Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
1
Method for providing a scrubber liquid and bacteria substrate for use in
biological desulphurisation of gases, particularly biogas.
The present invention relates to the use of water, such as waste
water, a liquid effluent from an anaerobic digestion and/or raw water for
the precipitation of sulphate originating from a biogas desulphurisation
process, the use as a substrate for sulphur-oxidizing bacteria and as a
scrubber liquid as well as methods for desulphurising of gases, such as
polluted air and particularly biogas, and providing a substrate for sul-
phur-oxidizing bacteria.
Anaerobic digestion is a biological process used to degrade or-
ganic matter in waste water and organic waste. During the anaerobic
degradation, biogas (typically comprising 50-70% methane, CH4, and
30-50% carbon dioxide, CO2, and 0-5% hydrogen sulphide, H25, equiva-
lent to 0-50.000 ppm (V/V)) is produced as a commercially important
by-product. The waste water and organic waste typically contains sul-
phur compounds, mainly bound as sulphate (SO4) compounds. The sul-
phur compounds originate from two main sources; i.e. sulphur from the
soil absorbed by plants during the growing season and sulphur used in
various production processes e.g. in the food industry. During the anae-
robic digestion, where no oxygen is present, sulphate is converted into
hydrogen sulphide (H25) which is absorbed in the biogas produced. H25,
contrary to sulphate, is not liquid and thus not bound in the liquid phase.
During combustion of the biogas, such as in engines and boilers,
the H25 will form sulphur dioxide (502), which will react with water and
form sulphuric acid (H2504). Thus, if the biogas is combusted without
prior H25 removal, severe corrosion will occur in the equipment. In addi-
tion, sulphur dioxide (502) causing acid rain, will be exhausted from the
chimney. Therefore, it is vital that the H25 is reduced to acceptable limits
before the biogas is used as a fuel in e.g. gas engines or boilers. Typical-
ly, gas engine manufacturers require max. 200-250 ppm H25 in the
clean gas if full warranty should be provided.
Thus, as hydrogen sulphide is highly unacceptable in the me-
thane gas provisions must be present for removing the hydrogen sul-
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
2
phide before the gas can be used as a commercial product.
Several means for removing hydrogen sulphide exist; both
chemical and biological systems.
Chemical removal of hydrogen sulphide can for example be per-
formed by scrubbing the gas with water and polyethylene glycol whereby
carbon dioxide is removed together with the majority of the hydrogen
sulphide. Hydrogen sulphide reacts readily with either iron oxide or iron
chloride to form removable insoluble iron sulphide. Also, activated car-
bon filters may be used to remove the hydrogen sulphide.
In biological removal of H25 from gases or polluted air, sulphur
oxidizing bacteria of the Thiobacillus family are used. These bacteria
convert, in the presence of oxygen, hydrogen sulphide to sulphate com-
pounds which are typically removed using an aqueous medium. The bac-
teria require water and nutrients in the correct amount and quality for
the process of oxidizing sulphur. This is for example described in DE 10
2009 014 221.
The aqueous medium used is typically soft water. If hard water
containing calcium and magnesium were used directly in the process
there would be precipitations of compounds formed by metal ions in the
water and sulphur oxides formed by the bacteria in the equipment. This
has a negative effect on the efficiency of the system because a high de-
gree of sludge formation inside the equipment will require frequent
cleaning thereof and result in many hours lost production. Therefore,
water used in the process has preferably to be low in i.a. calcium (Ca2 )
and magnesium (Mg2 ) and other metals capable of forming heavy salts.
Consequently, there is typically used soft demineralized water.
The source of nutrients is most often industrial fertilizers in the
form of agricultural or horticultural fertilizers typically containing at
least
nitrogen, phosphorous and potassium (NPK) diluted in water. One of the
main problems with this solution is that it can be difficult and expensive
to obtain the right mixture of fertilizers, since fertilizers often are made
for another purpose (e.g. farm use) and can contain chemicals and par-
ticles that are inhibitive for the bacteria and also cause sedimentation of
sludge and scaling inside the equipment, which is a problem for a
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
3
smooth operation and economical operation.
