Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
IMPROVED BIOCONT ROL THROUGH THE USE OF
CHLORINE-STABILIZER BLENDS
Cross-Reference to Related Applications
Not Applicable.
Statement Regarding Federally Sponsored Research or Development
Not Applicable.
Background of the Invention
At least one invention pertains to compositions and methods for reducing
biological activity in process streams, e.g. water based process streams.
Biological activity in
process streams is problematic for a variety of reasons, including, but not
limited to sanitation
problems, process equipment efficiency problems, and product quality problems.
For example, in
papennakiing processes, high biological activity levels have a deleterious
effect on equipment
operation. The problems associated with manufacturing certain paper types,
e.g. tissue/recycled
products, are more pronounced, because high fungal levels present the quandary
of providing a
biocide program that stabilizes the biocide well enough so that it is not
readily consumed (good
persistence) and providing a biocide at sufficient levels to combat periodic
spikes in biological
activity ¨ a need for less stabilization/decreased persistence. Moreover,
bleaching/processing of
recycled fiber presents the additional quandary for papermakers because
papermakers are
balancing the addition of sulfite post bleaching/processing of recycled
fibers, which quenches a
halogen, e.g. chlorine, with the need to maintain chlorine in the system, more
specifically, a
persistent level of chlorine in the paperrnaking system without having to add
more
halogen/chlorine than is necessary. Thus, there is a need for a further
refinement of biocide-
stabilizer formulations and delivery protocols, which can treat systems more
effectively and in an
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
environmental friendly manner, such as using less chlorine/halogen, which in
turn reduces
halogen by-product formation.
At least one invention relates to methods and compositions effective at
stabilizing
oxidant biocides. Oxidant biocides such as peroxide acid and halogen chemicals
like sodium
hypochlorite have been widely used in the pulp and paper industry. These
oxidant biocides are
highly effective at immediately killing large numbers of microorganisms.
Unfortunately, after
their introduction into process water systems, oxidant biocides are not
naturally stable and they
tend to oxidize rapidly and over time lose their effectiveness. In
environments with very high
populations of microorganisms such as in process water which is rich in
organic and inorganic
material on which the microorganisms can feast, sufficient numbers of
microorganisms can
survive until after the oxidant biocides have lost effectiveness. As a result,
unless there is
sufficient residual biocide present, the microorganism population will soon
recover from an
oxidant biocide treatment. In some cases, halogen tolerant bacteria strains
develop due to
repeated introduction of single oxidant biocide. This can result in systems
suffering from out of
control bacterial growth. (See for example the textbook: Disinfection,
Sterilization, and
Preservation, Fifth Edition, by Seymour S. Block, Lippincott Williams &
Wilkins, (2001) at least
in pp. 31-57).
This problem is compounded by the fact that repealed applications of oxidant
biocides is in many contexts, not commercially feasible. Many oxidant biocides
cause adverse
effects on paper brighteners, dyes, and other additives required to produce
commercially
acceptable paper products. Repeated introduction of oxidant biocides can also
corrode many
pieces of papermaking machinery.
One technique used to address this problem is to stabilize the oxidant
biocides
allowing them to suppress the viability of microorganisms over a long time
while weakening the
2
negative impact that the oxidant biocides have on the resulting paper and the
papennaking
equipment. As described in US Patents 3,328,294, 3,749,672, 3,170,883, 5565109
and 7651622
previous attempts at stabilizing oxidant biocides included the use of sulfamic
acid, sulfamate
stabilized chlorine, monochloramine, DMH stabilized halogen, AmBr-C12, and
organic nitrogen
stabilized chlorine. While somewhat stable, these attempts have proven to be
less effective
biocides than desired. N-hydrogen sources have also been used to stabilize
oxidant biocides but
they too have been unsatisfactory because they are volatile and too rigid in
their dosage
requirements. This rigidity prevents the kind of flexible molar ratio
adjustments that are often
required to suit the specific conditions of the particular water system they
are used to treat.
