Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02816869 2013-05-22
1
COMPOUND HAVING XANTHENE STRUCTURE, COLORING COMPOSITION,
INK FOR INKJET RECORDING AND INKJET RECORDING METHOD
BACKGROUND
1. Field
The present invention relates to a compound having a xanthene structure, a
coloring
composition containing a copper compound, an ink for inkjet recording and an
inkjet
recording method.
2. Description of the Related Art
An inkjet recording method is a method of printing by dispersing an ink
droplet and
attaching it to a recording medium such as paper as known in the related art.
By this printing
method, it is possible to print a high-resolution and high-quality image
conveniently at a high
speed. In particular, in color printing, a technical development has been
recently performed
for an image forming method which can replace photographs.
In the case of forming a color image by using an inkjet recording method, it
is common
to use a yellow ink, a magenta ink, a cyan ink and a black ink.
Conventionally, water-based
inks have been mainly used as these inkjet inks in terms of safety, such as
malodor and hazard
associated with fire-fighting. These inks are required to fail within suitable
ranges in
viscosity, surface tension, and the like, to be excellent in nozzle clogging
and storage stability,
to impart a recording image at a high concentration, and to be excellent in
light fastness, ozone
fastness, water fastness and moisture fastness.
Such a performance is mostly satisfied by using a water-based ink containing
water or
a mixture of water and a water-soluble organic solvent as a main solvent.
However,
characteristics such as color tone, brighteness, light fastness, ozone
fastness, water fastness
and moisture fastness are influenced considerably by colorants and additives,
and various dyes
have conventionally been studied.
In a color recording method using a plurality of color inks, uniform
characteristics are
required to all the constituting ink. Particularly, a magenta dye has a
problem in that
decolorization by ozone or light (sunlight, fluorescent light and the like) or
change in color
tone occurs remarkably, compared to other dyes (a cyan dye and a yellow dye).
Accordingly,
if the ozone fastness or light fastness of the magenta ink is inferior to that
of other inks,
decolorization of the magenta ink causes the color tone of the whole image of
printed matters
to be changed, resulting in deteriorating the quality.
CA 02816869 2013-05-22
2
Conventionally, an acidic dye having good color strength and high water
solubility,
such as C. I. Acid Red 52, 249 or 289, is known as a magenta dye for inkjet.
However, when
such a dye is used alone, clogging of a nozzle hardly occurs due to the high
water solubility,
but the performances of the ozone fastness, light fastness and moisture
fastness are
considerably low.
Japanse Patent Application Laid-Open No. H9-157562 (hereinafter JP-A-9-157562)
disloses a water-based ink for inkjet recording containing a xanthene
derivative colorant
having one sulfo group and one sulfonamide group, which is excellent in light
fastness.
Further, for a colorant for uses other than an inkjet ink, U.S. Patent
Application
Laid-Open No. 2006/0230545 (hereinafter US 2006/0230545) discloses a xanthenes
derivative
having one sulfo group and two sulfonamide groups, or a xanthenes derivative
having one
carboxyl group and one sulfonyl group having an amino acid group, as a
colorant suitably
used in a hair dye.
Japanse Patent Application Laid-Open No. 2005-250000 (hereinafter
JP-A-2005-250000) and Japanse Patent Application Laid-Open No. H7-179796
(hereinafter
JP-A-7-179796) describe a puporse of incorporating a sulfonamide group into a
xanthene
derivative having one sulfo group, as a colorant suitably used in a toner or a
color filter.
Howerver, in JP-A-9-157562, there is no description about a moisture fastness
of the
ink described therein, which is a performance needed to be used as an inkjet
ink. Therefore,
there is still room left for review. Also in US 2006/0230545, JP-A-2005-
250000, and
JP-A-7-179796, there are no description about a purpose of using as the
described colorant as
an inkjet ink and also about moisture fastness.
An object of the present invention is to provide a compound capable of forming
an
image which is excellent in moisture fastness as well as hue, print
concentration and ozone
fastness, a coloring composition containing the compound, an ink for inkjet
recording, and an
inkjet recording method using the compound.
In order to solve the above-mentioned problems, the present inventors have
studied
intensively and have found a coloring composition containing a compound
represented by
Formula (1) as described below. And, the present inventors have found out that
it is possible
to form an image which is excellent in moisture fastness as well as hue, print
concentration
and ozone fastness by employing the coloring composition.
That is, the present invention is as follows. Further, in the present
specification, "to"
indicates a range including the numerical values described before and after
"to" as a minimum
value and a maximum value, respectively.
CA 02816869 2013-05-22
3
SUMMARY
(1) A coloring composition containing a compound represented by
Formula (1):
SO3M)m
ISO2NRIR2) Formula (1)
n
wherein D represents a residue structure in which four hydrogen atoms are
removed
from the compound represented by Formula (2), R1 and R2 represent R1=R2=H, or
R1=R and
R2=-L-0O2M, R represents a hydrogen atom or a monovalent substituent, and when
a plurality
of R is present, R's may be the same or different, L represents a divalent
linking group, and
when a plurality of L is present, L's may be the same or different, M
represents a hydrogen
atom or a counteraction, and when a plurality of M is present, M's may be the
same or
different, and n represents the number of 1 to 4, and m represents the number
of 0 to 3,
provided that m+n is 4:
P-7(-R1 1)n101 R1021
/n102
= o) 40
Rio4 R105
Formula (2)
803¨
I 77* ---(R1 3)
= n103
wherein R101, Rto2 and ¨103
it each independently represent a monovalent
substituent,
R104 and R105
each independently represent a hydrogen atom or a monovalent substituent, and
nun and n102 each independently represent the number of 0 to 5, and n103
represents 0 to 4, and
when nwl, n102 and nun each represent the number of 2 or more, a plurality of
R' 1 may be the
same or different, a plurality of R1 2 may be the same or different, and a
plurality of R1 3 may
be the same or different (hereinafter, referred to as "each of pluralities of
Run, Rua and Rue
may be the same or different.", This expression may be applied to other
numerical references
in this specification).
(2) The coloring composition according to (1), wherein the compound
represented
by Formula (1) is a compound represented by the following Formula (3):
CA 02816869 2013-05-22
4
SO 2R*
SO2R3
I (R2a1)..-14R202) n202
n201
0 NH
HA
Formula (3)
R30 S S02R3
SO3-
"i"-}R.2 3 )n203
wherein when R1 and R2 are R1=R2=H, R3's each independently represent OM or
NH2,
provided that, when R1 and R2 are RI=R2=H, at least one of R3's represent NH2,
when R1 and
R2 are R1=R and R2=-L-0O2M, R3's each independently represent OM or NR-L-0O2M,
provided that when R1 and R2 are R1=R and R2=-L-0O2M, at least one of R3's
represent
NR-L-0O2M, R represents a hydrogen atom or a monovalent substituent, and when
a plurality
of R is present, R's may be the same or different, L represents a divalent
linking group, and
when a plurality of L is present, L's may be the same or different, R201, R202
and R.203 each
independently represent a monovalent substituent, n201 and n202 each
independently
represent the number of 0 to 4, n203 represents the number of 0 to 4, and when
n201, n202
and n203 each represent the number of 2 or more, each of pluralities of R201,
R202 and R203
may be the same or different, and M represents a hydrogen atom or a
counteraction, and when
a plurality of M is present, M's may be the same or different.
(3)
The coloring composition according to (1) or (2), wherein R1 1, R103, R201,
R202 and R203
each independently represent an alkyl group or an acylamino group.
(4) The coloring composition according to any one of (1) to (3), wherein
R101, Rio2,
R103, R201, R202 and K. ¨203
each independently represent an alkyl group having 1 to 6 carbon
atoms.
(5) The coloring composition according to any one of (1) to (4), wherein
n101,
n102, n201 n202 each independently represent the number of 2 to 4.
(6) The coloring composition according to any one of (1) to (5), wherein
n103 and
n203 represent 0.
(7) The coloring composition according to any one of (1) to (6), wherein M
is a
lithium ion, a sodium ion or a potassium ion.
CA 02816869 2013-05-22
(8) The coloring composition according to any one of (1) to (7), wherein
the
compound represented by Formula (1) is contained in an amount of 1% by mass to
20% by
mass.
(9) An ink for inkjet recording including the coloring composition
according to any
one of (1) to (8).
(10) An inkjet recording method including forming an image by using the
coloring
composition according to any one of (1) to (8) or the ink for inkjet recording
according to (9).
(11) A compound represented by Formula (1):
SO3M)m
Formula (1)
ItO2NRIR2)ri
wherein D represents a residue structure in which four hydrogen atoms are
removed
from the compound represented by Formula (2), RI and R2 represent RI=R2=H, or
R1=R and
R2=-L-0O2M, R represents a hydrogen atom or a monovalent substituent, and when
a plurality
of R is present, R's may be the same or different, L represents a divalent
linking group, and
when a plurality of L is present, L's may be the same or different, M
represents a hydrogen
atom or a counteraction, and when a plurality of M is present, M's may be the
same or
different, and n represents the number of 1 to 4, and m represents the number
of 0 to 3,
provided that m+n is 4:
n101 n102
0 N+
Ri 4 4111 NR1a5
Formula (2)
SO3-
-;;-%4FZID3)n103
wherein R101, R102 and R103 each independently represent a monovalent
substituent,
Rio4 and K-105
each independently represent a hydrogen atom or a monovalent substituent, and
n'0' and n102 each independently represent the number of 0 to 5, and n103
represents 0 to 4 and
when n101, n102 and niO3 each represent the number of 2 or more, each of
pluralities of R1 1,
tc and 103 may be the same or different.
According to the present invention, there are provided a compound capable of
forming
CA 02816869 2013-05-22
6
an image which is excellent in moisture fastness as well as hue, print
concentration and ozone
fastness, a coloring composition containing the compound, an ink for inkjet
recording, and an
inkjet recording method using the compound.
Hereinafter, the present invention will be described in detail.
First, in the present invention, Group A of substituents will be defuaed.
(Group A of substituents)
Examples may include a halogen atom, an alkyl group, an aralkyl group, an
alkenyl
group, an alkynyl group, an aryl group, a heterocyclic group, a cyano group, a
hydroxyl group,
a nitro group, an alkoxy group, an aryloxy group, silyloxy group, a
heterocyclic oxy group, an
acyloxy group, a carbamoyloxy group, an alkoxycarbonyloxy group, an
aryloxycarbonyloxy
group, an amino group, an acylamino group, an aminocarbonylamino group, an
alkoxycarbonylamino group, an aryloxycarbonylamino group, a sulfamoylamino
group, an
alkyl- or arylsulfonylamino group, a mercapto group, an alkylthio group, an
arylthio group, a
heterocyclic thio group, a sulfamoyl group, an alkyl- or arylsulfinyl group,
an alkyl- or
arylsulfonyl group, an acyl group, an aryloxycarbonyl group, an alkoxycarbonyl
group, a
carbamoyl group, an aryl- or heterocyclic azo group, an imide group, a
phosphino group, a
phosphinyl group, a phosphinyloxy group, a phosphinylamino group, a silyl
group and an
ionic hydrophilic group. These substituents may be further substituted, and
the further
substituent may be exemplified by Group A of substituents as described above.
