Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02830870 2013-10-21
STENT GRAFT SEALING ZONE CONNECTING STRUCTURE
REFERENCE TO RELATED APPLICATION
This application is a divisional of co-pending Canadian Patent Application No.
2,596,203 filed August 7, 2007.
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
[0001] This invention relates broadly to surgical implants. More
particularly, this
invention relates to connecting structure at the sealing zone for multi-part
stents
particularly useful in synthetic grafts, although it is not limited thereto.
STATE OF THE ART
[0002] An aneurysm is an abnormal dilation of a layer or layers of an
arterial wall,
usually caused by a systemic collagen or structural defect. An abdominal
aortic
aneurysm (AAA) is an aneurysm in the abdominal portion of the aorta, usually
located in
or near one or both of the two iliac arteries or near the renal arteries. The
aneurysm often
arises in the infrarenal portion of the diseased aorta, for example, below the
kidneys. A
thoracic aortic aneurysm is an aneurysm in the thoracic portion of the aorta.
When left
untreated, the aneurysm may rupture, usually causing rapid fatal hemorrhaging.
[0003] Aneurysms may be classified or typed by their position as well as by
the
number of aneurysms in a cluster. Typically, abdominal aortic aneurysms may be
classified into five types. A Type I aneurysm is a single dilation located
between the
renal arteries and the iliac arteries. Typically, in a Type I aneurysm, the
aorta is healthy
between the renal arteries and the aneurysm and between the aneurysm and the
iliac
CA 02830870 2013-10-21
arteries.
[0004] A Type II A aneurysm is a single dilation located between the renal
arteries
and the iliac arteries. In a Type II A aneurysm, the aorta is healthy between
the renal
arteries and the aneurysm, but not healthy between the aneurysm and the iliac
arteries. In
other words, the dilation extends to the aortic bifurcation. A Type II B
aneurysm
comprises three dilations. One dilation is located between the renal arteries
and the iliac
arteries. Like a Type II A aneurysm, the aorta is healthy between the aneurysm
and the
renal arteries, but not healthy between the aneurysm and the iliac arteries.
The other two
dilations are located in the iliac arteries between the aortic bifurcation and
the
bifurcations between the external iliacs and the internal iliacs. The iliac
arteries are
healthy between the iliac bifurcation and the aneurysms. A Type II C aneurysm
also
comprises three dilations. However, in a Type II C aneurysm, the dilations in
the iliac
arteries extend to the iliac bifurcation.
[0005] A Type III aneurysm is a single dilation located between the renal
arteries and
the iliac arteries. In a Type III aneurysm, the aorta is not healthy between
the renal
arteries and the aneurysm. In other words, the dilation extends to the renal
arteries.
[0006] A ruptured abdominal aortic aneurysm is presently the thirteenth
leading
cause of death in the United States. The routine management of abdominal
aortic
aneurysms has been surgical bypass, with the placement of a graft in the
involved or
dilated segment. Although resection with a synthetic graft via a
transperitoneal or
retroperitoneal procedure has been the standard treatment, it is associated
with significant
risk. For example, complications include perioperative myocardial ischemia,
renal
2
CA 02830870 2013-10-21
failure, erectile impotence, intestinal ischemia, infection, lower limb
ischemia, spinal
cord injury with paralysis, aorta-enteric fistula, and death. Surgical
treatment of
abdominal aortic aneurysms is associated with an overall mortality rate of
five percent in
asymptomatic patients, sixteen to nineteen percent in symptomatic patients,
and is as high
as fifty percent in patients with ruptured abdominal aortic aneurysms.
