Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
DYNAMIC CUSTOMIZABLE PERSONALIZED LABEL
BACKGROUND
1. Field
[0001] This
disclosure relates generally to the marketing, sale and distribution of
regulated
health care products to consumers or to health practitioners' patients, and,
more particularly, to
a computer-implemented health care product triage administered closed system.
[0002] This
disclosure also relates generally to the labeling of health care products so
that a
potential user of a particular health care product may understand information
about the product
and when and how to use the product, and, more particularly, to a computer-
implemented
Dynamic Customizable Personalized Label (DCPL), which takes into account and
is
subsequently reflective of information specific to an individual user that
could change the way
the user would use the product or whether that user would be an appropriate
candidate to use
the product at all.
2. Background
[0003] A plethora of protective restrictions are currently imposed by
regulatory agencies on the
sale of certain health care products. These restrictions may impose
significant financial and/or
social costs on sellers, buyers and intermediaries (such as physicians or
third party insurance
companies) that may assess and/or control the use of and payments associated
with these
products. Products falling under these restrictions are often categorized into
classes, some
having different, fewer or more associated restrictions as may be required by
regulatory body.
Generally, the restrictions relate to promoting the safe and effective use of
the product, but other
factors may impact these groupings, such as protecting the public (as in the
case of
pseudoephedrine products that were available over-the-counter, but were moved
behind-the-
counter to protect against the illicit preparation and sale of crystal
metharriphetamine). A similar
regulation applies to "Plan which
is a contraceptive that can prevent unwanted pregnancy if
it is taken with 72 hours of unprotected intercourse. The Food and Drug
Administration (FDA)
considers Plan B to be available "Over-the-Counter" (OTC) for women aged 17
or over, but a
prescription drug for women younger than 17. Furthermore, some "Schedule V"
controlled
substances may be classified as OTC products in certain U.S. States. Such
drugs are sold
without a prescription, but are subject to record-keeping rules, quantity
and/or age restrictions,
and must be dispensed by a pharmacy.
22368806.2 1
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
[0004] In the United States, pharmaceutical products, medical devices and
other healthcare
related products for human use and for animal use are legally grouped into two
distinct classes:
"Prescription" and "Non-Prescription." Prescription products are only
available to consumers
after receiving a prescription as a "patient" of an authorized prescriber
(such as a physician,
physician's assistant, podiatrist, dentist, nurse practitioner, optometrist,
ophthalmologist, etc.).
Non-prescription products can generally be purchased without a prescription.
OTC non-
prescription products are selected and purchased by the consumer themselves at
retail without
pharmacist intervention, or through various forms of e-commerce. These OTC
products may be
sold anywhere such as supermarkets, mass marketers, convenience stores, etc.
The FDA
requires that OTC products be labeled with an approved Drug Facts label to
educate consumers
about the proper use of these medications. Labels are standardized and must be
easy for
consumers to understand. Drug Facts labels include information on the
product's active
ingredient(s), inactive ingredients, indications and purpose, safety warnings,
directions for use,
etc.
[0005] A poorly defined, implied, but not legally defined, second category of
non-prescription
products in the United States, comprises products that are subject to other
restrictions on sale.
While these products are legally classified as non-prescription OTC drugs,
they are typically
stored behind the pharmacy counter and are only sold in stores employing a
registered
pharmacist. Such items are commonly referred to as "Behind-the-Counter" (BTC)
products and
are unavailable in convenience or grocery stores that stock other non-
restricted OTC
medications. BTC products, among other possible requirements, require a
consultation with
and/or authorization by a pharmacist prior to making a purchase. Ostensibly,
pharmacist
counseling aids in making determinations on a variety of factors, such as
authorizing a
consumer to buy a product, tracking product usage frequency, and limiting the
amount of
product sold to the consumer in a given time period (such as, for example, for
pseudoephedrine-based nasal decongestants). However, pharmacist delivered
counseling to
consumers in a retail setting, particularly in very busy stores, is frequently
an inadequate means
of providing fully appropriate screening of individuals to use certain
healthcare products and/or
information on the use of the products to maximize consumers health and safety
benefits when
using such products.
[0006] Certain non-prescription products may actually require an initial
prescription to specify a
product type and dose, but in some States are refillable without a
prescription thereafter (e.g.,
insulin and syringes). For example, although requirements may differ by State,
insulin products,
22368806.2 2
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
such as Beef, Pork, Beef-Pork, and the synthetic "human" insulins are usually
sold as BTC
products without a prescription. However, pharmacies in some States may
require an initial
prescription. Some States allow unlimited refills while others require a
prescription once
annually. The legal regulation of syringe access varies from state to state,
but takes one or
more of three forms: syringe prescription laws and regulations; other pharmacy
regulations or
miscellaneous statutes imposing a variety of restrictions on the sale of
syringes by pharmacists
or others; and drug paraphernalia laws prohibiting the sale or possession of
items intended to
be used to consume illegal drugs. Laws on drug possession may also be applied
in a manner
that, in practical terms, regulates the possession of syringes, and so must
also be considered
for their possible effects on syringe access. Generally, syringes do not
require a prescription,
but the buyer is usually required to demonstrate a legitimate medical purpose
for the purchase.
[0007] Similar distinctions exist in other countries as well, but many
countries have a formal
three tier categorization of health care products: Prescription Only, Pharmacy
Only (which may
also be known as "Counter Prescribing" or "Behind-the-Counter" in some
countries) and
General Sales List (GSL). In Canada, the national model consists of three
schedules or four
categories: Schedule I, Schedule II, Schedule III and Unscheduled, with
specific factors and
conditions for sale expected for each. Pharmacy Only products may require some
level of
pharmacist intervention or supervision to sell the product, but not a
physician's prescription.
The pharmacist determines whether the medicine is safe for the particular
customer based on
the customer's responses to a set of questions, but many pharmacies sell such
medicines with
no questions asked. Examples of these include some sleep aid tablets such as
Nytol , human
de-worming tablets such as Mebendazole, painkillers with small amounts of
codeine (up to 12.8
mg per tablet), and pseudoephedrine.GSL products or similar products are
available "off the
shelf" (similar to most OTC medicines in the United States) and require no
pharmacist
intervention to sell. As a result, they can generally be sold anywhere (e.g.,
retail outlets,
convenience stores, supermarkets, etc.).
[0008] Furthermore, in the United States, some non-prescription products and
nutritional
supplements are also currently sold directly to consumers through food stores,
convenience
stores, mass merchandisers, Internet websites and "direct-to-consumer
infomercials." Such
products are regulated so that they could also be sold in an open,
unrestricted manner for
consumer purchase in retail stores if the product distributor so desired.
22368806.2 3
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
[0009] Because of greater availability and fewer restrictions compared to
prescription drugs,
non-prescription products naturally service a far larger commercial market in
terms of the
number of actual users of many healthcare products. However, due to sizeable
pricing
premiums for prescription products, the commercial market for prescription
drugs is often valued
much higher than for non-prescription products, especially after a
prescription product is
"switched" to non-prescription regulatory status.
[0010] Restrictions on prescription products, while intended to keep patients
safe, may impose
a cost from a public health standpoint, because many consumers cannot afford
the cost of
prescription products, health insurance and/or physician's fees. They may
choose instead to
forego prescription medication and either leave their medical condition(s)
untreated or rely on
other, potentially less safe or potentially less effective remedies, such as
folk remedies,
Homeopathic medicine, Ayurvedic medicine and/or nutritional or other
supplementation.
[0011] As prescription-only products move toward the end of their patent
exclusivity period, it is
sometimes desirable for a pharmaceutical or medical device manufacturer to
move these
products from prescription to non-prescription status. When a prescription
product loses its
patent protection, it is highly vulnerable to generic drug competition. The US
Food and Drug
Administration defines an Rx-to-OTC switch as non-prescription marketing of a
drug product
that was once a prescription-only drug for the same indication, with the same
strength, dose,
duration of use, dosage form, treatment population and route-of-
administration. Once switched,
a product matching the identical specifications cannot be sold simultaneously
as both
prescription and non-prescription products. Therefore, a minufacturer may take
advantage of
this regulation by switching a drug from prescription to non-prescription
status, thereby deterring
generic forms of the prescription product from reaching the market.
Furthermore, in switching
prescription products to non-prescription status, the manufacturer can often
reach a larger
market for the product and lengthen the marketable lifespan of the product.
Therefore, it is clear
that there are various advantages for manufacturers who are able to switch
products from
prescription to non-prescription status.
[0012] In the United States, manufacturers may petition the FDA for a
regulatory status change
from prescription to non-prescription status. The FDA has outlined five
general requirements
governing whether a prescription product may be shifted to non-prescription
status. First, the
medicinal or medical benefits of the product must outweigh the expected health
risks involved
when using the product. Second, the potential for product abuse must be low.
Third, the effects
22368806.2 4
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
of the product should address consumer self-diagnosable issues without masking
an underlying
serious disease. Fourth, the product must have characteristics that allow it
to be adequately
labeled, such that consumers can safely use the product in a proper manner.
Fifth, the product
must be appropriately, safely and effectively useable without requiring
guidance or intervention
from a health care practitioner.
[0013] Two types of studies must be conducted to illuminate whether the
product meets these
last two requirements, often with a self-selection study added component, to
validate if
consumers can determine by themselves if they are an appropriate candidate to
use the
healthcare product. The first type of study, called a "Label Comprehension
Study," requires a
manufacturer to show that a designated proportion of consumers would clearly
understand the
"primary label communication points" on a label for the product in
consideration. The "primary
label communication points" constitutes information on the label that has the
greatest clinical
significance, and includes product usage indications, dose and dose interval,
contraindications,
warnings, drug interactions and when to stop using the product. In addition, a
manufacturer is
required to show that a designated proportion of consumers would clearly
understand the
"secondary label communication points," which address areas of less critical
importance (e.g.,
general health information). Other key communication points may also be
required. The
specific end points for a particular product may vary since "adequate" label
communication is an
issue of clinical judgment and varies depending upon the medical significance
of a particular
communication objective.
[0014] The second type of study, called an "Actual Product Use Study," may be
used to
investigate how a current prescription-only product would be used if it were
procured in an
actual non-prescription, buying situation. The Actual Product Use Study may
test for a plurality
of factors, such as adherence (which considers consumption of the drug and
monitors users for
efficacy and safety in accordance with the drug label), safety (which
considers experienced
negative effects of the drug, or adverse events (AE's) that occur during the
study), and efficacy
(which determines whether the clinical benefit in the prescription setting is
reproducible in the
non-prescription setting).
[0015] These studies must be conducted with scientific rigor, and include all
subject categories
that could potentially use the product, regardless of age, gender, underlying
medical condition
and use of concomitant medications. The Label Comprehension Study must test
label
comprehension even among market segments that have no expressed interest in
using the
22368806.2 5
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
product. It must also consider consumers with low literacy levels,
representing a range of
literacy below an eighth-grade reading level. As a result, these requirements
present a serious
problem for manufacturers seeking to shift their product lines from
prescription to non-
prescription status. Because of the large variation in consumer population
segments, it is
oftentimes impossible to create an adequate label that will satisfy all of the
FDA's requirements.
Consequently, a product that passes rigor in all other areas may fail to
qualify for non-
prescription status due to the difficulty inherent in creating a satisfactory
non-prescription label.
[0016] Given the aforementioned restrictions, which may be potentially
confusing to consumers,
and the limited time and training available for pharmacists to help consumers
diagnose
disorders and use disease and health disorder treatment, and health
maintenance products
properly as intended by regulatory authorities, there is need for an
alterative system that
addresses these systemic problems to ultimately improve consumer understanding
which
promotes the safe and effective use of such products, while also expanding the
range of health
products that are available to consumers without a prescription.
BRIEF SUMMARY
[0017] In one aspect of this disclosure, a controlled, closed marketing, sales
and distribution
system for health-care related products is disclosed. The system comprises a
processor, and
memory comprising program instructions. The program instructions are
executable by the
processor to store a set of health risk factors associated with the controlled
distribution product,
receive consumer requests to purchase the controlled distribution product,
retrieve consumer
information related to the set of health risk factors associated with the
controlled distribution
product, determine whether the consumer is authorized to purchase the
controlled distribution
product based on the consumer information, and sell the consumer the
controlled distribution
product when the consumer is authorized to purchase.
[0018] In another aspect of this disclosure, a computer-implemented system and
method is
disclosed for controlling the closed distribution of regulated health care
related products. A
database coupled to a processor includes a listing of at least one healthcare
related product of a
type that is access controlled based upon predefined medical criteria.
Qualification factors for
each of the listed healthcare related products is stored in the database, the
qualification factors
specifying medical information that must be satisfied before the healthcare
related product can
be distributed to an end user of the healthcare related product. A customer
interface is provided
through which requests can be made to obtain healthcare related products for
the end user. A
22368806.2 6
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
communication link is provided through which distribution of the healthcare
related products can
be directed. A triage module, implemented by program instructions executable
by the
processor, interacts with the customer interface to receive a request from a
requesting customer
to purchase at least one healthcare related product listed in the database.
The triage module
accesses the database to obtain the qualification factors for the requested
healthcare related
product and receives medical information relating to the end user of the
requested healthcare
related product that can be used to determine whether the qualification
factors for the requested
healthcare related product are satisfied. The triage module determines whether
the qualification
factors are satisfied based on the received medical information and issues an
indication via the
communication link that the requested healthcare related product should be
distributed to the
end user if the qualification factors are satisfied. If any of the
qualification factors are not
satisfied, the triage module causes the customer interface to indicate to the
requesting customer
that further action is required to satisfy the remaining qualification factors
before the requested
healthcare related product may be distributed to the end user.
[0019] In another aspect of this disclosure, a computer-implemented system and
method is
disclosed for controlling the closed distribution of regulated health care
related products. A
request to purchase a healthcare related product listed in a database is
received from a
requesting customer via a customer interface, the healthcare related product
of a type that is
access controlled based upon predefined medical criteria. Qualification
factors stored in the
database for the requested healthcare related product are accessed using the
processor. The
qualification factors specify medical information that must be satisfied
before the requested
healthcare related product can be distributed to an end user of the requested
healthcare related
product. Medical information relating to the end user of the requested
healthcare related
product is received using the processor and the processor determines whether
the qualification
factors for the requested healthcare related product are satisfied based on
the received medical
information. An indication is issued via a communication link that the
requested healthcare
related product should be distributed to the end user if the qualification
factors are satisfied.
[0020] In another aspect of this disclosure, a computer-implemented system and
method is
disclosed for generating a dynamic customizable personalized label for a
product. A request to
obtain a product listed in a database for use by the end user is received from
a requesting party
via a customer interface. Qualification factors stored in a database for the
product are
accessed, the qualification factors specifying conditions that must be
satisfied before the
product can be distributed for use by an end user. Information relating to the
end user of the
22368806.2 7
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
requested product is received to determine whether the qualification factors
are satisfied. A
dynamic customizable personalized label for the requested product is generated
by populating
the label with the received information and qualification factors for the
requested product to
enable the requesting party to determine whether the end user is qualified to
use the requested
product.
[0021] In another aspect of this disclosure, a computer-implemented system and
method is
disclosed for generating a dynamic customizable personalized label for a
healthcare related
product. The system and method includes receiving a request to purchase at
least one
healthcare related product listed in a database from a requesting customer via
a customer
interface, the at least one healthcare related product of a type that is
access controlled based
upon predefined medical criteria. The database is accessed to obtain
qualification factors
stored in the database for the requested healthcare related product, the
qualification factors
specifying medical information comprising end user medical conditions that
must be satisfied
before the healthcare related product can be distributed to an end user of the
healthcare related
product. Medical information is received including at least one current
medical condition relating
to the end user of the requested healthcare related product that can be used
to determine
whether the qualification factors for the requested healthcare related product
are satisfied. The
system and method determines whether the requested healthcare related product
is appropriate
to treat or diagnose the at least one current medical condition based on the
received medical
information relating to the end user, and, if so, whether the qualification
factors are satisfied
based on the received medical information. A dynamic customizable personalized
label is
generated for the requested healthcare related product, the dynamic
customizable personalized
label providing an indication as to whether or not the end user is qualified
to use the requested
healthcare related product based on whether the qualification factors are
satisfied.
[0022] The foregoing has outlined rather generally the features and technical
advantages of
one or more embodiments of this disclosure in order that the following
detailed description may
be better understood. Additional features and advantages of this disclosure
will be described
hereinafter, which may form the subject of the claims of this application.
