Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02848442 2014-04-08
POLYMER FLOOD WATER TREATMENT
FIELD OF THE INVENTION
The invention relates to a method for treating polymer flood waters and flood
waters used in oil industry
operations for the subsequent reuse thereof and, more specifically, to the
removal of compounds that
impact the efficiency of polymers used in these types of applications.
BACKGROUND OF THE INVENTION
In the oil industry, extraction of oil from oil wells will typically yield in
the range of 30% of the actual
content in the reservoir being exploited. The process of water flooding refers
to the method of injecting
water into a reservoir resulting in an increase in pressure and subsequent
increase in oil extraction. The
flood water is injected into a reservoir and allows to maintain or increase
the pressure inside the reservoir
and replaced the extracted oil. It also allows to displace oil within the
reservoir and push it towards a well.
The use of flood water allows for more production from a well and therefore
increased savings by the
extending the production expectancy of a well.
US2012/0152546A1 describes a process for water treatment specifically for SAGD
operations. There is
described a process which uses chemical oxidation (CO) or electromagnetic
treatment (ET) to destroy or
degrade organics in the produced water. It is stated a primary purpose of the
produced water treatment
steps described above is to provide water of suitable quality to the steam
generator.
US 7694736B2 generally describes a method and system for producing steam for
extraction of heavy
bitumen including the steps of mixing carbon or hydrocarbon fuel. It is stated
that with its simple direct
contact, above ground adiabatic nature, and its high pressure and temperature
solid removal, the invention
will minimize the amount of energy used to produce the mixture of steam and
gas injected into the
underground formation to recover heavy oil. It is stated that the present
invention adds the adiabatic direct
contact steam and carbon dioxide generation unit to reduce the disadvantages
of the prior art and to allow
for expansion with use of a low quality water supply, reject water from
existing facilities and the use of
low quality fuel supplies. Also, there is no need for high quality separation
of the oil from the produced
water and water purification processes with this invention. It is stated that
the mixture produced at the
EOR production well 65 is separated into gas (mainly carbon dioxide and
natural gas), oil and water. The
produced water contains heavy oil remains, dissolve minerals, sand and clay.
The separated low quality
produced water 64 is used for steam generation 61 without any additional
treatment.
1
CA 02848442 2014-04-08
SUMMARY OF THE INVENTION
Given the prior art, there is a need for an efficient and low cost process for
the treatment of polymer flood
and water flood waters. Accordingly, one object of the present invention
provides for a process for the
treatment of polymer flood water for subsequent reuse thereof, said process
comprising subjecting the
water to:
1) a mechanical separation step;
2) a fluid degassing step;
3) optionally, a second oil removal step;
4) a pH adjustment step, if necessary;
5) an electrocoagulation step;
6) an addition of chemical step for solids removal;
7) a third mechanical separation and oil removal step;
8) a multimedia filtration step; and
9) optionally, a bag filtration step.
According to another object of the present invention, there is provided a
process for the treatment of
polymer flood water for subsequent reuse for SAGD or CSS thermal water systems
thereof, said process
comprising subjecting the water to:
1) a mechanical separation step;
2) a fluid degassing step;
3) optionally, a second oil removal step;
4) a pH adjustment step, if necessary;
5) an electrocoagulation step;
6) an addition of chemical step for solids removal;
7) a third mechanical separation and oil removal step;
8) a multimedia filtration step;
9) optionally, a bag filtration step;
10) an addition of chemical for additional hardness removal;
11) a reverse osmosis step; and
12) optionally, an evaporation step to reduce waste volumes.
2
CA 02848442 2014-04-08
According to yet another aspect of the present invention, there is provided a
process for the treatment of
polymer flood water for use in alkaline surfactant polymer (ASP) alkaline
surfactant brine polymer
(ASBP) flood water, said process comprising subjecting the water to:
1) a mechanical separation step;
2) a fluid degassing step;
3) a second oil removal step;.
4) a multimedia filtration step comprising the addition of chemicals for fluid
viscosity
reduction, and coagulation of particles present;
5) a step of chemical addition for solids removal;
6) optionally, a fluid shearing step;
7) optionally, a water softening step by pumping the water through ion
exchangers;
8) optionally, a bag filtration step; and
9) optionally, a chemical addition step to adjust the conductivity with brine.
