Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02805908 2013-02-12
Attorney Docket MPPA006.29 Andrasi & Carkner
Thermal Barrier
In the United States Patent and Trademark Office
And in the Matter of a
Non-Provisional Patent Application by
Eric Andrasi and Steve Carkner for a
Hydrophilic Polymer Thermal Barrier System
RELATED APPLICATIONS
This Application claims the benefit of United States Provisional Patent
Application # 61610146
for the same invention filed by the same inventors in the USPTO on March 13,
2012
FEDERAL FUNDING
N/A
BACKGROUND OF THE INVENTION
Field of the Invention
This invention pertains to the field of batteries, and particularly to a
thermal barrier system that
resides between neighbouring cells of a battery pack in order to prevent
propagation of thermal
runaway through the cells of a battery pack.
1
CA 02805908 2013-02-12
Attorney Docket U:PPA006.29 Andrasi & Carkner
Thermal Barrier
Discussion of the Problem
A battery is generally constructed from one or more individual electrochemical
cells. Such cells
may be manufactured using a variety of systems including metal cylinders such
as industry
standard -AA" batteries or plastic jars such as the lead-acid batteries found
in automobiles.
Pouch cells are generally constructed by enclosing a flat laminate structure
of electrodes within a
pouch which is then sealed. These pouch cells may be referred to in the
industry as polymer
cells, fiat cells or laminate cells.
Pouch cell technology may also be applied in other areas such as the
construction of super-
capacitors and may be employed in future energy storage technology packaging
such as carbon-
tube, nano-wire. and other means that may be ionic, electrostatic or
electrochemical in nature.
In general, when energy is stored in a small space, the greater the energy
stored, the higher the
potential for a thermal event, such as fire. if the energy is released at a
rate that is much higher
than the system was designed for. For example, in the case of a Lithium
Polymer rechargeable
cell, if the cell is short circuited, it may develop very high internal
temperatures to the cell. The
connections and the shorting element may also get very hot with the shorting
element becoming
red or even white-hot during the event. External short circuits can be
protected to some extent by
using battery management electronics, fusible links and current-limiting
materials in the cell
construction. However, energy storage cells can also develop internal short
circuits. This
includes penetration of the cell by a foreign object such as a nail or a
bullet, and can also include
dendrite growth or manufacturing defects that will cause the conductive
electrodes inside the cell
to short together. These internal short circuits can cause the cell to become
extremely hot and
will sometimes result in thermal runaway of the energy storage system.
Thermal runaway is a condition where a reaction becomes exothermic to the
point where it
becomes self-sustaining and may even accelerate. When a lithium cell is
shorted, it can reach
temperatures in excess of 200 C. For some chemistries, a temperature of 200 C
will result in
chemical reactions that produce heat at a rate that cannot be dissipated by
the cell surface, as a
result the cell temperature may rise beyond that temperature that would be
caused purely by the
2
CA 02805908 2013-02-12
Attorney Docket #:PPA006.29 Andrasi & Carkner
Thermal Barrier
discharge of the electrical energy in the cell, this can cause the cell
temperature to rise far beyond
the 200 C, this is thermal runaway. The temperatures given are as an example
only, the actual
temperature a cell reaches when shorted and the temperature at which thermal
runaway occurs
will vary based on many conditions including the cell composition, charge
level, age,
environmental conditions, fault condition and packaging.
In some cases, a cell may also generate flammable gasses when it reaches very
high
temperatures. The self-generation of gasses such as hydrogen and oxygen,
coupled with high
point source temperatures and possibly sparking, can also result in ignition
of the system. Many
batteries contain large amounts of carbon and other materials that will
readily burn in the
presence of these gasses. as such the reaction may move from one of thermal
runaway to
complete system combustion.
The faulted cell can become so hot that, when such cell is part of a battery
assembly of multiple
cells, the heat from the faulted cell will bring neighbouring cells up to a
temperature where they
too enter thermal runaway, even if those cells have no faults. These
neighbouring cells may then
become so hot that in turn they cause other cells to enter thermal runaway,
with the end result of
the entire battery package entering thermal runaway.
