 3450 nm, such as 2800 nm to 3200 nm,
and 2930 nm
to 2950 nm. The laser may comprise a mid-infrared pulsed laser operating at a
wavelength from
2600 rim to 3450 nm, a repetition rate from 1 Hz to 100 Hz, and a pulse width
from 0.5 ns to 50
ns. In at least one embodiment, the laser may comprise a diode pumped Nd:YAG
laser-driven
optical parametric oscillator (OPO) (Opolette 100, Opotek, Carlsbad, CA)
operating at 2940 rim,
100 Hz repetition rate, and 5 ns pulse width. The optical fiber may comprise a
germanium oxide
(Ge02)-based optical fiber (450 pm core diameter, HP Fiber, Infrared Fiber
Systems, Inc., Silver
Spring, MD) with its tip etched to a radius of curvature from 1 m to 50 m,
such as 5 gm to 25
-9-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
gm, and 10 pm to 15 m, In at least one embodiment, the radius of curvature
may be 15 pm.
The optical fiber may deliver the laser pulse to the sample. The energy of a
laser pulse before
coupling into the optical fiber may be from 0.1 mJ to 6 mJ, thus the pulse-to-
pulse energy
stability generally corresponds to 2% to 10%. In at least one embodiment, the
energy of a laser
pulse before coupling into the optical fiber may be 5 54 26 J, thus the
pulse-to-pulse energy
stability generally corresponds to 5%. The laser system may be operated at 100
Hz from 0.01
seconds to 20 seconds to ablate a sample, In at least one embodiment, laser
system may be
operated at 100 Hz for 1 second to ablate a sample. In certain embodiments, 1
to 100 laser
pulses may be delivered to a sample for analysis.
In certain embodiments, the electrospray source may comprise a low noise
syringe pump
(Physio 22, Harvard Apparatus, Holliston, MA) to supply the electrospray
solution to a tapered
stainless steel emitter (inner diameter 50 pm, MT320-50-5-5, New Objective,
Woburn, MA).
The low noise syringe pump may supply the electrospray solution at a rate from
10 nL/min to 10
pL/min. In at least one embodiment, the low noise syringe pump may supply the
electrospray
solution at 200 nL/min. The tapered stainless steel emitter may have an
outside diameter from
100 pm to 500 m and an insider diameter from 10 pm to 200 pm. The power
supply may
comprise a regulated power supply (PS350, Stanford Research Systems,
Sunnyvale, CA), to
provide a stable high voltage from 2.5 to 5 kV to the electrospray emitter.
The power supply
may be mounted on a manual translation stage to optimize the LAESI signal by
adjusting the
relative position of the sample, electrospray emitter, and/or inlet orifice of
the mass
spectrometer. The electrospray solution may comprise at least one of 50%
methanol with 0.1%
(v/v) acetic acid, 50% methanol with 0.1 % (v/v) formic acid, 50% methanol
with 0.1 % (v/v)
trifluoroacetic acid, 50% methanol with 0.1% (v/v) ammonium acetate. The
electrospray
solution may be applied at an angle from 0 to 90 , such as 30 , 45 , and 60 ,
into the ablation
plume. The angle may be adjusted from 0 to 90 to optimize ion production. In
at least one
embodiment, the electrospray solution may be applied at a right angle (90 )
into the ablation
plume.
According to certain embodiments, the mass spectrometer orifice may be on the
same or
a different axis as the electrospray emitter of the LAESI ion source. The
angle between the mass
spectrometer orifice and electrospray emitter of the LAESI ion source may be
from 0 to 90 ,
such as 30 , 45 , and 60 . The distance from the mass spectrometer orifice to
the electrospray
emitter tip may be from 1 mm to 20 mm, such as 5 mm to 15 mm. In at least one
embodiment,
the distance from the mass spectrometer orifice to the electrospray emitter
tip may be 12 mm.
The sample may be placed onto a pre-cleaned microscope glass slide (catalog
no. 125496, Fisher
-10-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
Scientific, Pittsburgh, PA). The sample may be placed onto a stepper motor-
driven three axis
precision flexure stage (NanoMax TS, Thorlabs, Newton, NJ). The sample may be
1 mm to 30
mm below the spray axis, such as 5 mm to 25mm, and 10 mm to 20 mm. In at least
one
embodiment, the sample may be 15 mm below the spray axis. In one experiment,
no ions were
detected by the mass spectrometer when the ESI was off, indicating that no
ions directly induced
by the laser were collected. This observation may result from the large (>15
mm) distance
between the orifice of the mass spectrometer and the ablated sample.
The positive ions produced by the LAESI ion source may be analyzed by a mass
spectrometer. The mass spectrometer may comprise an orthogonal acceleration
time-of-flight
mass spectrometer (QTOF Premier, Waters Co., MA). The orifice of the mass
spectrometer
may have an inner diameter from 100 gm to 500 m, such as 225 m to 375 m. In
at least one
embodiment, the orifice of the mass spectrometer may have an inner diameter
from 100 m to
200 m, such as 127 p.m. The orifice of the mass spectrometer may be extended
by a straight or
curved extension tube having a similar inner diameter as the orifice of the
mass spectrometer
and a length from 20 mm to 500 mm. The interface block temperature may be from
ambient
temperature to 150 C, such as 23 C to 90 C, and 60 C to 80 C. In at least one
embodiment, the
interface block temperature may be 80 C. The potential may be from -100 V to
100 V, such as -
70 V to 70 V. In at least one embodiment, the potential may be -70 V. Tandem
mass spectra
may be obtained by collision activated dissociation (CAD) with a collision
gas, such as argon,
helium or nitrogen, at a collision cell pressure from 10-6 mbar to 10-2 mbar,
and with collision
energies from 10 eV to 200 eV. In at least one embodiment, the collision gas
may be argon, the
collision cell pressure may be 4x 10-3 mbar, and the collision energies may be
from 10 eV to 25
eV.
