Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
1
Gasification of bio-oil and alkali containing energy rich aqueous solutions
from
pulp mills
FIELD OF THE INVENTION
The present invention relates to the field of generating energy-rich synthesis
gas from
renewable energy sources, more specifically through gasification of alkali
containing
energy rich aqueous solutions from pulp mills. More specifically, the
invention relates
to a process for gasification of an alkali containing energy rich aqueous
solution from a
1() pulp mill in an entrained flow gasification reactor, the process
comprising the steps of
supplying said alkali containing energy rich aqueous solution to said
gasification
reactor, gasifying said alkali containing energy rich aqueous solution in the
reactor by
using an oxidizing medium at sub-stoichiometric conditions and at a
temperature below
1400 C in an outlet of said reactor; and producing a phase of a liquid
material and a
phase of a gaseous material in said reactor.
BACKGROUND INFORMATION
There is a need to find efficient technologies and to develop known
technologies further
to convert renewable energy sources to useful energy. Biomass is one of the
renewable
energy sources of great interest.
Biomass is biological material from living, or recently living organisms, such
as wood
or organic waste. Although fossil fuels have their origin in ancient biomass,
they are not
considered biomass by the generally accepted definition because they contain
carbon
that has been "out" of the carbon cycle for a very long time. Their combustion
therefore
increases the carbon dioxide content in the atmosphere.
Biomass energy is derived from a multitude of sources, such as wood, waste,
and
landfill gases. Wood energy is derived both from direct use of harvested wood
as a fuel
and from wood waste streams. An important source of energy derived from wood
is
pulping liquor or "black liquor," a by-product product from processes of the
pulp and
paper industry. Waste energy is another large source of biomass energy. The
main
contributors of waste energy are municipal solid waste (MSW), manufacturing
waste,
and landfill gas.
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
2
Industrial biomass can be grown from numerous types of plants, including
miscanthus,
switchgrass, hemp, corn, poplar, willow, sorghum, sugarcane, and a variety of
tree
species.
There are a number of technological options available to make use of a wide
variety of
biomass types as a renewable energy source. Conversion technologies may
release the
energy directly, in the form of heat or electricity, or may convert it to
another form,
such as liquid biofuel or combustible biogas. While for some classes of
biomass
resource there may be a number of usage options, for others there may be only
one
appropriate technology.
One of the technological options available is thermal conversion which
includes
processes in which heat is the dominant mechanism to convert the biomass into
another
chemical form. The basic alternatives of combustion, torrefaction, pyrolysis,
and
gasification are separated principally by the extent to which the chemical
reactions
involved are allowed to proceed (mainly controlled by the availability of
oxygen and
conversion temperature).
Gasification is a process that converts carbonaceous materials, such as coal,
petroleum,
biofuel, or biomass, into carbon monoxide and hydrogen by reacting the raw
material at
high temperatures with a controlled amount of oxygen and steam and/or water.
The
resulting gas mixture is called synthesis gas or syngas and is itself a fuel.
Gasification is
a method for extracting energy from many different types of organic or fossil
materials.
The advantage of gasification is that using the syngas is potentially more
efficient than
direct combustion of the original fuel because it can be combusted/utilized in
a more
flexible manner. Syngas may be burned directly in internal combustion engines
or gas
turbines to generate electricity, used to produce methanol, ammonia, hydrogen
or
synthetic diesel. The latter product is normally produced via the Fischer-
Tropsch
process.
Biomass gasification is expected to play a significant role in a renewable
energy
economy, because biomass production is neutral with respect to CO2 in the
atmosphere
and the net effect of using biomass for e.g. fuel production is that it lowers
CO2
concentration in the atmosphere compared to if fossil derived fuels would be
continued
to be used. While other biofuel technologies such as biogas and biodiesel
production are
also beneficial fuel sources for reducing carbon emissions, gasification runs
on a wider
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
3
variety of input materials, can be used to produce a wider variety of output
fuels and is a
very efficient method for extracting energy from biomass. Biomass gasification
is
therefore one of the most technically and economically viable energy
conversion routes
for a carbon emission constrained economy.
Three main types of gasifiers are currently available for commercial use:
fixed bed,
fluidized bed and entrained flow gasifiers. In the entrained flow gasifier a
dry
pulverized solid or a liquid fuel or fuel slurry is gasified with oxygen or
air in co-current
flow and the gasification reactions take place in a dense cloud of very fine
particles/droplets. Most coals are suitable for this type of gasifier because
of the high
operating temperatures and because the good contact achieved between the coal
particles and the gasifying agent. Entrained flow gasifiers have been
demonstrated as
highly effective units for the gasification of coal and other carbonaceous
fuels such as
residual oils and petcoke.
Black liquor, which is obtained from chemical pulping of wood chips in a
pulping
process, typically contains more than half of the energy content of the wood
chips fed
into the digester. Said black liquor needs to be concentrated, conventionally
by
evaporation, to a higher dry solids content, normally to 65 - 80 %, by multi-
effect
evaporators before being fed to either a recovery boiler or a gasification
plant to
produce energy and recover the cooking chemicals.