According to yet another aspect of the present invention, there is provided a
process for the treatment of
polymer flood water, said process comprising subjecting the water to:
1) a blending step with at least another water from a different source;
2) a mechanical separation step;
3) a fluid degassing step;
4) optionally, a second oil removal step;
5) a pH adjustment step, if necessary;
6) an electrocoagulation step;
7) an addition of chemical step for solids removal;
8) a third mechanical separation step and oil removal step;
9) a multimedia filtration step; and
10) optionally, a bag filtration step.
Preferably, the fluid degassing step is performed by using a double loop gas
bubbler. More preferably, the
double loop gas bubbler comprises apertures facing downward at an angle of 45
.
Preferably, the multimedia filtration step is performed by using a ceramic
media such as Macrolite .
3
CA 02848442 2014-04-08
Preferably, the mechanical separation steps are performed through the use of a
cone bottomed tank or any
other mechanical separation equipment such that is equipped with an oil
skimmer at the top of the tank or
overflow or standpipe.
Preferably, the solids removal step through the addition of chemicals such as
coagulant or flocculant.
Preferably, the second oil removal step comprises the use of a single or two
stage polymer packing vessel
for oil adsorption such as Mycelx .
Preferably, according to the process of the present invention, the treated
polymer flood water for reuse in
polymer flood water has the following specification:
Element Water Spec '
. ,
pH 8.5¨ 10.5
Calcium <20 ppm
Magnesium 100 ¨ 220 ppm
Total Hardness as CaCO3 400 ¨ 800 ppm
TDS 15000 - 25000 ppm
H2S <50 ppm
02 < 50 ppb
Sulphide <60 ppm
Sodium 6000 - 9000 ppm
Total alkalinity 1500¨ 2500 ppm
Turbidity <100 NTU
TSS <250 ppm
Iron <1 ppm
=
Preferably, according to the process of the present invention, the treated
polymer flood water specification
shown above for reuse in polymer flood water can be recycled and retreated
until the TDS reaches the
desired spec target then one can add additional equipment to create the
following SAGD or CSS thermal
water specification for steam flooding:
4
CA 02848442 2014-04-08
Element Wafer spec
PH 8.5 ¨ 10.5
Calcium <0.1 ppm
Magnesium <0.1 ppm
Total Hardness as CaCO3 <0.5 ppm
TDS < 12000 ppm
H2S Oppm
02 < 10 ppb
Sulphide n/a
= Sodium <9000 ppm
Total alkalinity <700 ppm
Turbidity <2 NTU
TSS <1 ppm
Iron <0.5 ppm
Preferably, according to the process of the present invention, the treated
polymer flood water for reuse in
alkaline surfactant polymer (ASP) or alkaline surfactant brine polymer (ASBP)
has the following
specification:
CA 02848442 2014-04-08
Elpmenr. , - Water Spec
PH 7.5 ¨ 13.0
Calcium <10 ppm
Magnesium <10 ppm
Total Hardness as CaCO3 30 - 70 ppm
TDS 8000 - 25000 ppm
H2 S 0 ppm
02 < 50 ppb
Sodium <8500 ppm
Total alkalinity <5000 ppm
Turbidity <10 NTU
TSS <20 ppm
Iron <1 ppm
Preferably, the process further comprises a step of polymer mixing and aging.
More preferably, the
process further comprises the use of a nitrogen blanket during the step of
polymer mixing and aging.
More preferably, the process comprises a step of addition of water to hydrate
the polymer, said water is
selected from the group consisting of: fresh water, treated saline water,
treated produced water and treated
blends of the waters.
Preferably, the process further comprises a step of tank gas bubbler,
electrocoagulation, and multimedia
filtration of the produced water, and fresh water hydration of the polymer
mixing solution.
Preferably, the treated water has the following specification: pH >8.5 and
<10.5 9.0; H2S <50 ppm and
02 < 50 ppb; TSS (total suspended solids) <250 ppm; and calcium ion <20 ppm.
Preferably, dependent on the water or polymer flood fluid characteristics, the
alkaline surfactant polymer
'15 (ASP) may be converted to an alkaline surfactant brine polymer where
brine is used instead of some of
the alkaline chemical to raise the fluid stream conductivity thus reducing the
chemical costs for the
mixture.
6
CA 02848442 2014-04-08
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 is a schematic representation of the process according to a preferred
embodiment of the present
invention where the produced water is treated to be reused as polymer flood
water.
Figure 2 is a schematic representation of the process according to a preferred
embodiment of the present
invention where the produced water is treated to be reused in steam assisted
gravity drainage operations.
Figure 3 is a schematic representation of the process according to a preferred
embodiment of the present
invention where the produced water is treated to be reused in alkaline
surfactant brine polymer flood
water.