Alternatively, waste water inherently containing nutrients could
be used in the process. However, waste water will besides organic mat-
ters, also known as the chemical oxygen demand (COD), typically also
contain calcium and magnesium and give major problems with sludge
formation inside the equipment if used directly in the process. Therefore,
the bacteria substrate normally used in biological gas cleaning systems
is demineralized water supplied with nutrients, often in the form of in-
dustrial NPK fertilizers. This will ensure the necessary supply of water
and nutrients for the bacteria and at the same time the problems with
sludge formation inside the equipment is avoided.
As described above the drawback of this solution is the limited
access to acceptable fertilizers and the significant investment and/or op-
eration costs for providing industrial fertilizers and demineralized water.
Though the methane gas produced is basically a by-product
from the degradation of organic matter in waste water and/or organic
waste typically imposed for environmental reasons, the opportunity of
creating a profitable business of for example the biogas drives the desire
for finding economical ways of treating the gas so it can be profitably
exploited as a valuable renewable energy source.
In the present invention a more productive and economical
process for biological removal of undesired sulphur compounds con-
tained in gases, such as polluted air, biogas or other gases is provided
which overcomes some or more of the problems mentioned above.
Summary of the invention
Thus, in a first aspect is provided a method for desulphurisation
of a gas, comprising the steps of
a) providing the gas to a main process tank, the gas comprising
at least H2S and the main process tank comprising sulphur oxidizing bac-
teria;
b) supplying a clean scrubber liquid to the main process tank,
providing in the presence of oxygen a desulphurized gas and a used
scrubber liquid comprising 5042-; wherein the clean scrubber liquid is
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
4
provided by the steps of:
i) supplying at least a part of the used scrubber liquid to a sedi-
mentation tank;
ii) contacting the used scrubber liquid in the sedimentation tank
with water wherein the water is not demineralized or deionized;
iii) allowing a precipitation comprising oxidized sulphur com-
pounds to form in the sedimentation tank providing a precipitation and
the cleaned scrubber liquid.
By the present invention a system has been developed where
waste water comprising the nutrients nitrogen, potassium and phospho-
rus, micro-nutrients and trace metals needed for the bacteria, as well as
organic matter, also known as the chemical oxygen demand (COD), can
be used in the desulphurisation process without causing problems with
the formation of sludge and precipitation and/or scaling in the main
process tank(s). This is achieved by the method of the invention where
the problems with sludge and precipitation and/or scaling are avoided by
the insertion of a loop where acidic sulphate containing water from the
main process tank(s), i.e. the used scrubber liquid, is contacted with un-
treated water, such as waste and/or raw water. Hereby undesired com-
pounds, possibly including a part of the sulphate formed, is removed in
one or more sedimentation steps before being re-circulated to the main
process tank(s) as a clean scrubber solution for scrubbing out sulphate
formed in the main process tank(s) by the bacteria. In a preferred em-
bodiment the gas is biogas or polluted air and more preferred biogas.
By this looping of the scrubber solution otherwise requiring fre-
quent cleaning of the main process tank(s) is avoided. In addition, the
requirement for soft, i.e. demineralized, water is avoided.
In a preferred embodiment the water is waste water whereby
addition of nutrients in the form of industrial fertilizers is superfluous
rendering the method even more economical.
In yet another embodiment the waste water is treated by anae-
robic digestion and the gas is biogas, whereby both treated waste water
and the biogas to be desulphurized is provided and both being usable in
the method of the invention.
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
The operating costs of the method of the invention is therefore
typically 5-15 % of the prior art methods using chemicals and typically
25-50 % of the prior art methods using biological systems with soft wa-
ter and industrial NPK fertilizer. In addition, the availability of the system
5 regarding operation hours is above 98%.
The method of the invention has several advantages, first of all
when the water used is waste water savings on operation costs for nu-
trients are achieved.
In addition, raw water comprising high levels of Ca2+ and Mg2+
can be used in the process instead of expensive demineralized or reverse
osmosis treated water. Thus, saving both the cost of using soft water or,
if combined with waste water, saving cost on both nutrients and soft wa-
ter.
By using the waste water for supplying nutrients, the risk of
adding unwanted chemicals or components such as dirt, soil and/or sand
normally present in industrial fertilizers is avoided. Such industrial ferti-
lizers are manufactured to be used in other applications, and conse-
quently such "unwanted" chemicals do not need to be removed. Thus,
availability of a suitable fertilizer for the purpose of supplying nutrients
to the sulphur oxidizing bacteria is never an issue.
