Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
1
Medical device for treating a heart valve insufficiency or stenosis
Field of Invention
This invention relates to a medical device for treating a heart valve
insufficiency or stenosis. The
medical device includes an endoprosthesis which can be introduced into a
patient's body with
minimal invasion and automatically expanded to position and secure a heart
valve prosthesis in the
patient's aorta.
Background of Invention
The expression "narrowing of a heart valve and/or heart valve insufficiency"
is intended to include
a functional defect of one or more heart valves which is either genetic or has
developed over time
due to age or disease. A valve defect of this type might affect each of the
four coronary valves, al-
though the valves in the left ventricle (aortal and mitral valves) are
affected much more often than
the right-hand part of the heart (pulmonary and tricuspid valves). The
functional defect can result
in narrowing (stenosis), inability to close (insufficiency) or a combination
of the two (combined
vitium).
The operating principle of medical devices for treating a heart valve
insufficiency or stenosis is al-
ready generally known in the field of medical technology. Biological or
mechanical valve models are
currently available as a means for replacing human heart valves. Replacement
valves are typically
stitched to the base of the native heart valve once the diseased valve has
been removed. The pro-
cedure requires an opening to be made in the thorax to undertake this
intervention, the patient's
circulation must be supported by a heart and lung machine and the heart
arrested whilst the heart
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
2
valve prosthesis is implanted. This is a risky surgical intervention which
places the patient at con-
siderable risk and involves a long post-operative phase of treatment. In multi-
morbid patients in
particular, the risk of carrying out such intervention is rarely justifiable.
In more recent times, minimally invasive treatment methods have been developed
which are dis-
tinctive due to the fact that the intervention can be carried out with a local
anaesthetic. This option
is based on the use of a self-expanding stent carrying a collapsible heart
valve prosthesis which is
implanted into the human body by means of an appropriate catheter system. A
self-expanding
heart valve endoprosthesis of this type can be fed by means of a catheter
system through a main
artery or vein to the implantation site at the heart. Once the implantation
site is reached, the endo-
prosthesis, such as a stent, is successively unfolded. Once unfolded, the
heart valve endoprosthesis
can be anchored in the blood vessel, for example, with the assistance of
anchoring hooks. The ac-
tual heart valve prosthesis is disposed directly in the proximal region of the
stent or endoprosthe-
sis.
Patent publication DE 100 10 074 Al discloses a device for securing and
anchoring heart valve
prostheses which essentially comprises shaped wire elements connected to one
another. Different
arches are used as a means of reliably securing and anchoring the heart valve
prosthesis. To this
end, the device described in this specification has three identical pairs of
arches respectively dis-
posed at a distance of 120 apart. These arches are connected to one another
by fixed body joints
which assume the function of pivot bearings. Arches bent in the opposite
direction are also pro-
vided, forming lever arms which are of identical length as far as possible, to
enable a reliable seating
of the arches, even in the event of peristaltic movements of the heart and
blood vessel, and afford
a reliable seal for an implanted and secured heart valve prosthesis.
With the known solutions there is still a risk of heart valves being
incorrectly implanted. in particu-
lar, the heart valve prosthesis must be exactly positioned and longitudinally
oriented. This requires
enormous skill on the part of the surgeon performing the treatment to position
a stent carrying a
heart valve prosthesis at its proximal end accurately enough in the vicinity
of the patient's diseased
heart valve to ensure both correct lateral and longitudinal positioning of the
heart valve prosthesis.
Amongst other things, incorrect or sub-optimal implantation and positioning of
a heart valve pros-
thesis can lead to inadequate sealing or valve insufficiency which places
considerable stress on the
CA 02822636 2013-07-31
W02008/125153 PCT/EP2007/061117
3
ventricle. For example, if a heart valve prosthesis is implanted too far above
the actual heart valve
plane, this can reduce or even cover and block the outlets of the coronary
vessels (coronaries) lead-
ing to fatal coronary ischaemia due to heart infarction. Thus, it is
absolutely vital that the require-
ments of both lateral and longitudinal positioning accuracy of a heart valve
prosthesis are met.
In the case of conventional minimally invasive implantation techniques where
self-expandable heart
valve prostheses are introduced to the implantation site at or in the heart
through a main artery of
the patient, the prosthesis is usually introduced by means of a guide wire and
with the aid of cathe-
ters. In such a case it is standard practice to use a balloon catheter to
expand and open the native
heart valves to allow insertion of a catheter. Although it is possible to
monitor and control the in-
troduction process during such an intervention, for example with the aid of an
X-ray system (heart
catheter laboratory = I-ICL) or with the aid of ultrasound (trans-oesophageal
echocardiagram =
TEE), the heart valve prosthesis is still of relatively large dimensions in
spite of being minimised
whilst it is being introduced. It is often not possible to obtain the required
positioning accuracy
due to restricted ability to manoeuvre, and in particular to ensure correct
longitudinal positioning,
of the heart valve prosthesis to be implanted with the fixing elements
attached to it. If there is a
risk that the coronary vessels might close, implanting the heart valve
prosthesis in a position angu-
larly offset from the optimum implantation site represents a particular risk
for thc patient.
When designing a heart valve prosthesis, allowance must specifically be made
for the considerable
forces which act on the prosthesis, including during the filling phase of the
heart cycle (diastole).
Reliable anchoring is necessary to prevent the implanted heart valve
prosthesis from becoming de-
tached or moving in any direction.
Accordingly, it must be possible to manoeuvre the heart valve prosthesis in
the relevant access ves-
sel as efficiently as possible during the implantation process to ensure
optimum positioning accu-
racy on the one hand and, on the other hand, the implanted heart valve
prosthesis must be firmly
anchored at the implantation site effectively to prevent the prosthesis from
subsequently shifting.
Known devices used for the transvascular implantation of heart valve
prostheses are often not suit-
able for easy implantation of a heart valve prosthesis due to the required
degree of positioning ac-
curacy. Furthermore, until now it has only been possible to correct an
incorrectly positioned heart
valve prosthesis that has already been partially implanted with great
difficulty - if at all.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
4
These problems have been overcome by means of the medical device of the
present invention
which has an integral structure cut from a metal tube to provide features that
allow accurate posi-
tioning and firm anchoring.
Summary of the Invention
In a first embodiment of the invention, a self expandable endoprosthesis for
treating a heart valve
insufficiency is provided, wherein the endoprosthesis comprises at least one
retaining means for
manoeuvring the endoprosthesis into and out of position within the patient.
In a particular aspect of the first embodiment, the self-expandable
endoprosthesis has a plurality of
positioning arches for locating the endoprosthesis in the correct position
within a patient and an
anchoring segment with retaining arches for accommodating a prosthetic heart
valve. The position-
ing arches and retaining arches have a co-operative shape and act in
combination to hold the flaps
of an incumbent heart valve between the positioning and retaining arches when
the endoprosthesis
is in situ within a patient.
The present invention resides in a medical device which comprises a self-
expandable endoprosthe-
sis (hereafter referred to simply as stent) which includes a valve-supporting
anchoring segment for
the accommodation of a heart valve prosthesis. The stent has a minimised
configuration in a first
mode that allows the stent to be introduced into the heart by way of a
catheter. The stent is 'pro-
grammed' to respond to a stimulus that allows the stent to expand to a second
mode having an
open or expanded configuration.
The stent design is distinctive due to the fact that the stent is provided
with at least three position-
ing arches that project radially outwards from the plane of the stent. The
positioning arches take
up an open position when the endoprosthesis assumes its second pre-definable
mode and, when in
situ within the body, sit in the pockets of the native heart valve. Correct
positioning of the stent is
thus determined by the siting of the positioning arches in the valve pockets
which the surgeon
should be able to feel.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
In a preferred embodiment, the stent is released in a staged manner such that
the positioning
arches are released and allowed to expand first. The positioning arches may
then be used as feelers
to identify pockets of the incumbent heart valves. The stent is designed and
shaped such that,
once the pockets have been identified by the feelers, the positioning arches
locate in the pockets,
thereby ensuring correct positioning of the stent. Once the positioning arches
have been located,
the anchoring segment, including the retaining arches carrying the prosthesis,
is then released.
In co-operation with the anchoring segment, the positioning arches engage the
original native (old)
flaps of the heart valve that are to be replaced, resulting in an automatic
fixing and positioning of
the medical device regarding axial rotation on one hand and the horizontal
position on the other
hand. Thus, the stent is anchored by means of both a radial force imparted by
the design and func-
tional properties of the stent, and a clipping action in a manner similar to a
paper clip, with the po-
sitioning arches on one side of the heart flaps and the anchoring segment on
the other. The native
heart valve flaps act as a seal and substantially minimise undesirable leakage
of blood around the
stent. While the device is referred to herein as a stent, it may also be
thought of and referred to as
a valve clip, within the frame of which is located a replacement valve.
The clipping action is provided by two surfaces, the positioning arches and
retaining arches, that
are effectively pressed towards one another by the elasticity of the material
from which the endo-
prosthesis is made. On initial implantation of the endoprosthesis, the
positioning and retaining
arches are forced apart as the positioning arches are released while the
anchoring segment is re-
tained in the catheter, while restoring forces act in the opposite (inward)
direction to return the
arches to their programmed position, pushing the arches together. Generally
these restoring forces
arc proportional to the distance separating the arches. On full release of
stent, the incumbent valve
flaps are located between the two sets of arches and the restoring forces
cause the arches to exert
inward, compressing forces on the valve flap, resulting in clipping or
clamping of the valve flap
between the two arches. When programming the shape of the endoprosthesis, the
radial angle of
the positioning and retaining arches can be set such that the forces applied
to, and friction forces
generated with, the valve flap are sufficient to hold the endoprosthesis in
place once fully im-
planted.
It will be appreciated that while the device of the present invention may be
used to replace native
heart valves, the device may also be used to replace a failing biological
prosthesis. Because the de-
,
CA 02822636 2013-07-31
W02008/125153 PCT/EP2007/061117
6
vice of the present invention clips onto the valve flaps already in place, the
device can be inserted
within an existing stent without modification or removal of the existing
stent.
Since the endoprosthesis (stent) of the medical device has a continuous
structure cut from a metal
tube incorporating the positioning arches on the one hand and the anchoring
segment with retain-
ing arches on the other hand, the endoprosthesis can be made particularly
inexpensively and in
large numbers. Specifically, it would be conceivable to cut the stent
structure from a metal tube by
means of a laser, after which the structure is subjected to an appropriate
shaping and heat treat-
ment process so that the endoprosthesis can be transferred from a minimised
state during implan-
tation to an expanded state at the implantation site. This shaping and heat
treatment process is ad-
vantageously operated in a series of steps to prevent damage to the stent
structure.
Since the endoprosthesis of the medical device has a continuous structure cut
from a metal tube,
each retaining arch is associated with a positioning arch and every end
portion of the positioning
arch at the distal end of the endopros thesis is joined to the terminal
portion of an associated retain-
ing arch. Thus, there is no need to provide fixed body joints or similar
connecting devices and the
complexity of the endoprosthesis is much reduced. Expressed in another way,
the endoprosthesis
of the medical device proposed by the invention is a stem or clip which, on
the one hand, offers a
positioning function due to positioning arches having a minimal longitudinal
extension and, on the
other hand, provides the function of retaining a heart valve prosthesis due to
the retaining arches.
As will be seen, when transferring the endoprosthesis from the first pre-
definable mode to the sec-
ond pre-definable mode by widening the cross-section of the entire stent, the
retaining arches on
the one hand and the positioning arches on the other hand arc opened out in a
radial direction. The
second mode of the endoprosthesis is advantageously selected so that, as the
retaining and posi-
tioning arches open up, they press against the internal vessel wall of the
aorta and form a positive
connection with it, thereby anchoring the medical device firmly at the
implantation site.
The structure of the endoprosthesis imparts a particularly short shape to the
medical device and so
the medical device is particularly easy to manoeuvre in its minimised state.
This is of particular ad-
vantage if the implantation route to the heart is via the aortic arch. The
minimum length of the
medical device is made possible because every end portion of the positioning
arch at the distal end
is joined to the end portion of an associated retaining arch and both the
positioning arch and the
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
7
retaining arch extend to the proximal retaining region of the medical device
or endoprosthesis.
The anchoring segment that accommodates the heart valve prosthesis therefore
lies at the proximal
retaining region of the endoprosthesis.
Expandable endoprostheses made of materials such as Stainless Steel 316L,
cobalt-chromium alloys
or Nitinol for example, may not possess a level of radio-opacity that enables
the stent to be suffi-
ciently visible during fluoroscopy or x-ray. However, during implantation it
is important for the
cardiologist or physician to visualise the position of an endoprosthesis or
catheter through the use
of fluoroscopes or similar radiological equipment. Similarly in subsequent
check-ups monitoring of
the position of an implanted endoprosthesis is also important Therefore,
although the endopros-
thesis of the present invention is easily and accurately positioned, in
another embodiment markers
may be applied to the endoprosthesis or insertion system to improve the radio-
opacity and visuali-
sation of the insertion system and/or endoprosthesis during and after
implantation.
In one embodiment coating processes such as sputtering, plating, or co-drawing
of metals are util-
ised to add a region(s) or layer(s) of material with higher radio-opacity to
the endoprosthesis. Al-
ternatively, radio-opaque markers can be attached to parts of the structure of
the endoprosthesis.
In this manner, materials which have higher radio-opacity than the stent
structure itself can be util-
ised as markers. For example, markers may be strategically placed along the
body of the endopros-
thesis to increase the visualisation characteristics of the stent. Preferably
the radio-opaque markers
comprise gold, tantalum or platinum. In an alternative embodiment a nickel
titanium alloy com-
prising a ternary element may be used. Such a ternary element is a material
having a high level of
radio-opacity. For example, the ternary element may be selected from the group
consisting of irid-
ium, platinum, gold, rhenium, tungsten, palladium, rhodium, tantalum, silver,
ruthenium, and haf-
nium, Preferably such markers are applied to the positioning arches.
Advantageous and preferred embodiments of the medical device are specified in
the dependent
claims.
In one particular embodiment every positioning arch and its associated
retaining arch has an ess.en-
tially U-shaped or V-shaped structure which is closed towards the proximal end
of the endopros-
thesis.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
8
In a preferred embodiment of the anchoring region or segment, it would be
conceivable for the
anchoring segment to be of an essentially U-shaped or V-shaped structure which
is closed at the
distal end of the endoprosthesis. In such a case, the distal region of the
anchoring segment consti-
tutes the tip of the anchoring segment and the respective arms of the
anchoring segment are joined
to the respective arms of two adjacent retaining arches at the proximal end of
the anchoring seg-
ment.
Alternatively and in another embodiment, the respective arms of the retaining
arches have con-
tinuous slots or elongate holes extending in the longitudinal direction of the
retaining arches. The
purpose of such a feature is to enable and assist the expansion of the
endoprosthesis from the
minimised state into the expanded state because these slots or elongate holes
are preferably de-
signed to permit a particularly easy cross-sectional expansion of the stent
(endoprosthesis) whilst
simultaneously reducing the length of the stent. Such slots or elongate holes
have the additional
advantage of saving on material.
In the case of the latter embodiment it would be conceivable for the
respective retaining arches to
be additionally provided with reinforcing portions which interrupt the slots
extending in the longi-
tudinal direction of the retaining arches. These reinforcing portions
essentially prevent components
of the retaining arches from projecting outwards from the circumferential
plane of the endopros-
thesis when the endoprosthesis is in an expanded state.
Each positioning arch is cut from the material blank of the metal tube which
is accommodated by
the essentially U-shaped or V-shaped structure of the associated retaining
arch. In this preferred
embodiment of the stent structure, therefore, the respective retaining arches
of the retaining seg-
ment form the proximal anchoring region of the endoprosthesis similarly, the
respective position-
ing arches are of a design symmetrical with the retaining arches but lie
slightly in front of the distal
retaining region of the medical device. The respective distal ends of the
positioning arches are
joined to the respective distal ends of the co-operating retaining arches in
the distal retaining region
of the endoprosthesis. When the endoprosthesis is in an expanded state, not
only the proximal an-
choring region with the heart valve prosthesis fitted to it, the positioning
arches disposed between
the proximal anchoring and the distal retaining regions of the medical device
open out, but also the
joining points between the respective positioning arches and retaining arches
at the distal end of
the medical device. This provides a radially acting force which is applied to
the vessel wall via the
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
9
distal retaining region of the medical device which further assists anchoring
of the medical device
at the implantation site.