DETAILED DESCRIPTION OF THE INVENTION
The process according to the present invention is intended for use in treating
various used waters
reclaimed from operations in the oil industry, more specifically, polymer
flood, SAGD and CSS thermal
flood, alkaline surfactant polymer flood, and alkaline surfactant brine
polymer flood waters, for their
subsequent reuse.
Polymer Flood Water Treatment
The polymer flood water treatment unit according to an embodiment of the
present invention, may
comprise an inlet mixing/solids tank with a gas bubbler; an elec.
trocoagulation unit; a solids
removal/handling system; one or more multimedia filtration units; and one or
more chemical injection
systems.
The polymer flood water treatment unit according to another embodiment of the
present invention, may
comprise an inlet mixing/solids tank (with or without a gas bubbler); an
electrocoagulation unit; a solids
removal/handling system; one or more multimedia filtration units; and one or
more chemical injection
systems.
One advantage of the process according to the present invention is the removal
of the residual recycled
polymer from the produced water stream. Another advantage is that the pH of
the water is adjusted to the
optimal range for each polymer to become viscous. Yet another advantage is the
removal of H2S from the
polymer produced and makeup water streams. H2S and 02 have a substantial
impact on polymer
7
CA 02848442 2014-04-08
degradation. Moreover, there is improved safety and handling of the water
system, i.e. safer for operations
when there is no H2S venting from the, plant polymer injection equipment.
Another advantage of the
process according to the present invention entails selective ion removal from
water streams to reach the
desired water specification. There is also bacteria removal from water, since
bacteria consume polymer
that is added to the flood water. The use of CDG gels require no bacteria if
gels were to be used in the
future instead of polymers. It is worthy of mention that the process according
to the present invention
allows for the removal of oil and grease residue from water as well as
reducing the total dissolved solids
(TDS) of the water each time it is processed. The intent is to have lower TDS
in the water stream for
future SAGD or CSS thermal water requirements. An advantage according to one
aspect of the present
invention is that benign water is created which, in turn, leads to savings on
materials for construction of
pipelines and polymer hydration and injection facilities. Other advantages
include the creation of a stable
polymer created when using a treated water stream; solids removal from water
streams - incoming solids
from makeup waters i.e. grosmont solids handled at one location versus the
multiple solids deposition
locations; and ability to blend the polymer produced water and makeup water
streams prior to treatment
system ¨ optimized with mixing.
The H2S and 02 reaction consumes polymer very aggressively and we found that
by reducing or removing
the H2S from the fluid this reaction does not occur so rapidly, therefore one
is capable of reducing the
amount of polymer usage with lower H2S in the fluid.
Another advantage of the treatment process according to an embodiment of the
present invention is the
reduction ranging up to 850 to 1000 ppm of polymer required for flood water.
Another advantage of the treatment process is the removal or deactivation of
the NORMS (naturally
occurring radioactive materials) that are present in the sour saline grosmont
water stream. The treatment
process removes the norms from the water precipitating with the solids sludge
stream that is created. This
makes the effluent treated water stream safer for handling for operations and
will decrease the norms
contamination levels of the downstream equipment. This also makes the sludge
disposal costs cheaper as
it costs 6 times more to dispose of NORMS contaminated sludge.
Polymer Flood Water Specification
The water specification for polymer hydration was determined through field
pilot scale testing at a rate of
275 m3/day.
8
CA 02848442 2014-04-08
One of the benefits of having determined a polymer flood water specification
is to optimize the polymer
consumption to meet the desired viscosity targets with the least amount of
polymer use. Another benefit is
the determination of optimal pH range for the polymer to function most
efficiently. It also allows the
analysis of other water sources and the determination of the most appropriate
water treatment process
required to allow the water to be used in the polymer systems. Further, it
allowed the determination of the
factors having the greatest impact on polymer loading, such as calcium
content, pH, H2S, 02, and solids
content. An advantage of having determined a polymer flood water specification
allowed reaching a
reduction in polymer usage ranging from 850 to 1000 ppm for floodwater uses.
Polymer Flood Water Pilot
in a pilot trial that was conducted, the five (5) main water streams were
tested in multiple equipment
configurations to achieve electrocoagulation and filtration/chemical treatment
during the pilot were Grand
Rapids, Quaternary, Sour Saline Grosmont, North Brintnell 7-27 produced water
and a 50/50 blend of the
sour saline grosmont and produced water streams. Polymer 'loading prior to the
implementation of
embodiments according to the present invention averaged 2200 ppm.