For large plants processing around 2,500 m3/h gas/air compris-
ing 30,000 ppm hydrogen sulphide in the raw gas and 250 ppm in the
desulphurized gas the total cost when using the method of the present
invention is approximately 20,000 Euro/year while plants of the same
size will cost approximately 80,000 Euro/year when using biological me-
thods with soft water and NPK, while chemical removal is the most ex-
pensive solution amounting to approximately 360,000 Euro/year. Thus,
a significant reduction in operation costs is achieved when using the me-
thod of the present invention.
The method of the invention is further economical in that a
complete recycling of nutrients and scrubber liquid in the plant is pro-
vided in a simple manner. Only small amounts of liquid is drawn from
the system in the form of a slurry comprising precipitated material, i.e.
heavy salts and sludge and possibly a part of the sulphate formed. This
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
6
slurry is taken from the sedimentation tank(s) where the precipitation
allows to sediment.
Also, the pH in the process is stabilized due to the reaction of
salts, organic matter and metals in the waste water. Biological prior art
H25 removal systems will frequently need neutralizing chemicals, such
as sodium hydroxide (NaOH) in order to increase and/or stabilize pH in
the system. The decreasing pH is due to the sulphate and acid formed in
the reaction facilitated by the bacteria. By stabilizing the pH without use
of external chemicals the cost of operation will further decrease and
problems when recycling the waste water are avoided.
In a particular embodiment the water used may be both waste
water and/or raw water. The contacting of ions in the raw water and the
sulphate in the used scrubber solution results in the formation of a pre-
cipitation whereby the ions are substantially removed from the water.
Therefore, the process of the invention also ensures that expensive de-
mineralized or reverse osmosis treated water can be replaced by un-
treated water. This will dramatically reduce the cost of operation and
consequently result in a more profitable production of biogas.
The sedimentation may take place in one or several steps such
as 2, 3, 4, 5 or more. Thus, the sedimentation step may take place in
one or several sedimentation tanks or in one compartmentalized sedi-
mentation tank. The sedimentation tank may then be lined with a suita-
ble material, preferably an acid proof material.
In a second aspect the scrubber liquid may be seen as a liquid
substrate for sulphur-oxidizing bacteria, and hence the method compris-
es the steps of
a) leading an acid liquid comprising sulphate (S042-) to a sedi-
mentation tank supplied with waste water and optionally raw water,
b) allowing the sulphate to react with chemical compounds
and/or organic matter contained in said waste water and/or raw water
such as to form a precipitation and a liquid substrate,
c) separating the liquid substrate from the precipitation.
In this aspect is provided a simple method for providing a sub-
strate for the bacteria used to convert the H25 to sulphate.
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
7
In both aspects, it is contemplated that one or more serial con-
nected sedimentation tanks may be present in order to optimize the pre-
cipitation. In this embodiment clean scrubber liquid from the first sedi-
mentation tank may be led to a second sedimentation tank for additional
precipitation and so on. Clean scrubber liquid may be taken from each of
the sedimentation tanks and supplied to the main process tank(s) as re-
quired.
It is also contemplated that more than one main process tank is
present in which simultaneous desulphurisation of the gas takes place.
The used scrubber liquid taken from the main process tanks may be
supplied to the same or different sedimentation tanks for precipitation.
In yet another aspect the present invention provides the use of
the precipitation formed in the methods of the invention as a liquid fertil-
izer.
In still another embodiment is provided the use of waste water
for precipitation of sulphate originating from a biogas desulphurisation
process, as a substrate for sulphur-oxidizing bacteria and as a scrubber
liquid.
From these aspect the multiple uses of the waste water accord-
ing to the invention illustrates all the benefits of the invention where the
supply of a waste product can be used as a multipurpose valuable prod-
uct.
All detailed and specific embodiments described herein for each
aspect equally apply to all aspect of the invention.