Other effluents comprising biomaterial waste from pulp mills are e.g. bleach
effluents
from bleaching of paper pulp. Typically, these effluents have low solids
content and
lower energy content than spent cooking liquors, such as black liquor. In
order to being
able to burn or gasify said effluents, the effluents would have to be
evaporated to such
an extent that the net amount of energy produced would be very low.
A major challenge for gasification technologies is to reach an acceptable
energy
efficiency for fuels with low energy content, low reactivity or other
undesired
properties. The high efficiency of converting syngas to fuels or electric
power may be
counteracted by significant power consumption in the feedstock preprocessing,
the
consumption of large amounts of pure oxygen (which is often used as
gasification
agent), and gas cleaning. Another challenge becoming apparent when
implementing the
processes in real life is to obtain long service intervals in the plants, so
that it is not
necessary to close down the plant every few months for cleaning or
maintenance.
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
4
In many gasification processes most of the inorganic components of the input
material,
such as metals and minerals, are retained in the ash. In some gasification
processes in
which the inorganic material is melted when passing the hot part of the
gasifier
(slagging gasification) this ash can have the form of a glassy solid with low
leaching
properties, but the energy efficiency in slagging gasification can be lower
due to the
higher temperature.
Furthermore, there are several problems associated with the use of biomass as
energy
source, some of which are a high bulk volume and a low calorie-value caused
e.g. by
1() high moisture content, high oxygen content or high inorganic content.
Biomass is
furthermore sensitive to moisture, difficult to feed to a pressurized
gasifier, costly to
grind, inhomogeneous and comprises metals which lead to a problematic handling
of
ashes when being gasified. Other problems associated with gasification of
biomass are
low ash melting point and a chemical composition with potentially high
chlorine
content. Several of said drawbacks and/or problems result in a decrease in
overall
efficiency, deposit formation (slagging and fouling), agglomeration, corrosion
and
difficult ash handling and also complicated and expensive process solutions in
order to
handle the various problems mentioned above.
In order to reduce said problems biomass may be pre-treated in some way before
the
gasification is performed. One way is to pyrolyze the biomass to provide a
biomass
pyrolysis oil, which is a dark, oily liquid. Pyrolysis is normally performed
at
temperatures between 400-600 C and generates a gas as well as a liquid and a
solid
fraction. The two latter ones can, depending on pyrolysis process, be combined
to form
a pyrolysis oil containing 80-85% of the energy in the biomass fed to the
pyrolysis
process.
Gasification of pyrolysis oil in an entrained flow gasifier has been carried
out in pilot
plant scale at 25 bar pressure and with oxygen and steam as gasification
media. In order
to achieve acceptable gasifier performance a minimum of 99% of the carbon
contained
in the bio-oil feedstock has to be converted to gas (CO and CO2) in the
gasifier. The
pilot plant tests have shown that a carbon conversion of 99% or more requires
a
gasification temperature of 1200-1600 C depending on the composition of the
pyrolysis
oil. This high temperature leads to a high consumption of oxygen and lowers
the cold
gas efficiency of the gasifier (cold gas efficiency defined as energy in
produced syngas
divided by energy in the fuel to the gasifier) which means lower content of
chemical
currency (CO + H2) in the produced syngas. A typical cold gas efficiency for
entrained
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
flow pyrolysis oil in pilot scale is 50-55%. Furthermore, handling of
pyrolysis oil ash
content is known to present difficulties in gasifier design. The normal ash
content of
pyrolysis oil is 0-5%.
5 Other renewable energy rich liquids are wood extractives, e.g. tall oil,
that are by-
products from pulping and glycerol that is for example produced as a by-
product from
bio-diesel production. Heating values are typically higher than for black
liquor.
Glycerol gasification is feasible, as described for example in document US
7,662,196.
The process described in that patent uses gasification in an entrained flow
reactor at
900-1000 C but due to the incomplete conversion of the feedstock to syngas, a
second
reaction step is required. The second step is a reformer wherein, also at
temperatures
above 900 C, the different partial oxidation/thermal cracking reactions in the
presence
of metal oxides are completed. Complete conversion in one step would require
significantly higher temperatures and hence lead to low efficiencies,
similarly as
described above for pyrolysis oil gasification.
Another pre-treatment alternative may be to torrefy the biomass. Torrefaction
of
biomass may results in a dry biomass which is grindable and of higher density
as well
as of a higher energy density. The torrefied biomass may be feedable, e.g. as
pellets or
powders, which are, furthermore, more homogenous in composition but feeding
torrefied solid biomass material to a pressurized gasifier may often be
difficult, and,
hence, it is preferred to have said torrefied solid biomass material pumpable
by fedding
it as a slurry. However, mixing torrefied solid biomass material with water
would
considerably lower the energy efficiency of the gasification.
With entrained flow gasifiers operating with coal-biomass mixture fuels, one
problem is
the delivery of the feedstock mixture of carbonaceous solids and biomass to
the gasifier.