Preferably the proximal ends of the positioning arches are shaped to minimise
damage to the base
of the valve pockets so that the arches position in the base of the valve
pocket without puncturing
or damaging the pocket. Accordingly, in one embodiment the proximal ends of
the positioning
arches have a curved shape. In an alternative embodiment, the proximal ends of
the positioning
arches are blunt, for example, flattened or in the form of a 'spade'. It will
be apparent to one
skilled in the art that the positioning arches may also have a shape that
allows the arches to pene-
trate the base of the valve pockets. For example, a pointed or barbed shape
allows penetration of
the base of the valve pockets whilst preventing withdrawal or dislodging of
the implanted endo-
prosthesis. However, it should be noted that although such a design imparts an
additional anchor-
ing feature, removal or repositioning of the endoprosthesis once the valve
pockets have been punc-
tured is generally precluded.
In a further embodiment, the length of one (or more) of the positioning arches
may be reduced to
take account of calcification of a native valve with a subsequent reduction in
the depth of the valve
pocket. Thus, such a feature enables the endoprosthesis to be positioned and
implanted correctly
even when, for example, one or more of the valve pockets is filled with
calcified material. In this
instance, shortening of one of the positioning arches prevents the
calcification from fouling the
end of the positioning arch.
Since the medical device is in a (an expanded) state in which the distal
retaining and proximal an-
choring regions as well as the positioning arches are opened out radially when
the endoprosthesis
assumes the second mode, the expanded medical device has a shorter length than
it does in its
minimised state. To enable the length of the medical device in its expanded
state to be set before-
hand, it would be conceivable to connect the respective distal end portions of
the positioning
arches to the distal end portions of the associated retaining arches using a
connecting web extend-
ing essentially in the longitudinal direction of the endoprosthesis rather
than directly. The length of
the medical device in the expanded state can therefore be adapted by selecting
the length of this
connecting web accordingly. However, it is preferable, especially with a view
to ensuring good ma-
noeuvrability of the medical device during the implantation process, i.e. when
the endoprosthesis is
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
in its first (minimised) mode, if the connecting web between the respective
end portions of the po-
sitioning arches and retaining arches is selected so that it is as short as
possible.
According to a second embodiment of the invention, a catheter tip is provided.
The tip is disposed
at the proximal end of the catheter system and accommodates the endoprosthesis
of the invention,
said catheter tip having a retaining mechanism for releasably securing at
least the distal end of the
endoprosthesis in the catheter tip.
In particular aspects of the second embodiment, the catheter tip has a
retaining mechanism shaped
to co-operate with the retaining means on the endoprosthesis. In another
particular aspect of the
second embodiment, the catheter tip includes a retaining mechanism having a
crown with at least
one pocket, the at least one pocket having a shape complementary to that of
the endoprosthesis
retaining means.
In one particularly preferred embodiment of the medical device, the
endoprosthesis has retaining
means at its distal end which can be engaged with corresponding retaining
means on an introduc-
tion catheter system, particularly a catheter tip or cartridge. In one
embodiment of the retaining
means, the means may bc in the form of an anchoring eye disposed between two
adjacent position-
ing arches. In which case, the arms of the adjacent positioning arches on the
one hand and the
arms of the retaining arches associated with the adjacent positioning arches
on the other hand are
connected to the anchoring eye. It would likewise be conceivable for the arms
of the adjacent posi-
tioning arches to be directly and the respective arms of the retaining arches
associated with the ad-
jacent positioning arches to be indirectly connected via a connecting web
extending essentially in
the longitudinal direction of the endoprosthesis. Generally speaking, the
purpose of the retaining
means provided on the distal end of the endoprosthesis is to accommodate
appropriate mecha-
nisms on the introduction catheter system which complement that of the
retaining means of the
endoprosthesis. The engagement between the catheter system on the one hand and
the retaining
means on the distal end of the endoprosthesis on the other hand can be
released by means of an
external manipulation to release the medical device at the implantation site,
thereby ensuring that
the medical device expands and is thus reliably anchored. It will be
appreciated that the retaining
means may be of any suitable shape or configuration such as eyes, loops,
fingers or imperforate
heads.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
11
The use of such retaining means enables the stent to remain in contact with
the catheter prior to
full release of the stent. By maintaining contact with the stent prior to its
full release, location and
implantation position of the stent can be controlled more accurately by a
physician. The function-
ing of the stent and heart valve prosthesis may also be checked and, if one or
neither is functioning
correctly, the physician can withdraw and remove the stent by virtue of the
retaining means re-
maining in contact with the catheter.
Problems or complications with a stent may occur post implantation. For
example, failure of the
stent to deploy properly, misalignment, dislodgement, or damage of the stern
after it has been de-
ployed may lead to valve leakage or other problems. In these cases, removal of
the stent is desir-
able. This may be accomplished up to four weeks after implantation and before
the stent has be-
come integrated with the implantation site by a covering or over-growth of
cells. Therefore, in an-
other embodiment, the retaining means preferably have a shape or configuration
which also allows
them to engage with parts of a tool that enables removal of the stent.
Preferably the retaining
means are caught by and engage or interact with parts of the tool so that the
stent can be pulled
into, for example, a catheter where the stem assumes its first compressed mode
and can be with-
drawn from the body with minimal or no tissue damage.
As an alternative to the embodiment of the medical device outlined above, it
would however also
be conceivable for the respective arms of the adjacent positioning arches to
be joined to the retain-
ing means indirectly via a connecting web extending essentially in the
longitudinal direction of the
endoprosthesis. In which case, the arms of the retaining arches associated
with the adjacent posi-
tioning arches are joined to the retaining means indirectly via a connecting
web extending in the
longitudinal direction of the endoprosthesis. The connecting web of the
retaining arches merges
into the connecting web of the positioning arches at the end portion of the
positioning arches.
Providing the respective connecting webs for connecting the arms of the
positioning arches to the
retaining means and for connecting the arms of the retaining arches to the end
portion of the posi-
tioning arches offers a particularly simple but effective way of adapting the
length of the endopros-
thesis to a patient's respective requirements and does so because the
respective lengths of the con-
necting webs can be selected appropriately.
In another embodiment of the solution proposed by the invention, the retaining
means may in-
clude at least one barb or hook, the tip of which points in the direction of
the proximal end of the
CA 02822636 2013-07-31
W02008/125153 PCT/EP2007/061117
12
endoprosthesis. This ensures that the distal retaining region of the
endoprosthesis can be retained
at the implantation sit in its expanded state particularly reliably. In this
preferred embodiment,
therefore, the endoprosthesis is secured at the implantation site due to the
radial force exerted on
the vessel wall by the endoprosthesis, in particular by the distal retaining
region of the endopros-
thesis, but also due to the barb hooking into the vessel wall. It would
naturally also be possible to
use other appropriate design options for the barb(s) or hook(s).
As an alternative to or in addition to the barbs or hooks, another conceivable
way of securing the
endoprosthesis reliably at the implantation site is for the respective arms of
the retaining arches of
the endoprosthesis to be provided with an anchoring support in the shape of a
bow which projects
out from the relevant arm of the retaining arch when the endoprosthesis is in
the expanded state.
The tip of the bow points in the direction of the distal end of the
endoprosthesis. This embodi-
ment provides additional fixing means for the endoprosthesis and additionally
secures the medical
device to prevent it from becoming dislocated after implantation.
As mentioned above, one main aspect of the invention is that the
endoprosthesis is provided with
retaining means at its distal end which can be moved into engagement with the
retaining mecha-
nism on the tip of an introduction catheter or insertion system. In one
embodiment these retaining
means are in the form of fixing eyes.
In an alternative embodiment, the retaining means comprises at least one
retaining element ar-
ranged at the distal region of the stent, the at least one retaining element
being designed to be
movable into a releasable engagement with a retaining mechanism of an
insertion system, such as a
catheter tip. Preferably the at least one retaining element engages with a
pocket or depression
formed in a crown of the retaining mechanism of the insertion system. Most
preferably, the at least
one retaining element of the stent has a retaining head, having a design which
complements at least
one pocket or depression formed in the crown of the insertion system, thereby
being adapted to
co-operate with the retaining mechanism of the insertion system by means of an
improved releas-
able engagement.
In this embodiment, there is less risk that the retaining mechanism of the
catheter tip can become
wedged or jammed with the distal region of the endoprosthesis. This can be
achieved because nei-
ther the retaining mechanism of the catheter tip nor the retaining means of
the endoprosthesis
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
13
have parts that protrude from the crown of the retaining mechanism when the
endoprosthesis is
fixed to the catheter tip. As a result, minimal shaking or moving of the
catheter tip should be re-
quired to release the engagement between the catheter system and the distal
region of the endo-
prosthesis.
Generally speaking, the retaining means provided on the distal end of the
endoprosthesis or stent
are to he accommodated in an appropriate mechanism on the insertion catheter
system. These
mechanisms should be of a design complementing the retaining means of the
stent. The engage-
ment between the retaining mechanisms of the catheter tip on the one hand and
the retaining
means at the distal end of the stent on the other hand can be released by
means of an external ma-
nipulation to release the stent at the implantation site and allow the stent
to expand thus ensuring
that the heart valve prosthesis is reliably secured. Naturally, it would also
be possible to consider
other solutions for the retaining means. For example, the retaining means may
have different
shapes and/or profiles, being convex or concave and forming a spoon or cup
shape. Profiling the
retaining means in this manner allows the retaining means to remain seated in
position without af-
fecting outward movement, for example as the stent expands radially during its
final release steps.
Alternatively, the retaining means may be of a ball-and-socket configuration
in co-operation with
the retaining mechanism at the catheter tip.
In a preferred embodiment of the retaining means, it is conceivable for the
means to be provided
in the form of a retaining head which is disposed between two adjacent
positioning arches. In this
embodiment, the respective arms of the adjacent positioning arches on the one
hand and the re-
spective arms of the retaining arches associated with the adjacent positioning
arches on the other
hand are joined to the retaining head. it will be apparent to one skilled in
the art that the use of
such fixing means is not limited to use with the disclosed stent design. Such
retaining means could
also be utilised with other stent designs where reliable release of the stent
from the implantation
means, such as a catheter, is required.
It is important that the stein with the heart valve prosthesis can be easily
released from the catheter
tip of a catheter system as soon as the heart valve prosthesis is optimally
positioned. The mecha-
nism described above has been found greatly to assist in this manoeuvre.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
14
In a third embodiment of the invention there is provided a catheter system for
use in treating a
heart valve defect, in particular a heart valve insufficiency or narrowing of
a heart valve, in a pa-
tient, said catheter system comprising a catheter tip, according to the second
aspect, and further
comprising a self-expandable endoprosthesis, according to the first aspect,
accommodated in the
catheter tip of the catheter system, and when the endoprosthesis is
accommodated in the catheter
tip of the catheter system it assumes a first pre-definable mode and outside
of the catheter tip and
in the implanted state it assumes a second pre-definable mode, and the
endoprosthesis is in a
folded state in its first mode and in an expanded state in its second mode.
Conventional catheter systems for inserting a self-expandable heart valve
stent typically comprise a
catheter tip with a retaining mechanism, the retaining mechanism being adapted
for releasably se-
curing the distal region of the stent on the catheter tip. The catheter tip
typically has a crown with a
plurality of projecting elements. The projecting elements of the crown are
designed so as to com-
plement retaining eyes provided at the distal region of the stent. In this
respect, the retaining
mechanism arranged in the catheter tip of the conventional catheter systems co-
operates with the
distal region of the stent by means of a releasable engagement. The engagement
between the retain-
ing mechanism of the catheter system, and the retaining means in the distal
region of the stent is
normally releasable by means of an external manipulation so that the stent
with the heart valve
prosthesis attached to it can be released from the catheter at the
implantation site.
However, the use of retaining eyes still pose a risk that the engagement
between the catheter sys-
tem and the distal region of the stent can only be released by means of
movement of the stent. In
particular, the retaining eyes provided in the distal region of the stent may
wedge with the project-
ing elements which protrude from the crown of the retaining mechanism of the
catheter tip. As a
result, shaking and/or moving of the catheter tip may be required to release
the engagement be-
tween the catheter system and the distal region of the stent. Such movement is
likely to dislodge
the stent from its desired position and may damage the prosthesis.
Thus, there is still a risk of heart valve prostheses being incorrectly
implanted. The heart valve
prosthesis must be exactly positioned and longitudinally oriented which
requires enormous skill on
the part of the surgeon performing the treatment. On the one hand the stem
must be accurately
positioned and on the other hand the stent must be released from the catheter
tip accurately
enough in the vicinity of the patient's existing heart valve to ensure both
correct lateral positioning
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
accuracy and a correct longitudinal position of the heart valve prosthesis.
Amongst other things,
incorrect implantation and/or sub-optimal positioning of a heart valve
prosthesis can lead to in-
adequate sealing or valve insufficiency which places considerable stress on
the ventricle. If a heart
valve prosthesis is implanted too far above the actual heart valve plane, for
example, this can cause
the outlets of the coronary vessels (coronaries) to close, leading to fatal
coronary ischaemia due to
heart infarction. This being the case, it is vital that both the lateral
positioning accuracy and longi-
tudinal positioning accuracy of a heart valve prosthesis meet these strict
requirements.
When such retaining heads described above are used, the catheter tip of the
insertion system ideally
includes a retaining mechanism for releasable securing at least the distal
region of the stent in the
catheter tip. Preferably, the retaining mechanism comprises a crown with at
least one pocket or de-
pression formed in the crown. The at least one pocket or depression is of a
design which comple-
ments the shape of the retaining means provided on the distal region of the
stent. Thus, the retain-
ing mechanism is adapted to co-operate with the distal region of the stent by
means of a releasable
engagement. In particular, this solution provides a reduced risk that the
retaining mechanism of the
catheter tip can become wedged or jammed with the distal region of the stent.
This can be achieved
because the retaining mechanism of the catheter insertion system has no parts
protruding or pro-
jecting from the crown of the retaining mechanism. As a result, there should
be no need to shake
or move the catheter tip to release the engagement between the catheter system
and the distal re-
gion of the stent.
In one particular embodiment, the at least one pocket or depression formed in
the crown of the
retaining mechanism has a shape which is adapted for accommodating the
retaining means pro-
vided on the distal region of the stent with positive locking, thereby
providing for releasable en-
gagement between the distal region of the stent and the catheter tip. The at
least one pocket or de-
pression may be integrally formed in the crown of the retaining mechanism of
the catheter tip.
Preferably, the at least one pocket or depression is formed as a mould or
inverse image of the re-
taining means of the stent even if the retaining means comprises, for example,
barbs or hooks
formed at a retaining head.
Preferably the crown of the retaining mechanism is generally cylindrical and
the at least one pocket
or depression formed in the crown has a shape adapted to completely
accommodate completely
the retaining means provided on the distal region of the stent such that there
are no parts of the
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
16
distal region of the stent protruding from the superficial surface of the
cylindrical crown. Hence,
this preferred embodiment leads to a catheter tip which has a very compact
retaining mechanism
with the surprising advantage that the diameter of the catheter tip can be
reduced.
In one embodiment, the catheter tip further comprises 'snap-on' means arranged
on the at least
one pocket or depression formed in the crown for releasable fixing of the
retaining means in the at
least one pocket or depression. Preferably, this snap-on means comprises a
projecting rim or
flange arranged on or near the outer edge of the at least one pocket or
depression formed in the
crown. The projecting rim or flange may be adapted to hold the stent retaining
means in the at least
one pocket or depression. Such snap-on means, for example in the form of a
clip mechanism,
serves to fix temporarily the stent retaining means during loading of the
catheter tip. Preferably the
snap-on means are designed such that the resisting force caused by the snap-on
means and acting
on the stent retaining means is smaller than the radial forces acting on the
distal portion of the
stent when the stem during expansion. This has the advantage that the snap-on
means should not
retard or inhibit the stem during its final release thus ensurfrig efficient
release of the stent.
