Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02845670 2014-02-13
. .
SYSTEMS AND METHODS FOR PREVENTING UNSAFE MEDICAL TREATMENT
Cross-Reference to Related Application
[0001] This application claims the benefit of U.S. Provisional
Application Serial No.
61/529,571, filed on August 31, 2011, the contents of which are herein
incorporated by
reference in their entirety.
Field of Invention
[0002] The present invention relates to systems and methods for
preventing the
unsafe administration of potentially hazardous treatments from medical
devices, and more
particularly, to systems and methods for quality assurance (QA) of radiation
therapy beam
delivery in clinical oncology.
Background of the Invention
[0003] There is a need for independent process control of
radiation oncology
(RADONC) treatment to help verify that a treatment about to be delivered to a
patient is not
unsafe or otherwise inappropriate. This need was echoed by recent RADONC
accidents as
reported January 23, 2010 in the New York Times (NYT): "The Radiation Boom:
Radiation
Offers New Cures, and Ways to Do Harm" by Walt Bogdanich. At least two of
these
accidents resulted in the eventual death of the patient. Quoting from the
article ¨ "...its
complexity has created new avenues for error ¨ through software flaws, faulty
programming, poor safety procedures or inadequate staffing and training. When
those
errors occur, they can be crippling. 'Linear accelerators and treatment
planning are
CA 02845670 2014-02-13
enormously more complex than 20 years ago,' said Dr. Howard I. Amols, chief of
clinical
physics at Memorial Sloan-Kettering Cancer Center in New York. But hospitals,
he said, are
often too trusting of the new computer systems and software, relying on them
as if they had
been tested over time, when in fact they have not."
[0004] Some disturbing facts particularly noted in the NYT article:
= On 133 occasions, devices used to shape or modulate radiation beams were
inadvertently omitted or used improperly, including wedges; and
= On 284 occasions, the radiation missed some or all of the intended
treatment area, in
some cases being directed to the entirely wrong area of the body (e.g.,
radioactive
seeds intended for a man's cancerous prostate were placed in the base of his
penis;
stomach cancer patient treated for prostate cancer. In 50 instances, this was
the
result of patients receiving a course of radiation treatment intended for a
different
patient (e.g., a brain cancer patient received radiation for breast cancer);
= In New York State alone, there were 21 accidents relating to beam-
modifying
devices, leading to a special alert in December 2004 and an additional alert
in April
2005, that hospitals need to ensure that radiation field size. and shape are
appropriate prior to delivery to the patient.
= An extremely serious accident occurred in New York in 2005, involving
improper and
unsafe radiation delivery to a patient, even after these warnings. Among other
=
issues, a radiation therapist incorrectly programmed the computer controlling
a linear
accelerator (LINAC) to "wedge out" rather than "wedge in" (as the plan
required).
The medical physics staff failed to notice it during weekly checks, and
therapists
during subsequent treatments (despite the fact that "wedge OUT" appeared on
their
screens). On 27 occasions, radiation was delivered with the improper
configuration.
= Investigations into this accident looked at the computer and software
that drove the
LINAC. The software required programming instructions be saved in the
following
order in the computer: a) the quantity or dose of radiation in the beam; b) a
digital
image of the treatment area; and c) instructions that guide the multileaf
collimator.
The computer crashed repeatedly and the medical physicist did not realize that
collimator instructions were not being saved, and proceeded on the assumption
that
2
CA 02845670 2014-02-13
they were. There was no fail-safe to ensure the proper radiation was being
administered.
[0005] Following the NYT article, the American Society for Radiation
Oncology
(ASTRO) issued a press release February 3, 2010, committing itself to a six-
point patient
protection plan, the fifth being "Further developing our Integrating the
Healthcare Enterprise
¨ Radiation Oncology (IHE-RO) connectivity compliance program to ensure that
medical
technologies from different manufacturers can safely transfer information to
reduce the
chance of a medical error."
[0006] Following the ASTRO press release, there were two patient safety
meetings:
1) FDA Public Meeting (March 30, 31, 2010) and 2) AAPM/ASTRO Safety Meeting
(June 9,
2010). Human error was considered the primary area where significant
improvement was
needed, followed by training and reducing device design complexity of use,
including
standardization and "human factors engineering" of these devices.