Different types of entrained flow gasifiers, feeding solid coal or coal-water
slurries,
have been reported to encounter feedstock delivery as one of the hurdles to
continuous
running. Failure of slurry pumps and the clogging of lock hoppers have been
observed.
It is therefore desirable to develop a way of feeding biomass to entrained
flow gasifiers
which does not suffer from these disadvantages.
Document WO 2010/046538 shows that the catalytic effect of black liquor alkali
can be
utilized to increase reaction rates in decomposition of organic material. The
process
described in this document is a hydrothermal treatment process in super-
critical or near-
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
6
critical conditions with water as oxidizing agent and hence not relevant for
gasification
processes utilizing oxygen or air as oxidizing agent i gaseous phase.
Document US 2010/0083575 relates to a process for co-gasification of
carbonaceous
solids, such as coal and coke, with biomass in which the biomass material is
pyrolyzed
to provide a biomass pyrolysis oil and biomass char or coke which are then
mixed with
the carbonaceous solid to form a slurry. However, the process still uses
carbonaceous
solids as part of the feedstock, i.e. the raw material is not a biomass raw
material
exclusively. Furthermore, this process does not give any advantages in terms
of lower
gasification temperatures and/or higher efficiencies. This means that said
document
does not deal with the problems associated with gasification of biomass
solely.
Taking the above into consideration there is a need to improve the process for
biomass
gasification and to increase the energy conversion efficiency.
SUMMARY OF THE INVENTION
It is an object of the present invention to overcome or at least minimize at
least one of
the drawbacks and disadvantages of the above described technologies to convert
renewable energy sources to useful energy. This can be obtained by a process
as defined
in the claims.
Thanks to the invention where alkali containing energy rich aqueous solution
from pulp
mills and bio-oil are gasified simultaneously, i.e. alkali containing energy
rich aqueous
solution and bio-oil are gasified together as a mixture in a reaction zone of
a gasification
reactor, it is possible to optimize the feedstock to be gasified so that said
feedstock has
appropriate properties for being efficiently gasified at lower temperature
than would be
required for gasification of only the bio-oil and preferably with less energy
consuming
pre-treatment for the alkali containing energy rich aqueous solution such as
evaporation.
This leads to a higher total energy efficiency of the process.
Furthermore, conventionally, the amount of available alkali containing energy-
rich
aqueous solution comprising material from the pulp mills restricts the size of
the
gasification plants. Thanks to a solution according to the invention,
gasification plants
of substantially higher capacity than conventional may be built at the pulp
mills since
bio-oil may be added to said alkali containing energy-rich aqueous solutions,
which
leads to substantially lowered specific investment costs.
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
7
According to one aspect of the invention, said alkali containing energy rich
aqueous
solution and said bio-oil are mixed and supplied as a feedstock mixture to the
gasification reactor. Thanks to this aspect feedstock inlets may be made
simpler.
According to another aspect, alkali containing energy rich aqueous solution
(120) and
said bio-oil (110) are supplied through separate inlets (3, 3') arranged on
the same
burner of said reactor (2) ensuring good mixing in the reactor close to the
inlets. Thanks
to this aspect also materials that cannot be mixed or does not form a
homogeneous
solution can be gasified together.
1()
According to a further aspect of the invention, the ratio (wt/wt) of alkali
containing
energy rich aqueous solution (120) and bio-oil (110) which are to be gasified
in said
reactor zone of the gasifier is between 95:5 and 20:80, more preferred between
90:10
and 40:60, and most preferred between 80:20 and 40:60. Thanks to this aspect
optimized water content, alkali content and viscosity of the liquid to be
gasified are
achieved at maximum cold gas efficiency.
According to another aspect, said bio-oil (110) comprises biomass pyrolysis
oil,
glycerol and/or liquid by-products, e.g. tall oil from the pulp mill, and said
alkali
containing energy rich aqueous solution (120) comprises spent liquor from a
pulping
step within the pulp mill and/or a bleach effluent from one or several
bleaching steps
within the pulp mill. Thanks to this aspect a flexible process is obtained
with a
possibility of gasifying a variety of liquids and mixtures according to the
specific
location of the gasification plant and situation at the site.
According to yet another aspects of the invention, the gasification process is
carried out
at an absolute pressure of the gasification process from about 1,5 to about
150 bar,
preferably from about 10 to about 80 bar, and most preferably from about 24 to
about
40 bar in the reaction zone and at a temperature which is at least 900 C,
preferably at
least 950 C but below 1400 C, preferably below 1200 C, in the reaction zone
during
the gasification. Thanks to these aspects optimal conditions are achieved
during the
gasification and subsequent heat recovery and maximal energy efficiency are
obtained.
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing aspects and many of the attendant advantages of this invention
will
become more readily appreciated as the same become better understood by
reference to
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
8
the following detailed description, when taken in conjunction with the
accompanying
drawings, wherein:
Fig. 1 shows a flow scheme for a set up of processes for carrying out
the
invention,
Fig. 2 shows a flow scheme for an alternative set up of processes for
carrying
out the invention,
Fig. 3 shows a general process scheme of a gasification plant of the
entrained-
flow, high temperature reactor type, and
Fig. 4 shows a modified version of the gasification plant as shown in Fig.
