Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
METHODS FOR PREDICTING SENSITIVITY TO TREATMENT WITH A
TARGETED TYROSINE KINASE INHIBITOR
RELATED APPLICATION INFORMATION
This application claims priority to U.S. Patent Application Serial No.
61/332,545 filed on
May 7, 2010, and is incorporated herein by reference by its entirety.
TECHNICAL FIELD
The present disclosure relates generally to the evaluation and/or treatment of
a subject
having or suspected of having a neoplastic condition, and in particular to the
use of biomarkers
for identifying patients receptive to a certain drug therapy, and which permit
monitoring of
patient response to such therapy.
BACKGROUND OF THE INVENTION
Genetic heterogeneity of cancer is a factor complicating the development of
efficacious
cancer drugs. Cancers that are considered to be a single disease entity
according to classical
histopathological classification often reveal multiple genomic subtypes when
subjected to
molecular profiling. In certain cases, different genomic subtypes appear to
have functional
relevance to the efficacy of certain drugs. For example, the efficacy of
certain targeted cancer
drugs has been correlated with the presence of a genomic feature, such as a
gene amplification.
(See, e.g., T.J. Lynch et al., "Activating mutations in the epidermal growth
factor receptor
underlying responsiveness of non-small-cell lung cancer to gefitinib", N.
Engl. J. Med., 350:
2129-2139, 2004.)
Clinical studies have also identified certain plasma and serum markers that
can be used to
sub-classify lung cancer patients. The National Association of Clinical
Biochemistry has
published practice guidelines and recommendations for use of tumor markers in
the clinic. (See
The NACB Practice Guidelines and Recommendations for Use of Tumor Markers in
the Clinic.
Lung Cancer Section 3P). A pattern of tumor marker release has been correlated
to the
histological background of the tumor and can reveal mixed histological
components. Table 1
summarizes the correlation of the markers CYFRA 21-1, CEA, NSE, and ProGRP
with tumor
histology.
1
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
Table 1:
H:i:stt fogy 6efiare Ã:k empy Pos=tf3erapy foffow.-up
U:rknnown CYFRA 21- CEn. N:5-E, Pry = P =.: suTge,y w I Mr histvogy
n c1 i rrecf d?ease us mg tt . e.athnr rnarke:
; enn:z3rc, nwia r c. 2" and CEN REF : ':-'T rc r
Sguamous cei c:3: rorla ^YFftA 21-` and tEA fafld SCCA) - 'R?i is 9`.^,vo C'E>
i.~.=k t'
Large ceI ra,;::nci r, CVRA2f-iaridC:EA C.`R;2=-1
vr:> ce::l ;z=.rdncrit. N_xE.m-Pr:i =uP õ_tEr:r?=v. ar o RP
E1, .. Li>: e ..? ~ r = n r ~...' r"tr, :-1 :eke, bt'.r. ?:~i. enf~. ~~:_, :e
r ~ ~x f.. r.:~l r.. r F:F. cas r n r l ~ i. 3 r .,1E;
While the correlation of certain markers with certain subclasses of lung
cancer may be helpful
for distinguishing among different histological subtypes, the functional
significance of such
markers is generally not well understood.
ABT-869 (Linifanib) [N-(4-(3-amino-lH-indazol-4-yl)phenyl)-N'-(2-fluoro-5-
methylphenyl)urea]), is a multi-targeted receptor tyrosine kinase inhibitor
that has been shown to
inhibit of all members of the VEGF and PDGF receptor families (e.g., KDR IC50
value of 4 nM),
and have less activity (IC50 values >1 M) against unrelated receptor tyrosine
kinases, soluble
tyrosine kinases and serine/threonine kinases. In addition, it exhibits potent
anti-proliferative and
apoptotic effects on tumor cells that are dependent on mutant, constitutively
active, FLT3 and
KIT kinases. Despite its potent anti-tumor activity, many malignant cell types
are refractory to
ABT-869. The cause of resistance is unknown.
Because of the potential therapeutic use of ABT-869 against various cancers,
companion
diagnostic assays that would identify those patients most receptive to ABT-869
therapy are
needed. Additionally, a need exists for diagnostic methods that can be used to
monitor the
efficacy of therapy with ABT-869. A further need exist for companion assays
using markers that
can be measured in readily obtainable tissue samples such as blood or a blood
plasma fraction.
SUMMARY OF THE INVENTION
The levels of the markers neuron-specific enolase (NSE), serum-soluble
fragments of
cytokeratin 19 (CYFRA 21-1), cancer antigen 125 (CA 125) and carcinoma
embryonic antigen
(CEA) have been found to be indicative of the sensitivity of a subject's
cancer to the
administration of the drug ABT-869. Methods and kits described herein are
based in part on the
finding that any one or more of. a level of NSE below a predetermined level
for NSE, a level of
2
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
CA125 below a predetermined level for CA125, a level of CEA above a
predetermined level for
CEA, and a level of CYFRA 21-1 below a predetermined level for CYFRA 21-1, or
any
combination thereof, indicates increased sensitivity of the subject's cancer
to the administration
of ABT-869, relative to a subject that does not have a comparable level for
any of the markers.
Accordingly, in one aspect, the present disclosure provides a method for
predicting the
sensitivity of a cancer in a subject to administration of ABT-869 to the
subject, the method
comprising the step of. determining in a sample obtained from the subject a
level of at least one
marker selected from the group consisting of. neuron-specific enolase (NSE),
serum-soluble
fragments of cytokeratin 19 (CYFRA 21-1), cancer antigen 125 (CA 125) and
carcinoma
embryonic antigen (CEA), wherein any one of. a level of NSE below a
predetermined level for
NSE, a level of CA125 below a predetermined level for CA125, a level of CEA
above a
predetermined level for CEA, a level of CYFRA 21-1 below a predetermined level
for CYFRA
21-1 or any combination thereof, indicates increased sensitivity of the
subject's cancer to the
administration of ABT-869 relative to a subject with a level of NSE, CYFRA 21-
1 or CA125
above the predetermined level for each marker, or to a subject with a level of
CEA below the
predetermined level for each marker. The cancer may be non small-cell lung
cancer. The
method can comprise, for example, determining the levels of at least two
markers selected from
the group consisting of. NSE, CA125, CYFRA21-1 and CEA. The method can
comprise
determining the levels of NSE, CA125, CYFRA21-1 and CEA. The method may
further
comprise, for example, generating a marker signature for the subject from the
levels of the two or
more markers, wherein a marker signature having a predetermined pattern
indicates an increased
sensitivity of the subject to administration of ABT-869, relative to a marker
signature lacking the
predetermined pattern. The method may further comprise comparing the levels of
the two or
more markers in the sample with levels of the same markers in a control sample
by applying a
classification tree analysis. The classification tree analysis may be
performed by a computer
process.