Examples of the halogen atom may include a fluorine atom, a chlorine atom, a
bromine
atom or an iodine atom.
Examples of the alkyl group may include a straight, branched or cyclic,
substituted or
unsubstituted alkyl group, including a cycloalkyl group, a bicycloalkyl group
and a tricycle
structure which has more cyclic structures. The alkyl group (for example, an
alkyl group in
an alkoxy group or an allcylthio group) in substituents as describred below
also represents an
alkyl group of such a concept.
Examples of the alkyl group may include preferably an alkyl group having 1 to
30
carbon atoms, such as a methyl group, an ethyl group, an n-propyl group, an i-
propyl group, a
t-butyl group, a n-octyl group, an eicosyl group, a 2-chloroethyl group, 2-
cyanoethyl group
and a 2-ethylhexyl group. Examples of the cycloalkyl group may include
preferably a
substituted or unsubstituted cycloalkyl group having 3 to 30 carbon atoms,
such as a
cyclohexyl group, a cyclopentyl group and a 4-n-dodecylcyclohexyl group.
Examples of the
bicycloalkyl group may include preferably a substituted or unsubstituted
bicycloalkyl group
having 5 to 30 carbon atoms, that is, a monovalent group in which one hydrogen
atom is
CA 02816869 2013-05-22
7
removed from a bicyloalkane having 5 to 30 carbon atoms, such as a bicyclo[1,
2,
2]heptan-2-y1 group and a bicyclo [2, 2, 2]octan-3-y1 group.
Examples of the aralkyl group may include a substituted or unsubstituted
aralkyl group,
and examples of the substituted or unsubstituted aralkyl group may include
preferably an
aralkyl group having 7 to 30 carbon atoms. Examples thereof may include a
benzyl group
and a 2-phenethyl group.
Examples of the alkenyl group may include a straight, branched or cyclic,
substituted
or unsubstituted alkenyl group, including a cycloalkenyl group and a
bicycloalkenyl group.
Examples of the alkenyl group may include a substituted or unsubstituted
alkenyl
group having 2 to 30 carbon atoms, such as a vinyl group, an allyl group, a
prenyl group, a
geranyl group and an oleyl group. Examples of the cycloalkenyl group may
include
preferably a substituted or unsubstituted cycloalkenyl group having 3 to 30
carobn atoms, that
is, a monovalent group in which one hydrogen atom is removed from a
cycloalkene having 3
to 30 carbon atoms, such as a 2-cyclopenten- 1 -y1 group and a 2-cyclohexen- 1-
yl group.
Examples of the bicycloalkenyl group may include a substituted or
unsubstituted
bicycloalkenyl group, preferably a substituted or unsubstituted bicycloalkenyl
group having 5
to 30 carbon atoms, that is, a monovalent group in which one hydrogen atom is
removed from
a bicycloakene having one double bond, such as a bicyclo[2, 2, 1] hept-2-en- 1
-y1 group and a
bicyclo[2, 2, 2] oct-2-en-4-y1 group.
Examples of the alkynyl group may include preferably a substituted or
unsubstituted
alkynyl group having 2 to 30 carbon atoms, such as an ethynyl group, a
propargyl group and a
trimethylsilylethynyl group.
Examples of the aryl group may include preferably a substituted or
unsubstituted aryl
group having 6 to 30 carbon atoms, such as a phenyl group, a p-tolyl group, a
naphthyl group,
a m-chlorophenyl group and an o-hexadecanoylaminophenyl group.
Examples of the heterocyclic group may include preferably a monovalent group
in
which one hydrogen atom is removed from a 5- or 6-membered substituted or
unsubstituted
aromatic or non-aromatic hetrocyclic compound, more preferably a 5- or 6-
membered
aromatic heterocyclic group having 3 to 30 carbon atoms, such as a 2-furyl
group, a 2-thienyl
group, a 2-pyrimidinyl group and a 2-benzothiazoly1 group.
Examples of the alkoxy group may include a substituted or unsubstituted alkoxy
group
having 1 to 30 carbon atoms, such as a methoxy group, an ethoxy group, an
isopropoxy group,
a t-butoxy group, an n-octyloxy group and a 2-methoxyethoxy group_
Examples of the aryloxy group may include preferably a substituted or
unsubstituted
CA 02816869 2013-05-22
8
aryloxy group having 6 to 30 carbon atoms, such as a phenoxy group, a 2-
methylphenoxy
group, a 4-t-butylphenoxy group, a 3-nitrophenoxy group and a 2-
tetradecanoylaminophenoxy
group.
Examples of the silyloxy group may include preferably a substituted or
unsubstituted
silyloxy group having 0 to 20 carbon atoms, such as a trimethylsilyloxy group
and a
diphenylmethylsilyloxy group.
Examples of the heterocyclic oxy group may include preferably a substituted or
unsubstituted heterocyclic oxy group having 2 to 30 carbon atoms, such as a
1-phenyltetrazol-5-oxy group and a 2-tetrahydropyranyloxy group.
Examples of the acyloxy group may include preferably a formyloxy group, a
substituted or unsubstituted alkylcarbonyloxy group having 2 to 30 carbon
atoms and a
substituted or unsubstituted arylcarbonyloxy group having 6 to 30 carbon
atoms, such as an
acetyloxy group, a pivaloyloxy group, a stearoyloxy group, a benzoyloxy group
and a
p-methoxyphenylcarbonyloxy group.
Examples of the carbamoyloxy group may include preferably a substituted or
=substituted carbamoyloxy group having 1 to 30 carbon atoms, such as an
N,N-dimethylcarbamoyloxy group, an N,N-diethylcarbamoyloxy group, a
morpholinocarbonyloxy group, an N,N-di-n-octylaminocarbonyloxy group and an
N-n-octylcarbamoyloxy group.
Examples of the alkoxycarbonyloxy group may include preferably a substituted
or
unsubstituted alkoxycarbonyloxy group having 2 to 30 carbon atoms, such as a
methoxycarbonyloxy group, an ethoxycarbonyloxy group, a t-butoxycarbonyloxy
group and a
n-octylcarbonyloxy group.
Examples of the aryloxycarbonyloxy group may include preferably a substituted
or
unsubstituted aryloxycarbonyloxy group having 7 to 30 carbon atoms, such as a
phenoxycarbonyloxy group, a p-methoxyphenoxycarbonyloxy group and a
p-n-hexadecyloxyphenoxycarbonyloxy group.
Examples of the amino group may include an alkylamino group, an arylamino
group
and a heterocyclic mina group, preferably an amino group, a substituted or
unsubstituted
alkylamino group having 1 to 30 carbon atoms and a substituted or
unsubstituted anilino group
having 6 to 30 carbon atoms, such as a methylamino group, a dimethylamino
group, an anilino
group, an N-methyl-anilino group, a diphenylamino group and a triazinylamino
group.
Examples of the acylamino group may include preferably a formylamino group, a
substituted or unsubstituted alkylcarbonylamino group having 1 to 30 carbon
atoms and a
CA 02816869 2013-05-22
9
substituted or unsubstituted arylcarbonylamino group having 6 to 30 carbon
atoms, such as an
acetylamino group, a pivaloylamino group, a lauroylamino group, a benzoylamino
group and a
3,4,5-tri-n-octyloxyphenylcarbonylaraino group.
Examples of the aminocarbonylamino group may include preferably a substituted
or
unsubstituted aminocarbonylamino group having 1 to 30 carbon atoms, such as a
carbamoylamino group, an N,N-dimethylaminocarbonylamino group, an
N,N-diethylaminocarbonylamino group and a morpholinocarbonylamino group.
Examples of the alkoxycarbonylamino group may include preferably a substituted
or
unsubstituted alkoxycarbonylamino group having 2 to 30 carbon atoms, such as a
methoxycarbonylamino group, an ethoxycarbonylamino group, a t-
butoxycarbonylamino
group, a n-octadecyloxycarbonylamino group and an N-methyl-
methoxycarbonylamino group.
Examples of the aryloxycarbonylarnino group may include preferably a
substituted or
unsubstituted aryloxycarbonylamino group having 7 to 30 carbon atoms, such as
a
phenoxycarbonylamino group, a p-chlorophenoxycarbonylamino group and a
m-n-octyloxyphenoxycarbonylamino group.
Examples of the sulfamoylamino group may include preferably a substituted or
unsubstituted sulfamoylamino group having 0 to 30 carbon atoms, such as a
sulfamoylamino
group, an N,N-dimethylarninosulfonylamino group and an N-n-
octylaminosulfonylamino
group.
Examples of the alkyl- or arylsulfonylamino group may include a substituted or
unsubstituted alkylsulfonylamino group having 1 to 30 carbon atoms and a
substituted or
unsubstituted arylsulfonylamino group having 6 to 30 carbon atoms, such as a
methylsulfonylamino group, a butylsulfonylamino group, a phenylsulfonylamino
group, a
2,3,5-trichlorophenylsulfonylamino group and a p-methylphenylsulfonylamino
group.
Examples of the alkylthio group may include preferably a substituted or
unsubstituted
alkylthio group having 1 to 30 carbon atoms, such as a methylthio group, an
ethylthio group
and a n-hexadecylthio group.
Examples of the arylthio group may include preferably a substituted or
unsubstituted
arylthio group having 6 to 30 carbon atoms, such as a phenylthio group, a p-
clalorophenylthio
group and a m-methoxyphenylthio group.
Examples of the heterocyclic thio group may include preferably a substituted
or
unsubstituted heterocyclic thio group having 2 to 30 carbon atoms, such as a
2-benzothiazolylthio group and a 1-phenyltetrazol-5-ylthio group.
Examples of the sulfamoyl group may include preferably a substituted or
unsubstituted
CA 02816869 2013-05-22
sulfamoyl group having 0 to 30 carbon atoms, such as an N-ethylsulfamoyl
group, an
N-(3 -dodecyloxypropyl) sulfamoyl group, an N,N-dimethylsulfamoyl group, an
N-acetylsulfamoyl group, an N-benzoylsulfarrioyl group and
an
N-(Nt-phenylcarbamoyl)sulfamoyl group.
Exmples of the alkyl- or arylsulfmyl group may include preferably a
substituted or
unsubstituted alkylsulfinyl group having 1 to 30 carbon atoms and a
substituted or
unsubstituted arylsulfinyl group having 6 to 30 carbon atoms, such as a
methylsulfinyl group,
an ethylsulfinyl group, a phenylsulfinyl group and a p-methylphenylsulfinyl
group.