The pilot allowed to determine a water specification for polymer flooding
activities and helped in finding
a more economical water treatment process that provided lower polymer loading.
Elemental analytical
results from the electrocoagulation testing were analyzed to determine the
impact of each element on the
polymer loading.
The following desired or preferred water specification for polymer floodwater
was determined as a result
of the pilot conducted:
9
CA 02848442 2014-04-08
' Element Water Spec
pH 8.5 ¨ 10.5
Calcium <20 ppm
Magnesium 100 ¨ 220 ppm
Total Hardness as CaCO3 400 ¨ 800 ppm
TDS 15000 - 25000 ppm
142S <50 ppm
02 < 50 ppb
Sulphide <60 ppm
Sodium 6000 - 9000 ppm
Total alkalinity 1500 ¨2500 ppm
Turbidity <100 NTU
TSS <250 ppm
Iron < 1 PPm
For the Brintnell waters tested, it was determined that the following
parameters impacted the polymer
loading the most:
- pH 5_ 9.0 or pH > 10.5, had an impact of about 200 - 300 ppm
polymer loading
increase
H2S and 02 reaction, - H2S > 50 ppm and 02> 50 ppb, had an impact of about 400
-
800 ppm polymer loading increase
- solids - TSS (total suspended solids) > 250 ppm, had an
impact of up to 500 ppm in
polymer loading increase
- calcium ion > 20 ppm, had an impact of up to 400 - 500 ppm in polymer
loading
increase.
- Total hardness level of 0 ppm (no calcium or magnesium
present) ¨ increased the
polymer loading by 200¨ 300 ppm.
From the trial results, it was determined that tank gas bubbler followed by
electrocoagulation (EC) water
treatment process then followed by multimedia filtration (MMF) provided
optimal efficiency with respect
to polymer loading in comparison to all other configurations. The combination
of tank gas
CA 02848442 2014-04-08
bubbler/EC,/MMF decreased polymer loading up to 1050 ppm range on all waters
tested, when all fluids
were adjusted to a pH range of 9.0 - 9.5.
Three other process steps resulted in improved polymer loading. The savings
noted for each individual
process enhancement cannot be necessarily combined for cumulative savings.
These three other processes
involved a gas bubbler, a nitrogen blanket, and fresh water for mother
solution hydration. The utilization
of a gas bubbler in the water inlet tank to degas out the gases H2S and CO2
from the water resulted in
polymer loading savings of up to 400 ppm. The use of a nitrogen gas blanket on
the polymer mixing and
aging tank in polymer injection skid resulted in polymer loading savings of up
to 300 ppm. The use of
fresh water to hydrate the polymer mother solution resulted in an additional
polymer loading savings of
up to 300 ppm.
Cumulatively, when creating the overall required polymer water specification
mixture for injection, the
testing found that one could also use treated water blended with some fresh
water (with tank gas
bubbler/EC/MMF treated water being used for the blend water and fresh water
being used for polymer
mother solution hydration) resulted in an additional 175 ppm in polymer
savings - from 1050 ppm down
to 875 ppm polymer loading
A separate system containing only filtration and chemicals was also tested for
comparison to the tank gas
bubbler/electrocoagulation/multimedia filtration unit. Filtration and
chemicals provided polymer
reduction but this reduction was lower at around 400 ppm. Although this
alternate system was very
effective as the filtration and chemical treatment utilizing ceramic Macroute
media with chlorine and
sulphite added were able to break up and remove the oil and grease, polymer,
and solids from the waters
effectively and reduced turbidity of the waters.
Additionally, for direct comparison to the electrocoagulation unit, the use of
Dow RSC resin was tested to
see if could remove NORMS with the resin product in a filter vessel. The Dow
resin tested allowed for
the reduction of radium levels in the waters by 36 to 59 % removal of inlet to
outlet stream.
The process according to the present is described with reference to specific
embodiments illustrated in
Figures 1 ¨3.
11
CA 02848442 2014-04-08
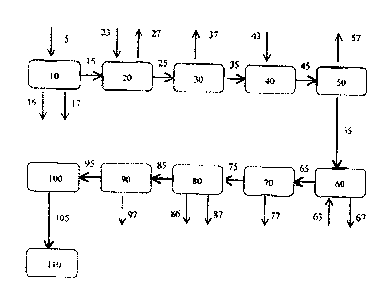
Example 1 - Polymer Flood Water Treatment Process
A preferred embodiment of the present invention relates to the treatment of
polymer flood water used in
oilfields. It will be better understood by referring to Figure 1. There is
provided a process where:
1) Polymer flood water flows (5) into a cone bottomed storage tank (10) (or
other mechanical
separation equipment which may be equipped with an oil skimmer at the top of
the tank or
overflow or oil removal standpipe) where the solids (16) are removed from
fluid as needed; and
the oil (17) is skimmed off of the storage tank (10) as needed.