[0007] Disadvantages associated with conventional surgery, in addition to
the high
mortality-rate, include an extended recovery period associated with the large
surgical
incision and the opening of the abdominal cavity, difficulties in suturing the
graft to the
aorta, the loss of the existing thrombosis to support and reinforce the graft,
the
unsuitability of the surgery for many patients having abdominal aortic
aneurysms, and the
problems associated with performing the surgery on an emergency basis after
the
aneurysm has ruptured. Further, the typical recovery period is from one to two
weeks in
'the hospital and a convalescence period, at home, ranging from two to three
months or
more, if complications ensue. Since many patients having abdominal aortic
aneurysms
have other chronic illnesses, such as heart, lung, liver and/or kidney
disease, coupled with
the fact that many of these patients are older, they are less than ideal
candidates for
surgery.
[0008] The occurrence of aneurysms is not confined to the abdominal region.
While
abdominal aortic aneurysms are generally the most common, aneurysms in other
regions
of the aorta or one of its branches are possible. For example, aneurysms may
occur in the
thoracic aorta. As is the case with abdominal aortic aneurysms, the widely
accepted
approach to treating an aneurysm in the thoracic aorta is surgical repair,
involving
3
CA 02830870 2013-10-21
=
replacing the aneurysmal segment with a prosthetic device. This surgery, as
described
above, is a major undertaking, with associated high risks and with significant
mortality
and morbidity.
[0009] Over the past five years, there has been a great deal of research
directed at
developing less invasive, endovascular, i.e., catheter directed, techniques
for the
treatment of aneurysms, specifically abdominal aortic aneurysms. This has been
facilitated by the development of vascular stents, which can and have been
used in
conjunction with standard or thin-wall graft material in order to create a
stent-graft or
endograft. The potential advantages of less invasive treatments have included
reduced
surgical morbidity and mortality along with shorter hospital and intensive
care unit stays.
[0010] Stent-gafts or endoprostheses are now Food and Drug Administration
(FDA)
approved and commercially available. Their delivery procedure typically
involves
advanced angiographic techniques performed through vascular accesses gained
via
surgical cut down of a remote artery, which may include the common femoral or
brachial
arteries. Over a guidewire, the appropriate size introducer will be placed.
The catheter
and guidewire are passed through the aneurysm. Through the introducer, the
stent-graft
will be advanced to the appropriate position. Typical deployment of the stent-
graft
device requires withdrawal of an outer sheath while maintaining the position
of the stent-
gaft with an inner-stabilizing device. Most stent-grafts are self-expanding;
however, an
additional angioplasty procedure, e.g., balloon angioplasty, may be required
to secure the
position of the stent-graft. Following the placement of the stent-graft,
standard
angiographic views may be obtained.
4
CA 02830870 2013-10-21
[00111 Due to the large diameter of the above-described devices, typically
greater
than twenty French (3F=1 mm), arteriotomy closure typically requires open
surgical
repair. Some procedures may require additional surgical techniques, such as
hypogastric
artery embolization, vessel ligation, or surgical bypass in order to
adequately treat the
aneurysm or to maintain blood flow to both lower extremities. Likewise, some
procedures will require additional advanced catheter directed techniques, such
as
angioplasty, stent placement and embolization, in order to successfully
exclude the
aneurysm and efficiently manage leaks.
[0012] While the above-described endoprostheses represent a significant
improvement over conventional surgical techniques, there is a need to improve
the
endoprostheses, their method of use and their applicability to varied
biological
conditions. Accordingly, in order to provide a safe and effective alternate
means for
treating aneurysms, including abdominal aortic aneurysms and thoracic aortic
aneurysms,
a number of difficulties associated with currently known endoprostheses and
their
delivery systems must be overcome. One concern with the use of endoprostheses
is the
prevention of endo-leaks and the disruption of the normal fluid dynamics of
the
vasculature. Devices using any technology should preferably be simple to
position and
reposition as necessary, should preferably provide an acute, fluid tight seal,
and should
preferably be anchored to prevent migration without interfering with normal
blood flow
in both the aneurysmal vessel as well as branching vessels. In addition,
devices using the
technology should preferably be able to be anchored, sealed, and maintained in
bifurcated
vessels, tortuous vessels, highly angulated vessels, partially diseased
vessels, calcified
vessels, odd shaped vessels, short vessels, and long vessels. In order to
accomplish this,
CA 02830870 2013-10-21
the endoprostheses should preferably be highly durable, extendable and re-
configurable
while maintaining acute and long-term fluid tight seals and anchoring
positions.
