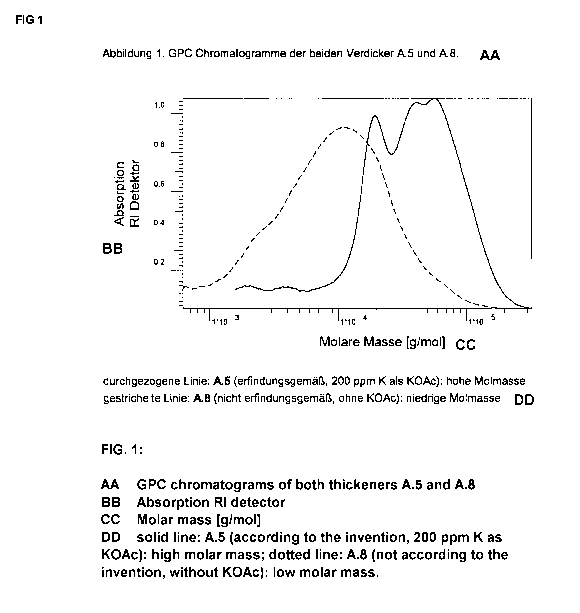
Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
W020121052383 CA 02815268 2013-04-19
PCT/EP2011/068072
1
Polyurethane thickeners
Description
The present invention relates to a single-stage process for preparing
polyurethanes
which comprise at least three hydrophilic sections, at least four hydrophobic
sections,
optionally allophanate segments and optionally isocyanurate segments, in the
presence of alkali(ne earth) metal carboxylates or zinc carboxylates.
Furthermore, the present invention relates to the polyurethanes themselves
obtainable
in this way, to the use thereof as thickeners for aqueous preparations, and to
aqueous
preparations comprising polyurethanes of this type.
Polyurethanes have been used for a long time in numerous fields of application
for
highly diverse purposes. Depending on the choice of starting materials and the
stoichiometric ratio of the starting materials, polyurethanes are obtained
with very
different physicochemical properties.
Thickeners are used widely for increasing the viscosity of aqueous
preparations, for
example in the fields of cosmetics, human and animal nutrition, pharmacy and
for
detergents, paints and coatings. Inter alia, polyurethanes are also known as
thickeners.
For example, polyurethane solutions or dispersions in water-dilutable aqueous
or
predominantly aqueous phase are referred to by the person skilled in the art
as HEUR
thickeners ("hydrophobically modified ethylene oxide urethane copolymer"), and
have
already been used for a relatively long time in highly diverse fields of
application for
thickening water-based emulsion paints.
The action principle of the thickening effect of the HEUR thickeners is
assumed to be
that the polyethylene glycol segments ensure the water compatibility and the
hydrophobic segments construct a viscosity-imparting three-dimensional
molecular
association via an association with one another and also with dispersed binder
particles of the emulsion paint to be thickened therein.
Thickeners are also used in the field of cosmetic preparations. Thickeners for
cosmetic
preparations are expected to have an adequate thickening effect even in
preparations
with a high content of salt. Furthermore, such thickeners should produce
cosmetic
preparations with a good texture and pleasant feel on the skin. Compatibility
with
numerous other auxiliaries, in particular with salts and surfactants, and also
incorporability of the thickener itself and also of the other auxiliaries
should be
provided.
W02012/052383 CA 02815268 2013-04-19
PCT/EP2011/068072
2
Moreover, the thickened preparations must have constant rheology and physical
and
chemical quality even upon long-term storage, and in the case of changes in
temperature and pH. Finally, it should also be possible to produce these
thickeners in a
cost-effective manner and without a notable impact on the environment.
US 4079028 and US 4155892 disclose, inter alia, linear polyurethane
thickeners. The
preparation of the polyurethanes specified therein takes place in two stages
in solution
and is catalyzed by the catalyst dibutyltin dilaurate (DBTL) customary in
polyurethane
chemistry.
EP 1584331 and EP 1013264 describe polyurethane thickeners for cosmetic
preparations. These are prepared in a single-step process from polyol,
polyisocyanate
and fatty alcohol, which may be ethoxylated, without use of a catalyst.
WO 2006/002813 describes polyurethane thickeners for various applications in
aqueous media. These thickeners are prepared in a single-stage process from
hydrophilic polyols having at least two hydroxy groups, one or more
hydrophobic
compounds, e.g. long-chain alcohols, and at least difunctional isocyanates.
Here, an
excess of NCO groups is used. The catalyst used is 1,8-diazabicyclo45-4-
0]undec-7-
ene (DABCO).
WO 02/88212 describes polyurethanes of ethoxylated long-chain alcohols and
cyclic
diisocyanate oligomers, for example isocyanurates. The polyurethanes described
are
prepared without using polyols and catalysts.
EP 725097 describes polyurethane thickeners, during the preparation of which a
polyether mixture of polyetherols and alkoxylated monoalcohols are reacted
with
polyisocyanates in a single-stage with catalysis by DBTL, diazabicyclooctane
or tin
dioctoate, the ratio of NCO to OH equivalents being in the range from 0.9:1 to
1.2:1.
These thickeners are proposed for use in the field of low shear forces, e.g.
for the flow
of water-based emulsion paints.
EP 1241198, EP 1241199, and EP 1241200 describe the preparation of
polyurethane
thickeners with DBTL catalysis and use of polyetherpolyols and urethane-group-
containing polyetherpolyols with functionalities greater than 2, such as, for
example,
ethoxylated sugars or glycerol.
EP 761780 and EP 1111014 describe polyurethane thickeners of polyol,
diisocyanate
and branched alcohols. The preparation takes place in one stage without a
diluent and
without using catalysts.
W02012/052383 CA 02815268 2013-04-19
PCT/EP2011/068072
3
WO 2009/135856 and WO 2009/135857 describe water-dispersible polyurethanes
with
an essentially linear backbone composed of alternating hydrophilic and
hydrophobic
sections and uses thereof. The polyurethane preparation takes place in two
steps and
is catalyzed by titanium or zinc compounds.
It was an object of the present invention to provide novel thickeners for
water-
comprising preparations, in particular cosmetic preparation. The preparation
process
should be easy and cost-effective to carry out and the novel thickeners should
have the
best thickening effect possible.
Moreover, the thickening effect of the novel thickeners should at least not be
diminished by the presence of salts in the aqueous preparations.
It is also desired for the thickeners to have the lowest possible stickiness
to surfaces
such as glass or steel since this leads to advantages during the preparation
of
formulations. Thus, in the event of low stickiness, it is possible to achieve
easier
emptying of production vessels during the production of cosmetic preparations.
Moreover, a good texture of the cosmetic preparations comprising the
thickeners
according to the invention is also important; in particular here, a smooth,
nonlumpy or
gritty structure is desired.
A further aim was to provide polyurethane thickeners with the properties
described,
which in addition tin-free, since this is desired for cosmetic applications.
The aforementioned objects were achieved by a process for preparing
polyurethanes
comprising
l) at least two hydrophilic sections S,
II) at least one hydrophilic section P different from S,
III) at least two terminal hydrophobic sections T,
IV) at least two hydrophobic sections D different from T,
where
a) to each section T is directly attached a section S,
b) to each section S on at least one side is attached at least one section
D,
c) to each section P are attached at least two sections D,
where the preparation takes place in the presence of at least one carboxylic
acid salt of
at least one metal selected from the group consisting of the alkali metals,
the alkaline
earth metals, zinc and mixtures thereof, wherein the method is single-stage.
By means of the process according to the invention, polyurethanes are also
obtained
which comprise
l) at least two hydrophilic sections S,
II) no hydrophilic section P different from S,
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
4
III) at least two terminal hydrophobic sections T,
IV) a hydrophobic section D different from T,
where
a) to each section T is directly attached a section S and
b) to each section S is attached a section D.
The polyurethanes obtainable by the process according to the invention are
preferably
dispersible in water. According to the invention, this comprises that they can
also be
emulsified in water or are completely or partially soluble in water.
The polyurethanes obtainable by the process according to the invention (also
referred
to hereinbelow as "polyurethanes according to the invention") are preferably
at least
partially branched. "At least partially branched" means that at least some of
the
polymer molecules are not linear, but have branching points.
Such branches may be present both in the hydrophobic sections and also the
hydrophilic sections.
In one embodiment of the invention, at least some of the terminal hydrophobic
sections
In one embodiment of the invention, at least some of the hydrophobic sections
D are
branched.
using alkali(ne earth) metal or zinc carboxylates it is possible to generate
branches of
the polyisocyanates in the form of isocyanurate or allophanate structures in-
situ and it
is therefore not necessary to rely on polyisocyanates with already prepared
isocyanurate or allophanate structures as starting compounds. Firstly, the
starting
The backbone of the polyurethanes according to the invention is composed of
alternating hydrophobic and hydrophilic sections, where the hydrophobic and
hydrophilic sections alternate in the sequence, but may be different in terms
of their
size, length and nature. In the polyurethanes according to the invention, a
hydrophilic
,
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
case, "attached to a section" is understood as meaning that the connection
takes place
directly, i.e. that the two sections in question are directly adjacent in the
polymer
molecule.
5 Hydrophilic sections
"Hydrophilic" is the term used here to refer to those sections which exhibit
marked
interaction with water. In general, hydrophilic sections consist of radicals
of substances
which are themselves hydrophilic.
Typical hydrophilic groups known to the person skilled in the art are, for
example,
nonionic polyether radicals. Polyether radicals may be homo-alkylene oxide
radicals, or
mixtures of different alkylene oxide radicals. These different alkylene oxide
radicals
may be present in the polyether radicals in random distribution or be present
in block
form. Preferred polyether radicals are homo-ethylene oxide radicals.
Hereinbelow,
ethylene oxide is also referred to as EO, and propylene oxide is also referred
to as PO.
According to another embodiment, the polyether radicals comprise mixtures of
EO
radicals and PO radicals. These may be present in the polyether radicals in
random
distribution or be present in block form. In one preferred embodiment, the EO
and PO
radicals are present in block form.
A particularly preferred embodiment includes polyether radicals which have at
least
50% by weight of ethylene oxide radicals, for example polyether radicals which
have
more than 50% by weight of ethylene oxide radicals, and propylene oxide
radicals as
further alkylene oxide radicals. The polyether radicals very particularly
preferably
consist of ethylene oxide radicals.
,
The hydrophilicity of a substance can be determined, for example, by means of
an
opacity measurement of an aqueous solution.
Preferred hydrophilic sections are water-soluble. For the purposes of this
invention, a
substance is referred to as being soluble in a liquid phase if at least 1 g,
preferably at
least 10 g, of the substance dissolved at 20 C and a pressure of 1 bar to give
a
solution that looks clear to the human eye, i.e. without visible clouding in 1
liter of the
liquid phase. Water-soluble substances are therefore substances which are
soluble in
an amount of at least 1 g, preferably at least 10 g, at 20 C and a pressure of
1 bar to
give a solution that looks clear to the human eye, i.e. without visible
clouding, in 1 liter
of water, preferably demineralized water.
Hydrophobic sections
,
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
6
By means of the process according to the invention, polyurethane molecules are
obtained which comprise in each case at least two terminal hydrophobic
sections T and
at least two further hydrophobic sections D.
By means of the process according to the invention, polyurethane molecules are
also
obtained which comprise at least two terminal hydrophobic sections T and only
one
hydrophobic section D.
In general, the hydrophobic sections consist of radicals of substances which
are
immiscible with water or only poorly miscible with water and are preferably
lipophilic at
the same time, i.e. are readily soluble in nonpolar solvents such as, for
example, fats
and oils.
Typical hydrophobic sections T are, for example, hydrocarbon radicals, in
particular
long-chain hydrocarbon radicals.
In one embodiment of the invention, the hydrocarbon radicals are unbranched.
In
another embodiment of the invention, the hydrocarbon radicals are branched.
In a further embodiment of the invention, the polyurethanes according to the
invention
comprise both branched and unbranched hydrocarbon radicals.
Long-chain aliphatic alcohols, aromatic alcohols and aliphatic diisocyanates
are
examples of hydrophobic substances, the radicals of which may be present in
the
hydrophobic sections of the polyurethanes according to the invention.
Polyurethanes prepared by the process according to the invention comprise at
least
two terminal hydrophobic sections (T) which, independently of one another, may
be
identical or different.
In one preferred embodiment, at least some of the polyurethanes according to
the
invention comprise more than two terminal hydrophobic sections (T).
The terminal hydrophobic sections T can be branched or unbranched. Preferably,
at
least one of the two terminal hydrophobic sections T is branched.
In one preferred embodiment, the terminal hydrophobic sections T comprise at
least
one alkyl radical. In one particularly preferred embodiment, this alkyl
radical comprises
4 to 30 carbon atoms, particularly 6 to 26 and very particularly preferably 8
to 20
carbon atoms.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
7
In another embodiment, this alkyl radical comprises 4 to 30 carbon atoms,
particularly 8
to 30 and very particularly preferably 12 to 30 carbon atoms.
Preferably, the chain length of the main chain of the alkyl radicals which are
present in
the sections T is 4 to 30 carbon atoms. These are for example radicals of
linear or
branched alkanes such as, for example, butane, isobutane, pentane, isopentane,
neopentane, hexane, heptane, octane, 2-ethylhexane, nonane, decane, undecane,
dodecane, tridecane, isotridecane, tetradecane, pentadecane, hexadecane,
heptadecane, octadecane, nonadecane, icosane, henicosane, docosane, tricosane,
isotricosane, tetracosane, pentacosane, hexacosane, heptacosane, octacosane,
nonacosane, triacontane, 2-octyldodecane, 2-dodecylhexadecane, 2-
tetradecyloctadecane, 2-decyltetradecane, or monomethyl-branched
isooctadecane.
The hydrophobic sections T can likewise also comprise radicals of cycloalkanes
and
-alkenes, as described for example in EP 761780 A2, p. 4, II. 56-58, radicals
of alkenes
as described for example in EP 761780 A2, p. 4, II. 51-52, or alkylaryl
radicals as
described for example in EP 761780 A2, p. 4, II. 53-55.
The sections T particularly preferably comprise the above-described alkyl
radicals with
a number of carbon atoms in the range from 8 to 30, very particularly
preferably in the
range from 12 to 30 carbon atoms.
The sections T preferably consist of aliphatic radicals, but may also comprise
aromatic
radicals.
Preferably, at least one section T is a branched alkyl radical.
The side chains are preferably also alkyl radicals or alkylene radicals,
particularly
preferably alkyl radicals, in particular unbranched alkyl radicals.
In one embodiment, the side chains of the branched alkyl radicals have a chain
length
of at most 6, preferably of at most 4, carbon atoms.
In one embodiment, the branches are considerably shorter than the main chain.
In one
embodiment, each branch of the sections T of the polyurethanes according to
the
invention has a chain length which corresponds at most to half of the chain
length of
the main chain of this section T. In one embodiment, the branches are
considerably
shorter than the main chain. In one preferred embodiment, the branched alkyl
radicals
are iso- and/or neo-alkyl radicals. In one preferred embodiment, radicals of
isoalkanes
are used as branched alkyl radicals. Particular preference is given to a C13-
alkyl
radical, in particular an iso-C13-alkyl radical.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
8
In another embodiment, the sections T comprise branched alkyl radicals, the
side
chains of which have a chain length of at least 4, preferably of at least 6,
carbon atoms.
The sections T can be introduced into the polyurethanes according to the
invention in
various ways.
In one preferred embodiment, the sections T are introduced, simultaneously and
together with hydrophilic sections S, through the use of alkoxylated alcohols.