A common method for preventing thermal runaway from propagating from cell to
cell within a
battery pack is to include some form of separator between cells that will
thermally insulate the
cells, such as fibreglass. The advantage of insulation is that it can be light
weight and
inexpensive. However, the major disadvantage of such material is that the cell
cannot easily
dissipate heat generated during normal operation. For this reason, use of
thermally insulating
materials between the cells may actually increase the probability of thermal
runaway, especially
in high-power battery packs that tend to generate significant heat when
operating normally.
Metal jackets or separators have been suggested as a method for removing heat
from battery
packs. A thermally conductive material such as aluminum is placed between the
cells and will
absorb and transmit heat. In some cases the jacket is filled with pumped
coolant that flows to a
reservoir and heat dissipater. The disadvantage of metallic systems is the
excessive weight and
cost of implementing such a system. In addition, metal systems are conductive
and therefore can
increase foreign object short-circuit potential during penetration, for
example if struck by a
3
CA 02805908 2013-02-12
Attorney Docket #:PPA006.29 Andrasi & Carkner
Thermal Barrier
bullet. A metal separator between cells may also increase conduction of heat
between cells and
can therefore promote thermal runaway by conducting very high temperatures
from a faulted cell
to a non-faulted cell.
The inclusion of flowing coolant further increases complexity, cost and system
weight and also
Thermally conductive systems which preferentially transmit heat in one
direction only have been
promoted as a compromise method to draw heat away from the cells, while
preventing heat
transfer between the cells. These systems suffer from high weight, complexity
and cost, and
typically they have poor thermal conductivity in the desired direction when
compared to metals.
The use of materials that transition from a solid to a liquid have also been
suggested for use as
cell separator materials. These solid phase change materials are often
composed of wax, carbon.
or other chemicals with transition temperatures in the order of 40 C to 60 C.
These materials
absorb on the order of 50 to 300J/g of energy during the transition phase.
While such materials
can absorb peak temperatures produced during normal operation, they provide no
protection
against thermal runaway if the battery system is operating above their
transition temperature. In
addition, the materials used will contribute to thermal runaway in situations
where flame is
involved, in effect the materials designed to absorb heat may actually add
fuel to the fire in
extreme thermal runaway conditions.
Solid materials do not absorb much energy when compared to the amount of
energy stored in a
lithium battery. As lithium batteries increase in energy density the amount of
solid phase change
materials required may actually exceed the original weight of the cells. For
example, a lithium
battery may have an energy density of 200 watt-hours-per-kilogram. A single
gram of material is
therefore capable of releasing 720 joules of energy. If the phase change
material is only capable
of absorbing 100 joules per gram. then 7.2 grams of material will be required
to absorb the
energy for every gram of battery. This increases the weight of the battery
system by more than
700%.
Battery systems may also be exposed to fire in situations where neighbouring
equipment that is
not associated with the battery catches on fire. In such situations, it is
important that the battery.
4
CA 02805908 2013-02-12
Attorney Docket N:PPA006.29 Andrasi & Carkner
Thermal Barrier
to the greatest extent possible, should resist catching on fire, and if it
does catch on fire, it is
desired that the battery contribute as little additional energy or fuel to
said fire.
There remains a need for a system that can absorb all of the energy released
by an energy storage
cell that is faulted to prevent the cell from entering thermal runaway, there
further exists a need
for a system that can absorb transient thermal heat from an energy storage
cell during normal
operation, there further exists a need for a thermal barrier system that can
prevent thermal
runaway propagation from cell to cell in a battery pack, there further exists
a need for a thermal
barrier system that is light weight and inexpensive, there further exists a
need for a thermal
barrier system that will not contribute significantly to the energy released
during a combustion
event in the case where a battery system is on fire.
SUMMARY OF THE INVENTION
In order to overcome the deficiencies noted above, we propose as a solution
our invention,
namely, a system which includes hydrophilic material disposed within a
hydrophobic material
and placed within a battery.