In certain embodiments, the laser beam may be steered by gold-coated mirrors
(PF10-03-
MO1, Thorlabs, Newton, NJ) and coupled into the cleaved end of the optical
fiber by a plano-
convex calcium fluoride lens (Infrared Optical Products, Farmingdale, NY)
having a focal length
from 2 mm to 100 mm, such as 25 mm to 75 mm, and 40 mm to 60 mm. In at least
one
embodiment, the focal length may be 50 mm. The optical fiber may be held by a
bare fiber
chuck (BFC300, Siskiyou Corporation, Grants Pass, OR). The optical fiber may
be positioned
by a five-axis translator (BFT-5, Siskiyou Corporation, Grants Pass, OR).
In certain embodiments, the optical fiber may comprise a Ge02-based glass
fiber, a
fluoride glass fiber, and a chalcogenide fiber. The optical fiber may have a
high laser-damage
threshold due to its high glass transition temperature. The Hytrel and
polyimide coatings may
be stripped off both ends of the fiber by the application of 1 -methyl-2-
pyrrolidinone (at 130 C to
-11-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
150 C for 1 min). After stripping off the Hytrel and the polyimide coatings,
the fiber ends may
be cleaved with a Sapphire blade (KITCO.Fiber Optics, Virginia Beach, VA) by
scoring and
gently snapping them. Chemical etching of the Ge02-based glass fiber tip may
be achieved by
dipping one of the cleaved fiber ends 0.5 mm deep into 24 C 1% HNO3 solution
in a wide
beaker to provide a low meniscus curvature. The meniscus formed at the fiber
end may
gradually etch the 450 m diameter core into a sharp tip having a radius of
curvature (R) of 15
m. Prior to use, the etched tips may be washed with deionized water. In
certain embodiments,
no visible change of the fiber tip may be observed after performing the LAESI
technique which
may indicate the absence of damage or contamination.
In certain embodiments, the etched end of the fiber may be attached to a
micromanipulator (MN-151, Narishige, Tokyo, Japan) to move the etched end of
the fiber closer
to the sample. The distance from the etched end of the fiber and the sample
may be from contact
(0 m) to 50 m. In at least one embodiment, the coordinate system may be
aligned so that the
x-y plane coincides with the sample and the x-axis is parallel with the
emitter, the optical fiber is
positioned at an azimuth angle from 20 to 160 and a zenith angle from 20 to
70 . In at least
one embodiment, the azimuth angle may be 135 and the zenith angle may be 45 .
The zenith
angle of 45 may provide an acceptable trade-off between the shape of the
ablation mark and
signal intensity reduction by blocking the expanding plume. A thin sample
material deposit may
be observed on the fiber tip after ablation. In these cases, the fiber may be
retracted from the
surface and elevated laser pulse energy may be used to clean the tip. In at
least one
embodiment, the distance between the fiber tip and the sample surface (h) may
be 2R. This
may result in an ablation mark with an average diameter of 2.5R. In at least
one embodiment,
the distance between the fiber tip and the sample surface may be 30 m,
resulting in an ablation
mark with an average diameter of 37.5 pm. Microscope images of the ablation
marks may be
obtained by an upright microscope (BX 51, Olympus America Inc., Center Valley,
PA) in either
reflected or transmitted mode and by an inverted microscope.
In certain embodiments, the mass spectrometer ion source may comprise a
visualization
system. The distance between the fiber tip and sample surface may be monitored
by a long
distance video microscope (InFocus Model KC, Infinity, Boulder CO) with a 5x
infinity
corrected objective lens (M Plan Apo 5 x, Mitutoyo Co., Kanagawa, Japan), and
the image may
be captured by a CCD camera (Marlin F131, Allied Vision Technologies,
Stadtroda, Germany).
With the environmental vibration in the low micrometer range, an approximate
distance from 30
m to 40 .tm may be maintained between the tip and the sample. A similar video
microscope
system may be used at a right angle to the sample surface to align the fiber
tip over the location
-12-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
of interest in the sample for ablation. The visualization system may comprise
a 7x precision
zoom optic (Edmund Optics, Barrington, NJ), fitted with a l Ox infinity-
corrected long working
distance objective lens (M Plan Apo lOx, Mitutoyo Co., Kanagawa, Japan) and a
CCD camera
(Marlin F131, Allied Vision Technologies, Stadtroda, Germany).
In certain embodiments, the sample may comprise any sample that comprises
water,
including, but not limited to, cells, tissues, organs, biofilms, aqueous
solutions, organic
materials, inorganic materials, synthetic materials, biomedical samples,
forensic samples,
biological warfare agents, and wetted surfaces. For example, the sample may be
selected from
the group consisting of biomolecules (such as metabolites, lipids, nucleic
acids, proteins,
peptides, and carbohydrates), organic and inorganic molecules (such as
pharmaceuticals,
polymers, dendrimers and other macromolecules), and mixtures thereof. The
sample may
comprise a biof lm comprising an aggregate of microorganisms in which cells
adhere to each
other and/or to a surface. The sample may comprise a single cell, cells, small
cell populations,
cell lines, and tissues. The single cell may have a smallest dimension less
than 100 micrometers,
such as less than 50 pm, less than 25 m, and/or less than 10 m. The single
cell may have a
smallest dimension from 1 pm to 100 [tm, such as from 5 pm to 50 m, and from
10 m to 25
m. In at least one embodiment, the single cell may have a smallest dimension
from 1 m to 10
m. The small cell population may comprise 10 to 1 million cells, such as 50
cells to 100,000
cells, and 100 cells to 1,000 cells. The sample may comprise a cell infected
with at least one of
a virus and a bacterium. The sample may comprise a virally infected living
cell and/or tissue.
The sample may comprise a cell having a metabolic disorder.
Referring to FIG. 4, according to certain embodiments, a method of mass
spectrometry
may generally comprise subjecting a sample comprising an indicator to laser
ablation
electrospray ionization mass spectrometry; determining a relative intensity of
the indicator; and
comparing the relative intensity of the indicator to a standard indicator
intensity. Subjecting the
sample to laser ablation electrospray ionization mass spectrometry may
comprise ablating the
sample with an infrared laser under ambient conditions to form an ablation
plume; intercepting
the ablation plume by an electrospray plume; and detecting the indicator by
mass spectrometry.
