Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CRYSTAL FORM OF RILAPLADIB
The present invention relates to a novel crystalline form of the Lp-PLA2
inhibitor
compound N41-(2-methoxyethyl)-piperidin-4-y1]-212-(2,3-difluorobenzylthio)-4-
oxo-4H-quinolin-
1-y1]-N-(4'-trifluoromethylbipheny1-4-ylmethypacetamide (also referred to
herein as rilapladib),
pharmaceutical formulations comprising this crystalline form, their use in
therapy and processes
for preparing the same.
BACKGROUND OF THE INVENTION
The compound rilapladib is described and exemplified, for example, in PCT
publication
W002/30904A1, published April 18, 2002 and its worldwide counterparts.
The compounds disclosed in this
application, including rilapladib, are inhibitors of the enzyme Lp-PLA2 and as
such are expected to be
of use in therapy in disorders mediated by Lp-PLA2 activity such as the
disorders disclosed therein.
For example, such diseases may be associated with increased involvement of
monocytes,
macrophages or lymphocytes, with the formation of lysophosphatidylcholine and
oxidised free fatty
acids, with lipid oxidation in conjunction with Lp-PLA2 activity, or with
endothelial dysfunction.
Examples of such diseases include atherosclerosis, diabetes, hypertension,
angina pectoris,
rheumatoid arthritis, stroke, inflammatory conditions of the brain such as
Alzheimer's Disease,
myocardial infarction, ischaemia, reperfusion injury, sepsis, acute
inflammation, chronic
inflammation, and psoriasis. Other disorders associated with Lp-PLA2 activity
have been described in
the art. Rilapladib is currently in a Phase 2 clinical study relating to
treatment of Alzheimer's Disease.
See, e.g., ClinicalTrials.gov, Study NCT01428453.
Prior to the present invention, two forms of rilapladib had been recognized. A
crystalline
form of rilapladib had been identified and for the sake of identification had
been termed "Form 1".
Another form, termed "Form 2" for the sake of identification, was less well
defined. Without
intending to be bound, Form 2 is thought to be a channel entity which is able
to incorporate a range
of solvents. While broadly similar and clearly related, solid state
characterizations such as XRPD
and others may vary for products termed "Form 2" depending on the solvent
composition.
W002/30904A1 Example 5 relating to a preparation of rilapladib does not
describe the
resulting crystalline form and in particular does not describe the new crystal
form according to the
present invention. The sample prepared in accordance with Example 5 has been
found by XRPD and
1
CA 2847066 2018-08-20
CA 02847066 2014-02-27
WO 2013/030374
PCT/EP2012/067027
Raman spectroscopy to comprise Form 1 and Form 2 rilapladib, with no evidence
within detection
limits of the novel form according to the present invention.
In the course of several experiments, it has now been unexpectedly discovered
that
rilapladib can be prepared as a new and advantageous crystalline form which
for the sake of
identification is termed herein as "Form 3".
BRIEF SUMMARY OF THE INVENTION
As one aspect, the present invention provides a crystalline form of rilapladib
characterized by an X-ray powder diffraction ("XRPD") pattern substantially in
accordance with
Figure 1.
In another aspect, the present invention provides a crystalline form of
rilapladib
characterized by an XRPD pattern comprising diffraction angles (2 theta) at
least at positions of
about 6.2, 7.6, 9.1, 11.2, and 14.3 degrees.
As another aspect, the present invention provides a crystalline form of
rilapladib
characterized by an XRPD pattern comprising diffraction angles (2 theta) at
least at positions of
about 6.2, 7.6, 9.1, 11.2, 11.7, 12.4, 13.1, 14.0, 14.3, 14.9, 15.3, 16.5,
16.8, 17.5, 17.8, 18.5, 18.9,
19.3, 20.0, 20.6, 21.1, and 22.1 degrees.
As another aspect, the present invention provides a crystalline form of
rilapladib
characterized by a Raman spectrum substantially in accordance with Figure 2.
As another aspect, the present invention provides a crystalline form of
rilapladib
characterized by a Raman spectrum comprising peaks at least at positions of
about 103, 276, 752,
1155, 1336, 1623, and 3075 cm-1.
As another aspect, the present invention provides a crystalline form of
rilapladib
characterized by a Raman spectrum comprising peaks at least at positions of
about 3075, 2952,
1623, 1611, 1576, 1528, 1467, 1336, 1288, 1179, 1155, 808, and 103 cm-1.
As a another aspect, the present invention provides a crystalline form of
rilapladib
characterized by a Raman spectrum comprising peaks at least at positions of
about 103, 159, 186,
276, 519, 524, 613, 628, 694, 736, 752, 766, 776, 808, 820, 1038, 1155, 1179,
1288, 1336, 1467,
1528, 1576, 1611, 1623, 2933, 2952, and 3075 cm-1.
In another aspect, the present invention provides a form of crystalline
rilapladib
characterized by a melting point of from about 165 C to about 185 C, e.g.,
from about 170 C to
about 180 C.
2
CA 02847066 2014-02-27
WO 2013/030374
PCT/EP2012/067027
In another aspect, the present invention provides a form of crystalline
rilapladib
characterized by infrared (IR) absorption spectrum (e.g., attenuated total
reflectance infrared or
"ATR-IR") substantially in accordance with Figure 7.
As another aspect, the present invention provides a form of crystalline
rilapladib
characterized by an IR (e.g. ATR-IR) absorption spectrum, comprising peaks at
least at about
wavenumber 2931, 1652, 1621, 1595, 1528, 1494, 1478 1423, 1403, 1327, 1317,
1286, 1237,
1204, 1187, 1166, 1140, 1109, 1066, 1024, 992, 969, 932, 865, 859, 813, 795,
767, 751,
708, and 693.
In another aspect, the present invention provides a form of crystalline
rilapladib
characterized by one or more of (i.e., at least one of) the aforementioned
XRPD patterns and
one or more of the aforementioned Raman spectra.
In another aspect, the present invention provides a form of crystalline
rilapladib
characterized by one or more of the aforementioned XRPD patterns, one or more
of the
aforementioned Raman spectra, and one or more of the aforementioned melting
points.
In another aspect, the present invention provides a form of crystalline
rilapladib
characterized by one or more of the aforementioned XRPD patterns, one or more
of the
aforementioned Raman spectra, one or more of the aforementioned melting
points, and one or
more of the aforementioned IR spectra.
In another aspect, the present invention provides a form of crystalline
rilapladib
characterized by at least two properties, selected from an XRPD pattern, a
Raman spectrum, a
melting point, and an IR spectrum, wherein those properties are as defined
according to any one
of the aforementioned embodiments.
As another aspect, the present invention provides a pharmaceutical composition
comprising one or more of the above mentioned crystalline forms of rilapladib,
referred to herein
as Form 3, and one or more pharmaceutically acceptable carriers. In some
embodiments, the
pharmaceutical composition further comprises another form of rilapladib.
In other aspects, the present invention provides: a method for the treatment
of a disease
or disorder mediated by Lp-PLA2in a subject (e.g. a mammal, e.g. a human)
comprising
administering to the subject an effective amount of rilapladib Form 3;
rilapladib Form 3 for use in
therapy; and the use of rilapladib Form 3 in the preparation of a medicament
for the treatment
of a disease or disorder mediated by Lp-PLA2.
As another aspect, the present invention provides a process for preparing
rilapladib Form
3.
Other aspects of the invention will be apparent in light of the present
disclosure.
3
,
BRIEF DESCRIPTION OF THE DRAWINGS
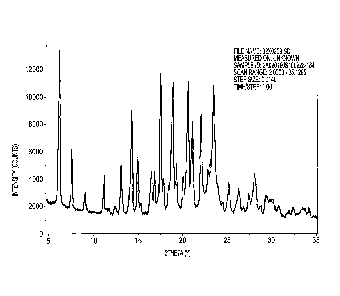
Figure 1. an example of an XRPD pattern of rilapladib Form 3 according to the
present invention.
Figure 2. an example of a Raman spectrum of rilapladib Form 3 according to the
present
invention.
Figure 3. Comparative - an example of an XRPD pattern of rilapladib Form 1.
Figure 4. Comparative - an example of a Raman spectrum of rilapladib Form 1.
Figure 5. Comparative - an example of an XRPD pattern of rilapladib Form 2.
Figure 6. Comparative - an example of a Raman spectrum of rilapladib Form 2.
Figure 7. an example of an ATR-IR absorption spectrum of rilapladib Form 3
according to the
present invention.
Figure 8. Comparative ¨ an example of an ATR-IR absorption spectrum of
rilapladib Form 1.
DETAILED DESCRIPTION OF THE INVENTION
The compound A/41-(2-methoxyethyl)-piperidin-4-y11-2[2-(2,3-
difluorobenzylthio)-4-oxo-4H
quinolin-1-y1]-N-(4.-trifluoromethylbiphenyl-4-ylmethypacetamide
=
F F
I so F
F
40 s N
1y F
.., ,..,.....,0,..N
0
4
CA 2847066 2018-08-20
CA 02847066 2014-02-27
WO 2013/030374
PCT/EP2012/067027
and methods of making and using it are disclosed, for example, in PCT
publication W002/30904.
The compound is alternatively known as 242-[(2,3-difluorophenypmethylsulfanyl]-
4-oxoquinolin-
1-y1]-N41-(2-methoxyethyl)piperidin-4-y1]-N-H444-
(trifluoromethyl)phenyllphenyl]methyl]acetamide (IUPAC name) and as rilapladib
(USAN name).
The present invention provides a novel crystalline form of rilapladib, "Form
3", exhibiting
one or more advantageous pharmaceutical properties or other advantages over
other forms of
rilapladib (e.g., Form 1). For example, rilapladib Form 3 is more
thermodynamically stable than
Form 1 and Form 2. Therefore, rilapladib Form 3 is more suitable for scale-up
and large-scale
manufacture associated with pharmaceutical products and reduces the risk of
conversion to
another form during manufacture, storage or use. In addition to having good
stability, rilapladib
Form 3 can be obtained in good yield and with reduced energy usage. Rilapladib
Form 3 also
exhibits very good chemical stability.