Fibures
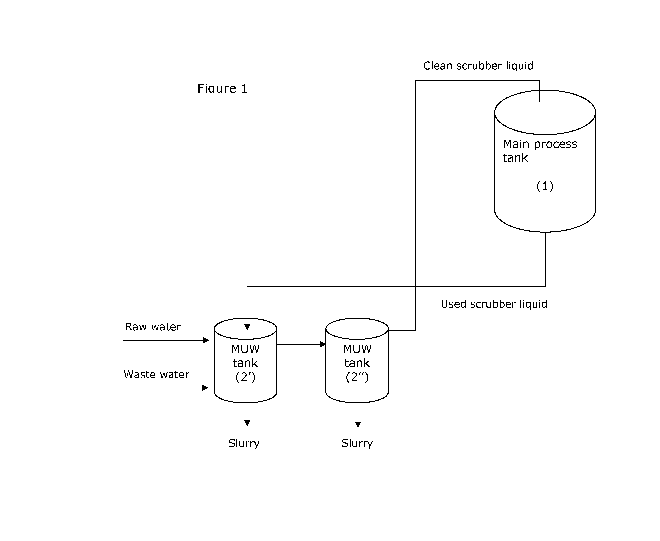
Figure 1 is a schematic illustration of an embodiment of the in-
vention in which two sedimentation tanks and one main process tank is
used.
Figure 2 is a schematic illustration of another embodiment of
the invention where one sedimentation tank and one main process tank
is used, the sedimentation tank being compartmentalized in two sec-
tions.
Detailed description of the invention
In the context of the present invention the water used in the
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
8
precipitation step comprise any type of water - no provisions need to be
present for deionizing or demineralizing the water. Preferably, the water
is waste water and more preferred treated waste water. Treated waste
water is in the context of the present invention waste water treated by
anaerobic digestion whereby methane gas, biogas, is produced. Most
preferred are embodiments where the water is treated waste water from
the same source producing the biogas to be treated in the method. This
will provide a complete process for recovering, often industrial, waste
water and producing desulphurized biogas without extra supply of ex-
pensive soft water. In this context soft water is deionized and/or demi-
neralized water.
The method of the invention can be seen as a biological gas
scrubber process. The main process tank or tanks house the reaction
where H25 is converted to sulphate by bacteria of the Thiobacillus family.
The main process tank(s) can be designed to meet any gas flow and H25
content in the raw gas. Typically, the H25 is reduced to below 300 PPm,
such as to 50-250 ppm but the method of the invention can reduce the
H25 content to lower values if required.
The process of the invention will now be described in details
with reference to the plant schematically shown in figure 1 showing a
main process tank (1) and two tanks, functioning as sedimentation tanks
or tanks for supplying make up water (2', 2" etc.). Herein the terms se-
dimentation tank and make up water (MUW) tank may be used inter-
changeably referring to the same type of tanks. It is contemplated that
there may be one or more main process tanks and that there may be
one or more sedimentation tanks, such as 2, 3 4 or 5. As embodied in
figure 2 it is also contemplated that the sedimentation tanks are com-
partmentalized thus capable of facilitating multi stage sedimentation in
one tank. The communication between tanks and/or compartments in
one tank can be in the form of pipe connections and by overflow from
one tank to another or compartment to another. Communication by
means of pipe connections is preferred when the system comprises more
than one tank. While overflow from one compartment to another is pre-
ferred when one compartmentalized tank is used.
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
9
The sedimentation tank(s) can be made of concrete, metal or fi-
ber glass. In the sedimentation tank(s) regeneration of the scrubber liq-
uid is performed, i.e. steps i)-iii) of the method of the first aspect as well
as the providing of the liquid substrate for the bacteria according to the
second aspect. In the sedimentation tank(s) chemical compounds of the
water used in the process, such as calcium (Ca2+), magnesium (Mg2+)
and COD, will be removed. These compounds will, if not removed, pro-
duce heavily dissolved salts with sulphate produced by the bacteria in
the main process tank(s). This would require frequent cleaning of the
main process tank(s) and thereby less efficient production of gas.
In the main process tank(s) H2S contained in the gas is oxidized
to S042- by the bacteria contained in the tank(s). The formed sulphate
will be transported into the scrubber liquid (the mixture of water and nu-
trients) and leave the tank(s) diluted in the scrubber liquid used for the
process, this solution is denoted the used scrubber liquid.
At least a part of the used scrubber liquid having a low pH typi-
cally pH 0.5 to 2.0 is pumped to the first sedimentation tank and mixed
with waste water and/or raw water. The amount of waste water pumped
to the sedimentation tank(s) is adjusted so that the correct amount of
nutrients are present for the process taking place in the main process
tank(s). The amount of effluent from the main process tank(s) pumped
to the sedimentation tank(s) is adjusted to ensure that sufficient 5042- is
present in the mixture to ensure that a full reaction between compounds
in the waste water that has to be removed before entering the main
process tank(s) will occur, and to ensure that an acceptable pH of the
solution is obtained. Such compounds that are undesired in the main
process tank(s) are for example Ca2+ (>100 mg/I), Mg2+ (>100 mg/I)
and organic matter (COD) (>1000 mg/I).