In certain embodiments, subjecting the sample to laser ablation electrospray
ionization mass
spectrometry may exclude pretreating the sample with a matrix material. In
certain
embodiments, determining the relative intensity of the indicator may comprise
determining the
relative intensity of an indicator comprising multiple charge states. The
standard indicator
intensity may comprise an internal reference and/or an external reference.
-13-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
According to certain embodiments, the indicator may comprise a biomolecule, an
organic molecule, a molecular complex and/or a xenobiotic. The indicator may
be selected from
the group consisting of metabolites, lipids, lipid precursors, lipid
components, nucleic acids,
proteins, peptides, carbohydrates, and combinations thereof. The indicator may
comprise
intracellular metabolites from a single cell. The indicator may comprise a
biomarker. The
biomarker may be related to a metabolic change. The biomarker may be related
to a disease
state. The disease state may be associated with a viral infection, a bacterial
infection, and/or a
metabolic disorder.
Viruses may include, but are not limited to, human immunodeficiency virus 1
(HIV-1),
human immunodeficiency virus 2 (HIV-2), human T-lymphotropic virus type 1
(HTLV 1),
human T-lymphotropic virus type 2 (HTLV2), human T-lymphotropic virus type 3
(HTLV3),
human papillomavirus (HPV), paramyxoviruses, hepatitis A, hepatitis B,
hepatitis C, viral
encephalitis, herpes virus, influenza, and/or severe acute respiratory
syndrome (SARS).
Bacteria may include, but are not limited to, Bacillus anthracis (the
causative agent of anthrax),
Yersinia pestis (the causative agent of bubonic plague), Mycobacterium
tuberculosis (the
causative agent of tuberculosis) and Vibrio cholera (the causative agent of
cholera). The
metabolic disorder may comprise diabetes, muscular dystrophy, phenylketonuria
(PKU), Tay
Sachs disease, leukodystrophies, lysosomal disorders, Wilson's disease, Lesch-
Nyhan
syndrome, urea cycle disorder, amyloidosis and/or lipid storage diseases. In
certain
embodiments, the disease state may comprise human immunodeficiency virus
(HIV), human T-
lymphotropic virus type 1 (HTLV 1), and/or human T-lymphotropic virus type 3
(HTLV3) as
well as Taxi and/or Taxi expressing cells, and the indicator may comprise
glutathione,
spermine, spermidine, putrescine, arginine, creatine, choline, phosphocholine,
glycerophosphocholine, glycerophosphocholine lipids, ATP, ADP, AMP, cAMP,
dopamine,
dopamine metabolites, and/or any combination thereof. In certain embodiments,
the indicator
may be related to a metabolic change caused by exposure to a toxin. For
example, the activity of
an enzyme, butyrylcholinesterase, in blood may be used as a biomarker for
nerve agent
exposure.
According to certain embodiments, the method of mass spectrometry may comprise
classifying the sample as belonging to or not belonging to the standard
indicator intensity. A
sample not belonging to the standard indicator intensity may indicate that the
sample comprises
a metabolic change. The metabolic change may be associated with a viral
infection, a bacterial
infection, and/or a metabolic disorder. A sample not belonging to the standard
indicator
-14-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
intensity may indicate that the sample is predicted to comprise a disease
state. The disease state
may be associated with a viral infection, a bacterial infection, and/or a
metabolic disorder.
In certain embodiments, the indicator may comprise a plurality of indicators.
The
method of mass spectrometry may comprise determining the relative intensity of
each indicator
to form a sample metabolite pattern and comparing the sample metabolite
pattern to a standard
metabolite pattern comprising the standard indicator intensity of each of the
plurality of
indicators. The method of mass spectrometry may comprise classifying the
sample as belonging
to or not belonging to the standard metabolite pattern. A sample not belonging
to the standard
metabolite pattern may indicate that the sample comprises a metabolic change.
A sample not
belonging to the standard metabolite pattern may indicate that the sample is
predicted to
comprise a disease state.
Referring to FIG. 5, according to certain embodiments, an in situ method of
determining
a metabolic state of a sample comprising an indicator may generally comprise
ablating the
sample with an infrared laser under ambient conditions to form an ablation
plume; intercepting
the ablation plume by an electrospray plume; detecting the indicator by mass
spectrometry;
determining a relative intensity of the indicator; comparing the relative
intensity of the indicator
to a standard indicator intensity; and classifying the sample as belonging to
or not belonging to
the standard indicator intensity. A sample not belonging to the standard
indicator intensity may
indicate that the sample comprises a metabolic change. A sample not belonging
to the standard
indicator intensity may indicate that the sample is predicted to comprise a
disease state. The
metabolic change may be associated with a viral infection, a bacterial
infection, and/or a
metabolic disorder.
In certain embodiments, determining the relative intensity of the indicator
may comprise
determining the relative intensity of an indicator comprising multiple charge
states. The
indicator may comprise a plurality of indicators. The method of mass
spectrometry may
comprise determining the relative intensity of each indicator to form a sample
metabolite pattern
and comparing the sample metabolite pattern to a standard metabolite pattern
comprising the
standard indicator intensity of each of the plurality of indicators. The
method of mass
spectrometry may comprise classifying the sample as belonging to or not
belonging to the
standard metabolite pattern. A sample not belonging to the standard metabolite
pattern may
indicate that the sample comprises a metabolic change. A sample not belonging
to the standard
metabolite pattern may indicate that the sample is predicted to comprise a
disease state. The
standard indicator intensity may comprise an internal reference and/or an
external reference.
-15-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
In certain embodiments, the metabolic state may be selected from the group
consisting of
developmental stages, for example, the stages of a cell cycle, environments,
nutritional supplies,
taxonomic units, genetic units, infected and uninfected states, diseased and
healthy states, and
different stages of pathogenicity.
According to certain embodiments, the method of determining a metabolic state
may
comprise ablating a second sample comprising the indicator with an infrared
laser under ambient
conditions to form a second ablation plume; intercepting the second ablation
plume by a second
electrospray plume; detecting the indicator of the second sample by mass
spectrometry;
determining the profile of the indicator of the second sample; comparing the
first indicator and
second indicator to each other and/or the standard indicator intensity; and
classifying at least one
of the first sample and the second sample as belonging to or not belonging to
the standard
indicator intensity. A sample not belonging to the standard indicator
intensity may indicate that
the sample comprises a metabolic change. A sample not belonging to the
standard indicator
intensity may indicate that the sample is predicted to comprise a disease
state. The metabolic
change and/or disease state may be associated with a viral infection, a
bacterial infection, and/or
a metabolic disorder. In certain embodiments, comparing the first indicator
and second indicator
to each other may indicate that the sample comprises a metabolic change. In
certain
embodiments, comparing the first indicator and second indicator to each other
may indicate that
the sample is predicted to comprise a disease state.