3.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The following detailed description, and the examples contained therein, are
provided for
the purpose of describing and illustrating certain embodiments of the
invention only and
are not intended to limit the scope of the invention in any way.
In Fig. 1 a flow scheme for a set up of processes for carrying out the
invention is
shown. Bio-oil 110 is fed to a feedstock mixing tank 100, where bio-oil 110 is
mixed
with alkali containing energy-rich aqueous solution 120 from a pulp mill. The
resulting
feedstock mixture 130 comprising the alkali containing energy rich aqueous
solution
120 and the bio-oil 110 is supplied to a gasification reactor 2 (shown in Fig.
3) of an
entrained bed gasification process 200, in which reactor 2 said feedstock
mixture 130 is
gasified and converted into a raw synthesis gas 210 and a so called green
liquor 220
comprising recovered pulping chemicals. After the raw synthesis gas 210 has
been
cleaned and conditioned in one or several after treatment units 300 the clean
syngas 310
may be used for efficient production of electric power and/or production of
fuels or
chemicals.
Said bio-oil 110 may be any type of liquid derived from biomass material,
preferably
pyrolysis oil from wood, glycerol from biodiesel production, or tall oil from
wood,
vegetable oils etc while said alkali containing energy-rich aqueous solution
120 may
preferably be a spent liquor from a pulping process in a pulp mill or an
effluent from the
pulp mill.
The term bio-oil is understood to comprise all renewable energy rich liquids
with origin
in biomass, e.g. pyrolysis oil, glycerol, tall oil etc. Different liquors
produced as waste
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
9
or by-products within the pulp mills when producing paper pulp, e.g. spent
liquors and
effluents, are not included in the term bio-oil throughout the text.
Depending on the chemical pulping process used, the resulting spent liquor
will have
different chemical composition and also be termed differently. For Kraft
pulping
processes the spent liquor is a so called black liquor. Typically, the black
liquor contains
more than half of the energy content of the wood chips fed into the digester.
Other
chemical pulping processes may be different sorts of sulphite pulping
processes, e.g.
sodium or potassium based sulphite processes, resulting in a sodium or
potassium based
sulphite spent liquor.
Alternatively, said alkali containing energy rich aqueous solution 120
comprising
energy-rich material may preferably be an effluent from the pulp mill, e.g. an
effluent
from a bleaching step within the mill. It may in some embodiments be a mixture
of
appropriate spent liquors and effluents so as to reach an appropriate total
solids content,
alkali content and/or viscosity. The alkali metal content of the alkali
containing energy
rich aqueous solution catalyzes the gasification and decomposition reactions,
enabling
very high carbon conversion at comparably low gasification temperature.
Hence, the alkali metal content of the mixture has to be high enough to give
sufficient
catalytic effect. Alternatively, alkali may be added, e.g. as NaOH, to the
mixture or to
the bio oil in embodiments where alkali containing energy rich aqueous
solution and the
bio oil are fed to the reactor through separate inlets (figs. 2 and 4) in
order to achieve
the catalytic effect. This addition may preferably be a part of the alkali
make-up to the
pulp mill chemical cycle.
Bio-oil normally has a higher heating value than said spent liquors, which in
turn have
higher heating values than said bleach effluents. Addition of bio-oil to spent
liquors may
increase cold gas efficiency of gasification of alkali containing energy rich
aqueous
solution. This is achieved e.g. by decreasing the relative losses of energy
from the
reactor or by lowering the relative amount of energy required to evaporate and
heat the
water content of the aqueous solution gasification in the gasification
reactor. Addition
of bio-oil to bleach effluents may indeed motivate gasification of bleach
effluents from
an economical point of view.
Said bio-oil may be manufactured from a biomass material comprising plant
matter but
said biomass material may also include discarded plant or animal matter which
has been
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
primarily used for other purposes such as production of food, production of
fibers,
chemical manufacturing or heat production. Furthermore, the biomass material
may also
biodegradable wastes that can be burnt as fuel including municipal wastes,
green waste
(the biodegradable waste comprised of garden or park waste such as grass or
flower
5 cuttings and hedge trimmings), byproducts of farming including animal
manures, food
processing wastes, sewage sludge or algae.
In Fig. 2 another preferred embodiment of the invention is shown, in which the
alkali
containing aqueous solution 120 and the bio-oil 110 are mixed inside the
gasification
10 reactor 2 (shown in Fig. 4) of the gasification process 200 after being
introduced/supplied via separate conduits through separate inlets of the
gasification
reactor 2.
Introduction of water and/or steam may often be required to counteract soot
formation
in bio-oil gasification. However, introducing water in the reactor generally
decreases the
cold gas efficiency. By mixing bio-oil 110 and the alkali containing aqueous
solution
120, the water in said aqueous solution 120 is utilized, meaning that no extra
water
and/or steam may have to be added that would otherwise decrease cold gas
efficiency
for gasification of the bio-oil 110.