Examples of the alkyl- or arylsulfonyl group may include preferably a
substituted or
unsubstituted allcylsulfonyl group having 1 to 30 carbon atoms and a
substituted or
unsubstituted arylsulfonyl group having 6 to 30 carbon atoms, such as a
methylsulfonyl group,
an ethylsulfonyl group, a phenylsulfonyl group and a p-methylphenylsulfonyl
group.
Examples of the acyl group may include preferably a formyl group, a
substituted or
unsubstituted alkylcarbonyl group having 2 to 30 carbon atoms, a substituted
or unsubstituted
arylcarbonyl group having 7 to 30 carbon atoms, a substituted or unsubstituted
heterocyclic
carbonyl group having 2 to 30 carbon atoms which is bound via a carbon atom to
a carbonyl
group, such as an acetyl group, a pivaloyl group, a 2-chloroacetyl group, a
stearoyl group, a
benzoyl group, a p-n-octyloxyphenylcarbonyl group, a 2-pyridylcarbonyl group
and a
2-furylcarbonyl group.
Examples of the aryloxycarbonyl group may include preferably a substituted or
unsubstituted aryloxycarbonyl group having 7 to 30 carbon atoms, such as a
phenoxycarbonyl
group, an o-chlorophenoxycarbonyl group, a m-nitrophenoxycarbonyl group and a
p-t-butylphenoxycarbonyl group.
Examples of the alkoxycarbonyl group may include preferably a substituted or
unsubstituted alkoxycarbonyl group having 2 to 30 carbon atoms, such as a
methoxycarbonyl
group, an ethoxycarbonyl group, a t-butoxycarbonyl group and a n-
octadecyloxycarbonyl
group.
Examples of the carbamoyl group may include preferably a substituted or
unsubstituted
carbamoyl group having 1 to 30 carbon atoms, such as a carbamoyl group, an
N-methylcarbamoyl group, an N,N-dimethylcarbamoyl group, an N, N-di-n-
octylcarbamoyl
group and an N-(methylsulfonyl)carbamoyl group.
Examples of the aryl- or heterocyclic azo group may include preferably a
substituted or
unsubstituted arylazo group having 6 to 30 carbon atoms and a substituted or
=substituted
heterocyclic azo group having 3 to 30 carbon atoms, such as phenylazo, p-
chlorophenylazo
CA 02816869 2013-05-22
11
and 5-ethylthio-1,3,4-thiadiazol-2-ylazo.
Examples of the imide group may include preferably an N-succinimide group and
an
N-phthalimide group.
Examples of the phosphino group may include preferably a substituted or
unsubstituted
phosphino group having 0 to 30 carbon atoms, such as a dimethylphosphino
group, a
diphenylphosphino group and a methylphenoxyphosphino group.
Examples of the phosphinyl group may include preferably a substituted or
unsubstituted phosphinyl group having 0 to 30 carbon atoms, such as a
phosphinyl group, a
dioctyloxyphosphinyl group and a diethoxyphosphinyl group.
Examples of the phosphinyloxy group may include preferably a substituted or
unsubstituted phosphinyloxy group having 0 to 30 carbon atoms, such as a
diphenoxyphosphinyloxy group and a dioctyloxyphosphinyloxy group.
Examples of the phosphinylamino group may include preferably a substituted or
unsubstituted phosphinylamino group having 0 to 30 carbon atoms, such as a
dimethoxyphosphinylamino group and a dimethylaminophosphinyla.mino group.
Examples of the silyl group may include preferably a substituted or
unsubstituted silyl
group having 0 to 30 carbon atoms, such as a trimethylsilyl group, a t-
butyldimethylsilyl group
and a phenyldimethylsilyl group.
Examples of the ionic hydrophilic group may include a sulfo group, a carboxyl
grup, a
thiocarboxyl group, a sulfino group, a phosphono group, a dihydroxyphosphino
group, a
quaternary ammonium group and the like. The ionic hydrophilic group is
particularly
preferably a sulfo group or a carboxyl group. Further, the carboxyl group, the
phosphono
group and the sulfo group may be in a form of a salt, and the paired cation
which forms a salt
includes an ammonium ion, an alkali metal ion (for example, a lithium ion, a
sodium ion and a
potassium ion) and an organic cation (for example, a tetramethylammonium ion,
a
tetramethylguanidium ion and tetramethylphosphonium), and is preferably a
lithium salt, a
sodium salt, a potassium salt or an ammonium salt, more preferably a sodium
salt or a mixture
salt containing a sodium salt being main component, and most preferably a
sodium salt.
Further, in the present invention, when the compound is a salt, the salt is
dissociated in
a water soluble ink, and is present as ions.
[Coloring composition]
The present invention relates to a coloring composition containing the
compound
represented by the following Formula (1).
CA 02816869 2013-05-22
12
D¨ESO3M)m
Formula (1)
\($02NR1R2)n
In Formula (1), D represents a residue structure in which four hydrogen atoms
are
removed from the compound represented by the following Formula (2).
R1 and R2 represent R1=R2=H, or R1=R and R2=-L-0O2M.
R represents a hydrogen atom or a monovalent substituent. When a plurality of
R is
present, R's may be the same or different.
L represents a divalent linking group. When a plurality of L is present, L's
may be
the same or different.
M represents a hydrogen atom or a countercation. When a plurality of M is
present,
M's may be the same or different.
n represents the number of 1 to 4, and m represents the number of 0 to 3,
provided that
m+n is 4.
f-.(R101)
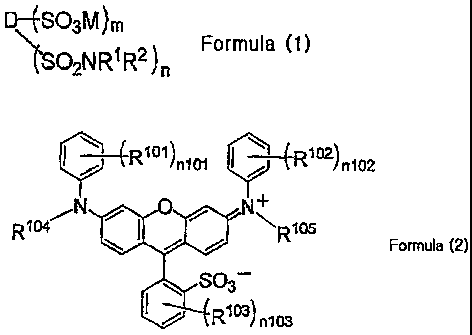
. n101 1 4R1 2)n102
RI04 0NR1 5
Formula (2)
SO3¨
n103
In Formula (2), R1 t) R102 and R103 each independently represent a monovalent
substituent, R1 4 and R1 5 each independently represent a hydrogen atom or a
monovalent
substituent, n101 and n102 each independently represent a number of 0 to 5,
and n1 3 represents
0 to 4. When n101, nIO2 and n103 each represents the number of 2 or more, each
of pluralities
of R101, R102 and ¨103
may be the same or different.
(Compound represented by Formula (1))
Hereinafter, the compound represented by Formula (1) will be described.
In Formula (1), D represents a residue structure in which four hydrogen atoms
are
removed in the compound represented by Formula (2).
In Formula (1), R1 and R2 represent R1=R2=1-1, or R1=R and R2=-L-0O2M.
In Formula (1), n represents a number of 1 to 4, and m represents the number
of 0 to 3,
provided that m+n is 4.
CA 02816869 2013-05-22
13
That is, the compound represented by Formula (1) is a xanthene dye in which
four
hydrogen atoms are substituted by sulfonamide groups or by sulfonamide groups
and sulfo
groups in the compound represented by Formula (2), or a xanthene dye which is
substituted
with substituted sulfonamide groups or with substituted sulfonamide groups and
sulfo groups.
It is considered that the dye represented by Formula (1) is excellent in hue,
and is
especially excellent in ozone fastness due to the electron-withdrawing
property possessed by
the sulfo groups.
Further, in the case of R1=R2=H, it is assumed that the possession of a
sulfonamide
group causes a hydrogen bonding between the sulfonamide group and a hydroxyl
group
possessed by silica or alumina present on the printing medium, resulting in a
dye having an
excellent moisture fastness. Also in the case of R1=-R and R2=-L-0O2M,
although its detailed
mechanism is not clear, a dye having an excellent moisture fastness is
obtained as well.
Further, in the compound represented by Formula (1) of the present invention,
the sum
of SO2NR1R2 and sulfo groups is 5. As a result, it is possible to combine
sufficient moisture
fastness with sufficient water solubility. Although the solubility by sulfo
groups and the
mechanism of the moisture fastness as described above by SO2NR1R2 are assumed,
if the sum
of SO2NR1R2 and sulfo groups is 3 or less as in the related art, there is a
problem that it is
impossible to balance the water solubility and the moisture fastness.
Further, by setting the sum of SO2NR1R2 and sulfo groups to 5 from the
viewpoint of
combining the water solubility with the moisture fastness, remarkably
excellent ozone fastness
is obtained. Although the mechanism is not clear, it would be because the
sulfo groups and
SO2NR1R2 act as electron-withdrawing groups to considerably delay the
oxidation reaction of
the dye.
R represents a hydrogen atom or a monovalent substituent. Examples of the
monovalent substituent represented by R may include an alkyl group or an aryl
group.
When R represents a monovalent substituent, the monovalent substituentis
preferably
an alkyl group, a phenyl group or a naphthyl group, more preferably an alkyl
group or a
phenyl group, and still more preferably an alkyl group.
When R represents an alkyl group, the alkyl group is preferably an alkyl group
having
1 to 6 carbon atoms, more preferably an alkyl group having 1 to 3 carbon
atoms, and still more
preferably a methyl group.
The monovalent substituent may have a substituent, and examples of the
substituent
may include a halogen atom, an alkoxy group, an aryloxy group and the like.
The monovalent substituent is preferably unsubstituted.
CA 02816869 2013-05-22
14
R is preferably a hydrogen atom or an unsubstituted alkyl group, and more
preferably a
hydrogen atom or a methyl group. When a plurality of R is present, R's may be
the same or
different.
L represents a divalent linking group. Examples of the divalent linking group
represented by L may include an alkylene group, a phenylene group, a
naphthylene group and
the like. From the viepoint of the availability of raw materials and the
material price, L is
preferably an alkylene group or a phenylene group, more preferably an alkylene
group or an
unsubstituted phenylene group, and still more preferably an alkylene group.
When L represents an alkylene group, the alkylene groupb is preferably an
alkylene
group having 1 to 6 carbon atoms, more preferably a methylene group, an
ethylene group or a
propylene group, and still more preferably a methylene group or an ethylene
group.
The divalent linking group may have a substituent, and examples of the
substituent
may include a hydroxyl group, a halogen atom, an alkoxy group, an aryloxy
group and the
like.
The divalent linking group is preferably unsubstituted.
When a plurality of L is present, L's may be the same or different.
n represents the number of 1 to 4, and m represents the number of 0 to 3,
provided that
m+n is 4.
When R1 and R2 are RI=R2=}1, n is preferably 1 to 3, and more preferably 2
from the
viewpoint of the moisture fastness and the water solubility of the dye.
When R1 and R2 are R1=R and R2=-L-0O2M, n is preferably 1 to 3, and more
preferably 2 from the viewpoint of the moisture fastness and the water
solubility of the dye.