In another embodiment, the crown of the retaining mechanism further comprises
at least one
groove formed therein. The at least one groove is assigned to the at least one
pocket or depression
and extends essentially in the longitudinal direction of the crown from said
pocket or depression to
one end of the crown. The at least one groove has a shape adapted to
accommodate a connecting
web of the stent. The connecting web of the stent extends essentially in the
direction of the stent
and connects the stent retaining means with respective arms of the stent.
Preferably, the groove
associated to the pocket or depression formed in the crown is formed as a
mould or inverse image
of the connecting web or other parts of the stent, extends essentially in the
direction of the stent
and connects the retaining means with respective arms of the stem.
Of course, a catheter tip of the kind as defined above may also comprise snap-
on means arranged
on the at least one groove formed in the crown of the retaining mechanism for
releasable fixing of
the stent connecting web which connects the stem retaining means with the
respective arms of the
stent. Preferably, this snap-on means comprises a projecting rim or flange
arranged on or near the
outer edge of the at least one groove formed in the crown of the retaining
mechanism, said project-
ing rim or flange being adapted to hold the connecting web of the stent in the
at least one groove.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
17
Thus, the catheter tip has an improved retaining mechanism for releasably
securing at least the dis-
tal region of the stent in the catheter tip. The catheter tip can be connected
to a catheter system by
means that allow manipulation of the catheter tip. Such catheter systems are
known in the art and
may, for example, comprise a handle which further comprises operating means
which co-operate
with the catheter tip so that when the operating means are operated, the stent
can be released from
the catheter tip in steps in a pre-definable sequence. In addition, the
catheter tip may further com-
prise a housing system for accommodating at least the proximal region of the
stent. The housing
system preferably comprises a first housing portion for accommodating first
functional compo-
nents of the stent, for example the retaining arches of the stent, and a
second housing portion for
accommodating second functional components of the stent, for example the
positioning arches.
In a yet further embodiment, the endoprosthesis has an external diameter of
approximately 5.0 mm
and a length of between 33.0 mm and 40.0 mm, preferably between 34.0 and 39.0
mm, even more
preferably between 34.37 mm and 38.37 mm, in its first mode. This means that
the medical device
can be introduced by means of a 21F introduction system, for example, and
heart valve prostheses
with a diameter of 21 mm to 28 mm may be used. The length specifications given
above are cur-
rently preferred values based on medical devices suitable for the majority of
patients requiring
treatment.
In order to obtain a particularly reliable anchoring of the implanted medical
device in its expanded
state, the endoprosthesis may be subjected to a shaping and heat treatment
process during its
manufacture so that when the endoprosthesis is in the finished state, it has a
slightly concave shape
tapering in the direction of the proximal anchoring region of the
endoprosthesis in its second
mode. In other words, the proximal anchoring region of the endoprosthesis,
i.e. the region to
which the heart valve prosthesis is attached, has a slightly narrower diameter
than the distal anchor-
ing region. It has been found that if the distal anchoring region of the
endoprosthesis in the second
mode has an approximately 10% to 25% bigger diameter than the proximal
anchoring region of the
endoprosthesis, radial forces are generated in particular at the distal
anchoring region of the endo-
prosthesis which enables the medical device to be securely anchored in the
vessel without causing
damage to the vessel wall. Due allowance is also made for the peristaltic
movements of the heart
and vessel wall. The slightly lower radial force expended by the proximal
anchoring region of the
endoprosthesis not only serves as a means of anchoring the medical device in
the aorta but in par-
ticular also opens out the heart valve prosthesis fitted on the proximal
anchoring region of the
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
18
endoprosthesis and imparts to it a reliable seal with respect to the vessel
wall. Naturally, however, it
would also be conceivable for the concave shape to be more or less pronounced
when the endo-
prosthesis assumes the second, expanded mode.
It is preferable if the anchoring region of the endoprosthesis has a diameter
of between 22 mm and
33 mm, and preferably between 25 mm and 31 mm, in the second mode. This being
the case, it
would be conceivable for the endoprosthesis to he made in two or more
differently dimensioned
sizes, in which case, an optimum size of endoprosthesis could be selected
depending on the patient
and the exact dimensions of the endoprosthesis adapted to the patient to be
treated - starting from
a pre-defined stent size - by an appropriate finishing treatment of the
endoprosthesis (stent), in par-
ticular by tempering. It is also preferable if the endoprosthesis is shaped
such that the retaining
arches curve or 'flare' outwards, i.e. in a radial direction, at the base to
provide an additional an-
choring force.
Stents in the prior art rely primarily on radial force alone to ensure that
they remain in position
once implanted. Such stents are constructed of a mesh or have a cylindrical
shape in order that as
much surface area of the stent is in contact with the blood vessel as
possible. Surprisingly, despite
its low profile, non-cyclindrical shape and lack of surface area, it has been
found that the endopros-
thesis of the present invention requires the application of in excess of 3-4kg
to dislodge once com-
pletely implanted.
In one, particularly preferred embodiment of the medical device, the device
comprises an endo-
prosthesis (stent) and a heart valve prosthesis, preferably a bio-heart valve
prosthesis, even more
preferably an aortic heart valve prosthesis. The valve is attached to the
anchoring segment of the
endoprosthesis by means of a thread, suture or similar. Orifices are provided
in the retaining
arches of the endoprosthesis through which the thread or similar is inserted.
It would be conceiv-
able for the heart valve prosthesis to be connected to the anchoring segment
of the endoprosthesis
immediately prior to the medical intervention. As a result, the medical device
can be made in a
modular design, which is of particular advantage in terms of transporting and
storing the device. A
bio-heart valve prosthesis may comprise material from a variety of sources
such as from human,
bovine, equine or porcine tissue. Bio-heart valves may be naturally occurring
valves or they may be
artificially derived or manufactured from suitable biological material, cells
or tissue. Alternatively a
heart valve prosthesis may be manufactured from biologically compatible
artificial materials, that is
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
19
non-biological sources such as, for example, from pyrolytic carbon, titanium,
Teflon, polyester,
Dacron and the like.
As regards the preferred material used for the endoprosthesis of the medical
device, a shape mem-
ory material is ideally used which is designed so that the endoprosthesis is
transformed from one
shape to another shape by means of an external stimulus. Thus, the
endoprosthesis assumes a
minimised shape in the first mode (when the medical device is in the minimised
state) and an open
shape in the second mode (when the medical device is in the expanded state).
Especially if a shape
memory material such as Nitinol is used, i.e. an equal atomic alloy of nickel
and titanium, the im-
plantation process will be particularly gentle during the operation of
implanting the medical device,
minimising the risk of tissue damage on insertion and implantation. Another
advantage of using a
shape memory metal is that the open shape can be transformed back to the
minimised shape sim-
ply by reversing the external stimulus.
During production of an endoprosthesis made from a shape memory material,
after the stent struc-
ture has been cut from the metal tube, it is deformed and fixed in the desired
open shape via a
process known as "programming". This operation may be performed on the one
hand by heating,
deforming and then cooling the stent structure. Alternatively, the stent
structure may also be de-
formed at low temperature by an operation known as cold stretching. As a
result, the open shape is
memorised whilst the minimised shape actually prevails. If the stent structure
is then subjected to
an external stimulus, the shape memory effect is triggered and the memorised
open shape is re-
stored.
In a particularly preferred embodiment, the external stimulus is a settable
switching temperature. It
is therefore conceivable for the endoprosthesis material to be heated to a
temperature higher than
the switching temperature in order to trigger the shape memory effect and thus
restore the memo-
rised open shape of the endoprosthesis. By selecting an appropriate chemical
composition of the
shape memory material, a specific switching temperature can be fixed before
the endoprosthesis is
programmed. This being the case, the switching temperature is set so that it
falls within the range
of room temperature and the body temperature of the patient. This is of
particular advantage in
applications where the medical device is to be implanted in a patient's body.
Accordingly, when
implanting the medical device, it is merely necessary to ensure that the
instrument is not heated
CA 02822636 2013-07-31
W02008/125153 PCT/EP2007/061117
until it is in the implanted state on the patient's body (36 C), at which
point the shape memory ef-
fect of the endoprosthesis material is triggered.
Preferred embodiments of an endoprosthesis of a medical device proposed by the
invention will be
described in more detail below with reference to the appended drawings:
Brief Description of Drawings
Fig. 1 a illustrates a first, preferred embodiment of a self-expandable
endoprosthesis
for the medical device proposed by the invention in its first predefined mode
in
which the medical device is in its minimised state;
Fig. lb shows the endoprosthesis illustrated in Fig. la but in a state
between its first
pre-definable mode and its second mode in which the medical device is in its
ex-
panded state;
Fig. lc shows the endoprosthesis illustrated in Fig. la but in its
second mode in
which the medical device is in its expanded state;
Fig. Id shows a first, preferred embodiment of the medical device
proposed by the
invention in its expanded state with an endoprosthesis of the type illustrated
in Fig.
lc and a heart valve prosthesis attached to it and opened out;
Fig. le is a flat projection of a cutting pattern which can be used
for the production
of the first, preferred, self-expandable endoprosthesis to cut the
endoprosthesis il-
lustrated in Fig. la integrally from a metal tube;
Fig. 2a shows a second, preferred embodiment of a self-expandable
endoprosthesis
for the medical device proposed by the invention in its first, pre-determined
mode
in which the medical device is in its minimised state;
Fig. 2b shows the endoprosthesis illustrated in Fig. 2a in a state
between its first,
pre-definable mode and its second mode in which the medical device is in its
ex-
panded state;
Fig. 2c shows the endoprosthesis illustrated in Fig. 2a in its second
mode in which
the medical device is in its expanded state;
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
21
Fig. 2d illustrates a second preferred embodiment of the medical
device proposed
by the invention in its expanded state, with an endoprosthesis of the type
illustrated
in Fig. 2c and a heart valve prosthesis attached to it and opened out;
Fig. 2e is a flat projection of a cutting pattern which can be used
for the production
of the second preferred, self-expandable endoprosthesis to cut the
endoprosthesis
illustrated in Fig. 2a integrally from a metal tube;
Fig. 3a shows a third, preferred embodiment of a self-expandable
endoprosthesis
for the medical device proposed by the invention in its first, pre-determined
mode
in which the medical device is in its minimised state;
Fig. 3b shows the endoprosthesis illustrated in Fig. 3a in a state
between its first,
pre-definable mode and its second mode in which the medical device is in its
ex-
panded state;
Fig. 3c shows the endoprosthesis illustrated in Fig. 3a in its second
mode in which
the medical device is in its expanded state;
Fig. 3d illustrates a third preferred embodiment of the medical device
proposed by
the invention in its expanded state with an endoprosthesis of the type
illustrated in
Fig. 3c and a heart valve prosthesis attached to it and opened out;
Fig. 3e is a flat projection of a cutting pattern which can be used
for the production
of the third preferred, self-expandable endoprosthesis to cut the
endoprosthesis il-
lustrated in Fig. 3a integrally from a metal tube;
Fig. 4a shows a fourth, preferred embodiment of a self-expandable
endoprosthesis
for the medical device proposed by the invention in its first, pre-determined
mode
in which the medical device is in its minimised state;
Fig. 4b shows the endoprosthesis illustrated in Fig. 4a in a state
between its first,
pre-definable mode and its second mode in which the medical device is in its
ex-
panded state;
Fig. 4c shows the endoprosthesis illustrated in Fig. 4a in its second
mode in which
the medical device is in its expanded state;
Fig. 4d illustrates a fourth preferred embodiment of the medical
device proposed
by the invention in its expanded state with an endoprosthesis of the type
illustrated
in Fig. 4c and a heart valve prosthesis attached to it and opened out;
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
22
Fig. 4e is a flat projection of a cutting pattern which can be used
for the production
of the fourth, preferred, self-expandable endoprosthesis to cut the
endoprosthesis
illustrated in Fig. 4a integrally from a metal tube;
Fig. 5a shows a fifth preferred embodiment of a self-expandable
endoprosthesis
for the medical device proposed by the invention in its first, pre-determined
mode
in which the medical device is in its minimised state;
Fig. 5b shows the endoprosthesis illustrated in Fig. 5a in a state
between its first,
pre-definable mode and its second mode in which the medical device is in its
ex-
panded state;
Fig. 5c shows the endoprosthesis illustrated in Fig. 5a in its second
mode in which
the medical device is in its expanded state;
Fig. 5d illustrates a fifth preferred embodiment of the medical device
proposed by
the invention in its expanded state with an endoprosthesis of the type
illustrated in
Fig. 5c and a heart valve prosthesis attached to it and opened out;
Fig. 5e is a flat projection of a cutting pattern which can be used
for the production
of the fifth, preferred, self-expandable endoprosthesis to cut the
endoprosthesis il-
lustrated in Fig. 5a integrally from a metal tube;
Fig. 6a shows a sixth preferred embodiment of a self-expandable
endoprosthesis
for the medical device proposed by the invention in its first, pre-determined
mode
in which the medical device is in its minimised state;
Fig. 6b shows the endoprosthesis illustrated in Fig. 6a in a state
between its first,
pre-definable mode and its second mode in which the medical device is in its
ex-
panded state;
Fig. 6c shows the endoprosthesis illustrated in Fig. 6a in its second
mode in which
the medical device is in its expanded state;
Fig. 6d illustrates a sixth preferred embodiment of the medical device
proposed by
the invention in its expanded state with an endoprosthesis of the type
illustrated in
Fig. 6c and a heart valve prosthesis attached to it and opened out;
Fig. 6e is a flat projection of a cutting pattern which can be used
for the production
of the sixth, preferred, self-expandable endoprosthesis to cut the
endoprosthesis il-
lustrated in Fig. 6a integrally from a metal tube;
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
23
Fig. 7a shows a seventh, preferred embodiment of a self-expandable
endoprosthe-
sis for the medical device proposed by the invention in its first, pre-
determined
mode in which the medical device is in its minimised state;
Fig. 7b shows the endoprosthesis illustrated in Fig. 7a in a state
between its first,
pre-definable mode and its second mode in which the medical device is in its
ex-
panded state;
Fig. 7c shows the endoprosthesis illustrated in Fig. 7a in its second
mode in which
the medical device is in its expanded state;
Fig. 7d illustrates a seventh preferred embodiment of the medical
device proposed
by the invention in its expanded state with an endoprosthesis of the type
illustrated
in Fig. 7c and a heart valve prosthesis attached to it and opened out;
Fig. 7e is a flat projection of a cutting pattern which can be used
for the production
of the seventh preferred, self-expandable endoprosthesis to cut the
endoprosthesis
illustrated in Fig. 7a integrally from a metal tube;
Fig. 8a shows an eighth, preferred embodiment of a self-expandable
endoprosthe-
sis for the medical device proposed by the invention in its first, pre-
determined
mode in which the medical device is in its minimised state;
Fig. 8b shows the endoprosthesis illustrated in Fig. 8a in a state
between its first,
pre-definable mode and its second mode in which the medical device is in its
ex-
panded state;
Fig. 8c shows the endoprosthesis illustrated in Fig. 8a in its second
mode in which
the medical device is in its expanded state;
Fig. 8d illustrates an eighth preferred embodiment of the medical
device proposed
by the invention in its expanded state with an endoprosthesis of the type
illustrated
in Fig. 8c and a heart valve prosthesis attached to it and opened out;
Fig. 8e is a flat projection of a cutting pattern which can be used
for the production
of the eighth preferred, self-expandable endoprosthesis to cut the
endoprosthesis il-
lustrated in Fig. 8a integrally from a metal tube;
CA 02822636 2013-07-31
W02008/125153 PCT/EP2007/061117
24
Fig. 9a shows a ninth, preferred embodiment of a self-expandable
endoprosthesis
for the medical device proposed by the invention in its first, pre-determined
mode
in which the medical device is in its minimised state;
Fig. 9b is a perspective side view of a connecting web between an end
portion of a
positioning arch and an end portion of an associated retaining arch of the
endo-
prosthesis illustrated in Fig. 9a in its second mode in which the medical
device is in
its expanded state;
Fig. 9c is a perspective side view of a positioning arch and the
associated retaining
arch of the endoprosthesis illustrated in Fig. 9a in its second mode in which
the
medical device is in its expanded state;
Fig. 9d is a perspective plan view of the distal region of the
endoprosthesis illus-
trated in Fig. 9a in its second mode in which the medical device is in its
expanded
state;
Fig. 9e is a flat projection of a cutting pattern which can be used
for the production
of the ninth preferred embodiment of the self-expandable endoprosthesis to cut
the
endoprosthesis illustrated in Fig. 9a integrally from a metal tube;
Fig. 10 is a flat projection of a cutting pattern which can be used
for the production
of another preferred embodiment of the self-expandable endoprosthesis to cut
an
endoprosthesis integrally from a metal tube;
Fig. 11 shows another preferred embodiment of a self-expandable
endoprosthesis
for the medical device proposed by the invention in its second mode in which
the
medical device is in its expanded state;
Fig. 12a shows a twelfth preferred embodiment of a self-expandable stent in
its first, pre-
determined mode in which the stent is in its minimised state;
Fig. 12b shows the stem illustrated in Fig. 12a in a state between its
first, pre-definable mode
and its second mode in which the stent is in its expanded state;
Fig. 12c shows the stent illustrated in Fig. 12a in its second mode in
which the stent is in its
expanded state;
Fig. 12d shows the stent illustrated in Fig. 12c with a heart valve
prosthesis attached to it
and opened out;
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
Fig. 12e is a flat projection of a cutting pattern which can be used for
the production of the
twelfth preferred embodiment of a self-expandable stent to cut the stent
illustrated
in Fig. 12a integrally from a metal tube;
Fig. 13a is a schematic view intended to illustrate one possible
implantation operation of the
medical device proposed by this invention; and
Fig. 13b is a schematic view of the medical device proposed by the
invention in the im-
planted state.