BRIEF DESCRIPTION OF THE DRAWINGS
[0023] This disclosure is further described in the detailed description that
follows, with reference
to the drawings, in which:
22368806.2 8
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[0024] FIG. 1 is a high level representation of the logical components
included or interacting
with an illustrative closed marketing, sales and distribution system;
[0025] FIG. 2 is a flow chart representing a preferred sequence of steps for
implementing the
illustrative closed marketing, sales and distribution system of FIG. 1;
[0026] FIG. 3A is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0027] FIG. 3B is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0028] FIG. 4A is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0029] FIG. 4B is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0030] FIG. 5 is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0031] FIG. 6 is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0032] FIG. 7 is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0033] FIG. 8 is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0034] FIG. 9 is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0035] FIG. 10A is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0036] FIG. 10B is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
22368806.2 9
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[0037] FIG. 10C is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0038] FIG. 11 is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0039] FIG. 12 is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0040] FIG. 13 is a flow chart representing a continued preferred sequence
of steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0041] FIG. 14 is a flow chart representing a continued preferred sequence of
steps for
implementing the illustrative closed marketing, sales and distribution system
of FIG. 1;
[0042] FIG. 15 is a high level representation of the logical components
included or interacting
with another illustrative closed marketing, sales and distribution system that
provides a Dynamic
Customizable Personalized Label;
[0043] FIG. 16 is flow chart representing a preferred sequence of steps for
implementing an
illustrative Dynamic Customizable Personalized Label using the closed
marketing, sales and
distribution system FIG. 15;
[0044] FIG. 17 is flow chart representing a continued preferred sequence of
steps for
implementing the illustrative Dynamic Customizable Personalized Label using
the closed
marketing, sales and distribution system FIG. 15;
[0045] FIG. 18 is flow chart representing a continued preferred sequence of
steps for
implementing the illustrative Dynamic Customizable Personalized Label using
the closed
marketing, sales and distribution system FIG. 15;
[0046] FIG. 19 is flow chart representing a continued preferred sequence of
steps for
implementing the illustrative Dynamic Customizable Personalized Label using
the closed
marketing, sales and distribution system FIG. 15;
[0047] FIG. 20 is flow chart representing a continued preferred sequence of
steps for
implementing the illustrative Dynamic Customizable Personalized Label using
the closed
marketing, sales and distribution system FIG. 15; and
22368806.2 10
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[0048] FIG. 21 is an illustrative representation of a Dynamic Customizable
Personalized Label
output from the closed marketing, sales and distribution system FIG. 15.
DETAILED DESCRIPTION
[0049] This application discloses a health care product "Triage Administered
Closed System"
(TACS) and method for enabling broader sale and classification of prescription
and/or non-
prescription health care products, (or for other health care product
regulatory classification(s)
which may not as yet be legislated) which are meant to regulate drugs,
biologics, medical
devices, diagnostic and/or genetic test(s) or any combinations of these for
human or veterinary
use. It is contemplated that TAGS Products sold in the manner to be described
will meet the
current or future criteria (consistent with this disclosure) for such products
under any regulatory
status including switching from one regulatory status to another. Moreover,
with the inclusion of
enhanced consumer education, comprehensive and fully capturable product
orders, sales and
product returns data, product usage tracking and analysis, pre- and post-
purchase product
counseling, health and health risk profiling and product compliance coaching,
TAGS is expected
to enable this regulatory status change for certain prescription products that
would not otherwise
be approved for sale under conventional OTC or BTC selling methods. In
addition, TAGS may
offer Regulators the opportunity to create new sub-classifications within the
current prescription
and non-prescription regulatory classifications (or, for example, in the case
of Canada, within
Unscheduled or Schedules I, II and III), which would allow the sale and
distribution of healthcare
products to individuals through a closed distribution system where this might
have been
previously prohibited in an Open distribution system or otherwise outside of
the TAGS system.
Also, TAGS is expected to potentially create, for all intents and purposes, a
new space to sell
healthcare products which may fall somewhere in between conventional
prescription-only and
OTC or BTO products which does not currently exist today.
[0050] TAGS may be considered a "closed" system and method because it
preferably enables
complete control, transparency and tracking of 100% of any and all TAGS
Product purchases,
which may include data on when those purchases were made, which products were
purchased
(including lot number, expiration date, etc.), what was actually dispensed,
where it was
dispensed, how it was dispensed and (with respect to the consumer's medical
condition) why it
was dispensed. A TAGS Triage Service may be implemented to provide a
centralized control
schema for organizing and storing this data. The richness of the data may
provide a high level
22368806.2 11
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
of control over consumer use authorization and distribution of authorized
products to
consumers.
[0051] As used herein, the terms "consumer" and "TAGS consumer" are intended
to relate to
the end user of the TAGS product. While it is envisioned that TACS products
may be
purchased by the end user or by other requesting customers on behalf of the
end user (e.g.,
parent, guardian, physician, etc.), medical information used by TAGS 100 to
qualify the
distribution of the TACS Product is intended to relate to the end user of that
healthcare related
product.
[0052] Examples of drug types that may be sold through TAGS as non-
prescription TAGS
Products include (but are not limited to) treatments for diabetes, thyroid
disease, hypertension,
high cholesterol, many diseases of the skin, etc. An example of a medical
device that may be
sold through TAGS as a non-prescription TACS Product may include (but is not
limited to)
contact lenses.Other products that may be switched from prescription to non-
prescription status
under TACS may include chronic use "maintenance" and disease prevention
products with a
benefit-to-risk therapeutic index somewhere in between the corresponding
therapeutic indexes
of current prescription products and the therapeutic indexes of current non-
prescription OTC
drugs.
[0053] TACS also preferably includes a Triage Service responsible for
determining purchasing
eligibility, and counseling consumers on products they purchase through TACS.
By
implementing the proposed TACS methodology, a Triage Service may adequately
identify
candidates who can safely and effectively use the product, and provide counsel
to them in the
safe and effective use of the product on an initial and possibly ongoing
basis. This may help
ensure appropriate drug use compliance, persistence and/or therapeutic
adherence. Therefore,
the usual need for (at least) some medical intervention from a physician or
other healthcare
professional may be eliminated. This may enable a broader range of consumers
(including
those with limited and/or poor education) to better comprehend appropriate
product usage in
tandem with or exclusive of any product labeling. The inclusion of product
candidate screening,
purchasing approval, product information counseling, informative product
advertising and/or
other support or product approval processes may be integrated into TAGS.
[0054] Because Triage Service-based counseling is not locked into any
particular format, it
offers superior education and instruction on use, advantages, and dangers of
the product, and it
may be adjusted to any segment of the population. Additionally, because Triage
Service is
22368806.2 12
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
envisioned as a nation-wide and globally available international service,
consumers with
questions may contact the Triage Service at any time, anywhere in the world,
long after sale of
the product has been completed. Therefore, TACS alleviates many communication
issues
because it introduces the possibility of medical education through informative
advertising, and/or
live guidance from a Triage service that would preferably be available on a 24-
hour a day,
seven day a week basis.
[0055] It is further anticipated that more robust and generally superior
consumer education will
be considered advantageous by a regulatory authority. As a result, a
regulatory authority may
approve of a practice where certain healthcare products are offered for sale
to consumers only
through TACS, preferably on an exclusive basis. As a result, conventional
channels, such as
OTC, BIG and prescribed products may be barred from selling these exclusive
products by the
same regulatory authority. Alternatively, TACS may be used to expand the
available channels
used to market, sell and distribute both prescription or non-prescription
healthcare products to
patients or to consumers directly. In cases where TAGS is the only available
channel,
consumers may preferably utilize TAGS to purchase the product and, in doing
so, will also gain
the healthcare benefits inherent to the channel described in this disclosure.
This exclusivity is
what may enable a regulatory authority to switch the product from prescription
regulatory
classification to a new non-prescription TACS "exclusive" (or on a semi-
exclusive basis, a TAGS
"preferred") status, thereby adopting new TACS protocols as implemented in the
system and
process described below.
[0056] Prices for non-prescription TAGS exclusive products may be reduced
compared to the
same products when they would be sold as prescription-only because direct sale
of exclusive
products to consumers would eliminate the need for wholesaler inventories,
logistics, third party
"payor" and/or other trade channel "middle-man" mark-ups. Prices may also be
lower because,
presumably, prescription-only products near the end of their patent protection
period will have
already recouped much of their product development costs, so the burden on
health care
companies to maintain disproportionately higher pricing compared to off-patent
generic
prescription drugs could be greatly reduced. These factors, in conjunction
with the greater
availability afforded by the TACS protocols, may result in a net public health
benefit, as
consumers have broader and easier access to former prescription products at
presumably lower
prices. Additionally, third party insurance payor programs may no longer be
expected to cover
all or part of the cost of non-prescription products, and physicians would be
freed from treating
patients with many conditions who could treat themselves instead through
accessing the
22368806.2 13
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
products from TACS, thereby alleviating administrative burdens and costs for
consumers, health
care providers, governmental agencies and insurance companies alike.
[0057] TACS may also utilize Electronic Medical Record (EMR) and/or Electronic
Health
Record (EHR) computer programs with data management technology specific to
heath care
records, which preferably enables TACS to maintain permanent (or semi-
permanent), but still
HIPAA compliant or other means of protected, confidential health records for
consumers who
purchase TACS products. This may allow TACS to store, retrieve, analyze,
ascertain, flag and
document changes in a consumer's health risk profile over time, thereby
enabling beneficial
modifications to be made with regard to the consumer's TACS Product regimen.
If
communicated to the consumer or the health care professional overseeing the
consumer's
health, TACS can also enable beneficial modifications to be made with regard
to the consumer's
prescription and/or non-prescription product regimen(s). Preventative
modifications may also
be made, including, for example, preventing the sale of certain TACS Products
to a consumer if
contraindicated, and urging the consumer to see a physician if a higher level
of supervised
medical attention is warranted.
[0058] Individual product purchases are preferably stored and tracked by
purchase date, stock-
keeping unit, lot number and health care product company control number, etc.,
thereby
allowing for analysis of consumer purchases and full control over future sales
or product returns.
This may be done to prevent, for example, product abuse by a consumer, or add
partial
physician oversight to product use. This captured information also enables
superior product
recall management compared to "open system" (prescription, OTC or BTC) sales
channel recall
methods that do not track purchases for each individual consumer in as
detailed a manner as
TAGS, which makes for fast and efficient communication to specific consumers
who actually
purchased recalled products. With respect to open system product
advertisements, notices or
more general messages may be sent to the households of consumers who may have
purchased or consumed the product without knowing whether or not these
consumers actually
did purchase or consume it. Therefore, the TAGS closed system is advantageous
relative to
current open product systems since all purchases are documented using TACS.
For example,
a product recall on a non-prescription product might currently reach only a
portion of the
consumers who purchased the product. Using TAGS, however, targeted
communications may
be made to each and every purchaser because buyer information would preferably
be available
in a centralized database accessed and only retrievable by TAGS.
22368806.2 14
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[0059] One of the most significant factors influencing poor treatment outcomes
of both
prescription and non-prescription products is related to poor usage compliance
of the products.
For example, users of drugs may under- or overdose themselves, or may not
remember to take
the medication at the correct dosing interval, or may stop using the
medication completely,
therefore failing to follow labeled dosing directions. TAGS may provide
product usage
compliance coaching and or follow-up communication with consumers as an
optional service.
Such consumer contacts can encourage the proper use of the product and,
therefore, enable
better treatment outcomes with potentially fewer adverse events.
[0060] Entities that may utilize or approve of the TAGS system and method may
include for
example, in the United States, the FDA, Center for Drug Evaluation and
Research (CDER)
and/or Center for Devices and Radiological Health (CDRH) and any other
regulatory authority,
and medical practices, clinics, hospitals, etc., situated in the United States
and in other
countries abroad. For animal health product applications of TACS, TAGS may be
utilized by the
Center for Veterinary Medicine (CVM), the branch of the FDA that regulates
food, food
additives, and drugs that are given to animals, including food animals and
pets or by the United
States Department of Agriculture that regulates vaccines for animals.
[0061] In sum, the disclosed TAGS methodology will benefit consumers by
enabling a larger
range of prescription and non-prescription healthcare products that can be
sold to consumers
for themselves, their family members, or animals under their care, while
simultaneously
improving consumer safeguards via superior product information, product
counseling,
monitoring of product purchases, and use and coaching for proper product
compliance post
purchase. TACS addresses many deficiencies with current "open system"
Prescription and
OTC or BTC sales methodologies and, in many respects, may provide superior
product safety
and product usage controls compared even to what is available for prescription-
only products.
Current systems with little or no consumer counseling are deficient because
pharmacists are not
particularly well trained or have the time to fully counsel patients and a
busy pharmacy is often
not an ideal setting for serious product counseling. Even if counseled
correctly, many
consumers forget the information they are provided or may become confused at a
later time.
Poor provisions exist for patient screening, follow-up and documentation of
product use, thereby
making it difficult to detect patient misuse or abuse (and indeed, sometimes
no such provisions
exist at all). In contrast, TACS Triage Service counseling, product sales and
TACS EMR/EHR-
enabled consumer record keeping may improve upon these factors for any
healthcare products
that may be sold through the TACS closed system.
22368806.2 15
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[0062] FIG. 1 is a high level representation of the preferred components of an
illustrative
embodiment of TAGS 100. TAGS 100 may be implemented utilizing one or more
computing
systems of varying configuration. Each computing system preferably includes
computing
components for executing computer program instructions and processes. These
components
may include a central processing unit (CPU), memory, input/output (I/O)
devices and a network
interface.
[0063] The CPU processes and executes computer program instructions. Random
access
memory (RAM) and/or fast access cache memory preferably provides fast data
supply to CPU.
Long-term storage may be provided as a more permanent form of computer memory,
and may
be, for example, a hard disk, optical disk, flash memory, solid-state memory,
tape, or any other
type of memory. The database may exist at an onsite TAGS facility (if one
exists) or, it may be
implemented via "cloud-computing" enabled data storage and retrieval.
[0064] The network interface device may provide the computing system with
access to a
Network 13, which may be a wireless or wired connection. Network 13 may be,
for example,
the Internet, a corporate intranet, or any other computer network through
which the computing
system may connect to or otherwise communicate with other computers and
databases for
specialized information that may be necessary for implementation of TAGS 100.
Network 13
may also represent other types of networks, such as telephone networks or
cable networks.
Any network suitable for communication between consumers, TACS 100 and TAGS-
related
components may be included as appropriate.
[0065] Regulatory Authority module 1 may represent one or more computer
systems and
processes controlled and/or utilized by a regulatory authority, such as (but
not limited to) the
FDA, CDER and/or the CDRH, etc. Regulatory Authority module 1 may be adapted
to meet any
requirements such an authority would desire for implementation of the TAGS
100. For example,
a regulatory authority may require full cataloguing and regular reporting of
all transactions
carried out under the TAGS 100, including details such as (but not limited to)
disease
classification, product type, consumer identification and purchaser's address,
phone number,
etc. Under this requirement, TAGS 100 may communicate that information
automatically to the
regulatory authority by communicating with Regulatory Authority module 1. Such
information
may be relayed to the Regulatory Authority module 1 by, for example,
electronic conveyance via
Network 13. TAGS 100 and Regulatory Authority module 1 may also be required to
communicate with a health care product company responsible for manufacturing,
marketing,
22368806.2 16
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
selling and/or distributing TAGS applications, processes and/or products sold
via TAGS 100.
TACS 100 may, therefore, communicate directly with Health Care Product Company
module 12,
a module responsible for supporting the health care product company
(encompassing at least
health care product manufacturing, marketing and/or selling), which may then
analyze the data
and subsequently communicate its findings and recommendations to Regulatory
Authority
module 1 in turn.
[0066] Triage Service module 2, Order Intake Service module 3, Order
Fulfillment & Shipping
Service module 4, Banking Service module 5, TACS Pharmacy Dispensing module 6A
and
Automated TACS Dispensing Machine module 6B are preferably be included as
components of
TACS 100. In accordance with the system description above, Triage Service
module 2, Order
Intake Service module 3, Order Fulfillment & Shipping Service module 4,
Banking Service
module 5, TACS Pharmacy Dispensing module 6A and Automated TAGS Dispensing
Machine
6B may be implemented as one or more computer systems, software processes
operating on
one or more computers concurrently, and/or database computer systems, etc.
Alternatively,
these modules may be implemented as distinct logistical components of TAGS
100, housed in
separate systems, intercommunicating with TACS 100 and each other over, for
example, a
Network 13.
[0067] Triage Service module 2 may be implemented as one or more computer
systems,
software processes operating on one or more computers concurrently, and/or
database
computer systems, etc. Triage Service module 2 may be tasked with supporting a
TAGS-
implemented Triage Service, which generally assists with consumer
qualification to purchase a
TAGS Product, pre- and post-purchase product counseling, physician scheduling,
diagnostic
test scheduling, product usage compliance coaching, consumer health profiling
and profile
tracking, pharmacovigilance and other direct customer communications.