[0007] Most recently, the Technical Committee of ASTRO's IHE-RO produced a
Framework titled "Quality Assurance with Plan Veto Profile" that aims to
prevent the most
serious errors as outlined in the NYT. Their goal is for independent QA
vendors to provide
this Quality Check role that would possess veto power over the treatment
delivery in the
event of a pending serious error, specifically excluding the Treatment
Delivery Device
vendors from this role. Complicating this Quality Assurance Plan Veto (QAPV
per the IHE-
RO Technical Committee) is the diversity of delivery machines and their
operating systems
that control beam delivery. Allowing a third party QA vendor to override a
beam delivery, let
alone designing for all these systems, is a daunting task facing many hurdles.
[0008] Systems for preventing unsafe beam operation already exist with most
ionizing
radiation treatment delivery devices (TDDs). It is available in the form of
external safety
interlocks provided by the institution. The most common is the door interlock
with a simple
3
CA 02845670 2014-02-13
electrical switch that is normally open when the door to the treatment room is
open and
closed when the door is closed. By way of example, a simplified schematic of a
door
interlock from a common LINAC system is illustrated with reference to FIG. 1.
In addition to
the door interlock, there may also be a visual warning indicator that the door
to the
treatment room is open. Notably, such systems have nothing to do with
preventing pending
radiation delivery to a patient based on some characteristics of that
radiation delivery being
improper and/or unsafe, of itself. Instead, these systems prevent delivery if
circumstances
exist which would result in hazards external to the patient treatment ¨ e.g.,
the danger of a
person walking into the treatment room during radiation delivery to a patient.
[0009] The entity operating the LINAC or other treatment delivery device is
ultimately
responsible for the safe use thereof, but conventionally these safety measures
have
focused on the many hazards associated with setup and use; for example, with
the LINAC
system, issues surrounding collision, beam portal imaging during treatment,
beam limiters
with certain accessories, and so on. The entity's assurance of safe treatment
beyond
hazard mitigation with safety features provided with RADONC equipment vendors
(e.g., the
door interlock) was limited to operator training of equipment use, visual and
audio
monitoring of the equipment. In particular, there have not been any means to
automatically
prevent unsafe treatments that help ensure an independent assessment of the
pending
treatment against the planned treatment.
Summary of the Invention
[0010] As can be seen from the foregoing, there has been for some time an
urgent
need for systems and methods that can prevent unsafe radiation delivery to
patients.
According to an embodiment of the present invention, a medical radiation
delivery system
comprises a treatment delivery device for delivering ionizing radiation to a
patient, a
4
CA 02845670 2014-02-13
treatment safety device including at least one of a warning indicator and an
operational
interlock for preventing improper operation of the treatment delivery device,
and at least one
processor and machine readable memory. The processor and memory are configured
to
execute a treatment delivery device control module for operating the treatment
delivery
device to deliver the ionizing radiation to the patient, and a treatment
verification module for
determining whether predetermined treatment verification criteria are met and
operating the
treatment safety device based thereon.
[0011] According to a method aspect, a method for preventing unsafe
delivery of
ionizing radiation to a patient comprises supplying at least one electronic
verification input to
a treatment verification module executed by at least one processor using
machine readable
code. Using the treatment verification module, it is determined whether the at
least one
electronic verification input satisfies at least one treatment verification
criteria. Based on the
determination of whether the at least one treatment verification criteria is
satisfied, a
verification output signal from the treatment verification module is
generated, and a
treatment safety device is operated based on the verification output signal to
allow or inhibit
the delivery of ionizing radiation by a treatment delivery device.
[0012] According to an advantageous aspect of the present invention, the
treatment
safety device includes an operational interlock that will prevent operation of
the treatment
delivery device. Preferably, the operational interlock is integrated into door
interlock circuitry
that will interrupt electrical power supply to the treatment delivery device.
[0013] These and other objects, aspects and advantages of the present
invention will
be better understood in view of the drawings and following detailed
description of preferred
embodiments.