2) When the resulting fluid (15) shows signs of being sour (H2S is present),
it flows into a tank
which is equipped with a double loop square gas bubbler inside (20) and gas
(23) (like natural
gas) is bubbled into the storage tank fluid reservoir as the fluid flows
in/out of the tank (20). This
permits the stripping out of H2S and other gases (27) present in the fluid.
Natural gas volume is
added at a 1 to 1 ratio to the fluid offgas volume.
3) If the resulting fluid (25) requires additional oil removal prior to
water treatment then a single or
two stage polymer packing vessel (30) for oil adsorption (37) are used (like
Mycelxe).
= 4) If the resulting fluid's (35) oil and grease level is sufficient, then
the fluid (35) is pumped and
undergoes a pH adjustment (40)(if necessary) where the pH is raised in the
fluid by adding a
chemical (like caustic - sodium hydroxide (43)).
5) The fluid (45) is then sent through an electrocoagulation unit (50) ¨ a
closed cell design (like
Waveionics ) this prevents gases from being released into the atmosphere
during the step. The
electrocoagulation step consists of metal plates with electrodes that are
electrified as the fluid
passes through the cell. During the step of electrocoagulation, the metal
plates are consumed and
the metal precipitates with the water solids (57).
6) Subsequently, there is another step of chemical addition (60) where
additional chemicals (63) like
caustic and coagulant, are added to the fluid (55) to assist with solids
removal (67) by further
raising the pH and promoting precipitation or agglomerating the particles
7) If necessary, the fluid (65) undergoes another step of bulk solids removal
stage (70Xwith a cone
bottomed tank and/or solids clarifier) is performed with oil recapture (77) if
applicable.
8) The resulting fluid (75) is sent to a cone bottomed tank (80) for surge
volume and additional
solids removal (86) and oil capture (87), if applicable.
9) The resulting fluid (85) is then pumped through multimedia filtration
(90)(like ceramic media
such as Macrolite ) in single or double filtration stages removing solids, oil
and polymer (97).
12
CA 02848442 2014-04-08
10) If fine micron particle size is required then the fluid (95) is sent
through bag filtration units (100)
in single or double follows the multimedia filtration with filtration bags
(such as 3M DuoFLO
followed by absolute 3M pillow bags)
11) The resulting fluid (105) is then sent into storage tanks (110) for
further use.
12) From the storage tank the treated fluid may be pumped and sent to a main
blend line and may also
be sent to the polymer mixing system.
13) A polymer mixing system is typically used to create a thick mother
solution and utilizes a
softened fresh, raw fresh or treated produced water supply for the hydration
of the polymer prior
to being blended into the main blend fluid stream.
14) The combined polymer water and blend water is then mixed to the desired
viscosity and is
injected into the wellbore.
It is preferable to use solids capture and separation system (such as, but not
limited to, cone bottom tanks)
so that solids can be removed from the water during the process.
The treated water to be used from storage tanks to send backwash water to the
filtration units and water
treatment as required must preferably meet the desired backwashing and water
properties for treatment.
Preferably, gas blanketing is desired on the process tanks and vessels to
ensure that there is no oxygen
ingress into the fluid.
A tank vapour recovery system is preferred to capture the offgases from the
process.
A tank gas bubbler as used in step 2) of the treatment process above (and in
examples 2 and 3) was used
in the inlet tank to degas the gases from the water requiring treatment.
The tank square double loop gas bubbler used in the treatment of polymer flood
water was made of linear
tubing the loops overlapping each other and positioned in an horizontal plane,
comprising holes
positioned to be at a 450 downward angle towards the walls of a tank in which
it is inserted. It has been
determined by the inventor that the above specification would allow for the
optimal removal, from the
waters to be treated, of H2S present and other gases which have deleterious
effects on polymers used in
polymer flood waters.
13
CA 02848442 2014-04-08
The tank gas bubbler used in the treatment of polymer flood waters allows to
effectively remove the 112S
from the grosmont and produced waters which, in turn, improves the polymer
loading for subsequent
polymer flood treatment. This leads to savings in polymer usage to meet
viscosity target.