Communication with
Triage module 2 may take any form desired, but preferably includes at least
both in-bound and
out-bound telephone calls, e-mails and electronic communications over Network
13 between
TAGS 100 and Consumer 11. Triage Service module 2 may, therefore, be equipped
with, for
example, a computer server for receiving and transmitting consumer information
and enabling
interaction with consumers over a Network 13.Alternatively, Triage Service
module 2 may be
equipped with a computerized automated telephone system that may issue voice
commands
and receive voice and/or keypad tones, such as dual-tone multi-frequency
(DTMF) tones. Other
forms of input may be implemented as required.
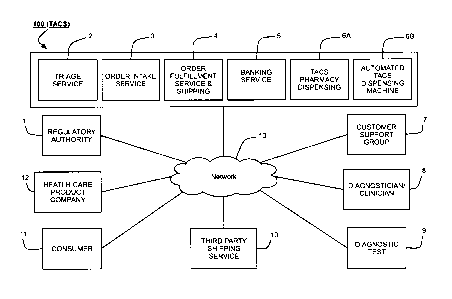
22368806.2 17
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[0068] Triage Service module 2 may also include support sub-components, such
as an
Electronic Medical and/or Health Records (EMR/EHR) software package or system.
EMR/EHR
systems enable doctors to write electronic prescriptions (i.e., e-prescribing)
and maintain
EMR/EHR databases. It is also envisioned that doctors may utilize TAGS 100 to
order TAGS
products on behalf of their patients. Sub-components, such as EMR/EHR packages
and
systems, may be implemented internally in TACS 100 or created using third
party solutions and
communicatively coupled to TACS 100. Non-local embodiments of EMR/EHR may be
interconnected with TACS 100 and Triage Service module 2 (or other TACS 100
components, if
desired) over Network 13. EMR/EHR packages generally include a repository of
patient
information viewable in clinically relevant ways, a workflow tool for patient
care process inside
an organization, a connectivity tool to exchange information outside the
organization, a tool for
clinical decision support and population management, and a clinical front-end
that is integrated
with core back-end billing processes. Such features may enable TAGS 100 to
provide users
with concurrent tracking and documentation of prescription products, TACS
products and other
non-prescription products.
[0069] Available data on a particular consumer's use of any drug, biologic,
medical device,
diagnostic or genetic test may be stored in an EMR/EHR system and routed
automatically to
pharmacies and to third-party payment providers (e.g., private or government
sponsored
medical insurance carriers that make payments to cover all or part of the cost
of the health care
products for covered individuals). Such data is preferably transmitted over
secure network
services, such as (but not limited to) an e-prescription network (e.g.,
Surescripte), thereby
enabling two-way data communication between TACS 100, EMR/EHR and all
authorized
parties. The EMR/EHR package, therefore, preferably assists TACS 100 in
tracking and
managing TACS purchases, TACS purchase authorizations and a plurality of other
related
aspects.
[0070] The EMR/EHR package may also facilitate the process used by Triage
Service module 2
to assess and qualify (or disqualify) purchase of a TACS Product. Given that
eventually,
virtually all health care providers will utilize EMR/EHR technology and
infrastructure, historical
prescription data for any individual consumer may be collected by EMR/EHR-
enabled
Pharmacies and stored on EMR/EHR systems. Therefore, implementation of an
EMR/EHR
software package or system may facilitate creation of a central repository for
consumer
prescription history. This may allow Triage Service module 2 to download a
consumer's
prescription drug and diagnostic test and other health related history through
EMR/EHR,
22368806.2 18
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
regardless of the physician who wrote the prescription, the provider who
performed the
diagnostic test, whether the drug was prescribed conventionally, or whether it
was prescribed
through an e-prescription process. EMR/EHR technology may also enable Triage
Service
based identification, storage and communication of product AE's for
pharmacovigilance
purposes, tracking drug-drug interactions, etc. This may enable TAGS 100 to,
for example,
determine and/or track the subsequent implications of allowing or disallowing
a TAGS Product
purchase, given other drug or diagnostic test products a TAGS consumer may be
using. Finally,
in embodiments where EMR/EHR is implemented non-locally, TAGS 100 (through
Triage
Service module 2) may require download of a consumer's product usage history
to TAGS 100 if
doing so would improve the performance of TAGS 100 or provide better server
for the consumer
in question.
[0071] EMR/EHR programs may present other uses as well. For example, product-
billing
services may also be managed through such an EMR/EHR package. Diagnostic
testing may
also benefit from EMR/EHR integration, as diagnostic testing information may
be captured and
tracked over a series of diagnostic test events for individual users.
Information stored in
diagnostic testing entity systems, such as a patient's diagnostic history and
genetic information,
may be coupled to the exemplary EMR/EHR component of Triage Service module 2.
This
information may be accessed automatically through Network 13 for direct use by
Triage Service
module 2. EMR/EHR may also be adapted to receive supplemental information from
consumers accessing Triage Service module 2. Information from consumers on a
variety of
topics may aid a Triage Service in addressing a variety of needs, concerns or
problems. For
example, EMR/EHR acceptance of information related to the use of non-TAGS open
system,
prescription, OTC or BTC products may aid the Triage service in identifying
potential AE's or
drug-drug interactions.
[0072] Order Intake Service module 3 may represent one or more computer
systems or
software processes associated with taking consumer orders, processing payment
and
transmitting information to begin order fulfillment. Order Intake Service
module 3 may be
implemented as one or more computers and/or software processes operating in
the framework
of TACS 100. Order Intake Service module 3 is preferably adapted to accept
order information
from a wide variety of formats, including at least telephone orders,
electronic orders (e.g.,
through an online interface, such as (but not limited to) a web page), or in-
store (presumably
after pre-authorizing the purchase through Triage Service counseling). Other
types and/or
combinations of formats may be implemented as necessary or as desired, such as
standardized
22368806.2 19
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
mail order forms accompanied by physical or electronic mail order catalogues.
Order Intake
Service module 3 is also preferably adapted to transmit the order information
to the Order
Fulfillment & Shipping Service module 4, which is preferably responsible for
shipping the
product directly to the consumer, or as described further below, to a TACS 100
affiliated secure
dispensing location of the consumer's choice for pick-up (such as at a TAGS
Pharmacy or a
TACS automated dispensing machine, either of which may preferably access TACS
100
through Network 13). Information for standing orders for a particular TACS
customer may, for
example, be stored in a queue in a TAGS database. When the TACS product is
picked up from
the TAGS retail location, the order for the person may be designated as
completed, and the
order information is preferably removed from the queue. During the purchase of
a TACS
product, Order Intake Service module 3 (and/or Order Fulfillment & Shipping
Service module 4)
may be tasked with transmitting the purchaser's payment information (e.g.,
credit card number,
bank information, etc.) to the appropriate handler, such as Banking Service
module 5.
[0073] Order Fulfillment & Shipping Service 4 preferably represents technical
systems for
implementing back-end logistical support for TACS 100. Order Fulfillment &
Shipping Service 4
is preferably tasked with packaging and transporting products directly to TACS
product buyers
or to TACS retail pick-up destinations (described further below). It may also
provide tracking
systems for orders, and field help-desk personnel for responding to customer
inquiries. Finally,
Order Fulfillment & Shipping Service 4 may provide technical documenting and
support for
warehousing, inventory and other related tasks.
[0074] Order Fulfillment & Shipping Service module 4 preferably operates in
conjunction with
Third Party Shipping Service module 10. Third Party Shipping Service module 10
may
represent technical systems and processes operating within third party
shipping providers that
may be utilized by TAGS 100 to deliver products to consumers or to pick-up
locations, such as
(but not limited to) UPS or FedExe. Third Party Shipping Service module 4
may, therefore,
represent one or more computer systems and/or processes provided by these
third parties for
receiving and forwarding customer inquiries, creating order information,
managing warehouse
inventory and providing shipping and tracking services. Therefore, Third Party
Shipping Service
module 10 preferably intercommunicates with Order Fulfillment & Shipping
Service module 4 to
provide TACS Product purchasers with tracking and shipment information.
[0075] Banking Service module 5 preferably represents computer systems and
processes
responsible for processing payments for purchases made through TACS 100.
Banking Service
22368806.2 20
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
module 5 may receive information pertinent to consumer payment, such as (but
not limited to)
information relating to bank accounts, credit cards, money orders, money
wires, cash or any
other suitable form of payment. Banking Service module 5 preferably has the
ability to utilize
this information to draw and deposit funds in a variety of financial systems,
including credit card
systems, bank accounts, etc., thereby giving Banking Service module 5 the
ability to process
payments, issue refunds and credits, place funds on temporary authorization
hold, etc.
[0076] TAGS Pharmacy Dispensing module 6A (as described below) preferably
represents one
or more computer systems and/or software processes associated with the
dispensing of
products to consumers, if such a distribution is allowed. For example, TAGS
Pharmacy
Dispensing module 6A and Automated TACS Dispensing Machine module 6B (as
described
below) may constitute computer terminals connected through Network 13 to TAGS
100.
Although TACS Pharmacy Dispensing module 6A and Automated TAGS Dispensing
Machine
module 6B may be situated in remote locations and may or may not be
interconnected
themselves, both modules may be considered components of the illustrative
embodiment of
TAGS 100, and have, therefore, been included within the preferred TACS 100
components
illustrated in FIG. 1. TACS Pharmacy Dispensing module 6A preferably operates
within a
pharmacy staffed by pharmacists or other authorized pharmacy store personnel
to coordinate
and control the secure dispensing of TACS Products to consumers authorized to
purchase a
TAGS Product. In contrast, Automated TACS Dispensing Machine module 6B may be
installed
in a variety of different locations. Preferably, Automated TACS Dispensing
Machine module 6B
operate adjacent to or within a retail location. However, they may
alternatively be located
outside of conventional retail environments, such as (but not limited to)
locations situated in
close proximity to high pedestrian traffic, thereby enabling high visibility
and convenient access
to the machine for many people (e.g., in airports, on cruise ships, in train
stations, etc.). Both
modules 6A and 6B may document and store detailed order information and
requirements for
dispensing TACS Products, provide access to consumer order information and
allow secured
and authenticated consumer pick-up of TACS Products from the pharmacy
(presumably from
behind the counter) or from a secure controlled vending machine that is linked
to TAGS 100.
[0077] In yet another embodiment, Automated TAGS Dispensing Machine module 6B
may
comprise stand-alone vending stations containing pre-filled, pre-packaged and
labeled
containers of the product (not unlike soda and snack machines). The stations
may alternatively
contain TACS Product consumption (dosage or usage) units that are
automatically counted,
filled, packaged and/or labeled by the machine itself (similar to Parata
Systems' automated
22368806.2 21
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
prescription medication dispensing machines), and subsequently dispensed to
consumers in
one or more fixed quantities or in quantities chosen by a TAGS 100 operator or
by the end
consumer at the point-of-purchase. The ability to purchase multiple packages
and/or large
consumption units at one time may be advantageous because it enables various
benefits, such
as the ability to offer price discounts for larger volume orders, or the
ability to purchase product
quantities sufficient to last multiple months at one time. However, it should
be noted that
quantity limitations may be set for individual purchases by the health care
company and/or
regulatory authority.
[0078] In yet another embodiment, the consumer may order the TAGS Product
directly from the
Automated TACS Dispensing Machine module 6B, which may contain a terminal that
communicates with Triage Service module 2, Order Intake Service 3 and/or Order
Fulfillment
Service & Shipping module 4 (or other components of TAGS 100). The consumer
may choose
to have the purchased product shipped by the Order Fulfillment Service &
Shipping module 4 to
an address of their choosing (preferably the consumer's residence) if, for
example, the product
is unavailable for pick-up at the Automated TAGS dispensing Machine module 6B.
[0079] The Automated TACS Dispensing Machine module 6B may utilize a video
screen to
provide the consumer with information about the TACS Product, and provide a
means for the
consumer to interact with Triage Service counseling. The machine module 6B may
also include
a microphone, telephone or other audio communications device that would allow
the consumer
to speak with a live Triage-based counselor, when desirable. Advertising for
TACS Products
and/or non-TACS Products may be displayed when the machine module 6B is not
being utilized
by a TACS consumer.
[0080] Additional modules are preferably utilized with TACS 100. For
instance,
Diagnostician/Clinician module 8 may represent one or more computer systems or
software
processes operating within a diagnostician or clinician's office or office
infrastructure tasked with
communicating with TAGS 100 over Network 13. Diagnostician/Clinician module 8
may allow a
diagnostician/clinician to perform a variety of tasks relevant to TACS 100,
such as (but not
limited to) approving or disapproving the initial purchase or repeat purchases
of products by a
consumer who is a patient of the diagnostician/clinician, overseeing the
products purchased by
a consumer to prevent product misuse, synchronizing consumer/patient
information with respect
to TACS Products used by patients, sending approval or requests for diagnostic
tests, etc.
Physician's, optometrists, ophthalmologists,
dentists, podiatrists, psychologists,
22368806.2 22
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
psychotherapists and other authorized diagnostician/clinician professionals
may be given
access to TAGS 100 and its information repositories, thereby allowing them to
potentially track
and influence which TAGS Products their patients may be using.
[0081] Diagnostician/Clinician module 8 may be implemented as one or more
computer
systems or processes operating independently of TACS 100.
Diagnostician/Clinician module 8
may interface with TAGS 100 via Network 13. Diagnosticians and clinicians may
encompass a
variety of health care professionals, including (but not limited to) medical
doctors, dentists,
podiatrists, psychologists, physician's assistants, nurse practitioners, oral
healthcare providers
and other health care providers who provide similar decision support commonly
related to
diagnosing and treating and preventing disorders that may result in
prescribing or
recommending prescription and/or non-prescription drugs, biologics, medical
devices and/or
genetic or diagnostic test usage. A diagnostician/clinician (or other health
care professional)
may access a remote Diagnostician/Clinician module 8 by utilizing, for
example, an independent
computer terminal or other network-enabled device and communicating with
Diagnostician/Clinician module 8 over Network 13. Alternative arrangements may
be utilized as
well. For example, Diagnostician/Clinician module 8 may be built into TACS 100
as a hardware
system or software process (or some combination therein). Information
pertinent to the
Diagnostician/Clinician module 8 may be stored locally within
Diagnostician/Clinician module 8.
Alternatively, information may be stored remotely on a separate database, or
even on a TACS
100 database, in which case, it is preferably remotely accessible over Network
13.
[0082] Diagnostic Test module 9 may represent one or more computer systems or
software
processes that facilitate communication between TAGS 100 and diagnostic
testing laboratories
that conduct health care related tests and procedures. Diagnostic Test module
9 may be
implemented as an integrated TAGS 100 component accessed remotely by
diagnostic facility
personnel over Network 13. A diagnostic examination and/or test result based
diagnosis may
be necessary initially and/or periodically thereafter to ensure proper use of
a TACS Product.
Diagnostic Test module 9 may, therefore, enable, for example, electronic
notification and
scheduling of incoming consumer patients and the tests to be conducted,
storage and
transmission of diagnostic lab test results to the Triage Service, physicians
and/or other
paramedical personnel (via Triage Service module 2 and/or
Diagnostician/Clinician module 8,
for instance), and the upload of such information to TACS 100 itself.
223688062 23
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
[0083] In another embodiment, Diagnostic Test module 9 may refer to
communicative systems
and processes contained within self-testing diagnostic devices. Self-testing
diagnostic devices
are test devices used to perform health or genetic tests, and can be usually
used personally by
consumers in their homes. Such devices may include, for example, blood
pressure monitors or
blood glucose level testers. Preferably, these personal-use Diagnostic Test
modules 9 contain
communicative systems and processes enabling them to communicate directly with
TAGS 100
over Network 13 to report the results of a diagnostic test conducted at home
by the consumer.
Alternatively, consumers may enter test values into TACS 100 manually by
accessing consumer
module 11.
[0084] Consumer module 11 may represent one or more computer systems that
facilitate
communication between TACS 100 and the consumer over Network 13. Consumer
module 11
may, therefore, represent telephones, personal computers, notebook computers,
network-
enabled televisions, tablets, smart phones and any other suitable device for
accessing TAGS
100 remotely. These devices may utilize Network 13 to access TAGS 100 or sub-
components
of TAGS 100.