In Formula (2), ell, Run and le 3 each independently represent a monovalent
substituent.
Examples of the monovalent substituent represented by R1 1, R102 and ¨103
x
may include
subsitutuents selected from Group A of substituents, and from the viepoint of
the availability
of raw materials and the material price, the monovalent substituent is
preferably a halogen
atom, an aryl group, an alkoxy group, an alkyl group or an acylamino group,
more preferably
an alkyl group or an acylamino group, and still more preferably an alyl group.
When R101, Run and E. ¨103
represent an alkyl group, the alkyl group is preferably an
alkyl group having 1 to 6 carbon atoms, and more preferably an alkyl group
having 1 to 3
carbon atoms from the viewpoint of the availability of raw materials. Further,
the alkyl
group is preferably a straight or branched alkyl group. Specific examples of
the alkyl group
may include a methyl group, an ethyl group, a n-propyl group, an i-propyl
group, a t-butyl
CA 02816869 2013-05-22
group and the like, and the alkyl group is preferably a methyl group, an ethyl
group or an
i-propyl group, more preferably a methyl group or an ethyl group, and still
more preferably a
methyl group.
The alkyl group may have a substituent, and examples of the substituent may
include a
halogen atom, a hydroxyl group and the like.
The alkyl group is preferably an unsubstituted alkyl group.
When Rio', R102 and Rt03 represent an acylamino group, the acyl group in the
acylamino group is preferably an aliphatic acyl group, and more preferably an
aliphatic acyl
group having 2 to 6 carbon atoms from the viewpoint of the availability of raw
materials and
the color strength. Specific examples thereof may include an acetylamino
group,
propionylamino group, butyrylamino group and the like, and particularly
preferably an
acetylamin.o group.
The acylamino group is preferably a monoacylamino group.
In Formula (2), le4 and R105 each independently represent a hydrogen atom or a
monovalent substituent group.
When R1 4 and le 5 represent a monovalent substituent, examples of the
monosubstituent may include subsituents selected from Group A of substituents,
and the
monovalent substituent is preferably a substituent or unsubstituent alkyl
group and more
preferably an unsubstituent alkyl group.
From the viewpoint of the absorption characteristic and ozone fastness, Ri 4
and 11.1 5
are preferably a hydrogen atom.
In Formula (2), n101 and n102 each independently represent the number of 0 to
5.
From the viewpoint of the availability of raw materials and the ease of
synthesis, n101 and
n102 is preferably the number of 1 to 5, more preferably 2 to 5, still more
preferably 2 to 4,
and particularly 2 or 3.
When n101 and n102 each represent the number of 2 or more, each of pluralities
of
RI ' and R102 may be the same or different.
In Formula (2), n103 represents the number of 0 to 4. From the viewpoint of
the
availability of raw materials, n103 is preferably a number of 0 to 3, more
preferably 0 to 2,
still more preferably 0 or 1, and particularly preferably 0.
When n103 represents the number of 2 or more, Itl 3's may be the same or
different.
In Formula (1), M represents a hydrogen atom or a countercation. When a
plurality
of M are present, M's may be the same or different.
In Formula (1), when M is a hydrogen atom, it is in a form of free acid, and
when M is
CA 02816869 2013-05-22
16
a countercation, it is in a form of a salt.
The countercation forming a salt may be exemplified by a monovalent
countercation,
and preferably an alkali metal ion, an ammonium ion, an organic cation and the
like.
Examles of the organic cation may include a tetramethylarnmonium ion, a
tetramethylguanidium ion, a tetramethylphospohonium ion and the like.
From the viewpoint of the availability of raw materials, the water solubility
of the dye,
and the suppression of gloss generation when forming a secondary color with
other dyes in a
case of using as an inkjet ink, the countercation is preferably an alkali
metal ion, and more
preferably a lithium ion, a sodium ion or a potassium ion. Particularly, a
sodium ion is
preferred because it is inexpensive.
In the present invention, the compound represented by Formula (1) is
preferably in a
form of a salt, more preferably a lithium salt, a sodium salt or a potassium
salt, and still more
preferably a sodium salt from the viewpoint of the ease of synthesis (ease of
handling as dye
powder).
In Formula (1), when a plurality of M are present, each M may be the same or
different.
That is, the compound represented by Formula (1) in a form of a salt includes
a case where all
sulfo groups are salts, and a case where some sulfo groups are in a form of
free acid and some
sulfo groups are salts. Further, the countercation forming a salt may be
present either alone
or in plurality.
In the present invention, the compound represented by Formula (1) is
preferably in a
form of a salt, and more preferably a case wehre all sulfo groups are salts
from the viewpoint
of the ease of synthesis (ease of handling as dye powder).
The compound represented by Formula (1) is preferably a compound represented
by
Formula (3).
It is considered that the compound represented by Formula (3) is particularly
excellent
from the viewpoint of the ozone fastness because it is possible to suppress
oxidative gas
(oxygen or ozone) from attacking a nitrogen atom by the steric hindrance of a
sulfo group
introduced in the vicinity of the nitrogen atom.
CA 02816869 2013-05-22
17
SO2R3 SO2Ra
'rX:1 I 201 \
R /n201 R202)
Y-11 n202
H,W 0111 N+
'H
Formula (3)
R3 0 2.S S02R3
SO3-
7¨ER243/1203
In Formula (3),
When R1 and R2 are R1=R2=H, R3's each independently represent OM or NH2,
provided that when R1 and R2 are R1=R2=H, at least one of R3's represent NH2.
WhenR1 and R2 are R1=R and R2=-L-0O2M, R3's each independently represent OM or
NR-L-0O2M, provided that when R1 and R2 are R1=R and R2=-L-0O2M, at least one
of R3's
represent NR-L-0O2M-
R represents a hydrogen atom or a monovalent substituent. When a plurality of
R is
present, R's may be the same or different.
L represents a divalent linking group. When a plurality of L is present, L's
may be
the same or different.
R201, R202 and R203
each independently represent a monovalent sub stituent.
n201 and n202 each independently represent the number of 0 to 4, and n203
represents
the number of 0 to 4.
When n201, n202 and n203 each represent the number of 2 or more, each of
pluralities
of- R201, R202 and R203
may be the same or different.
M represents a hydrogen atom or a countercation. When a plurality of M is
present,
M's may be the same or different.
In Formula (3), when R1 and R2 are R1=R2=H, R3's each independently represent
OM
or NH2, provided that when R1 and R2 are R1=R2=H, at least one of R3's
represent NH2.
From the viewpoint of the moisture fastness and the water solubility of the
dye, it is
preferred that one to three of R3's are NH2, and it is more preferred that two
of R3's are NH2.
When R1 and R2 are R1=R and R2=-L-0O2M, R3's each independently represent OM
or
NR-L-0O2M, provided that when R1 and R2 are R1=R and R2=-L-0O2M, at least one
of R3's
represent NR-L-0O2M.
From the viewpoint of the moisture fastness and the water solubility of the
dye, it is
CA 02816869 2013-05-22
18
preferred that one to three of R3's are NR-L-0O2M, and it is more preferred
that two of R3's
are NR-L-0O2M.
Specific examples and preferred ranges of R and L in NR-L-0O2M represented by
R3
are the same as the specific examples and preferred ranges of R and L in RI or
R2.
Specific examples and preferred ranges of R201, R202, R203, n203 and M in
Formula (3)
are the same as the specific examples and preferred ranges of Riol, R102,
R103, n103 and M in
Formula (2).
n201 and n202 in Formula (3) each independently represent the number of 0 to
4, and
from the viewpoint of the availability of raw materials and the ease of
synthesis, preferably the
number of 1 to 4, more preferably the number of 2 to 4, and still more
preferably the number
of 2 or 3.
Specific examples of the compound represented by Formula (1) are shown below,
but
the compound is not limited thereto. Further, in the following specific
examples, Me denotes
a methyl group, Et denotes an ethyl group, i-PR denotes an isopropyl group, t-
Bu denotes a
tertiary butyl (tert-butyl) group, and Ac denotes an acetyl group.
(1A) (1B)
(1D) Me Me
SO2R SO2R SO2R 102R (1 C) SO2R
SO2R
1, RO2S * 0 SO2R
1 ;
Me Me Me Me Me Me Me Me Me Me Me Me Me Me Me Me
H.N 0 N-.;ti
H-N 0 NZ'H H,N 00
1,1:1-H H-N 0 N:H
Oill 0 401 0
0 410) .-410
RO2S so2R RO2S soR RO2S 2 SO2R
RO2S
SO2R
*I SO; 40) so;
a SO; so SO;
R= ONa or NH2, ONa/NH2 = 2/2 R= ONa or NH2, ONa/NH2 = 1.5/2.5
R= ONa or NH2, ONa/NH2 = 3/1 R= ONa or
NH2, ONa/NH2 = 1/3
(-)
0
(1E) (1F) (1G)
(1H) N.)
co
1-,
Me
Me0,
SO2R SO2R SO2R
SO2R Me Me co
RO2S 0 ao SO2R .41,
"cis:: 1101 IP RO2S NHAc AcHN so SO2R
0,
ko
n.,
Et Et Et Et Et Et Et Et !Pr
i-Pr i-Pr i-Pr Me Me Me Me
0 "8
1-,
H,N 0 0,4 N:H H0 0 õN 0 N. H.!H ,N 4111 0 AO
NH
el
H,N 00 N.'Ft w
1
.
0
ol
1
RO2S SO2R R025 SO2R RO2S
SO2R RO2S SO2R N)
n.,
0 SO; 0 SO3 40 SO3
op SO3
R= ONa or NH2, ONa/NH2 = 2/2 Ft= ONa or NH2, ONa/NH2 = 2/2 R= ONa
or NH2, ONa/NH2 = 2/2 R= ONa or NH2, ONa/NH2 = 2/2
(2A) (28) (2C)
(2RD0) 2s iMe Me
SO2R
/SO2R JSO2R , iSO2R SO2R SO2R
SO2R
Me,,
__Cr/1
SI
MeMe Me Me Me Me Me Me Me Me Me Me Me Me Me
0
H"
N . le N:H H-N 0
411 0 N:H H,N 410 0 0 N:-
H õ
H N0
0 0 NH
RO2S SO2R RO2S SO2R RO2S
SO2R RO2S S02R
_
= SO; = SO;
lo SO; 0 SO3
R= ONa or NHCH2CO2Na R= ONa or NHCH2CH2CO2Na R= ONa or
N(CH3)CH2CO2Na R= ONa or NHCH2CO2Na 0
ONa/NHCH2CO2Na = 2/2 ONa/NHCH2CH2CO2Na = 2/2
ONa/N(CH3)CH2CO2Na = 2/2 0Na/NHCH2CO2Na = 1/3
c)
n.)
co
1-,
0,
co
(2E) (2F) (2G)
(2H) 0)
ko
Me Me SO2R
SO2R Me Me
SO2R SO2R
n.)