Forms of rilapladib, including Form 3 of the present invention, may be
characterized and
differentiated using a number of conventional analytical techniques, including
but not limited to
X-ray powder diffraction (XRPD) patterns, Raman spectra, Infrared (IR)
absorption spectra, and
melting point.
In one embodiment, the present invention provides a crystalline form of
rilapladib
characterized by an X-ray powder diffraction ("XRPD") pattern substantially in
accordance with
Figure 1.
In another embodiment, the present invention provides a crystalline form of
rilapladib
characterized by an XRPD pattern comprising diffraction angles (2 theta) at
least at positions of
about 6.2, 7.6, 9.1, 11.2, and 14.3 degrees (in some embodiments, 0.1 degrees
with respect to
each of the foregoing particular peaks).
In another embodiment, the present invention provides a crystalline form of
rilapladib
characterized by an XRPD pattern comprising diffraction angles (2 theta) at
least at positions of
about 6.2, 7.6, 9.1, 11.2, 11.7, 12.4, 13.1, 14.0, 14.3, 14.9, 15.3, 16.5,
16.8, 17.5, 17.8, 18.5, 18.9,
19.3, 20.0, 20.6, 21.1, and 22.1 degrees (in some embodiments, 0.1 degrees
with respect to
each of the foregoing particular peaks).
In another embodiment, the present invention provides a crystalline form of
rilapladib
characterized by a Raman spectrum substantially in accordance with Figure 2.
In another embodiment, the present invention provides a crystalline form of
rilapladib
characterized by a Raman spectrum comprising peaks at least at positions of
about 103, 276, 752,
5
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
1155, 1336, 1623, and 3075 cm-1 (in some embodiments, 1 cm-1. with respect to
each of the
foregoing particular peaks).
In another embodiment, the present invention provides a crystalline form of
rilapladib
characterized by a Raman spectrum comprising significant peaks at least at
positions of about
3075, 2952, 1623, 1611, 1576, 1528, 1467, 1336, 1288, 1179, 1155, 808, and 103
cm-1 (in some
embodiments, 1 cm-1 with respect to each of the foregoing particular peaks).
In another embodiment, the present invention provides a crystalline form of
rilapladib
characterized by a Raman spectrum comprising peaks at least at positions of
about 103, 159, 186,
276, 519, 524, 613, 628, 694, 736, 752, 766, 776, 808, 820, 1038, 1155, 1179,
1288, 1336, 1467,
1528, 1576, 1611, 1623, 2933, 2952, and 3075 cm-1 (in some embodiments, 1 cm-
1 with respect
to each of the foregoing particular peaks).
In another embodiment, the present invention provides a form of crystalline
rilapladib
characterized by one or more of (i.e., at least one of) the aforementioned
XRPD patterns and one
or more of the aforementioned Raman spectra.
In another embodiment, the present invention provides a form of crystalline
rilapladib
characterized by a melting point of from about 165 C to about 185 C, e.g.,
about 168 to
about182 C, e.g., from about 170 C to about 180 C.
In some embodiments, the present invention provides a form of crystalline
rilapladib
characterized by one or more of the aforementioned XRPD patterns, one or more
of the
aforementioned Raman spectra, and one or more of the aforementioned melting
points.
In another embodiment, the present invention provides a form of crystalline
rilapladib
characterized by infrared (IR) absorption spectrum (e.g. ATR-IR) substantially
in accordance with
Figure 7.
As another embodiment, the present invention provides a form of crystalline
rilapladib
characterized by an IR (e.g. ATR-IR) absorption spectrum, comprising
significant peaks at least at
about wavenumber 2931, 1652, 1621, 1595, 1528, 1494, 1478 1423, 1403, 1327,
1317, 1286,
1237, 1204, 1187, 1166, 1140, 1109, 1066, 1024, 992, 969, 932, 865, 859, 813,
795, 767,
751, 708, and 693 (in some embodiments, +/- 1 wavenumber with respect to each
of the
foregoing particular wavenumbers).
In some embodiments, the present invention provides a form of crystalline
rilapladib
characterized by one or more of the aforementioned XRPD patterns, one or more
of the
aforementioned Raman spectra, one or more of the aforementioned melting
points, and one or
more of the aforementioned IR absorption spectra.
6
CA 02847066 2014-02-27
WO 2013/030374
PCT/EP2012/067027
As used herein, "Form 3 rilapladib" or "rilapladib Form 3" refers to
crystalline rilapladib
characterized by at least one of the aforementioned XRPD patterns and/or at
least one of the
aforementioned Raman spectra and/or at least one of the aforementioned melting
points and/or
at least one of the aforementioned Infrared spectra.
In some embodiments, the Form 3 rilapladib is characterized by at least two
properties
selected from an XRPD pattern, a Raman spectrum, a melting point, and an IR
absorption
spectrum, wherein those properties are as defined according to any one of the
aforementioned
embodiments.
The X-ray powder diffraction pattern of forms of rilapladib, including Form 3,
can be
determined using conventional techniques and equipment known to those skilled
in the art of
analytical chemistry and physical characterization. In some embodiments, such
as used for
generating Figures 1, 3 and 5, the XRPD pattern is obtained with a
diffractometer equipped with a
diffracted beam graphite monochromator using copper Ka X-radiation.
The diffraction patterns of Figures 1, 3 and 5 were obtained with a Bruker D8
Advance X-
Ray powder diffractometer configured with a Cu anode (40 kV, 40 mA), variable
divergence slit,
primary and secondary SoIler slits, and a position sensitive detector. Data
were acquired over the
range 2 - 35 degrees 2-theta using a step size of 0.0145 degrees 2-theta (time
per step: 1 s).
Samples (lightly ground) were packed into top-filled cups that were rotated
during data
acquisition. In Figures 1, 3 and 5, 2-Theta angles in degrees (x-axis) is
plotted against peak
intensity in terms of the count rate per seconds (y-axis).
It is well known and understood to those skilled in the art that the apparatus
employed,
humidity, temperature, orientation of the powder crystals, and other
parameters involved in
obtaining an X-ray powder diffraction (XRPD) pattern may cause some
variability in the
appearance, intensities, and positions of the lines in the diffraction
pattern. An XRPD pattern
that is "substantially in accordance" with that of a Figure provided herein
(e.g., Figure 1) is an
XRPD pattern that would be considered by one skilled in the art to represent a
compound
possessing the same crystal form as the compound that provided the XRPD
pattern of that Figure
(e.g., Figure 1). That is, the XRPD pattern may be identical to that of the
Figure (e.g., Figure 1), or
more likely it may be somewhat different. Such an XRPD pattern may not
necessarily show each
of the lines of the diffraction patterns presented herein, and/or may show a
slight change in
appearance, intensity, or a shift in position of said lines resulting from
differences in the
conditions involved in obtaining the data. A person skilled in the art is
capable of determining if a
sample of a crystalline compound has the same form as, or a different form
from, a form
disclosed herein by comparison of their XRPD patterns. For example, one
skilled in the art can
7
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
overlay an XRPD pattern of a sample of rilapladib with Figure 1 and, using
expertise and
knowledge in the art, readily determine whether the XRPD pattern of the sample
is substantially
in accordance with the XRPD pattern of Figure 1 and rilapladib Form 3. If the
XRPD pattern is
substantially in accordance with Figure 1, the sample form can be readily and
accurately
identified as having the same form as rilapladib Form 3. Similarly, a person
skilled in the art is
capable of determining if a given diffraction angle (expressed in '20)
obtained from an XRPD
pattern is at about the same position as a value presented herein. The same
technique may be
employed using Figures 3 and 5 to determine whether a particular sample is
rilapladib Form 1 or
rilapladib Form 2 respectively.
One skilled in the art can readily determine the lattice spacings for an XRPD
pattern from
the diffraction angles and the source of radiation by derivation methods known
in the art.
The Raman spectrum of forms of rilapladib, including rilapladib Form 3, can be
determined using conventional equipment and techniques known to those skilled
in the art of
analytical chemistry and physical characterization. In some embodiments, such
as used for
generating Figures 2, 4 and 6, the spectrum is obtained using a FT-Raman
spectrometer at 4 crn4
resolution.
The Raman spectra of Figures 2, 4 and 6 were obtained using a Nicolet 960
E.S.P. FT-
Raman spectrometer. Data were acquired at 4cm-1 resolution. Laser excitation
was at 1064nm
(as is inherent by the use of an FT-Raman spectrometer) with a power of 400mW.
The number of
sample scans was 600, using an InGaAs detector and a CaF2 beamsplitter. Sample
was prepared
by placing the solid sample as received into a glass NMR tube. The sample was
rotated during the
measurement. Raman shift in cm4 (x-axis) is plotted against Raman intensity (y-
axis).
The power (mW) and minimum number of scans accumulation may be adjusted within
conventional knowledge to provide a spectrum of similar quality to that
provided in Figures 2, 4
or 6. For example, if a higher power is employed, a lower number of minimum
scans
accumulation may be required to achieve a spectrum of similar quality to that
reported in the
Figures. Similarly, if a lower power is employed, a higher number of minimum
scans
accumulation may be required to obtain a spectrum of similar quality. In some
embodiments, the
spectrum will be obtained using a power of 400 mW and a minimum of 600 scans
accumulation
(e.g., 1200 scans).
The choice of detector is not believed to be critical to obtaining a spectrum
suitable for
comparison with that provided in Figure 2, 4 or 6. As is known to those
skilled in the art, a
different detector will likely affect the intensity of the peaks. However,
peak positions should
8
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
remain relatively the same. In some embodiments, the spectrum will be obtained
using an
InGaAs detector.
Slight variations in observed peaks are expected based upon the specific
spectrometer
employed and acquisition parameters used. However, this variation should be no
more than + 1
wavenumber.