Figs. 14a to d illustrate a preferred embodiment of the insertion system of a
transapical design
proposed by the invention as a means of inserting a self-expandable heart
valve
stent in its four pre-definable functional modes with a view to illustrating
the pro-
cedure of loading a stent in the insertion system;
Figs. 15a to d shows the embodiment of insertion system illustrated in Fig. 14
in its four pre-
definable functional modes with a view to illustrating the procedure whereby a
stent
accommodated in the insertion system is released;
Fig. 16a shows a side view of a first preferred embodiment of the retaining
mechanism dis-
posed in the catheter tip of the insertion system proposed by the invention;
Fig. 16b is a view in cross-section of the retaining mechanism illustrated
in Fig. 16a, seen
along line A-A indicated in Fig. 16a;
Fig. 16c is a plan view of the distal retaining region of a stent, which
can be retained by
means of the retaining mechanism illustrated in Fig. 16a;
Figs. 17a to d illustrate a preferred embodiment of the medical device
proposed by the invention
with an insertion system of a transapical design, for example illustrated in
Fig. 14 or
Fig. 15, and a self-expandable heart valve stent in the four different
functional
modes of the insertion system;
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
26
Detailed Description
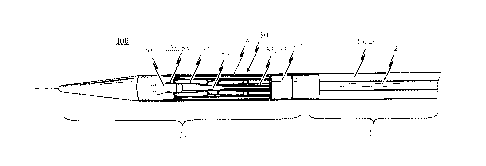
A first preferred embodiment of the self-expandable endoprosthesis 1 for the
medical device pro-
posed by the invention will be described first of all with reference to Figure
la to le. Fig. la illus-
trates the endoprosthesis 1 in its first pre-definable mode in which the
endoprosthesis is in a mini-
mised state and can therefore be introduced into a patient's body with minimal
invasion by means
of a catheter system. Fig. lc illustrates the endoprosthesis 1 in its second
mode in which the endo-
prosthesis is in its expanded state. Fig. lb illustrates the endoprosthesis 1
in a state between the
first mode (see Fig. la) and the second mode (see Fig. 1c). Fig. Id
illustrates the expanded endo-
prosthesis of Fig. lc with a heart valve prosthesis attached to it. The
endoprosthesis 1 of this em-
bodiment is distinctive due to the fact that it has a structure which is cut
integrally from a metal
tube. The cutting pattern used to produce the stem design is illustrated in a
flat projection in Fig.
le.
Specifically, the endoprosthesis 1 comprises a total of three positioning
arches 10 which assume the
function of automatically positioning the medical device in the patient's
aorta. The positioning
arches 10 have a rounded head portion 12 which engages in the pockets of the
insufficient heart
valve to be replaced by the medical device when the medical device is
positioned at the implanta-
tion site. Providing three positioning arches 10 in total ensures that the
requisite positioning accu-
racy can be obtained in the direction of rotation.
The head portions 12 of the positioning arches 10 pointing respectively
towards the proximal end 3
of the endoprosthesis 1 are appropriately rounded so that the vessel wall is
not damaged when the
positioning arches 10 engage in the pockets of the heart valve to be replaced.
Extending from the
head portion 12 of the positioning arch 10 to the distal end 2 of the
endoprosthesis 1 are two posi-
tioning webs or arms 11 in total for each positioning arch 10 which merge into
an eye-shaped ele-
ment 30 at the distal end 2 of the endoprosthesis 1. This eye-shaped element
30 serves as a retain-
ing means for attaching the endoprosthesis 1 and hence the medical device to
an introduction
catheter system.
Specifically, the respective retaining eyes 30 are disposed between the two
arms 11 of two mutually
adjacent positioning arches 10. Opening into the transition portion 13 between
the two arms 11 of
two mutually adjacent positioning arches 10 incorporating the retaining eye 30
is a connecting web
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
27
15 extending essentially in the longitudinal direction of the endoprosthesis
I. At the proximal end,
the connecting web 15 merges into the respective retaining arms 21 of two
mutually adjacent re-
taining arches 20.
As a result of this stent design, an axially symmetrical structure is obtained
whereby a retaining arch
20 is associated with each positioning arch 10. The endoprosthesis 1 in the
preferred embodiment
illustrated in Figs. la to lc therefore has a total of three retaining arms 20
which form the base for
an anchoring segment of the endoprosthesis 1 for accommodating a heart valve
prosthesis 40 (il-
lustrated in Fig. Id, for example). Providing the respective connecting webs
15 between the distally
lying transition portions 23 of two mutually adjacent retaining arches 20 and
the transition portions
13 of two mutually adjacent positioning arches 10 results in a stent structure
whereby the respec-
tive arms 11 of a positioning arch 10 extend essentially parallel with the
respective arms 21 of a re-
taining arch 21 associated with the positioning arch 10.
When the endoprosthesis 1 is in the state illustrated in Fig. la, in which it
assumes its first mode,
the respective arms 11 of the positioning arches 10 directly bound the
respective arms 21 of the
associated retaining arches 20.
Particular attention should be paid to Fig. lc in which the endoprosthesis 1
is illustrated in its sec-
ond mode. Particularly worth mentioning in respect of this diagram is the fact
that every position-
ing arch 10 and its associated retaining arch 20 has an essentially U-shaped
or V-shaped structure
which is closed towards the proximal end 3 of the endoprosthesis 1.
Specifically, every positioning
arch 10 is cut from the material portion of the metal tube which is
accommodated in the essentially
U-shaped or V-shaped structure of the associated retaining arch 20, as may be
seen from the cut-
ting pattern illustrated in Fig. le.
As may be seen by comparing Figures la and lc, during the transition from the
first mode into the
second mode, the endoprosthesis becomes shorter in the longitudinal direction
whilst the cross-
section simultaneously becomes wider, in particular at the distal and the
proximal anchoring
circumferential regions 2, 3. When the endoprosthesis 1 is in the expanded
state, the respective po-
sitioning arches 10 are specifically opened out to a more pronounced degree in
a radial direction
from the plane of the endoprosthesis than is the case at the distal anchoring
region 2 of the stent 1.
The positioning arches 10, which assume the function of positioning the
medical device in the irn-
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
28
planted state by engaging in the pockets of the heart valve to be replaced,
can therefore project fur-
ther out in a radial direction and can be inserted in the heart valve pockets
of the heart valve to be
replaced in a particularly easy manner.
Fig. 1d illustrates the embodiment in its expanded state with an
endoprosthesis 1 of the type illus-
trated in Fig. 1c and a heart valve prosthesis 40 attached with the aid of a
thread 41 and opened
out. As illustrated, opening out the proximal anchoring region 3 of the
endoprosthesis 1 in which
the heart valve prosthesis 40 is disposed causes the heart valve prosthesis 40
to open out. A radial
force is simultaneously applied to the vessel wall (not illustrated) by the
proximal end portions 22
of the retaining arches 21, thereby affording a reliable seal of the heart
valve prosthesis 40 with re-
spect to the vessel wall.
Although the force exerted by the retaining arches 21 in the radial direction
onto the vessel wall
causes the medical device to be secured at the implantation site to a certain
extent, the distal an-
choring region 2 is expanded by a further 10% to 25% in the radial direction
than the proximal an-
choring region 3 of the endoprosthesis 1 when the medical device is in the
expanded state. This
allows stable implantation of the medical device, especially in view of the
unavoidable peristaltic
movement of the vessel wall and the relatively high fluid pressures which
prevail. As a result, a
slightly concave shape is imparted to the endoprosthesis 1 which tapers in the
direction of the
proximal anchoring region 3 of the endoprosthesis 1, thereby ensuring that the
medical device is
firmly anchored in the vessel due to the distal anchoring region 2 of the
endoprosthesis 1 pressing
against the vessel wall.
In the embodiment illustrated, the respective arms 21 of the retaining arches
20 have uninterrupted
slots or elongate holes 24, the purpose of which is to enable or assist
expansion of the endopros-
thesis 1 from the minimised state into the expanded state. These slots or
elongate holes 24 make it
easy to widen the cross-section of the stent 1 whilst simultaneously reducing
its length. Naturally,
however, it would also be conceivable for these slots or elongate holes 24 to
accommodate a thread
41 or similar used to attach the heart valve prosthesis 40 (illustrated in
Fig. 1d) to the proximal re-
gion 3 of the endoprosthesis 1.
The medical device of the present invention is of a modular design essentially
comprising the two
separately manufactured components, endoprosthesis 1 and heart valve
prosthesis 40. The endo-
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
29
prosthesis 1 assumes the function of positioning and securing the heart valve
prosthesis 40 in the
patient's aorta. It may be preferable if the two components (endoprosthesis 1
and heart valve pros-
thesis 40) are not connected to one another until immediately prior to
performing the surgical in-
tervention; this is of advantage in terms of transporting and storing the
endoprosthesis 1 as such
since the endoprosthesis 1 is a relatively robust component from a mechanical
point of view and
can be stored for a significant period of time. This applies in particular if
the endoprosthesis 1 is
stored in its second mode, i.e. in the expanded state, and is not switched to
its first (minimised)
mode until immediately prior to undertaking the surgical intervention.
Fig. la shows the endoprosthesis 1 is in its first mode, in its minimised
state which is the so-called
"minimised" mode of the endoprosthesis structure made from a memory shape
material. When an
external stimulus acts on the endoprosthesis body illustrated in Fig. la, the
shape memory effect is
triggered and the fixed open shape memorised during production of the
endoprosthesis 1 illus-
trated in Figure lc is restored. This external stimulus is preferably a
settable switching temperature
and the body must be at a temperature higher than the switching temperature in
order to trigger
the shape memory effect and thus restore the memorised open shape of the
endoprosthesis 1. By
selecting the chemical composition of the material used for the endoprosthesis
1, a specific switch-
ing temperature can be fixed before the endoprosthesis is programmed. In the
case of the preferred
embodiment the switching temperature lies in a range of between 20 C and the
body temperature
of the patient.
Therefore, it would be conceivable for the medical device to be cooled
accordingly during the in-
troduction process. When the medical device has been moved to the desired
implantation site, in
other words in front of the native heart valve, preferably by means of an
appropriate introduction
system, cooling can be interrupted so that the endoprosthesis 1 of the medical
device is heated to
the body temperature (36 C) of the patient, thereby triggering the shape
memory effect of the
endoprosthesis material. Having triggered the self-expanding property of the
endoprosthesis 1, ra-
dial forces are generated which act on the individual components of the
endoprosthesis 1, in par-
ticular, on the respective positioning arches 10, 11 and retaining arches 20,
21 of the endoprosthe-
sis I. Since the endoprosthesis 1 of the medical device is still disposed in
the introduction catheter
system as before, the radial forces which build up once the critical switch
temperature is exceeded
and act on the individual components of the endoprosthesis 1 are compensated
by the introduction
port of the introduction catheter system so that - in spite of the shape
memory effect having been
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
triggered - the endoprosthesis 1 of the medical device is forcibly retained in
its first (minimised)
mode.
By releasing the endoprosthesis 1 from the introduction catheter system in
appropriate steps, it is
then possible to release the positioning arches 10, 11 of the endoprosthesis 1
through the introduc-
tion port of the introduction catheter system first, as a result of which it
opens up due to the radial
forces acting in the radial direction. The opened positioning arches 10, 11
can then be positioned in
the pockets of the native heart valve.
The remaining components of the endoprosthesis 1 and the medical device can
then be released
through the introduction port of the introduction catheter system. As this
happens, the retaining
arches 20, 21 open in the radial direction and the heart valve prosthesis 40
attached to the retaining
arches 20, 21 by means of a thread 41, etc., for example, thus unfolds in the
manner of an um-
brella. The radial forces acting on the retaining arches 20, 21 and also on
the distal anchoring re-
gion 2 of the endoprosthesis 1 cause the endoprosthesis 1 to be pressed in the
radial direction
against the vessel wall. In this way, a reliable anchoring of the medical
device is guaranteed at the
implantation site on the one hand and a reliable seal of the heart valve
prosthesis 40 is ensured at
the proximal anchoring region 3 of the endoprosthesis 1 on the other hand.
Figures 2a to 2c illustrate a second embodiment of a self-expandable
endoprosthesis 1 for the
medical device proposed by the invention in its first, pre-definable mode (see
Fig. 2a) in its second
pre-definable mode (see Fig. 2c) as well as in a state in between (see Fig.
2b).
Fig. 2d illustrates a second embodiment of the medical device proposed by the
invention in its ex-
panded state with an endoprosthesis of the type illustrated in Fig. 2c and a
heart valve prosthesis 40
attached to it and opened out. A flat projection of a cutting pattern which
may be used for the
production of the second embodiment of the self-expandable endoprosthesis is
illustrated in Fig.
2e. This cutting pattern is suitable for cutting the endoprosthesis
illustrated in Fig. 2a integrally
from a metal tube.
The endoprosthesis 1 of the second embodiment essentially corresponds to the
first embodiment
described above with reference to Figures la to le. Description of the various
components corre-
sponds to that described for Figs. la to le and will not be repeated. The
second embodiment dif-
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
31
fers from the first preferred embodiment of the endoprosthesis in that the
respective arms 11 of
the adjacent positioning arches 10 are joined indirectly via a connecting web
16 extending essen-
tially in the longitudinal direction of the endoprosthesis 1 to the retaining
eve 30. The respective
arms 21 of the retaining arches 20 are associated with the adjacent
positioning arches 10 are indi-
rectly joined via a connecting web 15 extending essentially in the
longitudinal direction of the
endoprosthesis 1 to the retaining eye 30. Specifically, the connecting web 15
of the retaining arches
20 merges into the connecting web 16 of the positioning arches 10 at the end
portion 13 of the po-
sitioning arches 10. By selecting the respective lengths of the two connecting
webs 15 and 16 ac-
cordingly, therefore, the overall length of the stent 1 can be adjusted in an
easy manner.