RO2S 0 401 SO2R
4
I
1; 4:1 1110)
lio RO2S 0 NHAc AcHN 40 so,R , .
,_
w
Et Et Et Et Et Et Et Et i-Pr i-Pr i-
Pr i-Pr Me Me Me Me 0
1-1-N 0
01
NH HN HN
IC
0 N:H , 0
'H H,N 0 N:H 1
0 0 0
0 t10 0 010 õ
õ
RO2S SO2R RO2S SO2R RO2S
802R RO2S SO2R
lb SO; is SO3
0 SO; 40 SO;
Ft= ONa or NHCH2CH2CO2Na R= ONa or NHCH2CH2CO2Na
R= ONa or NHCH2CH2CO2Na R= ONa or NHCH2CH2CO2Na
ONa/NHCH2CH2CO2Na = 3/1 ONa/NHCH2CH2CO2Na = 1/3
ONa/NHCH2CH2CO2Na = 1/3 ONa/NHCH2CH2CO2Na = 1.5/2.5
CA 02816869 2013-05-22
21
The synthesis of the compound represented by Formula (1) of the present
invention
will be described.
The compound can be synthesized by synthesizing a xanthene dye in accordance
to the
synthesis of a xanthene dye known in the related art or using a commercially
available
xanthene dye, chlorosulfonating by combination of chlorosulfonic
acid/phosphorous
oxychloride, reacting n equivalents of ammonia or amino acid (NHR-L-0O2M) with
the dye,
and then, alkaline hydrolyzing unreacted sulfonyl chloride (see the scheme
below). The
detailed descrption will be made in Examples as described below.
SO3Na SO2CI 50201 SO2Y
SO2Y
1
1
1
Me"--"MeI 1
Me y--.'sMe Me-Th---NMe , Me'¨`-rMe Me."¨.`-rMe
CISO 3H NHR
C102S 02CI YO2S
SO2Y
OS 3
40 SO 3
.!7". 3
Y = CI or NR1R2
NaOH
SO 2Y SO2Y
1
Me Me
H
YO2S
01 SO;
Y = ONa or NR1R2
The coloring composition of the present invention contains at least one kind
of the
compound represented by Formula (1). Although the compound represented by
Formula (1)
contains a compound of R1=R2=H and a comound of R1=R and R2=-L-0O2M, both may
be
contained either alone or in a mixture. Further, from the viewpoint of ease of
design for ink
prescription, it is preferred that the comound is contained alone.
The coloring composition of the present invention may include a medium.
Especially,
the coloring composition is suitable for ink for inkjet recording if a solvent
is used as the
medium. The coloring composition of the present invention may be prepared by
dissolving
and/or dispersing the compound of the present invention using an oleophilic
medium or an
aqueous medium as the medium. Preferably, the aqueous medium may be used. The
coloring composition of the present invention also includes a composition for
ink except the
medium.
CA 02816869 2013-05-22
22
In the present invention, the content of the compound represented by Formula
(1)
contained in the coloring composition is determined depending on, for example,
the kind of a
substituent in Formula (1) to be used or the kind of a solvent component used
for preparing the
coloring composition. However, the content of the compound represented by
Formula (1) in
the coloring composition is preferably 1% by mass to 20% by mass, more
preferably 1% by
mass to 10% by mass, and particularly preferably 2% by mass to 6 % by mass
based on the
total mass of the coloring composition.
If the content of the compound represented by Formula (1) contained in the
coloring
composition is 1% by mass or more, the color strength of the ink printed on a
recording
medium may be good and also a required image density may be secured. Also, if
the total
amount of the compound represented by Formula (1) contained in the coloring
composition is
20% by mass or less, there can be achieved effects in that a discharging
property of the
coloring composition is good, and further, an inkjet nozzle is suppressed from
being clogged
when used in an inkjet recording method.
The coloring composition of the present invention may contain other additives
in a
range not impairing the effects of the present invention, if necessary. Other
additives may
include additives that may be used in ink for inkjet recording as described
below.
[Ink for inkjet recording]
Hereinafter, ink for inkjet recording of the present invention will be
described.
The present invention also relates to an ink for inkjet recording which
containes the
coloring composition of the present invention.
The ink for inkjet recording may be prepared by dissolving and/or dispersing
the
compound (mixture) of the present invention in an oleophilic medium or an
aqueous medium.
Preferably, the ink is prepared by the aqueous medium.
If necessary, other additives may be contained in a range not impairing the
effects of
the present invention. For example, other additives may be known additives
such as, for
example, a drying preventing agent (wetting agent), a discoloration preventing
agent, an
emulsion stabilizer, a permeation promoting agent, a UV absorbent, a
preservative, an
antifungal agent, a pH adjusting agent, a surface tension regulator, an
antifoaming agent, a
viscosity regulator, a dispersant, a dispersion stabilizer, a rust inhibitor
and a chelating agent.
These various kinds of additives are directly added to an ink solution in the
case of a
water-soluble ink. In the case where an oil-soluble dye is used in a form of a
dispersant, the
additives are generally added to the dispersant after preparation of the dye
dispersant, but may
be added in an oil phase or an aqueous phase during the preparation.
CA 02816869 2013-05-22
23
The drying preventing agent is appropriately used for the purpose of
suppressing an ink
discharging hole of a nozzle used in an inkjet recording method from being
clogged due to the
dryness of the ink for inkjet recording.
The drying preventing agent is preferably a water-soluble organic solvent
having vapor
pressure lower than that of water. Specific examples thereof may include
polyhydric
alcohols represented by ethylene glycol, propylene glycol, diethylene glycol,
polyethylene
glycol, thiodiglycol, dithiodiglycol, 2-methyl-1,3-propanediol, 1,2,6-
hexanetriol, an acetylene
glycol derivative, glycerine and trimethylolpropan, lower alkyl ethers of
polyhydric alcohol
such as ethylene glycol monomethyl (or ethyl) ether, diethylene glycol
monomethyl (or ethyl)
ether and triethylene glycol monoethyl (or butyl) ether, heterocyclic rings
such as
2-pyrrolidone, N-methyl-2-pyrroli done, 1,3 -dimethy1-2-imid 7olidinone
and
N-ethylmorpholine, a sulfur-containing compound such as sulfolan,
dimethylsulfoxide and
3-sulfolene, a polyfunctional compound such as diacetone alcohol or diethanol
amine, and a
urea derivative. Among them, polyhydric alcohol such as glycerine or
diethylene glycol is
more preferred. Further, the drying preventing agent may be used either alone
or in
combination of two kinds or more thereof. It is preferred that the drying
preventing agent is
contained in the ink in an amount of 10% by mass to 50% by mass.
The permeation promoting agent is appropriately used for the purpose of
allowing the
ink for inkjet recording to be permeated through paper well. As the permeation
promoting
agent, alcohols such as ethanol, isopropanol, butanol, di(tri)ethylene glycol
monobutylether
and 1,2-hexanediol, sodium lauryl sulfate, sodium oleate and a non-ionic
surfactant, or the like
may be used. If the aforementioned permeation promoting agent is included in
the ink in an
amount of 5% by mass to 30% by mass in the ink, there is generally a
sufficient effect, and it
is preferred to use the permeation promoting agent in the range of the
addition amount not
causing spreading of print and print-through.
The UV absorbent is used for the purpose of improving a preservation property
of an
image. As the UV absorbent, a compound absorbing UV to emit fluorescence, that
is, a
so-called fluorescent brightening agent, which is represented by a
benzotriazole-based
compound described in Japalese Patent Application Laid-Open Nos. S58-185677,
S61-190537,
112-782, 115-197075 and H9-34057, a benzophenone-based compound described in
Japanese
Patent Application Laid-Open Nos. S46-2784, H5-194483 and U.S. Patent No.
3,214,463, a
cinnamic acid-based compound described in Japanese Patent Publication Nos. S48-
30492,
S56-21141 and H10-88106, a triazine-based compound described in Japanese
Patent
Application Laid-Open Nos. 114-298503, H8-53427, H8-239368, H10-182621 and 118-
501291,
CA 02816869 2013-05-22
24
a compound described in Research Disclosure No. 24239, or a stilbene-based or
benzooxazole-based compounds may be used.
The discoloration prevention agent is used for the purpose of improving a
preservation
property of an image. As the discoloration prevention agent, various kinds of
organic and
metal complex-based discoloration prevention agents may be used. The
organic
discoloration prevention agent is hydroquinones, alkoxyphenols,
dialkoxyphenols, phenols,
anilines, amines, indanes, cromanes, alkoxyanilines, heterocyclics or the
like, and the metal
complex is a nickel complex, a zinc complex or the like. More specifically,
the compound
described in the patent documents cited in Paragraphs Ito J of VII of Research
Disclosure No.
17643, Research Disclosure Nos. 15162, the left column on page 650 of Research
Disclosure
No. 18716, page 527 of Research Disclosure No. 36544, page 872 of Research
Disclosure No.
307105 and Research Disclosure No. 15162, or a compound included in the
formula of a
representative compound and examples of the compounds described on pages 127
to 137 of
Japanese Patent Application Laid-Open No. S62-215272 may be used.
The antifungal agent may be sodium dehydroacetic acid, sodium benzoate, sodium
pyridinethione- 1-oxide, ethylester p-hydroxybenzoate, 1,2-benzisothiazolin-3-
one or a salt
thereof. These may be preferably used in the ink in an amount of 0.02% by mass
to 1.00%
by mass.
As the pH adjusting agent, a neutralizing agent (organic base or inorganic
alkali) may
be used. The pH adjusting agent is added for the purpose of improving the
storage stability
of the ink for inkjet recording, so that the pH of the ink for inkjet
recording is preferably 6 to
10, and more preferably 7 to 10.
The surface tension regulator may be a non-ionic, cationic or anionic
surfactant. Also,
the surface tension of the ink for inkjet recording of the present invention
preferably ranges
from 25 mN/m to 70 mN/m, and more preferably ranges from 25 mN/m to 60 mN/m.
Also,
the viscosity of ink for inkjet recording of the present invention is
preferably 30 mPa.s or less,
and is more preferably adjusted to 20 mPa-s or less. Examples of the
surfactant preferably
include an anionic surfactant such as a fatty acid salt, an alkyl ester
sulfate salt, an
alkylbenzene sulfonate salt, an alkylnaphthalene sulfonate salt, a dialkyl
sulfosuccinate salt, an
alkyl ester phosphate salt, a naphthalene sulfonic acid formaline condensate
and a
polyoxyethylenealkyl ester sulfate salt, or a non-ionic surfactant such as
polyoxyethylenealkylether, polyoxyethylenealkylallylether, polyoxyethylene
fatty acid ester,
sorbitan fatty acid ester, polyoxyethylene sorbitan fatty acid ester,
polyoxyethylenealkylamine,
glycerine fatty acid ester and an oxyethyleneoxypropylene block copolymer.