The tank gas bubbler can be used for any water fluid requiring H2S removal
from system ¨ and it greatly
improves the downstream safety of fluid handling with reduced H2S levels.
Another advantage of the tank
gas bubbler is that the installation is simple and cost efficient and can be
adapted to accommodate wide
ranges of water : gas rates. The piping sizes can vary when used in this
design to meet the rigorous
process conditions and be adapted for any tank size. It is worth noting that
the process controls based on
water flow to gas flow rates ratio control program.
The use of a tank gas bubbler can lead to reductions in polymer usage for
subsequent polymer flooding
activities ranging from 100 to 400 ppm when conducting polymer flood water
operations.
The spacing between apertures on the tubing and the size of the apertures is
dependent on the tank size
(i.e. total volume) as well as the type of liquid being treated (i.e. the
content of gas to be extracted) and
the flow rate of the gas being used in the operation. =
Example 2 ¨ Steam Assisted Gravity Drainage (SAGD) or Cyclic Stimulation Steam
(CSS) Thermal
Water from Polymer Flood Water Treatment
If the TDS of the polymer flood returns water has reduced to the desired
levels after treating the fluids
with the process discussed in Example 1 then additional equipment can be added
downstream of the
process to make the water acceptable for thermal steam flood usage. The
desired or preferred water
specification for thermal steam flood usage is set out below:
Element ' - Water Spec '
pH 8.5 ¨ 10.5
Calcium <0.1 ppm
Magnesium <0.1 ppm
Total Hardness as CaCO3 <0.5 ppm
TDS < 12000 ppm
14
CA 02848442 2014-04-08
H2S 0 ppm
02 < 10 ppb
Sulphide n/a
Sodium <9000 ppm
Total alkalinity <700 ppm
Turbidity <2 NTU
TSS <1 ppm
Iron <0.5 ppm
According to a preferred embodiment of the process of the invention, there is
provided a process to
prepare Steam Assisted Gravity Drainage (SAGD) or Cyclic Steam Stimulation
(CSS) thermal water from
polymer flood water treatment. It will be better understood by referring to
Figure 3. The process
comprises the following steps where:
1) Process fluid (5) recovered from polymer flood activities flows into a cone
bottomed storage tank
(10) (or other mechanical separation equipment equipped with an oil skimmer at
the top of the
tank) where the solids (16) are removed from fluid as needed; and oil (17) is
skimmed off from
the storage tank as needed.
2) When the resulting fluid (15) shows signs of being sour (H2S is present),
the tank is equipped
with a double loop square gas bubbler inside (20) and gas (23Xlike natural
gas) is bubbled into
the storage tank fluid reservoir as the fluid flows in/out of the tank. This
permits the stripping out
the H2S and other gases (27) present in the fluid. Natural gas volume is added
at a 1 to 1 ratio to
the fluid offgas volume.
3) Then, follows a step of oil removal (30) prior to water treatment ¨ fluid
(25) flows through a
single or two stage polymer packing vessel(s) for oil adsorption/coalescence
(37) if such step is
necessary required (like Myceix ) there is recovery of an oil stream.
4) If the resulting fluid's (35) oil and grease level is sufficient, then
the fluid (35) is then pumped and
the pH is raised in the fluid by adding a chemical (like caustic - sodium
hydroxide (43)) if pH
adjustment (40) is needed.
5) The fluid (45) is then sent through an electrocoagulation unit (50) ¨ a
closed cell design (like
Waveionics ) this prevents gases from being released into the atmosphere
during the step. The
electrocoagulation step consists of metal plates with electrodes that are
electrified as the fluid
CA 02848442 2014-04-08
passes through the cell. The metal plates are consumed and the metal
precipitates with the water
solids (57).
6) Then, what follows is another step of chemical addition (60) where
additional chemicals (63) like
caustic and coagulant, are added to the fluid (55) to assist with solids
removal (67) by further
raising the pH and promoting precipitation or agglomerating the particles
7) Then, a chemical addition step is performed (370) where a chemical (like
phosphate or lime)(373)
is added to the fluid (65) to remove additional hardness (calcium and
magnesium) (377) not
removed by the electrocoagulation step above.
8) The fluid (375) then undergoes a bulk solids removal stage (380)(through
the use of a tank like a
cone bottomed tank and/or solids clarifier) where solids (386) are removed and
oil (387) is
recaptured, if applicable.