CA 02845670 2014-02-13
Brief Description of the Drawings
[0014] Figure 1 is a schematic diagram of a radiation oncology treatment
system with
a door interlock; and
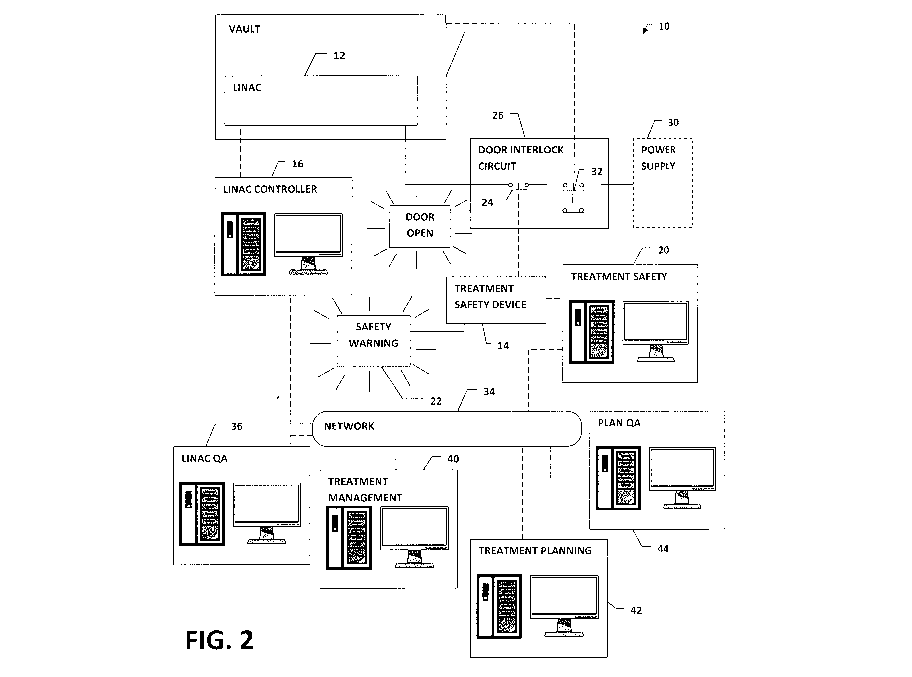
[0015] Figure 2 is a schematic diagram of a radiation oncology treatment
system,
according to an embodiment of the present invention.
Detailed Description of the Preferred Embodiments
[0016] The present invention will now be described more fully hereinafter
with
reference to the accompanying drawings, in which a preferred embodiment of the
invention
is shown. This invention may, however, be embodied in many different forms and
should
not be construed as limited to the embodiments set forth herein. Rather, this
embodiment is
provided so that this disclosure will be thorough and complete, and will fully
convey the
scope of the invention to those skilled in the art.
[0017] Referring to Figure 2, according to an embodiment of the present
invention, a
radiation oncology system 10 is adapted to prevent unsafe delivery of
radiation to a patient.
As used herein, radiation oncology (RADONC) refers to the study and treatment
of cancer
disease with ionizing radiation. RADONC will ordinarily involve personnel,
equipment and
facilities designed for imaging and diagnosis of cancer patients, then cancer
treatment
planning and delivery to the patient. However, aspects of the present
invention can readily
be applied to medical delivery of radiation for other purposes (e.g., medical
imaging
unconnected with cancer), as well as to the safe administration of medical
therapies
involving potentially dangerous modalities other than ionizing radiation
(e.g., magnetic
resonance imaging (MRI), robotic surgery, etc.)
[0018] The radiation oncology system 10 includes a treatment delivery
device (TDD)
12, a treatment safety device 14 and at least one processor and machine
readable memory
6
CA 02845670 2014-02-13
configured to execute a treatment delivery device control module 16 for
operating the
treatment delivery device and a treatment safety module 20. The treatment
safety module
20 determines whether predetermined treatment verification criteria are met
and, based on
the determination, operates the treatment safety device 14 to prevent improper
operation of
the treatment delivery device 12. Preferably, the treatment safety device 14
activates at
least one a warning indicator 22 and an operational interlock 24 to prevent
improper
operation.