Suitable alcohols are, for example, the alkoxylated
- linear alcohols from natural sources or from the Ziegler build-up reaction
of ethylene
in the presence of aluminum alkyl catalysts. Examples of suitable linear
alcohols are
linear C6-C30-alcohols, in particular C12-C30-alcohols. Particularly preferred
alcohols
which may be mentioned are: n-dodecanol, n-tetradecanol, n- hexadecanol,
n-octadecanol, n-eicosanol, n-docosanol, n-tetracosanol, n-hexacosanol,
n-octacosanol and/or n-triacontanol, and also mixtures of the aforementioned
alcohols,
for example NAFOL grades such as NAFOL 22+ (Sasol).
- oxoalcohols such as, for example, isoheptanol, isooctanol, isononanol,
isodecanol,
isoundecanol, isotridecanol (for example Exxal grades 7, 8, 9, 10, 11, 13).
- alcohols which are branched in the 2 position; these are the Guerbet
alcohols known
to the person skilled in the art which are accessible by dimerization of
primary alcohols
via the so-called Guerbet reaction. Particularly preferred alcohols which may
be
mentioned here are: Isofole12 (Sasol), RilaniteG16 (Cognis).
- alcohols which are obtained by the Friedel-Crafts alkylation with
oligomerized olefins
and which then comprise an aromatic ring as well as a saturated hydrocarbon
radical.
Particularly preferred alcohols which may be mentioned here are:
isooctylphenol and
isononylphenol.
- alcohols of the general formula (4) in EP 761780 A2, p. 4
4 H 6
R¨C¨R OH
\ 5
or alcohols of the general formula (5) in EP 761780 A2, p. 4
7 H
R¨C¨OH
R8
, where
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
9
- R4, Rs, R7 and R6, independently of one another, have the meaning
described in
EP 761780 A2, p. 4, lines 45 to 58; preferably, R4, R6, R7 and R8,
independently of
one another, are alkyl radicals having at least 4 carbon atoms and the total
number
of the carbon atoms in the alcohols is at most 30,
- R6 is an alkylene radical such as, for example, -CH2-, -CH2-CH2-, -CH2-
CH(CH3)-.
By way of example, mention may be made here of 2-decyI-1-tetradecanol as
suitable
alcohol.
In one embodiment, mixtures of ethoxylated C16-C18-fatty alcohols are used in
order to
introduce sections T into the polyurethanes.
In one embodiment, a linear, nonionic compound of the structural formula
RO(CH2CH20)xH, where R is a linear C16-C18-alkyl radical, and where x = 3, 5,
7, 8, 11,
13, 18, 25 or 80, preferably x = 11, is used as at least one of the alcohols
used.
Commercially, such an ethoxylated, linear fatty alcohol is available, for
example, as
LutensoloAT11.
In one embodiment of the invention, mixtures of ethoxylated linear and
ethoxylated
branched long-chain alcohols, in particular mixtures of the aforementioned
types, are
used.
In a further embodiment, ethoxylated iso-C13-oxo alcohols or mixtures thereof
are used
in order to introduce sections T into the polyurethanes.
In one embodiment, a branched, nonionic compound of the structural formula
RO(CH2CH20)H, where R is a C13-alkyl radical, preferably an iso-Ci3-alkyl
radical, and
where x = 3, 5, 6, 6.5, 7, 8, 10, 12, 15 or 20, preferably x = 10, is used as
at least one
of the alcohols used. Commercially, such an ethoxylated, alkyl-branched
alcohol is
available, for example, as LutensoleT010.
In a further embodiment, mixtures comprising ethoxylated C16-Ci8-fatty
alcohols and
ethoxylated iso-C13-oxo alcohols are used in order to introduce sections T
into the
polyurethanes.
In a further embodiment, the above-described alcohols of the general formula
(4) or (5)
in EP 761780 A2, p. 4, are used in their ethoxylated form in order to
introduce sections
T into the polyurethanes.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
It is of course possible to additionally also introduce other sections T into
the
polyurethanes.
The hydrophobic sections T can of course also be introduced into the
polyurethanes
5 through any desired mixtures of the aforementioned ethoxylated alcohols.
By means of the process according to the invention, as is customary in the
case of
polymerization reactions, mixtures of different polymers are obtained, in the
present
case thus mixtures of different polyurethanes.
The term "polyurethane" used here can refer either to any individual
polyurethane
molecule or to the totality of the polyurethane molecules obtainable by the
process
according to the invention.
The polyurethanes obtainable according to the invention are preferably
mixtures which
comprise the described polyurethane structures.
Accordingly, the preparation of mixtures of polyurethanes, the terminal
hydrophobic
sections T of which are branched and/or unbranched alkyl radicals is also in
accordance with the invention. The preparation of mixtures which comprise
polyurethanes which comprise both branched and unbranched terminal,
hydrophobic
sections T is also in accordance with the invention.
Preferably, at least some of the polyurethane molecules obtainable by the
process
according to the invention comprise allophanate segments.
The invention thus also provides a process according to the invention where at
least
some of the resulting polyurethanes comprise allophanate segments.
Preferably, at least some of the polyurethane molecules obtainable by the
process
according to the invention comprise isocyanurate segments.
The invention thus also provides a process according to the invention where at
least
some of the polyurethanes comprise isocyanurate segments.
Hydrophilic sections S
In the polyurethanes obtainable by the process according to the invention, to
the
terminal hydrophobic sections T are attached hydrophilic sections S.
In the polyurethanes, the sections S, independently of one another, may be
identical or
different.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
11
The sections S preferably comprise radicals of alkylene oxides. Preferably,
the number
is in the range from 2 to 150 alkylene oxide radicals, particularly preferably
in the range
from 2 to 100 alkylene oxide radicals and very particularly preferably in the
range from
2 to 50 alkylene oxide radicals.
The hydrophilic sections S comprise or consist preferably of ethylene oxide
radicals. In
one preferred embodiment, the hydrophilic sections S comprise ethylene oxide
radicals
(EO units), the number of which is in the range from 2 to 150 EO units,
particularly
preferably in the range from 2 to 100 EO units and very particularly
preferably in the
range from 2 to 50 EO units.
In one preferred embodiment, the sections S consist of 2 to 50, preferably 2
to 25 EO
units.
In another embodiment, the sections S consist of 25 to 100, preferably 40 to
100 EO
units.
The number of EO units per molecule of ethoxylated alcohol is also referred to
as
degree of ethoxylation.
The sections S can likewise comprise longer-chain alkylene oxide radicals,
with the
proviso that the sections S must be hydrophilic overall. The hydrophilicity
can be
controlled for example via the fraction of EO units in the sections S.
Hydrophobic sections D
To each hydrophilic section S is attached at least one hydrophobic section D.
Here, a
section S may also be present in the interior of the molecule of the
polyurethanes
according to the invention. In this case, this section S is connected not like
an edge-
position section S directly to a section D and a section T, but on at least
two sides to
sections D. Preferably, a section S is connected in the interior of the
molecule on both
sides to one section D in each case.
For all edge-position sections S, it is the case that they are directly
connected to an
end-position section T.
Should a section S be branched to a low extent, then it can be directly
connected at
two or more positions to hydrophobic sections D. Preferably, to each
hydrophilic
section S is connected a hydrophobic section D on at least one side.
In a particularly preferred embodiment, the sections S are unbranched and edge-
positioned and connected directly to a terminal hydrophobic section T on one
side and
to a hydrophobic section D on the other side.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
12
By means of the process according to the invention, polyurethane molecules are
obtained which comprise at least two hydrophobic sections D. In addition,
however,
polyurethane molecules are also obtained which comprise only one hydrophobic
section D.
In the polyurethane molecules with at least two hydrophobic sections D, these
may be
identical or, independently of one another, different.
The sections D can be branched with short-chain hydrophobic branches or be
unbranched. Preferably, at least some of the sections D are branched.
Preferably, the sections D comprise at least one hydrophobic chain of carbon
atoms,
the length of which is in the range from 2 to 20 carbon atoms, preferably 3 to
16 carbon
atoms and in particular in the range from 4 to 12 carbon atoms.
Preferably, the sections D comprise diisocyanate radicals. The sections D
particularly
preferably comprise radicals of aliphatic diisocyanates. Thus, for example, a
hydrophobic section D can consist of one or more aliphatic diisocyanate
radicals.
Preferably, a section D consists of one to ten aliphatic diisocyanate
radicals,
particularly preferably of one to five aliphatic diisocyanate radicals; very
particularly
preferably, it comprises one, two or three aliphatic diisocyanate radicals.
The hydrophobic sections D can comprise aliphatic diisocyanate radicals with
long,
mid-length or short aliphatic units.
In one of the preferred embodiments, the sections D of the polyurethanes
prepared by
the process according to the invention are cycloaliphatic or aliphatic
diisocyanate
radicals.
Aliphatic diisocyanate radicals are particularly preferred as sections D.
Aliphatic diisocyanates which may be mentioned by way of example are: 1,4-
butylene
diisocyanate, 1,12-dodecamethylene diisocyanate, 1,10-decamethylene
diisocyanate,
2-butyl-2-ethylpentamethylene diisocyanate, 2,4,4- or 2,2,4-
trimethylhexamethylene
diisocyanate and in particular hexamethylene diisocyanate (HD1).
By way of example, cycloaliphatic diisocyanates which may be mentioned are:
isophorone diisocyanate (IPD1), 2-isocyanatopropylcyclohexyl isocyanate,
4-methylcyclohexane 1,3-diisocyanate (H-TDI) and 1,3-
bis(isocyanatomethyl)cyclohexane. So-called H2-MDI or diisocyanates termed
"saturated MDI", such as, for example, 4,4'-methylenebis(cyclohexyl
isocyanate)
(alternatively also called dicyclohexylmethane 4,4'-diisocyanate) or 2,4'-
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
13
methylenebis(cyclohexyl) diisocyanate can also be present as radicals in
sections D of
the polyurethanes PU according to the invention.
It is of course possible, in the process according to the invention, to use
mixtures of the
abovementioned diisocyanates in order to prepare mixtures of different
polyurethanes.
In one preferred embodiment, some of the polyurethanes obtainable according to
the
invention comprise hydrophobic sections D with allophanate structures.
Allophanate
structures are formed as a result of the addition of an isocyanate group onto
a urethane
unit.
In one preferred embodiment, polyurethanes prepared by the process according
to the
invention comprise hydrophobic sections D with isocyanurate structures.
lsocyanurate
structures are formed by the addition of 3 isocyanate groups (trimerization).
In a further preferred embodiment, as a result of the process according to the
invention,
polyurethanes are obtained which comprise both hydrophobic sections D with
allophanate structures and also hydrophobic sections D with isocyanurate
structures.
In another embodiment, some of the polyurethanes prepared by the process
according
to the invention comprise hydrophobic sections D with biuret structures.
Biuret
structures are formed as a result of the addition of an isocyanate group onto
a urea
unit. Urea units in turn are formed as a result of the addition of primary
amines onto
isocyanate groups.
Hydrophilic sections P
As a result of the process according to the invention, polyurethane molecules
are
obtained which comprise at least one hydrophilic section P different from the
hydrophilic sections S. To a section P are directly attached at least two
hydrophobic
sections D. The sections P of the polyurethanes according to the invention
can,
independently of one another, be identical or different.
By the process according to the invention, polyurethanes are also additionally
obtained
which comprise no hydrophilic section P.
If more than one section P is present in a polyurethane according to the
invention, then
there is at least one hydrophobic section D between every two hydrophilic
sections P.
If more than one section P is present in a polyurethane according to the
invention, then
these may be identical or different.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
14
In one embodiment, polyurethanes obtainable according to the invention can
comprise
a sequence of sections in the order hydrophobic section D, then hydrophilic
section S,
then hydrophobic section D again between two hydrophilic sections P. Thus, if
in a
polyurethane according to the invention, more than one section P is present,
then in
such a case, the sections in the interior of the molecule can have a sequence
of P-D-P
or of P-D-S-D-P. Should more than two sections P be present, then both
sequences in
one molecule are possible.
Preferably, only one or two sections P are present in a molecule of the
polyurethanes
obtainable according to the invention.
The hydrophilic sections P are preferably introduced into the polyurethanes
through the
use of hydrophilic polyols. Per molecule, these comprise at least two OH
groups and at
least two functional groups which are selected from the functions -0- (ether
groups)
and -000- (ester groups), where the molecular weight of these hydrophilic
compounds
is at least 300 and preferably at least 1200.
One embodiment of the invention is a process according to the invention,
wherein the
at least one hydrophilic section P has a number-average molecular weight Mn of
from
1500 to 20 000 g/mol, preferably from 4000 to 12 000 g/mol.
Suitable hydrophilic polyols are, for example, the polymerization products of
ethylene
oxide, the copolymerization or graft polymerization products thereof, and the
polyethers
obtained by condensation of polyhydric alcohols or mixture thereof and the
polyethers
obtained by ethoxylation of polyhydric alcohols, amides, polyamides and amino
alcohols. Examples thereof are, for example, polyethylene glycols, addition
products of
ethylene oxide onto trimethylolpropane, EO-PO block copolymers, OH-terminated
polyesters, such as, for example, those of the multifunctional
polycaprolactone type.
Preferred hydrophilic polyols are polyetherpolyols. These are those
hydrophilic polyols
which comprise at least two OH groups and at least two -0- functions (ether
groups)
per molecule. These polyetherpolyols are generally so hydrophilic that they
are water-
soluble at room temperature (20 C).
Of suitability for preparing the polyurethanes by the process according to the
invention
are preferably those polyetherpolyols which comprise predominantly
polyethylene
glycol. It is particularly preferred if these polyethylene glycols have an
average amount
of EO units in the range from 30 to 450 per molecule.
Preference is given to polyols of the general formula HO-(CH2-CH2-0)n-H, where
n can
assume the values 30 to 450. These are polyethylene glycols, which are
condensation
products of ethylene oxide with ethylene glycol or water.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
Preferably, the molecular weight of these polyethylene glycols is adjusted to
values in
the range from 1500 to 20 000 g/mol, preferably from 4000 to 12 000 g/mol.
However, it is also possible to use EO-PO block copolymers in order to
incorporate the
5 sections P into the polyurethanes obtainable according to the invention.
For example, it
is possible to use EO-PO block copolymers of the general formula
H0-(E0)m-(PO)n-(E0)0-H, where m and o, independently of one another, are
integers
in the range from 10 to 100, preferably from 20 to 80, n is an integer in the
range from
5 to 50, preferably from 20 to 40, and where m, n and o are selected such that
HO-
10 (E0)m(PO)n(E0)0H is water-soluble.
According to the invention, the essentially linear polyether radicals which
form the
sections P preferably have a number-average molecular weight Mn of at least
1500 g/mol and at most 20 000 g/mol.
In one embodiment, these polyether radicals have number-average molecular
weights
Mr, in the range from 1500 g/mol to 15 000 g/mol.
In a further preferred embodiment, these polyether radicals have number-
average
molecular weights Mn in the range from 4000 g/mol to 12 000 g/mol.
In a particularly preferred embodiment, these polyether radicals have number-
average
molecular weights Mn in the range from 6000 g/mol to 12 000 g/mol.
The molecular weight Mn of the sections P is particularly preferably less than
or equal
to 10 000 g/mol and especially preferably in the range from 6000 g/mol to
10 000 g/mol.
In a particularly preferred embodiment, the linear polyether radicals have a
number-
average molecular weight Mn of about 10 000 g/mol.
In a further particularly preferred embodiment, the linear polyether radicals
have a
number-average molecular weight Mn of about 6000 g/mol.
In a further particularly preferred embodiment, the linear polyether radicals
have a
number-average molecular weight Mn of about 9000 g/mol.
All of the hydrophilic sections S and P of the polyurethanes obtainable
according to the
invention may be polyether radicals.
In one preferred embodiment, the hydrophilic sections of the polyurethanes
according
to the invention consist of
,
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
_
16
- polyalkylene oxide units (sections P) and
- polyethylene oxide units (sections S).
In one particularly preferred embodiment of the polyurethanes obtainable by
the
process according to the invention, all of the sections P and S consist of EO
units.