Hydrophilic polymers contain polar or charged functional groups, rendering
them soluble in
water. Most hydrophilic polymers arc grouped by the chemistry of their
structure. For example,
acrylics include acrylic acid, acrylamide. and maleic anhydride polymers and
copolymers.
Amine-functional polymers include allylaminc, ethyleneimine, oxazoline, and
other polymers
containing amine groups in their main- or side-chains. A hydrophilic polymer
that has been
mixed with water will often be referred to as a hydrogel.
In a preferred embodiment, sodium polyacrylate is used as the hydrophilic
substance and will be
mixed at a ratio of about I part sodium polyacrylate to 99 parts water. It is
a hydrophilic, or
water loving, polymer and can hold up 500 times its weight in water. Polymers
are long chains of
molecules linked together. The sodium polyacrylate forms chains around the
water molecules
and holds onto them like a net creating a hydrogel. A suitable ratio of water
to sodium
polyacrylate will depend on the application. Low ratios result in a material
that is very thick but
may not absorb as much heat as a very high ratio mixture which can have low
viscosity and very
high water content.
5
CA 02805908 2013-02-12
Attorney Docket #:PPA006.29 Andrasi & Carkner
Thermal Barrier
The resulting hydrogel will be held in a hydrophobic structure such as a foil
pouch. The
advantage of a foil pouch is that it may be constructed of the same materials
and on similar
equipment as the lithium polymer cell pouches. This may reduce manufacturing
costs. Foil will
retain the water content ratio, and therefore will maintain the viscosity of
the hydrogel for very
long periods.
The hydrophobic structure may include internal structures to spread-out and
maintain mechanical
integrity of the hydrogel. For example a porous sponge material may be used,
or the structure
may be divided up into a multitude of smaller segments each containing
hydrogel.
In another embodiment of the invention the hydrophilic material can be natural
cotton, sponge or
other material that does not contain polymers. In these cases the hydrophobic
structure is used to
ensure the water is kept in close contact with the energy storage cell.
In another embodiment of the invention the thermal barrier structure includes
a low viscosity,
non-flammable energy absorbing material which has an atmospheric boiling point
above the
maximum operating temperature of the battery and below the thermal runaway
temperature of
the battery, and such energy absorbing material is encased in a retaining
structure which retains
mechanical integrity at temperatures above the atmospheric boiling point of
the energy absorbing
material.
In another embodiment of the invention, the hydrophobic retaining structure
may include in the
design an intentional venting ability that will relieve excessive pressure
build-up within the
structure which could affect the atmospheric boiling point of the energy
absorbing material.
6 =
CA 02805908 2013-02-12
Attorney Docket #:PPA006.29 Andrasi & Carkner
Thermal Barrier
Description of the Figures
Figure I shows a cross section of the thermal barrier system.
Figure 2 is a graph of the expected performance of the thermal barrier system.
Figure 3 shows a cross-section the thermal barrier system under three
different operating
scenarios.
Figure 4 shows a cross-section of five difIbrent construction methods for the
thermal barrier
system.
Figure 5 shows the thermal barrier system installed in a battery pack.
Figure 6 shows the thermal barrier system installed in a battery pack with a
single cell
undergoing a thermal runaway condition.
Figure 7 shows a battery pack in a case being touched by a user.
7
CA 02805908 2013-02-12
Attorney Docket #:PPA006.29 Andrasi & Carkner
Thermal Barrier
Description of a Preferred Embodiment of the Invention
In a preferred embodiment, sodium polyacrylate is used as the hydrophilic
substance and will be
mixed at a ratio of about 1 part sodium polyacrylate (0 99 parts water. From
this point of view
the result substance can be treated as essentially pure water from a
thermodynamic point of view.
S Sodium polyacrylate also has the advantage of little or no health and
safety concerns around
humans. In particular the standard MSDN (Material Safety Data Sheet) lists
health, flammability,
reactivity, exposure and storage concerns all as "Zero" risk.