Handling of inorganic components (ash) is a key function of a spent cooking
liquor
gasifier, since inorganic chemicals in the black liquor have to be recovered
and recycled
to the mill. When the ash from the bio-oil 110 is mixed with the ash from
spent cooking
liquor 120 in the gasification reactor, a mixture with similar melting
temperature and
properties as the spent cooking liquor ash is formed. Thus, the ash handling
problem
that is present in bio-oil gasification may be solved if a feedstock
comprising a bio-
oil/spent cooking liquor mixture is gasified in a gasifier of the same type
that is
normally used for spent cooking liquor.
Fig. 3 shows a general process scheme of a gasification process of the
entrained-flow
reactor type for gasification at slagging conditions (high temperature) in
accordance
with the invention. Said process being a part of a chemical recovery cycle for
a kraft or
sulphite pulp mill.
The following description is to be seen as a general description of a
gasification process
and shall be interpreted as illustrative and not in a limiting sense. It is to
be understood
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
11
that numerous changes and modifications may be made to the below described
process,
without departing from the scope of the invention, as defined in the appending
claims.
The process scheme is however illustrating the embodiment as described in
relation to
Fig. 1 with a premix of the alkali containing energy rich aqueous solution 120
and bio-
oil 110 as the feedstock mixture 130.
Fig. 3 shows an equipment for down-draft gasification, i.e. gasification with
a
gasification burner positioned on top or substantially on top of the
gasification reactor.
1() Reference number 1 in Fig. 3 denotes a pressure vessel which comprises
a ceramically
lined gasification reactor 2 followed by a quench compartment 38 in which the
hot
media, i.e. a phase of liquid material and a phase of gaseous material, from
the reactor is
cooled by a cooling liquid. The reactor is provided with an inlet 3 for the
feedstock
mixture 130 and an inlet 4 for an oxidizing medium, e.g. oxygen or oxygen-
containing
gas and a burner (not shown). Said inlets 3, 4 are preferably arranged on the
upper
portion of the gasification reactor 2. In the embodiment shown in Fig. 3 said
inlets 3, 4
are arranged substantially on top of the dome of said gasification reactor 2.
However, in
other embodiments it may be preferred to place the inlets on other locations
of said
gasification reactor 2. There may also be an inlet for atomizing support
medium (not
shown). Said inlet for atomizing support medium may preferably be arranged in
vicinity
of the other said inlets so as making it possible to mix said atomizing
support medium
with the oxidizing medium and/or the fuel in the burner. The opening in the
bottom of
the reactor chamber is limited in size to give a recirculating flow pattern in
the reactor,
which is required to give high carbon conversion and sulphur reduction
efficiency. The
opening is in the form of a chute 5, which opens directly into the quench
compartment
38 above the surface 35 of the liquid in a green liquor liquid chamber 6 which
is
situated below. One purpose of the quench compartment 38 is to cool the gas
leaving
the reactor to a temperature at which gas phase chemical reactions does not
take place at
a significant rate.
A number of spray nozzles 7 for cooling liquid open out into the chute 5 and
the quench
compartment 38. Green liquor 220 which is produced is transported from the
chamber 6
through a conduit 8, via a pump 9 and a heat exchanger 10, to subsequent
process stages
for generating cooking liquor, e.g. white liquor, or to another process stage
in which
green liquor is employed. A partial flow of the green liquor transported in
conduit 8
may be returned to the green liquor liquid chamber 6 through a conduit 81 via
a pump
91. Cooling liquid that is not evaporated is collected in a volume 36 to be
reused.
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
12
Raw synthesis gas from the first vessel 1 is conveyed through a conduit 11 to
a second
pressure vessel 12 for gas treatment and sensible heat energy recovery. This
conduit 11
opens out in the pressure vessel 12 above the surface of a liquid in a washing
chamber
13 at the bottom of the vessel. The liquid in the washing liquid chamber of
the second
vessel may be conveyed, through a conduit 14 via a pump 15, to the first
vessel 1 in
order to serve as diluting liquid or as a cooling liquid which is provided via
the spray
nozzles 7. The pressure vessel 12 may comprise an indirect condenser of the
counter-
current falling-film condenser type 16 located above the chamber 13. Other
types of
condensers may be used without departing from the scope of the invention and
since
methods for gas washing and gas cooling are well known techniques it will not
be
described in detail here.
An outlet conduit 17 for cooled raw synthesis gas 210 is located at the top of
the second
pressure vessel 12. The outlet conduit 17 transports the cooled raw gas from
the
gasification plant 200 to an inlet 31 of the one or several after treatment
units 300 which
may comprise a plant 30 for further removal of sulphurous components and most
of the
CO2 (acid gas removal, AGR). The plant 30 comprises any gas separation
technology
for acid gas removal as well as gas conditioning technology as may be needed
to
produce high quality synthesis gas. In a preferred embodiment selective
removal of non-
desired gas components in raw syngas 210 is performed so that sulphur
containing
components, CO2 and traces of tar components which may be present in raw
syngas
210, are removed separately in conduits 33, 34, 37, respectively. A conduit 32
of the
plant 30 may transport the purified and cooled synthesis gas 310 now called
cleaned
synthesis gas, to any field of use of the synthesis gas, e.g. chemical
production, fuel
production, electricity generation and/or steam/heat generation.