As a result of the process according to the invention, polyurethane molecules
are
obtained which comprise at least three hydrophilic sections. In one preferred
embodiment, these are two sections S and at least one section P.
Additionally, however, polyurethane molecules with only two hydrophilic
sections S and
without hydrophilic section P are also obtained.
As a result of the process according to the invention, polyurethanes are also
preferably
obtained, according to the invention, which comprise
l) at least two hydrophilic sections S,
II) no hydrophilic section P,
III) at least two terminal hydrophobic sections T,
IV) at least one hydrophobic section D different from T,
where
a) to each section T is directly attached a section S,
b) to each section S is attached a section D.
The polyurethanes prepared by the process according to the invention which
additionally comprise allophanate structures preferably comprise at least
three sections
S and preferably also at least one section P.
The polyurethanes prepared by the process according to the invention which
additionally comprise isocyanurate structures preferably comprise at least
three
sections S and preferably also at least one section P.
At least some of the polyurethanes prepared by the process according to the
invention
are linear and have the following sequence of sections: T-S-D-P-D-S-T or T-S-D-
P-D-
P-D-S-T or T-S-D-S-T.
In one embodiment of the invention, at least some of the polyurethanes
prepared by
the process according to the invention comprise allophanate and/or
isocyanurate
structures and have the following sequence of sections:
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
17
T¨S¨ID¨P¨D¨S¨T
SI
In one embodiment of the invention, at least some of the polyurethanes
prepared by
the process according to the invention comprise allophanate and/or
isocyanurate
structures and have the following sequence of sections:
T¨S¨D¨ S¨T
For example, polyurethanes prepared by the process according to the invention
which
additionally comprise allophanate structures have the following structure:
0 0
N
0 N 0 ocnH2n
0 N
Y .1
o
OC H
n 2n41
o
For example, polyurethanes prepared by the process according to the invention
which
additionally comprise isocyanurate structures have the following structure:
H -
ocoI
NO
.z CCAno
0
0 0
CNIANNy 0'Lc
0
0 z
-r
0 N 0
.z OCIAno
0
For each section P, it is the case that its molecular weight Mr, is greater
than that of
each section S present in the same molecule.
The ratio of the molecular weights Mn of each hydrophilic section S of the
polyurethanes according to the invention to the molecular weight Mn of each
hydrophilic
section P is in the range from 1:1.4 to 1:140, preferably in the range from
1:1.7 to
1:120.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
18
In one preferred embodiment, the ratio is 1:x, where x is equal to or greater
than 2,
preferably equal to or greater than 2.3 and particularly preferably x is equal
to or
greater than 2.8.
The ratio is particularly preferably in the range from 1:2.8 to 1:115, very
particularly
preferably in the range from 1:3 to 1:110 and especially preferably in the
range from
1:3.4 to 1:105.
In the process according to the invention, the ratio (mol to mol) of the
polyols used to
diisocyanates used can be in the range from 1:1.1 to 1:1.9. Preferably, the
ratio is in
the range from 1:1.1 to 1:1.8. The ratio is particularly preferably in the
range from 1:1.1
to 1:1.75. The ratio is especially preferably in the range from 1:1.2 to
1:1.75. The ratio
can of course also be 1:x where x is greater than or equal to 1.3, preferably
x is greater
than or equal to 1.5.
In one embodiment, this results in no, one or two sections P preferably being
present in
one molecule of the polyurethanes according to the invention.
In one embodiment, as a result of the process according to the invention, a
mixture is
obtained comprising polyurethanes without sections P, polyurethanes with one
section
P and polyurethanes with two sections P.
In one embodiment of the process according to the invention, in addition to
the stated
ranges of the ratio of polyetherdiols to diisocyanates, the ratio of
polyetherdiols to
ethoxylated alkanols is additionally chosen such that the molar quantitative
ratio of
polyetherdiols used to ethoxylated alkanols used is in the range from 5:1 to
1:2.
Preferably, this ratio is in the range from 2:1 to 1:1.8, preferably in the
range from 1:1 to
1:1.6 and most preferably about 1:1.5.
For a particularly preferred process according to the invention, it is the
case that a
molar quantitative ratio of polyetherdiols to diisocyanates to ethoxylated
alkanols
1:1.75:1.5 is used.
As a result of the process according to the invention, mixtures of different
polyurethanes are generally obtained. Such a mixture can comprise e.g.
polyurethanes
which have the same sequence of the sections T, S, D and/or P, but differ from
one
another structurally in at least one of the sections. One example of this
which may be
mentioned is a different section structure or a different section chain
length. Thus, the
sections T in a mixture of the polyurethanes prepared according to the
invention can be
different. For example, a mixture according to the invention can comprise
polyurethanes, the sections T of which are all branched, and/or those, the
sections T of
,
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
19
which are all linear, and/or those polyurethanes which comprise both at least
one linear
section T and also at least one branched section T.
In one embodiment, the sum of the molecular weights of all sections T plus the
molecular weights of sections D should be kept less than or equal to the sum
of the
molecular weights of all of the sections P.
Catalyst
To prepare the polyurethanes by the process according to the invention, the
catalysts
used are carboxylic acid salts of alkali metals, carboxylic acid salts of
alkaline earth
metals, carboxylic acid salts of zinc or mixtures thereof.
The carboxylic acids, the alkali(ne earth) metal salts or zinc salts thereof
are used as
catalysts in the process according to the invention are preferably
monocarboxylic acids
of the general formula R-COOH, where R can be any desired organic, for example
an
aliphatic, an aromatic or a heterocyclic radical.
Preferably, R is an aliphatic radical, thus for example an alkyl radical, an
alkenyl radical
or an alkynyl radical. R can also comprise heteroatoms; for example, the
carboxylic
acid may be a hydroxycarboxylic acid.
In one embodiment of the invention, R is a hydrocarbon radical having 1 to 20,
preferably having 1 to 12, carbon atoms. R may be linear or branched,
saturated or
unsaturated.
In one embodiment of the invention, the carboxylic acid is acetic acid.
In a further embodiment of the invention, the carboxylic acid is octanoic
acid.
In a further embodiment of the invention, the carboxylic acid is 2-
ethylhexanoic acid.
In a further embodiment of the invention, the carboxylic acid is neodecanoic
acid.
In a further embodiment of the invention, the carboxylic acid is n-decanoic
acid.
In a further embodiment of the invention, the carboxylic acid is stearic acid.
In a further embodiment of the invention, the carboxylic acid is ricinoleic
acid
((9Z,12R)-12-hydroxy-9-octadecenoic acid).
In a further embodiment of the invention, the carboxylic acid is a
hydroxycarboxylic
acid, such as, for example, citric acid or lactic acid, in particular lactic
acid.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
,
If at least one carboxylic acid salt of an alkali metal is selected as
catalyst, then the
alkali metal is preferably selected from sodium and potassium, particularly
preferably
potassium.
5 If at least one carboxylic acid salt of an alkaline earth metal is
selected as catalyst, then
the alkaline earth metal is preferably selected from calcium and magnesium,
particularly preferably calcium.
A preferred embodiment of the invention is the process according to the
invention
10 wherein the preparation of the polyurethanes takes place in the presence
of potassium
carboxylate, preferably potassium acetate.
Another preferred embodiment of the invention is the process according to the
invention wherein the preparation of the polyurethanes takes place in the
presence of
15 potassium lactate.
A preferred embodiment of the invention is the process according to the
invention
wherein the preparation of the polyurethanes takes place in the presence of
zinc
carboxylate, preferably zinc neodecanoate.
It is of course also possible to use mixtures of two or more carboxylic acid
salts of
alkali(ne earth) metals or of zinc as catalysts for preparing polyurethanes PU
according
to the invention.
25 In one preferred embodiment, a mixture comprising at least one potassium
carboxylate
and at least one zinc carboxylate is used. In a further preferred embodiment,
a mixture
comprising potassium acetate and zinc neodecanoate is used. In another
preferred
embodiment, a mixture comprising potassium lactate and zinc neodecanoate is
used.
30 In addition to these catalysts, further catalysts known to the person
skilled in the art in
the field of polyurethane preparation can be used.
Such catalysts usually used in polyurethane chemistry are organic amines, in
particular
tertiary aliphatic, cycloaliphatic or aromatic amines, and Lewis-acidic
organic metal
35 compounds.
Suitable Lewis-acidic organic metal compounds are e.g. metal complexes such as
acetylacetonates of iron, titanium, zinc, aluminum, cobalt, manganese, nickel
and
zirconium, such as e.g. zirconium 2,2,6,6-tetramethy1-3,5-heptanedionate.
Further
40 suitable metal compounds are described by Blank et al. in Progress in
Organic
Coatings, 1999, 35, 19 ff.
CA 02815268 2013-04-19
WO 2012/052383 PCT/E
P2011/068072
21
Bismuth, cobalt or zinc catalysts and also cesium or titanium salts can also
be used as
catalysts.
In one embodiment of the invention, the amount of such further catalysts which
are not
carboxylic acid salts of alkali metals, carboxylic acid salts of alkaline
earth metals,
carboxylic acid salts of zinc or mixtures thereof, is at most 10% by weight,
preferably at
most 5% by weight, particularly preferably at most 1% by weight and in
particular at
most 0.1% by weight, of the total amount of catalyst.
One embodiment of the invention is the process according to the invention
wherein the
preparation of the polyurethanes takes place in the presence of less than 10
ppm of tin,
based on the reaction mixture.
In one embodiment of the invention, apart from the at least one carboxylic
acid salt of a
metal selected from alkali metals, alkaline earth metals, zinc and mixtures
thereof, no
further catalysts are used for preparing the polyurethanes.
The catalyst or the mixture of catalysts is preferably used in an amount in
the range
from 50 ppm to 5000 ppm, based on the total weight of all reacting compounds.
Preferably, the catalyst is used in an amount in the range from 50 to 2500
ppm,
particularly preferably in an amount in the range from 100 to 1000 ppm, based
on the
total weight of all reacting compounds.
The catalyst can be added to the reaction mixture in solid or liquid form,
depending on
the nature of the catalyst. Suitable solvents are non-aqueous solvents such
as, for
example, aromatic or aliphatic hydrocarbons, inter alia toluene, xylene, ethyl
acetate,
hexane and cyclohexane, and also carboxylic acid esters, such as, for example,
ethyl
acetate. Further suitable solvents are acetone, THF, DMSO, DMF, DMAc and
N-methylpyrrolidone and N-ethylpyrrolidone.
The catalyst/catalyst mixture is preferably used in dissolved form,
particularly
preferably dissolved in the polyetherdiols with which the hydrophilic sections
P are
introduced into the polyurethanes.
The catalyst may already be present, at least partially, in the
polyetherpolyols used for
the process according to the invention if, during the preparation thereof,
carboxylic acid
salts of alkali(ne earth) metals have been used or have been formed.
In one embodiment of the invention, the polyetherpolyols used in the process
according
to the invention thus comprise at least some of the catalyst, if appropriate
the total
required amount of catalyst.
-
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
,
22
In one embodiment of the invention, the polyetherpolyols used in the process
according
to the invention prior to the start of the process already comprise at least
some of the
required catalyst and the remainder is added to carry out the process.
5 In the process according to the invention for preparing the
polyurethanes, preference is
given to using the following starting materials:
A) Compounds which introduce the hydrophilic sections P into the
polyurethanes:
preferably polyols of the general formula HO-(Cl2-CH2-0)n-H, where n
10 preferably assumes the values 30 to 450; these are polyethylene
glycols which
are condensation products of ethylene oxide with ethylene glycol or water.
Preferred polyethylene glycols have a number-average molecular weight in the
range from 6000 to 12 000 g/mol and particularly preferred ones have a
number-average molecular weight of from 6000 to 10 000 g/mol.
6) Compounds which introduce the terminal hydrophobic sections T and the
hydrophilic sections S adjacent in each case to the sections T: preferably
ethoxylated Ci6-C18-fatty alcohols, ethoxylated iso-C13-oxo alcohols,
ethoxylated
branched alcohols as in Production Examples 1 to 24 of EP 761780 A2 and
20 mixtures thereof.
C) Compounds which introduce the hydrophobic sections D: aliphatic
diisocyanates, in particular hexamethylene diisocyanate (HDI).
25 D) Catalysts: potassium carboxylate and/or zinc carboxylate.
The invention provides a process according to the invention, wherein, to
prepare the
polyurethanes, in each case at least one C4-C30-alcohol ethoxylated with 2 to
100 mol
30 of ethylene oxide per mole, one polyetherdiol with a molecular weight Mn
in the range
from 4000 to 12 000 g/mol and one diisocyanate are used.
The process according to the invention for the preparation of the
polyurethanes
comprises, in one embodiment, the following steps:
l) preparation of a mixture comprising
a. at least one polyetherdiol with a molecular weight Mn
in the range from 1500
to 12 000 g/mol,
40 b. at least one, optionally alkyl-branched C8rC30-, preferably C12-
C30-alkanol
which has been ethoxylated with 2 to 150 mol, preferably with 2 to 100 mol,
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
23
further preferably with 2 to 50 mol, in particular with 2 to 25 mol, of
ethylene
oxide per mol of alkanol,
c. at least one carboxylic acid salt of at least one metal selected
from alkali
metals, alkaline earth metals or zinc, preferably potassium carboxylate or
zinc
carboxylate,
II) optionally heating the mixture from step l) to 60 to 120 C, preferably
to 80 to
100 C;
III) if appropriate, reducing the water content of the mixture, based on the
total weight
of the mixture, to at most 1000 ppm, preferably at most 300 ppm,
IV) addition of at least one diisocyanate, preferably hexamethylene
diisocyanate, to
the mixture;
V) leaving the resulting reaction mixture to react until the isocyanate
content is at
most 0.1% by weight, based on the total weight of the reaction mixture.
Reaction mixture is understood as meaning the totality of all substances which
are
present in the reaction space after the point at which the total amount of
catalysts,
isocyanates, substances reactive towards isocyanates and all further
substances, such
as for example solvents, have been completely supplied to the reaction space.
Solvents
Solvents is understood as meaning phases that are liquid at 20 C and a
pressure of
1 bar in which one or more of the starting materials for the polyurethanes,
i.e. the
substances which introduce the hydrophilic and/or hydrophobic sections into
the
polyurethanes or which act as catalyst, are soluble at 20 C and 1 bar.
A preferred embodiment of the invention is a process according to the
invention
wherein the amount of solvents, based on the reaction mixture, which are
different from
the substances which introduce the hydrophilic and hydrophobic sections into
the
polyurethanes or which act as catalyst is in the range from 0 to 10% by
weight, further
preferably from 0 to 5% by weight and in particular 0 to 1% by weight.
In one embodiment of the invention, the process according to the invention is
carried
out essentially in the absence of solvents which are different from the
substances
which introduce the hydrophilic and/or hydrophobic sections into the
polyurethanes or
which act as catalyst.
CA 02815268 2013-04-19
WO 2012/052383 PCT/E
P2011/068072
24
In one embodiment of the invention, the amount of substances which, following
the
complete addition of all substances during the reaction, are present in the
reaction
space and are neither catalysts, nor substances which are incorporated into
the
polyurethanes which form and consequently introduce the hydrophilic and
hydrophobic
sections into the polyurethanes, nor reaction products, is at most 10% by
weight,
preferably at most 5% by weight, further preferably at most 1% by weight and
particularly preferably at most 0.1% by weight, of the total amount of all of
the
substances present in the reaction space during the reaction.
In one preferred embodiment, apart from the substances which introduce the
hydrophilic and/or hydrophobic sections into the polyurethanes, the reaction
products, if
appropriate small amounts of water and catalyst, the reaction mixture
comprises no
further substances.
The preferred process in which, apart from the starting materials for the
polyurethane
formation, no further solvents are used, leads to a more rapid reaction and
solvents do
not have to be separated off.