The third embodiment of a self-expandable endoprosthesis for the medical
device proposed by the
invention illustrated in Figs. 3a to 3c essentially corresponds to the first
preferred embodiment il-
lustrated in Figures 1a to lc. The difference, however, is that in the third
preferred embodiment,
the retaining eyes 30 disposed between two adjacent positioning arches 10 are
provided with barbs
17, the respective tips of which point in the direction of the proximal end 3
of the endoprosthesis
1. With this modification to the design additional anchoring is provided for
the system to prevent
the stent 1 from being dislocated in the direction of the left ventricle.
Fig. 3d illustrates a third embodiment of the medical device proposed by the
invention in its ex-
panded state with an endoprosthesis of the type illustrated in Fig. 3c and a
heart valve prosthesis 40
attached to it and opened out. This diagram essentially corresponds to that of
Fig. Id. The differ-
ence, however, is that the barb elements 17 described above are provided on
the respective retain-
ing eyes 30.
A flat projection of a cutting pattern which may be used for the production of
the third embodi-
ment of the self-expandable endoprosthesis 1 is illustrated in Fig. 3e. This
cutting pattern is suitable
for cutting the endoprosthesis illustrated in Fig. 3a integrally from a metal
tube.
Figs. 4a to 4c illustrate a fourth embodiment of a self-expandable
endoprosthesis 1 for the medical
device proposed by the invention. A fourth embodiment of the medical device
proposed by the
invention is illustrated in its expanded state with an endoprosthesis in Fig.
4c and an opened out
heart valve prosthesis 40 attached to it is illustrated in Fig. 4d. Fig. 4e
illustrates a flat projection of
a cutting pattern which may be used for the production of the fourth
embodiment of the self-
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
32
expandable endoprosthesis 1. The cutting pattern illustrated in Fig. 4e is
specifically suitable for
cutting the endoprosthesis illustrated in Fig. 4a integrally from a metal
tube.
The fourth embodiment of the self-expandable prosthesis 1 corresponds to a
combination of the
second and third embodiments described above. Specifically, the respective
arms 11 of the adjacent
positioning arches 10 are indirectly joined via the connecting web 16
extending essentially in the
longitudinal direction of the endoprosthesis to the anchoring eye 30. Barbs 17
are provided on the
respective anchoring eyes 30, the tips of which point in the direction of the
proximal end 3 of the
endoprosthesis 1. The advantages which can be achieved as a result of the
features provided on the
fourth embodiment were described above and will not be reiterated at this
stage.
The fifth embodiment of a self-expandable endopros thesis 1 and a medical
device proposed by the
invention illustrated in Fig. 5a to Fig. 5e essentially corresponds to the
first embodiment described
with reference to Fig. la to Fig. le, except that, in this instance, the
respective retaining arches 21
of the endoprosthesis 1 are provided with reinforcing portions 26 which
interrupt the slots 24 ex-
tending in the longitudinal direction of the retaining arches 21. The purpose
of these reinforcing
portions 26 is to open out the individual components of the retaining arches
21 and, in particular,
to break thc anchoring support 25 radially out of the retaining arches 20.
Accordingly, a retaining
portion for the stent 1 can be obtained with the reinforcing portions 26 which
has no components
which might explant the medical device when it is in the expanded state.
Fig. 5e illustrates a flat projection of a cutting pattern which may be used
for production of the
fifth embodiment of the self-expandable endoprosthesis 1 to cut the
endoprosthesis 1 illustrated in
Fig. 5a integrally- from a metal tube.
The sixth embodiment of the self-expandable endoprosthesis and the medical
device proposed by
the invention illustrated in Figures 6a to 6e corresponds to a combination of
the second embodi-
ment illustrated in Figures 2a to 2e and the fifth embodiment described above
with reference to
Figures 5a to 5e. Specifically, therefore, the endoprosthesis 1 based on the
second embodiment is
provided with additional reinforcing portions 26 at the respective retaining
arches 21 which inter-
rupt the slots 24 extending in the longitudinal direction of the retaining
arches 21.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
33
The seventh embodiment of the endoprosthesis 1 and the medical device proposed
by the inven-
tion illustrated in Figures 7a to 7e corresponds to a combination of the third
and fifth embodi-
ments described above. In particular the respective retaining eyes 30 are
provided with barbs 17
and the respective retaining arches 21 are provided with reinforcing portions
26.
The eighth embodiment of the self-expandable endoprosthesis and the medical
device proposed by
the invention illustrated in Figures Sa to Se corresponds to a combination of
the fourth and fifth
embodiments, in which case the respective retaining arches 21 are provided
with reinforcing por-
tions 26. The retaining eyes 30 provided with barbs 17 are connected to the
respective arms 11 of
the adjacent positioning arches 10 by means of a connecting web 16 extending
essentially in the
longitudinal direction of the endoprosthesis 1.
The ninth embodiment of the self-expandable endoprosthesis for the medical
device proposed by
the invention illustrated in Figures 9a to 9d is of a slightly modified shape
compared with the first
embodiment (see Figures la to lc). The endoprosthesis 1 based on the ninth
embodiment is illus-
trated in its first pre-defined mode in Fig. 9a. Figures 9b and 9c
respectively show a perspective
side view of the endoprosthesis 1 based on the ninth embodiment in its second
mode. Specifically,
the connecting web 15 between the end portion 13 of a positioning arch 10, 11
and the end por-
tion 23 of an associated retaining arch 20, 21 is illustrated in Fig. 9b. Fig.
9c, on the other hand,
illustrates the positioning arches 10, 11 and the associated retaining arches
20, 21 of the endopros-
thesis 1 illustrated in Fig. 9a.
Fig. 9e illustrates a flat projection of a cutting pattern which may be used
to produce the ninth em-
bodiment of the self-expandable endoprosthesis to cut the endoprosthesis
illustrated in Fig. 9a in-
tegrally from a metal tube.
Unlike the first embodiment, the respective head portions 12 of the
positioning arches 10 pointing
towards the proximal end 3 of the endoprosthesis are of a slightly wider
design at the proximal end
in the ninth embodiment of the endoprosthesis 1. Although the head portions 12
of the position-
ing arches 10 have a slightly rectangular in shape compared with the first
embodiment, all the re-
spective corners of the head portions 12 are rounded so that the vessel wall
is not damaged when
the positioning arches 10 engage in the pockets of the heart valve to be
replaced. The advantage of
the slightly wider design of the head portions 12 of the positioning arches 10
is that the positioning
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
34
arches 10 can be placed in the pockets of the native heart valve with the
smallest possible clearance
during the implantation operation, thereby enabling even more accurate
positioning of the medical
device at the implantation site.
As with the embodiments described above, a total of two positioning webs or
arms 11 extend from
the head portion 12 of the positioning arches 10 to the distal end 2 of the
endoprosthesis 1 for
every positioning arch 10 in the ninth embodiment of the endoprosthesis 1 and
which merge at the
distal end 2 of the endoprosthesis 1 into an eye-shaped element 30. This eye-
shaped element 30
serves as a retaining means for attaching the endoprosthesis 1 and hence the
medical device to an
introduction catheter system.
Specifically, in the case of the ninth embodiment of the endoprosthesis 1, the
retaining eyes 30 are
disposed between the two arms 11 of two mutually adjacent positioning arches
10. The connecting
web 15 extends essentially in the longitudinal direction of the endoprosthesis
1 and opens into the
transition portion 13 between the two arms 11 of two mutually adjacent
positioning arches 10
where the retaining eye 30 is formed. At the proximal end of the connecting
web 15, the latter
merges into the respective retaining arms 21 of two mutually adjacent
retaining arches 20. This de-
sign is illustrated particularly clearly in Fig. 9d, which shows a perspective
plan view of the distal
region of the endoprosthesis illustrated in Fig. 9a in its second mode.
In contrast with the embodiments described above, the respective retaining
arms 21 of the retain-
ing arches 20 on the transition portion 23 between the two arms 21 of two
mutually adjacent re-
taining arches 20 are not provided with slots or elongate holes 24 in the
ninth embodiment of the
endoprosthesis 1. Due to the fact that only one arm web 21 actually opens into
the transition por-
tion 23 between the two arms 21 of two mutually adjacent retaining arches 20
for each retaining
arch, there are advantageously no components belonging to the retaining arches
20 which project
out from the respective retaining arches 20 in a radial direction when the
endoprosthesis 1 is in the
expanded state (see Fig. 9b for example). When the endoprosthesis 1 is in an
expanded state, no
anchoring support 25 usually extends through the slots 24 projecting out in a
radial direction at the
transition portions 23 between the two arms 21 of two mutually adjacent
retaining arches 20. It has
been found that, in this embodiment, the endoprosthesis 1 can be explanted
particularly easily and
removed from the patient's body.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
Although the ninth embodiment of the endoprosthesis 1 does not have slots or
elongate holes 24
at the respective transition portions 23 between the two arms 21 of two
mutually adjacent retaining
arches 20, the respective retaining arms 21 of the endoprosthesis 1 have
reinforcing portions 26
which are respectively provided on portions of the retaining arms 21 that are
not congruent with
the transition portions 23 between the two arms 21 of two mutually adjacent
retaining arches 20.
Fig. 10 illustrates a flat projection of a cutting pattern which may be used
for the production of an-
other embodiment of the self-expandable endoprosthesis 1 to cut an
endoprosthesis integrally
from a metal tube. The cutting pattern illustrated in Fig. 10 differs from the
cutting pattern illus-
trated in Fig. le due to the fact that the distally disposed slots 24
extending in the longitudinal di-
rection of the retaining arches 21 have been omitted from the respective
retaining arches 21 on the
one hand and a bigger space 27 is cut from between the adjacent retaining
arches 21 in order to
save on material on the other hand.
Fig. 11 illustrates another embodiment of a self-expandable endoprosthesis 1
for an alternative de-
sign of the medical device proposed by the invention. Specifically, the
endoprosthesis 1 of the em-
bodiment illustrated in Fig. 11 has assumed its second mode in which the
medical device is in its
expanded state. The endoprosthesis 1 illustrated in Fig. 11 differs from the
endoprosthesis 1 illus-
trated in Fig. lc due to the fact that the stent 1 illustrated in Fig. 11 has
an interconnecting web 16
extending essentially in the longitudinal direction of the endoprosthesis 1
between the retaining
eyes 30 and the transition portion 13 between the positioning arms 11 of two
adjacent positioning
arches 10. Thus, the total length of the endoprosthesis 1, and hence the
medical device, is made
longer. To ensure the optimum ability to manoeuvre the medical device in the
minimised state,
however, it is an advantage if the endoprosthesis 1 has as short a
longitudinal extension as possible,
especially if the implantation route to the heart valve leads through the arch
of the aorta. It is of
advantage if the medical device is as short as possible (and the
endoprosthesis 1 is also as short as
possible) so that the medical device can be manoeuvred easily around the arch.
The endoprosthesis 1 illustrated in Fig. 11 also differs from the
endoprosthesis of the embodi-
ments described above due to the fact that when the endoprosthesis 1 is in the
expanded state, a
barb portion 25 projects through the slots 24 in the radial direction at the
respective transition por-
tions 23 between the two arms 21 of two mutually adjacent retaining arches 20.
The tip of the barb
portion 25 points in the direction of the distal retaining region 2 of the
endoprosthesis 1.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
36
A yet further embodiment of the self-expandable endoprosthesis 1 for the
medical device proposed
by the invention will now be described with reference to Figs. 17a to 17d.
Fig. 17a illustrates the endoprosthesis 1 in its first pre-definable mode in
which the medical device
(not explicitly illustrated) is in a minimised state and can therefore be
introduced into a patient's
body with minimal invasion by means of a catheter system. Fig. 17c illustrates
the endoprosthesis 1
in its second mode in which the medical device is in its expanded state. Fig.
17b illustrates the stent
50 in a state between the first mode (see Fig. 17a) and the second mode (see
Fig. 17c). Fig. 17d il-
lustrates the endoprosthesis 1 in its expanded state with a heart valve
prosthesis 60 attached to it by
sutures.
This embodiment of the self expandable prosthesis 1 corresponds essentially to
the second em-
bodiment illustrated in Figs. 2a-2e. The difference with the embodiment
illustrated in Figs 12a to
12e is that the retaining eyes 30 are imperforate, taking the form of
retaining heads. It has been
found that an imperforate retaining eye allows the medical device to be
released more simply and
easily from an insertion catheter system. By way of clarification, it should
be noted that it is not the
presence or absence of perforations in the retaining means which directly
contribute to the ease of
release of the retaining means from the catheter. Rather, it is the absence of
parts on the catheter
on which the retaining means may become lodged, passing through or penetrating
the retaining
means, for example, such as a peg and hole.
It will be appreciated that the various optional features, including integral
or extended anchoring
eyes 30, barb elements 17, reinforcing portions 26, perforate or imperforate
retaining eyes 30, may
be used in any combination with the endoprosthesis structure.
A more detailed description will be given below with reference to Figures 12a
ad 12b, explaining
how the medical device proposed by the invention is used to treat a condition
of heart valve insuf-
ficiency.
The medical device proposed by the invention, and in particular the
endoprosthesis 1 with the
heart valve prosthesis 40 contained in it, is designed to be introduced into
the patient's body either
backwards (retrograde) or transapically, i.e. approaching from the heart apex
via a special catheter.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
37
The device is positioned percutaneously orthotopically in vivo and assumes the
function of an insuf-
ficient or narrowed (stenosed) heart valve. Fig. 13a provides a schematic
illustration of one possible
implantation operation for the medical device proposed by the invention,
whereby the medical de-
vice in this instance is introduced into the patient's body via a retrograde
approach from the femo-
ral artery using a special catheter. Fig. 13b provides a schematic view of the
medical device pro-
posed by the invention in the implanted state.
in the case of the implantation route illustrated in Fig. 13a, the catheter
system, which is not spe-
cifically illustrated, containing the medical device with the heart valve
prosthesis 40 and the endo-
prosthesis 1 serving as an anchoring stent, is introduced by puncturing the A.
femoris communis
(inguinal artery). This catheter system is preferably moved forward to the
aortal valve position as-
sisted by angiographic (vessel display) and echocardiographic (ultrasound)
control, where the actual
heart valve implantation then takes place.
Alternatively, a catheter system can be pushed transapically from the heart
apex through the left
ventricle to the aortal valve where a similar implantation of the
endoprosthesis 1 with the heart
valve prosthesis 40 is possible using a catheter suitably modified
accordingly.
As the catheter system is introduced, the medical device is preferably cooled,
for example by rins-
ing the interior of the catheter system with an appropriate coolant such as a
cooled salt solution.
When the medical device has been moved forward to the desired implantation
site, cooling is inter-
rupted, as a result of which the endoprosthesis 1 of the medical device is
warmed to the body tem-
perature of the patient (36 C), thereby triggering the shape memory effect of
the endoprosthesis
material.
Due to the triggering of the self-expanding property of the endoprosthesis 1,
radial forces develop
which act on the individual components of the endoprosthesis 1, in particular
on the respective
positioning arches 10, 11 and retaining arches 20, 21 of the endoprosthesis 1.
Since the endopros-
thesis 1 of the medical device is still disposed in the introduction catheter
system as before, the ra-
dial forces which develop once the critical switching temperature is exceeded
and act on the indi-
vidual components of the endoprosthesis 1 so that - in spite of the shape
memory effect having
been triggered - the endoprosthesis 1 of the medical device is forcibly held
in its first (minimised)
shape within the closed introduction port of the introduction catheter
system..
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
38
By releasing the endoprosthesis 1 from the introduction catheter system in
appropriate steps, the
positioning arches 10, 11 of the endoprosthesis 1 are moved out though the
introduction port of
the introduction catheter system once opened. The positioning arches 10, 11
open out due to the
radial forces within the endoprosthesis. The opened positioning arches 10, 11
are then positioned
in the pockets 50 of the native heart valve 51.