Further,
CA 02816869 2013-05-22
SURFYNOLS (AirProducts & Chemicals, Co., Ltd.) that is an acetylene-based
polyoxyethylene oxide surfactant is preferably used. Further, an amine oxide
type
ampholytic surfactant such as N,N-dimethyl-N-alkylamine oxide is preferred.
Moreover, a
matter exemplified as a surfactant on pp. 37 to 38 of Japanese Patent
Application Laid-Open
No. S59-157,636 and Research Disclosure No. 308119 (1989) may be used.
As the antifoaming agent, a fluorine-based or silicon-based compound, a
chelating
agent represented by EDTA, or the like may be used if necessary.
In the case where the compound of the present invention is dispersed in an
aqueous
medium, it is preferred that coloring fine particles containing the compound
and an oil-soluble
polymer are dispersed in the aqueous medium as described in Japanese Patent
Application
Laid-Open No. H11-286637, Japanese Patent Application Nos. 2000-78491, 2000-
80259 and
2000-62370, or the compound of the present invention dissolved in a high
boiling point
organic solvent is dispersed in the aqueous medium as described in Japanese
Patent
Application Nos. H2000-78454, 2000-78491, 2000-203856 and 2000-203857. In the
case
where the compound of the present invention is dispersed in the aqueous
medium, a specific
method, an oil-soluble polymer, a high boiling point organic solvent,
additives, and the
amounts thereof to be used may preferably refer to the description in the
aforementioned
patent documents. Otherwise, the compound of the present invention may be
dispersed in a
solid fine particle state. In the dispersion, a dispersant or a surfactant may
be used. As a
dispersion device, a simple stirrer or impeller agitation type, an inline
agitation type, a mill
type (for example, a colloid mill, a ball mill, a sand mill, an attritor, a
roll mill or an agitator
mill), an ultrasonic type, and a high pressure emulsification and dispersion
type (high pressure
homogenizer; Goehring homogenizer, microfluidizer, DeBEE2000 or the like as a
specific
commercially-available device) may be used. The aforementioned method of
preparing the
ink for inkjet recording is described in detail in Japanese Patent Application
Laid-Open Nos.
H5-148436, H5-295312, H7-97541, H7-82515, H7-118584 and H11-286637, and
Japanese
Patent Application No. 2000-87539, in addition to the aforementioned patent
documents, and
may be used for the ink for inkjet recording of the present invention.
As the aqueous medium, a mixture that contains water as a main component, and
if
necessary, is added with a water-miscible organic solvent may be used.
Examples of the
water-miscible organic solvent may include alcohol (for example, methanol,
ethanol, propanol,
isopropanol, butanol, isobutanol, sec-butanol, t-butanol, pentanol, hexanol,
cyclohexanol and
benzylalcohol), polyhydric alcohols (for example, ethylene glycol, diethylene
glycol,
triethylene glycol, polyethylene glycol, propylene glycol, dipropylene glycol,
polypropylene
CA 02816869 2013-05-22
26
glycol, butylene glycol, hexanediol, pentanediol, glycerine, hexanetriol and
thiodiglycol), a
glycol derivative (for example, ethylene glycol monomethyl ether, ethylene
glycol monoethyl
ether, ethylene glycol monobutyl ether, diethylene glycol monomethyl ether,
diethylene glycol
monobutyl ether, propylene glycol monomethyl ether, propylene glycol monobutyl
ether,
dipropylene glycol monomethyl ether, triethylene glycol monomethyl ether,
ethylene glycol
diacetate, ethylene glycol monomethyl ether acetate, triethylene glycol
monomethyl ether,
triethylene glycol monoethyl ether and ethylene glycol monophenyl ether),
amine (for
example, ethanolamine, diethanolamine, triethanolamine, N-
methyldiethanolamine,
N-ethyldiethanolamine, morpholine, N-ethylmorpholine, ethylenediamine,
diethylenetriamine,
triethylenetetramine, polyethyleneimine and tetramethylpropylenediamine), and
other polar
solvents (for example, formamide, N,N-dimethylformamide, N,N-
dimethylacetamide,
dimethylsulfoxide, sulfolane, 2-pyrrolidone, N-methyl-2-pynolidone, N-vinyl-2-
pyrrolidone,
2-oxazolidone, 1,3-dimethy1-2-imidazolidinone, acetonitrile and acetone).
Meanwhile, the
water-miscible organic solvents may be used in combination of two or more
thereof.
In 100 parts by mass of the ink for inkjet recording of the present invention,
the
compound represented by Formula (1) or Formula (4) is contained preferably in
an amount of
0.2 parts by mass to 10 parts by mass, and more preferably in an amount of 1
part by mass to 6
parts by mass. Also, in the ink for inkjet recording of the present invention,
the compound of
the present invention may be used in combination with other colorants. When
two or more
kinds of colorants are used in combination, the total content of the colorants
is preferably
within the aforementioned range.
The ink for inkjet recording of the present invention preferably has a
viscosity of 30
mPa.s or less. Also, the surface tension is preferably 25 mN/m to 70 mN/m. The
viscosity
and the surface tension may be adjusted by addition of various kinds of
additives, such as, for
example, a viscosity regulator, a surface tension regulator, a specific
fastness adjusting agent, a
film regulator, a UV absorbent, an antioxidant, a discoloration prevention
agent, an antifungal
agent, a rust inhibitor, a dispersant and a surfactant.
The ink for inkjet recording of the present invention may be used to form a
monochromic image or form an image of a full color. In order to form the full
color image, a
magenta color-tone ink, a cyan color-tone ink and a yellow color-tone ink may
be used, and
also a black color-tone ink may be further used so as to set up color-tones.
As an applicable yellow dye, arbitrary matters may be used. Examples thereof
may
include an aryl or heterylazo dye having heterocyclic rings such as phenols,
naphthols, anilines,
pyrazolone or pyridones, chain-opening active methylene compounds, or the like
as a coupling
CA 02816869 2013-05-22
27
component (hereinafter, referred to as "coupler component"); an azomethine dye
having
chain-opening active methylene compounds or the like as a coupler component; a
methine dye
such as, for example, a benzylidene dye and a monomethineoxonol dye; and a
quinine-based
dye such as, for example, a naphthoquinone dye and an anthraquinone dye.
Examples of
other kinds of the dye may include a quinophthalon dye, a nitro and nitroso
dye, an acridine
dye, an acrydinone dye and the like.
As an applicable magenta dye, arbitrary matters may be used. Examples thereof
may
include an aryl or heterylazo dye having phenols, naphthols, anilines or the
like as a coupler
component; an azomethine dye having pyrazolones, pyrazolotriazoles or the like
as a coupler
component; a methine dye such as an arylidene dye, a styryl dye, a melocyanine
dye, a
cyanine dye and an oxonol dye; a carbonium dye such as a diphenylmethane dye,
a
triphenylmethane dye and a xanthene dye, a quinone-based dye such as
naphthoquinone,
anthraquinone and anthrapyridone, and a condensed polycyclic ring-based dye
such as a
dioxadin dye.
As an applicable cyan dye, arbitrary matters may be used. Examples thereof may
include an aryl or heterylazo dye having phenols, naphthols, anilines or the
like as a coupler
component; an azomethine dye having heterocyclic rings such as phenols,
naphthols, and
pyrrolotriazoles, or the like as a coupler component; a polymethine dye such
as a cyanine dye,
an oxonol dye and a melocyanine dye; a carbonium dye such as a diphenylmethane
dye, a
triphenylmethane dye and a xanthene dye; a phthalocyanine dye; an
anthraquinone dye; indigo
and thioindigo dyes and the like.
Each of the aforementioned dyes may be a matter in which a portion of
chromophore is
dissociated to initially have each color of yellow, magenta and cyan, and in
this case, the
countercation may be an inorganic cation such as alkali metal or ammonium, an
organic cation
such as pyridinium or a quaternary ammonium salt, or a polymer cation having
the
aforementioned cations as a partial structure.
Examples of an applicable black coloring material may include a dispersing
element of
carbon black besides disazo, trisazo and tetrazo dyes.
The ink composition of the present invention may be used in a recording method
such
as, for example, sealing, copying, marking, writing, drawing and stamping, and
is particularly
suitably used in an inkjet recording method.
[Inkjet recording method]
The present invention also relates to an inkjet recording method of forming an
image
by using the coloring composition or the ink for inkjet recording of the
present invention.
CA 02816869 2013-05-22
28
The inkjet recording method of the present invention donates energy to the ink
for
inkjet recording, and forms an image on known image-receiving materials, that
is, plain paper,
resin-coated paper, exclusive inkjet paper described in, for example, Japanese
Patent
Application Laid-Open Nos. 118-169172, H8-27693, H2-276670, 117-276789, H9-
323475,
S62-238783, Japanese 1110-153989, 1110-217473, H10-235995, H10-337947, H10-
217597
and H10-337947, film, paper for use in electrophotography, fabric, glass,
metal, ceramic, or
the like.
When an image is formed, a polymer fine particle dispersion (also known as
polymer
latex) may be used in combination in order to impart a glossiness or a water
fastness, or
improve a weather fastness. The polymer latex may be added to the image-
receiving material
before, after or simultaneously with application of a colorant, and
accordingly, may be added
into image-receiving paper, or ink, or used alone as a liquid. Specifically,
the methods
described in Japanese Patent Application Nos. 2000-363090, 2000-315231, 2000-
354380,
2000-343944, 2000-268952, 2000-299465, 2000-297365, and the like may be
preferably used.
Hereinafter, the recording paper and the recording film used to perform inkjet
printing
by using ink of the present invention will be described.
In the recording paper and the recording film, a support is formed of a
chemical pulp
such as LBKP and NBKP, a mechanical pulp such as GP, PGW, RMP, TMP, CTMP, CMP
and
CGP, a used-paper pulp such as DIP, or the like, and, if necessary, a matter
manufactured by
various kinds of devices such as a fourdrinier paper machine or a rotoformer
paper machine by
mixing additives known in the art, such as a pigment, a binder, a sizing
agent, a settlement
agent, a cationic agent, a strength additive for paper, or the like may be
used. In addition to
the aforementioned support, any matter of a synthetic paper and a plastic film
sheet may be
used, and it is preferred that the thickness of the support is 10 p.m to 250
p.m, and the basis
weight thereof is 10 g/m2 to 250 g/m2.
The support may be provided with an ink-receiving layer and a backcoat layer
at once,
or may be provided with an ink-receiving layer and a backcoat layer after a
size press or an
anchor coat layer is formed by starch, polyvinyl alcohol or the like. Further,
the support may
be subjected to planarization treatment by a calender device such as a machine
calender, a TG
calender, or a soft calender. In the present invention, paper and plastic
films in which
polyolefins (for example, polyethylene, polystyrene, polyethylene
terephthalate, polybutene,
and a copolymer thereof) are laminated on both surfaces thereof are more
preferably used as
the support. It is preferred that a white pigment (e.g., titanium oxide or
zinc oxide) or a
coloring dye (e.g., cobalt blue, navy blue or neodymium oxide) is added to
polyolefins.