A Raman spectrum that is "substantially in accordance" with that of a Figure
provided
herein (e.g., Figure 2) is a Raman spectrum that would be considered by one
skilled in the art to
represent a compound possessing the same crystal form as the compound that
provided the
Raman spectrum of that Figure (e.g., Figure 2). That is, the Raman spectrum
may be identical to
that of the Figure (e.g., Figure 2), or more likely it may be somewhat
different. Such a Raman
spectrum may not necessarily show each of the lines of the diffraction
patterns presented herein,
and/or may show a slight change in appearance, intensity, or a shift in
position of said lines
resulting from differences in the conditions involved in obtaining the data. A
person skilled in the
art is capable of determining if a sample of a crystalline compound has the
same form as, or a
different form from, a form disclosed herein by comparison of their Raman
spectra. For example,
one skilled in the art can overlay a Raman spectrum of a sample of rilapladib
with Figure 2 and,
using expertise and knowledge in the art, readily determine whether the Raman
spectrum of the
sample is substantially in accordance with the Raman spectrum of Figure 2 and
rilapladib Form 3.
If the Raman spectrum is substantially in accordance with Figure 2, the sample
form can be
readily and accurately identified as having the same form as rilapladib Form
3. Similarly, a person
skilled in the art is capable of determining if a given peak obtained from a
Raman spectrum is at
about the same position as a value presented herein.
Since some margin of error is possible in the marking of peak positions, the
preferred
method of determining whether an unknown form of rilapladib is rilapladib Form
3 is to overlay
the Raman spectrum of the sample with the Raman spectrum of Figure 2. One
skilled in the art
can overlay a Raman spectrum of an unknown sample of rilapladib, obtained
using the methods
described herein, over Figure 2 and, using expertise and knowledge in the art,
readily determine
whether the Raman spectrum of the unknown sample is substantially in
accordance with Figure 2
and therefore of the same form as rilapladib Form 3. The same technique may be
employed using
Figures 4 and 6 to determine whether a particular sample is rilapladib Form 1
or rilapladib Form
2 respectively.
Melting points are determined by conventional methods such as capillary tube
and may
exhibit a range over which complete melting occurs, or in the case of a single
number, a melt
point of that temperature, +/- 1 degree.
9
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
It has been found that the melting point of rilapladib Form 3 is significantly
higher than
that of rilapladib Form 1. In some embodiments, Form 3 rilapladib is
characterized by a capillary
melting point in the range of from about 165 C to about 185 C, e.g., about 168
C to about 182 C,
e.g., from about 170 C to about 180 C, e.g. about 175 C. A melting point in
this range is
indicative of the presence of Form 3. In contrast, Form 1 provides a capillary
tube melting point
in the range of about 110 C to about125 C depending on the sample purity
(particularly in the
range of about 110 C to about120 C, e.g. about 116 C to a bout118 C). Form 2
tends to provide a
similar (capillary tube) melting point range of about 110 C to about 125 C.
DSC (differential scanning calorimetry) methods such as are known in the art
may also be
used to determine a phase or form change (e.g. melting) upon controlled
heating. Some
examples herein report the temperature(s) of the onset of endothermic activity
by DSC. For
example, depending e.g., on sample purity, Form 3 may have a DSC onset melt in
the range of
about 170 C to about 175 C. In contrast, Forms 1 and 2 tend to have at least
one DSC
endotherm onset which is significantly lower than Form 3 (e.g., in the range
of about 100 toabout
120 C).
Those skilled in the art will appreciate that depending on the composition of
a particular
sample, a material may exhibit a different melt point or endothernn onset, for
instance a higher
melt point/onset or multiple melt points/onsets including a higher melt
point/onset. One or
more other solid state characterization methods, such as those disclosed
herein, may be used to
determine the form(s) present.
The IR spectrum of forms of rilapladib, e.g., rilapladib Form 3, can be
determined using
conventional equipment and techniques known to those skilled in the art of
analytical chemistry
and physical characterization. The IR spectra of Figures 7 and 8 were obtained
by attenuated
total reflectance (ATR) infrared spectroscopy in accordance with USP, General
Chapter <197>,
Ph.Eur. 2.2.23, and JP General Test 2.25 recording the spectrum from
approximately 4000cm-1 to
650 cm-1. The wavenumber in cm-1 (x-axis) is plotted against percent
transmittance (y-axis).
More specifically, the IR spectra of Figures 7 and 8 were obtained using a
PerkinElmer
Spectrum One equiped with the PerkinElmer Universal Attenuated Total
Reflectance (ATR) pod
fitted with the Diamond/Zinc Selenide composite single bounce ATR (DATR)
crystal. The data
were acquired at 2 cm-1 resolution and 16 scans were acquired. The sample was
prepared by
crushing it as received using the ATR accessory.
Slight variations in observed IR peaks are expected based upon the specific
spectrometer
employed and acquisition parameters used. However, this variation should be no
more than + 1
wavenum ber.
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
An IR spectrum that is "substantially in accordance" with that of a Figure
provided herein
(e.g., Figure 7) is an IR spectrum that would be considered by one skilled in
the art to represent a
compound possessing the same crystal form as the compound that provided the IR
spectrum of
that Figure (e.g., Figure 7). That is, the IR spectrum may be identical to
that of the Figure (e.g.,
Figure 7), or more likely it may be somewhat different. A person skilled in
the art is capable of
determining if a sample of a crystalline compound has the same form as, or a
different form
from, a form disclosed herein by comparison of their IR spectra. For example,
one skilled in the
art can overlay an IR spectrum of a sample of rilapladib with Figure 7 and,
using expertise and
knowledge in the art, readily determine whether the IR spectrum of the sample
is substantially in
accordance with the IR spectrum of Figure 7 and rilapladib Form 3. If the IR
spectrum is
substantially in accordance with Figure 7, the sample form can be readily and
accurately
identified as having the same form as rilapladib Form 3. Similarly, a person
skilled in the art is
capable of determining if a given peak obtained from a IR spectrum is at about
the same position
as a value presented herein. The same technique may be employed using Figure 8
to determine
whether a particular sample is rilapladib Form 1.
Since some margin of error is possible in the marking of peak positions, the
preferred
method of determining whether an unknown form of rilapladib is rilapladib Form
3 is to overlay
the IR spectrum of the sample with the IR spectrum of Figure 7. One skilled in
the art can overlay
an IR spectrum of an unknown sample of rilapladib, obtained using the methods
described
herein, over Figure 7 and, using expertise and knowledge in the art, readily
determine whether
the IR spectrum of the unknown sample is substantially in accordance with
Figure 7 and therefore
of the same form as rilapladib Form 3. The same technique may be employed
using Figure 8 to
determine whether a particular sample is rilapladib Form 1.
In some embodiments, the rilapladib Form 3 has a water of hydration content of
not
more than 3% by weight (w/w), in more particular embodiments not more than 2%
w/w, 1.5%
w/w, 1% w/w, 0.7% w/w, or not more than 0.5% w/w. As used herein, "anhydrous"
refers to a
water of hydration content of 0.5% w/w. The water of hydration content may be
measured by
the Karl Fischer method which is well known in the art and is described, for
example, in the 1990
US Pharmacopoeia at pages 1619-1621, and the European Pharmacopoeia, second
edition (1992)
part 2, sixteenth fascicule at v. 3.5-6.1.
In some embodiments, Form 3 is non hygroscopic.
In some embodiments, the rilapladib Form 3 is substantially pure. As used
herein, the
term "substantially pure", when used in reference to a solid state form (such
as a polymorph or
polyamorph) of rilapladib, refers to the solid state form (e.g. Form 3) which
is at least 90% by
11
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
weight pure. This means that the form of rilapladib contains less than 10% by
weight of any
other compound and, in particular, contains less than 10% by weight of any
other form of
rilapladib. In particular embodiments, substantially pure refers to a solid
state form which is at
least 95% pure, at least 97% pure, or at least 99% pure by weight. This means
that the form of
rilapladib contains less than 5%, 3%, or 1% by weight respectively of any
other compound and, in
particular, contains less than 5%, 3%, or 1% by weight respectively of any
other form of rilapladib.
Substantial purity can be determined using conventional methods such as are
known in the art,
e.g. High Performance Liquid Chromatography (HPLC) and methods such as
disclosed herein for
identifying forms in a mixture of crystal forms.
In some embodiments, the rilapladib Form 3 comprises 1% by weight solvent(s).
Percent solvent can be readily determined using conventional methods such as
are known in the
art, e.g. Gas Chromatography. Thermogravimetric analysis by methods such as
known in the art
may also be used to determine the solvent or water content of a material, by
providing percent
weight loss on heating.
In some embodiments, the rilapladib Form 3 is characterized by the following
properties:
an IR spectrum substantially in accordance with Figure 7; a substantial purity
of 97 weight % (by
HPLC on water and solvent free basis);a total solvent content of < 1 weight %;
and a water of
hydration content of 0.5 weight %. In particular embodiments, such rilapladib
Form 3 is further
characterized by having an XRPD pattern substantially in accordance with
Figure 1 and/or a
melting point of about 175 C (e.g. by Differential Scanning Calorimetry or
"DSC").
The present invention includes Form 3 rilapladib both in substantially pure
form and in
admixture with one or more other forms of rilapladib (including, e.g., a
mixture comprising more
than 10 weight % total of one or more other forms) . Other forms of rilapladib
include one or
more other polymorphs, including but not limited to Form 1, and/or one or more
solvates
.. including but not limited to hydrates. As will be recognized by those
skilled in the art,
polymorphs may be essentially anhydrous or be solvated (e.g., hydrated).
Admixtures of Form 3 rilapladib with another form(s) of rilapladib may result
in the
masking or absence of one or more of the foregoing X-ray powder diffraction
peaks, Raman
spectrum peaks, and IR peaks disclosed herein for Form 3 rilapladib. Methods
are known in the
art for analyzing such admixtures of forms in order to provide for the
accurate identification of
the presence or absence of a particular form in the admixture. Suitable
methods for the
identification and/or quantitation of the particular forms in a mixture are
well known in the art,
e.g., IR, Raman, SSNMR, Near IR (NIR), XRPD and ATR-IR. Admixtures may also
result in multiple
melt points; presence of a melt point 1.65 C is indicative of the presence of
Form 3.
12
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
Processes for preparing rilapladib Form 3 of the invention are also within the
ambit of
this invention.
In some embodiments, a process for preparing rilapladib Form 3 comprises:
(a) forming a mixture of rilapladib in a solvent,
(b) crystallizing rilapladib from the mixture, where the crystallized material
comprises Form 3
rilapladib, and
(c) isolating the crystallized rilapladib comprising Form 3 rilapladib.