The other components of the endoprosthesis 1 and the medical device are then
released through
the introduction port of the introduction catheter system. As illustrated in
Fig. 13b, the retaining
arches 20, 21 open together in the radial direction and cause the heart valve
prosthesis 40 attached
to the retaining arches 20, 21 to open out in the manner of an umbrella.
However, the radial forces
acting on the retaining arches 20, 21 also act on the distal anchoring region
2 of the endoprosthesis
1, causing the endoprosthesis 1 to be pressed in a radial direction against
the vessel wall. This, on
the one hand, guarantees a reliable anchoring of the medical device at the
implantation site and, on
the other hand, ensures a reliable seal of the heart valve prosthesis 40 at
the proximal anchoring
region 3 of the endoprosthesis 1.
When the medical device is in the implanted state as illustrated in Fig. 13b,
the heart valve prosthe-
sis 40 is opened out at the proximal anchoring region 3 of the endoprosthesis
1 whilst the old (in-
sufficient) heart valve 51 is pressed towards the vessel wall due to the self-
expanding property of
the endoprosthesis 1. The distal anchoring region 2 of the endoprosthesis 1
affords additional me-
chanical support for the system and reliable anchoring.
As may be seen from Fig. 13b, when the endoprosthesis 1 is in its expanded
state, the respective
positioning arms 21 of the positioning arches 20 locate in the pockets of the
incumbent heart valve
and thus essentially guarantee secure and error-free positioning of the
medical device. The pocket
flaps of the incumbent heart valve are clamped between the positioning arches
10 and the retaining
arches 20 due to the expansion of the endoprosthesis 1. This further assists
in achieving optimum
positioning and a stable anchoring of the heart valve prosthesis 40. Optimum
lateral sealing of the
implanted valve prosthesis 40 is guaranteed at the same time.
The system is afforded additional mechanical support and reliable anchoring by
providing barbs 17
on the retaining eyes 30 disposed at the distal anchoring region 2 of the
endoprosthesis 1 and/or
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
39
by appropriate anchoring supports 23. When the endoprosthesis 1 is in the
expanded state, the an-
choring supports 25 stand proud of the co-operating arm 21 of the retaining
arches 20 and their
tips point in the direction of the distal end 2 of the endoprosthesis 1.
The design of the endoprosthesis 1 allows gripping of the endopros thesis 1 by
means of the retain-
ing eyes 30 and minimising the medical device by the longitudinal extension of
the endoprosthesis
1 so that the medical device is pulled back into the catheter and removed from
the patient's body.
Due to the modular integration of retaining elements (retaining eyes) on the
self-expandable endo-
prosthesis 1, it can also be explanted again by way of a catheter once the
endoprosthesis has been
implanted. To this end, the distal anchoring region 2 of the endoprosthesis 1
is pulled into a cathe-
ter by the several retaining eyes using guide wires within the catheter. In
other words, in the reverse
of the implantation operation, the endoprosthesis 1 is pulled from its
expanded state back into its
minimised state and released from the anchoring in the pockets of the
incumbent heart valve. As
will be described below the design of the endoprosthesis allows reversal of
the implantation at any
stage during the implantation process. Specifically explantation may be
carried out causing minimal
damage and/or stress to the heart, the vasculature and the patient.
Further preferred aspects of the invention will now be described with
reference to a catheter inser-
tion system.
Reference to the use of the catheter insertion system specifically disclosed
herein is not intended to
necessarily limit the use of the stent with this catheter insertion system
alone. It is the novel fea-
tures of the catheter tip, which could be supplied in the form of a modular
cartridge for example,
in combination with the retaining means, which afford the increased
reliability in terms of stent
release and positioning. Thus such a catheter tip, or cartridge, could be
affixed to catheter systems
already known in the art.
With reference to Figs. 14 and 15, use of the stent with a catheter insertion
system of a transapical
design will be explained for transapical insertion of a self-expandable heart
valve stent to a patient's
body. Figs. 14a to d and Figs. 15a to d respectively illustrate an insertion
system in its four different
pre-definable functional modes.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
The insertion system 1 is suitable for transapical access to a heart valve to
be replaced, such as an
aortal valve for example. Using the insertion system 1, it is possible to
implant a self-expandable
heart valve stem in a patient's body transapically, i.e. from the heart apex.
To this end, the insertion
system 1 has a catheter system 10, by means of which the heart valve stent,
not explicitly illustrated
in Figures 14 and 15, can be inserted in the patient's body in its minimised
mode. Fig. 16 illustrates
an embodiment of the catheter tip proposed by the invention.
In the insertion system 1 illustrated in Figs. 14 and 15, a catheter tip 20 is
provided at the proximal
end 11 of the catheter system 10, in which the heart valve stent to be
implanted in the patient's
body can be accommodated. At the distal end 12 of the catheter system 10, a
handle 30 is also pro-
vided, by means of which the catheter tip 20 can be manipulated.
Specifically, the catheter tip 20 of the insertion system 1 illustrated in
Figs. 14 and 15 has a retain-
ing mechanism 21 so that at least the distal region of the stent to be
implanted in the patient's body
can be releasably attached to the catheter tip. The retaining mechanism will
be described in more
detail, in particular, with reference to Fig. 16.
The catheter tip 20 also has a housing system for accommodating at least the
proximal region of
the stent. Specifically, the housing system comprises a first housing portion
23 for accommodating
first functional components of the stent, for example for accommodating the
retaining arches of
the stent, and a second housing portion 24 for accommodating second functional
components of
the stem, for example for accommodating positioning arches of the stent.
As regards the handle 30 of the insertion system 1, it has a first operating
means 33 co-operating
with the first housing portion 23 and an operating means 34 co-operating with
the second housing
portion. The first operating means 33 of the handle 30 co-operates with the
first housing portion
23 of the catheter tip 20 so that when the first operating means 33 is
operated, it causes a pre-
definable longitudinal movement of the first housing portion 23 relative to
the fixing mechanism
21. Secondly, the second operating means 34 of the handle 30 co-operates with
the second housing
portion 24 of the catheter tip 20 so that when the second operating means 34
is operated, it causes
a pre-definable longitudinal movement of the second housing portion 24 of the
catheter tip relative
to the fixing mechanism 21.
CA 02822636 2013-07-31
W02008/125153 PCT/EP2007/061117
41
With the insertion system 1, shown in Figs. 14 to 16 for example, the second
housing portion 24 of
the catheter tip 20 is disposed at the proximal end portion 25 of the catheter
tip 20. The first hous-
ing portion 23 is disposed between the housing portion 24 and the handle 30.
With this transapical
insertion system 1, when the associated second operating means 34 of the
handle is operated, the
second housing portion 24 of the catheter tip 20 can be moved in longitudinal
direction L of the
catheter tip 20 relative to the fixing mechanism 21 in the proximal direction,
i.e. away from the
handle 30. When the associated first operating means 33 of the handle 30 is
operated, the first
housing portion 23 can be moved in the longitudinal direction L of the
catheter tip 20 relative to
the fixing mechanism in the distal direction, i.e. towards the handle 30.
The insertion system 1 illustrated in Figs. 14 to 15 also has a gate system 13
co-operating with the
catheter system 10 which is connected on the one hand to the portion of the
catheter tip 20 facing
the handle and on the other hand to the portion of the handle 30 facing the
catheter tip. The gate
system 13, which is preferably of a hollow design, has in its interior a first
mechanism 26, such as a
wire system, to enable a force to be transmitted from the first operating
means 33 of the handle 30
to the first housing portion 23 of the catheter tip 20.
The gate system 13 is also provided with a second mechanism 27 for
transmitting force from the
second operating means 34 of the handle 30 to the second housing portion 24 of
the catheter tip
20. As with the first force transmitting mechanism 26, the second force
transmitting mechanism 27
may be provided in the form of a wire system.
In detail, in the insertion system 1, the second force transmitting mechanism
27, actively connect-
ing the second operating means 34 of the handle 30 with the second housing
portion 24 of the
catheter tip 20, is provided in the form of a wire extending through the
interior of the gate system
13. The wire is connected to the second operating means 34 of the handle 30 on
the one hand and
to the second housing portion 24 of the catheter tip 20 on the other hand. The
first force transmit-
ting mechanism 26 is provided in the form of a sleeve which extends through
the interior of the
gate system 13 and surrounds the wire constituting the second force
transmitting mechanism 27.
The second force transmitting mechanism 27 constitutes an extension of the
first housing portion
23 of the catheter tip 20 and is actively connected to the first operating
means 33 of the handle on
the one hand and to the first housing portion 23 of the catheter tip 20 on the
other hand. Natu-
rally, however, it would also be conceivable for the first force transmitting
mechanism 26 to be
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
42
provided in the form of a sleeve surrounding the wire constituting the second
force transmitting
mechanism 27. In which case the sleeve of the first force transmitting
mechanism 26 simultane-
ously also constitutes the gate system 13 and is an extension of the first
housing portion 23 which
is actively connected to the first operating means 33 of the handle on the one
hand and to the first
housing portion 23 of the catheter tip 20 on the other hand.
The gate system 13 or the sleeve of the first force transmitting mechanism 26
used with the cathe-
ter system 10 of the transapical design of the insertion system 1 is provided
in the form of an elon-
gate tube and the second force transmitting mechanism 27. Optionally, the
first force transmitting
mechanism 26 is disposed in the interior of this tube. The gate system 13 is
preferably designed so
that its length remains virtually unchanged, especially when subjected to the
compression or tensile
stress which occurs during the process of inserting the catheter system 10.
This function of the
gate system 13 is achieved by using an appropriate material for the elongate
tube and by an expedi-
ent choice of the wall thickness. In particular, it is preferable if the gate
system 13 or the sleeve of
the first force transmitting mechanism 26 is both resistant to buckling and
also flexible so that a
bending radius of at least 4 cm and preferably at least 3 cm can be achieved
with the gate system
13, at least in the proximal region 14 of the gate system 13.
As regards the fixing mechanism 21 belonging to the catheter tip 20 in the
illustrated embodiment
of the transapically designed insertion system 1, this fixing mechanism 21 is
provided in the form
of a crown 21a with a total of three pockets 22 formed therein. The pockets 22
of the crown 21a
are of a design complementing retaining elements, for example retaining heads,
which are disposed
at a distal region of the stent which is to be accommodated or can be
accommodated in the cathe-
ter tip 20 of the insertion system 1. Thc pockets 22 formed in the crown 21a
establish a releasable
engagement with the distal region of the stent so that the stent can be
releasably attached to the
retaining mechanism 21 of the catheter tip 20.
As may be seen in particular from Figs. 14 and 15, in the embodiment of the
illustrated transapi-
cally designed insertion system 1, both the first housing portion 21 of the
catheter tip 20 and the
second housing portion 24 of the catheter tip 20 are each provided in the form
of sleeves or sleeve-
type portions and are specifically designed to accommodate the functional
components of the
stent. Specifically, the internal diameter of the second sleeve-type housing
portion 24 is bigger than
the external diameter of the first sleeve-type housing portion 23.
Accordingly, in the case of the
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
43
embodiment of the illustrated transapically designed insertion system 1, the
second housing portion
24 of the catheter tip 20 is designed to accommodate, in addition to the
second functional compo-
nents of the stern, namely the positioning arches of the stent, the first
housing portion 23 of the
catheter tip 20 with the first functional components of the stem accommodated
in it, namely the
retaining arches of the stem.
Turning to the handle 30 used with the embodiment of the insertion system 1
illustrated in Figs. 14
and 15, the illustrated embodiment of the insertion system 1 is such that the
second operating
means 34, which co-operates with the second housing portion 24 of the catheter
tip 20 via the sec-
ond force transmitting mechanism 27, has a carriage 31 which is guided in a
guide 31' and actively
connected to a slide 31". Carnage 31 of the second operating means 34 is
actively connected to the
second housing portion 24 of the catheter tip 20 co-operating with the second
operating means 34
via the second force transmitting mechanism 27 so that when the second
operating means 34 is
operated, in particular the carriage 31 of the second operating means 34, a
force is transmitted di-
rectly from the carriage 31 of the second operating means 34 to the second
housing portion 24 of
the catheter tip 20. In the same way, the first operating means 33, which is
actively connected to
the first housing portion 23 of the catheter tip 20 via the first force
transmitting mechanism 26,
also has a carriage 32 which is guided in a guide 32' and is actively
connected to another slide 32".
Carriage 32 of the first operating means 33 is actively connected to the first
housing portion 23 of
the catheter tip 20 co-operating with the first operating means 33 via the
first force transmitting
mechanism 26. Thus, when the first operating means 33 is operated, in
particular when the car-
riage 32 of the first operating means 33 is operated, a force is transmitted
directly from the carriage
32 of the first operating means 33 to the first housing portion 23 of the
catheter tip 20.
As regards the first operating means 33 of the handle 30 used with the
insertion system 1 illustrated
in Figs. 14 and 15, the handle 30 also has a first and a second stop 35, 36.
These stops co-operate
with the first operating means 33 and are designed to fix the total stroke
length of the longitudinal
movement of the first housing portion 23 of the catheter tip 20 when the
operating means 33 is
operated. This is achieved by the fact that the displacement path which can be
covered by the slide
32 of the first operating means 33 on the guide 32' is fixed.
CA 02822636 2013-07-31
=
WO 2008/125153 PCT/EP2007/061117
44
The handle 30 also has a third and fourth stop 37, 38 which co-operate with
the second operating
means 34 and fix the total stroke length of the longitudinal movement. This
can be effected by the
second housing portion 24 of the catheter tip 20 when the second operating
means 34 is operated.
In addition to the third and fourth stops 37, 38, the handle 30 of the
embodiment of the insertion
system 1 illustrated in Figs. 14 and 15 also has another, fifth stop 39 co-
operating with the second
operating means 34. This stop co-operates with the third stop 37 on the one
hand and with the
fourth stop 38 on the other hand. When the second operating means 34 is
operated, a stepped lon-
gitudinal movement comprising two individual steps of the carriage 31 on the
guide 31' of the sec-
ond operating means 34 is effected and thus a stepped longitudinal movement
occurs comprising
two individual steps of moving the second housing portion 24 of the catheter
tip 20 relative to the
fixing mechanism or crown 21 of the catheter tip 20.
Since the fifth stop 39 co-operating with the second operating means 34 is
expediently positioned
on the guide 31' of the second operating means 34 between the third stop 37
and the fourth stop
38. The third and fifth stops 37, 39 on the one hand and the fourth and fifth
stops 38, 39 on the
other hand fix the stroke length of the longitudinal movement of the second
housing portion 24 of
the catheter tip 20 for each individual step when the second operating means
is operated.
As illustrated in Figs. 14 and 15, in the illustrated embodiment of the
insertion system 1, the above-
mentioned fifth stop 39 of the handle 30 co-operating with the second
operating means 34 of the
handle 30 is provided in the form of a stop element 44 releasably secured to
the guide 31' of the
carriage 31 belonging to the second operating means 34.
Finally, the handle 30 of the insertion system 1 illustrated in Figs. 14 and
15 is such that both the
first operating means 33 and the second operating means 34 are each assigned a
locking element
41, 42. Specifically, the first locking element 41, co-operating with the
first operating means 33 of
the handle 30, is provided in the form of a locking element which can be
removed from the car-
riage 32 or from the slide 32" of the first operating means 33. The locking
element 41 co-operates
with the first operating means 33 and with the carriage 32 of the first
operating means 33 so that a
longitudinal movement of the first housing portion 23 of the catheter tip 20,
which can be effected
by means of the first operating means 33, can be blocked. The second locking
element 42 on the
other hand, which co-operates with the second operating means 34, is also
provided in the form of
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
a locking element which can be removed from the carriage 31 or from the slide
31" of the second
operating means 34. The locking element co-operates with the second operating
means 34 so that a
longitudinal movement of the second housing portion 24 of the catheter tip 20,
which can be ef-
fected by means of the second operating means 34, can be blocked.
The four different functional modes which can be achieved with the insertion
system 1 will be de-
scribed below with reference to Figs. 15a to 15d.