[0085] FIG. 2 is a flow chart representing a preferred sequence of steps for
implementing TACS
100. In step 201, a consumer who has learned of a product available on TACS
100 may access
TACS 100 through Consumer module 11 to initiate purchase of the product by the
consumer or
by another requesting customer other than the consumer on behalf of, for
example, a
dependent child or elderly relative, or an animal. It is also envisioned that
a treating
diagnostician/clinician may order TACS Products on behalf of a patient. In
those instances
where the requesting customer is not the end user of the TAGS Product, it is
understood that all
health related information utilized by TAGS 100 specifically relates to the
end user of the TACS
Product.
[0086] TAGS Products may be advertised in a variety of ways, including (but
not limited to) all
traditional forms of advertisement, such as public broadcast media (e.g.,
television, radio, etc.),
Internet advertisements, e-mail-based mailing lists, physical and digital
product catalogues, etc.
TAGS related personnel and facilities, such as diagnostician/clinicians,
physician's offices,
hospitals, pharmacies, clinics, stores, etc., may also refer consumers to TAGS
100 through
advertisements, telephone numbers, internet websites or other locations from
which the
consumer may purchase a TAGS Product. In particular, highly informative
advertisements
(similar to some infomercials and preferably, more detailed and comprehensive
than
22368806.2 24
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
conventional direct-to-consumer (DTC) advertisements for prescription drugs in
the US) may be
employed to communicate information about TAGS Products, thereby educating
consumers
about the advantages/disadvantages of using certain TAGS Products, helping
consumers
understand whether they are qualified candidates to purchase the TAGS
Products, cautioning
consumers on which drugs or other products they should avoid when taking a
TAGS Product,
and directing consumers to purchase the product from a TAGS center, etc. These
marketing
efforts, alone or combined with other or related Customer Relationship
Management (CRM)
vehicles, methods, processes, etc., may help ensure that consumers are better
informed about
their choices for self diagnosing, self treating, preventing or otherwise self
managing certain
health issues.
[0087] Consumers are preferably given a single TACS account number for each
end user of the
TAGS Product, which is used for all subsequent TAGS activities, including
purchasing,
delivery/pick-up, adverse event reporting, product returns, order status
checking, general
inquiries, etc. The TACS account number may serve as a unique TAGS identifier
for that
consumer or end user, and all information stored relating to the consumer or
end user may be
associated with the TACS account number. The TACS account number may be
assigned (or
selected) upon the consumer's first use of TAGS 100.
[0088] TACS 100 may be accessed in a variety of ways. TACS 100 preferably has
a website
presence on the global Internet, not unlike many online retailers. A front-end
website or
specialized digital interface may be provided for interaction with the
consumer, backed by a
database that stores relevant Health Insurance Portability and Accountability
Act (HIPAA)
compliant information about the TAGS Consumer (e.g., a health history) and
product
information, including available inventory, associated purchasing
requirements, current available
stocks and prices, etc. Digitized graphical information, such as barcodes and
digital imprints
may also be utilized to deliver general or special information on TACS
Products to consumers
who have the means to read them. Such digitized graphical information may be
delivered to
Consumer module 11 over Network 13.
[0089] The database also preferably contains user information, including basic
identifiers such
as name, address and other identification. Additional features may be added to
increase the
level of consumer-identification security. These features may encompass
special identifiers,
such as biometrics, alphanumeric codes, graphical codes, social security
numbers, insurance
identification, etc.
22368806.2 25
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
[0090] The consumer or requesting customer may access the web-based interface
through
Network 13 via Consumer module 11, which may represent (as described above)
some
network-enabled computerized device (e.g., the consumer's personal computer, a
"smart"
phone, hand-held computing device, etc.). Consumers may also access TACS 100
through
other means, such as retail terminals (e.g., computer terminals located inside
a retail
pharmacies, food stores or even airports, train stations, other venues, etc.).
Other alternative
access methods may be implemented as desired. Automated TAGS Dispensing
Machines 6B
may also provide means for accessing TAGS 100. As described above, the
Automated TACS
Dispensing Machine 6B may contain display and communication systems that
enable full
functionality for the TACS functions (described below).
[0091] The consumer may also access TAGS 100 through a telephone, and issue
commands to
TACS 100 via Dual-Tone Multi Frequency (DTMF) tone input or voice command. A
toll free or
paid telephone number may be provided for this purpose. TACS 100 may
communicate with
the consumer via, for example, interactive computer program, interactive voice
response (IVR)
programs, etc.In yet another embodiment, TACS 100 may be accessed through
direct mail
order. Catalogues, fill-forms and other materials may be provided to enable
communication with
TAGS 100 via standard mail. Other methods, such as interactive networked
television menus,
interactive kiosk-mounted computers, etc. may also be implemented as desired
to permit the
consumer to access TAGS 100.
[0092] In step 202, TACS 100 may present the consumer with options. These
options
preferably correspond to the functions performed by TAGS 100, and may comprise
(at least)
TACS Product Ordering, TAGS AE Reporting, TAGS Product Returns, TACS Order
Fulfillment
Status Inquiries, TACS Product Recall Information and General Questions. These
options may
be presented, for example, via display on a computer display, if a consumer is
accessing TACS
100 from a remote terminal. Alternatively, these options may be communicated
by automated
voice if the consumer is accessing TAGS 100 by phone. TAGS 100 may then
determine, in step
203, which option was selected based on the input supplied by the consumer.
[0093] FIG. 3 is a flow chart representing a continued preferred sequence of
steps for
implementing TAGS 100, if the consumer selected Product Ordering from the TAGS
100
options. In step 300, the TACS ordering process may be initiated when the
consumer selects a
TAGS Product for purchase. If TACS 100 is being accessed from a remote
computer terminal,
the consumer is preferably provided with a graphics and text based display
that allows the
22368806.2 26
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
consumer to select one or more TACS Products. TACS Products may be organized
into
categories (preferably utilizing a back-end database) to enable faster and
more efficient
searching, as typical of many modern day online retailers. A search algorithm
may also be
provided, enabling the consumer to search by name, category, ailment and any
other factor or
descriptor that will aid the consumer in finding the desired TAGS Product.
[0094] If TACS 100 is being accessed through a phone interface, voice commands
and DTMF
tones are preferably utilized to navigate the menu. Because the system would
lack a visual
interface, information may be read aloud to the consumer (for example, through
IVR utilizing
voice or DTMF commands). As mentioned above, a physical TACS Product catalog
may be
provided to the consumer as well. The TACS Product catalog may comprise
different variants
for different consumers. For example, a primary product catalog may contain a
substantially full
list of available TAGS Products. Alternatively, a targeted product catalog may
be issued
uniquely to specific consumers, and list only those products for which the
consumer is currently
authorized to purchase. TAGS Product catalogs may be mailed to the consumer on
request, or
stocked, for example, in pharmacies, physician's offices, clinics, etc. TACS
Products may be
identified in the catalogue via an identifier, such as a product code. The
identifier may then be
utilized to select a product on TACS 100, preferably across all TAGS 100 input
methods (e.g.,
global product network internet site, text messaging, DTMF input and/or other
controlled
electronic means during ordering, etc.).
[0095] In step 301, TACS 100 preferably identifies the consumer.
Identification of the
consumer may occur before or after the consumer has selected a TACS Product
for purchase
(similar to the manner in which many online retailers function).
Identification may be performed
through a TACS-provided code or identifier if the consumer is already a
registered TAGS
purchaser. Alternatively, the information may be retrieved automatically from
TACS 100 data
storage based on some identifying token provided by the consumer or on behalf
of the end user
of the TACS Product. This token may include, for example, biometric
information (such as a
retina or fingerprint scan), identifying codes (such as a social security
number, driver's license,
etc.), or even something as simple as a TACS user name and password. lithe
consumer is not
already a registered TAGS purchaser, TACS 100 may present options allowing the
consumer to
register with TACS 100, preferably requesting basic information, such as name,
address and
phone number. Other forms of information may be requested as well. The
registration process
may be conducted through an Internet website, over telephone or through a
manual fill-out and
22368806.2 27
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
mail-in form. Registration may also be performed on behalf of the consumer at
health care
provider facilities, such as hospitals, diagnostician/clinician facilities,
etc.
[0096] In step 302, TACS 100 (through Triage Service module 2) preferably
determines if the
desired TAGS Product requires a prescription for purchase. Some TACS Products
may still
require a prescription for purchase despite being available on a non-
prescription basis since a
diagnostician may first need to determine what particular product strength,
type or characteristic
is appropriate to treat, manage or prevent a particular disorder. Thereafter,
the consumer may
purchase the TACS product on a non-prescription basis until such time that a
revised
prescription may be required. Examples of such products may include insulin
and syringes.
Contact lenses may also be included, as an example, if a regulatory authority
permits a
classification switch to a non-prescription TACS classification (in accordance
with this
disclosure). If the TACS Product requires a prescription for sale, the process
may proceed to
step 303. If the TACS Product does not require a prescription for sale, the
process may instead
progress directly to FIG. 3B. In step 303, TACS 100 may determine whether or
not the
consumer has obtained a prescription. If the consumer has not obtained a
prescription, then the
process may end and the consumer may be denied authorization to purchase the
TACS
Product until they obtain a valid prescription. Alternatively, if the consumer
has obtained a
prescription, then, in step 304, the prescription information is preferably
forwarded to TAGS 100,
and the process may progress to FIG. 3B.
[0097] Certain rules and limitations may be imposed on these process steps.
For example, if
contact lenses are switched from prescription to non-prescription status (due
to the adoption of
a TACS protocol), certain current legal restrictions may be levied against the
sale of contact
lenses on TAGS. For example, the Fairness to Contact Lens Consumers Act
(FCLCA)
promulgates certain rules for prescribers, requiring them to supply patients
with a copy of their
contact lens prescription(s) at the end of a contact lens fitting, even if the
patient has not
requested it.
[0098] FCLCA also stipulates that the prescription cannot be filled unless the
seller has either
received a copy of the prescription or has verified the prescription as
required by the Act. Time
constraints are also imposed, as the prescription cannot be filled if the
prescriber tells the seller
that the prescription is inaccurate, expired, or otherwise invalid by "direct
communication" within
"eight-business-hours" after receiving the complete verification request.
"Direct communication"
by telephone (with respect to TACS 100) may require reaching and speaking with
a TAGS
22368806.2 28
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
Triage Service provider or Prescriber recipient as the case may be, or leaving
a voice message
on a telephone answering machine of the intended recipient. "Direct
communication" by
facsimile or electronic mail requires that the intended recipient actually
receive the facsimile or
electronic mail message.
[0099] TACS 100 may, therefore, be required to comply with the requirements of
the Act if it
were to sell non-prescription classed contact lenses. In accordance with the
FCLCA, Triage
Service module 2 may be tasked with sending a verification request to the
prescriber
electronically by telephone, facsimile or electronic mail, or through other
legally suitable means.
An exemplary TACS contact lens ordering and verification process may function
as follows.
First, the consumer may send contact lens prescription information to Triage
Service module 2
in the form of a facsimile or as an electronically e-mailed secure scanned
image of the
prescription. If a prescription is not submitted to Triage for TACS approved
contact lenses,
Triage Service module 2 may deny sale authorization until a valid prescription
is secured from
an authorized provider. Second, Triage service module 2 may then submit the
prescription to
the prescriber in the form of a verification request. Third, the prescriber
has eight-business-
hours to respond, in accordance with the FCLCA. If the prescriber does not
respond within the
required time, then the prescription may be verified automatically, thereby
allowing Triage-
based authorization of the sale of contact lenses to the consumer. If the
prescriber responds in
the required time and approves the verification request, then the Triage
service may directly
authorize the sale of contact lenses to the consumer. However, if the
prescriber denies the
verification request, Triage Service module 2, may deny sale authorization
until a valid
prescription is secured from an authorized provider.
[00100] FIG. 3B is a flow chart representing a continued preferred sequence
of steps for
implementing TAGS 100. In step 305, based on the available consumer
identification
information, TAGS 100 preferably obtains the consumer's purchasing history.
The consumer's
TACS purchase history is preferably stored on a TACS 100 or TACS 100 related
database
(such as the EMR/EHR package system described above) and associated with
consumer
identification information. Once the consumer's identification is retrieved,
TACS 100 may
retrieve the consumer's TAGS purchase history. In step 306, TACS 100
preferably determines,
based on the consumer's purchase history, whether the current purchase is an
initial purchase
of a TAGS Product or a subsequent purchase of a TACS Product. TACS 100 may
accomplish
this step by algorithmically comparing the current to-be-purchased TAGS
Product to the
consumer's purchase history. Alternatively, TACS 100 may simply query the
consumer to
22368806.2 29
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
indicate whether the current purchase is an initial purchase or a subsequent
purchase. If no
match is found, the current purchase may be deemed an "initial" purchase, and
the process may
proceed to FIG. 4. If a match is found, then the current purchase may be
deemed a
"subsequent" purchase, and the process may proceed to FIG. 8.
[00101] FIG. 4A is a flow chart representing a continued preferred sequence of
steps for
implementing TACS 100 when it is determined that a current purchase is an
initial purchase.
Each TAGS Product preferably has a guideline algorithm that determines
consumer eligibility for
purchase. The guideline algorithm may take into account a variety of health
risk factors related
to the use of the product by the consumer, such as (but not limited to) age,
race, gender,
weight, personal medical history including (but not limited to) current
prescription and non-
prescription drug usage and current or past medical conditions, family medical
history, the
presence of one or more physical, genetic or biologic pre-existing conditions,
health-effecting
personal habits (e.g., cigarette or alcohol use), etc. A specific list may be
set, for example, by
the Health Care Product Company in accordance with guidelines approved by a
regulatory
authority for a particular TAGS Product.
[00102] In step 401, a TACS account number is preferably assigned to the
individual for
whom the product is being purchased. Tying account numbers to social security
number, date
of birth and home address may help ensure that the person supplying the TACS
number is the
person for whom the account was established. A unique TAGS identification card
or personal
identification code (TACS ID) may be provided to the consumer at this time as
well. The TACS
ID is preferably used to track all subsequent TACS activity undertaken by the
consumer.
[00103] In step 402, TAGS 100 may initiate collection of already extant
relevant consumer
information that is necessary to make an algorithmic determination of
purchasing eligibility.
TACS 100 may determine what information is required based on the guideline
algorithm for the
specific product to be purchased. TACS 100 may then retrieve the relevant
information for the
current consumer from a TACS data storage system, if any information exists.
[00104] It is anticipated that, oftentimes, some or all of the information
necessary to make
an algorithmic determination of eligibility will not be retrievable from TAGS
100 EMR/EHR
systems. Therefore, in step 403, TACS 100 may initiate Triage Service-enabled
information
collection. Triage-based information collection may be executed by Triage
Service module 2.
For many products and consumers, the information to be collected may be very
simple. This
may enable the use of a computer program to assist in its capture. Therefore,
Triage Service
22368806.2 30
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
module 2 may collect the pertinent information from the consumer utilizing
media (e.g.,
interactive websites, interrogatory voice programs, etc.) that presents the
users with questions
and stores the subsequent answers. This may be presented to the consumer in a
variety of
ways, depending on the format of the media. For example, interactive websites
may be
presented to the consumer over Network 13 onto a personal computer, smart
phone, or tablet,
etc. Similarly, interrogatory voice programs may question the consumer over
the telephone.
For more difficult information (or for more challenged consumers), live human
Triage
representatives may interact directly with consumers to obtain the necessary
information. In
any case, consumer responses (which would also be dependent on the format of
the media)
may then be interpreted, associated with the consumer and stored in TACS 100.
[00105] In step 404, TAGS 100 preferably determines whether the consumer
has the
appropriate pre-qualification candidacy for the desired TACS Product. A pre-
qualification
algorithm may determine whether the consumer has appropriate candidacy for the
TAGS
Product based on the information collected in steps 402 and 403. Naturally,
individual TACS
Products are directed towards treating, preventing or diagnosing specific
illnesses, disorders or
for alleviating symptoms. Consumers who lack the appropriate condition or lack
the likelihood
to develop the condition have little legitimate reason to purchase the TACS
Product. Therefore,
the pre-qualification algorithm may serve as a basic screening process to
ensure that the
consumer is an appropriate candidate to receive the TACS Product, and that the
consumer is
purchasing the TAGS Product for medically legitimate reasons. The pre-
qualification algorithm
may be supplemented by a TACS Product sale algorithm that determines product
sale
authorization based on more in-depth information (which is described further
below).
[00106] If the consumer does not have appropriate pre-qualification
candidacy for the
TAGS Product, then, in step 405, the consumer is preferably referred to a
diagnostician/clinician
for counseling and treatment of whatever malady the consumer may be concerned
about, and
the TACS process may end. Alternatively, if it is determined in step 404 that
the consumer does
have appropriate pre-qualification candidacy for the TAGS Product, then the
process may
progress to FIG. 48.