Therefore there is a clear need and utility in an enhanced stabilized halogen
biocide which is
effective, compatible with other biocides, and flexible in dosage and
concentration.
Another technique to address this problem is described in US Published Patent
Applications 2006/0231505A and 2003/0029812A1 where they disclose the use of
biocide
blends. Such blends typically include an oxidant halogen which provides an
initial large kill of
the organisms and another longer lasting but less effective biocide which
provides more long
term microorganism suppression. Unfortunately many biocides are themselves
incompatible with
other biocides and the use of multiple biocides, each having their own
preparation and
introduction issues, requires an inordinate investment in complex application
equipment.
Furthermore, multiple biocide feeding machines be installed at various points
along a
papertnaking production line thereby vastly increase the cost and complexity
of adding the
biocides. So there remains need for simplified making biocide and feeding
approach.
The art described in this section is not intended to constitute an admission
that any
patent, publication or other information referred to herein is "Prior Art"
with respect to this
invention, unless specifically designated as such.
3
CA 2844833 2018-10-17
=
Brief Summary of the Invention
At least one embodiment of the invention is directed to a composition
comprising:
a halogen source, urea, and an additional halogen stabilizer excluding urea,
optionally an alkali
in a concentration sufficient to provide said composition with a pIl of
greater than 10. Optionally
the composition excludes a stabilized bromine compound. The stabilizer may
comprise one item
from the list consisting of: an N-hydrogen compound, ammonia, ammonium salts,
ammonium
sulfamate, ammonium sulfate, sulfamic acid, sodium sulfamate, cyanuric acid,
succinimide, urea,
glycouril, glycine, amino acids, and any combination thereof. The stabilizer
may comprise at
least two compositions of matter each of which function as a halogen
stabilizer. The halogen
source may be selected from the group consisting of at least one of the
following: a chlorine
source, an alkaline hypohalite, C12 gas, Na0C1, Ca(0C1)2, and electrically
generated chlorine.
.. The composition may contain: an alkaline hypohalite, urea, and ammonium
sulfamate. The urea
and additional halogen stabilizer may be in a ratio of 50:50 with one another.
At least one embodiment of the invention is directed to a method for reducing
biological activity in a process stream comprising providing the composition
to a process stream.
The composition may be added to the process stream by the following mode of
addition: forming
a mixture of at least an alkali in a concentration sufficient to maintain a pH
of greater than 10 in
the final composition and an alkaline hypohalite, and secondarily mixing said
mixture with a
second mixture containing urea and said additional stabilizer, wherein said
secondary mixing is
optionally done with a T-mixer. The process stream may be a papermaking
process stream. The
papermaking process may be a process selected from the group consisting of:
tissue and/or
4
CA 2844833 2018-10-17
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
towel, board; packaging; pulping; and recycled pulping. The process stream may
contain fungus.
The process stream may have a sulfite concentration of between 2 ppm to 50
ppm. The method
may further comprise monitoring the biological activity in the process stream
prior to and
subsequent to the addition of said composition. The biological activity may be
monitored by
taking a sample of said process stream and plating said sample on a Petri dish
or similar
apparatus or by measuring ATP levels of a sample from the process stream or by
taking a sample
of said process stream and monitoring dissolved oxygen and optionally the
oxidation reduction
potential of said sample and optionally responding by adding or reducing the
amount of one or
more chemistries which are added to said process stream, wherein said
chemistries include said
lo composition. The method may further comprise adding a second composition
to said process
stream that contains a halogen, urea, and excludes an additional N-hydrogen
compound.
At least one embodiment of the invention is directed to a method of preventing
the
growth of microorganisms in a process water stream. The method includes the
step of:
introducing a composition into the process water stream. The composition
comprises: a halogen
source, a halogen stabilizer containing a mixture of a sulfur bearing species
with urea and/or
ammonium sulfate at any ratio, and optionally an alkali. The sulfur bearing
species includes
sulfamic acid or its salt equivalent. The molar ratio of sulfamic acid to
halogen atoms in the
halogen source is more than 2:1.