In some embodiments, the process further comprises seeding the mixture of
rilapladib
and solvent with rilapladib Form 3.
In some embodiments, the process further comprises the step of drying the
isolated
rilapladib comprising Form 3 rilapladib.
In step (a), forming a mixture of rilapladib and solvent, in some embodiments
the
rilapladib is a form other than Form 3, e.g., Form 1 rilapladib. In some
embodiments, the
rilapladib is Form 3 (for example, in a recrystallization of Form 3 previously
prepared, including
from another form such as Form 1).
Suitable solvents include acetonitrile, THF(tetrahydrofuran), ethyl acetate,
toluene,
heptane, acetone, aqueous ethanol, MIBK, DiMAC, and mixtures thereof. In some
particular
embodiments, the solvent is or comprises MIBK or a mixture of MIBK and DiMAC.
In some embodiments, the solvent is 1,4-dioxane,1-butanol, 1-propanol,
acetone,
acetone:water (1%), acetone:water (50%), cyclohexane, cyclohexanone, DEGDME
(diethylene
glycol), dimethylether, dimethyl carbonate, dioxane:water (1%), DMA (dimethyl
acetamide),
DMSO, Et0Ac (ethyl acetate), Et0Ac:cyclohexane (1:2), Et0Ac:toluene (1:2),
heptane, IPA
(isopropyl alcohol), IPA:iPrOAc (1:2), IPA:water (1%), isopropyl ether (IPE),
isopropyl acetate
(iPrOAc), MeCN (acetonitrile), MeCN:water (1%), MEK (methyl ethyl ketone),
Me0Ac (methyl
acetate), Me0H (methanol), MeOH:water (1%), MeOH:water (20%), MeOH:water
(50%), MIBK,
MIBK saturated with water, MIBK:DMA (8:1), NMP (methyl pyrrolidone),
PEG(polyethylene glycol)
400,TBME (t-butyl methyl ether),THF:Water (1%), toluene, water or a
combination thereof.
In some embodiments, the rilapladib is dissolved in the solvent. In other
embodiments,
the rilapladib/solvent mixture is a slurry.
The solvent and/or mixture may be heated, e.g., to facilitate dissolution. In
some
embodiments, the solvent and/or mixture are at ambient temperature, e.g.,
about 25 C. In other
embodiments, the solvent and/or mixture are cooled, e.g., to facilitate
crystallization, e.g. to a
temperature below ambient.
13
Step (b) of crystallizing rilapladib from the mixture may optionally comprise
cooling the
mixture, e.g., cooling from above ambient temperature, or cooling from ambient
temperature.
The process may involve heating and/or cooling, or temperature cycling of the
solvent
and/or mixture such as known in the art of crystallization and
recrystallization. In various
embodiments, Form 3 is recrystallized from one or more of the above mentioned
solvents by
preparing a slurry of rilapladib Form 3 in the solvent and temperature
cycling, e.g., from about
0 C to about 40 C or 50 C, e.g., for about 2-3 days (for instance 48-60
hours).
In some embodiments, the crystallization step is carried out over a period of
at least
about 12 hours, particularly e.g., at least about 15, 24, 48, 60, or 72 hours
(including about 12,
15, 24, 48, 60 and 72 hours).
Step (c) of isolating the crystallized rilapladib may be accomplished by
conventional
methods such as filtration.
Drying of the isolated material may be accomplished by conventional methods,
such as
vacuum drying with our without heat.
The present invention encompasses combinations of the aforementioned process
embodiments.
Rilapladib is an inhibitor of the enzyme lipoprotein associated phospholipase
A2 (Lp-
PLA2) and as such is expected to be of use in therapy. Therefore, in one
aspect the present
invention provides a method of inhibiting Lp-PLA2in a subject in need thereof,
comprising
.. administering to the subject an effective amount of rilapladib Form 3.
Therefore, in a further
aspect the present invention provides rilapladib Form 3 for use in therapy.
Diseases or disorders
for which rilapladib Form 3 may be used include those disclosed in
W002/30904A1,
W008/140449, W02008/141176, W02012/080497, W02012/037782, W02012/075917,
W02012/076435 or in US Patent Application Publication Nos. US 2008/0279846
(published Nov 13,
.. 2008), US2010/0239565 (published Sep 23, 2010), or US2008/0280829
(published Nov 13, 2008). I
In some embodiments, rilapladib Form 3 is used
for treating a disease or disorder selected from atherosclerosis, diabetes,
hypertension, angina
pectoris, rheumatoid arthritis, stroke, inflammatory conditions of the brain
such as Alzheimer's
Disease, myocardial infarction, ischaemia, reperfusion injury, sepsis, acute
inflammation, chronic
inflammation, psoriasis, diabetic retinopathy, diabetic macular edema,
vascular dementia,
multiple sclerosis, and skin ulcers.
14
CA 2847066 2018-08-20
CA 02847066 2014-02-27
WO 2013/030374
PCT/EP2012/067027
The invention accordingly includes a method for treating a disorder or disease
which is
mediated by Lp-PLA2in a subject in need thereof comprising administering to a
subject in need
thereof an effective amount of rilapladib Form 3 or a composition thereof.
In some embodiments, the disorder or disease which is mediated by Lp-PLA2is
selected
.. from atherosclerosis, diabetes, hypertension, angina pectoris, rheumatoid
arthritis, stroke,
inflammatory conditions of the brain such as Alzheimer's Disease, myocardial
infarction,
ischaemia, reperfusion injury, sepsis, acute inflammation, chronic
inflammation, psoriasis,
diabetic retinopathy, diabetic macular edema, vascular dementia, multiple
sclerosis, and skin
ulcers. In particular embodiments, the disorder or disease is Alzheimer's
Disease.
In some embodiments of the administration methods herein, the subject is a
mammal,
e.g. a human.
Administration methods include administering an effective amount of rilapladib
Form 3
(including as a pharmaceutical composition comprising rilapladib Form 3) at
different times
during the course of therapy. The rilapladib Form 3 may be administered in a
combination
.. therapy with one or more other pharmaceutical active agents as separate
administration of the
pharmaceutical active agents and/or administration with at least one other
pharmaceutical
active agent as a combination dosage form. The methods of the invention
include all known
therapeutic treatment regimens.
As used herein, "treatment", "treating" and the like includes prophylaxis and
refers to
.. ameliorating or alleviating the specified condition, eliminating or
reducing one or more
symptoms of the condition, slowing or eliminating the onset or progression of
the condition, and
preventing or delaying the reoccurrence of the condition in a previously
afflicted or diagnosed
subject. Prophylaxis (prevention or delay of condition onset) is typically
accomplished by
administering a drug in the same or similar manner as one would to a subject
with the developed
disease or condition.
"Effective amount" means that amount of drug substance (i.e. rilapladib Form
3,
optionally in combination with other pharmaceutical agents including other
forms of rilapladib)
that elicits the desired biological or medical response in a subject that is
being sought, for
instance, by a researcher or clinician. Such response includes alleviation of
the symptoms of the
condition being treated, ameliorating or alleviating the specified condition,
eliminating or
reducing one or more symptoms of the condition, slowing or eliminating the
onset or progression
of the condition, preventing or delaying the reoccurrence of the condition in
a previously afflicted
or diagnosed subject, and/or preventing or delaying onset of the condition
being treated.
CA 02847066 2014-02-27
WO 2013/030374
PCT/EP2012/067027
An embodiment of the invention includes administering rilapladib Form 3 or a
pharmaceutical composition containing the same in a combination therapy.
An embodiment of the invention includes administering rilapladib Form 3 or a
pharmaceutical composition containing the same in a combination therapy with
one or more
additional pharmaceutical agents for the treatment of the above-mentioned
diseases or
disorders. Such additional agents include any of the agents described herein.
Some embodiments of the invention include administering rilapladib Form 3 or a
pharmaceutical composition containing the same in a combination therapy with
one or more
additional agents selected from an anti-hyperlipidaemic, anti-atherosclerotic,
anti-diabetic, anti-
anginal, anti-inflammatory, or anti-hypertension agent or an agent for
lowering Lp(a). Examples
of the foregoing include cholesterol synthesis inhibitors such as statins,
anti-oxidants such as
probucol, insulin sensitisers, calcium channel antagonists, anti-inflammatory
drugs such as
NSAIDs, and Lp-PLA2inhibitors. Examples of agents for lowering Lp(a) include
the
aminophosphonates described in WO 97/02037, WO 98/28310, WO 98/28311 and WO
98/28312
(Symphar SA and SmithKline Beecham). Statins include atorvastatin,
simvastatin, pravastatin,
cerivastatin, fluvastatin, lovastatin and rosuvastatin. Examples of anti-
diabetic agents and insulin
sensitizers PPARgannma activators, e.g., GI262570 (GlaxoSmithKline), and the
glitazone class of
compounds such as rosiglitazone (AVANDIA, GlaxoSmithKline), troglitazone and
pioglitazone.
Examples of Lp-PLA2inhibitors include those disclosed in the above-mentioned
W002/30904,
W008/140449, W02008/141176, W02012/080497, W02012/037782, W02012/075917,
W02012/076435, US 2008/0279846, US2010/0239565 and U52008/0280829; other
additional
agents that may be used in combination therapy with rilapladib Form 3 are any
of the agents
disclosed in any of these publications for combined use with the Lp-
PLA2inhibitor.
Some embodiments of the invention include administering rilapladib Form 3 or a
pharmaceutical composition containing the same in a combination therapy with
one or more
additional agents for the treatment of Alzheimer's disease including agents
selected from
acetylcholinesterase inhibitors, NM DA receptor antagonists, and combinations
thereof.
Particular examples of such agents include tacrine, donepezil (e.g., ARICEPT),
rivastigmine (e.g.,
EXELON and EXELON PATCH), galantamine (e.g., RAZADYNE), and memantine (e.g.,
AKATINOL,
AXURA, EB1XA/ABIXA, MEMOX and NAMENDA).
Some embodiments of the invention include administering rilapladib Form 3 or a
pharmaceutical composition containing the same in a combination therapy with
one or more
other forms of rilapladib, for any of the uses of rilapladib Form 3 disclosed
herein.