[00107] FIG. 48 is a flow chart representing a continued preferred sequence
of steps for
implementing TACS 100 after it is determined that the consumer possesses
appropriate pre-
qualification candidacy for the TAGS Product. In step 406, TACS 100 may
initiate a Triage-
enabled product counseling session. Counseling sessions may be conducted to
educate
22368806.2 31
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
consumers on a variety of topics related to the product being purchased. The
topics may
include proper usage of the product, general product warnings, benefits of the
product, risks
associated with the product, or specific warnings on use of the product, such
as drug-drug
interactions, negative side effects or consumer-based risk factors (e.g.,
dangers due to age,
race, physical or genetic characteristics). The actual topics to be covered
and content
contained therein may be determined by, for example, the product manufacturer
and/or
regulatory authority. The counseling session, like Triage-based information
collection, may
utilize a variety of formats according to need. For example, if the consumer
is accessing TAGS
100 via a computer, the counseling session may take the format of an audio
voiceover, a video
multimedia presentation, or even something as simple as a readable text
displayed on the
consumer's computer screen. Alternatively, if the consumer is accessing TAGS
100 via
telephone, counseling may be limited to audio voiceover. In some instances, a
live Triage
representative may perform counseling. This may be advantageous in situations
where the
purchaser prefers discussing a particular TAGS Product with a live person or
if, for example, the
consumer has special needs, or where warnings and use of the product are
particularly complex
or potentially dangerous if the product is not used correctly.
[00108] TACS Product information relayed to or discussed with consumers is
preferably
scripted to ensure adherence to specific key communication points and health
management
algorithms. For example, Triage Service module 2 may provide (but is not
limited to) the
following services:
= assess the candidacy of the consumer to receive the TACS Product;
= educate the consumer regarding the appropriate use of the TACS Product;
= educate the consumer on the relative health benefits and risks associated
with using
the TACS Product;
= capture and document consumer "informed consent" as a prerequisite to
being
allowed to purchase the TACS Product;
= capture and document a list of factors, such as age, gender, lifestyle
habits,
diagnostic test results, hereditary risks, prior physician diagnosis, drug
history, etc.,
for particular disease states, documented disorders or potential disorders;
= capture and document consumer-provided list of prescription, OTC, BIG and
TACS
Products (such as drugs, biologics and medical devices, or diagnostic and
genetic
testing kits) currently being used by that consumer;
22368806.2 32
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
= capture and document a complete drug usage history, which may comprise a
list of
previously filled prescriptions for a particular consumer at pharmacies or
similar
vendors (whose records can preferably be automatically downloaded to Triage
Service module 2 through Network 13 utilizing specialized internet conduits,
such as
those offered in the United States through an e-prescription network);
= capture and document a liability waiver for utilizing the TAGS Product,
including
disclaimers (such as having had a physician consultation if required before
utilizing
the product);
= capture and document whether a physician's prior diagnosis has been
obtained for
the condition for which the TAGS Product is approved to treat;
= capture and document a list of clinical symptoms currently being
experienced by the
consumer which may or may not be traceable to the TAGS Product, and notifying
the
consumer if symptoms indicate that the intervention of a physician is
required;
= provide clinical decision support for drug interactions and allergies;
= capture and document a trending analysis of diagnostic test findings;
= provide preventive care protocols and reminders based on, for example,
symptoms,
prior medical diagnosis and self-reported medication;
= secure and structure communication between diagnosticians/clinicians,
Triage
Service computerized algorithms and/or Triage Service counselors and the
consumer;
= issue TAGS product compliance reminders;
= capture and document (or support) pharmacovigilance reporting; and
= provide consumer outreach services (automated or otherwise) for
preventative care
and/or chronic disease/disorder/marginal health management.
[001091 In step 407, a sales quantity allotment minimum and/or maximum
quantity may
be retrieved by Triage Service module 2 (in accordance with regulatory
requirements) for the
TAGS Product being purchased. Initial distributions of a TAGS Product may be
limited by
quantity limits as determined by Health Care Product Companies or by
Regulatory Authorities.
TAGS consumers are preferably restricted from purchasing more than these set
quantity limits.
[001101 In step 408, TACS 100 (through Triage Service module 2) may
determine
whether the consumer still wishes to purchase the TAGS Product. This may be
accomplished
by means of a simple prompt or query. The consumer preferably answers
affirmatively or
negatively. If the consumer answers negatively, the TACS Product purchasing
process may
22368806.2 33
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
end. However, if the consumer answers positively in step 408, the process
preferably proceeds
to FIG. 5.
[001111 FIG. 5 is a flow chart representing a continued preferred sequence
of steps for
implementing TACS 100. In step 501, TACS 100 preferably determines whether it
has sufficient
information to make an algorithmic determination of eligibility to purchase
the TAGS Product.
Oftentimes, the information collected in steps 402 and 403 (FIG. 4A) may be
insufficient for the
algorithm to determine whether the TAGS Product may be authorized for sale to
the consumer.
For example, with respect to the exemplary requirements for authorization of
sale of drugs
commonly known as "statins" (HMG-CoA reductase inhibitors), an additional risk
factor may be
imposed where people with certain health profiles having a fasting LDL-
Cholesterol level over a
certain threshold may be denied authorization to purchase the TAGS statin
product, because a
diagnostician/clinician's assessment may be required for more appropriate
treatment of the
individual than the treatment the individual would receive from merely taking
the TACS statin
product. However, the current LDL-Cholesterol level of a consumer may be
unavailable absent
diagnostic testing. Therefore, more intensive additional procedures may need
to be undertaken
before TACS 100 can make an algorithmic authorization determination. If TACS
100
determines in step 501 that it has sufficient information to make an
algorithmic authorization
determination, then the process may proceed to FIG. 7. If, however, TAGS 100
determines that
it does not have sufficient information to make an algorithmic authorization
determination, then
in step 502, TACS 100 may initiate a process for collecting additional
information, and progress
to FIG. 6.
[00112] FIG. 6 is a flow chart representing a continued preferred sequence
of steps for
implementing TAGS 100 in the event that TAGS 100 determines that additional
information
collection is required before a determination may be made on whether a TAGS
Product should
be authorized for sale to a consumer. In step 601, TAGS 100 preferably
determines what type
of service needs to be conducted to obtain the additional information.
Naturally, certain types of
information are available through specific forms of service. For example, LDL-
Cholesterol levels
are typically determined from a blood test (sometimes after a period of
enforced fasting).
Therefore, for the initial sale of a TACS statin product, a blood test may be
required including
a lipid panel screen to set a baseline level of Triglycerides, LDL and
cholesterol. Because liver
problems may develop without symptoms, people who take statins should have a
hepatic
function panel blood test six weeks after starting a statin medication to
check their liver function.
After that is completed, annual blood tests may be sufficient.
22368806.2 34
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[00113] Other
types of information may require other types of services. For example, a
form of diagnostic testing may be required, which may comprise tests performed
by the
consumer at home, tests conducted by health care professionals at the
consumer's home, tests
conducted by health care professionals at a diagnostician/clinician's office,
or even tests
conducted at diagnostic facilities with specialized equipment (such as a
magnetic resonance
imaging system).
Alternatively, a follow-on check-up and/or discussion with a
diagnostician/clinician may be required.
[00114] Once TACS 100 has determined which service is necessary, then, in step
602,
TACS 100 may optionally aid the consumer in scheduling the service.
Notification to the
consumer that a test is required may be sent, for example, via an e-mail
authorization, a fax
communication, a written/printed mailed letter, real-time notification on a
website or kiosk
display, a telephone call, telephone text message, etc. Any suitable
communications format
may be utilized as required.
[00115] Additionally, scheduling may be implemented in a number of ways. The
process
may be fully automated. For example, TACS 100 may communicate automatically
with
Diagnostic Test module 9 through Network 13, and retrieve a set of open time
slots for an
appointment. TACS 100 may then display these available time slots to the
consumer. Once the
consumer selects an available time slot, TACS 100 may reserve that time slot
for the consumer
by, for example, transmitting the consumer's selection to the Diagnostic Test
module 9 over
Network 13. Alternatively, a traditional scheduling scheme may be implemented.
For example,
TACS 100 may simply notify the consumer that a diagnostic test is required.
The consumer
would then be responsible for finding a qualified diagnostic center,
scheduling an appointment,
having the test completed and ensuring that the test results are received by
TACS 100. Mixed
solutions are also contemplated. For example, scheduling may be performed
manually, but the
test results may be automatically uploaded to a Diagnostic Test module 9.
Alternatively,
scheduling may be performed automatically, but TACS 100 may receive diagnostic
test results
through standard mail or, alternatively, the consumer may enter diagnostic
test results
themselves into TACS 100 through Consumer module 11. Multiple solutions may be
employed
uniquely, uniformly, or as a mixed system.
[00116] Identifying information, such as the consumer's name, date of birth,
address, driver's
license number, and/or social security number, etc., may be provided to the
Diagnostic Test
22368806.2 35
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
module 9 to aid scheduling and performing the test. Other types of unique
identifiers may be
assigned to the consumer as well.
[00117] In step 603, the service may be executed to obtain the necessary
information. The
consumer may execute self-administered tests, subject himself to tests
conducted by health
care professionals at the consumer's home, undergo tests conducted by health
care
professionals at a diagnostician/clinician's office, or be examined at a
diagnostic facility with
specialized equipment. In step 604, TACS 100 may receive the test result data.
For diagnostic
tests conducted at a diagnostician/clinician's office or at a designated
diagnostic facility, health
care professionals or automated processes at the diagnostic center preferably
analyze the
sample and upload the results of the diagnostic test into the Diagnostic Test
module 9. In
situations where interpretations of the diagnostic test results are simple,
TAGS 100 may
determine automatically from the uploaded data whether the diagnostic test
results satisfy the
requirements for purchasing the TAGS Product. More complex diagnostic tests
may require
interpretation from diagnostic facility personnel, or even outside third
parties, such as the
consumer's physician. In still other instances, the diagnostic test reading,
though complex, may
be modified either by the diagnostic test provider, by the Health Care Product
Company, by
TAGS 100 or by a third party (and preferably approved by a regulatory
authority), for example,
to two or more general classifications such as (but not limited to):
"Normal/Abnormal," or
"Normal/Abnormal High/Abnormal/Low." In these situations, the results of the
analysis (rather
than the diagnostic test results alone) may be uploaded to TAGS 100 by, for
example,
Diagnostic Test module 9. Alternatively, through an approved algorithm, TACS
100 may
convert the raw test data into more general classifications that can more
easily be interpreted by
Triage Service module 2.
[00118] For diagnostic tests administered in the consumer's home (whether by
the consumer
or by a health care professional), electronic data entry equipment may be used
to capture
diagnostic information and upload the information to TAGS 100 via Network 13.
Other means of
analyzing and transmitting the diagnostic test results to TACS 100 may be
implemented as
required. For example, the self-diagnostic testing device may transmit the
diagnostic test
results directly to TAGS 100. For example, the device may contain
communication technology,
such as a wired or wireless network connection. The device may, therefore,
automatically
upload results to TAGS 100 through Network 13. Alternatively, a storage
device, such as a
universal serial bus memory stick, may be removable from the device. Data may
then be
transferred to a personal computing terminal, and information may be uploaded
to TAGS 100
22368806.2 36
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
through Network 13. In yet another embodiment, consumers may manually input
the data into
their personal computer through Consumer module 11. A confirmation may be
required, forcing
the consumer to certify that the data is correct and true. The consumer may
then be allowed to
upload the data to TAGS 100 through Network 13. Other types of special
software and/or
hardware may be used to upload the results of diagnostic testing direct to
TACS 100 or to a
terminal connected to Network 13. TACS 100 may also receive diagnostic results
performed by
the consumer from alternate sources. For example, data may also be sent via
telephone
network (by text message, for example) or verbally related to a Triage Service
representative.
[00119] Subsequently, the process may return to FIG. 5, where, in step 501,
TACS 100 may
again determine whether it possess sufficient information to make an
algorithmic authorization
determination for the present consumer and current TACS Product.
[00120] FIG. 7 is a flow chart representing a continued preferred sequence of
steps for
implementing TACS 100 after it determines that sufficient information exists
to make an
algorithmic authorization determination. In step 701, TACS 100 may execute the
algorithmic
authorization determination for the current TAGS Product and the current
consumer. The
algorithm may analyze the set of health risk factors associated with the TACS
Product to be
purchased and determine, based on the information collected in the steps
above, whether the
consumer falls within an acceptable threshold for authorization based on the
risk factors for the
TACS Product.
[00121] For example, an algorithm for considering the eligibility of a
consumer for purchasing
a cholesterol-lowering statin may consider a number of risk factors. Primary
consideration risk
factors may include (but are not limited to) age, weight, gender, health-
effecting personal habits
(such as drinking alcohol or smoking), personal and family medical history,
obesity, race, and/or
the presence of one or more physical, genetic or biologic pre-existing
conditions. In one
embodiment, for example, the algorithm may dictate that the statin TACS
Product may be
appropriate for sale through TACS 100 for individuals with moderate risk of
Coronary Heart
Disease (CHD). Individuals with moderate risk of CHD may be defined to include
men between
the age of 45-54 and women of age 55-70 having one or more pre-identified risk
factors, and
men aged 55 to 70 with or without any listed risk factors. The listed risk
factors may also
include whether the consumer is currently a smoker or was a smoker during the
last four years,
and whether the consumer's family has a history of heart disease (e.g., a
father or brother had a
diagnosed heart attack or diagnosed angina before age 55, or a mother or
sister had a
22368806.2 37
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
diagnosed heart attack or diagnosed angina before age 65), whether the
consumer is obese
(defined by waist measurement. and/or Body Mass Index (BMI) indicators, which
may be
calculated by the TAGS 100 algorithm for the statin TAGS Product), and whether
the consumer
has a South Asian family origin (e.g., India, Pakistan, Bangladesh or Sri
Lanka). The presence
of one or more of these factors (in combination with the age and gender
information) may
determine algorithmically whether the consumer will be authorized to purchase
the statin TACS
Product. For example, if none of these categories apply, the statin TAGS
Product may not be
suitable to treat the condition since the individual may be at a low risk or
no risk of CHD. In
such instances, TAGS Triage Service may provide counseling on lifestyle
modifications for the
individual to reduce the future risk of CHD.
[00122] Even if the authorization criteria are met, certain people may
still be referred to a
medical doctor. The algorithm may list factors indicating such referral as
well. For example, a
family history of diabetes, angina, previous heart attacks, previous strokes,
hypertension,
abnormal liver function, hyperthyroidism, renal impairment and/or other
muscular disorders may
be barred from purchasing the statin TACS Product. Women who are pregnant or
breastfeeding may also be denied authorization. Secondary habits may be
considered as well,
such as a consumption of a certain quantity of alcohol per day, or whether the
consumer is a
smoker.
[00123] If the algorithm performed by TAGS 100 determines that the consumer is
authorized
to purchase the TACS Product, then the process may proceed to FIG. 10A
(subject to a
diagnostician/clinician override discussed below). lithe algorithm performed
by TAGS 100
determines that the consumer is not authorized to purchase the TACS Product,
then in step
702, TACS 100 may await overriding authorization from a health care
diagnostician/clinician. It
is anticipated that diagnosticians/clinicians may sometimes authorize their
patients to utilize
health care products and drugs even though TACS 100 has declined purchase
authorization,
because diagnosticians/clinicians may gain more nuanced and detailed
understandings of their
patients' conditions compared to the more rigid risk factor analysis
considered by TAGS 100.
Therefore, TACS 100 preferably includes a mechanism that allows a
diagnostician/clinician to
override the TACS 100 algorithmic authorization determination. If such an
override is provided,
TAGS 100 may authorize the sale of the TACS Product to the consumer and the
process may
proceed to FIG. 10A. If an override is not provided, then in step 703, the
consumer is denied
purchasing authorization and the process may end.
22368806.2 38
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[00124]
Similarly, diagnosticians/clinicians may override purchasing authorization as
well, as
it is anticipated that diagnosticians/clinicians may want to prohibit their
patients from receiving
certain TACS products (like statins in the above example) even though TAGS 100
determined
that consumer would be authorized to purchase and use the TACS Product.
Therefore, in step
704, if TAGS 100 receives a diagnostician/physician override for a TACS
Product purchasing
authorization, then, in step 705, TACS 100 may revoke purchasing authorization
for the TAGS
Product. If TACS 100 does not receive a diagnostician/physician override for a
TACS Product
purchasing authorization, then the process may proceed to FIG. 10A. A waiting
period of some
quantity may be imposed on certain TACS Products to create a sufficient window
of time for a
diagnostician/clinician to be notified of the sale, and determine if they wish
to override the TAGS
purchasing authorization or denial.