The sulfur bearing species may further comprises a nitrogen stabilizer. The
.. nitrogen stabilizer may be one item selected from the group consisting of
ammonium sulfate,
sodium sulfamate. and any combination thereof The molar ratio of halogen to
all of the sulfur in
the sulfur bearing species may be more than 2:1. The alkali may be sodium
hydroxide. The
halogen may be chlorine, sodium hypochlorite, 1,3,5-Trichloroisocyanuric acid
(TCCA), 1-
bromo-3-chloro-5,5-dimethy1-2,4-imidazolidedione (BCDMH) and 1,3-dichloro-5,5-
dimethyl-
5
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
2,4-imidazolidedione (DCDMH). The method may further comprise the steps of
first adding to
the sulfamic acid an alkali and then the adding urea and/or sodium sulfate.
The process water stream may be so rich in food for microorgasnisms that a
single
halogen oxidant biocide is not effective at exterminating the microorganisms
population but the
composition is. The process water stream may be one selected from the list
consisting of a
cooling tower water stream, and papermaking process water stream. The ratio of
sulfamie acid or
its salt to nitrogen stabilizer may be optimized at any ratio between the
concerns of biocidal
efficacy and impact on chemical additive present in the process water stream.
The ratio of
sulfamic acid or its salt to nitrogen stabilizer may be optimized at any ratio
between the concerns
to of biocidal efficacy and corrosion on equipment present in the process
water stream. The
composition when used in a papermaking process might not reduce the
effectiveness of OBA and
DYE additives on paper made from that process. The salt may be sodium
sulfamate.
Additional features and advantages are described herein, and will be apparent
from, the following Detailed Description.
Brief Description of the Drawings
A detailed description of the invention is hereafter described with specific
reference being made to the drawings in which:
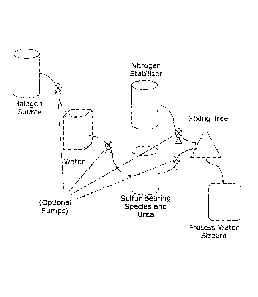
FIG. 1 is a flowchart illustrating one method of combining constituents of the
biocide composition.
FIG. 2 is a second flowchart illustrating one method of combining constituents
of
the biocide composition.
FIG. 3 is a third flowchart illustrating one method of combining constituents
of the
biocide composition.
6
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
FIG. 4 is a graph displaying data which demonstrates the effectiveness of the
invention.
Detailed Description of the Invention
The following definitions are provided to determine how terms used in this
application, and in particular how the claims, are to be construed. The
organization of the
definitions is for convenience only and is not intended to limit any of the
definitions to any
particular category.
-Alkali" means a composition of matter that functions as a pH altering
chemical
base.
"DYE' or "Dye- means one or more compositions used in the papermaking
industry to alter the optical properties of a substrate. Dyes often contain
chromophoric groups
and auxochrome and have good affinity to fiber and compatibility to other
additive in paper
industry.
"Nitrogen stabilizer- means a stabilizer which contains at least one nitrogen
atom.
"0134" means a dye or pigment based optical brightening agent which is a
component of a coating formulation commonly applied to a paper substrate. Dyes
or pigments
that absorb ultraviolet radiation and reemit it at a higher frequency in the
visible spectrum (blue),
thereby effecting a white, bright appearance.
"Pigment" means a solid material used in a papermaking process to alter the
optical properties of a substrate.
"Halogen Source" means a halogen atom by itself or a halogen atom associated
with a cationic counterpart.
7
"Halogen Stabilizer" means a halogen based material whose presence in
proximity to a composition of matter functioning as an oxidizing biocide
increases the amount of
time that the composition remains in a sufficient chemical state to continue
functioning as a
biocide, this includes but is not limited to materials which preserve (or slow
down the rate of loss
of) the oxidizing capability of the biocide composition.