[0013] The endoprostheses should also preferably be able to be delivered
percutaneously utilizing catheters, guidewires and other devices which
substantially
eliminate the need for open surgical intervention. Accordingly, the diameter
of the
endoprostheses in the catheter is an important factor. This is especially true
for
aneurysms in the larger vessels, such as the thoracic aorta. In addition, the
endoprostheses should preferably be percutaneously delivered and deployed such
that
surgical cut down is unnecessary.
[0014] Referring to Fig. 1, a typical percutaneously delivered
endoprosthesis 10 for
treating an abdominal aortic aneurism is a bifurcated device having a main
body 12 and
two legs 14, 16. Ideally, the device lines the aorta and extends from just
below the lowest
renal artery into both iliac arteries up to the juncture with the hypogastric
arteries. The
endoprosthesis 10 is generally comprised of a fabric graft material 18 coupled
to several
metallic stents. The stents support the graft and hold it open within the
vessels. The
main body 12 may include an upper supra renal stent 20 extending above the
graft
material 18 and typically provided with tissue anchors 20a, one or more
sealing stents 22,
24 generally extending circumferentially in a Z-shape and providing outward
force
against the graft to seal between the graft and the body tissue, and a tapered
stent 26 that
leads into the legs 14, 16.
[0015] The endoprosthesis is delivered to the aneurysm in the aorta by way of
a
delivery catheter. The delivery catheter containing the endoprosthesis is
inserted through
6
CA 02830870 2013-10-21
a small incision in the groin where it is threaded through the femoral artery
and advanced
to the location of the aneurysm. The surgeon uses fluoroscopy to guide the
endoprosthesis and the endoprosthesis has several markers to help the surgeon
visualize
the graft during placement of the endoprosthesis. It is desirable that there
be at least ten
mm of overlap between the endoprosthesis and a healthy vessel portion.
Otherwise, an
opening between the two can develop that can lead to leakage.
[0016]
Referring again to Fig. 1, the minimum sealing length 28, defined between the
top 30 of the graft material 18 and the bottom 32 of the first sealing stent
22, is a partial
determinative of how effective the endoprosthesis 10 will be in preventing or
at least
limiting leakage. The minimum sealing length is the length required to achieve
full
circumferential apposition of a portion of the stent against the vessel wall.
While a
longer sealing length is advantageous, the seal length must also be short
enough to allow
the endoprosthesis to fit the anatomy especially where the anatomy includes a
severe
bend.
[0017] Further, when the supra renal and sealing stents are crimped in
diameter for
loading and storage within a delivery device, it is recognized that due to
foreshortening
the effective length of the stents of the endoprosthesis will be increased.
Such
foreshortening could cause the stents to overlap each other, resulting in a
large profile.
To avoid any overlap between the supra renal and sealing stents, it is
necessary to
displace such stents within the graft material with a sufficiently large gap
34 so that when
crimped there is a sufficient gap between the stents to avoid undesirable
overlap. While
avoiding overlap allows a small profile to be achieved, the minimum sealing
length is
7
CA 02830870 2013-10-21
compromised since the gap increases the minimum sealing length.
100181 In
addition, axial blood pressure through the endoprosthesis can create
significant
loading at the attachments sites, particularly between the supra renal stent
and the graft
material thereabout. Over time, such loading can affect the durability and
integrity of the
endoprosthesis.
SUMMARY OF THE INVENTION
[0019-0023] The present invention is directed towards the provision of
endoprosthesis,
particularly adapted as an abdominal aortic aneurysm (AAA) device, is
bifurcated including a
main body portion and two le portions coupled thereto. The main
8
CA 02830870 2013-10-21
body portion includes a fabric graft material coupled to several metallic
stent structures at
attachment sites. The stent structures include an upper supra renal stent
extending above
the graft material, one or more sealing stents generally extending in a
repeating Z-shape
circumferentially within the graft and, when expanded, providing outward force
against
the graft, and a tapered stent that leads into the legs.