Fig. 15a illustrates the insertion system 1 in its first functional mode, in
which the catheter tip 20 is
completely closed. As mentioned above, a self-expandable heart valve stent can
he accommodated
in the catheter tip 20 or in the corresponding housing portions 23 and 24 of
the catheter tip 20.
In the first functional mode illustrated in Fig. 15a, the respective slides
31" and 32" and hence the
respective carriages 31 and 32 of the second and first operating means 34 and
33 are each in their
first position (Pos. 1). Specifically, the slide 32" of the first operating
means 33 lies against the first
top 35 provided at the catheter tip end of the guide 32'. In this first
position, the slide 32" is fixed
by means of the first locking element 41 so that a longitudinal movement of
the slide 32" and the
carriage 32 of the first operating means 33 on the guide 32' is blocked in the
direction of the sec-
ond stop 36 co-operating with the first operating means 33.
The slide 31" and the carriage 31 of the second operating means 34 are also
lying in the first func-
tional mode illustrated in Fig. 15a, likewise in the first position (Pos. 1)
on the third stop 37 of the
second operating means 34. The third stop of the second operating means 34 is
disposed at the
distal end of the guide 31' of the second operating means 34. In this first
position, the slide 31" and
the carriage 31 of the second operating means 34 are fixed by means of the
second locking element
42 in order to block a longitudinal movement of the slide 31" and carriage 31
along the guide 31' in
the direction towards the catheter tip.
As mentioned above, when the insertion system 1 is in the first functional
mode as illustrated in
Fig. 15a, the catheter tip 20 of the insertion system 1 is in a completely
closed mode. In this mode,
the first and second housing portions 23 and 24 of the catheter tip 20,
provided in the form of
sleeve-shaped elements, engage telescopically one inside the other. This
feature is achieved by
adapting the respective internal and external diameters of these sleeve-shaped
elements to one an-
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
46
other accordingly. Specifically, the sleeve-type second operating means 34 has
an external diameter
which is preferably identical to the external diameter of the proximal region
14 of the gate system
13. As will be described in more detail below, the sleeve-type first and
second housing portions 23
and 24 of the catheter tip 20 are adapted to one another in terms of their
respective internal and
external diameters so that the folded retaining arches of the stent to be
accommodated in the
catheter tip 20 with the heart valve prosthesis attached to it can be
accommodated in the sleeve-
shaped first housing portion 23 and can be held in their folded or minimised
mode. At the same
time, the folded positioning arches of the stent are accommodated between the
sleeve-shaped first
housing portion 23 and the sleeve-shaped second housing portion 24 of the
catheter tip 20 and
held in their folded mode.
In the first functional mode of the insertion system 1, the catheter tip 20 is
inserted in the patient's
body and guided to the desired implantation site. In the case of the insertion
system 1 based on the
first embodiment of the solution proposed by the invention, illustrated in
Fig. 15a, the implantation
site, i.e. the incumbent heart valve, can be accessed transapically, in other
words from the heart
apex, because at the proximal region of the catheter tip 20 the retaining
mechanism is provided in
the form of the crown 21, whilst the first housing portion 23 of the catheter
tip 20 is disposed dis-
tally with respect to it.
Fig. 15b shows the insertion system 1 illustrated in Fig. 15a in its second
functional mode. This
second functional mode is assumed immediately the catheter tip 20 of the
insertion system 1 has
reached the implantation site in the patient's body. As will be explained in
more detail with refer-
ence to Figs. 15b to 15d, once the catheter tip 20 has reached the
implantation site, the requisite
manipulations of the individual housing portions 23, 24 of the catheter tip
which are needed to re-
lease the stem accommodated in the catheter tip 20 in a predefined sequence of
events in steps are
effected so that the different functional components of the stent, in
particular the positioning
arches and the retaining arches of the stent, are released in sequence.
Stepped release of the endo-
prosthesis accommodated in the catheter tip 20 of the insertion system 1 by
specific movements of
the individual housing portions 23 and 24 of the catheter tip 20 will be
explained in detail below.
Once the catheter tip 20 has reached the implantation site, the insertion
system 1 is switched from
the first functional mode illustrated in Fig. 15a to the second functional
mode illustrated in Fig. 15b
by operating the second operating means 34. Specifically, the second locking
element 42 is re-
,
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
47
moved from the second operating means 34, as a result of which the
longitudinal movement of the
slide 31" and the carriage 31 of the second operating means 34 is no longer
blocked.
After removing the second locking element 42 from the second operating means
34 and after re-
leasing the lock of the slide 31" and the carriage 31 of the second operating
means 34, the slide 31"
and carriage 31 of the second operating means 34 are moved along the guide 31'
in the direction of
the catheter tip 20 from the first position (Pos. 1) to the second position
(Pos. 2). The second posi-
tion (Pos. 2) is determined by the fifth stop 30 disposed between the third
stop 37 (Pos. 1) and the
fourth stop 38.
By operating the second operating means 34 in this way, the second housing
portion 24 of the
catheter tip 20 co-operating with the second operating means 34 is moved in
the proximal direction
relative to the retaining mechanism 21 of the catheter tip 20. The amount of
movement, i.e. the
extent of the longitudinal movement of the second housing portion 24 of the
catheter tip 20 rela-
tive to the fixing mechanism 21 of the catheter tip 20 in the proximal
direction in this instance, is
fixed by the stroke length of the longitudinal movement which can be effected
between the first
position (Pos. 1) and the second position (Pos. 2) effected by the slide 31"
and the carriage 31 us-
ing the second operating means 34.
The resultant movement of the second housing portion 24 of the catheter tip 20
relative to the fix-
ing mechanism 21 causes release of the telescopic engagement between the two
sleeve-shaped first
and second housing portions 23 and 24. The extent of the movement of the
second housing por-
tion 24 relative to the fixing mechanism 21 or relative to the first housing
portion 23, and hence
the stroke of the longitudinal movement which can be effected by the slide 31"
and carriage 31 us-
ing the second operating means 34, is selected so that the sleeve-shaped
second housing portion 24
of the catheter tip 20 no longer surrounds the first housing portion 23 of the
catheter tip 20 tele-
scopically but still covers the retaining mechanism 21 or crown 21a, in
particular the pockets 22
formed in the crown 21a. As a result, in the second functional mode of the
insertion system 1, as
illustrated in Fig. 17b, the distal retaining region of the heart valve stent
accommodated in the
catheter tip 20 is held fixed by the catheter tip 20, in particular by the
retaining mechanism 21. This
is achieved because the retaining heads, etc., provided at the distal end of
the stent are engaged
with the pockets 22 formed in the crown 21a of the retaining mechanism 21.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
48
As will be explained in more detail below with reference to Fig. 17, when the
insertion system 1 is
in the second functional mode illustrated in Fig. 15b, the retaining arches of
the stem are still held
in their minimised mode by the first housing portion 23 of the catheter tip
20. This is because these
components of the stent are accommodated in the first housing portion 23 of
the catheter tip 20.
Engagement between the retaining heads at the distal end of the stent and the
pockets 22 formed
in the crown 21a of the retaining mechanism 21 is secured by means of the
distal end of the second
housing portion 24 so that the distal retaining region of the stent is also
still held in its minimised
mode by the second housing portion 24. As already explained, this is made
possible because the
distal end of the second housing portion 24 is still covering the crown 21a
and the retaining
mechanism 21 with the pockets 22 formed therein and the retaining heads of the
stent accommo-
dated in the pockets 22.
By manipulating the second operating means 34, the second housing portion 24
of the catheter tip
20 is moved away from the handle 30 in the proximal direction relative to the
retaining mechanism
21 and to the first housing portion 23 of the catheter tip 20 so that the
positioning arches of the
stem are no longer covered by the second housing portion 24. In other words,
due to the longitu-
dinal movement of the second housing portion 24, when the insertion system 1
is in the second
functional mode, the telescopic hold of the positioning arches of the stent
between the first and the
second housing portions 23 and 24 of the catheter tip 20 achieved in the first
functional mode (see.
Fig. 15a) is released. When the insertion system 1 is in the second functional
mode (see. Fig. 15b),
the second housing portion 24 of the catheter tip 20 no longer assumes the
function of holding the
positioning arches of the stent in their minimised mode so they are released
and are able to unfold
accordingly. As illustrated in detail in Fig. 17, the positioning arches of
the stent open up once they
have been released because of the inherent radial forces in the stent
structure. These opened posi-
tioning arches can then be positioned in the pockets of the incumbent heart
valve.
Once the positioning arches of the stent have been positioned in the pockets
of the incumbent
heart valve, the insertion system 1 is transferred from the second functional
mode illustrated in Fig.
151) to the third functional mode illustrated in Fig. 15c. This is achieved by
removing the first lock-
ing element 41 co-operating with the first operating means 33 of the handle 30
and thus releasing
the lock, allowing the slide 32" and the carriage 32 of the first operating
means 33 to effect a longi-
tudinal movement.
CA 02822636 2013-07-31
W02008/125153 PCT/EP2007/061117
49
Once the first locking element 41 has been removed, the slide 32" and carriage
32 of the first oper-
ating means 33 are moved along the guide 32' from the first position (Pos. 1)
into the second posi-
tion (Pos. 2). The stroke of the longitudinal movement is fixed by the second
stop 36 of the first
operating means 33 disposed at the distal end of the guide 32' of the first
operating means 33.
By manipulating the first operating means 33, the first housing portion 23 of
the catheter tip 20 co-
operating with the first operating means 33 is moved by the stroke of the
longitudinal movement.
This is effected by the slide 32" and carriage 32 of the first operating means
33 relative to the re-
taining mechanism 21 of the catheter tip 20 and to the second housing portion
24 in the distal di-
rection towards the handle 30. With this movement of the first housing portion
23 of the catheter
tip 20 relative to the retaining mechanism and crown 21a, the first housing
portion 23 is no longer
covering the proximal anchoring region of the stent and therefore releases the
retaining arches of
the stent, together with the heart valve prosthesis attached to it. This is
due to an expedient selec-
tion of the stroke of the longitudinal movement which can be achieved by the
first operating
means 33. The release of the proximal anchoring region of the stent causes the
proximal anchoring
region of the stent to unfold completely by virtue of the radial forces acting
on it.
When the insertion system 1 is in the third functional mode, as illustrated in
Fig. 15c, the distal end
of the second housing portion 24 of the catheter tip 20 is still covering the
retaining mechanism 21
or the crown 21a. The engagement of the stent retaining heads in pockets 22
formed in the crown
21a continues to exist so that the stent remains actively connected to the
catheter system 10 of the
insertion system 1. In spite of the fact that its proximal anchoring region
has unfolded, the stern
with the heart valve prosthesis attached to it can still be retracted back
into the catheter and ex-
planted. Explantation takes place in a corresponding sequence but in reverse,
whereby the insertion
system 1 is firstly switched from the third functional mode to the second
functional mode and then
to the first functional mode.
Once the proximal anchoring region of the stem has been fully released and
after checking that the
unfolded heart valve prosthesis is functioning correctly, the stent can be
released from the catheter
tip. This is achieved by switching the insertion system 1 from its third
functional mode illustrated
in Fig. 15c to the fourth functional mode illustrated in Fig. 15d. If any
abnormalities are found dur-
ing the check the stent may be explanted as described above.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
In the fourth functional mode, the catch element or locking element 44
provided between the third
stop 37 and fourth stop 38 on the guide 31' of the second operating means 34,
which defines the
fifth stop 39 in the second functional mode illustrated in Fig. 14b, has been
removed. As a result,
the slide 31" and carriage 31 of the second operating means 34 can be moved
farther in the direc-
tion of the catheter tip 20 of the insertion system 1 on the guide 31', from
the second position
(Pos. 2) into the third position (Pos. 3). This third position (Pos. 3) is
defined by the fourth stop 38
at the proximal end of the guide 31' of the second operating means 34.
Accordingly, a predefined
(additional) movement of the carriage 31 of the second operating means 34 is
effected. As a result,
the second housing portion 24 of the catheter tip 20 co-operating with the
second operating means
34 is moved relative to the retaining mechanism 21 further in the proximal
direction, away from
the handle 30, due to the stroke of the longitudinal movement caused by the
additional manipula-
tion of the second operating means 34.
The stroke of the longitudinal movement caused by the additional manipulation
of the second op-
erating means 34 is selected so that when the second housing portion 24 of the
catheter tip 20 is
moved relative to the retaining mechanism 21, at least the pockets 22 formed
in the crown 21a of
the retaining mechanism 21 are no longer covered by the distal end of the
second housing portion
24. This uncovering of the pockets 22 of the retaining mechanism 21 by the
second housing por-
tion 24 causes release of the engagement between the retaining heads provided
at the distal end of
the stent and the pockets 22 of the retaining mechanism 21. This, in turn,
causes the distal retaining
region of the stent to be released completely and thus leads to a complete
unfolding of the stent.
Figs. 14a to 14d illustrate the insertion system 1 in its four different
functional modes described
with reference to Figs. 15a to 15d. However, this time, the diagrams start
with the fourth functional
mode (Fig. 14a) and then show the third functional mode (Fig. 14b), the second
functional mode
(Fig. 14c), ending with the first functional mode (Fig. 14d). The sequence
illustrated in Fig. 14 is
used to load a stent, such as that illustrated in Fig. 17 for example, into
the catheter tip 20 of the
insertion system 1. The loading procedure, in the steps illustrated in Figs.
14a to 14d, corresponds
to the sequence of the procedure illustrated in Figs. 15a to 15d but in
reverse and is used to remove
a stent accommodated in the catheter tip 20 of the insertion system I. To
avoid repetition, refer-
ence may be made to the explanations given with respect to Figs. 15a to 15d.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
51
A preferred embodiment of the retaining mechanism 21 disposed in the catheter
tip 20 of the in-
sertion system proposed by the invention will be described in detail below
with reference to Fig.
16.
Fig. 16a is a side view showing a preferred embodiment of the retaining
mechanism 21. Fig. 16b is
a view in cross-section along line A-A indicated in Fig. 16a, illustrating the
embodiment of the re-
taining mechanism 21, whilst Fig. 16c shows a plan view of the distal
retaining region 52 of a stent
50 which can be retained in the catheter tip 20 of the insertion system
proposed by the invention
by means of the retaining mechanism 21 based on the embodiment illustrated in
Fig. 16a.
As illustrated, the retaining mechanism 21 has an essentially cylindrical body
21a, the axis of sym-
metry of which lies on the longitudinal axis L of the catheter tip 20. Several
cut-outs or pockets 22
- in Fig. 16b three in total - are spaced uniformly apart from one another in
the material of the re-
taining mechanism body 21a, preferably at the proximal end portion of the
cylindrical body 21a.
These pockets 22 are connected to the proximal-end surface of the cylindrical
body 21a by grooves
21b.
The shape and size of the pockets 22a in the material of the body or crown 21a
of the retaining
mechanism 21 are selected so that a retaining element 55 of the stent 50
complementing the pocket
22a can be accommodated, preferably positively, in each of the pockets 22a.
Thus, each retaining
element 55 of the stern 50 establishes a releasable engagement with a pocket
22a formed in the
crown 21a of the retaining mechanism 21.
As illustrated in Fig. 16c, it is preferable in this respect if the retaining
elements 55 of the stent 50
are provided in the form of projecting elements or projecting heads (retaining
heads) at the distal
end 52 of the stent 50. These retaining elements 55 of the stent 50 in the
form of projecting ele-
ments are each connected to the positioning arches 54 (and retaining arches
53) of the stent 50 via
a neck portion or connecting web 56. When the retaining elements 55 of the
stent 50 are positively
held in the pockets 22a of the retaining mechanism 21, at least the distal
ends of the neck portions
56 he in the grooves 22b.
Referring to Fig. 15b, the crown 21a of the retaining mechanism 21 is
cylindrical, wherein each of
the pockets 22a formed in the crown 21a of the retaining mechanism 21 has a
shape adapted for
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
52
substantially accommodating the retaining element 55 provided on the distal
region 52 of the stent
50 such that there are no parts of the distal region 52 of the stent 50
protruding from the superfi-
cial surface S of the cylindrical crown 21a.