"Stabilizer" means a composition of matter that increases the length of time
that
oxidizing halogen ions retain oxidant capacity and are capable of releasing
free ions slowly
thereby remaining an effective biocidal agent in a liquid environment.
"Substrate" means a sheet of paper, a sheet of paper precursor, a mass of
fibers, or
any other cellulose based or synthetic fibrous material that can be coverted
into a sheet of paper
by a papennaking process.
In the event that the above definitions or a description stated elsewhere in
this
application is inconsistent with a meaning (explicit or implicit) which is
commonly used, in a
dictionary, the application
and the claim terms in particular are understood to be construed according to
the definition or
description in this application, and not according to the common definition,
or the
dictionary definition. In
light of the above, in the event that a term
can only be understood if it is construed by a dictionary, if the term is
defined by the Kirk-Othmer
Encyclopedia of Chemical Technology, 5th Edition, (2005), (Published by Wiley,
John & Sons,
Inc.) this definition shall control how the term is to be defined.
As stated above, the present invention provides for a composition and a method
of
use for said composition, which reduces biological activity in a process
stream by providing a
more efficient application of a biocide. In turn the biocide is more
efficiently utilized, e.g.
increase in persistence of the biocide in the system when needed, which can
provide an
8
CA 2844833 2018-08-27
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
environmental benefit because a process operator can use less biocide to
combat various types of
microorganisms and bacteria that pervade process streams, e.g. including water
based systems,
wherein one water based system example is a papermaking system.
The composition contains at least the following components: halogen, urea, and
an
additional halogen stabilizer excluding urea. Stabilizers can be blended with
chlorine or bromine
to yield a milder oxidant. Benefits of halogen-stabilization include increased
persistence of the
halogen residual for improved control of microbial growth in biofilm or
surface deposits and in
systems with long residence times and high halogen demand.
Halogen-stabilization can also improve compatibility of the halogen with
sensitive
to process additives, including dyes, optical brightening agents, polymers,
and corrosion control
products. However, it has been observed in several instances that the halogen
becomes too
persistent when it is blended with stabilizers, for example urea. As a result,
the program may not
adequately control fungi and several types of bacteria, including
sphingomonads and spore-
forming bacteria. Some forms of stabilized-halogen are more volatile, reducing
the halogen
residual available in the water-phase and contributing to vapor-phase
corrosion.
In at least one embodiment optionally, there is an additional component: an
alkali
in a concentration sufficient to provide a pH of greater than 10. In at least
one embodiment, the
pH is greater than 12. In yet a further embodiment, the pH range is from 12 to
about 13.5. An
alkali can include one or more of the following chemistries: sodium hydroxide
and potassium
hydroxide.
Optionally, there is an additional component: excluding a stabilized bromine
compound from said composition.
With respect to the halogen, in at least one embodiment, the halogen is
selected
from at least one of the following: a chlorine source, alkaline hypohalite,
C12 gas (e.g. added to
9
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
H20 stream prior to blending), Na0C1, Ca(OC1)2, and electrically generated
chlorine.
In at least one embodiment the composition comprises urea in combination with
additional stabilizer, including ammonium Sulfamate, to stabilize halogens for
biocontrol
In at least one embodiment, the stabilizer is an N-hydrogen compound.
In at least one embodiment, the N-hydrogen compound is ammonium sulfamate.
In at least one embodiment, the N-hydrogen compound excludes ammonium
sulfate.
In at least one embodiment, the composition contains: an alkaline hypohalite,
urea,
and ammonium sulfamate.
to The ratios between urea and an additional stabilizer can vary
depending upon
system conditions, e.g. levels of fungus. For example, one could take into
account chemical
kinetics between: (a) urea with halogen; (b) additional stabilizer with
halogen; and (c) blend of
urea and additional stabilizer with halogen.
In at least one embodiment, the stabilizer blend between urea and the
additional
stabilizer is 50:50.