In another aspect, the present disclosure provides a method of predicting the
sensitivity of
a cancer in a subject to administration of ABT-869, the method comprising the
step of:
determining in a sample obtained from the subject levels of markers in a
marker panel
comprising NSE, CA125, CYFRA 21-1 and CEA, and comparing the level of each
marker in the
sample to a predetermined level for each marker, wherein the level of each
marker in the sample
3
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
relative to the predetermined level for each marker indicates sensitivity of
the cancer to
administration of ABT-869 to the subject. In the method, comparing the level
of each marker in
the sample to a predetermined level for each marker comprises comparing the
marker levels to a
level of each of the markers in a reference sample, wherein the reference
sample contains each of
the markers at a level corresponding to the predetermined level for each
marker. The cancer can
be non small-cell lung cancer. In the method, the NSE level in the subject's
sample can be, for
example, below the predetermined level for NSE, the CA125 level in the
subject's sample can be
below the predetermined level for CA125, the CYFRA 21-1 level in the subject's
sample can be
below the predetermined level for CYFRA 21-1, or the CEA level in the
subject's sample is
above the predetermined level for CEA, or any combination of all four
conditions may be
present. The method may further comprise generating a marker signature for the
subject from
the levels of the one or more markers, wherein a marker signature having a
predetermined
pattern indicates an increased sensitivity of the subject to administration of
ABT-869, relative to
a subject having a marker signature lacking the predetermined pattern. The
method may further
comprise comparing the levels of the markers in the subject's sample with
levels of the markers
in the reference sample by applying a classification tree analysis. The
classification tree analysis
may be performed, for example, by a computer process.
In another aspect, the present disclosure provides a method for classifying
one or more
subjects each having or suspected of having a cancer, for predicted efficacy
of administration of
ABT-869 for the treatment of the cancer, the method comprising determining in
a sample from
each subject, the level of at least one marker selected from the group
consisting of: NSE,
CYFRA 21-1, CA125 and CEA, wherein any one of: a reduced level of NSE relative
to the level
of NSE in a reference sample, a reduced level of CA125 relative to the level
of CA125 in the
reference sample, a reduced level of CYFRA 21-1 relative to the level of CYFRA
21-1 in the
reference sample, an elevated level of CEA relative to the level CEA in the
reference sample, or
any combination thereof, indicates sensitivity of the cancer to administration
of ABT-869 to the
subject. The method may further comprise classifying each subject as being
sensitive to
treatment with ABT-869 based on the level of at least one of NSE, CA125, CYFRA
21-1 and
CEA. In the method, the subject or subjects may have or may be suspected of
having non small-
cell lung cancer. According to the method, for example, the NSE level in the
subject's sample
can be reduced relative to the level of NSE in the reference sample. The CA125
level in the
4
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
subject's sample can be reduced relative to the level of CA125 in the
reference sample. The
CYFRA 21-1 level in the subject's sample can be reduced relative to the level
of CYFRA 21-1 in
the reference sample. The CEA level in the subject's sample can be elevated
relative to the level
of CEA in the reference sample. The method may further comprise generating a
marker
signature for each subject from the levels of the one or more markers, wherein
a marker
signature having a predetermined pattern indicates an increased sensitivity of
the subject to
administration of ABT-869, relative to a subject having a marker signature
lacking the
predetermined pattern. The method may further comprise comparing the levels of
the markers in
each subject's sample with levels of the same markers in the reference sample
by applying a
classification tree analysis, which may be performed by a computer process. In
any of the above
methods, the sample can be, a blood sample, including a serum or a plasma
sample. Any of the
above methods may further comprise the step of obtaining the sample from the
subject or
subject. In any of the above methods, the level of each marker can be
determined for example by
immunohistochemistry or immunoassay.
In another aspect, the present disclosure provides a kit for predicting the
sensitivity of a
cancer in a subject to administration of ABT-869 to the subject, the method
comprising: a) an
array comprising one or more binding reagents, each binding reagent having
independent
binding specificity for at least one marker selected from the group consisting
of NSE, CA125,
CYFRA 21-1 and CEA, wherein each binding reagent is independently bound to a
discrete
location on at least one substrate; and b) a control sample containing a
predetermined level of the
marker or markers in the array, wherein the predetermined level for each
marker is a level
relative to which a level for that marker indicates a sensitivity of the
subject's cancer to the
administration of ABT-869. The cancer for which the kit is configured to
predict the sensitivity
of administration of ABT-869 can be non small-cell lung cancer. In the kit,
the level of NSE in
the control sample can be, for example, a level below which a level of NSE in
a subject's sample
is indicative of sensitivity of the subject's cancer to the administration of
ABT-869. The level of
CA125 in the control sample can be a level below which a level of CA125 in a
subject's sample
is indicative of sensitivity of the subject's cancer to the administration of
ABT-869. The level of
CYFRA 21-1 in the control sample can be a level below which a level of CYFRA
21-1 in a
subject's sample is indicative of sensitivity of the subject's cancer to the
administration of ABT-
869. The level of CEA in the control sample can be a level above which a level
of CEA in a
5
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
subject's sample is indicative of sensitivity of the subject's cancer to the
administration of ABT-
869. In the kit, the one or more substrates may each comprise a solid support
coupled to a
detectable label. The detectable label can comprise, for example, a
fluorescent compound. The
kit may further comprise instructions for determining the level of each marker
in a sample from
the subject. The subject's sample can be a blood sample, including a plasma
sample or a serum
sample.
In another aspect, the present disclosure provides a kit for predicting the
sensitivity of a
cancer in a subject to administration of ABT-869 to the subject, comprising:
a) a microarray of
markers comprising one or more selected from the group consisting of NSE,
CA125, CYFRA
21-1, CEA and truncated forms thereof, and b) a control sample containing a
predetermined level
of the marker or markers, wherein the predetermined level for each marker is a
level relative to
which a level for that marker indicates a sensitivity of the subject's cancer
to the administration
of ABT-869. The cancer for which the kit is configured to predict the
sensitivity of
administration of ABT-869 can be non small-cell lung cancer. In the kit, the
level of NSE in the
control sample can be, for example, a level below which a level of NSE in a
subject's sample is
indicative of sensitivity of the subject's cancer to the administration of ABT-
869. The level of
CA125 in the control sample can be a level below which a level of CA125 in a
subject's sample
is indicative of sensitivity of the subject's cancer to the administration of
ABT-869. The level of
CYFRA 21-1 in the control sample can be a level below which a level of CYFRA
21-1 in a
subject's sample is indicative of sensitivity of the subject's cancer to the
administration of ABT-
869. The level of CEA in the control sample can be a level above which a level
of CEA in a
subject's sample is indicative of sensitivity of the subject's cancer to the
administration of ABT-
869. In the kit, the one or more substrates may each comprise a solid support
coupled to a
detectable label. The detectable label can comprise, for example, a
fluorescent compound. The
kit may further comprise instructions for determining the level of each marker
in a sample from
the subject. The subject's sample can be a blood sample, including a plasma
sample or a serum
sample.