Water has a specific heat capacity Cp of about 4.2J/gK where j is in joules, g
is in grams and k is
in degrees Kelvin or centigrade. Therefore. it will take 4.2 Joules of energy
to raise 1 gram of
water by 1 degree Kelvin (or centigrade). Water has one of the highest heat
capacities for a liquid
substance. Ammonia is another liquid with a high Cp of about 4.7J/gK.
Aluminum only has a heat capacity Cp of about 0.9J/gK and copper of 0.4J/gK.
Solid paraffin wax has a heat capacity Cp of about 2.5J/gK and liquid paraffin
of about 1.7.1/gK.
The latent heat of vaporization Lv of water is about 2260J/g. Therefore, when
water transitions
from being a liquid to a gas, it will absorb about 2260 joules of energy per
gram. Water will
remain at the atmospheric boiling point Tb, about 100 C, until all of the
water has been
converted to vapour, assuming standard atmospheric pressures.
Considering a Lithium energy storage cell, the maximum short term operating
temperature may
be specified as 70 C and the thermal runaway temperature can be as low as 150
C depending on
the depending on the chemistry and the manufacturer.
Denoting maximum operating temperature as Tmax and minimum thermal runaway
temperature
as Trun, and the amount of thermal absorption material as M in grams, and
also, assuming that
the vapour produced at the heat of vaporization temperature LA/, the following
formula can be
used to calculate the amount of energy Ea that will be absorbed as the thermal
material is heated
from Tmax to Th.
Provided Trun > Tb:
Ea = (((Tb ¨ Tmax) x Cp) + Lv) x M
8
CA 02805908 2013-02-12
Attorney Docket #:PPA006.29 Andrasi & Carkner
Thermal Barrier
One gram of a mixture of one part sodium polyacrylate with 99 parts water,
when used with a
lithium battery that has a maximum operating temperature of 70 C and the
thermal runaway
temperature of 150 C would therefore be able to absorb 2386 Joules of energy
per gram.
Ea = 0(100 ¨ 70) x 4.2) + 2260) = 2386J/g
If the lithium battery has an energy density of 200 wan-hours-per-kilogram.
The energy density
of the battery Ed is therefore capable of releasing 720 joules of energy.
In order to absorb all of the energy of the battery, the amount of energy
absorbing material Mr is
therefore Ed / Ea grams per gram.
Mr = 720 / 2386 = 0.3 grams per gram.
Therefore, as a worst-case, a lkg battery may require the addition of 300g of
energy absorbing
material in order to absorb all of the energy released by the battery during a
fault situation. In
reality a battery will require much less material because lithium battery
thermal runaway
temperatures typically increase as the battery becomes discharged. Also, the
other cells in the
battery pack will themselves absorb a significant amount of thermal energy as
they are raised to
their thermal runaway temperature.
Assuming the heat capacity of a lithium battery to be mostly dependent on the
graphite,
aluminum and copper materials that make up the electrode structures, it can be
assumed that a
lithium battery has a heat capacity Cp of about 0.7J/gK. Then the energy
absorption Ea of the
non-faulted lithium cells as they are heated from their maximum operating
point to the thermal
runaway point could be approximated as:
Ea = ((Trun ¨ Tmax) x Cp)
For the lithium battery used in the previous example:
Ea = ((150 ¨ 70) x 0.7) = 56 J/g
If we then assume that, on average, the lithium cells will have a mass that is
10 times higher than
the energy absorption material, and that we further assume that there will be
one cell on each
side of the faulted battery. then the total energy absorption contributed by
the non-faulted cells in
9
CA 02805908 2013-02-12
Attorney Docket #:PPA006.29 Andrasi & Carkner
Thermal Barrier
the immediate vicinity of the faulted cell would be 20 times the Ea value per
gram of energy
absorption materials.
If the energy absorption materials are used between each cell in the battery
pack. then we can
assume that the faulted cell will have two energy absorption elements
associated with it (one on
each side) and that each non faulted cell will also have an extra energy
absorption element
associated with it. Therefore, a total of four energy absorption elements will
be present per
faulted cell.