[0024]
According to one preferred aspect of the invention, the sealing length between
the top of the graft material and the bottom of the uppermost sealing stent is
minimized
while avoiding stent overlap or otherwise compromising the crimped profile.
The
invention includes connecting the apexes of the supra renal and sealing stents
with a
tether, preferably fixed in length, that is free to slide within the apexes.
When the
= endoprosthesis is crimped and thus the stents are compressed in diameter,
the tether has
sufficient length to displace the supra renal and sealing stents by a
sufficient gap to
prevent compromising the crimped profile. When the endoprosthesis is expanded
and
thus the stents are increased in diameter, the tether which is free to move
between the
apexes, is pulled taut drawing the stents toward each other to decrease the
minimum
sealing length.
[0025] According to another preferred aspect of the invention, additional
graft
material is heat set into a pleat at the top of the graft. When the
endoprosthesis is
crimped, the pleat is unfolded to accommodate stent separation. After
expansion of the
endoprosthesis, the heat set of the pleat results in at least partial
automatic re-gathering of
the material into the pleat. The additional graft material at the pleat
provides multiple
9
CA 02830870 2015-02-09
layers of graft material to increase tissue contact. The graft material is
thrombogenic.
The increased thickness of the material provides better sealing.
[0026] According to yet another preferred aspect of the invention, the loading
of the supra
renal stent is shared between the tether and the attachment sites that couple
the supra renal
stent to the graft. Initially all the force is carried by the tether, with the
force carried by the
attachment sites only after the pleat has unfolded as far as it is able. Only
thereafter is the
downward force from blood flow carried by the attachment sites between the
stent and graft.
[0026a] According to one aspect of the invention, there is provided an
endoluminal
prosthesis, comprises a tubular graft main body comprising a graft material
including inner
and outer surfaces and first and second ends, a first stent coupled at said
inner surface of said
graft material at said first end and extending out thereof, a second stent
coupled at said inner
surface of said graft material and extending completely therewithin and two
tubular leg
portions extending from said second end of said main body such that said
prosthesis assumes
a Y-configuration, wherein said graft material is multiple layers thick at a
junction of said
first and second stents when said main body portion is in an expanded state.
[0026b] According to another aspect of the invention, there is provided an
endoluminal
prosthesis, comprising: (a) a tubular graft main body comprising a graft
material including
inner and outer surfaces and first and second ends; (b) a first stent coupled
at said inner
surface of said graft material at said first end and extending out thereof;
(c) a second stent
coupled at said inner surface of said graft material and extending completely
therewithin; (d)
two tubular leg portions extending from said second end of said main body such
that said
prosthesis assumes a Y-configuration, wherein said graft material is multiple
layers thick at a
junction of said first and second stents when said main body portion is in an
expanded state;
and (e) a tether, wherein said first and second stents include respective sets
of apexes which
are nearest each other and said tether extends through and is freely movable
within said
apexes.
[0027] Additional advantages of the invention will become apparent to those
skilled in the
art upon reference to the detailed description taken in conjunction with the
provided figures.