[0019] The treatment delivery device 12 is a device for delivering a
treatment to a
patient. In the depicted embodiment, the treatment delivery device 12 is a
device that uses
ionizing radiation in a form that treats disease, and more particularly a
linear accelerator
(LINAC). As discussed above, a treatment delivery device may be used which
does not
deliver ionizing radiation, although the present invention is particularly
advantageous in that
context. In the context of treatment devices delivering ionizing radiation,
there are
teletherapy devices (radiation source external to the patient with an emitted
beam of
radiation directed toward the patient) and brachytherapy devices (radiation
source internal
to the patient with radiation directed toward the disease site). Examples of
teletherapy
devices are linear accelerators (LINAC) produced by Varian, Palo Alto, CA;
TomoTherapy,
Madison WI; Elekta, Crawley UK; Siemens, Erlangen, Germany; Accuray,
Sunnyvale, CA.
Radiation isotopes such as Cobalt 60 are also used for teletherapy, produced
by Viewray,
Cleveland OH and formerly by Atomic Energy of Canada (AECL). Examples of
brachytherapy devices are radioisotopes encapsulated in catheters and
manipulated in a
patient by a delivery system produced by Nucletron in Netherlands and Varian
in Palo Alto
CA. Electronic X-ray sources are also encapsulated for catheter delivery, as
exampled by
Xoft, Sunnyvale CA.
7
CA 02845670 2014-02-13
[0020] The treatment safety device 14 is a device that converts, as
necessary, the
logical determination of the treatment safety module 20 concerning treatment
verification
criteria into a form that can effect operation of the warning indicator 22
and/or operational
interlock 22. The treatment safety device 14 is not necessarily a physically
separate device
from the computer(s) executing the linac control and/or treatment safety
modules 20.
[0021] The warning indicator 22 is an indicator that is perceivable by an
operator of
the system 10. For example, the indicator 22 can incorporate visual, audible
and/or tactile
indicator elements. The warning indicator 22 could use a display associated
with the linac
control module 16 or some other system 10, and/or employ a special, purpose-
built device,
such as an illuminatable sign located in a control room for the treatment
delivery device 16.
[0022] The operational interlock 24 is not necessarily limited to a
particular interlock
type. For example, the operation interlock could include a mechanical
interlock and/or
electrical interlock effect to prevent operation of the treatment delivery
device 12 until the
interlock is satisfied. Advantageously, the operational interlock 24 is an
electrical interlock
located in a circuit 26 to interrupt the supply of electrical power to the
treatment delivery
device 12 from a power supply 30. In the depicted embodiment, the circuit 26
is a door
interlock circuit and the operational interlock 24 is a switch located
therein, preferably in
series with a door interlock switch 32, such that both switches must be closed
for the
treatment delivery device 12 to be operated. Alternately, where no existing
interlock is in
place, one could be added specifically for the system 10.
[0023] For clarity of illustration, the linac control module 16 and
treatment safety
module 20 are depicted as separate computers in communication via a network
24. While
these modules operate independently of one another, the present invention does
not
necessarily require that they be run by separate computers, but could be
executed by a
single machine. Likewise, various functions of either module 16, 20 could be
distributed
8
CA 02845670 2014-02-13
across a plurality of computers. As used herein, a "computer" is an electronic
device having
at least one processor that can execute program instructions stored in machine
readable
memories to perform a function. The present invention is not necessarily
limited to a
particular number, type or configuration of computers and/or processors, or to
a particular
type or format of memory, or to any particular programming language.
[0024] A "network" generally includes the electronic components that allow
two or
more computers to communicate data therebetween. A wireless or wired local
area network
and the Internet are examples of networks, although the present invention is
not necessarily
limited thereto, and moreover, the term "network" can encompass multiple types
and levels
of networks used in conjunction to transfer data between computers. In the
RADONC
context, data transfer is advantageously through network connectivity using a
NEMA
DICOM standard (Digital Imaging Communication in Medicine). NEMA, the
Association of
Electrical and Medical Imaging Equipment Manufacturers, maintains the DICOM
data
standard is in use by most of the RADONC systems.