In certain embodiments, the method of mass spectrometry may comprise
determining
temporal and/or spatial information from a sample. The second sample may
comprise the first
sample at a different time. For example, the first sample may comprise a cell
and/or tissue and
the second sample may comprise the same cell and/or tissue at a later time,
such as after a
predetermined period of time or at different stages in the cell cycle. The
second sample may
comprise a different portion of the first sample. For example, the first
sample may comprise a
first portion of a cell and/or tissue and the second sample may comprise
another portion of the
same cell and/or tissue.
According to certain embodiments, the metabolic changes in virally infected
cells/tissues
may be monitored using LAESI-MS. In certain embodiments, changes in
metabolites and lipids
may be directly detected from uninfected T lymphocytes, human T-lymphotropic
virus type 1
(HTLV1) transformed cells, and human T-lymphotropic virus type 3 (HTLV3)
transformed
cells, and Tax 1 and Taxi expressing cell lines T lymphocytes. The mass
spectra of uninfected
and infected cells may be compared to identify any metabolic changes.
Glycerophosphocholine
(PC) lipid components may be dominant in the non-HTLV 1 transformed cells and
PC(O-32:1)
-16-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
and PC(O-34: 1) plasmalogens may be displaced by PC(30:0) and PC(32:0) species
in the
HTLV 1 transformed cells. In HTLV1 transformed cells choline, phosphocholine,
spermine and
glutathione, among others, may be downregulated, whereas creatine, dopamine,
arginine and
AMP may be upregulated. In certain embodiments, individual measurements on the
T-cells may
take a few seconds enabling high throughput studies using LAESI-MS. Analysis
of different
cell lines transfected with either the HTLV3 molecular clone or Taxi may
reveal metabolite
changes that correlate to HTLV 1 and HTLV3 infected cells, whereas others may
be unique to
HTLV 1.
According to certain embodiments, the method of mass spectrometry may comprise
identifying virus-induced perturbations in the biochemical processes of the
host cell by high
throughput in situ metabolite profiling of HTLV 1 and HTLV3 transformed cells.
This method
may be used to better understand the molecular mechanisms of HTLV1 and HTLV3
infections,
which in turn may result in drug development and/or new treatment strategies.
According to certain embodiments, the method of mass spectrometry may comprise
analyzing cell-to-cell metabolic variations and/or analyzing cells at
different stages of the cell
cycle. In certain embodiments, a method of mass spectrometry may comprise
identifying
metabolic changes in HTLV 1 and Tax1 transformed T lymphocytes and HTLV3 and
Taxi
transformed kidney epithelial cells. The indicators may comprise glutathione,
spermine,
choline, phosphocholine, glycerophosphocholine, thioacetamide, proline,
taurine, carbamoyl
phosphate, methoxytyramine and 8-hydroxy guanosine, creatine, arginine,
dopamine,
homovanillic acid and AMP. The levels of glutathione, spermine, choline,
phosphocholine,
glycerophosphocholine, thioacetamide, proline, taurine, carbamoyl phosphate,
methoxytyramine
and 8-hydroxy guanosine may be downregulated in C81 cells. The levels of
creatine, arginine,
dopamine, homovanillic acid and AMP may be upregulated in infected cells.
In certain embodiments, the method of mass spectrometry may comprise comparing
metabolic changes detected in H9-Taxl cells and H9 cells as well as HUT102
cells and H9 cells.
Some of the key metabolic changes detected.in C81 cells were also observed in
H9-Taxl cells
vs. H9 cells as well as HUT102 cells vs. H9 cells. Without wishing to be bound
to any
particular theory, these indicators participate in biochemical pathways, such
as polyamine
biosynthesis, creatine biosynthesis, AMP biosynthesis, dopamine metabolism,
lipid metabolism,
redox reactions etc. Comparing HTLVI and HTLV3 transfected cells revealed that
part of the
metabolic response was similar, but there were several changes specific to
HTLV1. For
example, the changes in the levels of putrescine, taurine, arginine and AMP
were consistent
among HTLV I transformed cells.
-17-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
The various embodiments described herein may be better understood when read in
conjunction with the following representative examples. The following examples
are included
for purposes of illustration and not limitation,
D. Examples
The uninfected T lymphocyte cells (CEM and H9) and kidney epithelial cells
(293T),
HTLV1 infected cells (C81 and HUT102), and H9 cells stably transfected with
Taxi of HTLV1
(H9-Taxl) and 293T cells infected with HTLV3 (293T-HTLV3) and expressing Taxi
(293T-
Tax3) were maintained in RPMI 1640 medium containing fetal bovine serum, L-
glutamine
(2mM), penicillin (100 units/mL) and streptomycin (100 pg/mL). The medium
solutions and
buffers were procured from Quality Biological Inc. (Gaithersburg, MD) and the
solvents were
HPLC grade available from Acros Organics (Geel, Belgium). The glacial acetic
acid was
procured from Fluka (Munich, Germany).
According to certain embodiments, the method of mass spectrometry was
performed by a
infrared laser system. An optical parametric oscillator (OPO) (Opolette 100,
Opotek, Carlsbad,
CA) converted the output of a 100 Hz repetition rate Nd:YAG laser to mid-IR
pulses of 5 ns
duration at 2940 nm wavelength. Beam steering and focusing was accomplished by
gold coated
mirrors (PF10-03-MO1, Thorlabs, Newton, NJ) and a 150 mm focal length CaF2
lens (Infrared
Optical Products, Farmingdale, NY), respectively. At 5-6 mm downstream from
the tip of the
spray capillary, the laser beam having average output energy of 0.3 mJ/pulse
was used to ablate
the tissue sample at a right angle (90 ). The laser spot size was determined
by optical
microscopy of the bum pattern produced on a photographic paper. The laser spot
size had a 300
.m diameter.