Therefore the total energy absorption Et per gram of energy absorption
material disposed
between each cell in the battery pack can be multiplied by at least a factor
of 4, where Earn is the
energy absorption ability of the material and Eac is the energy absorption of
the lithium cells, the
total formula would become:
Et = (4 x Earn) + (20 x Eac) = (4 x 2386) + (20 x 56) = 10664 J/g
Therefore, returning to the formula for calculating grams-per-gram of material
we would find:
Mr = 720 / 10664 = 0.07 grams per gram.
Therefore, as a more reasonable case, a I kg battery may require the addition
of 70g of energy
absorbing material in order to prevent thermal runaway propagation within the
battery.
For a battery system with an energy capacity of 200Wh/kg, which is a primary
competitive
distinction between various energy storage technologies, it is expected that a
battery pack
containing thermal runaway propagation barriers may see the energy density
drop to about
187Whiltg. which, for most applications, is an insignificant difference,
especially when
considered against the dramatic increase in safety.
Other hydrophilic compounds can also be used including Polyacrylamide. for
example C3H5NO
in very long chains. Polyacrylamide-co-acrylic acid can also be super-
absorbent and therefore
meet the needs of providing thermal barrier. In particular. the addition of co-
acrylics or other
dopants can allow the atmospheric boiling point to be adjusted to higher or
lower boiling points,
therefore being tailored to suit the particular thermal operation and thermal
runaway points of the
given battery system.
CA 02805908 2013-02-12
Attorney Docket #:PPA006.29 Andrasi & Carkner
Thermal Barrier
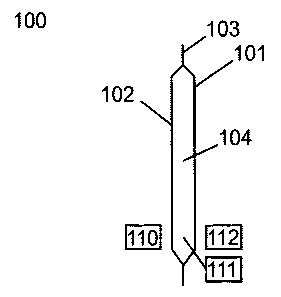
Referring to Figure 1, a thermal barrier system (100) is shown in cross-
section, which is
constructed from two hydrophobic sheets. A first hydrophobic sheet (102) and a
second
hydrophobic sheet (101) arc bonded together at a seal area (103) around the
perimeter of the
pouch. The interior of the pouch is filled with the hydrophilic material
(104).
Referring now to both Figure 1 and Figure 2 wherein Figure 2 includes a
vertical axis for
temperature (231) and a horizontal axis for time (230). When a hot body, such
as a faulted
lithium cell, is in contact with a first area (110) of the thermal barrier
system (100), the
hydrophilic material (104) will at first absorb the heat from the faulted
lithium cell The
hydrophilic material in the region of the heat (111) will rise in temperature
until it reaches its
atmospheric boiling point. In Figure 2, a graph (200) includes the ambient
operating temperature
(210) of the system. A fault occurs at time (201) at which point the
temperature of the faulted
battery (Figure 1-110, Figure 2 plot line 220) will rapidly rise. The un-
faulted lithium cell (112)
is located on the opposite side of the thermal barrier. The un-faulted lithium
cell (Figure 1-112.
Figure 2 plot line 221) will rise in temperature as the hydrophilic material
(111) also rises in
temperature. The un-faulted cell temperature rise will lag behind the rise in
temperature of the
faulted cell due to thermal absorption of the hydrophilic material and due to
thermal propagation
delay through the hydrophilic material and the thermal mass of the un-faulted
lithium cell itself.
Once the hydrophilic material (111) reaches its atmospheric boiling point, the
temperature seen
by the un-faulted lithium cell will stabilize (Figure 2, time 202, temperature
211). The
temperature of the faulted cell (Figure 1-110, Figure 2 plot line 220) may
continue to rise to a
point where the faulted cell rate of energy production equals the rate of
absorption of energy by
all materials that surround the faulted cell, including all packaging.
electronics and the thermal
barrier material itself. The maximum temperature (212) may be above or below
the thermal
runaway temperature of the faulted cell. Eventually the faulted cell will
exhaust its energy
capacity at time (203) and the temperature will then begin to fall. The
thermal energy of the
faulted cell will continue to be absorbed by the thermal barrier material
until the temperature of
the faulted cell is equal to the temperature of the thermal barrier material
at time (204). The
temperature of the entire system will then gradually fall as the energy is
absorbed and radiated
into the ambient environment.