[0025] In operation of the system 10 as described above, when a pending
treatment
is received by the linac control module 16, the treatment safety module 20
operates the
treatment safety device 14 such that the warning indicator 22 and/or
operational interlock 24
will be activated (i.e., to prevent treatment) until the predetermined
verification criteria are
satisfied. Once the verification criteria are satisfied, the treatment safety
device 14 releases
the indicator 22 and/or interlock 24.
[0026] A plurality of verification criteria can be advantageously
employed, either
singularly or in various combinations, in connection with the present
invention. The
treatment safety module 20 can include a user interface for receiving entry of
verification
criteria, and acceptable data, ranges, thresholds, etc. that satisfy the
criteria. In one
version, satisfaction of the verification criteria can include ensuring a
checklist procedure is
9
CA 02845670 2014-02-13
followed, completed and signed off on by the therapists prior to treatment
(i.e. 'right patient,
right site, right field size'). This checklist can be user-defined, and can
include any setup,
settings, or other visual checks the user may want but have no other way to
require be done
prior to treatment.
[0027] Via a user interface, a user can also enter general operating
thresholds for the
TDD 12 (e.g., thresholds that should generally not be exceeded for any use of
the TDD,
regardless of patient). If one or more such thresholds is not within the
acceptable range,
then the treatment verification criteria would not be satisfied, and the
treatment safety
device 14 will be operated to prevent treatment. A provision for operator
override, to allow
operation outside normal thresholds in special circumstances could also be
provided ¨ or in
the face of any other verification criteria that is not satisfied.
[0028] External software applications can interact with the treatment
safety module
20 as a further source of verification criteria. Examples of software modules
include a
treatment delivery device quality assurance (QA) module 36, a treatment
management
(TMS) module 40, a treatment planning (TPS) module 42 and a dose QA module 44.
As
with the control module 16 and treatment safety module 20, these additional
modules can
be executed by the same computer, or by different computers sharing data over
the network
34. There can also be integration with third party devices that require beam
control (i.e.
motion tracking cameras that will turn the beam off if patient moves beyond a
certain limit),
and the like, and can also provide a back-up to a door interlock to prevent
operation if the
treatment room door is opened during treatment.
[0029] An example of how an input from treatment device QA (e.g., as
received from
the module 36) can be a treatment verification criteria in the RADONC context
is if a specific
energy on the TDD had a measured daily output that was unsafe.
CA 02845670 2014-02-13
[0030] Using all of the data available from the modules 40-44, the system
10 can
effectively intercede to prevent unsafe treatment delivery from the TDD if the
plan pending
for delivery by the TDD is not the plan that was intended to be delivered to
the patient. With
respect to this distinction, it is useful to consider the relationship between
an intended
treatment and a pending treatment in the RADONC context.
[0031] The intended treatment is a TPS treatment plan after it has been
approved by
the RADONC personnel. A TPS treatment plan is pending in any state preceding
treatment.
There are several approval steps; the approval steps and criteria vary across
institutions,
depending upon the RADONC system. A first possible "approval step" is
typically a review
of several plan candidates at the TPS 42 console, resulting in selection of
one, which then
becomes the intended treatment. This selection may involve an independent plan
computation check using the selected plan data, which can also be a
verification criteria
considered by the treatment safety module 20.
[0032] The transfer of the selected plan to the TMS 40 and then TDD
control 16 and
TDD 12 via the RADONC system's network 34 is a common need, and represents
opportunity for data corruption, and if corruption occurs, the pending
treatment is no longer
the intended treatment. There is an opportunity for verification criteria to
be utilized after
transfer to the TMS 40; that is to compare the plan residing on the TMS 40 to
the plan from
the TPS 42, through the treatment safety module 20. Another similar
verification criteria
opportunity exists ¨ comparing the plan residing on the TDD control module 16
after the
transfer from TMS 40 using the treatment safety module 20. These two steps can
help
ensure that the pending treatment is equivalent to the intended treatment that
was approved
at the TPS 42.