A further advantage of dispensing with solvents, in particular organic
solvents which
are different from the substances which introduce the hydrophilic and/or
hydrophobic
sections into the polyurethanes, is the better acceptance of the resulting
polyurethanes
as ingredients of cosmetic preparations.
If solvents are nevertheless used in the process according to the invention
which are
different from the starting materials for the polyurethane formation, then
these are
removed as far as possible after the reaction. "After the reaction" means the
point at
which the content of isocyanate groups is at most still 0.1% by weight, based
on the
total weight of the reaction mixture.
Single-stage process
The process according to the invention is single-stage. "Single-stage" means
that the
different substances with groups reactive towards isocyanate groups, thus for
example
the polyols and the ethoxylated alkanols, are essentially not brought into
contact
successively and separately, but simultaneously and together with the
substances
carrying isocyanate groups, for example the polyisocyanates.
One characteristic of the single-stage process is that all types of substances
to be
polymerized with groups reactive towards isocyanate groups are present
alongside one
another in the mixture at every point in the reaction.
However, it is not obligatory that the quantitative ratio of the different
substances in this
mixture is constant. Thus, for example, at certain points in the reaction, the
amount of
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
substances with two or more groups reactive towards isocyanate groups, based
on the
total amount of these substances to be incorporated by polymerization, may be
greater
than the amount of substances with one group reactive towards isocyanate
groups that
are to be polymerized, based on the total amount thereof. At other points, the
5 quantitative ratios can be different therefrom.
One embodiment of the invention is the process according to the invention
comprising
the successive steps
10 1) preparation of a mixture comprising in each case at least some of the
total amount of
all of the substances with groups reactive towards isocyanate groups that are
to be
polymerized,
2) addition of at least some of the substances carrying isocyanate groups that
are to be
15 polymerized to the mixture prepared in step 1),
3) addition of those amounts of the substances to be polymerized which have
not
already been added in steps 1) and 2).
20 In the case of a two-stage reaction, firstly only the substances with
two or more groups
reactive towards isocyanate, i.e. for example the polyols, are brought into
contact with
an excess of polyisocyanate, giving prepolymers of the structure -D-P-D- and
¨D-P-D-
P-D- with isocyanate chain ends, which are reacted in the second step with the
substances with only one group reactive towards isocyanate groups, i.e. for
example
25 the ethoxylated monohydric alcohols, then giving the structures T-S-D-P-
D-S-T or
T-S-D-P-D-P-D-S-T. In the two-stage process, in the first stage, the majority
of the
substances with two or more groups reactive towards isocyanate is brought into
contact with the substances carrying isocyanate groups, and the substances
with only
one group reactive towards isocyanate groups react in the second stage with
the
remaining isocyanate groups and form the chain ends of the polyurethanes.
In the case of the single-stage reaction, already from the start, in addition
to the
substances with two or more groups reactive towards isocyanate, for example
the
polyols, also at least some of the substances with only one group reactive
towards
isocyanate groups, for example the ethoxylated alkanols, are reacted with the
substances carrying isocyanate groups. Consequently, in the one-stage
reaction, from
the start, as well as reaction products with the section sequences T-S-D-P-D-S-
T and
T-S-D-P-D-P-D-S-T, also those with the section sequence T-S-D-S-T are formed.
However, for the single-stage process, it is not obligatory that the entire
amounts of all
of the substances with groups reactive towards isocyanate groups that are to
be
polymerized are already provided at the start of the reaction. It is also
possible that, at
CA 02815268 2013-04-19
WO 2012/052383 PCT/E
P2011/068072
26
the start of the reaction, only in each case some of each substance with
groups
reactive towards isocyanate groups are provided in the reaction space and the
remainder is added during the reaction.
In the single-stage process, the amount of polymers with the structure T-S-D-S-
T is
larger than in the two-stage process, whereas in the two-stage process the
amount of
polymers with the structure T-S-D-P-D-S-T is greater.
In one preferred embodiment of the invention, the total amounts of all of the
substances with groups reactive towards isocyanate that are to be polymerized
are
provided as a mixture and the substances carrying isocyanate groups are added
to this
mixture. Preferably, thus, for example the total amounts of polyols and
alkoxylated
alkanols are introduced as initial charge in the form of a mixture and the
isocyanates
are added to this mixture.
The mixture comprising the substances with groups reactive towards isocyanate
that
are to be polymerized preferably also comprises the catalyst or the catalysts
and if
appropriate solvents.
A preferred embodiment of the invention is the process according to the
invention
comprising the successive steps
1) preparation of a mixture comprising the total amount of all of the
substances with
groups reactive towards isocyanate groups that are to be polymerized,
2) addition of the substances carrying isocyanate groups that are to be
polymerized to
the mixture prepared in step 1).
Such a single-stage process is also described for example in example 10 of
EP 761780 A2 on p. 14, lines 29 to 47 and the following examples 11 to 24.
In a further embodiment of the invention, the total amount of these substances
with two
or more groups reactive towards isocyanate that are to be incorporated by
polymerization and some of the substances with one group reactive towards
isocyanate are introduced as initial charge in the form of a mixture and the
total amount
of the substances carrying isocyanate groups is added to this mixture. The
remaining
part amount of the substances with one group reactive towards isocyanate that
are to
be incorporated by polymerization is likewise added to the mixture following
the
addition of the substances carrying isocyanate groups.
In a further embodiment of the invention, in each case only some of the
substances
with two or more groups reactive towards isocyanate and some of the substances
with
W020121052383 CA 02815268 2013-04-19
PCT/EP2011/068072
27
one group reactive towards isocyanate are introduced as initial charge in the
form of a
mixture and the substances carrying isocyanate groups are added to this
mixture. The
remaining part amounts of the substances with two or more groups or one group
reactive towards isocyanate that are to be incorporated by polymerization are
likewise
added to the mixture following the addition of the substances carrying
isocyanate
groups.
In one embodiment of the invention, the molar ratio of the groups reactive
towards
isocyanate: isocyanate groups following the addition of the total amount of
substances
carrying isocyanate groups is in the range from 0.7:1 to 1.3:1, preferably
from 0.8:1 to
1.2:1, particularly preferably in the range from 0.9:1 to 1.1:1.
In one preferred embodiment, the reaction products prepared by the process
according
to the invention are transferred to an aqueous phase. It is advantageous to
separate off
any solvents present prior to this transfer to the aqueous phase.
It is also advantageous to stabilize the aqueous mixture obtained after
transferring the
reaction products to water, for example by adding stabilizers (free-radical
scavengers)
and preservatives.
Suitable free-radical scavengers are for example hydroxy-TEMPO, 2,6-di-tert-
butyl-p-
kresol (Kerobit*TBK), hydroquinone monomethyl ether, tocopherol and mixtures
of
these compounds. These stabilizers are preferably added in an amount of from 5
to
500 ppm, based on the weight of the aqueous mixture.
It is also advantageous to use preservatives. Of suitability are, for example,
phenoxyethanol, methylisothiazolinone, ethylhexylglycerol, 3-acety1-6-methy1-
2H-pyran-
2,4(3H)-dione, benzoic acid, parabens and mixtures of these substances. The
preservatives are preferably added in amounts of from 0.1 to 1% by weight,
based on
the aqueous mixture.
Preferably, the substances with groups reactive towards isocyanate groups that
are
used in the process according to the invention are essentially water-free in
order to
prevent a competing reaction of the isocyanate groups with water.
The water can advantageously be separated off from these substances by
azeotropic
distillation, drying in vacuo or other methods known to the person skilled in
the art. For
example, the water is removed up to a water content of the substances of at
most 500
ppm, preferably at most 300 ppm, based on the total weight of the substances.
The preparation of the actual reaction can for example involve exposing the
substances with groups reactive towards isocyanate groups to reduced pressure,
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
28
preferably vacuum, and if appropriate elevated temperature, and thus largely
removing
the water.
The water-comprising substances can also be mixed with a solvent such as
xylene,
toluene or acetone and the water can be removed together with the added
solvent by
azeotropic distillation.
The invention further provides a process according to the invention wherein
the
substances with groups reactive towards isocyanate groups comprise at least in
each
case one alkoxylated C4-C30-alcohol, preferably a branched C12-C30-alkanol,
and at
least in each case one polyetherdiol preferably a polyetherdiol with a
molecular weight
Mn in the range from 4000 to 12 000 g/mol, and the substances carrying
isocyanate
groups comprise at least one diisocyanate, preferably hexamethylene
diisocyanate.
The invention also provides the use of the polyurethanes according to the
invention for
preparing aqueous preparations. Preference is given here to preparations which
comprise at least 5% by weight, in particular at least 20% by weight, very
particularly
preferably at least 30% by weight and most preferably at least 50% by weight,
of water.
Preference is given to preparations which comprise at most 95% by weight,
particularly
preferably at most 90% by weight and in particular at most 85% by weight, of
water.
The preparations comprising water are, for example, solutions, emulsions,
suspensions
or dispersions.
In addition to the polyurethanes obtainable by the process according to the
invention, it
is possible to use further substances for preparing the preparations, such as
e.g.
customary auxiliaries (for example dispersants and/or stabilizers),
surfactants,
preservatives, antifoams, fragrances, wetting agents, UV filters, pigments,
emollients,
active ingredients, further thickeners, dyes, softeners, humectants and/or
other
polymers.
The polyurethanes obtainable by the process according to the invention and
mixtures
thereof are preferably used for effectively and stably thickening preparations
with a
content of salts and pigments of more than 1% by weight, based on the
preparation.
Here, "stably" means maintaining an increased viscosity compared with the
unthickened state over a period of several weeks and/or when increasing the
temperature of the preparation, for example to up to 50 C.
The polyurethanes obtainable by the process according to the invention exhibit
their
thickening effect even at elevated temperatures up to about 50 C.
Furthermore, the polyurethanes obtainable by the process according to the
invention
exhibit a thickening effect in a broad pH from 2 to 13.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
29
The polyurethanes obtainable by the process according to the invention
furthermore
have an influence on the structure of the preparations in which they enlarge
the finely
divided nature of the particles dispersed therein, i.e. reduce the particle
size. Moreover,
the polyurethanes obtainable by the process according to the invention permit
the
preparation of cosmetic preparations with good texture, i.e. particularly
smooth, non-
lumpy or gritty structures can be obtained.
On account of the reduced stickiness of the polyurethanes obtainable by the
process
according to the invention on surfaces such as glass or steel, the thickeners
can be
used particularly advantageously in standard commercial production vessels for
producing cosmetic preparations.
The polyurethanes obtainable by the process according to the invention and
mixtures
thereof can also be used for preparing water-comprising preparations which
comprise
at least one salt or at least one surfactant or mixtures thereof.
In connection with the present invention, surfactants are also understood as
meaning
emulsifiers and also mixtures of surfactants and emulsifiers. In connection
with the
present invention, salt is understood as meaning salts and also salt-like
structures also
with a low pKa value and mixtures thereof.
The polyurethanes obtainable according to the invention are particularly
preferably
used in order to prepare preparations which comprise at least 0.05% by weight
of salt
and/or at least 0.5% by weight of surfactants, very particularly preferably
comprising at
least 0.1% (w/w) of salt and/or at least 1% by weight of surfactants.
In a further embodiment, the polyurethanes obtainable by the process according
to the
invention are used for preparing preparations which comprise at least 5% by
weight,
preferably at least 10% by weight, of salt.
In a further embodiment, the polyurethanes obtainable by the process according
to the
invention are used for preparing preparations which comprise up to 25% by
weight of
surfactants, preferably up to 20% by weight and particularly preferably 15% by
weight
or fewer surfactants.
In a further embodiment, the polyurethanes obtainable by the process according
to the
invention are used for preparing preparations which comprise at least 1% by
weight of
salt and up to 20% by weight of surfactants, preferably up to 15% by weight of
surfactants.
,
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
The polyurethanes obtainable by the process according to the invention are
preferably
used for preparing preparations which are oil-in-water emulsions.
Typically, oil-in-water emulsions comprise oil in the range from more than 0
to 40% by
5 weight. Preferably, according to the invention, oil-in-water emulsions
are prepared
which comprise an oil fraction in the range from 5 to 40% by weight,
particularly in the
range from 10 to 35% by weight and in particular from 15 to 30% by weight, of
oil.
The polyurethanes obtainable by the process according to the invention are
very
10 particularly preferably used for preparing preparations which are oil-in-
water emulsions
and moreover comprise at least one salt.
The preparations according to the invention which comprise at least one
polyurethane
obtainable by the process according to the invention may be, for example,
solutions,
15 emulsions, suspensions or dispersions.
In one embodiment, a preparation according to the invention is a dispersion,
preferably
an aqueous dispersion of the polyurethanes obtainable by the process according
to the
invention, as can be obtained from the reaction products by work-up after the
20 preparation process. For this, for example, water is added to the
reaction mixture after
the reaction to produce a dispersion. If desired, the addition of a
preservative and/or
stabilizer can also take place.
In one embodiment, a dispersion according to the invention comprises up to 50%
by
25 weight of the polyurethanes obtainable according to the invention.
In another embodiment, a dispersion according to the invention comprises 25%
by
weight of the polyurethanes obtainable according to the invention.
30 Very particular preference is given to aqueous dispersions comprising up
to 25% by
weight of the polyurethanes obtainable according to the invention, at least
one of the
above-described preservatives suitable for cosmetic applications and, if
desired, at
least one of the above-described stabilizers (free-radical scavengers)
suitable for
cosmetic applications.
In another embodiment, the polyurethane obtainable according to the invention
is in the
form of a powder. Such a powder can be obtained, for example, by spray-drying
or
freeze-drying the aqueous dispersion.
To prepare the preparations according to the invention, which may be, for
example,
solutions, emulsions, suspensions or dispersions, the polyurethanes according
to the
W02012/052383 CA 02815268 2013-04-19
PCT/EP2011/068072
31
invention are preferably used in the form of aqueous dispersions or as a
powder, as
can be obtained, for example, from the preparation process by appropriate work-
up.
Further ingredients may be present in the preparations according to the
invention
depending on the intended use.
The preparations comprising the polyurethanes according to the invention can
comprise further thickeners. Such further thickeners are known to the person
skilled in
the art. Suitable thickeners are specified for example in "Kosmetik und
Hygiene von
Kopf bis Full [Cosmetics and Hygiene from Head to Toe]", Ed. W. Umbach, 3rd
edition,
Wiley-VCH, 2004, pp. 235-236. Suitable further thickeners for the preparations
according to the invention are described for example also on page 37, line 12
to page
38, line 8 of WO 2006/106140. Reference is hereby made to the contents of the
cited
passages in their entirety.
In one embodiment of this invention, however, no further thickeners are used
besides
the polyurethanes according to the invention for preparing the preparations
according
to the invention.
Preference is given to polyurethanes according to the invention, the 10%
strength by
weight aqueous dispersions thereof having, at a shear rate of 100 1/s, a
dynamic
viscosity, measured as described below, of at least 100 mPa*s, particularly
preferably
of at least 200 mPa*s and very particularly preferably of at least 300 mPa*s.
The aqueous dispersions of the polyurethanes obtainable by the process
according to
the invention can exhibit Newtonian or non-Newtonian behavior.
Non-Newtonian 10% strength by weight aqueous dispersions which comprise the
polyurethanes obtainable by the process according to the invention have, at a
shear
rate of 100 1/s, dynamic viscosities of at least 1000 mPa*s, particularly
preferably of at
least 3000 mPa*s.
The person skilled in the art is aware that the effectiveness of many
thickeners in
aqueous preparations diminishes if the preparations comprise salts or
surfactants. By
contrast, the polyurethanes according to the invention permit a stable
viscosity of
aqueous preparations even upon the addition of salts and/or surfactants.