The S042- (sulphate) reacts with Ca2+, Mg2+ and COD under the
formation of Ca504, Mg504 and sludge; these compounds are allowed to
sediment in the sedimentation tank(s). Consequently, in the first sedi-
mentation tank the highest degree of sedimentation will occur while, the
optional subsequent sedimentation tank(s) serve to further allow for se-
dimentation.
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
The sediments are taken from the bottom section of the sedi-
mentation tank(s) as a slurry which can advantageously be used as a
liquid fertilizer.
The effluent from the main process tank(s), i.e. the used scrub-
5 ber liquid, comprising the waste water, and optionally raw water, will af-
ter the sedimentation contain the diluted nutrients functioning as a sub-
strate for the bacteria in the main process tank(s). This liquid is denoted
the clean scrubber liquid. This clean scrubber liquid then enters or re-
enters the main process tank(s). Consequently, the main process
10 tank(s) in which H2S is being converted to sulphate and removed, only
receives diluted nutrients with a very low content of heavy salts.
The amount of raw water and/or waste water added to the se-
dimentation tank(s) is adjusted to ensure both a sufficient supply of nu-
trients for the bacteria in the main process tank(s), but also to dilute and
remove S042- produced by the bacteria in order to adjust pH of the liq-
uid.
The supply of water for achieving the above will result in an in-
creased liquid level in the system why drainage from the system may
have to be done in order to maintain the liquid balance in the tank(s).
This water balance is mainly adjusted by removing the slurry of heavy
salts and sludge formed. The main point of drainage from the entire sys-
tem (i.e. main process tank(s) and make up water tank(s)) is at the bot-
tom of each of the sedimentation tank(s) where the sedimentation from
the chemical reaction between 5042-, Ca2 , Mg2+ and other reactive
compounds in the water and waste water ends up as a mixed sludge.
Preferably the sludge is removed from the sedimentation tank(s) in the
same frequency as it is produced.
The process parameters, including the liquid balance, addition of
raw water, waste water, drainage, supply of air and possibly heating are
preferably controlled by a programmable logic controller (PLC) system
making the process fully automated. PLC systems are commercially
available for example under the trade names TWD Compact, Micrologix
and Simatec available from Schneider Electric, Allan Bradley and Sie-
mens, respectively. The operation of such systems is within the skill of
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
11
the art.
The operation of the entire cleaning process is to be carried out
in a system comprising two main items; the at least one main process
tank (MPT) and one or more sedimentation tanks. The operation may be
automatic and may be controlled from a machine room comprising the
PLC system. In addition circulation/drain pump(s), air blower(s), water
supply, heating, etc. are present. The machine room may be housed in a
standard shipping container, housing of plastic or glass fiber or mounted
on a skid. The choice of these units is within the skill of the art.
The actual number and size of the sedimentation and main
process tank(s)s respectively, depends on the sulphur load. The main
process tank(s)s have internal pipe systems for gas distribution, supply
and distribution of the scrubber liquid and sludge removal and are filled
with packing media. The tank(s) are manufactured in PE (polyethylene),
FRP (reinforced fiberglass) or concrete or mild steel with an acid-proof
liner.
In operation, the clean scrubber liquid is pumped to the top of
the main process tank(s), where spray nozzles distribute the clean
scrubber liquid over the surface of the packing media containing sulphur
oxidizing bacteria. The scrubber liquid flows through the packed bed and
provides moisture and nutrients for the bacteria living on the surface of
the packing media.
The gas, such as biogas, may be introduced, both from the up-
per and the lower part of the main process tank(s), and passes upwardly
and/or downwardly, respectively, thereby bringing the gas into contact
with the bacteria. The bacteria oxidize the H25 to sulphate. Subsequent-
ly, the sulphate is absorbed in the scrubber liquid, said liquid flowing by
gravity to the bottom part of the main process tank(s).
The used scrubber liquid leaving the bottom part of the main
process tank(s) is re-circulated after being supplied with make-up water
and drainage of used scrubber liquid at certain intervals as detailed
above.