A method for reducing biological activity in a process stream is also
disclosed, e.g.
process stream contained in a water system. The method comprises: providing a
composition to a
process stream, wherein said composition contains: a halogen, urea, and an
additional stabilizer
excluding urea, optionally an alkali in a concentration sufficient to provide
said composition with
a pH of greater than 10; and optionally excluding a stabilized bromine
compound from said
composition.
In at least one embodiment, the composition is added to the process stream by
the
following mode of addition: forming a mixture of at least an alkali in a
concentration sufficient to
provide a pH of greater than 10 and an alkaline hypohalite, and secondarily
mixing said mixture
with a second mixture containing urea and said additional stabilizer, wherein
said secondary
mixing is optionally done with a T-mixer.
In at least one embodiment, the method comprises: adding a second composition
to said process stream that contains a halogen, urea, and excludes an
additional N-hydrogen
compound.
With respect to the order of addition of the components, In at least one
embodiment, the composition is added to the process stream by the following
mode of addition:
forming a mixture of at least an alkali in a concentration sufficient to
provide a plI of greater than
10, preferably 12 to 13.5, and an alkaline hypohalite, and secondarily mixing
said mixture with a
second mixture containing urea and an additional stabilizer. One of ordinary
skill in the art could
mix the first mixture and second mixture via a variety of techniques, e.g.
apparatuses.
In at least one embodiment, the first mixture and second mixture are mixed
together with a T-mixer. One of ordinary skill the art would understand what a
T-mixer is.
hi at least one embodiment, one of ordinary skill in the art can utilize a
mixing
Is chamber, such as the one disclosed in U.S. Patent No. 7,550,060,
to carry out a mixing protocol of the chemistries.
The methodology of the present invention is applicable to a variety of process
streams or aqueous based systems or water based systems or industrial based
systems or a
combination thereof
In at least one embodiment, the process stream is a papermaking process
stream.
In at least one embodiment, the papermaking process is a process selected from
the group consisting of: tissue and/or towel, board; packaging; pulping; and
recycled pulping.
In at least one embodiment, the process stream contains fungus.
11
CA 2844833 2018-08-27
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
In at least one embodiment, the process stream has a sulfite concentration of
between 2 ppm to 50 ppm.
The efficacy of the composition for reducing biological activity can be
measured
by a variety of analytical techniques and controls schemes.
In at least one embodiment, the process stream further comprises monitoring
said
biological activity in said process stream prior to and subsequent to the
addition of said
composition.
In at least one embodiment, the biological activity is monitored by taking a
sample
of said process stream and plating said sample on a Petri dish or similar
apparatus.
In at least one embodiment, the biological activity is monitored by measuring
ATP
(adenosine triphosphate) levels of a sample from said process stream.
In at least one embodiment, the biological activity is monitored by taking a
sample
of said process stream and monitoring dissolved oxygen and optionally the
oxidation reduction
potential of said sample and optionally responding to said biological activity
by adding or
reducing the amount of one or more chemistries which are added to said process
stream, wherein
said chemistries include said composition.
The compositions by themselves or compositions utilized to treat a process
stream
can be made outside of the process stream or within the process stream (in
situ) or a combination
thereof.
In at least one embodiment a composition comprising a halogen, a halogen
stabilizer, and optionally an alkali are provided for inhibiting the growth of
microorganisms in a
papermaking environment. The stabilizer is a composition comprising sulfur.
The sulfur bearing
species includes sulfamic acid (or its salt equivalent such as sodium
sulfamate). The molar ratio
of the halogen to the sulfamic acid is more than 2:1. By having such a large
ratio of halogen to
12
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
stabilizer, it has been observed that an unexpected biocidal effect occurs.
This was quite
surprising as at a molar ratio of 1:1 of halogen to sulfamic acid, no
significant anti-biological
efficacy was observed. Moreover because the stabilizers are needed to
stabilize the halogens, it
would be expected that more stabilizer relative to halogen would better
stabilize the halogen, yet
the opposite is the case.