According to certain embodiments, the electrospray system comprised a low-
noise
syringe pump (Physio 22, Harvard Apparatus, Holliston, MA) to feed a 50%
methanol solution
containing 0.1% (v/v) acetic acid through a stainless steel emitter with
tapered tip having an
outside diameter of 320 m and an inside diameter of 50 gm. (MT320-50-5-5, New
Objective
Inc., Woburn, MA). Stable high voltage (2800 V) was generated by a regulated
power supply
(PS350, Stanford Research Systems, Inc., Sunnyvale, CA). The regulated power
supply was
directly applied to the emitter. The orifice of the sampling cone was on-axis
with the
electrospray emitter at a distance of 12 mm from its tip.
According to certain embodiments, the cells of interest were grown to
populations to
produce 106 cells/pellet before subjecting the sample to LAESI-MS. The cells
were washed
twice with phosphate buffered saline (PBS) and pelleted by spindown (2000
rpm). The
supernatant PBS was removed without disturbing the pellet, and 10 JAL of the
pellet was loaded
-18-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
onto a microscope slide and presented to the mass spectrometer for direct
LAESI analysis. The
microscope slide with the cell pellet was positioned 15 mm below the spray
axis under ambient
conditions (in air at ambient temperature and pressure). The microscope slide
was mounted on a
computer-controlled stepper motor-driven three-axis precision flexure stage
(Nanomax TS,
Thorlabs, Newton, NY) for rastering and geometry optimization.
According to certain embodiments, the ion source was mounted on a Q-TOF
Premier
mass spectrometer (Waters, Milford, MA). Full scan mass spectra were recorded
over the mass
range of m/z 50-2,000 using a time-of-flight (TOF) analyzer at a resolution of
8,000 (FWHM).
Individual measurements on the samples generally took a few seconds. For
structure
identification of individual metabolites, collision activated dissociation
spectra was recorded by
selecting the precursor ion using a quadrupole analyzer (transmission window 2
Da) and the
product ions were resolved by the TOF analyzer. Argon was used as the
collision gas at a
collision cell pressure of 4x10"3 mbar and a collision energy set from 5 to 25
eV. Accurate
masses were determined using an internal standard method. Glycine, methionine,
N-acetyl
phenylalanine, leucine enkephalin and glufibrinopeptide were dissolved in the
electrospray
solution to concentrations from 50 M to 200 pM and used as internal
standards. Averages of
the LAESI spectra collected under similar experimental conditions for a fixed
time were
considered so that the approximate number of cells used for obtaining LAESI
spectra were
approximately the same for most of the samples.
The human metabolome database (HMDB; www.hmdb.ca), the MassBank high
resolution mass spectral database (www.massbank jp), the NIST/EPA/NIH mass
spectral library,
and the MetaCyc database (http://metacyc.org) were used with a mass tolerance
ranging from
0.1 Da to 0.01 Da for the metabolite searches and identifications.
For verification purposes, according to certain embodiments, arginase activity
was
measured using the QuantiChrom Arginase Assay Kit (BioAssay Systems, Hayward,
CA)
according to the manufacturer's instructions. CEM and C81 cell lysates (10 gg
and 100 g)
were measured in triplicates. The concentration of cAMP was measured using the
CatchPoint
Cyclic-AMP Fluorescent Assay Kit (Molecular Devices, Sunnyvale, CA) according
to the
manufacturer's instructions. CEM and C81 cell lysates (10 pg and 100 p,g) were
measured in
triplicates. Glutathione reductase from CEM and C81 cell lysates (10 pg and
200 g) were
measured utilizing the Glutathione Reductase Assay Kit (Sigma, St. Louis, MO)
according to
the manufacturer's instructions.
According to certain embodiments, the sample may comprise populations of
uninfected
(CEM) and HTLV 1 infected (C 81) T lymphocytes. These non-adherent cells were
grown in an
-19-
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
RPMI medium comprising inorganic salts, sugar, amino acids, vitamins and
antibiotics. To
minimize the interfering peaks from the medium in the LAESI spectra, the cells
were washed
with PBS and the cell pellet was loaded onto a microscope slide. The cells
were directly ablated
by multiple laser shots and the average LAESI spectra of 10-15 scans were
used. The resulting
positive ion spectra exhibited various cell related metabolite ions in the
range of m/z 20-1500,
but also included interfering peaks from PBS and the medium left in the cell
pellet. Referring to
FIG. 8, representative mass spectra according to certain embodiments are
shown. The
metabolite peaks observed in the mass spectra were identified based on the
accurate masses,
isotope distribution patterns, and structural information obtained from tandem
MS. The
background corrected spectra recorded from the cells consisted of protonated,
sodiated, and
potassiated species. The observed peaks may be due to small metabolites (< m/z
500), lipids
(between m/z 690 and 850), and multiply charged peaks (between m/z 700 and
1300).
Deconvolution of all multiply charged peaks (m/z 710, 828, 993 and 1241)
showed
correspondence to a single species with a nominal molecular weight of 4960.6,
probably related
to a peptide.
The spectra of CEM T lymphocytes and HTLV 1 infected C81 T lymphocytes with
Tax 1
expression cells showed a similar set of ions, except for the lipid peaks, but
consistent
differences were identified in their relative ion yields. Referring to FIGS.
6A and 6B, a
representative list of cell-specific metabolite ions and corresponding peak
assignments, based on
accurate mass and tandem mass spectral data and structure-specific fragment
ions, is shown.
The identification of the metabolites was confirmed by comparing their tandem
mass spectra
with the spectra of the corresponding standards from tandem MS databases.
Spermine (m/z
203.2), glutathione (m/z 308.1), a phosphocholine lipid (PC(34: 1), m/z 760.6)
and adenosine
monophosphate (m/z 348.1) were identified by the tandem mass spectra shown in
FIGS. 9A-D.