6
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
BRIEF DESCRIPTION OF THE DRAWINGS
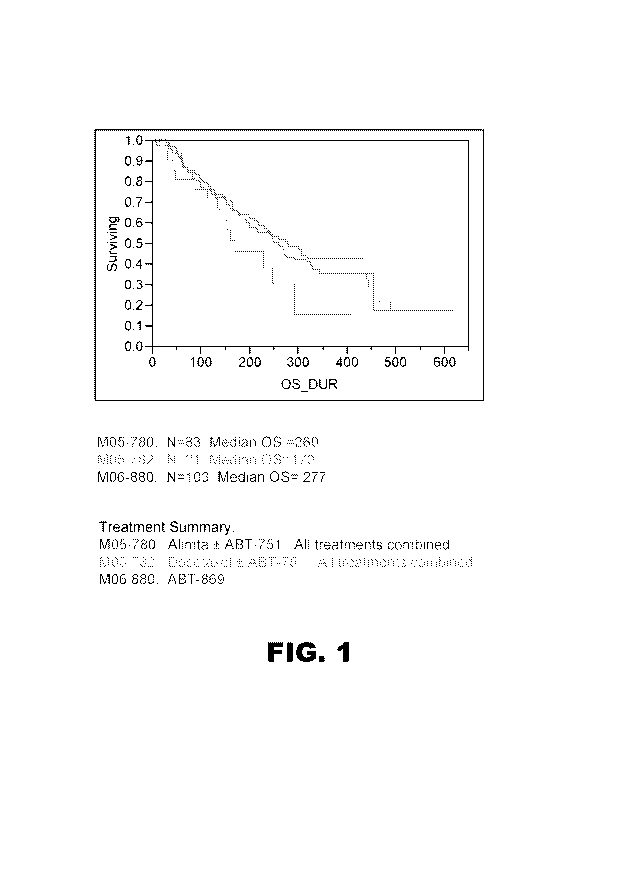
FIG. 1 is a Kaplan Meier plot showing the Overall Survival (OS) in days for
three
different cohorts of stage 3/4 NSCLC patients treated with or without ABT-869.
FIG. 2 is a set of Kaplan-Meier plots for a patient cohort (M05-780) treated
with
Alimta with or without ABT-75 1, plotting OS for each of eight markers
evaluated, according
to baseline plasma level of the marker in comparison to a NSCLC median
threshold.
FIG. 3 shows two Kaplan Meier plots based on analysis of a patient cluster
("Cluster 2")
characterized by increased OS following treatment with ABT-869 relative to
cluster patients not
treated with ABT-869.
DETAILED DESCRIPTION
A. Definitions
Section headings as used in this section and the entire disclosure herein are
not intended
to be limiting.
a) As used herein, the singular forms "a," "an" and "the" include plural
referents
unless the context clearly dictates otherwise. For the recitation of numeric
ranges herein, each
intervening number there between with the same degree of precision is
explicitly contemplated.
For example, for the range 6-9, the numbers 7 and 8 are contemplated in
addition to 6 and 9, and
for the range 6.0-7.0, the numbers 6.0, 6.1, 6.2, 6.3, 6.4, 6.5, 6.6, 6.7,
6.8, 6.9 and 7.0 are
explicitly contemplated.
b) Neuron-specific enolase ("NSE")
As used interchangeably herein, the terms "neurons-specific enolase" and "NSE"
refer to
a protein encoded by the human gene also known as enolase 2 (official symbol
ENO2), and
conservative variants thereof. As used herein, the term "official symbol"
refers to that used in
the EntrezGene database maintained by the United States National Center for
Biotechnology
Information.
c) Cancer antigen 125 ("CA125")
As used interchangeably herein, the terms "Cancer antigen 125" and "CA125"
refer to a
carbohydrate antigen recognized as a tumor marker for ovarian cancer, and
derived from Mucin
7
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
16, cell surface associated, also known as MUC 16, which is a protein encoded
by the human
MUC 16 gene (official symbol MUC 16), and conservative variants of CA 125.
d) Serum-soluble fragments of cytokeratin 19 ("CYFRA 21-1")
As used interchangeably herein, the terms "Serum-soluble fragments of
cytokeratin 19"
and "CYFRA 21-1" refer to an antigen recognized as a tumor marker for multiple
cancers
including lung cancer, and derived from cytokeratin 19, which is a protein
encoded by the human
keratin 19 gene (official symbol KRT19), and conservative variants of KRT19.
e) Carcinoembryonic antigen ("CEA")
As used interchangeably herein, the terms "Carcinoembryonic antigen" and "CEA"
refer
to the human protein having the amino acid sequence under GenBank Accession
No. CAE75559,
and conservative variants thereof.
f) Detectable Label
As used herein the term "detectable label" refers to any moiety that generates
a
measurable signal via optical, electrical, or other physical indication of a
change of state of a
molecule or molecules coupled to the moiety. Such physical indicators
encompass
spectroscopic, photochemical, biochemical, immunochemical, electromagnetic,
radiochemical,
and chemical means, such as but not limited to fluorescence,
chemifluorescence,
chemiluminescence, and the like.
g) Subject
As used herein, the terms "subject" and "patient" are used interchangeably
irrespective of
whether the subject has or is currently undergoing any form of treatment. As
used herein, the
terms "subject" and "subjects" refer to any vertebrate, including, but not
limited to, a mammal
(e.g., cow, pig, camel, llama, horse, goat, rabbit, sheep, hamsters, guinea
pig, cat, dog, rat, and
mouse, a non-human primate (for example, a monkey, such as a cynomolgous
monkey,
chimpanzee, etc) and a human). Preferably, the subject is a human.