In at least one embodiment the stabilizer is a composition comprising a
mixture
of sulfur bearing species with urea. The halogen is mixed with sulfamic acid
at molar ratio of
Nitrogen to Chlorine of more than 2:1. By having such a stabilizer mixture of
stabilized halogen,
it has been observed that an unexpected synergistic effect occurs which
results in the halogen
to remaining stabilized for a longer period of time, and without impairing
the quality of the
produced paper or corroding the papermaking equipment.
In at least one embodiment the stabilizer is a composition comprising a
mixture
of sulfur bearing species with ammonium sulfate.
In at least one embodiment the sulfur bearing species further comprises a
nitrogen
stabilizer.
In at least one embodiment the nitrogen stabilizer is one item selected from
the
group consisting of ammonium sulfate, sodium sulfamate, or any combination
thereof.
In at least one embodiment the molar ratio of halogen to all of the sulfur in
the
sulfur bearing species is more than 2:1.
In at least one embodiment the alkali is sodium hydroxide.
In at least one embodiment the halogen are chlorine, sodium hypochlorite.
1,3,5-
Trichloroisocyanuric acid (TCCA), 1-bromo-3-chloro-5,5-dimethy1-2,4-
imidazolidedione
(BCDMH) and 1,3-dichloro-5,5-dimethy1-2,4-irnidazolidedione (DCDMH).
13
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
In at least one embodiment the sulfamic acid is first amended with alkali and
then
the urea/ammonium sulfate is added. Sodium hypochlorite is added to above
mixture.
In at least one embodiment the sulfur baring nitrogen combined sodium
hypochlorite first at molar ratio more than 2:1 nitrogen to chlorine and then
is added to urea or
ammonium sulfate.
In at least one embodiment the urea or ammonium sulfate combined sodium
hypochlorite first then is added to sulfur baring nitrogen at different ratio.
The order is
significant because different stabilized halogen species are generated at
different rates due to
differing equilibrium constants. These differences can be accounted for by
dosing the halogens in
to different amounts and in different orders. Also chlorine is able to
transfer from stabilized
chlorine to other nitrogen species so the order of combinations can compensate
for that.
In at least one embodiment the composition contains no buffer.
In at least one embodiment the composition contains no alkali.
In at least one embodiment the composition can be formulated on site by mixing
the components together before mixing with halogen oxidant.
In at least one embodiment the composition can be formulated on site by mixing
the components as illustrated in any one of FIGs 1, 2, and/or 3.
In at least one embodiment the microorganisms killed by the biocide are
sessile.
In at least one embodiment the microorganisms killed by the biocide are
planktonic.
One noted benefit of the invention is the fact that the sulfamic acid and the
nitrogen stabilizer readily combine so when mixing the two a high product
yield is achieved with
little waste. In addition, unlike stand alone stabilizers containing inorganic
nitrogen stabilizers,
the mixture of sulfamic acid and nitrogen stabilizer functions at many
different ratio amounts. As
a result the relative amounts of sulfamic acid or nitrogen stabilizer can be
appropriately increased
14
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
or decreased depending on the particular environment it is to be used in. For
example in cases
where nitrogen stabilizer may interfere with particular paper additives such
as OBA or DYE, the
relative amount of sulfamic acid will be increased. In contrasts in contexts
where the sulfamic
acid has compatibility issues, the amount of nitrogen stabilizer can be
increased.
In at least one embodiment the details of the formulation is targeted towards
the
nature of the biological infestation. For example if bacteria are just
beginning to infiltrate one or
more items of process equipment, a formulation containing relatively equal
amounts of sulfamic
acid and the nitrogen stabilizer is used because it is optimized to causes low
impact on additives
and low degrees of corrosion which is more desirable than a highly effective
biocide when the
to infestation is weak. In contrast, when the contamination is intense or
long term colonization,
effectiveness of the biocide is more important than the one time effects on
additives or corrosion
and a therefore a formulation containing more sulfamic acid relative to the
molar amount of
nitrogen stabilizer is used. Thus by using a formulation having only two
variables, a number of
condition specific ratios can be provided which requires a simple input system
yet is capable of
.. dynamically responding to different conditions over the life cycle of the
industrial facility.