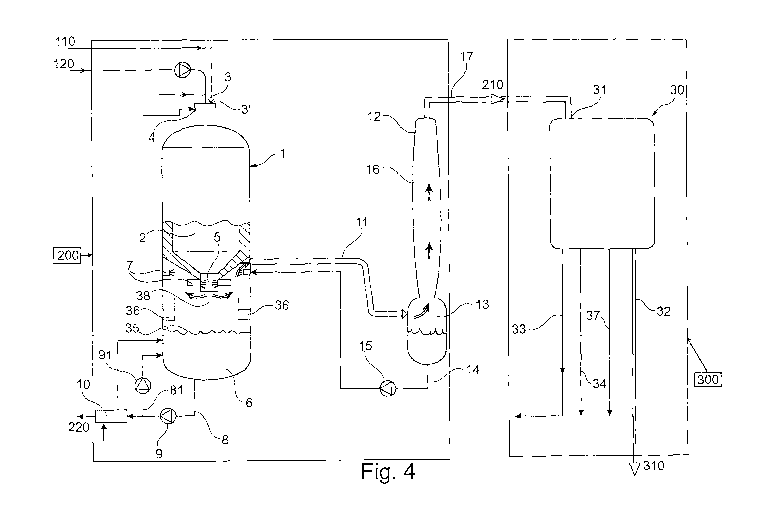
Fig. 4 shows a general process scheme of a modified version of the
gasification process
of the entrained-flow type for down-draft gasification at slagging conditions
(high
temperature) as shown in Fig. 3 and in accordance with another preferred
embodiment
of the invention. Said process being a part of a chemical recovery cycle for a
kraft or
sulphite pulp mill.
The process scheme is illustrating the embodiment as described in relation to
Fig. 2 in
which the bio-oil 110 and the alkali containing energy rich aqueous solution
120 are
brought separately via separate conduits to separate inlets 3, 3' of the
gasification
reactor 2 for feeding of the alkali containing energy rich aqueous solution
120 and the
bio-oil 110. The inlets 3, 3' are positioned, preferably in vicinity of each
other, so as to
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
13
achieve a good mixing of said feeds when they have entered the reactor. The
inlets 3, 3'
are preferably arranged on the upper portion of the gasification reactor 2. In
the
embodiment shown in Fig. 4 said inlets 3, 3' are arranged on top or
substantially on top
of the dome of the gasification reactor (2) but the invention is not limited
to the exact
location of the inlets on the upper half of the gasification reactor 2.
However, the
inventors have found that it may be beneficial to place the inlets 3, 3'
relatively close to
each other thereby enabling the use of the same gasification burner (not
shown) for
burning the feeds which may be beneficial since burning the feeds by using the
same
burner would ensure good mixing of the bio-oil and the alkali containing
energy rich
aqueous solution in the gasification reactor. This allows the catalytic effect
of the alkali
to be present also for the bio-oil gasification. By processes according to
this
embodiment it is possible to gasify combinations of the alkali containing
energy rich
aqueous solution 120 and the bio-oil 110 which are disadvantageous to mix or
does not
form homogeneous solutions.
A first preferred embodiment according to the invention is now to be
described.
Kraft black liquor 120 having a dry solids content of about 70-85% is fed to
the mixing
zone 100 where said liquor 120 is thoroughly mixed with pyrolysis oil 110 and
forming
a feedstock mixture 130. The dry solids content of the black liquor may be
lower than
what would normally be used for gasification of the Kraft black liquor
independently in
order to achieve appropriate gasification feedstock mixture properties, e.g.
water
content, alkali content and viscosity, that are optimal for the gasification
process of the
feedstock mixture. The black liquor 120 and the pyrolysis oil 110 are mixed so
as to
form a mixed gasification feedstock 130 having a ratio (wt/wt) of black liquor
120 to
pyrolysis oil 110 between 95:5 and 20:80, more preferred between 90:10 and
40:60 and
most preferred between 80:20 and 40:60.
Said mixed gasification feedstock 130 may be heated to a temperature of 100-
200 C
before entering the gasification reactor 2, if necessary, to achieve a
viscosity which may
more easily be processed conveniently in the gasifier 2. Said mixed
gasification
feedstock 130 is fed to the gasification unit 200 comprising the gasifier
reactor 2 of the
entrained flow type. Entrained flow gasifiers are known per se. However, in
the
preferred embodiments according to the invention the gasification may
preferably be
performed in an entrained flow type gasifier that may preferably be:
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
14
- Equipped with means for atomizing the gasification feedstock into small
droplets, preferably smaller than about 200 [tm
- Suited for gasification of a highly alkaline feedstock with high ash
content from
a material compatibility perspective
- Equipped to achieve handling and recovery of the ash content in the
gasification
feedstock.