CA 02830870 2015-02-09
BRIEF DESCRIPTION OF THE DRAWINGS
[0028] Fig. 1 is an expanded view of an exemplar endoprosthesis designed by
the inventors
for the purpose of describing certain issues with respect to prior art
endoprosthesis designs;
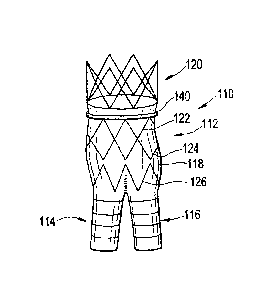
[0029] Fig. 2 is a side elevation of an endoprosthesis according to the
invention;
[0030] Fig. 3 is an enlarged broken schematic section of an upper portion of
an
endoprosthesis according to the invention, shown in an expanded state;
10a
CA 02830870 2013-10-21
[0031] Fig. 4 is an enlarged broken schematic section of an upper
portion of an
endoprosthesis according to the invention, shown in a crimped state;
[0032] Fig. 5 is graph of a the change in the gap between the supra
renal and
upper sealing stent as a result of increasing the diameter of the main body
portion of the
stent;
[0033] Fig. 6 is a partial section of an upper interior wall of an
endoprosthesis
according to the invention, shown in an expanded state; and
[0034] Fig. 7 is a schematic broken section view through an portion of
an
endoprosthesis according to the invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0035] Turning now to Figs. 2 through 4, a vascular endoprosthesis 110
suitable
for treatment of an abdominal aortic aneurysm (AAA) includes a main body
portion 112
and two smaller leg portions 114, 116 coupled thereto in a generally inverted
Y-
configuration. Each portion includes graft material 118 and a plurality of
expandable
stents coupled at the interior of the graft material, preferably with
stitching 119. The
graft material is preferably made of polyethylene terephthalate (sold under
the trademark
Dacron ), which is thrombogenic. The stents, described in more detail below,
are
preferably self-expanding, formed from a superelastic, shape memory material
such as
Nitinol or other nickel-titanium alloy, but may be pressure expandable such as
with a
11
CA 02830870 2013-10-21
balloon catheter. When the stents are expanded, they provide outward force of
the graft
material 118 against the body tissue.
[0036] The main body portion 112 preferably includes several discrete
stents: an
upper supra renal stent 120 preferably extending in a circular cylindrical
form (i.e.,
preferably non-helical) above a portion of the graft material 118 and
typically provided
with tissue anchors 120a, one or more sealing stents 122, 124 generally
extending
circumferentially about the graft in a repeating Z-shape (also preferably
extending non-
helically) and providing outward force against the graft to seal between the
graft and the
body tissue, and a tapered stent 126 that leads into the legs 114, 116.
[0037] According to one aspect of the invention, the supra renal and upper
sealing
stents 120, 122 are connected together with a preferably fixed length tether
130 that is
free to slide within the adjacent apexes 132, 134 of such stents. The
cylindrical
arrangement of the stents provides that the apexes 132, 134 define points that
lie on
respective circles. The tether 130 is preferably comprised of Dyneera
synthetic fiber
available from DSM of The Netherlands, or another flexible, lubricious, high
strength,
high fatigue resistance, and high wear resistance material such as an HDPE
fiber. When
the endoprosthesis 110 is crimped and thus the stents are compressed from the
expanded
diameter Di (Fig. 3) to the crimped diameter 1)2 (Fig. 4) for loading into a
delivery
device, the tether 130 has a length sufficient to allow the supra renal and
upper sealing
stents to be displaced relative to each other by a large enough gap 32 to
prevent
compromising the crimped profile. As will be described hereinafter, a pleat
140 helps
accommodate displacement of the stents 120, 122 relative to each other. When
the stents
12
CA 02830870 2013-10-21
are again increased in diameter to D2 once the endoprosthesis is expanded, the
tether 130,
which is free to move between the apexes 132, 134 through which it extends, is
pulled
taut, drawing the stents 120, 122 toward each other to significantly reduce or
even
completely eliminate the gap to G1 and decrease the length of the
endoprosthesis
required to achieve full circumferential apposition against the vessel wall,
i.e., the
minimum sealing length 136 (defined as the distance between the top 142 of the
graft
material 118 and the bottom 143 of the first sealing stent 122) relative to
prior art
endoprostheses suitable for treatment of abdominal aortic aneurism.
[0038] Fig. 5 illustrates the change in gap length as the diameter of the
stents are
increased in diameter from a crimped state (Di) to an expanded state (1)2).