Particular preference is given to polyurethanes according to the invention
which, in the
case of a salt concentration of at least 0.5% by weight following the
addition, lead to a
stabilization of the dynamic viscosity, measured as described below, of the
aqueous
preparations comprising them.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
32
Particular preference is given to those polyurethanes which permit a stable
dynamic
viscosity even upon the addition of at least 0.5% by weight of salt and
addition of at
least 1% by weight of surfactant.
In a further preferred embodiment, the presence of the polyurethanes according
to the
invention in salt-containing aqueous preparations leads to an increase in the
viscosity
compared to preparations which comprise only salt or only polyurethanes
according to
the invention.
The order in which polyurethane and salt are added is not important here.
Particular preference is given to polyurethanes according to the invention
which lead to
an increase in the dynamic viscosity of aqueous salt- and/or surfactant-
containing
preparations.
Preference is given to polyurethanes according to the invention which, in the
case of a
salt concentration of the aqueous preparation of at least 0.05% by weight,
based on the
aqueous preparation, lead to an increase in the dynamic viscosity.
Very particular preference is given to polyurethanes according to the
invention which,
in the case of a salt concentration of greater than or equal to 0.5% by
weight, based on
the aqueous preparation, lead to an increase in the dynamic viscosity.
Particular preference is given to those polyurethanes which lead to an
increase in the
dynamic viscosity compared to preparations which comprise less than 0.05% by
weight, preferably less than or equal to 0.01% by weight, of salt, or less
than 0.5% by
weight, preferably less than or equal to 0.1% by weight, of surfactant.
A further advantage of the polyurethanes obtainable by the process according
to the
invention is the micelle formation in water. The critical material
concentration for micelle
formation, also called critical micelle concentration (CMC) indicates the
concentration
of a substance, in most cases of a substance which has hydrophobic and
hydrophilic
sections on the inside, at which micelles with an average particle size of
less than or
equal to 200 nm, in particular less than or equal to 100 nm (determinable by
means of
dynamic light scattering) are spontaneously formed. The CMC of the
polyurethanes
according to the invention in water is preferably less than or equal to 1 g/I,
particularly
preferably less than or equal to 0.5 g/I, especially preferably less than or
equal to
0.25 g/I and very particularly preferably less than or equal to 0.1 g/I.
A further advantage of the process according to the invention, of the
polyurethanes
obtainable thereby and of the preparations according to the invention is the
use of
alkali(ne earth) metal carboxylate or zinc carboxylate catalysts and thus the
,
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
33
simultaneous omission of cosmetically unacceptable catalysts during the
preparation of
the polyurethanes.
In the field of cosmetic preparations, the known processes with tin are no
longer
desired since tin may also be present in the products and preparations
resulting
therefrom.
Moreover, the use of the alkali(ne earth) metal carboxylates or zinc
carboxylates as
catalysts permits the in-situ production of allophanate and isocyanurate
structures and
thus the economically advantageous production of branched hydrophobic sections
D of
the polyurethanes. By virtue of partially branched sections D, polyurethane
thickeners
with higher efficiency can be obtained.
On account of their tolerance toward high salt contents and simultaneously
high
surfactant contents even at extreme pH values, the polyurethanes according to
the
invention can advantageously also be used as thickeners in homecare
preparations,
such as, for example, liquid cleaners.
In particular, the polyurethanes according to the invention are also suitable
as rheology
modifiers for preparations containing hydrogen peroxide.
Cosmetic preparations
The polyurethanes obtainable according to the invention are preferably used in
cosmetic preparations. The invention thus provides cosmetic preparations
comprising
the polyurethanes obtainable according to the invention.
One embodiment of the invention is water-comprising cosmetic preparations
comprising polyurethanes obtainable according to the invention.
The preparations according to the invention can be in the form of aqueous or
aqueous-
alcoholic solutions, 0/W (preferably) and W/O emulsions, hydrodispersion
formulations, solids-stabilized formulations, stick formulations, PIT
formulations, in the
form of creams, foams, sprays (pump spray or aerosol), gels, gel sprays,
lotions, oils,
oil gels or mousses and accordingly be formulated with customary further
auxiliaries.
Preferably, the preparations according to the invention are in the form of a
gel, foam,
mousse, spray, ointment, cream, emulsion, suspension, lotion, milk or paste.
W020121052383 CA 02815268 2013-04-19
PCT/EP2011/068072
34
The invention preferably relates to cosmetic preparations which are selected
from gels,
gel creams, milks, hydroformulations, stick formulations, cosmetic oils and
oil gels,
mascara, self-tanning compositions, face care compositions, body care
compositions,
aftersun preparations. The term cosmetic preparations is also understood as
meaning
preparations for oral care.
Further cosmetic preparations according to the invention are skin cosmetic
preparations, in particular those for caring for the skin. These are present
in particular
as MO or preferably 0/W skin creams, day and night creams, eye creams, face
creams, antiwrinkle creams, mimic creams, moisturizing creams, bleaching
creams,
vitamin creams, skin lotions, care lotions and moisturizing lotions.
Further preferred preparations according to the invention are face masks,
cosmetic
lotions and preparations for the use in decorative cosmetics, for example for
concealing
sticks, stage make-up, mascara and eyeshadows, lipsticks, kohl pencils,
eyeliners,
make-ups, foundations, blushers, powders and eyebrow pencils.
Further preparations according to the invention are antiacne compositions,
repellents,
shaving compositions, hair removal compositions, intimate care compositions,
foot care
compositions and baby care products.
Further preferred preparations according to the invention are washing,
showering and
bathing preparations. Within the context of this invention, washing, showering
and
bathing preparations are soaps of liquid to gel-like consistency, transparent
soaps,
luxury soaps, deodorant soaps, cream soaps, baby soaps, skin protection soaps,
abrasive soaps and syndets, pasty soaps, soft soaps and washing pastes, liquid
washing, showering and bathing preparations, such as washing lotions, shower
baths
and shower gels, foam baths, oil baths and scrub preparations, shaving foams,
shaving
lotions and shaving creams.
Cosmetic preparations which comprise specific polyurethanes are described for
example in WO 2009/135857. The polyurethanes of the present invention
obtainable
according to the invention are generally also suitable for use in the
preparations
described in WO 2009/135857. Reference is hereby made expressly to the
disclosure
in WO 2009/135857.
Within the context of the present invention, the polyurethanes used in the
preparations
of WO 2009/135857 are replaced by the polyurethanes of this invention. The
polyurethanes according to the invention are thus used in the preparations of
WO 2009/135857 preferably in place of the polyurethanes used therein.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
_
Suitable ingredients for the preparations according to the invention are
described in
WO 2009/135857, p. 24 to p. 35, to which reference is made in its entirety.
Cosmetic UV photoprotective compositions comprising the polyurethanes obtained
5 according to the invention are also in accordance with the invention.
Within the context
of this invention, cosmetic photoprotective compositions are understood as
meaning
cosmetic preparations which comprise at least one, preferably two or more, UV
filter
substances.
10 UV photoprotective compositions corresponding to the UV photoprotective
composition
preparations according to the invention are described in WO 2009/135857, p. 35
to p.
42, to which reference is made in its entirety.
The invention also relates to cosmetic preparations, preferably in liquid or
pasty form,
15 for use on the skin, on semi mucosa, on mucosa and in particular on
keratin materials
such as hair, eyelashes and eyebrows, in particular for the shaping,
decoration,
coloring, beautifying of the same, and also for caring for the skin and the
skin
appendages. In principle, the preparations according to the invention, upon
suitable
adjustment and coloring, can also be used as make-up, concealer, camouflage,
20 eyeshadows, eyeliners, lip liners, blushers, lip blush, lip gloss, sun
protection
composition, sun block, temporary tattoo, colored effect sunscreen for surfers
and the
like.
A preferred embodiment of the present invention is thus cosmetic preparations
for
25 decorative cosmetics.
Preparations corresponding to the preparations according to the invention for
decorative cosmetics are described in WO 2009/135857, p. 43 to p. 46, to which
reference is made in its entirety.
The present invention provides aqueous preparations which, besides the
polyurethanes obtainable according to the invention, further comprise at least
one salt
or surfactant or both.
35 A further embodiment of the invention is shampoos and cosmetic cleaning
compositions comprising the polyurethanes obtainable according to the
invention.
Preparations corresponding to the shampoos and cosmetic cleaning compositions
according to the invention are described analogously in WO 2009/135857, p. 46
to
p. 55, to which reference is made in its entirety.
A further embodiment of the invention is deodorants or antiperspirants, in
particular
deodorant lotions and deodorant or antiperspirant sticks, comprising the
polyurethanes
W020121052383 CA 02815268 2013-04-19
PCT/EP2011/068072
36
obtainable according to the invention and based on oil-in-water dispersion or
emulsion
for the application of active ingredients, in particular of water-soluble
active ingredients,
to the skin.
Preparations corresponding to the deodorants and antiperspirants according to
the
invention are described analogously in WO 2009/135857, p. 55 to p. 59, to
which
reference is made in its entirety.
A further embodiment of the invention is hair colorants comprising the
polyurethanes
obtainable according to the invention.
Preparations corresponding to the hair colorants comprising the polyurethanes
obtainable according to the invention are described analogously in WO
2009/135857,
p. 59 to p. 65, to which reference is made in its entirety.
A further embodiment of the invention is hair care compositions, in particular
hair
conditioners, comprising the polyurethanes obtainable according to the
invention.
Hair care compositions corresponding to the hair care compositions comprising
the
polyurethanes obtainable according to the invention are described analogously
in
WO 2009/135857, p. 59 to p. 67, to which reference is made in its entirety.
A further embodiment of the invention is acidic preparations comprising the
polyurethanes obtainable according to the invention.
Numerous cosmetic preparations comprise active ingredients which develop their
desired effect in particular at acidic pHs. These include, for example,
preparations
which comprise alpha-hydroxycarboxylic acids (AHAs) and beta-hydroxycarboxylic
acids (BHAs) since these are not effective or not very effective in the
neutralized state.
Acidic preparations corresponding to the acidic preparations comprising the
polyurethanes obtainable according to the invention are described analogously
in
WO 2009/135857, p. 67 to p. 69, to which reference is made in its entirety.
A further embodiment of the invention is self-tanning products comprising the
polyurethanes obtainable according to the invention.
Standard commercial self-tanning products are generally 0/VV emulsions. In
these, the
water phase is stabilized by emulsifiers customary in cosmetics.
By applying the self-tanning products according to the invention, it is
possible to
achieve not only a uniform skin coloration, but it is also possible to
uniformly color
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
37
areas of skin that are differently colored by nature or as a result of a
pathological
change.
According to the invention, the self-tanning substances used are
advantageously
inter alia glycerol aldehyde, hydroxymethylglyoxal, y-d i a Id e h yde,
erythrulose,
5-hydroxy-1,4-naphtoquinone (juglone), and also 2-hydroxy-1,4-naphtoquinine as
occurs in henna leaves. Very particular preference is given to 1,3-
dihydroxyacetone
(DHA), a trivalent sugar which occurs in the human body. 6-Aldo-D-fructose and
ninhydrin can also be used as self-tanning agents according to the invention.
For the
purposes of the invention, self-tanning agents are also understood as meaning
substances which bring about a skin coloration other than a brown shade.
In one preferred embodiment of the invention, such preparations comprise self-
tanning
substances in a concentration of from 0.1 to 10% by weight and particularly
preferably
from 0.5 to 6% by weight, in each case based on the total weight of the
preparation.
Preferably, these preparations comprise 1,3-dihydroxyacetone as self-tanning
substance. These compositions further preferably comprise organic and/or
inorganic
light protection filters. The preparations can also comprise inorganic and/or
organic
and/or modified inorganic pigments.
Further ingredients preferably present in the preparations according to the
invention
are specified, for example, in DE 103 21 147 in paragraphs [0024] to [0132],
to which
reference is made at this point in their entirety.
The invention also provides the use of such preparations for coloring the skin
of
multicellular organisms, in particular the skin of humans and animals,
especially for
harmonizing the color of differently pigmented areas of skin.
A further embodiment of the invention is preparations for oral and dental care
and
cleansing which comprise the polyurethanes obtainable according to the
invention.
Preparations corresponding to the preparations for oral and dental care and
cleansing
comprising the polyurethanes obtainable according to the invention are
described
analogously in WO 2009/135857, p .69 to p. 70, to which reference is made in
its
entirety.
A further embodiment of the invention is preparations for hair removal which
comprise
the polyurethanes obtainable according to the invention.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
38
Preparations corresponding to the preparations for hair removal which comprise
the
polyurethanes obtainable according to the invention are described analogously
in
WO 2009/135857, p. 70 to p. 71, to which reference is made in its entirety.
A further embodiment of the invention is preparations for permanent hair
shaping
comprising the polyurethanes obtainable according to the invention.
Preparations corresponding to the preparations for permanent hair shaping
which
comprise the polyurethanes obtainable according to the invention are described
analogously in WO 2009/135857, p. 71 to p. 73, to which reference is made in
its
entirety.
Examples
The invention is illustrated in more detail by reference to the nonlimiting
examples
below.
Unless stated otherwise, the percentages are percentages by weight.
Determination of the dynamic viscosity
The dynamic viscosities of the polyurethanes according to the invention were
measured in the form of a 10 percent strength by weight aqueous dispersion at
23 C.
In the examples listed below, the dynamic viscosity was always determined at
shear
rates of 100 1/s and 350 1/s. These two values allow a statement about whether
the
polyurethanes according to the invention exhibit non-Newtonian or Newtonian
thickener
behavior in aqueous dispersion.
To determine the dynamic viscosity to DIN 53019, the following were used:
- rotary viscometer Physica Rheolab MCI Portable (Anton Paar);
- cylinder measurement system, Z4 DIN cylinder (diameter 14 mm).
The polyols, ethoxylated alcohols and isocyanates used below were used in
essentially
alkali-free form. The ppm data for potassium and/or zinc refer in each case to
the total
amount of substances reactive towards isocyanate groups plus the corresponding
potassium carboxylate or zinc carboxylate.
Synthesis example 1: Preparation of a PUR associative thickener A.1
Catalyst: 300 ppm of K as potassium acetate
120.00 g of polyethylene glycol Pluriol0E6000 (BASF SE, molecular weight
6000 g/mol), 9.60 g of LutensoloT010 (BASF SE), 11.10 g of Lutensol AT11 (BASF
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
39
SE), and 106 mg of potassium acetate (= 300 ppm of potassium) were introduced
as
initial charge under nitrogen in a 2 liter polymerization reactor and then
heated to
100 C. By applying a vacuum of ca. 10 mbar for 6 hours, traces of water were
removed
until ultimately the water content of the mixture was 230 ppm. The mixture was
then
cooled to 80 C. By adding 5.88 g of hexamethylene diisocyanate, the
polymerization
was started and the mixture was stirred at a temperature of 80 C for 50
minutes until
an isocyanate content of 0.0% was reached. The yellow colored residue was then
dissolved in 586.3 g of water and the aqueous solution was immediately admixed
with
7.33 g of EuxyleK701 (Schiilke & Mayr) and 70 mg of the stabilizer 4-hydroxy-
TEMPO.
The mixture was cooled to room temperature (25 C). The polymer A.1 (Mr, =
11 700 g/mol; M = 26 900 g/mol) was thus obtained in the form of a clear, pale
yellow
colored solution which had a solids content of 20.8%. The viscosity of a 10%
strength
by weight aqueous solution of the polyether polyurethane A.1 was 1550 mPa*s
(shear
rate 100 1/s) and 1360 mPa*s (shear rate 350 1/s).
Synthesis example 2: Preparation of a PUR associative thickener A.2
Catalyst: 600 ppm of K as potassium acetate
120.00 g of polyethylene glycol Pluriol0E6000, 9.60 g of LutensoloT010, 11.10
g of
LutensoleAT11, and 212 mg of potassium acetate (= 600 ppm of potassium) were
introduced as initial charge under nitrogen in a 2 l polymerization reactor
and then
heated to 100 C. By applying a vacuum of ca. 10 mbar for 5 hours, traces of
water
were removed until ultimately the water content of the mixture was 225 ppm.