h) test sample
As used herein, the term "test sample" generally refers to a biological
material being
tested for and/or suspected of containing one or more cancer markers. The
biological material
may be derived from any biological source. Examples of biological materials
include, but are
not limited to, a peripheral blood sample, a tumor or suspected tumor tissue,
a thin layer
cytological sample, a fine needle aspirate sample, a bone marrow sample, a
lymph node sample,
8
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
a urine sample, an ascites sample, a lavage sample, an esophageal brushing
sample, a bladder or
lung wash sample, a spinal fluid sample, a brain fluid sample, a ductal
aspirate sample, a nipple
discharge sample, a pleural effusion sample, a fresh frozen tissue sample, a
paraffin embedded
tissue sample or an extract or processed sample produced from any of a
peripheral blood sample,
a serum or a plasma fraction of a blood sample. The test sample may be used
directly as
obtained from the biological source or following a pretreatment to modify the
character of the
sample. For example, such pretreatment may include preparing plasma from
blood, diluting
viscous fluids and so forth. Methods of pretreatment may also involve
filtration, precipitation,
dilution, distillation, mixing, concentration, inactivation of interfering
components, the addition
of reagents, lysing, etc. If such methods of pretreatment are employed with
respect to the test
sample, such pretreatment methods are such that cancer cells remain in the
test sample
B. Markers Predictive of Cancer Sensitivity to ABT-869
The presently disclosed methods and kits are based in part on the surprising
finding that
levels of certain markers (or "biomarkers") found in a test sample obtained
from a subject are
predictive of the sensitivity of the subject's cancer to administration of ABT-
869. These
predictive markers include NSE, CA125, CYFRA 21-1 and CEA.
The inventive methods are particularly useful with the compound ABT-869
(Linifanib;
[N-(4-(3-amino-lH-indazol-4-yl)phenyl)-N'-(2-fluoro-5-methylphenyl)urea]),
which is an ATP-
competitive receptor tyrosine kinase (RTK) inhibitor that is a potent
inhibitor of members of the
vascular endothelial growth factor (VEGF) and platelet-derived growth factor
(PDGF) receptor
families. See Shankar D.B. et al., Blood, Apr 15: 109(8), 3400-8 (2007). The
chemical structure
of ABT-869 is:
NH2 N Y N
N CH3
I 1 HN F)1:
I
9
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
Other synthetic methods for ABT-869 have been described (see, e.g., A. Kruger
et al., Org.
Process Res. Dev.13 (6), 1419-25 (2009)). Pharmaceutical compositions
containing ABT-869
and routes and methods of its administration for cancer therapy are known and
described in
detail, for example, in U.S. Patent Application Serial No. 11/636,189 (US
2007/0135387), the
entire disclosure of which is hereby incorporated by reference.
A predictive marker is any marker that can be found and measured in a test
sample from
a subject, such as a blood sample which may be a plasma or a serum sample, the
level (i.e.
amount) of which marker in the sample is correlated with response of a cancer
to a specific
therapeutic compound and/or class of compounds. As described herein, the
markers NSE,
CA125, CYFRA 21-1 and CEA have been found to be predictive of a subject's
sensitivity, or
rather more specifically, the sensitivity of a subject's cancer, to treatment
with ABT-869 by
which is meant administration of ABT-869. To determine correlations of markers
with clinical
outcome, and more specifically with sensitivity to ABT-869, marker
concentrations in subjects
having a particular cancer of interest are measured for example at a starting
time point for a
baseline measure, and then at a second time point at about three weeks, for
example at day 21 or
22 following initiation of a treatment regimen. Marker thresholds or "cut-
offs" can be
established for example as the median for the particular cancer type, or by
using any other
statistical approach by which such a cut-off value within a distribution of
values may be selected.
For each marker, subjects are categorized as having a marker level above or
below the threshold.
Survival, as for example Overall Survival, is then determined as a function of
treatment class,
and compared for each marker and treatment.
Thus, for each marker, a predetermined cut-off level is identified and
provides a
reference level that can then be used according to the methods and kits
described herein. More
specifically, as described elsewhere herein, a level of NSE below a
predetermined level for NSE,
a level of CYFRA 21-1 below a predetermined level for CYFRA 21-1, a level of
CA125 below a
predetermined level for CA 125, a level of CEA above a predetermined level for
CEA, or any
combination thereof, indicates increased sensitivity of the subject's cancer
to the administration
of ABT-869, relative to a subject with a level of NSE, CA125 or CYFRA 21-1
above the
predetermined level for each marker, or to a subject with a level of CEA below
the
predetermined level for CEA.
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
Typically the level of each marker in the test sample from the subject is
determined using
an immunohistochemistry or immunoassay technique, such as for example an
enzyme
immunoassay (EIA), and for which kits are readily commerically available from
a number of
commercial suppliers. An exemplary microparticle enzyme immunoassay technology
is the
AXSYM System available from Abbott Laboratories. The assay may involve a
multiplex
technique so the levels of two or more markers can be determined from the
output of a single
assay process. The marker level of any two or more of the NSE, CA125, CYFRA 21-
1 and CEA
in a test sample can be combined to produce a marker signature (sometimes
referred to as a
"biomarker profile"), which is characterized by a pattern composed of at least
of the two or more
marker levels. An exemplary such pattern is composed of, for example, a level
of NSE below a
predetermined cut-off for NSE, together with one or more of a level of CA125
below the
predetermined cut-off for CA125, a level of CYFRA 21-1 below the predetermined
cut-off for
CYFRA 21-1, and a level of CEA above the predetermined cut-off for CEA. The
marker
signature may include the level of one or more markers other than NSE, CA125,
CYFRA 21-1
and CEA. A marker signature having a predetermined pattern, i.e. satisfying
certain criteria such
as a cut-off criterion for each at least two markers, indicates an increased
sensitivity of the
subject to administration of ABT-869, relative to a marker signature lacking
the predetermined
pattern.
Use of these markers in the methods and kits of the present disclosure
provides a basis for
developing targeted cancer therapy using ABT-869. The methods can be
especially useful, for
example, as a basis for companion assays for ABT-869 therapy, which is
administered to a
subject either as monotherapy or as part of combination therapy with other
chemotherapy, such
as conventional chemotherapy. The methods can be performed in relation to any
cancer type for
which it is determined that the marker levels are predictive of sensitivity of
the cancer to
administration of ABT-869. An exemplary such cancer is any carcinoma, such as
non-small cell
lung cancer, or any solid tumor.