Both protonated cyclic AMP (cAMP) and sodiated glutathione may be
theoretically assigned for
the m/z 330.0738 ion. Distinguishing between these two ions may be difficult
with the available
mass resolution of m/Am = 10,000, even if both the ions are contributing to
m/z 330 (as tested
with standards). Referring to FIG. 10, sodiated glutathione may be identified
by comparing the
tandem mass spectrum of the m/z 330 ion from the T lymphocytes and the tandem
mass
spectrum of the m/z 330 ions generated from the two standards. However, cAMP
at levels at or
below the detection limit may contribute to the mass spectrum of the m/z 330
ion. In one
experiment, cAMP in T lymphocytes was measured at levels of 6 pmoles/107
cells. The
interference from glutathione may cause difficulty in confirming the presence
of cAMP by
tandem MS.
-20-
RECTIFIED SHEET (RULE 91)
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
Representative mass spectra of degradation/fragmentation products of
putrescine,
spermidine and spermine are shown in FIG. 11 (designated as degradation
products a, b and c).
The degradation products a, b and c are shown in FIGS. 6A and 6B at serial
numbers 2 and 14.
The formation of these products was confirmed by comparing the LAESI data from
standard
polyamines with those detected in T lymphocytes at similar experimental
conditions. The m/z
72 ion primarily resulted from putrescine, and the m/z 129 and m/z 112 ions
formed from both
spermidine and spermine, probably mostly from the latter due to its higher ion
yields. The
abundances of these degradation products between the uninfected and infected
cells track those
of their precursors.
According to certain embodiments, the relative abundances of the detected ions
in the
mass spectra of non-HTLV 1 transformed cells and HTLV 1 transformed cells
maybe used to
determine the extent of metabolic changes between them. The background peaks
from
PBS/medium solution were used as internal standards. The relative abundance
ratios for each
ion detected in CEM and C81 are listed in FIGS. 6A and 6B. Some metabolites
may be detected
as more than one ionic species (i.e., protonated, sodiated and potassiated).
For example,
glutathione was detected as six different ionic species. In such cases, the
sum of the relative
abundances of all the related species was used to calculate the abundance
ratio. In case a
particular peak is absent in a spectrum, the background (base line signal) was
used to calculate
the ratio. Upregulation may be measured by the abundance ratio of ions from
HTLV 1
transformed cells over non-HTLV1 transformed cells whereas downregulation may
be measured
by the inverse ratio. A ratio of 1 may signify a small change, if any. The
changes in the levels
of metabolites between CEM and C81 cells from triplicate experiments according
to certain
embodiments are shown in FIG. 13.
According to certain embodiments, the mass spectra of T lymphocytes comprised
glucose (relative abundance < 3%), the major component of the medium (11 mM).
The mass of
protonated spermine (203.2236) was close to that of sodiated glucose species
(203.059), but
these two peaks may be well separated. This, however, may raise the issue of
possible
contribution of medium-related peaks to the spectra detected from T
lymphocytes. The mass
spectrum of the medium alone showed that arginine (m/z 175), choline (m/z
104), and
glutathione (m/z 308) contributed to the signal from the related metabolites
in T lymphocytes.
The glutathione and choline peaks were less than < 2% with respect to the
glucose peak (m/z
203, base peak), whereas the arginine peak was 25-30%. These ratios were
consistent with
values from diluted medium (100 times). The glucose peak appeared in both CEM
and C81
cells (< 3%) with an abundance ratio for m/z 203 close to unit value. The
arginine peak that was
-21-
RECTIFIED SHEET (RULE 91)
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
negligible in CEM cells may be much higher than the glucose peak in C81 cells.
This may
confirm that the arginine interferences from the medium are negligible and the
arginine levels
are indeed upregulated in C81 cells.
According to certain embodiments, the relative mass spectra in the low mass
region (<
m/z 500) may be corrected for the medium and electrospray related background.
After
correction, 43 ions were exclusively related to T lymphocytes, and 37 of these
ions
corresponded to the 21 metabolites shown in FIGS. 6A and 6B. The unassigned
ions showing
variations in their relative abundances between the non-HTLV 1 transformed
cells and HTLV 1
transformed cells were at m/z 158.1572 (abundance ratio C81/CEM = 2.5),
228.0363
(abundance ratio C81/CEM = 2.6), 260.0298 (abundance ratio C81/CEM = 3.6),
311.9216
(abundance ratio C81/CEM = 1.5), 333.9604 (abundance ratio C81/CEM = 2.2), and
346.0616
(abundance ratio C81/CEM = 1.2). As shown in FIGS. 6A and 6B, many metabolites
were
downregulated in the HTLVI transformed cells, e.g., spermine, choline,
phosphocholine,
glycerophosphocholine, and glutathione, and many other metabolites were
upregulated in the
infected cells, e.g., pyrrolidine, creatine, arginine, dopamine and adenosine
monophosphate.
According to certain embodiments, representative mass spectra of the T
lymphocytes
may comprise glycerophosphocholine (PC) lipids. Referring to FIG. 13,
significant changes
were observed in the lipid abundances and types between non-HTLV 1 transformed
cells and
HTLVI transformed cells. Tandem mass spectra of all major lipid peaks yielded
a single
product ion at m/z 184 (a typical spectrum of the m/z 760.6 ion is shown in
FIG. 9C) to confirm
PC lipids.
As shown in FIGS. 7A-C, the lipid peaks were assigned based on tandem mass
spectrometric and accurate mass information. FIGS. 7A-C includes diacyl
glycerophosphocholines (PC(C,,:dbn), where Cn represents the total number of
carbons and dbn
represents the total number of double bonds in the two fatty acid side chains
and the
alkylacyl/alkenylacyl glycerophosphocholines or plasmalogens, (PC(O-Cn:dbn)).
FIGS. 7A-C
also includes the relative abundance ratio values. Most of the lipids detected
in non-HTLV 1
transformed cells were downregulated in HTLV 1 transformed cells. Only a few
lipids were
retained in the HTLVI transformed cells, and the levels of PC(30:0), PC(0-
31:2), PC(32:3),
PC(32:0), and PC(0-33:3) were higher compared to the non-HTLVI transformed
cells.