[00125] FIG. 8 is a flow chart representing a continued preferred sequence of
steps for
implementing TAGS 100, if TAGS 100 determines that the current purchase is a
subsequent
purchase (from FIG. 3). In step 800, TAGS 100 preferably logs the TAGS account
number for
the consumer. The consumer would have received an account number during a
previous initial
purchase from TACS (as described in step 301). Logging the consumer's TAGS
account
number may aid in retrieving known consumer information and recording
information for the
present purchase.
[00126]
Subsequent purchases of a TACS Product may generally require less scrutiny
than
an initial TACS Product purchase because the consumer has already once been
authorized to
purchase the TACS Product. However, some screening may still be performed,
because there
may have been a change in one or more substantive risk factors associated with
the TAGS
Product. Therefore, in step 801, TACS 100 may collect both known and updated
relevant
information on the consumer. This step may at least partially mirror the
actions undertaken in
steps 402 and 403 of FIG. 4 and even steps 601 ¨ 604 of FIG. 6, if desired. As
before, TACS
100 may obtain consumer information from a variety of sources, including
extant information in
TACS data storage, Triage-managed self-identified consumer information, self-
administered
and professionally administered diagnostic testing, diagnostician/clinician
examinations, more
specialized diagnostic facility testing, etc. TACS 100 may also receive input
from any special
instructions or notations diagnostician/clinicians (or any authorized TAGS
affiliated person or
computerized program) may have made for the consumer in question. For example,
if a
diagnostician/clinician has limited the purchase quantity of a product, or
banned it outright for a
22368806.2 39
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
particular consumer, TACS 100 may translate these notations into purchasing
limitations or
deauthorizations.
[00127] In step 802, TACS 100 preferably determines whether a quantity
restriction has been
placed on the TACS Product for the particular consumer as of the present date.
As described
above, limitations may be placed on both the quantity and time within which a
re-order may be
allowed. For subsequent TAGS Product purchases, the sales quantity allotment
specifies how
many doses of the TACS Product can be purchased and/or consumed by the
consumer over an
allotted specified time period based on a per-product determination by a
regulatory authority.
For example, statin TACS Products may be authorized for up to a six-month
period (e.g., 180
tablets for 180 days, assuming once daily dosage). After the six-month period
expires, a
diagnostic test (with a corresponding diagnostic test result for lipid, LDL
and cholesterol levels
and a liver enzyme panel) may be required by the Triage Service prior to the
allowance of
another six-month purchasing renewal period.
[00128] If the re-order request occurs prior to a restricted date demarking
the end of a given
time period, then, in step 805, the consumer may be denied purchasing
authorization and
advised of a date on which fulfillment of the re-order is permissible.
Similarly, multiple re-orders
may be held in a queue in a TAGS pending order database. Once a restricted
date has passed,
automatic authorization and distribution of one or more queued orders may be
allowed.
[00129] If there is no current restriction on the allotment of a TAGS Product
re-order, then in
step 803, TAGS 100 may then determine, based on the information it has
collected, whether
there has been a change in the substantive risk factors associated with the
TAGS Product being
purchased. If there has been no change in the substantive risk factors
sufficient to de-authorize
future purchases, or if the change is substantive but does not require Triage-
based de-
authorization or a diagnostician/clinician follow-up, then, in step 804, the
repurchase of the
TACS Product may be authorized and the system may proceed to FIG. 10A. if,
however, there
has been a change in the consumer's substantive risk factors sufficient to de-
authorize future
purchases and for which a diagnostician/clinician visit is required, then the
system may progress
to FIG. 9.
[00130] FIG. 9 is a flow chart representing a continued preferred sequence of
steps for
implementing TACS 100, if TAGS 100 has determined that there has been a change
in the
consumer's substantive risk factors for a subsequent repurchase of a TAGS
Product. In step
901, TAGS 100 may require that the consumer undergo an examination and/or
assessment by
22368806.2 40
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
a diagnostician/clinician to determine whether the consumer may still utilize
the TAGS Product.
In step 902, TAGS 100 may await override authorization from the
diagnostician/clinician. lithe
diagnostician/clinician fails to provide override authorization in an allotted
time period, then, in
step 903, the consumer may be denied authorization to repurchase the TACS
Product, and the
process may end. If, however, the diagnostician/clinician does provide
override authorization in
an allotted time period, then, in step 904, the consumer may be authorized for
a repurchase of
the TACS Product. In this case, the diagnostician/clinician may be required to
provide a notice
of allowance to TACS 100. The notice preferably includes information including
a description of
the TACS Product, the diagnostician/clinician's name and address, the
consumer's identification
information and, of course, the actual allowance override authorization issued
by the
diagnostician/clinician.
[00131] FIG. 10A is a flow chart representing a continued preferred sequence
of steps for
implementing TAGS 100, if a consumer has obtained TAGS 100 authorization to
purchase a
TACS Product. In step 1001, TACS 100 may clear the TACS Product for sale to
the consumer
once all requirements have been met for selling the TAGS Product.
[00132] In step 1002, TAGS 100 may send the order information to the Order
Intake Service
module 3. Order Intake Service module 3 may also transmit information specific
to the order to
Order Fulfillment & Shipping Service Module 4 for shipment processing. A
preferred TACS
Product delivery method may be indicated by the consumer at this time. Upon
ordering a TAGS
Product, the consumer may choose to receive their TAGS Product either through
mail/parcel
shipment delivery or at a TAGS affiliated retail location connected to TAGS
100 through Network
13 for pick-up by an authorized individual (described in FIGS. 12 and 13).
[00133] Because a significant span of time may sometimes pass between the
commencement of the ordering process and the issuance of a TACS Product
purchasing
authorization, it may be necessary for Order Intake Service module 3 to
contact the consumer to
confirm the consumer's continued interest in purchasing the TAGS Product and
fulfilling the
order. The order may also be accepted automatically upon the issuance of
purchasing
authorization, if the consumer has approved of such an action at the time of
the original order
request.
[00134] FIG. 108 is a flow chart representing a continued preferred sequence
of steps for
implementing TAGS 100, if the consumer elects mail/parcel shipment delivery.
TAGS 100
preferably initiates execution of the payment process, which is preferably
completed before
22368806.2 41
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
shipping of the TAGS Product is commenced. The consumer's credit card is
preferably not
charged until the product order enters the actual shipping process within the
advertised time
period to the consumer indicating when shipping may be expected to occur and
be received.
Therefore, in step 1003, Order Intake Service module 3 may send consumer
payment
information to the Banking Service module 5, which is preferably the financial
institution or other
organization providing services to the Health Care Product Company for
purchase verification
so that the purchase on the credit card may be approved or denied. The Card
Association
(such as Visa , MasterCard , American Express , etc.) preferably acts as a
gateway between
the Banking Service module 5 and the Card Issuer (the financial institution or
organization that
issued the credit card to the consumer) for authorizing and funding the
transaction. In step
1004, the Banking Service module 5 may verify with the Card Issuer that the
card number and
transaction amount are both valid.After the transaction is authorized, in step
1005, notice of the
authorization may then be stored in a batch file by the Order Intake Service
module 3. In step
1006, Order Intake Service module 3 sends the batch file to Banking Service
module 5
preferably at the end of any given day. In step 1007, Order Fulfillment &
Shipping Service
module 4 may then authorize the TACS Product for retrieval and preparation for
shipment. The
TACS Product may then be retrieved from warehouse stock, or ordered from the
manufacturer if
stocks are depleted. Alternatively, a product kit may be assembled. Product
kits may contain
multiple purchased products, some of which are TACS Products and some of which
may be
other supplemental or secondary products not necessarily requiring TAGS sales
protocols or
authorizations. These secondary products may add value to the whole due to
some commercial
relationship with the TAGS product. For example, secondary product skin
hydrating creams
may be purchased along with a TACS Product for treating eczema.
[00135] FIG. 10C is a flow chart representing a continued preferred sequence
of steps for
implementing TACS 100. When product shipment occurs, in step 1008, Order
Fulfillment &
Shipping Service module 4 may concurrently send a data file to Banking Service
module 5
indicating that payment is ready to be accepted. In step 1008a, the order
information may also
be sent to and stored in EMR/EHR systems (as described above). In step 1009,
Banking
Service module 5 preferably sends any pending transactions in the batch file
through the Card
Association, which, in step 1010, may debit the Card Issuers for payment, and,
in step 1011,
credits the Banking Service module 5. Finally, in step 1012 the TAGS Product
preferably
reaches its target destination and is received by the consumer.
22368806.2 42
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[00136] Obviously, cash or other forms of payment may also be used to make
purchases
from TACS 100 as desired. If a money order is used instead of a credit card,
it is preferably
sent to and received by the Order Fulfillment Service & Shipping Company, and
subsequently
deposited in the Banking Service module 5 for processing.
[00137] As described above, rather than have the TAGS Product shipped or
mailed to the
consumer, the consumer may wish instead to purchase or pick up their TAGS
Product at a
TACS affiliated retail sales or pickup location. FIG. 11 is a flow chart
representing a continued
preferred sequence of steps for implementing TACS 100, depicting a preferred
sequence of
steps for acquiring a TAGS Product from either a TAGS affiliated Pharmacy or
from an
Automated TACS Product Dispensing Machine.
[00138] In step 1101, Order Fulfillment & Shipping Service module 4 may send
an
authorization to either a TAGS Pharmacy Dispensing module 6A or to an
Automated TAGS
Dispensing Machine 6B (or to both assuming that pick up at either location
will satisfy the sale).
Successful product pick-ups may be recorded by TACS 100, thereby preventing
repeated
pickups for the same product by the same consumer.
[00139] A TACS Pharmacy module 6A that has received an authorization may allow
a TACS
affiliated Pharmacy to release the TACS Product to the consumer in a quantity
not to exceed
the minimum and/or a maximum consumption allotment amounts per specified time
period (as
discussed previously). Similarly, an Automated TACS Product Dispensing machine
6B location
that has received such an authorization may release the TAGS Product in a
quantity not to
exceed the minimum and/or a maximum consumption allotment amounts per
specified time
period, as discussed previously.
[00140] If the TAGS Product is to be picked-up at a retail location, TAGS 100
may send a
notification through Network 13 to the consumer (which, for example, may take
the form of e-
mail, text messaging service, automatic phone notification, etc.), advising
the consumer that the
order is ready for pick-up at any TAGS Product pick-up location (e.g., TAGS
Pharmacy or any
Automated TACS Product Dispensing machine). The pick-up location may be pre-
designated
by the consumer, or suggested automatically by TACS 100 based on the
consumer's physical
address.
[00141] In step 1102, Triage Service module 2 may provide the consumer with an
authorization token or purchase code to purchase the TACS Product. The token
may take the
22368806.2 43
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
form of, for example, a purchase authorization card with an attached code, or
a code associated
with a user's TAGS ID. A TAGS ID or purchase authorization card may be encoded
with
information via, for instance, alphanumeric code, graphical code, magnetic
strip, etc.
Alternatively, the token may comprise an authorized purchase code sent to the
consumer
through the mail, transmitted orally over telephone, received digitally
through the Internet, or
through other appropriate means. The token is preferably secured against
counterfeiting,
electronic spoofing and other common forms of obtaining illicit access. The
token may enable
the consumer to purchase their TAGS Product from a retail TACS Pharmacy
(presumably from
a pharmacist or other authorized Pharmacy personnel) connected to TAGS
Pharmacy
Dispensing Module 6A, or from an Automated TACS Dispensing Machine connected
to Closed
System Automated TAGS Dispensing Machine Module 6B. The token may also allow
the
consumer to partake in Triage services, and make additional TACS purchases
through an
internet-enabled TAGS Product gateway on any computer or computing system. If
a token is
not readily available to the consumer, the TACS Pharmacist or Automated TAGS
Dispensing
machine may be able to look up the consumer's token with proper additional
identification or
other means. For example, the machine may be equipped with some form of
identification
confirmation system, such as (but not limited to) facial recognition, retina
scanning, fingerprint
scanning, etc.
[00142] FIG. 12 is a flow chart representing a continued preferred sequence of
steps for
implementing TACS 100, depicting a preferred sequence of steps for acquiring a
TACS Product
from a TAGS affiliated Pharmacy. In step 1200, the consumer preferably
presents the secure
authorized ID card (or other token) to authenticate the consumer for the
purpose of TACS
Product pick-up. When the consumer visits a TAGS Pharmacy to purchase and/or
pick-up a
pre-paid TACS Product, the consumer may show its authorized TAGS token to the
authorized
store personnel.
[00143] In step 1201, the TAGS authorization token associated with the
consumer may be
retrieved and, validated with a confirmation check against a TAGS 100
database. In step 1202,
the TAGS Pharmacy Dispensing module 6A may accept the purchase order as
authorized
(under any previously ordained restrictions) and determine whether the order
was prepaid or if
the order is to be paid in-store at the time of product pick-up. Subsequently,
in step 1203, the
pharmacist (or other store personnel, if permitted) preferably fills the
order. In step 1204,
payment is preferably completed at the point-of-purchase, unless, as
determined above, it was
pre-paid at the time of ordering. If the TACS Product was pre-paid during the
initial ordering
22368806.2 44
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
process, then the consumer may be required only to show appropriate
identification at pick-up.
A dispensing fee may be added to the cost of the TACS Product to compensate
the pharmacist
for filling the order. Finally, in step 1205, the consumer preferably receives
the TAGS Product.
In step 1206, the order information may be sent to an EMR/EHR system.
[00144] FIG. 13 is a flow chart representing a continued preferred sequence of
steps for
implementing TACS 100, if the consumer chooses to receive the product from an
Automated
TACS Dispensing Machine 6B. In step 1301, the consumer may approach an
Automated TAGS
Dispensing Machine 6B to purchase and/or pick-up the TACS Product. In step
1302, the TAGS
Dispensing Machine 6B preferably provides the Consumer with information about
the TAGS
Product.
[00145] In step 1303, the Consumer may input the consumer's TACS-issued token
authorization into the Automated TACS Dispensing Machine 6B. For example, the
consumer
may have a TAGS ID card read by a card reader integrated into the Automated
TACS
Dispensing Machine 6B. Alternatively, the consumer may input the TACS Product
code into a
kiosk keyboard or touch-input video screen. An accepted token may qualify the
consumer to
purchase TAGS Products from the Automated TACS Dispensing Machine 68
consistent with
any current TAGS authorization and restrictions on quantity and/or time
period.
[00146] In step 1304 the consumer preferably selects which TAGS Product(s) the
consumer
intends to purchase (up to a total approved allotment quantity for that TACS
Product specific to
the purchasing consumer), and specifies the quantity of the order. The
Automated TACS
Dispensing Machine 6B may show the consumer the price charged for the quantity
selected. In
step 1305, the consumer preferably pays for the purchase. If the order is a
pre-arranged and/or
pre-paid purchase (for example, if the order was input into TACS 100 by Order
Intake Service
module 3, Order Fulfillment & Shipping Service module 4, or by the consumer
through
Consumer module 11), then this step may be omitted since the order and/or
payment are
already stored in the TACS system 100. Payment may be made in any convenient
or desirable
format. Payment is preferably cleared by the Banking Service module 5 before
the Automated
TAGS Dispensing Machine 6B dispenses the TACS Product. For example, if a
credit card is
utilized as payment, the credit card information preferably clears the Banking
Service module 5
before actual provision of the TACS Product to the consumer begins.
Alternatively, if cash
tender is deposited in the machine, the machine may determine whether the
deposited amount
is sufficient to cover the cost of the TAGS Product (and may issue change if
necessary). Once
22368806.2 45
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
payment has been perfected, then the Automated TACS Dispensing Machine 6B may
dispense
the TAGS Product to the consumer.
[00147] TACS Product pick-up machines 6B may come in two or more formats. For
example, the machine may contain pre-packaged, sealed and labeled fixed
quantities of TACS
Products filled in one or more pre-filled unit quantities in each package. For
example, the
packages may come in 25, 100 and 200 count containers. Alternatively, the
Automated TACS
Dispensing Machine 6B may have the capability to automatically fill a
container with a variable
number of consumer selected dosage forms, which the consumer may select at the
machine at
the point of purchase. After filling the container, the Automated TAGS
Dispensing Machine 6B
may seal and label the bottle (or other container) for the individual consumer
and dispense it to
the consumer on demand after having cleared authorization and payment.
[00148] If the Automated TAGS Dispensing Machine 6B is a vend-style machine
containing
pre-packaged fixed quantity pre-labeled TACS Products, then, in step 1306, the
vend-style
Automated TAGS Dispensing Machine 6B may dispense the selected package(s) to
the
consumer.