9) If necessary, the fluid (385) undergoes another bulk solids removal
stage (390Xthrough the use of
a tank like a cone bottomed tank and/or solids clarifier) where solids (396)
are removed and oil
(397) is recaptured.
10) The resulting fluid (395) is then pumped through multimedia filtration
step (400)(like ceramic
media Macrolite ) in single or double filtration stages
11) If fine micron particle size desired is not attained, then the fluid (405)
is sent through bag
filtration units (410) in single or double with filtration bags (like the
nominal 3M DuoFLO
followed by absolute 3M pillow bags).
12) After filtration, the resulting fluid (415) will undergo a double pass
reverse osmosis (420) which
is performed with membranes in series. The waste stream, concentrated RO
reject, from the RO
system then needs to go to an evaporator to remove the contaminants, like
alkalinity and silica,
and reduce the overall waste volume. The resulting fluid (425) then flows into
storage tanks
(430) for future usage such as to make steam.
Alkaline Surfactant Polymer (ASP) or Alkaline Surfactant Brine Polymer (ASBP)
Flood Water Treatment
The following desired or preferred water specification for alkaline surfactant
polymer (ASP) or alkaline
surfactant brine polymer (ASBP) flood water was determined from laboratory
small scale fluid testing
and was further implemented onsite:
16
CA 02848442 2014-04-08
Element . Water Spec':
pH 7.5 ¨ 13.0
Calcium <10 ppm
Magnesium <10 ppm
Total Hardness as CaCO3 30 - 70 ppm
TDS 8000 - 25000 ppm
112S Oppm
02 < 50 ppb
Sodium <8500 ppm
Total alkalinity <5000 ppm
Turbidity <10 NTU
TSS <20 ppm
Iron <1 ppm
The site produced water was treated with oil removal system and water
treatment system as listed below
in Example 3.
The goal was to confirm the water specification for alkaline surfactant
polymer (ASP) or alkaline
surfactant brine polymer (ASBP) flooding activities determined in laboratory
and help in finding a more
economical water treatment process. It is estimated that the use of a process
of treating ASP or ASBP
polymer flood produced water prior to its reuse in for the same purpose can
yield reductions ranging from
200 to 400 ppm in the polymer usage on large scale projects.
Alkaline Surfactant Polymer (ASP) or Alkaline Surfactant Brine Polymer (ASBP)
Flood Water Spec
The alkaline surfactant polymer (ASP) or alkaline surfactant brine polymer
(ASBP) water specification
for polymers for hydration was determined through field pilot scale testing at
a rate of 450 m3/day.
According to an embodiment of the present invention, the alkaline surfactant
polymer (ASP) or alkaline
surfactant brine polymer (ASBP) flood water treatment unit comprises: a tank
gas bubbler, a Mycelx oil
water separator; a Mycelx backwash vessel; at least one multimedia filtration
unit (more preferably, in
17
CA 02848442 2014-04-08
double train of dual multimedia filter vessels in series); a double train
primary/polisher strong acid cation
ion exchange vessels with brine and caustic reagent step, and one or more of
chemical injection systems.
Some advantages of using the process according to the present invention for
the preparation of an
alkaline surfactant polymer (ASP) or alkaline surfactant brine polymer (ASBP)
flood water include; the
removal of H2S and other gases like CO2 by the tank gas bubbler (optional);
the removal and recovery of
oil from the ASP or ASBP polymer produced water stream and the creation of a
sales oil stream with the
MyceIxe green polymer packing technology vessels (OWS and BW) - revenue from
sales oil stream; the
removal of the solid particles from the water stream with filtration; the
removal of the hardness from the
water with strong acid cation resin exchangers down to 5 ¨ 10 ppm leakage
(designed for some hardness
leakage); the removal of the polymer, silicates, and oil and grease from the
strong acid cation resin and
multimedia filters with the addition of a caustic regeneration cycle step ¨ to
remove key foulants of other
ASP polymer flood systems resins and medias; the savings on polymer loading,
facilities and downhole
scaling of lines and injection wells which translates into less downtime and
less wellbore workover costs;
and the reduced cost of softening ¨ i.e. SAC/SAC regeneration with caustic
step added to the brine step is
cheaper than currently used WAC (weak acid cation) regeneration chemicals of
acid and caustic; and the
creation of a liquid waste to be disposed of from strong acid cation ion
exchange softeners versus other
technologies that may create a solids waste and liquid waste to deal with.