Collision induced dissociation (CID) products from in-source
fragmentation/degradation of
lipids appeared at ions m/z 184 and 104. When the mass spectrum is recorded
for a standard
lipid (PC(16:0/18:1)) under similar experimental conditions, the fragment ions
m/z 104 and 184
-22-
RECTIFIED SHEET (RULE 91)
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
were marginally observed (< 0.5%). This confirmed that the detected choline
peaks
corresponded to metabolites and not to CID artifacts.
According to certain embodiments, the changes in metabolite levels between the
non-
HTLV 1 transformed T lymphocytes and HTLV 1 transformed T lymphocytes may be
verified at
the protein level by quantifying the enzymes or proteins involved in the
related metabolic
pathway. The levels of cAMP, arginase, and glutathione reductase in CEM and
C81 cells were
measured using biochemical assays. Referring to FIG. 14, arginase and cAMP
levels were
upregulated and glutathione reductase levels were downregulated in the HTLV 1
transformed
cells (C81) as compared to non-HTLVI transformed cells (CEM). The assays
correlate with the
changes detected by LAESI-MS in the corresponding metabolites.
According to certain embodiments, the sample may comprise other cell lines,
e.g., non-
HTLV 1 transformed T lymphocytes (H9), their Tax l -transfected counterparts
(H9-Tax 1), and
HTLVI transformed cells (HUT 102 cells). The metabolic changes upon
transfection are listed
in FIG. 16. Referring to FIG. 15, metabolite abundance ratios indicating up or
down regulation
for HTLVI infected T cells (C81, HUT102) and HTLV3, Taxl or Tax3 transformed
cells (293-
HTLV3, H9-Taxl, 293-Tax3, respectively). The metabolites in these cells were
compared to the
CEM and C81 cells. The pattern of upregulation and downregulation of
metabolites, such as
glutathione and adenosine monophosphate, was similar to the CEM/C81 case.
These results
suggest that the metabolic changes observed in the HTLV 1 infected cells may
be partly
attributed to Tax 1 expression.
According to certain embodiments, the sample may comprise other cell lines to
determine the specificity of the observed metabolite changes to HTLV 1
transformation, e.g.,
uninfected 293T kidney epithelial cells, and on HTLV3 and Tax3 transfected
293T cells. The
metabolic changes upon HTLV 1 transformation and the presence of Tax3 are
listed in FIG. 15.
Referring to FIG. 15, metabolites detected in 293T, 293T-HTLV3 and 293T-Tax3
cells, and
their abundance ratios indicating up and down regulation due to HTLV3
transfection or the
presence of Tax3. As shown in FIGS. 15 and 16, the 293T cells showed a variety
of ions that
were not detected in the CEM, C81, H9 and HUT 102 cells, but there were some
metabolites
common to all these cells. The observed changes for HTLV3 and Tax3 transfected
293T cells
did not match those found in HTLVI transformed cells. As shown in FIGS. 18A
and 18B, the
lipid peaks that showed prominent changes in the HTLV 1 transformed cells were
found to be
unaltered in HTLV3/Tax3 affected 293T cells.
According to certain embodiments, multiple abundant ionic species of
glutathione may
be detected in non-HTLV 1 transformed T lymphocytes reflecting high
concentrations, whereas
-23-
RECTIFIED SHEET (RULE 91)
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
in HTLV 1 transformed cells there may be a 2 to 5-fold decrease in their
abundance. The reduced
form of glutathione (GSH) may be the most predominant thiol present in
mammalian cells with
concentrations up to 12 mM. GSH may serve several important functions, such as
antioxidant
(protection against oxidative stress), cofactor in isomerization reactions,
transport and storage
form of cysteine, and regulator of intracellular redox status, cell
proliferation and apoptosis.
Biologically, the oxidized glutathione (GSSG) may be converted to GSH by the
enzyme
glutathione reductase. The ratio of GSH and GSSG may serve as a representative
indicator of the
antioxidative capacity of the cell. Cellular GSH concentrations may be reduced
in response to
protein malnutrition, oxidative stress, and other pathological conditions.
Intracellular GSH
levels may regulate T lymphocyte function, and deficiency of GSH may be
associated with HIV
infection.
According to certain embodiments, the GSH level in HTLV 1 transformed T
lymphocytes
was decreased. The levels of glutathione reductase in both non-HTLV 1
transformed cells and
HTLV 1 transformed cells were measured using an enzyme assay, and GSH was
reduced in
HTLV1 transformed cells and GSH was reduced in HTLV3 transformed 293T cells.
According to certain embodiments, the sample may comprise spermine, spermidine
and
putrescine, which belong to the polycationic compounds named polyamines. These
polyamines
may be involved in genetic processes, such as DNA synthesis and gene
expression, and play a
major role in cell proliferation, cell differentiation, and programmed cell
death. Referring to
FIG. 16, the biosynthesis of polyamines is tightly regulated in cells, and
ornithine in the urea
cycle is their precursor. The level of these polyamines may indicate the
actual condition of the
cell, including whether the cell is virally infected. In one experiment,
spermine levels were
higher in all of the transformed cells except for the case of HUT 102 and the
putrescine level and
spermidine level, the precursors of spermine, were upregulated in HTLV 1
transformed cells.
Spermidine was also upregulated in the 293T-HTLV3 and the 293T-Tax3 cell
lines. Without
wishing to be bound to any particular theory, this may indicate that the
viruses and the Tax
transformation affect the tightly regulated biosynthesis of polyamines in the
cells, thereby
causing disturbances in the genetic processes. Although the trends for
individual amines were
not completely consistent among HTLV 1 transformed cells, the effect of
viruses on the overall
polyamine biosynthesis is reflected. In one experiment, arginine that was
converted into
ornithine, the precursor of polyamines in the urea cycle, was upregulated in
HTLV 1 transformed
cells, and particularly high in HUT102. Spermine, spermidine and putrescine
may associate
with nucleic acids due to electrostatic interactions between the positively
charged ammonium
groups of the polyamines and the negatively charged phosphates of nucleic
acids.