C. Methods
Methods for predicting the sensitivity of a cancer in a subject to
administration of ABT-
869 to the subject involve determining the level of at least one of the
predictive markers as
described herein, i.e. neuron-specific enolase (NSE), cancer antigen 125
(CA125), serum-soluble
fragments of cytokeratin 19 (CYFRA 21-1) and carcinoma embryonic antigen
(CEA). Any one
11
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
or more of. 1) a level of NSE below a predetermined level for NSE, 2) a level
of CA125 below a
predetermined level for CA125, CYFRA 21-1 below a predetermined level for
CYFRA 21-1 and
3) a level of CEA above a predetermined level for CEA, or any combination
thereof, indicates
increased sensitivity of the subject's cancer to the administration of ABT-869
as compared to a
subject having a level of NSE, CA125 or CYFRA 21-1 above the predetermined
level for each
marker, or to a subject with a level of CEA below the predetermined level for
CEA. The
methods can, for example, include determining the level of all four of NSE,
CA125, CYFRA 21-
1 and CEA. Cancers addressed by the present disclosure encompass any cancer
for which anti-
angiogenic therapy such as ABT-869 therapy is contemplated, and especially any
solid tumor
including breast tumors, and carcinomas including hepatocellular carcinoma,
renal cell
carcinoma, small cell and large cell carcinomas, and combinations thereof, and
include for
example non-small cell lung cancer (NSCLC).
A cancer or a subject (patient) may be described as sensitive to, or resistant
to a selected
therapeutic drug regimen including administration of ABT-869, based on the
ability of the drug
to kill cancer cells or decrease tumor size and/or reduce overall cancer
growth or spread
(metastasis). Cancer cells or tumors that are not sensitive are deemed
resistant to a therapeutic
regimen and are those that do not respond to the drug regimen, for example
those in which the
drug regimen fails to significantly decrease tumor size or slow tumor growth
or spread. Cancer
cells that are sensitive to the therapeutic regimen are those that do respond
to the drug regimen,
resulting in decreased tumor size and/or slowed tumor growth or spread, and
thus also in an
increase in overall survival ("OS"). Monitoring of a response to the drug
regimen can be
accomplished by numerous pathological, clinical and imaging methods such as
those described
elsewhere herein and as are generally well known in the medical field. For
example, tumor size
can be evaluated using any soft tissue imaging technique, such as ultrasound,
CT and/or DCE-
MRI. It will also be understood that the methods can further involve obtaining
the test sample
from the subject using any tissue sampling technique including but not limited
to blood draw and
fingerstick, and tissue biopsy techniques including needle biopsy.
When the levels of two or more markers are determined, the method may further
comprise generating a marker signature for the subject from the levels of the
two or more
markers. A marker signature may include for example the two or marker levels,
wherein each
level relative to a cut-off value for that marker defines a feature of the
marker signature, and the
12
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
features together form the signature. A signature sharing a predetermined
pattern, i.e. a pattern
that reflects marker levels each having a certain relationship relative to a
cut-off value for each
marker, indicates an increased sensitivity of the subject to administration of
ABT-869, relative to
a marker signature lacking the predetermined pattern. For example, a
predetermined signature
pattern indicative of increased sensitivity of the subject to administration
of ABT-869 and based
on marker levels for all of NSE, CA125, CYFRA 21-1 and CEA is a pattern
characterized by 1)
a level of NSE that is below a predetermined level for NSE, 2) a level of
CYFRA 21-1 that is
below a predetermined level for CYFRA 21-1, 3) a level of a level of CA125
that is below a
predetermined level for CA125, and 3) a level of CEA that is above a
predetermined level for
CEA. Any signature having all of these pattern features is exemplary of a
signature that is
indicative of sensitivity of the subject to administration of ABT-869.
Analysis of the marker levels may further involve comparing the levels of at
least two
markers with levels of the same markers in a control sample, which may be
performed by
applying a classification tree analysis. Classification tree analyses are
generally well-known and
can be readily applied to analysis of marker levels using a computer process.
For example, a
reference 3D contour plot can be generated that reflects the marker levels as
described herein
that correlate with sensitivity of a cancer to treatment with ABT-869. For any
given subject, a
comparable 3D plot can be generated and the plot compared to the reference 3D
plot to
determine whether the subject has a marker signature indicative of sensitivity
of the subject to
administration of ABT-869. Classification tree analyses are well-suited for
analyzing marker
levels because they are especially amenable to graphical display and are easy
to interpret. It will
however be understood that any computer-based application can be used that
compares multiple
marker levels from two different subjects, or from a reference sample and a
subject, and provides
an output that indicates sensitivity of a subject to administration of ABT-869
based on the
methods described herein.
The methods can be used to classify one or more subjects, each subject having
or
suspected of having a cancer, for predicted efficacy of administration of ABT-
869 for the
treatment of the cancer in the subject. Such an approach involves determining,
in a sample from
each subject, the level of at least one of the markers NSE, CA125, CYFRA 21-1
and CEA and
comparing the level of each marker to its level in a reference sample. The
reference sample
contains an amount of each marker that corresponds to predetermined cut-off
value for the
13
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
marker. Any one of. 1) a reduced level of NSE relative to the level of NSE in
a reference
sample, 2) a reduced level of CYFRA 21-1 relative to the level of CYFRA21-1 in
the reference
sample, an elevated level of CEA relative to the level CEA in the reference
sample, a reduced
level of CA125 relative to the level CA125 in the reference sample or any
combination thereof,
indicates sensitivity of the cancer to administration of ABT-869 to the
subject. Thus the methods
can be used for example to target a patient population in which treatment with
ABT-869 is likely
to produce superior results as compared to alternative therapies.
D. Kits
The present disclosure also provides kits for predicting the sensitivity of a
cancer in a
subject to administration of ABT-869 to the subject. The kit can comprise for
example an array
of one or more binding reagents, and a control sample containing a
predetermined level of the
marker or markers, wherein the predetermined level for each marker is a level
relative to which a
level for that marker indicates a sensitivity of the subject's cancer to the
administration of ABT-
869. The predetermined level for each marker is for example a cut-off or
threshold value
determined according to a statistical analysis, for example as described
elsewhere herein, such as
in the Examples. Each binding reagent has independent binding specificity for
at least one of
NSE, CA125, CYFRA 21-1, and CEA. Exemplary such binding reagents are
antibodies.
Alternatively, a kit may include an array of two or more of the markers or
truncated forms or
fragments thereof.
Antibodies
A binding reagent may be for example a polyclonal antibody, a monoclonal
antibody, a
chimeric antibody, a human antibody, an affinity maturated antibody or an
antibody fragment. A
sandwich immunoassay format may be used in which both a capture and a
detection antibody are
used for each marker. Antibodies may be bound, for example conjugated, to a
detectable label.