16
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
Combination therapy includes co-administration of rilapladib Form 3 and said
other
pharmaceutical agent, sequential administration of rilapladib Form 3 and the
other
pharmaceutical agent, administration of a composition containing rilapladib
Form 3 and the other
pharmaceutical agent, or simultaneous administration of separate compositions
containing
rilapladib Form 3 and the other pharmaceutical agent.
When combined in the same formulation it will be appreciated that the two (or
more)
pharmaceutical compounds must be stable and compatible with each other and the
other
components of the formulation and may be formulated together for
administration. When
formulated separately they may be provided in any convenient formulation, in
such a manner as
is known for such compounds in the art.
When Form 3 rilapladib is used in combination with a second therapeutic agent,
the dose
of each compound may differ from that when the compounds are used alone.
Appropriate doses
will be readily appreciated by those skilled in the art.
The invention includes the use of rilapladib Form 3 for the preparation of a
pharmaceutical composition for treating the aforementioned diseases or
disorders in a subject in
need thereof, wherein the composition comprises rilapladib Form 3 and a
pharmaceutically
acceptable carrier (e.g., as a mixture).
The invention further includes the use of rilapladib Form 3 as an active
therapeutic
substance, in particular in the treatment of one or more of the aforementioned
diseases or
disorders.
In another aspect, the invention includes the use of rilapladib Form 3 in the
manufacture
of a medicament for use in the treatment of one or more of the aforementioned
diseases or
disorders.
The rilapladib Form 3 (including as a pharmaceutical composition thereof) may
be
administered by ocular, oral, nasal, transdermal, topical with or without
occlusion, intravenous
(both bolus and infusion), or injection (intraperitoneally, subcutaneously,
intramuscularly,
intratumorally, or parenterally) routes.
"Pharmaceutically acceptable carrier" means any one or more compounds and/or
compositions that are of sufficient purity and quality for use in the
formulation of drug substance
that, when appropriately administered to a human, do not produce an adverse
reaction, and
that are used as a vehicle for a drug substance (e.g., rilapladib Form 3).
The invention further includes the process for making the pharmaceutical
composition
comprising combining (e.g., mixing) rilapladib Form 3 and a pharmaceutically
acceptable carrier;
and includes those pharmaceutical compositions resulting from such a process,
which process
17
includes conventional pharmaceutical techniques. For example, rilapladib Form
3 may be
nanomilled prior to formulation. Rilapladib Form 3 may also be prepared by
grinding, micronizing
or other particle size reduction methods known in the art. Such methods
include, but are not
limited to, those described in U.S. Pat. Nos. 4,826,689, 5,145,684, 5,298,262,
5,302,401,
5,336,507, 5,340,564, 5,346,702, 5,352,459, 5,354,560, 5,384,124, 5,429,824,
5,503,723,
5,510,118, 5,518,187, 5,518,738, 5,534,270, 5,536,508, 5,552,160, 5,560,931,
5,560,932,
5,565,188, 5,569,448, 5,571,536, 5,573,783, 5,580,579, 5,585,108, 5,587,143,
5,591,456,
5,622,938, 5,662,883, 5,665,331, 5,718,919, 5,747,001, PCT applications WO
93/25190, WO
96/24336, and WO 98/35666. The
pharmaceutical compositions of the invention may be prepared using techniques
and methods
known to those skilled in the art. Some of the methods commonly used in the
art are described
in Remington's Pharmaceutical Sciences (Mack Publishing Company).
Examples of pharmaceutically acceptable carriers
and pharmaceutical compositions suitable for use in the present invention
include the carriers
and compositions disclosed in the above-mentioned W002/30904A1, W02008/141176,
W008/140449, W02012/080497, W02012/037782, W02012/075917, W02012/076435, US
2008/0279846, US2010/0239565 and U52008/0280829.
The pharmaceutical compositions of the invention include ocular, oral, nasal,
transdermal, topical with or without occlusion, intravenous (both bolus and
infusion), and
injection (intraperitoneally, subcutaneously, intramuscularly, intratumorally,
or parenterally).
The composition may be in a dosage unit such as a tablet, pill, capsule,
powder, granule,
liposome, ion exchange resin, sterile ocular solution, or ocular delivery
device (such as a contact
lens and the like facilitating immediate release, timed release, or sustained
release), parenteral
solution or suspension, metered aerosol or liquid spray, drop, ampoule, auto-
injector device,
suppository, or a topical solution, ointment, transdermal patch or other
transdermal vehicle; for
administration ocularly, orally, intranasally, sublingually, parenterally,
topically, rectally, or by
inhalation or insufflation.
Compositions of the invention suitable for oral administration include solid
forms such as
pills, tablets, caplets, capsules (each including immediate release, timed
release, and sustained
release formulations), granules and powders; and, liquid forms such as
solutions, syrups, elixirs,
emulsions, and suspensions. Forms useful for ocular administration include
sterile solutions or
ocular delivery devices. Forms useful for parenteral administration include
sterile solutions,
emulsions, and suspensions.
18
CA 2847066 2018-08-20
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
The dosage form containing the pharmaceutical composition of the invention
contains an
effective amount of the drug substance (rilapladib Form 3 and optionally, one
or more other
pharmaceutical active agents, for instance as described herein) necessary to
provide a
therapeutic and/or prophylactic effect.
In some embodiments, the composition which is administered is in unit dose
form such
as a tablet or capsule. Each dosage unit for oral administration may contain,
e.g., from about 1 to
about 500 mg (e.g., about 250 mg)(and for parenteral administration from about
0.1 to about 25
mg) of rilapladib Form 3. The daily dosage regimen for an adult patient may
be, for example, an
oral dose of between about 1 mg and about 1000 mg, e.g., an oral dose of
between about 1 mg
and about 500 mg, e.g., about 250 mg, or an intravenous, subcutaneous, or
intramuscular dose of
between about 0.1 mg and about 100 mg, e.g. of between about 0.1 mg and about
25 mg, of
rilapladib Form 3, the compound being administered 1 to 4 times per day.
Suitably the
compound will be administered for a period of continuous therapy, for example
for a week or
more. It will be understood that in the foregoing dosages, "about" includes
the specifically
mentioned dosages.
Dosages will vary depending on factors associated with the particular patient
being
treated (e.g. age, weight, diet, and time of administration), the severity of
the condition being
treated, the compound(s) being employed, the mode of administration, and the
strength of the
preparation.
An oral composition is preferably formulated with a pharmaceutically
acceptable carrier
as a homogeneous composition, wherein the drug substance (rilapladib Form 3
and any optional
other pharmaceutical active agent) is dispersed evenly throughout the mixture,
which may be
readily subdivided into dosage units containing equal amounts of the drug
substance. The
pharmaceutically acceptable carrier may include one or more compounds known in
the art for
oral pharmaceutical dosage forms, including for example starch, sugar (e.g.,
natural sugars, e.g.
glucose and beta-lactose), corn sweeteners, diluents (e.g., water),
granulating agents, lubricants,
glidants, binding agents, disintegrating agents, gelatin, methyl cellulose,
agar, bentonite,
excipients (e.g., water, glycols, oils, alcohols, flavoring agents,
preservatives, coloring agents, and
syrup), conventional tableting ingredients (e.g., corn starch, lactose,
sucrose, sorbitol, talc, stearic
acid, magnesium stearate, dicalcium phosphate, and any of a variety of gums
including natural
and synthetic gums (e.g. acacia and tragacanth).
Tablets and capsules represent an advantageous oral dosage unit form. Tablets
may be
sugarcoated or film-coated using standard techniques. Tablets may also be
coated or otherwise
compounded to provide a prolonged, control-release therapeutic effect. The
dosage form may
19
CA 02847066 2014-02-27
WO 2013/030374
PCT/EP2012/067027
comprise an inner dosage and an outer dosage component, wherein the outer
component is in
the form of an envelope over the inner component. The two components may
further be
separated by a layer which resists disintegration in the stomach (such as an
enteric layer) and
permits the inner component to pass intact into the duodenum or a layer which
delays or
sustains release. A variety of enteric and non-enteric layer or coating
materials (such as
polymeric acids, shellacs, acetyl alcohol, and cellulose acetate or
combinations thereof) may be
used.
In some embodiments the oral dosage form is a tablet. Appropriate additives in
such a
tablet may comprise diluents (also known to the person skilled in the art as
fillers) such as
microcrystalline cellulose, mannitol, lactose (e.g. anhydrous lactose, lactose
monohydrate, and
mixtures thereof), calcium carbonate, magnesium carbonate, dicalcium phosphate
or mixtures
thereof; binders such as hydroxypropylmethylcellulose (also known as
hypromellose),
hydroxypropyl-cellulose, polyvinylpyrrolidone, pre-gelatinised starch or gum
acacia or mixtures
thereof; disintegrants such as microcrystalline cellulose (e.g. as both a
diluent and disintegrant),
cross-linked polyvinylpyrrolidone, sodium starch glycollate, croscarmellose
sodium or mixtures
thereof; lubricants, such as magnesium stearate or stearic acid, glidants or
flow aids, such as
colloidal silica, talc or starch, and stabilisers such as poloxamer,
desiccating amorphous silica,
colouring agents, flavours etc. In some embodiments, the tablet comprises a
diluent, binder,
lubricant, and a disintegrant, for instance lactose as diluent, a binder (for
instance,
hydroxypropylmethylcellulose), magnesium stearate as lubricant, croscarmellose
sodium as
disintegrant, and microcrystalline cellulose as diluent and/or disintegrant.
The tablet may be
optionally coated, e.g. with a one or more film, seal or enteric coats.
The diluent may be present in a range of 10¨ 80% by weight of the tablet core.
The
lubricant may be present in a range of 0.25 ¨ 2% by weight of the core. The
disintegrant may be
present in a range of 1 ¨ 10% by weight of the core. Microcrystalline
cellulose, if present, may be
present in a range of 10 ¨ 80% by weight of the core.
In some embodiments, the tablet core comprises microcrystalline cellulose,
lactose
monohydrate, hypromellose, croscarmellose sodium, and magnesium stearate. In
some
embodiments, the tablet is coated with a film coat, for instance comprising a
suitable polymer,
plasticizer and optional pigment, such as the OPADRY series of coatings. In
some embodiments
the film coat comprises hypromellose, polyethylene glycol (e.g., PEG400) and
titanium dioxide,
for instance, OPADRY 0Y-S-28876.