After passing the main process tank(s), for example desulphu-
rized biogas is ready for combustion in engine(s) or boiler(s).
CA 02821792 2013-06-14
WO 2012/079586 PCT/ K2011/050485
12
The desulphurization will now be explained in more details. The
biological oxidation of hydrogen sulphide to sulphate proceeds in two
stages. In the first stage, hydrogen sulphide is partially oxidized to sul-
phur (2) and in the second step, sulphur is completely oxidized to sul-
phate (3).
H2S <-> H+ + HS- (dissociation) (1)
2HS- + 0, 2S + 20H- (2)
2S + 302 + 20H- 2S024- + 2H+ (3)
The overall reaction can be described as follows:
2HS- + 402 2 S024- + 2H+ (4)
This reaction both desulphurizes the biogas and causes the pH
of the scrubber liquid to decrease.
The sulphate produced is discharged with the used scrubber liq-
uid from the main process tank(s) containing up to the equivalence of 1-
30 % 504, typically 2-10%; the chemical composition of the effluent will
depend on the raw water and/or waste water and/or treated digester ef-
fluent added to the process.
The sulphur oxidizing bacteria are mainly belonging to the Thi-
bacillus genus as the Thiobacillus thiooxidans (also known as Acidithi-
bacillus thiooxidans), Thiobacillus acidophilus (also known as Acidiphi-
lium) and Thiobacillus albertis (also known as Acidithiobacillus alberten-
sis) among others. These bacteria are well known in the art and are rea-
dily available from several suppliers, such as LCG Standards. The sul-
phur oxidising bacteria have the following requirements.
The bacteria need a place to live and multiply, which in the
present invention will be on the packed media inside the main process
tank(s). The bacteria require basically sulphur (sulphide), oxygen, nu-
trients (NPK) and water to grow. In the present invention these require-
ments are met by supplying the gas or polluted air containing H25, an
external supply of oxygen, preferably in the form of atmospheric air, and
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
13
the water supplied according to the invention, e.g. raw water and/or
waste water and/or treated effluent. When water is supplied as waste
water the nutrients are contained therein, when the water supplied is
raw water, liquid NPK fertilizer is supplied. The growth conditions of the
bacteria also requires a temperature between 25-60 C.
The desulphurisation process in the main process tank(s) re-
quires oxygen. The oxygen supply may be provided by the injection of
atmospheric air. This may be achieved by injecting atmospheric air into
the main process tank(s) or by adding air to the influent biogas.
The amount of air supplied depends on the H25 level in the raw
biogas.
When air is added the absolute volume of methane will remain
unchanged, but the injection of air will dilute the relative methane con-
tent in the clean gas.
The air may be injected by a frequency regulated air blower, so
that only air needed for the process is injected. The choice and operation
of injectors and blowers are within the skill of the art.
The nutrients for the bacteria may be supplied in several ways.
In particular embodiments of the invention the water is treated waste
water such as industrial waste water or raw water and NPK fertilizer,
preferably liquid.
The treated waste water is typically originating from industrial
waste water treatment plants (WWTPs) connected to palm oil mills, cas-
sava/tapioca production, ethanol production, breweries, food industries
etc., but may also originate from other types of biogas plants, incl. mu-
nicipal WWTP's and biogas plants treating animal manure. Such treat-
ment is often anaerobic digestion of organic matter in the waste water
and/or organic waste whereby treated waste water is produced as well
as the biogas, for example to be treated according to the invention.
If the treated waste water is not supplied from an end pond but
is supplied directly from anaerobic treatment, means for settlement is
required to avoid particles.
In yet another preferred embodiment the water is raw water to
which NPK fertilizer is added. Due to the sedimentation of heavy salts in
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
14
the sedimentation tank(s) the need for soft water is omitted.
In still another embodiment the water used is a mixture of raw
water and treated waste water. This will enable adjustment of the nu-
trients to be fed to the main process tank(s) without compromising the
cost of the process.
The used scrubber liquid leaving the main process tank(s) is in
the present invention mixed with the treated waste water and/or water,
such as raw water in the sedimentation tank(s). Here the heavy salts
and sludge formed by the contacting of sulphate containing used scrub-
ber liquid and metal containing water and COD containing waste water
respectively, cause the sedimentation of heavy salts and sludge in the
form of a waste slurry. This waste slurry will be high in NPK and S. The
slurry is continuously drawn from the sedimentations tank(s). Therefore,
the slurry drawn from the sedimentation tank(s) in a preferred embodi-
ment is used as a liquid fertilizer.