While monoclonal antibodies are highly specific to the marker/antigen, a
polyclonal antibody
can preferably be used as a capture antibody to immobilize as much of the
marker/antigen as
possible. A monoclonal antibody with inherently higher binding specificity for
the
marker/antigen may then preferably be used as a detection antibody for each
marker/antigen. In
any case, the capture and detection antibodies recognize non-overlapping
epitopes on each
marker, preferably without interfering with the binding of the other.
14
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
Polyclonal antibodies are raised by injecting (e.g., subcutaneous or
intramuscular
injection) an immunogen into a suitable non-human mammal (e.g., a mouse or a
rabbit).
Generally, the immunogen should induce production of high titers of antibody
with relatively
high affinity for the target antigen. If desired, the marker may be conjugated
to a carrier protein
by conjugation techniques that are well known in the art. Commonly used
carriers include
keyhole limpet hemocyanin (KLH), thyroglobulin, bovine serum albumin (BSA),
and tetanus
toxoid. The conjugate is then used to immunize the animal. The antibodies are
then obtained
from blood samples taken from the animal. The techniques used to produce
polyclonal
antibodies are extensively described in the literature (see, e.g., Methods of
Enzymology,
"Production of Antisera with Small Doses of Immunogen: Multiple Intradermal
Injections,"
Langone, et al. eds. (Acad. Press, 1981)). Polyclonal antibodies produced by
the animals can be
further purified, for example, by binding to and elution from a matrix to
which the target antigen
is bound. Those of skill in the art will know of various techniques common in
the immunology
arts for purification and/or concentration of polyclonal, as well as
monoclonal, antibodies (see,
e.g., Coligan, et al. (1991) Unit 9, Current Protocols in Immunology, Wiley
Interscience).
For many applications, monoclonal antibodies (mAbs) are preferred. The general
method
used for production of hybridomas secreting mAbs is well known (Kohler and
Milstein (1975)
Nature, 256:495). Briefly, as described by Kohler and Milstein, the technique
entailed isolating
lymphocytes from regional draining lymph nodes of five separate cancer
patients with either
melanoma, teratocarcinoma or cancer of the cervix, glioma or lung, (where
samples were
obtained from surgical specimens), pooling the cells, and fusing the cells
with SHFP-1.
Hybridomas were screened for production of antibody that bound to cancer cell
lines.
Confirmation of specificity among mAbs can be accomplished using routine
screening
techniques (such as the enzyme-linked immunosorbent assay, or "ELISA") to
determine the
elementary reaction pattern of the mAb of interest.
As used herein, the term "antibody" also encompasses antigen-binding antibody
fragments, e.g., single chain antibodies (scFv or others), which can be
produced/selected using
phage display technology. The ability to express antibody fragments on the
surface of viruses
that infect bacteria (bacteriophage or phage) makes it possible to isolate a
single binding
antibody fragment, e.g., from a library of greater than 1010 nonbinding
clones. To express
antibody fragments on the surface of phage (phage display), an antibody
fragment gene is
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
inserted into the gene encoding a phage surface protein (e.g., pIII) and the
antibody fragment-
pIII fusion protein is displayed on the phage surface (McCafferty et al.
(1990) Nature, 348: 552-
554; Hoogenboom et al. (1991) Nucleic Acids Res. 19: 4133-4137).
Since the antibody fragments on the surface of the phage are functional, phage-
bearing
antigen-binding antibody fragments can be separated from non-binding phage by
antigen affinity
chromatography (McCafferty et al. (1990) Nature, 348: 552-554). Depending on
the affinity of
the antibody fragment, enrichment factors of 20-fold-1,000,000-fold are
obtained for a single
round of affinity selection. By infecting bacteria with the eluted phage,
however, more phage
can be grown and subjected to another round of selection. In this way, an
enrichment of 1000-
fold in one round can become 1,000,000-fold in two rounds of selection
(McCafferty et al.
(1990) Nature, 348: 552-554). Thus, even when enrichments are low (Marks et
al. (1991) J.
Mol. Biol. 222: 581-597), multiple rounds of affinity selection can lead to
the isolation of rare
phage. Since selection of the phage antibody library on antigen results in
enrichment, the
majority of clones bind antigen after as few as three to four rounds of
selection. Thus only a
relatively small number of clones (several hundred) need to be analyzed for
binding to antigen.
Human antibodies can be produced without prior immunization by displaying very
large
and diverse V-gene repertoires on phage (Marks et al. (1991) J. Mol. Biol.
222: 581-597). In one
embodiment, natural VH and VL repertoires present in human peripheral blood
lymphocytes are
isolated from unimmunized donors by PCR. The V-gene repertoires can be spliced
together at
random using PCR to create a scFv gene repertoire which can be cloned into a
phage vector to
create a library of 30 million phage antibodies (Id.). From a single "naive"
phage antibody
library, binding antibody fragments have been isolated against more than 17
different antigens,
including haptens, polysaccharides, and proteins (Marks et al. (1991) J. Mol.
Biol. 222: 581-597;
Marks et al. (1993). Bio/Technology. 10: 779-783; Griffiths et al. (1993) EMBO
J. 12: 725-734;
Clackson et al. (1991) Nature. 352: 624-628). Antibodies have been produced
against self
proteins, including human thyroglobulin, immunoglobulin, tumor necrosis
factor, and CEA
(Griffiths et al. (1993) EMBO J. 12: 725-734). The antibody fragments are
highly specific for
the antigen used for selection and have affinities in the 1 nM to 100 nM range
(Marks et al.
(1991) J. Mol. Biol. 222: 581-597; Griffiths et al. (1993) EMBO J. 12: 725-
734). Larger phage
antibody libraries result in the isolation of more antibodies of higher
binding affinity to a greater
proportion of antigens.
16
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
As those of skill in the art readily appreciate, antibodies can be also
prepared by any of a
number of commercial services (e.g., Berkeley Antibody Laboratories, Bethyl
Laboratories,
Anawa, Eurogenetec, etc.).
Solid phase
In kits according to the present disclosure, each binding reagent may be bound
to a solid
phase. A solid phase can be any suitable material with sufficient surface
affinity to bind an
antibody, for example each capture antibody having a specific binding for one
of the markers.