In addition, the crown 21a of the illustrated retaining mechanism 21 comprises
snap-on means 21b
arranged on the at least one pocket 22a formed in the crown 21a of the
retaining mechanism 21 for
releasable fixing the retaining element 55 provided on the distal region 52 of
the stent 50 in the at
least one pocket 22a.
Turning now to Figs. 17a to 17d, a preferred embodiment of the medical device
100 proposed by
the invention will now be described. As illustrated, the medical device 100
has an insertion system
1 designed for transapical access, such as that described in detail above with
reference to Figs. 14
and 15. Naturally, however, it would be conceivable for the medical device to
be used with an in-
sertion system designed for transarterial access.
In addition to the insertion system 1, the medical device 100 has a self-
expandable heart valve stent
50 accommodated in the catheter tip 20 of the insertion system 1, to which a
heart valve prosthesis
to be implanted is attached, although this is not explicitly illustrated in
Fig. 17. In the first func-
tional mode of the insertion system 1 illustrated in Fig. 17a, the stent 50
has a first pre-definable
mode in which it is in a minimised mode. In the implanted mode, on the other
hand, the stent 50 is
designed to assume a second pre-definable mode in which it is in its expanded
configuration.
By using the insertion system 1 described above, the stent 30 is transferred
from its first predefined
mode into its second predefined mode sequentially on the basis of a pre-
definable sequence of
events, in steps, during the implantation process.
Specifically, the stent 50 used with the medical device 100 illustrated in
Fig. 16 has a proximal an-
choring region 51 to which a heart valve prosthesis is attachable. The stent
50 also has a distal re-
taining region 52 with three retaining elements 55 in the form of retaining
heads which can be
moved into a releasable engagement with the retaining mechanism 21 of the
insertion system 1 and
in particular with the pockets 22a formed in the crown 21a of the retaining
mechanism 21 of the
insertion system 1.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
53
In addition to the proximal anchoring and distal retaining regions 51, 52, the
stent 50 also has three
first functional components 53 in the form of retaining arches for
accommodating the heart valve
prosthesis and three second functional components 54 in the form of
positioning arches for auto-
matically positioning the stent 50 at the implantation site. The respective
positioning arches 54 of
the stent 50 are of a functional and structural design such that during the
implantation operation
and when the stent 50 is in the implanted mode, especially from the point when
the insertion sys-
tem 1 is in the second functional mode, they engage in the pockets of the
incumbent heart valve.
This being the case, each positioning arch 54 of the stent 50 co-operates with
a retaining arch 53,
and at the distal region 52 of the stent 50, each end portion 57 of the
respective positioning arches
54 is joined to the end portion 58 of the co-operating retaining arch 53.
Specifically, every positioning arch 54 and its co-operating retaining arch 53
of the stent 50 is re-
spectively of an essentially U-shaped or V-shaped structure, which is closed
towards the proximal
end 51 of the stunt 50.
The procedure involved in implanting the stent 50 will now be described in
detail with reference to
Figs. 17a to 17d. Specifically, Fig. 17a illustrates the proximal end of a
catheter system 10 with the
catheter tip 20 and the stunt 50 accommodated in the cathcter tip 20 with the
insertion system 1 in
the first functional mode. As already described in connection with Fig. 15a,
when the insertion sys-
tem 1 illustrated in Fig. 17a is in its first functional mode in which the
retaining heads 55 of the
stent 50 are engaged with the pockets 22a formed in the crown 21a of the
retaining mechanism 21
of the catheter tip 20 of the insertion system 1, the retaining arches 53 of
the stent with the heart
valve prosthesis attached to it are accommodated in the sleeve-shaped first
housing portion 23 of
the catheter tip 20. When the insertion system 1 is in the first functional
mode, the positioning
arches 54 of the stent 50 lie between the sleeve-shaped first housing portion
23 and the likewise
sleeve-shaped second housing portion 24 of the catheter tip 20. The two
housing portions 23 and
24 of the catheter tip 20 are of a mutually telescopic design. Specifically,
the sleeve-shaped second
housing portion 24 of the catheter tip 20 covers the distal retaining region
52 of the stent 50, the
positioning arches 54 of the stent 50 and the sleeve-type first housing
portion 23 of the catheter tip
20, in which the proximal anchoring region 51 of the stent 50 with the
retaining arches 53 and the
heart valve prosthesis 60 (not illustrated in Fig. 17) are accommodated.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
54
As explained above, the material used for the stem is a shape memory material
and the shape
memory effect and hence the open shape of the stent 50 is triggered by the
effect of an external
stimulus. By particular preference, this external stimulus is a pre-settable
switching temperature
which means that the stent material has to be heated to a temperature that is
higher than the
switching temperature to trigger the shape memory effect and thus restore the
memorised open
shape of the stent 50. In view of the application for which the medical device
100 is used, it is pref-
erable if the switching temperature is in the range of room temperature and
the patient's body
temperature. Accordingly, care must be taken when implanting the stent 50 that
the stent 50 is ap-
propriately cooled, for example by means of a syringe adapter 43 provided in
the handle 30,
thereby enabling the catheter system 10 and the catheter tip 20 of the
insertion system 1 to be
rinsed with an appropriate coolant, such as a salt solution.
In the mode illustrated in Fig. 17a, the catheter tip 20 is fed forwards
transapically, i.e. from the
heart apex, to the diseased or failing native heart valve.
When the catheter tip 20 with the stent 50 accommodated in the catheter tip 20
has been moved
forward to the desired implantation site, cooling is interrupted, as a result
of which the stent 50 is
heated to the patient's body temperature (36 C), thereby triggering the shape
memory effect of the
stein material.
Due to the self-expanding property of the stent 50 triggered as a result,
radial forces build up which
act on the individual components of the stent 50 and, in particular, on the
respective positioning
arches 54 and retaining arches 53 of the stent. Since the retaining arches 53
of the stent 50 are still
accommodated in the sleeve-type first housing portion 23 of the catheter tip
20 as before, the re-
taining arches 53 of the stent 50 and the proximal anchoring region 51 of the
stent 50 are held in
the minimised mode, in spite of the fact that the shape memory effect has been
triggered. The po-
sitioning arches 54 of the stent 50 and the distal retaining region 52 of the
stent 50 are therefore
forcibly retained in their folded mode by the sleeve-shaped second housing
portion 24.
On reaching the implantation site, the positioning arches 54 of the stent 50
are then released due to
an appropriate stepped release of the stein 50 from the insertion system 1.
This is done by transfer-
ring the insertion system 1 from its first functional mode (see Fig. 15a) to
its second functional
mode (see Fig. 15b) as described in detail above with reference to Fig. 15a
and Fig. 15b, for exam-
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
pie and individually illustrated in Fig. 17b. By manipulating the second
operating means 34 of the
handle 30 used with the insertion system 1, the second housing portion 24 of
the catheter tip 20 is
moved relative to the retaining mechanism 21 and to the distal retaining
region 52 of the stent 50 in
the proximal direction, in other words away from the handle. The stroke of the
longitudinal
movement of the sleeve-type second housing portion 24 effected as a result
relative to the retaining
mechanism 21 of the catheter tip 20 leads to a situation in which the
positioning arches 54 of the
stent 50 are no longer surrounded by the sleeve-shaped second housing portion
24 of the catheter
tip 20. As a result, the self-expanding property of the positioning arches 54
of the stent 50 opens
them due to the radial forces acting in the radial direction. The opened
positioning arches 54 of the
stent 50 are then positioned in the pockets of the incumbent heart valve. As
explained above, the
catheter tip 20 of the insertion system 1 can rotate about the longitudinal
axis L of the catheter tip
20, which makes positioning of the unfolded positioning arches 54 of the stent
50 in the pockets 70
of the native heart valve easier.
Once the partially expanded stent 50 has been positioned in the pockets of the
incumbent heart
valve, the insertion system 1 is switched from the second functional mode
illustrated in Fig. 17b to
the third functional mode illustrated in Fig. 17c. The way in which the
insertion system 1 is
switched from the second functional mode to the third functional mode was
explained in detail
above with reference to Fig. 15c. Fig. 17c illustrates how the proximal
anchoring region 51 of the
stent 50 is released from the first housing portion 23 of the catheter tip 20
when the insertion sys-
tem 1 is in the third functional mode. The released retaining arches 53 of the
stent 50 and the
proximal anchoring region 51 of the stent 50, released when the insertion
system 1 is in the third
functional mode, open due to the radial forces acting in a radial direction
and unfold the heart
valve prosthesis attached to the retaining arches 53 in the manner of an
umbrella.
In the mode illustrated in Fig. 17c, a check may be made to ensure that the
already unfolded heart
valve prosthesis is functioning correctly. Once the functioning of the heart
valve prosthesis 60 has
been checked, the insertion system 1 can then be switched from its third
functional mode (see Fig.
17c) to its fourth functional mode (see Fig. 17d) by another manipulation of
the second operating
means 34 of the handle 30 of the insertion system 1. The way in which the
insertion system 1 is
switched to the fourth functional mode was described above with reference to
Fig. 15d. Fig. 17d
illustrates the effect that switching of the insertion system 1 to the fourth
functional mode has on
the stela 50.
CA 02822636 2013-07-31
W02008/125153 PCT/EP2007/061117
56
When the second housing portion 24 of the catheter tip 20 is moved further in
the proximal direc-
tion, in other words away from the handle 30, the distal end portion of the
sleeve-type second
housing portion 24 of the catheter tip 20 is moved further in the proximal
direction so that this
distal part of the second housing portion 24 is no longer covering the pockets
22a formed in the
crown 21a of the retaining mechanism 21. Accordingly, the hold on the distal
retaining region 52 of
the stent 50 by the catheter tip 20 is released so that the distal retaining
region 52 of the stent 50
also expands in the radial direction, thereby causing the stent 50 to unfold
completely.
If, on checking the functioning of the already unfolded heart valve prosthesis
in the third func-
tional mode of the insertion system 1, it is ascertained that the implanted
heart valve prosthesis is
not able to fulfil its function or is not able to do so satisfactorily, or if
the stent 50 has not been or
can not be optimally positioned at the implantation site, it is possible to
switch the insertion system
1 back to the second and then the first functional mode again by moving the co-
operating housing
portions 23, 24 of the catheter tip 20 in the opposite direction. As a result,
the already released and
expanded components of the stent 50 can be moved back into the respective
housing portions 23,
24 of the catheter tip 20 so that the catheter tip 20 and the stent 50,
accommodated in the catheter
tip 20 again, can bc removed from the patient's body. By returning the stem
back into the catheter,
damage to vascular system on removal of the stent from the body is minimised.
As illustrated in Fig. 17d, when the stent 50 has been implanted, the
retaining arches 53 of the stent
open out in the radial direction, during which the radial forces acting on the
retaining arches 53 and
also on the distal retaining region 52 of the stent 50 cause the stent 50 to
be pressed in a radial di-
rection towards the vessel wall. This provides a reliable anchoring of the
stent 50 with the heart
valve prosthesis attached to it at the proximal anchoring region 51 at the
implantation site and sub-
stantially guarantees a reliable seal of the heart valve prosthesis at the
proximal anchoring region 51
of the stent 50.
When the heart valve prosthesis is in the implanted mode, the incumbent heart
valve is pressed
towards the vessel wall due to the self-expanding property of the stent 50.
Specifically, the pocket
flaps of the insufficient or stenosed heart valve are clamped between the
positioning arches 54 and
the retaining arches 53 due to the expansion of the stent 50. This permits
optimal positioning and
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
57
stable anchoring of the heart valve prosthesis disposed on the proximal
anchoring region 51 of the
stent 50.
The above-discussed stent designs, which constitute the basis of the medical
device 100 in conjunc-
tion with the insertion system 1, are particularly suitable for insertion in a
patient's body with
minimal invasion using the insertion system 1.
The solution proposed by the invention is distinctive due to an improved
insertion system with a
stent which can be accommodated in the catheter tip of the insertion system.
The stent may be in-
serted from a transarterial or transapical approach by means of the special
insertion system and op-
timally positioned so that the heart valve prosthesis, stitched to the
proximal anchoring region of
the stent, can assume the function of the insufficient, narrowed or calcified
native heart valve. The
radial forces which build up due to the self-expanding property of the stent
substantially guarantee
a reliable anchoring in the region of the aorta. The catheter system of the
insertion system is pref-
erably an 18 to 21F insertion unit, which is compatible with 21F insertion
gates and a 0.035"-guide
wire. The length of the catheter system should be at least 100 cm in the case
of the insertion system
designed for transarterial access. The deflecting mechanism which may
optionally be provided in
the proximal region of the gate system 13 is preferably approximately 30 cm.
The solution proposed by the invention is based on a metal endoprosthesis 1
with a heart valve
prosthesis which can be stitched to it or is stitched to it, designed for use
in treating diseases of the
heart valve which make replacement of the incumbent heart valve necessary. The
heart valve stent
(endoprosthesis) may be introduced in the inverted position and thus
positioned orthotopically in
vivo percutaneously and assumes the function of the insufficient or defective
native heart valve. The
radial forces created due to the self-expanding property of the endoprosthesis
1 substantially guar-
antee reliable anchoring in the region of the aorta.
A medical instrument comprising an endoprosthesis 1 for positioning and
securing a heart valve
prosthesis in the aorta of the patient is described, together with an
endoprosthesis 1 made from a
base of Nitinol as a means of accommodating a heart valve prosthesis for
implantation in the body,
particularly in the aorta at the site of an aortic heart valve that requires
replacement. The ready-to-
use medical device proposed by the invention consists of the components
comprising the self-
,
CA 02822636 2013-07-31
=
WO 2008/125153 PCT/EP2007/061117
58
expandable Nitinol stent 1 with the valve-supporting segment 20, valve and
system for introducing
it to the desired site in the body.
In terms of design, the endoprosthesis 1 has three positioning arches for
positioning and fixing the
medical device in the vessel of the patient and retaining webs for
accommodating/attaching the
heart valve prosthesis by means of a thread, for example. From a functional
point of view, the
endoprosthesis 1 exerts high radial forces in its second expanded mode to
ensure that the medical
device is anchored in the aorta. Eyes 30 are preferably provided on the distal
retaining region of the
endoprosthesis 1 or medical device, which can be releasably engaged with
corresponding compo-
nents of an introduction catheter system.
The material used to trigger the shape memory effect of the endoprosthesis has
a switching tem-
perature between 20 C and 36 C and is preferably 22 C. In the cooled state,
therefore, the medical
device can be introduced into the patient's body by means of a 21F
introduction system.
As regards the exact dimensions of the endoprosthesis 1, it is designed to
accommodate heart valve
prostheses with a valve diameter of 21 mm to 25 mm, in which case the distal
retaining region 2 of
the endoprosthesis 1 in particular has a diameter that is approximately 10% to
15% bigger than this
in order to ensure that the medical device is reliably anchored.
The medical device proposed by the invention has an endoprosthesis which is
readily visible by X-
ray, which can be achieved by applying markers at the proximal and/or distal
region of the endo-
prosthesis if necessary.
The materials used for the endoprosthesis 1 are materials that have been tried
and tested for im-
plantation purposes, for example Nitinol and Tantal. As regards the dimensions
of the endopros-
thesis, two different stent sizes are currently preferred, which are set out
in the table below together
with the diameter of the proximal anchoring region and the distal retaining
region.
CA 02822636 2013-07-31
WO 2008/125153 PCT/EP2007/061117
59
Stent size Diameter of the proximal an- Diameter
of the distal retain-
choring region ing region
Stent No. 1 21 to 25 mm 32 to 34 mm
Stem No. 2 26 to 31 mm 35 to 38 mm
By applying an appropriate finishing treatment, in particular tempering, other
dimensions of the
stent can be achieved - starting from the two currently preferred stent sizes.
The invention is not restricted to the features described in connection with
the preferred embodi-
ments illustrated in the drawings. All combinations of the features described
in the specification
would be conceivable.