The
mixture was then cooled to 80 C. By adding 5.88 g of hexamethylene
diisocyanate, the
polymerization was started and the mixture was stirred at a temperature of 80
C for 50
minutes until an isocyanate content of 0.0% was reached. The yellow colored
residue
was then dissolved in 586.3 g of water and the aqueous solution was
immediately
admixed with 7.33 g of EuxyloK701 and 70 mg of the stabilizer 4-hydroxy-TEMPO.
The
mixture was cooled to room temperature (25 C). The polymer A.2 (Mr, = 12 300
g/mol;
Mw = 29 700 g/mol) was thus obtained in the form of a clear, pale yellow
colored
solution which had a solids content of 20.1%. The viscosity of a 10% strength
by weight
aqueous solution of the polyether polyurethane A.2 was 3700 mPa*s (shear rate
100 1/s) and 3000 mPa*s (shear rate 350 1/s).
Synthesis example 3: Preparation of a PUR associative thickener A.3
Catalyst: 900 ppm of K as potassium acetate
120.00 g of polyethylene glycol Pluriol0E6000, 9.60 g of LutensoleT010, 11.10
g of
LutensoleAT11, and 318 mg of potassium acetate (= 900 ppm of potassium) were
introduced as initial charge under nitrogen in a 2 l polymerization reactor
and then
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
heated to 100 C. By applying a vacuum of ca. 10 mbar for 4 hours, traces of
water
were removed until ultimately the water content of the mixture was 242 ppm.
The
mixture was then cooled to 80 C. By adding 5.88 g of hexamethylene
diisocyanate, the
polymerization was started and the mixture was stirred at a temperature of 80
C for
5 55 minutes until an isocyanate content of 0.0% was reached. The yellow
colored
residue was then dissolved in 586.3 g of water and the aqueous solution was
immediately admixed with 7.33 g of the preservative EuxyleK701 and 70 mg of
the
stabilizer 4-hydroxy-TEMPO. The mixture was cooled to room temperature (25 C).
The
polymer A.3 (M0 = 12 700 g/mol; M = 33 000 g/mol) was thus obtained in the
form of a
10 clear, pale yellow colored solution which had a solids content of 20.6%.
The viscosity of
a 10% strength by weight aqueous solution of the polyether polyurethane A.3
was 6000
mPa*s (shear rate 100 1/s) and 4400 mPa*s (shear rate 350 1/s).
15 Synthesis example 4: Preparation of a PUR associative thickener A.4
Catalyst: 2000 ppm of K as potassium acetate
90.00 g of polyethylene glycol PlurioloE6000, 7.20 g of LutensoleT010, 8.33 g
of
LutensoleAT11, and 530 mg of potassium acetate (= 2000 ppm of potassium) were
20 introduced as initial charge under nitrogen in a 21 polymerization
reactor and then
heated to 100 C. By applying a vacuum of ca. 10 mbar for 5 hours, traces of
water
were removed until ultimately the water content of the mixture was 210 ppm.
The
mixture was then cooled to 80 C. By adding 4.41 g of hexamethylene
diisocyanate, the
polymerization was started and the mixture was stirred at a temperature of 80
C for
25 55 minutes until an isocyanate content of 0.0% was reached. The yellow
colored
residue was then dissolved in 439.7 g of water and the aqueous solution was
immediately admixed with 5.50 g of the preservative EuxyloK701 and 50 mg of
the
stabilizer 4-hydroxy-TEMPO. The mixture was cooled to room temperature (25 C).
The
polymer A.4 (Mn = 12 500 g/mol; My, = 31 300 g/mol) was thus obtained in the
form of a
30 clear, pale yellow colored solution which had a solids content of 20.6%.
The viscosity of
a 10% strength by weight aqueous solution of the polyether polyurethane A.4
was 5200
mPa*s (shear rate 100 1/s) and 3800 mPa*s (shear rate 350 1/s).
35 Synthesis example 5: Preparation of a PUR associative thickener A.5
Catalyst: 200 ppm of K as potassium acetate
100.00 g of polyethylene glycol (Merck KGaA, molecular weight 10 000 g/mol),
10.02 g
of 2-decy1-1-tetradecano1-20 EO, and 55 mg of potassium acetate (= 200 ppm of
40 potassium) were introduced as initial charge under nitrogen in a 21
polymerization
reactor and then heated to 100 C. By applying a vacuum of ca. 10 mbar for 6
hours,
traces of water were removed until ultimately the water content of the mixture
was
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
41
190 ppm. The mixture was then cooled to 80 C. By adding 2.52 g of
hexamethylene
diisocyanate, the polymerization was started and the mixture was stirred at a
temperature of 80 C for 40 minutes until an isocyanate content of 0.0% was
reached.
The yellow colored residue was then dissolved in 450.2 g of water and the
aqueous
solution was immediately admixed with 5.63 g of the preservative EuxyloK701
and
60 mg of the stabilizer 4-hydroxy-TEMPO. The mixture was cooled to room
temperature (25 C). The polymer A.5 (Mn = 19 100 g/mol; Mw = 48 500 g/mol) was
thus
obtained in the form of a clear, pale yellow colored solution which had a
solids content
of 20.7%. The viscosity of a 5% strength by weight aqueous solution of the
polyether
polyurethane A.5 was >20 000 mPa*s (shear rate 100 1/s) and >20 000 mPa*s
(shear
rate 350 1/s).
Synthesis example 6: Preparation of a PUR associative thickener A.6
Catalyst: Zinc neodecanoate; single-stage reaction
120.00 g of polyethylene glycol Pluriol8E6000 (BASF SE, molecular weight
6000 g/mol), dealkylated, 9.60 g of LutensoloT010 (BASF SE), dealkylated, and
11.10 g of LutensoleAT11 (BASF SE), dealkylated, were introduced as initial
charge
under nitrogen in a 2 I polymerization reactor and then heated to 100 C. By
applying a
vacuum of ca. 10 mbar for 6 hours, traces of water were removed until
ultimately the
water content of the mixture was 200 ppm. The mixture was then cooled to 80 C.
The
mixture was admixed with 675 mg of TIBeKat 616 (TIB Chemicals, Mannheim; =
zinc
neodecanoate, dissolved in a mixture of aliphatic hydrocarbons; = 900 ppm of
zinc). By
adding 5.88 g of hexamethylene diisocyanate, the polymerization was started
and the
mixture was stirred at a temperature of 80 C for 40 minutes until an
isocyanate content
of 0.0% was reached. The yellow colored residue was then dissolved in 589.0 g
of
water and the aqueous solution was immediately admixed with 7.36 g of the
preservative Euxyl8K701 (Schulke & Mayr) and 70 mg of the stabilizer 4-hydroxy-
TEMPO. The mixture was cooled to room temperature (25 C). The polymer A.1
(M,, = 11 200 g/mol; M = 26 400 g/mol) was thus obtained in the form of a
clear, pale
yellow colored solution which had a solids content of 21.0%. The viscosity of
a 10%
strength by weight aqueous solution of the polyether polyurethane A.6 was
2550 mPa*s (shear rate 100 1/s) and 2050 mPa*s (shear rate 350 1/s).
Comparative example 1: Preparation of a PUR associative thickener A.7
Without catalyst, single-stage reaction
120.00 g of polyethylene glycol Pluriol0E6000, 9.60 g of LutensoloT010, and
11.10 g of
Lutensol AT11 were introduced as initial charge under nitrogen in a 2 I
polymerization
reactor and then heated to 100 C. By applying a vacuum of ca. 10 mbar for 5
hours,
traces of water were removed until ultimately the water content of the mixture
was
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
42
250 ppm. The mixture was then cooled to 80 C. By adding 5.88 g of
hexamethylene
diisocyanate, the polymerization was started and the mixture was stirred at a
temperature of 80 C for a total of 32 hours until an isocyanate content of
0.0% was
reached. The yellow colored residue was then dissolved in 586.3 g of water and
the
aqueous solution was immediately admixed with 7.33 g of the preservative
EuxyleK701
and 70 mg of the stabilizer 4-hydroxy-TEMPO. The mixture was cooled to room
temperature (25 C). The polymer A.6 (M, = 10 000 g/mol; M, = 23 200 g/mol) was
thus
obtained in the form of a clear, pale yellow colored solution which had a
solids content
of 18.8%. The viscosity of a 10% strength by weight aqueous solution of the
polyether
polyurethane A.7 was 100 mPa*s (shear rate 100 1/s) and 100 mPa*s (shear rate
350 1/s).
Comparative example 2: Preparation of a PUR associative thickener A.8
Without catalyst, single-stage reaction
100.00 g of polyethylene glycol (Merck KGaA, molecular weight 10 000 g/mol),
and
10.02 g of 2-decy1-1-tetradecano1-20 EO were introduced as initial charge
under
nitrogen in a 2 I polymerization reactor and then heated to 100 C. By applying
a
vacuum of ca. 10 mbar for 6 hours, traces of water were removed until
ultimately the
water content of the mixture was 185 ppm. The mixture was then cooled to 80 C.
By
adding 2.52 g of hexamethylene diisocyanate, the polymerization was started
and the
mixture was stirred at a temperature of 80 C for a total of 12 hours until an
isocyanate
content of 0.0% was reached. The yellow colored residue was then dissolved in
450.2
g of water and the aqueous solution was immediately admixed with 5.63 g of the
preservative EuxyloK701 and 60 mg of the stabilizer 4-hydroxy-TEMPO. The
mixture
was cooled to room temperature (25 C). The polymer A.7 (Mn = 5200 g/mol; Mw =
14 500 g/mol) was thus obtained in the form of a clear, pale yellow colored
solution
which had a solids content of 18.5%. The viscosity of a 10% strength by weight
aqueous solution of the polyether polyurethane A.8 was <100 mPa*s (shear rate
100 1/s) and <100 mPa*s (shear rate 350 1/s).
Comparative example 3: Preparation of a PUR associative thickener A.9
Catalyst: Dibutyltin dilaurate, single-stage reaction
90.00 g of polyethylene glycol PluriolgE6000, 7.20 g of LutensoloT010, 8.33 g
of
LutensoloAT11, and 144 mg of dibutyltin dilaurate (DBTL) (= 300 ppm of Sn
based on
total mixture) were introduced as initial charge under nitrogen in a 2 I
polymerization
reactor and then heated to 100 C. By applying a vacuum of ca. 10 mbar for 5
hours,
traces of water were removed until ultimately the water content of the mixture
was
250 ppm. The mixture was then cooled to 80 C. By adding 4.41 g of
hexamethylene
CA 02815268 2013-04-19
WO 2012/052383
PCT/E P2011/068072
,
43
diisocyanate, the polymerization was started and the mixture was stirred at a
temperature of 80 C for 60 minutes until an isocyanate content of 0.0% was
reached.
The yellow colored residue was then dissolved in 440.3 g of water and the
aqueous
solution was immediately admixed with 5.50 g of the preservative Euxyl K701
and
5 60 mg of the stabilizer 4-hydroxy-TEMPO. The mixture was cooled to room
temperature (25 C). The polymer A.8 (Mr, = 10 100 g/mol; Mw = 22 000 g/mol)
was thus
obtained in the form of a clear, pale yellow colored solution which had a
solids content
of 20.4%. The viscosity of a 10% strength by weight aqueous solution of the
polyether
polyurethane A.9 was 720 mPa*s (shear rate 100 1/s) and 660 mPa*s (shear rate
10 350 1/s).
Comparative example 4: Preparation of a PUR associative thickener A.10
Catalyst: DABCO, singe-stage reaction
120.00 g of polyethylene glycol PlurioleE6000, 9.60 g of Lutensol0T010, 11.10
g of
Lutensol AT11, and 36 mg of 1,4-diazabicyclo[2.2.2]octane (DABCO; = 300 ppm
based on total mixture) were introduced as initial charge under nitrogen in a
2 l
polymerization reactor and then heated to 100 C. By applying a vacuum of ca.
10 mbar
20 for 5 hours, traces of water were removed until ultimately the water
content of the
mixture was 230 ppm. The mixture was then cooled to 80 C. By adding 5.88 g of
hexamethylene diisocyanate, the polymerization was started and the mixture was
stirred at a temperature of 80 C for a total of 13 hours until an isocyanate
content of
0.0% was reached. The yellow colored residue was then dissolved in 586.5 g of
water
25 and the aqueous solution was immediately admixed with 7.33 g of the
preservative
Euxyl K701 and 70 mg of the stabilizer 4-hydroxy-TEMPO. The mixture was cooled
to
room temperature (25 C). The polymer A.9 (Mr, = 8500 g/mol; Mw = 17 400 g/mol)
was
thus obtained in the form of a clear, pale yellow colored solution which had a
solids
content of 18.7%. The viscosity of a 10% strength by weight aqueous solution
of the
30 polyether polyurethane A.10 was 105 mPa*s (shear rate 100 1/s) and 105
mPa*s
(shear rate 350 1/s).
Comparative example 5: Preparation of a PUR associative thickener A.11
35 Catalyst: Zinc neodecanoate; two-stage reaction
120.00 g of polyethylene glycol Pluriol0E6000 (BASF SE, molecular weight 6000
g/mol)
were dissolved in 467.00 g of xylene under nitrogen in a 2 l polymerization
reactor (flat
flange glass vessel with anchor stirrer). After heating the solution to ca.
140 C (internal
40 temperature), exactly 200 g of xylene were distilled off. The water
content of the
reaction mixture was then only still ca. 50 ppm. The polymer solution was then
cooled
to 50 C (internal temperature) and admixed with 107 mg of acetic acid,
dissolved in
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
44
ml of xylene, in order to neutralize the amount of potassium acetate,
determined in
quantitative terms beforehand, within the polyethylene glycol. By adding 360
mg of
TIB Kat 616 (TIB Chemicals, Mannheim; = zinc neodecanoate, dissolved in a
mixture
of aliphatic hydrocarbons; = 400 ppm of zinc based on total mixture),
dissolved in 5 ml
5 of xylene, and 5.88 g of hexamethylene diisocyanate, dissolved in 10 ml
of xylene, the
polymerization was started and the mixture was run at an internal temperature
of 50 C
to an isocyanate content of 0.25%. A mixture of 9.60 g of LutensorT010 (BASF
SE)
and 11.10 g of Lutensol AT11 (BASF SE), dissolved in 20 ml of xylene, was then
added and the reaction mixture was further heated at 50 C until the isocyanate
content
was 0%. The solvent xylene was then largely removed by vacuum distillation at
elevated temperature (60 C) (residual content <100 ppm) and the residue was
dissolved in 586.3 g of water. After cooling to room temperature (25 C),
finally, 3.67 g
of the preservative Euxyl K500 were added to the aqueous solution. The polymer
A.11
(Mn = 17 700 g/mol; M = 31 800 g/mol) was obtained in the form of an aqueous
dispersion which had a solids content of 19.8%. The viscosity of a 10%
strength by
weight aqueous solution of the polyether polyurethane A.11 was 6400 mPa*s
(shear
rate 100 1/s) and 4600 mPa*s (shear rate 350 1/s).
Formulation example 1:
Cosmetic formulations based on Cremophor A6/Cremophor A25
The cosmetic formulations were prepared by adding the water phase B to the oil
phase
A and then admixing the resulting 0/W emulsion with the preservative (phase
C). This
gave the following formulations based on a Cremophor8A6/Cremophor0A25 base.
Table 1.