In at least one embodiment the composition is used as a biocidal agent in a
cooling
tower.
In at least one embodiment the composition is used to reduce biofilm on a
surface.
Biofilm is the accumulation of sessile organisms on the surfaces of equipment.
Such
accumulations often pose particular problems as the available exposed surface
area for the
biocide to work on is reduced. Moreover there is often a tradeoff between
biocide efficacy and
impact the biocide has on biofilms yet the invention avoids harmful effects on
process equipment
yet effectively neutralizes biofilms.
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
In at least one embodiment the composition is used to treat microorganisms in
a
membrane system. Membrane systems are often prone to biofilm colonization as
microorganisms find their surfaces (because of composition, shape, or both)
attractive. As they
are also very delicate relative to other forms of process equipment, the
general tradeoff issues are
even more pronounced in membranes. Fortunately the composition is effective at
treating
membrane biofilms without damaging them. In at least one embodiment the
membrane system is
a water permeable membrane. In at least one embodiment the membrane is a part
of a water
treatment system.
In at least one embodiment the composition has a particular pH before it is
introduced into the system. In at least one embodiment the pH is greater than
5 and less than 12,
and is most preferably between 8 and 10.
In at least one embodiment the ratio of the contents of the composition are
balanced to optimize the composition's effectiveness and utility. In the prior
art chlorosulfamate
was used in a ratio of 1:1 with chlorine. This resulted in stronger than
desired bonding of the
chlorine and as a result it reduced the rate of releasing sulfamate from
sulfate thereby reducing
the effectiveness of the composition. In at least one embodiment the ratio is
different and as a
result the composition is more effective. In at least one embodiment the ratio
of sulfamate to
stabilizer within the composition is between (less than 4):1 and (more than
1):1. Experimental
data has shown that in some circumstances ratios of 1:1 and 4:1 do not work at
all or at best work
poorly, ratios of 8:1 to 4:1 work somewhat and that 3:1 is highly effective as
a biocide. This
demonstrates that an unexpected sysnergistic effect based on more than just
concentration is at
work which is wholly novel and unexpected.
EXAMPLES
16
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
The foregoing may he better understood by reference to the following
examples, which are presented for purposes of illustration and are not
intended to limit
the scope of the invention.
A number of biocide formulations were prepared and were applied to
samples of process water from a paper mill. Their compositions and
effectiveness are
listed in FIG. 4 and in Table 1. Table 1 illustrates that a composition
comprising 12%
Sulfamic Acid and 3% Ammonium Sulfate is able to achieve high product yield
without
addition of NaOH. It also demonstrates that the addition of NaOH in bleach can
improve
feasibility of blending stabilizers at different rates.
Optimization of blending condition for inixiii!! stabilizer and N odium
ltypocidorite
Stabilizer formula [ Mclar Ratio TIRO*** FRO****, TRO Yield
FROITRO
C12: N2 P P m Fro % %
Caustic in Bleach= 0.5%
3%S.A' +12%Aa'' 1:1 22CC 51 29.32 2.77
4:1 15E0 620 19.26 33.55
7.5%5A+7,5%AS 1:1 3575 220 47.26 6.15
4:1 34C0 960 42.17 28.24
12 %SA+3 %AS 1:1 62E0 370 81.31 6.97
4:1 54C0 2330 86.78 37.69
sulfamic acid 4:1 65E0 121 9 80.93 18.61
Caustic in Bleach= 3.2%
3 %SA+12 %AS 1:1 62E6 810 70.16 16.38
4:1 49CE 1676 60.96 34.16
7_6% SA+7 5%AS 1:1 4050 146 53_54 3_60
4:1 8019 1330 99.45 12.47
12%8A+3%AS 1:1 3524 240 46.22 6.01
4:1 68E5 3000 85.14 43.57
sulfamic acid 4:1 6303 1450 77.84 23.02
*SA sulfatnic Acid
AS: ammonium sulfate
***TRO- total re sicical oxidant
****FRO: free residllal oxidat
Table 1
FIG. 4 illustrates that 12% Sulfamic Acid and 3% ammonium sulfate
showed more active on bioactivity inhibition than other combinations of
stabilizers.