For a device
having a 30 mm expanded diameter (i.e., an endoprosthesis sized for treatment
of an
abdominal aortic aneurism), the plotted points can be curve fit to the
following equation:
)2
[0039] Gap(y)=1134.7 (diameter(0)*
16
where the Gap(y) is the gap length between the lower apexes of the supra renal
and upper
apexes of the upper sealing stent, and the diameter(0) is the diameter of such
respective
stents.
[0040] By decreasing the minimum sealing length, the outward force of the
metal
stent material of the supra renal and sealing stents 120, 122, 124 is
maximized in the
region most subject to leakage. In addition, the endoprosthesis of the
invention has high
flexibility given that the tether 130 is free to move between the apexes 132,
134 of stents
120, 122. Such freedom of motion allows the main body portion 112 of the
13
CA 02830870 2013-10-21
endoprosthesis 110 to maintain flexibility across the minimum sealing length
136.
[0041] Referring to Figs. 2 through 4, 6 and 7, according to another aspect
of the
invention, and as previously mentioned, additional graft material is heat set
into a pleat
140 at the top of the graft 118. The lower apex 132 of the supra renal stent
120 is
stitched to an inner fold material 144 of the pleat 140, whereas the upper
apex 134 of the
upper sealing stent 122 is stitched to an outer fold material 148 of the
pleat. As such,
pleat material 150 is interposed between the respective lower and upper apexes
132, 134
of the supra renal and upper sealing stent 120, 122. The tether 130 is thread
in and out of
this pleat 140 as it is extends through the apexes of the supra renal and
upper sealing
stents. When the endoprosthesis 110 is crimped, the pleat 140 is preferably at
least
partially unfolded to accommodate stent separation. After expansion of the
endoprosthesis, the heat set of the pleat 140 results in at least partial
automatic re-
gathering of the material into the pleat. The additional graft material at the
pleat 140
provides multiple layers of graft material to increase bulk of the graft
material at the
location thereof and tissue contact thereat This increased thickness of graft
material
provides better sealing at a critical location, and such double layer of graft
material at the
pleat 140 is contacted against the body tissue without any gap (or any
significant gap)
between the supra renal and upper sealing stents 120, 122.
[0042] According to another aspect of the invention, after implantation, the
loading
on the supra renal and sealing stents 120, 122 is shared between the tether
130 and the
stitches 119 that couple the stents to the graft material 118. Such stitches
119 are
preferably at or adjacent the apexes of the stents and/or along the struts of
the stent.
14
CA 02830870 2013-10-21
Initially substantially all the downward force of blood flow through the
endoprosthesis
(particularly as the endoprosthesis narrows into the legs 114, 116) is carried
by the tether
130 rather than on the stitches that operate to attach the graft material 118
to the stents
120, 122. Upon being subject to such force, the tether is tensioned and loaded
incrementally. If the tether is fully loaded, the pleat 140 in the graft
material 118 then
absorbs force and may partially unfold. Only thereafter is the stitching 119
supporting
the graft material 118 to the stents 120, 122 loaded and subject to force.
[0043] The endoprosthesis of the invention has high flexibility because the
tether is
free to move between the apexes of the stents. Such freedom of motion allows
the main
body portion of the endoprosthesis to main flexibility across the minimum
sealing length.
[0044] There have been described and illustrated herein embodiments of an
endoluminal prosthesis, particularly suitable for an AAA device. However, it
is not
intended that the invention be limited thereto, as it is intended that the
invention be as
broad in scope as the art will allow and that the specification be read
likewise. Thus, the
tether and pleat may be used on endoluminal prostheses having no branches
and/or
intended for other purposes. In addition, while shape memory alloys, and
preferably
Nitinol have been disclosed as preferred materials for use in practicing the
invention, it
will be understood that other shape memory alloys and other shape set
materials
including biocompatible plastics may be used as well. Also, while the
preferred graft
material is the synthetic material polyethylene terephthalate, other graft
materials
including synthetic and natural materials can be used. It will therefore be
appreciated by
CA 02830870 2013-10-21
=
those skilled in the art that yet other modifications could be made to the
provided
invention without deviating from its scope as claimed.
16