11
CA 02805908 2013-02-12
Attorney Docket #:PPA006.29 Andrasi & Carkner
Thermal Barrier
Provided the thermal runaway temperature of the un-faulted lithium cell is
lower than the
atmospheric boiling point of the thermal barrier material. Figure 2
demonstrates that the
temperature of the un-faulted lithium cell (plot line 221) does not exceed the
atmospheric boiling
point (211) of the thermal barrier material.
Figure 3 shows three examples of the thermal barrier system (300) during three
different modes
of operation. The normal state (301) is similar to the concept shown in Figure
1. The thermal
barrier material (310) which preferably consists of a hydrophilic polymer that
is 99% water is
shown occupying most of the pouch. A small void (311) may be present which
will allow
normally expected levels of expansion and contraction of the thermal barrier
material to take
place without significantly affecting the pressure inside the pouch.
The pouch may swell as shown in the hot-state (302). When exposed to a
temperature which
exceeds the atmospheric boiling point of the thermal barrier material (320)
the pressure inside
the pouch will increase which may cause swelling. In addition, vapour may
evolve as the thermal
barrier material undergoes vaporization, this may cause the void area (321) to
increase in size.
The pouch may continue to swell as energy is absorbed from a faulted cell. The
pouch may enter
a vented state (303) when the vapour pressure inside the pouch exceeds the
design capacity of
the pouch itself. Elevated pressures inside the pouch may cause the boiling
point of the thermal
barrier material (330) to increase unacceptably. Therefore, the pouch may be
designed with a
specific weak area (332) which is designed to open at a specific pressure
level in order to reduce
pressure inside the pouch. As pressure instantly drops inside the pouch, the
thermal barrier
material will be super-heated when compared to its regular atmospheric boiling
point. As a
result. there will be a short period of higher than normal energy absorption
following such
venting action.
Figure 4 shows five examples of packaging methods for various thermal barrier
systems (400) all
shown in cross-section. The basic thermal barrier system (401) includes a
hydrophobic outer
material which may be in the form of a pouch, foil; plastic laminate; blow-
molded case, metal
shell, or any other method that retains the hydrophilic material (411)
contained and prevents it
from drying out during normal operation.
12
CA 02805908 2013-02-12
Attorney Docket 11:PPA006.29 Andrasi & Carkner
Thermal Barrier
A modified thermal barrier system (402) is shown with the pouch containing an
internal support
structure (412) which may be composed of a sponge, net, woven material,
cotton, nylon or any
other material that will ensure the hydrophilic material remains in its
intended location. Without
the internal support structure (412) the hydrophilic material may tend to pool
at the bottom of the
pouch due to gravity. This effect would be most pronounced if the walls of the
pouch are highly
flexible and the hydrophilic material is of low viscosity. The support
material may not be
required if the hydrophilic material is of high enough viscosity to support
itself, or if the barrier
is always installed in an orientation that prevents gravitational effects from
displacing the
hydrophilic material.
The thermal barrier may be constructed in a bubble-wrap form (403) where fluid
filled blisters
(413) are formed with sealed areas (414) between each blister. The blisters
ensure the
hydrophilic material remains constrained to a small localized area of the
lithium cell; it also
ensures that the loss of one blister due to mechanical damage will not
significantly impact the
operation of the entire sheet which may contain thousands of blisters.
The thermal barrier may be constructed with separated regions (404) isolated
by intermediate
walls (415) disposed between each region. This barrier is similar to the
bubble-wrap form (403)
with the exception that a higher volume of hydrophilic material and better
surface coverage may
be achieved.