- Connected with an acid gas removal unit that can remove / recover non-
desired
gas components such as traces of tars, sulphur containing components and CO2
from the raw synthesis gas produced in the gasifier
The gasifying reactor 2 is fed with the feedstock mixture 130 and a stream of
oxygen or
an oxygen containing gas. Said stream may have been pre-heated to 50-400 C.
The
feedstock mixture 130 is processed by gasification in the presence of an
oxidizing
medium, e.g. oxygen or air, whereby heat is released by the chemical reactions
taking
place to give a temperature in the outlet of the reactor 2 above 800 C,
preferably above
900 C, more preferred above 950 C but below 1400 C, preferably below 1200 C,
and
at an absolute pressure of about 1.5 to about 150 bar, preferably about 10 to
about 80
bar, and most preferably from about 24 to about 40 bar in the reaction zone (a
so called
high pressure gasification). An atomizing support medium may be used. Said
gasification takes place at reducing conditions, i.e. sub-stoichiometric
oxygen
conditions, whereby producing a mixture of partly at least one phase of a
liquid material
and partly at least one phase of a gaseous material. It is important that the
reactor
bottom outlet is designed to give a recirculating flow pattern in the reactor
in order to
achieve the desired process performance.
It is to be interpreted that the temperature in the outlet of the reactor 2
means the mean
temperature of the liquid material and the gaseous material when said
materials are to
leave the reactor 2, in the region adjacent the chute 5. The reaction
temperature within
the reactor 2 is usually considerably higher than the temperature in the
reactor outlet.
The phase of gaseous material comprising the raw synthesis gas, e.g. carbon
monoxide,
hydrogen, carbon dioxide, methane, hydrogen sulphide, and aqueous steam, and
the
phase of the liquid material comprising inorganic smelt and ash, e.g. sodium
sulphide,
carbonate and hydroxide, are cooled in the quench compartment 38 by spraying
cooling
liquor through a number of nozzles 7 in order to achieve maximum contact with
the
gas/smelt mixture. The cooling liquid may principally consist of water, some
of which
water will be evaporated when it makes contact with the hot gas and the smelt
at the
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
reactor temperature. The gas temperature drops to approx. 100-300 C in the
quench
compartment 38. The smelt drops are dissolved in the remaining part of the
cooling
liquid and falls into the green liquor liquid chamber 6 where it dissolves to
form green
liquor. Alternatively, the smelt drops fall down directly into the liquid
chamber and only
5 then dissolve in the green liquor which is already present in this
location. The smelt
drops are then possibly cooled by the evaporation of water in the green liquor
bath.
The green liquor comes out from the bottom of the quench compartment 38 of the
first
pressure vessel through the conduit 8 and may be pumped through a heat
exchanger, in
1() which heat energy is recovered from the green liquor by cooling the
latter.
Alternatively, green liquor heat energy may be recovered by other means. A
screen may
be used ahead of the pump to catch small particles. It is beneficial that the
amount of
unburnt charcoal in the smelt and in said green liquor is lower than 5%,
preferably
lower than 1% and more preferred lower than 0,2%, of the carbon in the sulfite
thick
15 liquor. i.e. that the carbon conversion in the reactor is at least 95%,
preferably at least
99% and more preferred at least 99,8%.
The green liquor sulphide may be recovered in the same manner as the sulphide
in the
green liquor from a recovery boiler. A high sulphur reduction efficiency
decreases the
total amount of sulphur that needs to be circulated by decreasing the so-
called dead-load
(i.e. inactive sulphur species such as sulphate). It is beneficial that the
green liquor is to
an extent of at least 90%, preferably at least 98% and more preferred at least
99%, free
from non-reduced sulphur, i.e. that the sulphur reduction efficiency is at
least 90%,
preferably at least 98% and more preferred 99%.
The raw synthesis gas 11, leaving the primary quench dissolver of the reaction
vessel 1,
now essentially free of smelt drops, is further cooled to saturation in the
second vessel
12, the gas cooler for particulate removal and gas cooling. Water vapour in
the raw
synthesis gas 11 is condensed, and the heat released may be used to generate
steam.
Traces of tars, hydrogen sulphide and carbon dioxide may be removed from the
cool
raw synthesis gas in a so called acid gas removal plant 300 - AGR. Several
known
commercial gas cleaning systems comprising units for absorption of acid gas
and
recovery of sulphur may be used. Said removed hydrogen sulphide may then be
conveyed to the cooking liquor preparation.
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
16
The table below shows typical properties for pyrolysis oil gasification, black
liquor
gasification and co-gasification of pyrolysis oil and black liquor in a 50/50
mixture.
Pyrolysis oil (PO) Black liquor (BL) 50/50 BL/PO
mix
gasification gasification
Heating value (wet basis) MJ/kg]
Typical 15-20 9-10 12-15
High 25 12
Ash content ro] 0-5 15-40 7-22
Presence of catalyzing Na And K Low 10-20 5-10
rol
Water ro] 5-30 20-35 10-30
Gasification temp 1200-1600 1000-1050 1000-1050
Carbon conversion About 99% >>99% >>99%
The pyrolysis oil 110 may be manufactured by pyrolysis of biomass material in
any
conventional manner resulting in a pyrolysis oil 110 that comprises primarily
a mixture
of organic chemicals and having a varying water quantity ranging from about 5
wt% to
about 50 wt%.