Without being limited in theory and the scope afforded in construing the
17
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
claims, it is believed that naturally the chlorine transfers back and forth
from one
chloronitrogen species to another chloronitorgen species according to the
equilibrium
equations below and the invention makes use of the different equilibrium
constants to
optimize the presence of the desired reactions that produce the particularly
desired
chloronitrogen species that is effective as a biocide.
H,NSO,H + Na0C1 <=> C1H1VS03H + NaOH
CLU1VSO3H +Na0C1<=> Cl,NSO,H + NaOH
(NH 4)2S0,+2Na0C1 <=>2iVfl 2C1 + Na2SO4+ 211 20
NH 2C1+ Ala0C1 <=> 2111-1C12+ Na01-1
H ,NS0,11 +NH 2C1 CIHNS0311 +
CHINA-VI +NiI2Cl C12N50311 +NH
In at least one embodiment the dosing sequence of the composition is
calibrated to
make optimal use of the relative equilibrium rates of the various chemical
reactions. Each of the
chemical reactions occurs at different rates and as a result Cl species are
constantly passing back
and forth between molecules and have different availabilities at different
times. In at least one
embodiment the reagents required for the lower occurring reactions are added
to the composition
first and are allowed to react somewhat or completely before the reagents
required for the faster
reactions are added. This avoids the faster reactions competing with the
slower reactions. In at
least one embodiment the reagents required to allow the chlorosulfamate
species to react with the
amine to form chloramine and ammonia is only added to the composition after
chloroamine has
been partially or completely formed.
In at least one embodiment the composition is diluted to produce a more
mild (and less violent, reactive, or destructive) biocide effect. In at least
one embodiment
18
the methods of diluting biocides disclosed in US Patents 6,132,628 and
7,067,063 are
employed. In at least one embodiment the composition is diluted so the species
exists
within the range of 100 ppm to 150,000 ppm.
While this invention may be embodied in many different forms, there are
shown in the drawings and described in detail herein specific preferred
embodiments of
the invention. The present disclosure is an exemplification of the background
and
principles of the invention and is not intended to limit the invention to the
particular
embodiments illustrated
to Furthermore, the invention encompasses any possible combination of
some or all of the various embodiments described herein and incorporated
herein.
The above disclosure is intended to be illustrative and not exhaustive. This
description will suggest many variations and alternatives to one of ordinary
skill in this art. All
these alternatives and variations are intended to be included within the scope
of the claims where
the term "comprising" means "including, but not limited to". Those familiar
with the art may
recognize other equivalents to the specific embodiments described herein which
equivalents are
also intended to be encompassed by the claims.
All ranges and parameters disclosed herein are understood to encompass any and
all subranges subsumed therein, and every number between the endpoints. For
example, a stated
range of "1 to 10" should be considered to include any and all subranges
between (and inclusive
of) the minimum value of 1 and the maximum value of 10; that is, all subranges
beginning with a
minimum value of 1 or more, (e.g. Ito 6.1), and ending with a maximum value of
10 or less,
(e.g. 2.3 to 9.4, 3 to 8, 4 to 7), and finally to each number 1, 2, 3, 4, 5,
6, 7, 8, 9, and 10 contained
within the range.
19
CA 2844833 2018-08-27
CA 02844833 2014-02-06
WO 2013/059074 PCT/US2012/059846
This completes the description of the preferred and alternate embodiments of
the
invention. Those skilled in the art may recognize other equivalents to the
specific embodiment
described herein which equivalents are intended to be encompassed by the
claims attached hereto.
20