CA 02816869 2013-05-22
29
The ink-receiving layer formed on the support contains a pigment or an aqueous
binder.
As the pigment, a white pigment is preferred, and examples of the white
pigment may include
an inorganic white pigment such as calcium carbonate, kaolin, talc, clay,
diatomite, synthetic
amorphous silica, aluminum silicate, magnesium silicate, calcium silicate,
aluminum
hydroxide, alumina, lithopone, zeolite, barium sulfate, calcium sulfate,
titanium dioxide, zinc
sulfide and zinc carbonate, and an organic pigment such as a styrene-based
pigment, an acrylic
pigment, a urea resin and a melamine resin. As the white pigment contained in
the
ink-receiving layer, a porous inorganic pigment is preferred, and in
particular, for example,
synthetic amorphous silica having a large fine pore area is appropriate. As
the synthetic
amorphous silica, any of silicic acid anhydride obtained by a dry
manufacturing method and
water-containing silicic acid obtained by a wet manufacturing method may be
used. In
particular, water-containing silicic acid is preferably used.
Examples of the aqueous binder contained in the ink-receiving layer may
include
water-soluble polymer such as polyvinyl alcohol, silanol denatured polyvinyl
alcohol, starch,
cationic starch, casein, gelatin, carboxymethyl cellulose, hydroxyethyl
cellulose,
polyvinylpyrrolidone, polyalkylene oxide and a polyalkylene oxide derivative,
and a water
dispersible polymer such as a styrenebutadiene latex and an acryl emulsion.
The aqueous
binder may be used either alone or in combination of two kinds or more
thereof. In the
present invention, among them, polyvinyl alcohol or silanol denatured
polyvinyl alcohol is
particularly suitable from the viewpoints of the attachment property to the
pigment and the
stripping fastness of an ink-receiving layer.
The ink-receiving layer may contain a mordant, an insolubilizer, a light
fastness
improving agent, a surfactant or other additives in addition to the pigment
and the aqueous
binder.
It is preferred that a mordant added to the ink-receiving layer is
immobilized. To this
end, a polymer-mordant is preferably used.
The polymer-mordant is described in Japanese Patent Application Laid-Open Nos.
S48-28325, S54-74430, S54-124726, S55-22766, S55-142339, S60-23850, S60-23851,
S60-23852, S60-23853, S60-57836, S60-60643, S60-118834, S60-122940, S60-
122941,
S60-122942, S60-235134 and H1-161236, and U.S. Patent Nos. 2,484,430,
2,548,564,
3,148,061, 3,309,690, 4,115,124, 4,124,386, 4,193,800, 4,273,853, 4,282,305
and 4,450,224.
An image-receiving material including the polymer-mordant described on pages
212 to 215 of
Japanese Patent Application Laid-Open No. H1-161236 is particularly preferred.
If the
polymer-mordant described in the aforementioned patent document is used, an
image having
CA 02816869 2013-05-22
an excellent image quality may be obtained, and the light fastness of the
image is improved.
The insolubilizer is effective to insolubilization of the image, and it is
particularly
preferred that a cation resin is an insolubilizer.
The cation resin may be
polyamidepolyamineepichlorohydrin, polyethyleneimine,
polyaminesulfone,
dimethyldiallylammonium chloride polymer, cation polyacrylatnide, colloidal
silica or the like.
Among the cation resins, polyamidepolyamineepichlorohydrin is particularly
appropriate.
The content of the cation resin is preferably 1% by mass to 15% by mass and
particularly
preferably 3% by mass to 10% by mass based on the total solid of the ink-
receiving layer.
Examples of the light fastness improving agent may include zinc sulfide, zinc
oxide,
hindered amine-based antioxidant, a benzotriazole-based UV absorbent such as
benzophenone,
and the like. Among them, zinc sulfide is particularly appropriate.
The surfactant serves as a coating aid, a stripping improving agent, a
slipping
preventing agent or an antistatic agent. The surfactant is described in
Japanese Patent
Application Laid-Open Nos. S62-173463 and S62-183457. An organic fluoro
compound
may be used instead of the surfactant. It is preferred that the organic fluoro
compound is
hydrophobic. Examples of the organic fluoro compound include a fluorine-based
surfactant,
an oil phase fluorine-based compound (for example, fluorine oil), and a solid
type fluorine
compound resin (for example, a tetrafluoroethylene resin). The organic fluoro
compound is
described in Japanese Patent Publication No. S57-9053 (8th to 17th columns),
and Japanese
Patent Application Laid-Open Nos. S61-20994 and S62-135826. Other additives to
be added
to the ink-receiving layer may include a pigment dispersant, a thickener, an
antifoaming agent,
a dye, a fluorescent brightening agent, a preservative, a pH adjusting agent,
a matting agent, a
hardening agent or the like. Also, the ink-receiving layer may have one layer
or two layers.
The backcoat layer may be provided into the recording paper and the recording
film,
and the component that may be added to the layer may be a white pigment, a
water-based
binder or other components. Examples of the white pigment contained in the
backcoat layer
may include a white inorganic pigment such as precipitated calcium carbonate,
ground
calcium carbonate, kaolin, talc, calcium sulfate, barium sulfate, titanium
dioxide, zinc oxide,
zinc sulfide, zinc carbonate, satin white, aluminum silicate, diatomite,
calcium silicate,
magnesium silicate, synthetic amorphous silica, colloidal silica, colloidal
alumina, pseudo
boehmite, aluminum hydroxide, alumina, lithopone, zeolite, hydrate halloysite,
magnesium
carbonate and magnesium hydroxide, an organic pigment such as a styrene-based
plastic
pigment, an acrylic plastic pigment, polyethylene, microcapsules, a urea resin
and a melamine
resin, and the like.
CA 02816869 2013-05-22
31
Examples of the water-based binder contained in the backcoat layer may include
a
water-soluble polymer such as a styrene/maleate copolymer, a styrene/acrylate
copolymer,
polyvinyl alcohol, silanol-modified polyvinyl alcohol, starch, cationic
starch, casein, gelatin,
carboxymethyl cellulose, hydroxyethyl cellulose and polyvinylpyrrolidone, a
water-dispersible
polymer such as a styrenebutadiene latex and an acryl emulsion, and the like.
Examples of
the other component contained in the backcoat layer may include an antifoaming
agent, a
defomaing agent an antifoaming agent, a dye, a fluorescent brightening agent,
a preservative,
an insolubilizer and the like.
Polymer latex may be added to a constitutional layer (including the backcoat
layer) of
the inkjet recording paper and the recording film. Polymerlatex is used for
the purpose of
improvement in physical properties of the layer, such as dimensional
stabilization, curling
prevention, attachment prevention, and crack prevention of the layer.
Polymerlatex is
described in Japanese Patent Application Laid-Open Nos. S62-245258, S62-136648
and
S62-110066. When polymerlatex having a low glass transition temperature (40 C
or less) is
added to the layer including the mordant, cracks or curling of the layer may
be prevented.
Further, even though polymerlatex having a high glass transition temperature
is added to the
backcoat layer, curling may be prevented.
The inkjet recording method using the ink of the present invention is not
limited, and is
used in a known manner, for example, a charge control manner discharging an
ink using
electrostatic force, a drop-on-demand manner (pressure pulse manner) using
vibration pressure
of a piezo element, a sound inkjet manner discharging an ink using radiation
pressure by
changing an electric signal into a sound beam and radiating the beam to the
ink, a thermal
inkjet manner using pressure generated by heating an ink to form bubbles, and
the like.
In the inkjet recording method, a manner of injecting ink that is called photo
ink at a
low concentration in a plurality of small volumes, a manner of improving an
image by using a
plurality of inks having substantially the same color and different
concentrations, and a
manner of using colorless transport ink are included.
[Compound represented by Formula (1)]
The present invention also relates to the compound represented by the
following
Formula (1).
SO3M )rn
Formula (1)
I'SO2NRIR2)rt
CA 02816869 2013-05-22
32
In Formula (1), D representes a residue structure in which four hydrogen atoms
are
removed from the compound represented by the following Formula (2).
R' and R2 are R1=R2=H, or R1=R and R2=-L-0O2M.
R represents a hydrogen atom or a monovalent substituent. When a plurality of
R is
present, R's may be the same or different.
L represents a divalent linking group. When a plurality of L is present, L's
may be
the same or different.
M represents a hydrogen atom or a countercation. When a plurality of M is
present,
M's may be the same or different.
n represents the number of 1 to 4, m represents a number of 0 to 3, provided
that m+n
is 4.
(j4R1CM L101 P4R102) n102
=
fil04 00) NR1 5
Formula (2)
SO3¨
R103) n103
In Formula (2), lem, R102 and R103 each independently represent a monovalent
substituent, le 4 and R1 5 each independently represent a hydrogen atom or a
monovalent
substituent, n101 and n102 each independently represent the number of 0 to 5,
n103 represents
the number of 0 to 4. When n101, n102 and n103 each represent the number of 2
or more,
each of pluralities of R101, and le 3 may be the same or different.
Preferred ranges of the compound represented by Formula (1) of the present
invention
and each substituent in Formula (1) are the same as the preferred ranges
defuaed with respect
to the compound represented by Formula (1) contained in the coloring
composition of the
present invention.
EXAMPLES
Hereinafter, the present invention will be described in detail by examples,
but the
present invention is not limited to those examples. In the examples, "%" and
"part" represent
"% by mass" and "parts by mass", respectively otherwise specified.
CA 02816869 2013-05-22
33
(Synthesis Example)
[Synthesis of Exemplary Compound (1B)]
120 g of chlorosulfonic acid and 12.4 g of phosphorous oxychloride were added
to a
500 mL three-necked flask, followed by stirring at room temperature. 19.1 g of
Acid Red
289 (manufactured by Chugai Kasei Co., Ltd., Purity: 71%) was added slowly in
portions
thereto, and then, reacted at an internal temperature of 70 C for 1 hour. The
reactant was left
standing to cool to room temperature and poured carefully to 600 g of ice.
Then, the
precipitated crystal was separated by filtration and washed with cold
saturated brine (Paste A,
about 81 g).