Traditionally, fertilizers are classified according to their content
of N (nitrogen), P (phosphorus) and K (potassium). However, S (sul-
phur) is as important, and in most countries the fertilizer manufacturers
must specify the content of N, P, K and S. For sugar cane production the
recommended yearly application is 30-50 kg S per hectare. For cereals
and oil seed rape the recommended yearly application per hectare is ap-
prox. 20 and 40 kg S, respectively.
Thus, the slurry drawn from the process of the invention, a liq-
uid fertilizer, may advantageously replace or supplement traditional ferti-
lizers, the price for such fertilizers is approx. 0.5 EUR per kg. The
process of the invention will produce approximately one kg S fertilizer
for each kg H25 removed.
The fertilizer produced according to the present invention is su-
perior over traditional waste water from desulphurization processes us-
ing soft water and NPK fertilizer because the pH is higher and conse-
quently the plants will be less exposed to acid damages on the leaves.
The present invention will now be illustrated by way of the fol-
lowing non limiting examples.
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
Examples
Calculation of operational costs for removing hydrogen
sulphide and comparison with prior art.
The following calculations are based on a similar plant as out-
5 lined in Figure 1 but with two main process tanks (1) and two sedimen-
tation (MUW) tanks (2' and 2"). The inlet of water will be either raw wa-
ter or waste water to the first sedimentation tank (2'). The water will be
further supplied to the second sedimentation tank (2").
The gas to be cleaned is biogas and the flow rate to the main
10 process tanks is set at 2,500m3/h and the biogas having an initial H25
content of maximum 30,000ppm. After the desulphurisation process in
the main process tanks, the content of H25 is reduced to maximum 250
ppm. Thus, illustrating that the degree of purity obtained by the method
of the present invention results in the required purity.
15 The
desulphurisation of the present invention was calculated to
be 113 kg H25 per hour corresponding to a desulphurization rate of the
biogas of approx. 2,700 kg H25 per day.
The calculations are based of the data summarized in the table
below.
Biogas flow per hour 2,500 m3/h
H25 max in raw biogas 30,000 ppm
H25 max in clean biogas 250 ppm
H25 to be cleaned 113 kg H25/h
Estimated costs of the present invention compared to
conventional plants
Based on the data obtained from the calculation example above, i.e. re-
moval of approximately 2,700 kg H25 per day providing a biogas with
maximum 250 ppm H25, the operation costs of the plant of the present
invention will be given below.
The cost of the electrical units is estimated to 0.1 EUR/kWh. The
cost of soft water is estimated to 0.5 EUR/m3, NPK nutrient to 0.4
EUR/kg, caustic soda to 0.35 EUR/kg, and the nutrient for partial rege-
CA 02821792 2013-06-14
WO 2012/079586 PCT/
K2011/050485
16
neration of the caustic soda in the chemical processes is estimated to
2.0 EUR/liter.
As the process of the present invention does not require soft
water, NPK nutrients, caustic soda or special nutrients, the only cost will
be electricity. Based on a plant as outlined above, the estimated demand
for electricity would be 22 kWh, which would result in operational costs
of approximately 20,000 EUR per year.
A prior art biological plant requires in addition to electricity soft
water and NPK nutrients, why the operational costs of such a plant
would amount to approximately 80,000 EUR per year. This is based on
an electricity consumption of 22.0 kWh, 4.0 m3 soft water and 12 kg
NPK nutrients per hour.
A state-of-the art chemical plant is estimated to use on average
158 kWh electricity, 2 m3 soft water, 52 kg caustic soda, and 3.3 liter
special nutrients which on a yearly basis results in operational cost of
approx. 360,000EUR. Thus, based on the presumptions above it is clear
that the process of the present invention provides a significant saving
without compromising the quality of the desulphurization process. Fur-
thermore, the present invention significantly reduces the need for fre-
quent cleaning of the main tanks which traditionally results in many lost
production hours. Therefore, the present invention will in addition to
substantial savings in operating costs also secure a very high availability
which will maximize revenues from generation of power and/or heat
from the biogas.