[00149] If the Automated TACS Dispensing Machine 6B is a variable quantity
dispensing
unit, with the ability to fill, package and label TACS Products in variable
quantities chosen by the
consumer, then, in step 1307, the consumer preferably selects which product
the consumer
intends to buy from the Automated TACS Dispensing Machine 6B, and what
quantity the
consumer wishes to purchase (up to the total approved TACS Product allotment
quantity if any
has been designated for the product).The Automated TACS Dispensing Machine 6B
may then
fill the product order from an existing (and presumably replenishable) stock
stored in the
machine by retrieving the specified units and installing them into a
container, capping or
otherwise sealing the container and labeling the container with a TACS
appropriate label. The
label may be printed on the container or on a self-adhesive label that may be
affixed to the
container by the Automated TAGS Dispensing Machine 6B prior to dispensing it
for consumer
pickup. The Automated TACS Dispensing Machine 6B preferably contains
replenishable stocks
of the container, label and closure materials. The Automated TACS Dispensing
Machine 6B
also preferably includes the ability to print text (or even images) on the
labels (through, for
example, ink to paper transfer, or a thermal heat printing process). Finally,
in step 1308, the
Automated TAGS Dispensing Machine 68 preferably dispenses the package(s) of
TACS
22368806.2 46
CA 02 8 18 052 2 0 13-0 6-0 6
Agent Ref.: 79305/00002
Product to be retrieved by the consumer. In step 1309, the order information
may be sent to an
EMR/EHR system.
[00150] Other enclosures, such as (but not limited to) sealed or otherwise
securely closed
pouches, may be used in the Automated TAGS Dispensing Machine 6B in place of
containers
(e.g., bottles or vials, etc.), which would not require capping. For example,
tablets may be
dispensed in individually pouched strips with a means to separate each dosage
unit, so the
strips can be separated without ripping open the pouch. This may be an
advantageous method
of dispensing certain TAGS Products, such as, for example, certain drugs or
medical devices
(e.g., contact lenses).
[00151] FIG. 14 is a flow chart representing a continued preferred sequence
for implementing
TAGS 100, illustrating a preferred sequence of steps for implementing
pharmacovigilance
capture and reporting. In step 1401, the consumer may experience an AE, which
is any
undesirable or unexpected experience associated with the use of a drug,
biologic, medicinal or
medical health care product, medical device and/or diagnostic or genetic test.
Reporting of AE's
is useful because it helps regulatory authorities, manufacturers, physicians
and other health
care related personnel or industries to identify, evaluate and manage a
plurality of factors,
including potential risks and complications, safety concerns, gaps in product
use and drug-drug,
drug-food or drug-test interaction education. Indeed, AE reporting has the
potential to remove
potentially dangerous products from the market AE reporting allows for the
capturing of trends
indicating the potential for such danger over time and for a particular usage
population. At a
minimum, labeling or counseling may be modified so that safer product usage
may be better
ensured. TAGS 100 may significantly improve AE reporting for drugs, biologics
and medical
devices, diagnostic tests, etc.
[00152] Pharmacovigilance departments are typically maintained within health
care
companies to identify, aggregate and analyze the data provided to them on
AE's. Therefore,
consumer reports on AE's may be reported to a regulatory authority and acted
upon if
necessary.
[00153] In steps 1402 and 1404, the consumer may contact TAGS 100 through the
Triage
Service module 2 and/or the Order Fulfillment & Shipping Service module 4,
respectively, to
report an AE. Alternatively, in step 1403, the consumer may directly contact
the health care
company or a third party service under contract to the health care company to
report an AE.
Reporting may be executed via, for example, calling a designated telephone
number listed on
22368806.2 47
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
the TAGS Product package or TAGS Product website. Alternatively, the AE
reporter may use a
hyperlink on a TAGS Product website to navigate to a web page linked directly
to a department
in the company responsible for pharmacovigilence. In step 1405, the consumer
is preferably
directed to Customer Support Group module 7 (i.e., the support group
associated with the
product that ostensibly caused the AE responsible for answering TACS Product
specific
questions on safety and/or efficacy) in order to conduct required
pharmacovigilance data
collection and processing. The Customer Support Group may also respond to
consumer
questions specific to the TAGS Product unrelated to AE's, such as proper usage
of the TAGS
Product.
[00154] Alternatively, or supplementally, TAGS 100 may include a TAGS Consumer
Support
Department separate from the health care company Customer Support Group. A
consumer
inquiry received by the TAGS Consumer Support Group may be transferred to the
health care
company Customer Support Group module 7 for handling relevant issues. For
example, the
TAGS Consumer Support Group may conduct pharmacovigilance on behalf of the
health care
company, assuming this service is approved by the health care company and/or
the regulatory
authority. Pharmacovigilance handlers in TAGS 100 may then record the relevant
information
(as required by the regulatory authority) and transmit that information to all
relevant parties,
such as (but not limited to) Regulatory Authority module 1 and the Health Care
Company
module 12.
[00155] If a TAGS Product return transaction is warranted, the TAGS Product
may be
returned through the mail or parcel shipping service by the consumer to an
authorized TAGS
Product Fulfillment Company through Order Fulfillment & Shipping Service
module 4. Once the
returned TAGS Product is logged in as having been received by the TAGS Order
Fulfillment &
Shipping Service module 4, the Banking Service module 5 may be instructed to
credit a relevant
consumer account, send the consumer a refund check, or otherwise refund money
to the
consumer.
[00156] An alternative embodiment of TAGS 100 is disclosed in FIGS. 15-21,
which provides
a Dynamic Customizable Personalized Label (DCPL) for enabling the dynamic
personalization
of affixed, integrated or stand-alone, non-affixed labeling of products. By
the integration of
personalized end user data in the label, it is expected that product users may
have a more
comprehensive and more understandable basis for the safe and effective use of
the products to
which the label applies. DCPL is expected to completely conform to United
States (and, where
22368806.2 48
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
implemented, international) regulatory requirements for labeling, particularly
as capabilities for
producing flatter and more flexible labels with built in electronic, magnetic,
radiofrequency, light-
based and/or other such capabilities are implemented.
[00157] With the advent of electronic ink, light emitting diode,
interactive television, wireless
electronic and radiofrequency technology or other signal-to-receiver based
technology, and
other electronic or radio frequency technology, a DCPL may be utilized either
affixed to or
otherwise integrated on (for example, through direct surface printing) the
product itself or shown
in physically or virtually non-affixed locations such as, for example, 1)
physically alongside the
product or 2) virtually, on a computerized or computer linked viewing device
(e.g., computer
monitor, cell phone screen, television, etc.), or 3) even as a computer-linked
personalizable text
print-out. A label shown on, near to or otherwise specifically related to a
product for sale, for
example, through an Internet web site at the time- or point-of-product
purchase, is essentially
considered by some regulatory bodies as a label, for example, in the United
States.
[00158] In certain circumstances, a product label may be manufactured to be
electronically or
radiofrequency controlled (or controlled through another connected or
disconnected means),
such that all of some of the information contained on the label can be
modified or changed
based on information sent to it by a computerized device or multiple devices.
Three sub-types
of DCPL (or any combination thereof) are contemplated: 1) Fixed DCPL where
there may be
two or more different fixed content versions of a label for a product which
may be selected by a
computer to be variably shown for viewing for a particular end user depending
on the output of
an algorithm, 2) Partial DCPL where some parts of the label contents are fixed
and do not
change and other parts of the label contents are able to be electronically or
otherwise changed
so as, for example, to be specific to an end user of the product, and 3) Full
DCPL where all of
the label contents are able to be electronically modified so as, for example,
to be specific to a
particular end user of the product.
[00159] Several governmental regulatory authorities in the United States and
elsewhere
publish guidelines and specifications on how labels must be presented and what
information a
label must contain in order to comply with the published guidelines. For non-
drug consumer
commodity products, certain labeling may be required, for example:
= The identity of the commodity;
= The name and place of business of the manufacturer, packer, or
distributor of the
commodity; and
22368806.2 49
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
= The number of servings of such commodity contained in such package and
the net
quantity (in terms of weight or mass, measure, or numerical count) of each
such
serving.
[00160] Controlled prescription drugs and non-controlled prescription drugs
dispensed
pursuant to a prescription must bear a label, permanently affixed to the
immediate container in
which the drug is dispensed or delivered and which is received by the
purchaser or patient,
which must include the following:
= The name and address of the dispenser or pharmacy;
= The serial number of the prescription;
= The current date of its filling or refilling;
= The name of the prescriber;
= The name of the patient;
= The directions for use, including precautions, if any, as indicated on
the prescription;
= The initials or name of the dispensing pharmacist;
= The telephone number of the pharmacy; and
= The drug name and strength and quantity.
[00161] The prescription label for controlled drugs, in addition to the above,
must comply with
the label requirements of the Federal and State Uniform Controlled Substances
Act, including
the transfer warning auxiliary label.
[00162] Consumer product labeling requirements fall under the Fair Packaging
and Labeling
Act (FPLA). The United States Code of Federal Regulations Title 21, Volume 4,
Chapter I,
Food and Drug Administration Department of Health and Human Services,
Subchapter C, Part
201 covers regulations governing prescription drug labeling. Specific labeling
components
covered by these regulations include, but are not limited to:
= Product Name
= Statement of ingredients
= The net quantity of contents (in terms of weight or mass, measure, or
numerical
count)
= Name and place of business of manufacturer, packer, or distributor
= National Drug Code numbers
= Adequate directions for use
22368806.2 50
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
= Misleading statements
= Prominence of required label statements
= Spanish-language version of certain required statements
= Location of expiration date
= Significance of control numbers
= Use of term "infant"
= Declaration of the presence of certain dyes in certain drugs for human
use
= Declaration of presence of phenylalanine as a component of aspartame in
over-the-
counter and prescription drugs for human use
= Prescription drugs containing sulfites; required warning statements
= Required pediatric studies
= Labeling for systemic antibacterial drug products
= Bar code label requirements
= Drug information shown on a website specific to the drug by a drug
manufacturer or
marketer is also considered constitute product labeling.
[00163] Along with fixed, non-changeable, non-dynamic information, DCPL in all
its forms
can communicate additional, specific personalized information that can be
tailored based on
factors, which may be uniquely specific to the end user. For example, the
following types of
information can be electronically or otherwise sent to the label itself to be
reflected in text and/or
pictures and/or symbols, which may be shown on an electronic viewing device
such as an
electronic telephone, computer screen, television or other such device:
= A particular person's age, sex, weight, height or other descriptive facts
= A particular person's pre-existing medical condition, such as
cardiovascular disease,
high blood pressure, diabetes, etc.
= A particular person's personal habits, such as drinking alcohol or
smoking tobacco,
which could impact the appropriateness of an individual using a drug product
or
diagnostic test, or could modify the way the product or test should be used or
read.
= The results of a particular person's blood testing (for example, oxygen
levels or
hematocrit) or blood levels of particular constituents (for example,
triglycerides, total
cholesterol, high density lipoprotein cholesterol (HDL) and low density
lipoprotein
cholesterol (LDL).
22368806.2 51
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[00164] A list of drugs which may have been or are currently being used by an
end user and,
potentially, an indication of whether or not the product intended for use by
end user can be used
concomitantly with any of these other drugs (for instance, by showing for
example, a green dot
or other symbol next to other drugs being used by an end user of a drug
product which are
acceptable to be used with the labeled product, and/or a red flashing dot or
other symbol next to
a certain other drugs which are contraindicated for concomitant use with the
labeled
product).Alternatively, rather than use colored or flashing lights, the
letters of the acceptable or
contraindicated drugs or other words may be colored or be associated with
other visual effects
to communicate acceptable or unacceptable use with the new product intended
for use by the
end user.
[00165] DCPL labeling may also be implemented to show other information in
addition to the
above examples. For example, a DCPL can dynamically indicate when a product
has expired.
For instance, the words iTHIS PRODUCT HAS NOW EXPIREDi may appear over the
front of
the label using, for example, electronic ink, which may be caused by an
embedded timed
program to appear on a certain expiration date of a particular batch or lot of
produced product.
[00166] DCPL may also be used in conjunction with a product so that the end
user can
view associated medical information and/or images of any kind (e.g.,
photographs, drawn,
animated, etc.) which can be dynamically modified such that content intended
for viewing by all
users of a product can be combined with special content which could be
personalized only for
viewing by the end user. For example, a special computer readable digitized
bar code, digital
imprint or other graphic interface could be shown by the system, printed on or
otherwise sent to
the DCPL label for viewing. A bar code reader, digital imprint reader or other
computerized
reading device may read the code and then by accessing a network, could send
an image
and/or written content browser to a particular website in order to show the
specialized label
content, which could be customized for viewing by the end user (or by a third
party on behalf of
the end user).
[00167] Referring to FIG. 15, TAGS 100 is similar to that described above and
illustrated in
FIG. 1. Like reference numerals in the drawings are intended to designate
identical or
corresponding components of TAGS 100. In contrast to FIG. 1, TAGS 100 in FIG.
15 also
includes an EMR/EHR Data module 14, a Diagnostic Test Benchmark module 15, and
a Drug
Interaction Database 16. These components of TAGS 100 may be implemented as
one or more
computer systems, software processes operating on one or more computers
concurrently,
22368806.2 52
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
and/or database computer systems, etc. Alternatively, these modules may be
implemented as
distinct logistical components of TAGS 100, housed in separate systems,
intercommunicating
with TAGS 100 and each other over, for example, a Network 13.
[00168] FIGS. 16-20 illustrate a preferred sequence of steps for implementing
an illustrative
DCPL using TAGS 100. As discussed above in step 402 of FIG. 4 and step 801 of
FIG. 8,
relevant information on the end user of a product is collected by TAGS 100.
Some information
may not have been automatically collected, such as information about the end
user (for
example, but not limited to, the end user's name, address, telephone number,
TAGS ID number,
sex, birth date and/or age, weight, allergies, a drug history, a medical
history and/or other
relevant information). In step 1600, this information may be input by the end
user or on behalf
of the end user by a third party. Other information may be automatically
retrieved specific to the
end user or independently provided to TAGS 100 by a third party on behalf of
the end user (for
example, data output from a diagnostic test provider, which may be stored by
TACS 100 in the
Diagnostic Test module 9, or may otherwise be collected and stored by TAGS 100
in, for
example, EMR/EHR Data module 14). Essentially, in this example, a
medical/health profile is
created for and specific to the end user of the product and is stored in one
or more TAGS 100
modules.
[00169] In step 1601, electronic medical health records and drug use history
records are
automatically pulled by TAGS 100 from EMR/EHR data retrieval module 14
containing
prescription drug use historical records and diagnostic testing records
specific to the end user of
the TAGS Product, which may come through an electronic information conduit
such as, but not
limited to, Surescripts . Surescripts is an operator of the United State's
largest health
information network, enabling the transmission of electronic prescribing of
controlled substances
and clinical messages, linking certified e-prescribing software, mail-order
pharmacies, Medicaid,
hospitals and acute care settings, State Departments of Health, EHR vendors,
IDNs, HIEs,
independent & chain pharmacies, health plans and PBMs.The Surescripts Network
for Clinical
Interoperability provides an opportunity for regional and proprietary networks
operated by HIEs,
IDNs and EMR/EHR vendors to connect with one another using a common, open and
secure
national network. Technical standards already exist to enable the secure,
electronic exchange
of laboratory test results. However, this disclosure goes a step further to
encompass the
transmission of EMR/EHR results to TAGS 100 so that some or all of the
information may
appear on the product label itself, or to a facsimile thereof, or as
supplemental information to the
label, where the information can be used by the end user of a TAGS Product, or
by a third party
22368806.2 53
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
on the end user's behalf, to make an informed decision on whether or not the
end user is an
appropriate candidate to use the product.
[00170] In step 1602, the product buyer or end user, as the case may be, may
resolve any
conflicts between self-input data from step 1600 and the data automatically
retrieved from step
1601.
[00171] Diagnostic Test module 9 may represent one or more computer systems or
software
processes that facilitate communication between TAGS 100 and diagnostic
testing laboratories
that conduct health care related tests and procedures. Diagnostic Test module
9 may be
implemented as an integrated TAGS 100 component accessed remotely by
diagnostic facility
personnel over Network 13, through which the consumer gets a diagnostic test
completed in
step 1603, the results of which are output or otherwise stored by TAGS 100 in
step 1604. The
resolved data from step 1602 and diagnostic test data from step 1604 are then
utilized by TAGS
100 for further processing in step 1700 of FIG. 17. Other relevant data may
also be collected in
step 1605 and utilized by TACS 100 for further processing in steps 1900 of
FIG. 19 and 2003 of
FIG. 20.