Formulations based on Cremophor A6/Cremophor0A25
Phase Ingredients F.1.1 F.1.2
F.1.3 F.1.4 F.1.5
Phase A CremophoreA 6 2.0 g 2.0 g
2.0 g 2.0 g 2.0 g
Cremophor0A 25 2.0 g 2.0 g
2.0 g 2.0 g 2.0 g
Lanette O 2.5 g 2.5 g 2.5 g 2.5 g
2.5 g
Paraffin oil 5.0 g 5.0 g 5.0 g 5.0 g
5.0 g
Luvitol EHO 5.0 g 5.0 g 5.0 g 5.0 g
5.0 g
A.1 A.2 A.3 A.4 A.5
Phase B PUR thickener
0.5 g 0.5 g 0.5 g 0.5 g 0.5 g
1,2-Propylene glycol 5.0 g 5.0 g 5.0 g 5.0 g
5.0 g
Water 77.5 g 77.5
g 77.5 g 77.5 g 77.5 g
Phase C Euxyl K300 0.5 g 0.5 g 0.5 g
0.5 g 0.5 g
CA 02815268 2013-04-19
WO 2012/052383 PCT/EP2011/068072
Table 2.
Formulations based on Cremophor0A6/Cremophor0A25
Phase Ingredients F.1.7 F.1.8 F.1.9 F.1.10
Phase A CremophoreA 6 2.0 g 2.0 g 2.0 g 2.0 g
CremophoreA 25 2.0 g 2.0 g 2.0 g 2.0 g
Lanette O 2.5 g 2.5 g 2.5 g 2.5 g
Paraffin oil 5.0 g 5.0 g 5.0 g 5.0 g
Luvitol EHO 5.0 g 5.0 g 5.0 g 5.0 g
A.7 A.8 A.9 A.10
Phase B PUR thickener
0.5 g 0.5g 0.5g 0.5 g
1,2-Propylene glycol 5.0 g 5.0 g 5.0 g 5.0 g
Water 77.5 g 77.5 g 77.5 g 77.5 g
Phase C Euxyl K300 0.5 g 0.5 g 0.5 g 0.5 g
5
Formulation example 2:
Cosmetic formulations based on stearate ester
10 The cosmetic formulations were prepared by adding the water phase B to
the oil phase
A and subsequently admixing the resulting OM emulsion with the preservative
(phase
C). This gave the following formulations based on a stearate ester base.
15 Table 3.
Formulations based on stearate ester
Phase Ingredients F.2.1 F.2.2
F.2.3 F.2.4 F.2.5
Phase A Cutina GMS 2.0 g 2.0 g
2.0 g 2.0 g 2.0 g
Lanette018 2.0 g 2.0 g 2.0 g 2.0 g
2.0 g
Dow Corning 345 Fluid 3.0 g 3.0 g 3.0 g 3.0 g
3.0 g
Cetio100E 3.0 g 3.0 g 3.0 g 3.0 g
3.0 g
Abil0350 2.0 g 2.0 g 2.0 g 2.0 g
2.0 g
Dry Flo PC 1.0 g 1.0 g 1.0 g 1.0 g
1.0 g
Myrj 52 2.0 g 2.0 g 2.0 g 2.0 g
2.0 g
A.1 A.2 A.3 A.4 A.5
Phase B PUR thickener
0.5 g 0.5 g 0.5 g 0.5 g
0.5 g
Glycerol 5.0 g 5.0 g 5.0 g 5.0 g
5.0 g
Water 79.0 g 79.0 g 79.0 g 79.0 g 79.0 g
Phase C Euxyl0K300 0.5 g 0.5 g 0.5 g 0.5 g 0.5 g
CA 02815268 2013-04-19
WO 2012/052383 PCT/EP2011/068072
46
Table 4.
Formulations based on stearate ester
Phase Ingredients F.2.7 F.2.8 F.2.9 F.2.10
Phase A Cutina GMS 2.0 g 2.0 g 2.0 g 2.0 g
Lanette818 2.0 g 2.0 g 2.0 g 2.0 g
Dow Corning 345 Fluid 3.0 g 3.0 g 3.0 g 3.0 g
Cetio100E 3.0 g 3.0 g 3.0 g 3.0 g
Abil0350 2.0 g 2.0 g 2.0 g 2.0 g
Dry Flo PC 1.0 g 1.0 g 1.0 g 1.0 g
Myrj 52 2.0 g 2.0 g 2.0 g 2.0 g
A.7 A.8 A.9 A.10
Phase B PUR thickener
0.5 g 0.5 g 0.5 g 0.5 g
Glycerol 5.0 g 5.0 g 5.0 g 5.0 g
Water 79.0 g 79.0 g 79.0 g 79.0 g
Phase C Euxyl0K300 0.5 g 0.5 g 0.5 g 0.5 g
Viscosities as a function of the salt concentration
Table 3.
Viscosity [mPa*s]
Formulation
with 2.0% NaCI addition
F.1.1 12 100
F.1.2 17 100
F.1.3 26 200
F.1.4 17 000
F.1.7 6800
F.1.9 11 400
Viscosities as a function of the salt concentration
Table 4.
Viscosity [mPa*s]
Formulation
with 2.0% NaCI addition
F.2.1 16 700
F.2.2 21 800
F.2.3 21 200
F.2.4 18 100
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
47
F.2.7 10 000
F.2.9 14 700
Viscosities as a function of shear rate and concentration
Table 5.
Viscosity Viscosity
Polymer concentration
Polymer in water [mPa*s] [mPa*s]
[% by weight] Shear rate 100 1/s Shear rate 350 1/s
A.1 10 1550 1360
A.2 10 3700 3000
A.3 10 6000 4400
A.4 10 5200 3800
A.5 5 >20000 >20000
,
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
µ
48
A.7 10 100
100
A.8 10 <100
<100
A.9 10 720
660
A.10 10 105
105
10
20
Formulation example 3:
Cosmetic formulations with associative thickeners A.6 and A.11
The cosmetic formulations were prepared by adding the water phase B to the oil
phase
A and subsequently admixing the resulting 0/W emulsion with the preservative
(phase
C). This gave the following formulations based on a Cremophore A6/Cremophor
A25
basis (Tab. 8).
Table 8.
Cosmetic formulations based on Cremophor0A6/A25
REPLACEMENT PAGE (RULE 26)
W020121052383 CA 02815268 2013-04-19
PCT/EP2011/068072
49
Phase Ingredients F.1.6 F.1.11
Phase A Cremophor A 6 2.0 g 2.0 g
Cremophor A 25 2.0 g 2.0 g
Lanette 0 2.5g 2.5g
Paraffin oil 5.0 g 5.0 g
Luvitol EHO 5.0 g 5.0 g
A.6 A.11
Phase B PUR thickener
0.5 g 0.5g
1,2-Propylene glycol 5.0 g 5.0 g
Water 77.5g 77.5g
Phase C Euxyl K300 0.5 g 0.5 g
Formulation example 4:
Cosmetic formulations with associative thickeners A.6. and A.11
The cosmetic formulations were prepared by adding the water phase B to the oil
phase
A and subsequently admixing the resulting 0/W emulsion with the preservative
(phase
C). This gave the formulations F.2.6 and CF.2.5 based on a stearate ester base
(Tab. 9).
Table 9.
Formula parameters for the cosmetic formulations F.2.6 and F.2.11 based on a
stearate ester base.
Phase Ingredients F.2.6 F.2.11
Phase A Cutina GMS 2.0 g 2.0 g
Lanette 18 2.0 g 2.0 g
Dow Corning 345 Fluid 3.0 g 3.0 g
Cetiol OE 3.0 g 3.0 g
Abil 350 2.0 g 2.0 g
Dry Flo PC 1.0 g 1.0 g
Myrj 52 2.0 g 2.0 g
A.6 A.11
Phase B PUR thickener
0.5 g 0.5g
Glycerol 5.0 g 5.0 g
Water 79.0 g 79.0 g
Phase C Euxyl K300 0.5 g 0.5 g
Viscosities and properties of the cosmetic formulations
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
Table 10. Viscosities of the cosmetic formulations admixed with 2% NaCI.
Viscosity
Formulation Properties
[Pas]
F 6 14 0 Polymer dissolved to give a clear solution in the
water
.1. .
phase (B), not sticky
F 8 Polymer dissolved to give a cloudy solution in the
water
.1.11 1.0
phase (B), sticky
F.2 6 22 2 Polymer dissolved to give a clear solution in the
water
. .
phase (B), not sticky
F.2.11 18.7 Polymer dissolved to give a cloudy solution in the
water
phase (B), sticky
For comparable thickening properties, the structure obtainable with
polyurethane A.6
5 can always be better processed (water-soluble to give a clear solution,
nonsticky
polymer) as polyurethane A.11.
Application examples:
At this point, reference is made in full to the application examples which are
disclosed
in WO 2009/135857 on pages 87 to 114.
The polyurethanes PU.1 to PU.11 used in the examples therein are replaced for
the
purposes of this invention by the polyurethanes A.1, A.2, A.3, A.4, A.5 or A.6
obtainable according to the invention, thus giving the corresponding cosmetic
preparations.
Further typical preparations according to the invention are described below,
without,
however, limiting the invention to these examples.
Besides the preparation of the cosmetic preparations described here, the
polyurethanes A.1, A.2, A.3, A.4, A.5 or A.6 and also combinations thereof can
be
added to the resulting emulsion also after combining the water phase and oil
phase at
60-80 C or to the cooled emulsion at about 40 C.
The invention also provides the subsequent addition of the polyurethanes
obtainable
according to the invention to a cosmetic preparation in order to establish the
desired
viscosity.
Unless expressly described otherwise, the percentages are % by weight.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
51
0/W emulsion
Phase Ingredient/INCI F.3.1 F.3.2 F.3.3 F.3.4 F.3.5
A Aqua ad 100 ad 100 ad 100 ad 100 ad 100
Glycerin 3.0 5.50 4.50 5.00 3.5
PUR thickener A.1 3.0 1.5 0.8 2.0 2.5
Hydroxyethyl
Acrylate/Sodium
Acryloyldimethyl Taurate 1.0 0.5
Copolymer, Squalane,
Polysorbate 60
Glyceryl Stearate Citrate 1.80 2.00 3.00 1.50 2
Sucrose Stearate 1.00 1.20 2.00 2.20 1.5
Cetearyl Alcohol 1.80 2.00 1.50 2.40 2.8
Ethylhexyl Palmitate 6.00 5.00 3.50 3.00 5.5
Caprylic/Capric Triglyceride 5.00 5.00 1.00 2.00 3.5
Octyldodecanol 1.50 3.00 2.40 5.0 4.6
Dimethicone 0.20 0.50 2.00 1.80 1.4
Ammonium
Acryloyldimethyltaurate/ VP 0.5 0.1
Copolymer
Carbomer 0.05 0.1
Sodium Hydroxide 0.02 0.04
Bisabolol 0.5 0.3 0.20 0.35 1.0
Phenoxyethanol, paraben
1.00 0.60 0.70 0.60 0.5
mixture
Perfume 0.05 0.10 0.10 0.05 0.05
Preparation:
Heat phases A and B separately to ca. 80 C. Stir phase C into phase B and then
stir
phase A into phase B/C and briefly homogenize.
Add phase D (when required) and cool with stirring to ca. 40 C. Add components
of
phase E to the emulsion in succession and cool with stirring to room
temperature.
Briefly homogenize.
Instead of the 0/W emulsion comprising polyurethane thickener A.1, 0/W
emulsions
comprising one or more of the polyurethanes A.2, A.3, A.4, A.5 or A.6 are also
prepared.
CA 02815268 2013-04-19
WO 2012/052383 PCT/EP2011/068072
52
Hydrodispersion
Phase Ingredients/INCI F.4.1
F.4.2 F.4.3 F.4.4 F.4.5
A Stearyl alcohol 0.5 1.5 2.0
Cetyl alcohol 1.00 2.5
C12-15 Alkyl benzoate 2.5 4.0
Dicapryl ether 4.0 6.0
Butylene glycol
4.0 2.0 1.0
dicaprylate/dicaprate
Dicapryl carbonate 2.0 3.0 4.0
Cyclopentasiloxane,
2.0 0.5
cyclohexasiloxane
Prunus Amygdalus Dulcis (Sweet
2.0 0.5
Almond) oil
Shea butter 2.0 1.0
Hydrogenated polyisobutene 3.0 1.0 7.0 0.5 2.0
Squalane 2.0 0.5
Vitamin E acetate 0.50 0.25 1.00
Acrylate/C10-30 alkyl acrylate
0.3 0.1 0.2 0.15 0.2
crosspolymer
Aqua ad
ad 100 ad 100 ad 100 ad 100
100
Polyacrylamide, C13-14 isoparaffin,
1.0 1.5 0.75
Laureth-7
PUR thickener A.1 2.5 2.0 0.9 1.5 3.0
Propylene Glycol 3.00 5.0 2.5 7.50
10.0
Sodium Hydroxide 0.12 0.04 0.08 0.06
0.08
Niacinamide 0.30 3.0 1.5 0.5 0.20
Aqua 2.0 10.0 5.0 2.0 2.0
DMDM Hydantoin 0.60 0.45 0.25
Methylparaben 0.50 0.25 0.15
Phenoxyethanol 0.50 0.40 1.00
Ethylhexylglycerin 1.00 0.80
Ethanol 3.00 2.00 1.50 7.00
Fragrance 0.20 0.05 0.40
Preparation:
Heat phases A and C separately to ca. 80 C.
CA 02815268 2013-04-19
WO 2012/052383 PCT/EP2011/068072
53
Stir phase B into phase A and then phase C into phase A/B. Briefly homogenize.
Add
phase D and cool with stirring to ca. 40 C. Add phase E and cool with stirring
to
ca. 30 C. Add phase F and G to the emulsion and cool to room temperature with
stirring. Briefly homogenize.
Instead of the hydrodispersion comprising polyurethane thickener A.1,
hydrodispersions comprising one or more of the polyurethanes A.2, A.3, A.4,
A.5 or A.6
are also prepared.
Solids-stabilized emulsion
Phase Ingredients/INCI F.5.1
F.5.2 F.5.3 F.5.4 F.5.5
A Mineral oil 4.0 6.0 16.0 10.0 6.0
Octyldodecanol 9.0 9.0 5.0
Ethylhexyl isononanoate 9.0 9.0 6.0 5.0 8.0
lsohexadecane 9.0 5.0 4.0 8.0
Dimethicone 0.5 2.0 1.0 1.5
Cera Microcristallina, Paraffinum
0.35 0.75 3.0
Liquidum
Phenyl trimethicone 2.0 1.0 2.5 3.0
Silica 2.5 6.0 2.5
Aluminum starch
2.0 1.0 0.5
octenylsuccinate
Tapioca starch 0.5
Titanium dioxide, coated 1.0 0.5 3.0 2.0 4.0
Zinc oxide 5.0 10.0 2.0 3.0
Ammonium
Acryloyldimethyltaurate/
0.2 1.0 0.5
Beheneth-25 Methacrylate
Crosspolymer
Aqua ad
100 ad 100 ad 100 ad 100 ad 100
Hydroxypropyl Methylcellulose 0.1 0.05
Sorbitol 5.0 7.0 8.5 3.0 4.5
PUR thickener A.1 3.0 5.0 0.9 1.4 2.0
Mixed parabens 0.3 0.6 0.2 0.4
Phenoxyethanol 0.2 0.3 0.4 0.5 0.4
Diazolidinyl urea 0.23 0.2
Perfume 0.2 0.3 0.1
Preparation:
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
54
Heat phase A to 80 C.
Add phase B to phase A and homogenize for 3 min. Stir in phase C.
Allow cellulose (if required) to preswell in water, then add the remaining
ingredient of
phase D and heat to 80 C.
Stir phase D into phase A+B+C and homogenize. Cool emulsion with stirring to
ca. 40 C and add phase E and F. With stirring, cool to RT and homogenize.
Instead of the solids-stabilized emulsion comprising polyurethane thickener
A.1, solids-
stabilized emulsions comprising one or more of the polyurethanes A.2, A.3,
A.4, A.5 or
A.6 are also prepared.