For ocular administration, a pharmaceutical composition is preferably in the
form of an
ophthalmic composition. The ophthalmic compositions are suitably formulated as
eye-drop
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
formulations and filled in appropriate containers to facilitate administration
to the eye, for
example a dropper fitted with a suitable pipette. Preferably, the compositions
are sterile and
aqueous based, using purified water. In addition to rilapladib Form 3, an
ophthalmic composition
may contain one or more of: a) a surfactant; b) a thickening agent; c) an anti-
oxidant; d) other
excipients such as an alcohol, isotonic agent, buffer, preservative, and/or pH-
controlling agent.
The pH of the ophthalmic composition is desirably within the range of 4 to 8.
Ophthalmic
compositions are also suitably formulated as an ointment.
Without further elaboration, it is believed that one skilled in the art can,
using the
preceding description, utilize the present invention to its fullest extent.
The following Examples
are, therefore, to be construed as merely illustrative and not a limitation of
the scope of the
present invention in any way.
Abbreviations are used in the following Examples and throughout this
disclosure. Unless
otherwise indicated, the following abbreviations are defined as indicated:
ATR Attenuated Total Reflectance
CD! 1,1'-carbonyldiimidazole
cGMP current good clinical manufacturing practice
DEGDME diethylene glycol
DiMAC dimethylacetamide
DMA dimethyl acetamide
DMSO dimethylsulfoxide
DSC Differential Scanning Calorimetry
Et0Ac ethyl acetate
HPLC High Performance Liquid Chromatography
IPA isopropyl alcohol
IPE isopropyl ether
iPrOAc isopropyl acetate
IR Infrared
MDC dichloronnethane
MeCN acetonitrile
MEK methyl ethyl ketone
Me0Ac methyl acetate
Me0H methanol
21
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
MIBK methylisobutylketone
MS (El) Mass Spectrometry (Electron Ionization)
NMP methyl pyrrolidone
NMR Nuclear Magnetic Resonance
PEG polyethylene glycol
TBME t-butyl methyl ether
TBTU (0-(Benzotriazol-1-y1)-N,N,N.,N'-tetramethyluronium
tetrafluoroborate)
TFA trifluoroacetic acid
TG thermogravimetry
THF tetra hyd rofura n
XRPD X-Ray Powder Diffraction
Other abbreviations used herein have their ordinary meaning.
Example 1 ¨ Comparative ¨ a preparation of Form 1 rilapladib
(a) Preparation of rilapladib to cGMP (current good clinical manufacturing
practice)
For a preparation of [2-(2,3-difluorobenzylthio)-4-oxo-4H-quinolin-1-yl]acetic
acid and
N-(1-(2-methoxyethyl)piperidin-4-yI)-4-(4-trifluoromethylphenyl)benzylamine
see, e.g.
W002/30904.
[2-(2,3-difluorobenzylthio)-4-oxo-4H-quinolin-1-yl]acetic acid (54.0g, 0.15
mol), N-(1-(2-
methoxyethyppiperidin-4-y1)-4-(4-trifluorornethylphenyl)benzylannine (58.6 g,
0.15mol, 1.0 eq)
and dichloromethane (MDC) (900m1) were charged to a 3L round-bottom flask with
stirring under
nitrogen. Triethyl amine (56.4 ml, 41.0g, 0.4 mol, 2.7 eq) and TBTU (0-
(Benzotriazol-1-y1)-
N,N,N',V-tetramethyluronium tetrafluoroborate) (52.7 g, 0.16 mol, 1.1 eq) were
added and the
mixture stirred at room temperature. After approximately 15 minutes a red
solution was
obtained. After about 2 hours the mixture was quenched by addition of 2M
aqueous HCI (500 ml)
and left to stir for 10 minutes. 2M aqueous NaOH (600 ml) was added slowly
with water bath
cooling. The organic layer was separated and washed with water (500 ml)(the
separation was
slow). The MDC solution was dried over sodium sulphate (100 g), filtered and
the residue washed
with MDC (100m1). Evaporation gave a red oil (130g). This was dissolved in
ethyl acetate (500 ml)
and filtered into a 2L round bottom flask. Heptane (500 ml) was added and the
mixture left to stir
for 1 hour at room temperature. After 1 hour at 0-5 C the product was filtered
and the residue
22
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
washed with MDC (100 ml). Separation gave a red oil (130 g). This was
dissolved in ethyl acetate
(500 ml) and filtered into a 2L round bottom flask. Heptane (500 ml) was added
and the mixture
left to stir for 1 hour at room temperature. After 1 hour at 0-5 C the product
was filtered and
washed with heptane (100m1). Drying in the vacuum oven at 35 C overnight gave
a pale pink solid
(89g, 81%). HPLC showed 98.46% rilapladib; total impurities 1.54%; largest
impurity 0.47%
(Column: 50 x 2.1 mm XTerra MS C18 3.5 um, Column temp:40 C, Eluent A:0.1% v/v
TFA
(trifluoroacetic acid) in H20, Eluent B:0.1% v/v TFA in MeCN, Flow rate:0.25
mL min-1, Gradient:
15-40% B in 15 mins; 20 mins @ 40%B, 40-90% B in 15 mins, 90-15%B in 1 min; 9
min re-
equilibration, Sample prep:-0.3 mg in 1.0 mL MeCN + 1.0 mL H20, lnj. vol:10
uL, Detection:UV at
254 nm).
(b) Recrystallization of rilapladib prepared in (a) above, to cGMP
Rilapladib from step (a) (85g) was dissolved in ethyl acetate (375 ml) with
heating and the
solution filtered using a Buchner funnel. The solution was charged to a 2L
flask and rinsed in with
ethyl acetate (50m1). Heptane (425 ml) was added and the mixture heated to 70
C. A clear
solution was not obtained so further ethyl acetate (75 ml, then 100 ml) was
added until a solution
was obtained. The solution was cooled to 40 C and seed (rilapladib prepared
under step (a)
above, 100mg) was added. The mixture was allowed to cool to room temperature
over
approximately 1 hour, then stirred at 0-5 C for 1 hour. The product was
filtered, washed with 1:1
ethyl acetate:heptane (100 ml) and dried overnight in a vacuum oven at 40 C.
11A NMR showed
some heptane remaining; HPLC showed 99.23% rilapladib; total impurities 0.77%;
largest
impurity 0.26% (HPLC conditions as for step (a)). After drying for a further
night NMR showed
virtually no heptane. The product rilapladib (76.2g, 90%) was analyzed and
found to be consistent
with Form 1 rilapladib.
(c) Preparation of rilapladib to cGMP
[2-(2,3-difluorobenzylthio)-4-oxo-4H-quinolin-1-yl]acetic acid
(70.0g, 0.193 mol), N-(1-
(2-methoxyethyl)piperidin-4-yI)-4-(4-trifluoromethylphenyl)benzylamine (76.0
g, 0.193m01) and
dichloromethane (MDC) (1.15L) were charged to a 3L round-bottom flask with
stirring under
.. nitrogen. Triethylamine (72.9m1, 52.9 g, 0.523 mol, 2.7 eq) and TBTU (68.4
g, 0.213 mol, 1.1 eq)
were added and the mixture stirred at ambient temperature for one hour. One
drop of the
reaction mixture was dissolved in 5 ml of methanol and analyzed by HPLC, which
showed 71%
rilapladib (Column ¨ symmetry C8, 51.1.m, 3.9x150 mm; flow ¨ 1 ml/min; Temp ¨
ambient;
Wavelength ¨ 254 nm; Gradient ¨ 90% of 0.1% TFA in water, 10% acetonitrile
ramped to 90%
23
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
acetonitrile over 10 minutes, held for 8 minutes and ramped back to 10%
acetonitrile). The
mixture was quenched by addition of 2M hydrochloric acid (500 ml) and left to
stir for half an
hour. 2M aqueous NaOH (600 ml) was added slowly with water bath cooling. The
organic layer
was separated and washed with water (500 ml). The MDC solution was dried over
sodium
sulphate (100g), filtered and the residue washed with MDC (100 ml).
Evaporation gave an orange
oil (190 g). This was dissolved in ethyl acetate (650 ml) and filtered into a
2L, 3 necked round
bottom flask. Heptane (650 ml) was added followed by rilapladib seed (100 mg,
prepared
according to step (b) above). After 1 hour at 20-25 C and 1 hour at 0-5 C the
product was filtered
and washed with 1:1 ethyl acetate:heptane (100 ml). Drying overnight in the
vacuum oven (18
hours) at 40 C gave rilapladib as a beige solid (100.0g, 70%).
(d) Purification and recrystallization of rilapladib from (c)
45g of rilapladib from step (c) was purified by silica gel chromatography in
25 and 20 g
lots. Each was columned with 250g of silica gel using 5% methanol in MDC (3L
each). The product
fractions were combined and evaporated to dryness. The yellow foam was
dissolved in ethyl
acetate (200 ml), filtered into a 1L 3-necked round-bottom flask and washed in
with ethyl acetate
(100 ml). The solution was warmed to 50 C and heptane (200 ml) added. At 40 C
rilapladib seed
(100mg) was added and the slurry stirred for 1 hour at ambient and 2 hours at
0-5 C. The product
was filtered, washed with heptane (50m1) and dried in a vacuum oven at 40 C.
This gave
rilapladib as an off-white solid (36.7g, 82%). HPLC showed 98.94% rilapladib;
1.06% impurities;
largest impurity 0.21% (H PLC conditions as in step (a)). N MR showed no
solvent.
The product was characterized in several ways and determined to be the form
termed
Form 1 rilapladib. It provided an XRPD pattern as represented in Figure 3, a
Raman spectrum as
represented in Figure 4, and a capillary melting point of 116-118 C. In
addition, it exhibited a DSC
endotherm of 110 C and a 0.04% weight loss by TG (thermogravimetry), and was
characterized
by Dynamic Vapor Sorption as a -non-hygroscopic, crystalline anhydrate.