The solid phase can take any of a number of forms, such as a magnetic
particle, bead, test tube,
microtiter plate, cuvette, membrane, a scaffolding molecule, quartz crystal,
film, filter paper, disc
or a chip. Useful solid phase materials include: natural polymeric
carbohydrates and their
synthetically modified, crosslinked, or substituted derivatives, such as agar,
agarose, cross-linked
alginic acid, substituted and cross-linked guar gums, cellulose esters,
especially with nitric acid
and carboxylic acids, mixed cellulose esters, and cellulose ethers; natural
polymers containing
nitrogen, such as proteins and derivatives, including cross-linked or modified
gelatins; natural
hydrocarbon polymers, such as latex and rubber; synthetic polymers, such as
vinyl polymers,
including polyethylene, polypropylene, polystyrene, polyvinylchloride,
polyvinylacetate and its
partially hydrolyzed derivatives, polyacrylamides, polymethacrylates,
copolymers and
terpolymers of the above polycondensates, such as polyesters, polyamides, and
other polymers,
such as polyurethanes or polyepoxides; inorganic materials such as sulfates or
carbonates of
alkaline earth metals and magnesium, including barium sulfate, calcium
sulfate, calcium
carbonate, silicates of alkali and alkaline earth metals, aluminum and
magnesium; and aluminum
or silicon oxides or hydrates, such as clays, alumina, talc, kaolin, zeolite,
silica gel, or glass
(these materials may be used as filters with the above polymeric materials);
and mixtures or
copolymers of the above classes, such as graft copolymers obtained by
initializing
polymerization of synthetic polymers on a pre-existing natural polymer. All of
these materials
may be used in suitable shapes, such as films, sheets, tubes, particulates, or
plates, or they may
be coated onto, bonded, or laminated to appropriate inert carriers, such as
paper, glass, plastic
films, fabrics, or the like. Nitrocellulose has excellent absorption and
adsorption qualities for a
wide variety of reagents including monoclonal antibodies. Nylon also possesses
similar
characteristics and also is suitable. Any of the above materials can be used
to form an array,
such as a microarray, of one or more specific binding reagents.
17
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
Alternatively, the solid phase can constitute microparticles. Microparticles
useful in the
present disclosure can be selected by one skilled in the art from any suitable
type of particulate
material and include those composed of polystyrene, polymethylacrylate,
polypropylene, latex,
polytetrafluoroethylene, polyacrylonitrile, polycarbonate, or similar
materials. Further, the
microparticles can be magnetic or paramagnetic microparticles, so as to
facilitate manipulation
of the microparticle within a magnetic field. In an exemplary embodiment the
microparticles are
carboxylated magnetic microparticles. Microparticles can be suspended in the
mixture of soluble
reagents and test sample or can be retained and immobilized by a support
material. In the latter
case, the microparticles on or in the support material are not capable of
substantial movement to
positions elsewhere within the support material. Alternatively, the
microparticles can be
separated from suspension in the mixture of soluble reagents and test sample
by sedimentation or
centrifugation. When the microparticles are magnetic or paramagnetic the
microparticles can be
separated from suspension in the mixture of soluble reagents and test sample
by a magnetic field.
The methods of the present disclosure can be adapted for use in systems that
utilize microparticle
technology including automated and semi-automated systems wherein the solid
phase comprises
a microparticle. Such systems include those described in pending U.S. App. No.
425,651 and
U.S. Pat. No. 5,089,424, which correspond to published EPO App. Nos. EP 0 425
633 and EP 0
424 634, respectively, and U.S. Pat. No. 5,006,309.
Other considerations affecting the choice of solid phase include the ability
to minimize
non-specific binding of labeled entities and compatibility with the labeling
system employed.
For, example, solid phases used with fluorescent labels should have
sufficiently low background
fluorescence to allow signal detection. Following attachment of a specific
capture antibody, the
surface of the solid support may be further treated with materials such as
serum, proteins, or
other blocking agents to minimize non-specific binding.
Detection Systems
Kits according to the present disclosure may include one or more detectable
labels. The
one or more specific binding reagents, e.g. antibodies, may be bound to a
detectable label.
Detectable labels suitable for use include any compound or composition having
a moiety that is
detectable by spectroscopic, photochemical, biochemical, immunochemical,
electrical, optical, or
chemical means. Such labels include, for example, an enzyme, oligonucleotide,
nanoparticle
chemiluminophore, fluorophore, fluorescence quencher, chemiluminescence
quencher, or biotin.
18
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
Thus for example, in an immunoassay kit configured to employ an optical
signal, the optical
signal is measured as an analyte concentration dependent change in
chemiluminescence,
fluorescence, phosphorescence, electrochemiluminescence, ultraviolet
absorption, visible
absorption, infrared absorption, refraction, surface plasmon resonance. In an
immunoassay kit
configured to employ an electrical signal, the electrical signal is measured
as an analyte
concentration dependent change in current, resistance, potential, mass to
charge ratio, or ion
count. In an immunoassay kit configured to employ a change-of-state signal,
the change of state
signal is measured as an analyte concentration dependent change in size,
solubility, mass, or
resonance.
Useful labels according to the present disclosure include magnetic beads
(e.g.,
DynabeadsTM), fluorescent dyes (e.g., fluorescein, Texas Red, rhodamine, green
fluorescent
protein) and the like (see, e.g., Molecular Probes, Eugene, Oreg., USA),
chemiluminescent
compounds such as acridinium (e.g., acridinium-9-carboxamide),
phenanthridinium, dioxetanes,
luminol and the like, radiolabels (e.g., 3H, 1251, 35S, 14C, or 32P),
catalysts such as enzymes
(e.g., horse radish peroxidase, alkaline phosphatase, beta-galactosidase and
others commonly
used in an ELISA), and colorimetric labels such as colloidal gold (e.g., gold
particles in the 40-
80 nm diameter size range scatter green light with high efficiency) or colored
glass or plastic
(e.g., polystyrene, polypropylene, latex, etc.) beads. Patents teaching the
use of such labels
include U.S. Pat. Nos. 3,817,837; 3,850,752; 3,939,350; 3,996,345; 4,277,437;
4,275,149; and
4,366,241.
The label can be attached to each antibody, for example to a detection
antibody in a
sandwich immunoassay format, prior to, or during, or after contact with the
biological sample.
So-called "direct labels" are detectable labels that are directly attached to
or incorporated into the
antibody prior to use in the assay. Direct labels can be attached to or
incorporated into the
detection antibody by any of a number of means well known to those of skill in
the art.
In contrast, so-called "indirect labels" typically bind to each antibody at
some point
during the assay. Often, the indirect label binds to a moiety that is attached
to or incorporated
into the detection agent prior to use. Thus, for example, each antibody can be
biotinylated before
use in an assay. During the assay, an avidin-conjugated fluorophore can bind
the biotin-bearing
detection agent, to provide a label that is easily detected.
19
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
In another example of indirect labeling, polypeptides capable of specifically
binding
immunoglobulin constant regions, such as polypeptide A or polypeptide G, can
also be used as
labels for detection antibodies. These polypeptides are normal constituents of
the cell walls of
streptococcal bacteria. They exhibit a strong non-immunogenic reactivity with
immunoglobulin
constant regions from a variety of species (see, generally Kronval, et al.