300 mL of water was added to a 1000 mL beaker, and its pH was adjusted to 8.5
with
2N sodium hydroxide. 2.64 g of ammonium chloride was added thereto, dissolved,
and then,
cooled to 5 C or lower. The previously prepared paste A was added in portions
at an internal
temperature of 5 C or lower. 50 mL of water was added, and the internal
temperature was
increased up to 55 C while maintaining the pH to 8.5 with an aqueous sodium
hydroxide
solution, followed by allowing to be reacted until change in pH was
disappeared. The
reaction solution was subjected to dust removal filtration with a GF/F fluter
manufactured by
Whattnan, Inc. About 25% by mass of sodium chloride was added to the obtained
filtrate,
the pH was adjusted to 2.0 with concentrated hydrochloric acid, and the
precipitated crystal
was separated by filtration. The obtained crystal was dissolved in 600 mL of
water while
addusting the pH to 9 with 2N sodium hydroxide, subjected to desalination by
using a dialysis
tube until the conductivity became 10 RS or less, and subjected to dust
removal filtration again
with a GF/F fluter manufactured by Whatman, Inc. The obtained filtrate was
concentrated to
dryness to obtain 10.0 g of a gloss crystal of Exemplary Compound (1B).
From MS spectrum, 894 corresponding to (M-H)" of m=2, n=2, 893 corresponding
to
(M-H). of m=1, n=3 were observed. Further, the ratio of Exemplary Compound
(1B) was
confirmed from the content of dye by using a measured value of water by Karl-
Fischer method
and the content of a sodium ion by ion chromatography.
Other exemplary compounds can be also synthesized in accordance to the
above-described method.
[Example 1]
Deionized water was added to the following contents to be amount of 100 g,
followed
by stirring for 1 hour while heating at 30 to 40 C. Thereafter, the solution
was prepared with
10mol/L of KOH so as to have pH=9, and subjected to a filtration under reduced
pressure with
a microfiltger having an average pore size of 0.25 pm to prepare a magenta ink
solution.
CA 02816869 2013-05-22
34
Composition of Ink Solution 1:
Dye (the following compound (1A)) 3.50 g
Diethylene glycol 10.65 g
Glycerin 14.70g
Diethylene glycol monobutyl ether 12.70 g
Triethanolamine 0.65 g
Olfin E1010 (acetylene glycol-based surfactant, manufactured by Nishin Kagalcu
Co.,
Ltd.) 0.9g
[Examples 2 to 16 and Comparative Examples 1 to 4]
Ink Solutions 2 to 8 and Comparative Ink Solutions 1 to 4 as ink solutions for
comparision were prepared in the same as in preparation of Ink Soultion 1,
except that the dye
was changed as shown in Tables 1 and 2 below.
Further, the ink solutions of Comparative Examples 3 and 4 could not be
evaluated
because the colorants were not dissolved.
(Image recording and evaluation)
The following evaluation was performed on each ink for inkjet recording in
Examples
and Comparative Examples. The results are shown in Tables 1 and 2.
Further, in Table 1 and 2, the hue, ozone fastness, print concentration and
moisture
fastness were evaluated after using each ink for inkjet recording to record
its image on a photo
gloss paper (PM Photo paper <Glossy> (KA420PSK, EPSON) manufactured by EPSON
Co.,
Ltd.) by an inkjet printer (PM-700C, manufactured by EPSON Co., Ltd.).
<Hue>
The hue was evaluated by a three-stage rating, that is, best, good and poor by
naked
eyes. In Tables 1 and 2 below, A denotes that the hue is best, B denotes that
the hue is good
and C denotes that the hue is poor.
<Ozone fastness>
In a box set to an ozone gas concentration of 0.5 0.1 ppm, room temperature
and dark
place using a Siemens-type ozonizer to which an a.c. voltage of 5 kV was
applied while
passing a dry air through the double glass tube, the photo gloss paper having
the image formed
thereon was left standing for 7 days. The image density before and after
standing in an ozone
gas atmosphere was measured by a reflection densitometer (X-Rite 310TR) and
evaluated as
the dye residual ratio. The reflection density was measured at three points of
1, 1.5 and 2Ø
The ozone gas concentration in the box was set using an ozone gas monitor
(Model
OZG-EM-01) manufactured by APPLICS.
CA 02816869 2013-05-22
The evaluation was performed by a three-stage rating, that is, A is a dye
residual ratio
of 70% or more at any density, B is less than 70% at one or two points, and C
is less than 70%
at all densities.
<Print concentration>
The concentration was measured by using X-lite (trade name, manufactured by X-
lite
Corp.) which is a 100% concentration portion of a printed sample, and
evaluated by a
three-stage rating, that is, A is 2.3 or more, B is 2.2 or more and less than
2.3, and C is 2.2 or
less.
<Moisture fastness>
A printed sample was stored under conditions of 35 C and 80% RH for a week,
and
evaluated by a three-stage rating for image spreading, that is, A is no
spreading or a case of
mostly unnoticeable level, B is a case of slight spreading, and C is a case of
clear spreading.
Table 1
Dye of Ozone Print Moisture
Hue
Remark
Formula (1) Fastness Concentration Fastness
Exampl 1 1A A A A A
Inventive
Exampl 2 1B A A A A
Inventive
Exampl 3 1C A A A A
Inventive
_
Exampl 4 1D A A A A
Inventive
Exampl 5 IE A A A A
Inventive
_
Exampl 6 1F A A A A
Inventive
Exampl 7 1G A A A A
Inventive
Exampl 8 111 A A A A
Inventive
Comparative Comparative
A A A C
Comparative
Example 1 Colorant A
Comparative C. I. Acid -
C A A C
Comparative
Example 2 Red 289
Comparative Comparative
- - - -
Comparative
Example 3 Colorant B
(1A) (1B)
(1D) Me Me
SO2R SO2R SO2R SO2R (1C) SO2R
SO2R
...,41
RO2S
so
0 SO2R
Me, Me Me Me Me Me Me Me Me Me Me Me Me Me Me Me
0
N:H KM 0
* 0 N:H H-N 41) 0 410
N,1 H,N 0 0, N:11
RO2S SO2R RO2S 50R R025 2
502R RO2S SO2R
0 SO; so so so SO;
op SO3
R= ONa or NH2, ONa/NH2 = 2/2 R= ONa or NH2, ONa/NH2 = 1.5/2.5
R= ONa or NH2, ONa/NH2 = 3/1 R= ONa or
NH2, ONa/NH2 = 1/3
o
0
F..,
co
(1E) (1F) (1G)
(1H)
0,
co
Me Me
SO2R SO2R SO2R
SO2R Me Me 0,
ko
RO2Sso . SO2R ...gi
RO2S 401 NHAc AcHN 40 SO2R
,
N,
.
1 ,--
w
Et Et Et Et Et Et Et Et i-Pr i-Pr
i-Pr i-Pr Me Me Me M
1-,
e
1
H-N
0 0, N HN
Zii 0 , 0 0 WH H ,
N s,.. Apo N:H
--"I
H 0 -N 00 N
SO2R RO2S
:H 0
ol
1
iv
iv
RO2S SO2R RO2S
SO2R RO2S 902R
õI SO; 0 so3 to
SO3 0 SO;
R= ONa or NH2, ONa/NH2 = 2/2 R= ONa or NH2, ONa/NH2 = 2/2 R= ONa or
NH2, ONa/NH2 = 2/2 R= ONa or NH2, ONa/NH2 = 2/2
CA 02816869 2013-05-22
37
(Comparative Colorant A)
(C. I. Acid Red 289)
ISO3Na SO3Na
Me
c.crl, 1 /503Na
Me Me me Me . Me Me me
H,N 0 Nt ,W 0 NtH
H
411
a033 ..--1101 H
N 303Na 411/ õOP
=io so; 803-
(Comparative Colorant B)
SO2NH2
110
.----
H3c cH, .3c4 c.,
N 0 k I
1-1' I.
.---"
so so;
Table 2
Dye of Ozone Print Moisture
Hue
Remark
Formula (1) Fastness Concentration Fastness
Exampl 9 2A A A A A
Inventive
Exampl 10 2B A A A A
Inventive
Exampl 11 2C A A A A
Inventive
Exampl 12 2D A A A A
Inventive
Exampl 13 2E A A A A
Inventive
Exampl 14 2F A A A A
Inventive
Exampl 15 2G A A A A
Inventive
Exampl 16 2H A A A A
Inventive
Comparative Comparative
A A A C Comparative
Example 1 Colorant A
Comparative C. I. Acid
C A A C Comparative
Example 2 Red 289
Comparative Comparative
- - - - Comparative
Example 4 Colorant B
(2A) (28) (2C)
(20) Me Me
502R SO2R SO2R SO2R LSO2R
SO2R
,,,,c6,,Fca. ...I ../
.....,...
Si RO2S
0
0 502R
Mel Me Me Me Me Me Me Me Me,,,c Me Me
Me Me Me Me Me
H,N 00 N.!.H
0
H,N 0
01 0 N;H1,4 0 0 0 N:".H
HM 0 0,41
RO2S SO2R RO2S SO2R R025
SO2R RO2S SO2R
0 SO; 01 SO; 0 SO;
0 503
cl
P
R-= ONa or NHCH2CO2Na R= ONa or NHCH2CH2CO2Na R= ONa
or N(CH3)CH2CO2N8 R= ONa or NHCH2CO2Na 0
ONaIN1ICH2CO2Na = 2/2 ONa/NHCH2CH2CO2Na = 2/2
ONa/N(CH3)CH2CO2Na = 2/2 ONa/NHCH2CO2Na = 1/3 N)
co
1-,
0,
co
a,
ka
(2E) (2F) (2G)
(2H) N)
Me Me
0
SO2R ../SO2R SO2R
SO2R Me Me w 1-=
RO2S 40 401 SO2R
R025 ill NHAc AcHN aoi 502R 00 u.)
, ..,"õcl. 1110
SO ,
,,
,
Et Et Et Et Et70 Et Et Et i-Pr i-Pr
I-Pr i-Pr Me Me Me Me tv
H 0 0 ...N
N:H H,N
= 0 1,1;H
HõN . 010 W..H H,N 0 0, N:.'11 N)
R025 SO2R R02 SO2R R025
SO2R RO2S 502R
so SO3 SO;
I. "3
0 SO3
R= ONa or NHCH2CH2CO2Na R-= ONa or NHCH2CH2CO2Na
R.= ONa or NHCH2CH2CO2Na R= ONa or NHCH2CH2CO2Na
ONa/NHCH2CH2CO2Na = 3/1 ONa/NHCH2CH2CO2Na = 1/3
ONa/NHCH2CH2CO2Na = 1/3 ONaINHCH2CH2CO2Na = 1.5/2.5
CA 02816869 2013-05-22
39
(Comparative Colorant C)
802NH".-N002H
H30 H3 H3C It H3
0 abh,NtH
4111
soi
As seen clearly from the results in Tables 1 and 2, it is understood that the
ink in
Examples using the coloring composition of the present invention is able to
form an image
which is excellent in moisture fastness as well as hue, print concentration
and ozone fastness.
While the present invention has been shown and described with reference to
certain
exemplary embodiments thereof, it will be understood by those skilled in the
art that various
changes modifications may be made therein without departing from the spirit
and scope of the
present invention as defined by the appended claims.