-24-
RECTIFIED SHEET (RULE 91)
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
According to certain embodiments, the arginine levels in HTLV1 transformed
cells were
increased. The transcriptional upregulation of arginase in infected cells,
which may be
confirmed by the enzyme assay, was consistent with elevated levels of arginine
upon viral
infection. The role of arginine may play a role in the survival of endothelial
cells during
oxidative stress. Deprivation of arginine may cause serious disturbances in
cellular function and
enhances apoptosis. Arginine availability may contribute to the regulation of
T lymphocyte
function in cancer. Referring to FIG. 17, arginine is a precursor in the
biosynthesis of creatine,
and may be an important molecule in energy supply. The blood of HTLV2 infected
patients
may have abnormal creatine phosphokinase levels. The enzymes related to
creatine and arginine
metabolism were found to be significantly upregulated in malignant cells.
Accordingly, a
finding of upregulation of arginine levels in HTLV 1 transformed cells is in
line with other
biological systems.
According to certain embodiments, the mass spectra of T lymphocyte cells and
kidney
epithelial cells show that choline containing metabolites, e.g., choline,
phosphocholine,
glycerophosphocholine, and several glycerophosphocholine lipids, may be
downregulated upon
transformation by HTLV 1, HTLV3, Tax1 or Tax3 when compared to non-transformed
cells,
except in the HUT102 case. Referring to FIG. 19, choline containing
metabolites may have a
role in lipid metabolism. Choline may be a precursor of various metabolites
and the intracellular
routing of choline to its various metabolic pathways, phosphorylation,
oxidation, and acetylation
may be cell specific. Choline and choline metabolites may be regenerated by
controlled
breakdown of choline phospholipids through several pathways. Increases in
choline containing
metabolites may be associated with a number of disorders, including malignant
cell growth.
Changes in the lipid levels in HIV and HCMV infected cells may be detected
using proteomic
and metabolomic platforms, respectively. Fatty acid biosynthesis in infected
cells may also be
considered as an antiviral response. An increase in the levels of choline
containing metabolites
may be associated with a number of disorders, for example, substantial
upregulation of fatty acid
synthesis in HCMV infected fibroblast cells.
In vivo NMR spectroscopy may be used to monitor choline containing
metabolites,
however, it may be difficult to determine which specific metabolites are
altered. According to
certain embodiments, choline containing metabolites may be directly subjected
to LAESI-MS to
determine which specific metabolites are altered. The mass spectra of non-
transformed cells and
transformed cells may provide information about the precursors and lipid
components
simultaneously. In one experiment, a decrease in the glycerophosphocholine
lipid content in
HTLV 1 transformed cells confirmed increased lipid catabolism to produce fatty
acids. Apart
-25-
RECTIFIED SHEET (RULE 91)
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
from a few lipids (PC(30:0), PC(32:5), PC(32:3), PC(32:0), and PC(34:6) that
remained at
higher levels in HTLV 1 transformed cells, most of the glycerophosphocholine
lipids present in
non-HTLV 1 transformed cells were downregualated in the HTLV 1 transformed
cells.
Levels of phosphorylated adenosine nucleotides, including ATP, ADP, and AMP,
may
define the energy state in living cells. Quantitation of individual adenine
nucleotides may be
used for the assessment of the energy state of cells. The level of exogenous
ATP in the body
may be increased in various inflammatory and shock conditions. Extracellular
ATP may be
important for cell-to-cell communication and in the immune system. In one
experiment, AMP
was detected directly from cells subjected to LAESI-MS. Significantly elevated
AMP
abundance was observed in HTLV 1 and Taxi transformed cells. Referring to FIG.
19A, AMP
may be formed by the dephosphorylation of ATP/ADP or by the hydrolysis of
cAMP. Apart
from being a degradation product of ATP, AMP may activate the AMP-activated
kinase
(AMPK) system that is ubiquitously expressed in mammalian cells. Without
wishing to be
bound to any particular theory, it may be involved in the response to a
variety of metabolic
stresses that disturb the cellular energy homeostasis.
According to certain embodiments, the sample may comprise cAMP. cAMP is a
second
messenger and activates several protein kinases that may be involved in
significant biochemical
processes. The amount of cAMP known to be present in T lymphocytes is 6
pmol/107 cells.
In one experiment, glutathione interfered with the detection of cAMP.
Referring to FIG. 14,
cAMP was measured by an immunoassay. Referring to FIG. 20A, the cAMP levels
(adenylyl
cyclase activity) were increased in HTLV 1 transformed cells compared with non-
HTLV 1
transformed cells. The changes in cAMP levels may indicate HIV and in HTLV 1
infected T
lymphocytes.
According to certain embodiments, the sample may comprise dopamine, a
neuromodulator, and its metabolites, methoxytyramine and homovanillic acid, in
T
lymphocytes. Dopamine belongs to the group of catecholamines, and may be
involved in the
neuroimmunological network. T lymphocytes may be activated by
neurotransmitters via
neurotransmitter receptors that may elicit crucial functions. Catecholamines
may be synthesized
in mouse lymphocytes. Increased levels of catecholamines may indicate an
activated state. As
shown in FIG. 20B, dopamine may be biosynthesized in the body from tyrosine,
and related
metabolic pathways. In one experiment, dopamine and homovanillic acid levels
were
upregulated and methoxytyramine was downregulated in HTLV1 transformed cells.
All documents cited herein are, in relevant part, incorporated herein by
reference, but
only to the extent that the incorporated material does not conflict with
existing definitions,
-26-
RECTIFIED SHEET (RULE 91)
CA 02798182 2012-11-01
WO 2011/139274 PCT/US2010/033757
statements, or other documents set forth herein. To the extent that any
meaning or definition of
a term in this document conflicts with any meaning or definition of the same
term in a document
incorporated by reference, the meaning or definition assigned to that term in
this document shall
govern. The citation of any document is not to be construed as an admission
that it is prior art
with respect to this document.
While particular embodiments of mass spectrometers and methods of making and
using
the same have been illustrated and described, it would be obvious to those
skilled in the art that
various other changes and modifications can be made without departing from the
spirit and
scope of the invention. Those skilled in the art will recognize, or be able to
ascertain using no
more than routine experimentation, numerous equivalents to the specific
apparatuses and
methods described herein, including alternatives, variants, additions,
deletions, modifications
and substitutions. This disclosure including the appended claims is therefore
intended to cover
all such changes and modifications that are within the scope of this
invention.
-27-
RECTIFIED SHEET (RULE 91)