(1973) J. Immunol., 111:
1401-1406, and Akerstrom (1985) J. Immunol., 135: 2589-2542). Such
polypeptides can thus be
labeled and added to the assay mixture, where they will bind to each capture
and detection
antibody, as well as to the autoantibodies, labeling all and providing a
composite signal
attributable to analyte and autoantibody present in the sample.
Some labels may require the use of an additional reagent(s) to produce a
detectable
signal. In an ELISA, for example, an enzyme label (e.g., beta-galactosidase)
will require the
addition of a substrate (e.g., X-gal) to produce a detectable signal. In an
immunoassay kit
configured to use an acridinium compound as the direct label, a basic solution
and a source of
hydrogen peroxide can also be included in the kit.
Test kits according to the present disclosure preferably include instructions
for
determining the level of each marker in a sample from the subject, for example
by carrying out
one or more immunoassays. The instructions may further include instructions
for analyzing a
test sample of a specific type, such as a blood sample, or more specifically a
serum sample or a
plasma sample. Instructions included in kits of the present disclosure can be
affixed to
packaging material or can be included as a package insert. While the
instructions are typically
written or printed materials they are not limited to such. Any medium capable
of storing such
instructions and communicating them to an end user is contemplated by this
disclosure. Such
media include, but are not limited to, electronic storage media (e.g.,
magnetic discs, tapes,
cartridges, chips), optical media (e.g., CD ROM), and the like. As used
herein, the term
"instructions" can include the address of an internet site that provides the
instructions.
E. Adaptations of the Methods of the Present Disclosure
One skilled in the art would readily appreciate that the biomarkers,
oligonucleotides,
methods, kits and related compositions described herein are representative of
exemplary
embodiments, and not intended as limitations on the scope of the invention. It
will be readily
apparent to one skilled in the art that varying substitutions and
modifications may be made to the
present disclosure disclosed herein without departing from the scope and
spirit of the invention.
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
All patents and publications mentioned in the specification are indicative of
the levels of
those skilled in the art to which the present disclosure pertains. All patents
and publications are
herein incorporated by reference to the same extent as if each individual
publication was
specifically and individually indicated as incorporated by reference.
The present disclosure illustratively described herein suitably may be
practiced in the
absence of any element or elements, limitation or limitations that are not
specifically disclosed
herein. Thus, for example, in each instance herein any of the terms
"comprising," "consisting
essentially of' and "consisting of' may be replaced with either of the other
two terms. The terms
and expressions which have been employed are used as terms of description and
not of
limitation, and there is no intention that in the use of such terms and
expressions of excluding
any equivalents of the features shown and described or portions thereof, but
it is recognized that
various modifications are possible within the scope of the present disclosure
claimed. Thus, it
should be understood that although the present disclosure has been
specifically disclosed by
preferred embodiments and optional features, modification and variation of the
concepts herein
disclosed may be resorted to by those skilled in the art, and that such
modifications and
variations are considered to be within the scope of this invention as defined
by the appended
claims.
EXAMPLE
By way of example, and not of limitation, examples of the present disclosures
shall now
be given.
Example 1: Correlation of Markers with Clinical Outcome based on Data across
Multiple
NSCLC Trials with Differing Therapeutics
Three patient cohorts distinguished by treatment regimen were evaluated for
overall
survival. All patients were diagnosed with stage 3/4 NSCLC. Marker
concentrations were
measured by immunoassay at baseline in NSCLC trials. NSCLC subjects were
assigned to one
of three cohorts as follows: M05-780 (N=83) in which subjects received
pemetrexed (Alimta
available from Eli Lilly and Company, Indianapolis, IN) with or without ABT-
751 ((N-[2-[(4-
Hydroxyphenyl)amino]-3-pyridinyl]-4-methoxybenzenesulfonamide, available from
Abbott
Laboratories, Abbott Park, IL); M05-782 (N=21), in which subjects received
Docetaxel with or
without ABT-75 1;and M06-880 (N=103), in which subjects received only ABT-869.
21
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
Patients were categorized as having a marker level above or below the
threshold marker
level. Survival as a function of classification was compared for each marker
and treatment.
Marker thresholds were assessed by multiple methods including but not limited
to, median value
determination, statistical modeling for optimal thresholds, values determined
in the community
to be predictive for NSCLC vs. benign lung disease and comparison of relative
concentration of
the marker in patients with stable disease vs. rapid progression on therapy
with ABT-869.
FIG. 1 is a Kaplan Meier plot showing the Overall Survival (OS-DUR) in days
for the
three different cohorts of stage 3/4 NSCLC patients, showing the results for
M05-780 in red, for
M05-782 in green and for M06-880 in blue. FIG. 2 is a set of Kaplan-Meier
plots for the patient
cohort (M05-780) that was treated with Alimta with or without ABT-75 1,
plotting OS for each
of eight plasma markers evaluated, according to baseline plasma level of each
marker in
comparison to an NSCLC median threshold. Table 2 provides a summary of the raw
marker
levels observed and thresholds for seven of the eight markers in FIG. 2
(Cyfra21-1, NSE, CEA,
SCC, ProGRP, CA 15-3, and CA125), in patients treated with ABT-869 or treated
with ABT-
751.
FIG. 3 shows two Kaplan Meier plots, both based on further analysis of a
patient cluster
identified as Cluster 2. As can be seen in FIG. 3, Cluster 2 patients were
those across the
NSCLC trials who showed a pronounced increase in OS following treatment with
ABT-869
when compared to patients treated with Alimta with or without ABT-75 1.
Cluster 2 patients
were characterized in terms of baseline plasma marker levels and all showed
one or more of a
level of NSE below the threshold for NSE, a level of CYFRA 21-1 below the
threshold for
CYFRA 21-1, a level of CA125 below the threshold for CA125, a level of CEA
above the
threshold for CEA.
22
CA 02798441 2012-11-05
WO 2011/140234 PCT/US2011/035213
Table 2:
Cox model (raw data) logrank (threshold data)
Marker ABT869 ABT751 ABT869 ABT751
CYFRA21 0.0005 0.0004 0.0039 0.0065
NSE 0.0006 0.8684 0.0005 0.5489
CEA 0.6331 0.8115 0.8190 0.8558
SCC 0.4554 0.0212 0.2258 0.1539
ProGRP 0.7279 0.5873 0.0811 0.7121
CA15.3 0.0063 0.0580 0.0431 0.6802
CA125 0.0004 0.0004 0.0008 0.0004
23