It is estimated that the use of a process of polymer flood water according to
the present invention can
yield reduction in 200 to 400 ppm of polymer usage which is substantial given
the cost of polymer and
the amounts of water treated. These savings can amount to several million
dollars yearly on a large scale
project.
By having an optional chemical addition step at the end of the water treatment
process one can adjust the
conductivity of the fluid with brine to reduce amount of alkaline required for
best surfactant activity.
The way to pretreat ASP or ASBP polymer flood water according to an embodiment
of the present
invention, allows one to utilize produced water for polymer mixing and
reinjection versus disposal and
using makeup waters.
Example 3 ¨ Alkaline Surfactant Polymer (ASP) or Alkaline Surfactant Brine
Polymer (ASBP) Flood
Water Treatment
18
CA 02848442 2014-04-08
According to another preferred embodiment of the process of the invention,
there is provided a process to
prepare an alkaline surfactant polymer (ASP) or an alkaline surfactant brine
polymer (ASBP) flood water.
It will be better understood by referring to Figure 2. The process comprises
the following steps where:
1) Process fluid (5) recovered from polymer flood activities flows into a cone
bottomed storage tank
(10) (or other mechanical separation equipment equipped with an oil skimmer at
the top of the
tank) where the solids (16) are removed from fluid as needed; and oil (17) is
skimmed off from
the storage tank as needed.
2) When the resulting fluid (15) shows signs of being sour (H2S is present),
the tank is equipped
with a double loop square gas bubbler inside (20) and gas (23Xlike natural
gas) is bubbled into
the storage tank fluid reservoir as the fluid flows in/out of the tank. This
permits the stripping out
the I-12S and other gases (27) present in the fluid. Natural gas volume is
added at a 1 to 1 ratio to
the fluid offgas volume.
3) Then, follows a step of oil removal (30) prior to water treatment ¨ fluid
(25) flows through a
single or two stage polymer packing vessel(s) for oil adsorption/coalescence
(37) if such step is
necessary (like Mycelx ) ¨ there is recovery of an oil stream.
4) The fluid (35) is then pumped through multimedia filtration (90)(like
ceramic media Macroute )
in single or double filtration stages:
a. Oxidant Chemical (91)(like bleach) is added upfront of filters to reduce
fluid viscosity
(destroy the remaining polymer) and to kill bacteria;
b. Coagulating Chemical (92Xlike polyaluminum chloride PAC) is added upfront
of filters
to coagulate particles ¨ which aids in the filtration; and
c. Reducing Chemical (93)(like sulphite) is added in downstream of
first filter (upfront of
second filter) to remove the oxidant chemical residuals (i.e. consume the
bleach, if
present);
d. There is a filter backwash step to include an additional step of addition
of alkaline
chemical (94)(like caustic) for polymer, silica, and oil removal from the
filtration media;
5) Then, the fluid (115) passes through a shearing stage (120) of a inline
static mixer followed by a
inline jet nozzle and into a storage tank;
6) Then, the fluid (125) is pumped through anion exchangers (130), two strong
acid cation resin
vessels in series called SAC/SAC,
a. Due to the higher fluid total dissolved solids, the SAC/SAC is designed to
leak from 5
ppm to 10 ppm hardness (calcium and magnesium) in effluent - to not achieve
normal 0
ppm hardness leakage.
19
CA 02848442 2014-04-08
b. Optionally, it has an additional alkaline chemical injection step (like
caustic) as part of
the regeneration cycle ¨ the alkaline chemicals are being utilized to remove
polymer,
silica, and oil from the strong acid cation resin beads.
7) In the event that fine micron particle size is desired, then the fluid
(135) flows through bag
filtration units (100) in single or double will follow the anion exchangers
(130) with filtration
bags (like the nominal 3M DuoFLOe followed by absolute 3M pillow bags)
8) The resulting fluid 145 is then sent into a storage tank (140).
9) From the storage tank (140), the fluid (145) is pumped and chemicals 143
(like caustic,
surfactant, brine, and polymer) are added to create the required alkaline
surfactant polymer (ASP)
or alkaline surfactant brine polymer (ASBP) mixture (155) to be injected
downhole. In alkaline
surfactant brine polymer (ASBP), the brine is added to increase the
conductivity and reduce the
alkaline volume required for the overall alkaline surfactant brine polymer
mixture.
10) A polymer mixing system (150) is used to create a thick mother solution
and utilizes a softened
fresh (175), raw fresh or treated produced water supply (5) for the hydration
of the polymer (165)
prior to being blended into the main chemical fluid stream (155).