Sunscreen cream
Phase Ingredients/INCI F.6.1 F.6.2 F.6.3 F.6.4 F.6.5
A Aqua ad 100 ad 100 ad 100 ad 100 ad 100
Disodium EDTA 0.1 0.1 0.1 0.1 0.1
Glycerin 3.00 7.50 8.0 7.50 5.00
Benzophenone-4 2.0 4.0
Phenylbenzimidazole
0.50 4.00 8.0
sulfonic acid
Triethanolamine 1.0 0.25 2.0 2.0 4.0
Panthenol 0.5 0.75 1.0
PUR thickener A.1 2.5g 0.5g 2.0 g 4.0 1.5
Xanthan gum 0.3 0.15 0.2
Octocrylene 8.0 7.5
Ethylhexyl
methoxycinnamate,
5.0 10.0 8.0 3.0 7.0
diethylamino hydroxybenzoyl
hexyl benzoate
Steareth-21 2.0 3.0 2.5
Steareth-2 1.5
PEG-40 stearate 1.0 2.0
Glycerin monostearate SE 1.0 3.0 1.5 1.5
Dibutyl adipate 3.0 5.0 3.5 2.5 2.0
Cetearyl alcohol 2.0 0.5 3.0
Stearyl alcohol 1.5 3.0 2.5 0.6 2.0
Butyrospermum Parkii
1.0 0.5 1.0 1.5
(Shea Butter)
Dimethicone 1.0 0.5 1.5 0.8 2.0
W02012/052383 CA 02815268 2013-04-19
PCT/EP2011/068072
PVP hexadecane copolymer 0.20 0.50 0.8 1.00
Bisabolol 0.2 0.1 0.3
DMDM hydantoin 0.5 0.5 0.5 0.5 0.75
Water, Aloe Barbadensis
0.5 1.0
Leaf Juice
Alpha-glucosylrutin 0.60 0.5 0.4 0.25 0.3
Perfume 0.10 0.25 0.30 0.40 0.20
Preparation:
5 Heat phases A and B separately to ca. 80 C.
Stir phase A into phase B and briefly homogenize.
Cool to ca. 40 C with stirring. Add components of phase C to the emulsion in
10 succession and cool to room temperature with stirring. Briefly
homogenize.
Instead of the sunscreen cream comprising polyurethane thickener A.1,
sunscreen
creams comprising one or more of the polyurethanes A.2, A.3, A.4, A.5 or A.6
are also
prepared.
Face cream with sodium ascorbyl phosphate
Phase Ingredients/INCI F.7.1 F.7.2 F.7.3 F.7.4 F.7.5
A Water ad 100 ad 100 ad 100 ad 100 ad 100
Butylene glycol 5.0 6.5 5.5 3.5 4.0
PUR thickener A.1 3.5 1.5 2.5 5.0 2.0
Xanthan gum 0.25 0.2 0.1
Potassium sorbate 0.1 0.1 0.1 0.1 0.1
I midazolidinyl urea 0.3 0.2
Potassium cetyl phosphate 1.5 2.0 1.9 2.5 1.0
Caprylic/capric triglyceride 2.0 5.0 3.0 4.0 2.5
Stearyl alcohol 0.5 1.5 2.0 1.0 3.0
Cetearyl alcohol, dicetyl
phosphate, Ceteth-10 1.5 2.0 1.8 1.9 2.1
phosphate
Simmondsia Chinensis
3.0 1.5 0.5 1.0 2.5
(Jojoba) seed oil
Mineral oil 2.0 5.0 10.0 7.5 4.0
Paraben mixture 0.2 0.2 0.2 0.2 0.2
Sodium ascorbyl phosphate 5.0 2.0 3.0 4.0 1.5
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
56
Lactic acid q.s. q.s. q.s. q.s. q.s.
Water 10.0 4.0 5.0 10.0 3.0
Tocopherol 0.1 0.2
Retinol 0.03 0.025 0.05
Fragrance 0.1 0.05 0.05 0.1 0.15
Preparation
Heat phases A and B separately to 80 C.
Stir phase A into phase B and homogenize.
Stir phase C into phase A+B and homogenize.
Cool to ca. 40 C with stirring. Adjust pH of phase C to < 6.5 with lactic
acid. Add phase
C and cool to ca. 30 C with stirring. Add phase D and E. Cool to room
temperature with
stirring and briefly homogenize.
Note: adjust pH of the emulsion to < 6.5 with lactic acid.
Instead of the face cream comprising polyurethane thickener A.1, face creams
comprising one or more of the polyurethanes A.2, A.3, A.4, A.5 or A.6 are also
prepared.
Hydroxycarboxyclic acid cream
Phase Ingredients/INCI F.8.1 F.8.2 F.8.3
A Ceteareth-6, stearyl alcohol 2.0 2.5
Ceteareth-25 2.0 2.5
PEG-100 stearate, glyceryl
3.5 0.5
stearate
Polyglycery1-3 distearate 2.0
Mineral oil 8.0 3.5 5.0
Cetearyl ethylhexanoate 7.0 5.5 4.0
Sorbitan stearate 0.5 1.5 0.5
Cera Alba 0.5 1.0
Cetyl alcohol 1.5 3.5 4.0
Dimethicone 0.2 2.0 0.5
Panthenol 1.0 0.5 0.3
Propylene glycol 3.0 2.0 5.0
PUR thickener A.1 1.0 3.0 5.0
. .
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
s
57
Hydroxy acid 3.0 7.0
10.0
Aqua ad 100 ad 100 ad
100
C Sodium hydroxide q.s. q.s.
q.s.
D Bisabolol 0.2 0.1
0.3
Preservative q.s. q.s.
q.s.
Fragrance q.s. q.s.
q.s.
Note
Alpha-hydroxy acids: lactic acid, citric acid, mac acid, glycolic acid
Dihydroxy acid: tartaric acid
Beta-hydroxy acid: salicylic acid
Adjust pH to > 3
Preparation
Heat phase A and B separately to ca. 80 C. Adjust pH of phase B to >3 if
necessary
using NaOH. Stir phase B into phase A, briefly homogenize.
Cool to ca. 40 C with stirring, add components of phase D in succession,
homogenize
again.
Instead of the hydroxycarboxylic acid cream comprising polyurethane thickener
A.1,
hydroxycarboxylic acid creams comprising one or more of the polyurethanes A.2,
A.3,
A.4, A.5 or A.6 are also prepared.
Emulsion with deodorant active ingredient
Phase Ingredients/INCI
F.9.1 F.9.2 F.9.3 F.9.4 F.9.5
Ceteareth-6, stearyl alcohol 1.5 2.0
1.0
Ceteareth-25 1.5 0.5
1.0
PEG-40 hydrogenated castor
0.5 1.0 2.0
oil
Glyceryl stearate 0.5 2.0 1.0
Cetyl alcohol 2.0 1.0 0.5 2.5
0.2
Hydrogenated coco-glycerides 2.0 1.0
0.5
Hydrogenated polyisobutene 10.0 20.0
5.0 3.0
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
58
Decyl oleate 3.0 2.0 8.0 5.0
Bis-PEG/PPG-14/14
dimethicone, 3.0 3.5 4.0 2.0 1.5
cyclopentasiloxane
Talc 3.0 2.5 1.5
Magnesium aluminum silicate 1.0 0.5 1.0 1.5
Propylene glycol 10.0 5.0 7.5 20.0 15.0
PUR thickener A.1 0.5 1.0 3.0 3.5 2.0
Xanthan gum 0.2 0.1 0.05
Cetyl hydroxyethylcellulose 0.3 0.1
Aluminum chlorohydrate 5.0 10.0 20.0
Aluminum zirconium
15.0 50.0 20.0
tetrachlorohydrex GLY
Aqua ad 100 ad 100 ad 100 ad 100 ad 100
C Neutralizing agent q.s. q.s. q.s. q.s. q.s.
Alcohol 5.0 10.0 25.0 7.5 6.0
Allantoin 0.1 0.1 0.1 0.1 0.1
Preservative q.s. q.s. q.s. q.s. q.s.
Fragrance q.s. q.s. q.s. q.s. q.s.
Preparation
Heat phases A and B separately to ca. 80 C.
Stir phase B into phase A with homogenization. If appropriate, adjust to pH 4-
5 using
phase C. Cool to ca. 40 C, add phase D and allow to cool to room temperature
with
stirring. Briefly homogenize.
Note: adjust pH of the emulsion to 4-5
Instead of the emulsion with deodorant active ingredient comprising
polyurethane
thickener A.1, emulsions with deodorant active ingredient comprising one or
more of
the polyurethanes A.2, A.3, A.4, A.5 or A.6 are also prepared.
Hair removal cream
Phase Ingredients/INCI F.10.1 F.10.2 F.10.3
A Glyceryl stearate 1.0
Ceteareth-12 1.0 2.0
Ceteareth-20 1.0 2.0
Stearyl alcohol 4.0 1.0
W020121052383 CA 02815268 2013-04-19
PCT/EP2011/068072
59
Cetyl alcohol 4.0 1.0
Mineral oil 6.0 4.0
Prunus Armeniaca (Apricot)
3.0 1.0 2.0
kernel oil
Propylene glycol 1.0 2.0 10.0
Calcium carbonate 10.0
Calcium hydroxide 7.0
Sodium hydroxide 0.4 0.6
Calcium thioglycolate 5.0 3.0 5.0
PUR thickener A.1 3.0 1.5 2.0
Aqua ad 100 ad 100 ad 100
Tocopherol 0.1 0.2 0.15
Bisabolol 0.2 0.1 0.3
Fragrance q.s. q.s. q.s.
Preparation
Heat phase A and B separately to ca. 80 C.
Stir phase B into phase A with homogenization, briefly homogenize.
Cool to ca. 40 C, add phase C, cool to RT with stirring and homogenize again.
Note: adjust pH of the emulsion to > 10.
Instead of the hair removal cream comprising polyurethane thickener A.1, hair
removal
creams comprising one or more of the polyurethanes A.2, A.3, A.4, A.5 or A.6
are also
prepared.
, * CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
Self-tanning emulsion
Phase Ingredients/INCI F.11.1 F.11.2
F.11.3 F.11.4
A Isohexadecane 4.0 2.0 3.0
1.0
Dimethicone 1.0 1.0 0.5
1.5
Cetearyl alcohol 2.0 2.5 1.5
2.5
Isopropyl myristate 1.0 2.0
3.0
Simmondsia Chinensis (Jojoba)
2.0 1.0 0.5
0.5
seed oil
Polyglycery1-3 methylglucose
3.0 3.5
distearate .
PEG-40 stearate 2.5
2.0
Lecithin 0.5
1.0
Cetearyl glucoside 0.5 0.5
Sorbitan oleate 0.5 0.5
0.3
B Glycerin 4.0
5.0
Butylene glycol 4.0 3.0
PUR thickener A.1 1.0 3.0 1.5
2.5
Xanthan gum 0.1
0.1
Aqua
ad 100 ad 100 ad 100 ad 100
C Dihydroxyacetone 1.5 5.0
Erythrulose 2.0
4.0
Aqua 5.0 10.0 5.0
8.0
Citric acid q.s. q.s. q.s.
q.s.
D Bisabolol 0.3 0.5 0.2
0.4
Tocopheryl acetate 0.7 0.5 0.6
1.0
Preservative q.s. q.s. q.s.
q.s.
Fragrance q.s. q.s. q.s.
q.s.
Preparation
5
Heat phases A and B separately to ca. 80 C.
Stir phase B into phase A and briefly homogenize.
10 With stirring, cool to ca. 40 C, add phase C and cool to 30 C
with stirring. Add
components of phase D in succession and cool to RT with stirring. Briefly
homogenize.
Note: adjust pH of the emulsion to 4-5.5
W02012/052383 CA 02815268 2013-04-19
PCT/EP2011/068072
61
Instead of the self-tanning emulsion comprising polyurethane thickener A.1,
self-
tanning emulsions comprising one or more of the polyurethanes A.2, A.3, A.4,
A.5 or
A.6 are also prepared.
Conditioner shampoo
Ingredients/INCI F.12.1 F.12.2 F.12.3 F.12.4
Aqua ad 100 ad 100 ad 100 ad 100
Sodium laureth sulfate 35.7 30.0 12.0
Cocamidopropyl betaine 13.5 15.0
Disodium
10.0
cocoamphodiacetate
Sodium cocoamphoacetate 6.0
Polysorbate 20 5.0
Decyl glucoside 5.0 1.5
Laureth-3 2.0
Sodium laureth sulfate,
glycol distearate, cocamide 3.0 2.0
MEA, laureth-10
Coco-glucoside, glyceryl
5.0
oleate
Dimethicone 2.0
Conditioning polymer 2.0 0.5 0.75 0.4
PUR thickener A.1 0.75 1.2 0.5 1.0
PEG-150 distearate 3.0
Citric acid q.s. q.s.
Preservative q.s. q.s. q.s. q.s.
Fragrance q.s. q.s. q.s. q.s.
Dye q.s. q.s. q.s. q.s.
Sodium chloride 1.0 1.0
Conditioning polymer is understood as meaning polyquaternium-7, PQ-10, PQ-16,
PQ-39, PQ-44, PQ-46, PQ-67, guar hydroxypropyltrimonium chloride, PQ-87, and
combinations of these.
Instead of the conditioner shampoo comprising polyurethane thickener A.1,
conditioner
shampoos comprising one or more of the polyurethanes A.2, A.3, A.4, A.5 or A.6
are
also prepared.
CA 02815268 2013-04-19
WO 2012/052383
PCT/EP2011/068072
62
Hair conditioner
Phase I ngredients/INCI F.13.1 F.13.2 F.13.3 F.13.4 F.13.5
A Water ad 100 ad 100 ad 100 ad 100 ad 100
PUR thickener A.1 2.5 1.5 3.0 0.6 2.0
Hydroxyethylcellulose 0.05 0.1 0.2
Propylene glycol 1.0 2.0 0.8 0.5
Panthenol 0.5 0.75 0.25
0.3
B Quaternium-91, cetearyl alcohol,
2.0 1.5
cetrimonium methosulfate
Distearoyethyl hydroxyethylmonium
3.0 4.0
methosulfate, cetearyl alcohol
Hydrogenated polyisobutene 1.0 1.5 1.0
Cyclopentasiloxane 2.0 1.0 0.5
Isopropyl palmitate 1.0 2.0
Persea Gratissima (Avocado) oil 2.5
Steareth-2 0.75 0.5
Ceteareth-6, stearyl alcohol 1.5 0.5
Ceteareth-25 1.5
Cetearyl alcohol 2.0 1.5 0.5 4.0
C Acrylate/C10-30 alkylacrylate
0.1 0.2 0.15
copolymer
D Cetrimonium chloride 1.5 3.0
Conditioning polymer 2.0 6.0 3.0 1.5 0.8
E Preservative q.s. q.s.
q.s. q.s. q.s.
Fragrance q.s. q.s. q.s. q.s.
q.s.
Conditioning polymer is understood as meaning polyquaternium-7, PQ-10, PQ-16,
PQ-39, PQ-44, PQ-46, PQ-67, guar hydroxypropyltrimonium chloride, PQ-87, and
combinations of these.
Preparation
Heat phases A and B separately to ca. 80 C.
Stir phase C into phase B, then stir phase A into phase B/C and briefly
homogenize.
,
CA 02815268 2013-04-19
, WO 2012/052383
PCT/EP2011/068072
63
With stirring, cool to ca. 50 C, add components of phase D in succession and
cool to
ca. 30 C with stirring. Add components of phase E in succession and cool to RT
with
stirring. Briefly homogenize.
Instead of the hair conditioner comprising polyurethane thickener A.1, hair
conditioners
comprising one or more of the polyurethanes A.2, A.3, A.4, A.5 or A.6 are also
prepared.