[00172] Referring to FIG. 17, in step 1700, the data collected in steps
1600, 1601, 1602 and
1604 of FIG. 16 are sorted by TAGS 100 into several groups and sub-groups of
data. These
groups and sub-groups of data may include: 1) in step 1701, End User Personal
and Physical
Data (e.g., sex (1701a), birth date or age (1701b), weight (1701c), height
(1701d), blood
pressure (1701e), or other personal or physical information (1701f) such as a
TACS ID number,
address, phone number and other identifying information; 2) in step 1702, the
end user's Body
Mass Index ("BMI") value is calculated from the end user's reported physical
weight and height.
The BMI value, which provides a reliable indicator of a person's "body
fatness" to screen for
weight categories that may lead to certain health problems, may be modified by
the end user's
sex and age; 3) in step 1703, the end user's personal habitual data (e.g.,
smoking tobacco,
using certain recreational drugs, drinking alcohol, etc.), 4) in step 1704,
data on the end user's
known, pre-existing medical condition(s), which may include diseases,
disorders, allergies,
hypersensitivies and other conditions; 5) in step 1705, end user Drug
Treatments, which may
include a list of prescription and/or non-prescription drugs historically
and/or currently taken by
an end user; and 6) in step 1706, a list of end user diagnostic test results.
All of this information
may be sent to the TACS Product candidacy algorithm in step 1900 of FIG. 19.
22368806.2 54
CA 02818052 2013-06-06
Agent Ref.: 79305/00002
[00173] In step 1707, the relevant above factors may be collected for a
particular end user,
related to a particular medical condition that the end user is trying to treat
or diagnose. Some of
these captured factors may render a diagnostic test standard reference
"normal" value or range
of values unsuitable for use as a benchmark, then an alternative adjusted
benchmark value or
range of values may be used as a benchmark instead.
[00174] In step 1708, these factors may be combined with standard benchmark
value(s),
which may be stored in Diagnostic Test Benchmark Database 15.An algorithm may
adjust or
otherwise modify the benchmark values if necessary based on the retrieved
factors. The
modification may, for example, be made as an automatic change or calculation
based on an
algorithm, or as an independent example, the modification may be based on the
presence of
certain pre-defined captured factors, whereby if such factors are present, an
algorithm may
cause TAGS 100 to use alternative pre-defined benchmark factors that may have
already been
stored in Diagnostic Test Benchmark Database module 15.
[00175] A diagnostic test end user baseline value (or multiple baseline
values) may also be
captured and stored in Diagnostic Test module 9 from, for example, a prior
diagnostic test
performed on an end user. Such baseline value(s) can be retrieved from the
TAGS 100
database on demand. Using the baseline value, TACS 100 calculates how the most
current
diagnostic test value(s) compare(s) to the baseline value(s). For example, the
standard
unadjusted benchmark reference value range for fasting total blood cholesterol
is assumed for
this example to be any level between 125 mg/dL to 200 mg/dL, and that any
total cholesterol
level above 200 mg/dL will justify treatment by a TAGS cholesterol lowering
treatment product.
This example will assume that just prior to starting a course of daily
medication treatment with a
statin drug to lower an end user's high cholesterol, an end user may have had
their fasting total
cholesterol measured through a laboratory blood test and that the result from
the test is 280
mg/dL. Such a value may be preferentially stored in Diagnostic Test module 9,
and labeled by
TAGS 100 to be the "baseline value" since it was taken just prior to
initiating treatment with the
TAGS Product. This example will further assume that fasting total cholesterol
blood levels will
be drawn every 30 days and that after 60 days of treatment with a cholesterol
reducing
treatment, the end user's fasting total cholesterol blood level was measured
at 183 mg/dL,
which is within the standard unadjusted benchmark reference value range for
fasting total
cholesterol. Continuing with this example, after 90 days of treatment, the end
user's fasting
total cholesterol is assumed to be measured at 175 mg/dL. Again, such a value
may be
preferentially stored in Diagnostic Test module 9, and labeled by TAGS 100 as
the "latest
22368806.2 55
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
treatment" diagnostic test value (equivalent to 175 mg/dL). The value of the
diagnostic test
taken at 30 days prior to the 90-day period. Such a value may be
preferentially stored in
Diagnostic Test module 9, and labeled by TACS 100 as the "prior period"
diagnostic test value
(equivalent to 183 mg/dL).
[00176] In step 1710, TACS 100 may preferentially calculate point and/or
percentage
differences comparing latest period diagnostic test results from step 1706 to
benchmark or
adjusted benchmark diagnostic test values, as the case may require (from step
1709) and/or
prior period test results (stored by TAGS 100 in Diagnostic Test module 9).
[00177] Continuing with the same example, the percentage difference between
the latest
treatment diagnostic test value and the baseline value would be calculated as -
34.6% (after
rounding), signifying that the end user's cholesterol was reduced by that
amount after 90 days
of treatment with the cholesterol lowering drug. The percentage difference
between the latest
treatment diagnostic test value and the prior period diagnostic test value
would be calculated as
-4.3% (after automatic rounding), signifying that the drug had a continuing
cholesterol reduction
benefit during the last 30 days. It is expected that after a continuous period
of time on the drug,
a person's fasting blood cholesterol measurement would flatten out and
stabilize. The algorithm
may be preferentially written so that the end user can continue to use the
TACS Cholesterol
reducing product for as long as the end user's fasting total cholesterol while
on the medication is
measured at, for example, 33% less than baseline cholesterol level, but not
more than, for
example, 50% of baseline. An alternative approach might set the cholesterol
level values at
between 150 mg/dL and 180 mg/dL (or whatever other fixed cholesterol blood
levels may be
set) for use by the algorithm to allow automatic product use renewal for the
end user.
[00178] In step 1711, TAGS 100 may also assign specific colors (e.g., green
for test values
falling within a particular reference range, signifying, for example, a normal
test outcome; red for
test values falling higher than the reference range, signifying, for example,
that the cholesterol
level is too high; and yellow for test values falling lower than the reference
range, signifying, for
example, that the cholesterol level is too low). Alternatively, TACS 100 may
assign any
variation of visual and/or audible effects to visibly and/or audibly
communicate and flag such
test outcomes (e.g., blinking lights or lights of fluctuating increasing
and/or decreasing intensity
or an audible siren or beeping noise, etc.). As yet another alternative
example, TAGS 100 may
assign different symbols, such as, but not limited to "=" for test values
falling within a particular
reference range signifying a normal test outcome, "+" or ">" for test values
higher than the
22368806.2 56
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
reference range, and "¨" or "<" for test values lower than the reference
range). The TACS 100
algorithm may also assign a combination of colors and/or visual and/or audible
effects to certain
diagnostic test value outcomes to further distinguish between the test
outcomes. The meaning
of the color(s) and/or visual effect(s) and/or audible effect(s) can be
explained on the DCPL so
that the end user can interpret the results correctly.
[00179] The point and/or percentage or other differences between the latest
and comparator
values (i.e., baseline and/or prior test value) may be output to step 1900 of
FIG. 19.TACS 100
may use these values to determine end user product use candidacy in step
1900.The values
may also be captured as DCPL outputs in step 2003.
[00180] Referring to FIG. 18, TAGS 100 may contemporaneously access Drug
Interaction
Database module 16 and, in steps 1800 and 1801, TAGS 100 may flag or otherwise
identify
known drug interactions stored in the drug interaction database between 1) the
TAGS Product
intended for use by the end user, and 2) other drug treatments already being
used by the end
user according to the end user's drug use history, which may be stored or
otherwise accessed
by TAGS 100 in step 1705 of FIG. 17. Any drug interaction(s) noted by the TAGS
100 algorithm
for the TAGS Product may be output to the TAGS Product candidacy algorithm in
step 1900
(FIG. 19) and may concomitantly be captured as DCPL outputs in step 2003 of
FIG 20.
[00181] Referring to step 1900 in FIG. 19, the TAGS Product usage candidacy
algorithm may
be run by TAGS 100, using the TAGS 100 data inputs heretofore described. Based
on the
output of the algorithm, the end user will either not be cleared to use the
TAGS Product or be
cleared to use the TAGS Product in step 1901.
[00182] Referring to FIG 20, TAGS 100 may generate a TAGS Product label
template in step
2000, which may contain pre-determined fixed and/or extemporaneous data entry
placeholders
that may be populated with data from the aforementioned steps. The DCPL label
for the
product may be generated or otherwise prepared by TAGS 100 based on some
fixed, pre-
determined information, for example, information that is not specific to a
particular end user of
the product, but is generally applicable to any possible user of the product.
In step 2001, TAGS
100 separates instances of product clearance and product denial. In step 2002,
TAGS 100
sends all relevant static and dynamic label data to physical and/or virtual
label placeholder
locations. TAGS 100 may either print the label in step 2003 or provide a
virtual screen output of
a virtual label in step 2004.
22368806.2 57
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[00183] FIG. 21 is an illustrative representation of a DCPL, which is an
example of the
label output from TAGS 100 to the end user (either virtually on a display of a
computer, hand
held device or other electronic device, or physically affixed to the TACS
Product). TACS 100
generates the DCPL and populates fields in the DCPL based on information
received by TAGS
100 for the end user of the TAGS Product. Generally, the dynamic portion of
the DCPL may
help the end user determine whether they are a candidate for using the TAGS
Product by
showing the end user information specific to their own personal health
profile. An indication of
TACS Product use candidacy may be provided on the DCPL, such as in the area
designated
1901. More specifically, TACS 100 may populate the DCPL with information on
end user
physical data 1600, family history 1604, habitual data 1703, medical
conditions 1601, drug
treatment 1602, diagnostic test results 1710, etc. TACS 100 may compare
selected end user's
data against benchmark values related to using the TACS Product and the
results of that
comparison may be provided on the DCPL. TAGS 100 may provide a visual
indication on the
DCPL to indicate whether the end user's diagnostic test results or other
medical information falls
into, above or below a normal range. For instance, a value may be provided on
the DCPL in
green to indicate a normal level, in flashing red to indicate a level above
the normal range, and
in flashing blue to indicate a level that is below normal.
[00184] TACS 100 may also provide an indication on the DCPL if any
medications taken
by the end user are contraindicated for use with the TACS Product. Information
regarding other
medications taken by the end user is received by TACS 100. TAGS 100 accesses
the Drug
Interaction Database 16 to determine whether there are any contraindications.
If so, TACS 100
may provide a visual indication of the contraindication to the end user on the
DCPL.
[00185] The DCPL may also include, among other things, directions for use
of the TAGS
Product. TAGS 100 may cause the TAGS Product directions to be dynamically
changed on the
DCPL based on medical information received regarding the end user of the TACS
Product. For
instance, if the desired reduction in blood cholesterol is not being achieved,
TACS 100 may
dynamically change the DCPL to direct the end user to increase the dosage of
the TACS
Product, or the DCPL may indicate that the end user should change to a higher
strength product
in the future.
[00186] The DCPL may be produced by TACS 100 so that it is a two or three
dimensional
viewable DCPL label, which may be made to dynamically change its contents
under certain
conditions to customize the label for a particular viewer.
22368806.2 58
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[00187] For
example, the DCPL may utilize printed electronics to create electrical devices
on
various substrates such as screen printing, lexography, gravure, offset
lithography, inkjet and
others. The DCPL may capture and show images in more than two dimensions, for
example,
using light patterns to display three dimensional holograms. Two or three
dimensional
functional electronic or optical inks, or light pattern based images can be
deposited on a
substrate, creating active or passive devices, for example, thin film
transistors or resistors.
[00188]
Electrically functional electronic or optical inks can be deposited by
solution-
based, vacuum-based or some other method, creating active or passive devices,
such as thin
film labels composed, in part, for example, of transistors or resistors. Other
solution-based
materials can also be used, including organic semiconductors, inorganic
semiconductors,
metallic conductors, nanoparticles, nanotubes, etc. Printed electronics
technology differs from
traditional electronics in that certain components and interconnects for the
former can be
fabricated on flexible substrates such as plastic, paper and foil. The
components and systems
are thin, lightweight and rugged. Printed electronics can also enable the
manufacture of wide-
area and distributed components such as sensor networks. Together, these
attributes are
attractive for the application of DCPL, especially since thin, flexible and,
if need be, rugged
form-factors can be created by the technology.
[00189] Radio frequency labeling may include the use of a wireless non-contact
system that
uses radio-frequency electromagnetic fields to transfer data to a DCPL tag or
label attached to
an object to change the text or other visual components of a label. Such
labels may require no
battery as they may be powered by the electromagnetic fields used to read them
or to send data
to them.
[00190] Holographic images may be projected onto a DCPL label or otherwise
viewed
independently for the purpose of communicating label information and related
content. By using
film with holographic properties and creating customized computer animations,
some
companies have created holographic illusions that can be seen in three
dimensions without the
need of special glasses. Over time, holographic projectors may be able to
render sharp
projected images from relatively small projection devices for example,
designed into cell-
phones, because they may not require high intensity, high-temperature light
sources. Indeed,
some companies are working toward applied science that could make television
with
holographic projections that can project moving, three-dimensional pictures
outside of the
screen.
22368806.2 59
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
[00191] Any of the above-described methods may be used to generate two or
three
dimensional viewable DCPL labels, which may be made to dynamically change
their contents
under certain conditions to customize the label for a particular viewer.
[00192] Software process or processes and executables on the computing
system may
be used to provide human interfaces and to store and initiate computer program
instructions
used to process and analyze data. Computer program code for carrying out
operations
described herein may be written in any combination of one or more programming
languages,
including an object oriented programming language such as Java, SaaS, C++, C#
or the like
and conventional procedural programming languages, such as the "C" programming
language
or similar programming languages. The program code may execute entirely on the
computing
system, partly on the computing system, as a stand-alone software package,
partly on the
computing system and partly on a remote computer or server, or entirely on a
remote computer
or server.
[00193] This application was described above with reference to flow chart
illustrations
and/or block diagrams of methods, apparatus (systems) and computer program
products
according to one or more embodiments. It is understood that some or all of the
blocks of the
flow chart illustrations and/or block diagrams, and combinations of blocks in
the flow chart
illustrations and/or block diagrams, can be implemented by computer program
instructions. The
computer program instructions may also be loaded onto the computing system to
cause a series
of operational steps to be performed on the computer to produce a computer
implemented
process such that the instructions that execute on the computer provide
processes for
implementing the functions/acts specified in the flowchart and/or block
diagram block(s). These
computer program instructions may be provided to the CPU of the computing
system such that
the instructions, which execute via the CPU of the computing system, create
means for
implementing the functions/acts specified in the flowchart and/or block
diagram block(s).
[00194] These computer program instructions may also be stored in a computer-
readable
medium that can direct the computing system to function in a particular
manner, such that the
instructions stored in the computer-readable medium implement the function/act
specified in the
flowchart and/or block diagram block or blocks. Any combination of one or more
computer
usable or computer readable medium(s) may be utilized. The computer-usable or
computer-
readable medium may be, for example (but not limited to), an electronic,
magnetic, optical,
electromagnetic, infrared, or semiconductor system, apparatus, device, or
propagation medium.
22368806.2 60
CA 02 818 052 2 013-0 6-0 6
Agent Ref.: 79305/00002
More specific examples (a non-exhaustive list) of the computer-readable medium
include the
following: an electrical connection having one or more wires, a portable
computer diskette, a
hard disk, a random access memory, a read-only memory, an erasable
programmable read-only
memory (e.g., EPROM or Flash memory), an optical fiber, a portable compact
disc read-only
memory, an optical storage device, a transmission media such as those
supporting the Internet
or an intranet, or a magnetic storage device. Any medium suitable for
electronically capturing,
compiling, interpreting, or otherwise processing in a suitable manner, if
necessary, and storing
into computer memory may be used. In the context of this disclosure, a
computer-usable or
computer-readable medium may be any medium that can contain, store,
communicate,
propagate, or transport the program for use by or in connection with the
instruction execution
system, apparatus, or device. The computer-usable medium may include a
propagated data
signal with the computer-usable program code embodied therewith, either in
base band or as
part of a carrier wave. The computer usable program code may be transmitted
using any
appropriate medium, including (but not limited to) wireless, wire line,
optical fiber cable, RF, etc.
[00195] Having described and illustrated the principles of this application
by reference to
one or more preferred embodiments, it should be apparent that the preferred
embodiment(s)
may be modified in arrangement and detail without departing from the
principles disclosed
herein and that it is intended that the application be construed as including
all such
modifications and variations insofar as they come within the spirit and scope
of the subject
matter disclosed herein.
22368806.2 61