[0033] Prior to the patient treatment, the Intended Treatment may be
delivered to a
dosimetry phantom, a QA measurement with the phantom being a surrogate
patient.
11
CA 02845670 2014-02-13
Following treatment, the dose delivered to the phantom (computed via the dose
QA module
44) is compared to the planned dose associated with the intended treatment as
another
verification criteria; and if equivalent, then the Intended Treatment was
delivered, confirming
the TDD contains the approved plan.
[0034] A final verification criteria can involve the technician during
the patient
sessions, or fractions. Visual inspection of the patient setup and TDD
parameters prior to
treatment can prevent personnel errors, oversights, and incomplete processes
before the
pending treatment is delivered with more assurance that it is the intended
treatment ¨ this
step can be accomplished via checklist procedure as described above.
[0035] There are other data sources, such as the log files following a
treatment which
provide the treatment data that was delivered. These data can also be captured
and
compared to the intended treatment data. This is after the fact, but for most
RADONC
treatments, the treatment course is over many fractions (e.g., 30 fractions)
and the
opportunity for verification after the pending treatment delivery was
delivered and found to
= be incorrect still allows for the treatment safety module 20 to prevent
delivery of the next
= fraction (typically the following day) via operation the treatment safety
device 14.
[0036] The present invention makes is possible to evaluate a pending
treatment
against an intended treatment using the comparison of this data from these
sources by the
treatment safety module 20. As described above, in the RADONC context, the
data
transfer is preferably through network connectivity using a NEMA DICOM
standard (Digital
Imaging Communication in Medicine), which also allows communication with the
linac
control module 16, TMS 40 and TPS 42. For example, the treatment safety module
20
subscribes to the DICOM data traffic as a listener and captures intended and
pending
treatments when the data is pushed onto the network from the appropriate
systems. The
12
CA 02845670 2014-02-13
approved TPS treatment plan (intended treatment) data is available immediately
after plan
approval, and is stored by the treatment safety module 20 for later
comparisons.
[0037] However, there is more than one method of treatment data
transfer,
depending on the RADONC system. The module 20 interprets the data, pending and
intended, by means of software that compares the TDD 12 setup and
characteristics to the
TPS 42 plan. The TDD setup and characteristics may not be directly accessible
by the
treatment safety module 20, but can receive its setup instruction just prior
to treatment from
the TMS 40. The TDD controller 16 (controlling TDD/LINAC 12) may receive the
TMS 40
instruction just prior to patient mode-up or it may recall it from its own
database. The TDD
controller 16 may be arranged to export its setup to the treatment safety
module 20.
[0038] TMS (Treatment Management System ¨ from the IHE-RO Technical
Committee) is used as a descriptive acronym, although the planning to
treatment interface
(PTI) accomplished thereby can go by other names, such as Record and Verify
(RV) (an
early name), Oncology Information System (01S) and Electronic Medical Record
(EMR)
. which describe global departmental features. TMS incorporates multiple
data points that
can be used in the treatment safety module 20 determination, including the
automatic setup
parameters for the TDD 12, such as MLC positions, gantry angle, collimator
position, beam
energy, and beam monitor units. (An example of a TMS system that can work
advantageously in connection with the current invention is the MOSAIQ system
from
Elekta.)
[0039] The present invention provides great flexibility and adaptability
in preventing
the delivery of an unsafe treatment from a TDD, and particularly in the case
of the delivery
of ionizing radiation. This allows entities that use such devices to
automatically ensure that
proper standards are being met, from an evaluation of tolerance values for a
particular test
that is monitored during a QA check to a treatment plan review of dose volume
histograms
13
CA 02845670 2014-02-13
(DVH) of anatomical structures (treatment targets and critical organs) as
compared to an
institution's standard of care.
[0040] Many
modifications and other embodiments of the invention will come to the
mind of one skilled in the art having the benefit of the teachings presented
in the foregoing
descriptions and the associated drawings. Therefore, it is understood that the
invention is
not to be limited to the specific embodiments disclosed, and that
modifications and
embodiments are intended to be included within the scope of the claims
supported by this
disclosure.
14