In a second preferred embodiment sulphite spent liquor is mixed with pyrolysis
oil.
Sulphite spent liquor 120 having a dry solids content of about 60-80% is fed
to the
mixing zone 100 where said liquor 120 is thoroughly mixed with pyrolysis oil
110 and
forming a mixed gasification feedstock 130.
The sulphite spent liquor and pyrolysis oil are mixed so as to form a
feedstock mixture
130 having a ratio (wt/wt) of sulphite spent liquor 120 to pyrolysis oil
between 95:5 and
20:80, preferably between 90:10 and 40:60 and most preferred between 80:20 to
about
40:60.
The generally lower alkali content of sulphite spent liquor compared to Kraft
black
liquor may allow a lower proportion of pyrolysis oil to be mixed in without
reaching too
low alkali content in the mixed gasification feedstock 130.
Said mixed gasification feedstock 130 may be heated to a temperature of 100-
200 C
before entering the reactor 2, if necessary, to achieve a viscosity which can
more easily
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
17
be processed conveniently in the gasifier and is then fed to the gasification
unit 200 and
being gasified in accordance to what is earlier described. The gasification
process may
preferably be similar to the one given for the description of the first
preferred
embodiment above.
Recovery of green liquor and sulphur in syngas is different for a sulphite
pulping
process, giving sulphite spent liquor, compared to a kraft pulping process,
giving kraft
black liquor as described above.
1() In a third preferred embodiment a bleach effluent 120 is mixed with
glycerol 110.
Bleach effluent 120 from one or several bleaching steps is fed to the mixing
zone 100
and mixed with glycerol 110. Depending on the properties of the bleach
effluent, said
effluent may be evaporated to some extent before being mixed with the glycerol
110
and forming the feedstock mixture 130. Bleach plant effluents typically has a
dry solids
content of about 40-70% after concentration by evaporation.
Bleach plant effluents are known to frequently be difficult to evaporate to
high dry
solids content. Furthermore, the heating value of the bleach plant effluents
is often
lower than for spent liquors from the pulping process. Both low dry solids
content and
low heating value decreases gasification process efficiency. Hence,
gasification of
bleach plant effluents separately may be difficult with acceptable process
performance.
As described above, the alkali content in the bleach plant effluent may be
used to
increase efficiency of bio-oil gasification reactions and the higher heating
value of the
bio-oil leads to higher efficiency in the gasification of a mixed gasification
feedstock.
The bleach plant effluent 120 and glycerol 110 are mixed so as to form a
feedstock
mixture 130 having a ratio (wt/wt) bleach plant effluent 120 to glycerol 110
between
95:5 and 20:80, preferably between 90:10 and 40:60 and most preferred between
80:20
to about 40:60.
Said feedstock mixture 130 is fed to the gasification unit 200 and being
gasified in
accordance to what is earlier described. The gasification process is similar
to the one
given for the description of the other preferred embodiments above.
It is understood that the objects of the present invention set forth above,
among those
made apparent by the detailed description, shall be interpreted as
illustrative and not in a
limiting sense. Within the scope of the following claims the set-up of various
alterations
CA 02834052 2013-10-22
WO 2012/150899 PCT/SE2012/050441
18
of the present invention may be possible, for instance to use a
combination/mixture of
spent liquors and bleach effluents as said aqueous solution comprising energy-
rich
material. A combination/mixture may give an opportunity to more exactly adjust
the
viscosity and the water content of the slurry to be gasified.
The gasifier in the different embodiments of the invention is of the down-
draft
entrained-flow type but it is understood that other kinds of entrained flow
gasifier may
as well be used according to the invention, e.g. an up-draft type gasifier
with the
gasification burner located on the lower portion of the gasification reactor.
Furthermore, it is understood that other materials of biomass material origin
than bio-oil
may be gasified together with the alkali containing energy rich aqueous
solution, e.g.
torrefied biomass material in powdered or granular form.
It is also understood that for reactors having more than one inlet for
feedstock there may
be possible not only to feed different feedstocks through different inlets but
to feed a
mixture of feedstock through one inlet while simultaneously feed other
feedstocks
having other compositions through other inlets so as to reach e.g. appropriate
viscosity
and alkali content of the energy-rich materials to be simultaneously gasified,
i.e. a
mixture of two or more feedstocks, in the reactor zone. In embodiments where
there are
two inlets arranged on the gasifier it may for instance be preferred to feed a
feedstock of
black liquor through one inlet and a feedstock mixture of bio-oil and bleach
effluent
through a second inlet. Moreover, in embodiments where the gasification
reactor is
provided with a third inlet a feed of e.g. bio-oil may be added through said
third inlet.
Furthermore, the wording "simultaneously gasifying" should be interpreted as
being
gasified together under the same gasification conditions in the reactor zone
of the
gasifier.