XRPD instrument/acquisition details were as follows. Powder patterns were
obtained
using a Bruker D8 Advance X-Ray powder diffractometer configured with a Cu
anode (40 kV, 40
mA), variable divergence slit, primary and secondary SoIler slits, and a
position sensitive detector.
Data were acquired over the range 2 - 35 degrees 2-theta (Copper K-alpha
radiation) using a step
size of 0.0145 degrees 2-theta (time per step: 1 s). Samples (lightly ground)
were packed into top-
filled cups that were rotated during data acquisition.
The XRPD pattern is shown in Figure 3 and is representative of Form 1
rilapladib. The
following peaks may be particularly useful for identifying Form 1: 5.5, 5.9,
8.8, 10.9, 11.5, 11.8,
24
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
12.3, 14.5, 15.7, 16.6, 17.4, 18.2, 18.5, 20.4, 22.0, 23.3, and 24.2 degrees (
0.1 degrees
respectively); peaks 5.5, 8.8, 10.9, 14.5 and 18.2 degrees ( 0.1 degrees
respectively) may be
particularly useful. As will be appreciated by those skilled in the art, these
peaks do not
represent an exhaustive list of peaks exhibited by Form 1 rilapladib. In
addition, while the
aforementioned peaks may be useful for identifying Form 1, identification of
an unknown sample
of rilapladib is preferably carried out by comparison of the full XRPD to that
of Figure 3 as
described herein above.
Raman data were acquired on a Nicolet FT-Raman 960 E.S.P., using 1064nm
excitation at
400mW power. The resolution of the scans was 4cm-1. Specific details are
described herein
above.
The Raman spectrum is shown in Figure 4 and is representative of Form 1
rilapladib.
Bands which may be useful for identifying Form 1 include band positions at 86,
112, 158, 181,
263, 519, 528, 615, 629, 694, 739, 746, 764, 777, 808, 822, 1036, 1164, 1180,
1290, 1344, 1471,
1526, 1576, 1613, 1619, 2928, 2955, 3064, and 3080 cm' ( 1 cm' respectively);
band positions at
86, 112, 263, 746, 1164, 1344, 1619, 3064, and 3080 cm-1 ( 1 cm-1
respectively) may be
particularly indicative. Peak positions of significant peaks rounded to the
nearest whole
wavenumber include 3064, 2955, 1619, 1576, 1526, 1344, 1290, 1180, 808, 112,
and 86 cm'.
These bands, 1 cm-1 respectively, may be useful for identifying Form 1. As
will be appreciated by
those skilled in the art, these bands do not represent an exhaustive list of
peaks exhibited by
Form 1 rilapladib. In addition, while the aforementioned bands may be useful
for identifying
Form 1, as described above identification of an unknown sample of rilapladib
is preferably carried
out by comparison of the full Raman spectrum to that of Figure 4.
Example 2 - Comparative - a preparation of Form 2 rilapladib
Form 1 rilapladib was recrystallized by slow evaporation from toluene. 500 mg
of
rilapladib Form 1 prepared in accordance with Example 1(d) was dissolved in
toluene (5-10
volumes) with heating. The solution was allowed to evaporate over a weekend.
The solids were
isolated and properties determined. The resulting rilapladib solids provided
Raman and XRPD
spectra different from that of Example 1(d), and was designated Form 2
rilapladib. As noted
above, Form 2 is less well defined than Form 1 and, while broadly similar and
clearly related,
"Form 2" solid state characterizations may vary depending on the solvent
composition.
This preparation of rilapladib provided a Raman spectrum represented by Figure
6, an
XRPD pattern represented by Figure 5, and a capillary melt point of 114-116 C.
In addition, the
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
product exhibited a DSC endotherm of 109 C and a weight loss of 1.7% by
thernnogravimetry.
NMR showed 3.4% toluene.
Figure 5 represents an XRPD spectrum of this sample of Form 2 rilapladib.
Figure 6
represents a Raman spectrum of this sample of Form 2 rilapladib. XRPD and
Raman
instrument/acquisition details were as in Example 1. Raman peak positions of
significant peaks
rounded to the nearest whole wavenumber include: 3063, 2947, 1612, 1576, 1528,
1343, 1290,
1180, 1164, 806, 112, and 80 cm'. These bands, 1 cm' respectively, may be
useful for
identifying Form 2. However, identification of an unknown sample of
rilapladib is preferably
carried out by comparison of the full spectrum to that of Figure 5 or 6, as
applicable, as described
herein above.
Example 3 ¨ Preparation of rilapladib Form 3 and other forms
To mini-reaction vials (2 per each solvent used), approximately 1 mL of
solvent was
added. The solvents used were acetonitrile, THF(tetrahydrofuran), ethyl
acetate, toluene,
heptane, acetone, and aqueous ethanol. Rilapladib Form 1 prepared in
accordance with Example
1(d) was added and stirred at 10 C until a slurry formed (in the case of
aqueous ethanol, a
biphasic mixture formed with a gel-like bottom layer). The mixtures were
stirred overnight at
10 C, then the temperature was increased to 30 C, causing dissolution of the
crystals that had
formed in the solvents other than aqueous ethanol. The mixtures were then
cooled to 10 C,
.. which resulted in recrystallization of the solids.
For one set of vials (A) (1 per each solvent used), the solids were isolated
after further
aging for 3 hours at 10 C. The second set (B) of vials was seeded with
crystals from set 1 and held
at 10 C over the weekend (about 3 days).
The solids were isolated and found to exhibit the following melting points
(capillary tube)
and DSC onset endotherms:
1st set (A) 2nd set (B)
Solvent Melting point C DSC C Melting point
C DSC C
Acetonitrile Al: 179.9 170 B1: 180.9-182.2 170
THF A2: 112.3-121.8 103 B2: 2 melts: 113.8- 104; 161
120.6; 175.3-
176.6
ethyl acetate A3: 2 melts: 112.1- 110; B3: 174.5-177.3
171
26
CA 02847066 2014-02-27
WO 2013/030374
PCT/EP2012/067027
118.9; 176.5- 175
179.9
Toluene A4: 115.5 104 B4: 173.9-178.3 100;
170
Heptane A5: 112.5-122.2 105; B5: 116.4-126.6
108; 162
160
Acetone A6: 178.2-179.5 171 B6: 173.3-176.6
172
The X-ray powder diffraction pattern of the material from the 1st set,
acetonitrile
crystallized product (Al) is shown in Figure 1 and is representative of Form 3
rilapladib. The
following peaks may be particularly useful for identifying Form 3: diffraction
angles (2 theta) at
positions of 6.2, 7.6, 9.1, 11.2, 11.7, 12.4, 13.1, 14.0, 14.3, 14.9, 15.3,
16.5, 16.8, 17.5, 17.8, 18.5,
18.9, 19.3, 20.0, 20.6, 21.1, and 22.1 degrees ( 0.1 degrees respectively);
diffraction angles (2
theta) at positions 6.2, 7.6, 9.1, 11.2, and 14.3 degrees ( 0.1 degrees
respectively) may be
particularly useful. Those skilled in the art will appreciate that these peaks
do not represent an
exhaustive list of peaks exhibited by Form 3 rilapladib. In addition, while
the aforementioned
peaks may be useful for identifying Form 3, identification of an unknown
sample of rilapladib is
preferably carried out by comparison of the full XRPD to that of Figure 1 as
described herein
above. Figure 1 does not indicate the presence of Form 1 or Form 2 rilapladib,
considering a
detectability of about 2% of either form in Form 3.
The Raman spectrum of the material from the 1st set, acetonitrile crystallized
product
(Al) is shown in Figure 2 and is representative of Form 3 rilapladib. The
following bands may be
particularly useful for identifying Form 3: bands at positions of 103, 159,
186, 276, 519, 524, 613,
628, 694, 736, 752, 766, 776, 808, 820, 1038, 1155, 1179, 1288, 1336, 1467,
1528, 1576, 1611,
1623, 2933, 2952, and 3075 cm' ( 1 cm' respectively)(e.g., bands at positions
of 103, 808, 1155,
1179, 1288, 1336, 1467, 1528, 1576, 1611, 1623, 2952, and 3075 cm 1( 1 cm 1
respectively)).
Bands at positions 103, 276, 752, 1155, 1336, 1623, and 3075 cm-1 ( 1 cm'
respectively) may be
particularly indicative. Those skilled in the art will appreciate that these
bands do not represent
an exhaustive list of bands exhibited by Form 3 rilapladib. In addition, while
the aforementioned
bands may be useful for identifying Form 3, as described above identification
of an unknown
sample of rilapladib is preferably carried out by comparison of the full Raman
spectrum to that of
Figure 2.
XRPD and Raman analysis of the other isolated solids is summarized below:
(A2) - Raman and XRPD spectra were similar to those of Example 2.
27
CA 02847066 2014-02-27
WO 2013/030374
PCT/EP2012/067027
(A3) ¨ the Raman spectrum was like that of Example 1(d) and 2 combined.
(A4) - Raman and XRPD spectra were similar to that of Example 2.
(A5) - Raman and XRPD spectra was consistent with that of Example 1(d).
(A6) ¨ Raman and XRPD spectra were consistent with that of (Al).
.. (B1) - Raman and XRPD spectra were consistent with that of (Al).
(B2) - Raman and XRPD spectra were similar to that of Example 2.
(B3) - Raman and XRPD spectra were consistent with that of (Al).
(B4) ¨ The Raman spectrum indicated a mixture of forms. XRPD was like that of
(Al) and Example
2 combined.
(B5) - the Raman spectrum was like that of Example 1(d) and 2 combined. XRPD
was consistent
with that of Example 1(d).
(B6) - Raman and XRPD spectra were consistent with that of (Al).
XRPD and Raman instrument/acquisition details for the products of Example 3
were as in
Example 1.
The XRPD, Raman and/or melting points of the 1st set acetonitrile and acetone
crystallizations (Al and A6) and the 2nd set acetonitrile, ethyl acetate,
toluene and acetone (B1,
B3, B4, B6) crystallizations are indicative of the presence of Form 3
rilapladib. Where 2 melting
points were observed, a mixture of rilapladib forms, including Form 3, may be
present. The lower
melt points of about 110-130 C (e.g., about 112-127 C) are indicative of the
presence of Form 1
or another form (other than Form 3). All samples may include a form of
rilapladib other than
Form 3.