The thermal barrier may be constructed by utilizing a laminated concept (405).
This concept
works best with high viscosity hydrophilic materials. The high viscosity
hydrophilic material
(417) is disposed between alternating sheets of hydrophobic material (416). If
the thickness of
the laminates is made sufficiently thin compared to the overall area of the
laminates, the system
may require no other mechanical seals or supports, otherwise the edges of the
laminate may be
sealed with glue, tape, paint or by other conventional methods.
Figure 5 shows a preferred embodiment installed in a three cell battery pack
(500). The side
cross-section (510) of the battery pack shows a lithium polymer cell (501)
which includes an
electrical connection tab (502). Additional cells (503. 504) are included in
the pack. The thermal
barriers (505. 506) are installed between each cell (501, 503. and 504). A
front cross-section
13
CA 02805908 2013-02-12
Attorney Docket ti:PPA006.29 Andrasi & Carkner
Thermal Barrier
(520) of the battery pack shows the lithium polymer cell (501) in front of the
thermal barrier
(505) and shows two electrical connection tabs (502) on the cell.
In the case of the previous descriptions of a faulted lithium cell, the
faulted cell (503) would try
to transfer heat to the neighbouring cells (501, 504) but will be prevented
from transmitting
temperatures beyond the atmospheric boiling point of the hydrophilic material
due to the thermal
barriers (505. 506).
Figure 6 shows a side cross-section of a faulted five-cell lithium battery
pack (600). In this case
the faulted cell (601) has increased in temperature and is starting to swell:
this is common for
lithium polymer cells in foil pouches. The thermal barriers (602) on each side
of the faulted cell
have also reached their boiling temperatures and are therefore evolving vapour
which is causing
them to intentionally swell. In this example the thermal barriers are made in
a similar pouch
format to the lithium cells themselves. This battery pack includes four un-
faulted cells (603)
which remain at or below the atmospheric boiling point of the hydrophilic
material and therefore
they are not swelling. The two thermal barriers (604) which separate the un-
faulted cells will
continue to cool the un-faulted cells due to their contact with one side of
each un-faulted cell.
Provided the battery pack outer housing provides for some expansion room, it
can be seen in
Figure 6 that allowing the thermal barriers to swell will also cause the un-
faulted cells to be
physically pushed away from the faulted cell. This increased gap will further
reduce the
conduction of heat into the un-faulted cells and will further reduce the
probability of thermal
runaway propagation through the pack.
Figure 7 shows a typical complete battery pack system (700) made up of three
cells (701)
installed within housing (702). The cells each have the hydrophilic polymer
thermal barrier
installed between them. In addition, a hydrophilic polymer thermal barrier is
installed between
the outer cells and the casing (703). In the even of a thermal runaway
situation, or any situation
where the temperature of the cells within the battery pack housing (702) rises
above the
atmospheric boiling temperature, the thermal barrier between the cells and the
housing (702) will
ensure that the housing temperature does not rise above the atmospheric
boiling temperature.
Contact with the housing by an operator (704) or by neighbouring equipment
(including other
complete battery packs) will be dramatically safer due to the relatively low
temperature of the
14
CA 02805908 2013-02-12
Attorney Docket #:PPA006.29 Andrasi & Carkner
Thermal Barrier
housing compared with the possible high temperature of the faulted cells
within the battery
housing.
An advantage of the hydrophilic polymer thermal barrier is that it will
conduct thermal energy up
to the point where it reaches the atmospheric boiling temperature. During
normal operation,
thermal barrier itself (through the use of packaging such as those shown in
Figure 4 (403. 404 or
405) can allow the battery pack thermal characteristics to be optimized for
safety and
performance.
The thermal barrier system may also be applied to electronics to prevent
overheated components
The thermal barrier can also reduce thermal signatures, often called an
infrared signature that
Although the description above contains much specificity. these should not be
construed as
limiting the scope of the invention but as merely providing illustrations of
the presently preferred
embodiment of this invention. Thus the scope of the invention should be
determined by the