Example 4¨ a preparation of Form 3 rilapladib
Form 1 rilapladib was slurried in the seven solvents used in Example 3 for 2
days at 50 C.
Rilapladib Form 1 prepared in accordance with Example 1(d) was added to
solvent until a slurry
formed and the solids were isolated. Capillary melting points and DSC onset
endotherms for the
isolated crystalline materials were as follows:
Solvent Melting point C DSC C
Acetonitrile 175.1-177.8 172
THF 175.7-177.7 172
ethyl acetate 173.1-175.9 172
Toluene 173.4-176.8 170
28
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
Heptane 171.9-174.6 172
Acetone 175.6-178.4 173
Aqueous ethanol 175.9-178.6 172
XRPD and Raman patterns for the isolated crystalline materials were consistent
with
those described in Example 3, indicating that rilapladib Form 3 had formed.
The melting points of
the isolated materials are also indicative of the presence of rilapladib Form
3.
Example 5 - a preparation of Form 3 rilapladib
1 gram of Form 1 rilapladib prepared in accordance with Example 1(d) was
dissolved in 5
volumes ethyl acetate at 50 C. The solution started to crystallize. It was
cooled to 0 C and the
crystalline material was isolated and dried in a vacuum oven for 1 hour
(recovered 0.87g,
capillary melt point 173.8-180.0 C, DSC onset endotherm 171 C).
XRPD and Raman patterns for the isolated crystalline material were consistent
with those
described in Example 3, indicating that rilapladib Form 3 had formed. The
melting point of the
isolated material is also indicative of the presence of rilapladib Form 3.
Example 6- a preparation of Form 3 rilapladib
1 gram of Form 1 rilapladib prepared in accordance with Example 1(d) was
dissolved in 5
mL ethyl acetate at 60 C. 5 mL heptane was added, the mixture was cooled to 50
C and then
seeded with Form 3 rilapladib crystallized from Form 1 slurried in
acetonitrile at 50 C for 2 days.
The mixture was cooled to 0 C and the crystalline material was isolated and
dried in a vacuum
oven for 1 hour (recovered 0.93g, capillary melt point 173.8-175.1 C, DSC
onset endotherm
170 C).
XRPD and Raman patterns for the isolated crystalline materials were consistent
with
those described in Example 3, indicating that rilapladib Form 3 had formed .
The melting point of
the isolated material is also indicative of the presence of rilapladib Form 3.
Example 7 - a preparation of Form 3 rilapladib
1 gram of Form 1 prepared in accordance with Example 1(d) was dissolved in 10
mL ethyl
acetate at 50 C. The mixture was seeded with Form 3 rilapladib crystallized
from Form 1 slurried
in acetonitrile at 50 C for 2 days. The mixture was cooled to 0 C and the
crystalline material was
29
CA 02847066 2014-02-27
WO 2013/030374
PCT/EP2012/067027
isolated and dried in a vacuum oven for 1 hour (recovered 0.85g, capillary
melt point 174.0-
176.0 C, DSC onset endotherm 171 C).
XRPD and Raman patterns for the isolated crystalline material were consistent
with those
described in Example 3, indicating that rilapladib Form 3 had formed. The
melting point of the
isolated material is also indicative of the presence of rilapladib Form 3.
Example 8 - a preparation of Form 3 rilapladib
Rilapladib Form 1 prepared in accordance with Example 1(d) (1 gram) was
charged to a
mL round-bottomed flask with ethyl acetate (5mL). The mixture was heated to 50
C to give a
10 solution, then left to stir at 50 C. After several hours, the mixture
was cooled to 0-5 C and stirred
for one hour. The product was filtered, washed and dried in the vacuum oven to
give a white
solid (960 mg, 94%; capillary melting point 169-170 C; DSC onset endotherm 172
C). XRPD and
Raman patterns for the isolated material were consistent with those described
in Example 3,
indicating that rilapladib Form 3 had formed. The melting point of the
isolated product is also
indicative of the presence of rilapladib Form 3.
Example 9 ¨ a preparation of Form 3 rilapladib
Rilapladib is prepared from an amide coupling reaction between the
corresponding
amine and acid (see, e.g. W002/30904) in accord with the following: a) The
acid is heated initially
with 1,1'-carbonyldiimidazole ("CDI") in a mixture of MIBK and DiMAC. The
amine is added and
the rilapladib so formed is isolated by washing the reaction mixture with
water and aqueous
sodium bicarbonate, concentrating the MIBK solution and crystallization (a
rilapladib Form 3 seed
is optionally used); b) the rilapladib is recrystallized from MIBK (a
rilapladib Form 3 seed may be
used).
a) Preparation of rilapladib
[2-(2,3-difluorobenzylthio)-4-oxo-4H-quinolin-1-yl]acetic acid (15.0g) as a
solution in a
mixture of dimethylacetamide (DiMAC, 15mL) and methylisobutylketone (MIBK,
95mL) at 60 C is
treated with 1,1'-carbonyldiimidazole CDI (7.41g) portionwise over ca. 30
minutes and then
stirred for a further 30 minutes to form the imidazolide.
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
N-(1-(2-methoxyethyppiperidin-4-y1)-4-(4-trifluoronnethylphenypbenzylamine
(16.29g),
dissolved in MIBK (30mL), is added to the above innidazolide and the mixture
heated and stirred
at 83 C until the reaction is complete (2-4h). The DiMAC/MIBK solution
containing the crude
reaction product (N41-(2-methoxyethyl)-piperidin-4-y1]-242-(2,3-
difluorobenzylthio)-4-oxo-4H-
quinolin-1-01-N-(4'-trifluoromethylbipheny1-4-ylmethypacetamide) is then
cooled (45QC), diluted
with MIBK (45mL), washed with water (1 x 60mL), 5%w/v aqueous Na2CO3 (2 x
60mL) and finally
water (1 x 60mL). The MIBK solution is then concentrated to ca. 120mL (88-
118QC, at
atmospheric pressure). The solution is cooled to 45 C, seeded with rilapladib
Form 3 (15mg) and
stirred for at least 12 hours at 45 C. The resulting slurry is cooled to 20 C
over at least an hour,
stirred for at least a further 3 hours and filtered off. The filter cake is
washed with MIBK (lx
30mL) and dried in vacuo at 50 C to yield crystalline rilapladib, Form 3:
25.90g, 85%. HPLC
99.85% purity (the HPLC method used the following
equipment/conditions:Detection: UV;
Column: Phenomenex Luna C18 50 x 2.1 mm id 5 micron; Temperature: 40-60 C;
Flow Rate: 1-2
ml/min; Mobile Phase: A = Water 0.05% TFA; B = Acetonitrile 0.05% TFA;
Gradient elution 0%13 to
95%3 over 8 minutes; Equilibration Time: 0.5 -2 min (within gradient
programme); Injection
Volume: 1 LEL; Sample Prep: 2-3 drops in 5m1 of 1:1 acetonitrile:water).
Hplc retention time: 5.0 min (detection at X = 254nnn).
1H nmr (400MHz, D4-Me0H): 5 8.2-8.35 (1H, m), 7.35-7.85 (11H, m), 6.95-7.3
(3H, m), 6.44 + 6.52
(1H, 2 x s), 5.31 + 5.68 (2H, 2 x s), 4.71 + 4.87 (2H, 2 x br s), 4.37 + 4.48
(2H, 2 x s), 4.4-4.5 + 3.9-
4.05 (1H, 2 x m), 3.45-3.55 (2H, m), 3.3 (3H, s), 2.95-3.1 (2H, m), 2.5-2.65
(2H, m), 2.05-2.3 (2H, 2
x t), 1.7-2.05 (4H, m).
MS (El): found (M+1): 736; C40H38F5N303S requires 735.
b) Recrystallization of rilapladib
Rilapladib Form 3 (30.0 g, 40.77 mmol, 1 wt) and MIBK (210 mL, 7 vol) are
heated at 90 C
to obtain a solution. The solution is filtered and the filter washed with
further hot (70-90 C) MIBK
(30 mL, 1 vol). The combined filtrates are cooled to 45 C, seeded with
rilapladib Form 3 (30 mg,
0.1% wt) and stirred at 45 C for at least 15h. The resultant slurry is cooled
to 20 C over at least
one hour and stirred at 20 C for at least 3 hours. The product is isolated by
filtration, washed with
MIBK (1 x 36 mL, 1 x 1.2 vol) and dried in vacuo at 50 C to yield rilapladib
Form 3 as a white
crystalline solid: 26.55 g, 88.5%. HPLC 99.9% (conditions as in part a) of
this Example 9). The
product characterization data/methodology is the same as described in part a)
of this Example 9.
31
CA 02847066 2014-02-27
WO 2013/030374 PCT/EP2012/067027
Example 10 - ATR-IR Form 3 and Form 1 (comparative)
Figure 7 is an ATR-IR spectrum of another preparation of rilapladib Form 3.
Peak
positions of significant peaks rounded to the nearest whole wavenumber
include: 2931, 1652,
1621, 1595, 1528, 1494, 1478 1423, 1403, 1327, 1317, 1286, 1237, 1204, 1187,
1166, 1140,
1109, 1066, 1024, 992, 969, 932, 865, 859, 813, 795, 767, 751, 708, and 693
(+1- 1
wavenumber for each of the foregoing particular wavenumbers).
Figure 8 is an ATR-IR spectrum of another preparation of rilapladib Form 1.
Peak positions
of significant peaks rounded to the nearest whole wavenumber include: 2928,
1652, 1620,
1598, 1527, 1495, 1478, 1468, 1401, 1329, 1297, 1271, 1240, 1201, 1187, 1164,
1143,
1112, 1071, 1030, 1016, 1005, 994, 973, 933, 845, 822, 789, 755, 746, 712,
693, and 665 (+/- 1
wavenumber for each of the foregoing particular wavenumbers).
The equipment and acquisition details were as described herein above in
reference to
Figures 7 and 8. Those skilled in the art will appreciate that slight
variations in observed peaks
.. are expected based upon the specific spectrometer employed and acquisition
parameters used.
However, this variation should be no more than + 1 wavenumber.
32